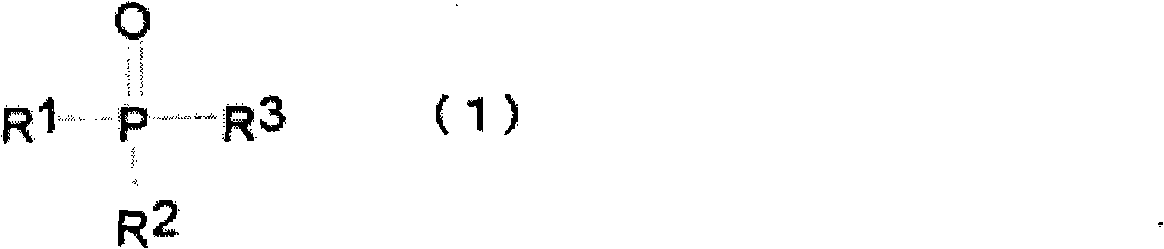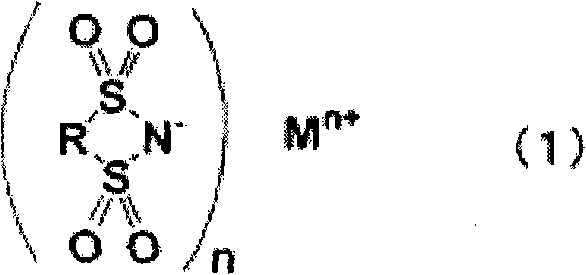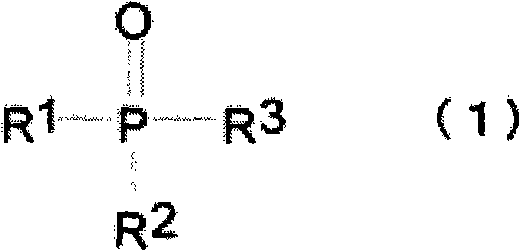Nonaqueous electrolyte for rechargeable battery, and rechargeable battery with nonaqueous electrolyte
A non-aqueous electrolyte and non-aqueous electrolyte technology, which is applied in non-aqueous electrolyte batteries, non-aqueous electrolytes, secondary batteries, etc., and can solve problems such as high resistance and reduced discharge load characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1023]
[1024] The non-aqueous electrolytic solution 4 of the present invention is prepared by dissolving an electrolyte, a cyclic sulfone compound, a "compound having a viscosity coefficient at 25°C of 1.5 mPa·s or less", a specific compound, and optionally added "other compounds" . When preparing the non-aqueous electrolytic solution 4, it is preferable to dehydrate each raw material in advance in order to reduce the water content when preparing the electrolytic solution. Usually, it can be dehydrated to 50 ppm or less, preferably 30 ppm or less, particularly preferably 10 ppm or less. In addition, dehydration, deacidification treatment, etc. may be performed after preparing the electrolytic solution.
[1025] In a nonaqueous electrolyte battery, the nonaqueous electrolyte solution 4 of the present invention is preferably used as an electrolyte solution for secondary battery applications, such as lithium secondary battery applications. Next, the non-aqueous electrolyte ...
Embodiment approach 5-1
[1043] The non-aqueous electrolytic solution 5 of the present invention contains a lithium salt and a non-aqueous organic solvent in which the lithium salt is dissolved. The non-aqueous organic solvent contains a cyclic polyamine compound and / or a cyclic polyamide compound, and further contains at least one compound selected from unsaturated carbonates, fluorine-containing carbonates, monofluorophosphates, and difluorophosphates . Let this be "Embodiment 5-1".
[1044] [1. Cyclic polyamine compound]
[1045] [1-1. Type]
[1046] The cyclic polyamine compound contained in the non-aqueous electrolytic solution 5 of the present invention (hereinafter, appropriately referred to as "the cyclic polyamine compound of the present invention 5") is a cyclic compound having a structure in which amines are condensed and derivatives thereof. thing. That is, a cyclic compound in which a plurality of nitrogen atoms are bonded via an alkylene group, and a derivative in which a hydrogen at...
Embodiment approach 5-2
[1136] In addition, another gist of the present invention 5 is a non-aqueous electrolytic solution containing a lithium salt and a non-aqueous organic solvent in which the lithium salt is dissolved, wherein the non-aqueous organic solvent contains a cyclic polyamine compound and is The liquid contains 5 to 40% by mass of cyclic carbonate. Let this be "Embodiment 5-2".
[1137] [1. Cyclic polyamine compound]
[1138] [1-1. Type]
[1139] Same as above.
[1140] [1-2. Composition]
[1141] Same as above.
[1142] [2. Cyclic carbonate]
[1143] The cyclic carbonate referred to in the present invention 5 is not particularly limited as long as it is a cyclic carbonate, and part or all of its hydrogen atoms may be replaced by halogens such as fluorine and chlorine. To give examples, ethylene carbonate, propylene carbonate, butylene carbonate, fluoroethylene carbonate, difluoroethylene carbonate, fluoropropylene carbonate, trifluoromethylethylene carbonate, etc. . The cyclic ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity coefficient | aaaaa | aaaaa |
| Viscosity coefficient | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com