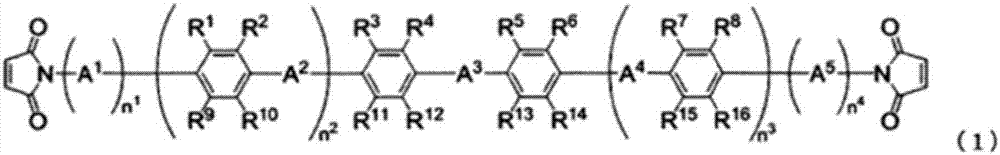
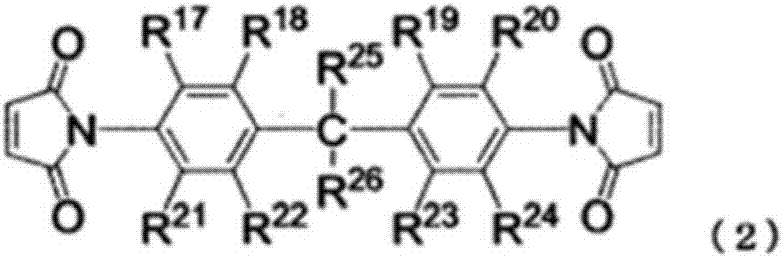
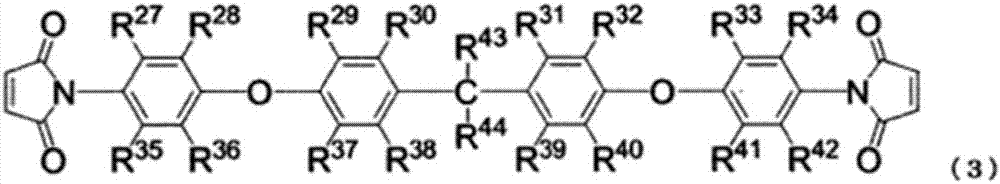
Non-aqueous electrolyte solution, and non-aqueous-electrolyte secondary cell using same
A non-aqueous electrolyte, secondary battery technology, applied in non-aqueous electrolyte batteries, secondary batteries, non-aqueous electrolytes, etc., can solve the problems of battery stability decline, battery capacity reduction, deterioration, etc. Excellent effect of discharge load characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0323] Under a dry argon atmosphere, ethylene carbonate (hereinafter, EC), ethylmethyl carbonate (hereinafter, EMC) and diethyl carbonate (hereinafter, DEC) were mixed so as to be 30% by volume, 40% by volume, and 30% by volume, respectively. volume % to make LiPF 6 Dissolved in the non-aqueous solvent thus mixed, LiPF 6 The concentration was 1.2M, and 2% by mass of vinylene carbonate and 2% by mass of fluoroethylene carbonate were added. Further, 0.3% by mass of Compound 2-10 was added to prepare a non-aqueous electrolytic solution.
Embodiment 2-1
[0344] Under a dry argon atmosphere, ethylene carbonate (hereinafter, EC), ethylmethyl carbonate (hereinafter, EMC) and diethyl carbonate (hereinafter, DEC) were mixed so as to be 30% by volume, 40% by volume, and 30% by volume, respectively. volume % to make LiPF 6 Dissolved in the non-aqueous solvent thus mixed, LiPF 6 The concentration was 1.2M, and 2% by mass of vinylene carbonate, 2% by mass of fluoroethylene carbonate, and 1% by mass of adiponitrile were added. Furthermore, 0.3% by mass of Compound 2-10 was added to prepare a non-aqueous electrolyte solution. Except having used this non-aqueous electrolytic solution, it carried out similarly to Example 1-1, and produced the sheet-shaped battery.
Embodiment 3-1
[0354] Under a dry argon atmosphere, ethylene carbonate (hereinafter, EC), ethylmethyl carbonate (hereinafter, EMC) and diethyl carbonate (hereinafter, DEC) were mixed so as to be 30% by volume, 40% by volume, and 30% by volume, respectively. volume % to make LiPF 6 Dissolved in the non-aqueous solvent thus mixed, LiPF 6 The concentration was 1.2M, and 2% by mass of vinylene carbonate, 2% by mass of fluoroethylene carbonate, and 1% by mass of adiponitrile were added. Furthermore, 0.3% by mass of Compound 2-10 was added to prepare a non-aqueous electrolyte solution. Except having used this non-aqueous electrolytic solution, it carried out similarly to Example 1-1, and produced the sheet-shaped battery.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com