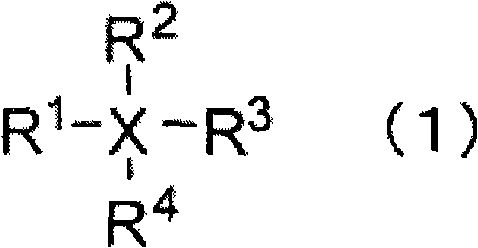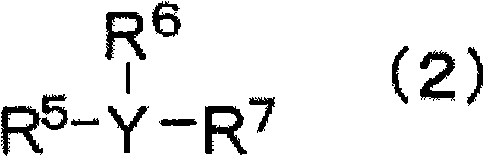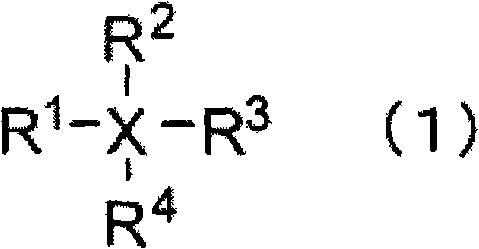Nonaqueous electrolyte, and rechargeable battery with the nonaqueous electrolyte
A non-aqueous electrolyte, non-aqueous electrolyte technology, applied in secondary batteries, circuits, electrical components, etc., can solve problems such as high risk, easy ignition, fire and explosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0339]
[0340] The non-aqueous electrolyte of the present invention 1 can be prepared by dissolving the electrolyte, the "monofluorophosphate and / or difluorophosphate" of the present invention 1, the "iron group compound" and the "other additives" used as required in a non-aqueous solvent. " to prepare.
[0341] When preparing non-aqueous electrolytic solution, it is preferable to use various raw materials of non-aqueous electrolytic solution, i.e. lithium salt, monofluorophosphate and / or difluorophosphate of the present invention 1, iron group compound, non-aqueous solvent, and other auxiliary The agent is dehydrated in advance. The degree of dehydration is generally desirably dehydrated to 50 ppm or less, preferably 30 ppm or less. In addition, the ppm mentioned in this specification means the ratio based on weight basis.
[0342] If water exists in the non-aqueous electrolytic solution, electrolysis of water, reaction of water and lithium metal, hydrolysis of lithium s...
Embodiment 1
[1117] The electrolyte LiPF was dissolved in a mixed solvent of ethylene carbonate (EC) and ethyl methyl carbonate (EMC) (mixing volume ratio 2:8) at a ratio of 1mol / L. 6 The electrolyte is used as the basic electrolyte (I), and lithium difluorophosphate (LiPO 2 f 2 ), and nickel(II) hexafluorophosphate (Ni(PF 6 ) 2 ), so that their concentrations relative to the non-aqueous electrolytic solution are 0.5% by weight and 20 ppm (in terms of the concentration of Ni element, equivalent to 3.4 ppm), and the non-aqueous electrolytic solution is made. Using the obtained nonaqueous electrolyte solution, a nonaqueous electrolyte secondary battery was manufactured according to the above-mentioned method, and capacity evaluation and cycle characteristic evaluation were performed. The results are shown in Table 1.
Embodiment 2
[1119] Lithium difluorophosphate (LiPO 2 f 2 ), and nickel(II) hexafluorophosphate (Ni(PF 6 ) 2 ), so that their concentrations relative to the non-aqueous electrolytic solution are 0.5% by weight and 50ppm (in terms of the concentration of Ni element, equivalent to 8.4ppm), the non-aqueous electrolytic solution is made, and the non-aqueous electrolytic solution is used to make according to the above method Non-aqueous electrolyte secondary batteries, and conduct capacity evaluation and cycle characteristic evaluation. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com