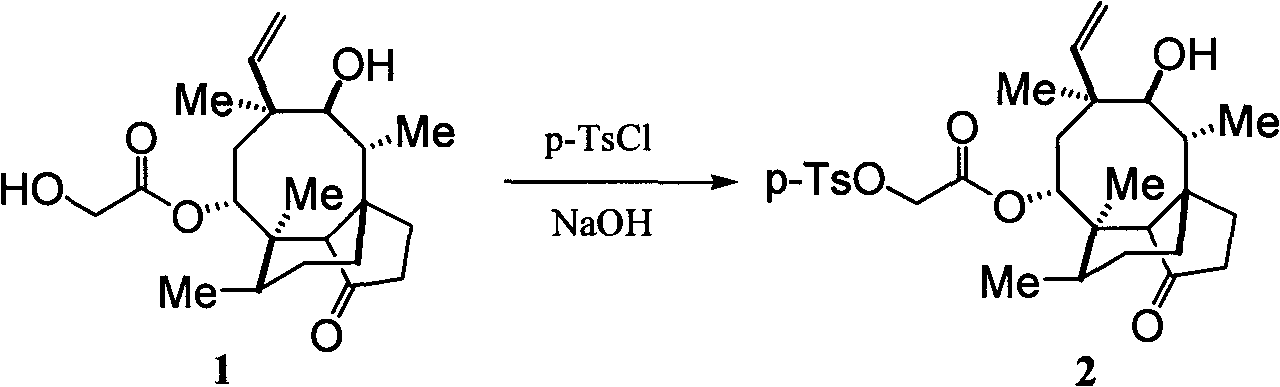Patents
Literature
38 results about "Valnemulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
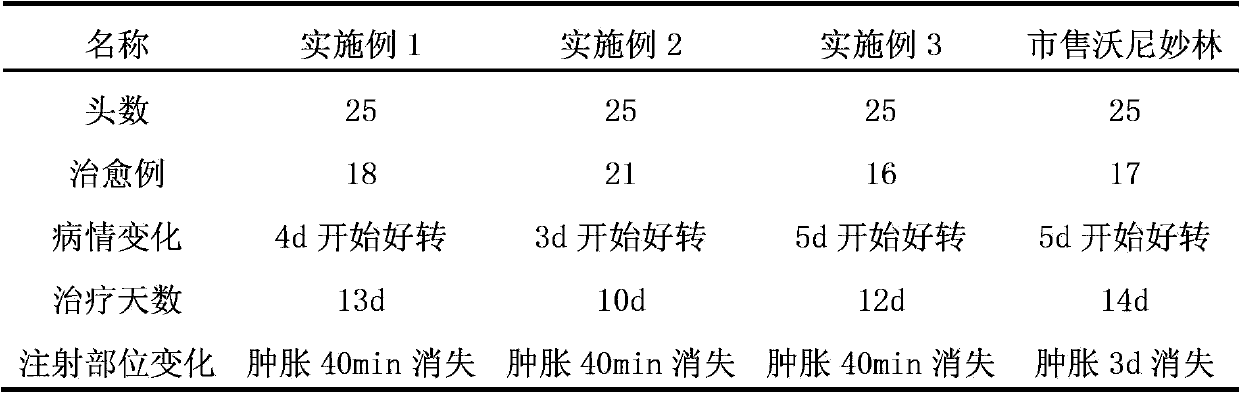
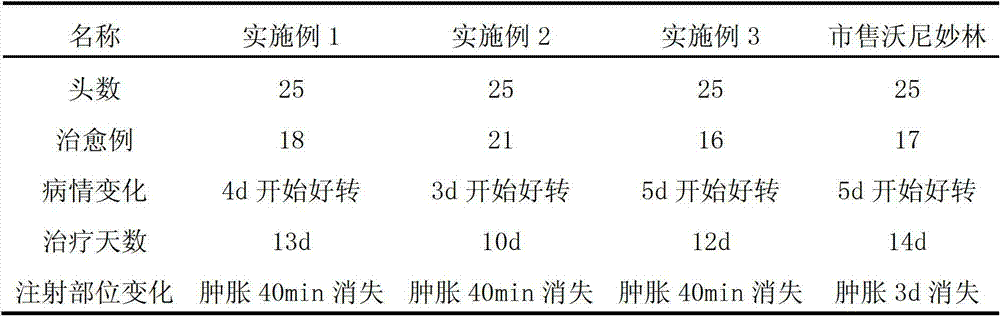
Valnemulin (trade name Econor) is a pleuromutilin antibiotic used to treat swine dysentery, ileitis, colitis and pneumonia. It is also used for the prevention of intestinal infections of swine. Valnemulin has been observed to induce a rapid reduction of clinical symptoms of Mycoplasma bovis infection, and eliminate M. bovis from the lungs of calves.
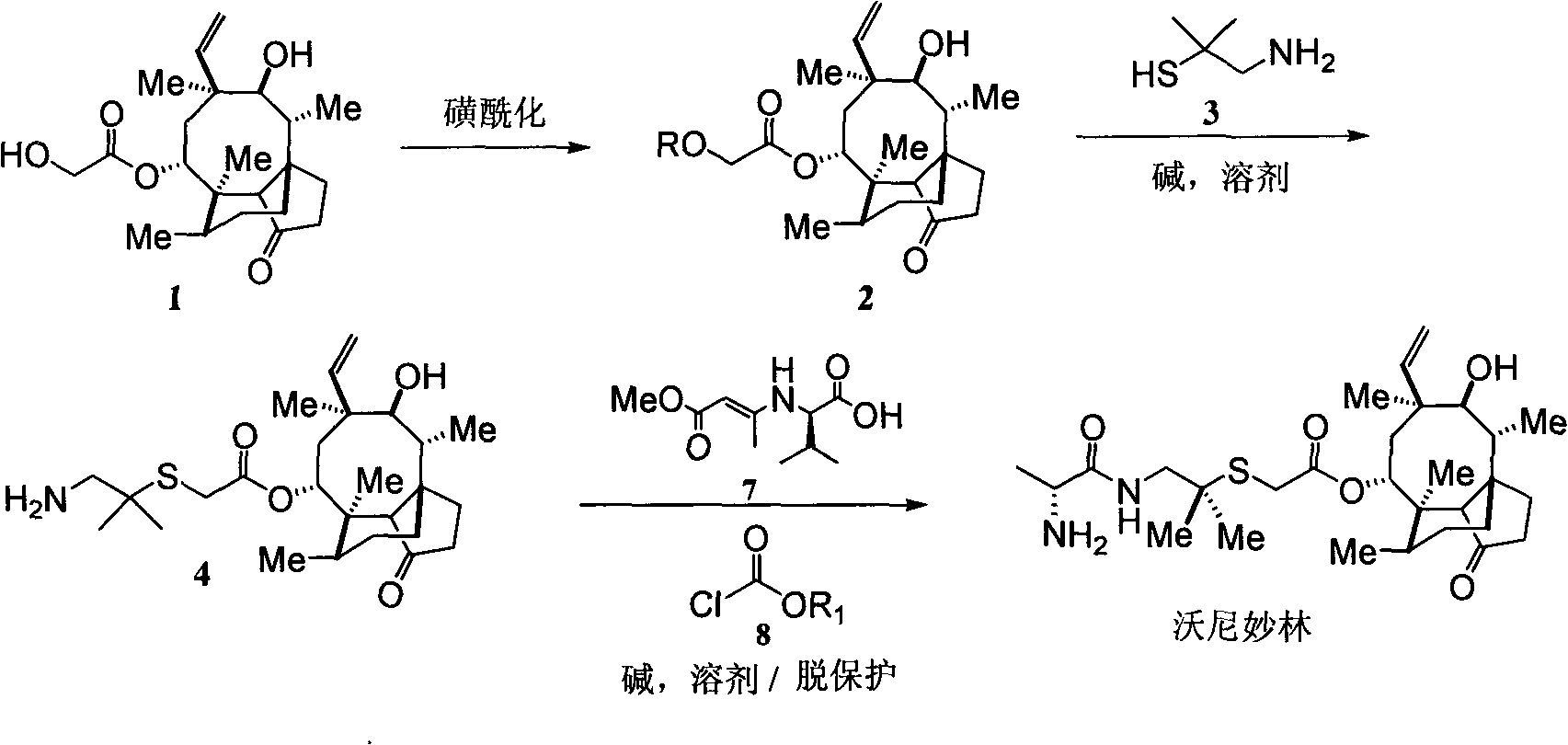
Method for preparing valnemulin
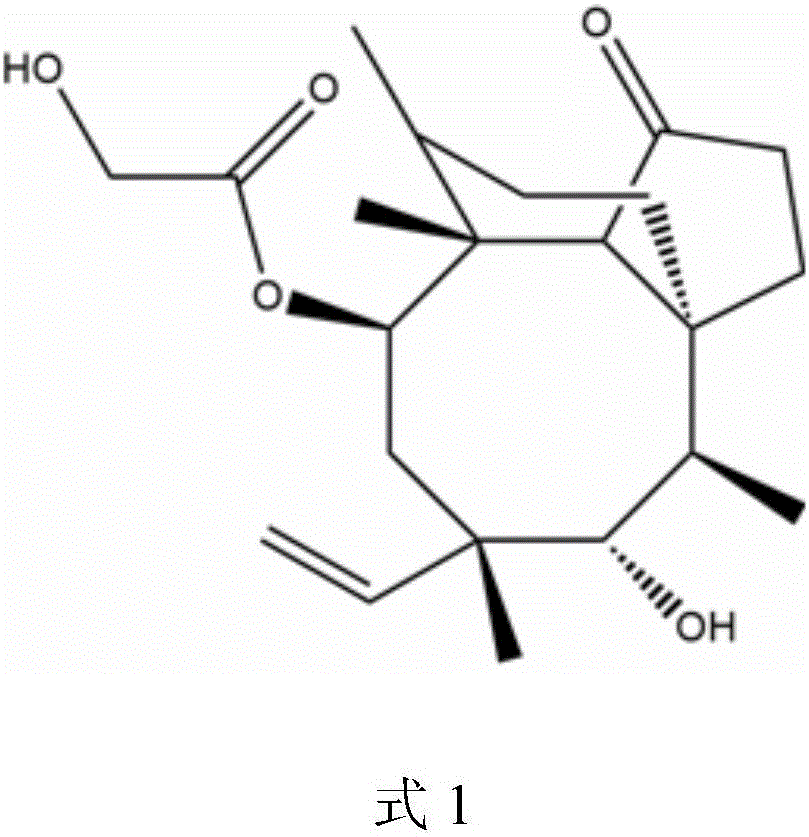
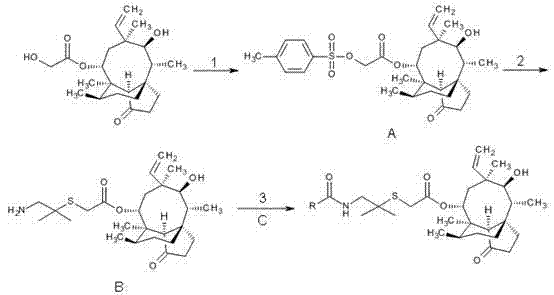
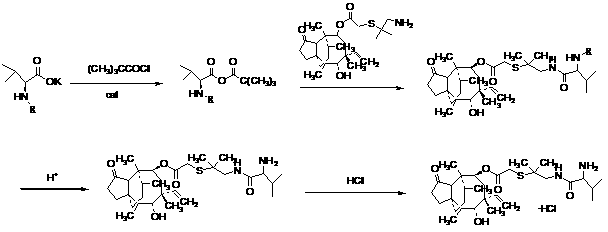
The invention discloses a method for preparing valnemulin. The method comprises the following steps of: preparation of chloridized pleuromutilin, preparation of N-allyloxycarbonyl-valine; preparation of 1, 1-dimethyl-2-(N-allyloxycarbonyl valyl amino) ethanethiol and preparation of the valnemulin. The method can effectively improve the product quality and the yield.
Owner:江苏赛奥生化有限公司
Valnemulin synthesis method
InactiveCN101993400AEasy to operateNot dangerousSulfide preparationBulk chemical productionPenicillamineSynthesis methods
The invention discloses a valnemulin synthesis method which comprises the following steps of: 1. pleuromutilin sulphonic acid ester synthesis: using pleuromutilin as raw materials to react with sulfonic acid chloride under the alkaline condition for converting the pleuromutilin into pleuromutilin sulphonic acid ester; 2. sulpho-pleuromutilin synthesis: using penicillamine to substitute sulphonic acid ester in the product in the step 1 under the alkaline condition to obtain sulpho-pleuromutilin; 3. amino-protected D-R-valine synthesis; and 4. valnemulin synthesis. The mainly used raw materials of the valnemulin synthesis method are fermentation products of the pleuromutilin and cysteine which can be easily obtained in the market and have low cost, and in addition, the valnemulin synthesis method has simple process operation, has no danger, has the characteristics of low cost, high yield and the like, is applicable to enlargement and industrial production and has industrialization value.
Owner:BEIJING ZHONGMU TECH SERVICE
Preparation method of tartaric acid valnemulin premixing agent
InactiveCN102813629ALower metabolismImprove bioavailabilityAntibacterial agentsDigestive systemPoultry diseaseFluidized bed
The invention provides a preparation method of a tartaric acid valnemulin premixing agent, belonging to the field of veterinary antibiotic preparations. The preparation method comprises the following steps of: preparing a coating liquid from a coating material, an anti-sticking agent and purified water; atomizing the coating liquid and spraying the atomized coating liquid into a fluidized bed; coating tartaric acid valnemulin to prepare coated grains; and screening the coated grains and then mixing the screened grains with a diluent, so as to prepare the tartaric acid valnemulin premixing agent. The tartaric acid valnemulin premixing agent prepared by the preparation method is stable in medicine quality, increases the bioavailability of medicines, has outstanding medicine effect, and is mainly used for treating and preventing livestock and poultry diseases.
Owner:HUBEI LONGXIANG PHARMA TECH CO LTD
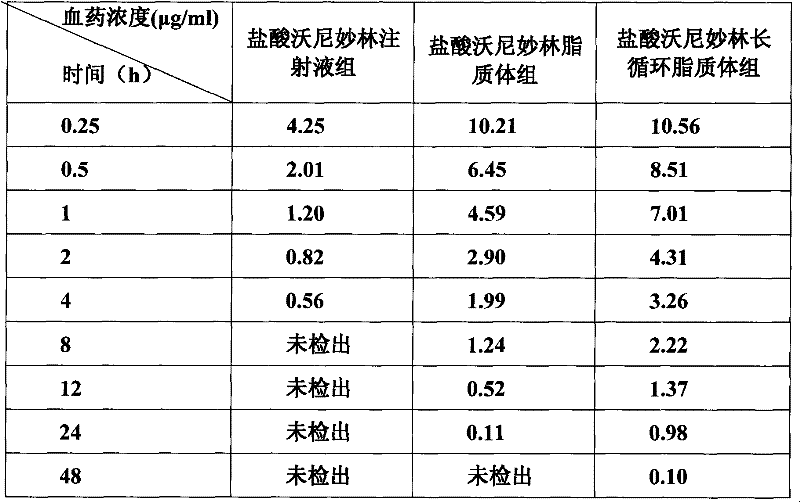
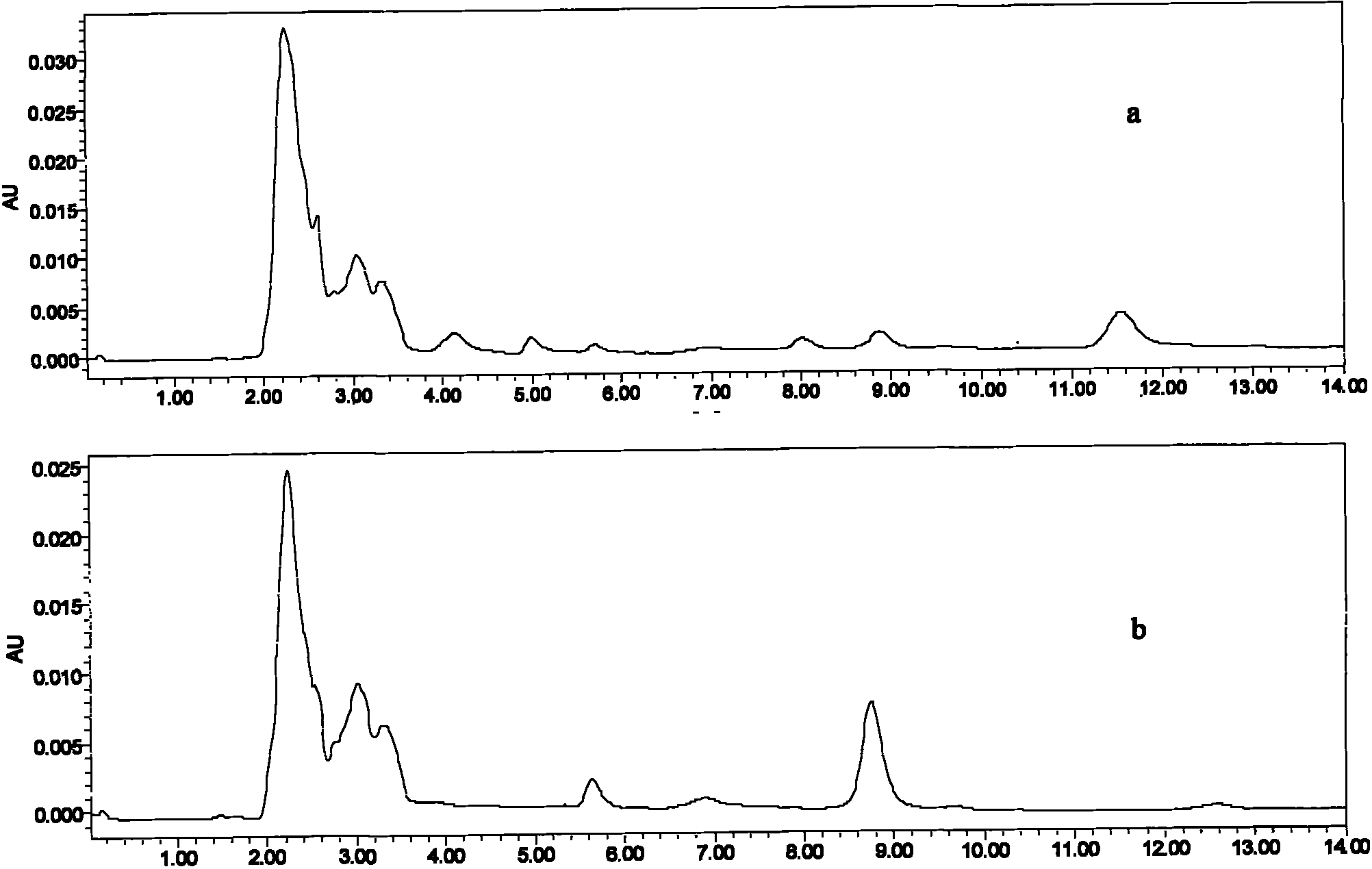
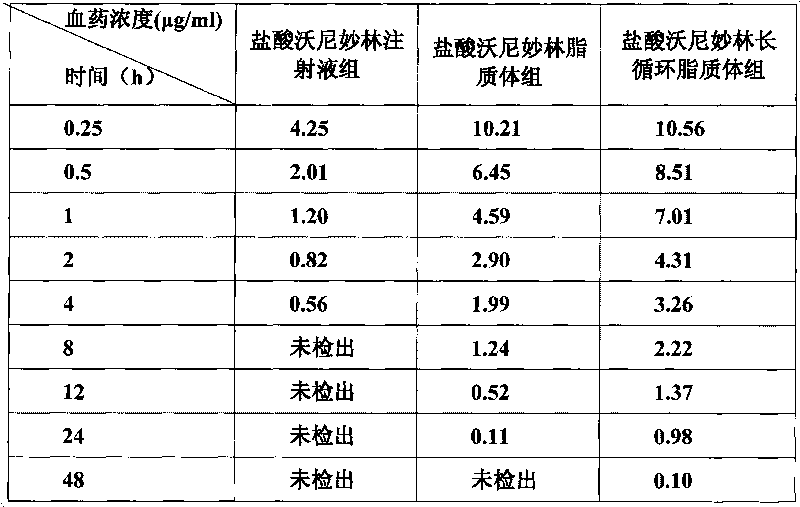
Veterinary valnemulin, novel liposome formulation of the salt thereof and preparation method thereof
ActiveCN101744799BExtend cycle timeImprove tissue selectivityAntibacterial agentsEster active ingredientsSide effectCholesterol
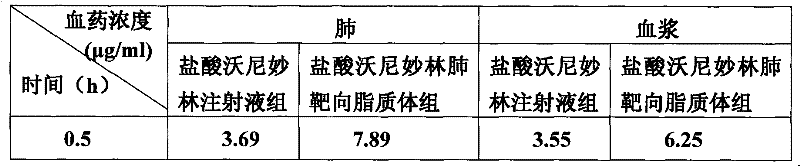
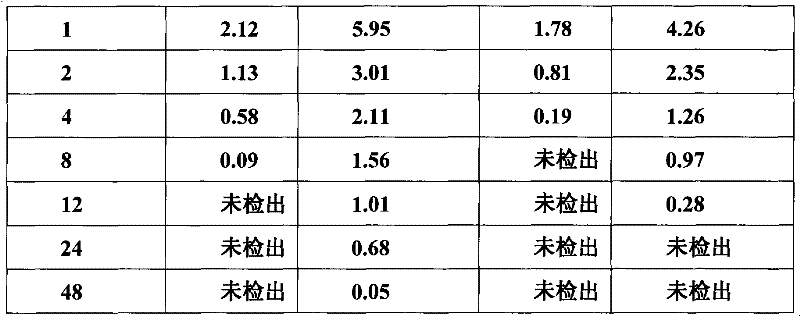
The invention relates to veterinary valnemulin, a novel liposome formulation of the salt thereof and a preparation method thereof, which belongs to the field of veterinary antibiotic preparations. In the preparation, valnemulin and the salt thereof serve as the main components, phospholipid and cholesterol serve as membranes, and the common liposomes, the lung targeting liposomes or the long circulating liposomes of valnemulin or valnemulin salt can be prepared. The valnemulin and the liposomes of the salt thereof have better tissue selectivity compared with the common preparation, are effective to the bacterial and mycoplasma infection in cells, and can reduce the toxic and side effect. The lung targeting liposomes targets the medicine to the lung, and has significant effect to the mycoplasma infection of the lung, bacterial infection and the like, and reduces the toxic and side effect. The long circulating liposomes significantly improves the circulation time of the liposomes in thebody, so that the medicines displays the efficacy for a longer time more effectively, so s to reduce the delivery times and the dosage.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH +1
Pleuromutilin derivatives with piperazine side chain, and preparation method and application thereof
ActiveCN105837530AGood in vitro antibacterial activityReduce manufacturing costAntibacterial agentsOrganic active ingredientsHydrogen atomSide chain
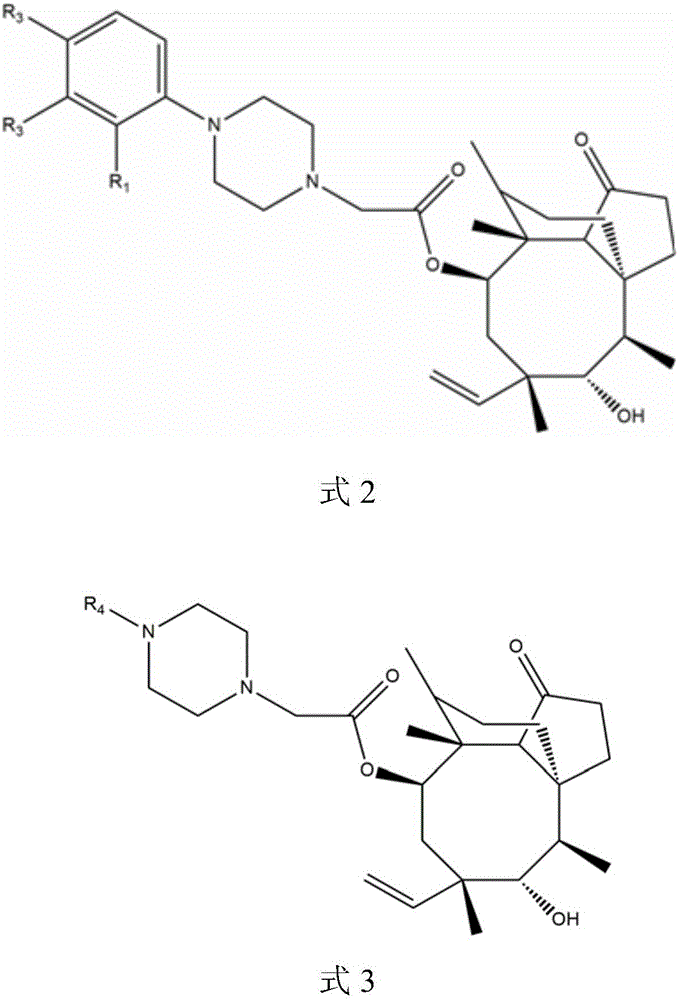
The invention belongs to the field of pharmaceutical chemistry, and discloses pleuromutilin derivatives with piperazine side chain, and a preparation method and application thereof. The derivatives have the structure disclosed as Formula 2 or Formula 3, wherein R1 is hydrogen atom, methoxy, methyl, hydroxy, nitro or chlorine atom; R2 is hydrogen atom, methoxy, methyl, hydroxy, nitro or chlorine atom; R3 is hydrogen atom, methoxy, methyl, hydroxy, nitro or chlorine atom; and R4 is phenyl or methyl. The pleuromutilin derivatives with piperazine side chain have favorable in-vitro antimicrobial activity, have the advantage of lower preparation cost than valnemulin and retapamulin, and thus, are specially suitable to be used as a novel antibacterial drug for preventing and treating human or animal bacterial infective diseases.
Owner:SOUTH CHINA AGRI UNIV
Pleuromutilin derivatives, and preparation method and application thereof
ActiveCN102924350ARaw materials are easy to getLow priceAntibacterial agentsEster active ingredientsStreptococcus mastitidisStaphyloccocus aureus
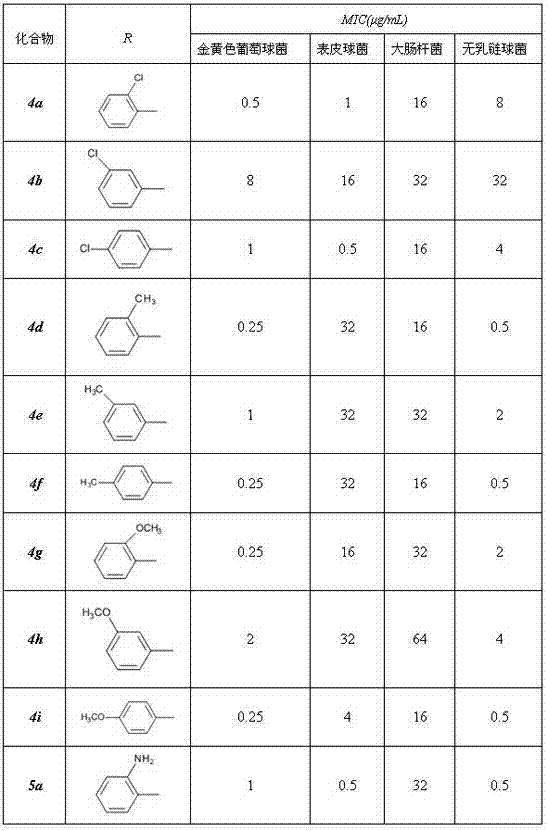
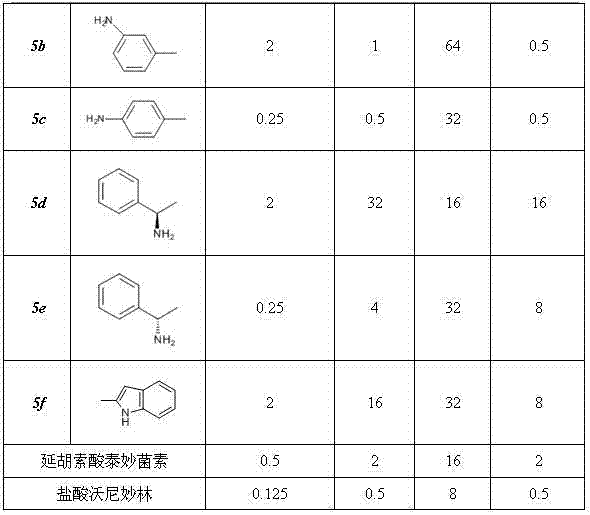
The invention discloses new pleuromutilin derivatives which have a structural formula (I) or a structural formula (II) shown in the specification, wherein in the formula (I), when n=0, R1 is Cl, CH3, OCH3 or NH2; and when n=1, R1=H, and R2=NH2. The compounds have favorable inhibiting action on Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli and Streptococcus mastitidis; the antibacterial activity of one compound having a phenyl group with ortho-substituting groups or para-substituting groups (such as Cl, CH3, OCH3 or NH2) is superior to the antibacterial activity of one compound having a phenyl group with meta-substituting groups; and part of compounds having para-substituting aryl groups or ortho-substituting aryl groups are the same with valnemulin in the antibacterial activity on Staphylococcus epidermidis or Streptococcus mastitidis, and can be used for the preparation of antibacterial drugs. The synthesis method of the compounds has the advantages that the raw materials are accessible, the price is low, the operation process is simple, the products are easy to separate and purify, the yield is high and the total yield is 35-45%.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Method for preparing molecularly imprinted polymer used for detecting valnemulin
InactiveCN101921370AStrong specificityStrong water absorptionOther chemical processesPolymer scienceBulk polymerization
The invention relates to the compound detection field, in particular to a method for preparing a molecularly imprinted polymer used for detecting valnemulin which is prepared by adopting a bulk polymerization method, a precipitation polymerization method, an in-situ polymerization method or a suspension polymerization method and taking a valnemulin precursor as a template; and the prepared molecularly imprinted polymer has high selectivity on the valnemulin in an aqueous solution system, can identify the valnemulin in high specificity, and has wide application prospect as a sample pretreatment material analyzing the valnemulin in the matrixes such as feeds and tissues and the like.
Owner:SOUTH CHINA AGRI UNIV
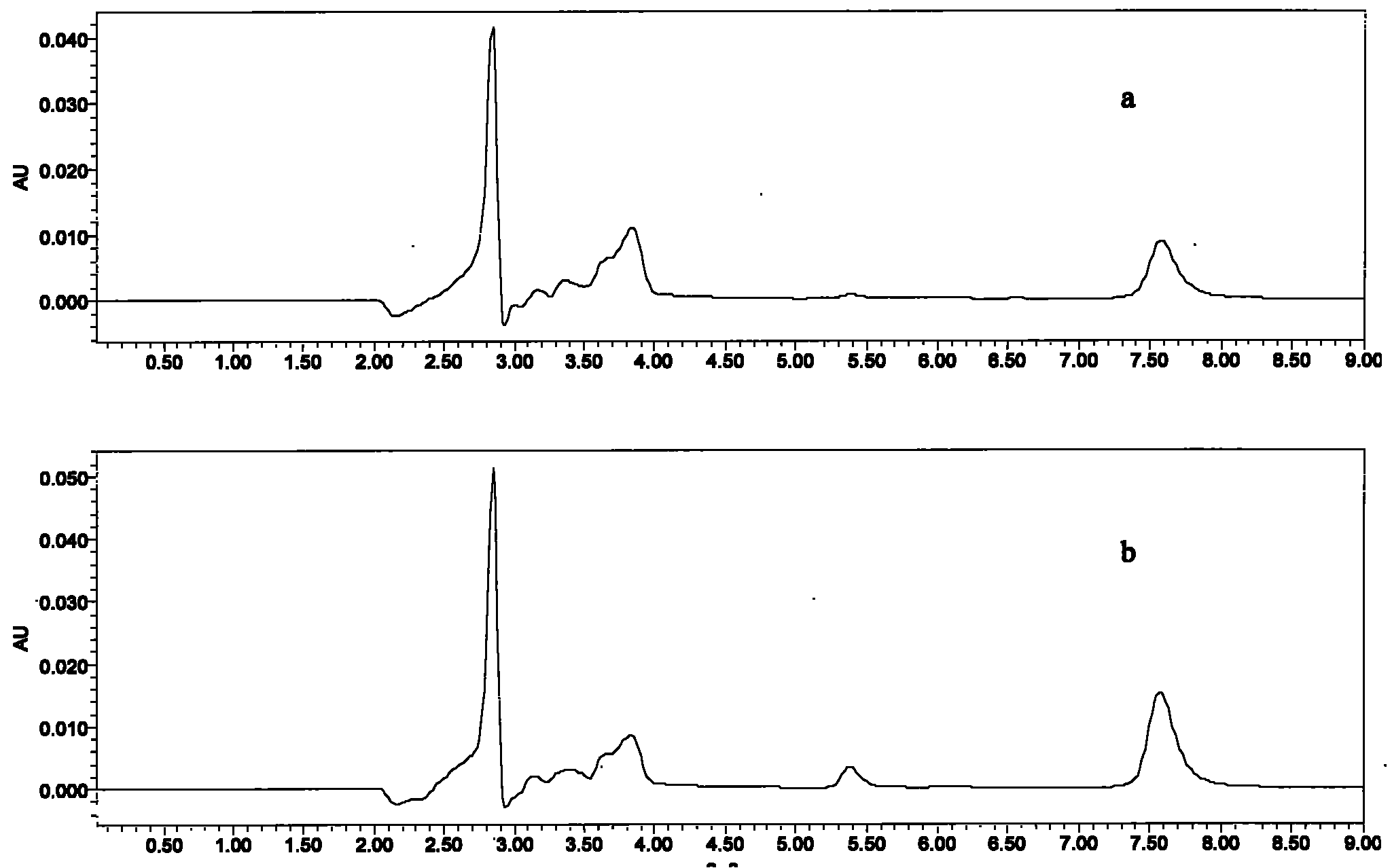
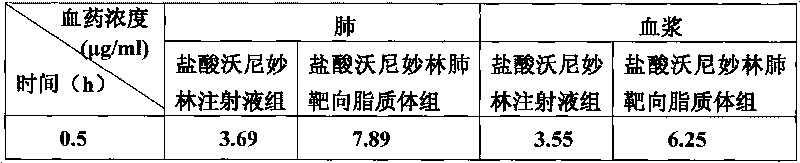
Veterinary valnemulin, novel liposome formulation of the salt thereof and preparation method thereof
ActiveCN101744799ASmall toxicityImprove the effectiveness of treating lung infectionsAntibacterial agentsEster active ingredientsSide effectCholesterol
The invention relates to veterinary valnemulin, a novel liposome formulation of the salt thereof and a preparation method thereof, which belongs to the field of veterinary antibiotic preparations. In the preparation, valnemulin and the salt thereof serve as the main components, phospholipid and cholesterol serve as membranes, and the common liposomes, the lung targeting liposomes or the long circulating liposomes of valnemulin or valnemulin salt can be prepared. The valnemulin and the liposomes of the salt thereof have better tissue selectivity compared with the common preparation, are effective to the bacterial and mycoplasma infection in cells, and can reduce the toxic and side effect. The lung targeting liposomes targets the medicine to the lung, and has significant effect to the mycoplasma infection of the lung, bacterial infection and the like, and reduces the toxic and side effect. The long circulating liposomes significantly improves the circulation time of the liposomes in the body, so that the medicines displays the efficacy for a longer time more effectively, so s to reduce the delivery times and the dosage.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH +1
Preparation of valnemulin and hydrochloride of valnemulin
InactiveCN103193692AReduce usageHigh yieldSulfide preparationBulk chemical productionValineProtecting group
The invention relates to a preparation method of valnemulin and hydrochloride of valnemulin. The preparation method comprises the following steps of: reacting amino-protected D-valine and pivaloyl chloride to obtain mixed anhydride; carrying out condensation reaction to the mixed anhydride and 14-O-[(2-amino-1, 1-dimethyl ethyl) thiomethylcarbonyl] mutilin to prepare valnemulin with an amino protecting group; carrying out acid treatment to remove the protecting group; crystallizing to obtain valnemulin; and then reacting with an acid to obtain the hydrochloride. The preparation method is scientific and advanced in technology, and has the advantages of being high in cost, and high in product yield and purity.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Pig respiratory tract diseases prevention and treatment drug composition, premix and matching material thereof
InactiveCN104784411AReasonable compositionFormulation ScienceAnimal feeding stuffRespiratory disorderDiseaseRespiratory tract disease
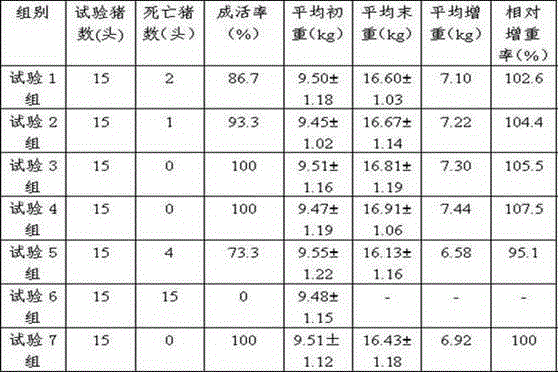
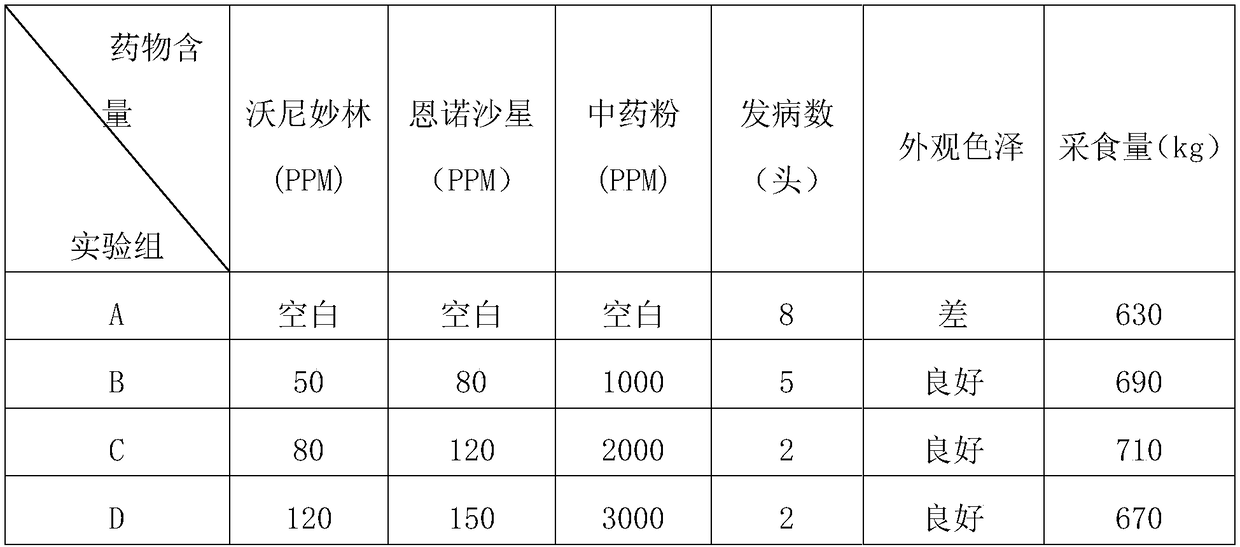
The present invention relates to a pig respiratory tract diseases prevention and treatment drug composition, a premix and a matching material thereof, and belongs to the field of veterinary drugs and premixes. The drug composition of the present invention comprises, by weight, 40-95 parts of a traditional Chinese medicine component, and 10-60 parts of a chemical drug component, wherein the traditional Chinese medicine component is prepared from the fructus arctii, roripa montana and common yam rhizome, and the chemical drug component is selected from florfenicol, tilmicosin, tiamulin, valnemulin, acetylisovaleryltylosin, tylosin, kitasamycin and a salt thereof. The drug compositions of the present invention is suitable for pig respiratory tract diseases prevention and treatment, has characteristics of significant treatment effect, high safety, stable formulation, controllable quality, simple preparation process and the like, is suitable for industrial production, and can further be added to the feed so as to be used for preparation of pig growth promoting feed such as premixes, matching materials and the like.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH +2
Method for preparing valnemulin salt premix
ActiveCN101984957AEasy to takeTo achieve the purpose of taste maskingAntibacterial agentsPowder deliveryFluidized bedIrritation
The invention discloses a method for preparing a valnemulin salt premix, which comprises the following steps of: preparing coating liquid by using a coating material, an antisticking agent and water, mixing valnemulin salt with a lubricating agent and fluidizing; then atomizing the coating liquid and injecting into a fluidized bed to coat the valnemulin salt and obtaining microcapsules of the valnemulin salt; and screening the microcapsules and uniformly mixing with a stabilizing agent, a lubricating agent and a diluent so as to prepare the valnemulin salt premix. The preparation process is simple, the prepared premix can effectively overcome the defect that raw material medicaments of the valnemulin salt have humidity, high powder irritation and optical instability, are easy to decompose when contacted with feed and the like, and the premix is convenient for animal administration.
Owner:HUBEI LONGXIANG PHARMA TECH CO LTD
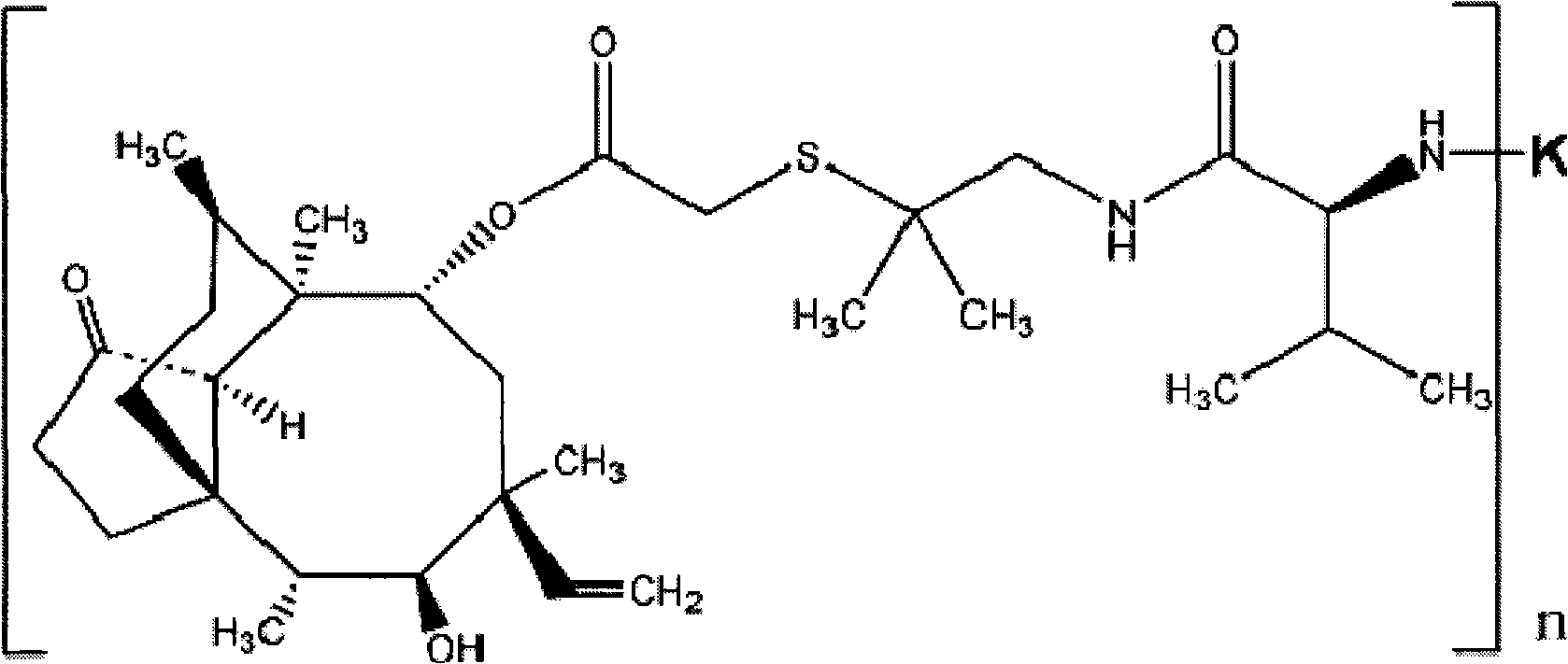
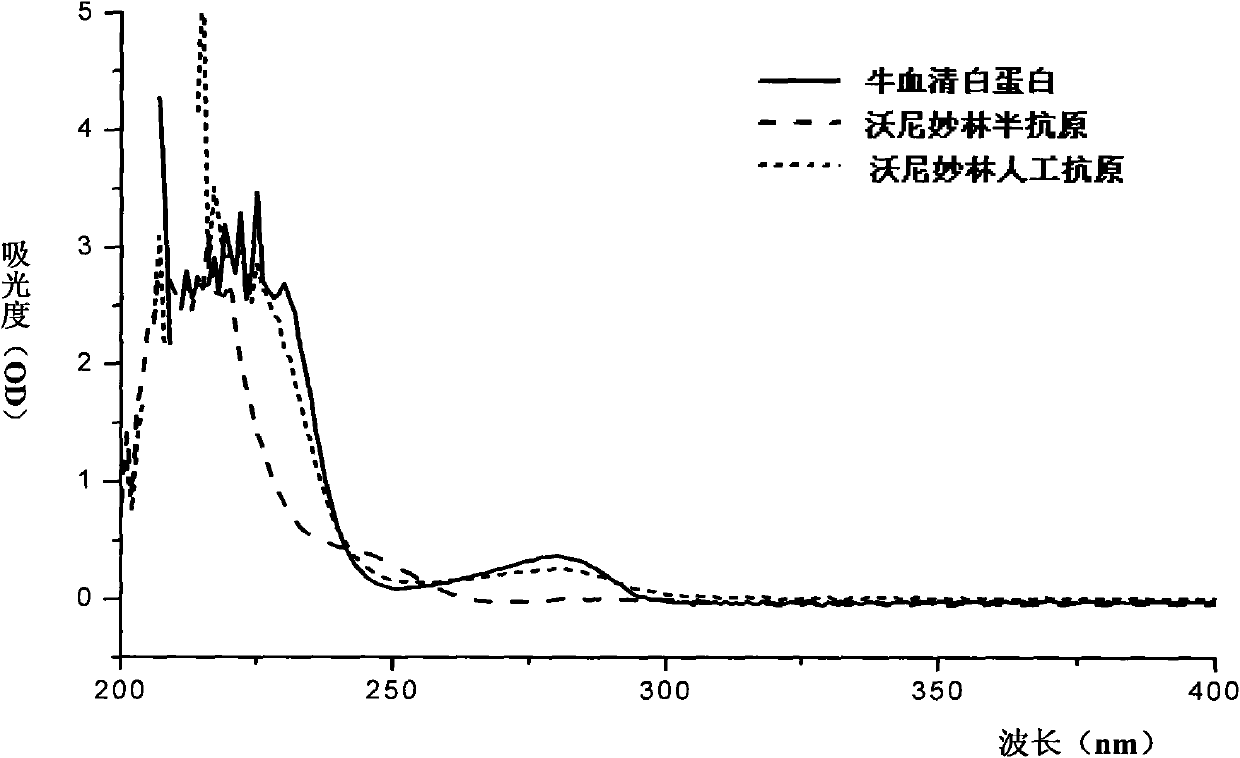
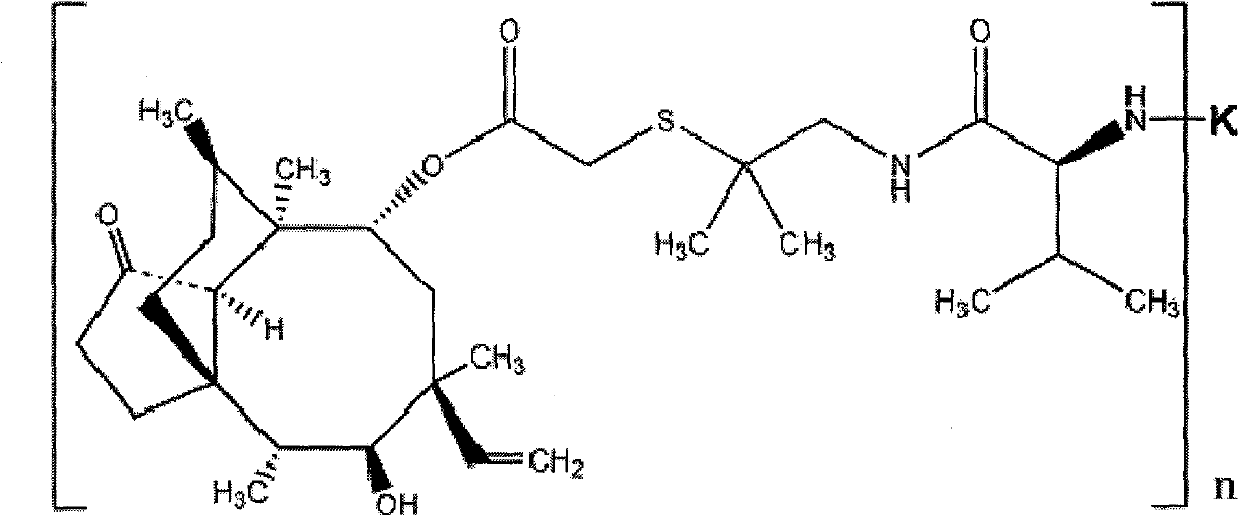
Valnemulin artificial antigen and preparation method and application thereof
ActiveCN101812128AStrong specificityHigh sensitivityOvalbuminSerum albuminSerum igeNew Zealand white rabbit
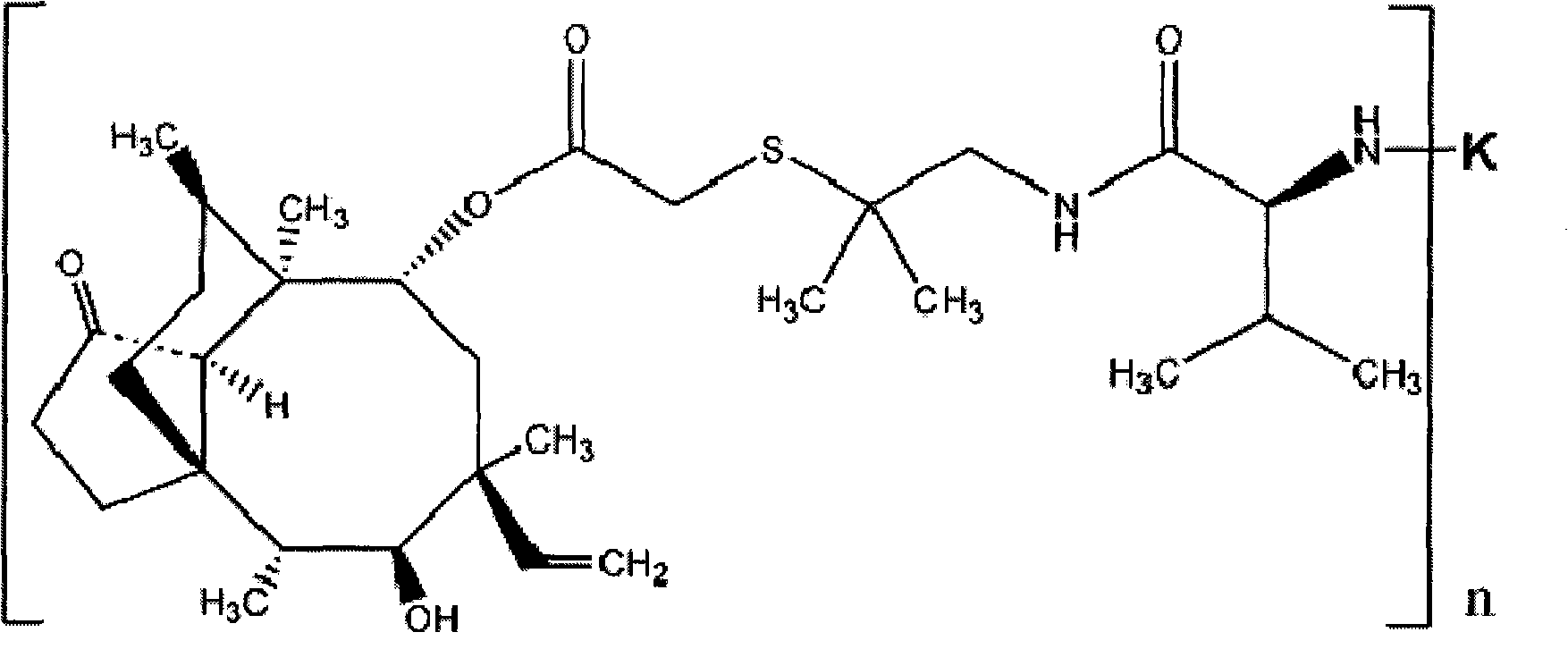
The invention discloses a valnemulin artificial antigen and a preparation method and application thereof. The valnemulin artificial antigen is a compound as shown in a formula I, wherein n denotes a natural number from 1 to 25; and K denotes carrier protein. When the artificial antigen is used to immunize a New Zealand white rabbit, an antibody with specific reaction to valnemulin can be obtained. According to ELISA experiments, the titer of the obtained antiserum reaches 1:102400. The invention provides a wide application and development space for the development of detection reagent used in the enzyme-linked immunoassay method of the valnemulin and the preparation of the enzyme-linked immunoassay reagent kit of the valnemulin. When the antibody obtained by using the artificial antigen is used for the detection of the valnemulin, the operation is simple and convenient, the specificity is good and the accuracy is high. The antibody can be used for the rapid and high-efficiency screening of the valnemulin in animal-based foods and a technical foundation is laid for the rapid detection of the valnemulin. The formula I is as shown is the accompanying drawing.
Owner:北京明日达科技发展有限责任公司
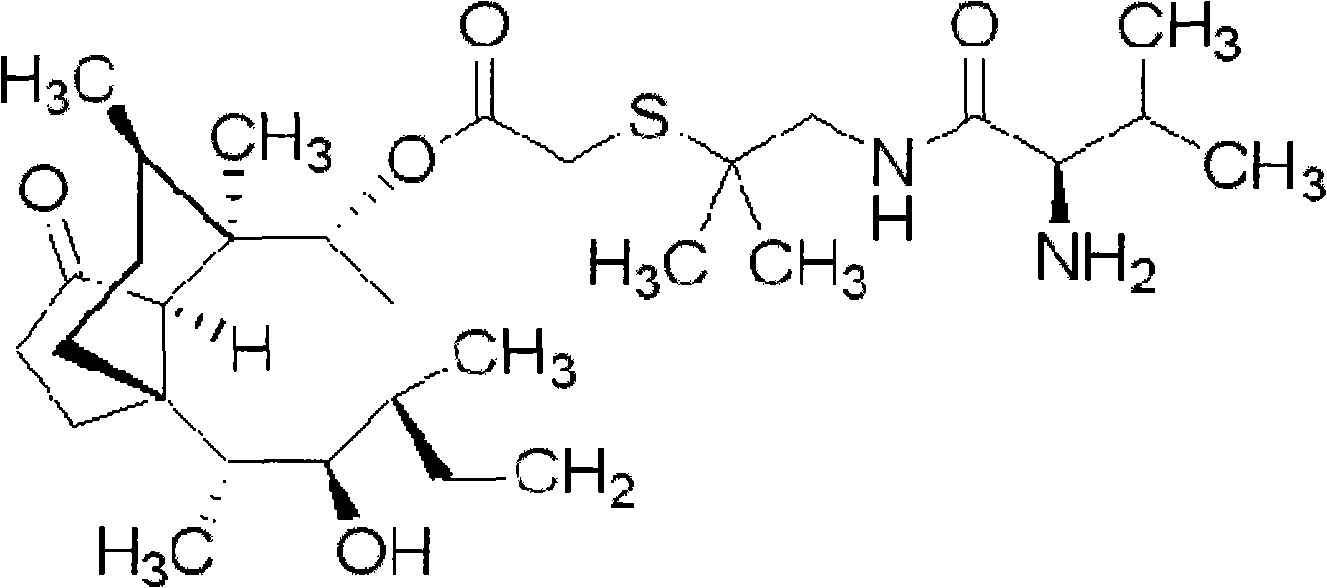
Valnemulin prodrug as well as preparation method and detection method thereof
InactiveCN104927041AImprove stabilityOvercome the shortcoming that the half-life is only 1.3-2.7 hoursPharmaceutical non-active ingredientsEster active ingredientsDrug loading doseOrganic chemistry
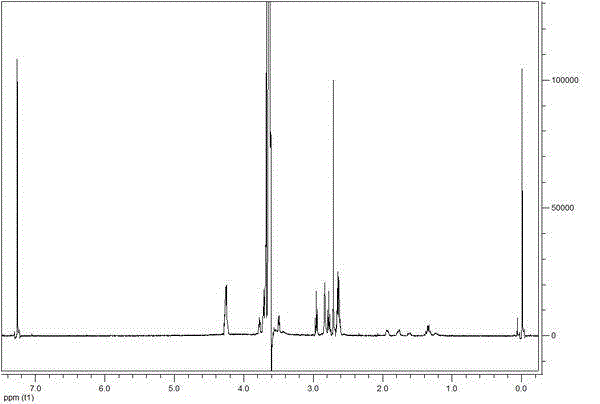
The invention discloses a valnemulin prodrug as well as a preparation method and a detection method thereof. The provided valnemulin prodrug has good stability and overcomes the weakness that the half attenuation period of the valnemulin prodrug is only 1.3 to 2.7 hours, the prepared prodrug has a controlled-release action, and the in-vitro release experiment shows that the valnemulin prodrug can reach the maximum value in 8 hours. Since valnemulin molecules have a plurality of active reaction sites, a method for determining a reaction site for connecting the carrier and valnemulin is a method for adopting a single-molecular carrier to react with valnemulin, the obtained single-molecular prodrug is determined as the reaction site of the valnemulin by virtue of nuclear magnetic detection, and the prodrug reaction site obtained in the method is two amino acids of the valnemulin; the valnemulin prodrug with relatively high drug loading capacity can be prepared; the drug loading capacity of the prepared PEG-valnemulin prodrug is determined by adopting a UV, HPLC-UV and HPLC-ELSD combination method, so that the measured drug loading capacity is more accurate. The method is suitable for preparing and detecting the valnemulin prodrug.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Valnemulin gel microballoon and preparation method thereof
InactiveCN102512376AEasy to makeReduce manufacturing costAntibacterial agentsPharmaceutical non-active ingredientsOrganic solventMicrosphere
The invention belongs to the technical field of veterinary medicine and particularly relates to a valnemulin gel microballon and a preparation method thereof. The grain diameter of the valnemulin gel microballoon is 1 mum to 50 mum and is composed of the following materials by mass: 1 part to 10 parts of valnemulin, 1 part to 10 parts of sodium alginate tech grade and 2 parts to 5 parts of calcium chloride. The valnemulin gel microballon and the preparation method thereof have the advantages of using the characteristic that when sodium alginate tech grade contacts calcium ions, gelation can happen instantly to embed the valnemulin, being simple in preparation processes, free of high temperature, organic solvent or other harmful compounds and low in production cost, meeting requirements of industrialization production and having good market prospects.
Owner:HENAN SOAR VETERINARY PHARMA
Preparation method of tartaric acid valnemulin premixing agent
InactiveCN102813625AEasy to solveGood treatment effectAntibacterial agentsPowder deliverySwine dysenteryEnteropathy
The invention provides a preparation method of a tartaric acid valnemulin premixing agent. According to the invention, tartaric acid valnemulin is directly mixed with accessories to prepare the tartaric acid valnemulin premixing agent. The method comprises four steps of: carrying out dry processing on the accessories; mixing the crushed and screened accessories with tartaric acid valnemulin and performing sampling inspection; packaging; and warehousing. The required tartaric acid valnemulin premixing agent can be obtained conveniently and quickly through the four steps. The tartaric acid valnemulin premixing agent obtained by using the preparation method can well prevent and treat swine dysentery, enzootic pneumoniae, swine colonic spirochetosis, swine hyperplastic enteropathy and other animal diseases, and is simple in processing technique. Not only is production equipment simplified, but also manufacturing cost is reduced, and therefore, the premixing agent can be rapidly popularized in the market at lower price.
Owner:HUBEI LONGXIANG PHARMA TECH CO LTD
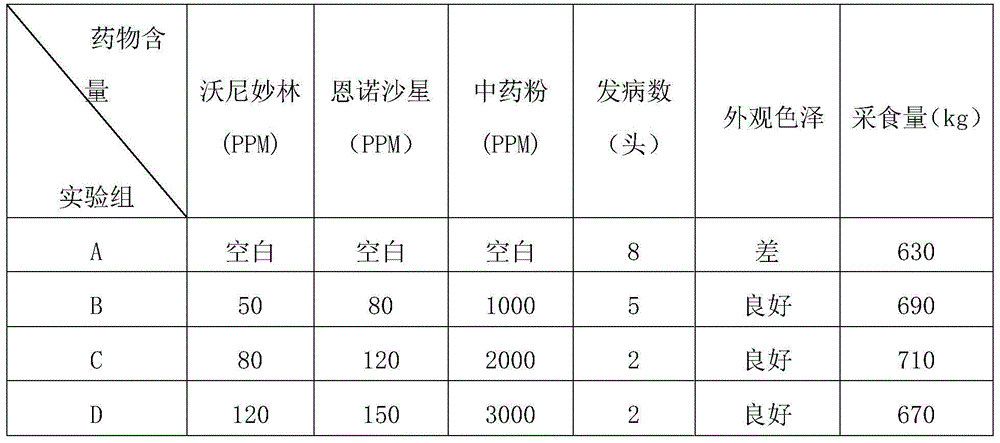
Drug formula for preventing and treating chronic PRDC (porcine respiratory disease complex)
ActiveCN104666878AHigh antibacterial activityEasy to controlAntibacterial agentsInorganic active ingredientsWater vaporRespiratory disease
The invention relates to a drug formula for preventing and treating the chronic PRDC (porcine respiratory disease complex). The drug formula comprises raw materials, namely, valnemulin, enrofloxacin and traditional Chinese medicine powder, wherein the traditional Chinese medicine powder comprises raw materials, namely, codonopsis pilosula, liquorice roots, poria cocos, largehead atractylodes rhizomes, lily bulbs, dwarf lilyturf tubers, purified pinellia tubers, tatarian aster roots, ephedra herbs, thinleaf milkwort roots, common anemarrhena rhizomes, coastal glehnia roots, dried tangerine peels and pumice, the Chinese herbal medicines are distilled with water vapor, an extract is obtained, the extract and fine pumice powder produced through milling by an efficient ball mill are evenly mixed, then dried at the low temperature and packaged in a vacuum manner, and the traditional Chinese medicine powder is prepared. The formula is significant in curative effect on the chronic PRDC, less in traditional Chinese medicine addition amount and good in palatability, takes effect quickly and is high in cure rate according to clinical usage.
Owner:SICHUAN CHENGKANG ANIMAL PHARMA
Preparation method of fumaric acid valnemulin
InactiveCN102180818AHigh yieldSimple processCarboxylic acid salt preparationSulfide preparationAnhydrous ethanolMethyl isobutyl ketone
The invention relates to a preparation method of fumaric acid valnemulin. The preparation method comprises the following steps: firstly adding valnemulin in a methyl isobutyl ketone solution, then heating and stirring; after dissolving, adding absolute ethanol, adding fumaric acid until a reaction solution is clear; reducing the temperature with ice bath so as to separate out crystal; filtering, and washing filter cake with methyl isobutyl ketone; and drying so as to obtain the fumaric acid valnemulin, wherein the melting point of the fumaric acid valnemulin is larger than 130 DEG C, and the yield of the fumaric acid valnemulin reaches 85%.
Owner:QINGDAO UNIV OF SCI & TECH
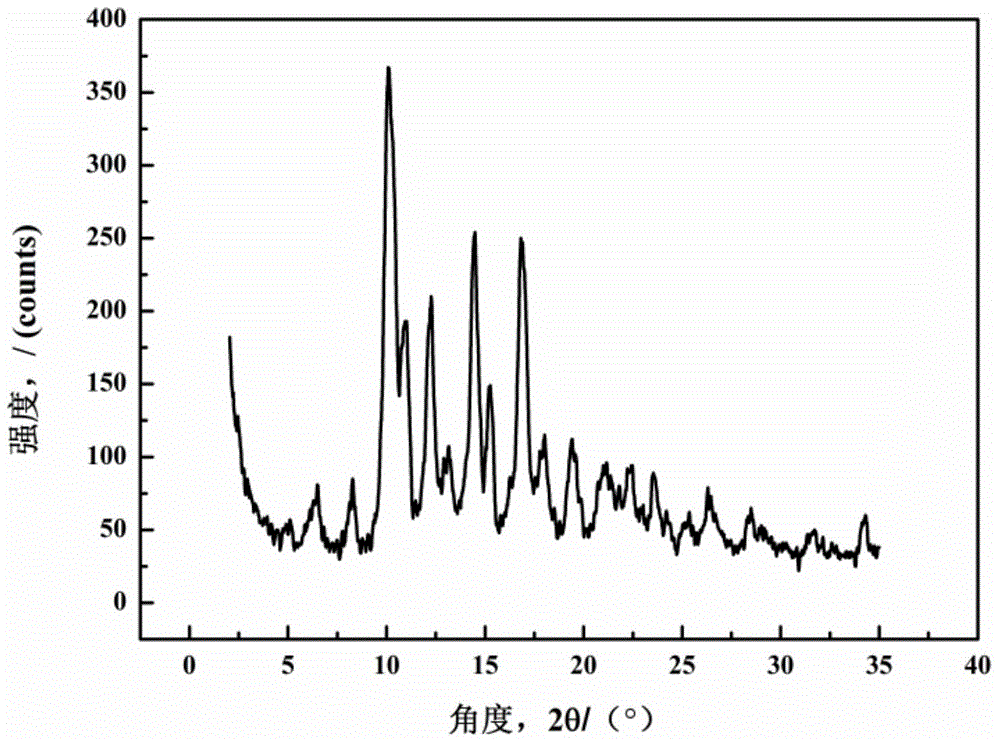
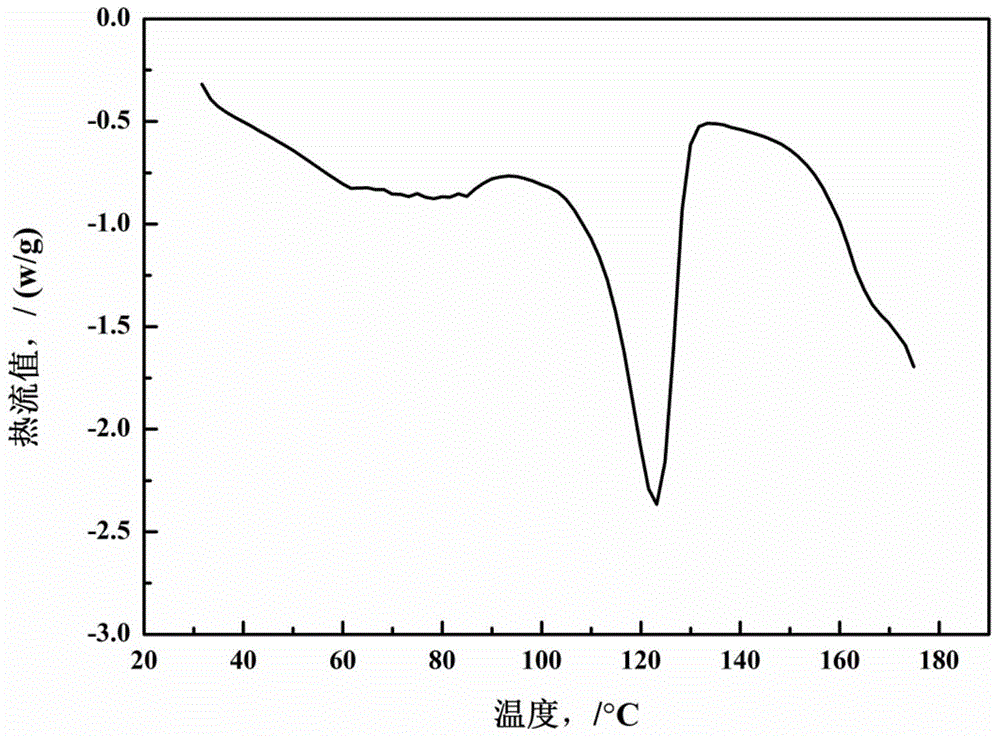
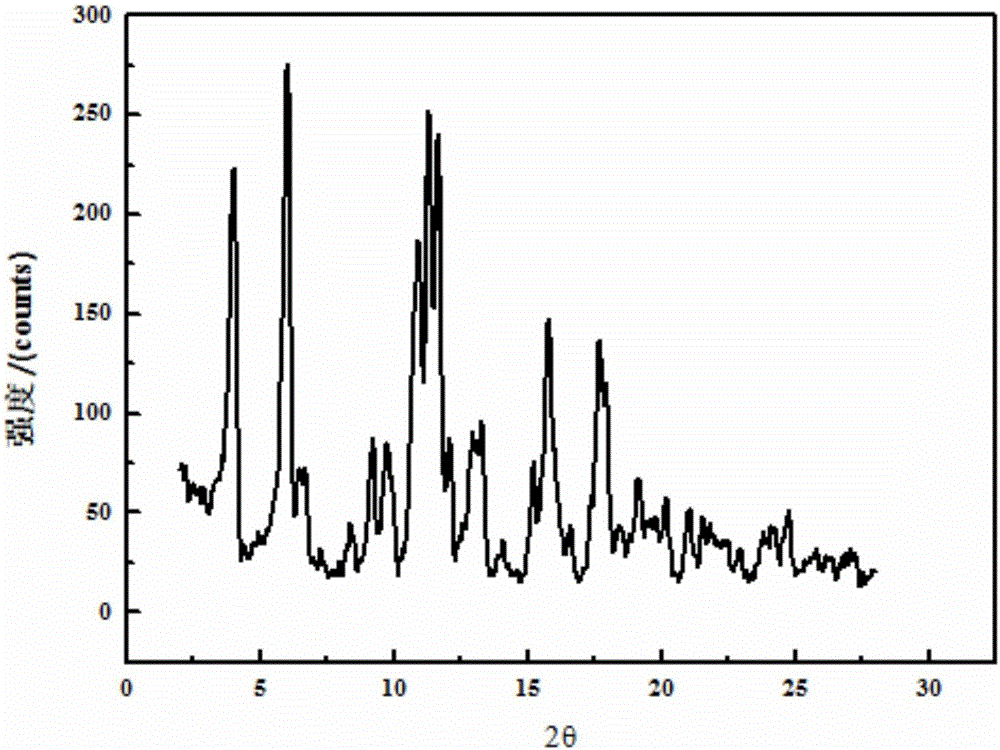
Valnemulin oxalate crystal and preparation method thereof
ActiveCN104130168AHigh purityHigh yieldOrganic chemistryOrganic compound preparationX-rayCrystallinity
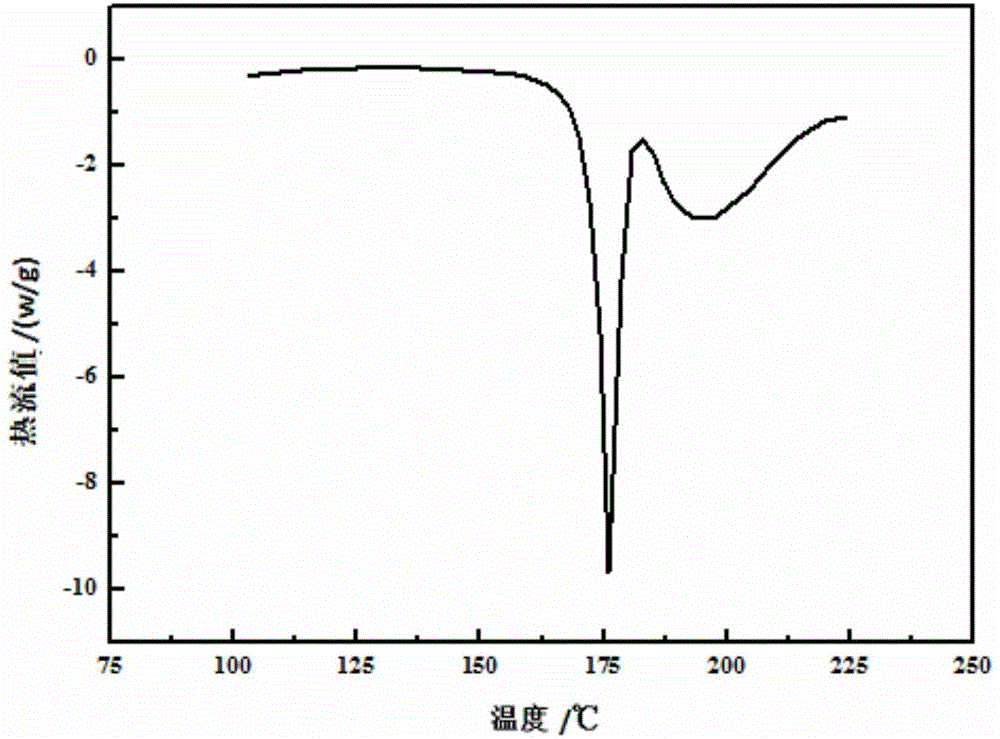
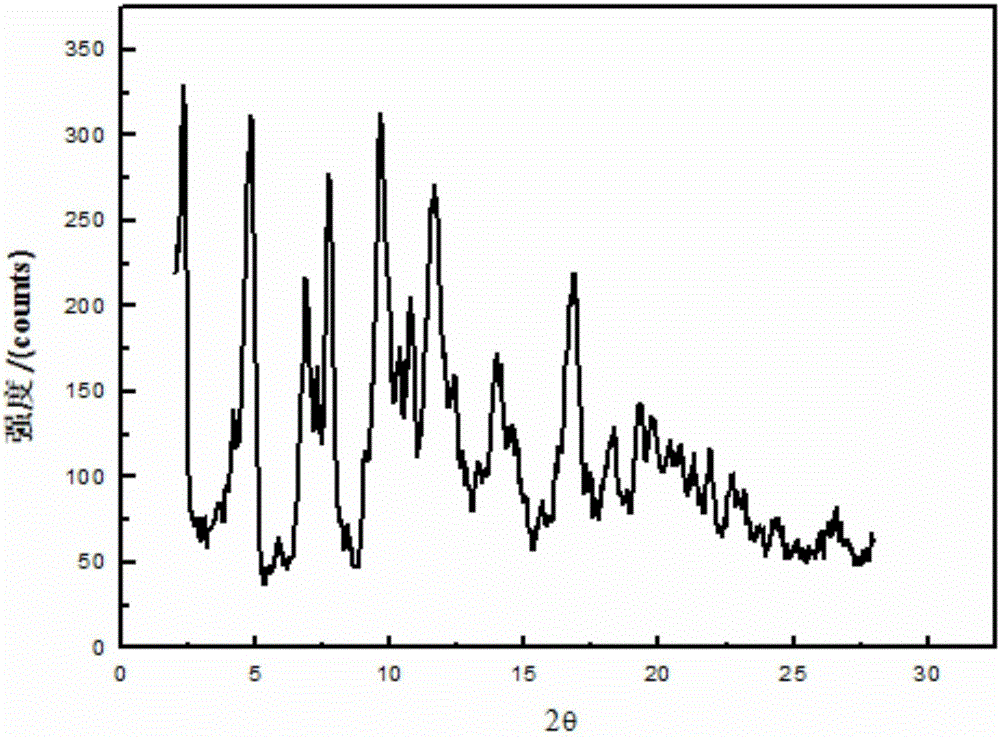
The invention relates to a valnemulin oxalate crystal and a crystallization preparation method thereof. According to an X-ray powder diffraction pattern of the crystal, characteristic peaks appear at diffraction angle 2theta of 6.2, 8.0, 10.5, 11.2, 12.5, 13.2, 14.6, 15.0, 17.0, 18.0, 19.7 and 21.1 degrees. According to the invention, valnemulin is dissolved in an ester type solvent, wherein a solution concentration is 0.1-0.4g / mL; oxalic acid is added according to a molar ratio that oxalic acid to valnemulin is 1:1-1:2; under a stirring effect, a reaction is carried out under a temperature of 40-60 DEG C until the solution is clear; the temperature of the solution is reduced to 10-20 DEG C; and filtering, washing, and drying are carried out, such that a valnemulin oxalate crystal product is obtained. The crystallization process has a yield higher than 90%. The valnemulin oxalate product has high crystallinity, high purity, and low fly-off. The valnemulin oxalate product has good stability against light, heating, and moisture.
Owner:TIANJIN UNIV +1
Pleuromutilin derivative with amide side chain as well as preparation and application of pleuromutilin derivative
ActiveCN111574395AGood in vitro antibacterial activityReduce manufacturing costAntibacterial agentsOrganic active ingredientsAntimicrobial drugSide chain
The invention belongs to the field of medicinal chemistry, and particularly relates to a pleuromutilin derivative with an amide side chain as well as preparation and application of the pleuromutilin derivative. The pleuromutilin derivative with an amide side chain is a compound shown as a formula 2 (See the specification) or a pharmaceutically acceptable salt thereof, and a solvate, an enantiomer,a diastereoisomer, a tautomer or a mixture of the solvate, the enantiomer, the diastereoisomer and the tautomer of the compound of formula 2 or the pharmaceutically acceptable salt thereof in any proportion, including a racemic mixture. The compound not only has good in-vitro antibacterial activity and water solubility, but also has the advantage of low preparation cost compared with valnemulin and retapamulin, so that the compound is particularly suitable for being used as a novel antibacterial agent for preventing and treating human or animal bacterial infectious diseases, particularly infectious diseases caused by drug-resistant staphylococcus aureus.
Owner:SOUTH CHINA AGRI UNIV
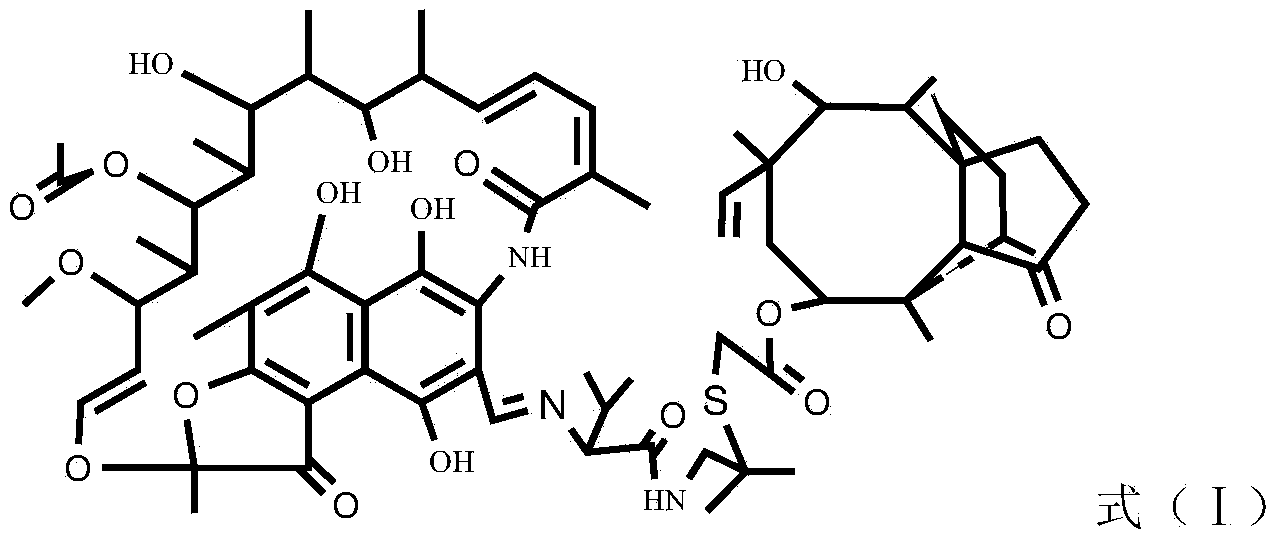
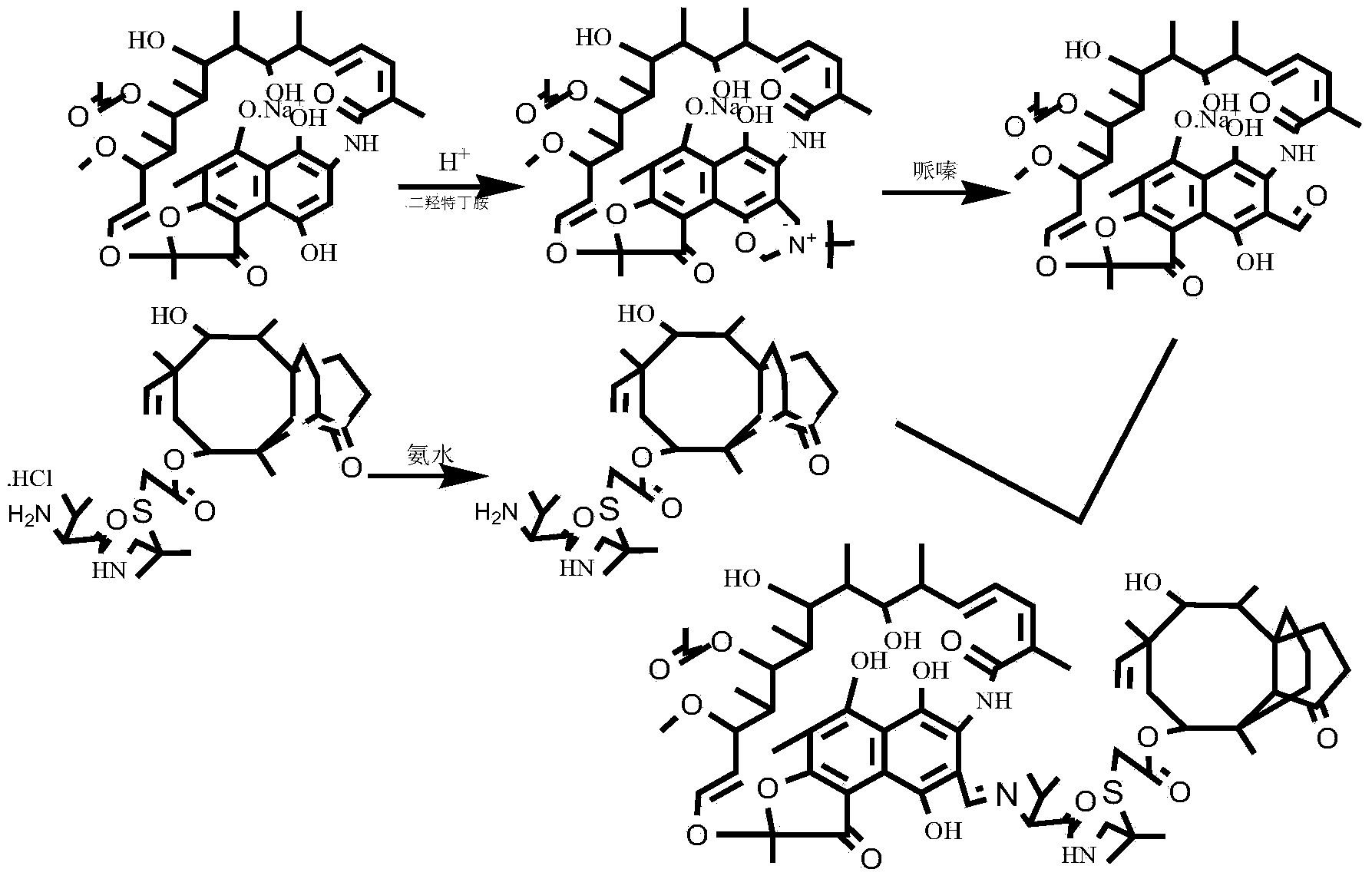
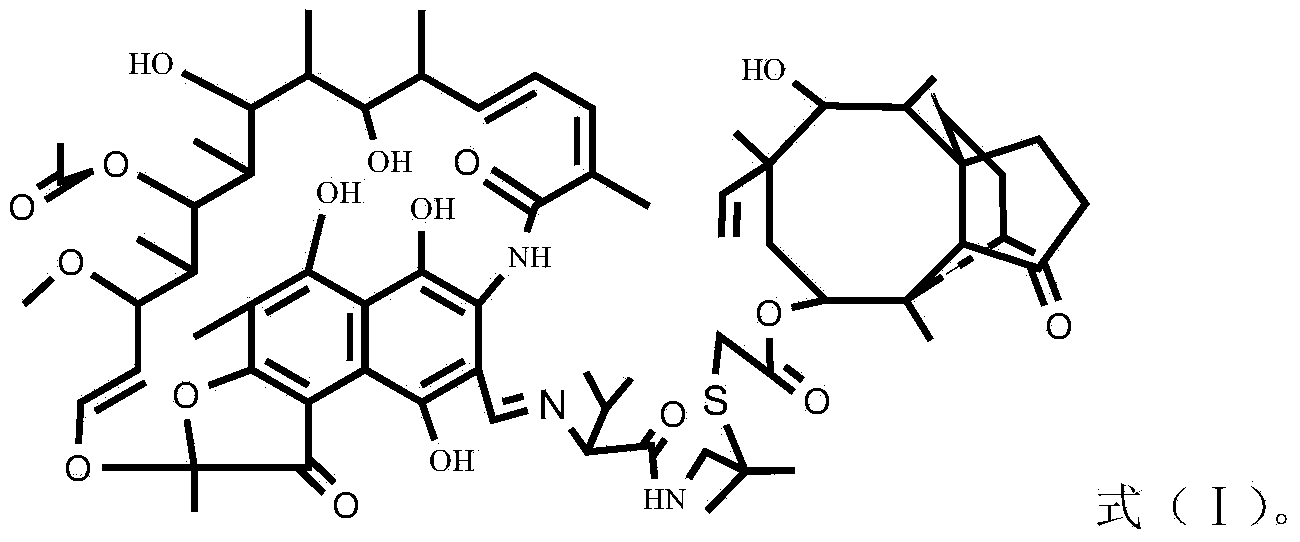
Rifamycin and valnemulin hybrid antibiotic and preparation method thereof
InactiveCN103709177ALinker stabilizationImprove antibacterial propertiesAntibacterial agentsOrganic chemistryDimethyl formamideValnemulin
The invention discloses a rifamycin and valnemulin hybrid antibiotic compound and a preparation method thereof. The rifamycin and valnemulin hybrid antibiotic has a structure as shown in the formula (I). The preparation method comprises the following steps: (1) adding concentrated sulfuric acid into a DMF (Dimethyl Formamide) solution of rifamycin s and sodium salt to acidize for 0.5h, and then, dropwise adding dihydroxy-tert-butylamine to react to obtain an intermediate, i.e., oxazine rifamycin II; (2) mixing the intermediate, i.e., the oxazine rifamycin II and piperazine anhydrous in a protonic solvent, and reacting to obtain an intermediate, i.e., 3-formyl rifamycin III; and (3) mixing and dissolving the 3-formyl rifamycin III and valnemulin in a solvent, stirring at the temperature of 15-30 DEG C for 2h under the protection of nitrogen gas, and cooling to obtain the rifamycin and valnemulin hybrid antibiotic. Two drug molecules with different action mechanisms are connected together, the combination way of the rifamycin and valnemulin hybrid antibiotic compound is similar to that of a prodrug with dual functions, the linking group is stable, and the rifamycin and valnemulin hybrid antibiotic compound can take a very good treating effect.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Preparation method for valnemulin liposome
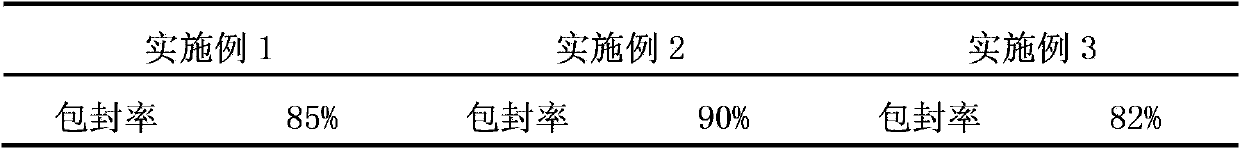
InactiveCN102846548BProlong the effective timeImprove stabilityAntibacterial agentsEster active ingredientsWater bathsSolubility
The invention discloses a preparation method for a valnemulin liposome. The method comprises the following steps: 1) preparation of a NaHCO3 solution; 2) preparation of a citric acid buffer solution; 3) preparation of a blank liposome: a step of dissolving phosphatide and cholesterol in absolute ethyl alcohol in a water bath, allowing ethanol to volatilize and a film to be formed, then adding the citric acid buffer solution, carrying out complete hydration and filtering an obtained liposome solution with a millipore filter to realize whole grain filtering; 4) preparation of valnemulin fluid by dissolving raw valnemulin powder in ethanol; 5) active loading: a step of successively adding the valnemulin fluid and the NaHCO3 solution into the blank liposome and carrying out uniform mixing so as to obtain a crude liposome product; and 6) low temperature high speed centrifugation of the crude product obtained in step 5) so as to prepare the valnemulin liposome. According to the invention, through preparation of the blank liposome and active loading, the valnemulin liposome with a high entrapment rate and good clinical effects is obtained; the method is simple and easily practicable, and solubility of valnemulin in water and effective acting time, stability and targeting of valnemulin are improved.
Owner:ZHENGZHOU HOUYI PHARMA
Preparation method for valnemulin liposome
InactiveCN102846548AProlong the effective timeImprove stabilityAntibacterial agentsEster active ingredientsSolubilityWater baths
The invention discloses a preparation method for a valnemulin liposome. The method comprises the following steps: 1) preparation of a NaHCO3 solution; 2) preparation of a citric acid buffer solution; 3) preparation of a blank liposome: a step of dissolving phosphatide and cholesterol in absolute ethyl alcohol in a water bath, allowing ethanol to volatilize and a film to be formed, then adding the citric acid buffer solution, carrying out complete hydration and filtering an obtained liposome solution with a millipore filter to realize whole grain filtering; 4) preparation of valnemulin fluid by dissolving raw valnemulin powder in ethanol; 5) active loading: a step of successively adding the valnemulin fluid and the NaHCO3 solution into the blank liposome and carrying out uniform mixing so as to obtain a crude liposome product; and 6) low temperature high speed centrifugation of the crude product obtained in step 5) so as to prepare the valnemulin liposome. According to the invention, through preparation of the blank liposome and active loading, the valnemulin liposome with a high entrapment rate and good clinical effects is obtained; the method is simple and easily practicable, and solubility of valnemulin in water and effective acting time, stability and targeting of valnemulin are improved.
Owner:ZHENGZHOU HOUYI PHARMA
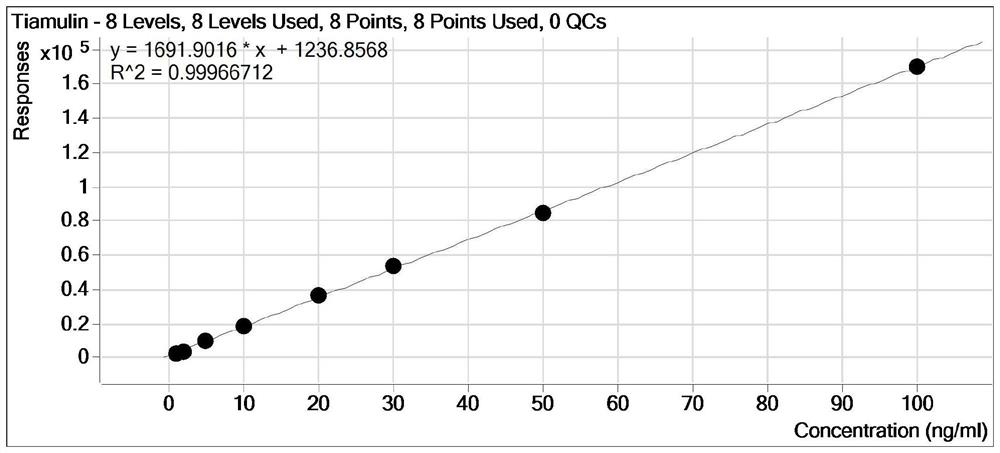
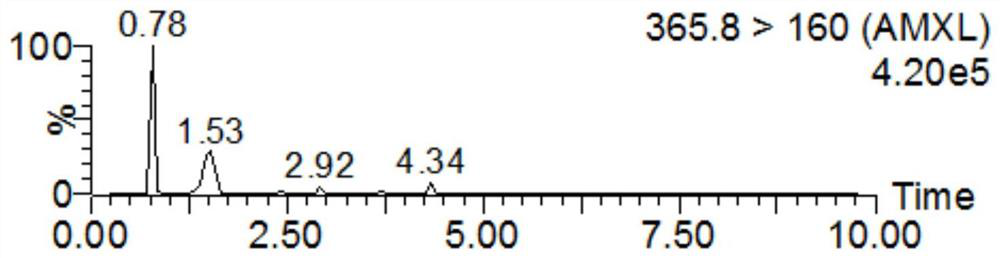
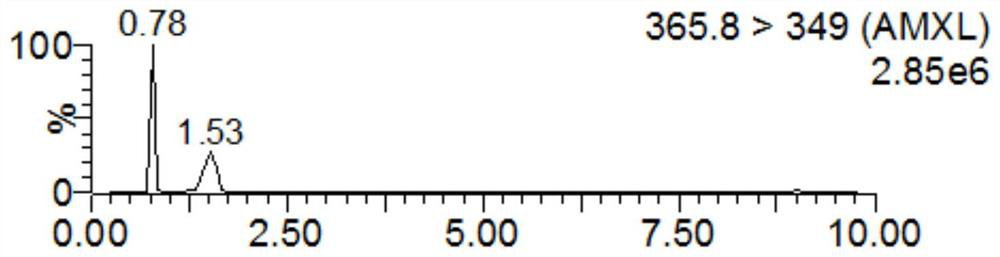
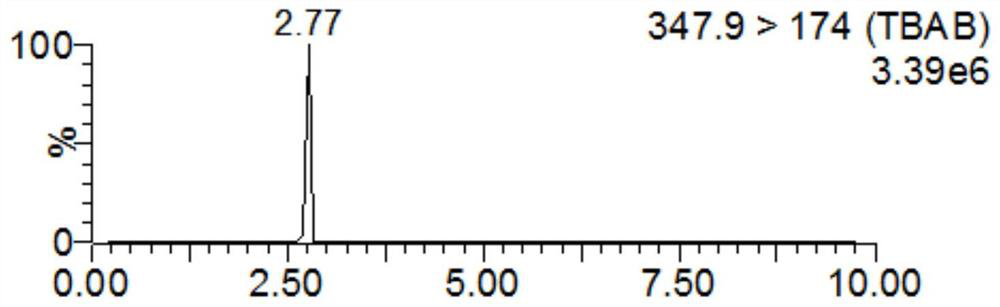
Detection method for tiamulin and valnemulin in aquatic product
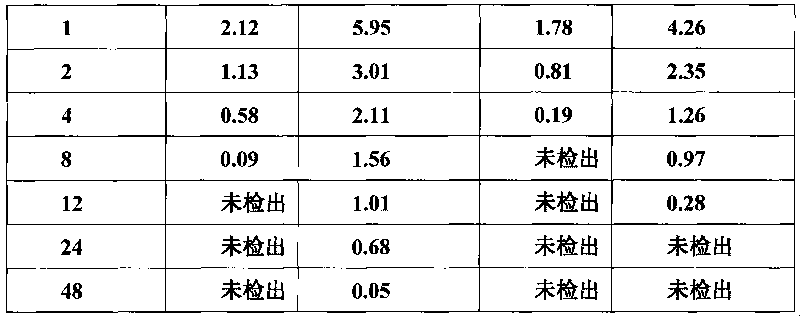
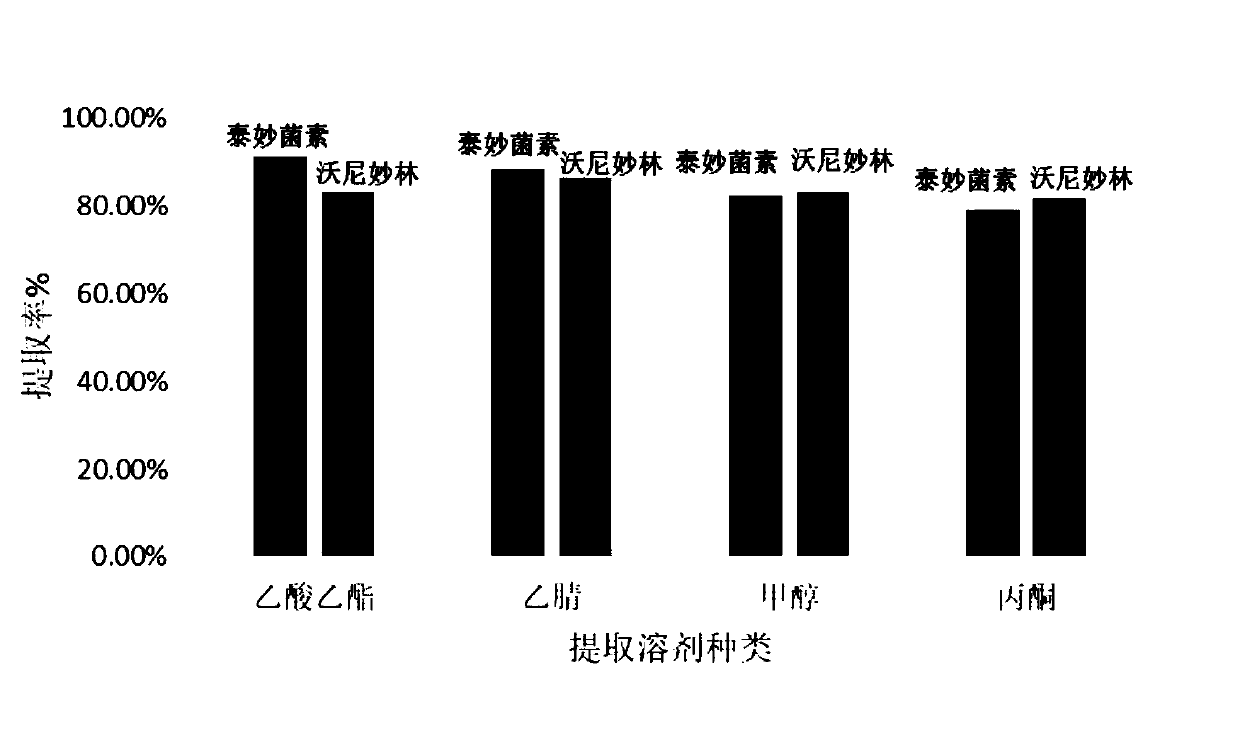
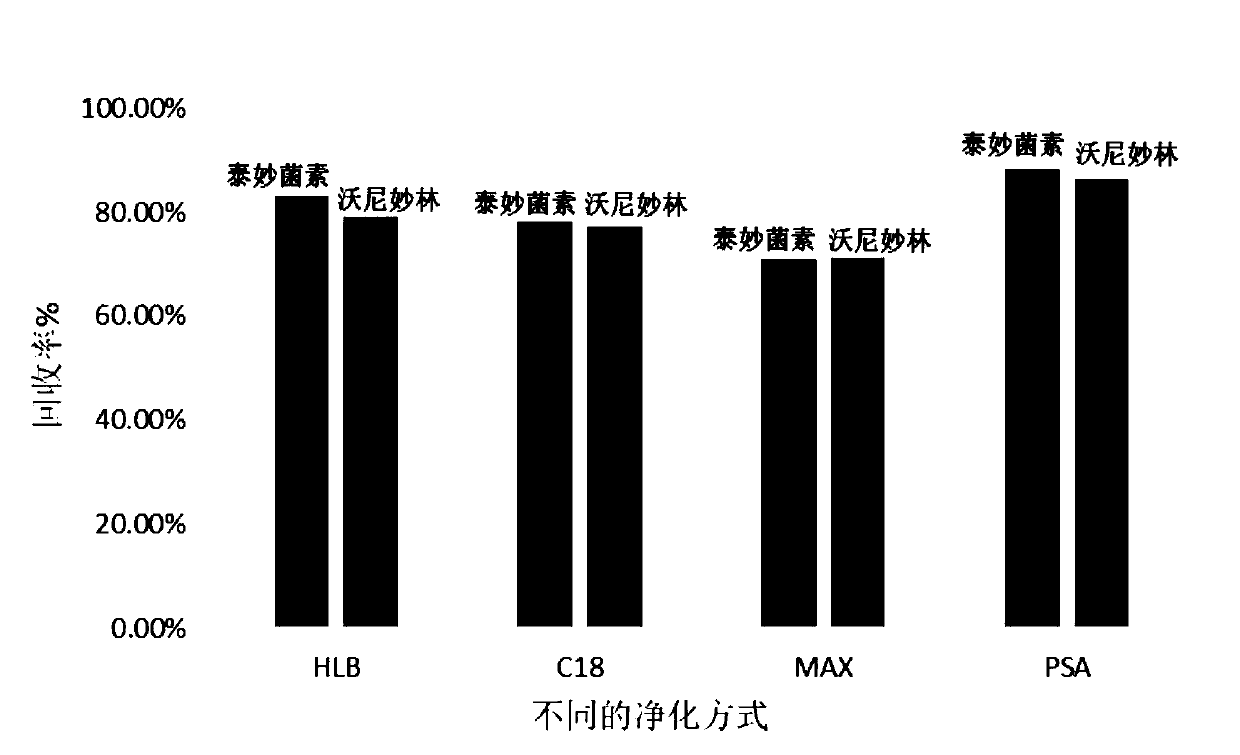
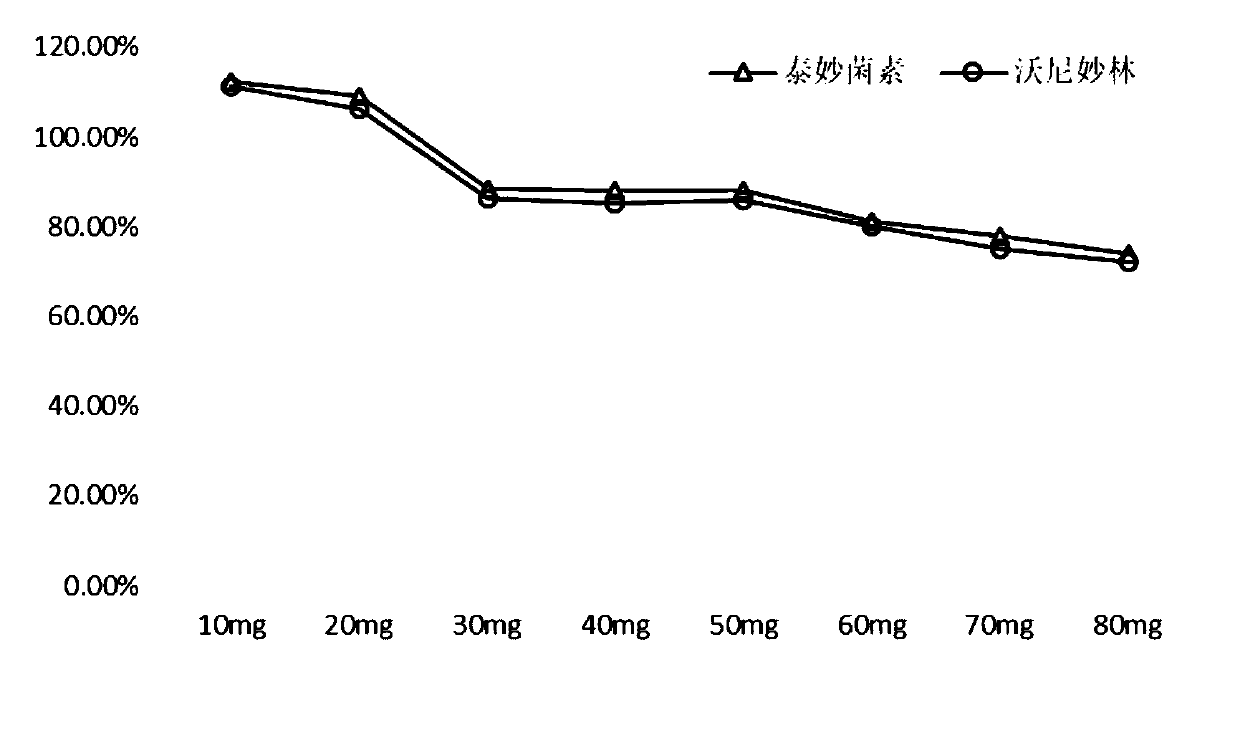
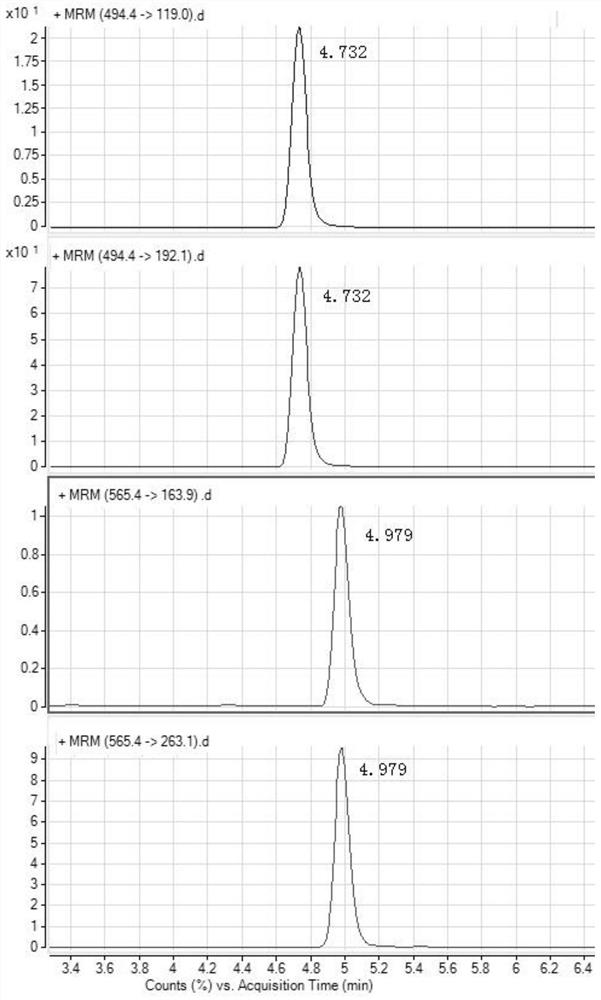
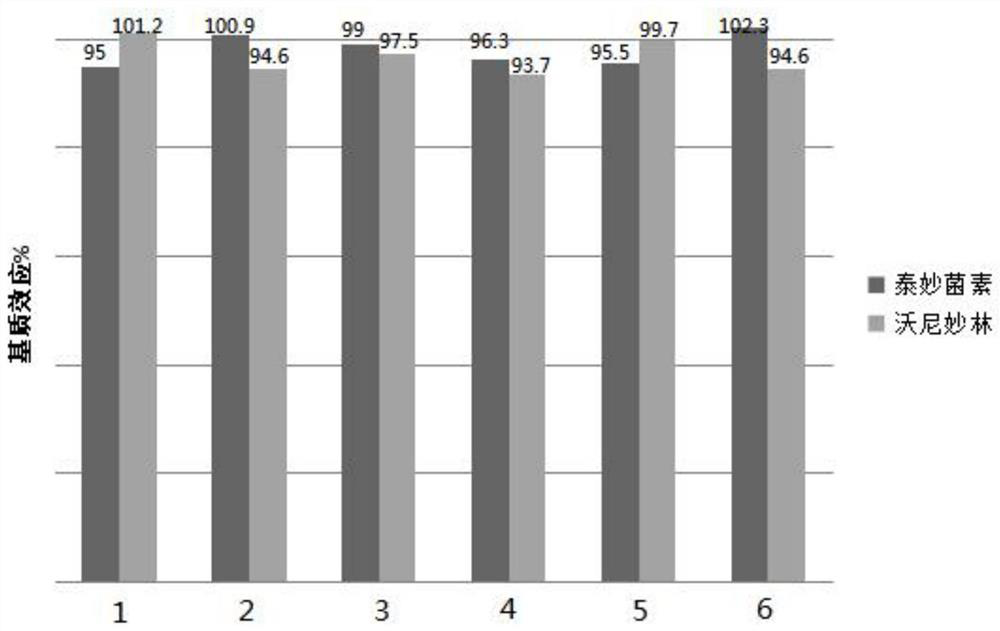
The invention discloses a detection method for tiamulin and valnemulin in an aquatic product. The detection method comprises the following steps of (1) sample pre-processing; (2) extraction; (3) purification; and (4) concentration and conversion solution. A dispersive solid-phase extraction-ultra high performance liquid chromatography-tandem mass spectrometry measurement technology is employed, the experiment efficiency is improved by optimizing instrument condition, sample pre-processing, extraction mode and PSA purification condition, the detection limit of the method is 0.1 microgram / kg, the recycling rate is 82.5-95.4%, the method is high in sensitivity, good in accuracy and reproducibility and high in applicability, the detection limit, the quantization limit and the recycling rate all can conform to the quality detection requirement of the aquatic product, and rapid detection of residue of veterinary drugs such as the tiamulin and the valnemulin is achieved.
Owner:MARINE FISHERIES RES INST OF ZHEJIANG
A medicinal prescription for preventing and treating porcine chronic respiratory syndrome
ActiveCN104666878BHigh antibacterial activityEasy to controlAntibacterial agentsInorganic active ingredientsWater vaporRespiratory disease
Owner:SICHUAN CHENGKANG ANIMAL PHARMA
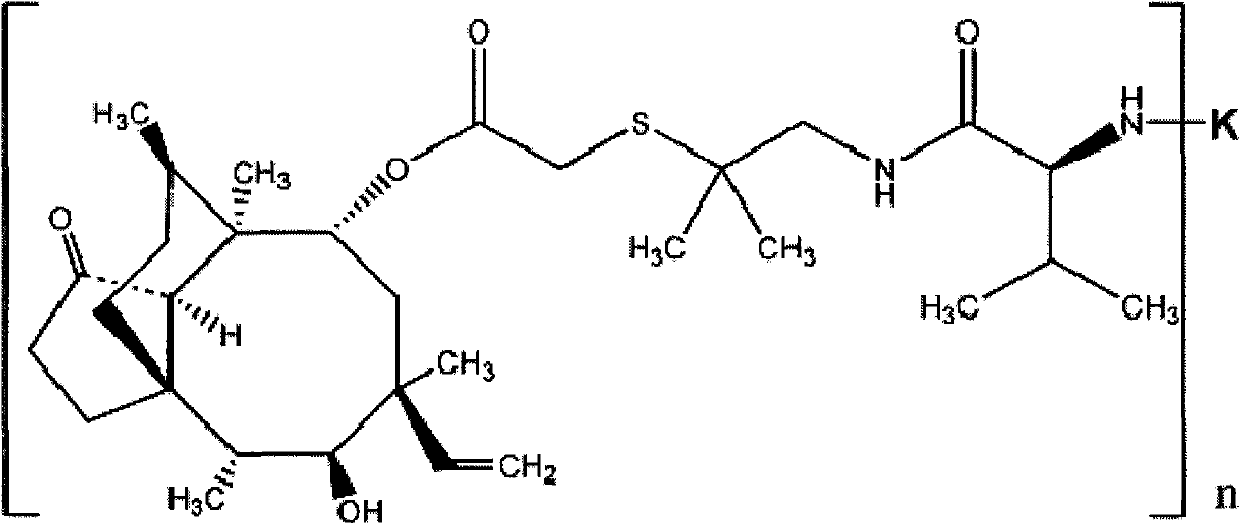
Valnemulin artificial antigen and preparation method and application thereof
ActiveCN101812128BStrong specificityHigh sensitivityOvalbuminSerum albuminSerum igeNew Zealand white rabbit
Owner:北京明日达科技发展有限责任公司
Purification method of valnemulin salt
ActiveCN104230774ALow costImprove solubilityOrganic chemistryOrganic compound preparationSalt waterSolvent
The invention relates to a method for preparing a solid valnemulin salt refined product by purifying a valnemulin salt crude product, which comprises the following steps: (1) taking a 1-15 wt% valnemulin salt water solution, adding alkali to regulate the pH value to 7-14, filtering, adding a solvent I for washing, and drying the filter cake to obtain a valnemulin alkali, wherein the solvent I is water or a low-concentration methanol solution or ethanol solution or isopropanol solution; (2) adding the valnemulin alkali obtained in the step (1) into a solvent II, dissolving, dropwisely adding water while stirring, crystallizing, filtering, washing and drying to obtain a valnemulin alkali refined product, wherein the solvent II is methanol, ethanol, isopropanol, n-butanol or any other alcohol solvent, and the volume of the solvent II is 5-100 times of the mass of the valnemulin alkali and preferably 10-20 times; and (3) adding the valnemulin alkali refined product obtained in the step (2) into an acid solution, reacting completely, and drying to obtain the pure valnemulin salt solid product. The method has the advantages of simple technique, practical production, low cost, no pollution and higher yield.
Owner:ZHEJIANG ESIGMA BIOTECH CO LTD
Tartaric acid valnemulin cyclodextrin inclusion compound and preparation method thereof
InactiveCN104415346AGood water solubilityImprove stabilityAntibacterial agentsPharmaceutical non-active ingredientsVeterinary DrugsProliferative enteropathy
The invention relates to a tartaric acid valnemulin cyclodextrin inclusion compound and a preparation method thereof, and belongs to the technical field of veterinary medicines. The tartaric acid valnemulin cyclodextrin inclusion compound provided by the invention can be used for preparing a tartaric acid valnemulin injection, soluble powder, a solvent and the like, and is used for preventing and treating animal diseases such as swine dysentery, enzootic pneumoniae, pig colon spirochetosis and proliferative enteropathies.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Crystal form of warnemulin tartrate and preparation method thereof
ActiveCN103755609BHigh crystallinityXRD pattern peak intensityOrganic compound preparationCarboxylic acid salt preparationSolubilityN dimethylformamide
The invention relates to a novel crystal form of valnemulin hydrogen tartrate and a preparation method thereof. The method comprises the steps of dissolving valnemulin hydrogen tartrate with the purity being over 95% into a mixed solvent of an ester solvent and N,N-dimethylformamide, adding an elution agent after complete dissolution so as to carry out elution crystallization, then, cooling a solution to the temperature of 5-15 DEG C, and washing, filtrating and drying crystal slurry, thereby obtaining a valnemulin hydrogen tartrate product. An X-ray powder diffraction atlas of the crystal has characteristic peaks when a diffraction angle 2[theta] is equal to 2.5, 4.8, 7.4, 7.5, 10.1, 11.2, 11.7, 13.8 or 17.1 degrees. According to the novel crystal form, during crystallization, the yield reaches over 90%, the product purity reaches over 98%, the product is high in crystallinity, good in stability, difficult in moisture absorption and high in solubility, the water solubility at normal temperature reaches 6.5g / 100mL and is increased by about 30% compared with that of the currently marketed products, and the water stability is good, so that the product can be conveniently prepared into a premix.
Owner:TIANJIN UNIV
Method for determining content of tiamulin and valnemulin in veterinary drug preparation through solid phase extraction-high performance liquid chromatography-tandem mass spectrometry
PendingCN114755317ABest purification methodEasy to operateComponent separationGradient elutionVeterinary Drugs
The invention provides a method for determining the content of tiamulin and valnemulin in a veterinary drug preparation through solid phase extraction-high performance liquid chromatography-tandem mass spectrometry, and belongs to the technical field of drug detection. The invention provides a method for rapidly detecting tiamulin and valnemulin in a veterinary drug preparation through solid phase extraction / high performance liquid chromatography-tandem mass spectrometry. The method comprises the following steps: extracting a sample by using acetonitrile, purifying by using an MCX solid-phase extraction column, carrying out gradient elution on a C18 chromatographic column by using acetonitrile and a 0.1% formic acid aqueous solution as a mobile phase, and carrying out quantitative analysis in a liquid chromatography-tandem mass spectrometry MRM mode. The linear relation of the two detected compounds in the range of 1.0-100 ng / mL is good, the correlation coefficient is larger than 0.999, the detection limit of the method is 0.05 mg / kg, and the quantitation limit is 0.1 mg / kg. The method provided by the invention is simple, rapid and accurate, and can realize rapid determination of the veterinary drug preparation.
Owner:石家庄海关技术中心
UPLC-MS/MS detection method for multiple medicines added in traditional Chinese veterinary medicine powder
The invention relates to a UPLC-MS / MS (Ultra Performance Liquid Chromatography-Mass Spectrometry / Mass Spectrometry) detection method for multiple medicines added in traditional Chinese veterinary medicine powder, and belongs to the technical field of detection methods for medicines added in traditional Chinese medicines. According to the method, the defects of microscopic identification and thin-layer identification are broken through, ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS / MS) is adopted, parent ions and daughter ions are optimized, and mass spectrometry parameters are adjusted, 10 illegally added drugs such as amoxicillin, penicillin-V, ampicillin, ceftiofur hydrochloride, oxacillin, cloxacillin, valnemulin, metronidazole and rimantadine can be qualitatively and quantitatively detected. According to the method, parent ions and specific daughter ion fragments are used as identity characteristics of different compounds, uniqueness and specificity are achieved, the sensitivity is one thousandth to one ten thousandth of that of a liquid chromatography method, and the detection limit is low.
Owner:魏秀丽 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com