Preparation of valnemulin and hydrochloride of valnemulin
A technology of vonimulin and valine salt, which is applied in the field of preparation of vonimulin and its hydrochloride, can solve the problems such as the target product cannot meet the requirement of purity, it is difficult to realize industrialization, and the danger is increased, Achieve the effect of reducing energy consumption, reducing operating time, and simplifying process operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
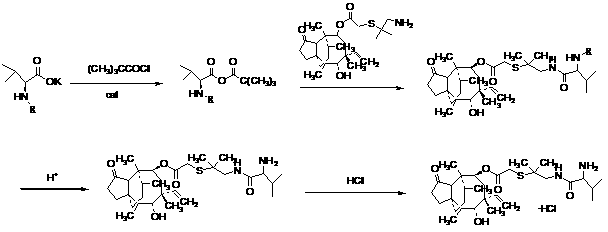
[0023] The preparation of embodiment 1 warnemulin
[0024] Add 35 g of cbz-D-valine potassium salt and 450 mL of ethyl acetate to a four-necked flask, cool down to -15°C, add 16 mL of N-methylmorpholine, and slowly add 18 mL of pivaloyl chloride dropwise to react 2 h, when cooling down to -20 ℃. Within 10 min, 30 mL of ethyl acetate solution containing 56 g of 14-O-[(2-amino-1, 1-dimethylethyl)thiomethylcarbonyl]muthrin was added dropwise, and stirred for 2 h.
[0025] Pour 500 mL of water into the solution system, adjust the pH to 1~2 with HBr aqueous solution at 20 °C, and stir for 2 h. The aqueous phase was taken, and the pH was adjusted to 9-10 with saturated NaHCO3 solution, and the obtained vonemulin solid had a yield of 75%.
Embodiment 2
[0026] The preparation of embodiment 2 warnemulin hydrochloride
[0027] Dissolve the solid obtained in Example 1 with 200 mL of methyl tert-butyl ether, adjust the pH to 1~2 with hydrochloric acid, add saturated NaCl aqueous solution to precipitate the solid, then dissolve it with ethyl acetate, add cyclohexane dropwise, crystallize, and filter , vacuum-dried at 40 °C, weighed 49.9 g, the total yield of Example 1 and Example 2 was 69%, and the purity was 98.6%.
Embodiment 3
[0028] The preparation of embodiment 3 warnemulin hydrochloride
[0029] Add 35 g of cbz-D-valine potassium salt and 500 mL of dichloromethane into a four-neck flask, cool down to -10°C, add 12 mL of pyridine, slowly add 19 mL of pivaloyl chloride dropwise to react for 1 h, and cool down to -20 °C ℃. Within 10 min, 30 mL of ethyl acetate solution containing 56 g of 14-O-[(2-amino-1, 1-dimethylethyl)thiomethylcarbonyl]muthrin was added dropwise, and stirred for 2 h.
[0030] Pour 1000 mL of water into the solution system, adjust the pH to 1~2 with HBr aqueous solution at 20 °C, and stir for 2 h. Take the water phase, use saturated NaHCO3 solution to adjust the pH to 9~10, dissolve it with 200 mL of methyl tert-butyl ether, adjust the pH to 1~2 with hydrochloric acid, add saturated NaCl aqueous solution to precipitate a solid, then dissolve it with ethyl acetate, add ring Hexane, filtered, dried under vacuum at 40 °C, weighed 53.2 g, yield 71%, purity 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com