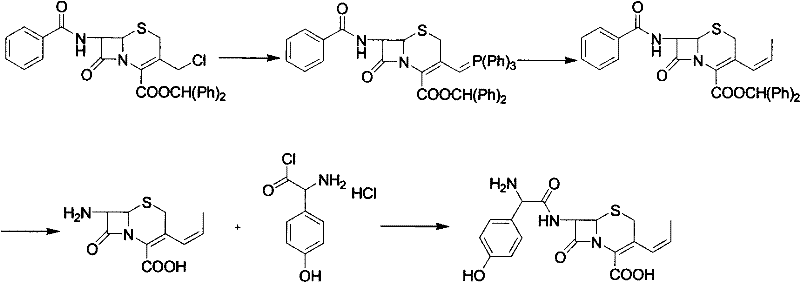
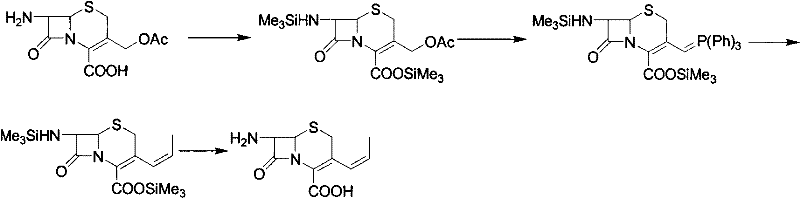
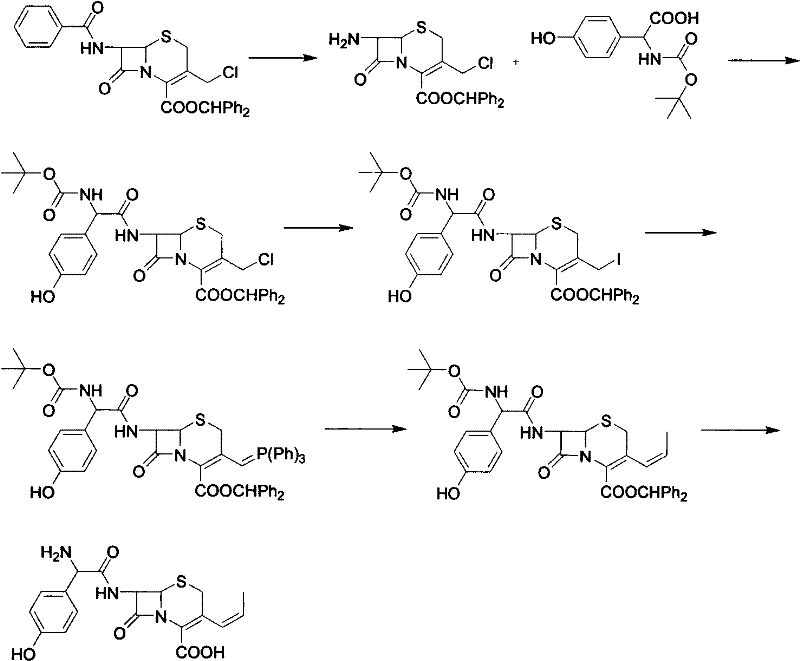
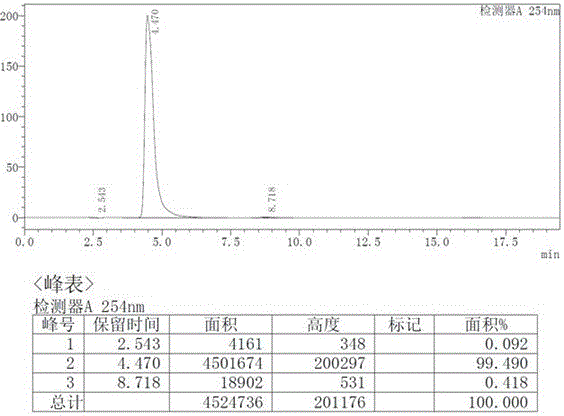
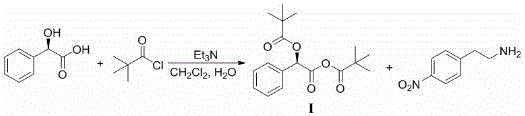
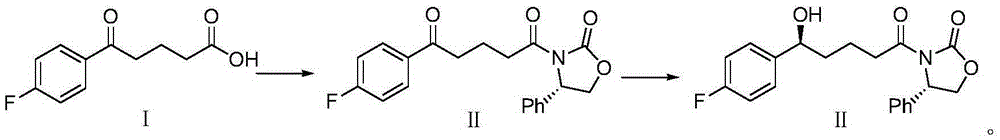
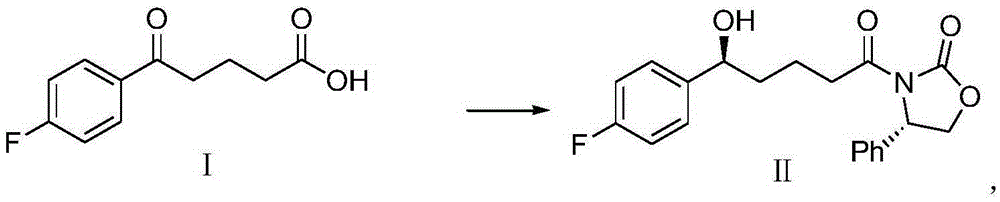
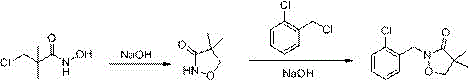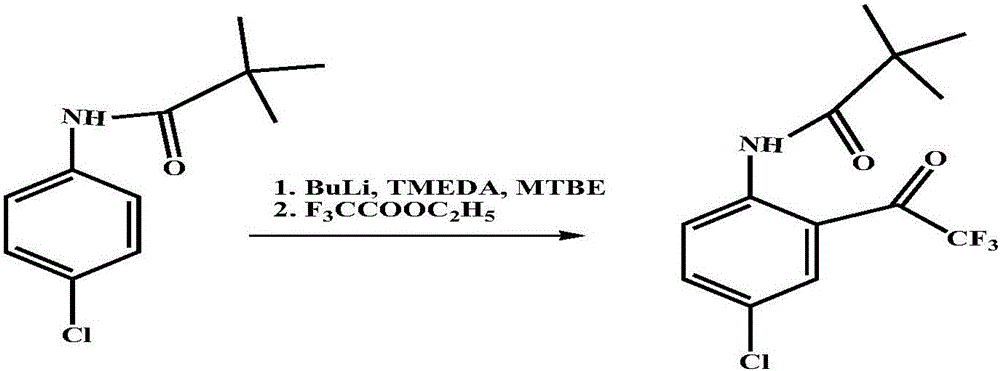Patents
Literature
100 results about "Pivaloyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
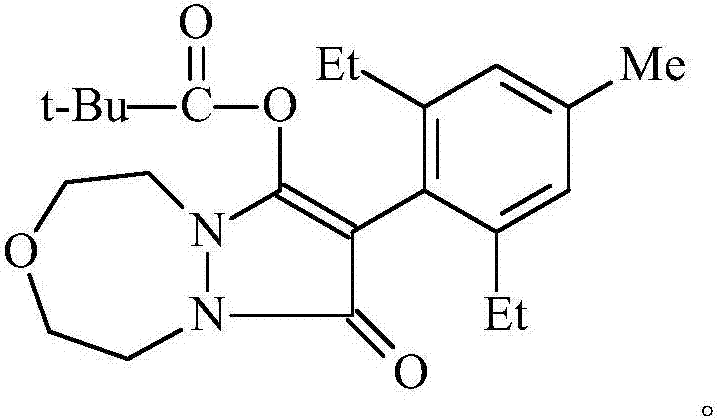
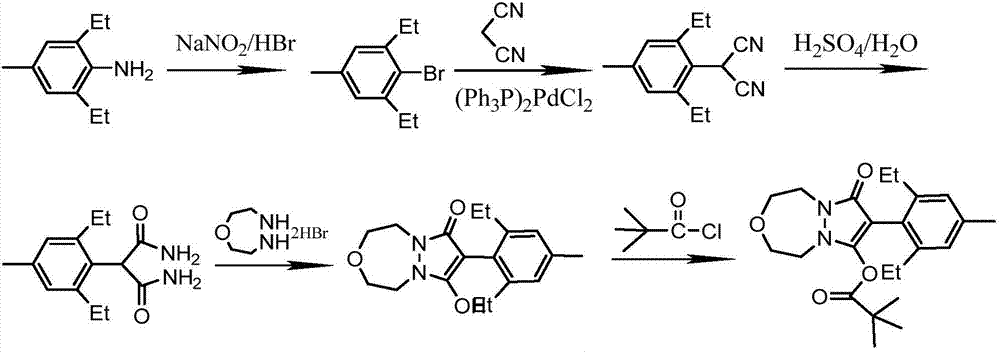
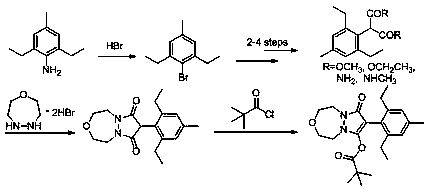
Preparation method of pinoxaden
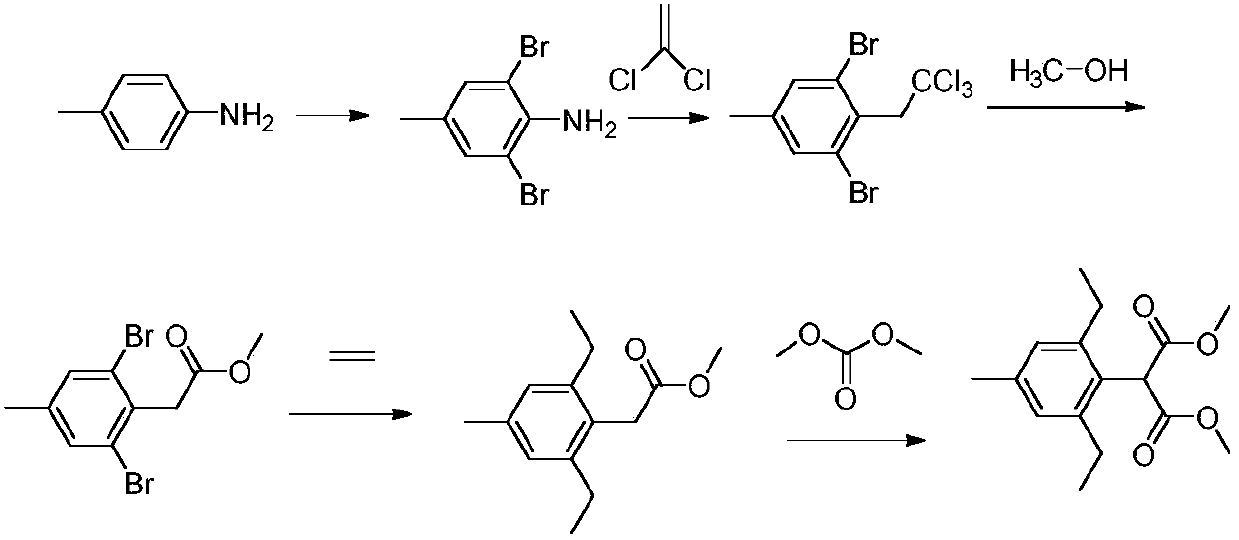
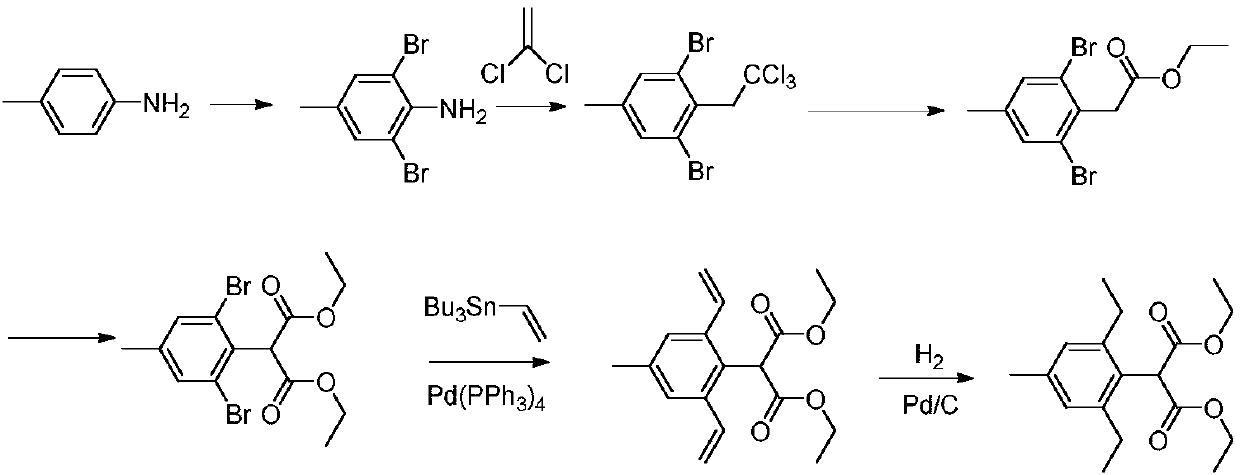
InactiveCN106928253AReduce pollutionEmission reductionOrganic chemistrySandmeyer reactionMethylaniline
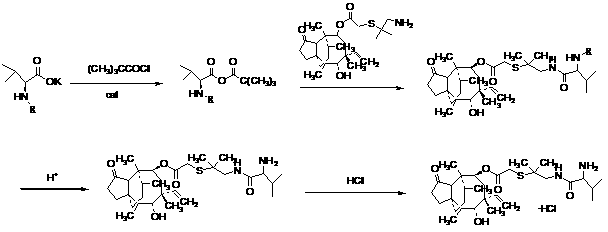
The invention relates to a method for preparing pinoxaden. The steps are as follows: 2,6-diethyl-4-methylaniline undergoes a sandmeyer reaction to obtain 2,6-diethyl-4-methylbromobenzene, and then reacts with Malononitrile is coupled under the catalysis of copper iodide to obtain 2,6-bis-ethyl-4-methylphenylmalononitrile, and finally hydrolyzed in hydrogen peroxide solution to obtain 2,6-diethyl-4 -Methylphenylmalonamide; subsequent reaction with [1,4,5]-oxadiazepine dihydrobromide in the presence of triethylamine gives 8-(2,6-diethyl- 4-methylbenzene)tetrahydropyrazole[1,2d][1,4,5]-oxadiazepine-7,9-dione, preferably reacted with pivaloyl chloride to obtain pinoxaden ester. The present invention uses low-priced catalysts to reduce production costs. In addition, hydrogen peroxide-alkali is used as the hydrolysis system in the preparation process, which greatly reduces environmental pollution.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
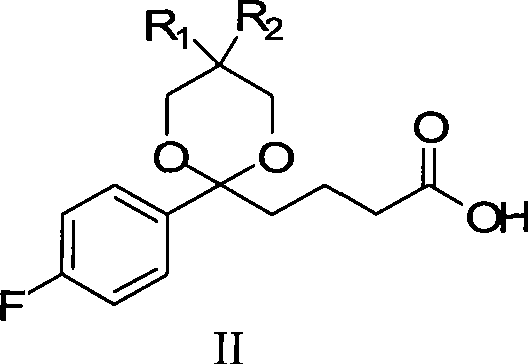
Ezetimible intermediate and synthetic method of ezetimible
InactiveCN101423511AReduce pollutionEasy to operateOrganic chemistryMetabolism disorderCompound aSynthesis methods
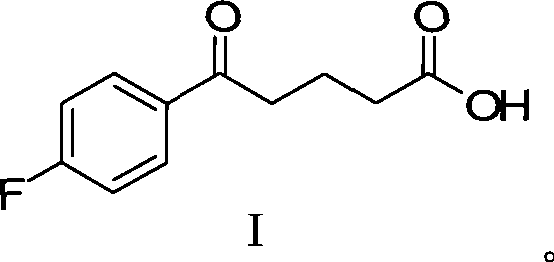
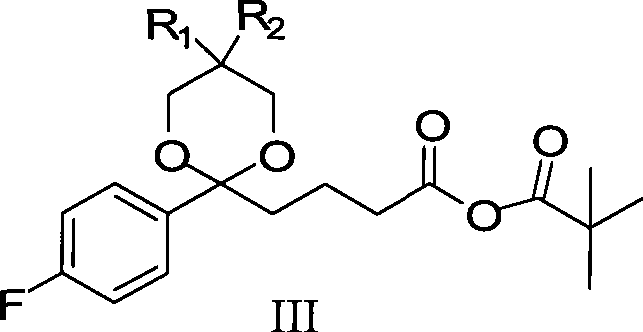
The invention provides an intermediate for synthesizing ezetimibe and a preparation process thereof, and also provides a method for synthesizing the ezetimibe by the intermediate. The synthesizing method has short route, and comprises the following concrete steps: a compound I and substituted 1, 3-propanediol react to generate a compound II; the compound II and pivalyl chloride react to generate a compound III; the compound III and a compound A react to generate a compound IV; the compound IV and a compound V react to generate a compound VI under the condition of a titanium compound catalyst; the compound VI is re-ringed to generate a compound VII with beta-lactam; the compound VII is hydrolyzed to produce a compound VIII; and the compound VII is reduced to a compound IX ezetimibe by a borane chiral reducing agent. The synthesization has short route and mild reaction condition; and the produced intermediate and final product has high yield and high purity.
Owner:ENANTIOTECH CORP
New synthesis process of canagliflozin
The present invention discloses a new synthesis process of canagliflozin. The new synthesis process comprises: adopting 2-methyl benzoic acid as a starting raw material, and adopting a self-made catalyst, iodic acid and iodine to carry out a reaction to produce an intermediate 1, or adopting 2-methyl benzoic acid as a starting raw material, and adding liquid bromine under effects of a metal reagent and a catalyst to synthesize an intermediate 2; optionally selecting the intermediate 1 or 2 to carry out an acylation reaction with thionyl chloride, and then carrying out a Friedel-Crafts reaction to produce an intermediate 3; adopting ALPHA-D-glucose as a raw material, carrying out a reaction with pivaloyl chloride to protect all hydroxyl, and carrying out a reaction with zinc bromide and bromotrimethylsilane to produce an intermediate 4; linking the intermediate 3 and the intermediate 4 to produce an intermediate 5; and finally under an acid condition, removing the pivaloyl to produce the target compound. The new synthesis process has characteristics of high yield, mild condition, safety, reliability, cheap and easily available raw material, and easy production cost control, and is suitable for industrial production.
Owner:HAIMEN RUIYI MEDICAL TECH
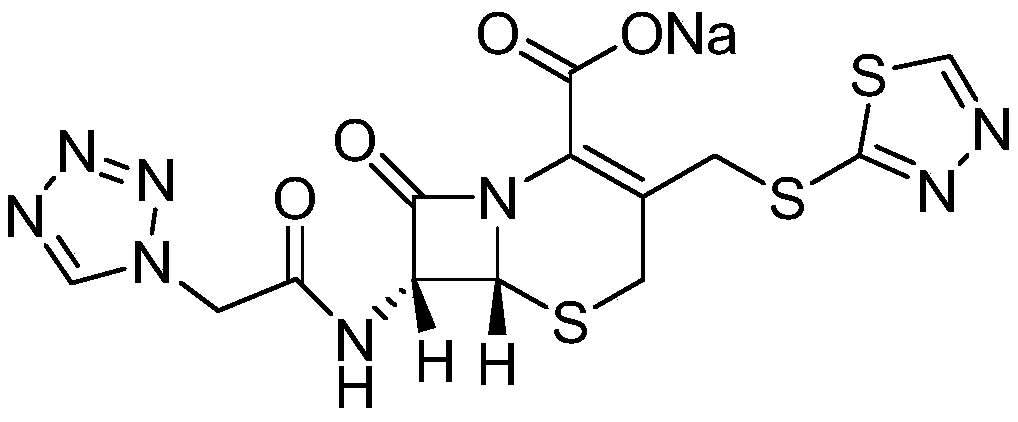
Method for preparing ceftezole sodium compound
ActiveCN102617606AReduce pollutionHigh yieldAntibacterial agentsOrganic chemistryCeftezole SodiumMethyl carbonate
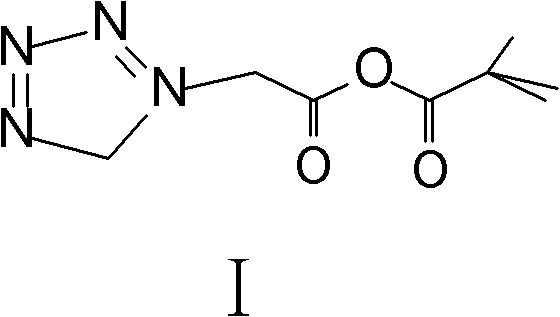
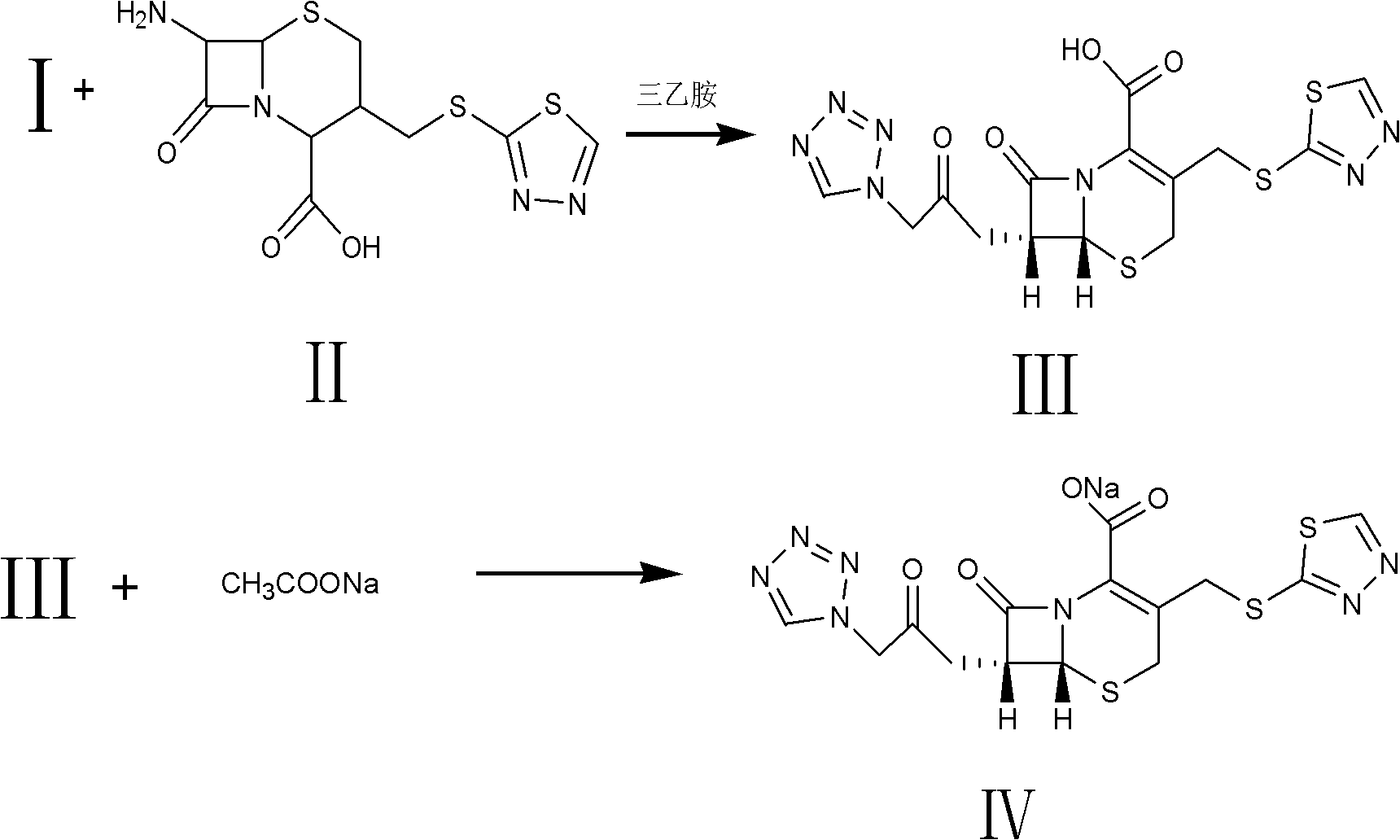
The invention relates to a method for preparing an antibacterial compound, in particular to a method for preparing a ceftezole sodium compound. The method comprises the following steps of: 1, synthesizing TZT II, and reacting 2-mercapto-1,3,4 thiadiazole and 7-aminocephalosporanic acid (ACA) to obtain TZT, wherein dimethyl carbonate is used as a reaction solvent; a boron trifluoride-dimethyl carbonate complex is used as a catalyst; after reaction, the agent used by adjusting pH of reaction liquid is sodium carbonate; the weight ratio of boron trifluoride to 7-ACA is 0.7 to 1.3; and 2, synthesizing anhydride I, namely reacting 1H-tetrazole-1-acetic acid and pivaloyl chloride to obtain anhydride; 3, synthesizing ceftezole III, namely reacting TZT II and anhydride I to obtain ceftezole; and 4, synthesizing ceftezole sodium IV, namely reacting ceftezole and sodium salt to obtain ceftezole sodium IV, wherein salt is sodium hydroxide.
Owner:哈药集团股份有限公司 +1
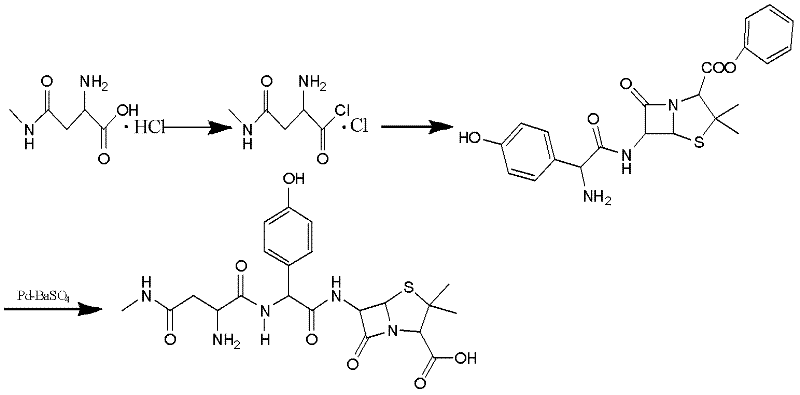
Preparation method for Aspoxicillin
The invention discloses a preparation method for Aspoxicillin. D-aspartic acid is added into a mixture liquid of sulfuryl chloride and carbinol under a low temperature of zero to prepare D-aspartic acid methyl ester hydrochloride; the obtained D-aspartic acid methyl ester hydrochloride and triethylamine are reacted in ethanol to obtain D-aspartic acid methyl ester educt; the D-aspartic acid methyl ester educt and methylamine aqueous liquid with a concentration of 40 percent are reacted in a room temperature to prepare aspartic formamide; the aspartic formamide, ethyl acetoacetate and potassium hydroxide are reacted in isopropanol to prepare diene salt (D-2-amino-3N-methylamino oxo-propionic acid diene formamide); the diene salt and pivaloyl chloride are reacted in acetone under the catalysis of pyridine to obtain active anhydride; then the active anhydride is condensed and further protected by deacidification to obtain a target product-crude product of Aspoxicillin. The preparation method for Aspoxicillin has the advantages of cheap and easily-obtained reagent, lower toxicity and lower environment pressure, stable and simple technological operation and high yield.
Owner:SOUTHWEST JIAOTONG UNIV
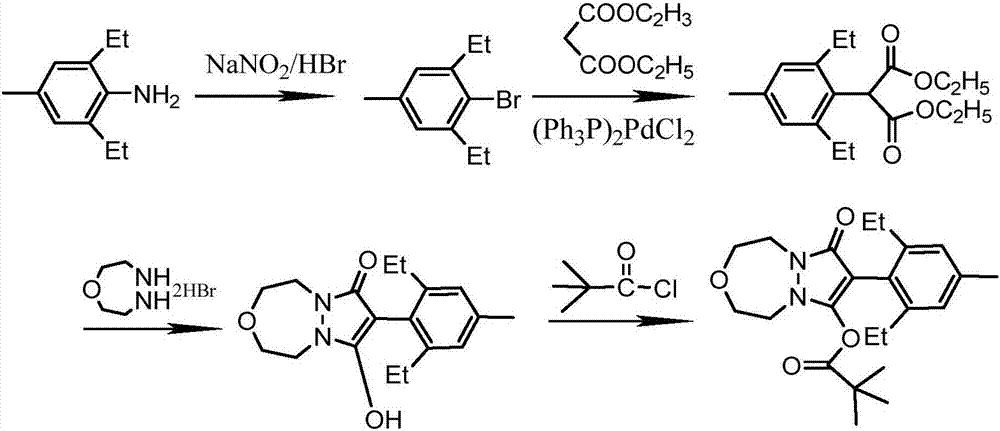
Synthesis method of pinoxaden
The invention discloses a synthesis method of pinoxaden, and belongs to the field of chemical engineering. The method comprises the following steps that 2,6-diethyl-4-methylaniline is subjected to sodium nitrite diazotization and thermal decomposition to generate 2,6-diethyl-4-methylbromobenzene; diethyl malonate is added into [1,4,5]-oxadiazepine dihydrobromide hydrate and an alkaline catalyst tosynthesize dihydro-1H-pyrazolo[1,2-d][1,4,5]-oxadiaza-7,9(2H, 8H)-diketone, the dihydro-1H-pyrazolo[1,2-d][1,4,5]-oxadiaza-7,9(2H, 8H)-diketone and 2,6-diethyl-4-bromomethyl benzene are subjected tocoupling reaction to obtain 8-(2,6-diethyl-4-methyl phenyl)tetrahydropyrazolo[1,2-d][1,4,5]xazepine-7, 9(2H, 8H)-diketone; the 8-(2,6-diethyl-4-methyl phenyl)tetrahydropyrazolo[1,2-d][1,4,5]xazepine-7, 9(2H, 8H)-diketone is reacted with pivaloyl chloride to synthesize the final product pinoxaden. According to the synthesis method of the pinoxaden, the synthesis steps are shortened, operation is simple and convenient, the cost is reduced, the economic efficiency is high, and environmental pollution is little.
Owner:兰州精细化工有限责任公司 +1
Preparation method of clomazone
The invention relates to a preparation method of clomazone, wherein an intermediate, 4,4-dimethylisoxazole-3-one, is synthesized by dropwise adding chloro-pivaloyl chloride to a raw material, hydroxylamine hydrochloride, under the effects of a self-made ether catalyst; and then the intermediate is condensed with o-chlorobenzyl chloride under the effects of a caustic soda flake catalyst to synthesize a raw drug of the clomazone. The preparation method uses easy-to-obtained raw materials and mild reactions, is high in yield and is simple in separation and purification, has low cost, is environment-friendly, and has great industrial application prospect.
Owner:江苏长青生物科技有限公司
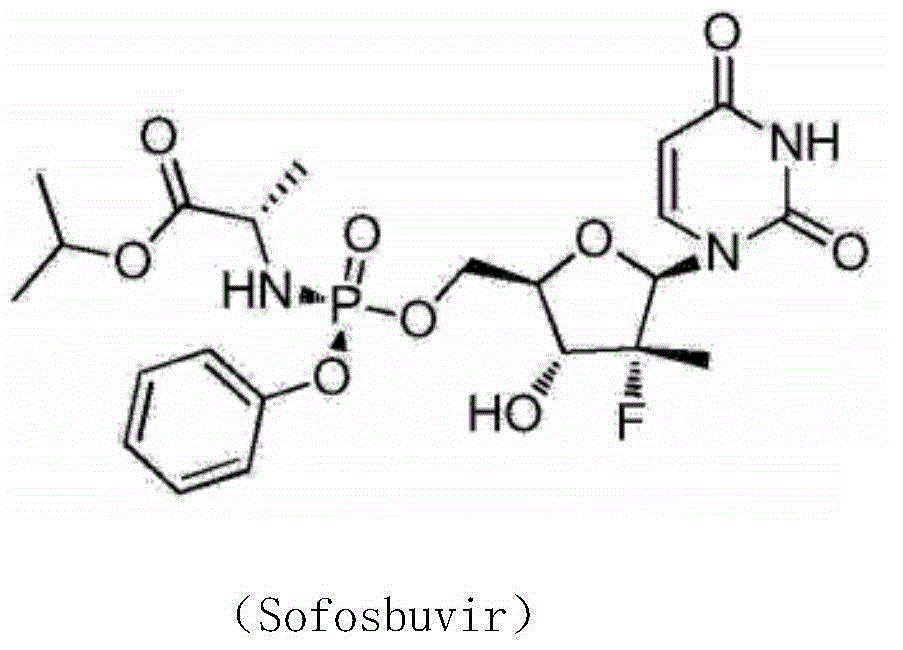
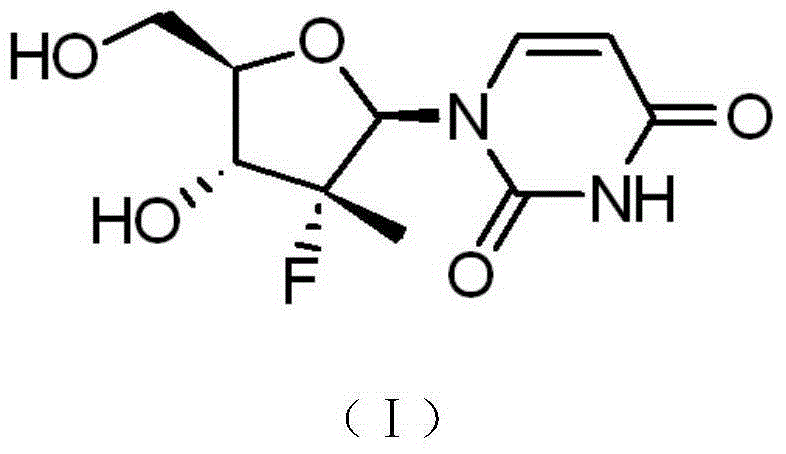
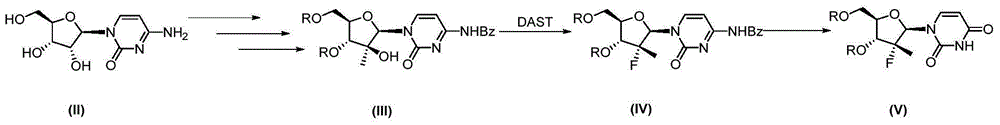
Synthesis method of intermediate compound of sofosbuvir
InactiveCN104987355AHigh yieldMild reaction conditionsSugar derivativesSugar derivatives preparationSodium methoxideSynthesis methods
The invention provides a synthesis method of an intermediate compound of sofosbuvir as shown in formula (I) (see specification). The synthesis method of the intermediate compound of sofosbuvir, a synthetic route of which is as follows: (see specification) the synthesis method comprises the following steps: taking (3R, 4R, 5R)-3-fluoro-dihydro-4-hydroxl3-methyl furan-2(3H)-ketone as an initial raw material to react with pivaloyl chloride to generate an intermediate product (XII); reducing the intermediate product to obtain an intermediate product (XIII); enabling the intermediate product (XIII) to react with pivaloyl chloride to generate an intermediate product (XIV); generating an intermediate product (XV) by virtue of the reaction of the intermediate product (XIV) and a hydrogen bromide; performing silyl-hilbert-johnson reaction for the intermediate product (XV) and uracil to generate an intermediate product (XVI); enabling the intermediate product (XVI) to react with sodium methoxide to obtain a target product. The synthesis method of the intermediate compound (I) is moderate in reaction condition, simple in procedure, low in cost, environment-friendly and favorable for the industrialized production.
Owner:SHANGHAI TWISUN BIO PHARM
Method for preparing cefazolin compounds
ActiveCN102617607ASimple recycling processHigh recovery rateAntibacterial agentsOrganic chemistryCefazolin SodiumMethyl carbonate
The invention belongs to the field of pharmacy and relates to a method for preparing cefazolin compounds. The method comprises the following steps that: 1, cefazolin sodium imidazo (toluene diamine TDA) is synthetized, thiadiazole and 7-ACA are obtained through reaction, dimethyl carbonate is used as solvents in the reaction, boron trifluoride-dimethyl carbonate is used as catalysts, and reagents used for regulating the pH of the reaction liquid are inorganic alkali after the reaction is completed; 2, anhydride is prepared, and the anhydride is obtained through the reaction between tetrazole acetic acid and pivaloyl chloride, and 3, the cefazolin is synthesized, TDA solution reacts with the anhydride, and the reaction solution is subjected to decoloration and purification through an aluminium oxide column.
Owner:哈药集团股份有限公司 +1
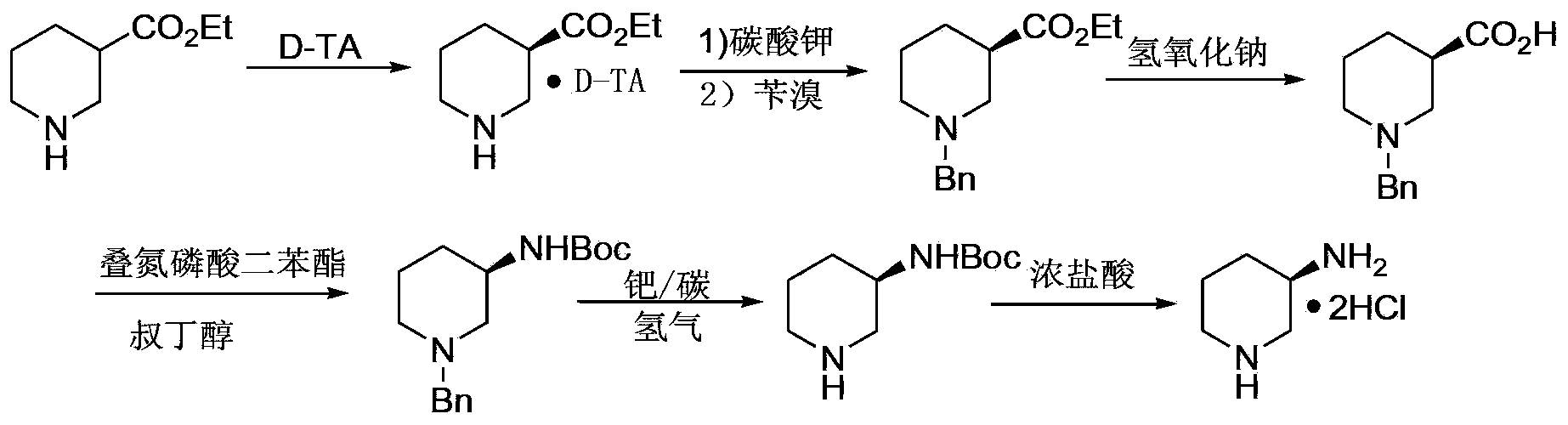
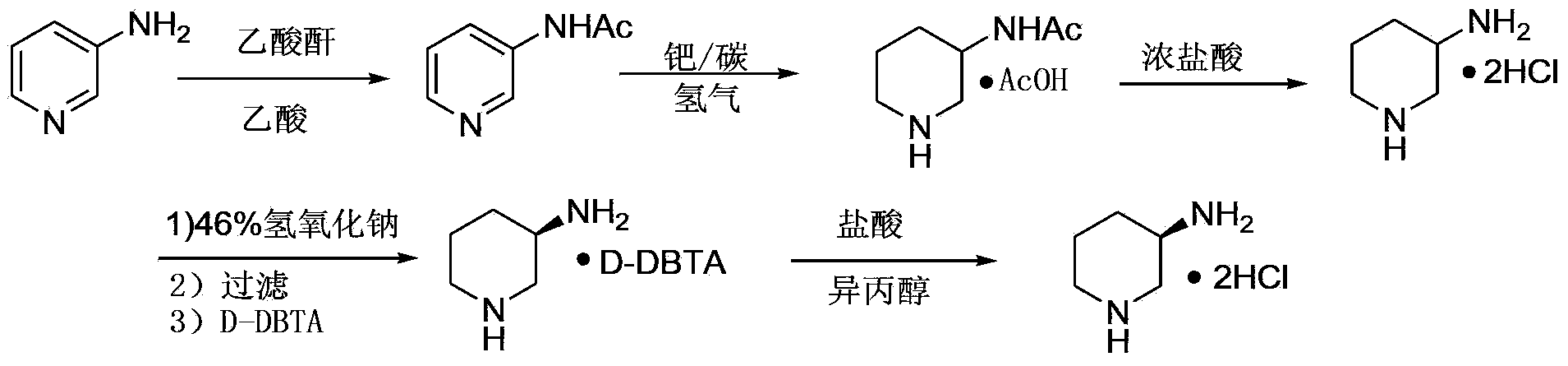
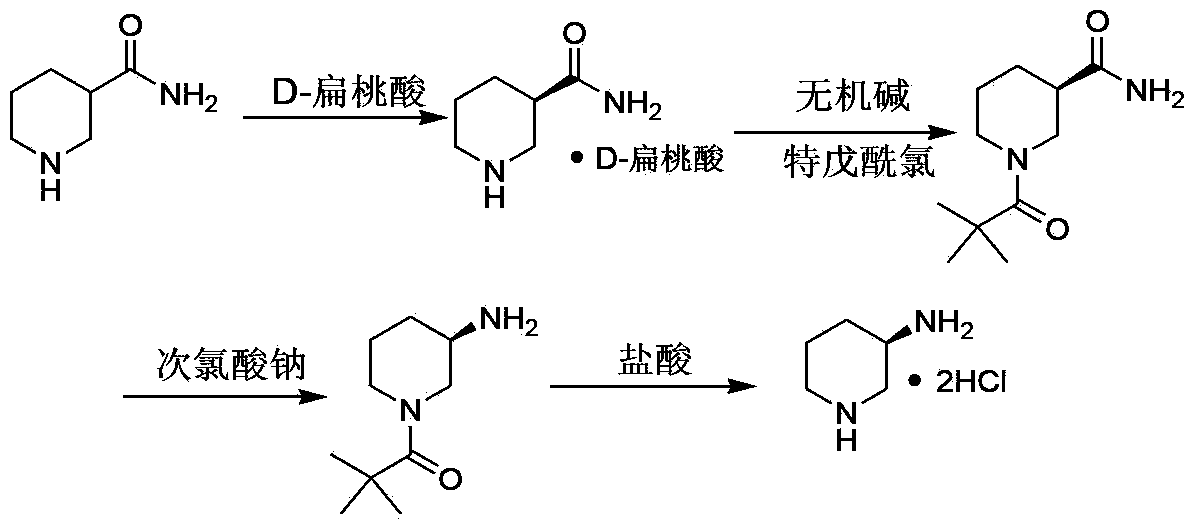
(R)-3-amino piperidine hydrochloride preparation method
ActiveCN103435538AMild reaction conditionsEase of industrial productionOrganic chemistryAlcoholOrganic solvent
The invention belongs to the field of organic synthesis, particularly relates to an (R)-3-amino piperidine hydrochloride preparation method, and aims to solve the technical problems about high cost, relatively low yield and difficulty in industrialization in an existing synthesis method. The technical scheme to solve the technical problems by the invention is to provide the (R)-3-amino piperidine hydrochloride preparation method. The preparation method comprises the following steps: a, enabling D-mandelic acid and racemic 3-piperidine amide to react in an organic solvent to generate D-mandelic acid organic salt of (R)-3-piperidine amide; b, regulating the pH of the product prepared in the step a to 10-11 and enabling the product to react with pivaloyl chloride to prepare (R)-N-valeryl-3-piperidine amide; c, enabling the (R)-N-valeryl-3-piperidine amide to react in a sodium hypochlorite solution to prepare the product (R)-N-valeryl-3-amino piperidine; d, enabling the (R)-N-valeryl-3-amino piperidine to react in a mixed solution of hydrochloric acid and alcohol to prepare the (R)-3-amino piperidine hydrochloride. The invention provides the novel method with low cost and high yield for preparing the (R)-3-amino piperidine hydrochloride.
Owner:ASTATECH CHENGDU BIOPHARM CORP
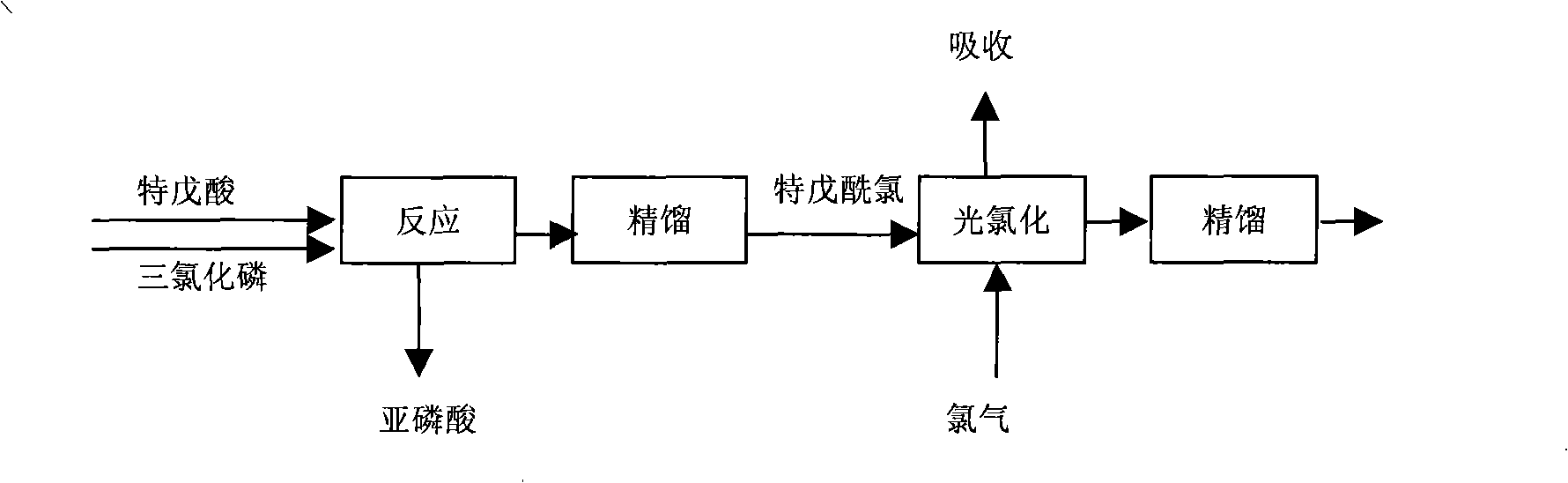
Process for preparing chloro-pivalyl chloride
ActiveCN101311155AMild process conditionsSimple and fast operationOrganic compound preparationChemical industryPivalic acidGas phase
The invention relates to a preparation method for chloro-pivaloyl chloride, in particular to a method that pivalic acid is taken as starting material to synthesize pivaloyl chloride by phosphorus trichloride acyl chlorination; then, gas-phase photocatalysis chlorination is carried out on the pivaloyl chloride by adopting reactive rectification technique; finally the chloro-pivaloyl chloride is prepared by vacuum rectification. The method of the invention can effectively inhibit the generation of multi chlorine substitutes and has simple production technique, safe operation, no pollution, low production cost and high production quality.
Owner:JIANGSU JIANNONG PLANT PROTECTION CO LTD
Preparation method of cefprozil
The invention discloses an economical and effective method for preparing cefprozil, which comprises the following steps: carrying out acylation reaction on 7-amino-3-propenylcephalosporanic acid, which is used as an initial raw material, and mixed anhydrides of p-hydroxyl phenyl glycine potassium salt and pivaloyl chloride in a solvent, hydrolyzing, and refining to obtain the cefprozil.
Owner:吴彬 +1
Synthesis technology of dutasteride
The invention discloses a synthesis technology of dutasteride, comprising the following steps of: using the acidity of DT4 carboxyl to directly react with ammonia to generate DT4-ammonium salt; dehydrating the obtained ammonium salt into DT4-amide, and performing an amino exchange reaction between amide and BTFMA in the presence of a catalyst so as to prepare dutasteride. With DT4 as a starting material, dutasteride is synthesized by three reactions of salt formation, dehydration and amino exchange. During the reactions, thionyl chloride, oxalyl chloride, pivaloyl chloride or methylsulfonyl chloride which is sensitive to the environment is avoided, special expensive catalysts such as DBU, copper powder and the like are not needed, and harmful and poisonous chemical materials are avoided. The product has good product yield and high purity, and is easy to refine. The synthesis technology has advantages of low cost and few ''three wastes'', is easy and simple to operate, conforms to green chemical synthesis requirements, and lays a good industrial foundation for realizing large-scale green clean production of high-yield and high-purity dutasteride.
Owner:HUBEI TIANSHENG PHARMA
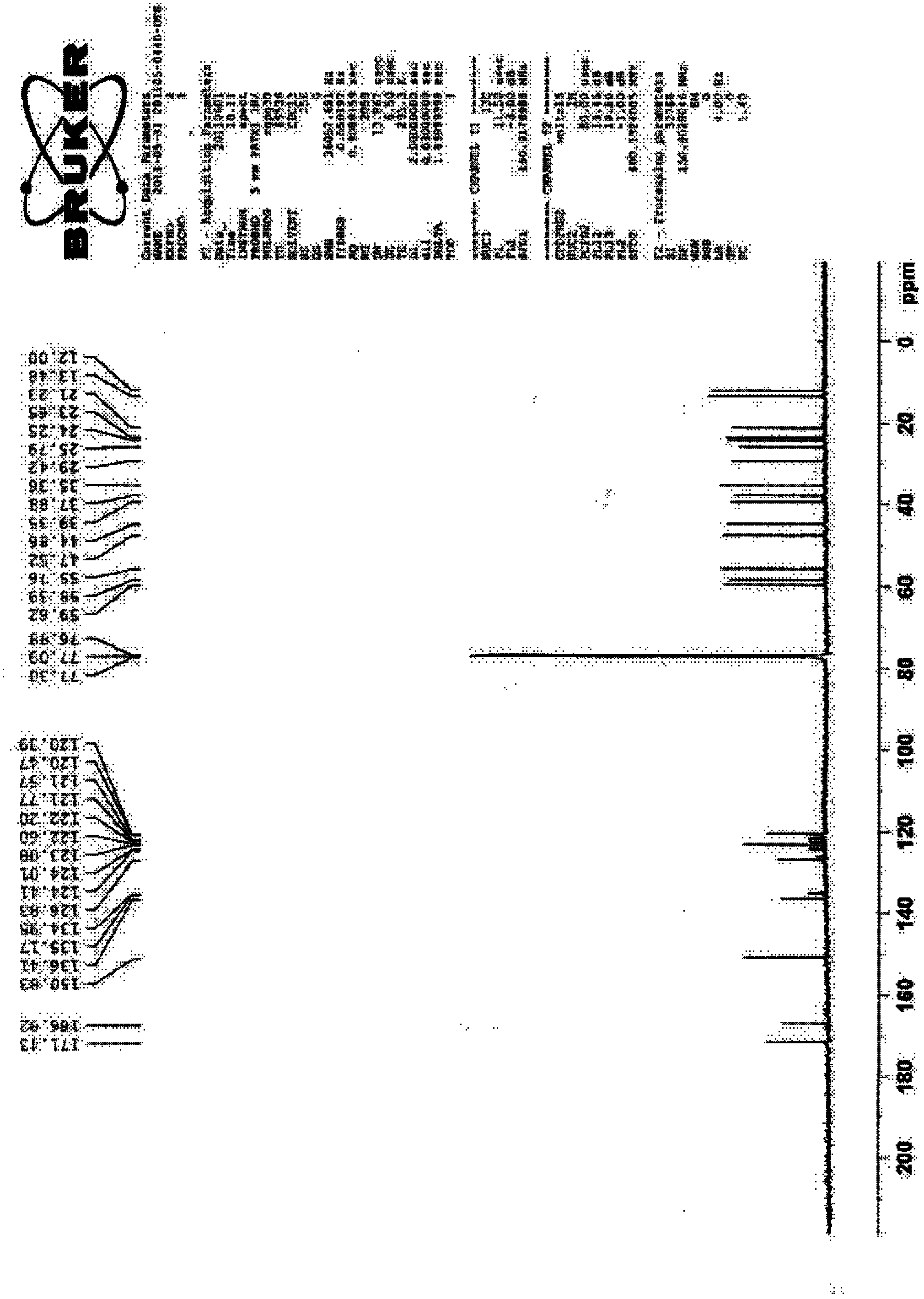
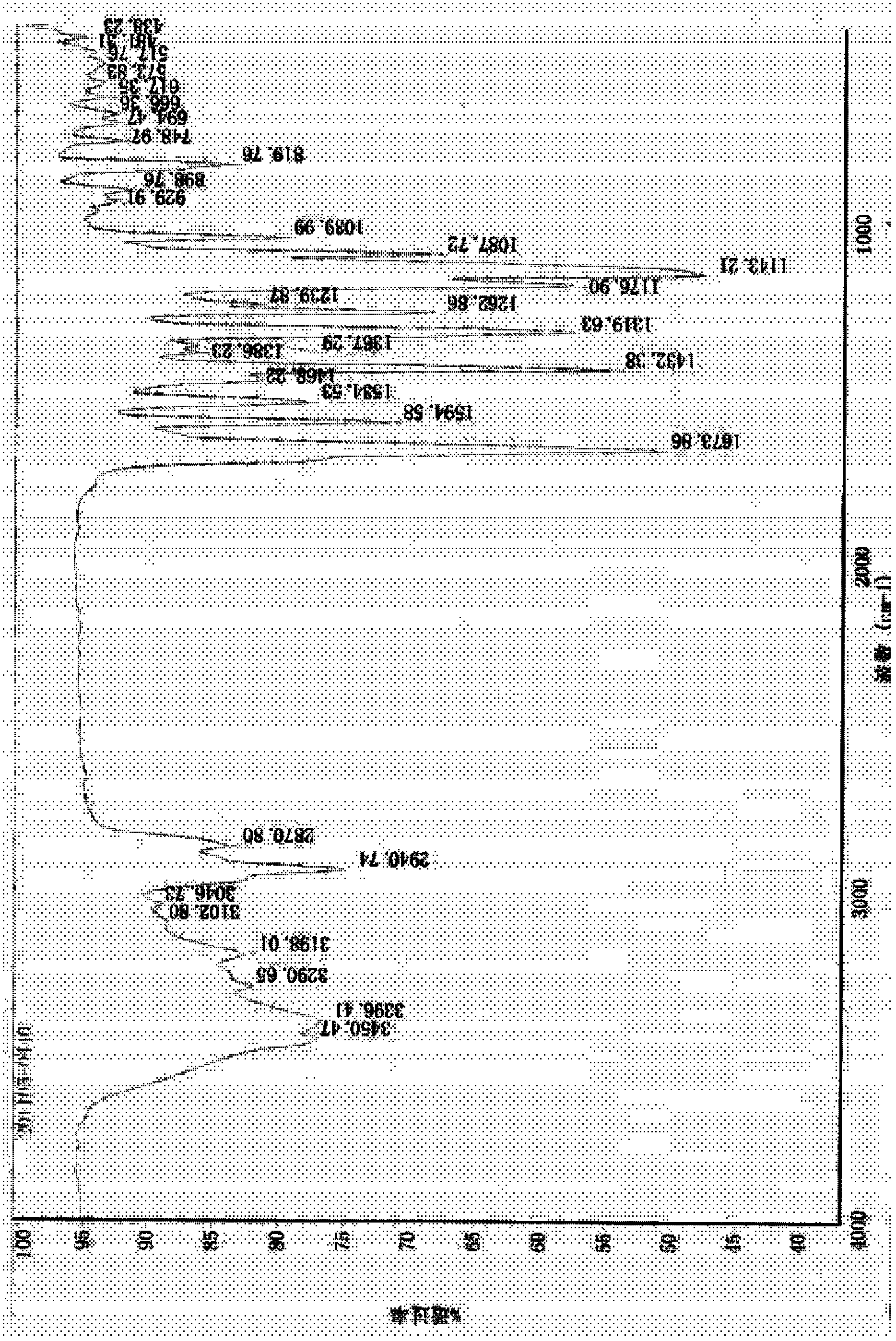
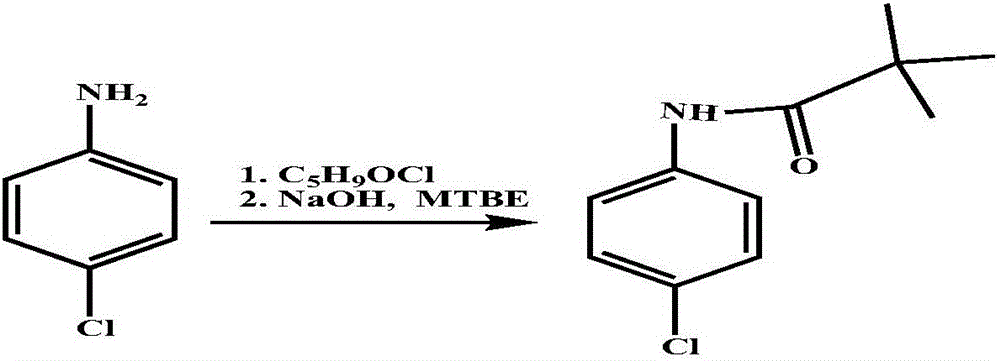
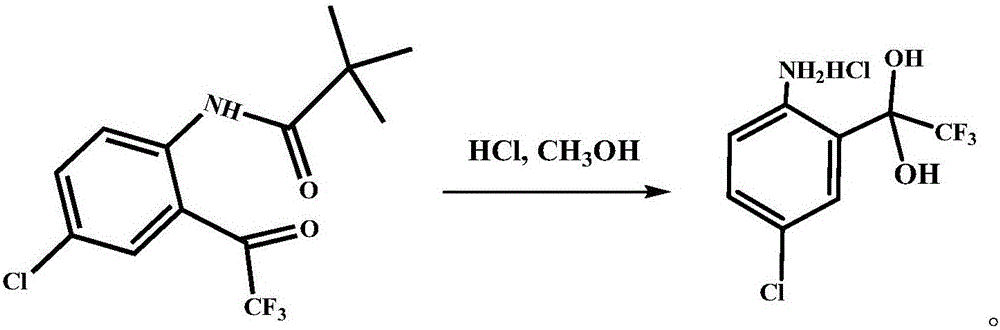
Preparation method of 4-chloro-2-(trifluoroacetyl) aniline hydrochloride hydrate
InactiveCN105801442AEmission reductionReduce processing difficultyOrganic compound preparationCarboxylic acid amides preparationN-ButyllithiumTrifluoroacetic acid
The invention discloses a preparation method of a 4-chloro-2-(trifluoroacetyl) aniline hydrochloride hydrate and belongs to the field of compound synthesis. The method adopts parachloroaniline as a raw material and comprises steps as follows: 1, parachloroaniline is subjected to a reaction with pivaloyl chloride, and 4-chloro-N-pivaloyl aniline is obtained; 2, 4-chloro-N-pivaloyl aniline is subjected to a reaction with ethyl trifluoroacetate under the action of n-butyllithium, and N-(4-chloro-2-trifluoroacetyl phenyl) pivaloyl amide is obtained; 3, N-(4-chloro-2-trifluoroacetyl phenyl) pivaloyl amide is subjected to a reaction with acid, pivaloyl is removed, and meanwhile, the 4-chloro-2-(trifluoroacetyl) aniline hydrochloride hydrate is obtained. The operation is simple, discharge of three wastes is greatly reduced, the treatment difficulty of the three wastes is reduced, the cost is low, the total recovery is up to 86.7%, and the preparation method of the 4-chloro-2-(trifluoroacetyl) aniline hydrochloride hydrate is suitable for industrial production.
Owner:SHAXING CHEM TAIZHOU CITY
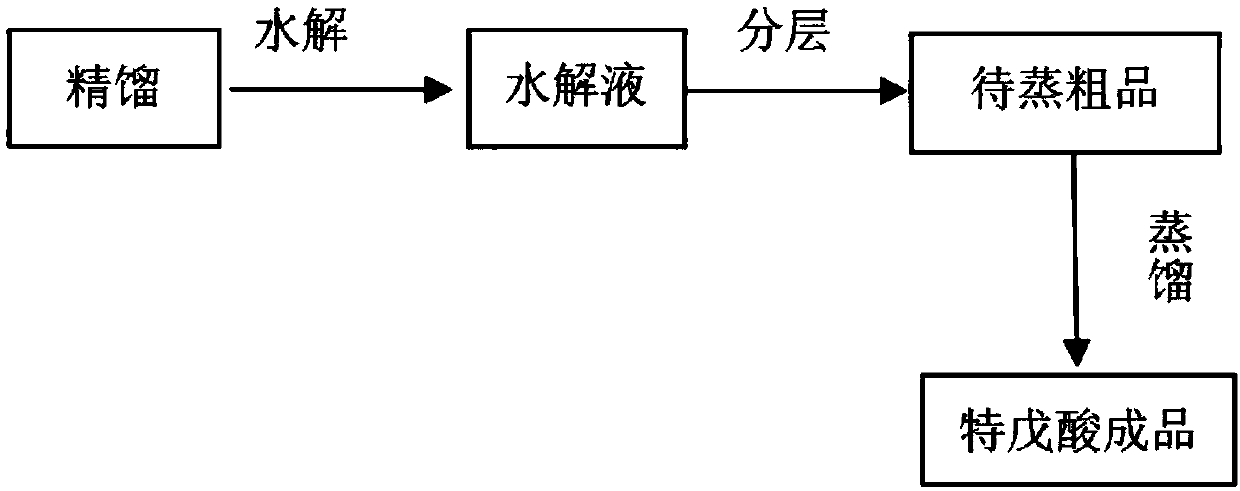
Method for recycling trimethylacetic acid from pivaloyl chloride rectification raffinate
PendingCN107721841AIncrease profitReduce pressure on environmental protectionPreparation from carboxylic acid halidePreparation from carboxylic acid anhydridesPhosphorous acidRaffinate
The invention provides a method for recycling trimethylacetic acid from pivaloyl chloride rectification raffinate. According to the technical scheme, the method has the experiment ideas that components and contents of pivaloyl chloride rectification raffinate are analyzed firstly on the basis of experiment means, on the basis, procedures such as hydrolysis, layering and rectification are confirmedaccording to chemical properties of pivaloyl chloride and trimethylacetic anhydride, and trimethylacetic acid is recycled. Specifically, the method comprises the following steps: firstly, adding a certain amount of pivaloyl chloride rectification raffinate, controlling specific temperature and pressure, adding a proper amount of water for hydrolysis reactions for multiple times to sequentially convert pivaloyl chloride and trimethylacetic anhydride into trimethylacetic acid, leaving to stand to layer water-containing phosphorous acid and a crude trimethylacetic acid product, feeding back thecrude trimethylacetic acid product to a trimethylacetic acid rectification procedure, and performing decoloring refining. By adopting the method provided by the invention, more than 90% of the pivaloyl chloride rectification raffinate can be converted into trimethylacetic acid, so that the utilization rates of raw materials are increased, the wastes are reduced, the environment protection burden of companies is alleviated, and meanwhile the production cost of products is effectively reduced.
Owner:山东民基新材料科技有限公司
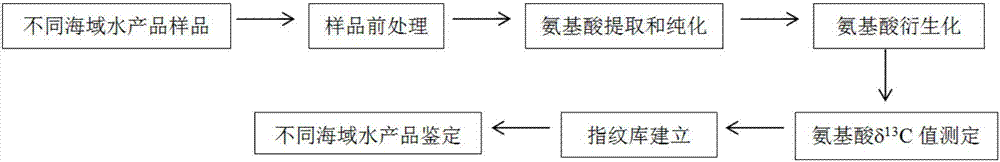
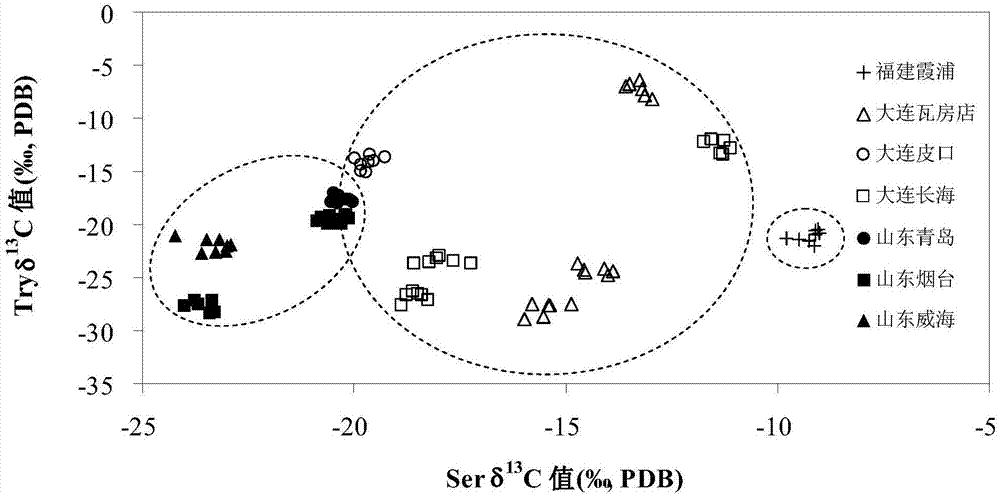
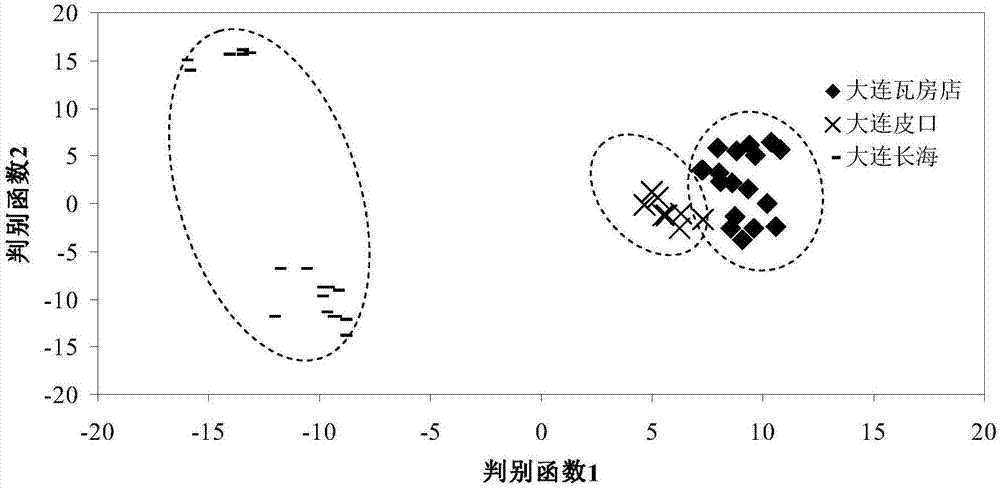
Method of tracing places of origin of aquatic products based on amino acid carbon stable isotopes
InactiveCN107255693AThe identification result is accurateSingle ingredientComponent separationEthyl acetateSilica gel
The invention discloses a method of tracing places of origin of aquatic products based on amino acid carbon stable isotopes; the method comprises the steps of subjecting a pretreated sample to amino acid extraction before purification; adding hydrochloric acid into the sample, hydrolyzing, centrifuging, subjecting supernate to purification of strong cation exchange columns, concentrating the purified liquid via nitrogen blowing process to obtain sample amino acid; adding amino acids into thionyl chloride-isopropanol solution by means of N-pivaloyl and O-isopropanol ester (NPP) process, performing amino acid derivation, blow-drying by nitrogen, acylating by pivaloyl chloride, passing through silica gel chromatographic columns, concentrating the obtained liquid by a nitrogen blowing process to obtain a derivative, dissolving in ethyl acetate for cryopreservation, and performing amino acid C stable isotope measurement, to be specific, analyzing and identifying an amino acid ester sample via a gas chromatograph-mass spectrometer; comparing amino acid delta 13C values of samples from different sea areas to identify different types of aquatics from different sea areas.
Owner:DALIAN MARITIME UNIVERSITY
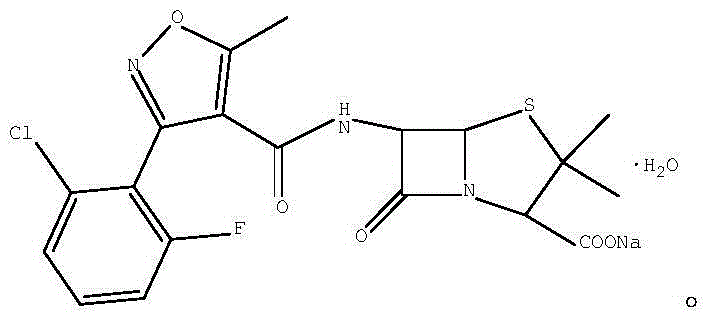
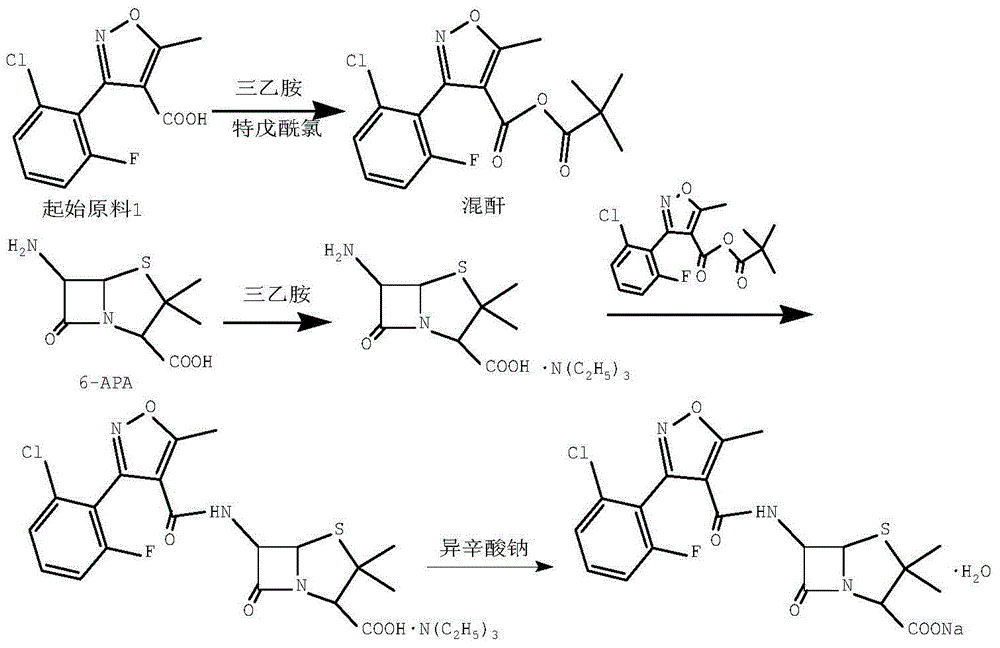
Preparation method for flucloxacillin sodium
The invention discloses a preparation method for flucloxacillin sodium. The method comprises: (1) adding an initial raw material 1 into dichloromethane, dropwise adding triethylamine, and then dropwise adding pivaloyl chloride for reaction, so as to obtain a mixed anhydride; (2) adding 6-APA into dichloromethane, then adding triethylamine, reacting until the material solution is clear, so as to obtain a 6-APA triethylamine salt solution; dropwise adding the mixed anhydride into the 6-APA triethylamine salt solution for reaction, then distilling at a reduced pressure to remove dichloromethane, so as to obtain an oily substance, then adding acetone into the oily substance, stirring, and filtering off triethylamine hydrochloride, so as to obtain an acetone solution of flucloxacillin triethylamine salt; and (4) adding water and an ethyl acetate solution of sodium 2-ethylhexanoate into the acetone solution of flucloxacillin triethylamine salt, controlling the temperature to be 0-40 DEG C after adding is finished, and crystallizing, so as to obtain flucloxacillin sodium monohydrate. The preparation method has the advantages of mild reaction conditions, high yield, good purity, simple operation, friendliness to environment, and the like.
Owner:山东安信制药有限公司
Preparation of valnemulin and hydrochloride of valnemulin
InactiveCN103193692AReduce usageHigh yieldSulfide preparationBulk chemical productionValineProtecting group
The invention relates to a preparation method of valnemulin and hydrochloride of valnemulin. The preparation method comprises the following steps of: reacting amino-protected D-valine and pivaloyl chloride to obtain mixed anhydride; carrying out condensation reaction to the mixed anhydride and 14-O-[(2-amino-1, 1-dimethyl ethyl) thiomethylcarbonyl] mutilin to prepare valnemulin with an amino protecting group; carrying out acid treatment to remove the protecting group; crystallizing to obtain valnemulin; and then reacting with an acid to obtain the hydrochloride. The preparation method is scientific and advanced in technology, and has the advantages of being high in cost, and high in product yield and purity.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Preparation method of pinoxaden
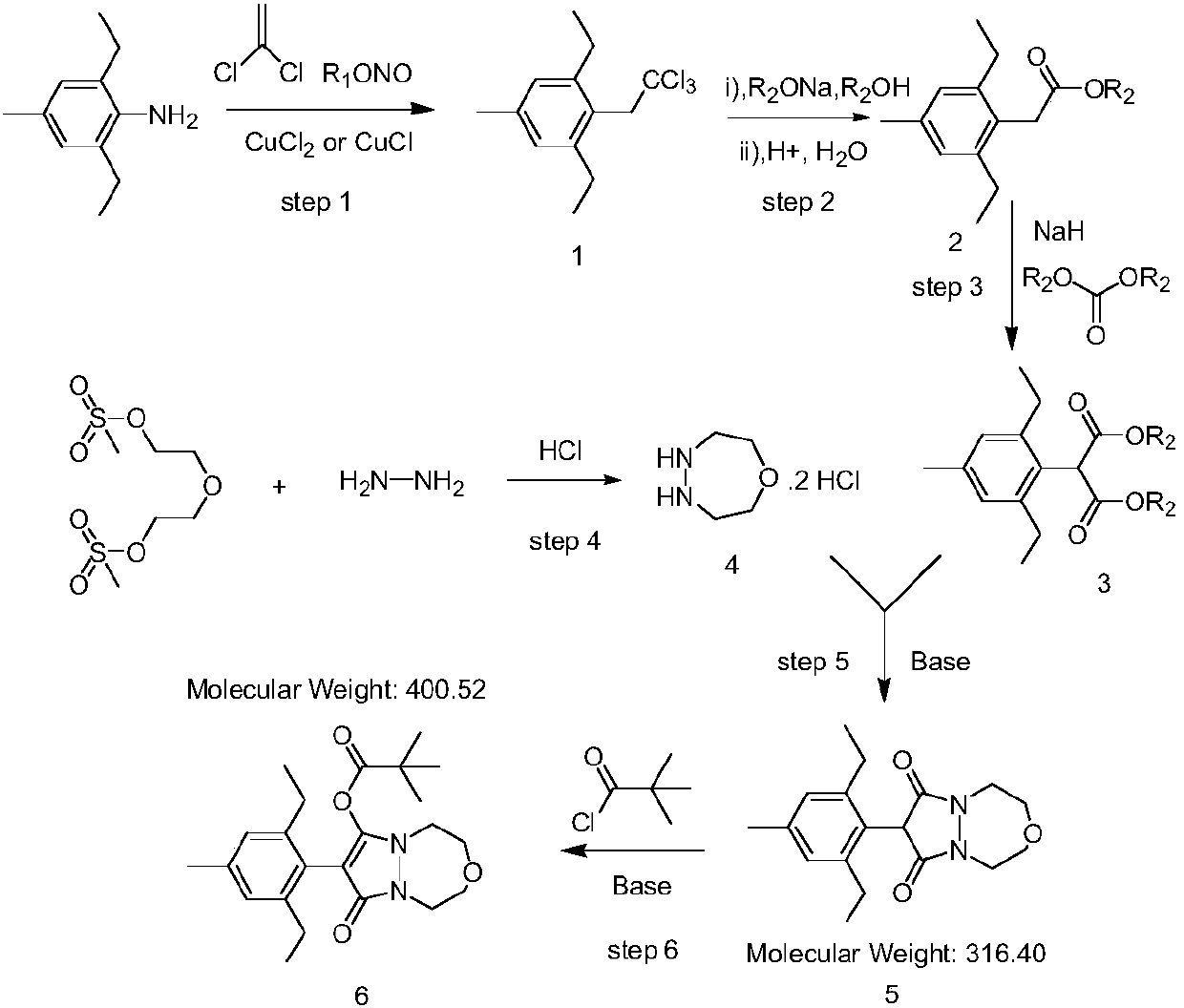
The invention relates to the field of organic synthesis, in particular to a synthesis method of pinoxaden. The synthesis method comprises the following steps that under catalysis of copper chloride orcuprous chloride, 4-methyl-2,6-diethyl aniline reacts with 1,1-dichloroethylene and nitrous acid ester to generate a compound 1; the compound 1 reacts with sodium alcoholate, and a reaction product is subjected to acidic hydrolysis to obtain a compound 2; the compound 2 reacts with diester carbonate and strong base to obtain an intermediate 3; after diethylene glycol dimethanesulfonate reacts with hydrazine hydrate, salifying with hydrochloric acid is conducted to obtain an intermediate 4; the intermediate 3 and the intermediate 4 are subjected to ring forming under the effect of base to obtain a compound 5; and the compound 5 and pivaloyl chloride react under the effect of the base or base / DMAP to obtain the pinoxaden. The synthesis method of the pinoxaden does not use expensive or toxiccatalysts, does not need to adopt a protection / deprotection strategy, and has the characteristics of low cost and high atomic economy.
Owner:JIANGSU FLAG CHEM IND
A kind of synthetic method of cefadroxil
The invention discloses a synthetic method of cefadroxil. The synthetic method of cefadroxil comprises the following steps of: dissolving 7-aminodesacetoxycephalosporanic acid by adopting organic base at the temperature ranging from minus 10 DEG C to minus 50 DEG C to obtain 7-aminodesacetoxycephalosporanate; carrying out reaction on HPCDane salt and pivaloyl chloride at the temperature ranging from minus 20 DEG C to minus 70 DEG C to generate mixed anhydride and carrying out condensation reaction on the mixed anhydride and 7-aminodesacetoxycephalosporanate, extracting and regulating pH value of a water layer, then separating salt and regulating pH value, and crystallizing to obtain cefadroxil. By adopting the manner, the synthetic method of cefadroxil has the advantages that a process is simple, solvate does not need to be formed in a synthetic process, crystallization can be directly carried out in water, consumption of an organic solvent is reduced, production cost is reduced, yield of cefadroxil is high, various quality indexes are qualified, pharmacopeia standards are met, and implementation of industrial production can be facilitated.
Owner:苏州盛达药业有限公司
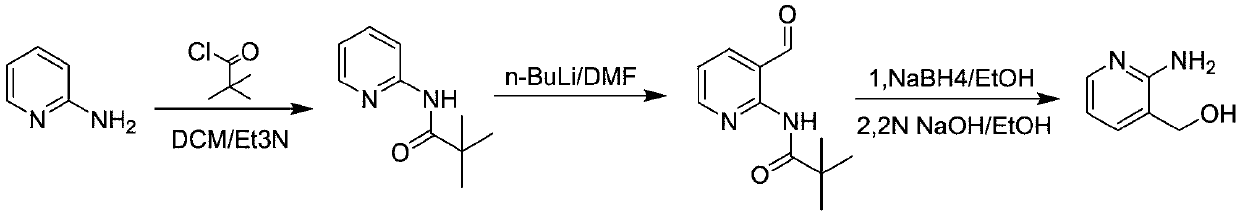
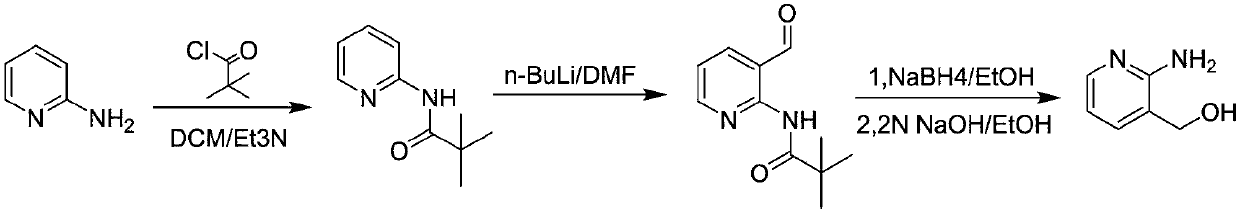
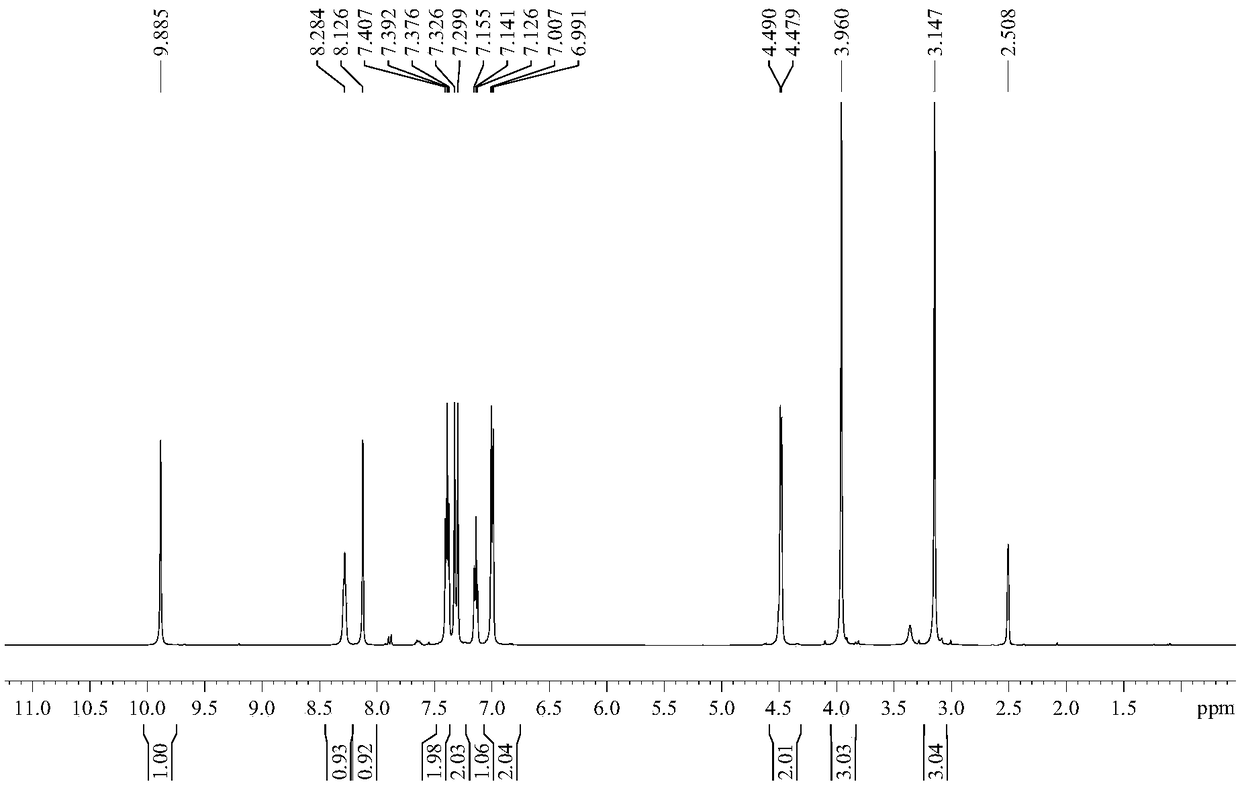
Method for preparing 2-amino-3-hydroxymethylpyridine
InactiveCN108675954ALow costShort synthesis processOrganic chemistryChemical synthesis2-Aminopyridine
The invention discloses a method for preparing 2-amino-3-hydroxymethylpyridine, and belongs to the field of chemical synthesis. 2-aminopyridine and pivaloyl chloride are used as initial reactants to form intermediate product 2-pivalamidopyridine under the action of triethylamine, after hydrogen extraction by use of n-butyllithium, dimethylformamide is added for hydroformylation, and target product2-amino-3-hydroxymethylpyridine can be obtained by reduction with sodium borohydride. Raw materials used in the method are low in cost, and the method is simple in operation, short in synthesis process, mild in reaction conditions, high in yield, and suitable for large-scale industrial production.
Owner:ASTATECH CHENGDU BIOPHARM CORP
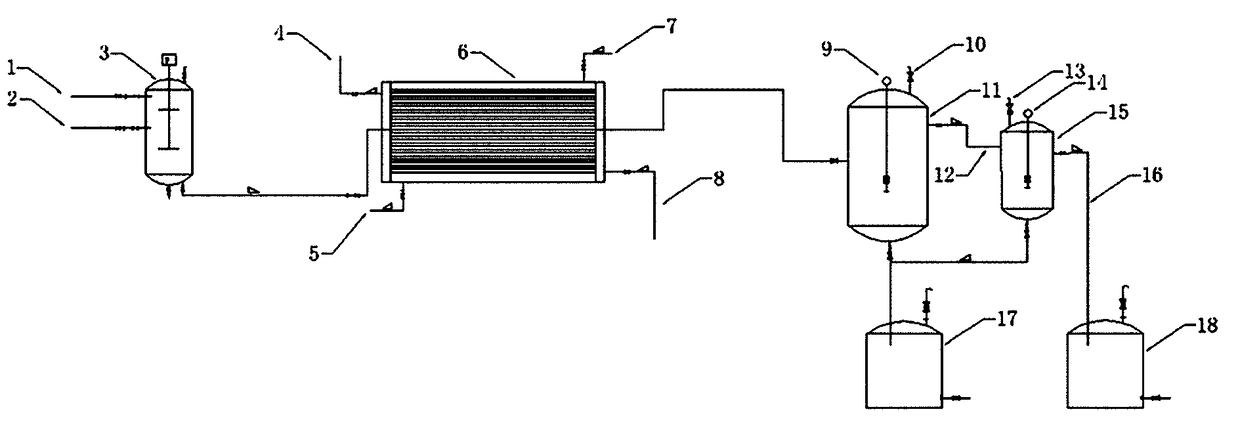
Method and device for continuously preparing pivaloyl chloride
ActiveCN109438223AHigh purityThorough responseCarboxylic acid anhydrides preparationPivalic acidPhosphorus trichloride
The invention relates to a method and a device for continuously preparing pivaloyl chloride. The specific operation method comprises the following steps: adding raw materials pivalic acid and phosphorus trichloride to a raw material mixer respectively, uniformly stirring the raw materials, and then conveying the raw materials into a microchannel reactor with a solid catalyst to prepare a chlorination solution; feeding the prepared chlorination solution into two chromatography tanks for chromatography respectively to prepare a crude product of pivaloyl chloride; finally, feeding the crude product of pivaloyl chloride into a rectification section to obtain a pure product of pivaloyl chloride. The microchannel reactor is adopted to replace an original chlorination reactor, so that the reaction is more thorough, the conversion rate is high, and purity is high; a continuous reaction is adopted to replace the original intermittent reaction, and reaction time is greatly shortened to 0.5 hoursfrom 12 hours in the prior art.
Owner:山东民基新材料科技有限公司
Preparation method of Iguratimod formylation intermediates
ActiveCN108727232AQuick responseImprove conversion rateSulfonic acid amide preparationSide reactionImpurity
The invention relates to the technical field of synthesis of chemical drugs, in particular to a preparation method of Iguratimod formylation intermediates. The preparation method comprises the following steps: mixed acid anhydride is prepared from formic acid, sodium formate and pivaloyl chloride, a compound I is added for carbamylation, and the Iguratimod formylation intermediates are obtained. Mixed acid anhydride is prepared from formic acid and pivaloyl chloride, the reaction with pivaloyl chloride is a homogeneous reaction, reaction speed is high, and conversion rate is high; the relatively acidic environment of the system is kept by formic acid, side reactions in which piperazine compounds are generated from alpha-aminoketone compounds in raw materials through own condensation reaction under alkaline conditions are avoided, yield is increased, impurities are reduced, and purity is improved. The Iguratimod formylation intermediates prepared with the method are high in purity and can be used for preparing Iguratimod.
Owner:康美(北京)药物研究院有限公司 +2
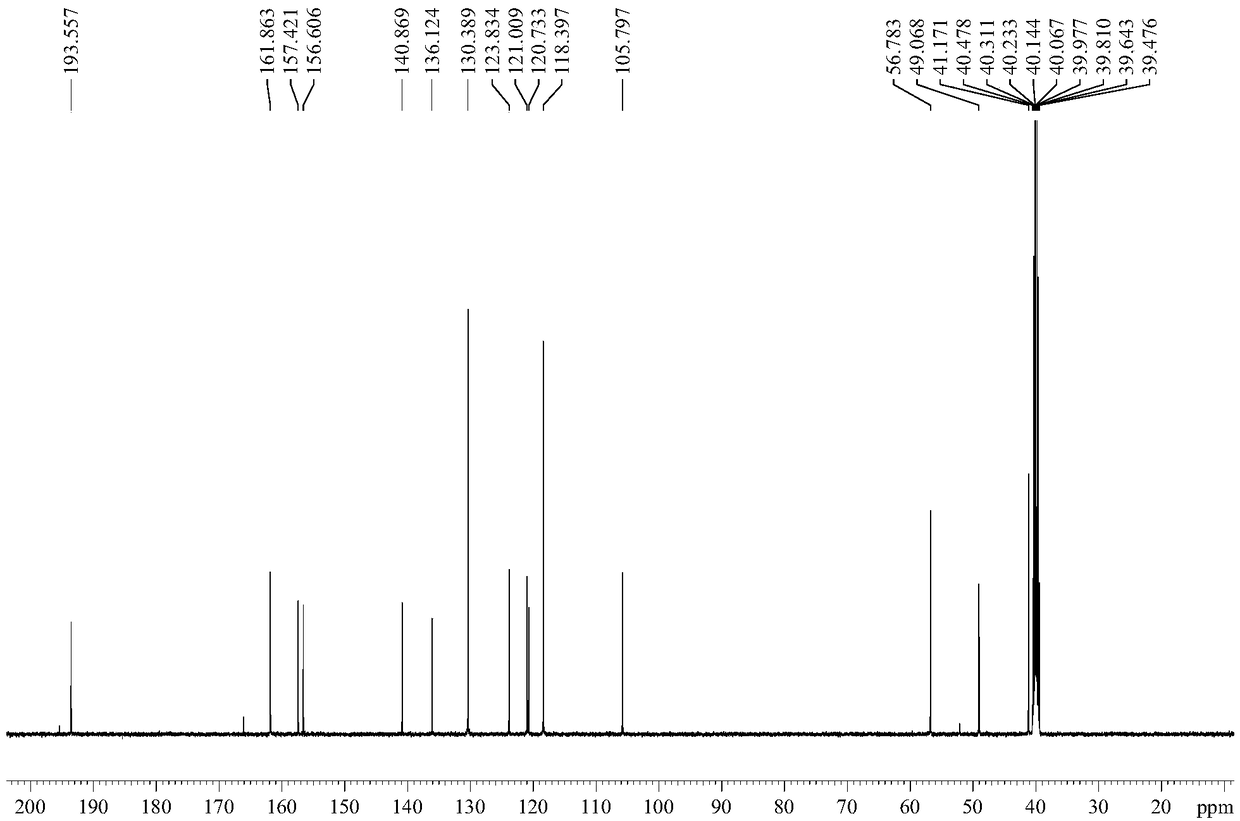
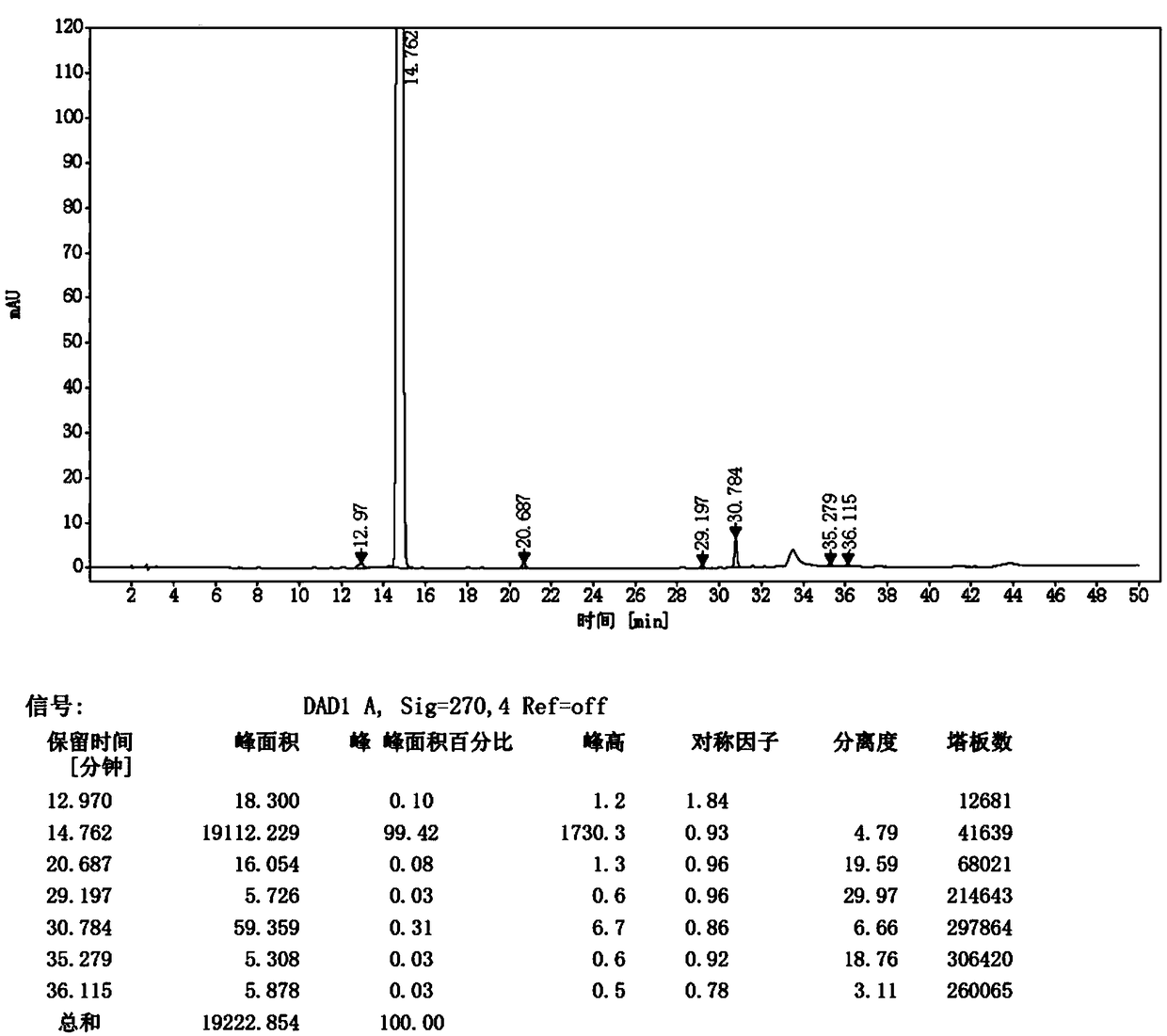
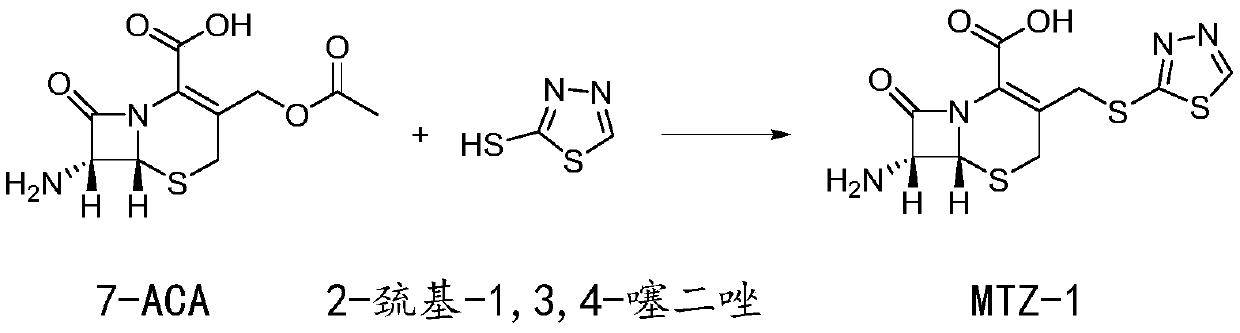
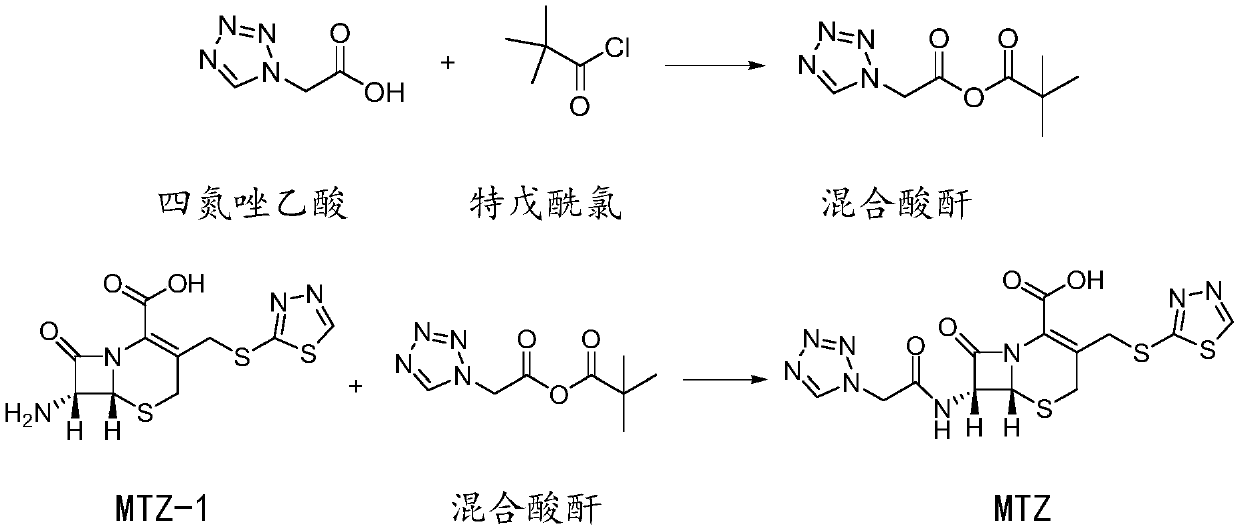
Preparation method of ceftezole acid
PendingCN109824697AReduce manufacturing costReduce lossOrganic chemistryLewis acid catalysisTriethylamine
The invention discloses a preparation method of ceftezole acid and belongs to the field of pharmaceutical technology. The preparation method of the ceftezole acid comprises the following steps of producing an intermediate of MTZ-1, specifically, adding in dimethyl carbonate, anhydrous formic acid, 7-ACA (7-aminocephalosporanic acid) and 2-mercapto-1, 3, 4-thiadiazol, then adding in Lewis acid as the catalyst for reaction, and then performing centrifugation and drying to obtain the intermediate of MTA-1; producing MTZ, specifically, adding in dichloromethane and tetrazole-1-acetic acid, coolingdown, dropwise adding in triethylamine, cooling down, adding in 4-methylpyridine and pivaloyl chloride for reaction, cooling down to obtain a mixed anhydride, adding in the TMZ-2, dropwise adding thetriethylamine for reaction, in a stirring state, adding in water for extraction, mixing for layering, decoloring the extracted aqueous phase, regulating the pH of and crystalizing the decolored solution, then performing centrifugation, washing and drying to obtain refined MTZ, namely, the ceftezole acid. The preparation method of the ceftezole acid changes a three-step method in the prior art into a two-step method, thereby effectively improving the yield, saving the cost and reducing sewage emission.
Owner:GUANGXI KELUN PHARMA
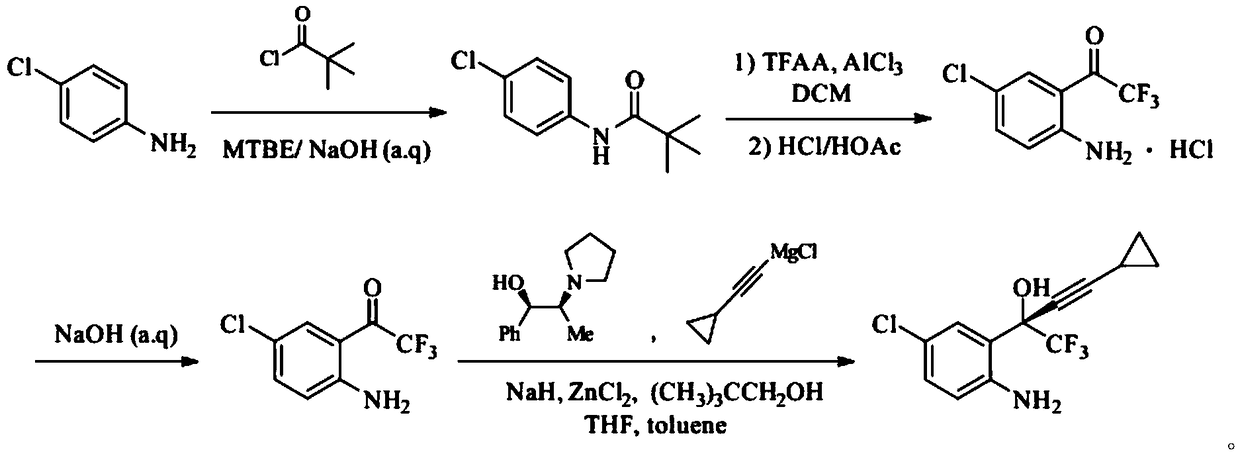
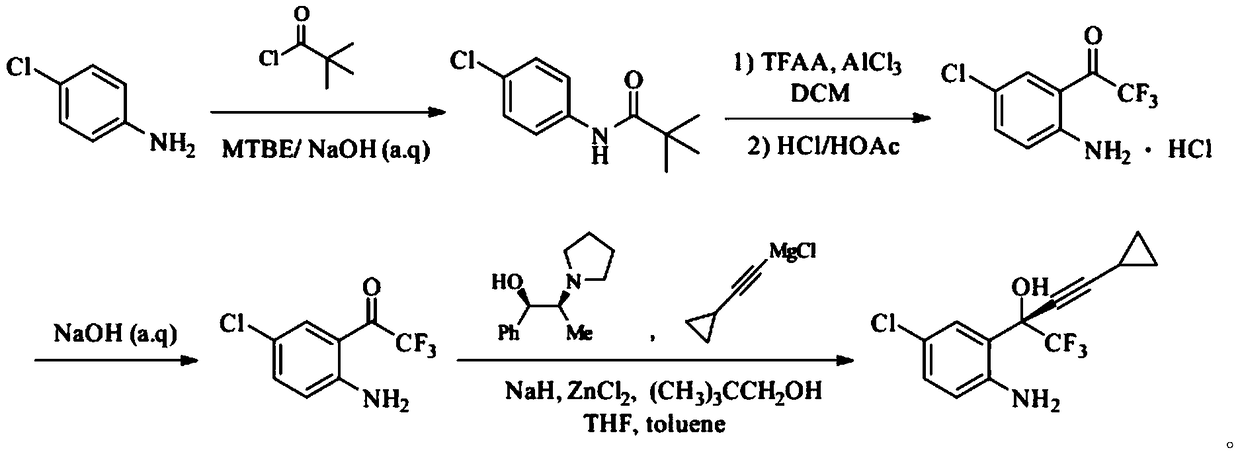
Synthetic method of efavirenz key intermediate
ActiveCN108947855ALow toxicityRaw materials are cheap and easy to getOrganic compound preparationOrganic chemistry methodsAnilineAniline hydrochloride
The invention provides a synthetic method of an efavirenz key intermediate. The synthetic method comprises the following steps: carrying out reaction on parachloroaniline and pivaloyl chloride to protect amino to obtain N-(4-chlorphenyl)-2,2-dimethyl propanamide; carrying out Friedel-Crafts acylation reaction on the product and Friedel-Crafts acylation under action of aluminum trichloride to hydrolyze to obtain 4-chloro-2-trifluoroacetyl aniline hydrochloride in an acidic condition; and then carrying out alkalization to obtain 4-chloro-2-trifluoroacetyl aniline, carrying out reaction with cyclopropyl acetylene magnesium chloride in a catalytic system formed by a ligand (1R, 2S)-1-phenyl-2-(1-pyrrolidyl)-1-propyl alcohol, and carrying out an asymmetrical self-catalytic reaction to obtain the efavirenz key intermediate. The synthetic method of the efavirenz key intermediate, provided by the invention, is cheap and easily available in raw material, low in toxicity of reagent and mild in reaction condition, amino protection and deprotection are not carried out frequently, the line is concise, the yields of reaction of each step are excellent, and the total yield is high.
Owner:JIANGSU SHAXING CHEM
Synthetic method of mirabegron intermediate
InactiveCN105801438AReduce manufacturing costSimple and fast operationOrganic compound preparationCarboxylic acid amides preparationNitrobenzeneMandelic acid
The invention discloses a synthetic method of a mirabegron intermediate (R)-2-(4-nitrobenzene ethyl amino)-1-phenyl ethanol hydrochloride (M-02) and belongs to the field of drug synthesis. The method comprises steps as follows: (1) R-mandelic acid and pivaloyl chloride produce a mixed anhydride intermediate (I); (2) the mixed anhydride intermediate (I) and 4-nitrobenzene ethylamine are subjected to an acrylation reaction to produce an intermediate (II); (3) a mirabegron intermediate (M-01) is obtained through hydrolysis of the intermediate (II); (4) an amido bond of the intermediate (M-01) is reduced and the mirabegron intermediate (M-02) is obtained. The reaction condition is mild, the yield is high, few impurities exist, the production cost is low, and the method is suitable for mass industrial production.
Owner:UNIV OF JINAN
Synthetic method for ezetimibe intermediate
The invention discloses a synthetic method for an ezetimibe intermediate. The synthetic method comprises: by taking a compound I as a raw material, mixing the compound I with a reaction solution; under the action of an acid-binding agent, firstly activating the compound I by pivaloyl chloride; then coupling the compound with S-4-phenyl-2-oxazolidinone; then carrying out reduction reaction through (R)-2-mehtyl-CBS-oxazole borane; and then carrying out post-treatment to prepare (4S)-3-[(5S)-5-(4-fluorophenyl-5-hydroxyl valeryl)-4-phenyl-1,3- azacyclocyclopentane-2-(one) (II), wherein the formula is as shown in the description, and the reaction solution comprises tetrahydrofuran, chloroform, dioxane or dichloromethane. The synthetic method for the ezetimibe intermediate disclosed by the invention has the advantages of being simple to operate, short in synthetic line and relatively low in synthetic cost, and is suitable for large-scaled industrial production.
Owner:WUXI FORTUNE PHARMA
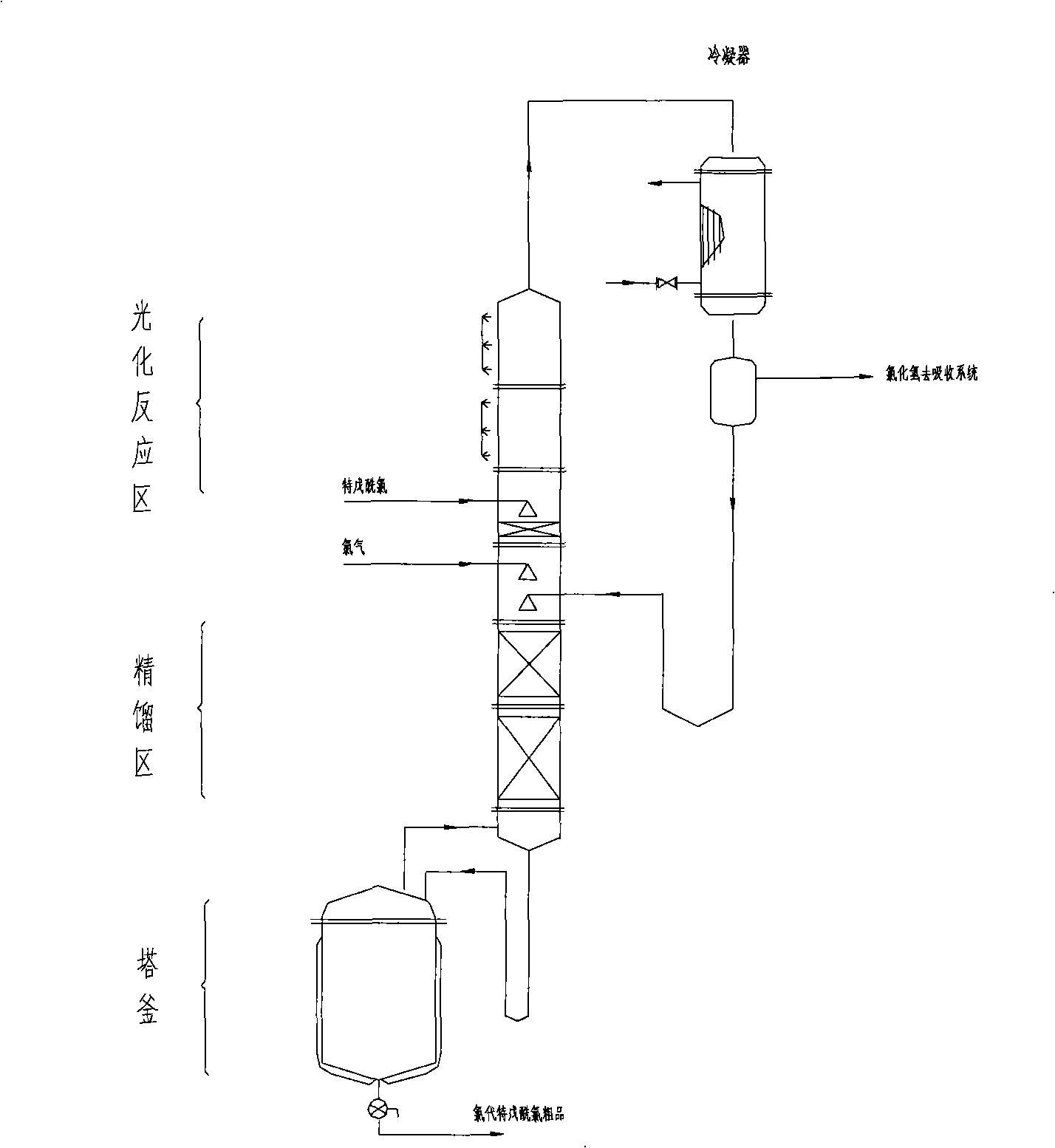
Method and device for continuously preparing chloropivaloyl chloride by using micro-channel
ActiveCN110818558AImprove utilization efficiencyHarm reductionOrganic compound preparationChemical/physical/physico-chemical microreactorsChemical synthesisDistillation
The invention belongs to the technical field of chemical synthesis, and particularly relates to a method and device for continuously preparing chloropivaloyl chloride by using a micro-channel. The method comprises the steps: mixing pivaloyl chloride and chlorine, then adding the mixture to a preprocessor with a composite photocatalyst attached to the inner wall, pretreating, allowing the pretreated mixture to enter a micro-channel photochlorination reactor, carrying out a photochlorination reaction under an ultraviolet irradiation condition to prepare a chloropivaloyl chloride reaction solution, and rectifying the chloropivaloyl chloride reaction solution to obtain the chloropivaloyl chloride finished product. The inner wall of the preprocessor is lined with a nano MnS-ZnS composite photocatalyst to enhance the photochlorination reaction efficiency; the multi-wave band ultraviolet lamp adopts a surrounding mode, so that the harm of light diffusion to the surrounding environment is reduced while the utilization efficiency of ultraviolet light is enhanced; by adopting the micro-channel photochlorination reactor, the reaction time is shorter, the reaction is more thorough, and the impurities are fewer; a middle distillation tower is added in the rectification section, so that middle distillation impurities are separated more thoroughly, and the product quality is higher.
Owner:山东民基新材料科技有限公司
Method for synthesizing cephalosporin
The invention provides a method for synthesizing cephalosporin. The method comprises the following steps that 1, 7-ADCA dissolving liquid is prepared, wherein a solution and 7-ADCA submicron powder are put in a reaction tank, cooling is carried out, at the temperature of 10 DEG C or below, a solvent is added, heat preservation and reacting are carried out, and the 7-ADCA dissolving liquid is obtained; b, N-Phenylglycine potassium salt and pivaloyl chloride react in advance to prepare a mixed anhydride solution; c, the 7-ADCA dissolving liquid prepared in step a is added to the mixed anhydridesolution, a condensation reaction is carried out at the temperature of -40 DEG C to -30 DEG C, and a condensed solution is obtained; d, the condensed solution obtained in step c is subjected to hydrolysis and crystallization, and cephalosporin is obtained. According to the method for synthesizing cephalosporin, the 7-ADCA submicron powder raw material is used, the specific dissolving temperature is adopted in cooperation, the yield of the final cephalosporin product is greatly increased, the purity of the final cephalosporin product is greatly improved, and the method is suitable for industrial popularization and application.
Owner:NORTH CHINA PHARMA COMPANY
Method for preparing crystal high-purity phosphorous acid by purifying byproduct phosphorous acid in pivaloyl chloride production
PendingCN111606313ASolve the problem of low concentration and difficulty in further utilizationImprove use valuePhosphorous acidPhosphorous acidO-Phosphoric Acid
The invention belongs to the field of chemical product purification, and particularly relates to a method for preparing crystal high-purity phosphorous acid by purifying byproduct phosphorous acid inpivaloyl chloride production, which comprises the following steps of: 1, diluting phosphorous acid serving as a byproduct of pivaloyl chloride production by using evaporation condensate water; 2, adding porous adsorbents such as activated carbon into the diluted solution, and performing filtering to separate the activated carbon and impurities; 3, vacuumizing the filtered clear liquid into an evaporation and concentration kettle with a stirring function, adopting a steam medium to perform heating in a jacketed manner, and performing evaporating and concentrating under a vacuum condition; 4, performing water evaporation, namely pumping the material into a cooling kettle with a stirring function, introducing circulating cooling water into a jacket, cooling the material, and putting the material liquid into a crystallization barrel for natural crystallization; and 5, naturally cooling the material, crushing the material, and centrifugally separating impurities containing uncrystallized phosphoric acid to obtain a crystal high-purity phosphorous acid product. The method effectively solves the problem that the byproduct phosphorous acid in pivaloyl chloride production is low in concentration and difficult to further utilize.
Owner:民乐县锦世建材新材料有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com