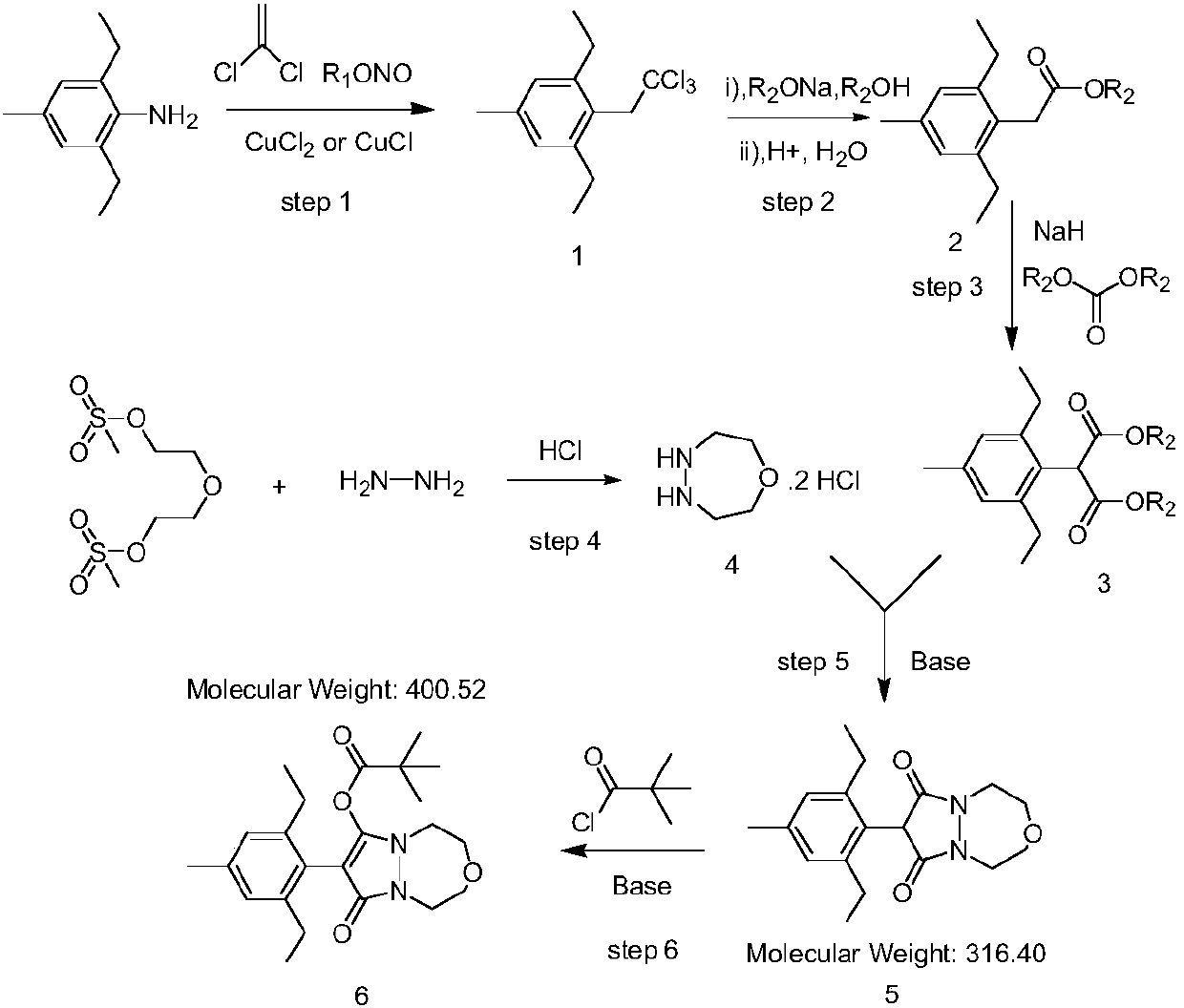
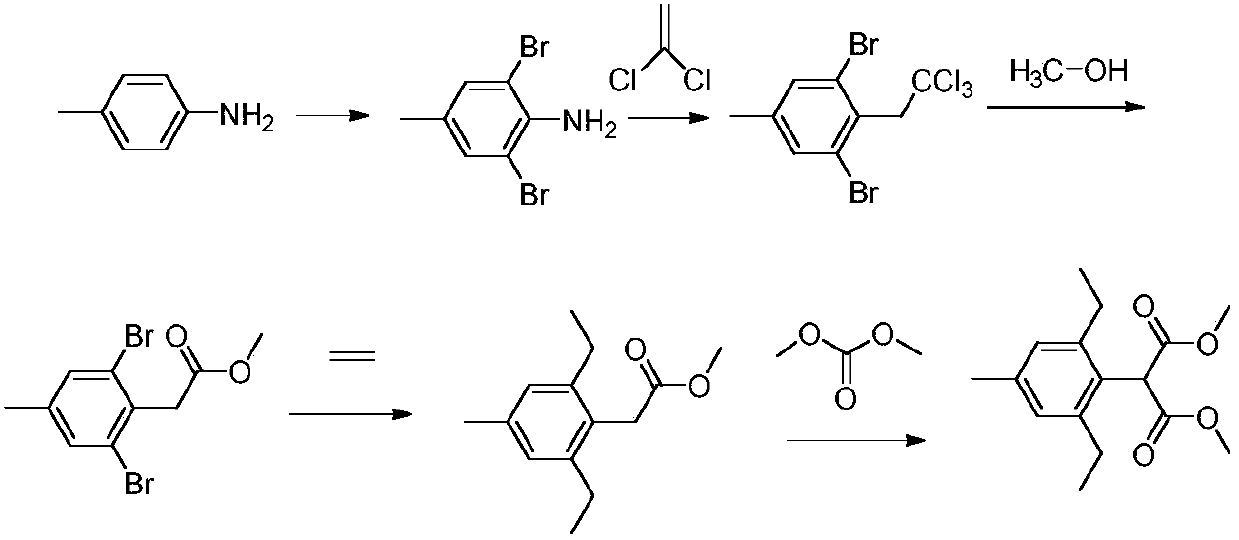
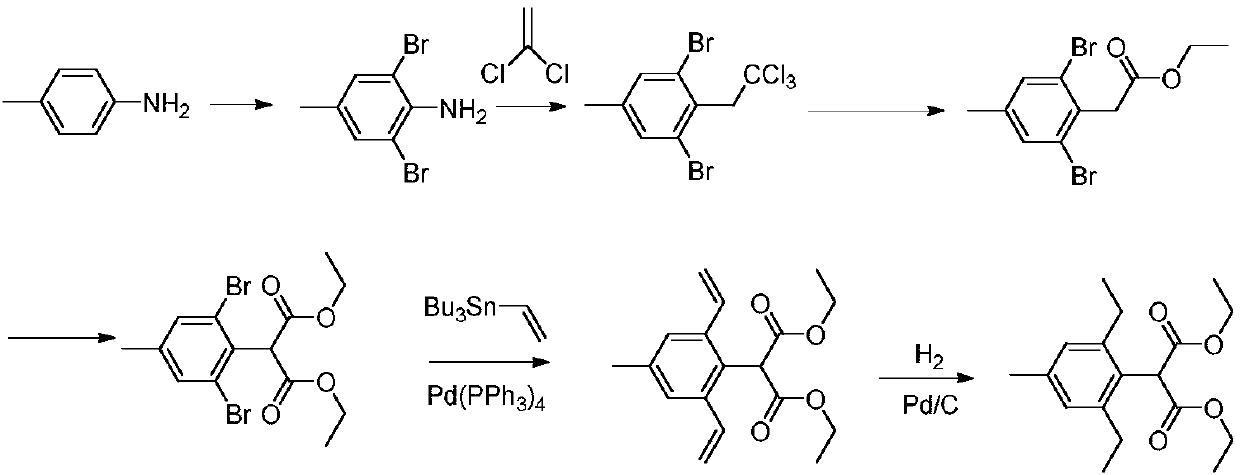
Preparation method of pinoxaden
A technology of pinoxaden and nitrite, which is applied in the field of pinoxaden preparation, can solve the problems of not being environmentally friendly, expensive hydrobromic acid, and expensive Boc anhydride, and achieves low cost and high atom economy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Step 1: Add 200 grams of acetonitrile, 53.5 grams of anhydrous copper chloride (0.4mol), 445.3 grams of 1,1-dichloroethylene (4.6mol) and 47.4 grams of tert-butyl nitrite in a 1-liter four-necked bottle (0.46mol), the reaction system was cooled to 10 degrees, and 50 grams of raw material 4-methyl-2,6-diethylaniline (0.3mol) was slowly added dropwise. After the addition was complete, the reaction was stirred at room temperature for 16 hours. The reaction system was suction-filtered to remove copper salts, and the filter cake was washed with 200 g of ethyl acetate. The filtrate was washed once with 100 grams of 10% hydrochloric acid, and then washed once with 80 grams of water. The organic phase was evaporated to dryness under normal pressure to obtain a crude product, which was slurried with 48 g of n-hexane to obtain 73.5 g of off-white solid 1, with a yield of 85.8%. 1 HNMR (CDCl 3 ) δppm: 1.19 (t, 6H), 2.30 (s, 3H), 2.62 (q, 4H), 3.85 (s, 2H), 6.93 (s, 2H).
[004...
Embodiment 2
[0049] Step 1: In a 1-liter four-neck flask, add 180 grams of acetonitrile, 48.2 grams of anhydrous copper chloride (0.36mol), 290.4 grams of 1,1-dichloroethylene (3mol) and 37.1 grams of tert-butyl nitrite ( 0.36mol), the reaction system was cooled to 10 degrees, and 50 grams of raw material 4-methyl-2,6-diethylaniline (0.3mol) was slowly added dropwise. After the addition was complete, the reaction was stirred at room temperature for 16 hours. The reaction system was suction-filtered to remove copper salts, and the filter cake was washed with 150 g of ethyl acetate. The filtrate was washed once with 100 grams of 10% hydrochloric acid, and then washed once with 80 grams of water. The organic phase was evaporated to dryness under normal pressure to obtain a crude product, which was slurried with 50 g of n-hexane to obtain 72.9 g of off-white solid 1, with a yield of 85.1%.
[0050] Step 2: Synthesis of 2,6-diethyl-4-methylphenylacetate: In a 1-liter four-neck flask, add 160 ...
Embodiment 3
[0056] Step 1: In a 2-liter four-necked flask, add 250 grams of acetonitrile, 51.8 grams of anhydrous cuprous chloride (0.52mol), 580.8 grams of 1,1-dichloroethylene (4.6mol) and 55.6 grams of tert-butyl nitrite ester (0.54mol), the temperature of the reaction system was lowered to 10°C, and 50 g of raw material 4-methyl-2,6-diethylaniline (0.3mol) was slowly added dropwise. After the addition was complete, the reaction was stirred at room temperature for 16 hours. The reaction system was suction-filtered to remove copper salts, and the filter cake was washed with 200 g of ethyl acetate. The filtrate was washed once with 100 grams of 10% hydrochloric acid, and then washed once with 90 grams of water. The organic phase was evaporated to dryness under normal pressure to obtain a crude product, which was slurried with 48 g of n-hexane to obtain 74.6 g of off-white solid 1, with a yield of 87.1%.
[0057] Step 2: Synthesis of 2,6-diethyl-4-methylphenylacetate: In a 2-liter four-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com