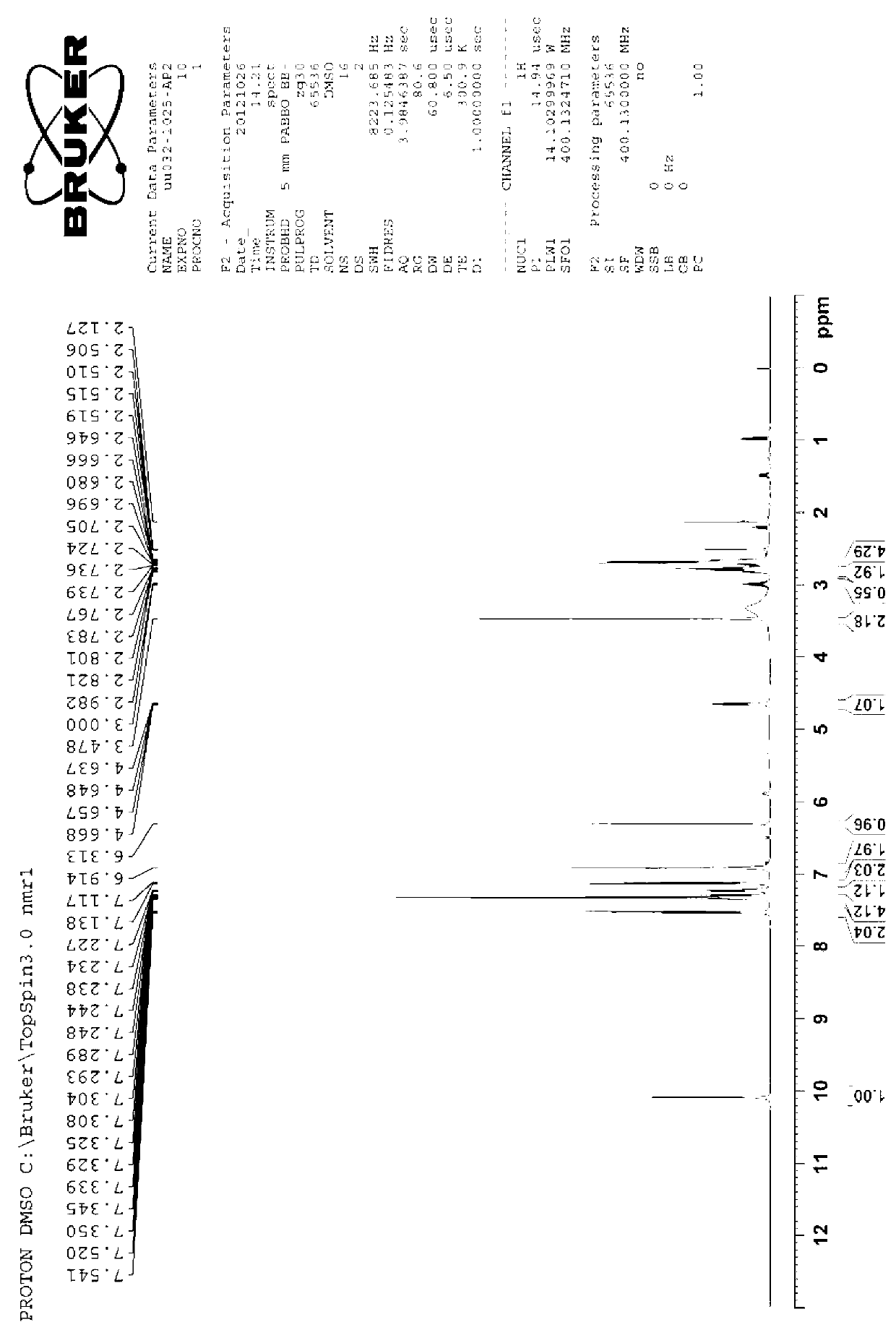
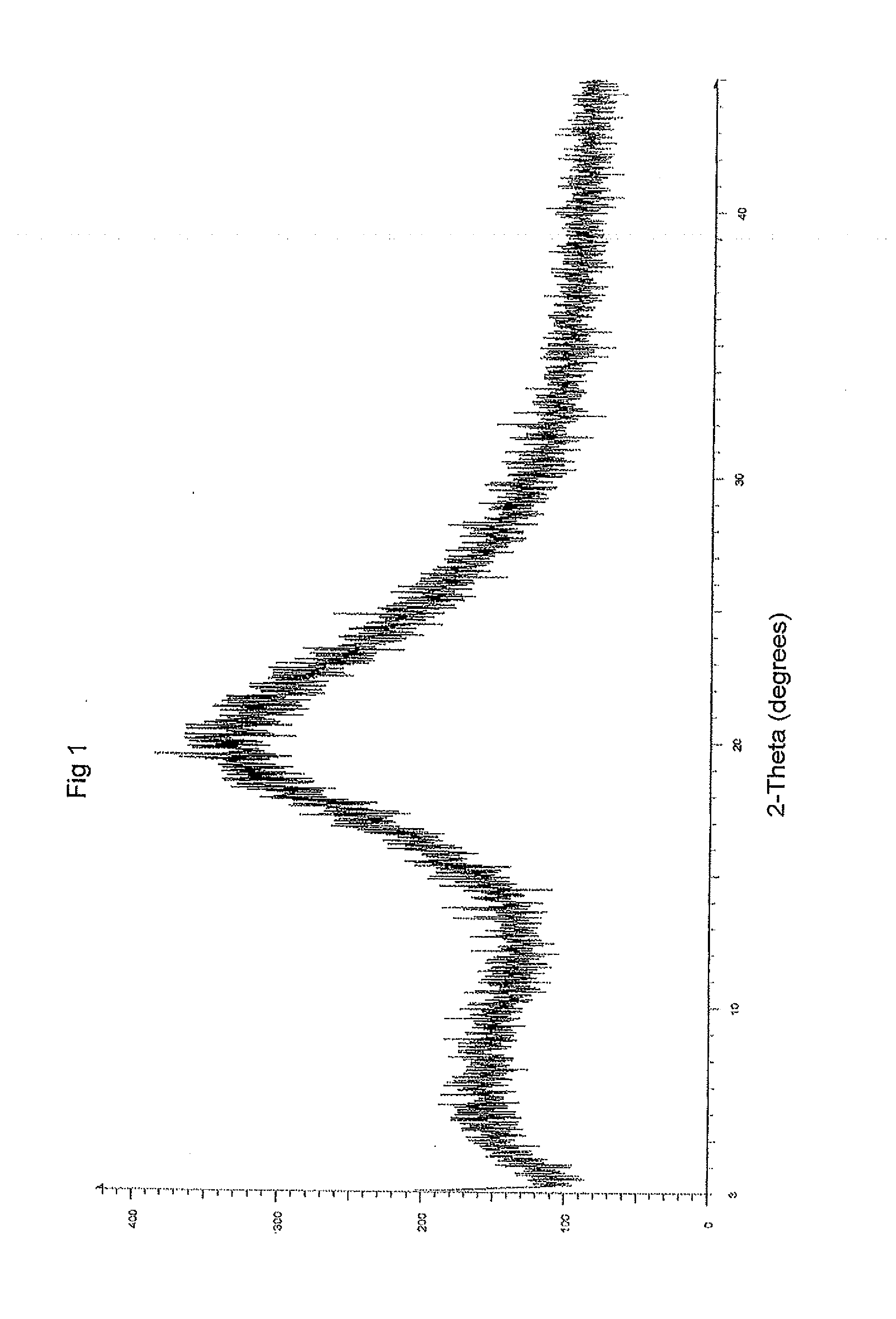
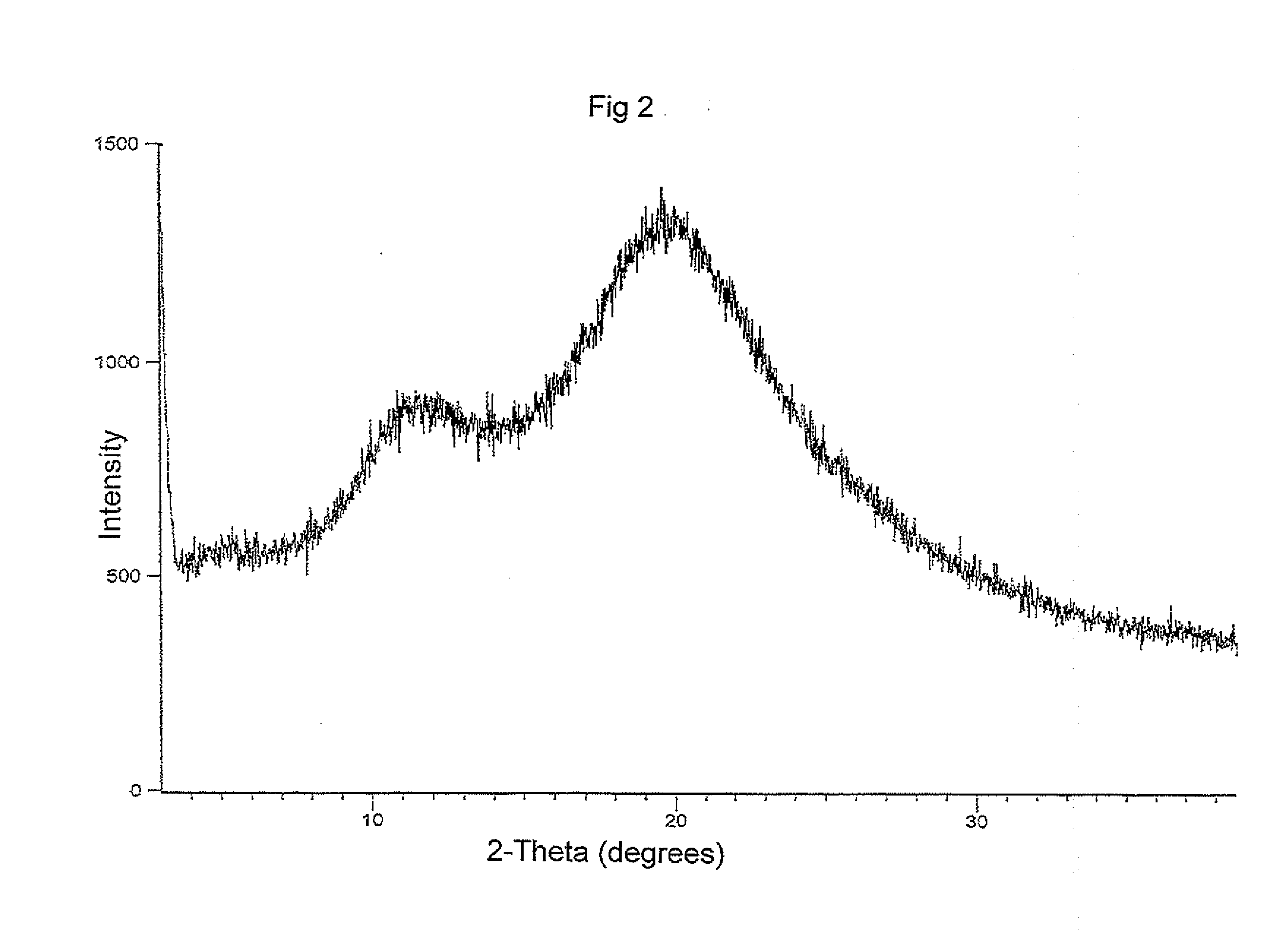
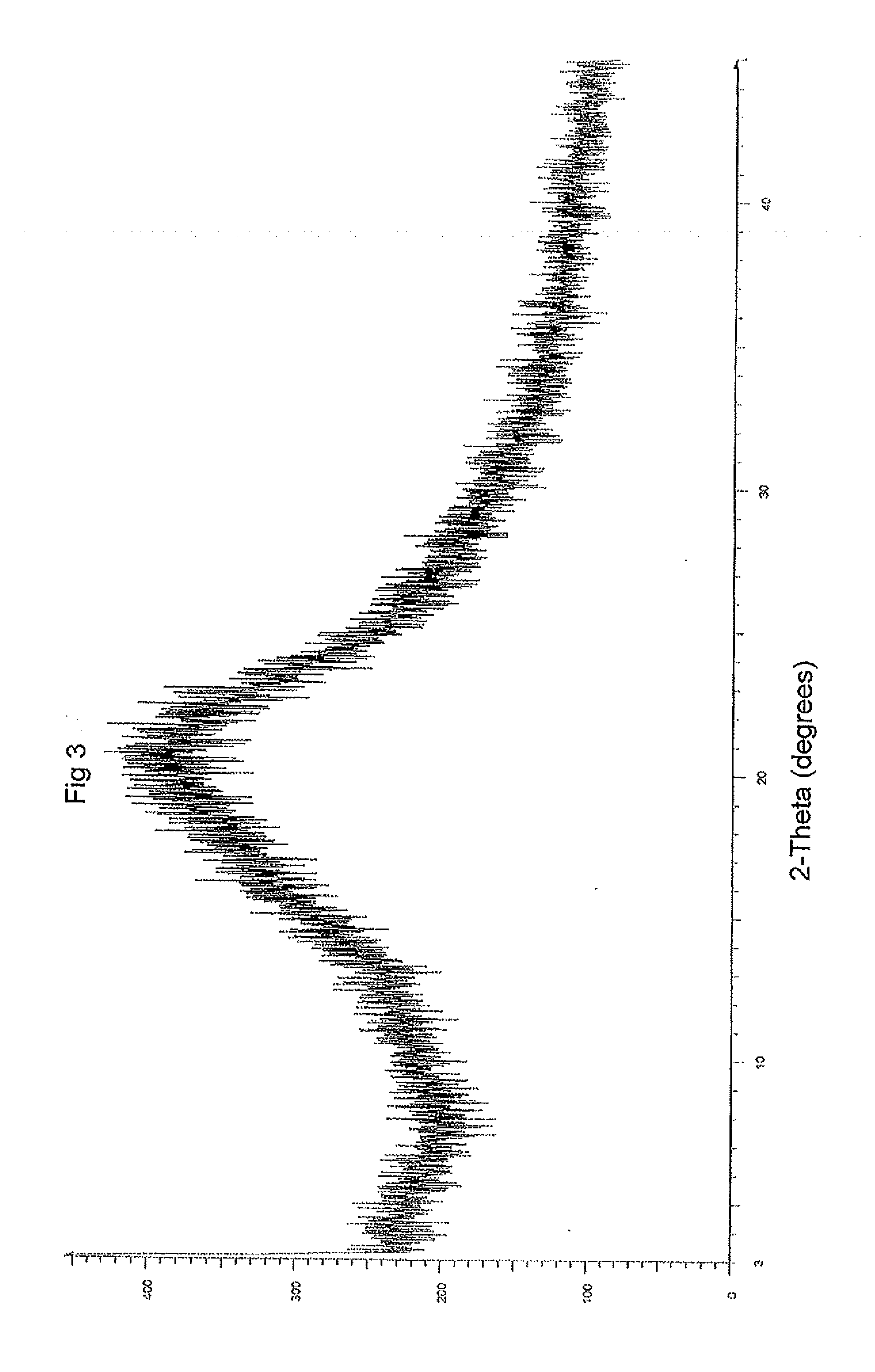
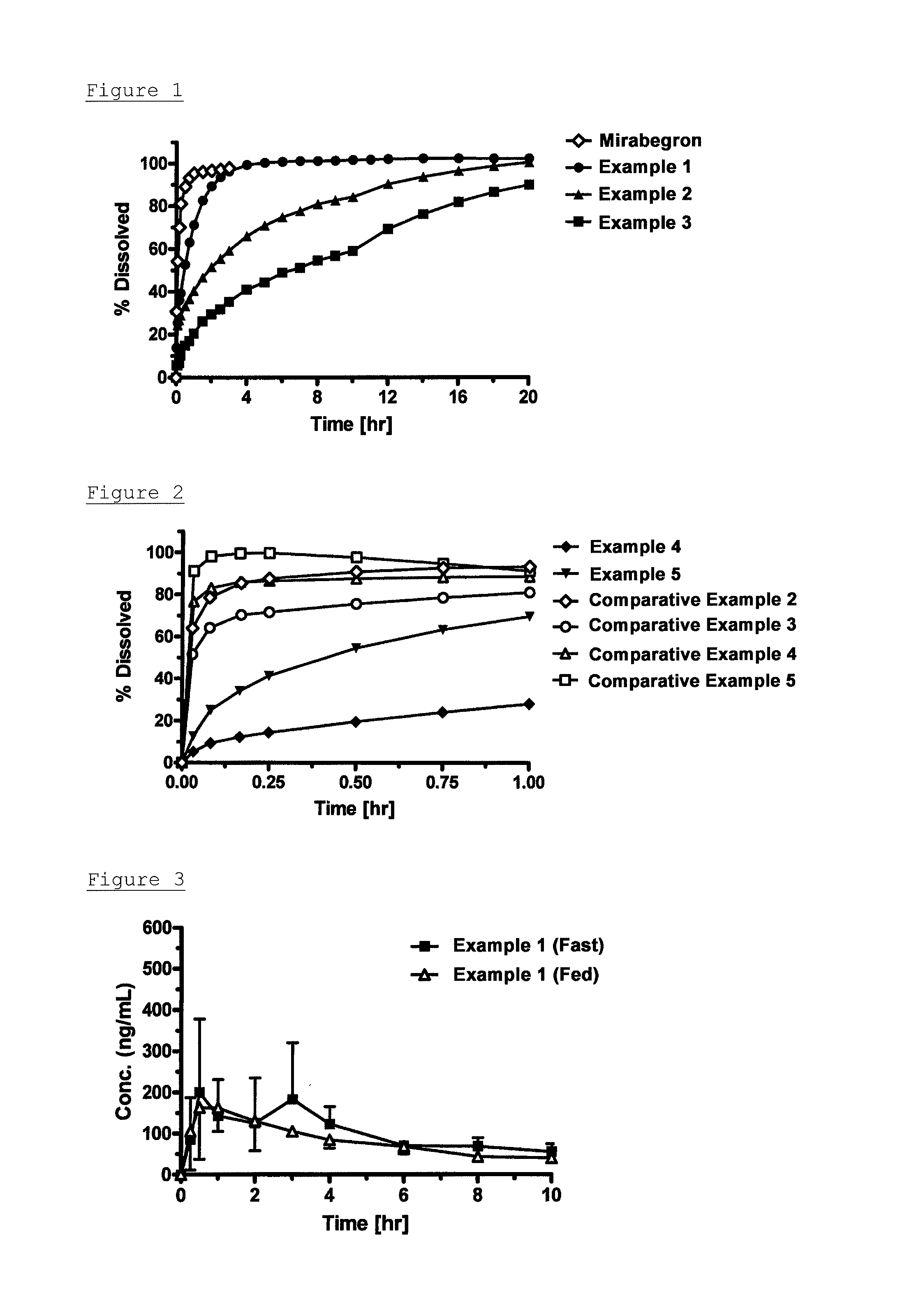
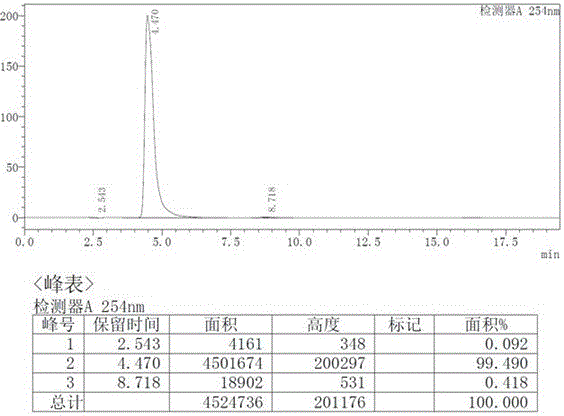
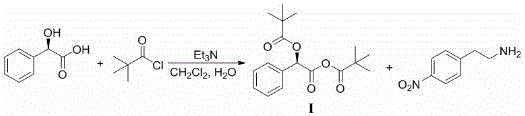
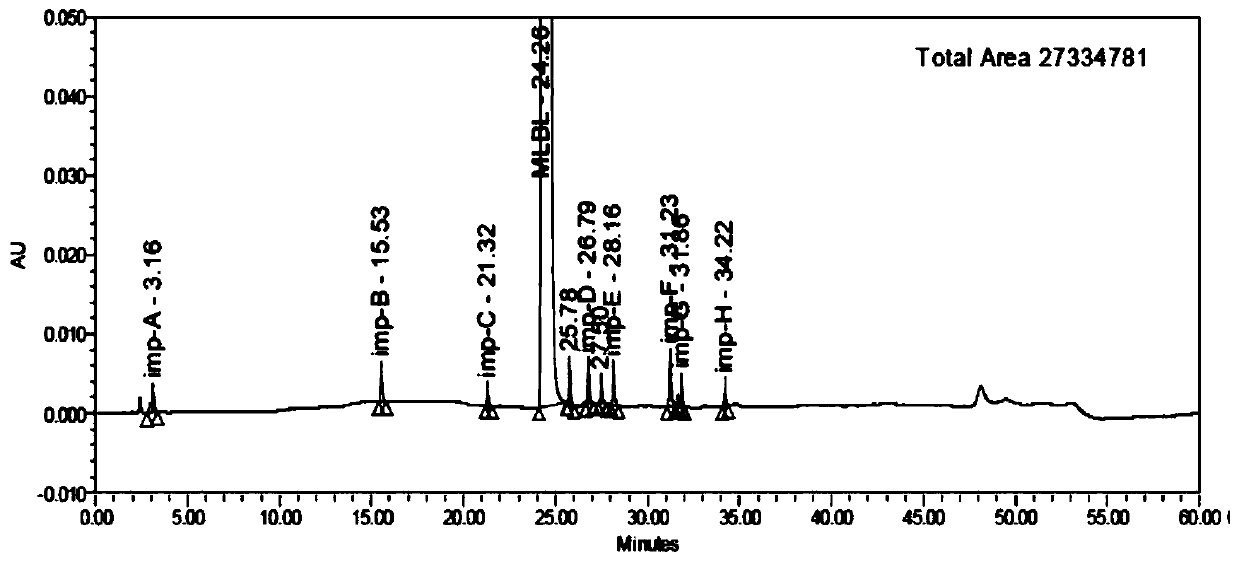
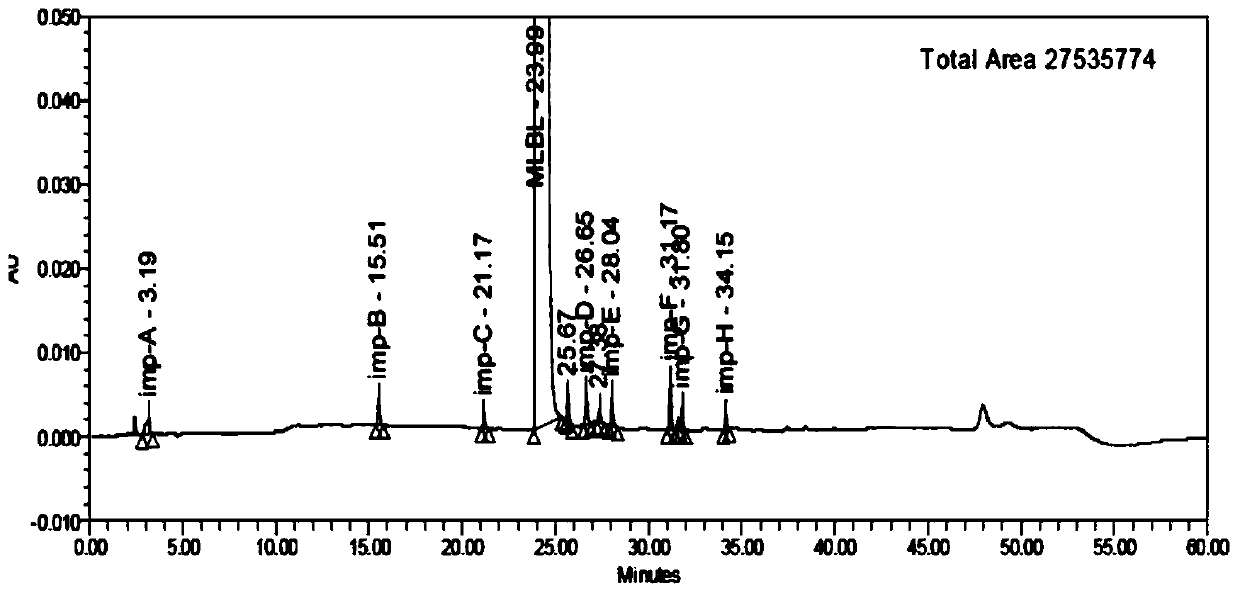
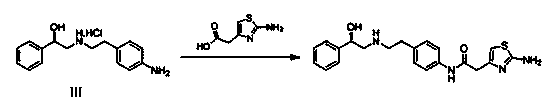Patents
Literature
66 results about "Mirabegron" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
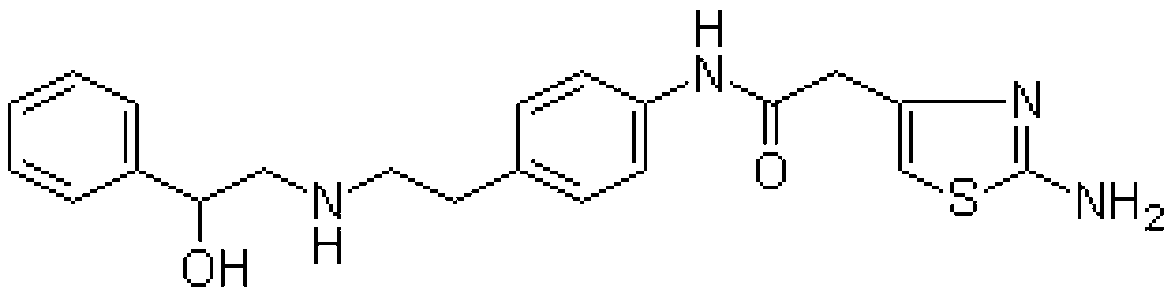
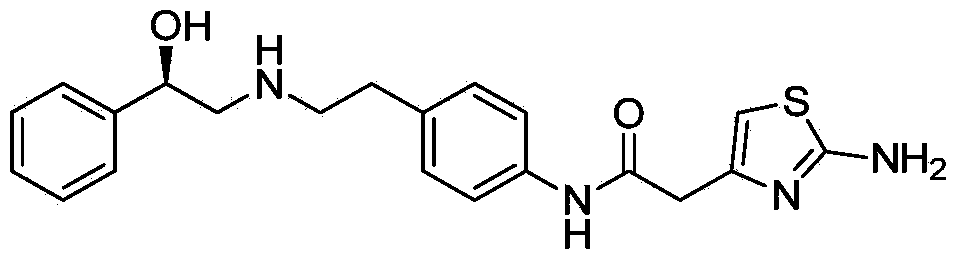
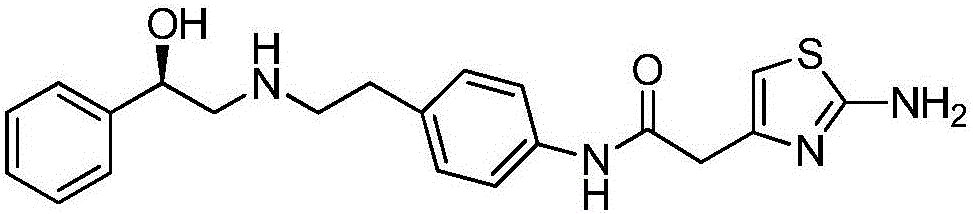
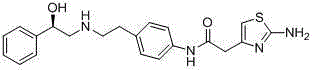
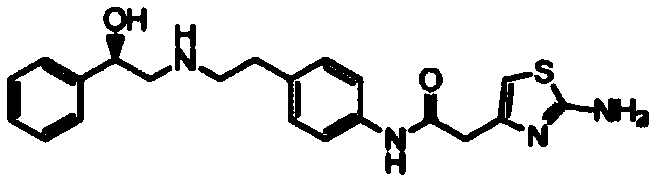
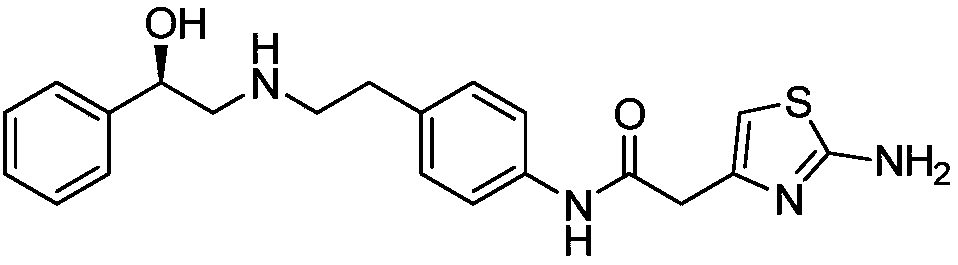
This medication is used to treat overactive bladder.
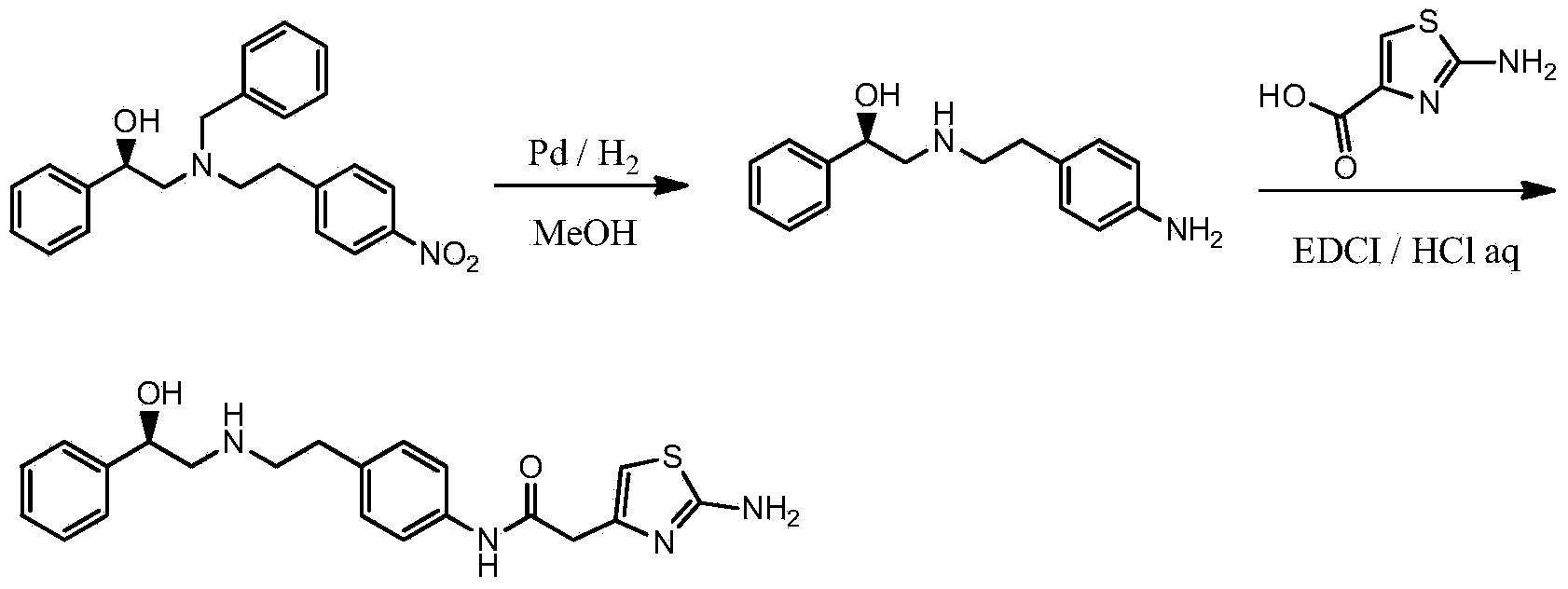
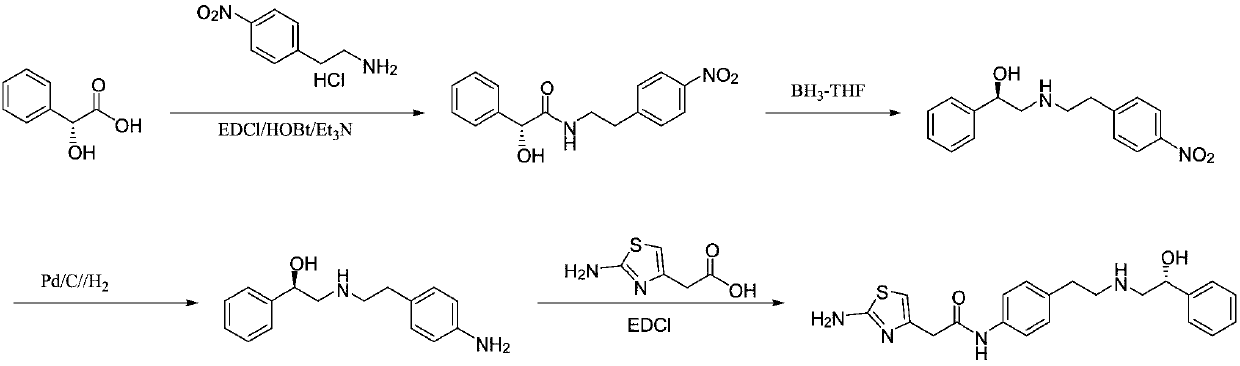
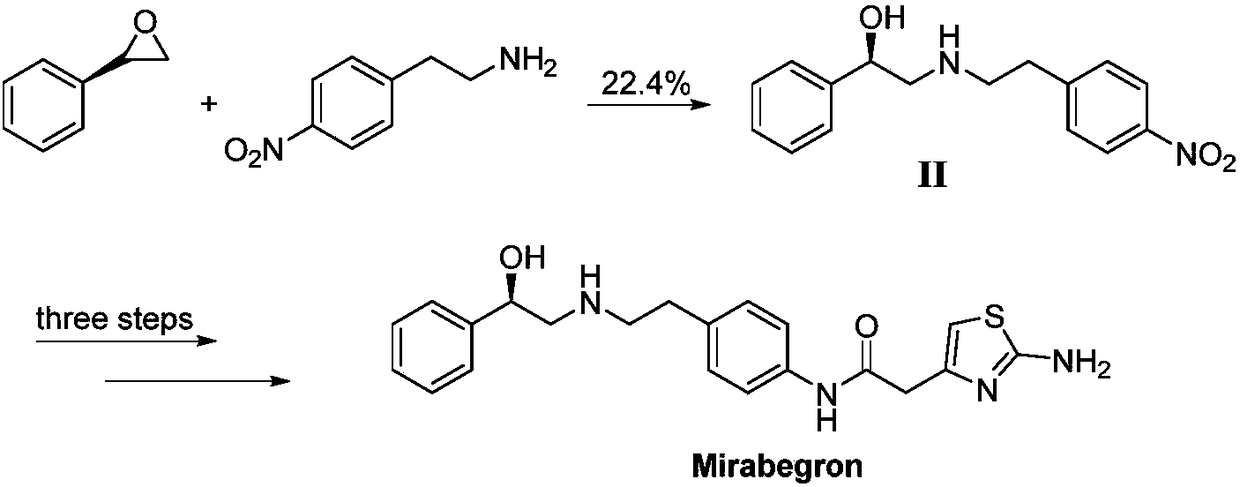
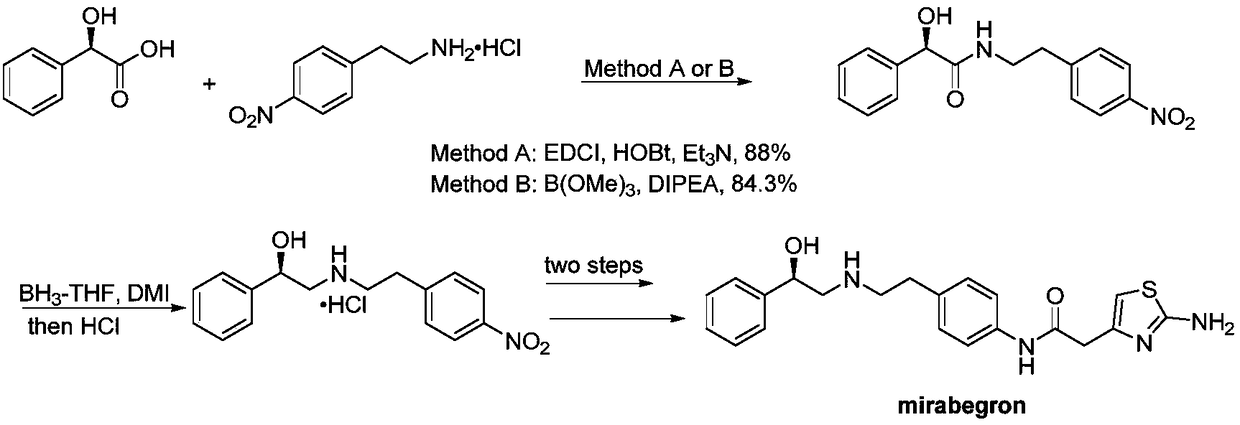
Synthesis method of mirabegron
InactiveCN103193730ALow costRealize safe and clean productionOrganic chemistryBulk chemical productionSynthesis methodsPhenethyl alcohol
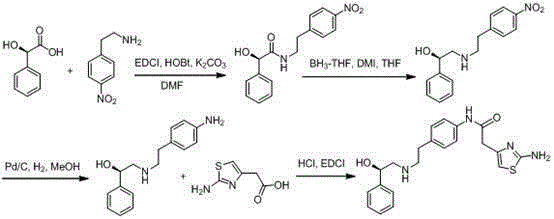
The invention provides a synthesis method of mirabegron and belongs to the technical field of medicine synthesis. The synthesis method solves the problems that the synthesis method of the mirabegron is low in product yield and is not suitable for large-scale industrialized production in the prior art. The synthesis method comprises the following steps: 1) amino protection: reacting 2-aminothiazole-5-acetic acid with an amino protective agent to obtain a mirabegron intermediate product A; 2) condensation reaction: performing condensation reaction on the mirabegron intermediate product A and 4-amino phenethyl alcohol to obtain a mirabegron intermediate product B; 3) oxidation reaction: performing oxidation reaction on the mirabegron intermediate product B and an oxidant to obtain a mirabegron intermediate product C; and 4) reductive amination and protecting group removal: reacting the mirabegron intermediate product C with (R)-2-amino-1-phenethyl alcohol and removing the protecting group from the mirabegron intermediate product C to obtain the mirabegron. The synthesis method of the mirabegron is low in cost, high in product yield and suitable for large-scale industrialized production.
Owner:SUZHOU UUGENE BIOPHARMA
Novel synthesis method of mirabegron
ActiveCN103304511ALow costRealize safe and clean productionOrganic chemistryBulk chemical productionSynthesis methodsPhenethyl alcohol
The invention provides a novel synthesis method of mirabegron, and belongs to the technical field of drug synthesis. The problems that the yield of synthesized mirabegron is low in the prior art, and the method is unsuitable for large-scale industrial production are solved. The synthesis steps are as follows: 1) performing amino protection, namely reacting 2-aminothiazole-4-acetic acid with an amino protective agent to obtain a mirabegron intermediate product A; 2) performing condensation reaction, namely performing the condensation reaction on the mirabegron intermediate product A and the 4-aminophenethanol to obtain the mirabegron intermediate product B; 3) performing oxidizing reaction, namely performing the oxidizing reaction on the mirabegron intermediate product B and an oxidizing agent to obtain the mirabegron intermediate product C; 4) removing protective agent while performing reductive amination, namely reacting the mirabegron intermediate product C with (R)-2-amino-1-phenethanol under the effect of a reducing agent, and meanwhile removing the protective group on the mirabegron intermediate product C to obtain the mirabegron. The novel synthesis method of mirabegron provided by the invention is low in cost, high in product yield and suitable for large-scale industrial production.
Owner:江苏欣德瑞医药科技有限公司
Method for synthesizing mirabegron
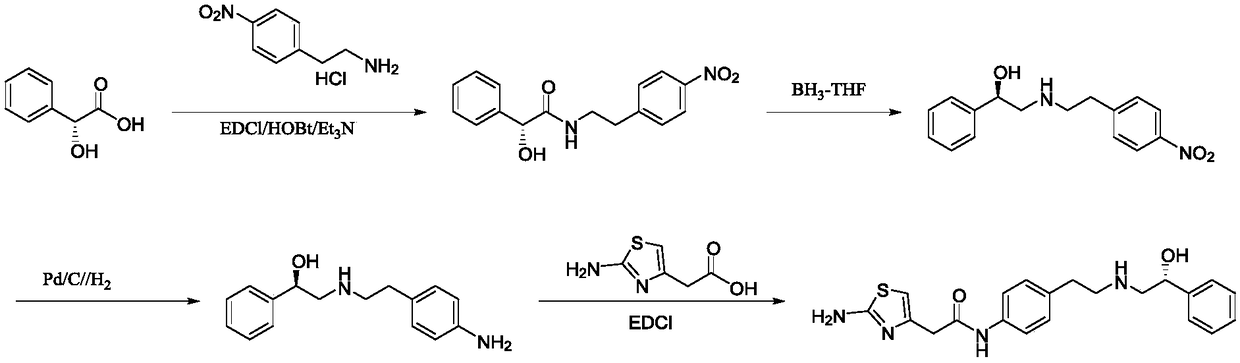
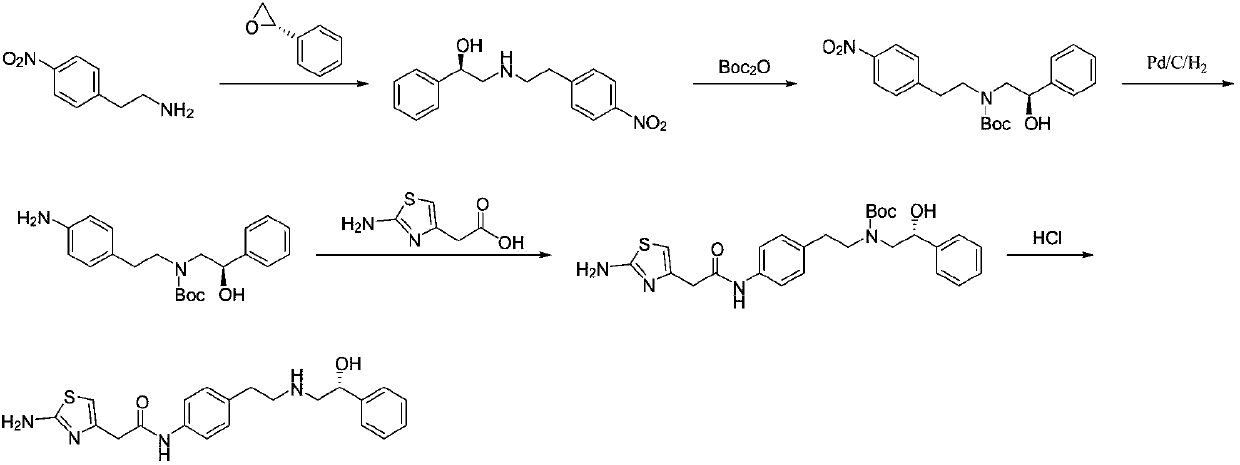
The invention discloses a method for synthesizing mirabegron, relates to a method for preparing mirabegron, and aims at solving the problems that the existing method for synthesizing the mirabegron is not friendly to environment, more in reaction steps, low in yield, and complicated in post-treatment, and cannot be amplified to produce. The method disclosed by the invention comprises the following steps: 1, carrying out ring-opening reaction by taking p-nitrophenylethylamine and (R)-phenylethylene oxide as initial raw materials; 2, carrying out reduction reaction; 3 carrying out condensation reaction. The method disclosed by the invention is cheap and available in selected raw materials, high in yield, low in cost, friendly to environment and applicable to industrial amplifying production. The method is applied to synthesis of the mirabegron.
Owner:HEILONGJIANG UNIV
Mirabegron related substance or salt thereof, and preparation method and use thereof
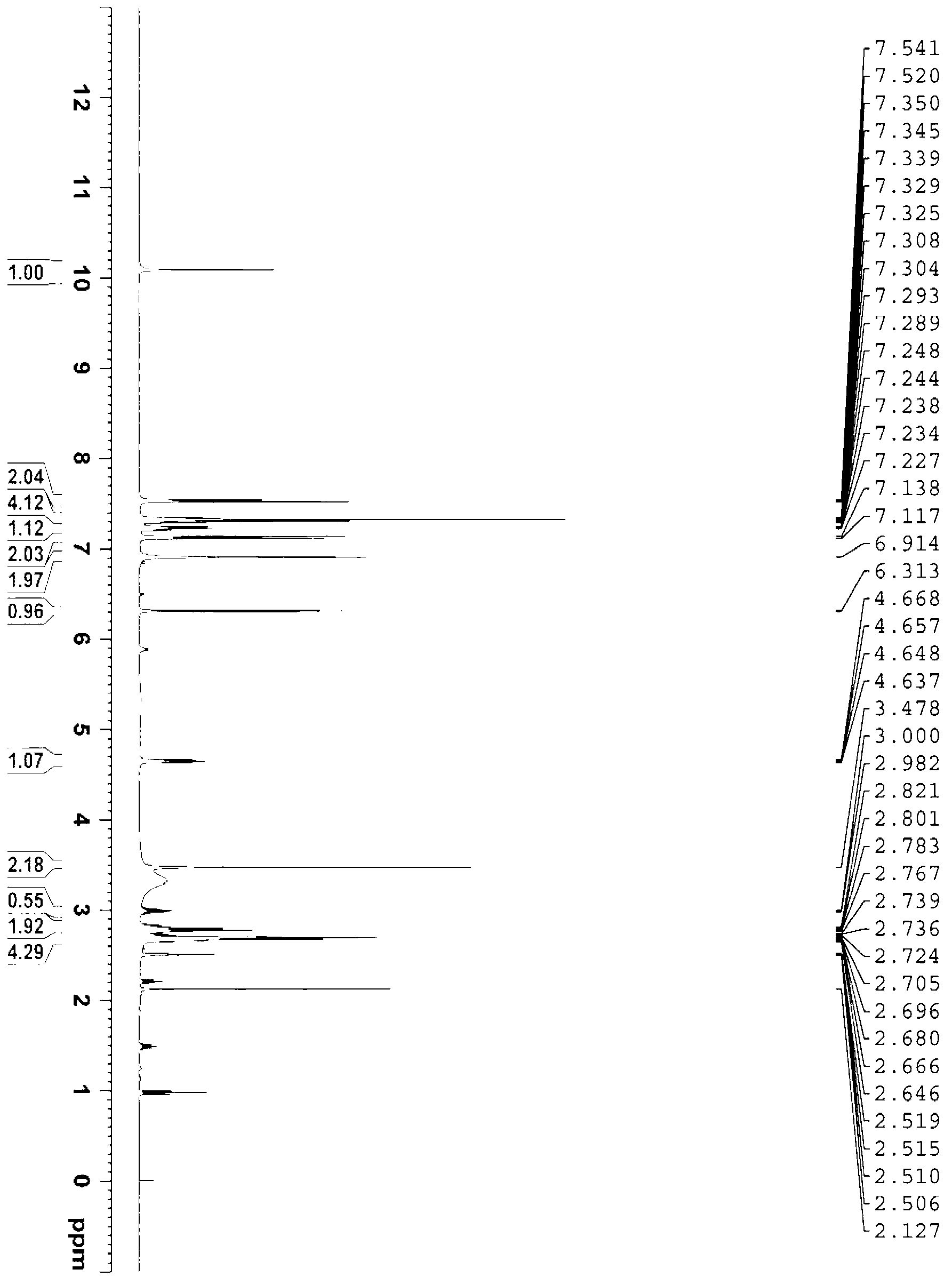
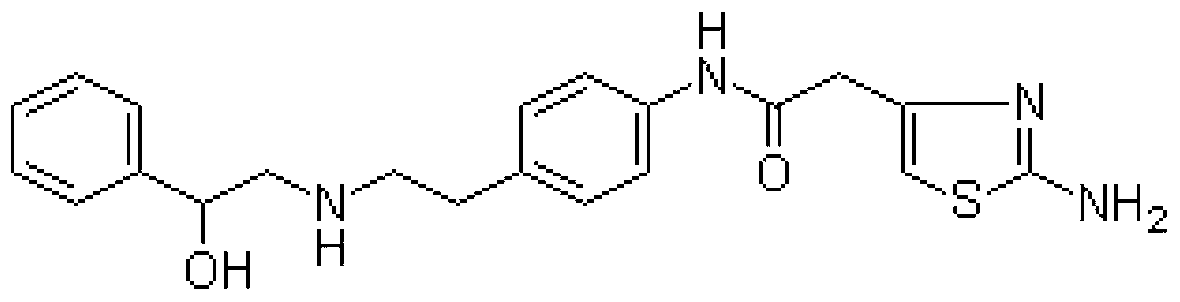
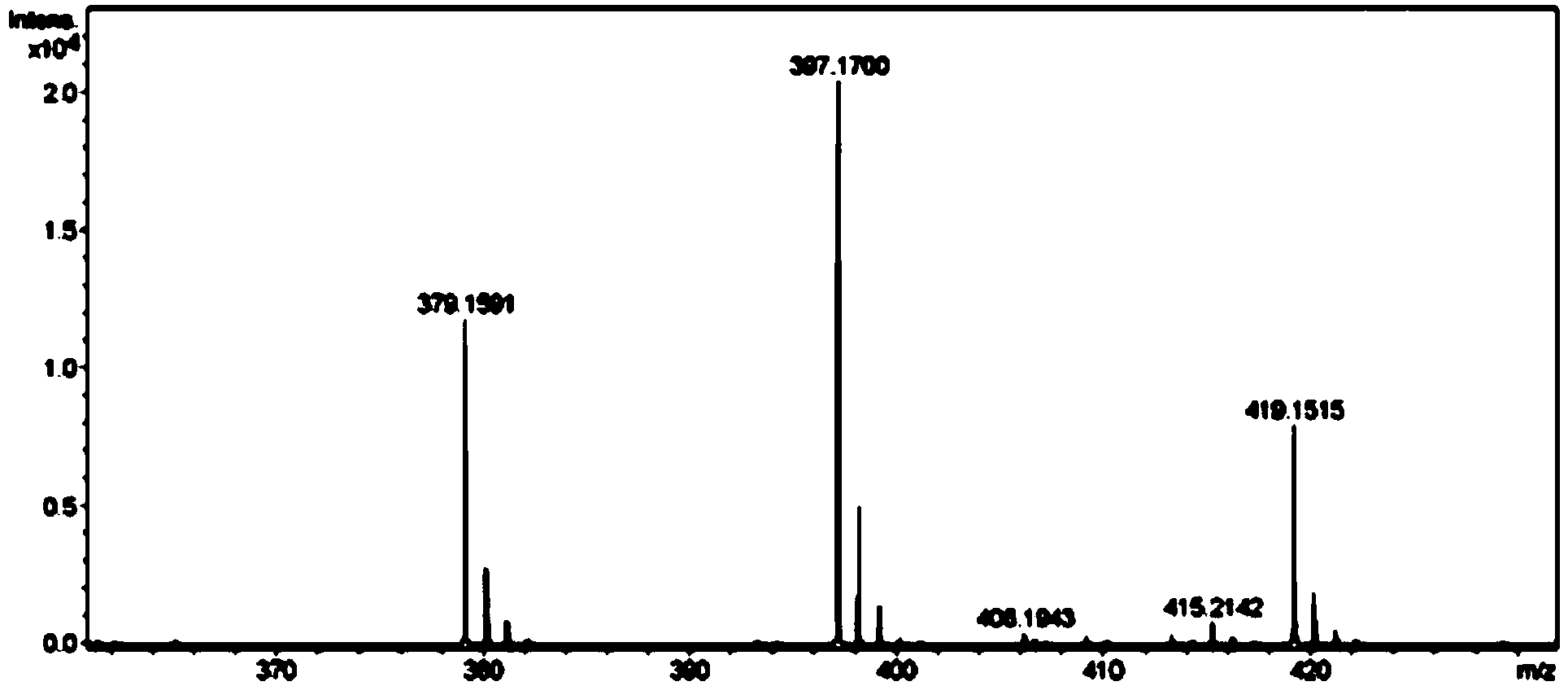
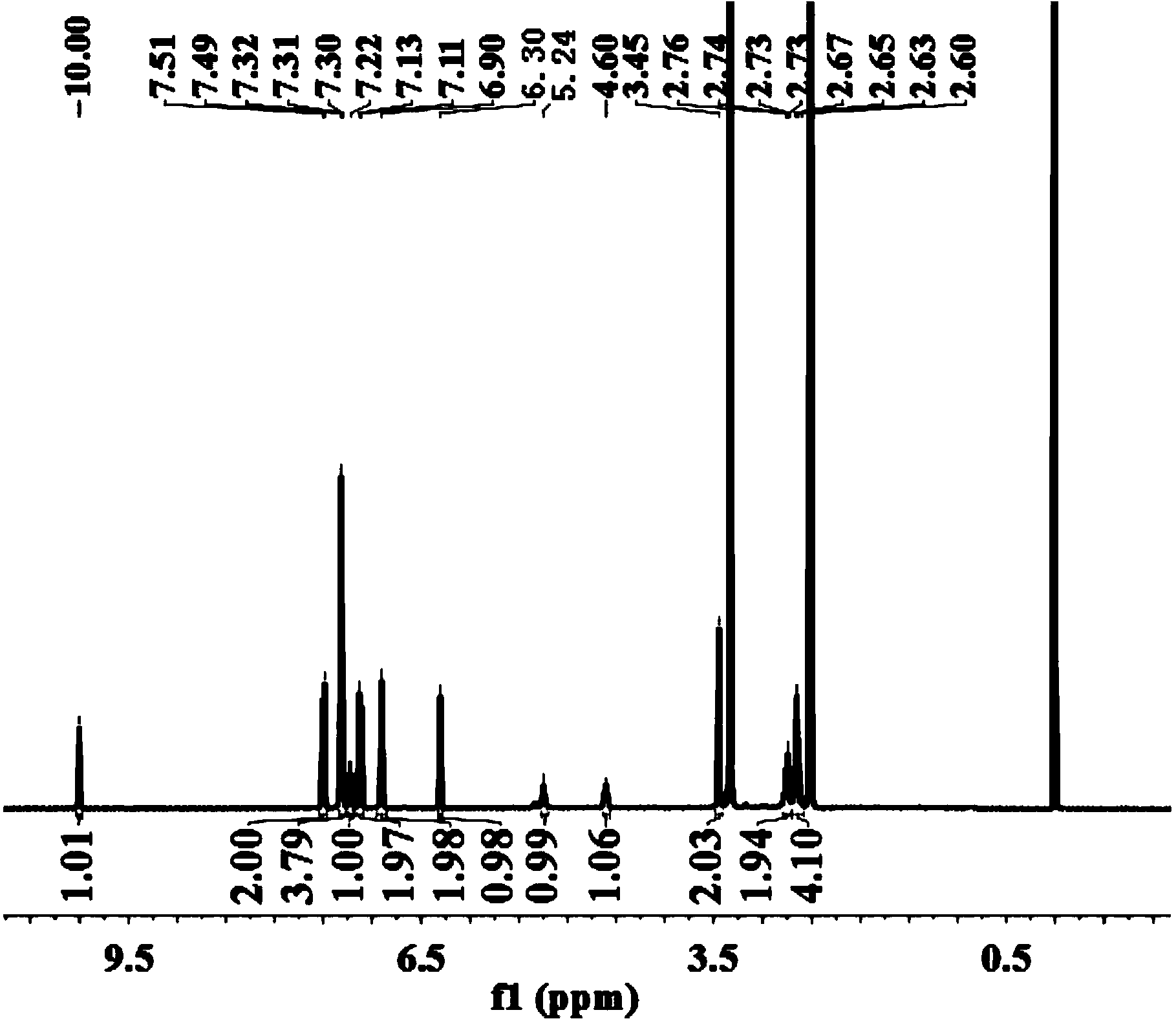
The invention discloses a mirabegron related substance as shown in a formula I or a salt thereof, and a preparation method and a use thereof. The preparation method of the mirabegron related substance or the hydrochloride thereof disclosed by the invention comprises the following step: in a solvent, carrying out a condensation reaction as shown in specification between a compound as shown in a formula 6 and a compound as shown in a formula 7 or an active ester thereof under the action of a condensating agent to obtain the mirabegron related substance as shown in the formula I; or in the solvent, carrying out the condensation reaction between the hydrochloride of the compound as shown in the formula 6 and the compound as shown in the formula 7 or the active ester thereof under the action of the condensating agent to obtain the hydrochloride of the mirabegron related substance as shown in the formula I. The mirabegron related substance disclosed by the invention is capable of effectively identifying impurities generated in the synthesis of the mirabegron so as to control the medicine quality of the mirabegron.
Owner:CHINA NAT MEDICINES GUORUI PHARMA +1
Amorphous mirabegron and processes for crystal forms of mirabegron
Aspects of the present invention relate to amorphous form of mirabegron, amorphous solid dispersion of mirabegron, process for its preparation, processes for preparation of α form crystal and β form crystal of mirabegron and pharmaceutical composition thereof.
Owner:DR REDDYS LAB LTD
Pharmaceutical composition containing mirabegron
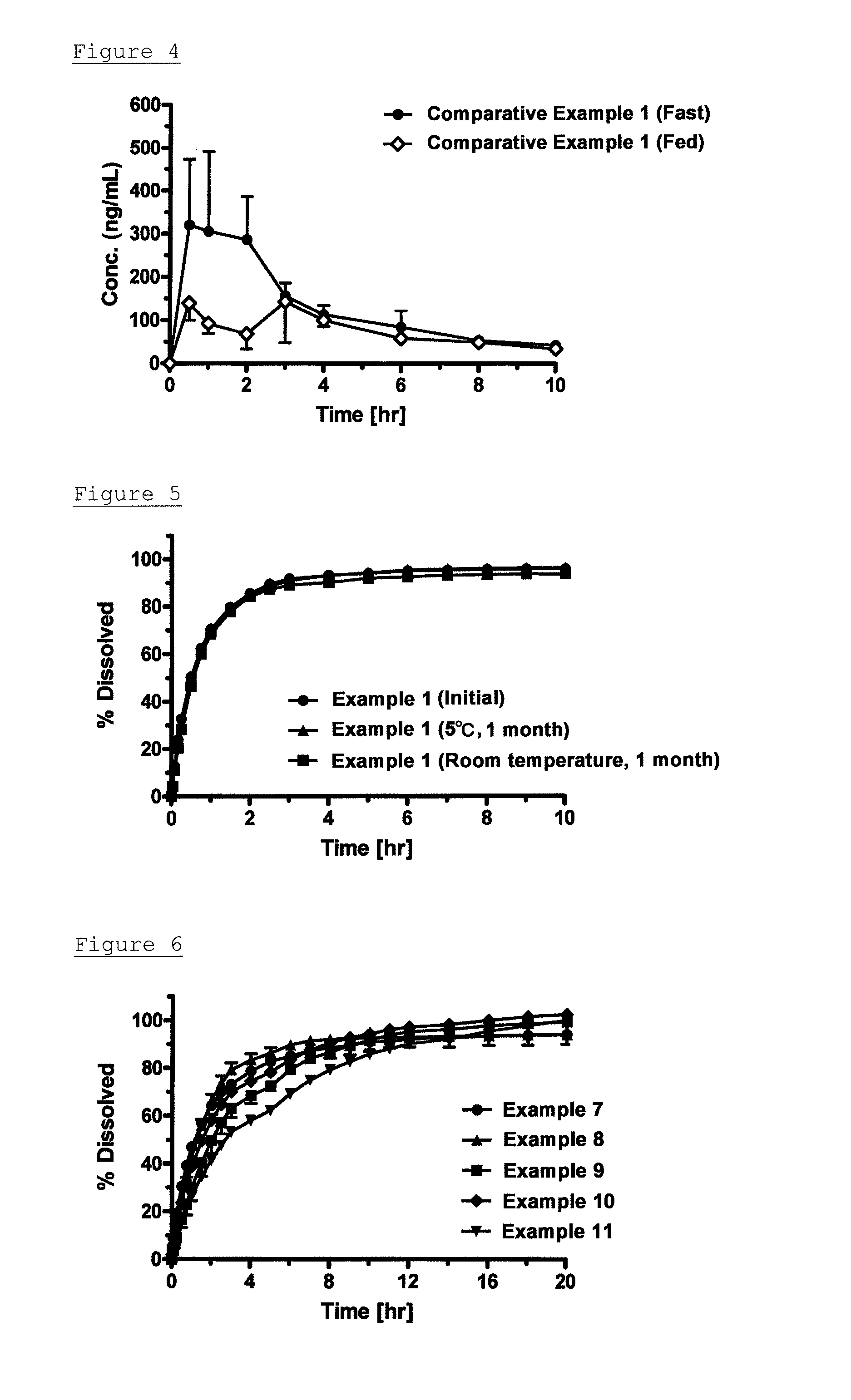
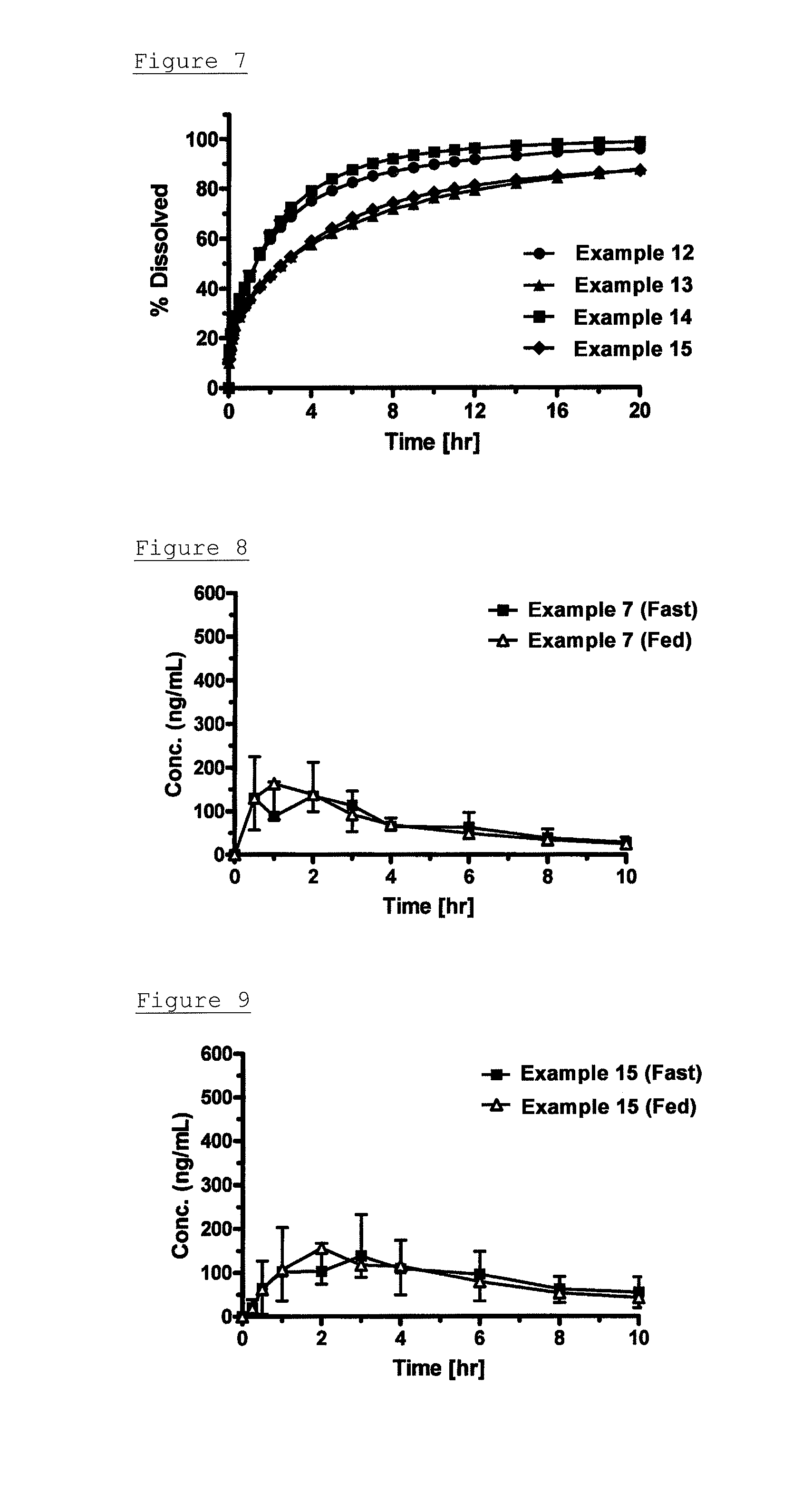
InactiveUS20150031734A1Rate decreased and reducedSolubility and decreased and reducedOrganic active ingredientsBiocideMirabegronFood intakes
Provided is a mirabegron-containing pharmaceutical composition in which the leakage of mirabegron can be inhibited when the pharmaceutical composition is dispersed in a liquid, and in which the change in pharmacokinetics caused by the presence or absence of food intake is decreased. The pharmaceutical composition comprises an acid addition salt of alkyl sulfuric acid and mirabegron, and a base for modified release.
Owner:ASTELLAS PHARMA INC
Acetylaniline compounds and application thereof in preparation of mirabegron
ActiveCN104016877AOrganic compound preparationCarboxylic acid amides preparationMirabegronMedicinal chemistry
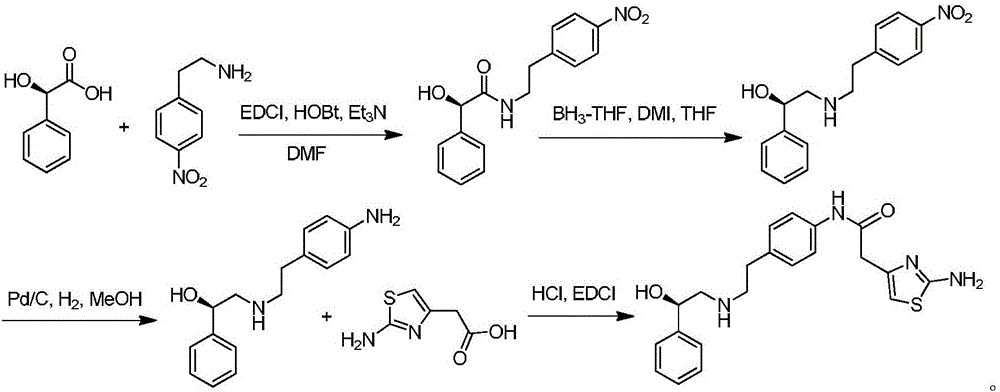
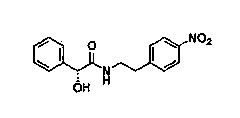
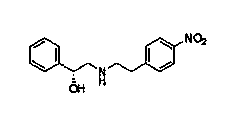
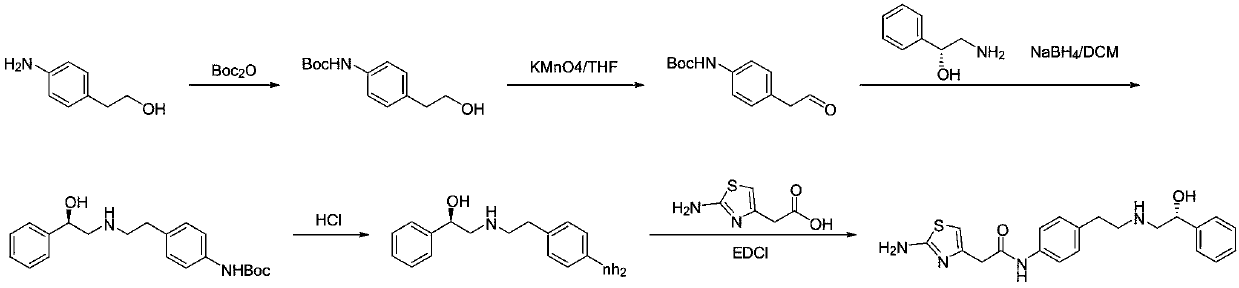
The invention discloses a new intermediate (R)-N-(4-amino phenethyl)-2-hydroxy-2-acetylaniline for preparing mirabegron, and a method for preparing the mirabegron through the intermediate. The method is simple and convenient to operate, has low cost, and is applicable to industrial production.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD
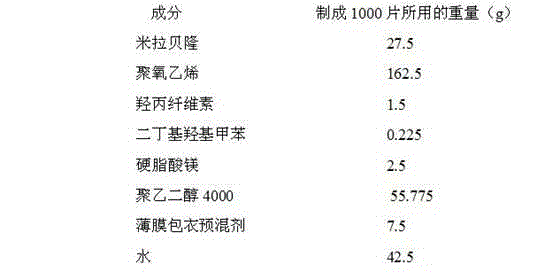
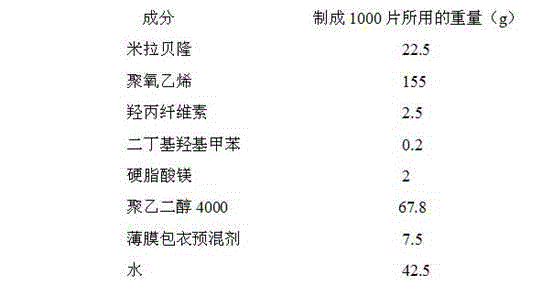
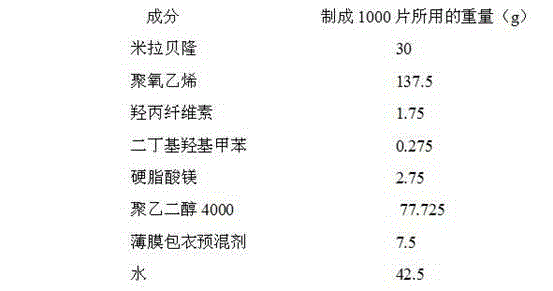
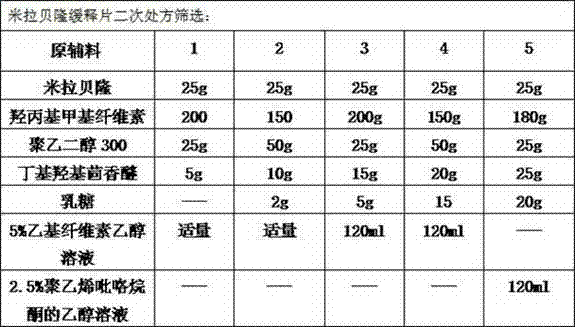
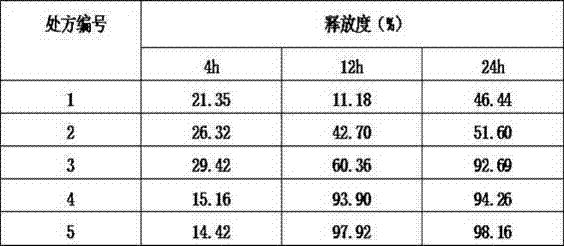
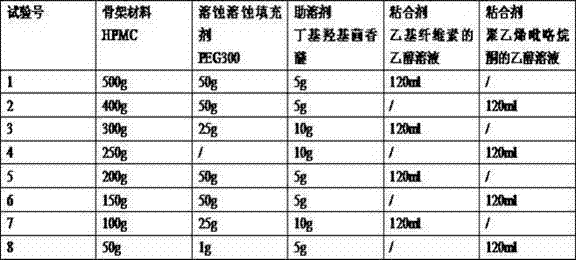
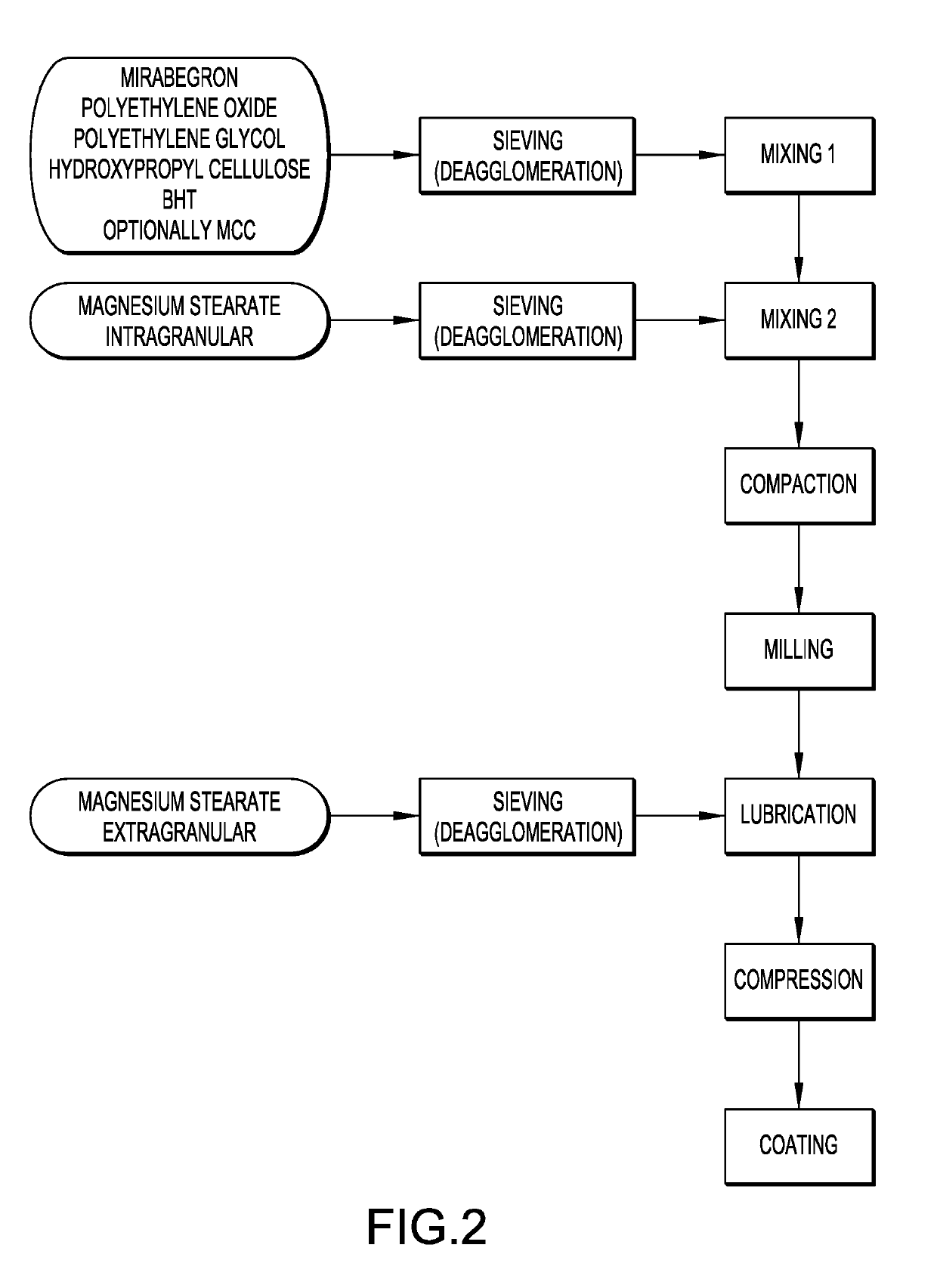
Mirabegron sustained release tablet and preparation method thereof
InactiveCN104352475ARelease influenceSimple and fast operationOrganic active ingredientsUrinary disorderSustained Release TabletMirabegron
The invention relates to a mirabegron sustained release tablet and a preparation method thereof. The mirabegron sustained release tablet comprises the following components in percentage by mass: 8%-12% of mirabegron, 55%-65% of polyoxyethylene, 0.6%-1.0% of hydroxypropylcellulose, 0.08%-0.12% of butylated hydroxytoluene, 0.7%-1.1% of magnesium stearate and the balance of polyethylene glycol. The preparation method comprises the following steps: 1, carrying out sieving pretreatment on raw materials; 2, adding polyoxyethylene, polyethylene glycol and hydroxypropylcellulose to the mirabegron, sieving and mixing evenly to obtain a premix; and 3, adding butylated hydroxytoluene to the premix, sieving, adding magnesium stearate, sieving, mixing evenly, carrying out powder vertical compression to obtain the tablet, so as to obtain the mirabegron sustained release tablet. The mirabegron sustained release tablet provided by the invention does not contain an adhesive; the tablet is directly obtained from the powder in a vertical compression manner; and the operation is simple and convenient.
Owner:安徽联创生物医药股份有限公司
Efficient synthesis method of mirabegron
InactiveCN106083758AThe reaction route is simpleLower reaction costOrganic chemistryPalladium on carbonHydrazine compound
The invention discloses an efficient synthesis method of mirabergron. The method comprises the specific steps that nitrophenylacetonitrile is subjected to a nitroreduction and nitrile group reduction reaction in hydrazine hydrate under the catalysis effect of a catalyst of palladium on carbon hydrogenation to obtain ethylamine; (R)-1-phenyl-1,2-ethanediol and methylsulfonyl chloride react under the catalysis effect of a basic catalyst of piperidine or triethylamine to obtain (R)-1-phenyl-1-hydroxy-2-mesyl-ethane; the (R)-1-phenyl-1-hydroxy-2-mesyl-ethane and the ethylamine react under the catalysis effect of a basic catalyst of potassium carbonate or triethylamine to obtain (R)-2-((4-amino phenyl ethyl amine)-1-phenethyl alcohol; the (R)-2-((4-amino phenyl ethyl amine)-1-phenethyl alcohol and 2-aminothiazole-4-ethyl acetate are subjected to a condensation reaction under the effect of potassium methoxide to obtain mirabergron. The method is simple and easy to implement, the raw materials are cheap and easy to obtain, the reaction efficiency is high, and the repeatability is good.
Owner:HENAN NORMAL UNIV
Synthetic method of mirabegron
InactiveCN104016943ALow costThe synthesis process is simpleOrganic chemistry4-nitrophenethylamineAcetic acid
The invention discloses a synthetic method of mirabegron, which comprises the following steps: 1)amine-ester exchange reaction: taking (R)-mandelic ester and 4-nitrophenethylamine as raw materials for an ester interchange reaction to obtain a mirabegron intermediate A; 2)amide reduction: reducing amide of the mirabegron intermediate A to obtain a mirabegron intermediate B; C)nitro reduction: performing a reduction reaction of the mirabegron intermediate B and a reducing agent stannous chloride to obtain a mirabegron intermediate C; and D)condensation reaction: performing a condensation reaction of the mirabegron intermediate C and 2-aminothiazole-4-acetate under the effect of a condensing agent to obtain mirabegron. The synthetic method has the advantages of low cost of raw material and high yield of product, and is suitable for large scale industrial production.
Owner:苏州凯瑞医药科技有限公司
Synthesis method of mirabegron
The invention provides a synthesis method of mirabegron. The synthesis method comprises the following steps: (1) in a tetrahydrofuran solvent and a nitrogen atmosphere, reducing a compound I into a compound II by adopting a reducing agent; (2) in an alcohol solvent and a hydrogen environment, reducing the compound II into a compound III by adopting palladium-on-carbon catalytic hydrogenation; and (3) in a water solvent and under an acidic condition, mixing the compound III with 2-amino-4-thiazole acetic acid, and performing condensation reaction under the action of a condensing agent, to obtain mirabegron. The method has the advantages of being mild in reaction conditions, high in yield and low in cost, and the provided mirabegron is low in the content of impurities, therefore, the synthesis method is suitable for large-scale industrial production.
Owner:安徽联创生物医药股份有限公司
Preparation method of Mirabegron
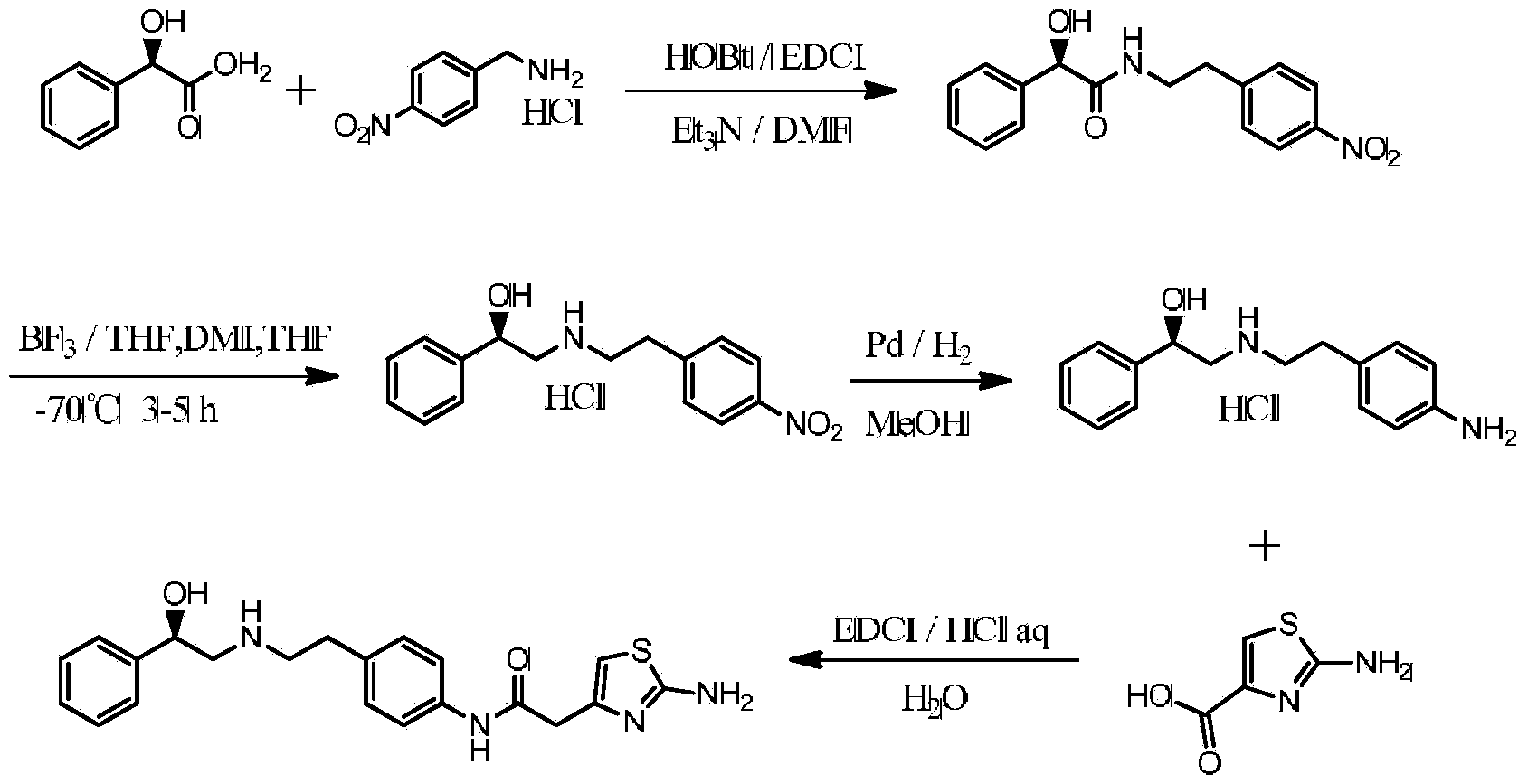
The invention discloses a preparation method of Mirabegron and relates to the technical field of medicine preparation. The preparation method includes the steps of subjecting R-mandelic acid and 4-nitrophenylethylamine to amide condensation reaction to obtain an intermediate (R)-N-(4-nitrophenethyl)-2-hydroxy-2-phenylacetamide, reducing amide carbonyl through diisobutyl aluminium hydride to obtainan intermediate (alphaR)-alpha-[[[2-(4-Nitrophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride, reducing nitryl through a ammonium formate-Pd / C reduction system to obtain an intermediate 2-[2-(4-amino-phenyl)-ethylamino]-1-phenyl-ethanol, and finally subjecting the intermediate and (2-aminothiazole-4-yl)acetic acid to condensation reaction to obtain Mirabegron. The Mirabegron prepared by themethod has high purity and high yield; the preparation method needs fewer steps of synthesis route and mild and controllable reaction conditions, is easy and convenient in operation, low in cost andadaptable to industrial production, and has broad prospect and high industrial application value.
Owner:ANHUI QINGYUN PHARMA & CHEM
Preparation method of mirabegron
ActiveCN103864713AReduce pollutionEase of industrial productionOrganic chemistryBulk chemical productionThiazolePhenethyl alcohol
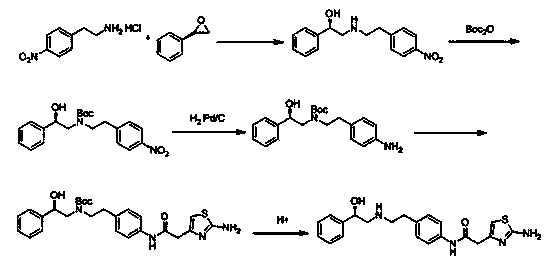
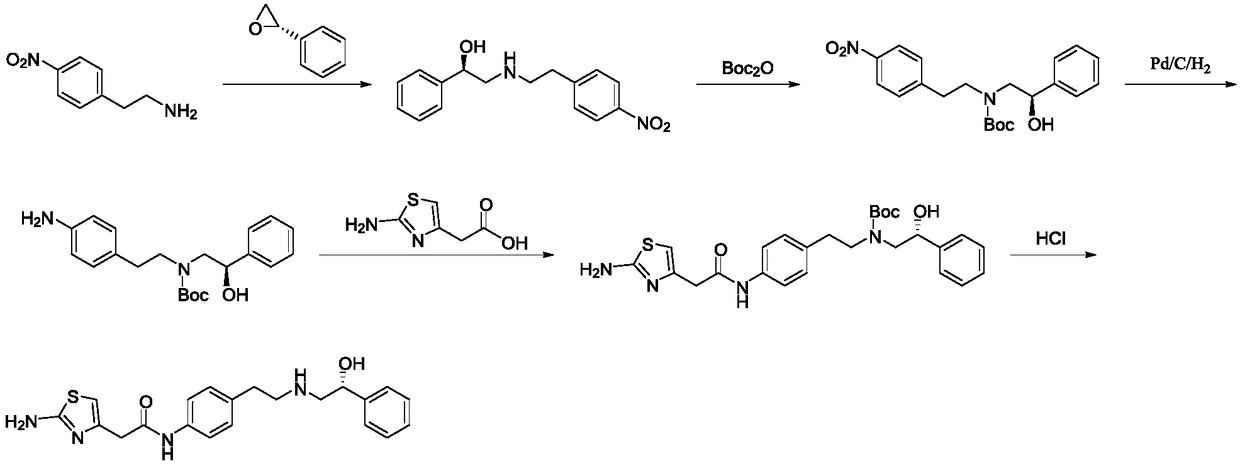
The invention discloses a preparation method of mirabegron. The preparation method comprises the following steps: I, carrying out a reaction on 2-(2-aminothiazole-4-) acetic hydrochloride shown in a structural formula 5 and an amino protective agent to obtain a compound shown in a structural formula 4; II, carrying out a condensation reaction on the compounds shown in the structural formulae 3 and 4 in the presence of a condensating agent and an acid-binding agent to obtain a compound shown in the structural formula 2; and III, carrying out a substitution reaction on the compound shown in the structural formula 2 and (R)-2-amino-1-phenethyl alcohol under the effect of the acid-binding agent, and after reaction, adding a deprotection group agent to remove the protective group to obtain mirabegron shown in a structural formula 1. The method provided by the invention is few in technological step in the synthetic process, simple, high in yield and free from highly toxic products and suitable for industrialized production on a large scale.
Owner:JIANGXI SYNERGY PHARMA
Mirabegron sustained-release tablet composition
InactiveCN104288116AQualified and stableUniform release rateOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMirabegron
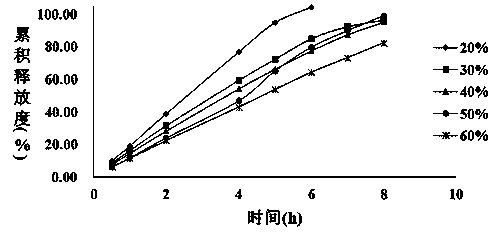
The invention provides a Mirabegron sustained-release tablet composition which contains Mirabegron, a non-polyoxyethylene sustained-release framework material, an antioxidant and a lubricant. The quality of the Mirabegron sustained-release tablet prepared after Mirabegron and the non-polyoxyethylene sustained-release framework material are mixed is qualified and stable, and uniform release speed and high release rate can be achieved.
Owner:NANJING HUAWE MEDICINE TECH DEV
Tablet composition with solubilizing and slow release effects
InactiveCN104288117ASimple processLower control costsOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMirabegron
The invention relates to the technical field of medicines, in particular relates to a tablet composition with solubilizing and slow release effects and a preparation method thereof and particularly relates to mirabegron sustained release tablets. The preparation method comprises the following steps: mixing proper medical auxiliary materials comprising a framework material, a solubilizer and a filling agent with mirabegron, and preparing a mirabegron pharmaceutical preparation which is fully and slowly released. The tablet composition is suitable for single dose and has the service characteristic of lasting slow and full release.
Owner:北京博泽德润医药科技开发有限公司
Orally administered medical composition
InactiveUS20150306090A1Improve drug dosing complianceReduce in quantityPowder deliveryBiocideImmediate releaseDissolution
In order to provide the medical field with a single formulation comprising a modified release portion containing mirabegron or a pharmaceutically acceptable salt thereof and an immediate release portion containing solifenacin or a pharmaceutically acceptable salt thereof, (1) a single formulation having dissolution rates of both drugs similar to those of the current single drug formulations is provided, and (2) a single formulation having maximum percentages of dissolution of both drugs of 90% or more, and having a bioavailability equivalent to those of the current single drug formulations. Further, in order to provide a single formulation, (3) a single formulation having good productivity whereby failures in tabletting are reduced, and having good storage stability whereby the coloration of the immediate release portion is suppressed is provided. The pharmaceutical composition for oral administration of the present invention contains (1) a modified release portion comprising mirabegron or a pharmaceutically acceptable salt thereof, and (2) an immediate release portion comprising solifenacin or a pharmaceutically acceptable salt thereof, and calcium stearate.
Owner:ASTELLAS PHARMA INC
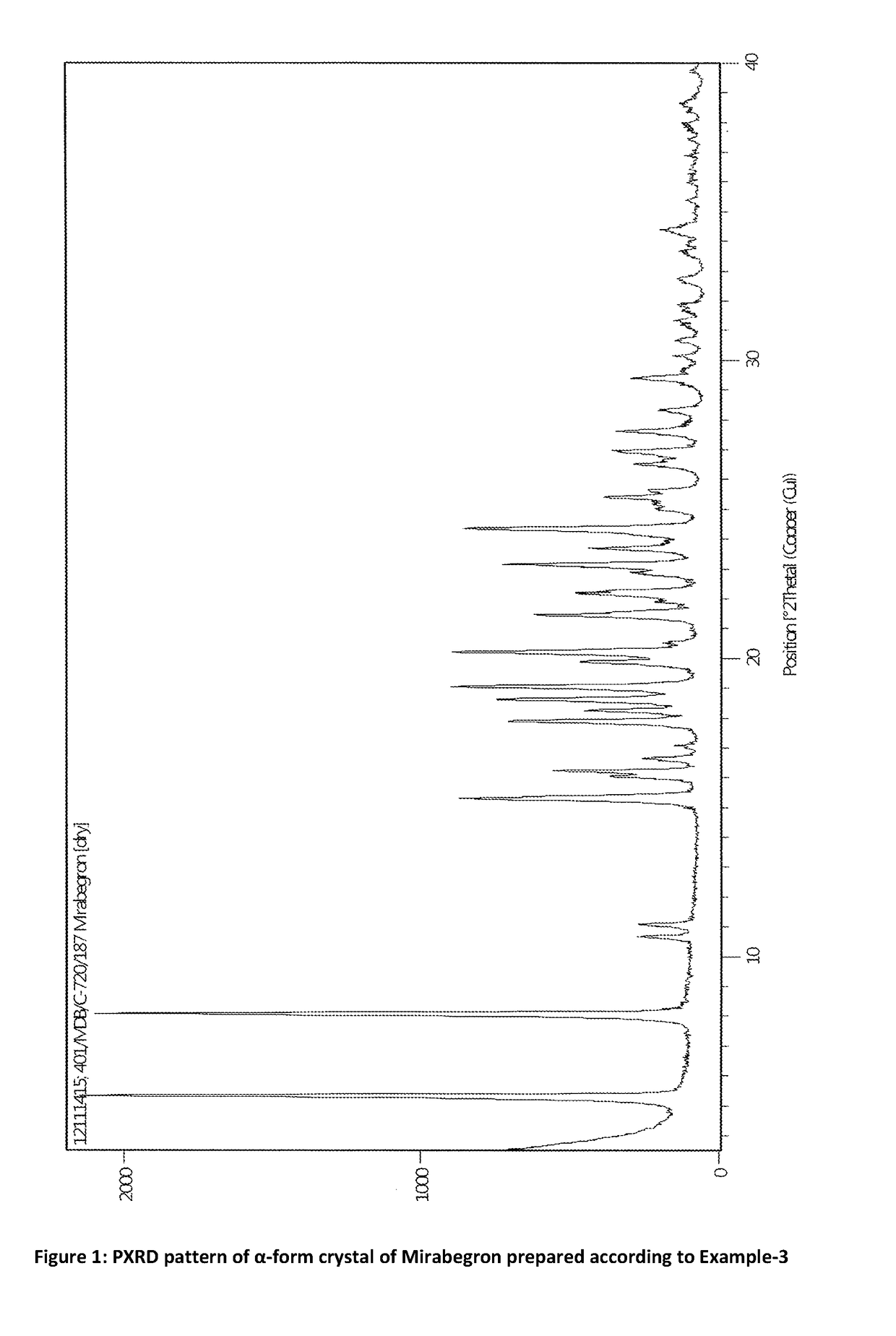
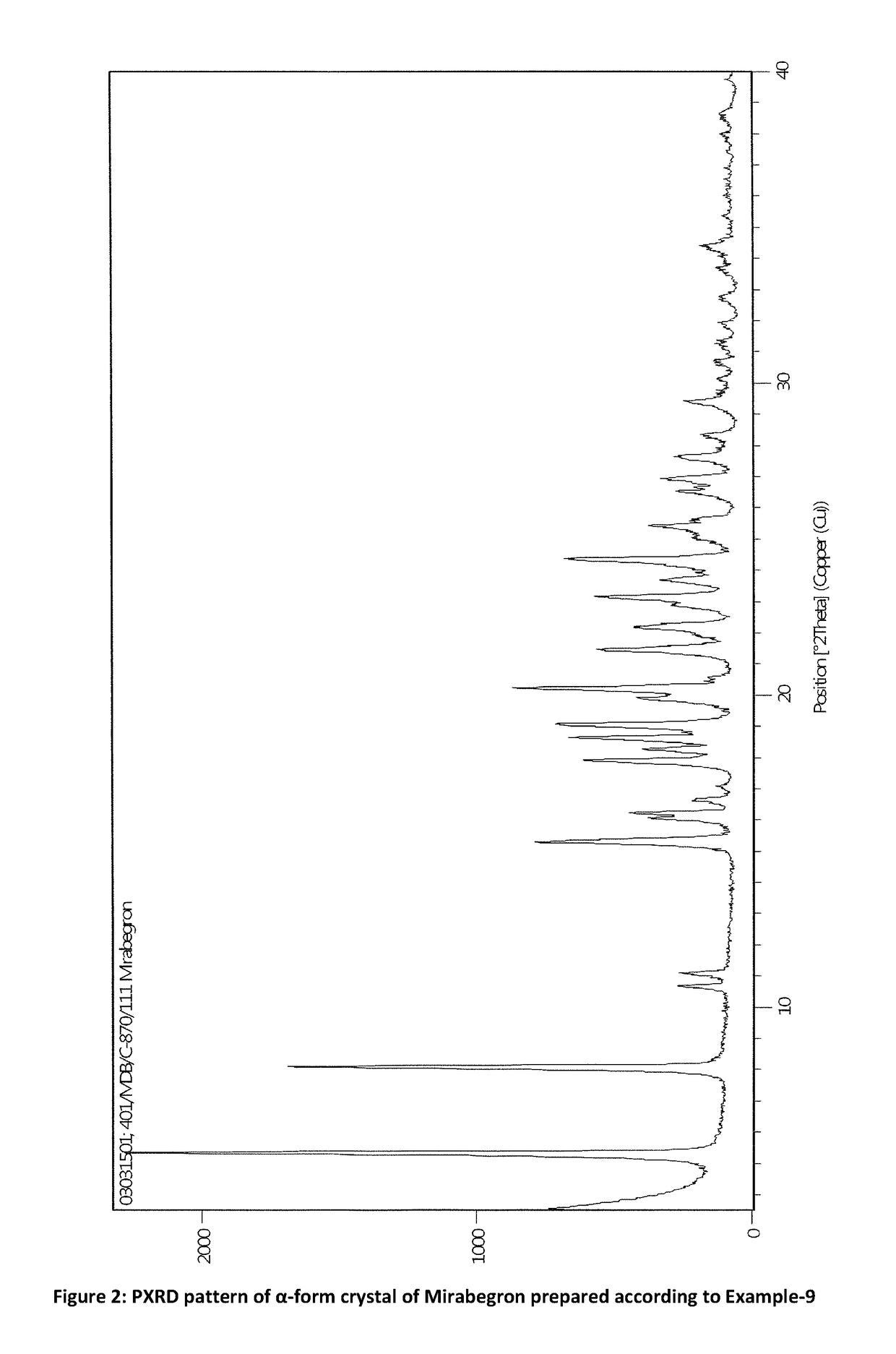
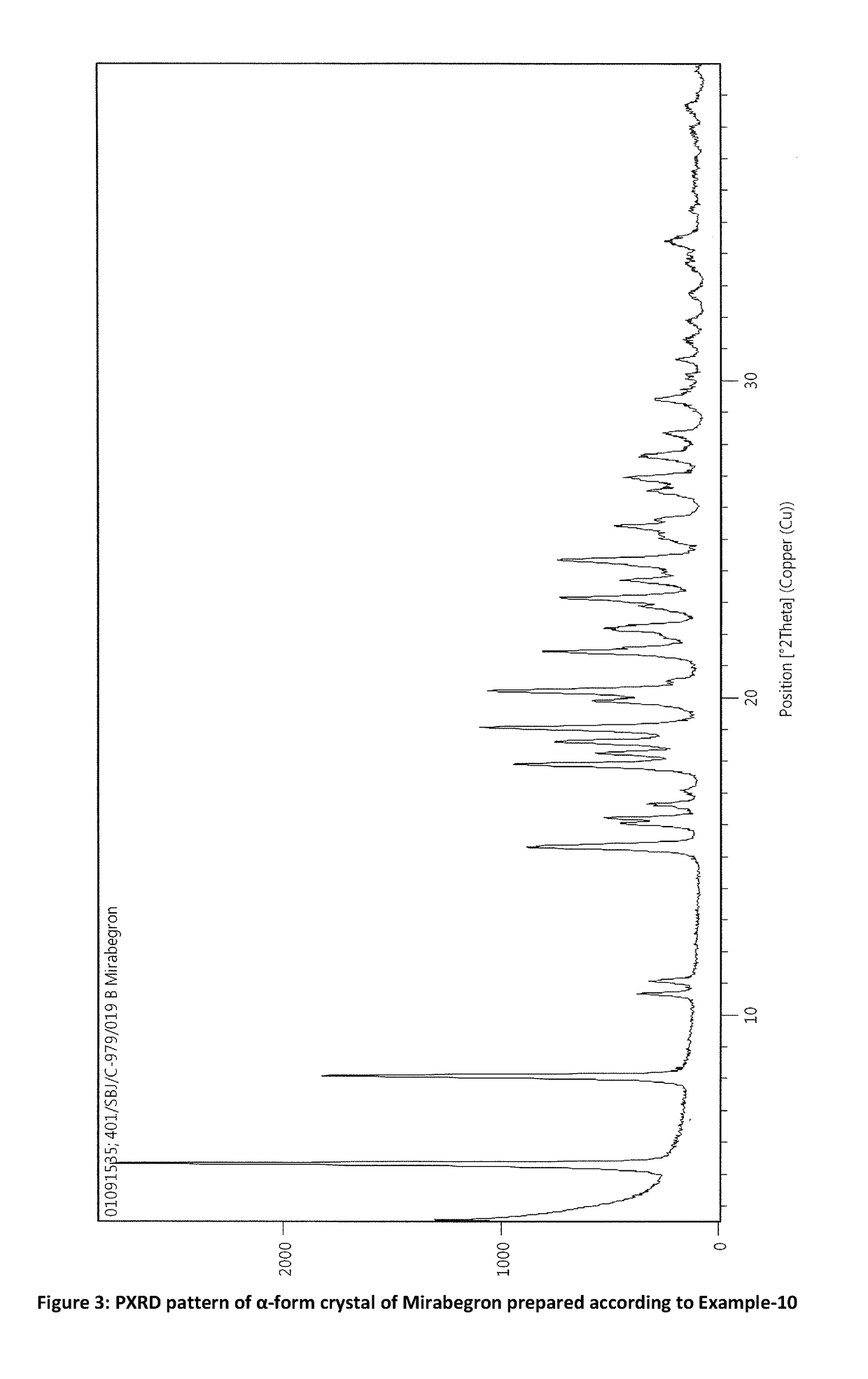
Process for preparation of polymorphic form of mirabegron
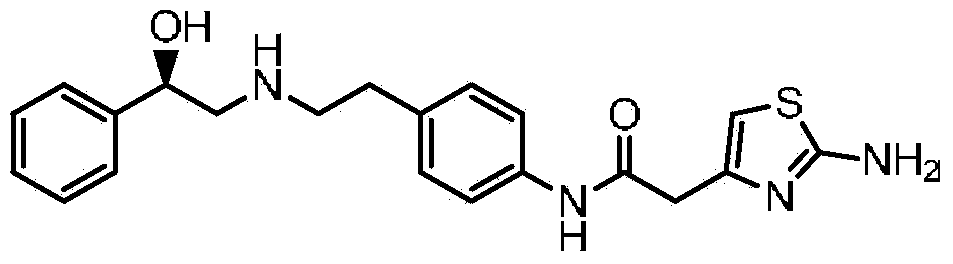
ActiveUS20180016246A1Prevent discolorationSmall particle sizeOrganic chemistry methodsUrinary disorderMirabegron2-aminothiazole
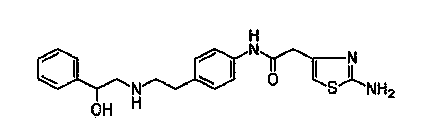
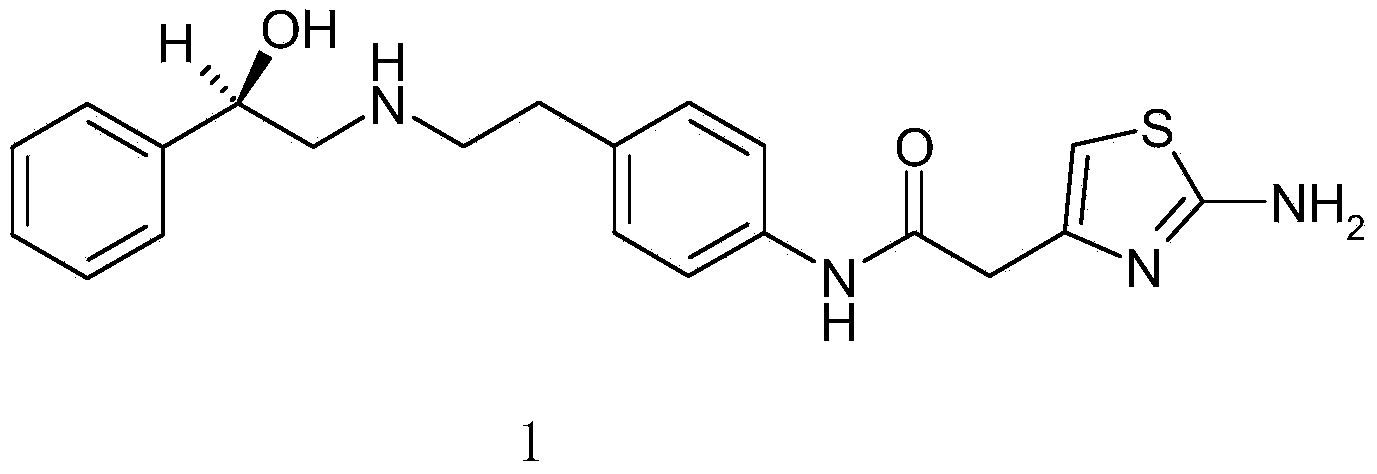
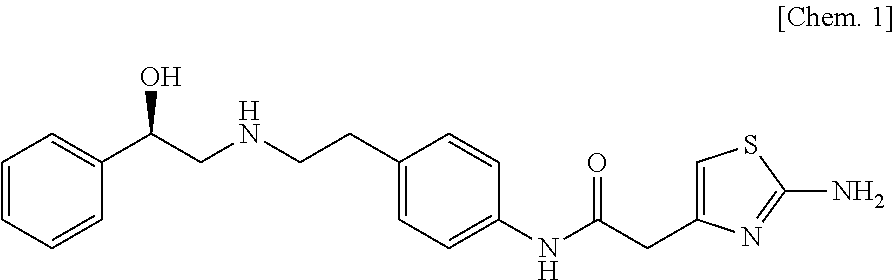
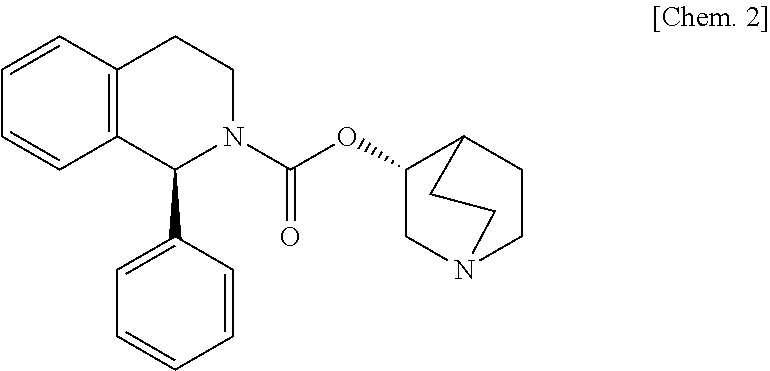
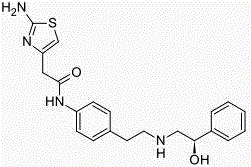
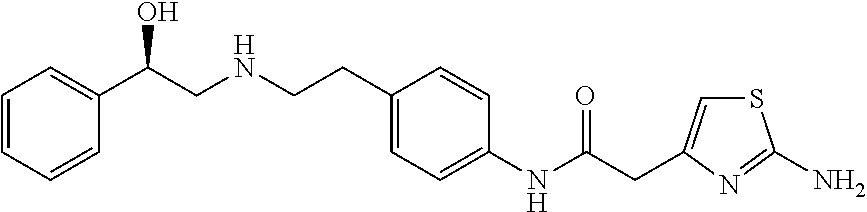
The present invention is directed to process for preparation of α-form crystal of Mirabegron, (R)-2-(2-aminothiazol-4-yl)-N-(4-(2-((2-hydroxy-2-phenylethyl) amino) ethyl) phenyl) acetamide of formula (1).
Owner:LUPIN LTD
Mirabegron preparation method
InactiveCN105198830AEasy to operateRaw materials are cheap and easy to getOrganic chemistrySulfonyl chlorideNitrobenzene
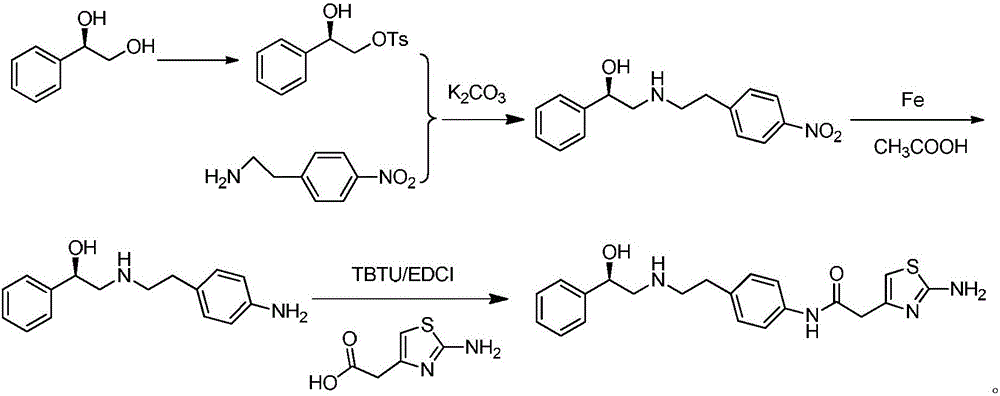
The invention discloses a mirabegron preparation method. The mirabegron preparation method comprises the following steps of a, hydroxy protection: obtaining an (R)-1-phenyl-1,2-hydroxyl-sulfonate compound through an (R)-1-phenyl-1,2-glycol and sulfonyl chloride compound, namely methylsulfonyl chloride or paratoluensulfonyl chloride, under a catalytic action of a basic catalyst-piperidine or triethylamine; b, condensation reaction: obtaining (R)-2-((4-nitrobenzene ethyl) amino)-1-phenethyl alcohol through the (R)-1-phenyl-1,2-hydroxyl-sulfonate compound and p-nitrophenyl ethylamine under a catalytic action of a basic catalyst-potassium carbonate or triethylamine; c, nitro reduction: obtaining a compound, namely (R)-2-((4-aminobenzene ethyl) amino)-1-phenethyl alcohol by carrying out nitro catalyzing and amino reducing on the (R)-2-((4-nitrobenzene ethyl) amino)-1-phenethyl alcohol through reduced iron powder; d, condensation reaction: obtaining a target product-mirabegron through the (R)-2-((4-aminobenzene ethyl) amino)-1-phenethyl alcohol and 2-aminothiazole-4-acetic acid under the action of a coupling agent. The mirabegron preparation method disclosed by the invention has the advantages that the operation is simple and easy, raw materials are low in cost and easily obtained, the reaction efficiency is high, and the repeatability is good.
Owner:HENAN NORMAL UNIV
Preparation method of mirabegron
The invention discloses a preparation method of mirabegron, and the method comprises: S1, carrying out reduction reaction on p-nitrophenylacetonitrile to obtain p-nitrophenylacetaldehyde; S2, carryingout condensation reduction on the p-nitrophenylacetaldehyde and (R) 2-amino-1-phenethyl alcohol to obtain (R) 2-(4-nitrophenethyl) amino)-1-phenyl ethyl alcohol; S3, carrying out reduction on the (R)2-amino-1-phenethyl alcohol to obtain (R) 2-(4-nitrophenethyl) amino)-1-phenyl ethyl alcohol to obtain an intermediate (R) 2-((4-aminophenyl ethyl) amino)-1-phenyl ethyl alcohol; and S4, carrying outcondensation on the (R) 2-((4-aminophenyl ethyl) amino)-1-phenyl ethyl alcohol and aminothiazole acetic acid to obtain the mirabegron. According to the method, the starting raw materials are cheap and easy to obtain, the reaction conditions are controllable, the synthetic route steps are few, the yield is high, the cost is low, and the prepared mirabegron is high in purity.
Owner:ANHUI QINGYUN PHARMA & CHEM
Recrystallization method of mirabegron and preparation method thereof
ActiveCN111072589AReduce the content of imAReduce solubilityOrganic chemistry methodsSolution crystallizationAlcoholPharmaceutical drug
The invention relates to the technical field of purification of chemical drugs, in particular to a recrystallization method of mirabegron and a preparation method thereof. The recrystallization methodof mirabegron comprises the following steps: dissolving a mirabegron crude product in a mixed solvent under a reflux temperature condition, cooling for crystallization, and collecting a solid; wherein the mixed solvent comprises an alcohol solvent and dichloromethane. According to the recrystallization method disclosed by the invention, by regulating and controlling the mixed solvent, the mirabegron in the beta crystal form can be rapidly converted into the alpha crystal form after being dissolved in the specific mixed solvent, the solubility of the mirabegron in the specific mixed solvent system is reduced after crystal transformation, and the mirabegron is slowly separated out, so that other impurity components are separated from the mirabegron, and the purification purpose is achieved;the method has the advantages of simple operation process, low production cost, reduction of the impurity imA content in the product especially for the imA impurity in the mirabegron crude product, simple crystallization and purification of the crude product obtained on the basis of the original process route, and important meaning for the research, development and application of mirabegron medicines.
Owner:BEIJING ZHENDONG GUANGMING PHARMA RES INST
Mirabegron sustained-release pharmaceutical composition
ActiveCN104523635AGuaranteed efficacyEnsure safetyOrganic active ingredientsUrinary disorderSustained Release TabletAntioxidant
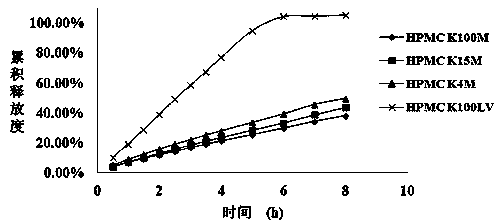
The invention provides a mirabegron sustained-release tablet. The core of the sustained-release tablet is made of hydroxypropyl methyl cellulose serving as a framework material; the mirabegron sustained-release tablet can be continuously released for 8 hours after being orally taken by a human body, and the release amount is not smaller than 90%; and no antioxidants are adopted, so that the pharmacological function and the safety are ensured.
Owner:深圳万乐药业有限公司
Mirabegron sustained release tablet and preparation method thereof
ActiveCN105769786AImprove use complianceEasy to useOrganic active ingredientsUrinary disorderSustained Release TabletBlood concentration
The invention provides a mirabegron sustained release tablet. The sustained release tablet is characterized by consisting of the following components in percentage by weight (as shown in description). The invention can overcome shortcomings of the prior art; the mirabegron can slowly release and absorb in vivo, so that a stable blood concentration is kept and patient's compliance is enhanced, and the sustained release tablet is good in clinical application prospect. The preparation method disclosed by the invention is simple and convenient, applicable to industrial production and is relatively high in practical value.
Owner:SHANGHAI SUNTECH PHARMA +1
Mirabegron medicine composition and preparation method thereof
InactiveCN105641706AHigh yieldReduce manufacturing costOrganic active ingredientsPharmaceutical non-active ingredientsPharmaceutical drugMirabegron
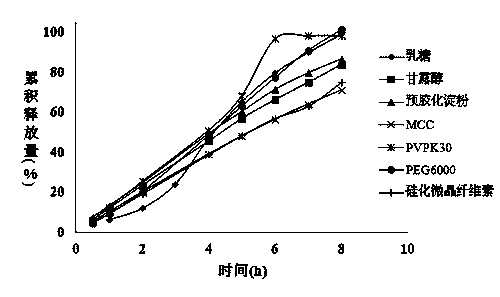
The invention discloses a mirabegron medicine composition. A recipe comprises framework materials; and 20 weight percent to 25 weight percent of mannitol and 10 weight percent to 20 weight percent of microcrystalline cellulose are used as filling agents. The mirabegron medicine composition has the beneficial effects that the stability is high; and the obvious advantages are realized on product yield improvement, cost reduction, industrialization realization and good clinical application.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Mirabegron composition
The invention relates to a Mirabegron composition and a preparing method thereof, and belongs to the technical field of pharmaceuticals. According to the technical scheme, a Mirabegron sustained release tablet is characterized in that every one thousand compositions are prepared from 25 g-50 g of Mirabegron, 80 g-150 g of hydroxypropyl methylcellulose (K4M), 0.15 g-0.3 g of butylated hydroxytoluene, 50 g-80 g of polyoxyethylene (POLYOX N80), 8 g-26 g of polyethylene glycol6000, 18 g-25 g of lactose, 0.8 g-1.4 g of magnesium stearate and 8 g-15 g of powdery silicon dioxide. According to the technical scheme, the Mirabegron sustained release tablet with the even content is obtained, and the safe medicine is provided for clinic.
Owner:DISHA PHARMA GRP +1
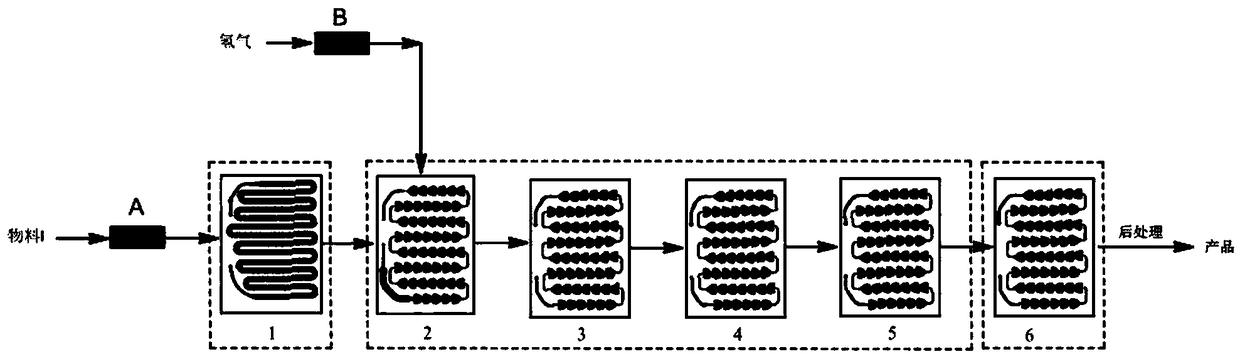
Method for synthesizing Mirabegron intermediate by microchannel reactor
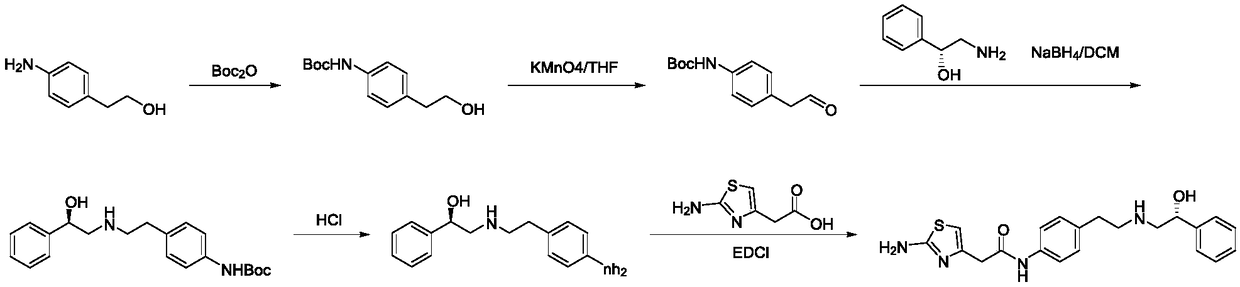
InactiveCN108947853AQuick responseShort reaction timeOrganic compound preparationChemical/physical/physico-chemical microreactorsActivated carbonOrganic solvent
The invention relates to a method for synthesizing a Mirabegron intermediate by a microchannel reactor, and belongs to the field of drug synthesis in organic synthesis. The invention aims to solve problems of existing Mirabegron intermediate synthesis process, such as large potential safety hazard, low yield, poor purity, low frequency for recycling and reusing of a catalyst and the like. According to the method, a raw material, an organic solvent and an activated carbon-supported precious metal catalyst are mixed and then react with hydrogen in a microchannel reactor to obtain the Mirabegronintermediate (ALPHAR)-ALPHA-[[[2-(4-aminophenyl)ethyl]amino]methyl]benzyl alcohol. The concentration of the raw material in the organic solvent is 0.1 mol / L-0.5 mol / L; mass ratio of the raw material to the activated carbon-supported precious metal catalyst is 1: (0.01-0.10); and the molar ratio of the raw material in the material I to hydrogen is 1: (3.0-3.5). The method of the invention is suitable for long-term safe and stable online production of the Mirabegron intermediate.
Owner:HEILONGJIANG XINCHUANG BIOLOGICAL TECH DEV CO LTD
Synthetic method of mirabegron intermediate
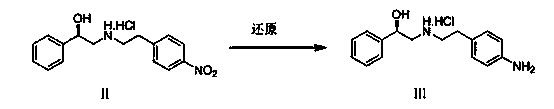
InactiveCN105801438AReduce manufacturing costSimple and fast operationOrganic compound preparationCarboxylic acid amides preparationNitrobenzeneMandelic acid
The invention discloses a synthetic method of a mirabegron intermediate (R)-2-(4-nitrobenzene ethyl amino)-1-phenyl ethanol hydrochloride (M-02) and belongs to the field of drug synthesis. The method comprises steps as follows: (1) R-mandelic acid and pivaloyl chloride produce a mixed anhydride intermediate (I); (2) the mixed anhydride intermediate (I) and 4-nitrobenzene ethylamine are subjected to an acrylation reaction to produce an intermediate (II); (3) a mirabegron intermediate (M-01) is obtained through hydrolysis of the intermediate (II); (4) an amido bond of the intermediate (M-01) is reduced and the mirabegron intermediate (M-02) is obtained. The reaction condition is mild, the yield is high, few impurities exist, the production cost is low, and the method is suitable for mass industrial production.
Owner:UNIV OF JINAN
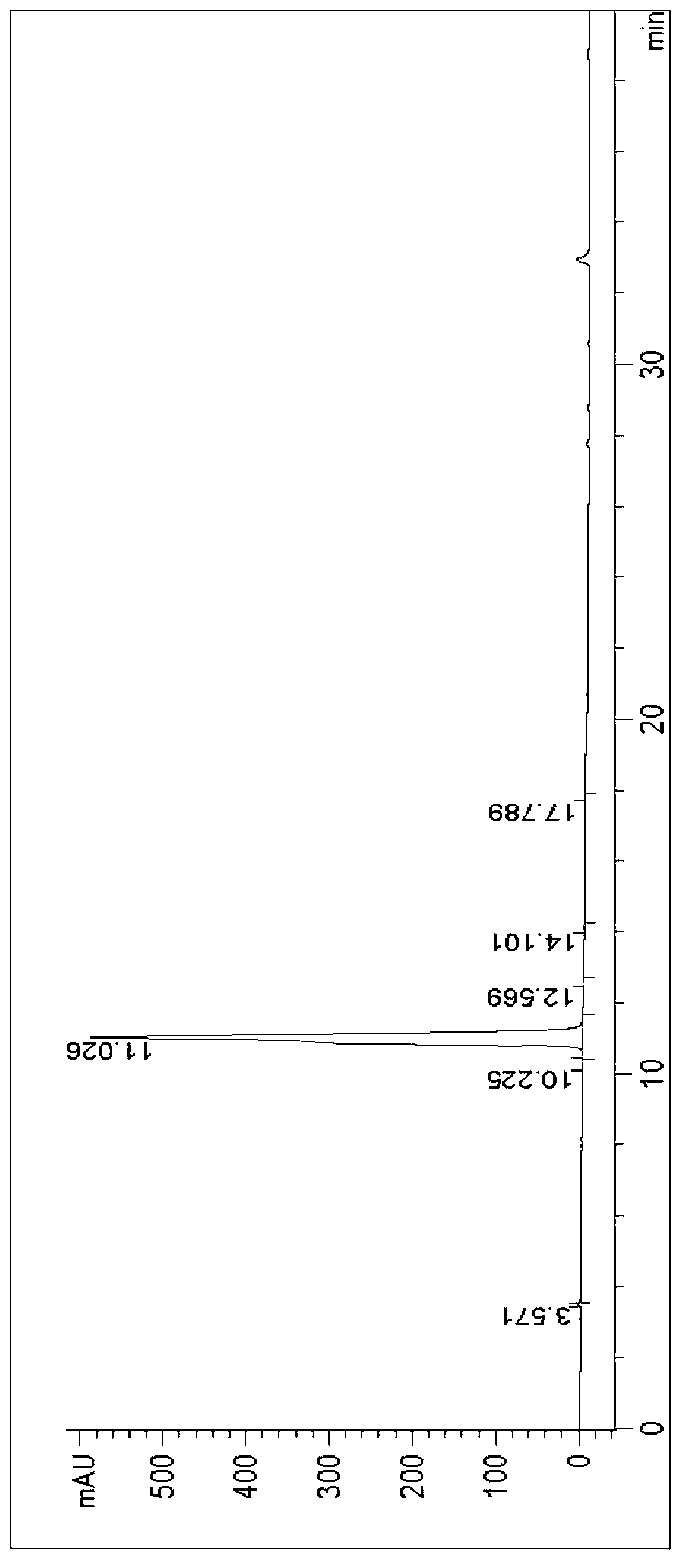
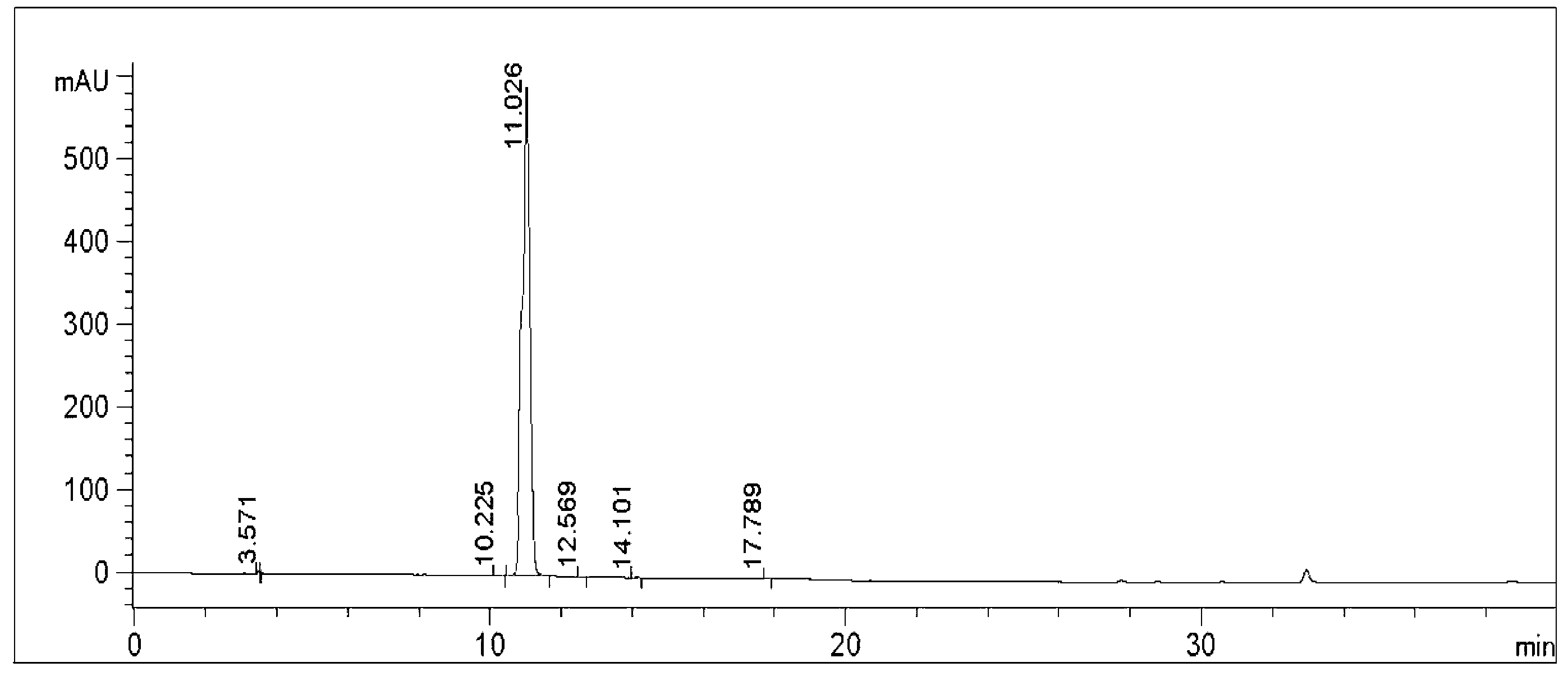
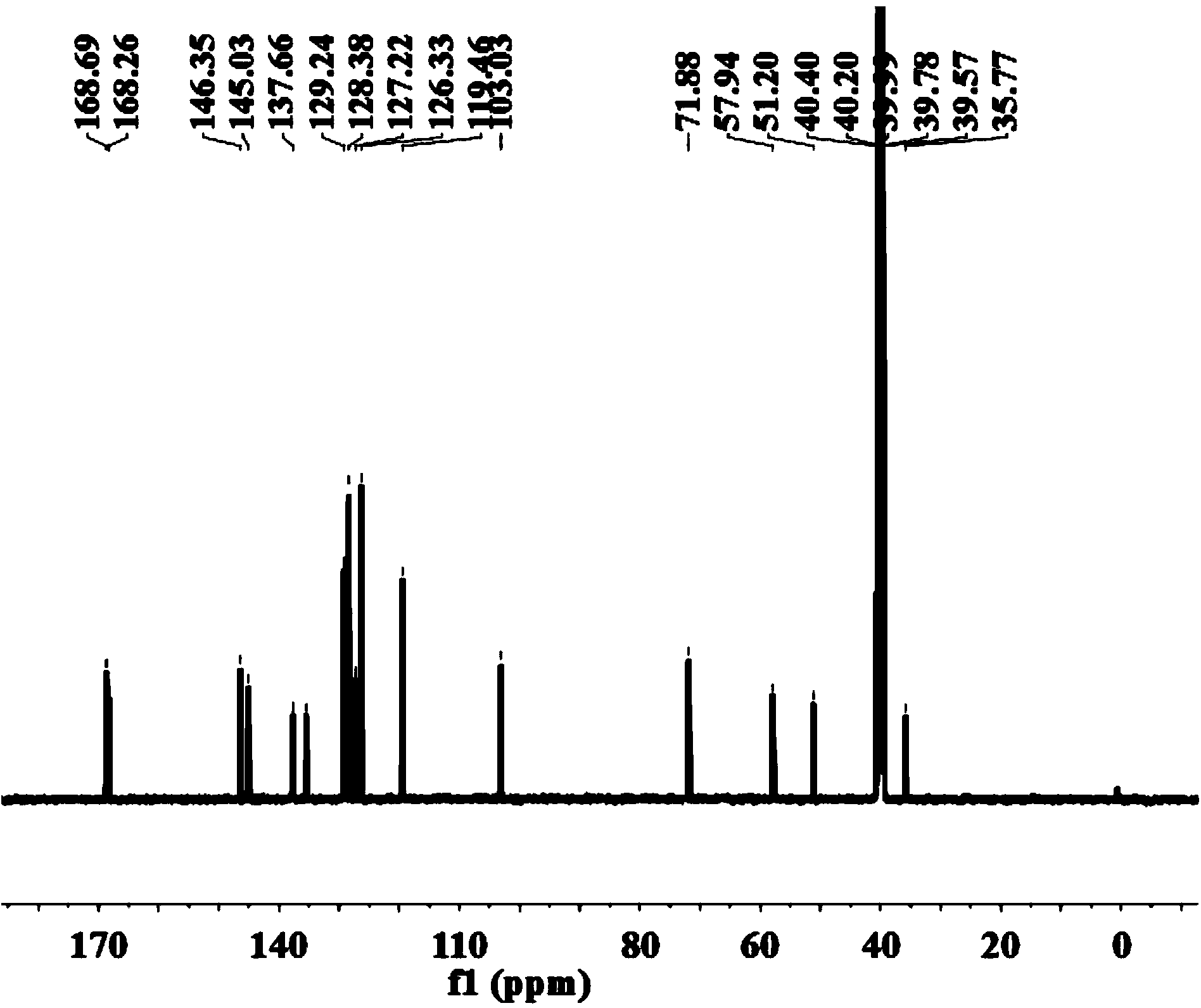
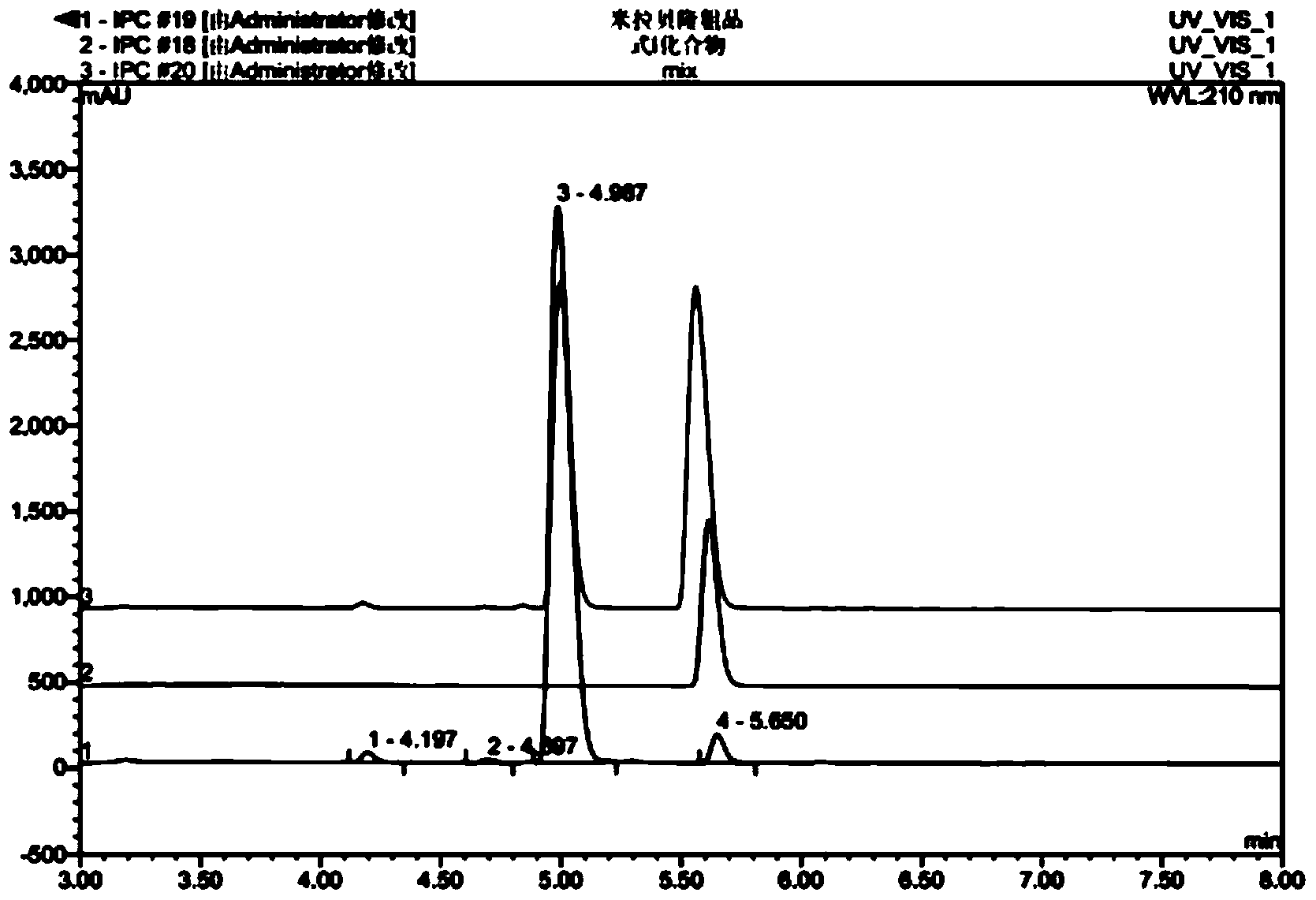
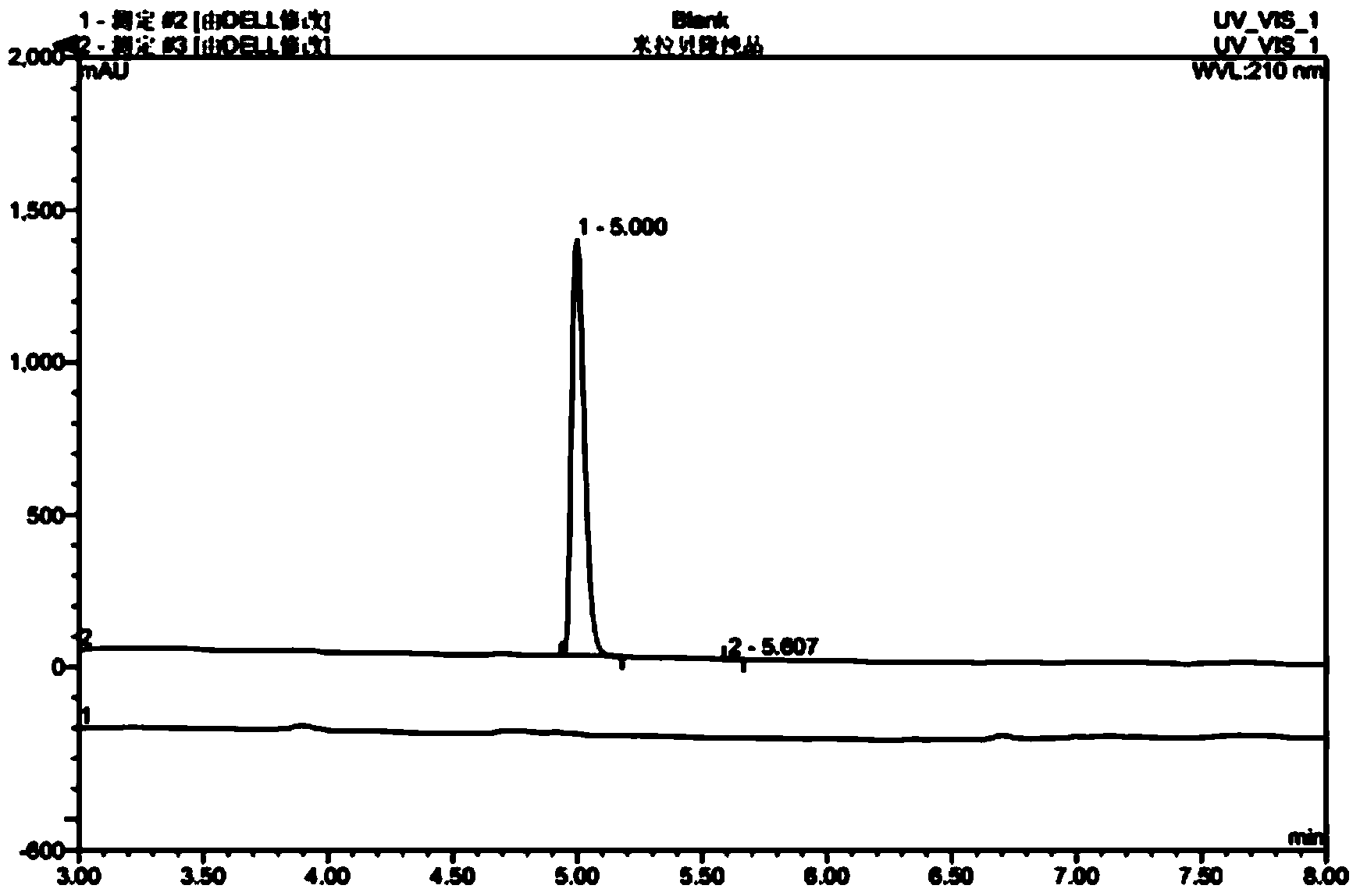
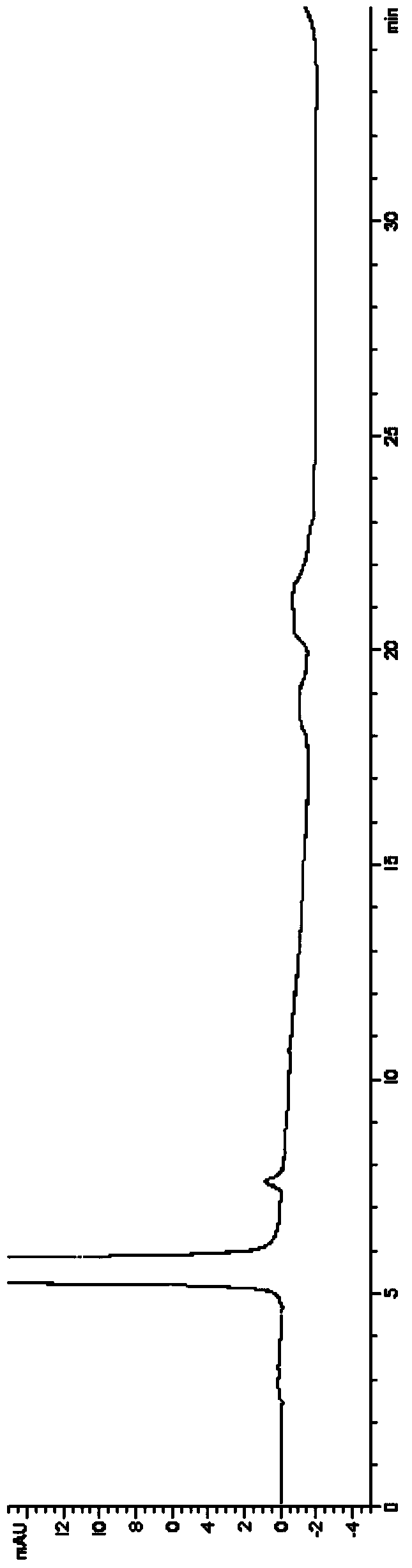
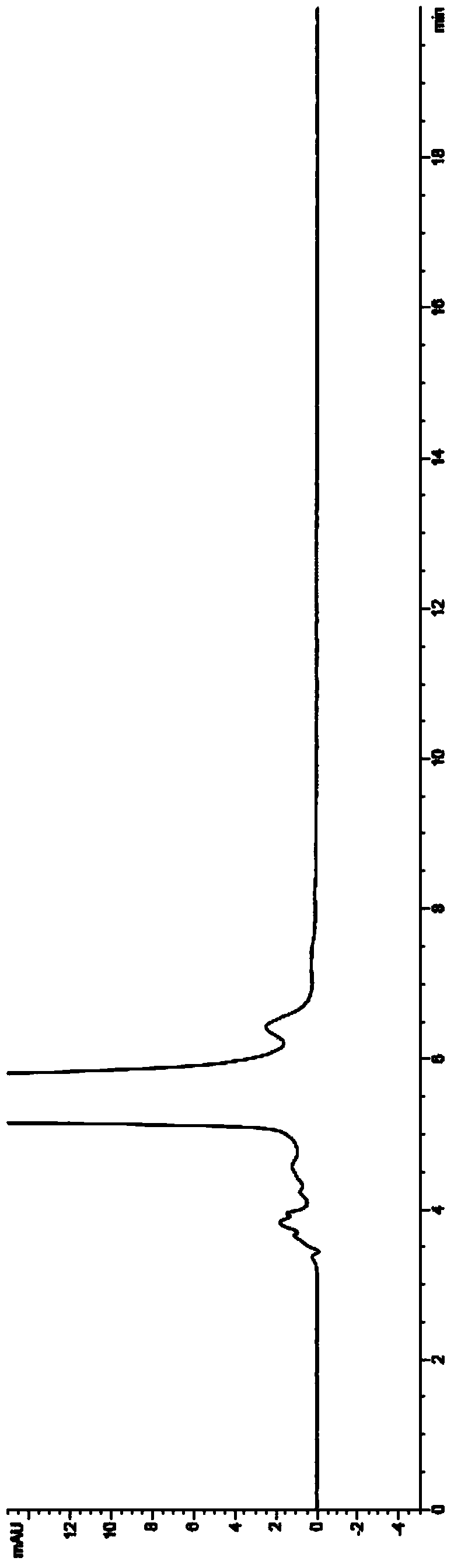
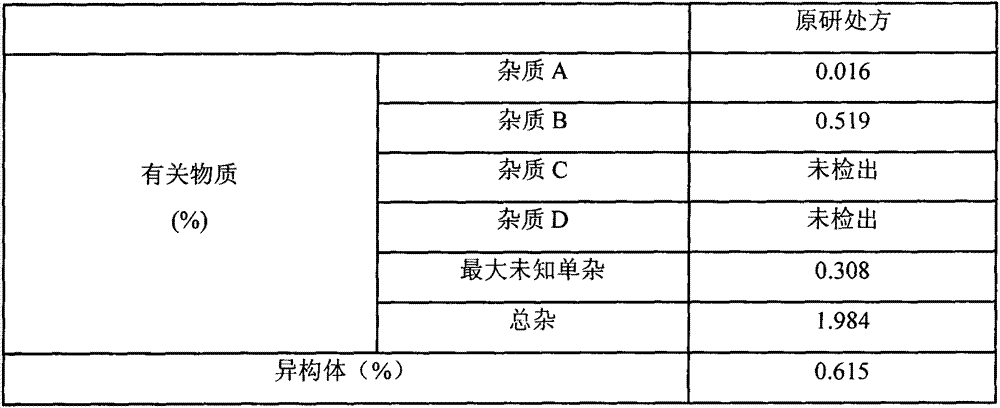
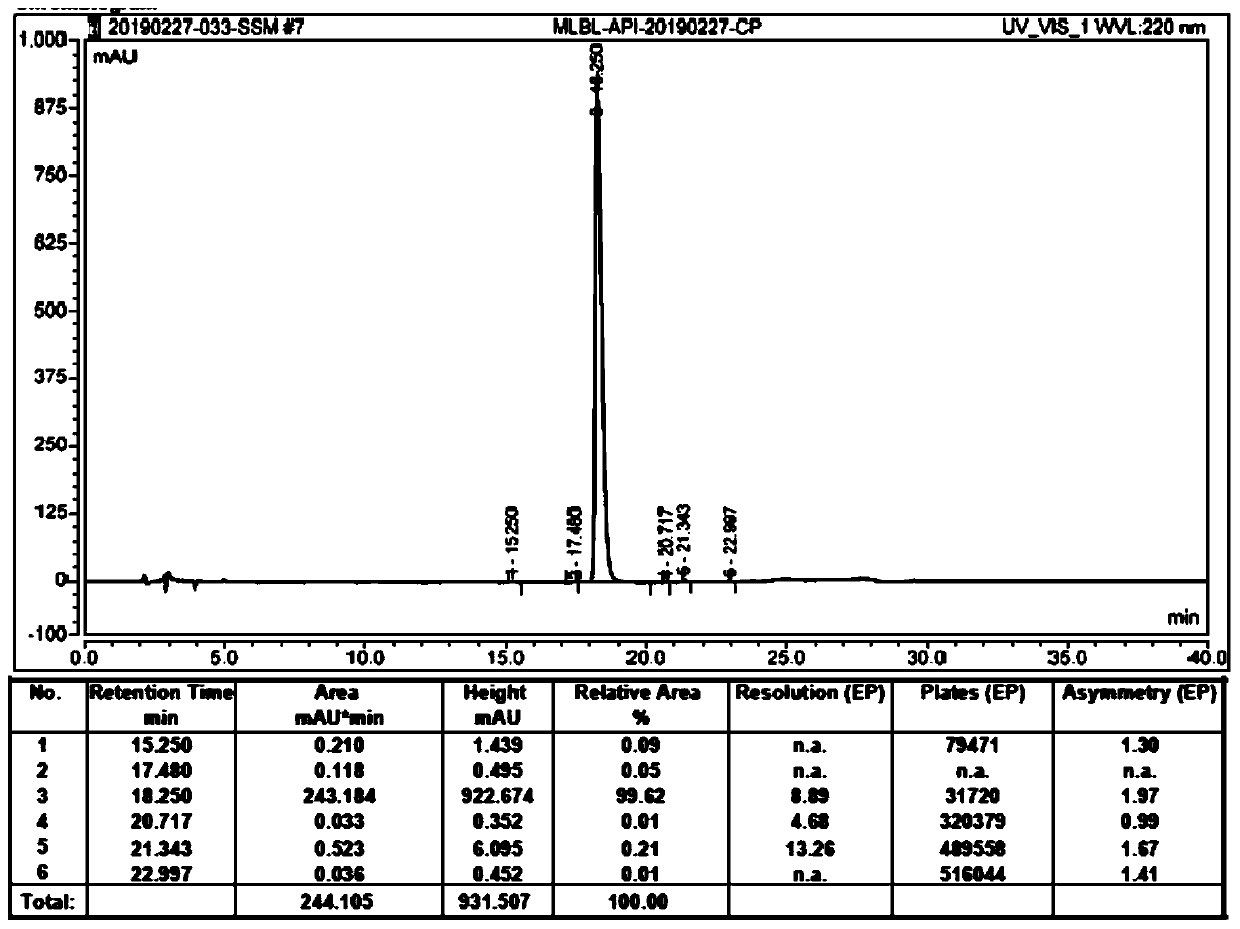
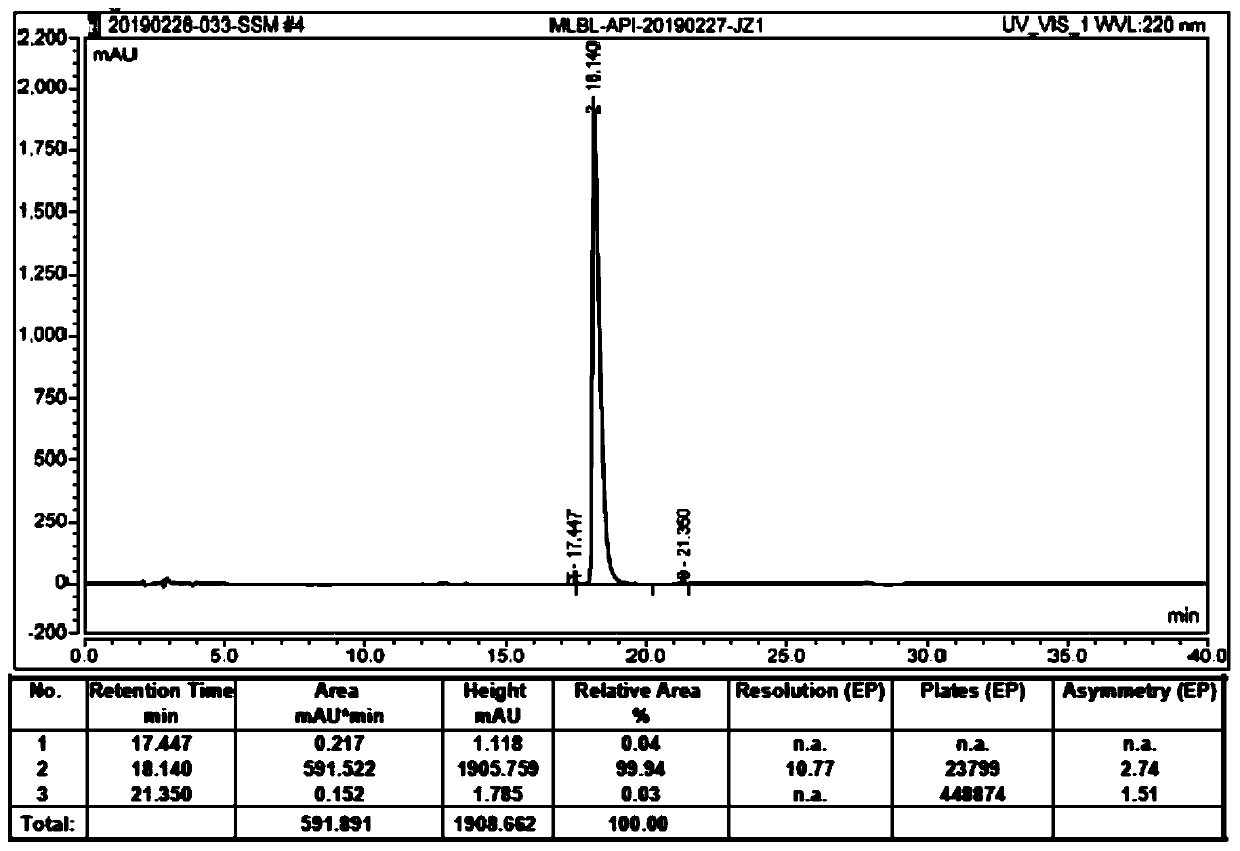
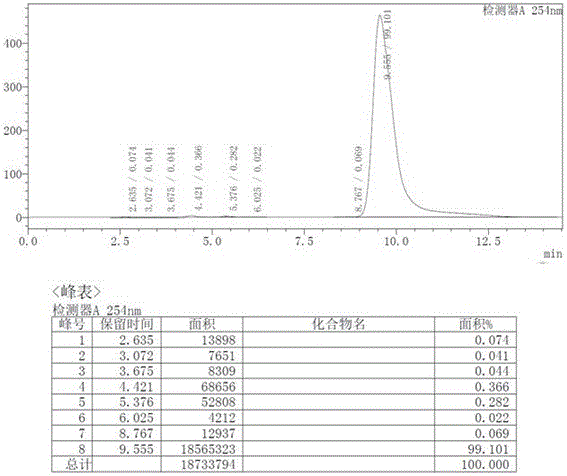
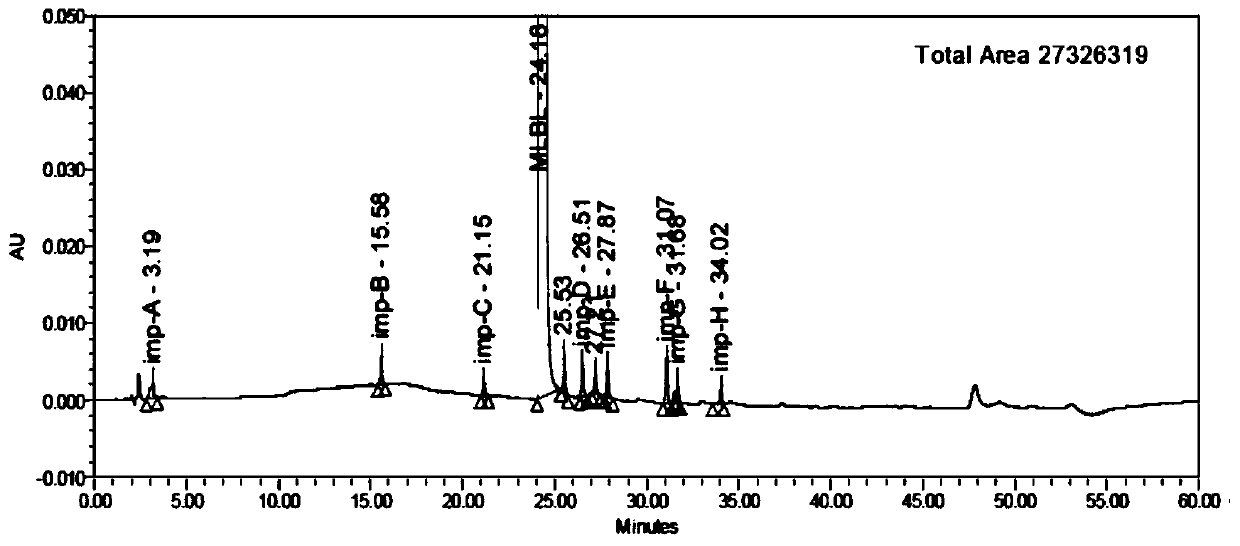
HPLC analysis method of mirabegron-related substances
The invention discloses an HPLC analysis method of mirabegron-related substances. The HPLC analysis method includes the steps that octadecyl silane chemically bonded silica is selected to be taken asa chromatographic column of a filler; a mobile phase is adopted for carrying out gradient elution, and the mobile phase is prepared from the compositions of a potassium dihydrogen phosphate buffer solution, methanol and acetonitrile; and high performance liquid chromatograph is carried out at appropriate velocity and column temperature, and a chromatogram is recorded. Impurities in a mirabegron raw material drug can be effectively eluted, separated and quantified, an impurity peak is completely separated from a main peak, the analysis speed is fast, and the detection effect is good.
Owner:ZHEJIANG HUAYI PHARMA CO LTD OF HANGZHOU HUADONG PHARMA GRP
Synthesis of mirabegron intermediate (R)-2-(4-nitrophenethylamino)-1-phenylethanol hydrochloride
InactiveCN108658797AFeasibility of large industrial operationReduce manufacturing costOrganic compound preparationOrganic chemistry methodsStyrene oxideSynthesis methods
The invention discloses synthesis of a mirabegron intermediate (R)-2-(4-nitrophenethylamino)-1-phenylethanol hydrochloride. The synthesis comprises the following steps: firstly, enabling alpha-bromoacetophenone and 4-nitro-phenethylamine to react in a solvent in the presence of alkali to generate an intermediate I; then carrying out reduction reaction on the intermediate I under the action of a chiral inducing agent and a reducing agent to generate an intermediate II; enabling the intermediate II to react with concentrated hydrochloric acid in a solvent to generate a target product. Accordingto the synthesis method disclosed by the invention, EDCI (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide), HOBt (Hydroxybenzotriazole) and DMI (1,3-dimethyl-2-imidazolidinone) which have a relatively high price, and hypertoxic R-styrene oxide can be avoided; the synthesis method has the advantages of easiness for obtaining raw materials, low cost, simplicity in operation and few impurities and is suitable for industrial production.
Owner:ANHUI DEXINJIA BIOPHARM
Mirabegron sustained-release pharmaceutical composition
ActiveCN104523635BOrganic active ingredientsPharmaceutical non-active ingredientsSustained Release TabletAntioxidant
Owner:深圳万乐药业有限公司
Modified release tablet composition comprising mirabegron
The present invention relates to a pharmaceutical composition, particularly a modified release tablet composition comprising mirabegron or a pharmaceutically acceptable salt thereof and to a process for preparing such a composition
Owner:SYNTHON BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com