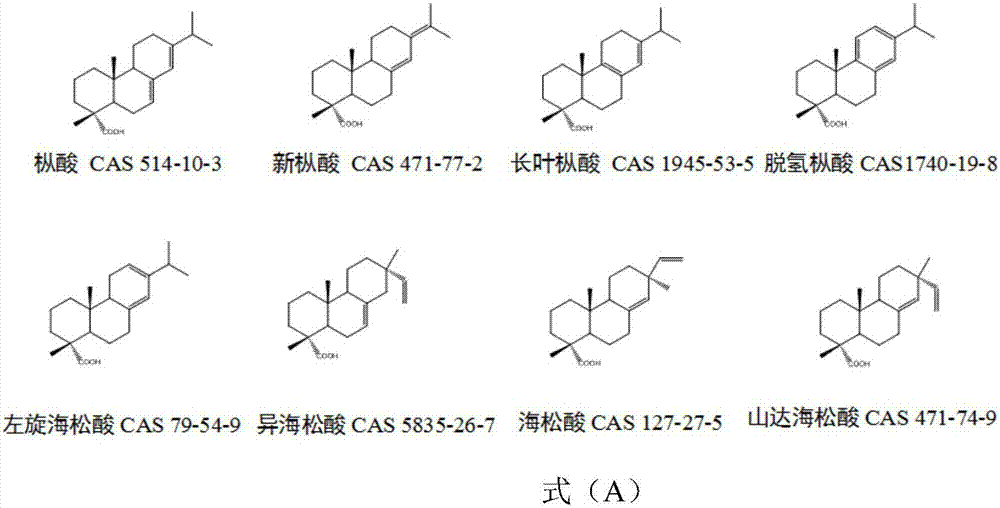
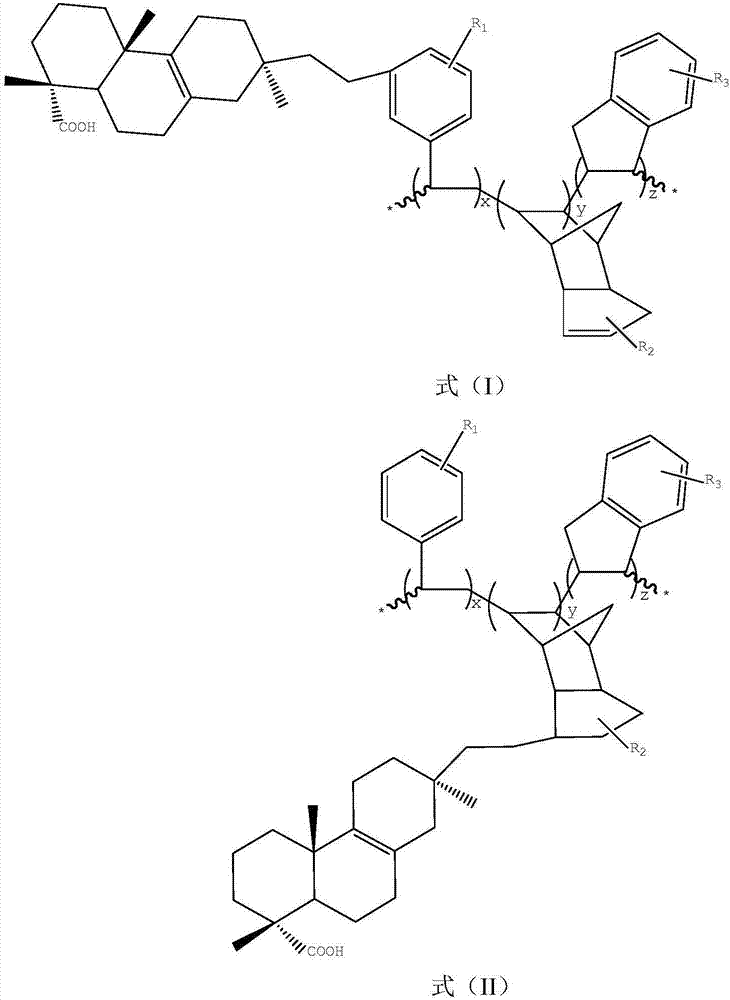
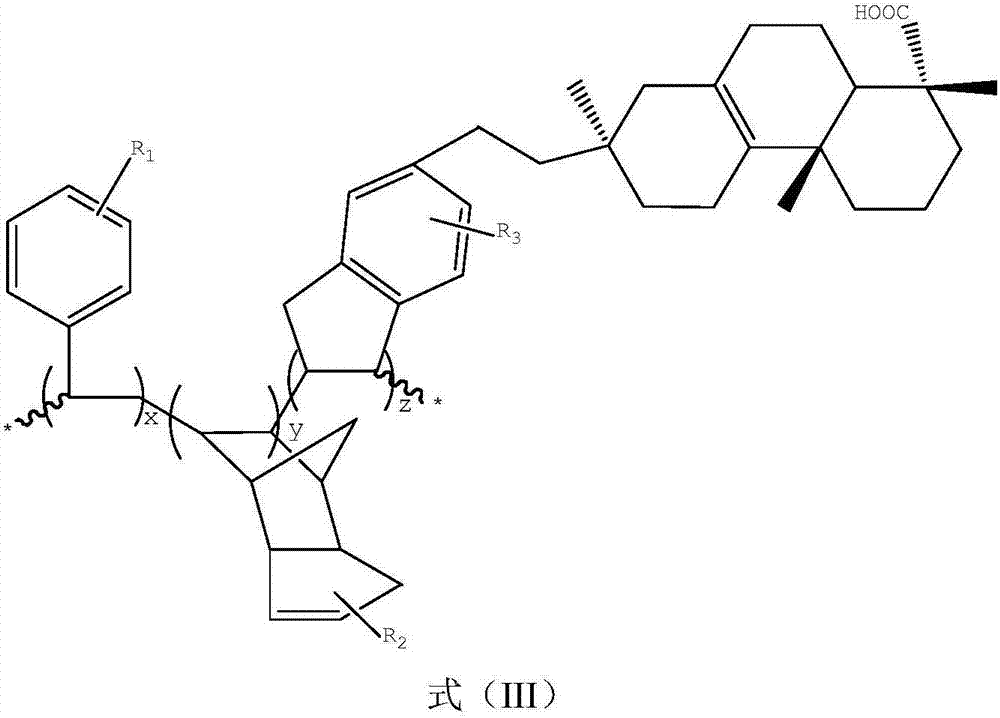
Patents
Literature
161results about How to "The reaction route is simple" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
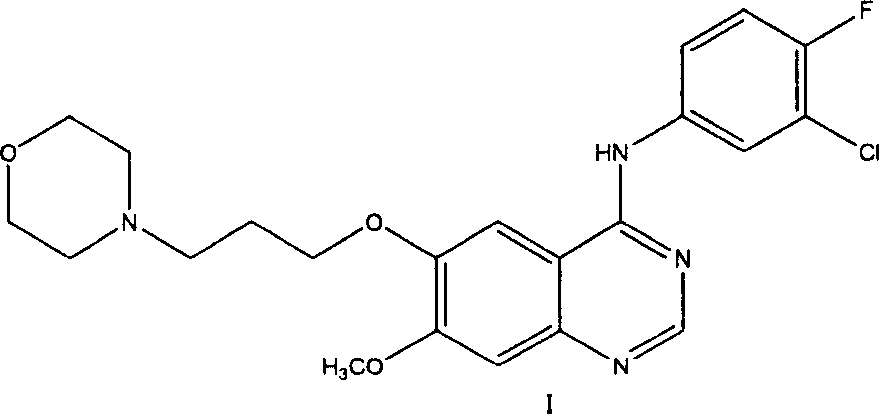
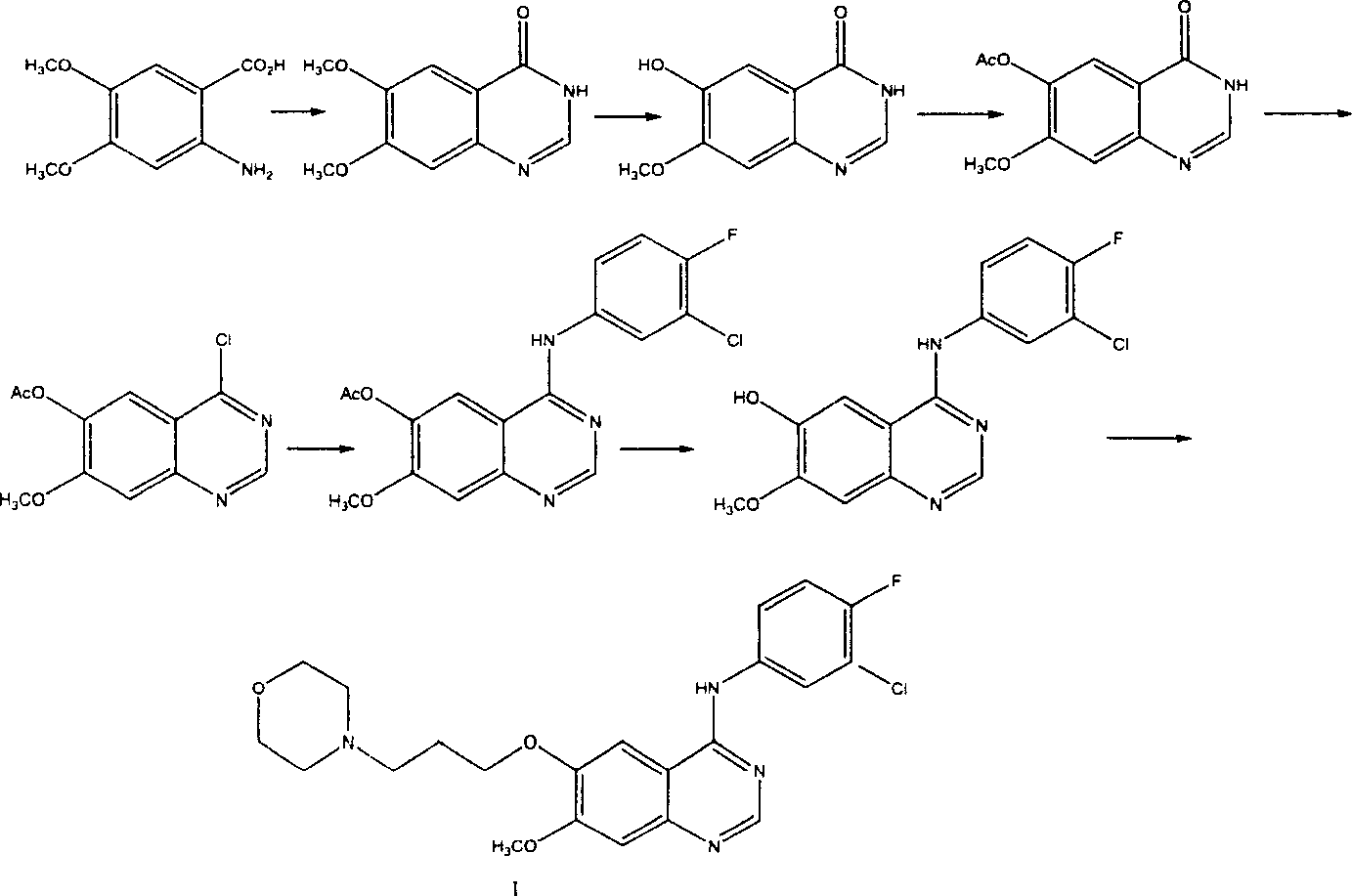
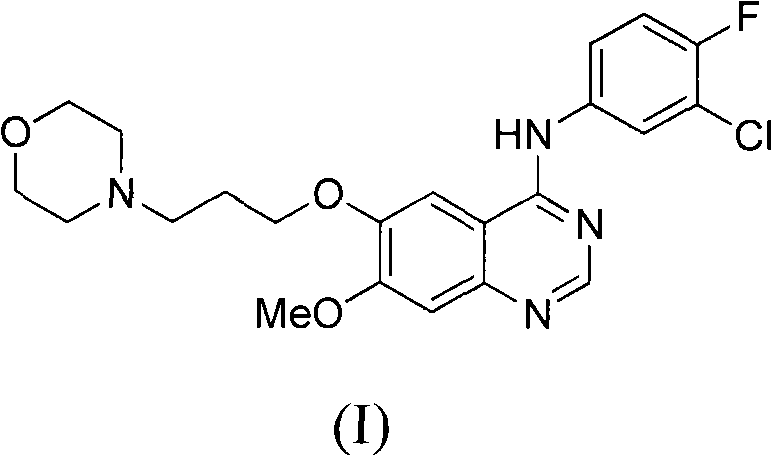
Preparation method of 4-(3-chlor-4-fluorobenzeneamidocyanogen)-7-methoxy-6-(3-morpholine oxypropyl)quinazoline
InactiveCN1733738AReduce pollutionReduce manufacturing costOrganic chemistryAntineoplastic agentsMorpholine3-chloro-4-fluoroaniline
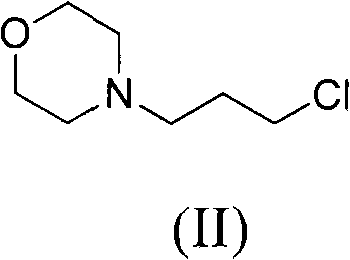
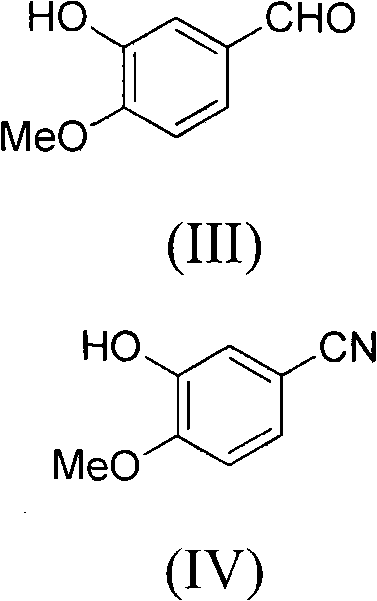
The invention relates to a preparation method of 4-(3-chlor-4-fluorobenzeneamidocyanogen)-7-methoxy-6-(3-morpholine oxypropyl)quinazoline, which comprises using 3,4-dimethoxybenzoic acid (II) as raw material, synthesizing 2-amido-4-methoxy-5-hydroxybenzoic acid (V), cyclizing to obtain 6-hydroxy-7-methoxy-3,4-dihydroquinazolin-4-one (VI), directly chloridizing to obtain 4-chloro-hydroxy-7-methoxy-quinazoline (VII), reacting directly with 3-chloro-4-fluoroaniline, carrying out amination to obtain 4-(3-chloro-4-fluoroanilino)-6-hydroxy-7-methoxy-quinazoline (VIII), finally reacting with morpholinyl chloropropane to obtain Geftinat (I).
Owner:江苏吴中苏药医药开发有限责任公司
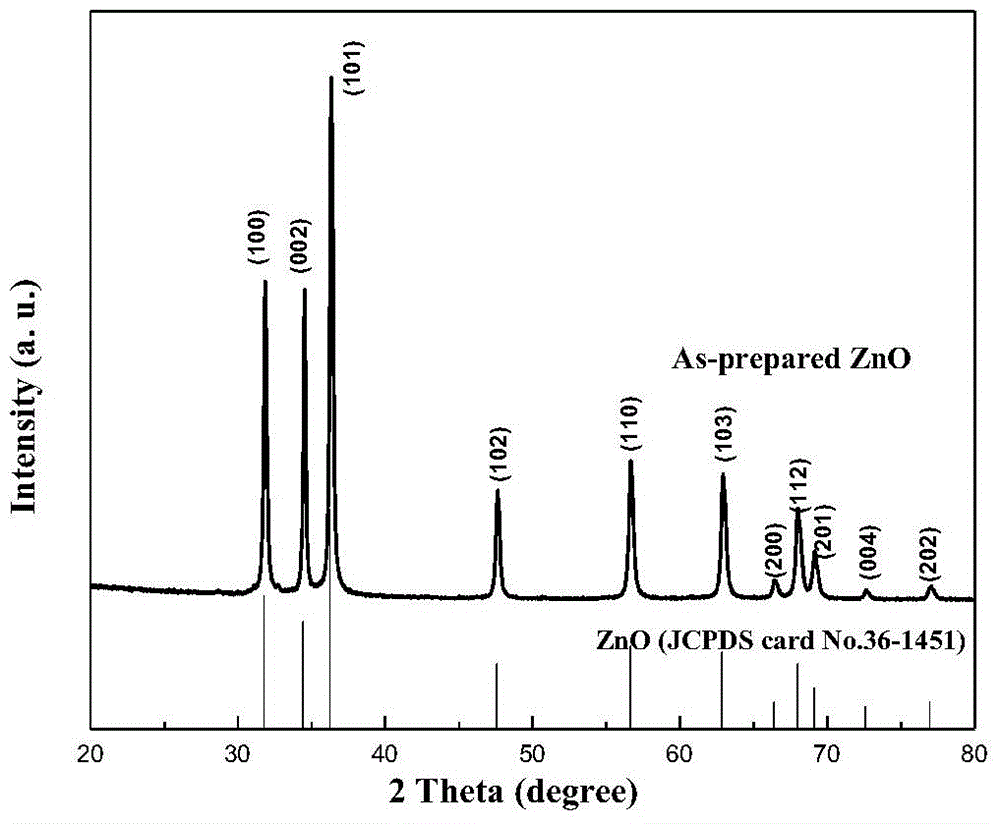
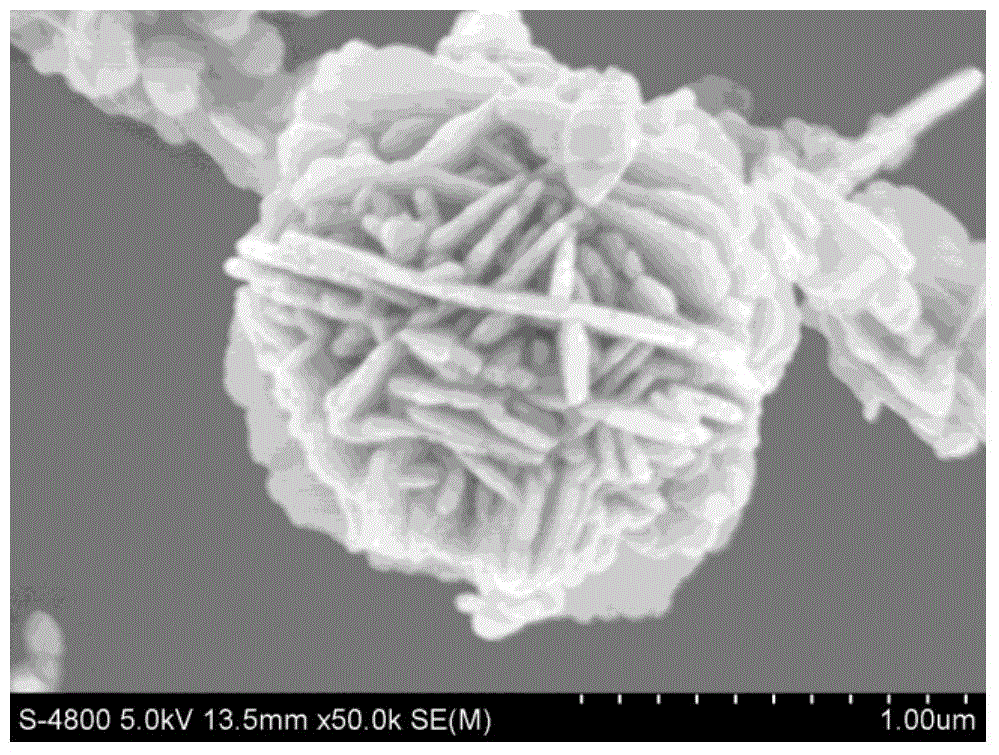
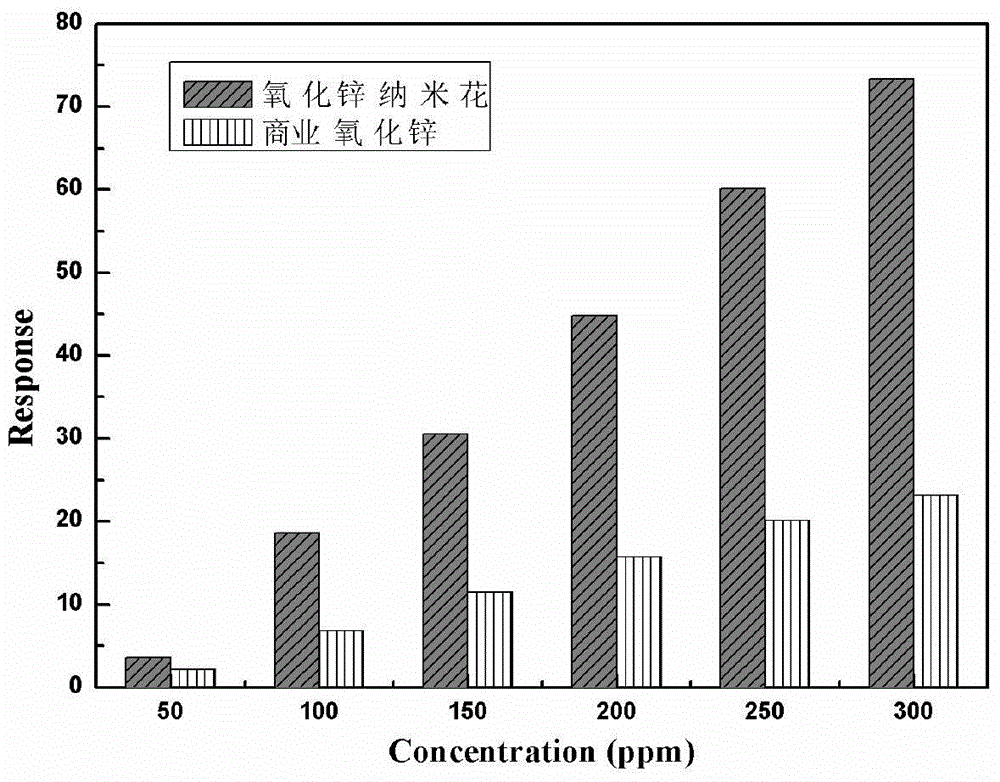
Preparation method of nanoflower-shaped zinc oxide
InactiveCN104445367AUniform particle sizeHigh crystallinityMaterial nanotechnologyZinc oxides/hydroxidesSaline waterAlcohol
The invention discloses a preparation method of nanoflower-shaped zinc oxide. The preparation method specifically comprises the following steps: preparing a zinc salt aqueous solution; dropwise adding an alkaline solution into the zinc salt aqueous solution till the zinc salt aqueous solution is strongly alkaline to form a reaction liquid; putting the reaction liquid into a liner of a hydrothermal reaction kettle; screwing the hydrothermal reaction kettle and putting the kettle into an oven to react; taking out the hydrothermal reaction kettle and cooling at room temperature, centrifugalizing the reaction liquid to obtain a white precipitate and respectively cleaning the white precipitate alternately by using absolute ethyl alcohol and deionized water; and putting the cleaned white precipitate into the oven to be dried at 60-100 DEG C to obtain ZnO powder in a nanoflower-shaped structure, wherein the pedal is a nanosheet which is about 30nm thick. The raw materials are easily available, and the preparation method has the characteristics of simple equipment process, high yield, low preparation cost and the like and is suitable for industrial production on a large scale.
Owner:HUAQIAO UNIVERSITY
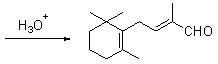
Preparation method of C-14 enol ether
InactiveCN102180774AThe reaction route is simpleRaw materials are easy to obtainEther preparationDimethyl methylphosphonateDiethyl methylphosphonate
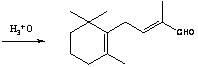
The invention relates to a preparation method of C-14 enol ether. The C-14 enol ether is an intermediate of vitamin A, and the chemical name of the C-14 enol ether is 1-methoxy-2-methyl-4-(2,6,6-trimehtyl-1-cyclohexene-1-ly)-1,3-butadiene. The preparation method comprises the step of carrying out Wittig-Horner condensation reaction on beta-irisone as shown in a formula 4 and diethyl methylphosphonate as shown in a formula 8 at the temperature of minus 40-30 DEG C in an ether solvent or dipolar aprotic solvent in the presence of alkali so as to prepare the C-14 enol ether as shown in a formula7. According to the preparation method, reaction route is simple, and raw materials are simple and available.
Owner:SHAOXING UNIVERSITY
Preparing method for organic silicon compound containing quaternary ammonium group
InactiveCN101016310AThe reaction route is simpleImprove bindingGroup 4/14 element organic compoundsOrganic compoundAntibacterial agent
The invention discloses a making method of organic silicon compound with little-surface tension quaternary ammonium group, which is characterized by the following: reacting (RO)2CH3Si(CH2)3X and secondary amine to produce organic silicon compound with tertiary amino; reacting with halogenated alkyl to do quaternised reaction; making the organic silicon compound with structure as (RO)2CH3Si(CH2)3N+R1R2R3X-; fitting for surface activator of organic silicon cation, sterilizer and antibacterial agent; lengthening sterilizing time.
Owner:QILU UNIV OF TECH
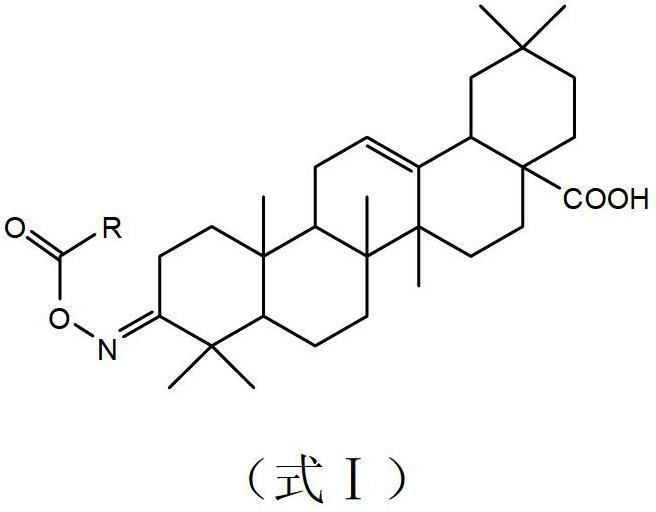
Oleanolic acid oxime ester derivate, preparation method and application thereof
The invention discloses an oleanolic acid oxime ester derivate compound, a preparation method and an application thereof. The structural formula of the compound is shown in formula I, and in the formula I, R refers to furyl, pyridyl, 1-menaphthyl, 4-chlorophenoxy methyl or phenyl with 1 to 2 substitutional groups; and the substitutional groups in phenyl with 1 to 2 substitutional groups are halogen (such as fluorine, chlorine, bromine and iodine) or nitryl. The preparation method comprises the following steps: 1) performing the esterification reaction between oleanolic oxime subject to benzylation protection and substituted carboxylic acids to prepare an oxime ester compound; and 2) removing benzylation protection on the oxime ester compound by Pd / C-H2 to prepare the compound in the formula I. The bactericidal activity test result shows that CAU2012-A has a favorable effect of inhibiting growth of sclerotinia scleotiorum, phytophthora melongenae, botrytis cintrea, pyricularia grisea, rhizoctonia solani and cotton fusarium wilt.
Owner:CHINA AGRI UNIV
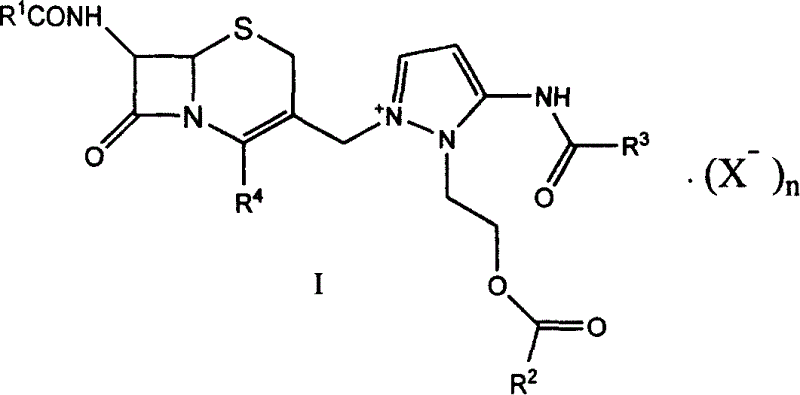
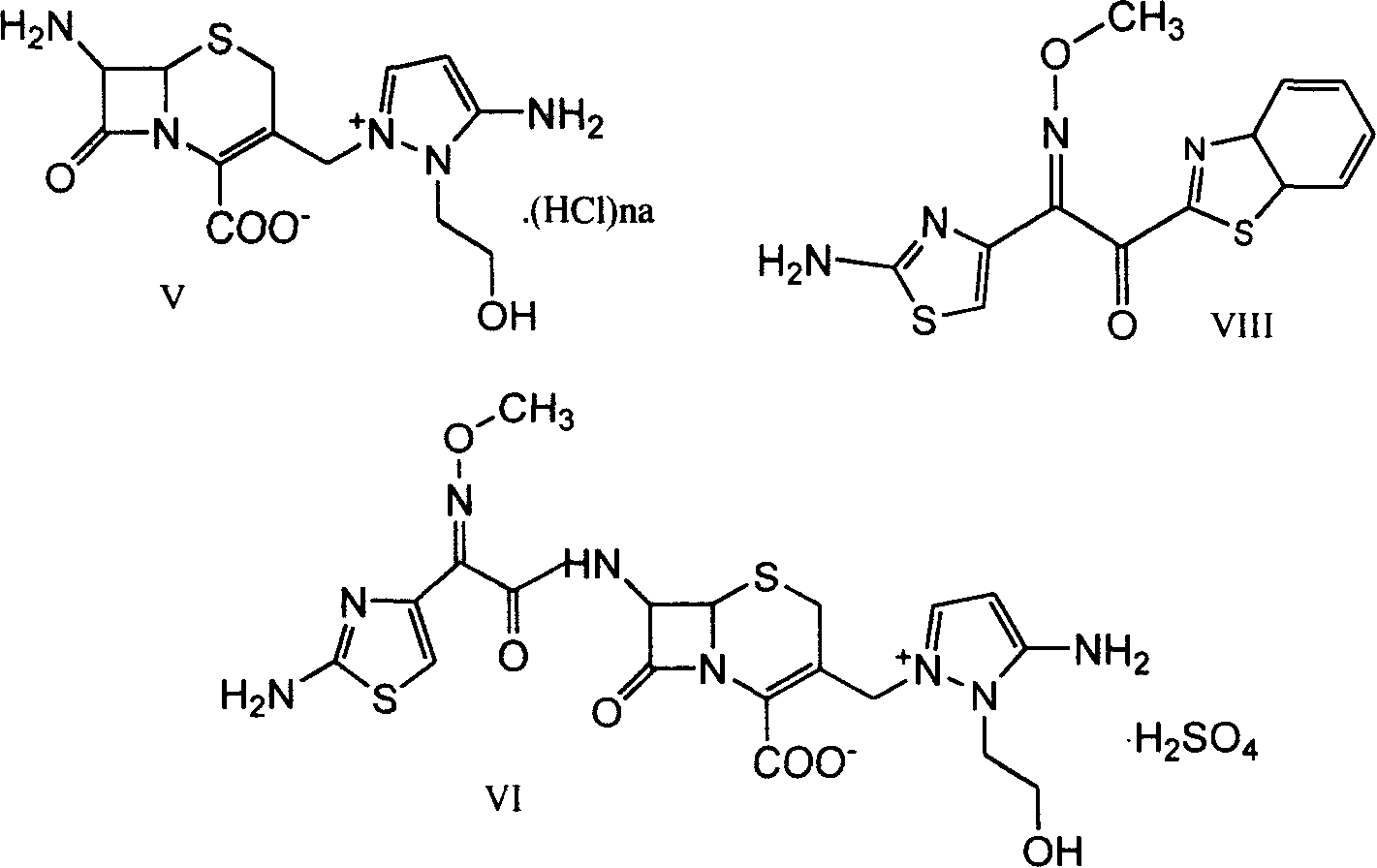
Cephaene onium salt compound and its preparation, and synthesis of cephapyrazde sulfate therefrom
This invention relates to cephalene onium salt compound and its preparation and synthesis of cephapyrazole sulfate therefrom. It is produced from 7beta-alkyl amido-3-[3-alkyl amido-2-(2-alkyl acyl oxethyl)-1-pyrazole onium group]methyl-3-cephalene- 4-carboxylic salt as intermediate by hydrolyzing it and reacting with alpha-(2-amino thiazole-4-radicle)-alpha-(Z)-methoxy imino acetic acid(benzo thiazole-2-radicle)hydrosulfate. The reacting condition is moderate, its raw materials can be obtained easily, and no expensive and strong irritant reagent is needed.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
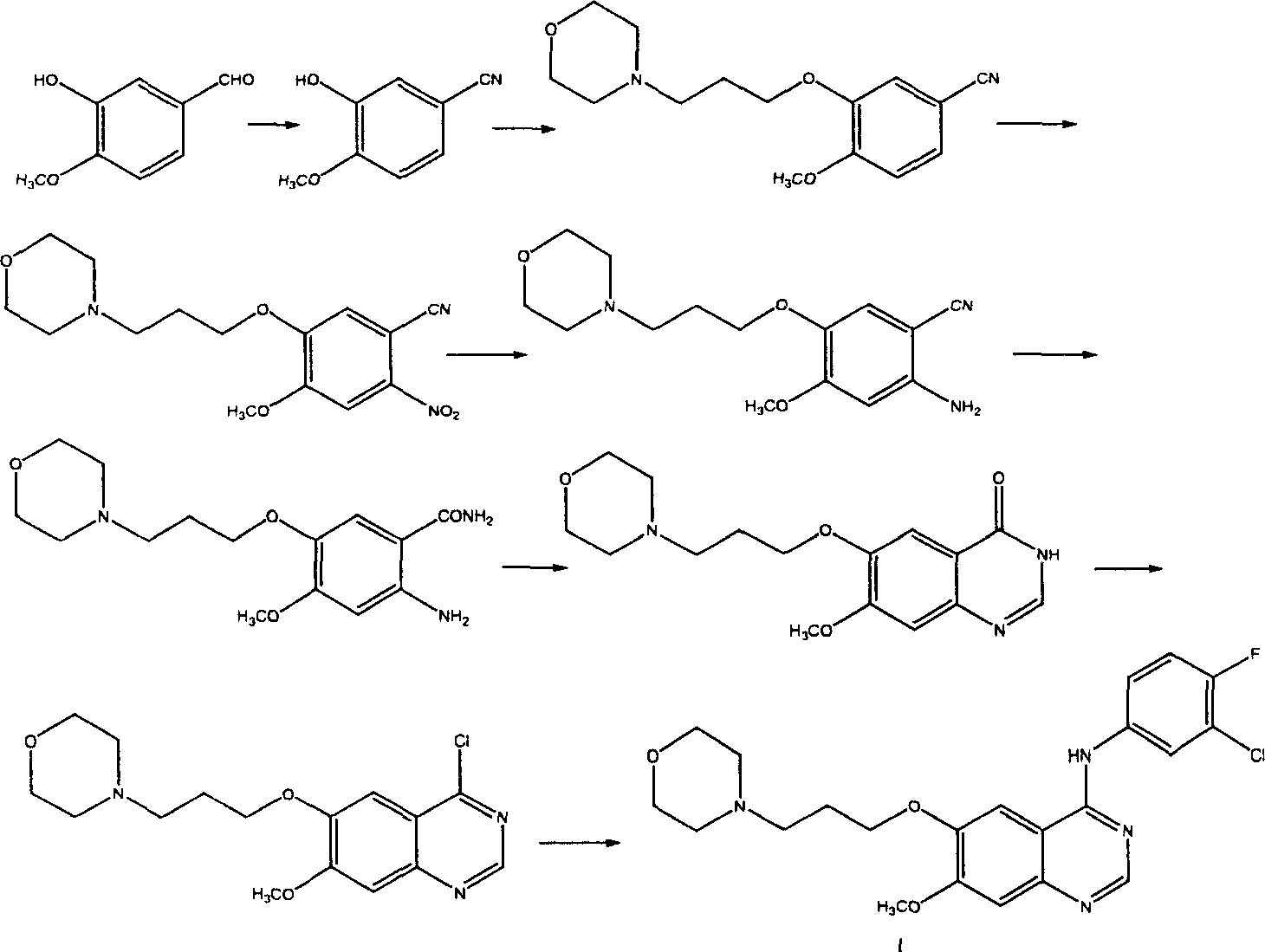
Novel method for preparing 4-(3-chlorine-4-fluorophenylamino)-7-methoxyl-6-(3-morpholinepropoxy)quinazoline
The invention discloses a novel method for preparing 4-(3-chlorine-4-fluorophenylamino)-7-methoxyl-6-(3-morpholinepropoxy)quinazoline (gefitinib, I). The method comprises the following steps of: preparing an intermediate (VI), performing functional group transformation twice under a hydrogenation condition to generate 2-(N,N-dimethylformylimido)-4-methoxyl-5-(3-morpholinepropoxy)cyanophenyl (VIII), and performing ring closure rearrangement on the obtained (VIII) and 3-chlorine-4-fluoroaniline to generate the gefitinib (I). The method is short in steps and high in yield in each step, the intermediate is convenient to purify, a target product has high purity, and the method is suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
Rosin modified C9 petroleum resin, preparation method and application thereof
ActiveCN106967201AIncreased branched structureHigh strengthSpecial tyresRolling resistance optimizationPetroleum resinTear resistance
The invention discloses a rosin modified C9 petroleum resin and a preparation method thereof. Rosin and C9 petroleum resin react by high temperature and high pressure polymerization to prepare the rosin modified C9 petroleum resin. The rosin modified C9 petroleum resin at least comprises the structure shown as formula (I), formula (II) and formula (III). The invention also puts forward application of the rosin modified C9 petroleum resin in rubber tyre treads. The rosin modified petroleum resin can improve the tear resistance of tyre treads, enhance the rigidity of C9 petroleum resin and the wet skid resistance of tyre treads, and has broad application prospects in the field of rubber preparation.
Owner:RACHEM CHINA CO LTD +1
Method for preparing 2-aminopyridine and alkyl derivative
A process for preparing 2-amino pyridine and its alkyl derivative from 2-cyanopyridine or its alkyl derivative includes incomplete hydrolysis and Hofmann degradation. Its advantage is high output rate (80%) and purity up to 99% or more.
Owner:ZHEJIANG UNIV
Method for preparing difluoroacetyl fluoride
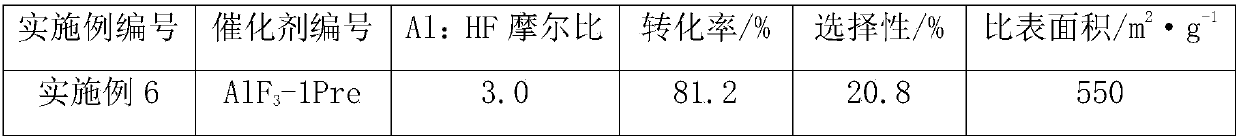
ActiveCN107867997AImprove stabilityHigh catalytic activityPhysical/chemical process catalystsCarboxylic acid halides preparationAluminum fluorideGas phase
The invention discloses a method for preparing difluoroacetyl fluoride from 1-C1-C8-alkoxy-1,1,2,2-tetrafluoroethane as a raw material by gas-phase catalytic cracking reaction in the presence of an aluminum fluoride catalyst. According to the disclosed preparation method, the reaction route is concise and efficient, fewer by-products are generated, catalytic activity is high, catalyst stability ishigh, and industrial production is facilitated.
Owner:SINOCHEM MODERN ENVIRONMENTAL CHEM INDAL XI ANCO +1
Synthesis method of high-grade intermediate R-1 of rosuvastatin calcium
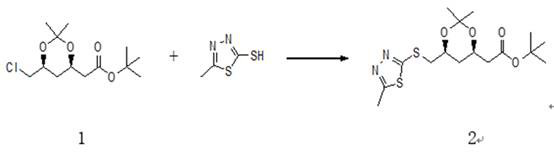
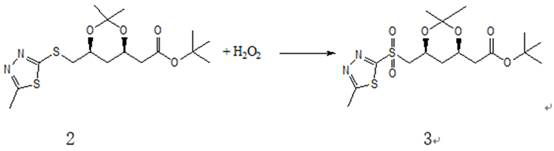
ActiveCN112028881ALow priceTo avoidOrganic chemistryBulk chemical productionRosuvastatin CalciumThiadiazoles
The invention relates to a synthesis method of a high-grade intermediate R-1 of rosuvastatin calcium. The synthesis method comprises the following steps: condensing a compound 1 serving as an initialraw material with 2-mercapto-5-methyl-1,3,4-thiadiazole to generate a compound 2, oxidizing the compound 2 with hydrogen peroxide to obtain a compound 3, and carrying out Julia-Kocienski Olefination condensation reaction on the compound 3 and a compound 4 to obtain the R-1, wherein the compound 1 is (4R-cis)-6-chloromethyl-2,2-dimethyl-1,3-dioxolane-4-acetic acid tert-butyl ester, and the compound4 is pyrimidine aldehyde. The synthesis method has the advantages of simple reaction route, mild reaction conditions, cheap raw materials, high reaction selectivity and almost no generation of cis-isomers; the method avoids the use of a phosphine salt in the route, so the generation of a large-polarity byproduct triphenylphosphine oxide in the product is avoided; the cost for synthesizing a rosuvastatin calcium bulk drug is greatly reduced; and no cis-rosuvastatin calcium impurity is formed in a subsequent reaction, so the influence of the impurity on the medicine effect of rosuvastatin calcium is avoided, and the method has great significance for reducing the medication burden of residents.
Owner:NENTER & CO
Pyridine-ethylbenzene-ether oxime esterPyrene ethylbenzene oxime-ethers esters compound, and preparation method and application thereof
InactiveCN105503712ASave raw materialsThe reaction route is simpleBiocideOrganic chemistryBenzaldehydeCarboxylic acid
The invention discloses a pyridine-ethylbenzene-ether oxime esterpyrene ethylbenzene oxime-ethers esters compound, and a preparation method and application thereof, belonging to the technical field of agricultural chemistry. The preparation method comprises the following steps of adopting p-hydroxy benzaldehyde as a raw material, and reacting with potassium carbonate in DMF (Dimethyl Formamide) to obtain an intermediate product II; reacting enabling the intermediate product II to react with sodium hydride to obtain sodium alkoxide and then reacting with 2-fluoropyridine to obtain an intermediate product III; reacting enabling the intermediate product III to react with pyridine and hydroxylamine hydrochloride in methyl alcohol to obtain an intermediate product IV; reacting enabling the intermediate product IV to react with various carboxylic acid, DCC and pyridine in dichloromethane to obtain the pyridine-ethylbenzene-ether oxime esterpyrene ethylbenzene oxime-ethers esters compound as shown by an end product I. The compound and a preparation thereof provided by the invention has have insecticide and mite ovicidal activity, favorable ovum killing activity, and higher pesticide research value.
Owner:CHINA AGRI UNIV
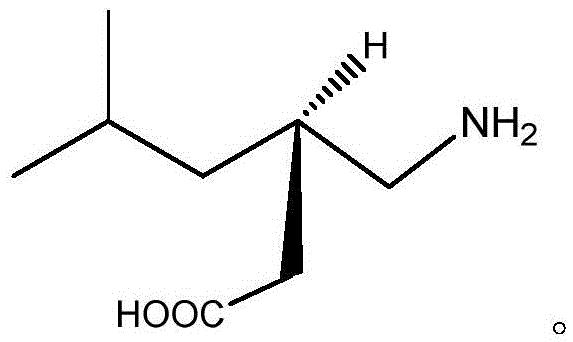
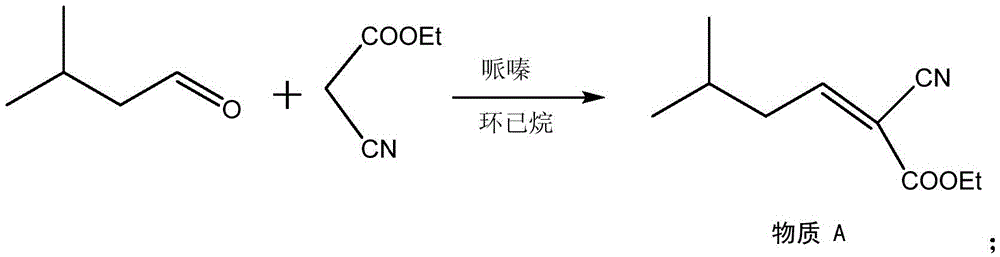
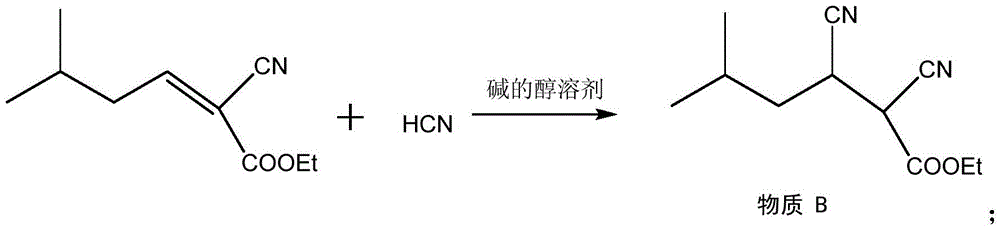
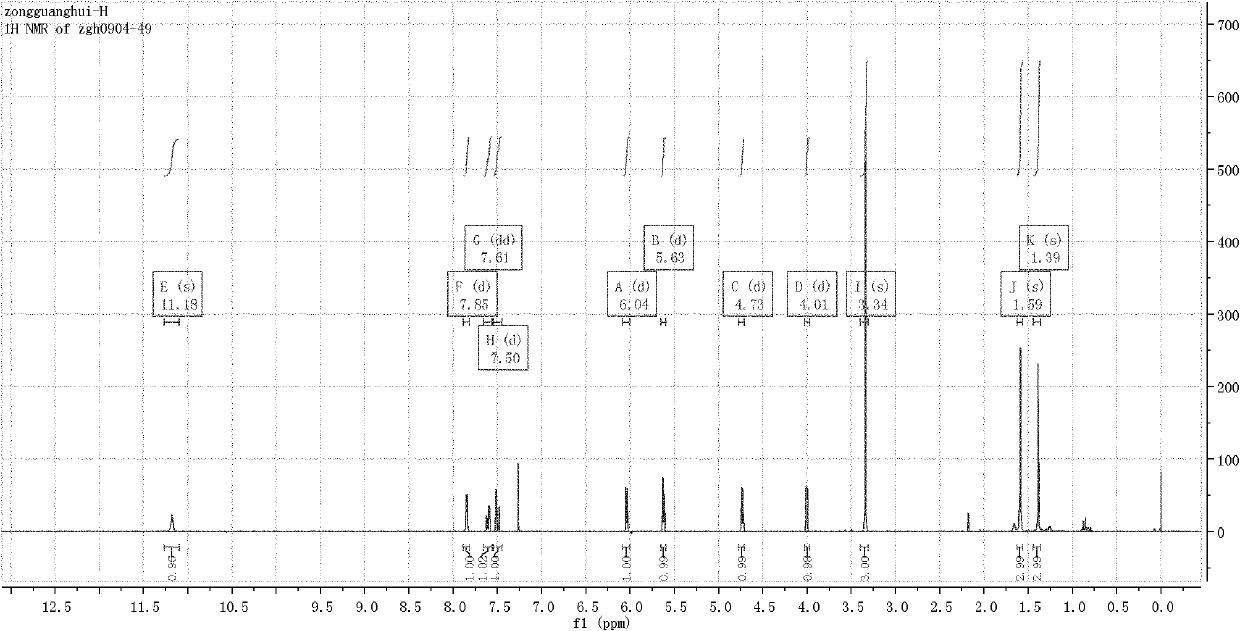
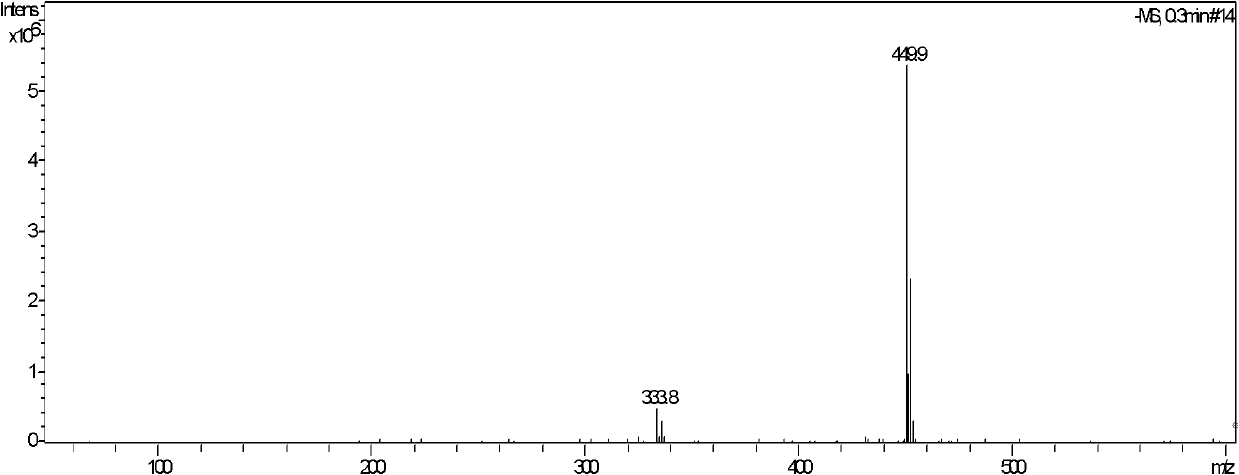
Method for synthesizing pregabalin with isobutyl butanedinitrile as intermediate
InactiveCN105463037AGuaranteed yieldGuaranteed purityOrganic compound preparationOrganic chemistry methodsCyanide hydrataseSolvent
The invention discloses a method for synthesizing pregabalin with isobutyl butanedinitrile as the intermediate. The method includes the steps of conducting the Knoevenagel condensation reaction on isovaleraldehyde and ethyl cyanoacetate in cyclohexane solvent with piperazine as the catalyst, conducting Michael addition on the product obtained in the first step and cyanic acid in alkaline alcohol solvent, conducting the decarboxylic reaction on the product obtained in the second step in isopropanol solvent under the heating condition to obtain the isobutyl butanedinitrile solvent, conducting hydrolysis on the intermediate under catalysis of cyanide hydratase AtNiTl, and conducting catalytic hydrogenation with raney nickel as the catalyst. According to the method, isoamyl aldehyde and ethyl cyanoacetate which are low in price and easy to obtain are used as raw materials, and the isobutyl butanedinitrile intermediate is obtained through the Knoevenagel condensation reaction, Michael addition and the decarboxylic reaction. The intermediate is then catalyzed, hydrolyzed and reduced through cyanide hydratase AtNiTl, and pregabalin is obtained. The reaction route is simple, the yield of each step of reaction is high, and therefore the final total recovery and purity of pregabalin are ensured.
Owner:TAICANG YUNTONG BIOCHEM ENG
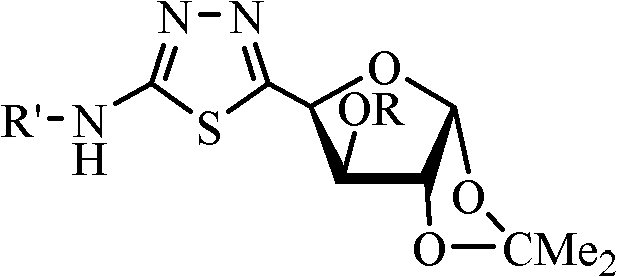
Furanosyl modified 1,3,4-thiadiazole derivative and preparation method thereof as well as application of derivative as bactericide
InactiveCN102153602ABroad spectrumSave raw materialsBiocideSugar derivativesStructural formulaThiadiazoles
The invention discloses a furanosyl modified 1,3,4-thiadiazole derivative and a preparation method thereof as well as application of the derivative as bactericide. The structural formula of the derivative CAU-2010-ZJ is as shown in a formula I. In the formula, R is acetyl, allyl, propargyl, methyl or ethyl; and R' is isopropyl, naphthaline, phenyl or phenyl having 1-2 substituted groups, wherein the substituted groups are halogen, methyl, methoxy, nitro, trifluoromethyl, or heptafluoro isopropyl. The preparation method comprises the following steps: carrying out a condensation reaction on a compound as shown in a formula II and N-substituted thiosemicarbazide so as to obtain thiosemicarbazone; and then carrying out a MnO2 ring-closing reaction so as to obtain a compound as shown in the formula I. The result of a bactericidal activity assay indicates that the compound CAU-2010-ZJ and a preparation thereof have good growth inhibition effect on sclerotium germs, eggplant early blight germs, gray mold germs, seedbed rhizoctonia solani germs, rice blast germs, asparagus stem blight germs, pythium germs, damping-off fungi, peanut black spot germs, white rot germs, tomato leaf mildew germs and watermelon anthrax. The formula I and formula II are as shown in the specification.
Owner:CHINA AGRI UNIV
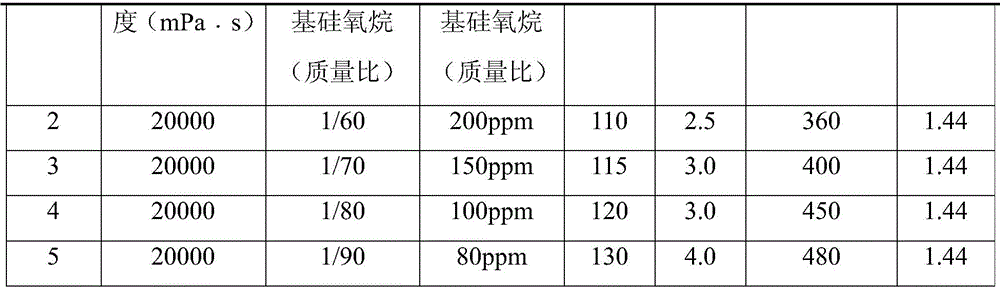
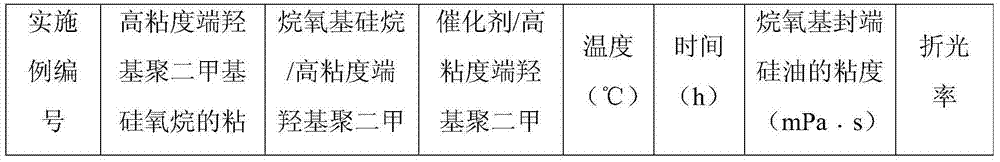
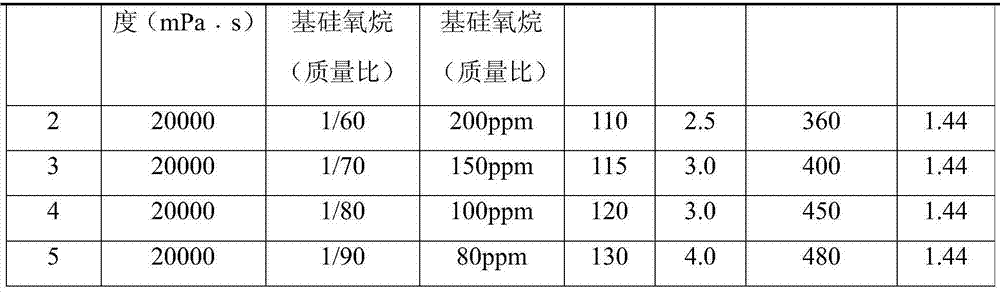
Preparation method of alkoxy end capping silicone oil
The invention belongs to the field of modified silicone oil, specifically relates to a preparation method of alkoxy end capping silicone oil, and aims at solving the technical problems of the existing preparation that the process is complex because of starting from chlorosilane monomer, hydrogen chloride produced by hydrolyzing is difficult to remove, and therefore, the storage stability of a product is poor. The preparation method of the alkoxy end capping silicone oil comprises the following steps: adding a catalyst to high-viscosity-end hydroxyl polydimethylsiloxane and alkoxy silane under the protection of nitrogen, heating to be 80 to 130 DEG C, and reacting for 1 to 6 hours; b, neutralizing to be neutral after the reaction is done; c, filtering to obtain the filtrate that is the crude alkoxy end capping silicone oil product; d, distilling the crude product under a temperature of 120 to 170 DEG C and at a pressure of -0.06 to -0.09Mpa to obtain the alkoxy end capping silicone oil. According to the preparation method of the alkoxy end capping silicone oil, the reaction line is simple, the alkoxy end capping silicone oil is high in stability and high in end capping rate, and a new method is provided to the preparation of the alkoxy end capping silicone oil.
Owner:江苏科幸新材料有限公司
compounds with novel 5-oxime ester B2a structures, preparation methods and applications of compounds
ActiveCN107266511ABroad insecticidal activity assayGood effectBiocideSugar derivativesAvermectinStructural formula
The invention provides a class of compounds with novel 5-oxime ester B2a structures, preparation methods of the compounds and applications that the compounds are used as insecticides, acaricides and nematicides. Structural formulae of the compounds are represented as a formula I, a formula II or a formula III. According to the invention, the structure of B2a, a byproduct of avermectin productive process, is optimized; a series of 5-oxime ester B2a compounds with novel structures is designed and synthesized; comprehensive insecticidal activity determination is performed on the compounds; and compounds with good pesticide effects are found. The reaction route is simple; the products have good insecticidal activity; the biological activity ratio of most compounds surpasses a control insecticide avermectin; and the biological activity increases obviously, and therefore, the compounds provided by the invention have very high pesticide researching value.
Owner:CHINA AGRI UNIV
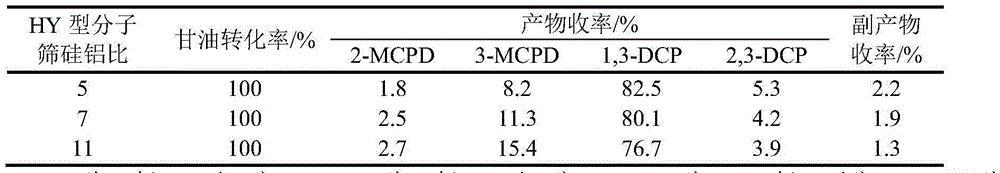
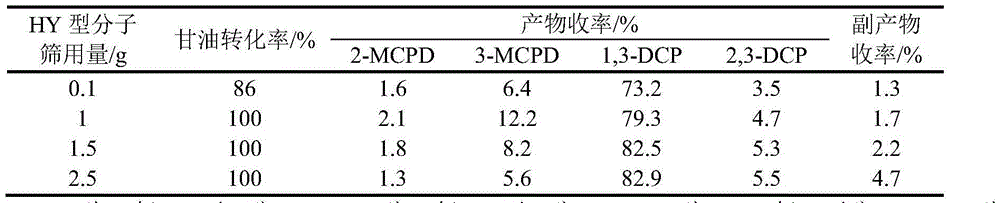
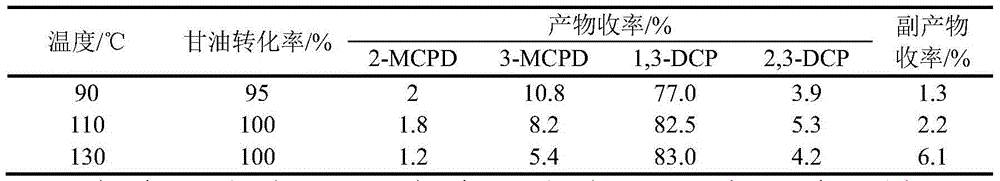
Method for preparing dichlorohydrin through glycerinum chlorination under catalysis of HY type molecular sieve
InactiveCN104151139ARaw materials are easy to getLow costPreparation by halogen introductionMolecular sieveBiodiesel
The invention provides a method for preparing dichlorohydrin through glycerinum chlorination under catalysis of an HY type molecular sieve. According to the method, dichlorohydrin is prepared by taking a biodiesel byproduct glycerinum as a raw material, a normal hydrogen chloride gas as a chlorinating agent and the HY molecular sieve of different silicon-aluminum ratios as chlorination catalysts by using a gas-liquid two-phase method. The method for preparing dichlorohydrin is low in raw material cost, relatively gentle in reaction condition and small in amount of byproducts, the catalyst HY molecular sieve is high in catalysis activity and good in selectivity, appropriate reaction conditions are selected, and the yield of dichlorohydrin is greater than 87%. In addition, after the catalyst is repeatedly used for 5 times, the yield of dichlorohydrin is still greater than 80%, and the circulation effect is relatively good. By adopting the method, a novel way is provided for industrial production of dichlorohydrin through glycerinum chlorination.
Owner:JIANGSU UNIV
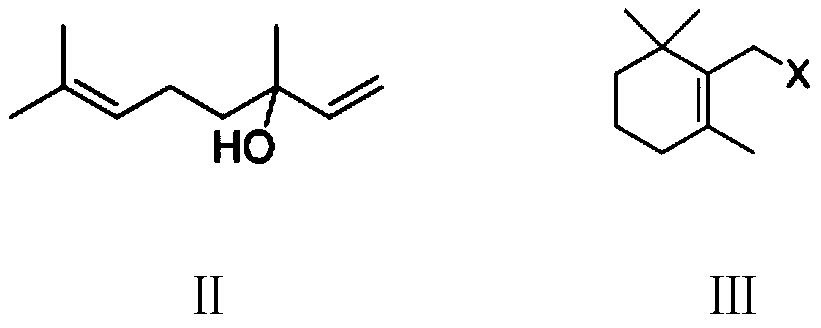
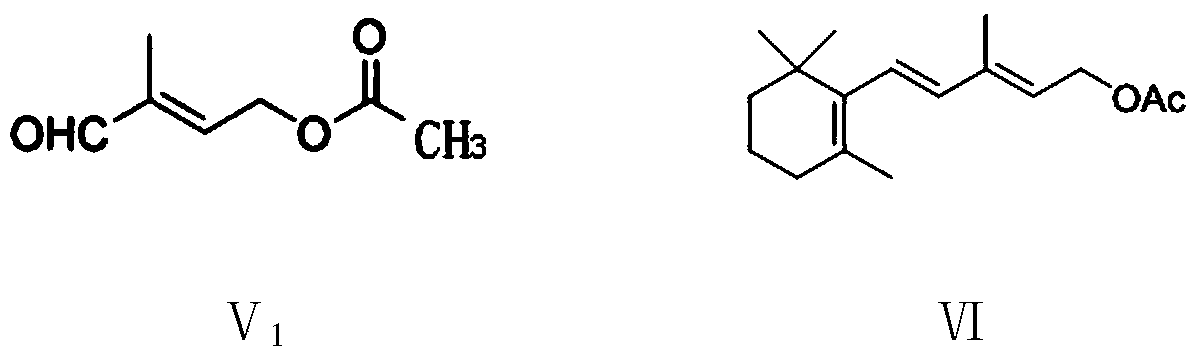
Preparation method of vitamin A ester intermediate C15 and vitamin A ester
ActiveCN111484525AHigh purityHigh yieldGroup 5/15 element organic compoundsHalogenated hydrocarbon preparationPhosphorous acidHalogenation
The invention provides a preparation method of a vitamin A ester intermediate C15 and vitamin A ester. The method comprises the following steps: carrying out a halogenation reaction and a cyclizationreaction on 3, 7-dimethyl-3-hydroxy-1, 6-octadiene as an initial raw material, carrying out a substitution reaction on the obtained product and triphenylphosphine or triester phosphite to prepare a corresponding Wittig reagent, carrying out a Wittig reaction on the Wittig reagent and 2-methyl-4-acetoxy-2-butenal, performing acidifying, hydrolyzing and acidifying the obtained product, and carryingout a substitution reaction on the hydrolyzed and acidified product and triphenylphosphine or triester phosphite to prepare C15. The vitamin A ester can be prepared by carrying out a Wittig reaction on the obtained C15 and 2-methyl-4-R3 substituent carbonyloxy-2-butenal. The method has the advantages of single reaction type, easy operation and realization of reaction conditions, safe and environment-friendly operation, simple post-treatment and low cost; and the reaction activity is strong, the reaction selectivity is high, the atom economy is high, and the target product yield and purity arehigh.
Owner:XINFA PHARMA
Phosphate compound containing 1,2,3-triazole ring as well as preparation method and application thereof
InactiveCN104311598ARaw materials are cheap and easy to getThe reaction route is simpleBiocidePlant growth regulatorsPhosphateGrowth plant
The invention discloses a phosphate compound containing a 1,2,3-triazole ring as well as a preparation method and an application thereof. The structural formula of the phosphate compound containing the 1,2,3-triazole ring is shown as a formula (I). According to the method for preparing the phosphate compound containing the 1,2,3-triazole ring disclosed by the invention, the raw materials are low in price and readily available, the reaction route is simple, and the phosphate compound containing the 1,2,3-triazole ring is high in herbicidal activity. The measurement result of the herbicidal activity plating method proves that the phosphate compound has strong effects of inhibiting growth of roots and stems of wheat and oilseed rapes. The pot experiment proves that the phosphate compound has a good effect of inhibiting growth of barnyard grass. The phosphate compound containing the 1,2,3-triazole ring has high plant growth regulation activity and high herbicidal activity during low concentration.
Owner:CHINA AGRI UNIV
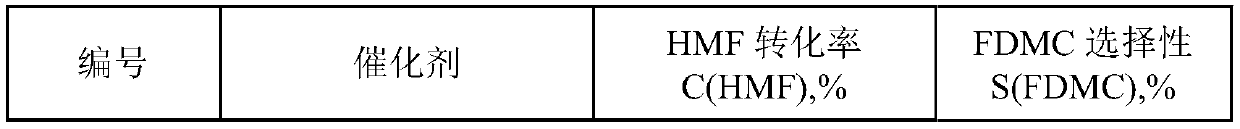
Method for preparing dimethyl furan-2,5-dicarboxylate by oxidizing and esterifying 5-hydroxymethyl furfural
The invention discloses a method for preparing dimethyl furan-2,5-dicarboxylate by oxidizing and esterifying 5-hydroxymethyl furfural. The method comprises the following steps: using air and / or oxygenas an oxidant and using methanol as a solvent and a reactant without adding alkali or bromine; using a gold-based catalyst for catalyzing oxidation and esterification of 5-hydroxymethylfurfural to synthesize dimethyl furan-2,5-dicarboxylate. The method provided by the invention has high selectivity for dimethyl furan-2,5-dicarboxylate, and can ensure that a conversion rate of 5-hydroxymethyl furfural is greater than 95% and the selectivity of dimethyl furan-2,5-dicarboxylate is greater than 99%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
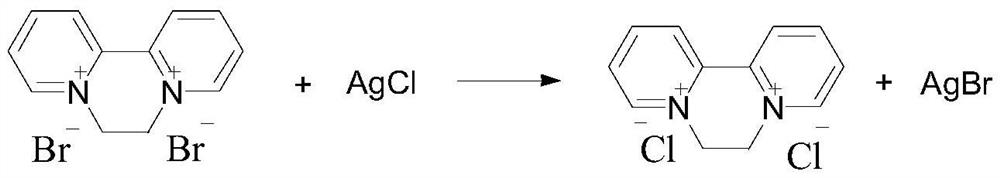
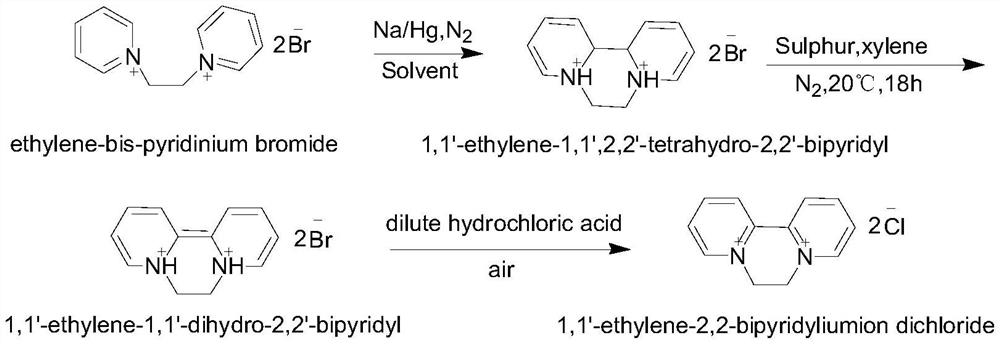
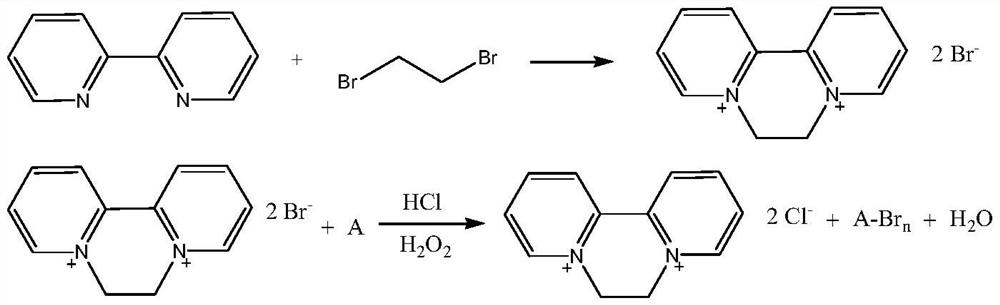
Preparation method of 1, 1 '-ethylene-2, 2'-dipyridyl dichloride
ActiveCN112500411AOvercoming long reaction routesSimple processOrganic chemistryDichloroethanePtru catalyst
The invention discloses a preparation method of 1, 1 '-ethylene 2, 2'-dipyridyl dichloride, which comprises the following steps: by using 2, 2 '-dipyridyl and dichloroethane as raw materials and a solvent A or a solvent B as a solvent, carrying out cyclization reaction at 120-300 DEG C to obtain 1, 1'-ethylene 2, 2 '-dipyridyl dichloride, wherein the solvent A is selected from dichloroethane; thesolvent B meets the following conditions: (1) the solvent B does not chemically react with reaction raw materials or reaction products; 2, reaction raw materials of dichloroethane and 2, 2 '-dipyridylcan be dissolved; compared with the prior art, the method has the advantages of simple process, short reaction route and low cost, and is suitable for industrial production. The catalyst is added into the reaction system, so that the reaction rate can be remarkably increased, and the raw material conversion rate and the target product selectivity are improved.
Owner:NANJING REDSUN BIOCHEM CO LTD
Method for synthesizing organic fluorine modified polysiloxane linen finishing agent
The invention relates to a method for synthesizing organic fluorine modified polysiloxane linen finishing agent, belonging to the technical field of linen finishing agent. Polymethyl hydrogen polysiloxane, methyl methacrylate and fluorinated olefin are used as raw materials which have wide sources and moderate price and ensure sufficient addition reaction, easy reaction product separation and purification and high yield of organic fluorine modified polysiloxane linen finishing agent. Being the organic fluorine modified product with a stable structure, the finishing agent has good biological aging resistance, dose not repel the animal body, has no harmful effect on the environment, is green and environmental friendly and ensures that the linen fabric has durable finishing effect, resists water, oil and foul. The method adopts the hydrosilylation reaction and the simple reaction route and ensures high conversion rate.
Owner:QIQIHAR UNIVERSITY
Method for synthesizing pregabalin
InactiveCN105085290AThe reaction route is simpleHigh yieldOrganic compound preparationAmino-carboxyl compound preparationSolventChiral resolution
The invention discloses a synthesis method of pregabalin. The synthesis method comprises the following steps: carrying out addition elimination reaction on isovaleraldehyde and diethyl malonate in a solvent composed of DMSO (dimethyl sulfoxide) and carbon tetrachloride by using vanadium tetrachloride as a catalyst; carrying out Michael addition on the product obtained in the first step in an alkaline alcohol solvent; carrying out hydrogenation reaction on the product obtained in the second step with hydrogen gas by using a carbon-supported Pd catalyst; carrying out hydrolysis reaction on the product obtained in the third step in hydrochloric acid; and carrying out chiral resolution on the product obtained in the fourth step in an alcohol-water mixed solvent by using L-malic acid as a resolving agent. The cheap and accessible isovaleraldehyde used as the raw material is subjected to addition elimination, Michael addition, hydrogenation reaction, hydrolysis and chiral resolution to obtain the pregabalin. The method has the advantages of simple reaction route and higher yield of each step, thereby ensuring the total yield and purity of the final pregabalin.
Owner:TAICANG YUNTONG BIOCHEM ENG
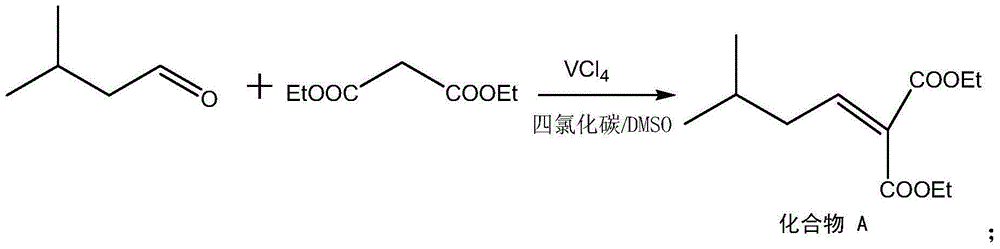
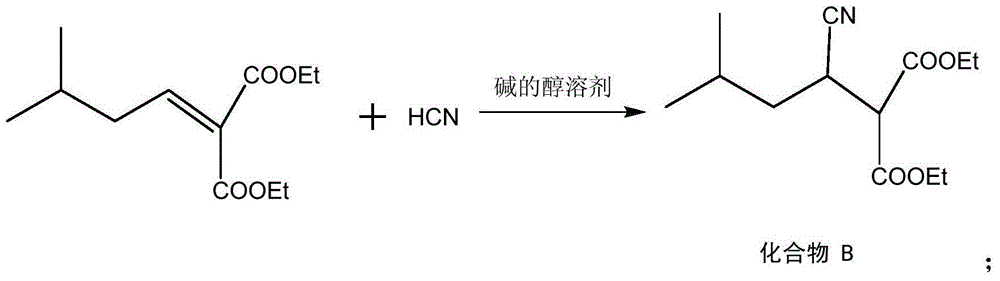
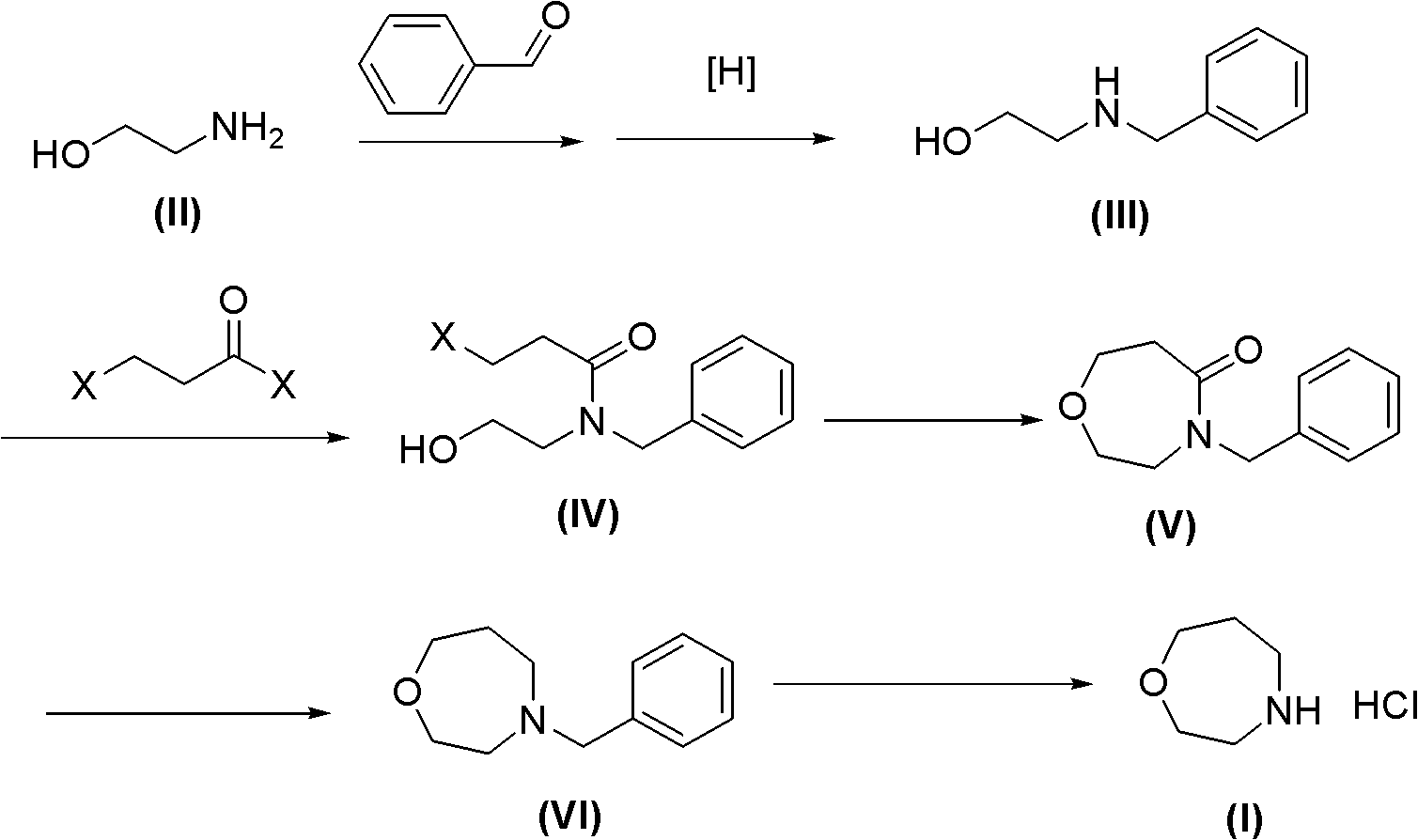
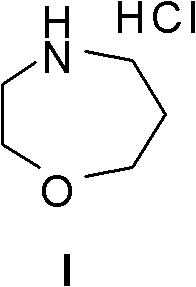
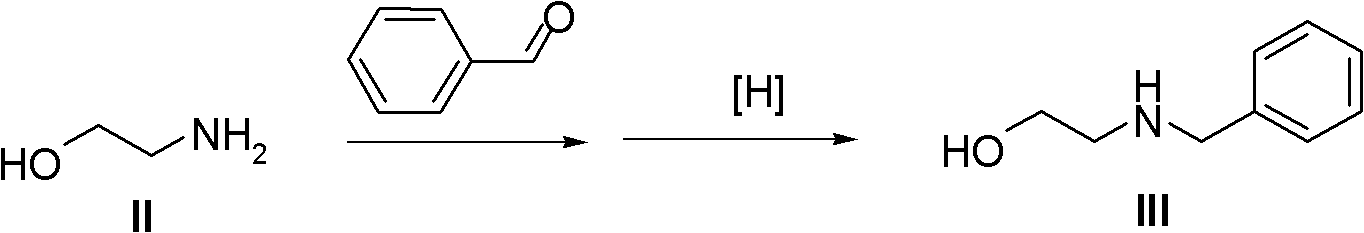
Method for preparing high morphine hydrochloride
InactiveCN102321045AEasy to manufactureHigh purityOrganic chemistryBenzaldehydeMorphine hydrochloride
The invention discloses a method for preparing high morphine hydrochloride I. The method comprises the following steps of: taking ethanol amine as a starting material and generating N-benzyl ethanol amine III through a condensation reaction with phenyl aldehyde; reacting the obtained compound III with chlorpromazine chloride or bromidepropionyl bromide to generate homologous N-halogenated propionyl group-N-benzyl ethanol amine IV; generating a seven-membered ring intermediate body V through performing a ring closing reaction on the obtained compound IV; converting the obtained compound V under a reducing condition to a compound VI; and generating a target compound I from the obtained compound VI under a oxidation-reduction condition. The method provided by the invention has the advantagesof high overall yield, convenience for purifying the intermediate body, high purity of the target product and suitability for industrialized production and the like.
Owner:陕西瑞科新材料股份有限公司
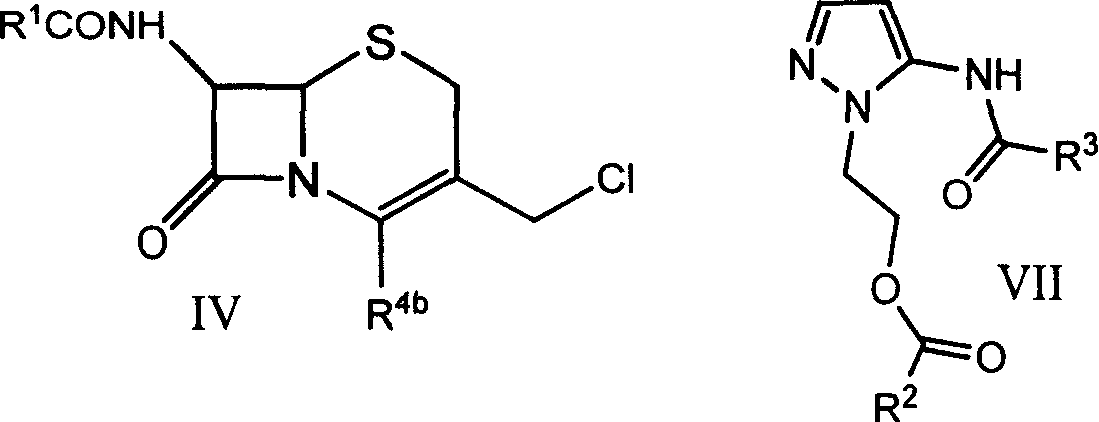
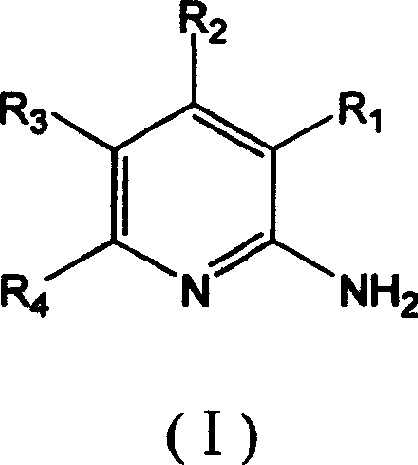
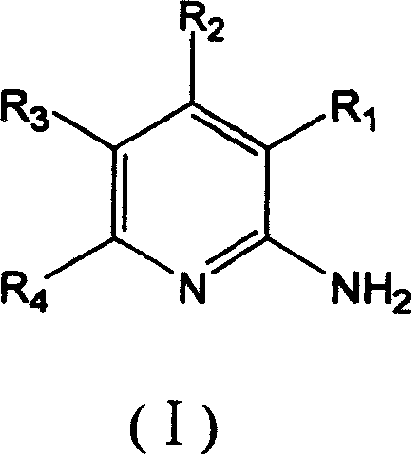
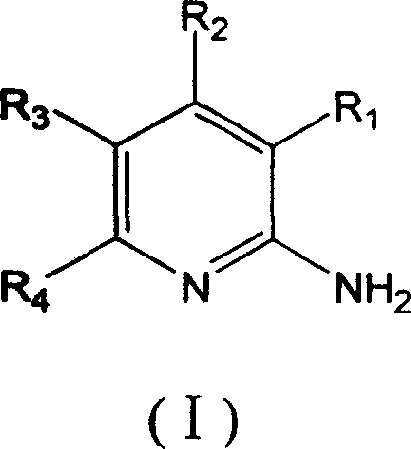
7-oxo-diazabicyclo [3, 2, 1] octane derivative compound, preparation method thereof and application
ActiveCN107501265ASimple processThe reaction route is simpleAntibacterial agentsOrganic active ingredientsEscherichia coliPhosphorylation
The invention relates to a 7-oxo-diazabicyclo [3, 2, 1] octane derivative compound, a preparation method thereof and an application, and belongs to the technical field of drug synthesis, and aims to solve the problem of how to obtain high-activity antibacterial drugs. The method includes the steps: performing condensation reaction of a compound in a formula IV and RH to obtain an intermediate product compound in a formula V in the presence of acid-binding agents; removing benzyl by hydrogenation deprotection treatment and then performing sulfonation or phosphorylation reaction to obtain the 7-oxo-diazabicyclo [3, 2, 1] octane derivative compound in a formula I. The RH is selected from substituted or unsubstituted 1H-1, 2, 3-triazole or 1H-1, 2, 4-triazole compound, and compounds in the formula VI or VII are in one-to-one correspondence to R2, R3 and R4 in the compound in the formula I. The derivative compound has good antibacterial activity and particularly has good inhibiting effects on pseudomonas aeruginosa and Escherichia coli, the method is simple in operation, and conditions are mild.
Owner:上海海宸医药科技有限公司
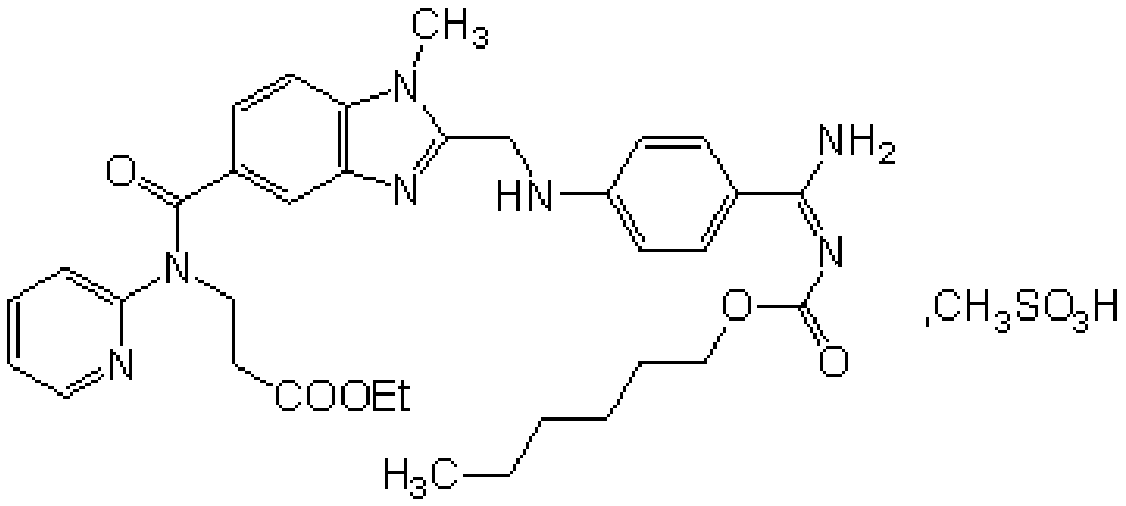
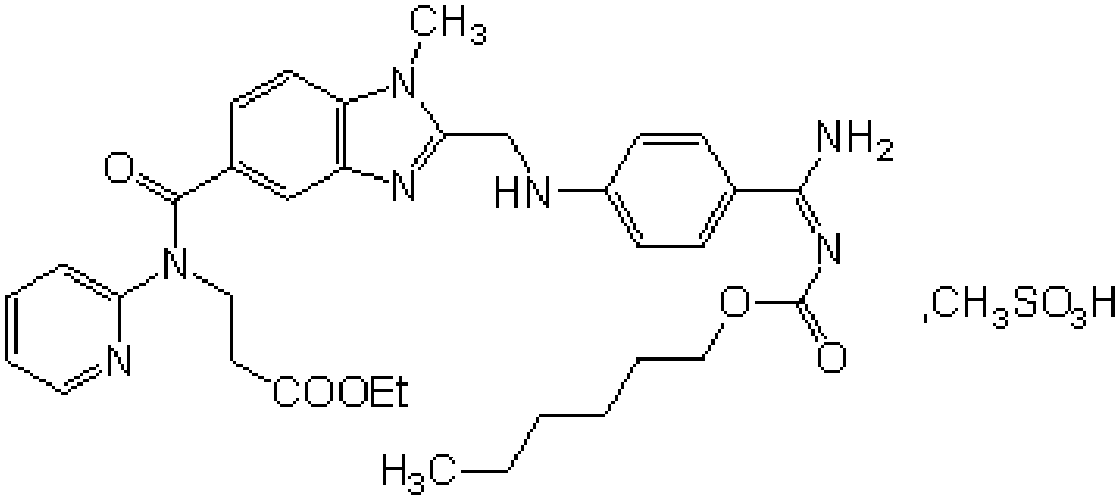
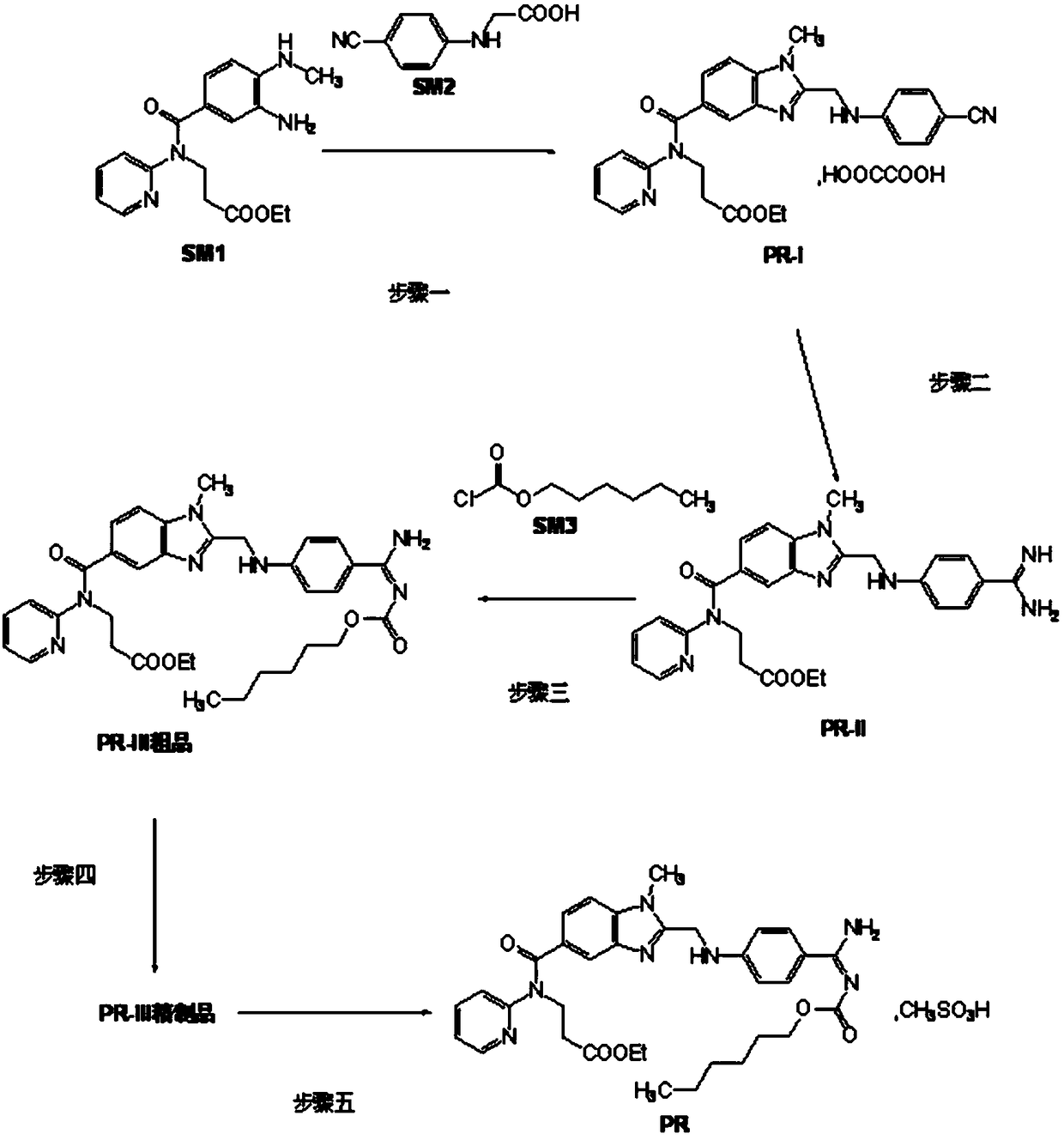
Production process of pradaxa mesylate
The invention discloses a production process of pradaxa mesylate. The production process comprises the following steps: (1) preparing an intermediate PR-I; (2) preparing an intermediate PR-II; (3) preparing pradaxa PR-III; (4) refining the pradaxa PR-III; and (5) preparing pradaxa mesylate. The production process is mild in reaction condition, simple in reaction route, convenient in operation, high in selectivity, and capable of shortening the production period; and the obtained pradaxa intermediate is low in water content, the prepared pradaxa mesylate is high in yield and purity, and the maximum impurity is low in impurity content; and the production process is less in emission of three wastes, environmentally friendly, free from requiring the columnar chromatography purification, suitable for the industrialized production, capable of avoiding the requirement of palladium-on-carbon high-pressure hydrogenation on equipment and capable of reducing the risk.
Owner:JIANGXI GUOYAO PHARMA LLC
Preparation method of 2-piperidinecarboxylic acid, 3-piperidinecarboxylic acid and 4-piperidinecarboxylic acid
InactiveCN102174011AThe reaction route is simpleLess side effectsOrganic chemistryHydrogenPipecolic acid
The invention discloses a preparation method of 2-piperidinecarboxylic acid, 3-piperidinecarboxylic acid and 4-piperidinecarboxylic acid. The method is as follows: 2-pyridinecarboxylic acid, 3-pyridinecarboxylic acid and 4-pyridinecarboxylic acid are used as the raw material respectively, hydrogen is used to reduce the raw material to the corresponding piperidinecarboxylic acid in the presence of palladium-carbon catalyst. The method has the advantages of simple route, less side reactions, low hydrogenation pressure and hydrogenation temperature, short hydrogenation time and simple treatment of solid wastes, and is easy to realize industrialization.
Owner:CHANGZHOU DAOU CHEM IND
Synthesis process for DL-p-methyl sulfone benzene ethyl serinate water-carrying esterification
ActiveCN108033903AThe reaction route is simpleHigh yieldOrganic chemistryOrganic compound preparationVeterinary DrugsEthyl fumarate
The invention discloses a synthesis process for DL-p-methyl sulfone benzene ethyl serinate water-carrying esterification. The synthesis process comprises the following steps: (1) putting a copper saltand absolute ethyl alcohol into a reactor, dropping sulfuric acid to carry out an esterification reaction, and after the reaction is completed, filtering so as to obtain esterification filtrate; (2)heating the esterification filtrate obtained in the step (1), concentrating, performing water-carrying esterification with the absolute ethyl alcohol, and after the water-carrying esterification is completed, performing aftertreatment, thereby obtaining DL-p-methyl sulfone benzene ethyl serinate. The synthesis process is simple in reaction route, high in product yield, good in synthesis product stability and high in purity, and can be adopted to synthesize a key intermediate of a veterinary drug florfenicol and an antibacterial raw material medicine thiamphenicol.
Owner:SHANDONG HANXING PHARM TECH CO LTD +1
Synthesis method of picolinafen
InactiveCN107382847AThe reaction route is simpleReaction conditions are easy to reachOrganic chemistryTrifluoromethylPhenols
The invention provides a synthesis method of picolinafen. The synthesis method comprises the following steps: reacting 2-chloro-6-trichloromethyl pyridine (formula II) with 3-trifluoromethyl phenol (formula III) to generate 2-(3-trifluoromethylphenoxyl)-6-trichloromethyl pyridine (formula IV); converting 2-(3-trifluoromethylphenoxyl)-6-trichloromethyl pyridine (formula IV) into 2-(3-trifluoromethylphenoxyl)-6-pyridine formyl chloride (formula V); and reacting 2-(3-trifluoromethylphenoxyl)-6-pyridine formyl chloride (formula V) with 4-fluoroaniline to generate picolinafen (formula I). By synthesizing a key intermediate namely 2-(3-trifluoromethylphenoxyl)-6-trichloromethyl pyridine (formula IV), the reaction route is well simplified, moreover, the reaction conditions can be reached easily, the product yield is high, and the synthesis method has a good value for scientific research and can be used in industry.
Owner:南通嘉禾化工有限公司 +1
Preparation method of alkoxy-terminated silicone oil
The invention belongs to the field of modified silicone oil, specifically relates to a preparation method of alkoxy end capping silicone oil, and aims at solving the technical problems of the existing preparation that the process is complex because of starting from chlorosilane monomer, hydrogen chloride produced by hydrolyzing is difficult to remove, and therefore, the storage stability of a product is poor. The preparation method of the alkoxy end capping silicone oil comprises the following steps: adding a catalyst to high-viscosity-end hydroxyl polydimethylsiloxane and alkoxy silane under the protection of nitrogen, heating to be 80 to 130 DEG C, and reacting for 1 to 6 hours; b, neutralizing to be neutral after the reaction is done; c, filtering to obtain the filtrate that is the crude alkoxy end capping silicone oil product; d, distilling the crude product under a temperature of 120 to 170 DEG C and at a pressure of -0.06 to -0.09Mpa to obtain the alkoxy end capping silicone oil. According to the preparation method of the alkoxy end capping silicone oil, the reaction line is simple, the alkoxy end capping silicone oil is high in stability and high in end capping rate, and a new method is provided to the preparation of the alkoxy end capping silicone oil.
Owner:江苏科幸新材料有限公司
Popular searches
Improve protection Reduced response Suitable for industrialized mass production Raw materials are abundant and easy to obtain Suitable for large-scale industrial production Strong sterilization Improve antibacterial properties Improve performance Strong growth inhibitory effect Simple waste treatment
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
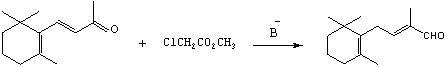
![7-oxo-diazabicyclo [3, 2, 1] octane derivative compound, preparation method thereof and application 7-oxo-diazabicyclo [3, 2, 1] octane derivative compound, preparation method thereof and application](https://images-eureka.patsnap.com/patent_img/c2775399-90fb-4365-9158-ff420751b53b/BDA0001428376400000011.png)
![7-oxo-diazabicyclo [3, 2, 1] octane derivative compound, preparation method thereof and application 7-oxo-diazabicyclo [3, 2, 1] octane derivative compound, preparation method thereof and application](https://images-eureka.patsnap.com/patent_img/c2775399-90fb-4365-9158-ff420751b53b/BDA0001428376400000022.png)
![7-oxo-diazabicyclo [3, 2, 1] octane derivative compound, preparation method thereof and application 7-oxo-diazabicyclo [3, 2, 1] octane derivative compound, preparation method thereof and application](https://images-eureka.patsnap.com/patent_img/c2775399-90fb-4365-9158-ff420751b53b/BDA0001428376400000031.png)