Synthesis method of high-grade intermediate R-1 of rosuvastatin calcium
A technology for rosuvastatin calcium and a synthesis method, which is applied in the field of fine chemical synthesis, can solve the problems of Z-configuration compound residues, drug efficacy effects, etc., and achieves the effects of simple reaction route, reduced cost, and reduced drug burden on residents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
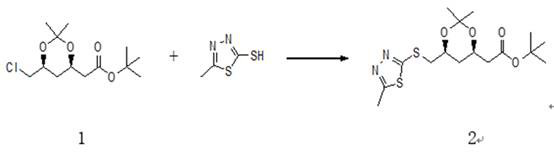
[0049] Add 70 grams of NMP, 35 grams of compound 1 and 23.8 grams of 2-mercapto-5-methyl-1,3,4-thiadiazole into a 500ml three-necked reaction flask, then add 3.5 grams of benzyltriethylammonium chloride and 21.2 grams of sodium carbonate, three times of nitrogen vacuum displacement, then the temperature was raised in an oil bath under nitrogen protection, the temperature was controlled at 115±5°C, and the reaction was completed in about 20 hours (HPLC analysis + spot plate analysis). After taking a sample to confirm that the reaction is complete, the reaction solution is cooled to room temperature. After cooling, 140 grams of water and 140 grams of ethyl acetate are added to the reaction solution to extract the product. After layering, 140 grams of ethyl acetate is added to the water phase to re-extract the water phase. Once, combine the organic phases and add 140 grams of water to wash once, concentrate at normal pressure to recover ethyl acetate, after the concent...
Embodiment 2
[0051] Add 700gDMSO, 350g compound 1 and 238g 2-mercapto-5-methyl-1,3,4-thiadiazole to a 5000ml three-neck reaction flask, then add 35g tetrabutylammonium bromide and 212g sodium carbonate , Nitrogen vacuum replacement three times, the temperature was raised in an oil bath under nitrogen protection, the temperature was controlled at 115±5°C, and the reaction was completed in about 20 hours (HPLC analysis + spot plate analysis). After taking a sample to confirm that the reaction is complete, the reaction solution is cooled to room temperature. After cooling, 1400 grams of water and 1400 grams of ethyl acetate are added to the reaction solution to extract the product. After layering, 1400 grams of ethyl acetate is added to the water phase to re-extract the water phase. Once, combine the organic phases and add 1400 grams of water to wash once, concentrate and recover ethyl acetate under normal pressure, weigh a total of 587 grams after the concentration is completed, the content i...
Embodiment 3
[0053]
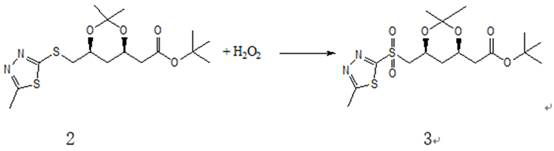
[0054] Add 300 grams of acetonitrile to the concentrated solution of 61.2 grams of compound 2 to dissolve, transfer the solution to a 1000 ml three-necked flask after dissolving, add 10 grams of phosphomolybdic acid, and add 300 grams of 30% hydrogen peroxide dropwise to the reaction flask. During the process, the temperature is controlled at 30-40°C. After the drop is completed, it is stirred at room temperature until the reaction is complete (the raw materials + intermediates are all converted into products), and the HPLC analysis is completed in about 20 hours. After the reaction is completed, add 300 grams of 10% sodium bisulfite aqueous solution dropwise to the reaction liquid to quench the hydrogen peroxide. After the dropwise addition, stir and detect that there is no hydrogen peroxide, start to recover acetonitrile under normal pressure, concentrate until the internal temperature reaches 100°C, stop heating, and cool down to room temperature. Finally, add 60...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com