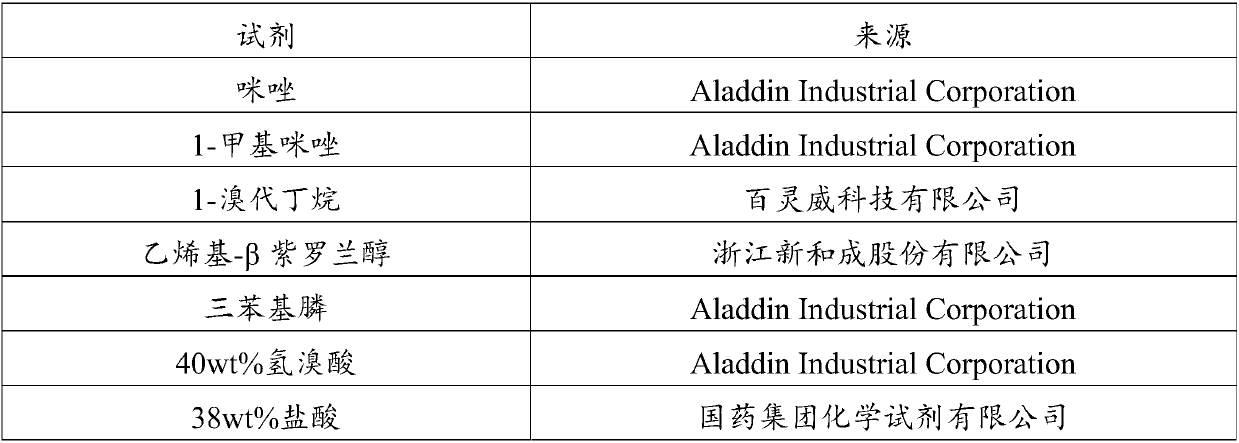
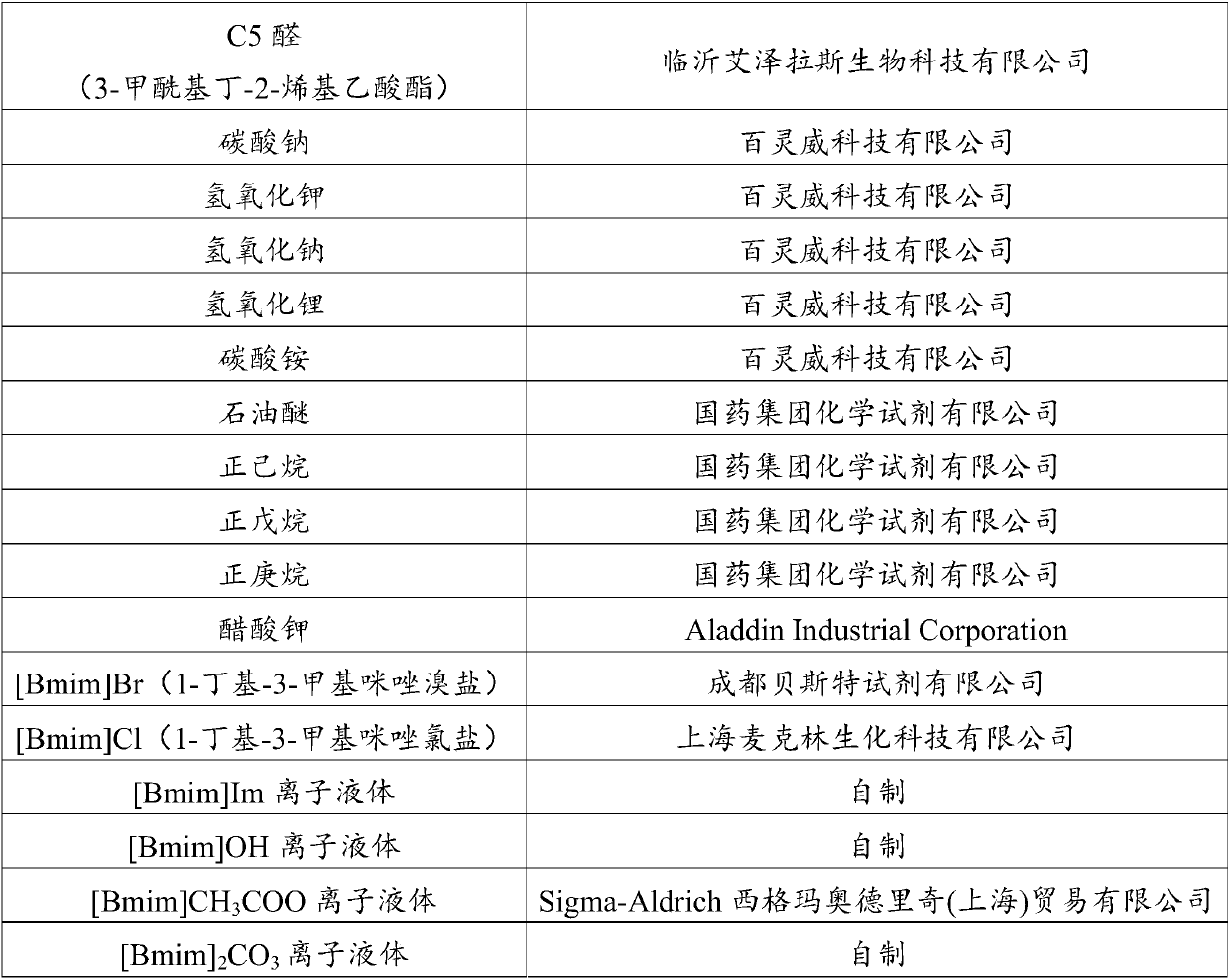
Patents
Literature
417 results about "Wittig reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide (often called a Wittig reagent) to give an alkene and triphenylphosphine oxide.
Gathering induced luminescence material containing triphenyl thylene structure, synthesis method and application thereof
ActiveCN101659865AImprove thermal stabilityHigh glass transition temperatureStyryl dyesLight-sensitive devicesOrganic solar cellSynthesis methods
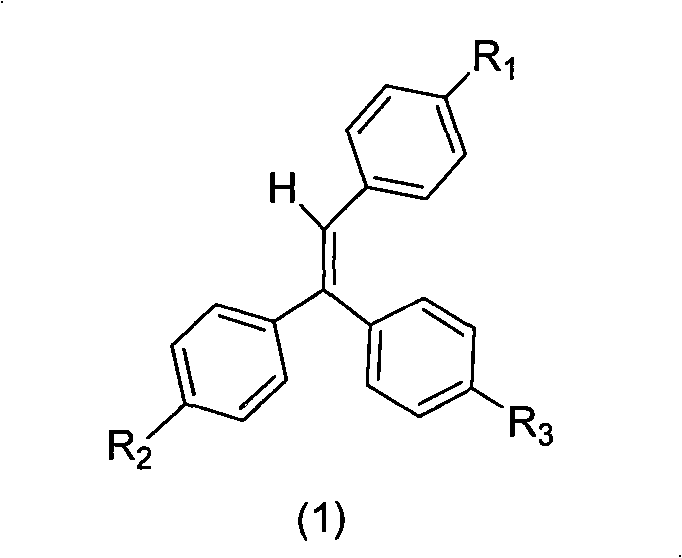
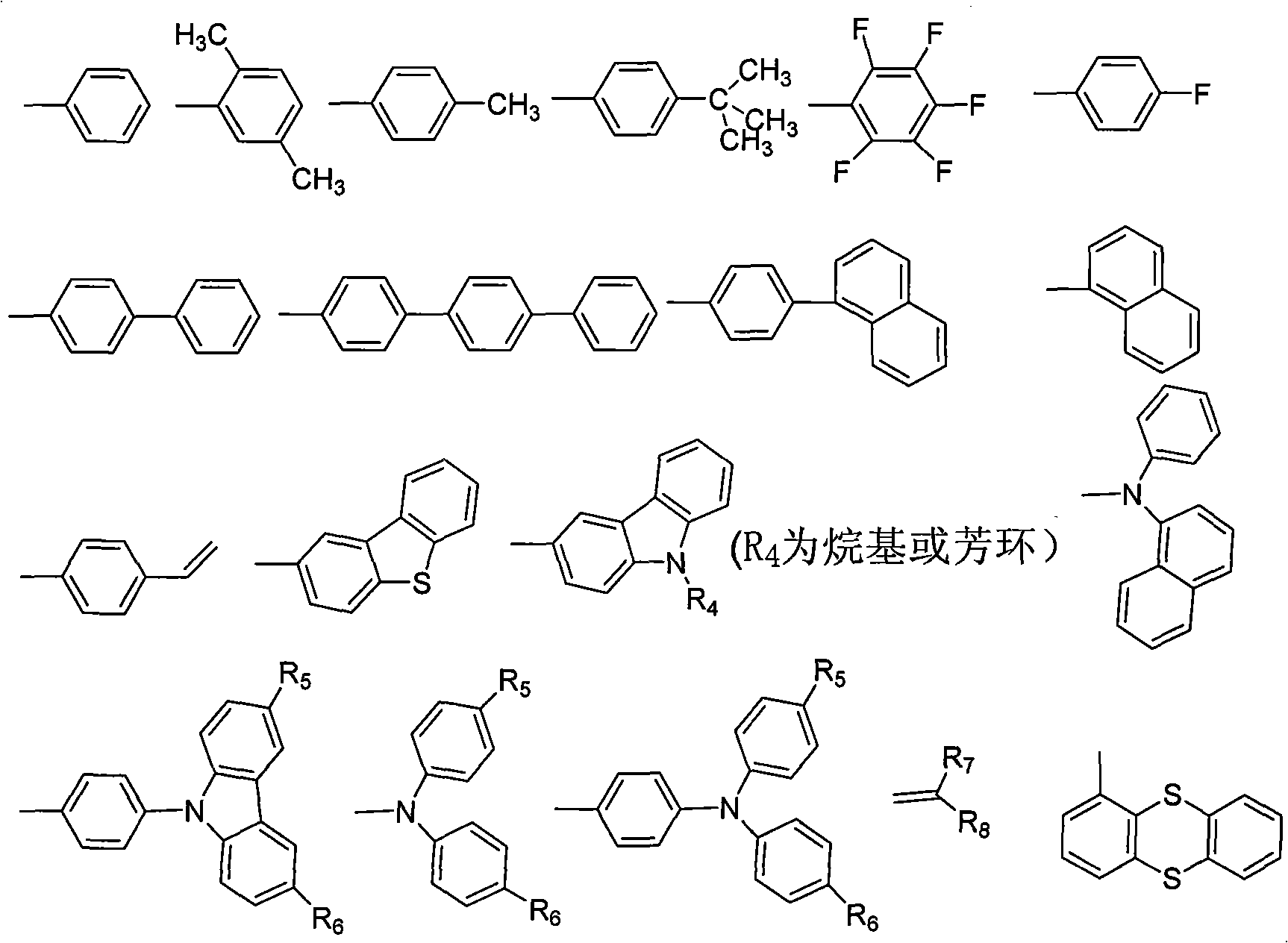
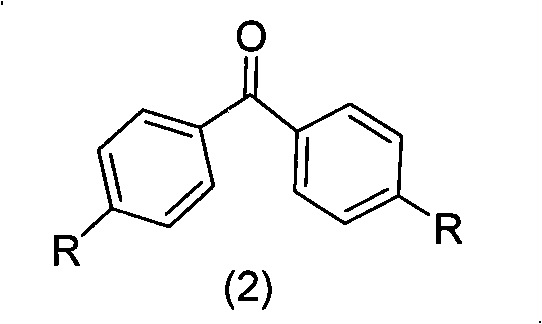
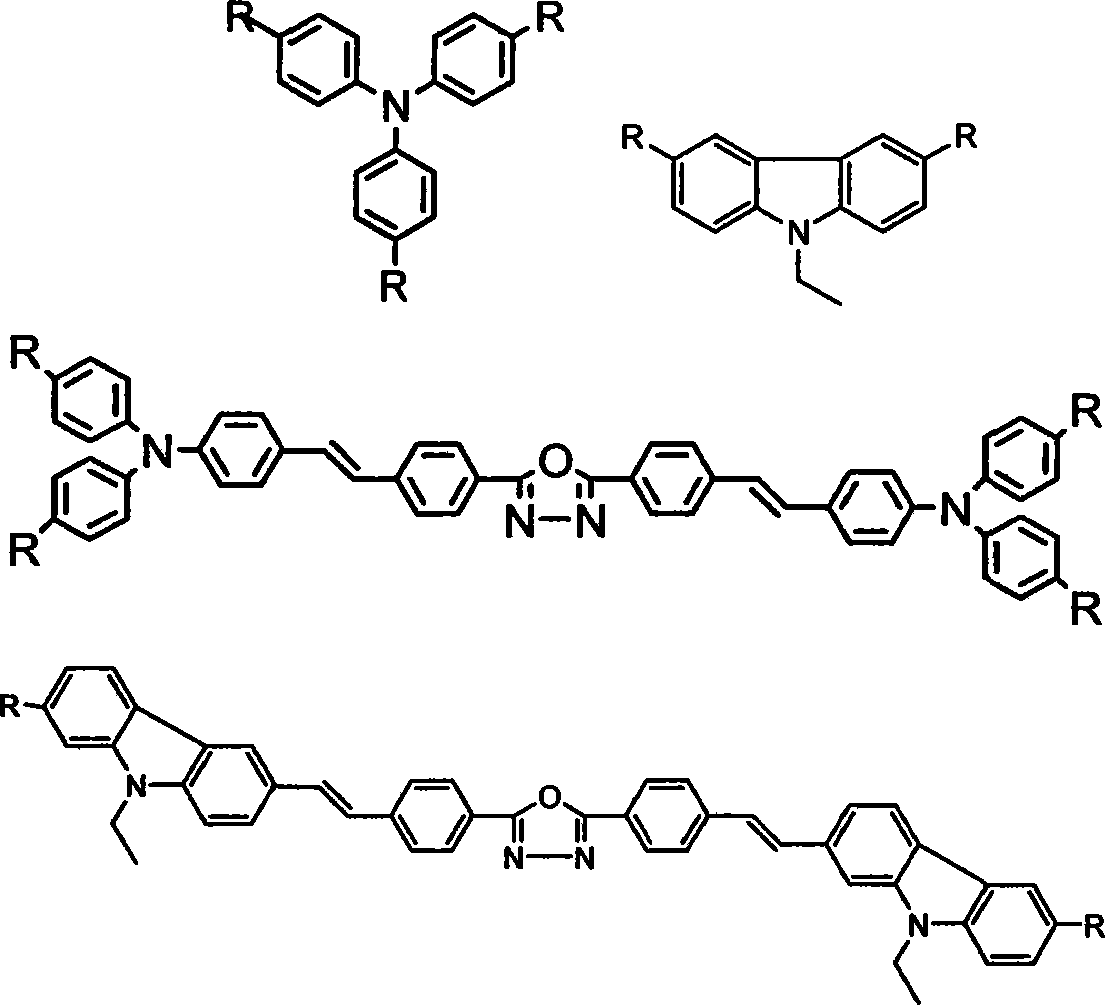
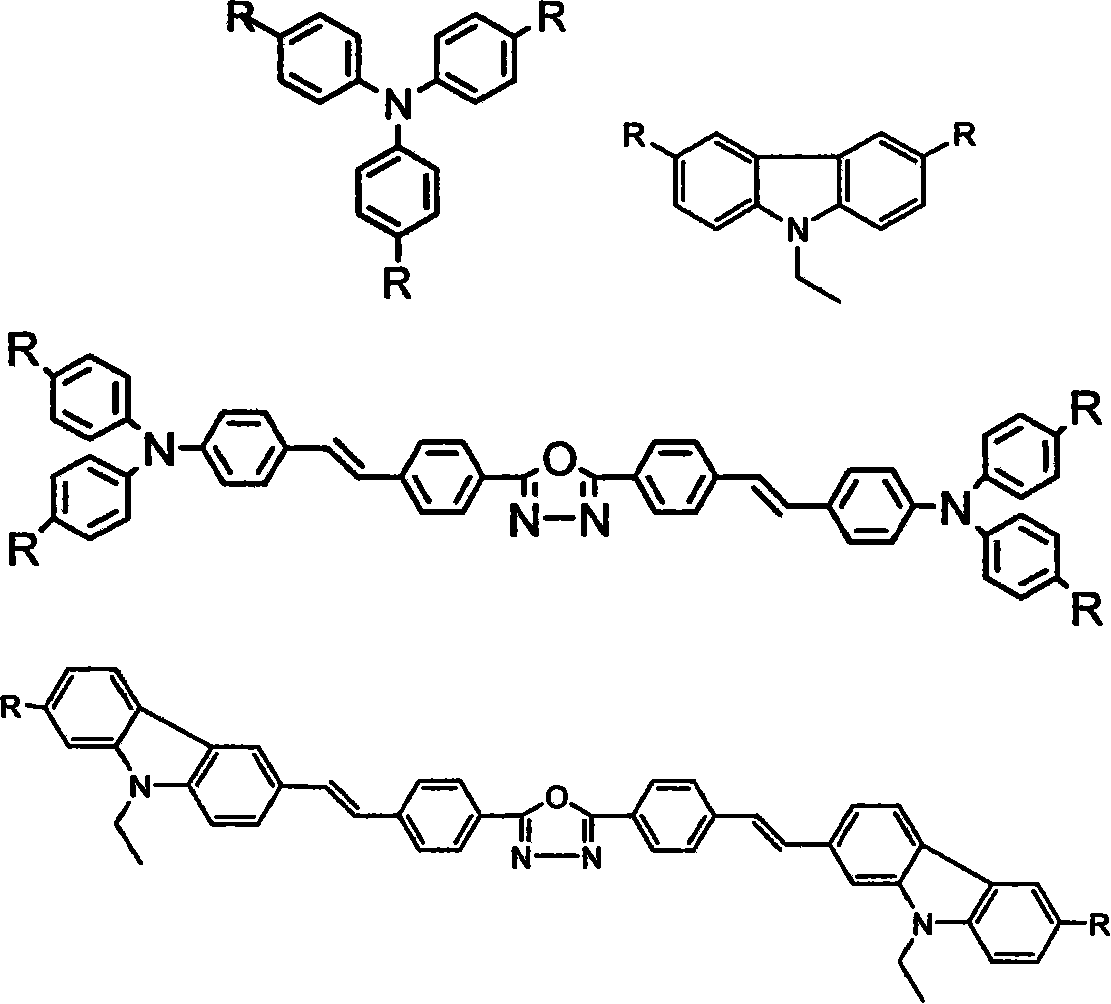
The invention belongs to the technical field of organic luminescence materials. The gathering luminescence material of the invention contains a triphenyl thylene structure, ketone carbonyl is converted into double bonds by substituent phenyl ketone through a Wittig reaction or a Wittig-Horner reaction during synthesis, and the triphenyl thylene structure is formed and then is connected with otheraromatic base groups. The synthesis method has simple technique and easy purification, and the synthesized organic luminescence material containing the triphenyl thylene structure not only has obvious gathering induced luminescence performance, high thermal stability, high vitrifaction transformation temperature and high luminescence intensity, but also is suitable for preparing a luminescence layer material in an organic electroluminescence material component; by introducing proper base groups, and the organic luminescence material also can be used as a fluorescent probe and organic solar battery sensitizing dyestuff.
Owner:SUN YAT SEN UNIV
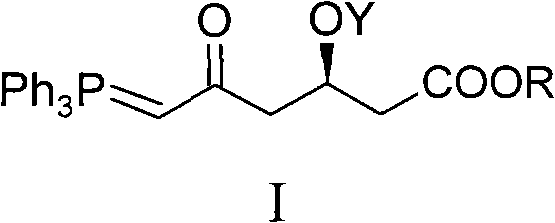
Method for preparing rosuvastatin calcium midbody
ActiveCN101735272AHigh yieldReduce pollutionGroup 5/15 element organic compoundsBulk chemical productionWittig reactionGrignard reaction
The invention discloses a method for preparing a rosuvastatin calcium midbody, namely a compound (R is C1-C10 alkyl, and Y is a hydroxyl protecting group) shown as the general formula I. Chloroethylene and R-epoxy chloropropane as initial raw materials are carried out seven steps of reaction, such as Grignard reaction, sodium cyanide nucleophilic substitution reaction, alcoholysis reaction, hydroxyl protection, oxidizing reaction, methylchloroformate acylation reaction and Wittig reaction to prepare the compound shown as the general formula I. The method has mild condition, simple and convenient operation, stable process, low cost and easy acquisition of raw materials, high product yield, easy disposal of the three wastes, less environmental pollution, low preparation cost and suitability for industrialized large-scale production.
Owner:JIANGXI DONGBANG PHARMA
Method for preparing cephalosporin propylene
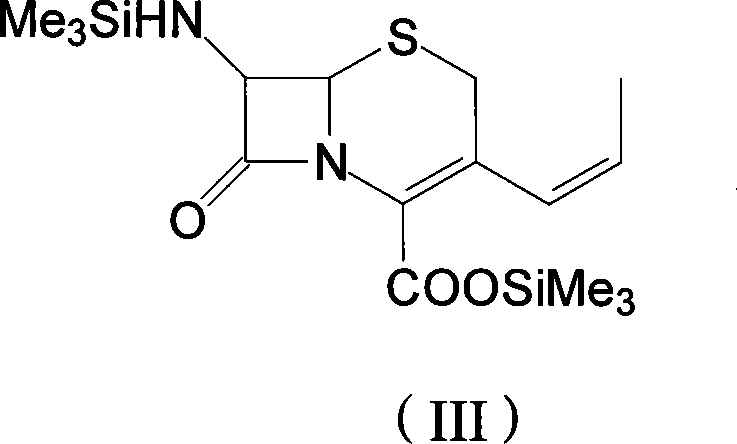
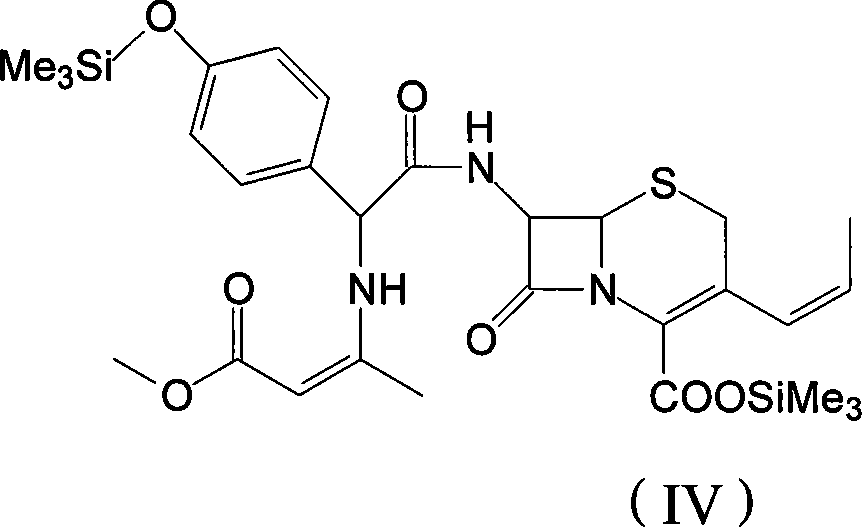
The invention discloses a preparation method of cefprozil, which comprises: 7-amin cethalosporanic acid (7-ACA) reacts with triphenyl phosphine to get 7- trimethylsilyl amino-3-triphenyl phosphate methylene-4-cethalosporanic acid trimethylsilyl ester through silanization protection and iodination reagent replacing under the condition of catalyst existing; WITTIG reaction is made for the product and acetaldehyde to get 7-trimethylsilyl amino -3-(propenyl-1-alkenyl)-4-cethalosporanic acidtrimethylsilyl ester; then the compound reacts with D-para hydroxybenzene glycine dane potassium salt to geta compound (6R, 7R)-7-[(2R)-2- ethoxycarbonyl-1-methyl - ethylene amino (4-trimethylsilyl oxyphenyl) acetamido group(amide)]-8-oxo-3- (1- propenyl)-5-thio-1- heterobicycle [4.2.0] octylene-2-ene-2-carboxylic acid trimethylsilyl ester; hydrolytic treatment is then used to get the cefprozil. The invent adopts the method of one pot and can participate in next reaction without separating intermediateproducts. The preparation method of cefprozil has the advantages of low cost, convenient operation and high overall yield, adapting to demands of industrial production.
Owner:南通康鑫药业有限公司
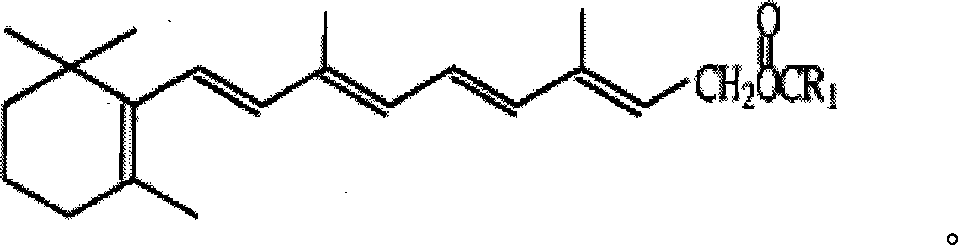
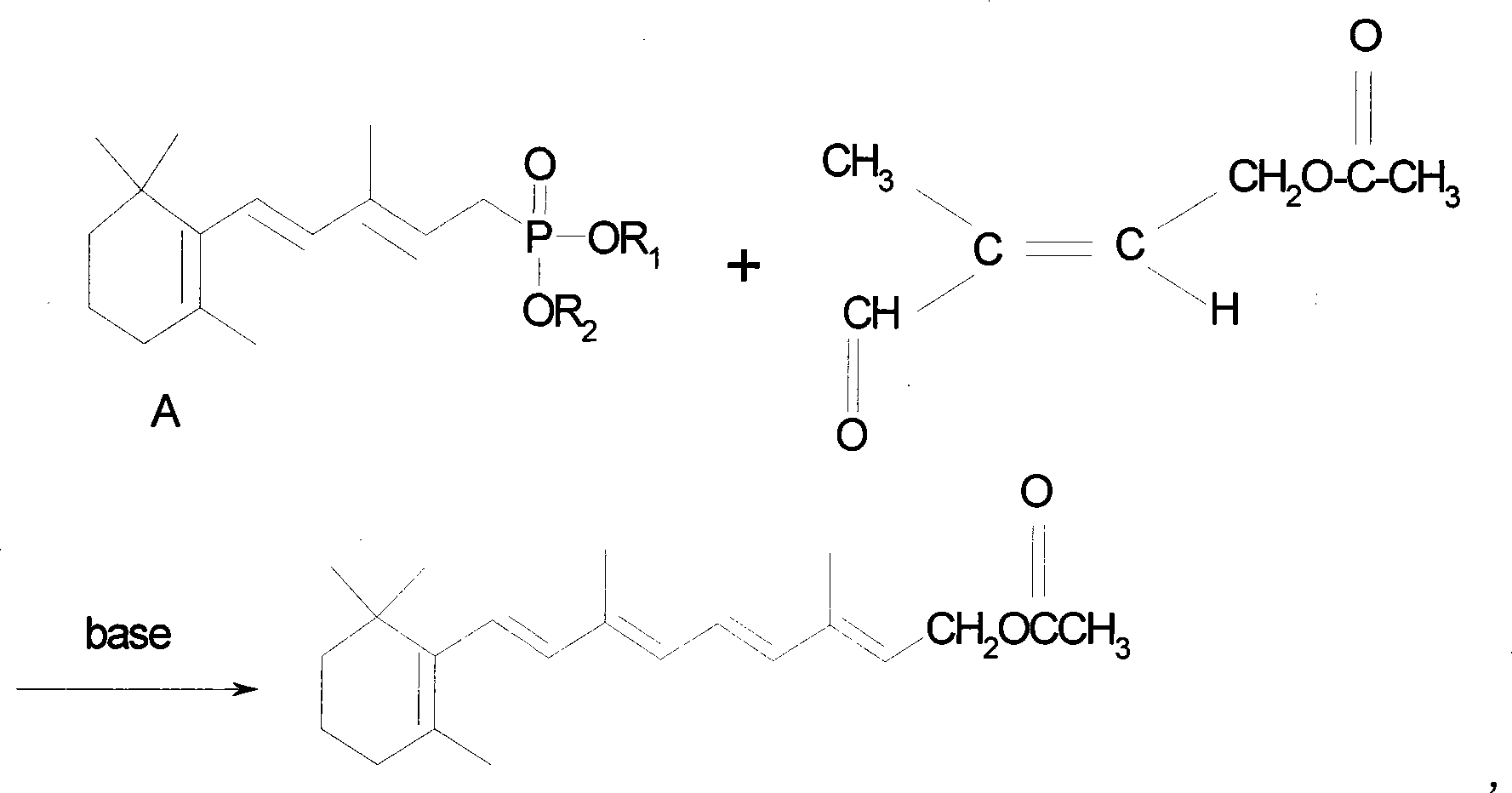
Method for preparing vitamin A acetate through one-pot method
ActiveCN103044302APrevent drynessRaise the level of industrial productionOrganic chemistryWittig reactionWater discharge
The invention relates to a method for preparing vitamin A acetate through a one-pot method, which comprises the following steps: reacting C14 aldehyde and an intermediate compound C1 (tetraethyl methylenediphosphonate or tetramethyl methylenediphosphonate) under alkaline conditions to generate C15 phosphonate; and under the condition of not separating the C15 phosphonate, directly reacting the C15 phosphonate and C5 aldehyde through a one-pot method, thus preparing the vitamin A acetate. According to the method, two Wittig reaction steps are combined and performed in one pot, the technical operation is simple, the use and separation of solvent are reduced, the drying of the C15 phosphonate is avoided, the technical process is short, and the reaction is performed at a preselected temperature, thereby improving the reaction selectivity, avoiding the cryogenic operation environment and lowering the energy consumption; and meanwhile, the waste water discharge is reduced, and the method is economical and environment-friendly. The obtained vitamin A acetate has high product purity, high yield and low cost, and is beneficial to industrial production.
Owner:XINFA PHARMA
Method for producing improved vitamin A acetic ester
The invention provides an improved preparation method for vitamin A acetate, which adopts a route of C15+C5 with Wittig reaction as characteristics, namely, using a carbon 15 phosphonic acid dialkyl ester and a C 5-aldehyde to react under the function of alkali to generate the vitamin A acetate. The invention is characterized in that a mixture of toluene and pyridine (or the methyl substitutes of pyridine) is used as a reaction solvent to enable the reaction to be carried on in an environment close to a room temperature and to reach the yield that can be achieved only under extremely low temperature, which greatly reduces the energy consumption and the difficulties of production operation and is beneficial to industrial production.
Owner:安徽智新生化有限公司
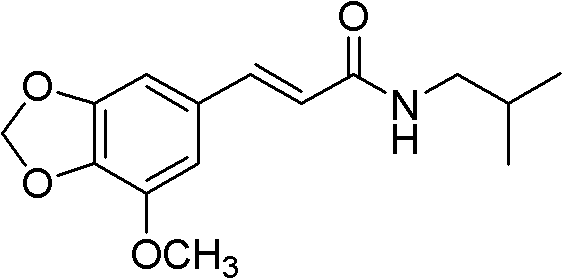
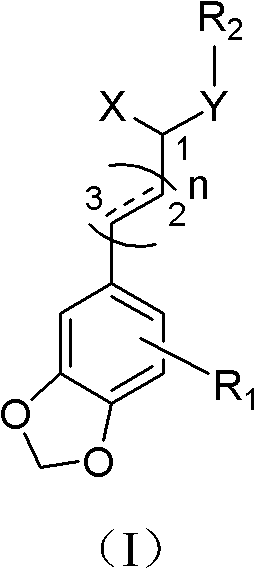
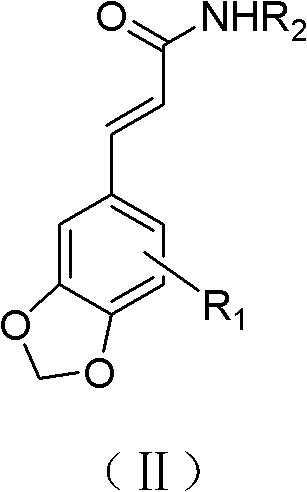
Substituted cinnamide derivative, its preparation method and application
ActiveCN102850317AGood antidepressant activityOrganic chemistryNervous disorderWittig reactionRaw material
The invention relates to a substituted cinnamide derivative of formula (i), its preparation method and application. The invention further discloses a method for preparing substituted cinnamide and its derivative by using a substituted heliotropin derivative as a raw material and conducting Wittig reaction and acid amine condensation, and an application of substituted cinnamide and its derivative in medicines for treating and preventing depressive psychosis.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD +1
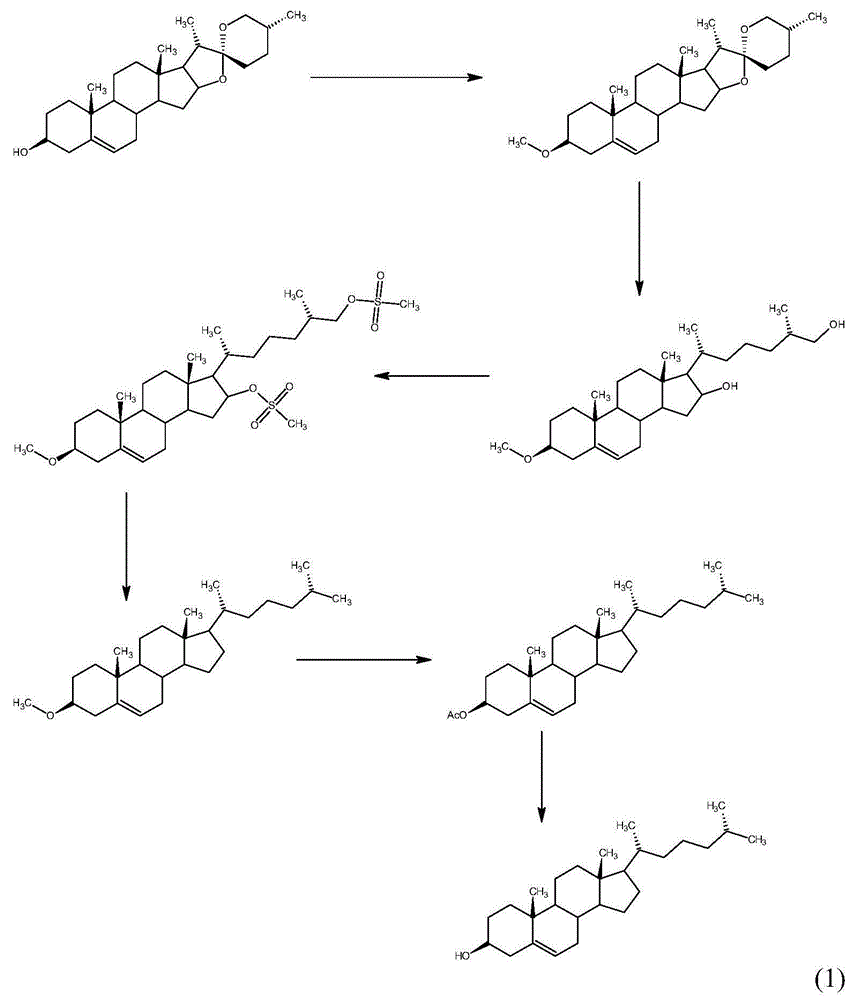
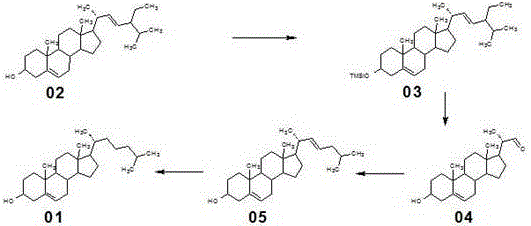
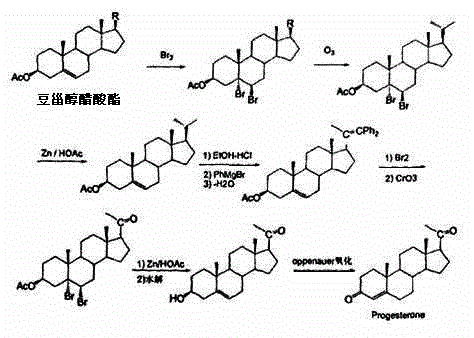
Method for synthesizing cholesterol by using stigmasterol degradation products as raw materials
The invention provides a method for synthesizing cholesterol by using stigmasterol degradation products as raw materials. The method comprises the following steps: 1) performing an etherification reaction on 3-carbonyl-4-pregnene-22-aldehyde and triethyl orthoformate to obtain 3-ethyoxyl-3,5-pregnadiene-22-aldehyde; 2) preparing a 3-methylbutyltriphenyl phosphonium chloride solution; 3) adding potassium tert-butoxide into the 3-methylbutyltriphenyl phosphonium chloride solution, performing a wittig reaction on the 3-methylbutyltriphenyl phosphonium chloride solution and the 3-ethyoxyl-3,5-pregnadiene-22-aldehyde to obtain 3-ethyoxyl-3,5,22-triene cholestane; 4) catalyzing the 3-ethyoxyl-3,5,22-triene cholestane to perform a selective hydrogenation reaction to obtain 3-ethyoxyl-5-ene cholestane; 5) performing a reaction on the 3-ethyoxyl-5-ene cholestane and acetic anhydride to obtain 3-acetyl-5-ene cholestane; 6) performing a hydrolysis reaction on the 3-acetyl-5-ene cholestane to obtain the cholesterol. The synthesizing method is simple in process, and the mole yield of the cholesterol exceeds 85 percent by using the stigmasterol degradation products which are cheap and easily obtained as the raw materials; the production cost is low, the process is environmentally friendly, and the method is economical and environmentally friendly, and facilitates industrial implementation.
Owner:HUNAN KEREY BIOTECH
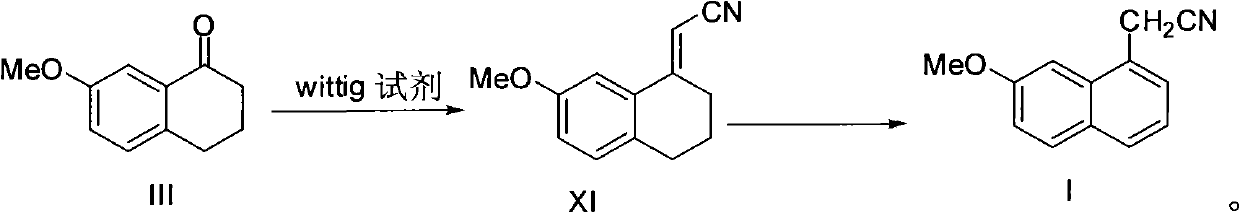
Preparation method of 2-(7-methoxy-1-naphthyl) acetonitrile
InactiveCN102336686ACarboxylic acid nitrile preparationOrganic compound preparationWittig reactionAromatization
The invention provides a new preparation method of an important intermediate of the antidepressant agomelatine, i.e. 2-(7-methoxy-1-naphthyl) acetonitrile. In the method, a compound of formula III is subjected to a Wittig reaction so as to obtain a compound of formula XI, which then undergoes an aromatization reaction, so that 2-(7-methoxy-1-naphthyl) acetonitrile can be obtained. The preparation method of the invention has a short reaction route, the Wittig reaction and the aromatization reaction both have high yield. With mild reaction conditions and without anything to do with reagents and solvents etc. of high toxicity, the preparation method provided in the invention is suitable for industrial production.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Methyl pyridinium with organic two-photon absorption materia, and preparation method and application thereof
InactiveCN102702090APromote absorptionGood water solubilityOrganic chemistryMaterial analysis by observing effect on chemical indicatorSolubilitySolvatochromism
The invention relates to the field of organic nonlinear optical materials and relates to methyl pyridinium with an organic two-photon absorption material, and a preparation method and the application of the methyl pyridinium. The structural formula of the product is described in structural formula (I); the preparation method comprises the steps of taking 2,6-dimethyl pyridine as a raw material and finally obtaining a compound (I) by conducting oxidizing reaction, esterification reaction, methylolation reaction, halogenation reaction, nucleophilic addition reaction, Wittig reaction and the final methylation reaction. The raw materials are easy to access, and the synthesis steps are simple. The compound (I) has excellent two-photon absorption performance and good water solubility and is an ideal biological fluorescence probe; an obvious solvatochromism phenomenon easily occurs, the compound can be used for detecting water content in a common organic solvent, can also be used as a fluorescent probe for detecting the PH value in a solution system, has a series of advantages of high sensibility, quick response, identification by naked eyes and the like, and has an excellent application prospect.
Owner:SHANGHAI NORMAL UNIVERSITY
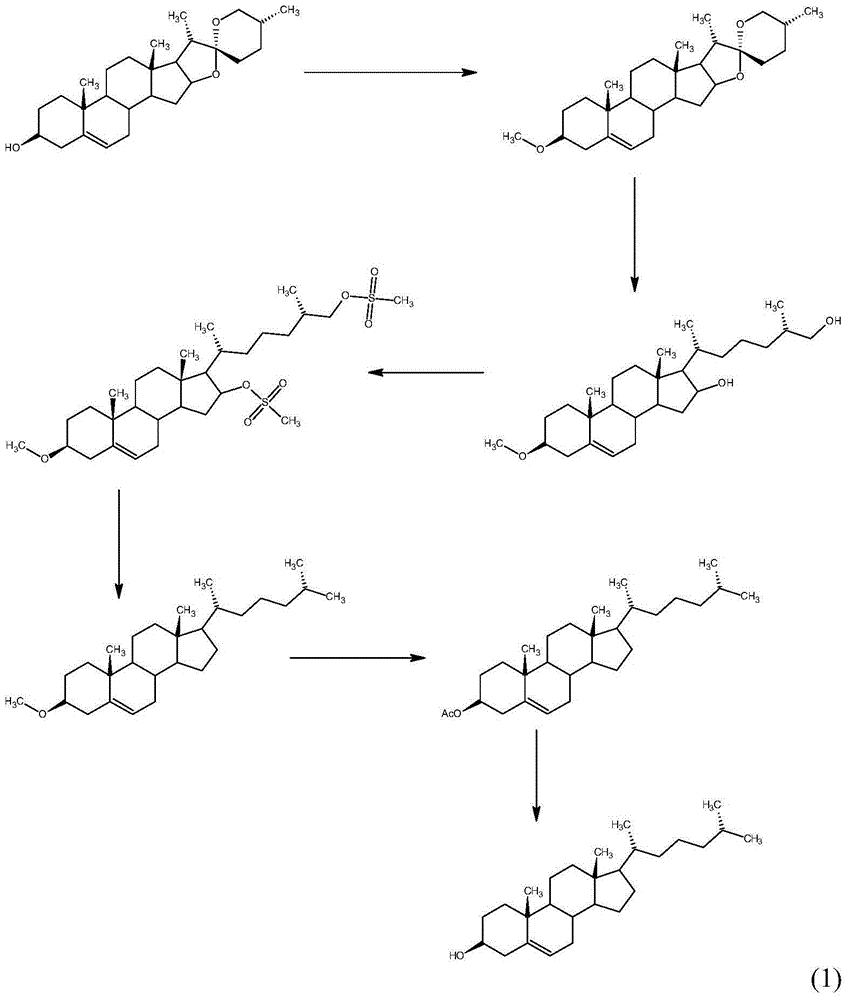
Method for synthesizing cholesterol by using pregnenolone as raw material
The invention provides a method for synthesizing cholesterol by using pregnenolone as a raw material. The method comprises the following steps: 1) adding potassium acetate into methyl alcohol, and performing a reaction on sulfonate to obtain 6-methoxyl-3,5-cyclo-5alpha-pregn-20-one; 2) performing a reaction on triphenylphosphine and 1-chloro-4-methylpentane in an aprotic solvent to obtain a 4-methylbutyltriphenyl phosphonium chloride solution; 3) adding potassium tert-butoxide into the 4-methylbutyltriphenyl phosphonium chloride solution, and performing a wittig reaction; 4) under the catalysis of a rhodium catalyst, performing an asymmetric hydrogenation reaction to obtain 6-methoxyl-3,5-cyclo-5alpha-cholestane; 5) performing a catalytic hydrolysis deprotection reaction by using sulfuric acid to obtain the cholesterol. The method provided by the invention has the advantages that six-step reactions in the conventional method are simplified into four-step reactions, and a ring-opening reaction in which a great number of hydrochloric acid and a large number of zinc powder are consumed in a route of using saponin as an initial raw material. The synthesizing method is simple in process, the consumption of the raw material and auxiliary materials is low, and the mole yield is high; the method is economical and environmentally friendly, and facilitates industrial implementation.
Owner:HUNAN KEREY BIOTECH
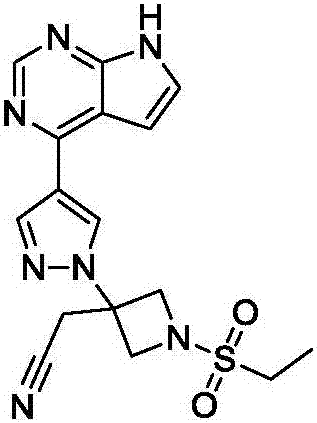
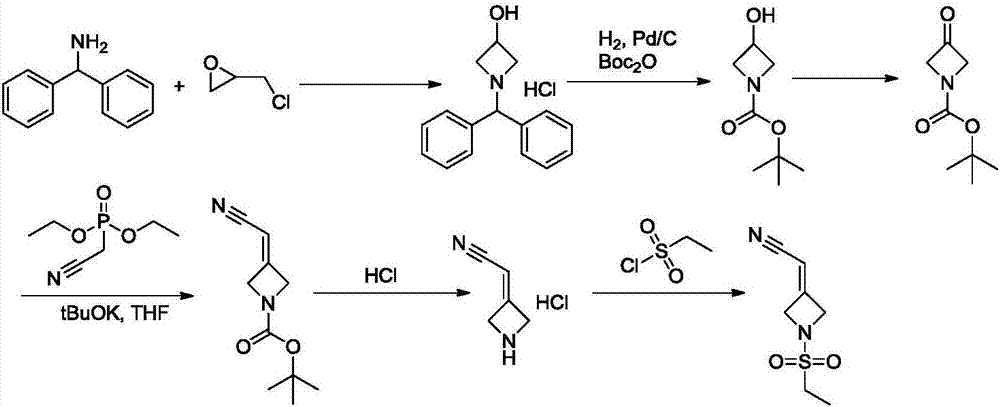
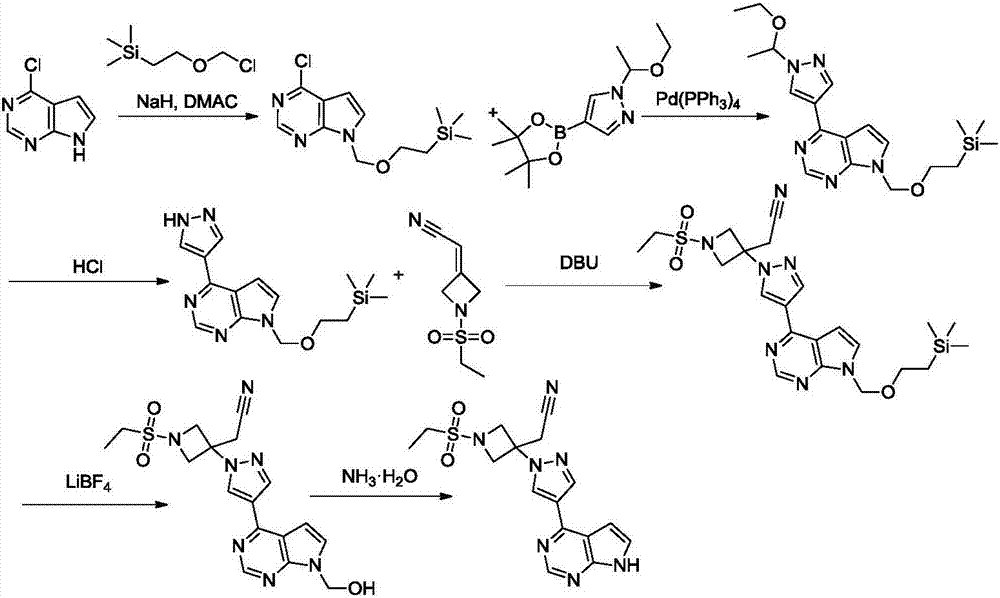
New synthesis methods of JCK inhibitor baricitinib and intermediate thereof
ActiveCN106946917AImprove efficiencyAtom economy is highGroup 3/13 element organic compoundsBulk chemical productionWittig reactionSynthesis methods
The present invention provides a new synthesis method of a baricitinib compound 11. According to the present invention, by using a compound 1 as a staring raw material, the amino group is protected by directly using ethanesulfonyl chloride, and direct cyclization is directly performed by using the effect of an alkali to obtain a key intermediate compound 3 so as to avoid the use of other protection groups and substantially improve the route efficiency and the atomic economy; during the compound 5 preparation, a Wittig reaction is performed by using triphenylphosphine acetonitrile so as to avoid the use of strong alkali and improve the reaction yield; the completely-new neopentyl glycol borate derivative compound 8 has good stability and good crystallinity so as to simplify the separation and purification process; and the route is simple to operate and has the high yield, the purity of the obtained product is high, and the synthesis method is suitable for amplification production. The formulas 1-11 are defined in the specification.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Divinyl liquid crystal compound and preparation method thereof
ActiveCN101928199AWide phase change rangeGood chemical stabilityLiquid crystal compositionsOrganic compound preparationStructural formulaPhosphine
The invention relates to a divinyl liquid crystal compound and a preparation method thereof. A structural formula of the divinyl liquid crystal compound is expressed as the following general formula I, wherein R1 represents hydrogen, C1-8 straight-chain alkyl or C2-8 straight-chain vinyl; R2 represents hydrogen or C1-8 straight-chain alkyl; A represents benzene ring or 1,4-cyclohexane ring; and nis equal to 1 to 2 and m is equal to 0 to 2. The preparation method for the divinyl liquid crystal compound comprises that: corresponding phosphine salt and aldehyde undergo wittig reaction in the presence of alkali to generate cis and trans mixed alkene, and then the cis and trans mixed alkene is transformed into trans alkene. The invention has the advantages that: a series of negative liquid crystals are developed, have wider liquid crystal phase change range, good chemical stability and excellent use value and can reduce the freezing point and improve the clearing point when added into mixed liquid crystal and increase the absolute value of a negative dielectric constant.
Owner:VALIANT CO LTD
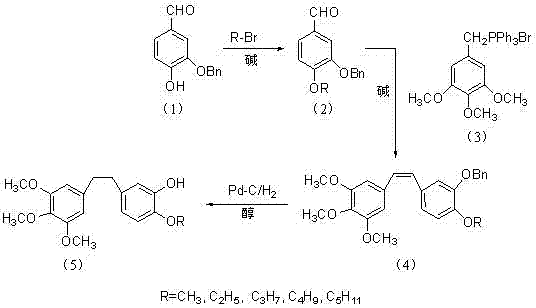
Method for preparing 3,4,5-trimethoxy-3'-hydroxy-4'-alkoxy diphenylethane
InactiveCN103539642AImprove protectionSignificant technological progressOrganic compound preparationCarbonyl compound preparationPalladium on carbonBenzaldehyde
Owner:SHANGHAI INST OF TECH
Method for preparing vitamin A acetate
ActiveCN109651150AReduce separation and purification operationsSmooth responseOrganic compound preparationGroup 5/15 element organic compoundsWittig reactionVitamin A Alcohol
The invention belongs to the technical field of production of vitamin A and its derivatives, in particular to a method for preparing vitamin A acetate. The method comprises the following steps: (1) mixing triphenylphosphine and acid in supercritical CO2, and then contacting the obtained mixture with vinyl-beta-ionol for salt formation to obtain C15 phosphine salt; (2) after adding solvent water, ionic liquid and C5 aldehyde to the finished system of step (1) , CO2 is released for solvent replacement, and the materials are uniformly mixed; (3) adding an extractant to the system after the solvent replacement of step (2), stirring and adding an alkali solution for Wittig reaction to obtain the vitamin A acetate. The method of the invention has mild reaction conditions, safe production and easy control, and the purity and reaction yield of the final product are high.
Owner:WANHUA CHEM GRP CO LTD
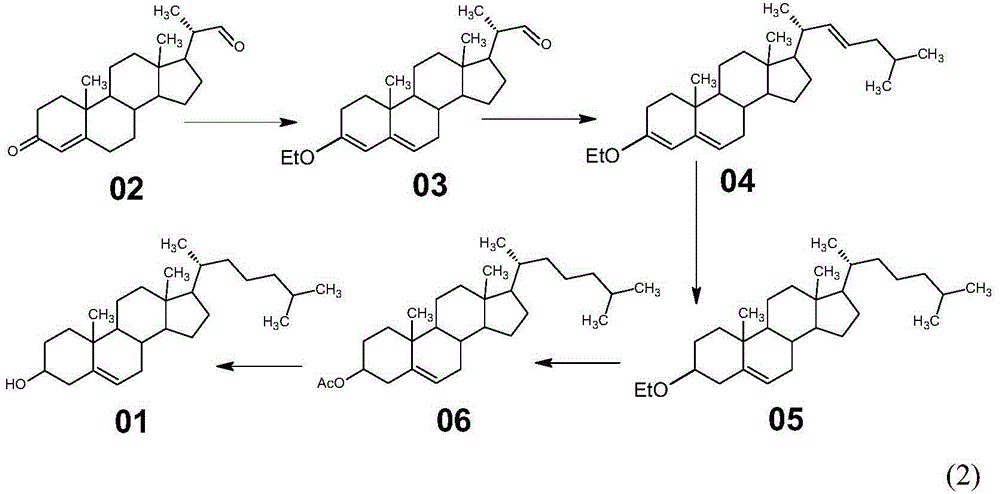
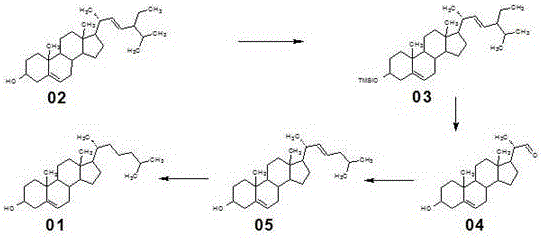
Novel method used for synthesizing cholesterol
The invention discloses a novel method used for synthesizing cholesterol. The novel method comprises following steps: 1, stigmasterol 02 is dissolved in an organic solvent I, and is subjected to silicon etherification reaction with trimethylchlorosilane under the effect of a catalyst, and a compound 03 is obtained via water washing and condensation; 2, the compound 03 is dissolved in an organic solvent II for ozone oxidation, zinc powder and acetate acid gracial are added for reduction, after reduction, filtering, washing, and condensation are carried out so as to obtain a compound 04; 3, triphenylphosphine is reacted with 1-halogenated-3-methyl butane so as to obtain 3-methyl butyl triphenyl halogenated phosphorus, the 3-methyl butyl triphenyl halogenated phosphorus is added into an aprotic solvent, a strong base is added, wittig reaction with a compound 4 is carried out, and neutralizing, condensation, and filtering are carried out so as to obtain a compound 05; 4, the compound 05 is subjected to selective hydrogenation in a solvent III under the action of a catalyst and a passivator, and filtering, condensation, and crystallization are carried out so as to obtain cholesterol 01. The novel method is simple; raw materials are cheap; the novel method is economic and is friendly to the environment, and is convenient for industrialized production.
Owner:HUNAN KEREY BIOTECH
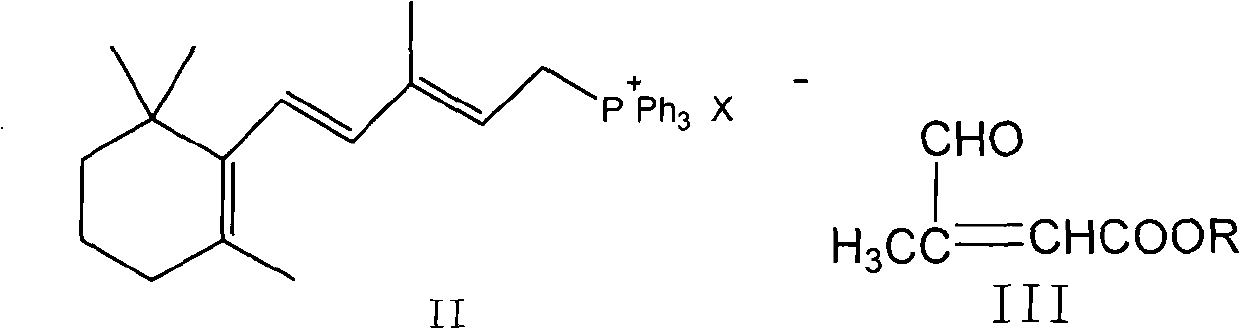
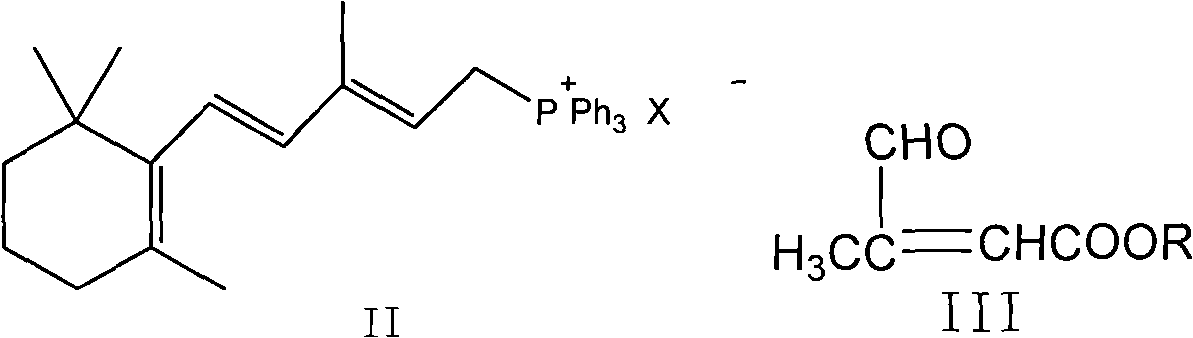
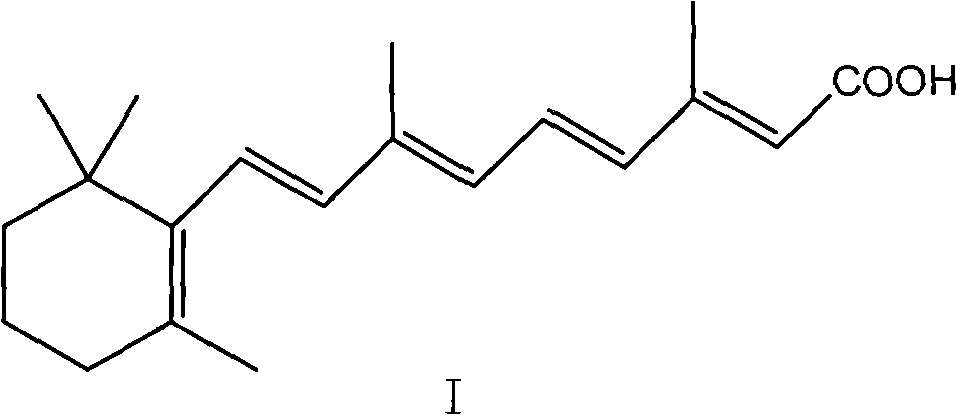
Method for preparing all-trans tretinoin
ActiveCN101774954AEasy to separateThorough responseOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsWittig reactionTretinoin
The invention provides a method for transforming 11-cis-tretinoin into all-trans tretinoin, which is characterized by using a palladium nitrate composite catalyst. The invention also provides a method for synthesizing the all-trans tretinoin, which comprises the following steps of: using compounds (II) and (III) as raw materials to perform WITTIG reaction under the action of alkali so as to obtain a mixture of all-trans retinoic acid ester and 11-cis-retinoic acid ester; performing hydrolysis and acidification to obtain a mixture of the all-trans tretinoin and an isomer 11-cis-tretinoin; and transforming the isomer to obtain the target product namely the all-trans tretinoin. The method can be finished by two steps and has the advantages of complete reaction, high yield, less isomer content in the finished product and low cost, and is suitable for industrial production.
Owner:CHONGQING HUABANGSHENGKAI PHARM
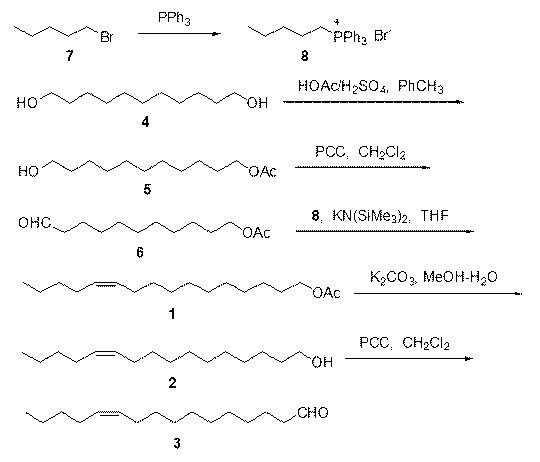
Synthetic method of diamondback moth sex pheromone compound
InactiveCN102795997ALow priceReduce manufacturing costOxygen-containing compound preparationOrganic compound preparationHydrolysisToxicology
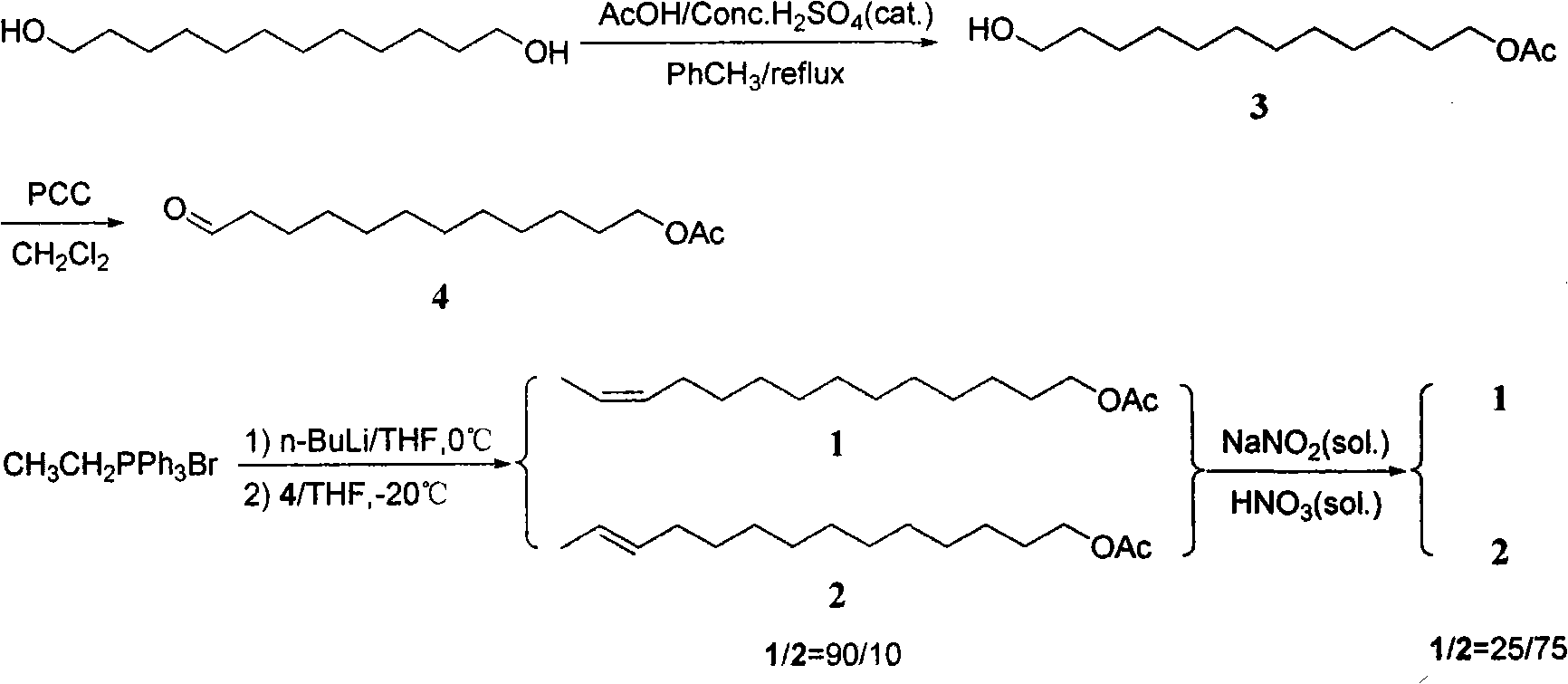
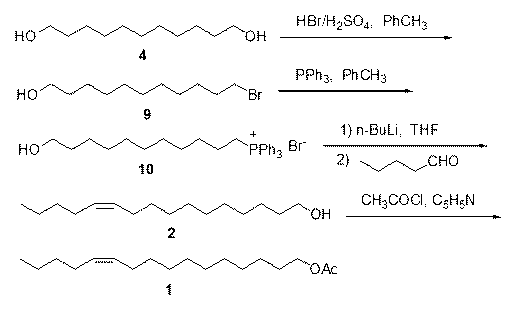
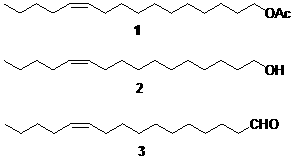
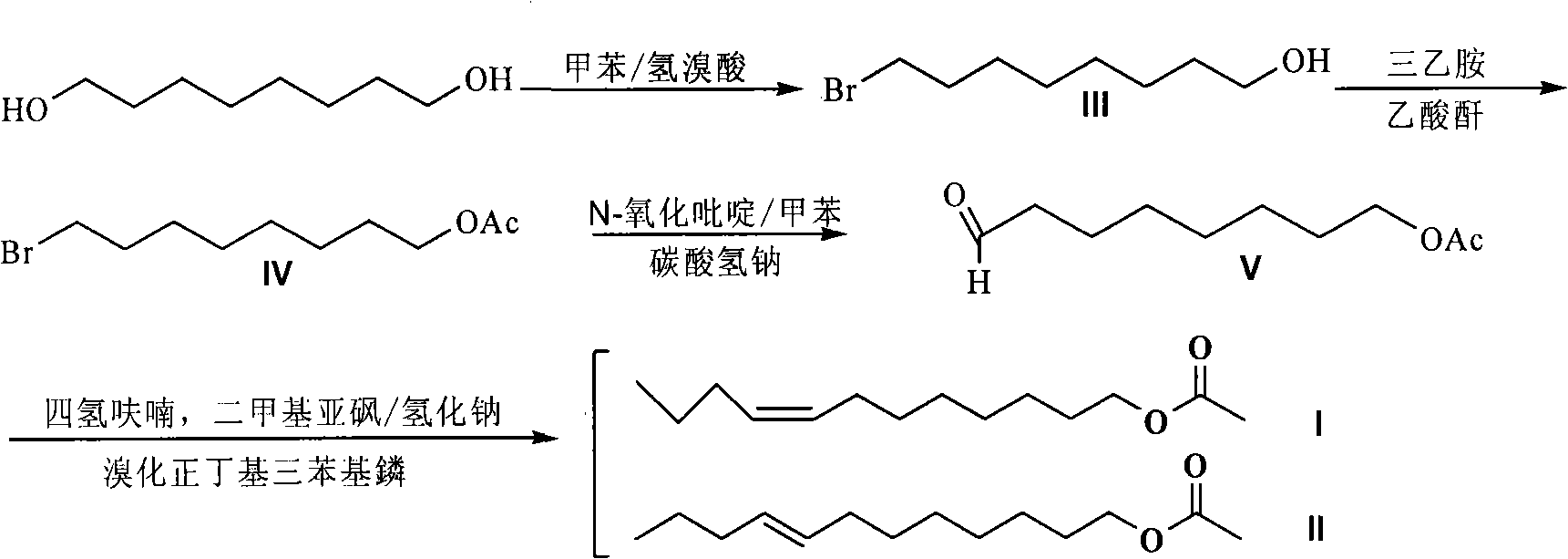
The invention discloses two synthetic methods of a diamondback moth sex pheromone compound. The first method adopts 1,11-undecadiol and n-amyl bromide as initial raw materials, and comprises four-step reactions including a critical Wittig reaction to synthesize (Z)-11-hexadecen-1-yl acetate, a hydrolytic reaction to obtain (Z)-11-hexadecen-1-ol, and a pyridinium chlorochromate oxidation reaction to obtain (Z)-11-hexadecenal. The second method adopts 1,11-undecadiol and valeraldehyde as initial raw materials, and comprises three-step reactions including a critical Wittig reaction to synthesize (Z)-11-hexadecen-1-ol, a pyridinium chlorochromate oxidation reaction to obtain (Z)-11-hexadecenal, and an esterification reaction to obtain (Z)-11-hexadecen-1-yl acetate. The invention has the advantages of cheap and easily available raw materials, simple synthetic route, mild reaction condition, convenient and safe operation, high yield, and low cost.
Owner:昆明博鸿科技有限公司
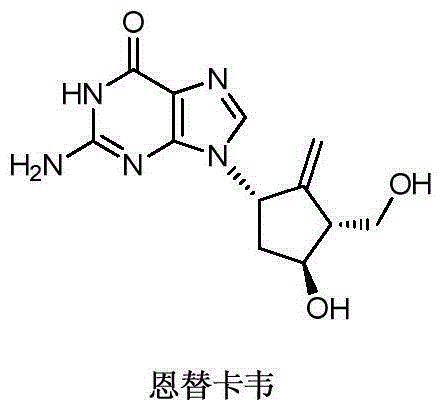
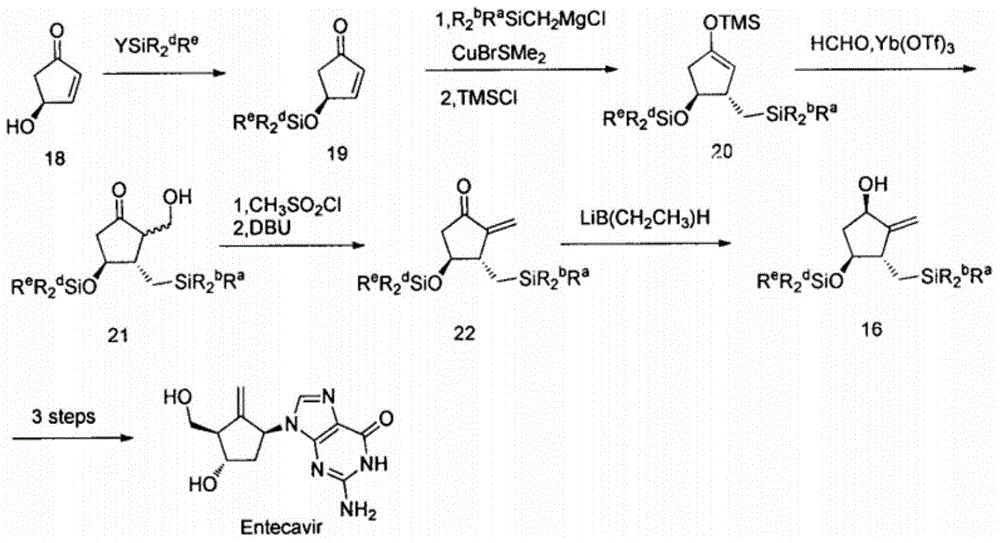
Novel synthetic method for entecavir compound
ActiveCN105037363ASolve rare problemsDosage controllableGroup 4/14 element organic compoundsKetoneAdipate
The invention belongs to the field of drug synthesis and relates to an entecavir compound and a synthetic method of an intermediate of the entecavir compound. The novel synthetic method comprises: by taking (S)-3-hydroxyl dimethyl adipate as an initial raw material, preparing an intermediate 9 through hydroxyl TBS protection, Dieckmann condensation reaction, ketone protection to ketal, ester group reduction to hydroxyl, hydroxyl protection, deprotection, ketone to silyl enol ether and Rubottom oxidizing reaction; and preparing entecavir from the intermediate 9 through wittig reaction, Mitsunobu reaction, silicon preventing radical group removal and basic hydrolysis. The novel synthetic method provided by the invention is mild and easily controllable in reaction condition, simple to operate, high in product yield, high in purity and suitable for industrialized mass production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
Method of preparing cydia molesta sex pheromone
InactiveCN101270049AThe preparation method is easy to obtainEasy to prepareOrganic compound preparationPest attractantsFruit treeWittig reaction
The present invention relates to a preparation method of 8 (Z / E)-dodecylene-1-yl acetate of pheromone of oriental fruit moth of pests of fruit trees of the lep-idoptera tortricidae. In the method, 1, 8-octandiol is used as raw material, treated through unilateral bromination with hydrobromic acid, acetylated and transformed into aldehyde with N-oxidated pyridyl; finally the 1, 8-octandiol and bromide butyl triphenyl have the Scholer-Wittig reaction to synthesize the target compound. The method has the advantages of raw materials that can be easily prepared, simple process, mild conditions, high yield, low cost, suitability for large-scale production, and so on. The method provides necessary conditions for the wide application of the pheromone of oriental fruit moth to replace chemical pesticides so as to prevent and treat the pests of fruits, and has higher theoretical significance and excellent application value.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
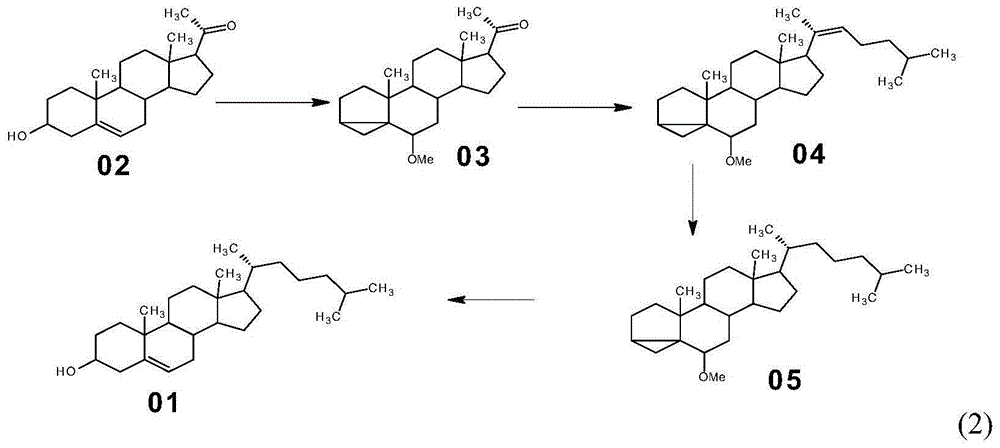
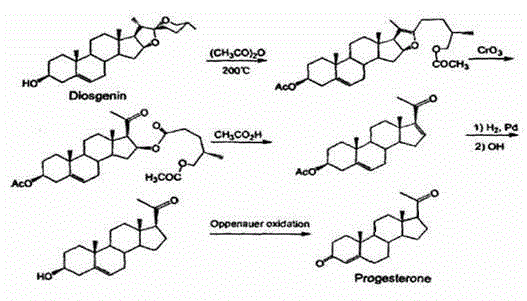
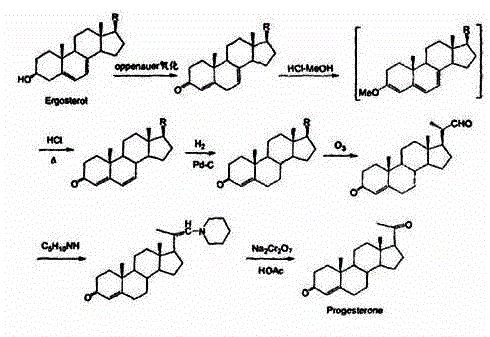
Method for preparing progesterone by taking 1,4-androstenedione as raw material
The invention discloses a method for preparing progesterone by taking 1,4-androstenedione as a raw material, which comprises the following steps: 1) dissolving 1,4-androstenedione into an organic solvent, adding the acid of trimethyl orthoformate or triethyl orthoformate, and introducing nitrogen to protect the 1,4-androstenedione to synthesize the enol ether of 1,4-androstenedione, namely 3-methoxy-androstane 3,5-diene-20-ketone; and 2) dispersing (1-methoxy ethyl)-triphenylphosphine salt in a reaction medium, an organic solvent, adding alkali at low temperature, performing a Wittig reaction of the 3-methoxy-androstane 3,5-diene-20-ketone synthesized in the step 1), and purifying and crystallizing to obtain progesterone. By adopting the 1,4-androstenedione as the raw material, the method solves the problem that of lack in raw materials for synthesizing steroid drugs such as progesterone, and improves the utilization rate of 1,4-androstenedione and the yield of progesterone; the preparation process is simple.
Owner:HUNAN KEYUAN BIO PRODS
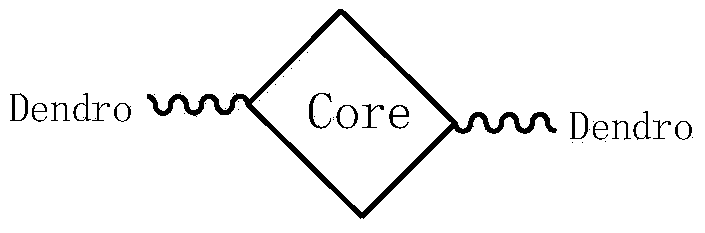
Soluble triphenylamine type organic micromolecular hole transport material and preparation method and application thereof
ActiveCN103626666AEasy to synthesizeEasy to purifyAmino preparation from aminesSolid-state devicesSolubilityWittig reaction
The invention discloses a soluble triphenylamine type organic micromolecular hole transport material and a preparation method and application thereof. According to the triphenylamine type organic micromolecular hole transport material, dendrons containing triphenylamine groups are introduced onto different cores, so that the prepared hole transport material has certain solubility and can be purified by a solution method. The triphenylamine type organic micromolecular hole transport material disclosed by the invention can be prepared through firstly synthesizing different modules, namely cores and dendrons, and then correspondingly synthesizing respectively by adopting Wittig reaction, so that routes of synthesis are relatively simple, and the purification is convenient. The triphenylamine type organic micromolecular hole transport material has an important application prospect in organic electroluminescent devices.
Owner:TIANJIN UNIV
Conjugation arborization electroinduced ethereal blue light material, preparation method and application thereof
InactiveCN101219921AImprove solubilityExtended conjugate lengthOrganic chemistrySolid-state devicesWittig reactionOrganic electroluminescence
The invention provides a pure blue light material of a conjugated branchlike molecular and an organic electroluminescence device by using the blue light material as an emitting layer. The blue light material of the invention is a conjugated branchlike molecule, which takes a trimeric indene as a nuclear and a trans 1, 2-stilbene as a bridge; the blue light material is obtained through Homer-Wadsworth-Emmons reaction or Wittig reaction of three phosphonate and single-aldehyde derivative of the trimeric indene; conjunction length and molecular weight of the material are rapidly increased, and emission of the pure blue light and film forming capability of the material are correspondingly regulated. Furthermore, the branchlike structure of the material and an replaced alkyl chain effectively reduce molecular aggregation, thereby obtaining high-purity blue light. By using the conjugated branchlike molecular as the emitting layer material of the organic electroluminescence diode device, the organic electroluminescence diode device can be prepared by simple spin coating process and membrane making; the invention can successfully emit the pure light, with good photoelectric stability.
Owner:PEKING UNIV
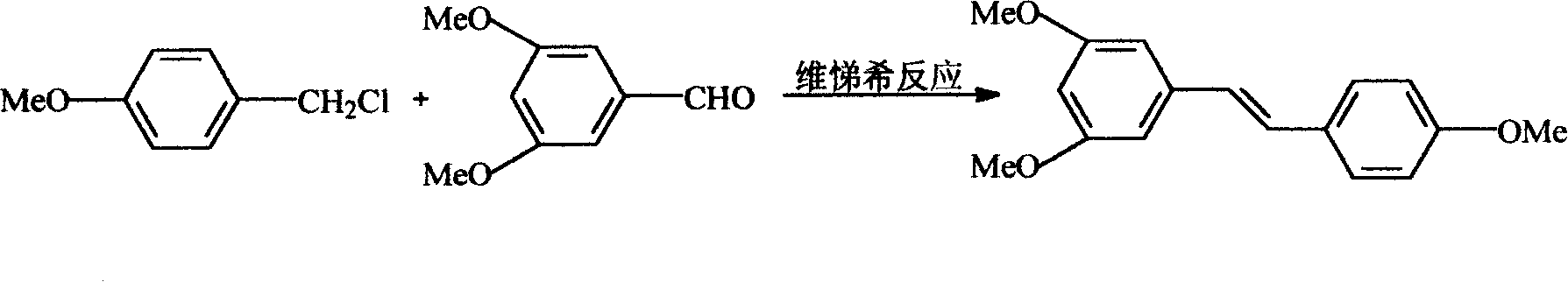
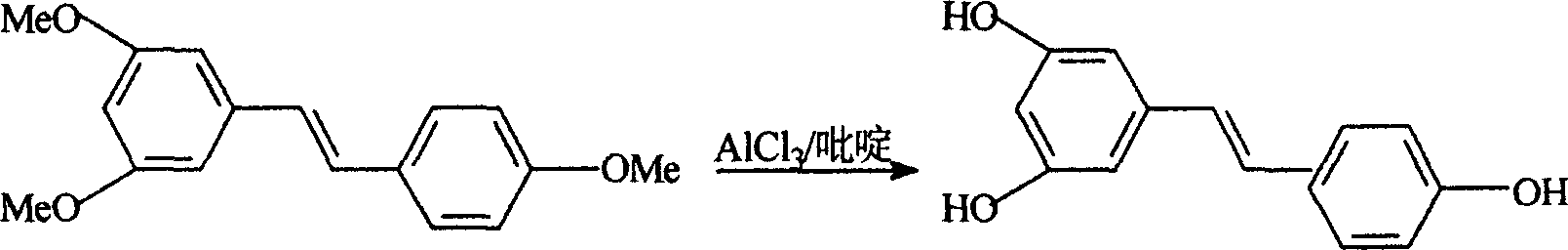
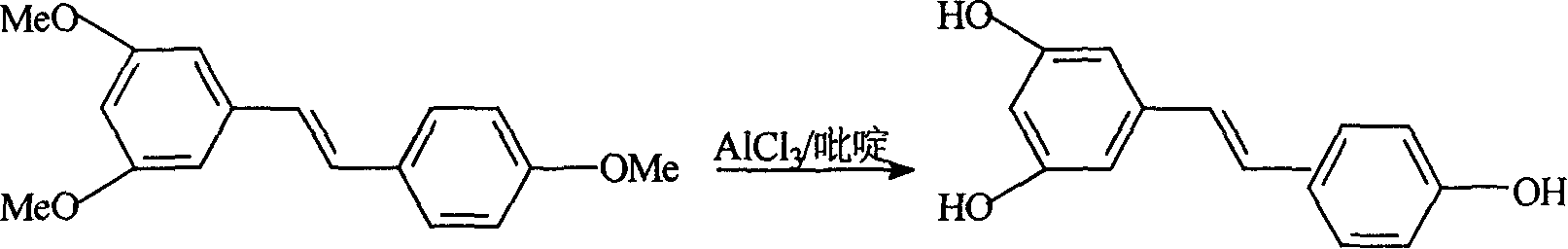
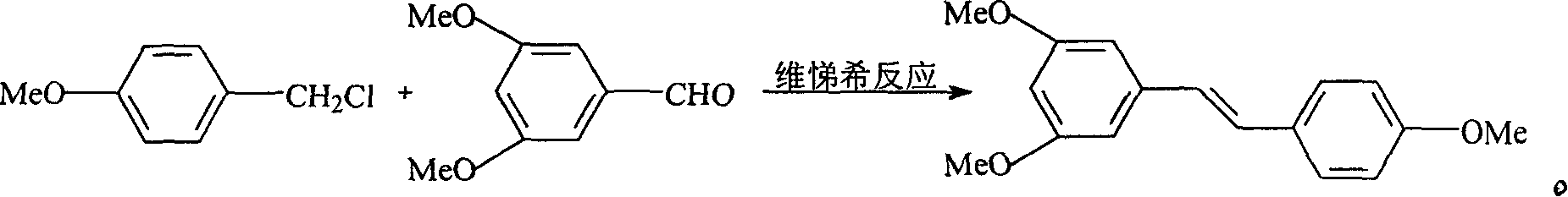
Process for synthesizing resveratrol by using de-methylation technology
InactiveCN1663939AExcellent qualityExcellent yieldOrganic chemistryOrganic compound preparationAluminium chlorideWittig reaction
The invention relates to a process for synthesizing resveratrol by using de-methylation technology, which comprises using 3,5,4'-trimethoxyl diphenyl ethylene as raw material, using aluminium chloride / pyridine as catalyst for synthesizing Resveratrol through demethyl process, and employing market available methoxyl benzyl chloride for Wittig reaction with 3,5- dimethoxybenzaldehyde, thus preparing intermediate 3,5,4'-trimethoxyl diphenyl ethylene.
Owner:NANJING RALLY BIOCHEM
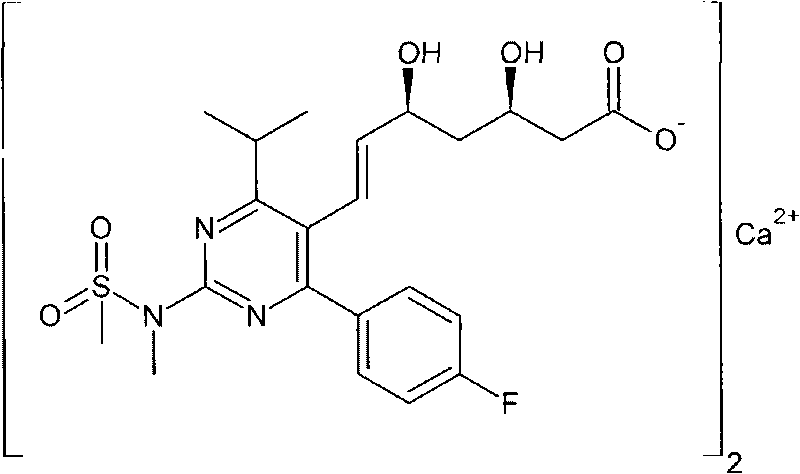
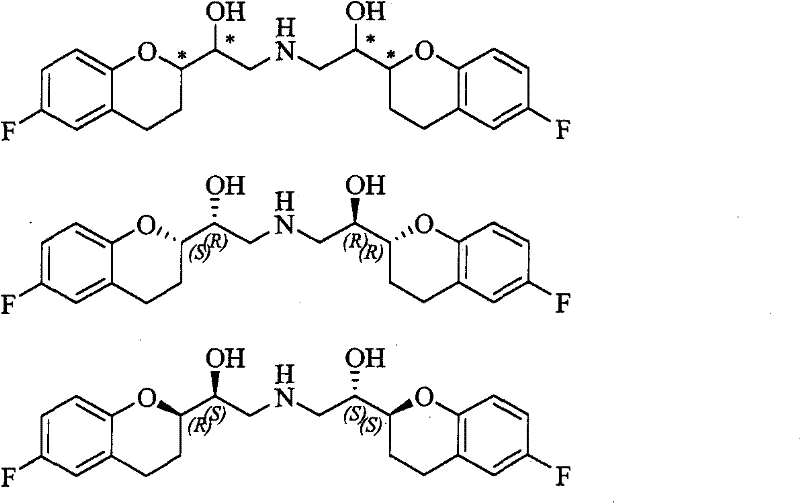
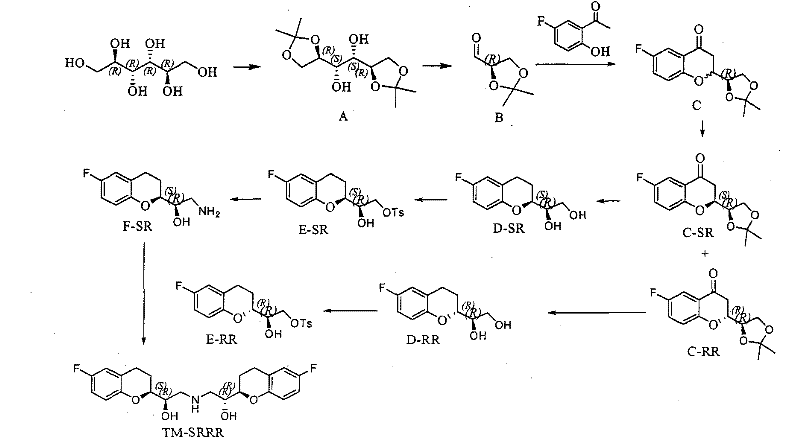
Method for preparing nebivolol hydrochloride
ActiveCN102344431ANo pollution in the processRaw materials are cheap and easy to getOrganic chemistryWittig reactionSinistral and dextral
Owner:SICHUAN JISHENGTANG PHARMLS
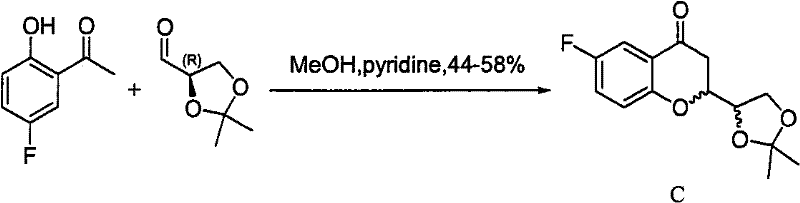
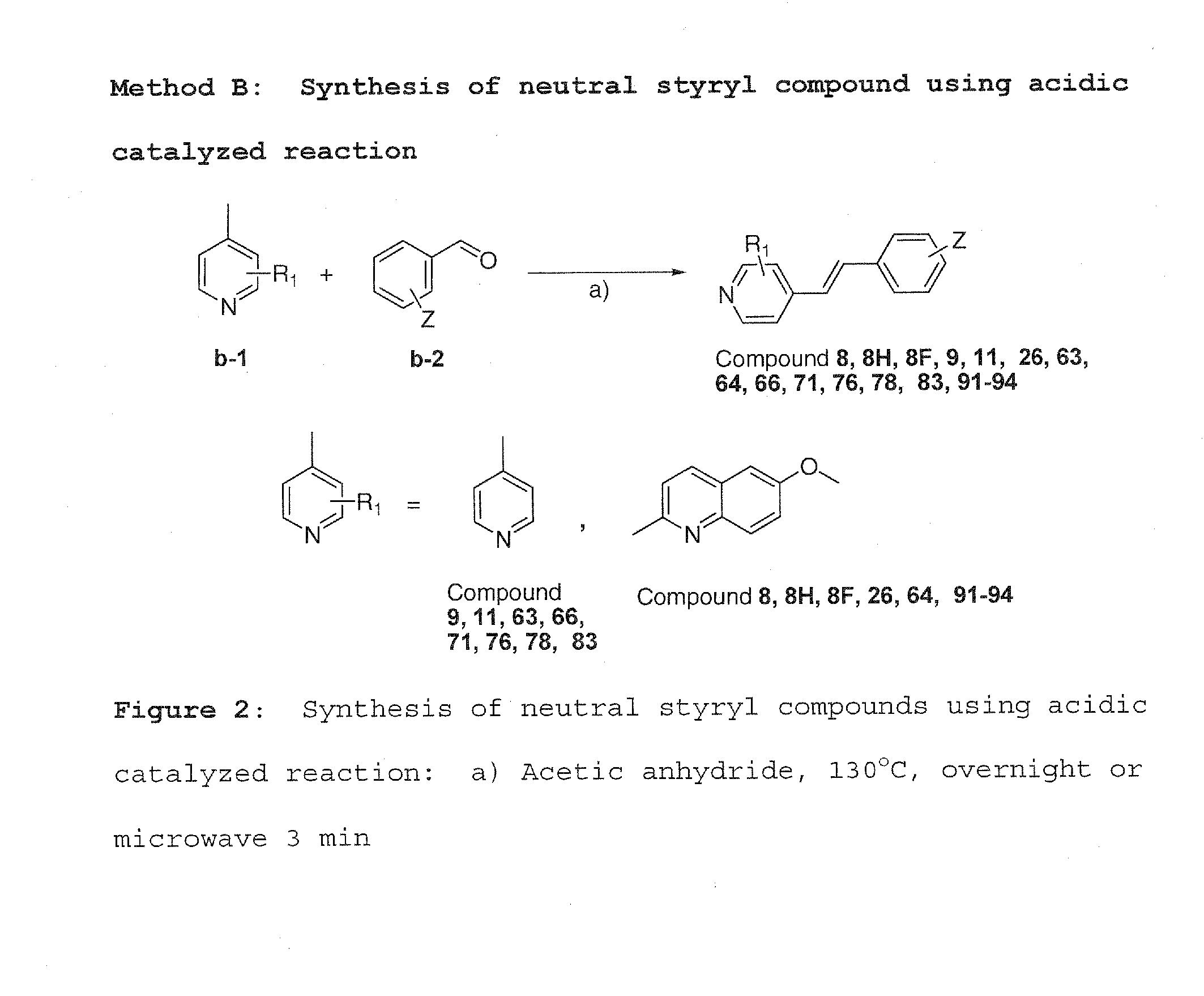
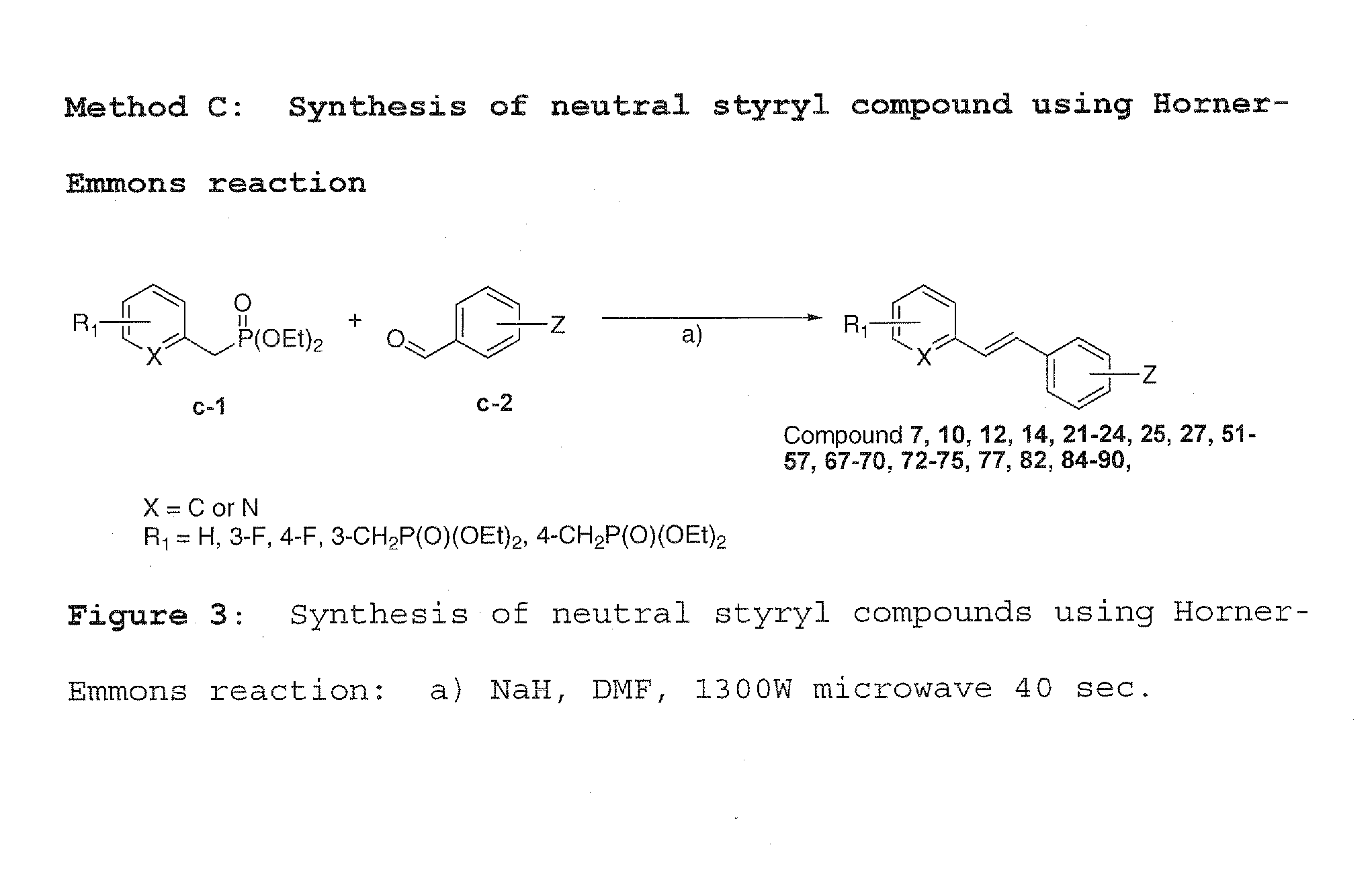
Imaging agents for protein misfolding
Charged and neutral small fluorescent molecules based upon the styryl scaffold are useful as imaging agents for misfolded proteins such as amyloid plaque. Charged molecules are prepared using pyrrolidine catalyzed reactions by solution-phase synthesis. Neutral styryl molecules are prepared using acetic anhydride catalyzed reactions, Horner-Emmons reactions or Wittig reactions.
Owner:NEW YORK UNIV
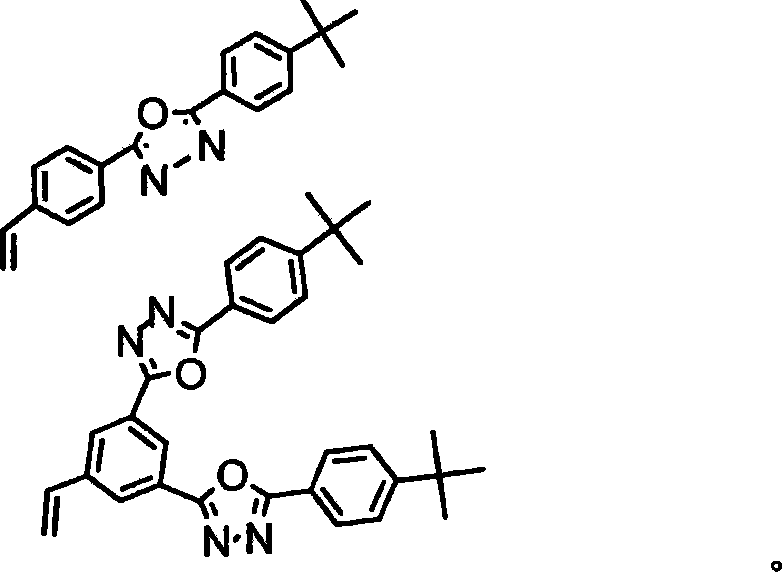
Multi-branched oxdiazole two-photon pump fluorescent material and preparation method thereof
InactiveCN101081978AAbsorbentLarge optical clipping factorLuminescent compositionsFluorescencePhoton upconversion
The present invention is multiramose double photon pumped fluorescent oxdiazole material and its preparation process. The oxdiazole material is prepared with polycyclic arene bromide and 3, 5-bis{2-(4-tertiary butyl phenyl)-5-[1, 3, 4]-oxdiazolyl}-styrene or 2-(4-tertiary butyl phenyl)-5-[1, 3, 4]-oxdiazolyl-styrene, and through Heck reaction inside mixed solvent of triethylamine and N, N-dimethyl formamide catalyst under the action of catalyst palladium acetate and tri-o-methylphenyl phosphide; or prepared with triphenylamido aromatic aldehyde derivative and benzyl bromo triphenyl phosphine containing [1, 3, 4]-oxdiazole structure, and through solid and liquid Wittig reaction. The oxdiazole material possesses sufficient double photon absorption cross section, high double photon fluorescence efficiency and high heat stability, and can emit strong double photon up converted green and blue fluorescence under the excitation of 800 nm laser beam.
Owner:SOUTHEAST UNIV
Method for modifying synthetic veratric alcohol
InactiveCN101033172AExcellent qualityExcellent yieldOrganic chemistryOrganic compound preparationAlcoholWittig reaction
This invention relates to an improved method for synthesizing resveratrol, which takes 3, 5, 4'-methylate toluylene as the raw material, 1, 3, 5-trimethylbenzene as the solvent and hydroquinone as the antioxidant to synthesize resveratrol with alchlor / pyridine as the catalyst and applies commercial cheap anisyl chlorben and 3, 5-dimethoxy benzene formaldehyde for wittig reaction to prepare an intermediate 3, 5, 4'-methylate toluylene.
Owner:NANJING RALLY BIOCHEM
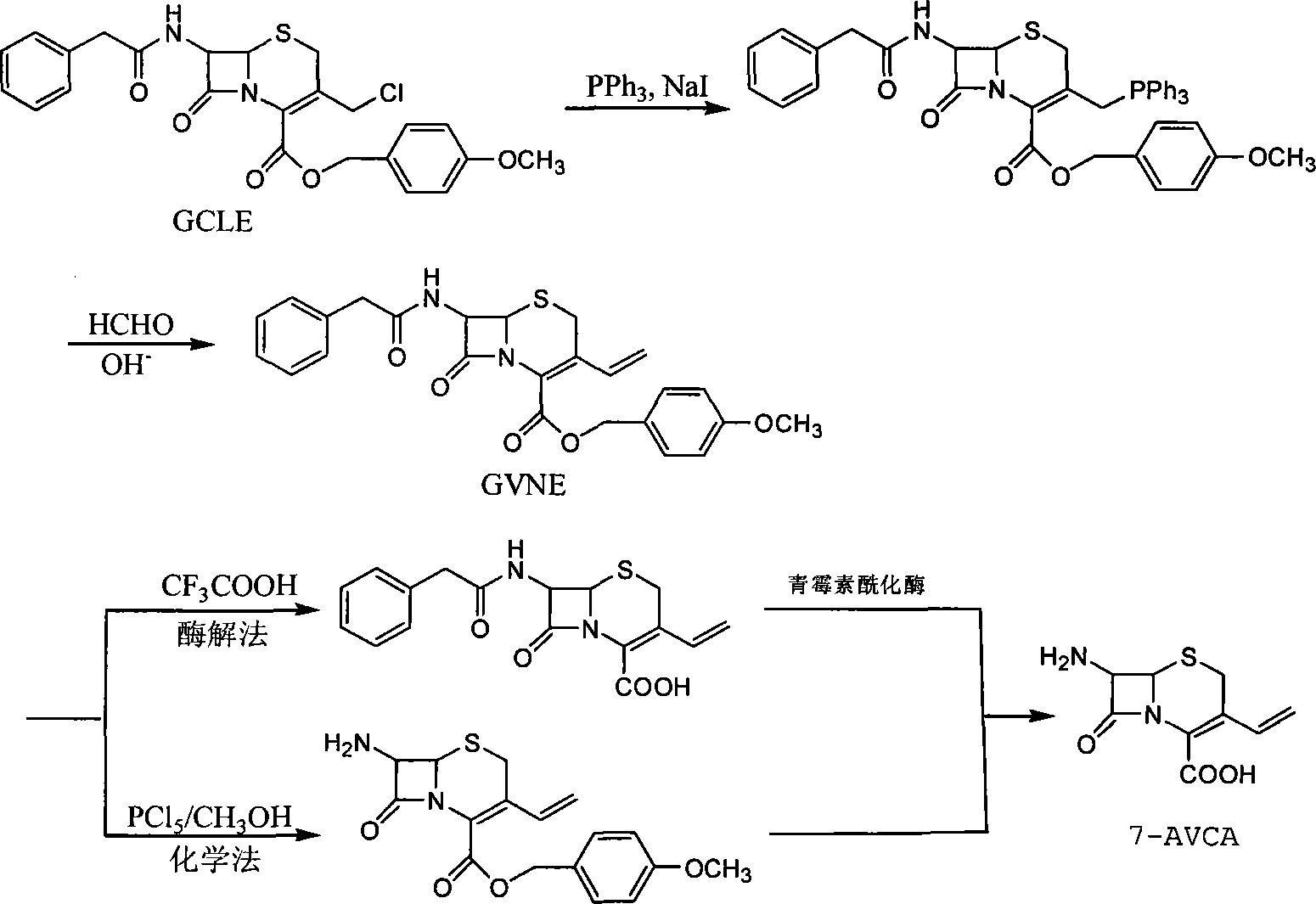
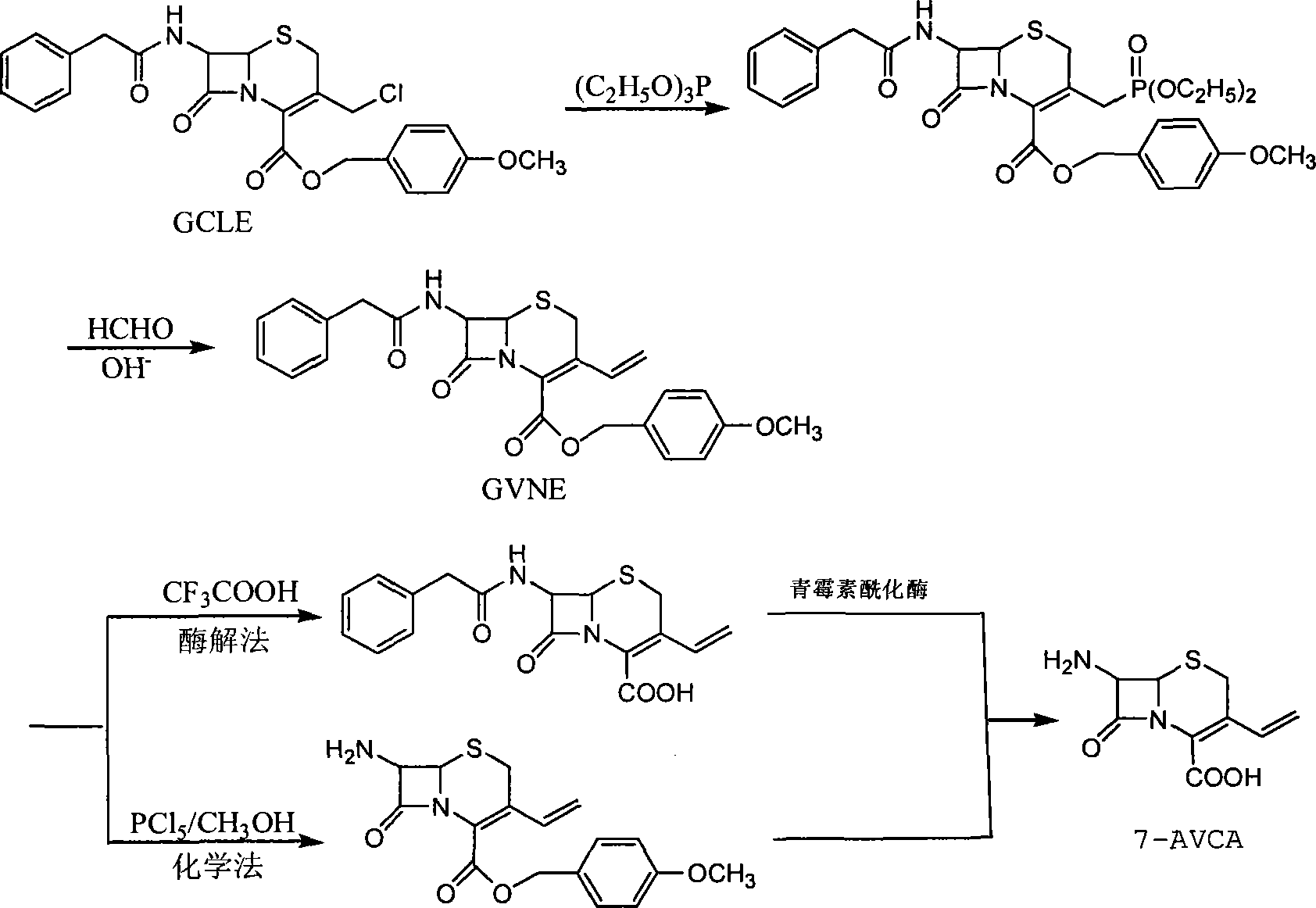
Method for preparing 7-amido-3-vinyl cethalosporanic acid
InactiveCN101182326ALow costSimple production methodOrganic chemistryWittig reactionTriethylphosphite
A method of preparing for 7-amido-3-ethylene cephalosporanic acid considers 7-phenylacetyl amino-3-chloromethyl cephalosporanic acid p-methoxybenzyl ester (GCLE) as a starting material and adopts the Wittig reaction. Ethylene is introduced on position 3 to obtain 7-phenylacetyl amino-3- ethylene-4- cephalosporanic acid p-methoxybenzyl ester, and a product GVNE is obtained. The GVNE is used to prepare for 7-amido-3-7 alkenyl cephalosporanic acid (7-AVCA). The present invention has the main technical characteristic that the triethyl phosphite with quite low price is used to replace triphenyl-phosphonium in the preparation of the GVNE to realize the purpose of introducing the ethylene on the position 3 of GCLE to prepare for the GVNE. The production method is simple; the cost of the main materials is low; the yield is high; the comprehensive benefit efficiency is high; the present invention is applicable to the large scale industrialized preparation of 7-AVCA.
Owner:SHANDONG JINCHENG PHARMA & CHEM
Method for preparing 2-(1-methylethyl)-5-[(E)-2-phenylethenyl]benzene-1,3-diol
InactiveCN105884581AMild reaction conditionsReduce generationOrganic chemistryOrganic compound preparationSodium methoxideBenzene
The invention provides a method for preparing 2-(1-methylethyl)-5-[(E)-2-phenylethenyl]benzene-1,3-diol, belongs to the technical field of medicine preparation and particularly provides a simple method for preparing 2-(1-methylethyl)-5-[(E)-2-phenylethenyl]benzene-1,3-diol. The method includes the following steps that 4-isopropylbenzaldehyde serves as the starting material, 3,5-dibromo-4-isopropylbenzaldehyde is obtained through 3,5 position bromination, 3,5-dimethoxy-4-isopropylbenzaldehyde is synthesized with sodium methoxide, then 3,5-dyhydroxy-4-isopropylbenzaldehyde is obtained through demethylation with pyridine, 3,5-dialkyl ester-4-isopropylbenzaldehyde is obtained through esterification, 2-(1-methylethyl)-5-[(E)-2-phenylethenyl]benzene-1,3-diol protected by carbethoxy is obtained through wittig reaction, and the target compound, namely 2-(1-methylethyl)-5-[(E)-2-phenylethenyl]benzene-1,3-diol, is obtained through hydrolysis reaction. The method has the advantages that the number of reaction steps is small, reaction conditions are mild and industrialization is easy, and can meet the synthesis requirement of 2-(1-methylethyl)-5-[(E)-2-phenylethenyl]benzene-1,3-diol raw materials.
Owner:SHANGHAI FOSUN PHARMA DEV CO LTD
Method for synthesizing tetradecene alcohol acetic ester in ostrinia nubilalis sex pheromone
InactiveCN101665430AIncrease profitHigh yieldOrganic compound preparationCarboxylic acid esters preparationAlcoholWittig reaction
The invention discloses a method for synthesizing tetradecene alcohol acetic ester in ostrinia nubilalis sex pheromone, which takes ethyltriphenylphosphonium bromide and 1, 12-codlemone as starting materials. The method comprises the following steps: the 1, 12-codlemone is subjected to a unilateral transesterification reaction to obtain 12-hydroxy dodecanol acetic ester, and then subjected to an oxidation reaction to obtain 12-oxo dodecanol acetic ester which is in a Wittig reaction with the ethyltriphenylphosphonium bromide to obtain (Z / E)-12 tetradecenoic alcohol acetic ester of high cis-trans ratio in Asian ostrinia nubilalis sex pheromone. The invention has the advantages of simple and reasonable synthesis route, convenient and safe operation, high usage ratio of raw materials, short synthesis time and the like.
Owner:WENZHOU MEDICAL UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
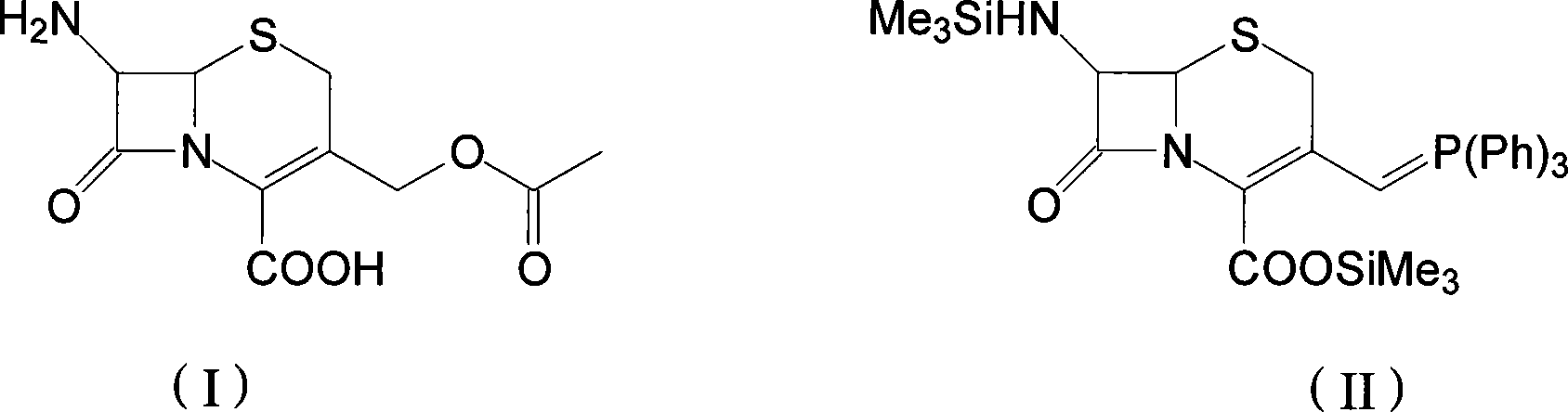
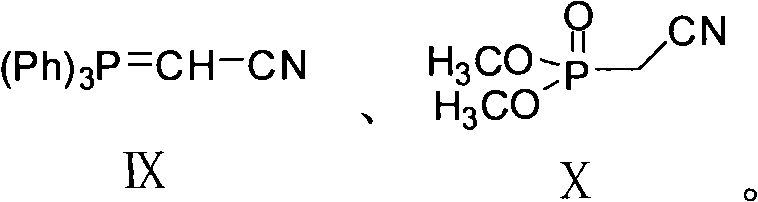
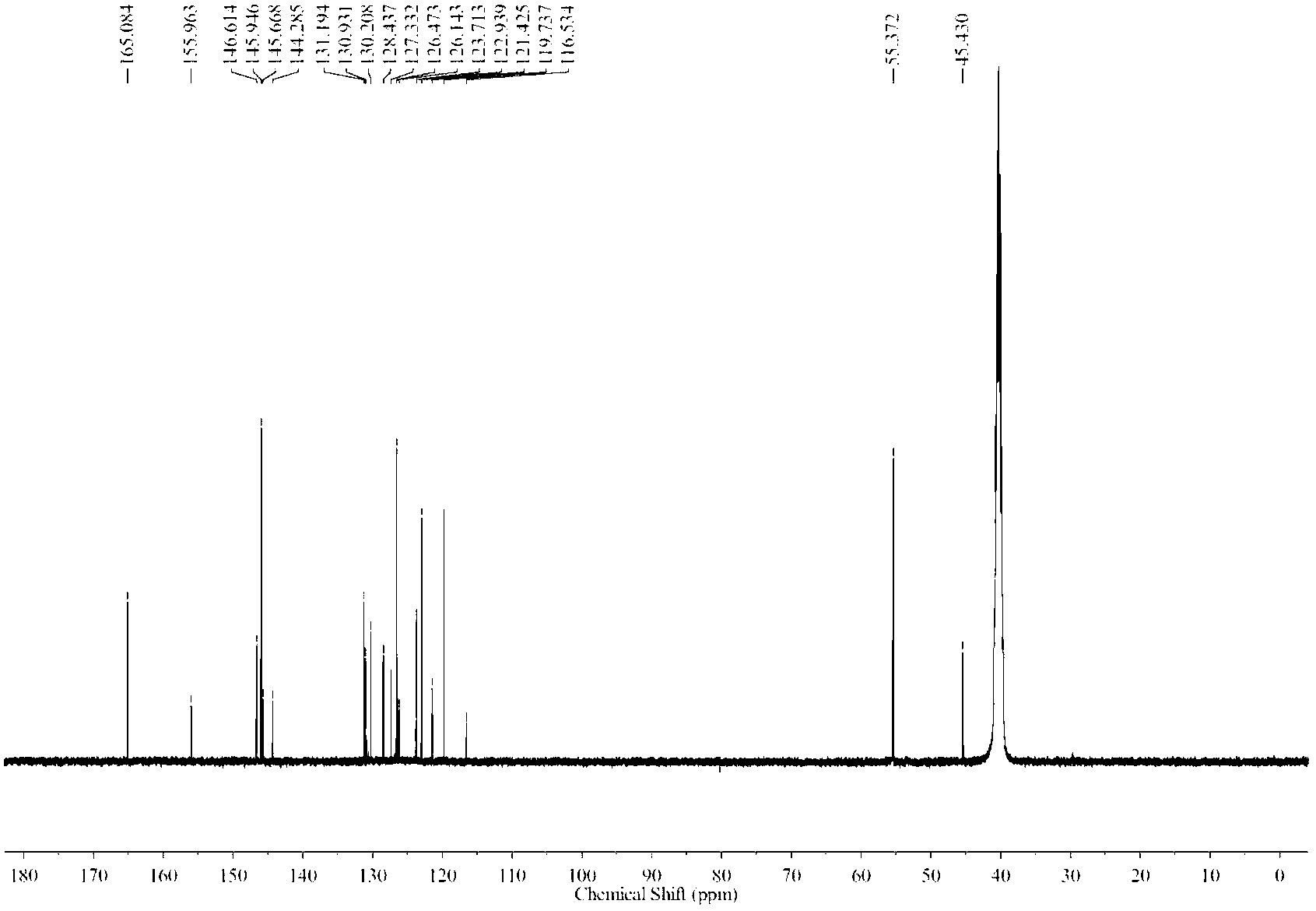
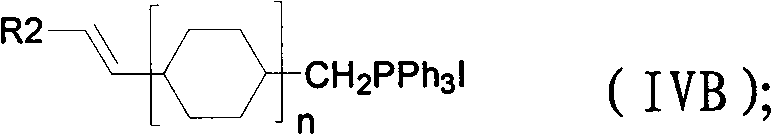
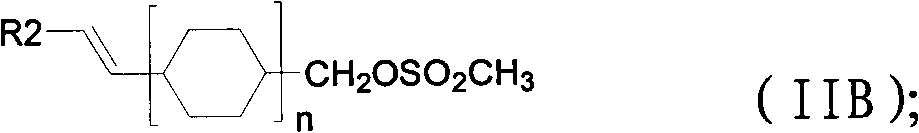
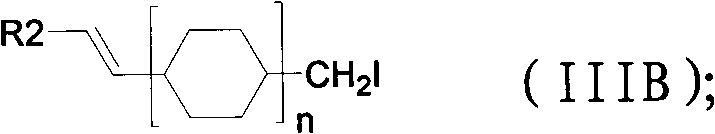
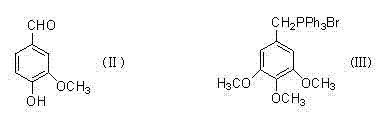

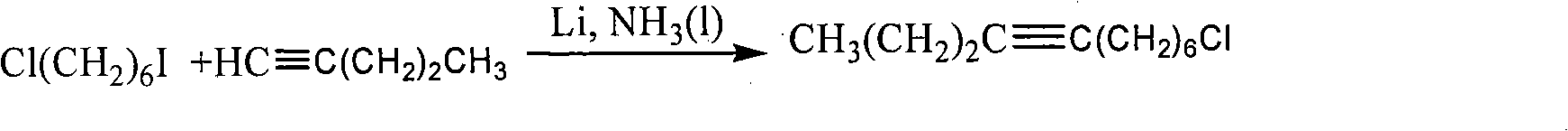
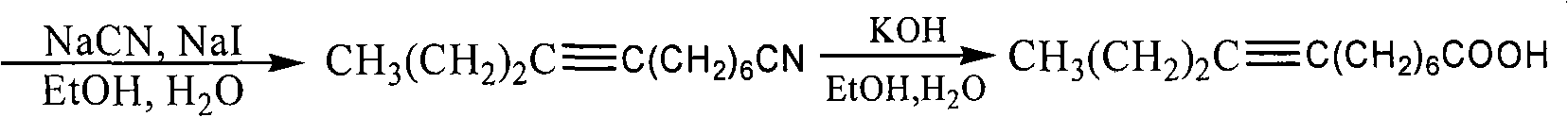

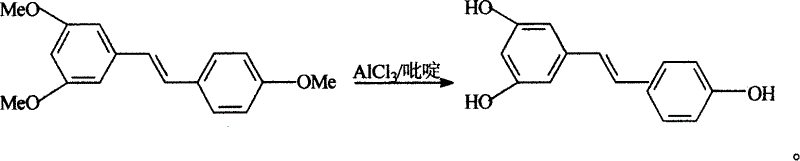
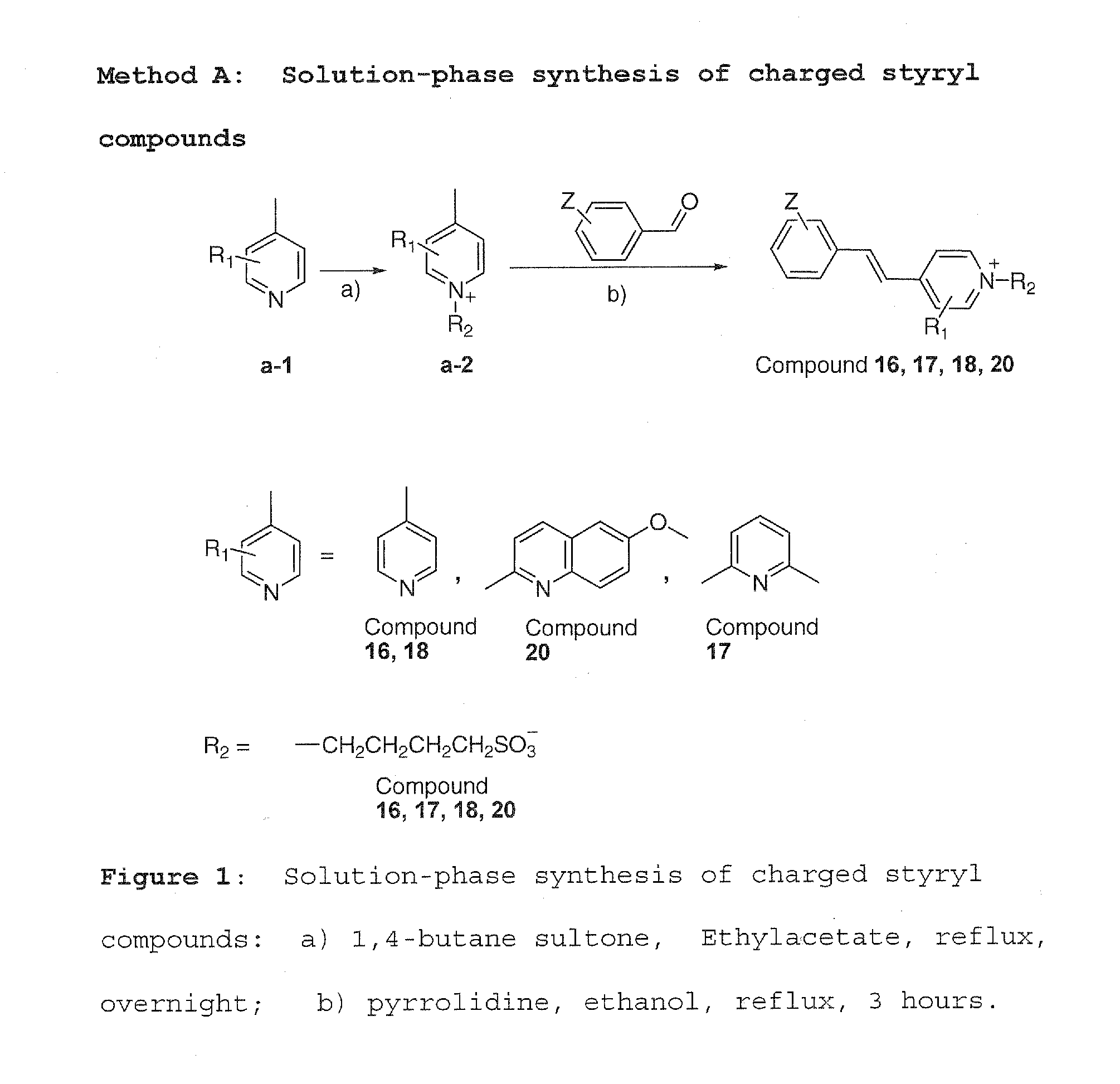
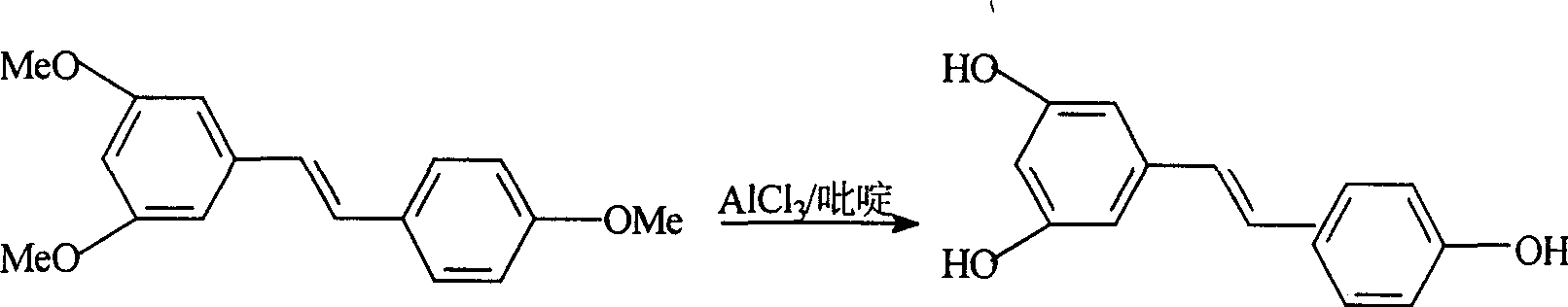
![Method for preparing 2-(1-methylethyl)-5-[(E)-2-phenylethenyl]benzene-1,3-diol Method for preparing 2-(1-methylethyl)-5-[(E)-2-phenylethenyl]benzene-1,3-diol](https://images-eureka.patsnap.com/patent_img/14244be1-4576-4d64-a71b-15753277c61d/BSA0000112408310000011.PNG)
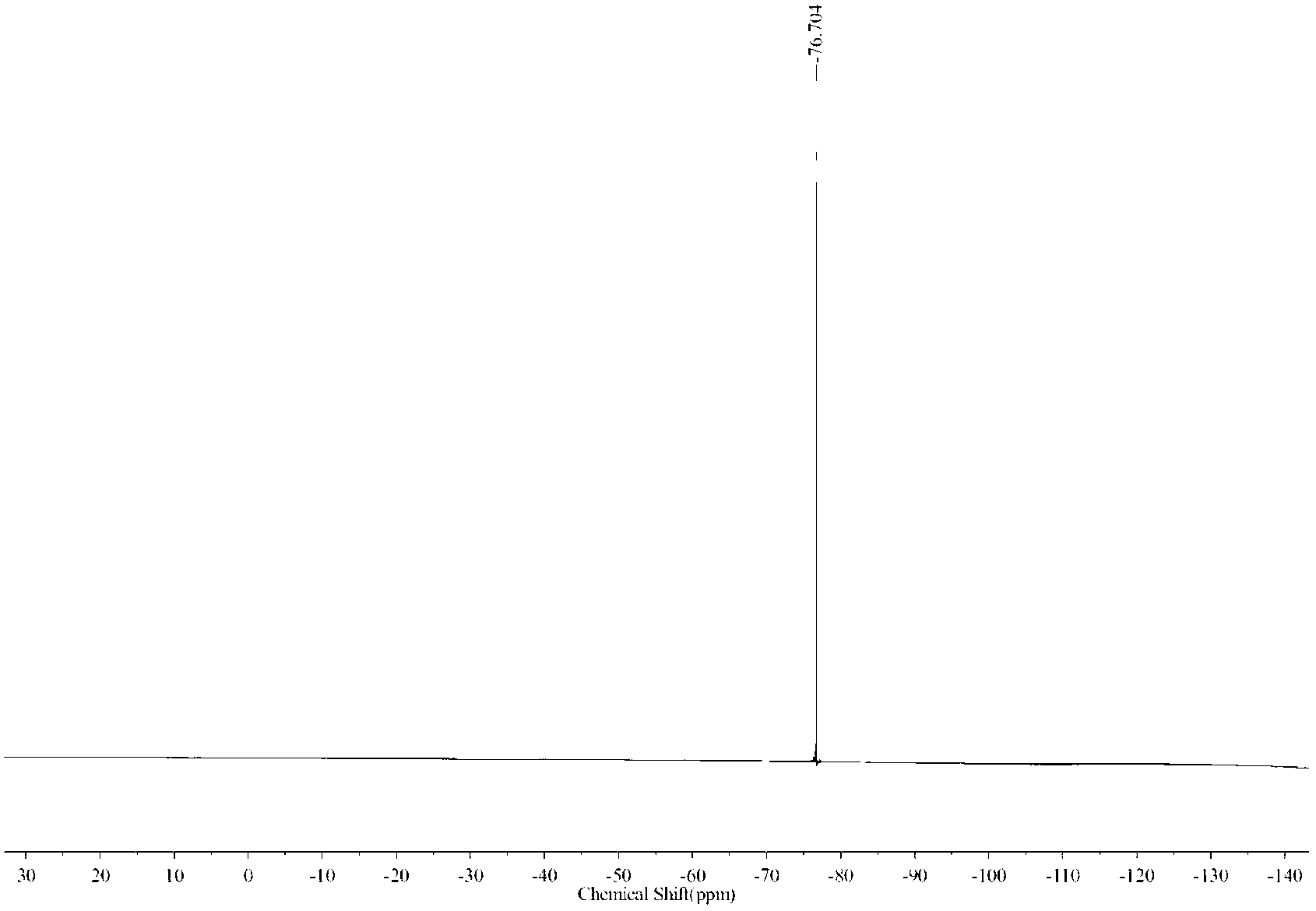
![Method for preparing 2-(1-methylethyl)-5-[(E)-2-phenylethenyl]benzene-1,3-diol Method for preparing 2-(1-methylethyl)-5-[(E)-2-phenylethenyl]benzene-1,3-diol](https://images-eureka.patsnap.com/patent_img/14244be1-4576-4d64-a71b-15753277c61d/BSA0000112408310000021.PNG)
![Method for preparing 2-(1-methylethyl)-5-[(E)-2-phenylethenyl]benzene-1,3-diol Method for preparing 2-(1-methylethyl)-5-[(E)-2-phenylethenyl]benzene-1,3-diol](https://images-eureka.patsnap.com/patent_img/14244be1-4576-4d64-a71b-15753277c61d/BSA0000112408310000031.PNG)