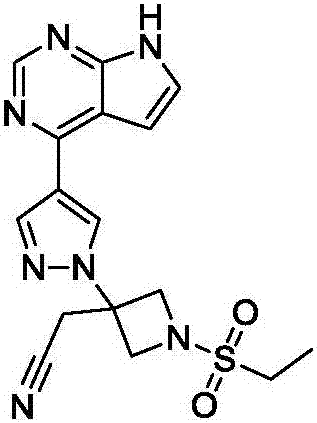
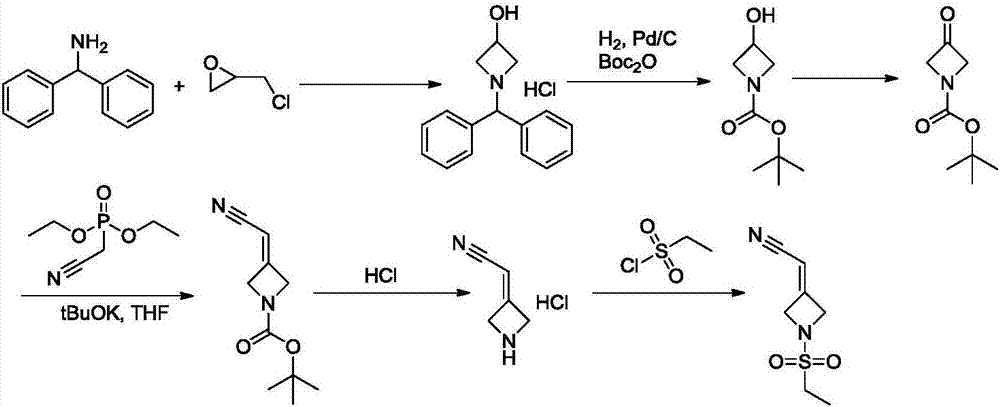
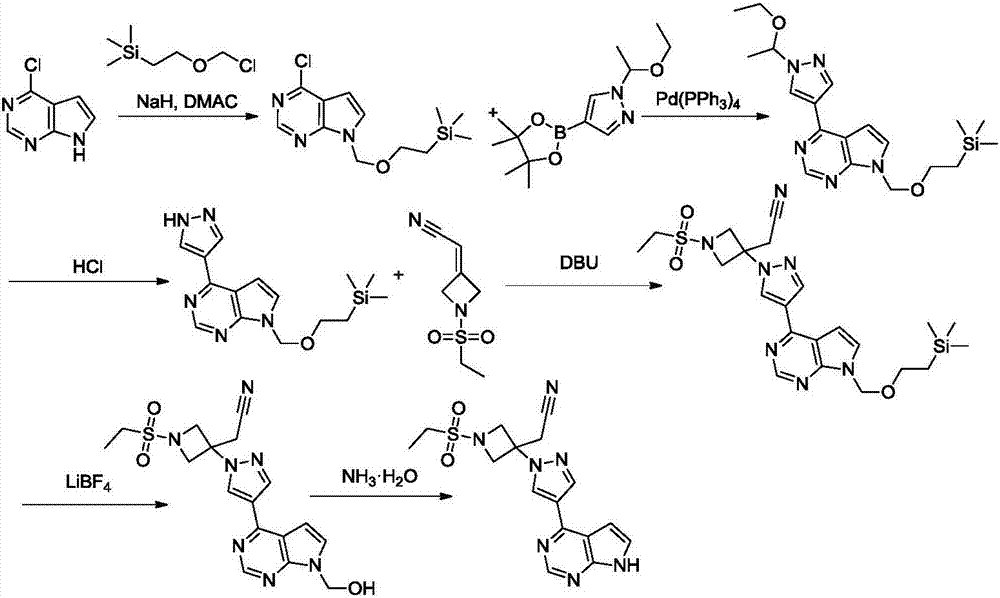
New synthesis methods of JCK inhibitor baricitinib and intermediate thereof
A technology of baricitinib and synthetic methods, applied in the field of medicine and chemical industry, can solve the problems of expensive 3-hydroxyazetidine starting raw materials, unsatisfactory product yield and purity, poor stability of acetonitrile, etc., and achieve improvement The effect of route efficiency and atom economy, simplified separation and purification process, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: N-(3-chloro-2-hydroxypropyl)ethanesulfonamide
[0048]
[0049] Add 1-amino-3-chloropropyl-2-ol hydrochloride (14.60g, 100mmol), tetrahydrofuran (73mL), water (73mL) into a three-necked flask, stir to dissolve and cool to 0-5°C, add hydrogen phosphate Dipotassium (34.84g, 200mmol), after stirring for 5 minutes, add ethylsulfonyl chloride (13.50g, 105mmol) dropwise. After the addition, warm up to room temperature and react for 3-4 hours. After the reaction, add 73mL of 0.5mol / L dilute hydrochloric acid to quench reaction, stirred and separated, the aqueous phase was extracted twice with ethyl acetate 35mL, the combined organic phase was washed once with saturated brine (73mL), dried over anhydrous sodium sulfate, and concentrated to obtain N-(3-chloro-2-hydroxypropane Base) The crude product of ethanesulfonamide was directly put into the next reaction (GC purity about 92%).
[0050] ESI m / z=202.1(M+H) + , 1 H NMR (400MHz, CDCl 3 )δ5.10-4.95(m,1H),4.00-3...
Embodiment 2
[0052] Example 2: 1-(Ethylsulfonyl)azepin-3-ol
[0053]
[0054] Add N-(3-chloro-2-hydroxypropyl)ethanesulfonamide (prepared from Example 1) and N,N-dimethylformamide (100 mL) into a three-necked flask, stir to dissolve, cool to 0-5°C, Add potassium tert-butoxide (11.22g, 100mmol), keep stirring at 0-5°C for 15-20 minutes after the addition is complete, then raise the temperature to room temperature and react for 3-4 hours, add 1mol / L dilute hydrochloric acid 100mL to quench the reaction, stir and separate, The aqueous phase was extracted twice with 50 mL of ethyl acetate, the combined organic phase was washed once with saturated brine (100 mL), dried over anhydrous sodium sulfate, and concentrated to obtain crude 1-(ethylsulfonyl)azidine-3-ol, which was directly Submit the next reaction (GC purity about 86%). ESI m / z=166.3(M+H) + .
[0055] In Example 2, the alkaline substance potassium tert-butoxide can be sodium hydride, sodium tert-butoxide, lithium diisopropylamide,...
Embodiment 3
[0056] Example 3: 1-(Ethylsulfonyl)azepin-3-one
[0057]
[0058] Add 1-(ethylsulfonyl)azepin-3-ol (prepared by Example 2), sodium bromide (10.29g, 100mmol), sodium bicarbonate (12.6g, 150mmol), dichloromethane in the three-necked flask (83mL) and water (123mL), stirred evenly and cooled to -5~0℃, added TEMPO (312mg, 2mmol), added dropwise 10% sodium hypochlorite solution (81.9g, 110mmol), after the reaction was completed, the water The phase was extracted with dichloromethane (83mL) once more, the combined organic phase was washed once with sodium bisulfite solution (5%, 42mL), washed once with saturated brine (83mL), dried over sodium sulfate, and concentrated to give 1-( The crude ethylsulfonyl)azepin-3-one (GC purity about 83%) was directly put into the next reaction. ESI m / z=164.0(M+H) +
[0059] Additive sodium bromide and sodium bicarbonate can be replaced by one or more combinations of triethylamine, diisopropylethylamine, sodium acetate, sodium bicarbonate, sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com