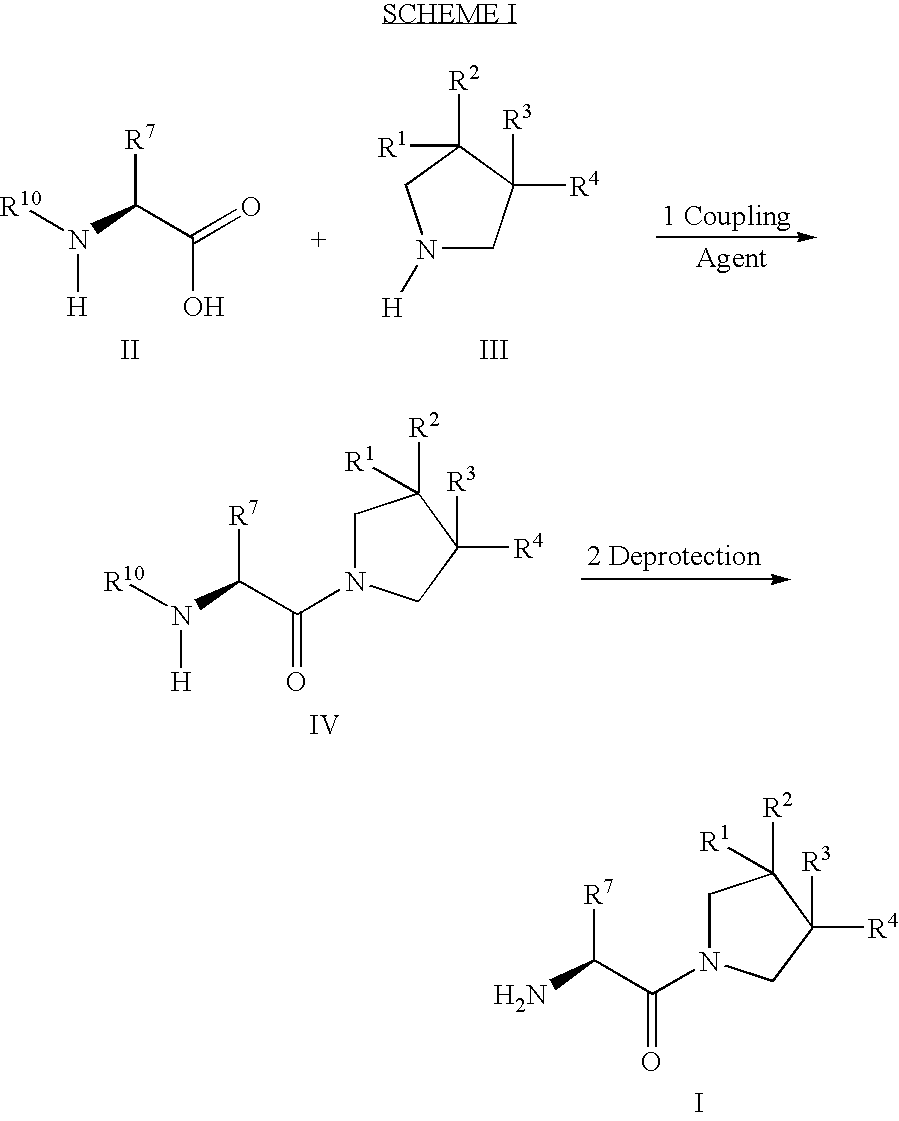
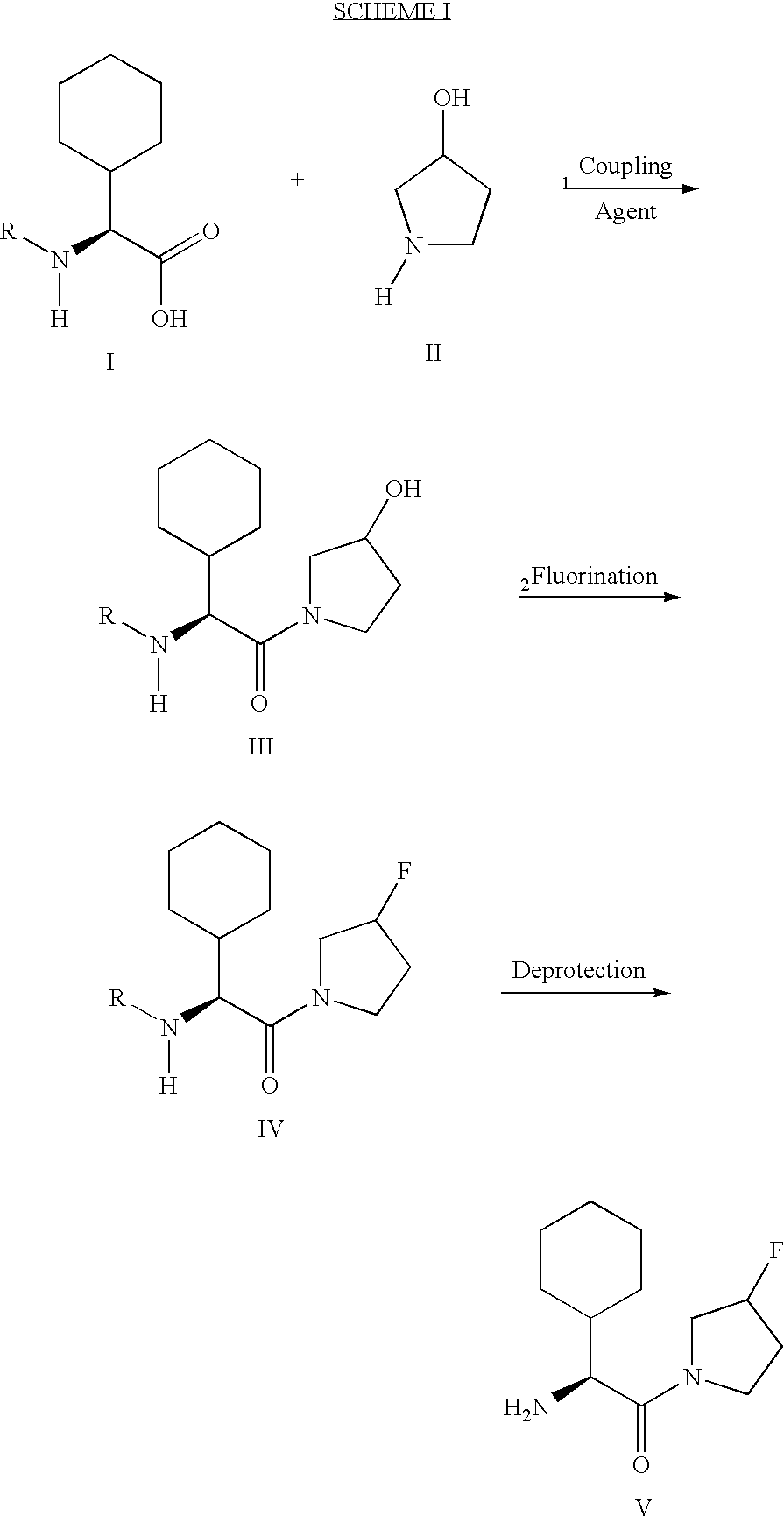
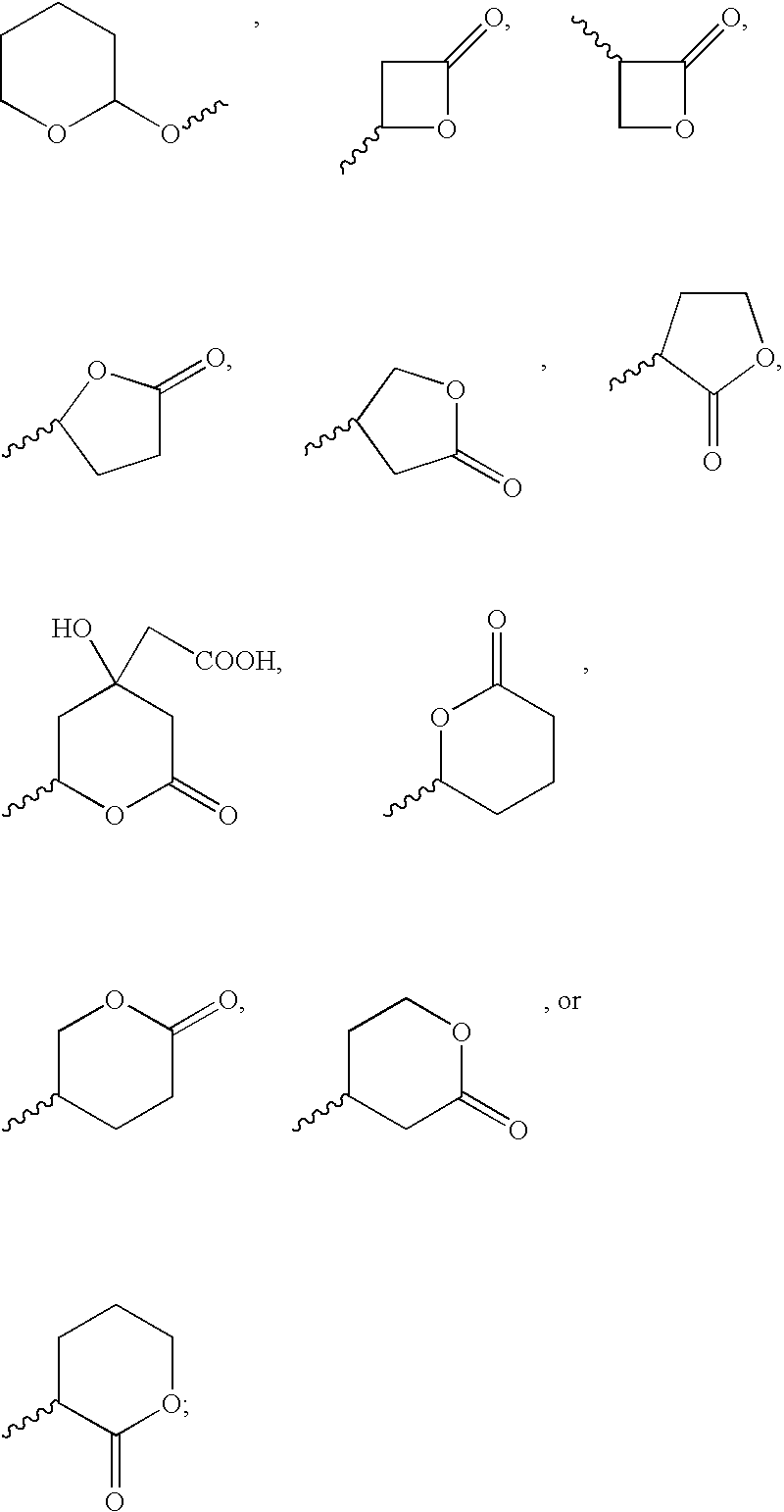
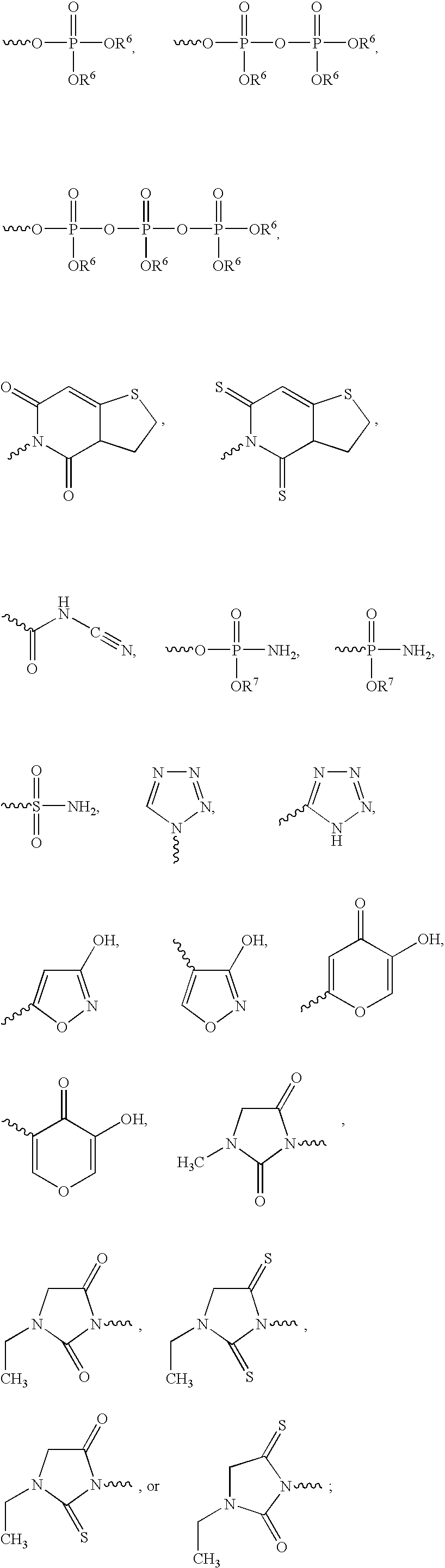
Patents
Literature
818 results about "Diabetic nephropathy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A serious kidney-related complication of type 1 diabetes and type 2 diabetes.
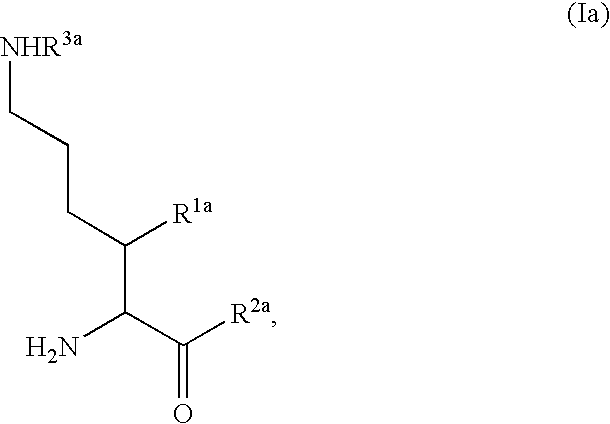
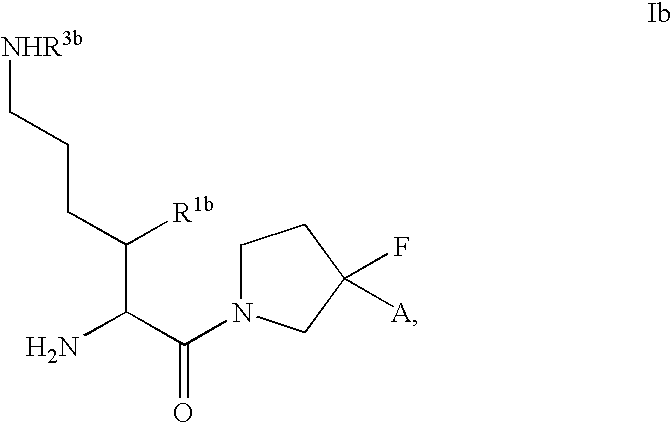
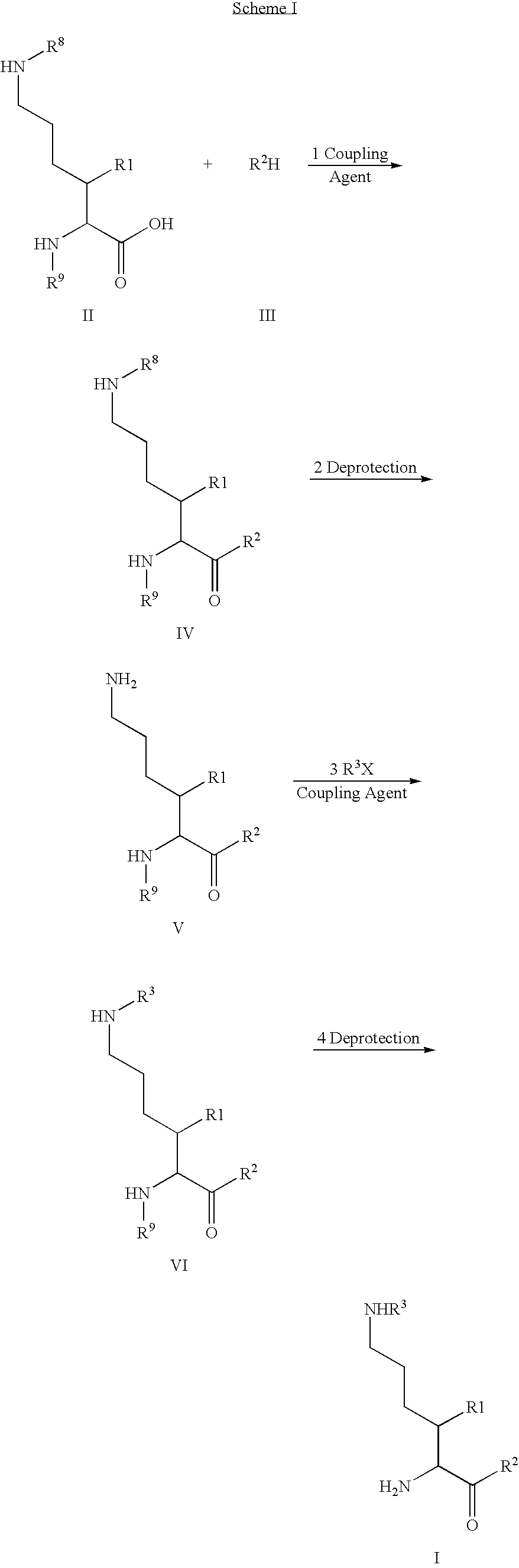
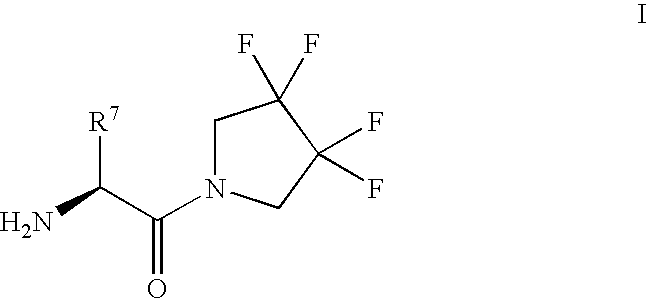
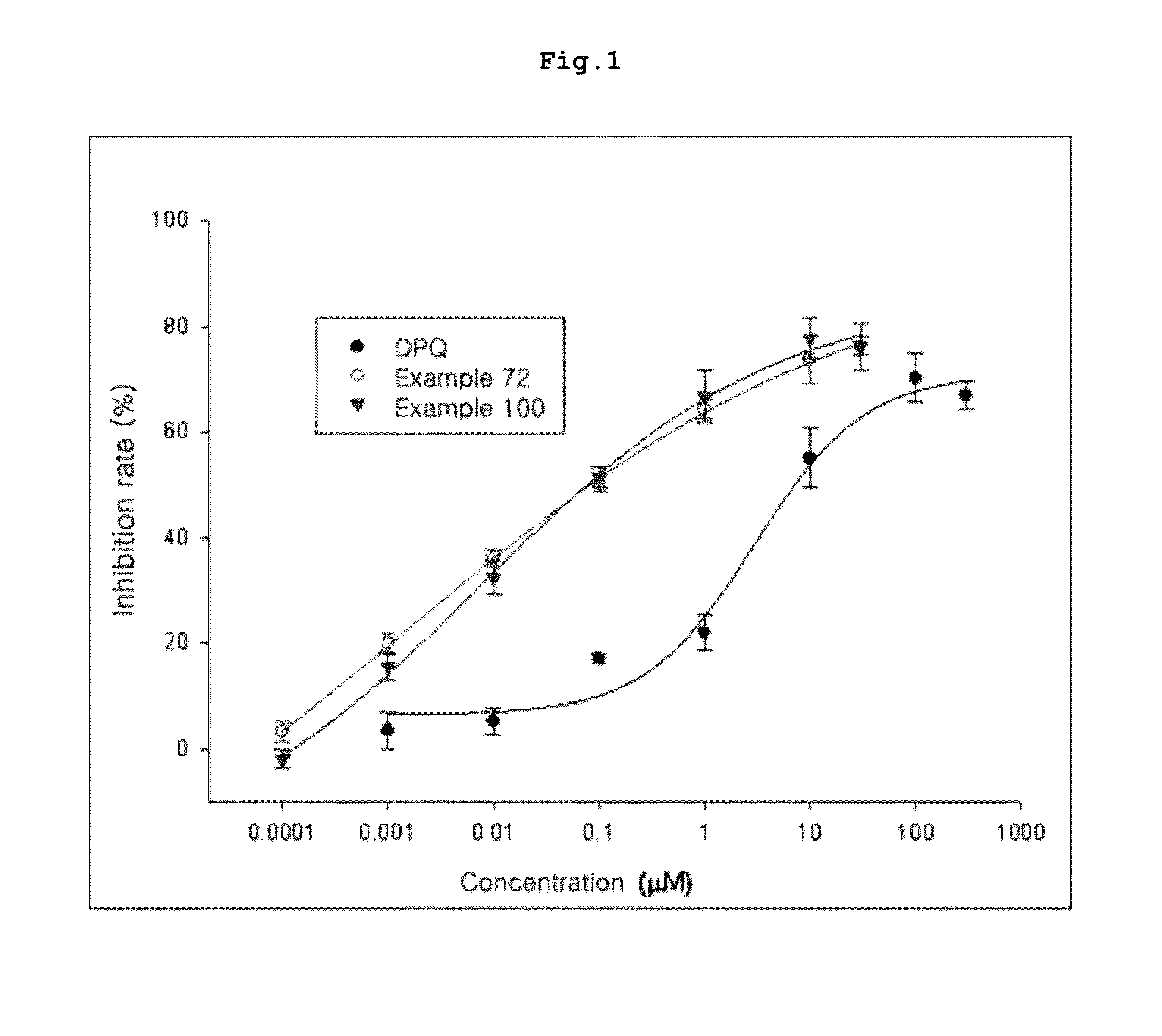
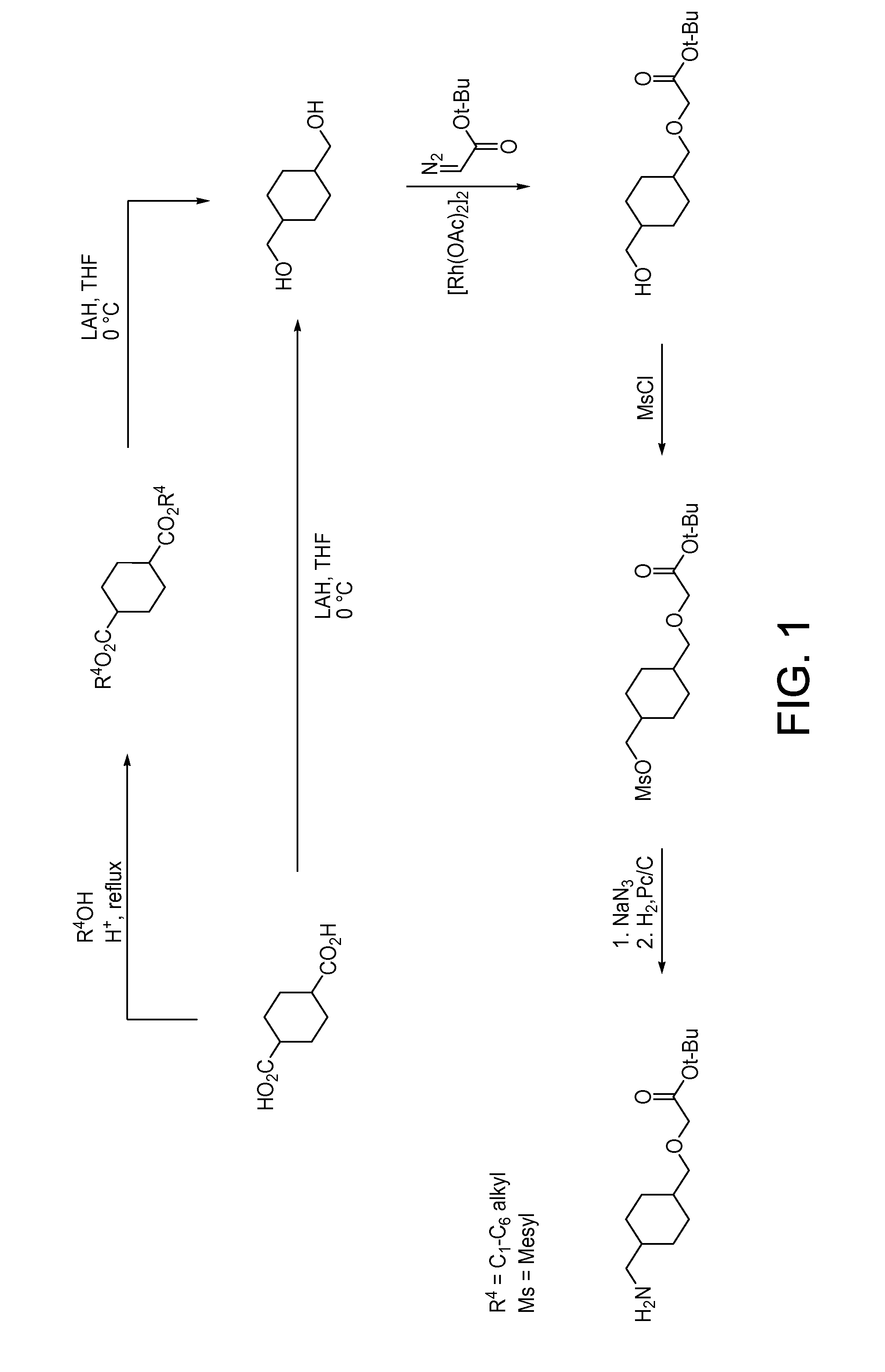
Fluorinated lysine derivatives as dipeptidyl peptidase IV inhibitors
InactiveUS20050043292A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDiabetic retinopathyArthritis
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV (“DPP-IV”), pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes, metabolic syndrome (syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, arthritis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
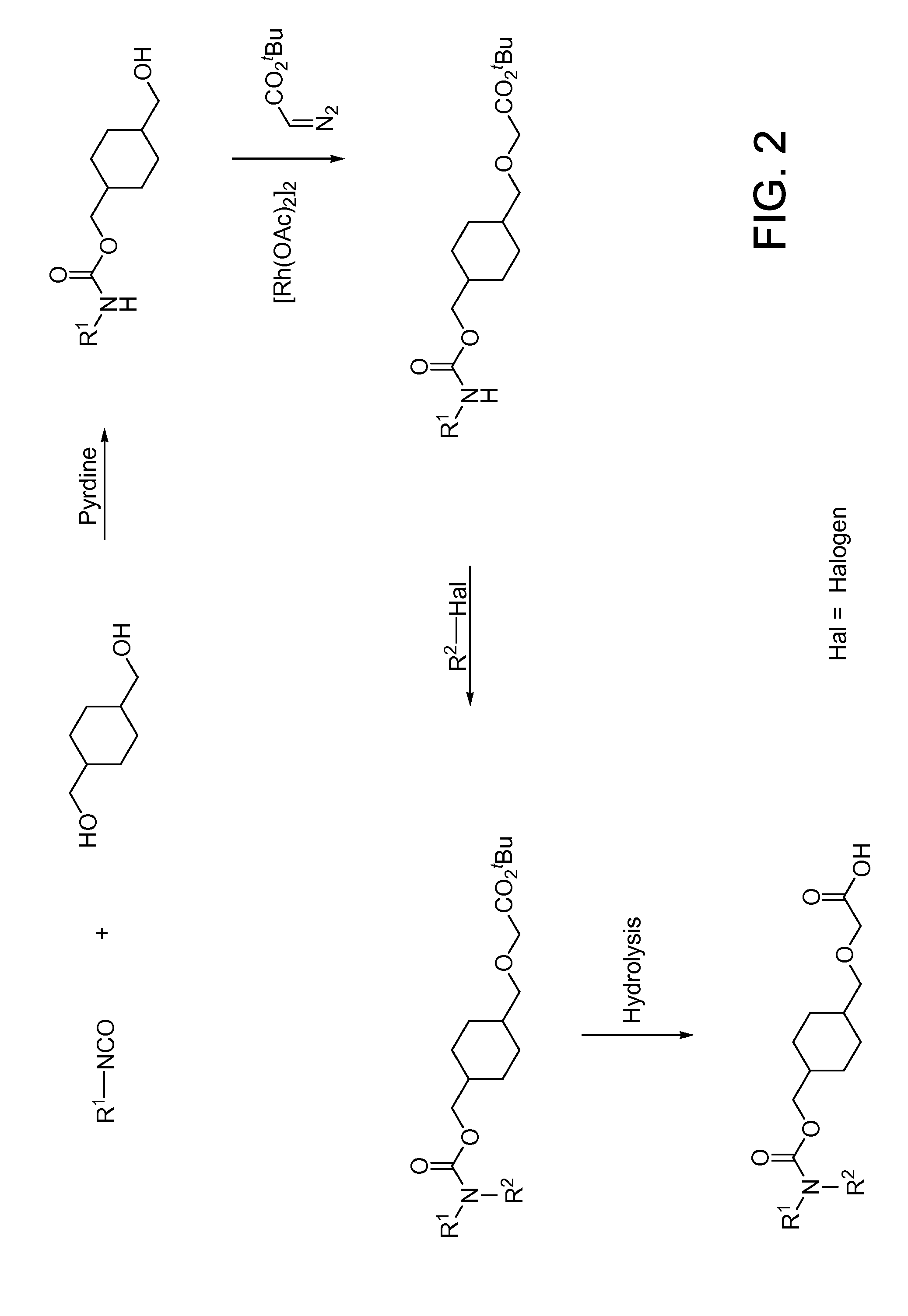
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS6710040B1Easy to prepareEase of detectabilityBiocideOrganic chemistryAcute coronary syndromeDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
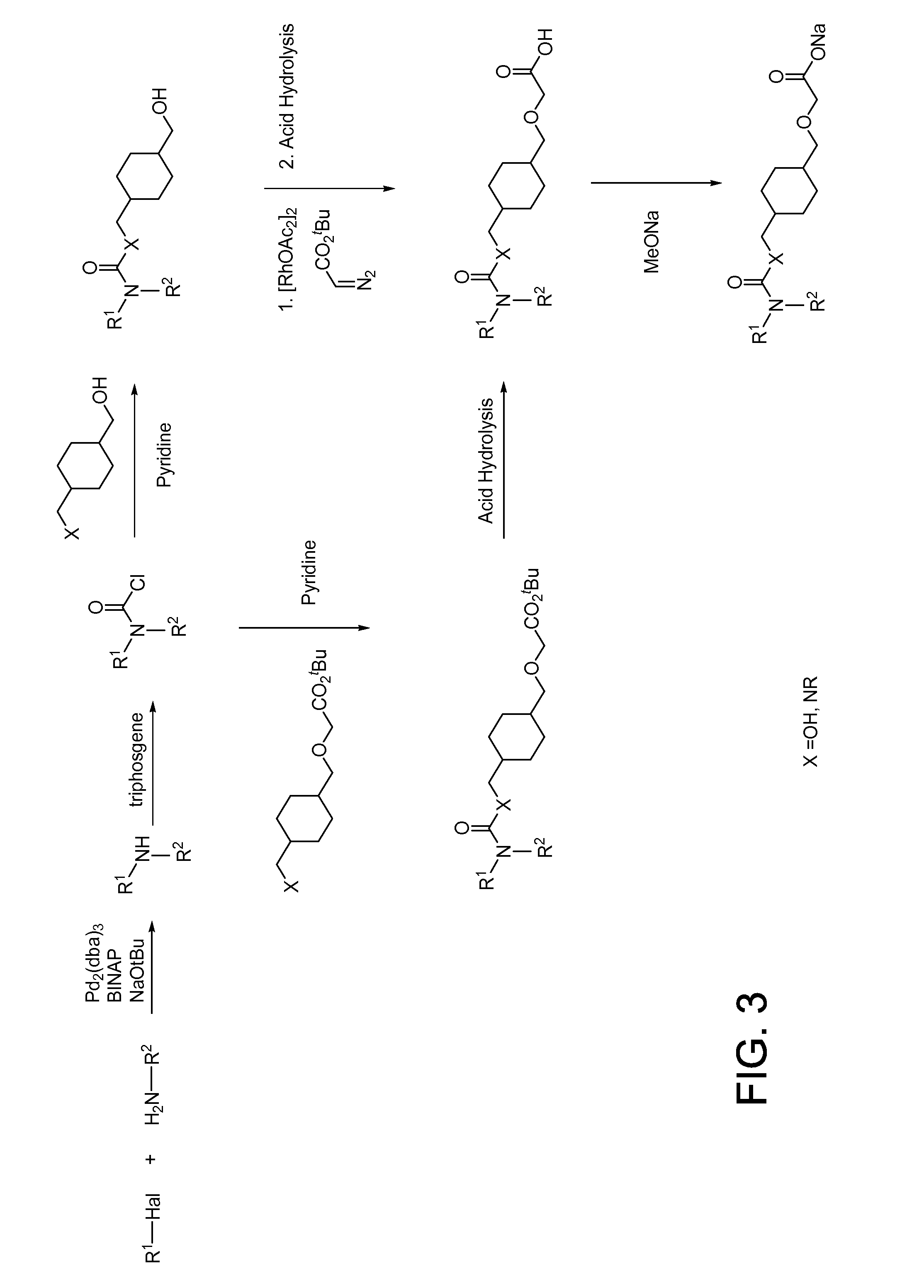
Dipeptidyl peptidase IV inhibiting fluorinated cyclic amides
InactiveUS20040110817A1Ease of preparation and detectabilityGood metabolic stabilityBiocideSenses disorderDiabetic retinopathyDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Morphinan-derivatives for treating diabetes and related disorders
ActiveUS20150087669A1Risk of developingDelay progressBiocideMetabolism disorderDiseaseNR1 NMDA receptor
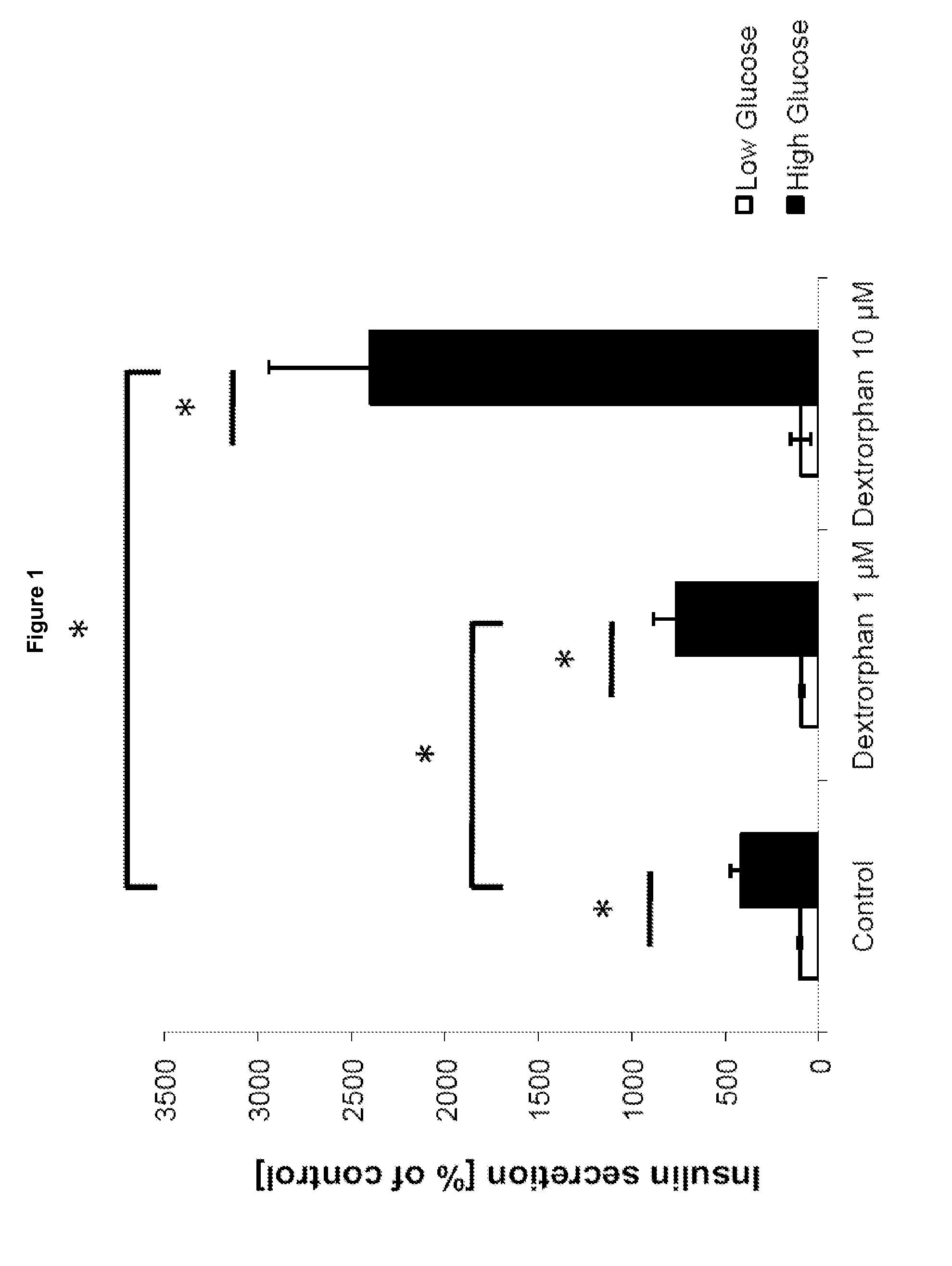
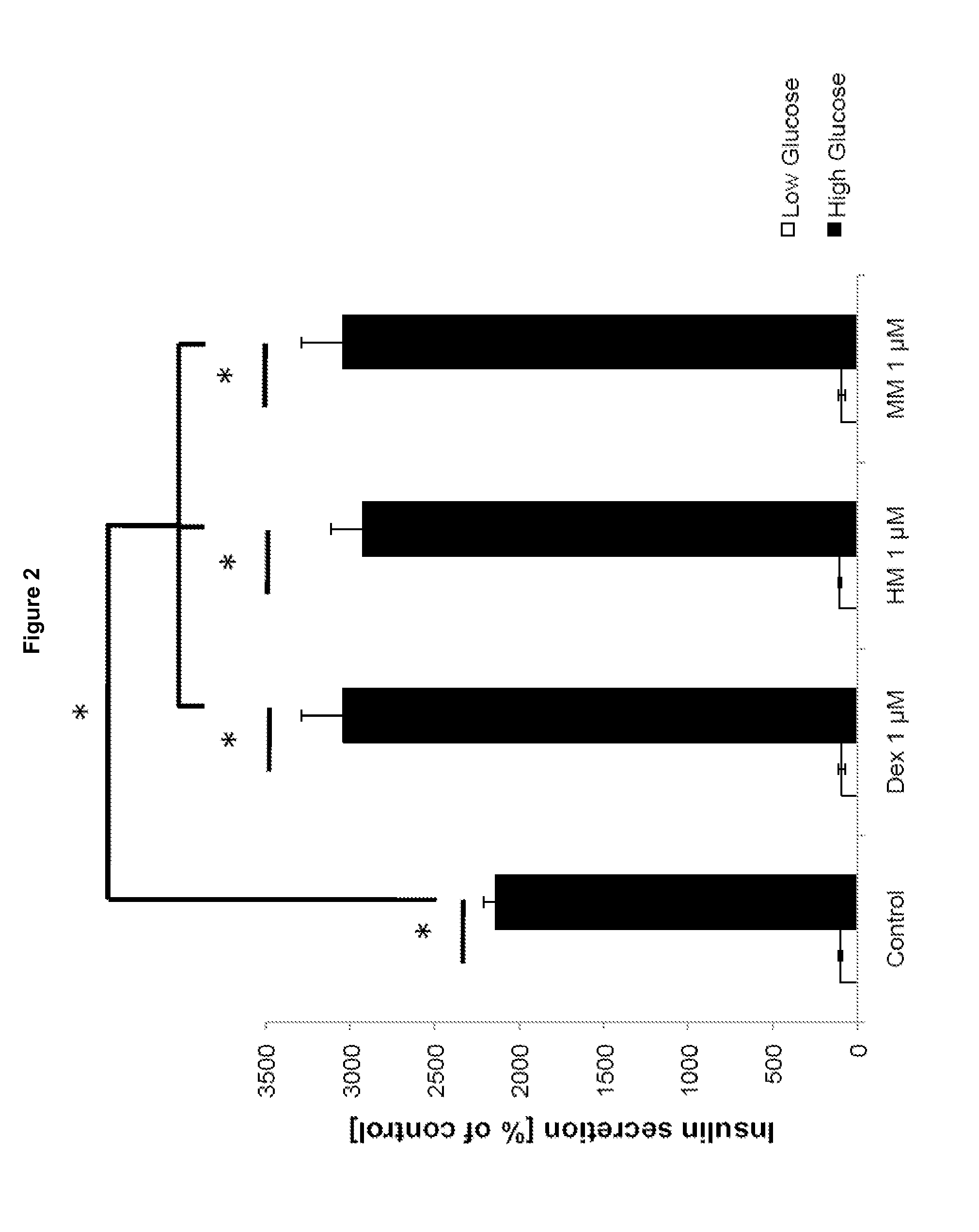
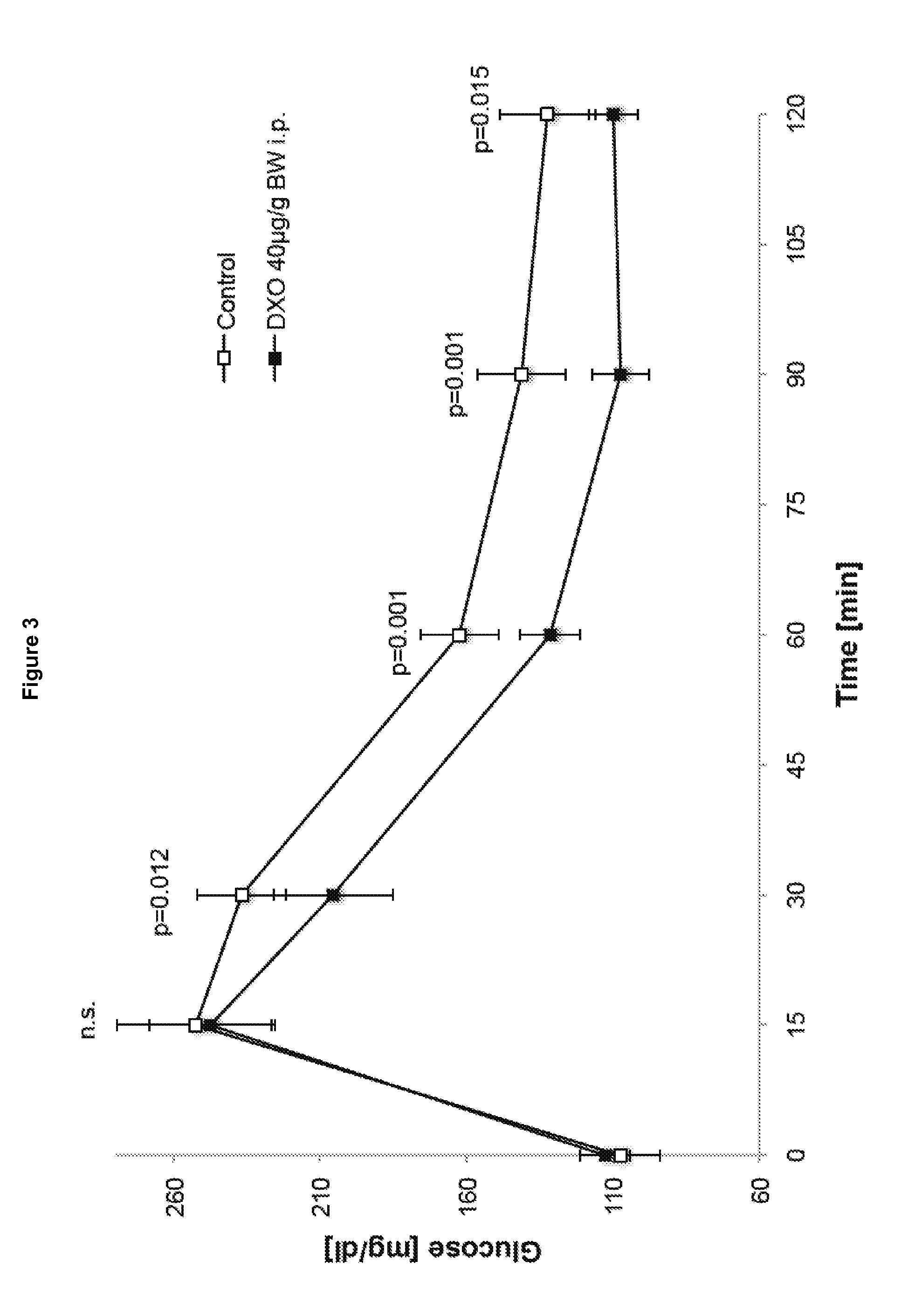
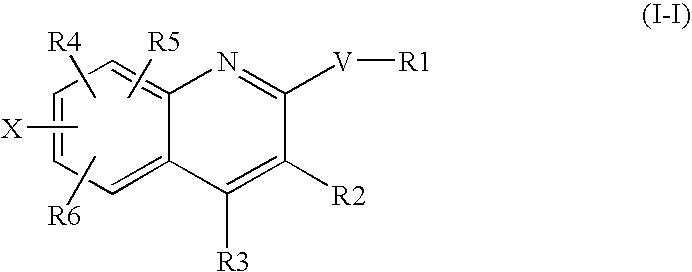
The invention relates to a morphinan-derivative that targets NMDA receptors on pancreatic islets and has the general formula (I)whereinR1 is selected from —OH, —CO2H, —R0, —OR0, —OC(═O)R0, —OC(═O)OR0 or —OC(═O)NHR0; and R2 is selected from —H, —R0, —C(═O)R0, —C(═O)OR0, —C(═O)NHR0 or —C(═NH)—NH—C(═NH)—NH2; wherein R0 is in each case independently selected from —C1-C6-alkyl, -aryl, -heteroaryl, —C1-C6-alkyl-aryl or —C1-C6-alkyl-heteroaryl, in each case independently unsubstituted or substituted;or its physiologically acceptable salt and / or stereoisomer, including mixtures thereof in all ratios, for use in the treatment of a disease or condition, where the disease or condition is insulin-dependent diabetes mellitus, non-insulin-dependent diabetes mellitus, obesity, and / or diabetic nephropathy.
Owner:DEUTE DIABETES FORSCHUNGSGES
Selective androgen receptor modulators for treating diabetes
InactiveUS20070281906A1Reduce severityReduce morbidityBiocideSenses disorderSelective androgen receptor modulatorDiabetes retinopathy
This invention provides use of a SARM compound or a composition comprising the same in treating a variety of diseases or conditions in a subject, including, inter-alia, a diabetes disease, and / or disorder such as cardiovascular disease, atherosclerosis, cerebrovascular conditions, diabetic nephropathy, diabetic neuropathy and diabetic retinopathy.
Owner:UNIV OF TENNESSEE RES FOUND
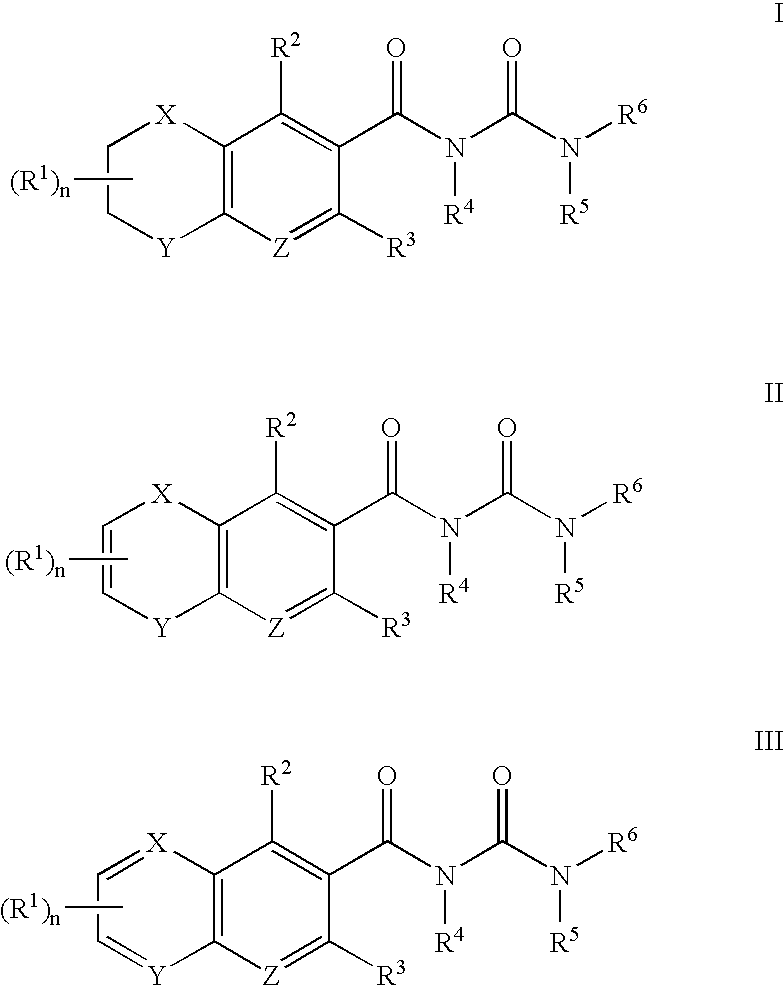
Therapeutic compounds
InactiveUS20050256310A1High plasma concentrationHigh activityOrganic active ingredientsSenses disorderMedicineDiabetic complication
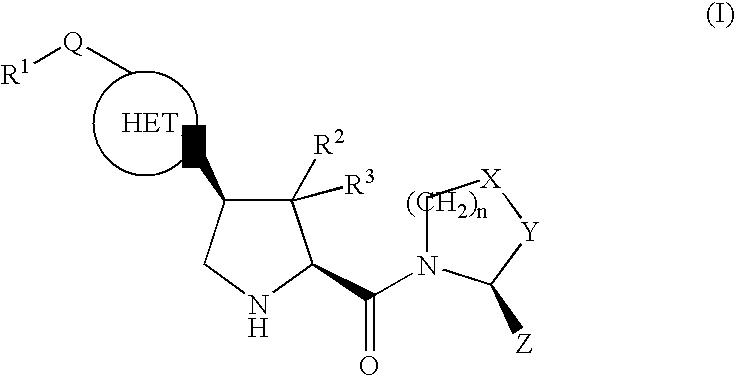
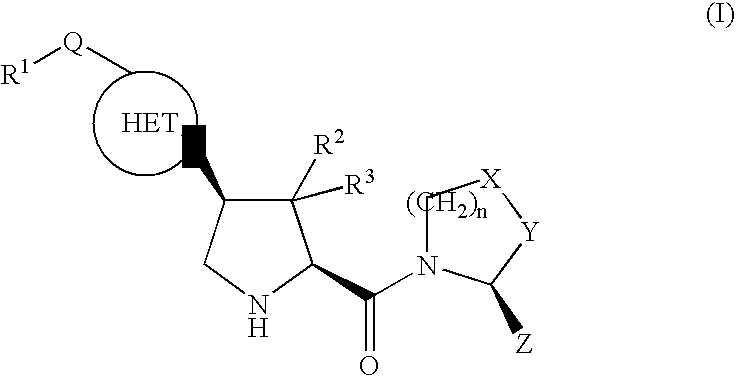
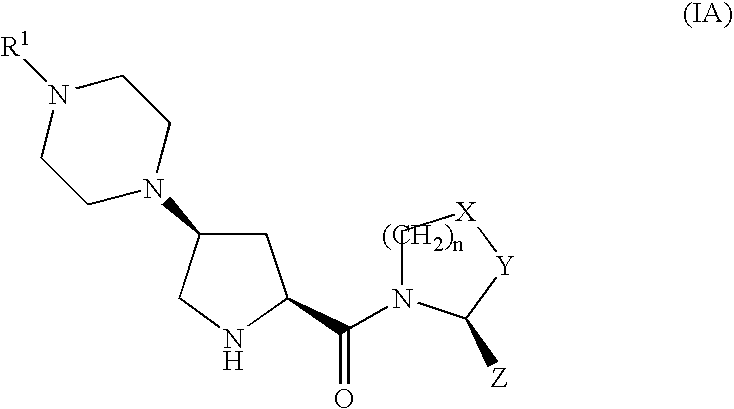
The invention provides compounds of formula (I), prodrugs and stereoisomers thereof, and the pharmaceutically acceptable salts of the compounds, prodrugs, and stereoisomers, wherein R1, R2, R3, HET, n, Q, X, Y, and Z are as described herein; compositions thereof; and uses thereof in treating diabetic complications including diabetic neuropathy, diabetic nephropathy, diabetic microangiopathy, and the like.
Owner:PFIZER INC
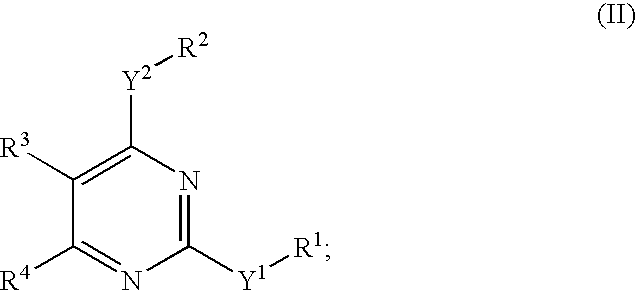
Novel pyrimidine compounds, process for their preparation and compositions containing them
InactiveUS20060084645A1Inhibit inflammationInhibition is effectiveBiocideOrganic chemistryDyslipidemiaNephrosis
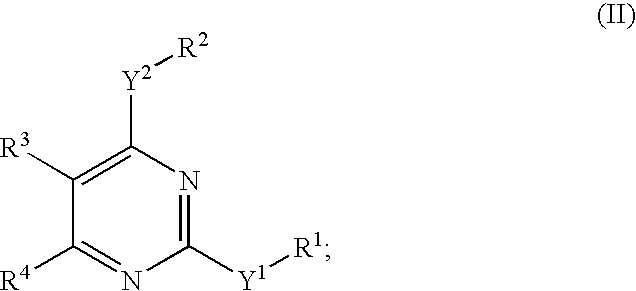
The present invention provides new heterocyclic compounds, particularly substituted pyrimidines, methods and compositions for making and using these heterocyclic compounds, and methods for treating a variety of diseases and disease states, including atherosclerosis, arthritis, restenosis, diabetic nephropathy, or dyslipidemia, or disease states mediated by the low expression of Perlecan.
Owner:DR REDDYS LAB LTD
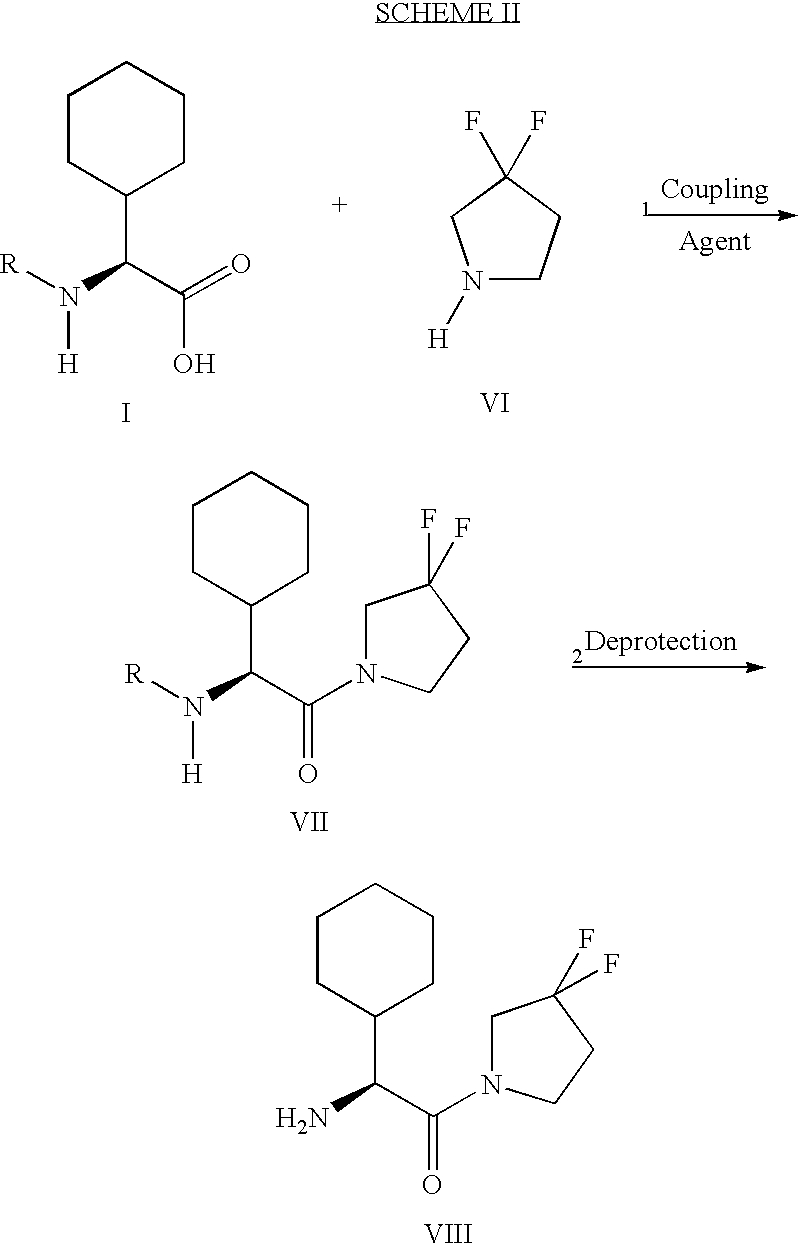
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
The present invention relates to a method of making novel dipeptidyl peptidase-IV ("DPP-IV') inhibitor compounds useful for treating, inter alia, diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, metabolic syndrome (Syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of making 3,3,4,4-tetrafluoropyrrolidine, a starting material utilized in the afore-mentioned method for preparing DPP-IV compounds.
Owner:PFIZER INC
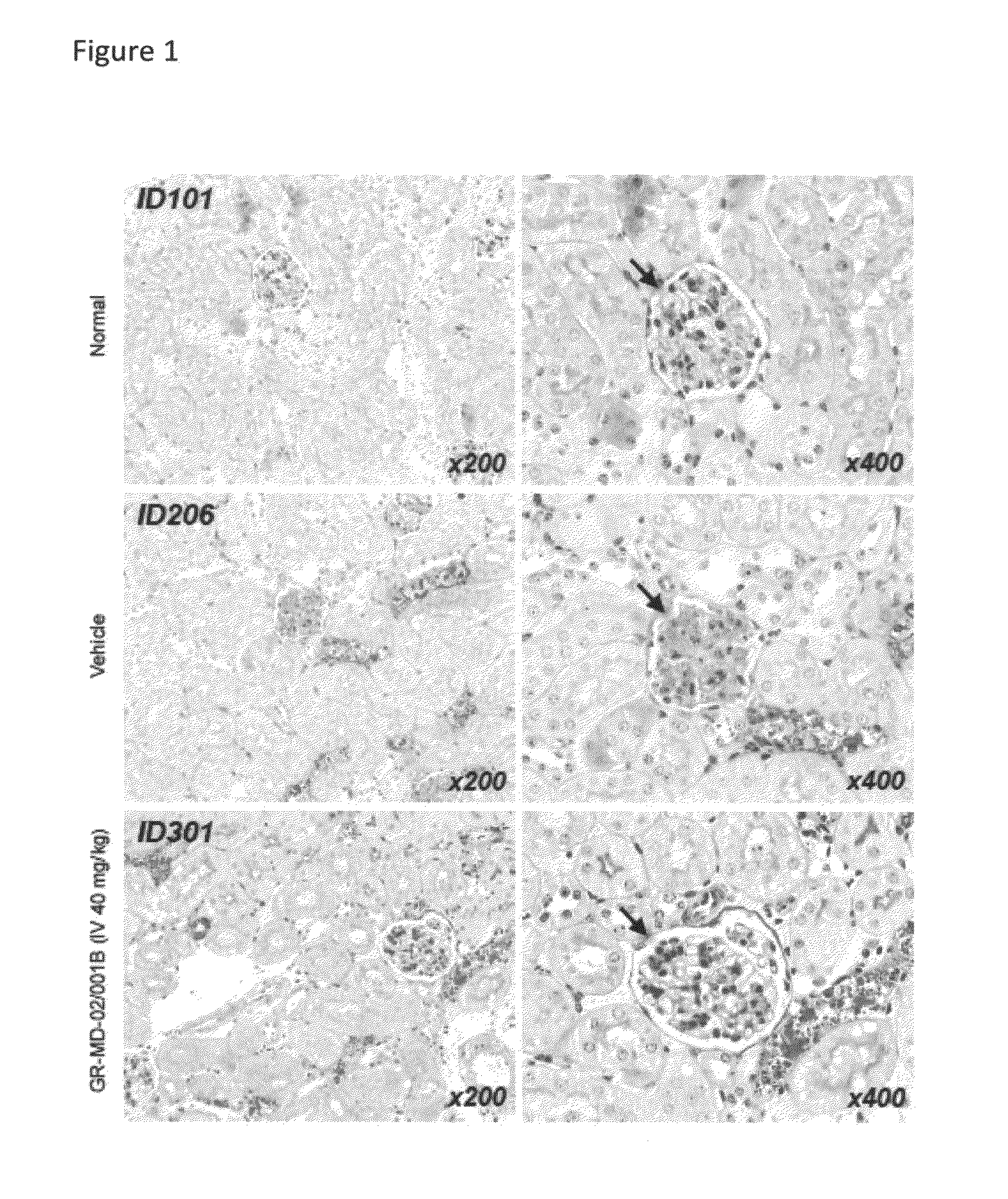
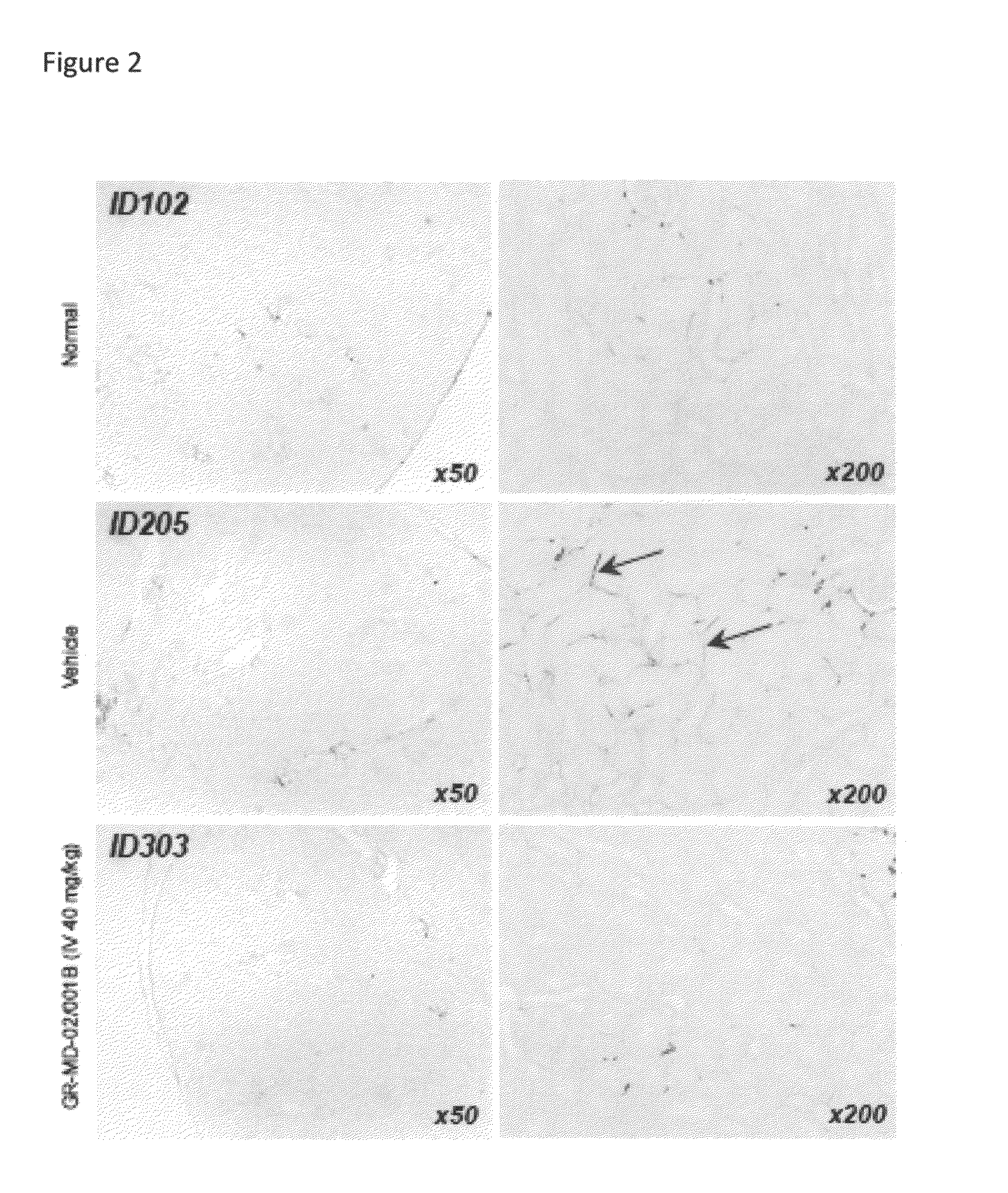
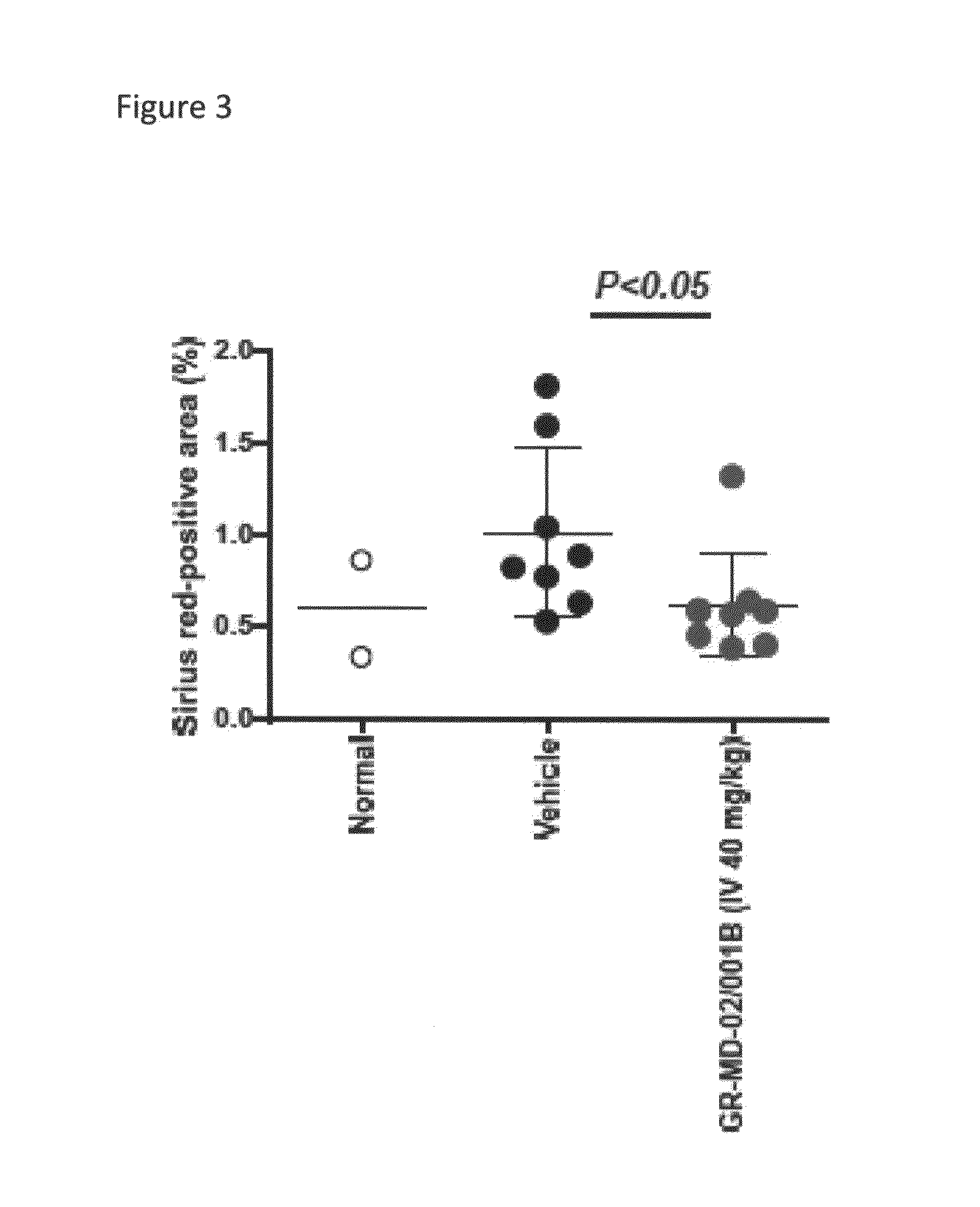
Galactose-pronged carbohydrate compounds for the treatment of diabetic nephropathy and associated disorders
Methods and compositions of the invention relate to the treatment of diabetic nephropathy and associated disorders. In particular, the methods and compositions use a pharmaceutical-grade galactose-pronged carbohydrate or pharmaceutical compositions thereof alone or in combination with other therapeutic agents.
Owner:GALECTIN THERAPEUTICS
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
InactiveUS6812350B2Metabolism disorderPhosphorus organic compoundsDisease progressionDiabetic nephropathy
Owner:PFIZER INC
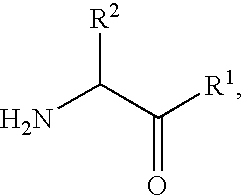
Antidiabetic agents
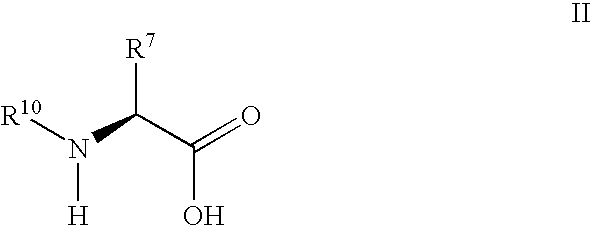
A compound of the formula wherein R1 is: R5 is: and n, m, Z, R8, R9, R10 and R11 are as defined herein, useful in the treatment of diabetes, insulin resistance, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, cataracts, hyperglycemia, hypercholesterolemia, hypertension, hyperinsulinemia, hyperlipidemia, atherosclerosis, and tissue ischemia, particularly, myocardial ischemia.
Owner:GAMMILL RONALD B
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
InactiveUS20040002609A1Easy to cutMetabolism disorderPhosphorus organic compoundsDisease progressionDisease cause
The present invention relates to a method of making novel dipeptidyl peptidase-IV ("DPP-IV') inhibitor compounds useful for treating, inter alia, diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, metabolic syndrome (Syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of making 3,3,4,4-tetrafluoropyrrolidine, a starting material utilized in the afore-mentioned method for preparing DPP-IV compounds.
Owner:PFIZER INC
Benzene compounds
InactiveUS20070105899A1Preventing, treating, and arresting the development of these diseasesExcellent ACC inhibiting activityBiocideSenses disorderDiabetic retinopathyDiabetic complication
The present invention provides novel benzene compounds presented by the following formulas, and analogs thereof, that exert an ACC activity-inhibiting effect that is effective in the treatment of obesity, hyperlipemia, fatty liver, hyperglycemia, impaired glucose tolerance, diabetes, diabetic complications (diabetic peripheral neuropathy, diabetic nephropathy, diabetic retinopathy, and diabetic macroangiopathy, hypertension, arteriosclerosis), hypertension, and arteriosclerosis.
Owner:AJINOMOTO CO INC
Dihydroxyl compounds and compositions for cholesterol management and related uses
The present invention relates to novel dihydroxyl compounds, compositions comprising hydroxyl compounds, and methods useful for treating and preventing a variety of diseases and conditions such as, but not limited to aging, Alzheimer's Disease, cancer, cardiovascular disease, diabetic nephropathy, diabetic retinopathy, a disorder of glucose metabolism, dyslipidemia, dyslipoproteinemia, hypertension, impotence, inflammation, insulin resistance, lipid elimination in bile, obesity, oxysterol elimination in bile, pancreatitis, Parkinson's disease, a peroxisome proliferator activated receptor-associated disorder, phospholipid elimination in bile, renal disease, septicemia, metabolic syndrome disorders (e.g., Syndrome X), thrombotic disorder. Compounds and methods of the invention can also be used to modulate C reactive protein or enhance bile production in a patient. In certain embodiments, the compounds, compositions, and methods of the invention are useful in combination therapy with other therapeutics, such as hypocholesterolemic and hypoglycemic agents.
Owner:ESPERION THERAPEUTICS
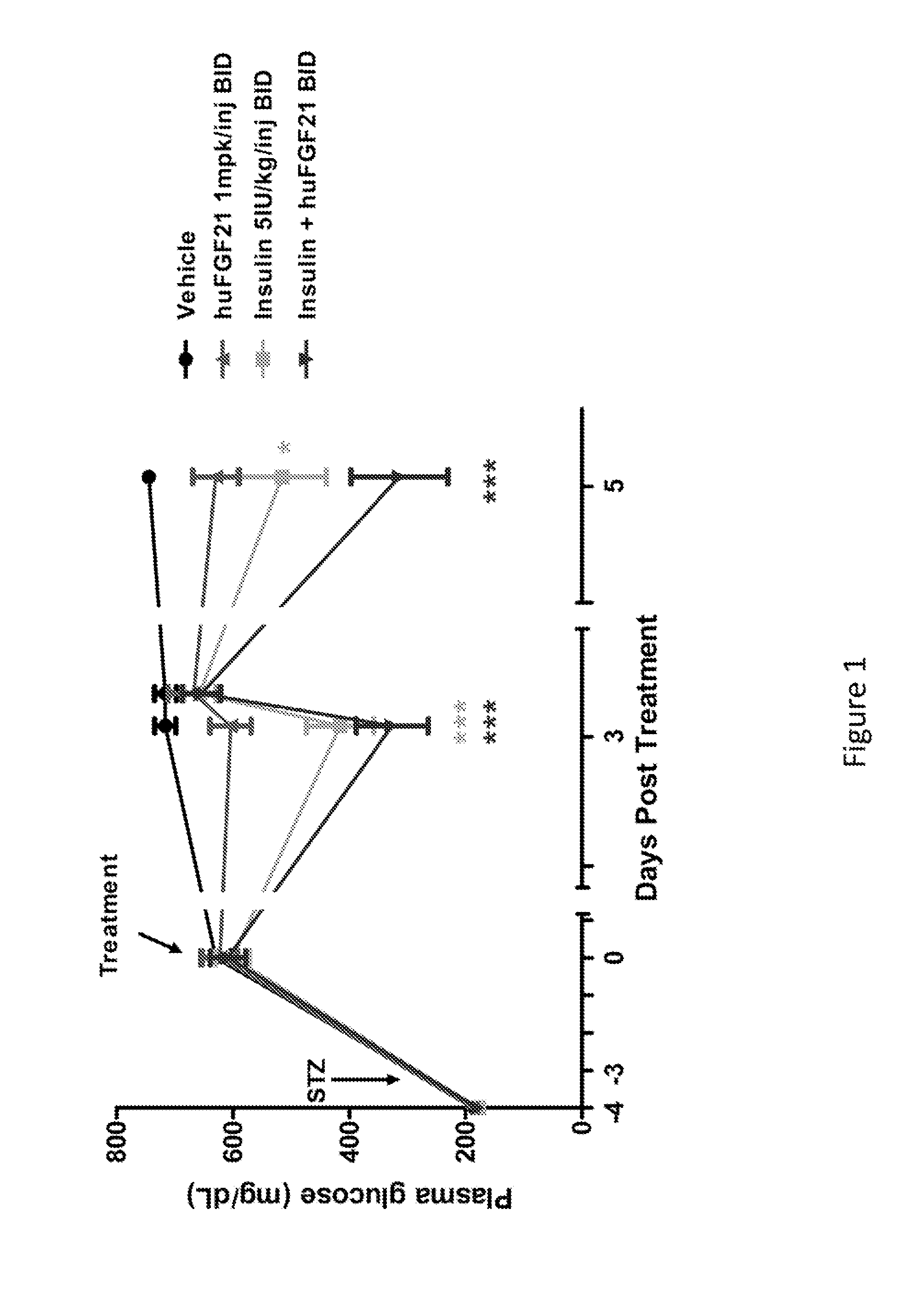
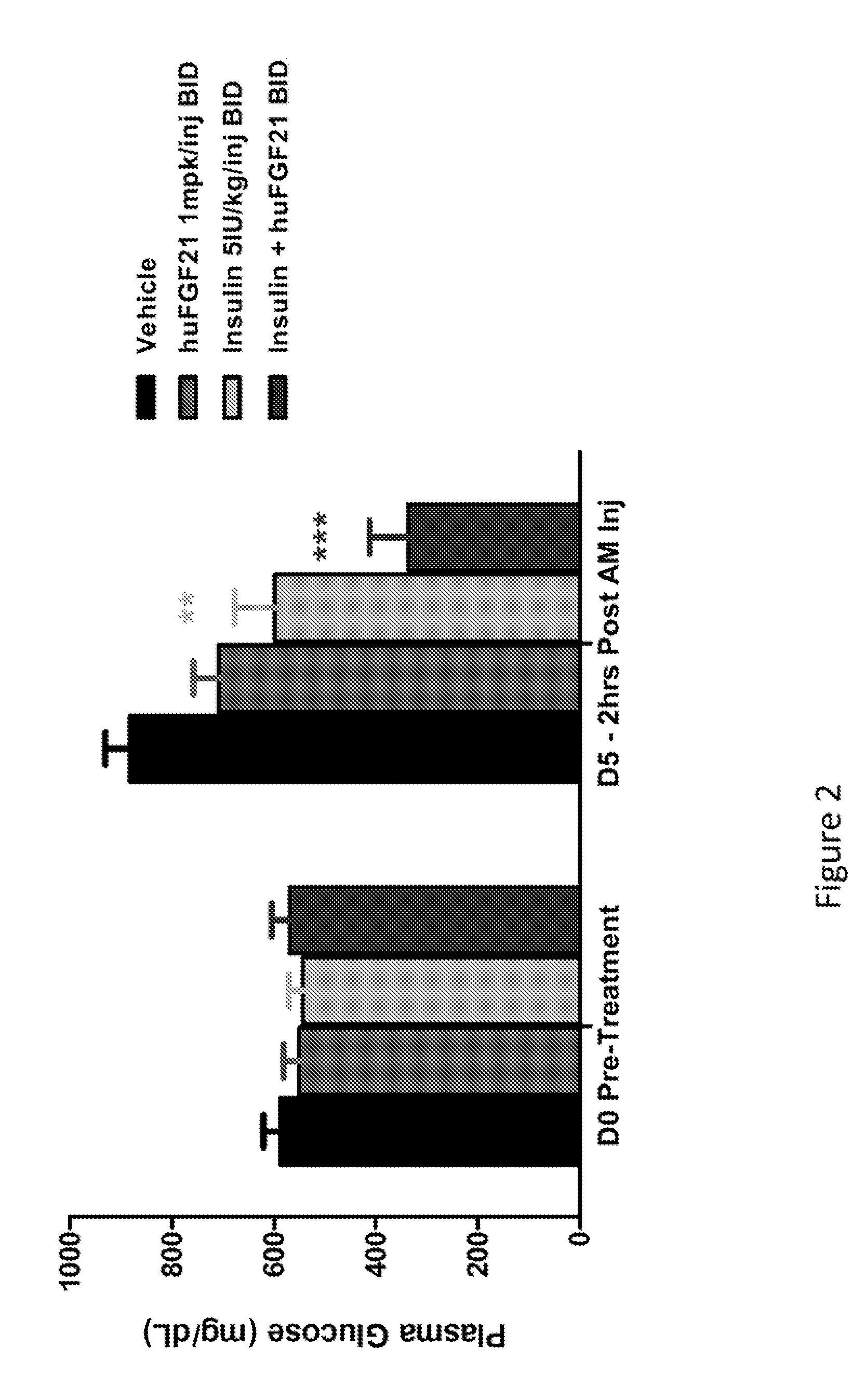
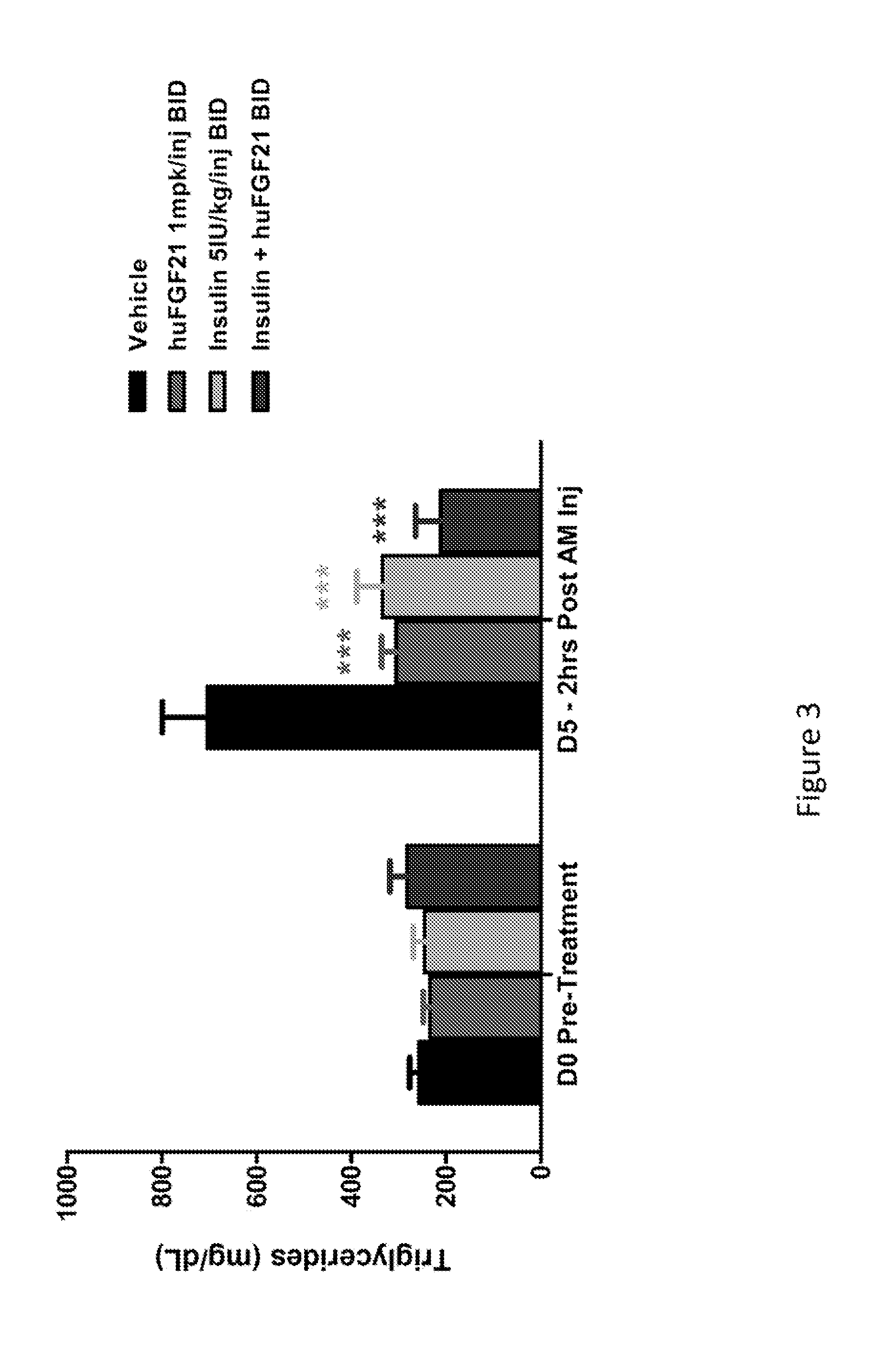
Method of Treating or Ameliorating Type 1 Diabetes Using FGF21
Methods of treating metabolic diseases and disorders using a FGF21 polypeptide are provided. In various embodiments the metabolic disease or disorder is type 1 diabetes, obesity, dyslipidemia, elevated glucose levels, elevated insulin levels, diabetic nephropathy, neuropathy, retinopathy, ischemic heart disease, peripheral vascular disease and cerebrovascular disease
Owner:AMGEN INC
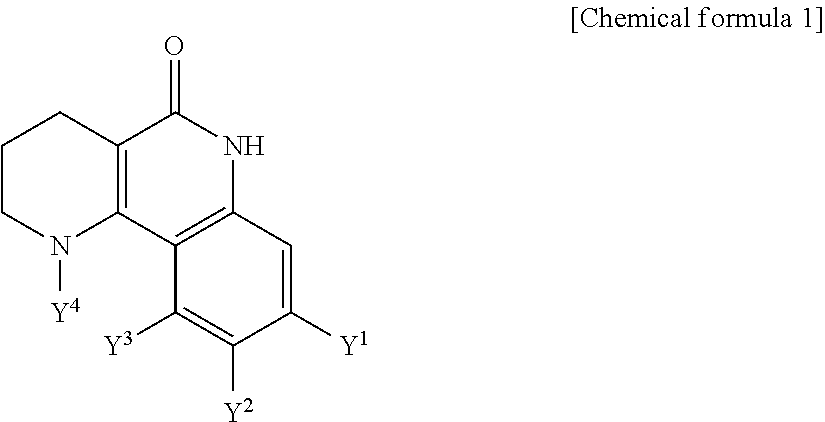
Tricyclic derivative or pharmaceutically acceptable salts thereof, preparation method thereof, and pharmaceutical composition containing the same
Owner:JEIL PHARM CO LTD
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS20040132713A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDisease progressionDiabetic nephropathy
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Dipeptidyl peptidase-IV inhibitors
InactiveUS20050234065A1High activityImproved gastrointestinal permeabilityBiocideNervous disorderDiabetic retinopathyArthritis
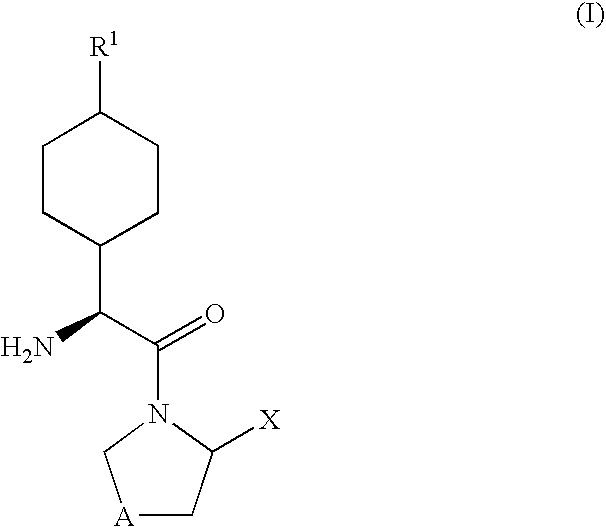
The invention provides compounds of Formula (I) or prodrugs thereof, or pharmaceutically acceptable salts of said compounds or prodrugs, or solvates of said compounds, prodrugs or salts, wherein A, N, X and R1 are as defined herein; pharmaceutical compositions thereof; and methods of using the pharmaceutical compositions for the treatment of diseases, including Type 2 diabetes, Type 1 diabetes, impaired glucose tolerance, hyperglycemia, metabolic syndrome (syndrome X and / or insulin resistance syndrome), glucosuria, metabolic acidosis, arthritis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, short stature due to growth hormone deficiency, infertility due to polycystic ovary syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome; short bowel syndrome; and the prevention of disease progression in Type 2 diabetes.
Owner:PFIZER INC
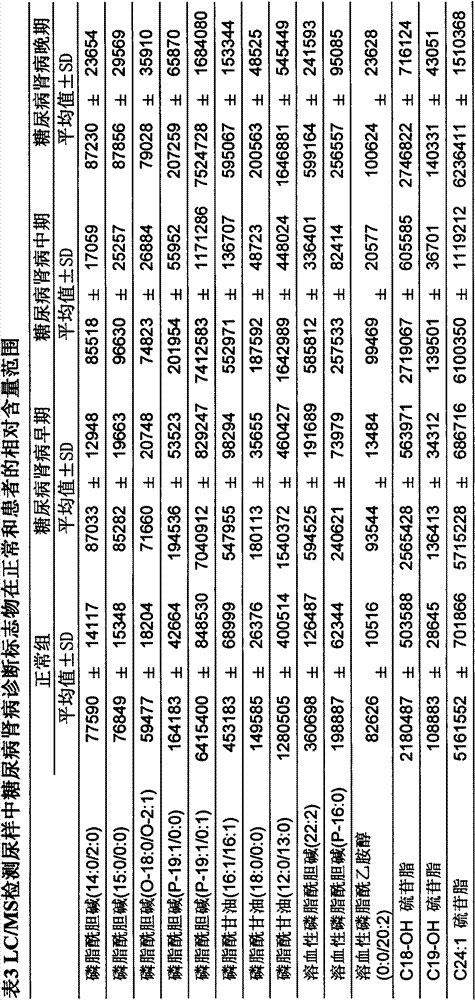
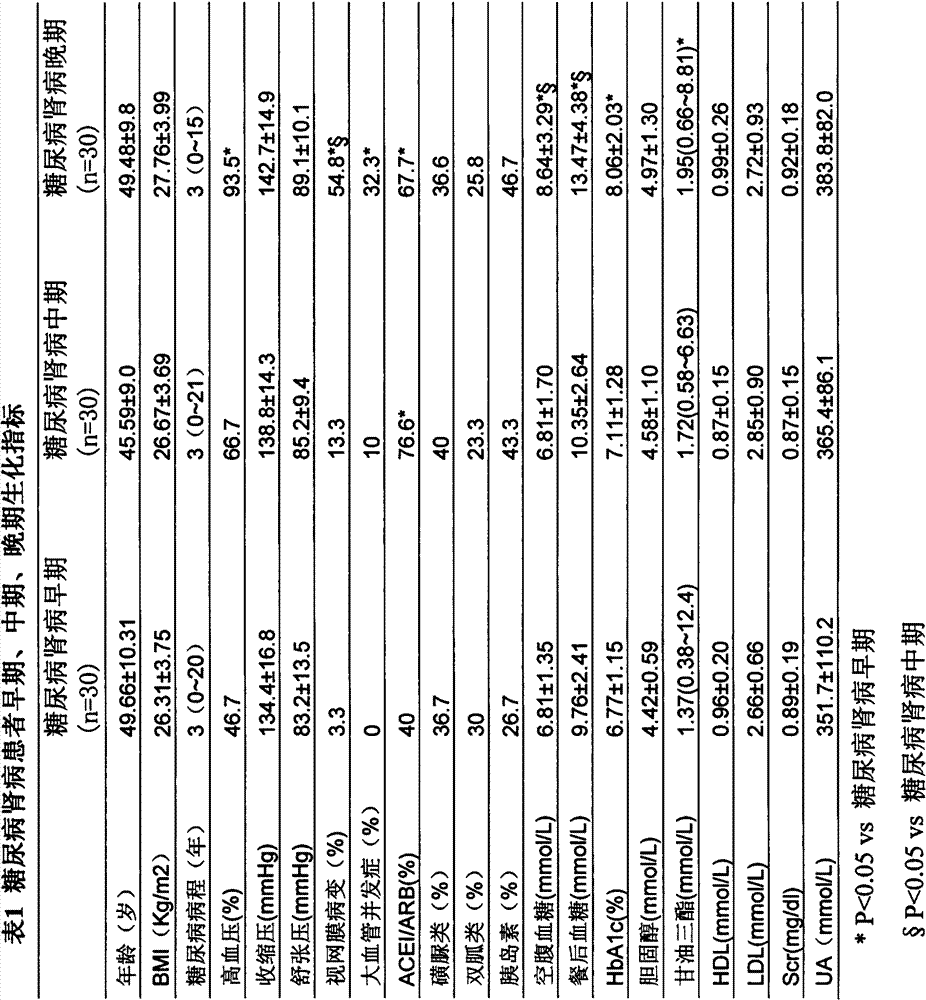
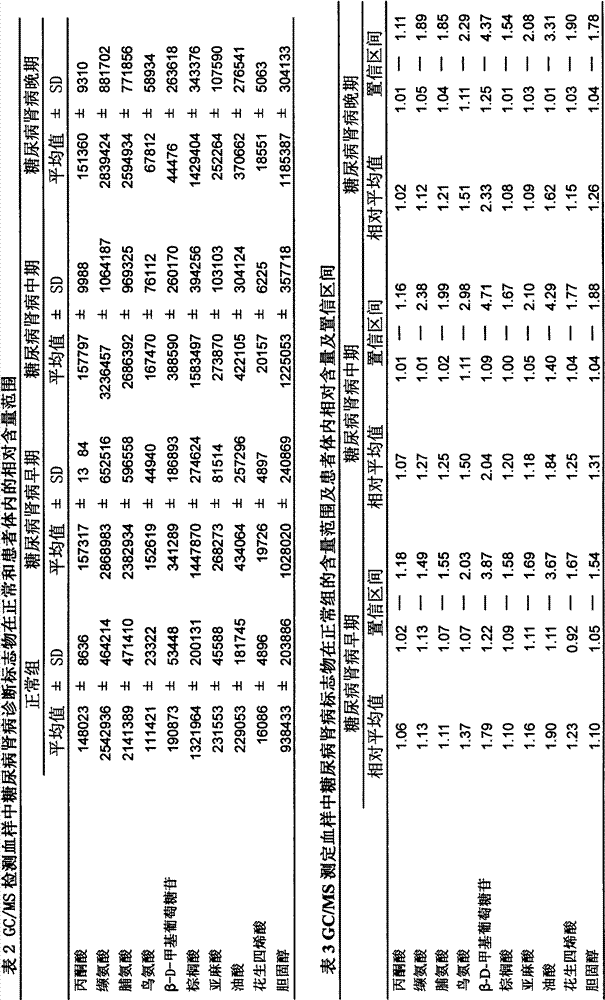
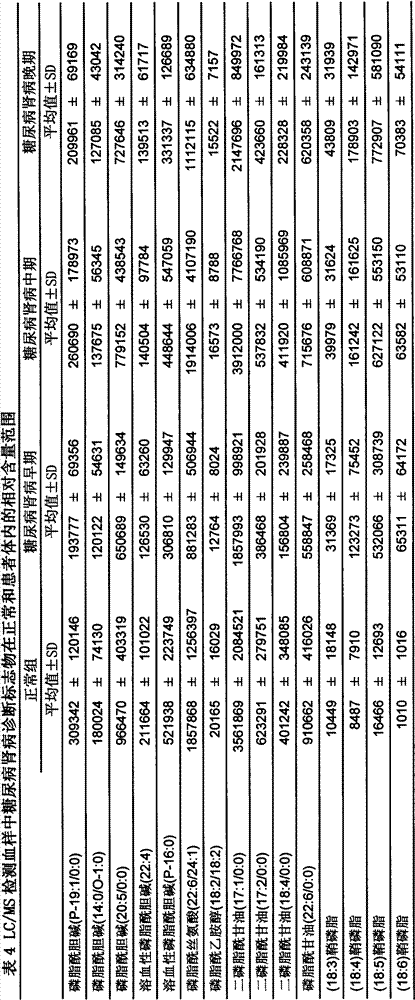
Determination method of urine metabolic marker for early diagnosis of diabetic nephropathy.
InactiveCN102901790AAvoid irreversible changesBest time to treatComponent separationTandem mass spectrometryDiabetic nephropathy
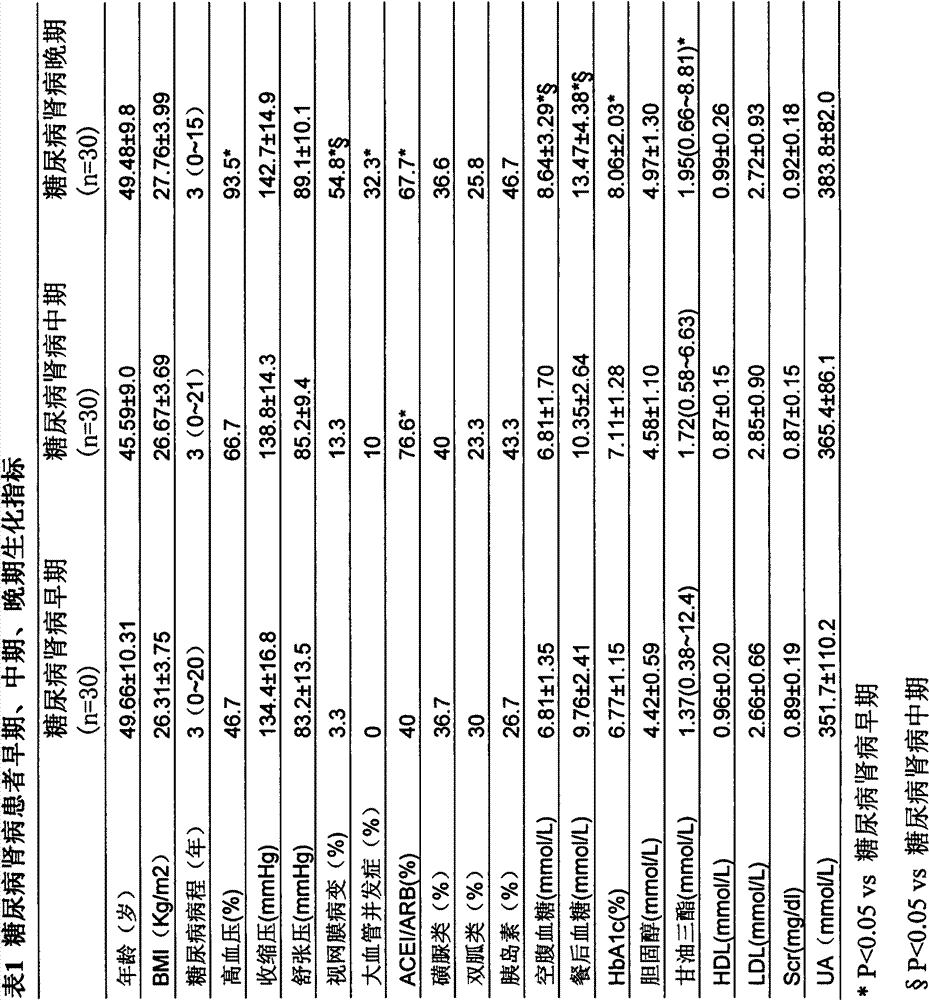
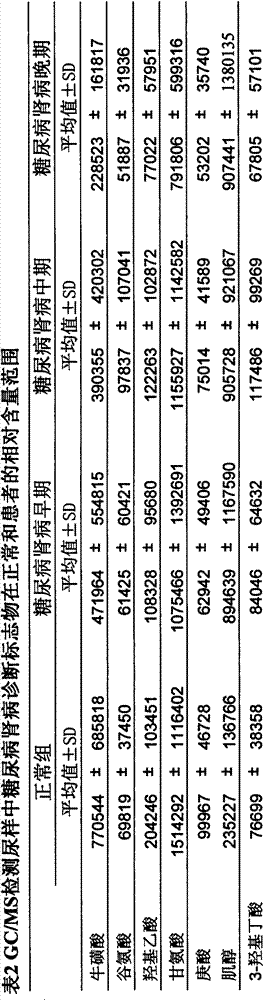
The invention discloses a determination method of a urine metabolic marker for early diagnosis of diabetic nephropathy. The method mainly utilizes analysis technique and method such as gas mass spectrometry and mass liquid spectrometry for quantitative determination of endogenous small molecule compounds in urine of patients with diabetes and diabetic nephropathy; and relative concentration difference between endogenous small molecule compounds in a pathological group and a normal group is calculated and compared, so as to be applied to clinical early diagnosis of diabetic nephropathy. Compared with other existing clinical diagnosis indexes, the method has advantages of wide adaptation range, sensitivity, simple sampling and operation, and no harm on the body. The method is more suitable for screening of diabetic nephropathy in early stage with some uncertain physiological and biochemical indexes, so as to avoid missing of the best treatment time due to delayed diagnosis.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A +1
Determination method of serum metabolic marker for early diagnosis of diabetic nephropathy.
InactiveCN102901789AAvoid irreversible changesBest time to treatComponent separationDiabetic nephropathyDiabetic nephropathy syndrome
The invention discloses a determination method of a serum metabolic marker for early diagnosis of diabetic nephropathy. The method mainly utilizes analysis technique and method such as gas mass spectrometry and mass liquid spectrometry for quantitative determination of endogenous small molecule compounds in urine of patients with diabetes and diabetic nephropathy; and relative concentration difference between endogenous small molecule compounds in a pathological group and a normal group is calculated and compared, so as to be applied to clinical early diagnosis of diabetic nephropathy. Compared with other existing clinical diagnosis indexes, the method has advantages of wide adaptation range, sensitivity, simple sampling and operation, and no harm on the body. The method is more suitable for screening of diabetic nephropathy in early stage with some uncertain physiological and biochemical indexes, so as to avoid missing of the best treatment time due to delayed diagnosis.
Owner:CHINA PHARM UNIV +1
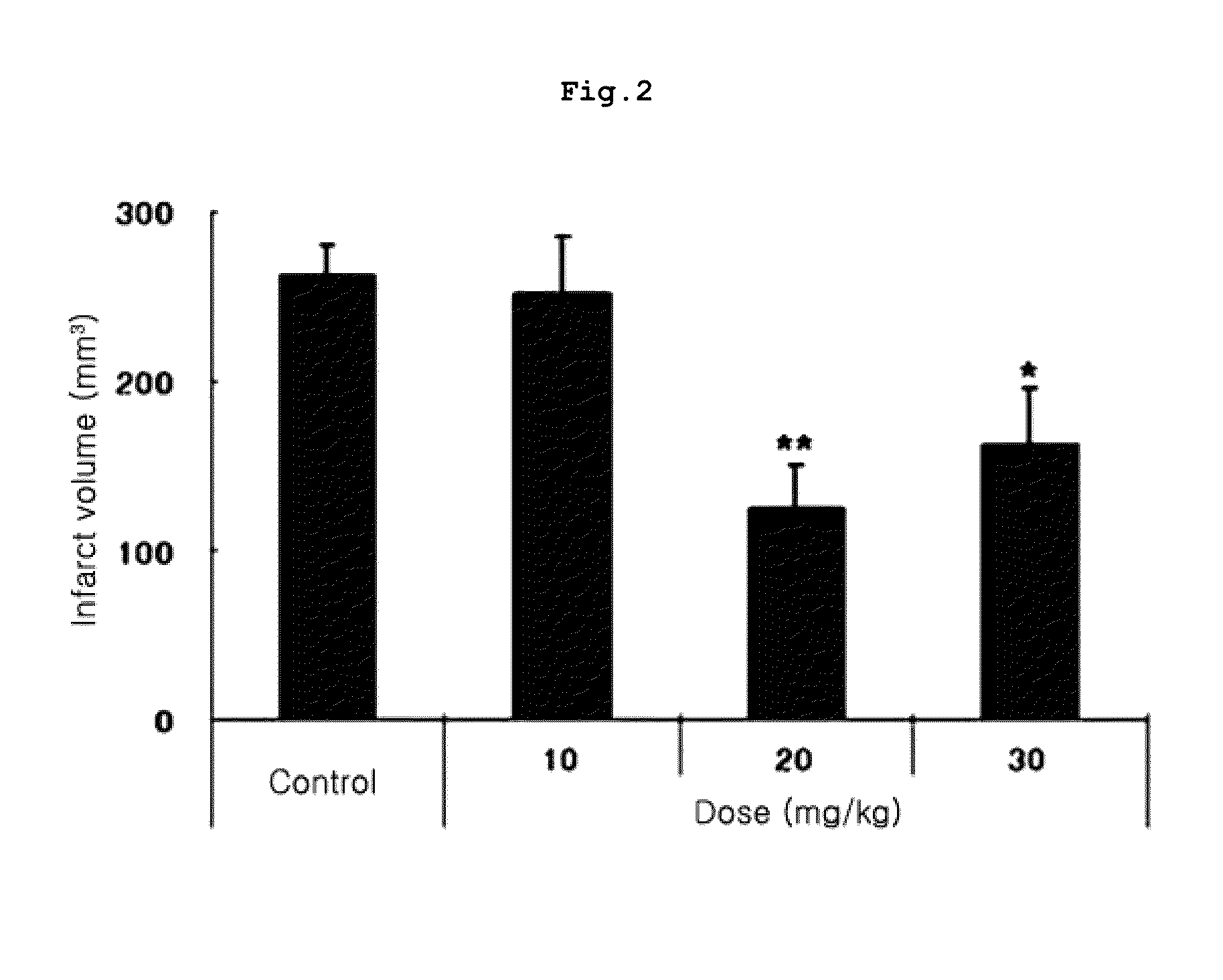
Method for treating diseases associated with alterations in cellular integrity using Rho kinase inhibitor compounds
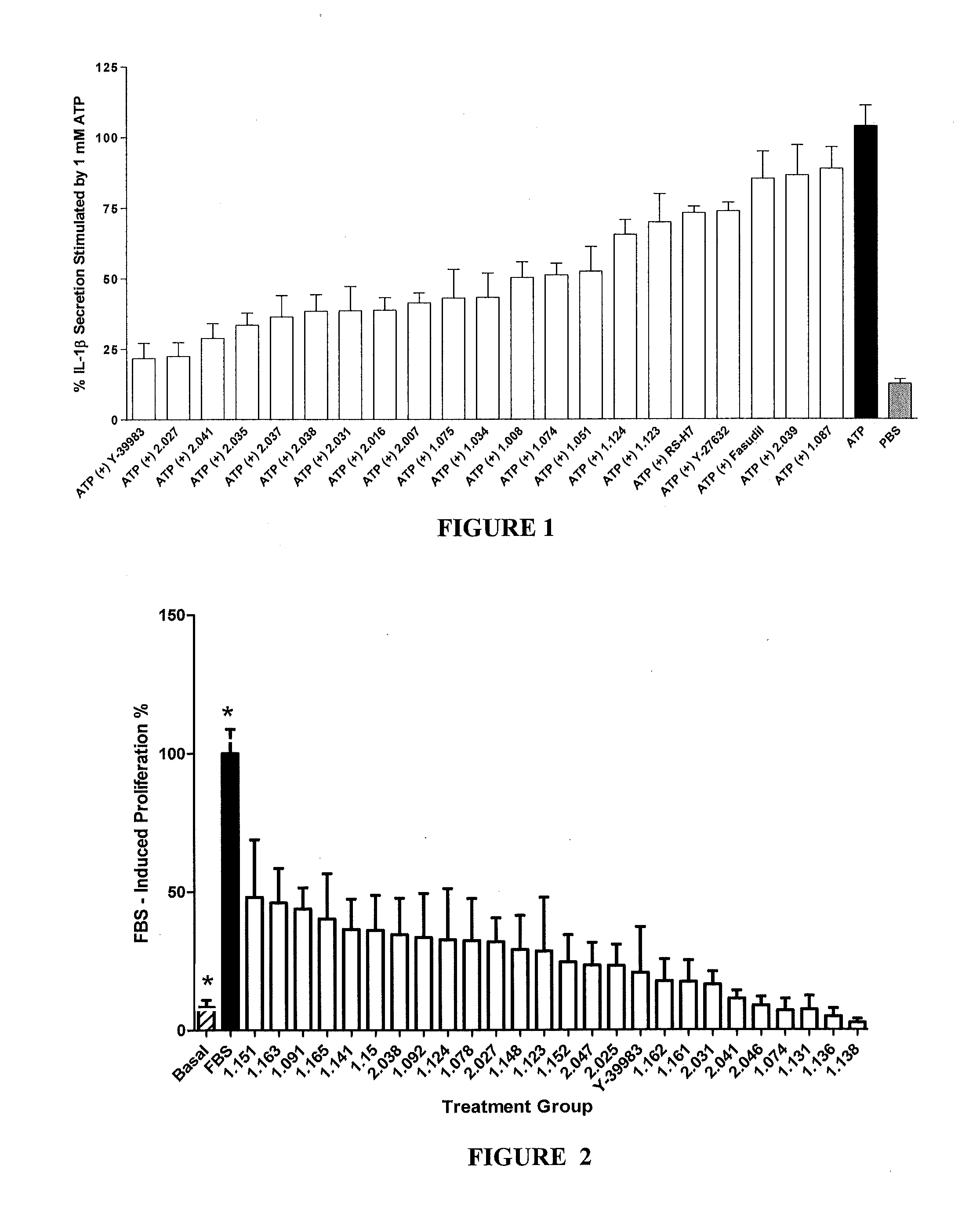
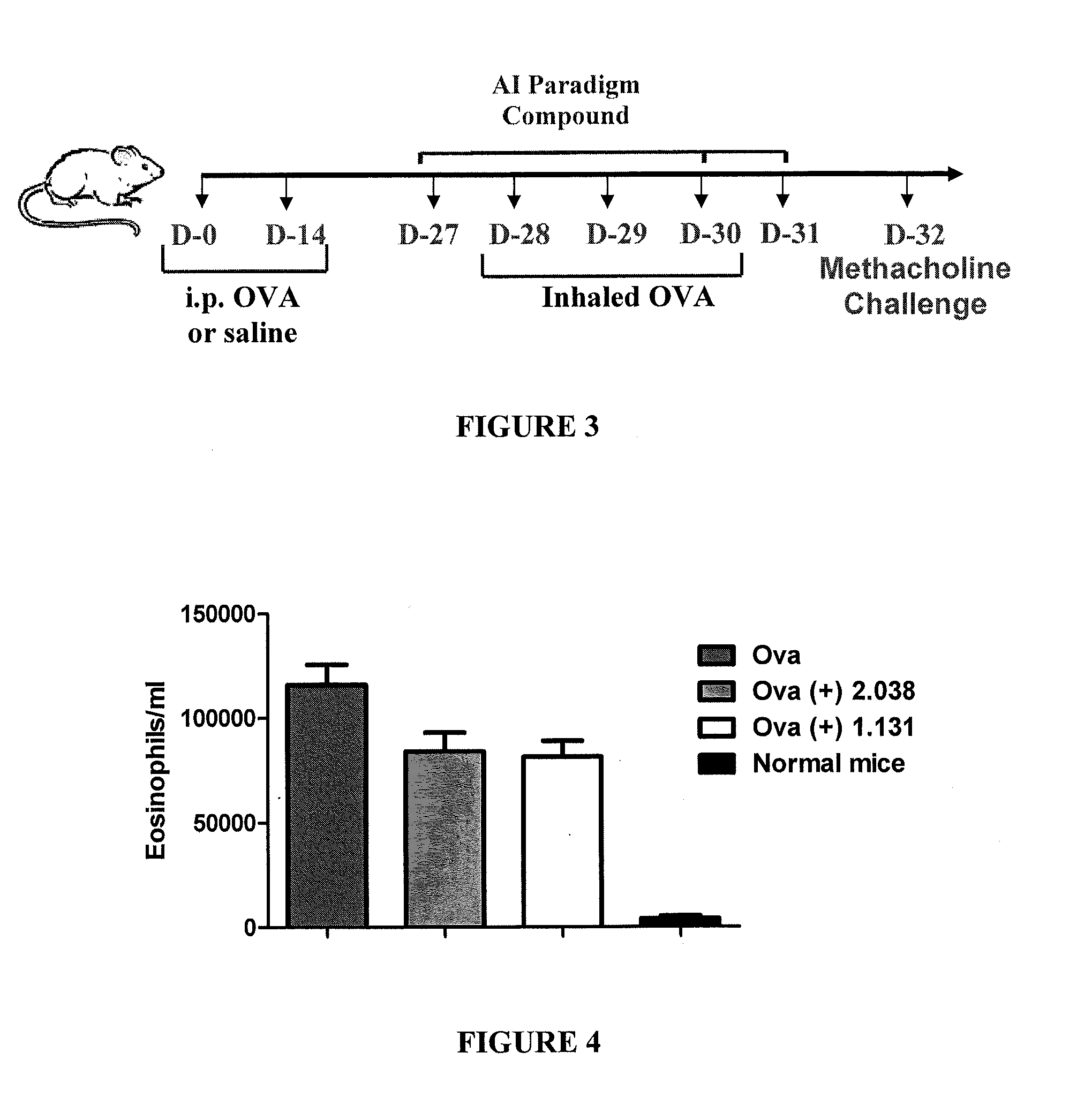
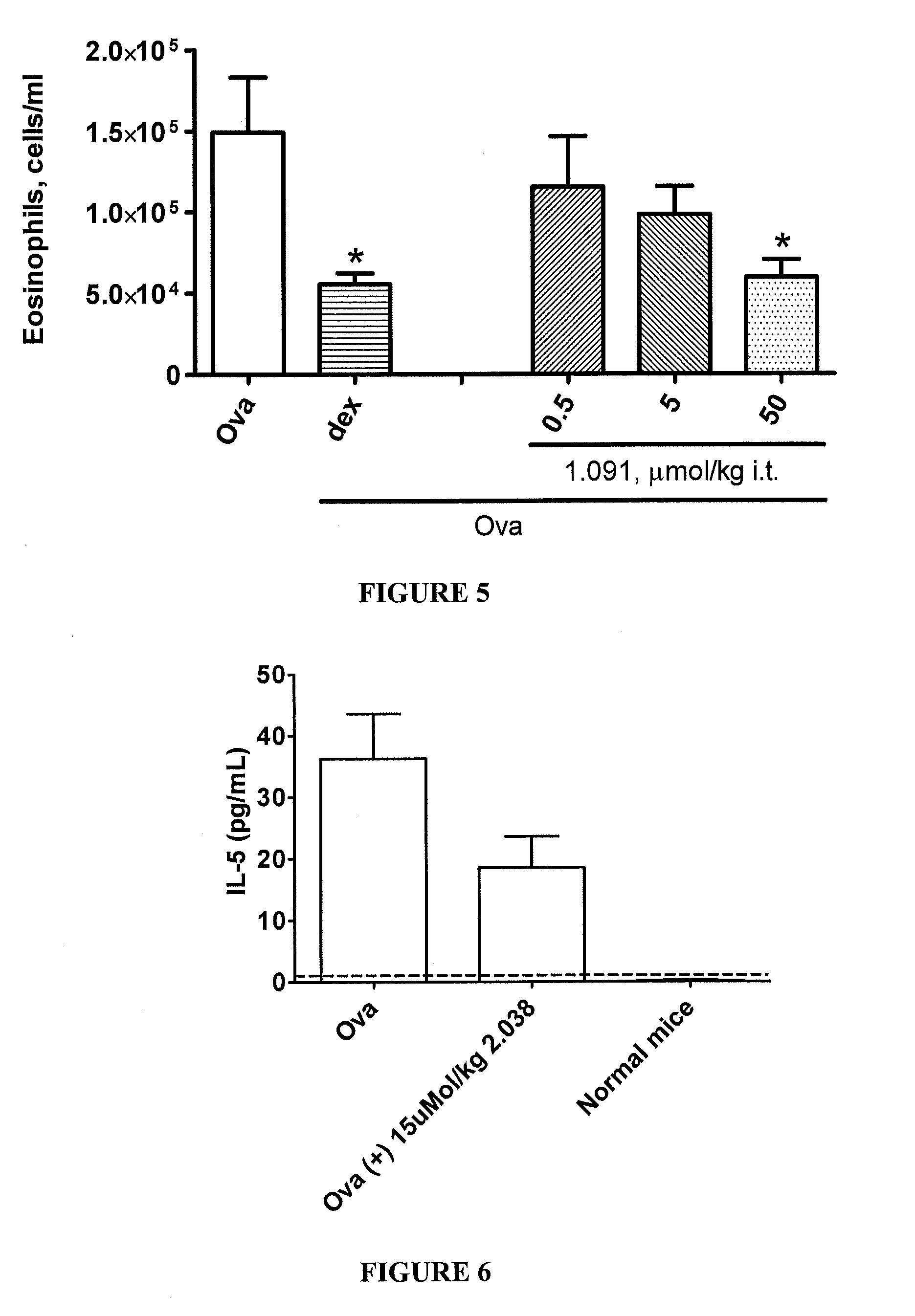
This invention is directed to methods of preventing or treating diseases or conditions associated with alterations in cellular integrity including alterations in endothelial permeability, excessive cell proliferation or tissue remodeling. Particularly, this invention is directed to methods of treating diabetic nephropathy, malaria, or cancer. The method comprises identifying a subject in need of the treatment, and administering to the subject an effective amount of a novel rho kinase inhibitor compound to treat the disease.
Owner:INSPIRE PHARMA
Antagonists of MCP-1 function and methods of use thereof
Compounds which are antagonists of MCP-1 function and are useful in the prevention or treatment of chronic or acute inflammatory or autoimmune diseases, especially those associated with aberrant lymphocyte or monocyte accumulation such as arthritis, asthma, atherosclerosis, diabetic nephropathy, inflammatory bowel disease, Crohn's disease, multiple sclerosis, nephrtitis, pancreatitis, pulmonary fibrosis, psoriasis, restenosis, and transplant rejection; pharmaceutical compositions comprising these compounds; and the use of these compounds and compositions in the prevention or treatment of such diseases.
Owner:TELIK INC +1
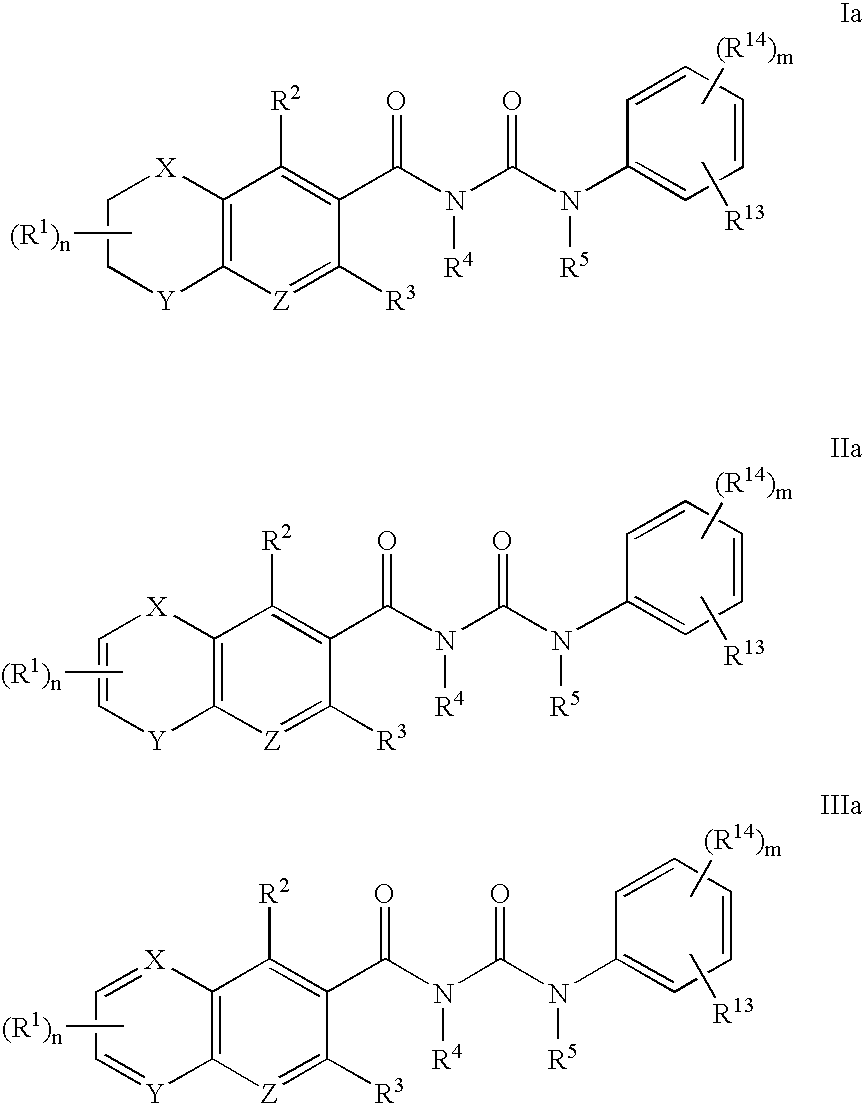
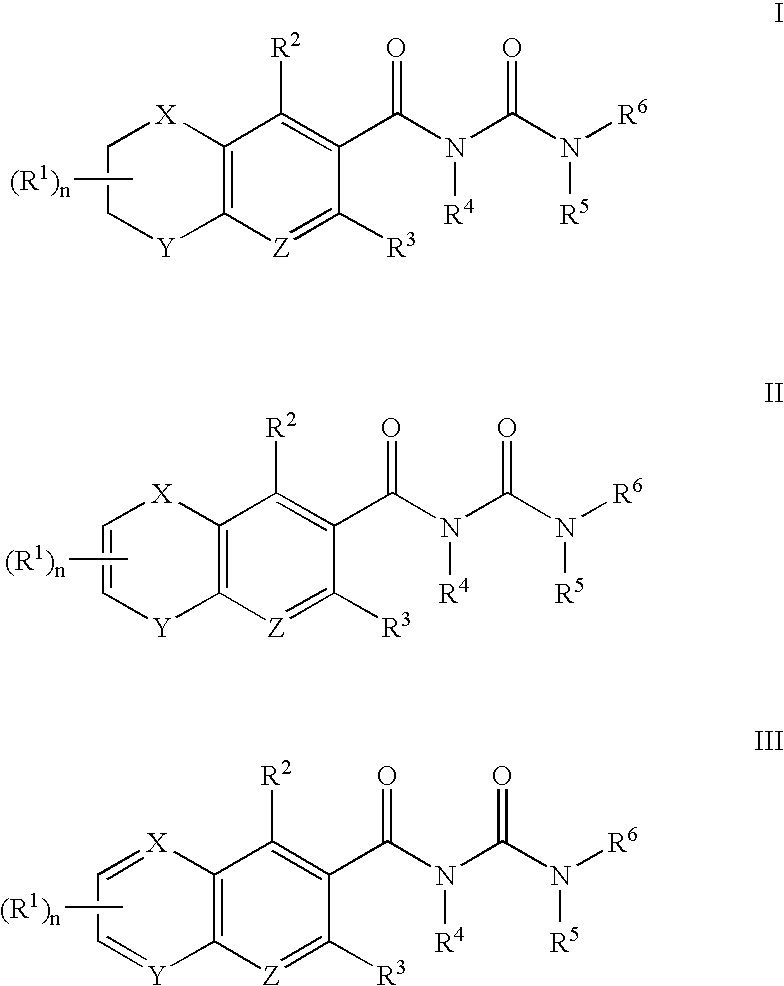
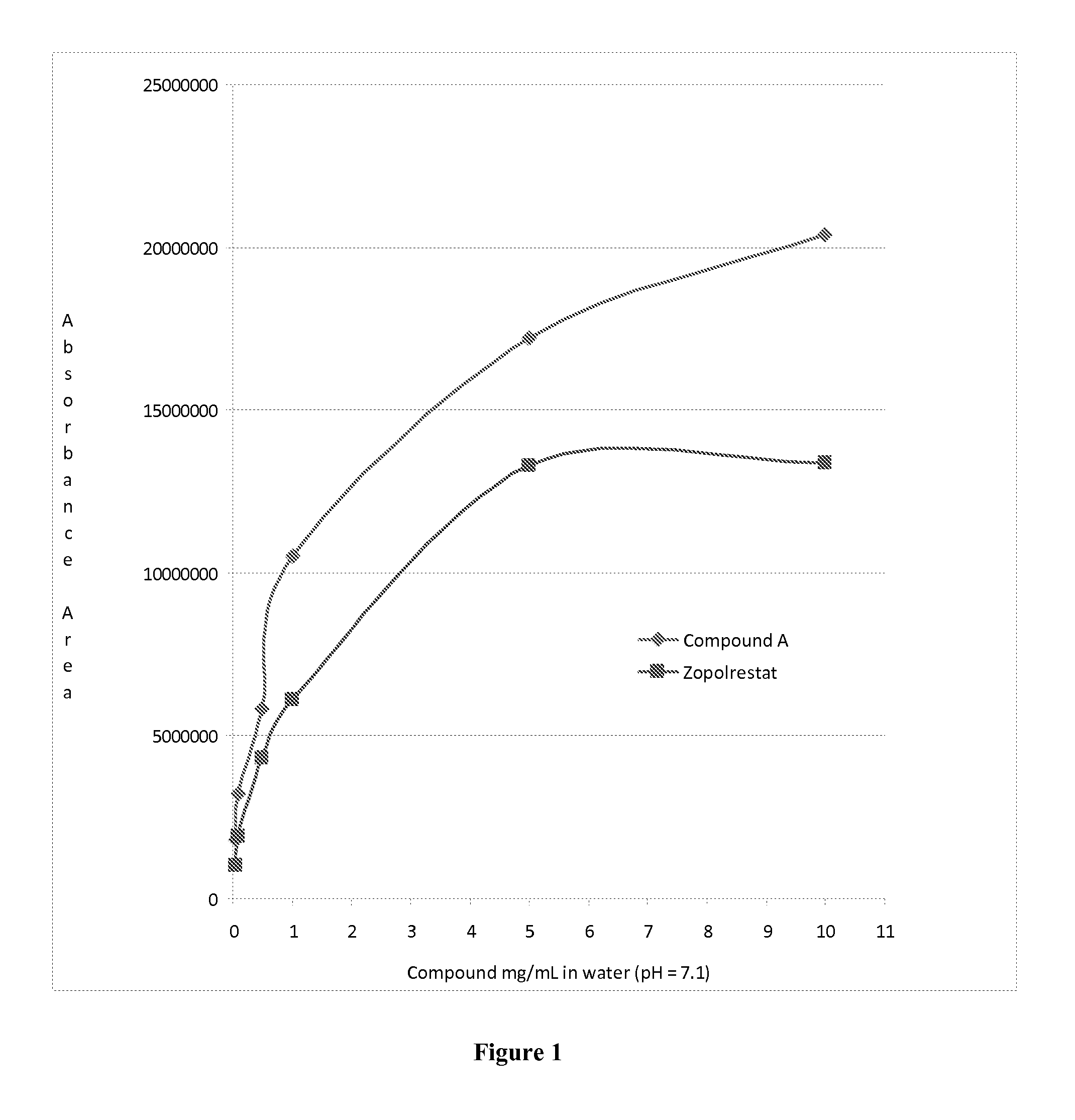
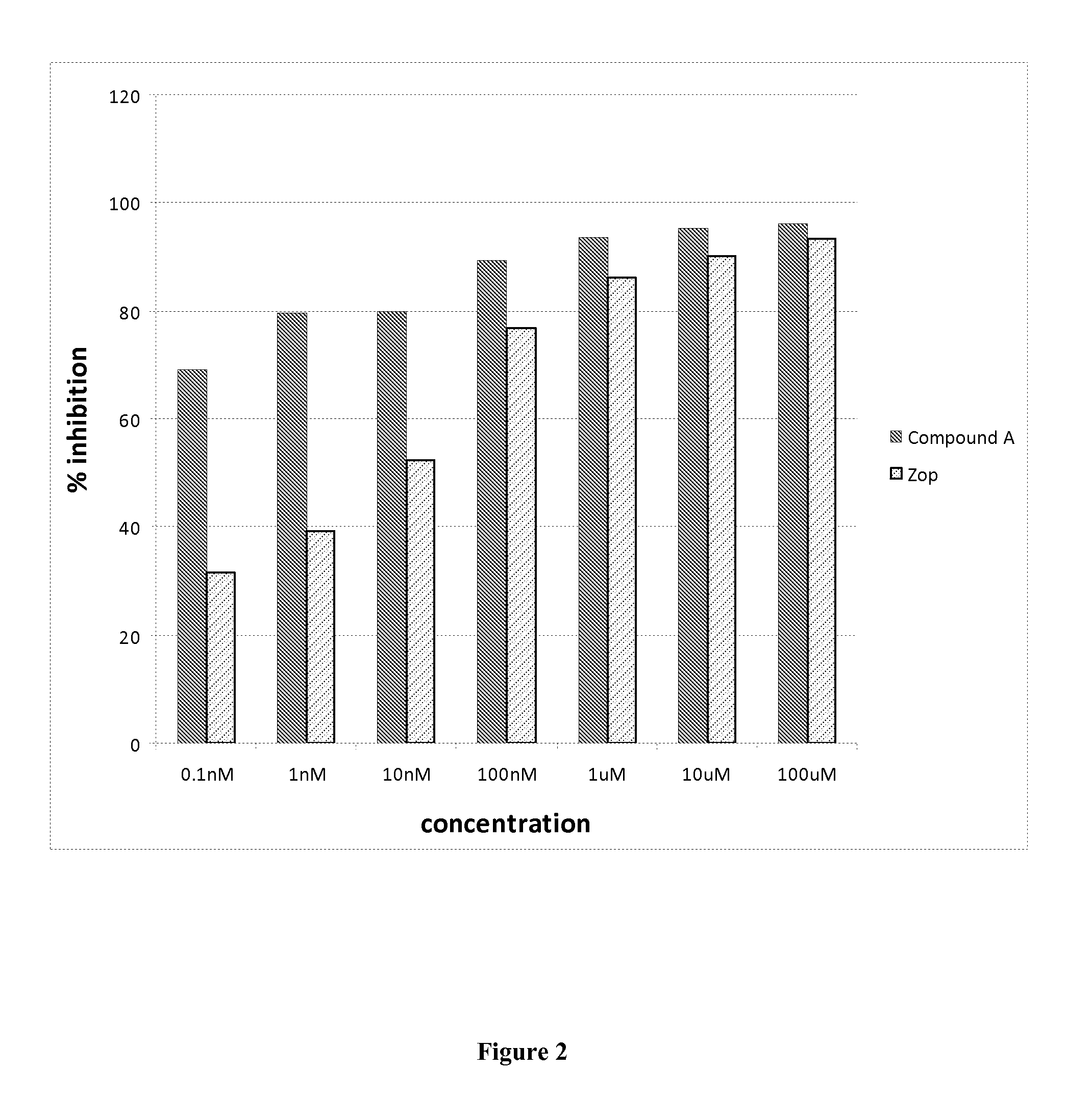
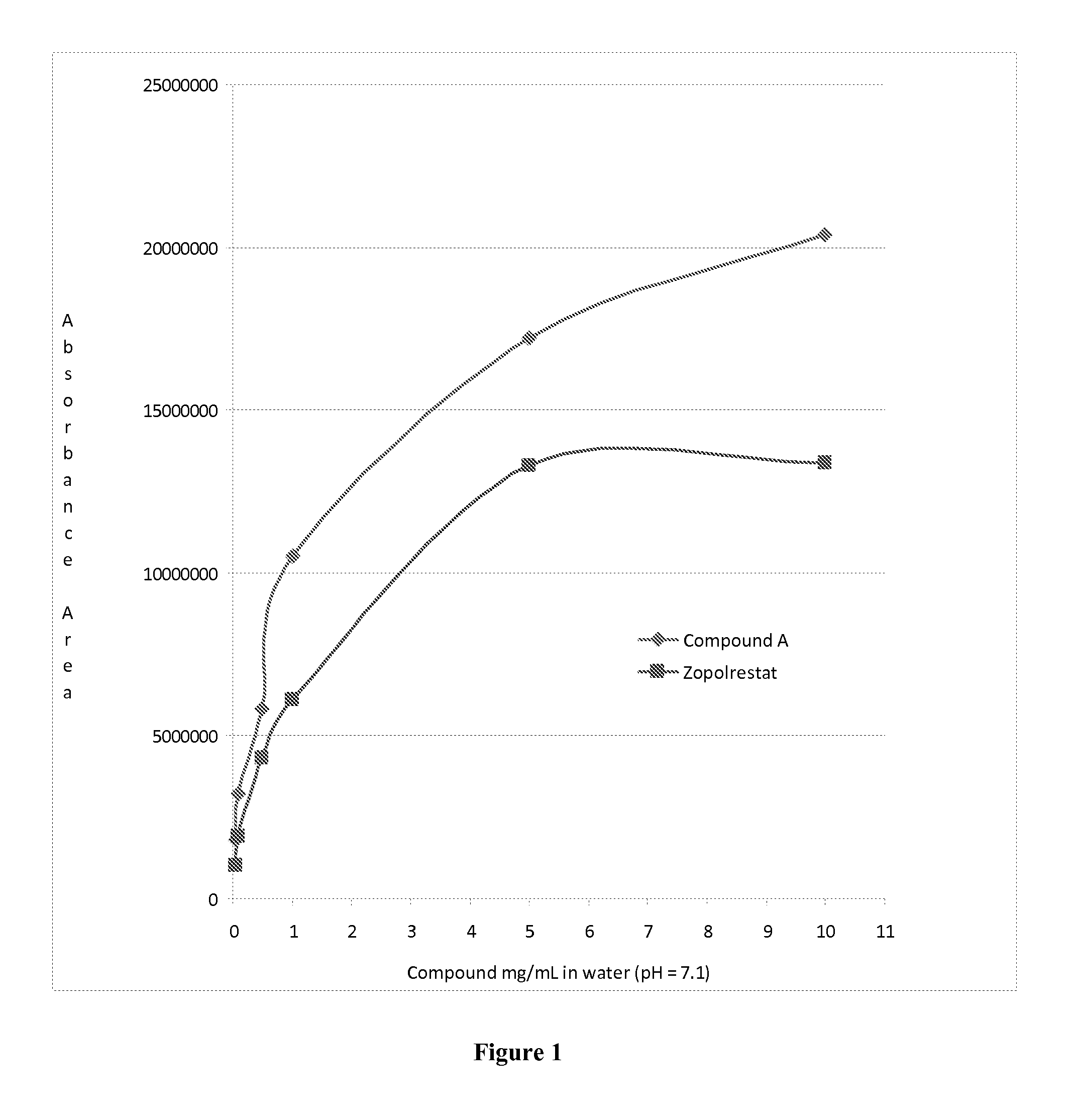
Aldose reductase inhibitors and uses thereof
The present invention relates to novel compounds and pharmaceutical compositions thereof, and methods for promoting healthy aging of skin, the treatment of skin disorders, the treatment of cardiovascular disorders, the treatment of renal disorders, the treatment of angiogenesis disorders, such as cancer, treatment of tissue damage, such as non-cardiac tissue damage, the treatment of evolving myocardial infarction, and the treatment of various other disorders, such as complications arising from diabetes with the compounds and compositions of the invention. Other disorders can include, but are not limited to, atherosclerosis, coronary artery disease, diabetic nephropathy, diabetic neuropathy, diabetic retinopathy, infections of the skin, peripheral vascular disease, stroke, and the like.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Antagonists of MCP-1 function and methods of use thereof
InactiveUS20050054668A1Useful in treatmentBiocideOrganic chemistryAutoimmune conditionAutoimmune disease
Compounds which are antagonists of MCP-1 function and are useful in the prevention or treatment of chronic or acute inflammatory or autoimmune diseases, especially those associated with aberrant lymphocyte or monocyte accumulation such as arthritis, asthma, atherosclerosis, diabetic nephropathy, inflammatory bowel disease, Crohn's disease, multiple sclerosis, nephrtitis, pancreatitis, pulmonary fibrosis, psoriasis, restenosis, and transplant rejection; pharmaceutical compositions comprising these compounds; and the use of these compounds and compositions in the prevention or treatment of such diseases.
Owner:TELIK INC +1
Preparation method of 2-type diabetic nephropathy model
InactiveCN101766149AIncrease concentrationEasy to operateAnimal feeding stuffIntraperitoneal routeSucrose
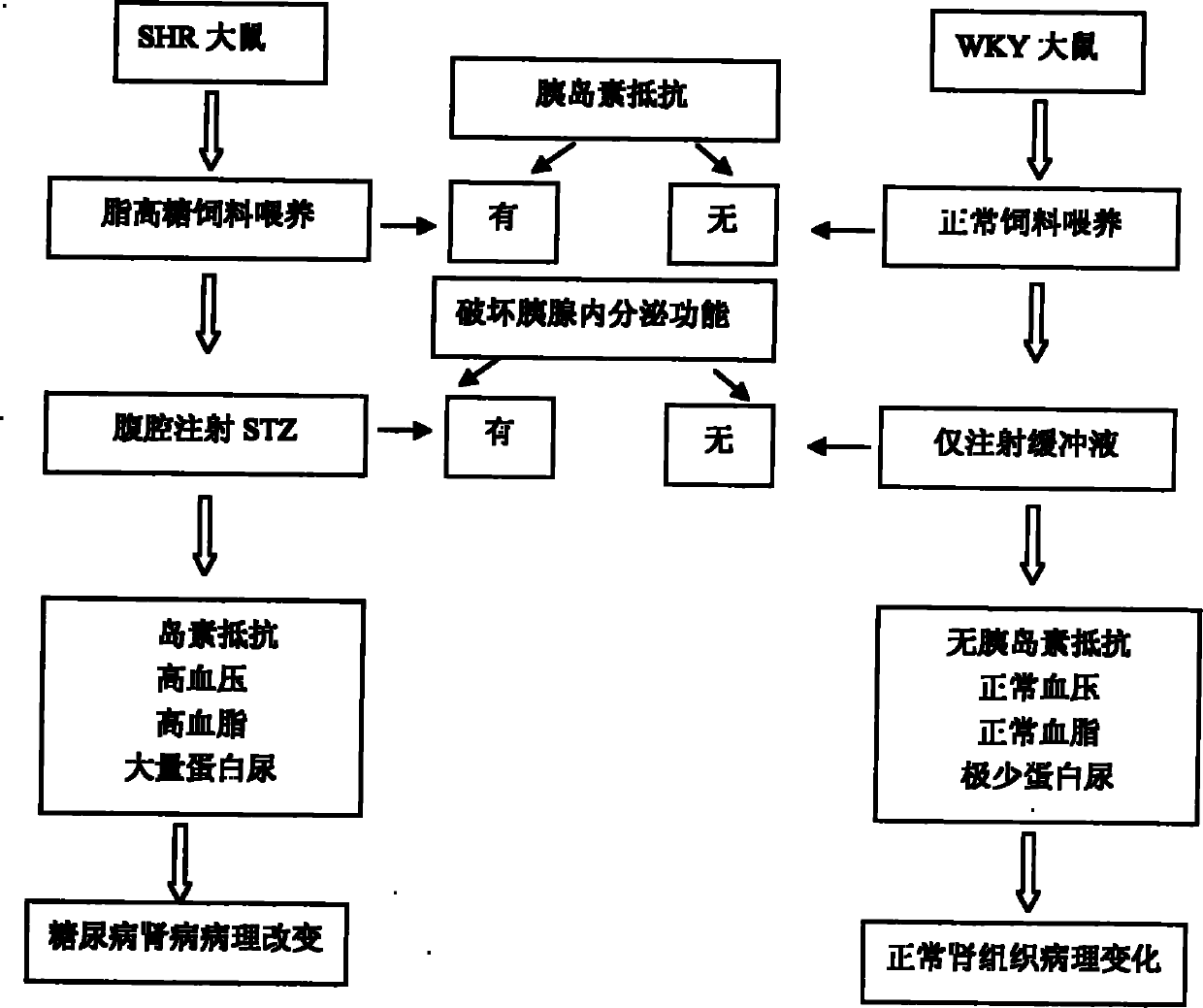
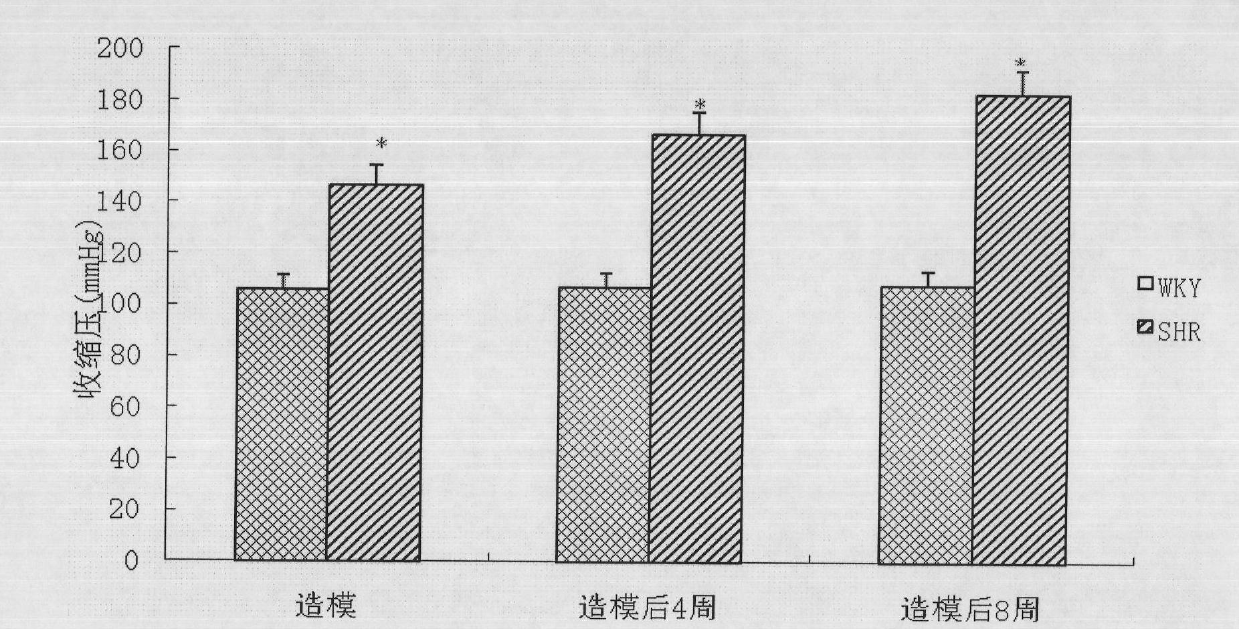
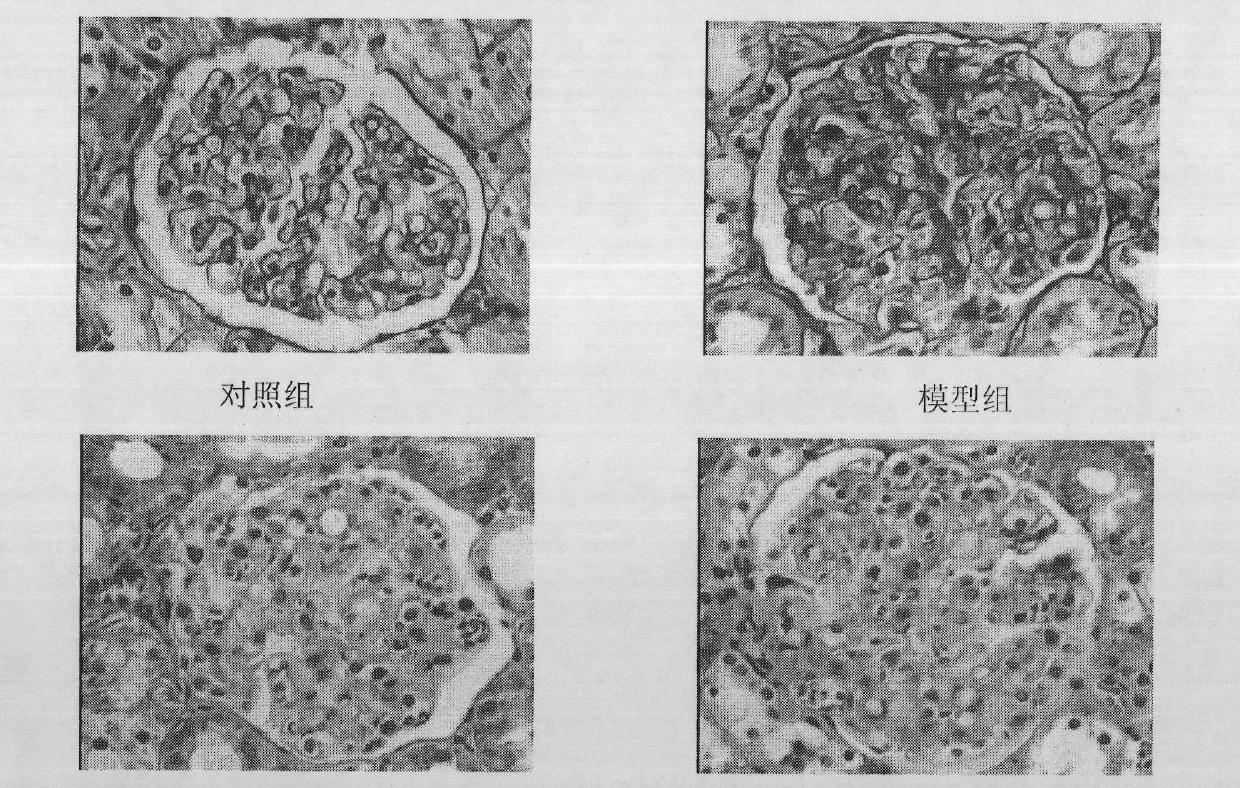
The invention relates to a preparation method of a 2-type diabetic nephropathy model. The method comprises the following steps of: selecting a male SHR (spontaneously hypertensive rat) rat with an age of 6 weeks, wherein the blood pressure of the male SHR rat at birth is normal, and at the time of 4 weeks, the blood pressure begins to rise; after adaptively feeding the SHR rat selected in the step (1) for one week, feeding a high sugar and high fat feed for 2 weeks; detecting the weight, the blood fat, the blood sugar and the insulin concentration and calculating an insulin resistance index evaluated by a steady-state model, wherein the insulin resistance index is not smaller than 3.8, and the high fat and high sugar feed comprises the following components in percentage by weight: 10.0 percent of lard, 20.0 percent of cane sugar, 2.5 percent of cholesterol, 2.0 percent of cholate and 66.5 percent of conventional feed; after fasting the insulin resistant SHR rat for 12 hours, carrying out a small-dose one-time STZ (streptozocin) (35mg / kg) intraperitoneal injection on the SHR rat, wherein the small dose is 35mg / kg; after 72 hours, measuring the random blood sugar; and judging the 2-type diabetic nephropathy model to be successfully prepared if the plasma glucose is not smaller than 16.7mmol / L.
Owner:SOUTHEAST UNIV
Therapy for complications of diabetes
InactiveUS20090054473A1High control sensitivityEnhancing glycemic controlBiocideOrganic active ingredientsDyslipidemiaDiabetes Mellitus Complications
A method for enhancing glycemic control and / or insulin sensitivity in a human subject having diabetic nephropathy and / or metabolic syndrome comprises administering to the subject a selective endothelin A (ETA) receptor antagonist in a glycemic control and / or insulin sensitivity enhancing effective amount. A method for treating a complex of comorbidities in an elderly diabetic human subject comprises administering to the subject a selective ETA receptor antagonist in combination or as adjunctive therapy with at least one additional agent that is (i) other than a selective ETA receptor antagonist and (ii) effective in treatment of diabetes and / or at least one of said comorbidities other than hypertension. A therapeutic combination useful in such a method comprises a selective ETA receptor antagonist and at least one antidiabetic, anti-obesity or antidyslipidemic agent other than a selective ETA receptor antagonist.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Method for Treatment of Inflammatory Disorders Using Triptolide Compounds
InactiveUS20070244080A1Inhibiting cytokine productionRelieve symptomsOrganic active ingredientsAntipyreticHepatic fibrosisTriptolide
Inflammatory disorders, including obliterative airway disease, renal fibrosis, diabetic nephropathy, and liver fibrosis are treated with immunosuppressive triptolide compounds, in particular triptolide compounds effective to inhibit TGF-β production in a patient afflicted with such a disorder.
Owner:PHARMAGENESIS
Modulators of the prostacyclin (PGI2) receptor useful for the treatment of disorders related thereto
The present invention relates to amide derivatives of Formula (XIIIa) and pharmaceutical compositions thereof that modulate the activity of the PGI2 receptor. Compounds of the present invention and pharmaceutical compositions thereof are directed to methods useful in the treatment of: pulmonary arterial hypertension (PAH); idiopathic PAH; familial PAH; PAH associated with a collagen vascular disease, a congenital heart disease, portal hypertension, HIV infection, ingestion of a drug or toxin, hereditary hemorrhagic telangiectasia, splenectomy, pulmonary veno-occlusive disease (PVOD) or pulmonary capillary hemangiomatosis (PCH); PAH with significant venous or capillary involvement; platelet aggregation; coronary artery disease; myocardial infarction; transient ischemic attack; angina; stroke; ischemia-reperfusion injury; restenosis; atrial fibrillation; blood clot formation in an angioplasty or coronary bypass surgery individual or in an individual suffering from atrial fibrillation; atherosclerosis; atherothrombosis; asthma or a symptom thereof; a diabetic-related disorder such as diabetic peripheral neuropathy, diabetic nephropathy or diabetic retinopathy; glaucoma or other disease of the eye with abnormal intraocular pressure; hypertension; inflammation; psoriasis; psoriatic arthritis; rheumatoid arthritis; Crohn's disease; transplant rejection; multiple sclerosis; systemic lupus erythematosus (SLE); ulcerative colitis; ischemia-reperfusion injury; restenosis; atherosclerosis; acne; type 1 diabetes; type 2 diabetes; sepsis; and chronic obstructive pulmonary disorder (COPD).
Owner:ARENA PHARMA
Aldose reductase inhibitors and uses thereof
The present invention relates to novel compounds and pharmaceutical compositions thereof, and methods for promoting healthy aging of skin, the treatment of skin disorders, the treatment of cardiovascular disorders, the treatment of renal disorders, the treatment of angiogenesis disorders, such as cancer, treatment of tissue damage, such as non-cardiac tissue damage, the treatment of evolving myocardial infarction, and the treatment of various other disorders, such as complications arising from diabetes with the compounds and compositions of the invention. Other disorders can include, but are not limited to, atherosclerosis, coronary artery disease, diabetic nephropathy, diabetic neuropathy, diabetic retinopathy, infections of the skin, peripheral vascular disease, stroke, and the like.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
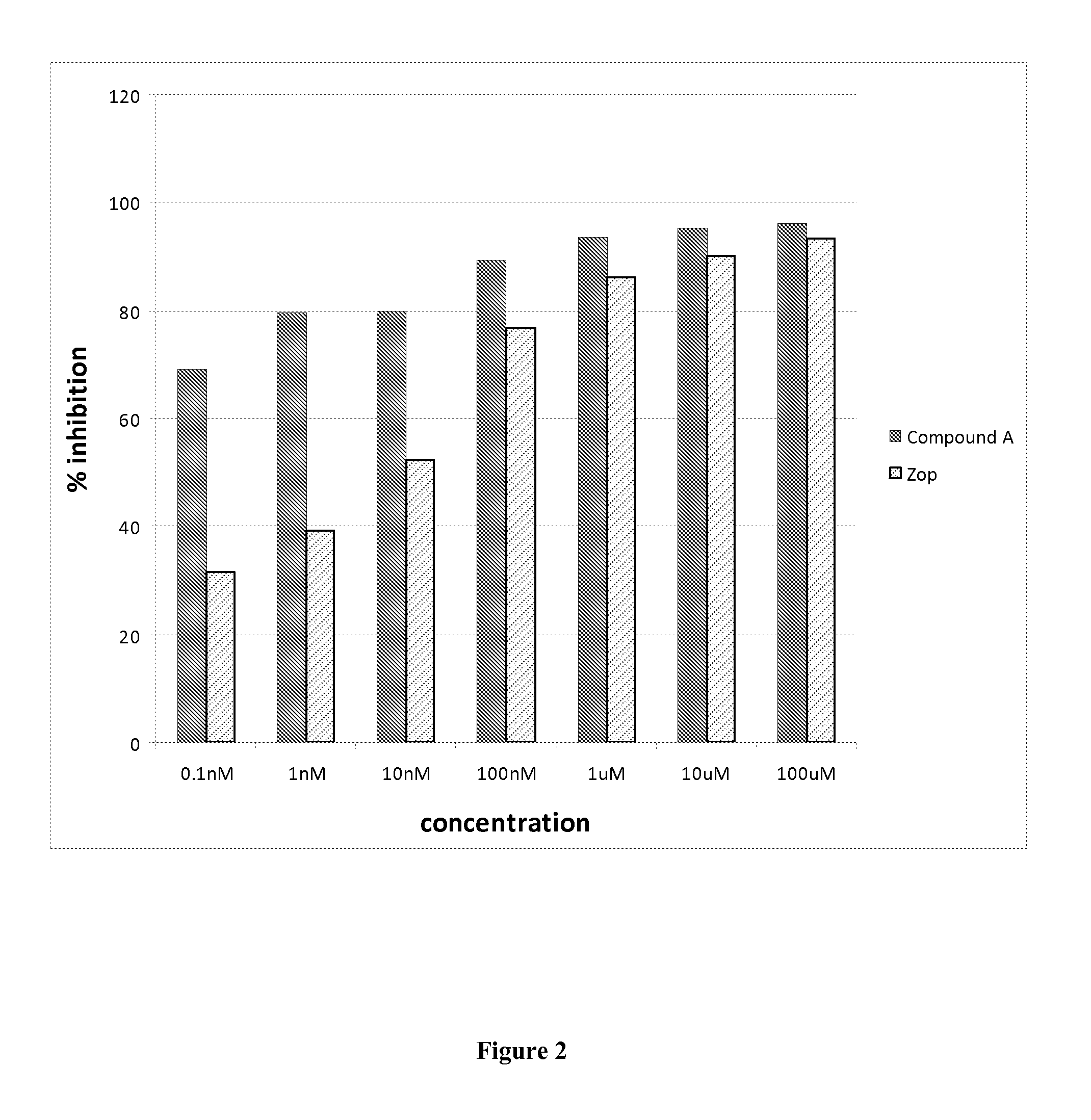
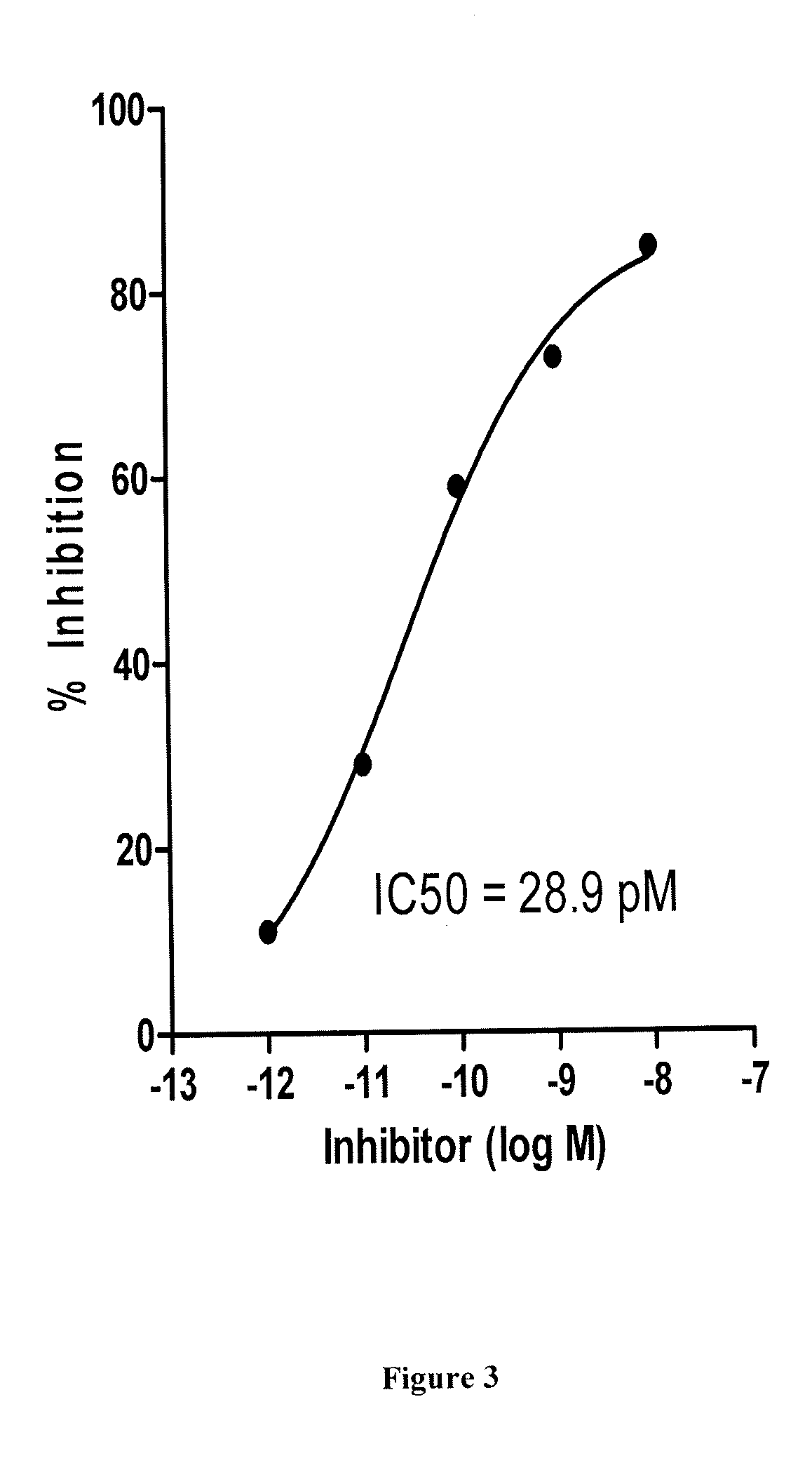
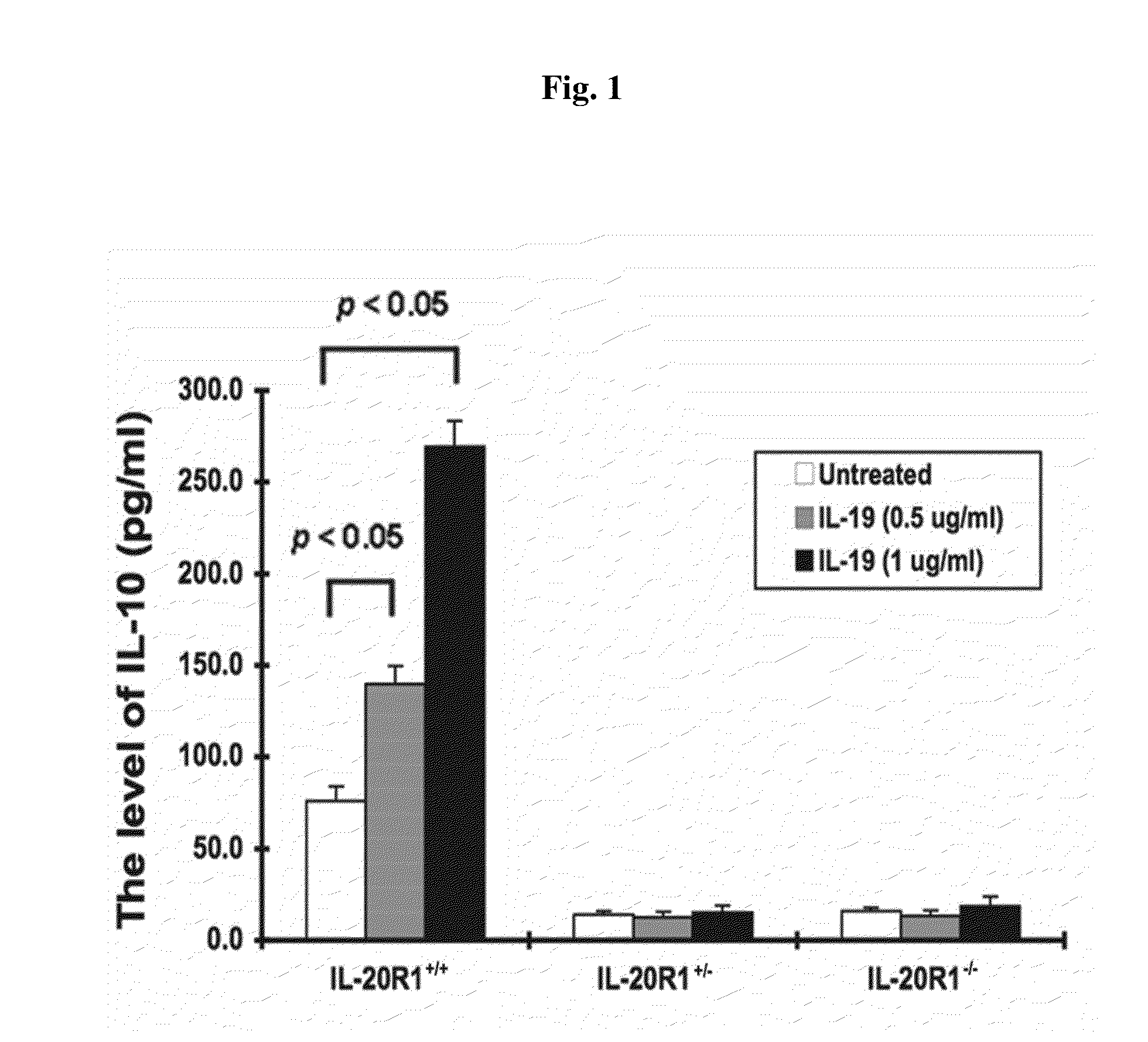
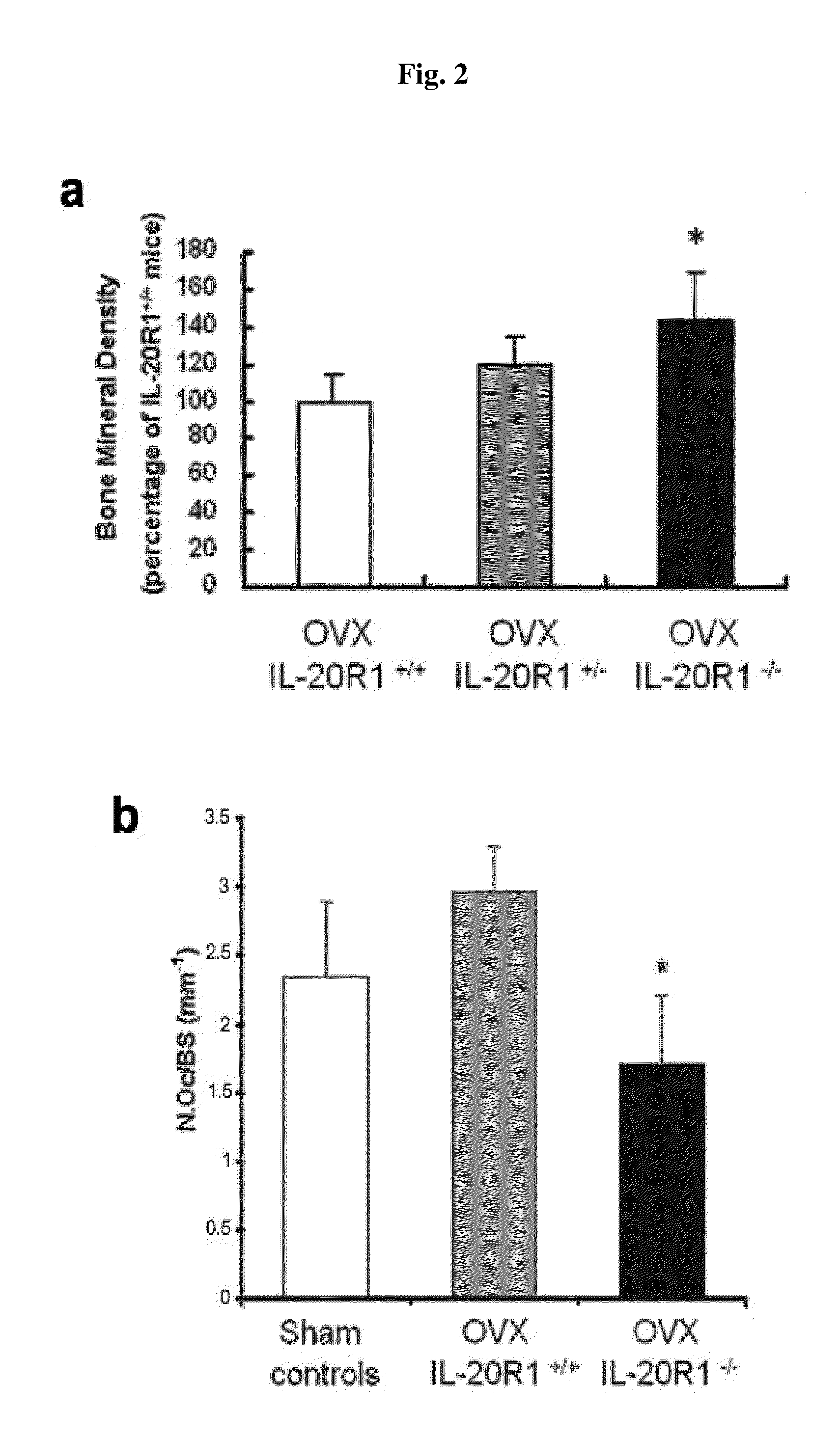
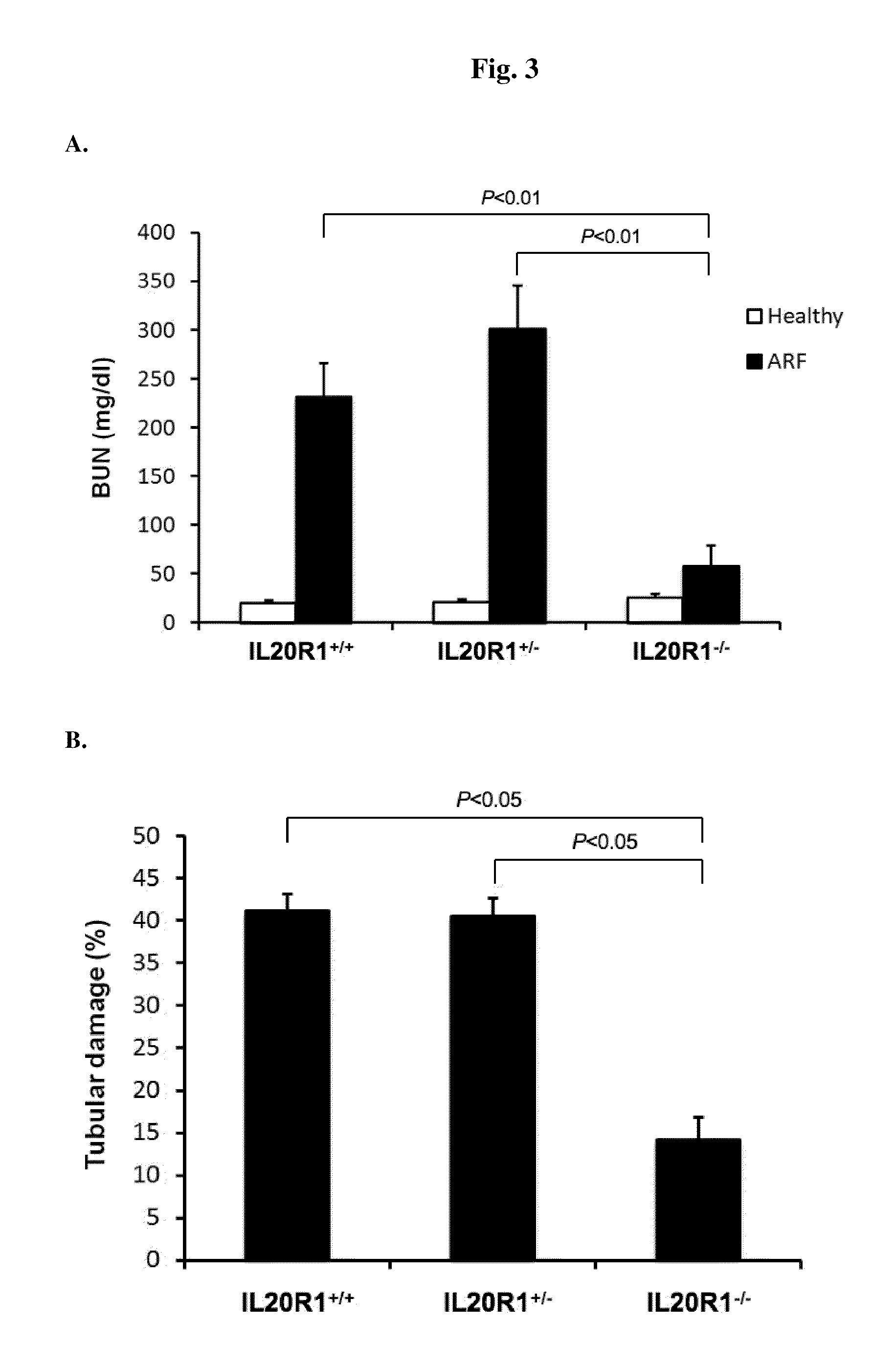
Treating Disorders Associated with IL-20 Receptor-Mediated Signaling Pathway by Blocking IL-20 Receptor Activity
ActiveUS20110256093A1Less severe osteoporosisLess severe renal failureOrganic active ingredientsPeptide/protein ingredientsDiseaseAntisense nucleic acid
Treating a disorder (e.g., osteoporosis, renal failure, or diabetic nephropathy) associated with a signaling pathway mediated by IL-20 receptor with an agent that suppresses IL-20 receptor activity, e.g., an antibody that neutralizes IL-20 receptor via binding to IL-20R1, an antisense nucleic acid that suppresses expression of IL-20R1, a small molecule that inhibits IL-20 receptor activity, or a dominant negative mutant of IL-19, IL-20, or IL-24.
Owner:LBL BIOTECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com