Patents
Literature
59 results about "Short bowel syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inability to absorb nutrients from food due to having a short small intestine.
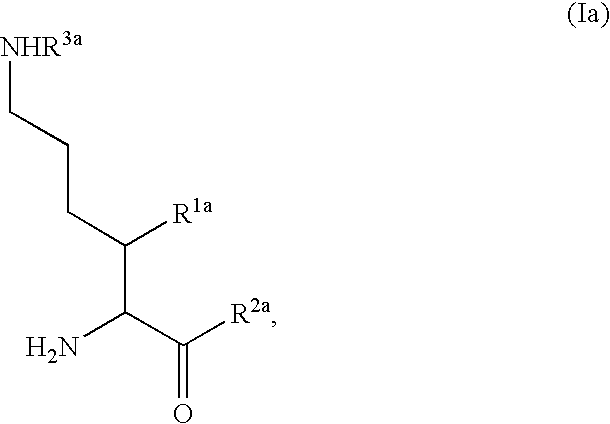
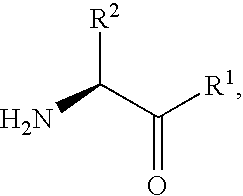
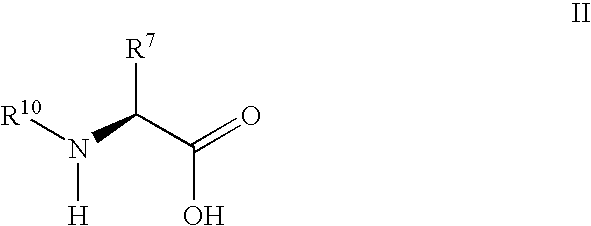
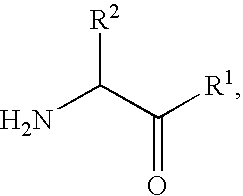
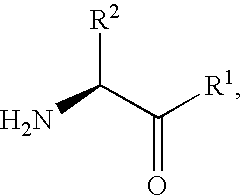
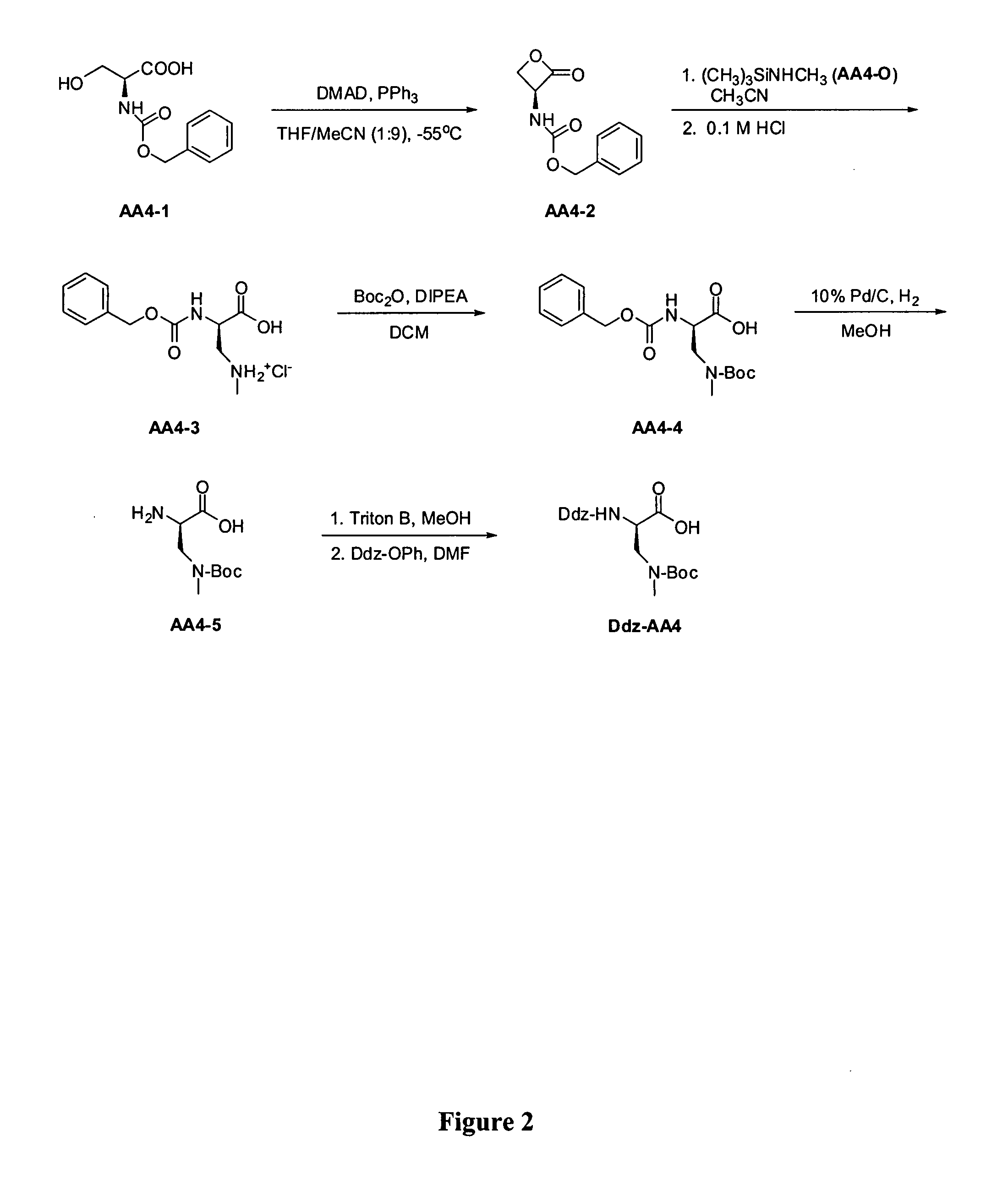
Fluorinated lysine derivatives as dipeptidyl peptidase IV inhibitors
InactiveUS20050043292A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDiabetic retinopathyArthritis
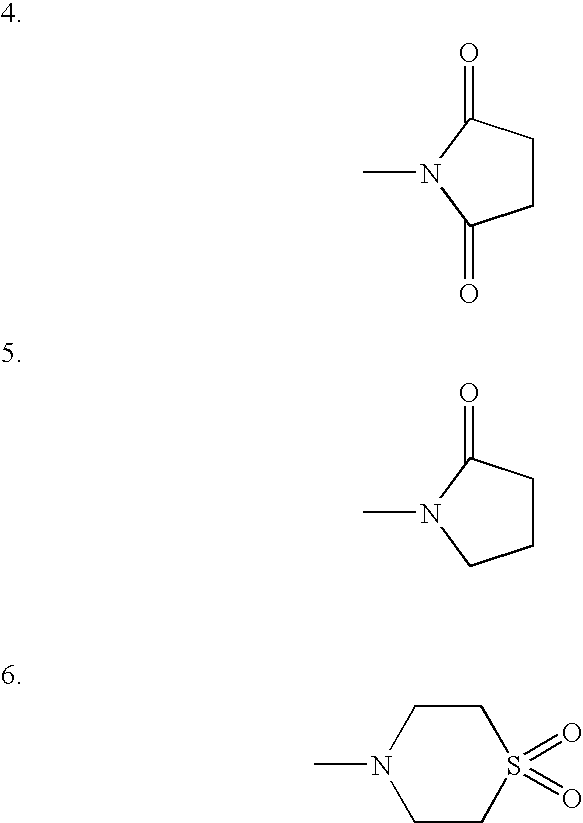
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV (“DPP-IV”), pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes, metabolic syndrome (syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, arthritis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
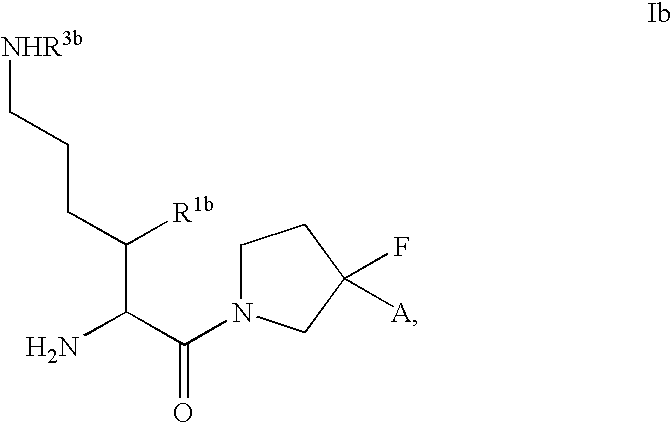
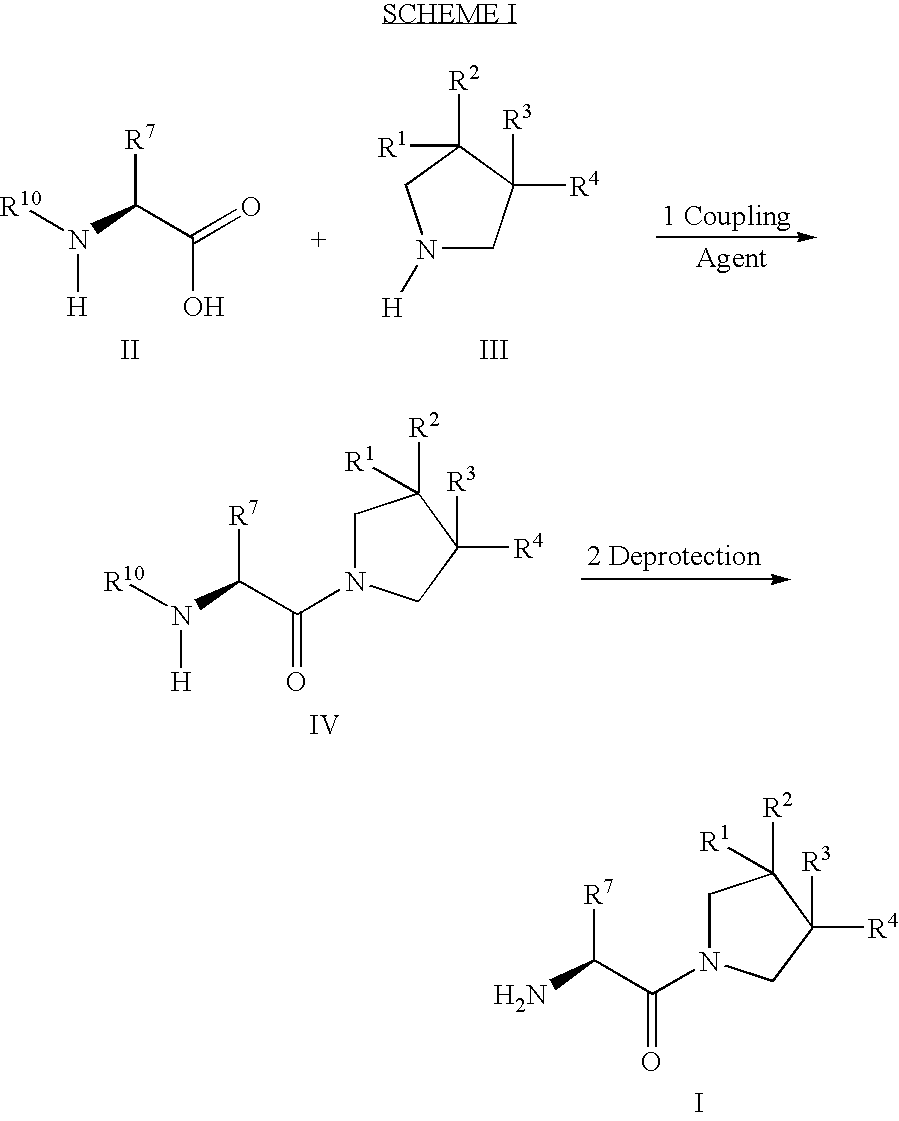
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS6710040B1Easy to prepareEase of detectabilityBiocideOrganic chemistryAcute coronary syndromeDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Dipeptidyl peptidase IV inhibiting fluorinated cyclic amides
InactiveUS20040110817A1Ease of preparation and detectabilityGood metabolic stabilityBiocideSenses disorderDiabetic retinopathyDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
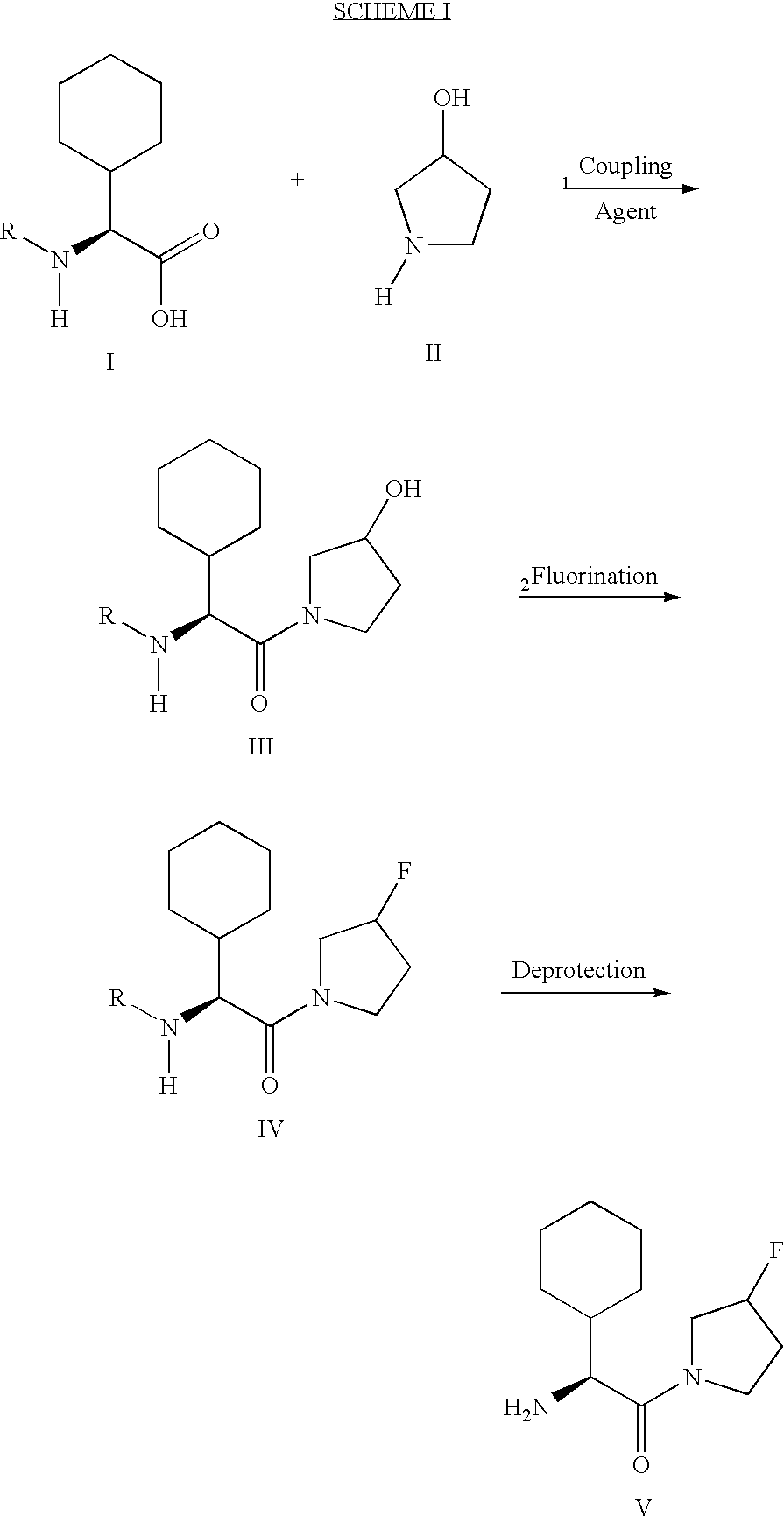
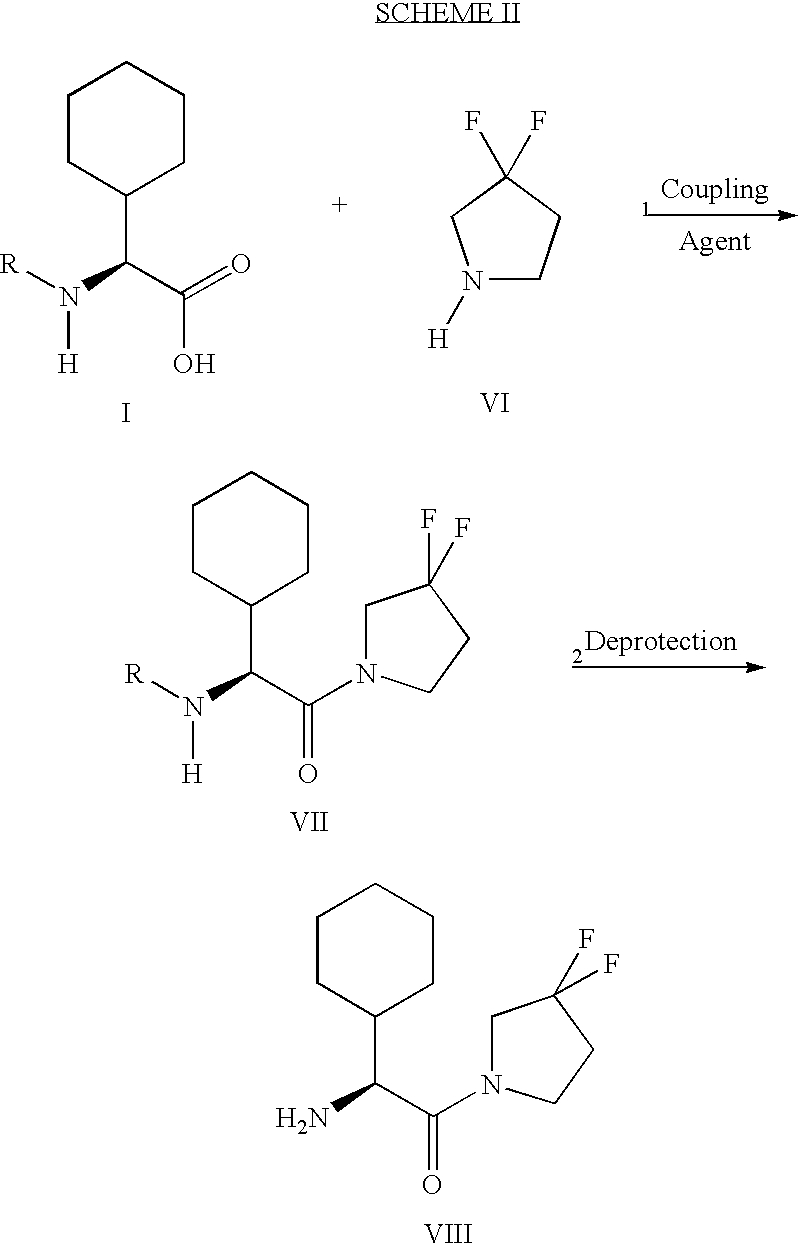
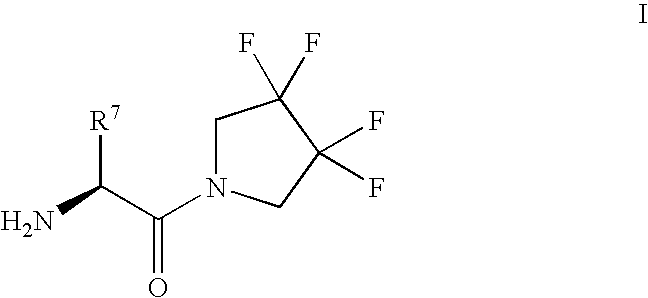
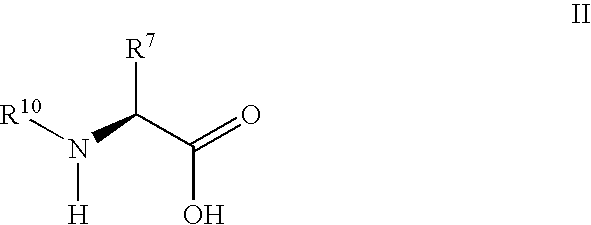
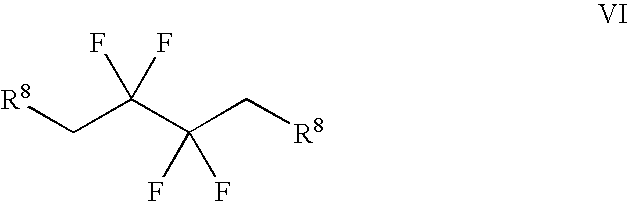
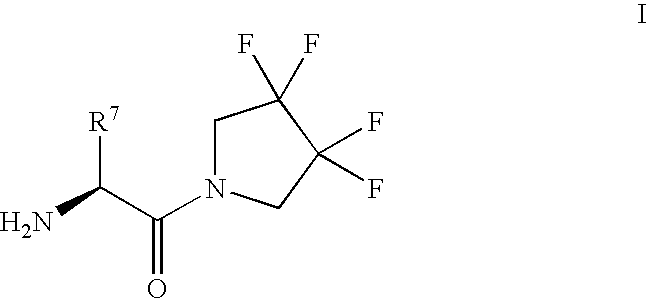
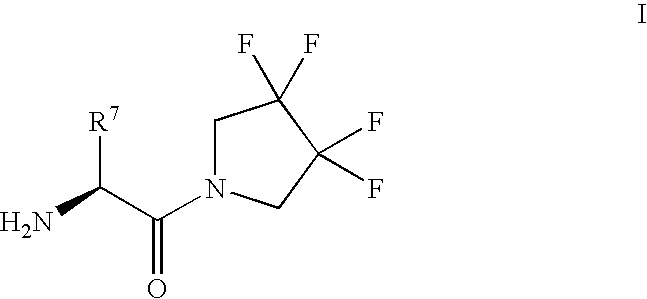
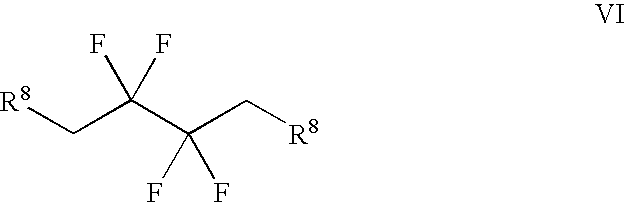
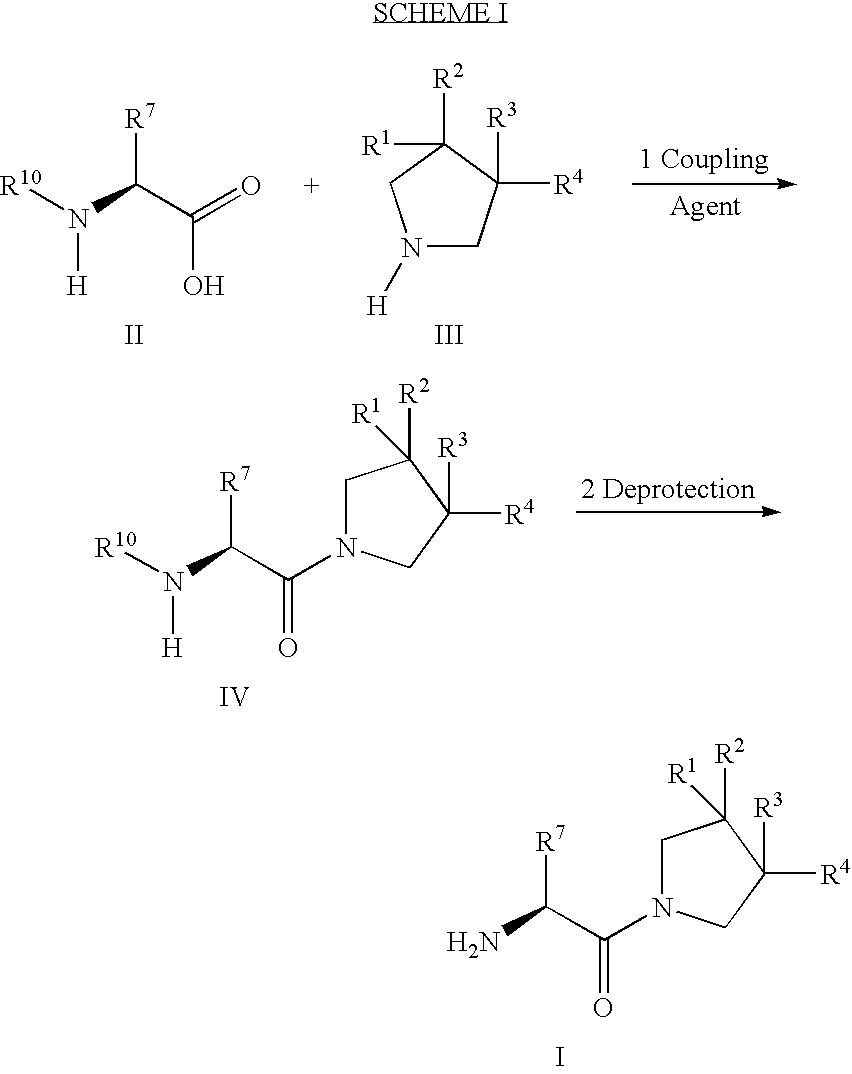
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
The present invention relates to a method of making novel dipeptidyl peptidase-IV ("DPP-IV') inhibitor compounds useful for treating, inter alia, diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, metabolic syndrome (Syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of making 3,3,4,4-tetrafluoropyrrolidine, a starting material utilized in the afore-mentioned method for preparing DPP-IV compounds.
Owner:PFIZER INC
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
InactiveUS6812350B2Metabolism disorderPhosphorus organic compoundsDisease progressionDiabetic nephropathy
Owner:PFIZER INC
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
InactiveUS20040002609A1Easy to cutMetabolism disorderPhosphorus organic compoundsDisease progressionDisease cause
The present invention relates to a method of making novel dipeptidyl peptidase-IV ("DPP-IV') inhibitor compounds useful for treating, inter alia, diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, metabolic syndrome (Syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of making 3,3,4,4-tetrafluoropyrrolidine, a starting material utilized in the afore-mentioned method for preparing DPP-IV compounds.
Owner:PFIZER INC
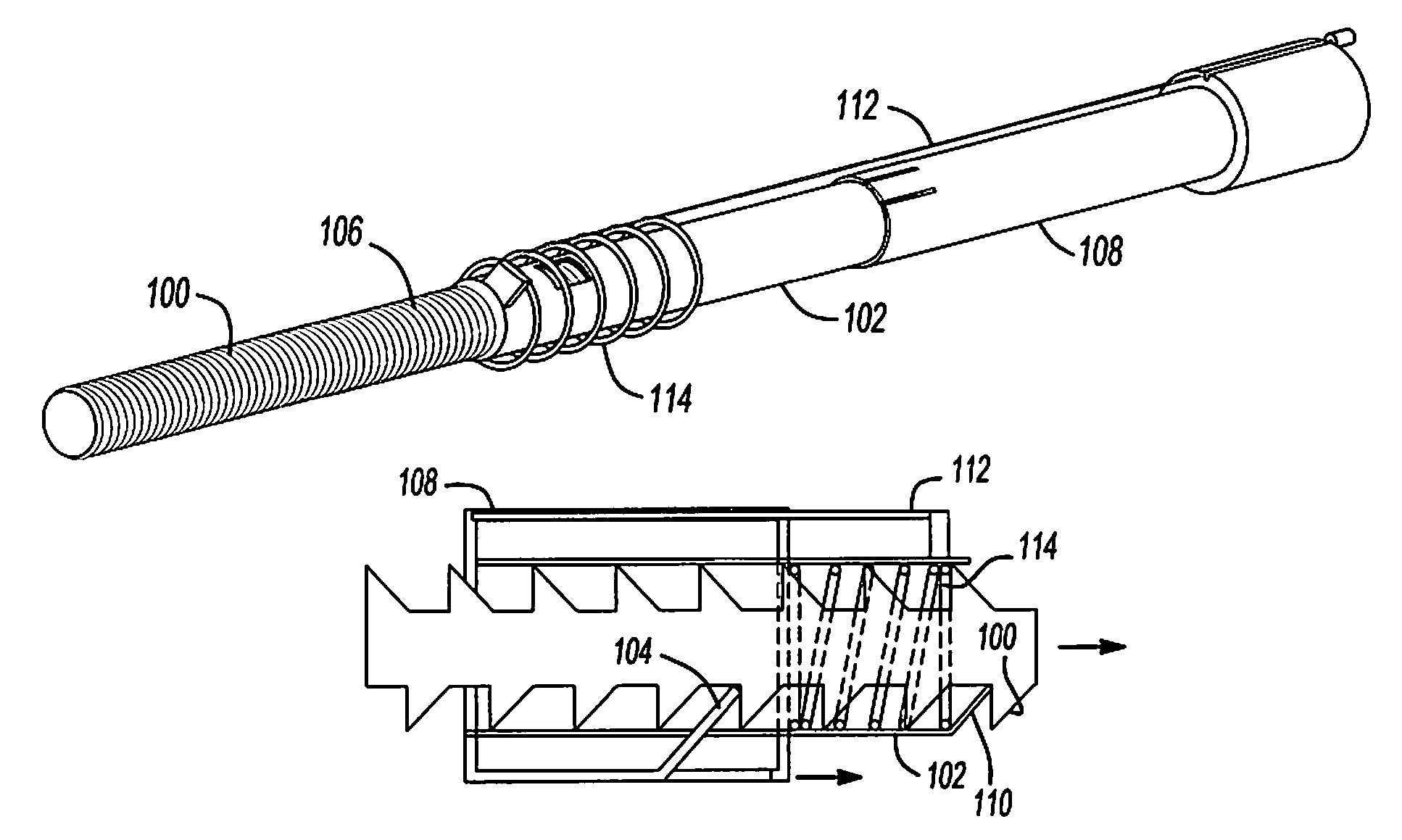
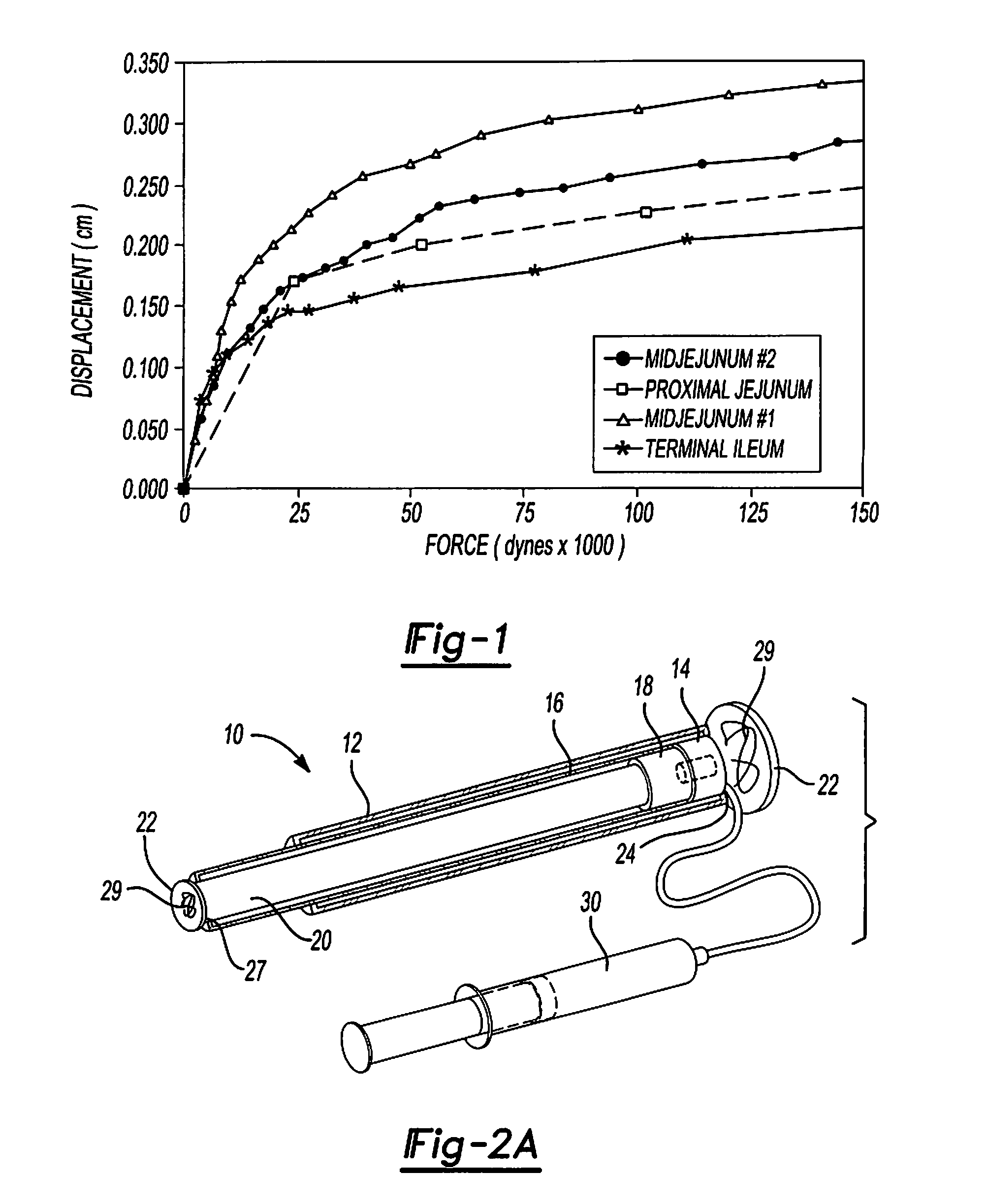
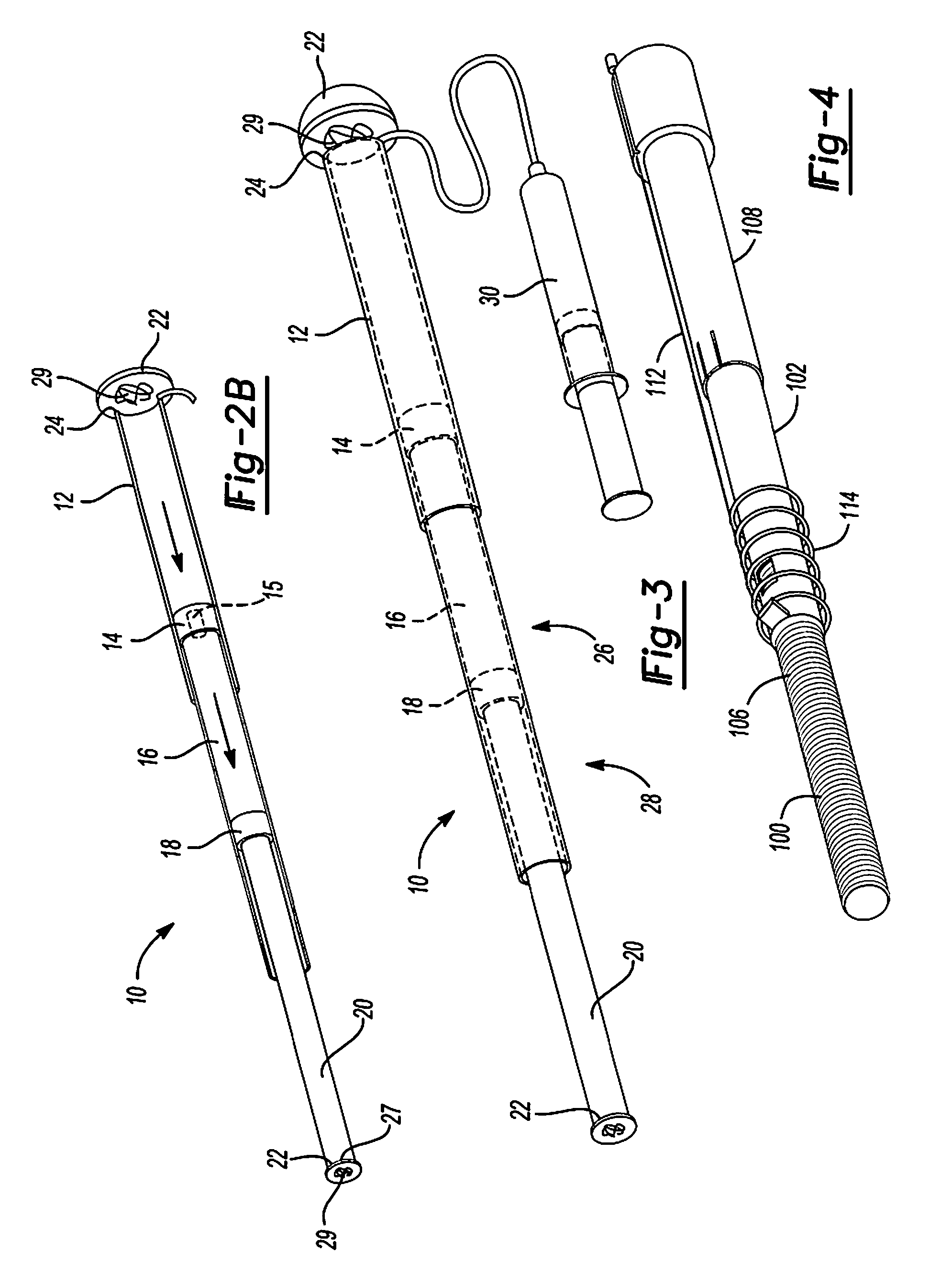
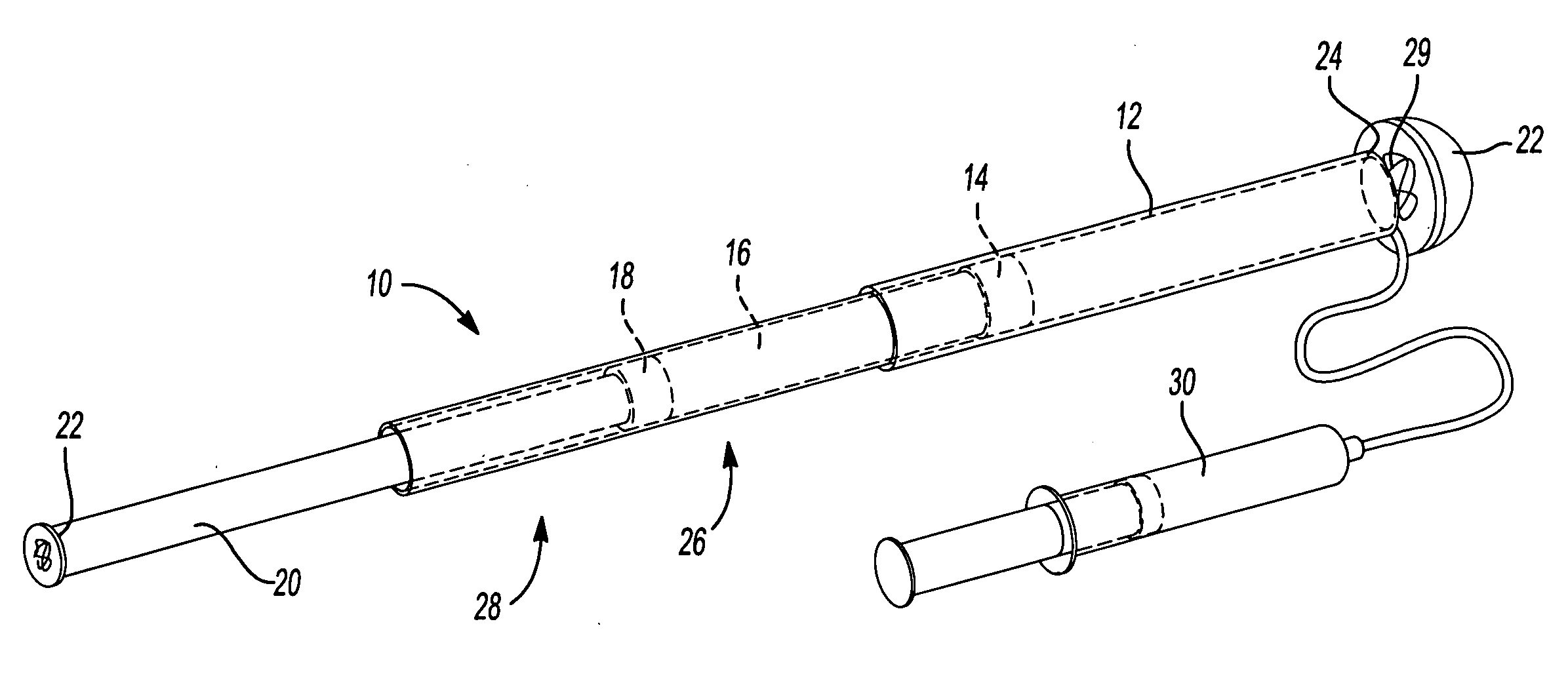
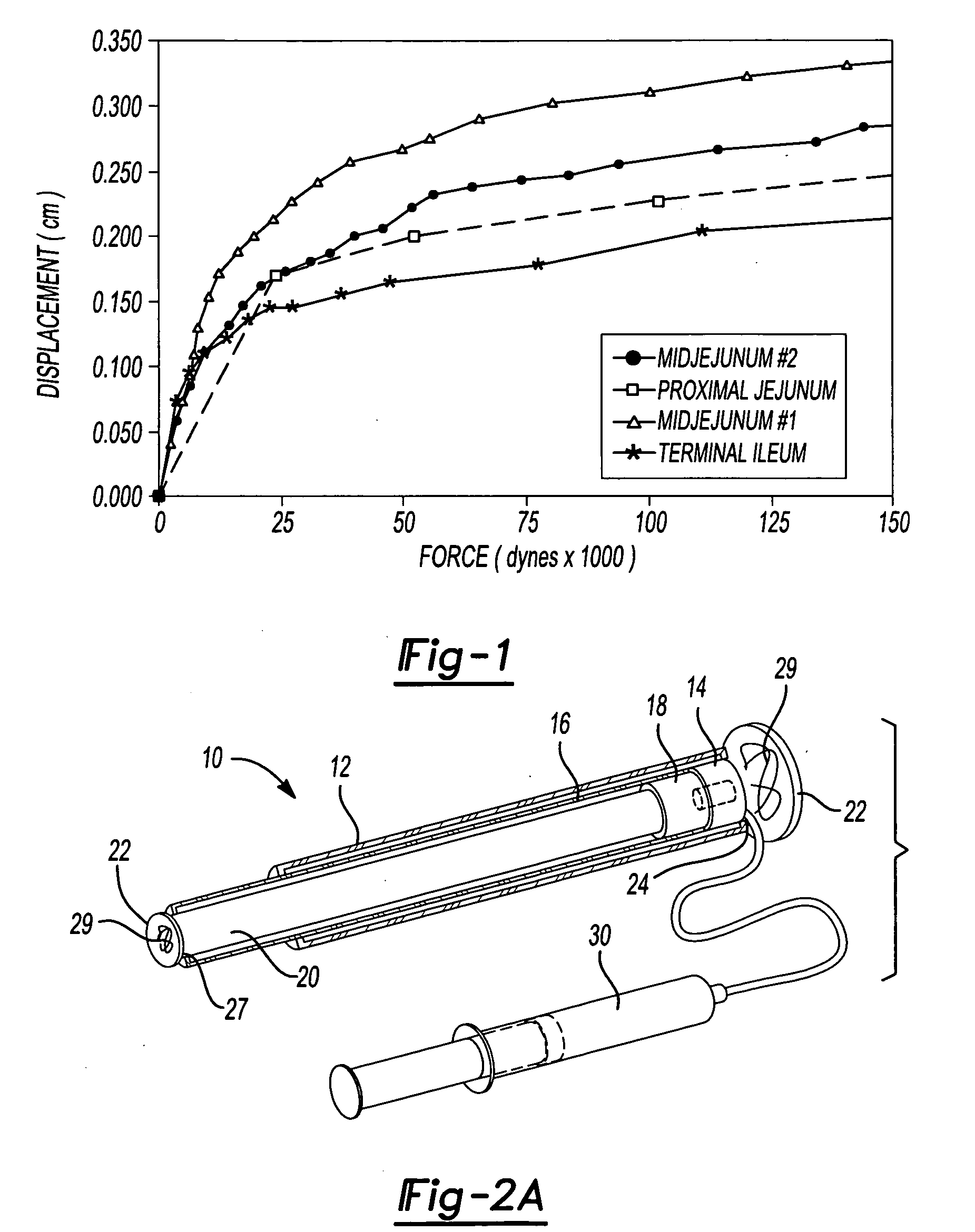
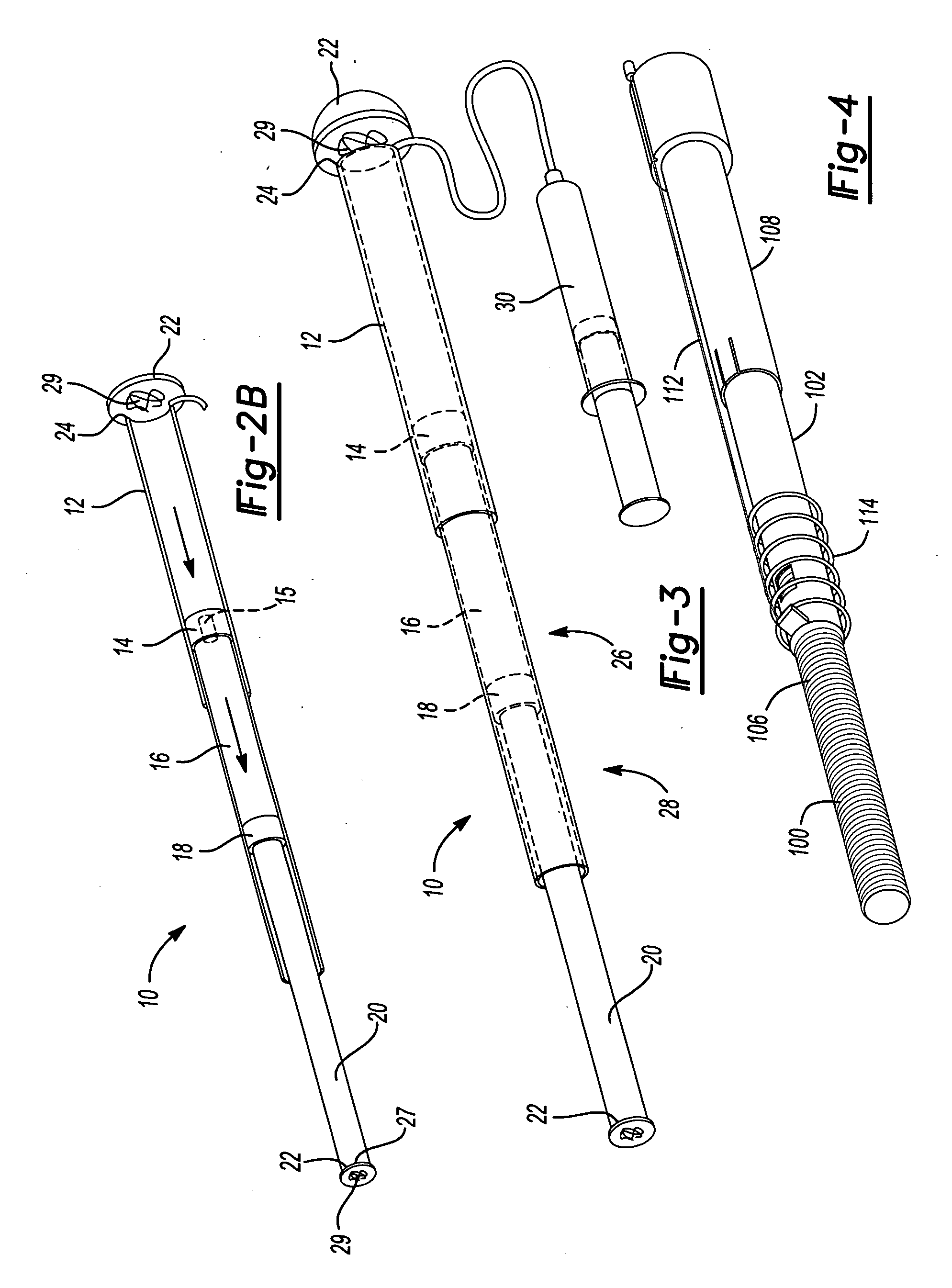
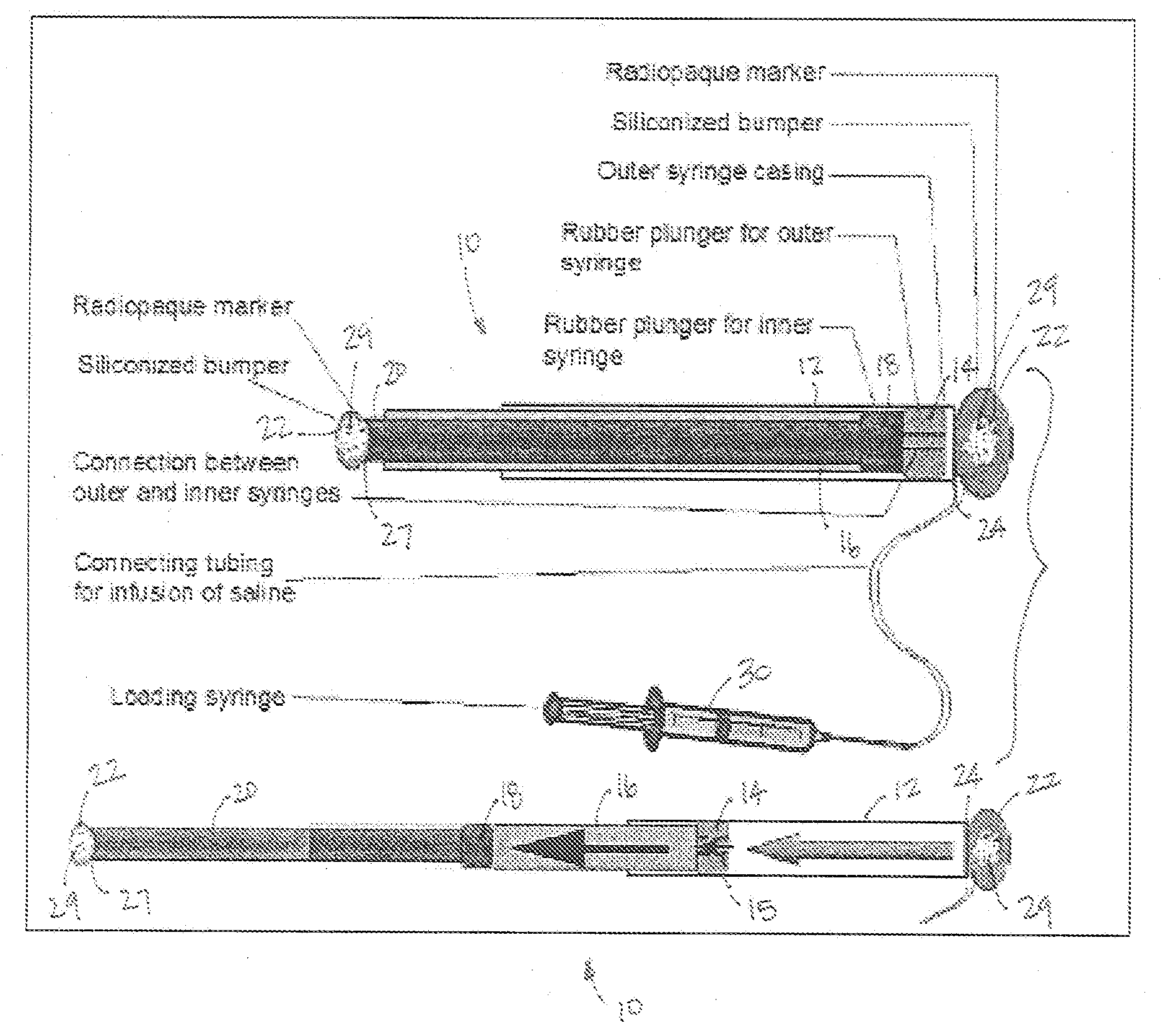
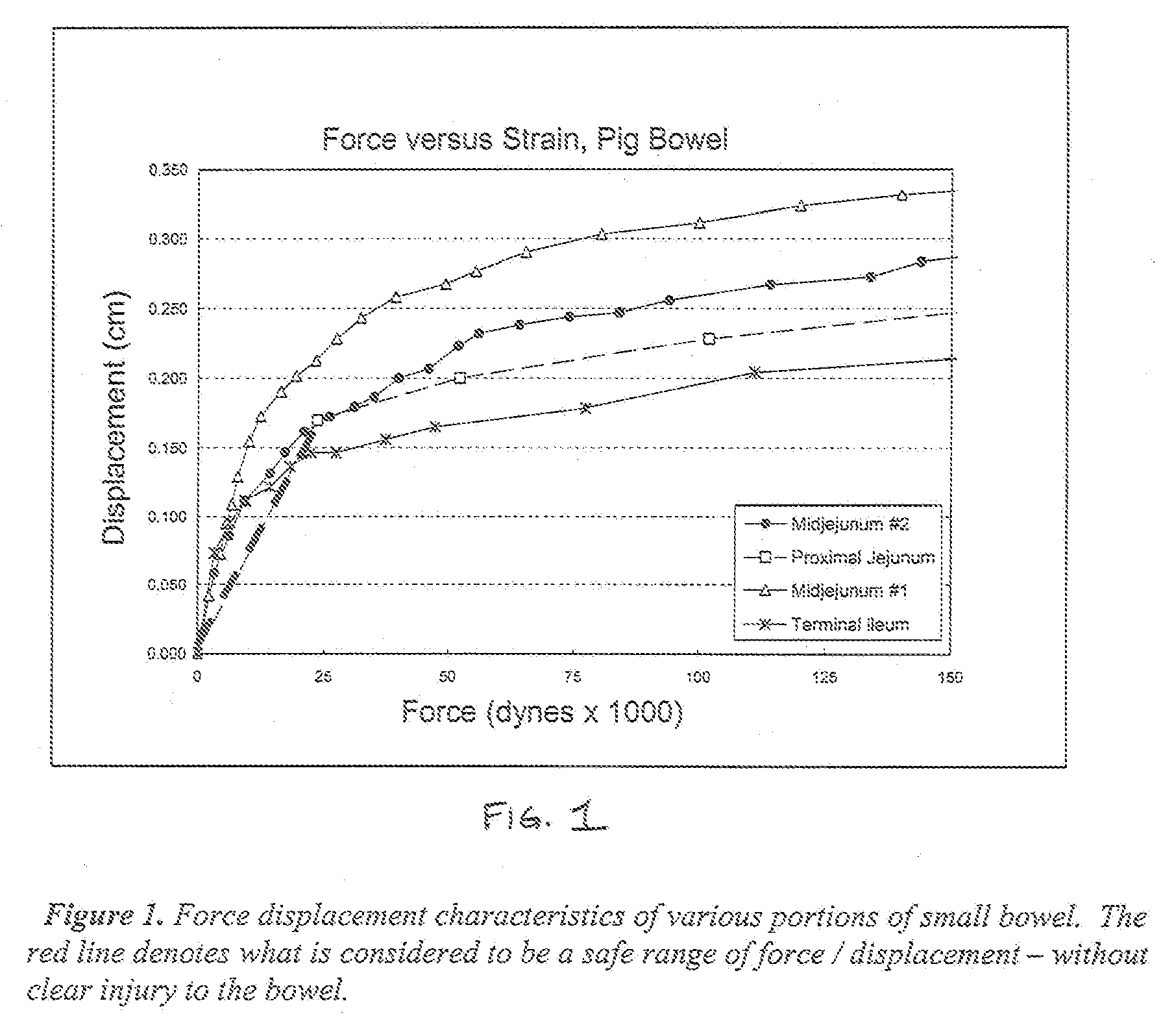
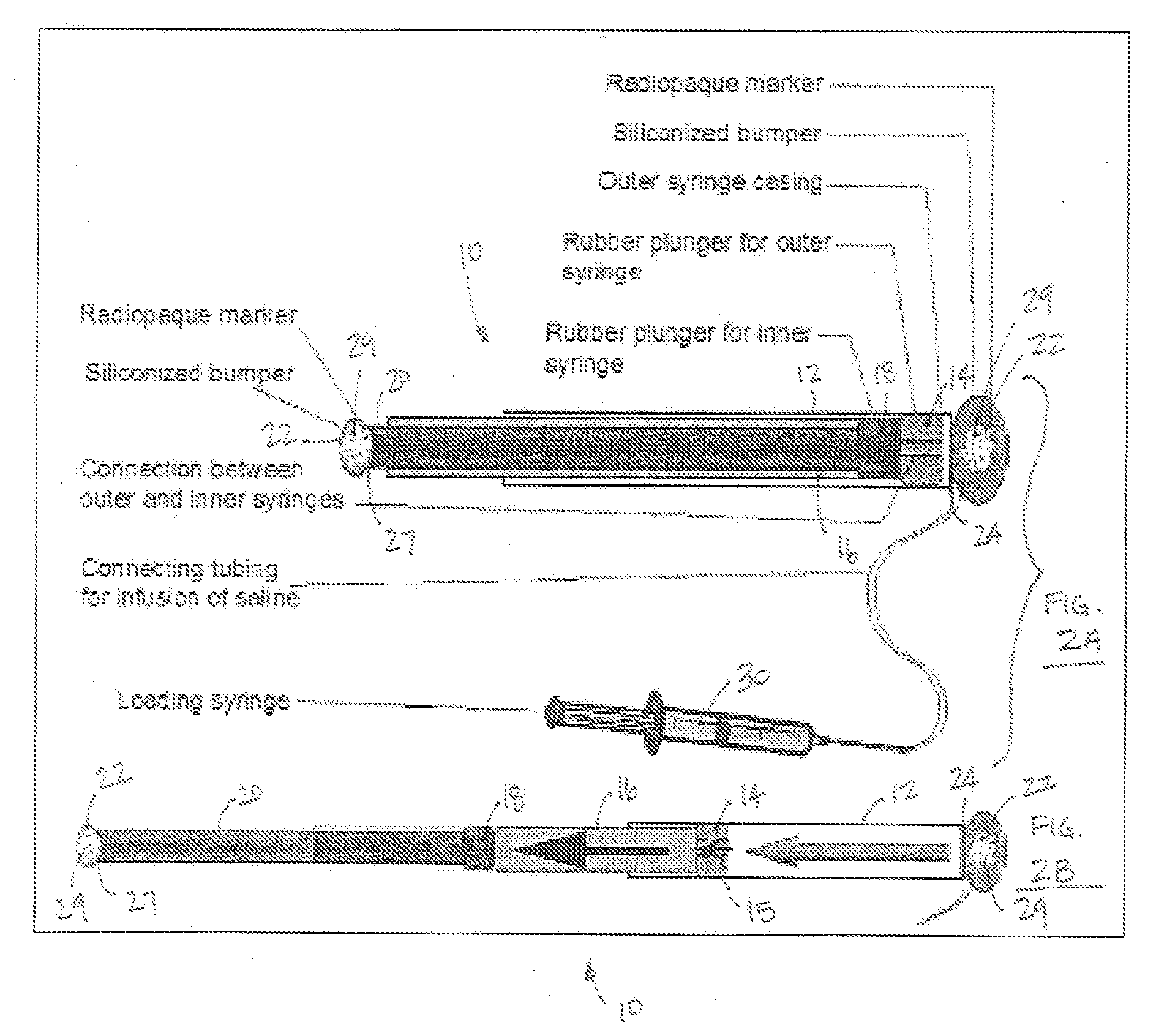
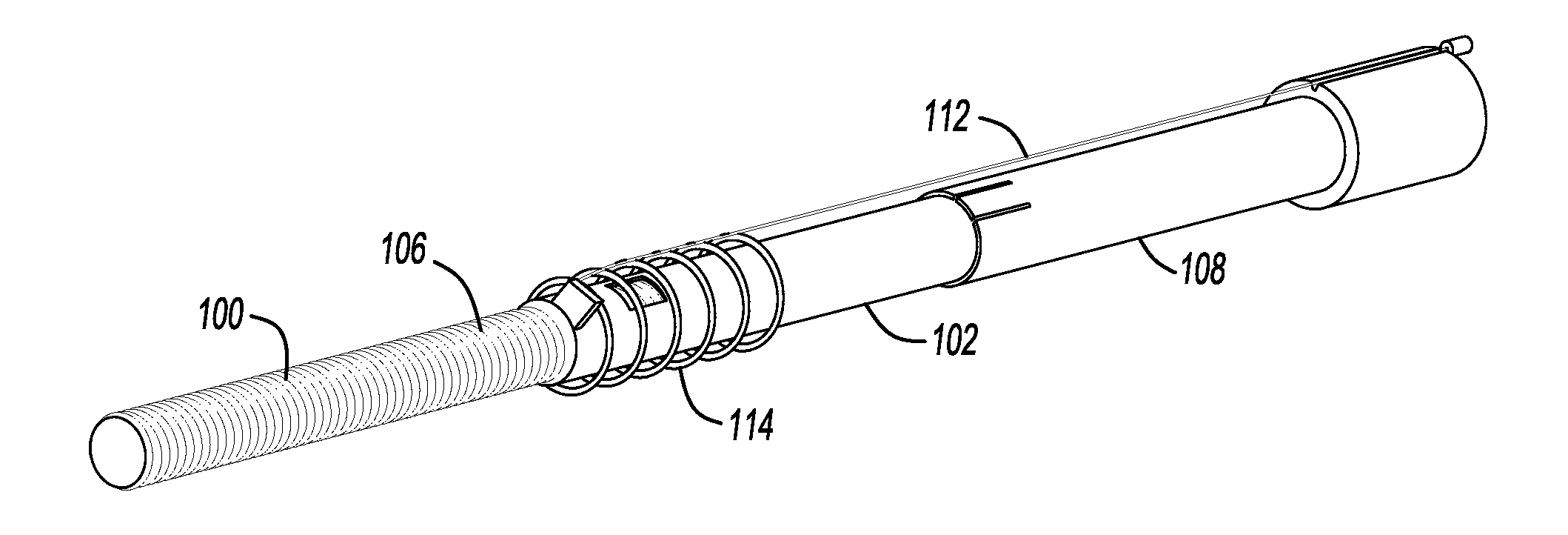
Mechanical extension implants for short bowel syndrome
A bowel extension device implantable into a body for treatment of short bowel syndrome. The bowel extension device comprises a housing and a displaceable member coupled to the housing. The bowel extension device is configured to apply a tensile force sufficient to promote bowel growth without causing damage to the bowel. In some embodiments, the bowel extension device can be completely contained with the body.
Owner:RGT UNIV OF MICHIGAN
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS20040132713A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDisease progressionDiabetic nephropathy
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
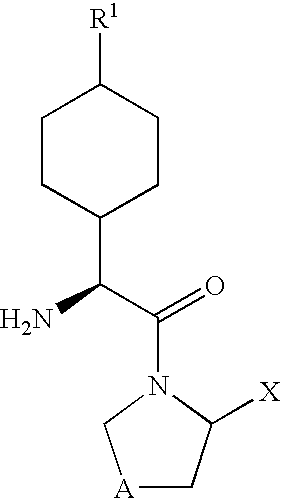
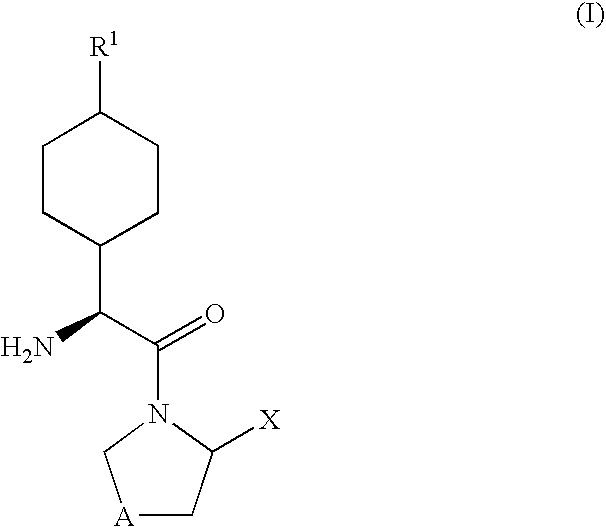
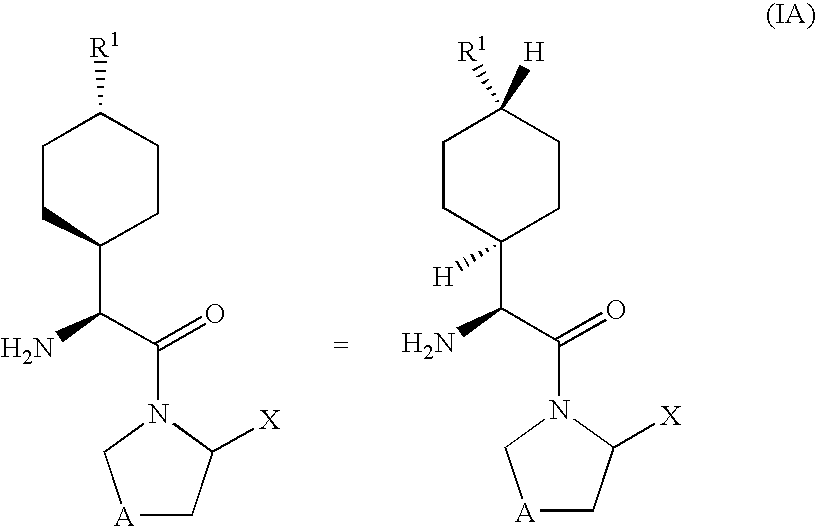
Dipeptidyl peptidase-IV inhibitors
InactiveUS20050234065A1High activityImproved gastrointestinal permeabilityBiocideNervous disorderDiabetic retinopathyArthritis
The invention provides compounds of Formula (I) or prodrugs thereof, or pharmaceutically acceptable salts of said compounds or prodrugs, or solvates of said compounds, prodrugs or salts, wherein A, N, X and R1 are as defined herein; pharmaceutical compositions thereof; and methods of using the pharmaceutical compositions for the treatment of diseases, including Type 2 diabetes, Type 1 diabetes, impaired glucose tolerance, hyperglycemia, metabolic syndrome (syndrome X and / or insulin resistance syndrome), glucosuria, metabolic acidosis, arthritis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, short stature due to growth hormone deficiency, infertility due to polycystic ovary syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome; short bowel syndrome; and the prevention of disease progression in Type 2 diabetes.
Owner:PFIZER INC
Methods using proton pump inhibitors
The invention provides methods of treating and preventing asthma, laryngitis, symptomatic gastroesophageal reflux disease, pregnancy-induced gastroesophageal reflux disease, noncardiac chest pains, coughing, apnea, dyspepsia, inflammatory bowel disease, irritable bowel syndrome, gastritis, stress ulcers, bleeding peptic ulcers, acute gastrointestinal bleeding, infectious enteritis, collagenous colitis, lymphocytic colitis, chronic diarrhea in immunocompromised patients, esophageal ulcers in immunocompromised patients, idiopathic gastric acid hypersecretion, gastroparesis, gastrointestinal motility disorders, Zollinger-Ellison syndrome, short bowel syndrome, emesis, regurgitation, early satiety, chronic sore throat, abdominal pain, abdominal bloating, nausea, sour stomach, diarrhea, constipation, bacterial infections, refractory ulcers, gastrointestinal disorders induced by NSAIDs, Barrett's esophagus, gastrointestinal disorders caused by steroids, gastrointestinal disorders induced by cholinergic compounds, and fungal or viral-induced ulcers in the gastrointestinal tract by administering a therapeutically effective amount of at least one proton pump to a patient in need thereof. The invention also provides on demand relief of symptoms associated with gastroesophageal reflux disease (GERD), and provides relief from symptoms caused by the consumption of excessive amounts of food and / or alcohol by administering a therapeutically effective amount of at least one proton pump inhibitor to a patient in need thereof. The invention also provides methods for treating parasitic infections, such as malaria, by administering a therapeutically effective amount of at least one proton pump inhibitor to a patient in need thereof.
Owner:EISAI CO LTD
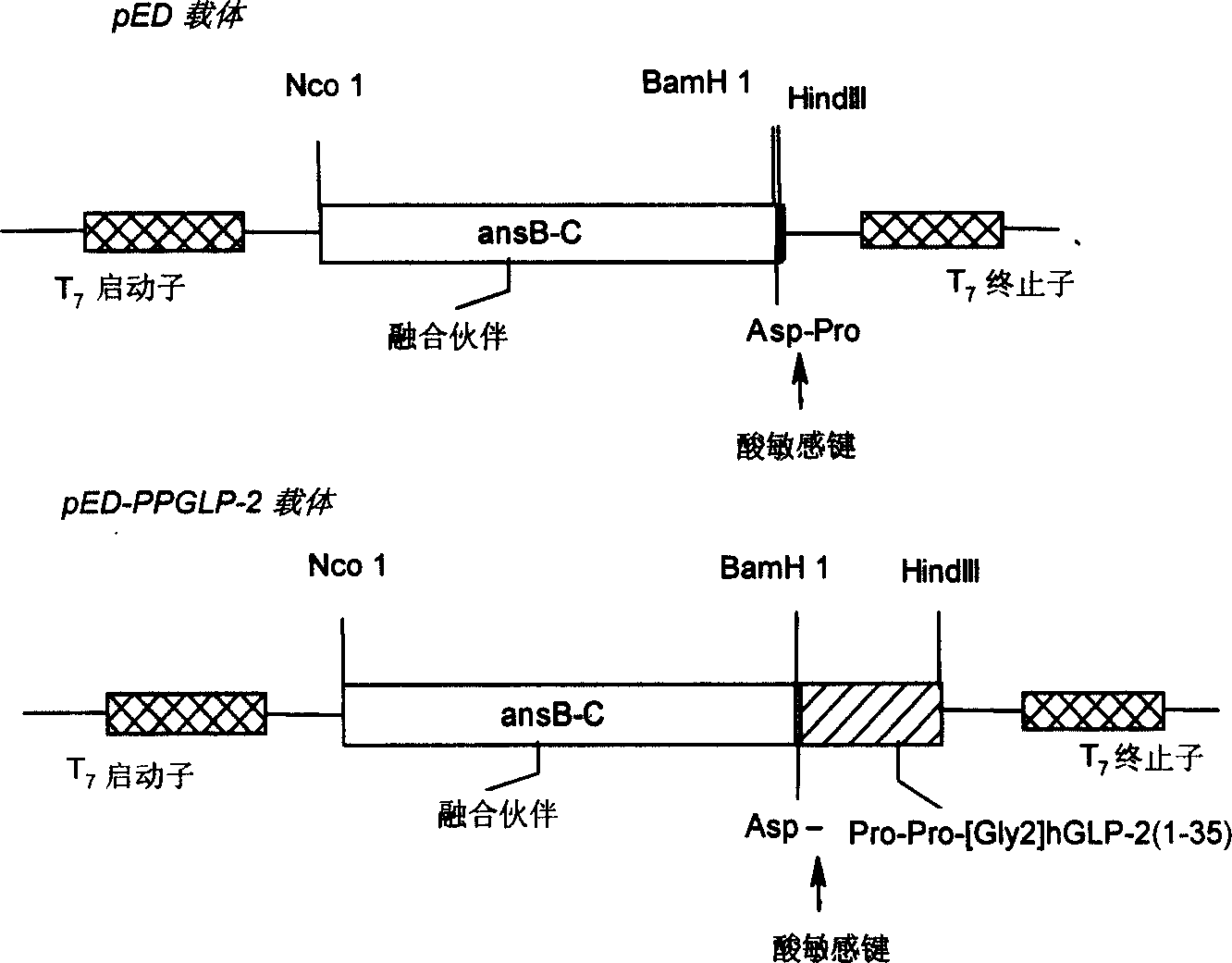
Human pancreas hyperglycemiacin relative peptide-2 analogue
A human glucagon associated peptide-2 analog Pro-Pro-h[Gly2]GLP-2(1-35) for treating the short bowel syndrome and malabsorption of stomach and intestine is prepared through configuring genetic engineering bacterium for effective expression of Pro- Pro-h[Gly2]GLP-2(1-35) in colibacillus cell, splitting, washing, dissolving urea, depositing in alcohol, separating fusion protein, hydrolyzing it, chromatography by DEAE-52 column, separating by HPLC, and freeze drying.
Owner:CHINA PHARM UNIV
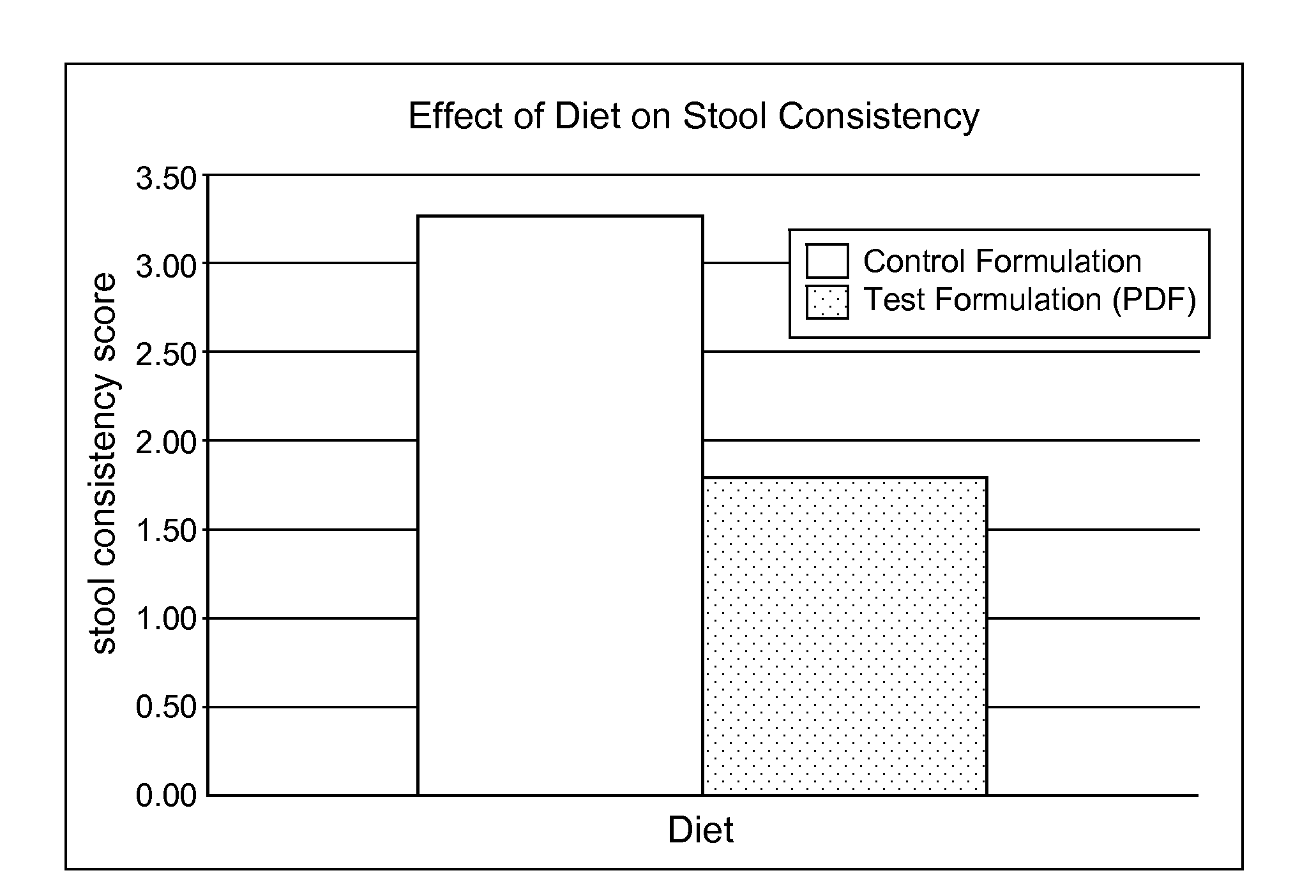
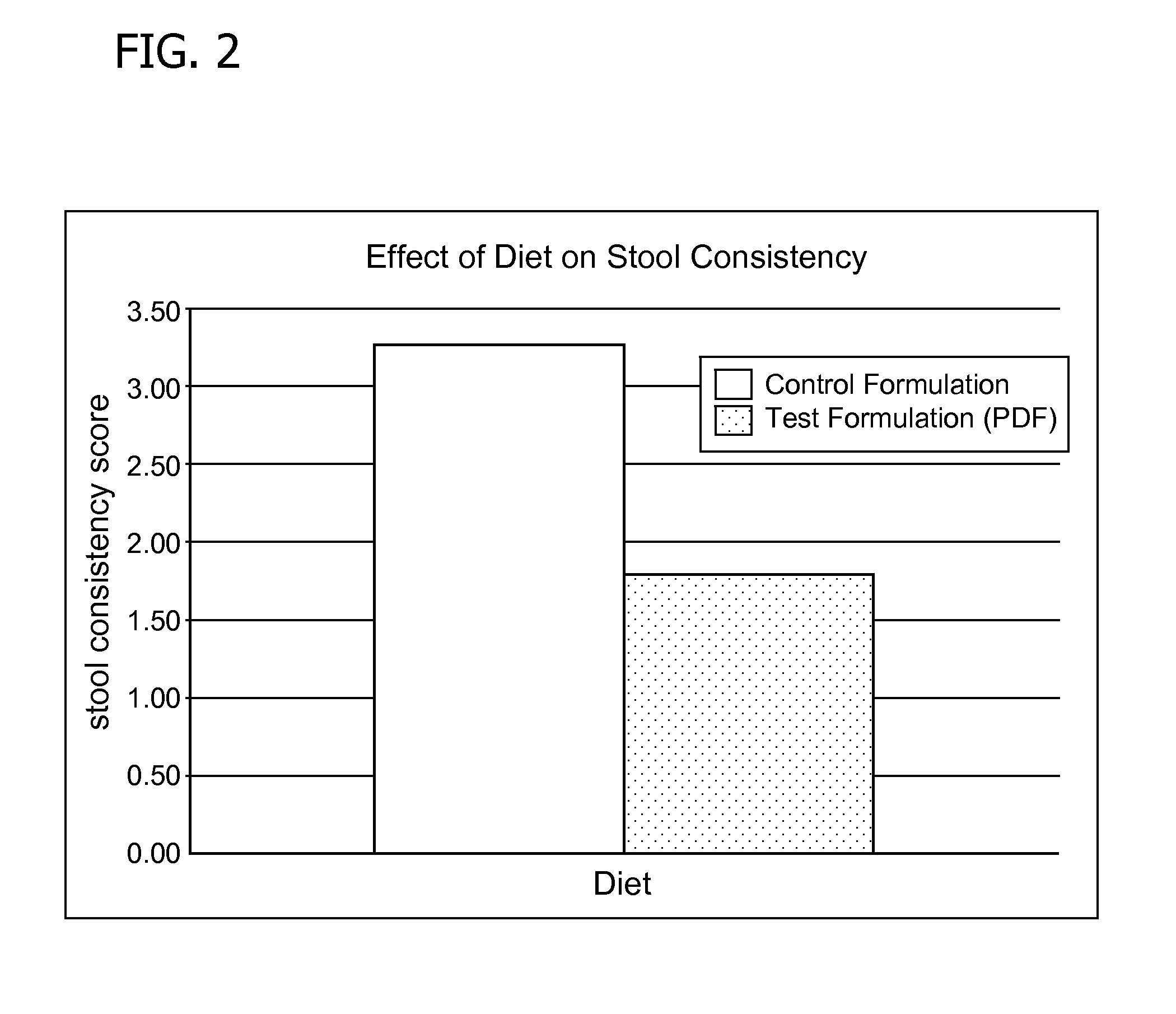
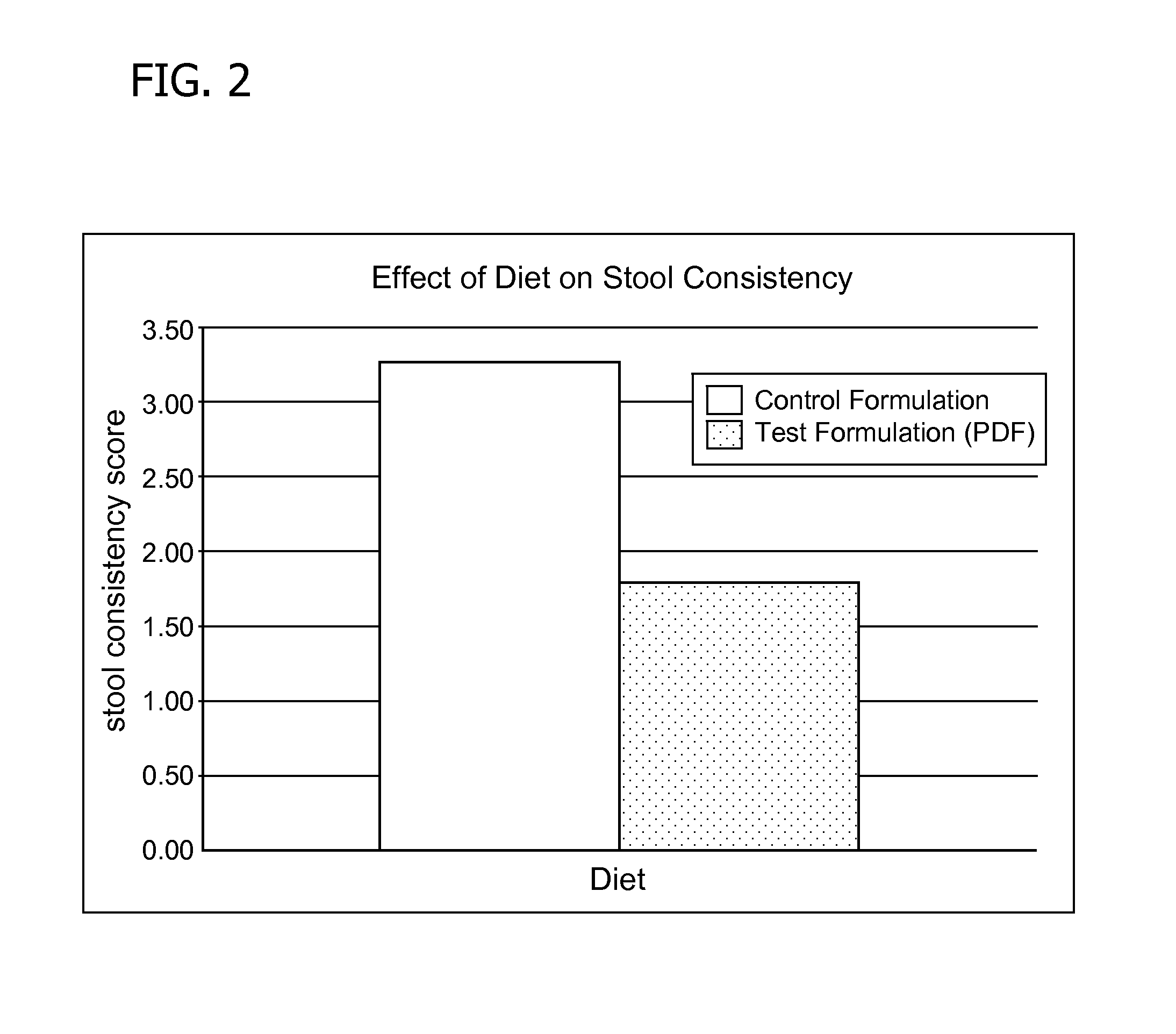
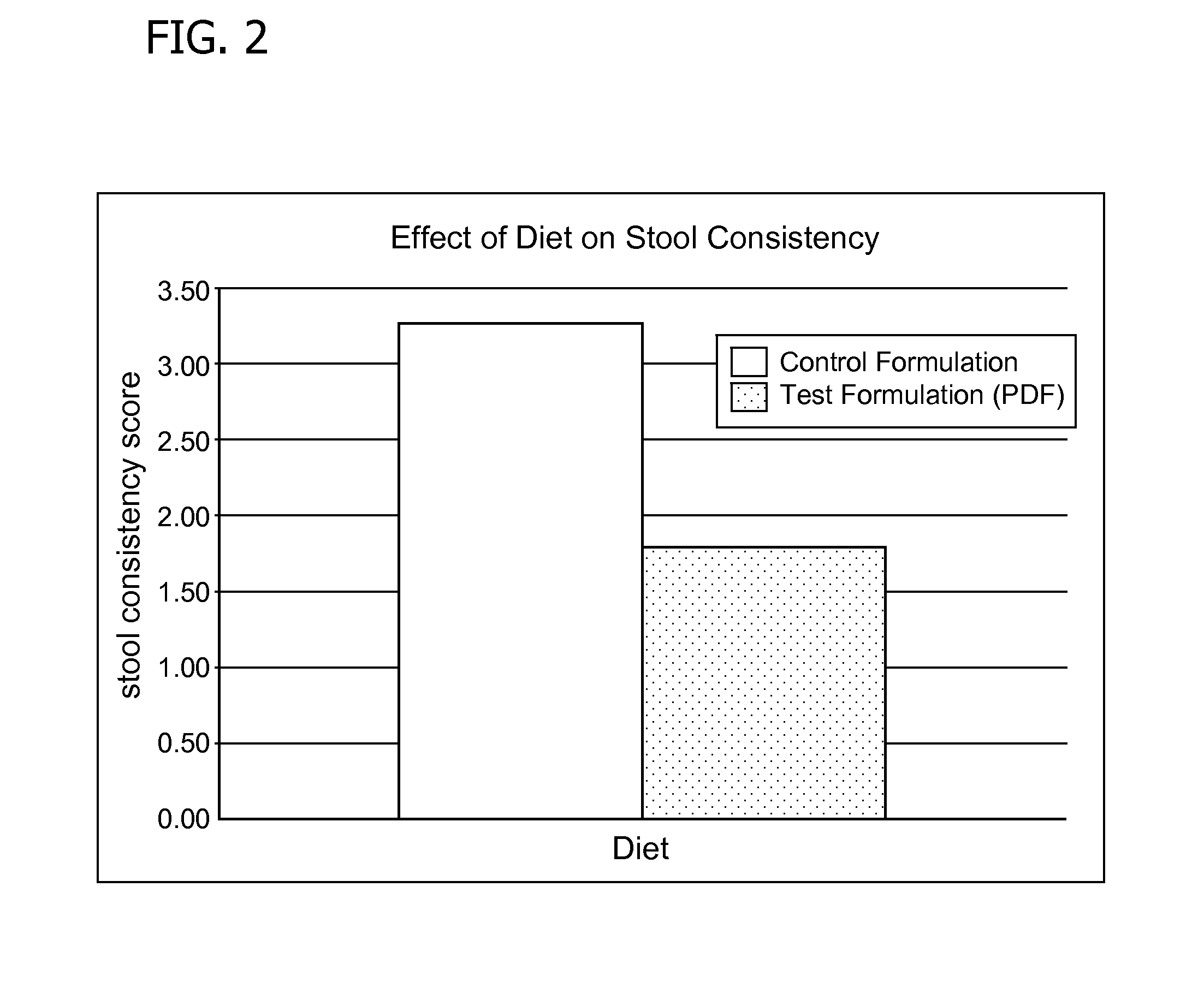
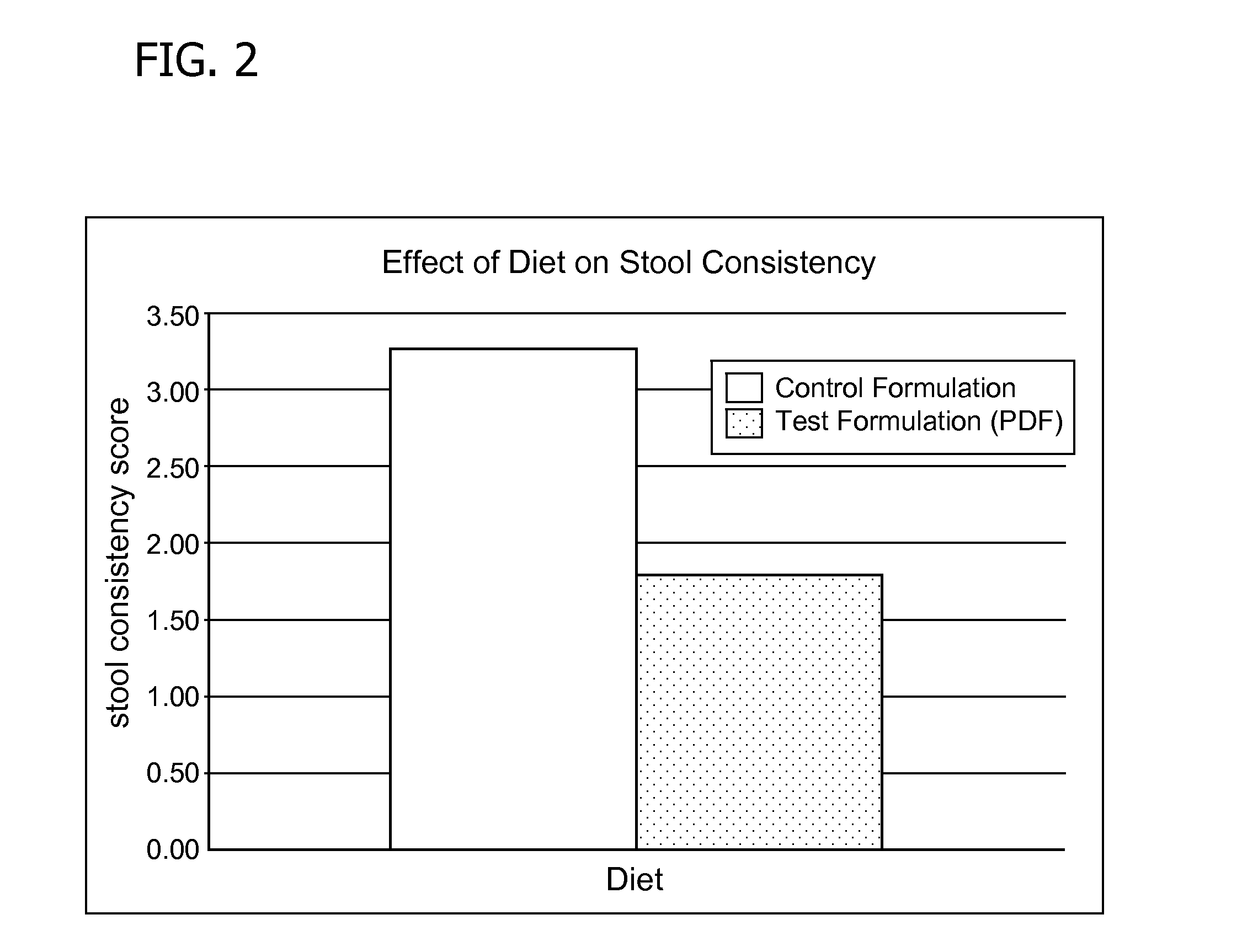
Methods for improving tolerance, digestion, and lipid soluble nutrient absorption in an infant, toddler, or child
ActiveUS20120172434A1Reduce the burden onImprove infant fat digestionBiocideHydroxy compound active ingredientsPremature thelarcheMonoglyceride
Disclosed are nutritional formulations including predigested fats that can be administered to preterm infants, infants, toddlers, and children for improving tolerance, digestion, and absorption of nutrients and for reducing the incidence of necrotizing enterocolitis, colic, and short bowel syndrome. The predigested fats include fatty acid-containing monoglycerides and / or a fatty acid component.
Owner:ABBOTT LAB INC
Methods for improving tolerance, digestion, and lipid soluble nutrient absorption in an infant, toddler, or child
ActiveUS8754126B2Improved tolerance and digestion and absorptionReduce morbidityBiocideHydroxy compound active ingredientsPremature thelarcheMonoglyceride
Disclosed are nutritional formulations including predigested fats that can be administered to preterm infants, infants, toddlers, and children for improving tolerance, digestion, and absorption of nutrients and for reducing the incidence of necrotizing enterocolitis, colic, and short bowel syndrome. The predigested fats include fatty acid-containing monoglycerides and / or a fatty acid component.
Owner:ABBOTT LAB INC
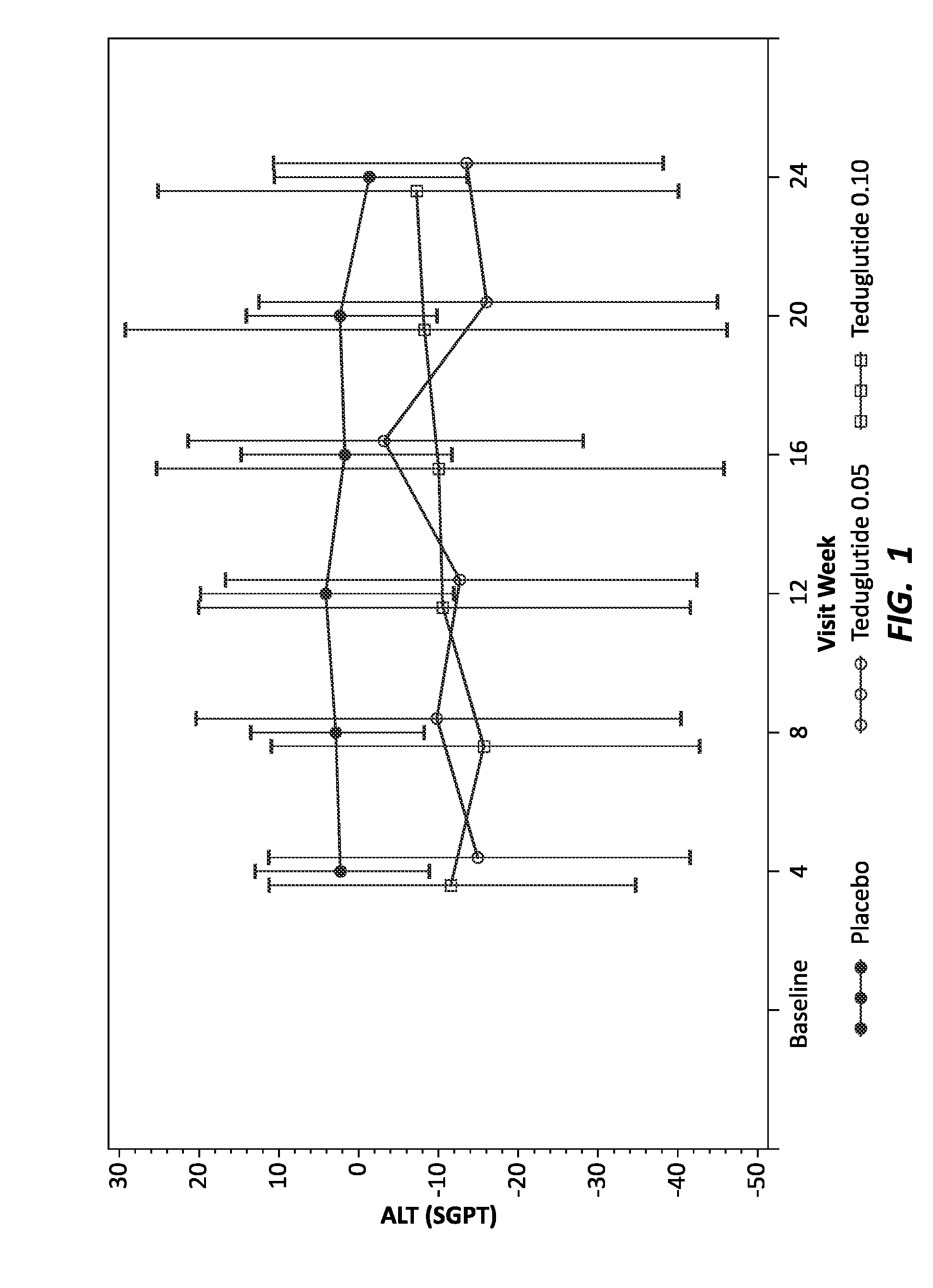
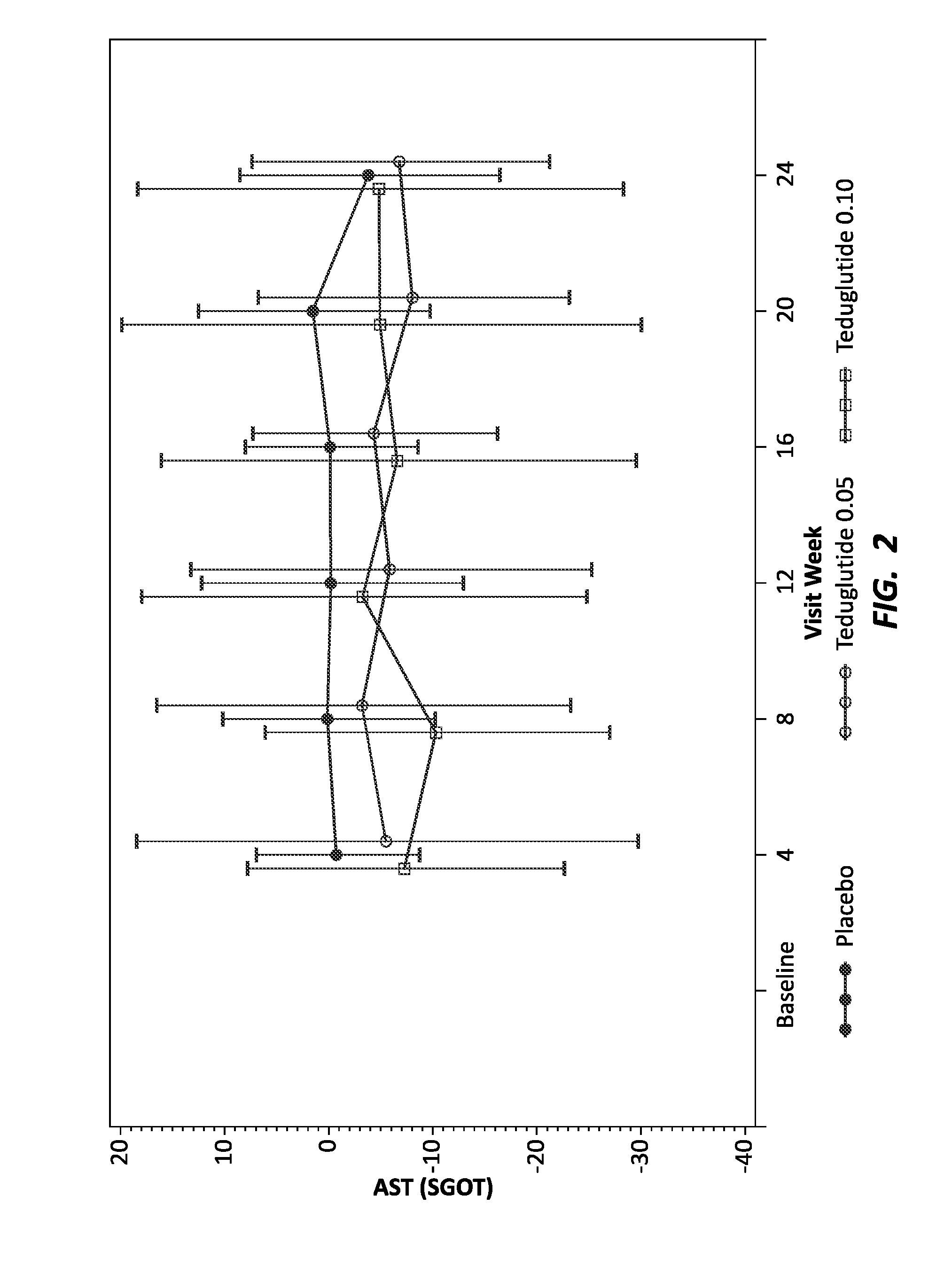
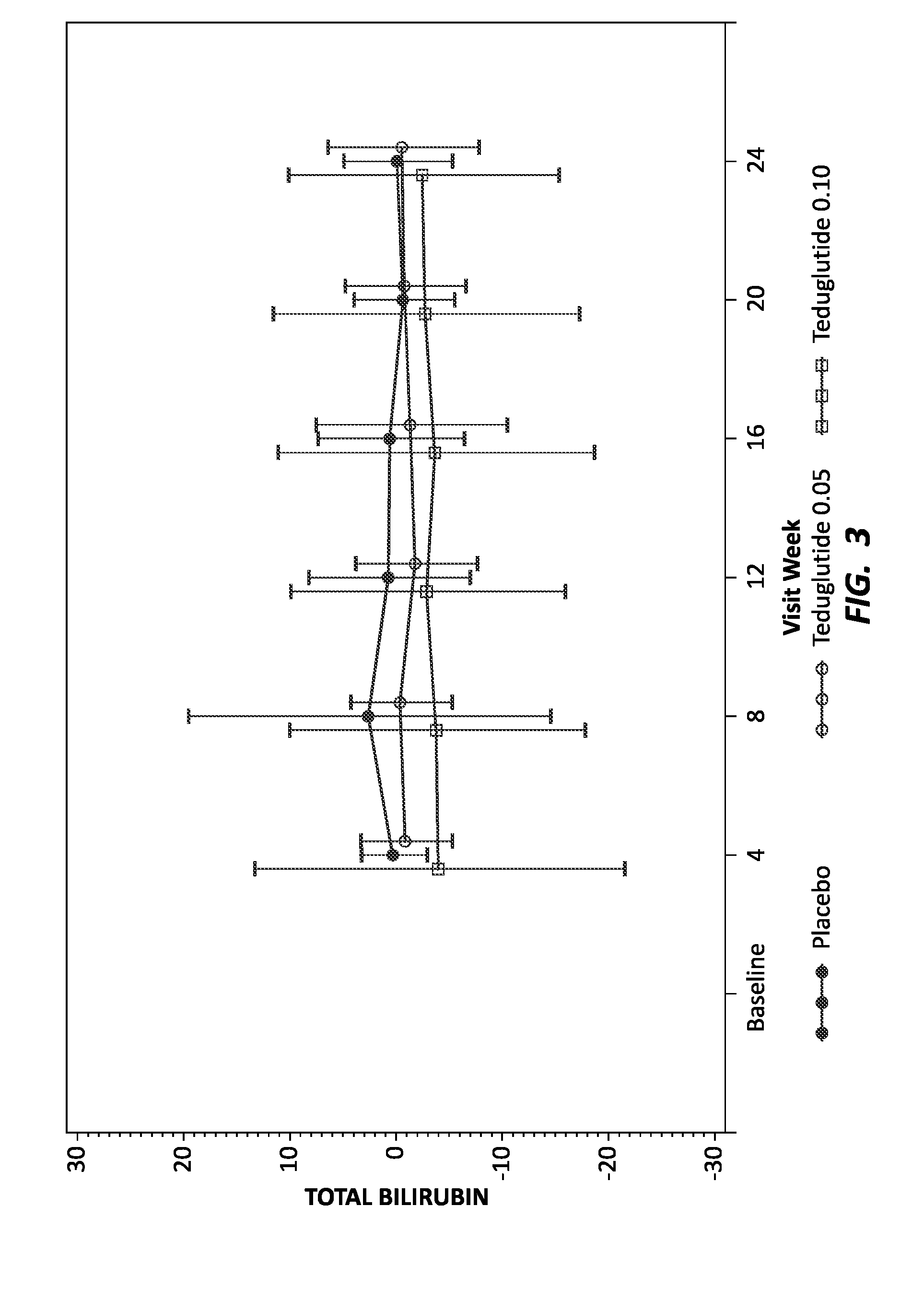
Use of GLP-1 receptor agonists for the treatment of short bowel syndrome
ActiveUS8236760B2Peptide/protein ingredientsMetabolism disorderEsophageal motor disorderIncretin Hormone
Owner:CEDARS SINAI MEDICAL CENT
Macrocyclic antagonists of the motilin receptor for treatment of gastrointestinal dysmotility disorders
The present invention provides conformationally-defined macrocyclic compounds that bind to and / or are functional modulators of the motilin receptor including subtypes, isoforms and / or variants thereof. These macrocyclic compounds, at a minimum, possess adequate pharmacological properties to be useful as therapeutics for a range of disease indications. In particular, these compounds are useful for treatment and prevention of disorders characterized by hypermotilinemia and / or gastrointestinal hypermotility, including, but not limited to, diarrhea, cancer treatment-related diarrhea, cancer-induced diarrhea, chemotherapy-induced diarrhea, radiation enteritis, radiation-induced diarrhea, stress-induced diarrhea, chronic diarrhea, AIDS-related diarrhea, C. difficile associated diarrhea, traveller's diarrhea, diarrhea induced by graph versus host disease, other types of diarrhea, dyspepsia, irritable bowel syndrome, chemotherapy-induced nausea and vomiting (emesis) and post-operative nausea and vomiting and functional gastrointestinal disorders. In addition, the compounds possess utility for the treatment of diseases and disorders characterized by poor stomach or intestinal absorption, such as short bowel syndrome, celiac disease and cachexia. The compounds also have use for the treatment of inflammatory diseases and disorders of the gastrointestinal tract, such as inflammatory bowel disease, ulcerative colitis, Crohn's disease and pancreatitis. Accordingly, methods of treating such disorders and pharmaceutical compositions including compounds of the present invention are also provided.
Owner:OCERA THERAPEUTICS INC
Nutritional products including monoglycerides and fatty acids
ActiveUS20120171350A1Reduce the burden onImprove infant fat digestionHydroxy compound active ingredientsMetabolism disorderMonoglycerideMedicine
Disclosed are nutritional formulations including predigested fats that can be administered to preterm infants, infants, toddlers, and children for improving tolerance, digestion, and absorption of nutrients and for reducing the incidence of necrotizing enterocolitis, colic, and short bowel syndrome. The predigested fats include fatty acid-containing monoglycerides and / or a fatty acid component.
Owner:ABBOTT LAB INC
Mechanical extension implants for short bowel syndrome
ActiveUS20090240339A1Induce intestinal growthInduced growthDiagnosticsSurgeryShort bowel syndromeGeneral surgery
A bowel extension device implantable into a body for treatment of short bowel syndrome. The bowel extension device comprises a housing and a displaceable member coupled to the housing. The bowel extension device is configured to apply a tensile force sufficient to promote bowel growth without causing damage to the bowel. In some embodiments, the bowel extension device can be completely contained with the body.
Owner:RGT UNIV OF MICHIGAN
Methods and means to promote gut absorption
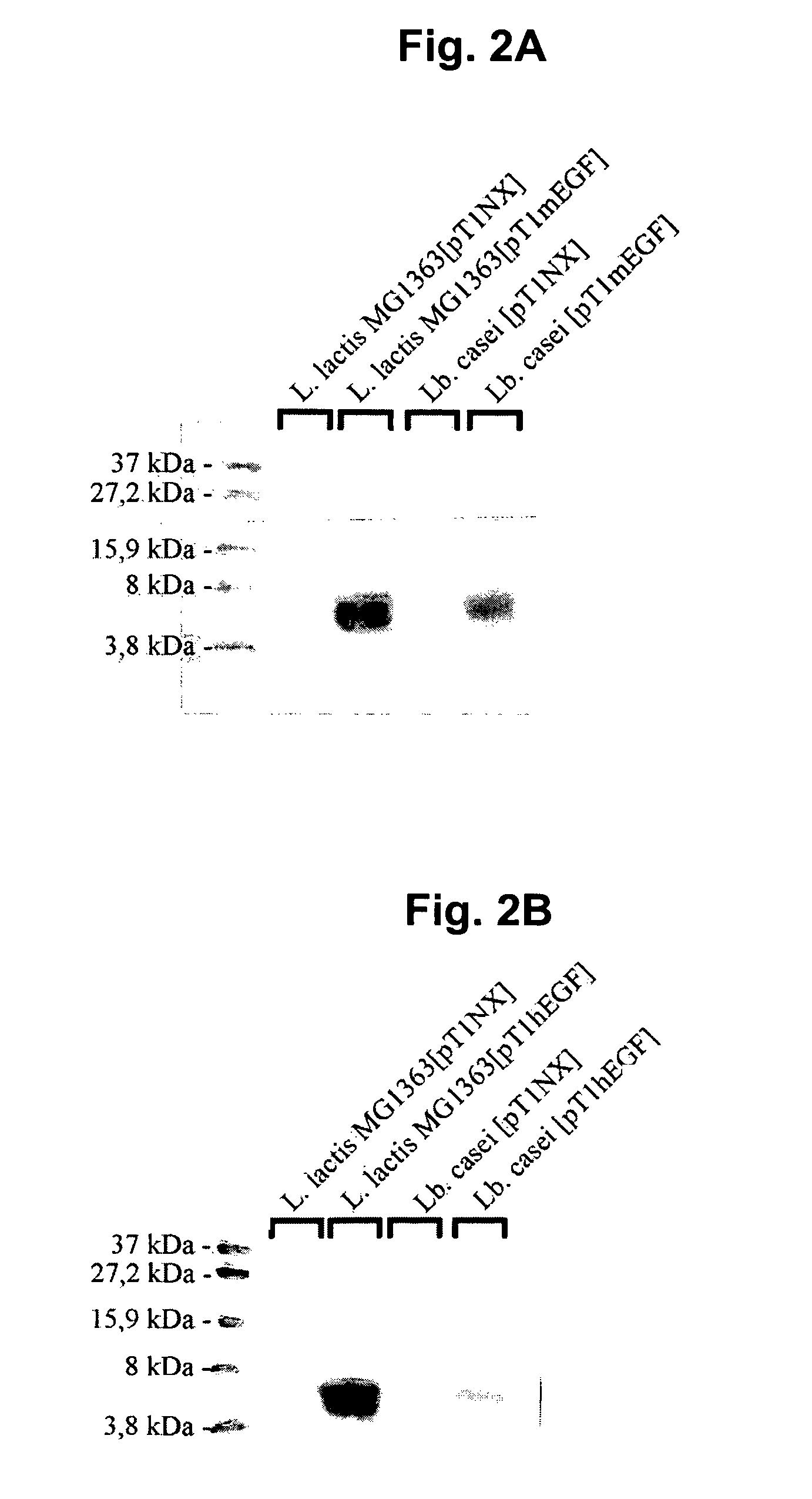
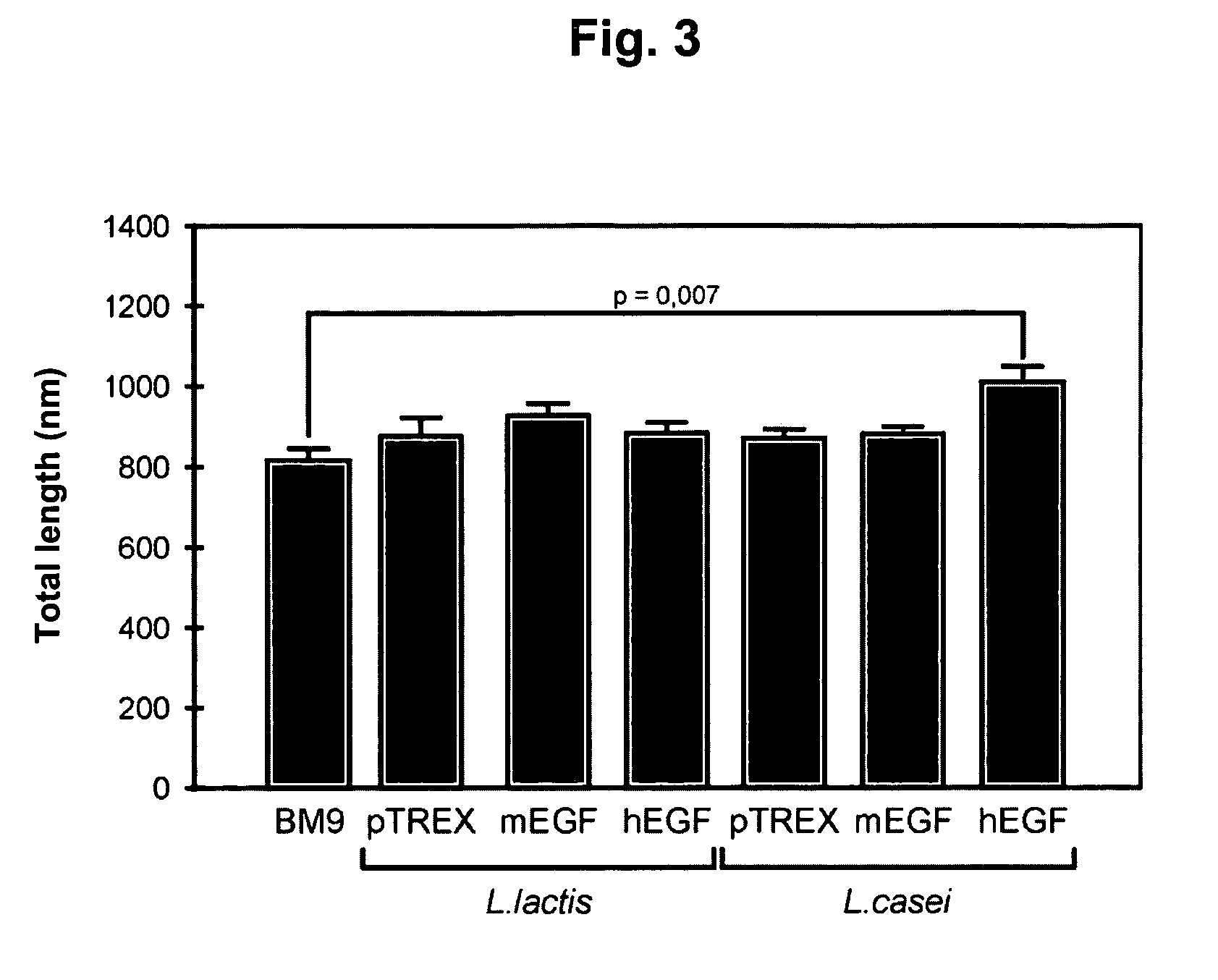
The present invention relates to epidermal growth factor (EGF) producing lactic acid bacteria and their use to increase intestinal villi height and to promote gut absorption. In particular, the invention relates to EGF producing Lactococcus lactis and Lactobacillus casei. The organisms may be especially useful to treat Short Bowel Syndrome.
Owner:INTREXON ACTOBIOTICS NV
Nutritional products including a novel fat system including fatty acids
InactiveUS20120172445A1Reduce the burden onImprove infant fat digestionBiocideHydroxy compound active ingredientsMonoglycerideMedicine
Disclosed are nutritional formulations including predigested fats that can be administered to preterm infants, infants, toddlers, and children for improving tolerance, digestion, and absorption of nutrients and for reducing the incidence of necrotizing enterocolitis, colic, and short bowel syndrome. The predigested fats include fatty acid-containing monoglycerides and / or a fatty acid component.
Owner:ABBOTT LAB INC
Nutritional products including a novel fat system including monoglycerides
ActiveUS20120172443A1Reduce the burden onImprove infant fat digestionBiocideHydroxy compound active ingredientsMonoglycerideMedicine
Disclosed are nutritional formulations including predigested fats that can be administered to preterm infants, infants, toddlers, and children for improving tolerance, digestion, and absorption of nutrients and for reducing the incidence of necrotizing enterocolitis, colic, and short bowel syndrome. The predigested fats include fatty acid-containing monoglycerides and / or a fatty acid component.
Owner:ABBOTT LAB INC
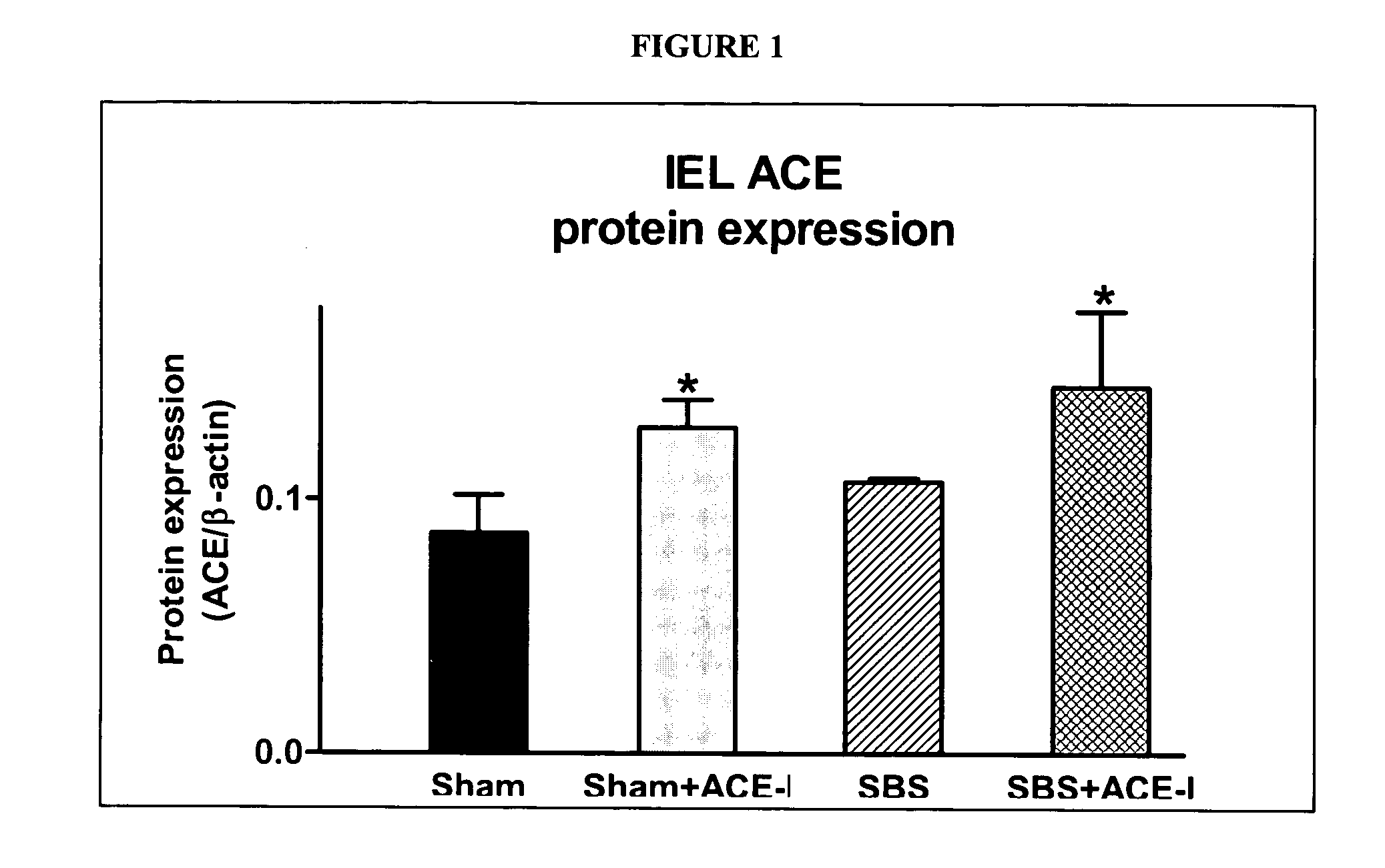
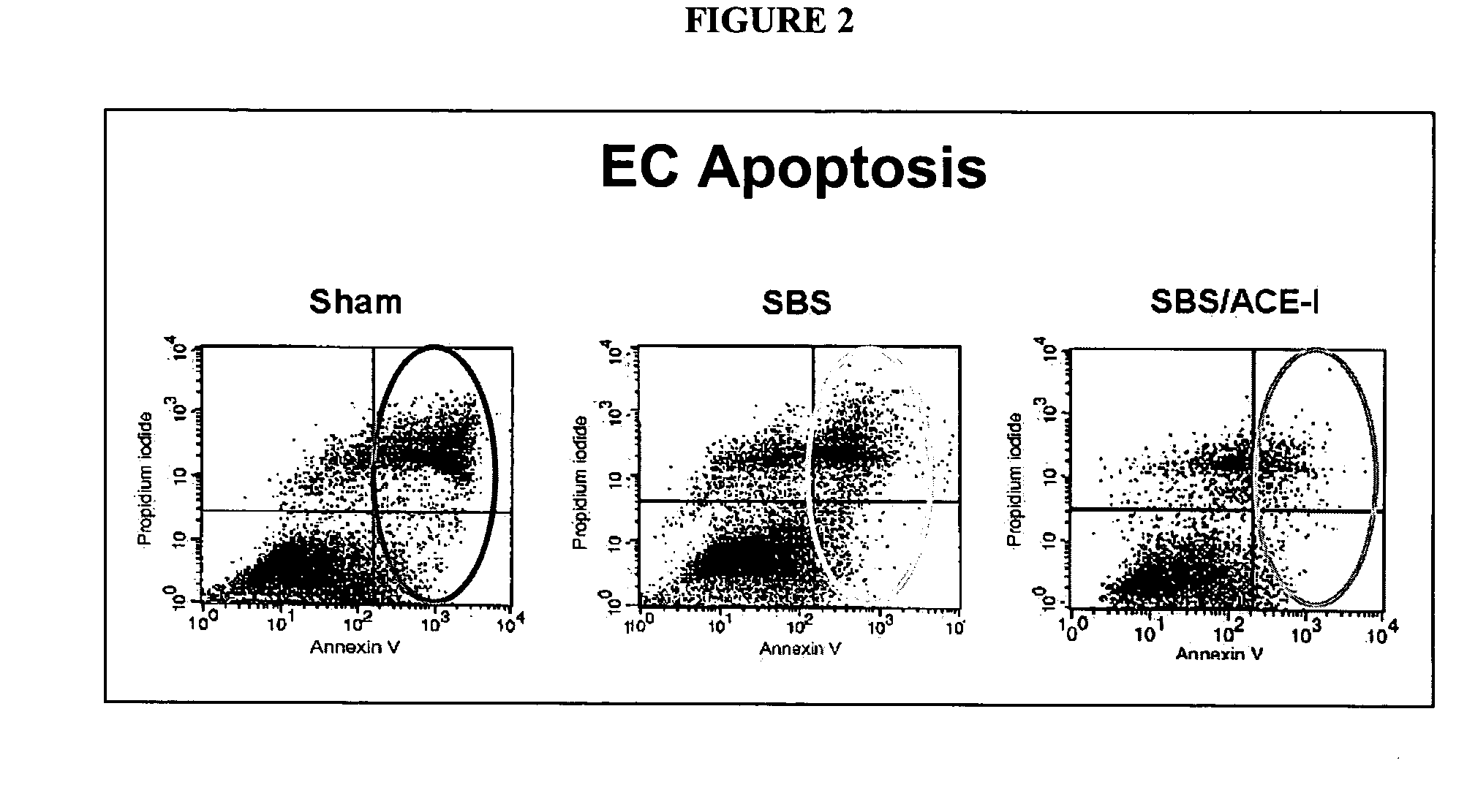
Compositions and methods for treatment and prevention of gastrointestinal disorders
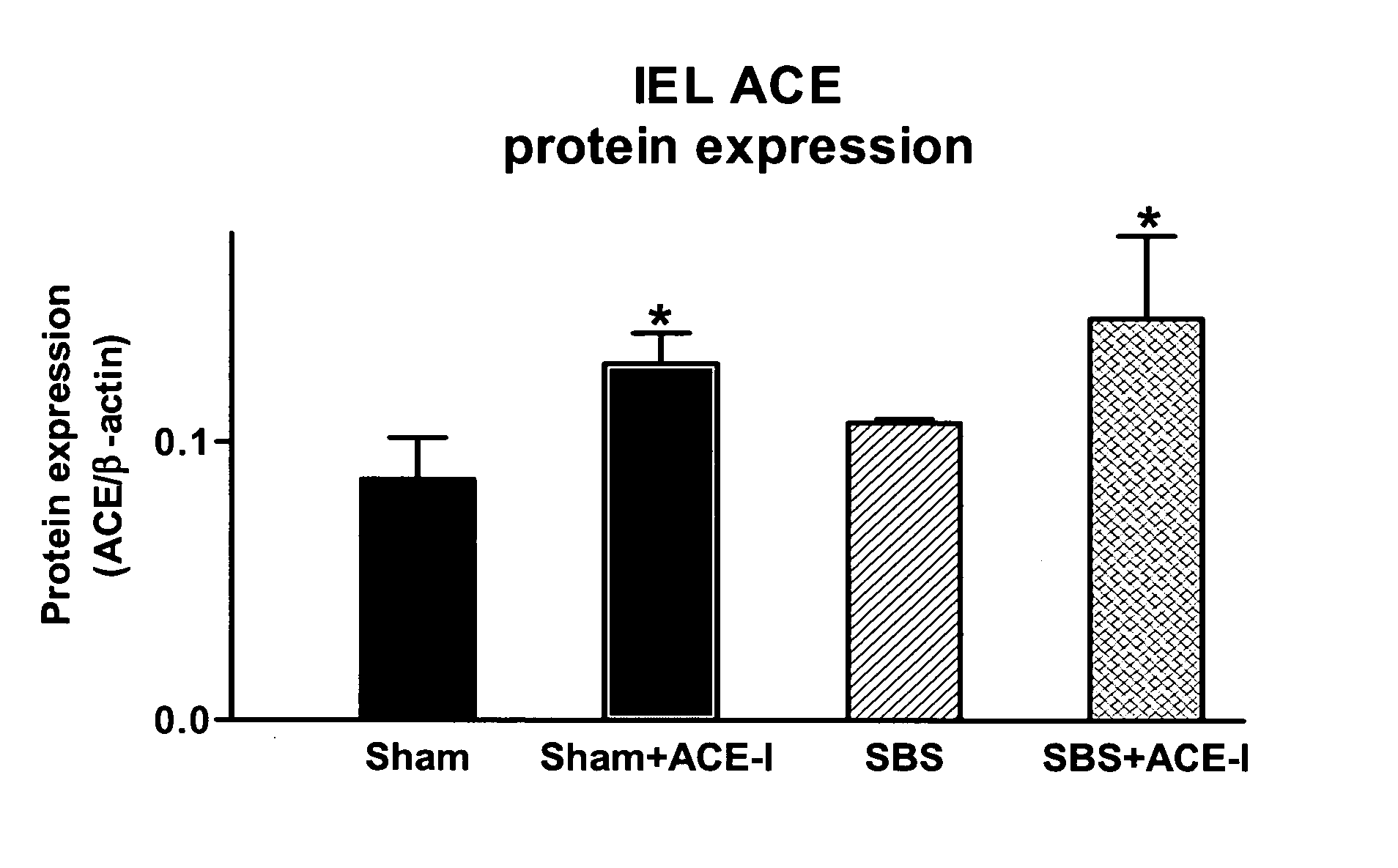
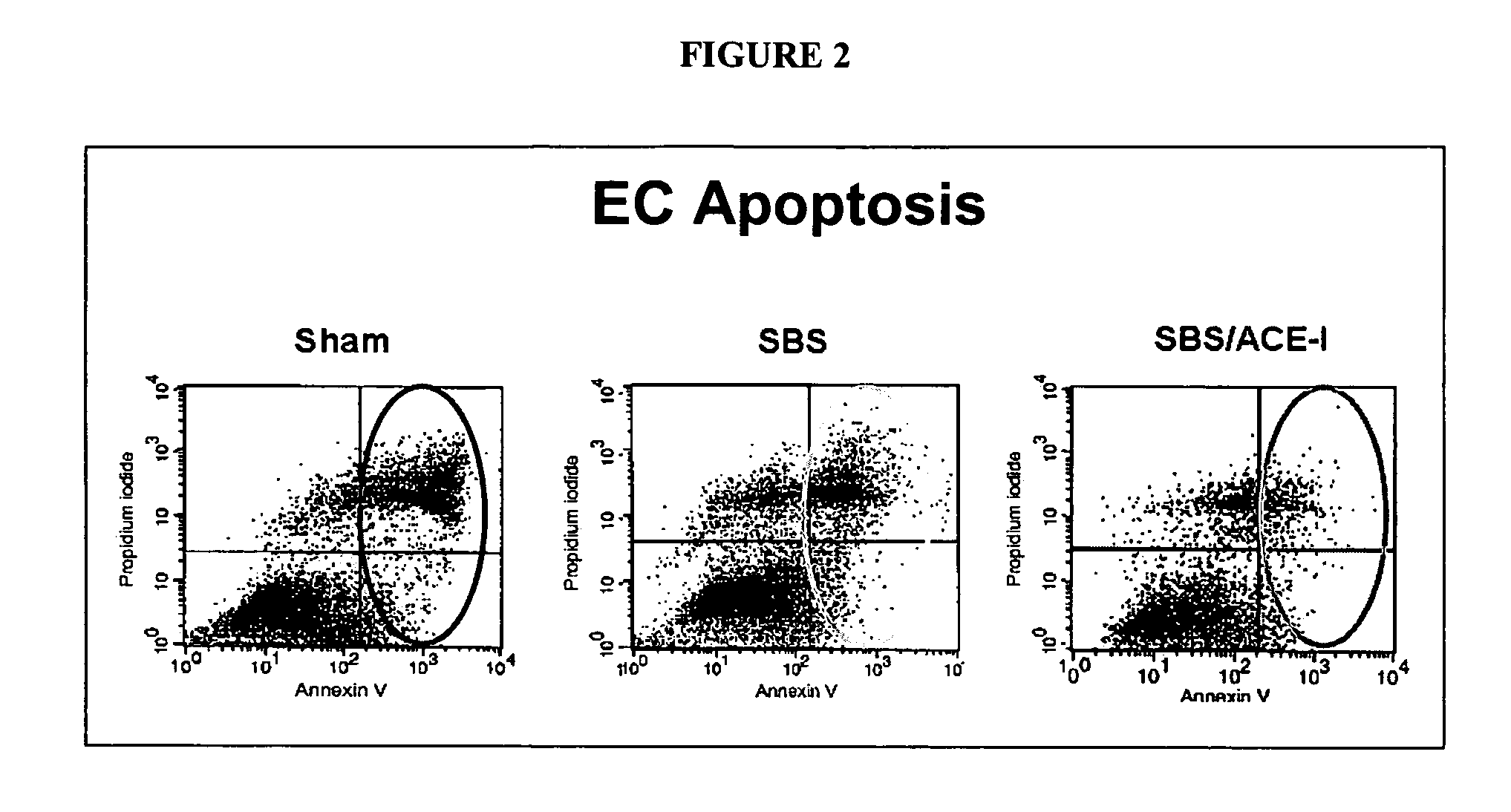
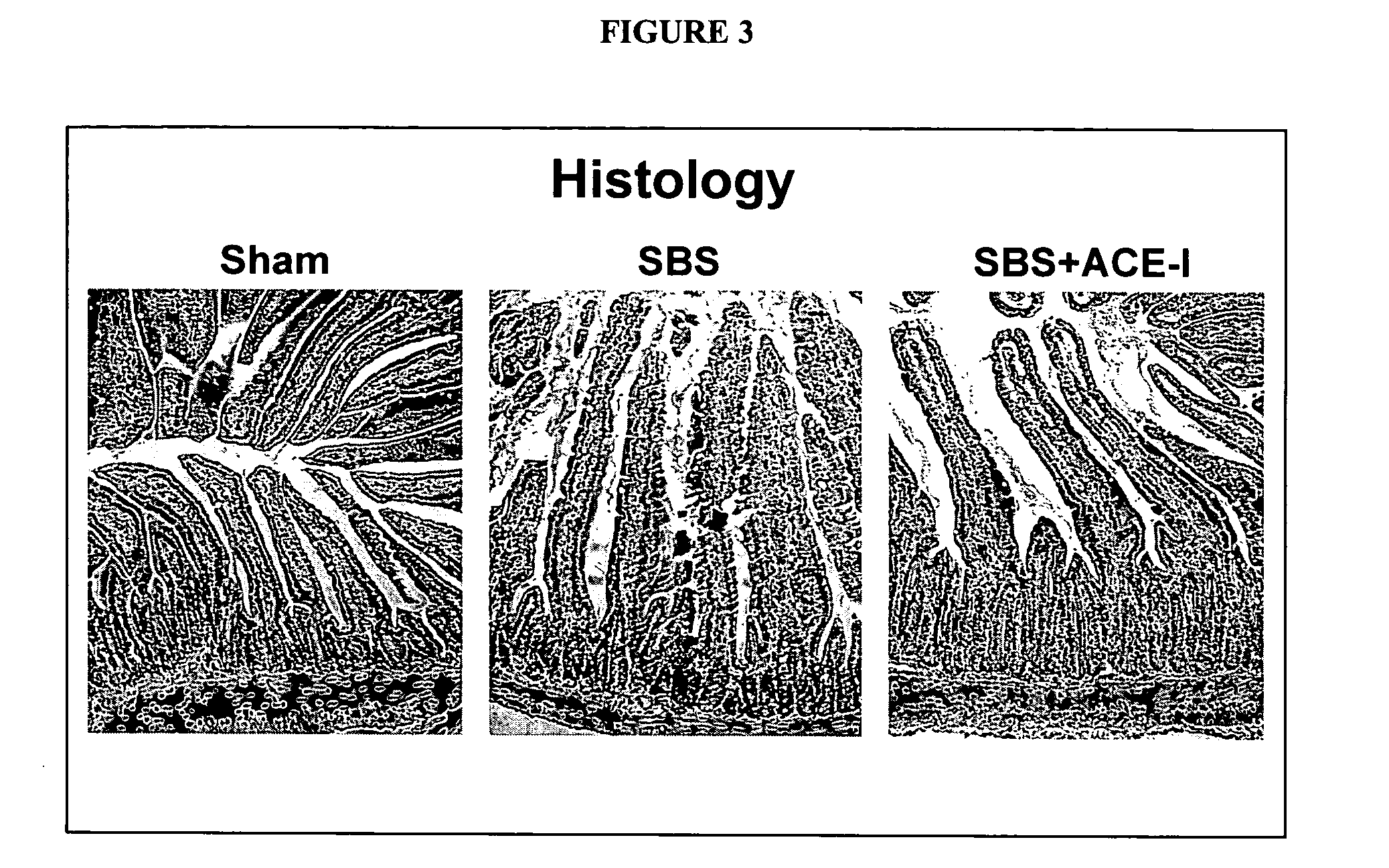
ActiveUS20070123499A1Clinical severity of was improvedLoss preventionBiocidePharmaceutical delivery mechanismAngiotensin-converting enzymeInflammatory Bowel Diseases
The present invention provides compositions and methods for treating and preventing inflammatory bowel disease and other gastrointestinal disorders (e.g., mucositis, radiation induced enteritis, and short bowel syndrome). In particular, the present invention provides methods of treating and preventing symptoms of inflammatory bowel disease and other gastrointestinal disorders using compositions comprising an angiotensin converting enzyme (ACE) inhibitor and polyethylene glycol.
Owner:RGT UNIV OF MICHIGAN
Mechanical extension implants for short bowel syndrome
ActiveUS20080195224A1Induce intestinal growthInduced growthInfusion syringesSurgeryShort bowel syndromeGeneral surgery
A bowel extension device implantable into a body for treatment of short bowel syndrome. The bowel extension device comprises a housing and a displaceable member coupled to the housing. The bowel extension device is configured to apply a tensile force sufficient to promote bowel growth without causing damage to the bowel. In some embodiments, the bowel extension device can be completely contained with the body.
Owner:RGT UNIV OF MICHIGAN
Angiotensin converting enzyme inhibitor use for treatment and prevention of gastrointestinal disorders
InactiveUS20050203168A1Shorten the progressRelieve symptomsBiocideAnimal repellantsAngiotensin-converting enzymeGastrointestinal disorder
The present invention provides compositions and methods for treating and preventing inflammatory bowel disease and other gastrointestinal disorders (e.g., short bowel syndrome). In particular, the present invention provides methods of treating symptoms of inflammatory bowel disease and other gastrointestinal disorders using angiotensin converting enzyme (ACE) inhibitors.
Owner:RGT UNVERSITY OF MICHIGAN THE
Methods for decreasing the incidence of necrotizing enterocolitis, colic, and short bowel syndrome in an infant, toddler, or child
ActiveUS20120172442A1Reduce morbidityReduce the burden onBiocideHydroxy compound active ingredientsMedicineNecrotizing enterocolitis
Disclosed are nutritional formulations including predigested fats that can be administered to preterm infants, infants, toddlers, and children for improving tolerance, digestion, and absorption of nutrients and for reducing the incidence of necrotizing enterocolitis, colic, and short bowel syndrome. The predigested fats include fatty acid-containing monoglycerides and / or a fatty acid component.
Owner:ABBOTT LAB INC
Substituted 4-phenyl pyridine compounds as non-systemic tgr5 agonists
ActiveUS20170174718A1Improved efficacy profileImproved safety profileOrganic active ingredientsNervous disorderAcute hyperglycaemiaUlcerative colitis
The invention relates to non-systemic TGR5 agonist useful in the treatment of chemotherapy-induced diarrhea, diabetes, Type II diabetes, gestational diabetes, impaired fasting glucose, impaired glucose tolerance, insulin resistance, hyperglycemia, obesity, metabolic syndrome, ulcerative colitis, Crohn's disease, disorders associated with parenteral nutrition especially during short bowel syndrome, and irritable bowel syndrome (IBS), and other TGR5 associated diseases and disorders, having the Formula:where R1, R2, R2′, R3, R4, X1, X2, X3, X4, Q, and n are described herein.
Owner:ARDELYX
Nutritional compositions for promoting gut barrier function and ameliorating visceral pain
ActiveUS20170128500A1Promote regenerationSupporting gut barrier resistancePeptide/protein ingredientsAntipyreticPresent methodVisceral pain
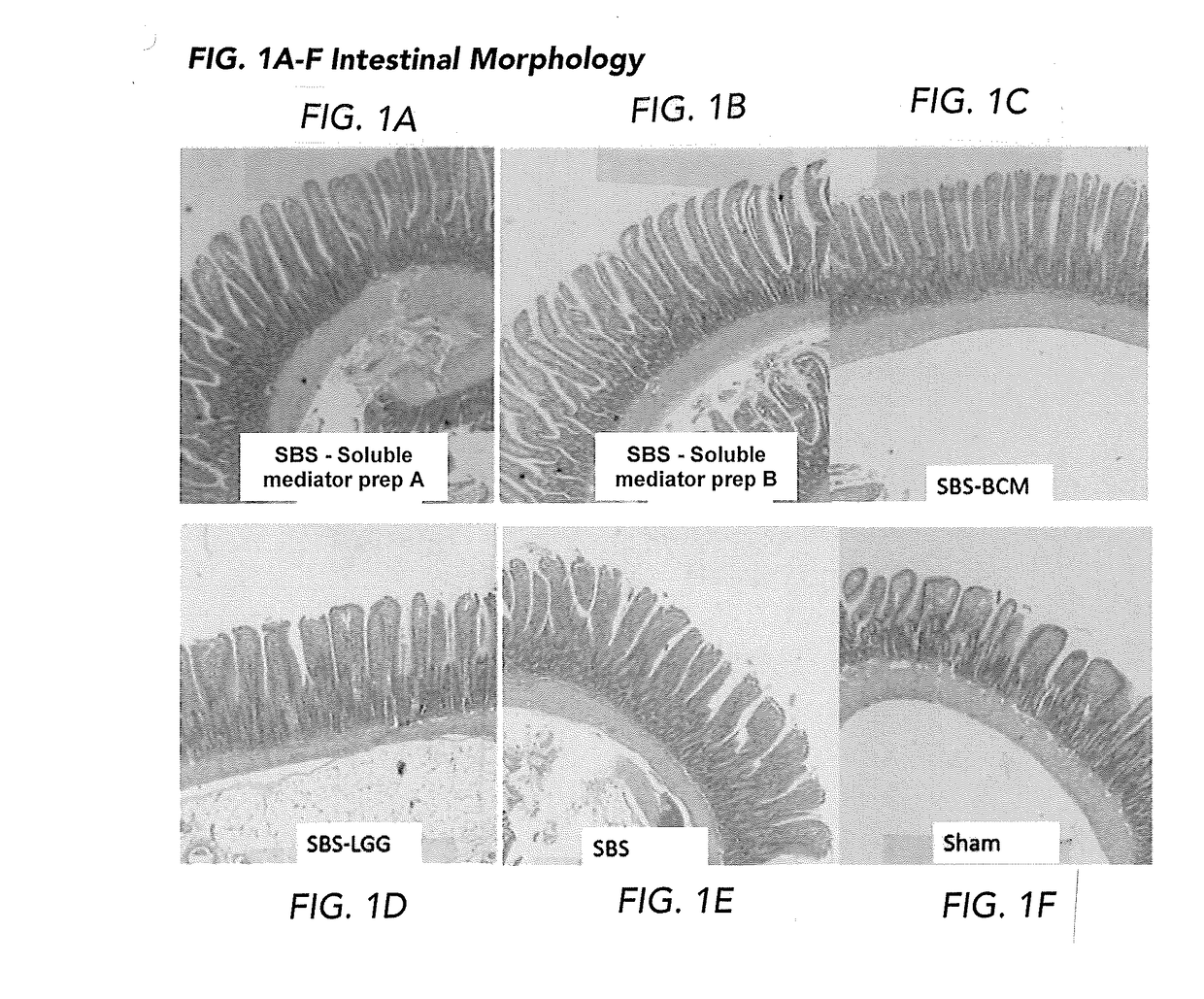
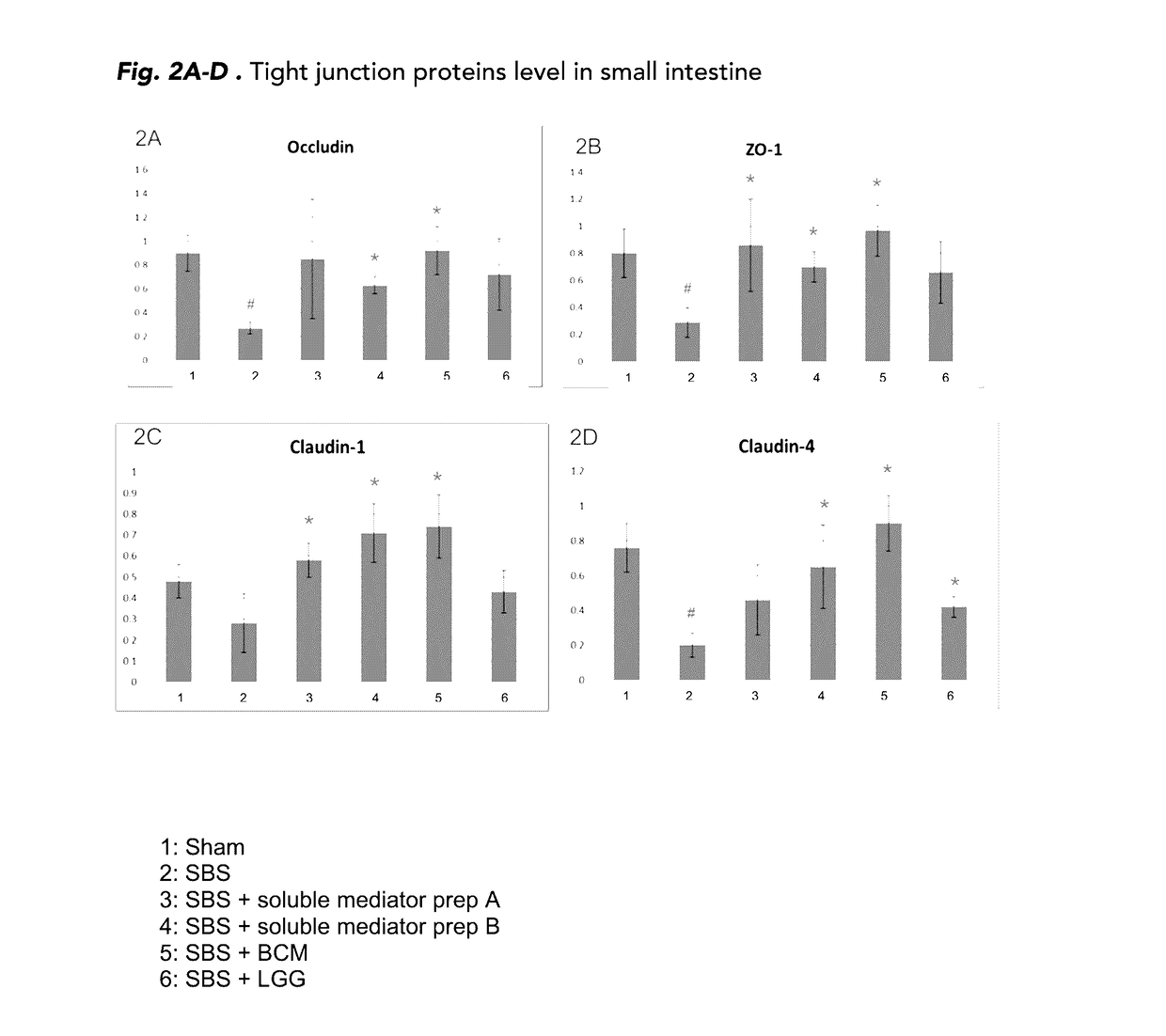
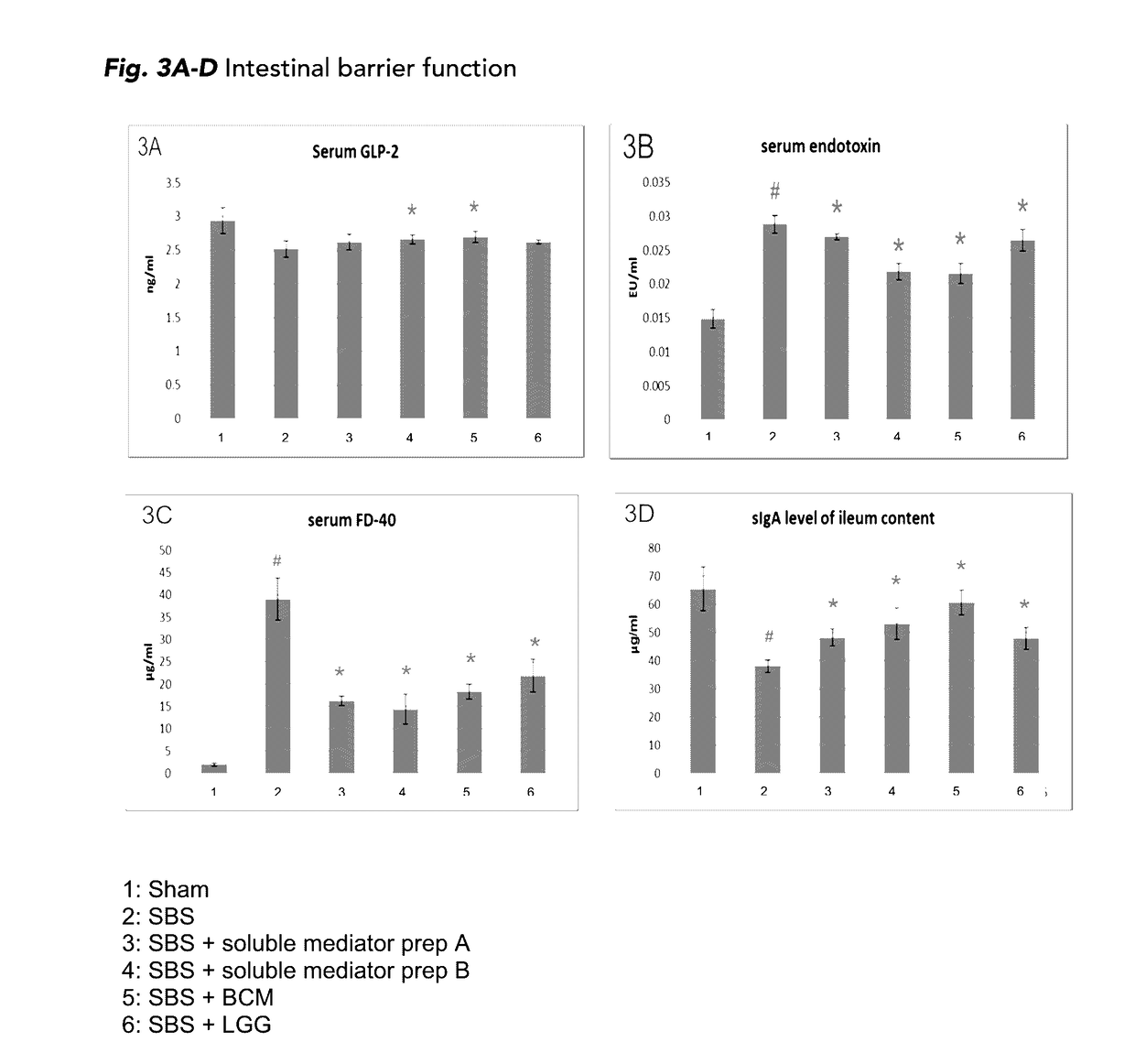
The present disclosure is directed to methods for i) promoting gut regeneration, ii) promoting gut maturation and / or adaptation, iii) supporting gut barrier resistance and / or iv) protecting gut barrier function in a pediatric subject comprising administering to the pediatric subject a composition comprising an effective amount of a soluble mediator preparation derived from a late-exponential growth phase of a probiotic batch-cultivation process, such as Lactobacillus rhamnosus GG (LGG). The present methods advantageously provide the gut-protection benefits of LGG soluble mediators without introducing live bacterial culture to individuals with impaired gut barrier function. In some embodiments, the pediatric subject has impaired gut barrier function and / or short bowel syndrome. The present disclosure in other embodiments, directed methods for reducing visceral pain in a pediatric subject comprising administering to the pediatric subject a composition comprising an effective amount of a soluble mediator preparation from a late-exponential growth phase of a probiotic batch-cultivation process, such as LGG.
Owner:MEAD JOHNSON NUTRITION
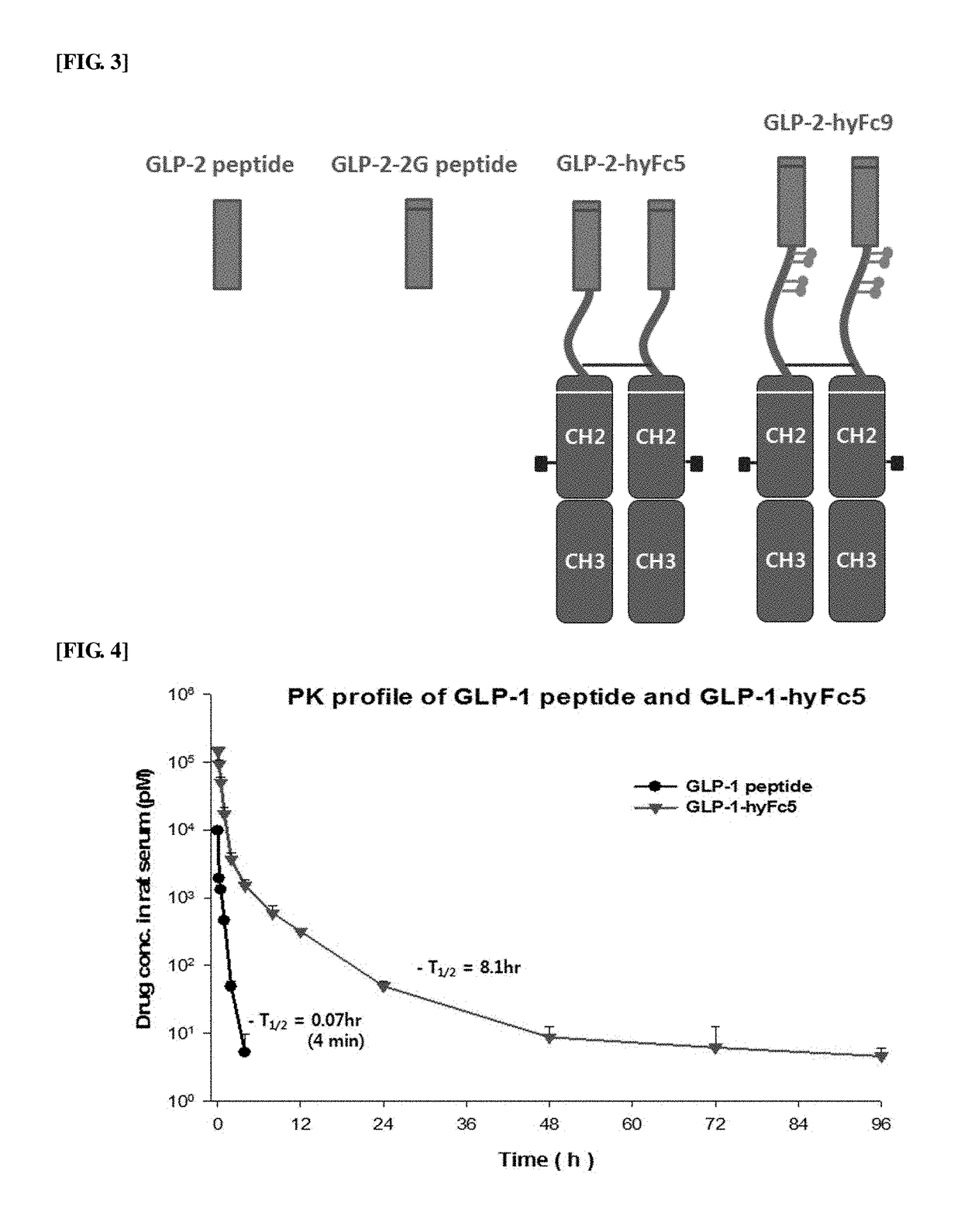
Fusion polypeptide containing GLP and immunoglobulin hybrid fc and use thereof
ActiveUS20170362293A1Extended half-lifeImprove the immunityPeptide/protein ingredientsMetabolism disorderAnticancer chemotherapyHalf-life
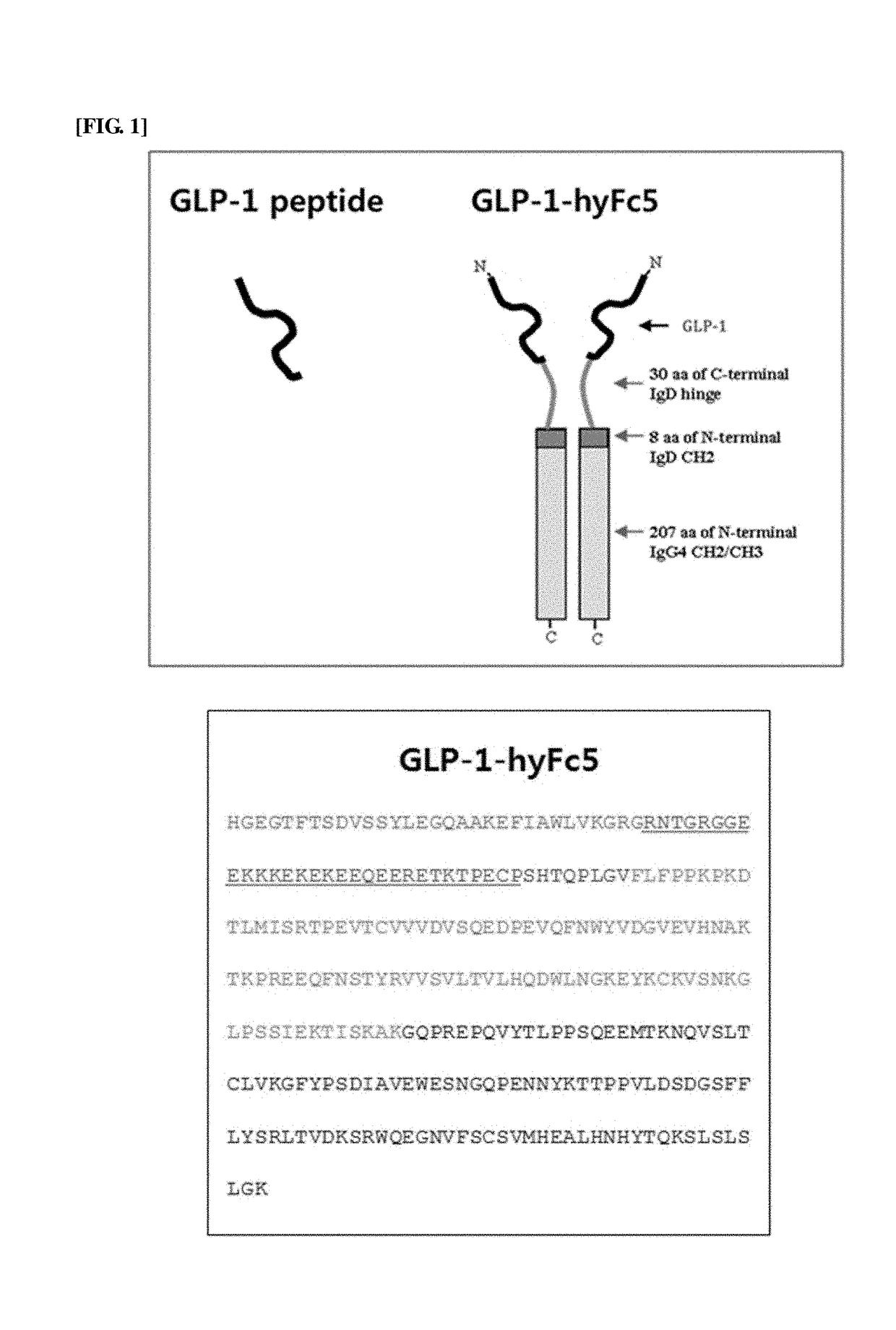
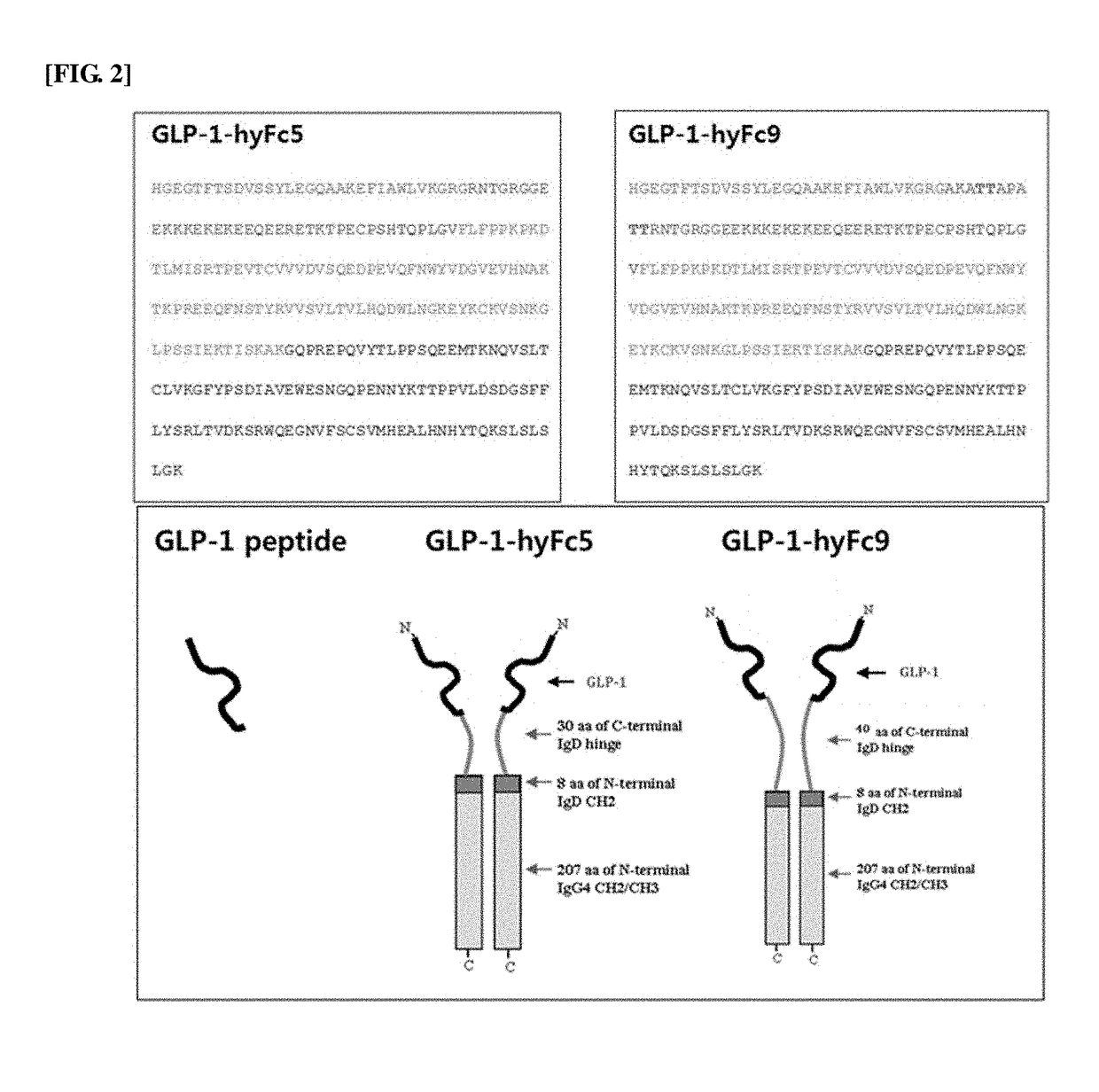
The present invention relates to a fusion polypeptide containing a glucagon-like peptide (GLP) and an immunoglobulin hybrid Fc, and more specifically, to a fusion polypeptide with an increased half-life and improved efficacy compared to the conventional fusion polypeptide based on the discovery of an immunoglobulin hybrid Fc suitable for GLP or analogs thereof, and a pharmaceutical composition for treating diabetes, inflammatory bowel disease, endoenteritis or diarrhea caused by anticancer chemotherapy, or short bowel syndrome, containing the fusion polypeptide. The fusion polypeptide of the present invention has an increased half-life and improved resistance to DPP-4 enzyme compared to those of GLP-1 and GLP-2, and it thus has improved drug efficacy in treating diabetes, inflammatory bowel disease, endoenteritis or diarrhea caused by anticancer chemotherapy, or short bowel syndrome, compared to those of the conventional drugs. Accordingly, the fusion polypeptide of the present invention can be effectively applied to pharmaceutical drugs.
Owner:GENEXINE INC
Mechanical extension implants for short bowel syndrome
A bowel extension device implantable into a body for treatment of short bowel syndrome. The bowel extension device comprises a housing and a displaceable member coupled to the housing. The bowel extension device is configured to apply a tensile force sufficient to promote bowel growth without causing damage to the bowel. In some embodiments, the bowel extension device can be completely contained with the body.
Owner:RGT UNIV OF MICHIGAN
Methods for treatment or prophylaxis of kidney or liver dysfunction
InactiveUS20130157954A1Peptide/protein ingredientsMetabolism disorderLiver functionShort bowel syndrome
Methods are provided for treatment or prophylaxis of liver or kidney dysfunction in individuals experiencing one or more of intestinal failure, short bowel syndrome or parenteral nutrition by the administration of GLP-2 or GLP-2 analogs.
Owner:NPS PHARM INC
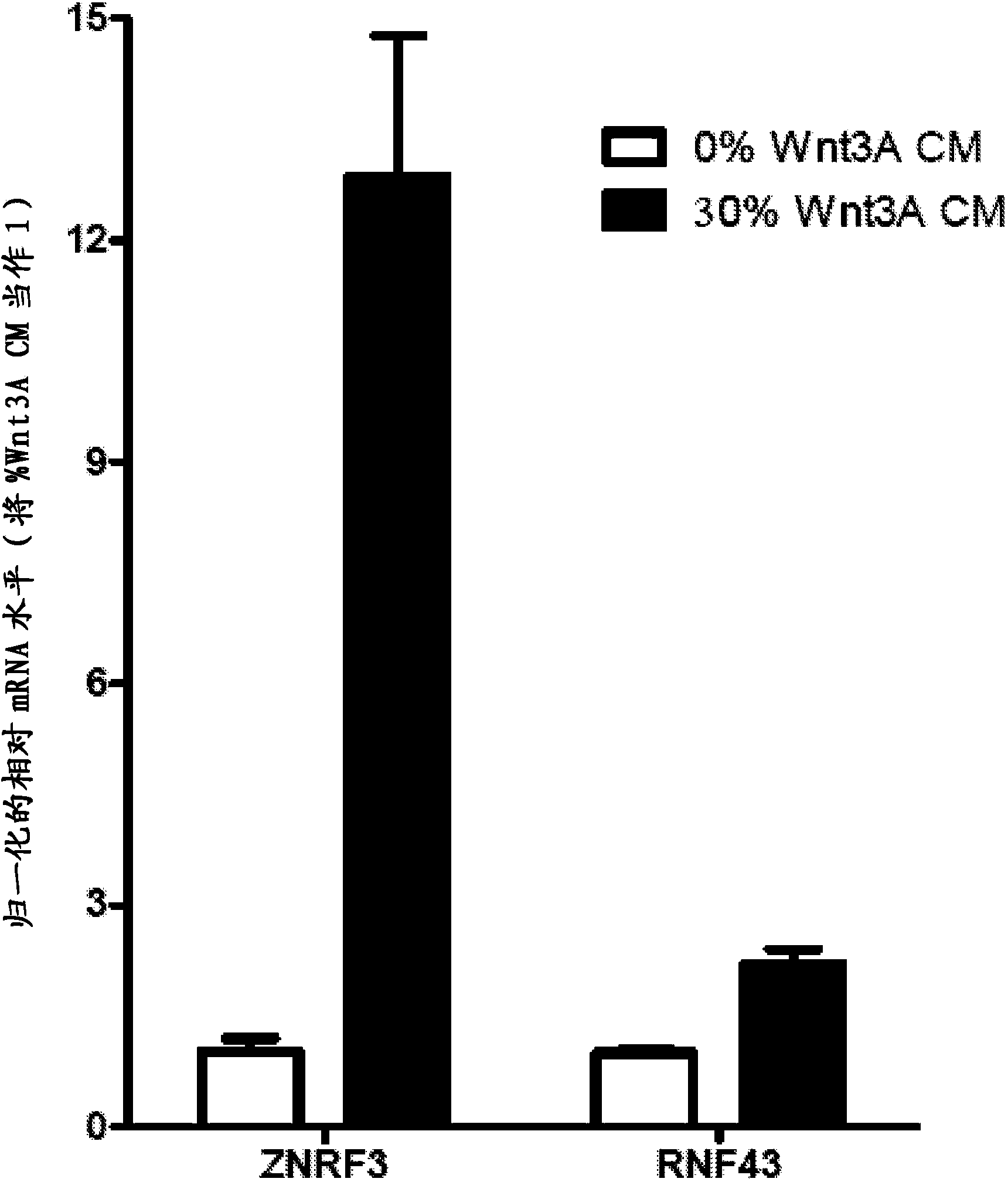
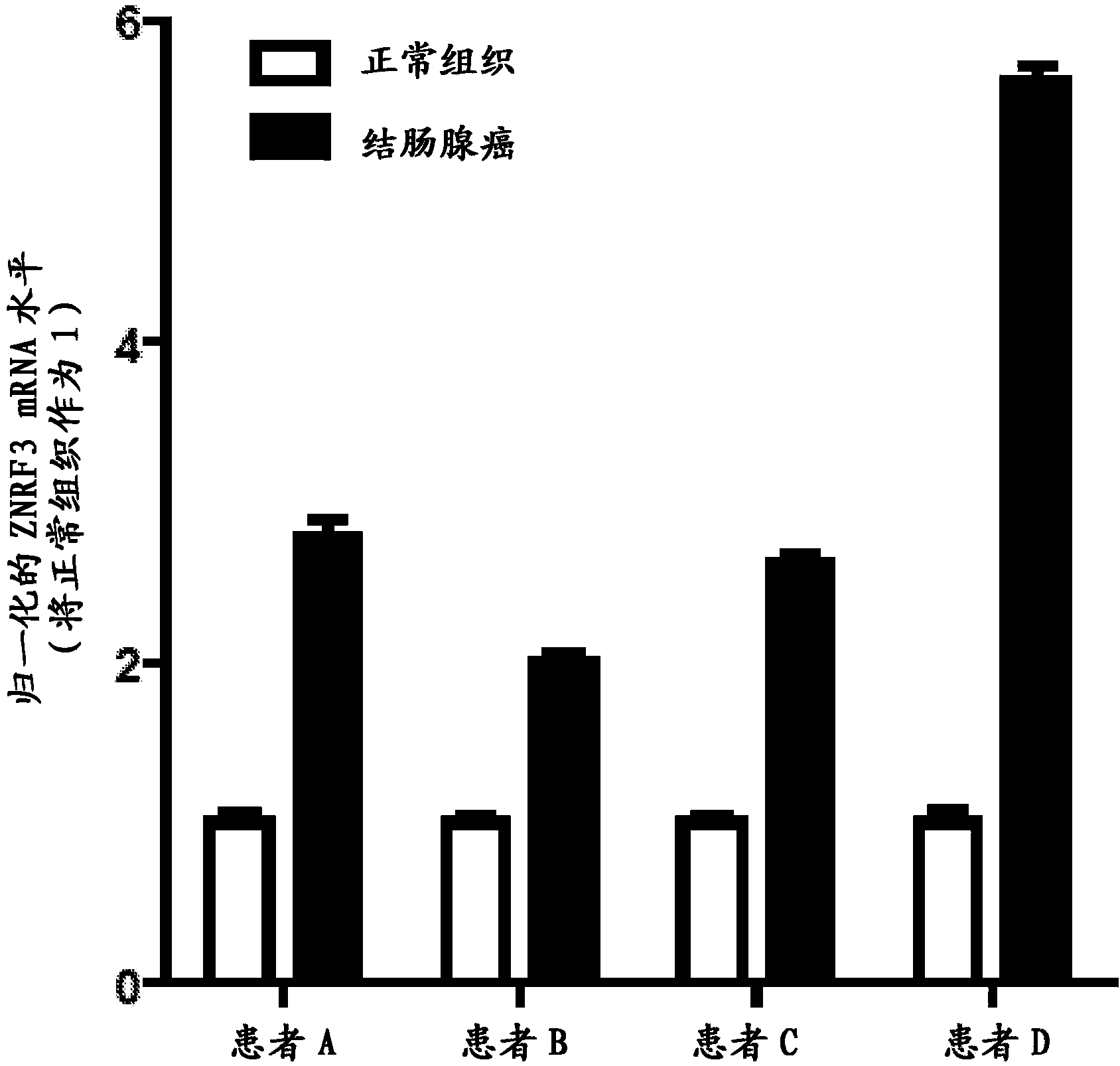
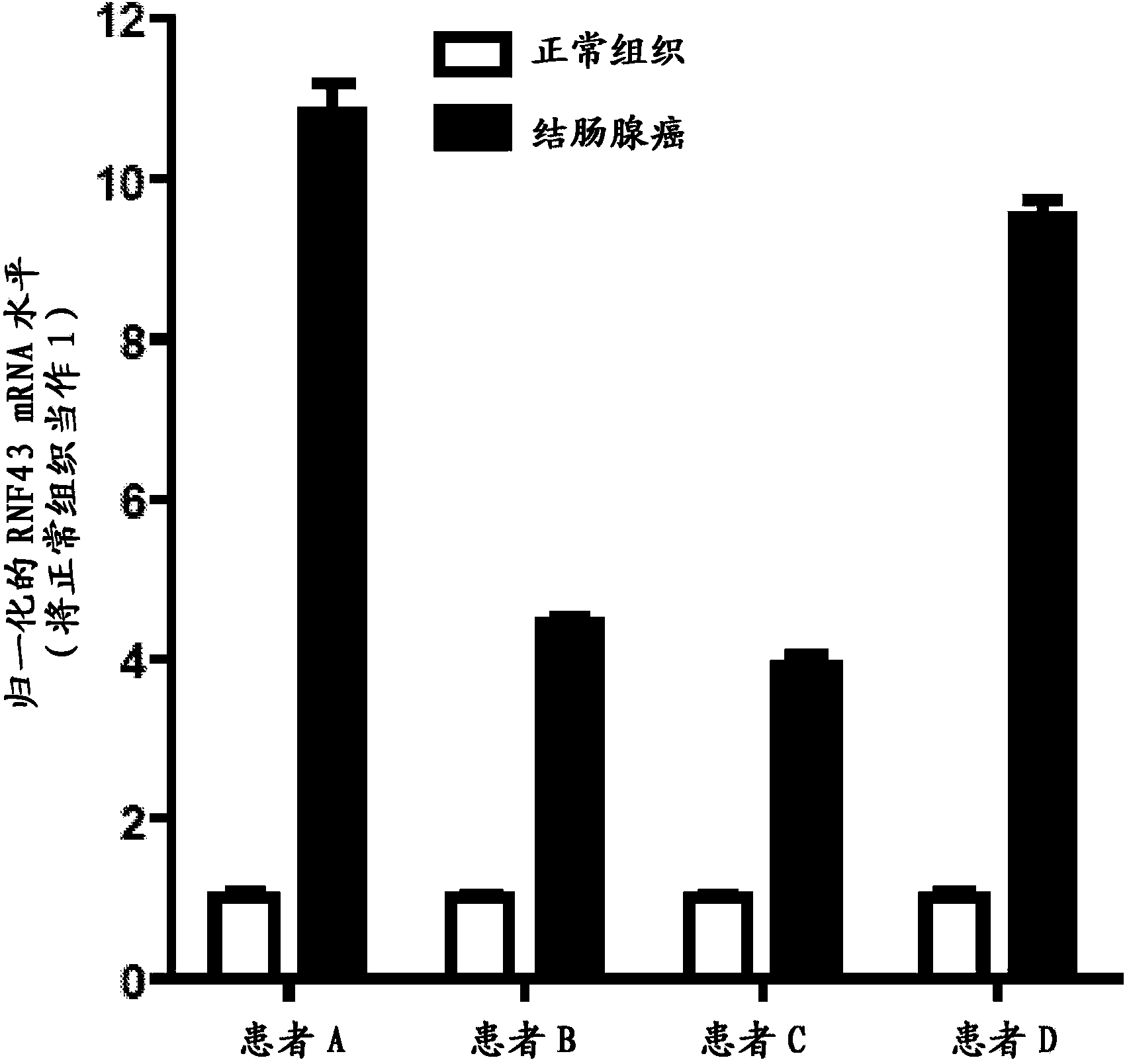
Antibodies and methods for Wnt pathway-related diseases
The transmembrane E3 ubiquitin ligases ZNRF3 and RNF43 are negative regulators of [beta]-catenin and the Wnt signaling pathway in eukaryotic cells. The activity of ZNRF3 can be modulated by antibody binding to its extracellular domain, thus causing an increase in Wnt signaling. The ZNRF3 antagonizing antibodies can be used to treat diseases with low Wnt signaling, such as short bowel syndrome, osteoporosis, diabetes, neurodegenerative diseases, and mucositis. In addition, the antagonizing antibodies of the invention can be used to enhance Wnt signaling for tissue repair and wound healing.
Owner:NOVARTIS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com