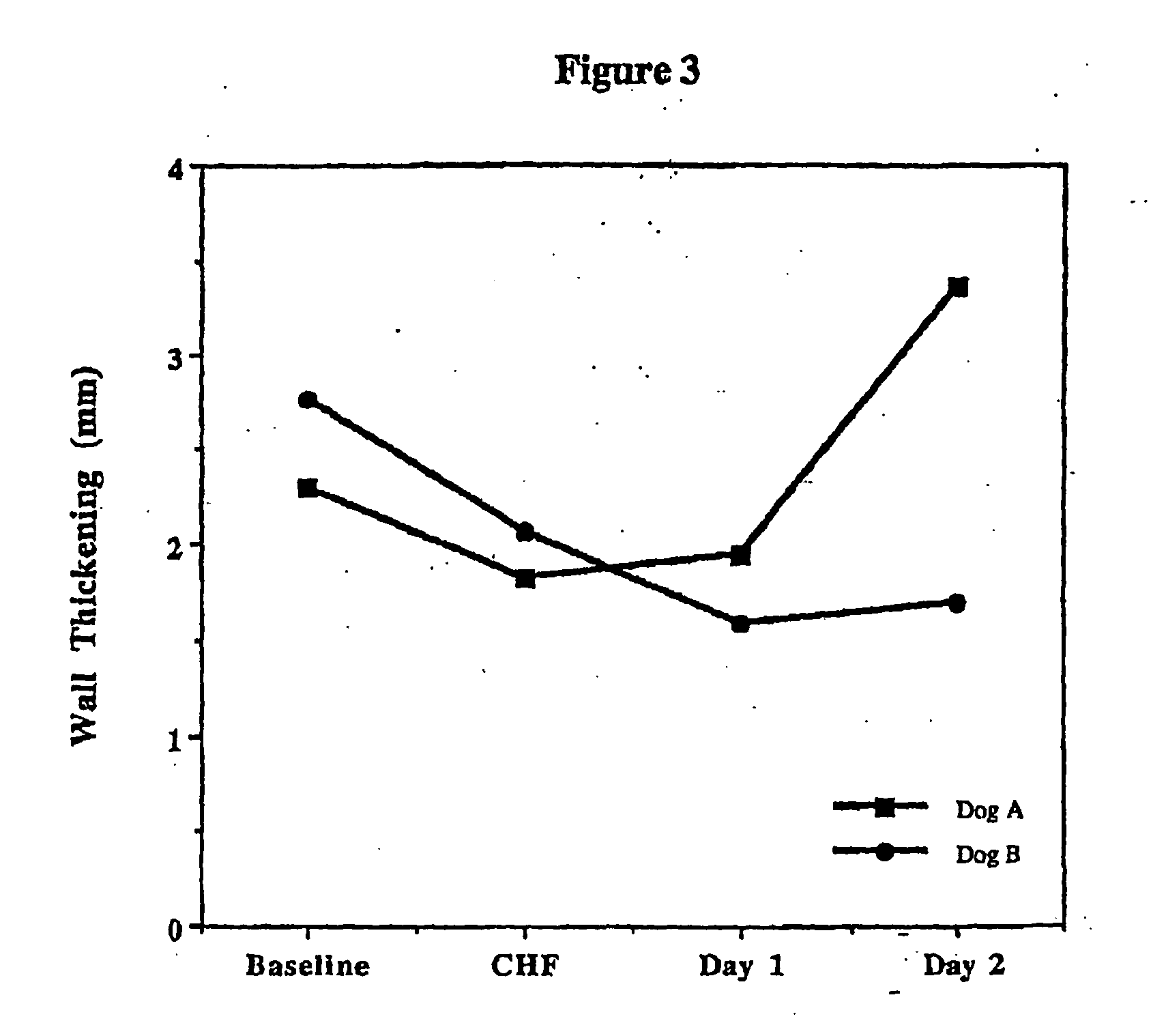
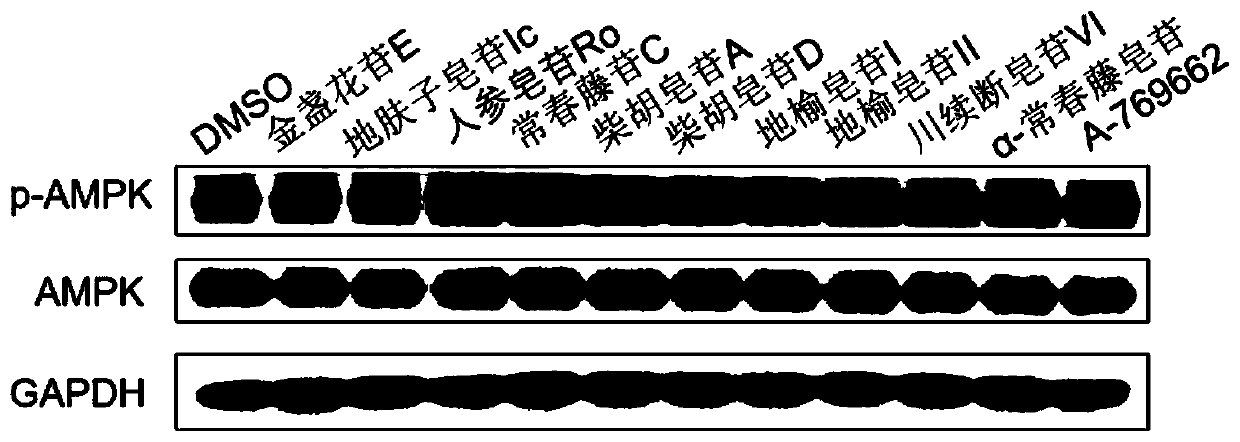
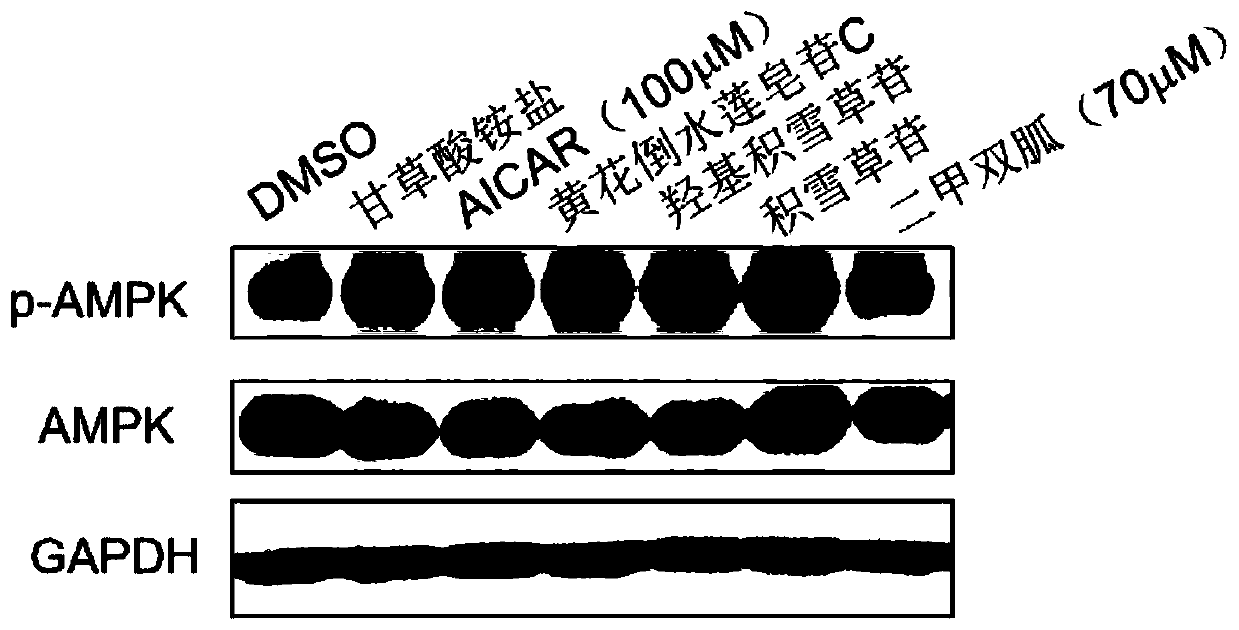
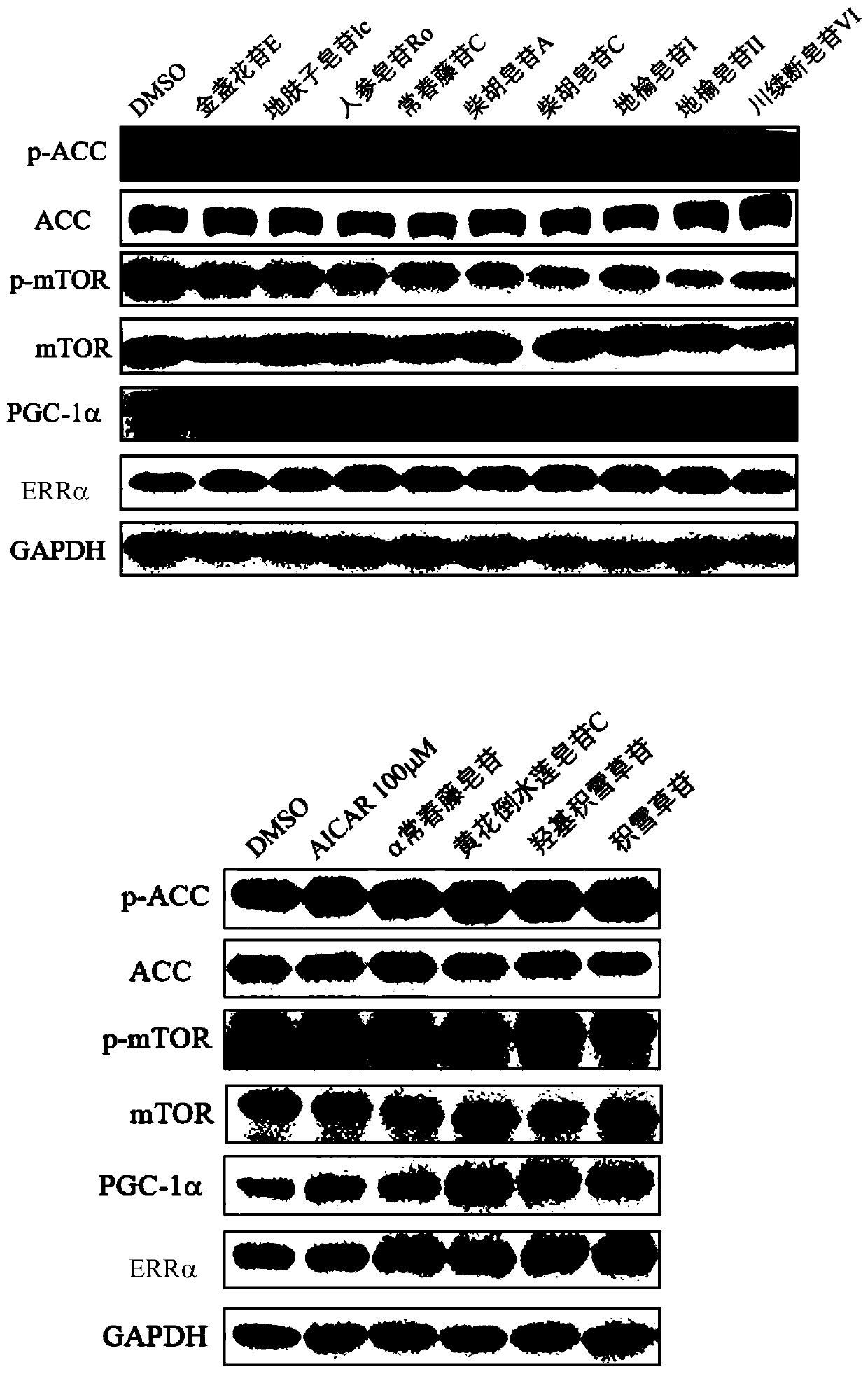
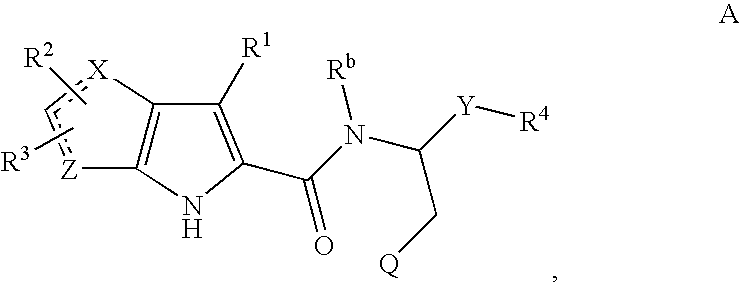
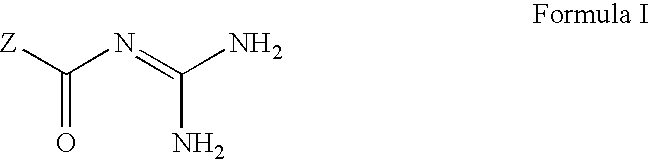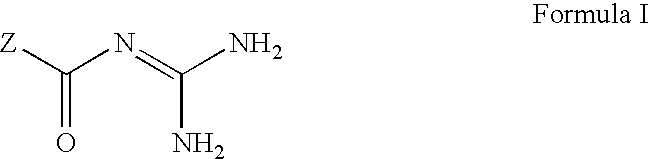Patents
Literature
85 results about "Diabetic cardiomyopathy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diabetic cardiomyopathy is a disorder of the heart muscle in people with diabetes. It can lead to inability of the heart to circulate blood through the body effectively, a state known as heart failure, with accumulation of fluid in the lungs (pulmonary edema) or legs (peripheral edema). Most heart failure in people with diabetes results from coronary artery disease, and diabetic cardiomyopathy is only said to exist if there is no coronary artery disease to explain the heart muscle disorder.
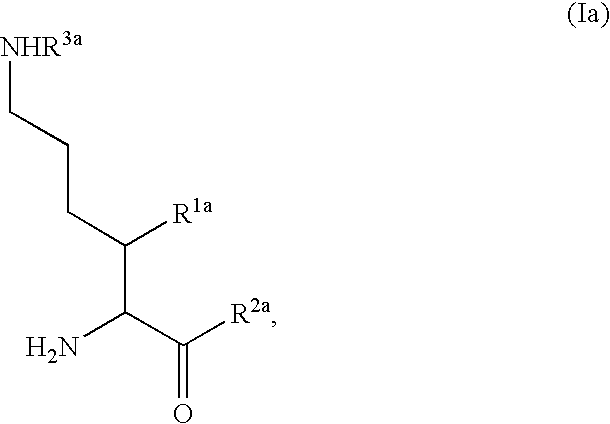
Fluorinated lysine derivatives as dipeptidyl peptidase IV inhibitors
InactiveUS20050043292A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDiabetic retinopathyArthritis
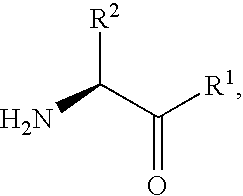
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV (“DPP-IV”), pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes, metabolic syndrome (syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, arthritis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
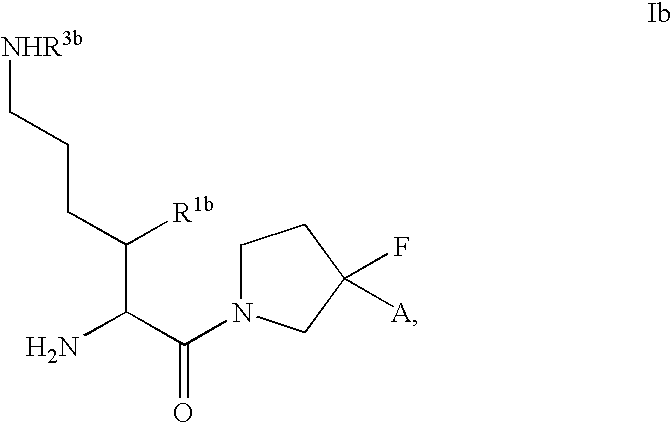
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS6710040B1Easy to prepareEase of detectabilityBiocideOrganic chemistryAcute coronary syndromeDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Dipeptidyl peptidase IV inhibiting fluorinated cyclic amides
InactiveUS20040110817A1Ease of preparation and detectabilityGood metabolic stabilityBiocideSenses disorderDiabetic retinopathyDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Treatment of hibernating myocardium and diabetic cardiomyopathy with a GLP-1 peptide
InactiveUS6894024B2Suppress plasma blood levelReducing norepinepherine levelPeptide/protein ingredientsMetabolism disorderHigh energyMortality rate
Hibernating myocardium is characterized by viable myocardium with impaired function due to localized reduced perfusion. Hibernating myocytes retain cellular integrity, but cannot sustain high-energy requirements of contraction. High plasma levels of catecholamines, such as norepinepherine, are believed to be predictive of mortality from hibernating myocardium. Likewise, high levels of catecholamines lead to cardiomyopathy in patients with diabetes. GLP-1 reduces plasma norepinepherine levels, and it thus is useful in a method of treating hibernating myocardium or diabetic cardiomyopathy.
Owner:ASTRAZENECA PHARMA LP
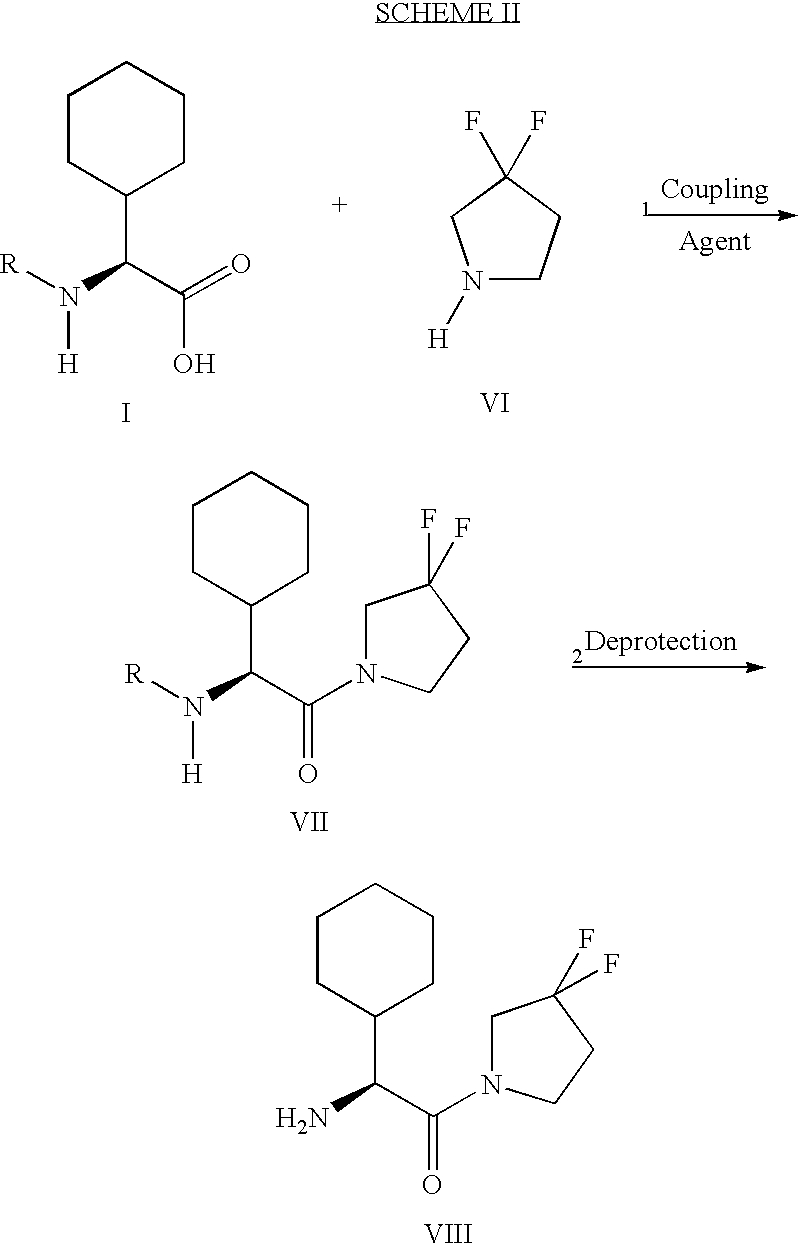
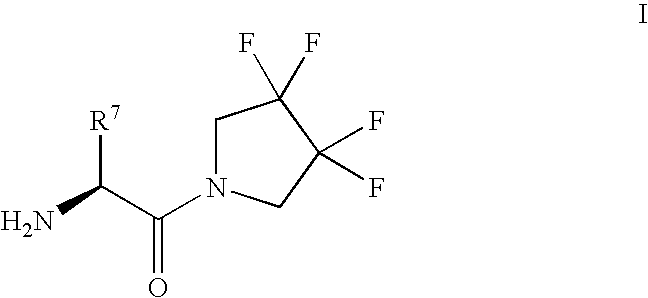
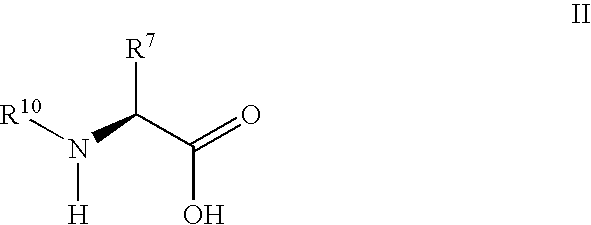
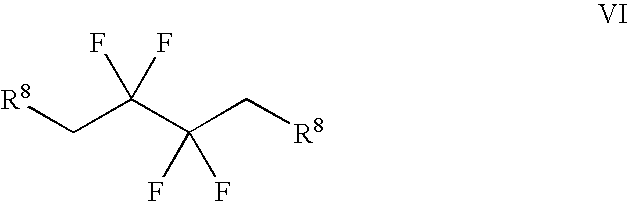
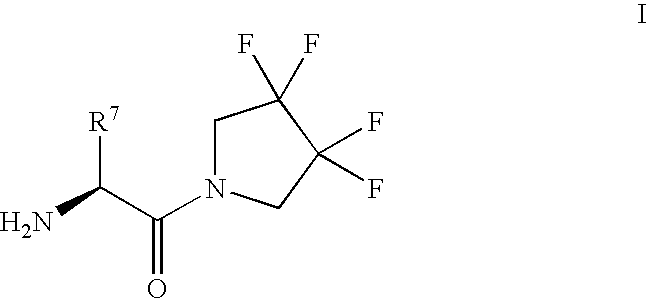
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
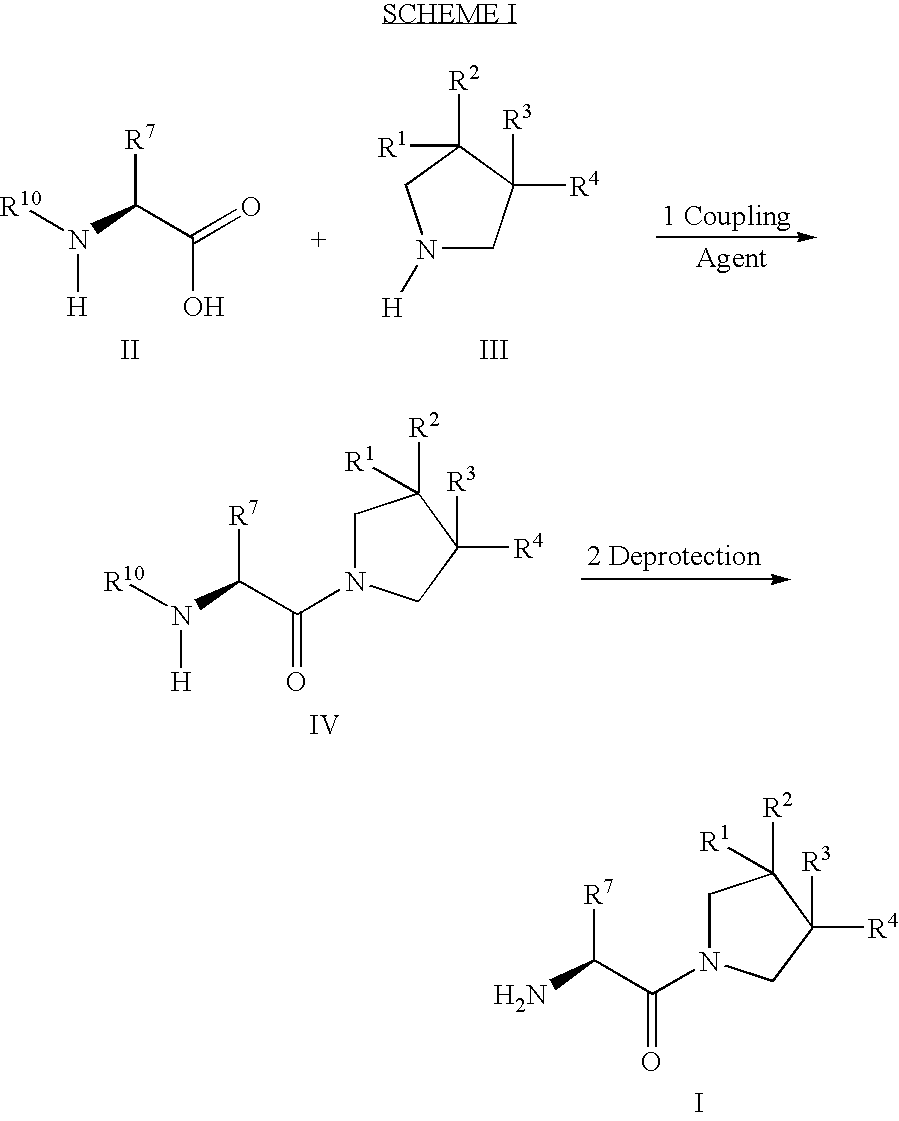
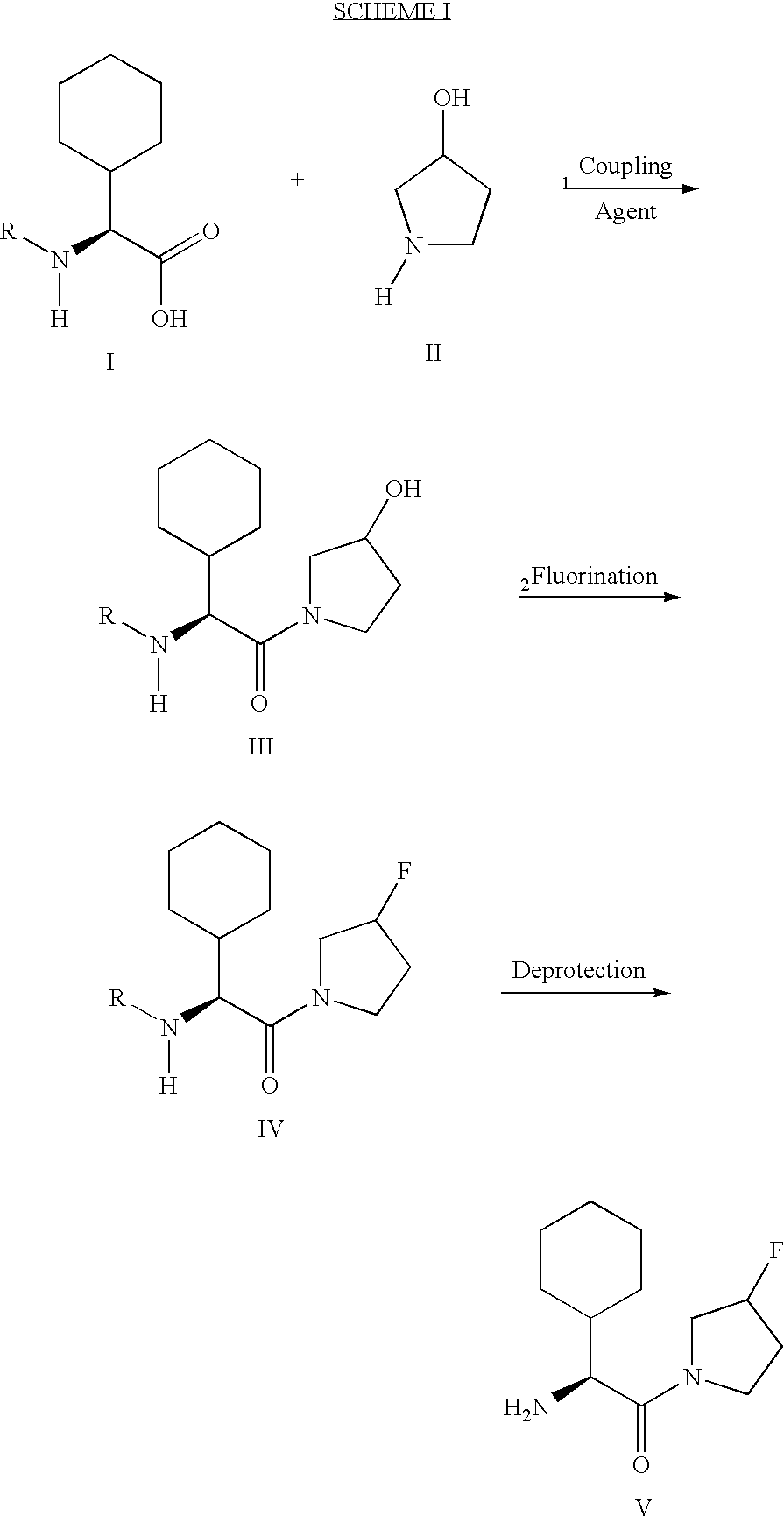
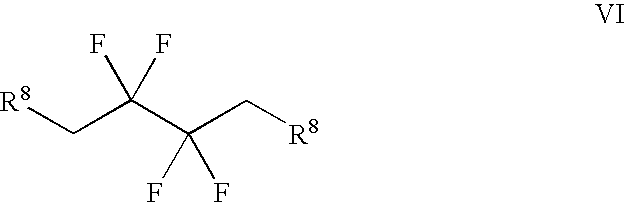
The present invention relates to a method of making novel dipeptidyl peptidase-IV ("DPP-IV') inhibitor compounds useful for treating, inter alia, diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, metabolic syndrome (Syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of making 3,3,4,4-tetrafluoropyrrolidine, a starting material utilized in the afore-mentioned method for preparing DPP-IV compounds.
Owner:PFIZER INC
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
InactiveUS6812350B2Metabolism disorderPhosphorus organic compoundsDisease progressionDiabetic nephropathy
Owner:PFIZER INC
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
InactiveUS20040002609A1Easy to cutMetabolism disorderPhosphorus organic compoundsDisease progressionDisease cause
The present invention relates to a method of making novel dipeptidyl peptidase-IV ("DPP-IV') inhibitor compounds useful for treating, inter alia, diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, metabolic syndrome (Syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of making 3,3,4,4-tetrafluoropyrrolidine, a starting material utilized in the afore-mentioned method for preparing DPP-IV compounds.
Owner:PFIZER INC
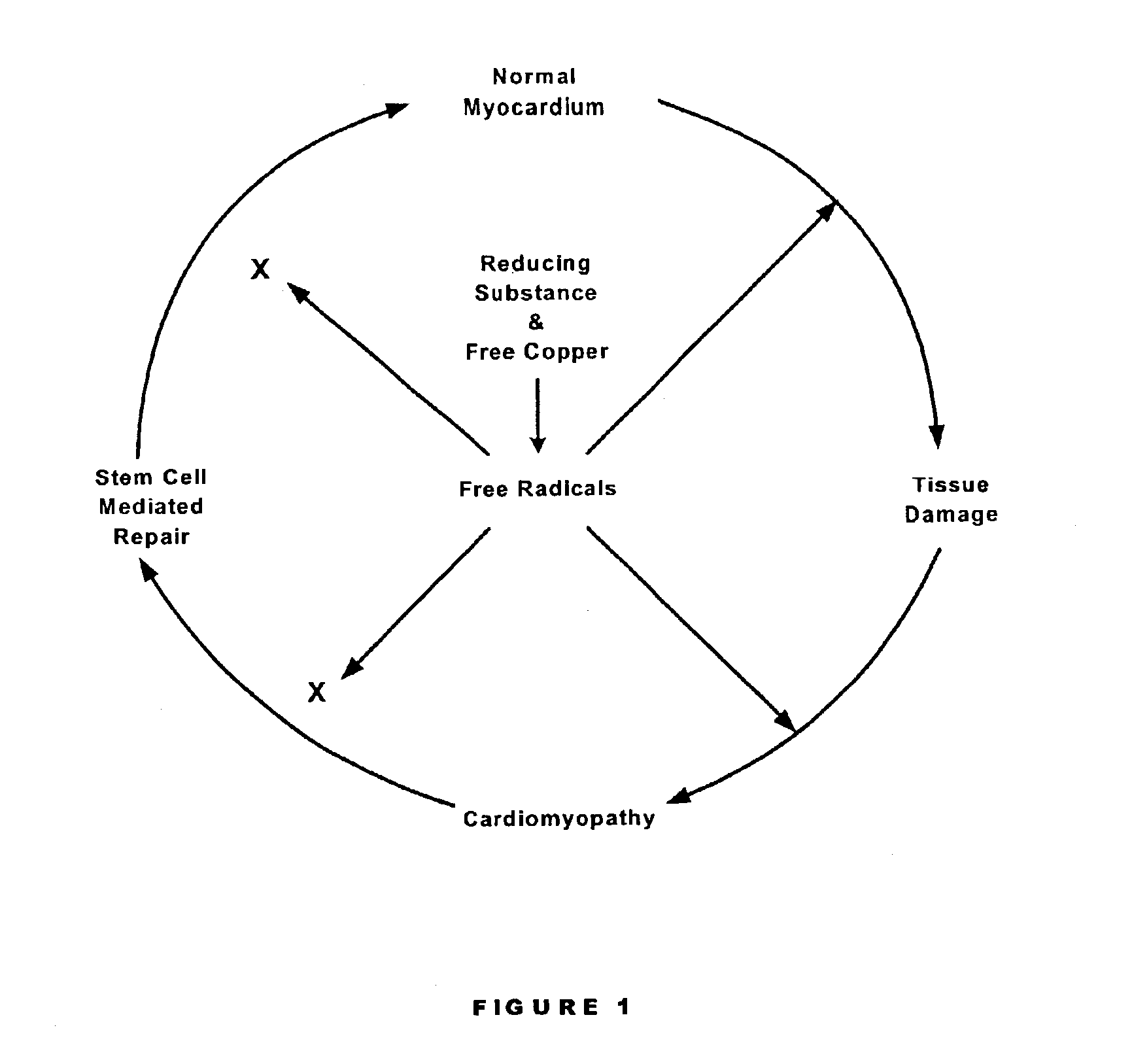
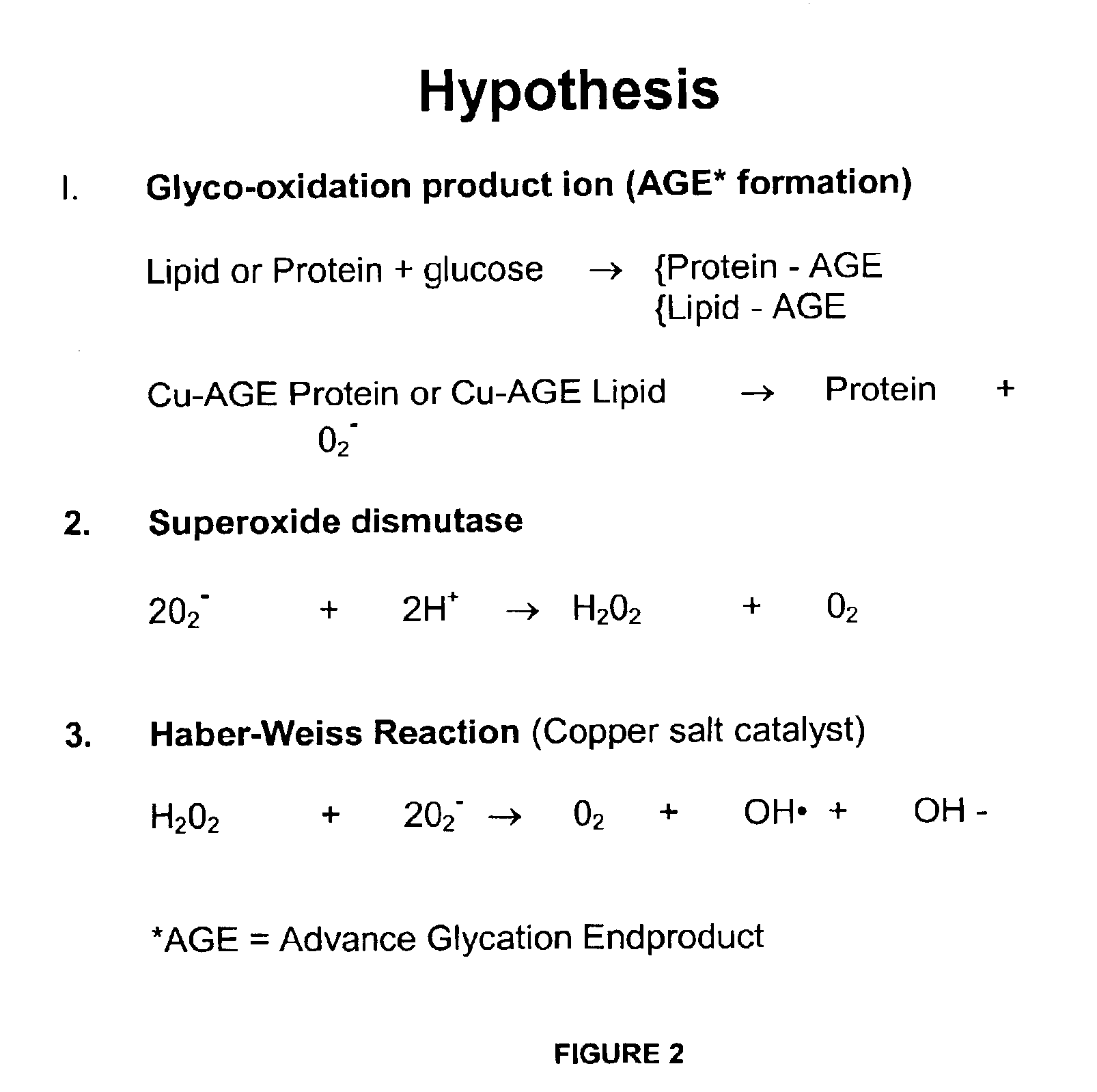
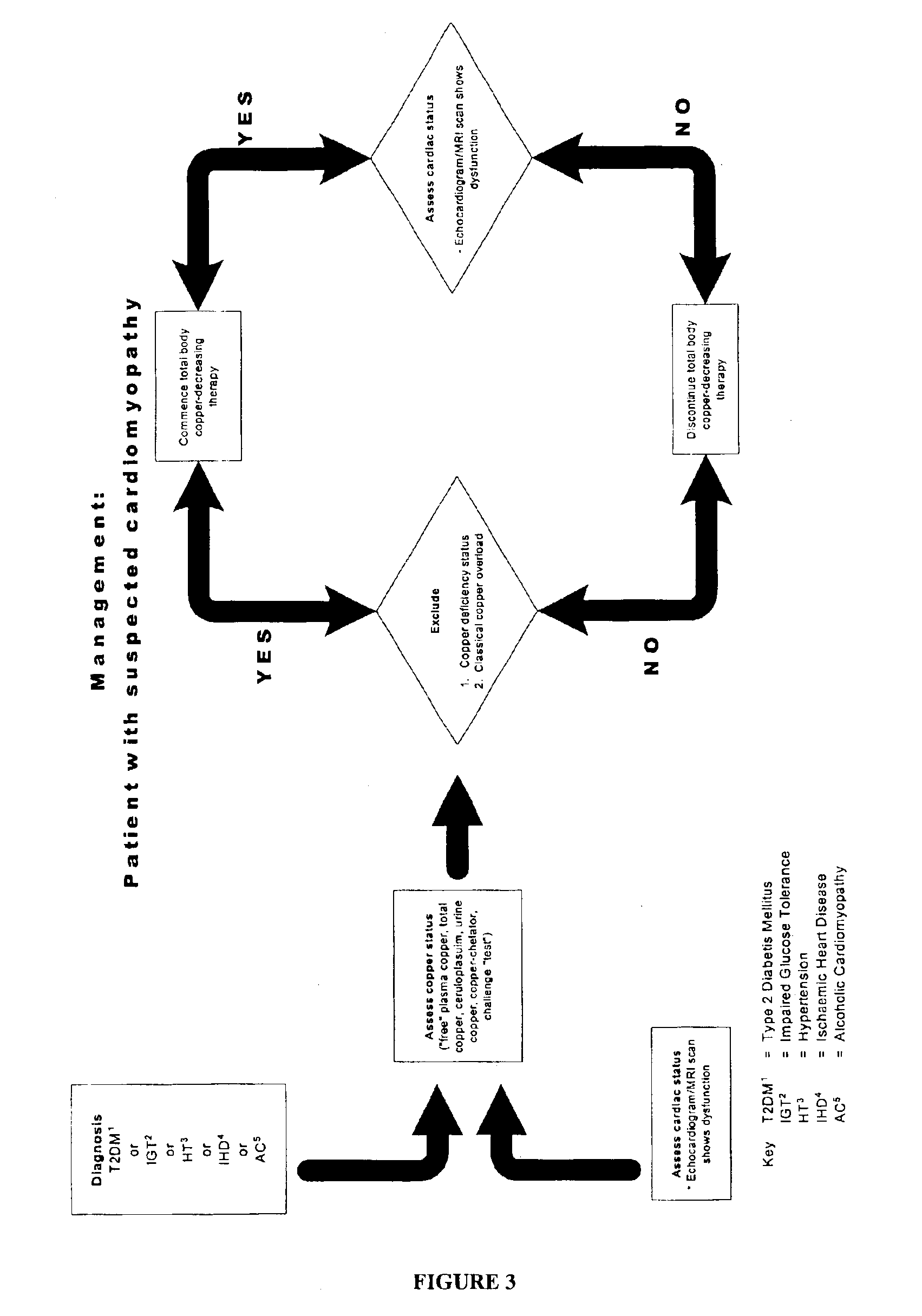
Preventing and/or treating cardiovascular disease and/or associated heart failure
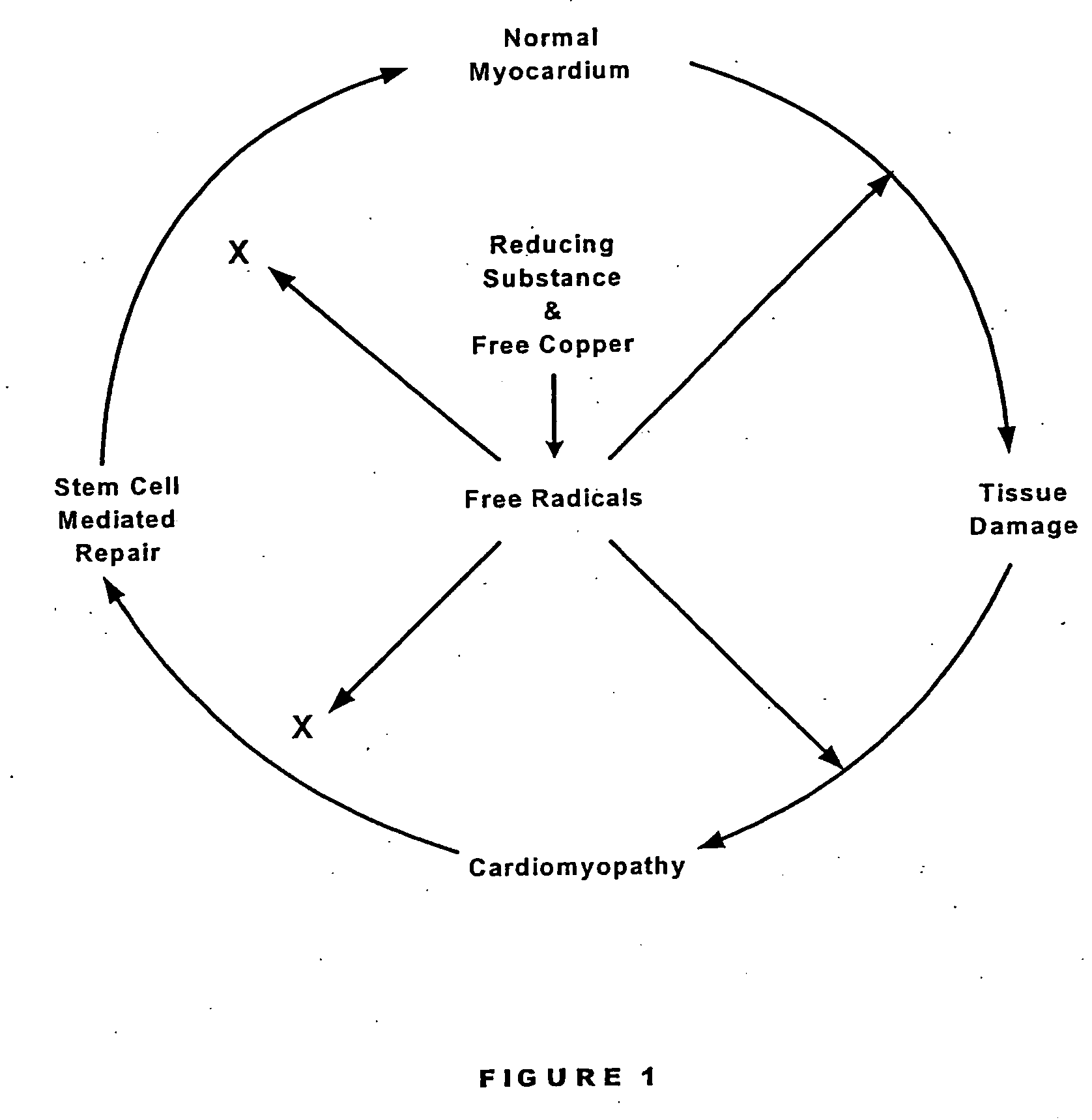
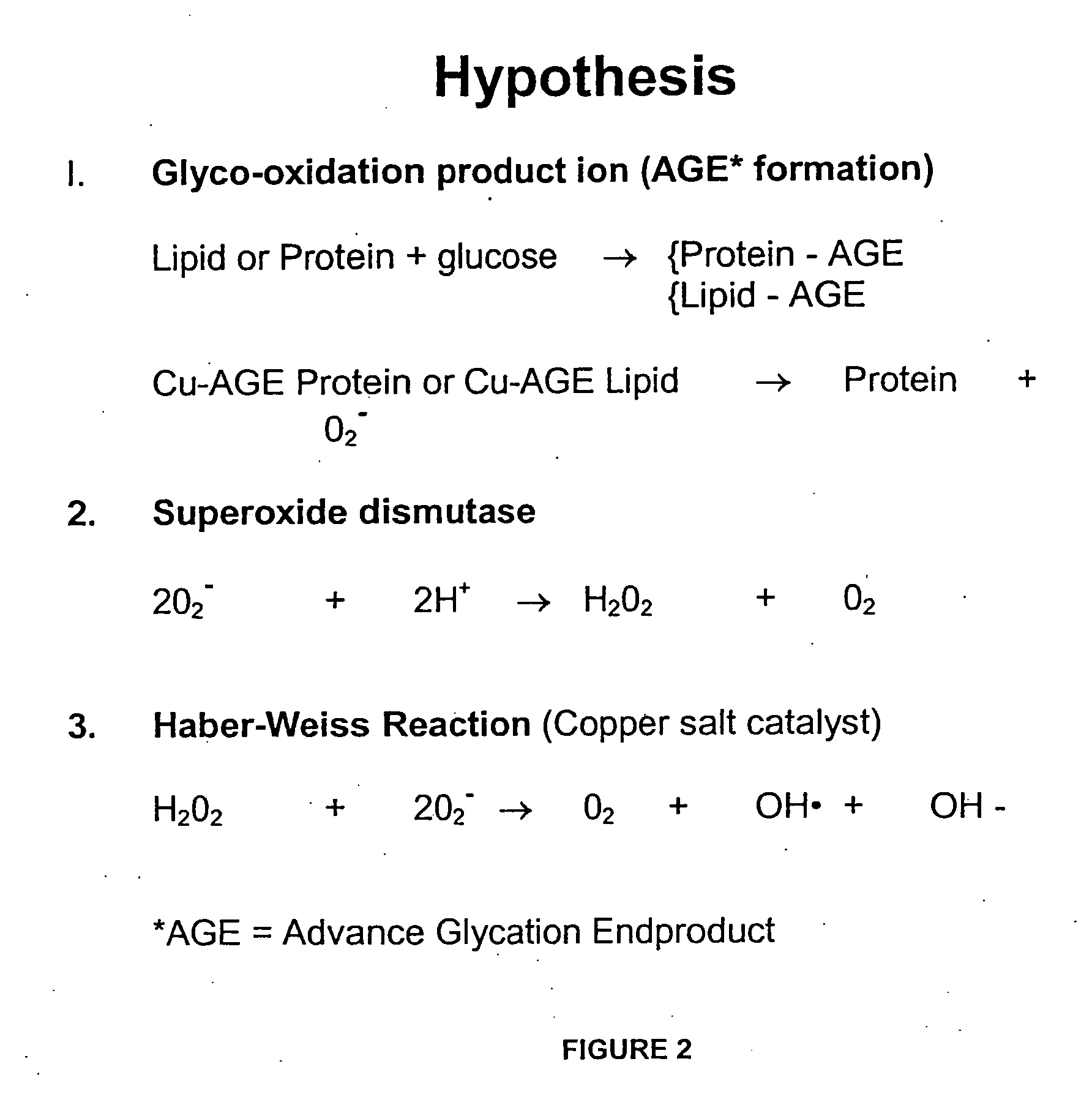
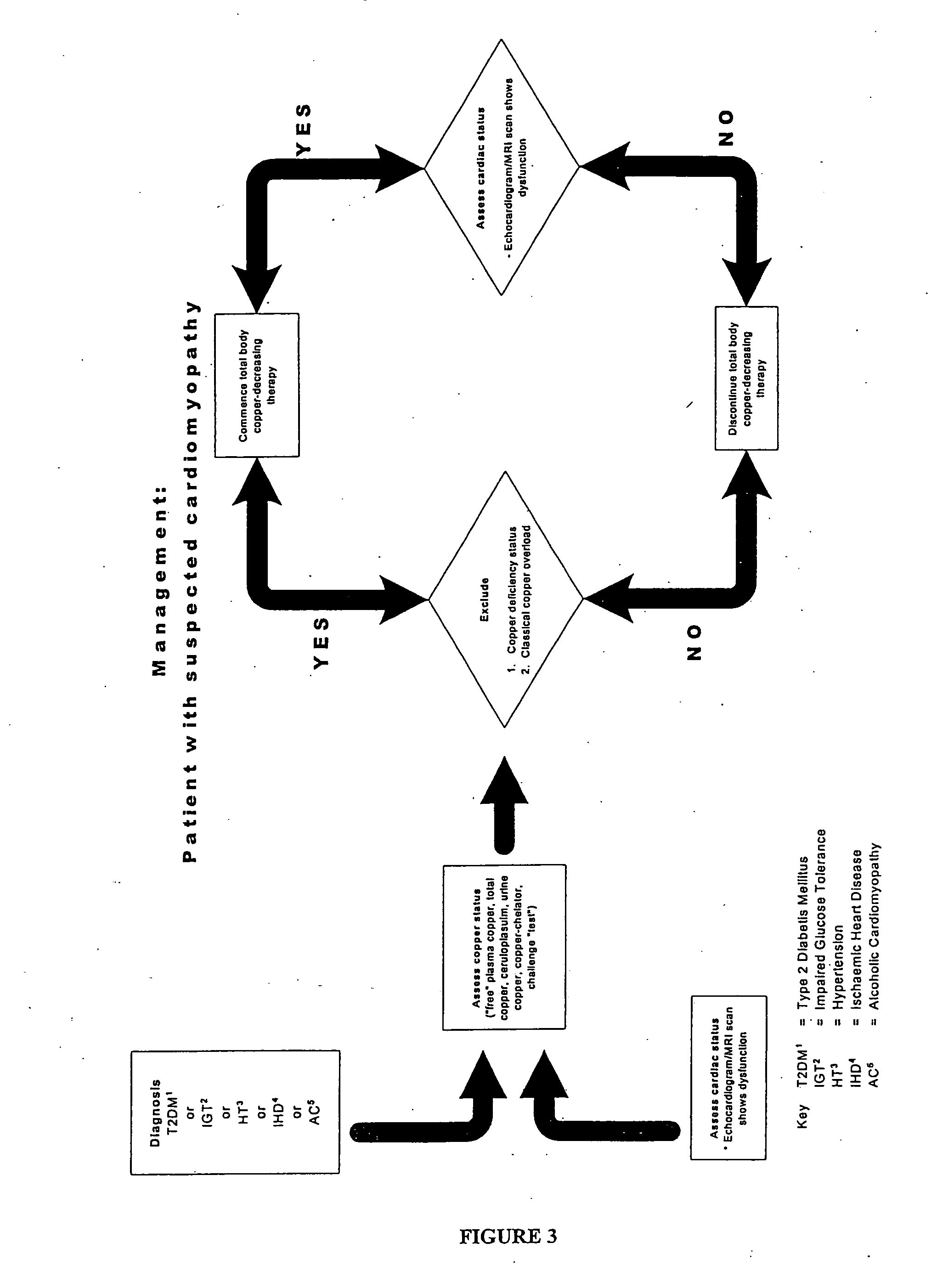
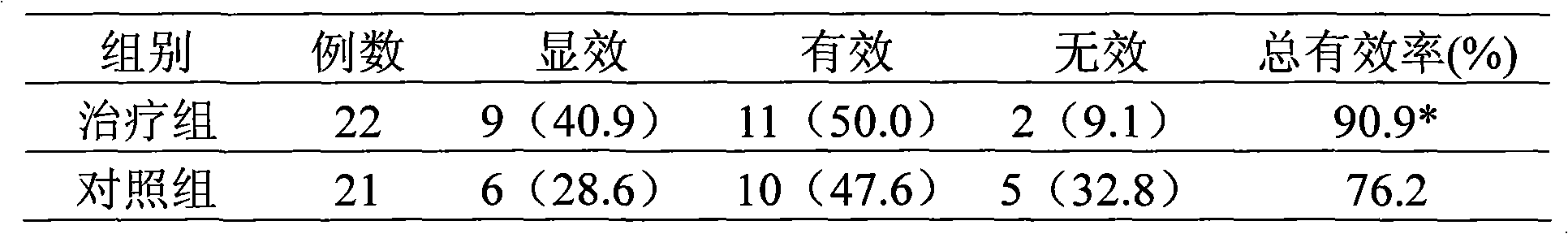
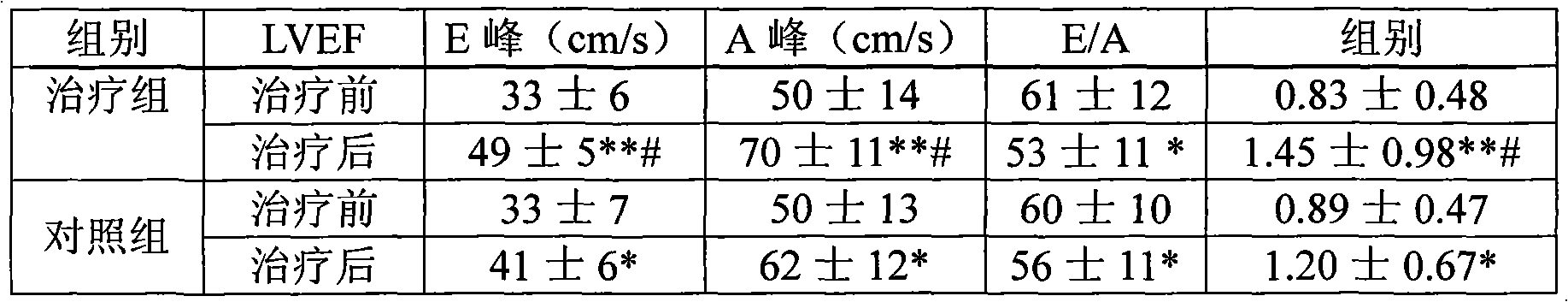
Methods are provided for reducing copper values for, by way of example, treating, preventing or ameliorating tissue damage such as, for example, tissue damage that may be caused by (i) disorders of the heart muscle (for example, cardiomyopathy or myocarditis) such as idiopathic cardiomyopathy, metabolic cardiomyopathy which includes diabetic cardiomyopathy, alcoholic cardiomyopathy, drug-induced cardiomyopathy, ischemic cardiomyopathy, and hypertensive cardiomyopathy, (ii) atheromatous disorders of the major blood vessels (macrovascular disease) such as the aorta, the coronary arteries, the carotid arteries, the cerebrovascular arteries, the renal arteries, the iliac arteries, the femoral arteries, and the popliteal arteries, (iii) toxic, drug-induced, and metabolic (including hypertensive and / or diabetic disorders of small blood vessels (microvascular disease) such as the retinal arterioles, the glomerular arterioles, the vasa nervorum, cardiac arterioles, and associated capillary beds of the eye, the kidney, the heart, and the central and peripheral nervous systems, (iv) plaque rupture of atheromatous lesions of major blood vessels such as the aorta, the coronary arteries, the carotid arteries, the cerebrovascular arteries, the renal arteries, the iliac arteries, the fermoral arteries and the popliteal arteries, (v) diabetes or the complications of diabetes.
Owner:PHILERA NEW ZEALAND +1
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS20040132713A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDisease progressionDiabetic nephropathy
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
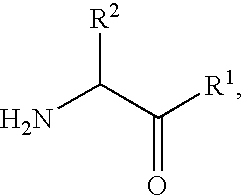
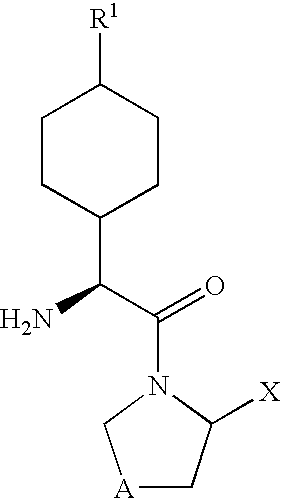
Dipeptidyl peptidase-IV inhibitors
InactiveUS20050234065A1High activityImproved gastrointestinal permeabilityBiocideNervous disorderDiabetic retinopathyArthritis
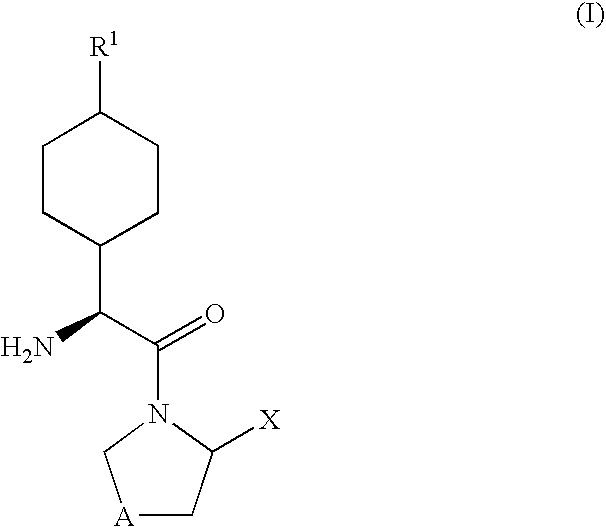
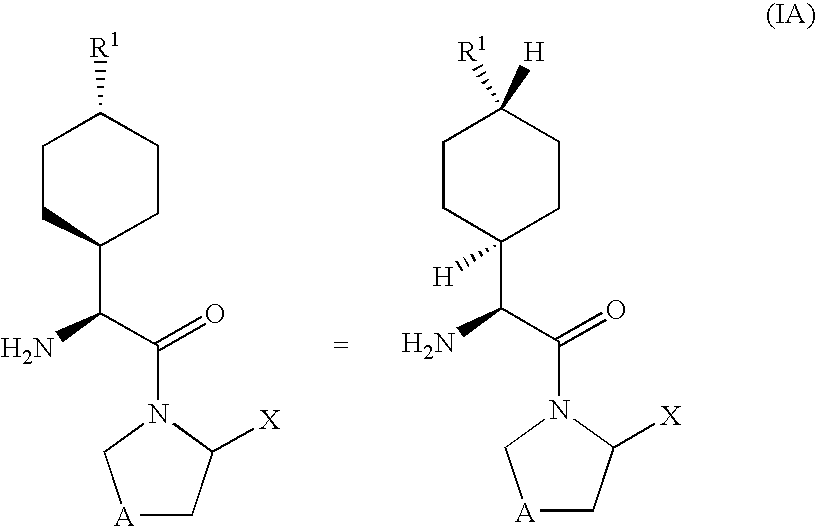
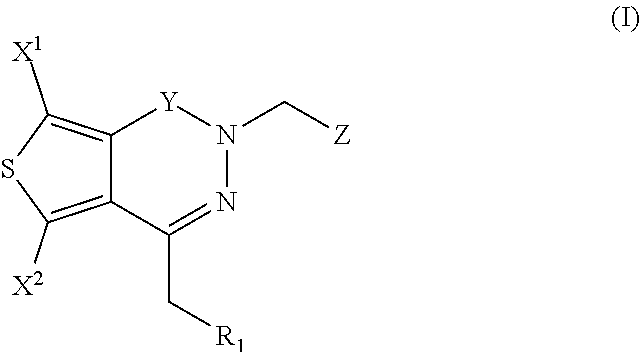
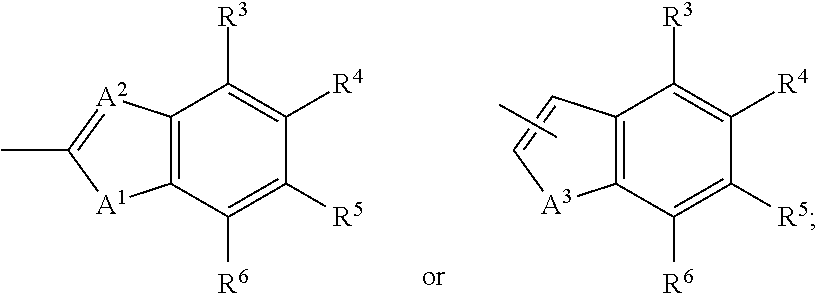
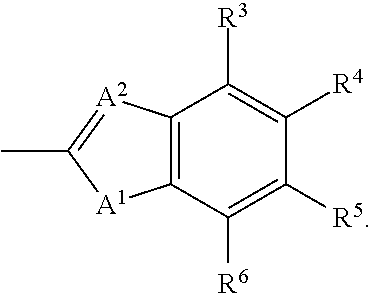
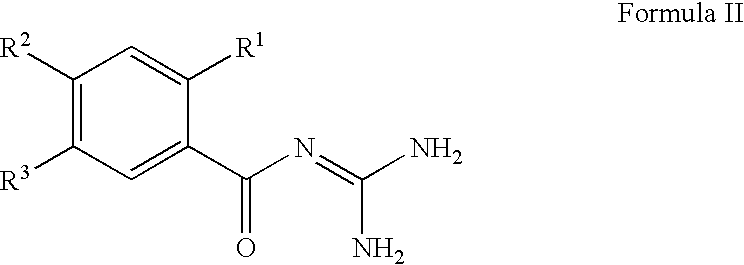
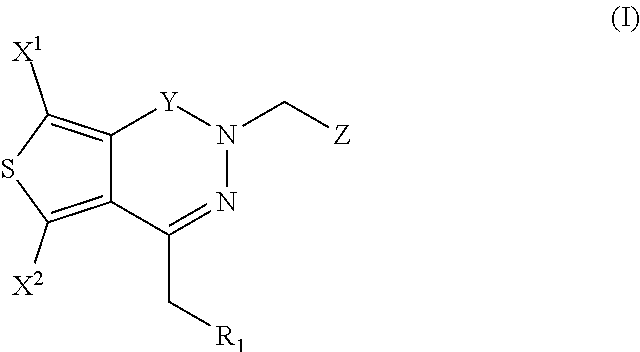
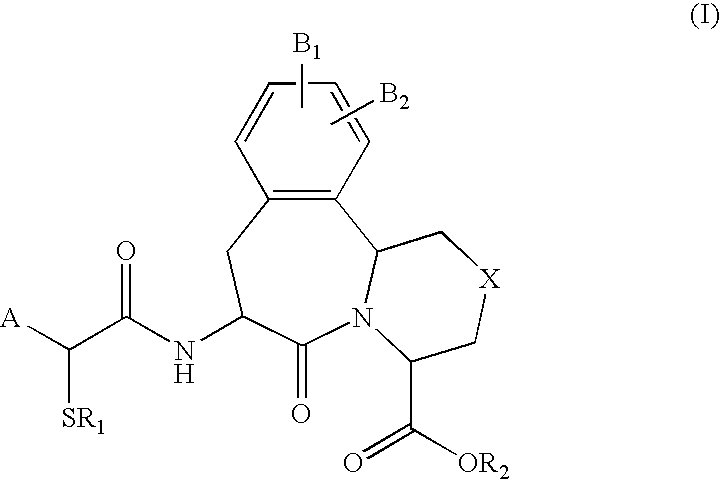
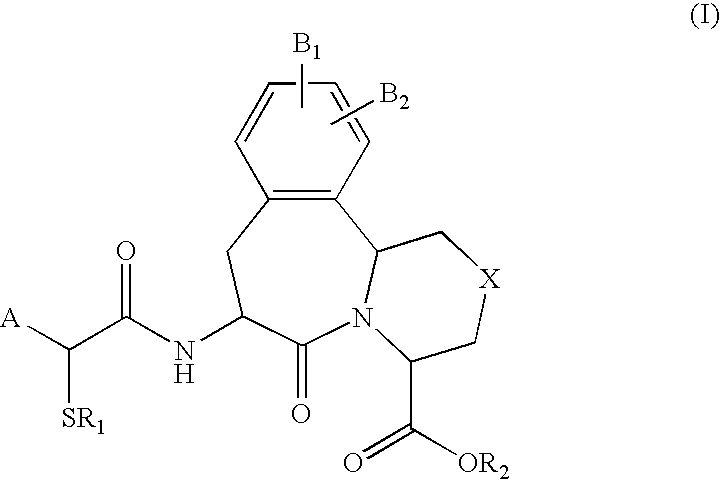
The invention provides compounds of Formula (I) or prodrugs thereof, or pharmaceutically acceptable salts of said compounds or prodrugs, or solvates of said compounds, prodrugs or salts, wherein A, N, X and R1 are as defined herein; pharmaceutical compositions thereof; and methods of using the pharmaceutical compositions for the treatment of diseases, including Type 2 diabetes, Type 1 diabetes, impaired glucose tolerance, hyperglycemia, metabolic syndrome (syndrome X and / or insulin resistance syndrome), glucosuria, metabolic acidosis, arthritis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, short stature due to growth hormone deficiency, infertility due to polycystic ovary syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome; short bowel syndrome; and the prevention of disease progression in Type 2 diabetes.
Owner:PFIZER INC
Treatment of hibernating myocardium with a GLP-1 peptide
InactiveUS20050096276A1Suppress plasma blood levelEase ischemic stressPeptide/protein ingredientsMetabolism disorderCatecholamineHigh energy
Hibernating myocardium is characterized by viable myocardium with impaired function due to localized reduced perfusion. Hibernating myocytes retain cellular integrity, but cannot sustain high-energy requirements of contraction. High plasma levels of catecholamines, such as norepinepherine, are believed to be predictive of mortality from hibernating myocardium. Likewise, high levels of catecholamines lead to cardiomyopathy in patients with diabetes. GLP-1 reduces plasma norepinepherine levels, and it thus is useful in a method of treating hibernating myocardium or diabetic cardiomyopathy.
Owner:COOLIDGE THOMAS +1
Medical use of pentacyclic triterpenoid saponin and pharmaceutical composition thereof
InactiveCN110200981AImprove securityGood curative effectOrganic active ingredientsSenses disorderBovine respiratory diseaseDiabetic complication
The present invention discloses an application of pentacyclic triterpenoid saponin represented by formulas (I) to (XIII) for the preparation of a medicine for preventing or treating AMPK-mediated diseases including fatty liver disease, inflammatory bowel disease, respiratory disease, diabetic complications and polycystic kidney disease. The compounds of formula (I) to (XIII) are especially usefulfor nonalcoholic simple fatty liver, nonalcoholic steatohepatitis, non-alcoholic steatohepatitis-induced cirrhosis, ulcerative colitis, Crohn's disease, chronic obstructive Pulmonary diseases, asthma,idiopathic pulmonary fibrosis, cystic fibrosis, allergic rhinitis, diabetic nephropathy, diabetic cardiomyopathy, diabetic ulcers, and autosomal dominant polycystic kidney disease. A pharmaceutical composition for preventing and treating the AMPK-mediated diseases of the present invention comprises a therapeutically effective amount of the compounds of the formula (I) to (XIII) or a pharmaceutically acceptable salt or a solvate thereof as an active ingredient and a pharmaceutically acceptable excipient.
Owner:CHINA PHARM UNIV
Enhanced medical treatment in diabetic cardiomyopathy
InactiveUS20070265216A1Efficient and accurate to quantifyEfficient identificationOrganic active ingredientsMetabolism disorderTissue biopsyMammal
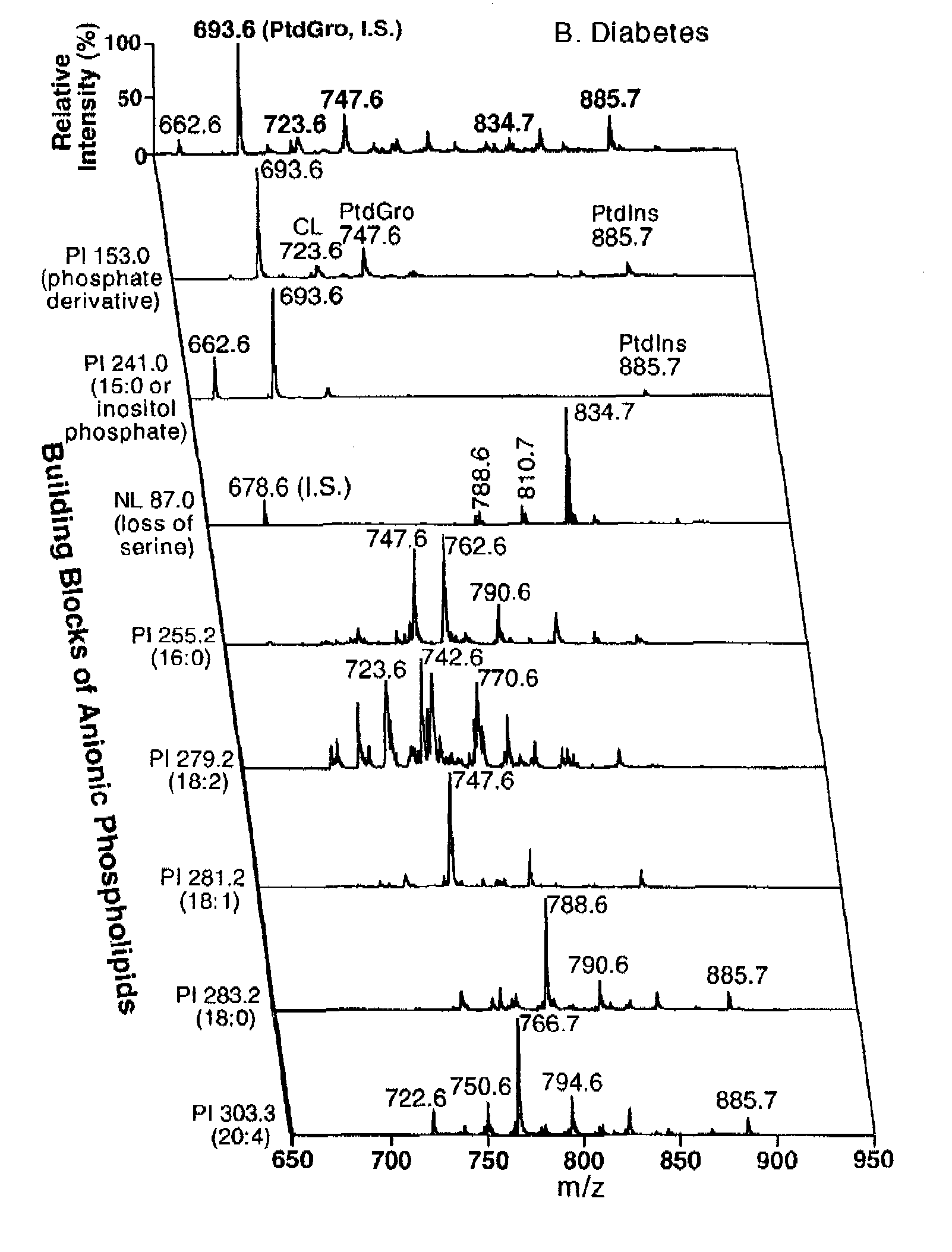
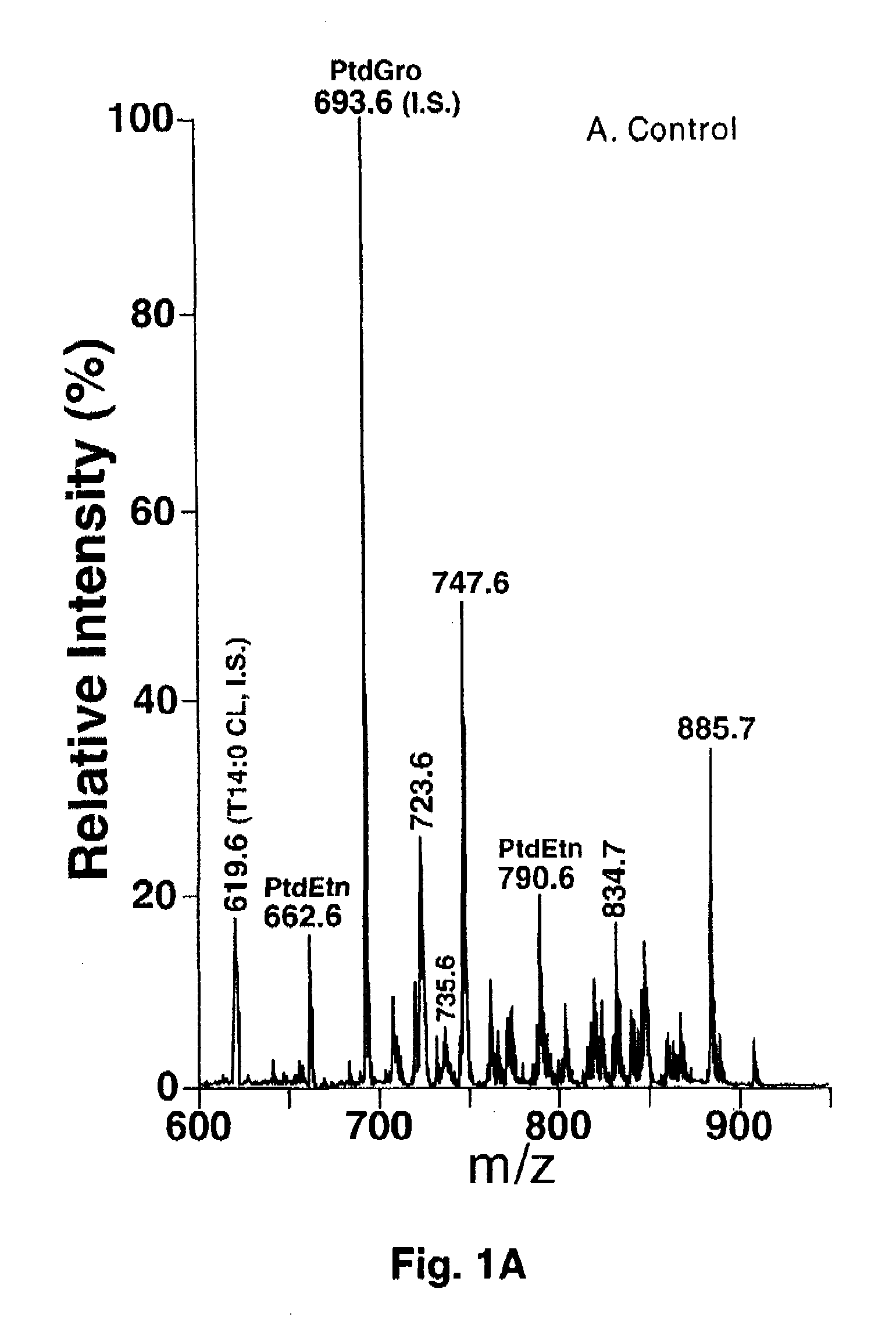
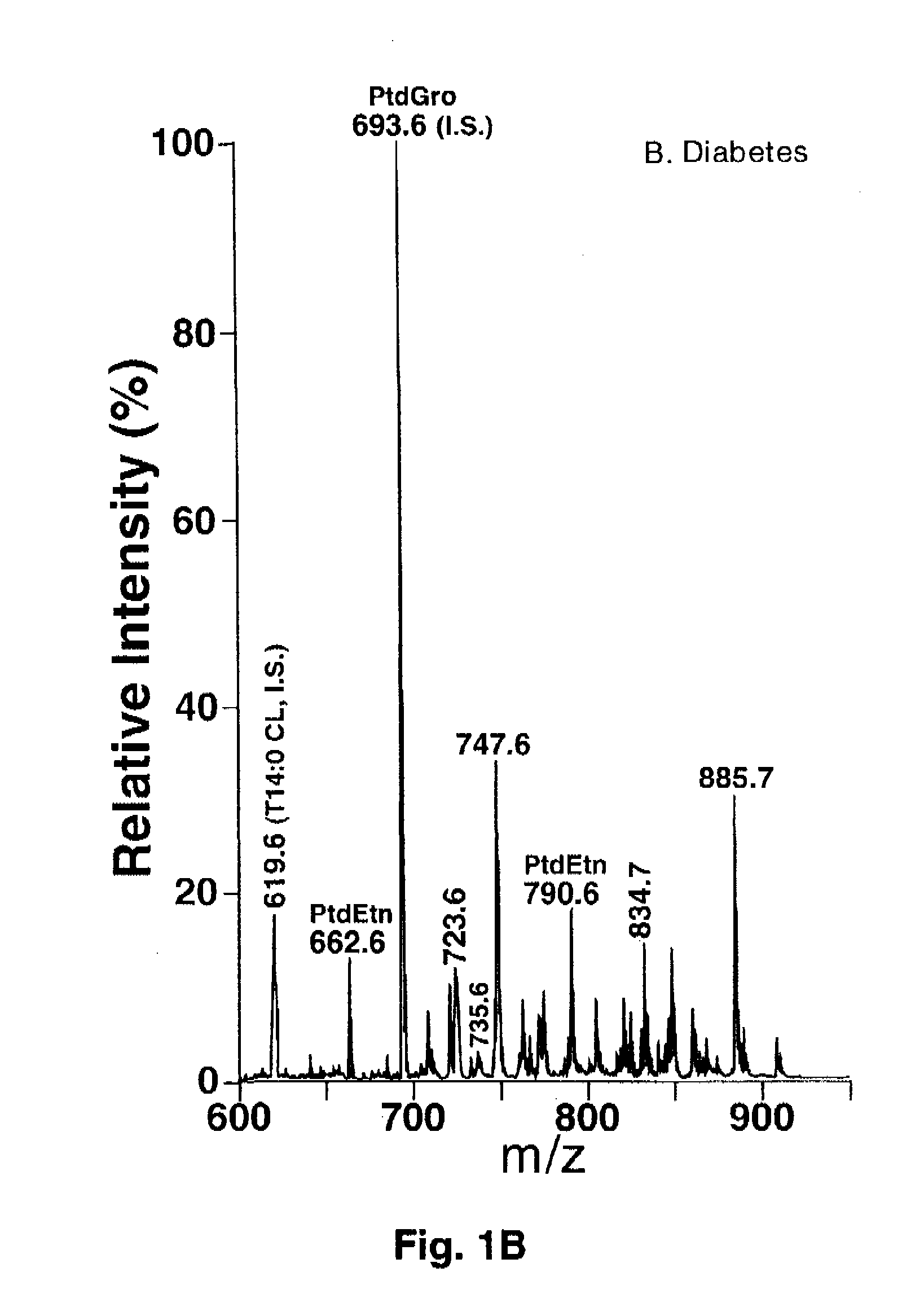
A method of treating a living mammal having diabetic cardiomyopathy comprises administering an effective amount of an inhibitor to the mammal performing a shotgun lipidomics analysis on the mammal and determining that the treatment was successful when and if serum or tissue biopsy cardiolipin levels are increased and / or lysocardiolipin levels are decreased.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Crystalline ARB-NEPi dicationic compound and preparation method and application thereof
The present invention discloses a crystalline ARB-NEPi dicationic compound and a preparation method and application thereof. In particular, the present invention relates to the crystalline ARB-NEPi dicationic compound having the formula of NEPi.Nax.Ky.ARB.ZH2O. The present invention also relates to a pharmaceutical composition containing an effective amount of the crystalline dicationic compound and application of the pharmaceutical composition in the preparation of drugs for treatment or prevention of neutral-endopeptidase-related diseases, cardiovascular diseases, hypertension, acute and chronic heart failures, congestive heart failure, left ventricular dysfunction, hypertrophic cardiomyopathy, diabetic cardiomyopathy, supraventricular arrhythmia and ventricular arrhythmia, atrial fibrillation, atrial flutter or detrimental vascular remodeling, and the like, and the pharmaceutical composition has broad application prospects.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
Methods of treatment and pharmaceutical composition
The invention relates a pharmaceutical composition comprising a combination of:(i) the AT 1-antagonist valsartan or a pharmaceutically acceptable salt thereof; and(ii) a NEP inhibitor or a pharmaceutically acceptable salt thereof and optionally a pharmaceutically acceptable carrier and to a method for the treatment or prevention of a condition or diseaseselected from the group consisting of hypertension, heart failure, such as (acute and chronic) congestive heart failure, left ventricular dysfunction and hypertrophic cardiomyopathy, diabetic cardiac myopathy, supraventricular and ventricular arrhythmias, atrial fibrillation, atrial flutter, detrimental vascular remodeling, myocardial infarction and its sequelae, atherosclerosis, angina (whether unstable or stable), renal insufficiency (diabetic and non-diabetic), heart failure, angina pectoris, diabetes, secondary aldosteronism, primary and secondary pulmonary hypertension, renal failure conditions, such as diabetic nephropathy, glomerulonephritis, scleroderma, glomerular sclerosis, proteinuria of primary renal disease, and also renal vascular hypertension, diabetic retinopathy, the management of other vascular disorders, such as migraine, peripheral vascular disease, Raynaud's disease, luminal hyperplasia, cognitive dysfunction, such as Alzheimer's, glaucoma and stroke, comprising administering a therapeutically effective amount of the pharmaceutical composition to a mammal in need thereof.
Owner:NOVARTIS PHARM CORP
Aldose reductase inhibitors and methods of use thereof
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Methods of treating diabetic cardiomyopathy using glycogen phosphorylase inhibitors
The present invention provides methods of treating diabetic cardiomyopathy, the methods comprising administering to a patient having or at risk of having diabetic cardiomyopathy a therapeutically effective amount of a glycogen phosphorylase inhibitor. The present invention also provides methods of treating diabetic cardiomyopathy, the methods comprising administering to a patient having 1) diabetes and 2) having cardiovascular disease, ischemic heart disease, congestive heart failure, congestive heart failure but not having coronary arteriosclerosis, hypertension, diastolic blood pressure abnormalities, microvascular diabetic complications, abnormal left ventricular function, myocardial fibrosis, abnormal cardiac function, pulmonary congestion, small vessel disease, small vessel disease without atherosclerotic cardiovascular disease or luminal narrowing, coagulopathy, cardiac contusion, or having had or at risk of having a myocardial infarction a therapeutically effective amount of a glycogen phosphorylase inhibitor.
Owner:PFIZER INC
Preventing and/or treating cardiovascular disease and/or associated heart failure
Owner:PHILERA NEW ZEALAND
Application of Chinese medicinal composition in preparation of medicament for treating diabetic cardiomyopathy
The invention discloses application of a Chinese medicinal composition in preparation of a medicament for treating diabetic cardiomyopathy. The Chinese medicinal composition consists of 11 gouts of Chinese medicaments such as astragalus, ginseng, root of red rooted salvia and the like. The medicaments for tonifying qi and warming yang are used as radical components for treating channels and strengthening heart, and the medicaments for promoting blood circulation and removing obstruction in channels are used as auxiliary medicaments, so the qi is vigorous, the blood is activated, the channels are dredged, and blood stasis and channel obstruction are blocked. Experiment researches prove that the Chinese medicinal composition can effectively treat the diabetic cardiomyopathy.
Owner:HEBEI YILING MEDICINE INST
Medicine composition for preventing and/or treating diabetes and complication of diabetes
InactiveCN104337884ASignificant progressLower blood sugarCosmetic preparationsSenses disorderSide effectPeripheral neuritis
The invention discloses a medicine composition for preventing and / or treating diabetes and complication of diabetes. The medicine composition is prepared from Chinese herbaceous peony and cassia bark in a weight ratio of (10-1) to 1. The medicine composition comprises the following effective components in parts by weight: 50-150 parts of a pinane monoterpene glycoside compound in Chinese herbaceous peony, and 1-5 parts of a cinnamic acid / aldehyde compound. The medicine composition not only has a good and stable blood sugar reduction and weight loss function, but also can effectively prevent and treat multiple diabetes complications, such as fatty liver, diabetic cardiomyopathy, diabetes peripheral neuritis, diabetes eye diseases and diabetic nephropathy. The composition is low in toxicity, small in side effect, low in cost and safe to use.
Owner:颜明
Treatment of diabetes and diabetic complications with NHE-1 inhibitors
This invention relates to methods of treating or preventing type 2 diabetes, diabetic neuropathy, diabetic cardiomyopathy, cataracts, diabetic retinopathy, foot ulcers, diabetic microangiopathy, diabetic macroangiopathy, diabetic ischemia reperfusion injury, diabetic cardiac ischemia reperfusion injury and / or insulin resistance syndrome (IRS) in mammals, particularly in humans, by administering a sodium-hydrogen exchanger type 1 (NHE-1) inhibitor or a pharmaceutical composition containing such an inhibitor. This invention also relates to combinations comprising NHE-1 inhibitors and a second pharmaceutical agent, said combinations being useful in treating type 2 diabetes, IRS, diabetic neuropathy, diabetic cardiomyopathy, cataracts, diabetic retinopathy, foot ulcers, diabetic ischemia reperfusion injury, diabetic cardiac ischemia reperfusion injury, diabetic microangiopathy and / or diabetic macroangiopathy.
Owner:PFIZER INC
Application of rhein or compound of rhein compound and arginine in preparation of medicine for treating diabetic complications
InactiveCN102258509AWide range of choicesImprove securityOrganic active ingredientsSenses disorderDiseaseArginine
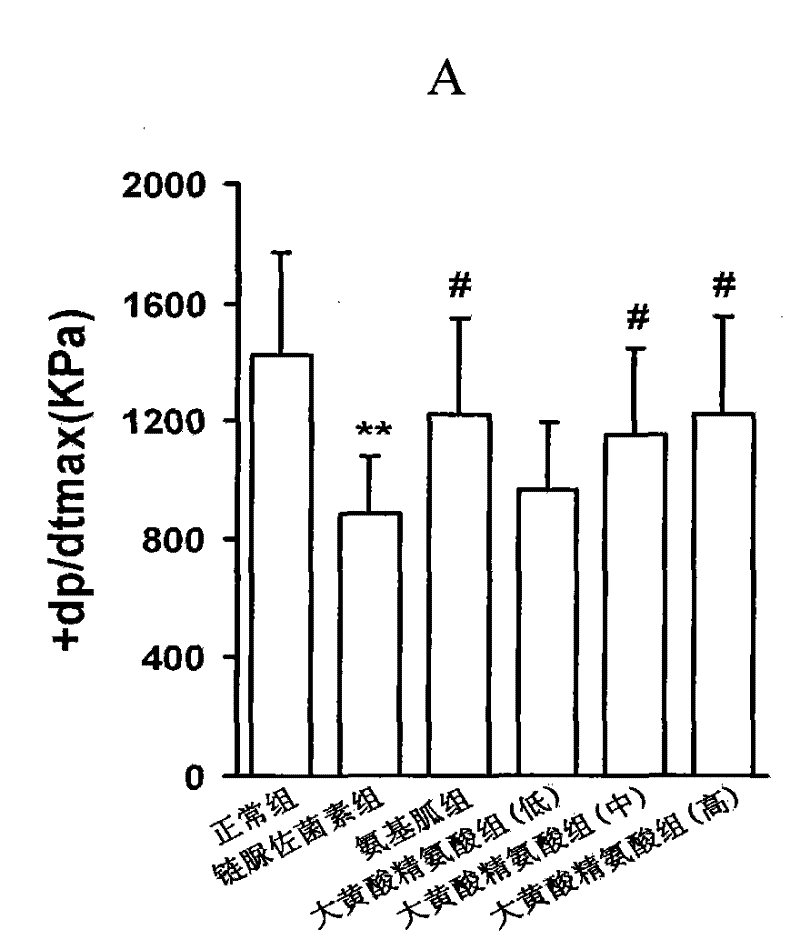
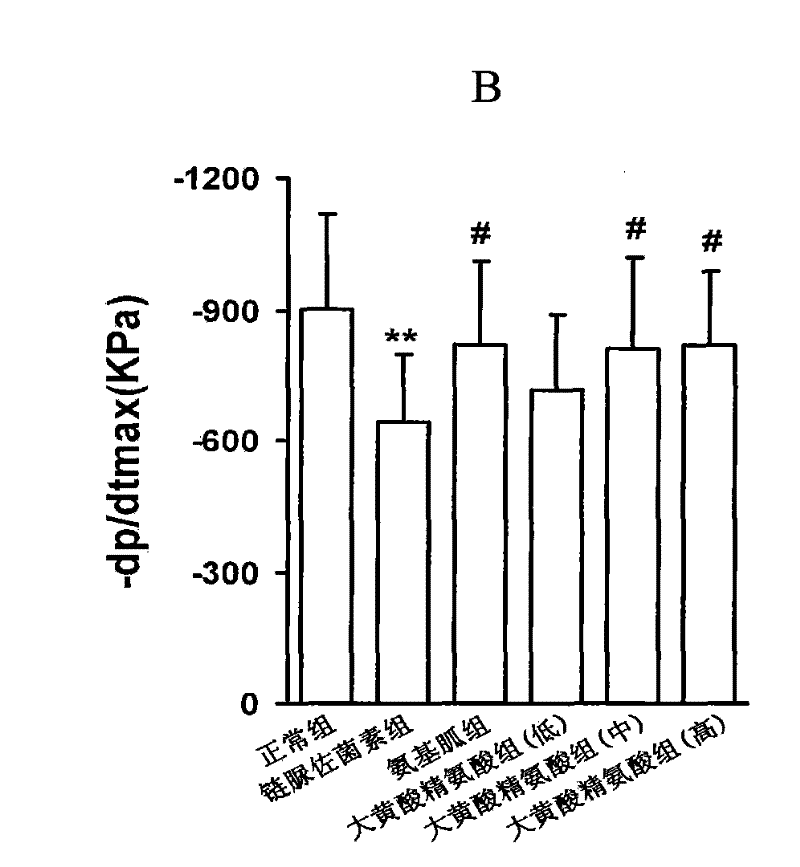
The complex of rhein or rhein compound and arginine is used in the preparation of medicines for treating diabetic complications. The drug for the treatment of diabetic complications refers to the drug for the treatment of diabetic cardiomyopathy, the drug for the treatment of diabetic macrovascular disease, the drug for the treatment of diabetic nephropathy, the drug for the treatment of diabetic orchopathy, the drug for the treatment of diabetes complicated by male hypofunction, Or drugs for diabetic impotence. The new drug with good safety provided by the present invention; its drug effect will be better than traditional therapeutic drugs and treatment methods; it will open the paradigm of Chinese medicine supramolecular technology in the transformation of important active ingredients, and expand supramolecular technology in the research and development of innovative Chinese medicine drugs. application. The new drug is used for the treatment of diabetic complications, including: diabetic cardiomyopathy; diabetic macroangiopathy; diabetic nephropathy;
Owner:ZHEJIANG CHINESE MEDICAL UNIV MEDICAL PIECES
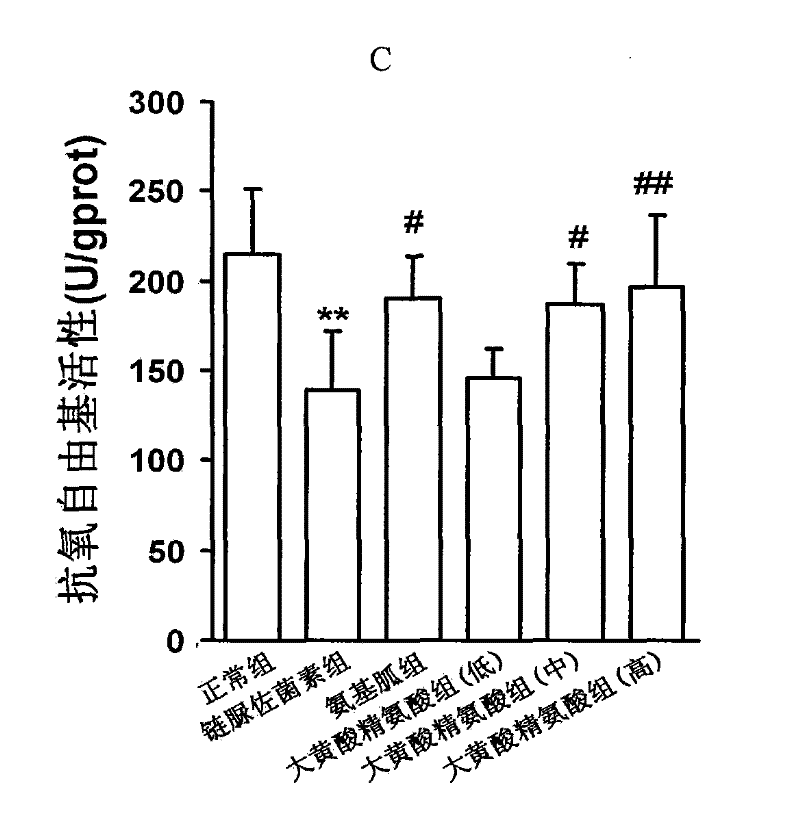
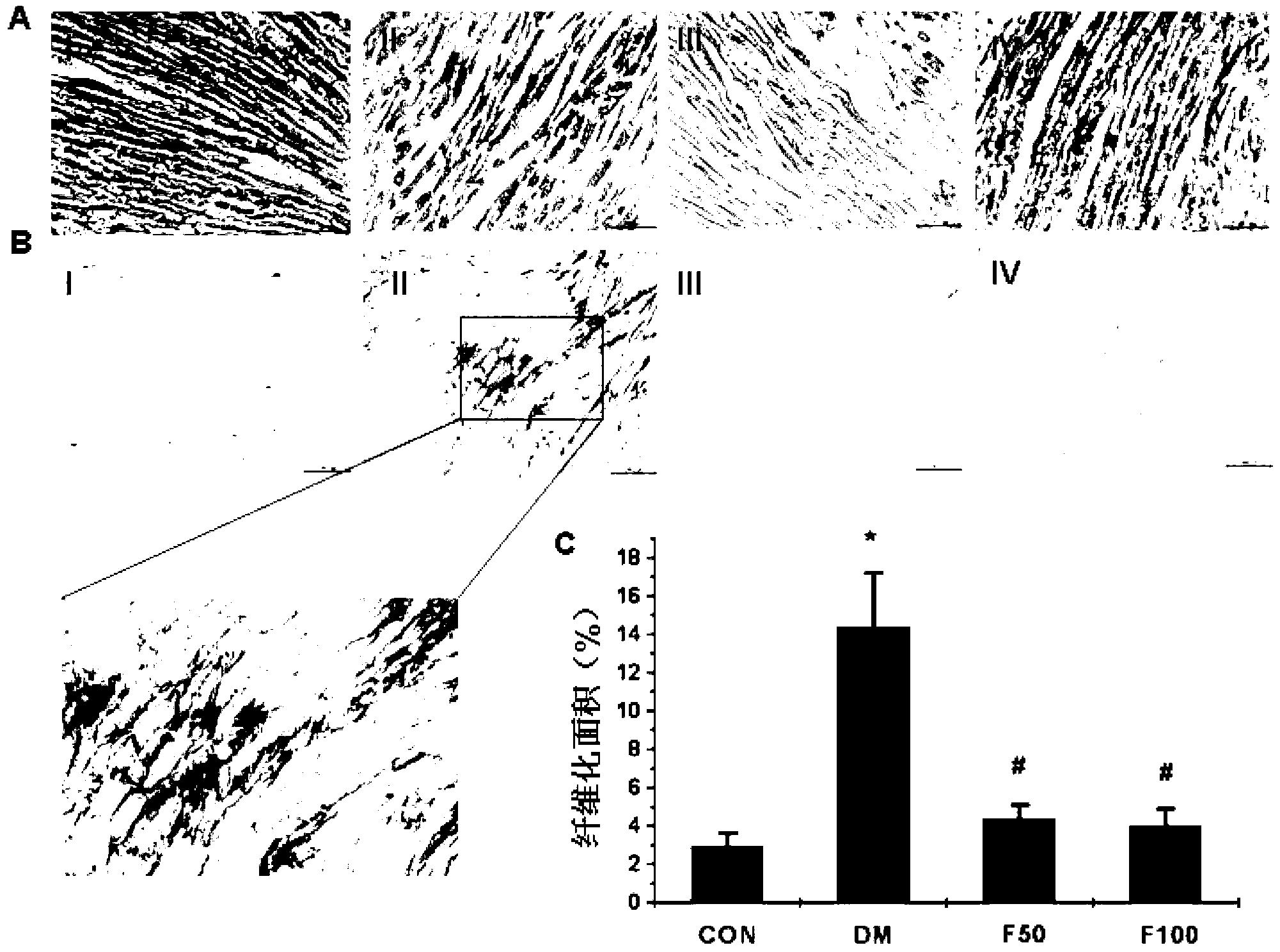
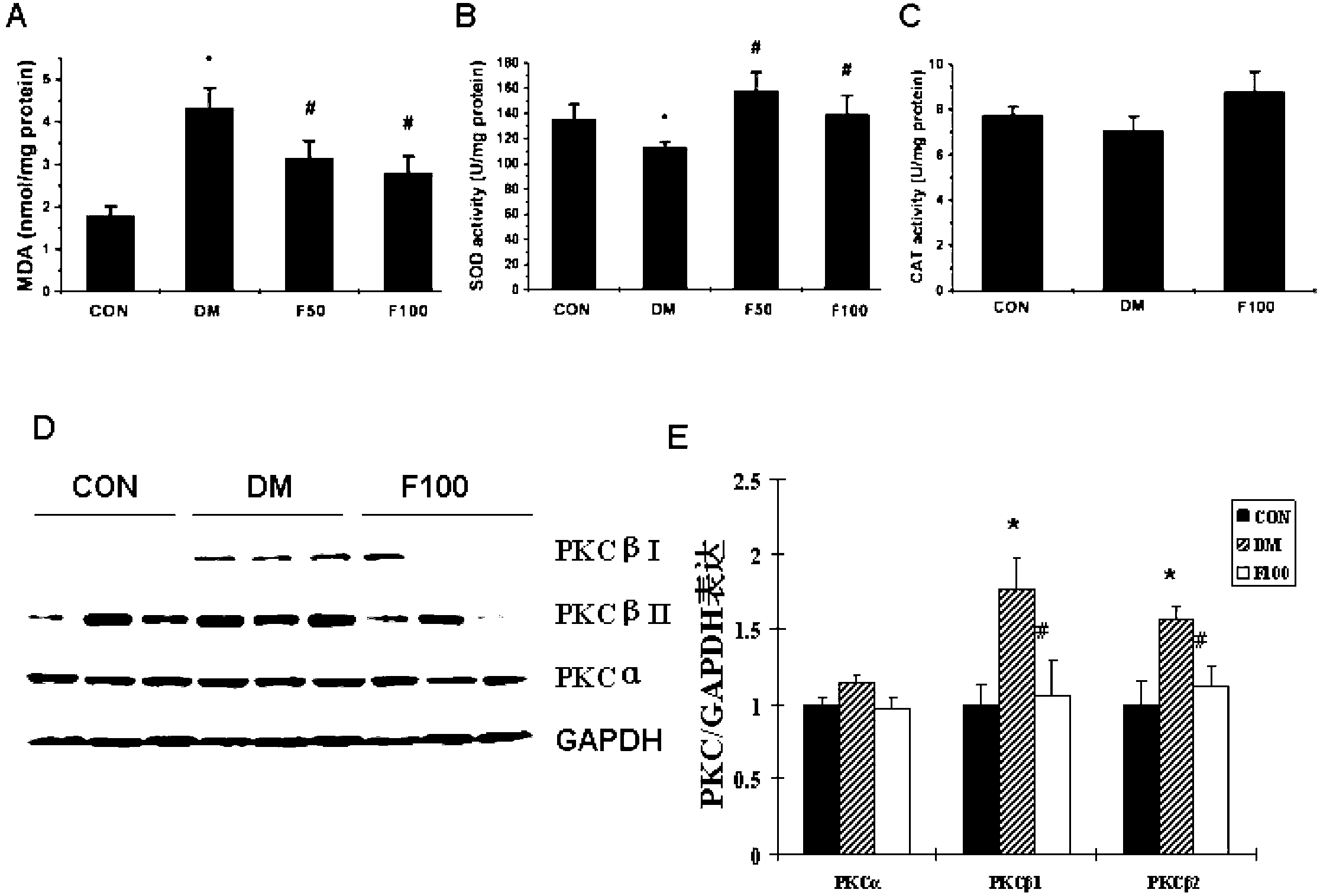
Application of fucoidan polysaccharide sulfate in preparing drug for preventing and/or treating diabetic cardiomyopathy
ActiveCN103230411ALittle side effectsIncreased systolic functionOrganic active ingredientsMetabolism disorderCardiac fibrosisAntioxidative enzyme
The invention discloses a drug novel use of fucoidan polysaccharide sulfate. The use is the application of the fucoidan polysaccharide sulfate in preparing the following products: (1), a product for preventing and / or treating diabetic cardiomyopathy; (2), a product for inhibiting diabetic myocardial oxidative stress; and (3), a product for inhibiting expression and activation of PKC (Protein Kinase C) beta in diabetic cardiomyopathy; and (4), a product for relieving cardiac interstitial fibrosis of a diabetic. The fucoidan polysaccharide sulfate is preferably LMWF (Fucoidan Polysaccharide Sulfate) with weight-average molecular weight of 3-30KD. According to the pharmacodynamic experiment, the LMWF can be used for generating beneficial interference to the development of pathogenetic condition of the diabetic cardiomyopathy by strengthening the myocardial systolic function and relieving cardiac fibrosis. More importantly, the LMWF can be used for effectively inhibiting oxidative stress by regulating the activity of antioxidase, and can be used for inhibiting the expression of protein kinase C beta (PKCbeta) subtype. The LMWF is from the natural existing kelp, so that the availability and safety of the kelp are beneficial to using the fucoidan polysaccharide sulfate as a potential clinical treatment drug and preventing the diabetic cardiomyopathy.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Transgenic Non-Human Animal Models of Ischemia-Reperfusion Injury and Uses Thereof
The present invention relates to a nucleic acid molecule encoding a K94A / K447A mutant of wild type p90 ribosomal S6 kinase (p90RSK) and DNA constructs, expression vectors, and hosts including the mutant p90RSK-encoding molecule. The present invention also relates to two transgenic non-human animal models of ischemic reperfusion (I / R) damage, the first animal having a transgene encoding a mutant p90RSK that is rendered kinase inactive for S703 phosphorylation of NHE1 and the second animal having a transgene encoding for cardiac-specific overexpression of wild type p90RSK in the animal that provides a model for diabetic cardiomyopathy. Also provided are methods for generating transgenic non-human animal models of ischemic reperfusion (I / R) damage; for using the transgenic cells for identifying an agent capable of inhibiting p90RSK-induced I / R damage; for identifying agents that modulate I / R injury resulting from an ischemic event; and for treating individuals to inhibit I / R injury following an ischemic event.
Owner:UNIVERSITY OF ROCHESTER
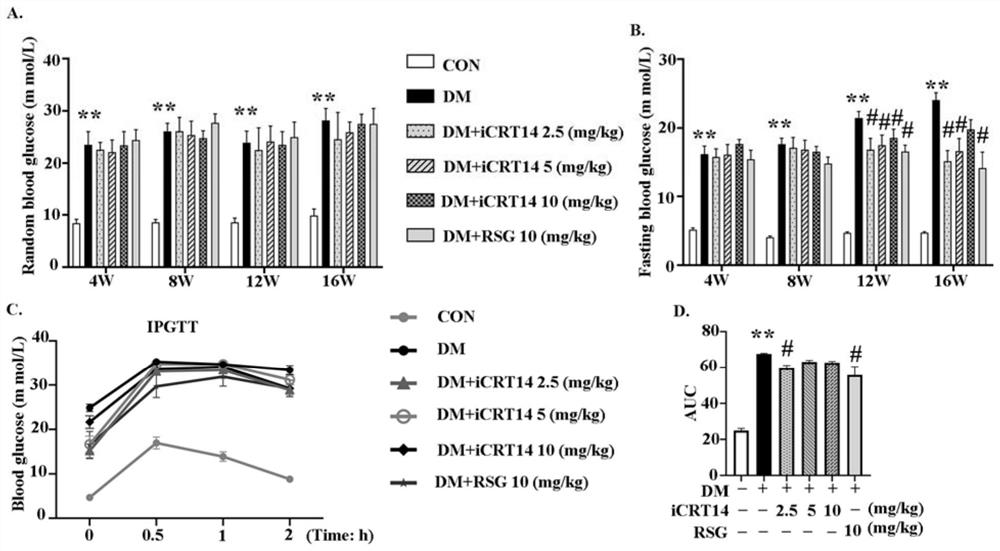
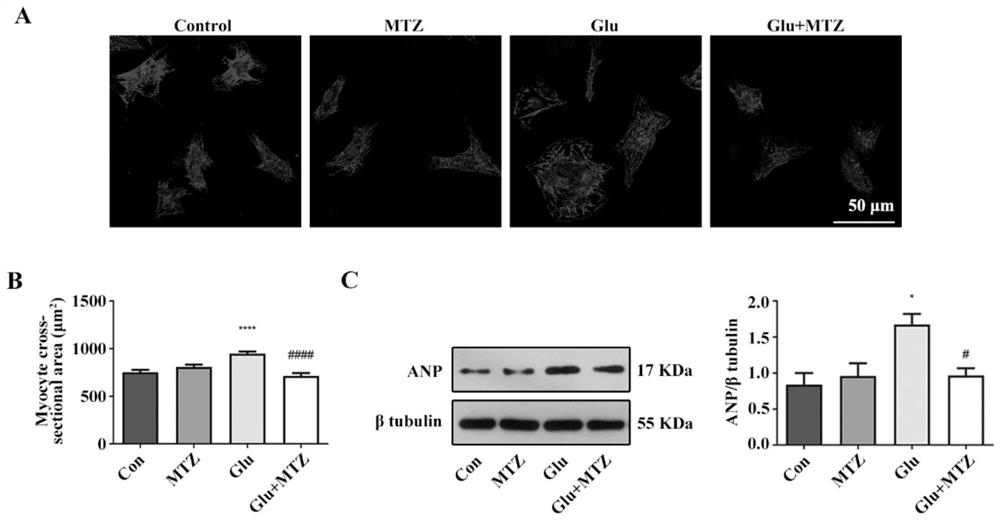
Application of compound in preparation of drug for treating type 2 diabetic cardiomyopathy
InactiveCN113304149AMolecular smallIncrease fat solubilityOrganic active ingredientsMetabolism disorderPharmacologyInsulin humulin
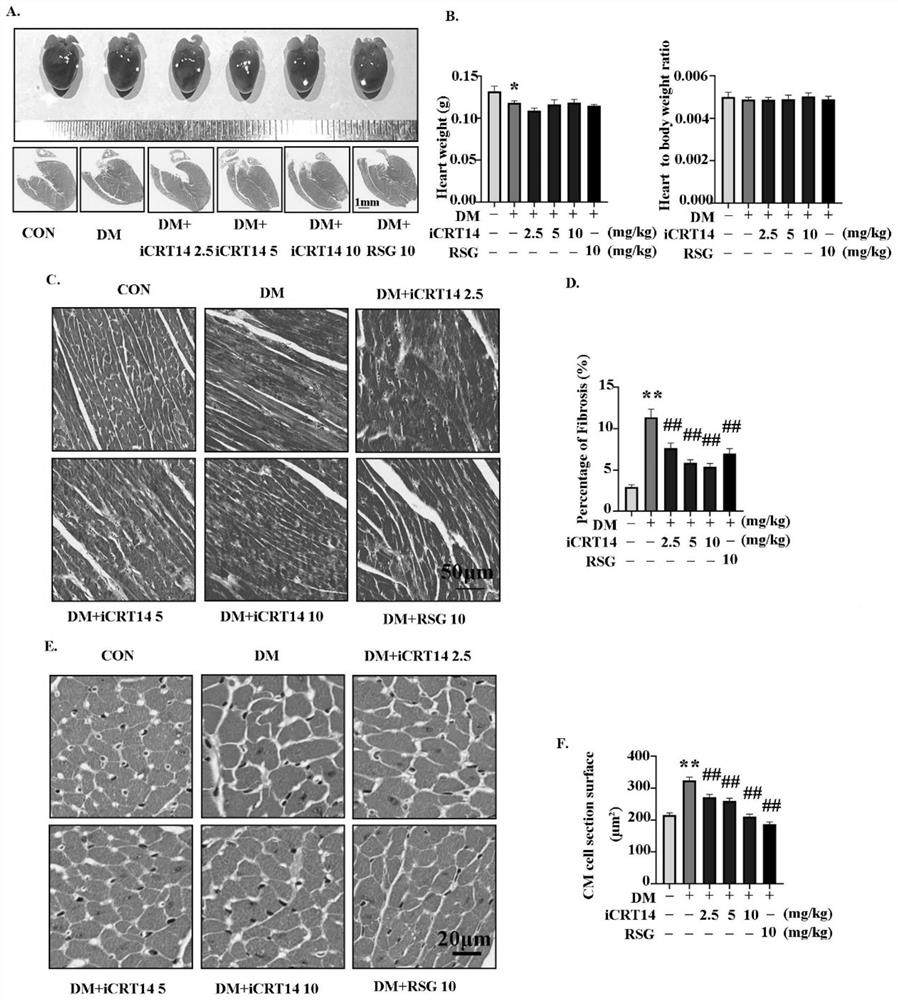
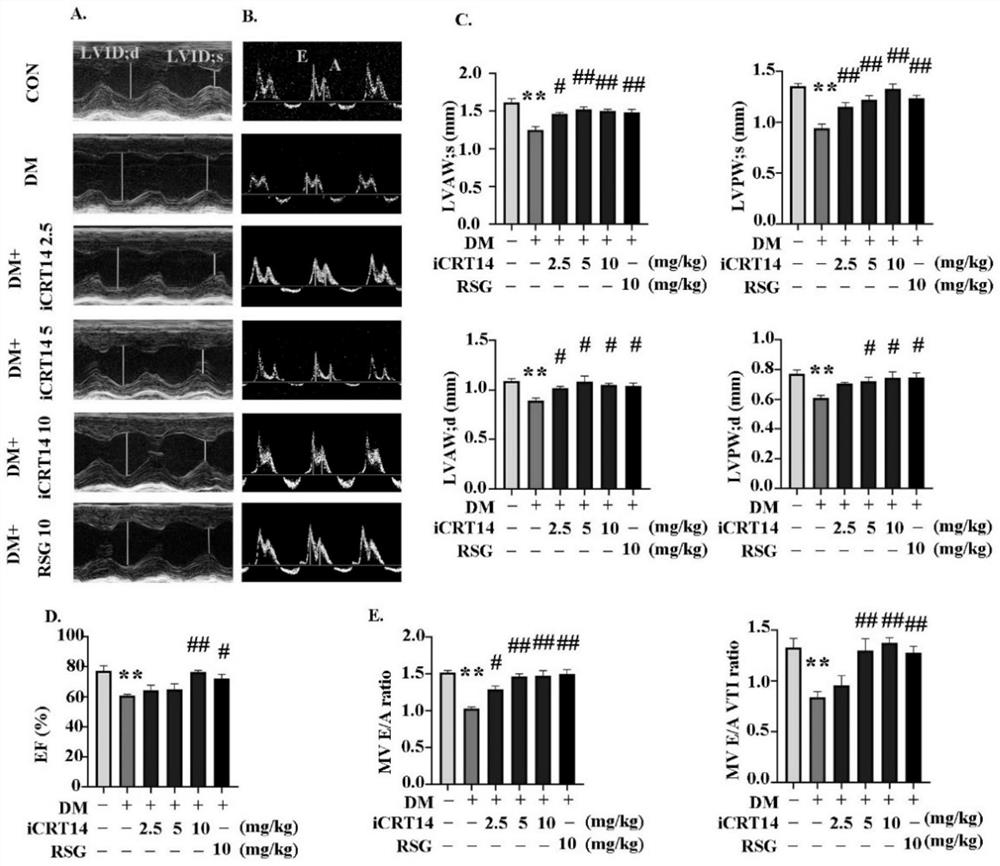
The invention discloses a novel application of iCRT14, and particularly relates to a novel application of iCRT14 in preparation of a drug for treating type 2 diabetic cardiomyopathy. In the research, the inventor of the application finds that: the iCRT14 can reduce the blood sugar of the type 2 diabetic cardiomyopathy, improve myocardial remodeling of the type 2 diabetic cardiomyopathy, improve cardiac contraction and diastolic dysfunction caused by the type 2 diabetic cardiomyopathy and inhibit myocardial cell hypertrophy induced by high glucose and high insulin, and has a good treatment effect on the type 2 diabetic cardiomyopathy. The invention provides a new drug choice for treatment of the type 2 diabetic cardiomyopathy.
Owner:GUANGZHOU MEDICAL UNIV
Aldose reductase inhibitors and methods of use thereof
The present disclosure relates to novel compounds and pharmaceutical compositions thereof, and methods for promoting healthy aging of skin, the treatment of skin disorders, the treatment of cardiovascular disorders, the treatment of renal disorders, the treatment of angiogenesis disorders, such as cancer, treatment of tissue damage, such as non-cardiac tissue damage, the treatment of evolving myocardial infarction, the treatment of ischemic injury, and the treatment of various other disorders, such as complications arising from diabetes with the compounds and compositions of the invention. Other disorders can include, but are not limited to, atherosclerosis, coronary artery disease, diabetic nephropathy, diabetic neuropathy, diabetic retinopathy, diabetic cardiomyopathy, infections of the skin, peripheral vascular disease, stroke, asthma, and the like.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Application of oral hypoglycemic peptide to preparation of medicine for treating or preventing diabetes complicated with cardiovascular diseases
ActiveCN111905097AImproves oxidative stressReduce contentPeptide/protein ingredientsMetabolism disorderFibrosisGlycopeptide
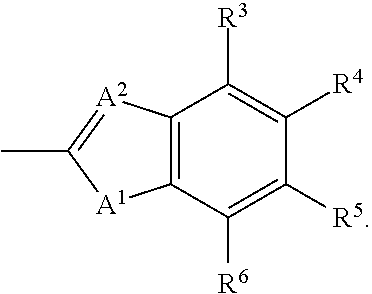
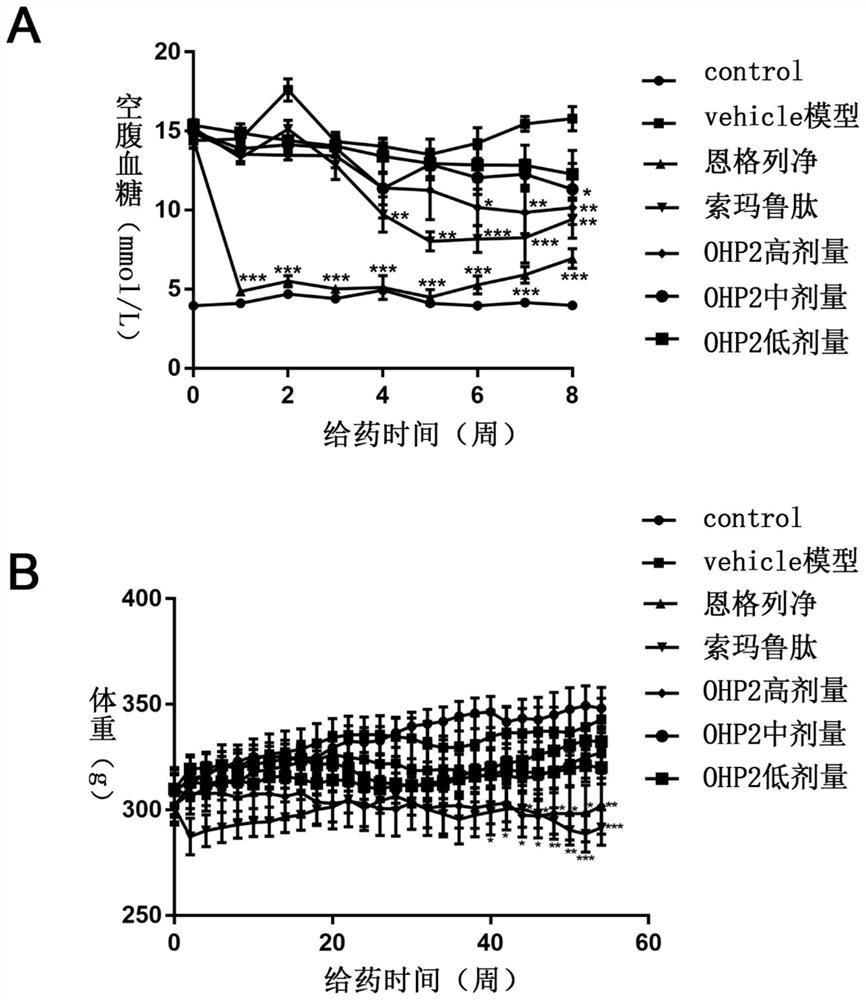
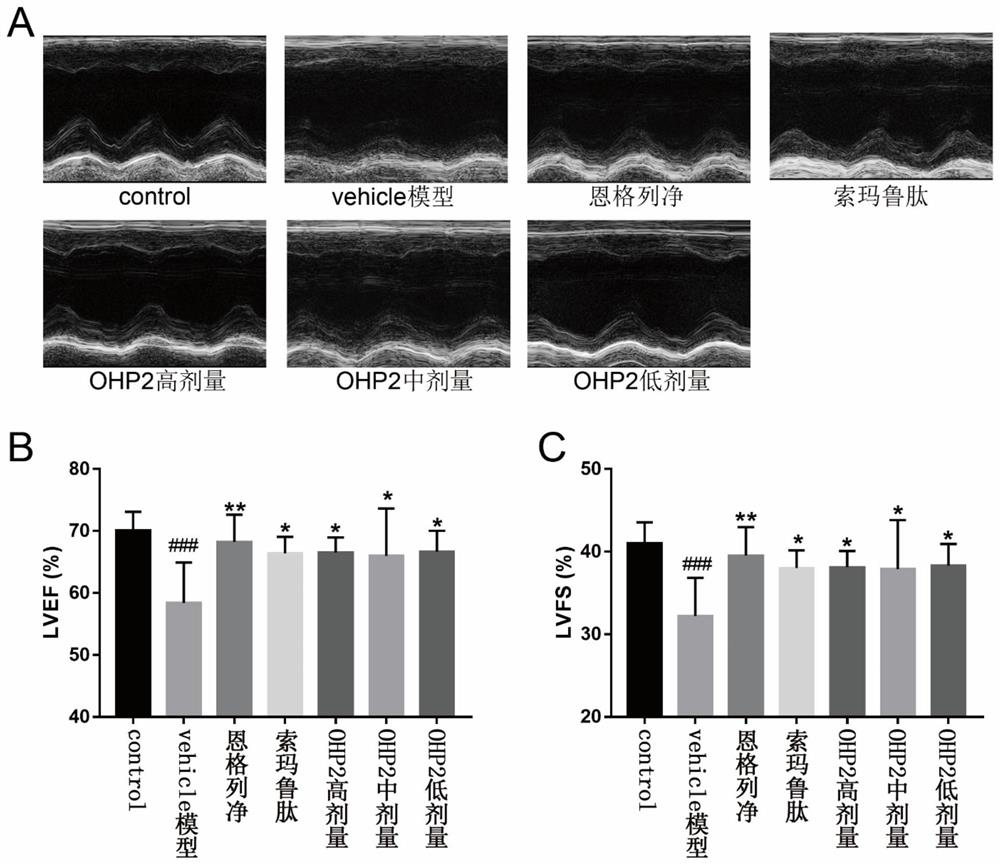
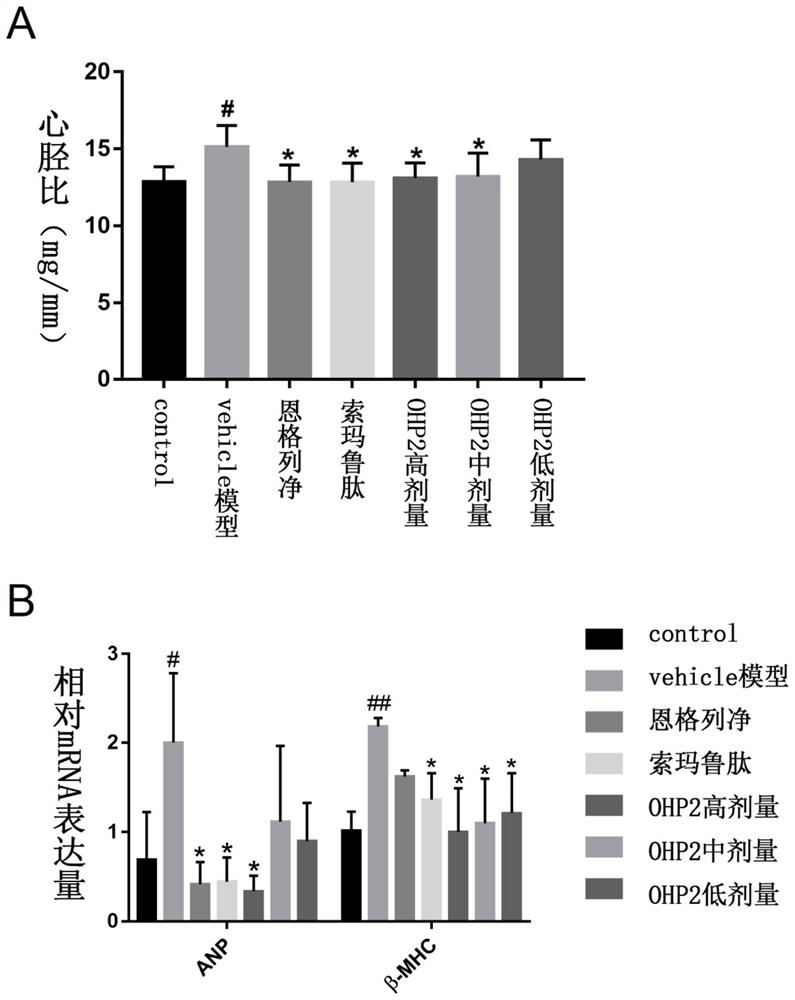
The invention relates to application of an oral hypoglycemic peptide. The application is used for preparing a medicine or a medicine composition for treating or preventing diabetes complicated with cardiovascular diseases. The oral hypoglycemic peptide is polypeptide OHP2. The polypeptide can effectively improve myocardial hypertrophy and fibrosis damage caused by the diabetes, reduce the contentof related myocardial enzymes, adjust the lipid metabolism level and improve myocardial cell oxidative stress and mitochondrial damage, so that the effect of protecting the heart is achieved; and therefore, the polypeptide can be used as a candidate molecule for treating or preventing the diabetes complicated with the cardiovascular diseases (especially diabetic cardiomyopathy), and has a good application prospect.
Owner:CHINA PHARM UNIV
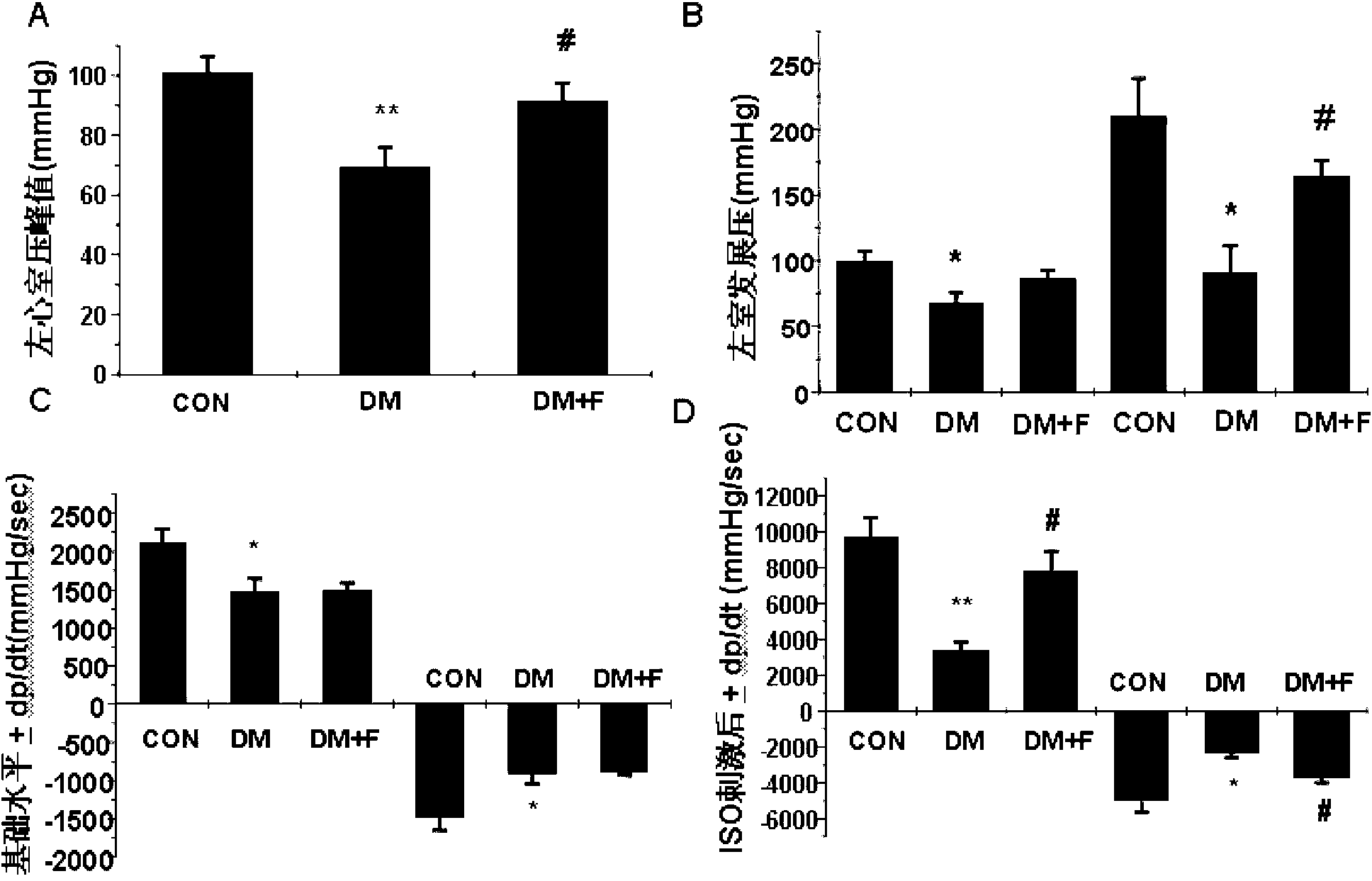
Construction method of diabetic cardiomyopathy animal model
PendingCN109481428AReduce mortalityEasy to operateOrganic active ingredientsAccessory food factorsLeft ventricular sizeRight ventricular hypertrophy
The invention discloses a construction method of a diabetic cardiomyopathy animal model. An SD (Sprague Dawley) rat under high-sugar and high-fat feeding is subjected to multiple abdominal injection with STZ (streptozocin); after the diabetics diagnosis standard is reached, subcutaneous injection with isoprenaline is performed; after 2 weeks of continued feeding, left ventricular cannulation and three-lead electrocardiogram test of ventricular hemodynamic parameters and electrocardiogram parameters shows that the rat experiences cardiac dysfunction; heart weight index and left ventricular index show that the rat experiences evident ventricular hypertrophy; biochemical analysis on rat blood shows that the rat has evident myocardial damage; HE (hematoxylin-eosin) pathological staining satisfies pathological changes in diabetic cardiomyopathy; Masson collagen staining satisfies myocardial connective tissue proliferation of diabetic cardiomyopathy. The construction method has the advantages of good operational simplicity, short construction time, low mortality in construction phase, high construction success rate and the like, and the pathological process of myocardial damage due to human type 2 diabetes mellitus can be well simulated.
Owner:陕西中药研究所
Use of vasopeptidase inhibitors in the treatment of metabolic diseases, nephropathy and advanced glycation end-product associated diseases
The invention describes and claims the use of vasopeptidase inhibitors of formula (I) for the treatment of nephropathy in diabetic or non-diabetic patients, including diabetic or non-diabetic nephropathy, glomerulonephritis, glomerular sclerosis, nephrotic syndome, hypertensive nephrosclerosis, microalbuminuria or end stage renal disease, or insulin resistance or of metabolic diseases associated with advanced glycation end-products, such as diabetic complications, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, cataracts, myocardial infarction and / or diabetic cardiomyopathy, or atherosclerosis or endothelial dysfunction.
Owner:SANOFI AVENTIS DEUT GMBH
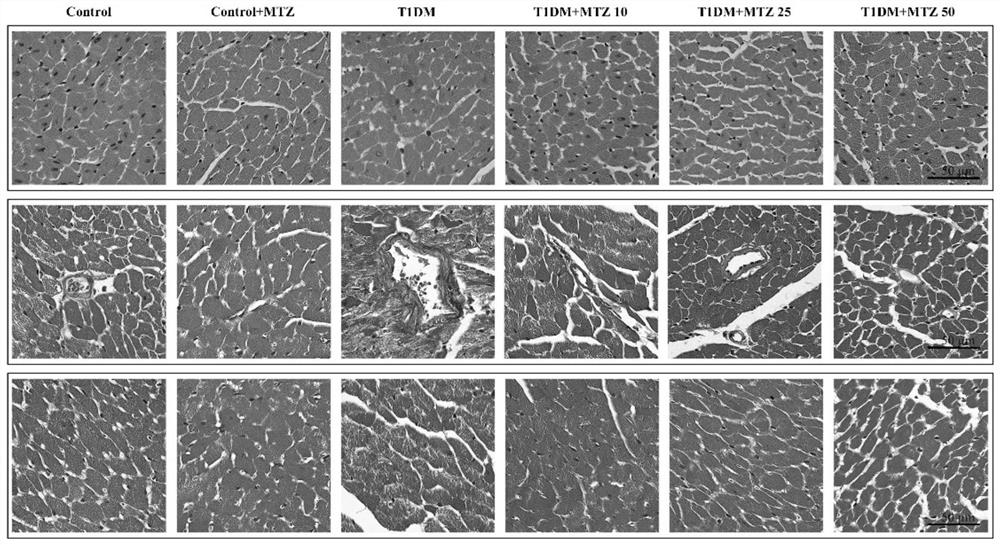
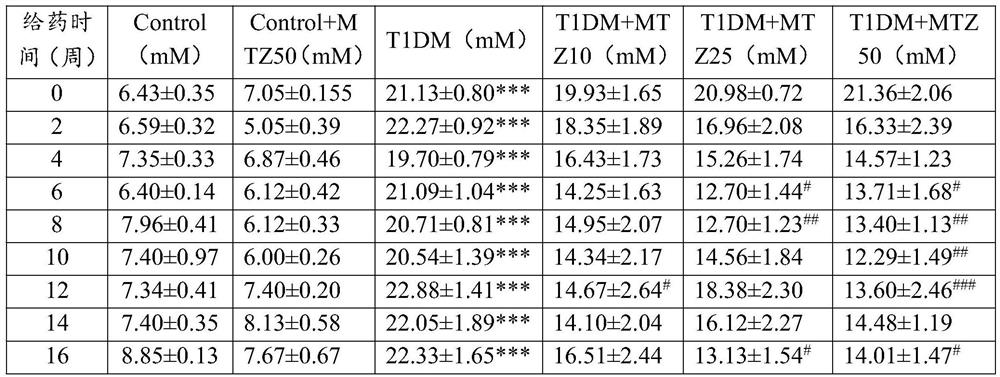
Application of methazolamide in preparation of medicine for treating type 1 diabetic cardiomyopathy
PendingCN112773796AImprove enduranceEasy to shapeOrganic active ingredientsMetabolism disorderPharmacologyGlucose blood
The invention discloses a new application of methazolamide, and particularly relates to a new application of methazolamide in preparation of a medicine for treating type 1 diabetic cardiomyopathy. In the research, the inventor of the application finds that the methazolamide can reduce the blood sugar of the type 1 diabetic cardiomyopathy, improve the heart morphological structure abnormality and cardiac contraction and diastolic dysfunction of the type 1 diabetic cardiomyopathy, can improve myocardial hypertrophy caused by high glucose, and has a good treatment effect on the type 1 diabetic cardiomyopathy. The invention provides a new drug choice for the treatment of type 1 diabetic cardiomyopathy.
Owner:GUANGZHOU MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com