Crystalline ARB-NEPi dicationic compound and preparation method and application thereof
A dual-cation, crystalline technology, applied in organic chemical methods, active ingredients of heterocyclic compounds, drug combinations, etc., can solve problems such as increasing the risk of cardiovascular burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
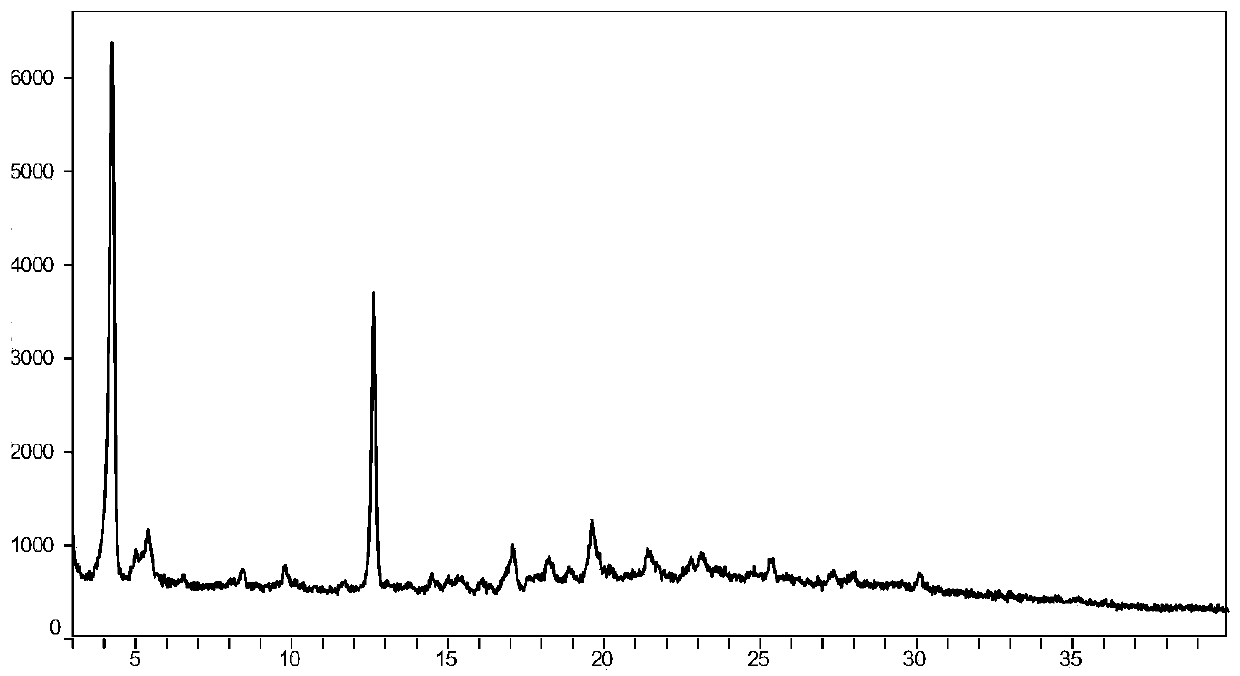
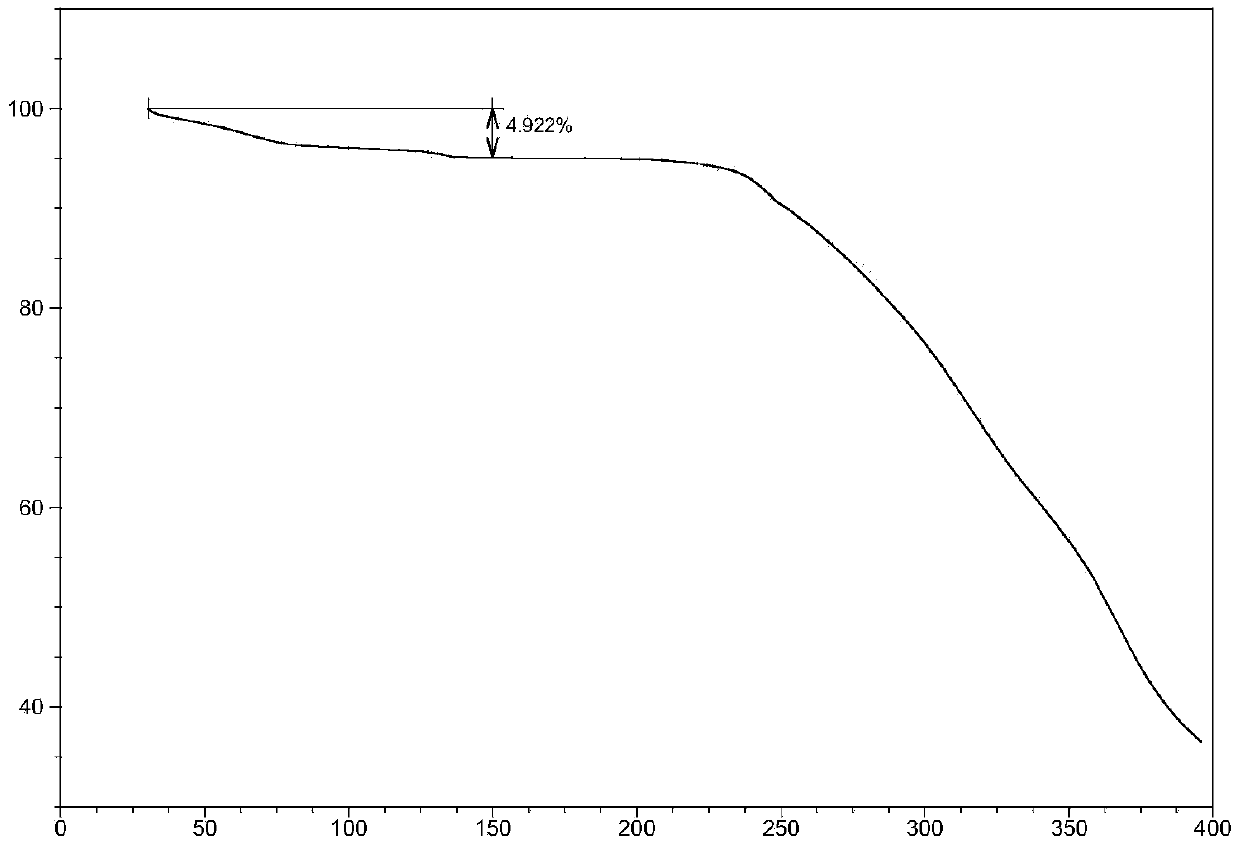
[0101] Weigh 500mg of AHU-377 free acid (oil) and 529mg of valsartan into a 100-mL round bottom flask, then add 50mL of acetone to dissolve, and stir at room temperature. In addition, 97 mg of sodium hydroxide and 68 mg of potassium hydroxide were weighed and dissolved in 0.4 mL of water, then added to the acetone solution of AHU377 and valsartan, and stirred overnight at room temperature. Distill about half of the acetone under reduced pressure, then add 25 mL of isopropyl acetate, continue stirring for 4 hours, distill off about half of the solvent volume again under reduced pressure, then add 25 mL of isopropyl acetate, and stir for 4 hours. Filter under the protection of nitrogen, wash the filter cake with isopropyl acetate, and dry to obtain a powdery solid. Its powder X-ray diffraction pattern (XRPD) is as figure 1 shown. See the TGA analysis chart figure 2 . The content of each component in the crystal powder is shown in the table below:
[0102]
[0103] The r...
Embodiment 2
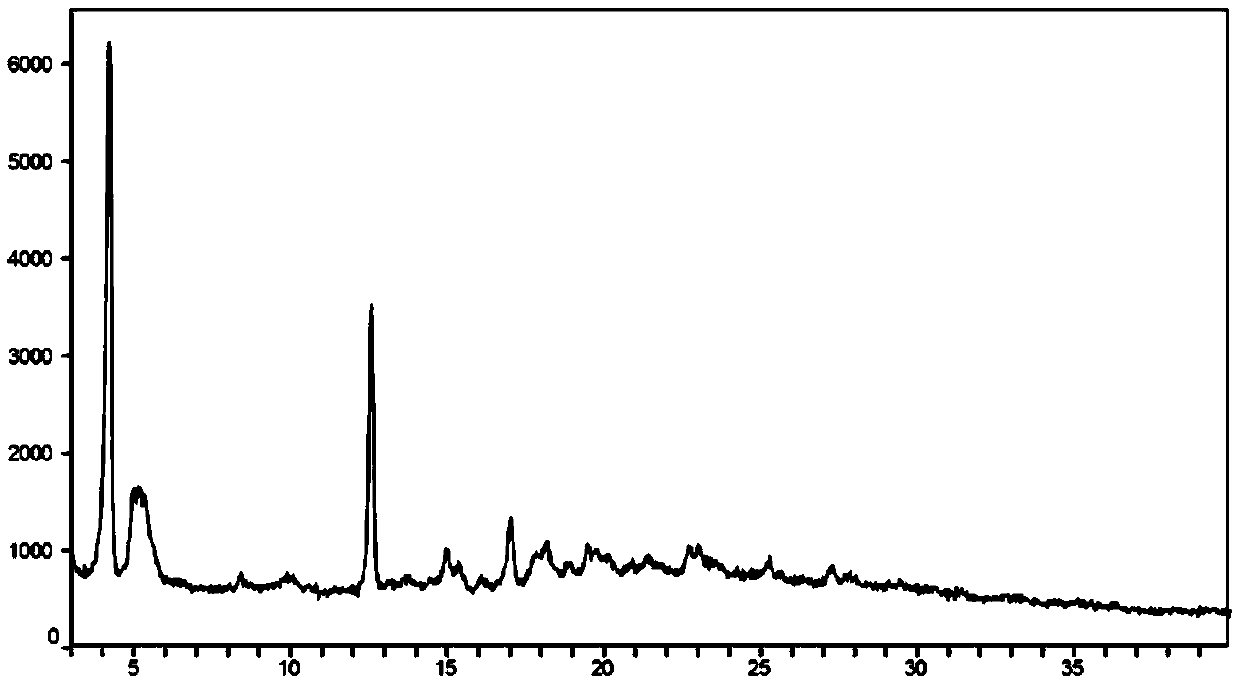
[0108] Weigh 500mg of AHU-377 free acid (oil) and 529mg of valsartan into a 100-mL round bottom flask, then add 50mL of acetone to dissolve, and stir at room temperature. In addition, 121.5 mg of sodium hydroxide and 34 mg of potassium hydroxide were weighed and dissolved in 0.4 mL of water, then added to the acetone solution of AHU377 and valsartan, and stirred overnight at room temperature. Distill about half of the acetone under reduced pressure, then add 25 mL of isopropyl acetate, continue stirring for 4 hours, distill off about half of the solvent volume again under reduced pressure, then add 25 mL of isopropyl acetate, and stir for 4 hours. Filter under the protection of nitrogen, wash the filter cake with isopropyl acetate, and dry to obtain a powdery solid. Its powder X-ray diffraction pattern (XRPD) is as image 3 shown. See the TGA analysis chart Figure 4 . The content of each component in the crystal powder is shown in the table below:
[0109]
[0110] Th...
Embodiment 3
[0115] Weigh 500mg of AHU-377 free acid (oil) and 529mg of valsartan into a 100-mL round bottom flask, then add 50mL of acetone to dissolve, and stir at room temperature. In addition, 24.3 mg of sodium hydroxide and 170.1 mg of potassium hydroxide were weighed and dissolved in 0.4 mL of water, then added to the acetone solution of AHU377 and valsartan, and stirred overnight at room temperature. Distill about half of the acetone under reduced pressure, then add 25 mL of isopropyl acetate, continue stirring for 4 hours, distill off about a normal volume of solvent again under reduced pressure, then add 25 mL of isopropyl acetate, and stir for 4 hours. Filter under the protection of nitrogen, wash the filter cake with isopropyl acetate, and dry to obtain a powdery solid. Its powder X-ray diffraction pattern (XRPD) is as Figure 5 shown. See the TGA analysis chart Figure 6 . The content of each component in the crystal powder is shown in the table below:
[0116]
[0117]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com