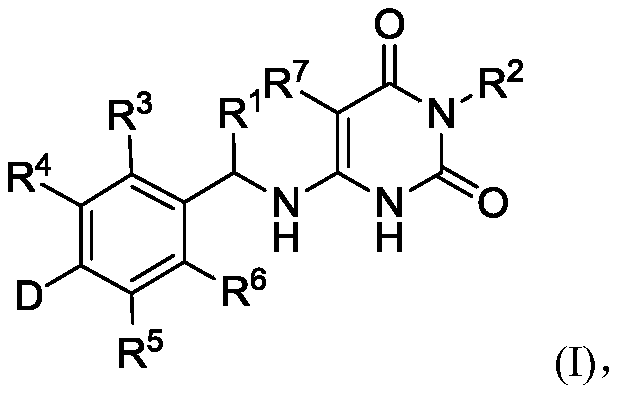
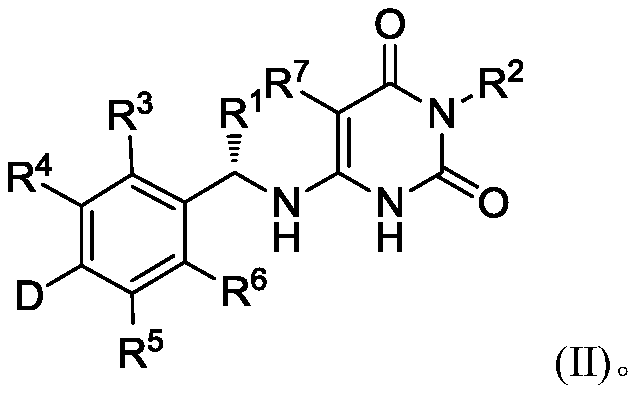
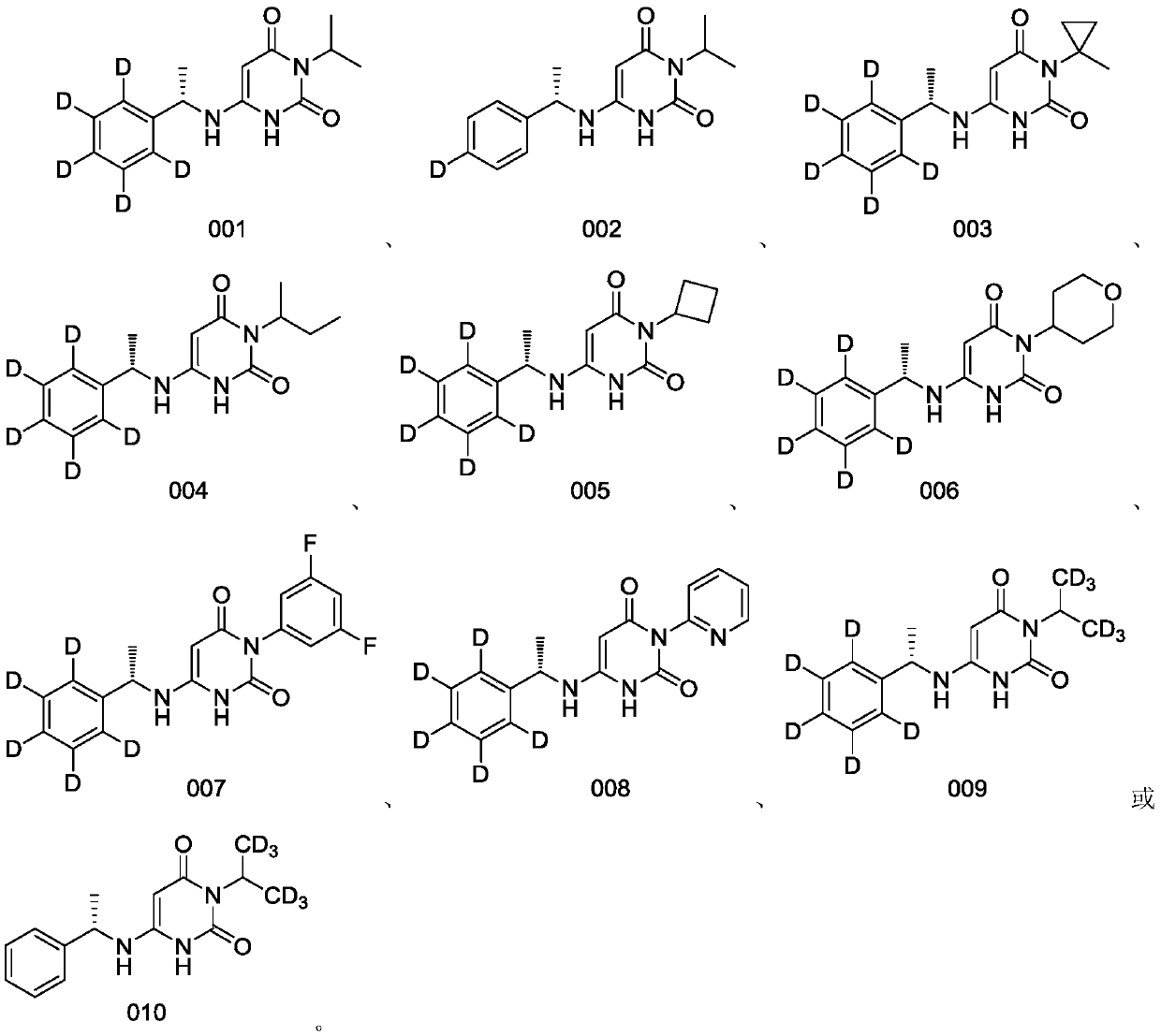
Deuterated benzylaminopyrimidine diketone derivative and application thereof
A compound and hydrate technology, applied in the field of medicine, can solve the problem that there is no marketed drug for hypertrophic cardiomyopathy, and achieve the effects of good clinical application prospects, good metabolic stability and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Example 1 (S)-3-isopropyl-6-((1-(phenyl-d5)ethyl)amino)pyrimidine-2,4(1H,3H)-dione
[0149]
[0150] The first step: (R)-2-methyl-N-((phenyl-d5)methylene)propane-2-sulfinamide
[0151] Under nitrogen protection, dichloromethane in (R)-2-methylpropane-2-sulfinamide (4.91g, 40.48mmol), PPTS (339.12mg, 1.35mmol) and magnesium sulfate (16.24g, 134.95mmol) (200mL) benzaldehyde-2,3,4,5,6-d5 (3g, 26.99mmol) was added into the mixed system, stirred overnight at room temperature, TLC monitored the completion of the reaction, filtered to remove insoluble matter, and the filter cake was washed with dichloromethane ( 50 mL), the filtrate was concentrated, and the resulting residue was purified by silica gel column chromatography (PE / EA (v / v)=20 / 1) to obtain 3 g of the title compound as a pale yellow solid, with a yield of 51.8%.
[0152] The second step: (R)-2-methyl-N-((S)-1-(phenyl-d5)ethyl)propane-2-sulfinamide
[0153] Under nitrogen protection, (R)-2-methyl-N-((phenyl-d5)...
Embodiment 2
[0161] Example 2 (S)-3-isopropyl-6-((1-(phenyl-4-d)ethyl)amino)pyrimidine-2,4(1H,3H)-dione
[0162]
[0163] The first step: (R)-2-methyl-N-((phenyl-4-d)methylene)propane-2-sulfinamide
[0164] Under nitrogen protection, benzaldehyde-4-d (0.7g, 6.53mmol), (R)-2-methylpropane-2-sulfinamide (1.58g, 13.07mmol) and anhydrous copper sulfate (3.13g, 19.60mmol) of dichloromethane (200mL) solution was stirred overnight at room temperature, filtered through celite to remove insoluble matter, the filter cake was washed with dichloromethane (50mL), the filtrate was concentrated, and the resulting residue was purified by silica gel column chromatography (PE / EA( v / v)=20 / 1), 0.8 g of the title compound was obtained as a light yellow solid with a yield of 58.4%.
[0165] The second step: (R)-2-methyl-N-((S)-1-(phenyl-4-d)ethyl)propane-2-sulfinamide
[0166]Under nitrogen protection, (R)-2-methyl-N-((phenyl-4-d)methylene)propane-2-sulfinamide (0.8g, 3.8mmol) was dissolved in anhydrous di...
Embodiment 3
[0173] Example 3 (S)-3-(3,5-difluorophenyl)-6-((1-(phenyl-d5)ethyl)amino)pyrimidine-2,4(1H,3H)-dione
[0174]
[0175] Step 1: 1-(3,5-Difluorophenyl)urea
[0176] Slowly drop trimethylsilylisocyanate (4.45g, 38.73mmol) into a solution of 3,5-difluoroaniline (5g, 38.73mmol) in dichloromethane (100mL) at room temperature, and the resulting reaction solution was stirred overnight at room temperature . The reaction solution was cooled to 0°C, slowly dropped into methanol (40mL) to quench the reaction, the resulting reaction solution was raised to room temperature and stirred for 1 hour, then concentrated under reduced pressure, the obtained residue was stirred overnight with methanol / ether at room temperature, filtered to obtain a yellow solid The title compound is 3.2g, the yield is 48%.
[0177] The second step: 1-(3,5-difluorophenyl)pyrimidine-2,4,6(1H,3H,5H)-trione
[0178] To a solution of 1-(3,5-difluorophenyl)urea (2.3g, 13.36mmol) in methanol (40mL) was added a solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com