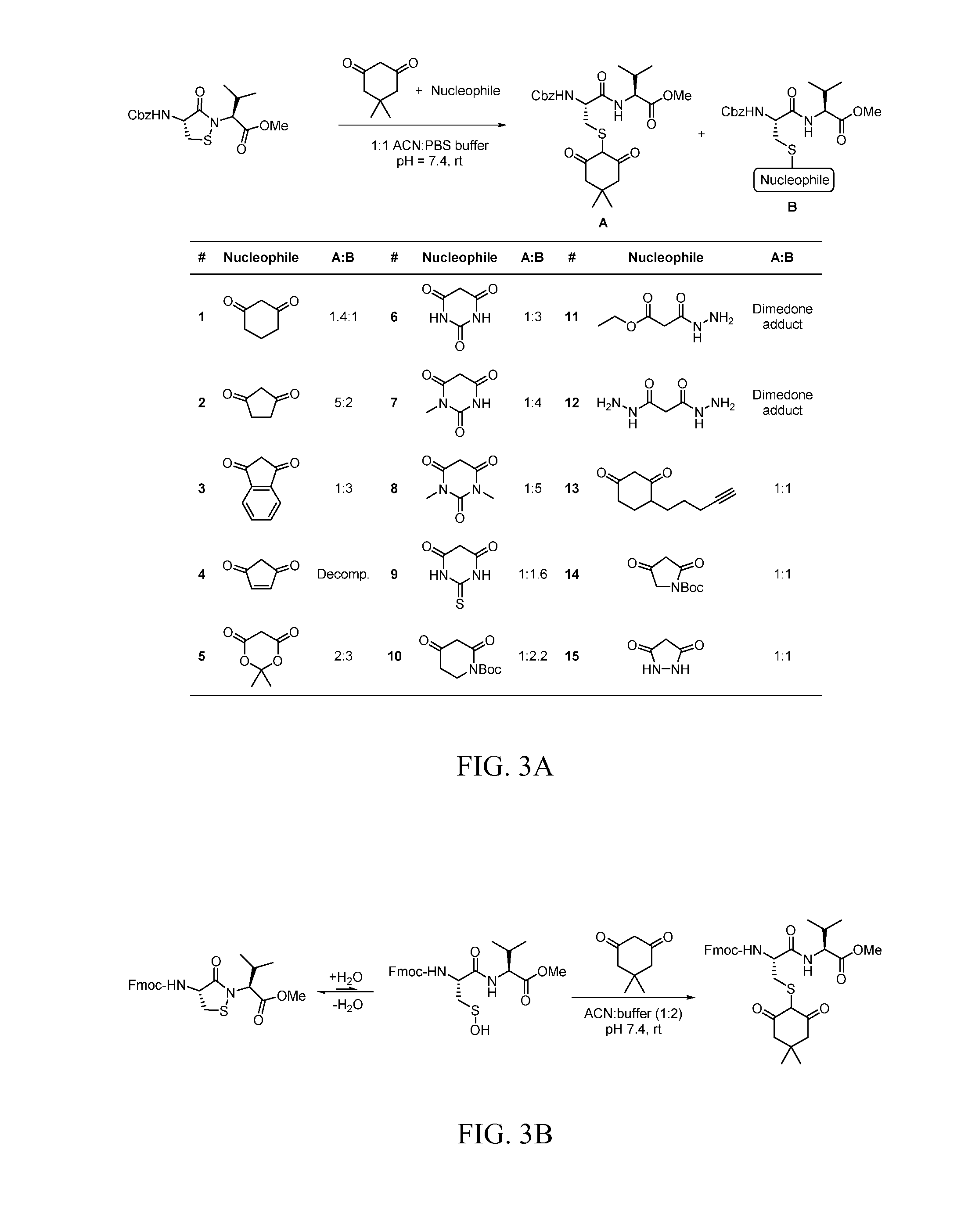
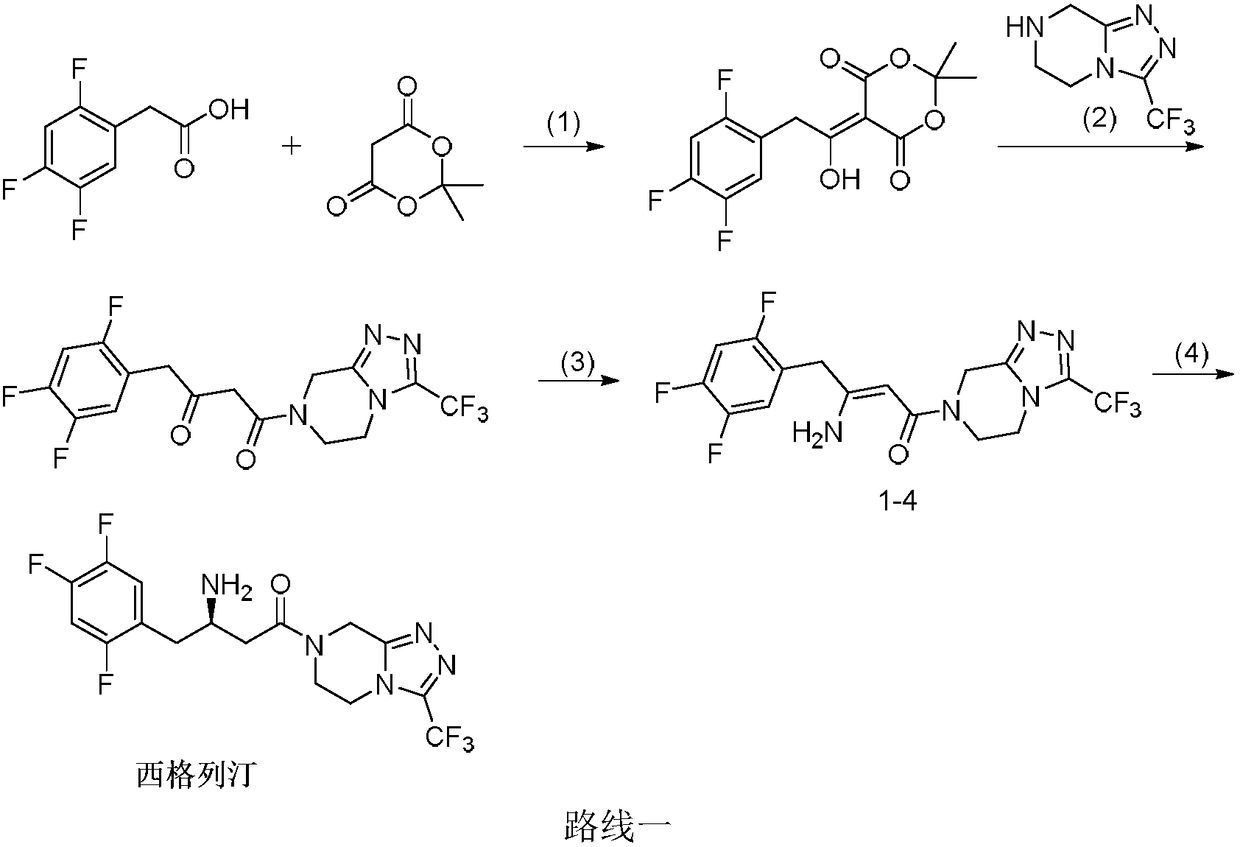
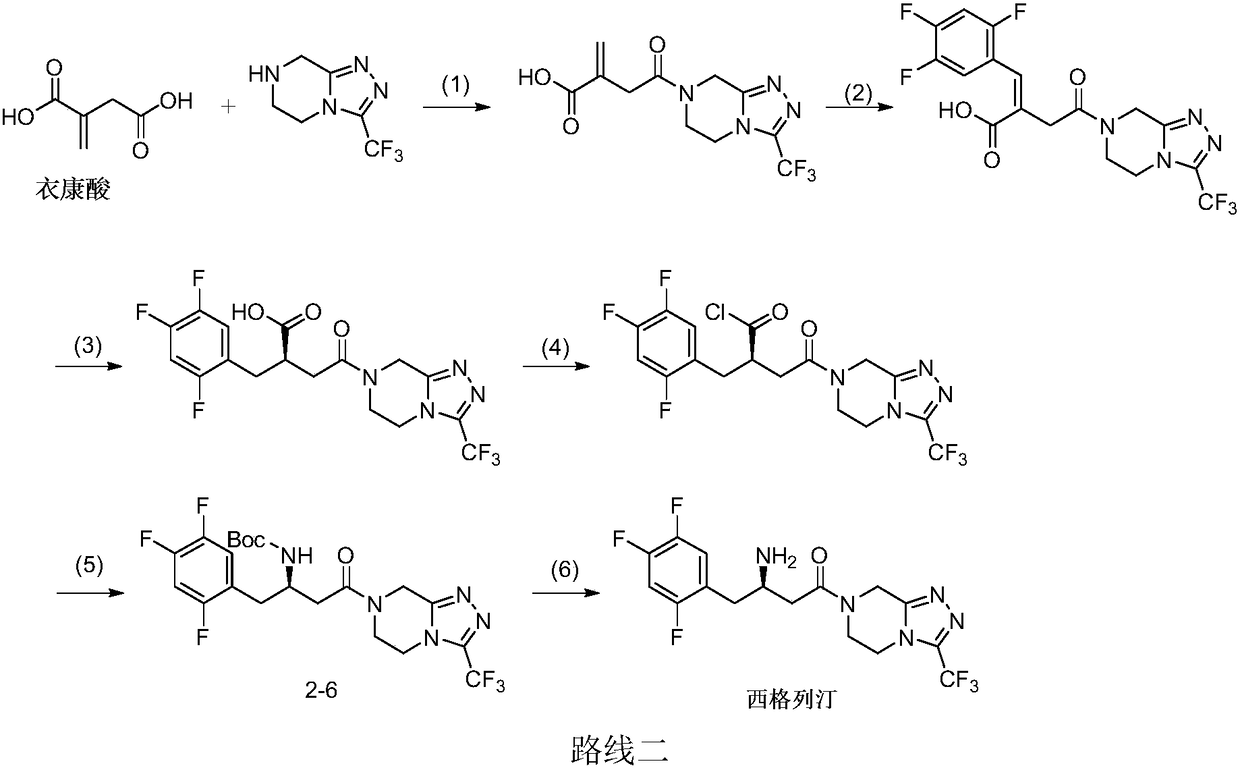
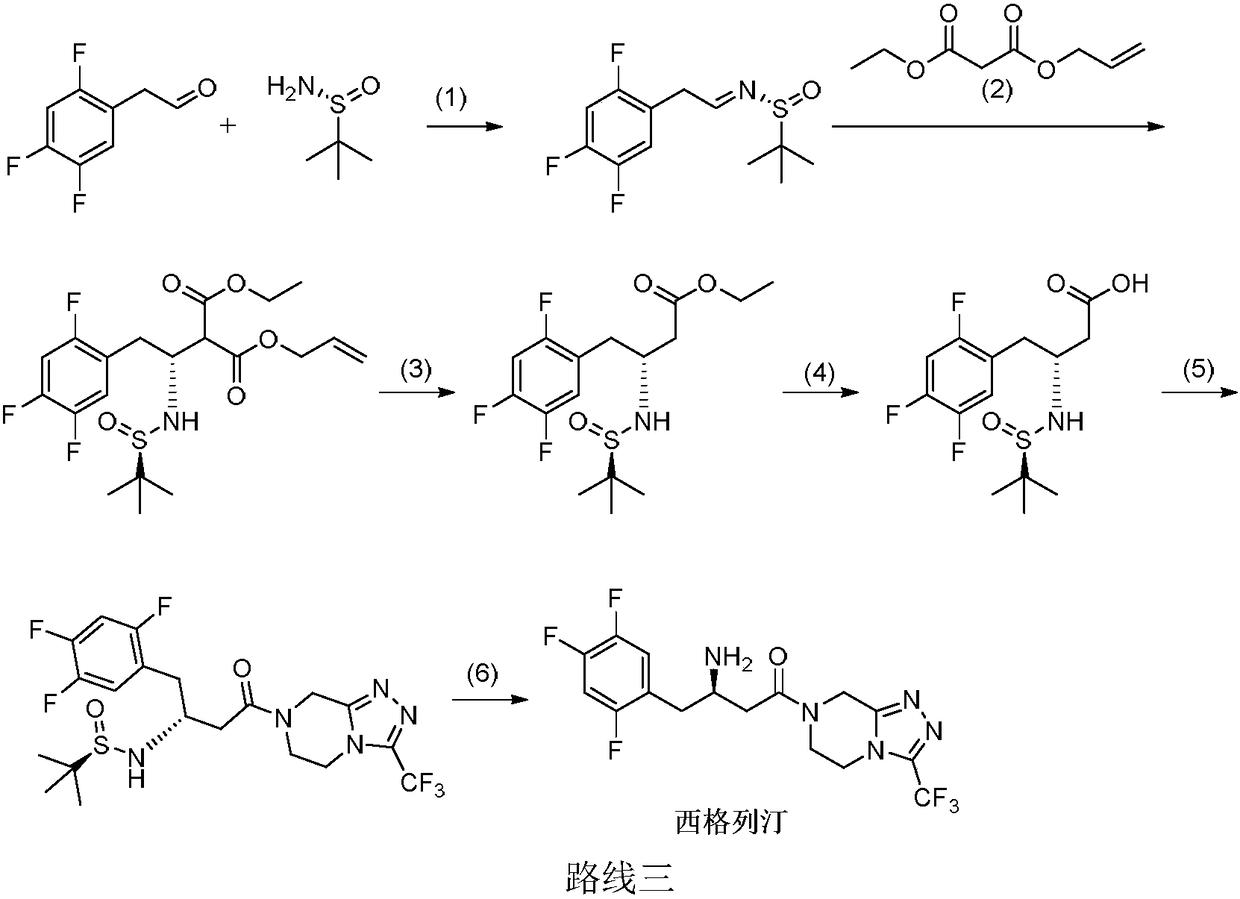
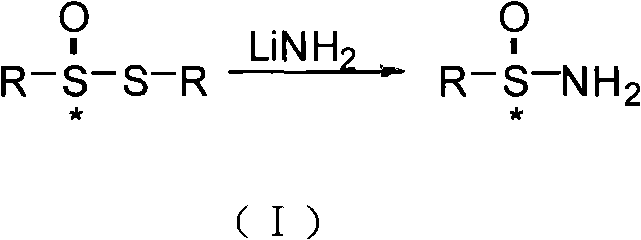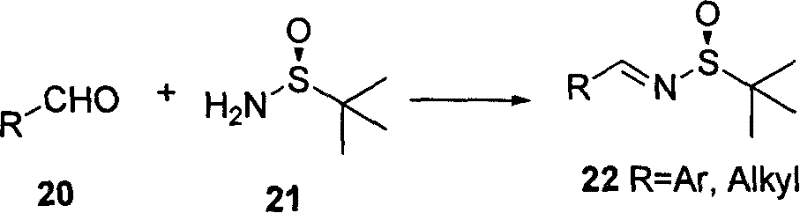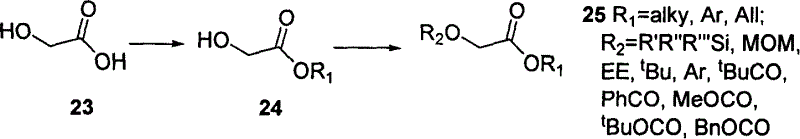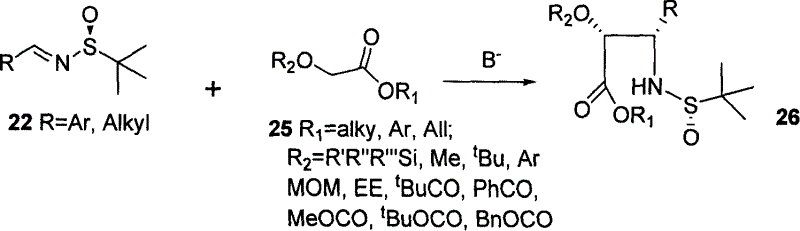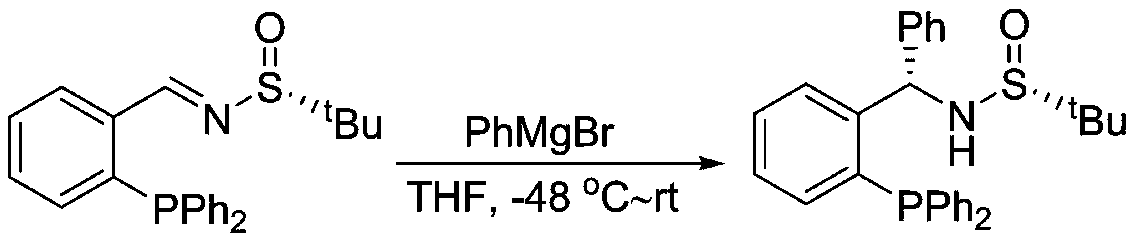Patents
Literature
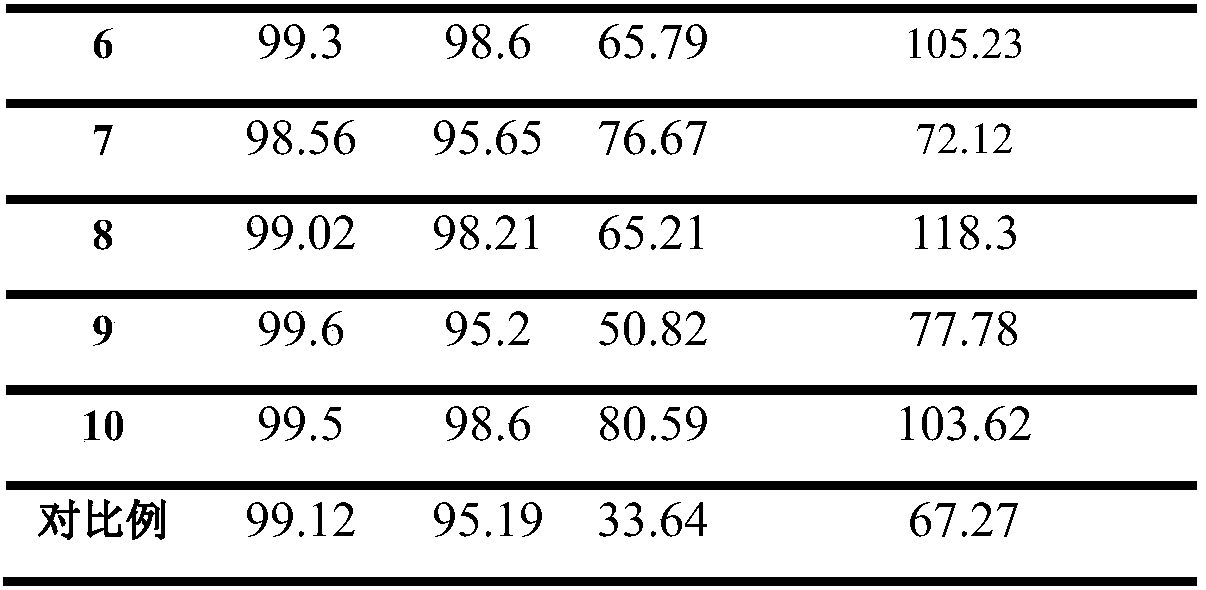
122 results about "Sulfinamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
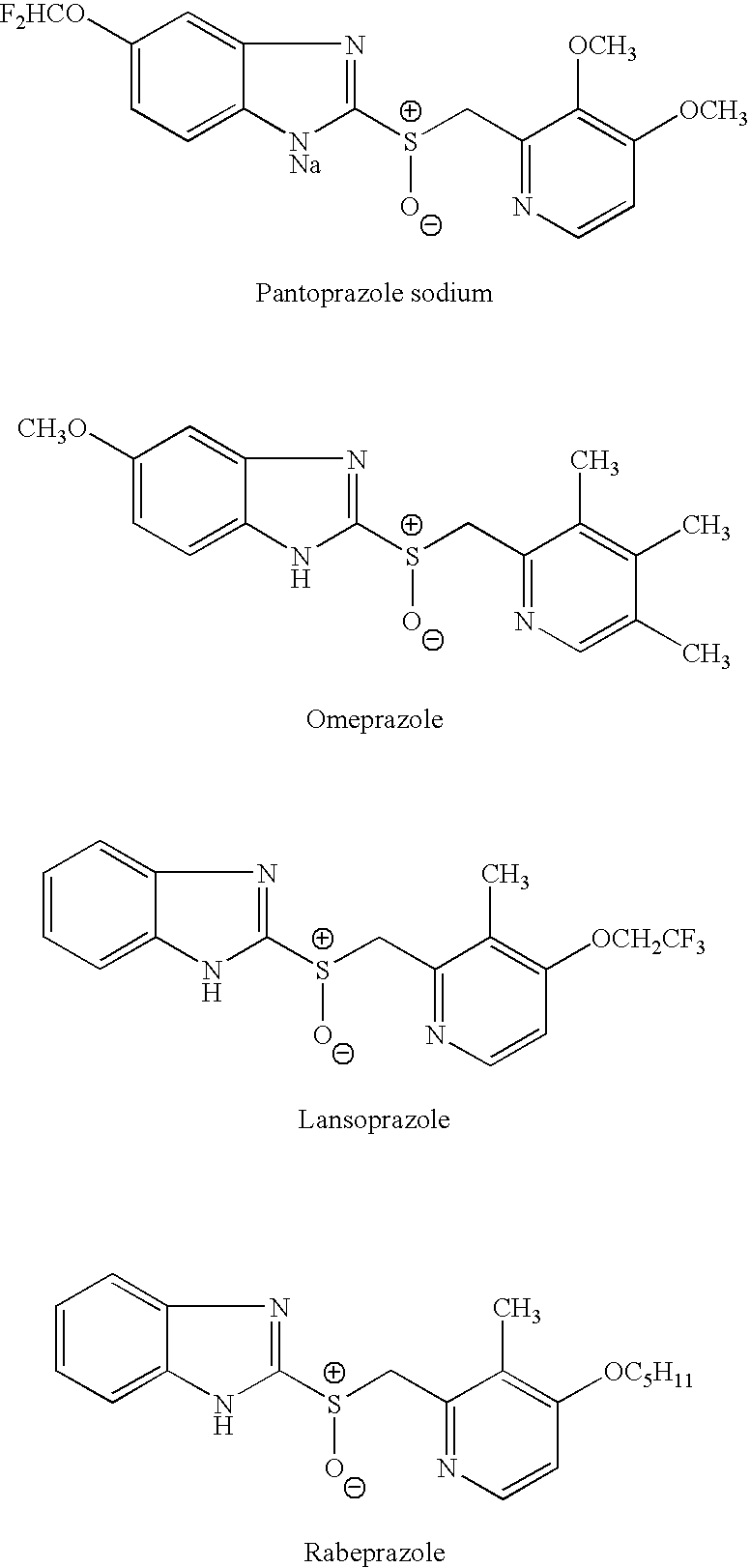
Sulfinamide is a functional group in organosulfur chemistry with the formula RS(O)NR'₂ (R and R' = organic substituent). With a sulfur-oxygen double bond as well as S-C and S-N single bonds, the compounds are chiral. Sulfinamides are amides of sulfinic acid (RS(O)OH).
Hetero diels-alder adducts of pentacene as soluble precursors of pentacene
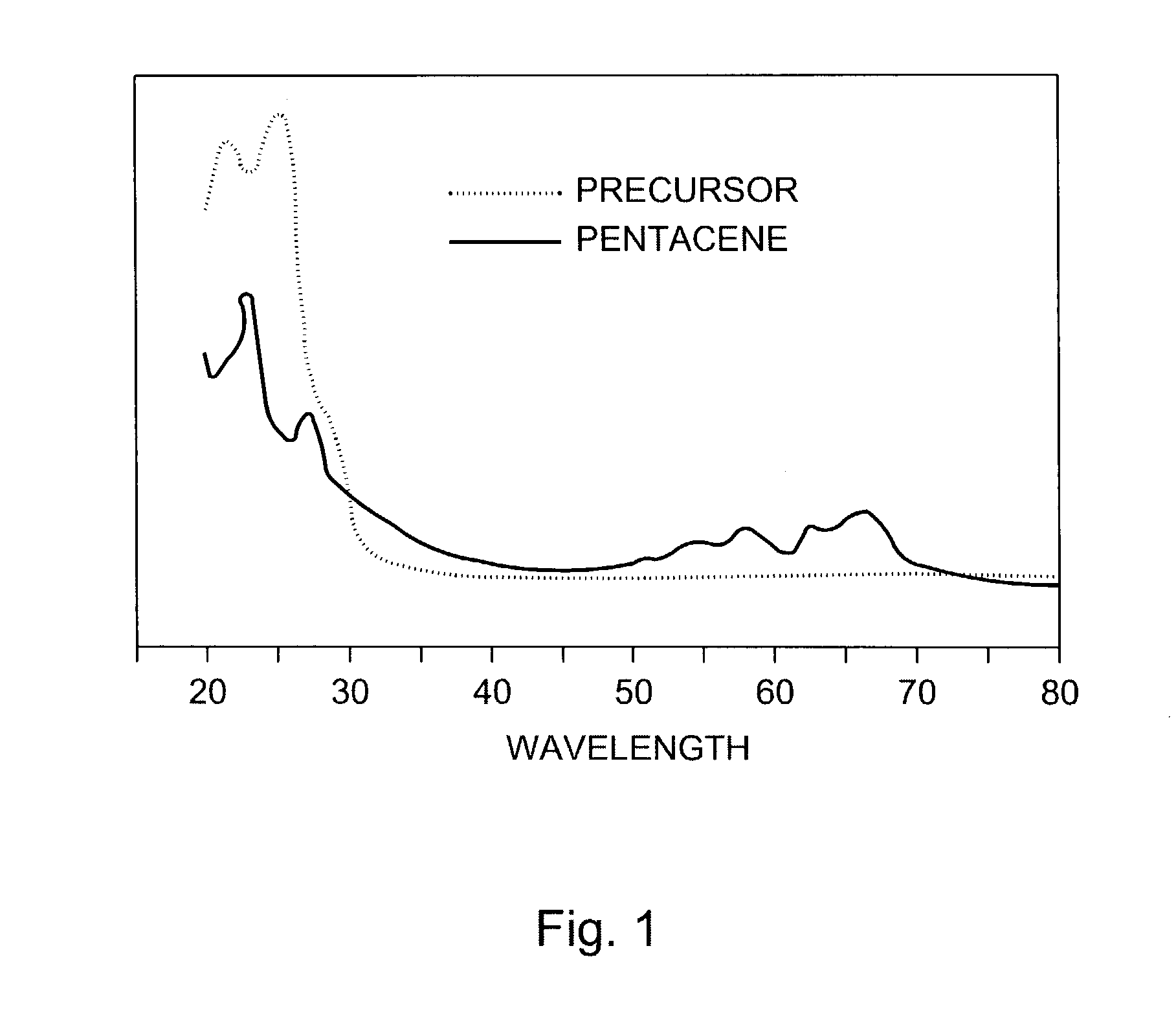
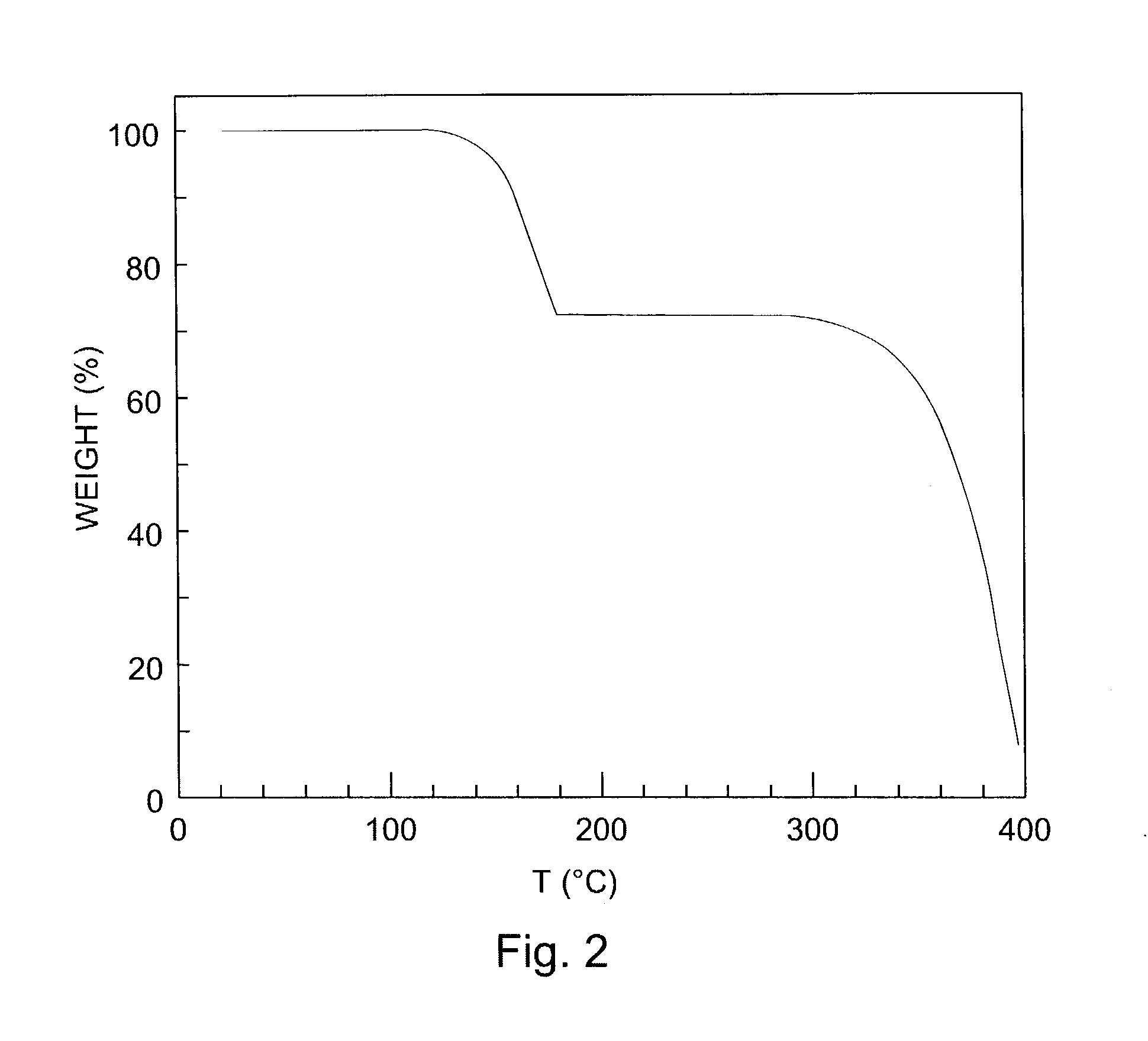
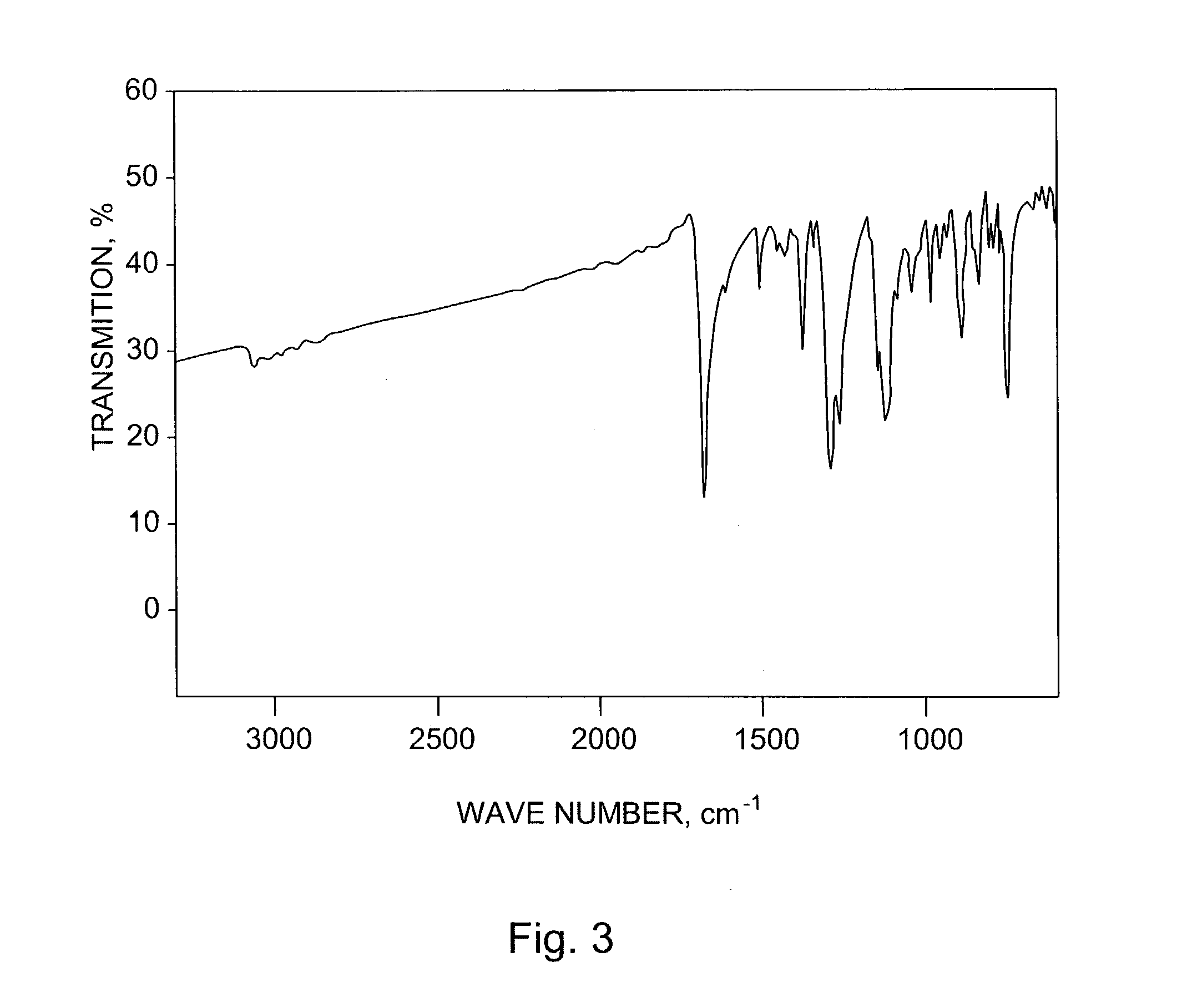
The present invention describes organic solvent-soluble Diels-Alder adducts of polycyclic aromatic compounds, such as, oligothiophene, perylene, benzo[ghi]perylene, coronene and polyacenes, with variety of dienophiles containing at least one heteroatom and in some cases two heteroatoms bonded to aromatic moiety, such as, thioxomalonates, azodicarboxylates, thialdehyde, acylnitroso and N-sulfinylamides. The Diels-Alder adducts are prepared by a simple, one step cycloaddition reaction of the polycyclic aromatic compounds, such as, pentacene, or other fused aromatic compounds, with heterodienophiles. The Diels-Alder adducts according to the present invention all form soluble adducts with pentacene and can be converted back to pentacene by retro-Diels-Alder reaction at moderate (60–250° C.) temperatures both in bulk, in solution or as thin-films.
Owner:GLOBALFOUNDRIES INC
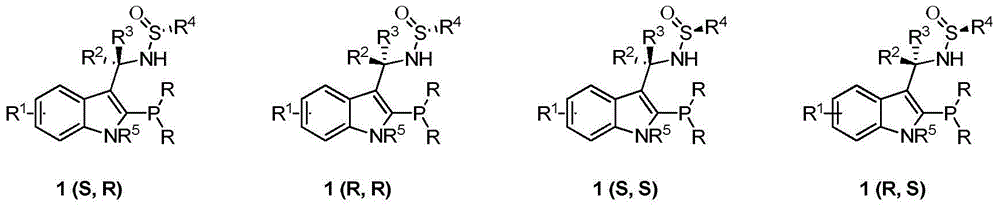
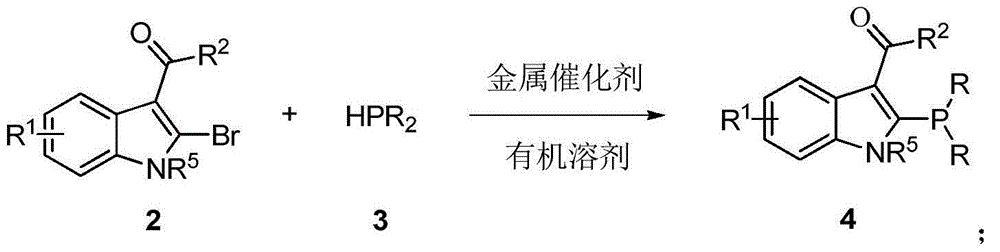
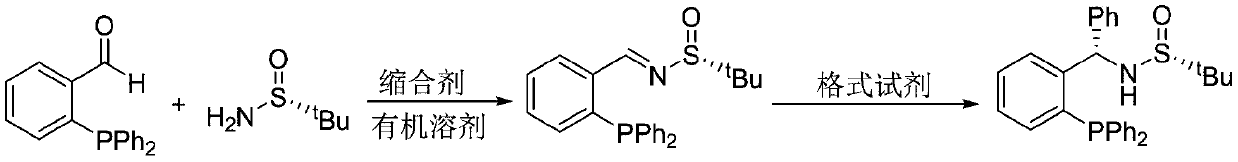
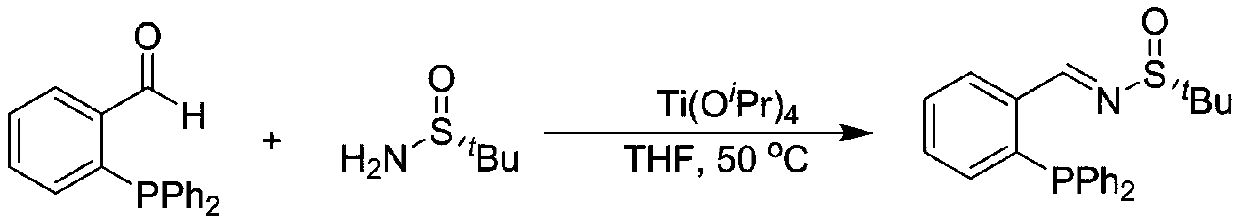
Chiral sulfinylamine monophosphine, and full-configuration preparation method and application thereof
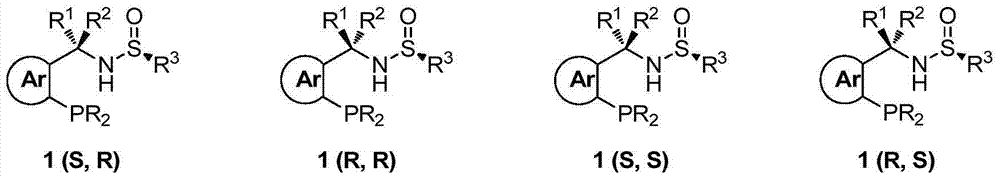
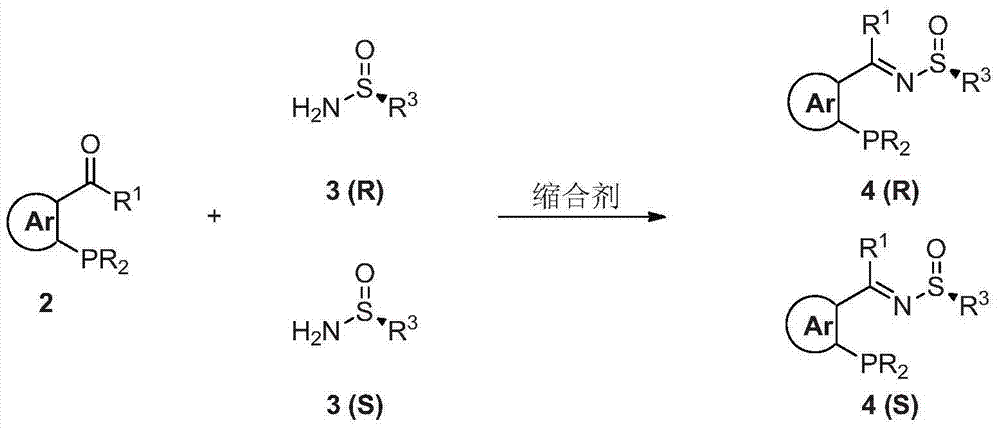
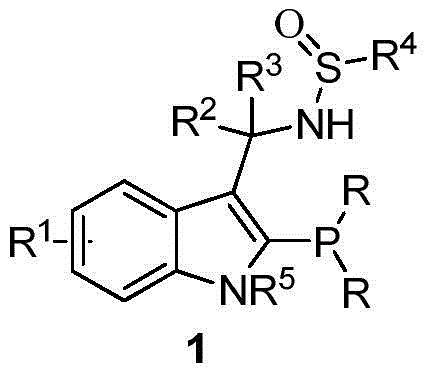
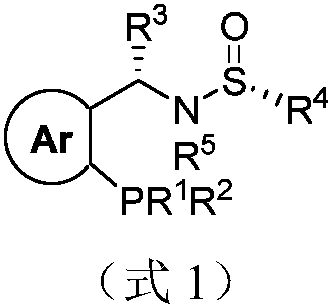
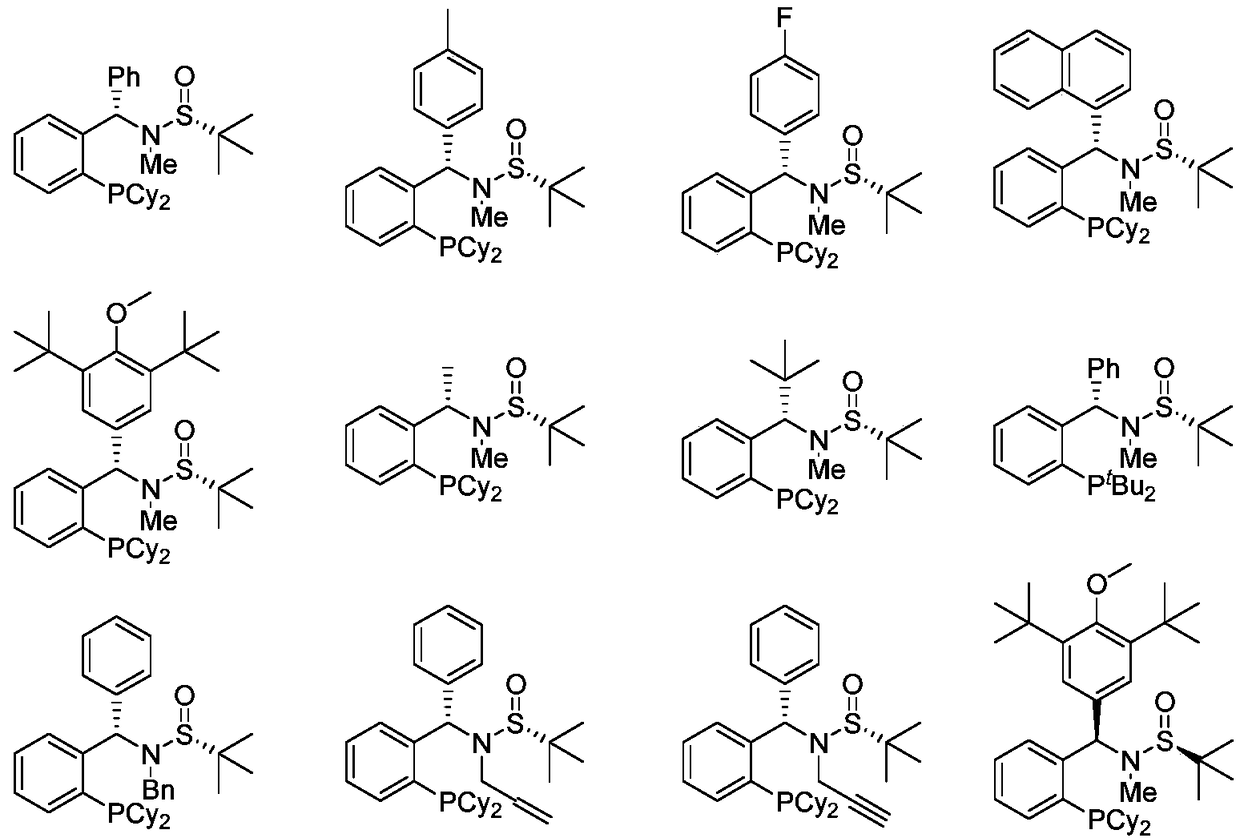
The invention provides a chiral sulfinylamine monophosphine and a preparation method thereof. The preparation method comprises the following steps: carrying out a condensation reaction of 2-disubstituted phosphinoaryl(heteroaryl)formaldehyde (ketone) 2 and chiral sulfonamide 3 to obtain a compound 4, and reacting the compound 4 with a nucleophilic reagent to prepare a compound 1; or carrying out a condensation reaction of aldehyde (ketone) 5 and the chiral sulfonamide 3 to obtain imine 6, and reacting the imine 6 with a 2-disubstituted phosphinoaryl(heteroaryl) metal reagent to obtain the compound 1; or reacting the imine 6 with the 2-disubstituted phosphinoaryl(heteroaryl) metal reagent to obtain a compound, and reducing the compound to obtain the compound 1; or carrying out a condensation reaction of 2-substituted phosphinoaryl(heteroaryl)formaldehyde (ketone) 8 and the chiral sulfonamide 3 to obtain an imine compound 9, reacting the imine compound 9 with the nucleophilic reagent to obtain a compound 7, and reducing the compound 7 to obtain the compound 1. Different chiral sulfenamides and different metal reagents are used to conveniently obtain the optically pure compound of four configurations comprising (R,R), (R,S), (S,S) and (S,R). The above ligand has the advantages of simple skeleton, synthesis convenience and easy reconstruction, can be applied in various metal-catalyzed asymmetric reactions, and has a very high reaction activity and stereoselectivity.
Owner:苏州凯若利新材料科技有限公司
High-throughput polyamide nanofiltration composite membrane and preparation method thereof
InactiveCN108187512ALow operating pressureImprove throughputSemi-permeable membranesWater desalinationChemical oxygen demand
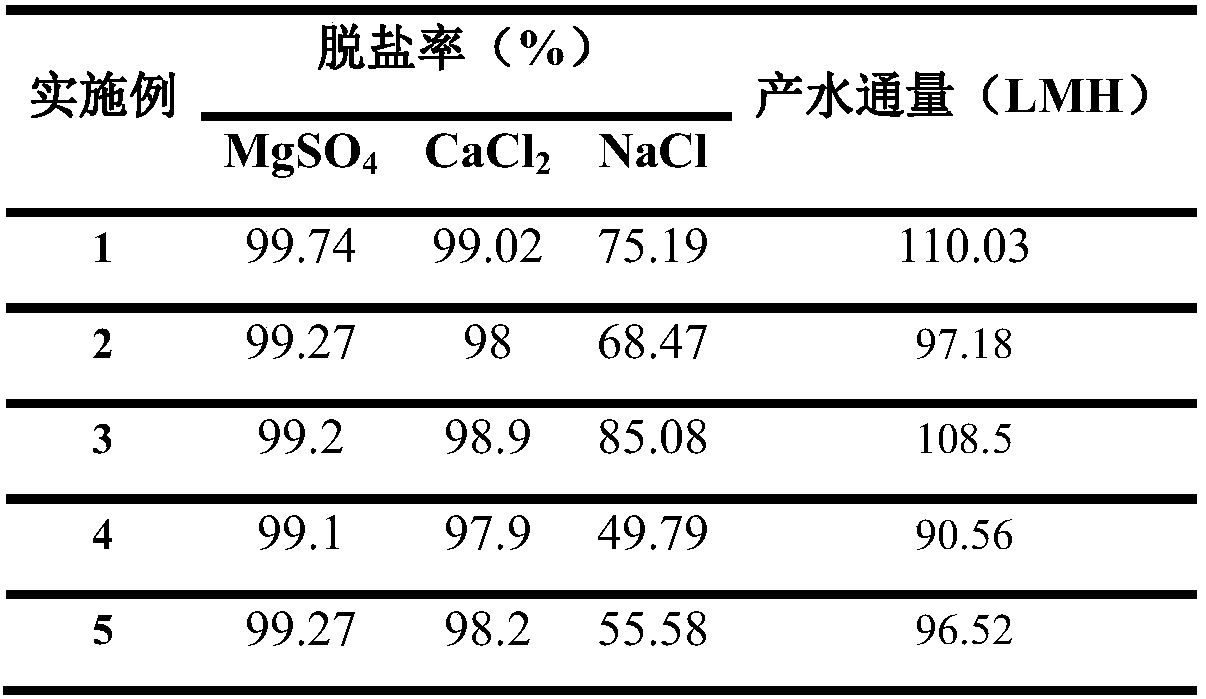
The invention discloses a high-throughput polyamide nanofiltration composite membrane and a preparation method thereof, which belong to the field of polymeric membrane materials. The composite membrane is prepared by the interfacial polymerization reaction between 5-(N-sulfonamido)isophthaloyl dichloride or a mixture of 5-(N-sulfonamido)isophthaloyl dichloride and 1,3,5-trimesoyl chloride and piperazine monomer, and can be widely used in a water softening process for removing bivalent cations such as Ca<2+> and Mg<2+>. The high-throughput polyamide nanofiltration composite membrane is structurally provided with an ultrathin (10nm) release layer, surface charge are low in electronegativity, the membrane surface property is smooth and hydrophilic, high throughput is shown in terms of properties, the high-throughput polyamide nanofiltration composite membrane has an excellent trapping effect on bivalent and polyvalent cations and good pollution resistance, and the high-throughput polyamide nanofiltration composite membrane can be widely applied in high-throughput domestic water purifiers, sea water desalination, brackish water pretreatment, removal of COD (chemical oxygen demand) suchas dye molecules and phenol molecules in wastewater, separation of dye / salt and material separation and concentration processes in pharmaceutical and food industries.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Method for synthesizing chiral sulfenamide
InactiveCN101525308AReduce dosageLower synthesis costOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesThiosulfinateSalicylaldehyde
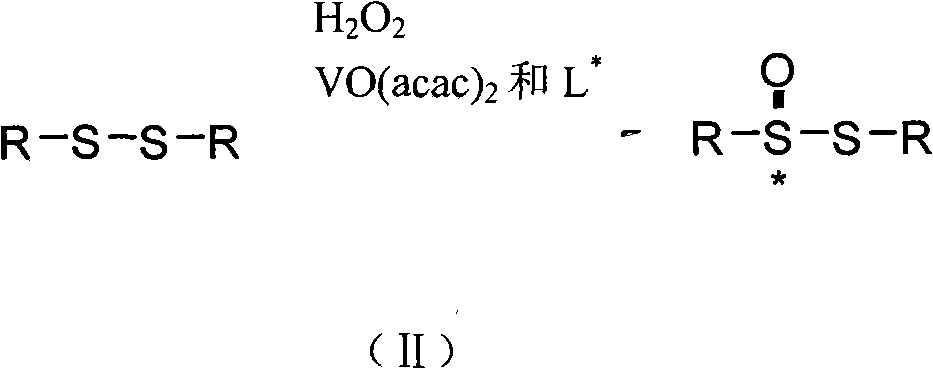
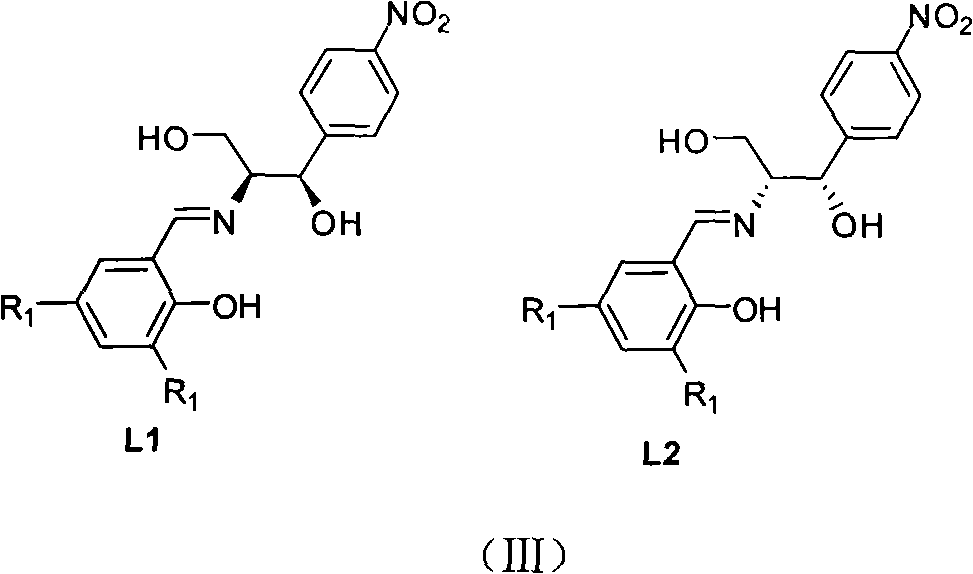
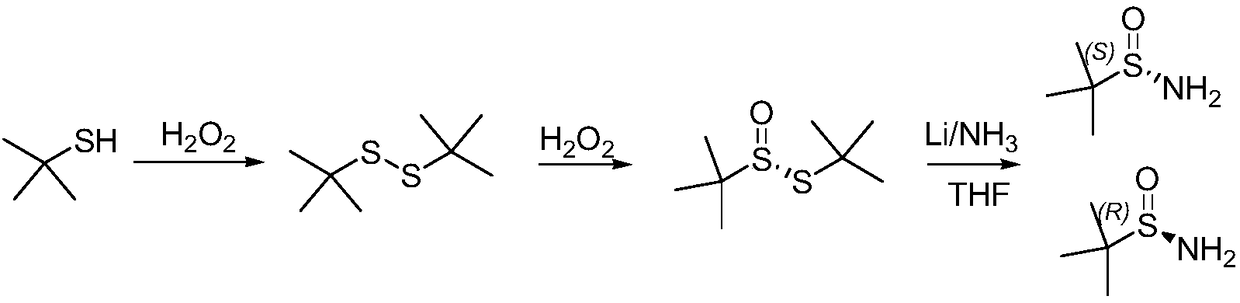
The invention discloses a method for synthesizing chiral sulfonamide. In the method, chiral thiosulfinate is taken as a raw material and reduced with liquid ammonia containing lithium amide to obtain the chiral sulfonamide, and a reaction formula is as above, wherein, R is one of C1-C10 alkyl, phenyl, p-cresyl, o-cresyl, metal-cresyl, p-ethyl benzyl, p-tertiary-butyl phenyl, p-acetyl phenyl, o-acetyl phenyl and naphthyl. The method sequentially comprises the following steps: preparing the lithium amide; dropwise adding the lithium amide to chiral thiosulfinate solution and stirring for reaction; and adding ice to obtained mixture after the reaction, extracting, combining organic phases and eliminating solvents, and crystallizing to obtain the chiral sulfonamide. The method has the advantages that synthesis cost is reduced by an oxidation method in the presence of a chiral catalyst; a ligand is a condensation product of cheap and available chloromycetin derivative and salicylaldehyde derivative; and reaction steps are less, the reaction conditions are mild and post-treatment is simple.
Owner:上海立科化学科技有限公司
Taxol and its analogue side chain synthesizing method
InactiveCN1709864AGood asymmetric induction abilityEasy to removeOrganic chemistryBulk chemical productionSide chainChemical products
This invention has disclosed a kind of synthetic method to paclitaxol and side chain of its analogue. This invention using low-cost alpha- glycolic acid as raw material, the asymmetry addition reaction induced by chirality tertiary butyl sulfinamide as committed step, prepares paclitaxol and polyene paclitaxol side chain. The chemical product quotiety of key reaction is high, protecting group is easy to control and operate, synthesis process is short.
Owner:成都科杰高新技术发展有限公司
Indole framework based center chirality sulfonamides monophosphine ligand and preparation method
ActiveCN104610355ASave raw materialsEfficient synthesisGroup 5/15 element organic compoundsKetonePhosphorine
The invention discloses an indole framework based center chirality sulfonamides monophosphine ligand and a preparation method thereof. The monophosphine ligand adopts the structure of a formula I, and is prepared through adopting 2-halogenated indole formaldehyde (ketone) to obtain 2-disubstituted phosphine indole formaldehyde (ketone), and taking the 2-disubstituted phosphine indole formaldehyde (ketone) and chirality sulfonamide as raw materials to react with a nucleophilic reagent or a reducing reagent to prepare the compound of the formula I; optical pure compounds of four configurations of (R,R), (R,S), (S,S) and (S,R) can be obtained conveniently through adopting different chirality sulfonamides and different nucleophilic reagents. The monophosphine ligand is simple in framework, convenient to synthesize, easy to transform, can be applied to various metal catalyzed asymmetric reactions as well as the tertiary phosphine catalyzed reaction, and has good application prospect.(The formula I is shown in the description).
Owner:苏州凯若利新材料科技有限公司
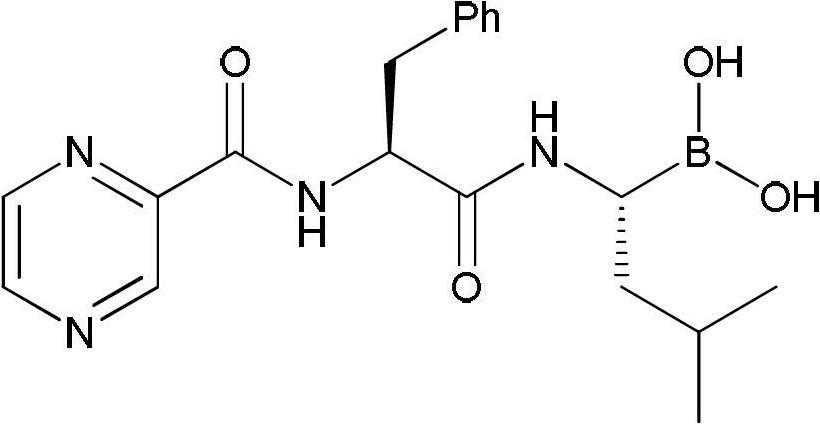
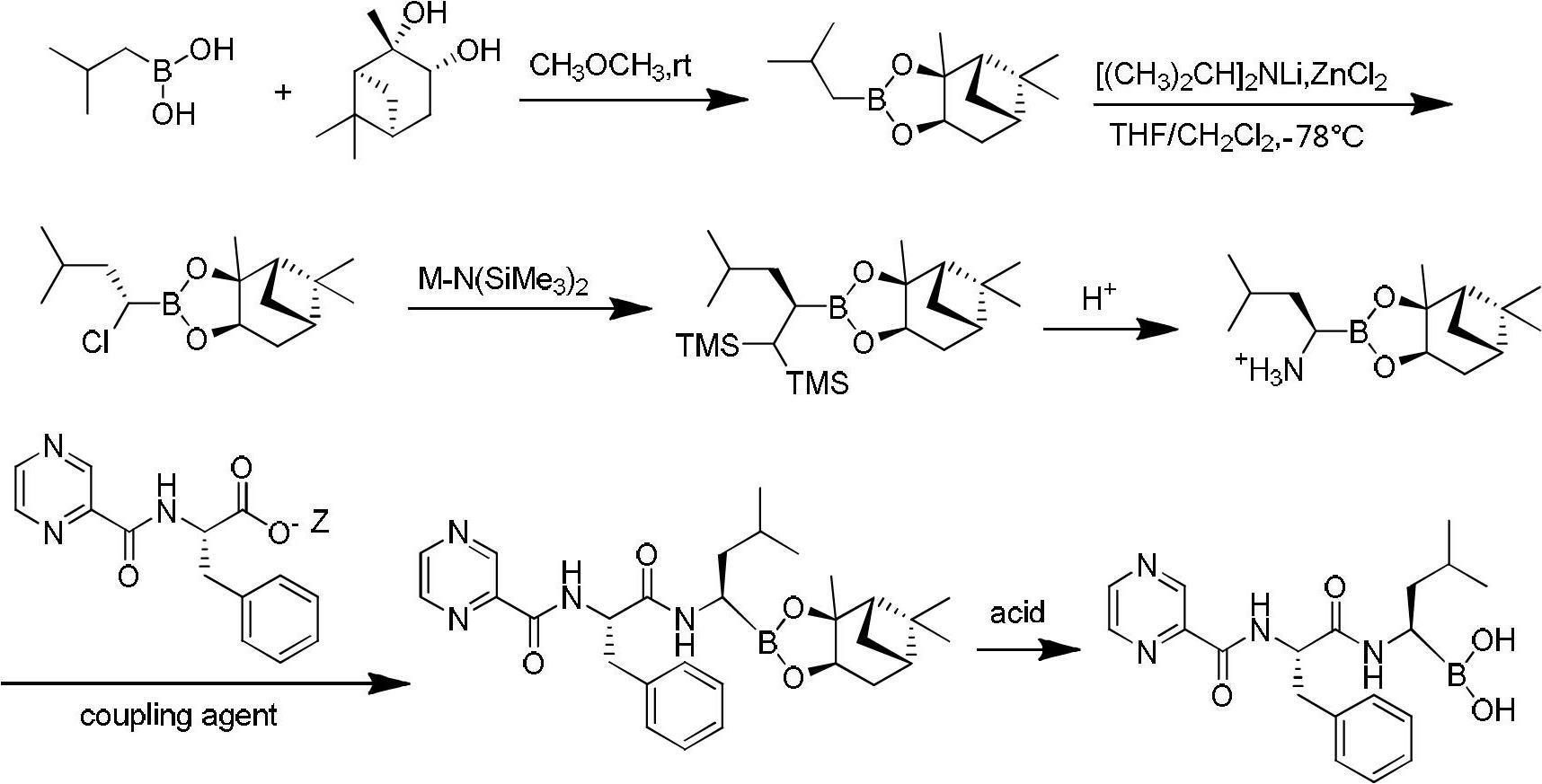
Synthetic method of bortezomib
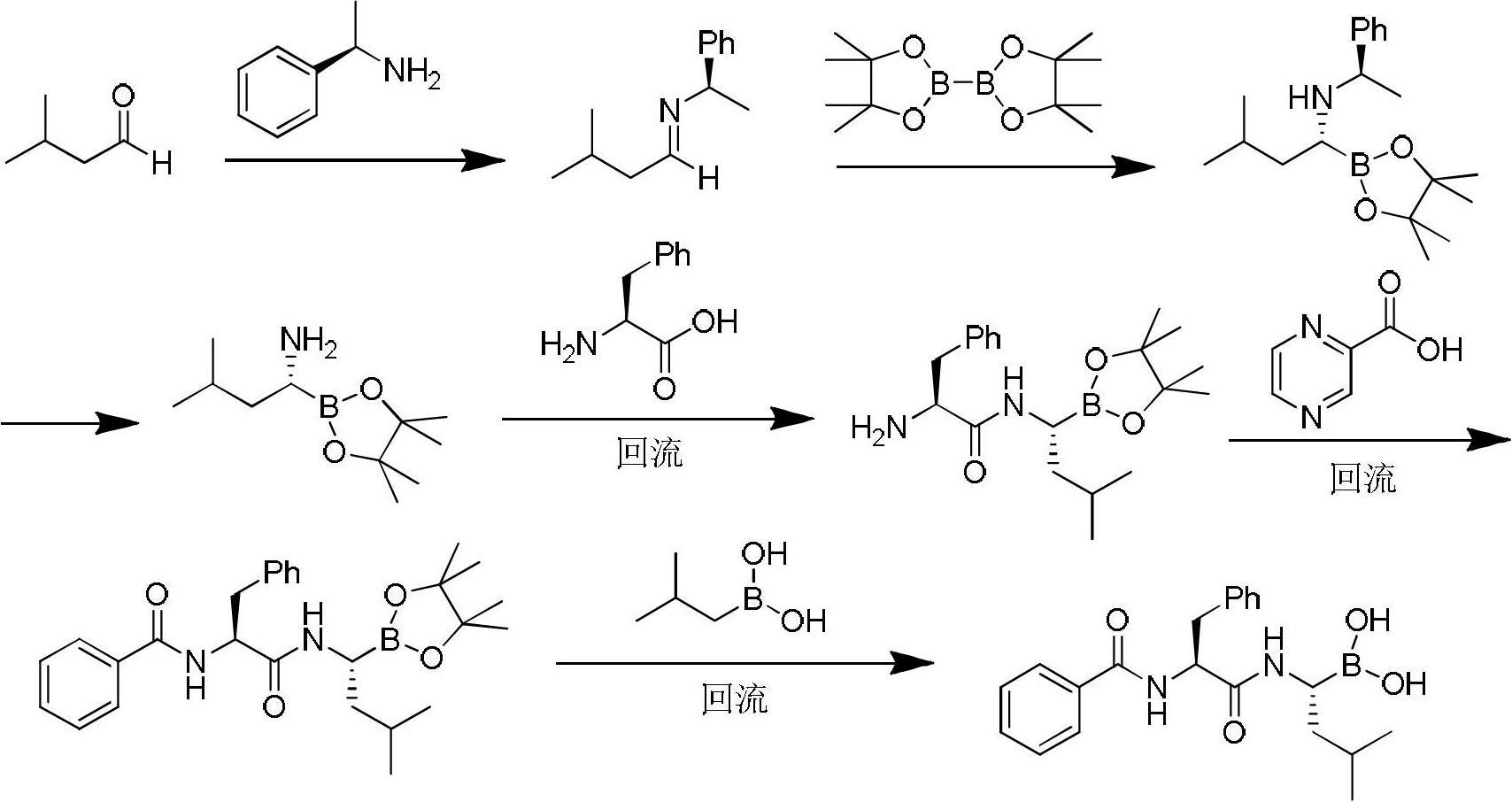
The invention discloses a synthetic method of bortezomib, which comprises the following steps of: taking isovaleraldehyde as an initial raw material, taking (R)-methylpropane-2-sulfinamide as a chiral reagent, generating (R,E)-2-methyl-N-(3-methyl butylidene) propane-2-sulfinamide by a condensation and dehydration reaction, then carrying out a nucleophilic addition reaction with pinacol diboron so as to generate (R)-1-N-methylpropane sulfinyl-3-methyl butane-1-pinacol borate ester, afterwards hydrolyzing under an acidic condition so as to obtain pinacol-(R)-1-amino-3-methyl butane-1-borate ester hydrochloride, then reacting with (S)-3-phenyl-2-(pyrazine-2-formamido) propionic acid under the existence of a coupling agent and also hydrolyzing under the action of isobutyl borate so as to generate a final product of the bortezomib. According to the synthetic method of the bortezomib, the (R)-methylpropane-2-sulfinamide which is easy to obtain is used as the chiral induction reagent, so that an obtained intermediate enantiomorph has higher purity, and a bulk drug which is finally obtained has better quality.
Owner:HEFEI UNIV OF TECH
Sulfonamide type chiral monophosphine ligand as well as preparation method and application thereof
InactiveCN108864189AHigh reactivityHigh stereoselectivityOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsArylEnantiomer
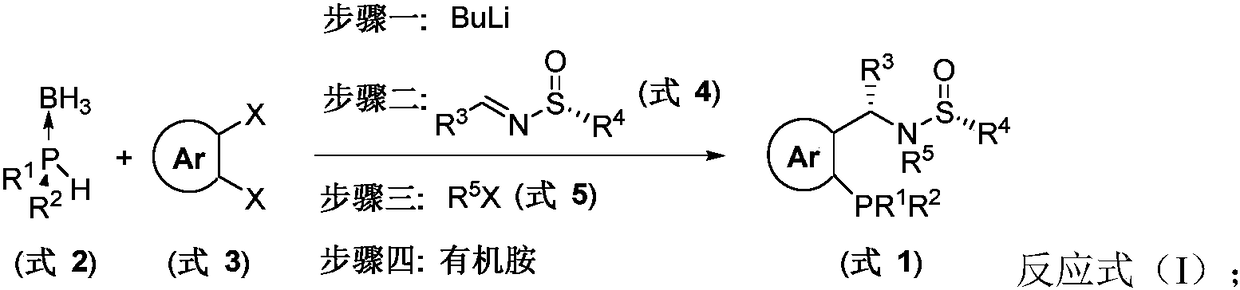
The invention discloses a sulfonamide type chiral monophosphine ligand. The chiral monophosphine ligand is a compound shown as a formula I in the description. The invention also discloses a syntheticmethod of the ligand. The method comprises the following steps of using compounds shown as a formula (2) R<1>R<2>PH.BH3, a formula (3) in the description, a formula (4) in the description and a formula (5) R<5>X as raw materials; performing substitution reaction and addition reaction for preparing the ligand by a one-pot method. The enantiomer (as shown in the description) of the formula (1) of the ligand can be realized by using the enantiomer (as shown in the description) of the formula (4) of the ligand. By using the chiral sulfoimide of two structures, the two kinds of optical pure chiralmonophosphine ligands (S, Rs) and (R, Ss) can be obtained. The invention also discloses application of the ligand to catalysis of asymmetrical reduction Heck reaction in O-halogenated aryl allyl ethermolecules; high reaction activity and three-dimensional selectivity are realized; wide application values are realized.
Owner:EAST CHINA NORMAL UNIV
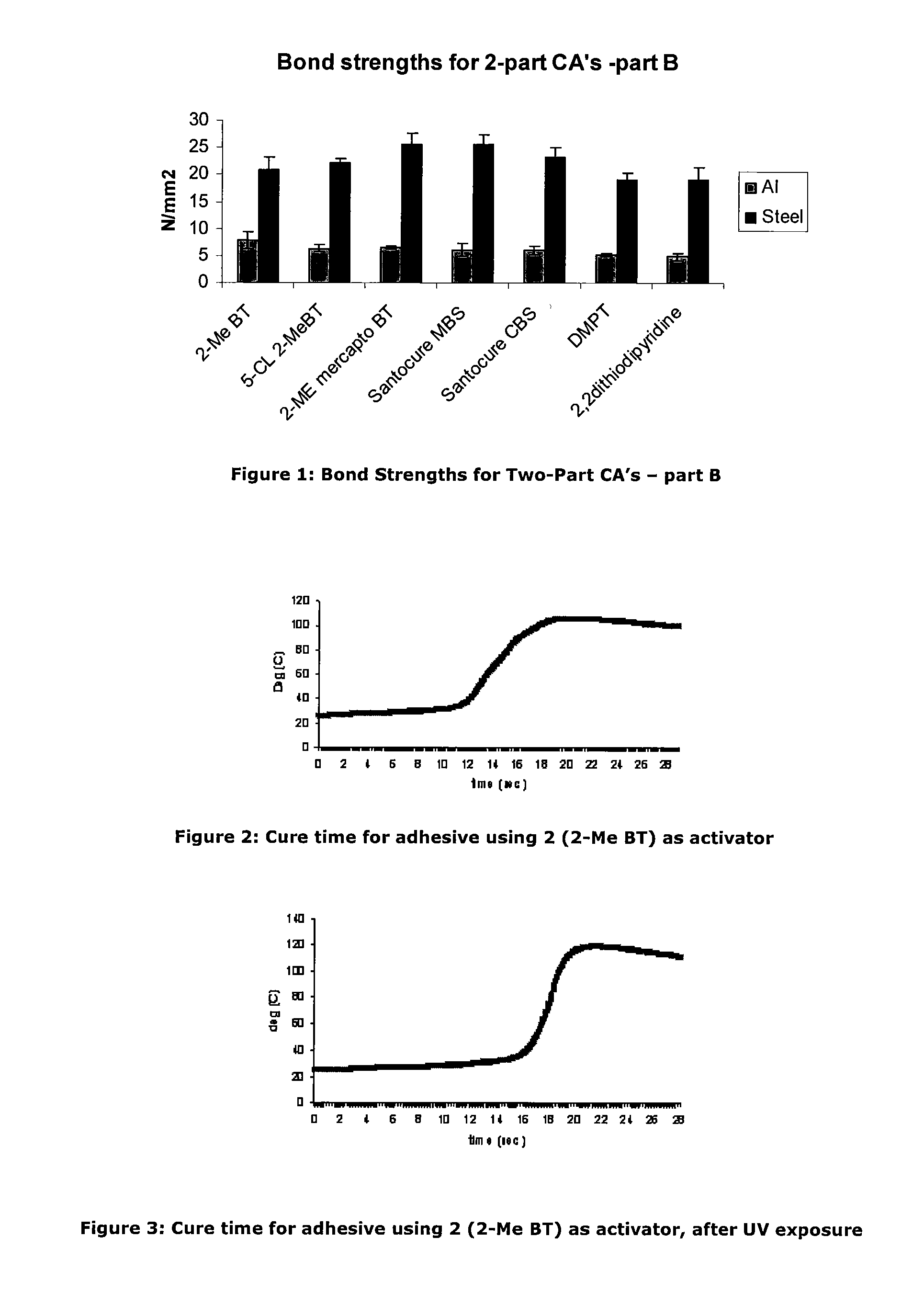
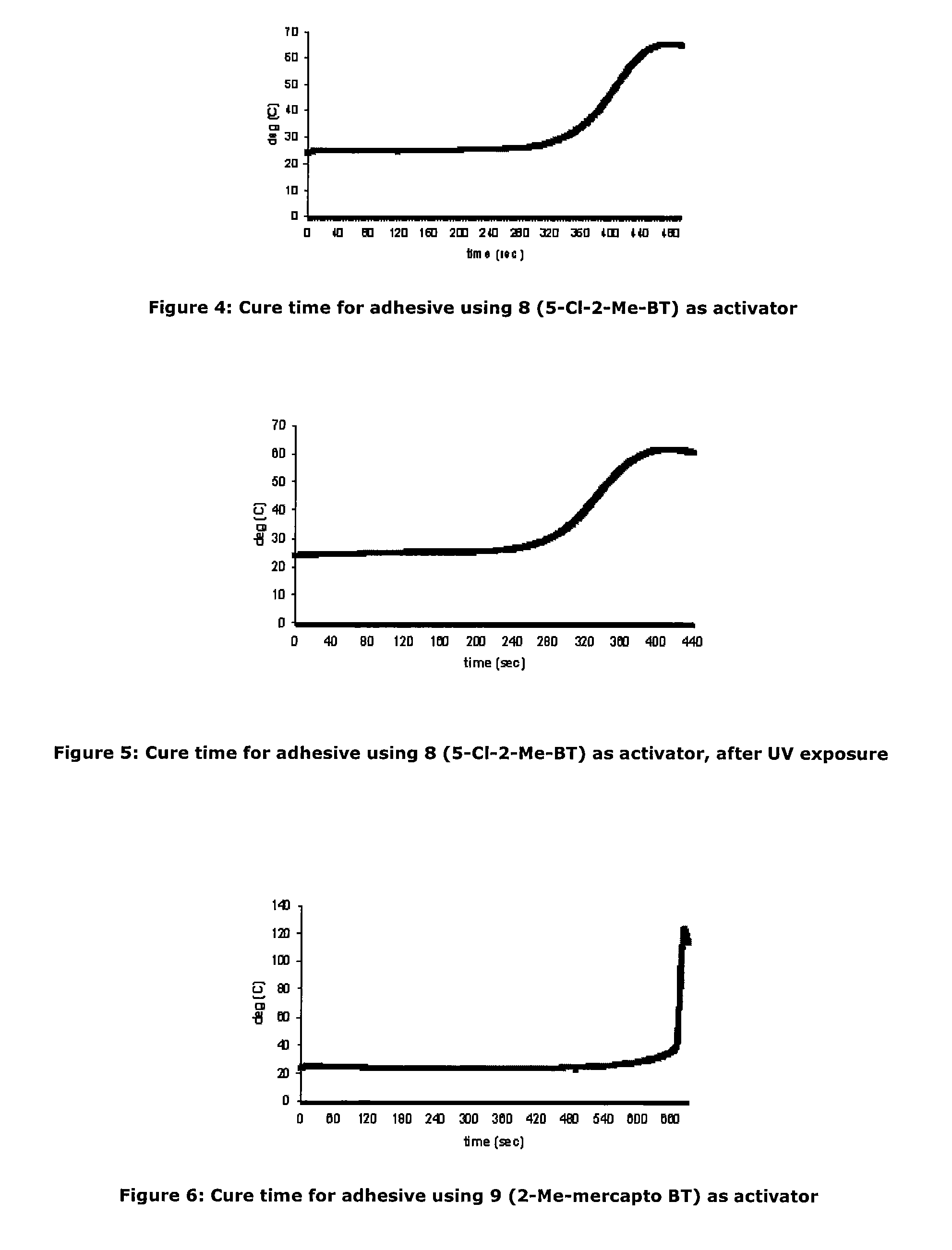
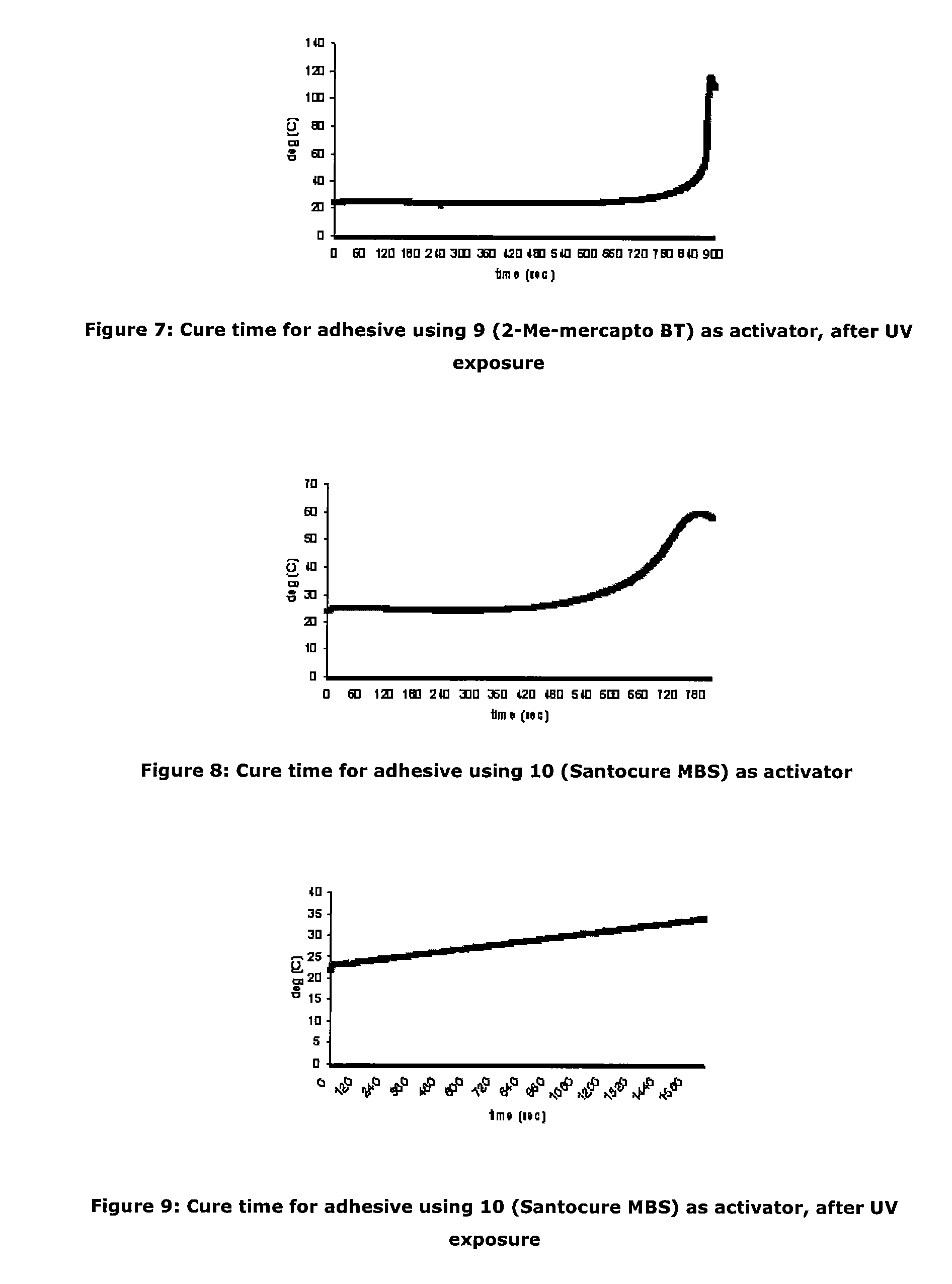
Activators for two part cyanoacrylate adhesives
ActiveUS20110196092A1Rapid adhesive bond formationLess-prone to moisture sensitivityNon-macromolecular adhesive additivesMixingMorpholineSulfenamide
There is provided a cyanoacrylate composition comprising:a cyanoacrylate; anda 2-substituted benzothiazole or a derivative thereofwherein the 2-substituent is an alkyl, an alkene, an alkylbenzyl, an alkylamino, an alkoxy, an alkylhydroxy, an ether, a sulfenamide, a thioalkyl or a thioalkoxy group, with the proviso that an amide portion of the sulfenamide does not have a tert butylamino or a morpholine group.
Owner:HENKEL KGAA
Sitagliptin chiral intermediate and asymmetric synthesis method thereof
The invention relates to a sitagliptin chiral intermediate and an asymmetric synthesis method thereof. The asymmetric synthesis method comprises the steps: with 2,4,5-trifluorophenyl acetic acid as a starting material, carrying out a reduction reaction to obtain 2-(2,4,5-trifluorophenyl)ethanol, then carrying out a reaction with an oxidant, carrying out condensation of the product without separation and (R)-(+)-tert-butyl sulfinamide to obtain corresponding acetal, carrying out a reaction of the obtained product with dialkyl malonate to obtain a key chiral intermediate, hydrolyzing to obtain a corresponding organic amine, carrying out a reaction of the amine with caustic alkali and then acidifying to obtain a corresponding carboxylic acid, then carrying out condensation with 3-(trifluoromethyl)-5,6,7,8- tetrahydro-[1,2,4] triazolo[4,3-a]pyrazine hydrochloride to obtain sitagliptin tert-butyl oxanamide, and finally deprotecting with hydrochloric acid to obtain sitagliptin. The yields of all the steps are all higher, the used reagents are all conventional cheap reagents, no expensive chiral catalysts are used, the reaction conditions are quite mild, and the asymmetric synthesis method is suitable for industrialization.
Owner:成都四面体药物研究有限公司
N-aryl t-butyl sulfonamide, synthetic method and application thereof
InactiveCN102516138AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCarboxyl radicalAcyl group
The invention relates to N-aryl t-butyl sulfonamide, a synthetic method and an application thereof. N-aryl t-butyl sulfonamide has the general molecular formula as shown in the invention, wherein the structure of sulfur atom is R structure, S structure or an arbitrary mixture of the two structures; R1 and R5 are H, C1-C6 alkyl, and R1 and R5 can be the same or different; R2, R3 and R4 are H, C1-C6 alkyl, aryl, nitro, hydrocarbon acyl, formyl, carboxyl, alkoxy and halogen, and R2, R3 and R4 can be the same or different; and R1 and R2 or R2 and R3 form naphthalene ring with A ring. The compound is obtained by a coupling reaction between t butyl sulfenamides and aryl halide C-N under the catalysis of palladium. Under the condition of the catalytic reaction, the chiral structure of the raw material t butyl sulfonamide sulfur atom is maintained in the product. Obvious racemization phenomenon is not generated during the reaction process. The compound provided by the invention can be used as a ligand to promote the addition reaction of zinc ethyl and aromatic aldehyde.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Low temperature detachable anaerobic adhesive and method for preparing the same
InactiveCN1803959AReduce bond strengthLittle effect on bonding strengthGraft polymer adhesivesPolyesterBenzene
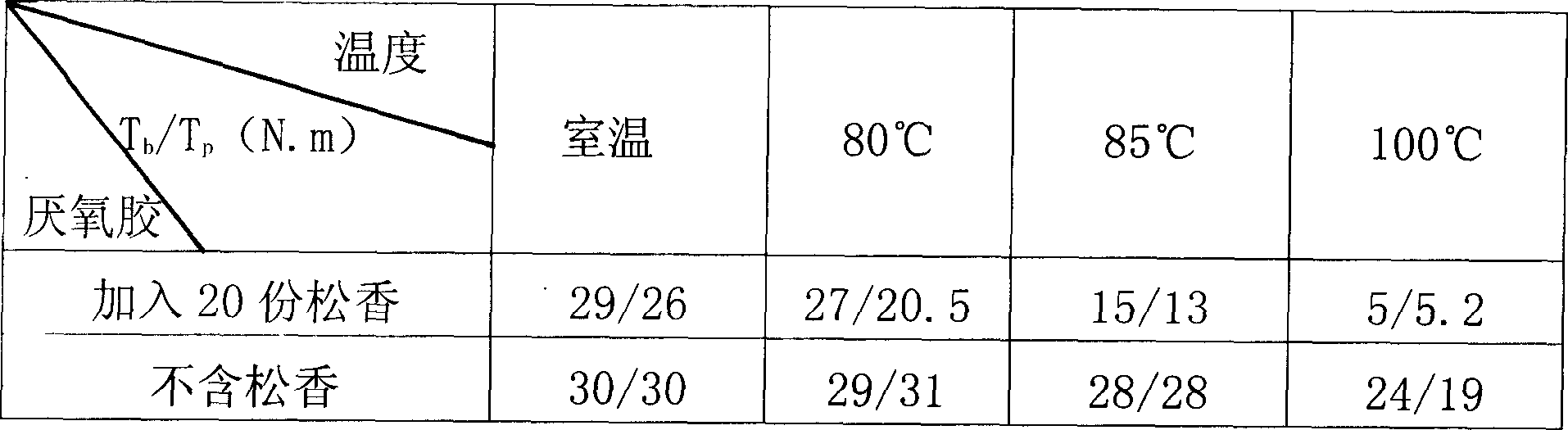
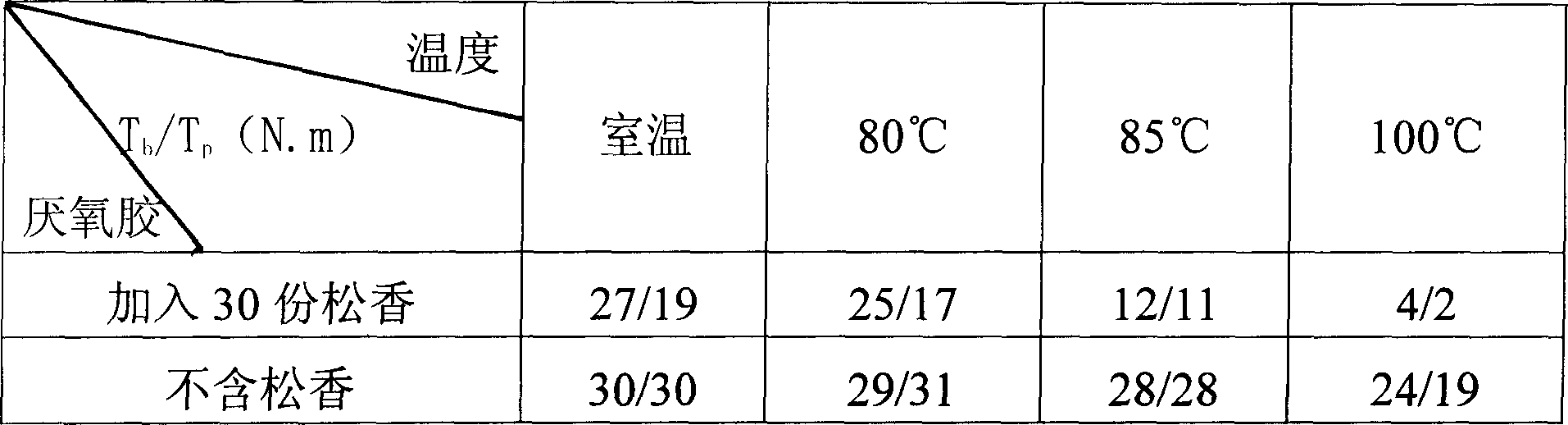
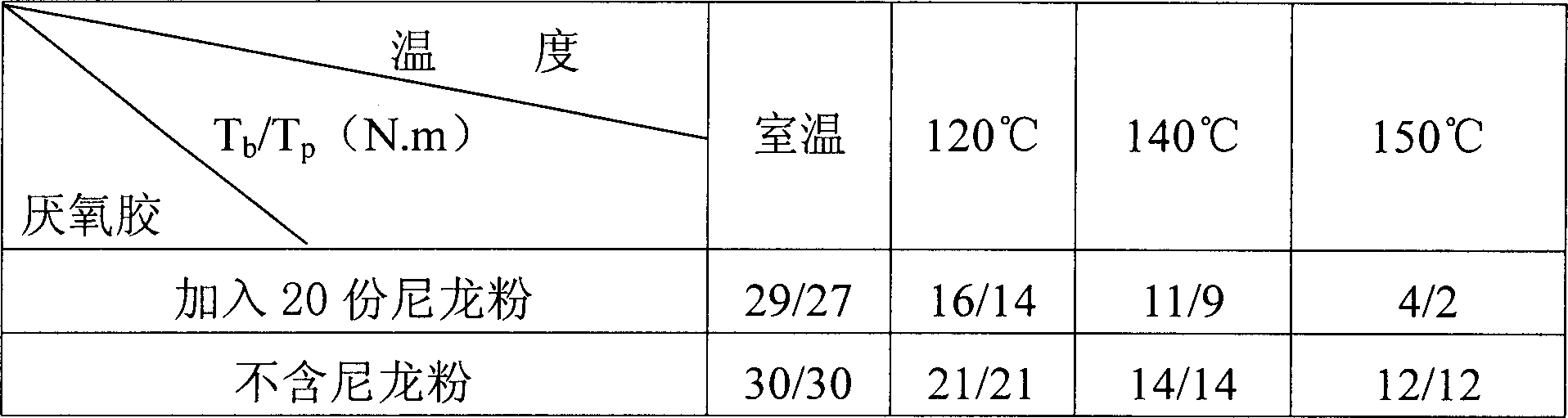
The preparation method for a low temperature detachable anaerobic glum overcomes the poor maintainability, hard assembly to non-resistance heat material and part and depressed reliability, and comprises: A. by weight share, mixing 50-95 methyl dimethacrylate and 0.01-2 stabilizer to dissolve at 45-55Deg; B. adding 0.1-5 accelerant and 0.1-5 o-benzene sulfinamide; C. adding 10-15 unsaturated polyester, 5-50 modifier and 0.1-5 gas-phase SiO2 into the product in B, and cooling to room temperature; D. adding 1-10 oxide to stir evenly. This invention has little effect to adhesive strength and high benefit with low cost.
Owner:HEILONGJIANG INST OF PETROCHEM
Method for manufacturing rubber composition
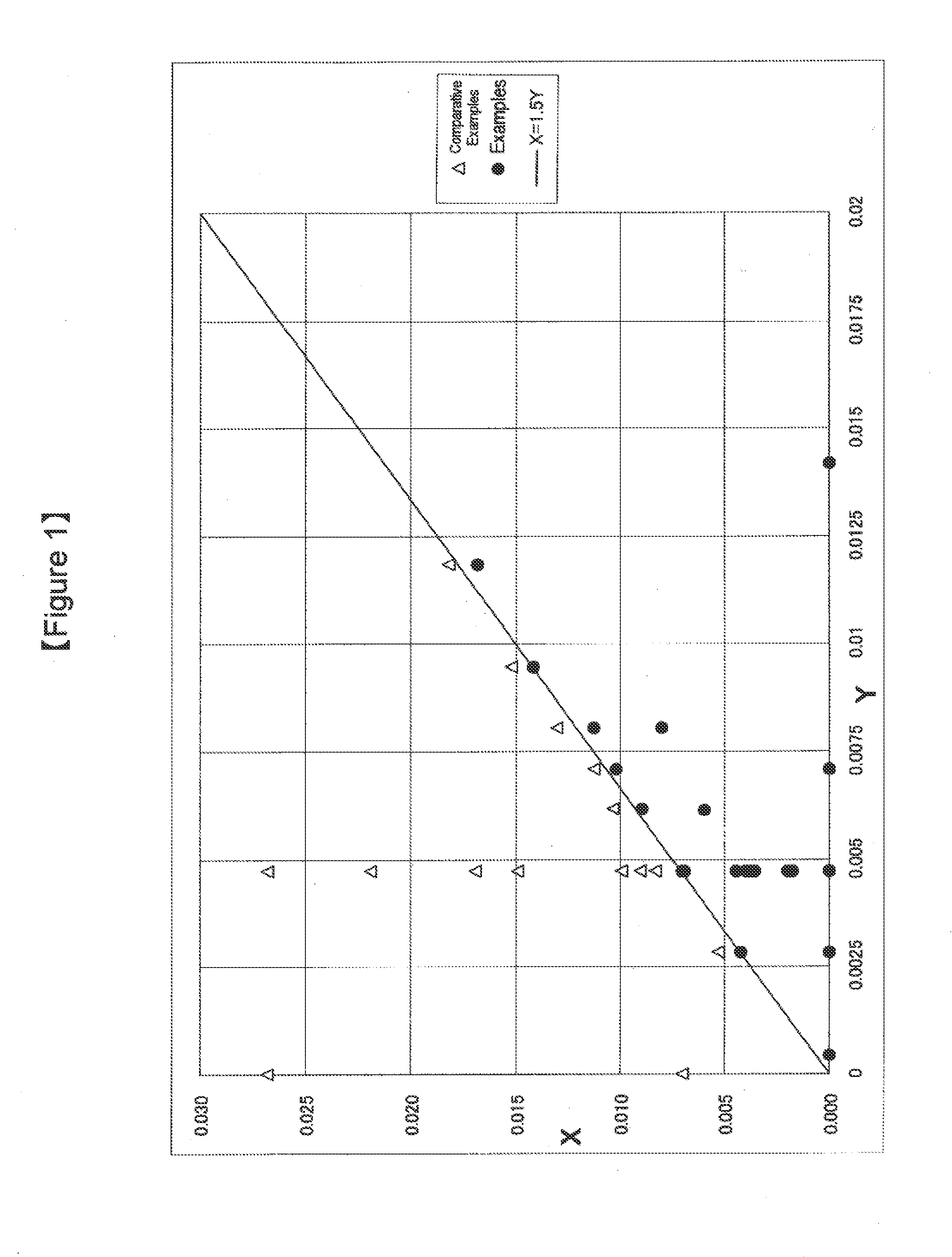
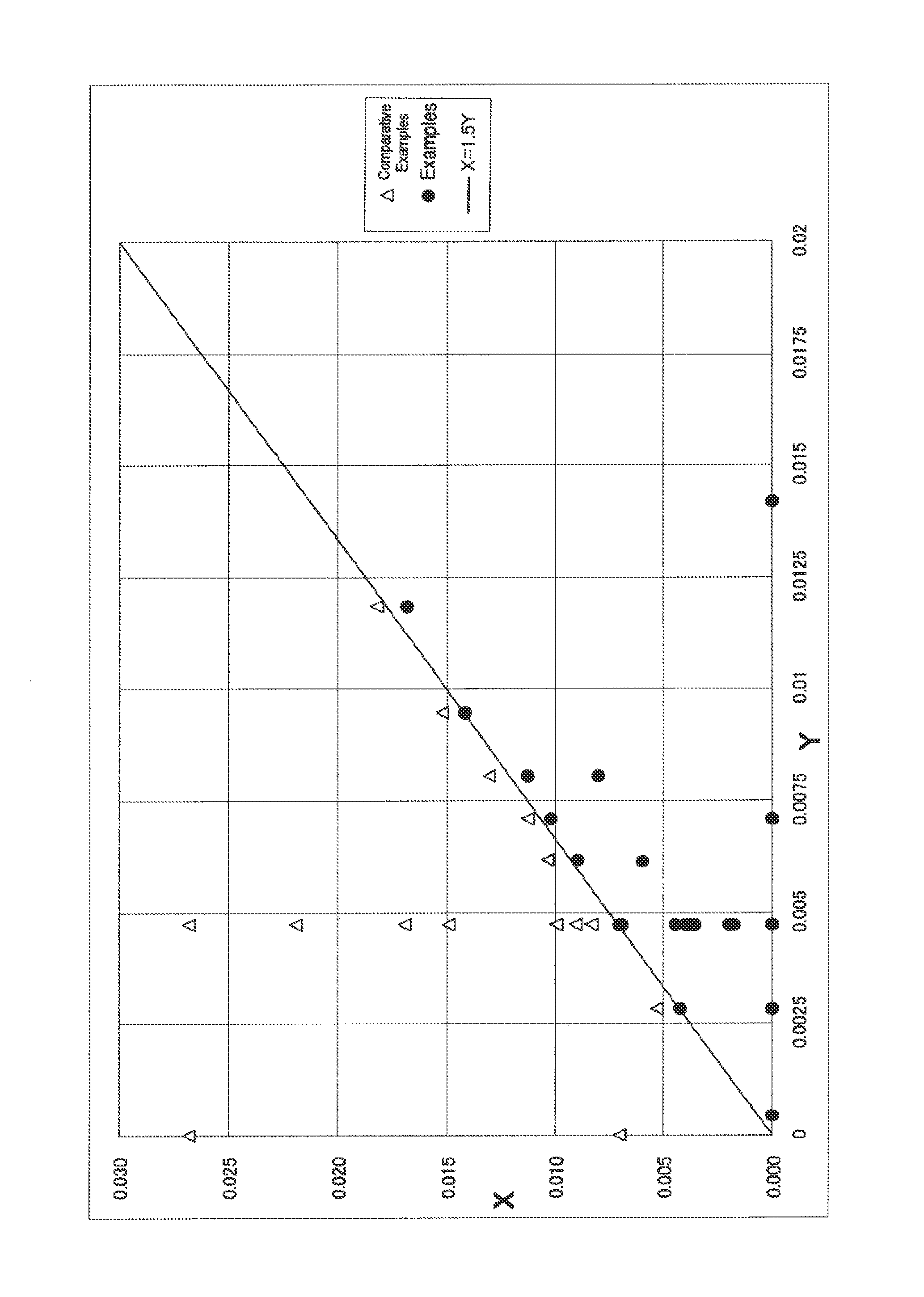
The present invention is a method for producing a rubber composition containing a rubber component (A) of at least one selected from natural rubbers and synthetic dienic rubbers, a filler containing an inorganic filler (B), a silane coupling agent (C), at least one vulcanization accelerator (D) selected from guanidines, sulfenamides and thiazoles, and an organic acid compound (E), wherein the rubber composition is kneaded in plural stages, the rubber component (A), all or a part of the inorganic filler (B), all or a part of the silane coupling agent (C), the vulcanization accelerator (D) and the organic acid compound (E) are kneaded in the first stage of kneading, and the number of molecules X of the organic acid compound (E) in the rubber composition in the first stage is in a relation of the following formula [1] relative to the number of molecules Y of the vulcanization accelerator (D) therein. The production method enables production of a rubber composition having a low-heat-generation property while successfully preventing the coupling function activity of the silane coupling agent from lowering.0≦X≦1.5×Y [1]
Owner:BRIDGESTONE CORP
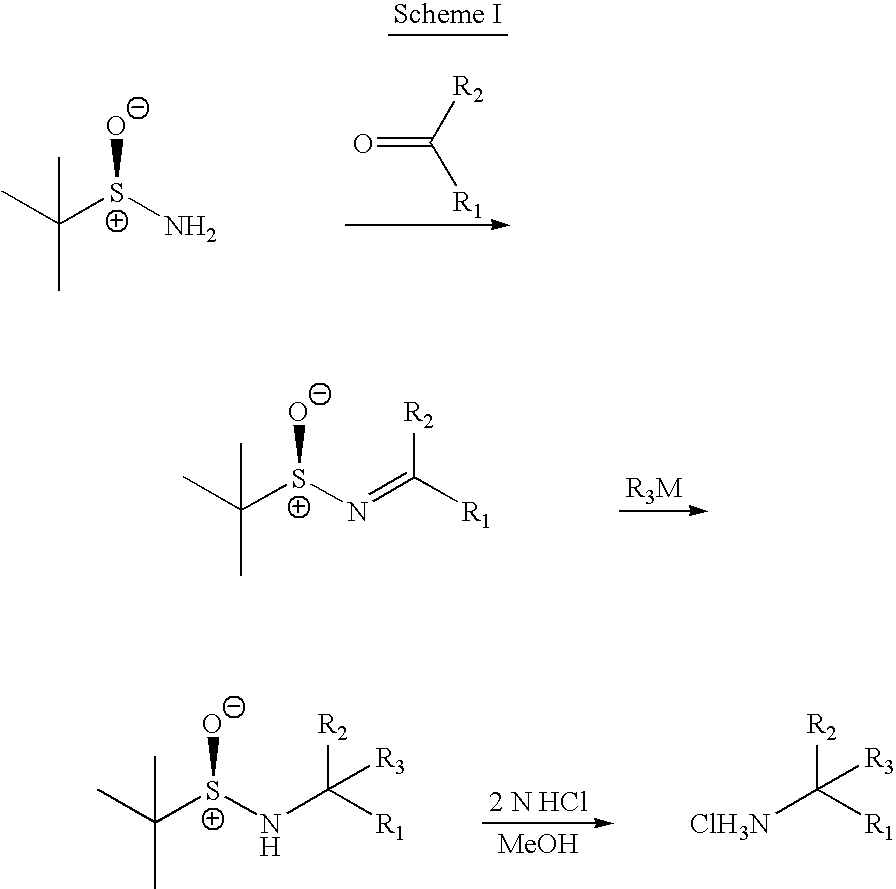
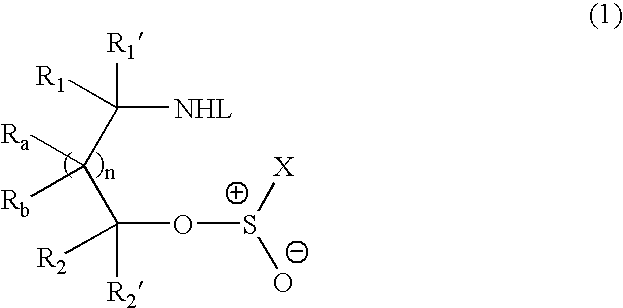
Methods of preparing sulfinamides and sulfoxides
Owner:APSINTERM
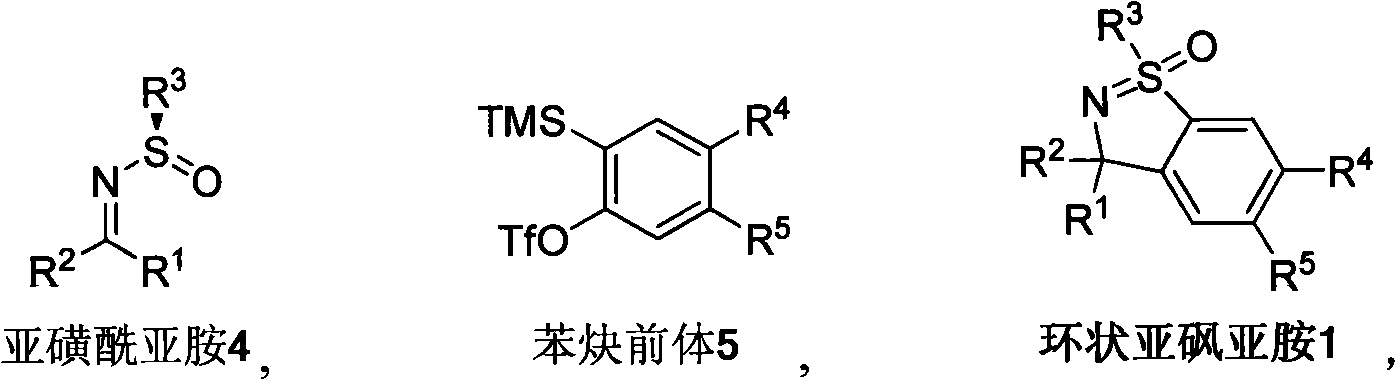
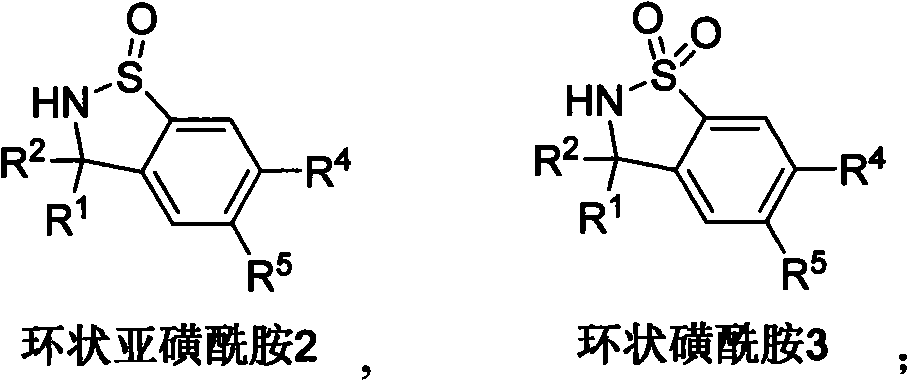
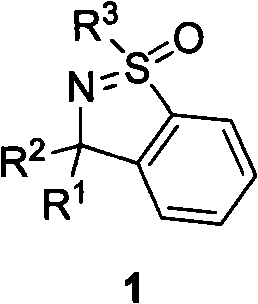
Method for synthesizing cyclic sulphoxide imine, sulfenamide and sulfamide by stereospecificty
InactiveCN101665470AThe reaction method is simpleLow costOrganic chemistryOrganic solventRoom temperature
The invention relates to a method for synthesizing cyclic sulphoxide imine, sulfenamide and sulfamide by stereospecificty; the method comprises the following steps of: under the condition of room temperature, sulphinyl imine 4 and benzyne precursor 5 have reaction for 1-15h under the initiation of organic solvent and CsF, the cyclic sulphoxide imine 1 is generated; when the cyclic sulphoxide imine1 contains sulfonyl, the sulfonyl inside a molecule can be successfully removed under the condition of Mg / HOAc / AcONaDMF / H2O, and the cyclic sulphoxide imine 1 is obtained; under the condition of HCl(dioxane), the cyclic sulphoxide imine 1 is successfully transformed into the cyclic sulfenamide 2; and after the cyclic sulfenamide 2 is oxidized by m CPBA, the cyclic sulfamide 3 is obtained. The method has the characteristics of low cost, high yield, mild reaction condition, environmental protection, good repeatability and the like.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
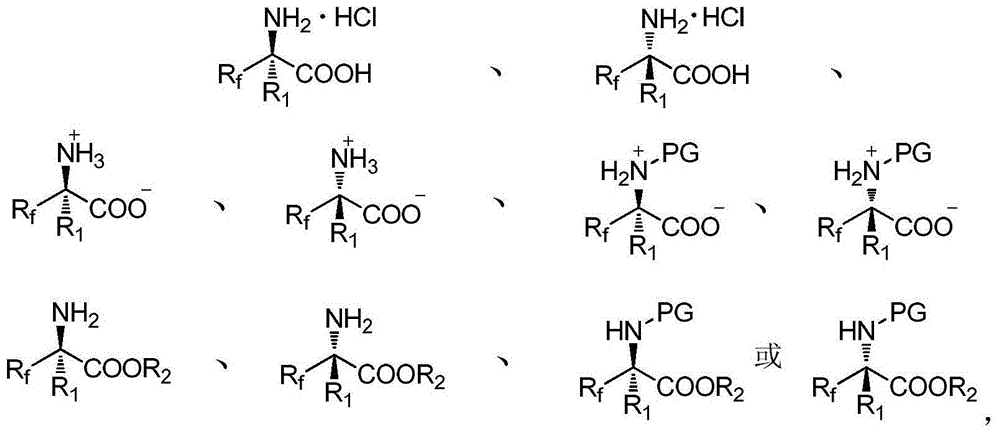
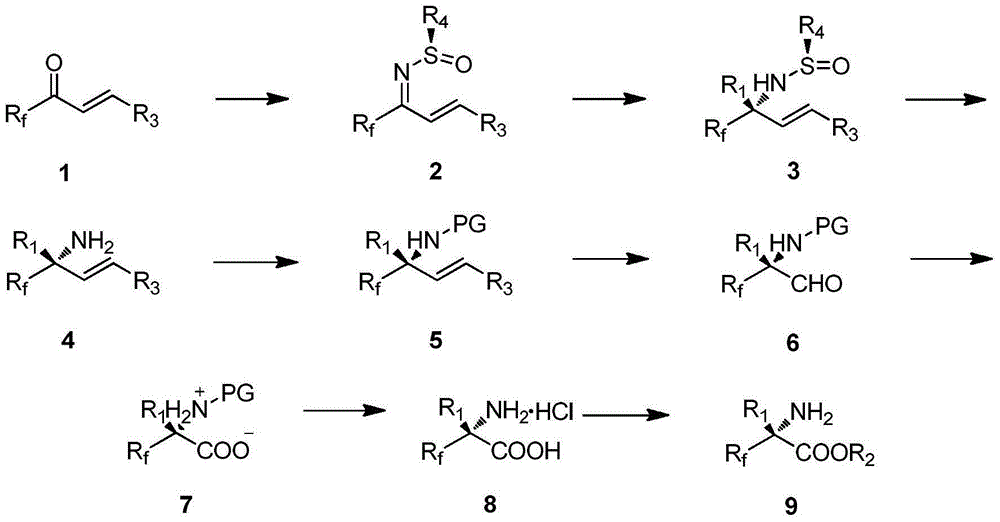
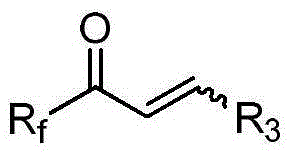
Alpha-fluoroalkyl-alpha-amino acid compound containing tetrasubstituted carbon chiral center and preparation method thereof
InactiveCN105017048AHigh enantiopurityEasy to operateOrganic compound preparationAmino-carboxyl compound preparationOrganic synthesisSynthesis methods
Belonging to the field of organic synthesis, the invention provides an alpha-fluoroalkyl-alpha-amino acid compound containing a tetrasubstituted carbon chiral center, and also discloses a preparation method of the compound. The method utilizes cheap and easily available fluoroalkyl-containing unsaturated ketone and chiral prosthetic group sulfinamide as the starting raw material, and by means of preparation of alpha, beta-unsaturated fluoroalkyl sulfenyl ketoimine, selective addition of an organic metallic reagent and chiral alpha, beta-unsaturated imine, removal of chiral prosthetic group, protection of amino group, and finally double-bond simple oxidation in order, the alpha-fluoroalkyl-alpha-amino acid compound containing the tetrasubstituted carbon chiral center can be obtained by entiomeric purity. The method provided by the invention has the advantages of cheap and easily available raw materials, simple operation of the synthesis method, good selectivity, high yield, and reaction with universal applicability, is a universal method for synthesis of the alpha-fluoroalkyl-alpha-amino acid compound containing the tetrasubstituted carbon chiral center, and has very good application prospects.
Owner:SHANGHAI INST OF TECH
Process method for synthetizing tert-butyl sulfinamide
The invention relates to a method for synthetizing an organic compound. A process method for synthetizing tert-butyl sulfinamide comprises the following steps: (a) under the temperature of between 50 DEG C below zero to 70 DEG C below zero, adding triphenyl methyl halide into liquid ammonia to obtain N methyl triphenyl ammonia; (b) under the temperature of between 60 DEG C below zero and 70 DEG Cbelow zero, adding n-butyl lithium into organic solution of N methyl triphenyl ammonia to obtain organic solution of N methyl (triphenyl) lithium amide; (c) at the temperature of between 50 DEG C below zero and 70 DEG C below zero, dropwise adding organic solution of (S)-tert-butyl sulfinic acid tert-butyl thioester into the N-methyl (triphenyl) lithium amide, quenching the mixture by using water, carrying out deodorization by using dimethyl sulfate, destroying excessive dimethyl sulfate by using ammonia and carrying out post treatment to obtain (S)-N-methyl (triphenyl) tert-butyl sulfinamide; and (d) taking the product in the step (c), adding dilute acid into the product, adjusting the pH value of the mixture to 3, neutralizing the mixture by using alkaline solution until the pH value isbetween 13 and 14 and carrying out post treatment to obtain (S)-tert-butyl sulfinamide. The process method for synthetizing the tert-butyl sulfinamide has simple and convenient operation and good process stability, avoids copious cooling at the low or ultralow temperature, reduces the using amount of ammonia, removes foul odour and is easy to realize industrial production.
Owner:DALIAN NETCHEM CHIRAL TECH
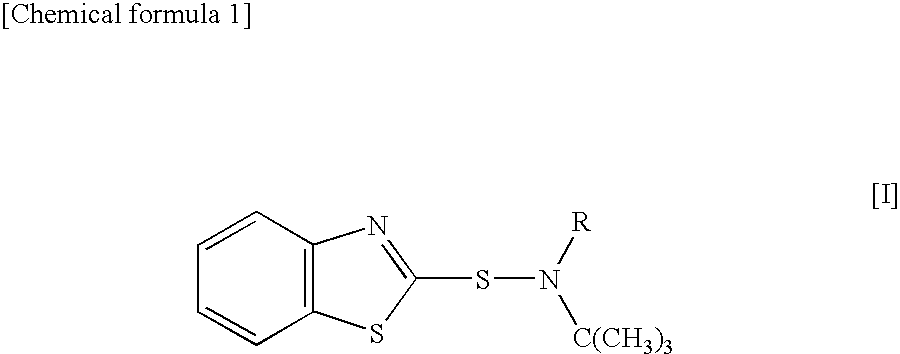
Sulfenamide, vulcanization accelerator containing the sulfenamide for rubber, and process for producing the vulcanization accelerator
ActiveUS20100324301A1Slow effectReduce the possibilityOrganic chemistryPolymer scienceEnvironmental hygiene
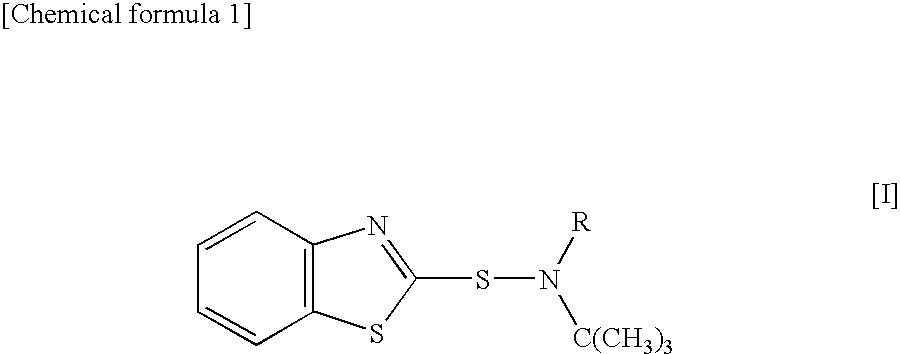
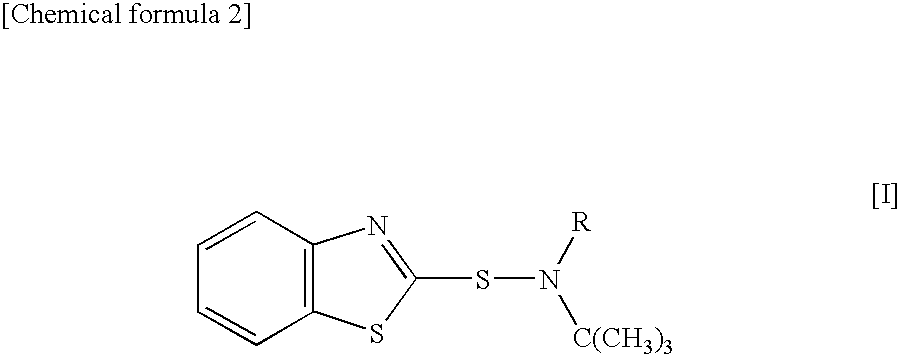
A sulfenamide vulcanization accelerator is provided that acts satisfactorily slowly on a vulcanization reaction, produces no carcinogenic nitrosamine, and is free from environmental hygiene problems such as bioaccumulation. Also provided is an N-alkyl-N-t-butylbenzothiazole-2-sulfenamide represented by formula [I]. The vulcanization accelerator is a vulcanization accelerator for rubber, containing this compound. Furthermore provided is a process for producing the vulcanization accelerator.wherein R represents methyl, ethyl, n-propyl, or n-butyl.
Owner:OUCHI SHINKO CHEM IND
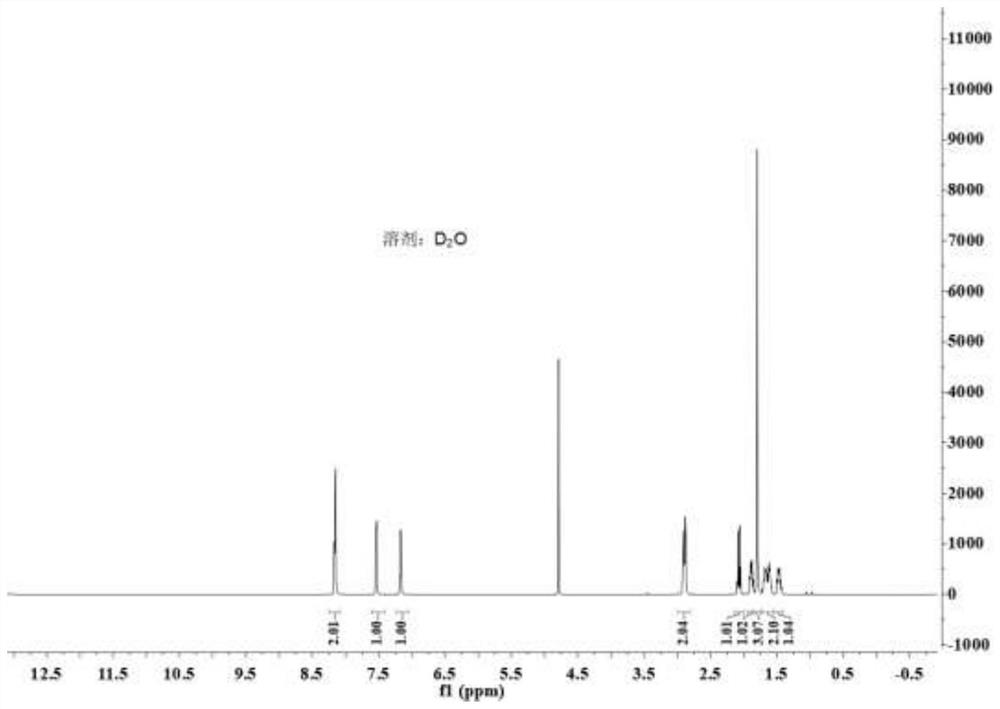
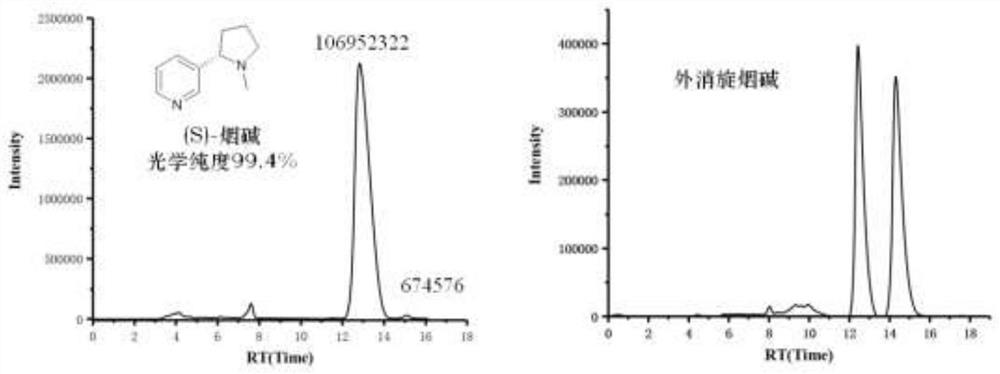
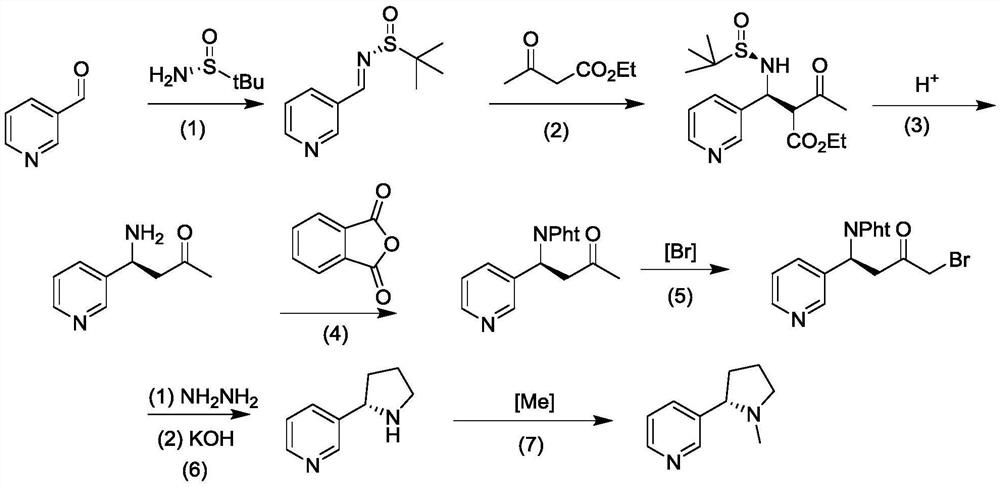
Preparation method for synthesizing chiral nicotine from chiral tert-butyl sulfinamide
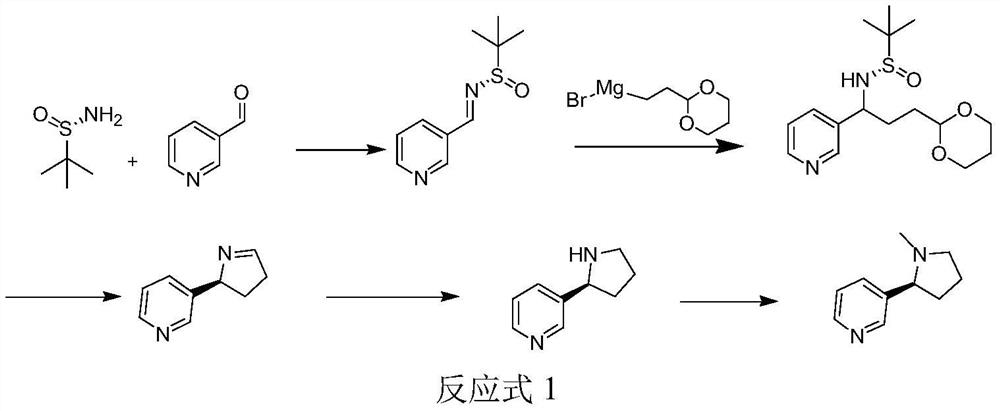
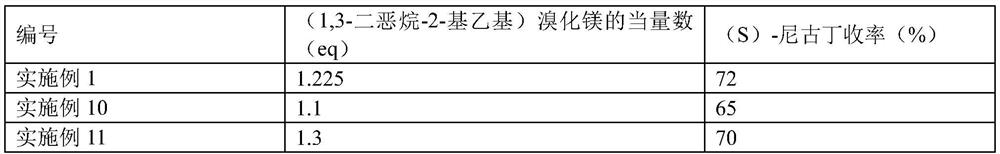
PendingCN113444070AHigh yieldHigh ee valueOrganic chemistry methodsPtru catalystCombinatorial chemistry
The invention discloses a preparation method for synthesizing chiral nicotine from chiral tert-butyl sulfinamide. The preparation method comprises the following steps: carrying out a condensation reaction on 3-pyridylaldehyde and chiral tert-butyl sulfinamide under the action of titanate; then carrying out a reaction on a condensation product and (1,3-dioxan-2-ylethyl)magnesium bromide; performing cyclization under an acidic condition; and finally, carrying out reduction and aminomethylation to obtain the chiral nicotine. The novel synthesis method of chiral nicotine provided by the invention has the advantages that a reaction route is short, raw materials are easy to obtain and low in price since chiral tert-butyl sulfinamide is taken as a starting raw material, expensive or complex chiral catalysts do not need to be prepared, each step of reaction operation is simple, and the chiral nicotine generated by the reaction is high in yield, high in ee value and reduced in production cost.
Owner:SHENZHEN ZINWI BIO-TECH CO LTD
Method for manufacturing rubber composition
The present invention is a method for producing a rubber composition containing a rubber component (A) of at least one selected from natural rubbers and synthetic dienic rubbers, a filler containing an inorganic filler (B), a silane coupling agent (C), at least one vulcanization accelerator (D) selected from guanidines, sulfenamides and thiazoles, and an organic acid compound (E), wherein the rubber composition is kneaded in plural stages, the rubber component (A), all or a part of the inorganic filler (B), all or a part of the silane coupling agent (C), the vulcanization accelerator (D) and the organic acid compound (E) are kneaded in the first stage of kneading, and the number of molecules X of the organic acid compound (E) in the rubber composition in the first stage is in a relation of the following formula [1] relative to the number of molecules Y of the vulcanization accelerator (D) therein. The production method enables production of a rubber composition having a low-heat-generation property while successfully preventing the coupling function activity of the silane coupling agent from lowering.0≦X≦1.5×Y [1]
Owner:BRIDGESTONE CORP
Antimicrobial compositions
The present invention relates to antimicrobial compositions useful in industrial applications. More specifically, it relates to a process by combining 3-benzo[b]thiophen-2-yl-5,6-dihydro-1,4,2-oxothiazine-4-oxide and one or more options. From 1-[[(3-iodo-2-propynyl)oxy]methoxy]-4-methoxybenzene, 1-chloro-4-[[(3-iodo-2-propynyl)oxy] Methoxy]benzene, zinc 2-pyridinethiol-1-oxide, copper 2-pyridinethiol-1-oxide, sodium salt of 2-pyridinethiol-1-oxide, 2,2-dithio - Bis(pyridine-1-oxide), 2-methylthio-4-tert-butylamino-6-cyclopropylamino-cis-triazole, 2,4,5,6-tetrachloro-1 , 3-phthalonitrile (chlorothalonil), 1,1-dichloro-N-[(dimethylamino)sulfonyl]-1-fluoro-N-phenyl-methanesulfenamide (bacteriozolin) , or 1,1-dichloro-N-[(dimethylamino)sulfonyl]-1-fluoro-N-(4-methylphenyl)-methanesulfenamide (tolylfluanid) combined with Antibacterial compositions of these compounds acting synergistically.
Owner:JANSSEN PHARMA NV
Rubber prepared with pre-treated precipitated silica and tire with component
The invention relates to a rubber composition prepared with pre-treated precipitated silica and tire with component comprised of such rubber composition. The rubber composition is comprised of at least one diene-based elastomer and said pre-treated precipitated silica together with a sulfur cure package comprised of elemental sulfur together with 1,6-bis (N,N′-dibenzylthiocarbamoyldithio)-hexane crosslinking agent and sulfenamide accelerator.
Owner:THE GOODYEAR TIRE & RUBBER CO
Method for preparing pure enantio-methylpropane-2-sulfinamide
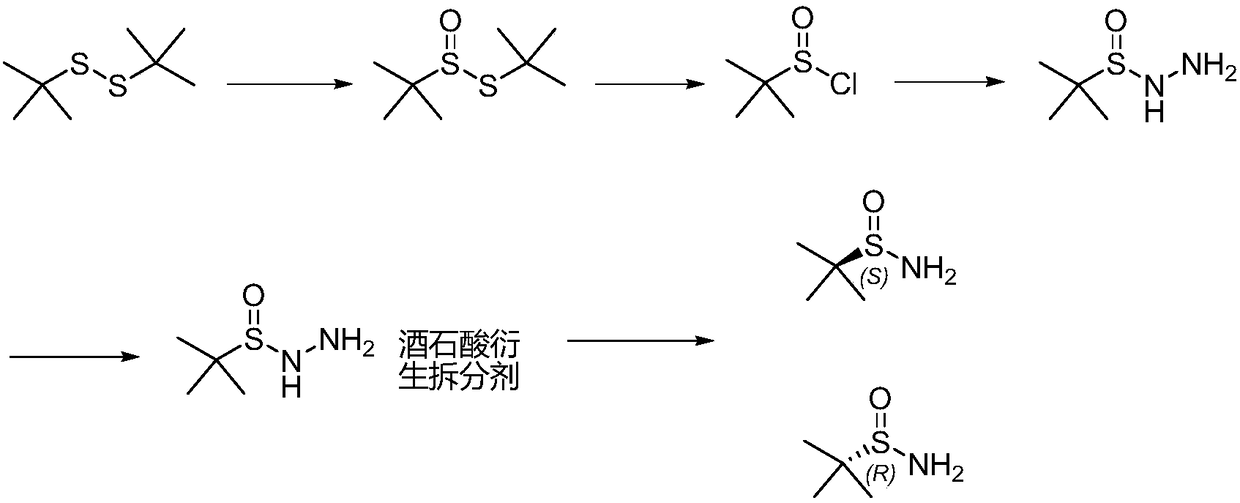
ActiveCN108558715AShort reaction timeThe synthetic route is simpleOptically-active compound separationOrganic racemisationChlorideTert butyl
The invention discloses a method for preparing pure enantio-methylpropane-2-sulfinamide. The method comprises the following steps: carrying out selective oxidation on di-tert-butyl disulfide and hydrogen peroxide, reacting with an acylation reagent so as to obtain tert-butylsulfinyl chloride and tertiary butyl sulfonyl bromide, further reacting with hydrazine hydrate so as to obtain tertiary butylhydrazide, further carrying out resolution and separation with a DBTA resolving agent, and carrying out cracking with zinc acetate, thereby obtaining the pure enantio-methylpropane-2-sulfinamide. Themethod is simple, convenient and stable in process operation, high in yield and good in environment protection, and compared with a conventional process, the method is cheap and easy in raw materialobtaining, low in production cost of pure enantio-methylpropane-2-sulfinamide, and beneficial to industrial on-scale production.
Owner:安庆融创生物科技有限公司
Method for asymmetrically synthesizing (S)-nicotine
PendingCN113979993ALow priceReduce manufacturing costOrganic chemistry methodsBulk chemical productionNornicotineAcyl group
The invention provides a method for asymmetrically synthesizing (S)-nicotine. The method comprises the following steps: condensing 3-pyridylaldehyde and (R)-tert-butanesulfinamide to obtain chiral imine; adding ethyl acetoacetate to react with the chiral imine to obtain chiral amine; removing tert-butyl sulfinyl and ester group from the chiral amine to obtain chiral aminoketone; protecting the amino group of the chiral aminoketone by using phthalic anhydride; adding a brominating agent to brominate the chiral aminoketone to obtain bromide; and adding hydrazine hydrate to remove a Pht protecting group and form a tetrahydropyrrole ring at the same time by utilizing a one-pot method, reducing carbonyl into methylene to obtain (S)-nornicotine, and carrying out N-methylation on the (S)-nornicotine to obtain the final product (S)-nicotine. According to the method, direct construction of chiral carbon is realized by utilizing a cheap chiral reagent (R)-tert-butanesulfinamide, (S)-nicotine with high chiral purity is obtained at a relatively high yield through subsequent simple reaction steps, and the method is simple, convenient, feasible and suitable for industrial production.
Owner:云南萃精生物科技有限责任公司
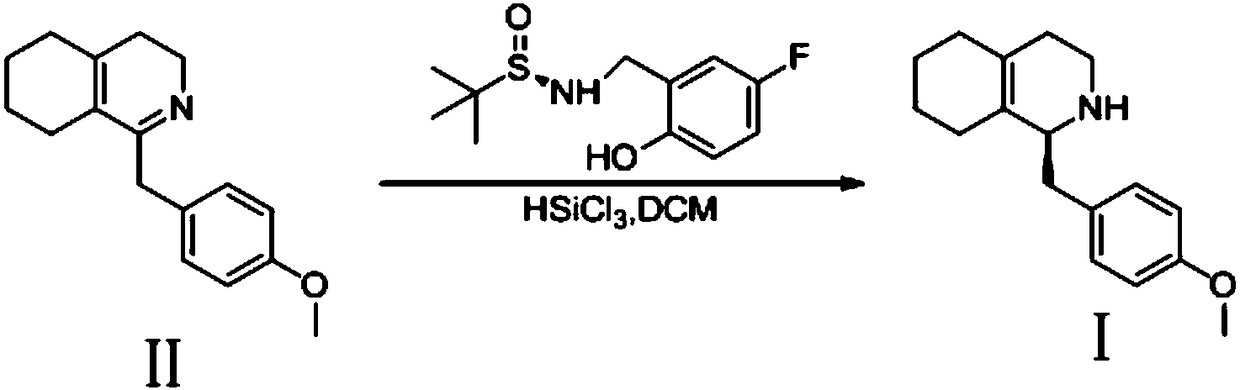
Method for preparing (S)-1-(4-methoxy benzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydro isoquinoline
InactiveCN108383786ASave raw materialsRaw material safetyOrganic chemistry methodsIsoquinolineReaction temperature
The invention discloses a method for preparing (S)-1-(4-methoxy benzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydro isoquinoline which is a dextromethorphan intermediate. The method includes selectively hydrogenating 1-(4-methoxy benzyl)-3, 4, 5, 6, 7, 8-octahydro isoquinoline (II) under the condition of (R)-N-(5-fluorine-2-hydroxyl benzyl)-2-methylpropane-2-sulfinamide and trichlorosilane to obtain the (S)-1-(4-methoxy benzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydro isoquinoline (I). The 1-(4-methoxy benzyl)-3, 4, 5, 6, 7, 8-octahydro isoquinoline (II) is used as a raw material, and the (R)-N-(5-fluorine-2-hydroxyl benzyl)-2-methylpropane-2-sulfinamide is used as an organic chiral ligand. The method has the advantages that the organic chiral ligand is used, and the raw material is inexpensive, safe, simpleand easily available; the reaction temperatures range from -20 DEG C to -15 DEG C, and accordingly the method can be implemented in industrial production; an ee (enantiomeric excess) value of the (S)-1-(4-methoxy benzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydro isoquinoline which is a product can reach 63%.
Owner:启东东岳药业有限公司 +1
Rubber composition
InactiveUS20110124787A1Reduce generationReduced durabilityConductive materialOrganic conductorsHydrogen atomPolymer science
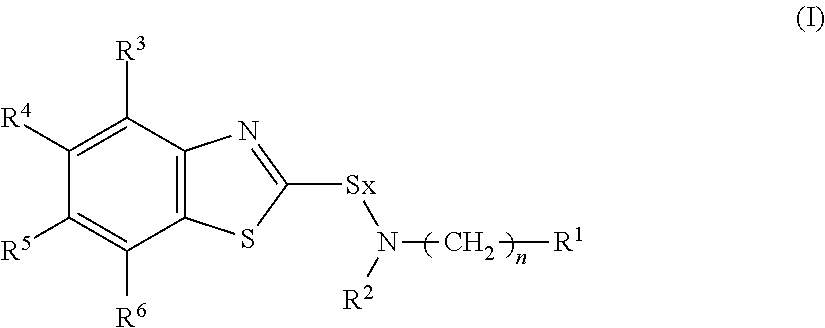
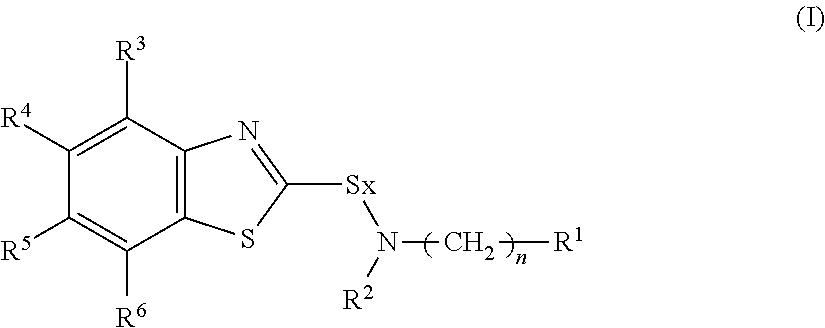
Provided are a rubber composition which is remarkably reduced in generation of rubber burning and excellent in degradation durability and which has a high elastic modulus and a rubber composition which exerts a high heat resistant adhesive property and a wet heat adhesive property and which is excellent in an adhesive property with metal such as a steel cord.The above rubber composition is characterized by comprising a rubber component, a sulfenamide base vulcanization accelerator represented by the following Formula (I), a compound having a disubstituted or trisubstituted benzene ring in which at least one of the substituents is a hydroxyl group and a methylene group donor:(wherein R1 is a branched alkyl group having 3 to 12 carbon atoms; R2 is a linear alkyl group having 1 to 10 carbon atoms or a branched alkyl group having 3 to 10 carbon atoms; R3 to R6 are a hydrogen atom, a linear alkyl group or alkoxy group having 1 to 4 carbon atoms or a branched alkyl group or alkoxy group having 3 to 4 carbon atoms, and they may be the same or different; x represents an integer of 1 or 2, and n represents an integer of 0 or 1).
Owner:BRIDGESTONE CORP
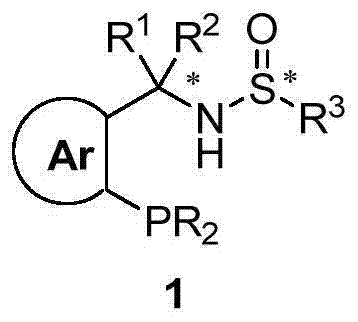
Large-scale preparation method of chiral sulfinamide monophosphine ligand
ActiveCN110615811AHigh selectivityHigh synthesis efficiencyGroup 5/15 element organic compoundsOrganic chemistry methodsImideBenzaldehyde
The invention discloses a large-scale preparation method of a chiral sulfinamide monophosphine ligand. The method includes condensing chiral sulfinamide and 2-(diphenylphosphino)benzaldehyde which areadopted as raw materials to obtain an intermediate that is chiral sulfinyl imide, and carrying out a nucleophilic addition reaction with a phenylmagnesium bromide reagent to generate the chiral sulfinamide monophosphine ligand. The intermediate imine product and a final product chiral sulfinamide monophosphine ligand are purified by adopting a crystallization method, and column chromatography isnot needed. The method has the characteristics of cheap and accessible raw materials, simple synthesis steps, large-scale synthesis and preparation, a specific three-dimensional structure of the product and the like. The method is mild in condition, and is of important significance for future process and commercialization development of chiral sulfinamide monophosphine ligand products.
Owner:EAST CHINA NORMAL UNIV
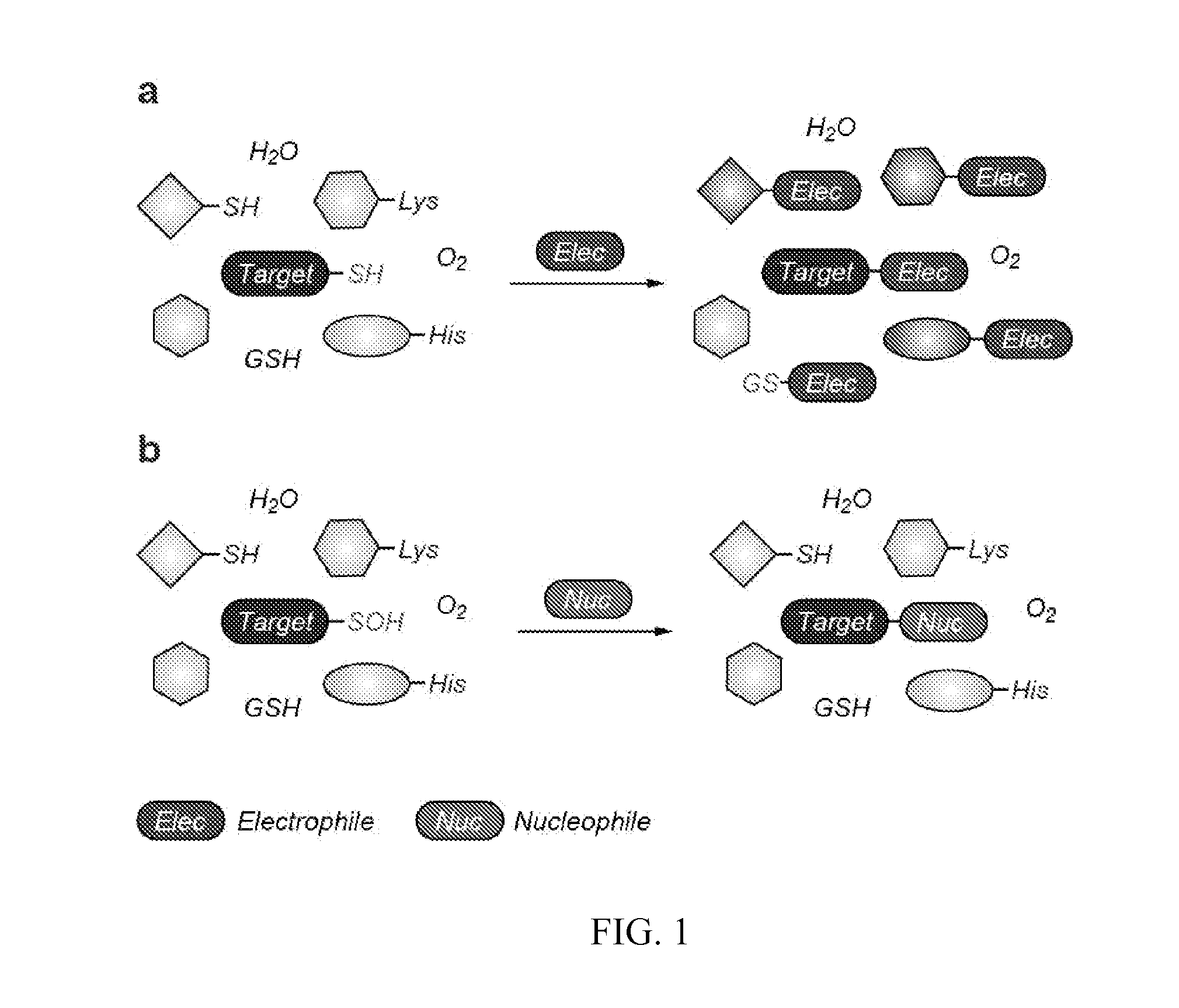
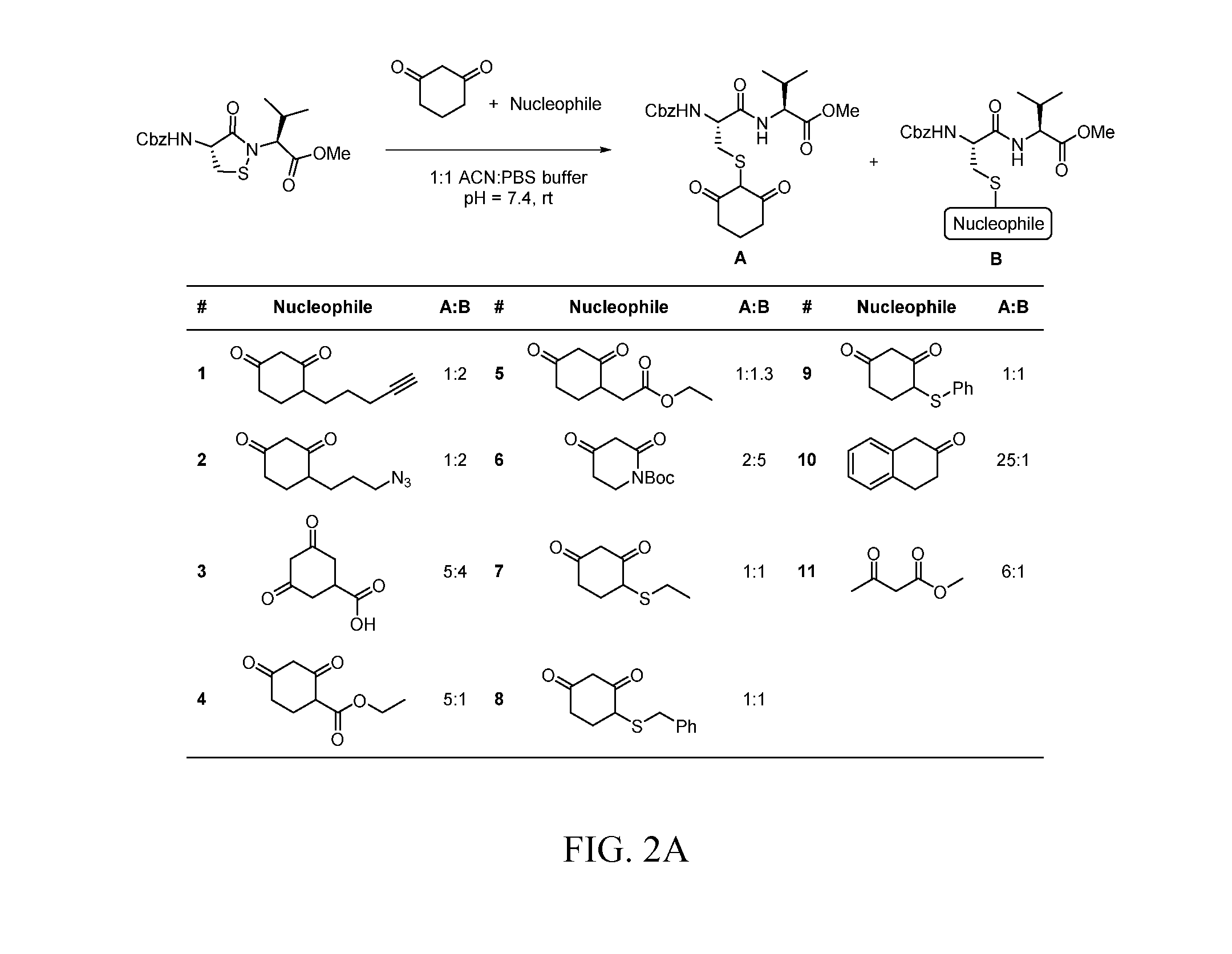
Targeted Covalent Probes and Inhibitors of Proteins Containing Redox-Sensitive Cysteines
ActiveUS20160195532A1Strong specificityHigh selectivityCompound screeningBiocideReaction centreProtein
Covalent, irreversible small-molecule inhibitors that modify the sulfenyl form (i.e., sulfenic acid, RSOH and sulfenamide, RSNR′2) of therapeutically important proteins (particularly kinases and phosphatases) are disclosed, where the compositions include a compound having a substituted aryl or heterocyclic core structure that promotes binding interactions with a specific protein, and a nucleophilic reaction center (carbon, nitrogen, sulfur, or phosphorous) that is capable of forming a covalent bond with a sulfenic acid- or sulfenamide-modified cysteine residue in the protein. Methods for synthesizing these compounds are also disclosed, as well as methods of using them for determining the bioactivity of a chemical composition comprising an active compound toward a specific protein and for determining the potency of an inhibitor against a specific protein.
Owner:THE SCRIPPS RES INST
Sitagliptin synthesis method
The invention discloses a sitagliptin synthesis method, which comprises: carrying out a reaction on a compound represented by a formula IV, (R)-(+)-tert-butyl sulfinamide and hydrogen under the catalysis of a catalyst to obtain a compound represented by a formula V; and carrying out a hydrolysis reaction on the compound represented by the formula V to obtain a compound represented by a formula VI,ie., sitagliptin. According to the present invention, the method has advantages of easily available raw materials, simple steps, high yield and mild reaction conditions, and is suitable for industrial production. The formulas IV, V and VI are defined in the specification.
Owner:安庆奇创药业有限公司
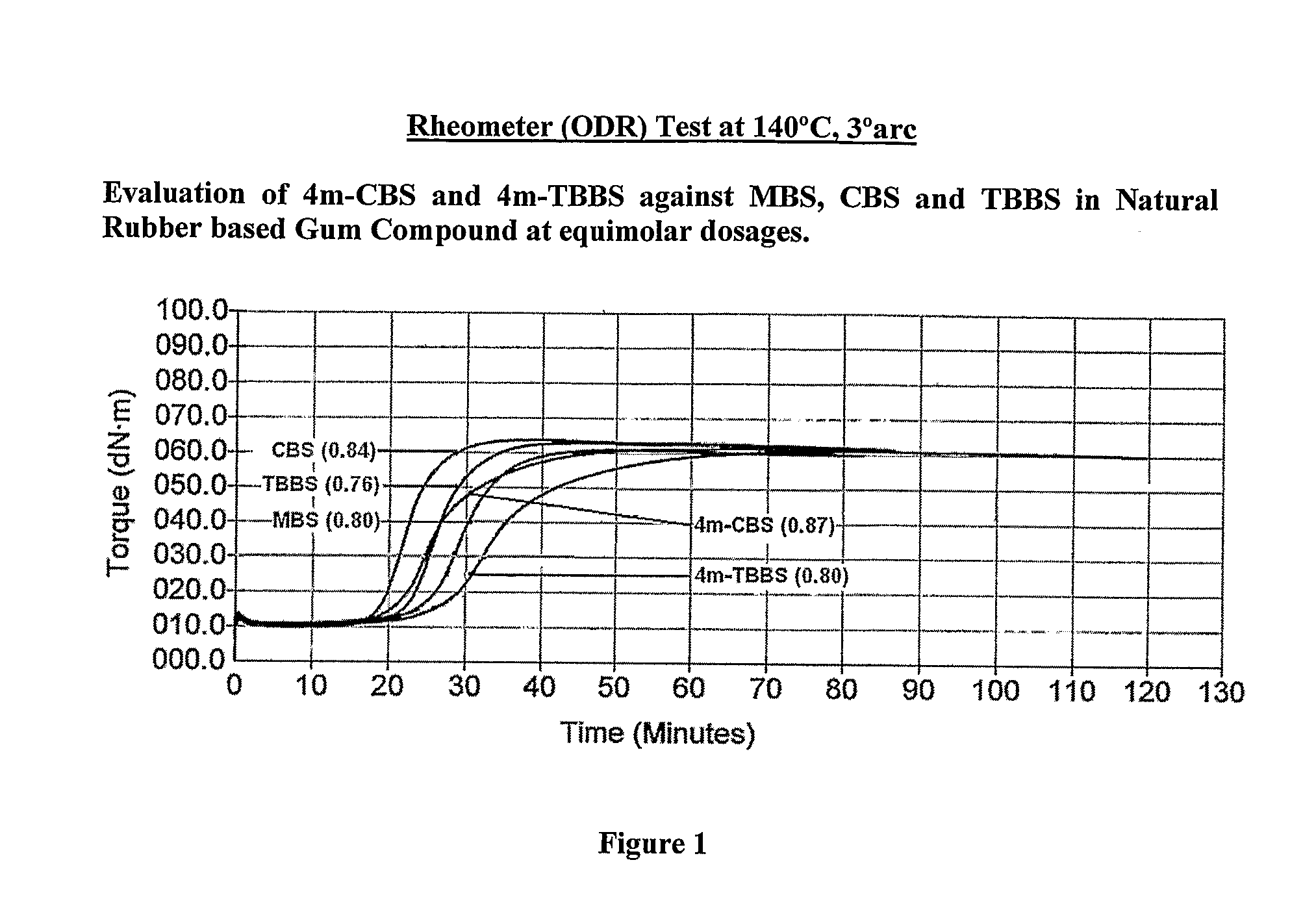
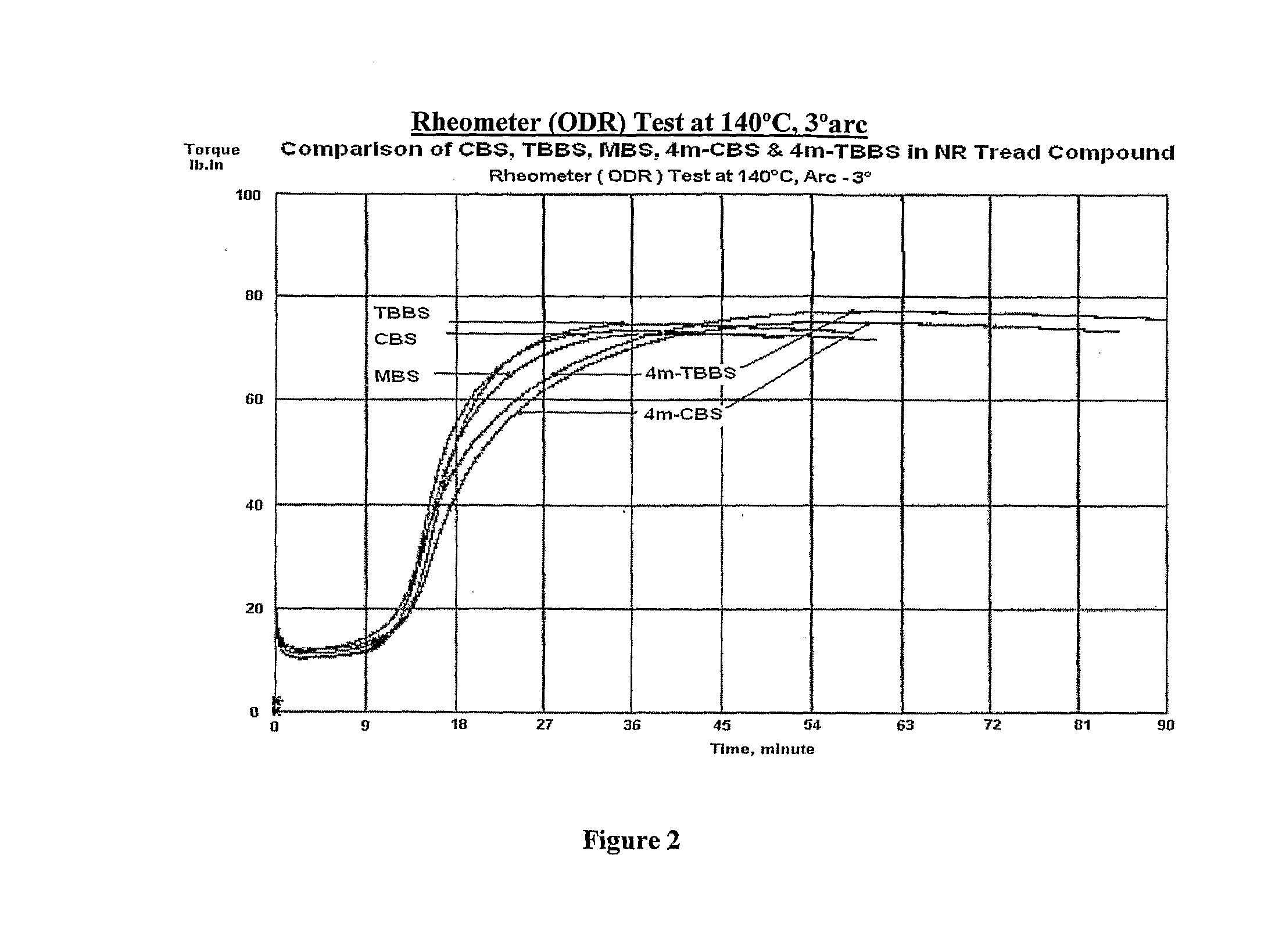
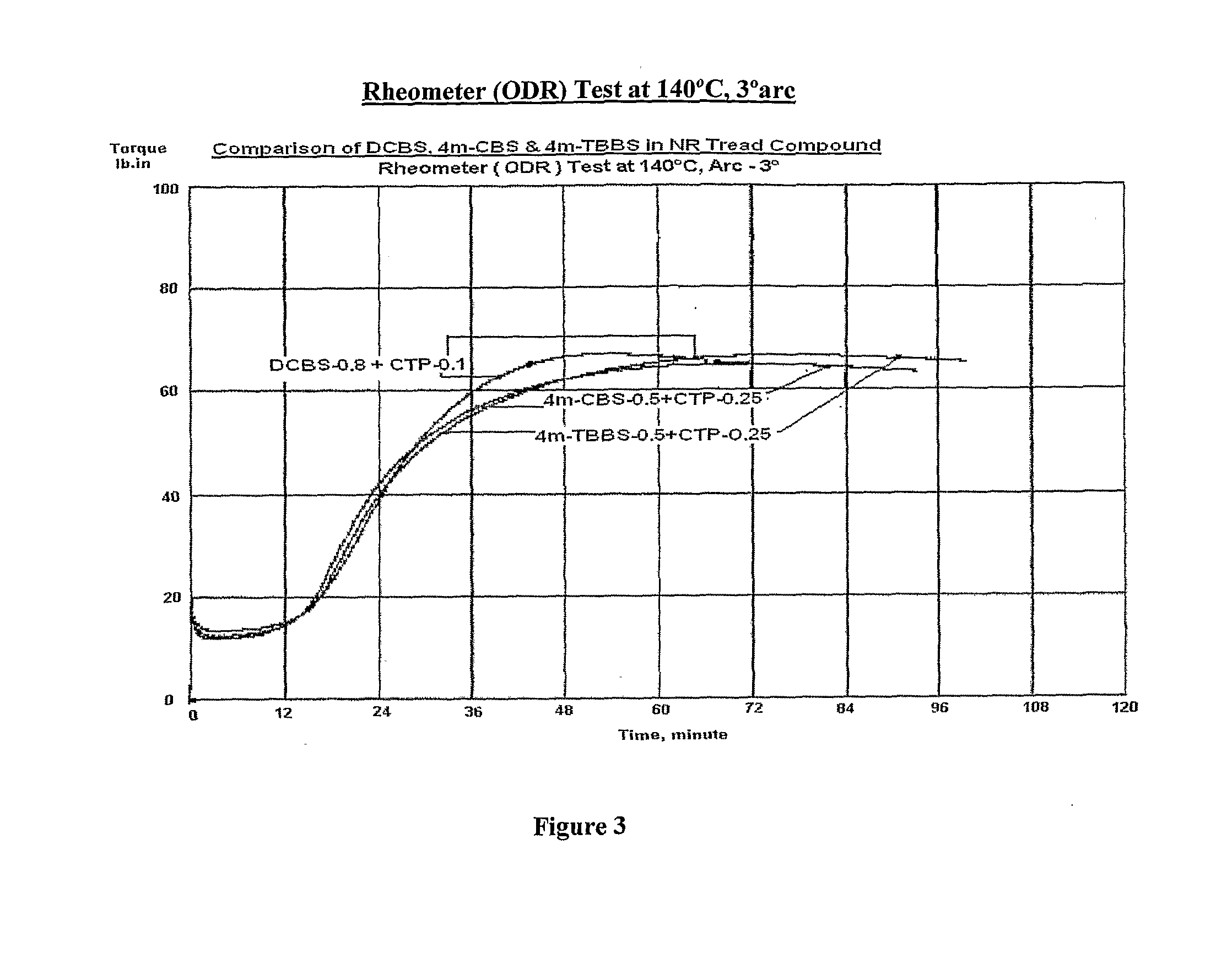
Novel sulfenamide accelerators for improved network stabilization of rubber vulcanizates
Sulfenamide derivatives of 4-alkyl substituted 2-mercapto benzothiazole (4m-MBT) as accelerators used in vulcanizable rubber composition having improved ‘Reversion Resistance’ and ‘Modulus & Hardness Stabilization’ properties for sulphur vulcanized tire compounds predominantly based on Natural Rubber or its blends with Polybutadiene (BR) and styrene butadiene rubber (SBR) in which Natural Rubber is the major component thereby improving the overall tire performance, ride safety and tyre service life.
Owner:NOCIL
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com