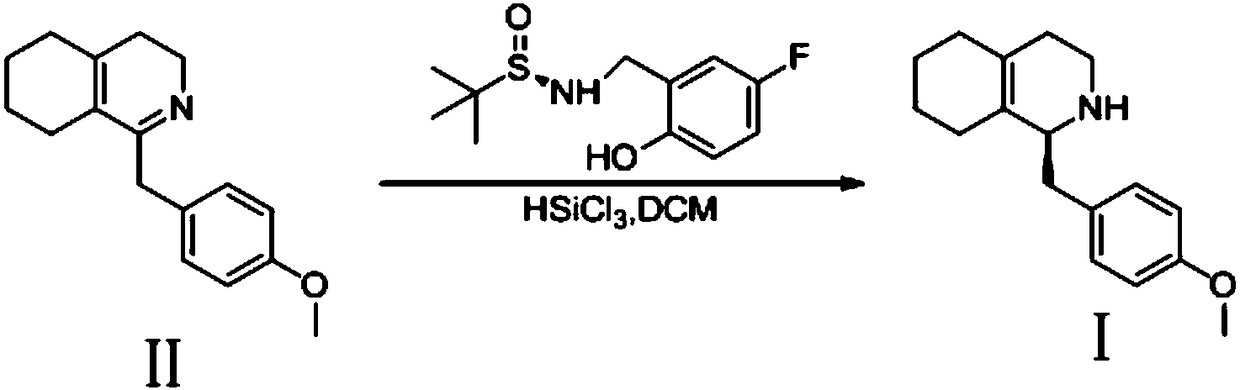
Method for preparing (S)-1-(4-methoxy benzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydro isoquinoline
A technology of methoxybenzyl and octahydroisoquinoline, applied in the directions of organic chemistry, organic chemistry, etc., can solve the problems of harsh reaction conditions, waste of raw materials, expensive catalysts, etc., and achieve the effect of cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: 1-(4-methoxybenzyl)-3,4,5,6,7,8-hexahydroisoquinoline (1.28g, 5mmol), (R)- N-(5-fluoro-2-hydroxybenzyl)-2-methylpropane-2-sulfinamide (0.13g, 0.5mmol) and 20mL redistilled dichloromethane, cooled to -20°C under nitrogen protection and stirred . Then trichlorosilane (1.35g, 10mmol) was slowly added dropwise into the reaction solution, keeping the temperature in the range of -20 to -15°C, the drop was completed in 0.5 hours, and the temperature was kept at -15°C to continue stirring the reaction. After 12h, slowly add saturated sodium bicarbonate solution until no bubbles are generated, continue stirring for 0.5h and then filter. After the filtrate was separated, the aqueous phase was extracted twice with 20 mL of dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The crude product was subjected to silica gel column chromatography to obtain 1.15 g of yellow oil, with a yie...
Embodiment 2
[0015] Example 2: 1-(4-methoxybenzyl)-3,4,5,6,7,8-hexahydroisoquinoline (1.28g, 5mmol), (R)- N-(5-fluoro-2-hydroxybenzyl)-2-methylpropane-2-sulfinamide (0.25 g, 1 mmol) and 20 mL redistilled dichloromethane were stirred at -20°C under nitrogen protection. Then trichlorosilane (1.35g, 10mmol) was slowly added dropwise into the reaction solution, keeping the temperature in the range of -20 to -15°C, the drop was completed in 0.5 hours, and the temperature was kept at -15°C to continue stirring the reaction. After 12h, slowly add saturated sodium bicarbonate solution until no bubbles are generated, continue stirring for 0.5h and then filter. After the filtrate was separated, the aqueous phase was extracted twice with 20 mL of dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. After using silica gel to pass through the column, 1.12 g of yellow oil was obtained, with a yield of 86.8% and an ee...
Embodiment 3
[0017] Example 3: 1-(4-methoxybenzyl)-3,4,5,6,7,8-hexahydroisoquinoline (1.28g, 5mmol), (R)- N-(5-fluoro-2-hydroxybenzyl)-2-methylpropane-2-sulfinamide (0.50 g, 2 mmol) and 20 mL redistilled dichloromethane were stirred at -20°C under nitrogen protection. Then trichlorosilane (1.35g, 10mmol) was slowly added dropwise into the reaction solution, keeping the temperature in the range of -20 to -15°C, the drop was completed in 0.5 hours, and the temperature was kept at -15°C to continue stirring the reaction. After 12h, slowly add saturated sodium bicarbonate solution until no bubbles are generated, continue stirring for 0.5h and then filter. After the filtrate was separated, the aqueous phase was extracted twice with 20 mL of dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The crude product was subjected to silica gel column chromatography to obtain 1.14 g of a yellow oil, with a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com