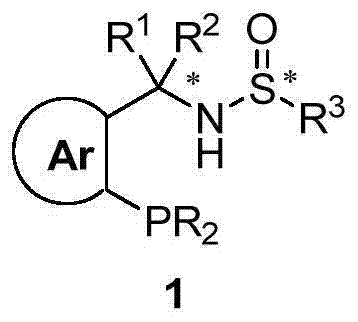
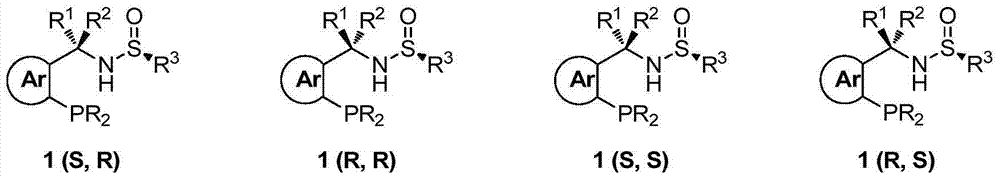
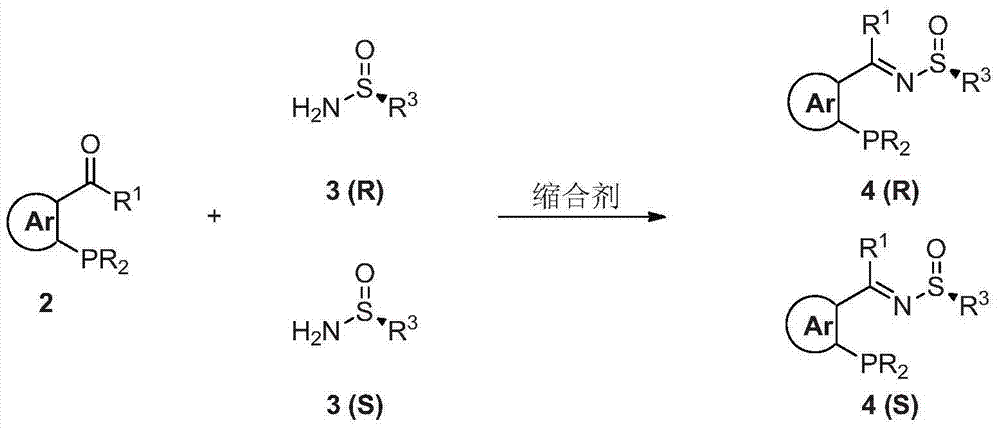
Chiral sulfinylamine monophosphine, and full-configuration preparation method and application thereof
A technology of sulfenamide and phosphine ligands, which is applied in the chemical industry and can solve the problems of long reaction time, expensive raw materials, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] The synthesis of embodiment 1 (Rs, R)-tert-butylsulfinyl-1-(2-diphenylphosphino) phenethylamine [1a (R, R)]
[0079]
[0080] Wherein, THF is tetrahydrofuran; N 2 is nitrogen; Ti( i PrO) 4 For tetraisopropyl titanate.
[0081] Refer to Route 1. The first step: In a 500mL three-necked flask, add 2-diphenylphosphinebenzaldehyde (50mmol) and (R)-(+)-tert-butylsulfinamide (50mmol), add 150mL under nitrogen atmosphere Add tetraisopropyl titanate (100 mmol) to THF, stir at 50° C. for 10 hours, and the yield is 85%. Infrared: 1087cm -1 .Proton NMR (400MHz, CDCl 3 ): δ1.08(s, 9H), 6.94-6.98(m, 1H), 7.23-7.48(m, 12H), 7.98-8.02(m, 1H), 9.11(d, 1H, J=4.8). Phosphine Spectrum NMR (160MHz, CDCl 3 ): δ-11.7. Mass Spectrum (FAB): m / z 394 (MH + ). Elemental analysis theoretical data C 23 h 24 NOPS: C, 70.21; H, 6.15; measured data N, 3.56.: C, 70.03; H, 6.27; N, 3.36.
[0082]
[0083] The second step: add the imine (1.91 g, 5 mmol) prepared in the first step into a ...
Embodiment 2
[0084] Synthesis of Example 2 (Rs, S)-tert-butylsulfinyl-1-(2-diphenylphosphino) phenethylamine [1a(R, S)]
[0085]
[0086] Refer to Route 1. Other operations refer to Example 1, the metal reagent used is methylmagnesium bromide, and the total yield is 60%. Proton NMR (400MHz, CDCl 3 )δ=7.58~7.51(m, lH), 7.41~7.18(m, 12H), 6.96~6.92(m, 1H), 5.49~5.46(m, 1H), 3.58(d, J=3.3Hz , 1 H), 1.38(d, J=6.6Hz, 3H), 1.13(t, 9H); carbon NMR (100MHz, CDCl 3 )δ=23.60, 24.56, 52.21, 53.56, 56.58, 126.10, 127.88, 128.05, 128.60, 129.49, 133.56, 133.99, 134.20; Phosphine NMR (162MHz, CDCl 3 )δ=-17.59ppm. High resolution mass spectrometry theoretical data C 24 h 28 NOPS: 409.5240; experimental data: 409.5233.
Embodiment 3
[0087] Synthesis of Example 3 (Ss, R)-tert-butylsulfinyl-1-(2-diphenylphosphino) phenethylamine [1a(S, R)]
[0088]
[0089] Refer to Route 1. Other operations refer to Example 1, the metal reagent used is methylmagnesium bromide, and the total yield is 60%. Proton NMR (400MHz, CDCl 3 )δ=7.58~7.51(m, 1H), 7.41~7.18(m, 12H), 6.96~6.92(m, 1H), 5.49~5.46(m, 1H), 3.58(d, J=3.3Hz, 1H) , 1.38(d, J=6.6Hz, 3H), 1.13(t, 9H); carbon NMR (100MHz, CDCl 3 )δ=23.60, 24.56, 52.21, 53.56, 56.58, 126.10, 127.88, 128.05, 128.60, 129.49, 133.56, 133.99, 134.20; Phosphine NMR (162MHz, CDCl 3 )δ=-17.59ppm. High resolution mass spectrometry theoretical data C 24 h 28 NOPS: 409.5240; experimental data: 409.5233.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com