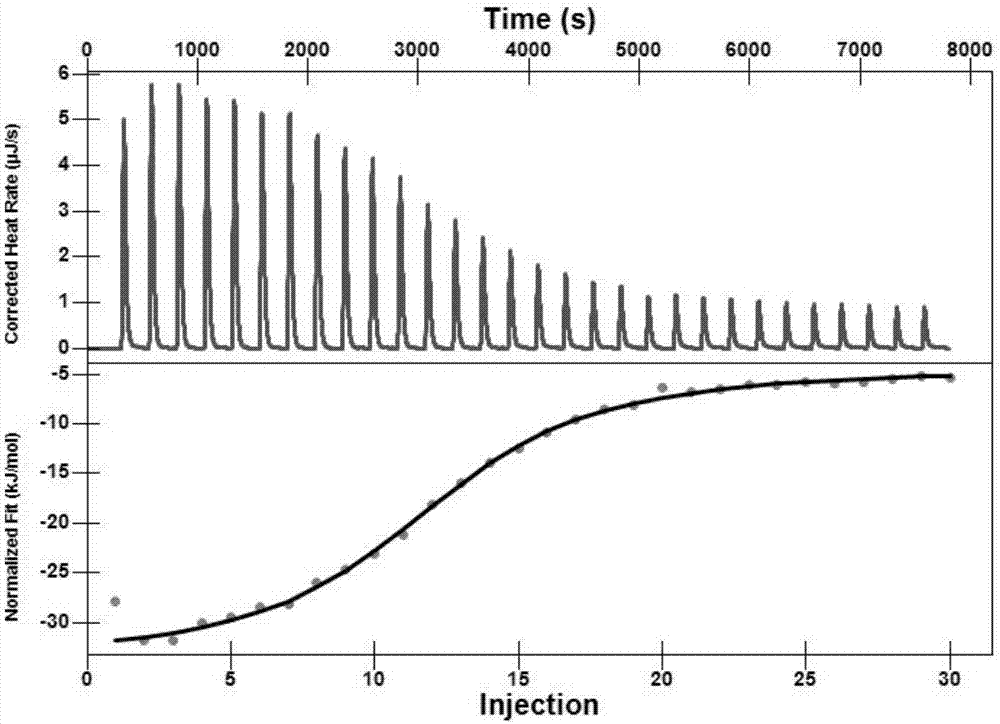
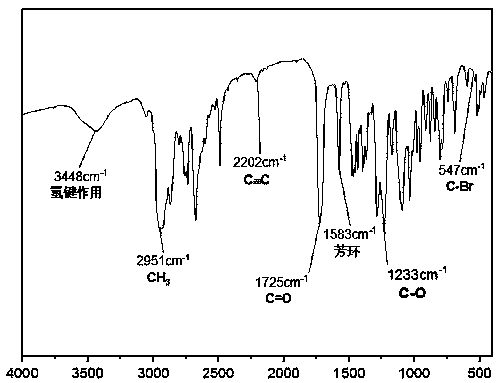
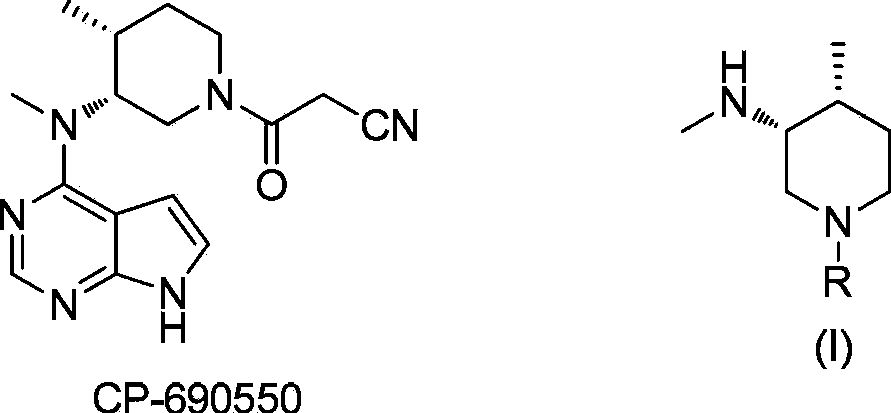
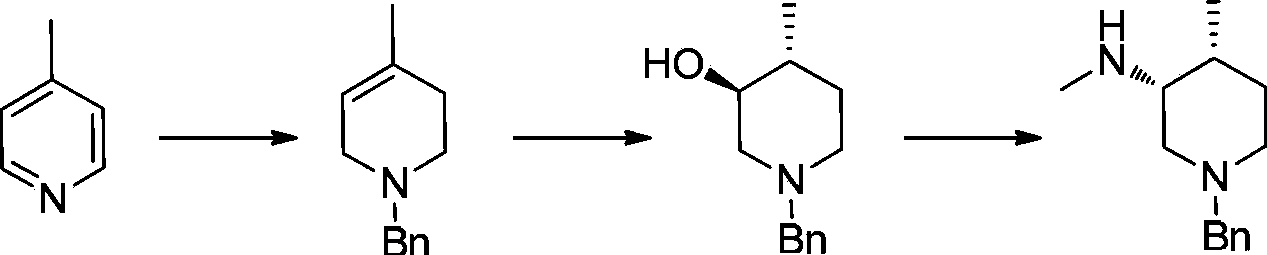
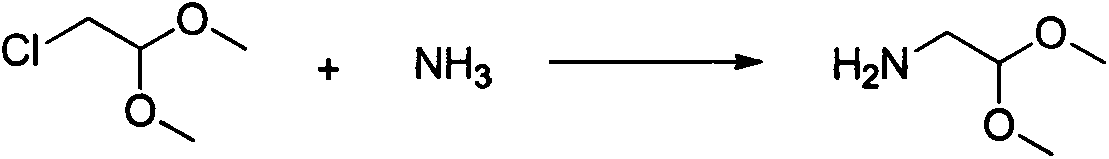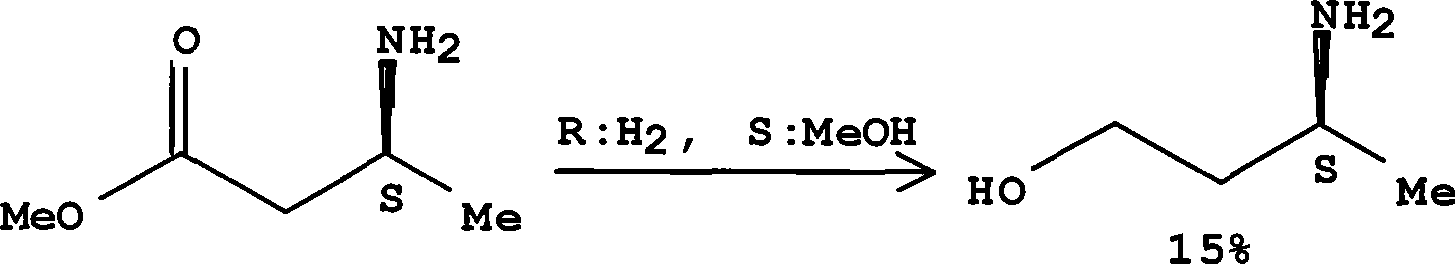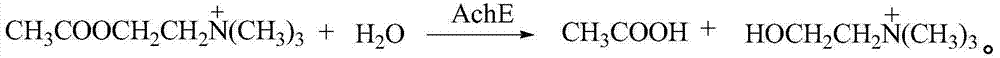Patents
Literature
143 results about "Phenethylamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons; to a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.
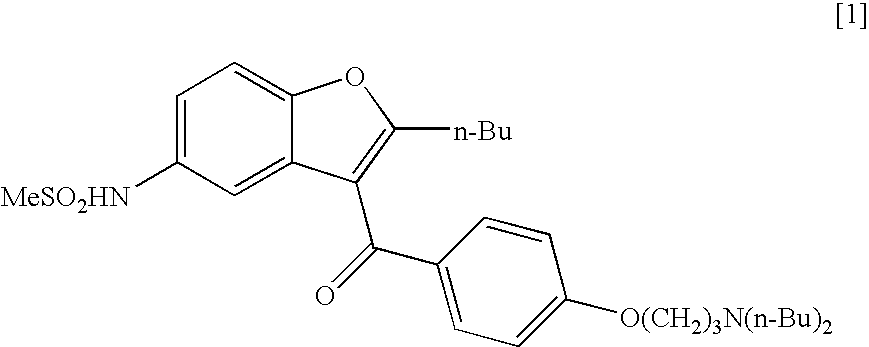
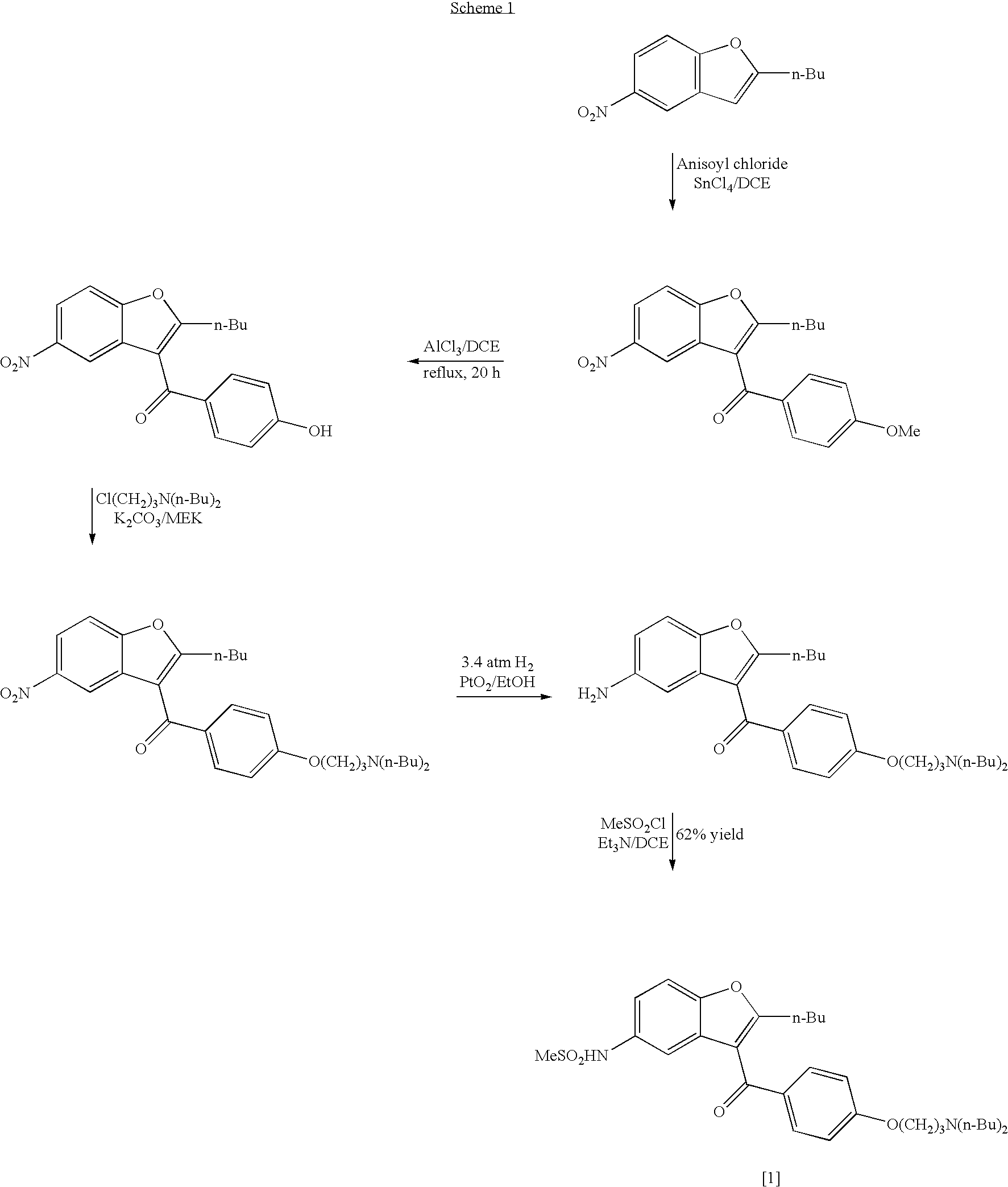
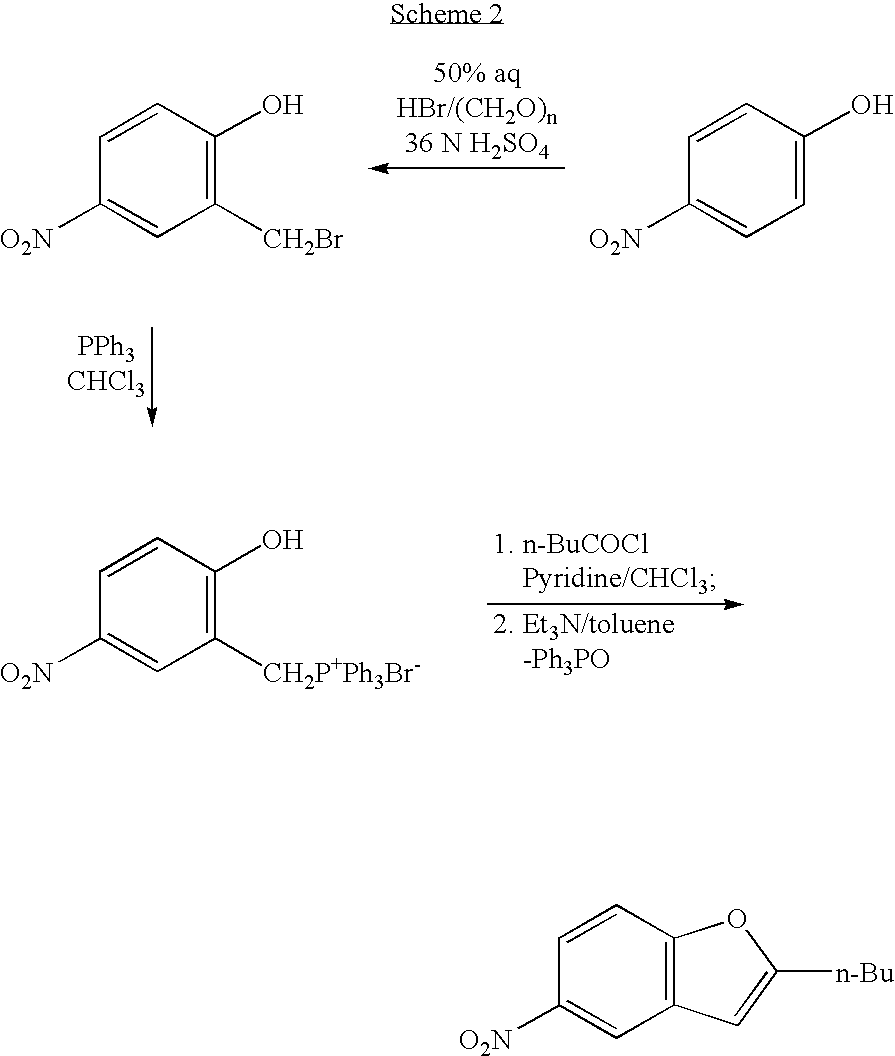
Process for the preparation of dronedarone
InactiveUS20050049302A1High yieldLow costBiocideCarbamic acid derivatives preparationAluminium chlorideDronedarone
The present invention provides, according to an aspect thereof, a novel process for the preparation of dronedarone [1] and pharmaceutically acceptable salts thereof. According to a preferred embodiment, the process comprises N-acetylating of p-anisidine or p-phenetidine with acetic anhydride, reacting of the obtained N-(4-alkoxyphenyl)acetamide with 2-bromohexanoyl chloride or bromide in the presence of aluminum chloride or bromide to obtain N-[3-(2-bromohexanoyl)-4-hydroxyphenyl]acetamide [6a], converting the compound [6a] into 2-butyl-5-benzofuranamine hydrochloride [12a] and subsequently converting [12a] into [1] or pharmaceutically acceptable salts thereof. In accordance with another aspect of this invention, there are provided novel intermediates, inter alia the novel compounds [6a] and [12a]. The novel intermediates of the present invention are stable, solid compounds, obtainable in high yields, which can be easily purified by crystallization and stored for long periods of time.
Owner:ISP INVESTMENTS LLC
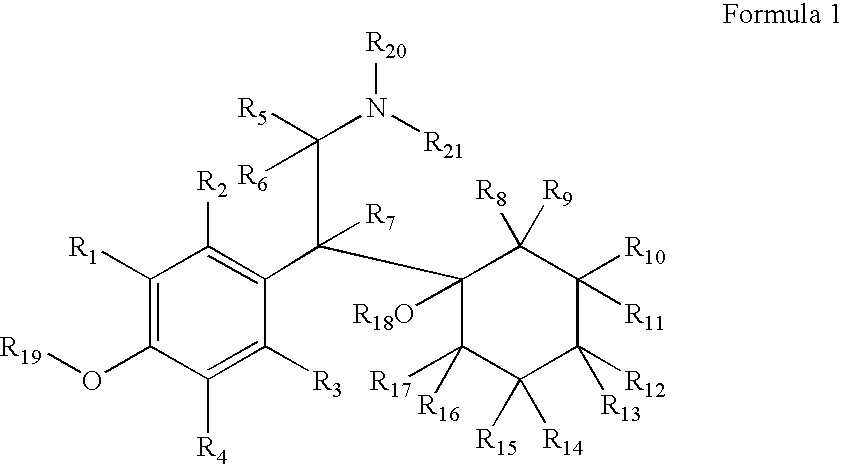
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Owner:ACADIA PHARMA INC
Phenylethylamine analogs and their use for treating glaucoma
Owner:ALCON INC
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:ACADIA PHARMA INC
Method for preparing praziquantel
InactiveCN103739601AThe synthesis process is simpleImproved post-treatment processOrganic chemistryDimethyl acetalCyclohexanecarboxylic acid
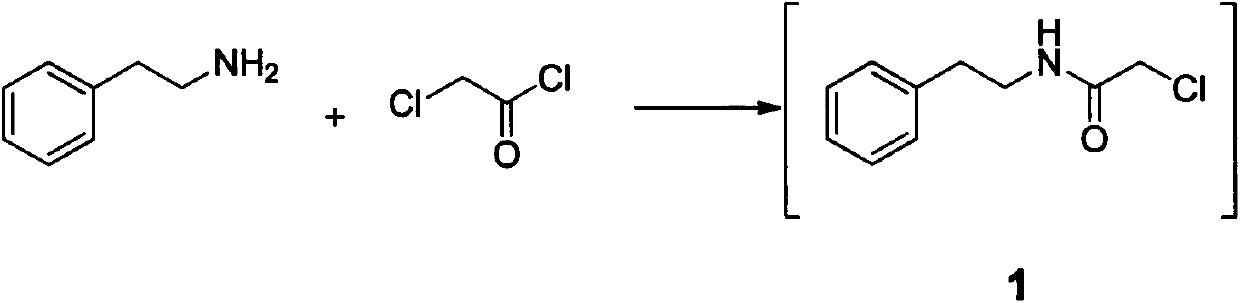
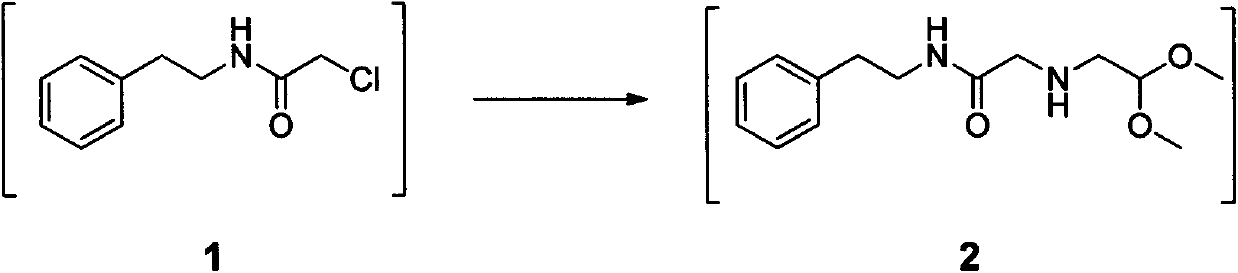
The invention relates to a method for preparing praziquantel, which is a one-pot method and comprises the following steps: performing an ammonolysis reaction of chloroacetaldehyde dimethyl acetal and an ammonia aqueous solution to generate aminoacetaldehyde dimethyl acetal; performing a condensation reaction of beta-phenylethylamine and chloroacetyl chloride in an organic solvent in alkaline environment to generate an intermediate 1; performing a condensation reaction of the intermediate 1 and the aminoacetaldehyde dimethyl acetal in an organic solvent to generate an intermediate 2; performing cyclization of the intermediate 2 in the presence of an acidic catalyst to generate an intermediate 3; performing a reaction of the intermediate 3 and cyclohexanecarboxylic acid chloride in an organic solvent in alkaline environment, and performing solvent crystallization to obtain the target product of praziquantel.
Owner:JIANGSU CHENGXIN PHARMA
Method for preparing optically pure 3-amino butyl alcohol
ActiveCN101417954ASuitable for industrial productionStereoselectiveOrganic compound preparationAsymmetric synthesesAcetoacetatesPotassium borohydride
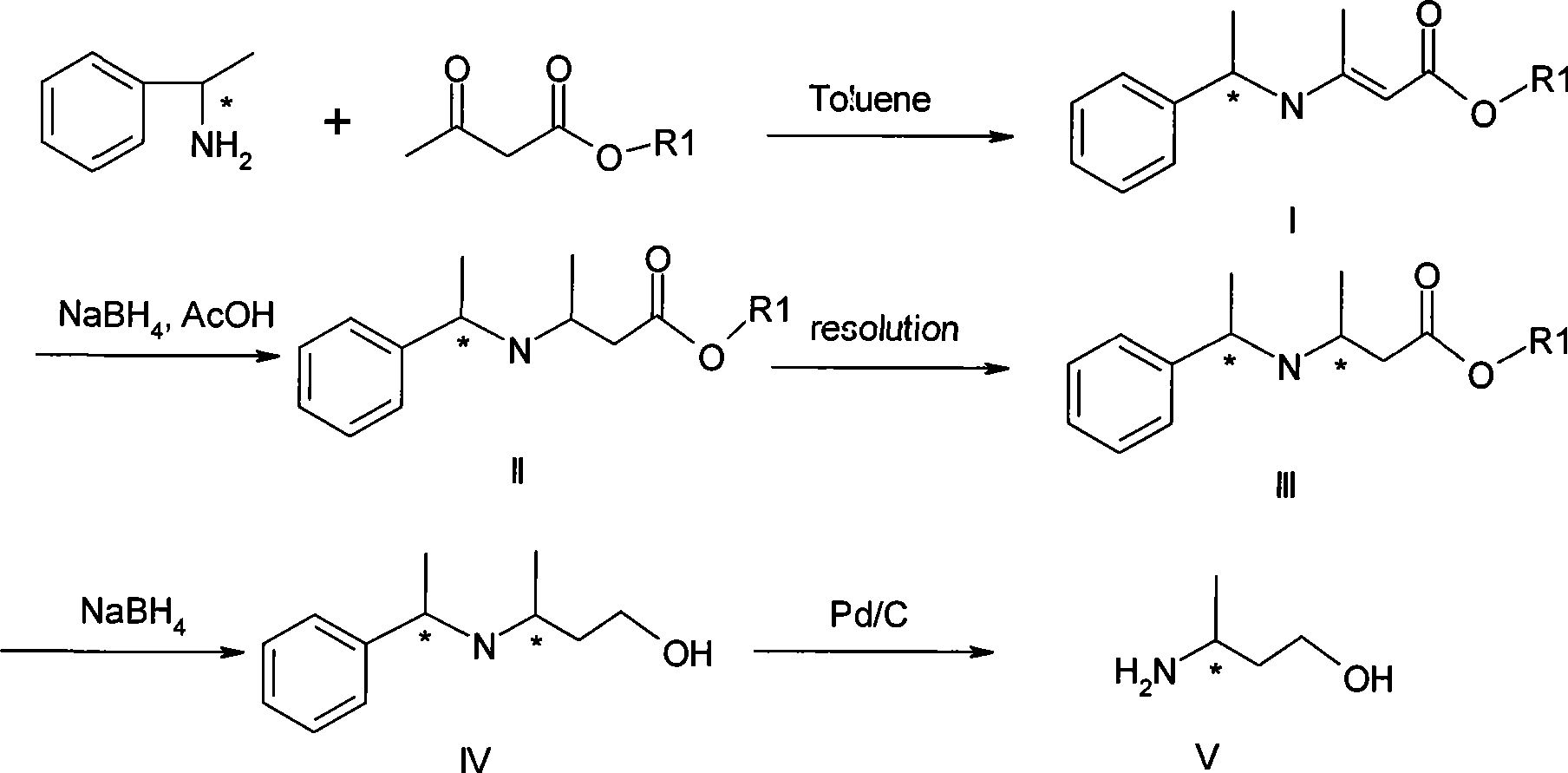
The invention discloses a method for preparing optical pure 3-aminobutanol, which comprises the following steps: acetylacetic ester and chiral phenethylamine react to generate 3-(1'-benzylmethylamine)-2-crotonate enantiomer, namely 3-(1'-benzylmethylamine)-2-crotonate; potassium borohydride triacetate or sodium borohydride triacetate is used to reduce the 3-(1'-benzylmethylamine)-2-crotonate enantiomer into a 3-(1'-benzylmethylamine)-2-butyric ester enantiomer; then chiral pure 3-(1'-benzylmethylamine)-2-butyric ester is obtained through salification and resolution; and the 3-(1'-benzylmethylamine)-2-butyric ester is subject to reducing debenzylation by palladium-carbon to obtain the optical pure 3-aminobutanol. The method has low cost and high product purity, and is suitable for industrialized production.
Owner:ABA CHEM SHANGHAI +1
Omega-transaminase from bacillus pumilus and application in biological amination
ActiveCN109402188AIncrease productionCannot meet the requirements of industrial applicationTransferasesMicroorganism based processesEscherichia coliBacillus pumilus
The invention discloses Omega-transaminase from bacillus pumilus and application in biological amination, and belongs to the technical field of bioengineering. In the invention, an Omega-transaminasegene is separated and identified from bacillus pumilus for the first time, and by adopting an escherichia coli recombination expression system to produce codon optimized Omega-transaminase, the yieldcan be remarkably increased, and the requirements of industrial application are met more easily. The invention discovers that the most proper action pH of the Omega-transaminase is 7.0 and the most proper action temperature is 45 DEG C when (R)-Alpha-phenethylamine serves as a substrate; meanwhile, the R-Omega-transaminase has excellent pH stability and temperature stability, and the catalytic activity on the experimental group (taking (R)-Alpha-phenethylamine as a substrate) is higher than that on the control group (taking (S)-Alpha-phenethylamine as a substrate), indicating that the recombinant Omega-transaminase has a function of synthesizing R-chiral amine with perfect selectivity and has relatively great application potential.
Owner:JIANGNAN UNIV
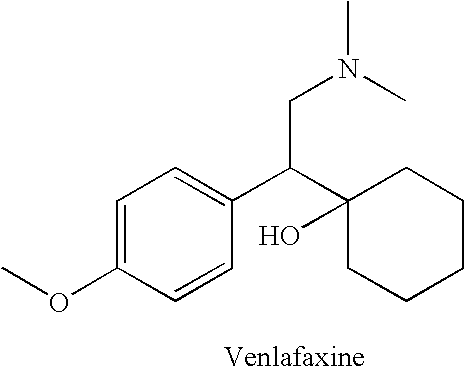
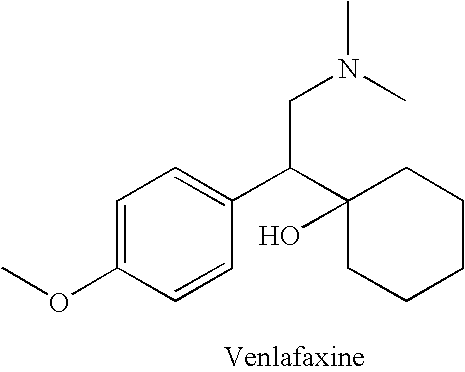
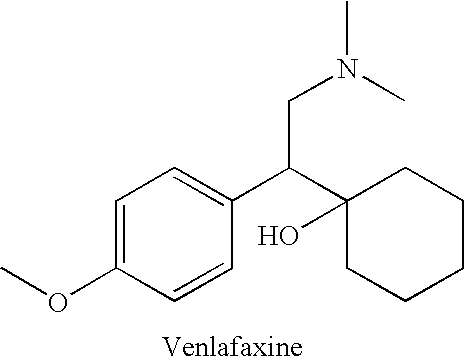
Process for the preparation of phenethylamine derivative, an intermediate of Venlafaxine hydrochloride
InactiveUS20050033088A1High yieldOrganic compound preparationCarboxylic acid amides preparationPalladium on carbonPhenethylamine derivative
The present invention relates to an improved process for the preparation of phenethylamine derivatives or salts by hydrogenation of phenylacetonitriles in the presence of heterogeneous palladium on carbon catalyst.
Owner:DR REDDYS LAB LTD +1
Optical active compound of 1-(3-benzoyloxy-propyl)-5-(2-(1-phenyl ethyl amine) propyl-7-cyano indoline as well as preparation method and application thereof
The invention provides an optical active compound of 1-(3-benzoyloxy-propyl)-5-(2-(1-phenyl ethyl amine) propyl-7-cyano indoline as well as a preparation method and the application thereof. The optical active compound is shown in a formula (1), comprises a (R,R) configuration and a (S,S) configuration and can be used as an intermediate for synthetizing silodosin. The optical active compound of the single formula (1) can be used for preparing an optical high-purity product and has good yield on the premise of ensuring good optical purity. The preparation method of the optical active compound uses low-cost chiral assistant agent of alpha-phenethylamine and derivatives thereof, has mild reaction conditions, low cost and controllable optical purity and is easy for industrialized production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
Process for producing S-tetrahydrochysene furoic acid
ActiveCN101429180AEasy to recycleGood crystal formOptically-active compound separationOrganic racemisationDiastereomerAmmonia gas
The invention discloses a method for preparing S-tetrahydrofurfuryl acid. The method comprises the following steps: using R-phenethylamine and racemic tetrahydrofurfuryl acid to obtain tetrahydrofurfuryl acid phenethylamine salt, and obtaining pure diastereoisomer salt after the tetrahydrofurfuryl acid phenethylamine salt is refined; after ammonia gas is introduced into the salt, obtaining tetrahydrofurfuryl acid ammonia salt, and reclaiming phenethylamine; and adjusting pH value of the tetrahydrofurfuryl acid ammonia salt to be acidic, extracting free tetrahydrofurfuryl acid by using a continuous extraction method, and decompressing and distilling the free tetrahydrofurfuryl acid to obtain the optically pure finished product of the S-tetrahydrofurfuryl acid. Moreover, a by-product containing the R-tetrahydrofurfuryl acid, which is generated after separated mother liquor is dissociated by the ammonia gas, is racemized by alkali to obtain the racemic tetrahydrofurfuryl acid which can be used as a raw material for reutilization. The method has the advantages of low cost and high purity of the product, and is suitable for industrialized production.
Owner:ABA CHEM CORP
Double perovskite single crystal photodetector and preparation method thereof
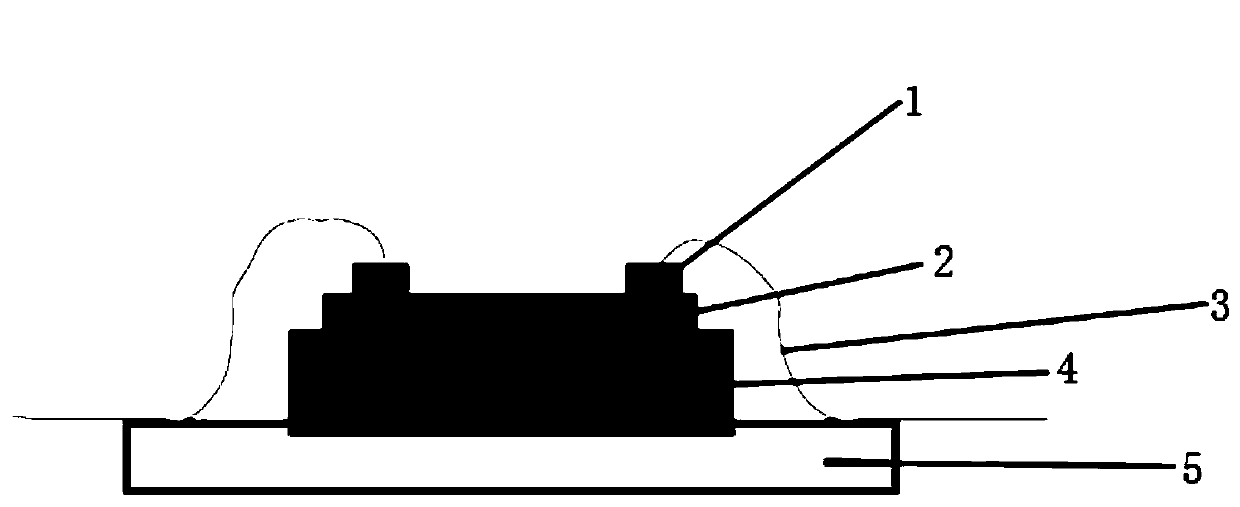
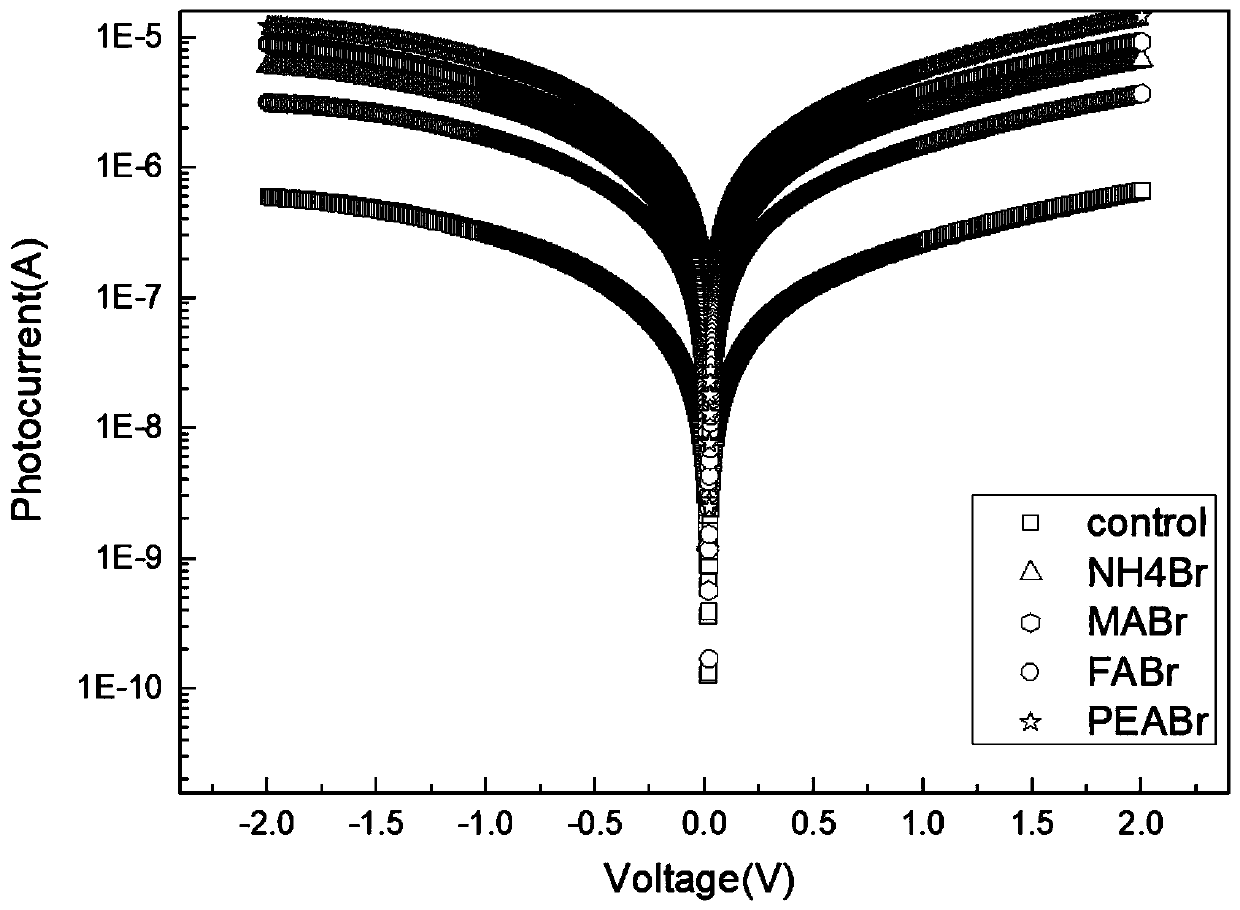
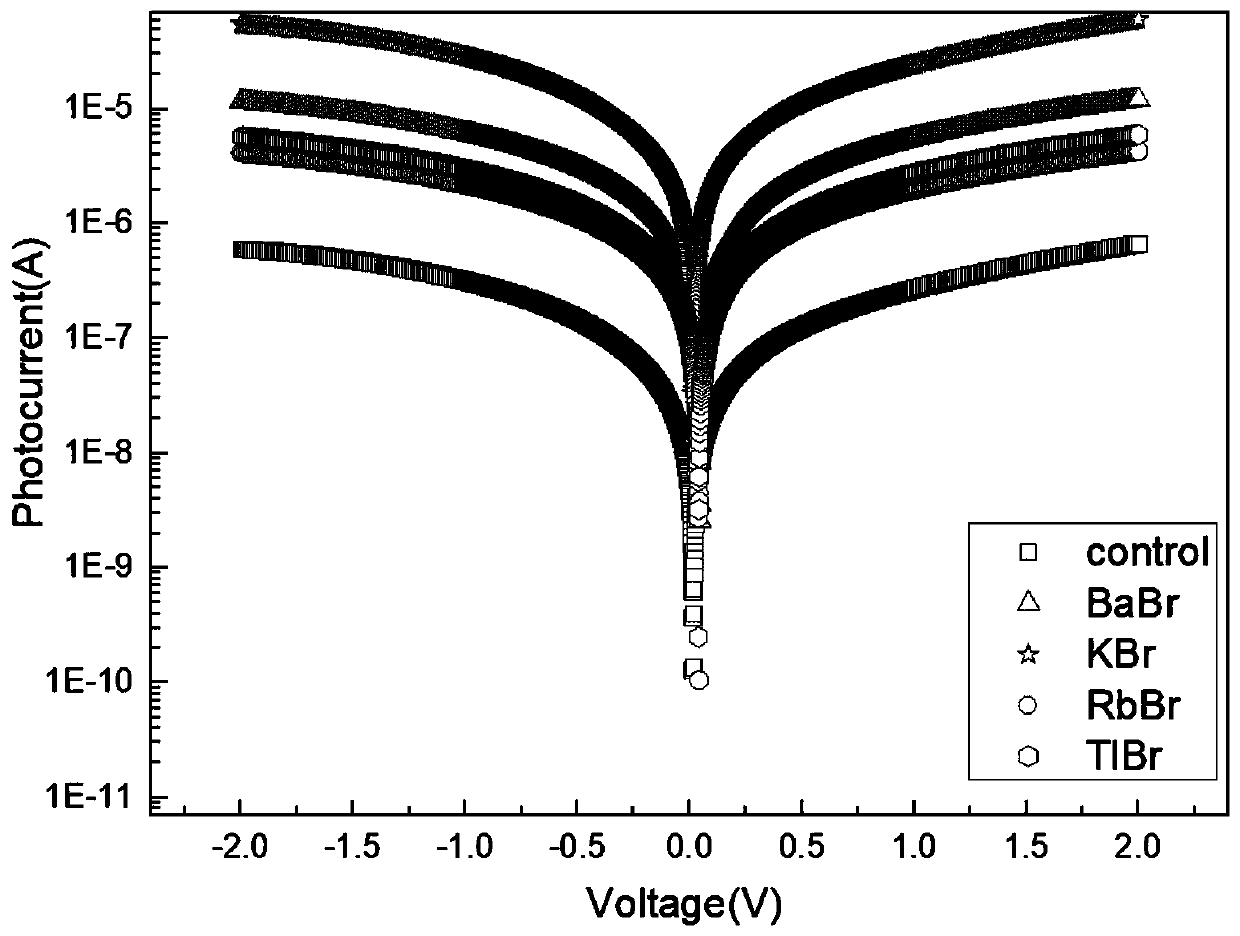
InactiveCN109786486AEasy to detectIncrease the carrier concentrationFinal product manufactureSemiconductor devicesPhotovoltaic detectorsPhotodetector
The invention discloses a double perovskite single crystal photodetector and a preparation method thereof. The double perovskite single crystal photodetector comprises a substrate, wherein the substrate is provided with a double perovskite single crystal, electrodes and a silver glue in sequence, the two electrodes are respectively connected to a conductive gold wire, the double perovskite singlecrystal is in a double perovskite structure formed by adding different cations in a solution of the perovskite growth single crystal, and each of the added cations is one of methylamine ion (MA+), formamidine ion (FA+), phenethylamine ion (PEA+), NH4+, butylamine ion (Ba+), K+, Rb+ and Tl+. By doping different cations in the double perovskite single crystal photodetector, the carrier concentrationof the device is increased under illumination conditions, which leads to an increase in photocurrent and enhances the detection performance of the photodetector.
Owner:JINAN UNIVERSITY
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:ACADIA PHARMA INC
Synthesis process of palmatine and its salts
InactiveCN1733763ARaise quality standardsContent increased and stabilizedOrganic chemistryBenzeneAcetonitrile
The invention discloses a synthesis process of palmatine and its salts mainly comprising the following steps, (1) etherification, preparation of o-dimethoxybenzene, (2) acetonitrilizaiton, preparation of methylenedioxy benzene acetonitrile, (3) hydrogenization, preparation of o-dimethoxy-phenethylamine, (4) condensation, preparation of condensate hydrochlorates, (5) recondensation, preparation of palmatine, (6) preparation of corresponding salts from palmatine with related acids.
Owner:余娟
Formulation for enhanced delivery of phenethylamine
InactiveUS20070190187A1Promote absorptionTo promote metabolismBiocideOrganic chemistryPhenethylamineInternal medicine
The present invention is directed to a formulation comprising phenethylamine or a derivative thereof, and an MAO-B enzyme inhibitor. The present invention is also directed to a method of increasing the absorption of phenethylamine or a derivative thereof, into the bloodstream, cells and tissue. The method includes administering phenethylamine or a derivative thereof, in combination with an MAO-B enzyme inhibitor.
Owner:KNELLER BRUCE W +1
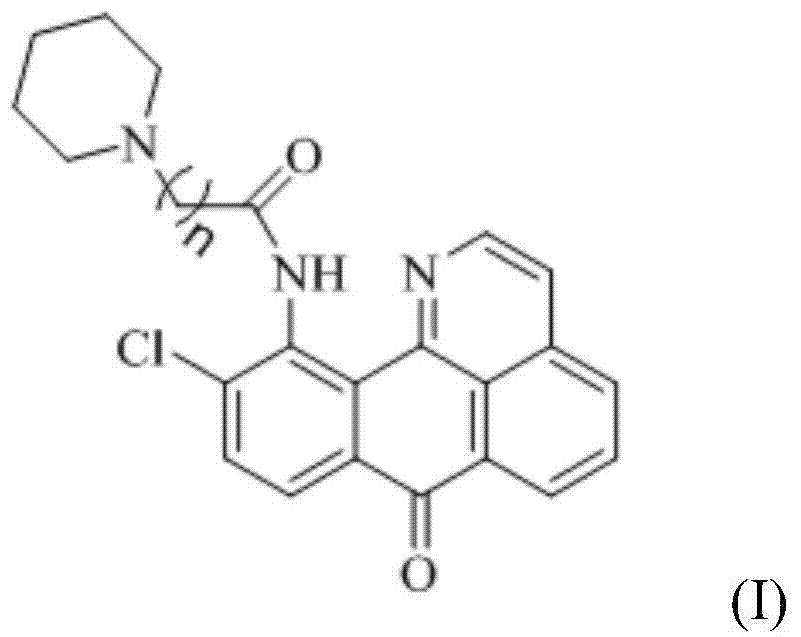
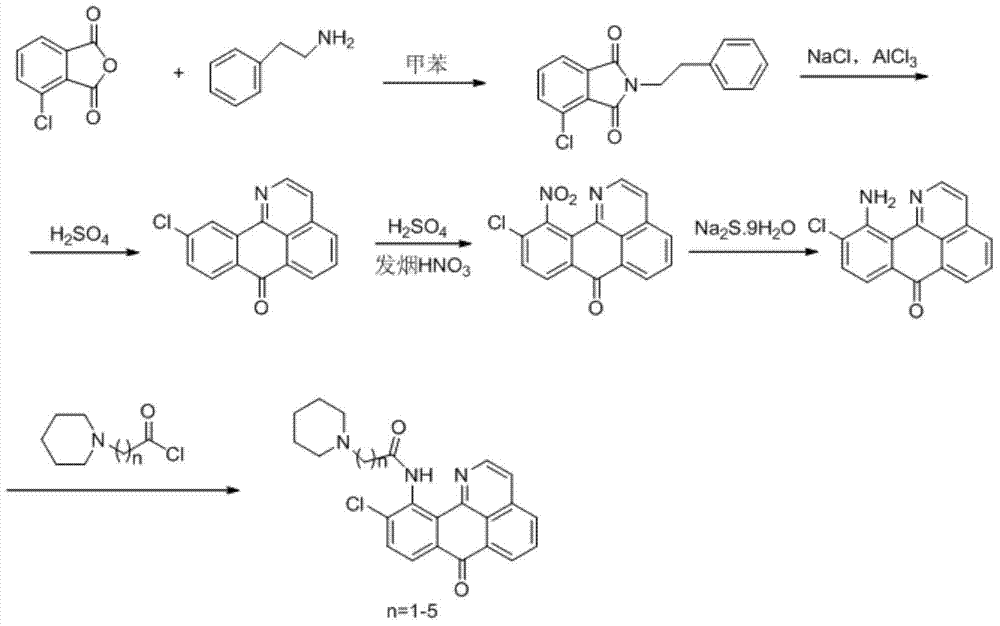
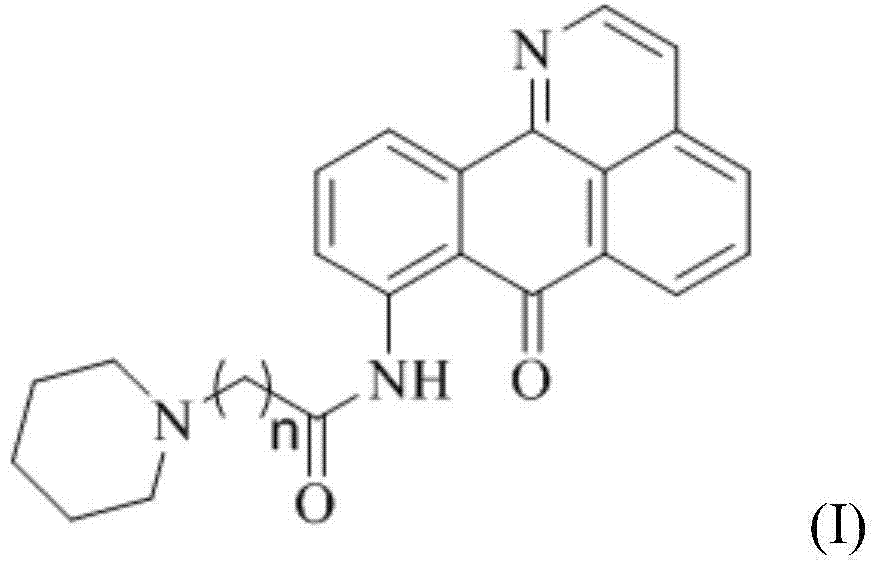
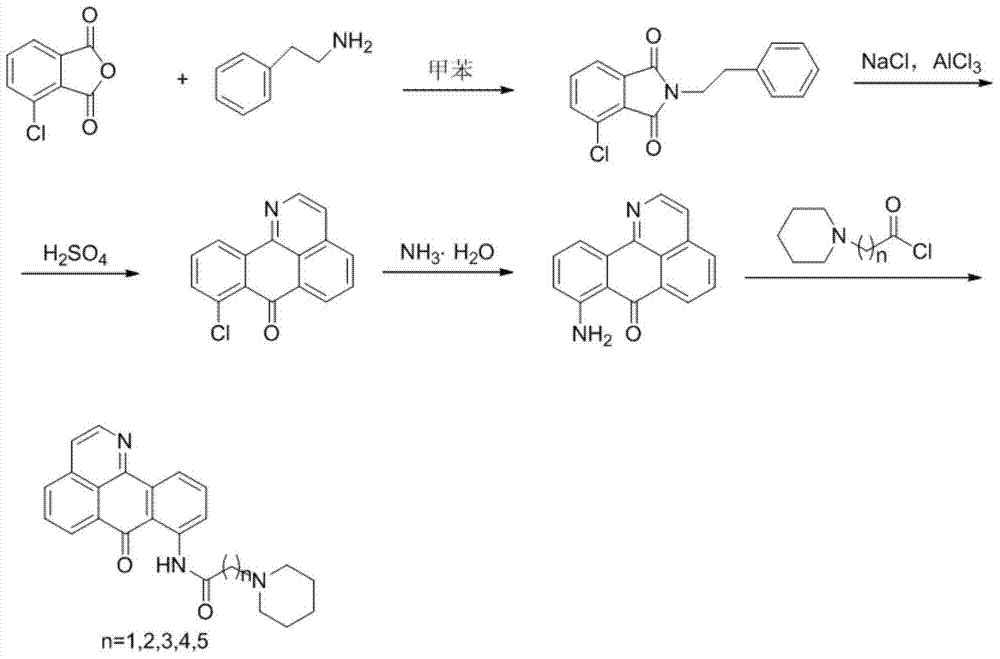
11-replaced oxoisoaporphine derivatives as well as synthetic method and application thereof
InactiveCN103923010AStrong inhibitory activityGood potential medicinal valueOrganic active ingredientsSenses disorderKetoneStructural formula
The invention discloses a series of 11-replaced oxoisoaporphine derivatives as well as a synthetic method and an application thereof. The synthetic method comprises the following steps: (1) carrying out ring closing reaction on 3-chlorophthalic anhydride and phenylethylamine as raw materials so as to construct a 10-Cl-1-azabenzanthrone parent body; (2) nitrating the parent body compound so as to obtain a 11-site nitrated product, and reducing the 11-site nitrated product so as to obtain 11-amino-10-chlorine-7H-dibenzoquinoline-7-ketone; and (3) reacting the 11-amino-10-chlorine-7H-dibenzoquinoline-7-ketone with an acyl chloride compound connected with piperidine so as to obtain a corresponding target product. Through study, the applicant finds that the series of derivatives have very strong inhibitory activity on acetylcholin esterase and are expected to be used for treating AD (Alzheimer Disease), cerebrovascular dementia and related diseases caused by cholinergic neurotransmitter reduction. The structural formula of the 11-replaced oxoisoaporphine derivatives is shown in descriptions.
Owner:GUANGXI NORMAL UNIV
1-phenyl-1, 2, 3, 4-tetrahydroisoquinoline preparation method
ActiveCN103159677AAvoid pollutionAvoid it happening againOrganic compound preparationCarboxylic acid amides preparationBenzoic acidSolvent
The invention provides a 1-phenyl-1, 2, 3, 4-tetrahydroisoquinoline preparation method. The 1-phenyl-1, 2, 3, 4-tetrahydroisoquinoline preparation method comprises the following steps of: mixing benzoyl chloride or benzoic acid, phenethylamine and alkali metal hydroxide with water, and reacting to obtain N-(2-phenethyl) benzamide; then mixing the N-(2-phenethyl) benzamide with phosphorus pentoxide, chloride phosphorus and a benzene solvent, heating and reacting to obtain 1-phenyl-3, 4-dihydro-isoquinoline; and further mixing the 1-phenyl-3, 4-dihydro-isoquinoline with a first alcohol solvent and hydroboron, and reacting to obtain the product. Compared with the prior art, the 1-phenyl-1, 2, 3, 4-tetrahydroisoquinoline preparation method has the advantages that firstly, no organic solvents are added, the product N-(2-phenethyl) benzamide is insoluble in an aqueous solution, and therefore, the steps of skimming and the like are avoided in the after-treatment process, and the after-treatment operation is simplified; secondarily, as the organic solvents are not added, the cost is reduced, and the pollution to environments is avoided; and thirdly, as the phosphorus pentoxide and the chloride phosphorus are subjected to oxidization cyclization reaction, polyphosphoric acids are prevented from being heated and decomposed to generate hypertoxic phosphorus oxide exhaust gas.
Owner:SHENGQUAN HEALTANG
Fishy flavor agent for feed and preparation method of fishy flavor agent
The invention discloses a fishy flavor agent for a feed and a preparation method of the fishy flavor agent. The fishy flavor agent contains the following perfume materials in percentage by weight: 0.5-2% of glycine betaine, 1-3% of trimethylamine, 0.1-2% of 2-methyl-3-sulfydrylfuran, 0.1-1% of pyrrolidine, 0.1-1% of piperidine, 0.1-2% of phenylethylamine, 0.01-1% of 2-acetylpyridine, 0.1-1% of furanone, 1-3% of ethyl maltol, 1-3% of 2-acetylpyrazine, 1-3% of 2,3,5-trimethylpyrazine, 0.01-2% of 3-methylthiopropanal, 30-70% of fishy smell reactants and 10-60% of glycerol triacetate. The fishy flavor agent is prepared by carrying out enzymolysis on animal wastes, then adding reducing sugars to carry out Maillard reaction, and then, adding artificial synthetic essence to blend. The fishy flavor agent has the advantages that the animal wastes are reasonably utilized, the preparation process is simple, and the product is strong in fragrance, stable and easy to store.
Owner:成都大帝汉克生物科技有限公司
Process for the preparation of substituted pyrrolidine derivatives and intermediates
ActiveUS20060128789A1Easy to operateHigh yieldBiocideOrganic chemistryCombinatorial chemistryPyrrolidine
Two syntheses are provided; one for the preparation of (3R,4R)-4-(hydroxymethyl)pyrrolidin-3-ol, and other for the preparation of (3S,4R)-4-(hydroxymethyl)pyrrolidin-3-ol. (3R,4R)-4-(hydroxymethyl)pyrrolidin-3-ol is prepared using the achiral ylide prepared from benzylamine instead of phenethylamine (Scheme 3) which provides a crystalline intermediate. The synthesis of (3S,4R)-4-(hydroxymethyl)pyrrolidin-3-ol is achieved from (S)-diethylmalate as described in Scheme 4. A process for preparing camphor sultam is also provided.
Owner:BIOCRYST PHARM INC
Preparation method of R-5-(2-Aminopropyl)-2-methoxybenzenesulfonyl with high optically active purity
InactiveCN101037402AOperational securityMild reaction conditionsSulfonic acid amide preparationAsymmetric synthesesOrganic solventHydrogenation reaction
A producing method for a R-5-(2-aminopropyl)-2-methoxybenzsulfamide is provided, which is including: catalyzed by Pd / C, doing an ammoniation hydrogenation reaction in the organic solvent making use of the chiral phenethylamine and 2-methoxy-5-(2-oxopropyl)benzsulfamide to get a product and acidifying to a hydrochlorate; removing the eshyl phenyl in a condition of hydrogen to get a R-5-(2-aminopropyl)-2-methoxybenzsulfamide hydrochlorate; further reacting with alkali to produce R-5-(2-aminopropyl)-2-methoxybenzsulfamide. The producing method is simple with a good yield and can obtain the product with a high optical purity and can be an industrial production method as the intermediate of the tamsulosin medicine.
Owner:ZHEJIANG UNIV
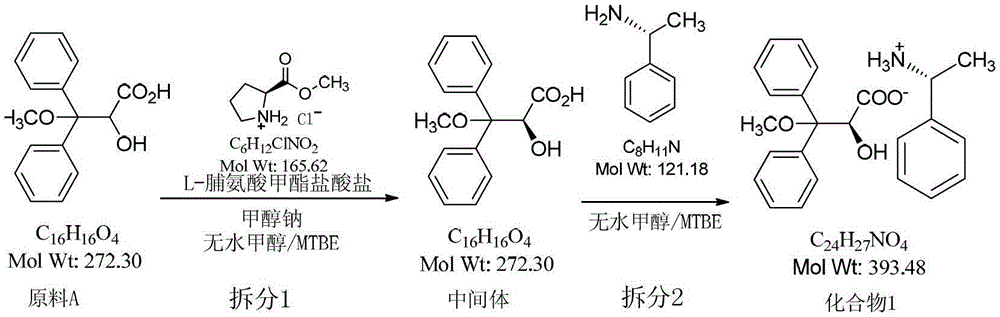
Resolution method of 2-hydroxy-3-methoxy-3,3-dibenzylpropionic acid racemate
ActiveCN104098462AGood reproducibilityEase of industrial implementationOrganic compound preparationOrganic chemistry methodsHydrochlorideReagent
The invention relates to a resolution method of 2-hydroxy-3-methoxy-3,3-dibenzylpropionic acid racemate. The method comprises the following steps: reacting racemic acid with an optical alkali, and separating non-enantiomeric salts of acid and alkali. L-proline methyl ester hydrochloride and R-(+)-alpha-phenylethylamine are used as optically active alkalis. Highly pure target compounds can be obtained in a high yield mode by using an efficient and cheap resolution reagent, and the method has good reappearance in industrial production and has a wide industrial application prospect.
Owner:CHANGZHOU HANSOH PHARM CO LTD +1
Method for recovering pregabalin intermediate resolving agent (R)-(+)-alpha-phenylethylamine
ActiveCN104086439AEmission reductionSimple and fast operationAmino compound purification/separationOrganic solventDistillation
The invention discloses a method for recovering pregabalin intermediate resolving agent (R)-(+)-alpha-phenylethylamine. The method comprises the following steps: a) adding an alkali to free mother liquor at a certain temperature for regulating the pH until the mother liquor is alkaline; b) adding an organic solvent to the system of which the pH is regulated previously for extraction; and c) blending the organic layer, carrying out reduced pressure distillation at a relatively low temperature to remove an extracting agent first, collecting the preceding fraction and then heating to carry out reduced pressure distillation again, thereby obtaining a fraction, namely the resolving agent (R)-(+)-alpha-phenylethylamine. The method has the advantages that the atom utilization rate is increased, the environmental pollution due to direct emission of materials in the mother liquor is avoided, and the production cost is greatly reduced, and the method has the characteristics of green chemistry. In a word, the method for recovering and recycling the (R)-(+)-alpha-phenylethylamine is green and environment-friendly, and low in cost and pollution.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Nesting method for pregabalin intermediate mother liquor
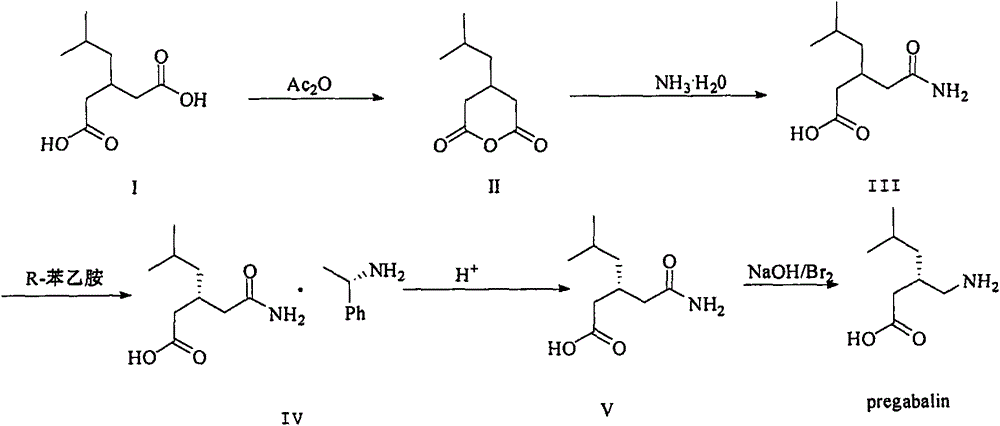
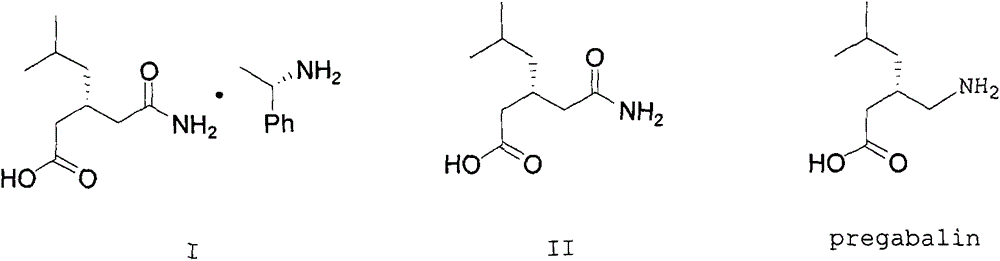
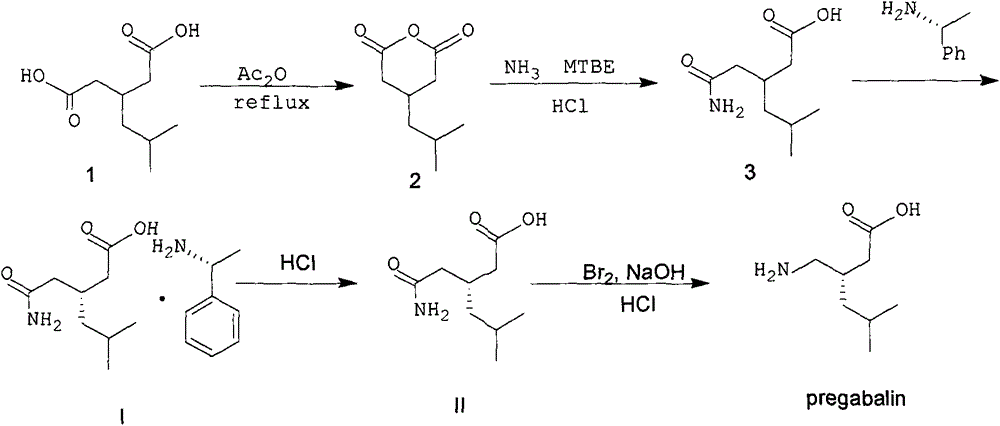
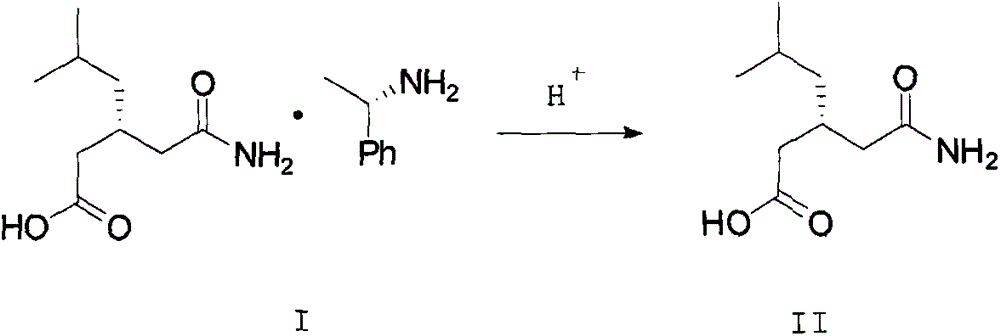
ActiveCN103980144AEmission reductionSimple and fast operationOrganic compound preparationCarboxylic acid amides preparationPregabalinSolvent
The invention discloses a nesting method for a mother liquor of a free pregabalin intermediate (R)-(-)-3-(carbamyl methyl)-5-methylhexanol-(R)-(+)-alpha-phenethylamine salt. The method comprises the following steps: (1) feeding a phenethylamine salt (R)-(-)-3-(carbamyl methyl)-5-methylhexanol-(R)-(+)-alpha-phenethylamine salt to the mother liquor filtered by dissociation in the procedure, and adding a certain amount of solvent to agitate, heat and dissolve; (2) cooling, dropwise adding an acid to adjust the pH; (3) devitrifying at certain temperature, filtering, wherein the filtrate is the mother liquor, and baking the filter cake to obtain (R)-(-)-3-(carbamyl methyl)-5-methylhexanol. The atom utilization rate of the reaction is improved, environmental pollution caused by direct emission of the material in the mother liquor is avoided, the nesting method is mild in reaction condition, has no demands on special equipment and instrument and has green chemistry characteristics, and the production cost is greatly reduced.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
8-substitued oxoisoaporphine derivatives as well as synthetic method and application thereof
InactiveCN103923009AStrong inhibitory activityGood potential medicinal valueOrganic active ingredientsSenses disorderDiseaseKetone
Owner:GUANGXI NORMAL UNIV
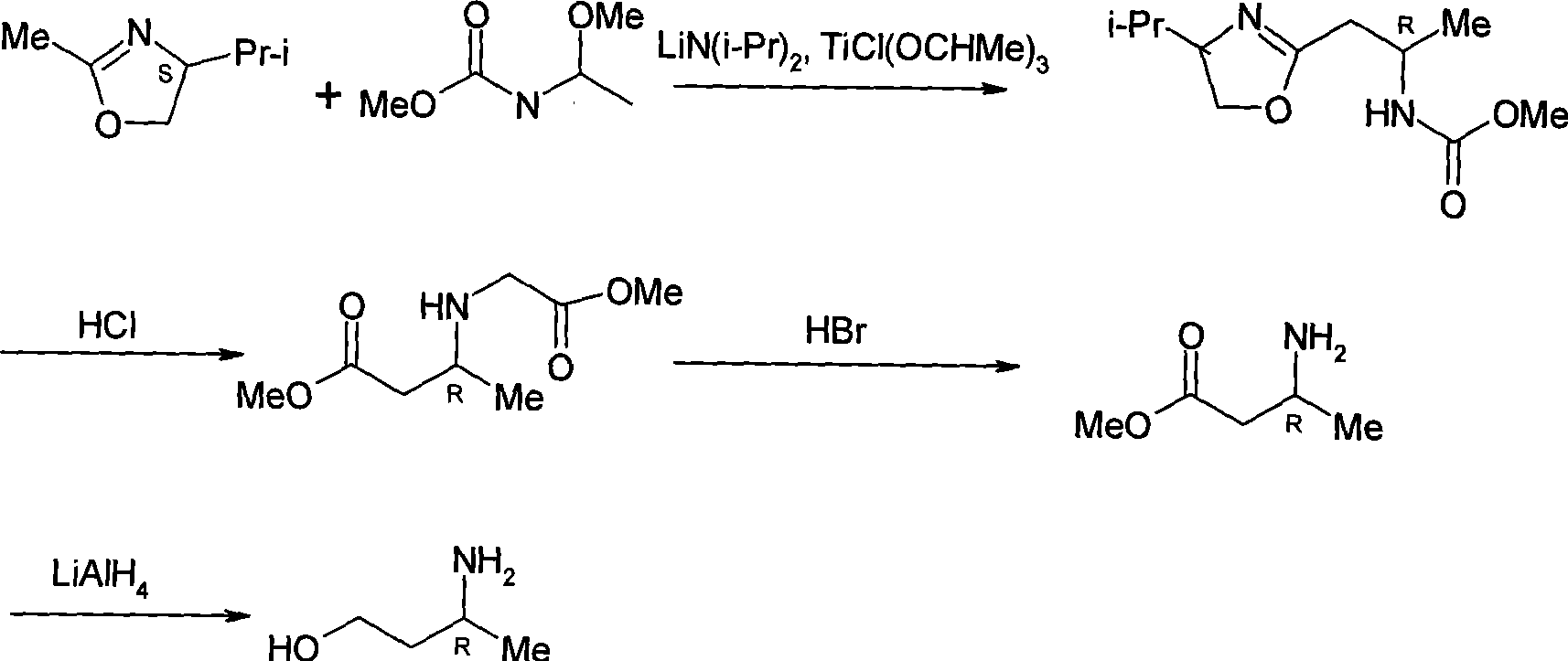
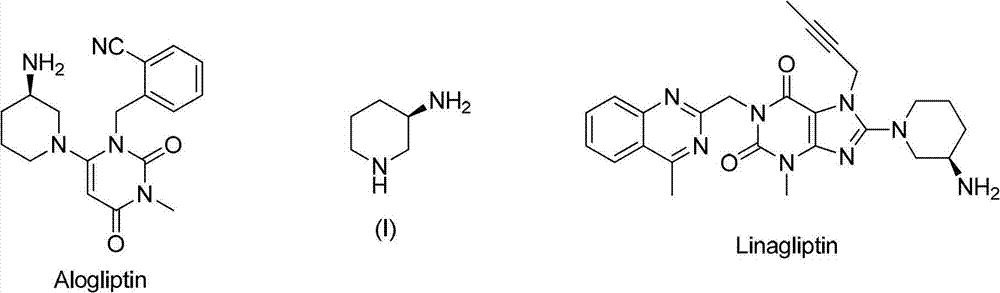
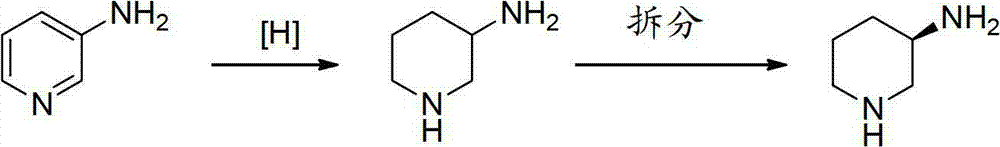
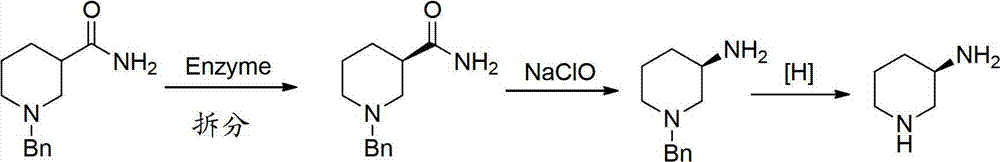
Asymmetric syntheses method and correlated intermediate of (R)-3-aminopiperidine (I)
ActiveCN103588699AHigh enantiopurityEasy to operateOrganic chemistryBulk chemical production1-phenylethanamineSynthesis methods
The invention relates to an asymmetric syntheses method of (R)-3-aminopiperidine (I). The method comprises: reducing a formula (III) compound to obtain a formula (II) compound, and then removing a chiral prosthetic group and an amino protective group from the formula (II) compound to obtain (R)-3-aminopiperidine (I), wherein R is the amino protective group, and specially is C1-4 alkoxycarbonyl or benzyl removable by hydrolysis or hydrogenation. Preferably, the formula (III) compound is obtained by performing a dehydration reaction on 3-piperidone and a chiral amine (R)-1-phenylethylamine. The invention also relates to a new compound (II). The asymmetric syntheses method of (R)-3-aminopiperidine (I) is reasonable in technology and concise in route, the needed product with relatively high ee value is obtained by utilizing chiral induction, the raw material is cheap and does not need resolving, no waste isomer is discharged, and the asymmetric synthesis method is applicable to large-scale industrial production.
Owner:SHANGHAI PUYI CHEM CO LTD
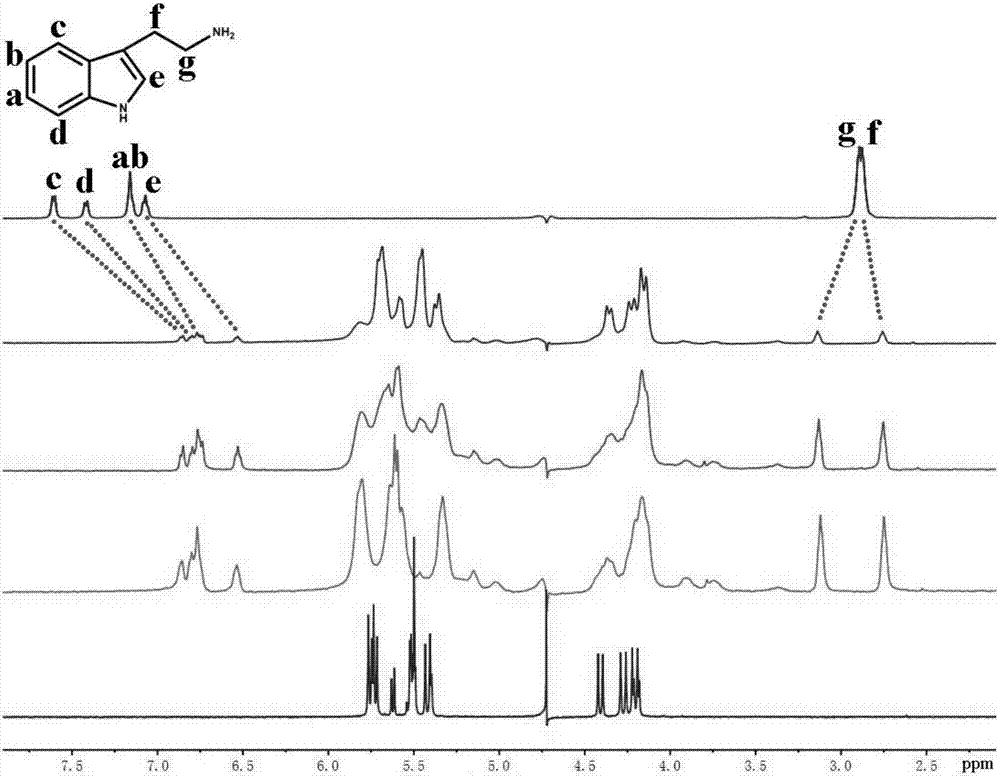
Application of trans-cucurbit(7)uril in recognition of biogenic amines
ActiveCN107064199AStable inclusionsAnalysis using nuclear magnetic resonanceFrozen storageTryptamine
The invention discloses application of trans-cucurbit(7)uril in recognition of biogenic amines. Trans-cucurbit(7)uril can be used for recognizing tryptamine by the following steps: (a) adding trans-cucurbit(7)uril into a nuclear magnetism tube, adding D2O, and vibrating to dissolve, so as to obtain a product A; (b) storing tryptamine into a frozen storage pipe, adding D2O to dissolve, so as to obtain a product B; and (c) successively adding the product B into the product A so as to obtain a corresponding nuclear magnetism spectrogram in each dropwise adding process until a series of nuclear magnetism spectrograms of a host-guest complex, making comparison on the nuclear magnetism spectrograms and a nuclear magnetism spectrogram of tryptamine, and determining biogenic amines as tryptamine when two groups of signal peaks Hg and Hf appear in the spectrograms, Hgs move towards a low field, Hfs move to a high field, peak forms of signal peaks of other Ha-He protons are widened and signal peaks move towards the high yield. Trans-cucurbit(7)uril can be used for recognizing six biogenic amines including tryptamine, spermine, spermidine, tyramine and phenylethylamine and is of a great significance to reveal of biological phenomena and processes.
Owner:GUIZHOU UNIV
Seafood flavor agent for cats and preparation method thereof
ActiveCN104187177AIncrease profitStimulate interest in predationAnimal feeding stuffCysteamineHydrolysate
The invention discloses a seafood flavor agent for cats and a preparation method of the seafood flavor agent for the cats. The seafood flavor agent comprises the following raw materials: 30%-60% of a seafood reactant, 1%-5% of dimethyl sulfide, 0.1%-2% of pyrrolidine, 1%-3% of phenethylamine, 0.2%-1% of sulfurol, 0.1%-0.5% of 3-methylthiopropanal, 0.2%-1% of trimethylamine, 0.2%-0.5% of 2-methylpyrazine, 0.1%-2% of hexadecanoic acid ethyl ester, 0.5%-3% of isoamylamine, 1%-3% of 3-methyl-3-furanthiol, and 20%-70% of salad oil. The preparation method of the seafood flavor agent comprises the following steps: after sea-fishes and wastes of the sea-fishes are milled by a colloid, adding protease, carrying out enzymolysis at a temperature of 55 DEG C for 3 to 3.5 hours, then rising the temperature to be 100 DEG C, and inactivating enzyme for 30 to 40 minutes to obtain an enzymatic hydrolysate, and then taking the following ingredients as raw materials in percentage by weight: 85% of the enzymatic hydrolysate, 3%-5% of xylose, 3%-5% of arabinose, 1%-2% of L-cysteamine acid, 3%-5% of yeast extract, 1%-2% of thiamine, 1%-3% of alanine and 1%-3% of methionine, and reacting for 1.5 to 2 hours at the temperature of 95 DEG C, adding remaining fragrance raw materials after cooling, and stirring uniformly to prepare the seafood flavor agent. According to the seafood flavor agent provided by the invention, the fragrance is strong and stable, and the product has stable fragrance and does not go bad under the condition of sealed packaging, thereby being easily stored.
Owner:成都大帝汉克生物科技有限公司
Preparation of chiral covalent organic framework material having L-menthol as chiral source
ActiveCN109134875AHigh efficiency of chiral resolutionReduce energy consumptionOther chemical processesL mentholTwo step
The invention discloses a preparation method of a hyperbranched polymer having (s)-(-)-alpha-phenethylamine as a chiral source. Thionyl chloride, 3,5-dibromobenzoic acid and (S)-(-)-alpha-phenethylamine are subjected as materials to two-step organic synthetic reaction to obtain white needle crystal A; 1,3,5-tribromophenyl, Pd(PPh3)2Cl2, CuI, PPh3 and ethynyl trimethylsilane are subjected as raw materials to two-step reaction to synthesize compound B; the white needle crystal A, the compound B, Pd(PPh3)2Cl2, PPh3 and CuI are subjected as materials to heating via a microwave reactor by using triethylamine and dimethylformamide as solvents, and stirring is carried out for 17 min; the crude product is purified and dried to obtain yellow solid; the finished product is tested. The novel nano-level hyperbranched polymer herein can provide massive catalytic active sites containing chiral groups in asymmetric catalytic reaction, and asymmetric synthetic reactive catalytic efficiency is improved.
Owner:QIQIHAR UNIVERSITY
Method for enzymatic resolution of phenylethylamines by using novel acyl donor
The invention discloses a method for enzymatic resolution of phenethylamines by using a novel acyl donor, which comprises following steps: (1) adding parachlorophenol, organic acid, dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) with a molar ratio of 1:(1-2):(1-2):(0.03-0.05), reacting under stirring, filtering, concentrating and separating by column chromatography to obtain the acyl donor; alternatively adding acetophenone and sodium borohydride with a molar ratio of 1:1.2 into methanol, reacting under stirring, distilled under reduced pressure, water washing, carrying out extraction with dichloromethane, drying and concentrating to obtain phenethyl alcohol, adding phenethyl alcohol, acetyl chloride and triethylamine with a molar ratio of 1:(1-1.2):(1-1.3), reacting under stirring, washing with saturated sodium bicarbonate solution, drying, concentrating and separating by column chromatography to obtain the acyl donor; and (2) adding phenylethylamine and the acyl donor with a molar ratio 1:(0.6-1) into 2-4mL organic solvent, and reacting with 10-20 mg / mL lipase to obtain amide. The method of the invention has advantages of mild reaction conditions, fast reaction, high conversion rate and high optical purity of the product, and has great application value.
Owner:ZHEJIANG UNIV
Asymmetric synthesis method of nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine, and relevant intermediate and raw material preparation method
ActiveCN103896826ANo emissionsSave raw materialsOrganic chemistryBulk chemical production4-methylpiperidineSynthesis methods
The invention relates to a preparation method of nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine (I). The method comprises the following steps: carrying out a reductive amination reaction on a compound of formula (III) and (R)-1-phenylethylamine to obtain a compound of formula (II), removing chiral prosthetic groups from the compound of formula (II), and adding a methyl group to the amino group of the compound of formula (II) in order to obtain nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine (I), wherein R in each of the formula (I), the formula (II) and the formula (III) is an amino protection group or hydrogen, and the amino protection group can be C1-4 alkoxycarbonyl, benzyloxycarbonyl or benzyl groups which can be removed through hydrolysis or hydrogenation. The asymmetric synthesis method of nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine (I) has the advantages of reasonable technology, concise route, obtaining of the required product in a high ee value manner by constructing two chiral centers through chiral induced one-step reductive amination, cheap raw materials, and no waste isomer emission, and is suitable for large-scale industrialized production.
Owner:SHANGHAI PUYI CHEM CO LTD
Method for splitting gamma-dodecalactone chiral molecule
InactiveCN102442981APure fragranceQuality improvementEssential-oils/perfumesOptically-active compound separationGamma-dodecalactoneLactone
The invention discloses a method for splitting a gamma-dodecalactone chiral molecule. The method comprises the following steps of: under the alkaline condition, hydrolyzing gamma-dodecalactone into hydroxy acid; under the action of a catalyst, reacting a chiral splitting agent R-(+)-alpha-phenyl ethylamine with optical activity and the hydroxy acid to form a hydroxyl-acid salt complex; splitting and purifying the racemized hydroxyl-acid salt complex by using a recrystallization method; and under the action of the catalyst, acidifying and cyclizing the purified product to obtain chiral gamma-dodecalactone. According to the method disclosed by the invention, the problems of low half-quantity yield and high splitting cost in the splitting process of the gamma-dodecalactone chiral molecule inthe prior art are solved; and the method for splitting the gamma-dodecalactone chiral molecule with the advantages of high half-quantity yield, chromatogram content as high as over 99.9 percent and higher utilization value in scale production is provided.
Owner:JINGJIANG TAIDA PERFUME CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com