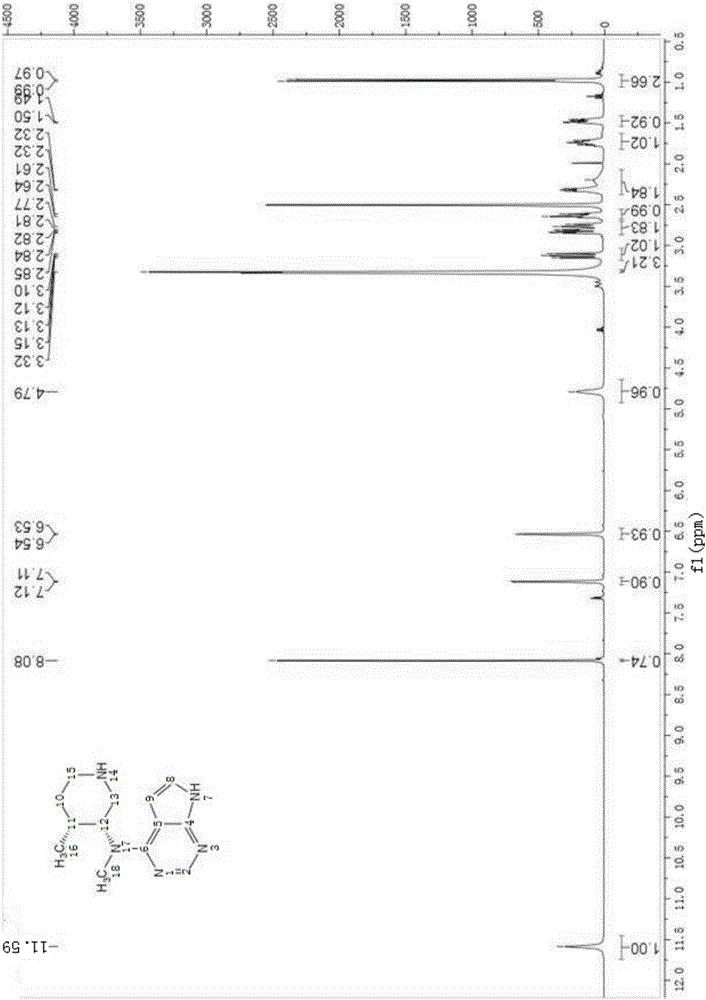
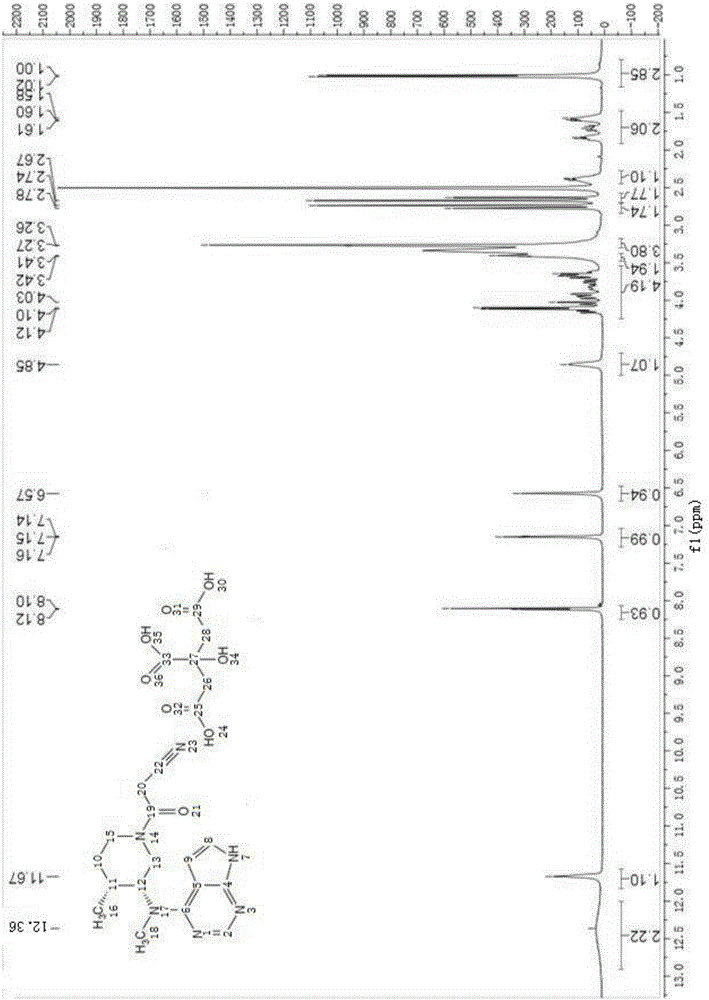
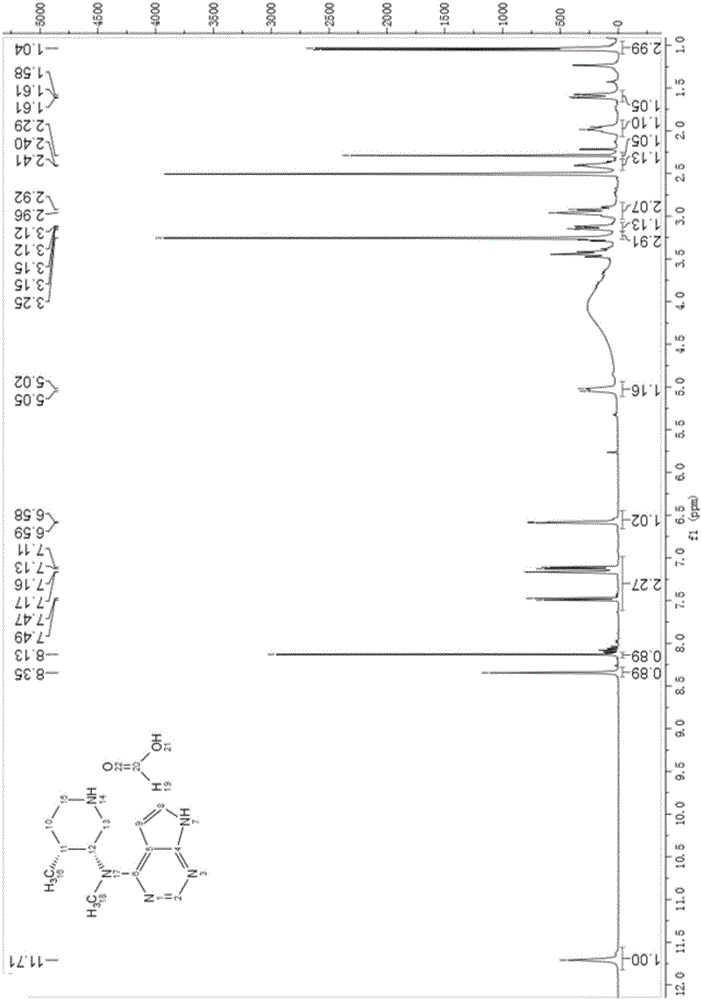
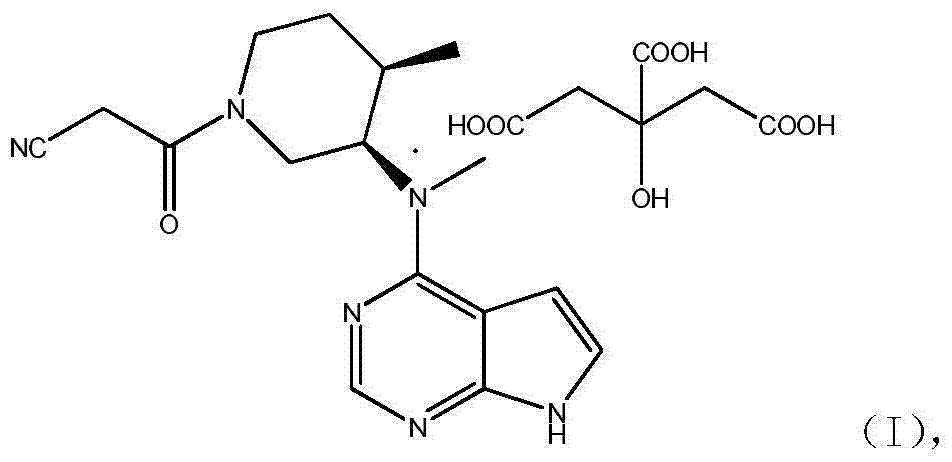
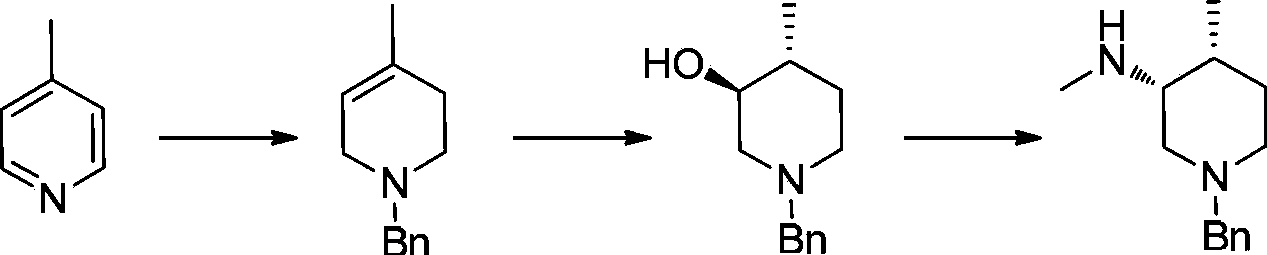
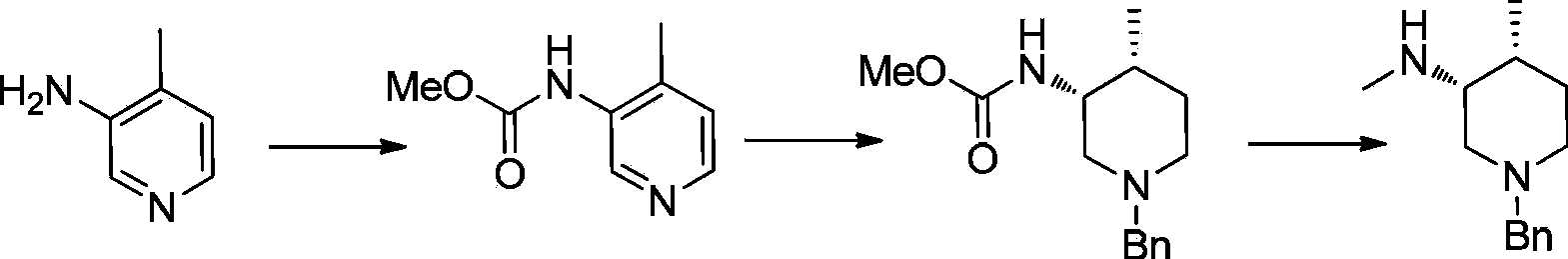
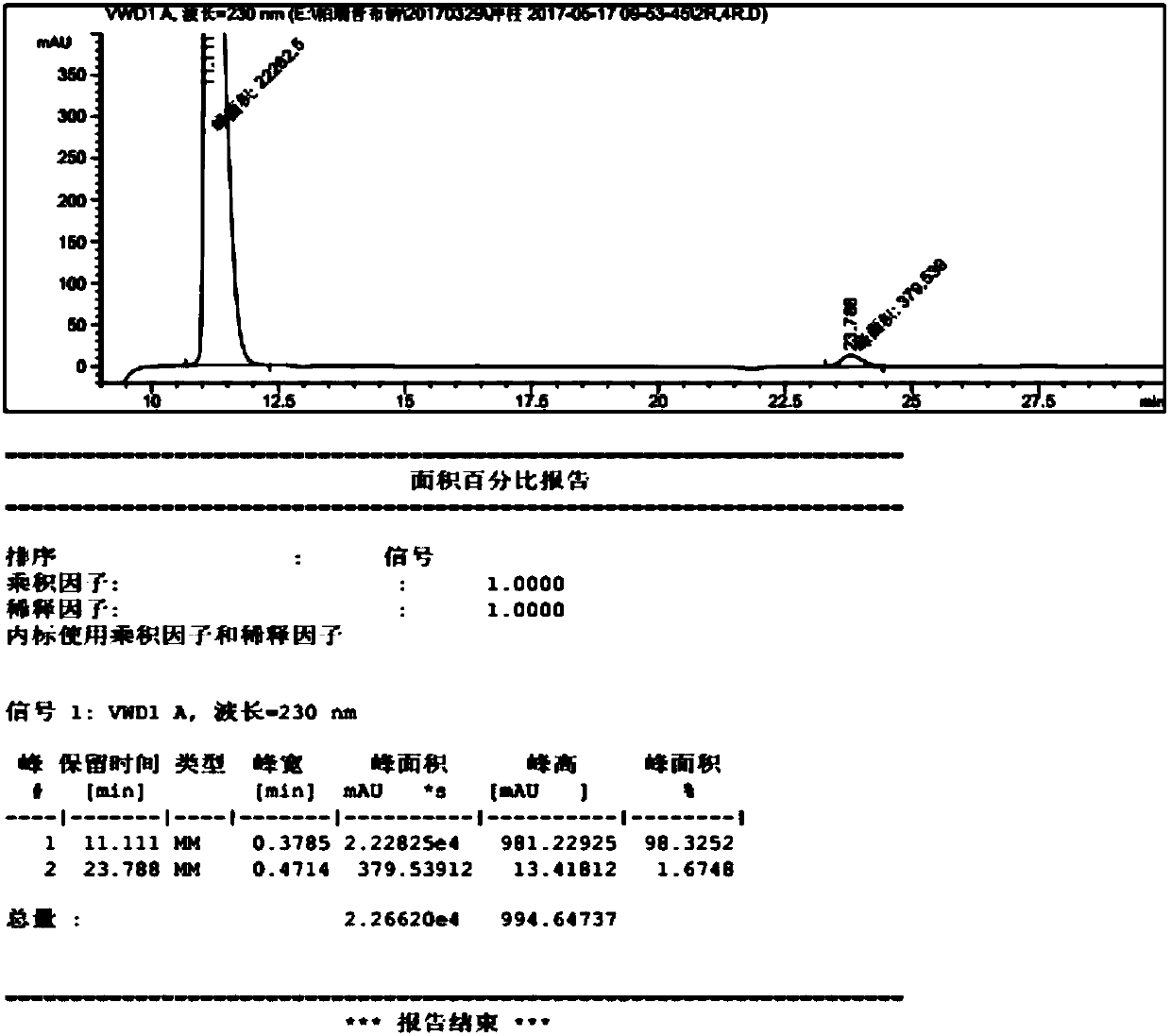
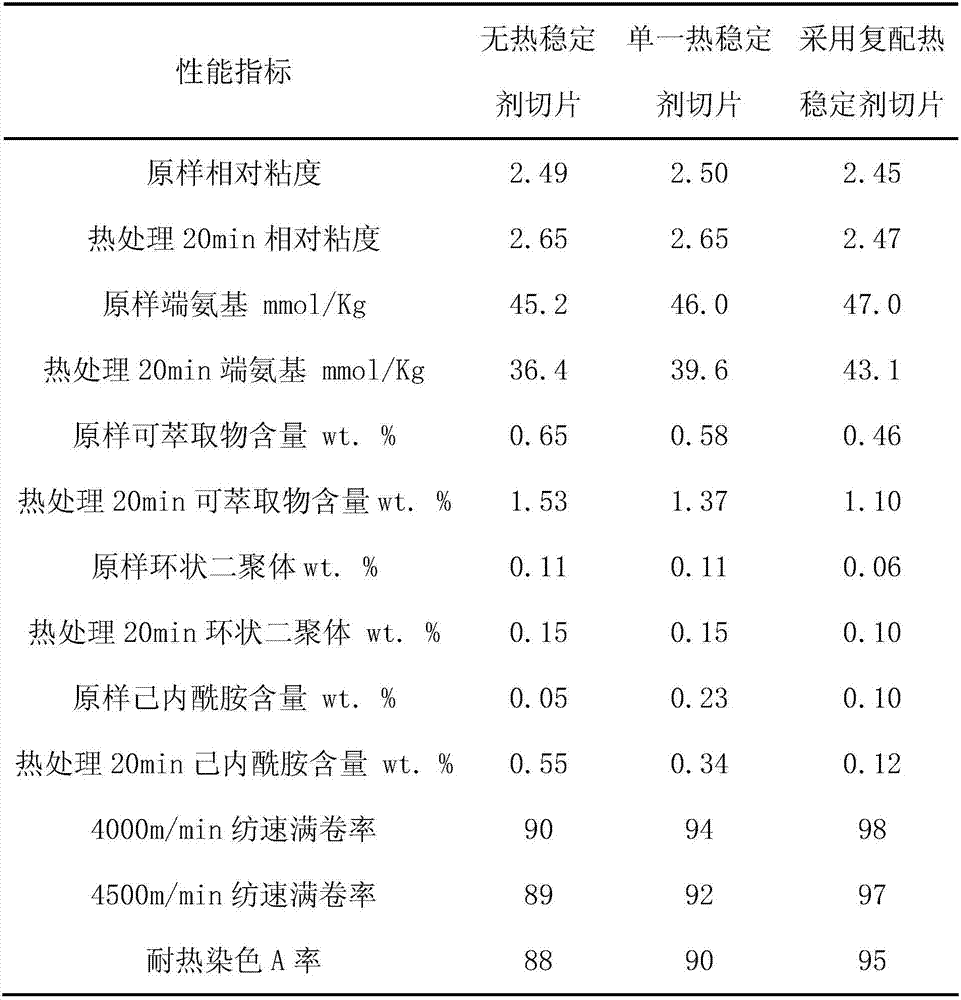
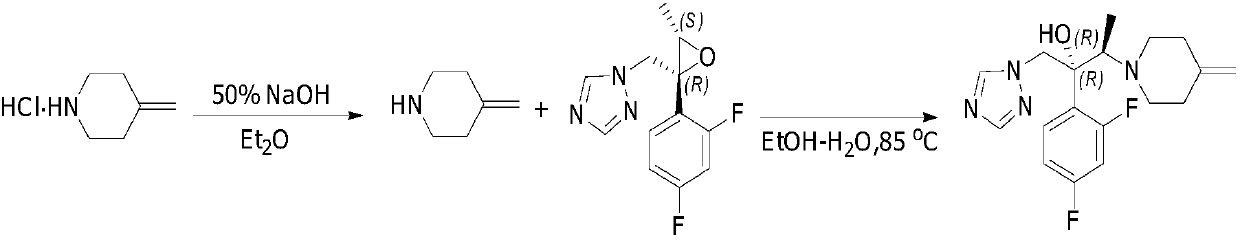
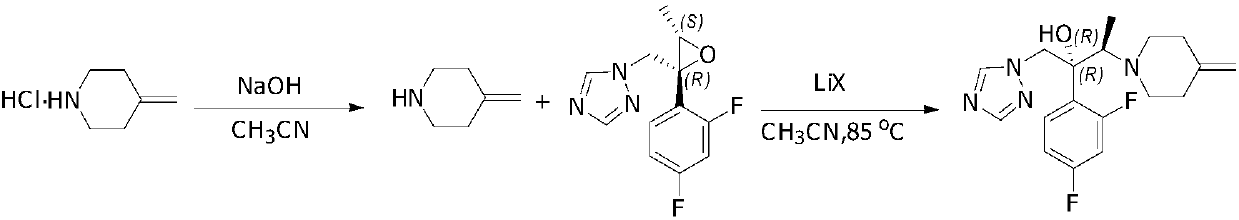
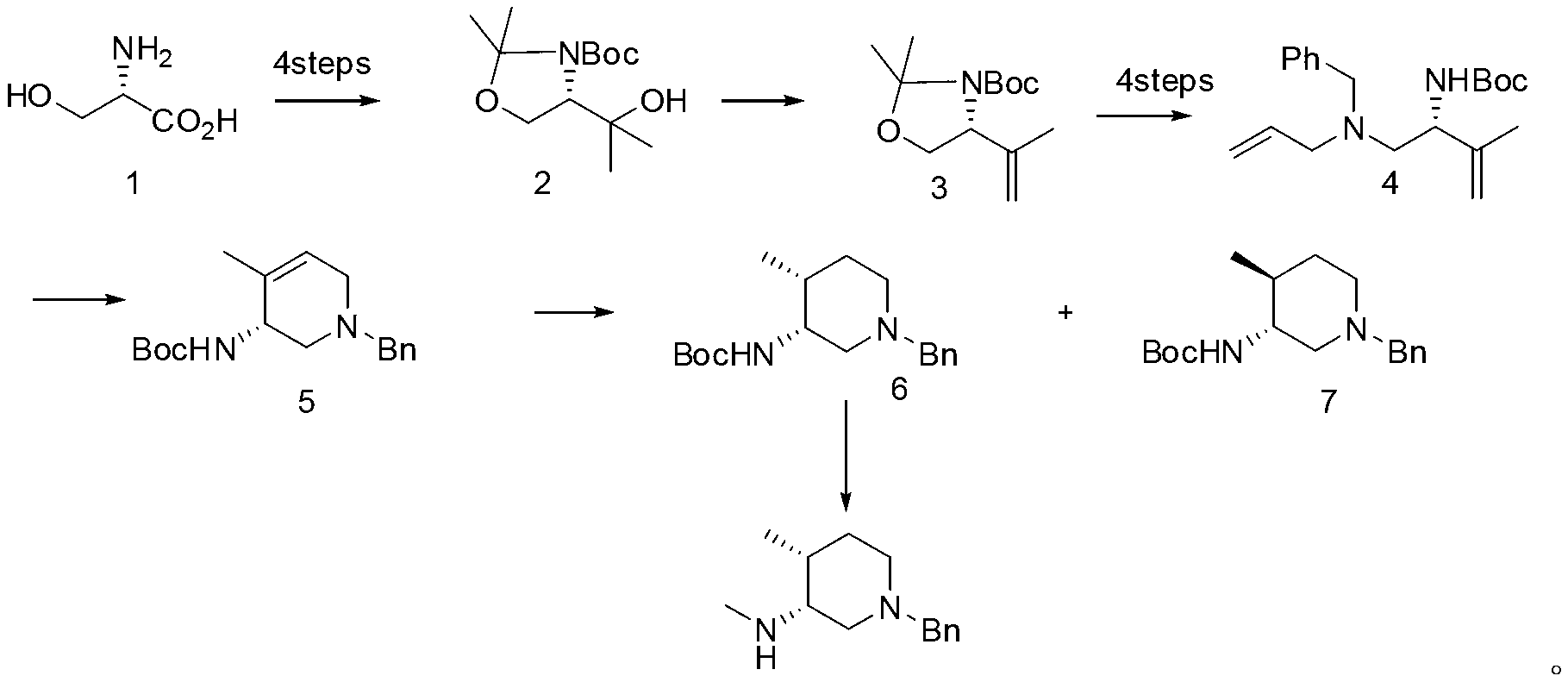
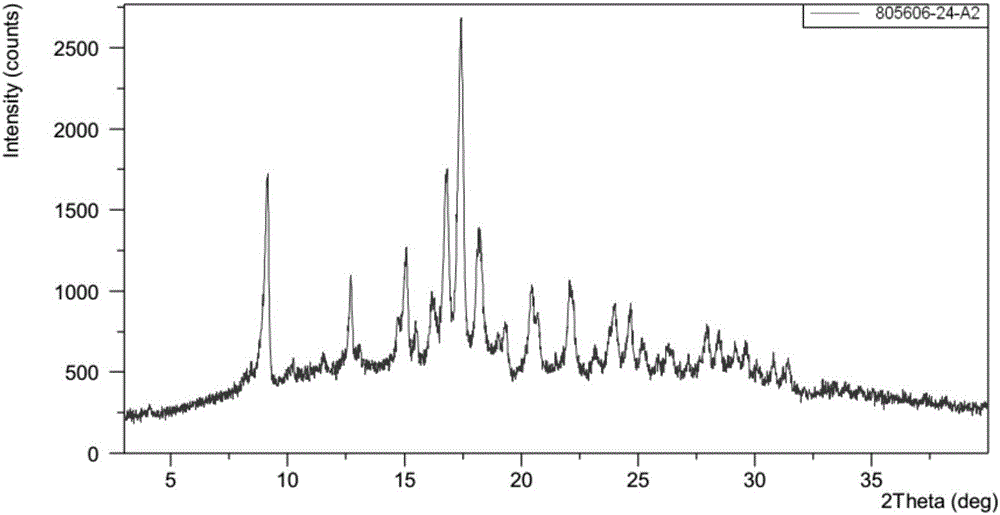
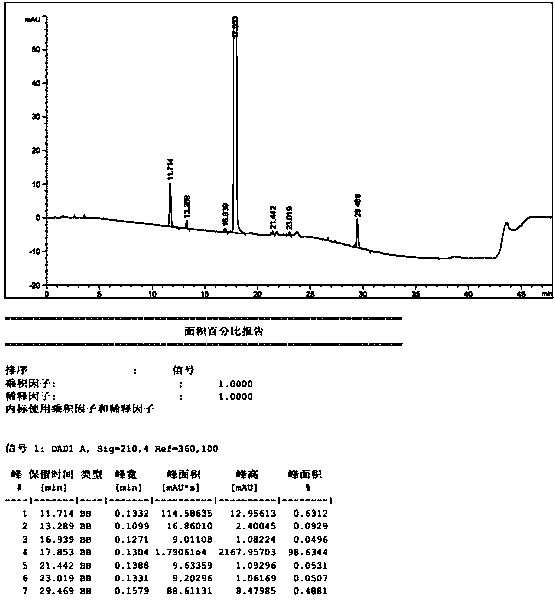
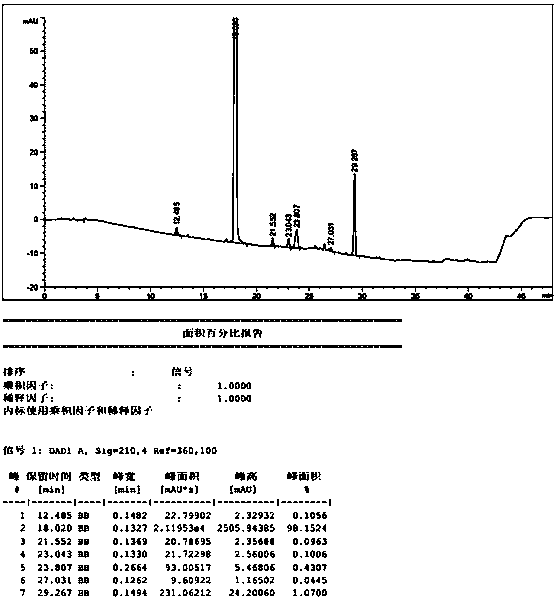
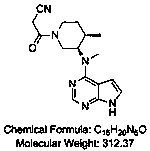
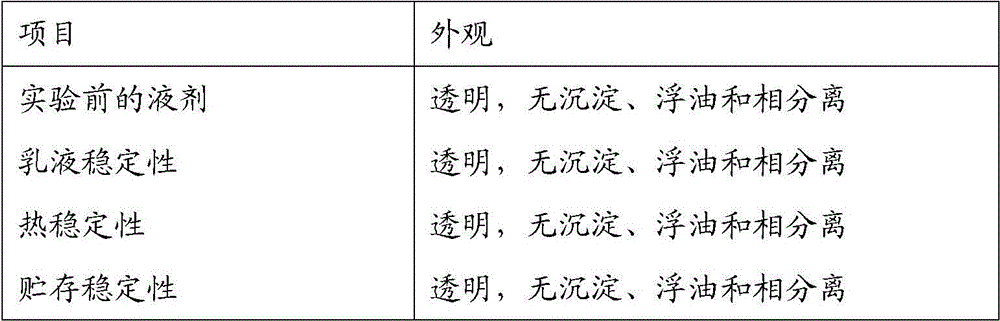
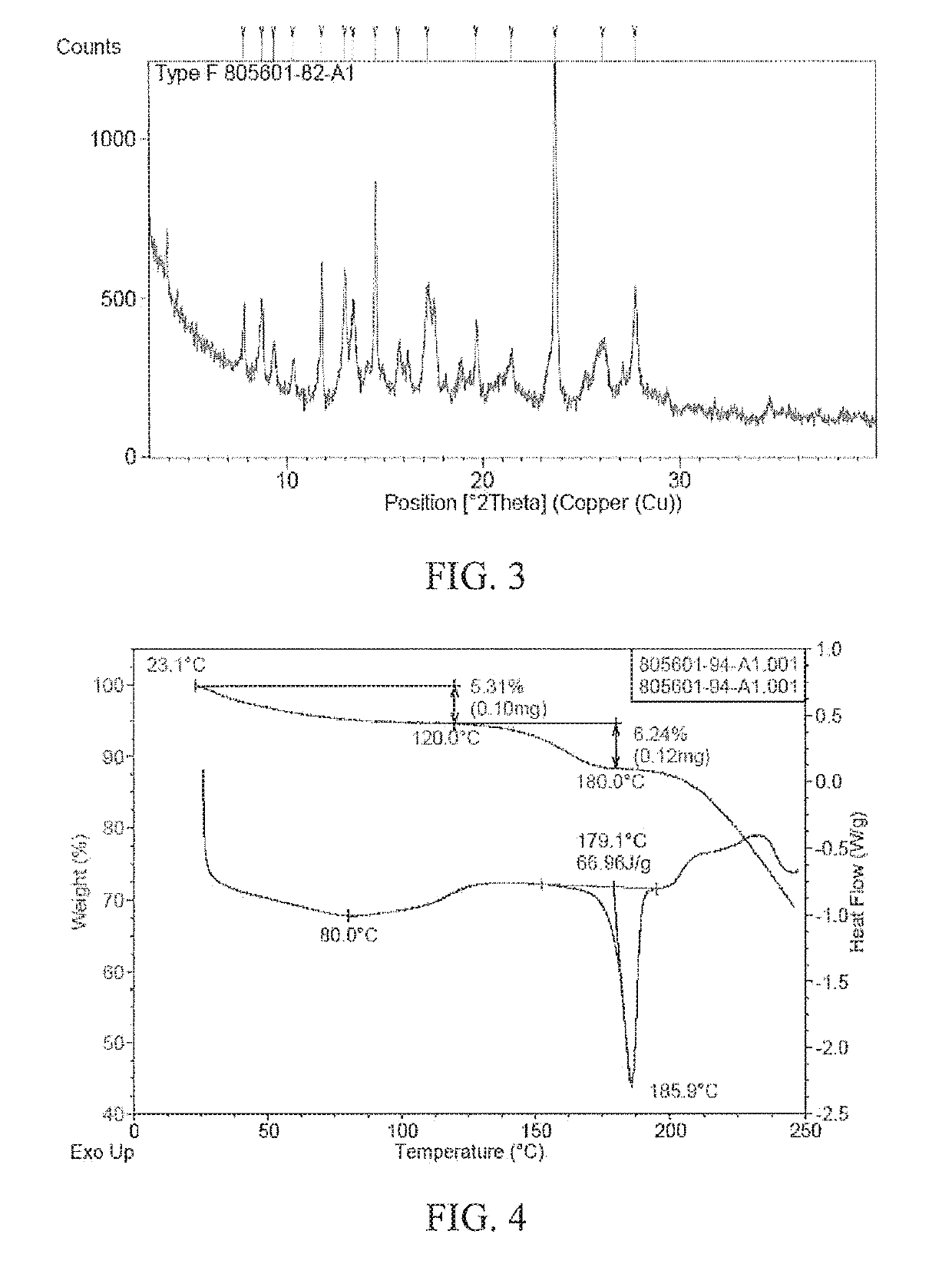
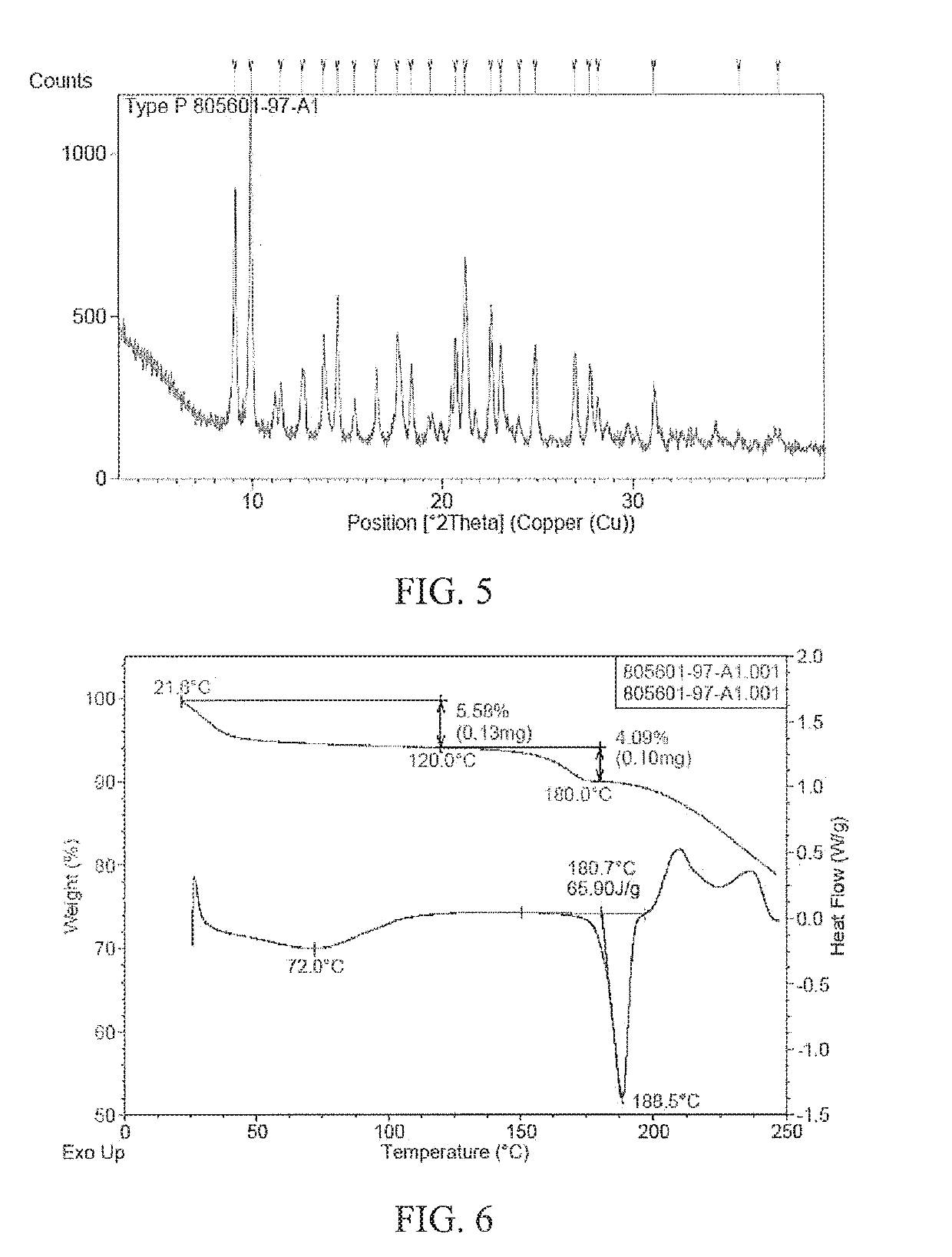
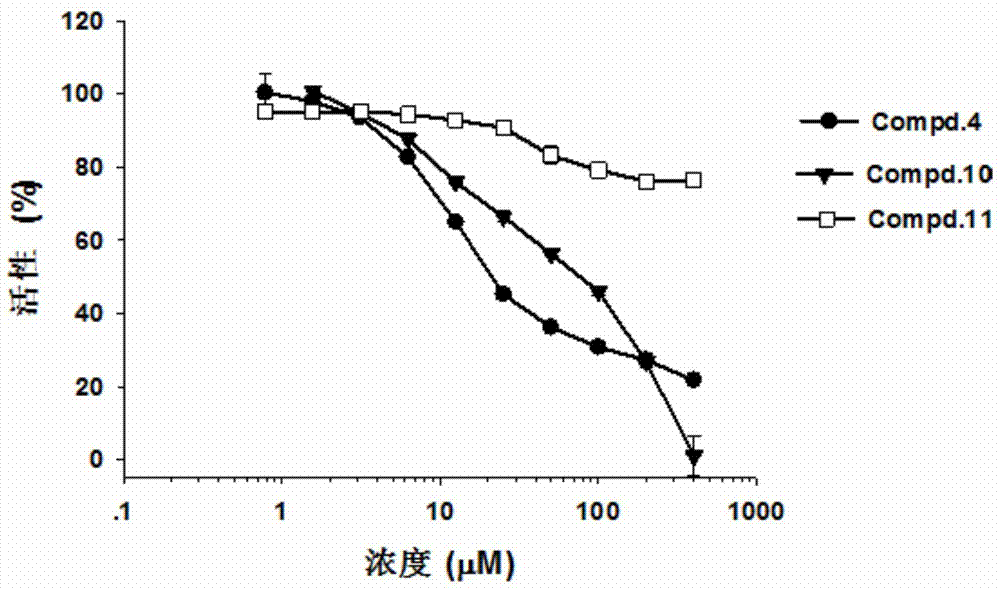
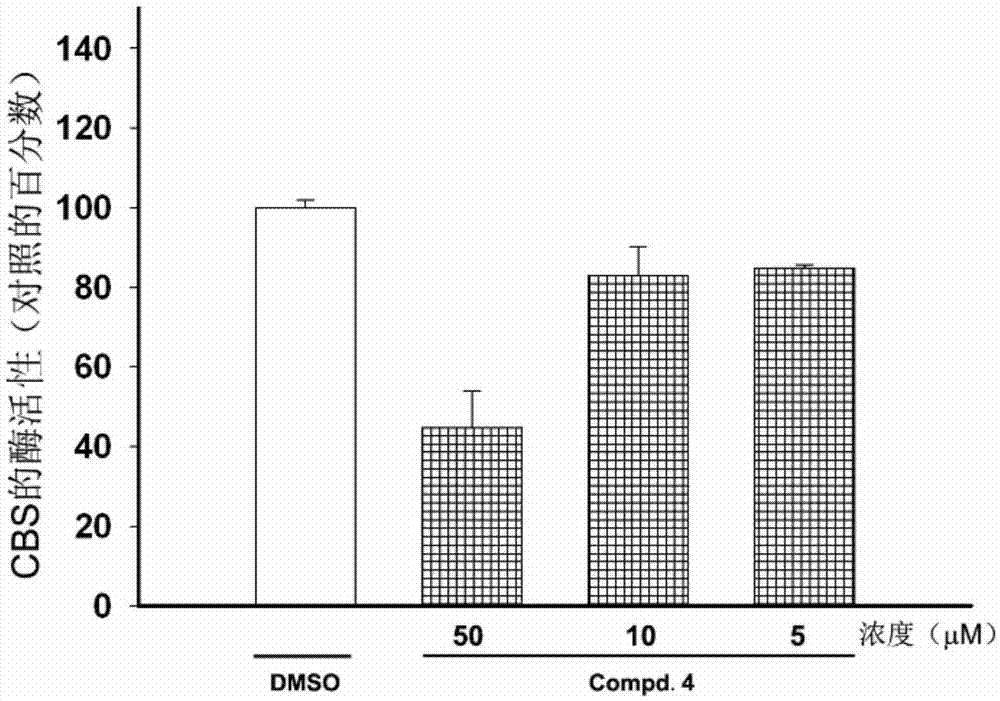
Patents
Literature
40 results about "4-methylpiperidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
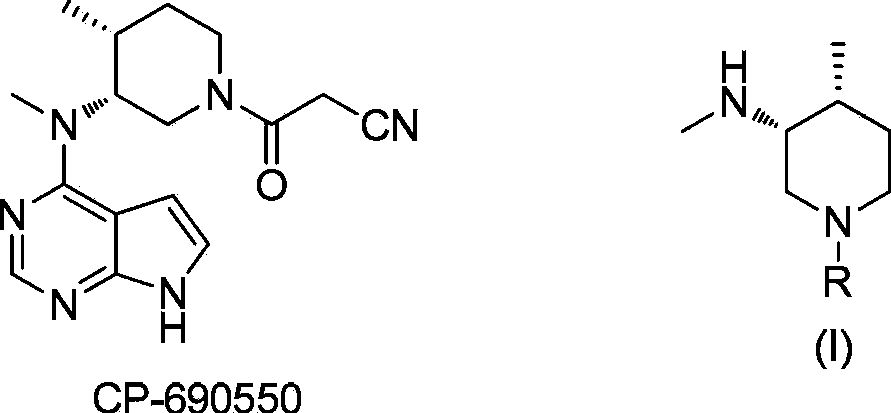
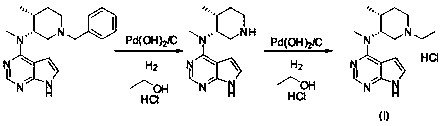
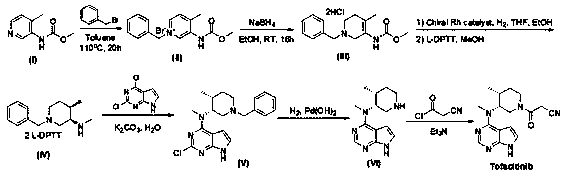
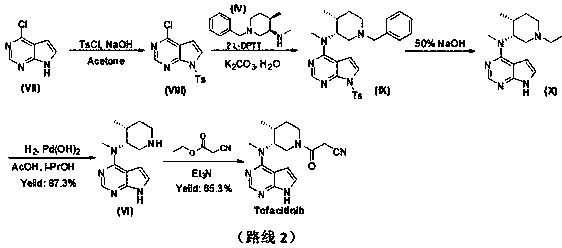
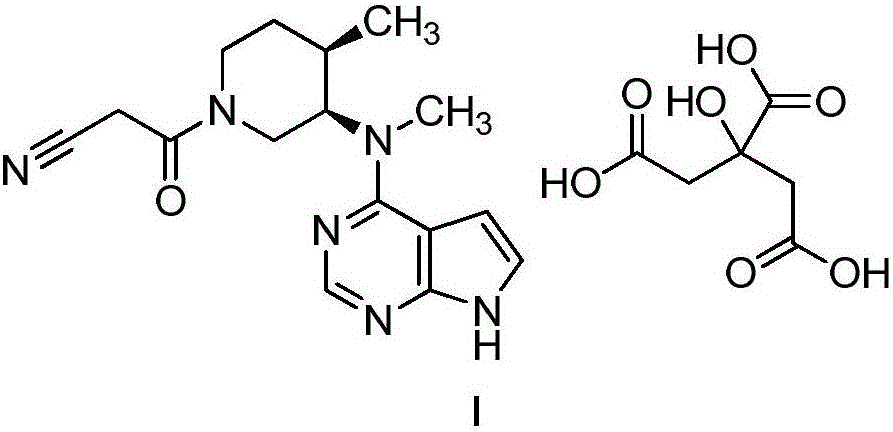
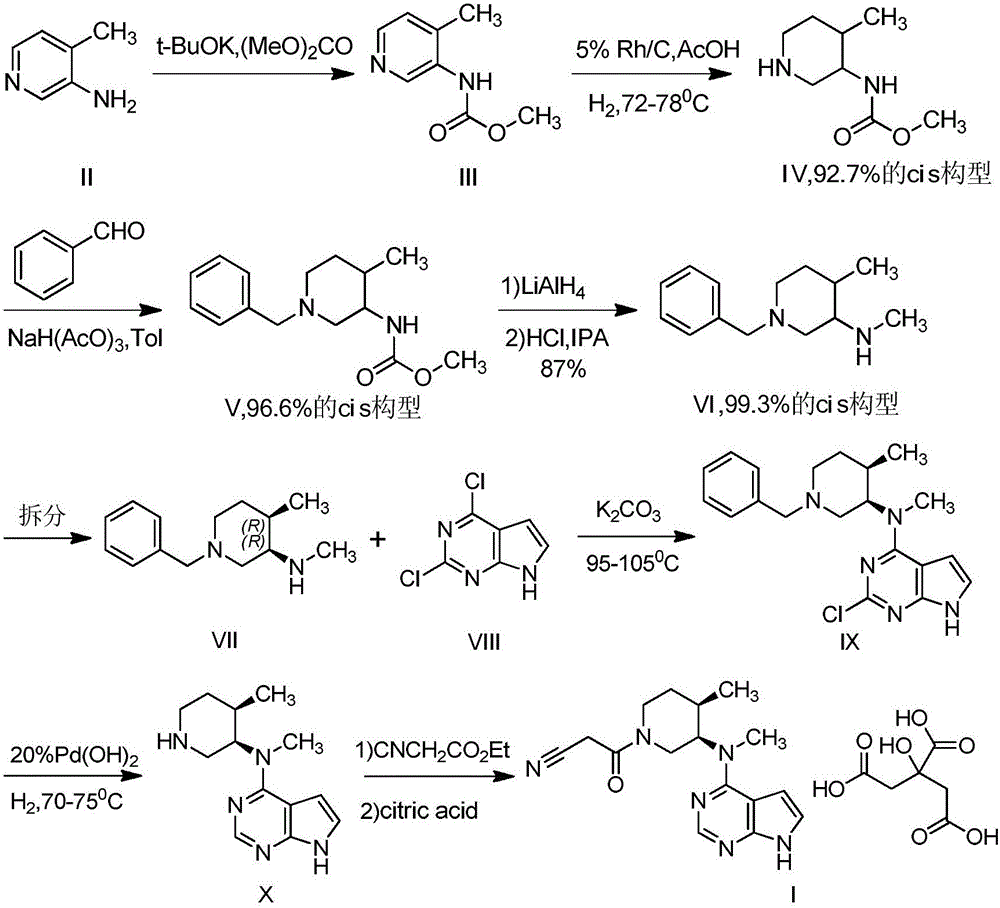
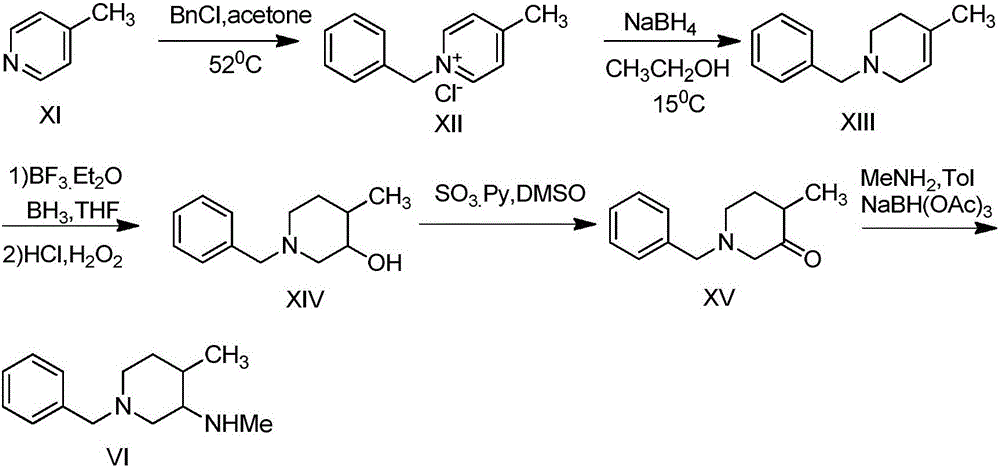
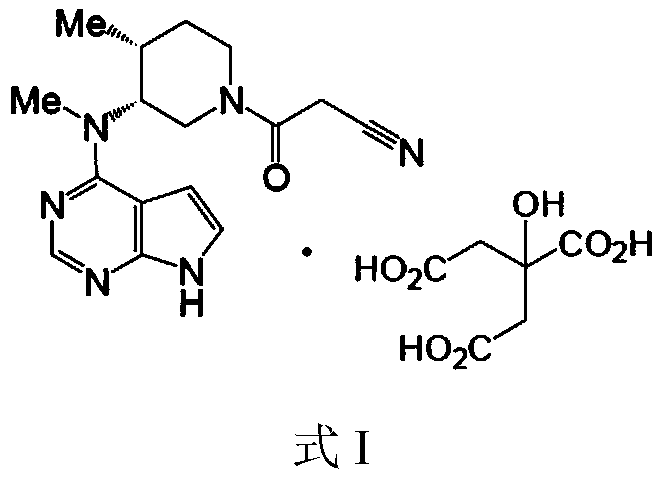
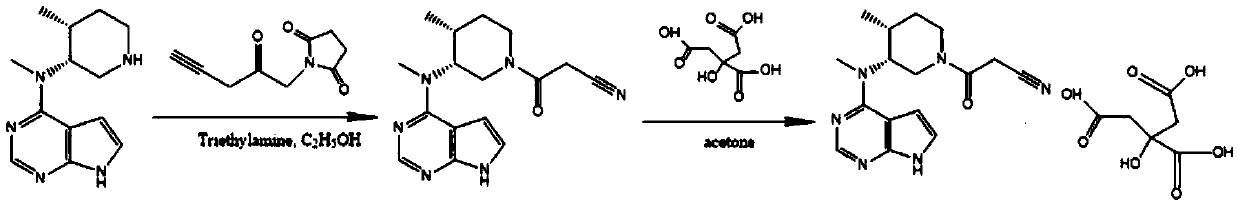
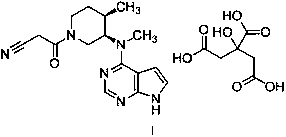
Method for synthesizing citric acid tofacitinib
ActiveCN106146517AEliminate potential safety hazardsShort reaction timeCarboxylic acid salt preparation4-methylpiperidineTofacitinib
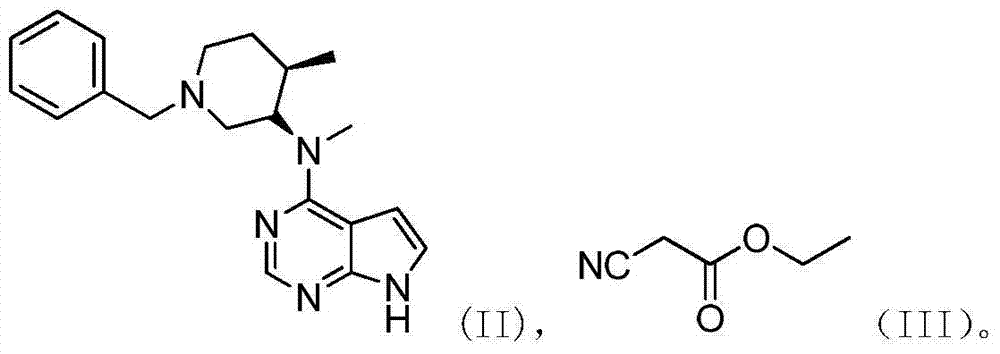
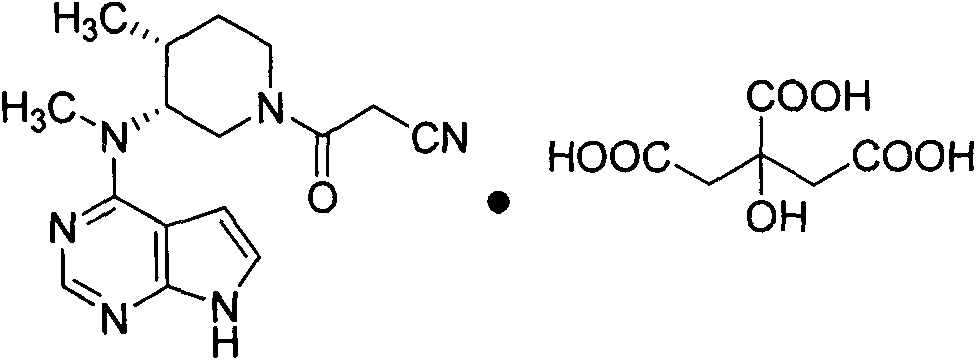
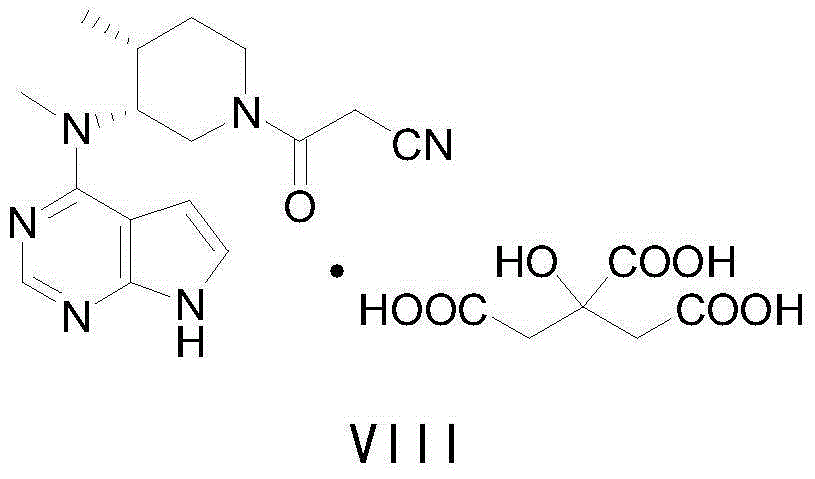
The invention discloses an efficient and safe method for synthesizing citric acid tofacitinib. N-[(3R,4R)-1-benzyl-4-methylpiperidine-3-base]-N-methyl-7H-pyrrolo[2,3-d]pyrimidine-4-amine is used as the raw material, Pd / C and HCOOH reduction debenzylation is conducted, condensation is conducted under the catalysis of an EDCI or EDCI, HOBT and triethylamine compound system with cyanoacetic acid, and salifying is conducted with citric acid in acetone to obtain citric acid tofacitinib. By the adoption of the synthesis method, potential safety hazards caused by hydrogen and ammonium formate are avoided, debenzylation reaction and amidation are thorough, no side reaction is caused basically, reaction time is shortened greatly, yield is high, aftertreatment is easy, and citric acid tofacitinib can be prepared efficiently and safely.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Preparation method of tofacitinib citrate
ActiveCN104292231AOrganic chemistry methodsBulk chemical productionAfter treatment4-methylpiperidine
The invention relates to a method for preparing tofacitinib citrate. The method comprises the following steps: by taking N-methyl-N-((3R, 4R)-1-benzyl-4-methylpiperidine-3-yl)-7H-pyrrolo (2, 3-d) pyrimidin-4-amine as a raw material, performing hydrogenation to remove an amino protecting group, performing amidation reaction and salt-forming reaction and finally drying to obtain tofacitinib citrate. In the preparation process, after-treatment is not required, the separation of a target intermediate is not required, and only reactants need to be added sequentially. The preparation method of tofacitinib citrate provided by the invention has the advantages of simplicity in operation, high yield, mild conditions and the like, and is suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method of tofacitinib citrate
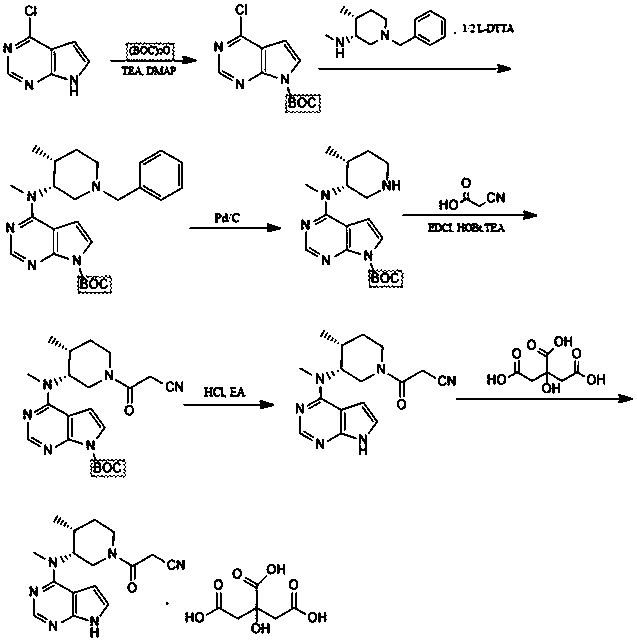
The invention relates to a preparation method of tofacitinib citrate, in particular to high-yield synthesis of tofacitinib citrate. The tofacitinib citrate is synthesized from raw materials including4-chloro-7-pyrrolo[2,3-d]pyrimidine, (BOC)2O, (3R,4R)-(1-benzyl-4-methylpiperidine-3-yl)methylamine-L-di-p-toluoyl tartrate, Pd / C, cyanoacetic acid and citric acid through six steps including an aminoprotection reaction, an amination reaction, a debenzylation reaction, a condensation reaction, a deprotection reaction and a salt forming reaction. The synthesis route provides the preparation methodof tofacitinib citrate, and the preparation method is high in yield, low in cost, easy to operate and suitable for industrialization.
Owner:南京法恩化学有限公司
Synthesis method for tofacitinib
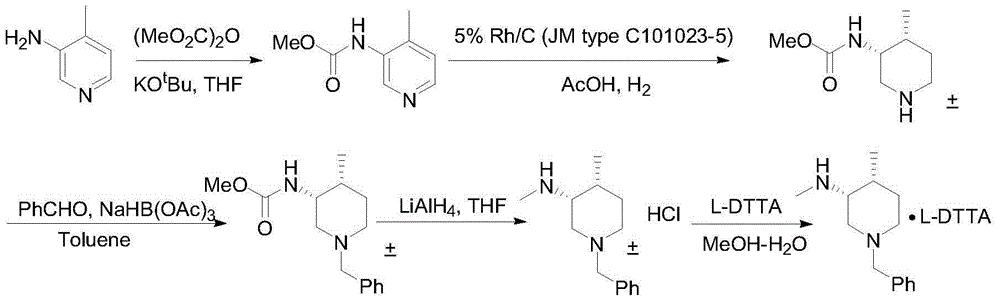
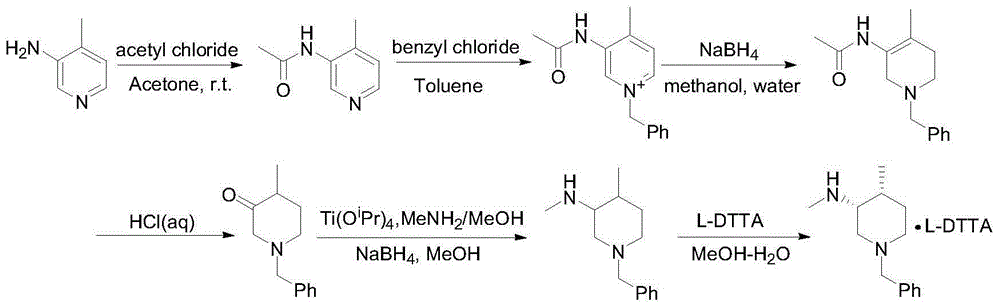
The invention relates to a synthesis method for tofacitinib serving as a JAK inhibitor. According to the method, the tofacitinib is prepared by taking (4-picoline-3-yl)methyl carbamate as a raw material, and performing catalytic hydrogenation, benzyl protection, reduction, salification, separation, deprotection and amidation salification. The method specifically comprises the following steps: (1) performing catalytic hydrogenation and reduction on (4-picoline-3-yl)methyl carbamate in sulfuric acid and Pd / C; (2) reacting cis-(4-picoline-3-yl)methyl carbamate and benzyl chloride to obtain cis-(1-benzyl-4-methylpiperidine-3-yl)methyl carbamate; (3) performing HOBT catalytic condensation on N-[(3R,4R)-4-methylpiperidine-3-yl]-N-methyl-7H-pyrrolo[2,3-d]pyrimidine-4-amine and cyanoacetic acid to obtain tofacitinib free alkali. The preparation method provided by the invention has easily obtained raw materials, mild reaction conditions, easiness and convenience in operation and high yield, and is suitable for industrial production.
Owner:济南扬诺生物科技有限公司
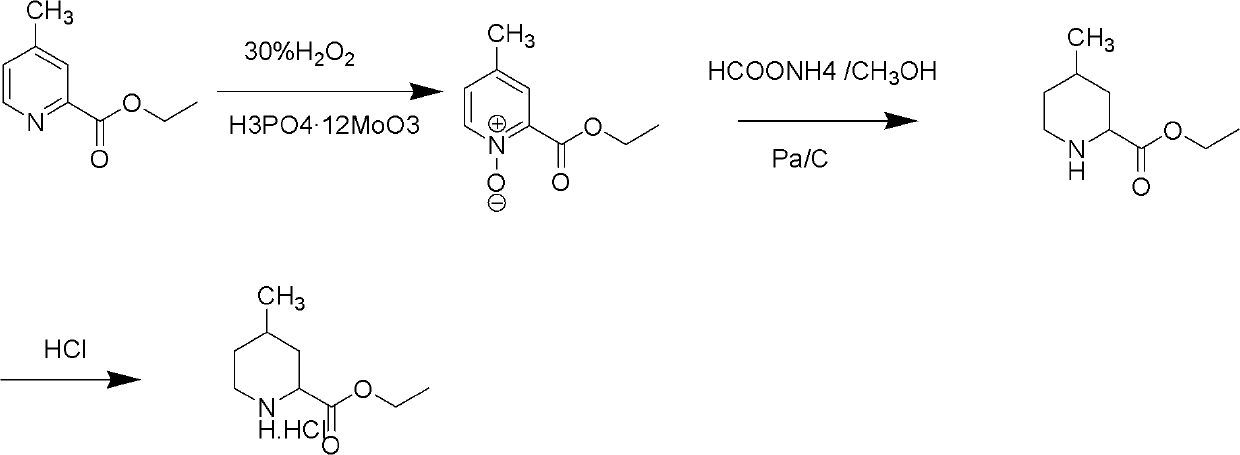
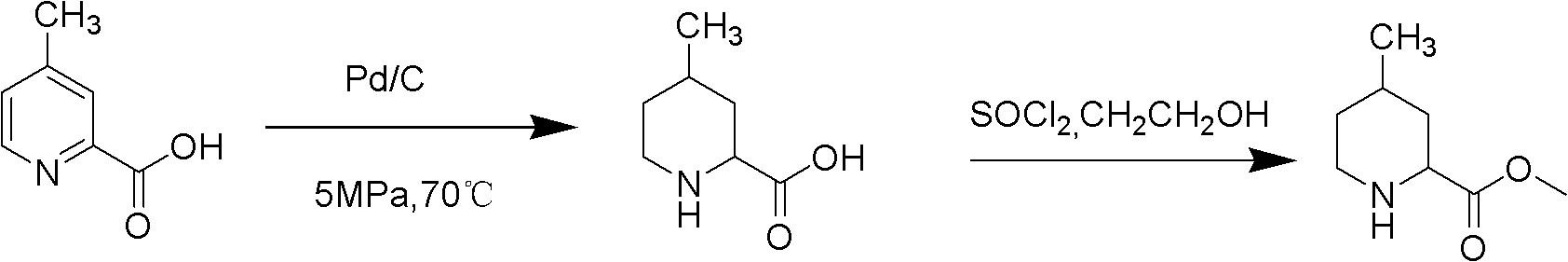
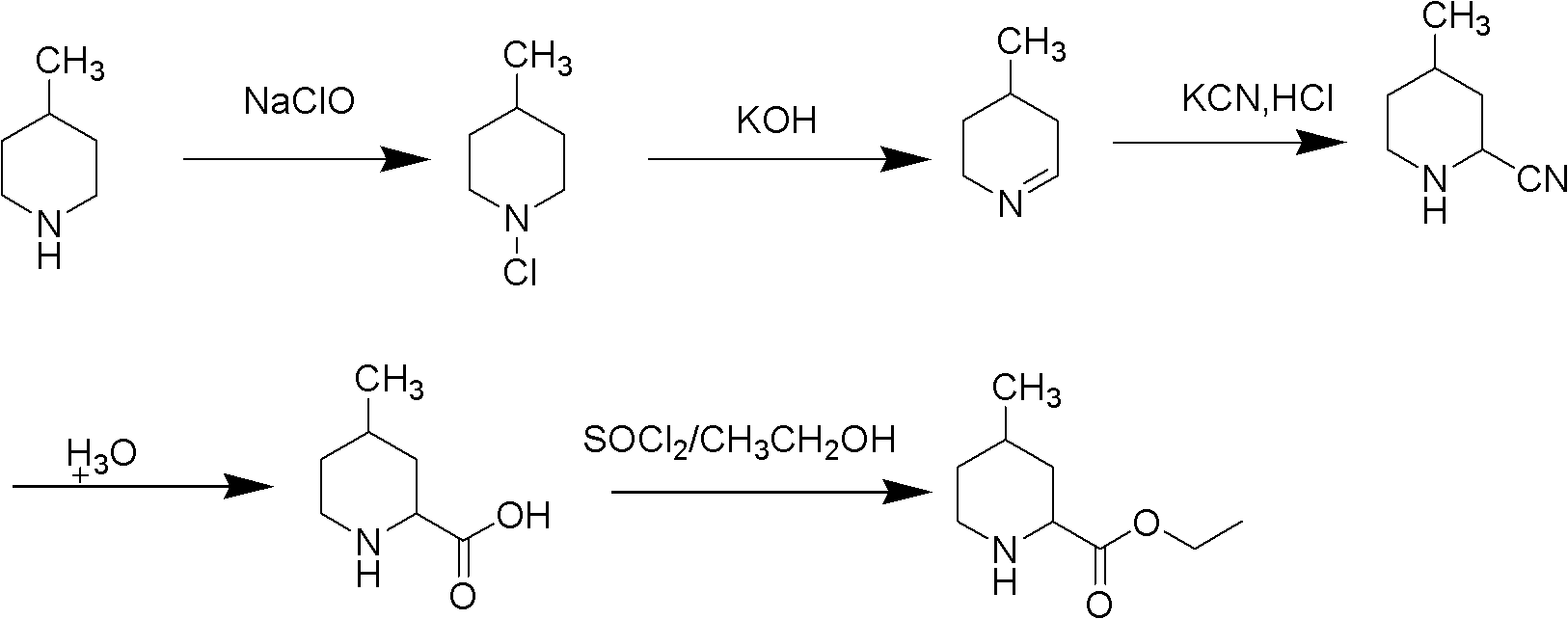
Method for preparing 4-methylpiperidine-2-carboxylate hydrochloride
ActiveCN102887854AEasy to operateMild conditionsOrganic chemistryPhosphomolybdic acid4-methylpiperidine
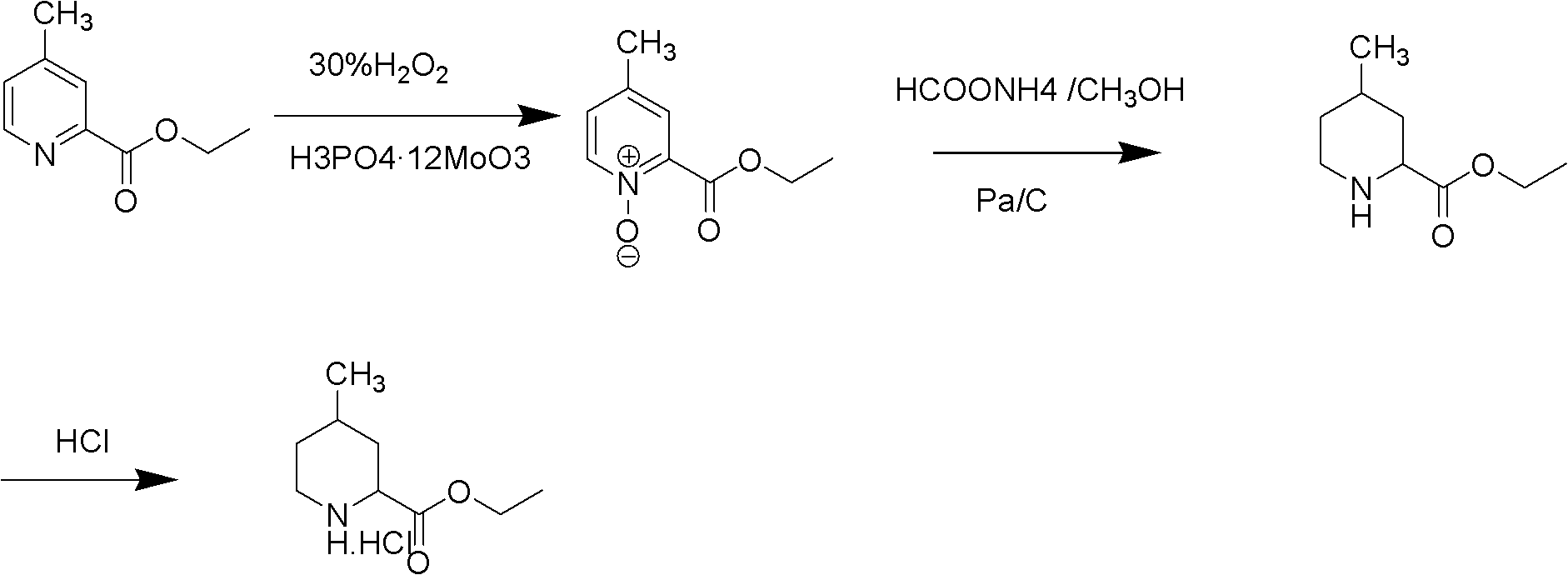
The invention relates to a method for preparing 4-methylpiperidine-2-carboxylate hydrochloride which is an intermediate of argatroban active precursor (2R, 4R)-4-methylpiperidine-2-carboxylic acid ethyl ester. 4-methylpiperidine-2-carboxylic acid ethyl ester is adopted as a raw material, phosphomolybdic acid is used as a catalyst, oxidizing is performed to obtain 4-methylpiperidine-2-carboxylic acid ethyl ester nitrogen oxide, methanol or ethanol is taken as a solvent, and 4-methylpiperidine-2-carboxylate hydrochloride is produced through reduction reaction. The method is simple to operate and has moderate conditions; phosphomolybdic acid is adopted to increase the activity of an oxidizing agent of hydrogen peroxide, the usage of the oxidizing agent is reduced, and the reaction yield is increased; the purification is convenient, the product quality is excellent, and good safety is obtained; and pollution is reduced, and industrial production is benefited.
Owner:CHANGSHAN BIOCHEM PHARM JIANGSU CO LTD
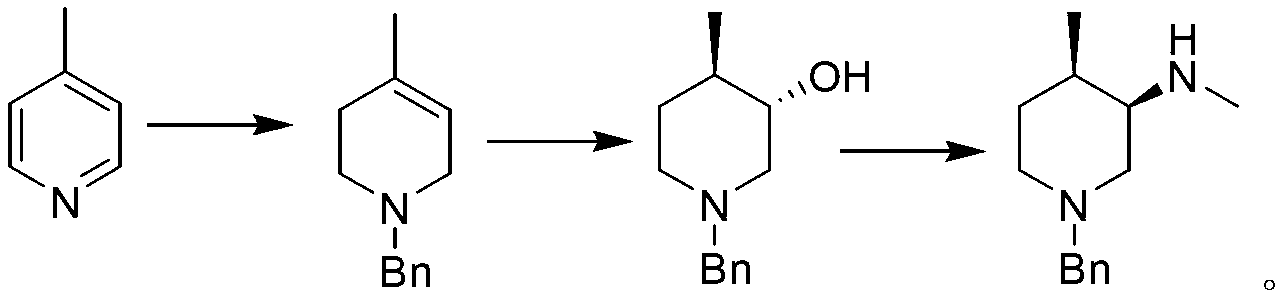
Asymmetric synthesis method of nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine, and relevant intermediate and raw material preparation method
ActiveCN103896826ANo emissionsSave raw materialsOrganic chemistryBulk chemical production4-methylpiperidineSynthesis methods
The invention relates to a preparation method of nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine (I). The method comprises the following steps: carrying out a reductive amination reaction on a compound of formula (III) and (R)-1-phenylethylamine to obtain a compound of formula (II), removing chiral prosthetic groups from the compound of formula (II), and adding a methyl group to the amino group of the compound of formula (II) in order to obtain nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine (I), wherein R in each of the formula (I), the formula (II) and the formula (III) is an amino protection group or hydrogen, and the amino protection group can be C1-4 alkoxycarbonyl, benzyloxycarbonyl or benzyl groups which can be removed through hydrolysis or hydrogenation. The asymmetric synthesis method of nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine (I) has the advantages of reasonable technology, concise route, obtaining of the required product in a high ee value manner by constructing two chiral centers through chiral induced one-step reductive amination, cheap raw materials, and no waste isomer emission, and is suitable for large-scale industrialized production.
Owner:SHANGHAI PUYI CHEM CO LTD
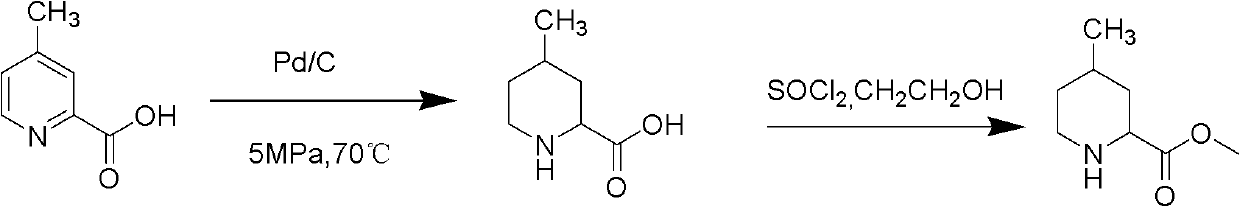
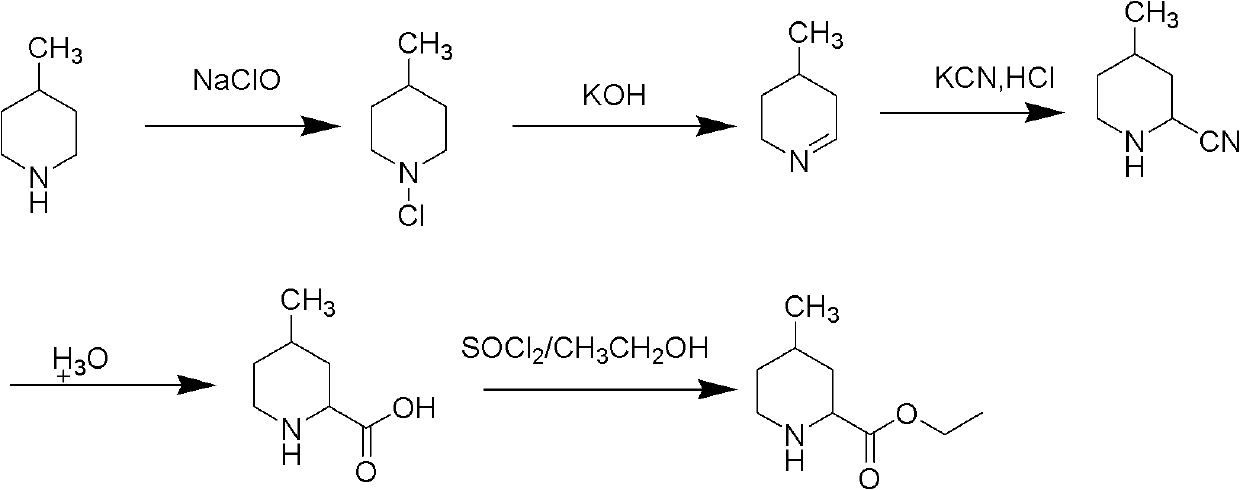
Preparing method of (2R,4R)-4-methylpiperidine-2-ethyl formate compound
InactiveCN108047125AEasy to routeSimple and fast operationOrganic chemistry methods4-methylpiperidineEthyl nipecotate
The invention relates to a preparing method of a (2R,4R)-4-methylpiperidine-2-ethyl formate compound. The method comprises the following steps of 1, regarding 4-methyl-2-cyanopiperidine as an initialraw material to be subjected to a hydrolysis reaction through hydrochloric acid to obtain 4-methyl-2-piperidinecarboxylicacid hydrochloride; 2, using ethyl alcohol to esterify 4-methyl-2-piperidinecarboxylicacid hydrochloride to obtain 4-methyl-2-ethyl nipecotate hydrochloride; 3, adding a mixed solvent of methyl tertiary butyl ether and ethyl alcohol, reacting the mixed liquid for pulping, filtering to remove cis-form 4-methyl-2-ethyl nipecotate hydrochloride solid, and collecting mother liquor to obtain anti-form 4-methyl-2-ethyl nipecotate hydrochloride; 4, using L-tartaric acid to split anti-form 4-methyl-2-ethyl nipecotate to obtain the target product (2R,4R)-4-methylpiperidine-2-ethyl formate.
Owner:BEIJING VOBAN PHARMA TECH CO LTD
Chinlon 6 for spinning and manufacturing method thereof
InactiveCN106977712AImprove thermal stabilityReduce concentrationMonocomponent polyamides artificial filamentWeight control agent4-methylpiperidine
The invention relates to chinlon 6 for spinning. The chinlon 6 is prepared from the following raw materials in parts by mass: 96 to 98 parts of caprolactam, 2 to 4 parts of desalted water, 0.28 to 0.3 part of molecular weight controlling agents and 0.05 to 0.15 part of thermal stabilizers, wherein the molecular weight controlling agents are any one kind of materials from terephthalic acid, m-phthalic acid and 1.4-phenylenediacetic acid; the thermal stabilizers are 4-amino-2,2,6,6-4 methyl piperidine and N,N'-bis(2,2,6,6- tetramethyl-4 piperidyl)-1,3-benzenedicarboxamide.
Owner:FUJIAN ZHONGJIN NEW MATERIALS
Preparing method of 1-triazole-2-butanol derivative
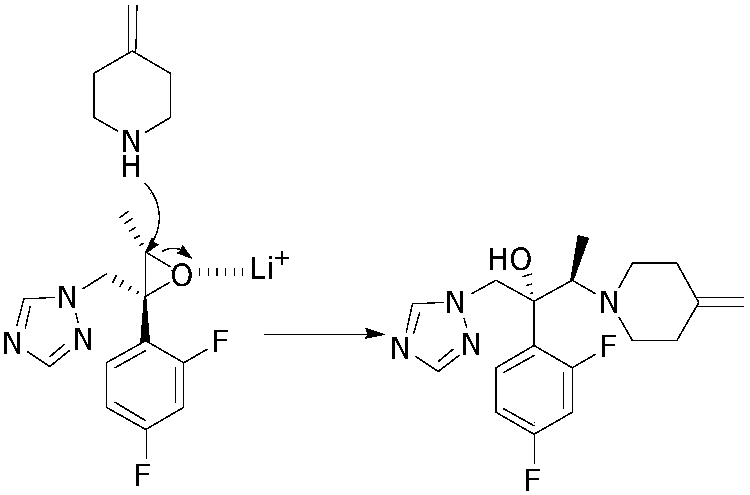
The invention relates to a preparing method of a 1-triazole-2-butanol derivative. The preparing method comprises the steps of making 4-methylpiperidine addition salt react with (2R,3S)-2-(2,4-di-fluorophenyl)-3-methyl-2-[(1H-1,2,4-traizole-1-radical)methyl]oxirane in a solvent in the presence of lithium halide and / or magnesium halide and alkaline to generate the 1-triazole-2-butanol derivative. The reaction is complete, the selectivity is high, and the product of the 1-triazole-2-butanol derivative of which the purity is larger than 99% can be obtained through simple aftertreatment. Besides, the yield is high no matter which form of 4-methylpiperidine addition salt is adopted as raw materials.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD
Polymorphic substance of triarylated dimethylpiperazine di-hydrochloride and preparation method and application thereof
The invention discloses four polymorphic substances B, E, H and D of (4-((R)-((2S, 5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazine-1-base)(3-hydroxyphenyl)methyl)phenyl)(4-methylpiperidine-1-base)ketone di-hydrochloride, a preparation method thereof, and application in preparing a drug.
Owner:YUNNAN INST OF MATERIA MEDICA
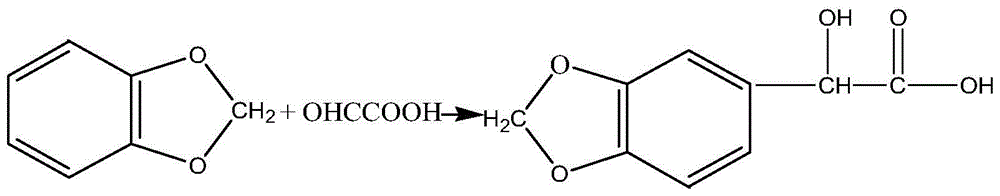
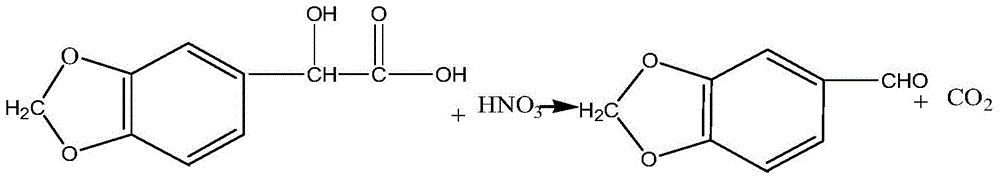
Synthetic method of piperonal
InactiveCN103936709ARaw materials are easy to getLow costOrganic chemistrySodium bicarbonate4-methylpiperidine
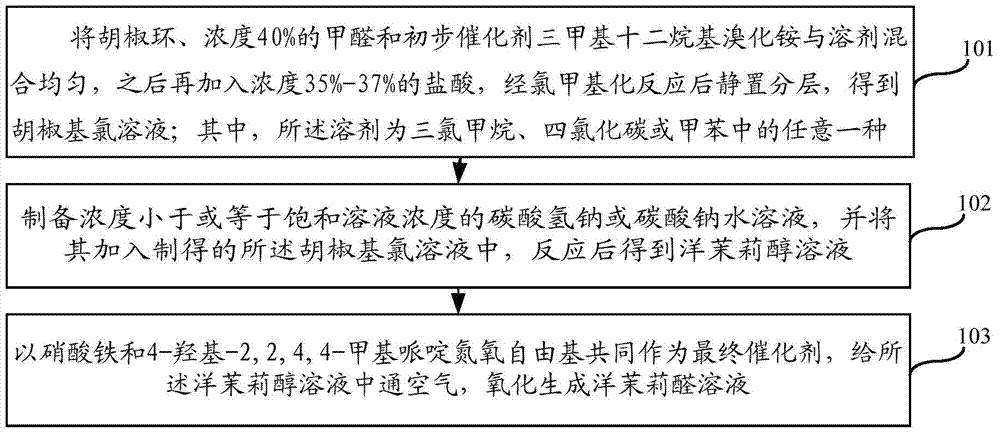
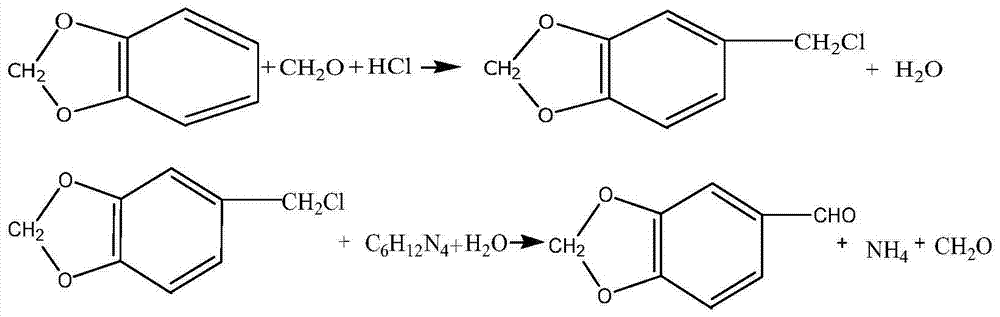
The invention relates to the technical field of the medical intermediate fine chemical industry and in particular relates to a synthetic method of piperonal. The synthetic method comprises the following steps: 1 uniformly mixing benzodioxole, formaldehyde with concentration of 40% and a primary catalyst dodecyl trimethyl ammonium bromide with a solvent, then adding hydrochloric acid with concentration of 35-37% and standing for layering after reaction, thus obtaining a piperonyl chloride solution, wherein the solvent is trichloromethane, tetrachloromethane or methylbenzene; 2 preparing a sodium bicarbonate or sodium carbonate water solution with concentration less than or equal to that of a saturated solution, adding the sodium bicarbonate or sodium carbonate water solution to the piperonyl chloride solution and reacting to obtain a piperonyl alcohol solution; 3 introducing air into the piperonyl alcohol solution to oxidize the piperonyl alcohol solution to generate a piperonal solution by using both ferric nitrate and 4-hydroxy-2,2,4,4-methylpiperidine nitroxyl free radical as final catalysts. The synthetic method provided by the invention has the advantages of accessible raw materials, mild reaction conditions, stable and high yield, few byproducts and low cost.
Owner:李东风
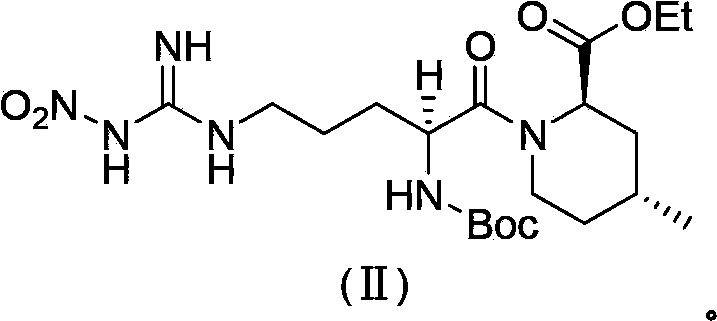
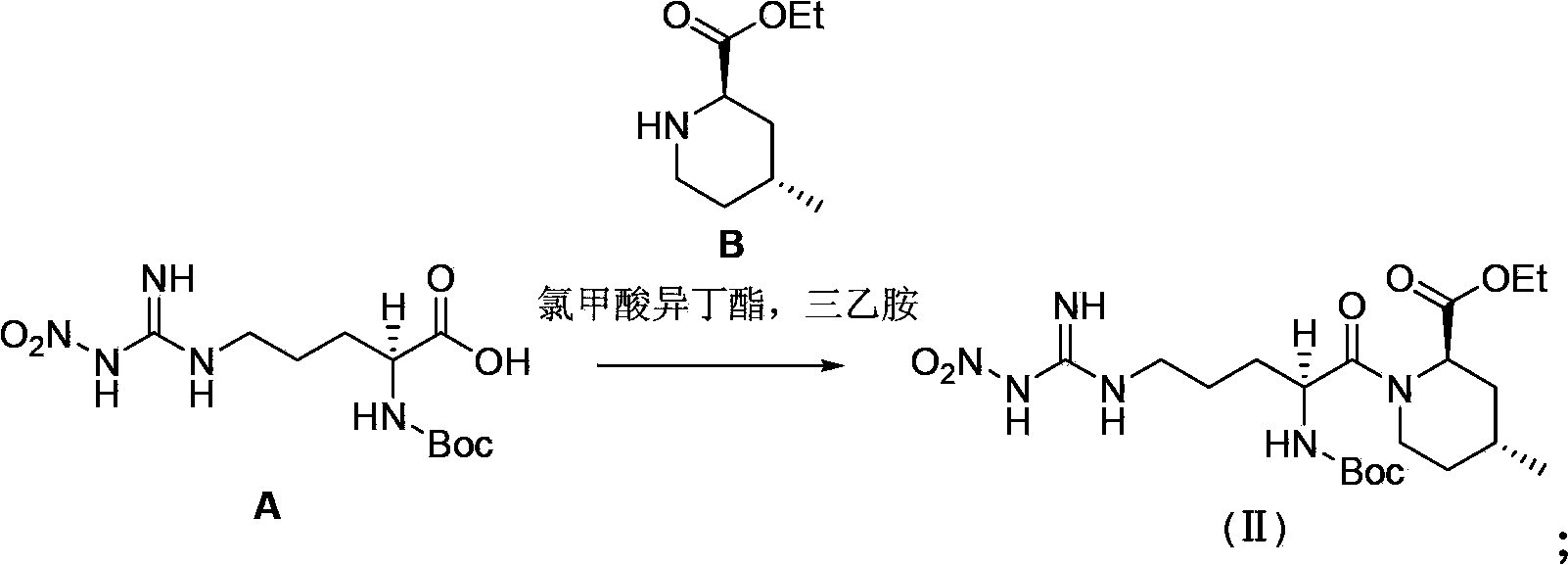
Preparation method of argatroban intermediate
The invention relates to a preparation method of argatroban intermediate (2R,4R)-ethyl-1-((S)-2-(tert-butoxy amido)-5-(3-nitroguanidine)valery)-4-methylpiperidine-2-ethyl carboxylate. The method comprises the following steps: enabling NG-nitro-N2-t-Boc-L-arginine and (2R,4R)-4-methyl-2-ethyl nipecotate to perform condensation reaction in the presence of a condensing agent selected from 1-ethyl-3-(3-dimethylamine propyl)carbodiimide hydrochloride, N,N-diisopropyl carbodiimide and N,N'-carbonyl diimidazole and an aprotic solvent to generate the argatroban intermediate. The raw material for the method is wide in source, cheap and easily-available; the method is mild in reaction condition, short in step, simple in operation, easy to purify the product, low in production cost and environment-friendly, not only suitable for laboratory synthesis, but also suitable for large-scale industrial production.
Owner:SHANGHAI SYNCORES TECH INC
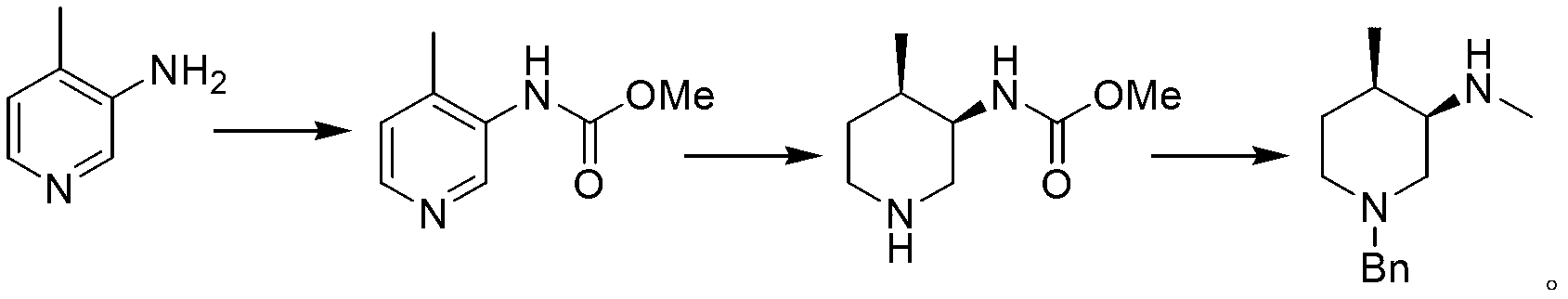
Synthesis method of (3R, 4R)-N-PG-4-methyl-3-methylaminopiperidine
InactiveCN103254121ALow priceThe synthetic route is simpleOrganic chemistryBulk chemical productionOrganic acid4-methylpiperidine
The invention relates to a synthesis method of (3R, 4R)-N-PG-4-methyl-3-methylaminopiperidine, the structural formula of which is as the following, wherein PG is an amino protecting group. The synthesis steps comprise: 1) cyclization: putting 2-butenal, N-PG-2-nitroethylamine, a catalyst and an organic acid into a reaction medium to react at a temperature ranging from -10 to 50DEG C for 0.5-8h; at the end of the reaction, adding an acid into the system to undergo dehydration, and letting the substances further react for 1-5h at 10-50DEG C so as to obtain (3R, 4R)-N-PG-4-methyl-3-nitro-3, 4-dihydropyridine, with the structure of the catalyst shown as the following; 2) subjecting (3R, 4R)-N-PG-4-methyl-3-nitro-3, 4-dihydropyridine to catalytic hydrogenation reduction so as to obtain (3R, 4R)-N-PG-3-amino-4-methylpiperidine; and 3) subjecting the (3R, 4R)-N-PG-3-amino-4-methylpiperidine to a methylation reaction so as to generate the target product.
Owner:LUOYANG NORMAL UNIV
Spinning polyamide 6 and manufacture method thereof
InactiveCN106905517AWith strengthHigh strengthMonocomponent polyesters artificial filamentArtifical filament manufactureAcetic acidWeight control agent
Spinning polyamide 6 comprises the following raw materials, by mass part, 96-98 parts of caprolactam, 2-4 parts of desalted water, 0.28-0.3 part of a molecular weight control agent, and 0.05-0.15 part of a heat stabilizer, wherein the molecular weight control agent is any two of adipic acid, terephthalic acid and acetic acid, and the heat stabilizer is any one of 4-amino-2,2,6,6-4-methyl piperidine and N,N'-di(2,2,6,6-tetramethyl-4-piperidyl)-1,3-benzenedicarboxamide.
Owner:FUJIAN ZHONGJIN NEW MATERIALS
Triaryldimethylpiperazine dihydrochloride polymorphic substance, preparation method and application thereof
The application discloses five polymorphic substances: B, C, A, M and I of (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazine-1-yl)(3-hydroxyphenyl)methyl)phenyl)(4-methylpiperidine-1-yl)methyl ketone dihydrochloride, a preparation method thereof, and applications thereof in preparation for medicines.
Owner:YUNNAN INST OF MATERIA MEDICA
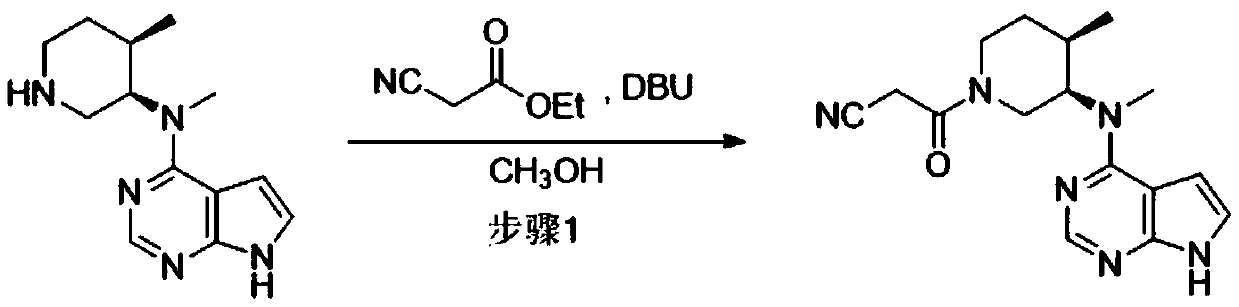
Preparation process of Xeljanz intermediate
The invention belongs to the technical field of pharmaceutical synthesis, in particular to a preparation process of a Xeljanz intermediate. The preparation process comprises the following steps: 1) adding ethyl cyanoacetate into n-butyl alcohol, and uniformly stirring; 2) dropwise adding DBU into a solution prepared in the step 1) at a temperature of or below 30 DEG C; 3) adding N-methyl-N-((3R,4R)-4-methylpiperidine-3-yl)-7H-pyrrolo[2,3-D]pyrimidine-4-amine into a solution prepared in the step 2) in batches for reacting; 4) dropwise adding water into a system reacting completely in the step 3), stirring, filtering and washing to obtain the Xeljanz intermediate, namely, (3R,4R)-4-methyl-3-(methyl-1H-pyrrolo[2,3-d]pyridine-4-amino)-beta-oxy-1-piperidine propionitrile. The intermediate prepared by the invention has higher yield and purity, and the decrease of the purity of the intermediate in expanded production is less.
Owner:国药集团容生制药有限公司 +1
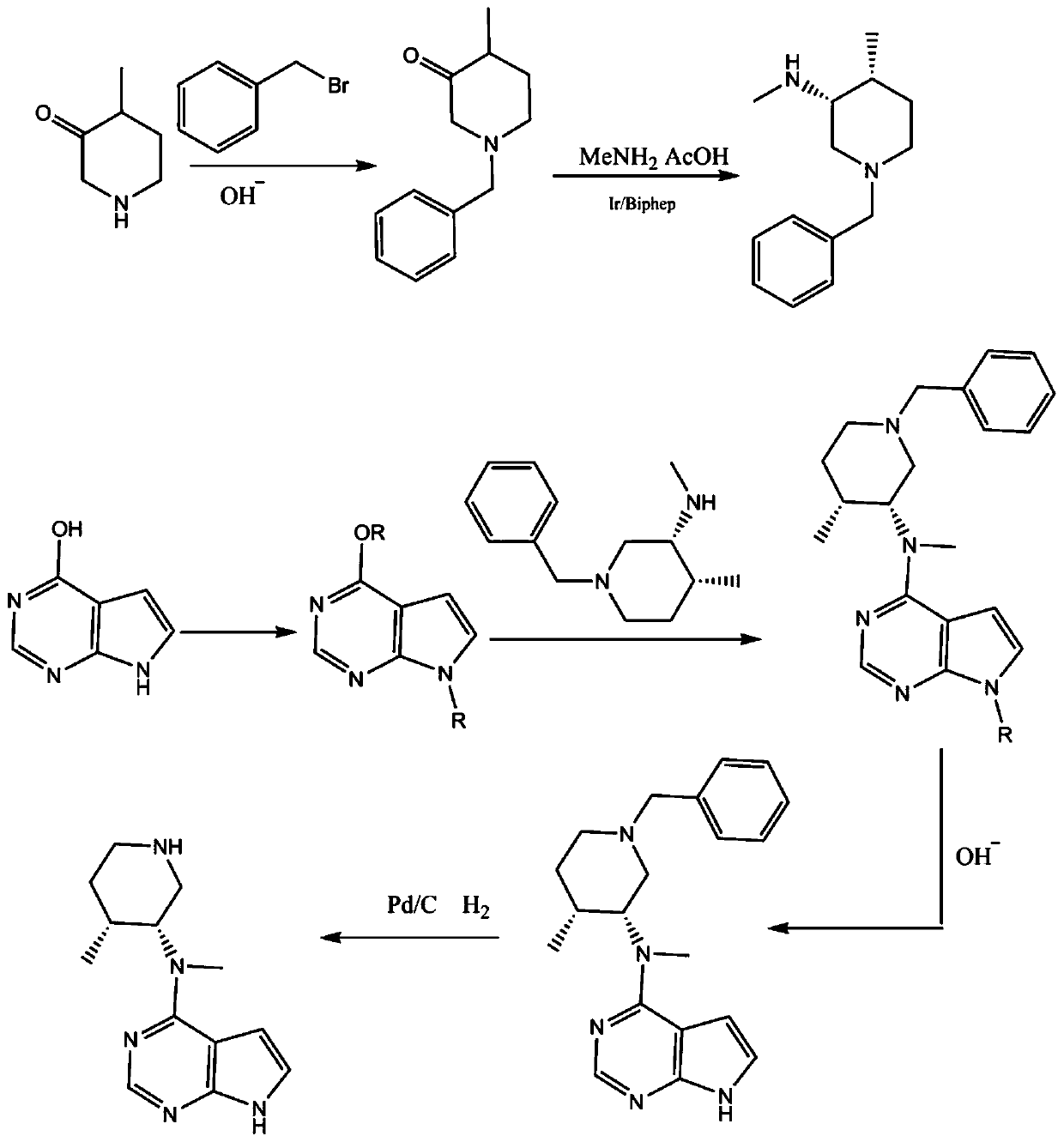
Preparing method of N-methyl-N-(4-methylpiperidine)-3-yl-7H-pyrropyrimidine-4-amine
Owner:南京焕然生物科技有限公司
Preparation method for Xeljanz related substance
InactiveCN107698595AMild reaction conditionsEasy to operateOrganic chemistry4-methylpiperidineCombinatorial chemistry
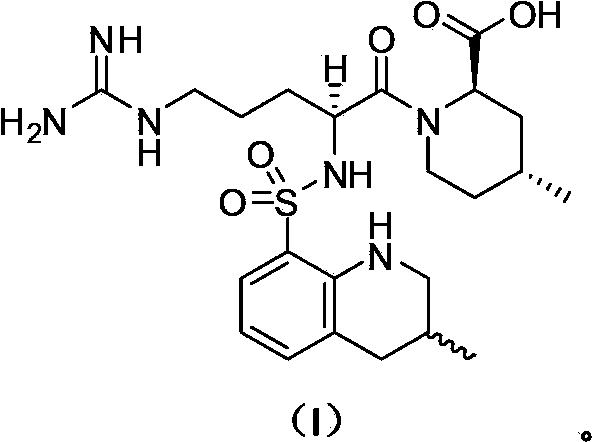
The invention discloses a preparation method for a Xeljanz related substance. The preparation method is characterized by comprising the following steps: with N-methyl-N-((3R,4R)-4-methylpiperidine-3-yl)-7H-pyrrolo[2,3-d]pyrimidine-4-amine hydrochloride and acetaldehyde as starting materials, preparing N-((3R,4R)-1-ethyl-4-methylpiperidine-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidine-4-amine under the action of a reducing agent, and carrying out salification so as to obtain the Xeljanz related substance namely N-((3R,4R)-1-ethyl-4-methylpiperidine-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidine-4-amine hydrochloride as shown in a formula (I) which is described in the specification.
Owner:JIANGSU QINGJIANG PHARMA
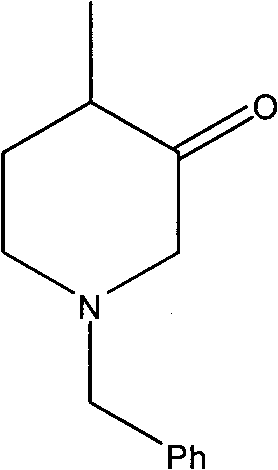
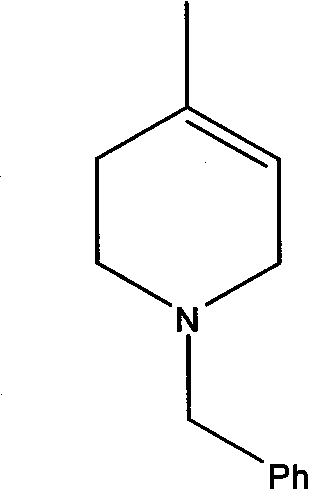
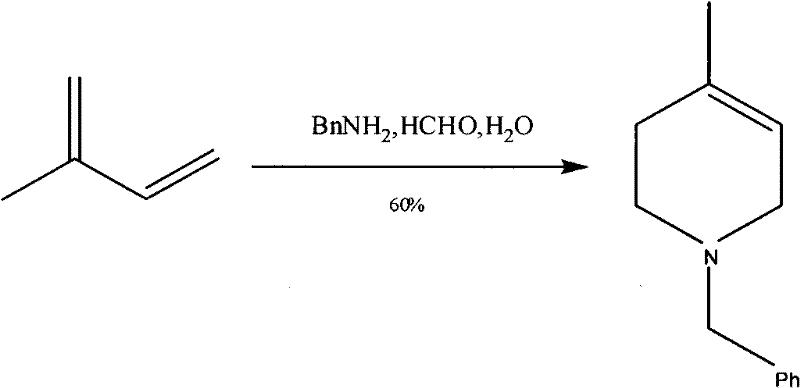
Synthesis method of N-benzyl-4-methylpiperidine-3-one hydrochloride
The invention discloses a synthesis method of N-benzyl-4-methylpiperidine-3-one hydrochloride. The synthesis method includes the steps of: a) with N-benzyl glycine ethyl ester as a raw material, performing a condensation reaction to the raw material with 2-methyl-4-halogenated ethyl acetate; and b) after the reaction is finished, performing an intramolecular cyclization reaction and finally performing hydrolytic decarboxylation to prepare the product. Compared with methods reported in references, the method establishes a novel synthesis route which is short in process, is novel in technology, employs low-cost raw materials and simple process operations, has good product quality and high total yield, and is suitable for industrial production.
Owner:CHONGQING WORLD HAORUI PHARM CHEM
Tofacitinib citrate impurity as well as analysis method and application thereof
InactiveCN110606846AHigh purityGuarantee quality and safetyOrganic chemistryComponent separation4-methylpiperidineMethyl group
The invention provides a novel impurity in tofacitinib citrate, namely, 3-((3R,4R)-3-((7-hydroxy-7H-pyrrolo[2,3-d]pyrimidine-4-yl)(methyl)amino)-4-methylpiperidine-1-yl)-3-oxopropionitrile. The preparation method of the compound is simple to operate, the obtained product is high in yield and purity, the invention further provides an analysis method of the compound, the method is sensitive, rapid and good in specificity, and the compound can be used as a reference substance for researching impurities in tofacitinib citrate raw materials and preparations, so that the product quality is effectively controlled.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
The synthetic method of n-benzyl-4-methyl-3-piperidone
The invention discloses a synthesis method of N-benzyl-4-methyl-3-piperidone, using 3-hydroxy-4-methylpyridine and benzyl chloride as starting materials, comprising the following steps: 1) mixing 3-Hydroxy-4-picoline is added to the reaction solvent I, and the mixture of benzyl chloride and reaction solvent I is added dropwise at room temperature, and the temperature is raised to reflux; 2) Add the 3-hydroxyl obtained in step 1) to the alkaline aqueous solution -benzyl chloride salt and reducing agent of 4-picoline, then warming up to reflux; 3) adding the N-benzyl-3-hydroxyl-4-methylpiperidine obtained in step 2) into the reaction solvent II, dropwise Add CrO 3 solution and concentrated sulfuric acid, and react at room temperature to obtain N-benzyl-4-methyl-3-piperidone. The synthesis method has the characteristics of high total yield and the like.
Owner:ZHEJIANG UNIV
Method for preparingtofacitinib citrate
ActiveCN110343111ALow priceEasy to getOrganic compound preparationCarboxylic acid salt preparation4-methylpiperidineFiltration
The invention provides a method for preparing tofacitinib citrate. The method for preparing tofacitinib citratecomprises the following steps that1, N-methyl-N-((3R,4R)-4-methylpiperidine-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine and ethyl cyanoacetate are added into methyl alcohol for condensation reaction to prepare and obtain 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile;and 2,3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrileis added to a mixed solvent and subjected to 80+ / -5 DEG reflux, additionally, citric acid is dissolved in the mixed solvent and then added slowly to a reflux system forsalt forming reaction, cooling, filtration, washing, and drying to obtain 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile citrate. According to the method for preparing tofacitinib citrate, operation isconvenient, time consumption isless, the yield rate is high, product impurity is low,and purity is good.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Methods and compositions for repelling arthropods
A method for repelling arthropods involving treating an object or area with an arthropod repelling effective amount of at least one compound having the formulawherein X is O, S, NH, N-NH2, N-CH3 or CH2, R′ is H or alkyl, R″ is alkyl, n is 0, 1, 2, 3 or 4, and mixtures thereof, optionally including a carrier material or carrier. The compound is preferably selected from homopiperazine, 1-methylhomopiperazine, 1-methylpyrrolidine, (R)-(−)-2-methylpiperazine, (S)-(+)-2-methylpiperazine, 2-methylpiperazine, 1-methylpiperazine, pyrrolidine, 1-methylpiperidine, piperidine, 1-ethylpiperazine, 1-methylimidazolidine, 1-methylthiomorpholine, 1,4-dimethylpiperazine, homopiperidine, imidazolidine, 4-methylpiperidine, thiomorpholine, 1-amino-4-methylpiperazine, 4-methylmorpholine, azocane, 2,6-dimethylpiperazine, 2,5-dimethylpiperazine, piperazine, 1-methlyhomopiperidine, or mixtures thereof.
Owner:US SEC AGRI
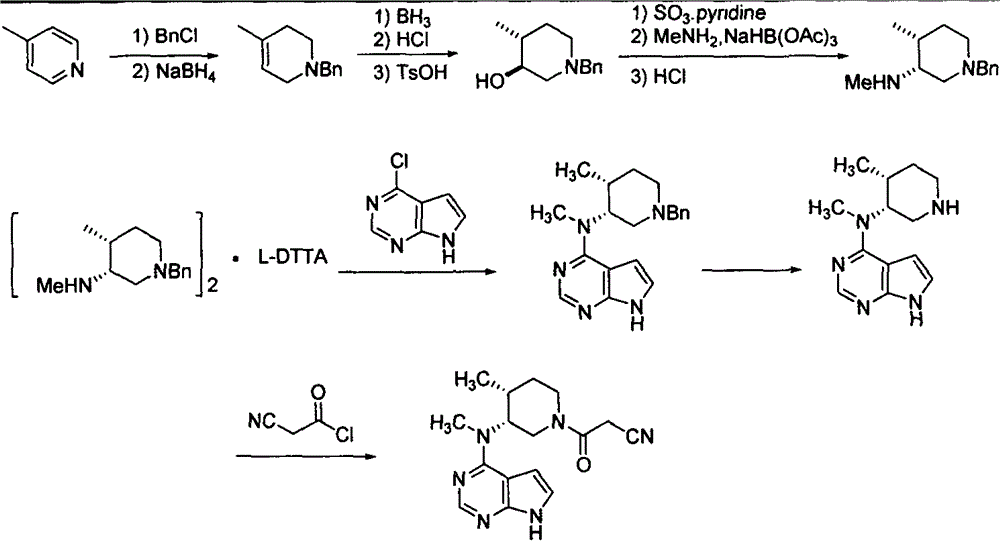
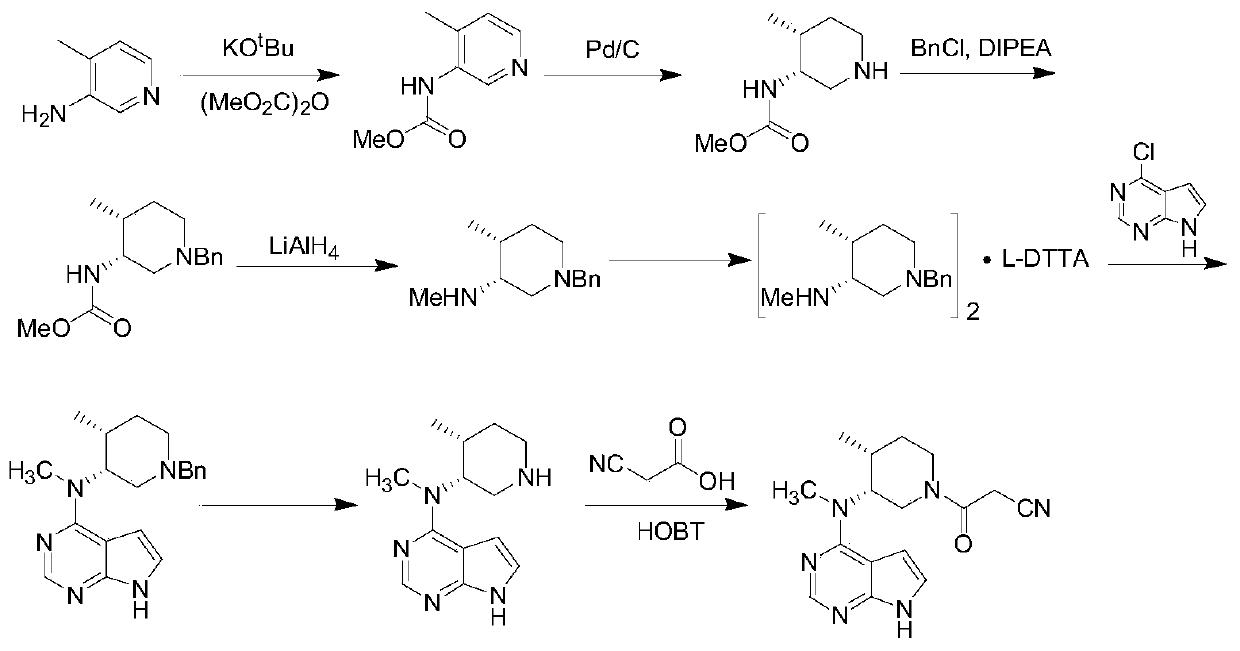
Preparation method of (3R,4R)-(1-benzyl-4-methylpiperidine-3-yl)methylamine-L-di-p-toluoyltartaric acid salt
InactiveCN105237463ANovel process designHigh yieldOrganic chemistryCondensation process4-methylpiperidine
The invention discloses a preparation method of (3R,4R)-(1-benzyl-4-methylpiperidine-3-yl)methylamine-L-di-p-toluoyltartaric acid salt prepared from the following reaction steps shown in the description. The preparation method of (3R,4R)-(1-benzyl-4-methylpiperidine-3-yl)methylamine-L-di-p-toluoyltartaric acid salt has the following advantages that the process is novel in design, extremely dangerous chemicals (such as lithium aluminum hydride) are not required to be used, a folding condensation process is adopted, and the total yield is high; more importantly, the starting materials are cheap and easy to obtain, and the cost is low; and the maneuverability is high, and industrialization is easy.
Owner:刘卫国
Method for preparing 4-methylpiperidine-2-carboxylate hydrochloride
ActiveCN102887854BReduce dosageEasy to operateOrganic chemistryPhosphomolybdic acid4-methylpiperidine
The invention relates to a method for preparing 4-methylpiperidine-2-carboxylate hydrochloride which is an intermediate of argatroban active precursor (2R, 4R)-4-methylpiperidine-2-carboxylic acid ethyl ester. 4-methylpiperidine-2-carboxylic acid ethyl ester is adopted as a raw material, phosphomolybdic acid is used as a catalyst, oxidizing is performed to obtain 4-methylpiperidine-2-carboxylic acid ethyl ester nitrogen oxide, methanol or ethanol is taken as a solvent, and 4-methylpiperidine-2-carboxylate hydrochloride is produced through reduction reaction. The method is simple to operate and has moderate conditions; phosphomolybdic acid is adopted to increase the activity of an oxidizing agent of hydrogen peroxide, the usage of the oxidizing agent is reduced, and the reaction yield is increased; the purification is convenient, the product quality is excellent, and good safety is obtained; and pollution is reduced, and industrial production is benefited.
Owner:CHANGSHAN BIOCHEM PHARM JIANGSU CO LTD
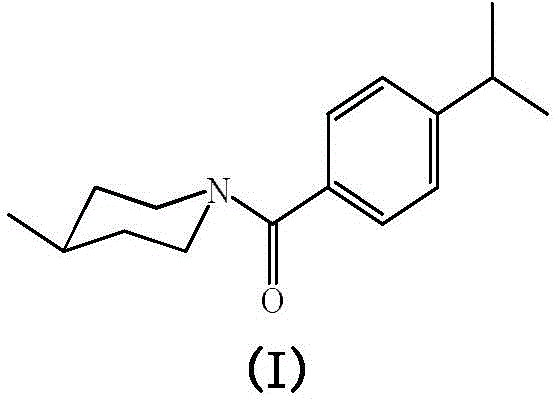
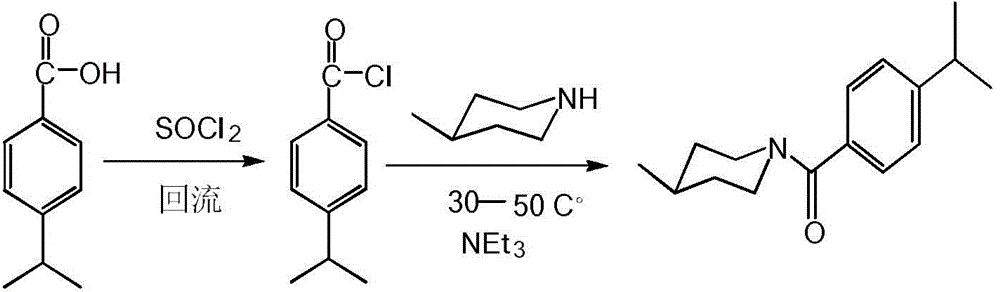
A compound with antibacterial activity and preparation method and application of water-soluble liquid thereof
InactiveCN104478792BGood environmental compatibilityHigh inhibition rateBiocideOrganic chemistryBenzoic acid4-methylpiperidine
The invention discloses preparation methods and application of a compound with antibacterial activity and water-soluble liquid thereof, and relates to the field of pesticides. The compound is N-(4-isopropyl benzoyl)-4-methylpiperidine, and the structural formula of the compound is the formula (I) shown in the description. The preparation method of the compound comprises the following steps: enabling 4-isopropyl benzoic acid to react with thionyl chloride under a heating reflux condition to obtain p-isopropyl benzoyl chloride; adding a catalyst, namely, pyridine or triethylamine into 4-methylpiperidine and dichloromethane; agitating under the room temperature; dropwise adding p-isopropyl benzoyl chloride to react; filtering, washing and recrystallizing to obtain a white solid namely the compound. The compound has excellent inhibitory activity for bipolaria maydis, sclerotinia sclerotioru, botrytis cinerea and other plant pathogenic fungi; in addition, an emulsifier, an anti-foaming agent and a solvent can be added into the compound to prepare into the water-soluble liquid and other pesticide preparations for developing and application.
Owner:XIHUA UNIV
A kind of synthetic method of jasmonal
InactiveCN103936709BRaw materials are easy to getLow costOrganic chemistrySodium bicarbonate4-methylpiperidine
Owner:李东风
Polymorph of triaryldimethylpiperazine dihydrochloride and preparation method and application thereof
ActiveUS20190270720A1Improve solubilityPromote absorptionOrganic active ingredientsNervous disorder4-methylpiperidinePharmacology
The application discloses five polymorph forms B, P, F, J, O of (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazine-1-yl)(3-hydroxyphenyl)methyl)phenyl)(4-methylpiperidine-1-yl)methanone dihydrochloride, preparation methods thereof and application thereof in the manufacture of a medicament for preventing or treating a mood disorder or a disease related to a δ opioid receptor.
Owner:YUNNAN INST OF MATERIA MEDICA
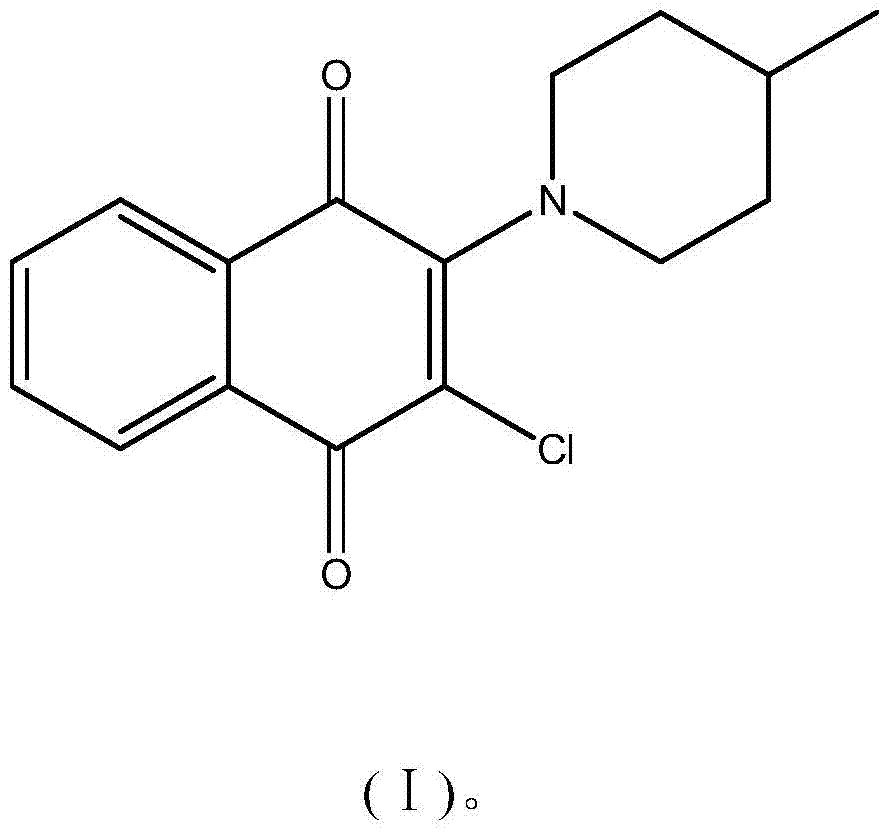
Application of a naphthalene-1,4-diketone compound as an hcbs enzyme inhibitor
The invention discloses the application of a naphthalene-1,4-diketone compound as an hCBS enzyme inhibitor, in particular to the compound 2-chloro-3-(4-methylpiperidin-1-yl)naphthalene-1,4 - Use of diketones as hCBS enzyme inhibitors. The IC50 of the small molecular compound on the hCBS enzymatic reaction in vitro is 20 μM. The small molecular compound can be used as a tool drug for studying the H2S signaling pathway, and a lead compound for developing drugs for treating circulatory shock, stroke, Down syndrome and tumors and other diseases related to H2S.
Owner:SHANGHAI JIAO TONG UNIV
A kind of synthetic method of n-benzyl-4-methylpiperidin-3-one hydrochloride
The invention discloses a synthesis method of N-benzyl-4-methylpiperidine-3-one hydrochloride. The synthesis method includes the steps of: a) with N-benzyl glycine ethyl ester as a raw material, performing a condensation reaction to the raw material with 2-methyl-4-halogenated ethyl acetate; and b) after the reaction is finished, performing an intramolecular cyclization reaction and finally performing hydrolytic decarboxylation to prepare the product. Compared with methods reported in references, the method establishes a novel synthesis route which is short in process, is novel in technology, employs low-cost raw materials and simple process operations, has good product quality and high total yield, and is suitable for industrial production.
Owner:CHONGQING WORLD HAORUI PHARM CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com