A compound with antibacterial activity and preparation method and application of water-soluble liquid thereof
A technology for bacteriostatic activity and compound is applied in the field of preparation of compounds and water-soluble liquid preparations thereof, and can solve the problem that the bacteriostatic activity of N-(4-isopropylbenzoyl)-4-methylpiperidine is unknown. The use of chemical compounds and other problems can achieve the effect of good control effect, good environmental compatibility and simple structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
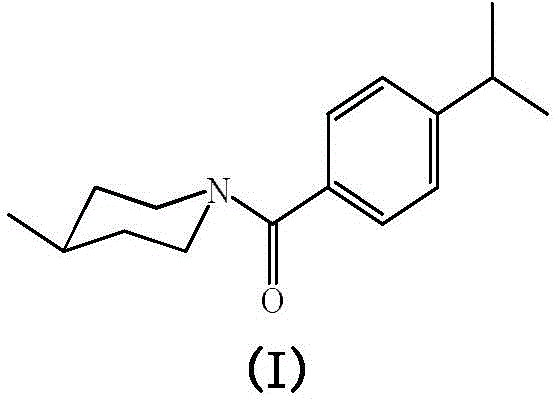
[0028] Preparation of compound N-(4-isopropylbenzoyl)-4-methylpiperidine
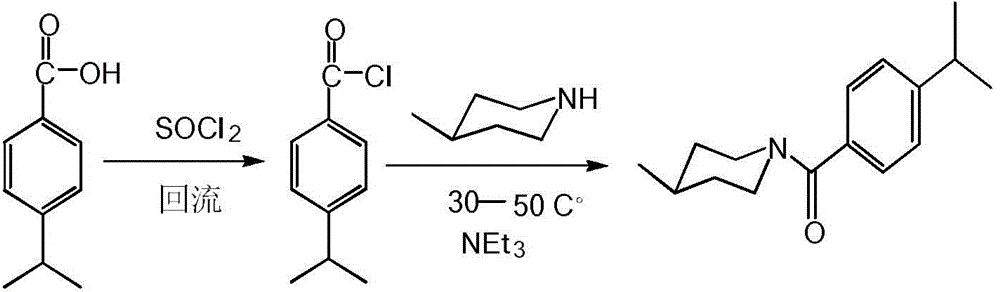
[0029] Add 0.01mol p-isopropylbenzoic acid and 15mL thionyl chloride to a 50mL three-neck flask, heat to 80°C and reflux for 2 hours, distill off excess thionyl chloride until no liquid flows out, and cool to At room temperature, p-isopropylbenzoyl chloride can be obtained.
[0030] Add 0.01mol 4-methylpiperidine and 10mL dichloromethane into a 50mL three-necked flask, add 3mL pyridine or triethylamine, and add freshly prepared p-isopropylbenzoyl chloride dropwise while stirring at room temperature. After the dropwise addition, continue the reaction at 30-50°C for 5 hours. After the reaction is complete, wash the reaction mixture with 10% hydrochloric acid, 10% NaOH and distilled water to neutrality, and place it in a refrigerator at 2-6°C until the solid precipitates Afterwards, filtered, washed, and recrystallized with absolute ethanol to obtain a white solid product with a yield of 81%. The spectra...
Embodiment 2
[0034] Preparation of 5% N-(4-isopropylbenzoyl)-4-methylpiperidine water-soluble solution
[0035] Weigh 5kg of N-(4-isopropylbenzoyl)-4-methylpiperidine into 86.99kg of ethylene glycol, add 0.01kg of dibutyl phthalate, stir at a constant temperature of 30°C, slowly Add 8 kg of Tween-20 until the solution is completely clear and transparent, and then stir at constant temperature for 20 minutes to obtain a 5% N-(4-isopropylbenzoyl)-4-methylpiperidine water-soluble liquid.
Embodiment 3
[0037] Preparation of 10% N-(4-isopropylbenzoyl)-4-methylpiperidine water-soluble solution
[0038] Weigh 10kg of N-(4-isopropylbenzoyl)-4-methylpiperidine into 74.99kg of diethylene glycol, add 0.01kg of dibutyl phthalate, stir at a constant temperature of 50°C, slowly Add 15kg of OP-7 until the solution is completely clear and transparent, and then stir at constant temperature for 30 minutes to obtain a 10% N-(4-isopropylbenzoyl)-4-methylpiperidine water-soluble liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com