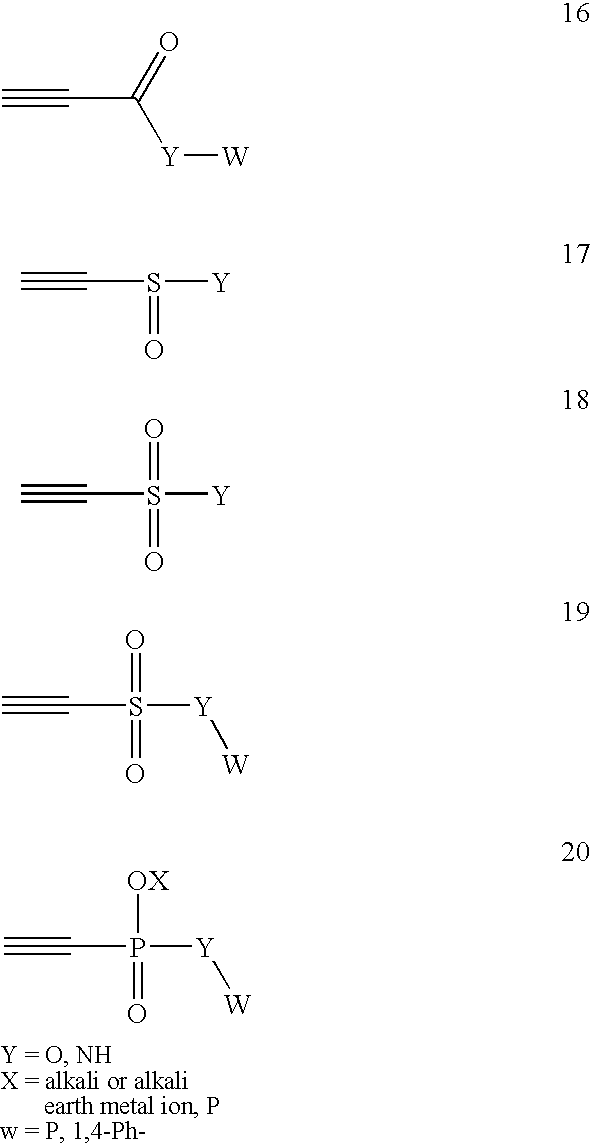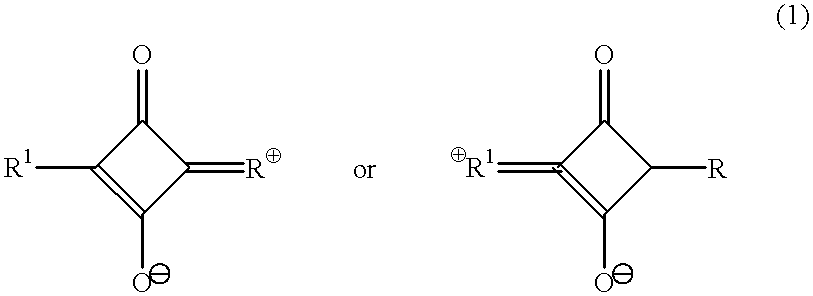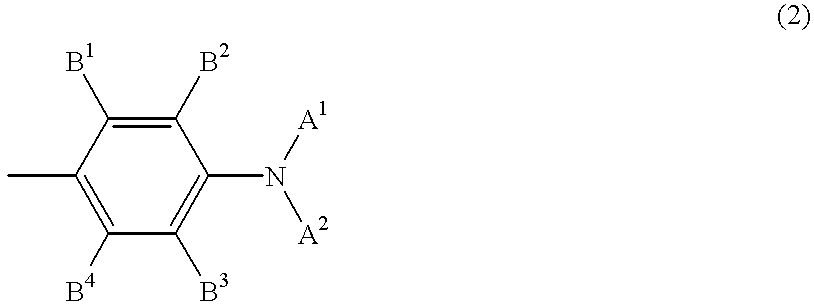Patents
Literature
4049 results about "Active compound" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Miniaturized cell array methods and apparatus for cell-based screening
InactiveUS6103479AImprove throughputIncrease contentBioreactor/fermenter combinationsMaterial nanotechnologyTemporal informationHigh-Throughput Screening Methods
The present invention discloses devices and methods of performing high throughput screening of the physiological response of cells to biologically active compounds and methods of combining high-throughput with high-content spatial information at the cellular and subcellular level as well as temporal information about changes in physiological, biochemical and molecular activities. The present invention allows multiple types of cell interactions to be studied simultaneously by combining multicolor luminescence reading, microfluidic delivery, and environmental control of living cells in non-uniform micro-patterned arrays.
Owner:CELLOMICS
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
One component EUV photoresist
In one embodiment, a photoactive compound may be attached to a polymer backbone. This embodiment may be more resistant to the generation of reactive outgassing components and may exhibit better contrast.
Owner:INTEL CORP
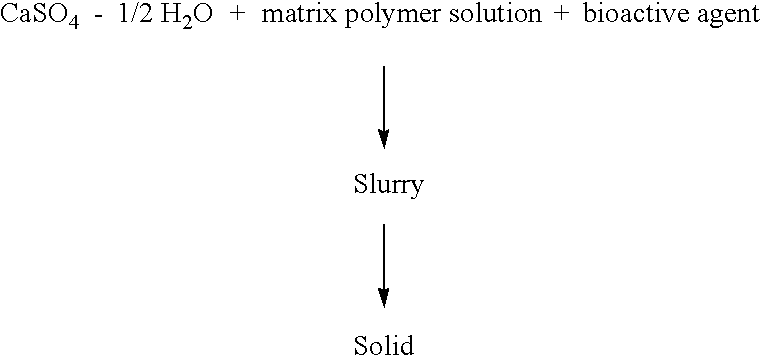
Resorbable matrices for delivery of bioactive compounds
This invention relates generally to the production and use of inorganic-conditioning agent complexes for the controlled release of compounds including medicinals. Advantageously, the inorganic used is calcium sulfate and the conditioning agent is calcium stearate.
Owner:ROYER BIOMEDICAL INC
Methods of using and compositions comprising (+) sibutramine optionally in combination with other pharmacologically active compounds
This invention encompasses methods for the treatment and prevention of disorders that include, but are not limited to, eating disorders; weight gain; obesity; irritable bowel syndrome; obsessive-compulsive disorders; platelet adhesion; apnea; affective disorders such as attention deficit disorders, depression, and anxiety; male and female sexual function disorders; restless leg syndrome; osteoarthritis; substance abuse including nicotine and cocaine addiction; narcolepsy; pain such as neuropathic pain, diabetic neuropathy, and chronic pain; migraines; cerebral function disorders; chronic disorders such as premenstrual syndrome; and incontinence. The invention further encompasses pharmaceutical compositions and dosage forms which comprise optically pure (+) sibutramine, optionally in combination with a phosphodiesterase inhibitor or a lipase inhibitor.
Owner:SEPACOR INC
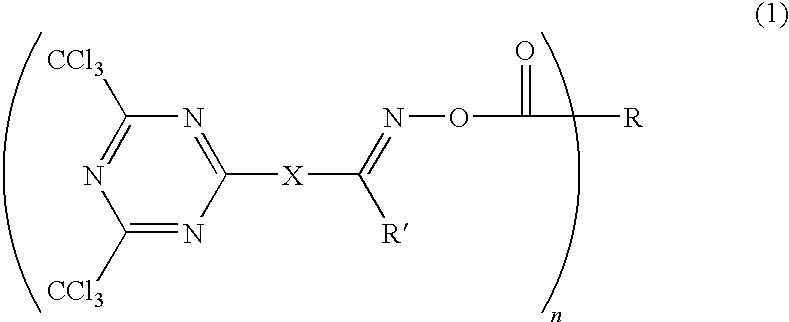
Photosensitive composition comprising triazine-based photoactive compound containing oxime ester
ActiveUS7556910B2Effective absorptionDevelopment durabilityOrganic chemistryPhotosensitive materialsOximePhotochemistry
The present invention relates to a photosensitive composition comprising a triazine-based photoactive compound containing oxime ester. The photosensitive composition according to the present invention has good sensitivity, retention rate, mechanical strength, heat resistance, chemical resistance and developing durability since it contains, as photopolymerization initiator, a compound having an oxime ester group and a triazine group in one molecule and thus effectively absorbs UV radiation. Therefore, the photosensitive composition according to the present invention is advantageous not only in curing of materials for color filters, resin black matrixes, column spacers, overcoats and passivation films of liquid crystal displays, but also in high temperature process characteristics.
Owner:LG CHEM LTD
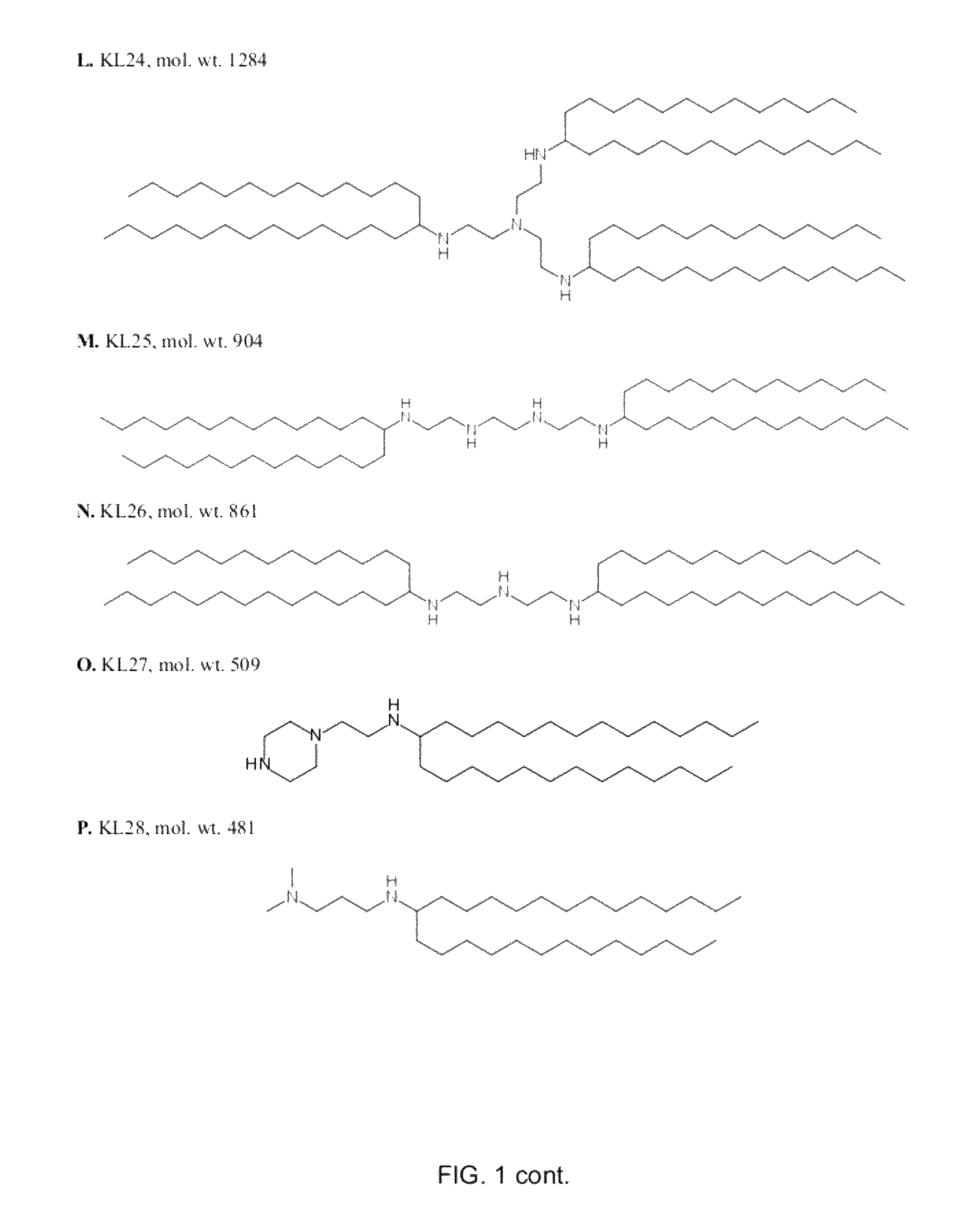
Lipids and compositions for intracellular delivery of biologically active compounds
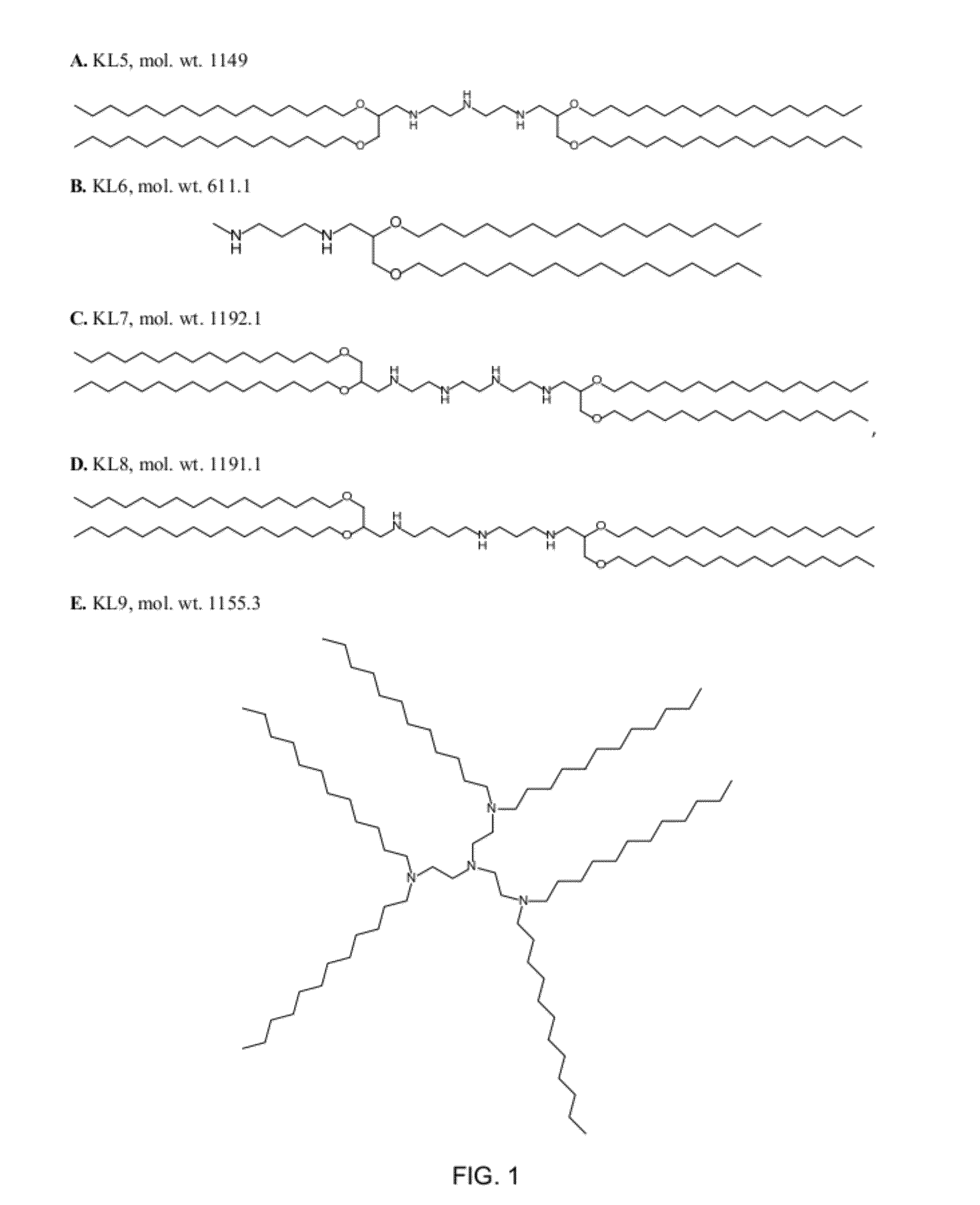
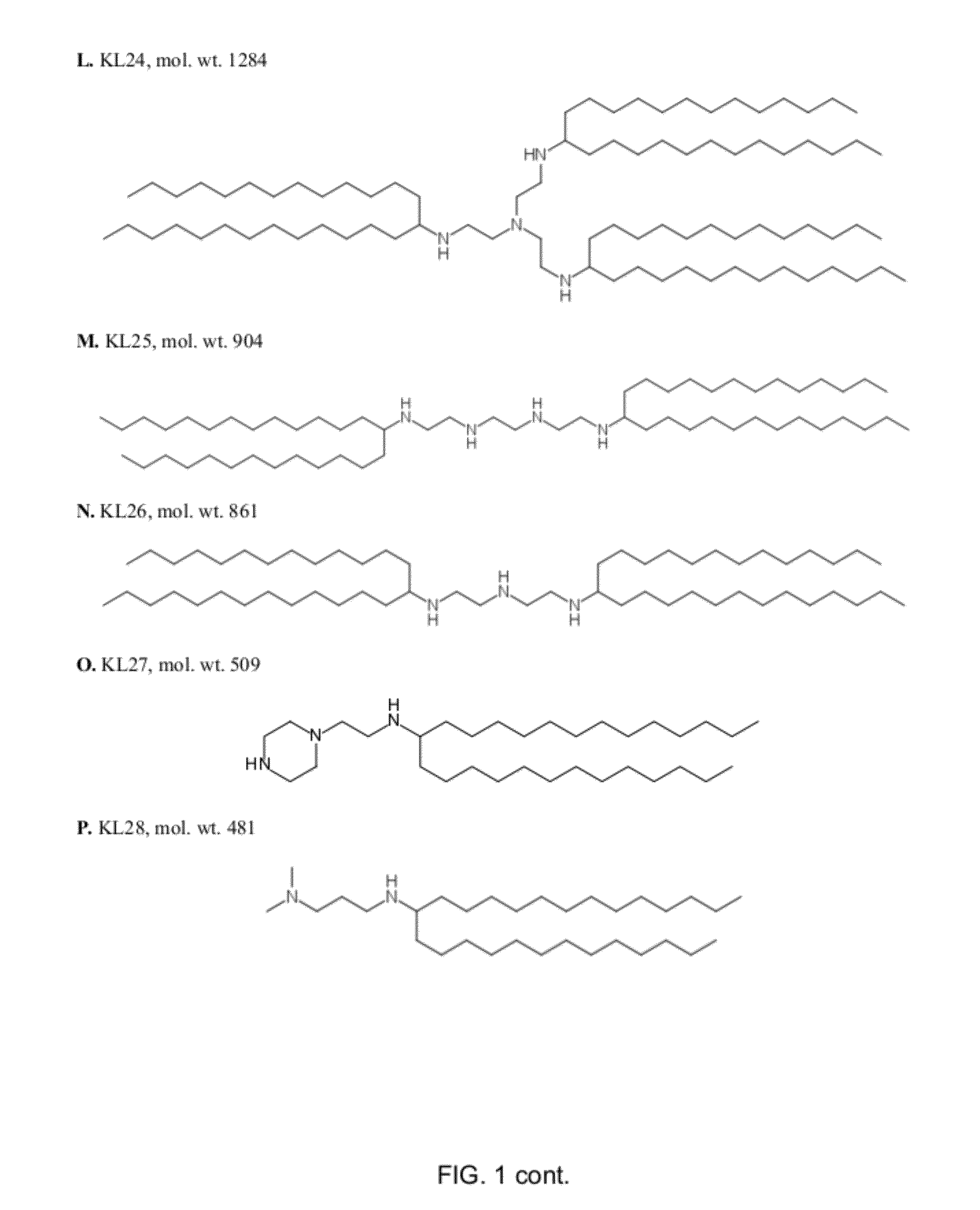
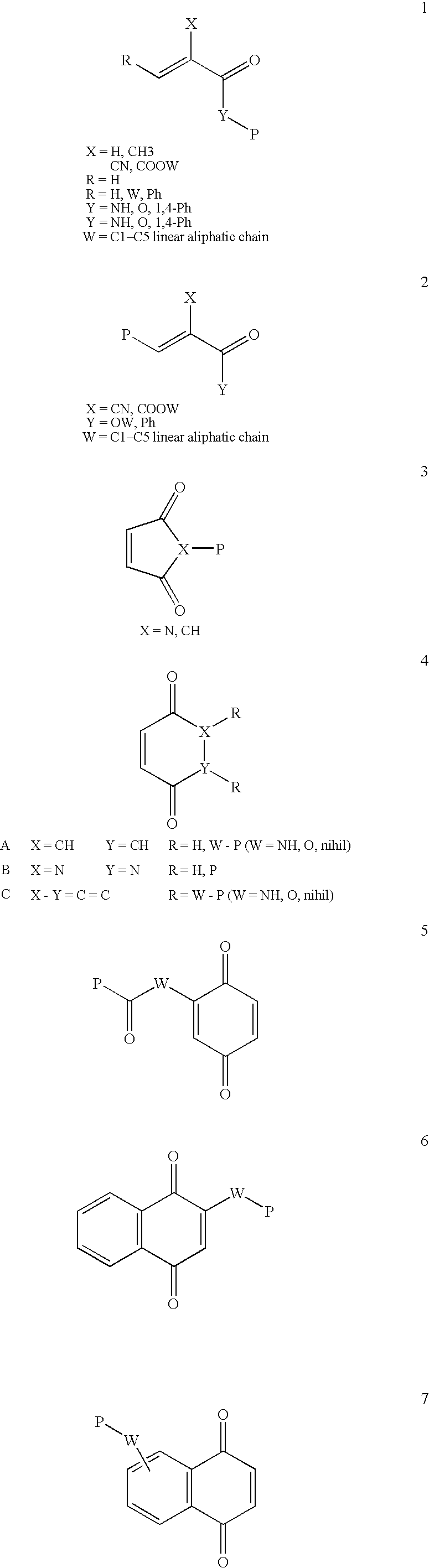
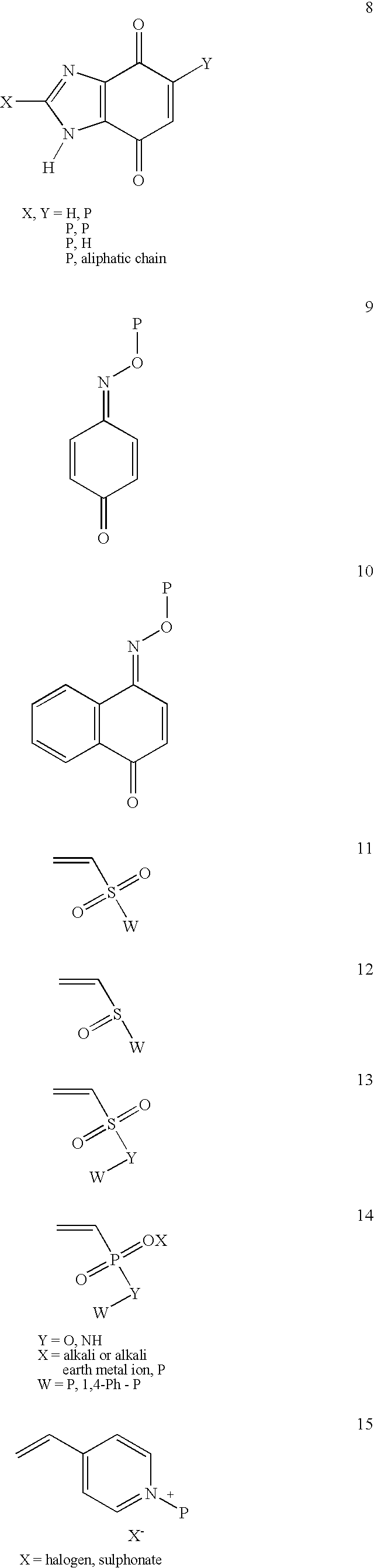
ActiveUS8691750B2High yieldHigh purityPowder deliveryMaterial nanotechnologyLipid formationIntracellular drug delivery
The present invention provides novel amino-lipids, compositions comprising such amino-lipids and methods of producing them. In addition, lipid nanoparticles comprising the novel amino-lipids and a biologically active compound are provided, as well as methods of production and their use for intracellular drug delivery.
Owner:AXOLABS
Ocular plug formed from placenta derived collagen biofabric
The present invention relates to ocular plugs formed from a biodegradable material. The plugs comprises a shaft and, optionally, a cap. The ocular plugs are intended to occlude, and to repair, discontinuities in the sclera, whether formed deliberately during injection or surgical foray into the eye, or accidentally. The method further provides methods of making the ocular plug. the invention also provides methods of using the ocular plugs to occlude and repair discontinuities in the sclera, or to deliver biologically active compounds to the sclera or the eye. Finally, the invention provides kits comprising one or more ocular plugs in a container.
Owner:LIU QING +1
Novel Lipids and Compositions for Intracellular Delivery of Biologically Active Compounds
ActiveUS20120295832A1High yieldHigh purityPowder deliveryBiocideLipid formationIntracellular drug delivery
The present invention provides novel amino-lipids, compositions comprising such amino-lipids and methods of producing them. In addition, lipid nanoparticles comprising the novel amino-lipids and a biologically active compound are provided, as well as methods of production and their use for intracellular drug delivery.
Owner:AXOLABS
Conjugate addition reactions for the controlled delivery of pharmaceutically active compounds
InactiveUS6958212B1Reducing and delaying onsetGood water solubilitySugar derivativesPeptide/protein ingredientsBiological materialsPolymer
The invention features polymeric biomaterials formed by nucleophilic addition reactions to conjugated unsaturated groups. These biomaterials may be used for medical treatments.
Owner:ETH ZZURICH +1
Abuse-resistant pharmaceutical compositions
An abuse-resistant controlled release pharmaceutical composition comprising a pharmaceutically effective amount of discrete particles of an active capable of abuse, wherein surfaces of said particles are wetted with a water insoluble coating material, and preferably wherein said composition comprises a matrix, in which said particles are distributed, and which renders the abuse-capable compound within the matrix difficult to separate from the matrix; and a method for the preparation of a controlled release pharmaceutical composition having a reduced potential for abuse, comprising applying a pressure force to a mixture comprising a water insoluble material, and particles of a pharmaceutically active compound capable of inducing in a subject a reaction that is physiologically or psychologically detrimental if administered in an immediate release dosage form, thereby resulting in surface coated particles, and incorporating said surface coated particles into a pharmaceutical composition
Owner:ELAN PHRMA INT LTD
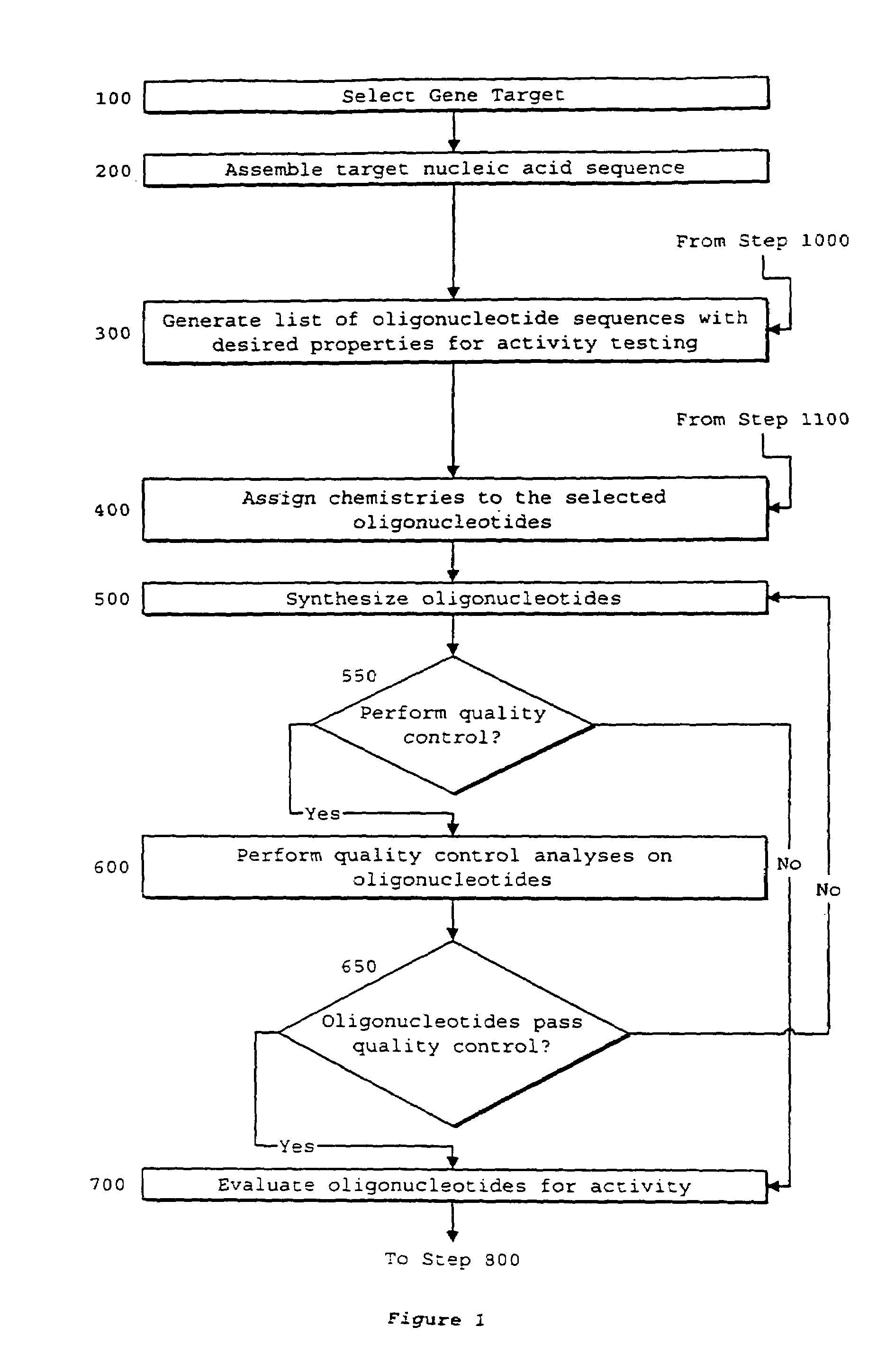
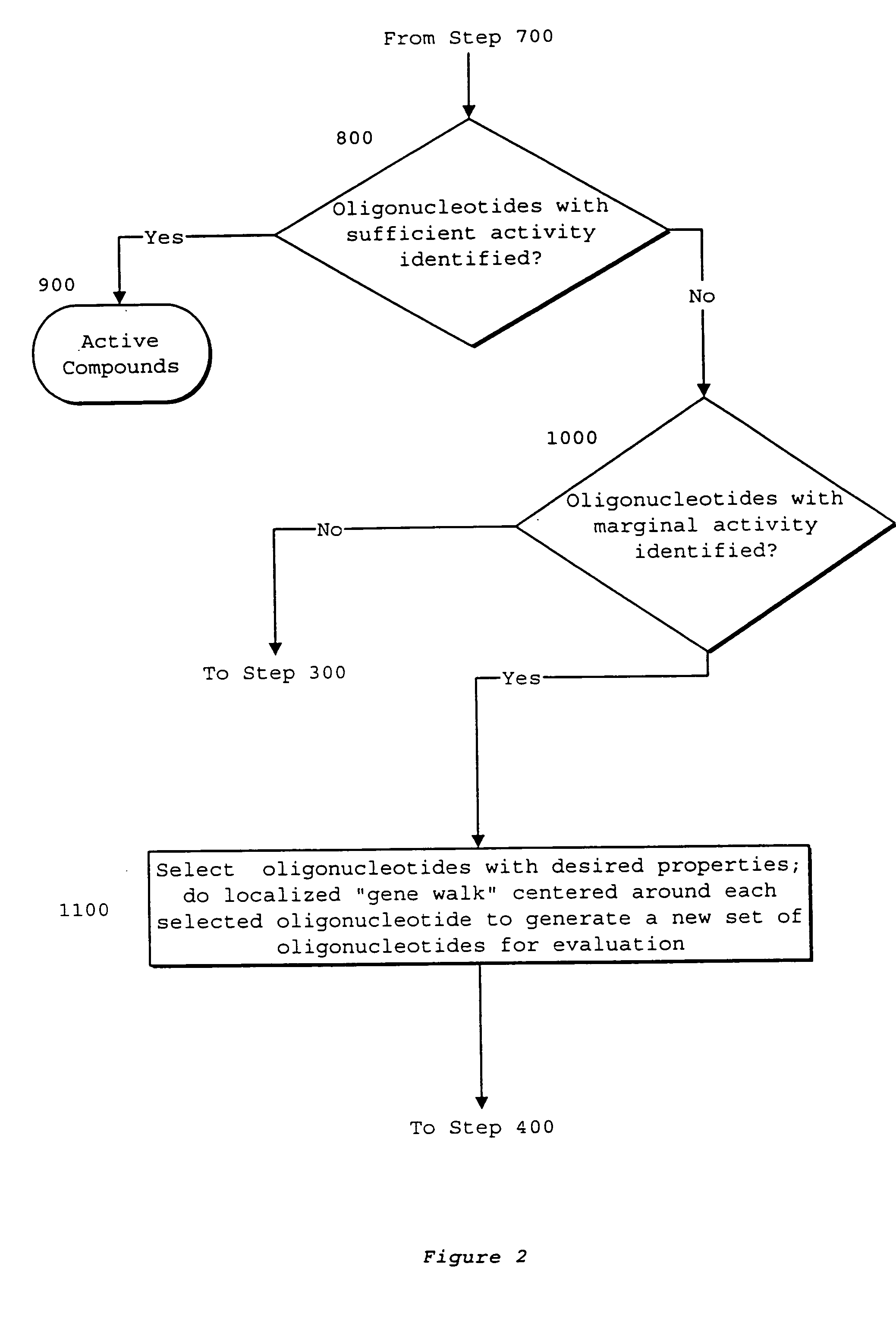
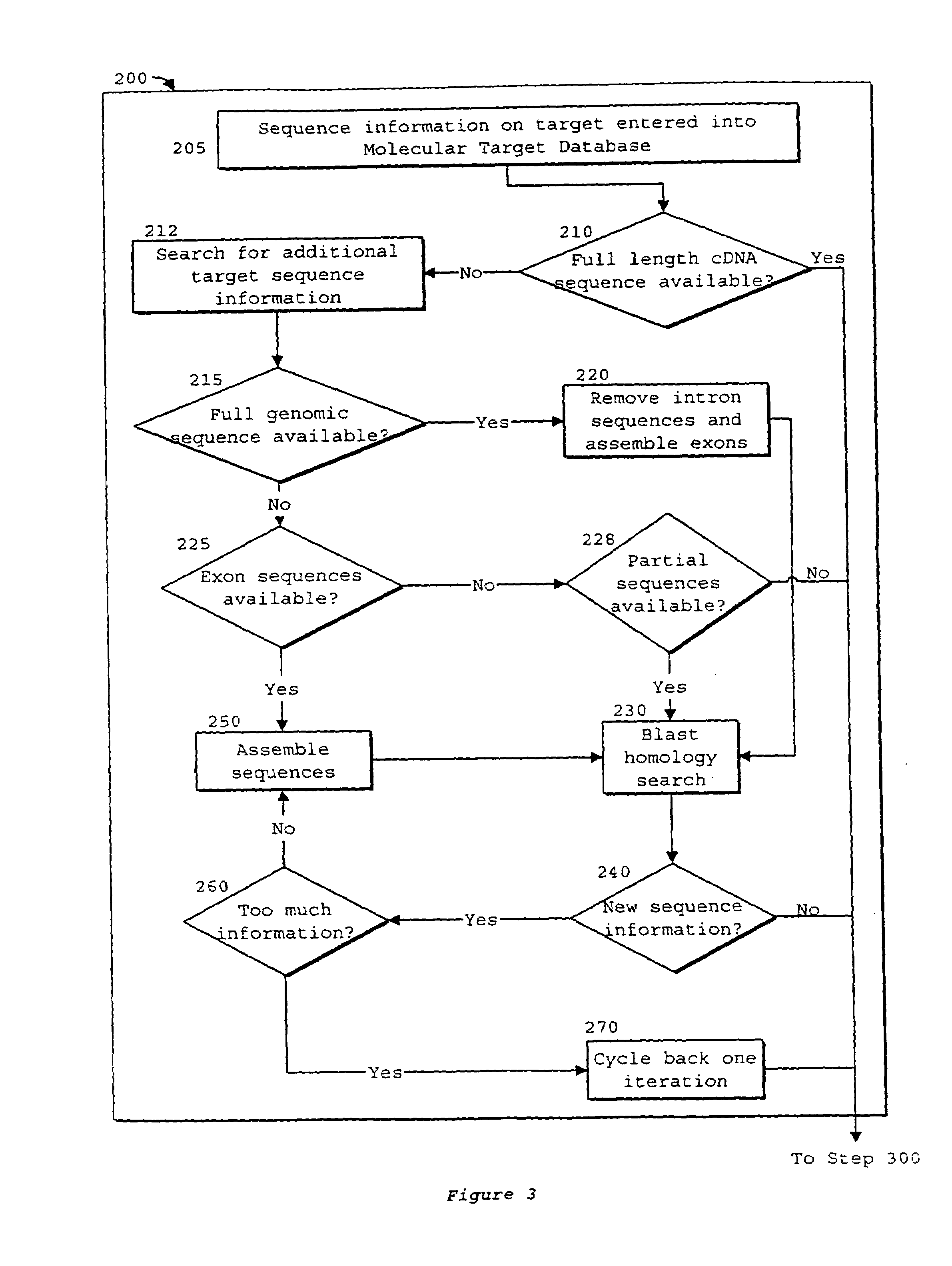
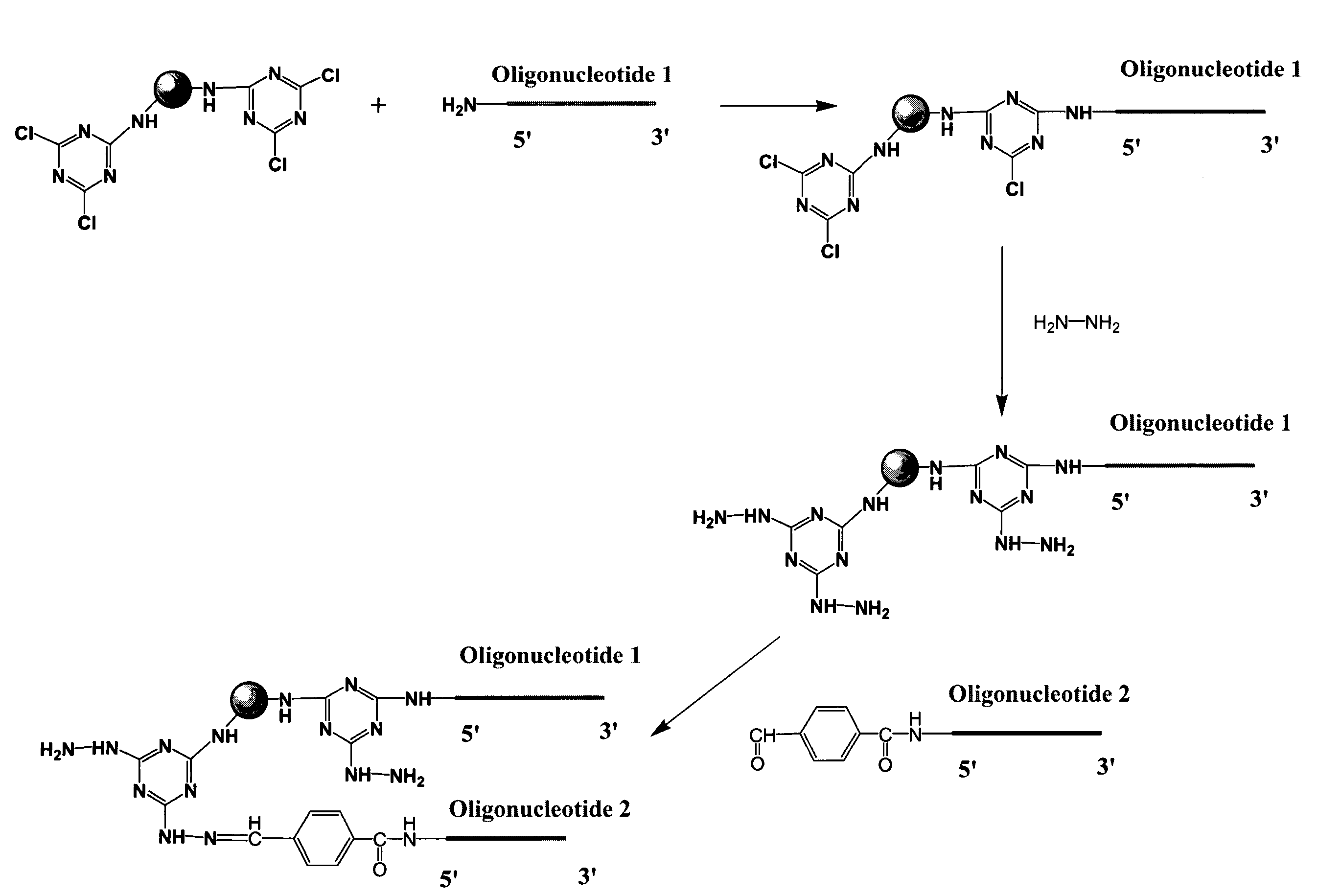
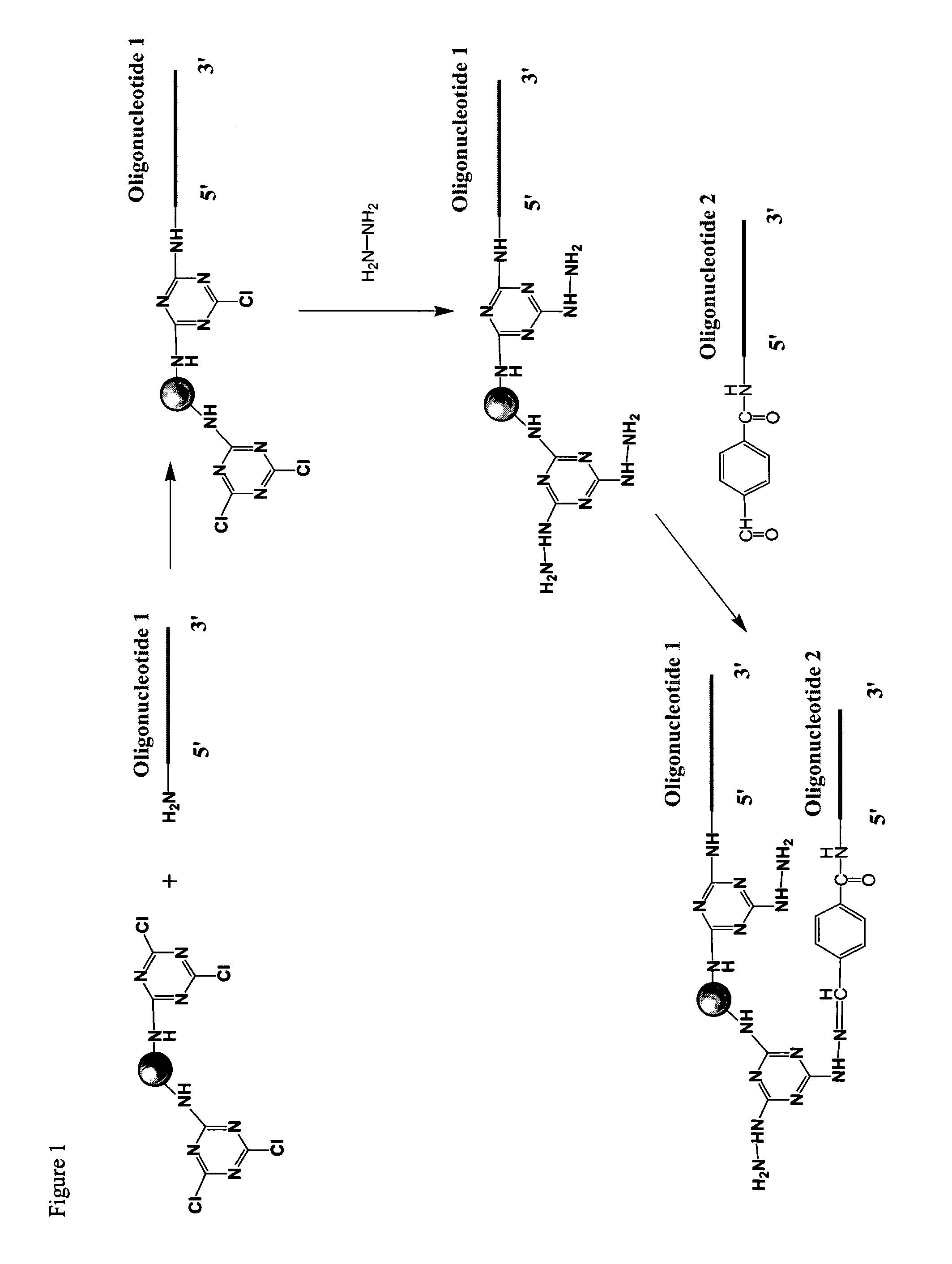
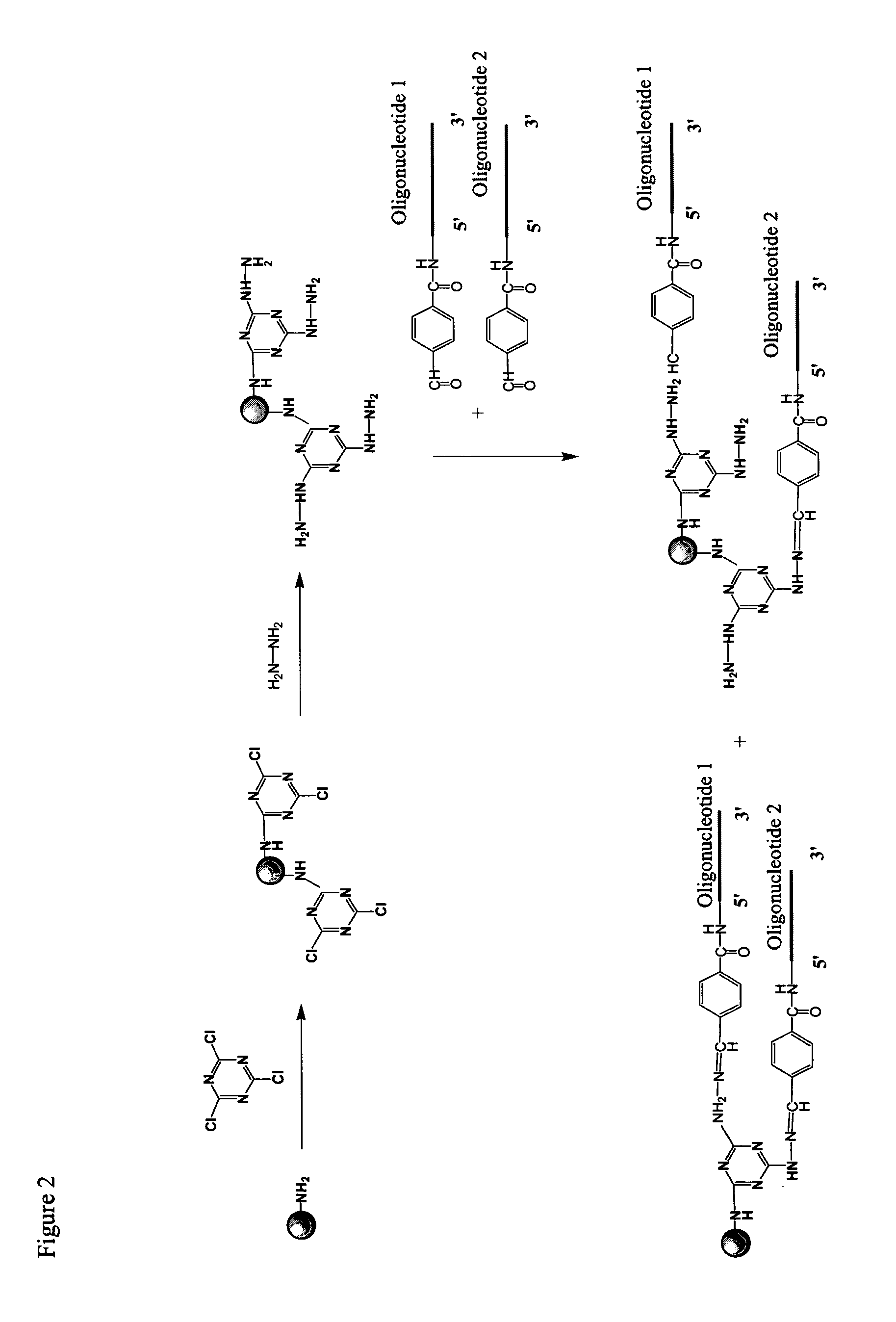
System of components for preparing oligonucleotides
Interative, preferably computer based iterative processes for generating synthetic compounds with desired physical, chemical and / or bioactive properties, i.e., active compounds, are provided. During iterations of the processes, a target nucleic acid sequence is provided or selected, and a library of candidate nucleobase sequences is generated in silico according to defined criteria. A “virtual” oligonucleotide chemistry is chosen and a library of virtual oligonucleotide compounds having the selected nucleobase sequences is generated. These virtual compounds are reviewed and compounds predicted to have particular properties are selected. The selected compounds are robotically synthesized and are preferably robotically assayed for a desired physical, chemical or biological activity. Active compounds are thus generated and, at the same time, preferred sequences and regions of the target nucleic acid that are amenable to oligonucleotide or sequence-based modulation are identified.
Owner:IONIS PHARMA INC
Slow-release matrix pellets and the production thereof
InactiveUS6290990B1Simple and low-cost productionHigh content of active substancesPharmaceutical non-active ingredientsGranular deliveryMaximum diameterPlasticizer
Slow-release matrix pellets with a spherical or lenticular shape and uniform maximum diameters in the range from 0.5 to 4 mm, composed ofa) 0.1-87% by weight of at least one biologically active compound,b) 5-50% by weight of at least one water-insoluble polymer,c) 5-45% by weight of at least one lipophilic component as plasticizer for polymer b),d) 3-40% by weight of a natural or semisynthetic gel former,e) 0-50% by weight of one or more conventional formulation aids.
Owner:BASF AG
Raman-active taggants and their recognition
InactiveUS6610351B2Easy to useQuality improvementMaterial nanotechnologyRadiation applicationsMaximum dimensionActive component
An organic or organoelement, linear or branched, monomeric or polymeric composition of matter having a Raman-active component in the form of particles. The particles having a maximum dimension of 50 mum. The Raman-active compound is applied to a substrate. When the Raman-active compound is exposed to a laser light wavelength which is batochromically well beyond a spectral region of maximum absorbance of said Raman-active compound, Raman scattering can be detected.
Owner:QUANTAG SYST
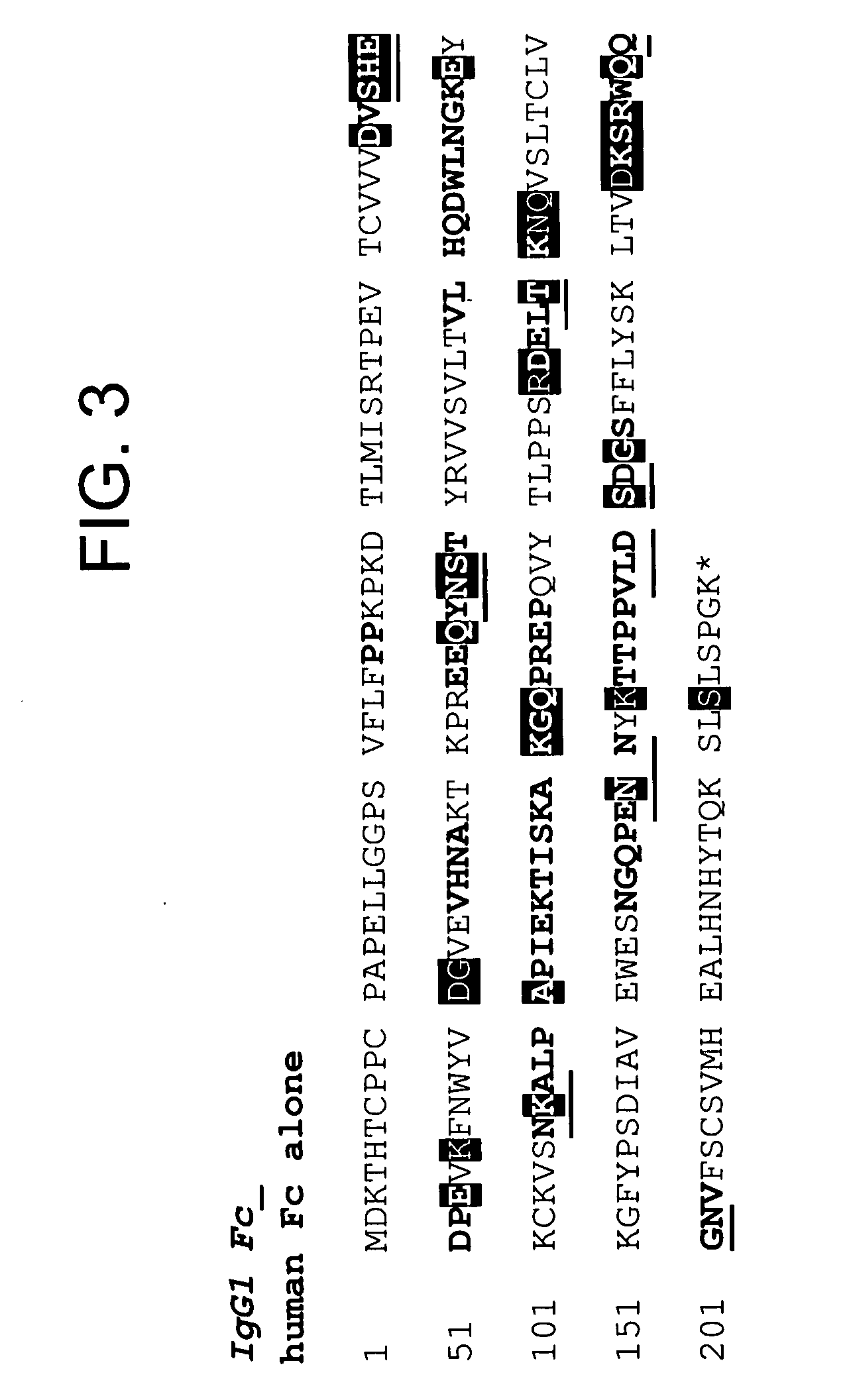
Modified Fc molecules
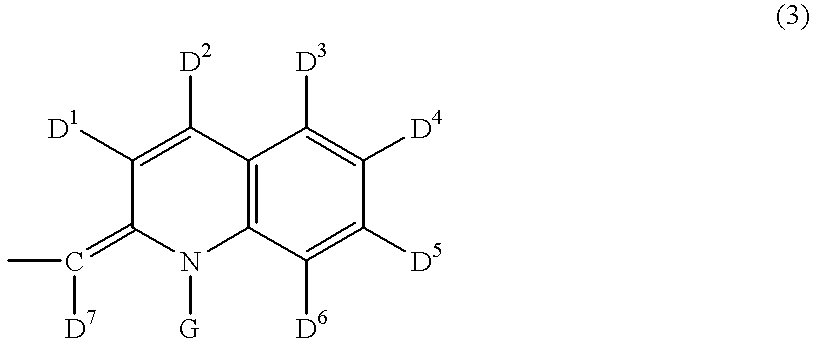
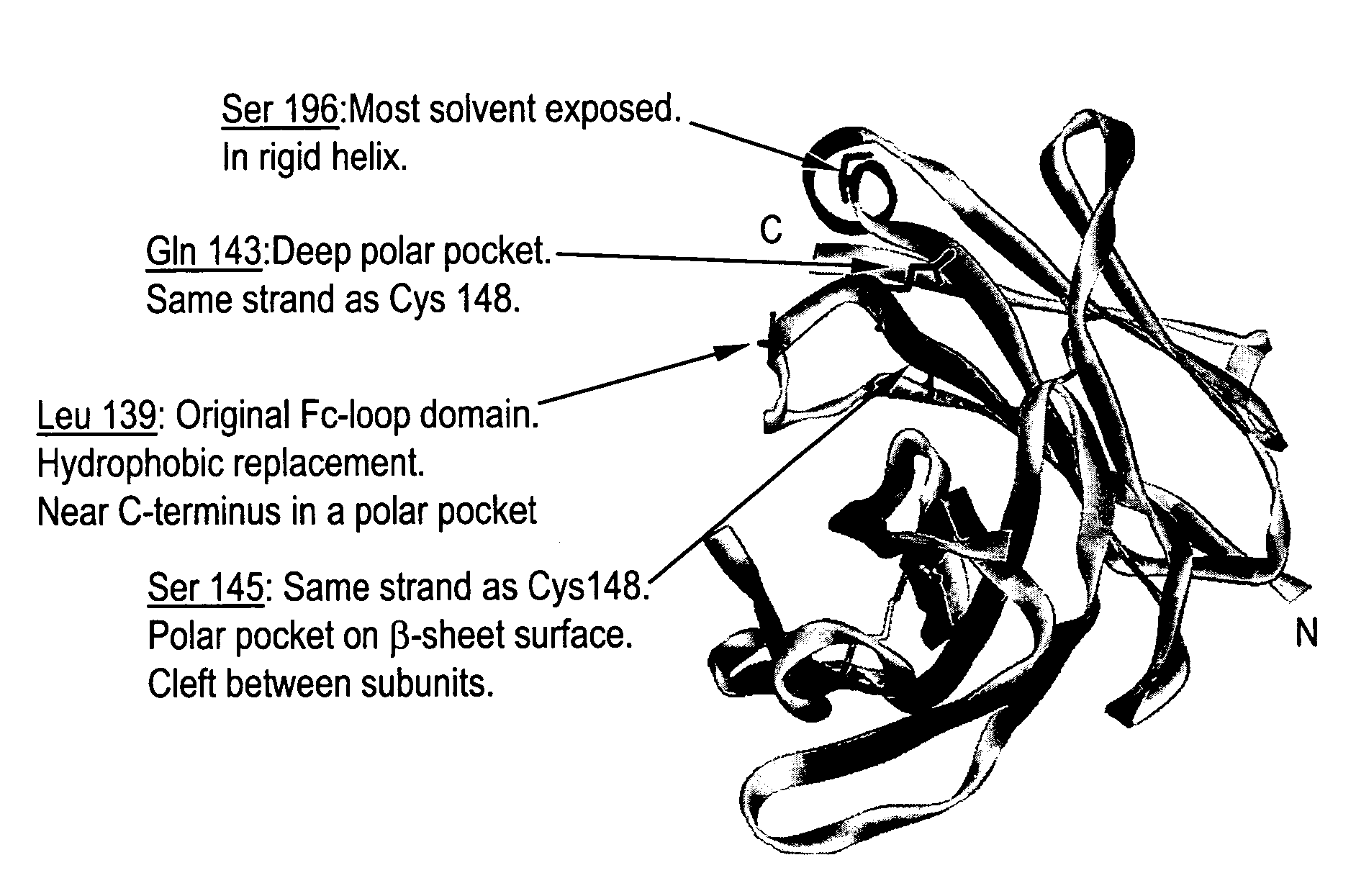
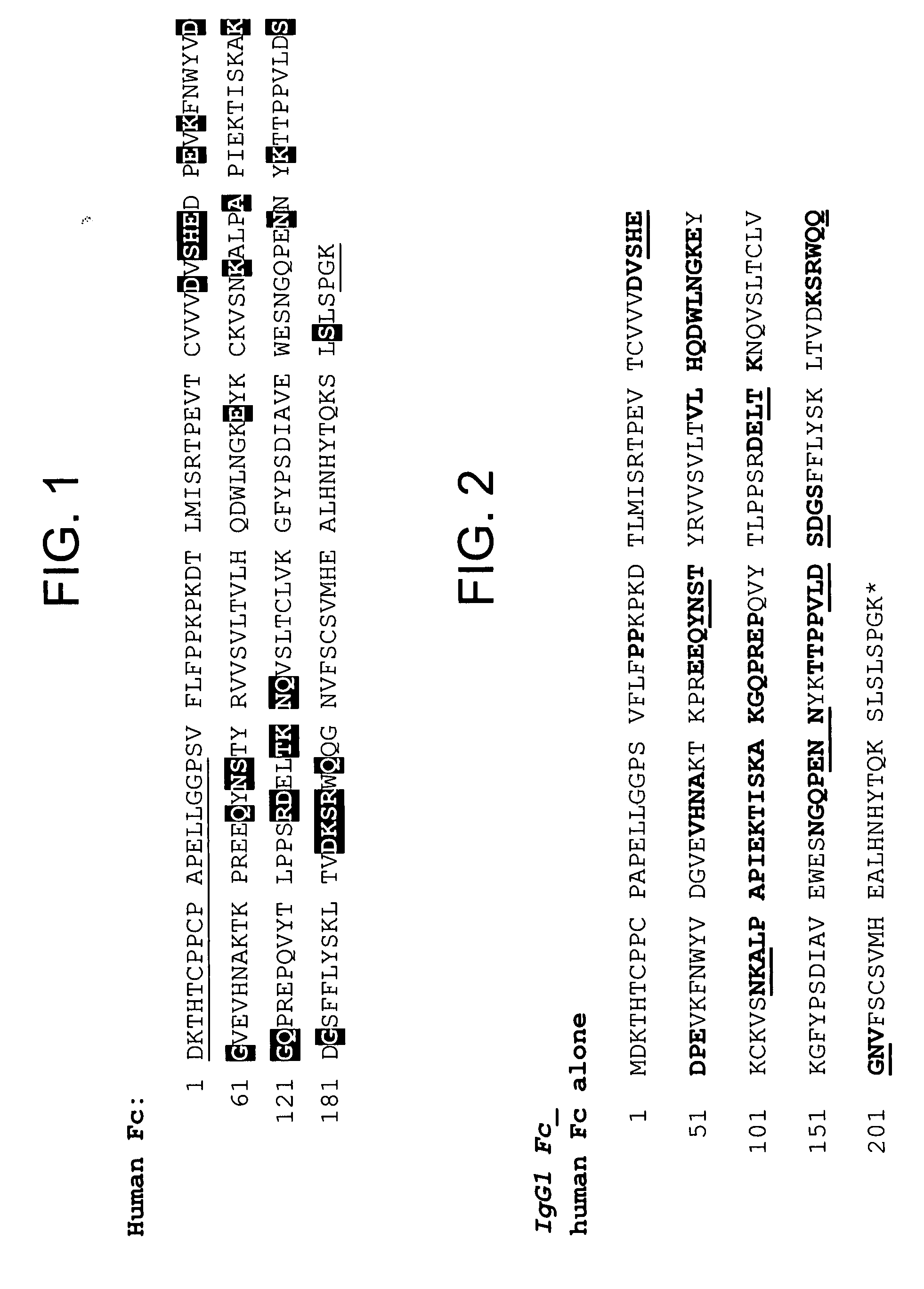
Disclosed is a process for preparing a pharmacologically active compound, in which at least one internal conjugation site of an Fc domain sequence is selected that is amenable to conjugation of an additional functional moiety by a defined conjugation chemistry through the side chain of an amino acid residue at the conjugation site. An appropriate amino acid residue for conjugation may be present in a native Fc domain at the conjugation site or may be added by insertion (i.e., between amino acids in the native Fc domain) or by replacement (i.e., removing amino acids and substituting different amino acids). In the latter case, the number of amino acids added need not correspond to the number of amino acids removed from the previously existing Fc domain. This technology may be used to produce useful compositions of matter and pharmaceutical compositions containing them. A DNA encoding the inventive composition of matter, an expression vector containing the DNA, and a host cell containing the expression vector are also disclosed.
Owner:AMGEN INC
Pharmaceutical dosage form
ActiveUS20090202634A1Improved controlled releaseEasy to adjustBiocideOrganic active ingredientsControlled releaseBreaking strength
The invention relates to a pharmaceutical dosage form, preferably with controlled release of a pharmacologically active compound (A) contained therein, the pharmaceutical dosage form very preferably being tamper-resistant and most preferably having a breaking strength B1 of at least 500 N in direction of extension E1 and having a breaking strength B2 of less than 500 N in direction of extension E2.
Owner:GRUNENTHAL GMBH
Compositions comprising lipophilic active compounds and method for their preparation
ActiveUS20090098200A1Quick releaseImprove bioavailabilityPowder deliveryBiocideHydrophilic polymersCompound (substance)
Compositions are provided comprising a lipophilic active compound, e.g., a human or veterinary drug or a nutraceutical, interwoven with a polymeric matrix formed by two or more polymers, wherein one of the polymers is an amphiphilic polymer and the other polymer is either an amphiphilic polymer with a different hydrophobic-hydrophilic balance or a hydrophilic polymer, and the active lipophilic compound has modified physicochemical properties. The composition forms colloidal nanodispersion upon contact with aqueous media.
Owner:SOLUBEST
Transdermal delivery of pharmaceutical agents
InactiveUS20070243132A1Improve permeabilityEfficient use ofAntibacterial agentsOrganic active ingredientsActive agentGenetic molecular
The present invention generally relates to a vehicle useful for delivering a pharmaceutically active compound including a genetic molecule or composition. More particularly, the present invention provides microemulsions for transdermal delivery of pharmaceutically active agents to a subject.
Owner:APOLLO LIFE SCI
Microparticles for Oral Delivery
The invention provides microbeads containing oil-associated biologically active compounds and methods for their manufacture and use. The microbeads consist of a soluble complex of non-digestible polymer and emulsifier with oil-associated biologically active compounds embedded in a matrix of digestible polymer. The disclosed microbead complex protects the biologically active compounds, such as vitamins, fish oil and carotenoids, from oxidation, taste and odor degradation. The disclosed microbeads also provide protection from the stomach digestive distraction, and allows for the delivery of the biologically active compounds in the intestine.
Owner:INTERVET INC
Medical aerosol formulations
InactiveUS6461591B1Improve dosage accuracyIncrease doseBiocideDispersion deliveryAlkaneAerosol spray
A pressure-liquefied propellant mixture for aerosols, comprising a fluorinated alkane, in particular 1,1,1,2-tetrafluoroethane and / or 1,1,1,2,3,3,3-heptafluoropropane, and carbon dioxide, makes possible an improvement of the wetting properties of pharmaceutically active compounds, with which the formulation problems existing with hydrofluoroalkanes in relation to suspension as well as solution aerosols can be overcome and thus improved medicinal aerosol formulations can be obtained. With the aid of carbon dioxide, it is also possible to specifically influence the pressure and thus the particle size distribution and also by displacement of oxygen from the hydrofluoroalkanes to improve the storage stability of oxidation-sensitive active compounds.
Owner:JAGOTEC AG
Raman-active taggants and their recognition
InactiveUS20020025490A1Easy to useQuality improvementOptical radiation measurementMaterial nanotechnologyLaser lightLasing wavelength
An organic or organoelement, linear or branched, monomeric or polymeric composition of matter having a Raman-active component in the form of particles. The particles having a maximum dimension of 50 mum. The Raman-active compound is applied to a substrate. When the Raman-active compound is exposed to a laser light wavelength which is batochromically well beyond a spectral region of maximum absorbance of said Raman-active compound, Raman scattering can be detected.
Owner:QUANTAG SYST
Method of deacidizing a gaseous effluent with extraction of the products to be regenerated
The present invention relates to a method of deacidizing a gaseous effluent comprising at least one of the acid compounds as follows: H2S, mercaptans, CO2, COS, SO2, CS2, wherein the following stages are carried out: a) contacting the acid compounds contained in said effluent with reactive compounds forming a liquid, so as to obtain a gaseous effluent depleted in acid compounds and a first liquid fraction comprising products formed by reaction of the reactive compounds with acid compounds, and reactive compounds that did not react with acid compounds, b) contacting said products contained in the first liquid fraction with extraction compounds forming a second liquid fraction so as to obtain a product-depleted first liquid fraction and a product-enriched second liquid fraction, c) recycling to stage a) the first liquid fraction obtained in stage b), said first liquid fraction obtained making up at least part of said liquid, d) regenerating the second liquid fraction obtained in stage b) so as to release acid compounds in gaseous form and to obtain a mixture of reactive compounds and of extraction compounds.
Owner:CARRETTE PIERRE LOUIS +6
Bactericide pesticide composite containing oligochitosan
InactiveCN101816305AEnhanced application safetyReduce dosageBiocideFungicidesEcological environmentDrug resistance
The invention discloses a bactericide pesticide composite containing oligochitosan, which comprises a component A and a component B, wherein the component A is oligochitosan or a derivative of the oligochitosan; and the component B is one or several of active compounds. The pesticide composition can produce a synergistic action, reduce the dosage of a chemically synthetic bactericide, prevent or delay the medicament resistance of the bactericide, improve crop stress resistance and yield, regulate metabolic balance in plant bodies and improve quality of agricultural products, improve the application safety of the bactericide and is more safe to ecological environmental and human bodies.
Owner:HAINAN ZHENGYE ZHONGNONG HIGH TECH
Raman-active taggants and thier recognition
InactiveUS20040058058A1Easy to useQuality improvementMaterial nanotechnologyRadiation pyrometryLasing wavelengthLaser light
An organic or organoelement, linear or branched, monomeric or polymeric composition of matter having a Raman-active component in the form of particles. The particles having a maximum dimension of 50 mum. The Raman-active compound is applied to a substrate. When the Raman-active compound is exposed to a laser light wavelength which is batochromically well beyond a spectral region of maximum absorbance of said Raman-active compound, Raman scattering can be detected.
Owner:SHCHEGOLIKHIN ALEXANDER NIKITOVICH +4
Substituted diaryl compound and preparation method and antiviral application thereof
ActiveCN102206172ALess likely to develop drug resistanceImprove securityCarbamic acid derivatives preparationSulfonic acid esters preparationMechanism of actionStructure–activity relationship
The invention provides substituted diaryl compounds as shown in general formula (I) or their pharmaceutically acceptable salts, and also provides a preparation method; a class of novel broad-spectrum antiviral compounds and pharmaceutical salts targeting cytokines are screened and obtained through studies on structure-activity relationship and action mechanism of active compounds; the compounds not only have significant broad-spectrum antiviral activity, but also have the advantages of low toxicity and good pharmaceutical properties.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Apparatus for heating to a desired temperature for improved administration of pharmaceutically active compounds
Methods and apparatus for improving administration of drugs through the use of heat and other physical means. The present invention relates to the use of heat and other physical means in conjunction with specially designed dermal drug delivery systems, conventional commercial dermal drug delivery systems, or drugs delivered into a sub-skin depot site via injection and other methods to alter, mainly increase, the drug release rate from the dermal drug delivery systems or the depot sites to accommodate certain clinical needs.
Owner:NUVO RES
Methods and compositions for stimulating osteoblast proliferation or treating malignant cell proliferation and methods for selecting osteoblast proliferation stimulants
The present invention relates to methods for stimulating osteoblast proliferation and methods for selecting pharmacologically active compounds useful for stimulating osteoblast proliferation.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Transporters comprising spaced arginine moieties
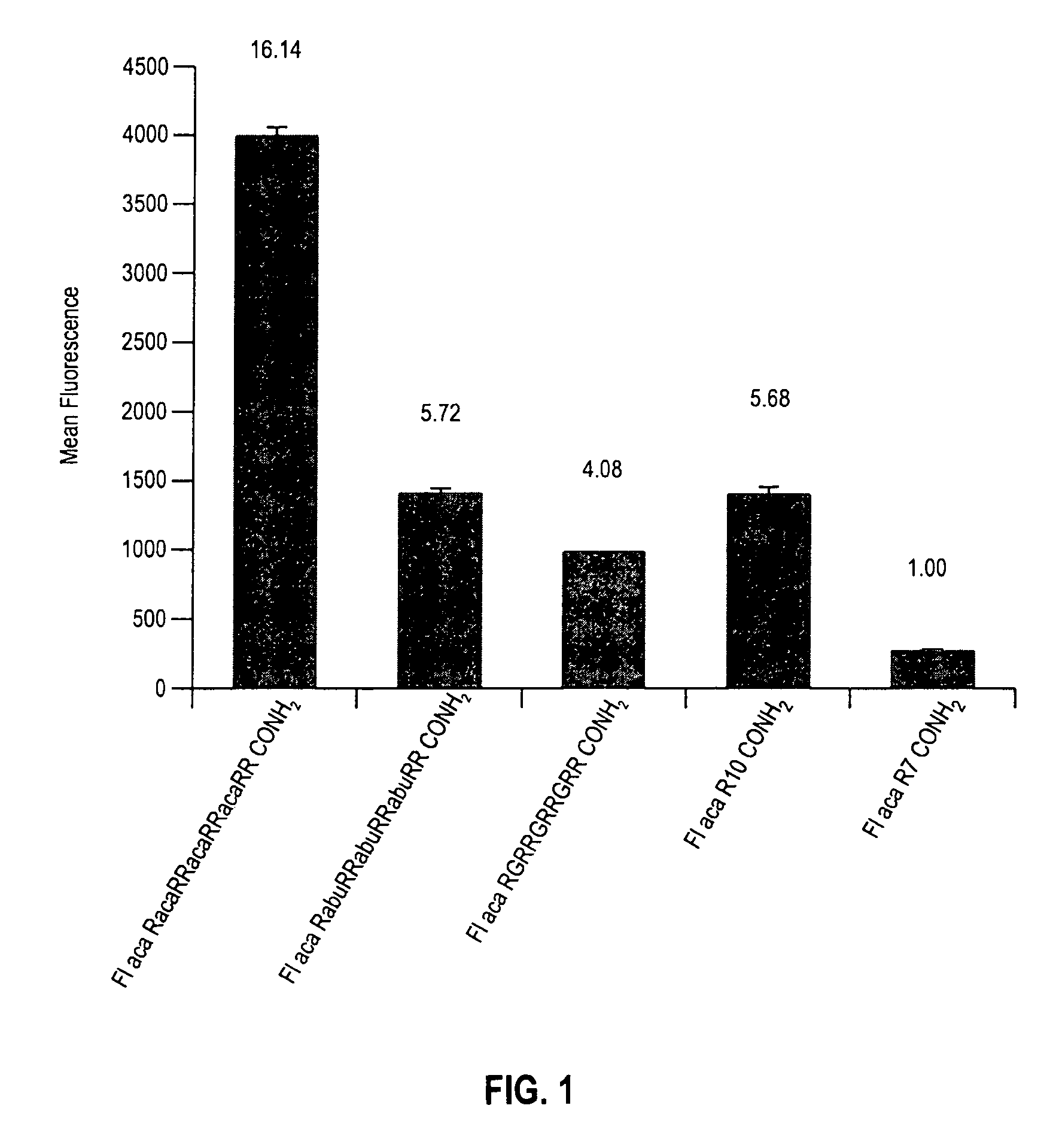
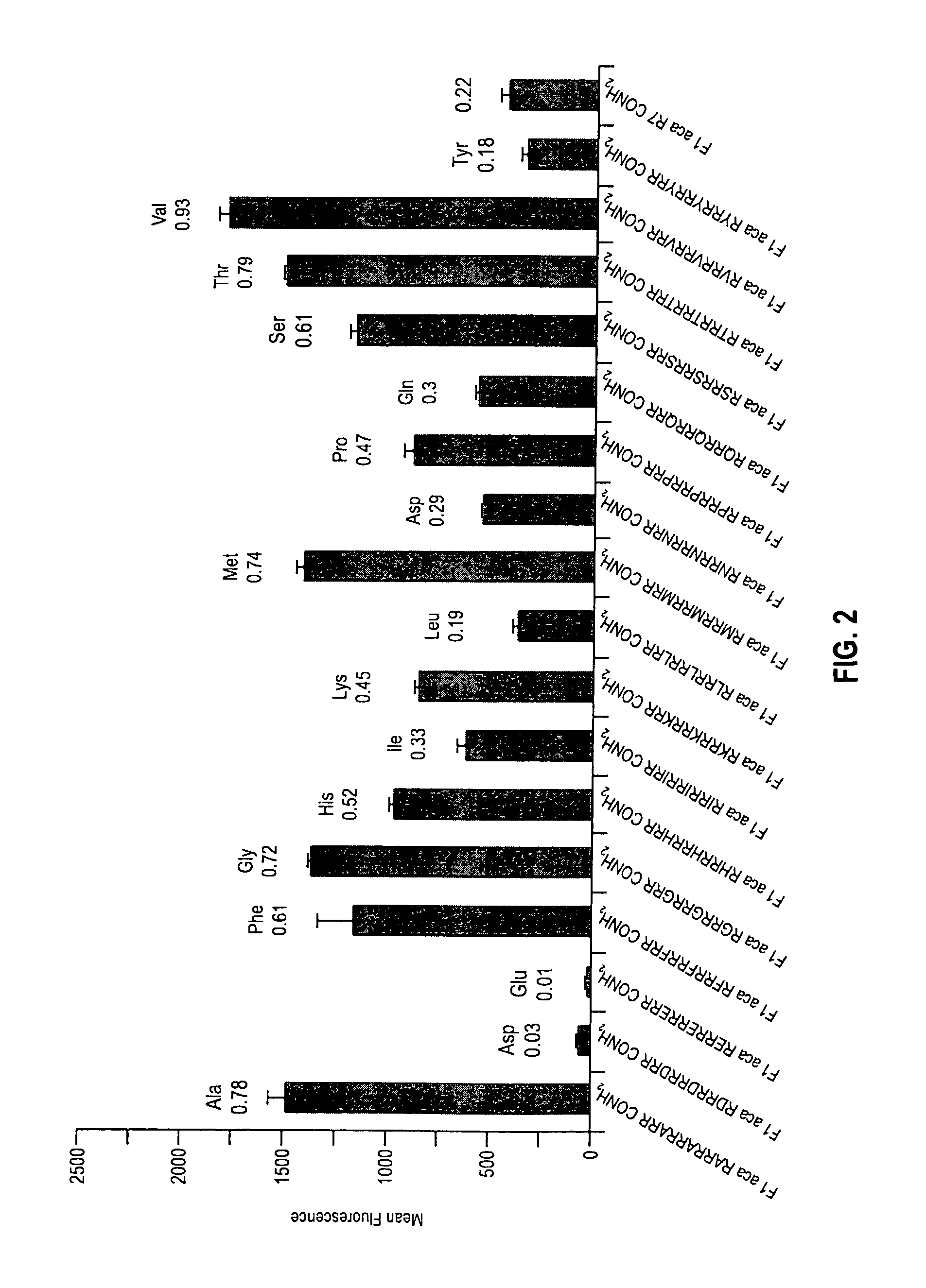
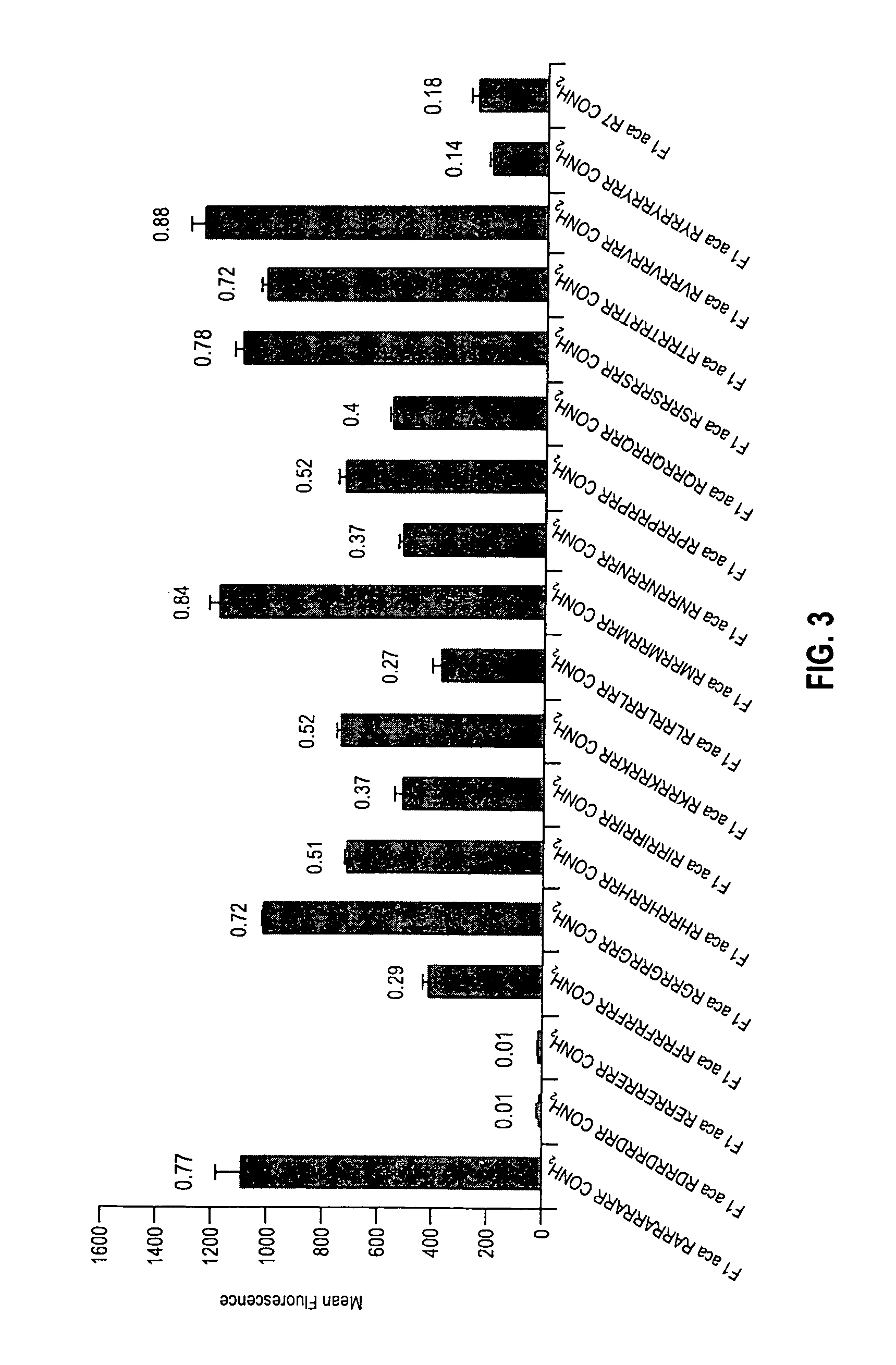
The present invention provides compositions and methods for enhancing transport of biologically active compounds across biological membranes and across and into animal epithelial or endothelial tissues. The composition includes a biologically active agent and a transport moiety. The transport moiety includes a structure selected from the group consisting of (ZYZ)nZ, (ZY)nZ, (ZYY)nZ and (ZYYY)nZ. Subunit “Z” is L-arginine or D-arginine, and subunit “Y” is an amino acid that does not comprise an amidino or guanidino moiety. Subscript “n” is an integer ranging from 2 to 10. The method for enhancing transport involves the administration of the aforementioned composition.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
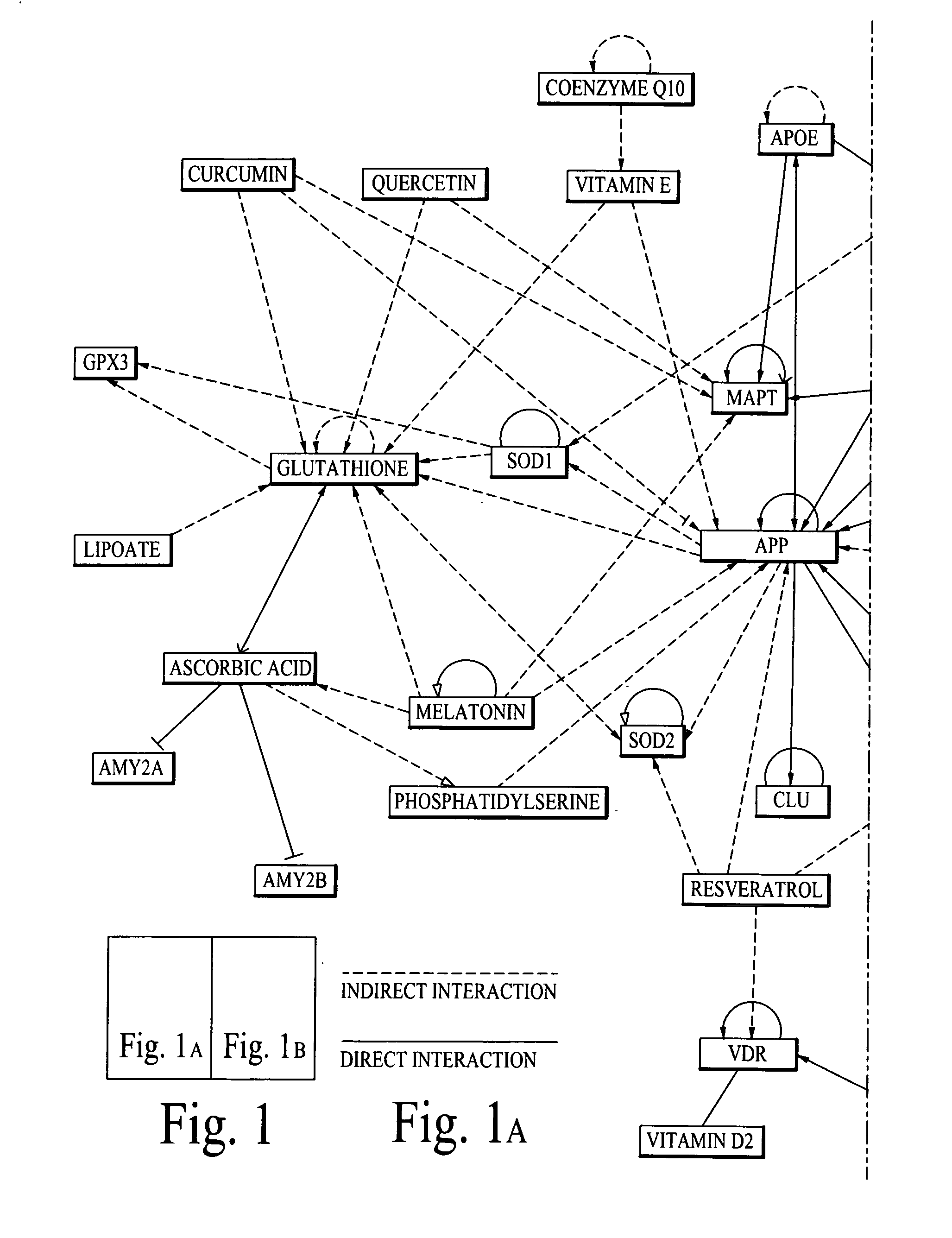
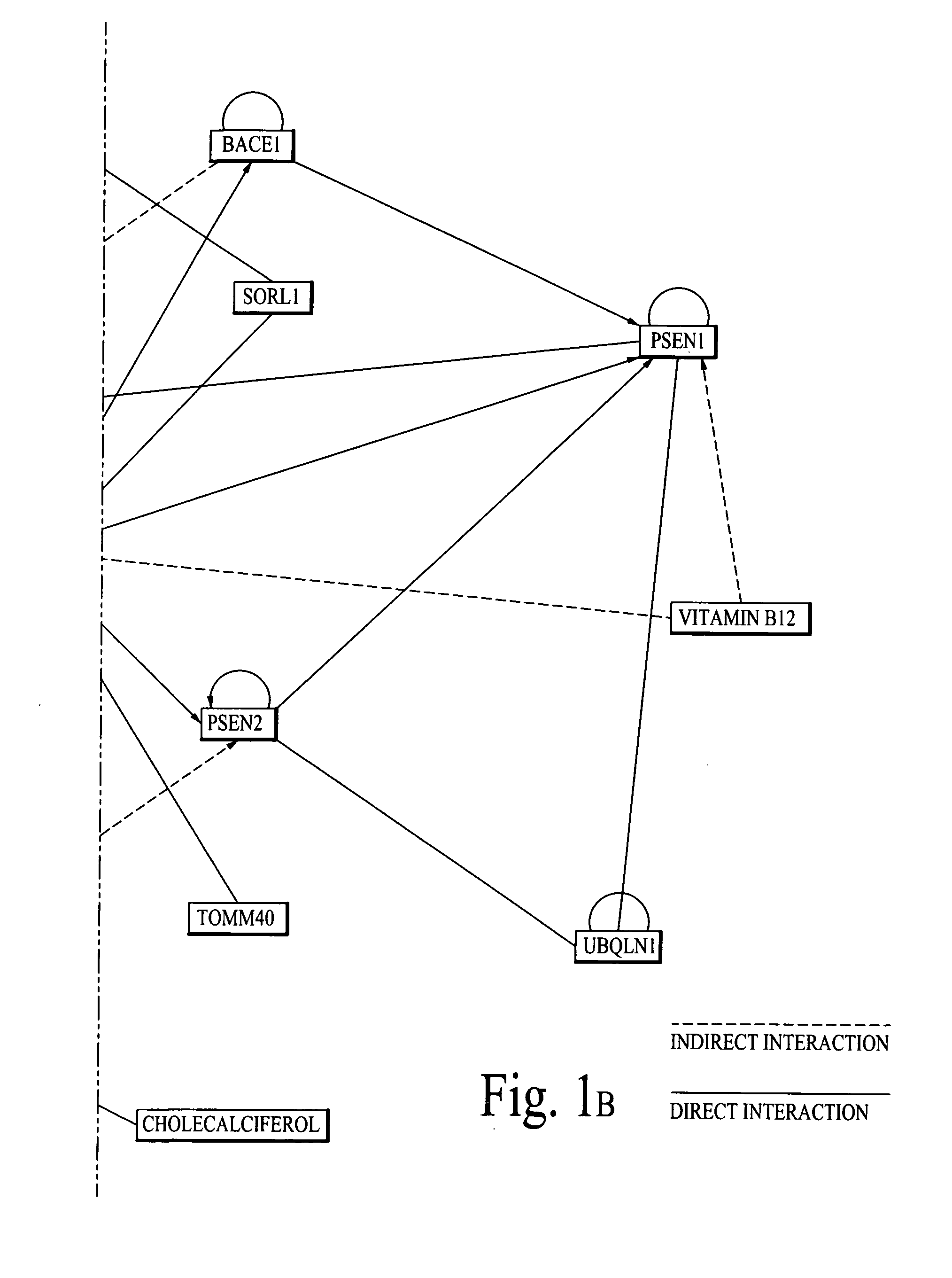
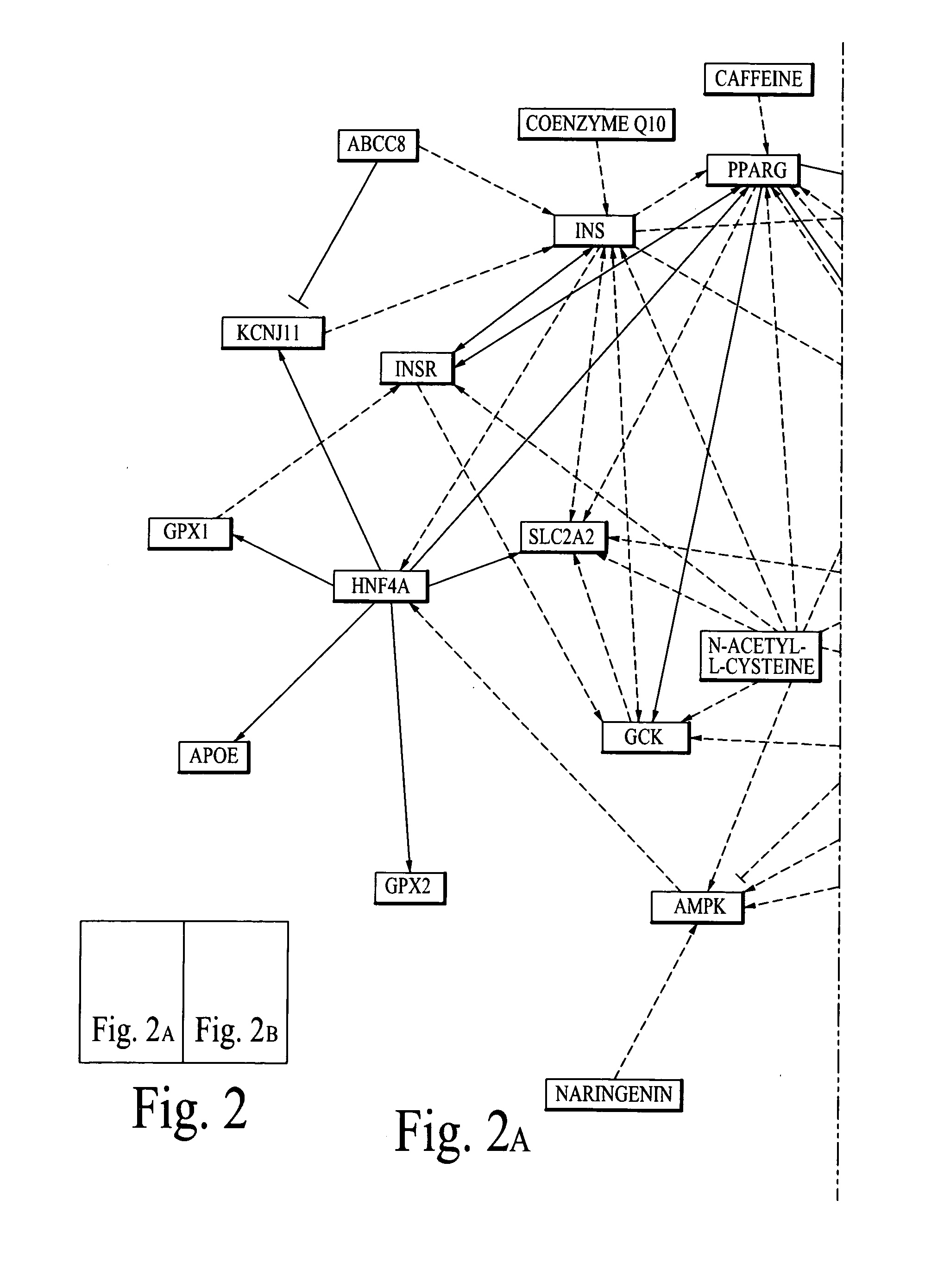
Chemical composition and its delivery for lowering the risks of alzheimer's, cardiov ascular and type-2 diabetes diseases
Synergistic chemical compositions of bioactive compounds in a dietary supplement for lowering the risks of Alzheimer's, Cardiovascular and Diabetes diseases; chemical compositions of a sugar free super sweetener for people with Type-2 Diabetes disease and a targeted nano delivery of bioactive compounds and / or bioactive molecules are described. Furthermore, microelectro-mechanical system (Mems) based passive and active (based on feedback diagnostics data) delivery systems and methods of bioactive compounds and / or bioactive molecules are also described.
Owner:MAZED MOHAMMAD A +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com