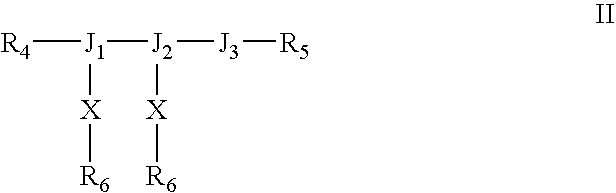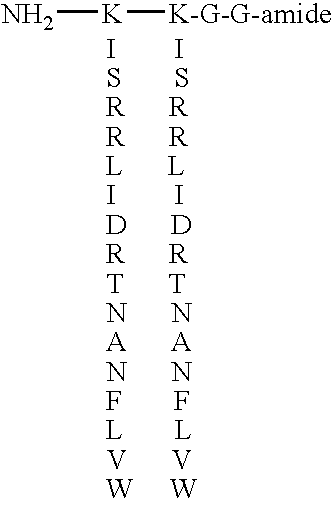Patents
Literature
792 results about "Nerve growth factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nerve growth factor (NGF) is a neurotrophic factor and neuropeptide primarily involved in the regulation of growth, maintenance, proliferation, and survival of certain target neurons. It is perhaps the prototypical growth factor, in that it was one of the first to be described. Since it was first isolated by Nobel Laureates Rita Levi-Montalcini and Stanley Cohen in 1956, numerous biological processes involving NGF have been identified, two of them being the survival of pancreatic beta cells and the regulation of the immune system.
Application of lipid vehicles and use for drug delivery
InactiveUS7063860B2Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticAnticarcinogenCapsaicin
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Biodegradable polymer coils for intraluminal implants
An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location of a separable coil comprised at least in part of at least one biocompatible and absorbable polymer or protein and growth factors. Typically a catheter associated with the separable coil is used to dispose the coil into a selected body lumen. The biocompatible and absorbable polymer or protein is thrombogenic. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and absorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides. The biocompatible and absorbable protein is at least one protein selected from the group consisting of collagen, fibrinogen, fibronectin, vitronectin, laminin, and gelatin. In one embodiment the coil is composed of the biocompatible and absorbable polymer or protein with a radio-opaque material is disposed thereon. Alternatively, the coil is composed of a radio-opaque material, and the biocompatible and absorbable polymer or protein is disposed thereon. This apparatus may be positioned within intracranial aneurysms or any aneurysm in the body as well as within other body cavities.
Owner:RGT UNIV OF CALIFORNIA
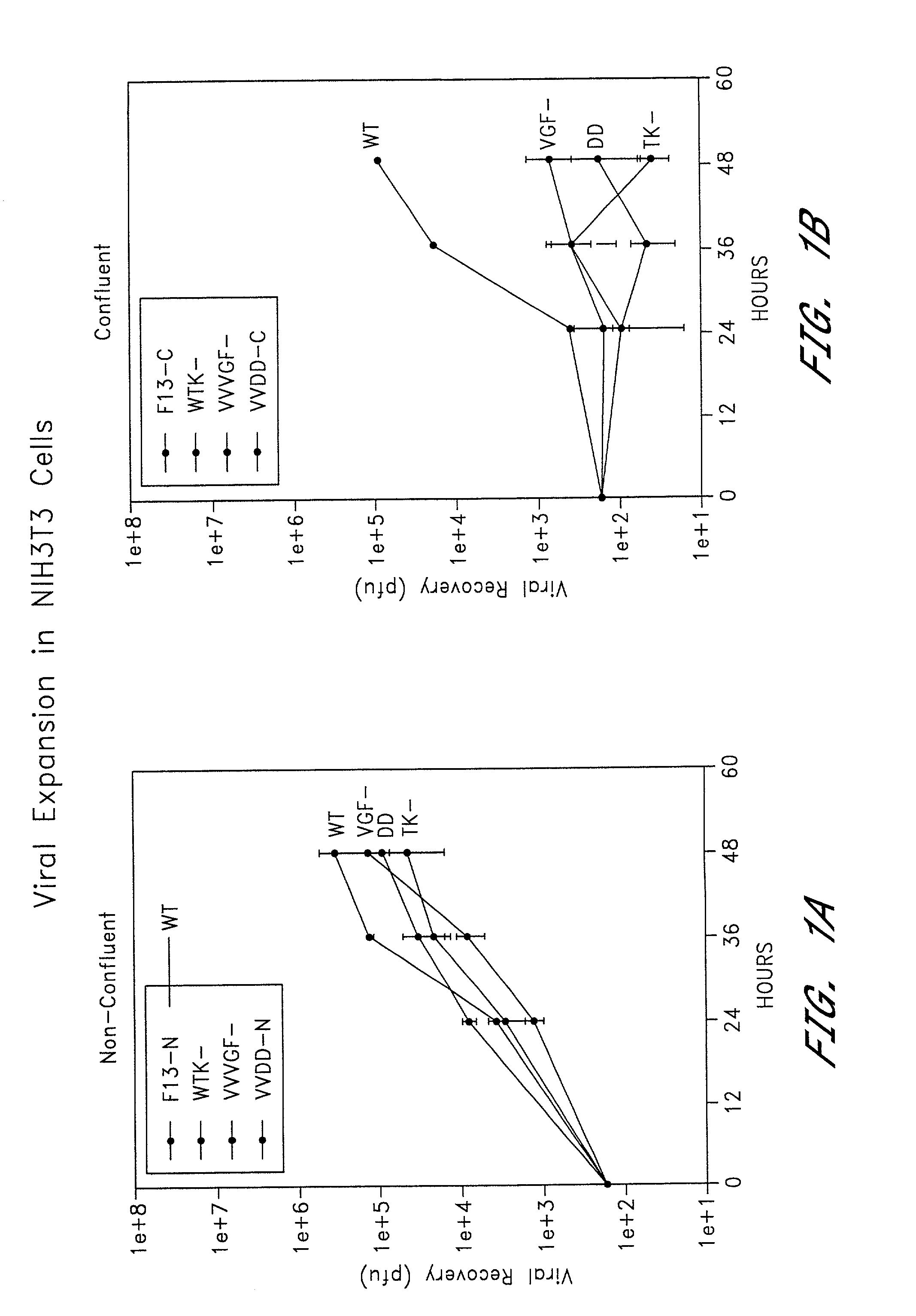
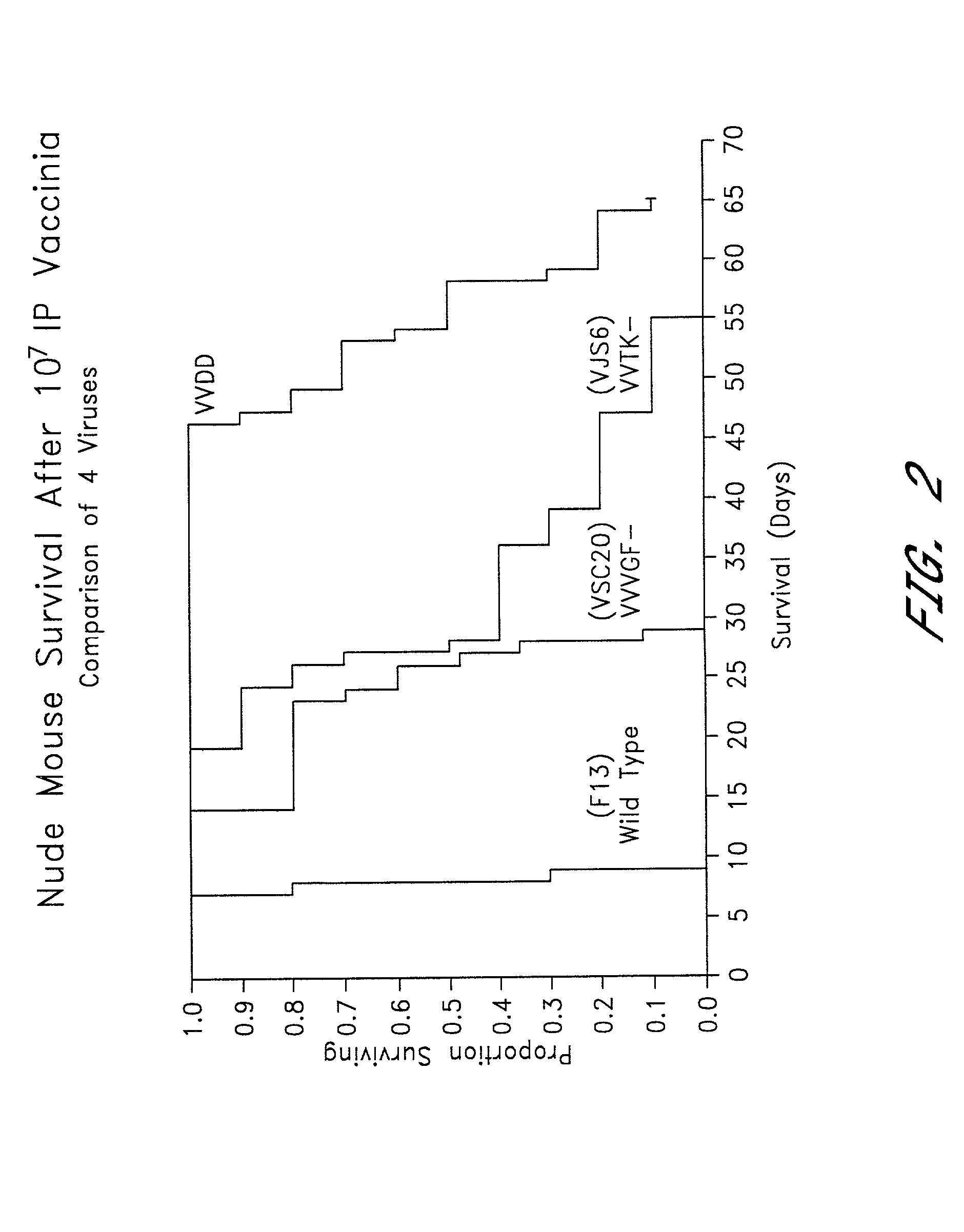
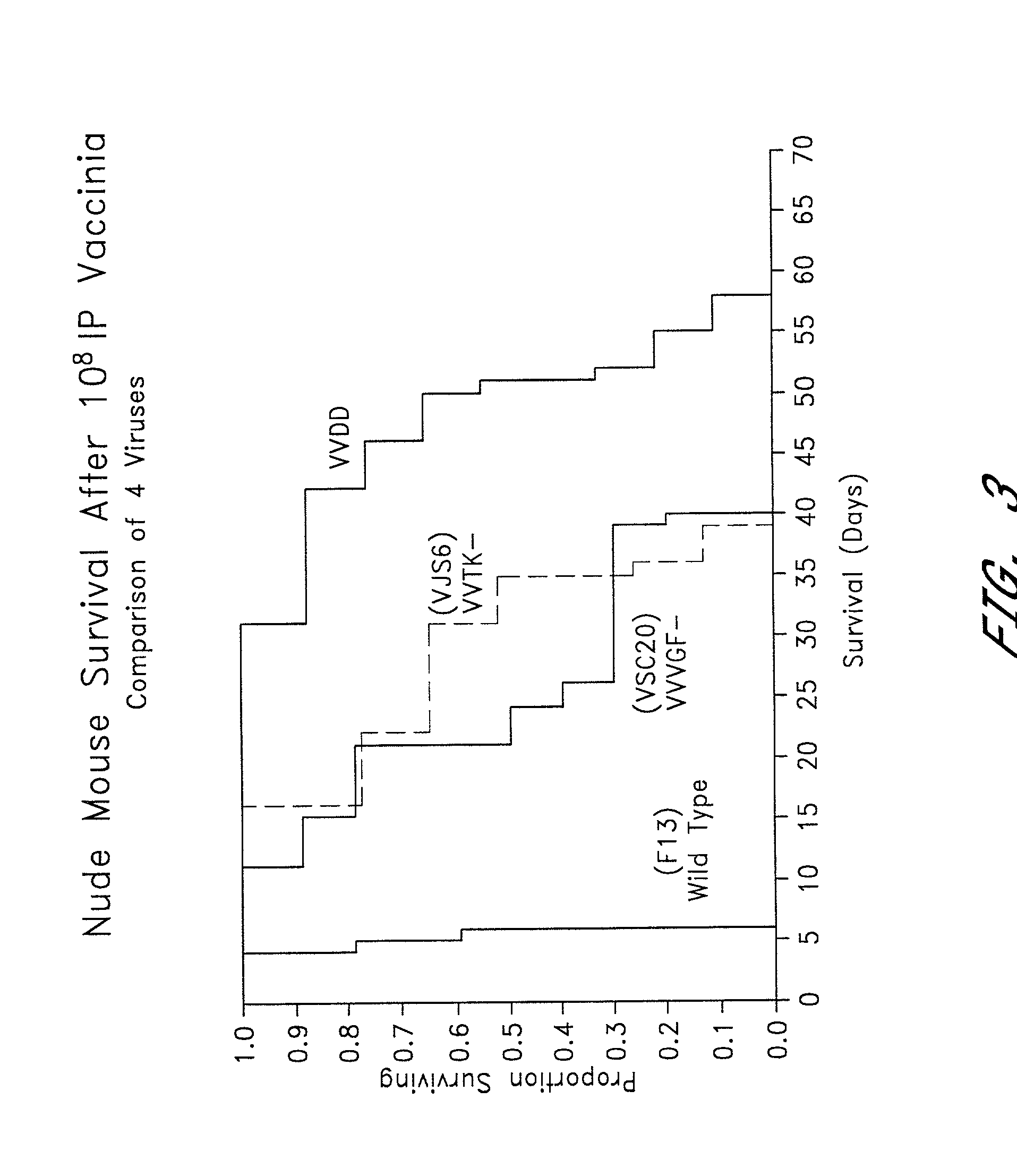
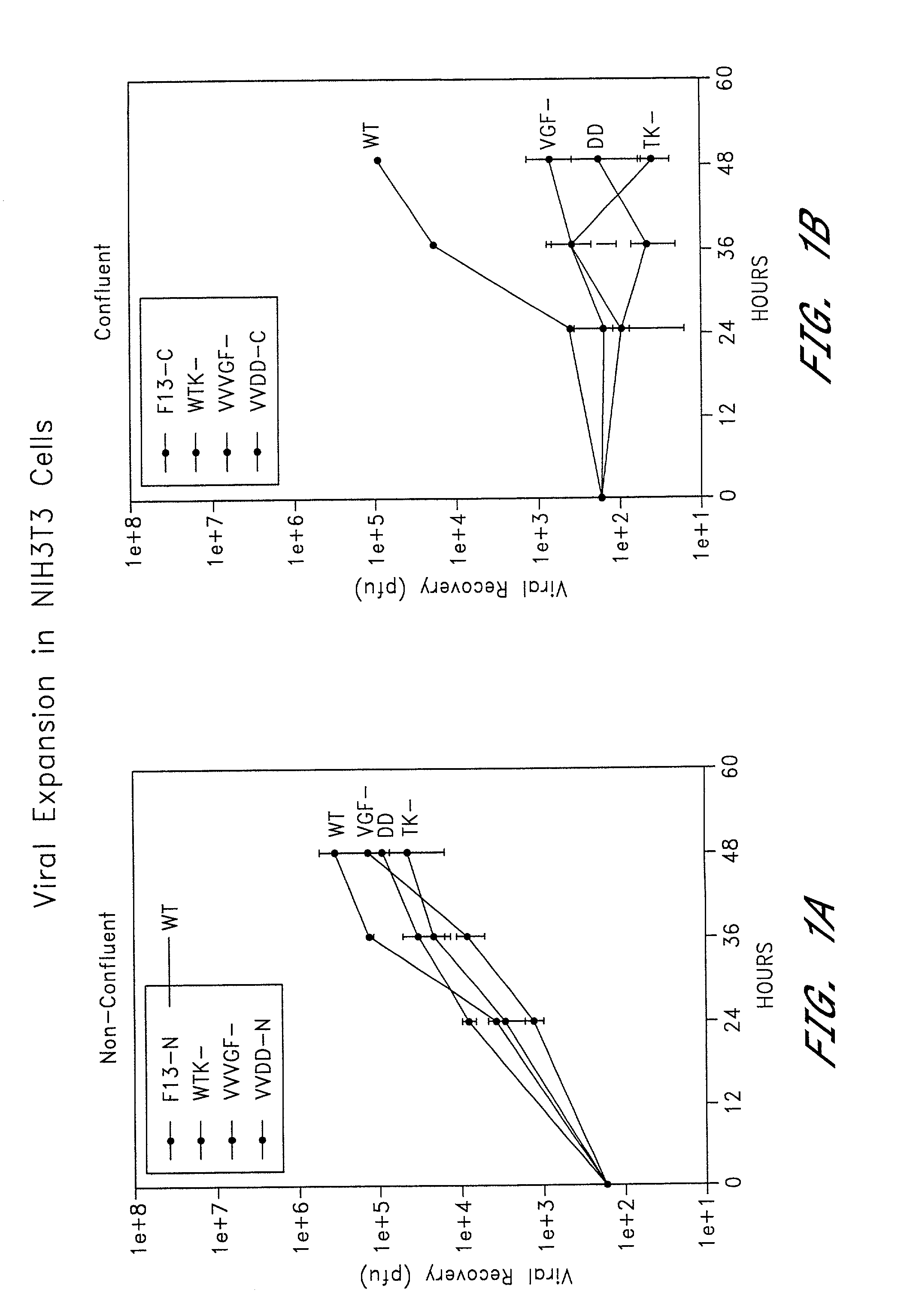
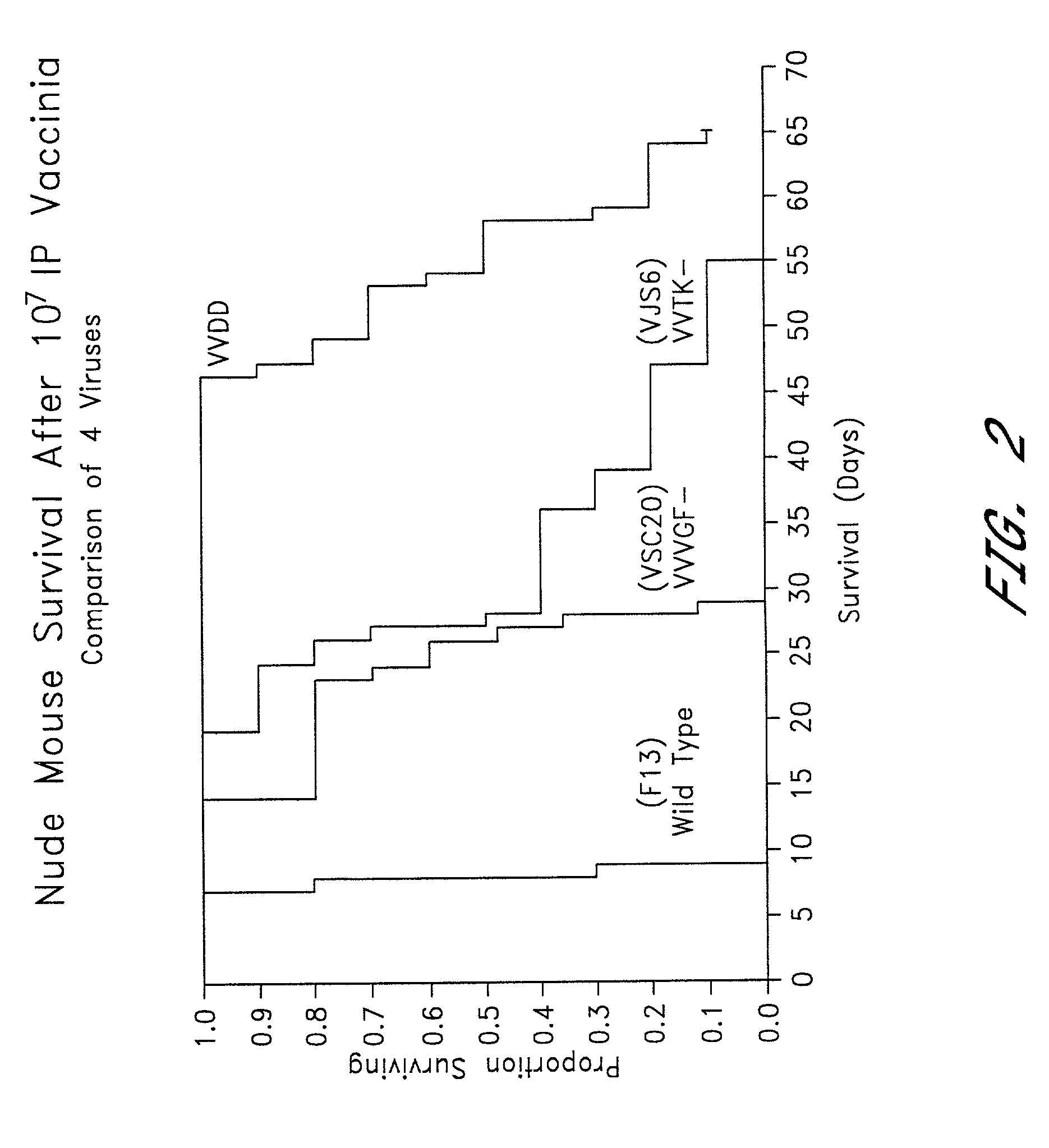
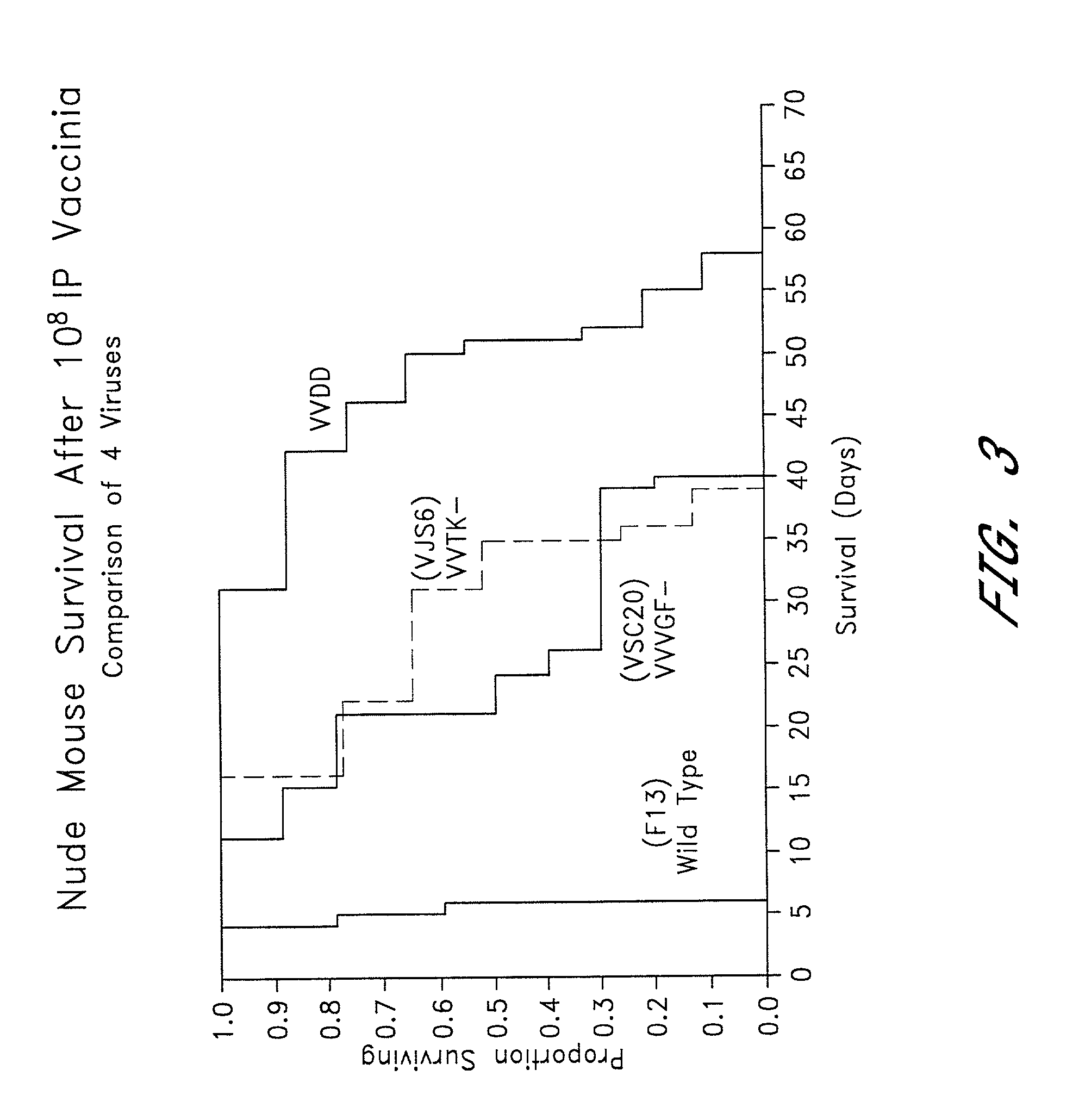
Combined growth factor-deleted and thymidine kinase-deleted vaccinia virus vector
A composition of matter comprising a vaccinia virus expression vector with a negative thymidine kinase phenotype and a negative vaccinia virus growth factor phenotype.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT HEALTH & HUMAN SERVICES THE
Composition and method for treating impaired or deteriorating neurological function
InactiveUS6964969B2Impair actionHigh affinityBiocideHydrocarbon active ingredientsPhosphateAntioxidant
A nutritional supplement composition for normalizing impaired or deteriorating neurological function in humans is composed of: at least one agent which promotes synthesis of ATP and / or creatine phosphate in the body, at least one antioxidant for scavenging free radicals in at least one pathway in the body; at least one agent for normalizing or maintaining membrane function and structure in the body; at least one agent for normalizing or maintaining normal neurotransmitter function in the body; at least one agent for down-regulating cortisol action; and at least one agent for suppressing activation of apoptotic pathways in the body. The composition may further contain one or more of: at least one agent for suppressing inflammation in the body; at least one agent for normalizing or maintaining vascular wall function and structure in the body; at least one agent for normalizing or maintaining function of nerve growth factors and / or neurotropic factors in the body; at least one agent for suppressing toxic metal ionic effects; at least one agent for normalizing or maintaining methyl metabolism in the body; at least one agent for normalizing or maintaining metabolism of insulin and glucose in the body; and at least one agent for up-regulating activity of heat shock proteins in the body. A method for normalizing impaired neurological function in humans modulating nutrient partitioning in a human involves administering the aforementioned composition to the human, preferably on a daily basis, for a therapeutically effective period of time. Preferably, the method further involves having the human follow a stress reduction program, and / or a cognitive retraining program, and / or a dietary program designed to maximize insulin and glucose metabolism.
Owner:MCCLEARY EDWARD LARRY
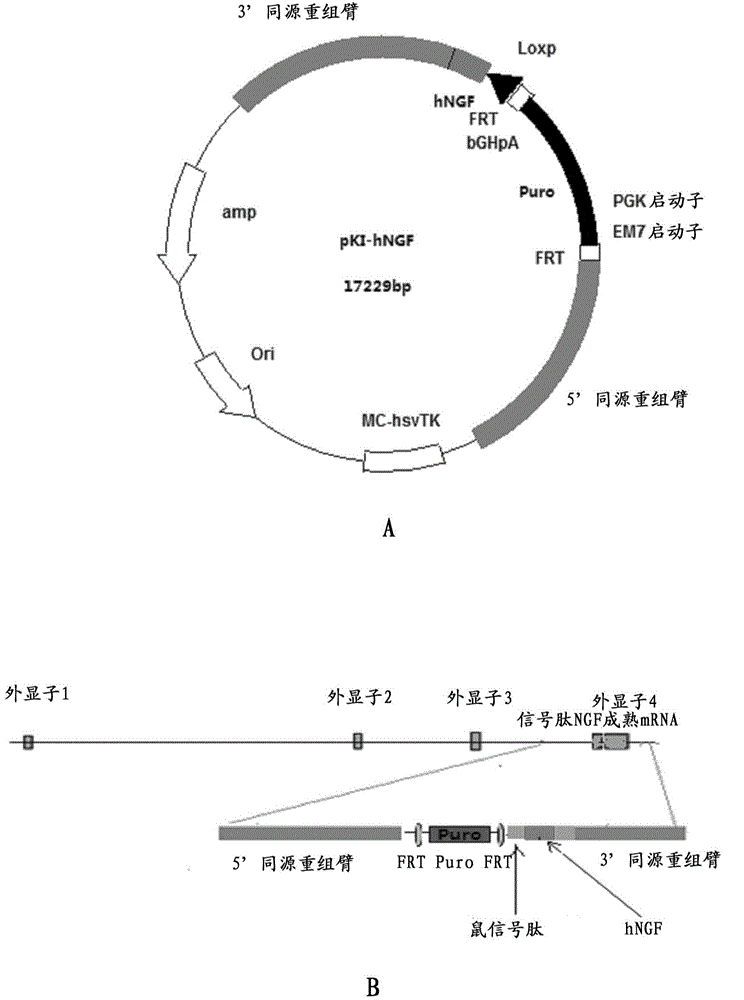
Preparation method for transgenic mice capable of producing human nerve growth factor
ActiveCN104561095AEasy to operateIncrease success rateAnimals/human peptidesVector-based foreign material introductionPlasmid dnaGenetically modified mouse
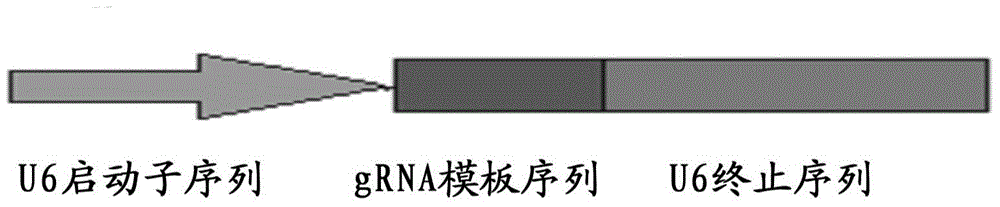
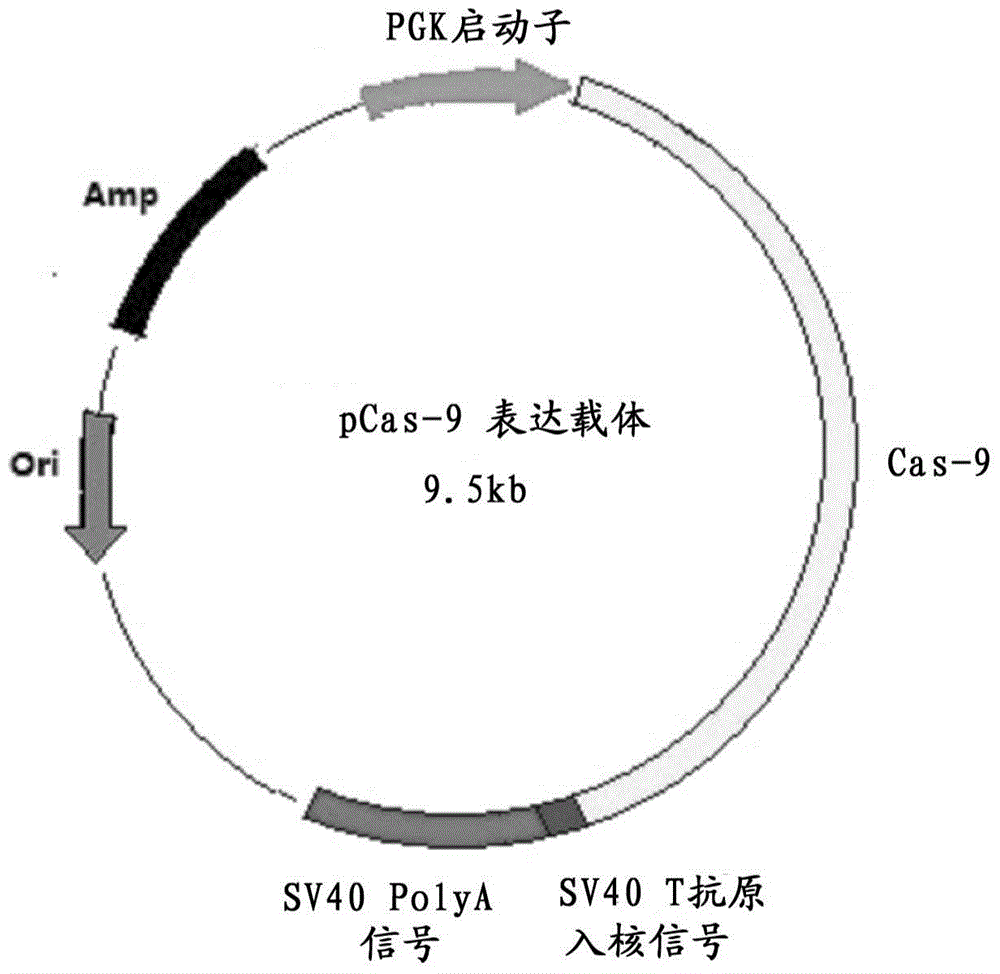
The invention discloses a method for producing transgenic mice through a homologous recombination technology. The method comprises the following steps: replacing mouse NGF genes on mouse chromosomes by human NGF genes through a Cas-9 / CRISPR gene knock-in technology, and obtaining the genes with homozygous human NGF genes through breeding and knocking the genes in mice, thus obtaining filial generation mice with salivary glands capable of secreting human NGF. Compared with the conventional targeting technology utilizing positive and negative double screening homologous recombination genes, the method disclosed by the invention is simple to operate, capable of being realized only by transfecting mouse embryonic stem cells by three plasmid DNAs or carrying out pronucleus injection on the zygotes of mice, and high in success rate which is up to 2-5% and remarkably higher than the mouse embryonic stem cell positive rate of 0.1% of the common gene targeting technology.
Owner:深圳市国创纳米抗体技术有限公司
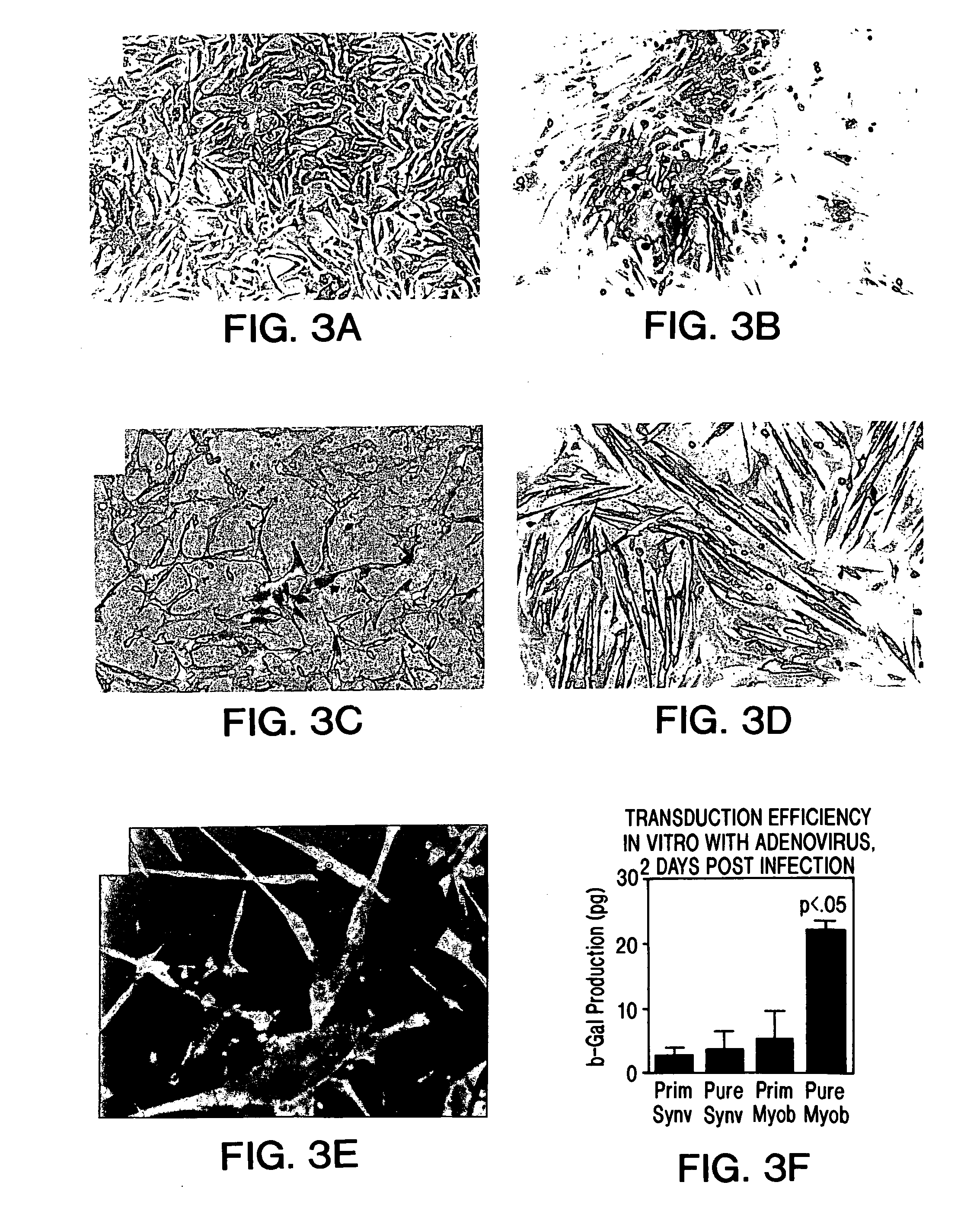
Muscle-derived cells (MDCs) for treating muscle- or bone-related injury or dysfunction
The present invention provides muscle-derived cells, preferably myoblasts and muscle-derived stem cells, genetically engineered to contain and express one or more heterologous genes or functional segments of such genes, for delivery of the encoded gene products at or near sites of musculoskeletal, bone, ligament, meniscus, cartilage or genitourinary disease, injury, defect, or dysfunction. Ex vivo myoblast mediated gene delivery of human inducible nitric oxide synthase, and the resulting production of nitric oxide at and around the site of injury, are particularly provided by the invention as a treatment for lower genitourinary tract dysfunctions. Ex vivo gene transfer for the musculoskeletal system includes genes encoding acidic fibroblast growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor, platelet derived growth factor, transforming growth factor-β, transforming growth factor-α, nerve growth factor and interleukin-1 receptor antagonist protein (IRAP), bone morphogenetic protein (BMPs), cartilage derived morphogenetic protein (CDMPs), vascular endothelial growth factor (VEGF), and sonic hedgehog proteins.
Owner:UNIVERSITY OF PITTSBURGH
Growth factor releasing biofunctional dental implant
A biofunctional dental implant system for affixing a crown to an implant socket that is made in the bone structure within the mouth of a dental patient. A root portion is initially anchored within the implant socket made in the patient's bone structure. According to a first embodiment of this invention, the crown is secured to the root portion by a flexible abutment post. According to a second embodiment, the crown is secured to the root portion by a hollow abutment tube. The dental implant system of this invention is capable of increasing bone / implant stabilization and providing a supply path through which to evenly distribute human growth factor to the bone structure surrounding the root portion. The system also enables the crown to have a selectively controllable mobility relative to the root portion that is anchored within the implant socket so as to advantageously function like a natural tooth.
Owner:SAPIAN SCHUBERT L
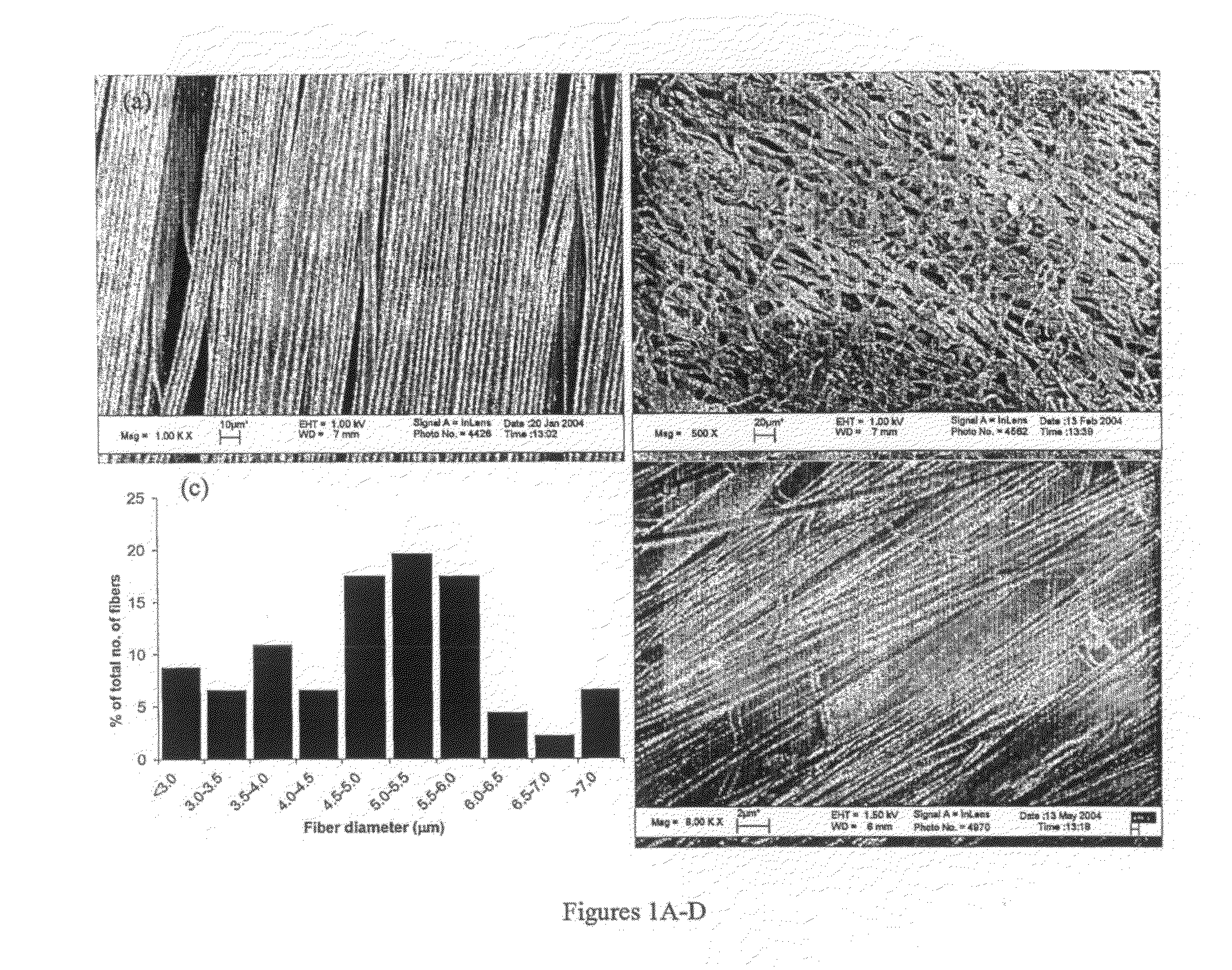
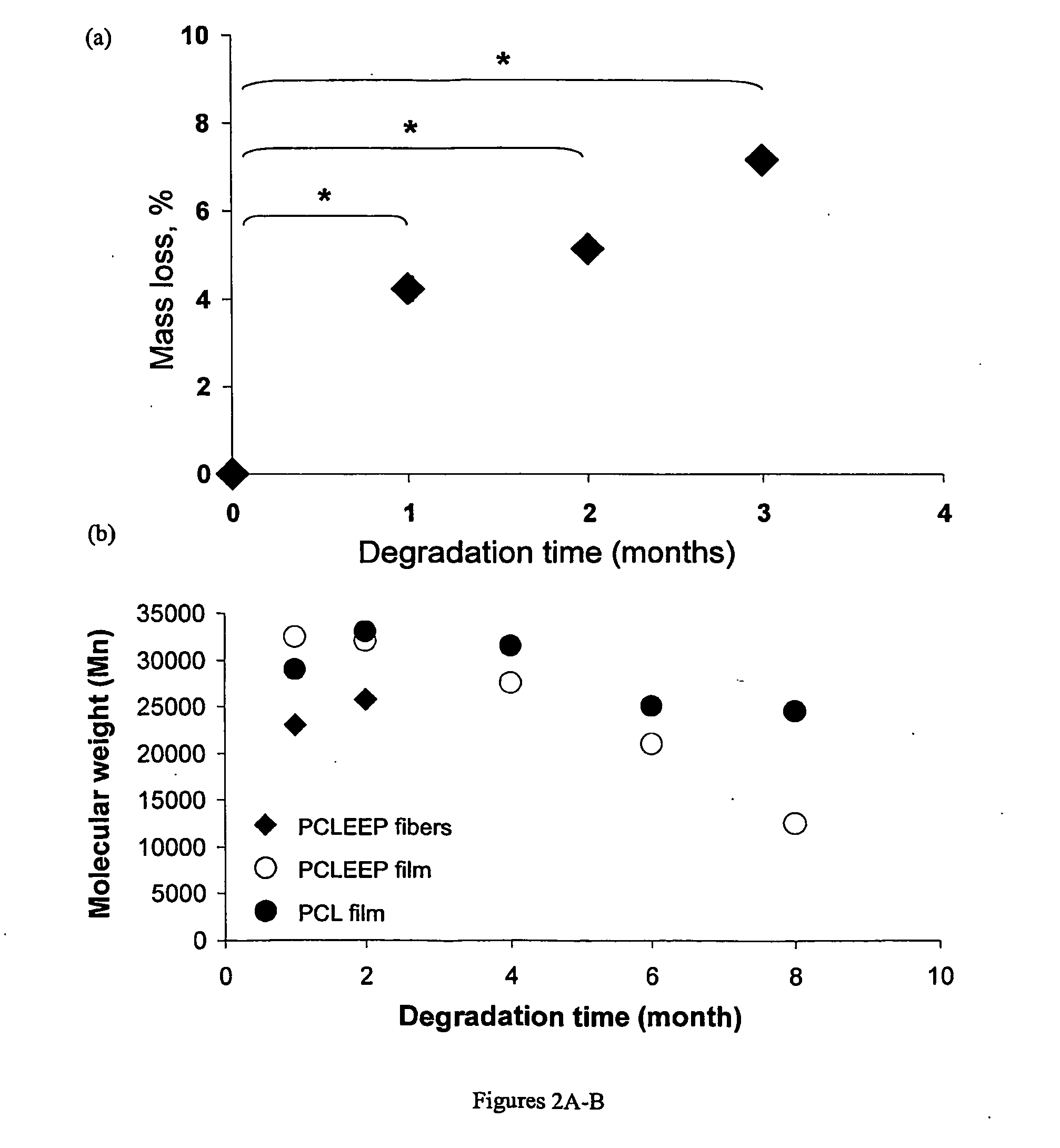
Therapeutic Electrospun Fiber Compositions
InactiveUS20100303881A1Promote nerve growthEfficient releaseBiocideNervous disorderFiberActive agent
The instant invention provides electrospun fiber compositions comprising one or more polymers and one or more biologically active agents. In specific embodiments, the biologically active agents are nerve growth factors. In certain embodiments, the electrospun fiber compositions comprising one or more biologically active agents are on the surface of a film, or a tube. The tubes comprising the electrospun fiber compositions of the invention can be used, for example, as nerve guide conduits.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Chitosan collagen and calcium alginate compounded spongy biological dressing and its preparation process
The composite spongy biological dressing contains chitosan, collagen and calcium alginate with weigh tmixing ratio o f 0.5-8:0.5-8:0.1-8. Its preparation method includes the following steps: selecting chitosan and collagen type I, adding calcium alginate, compounding and cross-linking, using buffer solution to make neutralization, emulsifying, prefreezing and one-step freeze-drying so as to obtain the invented dressing with good biological compatibility and strong adhesion property. Said invented dressing possesses active function of promoting wound healing and hemostatic action, can be combined with anti-bacterial medicine to obtain gene engineeirng dressing for curing wound surface infection, also can be combined with active growth factor or active cell to form gene engineering dressingfor curing intractable ulcer and burn wound surface.
Owner:JIANGXI RUIJI BIOTECH CO LTD
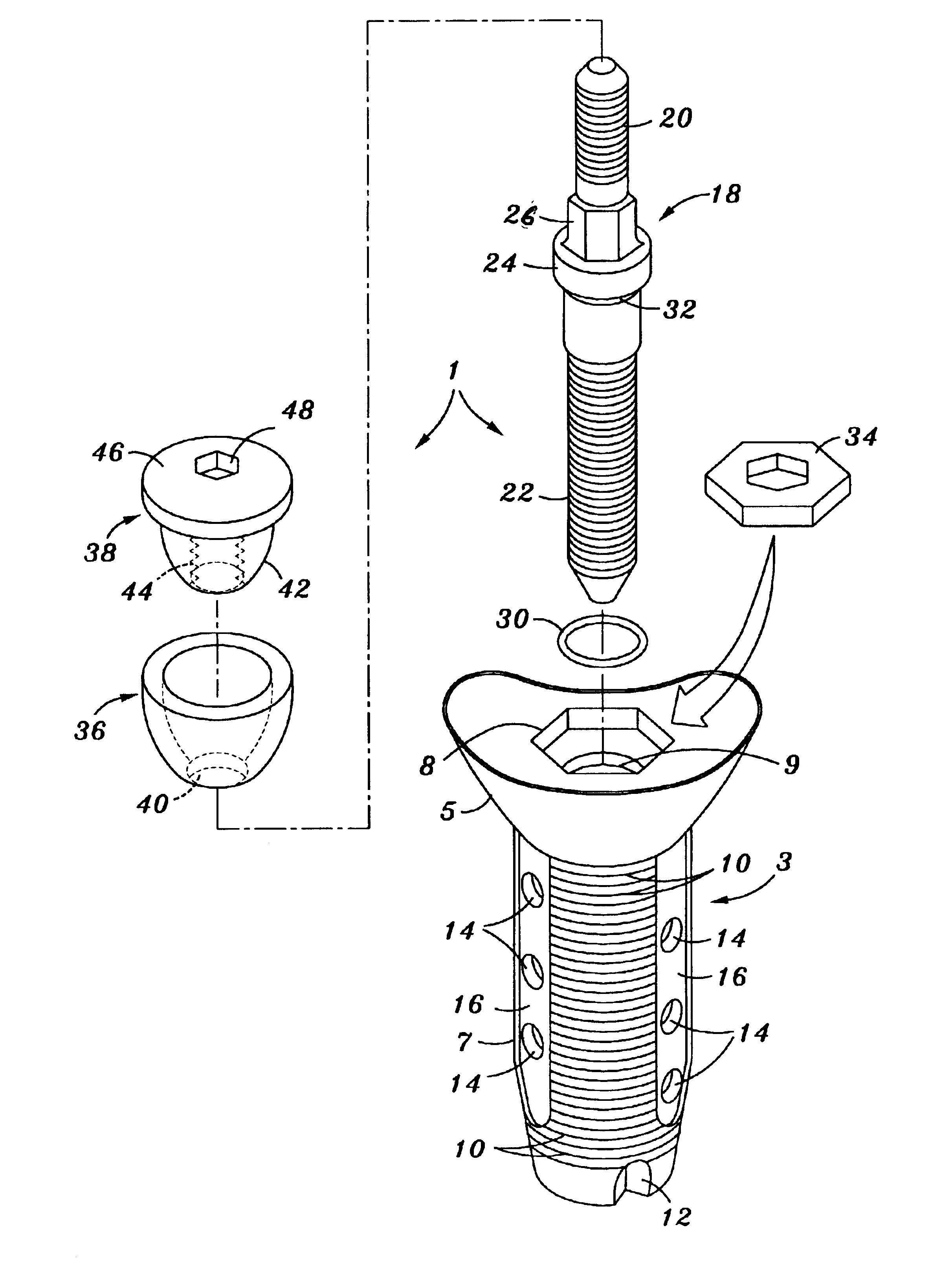
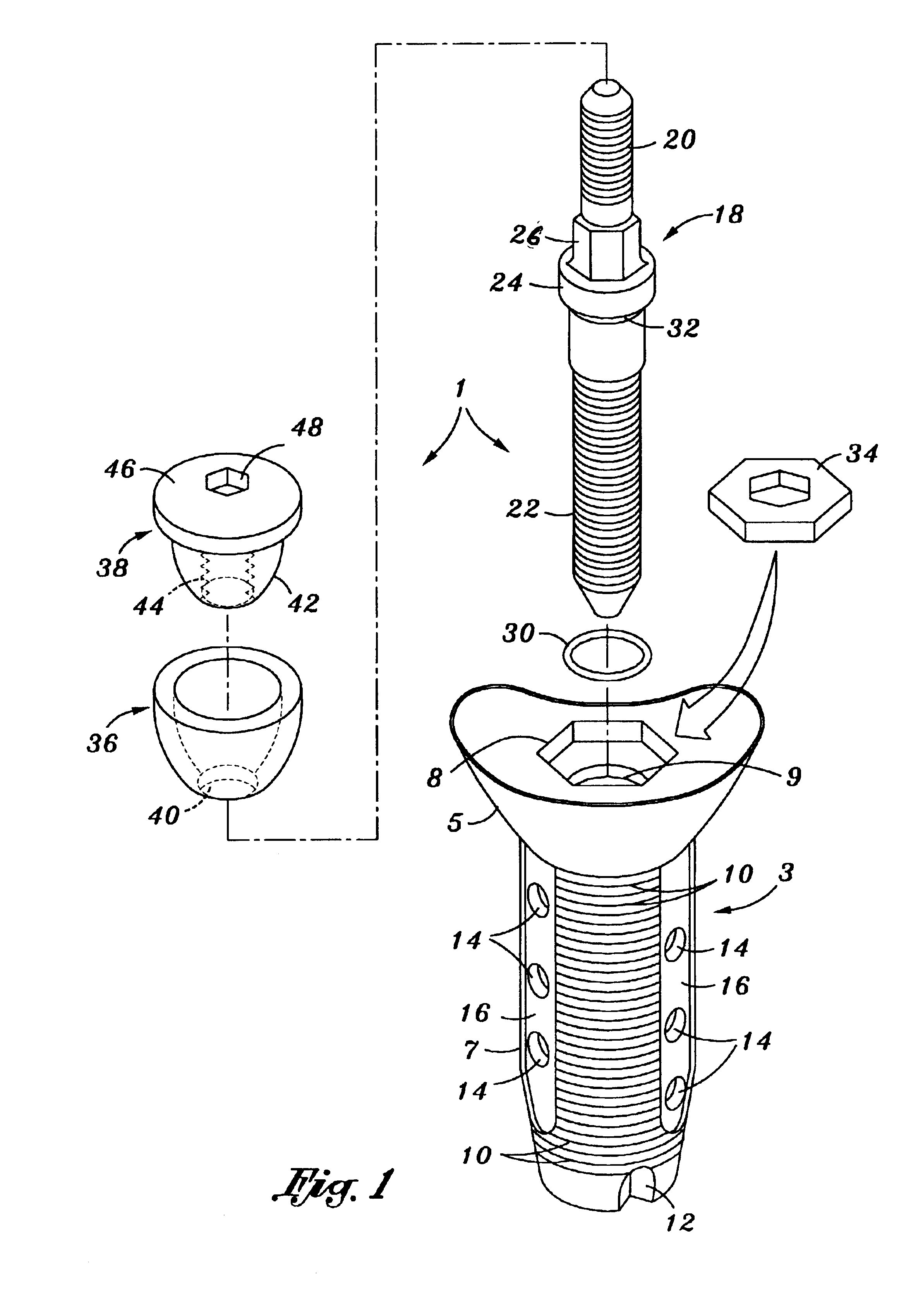
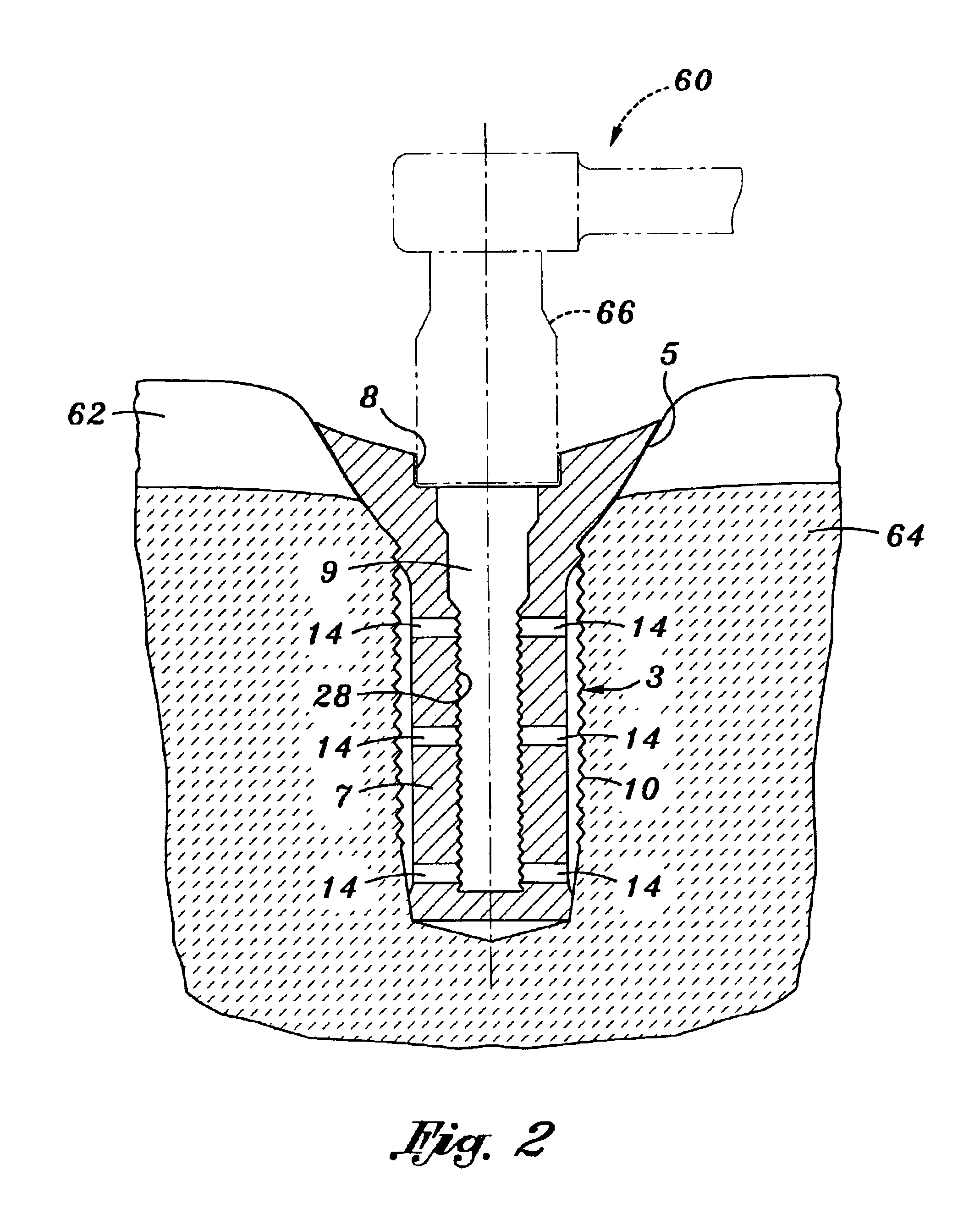
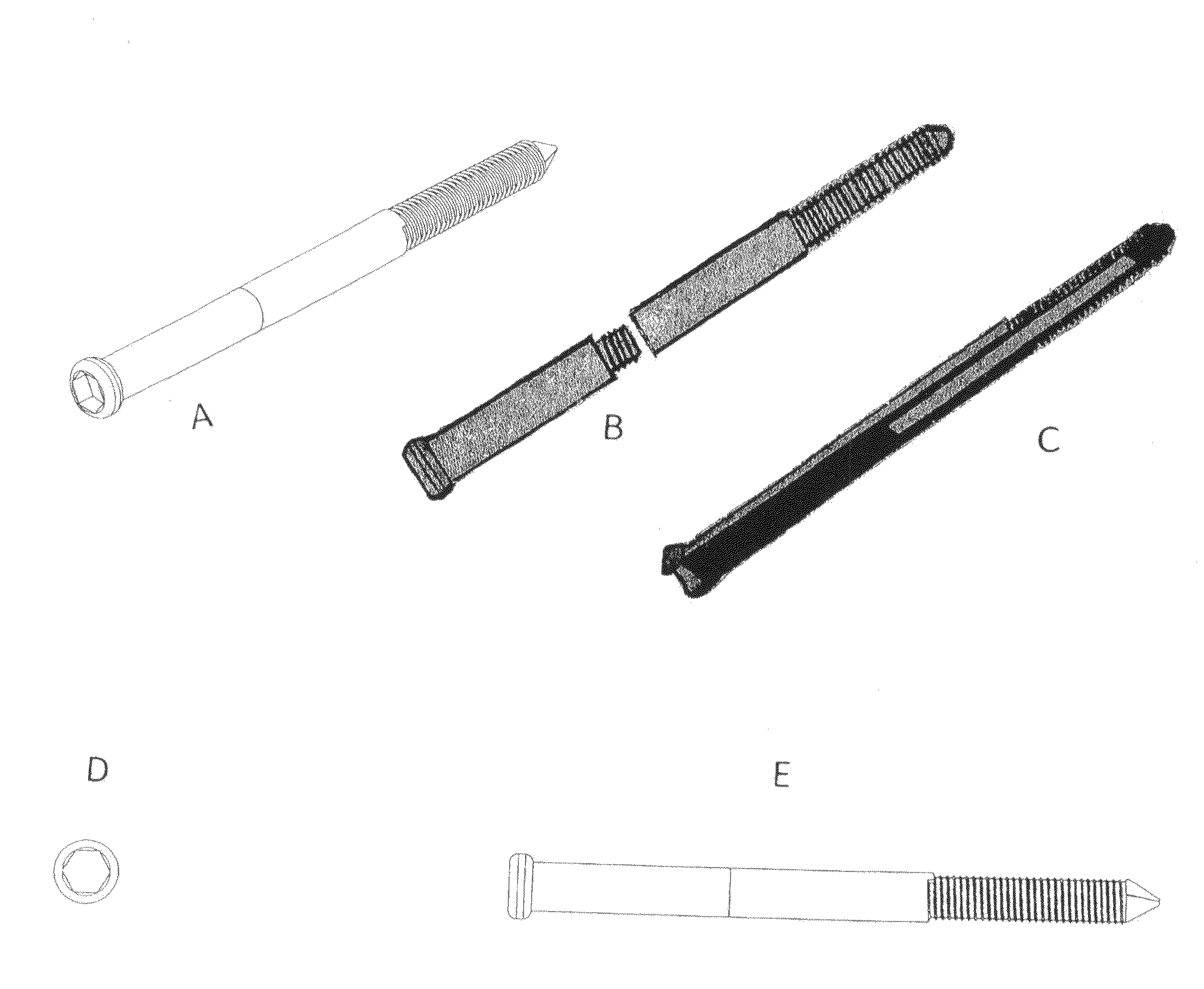
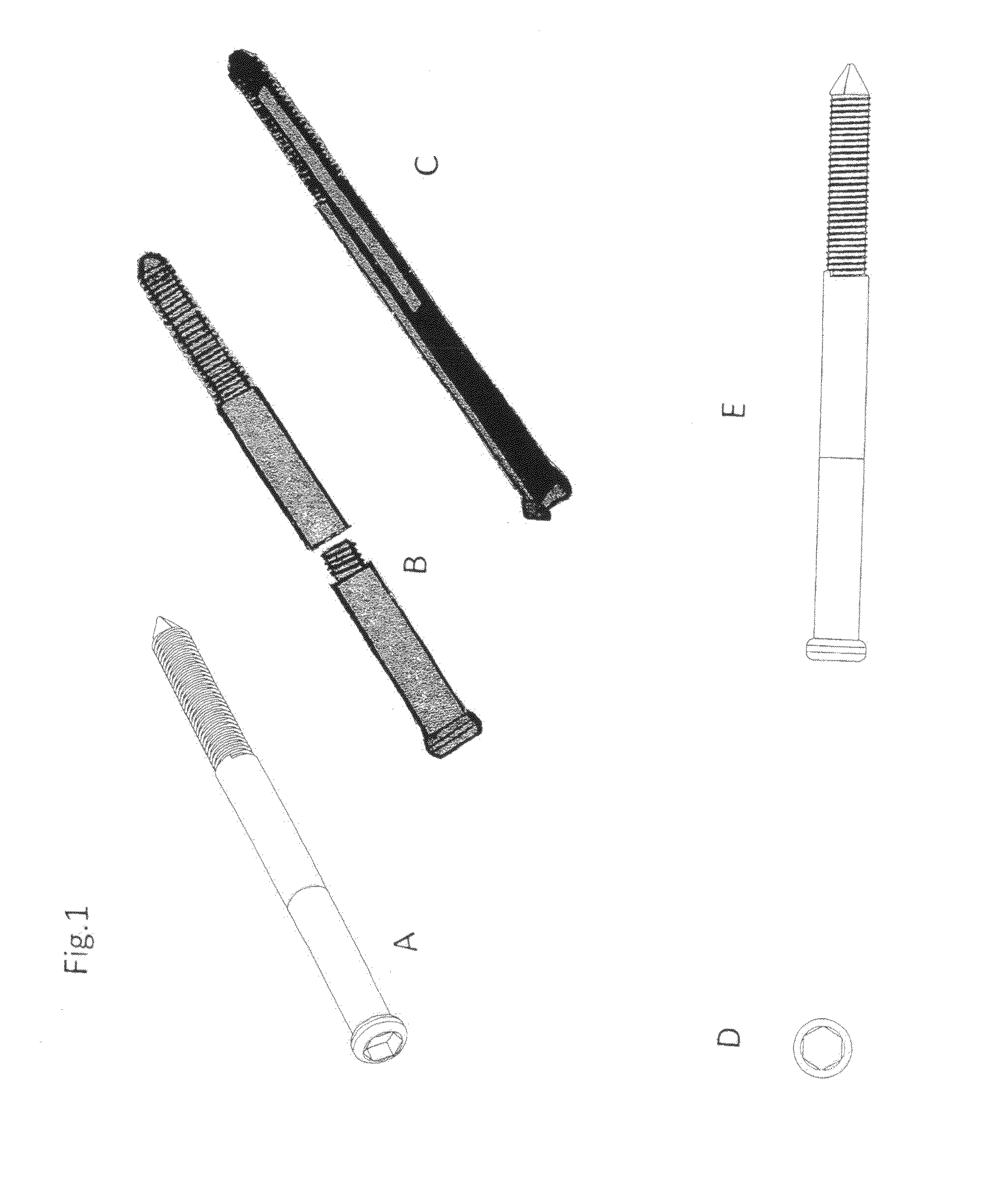
Poly-porous hollow screw for target delivery of growth factors and stem cells:the design and potential clinical application
Present invention depicts a poly-porous (micropore) hollow screws as diffusion chamber filled with core matrix for targeted delivery of growth factors and bone marrow stem cells. The screws comprise at least two parts: the distal part of the screw consists of the tip of the screw made of poly porous material and hollow inside proximally. It has threaded navel attached to the threaded nipple of the distal part of the proximal screw which has the screw head and is made of the solid material of the same kind. The screw head had hexagonal recess targeted for screw driver insertion. Assembly of screw created a chamber in the middle of the screw. The chamber is filled with core matrix consisting of gelatin nano-particles pre-impregnated with BMPs (BMP2 / BMP7 for bone or BMP12 for tendon, ligament) and fibrin sealants or Chitosan dispersed with bone marrow stem cells and / or other growth factors. Bioactive protein core material is prepared during the surgery and filled the chamber of the screw by the surgeon. Fibrin sealants or Chitosan will polymerize to form a gel to hold the growth factors and stem cell in place. The screw can be used as the lag screw or other function to provide mechanical fixation in variety of condition. Once the screw implanted in the human body, the fibrin sealant or Chitosin / gelatin nano-particles are gradually degraded and slowly release growth factors and stem cells via micropores of screw to facilitate the bone healing and regeneration. The gelatin nanoparticles and fibril sealant / or Chitosan matrix also serve as the scaffold and platform for bone in-growth to the screw or alternatively, the stem cell inside of screw can regenerate new bone, providing the biological fixation. At the mean time as the bone regenerate and / or in growth, mechanical strength of the screw increased.
Owner:WU YANGGUAN
Aneurysm stent with growth factor
The present invention relates to an aneurysm stent having a base and connector. The base has a vessel facing side and an aneurysm facing side, and is shaped to cover an aneurysm sufficiently. The connector is coupled to the aneurysm facing side of the base such that when deployed the connector is adapted to extend partially into the aneurysm to anchor the base about the aneurysm and alter flow into the aneurysm. Further, the stent can be used as a delivery mechanism to deliver growth factor.
Owner:THRAMANN JEFREY
Stabilized formulations containing Anti-ngf antibodies
The present invention provides pharmaceutical formulations comprising a human antibody that specifically binds to human nerve growth factor (hNGF). The formulations may contain, in addition to an anti-hNGF antibody, at least one non-ionic surfactant, at least one sugar, and acetate. The pharmaceutical formulations of the present invention exhibit a substantial degree of antibody stability after storage for several months.
Owner:REGENERON PHARM INC
Combined growth factor-deleted and thymidine kinase-deleted vaccinia virus vector
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT HEALTH & HUMAN SERVICES THE
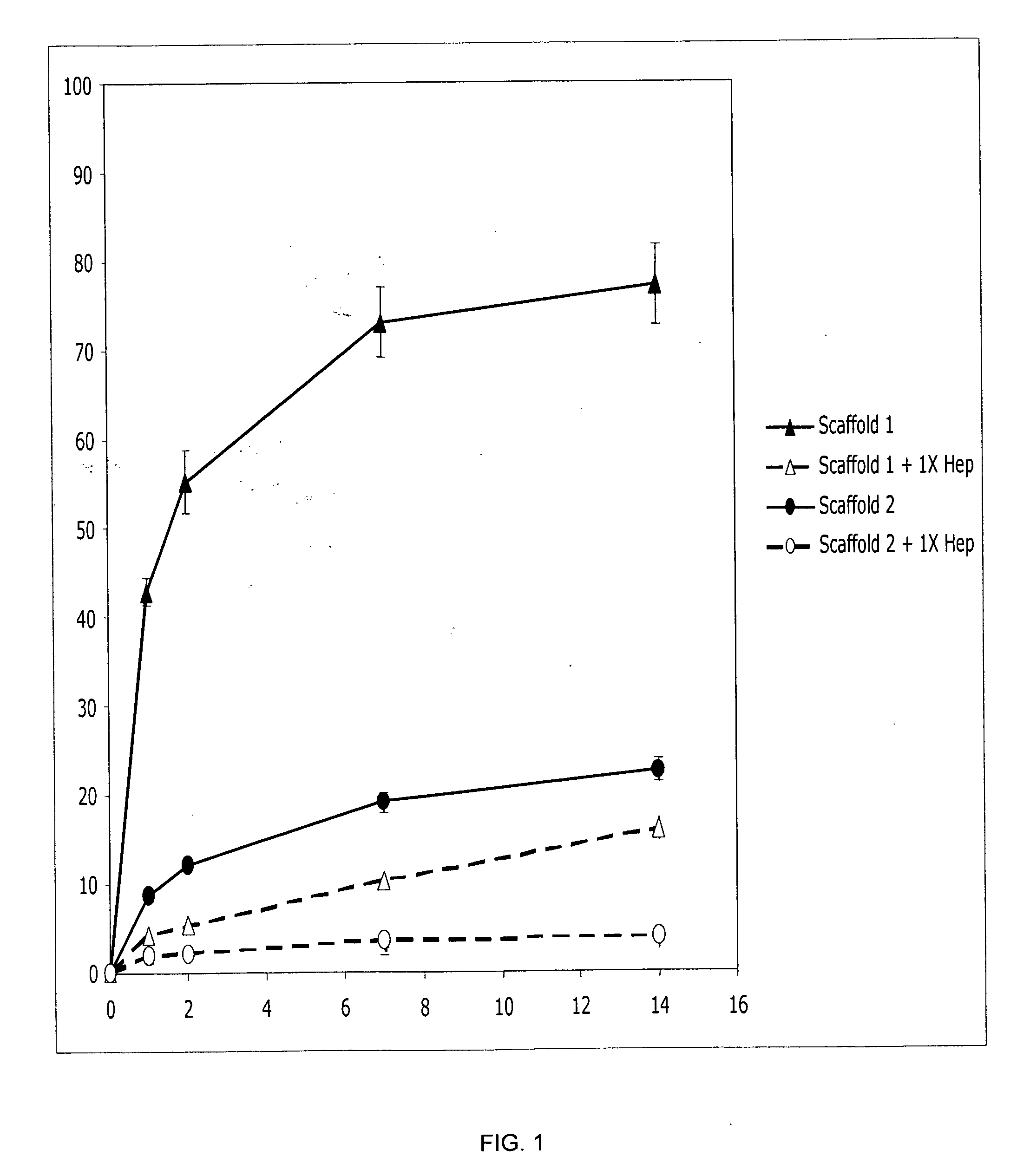
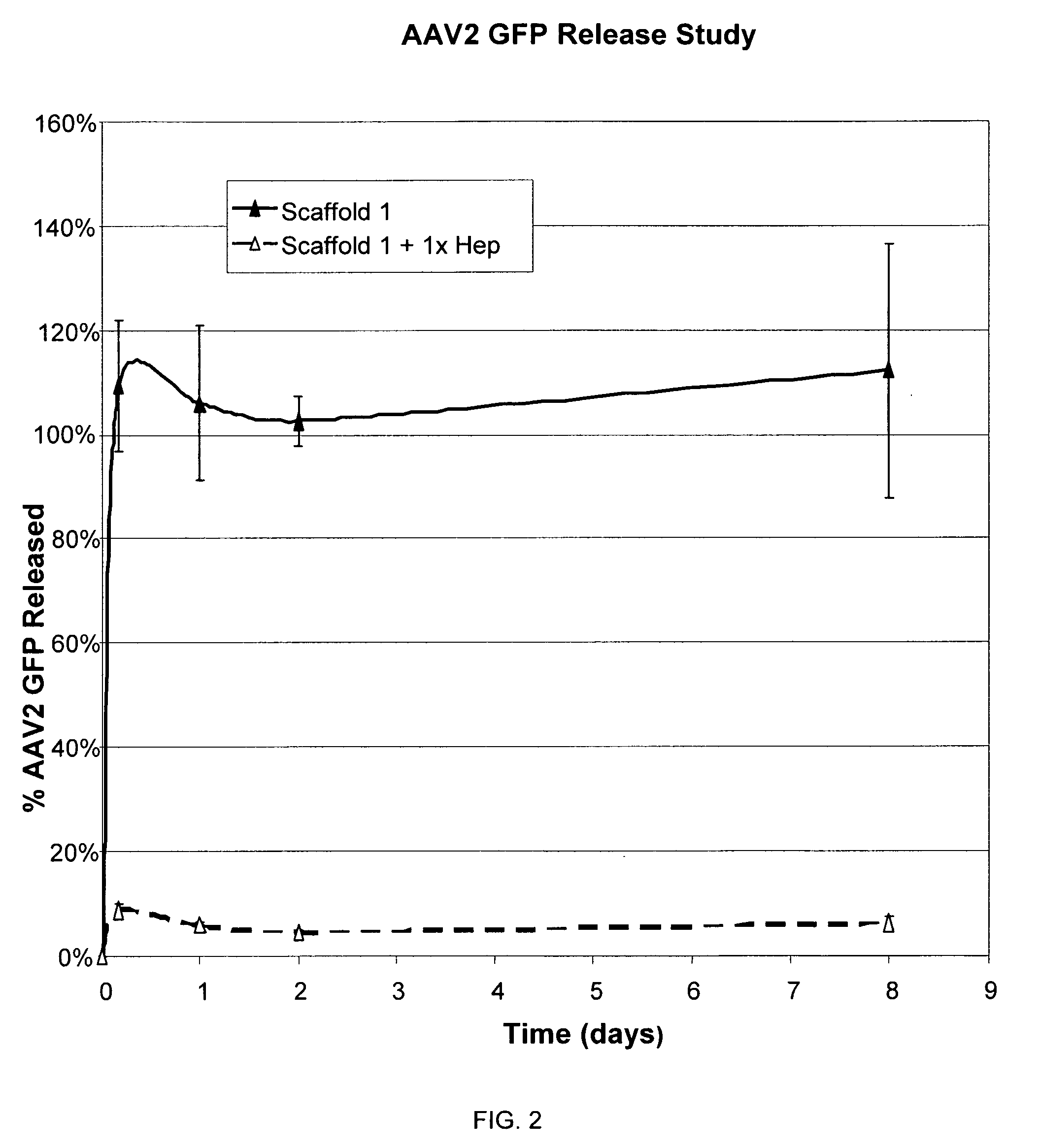
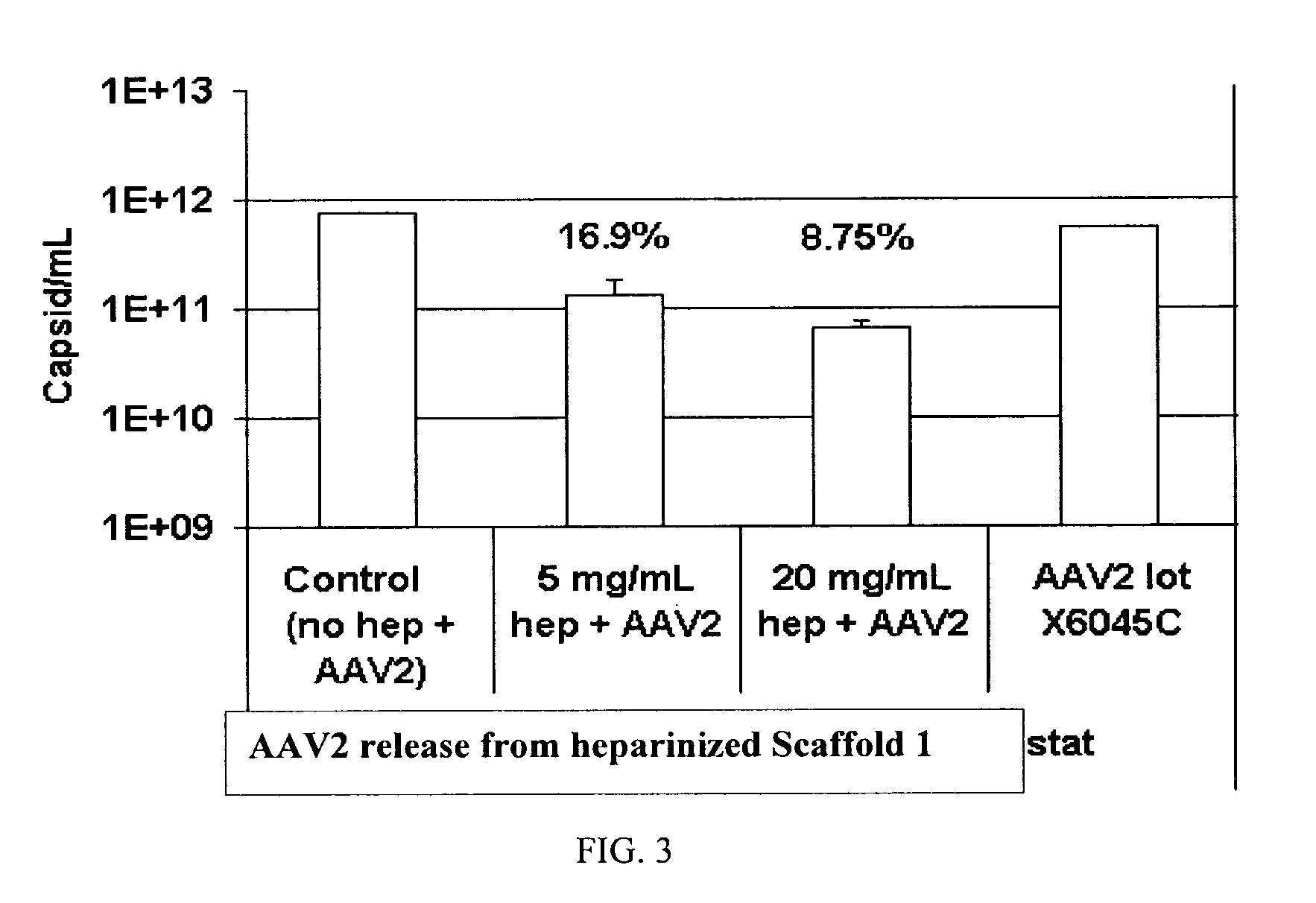
Prolonged delivery of heparin-binding growth factors from heparin-derivatized collagen
InactiveUS20090192079A1Improve biological activityPeptide/protein ingredientsGenetic therapy composition manufactureMuscle tissueCollagen scaffold
The present invention relates to a heparin-derivatized collagen matrix comprising a fragment of heparin covalently linked to a collagen scaffold, wherein the fragment of heparin has molecular weight of less than about 15 kDa, and at least one heparin-binding growth factor (HBGF) or heparin-binding adeno-associated virus (HB-AAV) or a combination thereof and methods for promoting bone growth, bone repair, cartilage repair, bone development, neo-angiogensis, wound healing, tissue engraftment and muscle tissue regeneration and / or tissue augmentation comprising administering a heparin-derivatized collagen matrix that includes at least one heparin-binding growth factor or heparin-binding adeno-associated virus or a combination thereof.
Owner:GENZYME CORP
Stabilized formulations containing Anti-ngf antibodies
InactiveUS20120014968A1Improve adverse effectsNervous disorderSurgical needlesPharmaceutical formulationSURFACTANT BLEND
The present invention provides pharmaceutical formulations comprising a human antibody that specifically binds to human nerve growth factor (hNGF). The formulations may contain, in addition to an anti-hNGF antibody, at least one non-ionic surfactant, at least one sugar, and acetate. The pharmaceutical formulations of the present invention exhibit a substantial degree of antibody stability after storage for several months.
Owner:REGENERON PHARM INC
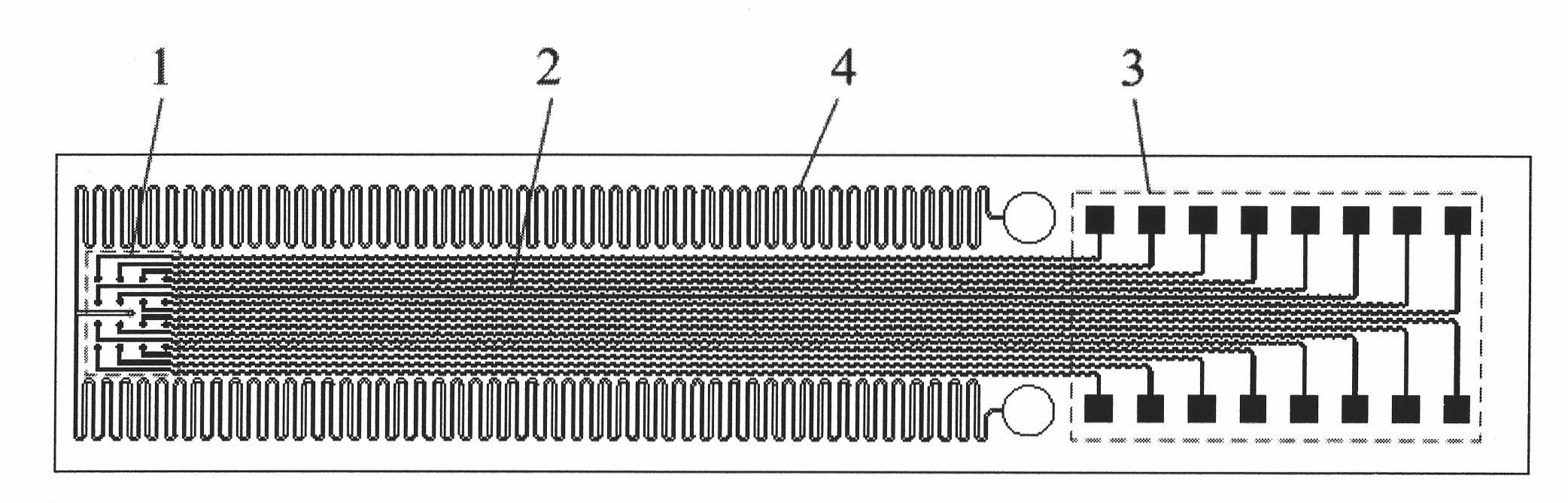
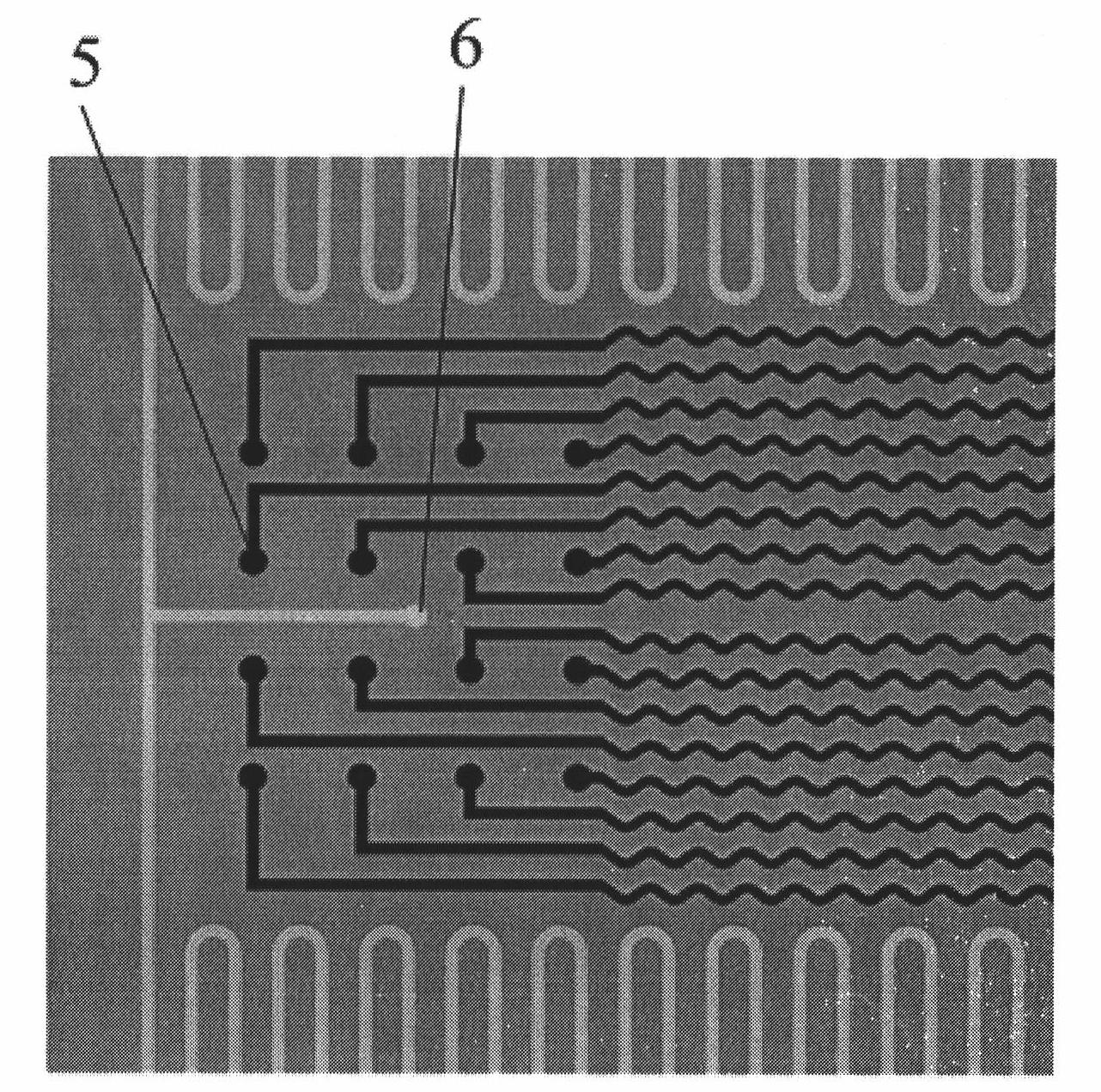
PDMS-based flexible implanted neural microelectrode and manufacturing method
ActiveCN101912666AGood biocompatibilityIncrease elasticityInternal electrodesMedical devicesBiocompatibility TestingMicroelectrode
The invention discloses a polydimethylsiloxane-based (PDMS) flexible implanted neural microelectrode and a manufacturing method. The electrode is characterized in that the PDMS with high biocompatibility and mechanical elasticity is used as a substrate material for the neural microelectrode, wherein the implanted flexible neural microelectrode which comprises an electrode site region, a connecting line region, a welding spot region and a micro-pipeline region is formed by electroplating technology, PDMS injection molding technology and bonding technology; the electrode site, the connecting line and the welding spot are structurally formed of an electroplated metal layer, so that the tensile resistance and the reliability of the metal structure of the PDMS microelectrode are enhanced; and the micro-pipeline integrated on the electrode can be used for pouring a curable liquid material which contains medicament or nerve growth factor, so that the operability of the operation implantation of the PDMS neutral microelectrode and the biocompatibility after the implantation are improved. Meanwhile, the preparation method of the PDMS microelectrode provided by the invention has the characteristics of simple process, low cost and standard batch manufacturing.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
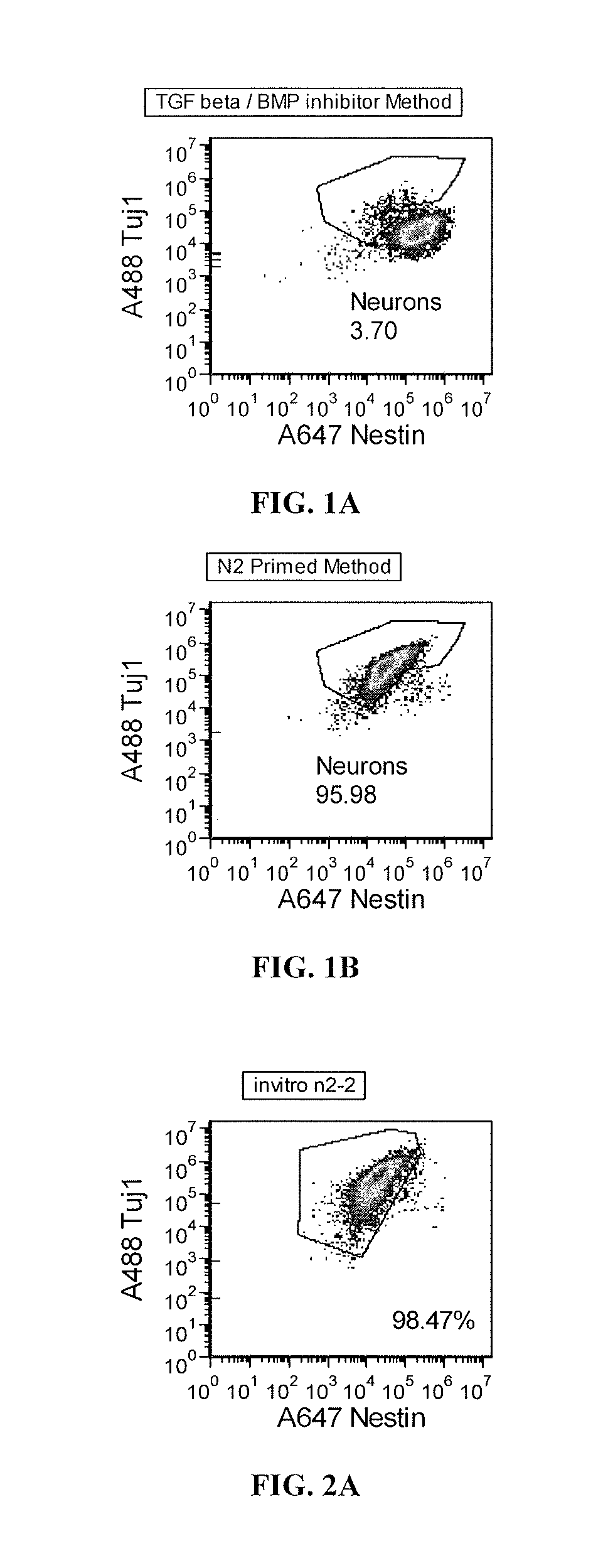
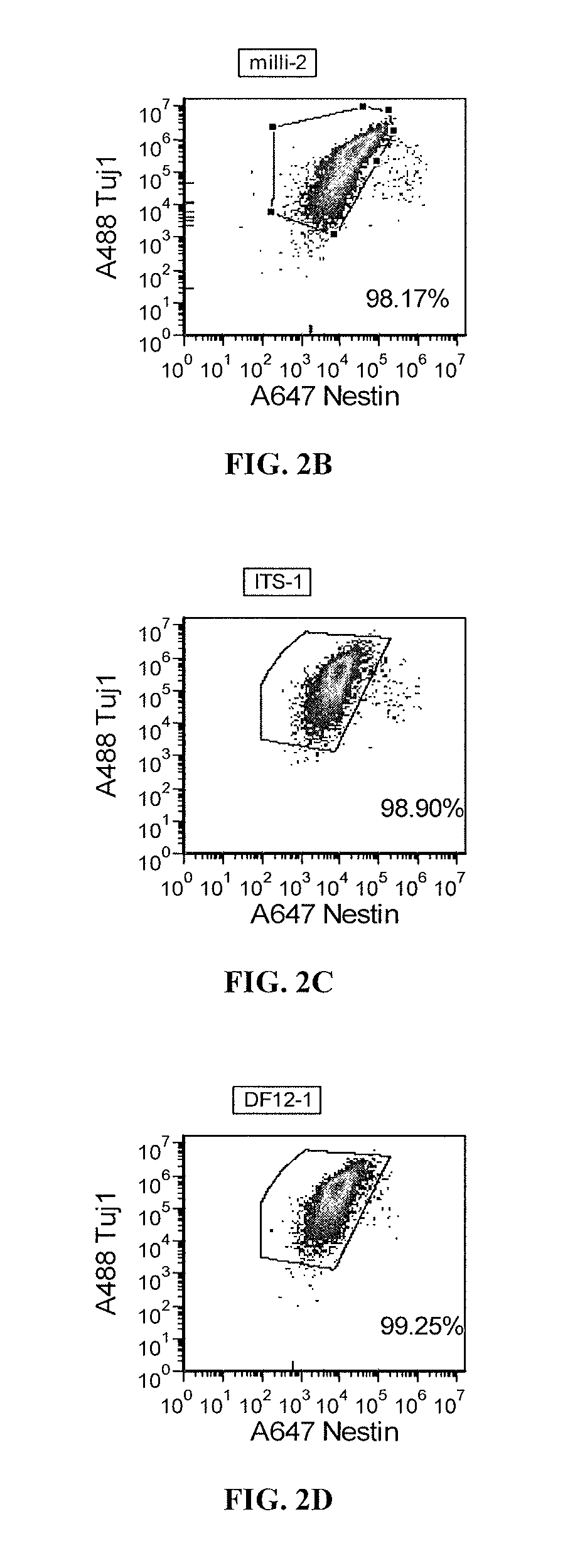
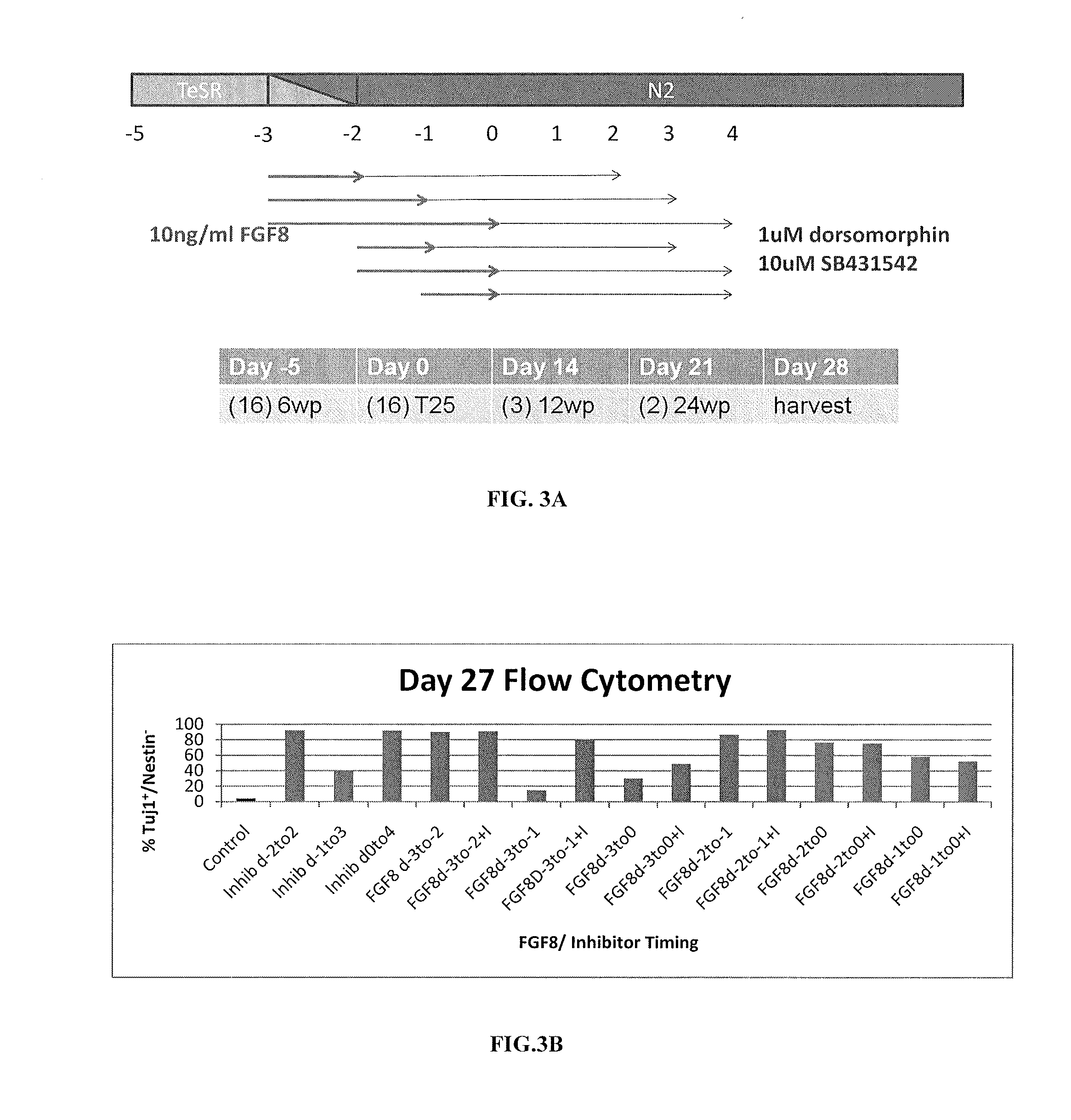
Priming of pluripotent stem cells for neural differentiation
ActiveUS20120276063A1Improve efficiencyMass productionBiocideSenses disorderPluripotential stem cellNeurulation
Methods and composition for differentiation of pluripotent stem cells are provided. For example, in certain aspects methods including priming stem cells for neural differentiation in a culture medium essentially free of growth factors such as FGF and TGFβ. As an advantage, the neural cells may be provided with improved consistency and purity.
Owner:FUJIFILM CELLULAR DYNAMICS INC
Multiplex composite bone tissue engineering bracket material capable of degrading gradiently and preparation method thereof
The invention discloses a multiplex composite engineering scaffold material capable of gradually decomposing bone tissue and a preparation method thereof. The composite scaffold material consists of calcium phosphate bone cement, biological compatible degradable synthetic high polymer and biological compatible degradable natural high polymer, has better mechanical property and gradient degradation characteristic, and can achieve the aim of regenerating and repairing bone tissue defect by implanting a bone growth factor to induce in-vivo stem cells to be differentiated into bone cells, thereby obviously improving initial strength and toughness of the scaffold material, and ensuring enough strength and toughness of the scaffold material during operating and implanting. After compounded with the high polymer material, the scaffold has excellent flexibility, so that the scaffold can be subjected to certain machining, such as cutting and the like.
Owner:SOUTH CHINA UNIV OF TECH
Device which enhances the biological activity of locally applied growth factors with particular emphasis on those used for bone repair
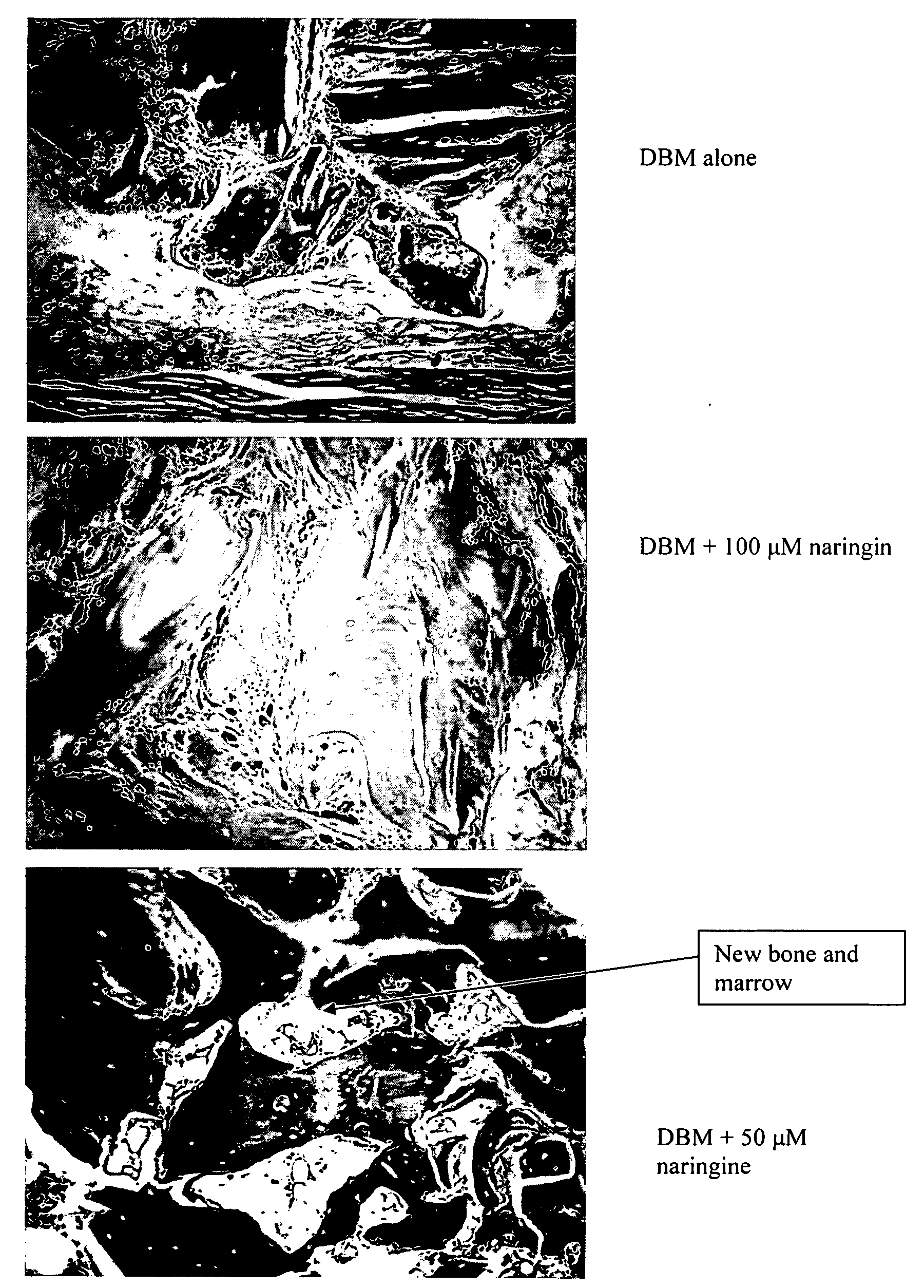
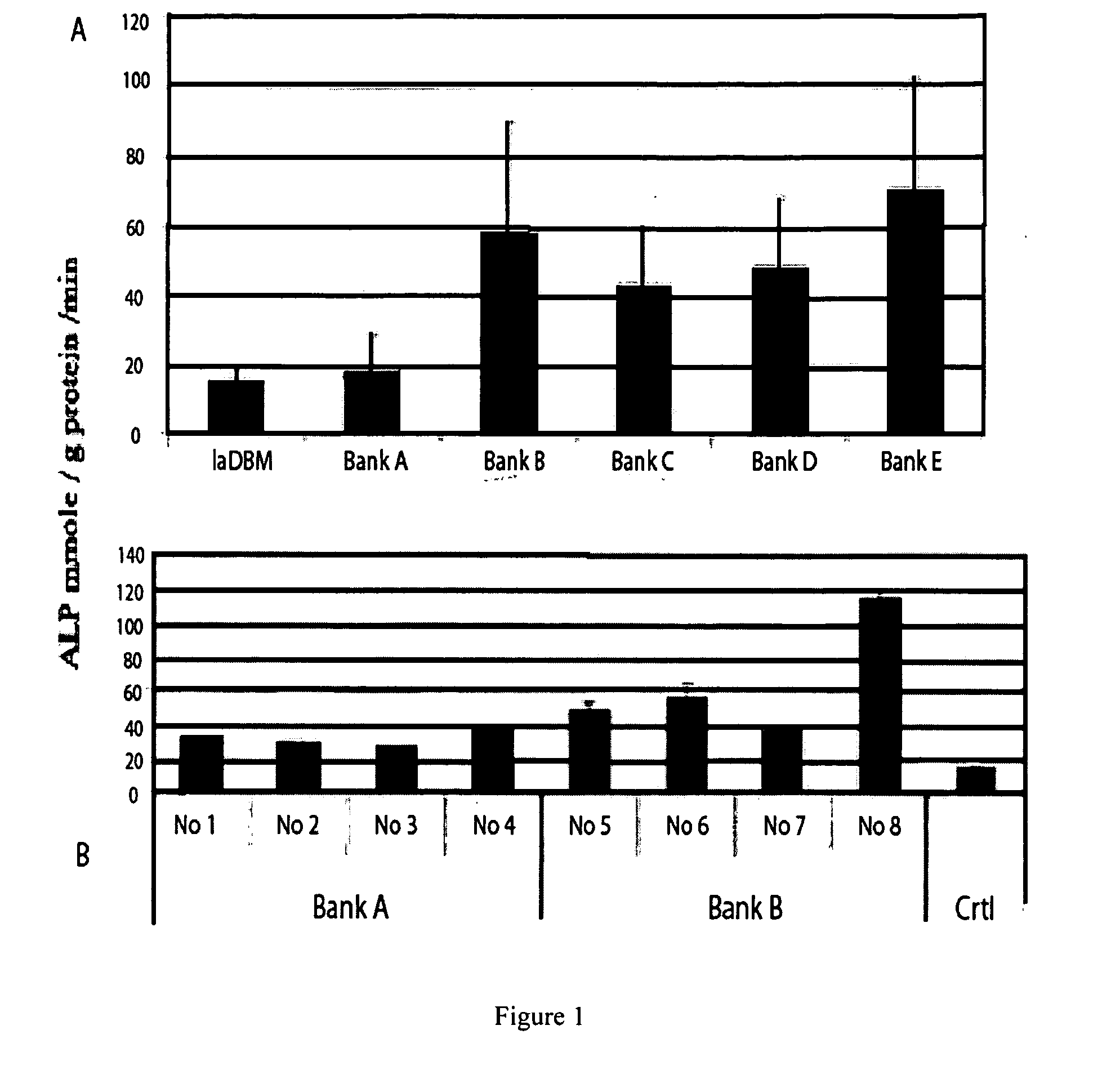
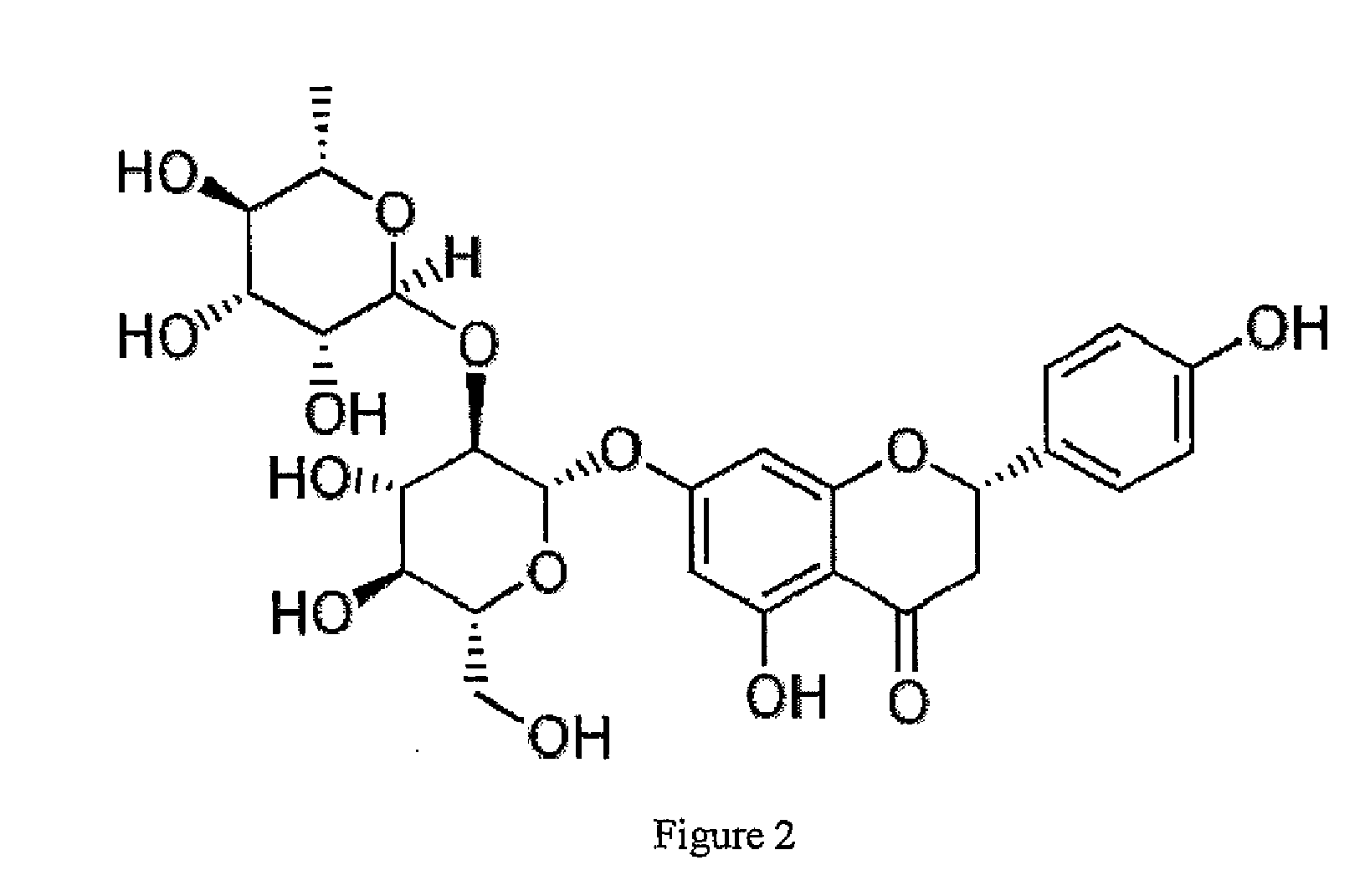
InactiveUS20080241211A1Improve biological activityReduce dose requiredBiocidePowder deliveryNaringinMedicine
This invention provides a novel medical appliance for repairing, regenerating, maintaining, and / or augmenting a bone. The medical appliance generally includes an osteoinductive agent, an osteoinductive enhancer, and a carrier matrix. Also disclosed are methods, compositions, kits, and bone matrix formulations for regenerating, maintaining, and / or augmenting a bone. Exemplary preferred osteoinductive agents include growth factors such as BMP and TGF-β. Exemplary preferred osteoinductive enhancers include phytoestrogens such as naringin.
Owner:UNIV OF SOUTHERN CALIFORNIA
Nerve growth factor as a vaccine adjuvant
A vaccination method utilizes a pharmaceutical combination for enhancing vaccine effectiveness. The method utilizes an immune response-triggering vaccine capable of stimulating production in an immunodeficient animal antibodies to a disease-causing agent foreign to the animal. As an adjuvant, a vaccine effectiveness-enhancing amount of Nerve Growth Factor (NGF) is administered, which enhances production and affinity of the antibodies in the animal, in response to the vaccine.
Owner:PROTECHTION UNLIMITED
Cosmetic or skin care product preparation rich in growth factors and preparation method therefor
ActiveCN104856892APromote growth and developmentPromote epithelializationCosmetic preparationsToilet preparationsWrinkle skinAdditive ingredient
The invention relates to the cosmetic technology field, and especially relates to a cosmetic or skin care product preparation rich in growth factors and a preparation method therefor. A cosmetic or skin care product preparation rich in growth factors and composed of main ingredients: cell growth factors, micromolecule polypeptides, water, grease, surfactants, humectants, anti-oxidants and preservatives and a preparation method are provided. Through mutual cooperation of ingredients, the composition preparation can accelerate metabolism of skin cells, restore fractured shallow epidermal layer fibrocytes, has protruding rapid restoration function to skin wound surfaces, and has a plurality of functions of whitening, wrinkle removing, calmness, anti-allergy, skin smoothing, anti-wrinkle, brightening, refreshing, anti-aging, anti-radiation, stain prevention and reduction and the like.
Owner:广州远想生物科技股份有限公司 +1
Implant depots to deliver growth factors to treat avascular necrosis
ActiveUS20070259018A1Treat and alleviate AVN bone problemIncreasing bone mineral densityPeptide/protein ingredientsAntipyreticPorosityBone structure
The present invention relates to the design and composition of a depot implant for optimal delivery of growth factors to treat bone avascular necrosis, in that such depot implant is constructed to be in a cylinder (rod) or sphere shape and have a natural or synthetic polymer scaffold with or without impregnated calcium phosphate particles. The density of the depot is higher than a typical BMP sponge carrier to facilitate it's implantation and slower release of the growth factor. The scaffold is such that it has adequate porosity and pore size to facilitate growth factor seeding and diffusion throughout the whole of the bone structure resulting in increased new blood vessel growth and density in the avascular necrotic bone. In addition, the shape of the depot implant allows for delivery through a cannula or large bore needle.
Owner:WARSAW ORTHOPEDIC INC
Methods for therapy of neurodegenerative disease of the brain
InactiveUS6451306B1Reduce adverse effectsAvoid restrictionsBiocideVirusesBasal forebrainTherapeutic Area
The invention provides a specific protocol for use in grafting donor cells genetically modified to produce nerve growth factors into grafting sites within the cholinergic basal forebrain and is especially useful in treating neurodegenerative conditions such as Alzheimer's Disease. Grafting sites are selected for proximity to previously identified defective, diseased or damaged brain cells. Each graft is situated no more than about 550 mum from a targeted cell and no more than about 5 mm from another graft. Depending on the size of the region to be treated, the number of grafting sites will vary upwards of 10 sites, with between 5 and 10 sites serving to deliver a therapeutically significant dosage of nerve growth factors to targeted cells. Donor cells are delivered in a composition concentration of at least 1x105 cells / mul, wherein each graft is comprised of between 2 and 20 mul of the donor cell composition. The composition is delivered to each grafting site over a period of about 5-10 minutes.
Owner:RGT UNIV OF CALIFORNIA
Administration of growth factors for the treatment of CNS disorders
ActiveUS7922999B2Increased growth factor mobilityAvoid delayHeavy metal active ingredientsSenses disorderNeuron deathNeuron
A method and system that is directed to the local delivery of growth factors to the mammalian CNS to treat CNS disorders associated with neuronal death and / or dysfunction is described.
Owner:RGT UNIV OF CALIFORNIA
Cloning and recombinant production of CRF receptor(s)
InactiveUS20050186593A1Peptide/protein ingredientsAntibody mimetics/scaffoldsBinding siteSerine Kinase
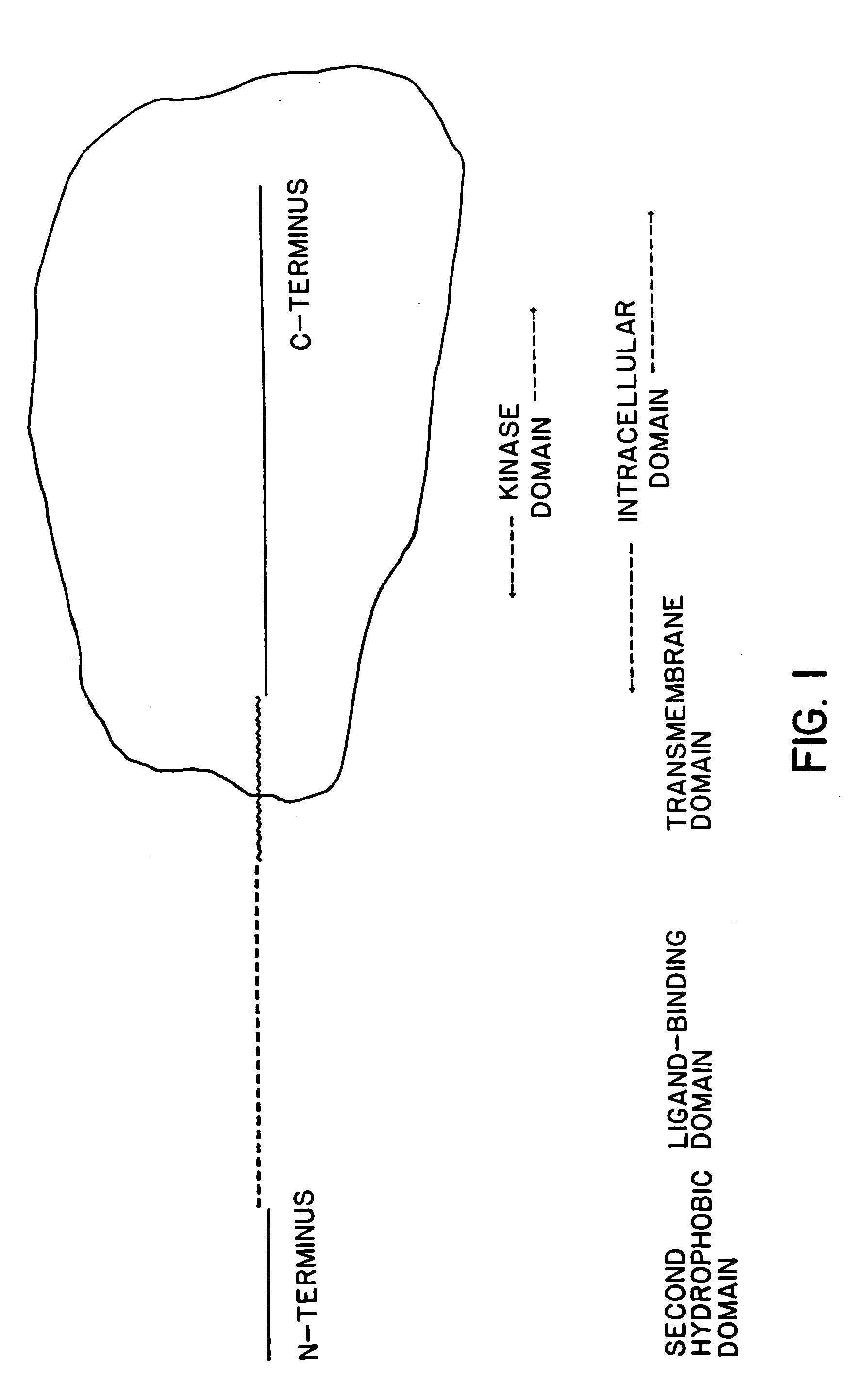
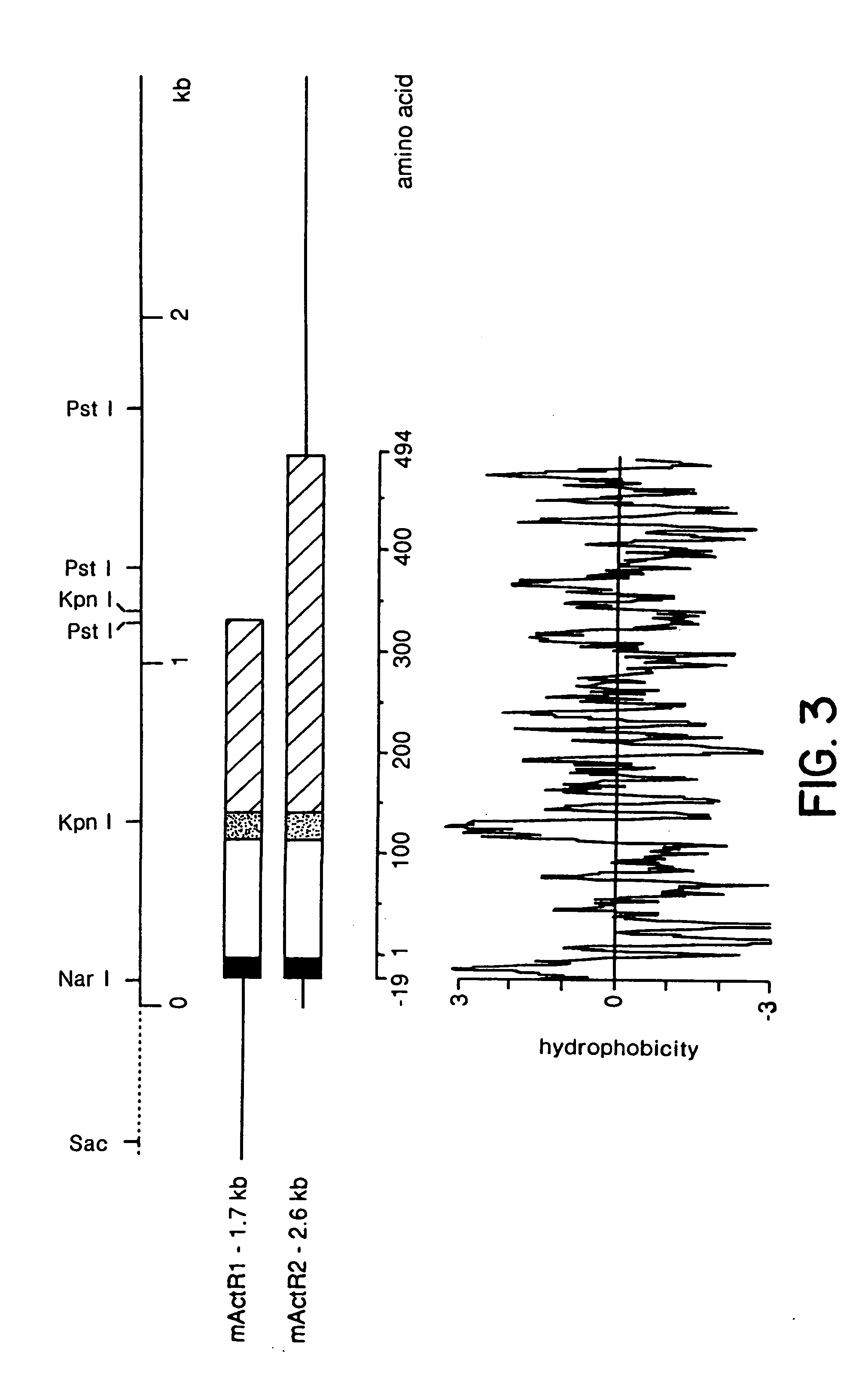
In accordance with the present invention, there are provided novel receptor proteins characterized by having the following domains, reading from the N-terminal end of said protein: an extracellular, ligand-binding domain, a hydrophobic, trans-membrane domain, and an intracellular, receptor domain having serine kinase-like activity. The invention receptors optionally further comprise a second hydrophobic domain at the amino terminus thereof. The invention receptor proteins are further characterized by having sufficient binding affinity for at least one member of the activin / TGF-β superfamily of polypeptide growth factors such that concentrations of ≦10 nM of said polypeptide growth factor occupy ≦50% of the binding sites of said receptor protein. A presently preferred member of the invention superfamily of receptors binds specifically to activins, in preference to inhibins, transforming growth factor-β, and other non-activin-like proteins. DNA sequences encoding such receptors, assays employing same, as well as antibodies derived therefrom, are also disclosed.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Orientated nano fiber bionic nerve conduit and manufacturing method thereof
ActiveCN102525689APromote regenerationHigh tensile strengthCatheterTubular organ implantsFiberSpinning
The invention provides an orientated nano fiber bionic nerve conduit and a manufacturing method thereof. A nano fiber membrane with orientation degree larger than or equal to 75% is prepared by means of coaxial electrostatic spinning, and then is curved to form the nerve conduit, NGF (nerve growth factor) is added into fibers so that the bionic nerve conduit is combined with various factors promoting nerve regeneration, such as 'nano bionics', 'contact guiding', 'drug release' and the like, and the bionic nerve conduit can effective promote nerve regeneration as proved by animal experiments.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Surface treatments of an allograft to improve binding of growth factors and cells
A method for enhancing the binding of growth factors and / or cells to an allograft structure by applying an effective quantity of a coating material to the surface of the allograft structure, producing a thin coated allograft structure, administering to the thin coated allograft structure a growth factor, cells or a combination thereof, and implanting the thin coated allograft structure into a host bone.
Owner:WARSAW ORTHOPEDIC INC
Growth factor analogs
InactiveUS7414028B1Promote cell proliferationPromote growthPeptide/protein ingredientsFibroblast growth factorFactor iiChemistry
The invention provides synthetic heparin-binding growth factor analogs of formulas I or II as given in the specification, having two peptide chains branched from a dipeptide branch moiety composed of at least one and preferably two trifunctional amino acid residues, which peptide chain or chains bind a heparin-binding growth factor receptor. The synthetic heparin-binding growth factor analogs are useful as pharmaceutical agents, soluble biologics or as surface coatings for medical devices.
Owner:BIOSURFACE ENG TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com