Patents
Literature
2389 results about "Urinary bladder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
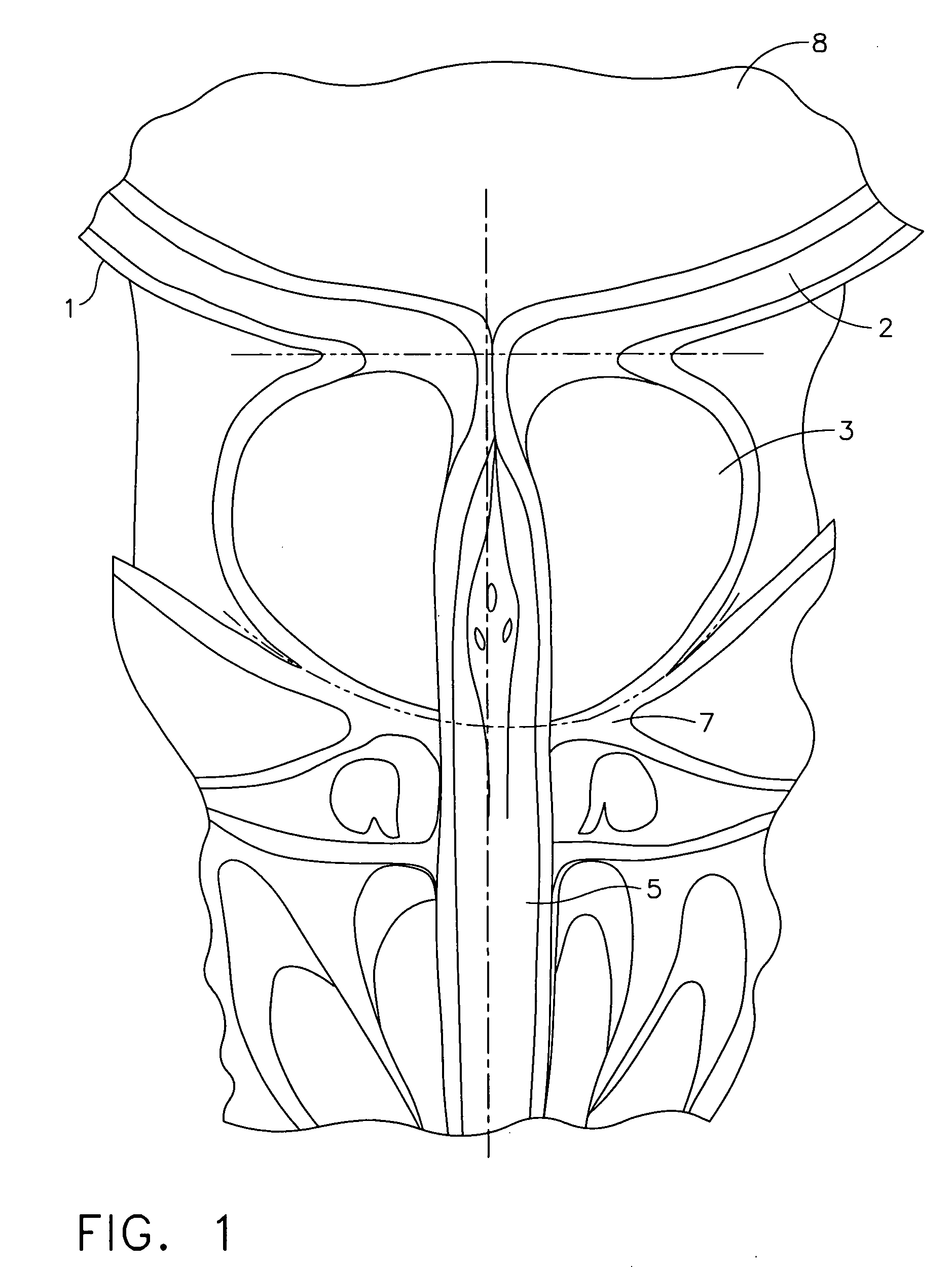
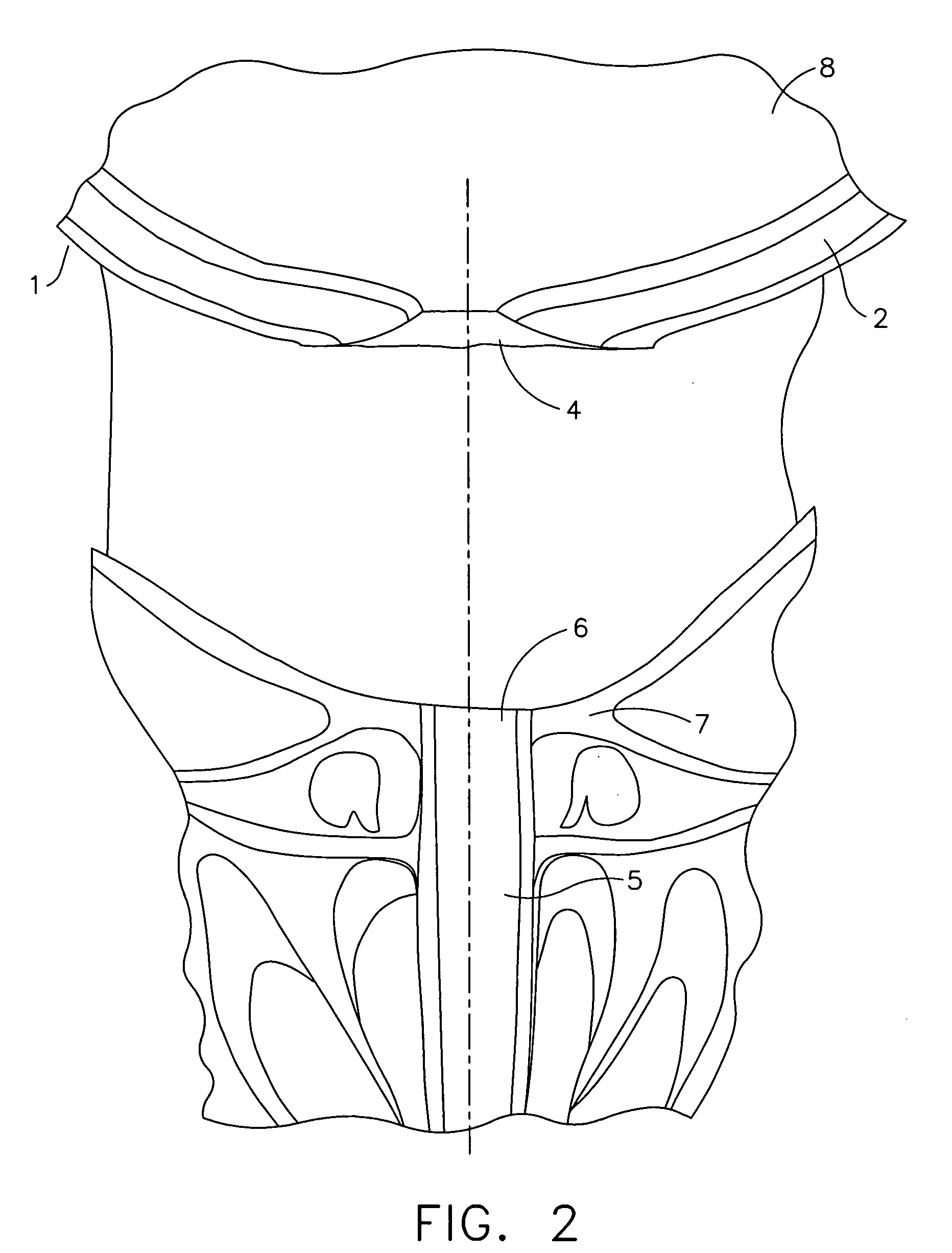
The urinary bladder is a hollow muscular organ in humans and some other animals that collects and stores urine from the kidneys before disposal by urination. In the human the bladder is a hollow muscular, and distensible (or elastic) organ, that sits on the pelvic floor. Urine enters the bladder via the ureters and exits via the urethra. The typical human bladder will hold between 300 and 500 mL (10.14 and 16.91 fl oz) before the urge to empty occurs, but can hold considerably more.
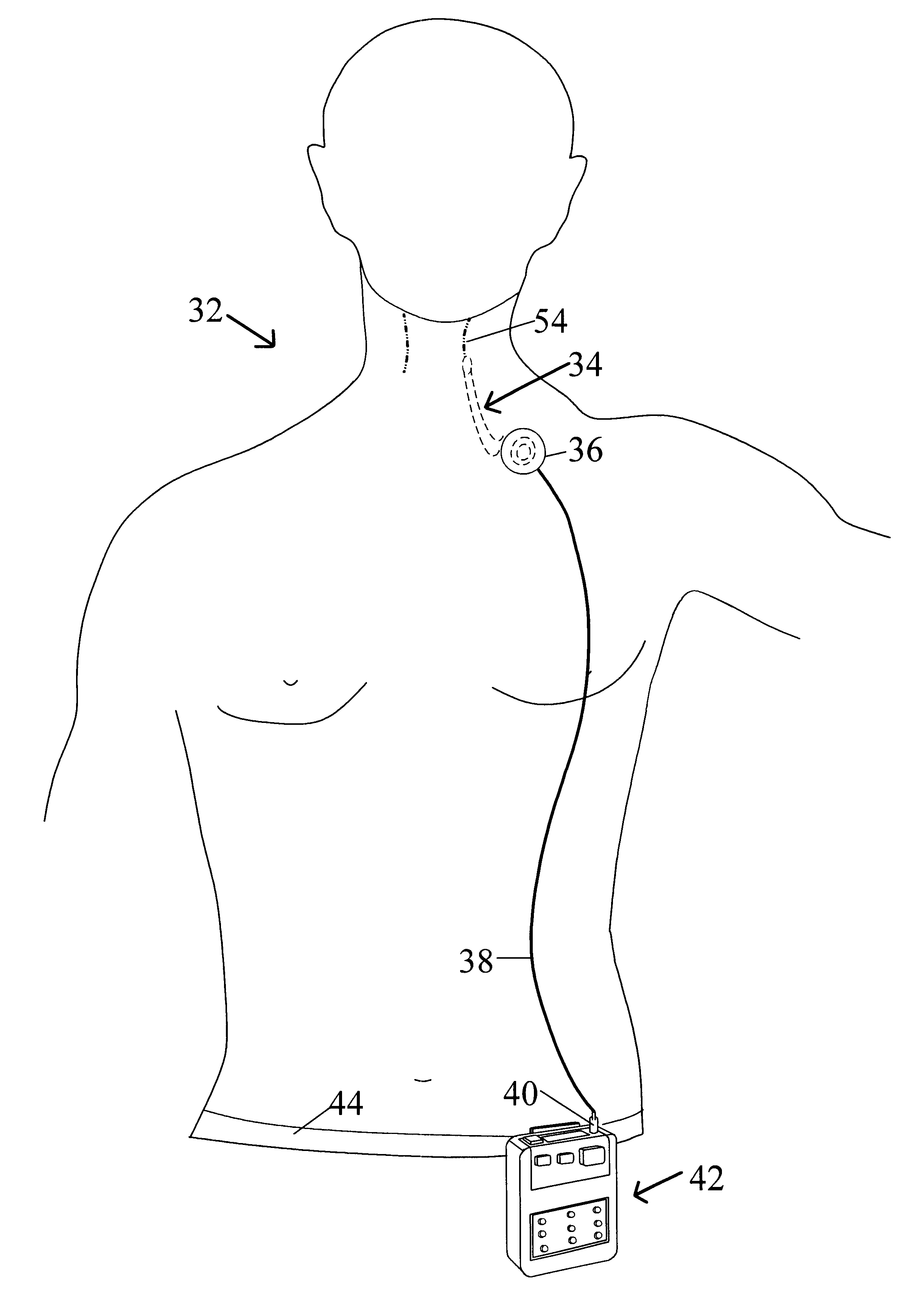
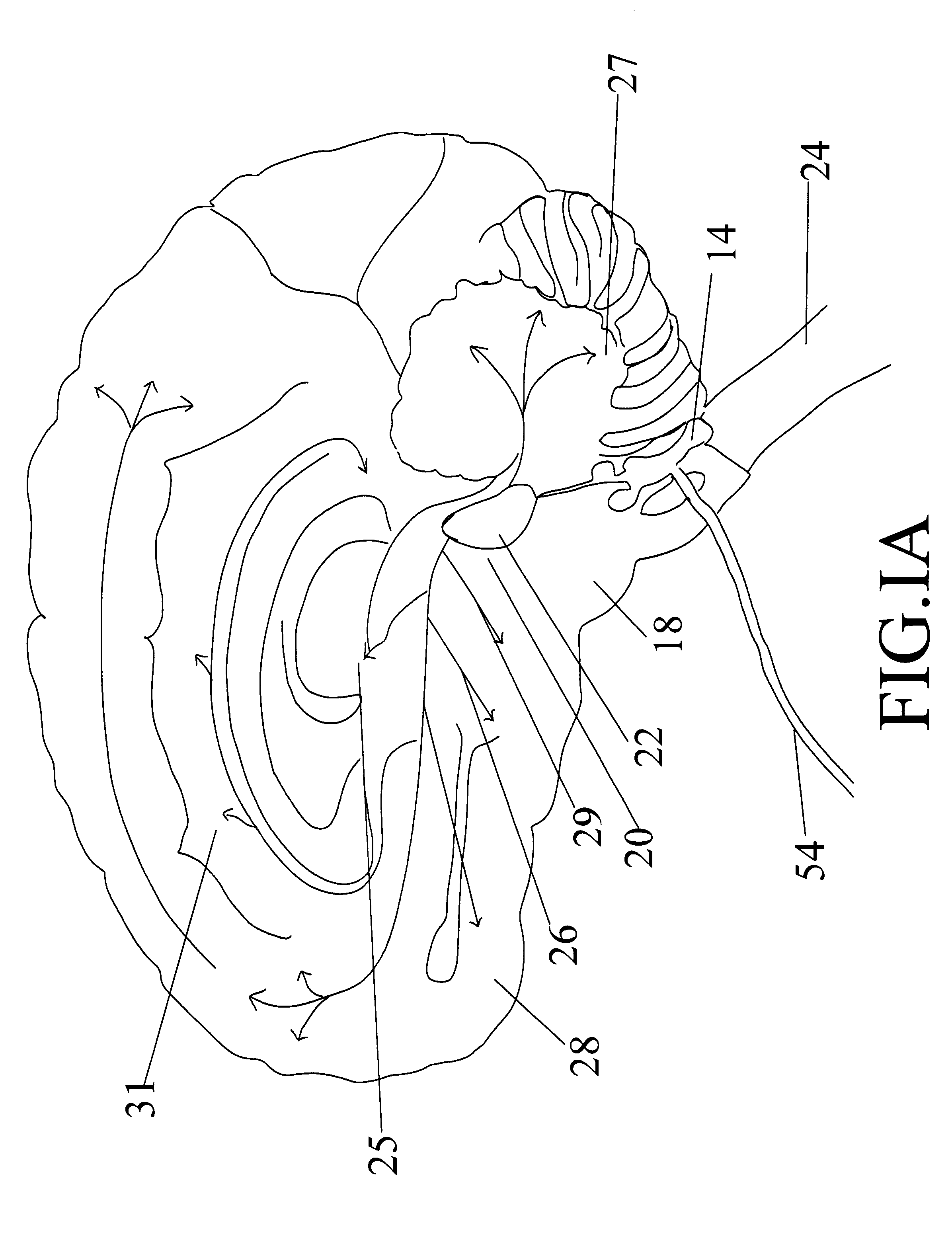
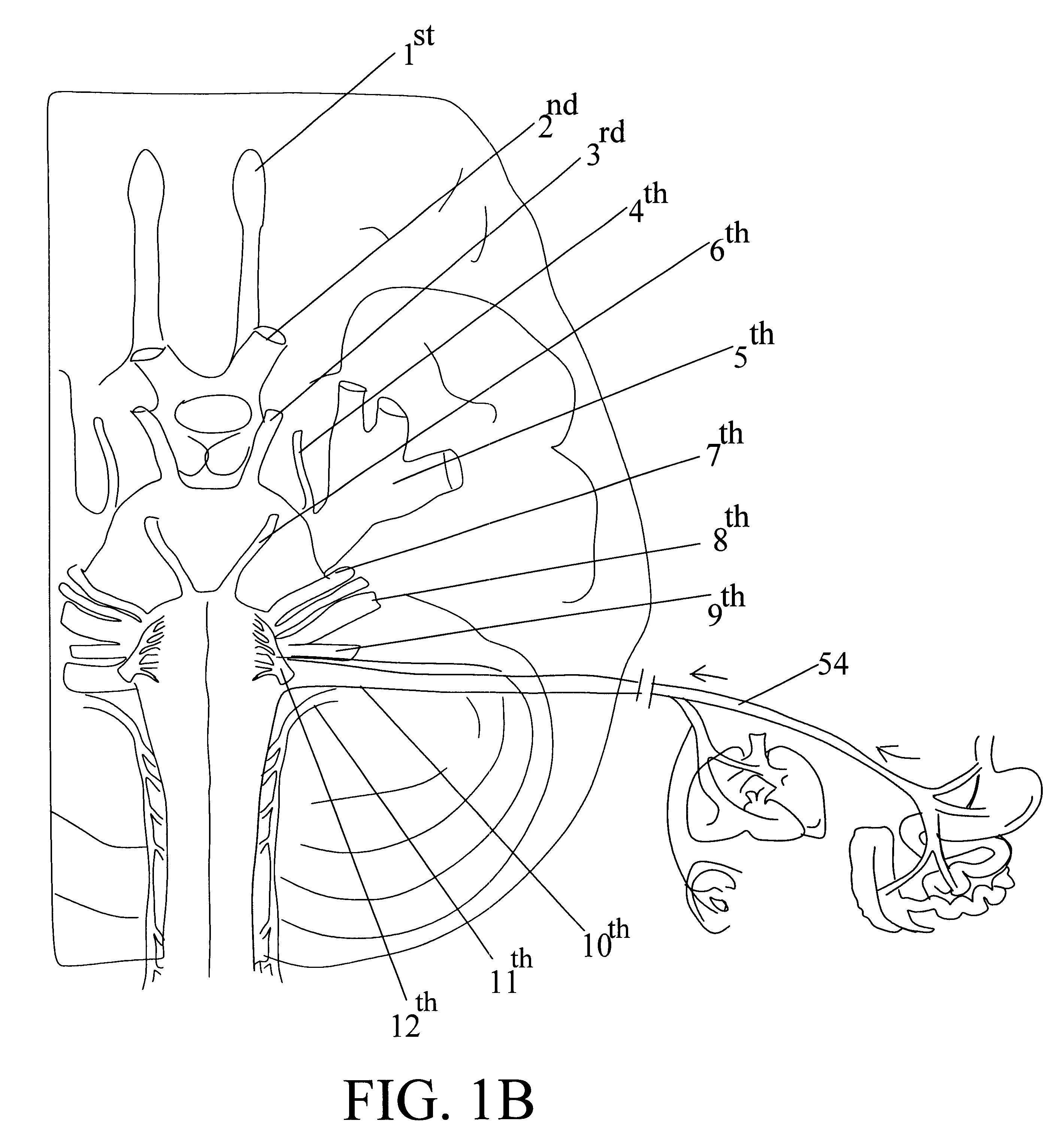
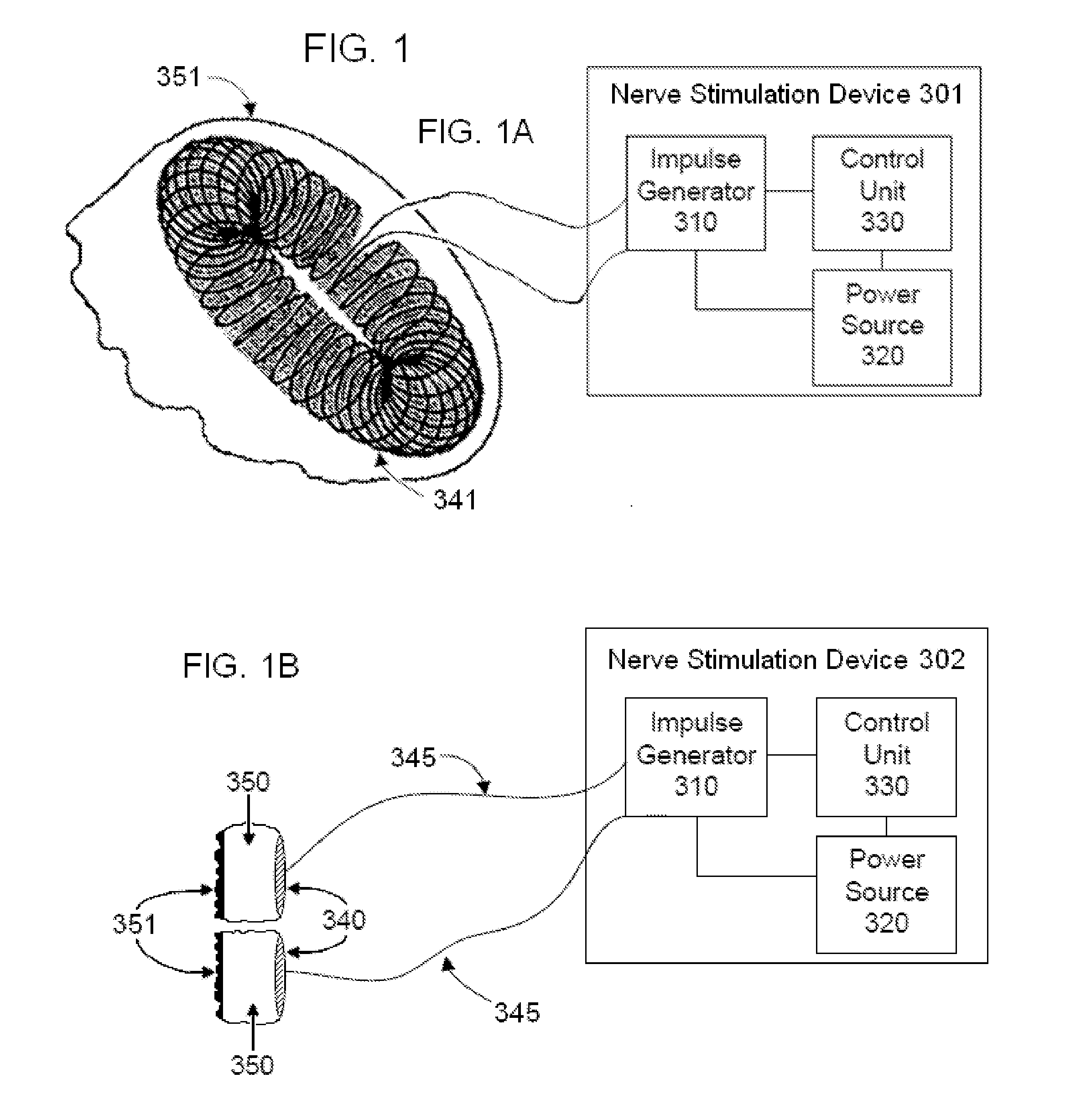
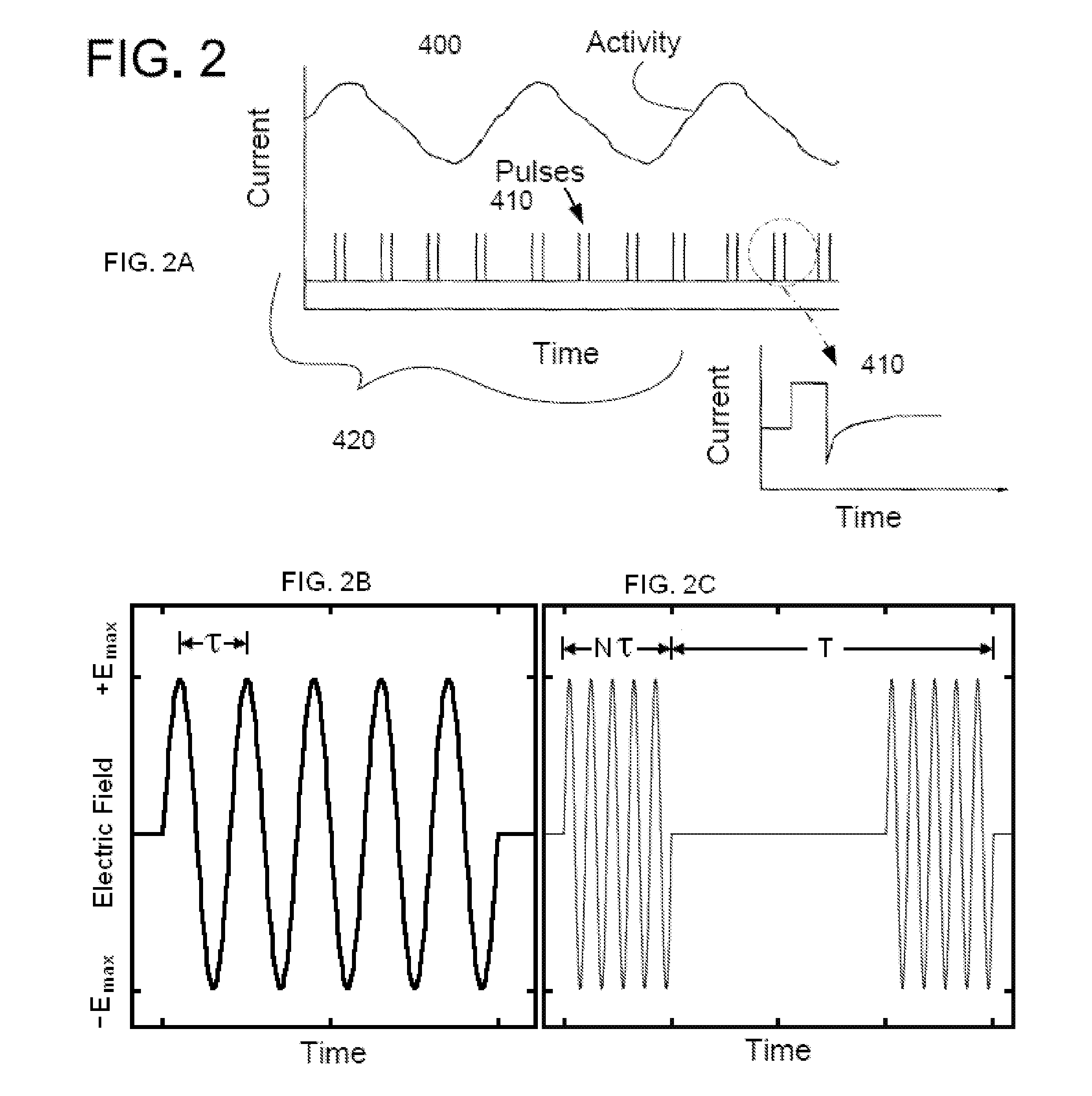
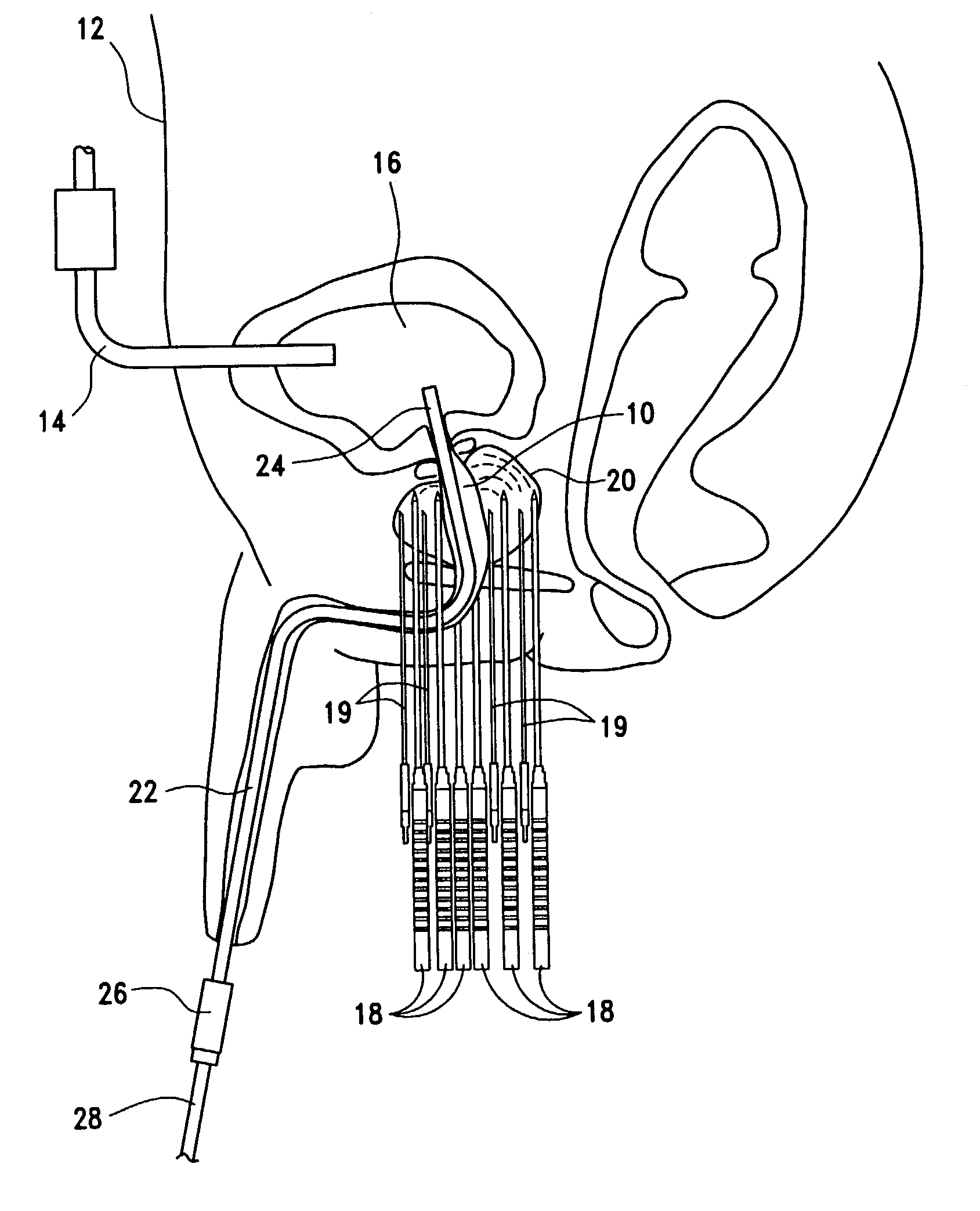
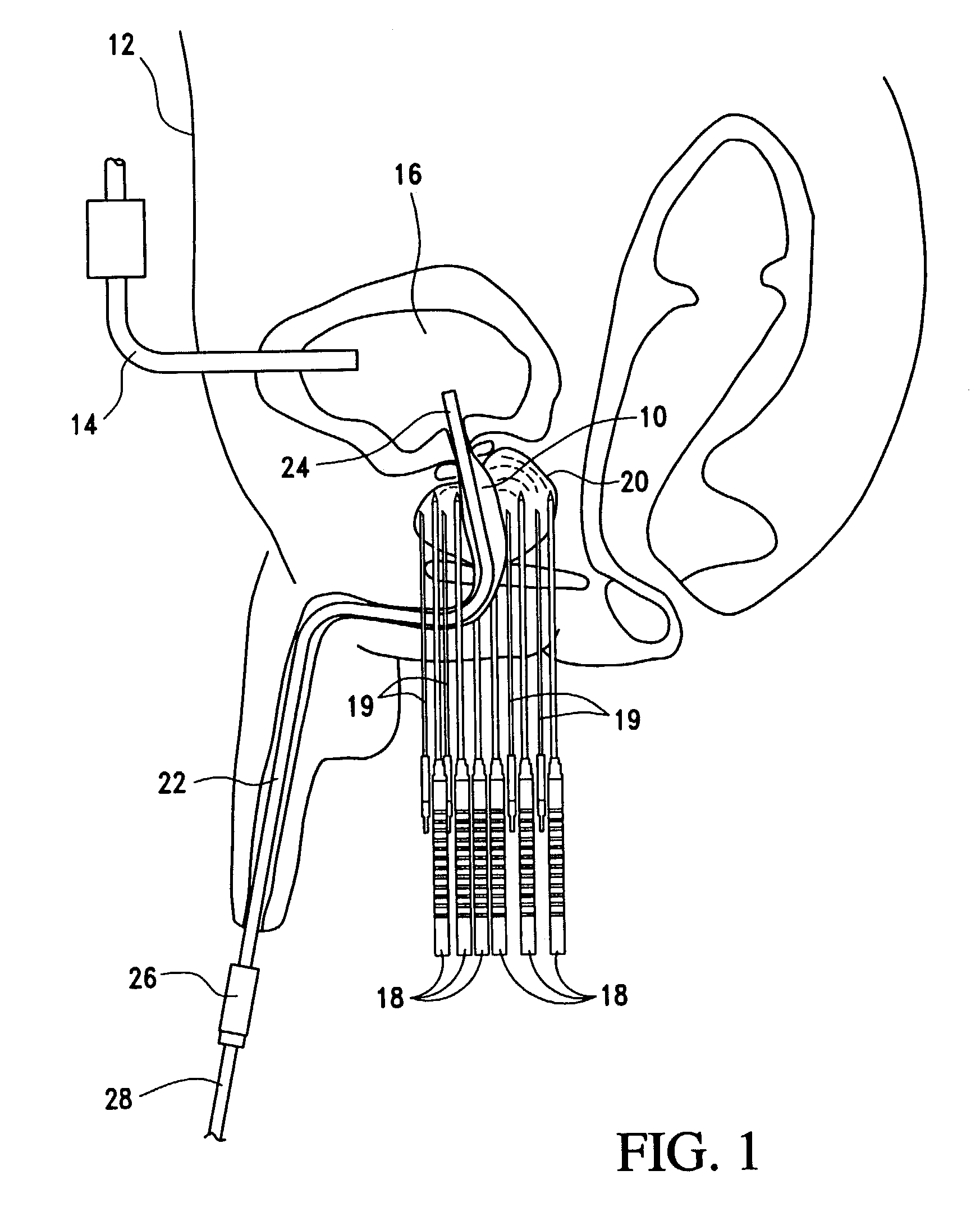
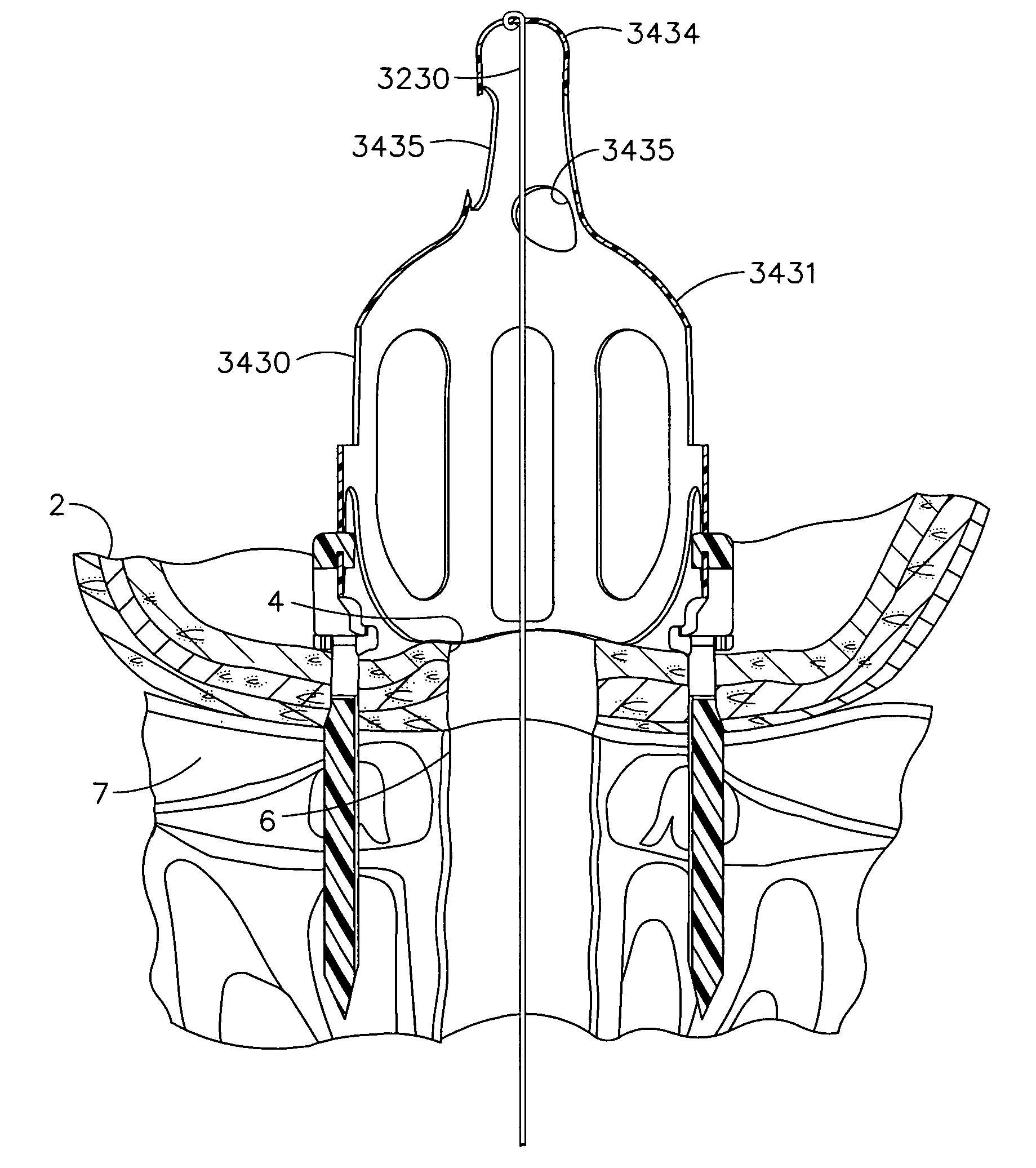
External stimulator for adjunct (add-on) treatment for neurological, neuropsychiatric, and urological disorders
An external stimulator adapted to be inductively coupled with an implanted lead-receiver is designed to deliver neuromodulation therapy for disorders including depression, migraine, partial complex epilepsy, generalized epilepsy, involuntary movement disorders, dementia, obsessive compulsive disorders, urinary incontinence, neurogenic / psychogenic pain and bladder control. The external stimulator containing limited number of predetermined programs packaged into the stimulator, giving the patient or caretaker a way to adjust the therapy within confined limits, or turn the device off. The pre-packaged programs contain unique combination of pulse amplitude, pulse width, frequency of stimulation, and on-off time. The programs are capable of being modified with a programming station connected to the pulse generator with a RS232-C serial connection.
Owner:NEURO & CARDIAC TECH
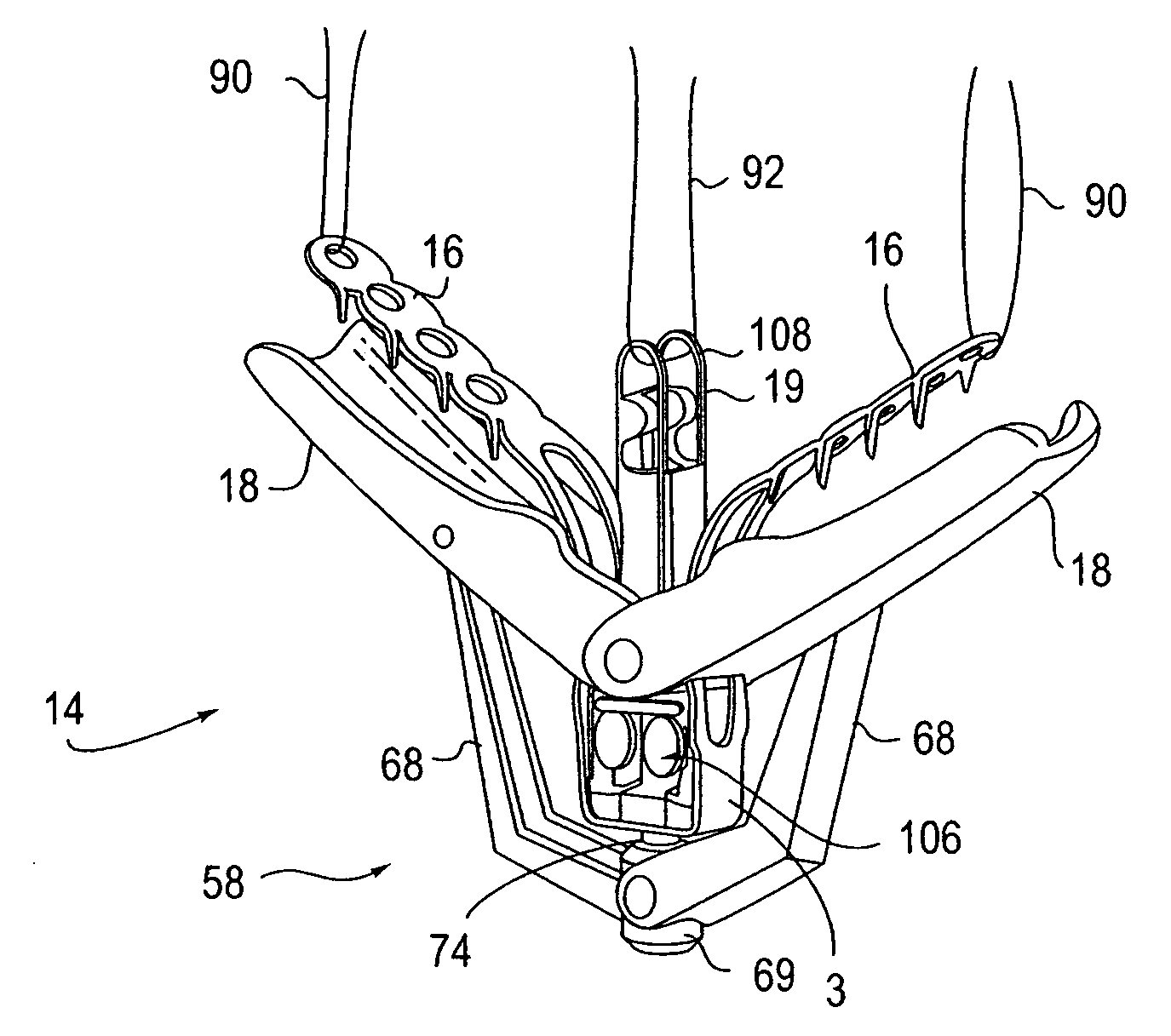
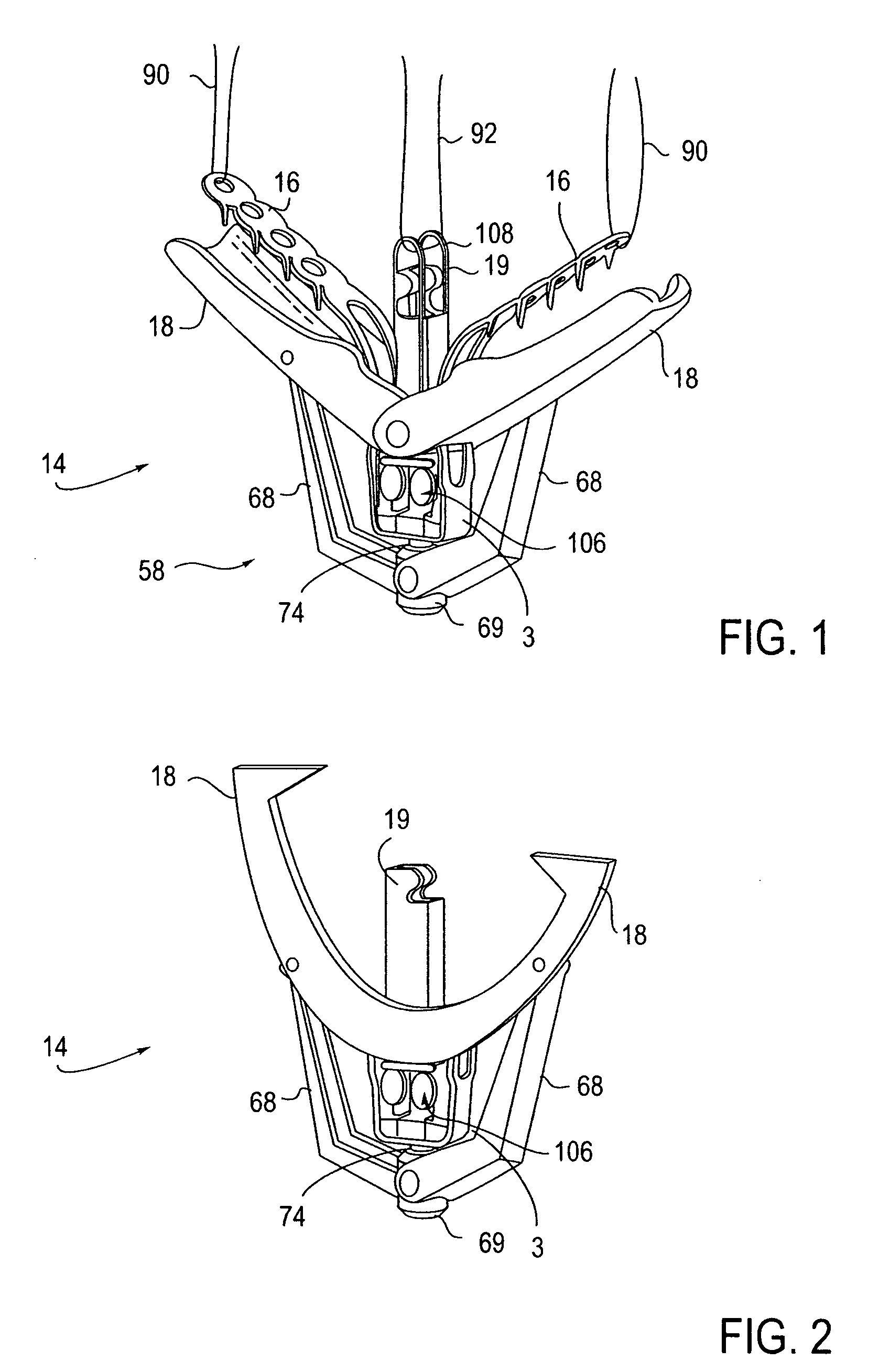
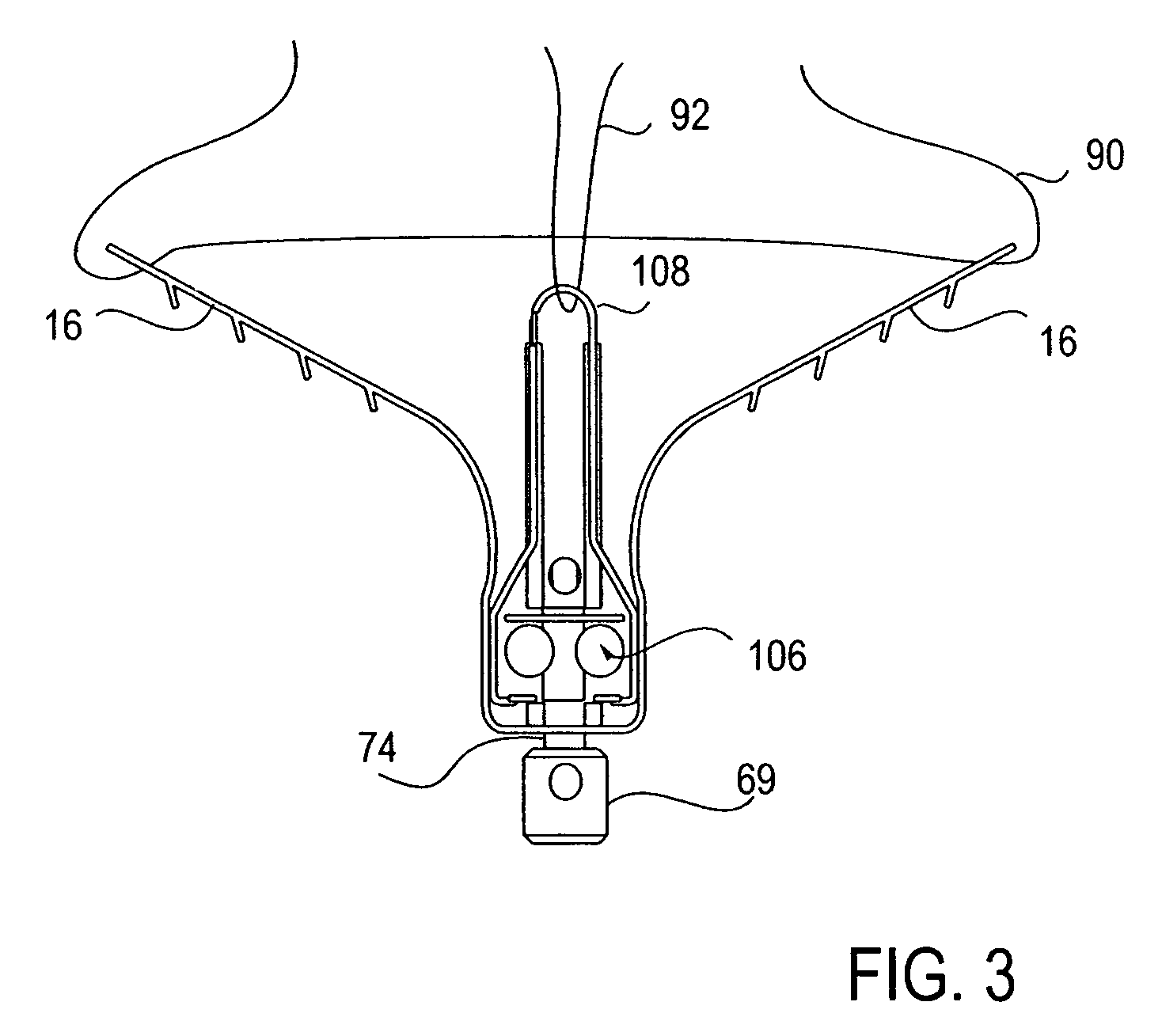
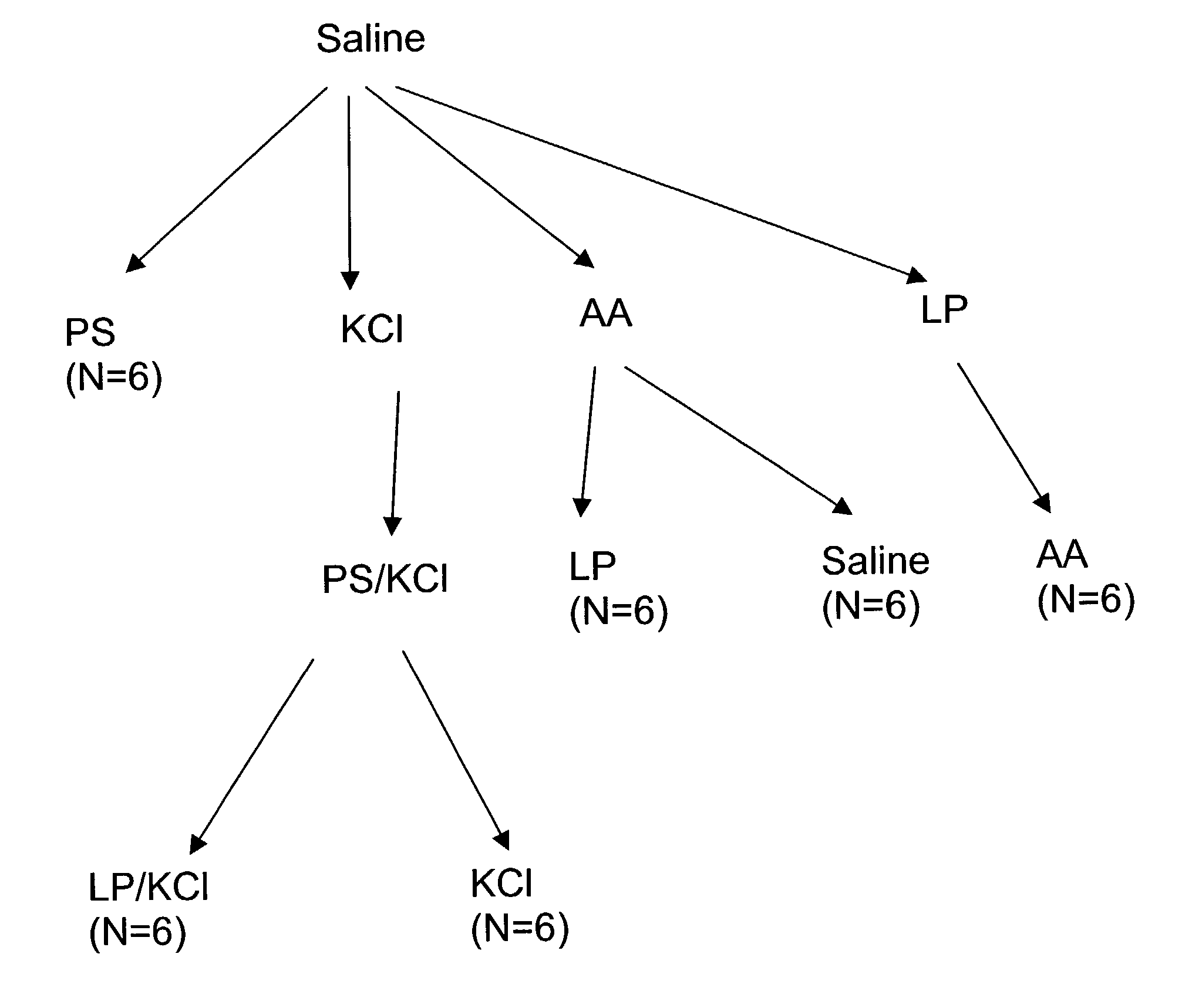
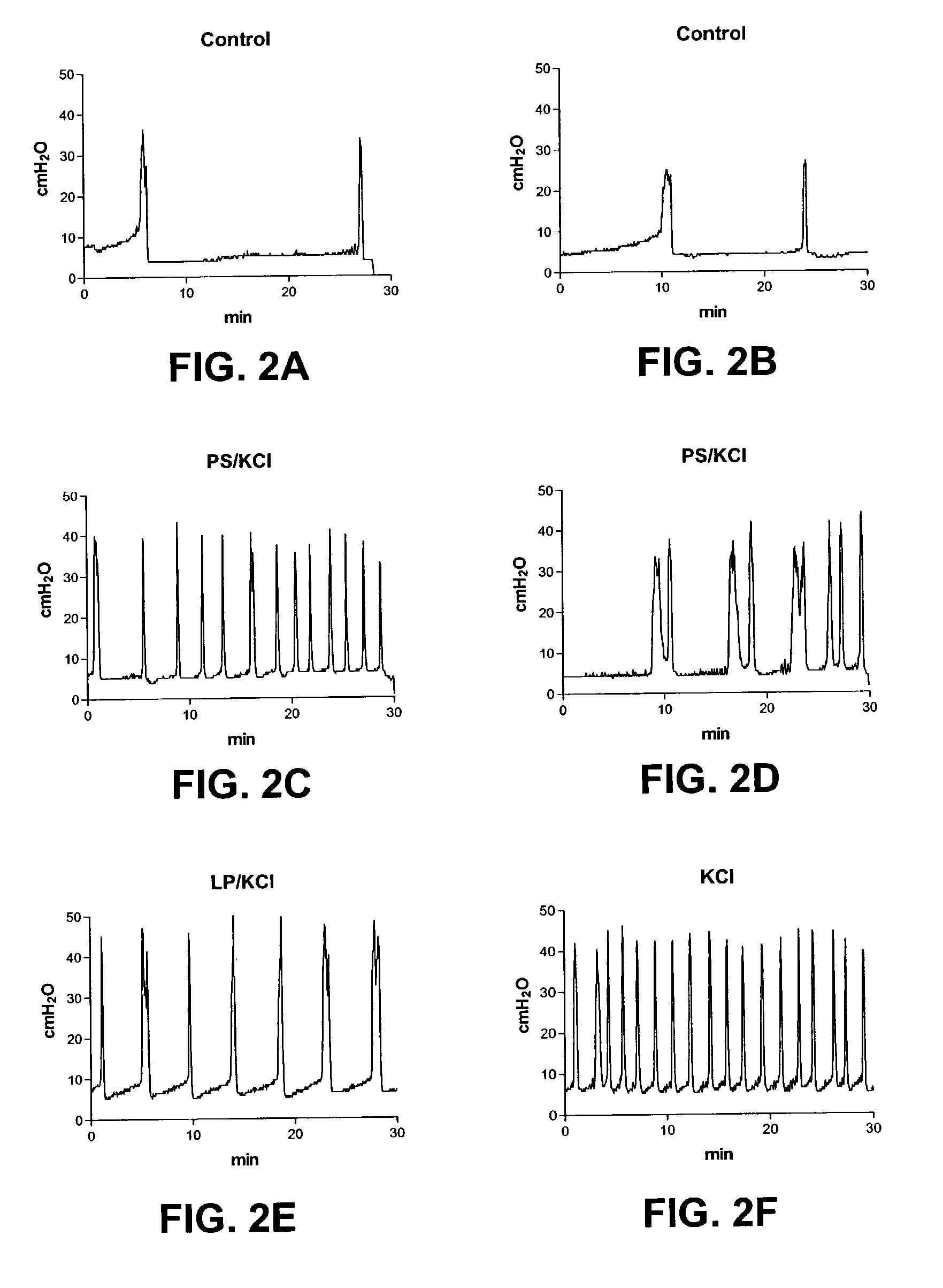
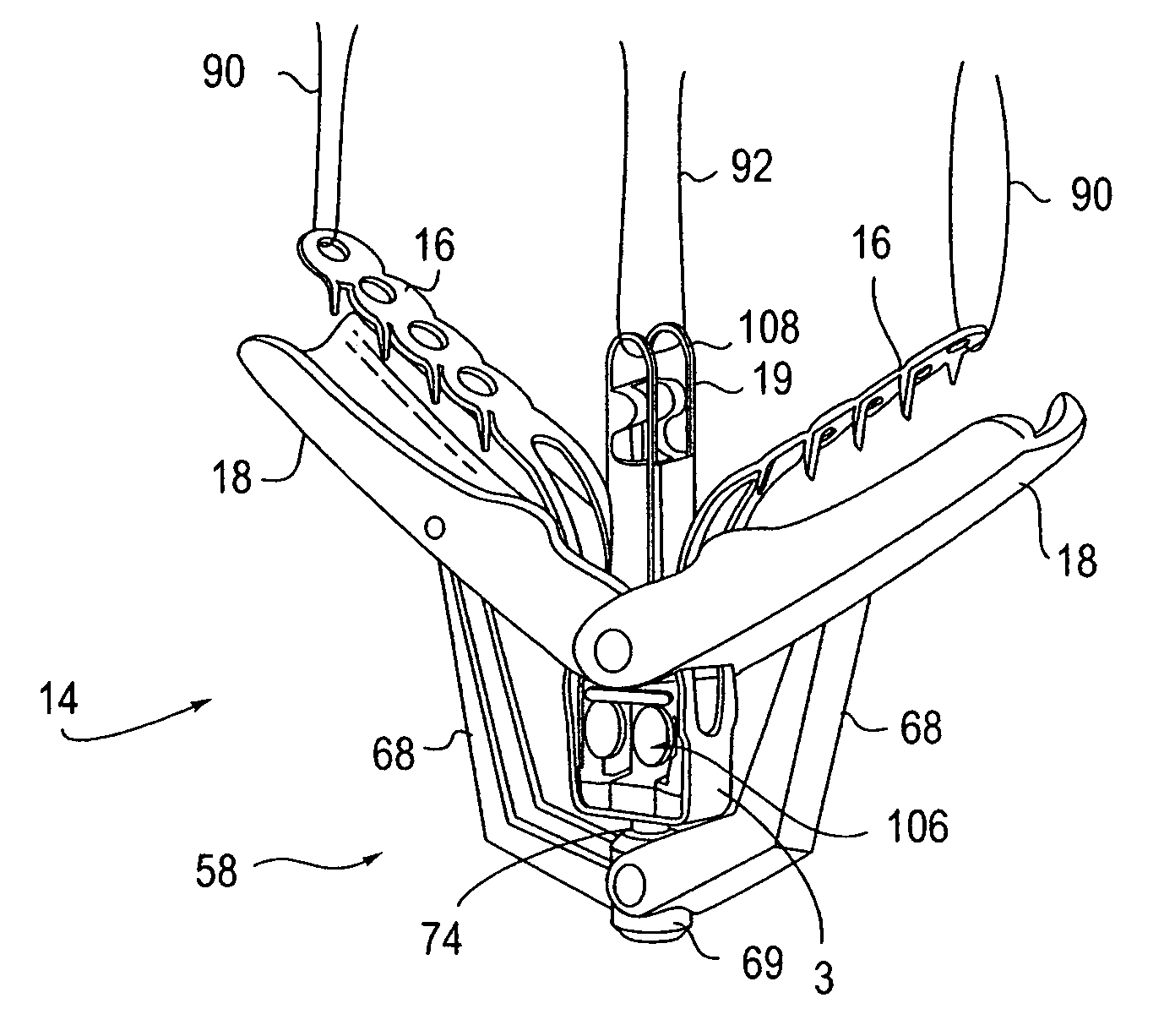
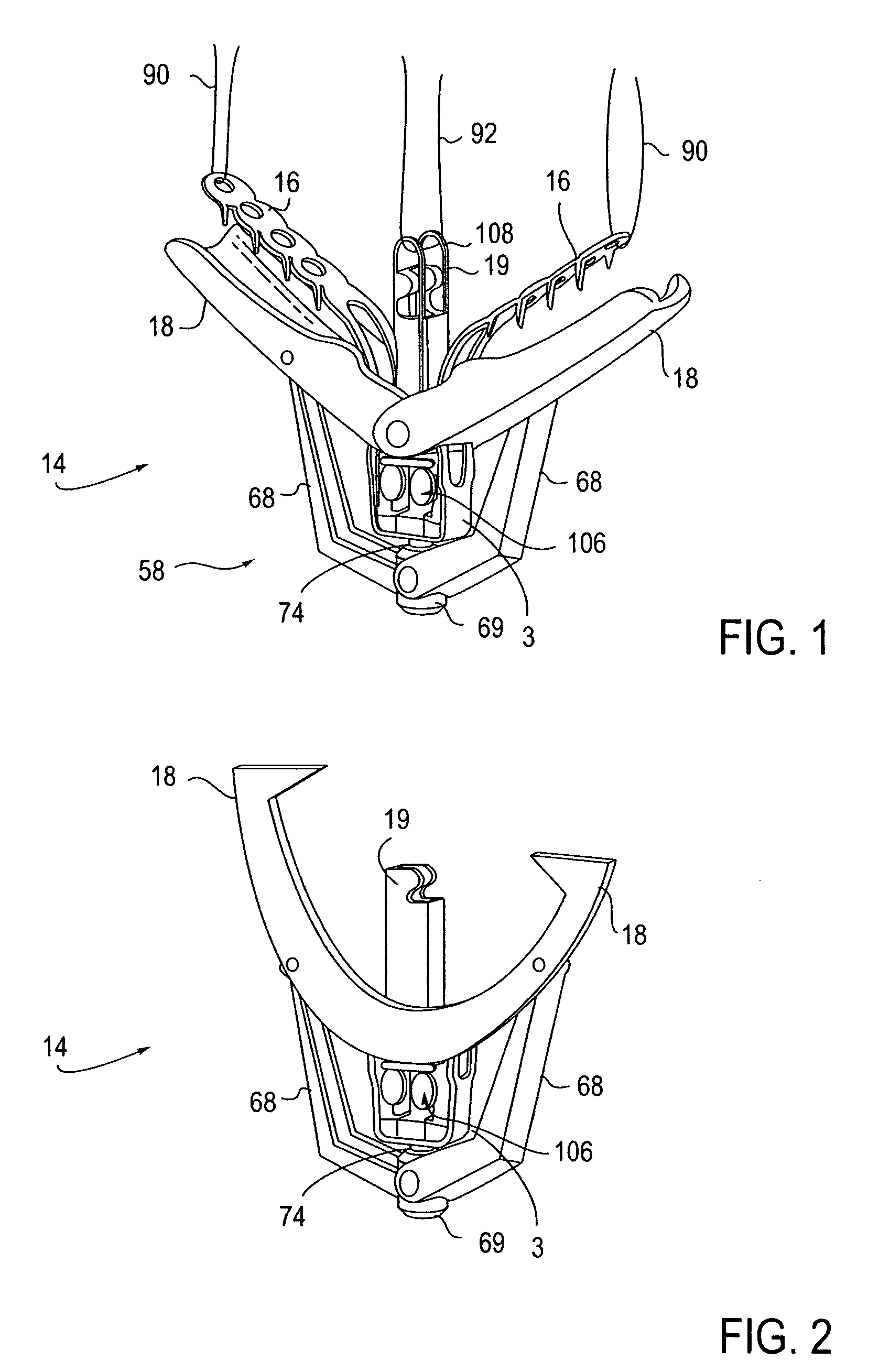
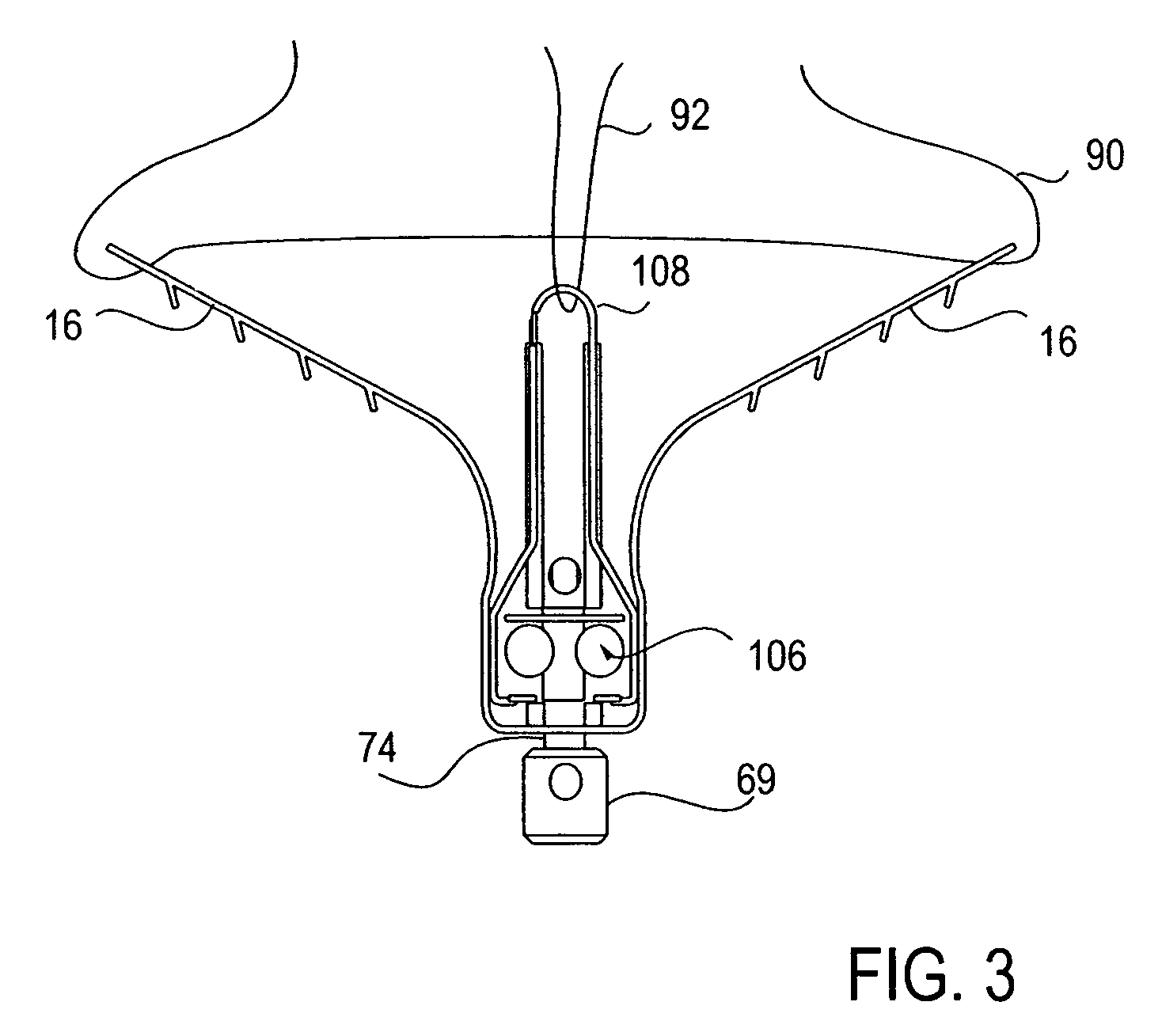
Locking mechanisms for fixation devices and methods of engaging tissue
InactiveUS20060020275A1Reduce frictional contactRestrict movementSuture equipmentsAnnuloplasty ringsEngineeringThoracic cavity
Devices, systems and methods are provided for tissue approximation and repair at treatment sites. In particular, fixation devices are provided comprising a pair of elements each having a first end, a free end opposite the first end, and an engagement surface therebetween for engaging the tissue, the first ends being moveable between an open position wherein the free ends are spaced apart and a closed position wherein the free ends are closer together with the engagement surfaces generally facing each other. The fixation devices also include a locking mechanism coupled to the elements for locking the elements in place. The devices, systems and methods of the invention will find use in a variety of therapeutic procedures, including endovascular, minimally-invasive, and open surgical procedures, and can be used in various anatomical regions, including the abdomen, thorax, cardiovascular system, heart, intestinal tract, stomach, urinary tract, bladder, lung, and other organs, vessels, and tissues. The invention is particularly useful in those procedures requiring minimally-invasive or endovascular access to remote tissue locations, where the instruments utilized must negotiate long, narrow, and tortuous pathways to the treatment site.
Owner:EVALVE
Application of lipid vehicles and use for drug delivery
InactiveUS7063860B2Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticAnticarcinogenCapsaicin
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
Locking mechanisms for fixation devices and methods of engaging tissue
InactiveUS7604646B2Prevent movementReduce frictional contactSuture equipmentsAnnuloplasty ringsEngineeringSurgical department
Owner:EVALVE
Method and apparatus for correction for gynecological pathologies including treatment of female cystocele
Owner:BOSTON SCI SCIMED INC
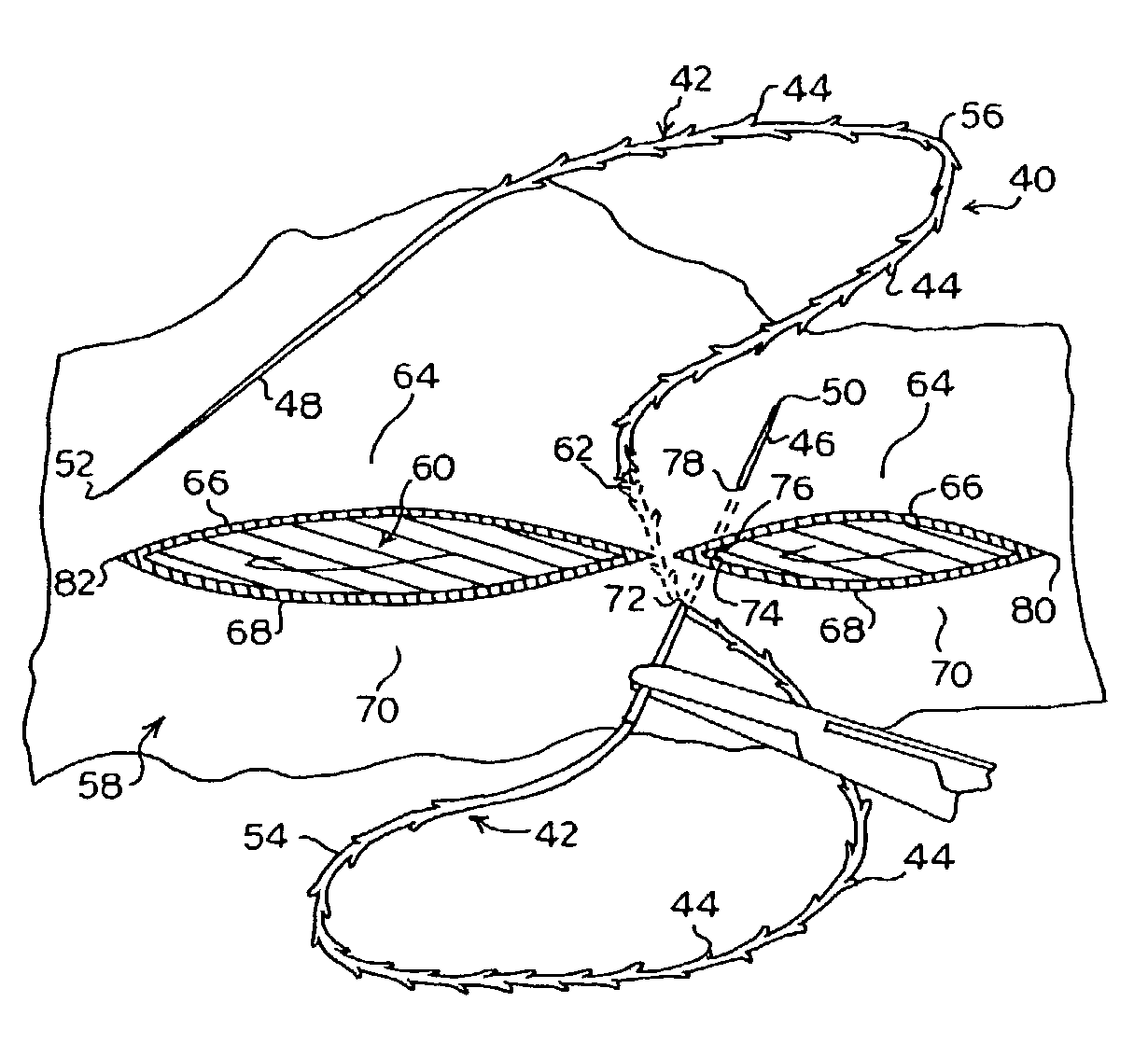
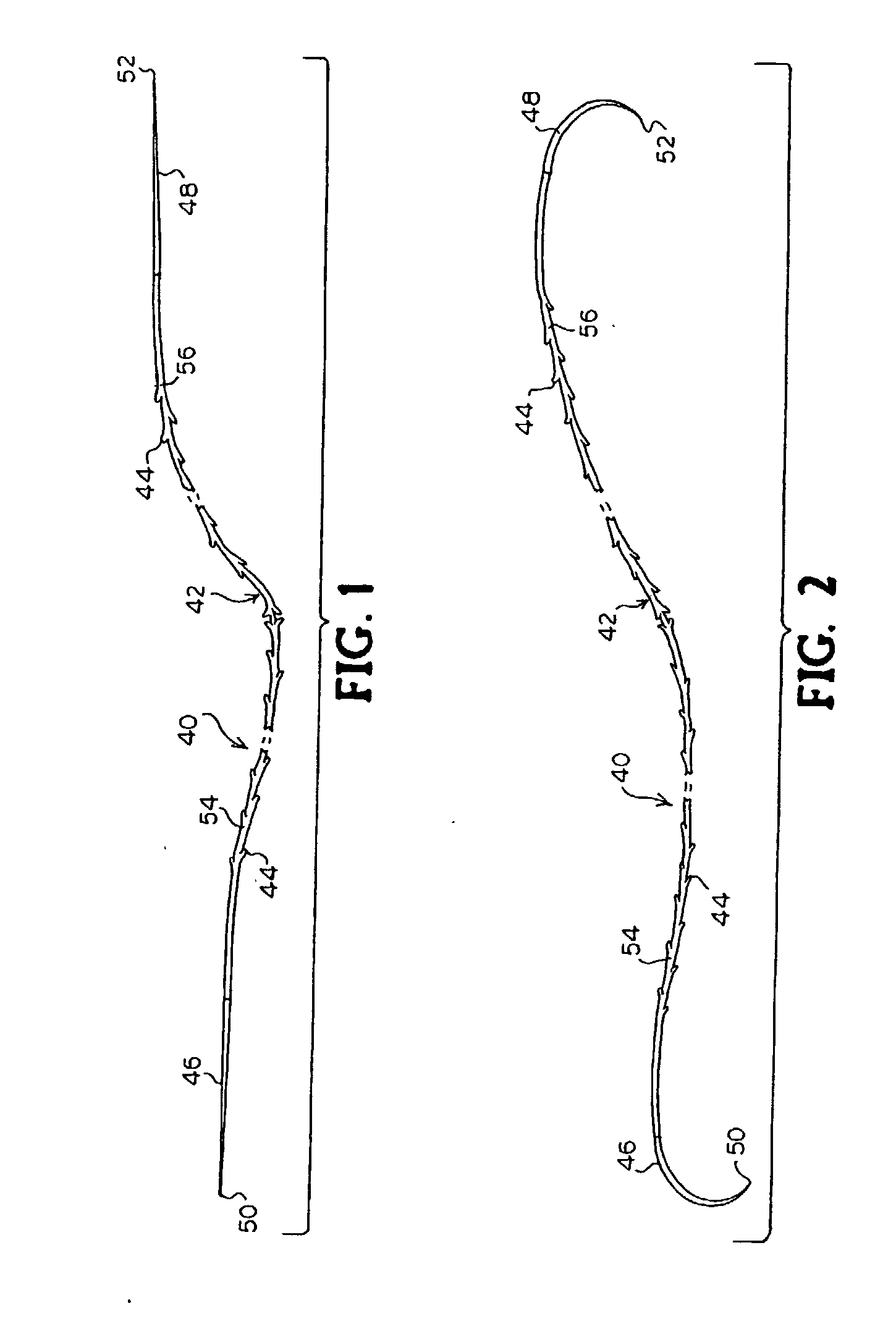
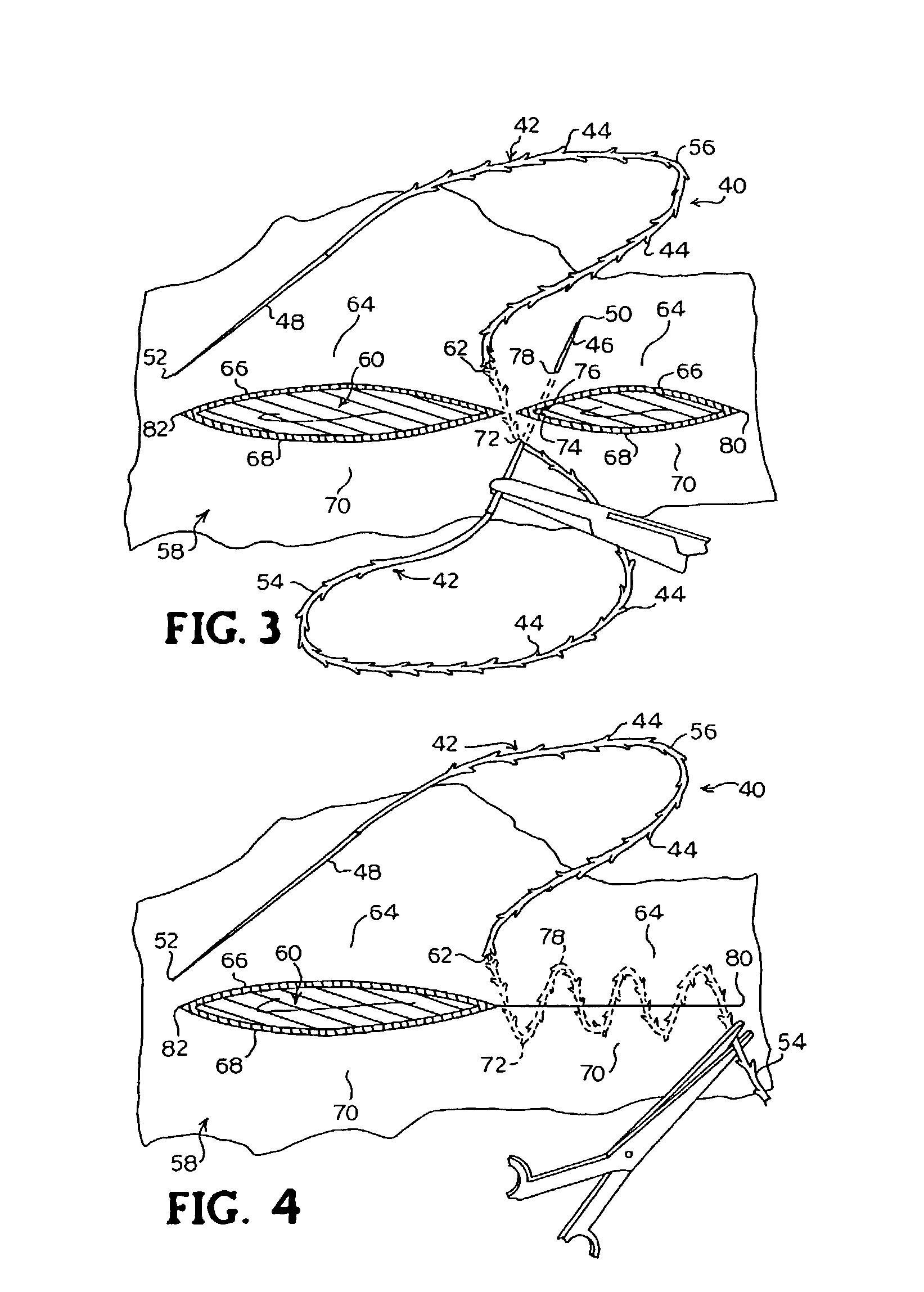
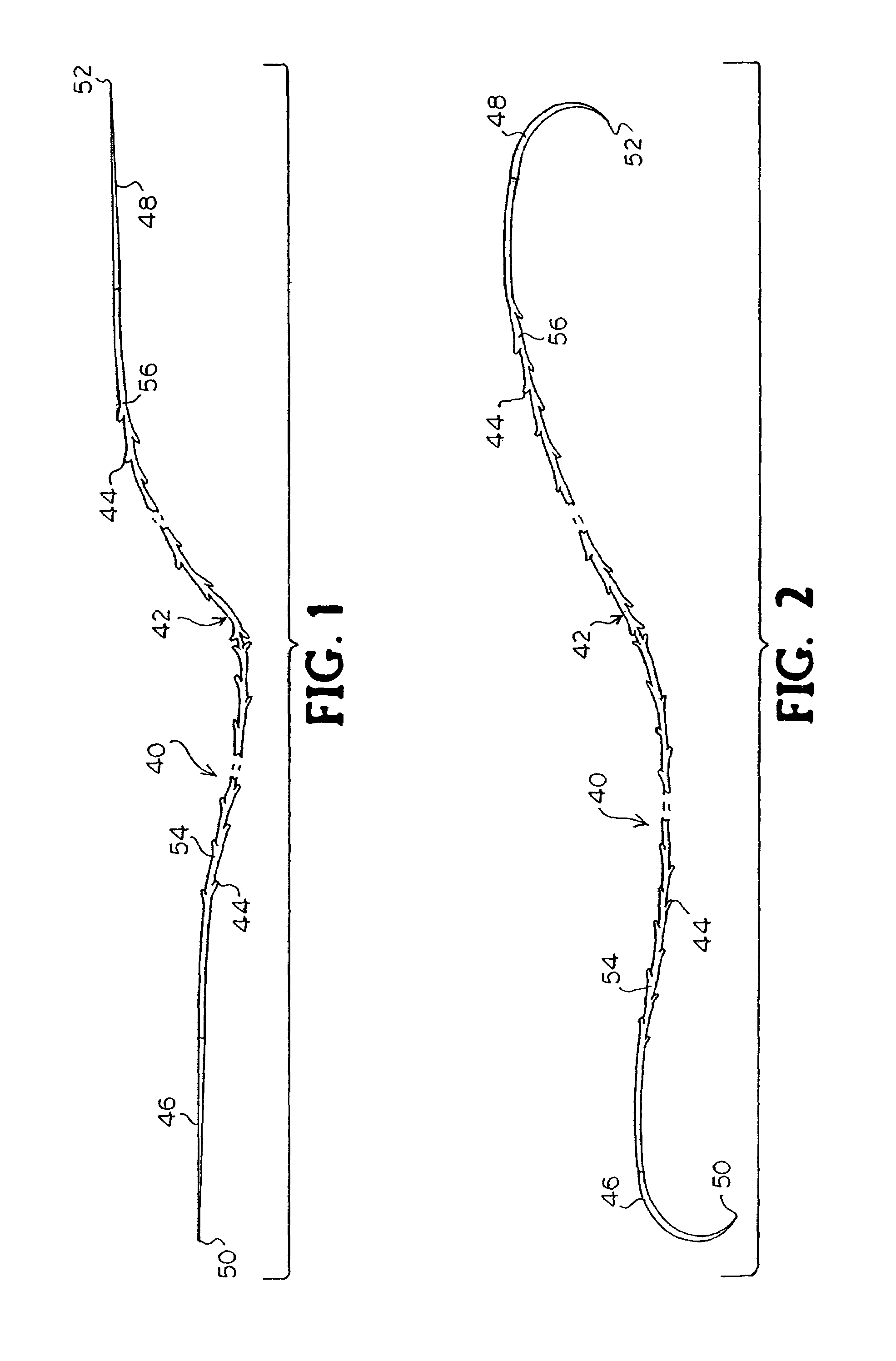
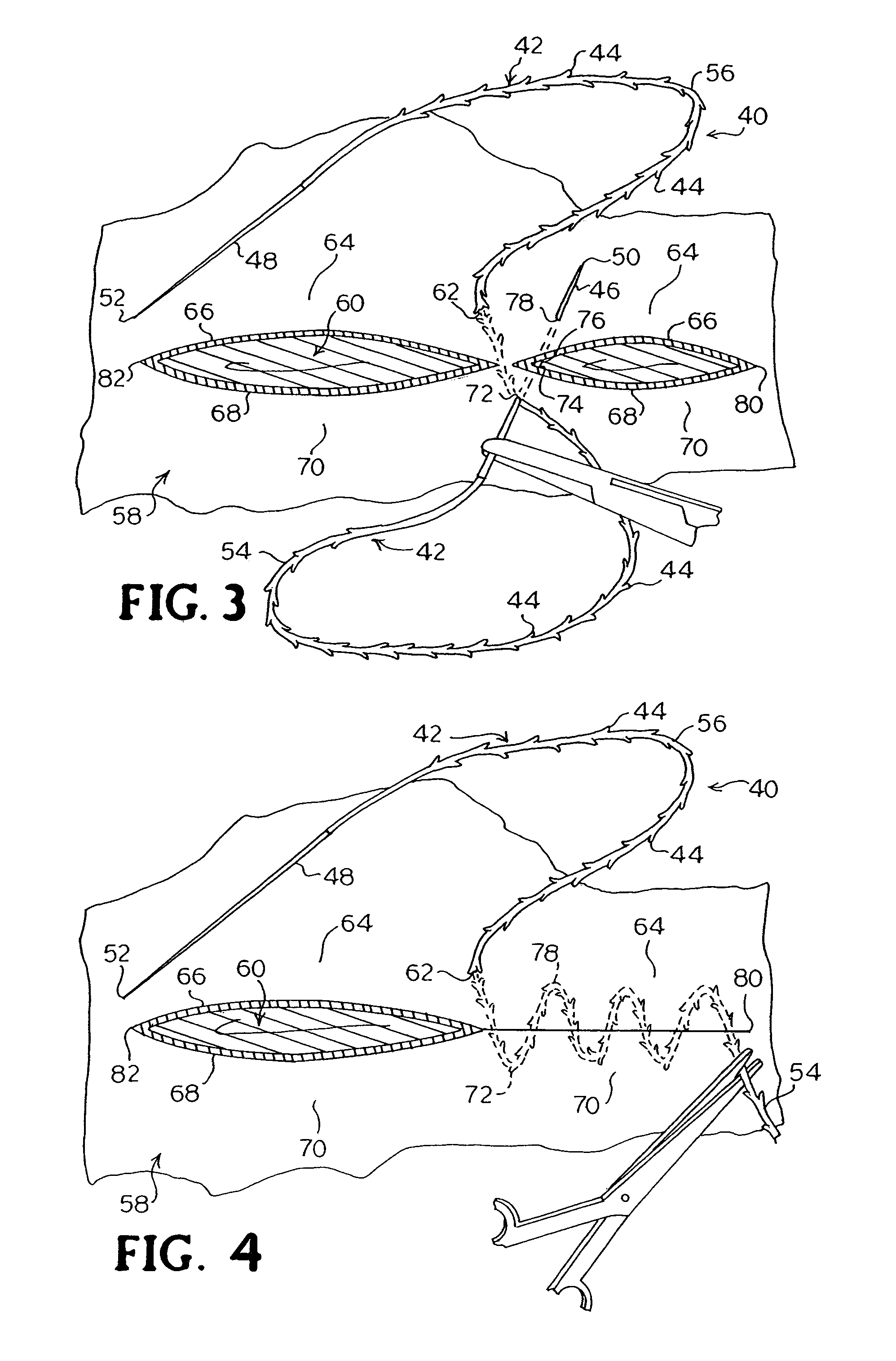
Suture method
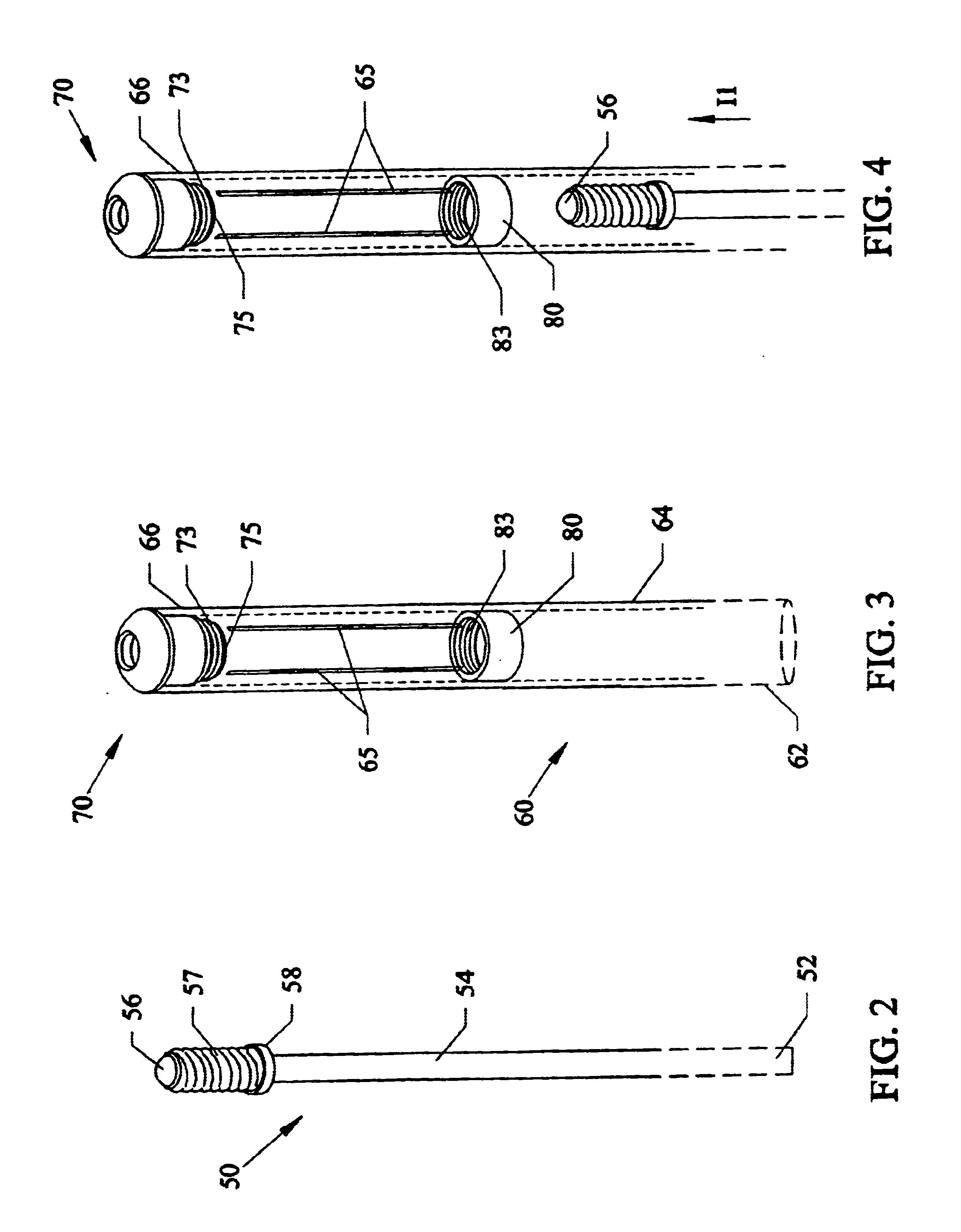
A method for joining and holding closed a wound in bodily tissue, fastening junctions of wounds, tying off wounds, joining a foreign element to tissue, and altering the position of tissue using a barbed suture including sharp pointed ends. Each end of the suture includes barbs on that permit movement in an opposing direction to the barbs on the other end of the suture. This two-way barbed suture is used by the method of the present invention in applications including abdominal surgeries such as a Nissen fundoplication, laparoscopic uses such as stabilizing a bowel structure and performing a closure of a cystostomy, liver to bowel anastomosis, closure of an orifice of a Zenker's Diverticulum, endoscopic uses such as closure of ulcerative lesions or and post-procedural tissue defects, bladder wound closure, valve replacement surgery, device attachment, cosmetic surgery, and blood vessel wound closure.
Owner:ETHICON INC
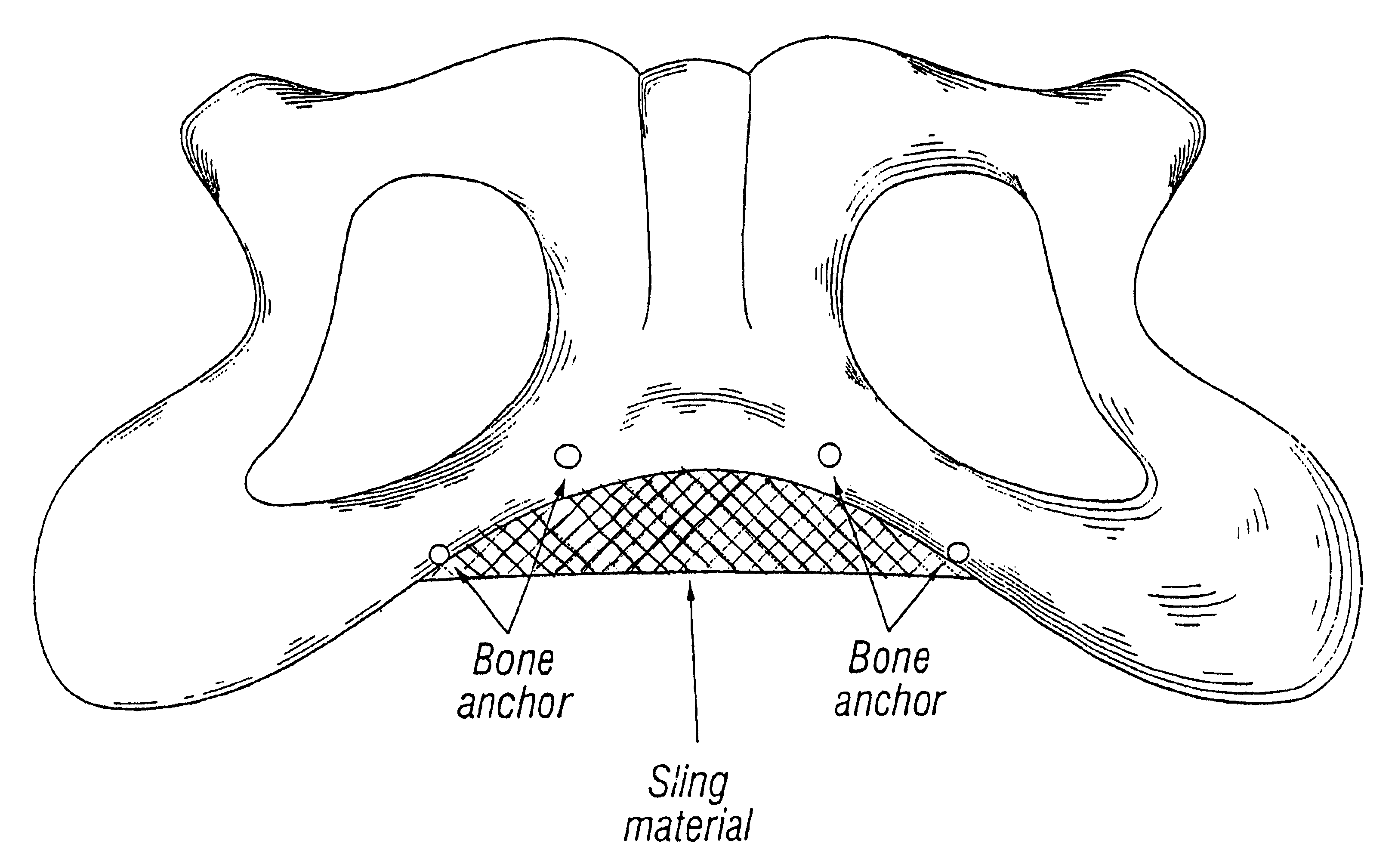
Method and apparatus for cystocele repair
A method for cystocele repair comprising the steps of: establishing four pathways in tissue around a bladder of a patient, introducing a strap into each of said pathways, and positioning beneath said bladder of said patient a support member having each said strap connected thereto such that said bladder of said patient is supported by said support member and a bulge of said bladder into a vagina of said patient is reduced.
Owner:ASTORA WOMENS HEALTH
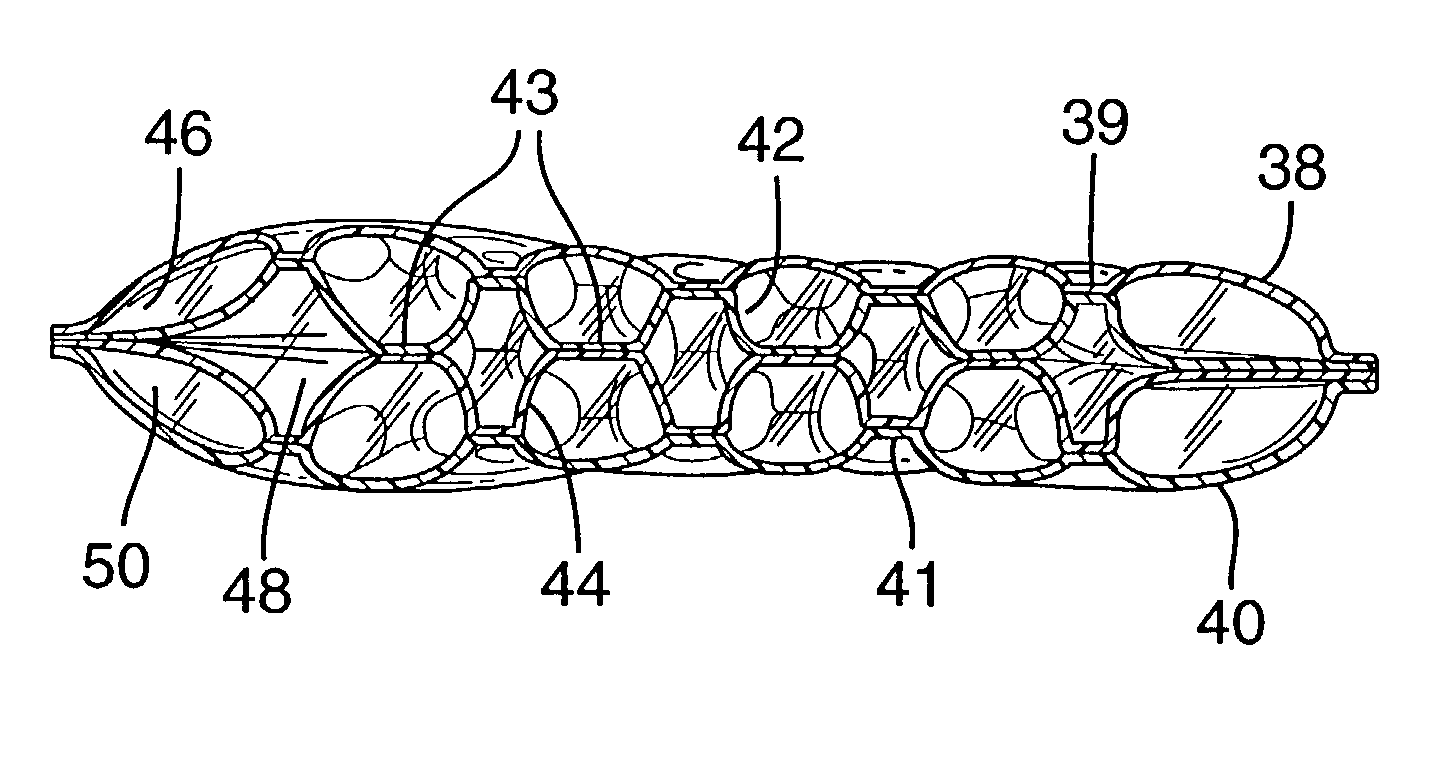
Insertion and retrieval system for inflatable devices
InactiveUS20050131442A1Reduce chanceEliminate chanceAnti-incontinence devicesSurgical needlesUrethraCoupling
A system for deploying and retrieving implantable articles, such as balloons, typically for treating incontinence, from the bladder includes a urethral module, this deployed in the urethra and bladder with the coupling of a mandrel. The deployed urethral module, with the mandrel removed therefrom, provides a channel to the bladder for a magazine unit, that deploys the balloon, and a retriever, that retrieves the balloon, and is ultimately removed from the body with the balloon in a deflated or contracted state.
Owner:INNOVENTIONS INC
Suture method
A method for joining and holding closed a wound in bodily tissue, fastening junctions of wounds, tying off wounds, joining a foreign element to tissue, and altering the position of tissue using a barbed suture including sharp pointed ends. Each end of the suture includes barbs on that permit movement in an opposing direction to the barbs on the other end of the suture. This two-way barbed suture is used by the method of the present invention in applications including abdominal surgeries such as a Nissen fundoplication, laparoscopic uses such as stabilizing a bowel structure and performing a closure of a cystostomy, liver to bowel anastomosis, closure of an orifice of a Zenker's Diverticulum, endoscopic uses such as closure of ulcerative lesions or and post-procedural tissue defects, bladder wound closure, valve replacement surgery, device attachment, cosmetic surgery, and blood vessel wound closure.
Owner:ETHICON INC
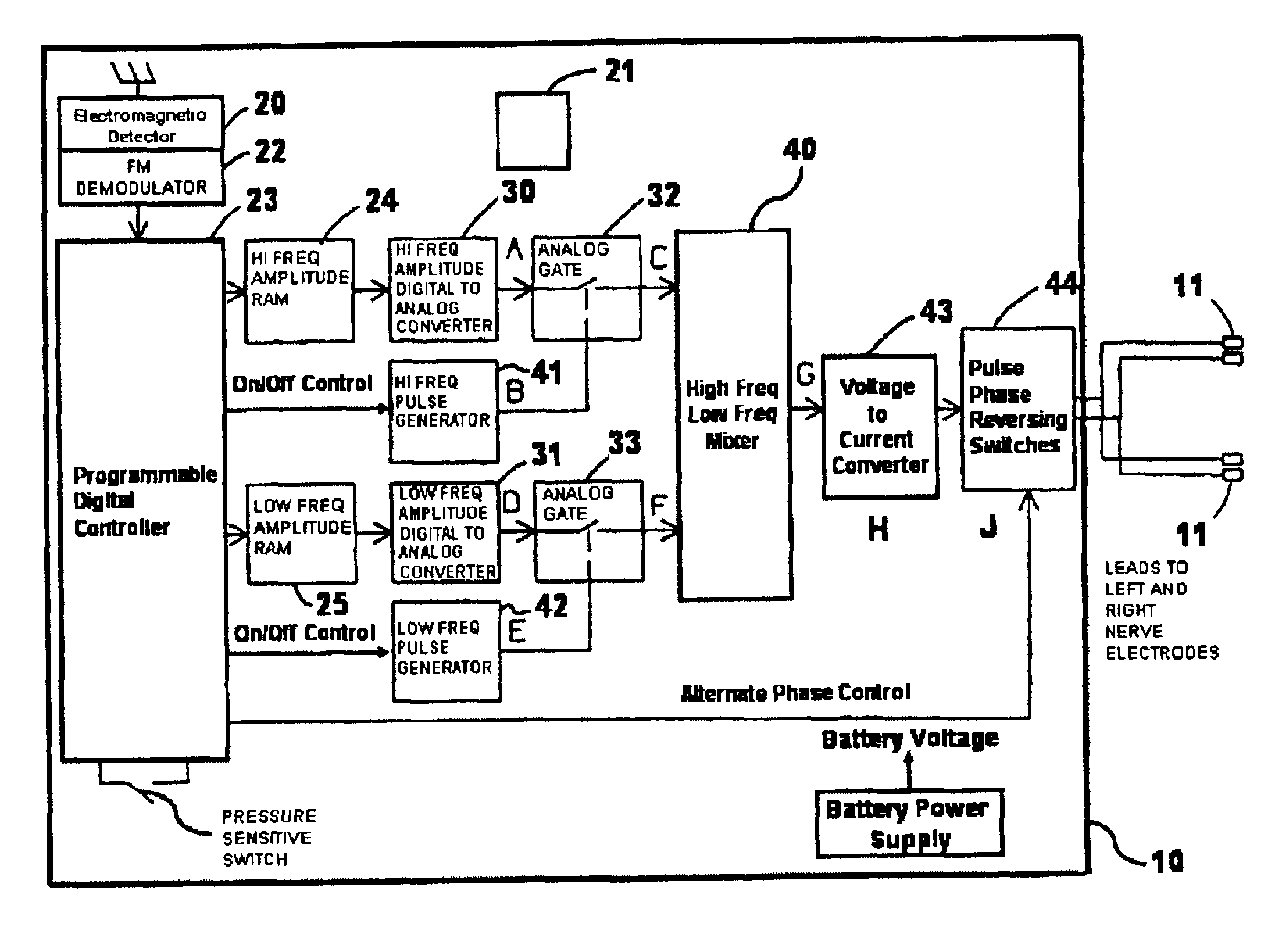
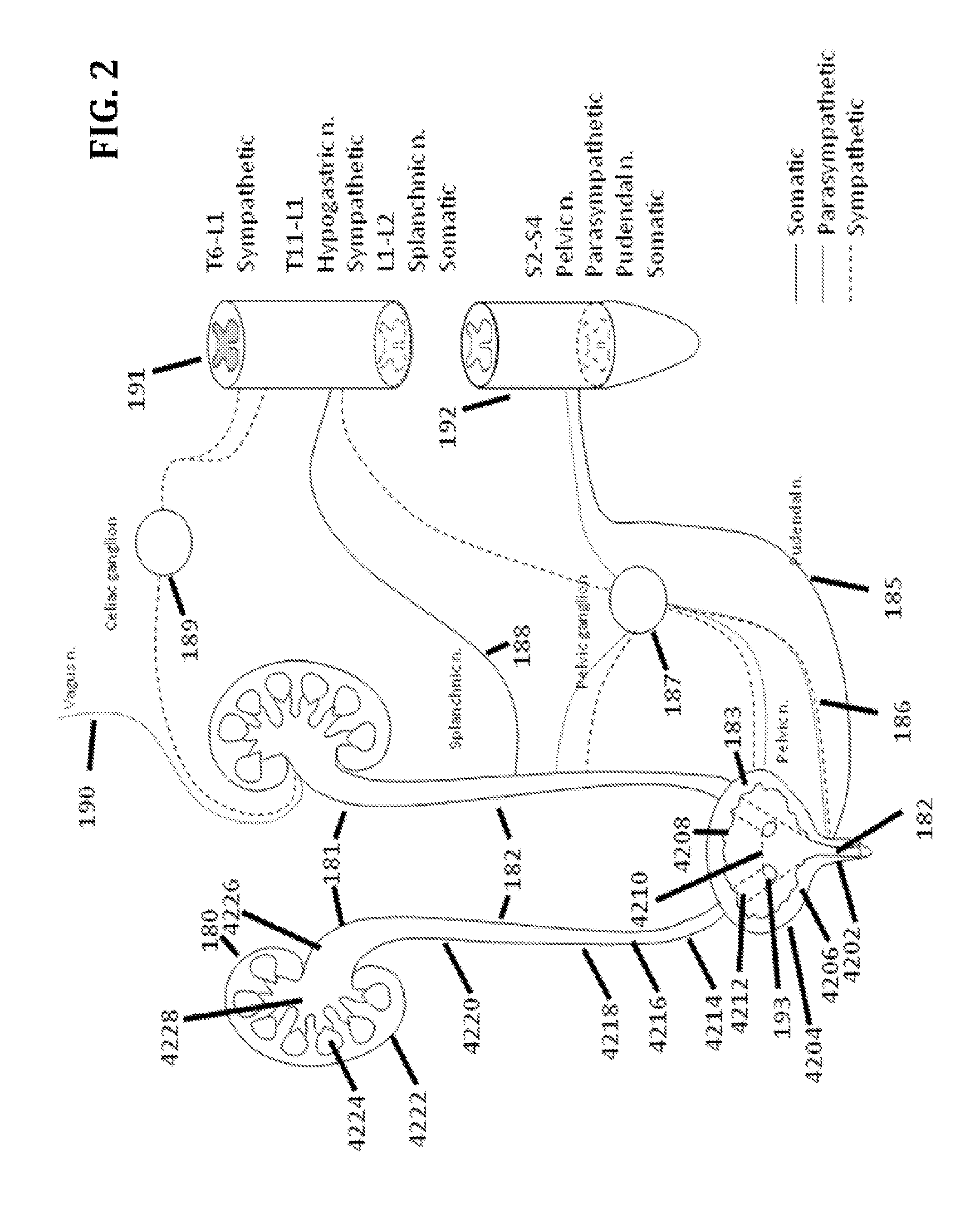
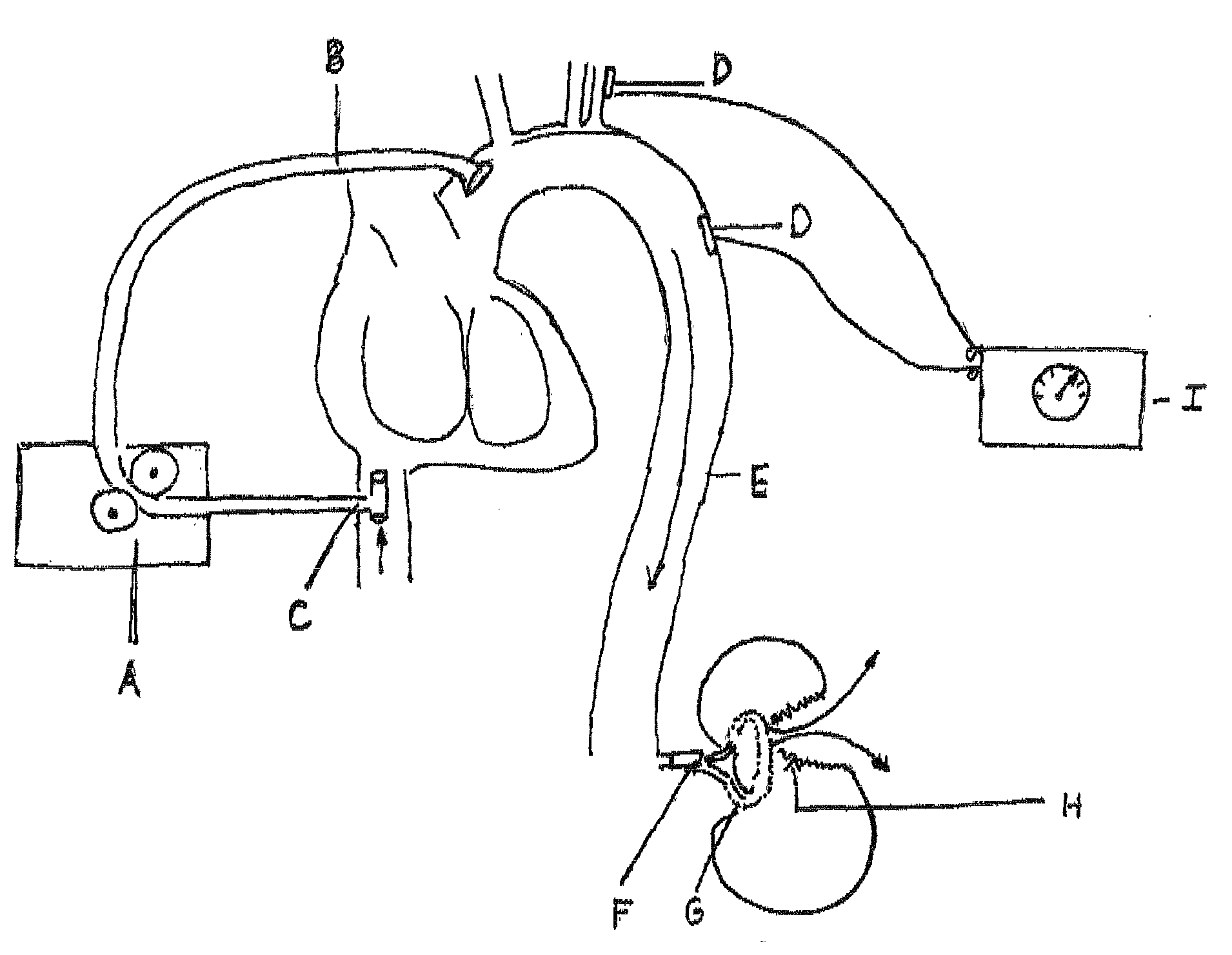
Methods and systems for selective control of bladder function
InactiveUS6990376B2Inhibiting neural transmissionStimulating neural transmissionElectrotherapyArtificial respirationNerve fibreHigh amplitude
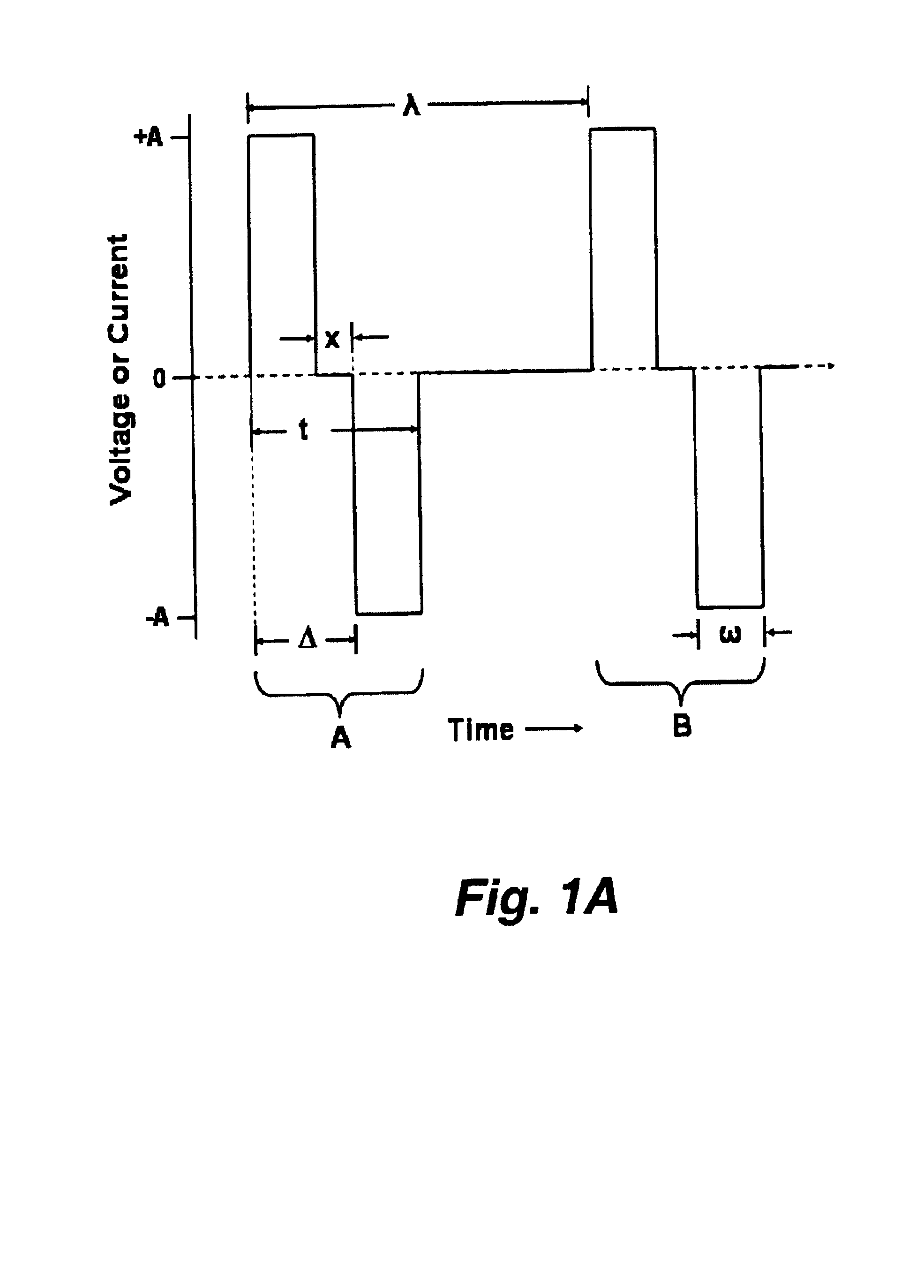
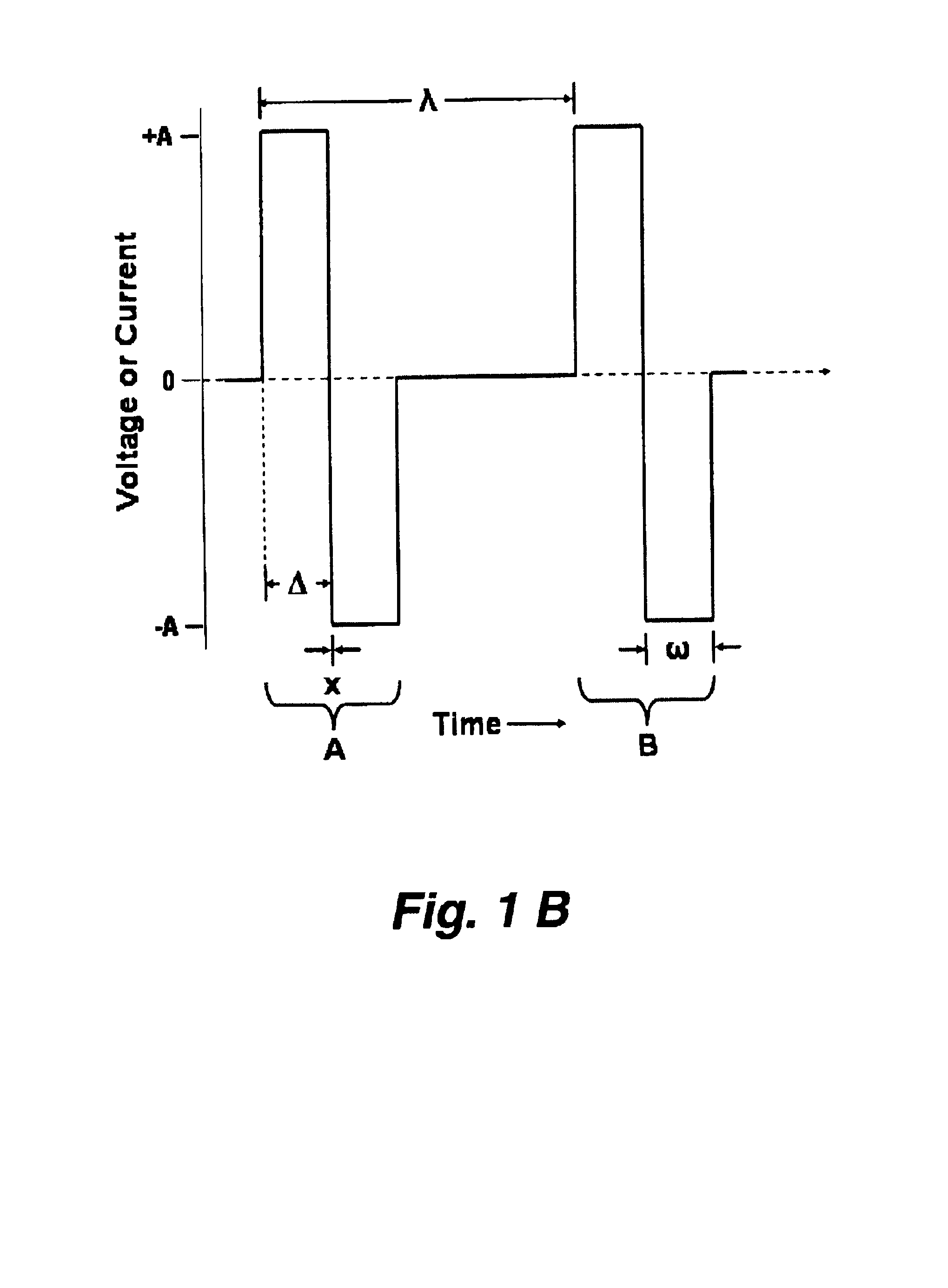
A method and system for selective inhibition of somatic nerve fibers in a mixed nerve containing both somatic and autonomic nerve fibers where the method finds use in treatment of chronic pain, spastic muscles and for sensory and motor control of a bladder. The methods and systems utilize alternate phase rectangular electrical pulses. An electrical pulse generator is coupled to a nerve. An alternate phase high frequency, low amplitude pulse is first applied to selectively inhibit somatic nerves when present in a mixed nerve. An alternate phase low frequency, high amplitude phase pulse subsequently supplied to stimulate the autonomic nerve fibers and in the case of the sacral root will permit a controlled voiding of the bladder and bowel.
Owner:RGT UNIV OF CALIFORNIA
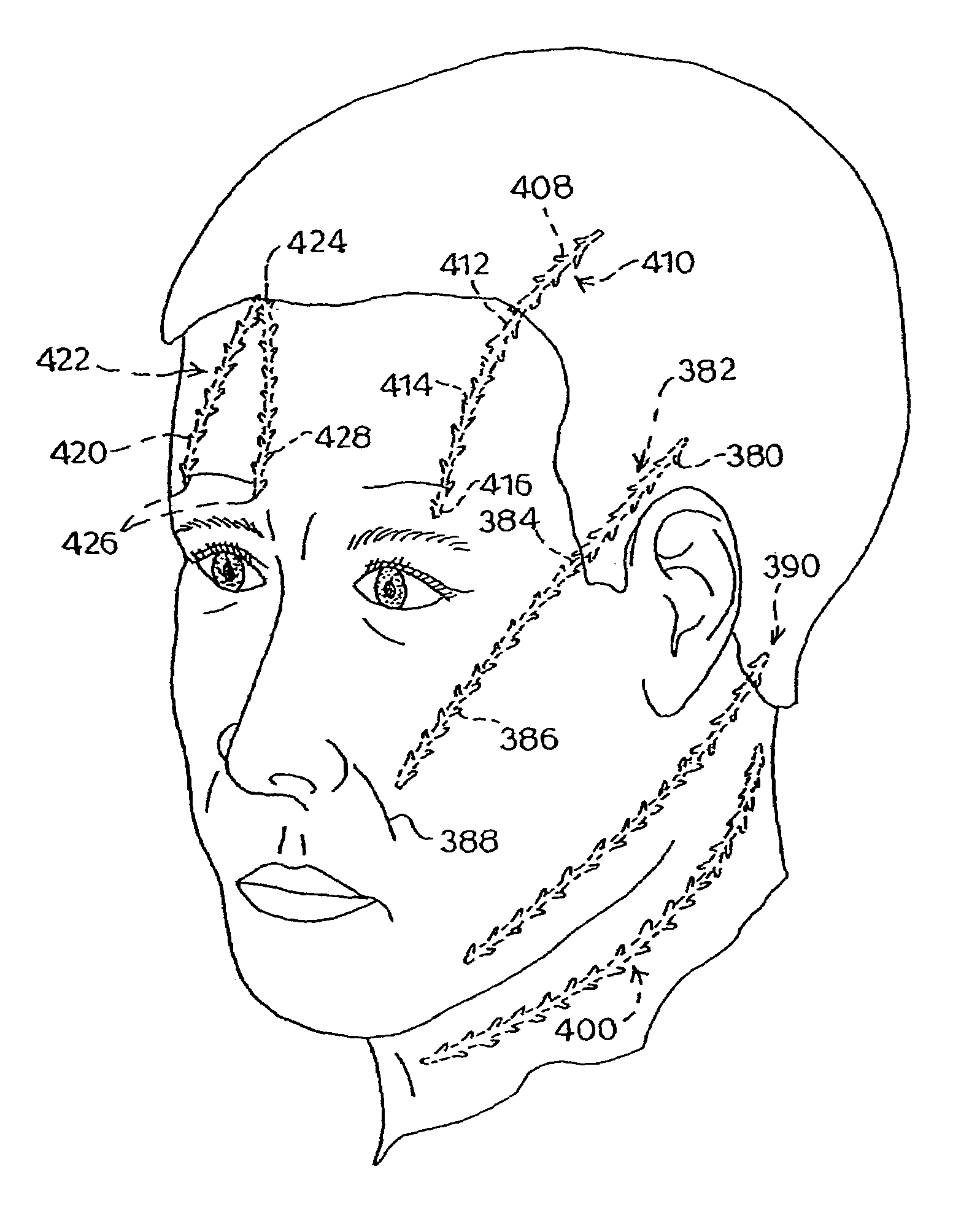
Non-invasive electrical and magnetic nerve stimulators used to treat overactive bladder and urinary incontinence
Transcutaneous electrical and magnetic nerve stimulation devices are disclosed, along with methods of treating lower urinary tract disorders using energy that is delivered noninvasively by the devices. The disorders comprise overactive bladder, urge incontinence, stress, incontinence, urge frequency, non-obstructive urinary retention and interstitial cystitis / painful bladder syndrome. In preferred embodiments of the disclosed methods, a posterior tibial nerve of a patient is stimulated non-invasively. Methods are disclosed for selecting protocol parameters for a nerve stimulation session for treating each individual patient, wherein modules of the bladder of the patient are represented as coupled non-linear oscillators.
Owner:ELECTROCORE
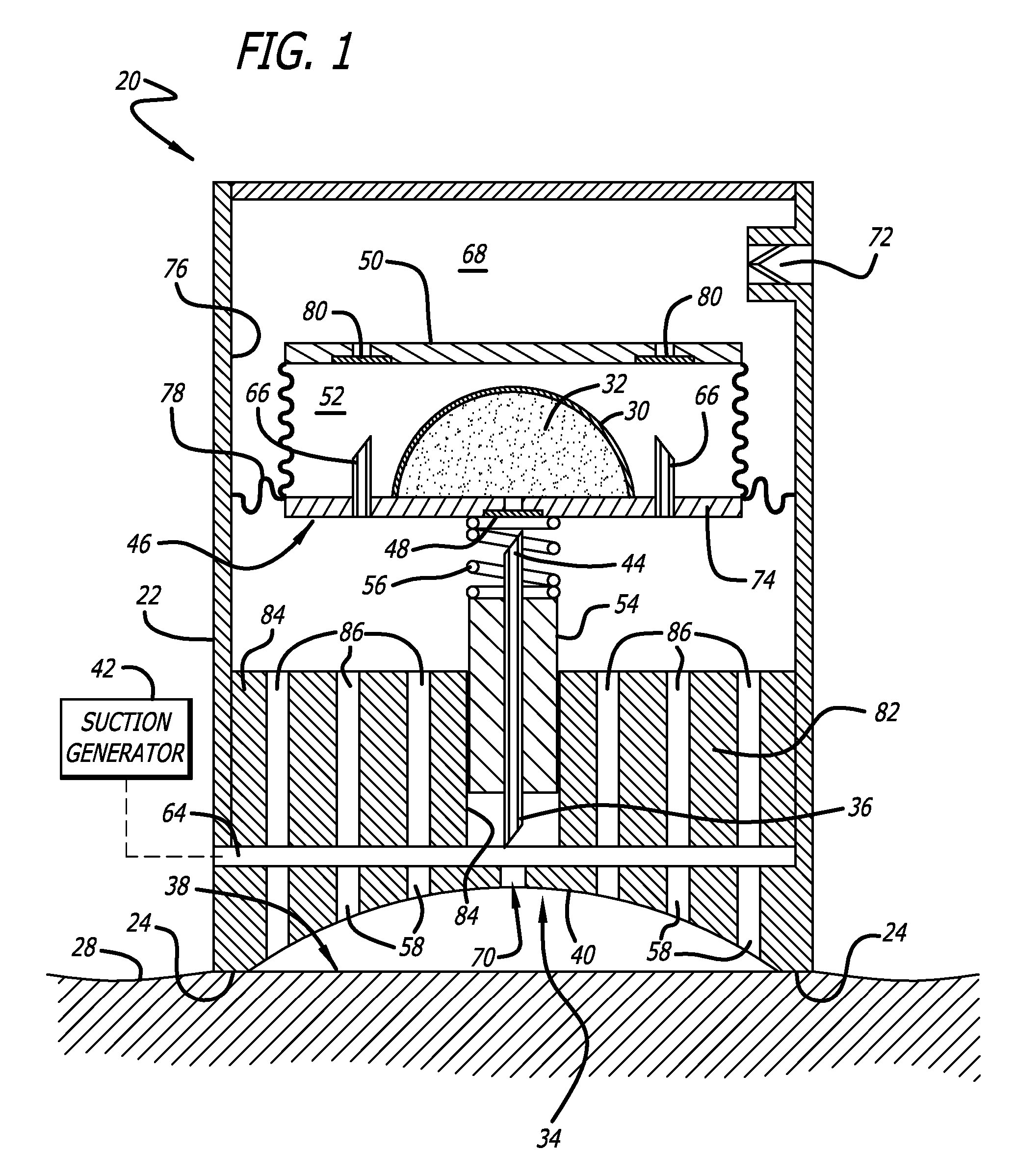
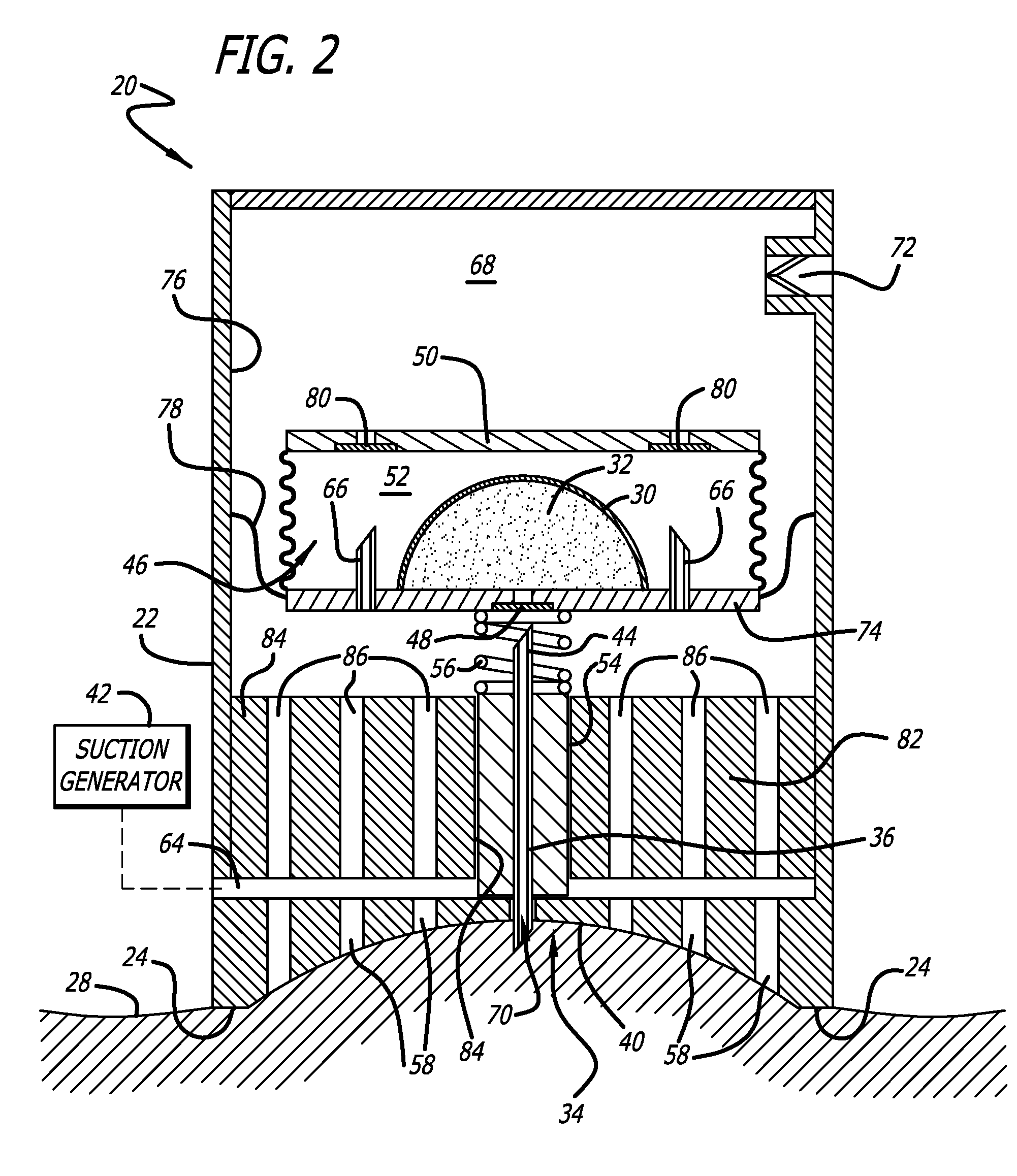
Implantable Drug Delivery Device and Methods for Treatment of the Bladder and Other Body Vesicles or Lumens
ActiveUS20090149833A1High plasma concentrationMinimize irritationBiocideMedical devicesDrug reservoirControlled drugs
An implantable medical device is provided for controlled drug delivery within the bladder, or other body vesicle. The device may include at least one drug reservoir component comprising a drug; and a vesicle retention frame which comprises an elastic wire having a first end, an opposing second end, and an intermediate region therebetween, wherein the drug reservoir component is attached to the intermediate region of the vesicle retention frame. The retention frame prevents accidental voiding of the device from the bladder, and it preferably has a spring constant selected for the device to effectively stay in the bladder during urination while minimizing the irritation of the bladder.
Owner:MASSACHUSETTS INST OF TECH
Method and apparatus for cystocele repair
ActiveUS20050250977A1Reduce bumpsSuture equipmentsAnti-incontinence devicesCystocele repairEngineering
A method for cystocele repair comprising the steps of: establishing four pathways in tissue around a bladder of a patient, introducing a strap into each of said pathways, and positioning beneath said bladder of said patient a support member having each said strap connected thereto such that said bladder of said patient is supported by said support member and a bulge of said bladder into a vagina of said patient is reduced.
Owner:ASTORA WOMENS HEALTH
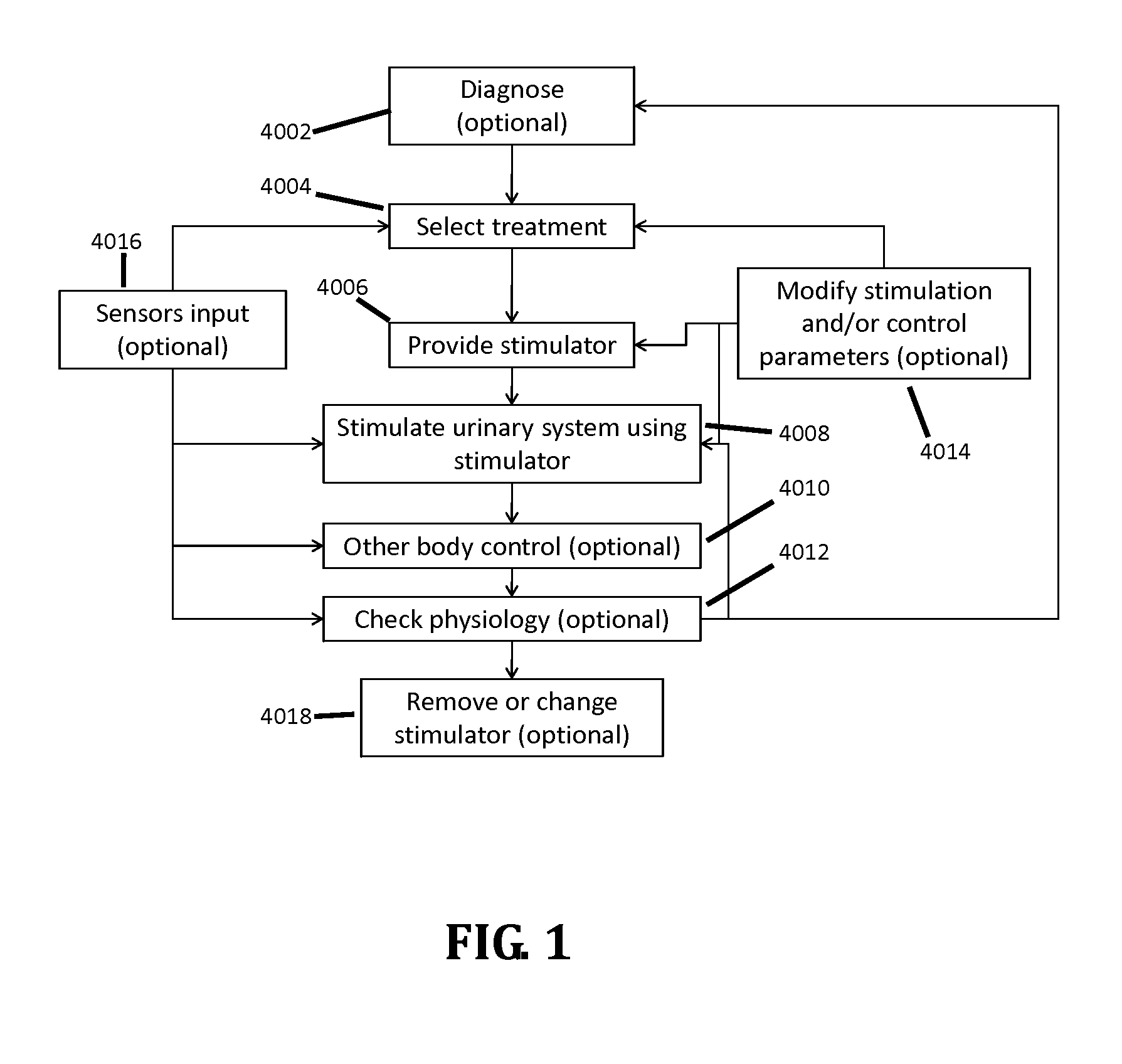
Stimulation of the urinary system
InactiveUS20110301662A1Prevent rotationNot interfere with mobilitySpinal electrodesDigestive electrodesUrethraShort urethra
Apparatus and methods are provided, including a bladder stimulator that includes an elongate element adapted to pass through a urethra or adapted to pass through another opening in the bladder, an expandable body coupled to said elongate element, and an array of one or more stimulator contacts coupled to the expandable body, the array including at least one contact adapted to contact a portion of a bladder of a subject when the expandable body is inserted in the bladder and expanded. A controller stimulates the portion of the bladder by driving a pulse into the bladder via the contact, the pulse having a frequency of 5 Hz-1 kHz. Other applications are also described.
Owner:NEPHERA LTD
Conformable balloonless catheter
InactiveUS6855126B2Relieve fluid pressure buildupNormal body functionEngine diaphragmsSurgeryBladder drainageUrethra
Indwelling catheter having an upper distal end having a portion that can expand within a bladder type spaces without having to be inflated. An embodiment allows for at least one slit on an upper side of the catheter tube and a head member that when pulled down by a stylette moving inside the catheter causes a bulge wing portion(s) that holds the catheter safely and painlessly within the bladder. Magnetic and electret valves can be included inside the tube of the catheter that can cycle between open and closed positions when activated by normal bladder pressure when urination is desired. The novel catheter tube can naturally conform to an opening and closing urethra during natural bladder drainage. The catheter tube surface can include an anti-microbial layer that is either or both coated and impregnated thereon with either an antibacterial and / or hydrophyllic materials. Sampling ports can be located on both inside catheter tube valves and on an externally attached magnetic valve.
Owner:FLINCHBAUGH DAVID E DR
Surgical adhesive and uses therefore
InactiveUS20050129733A1Controlled strengthLess erosiveSurgical adhesivesPharmaceutical delivery mechanismIn situ polymerizationEnteroceles
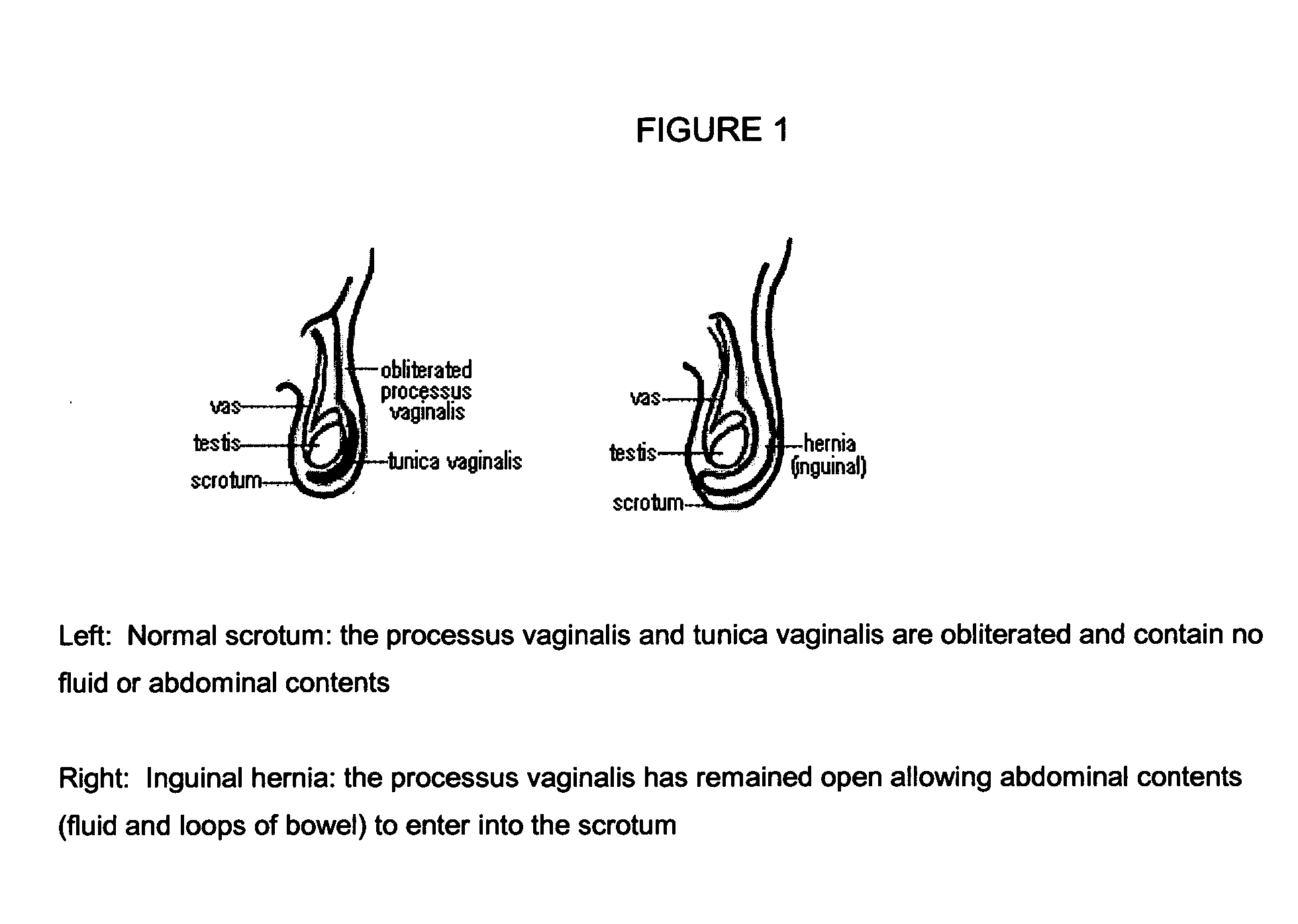
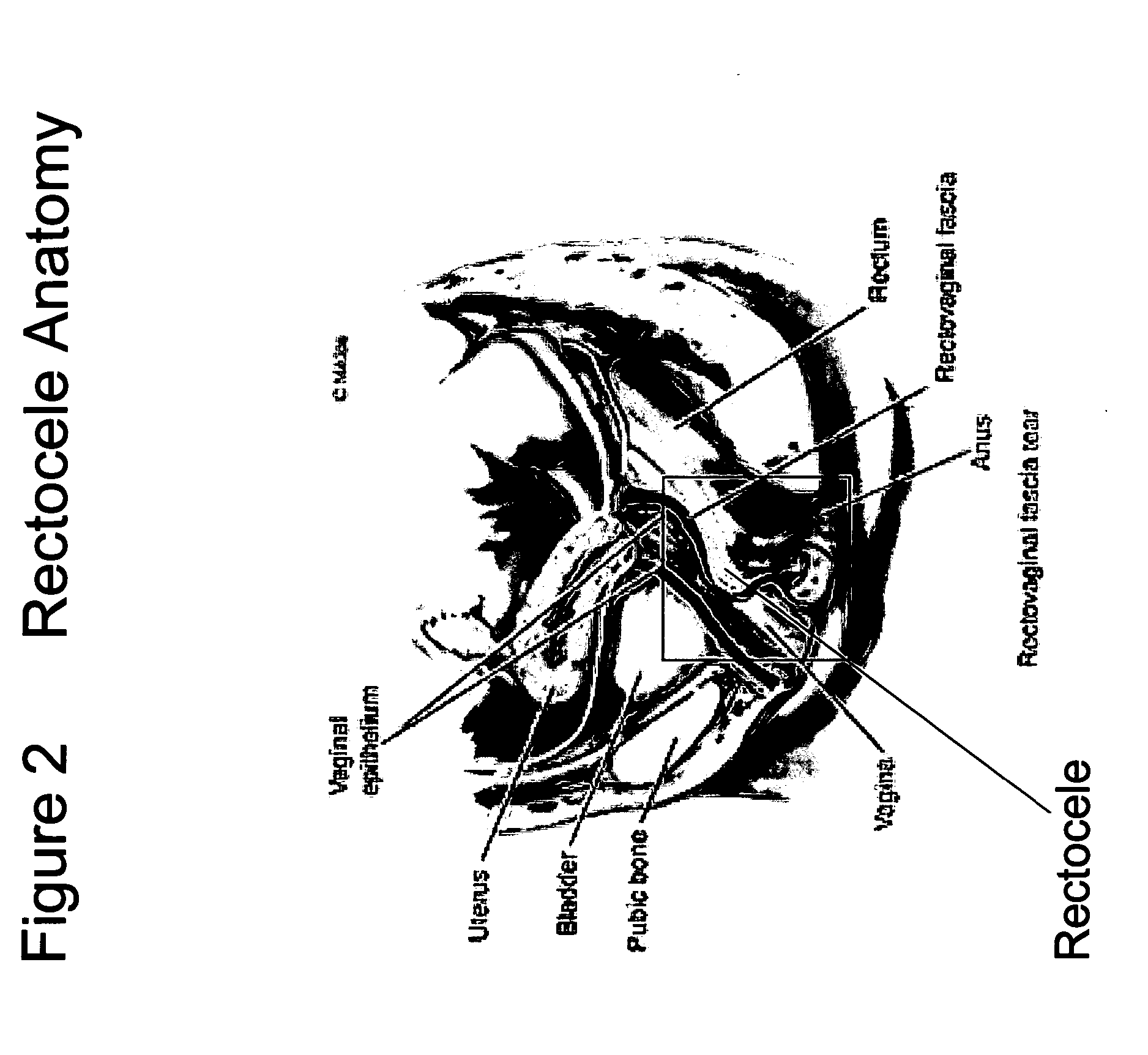
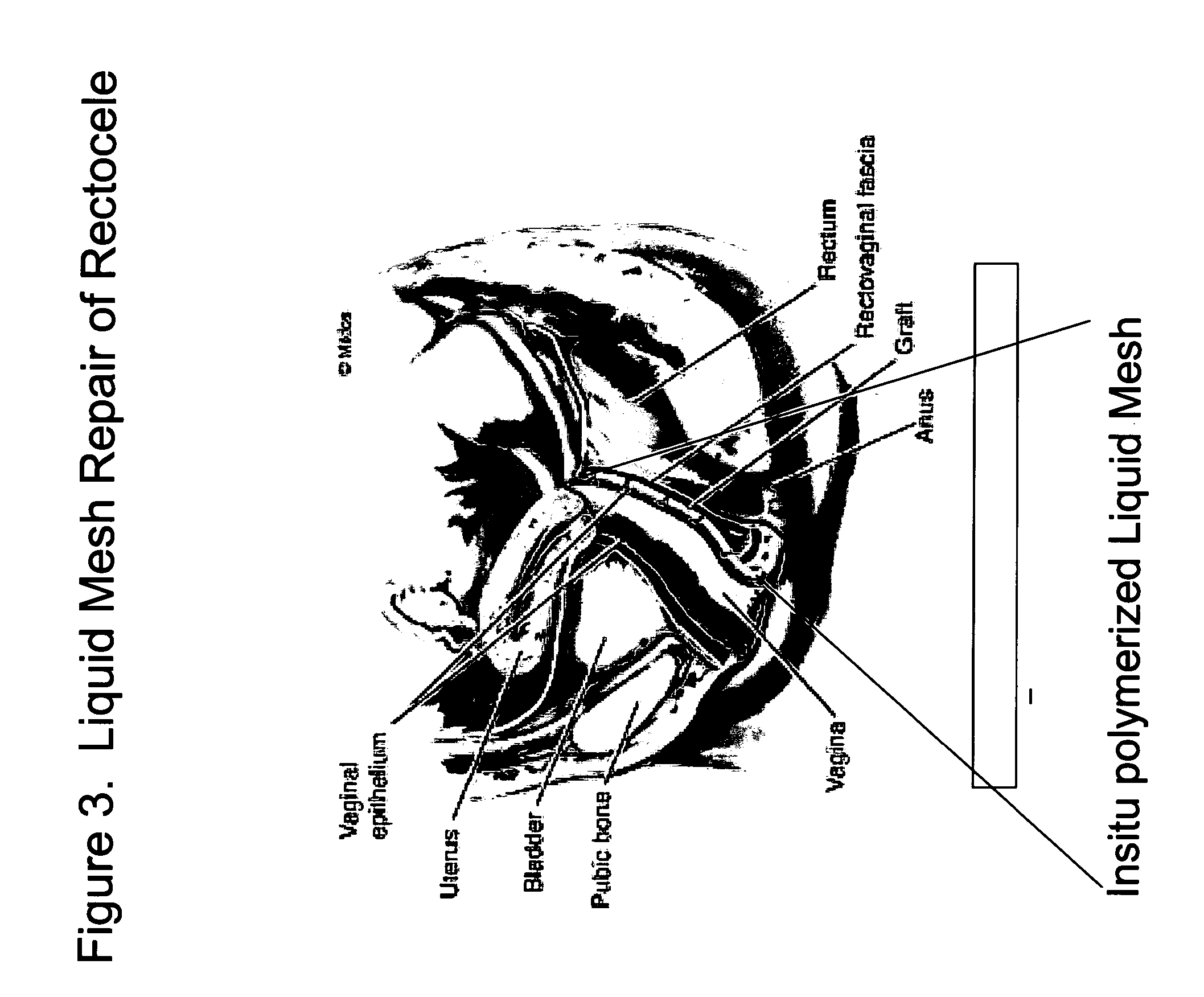
The present invention provides a liquid polymer composition which can be implanted into a living mammal and which forms a solid hydrogel by in situ polymerization upon contact with body fluid and tissue. The composition also can be used as a coating on a medical device, or for the formation of a medical device. Formation of a solid implant or coating involves crosslinking of the adhesive with itself and with surrounding tissue. The liquid implant, by itself or in conjunction with various prostheses, can be used for many purposed, including fixation of the urethra for providing treatment for incontinence, and repair of herniations in the abdominal cavity, including rectocele, cystocele, enterocele, and inguinal hernia. The adhesive may be used to establish adhesion prevention during such repairs, in part by coating or being the material of a repair mesh.
Owner:PROMETHEAN SURGICAL DEVICES
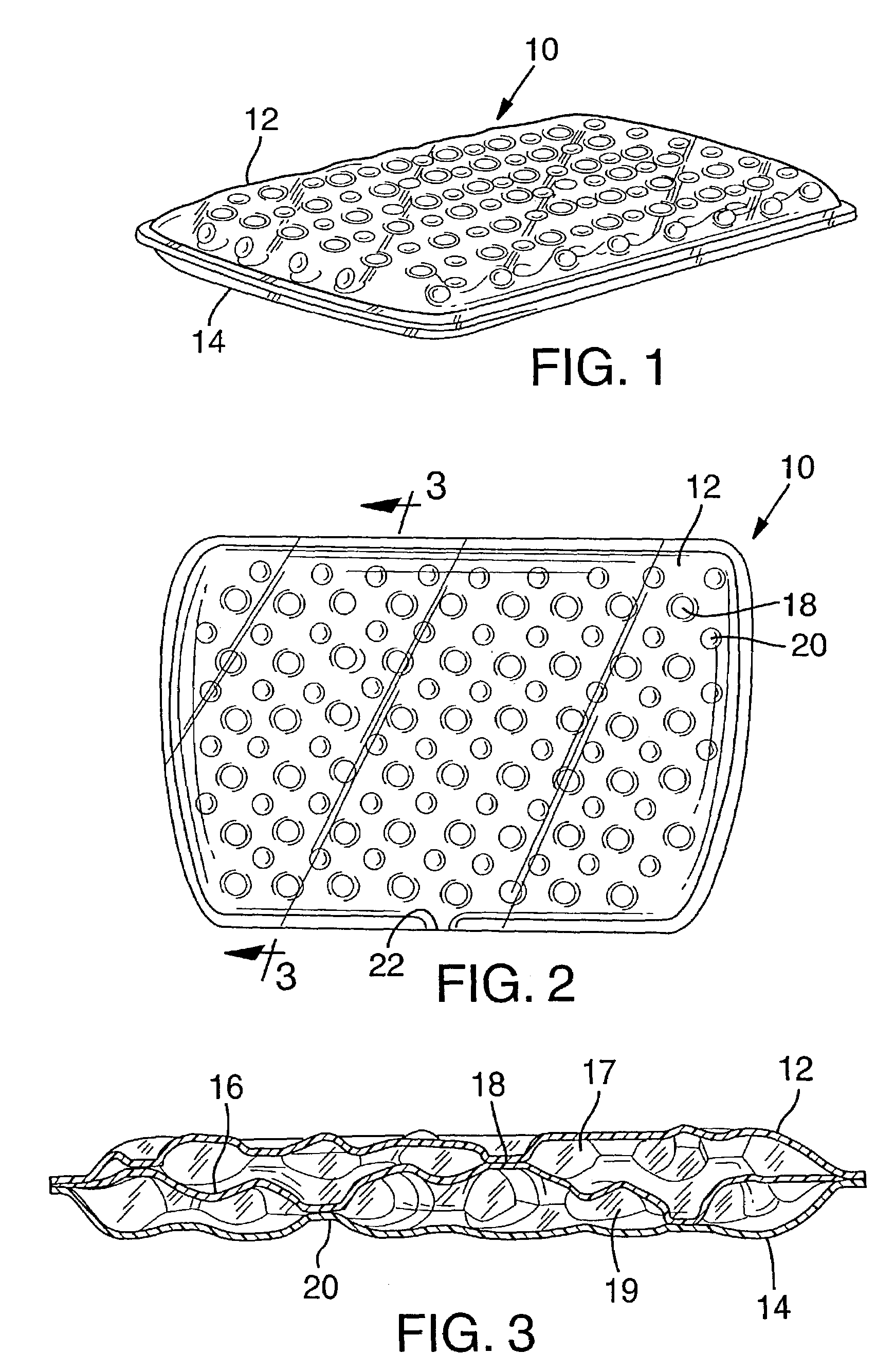
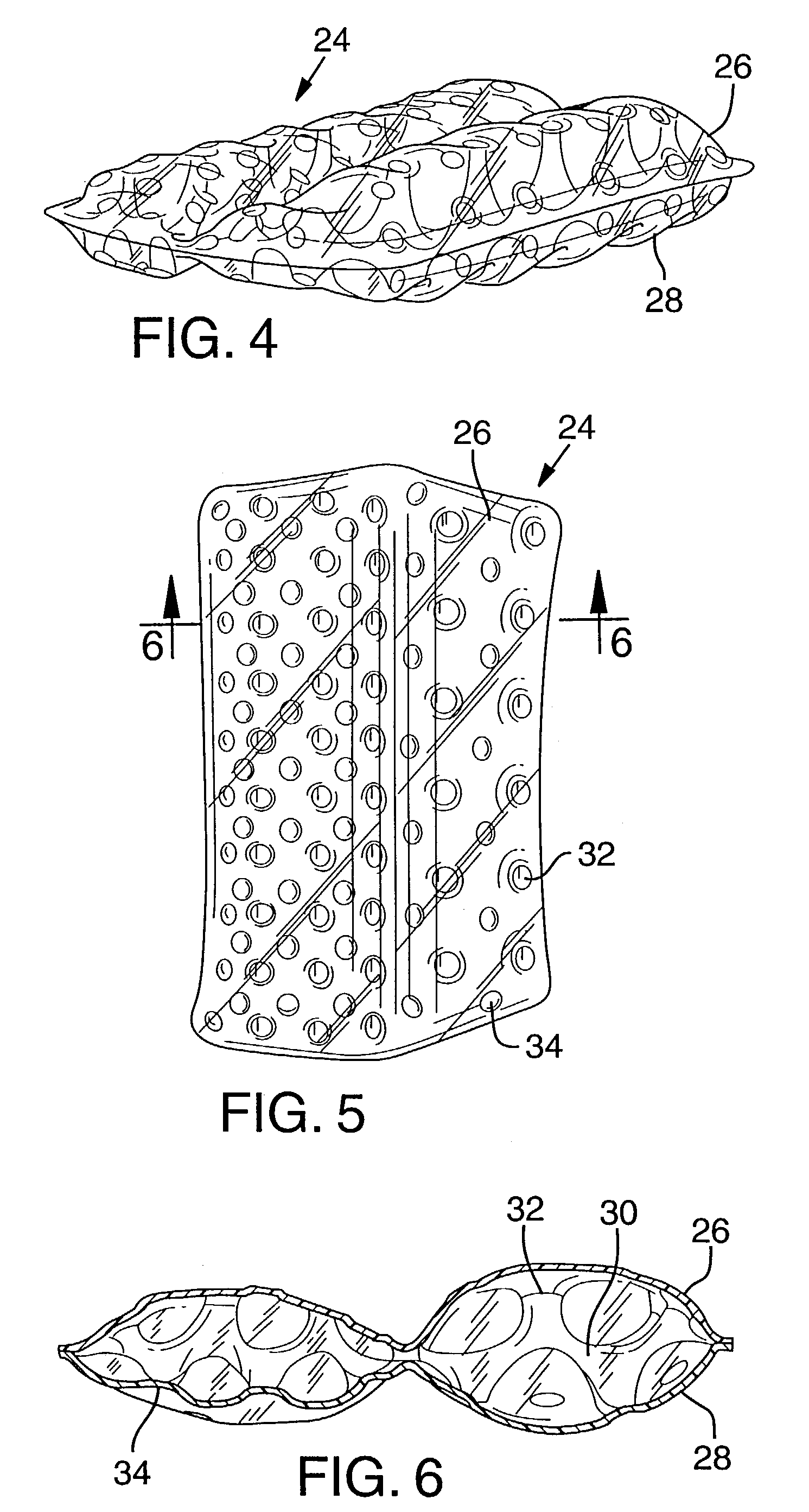
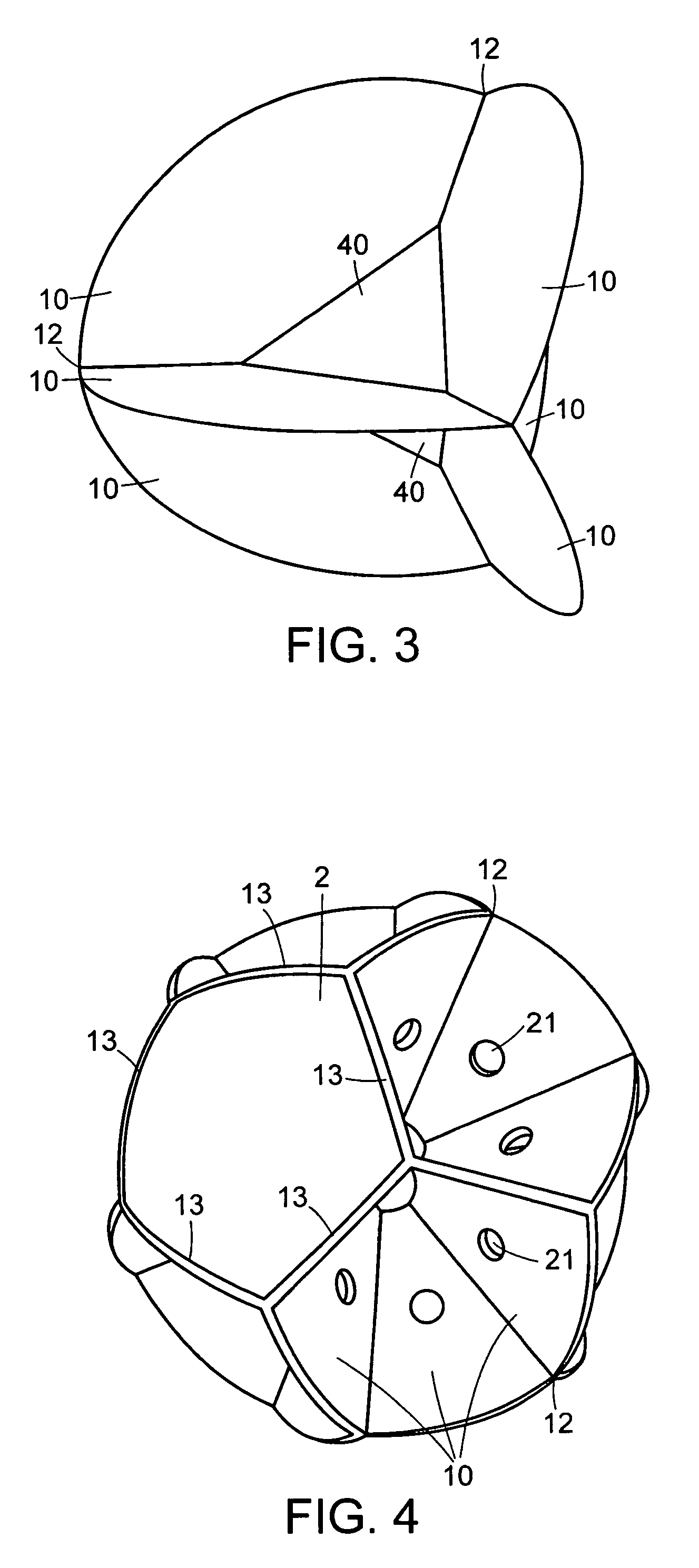
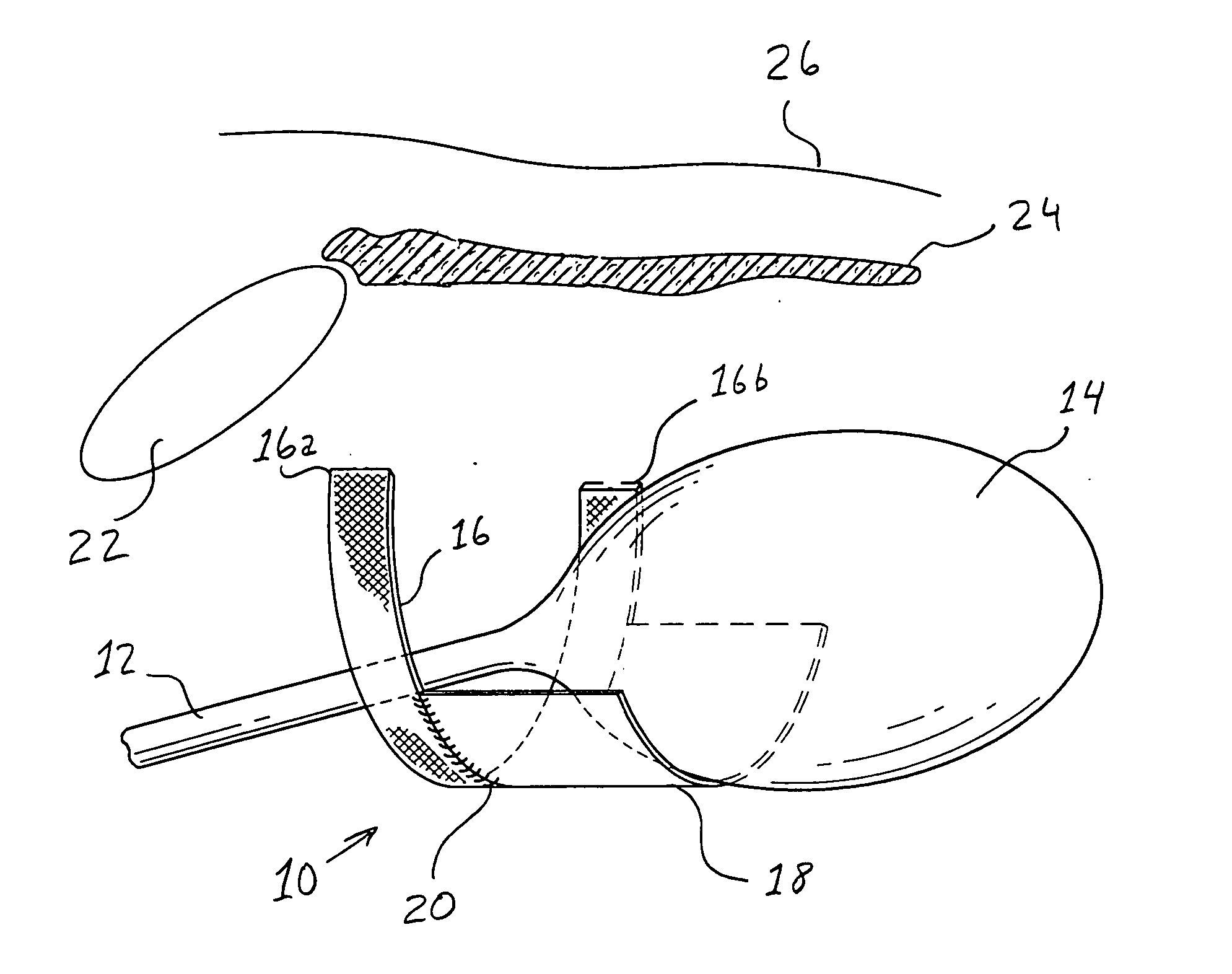
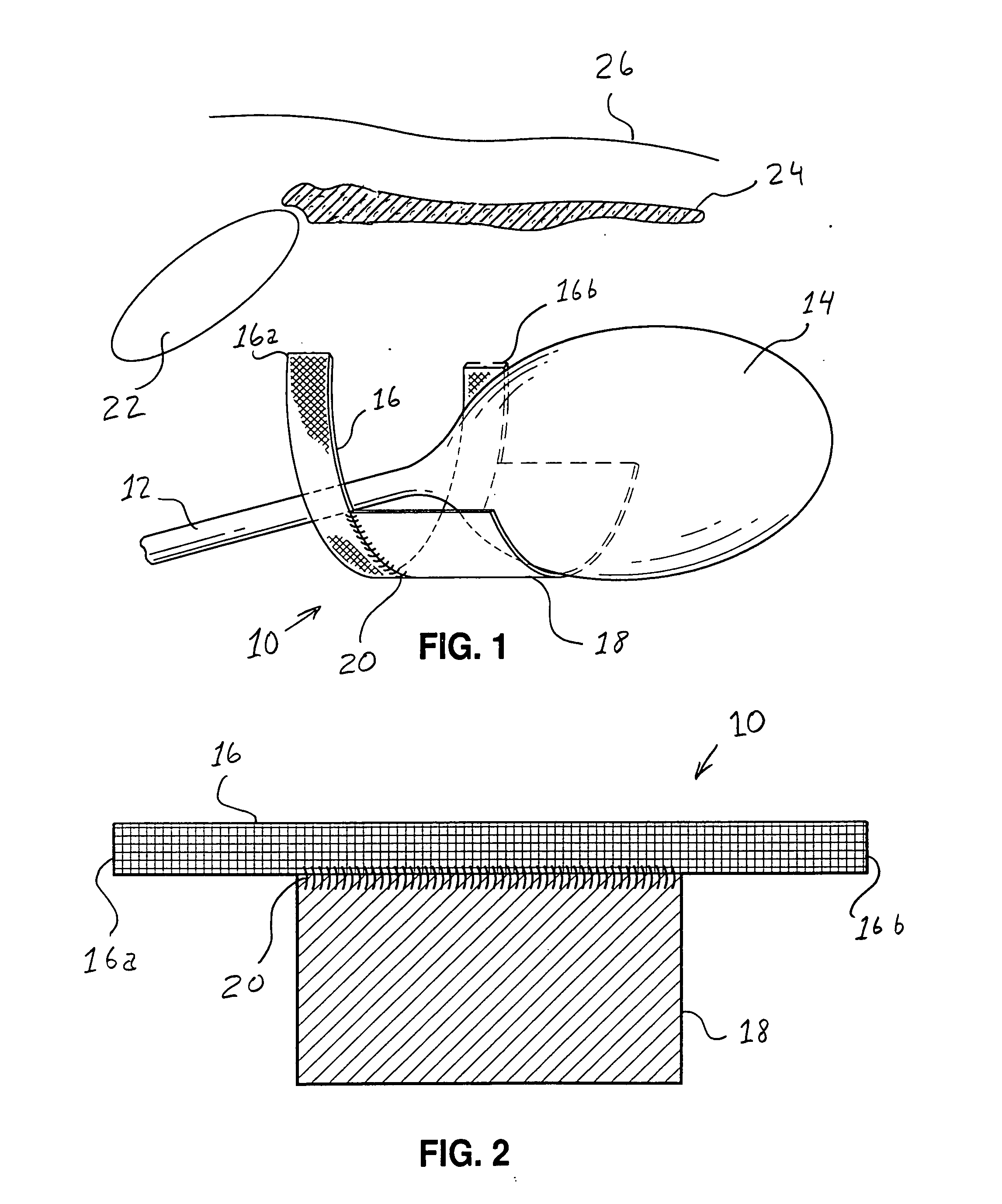
Bladder with multi-stage regionalized cushioning
InactiveUS7132032B2Overcome problemsEnhances cushioning responseSolesHollow inflatable ballsCushioningEngineering
A bladder which is particularly useful for a sole assembly of a shoe is formed of multiple layers of barrier film to provide multiple pressurized layers of cushioning fluid or gas when the bladder is filled. A multiple gas layer bladder enhances cushioning response by relying more on the response characteristics of the gas and reducing the amount of foam and the dependence on foam as a cushioning material. The internal film layers provide a truss-like geometry in cross section and act as tensile members to impart a generally smooth surface contour to the bladder. The bladder is constructed to provide complex regionalized cushioning profiles which are coupled to the anatomy of the foot and expected loads at known points.
Owner:NIKE INC
Noninvasive devices, methods, and systems for shrinking of tissues
InactiveUS20100049186A1Sufficient energyPrevent shrinkageUltrasound therapyDiagnosticsUrethraNon invasive
The invention provides improved methods for modifying collagenous tissues, particularly for treating urinary incontinence in a noninvasive manner. The methods typically include inserting a probe into a patient's urethra, transmitting electromagnetic energy from the probe to a collagneous target tissue, and cooling the tissue in the vicinity of the probe. The probe may include an expandable element at or near the distal end that is configured to expand within the patient's bladder. The expandable element may anchor the probe before or during treatment.
Owner:BOSTON SCI SCIMED INC +1
Method and apparatus for treating pelvic organ prolapses in female patients
An anterior implant adapted to treat central and lateral cystoceles present in a female patient includes laterally extending stabilizing straps for supporting the implant between the patient's bladder and vagina independently of the patient's arcus tendineous fascia pelvis. Rectocele and hysterocele repairs can be carried out using a single posterior implant which, like the anterior implant, is provided with laterally extending stabilizing straps for supporting the implant between the patient's rectum and vagina.
Owner:ETHICON INC
Patch-Like Infusion Device
InactiveUS20070203454A1Conveniently worn against skinAutomatic syringesMedical devicesInfusion setInfusion solution
A system and method for a patch-like, self-contained substance infusion device which provides one or more substantially hidden patient needles which can be placed in fluid communication with a fluid reservoir subassembly that includes a rigid bladder portion used in conjunction with a non-distensible bladder film, such as a metallized film. Simple removal of an interlock allows a disk, or Belleville spring assembly to apply an essentially even and constant pressure to the contents of the fluid reservoir assembly, and allows the device to then be attached to a skin surface via an adhesive contact surface. A push button activation assembly is provided which can then be used to release and seat one or more spring-loaded patient needles into the skin surface, and establish a fluid communication path between the patient needles and the pressurized fluid reservoir contents thereby delivering an infusion into the skin.
Owner:BECTON DICKINSON & CO
Open system heat exchange catheters and methods of use
InactiveUS6972014B2Eliminate needDiagnosticsSurgical instruments for heatingUrethraSuprapubic aspirate
Various embodiments of open system heat exchange catheters and methods of use are disclosed. The various catheters can be used with various ablative surgical devices. One specific exemplary use is in conjunction with cryosurgical probes involving ablation of the prostate, in which the integrity of the urethra is desired to be maintained. Other uses involve various heating ablative devices. In one embodiment an injection tube assembly is used to provide heat exchange fluid through the urethra to the bladder where it is then expelled via a suprapubic suction tube. In other embodiments a coaxial tube assembly is utilized which defines a passageway for either expelling the bladder fluid or for providing access to an endoscope. In other embodiments a double lumen assembly is utilized that defines a passageway for expelling bladder fluid.
Owner:VARIAN MEDICAL SYSTEMS
Cutaneous stabilization by vacuum for delivery of micro-needle array
InactiveUS20060264926A1Add depthImprove uniformityAmpoule syringesSurgical needlesMicro-needleSecondary layer
Owner:MEDICAL MICRODEVICES
Bladder
ActiveUS7740551B2Maintain positionEasy to produceHollow inflatable ballsHollow non-inflatable ballsCushioningEngineering
Owner:ADIDAS
Simulator for major surgical operations
The invention consists of a hands-on, physically simulated human or animal body interior, containing physically simulated representations of all of the solid organs, hollow viscera, bladders, glands, major ducts, large and medium-sized blood vessels, muscle groups and interstitial tissues. The organs and vessels are life-sized and are composed of molded or sculpted open cell or closed cell foam rubber of varying density and load deformation, matching the physical properties of the specific biologic organs or tissues simulated. The organs, tissues and vessels may be treated with pigments, sealants and / or hardening agents to reflect the contours, appearances, densities, textures, elasticity, and deformability of normal or pathologically-altered internal organs and tissues. Organs contain molded vascular channels, reversibly attached to the larger blood vessels of the simulator. The model has a pressurized, watertight, simulated vascular system, monitored by electronic sensors.
Owner:OPERATIVE EXPERIENCE
Harness and balloon catheter assembly and method for use in anastomosis procedures
Disclosed is an instrument, assembly, and method for use in a procedure to effect anastomosis of a patient's bladder and urethra following a prostatectomy. The instrument comprises a tube assembly having an end effector assembly operably supported thereby, where the end effector assembly includes a harness adapted to receive a balloon portion of a balloon catheter assembly.
Owner:ETHICON ENDO SURGERY INC
Implantable sling having bladder support
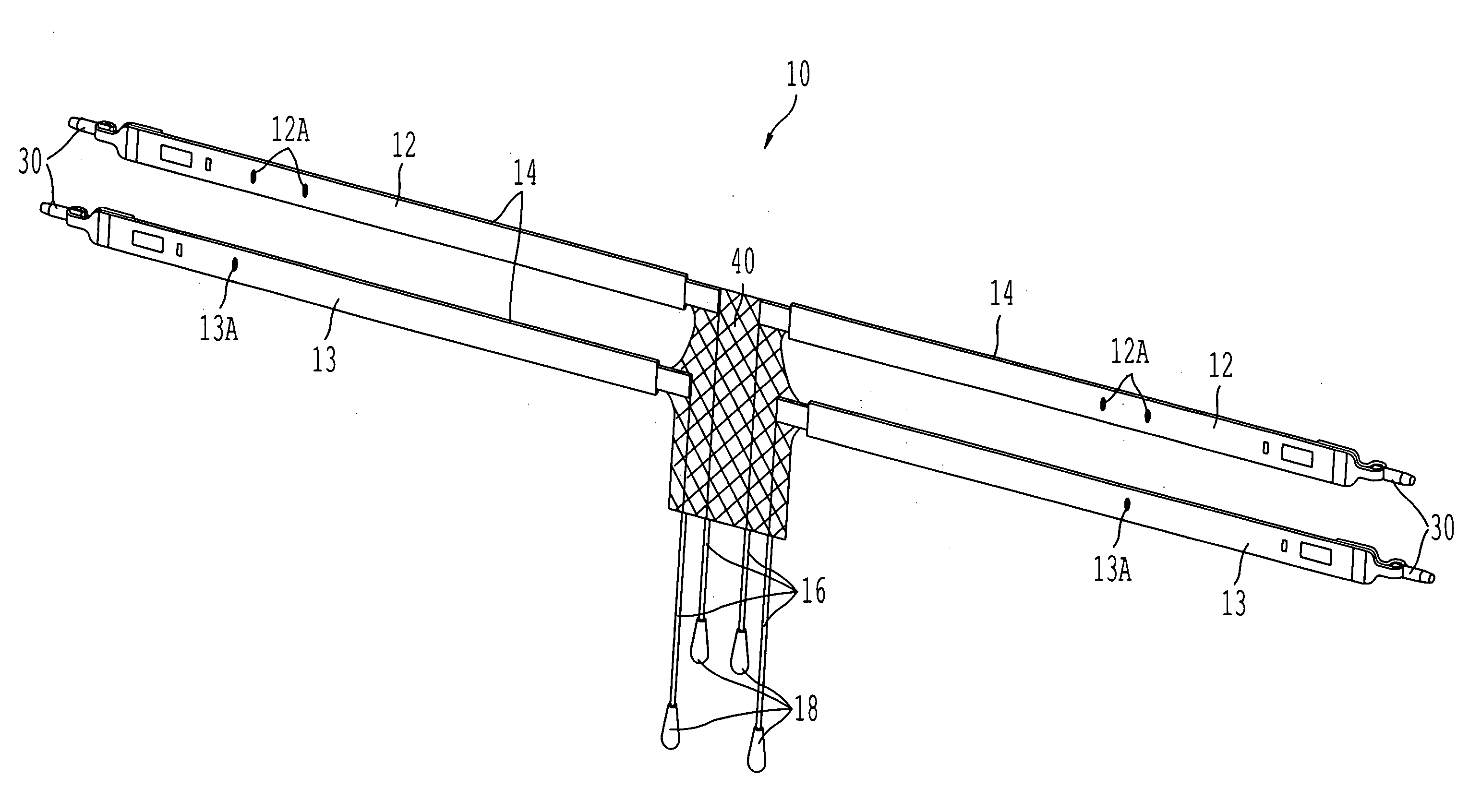
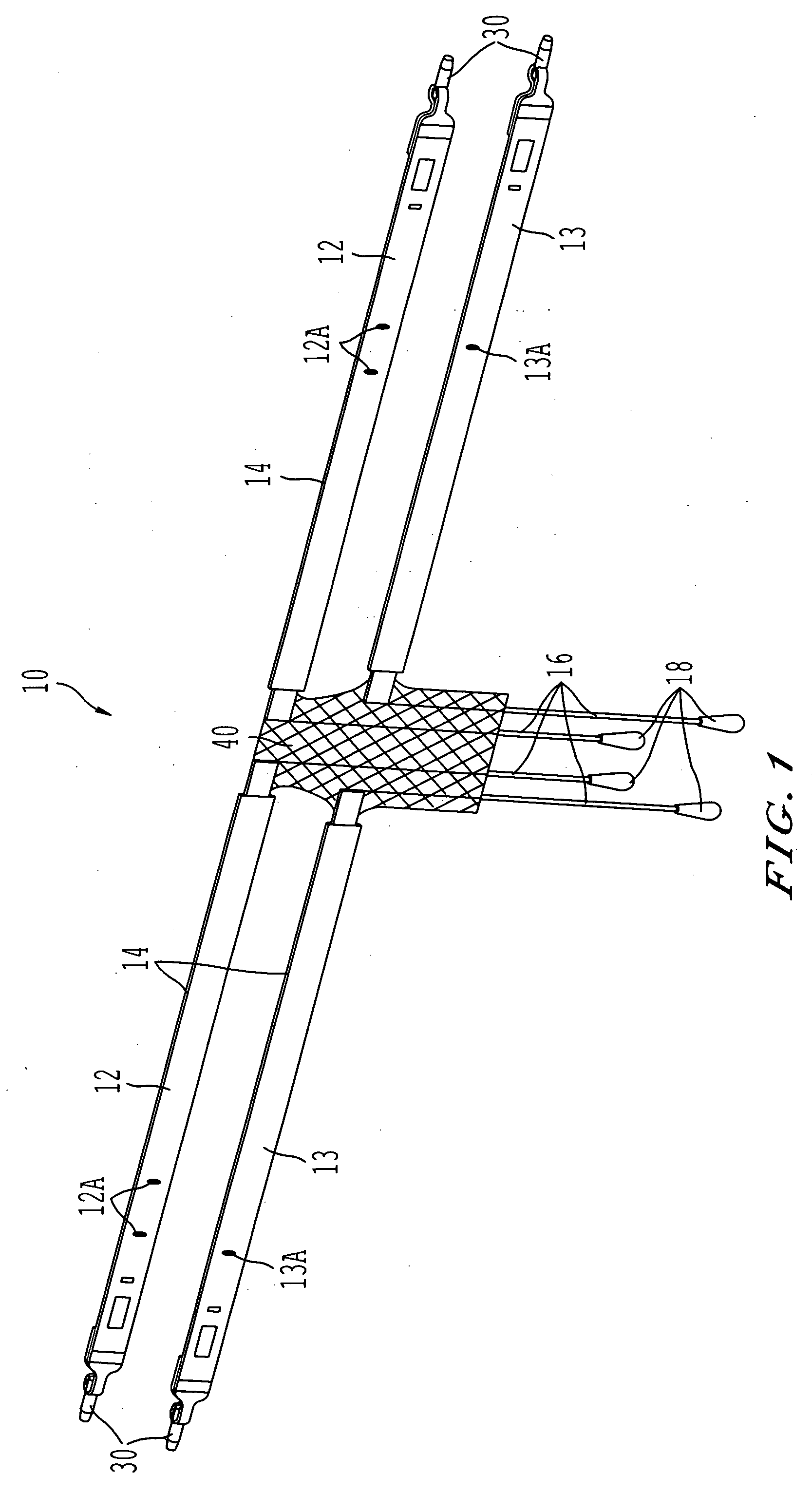
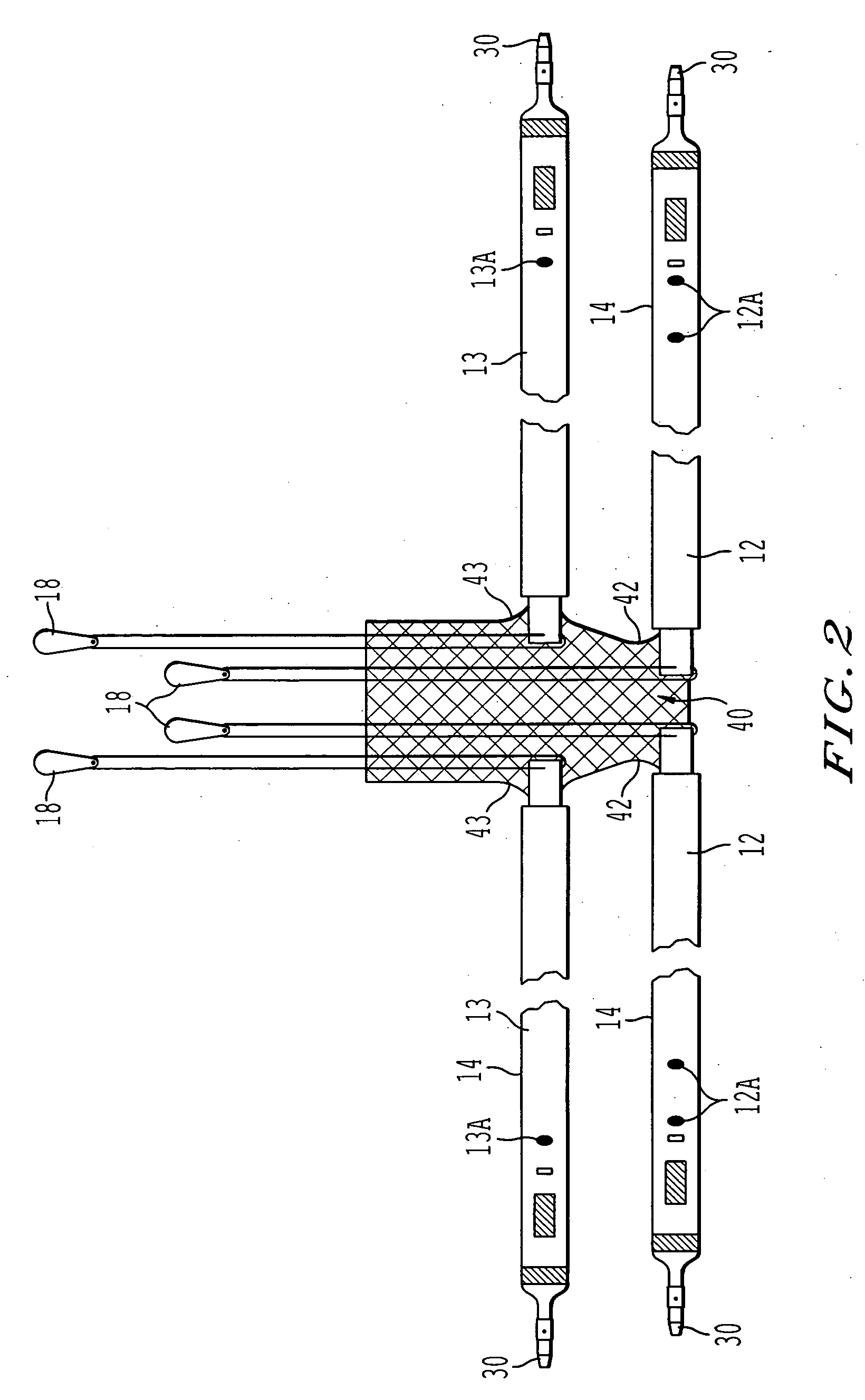
ActiveUS20050080317A1Sufficient degree of supportEliminate dependenciesAnti-incontinence devicesWound clampsUrethraCystocele repair
Surgical implants operative to simultaneously function as a pubovaginal sling for the treatment of incontinence and as a support member to effectuate cystocele repair. The implant comprises a first sling portion operative to be positioned beneath the urethra, per conventional pubovaginal sling surgery. The implant further includes a second bladder support portion extending from the sling support portion that is oriented to extend beneath and be surgically attached to a portion of the bladder to thus enable the same to be supported to a degree necessary to effectuate cystocele repair. The implant may be fabricated from a unitary piece of harvested tissue, synthetic material or combinations thereof. Preferably, the sling portion of the implant is fabricated from a synthetic material whereas the bladder support portion of the implant comprises a segment of harvested tissue sewn to the sling portion.
Owner:CALDERA MEDICAL
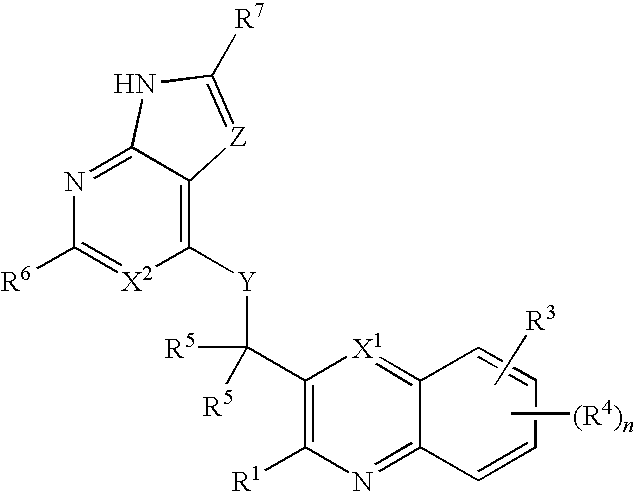
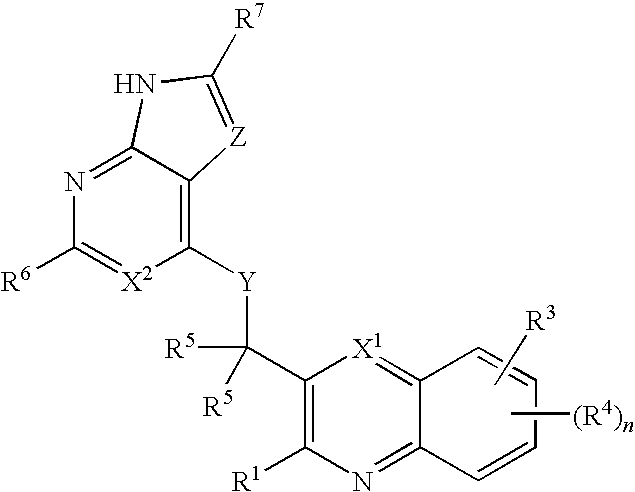
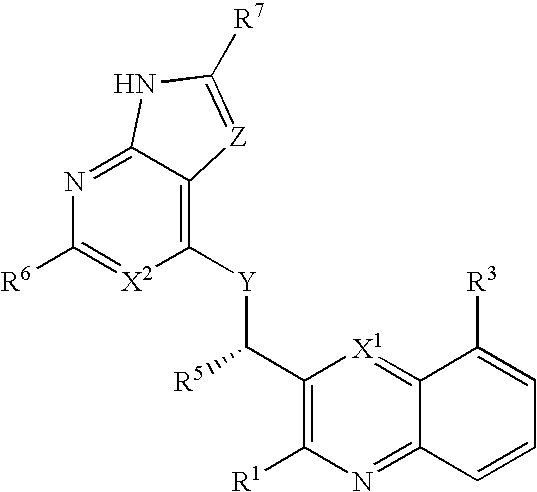
Heterocyclic compounds and their uses
ActiveUS20090137581A1Low inhibitory potencyInhibitory activityBiocideSenses disorderDiseaseMyeloid leukemia
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Intravesical drug delivery device and method
ActiveUS20070202151A1Easy to keepMedical devicesPharmaceutical delivery mechanismImplanted deviceBody cavity
Implant devices for intravesical administration and local drug delivery. The device has a body which includes a hollow tube formed of a biocompatible material; at least one reservoir in the tube which contains a drug; and one or more apertures through which the drug can be released. The device is configured for minimally invasive insertion into a body cavity, such as the bladder. The hollow tube may be elastomeric to permit the device to be elastically deformed from its initial shape into an elongated shape for passage through a catheter, where following such passage the device can return to or toward its initial shape to facilitate retention of the device in the body cavity. The body may have a narrow, elongated shape effective to permit insertion of the drug delivery device through a catheter without necessarily deforming the body, yet include flexible projections which effect retention within the body cavity.
Owner:MASSACHUSETTS INST OF TECH +1
Method and Apparatus for Treatment of Vaginal Anterior Repairs
ActiveUS20080039678A1DistanceSurgical furnitureDiagnosticsAnterior repairPhysical medicine and rehabilitation
Owner:BOSTON SCI SCIMED INC
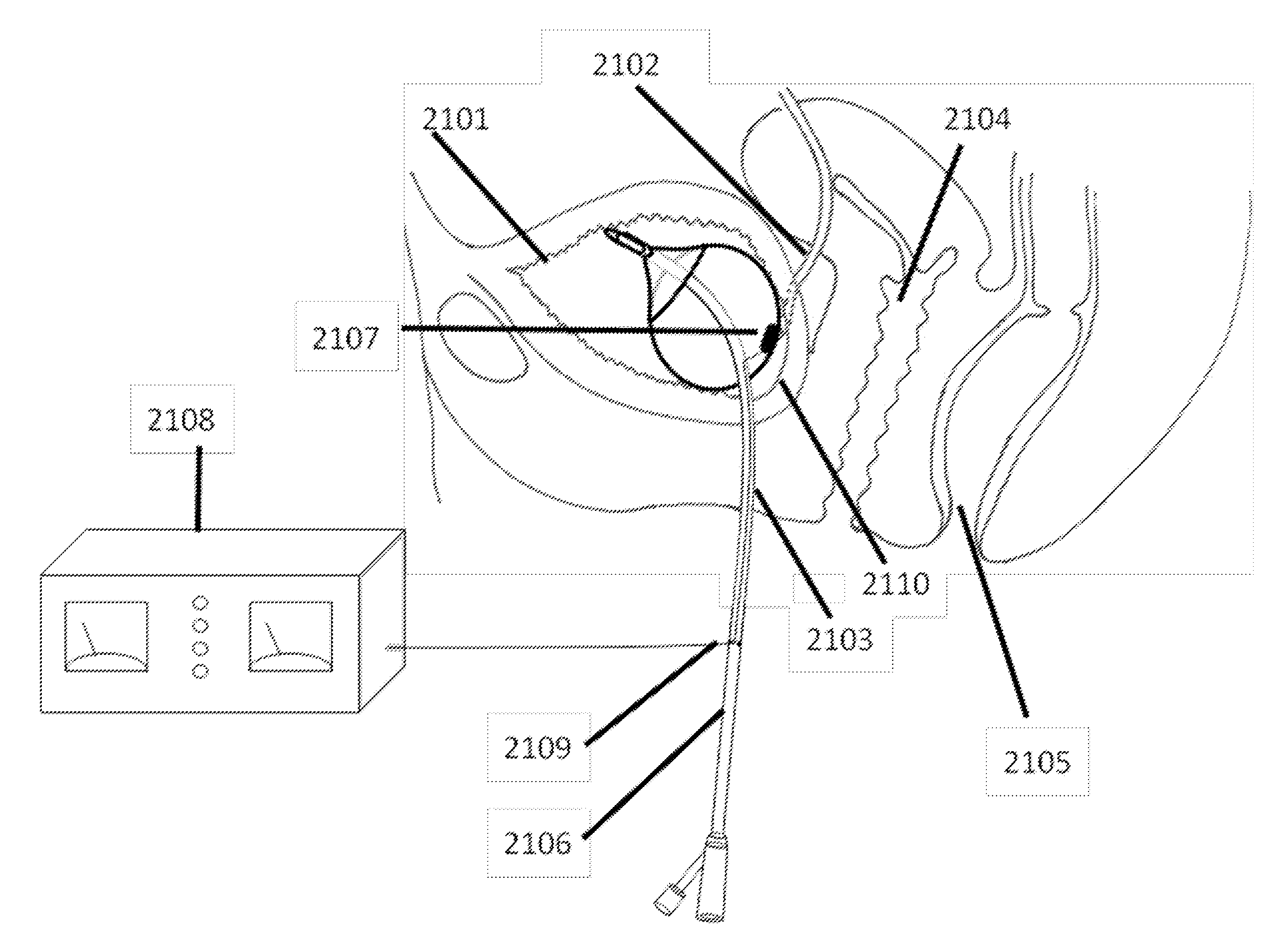
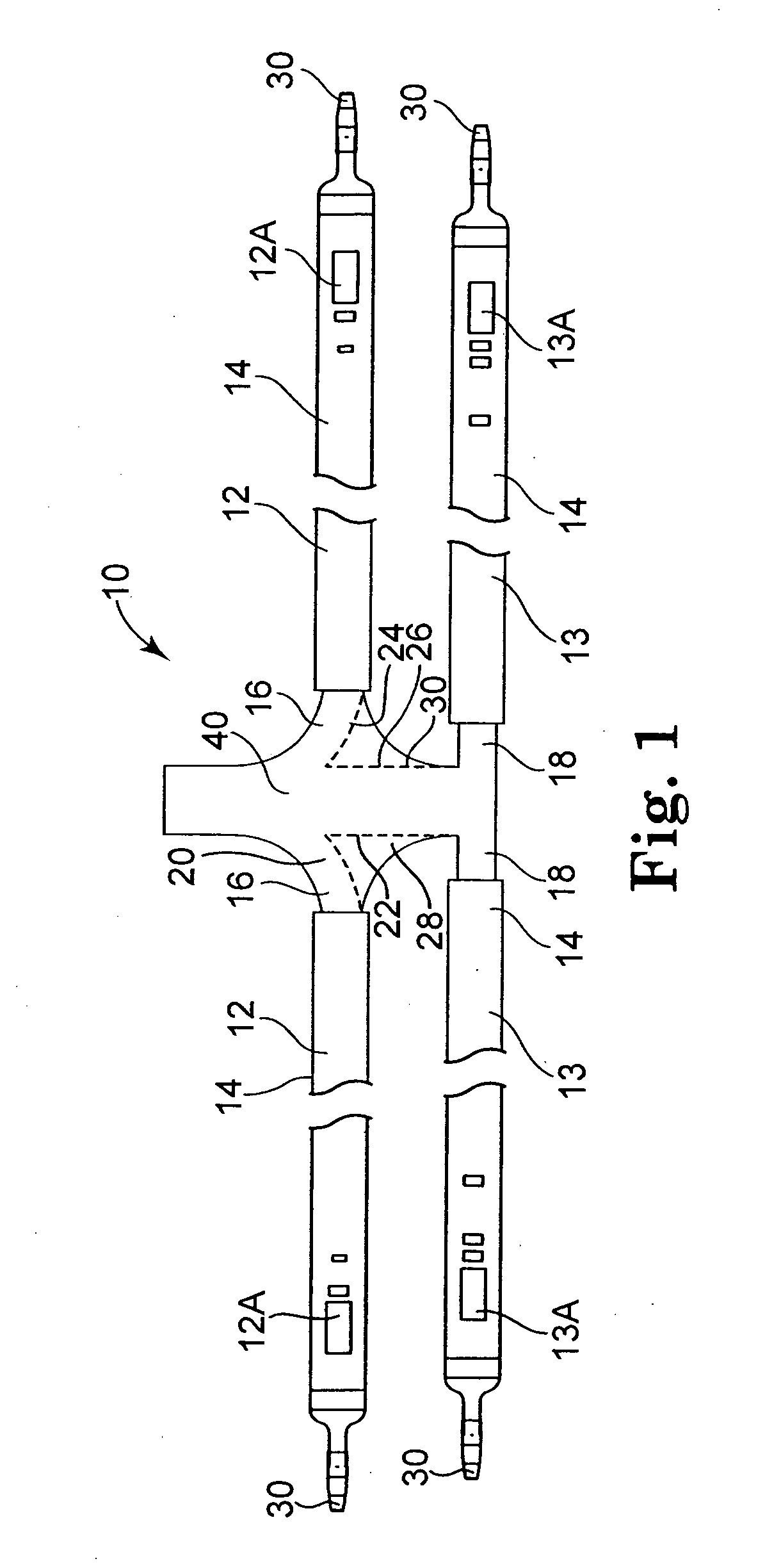
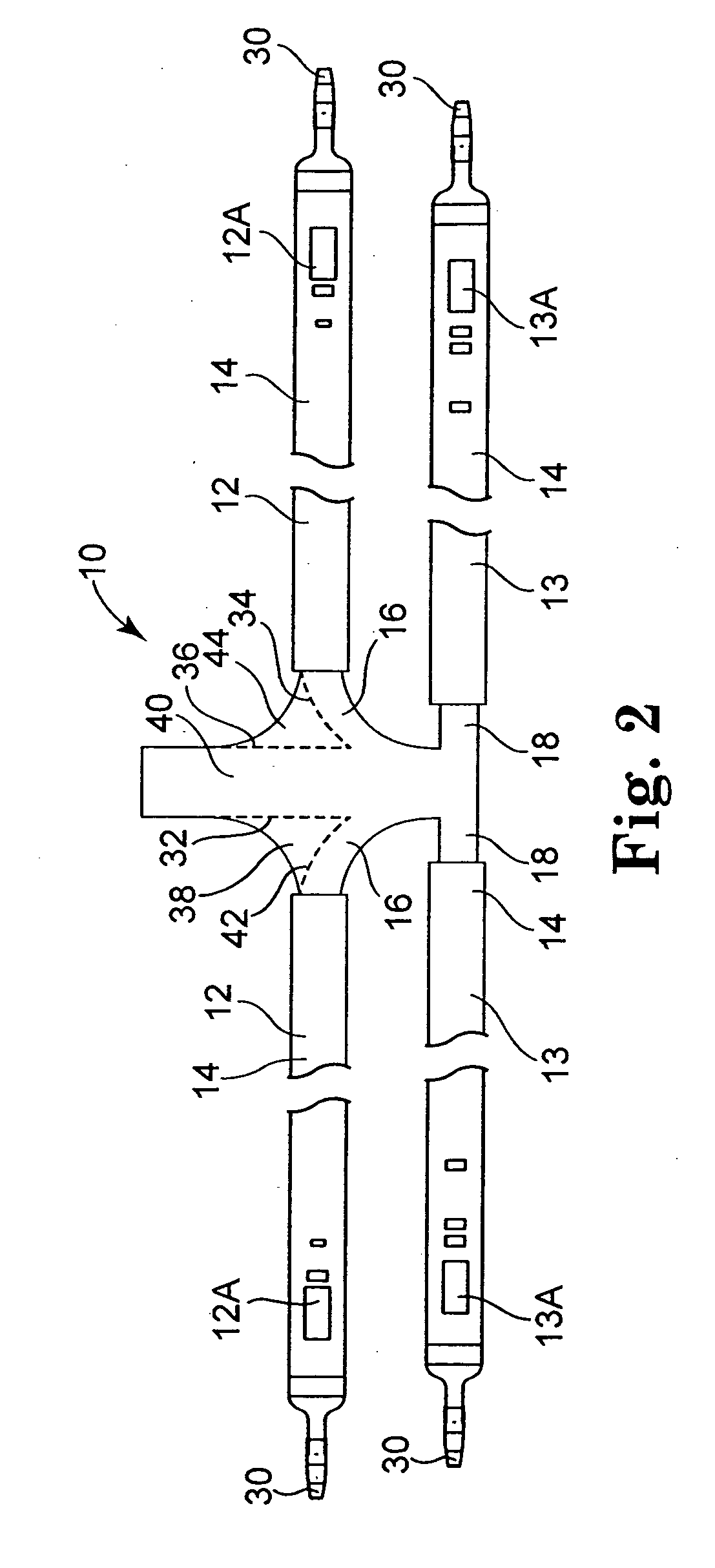
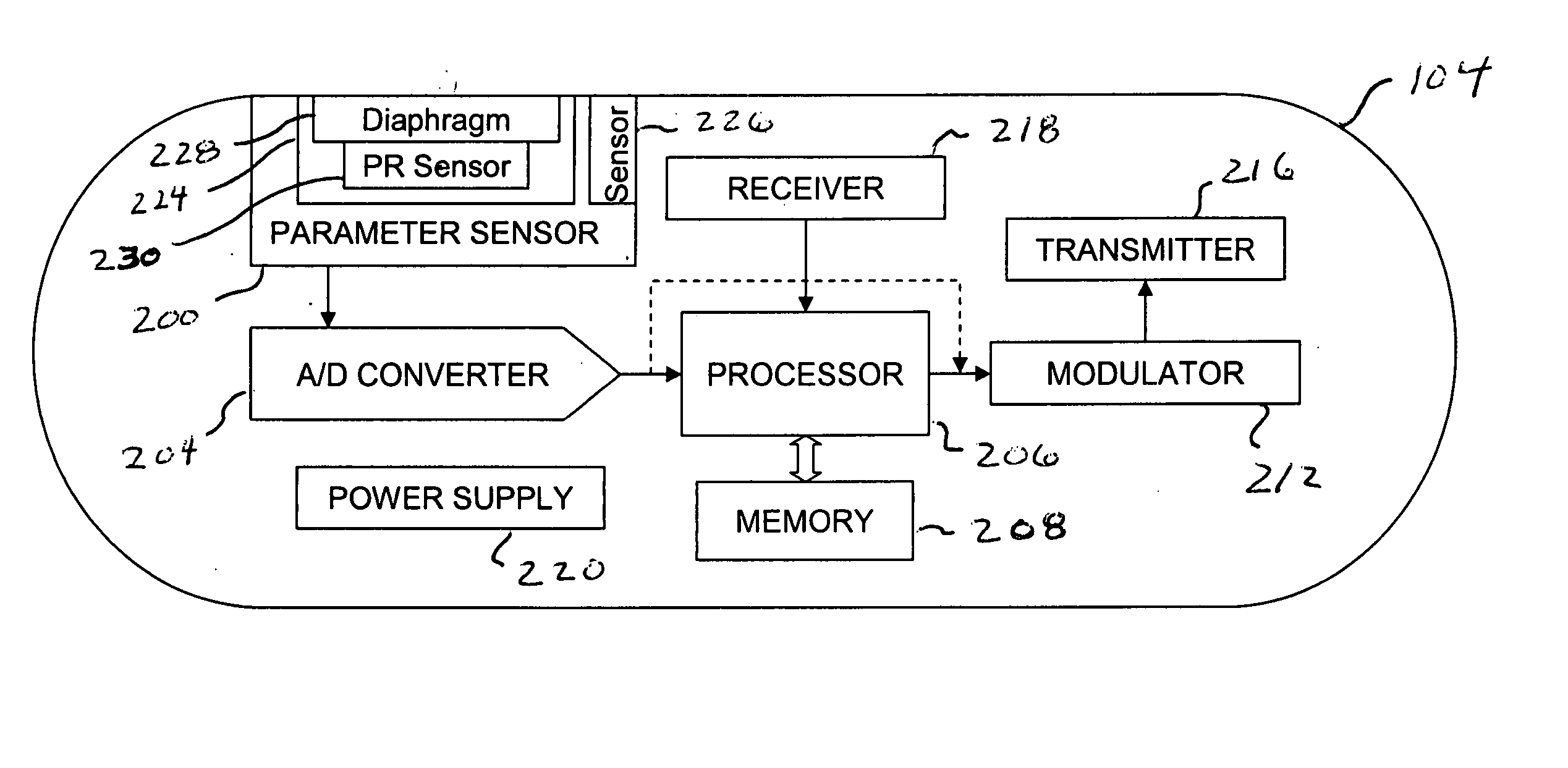
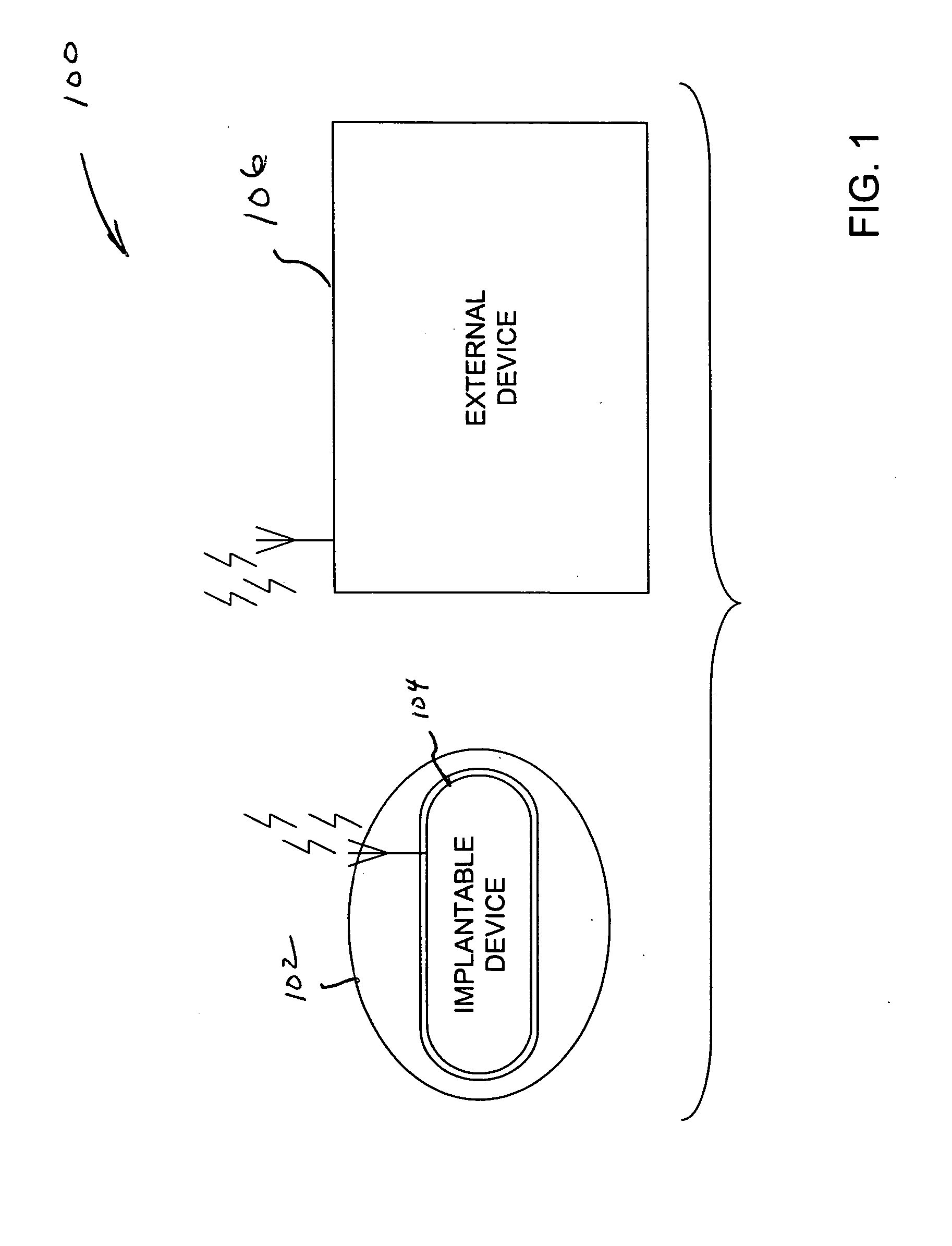
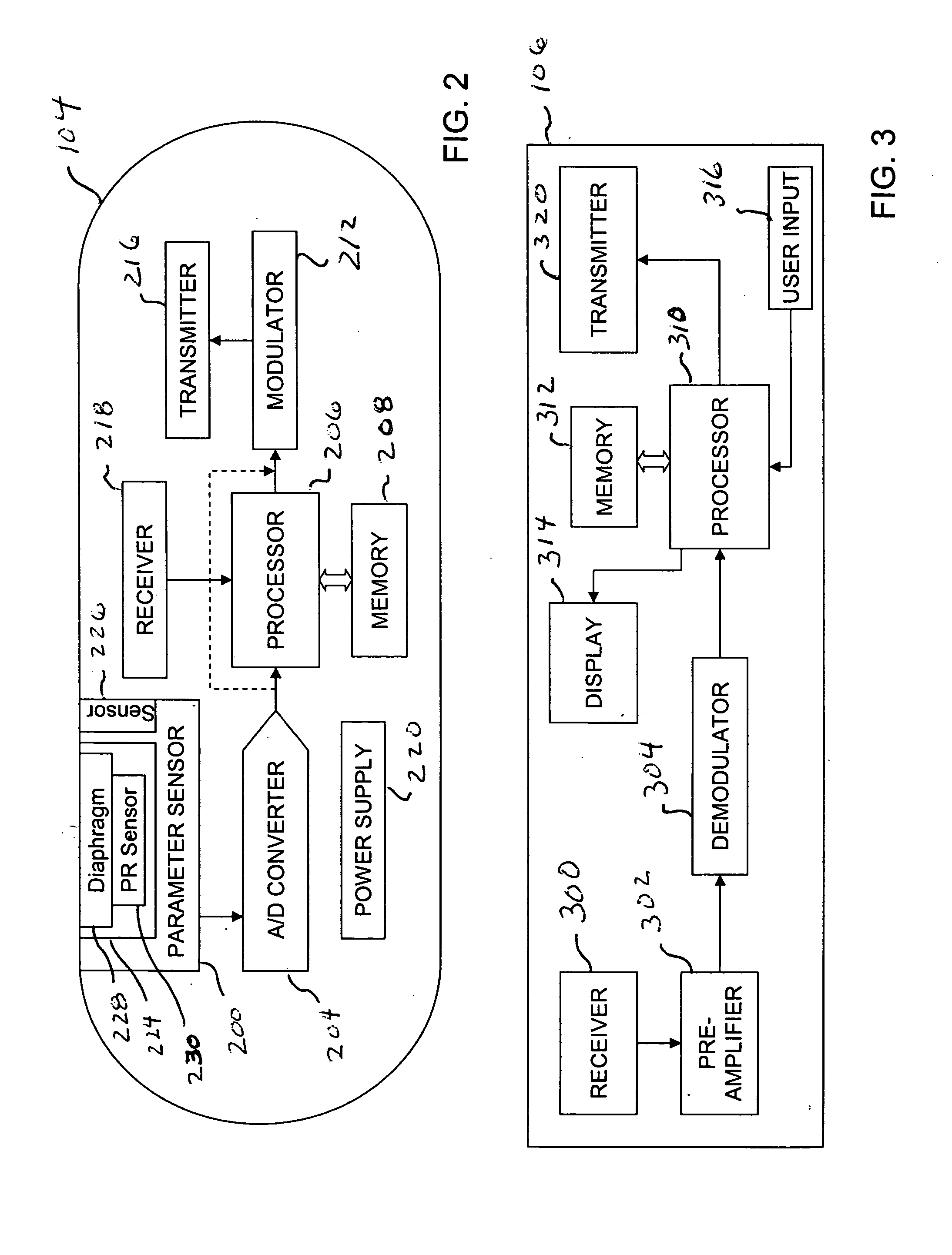
Implantable telemetric monitoring system, apparatus, and method
Systems, apparatus, and methods are disclosed for telemetrically monitoring parameters within a body of an animal such as a within a urinary bladder of a human. The system includes an implantable device configured for insertion within the body and an external device for use external to the body. The implantable device senses and stores one or more bodily parameters and transmits the stored bodily parameters for receipt by the external device responsive to receipt of a parameter transfer signal. The external device communicates with the implantable device, generates the parameter transfer signal, and receives the bodily parameters transmitted by the implantable device.
Owner:TEMPLE UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com