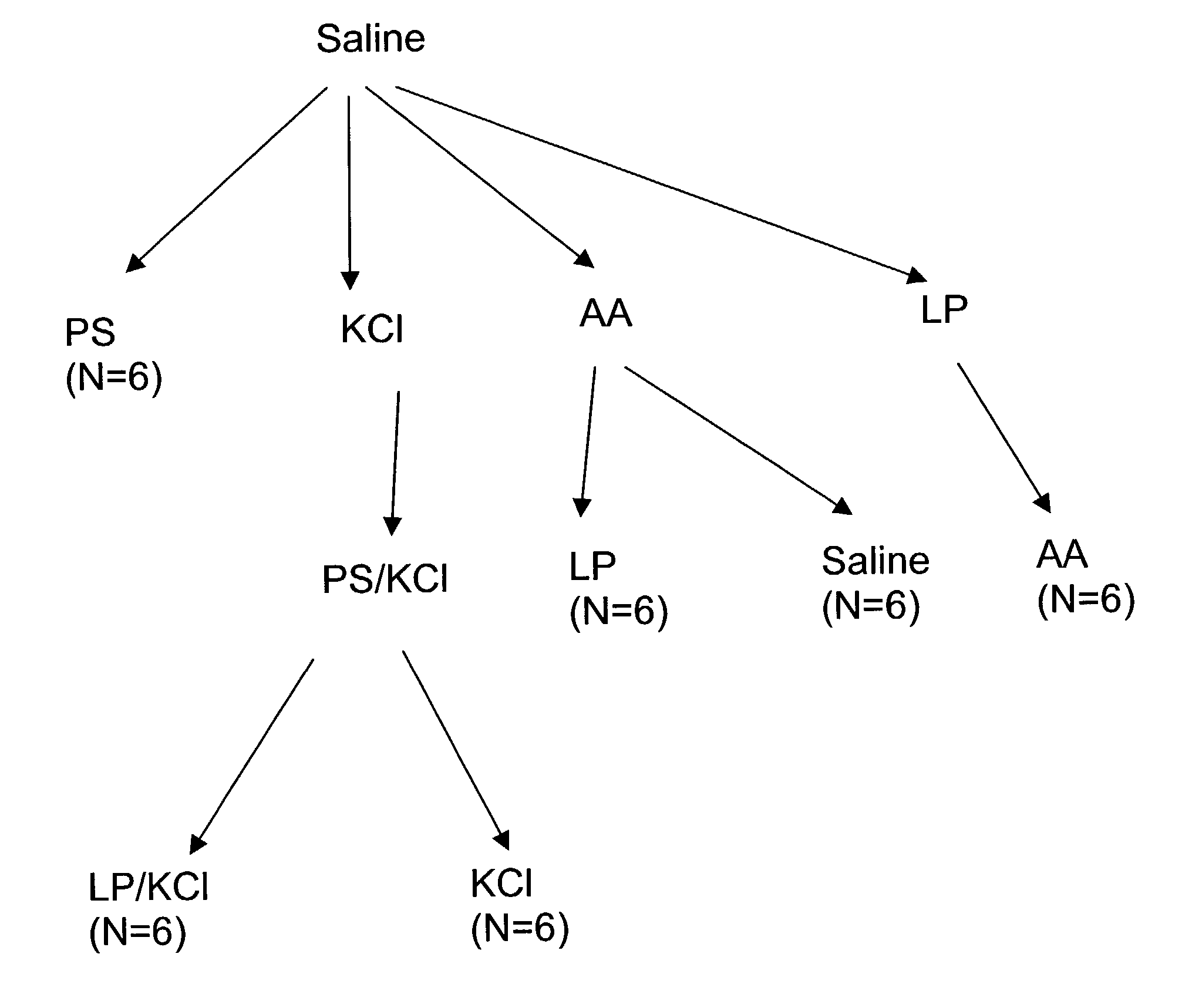
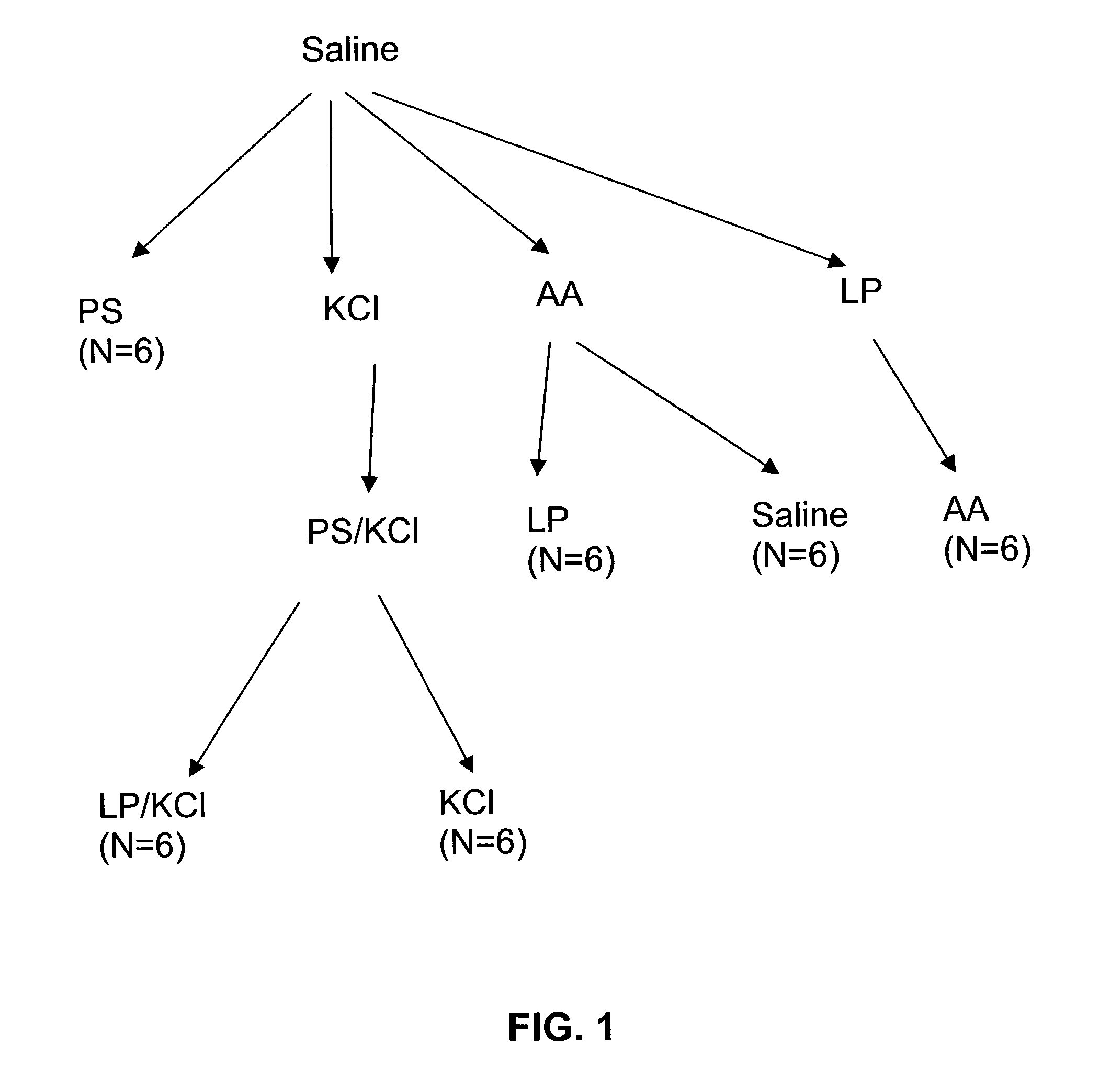
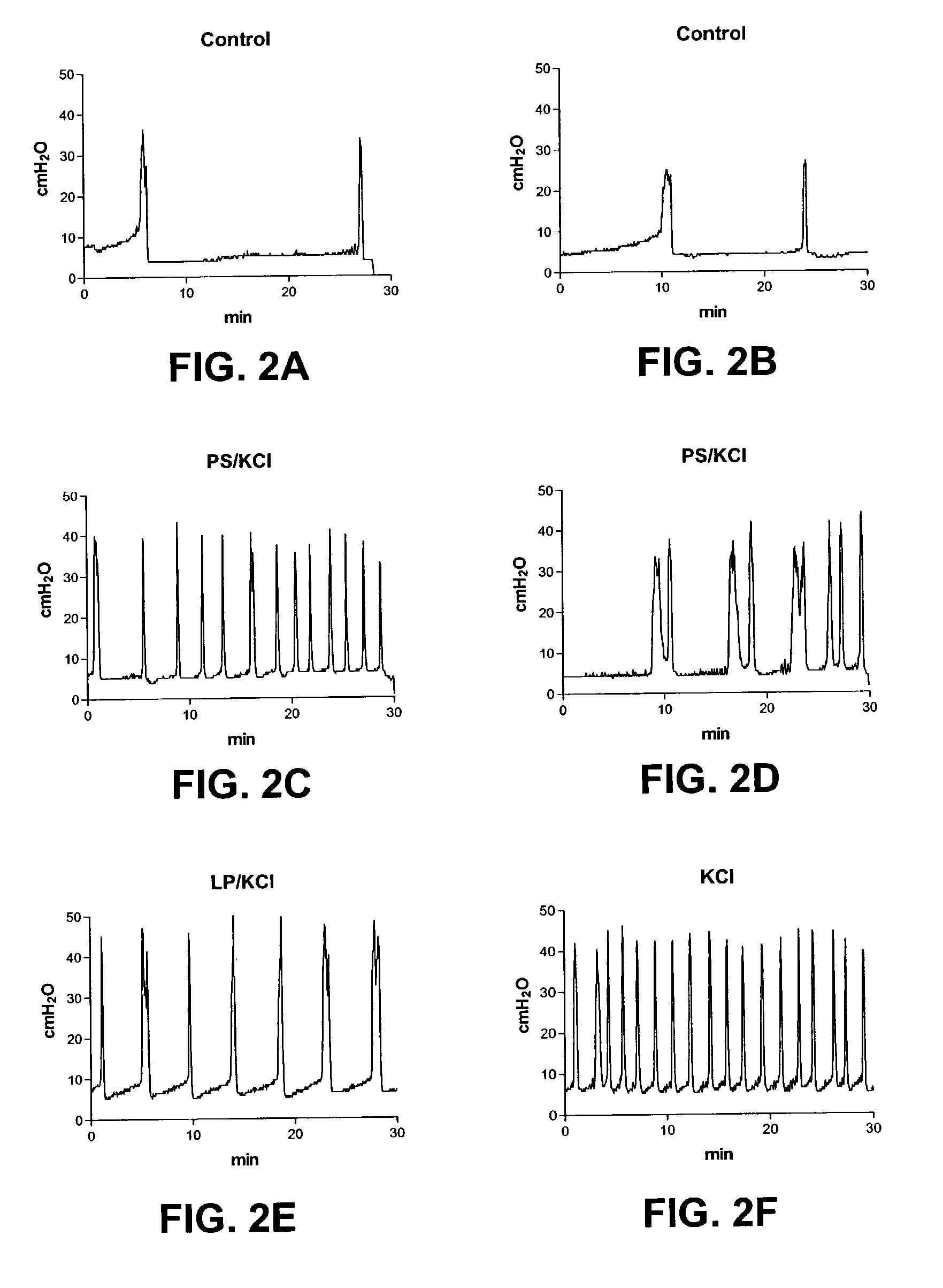
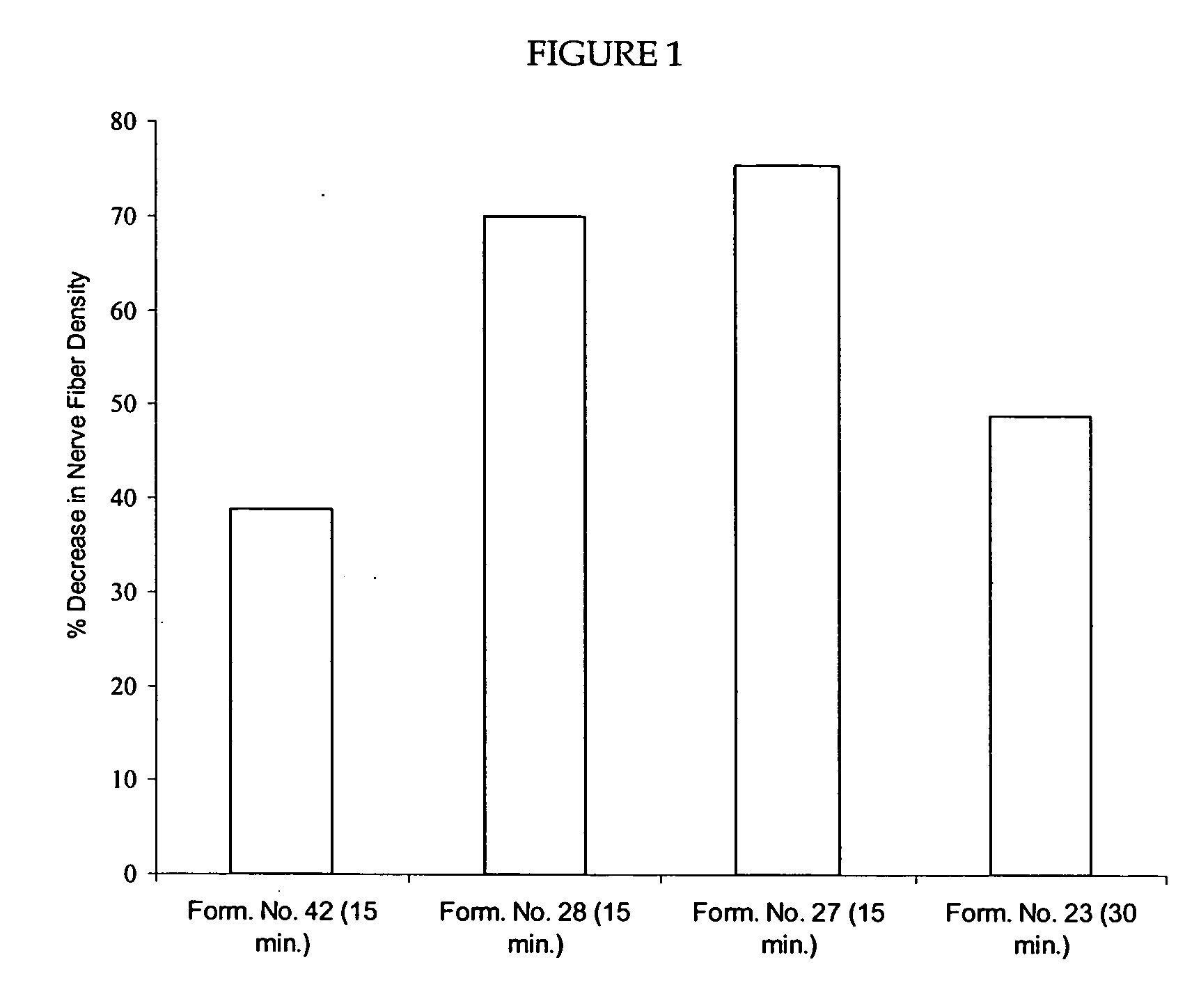
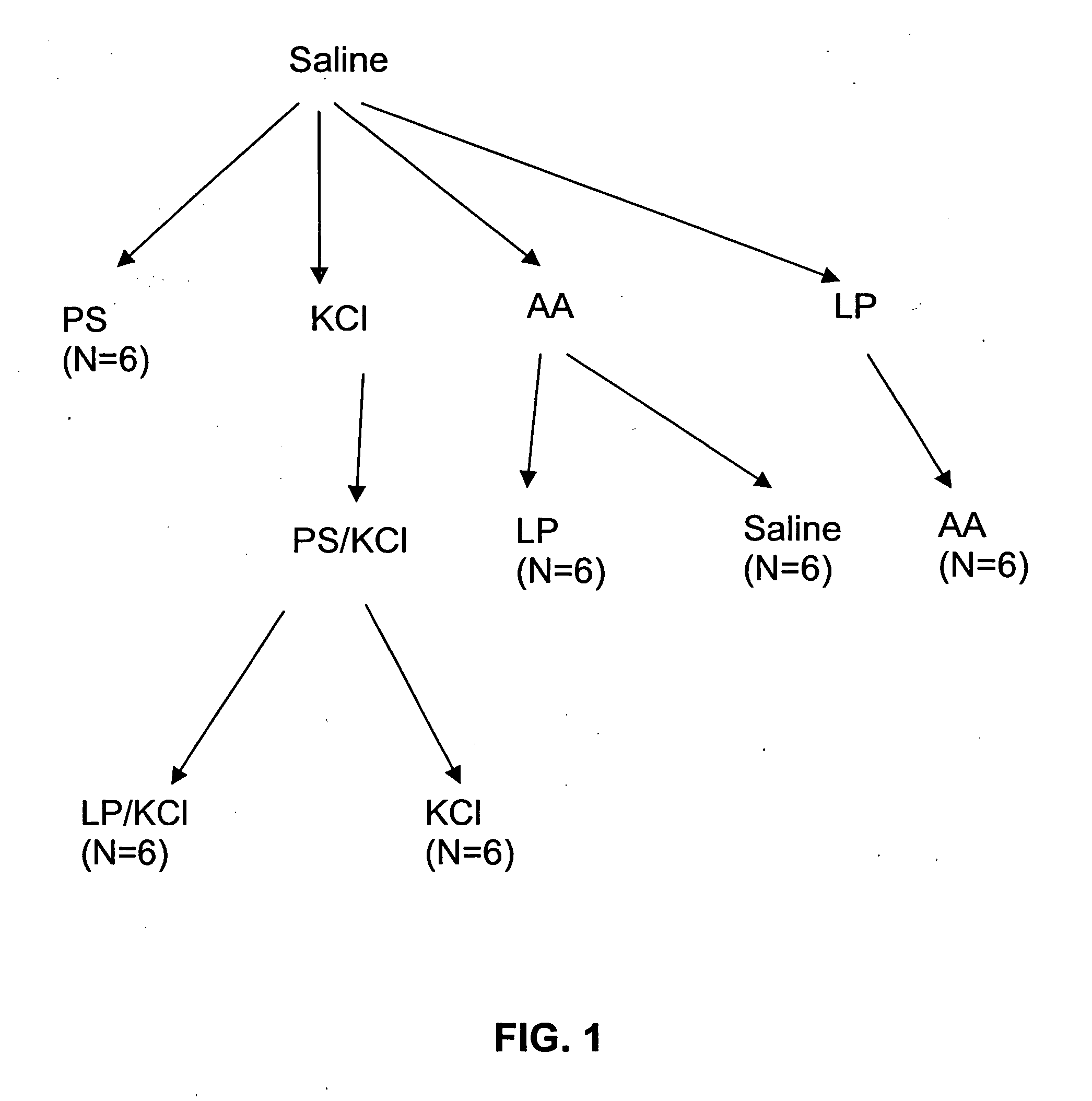
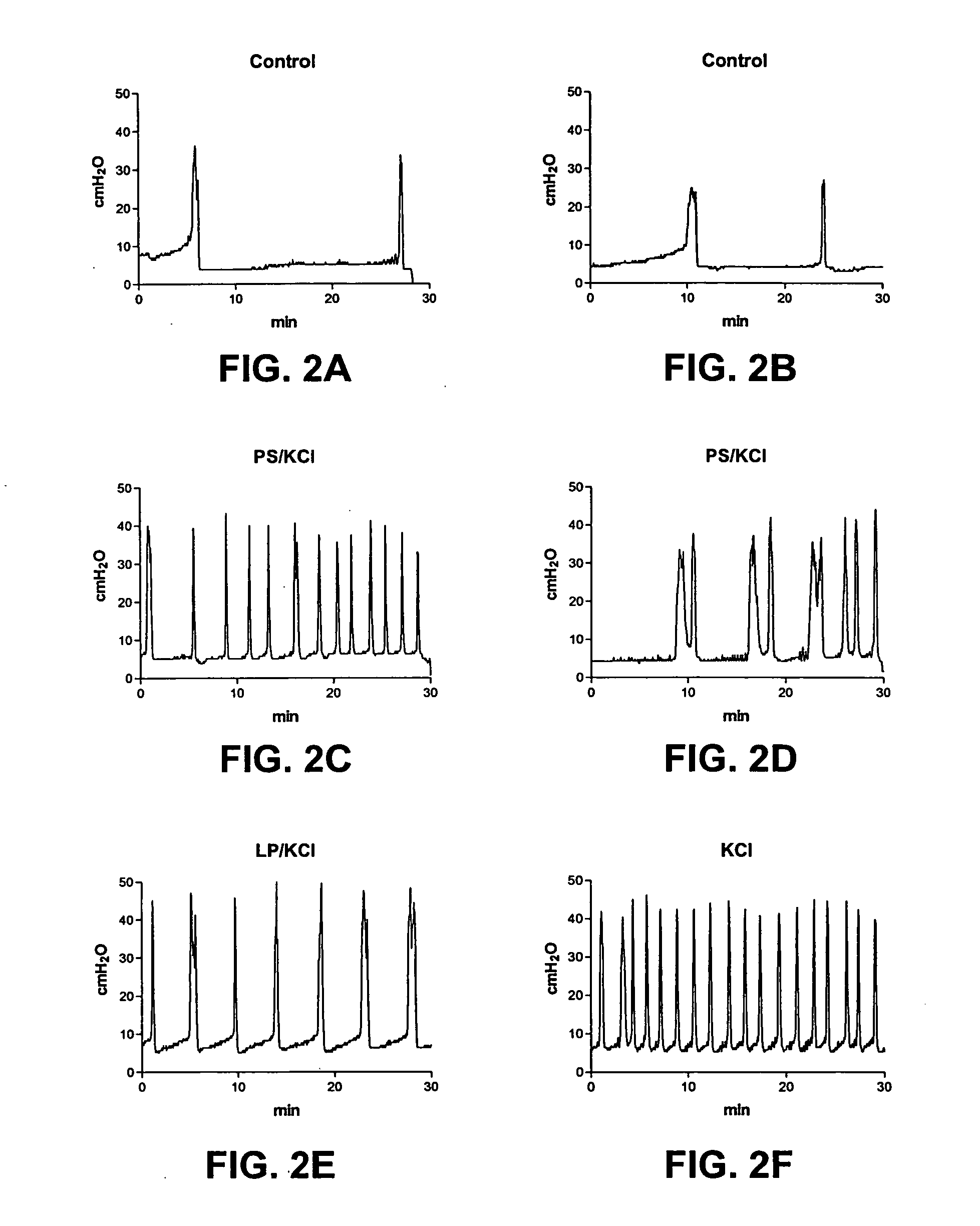
Patents
Literature
933 results about "Capsaicin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat minor aches and pains of the muscles/joints (e.g., arthritis, backache, sprains).
Application of lipid vehicles and use for drug delivery
InactiveUS7063860B2Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticAnticarcinogenCapsaicin
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
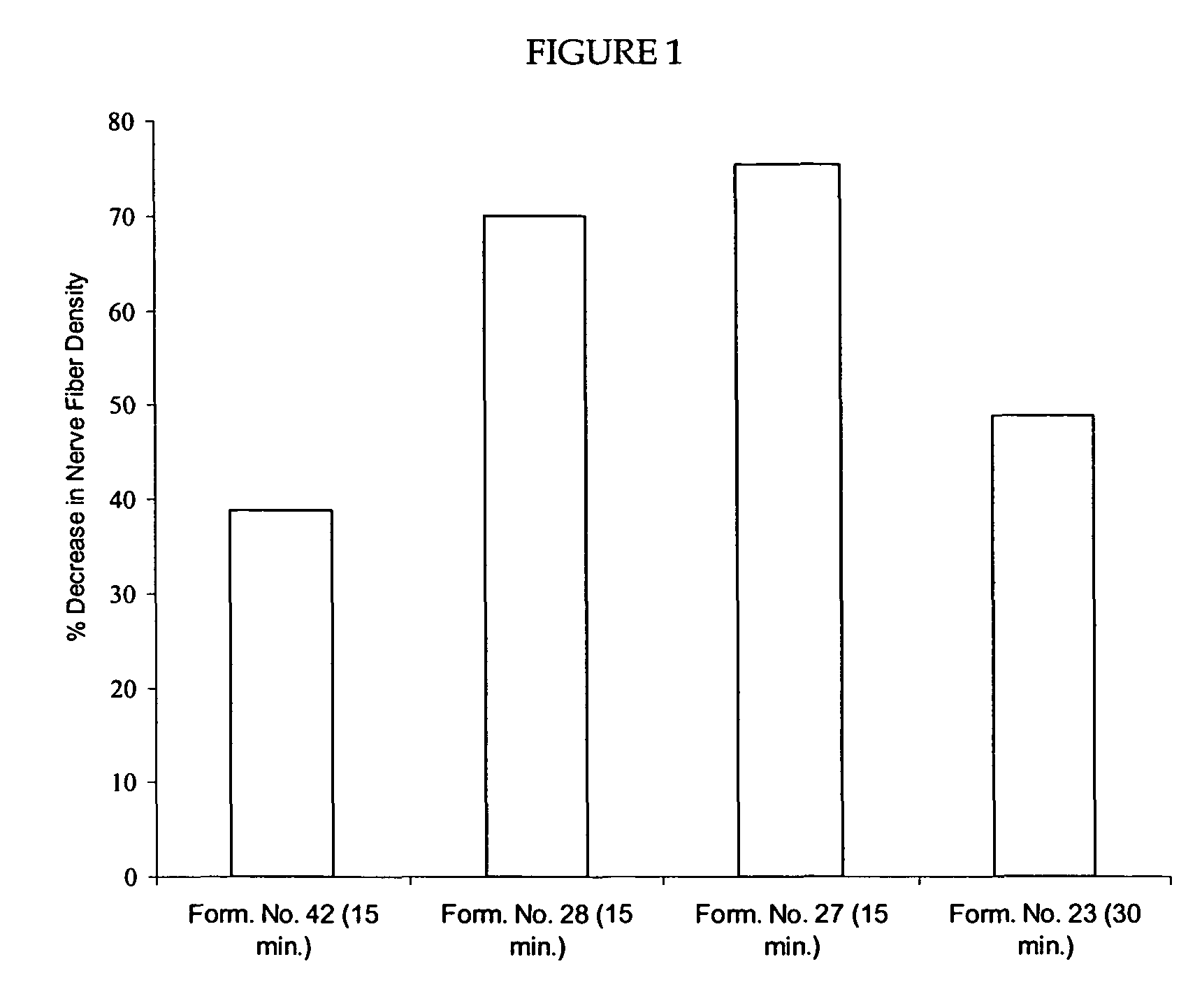
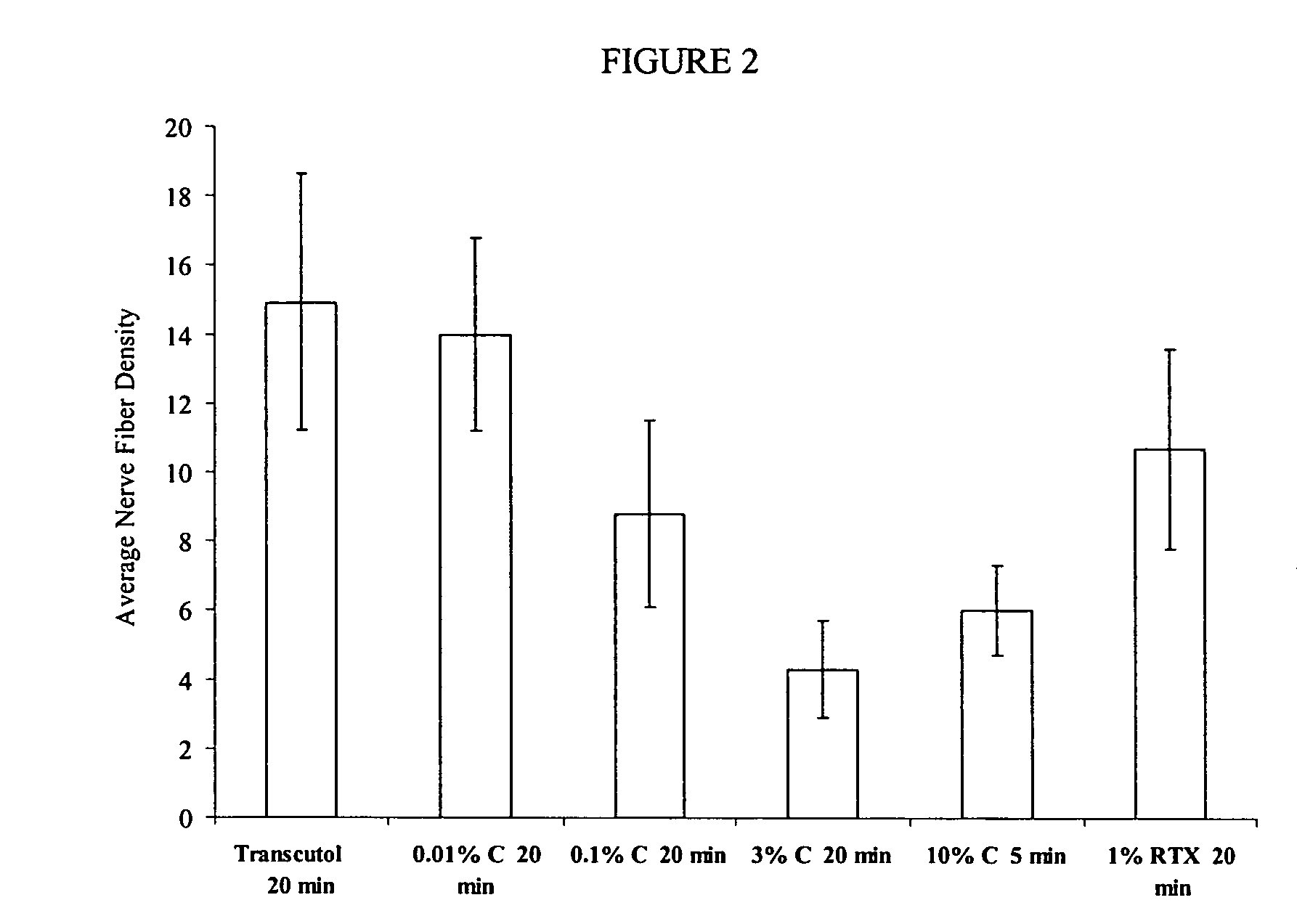
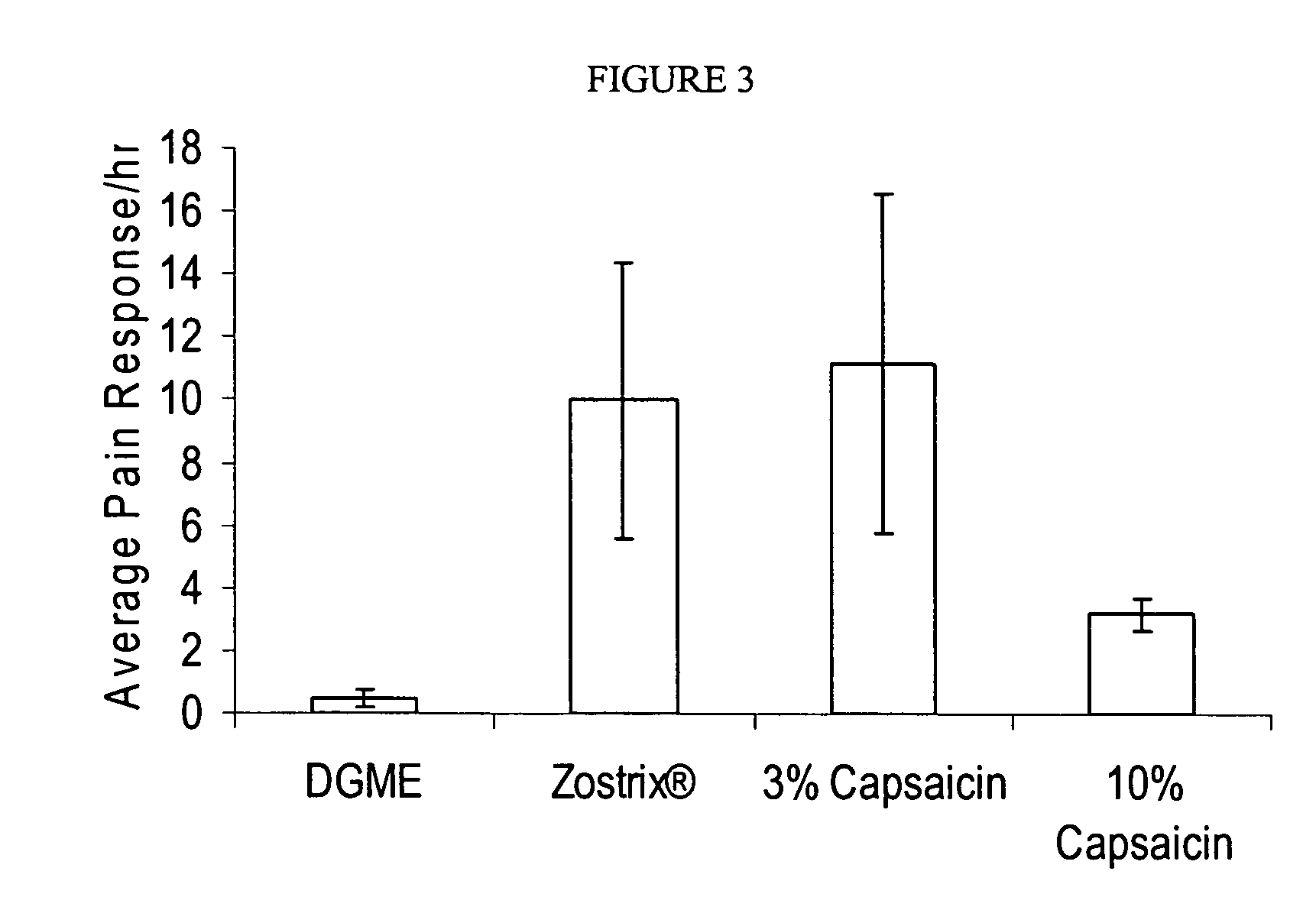
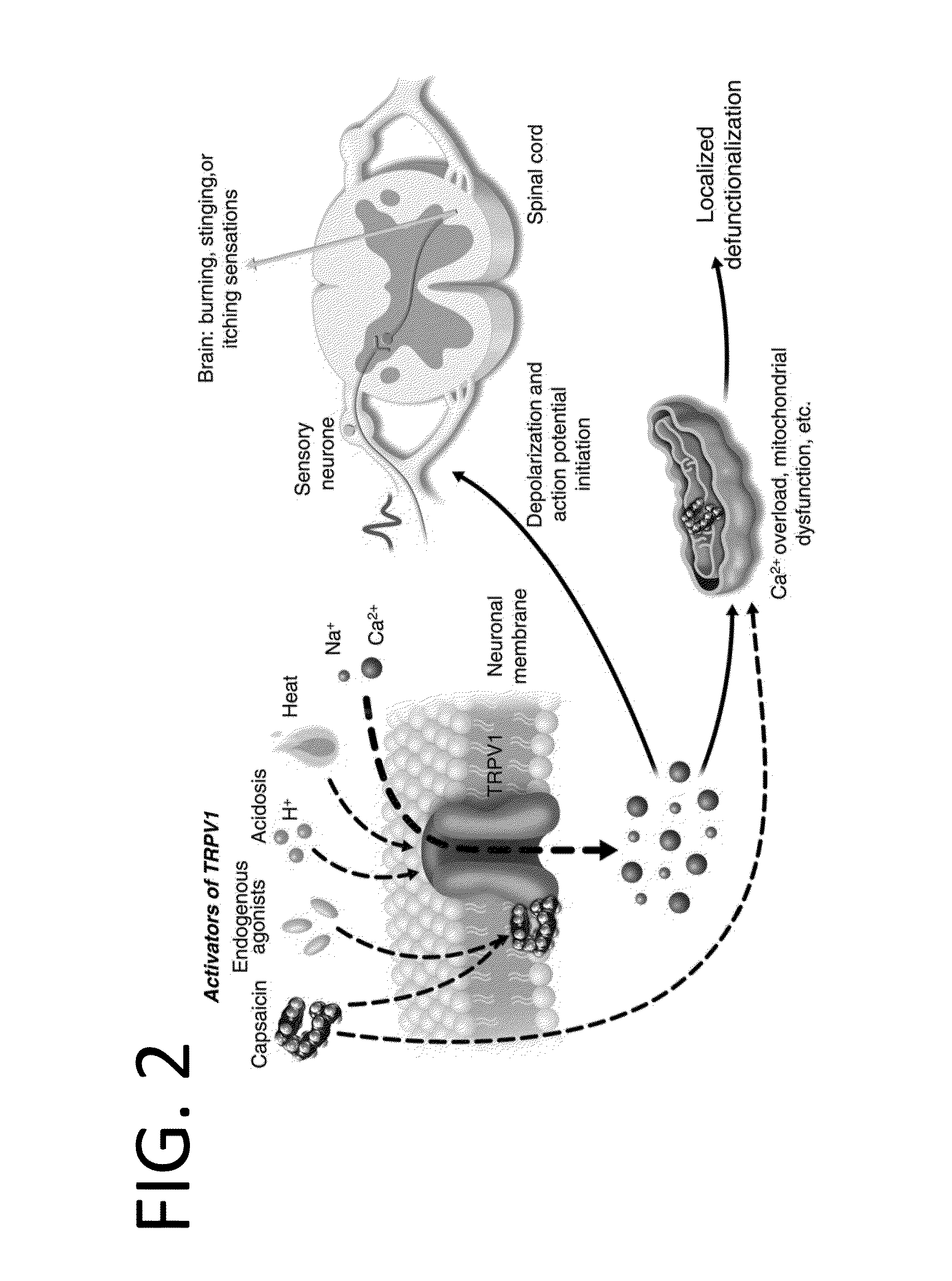
Methods and compositions for administration of TRPV1 agonists
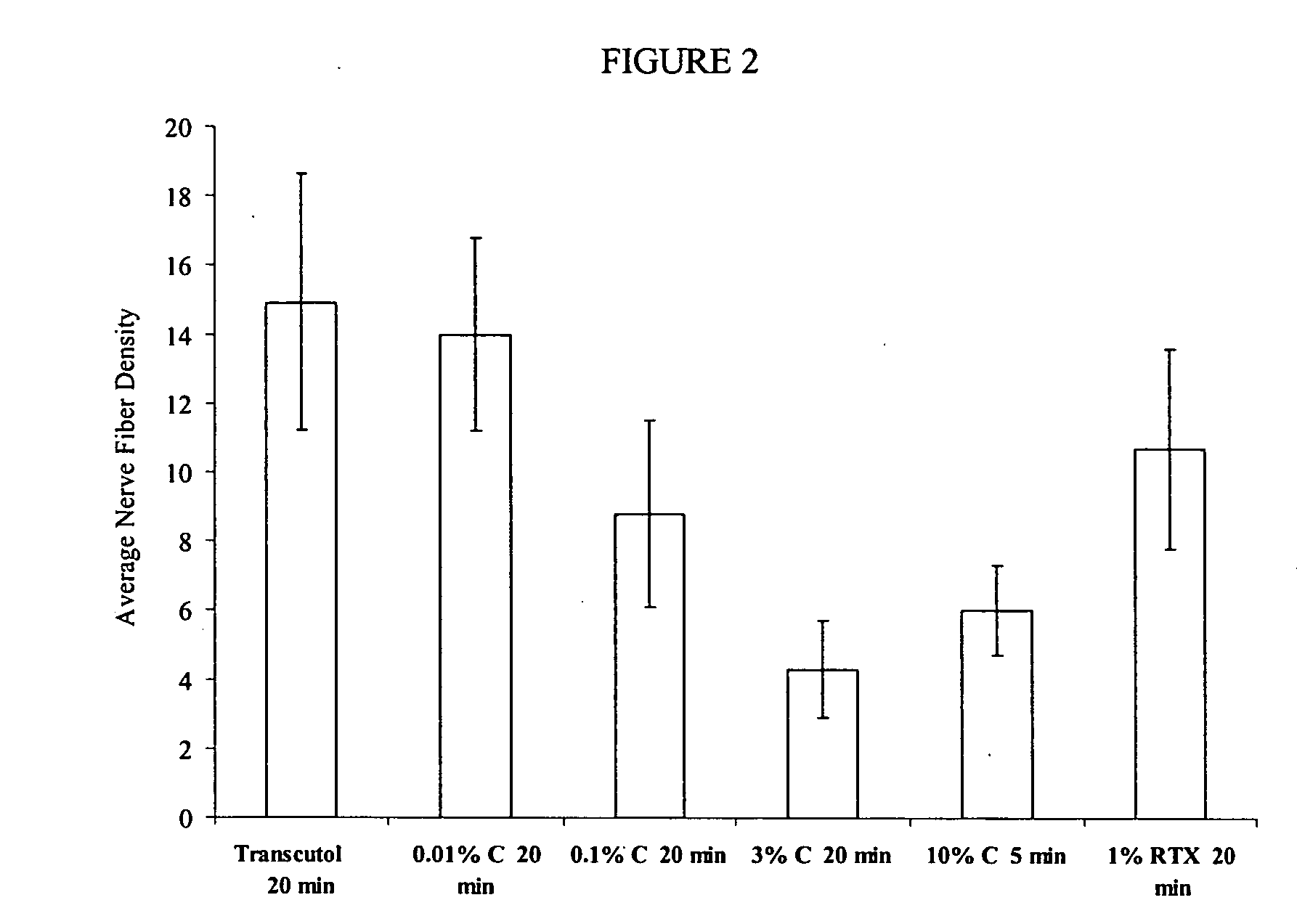
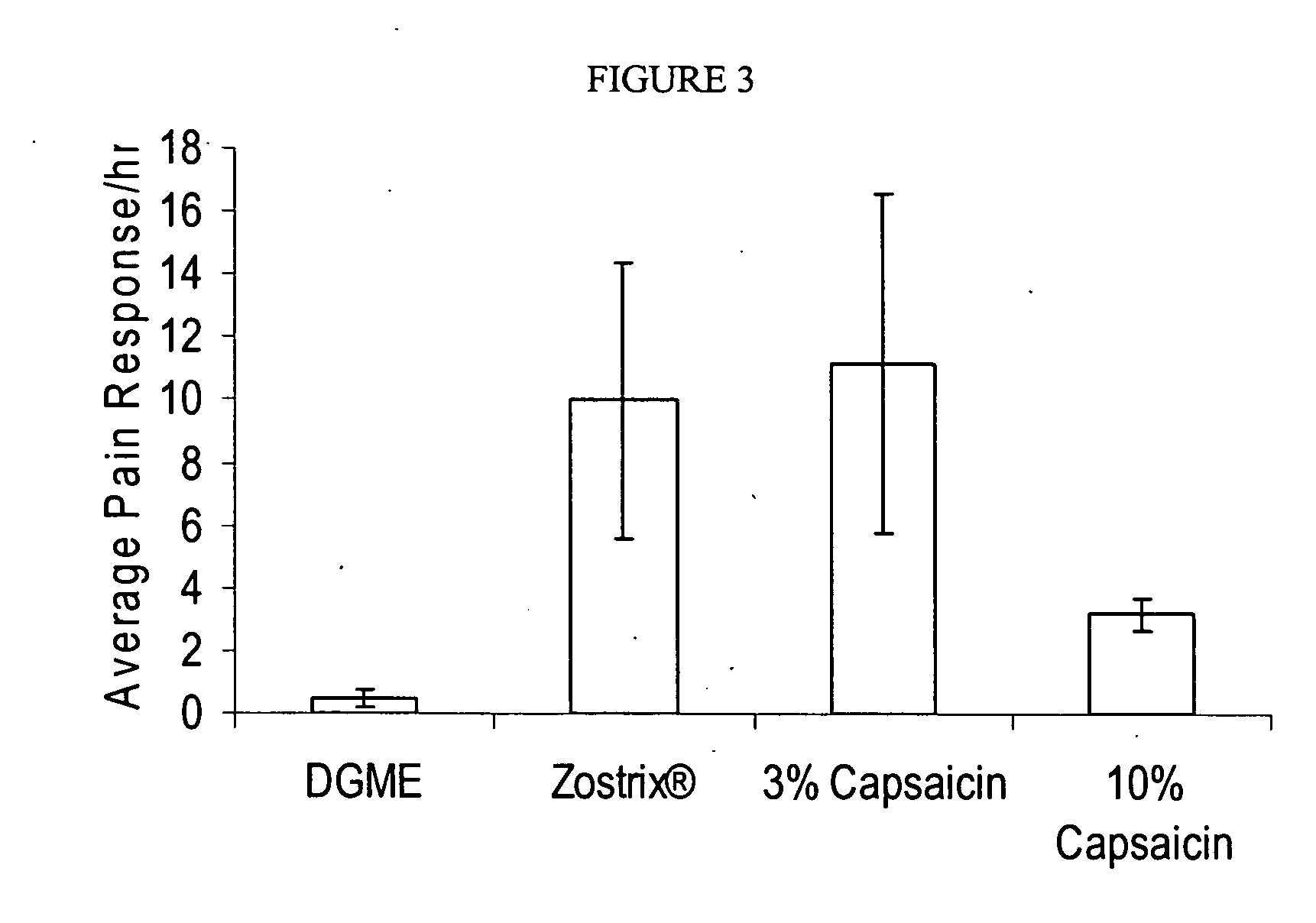
Compositions are provided that contain a TRPV1 agonist, such as capsaicin, and a solvent system. Topical application of the composition results in rapid delivery of agonist to the dermis and epidermis. Method of using the compositions for reducing nociceptive nerve fiber function in subjects, and for treatment of capsaicin-responsive conditions are also provided.
Owner:GRT US HLDG INC
Diterpene Glycosides as Natural Solubilizers
InactiveUS20110033525A1Improve solubilityRetain activityBiocideHydroxy compound active ingredientsItraconazoleCapsaicin
Several diterpene glycosides (e.g., rubusoside, rebaudioside, steviol monoside and stevioside) were discovered to enhance the solubility of a number of pharmaceutically and medicinally important compounds, including but not limited to, paclitaxel, camptothecin, curcumin, tanshinone HA, capsaicin, cyclosporine, erythromycin, nystatin, itraconazole, and celecoxib. The use of the diterpene glycoside rubusoside increased solubility in all tested compounds. The diterpene glycosides are a naturally occurring class of water solubility-enhancing compounds that are non-toxic and that will be useful as new complexing agents or excipients in the pharmaceutical, agricultural (e.g., solubilizing pesticides), cosmetic and food industries. Aqueous solutions by using rubusoside to increase the solubility of otherwise insoluble drugs will have several new routes of administration. In addition, aqueous solutions of therapeutic compounds with rubusoside were shown to retain the known pharmacological activity of the compounds.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
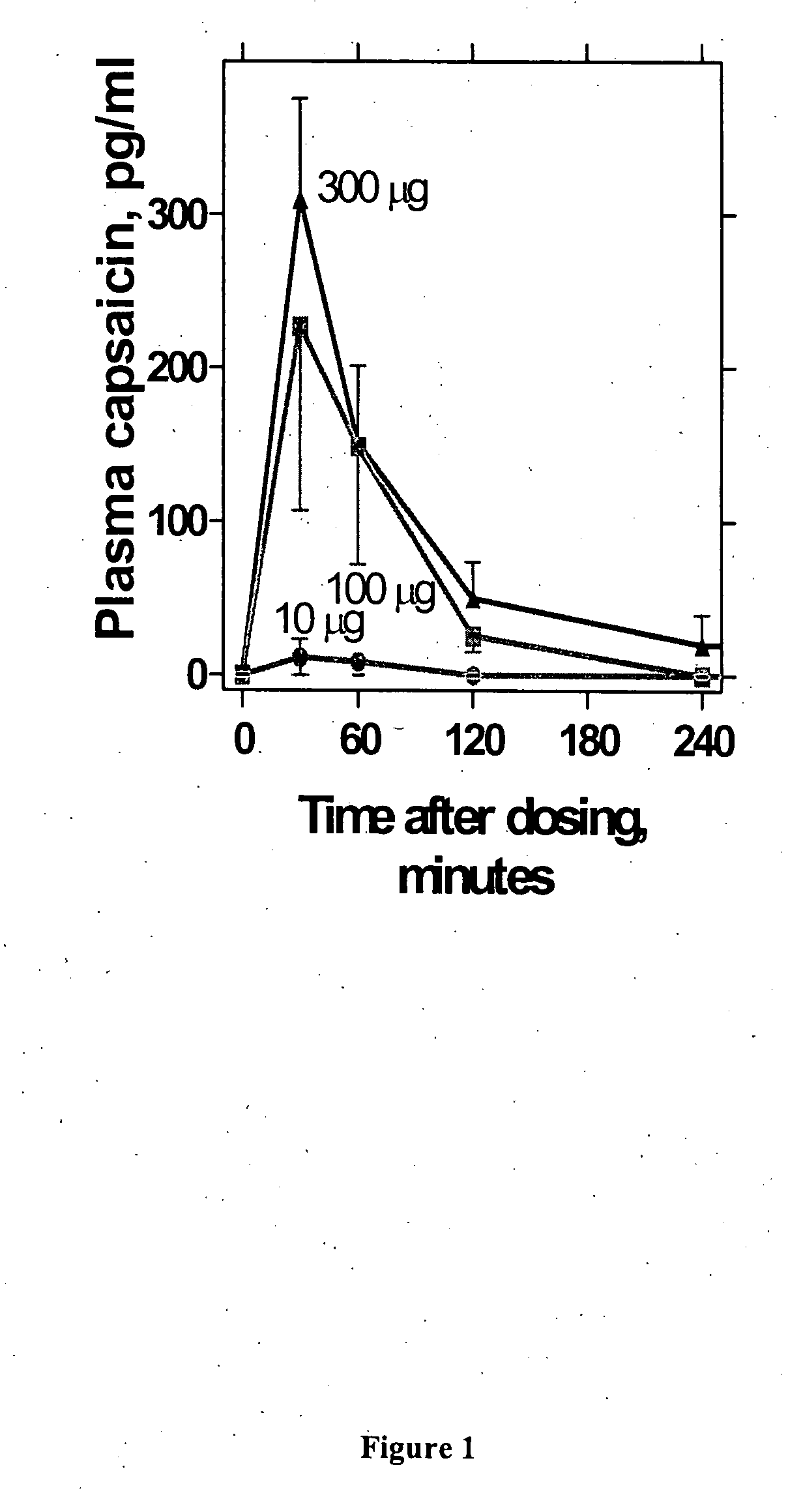
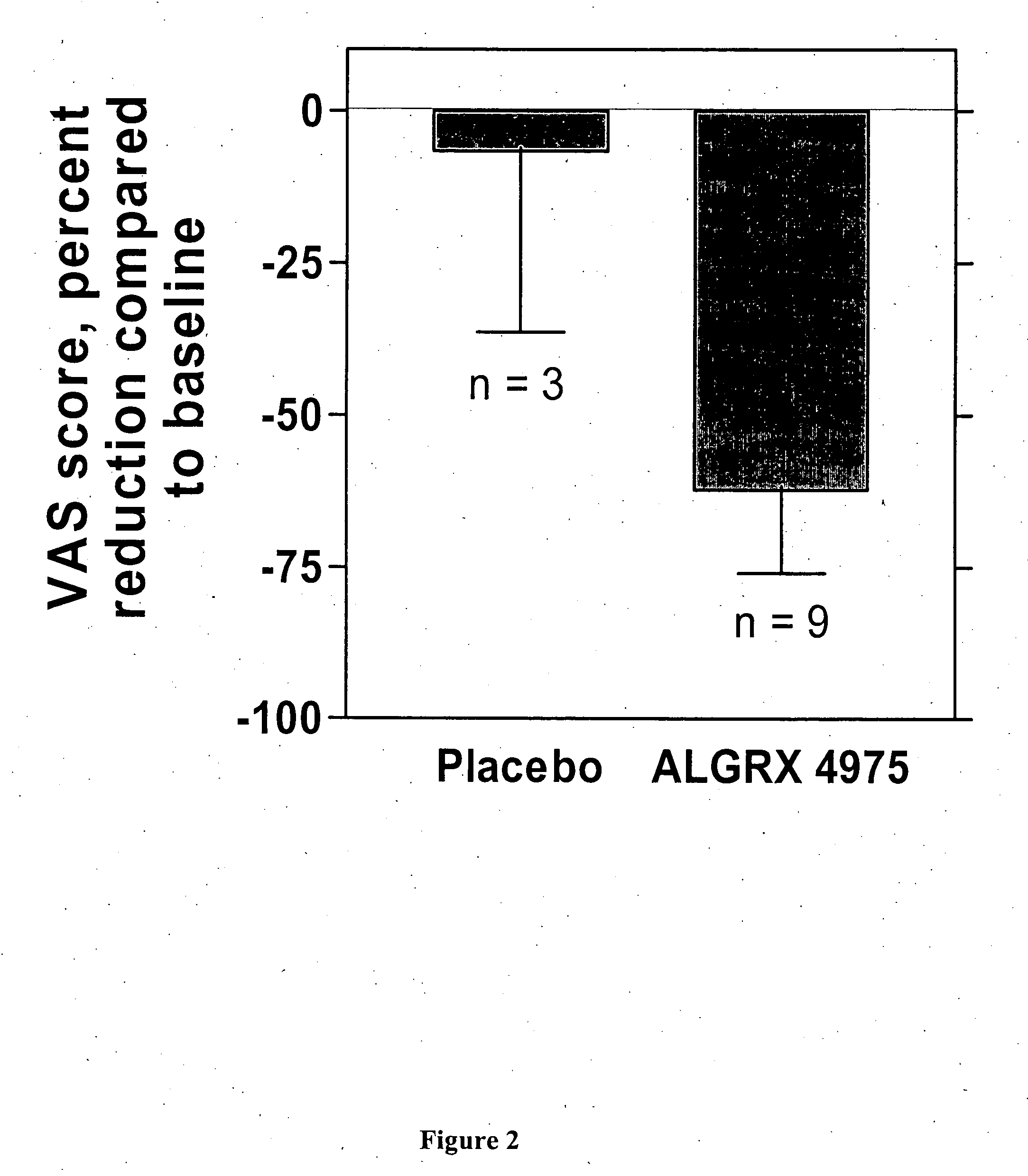
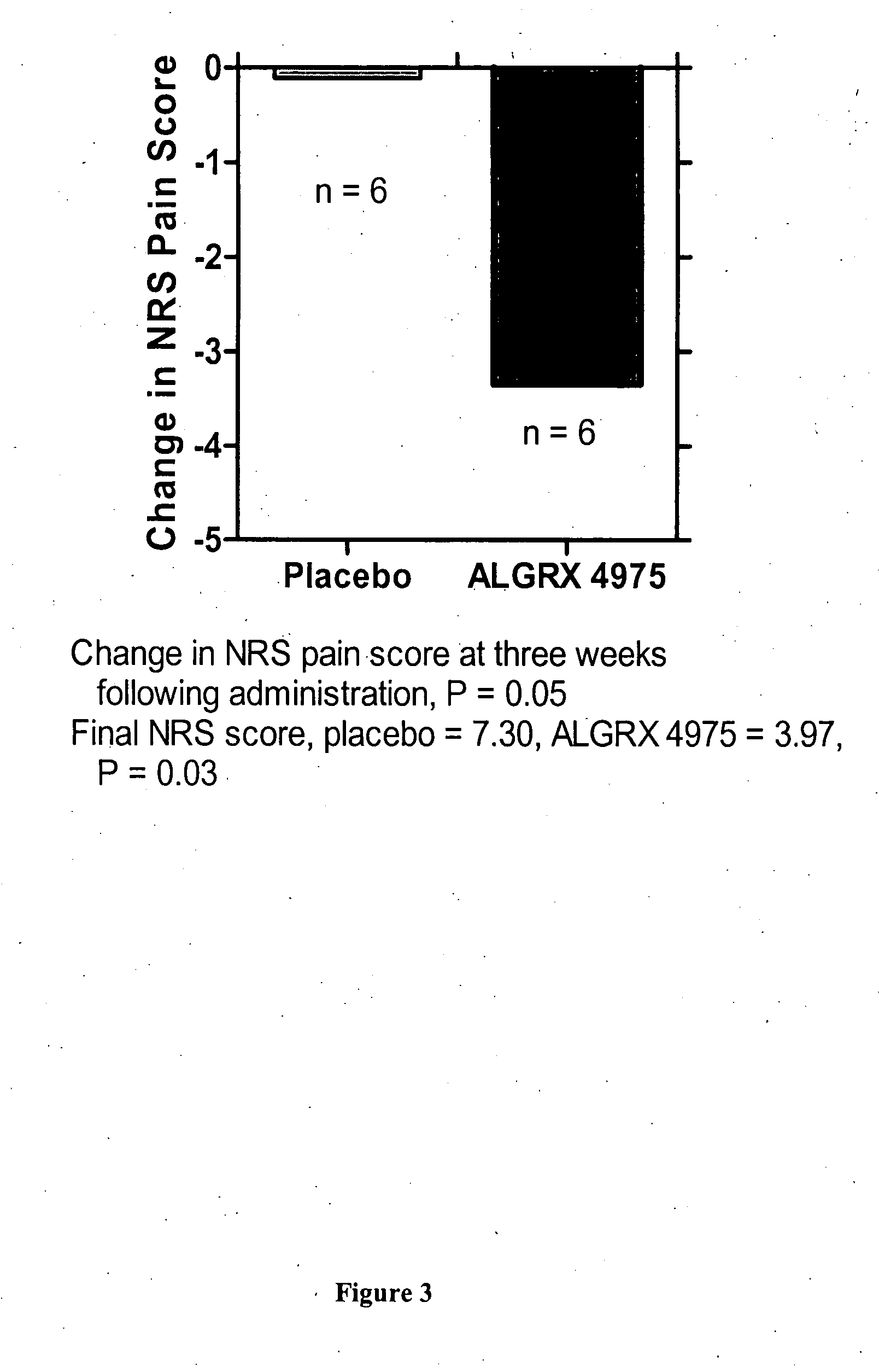
Injectable capsaicin
InactiveUS20050019436A1Reducing and eliminating painMinimizing adverse consequenceBiocideNervous disorderCapsaicinAnesthesia
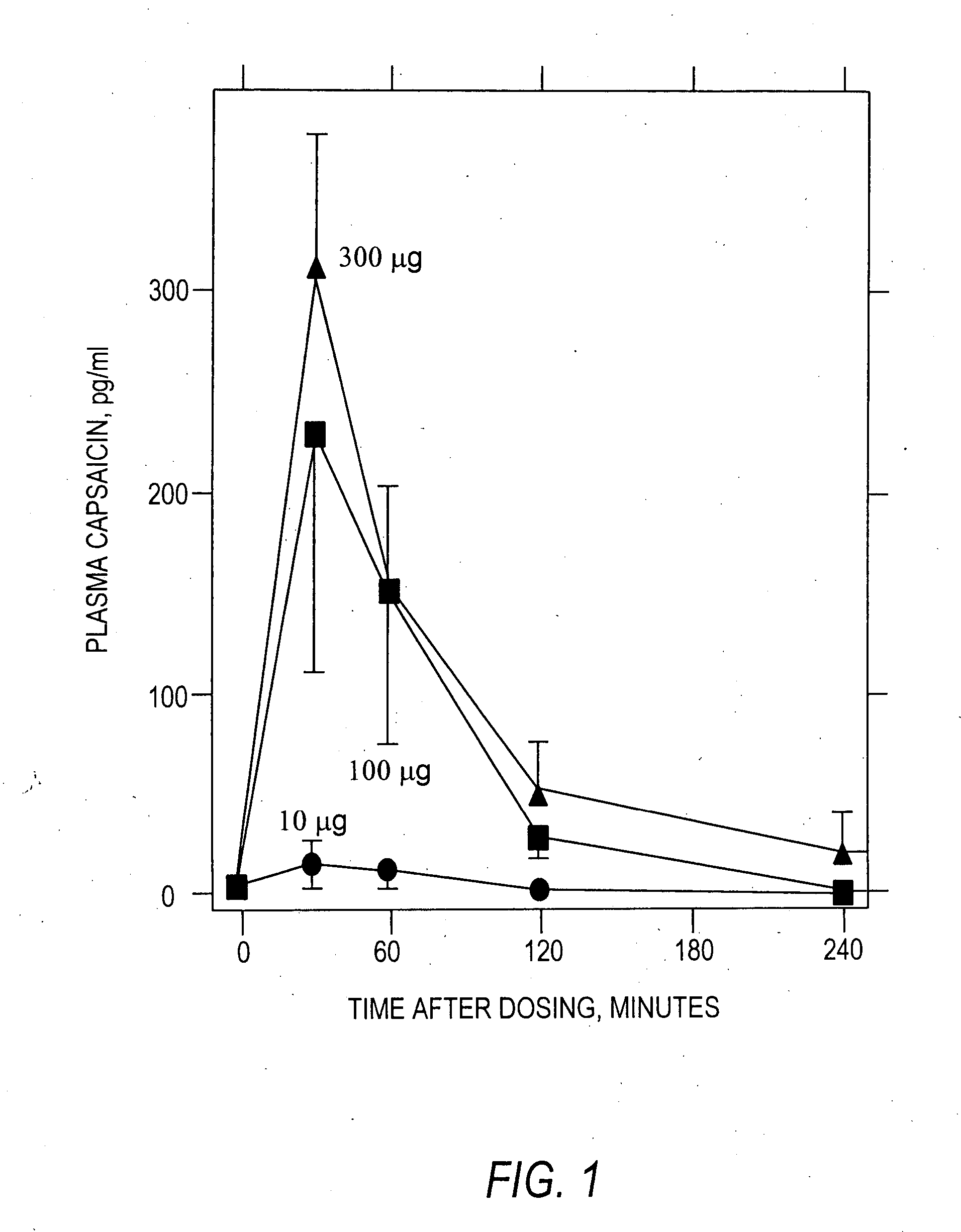
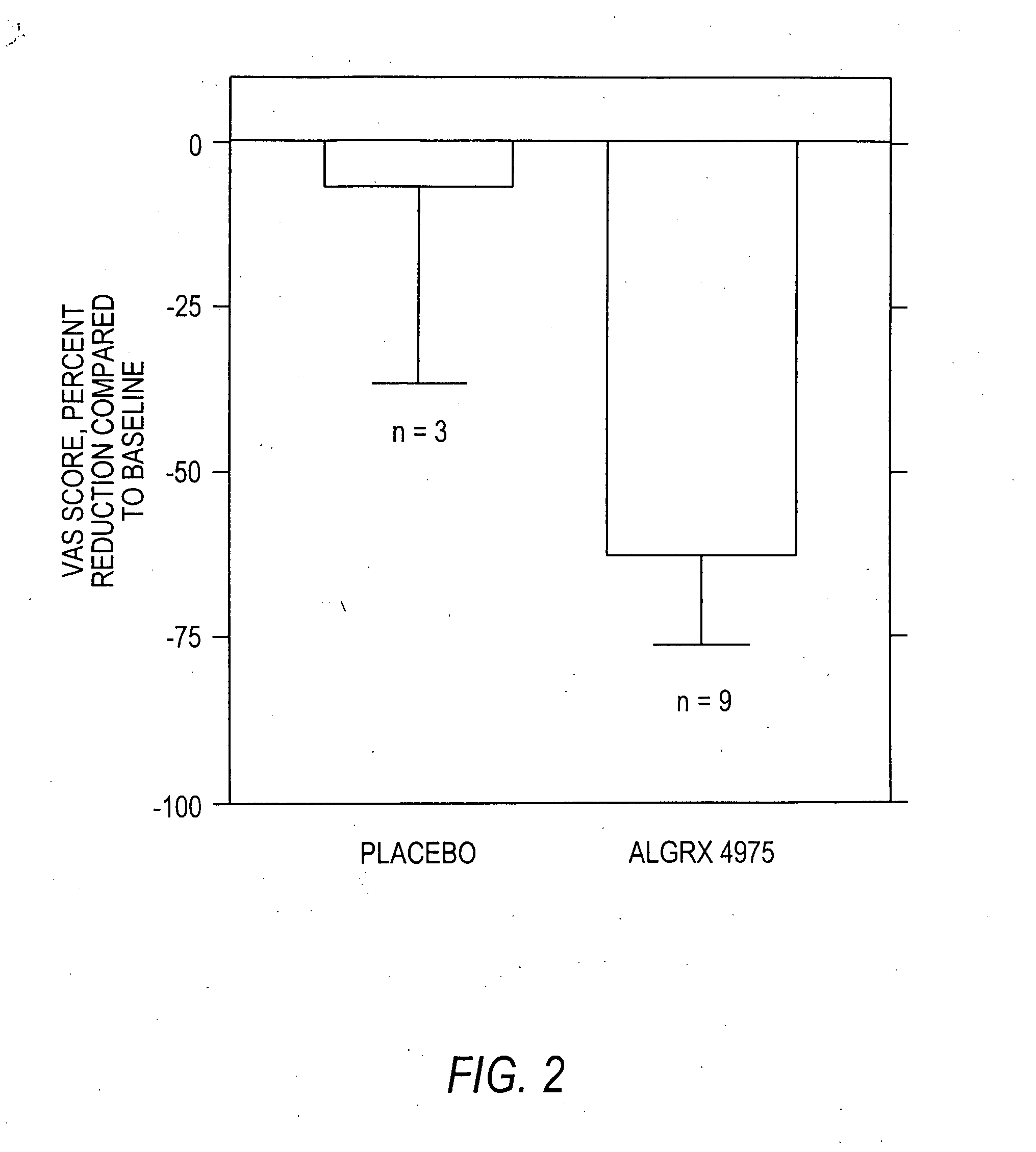
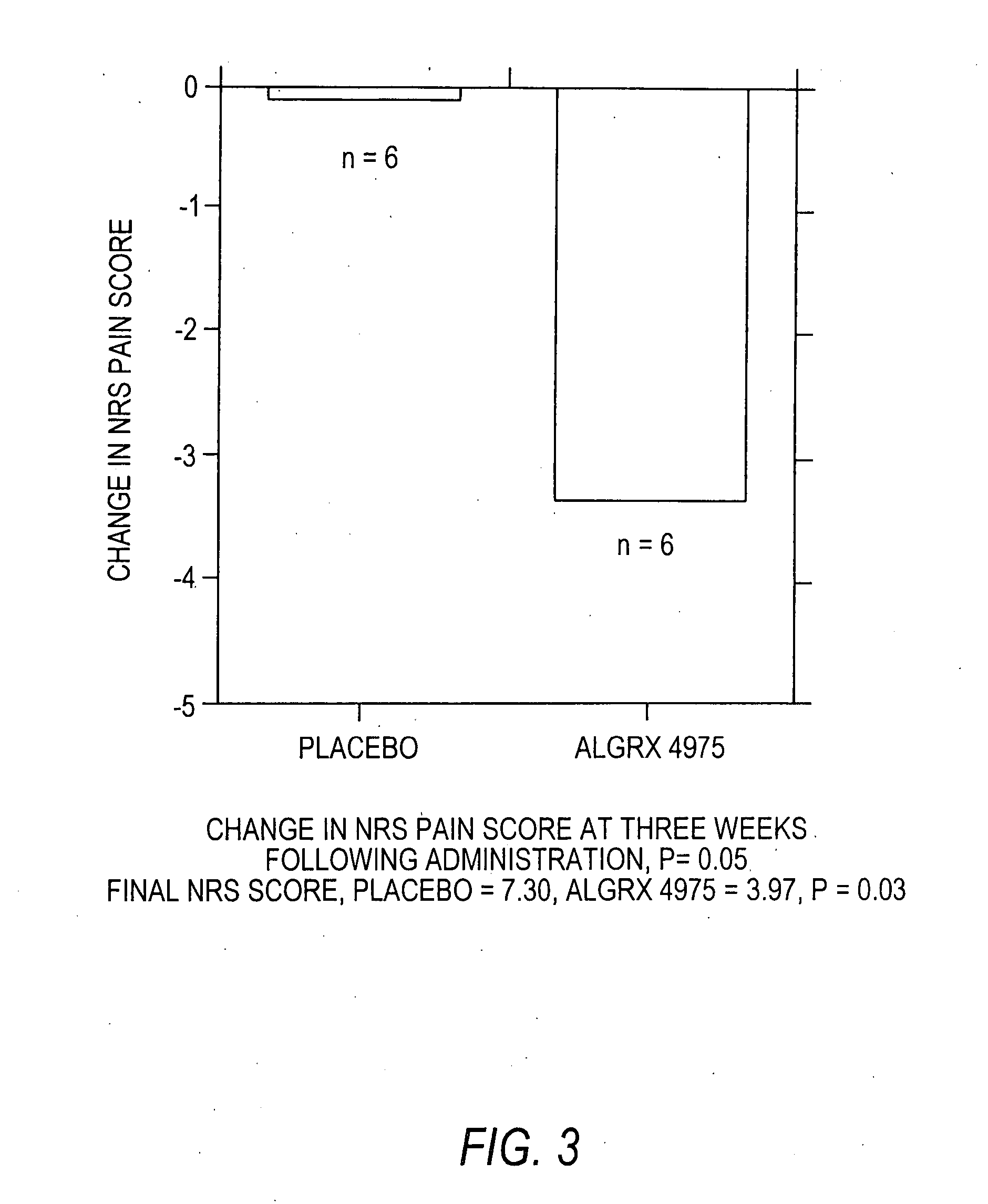
The present invention provides compositions and methods for relieving pain at a site in a human or animal in need thereof by administering at a discrete site in a human or animal in need thereof a dose of capsaicin in an amount effective to denervate a discrete site without eliciting an effect outside the discrete location, the dose of capsaicin ranging from 1 μg to 3000 μg.
Owner:ALGORX PHARMA INC
Novel pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS20080058362A1Reduced plasma concentrationEffective pain managementBiocideAmide active ingredientsDextrorphanSustained release drug
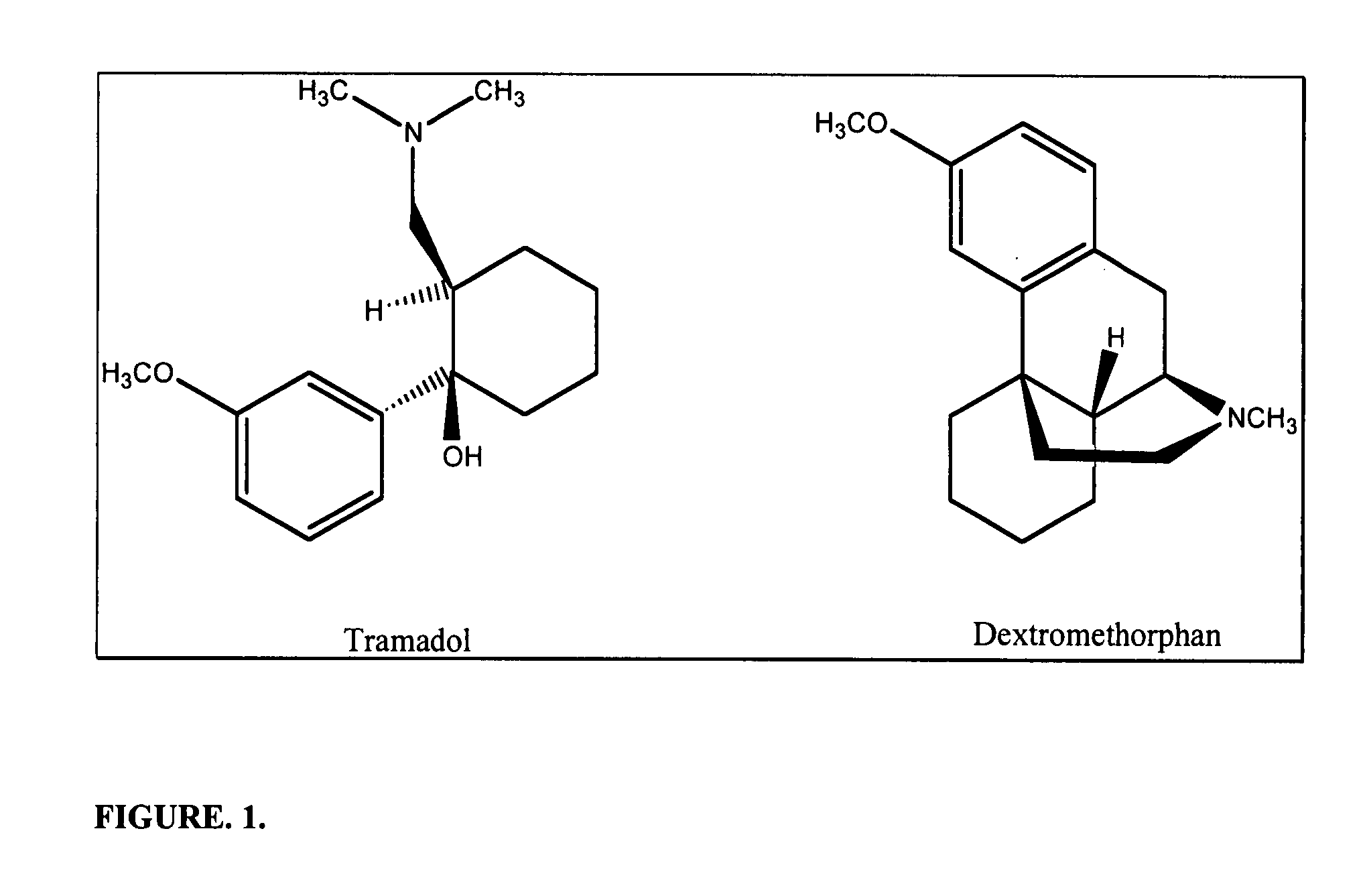
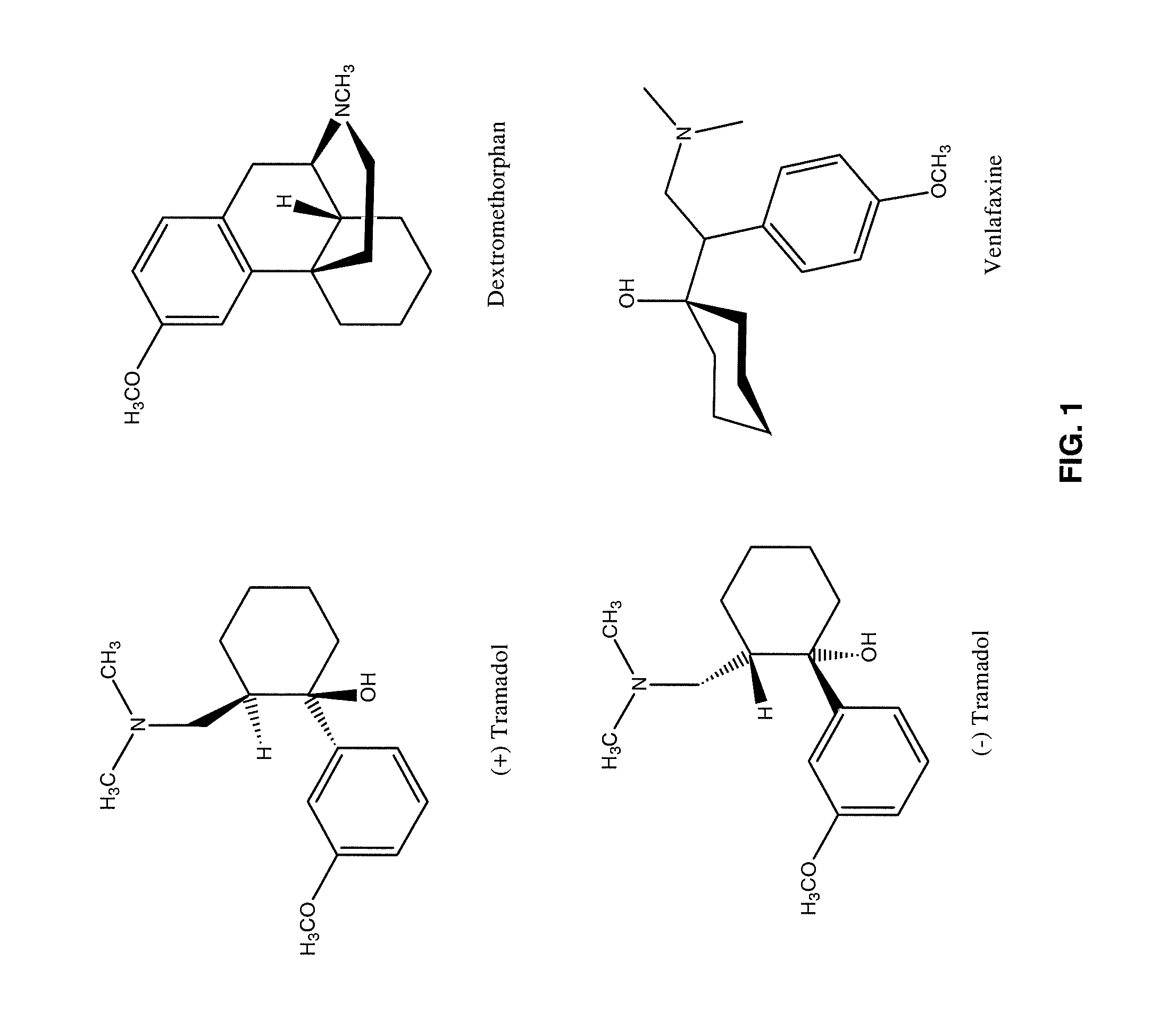
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
Esters of capsaicin for treating pain
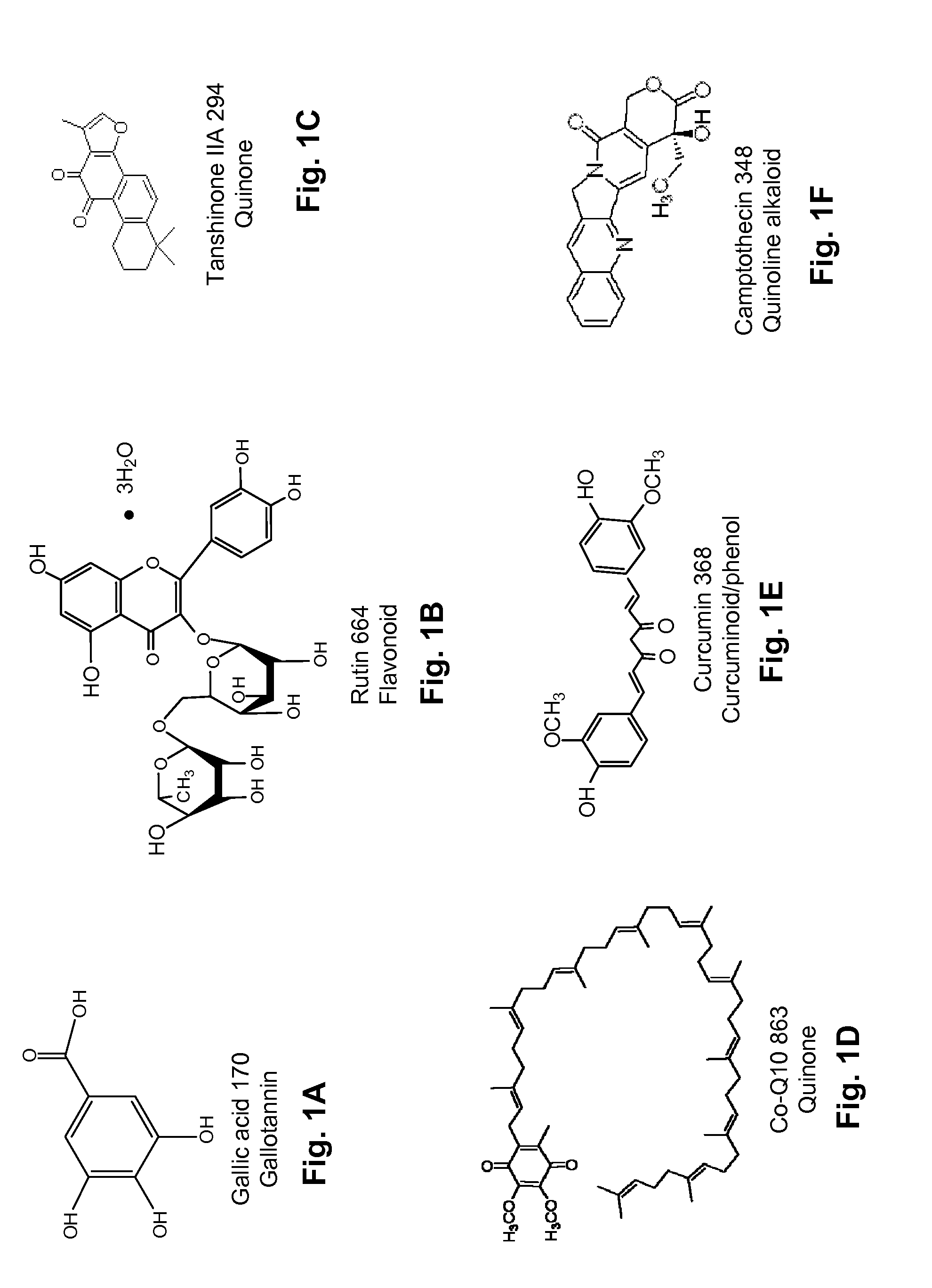
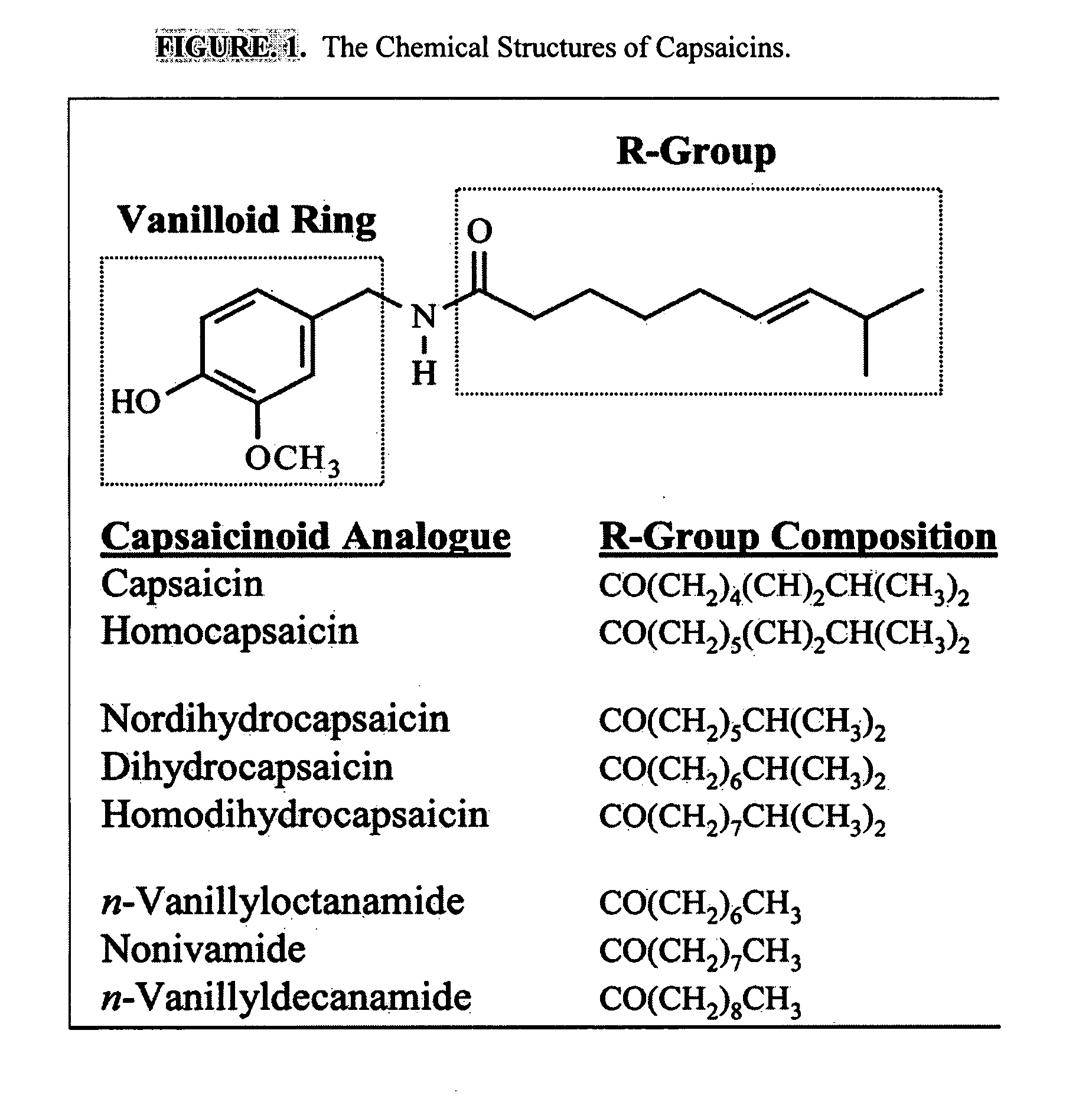
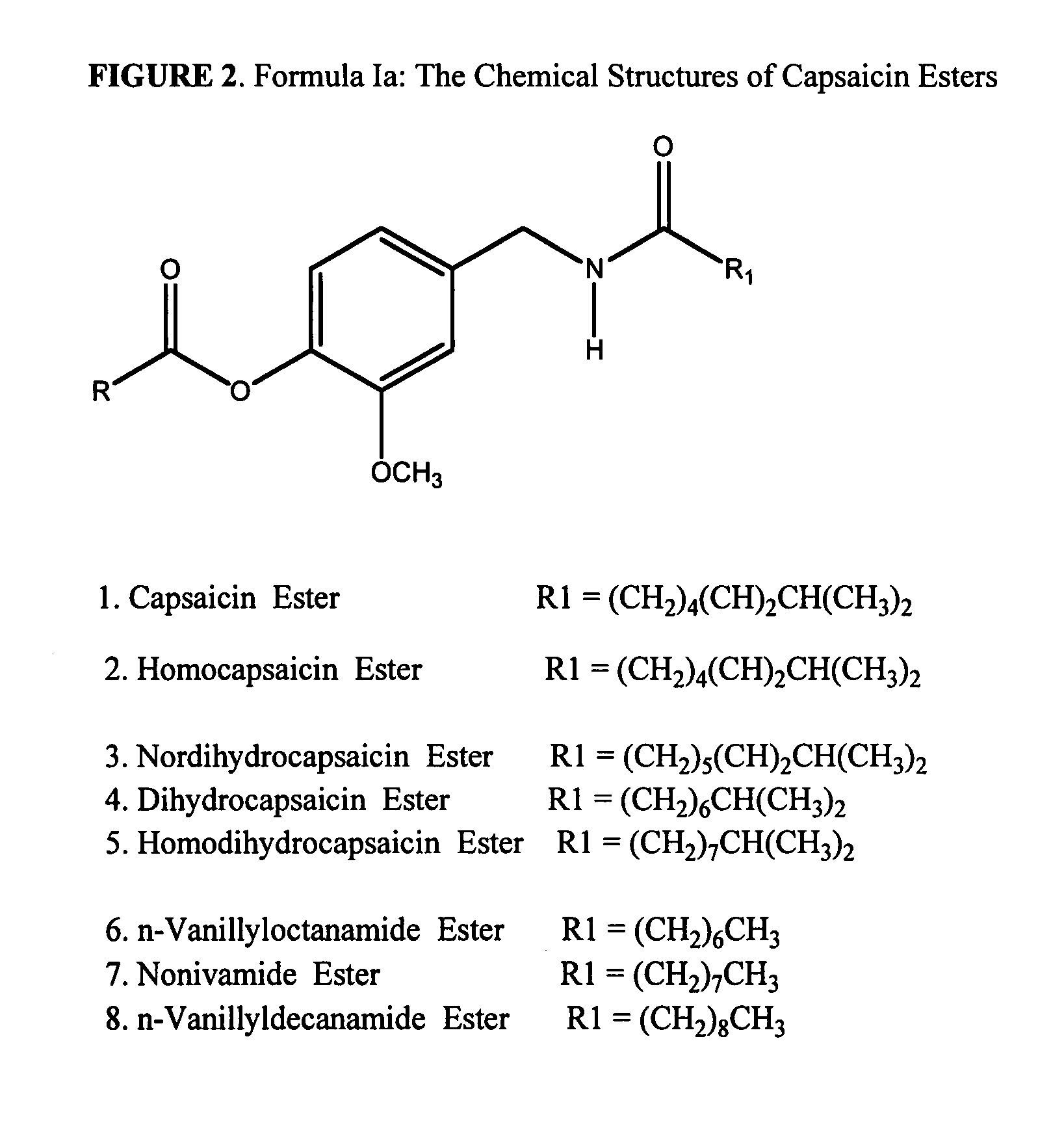
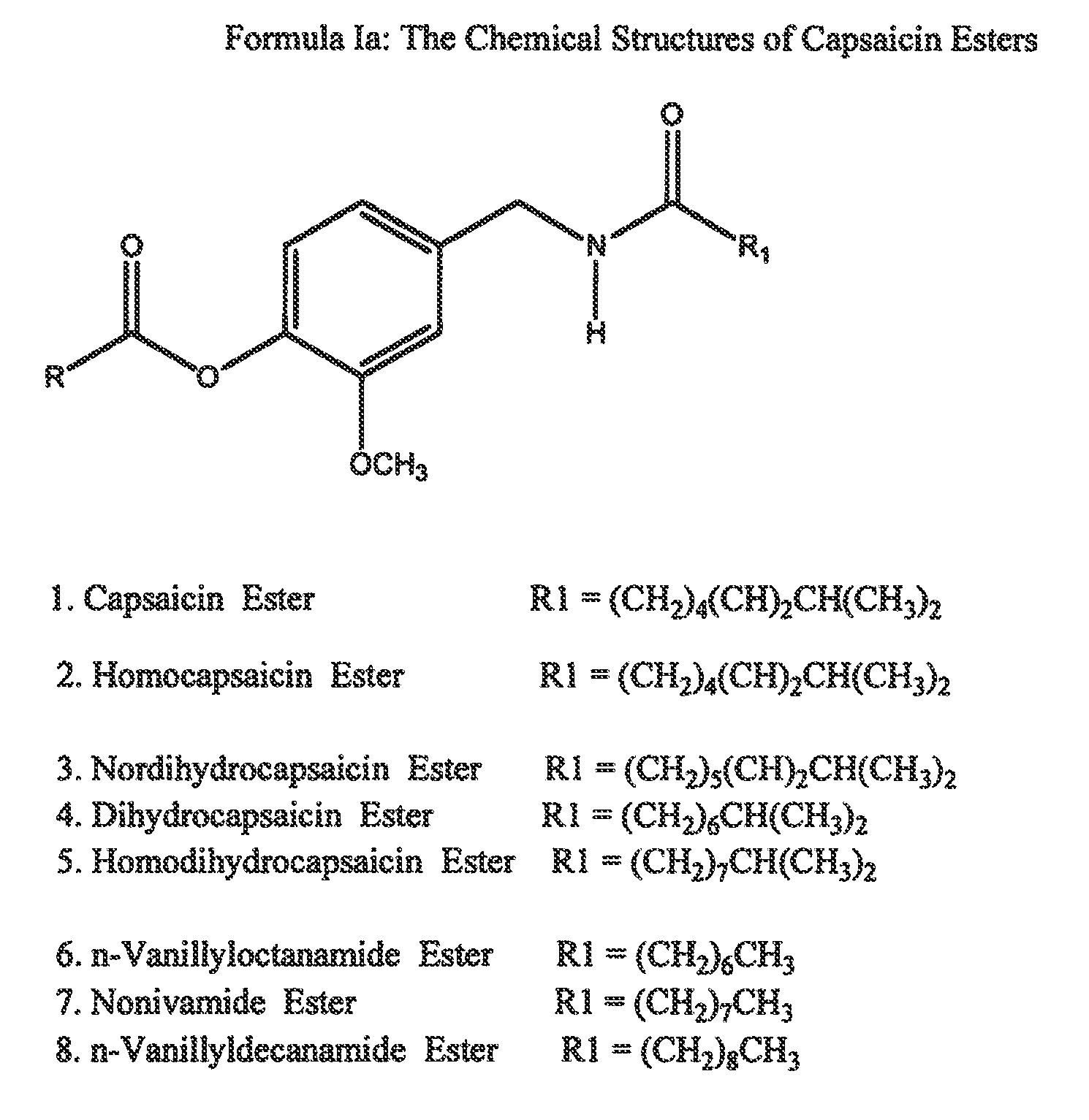
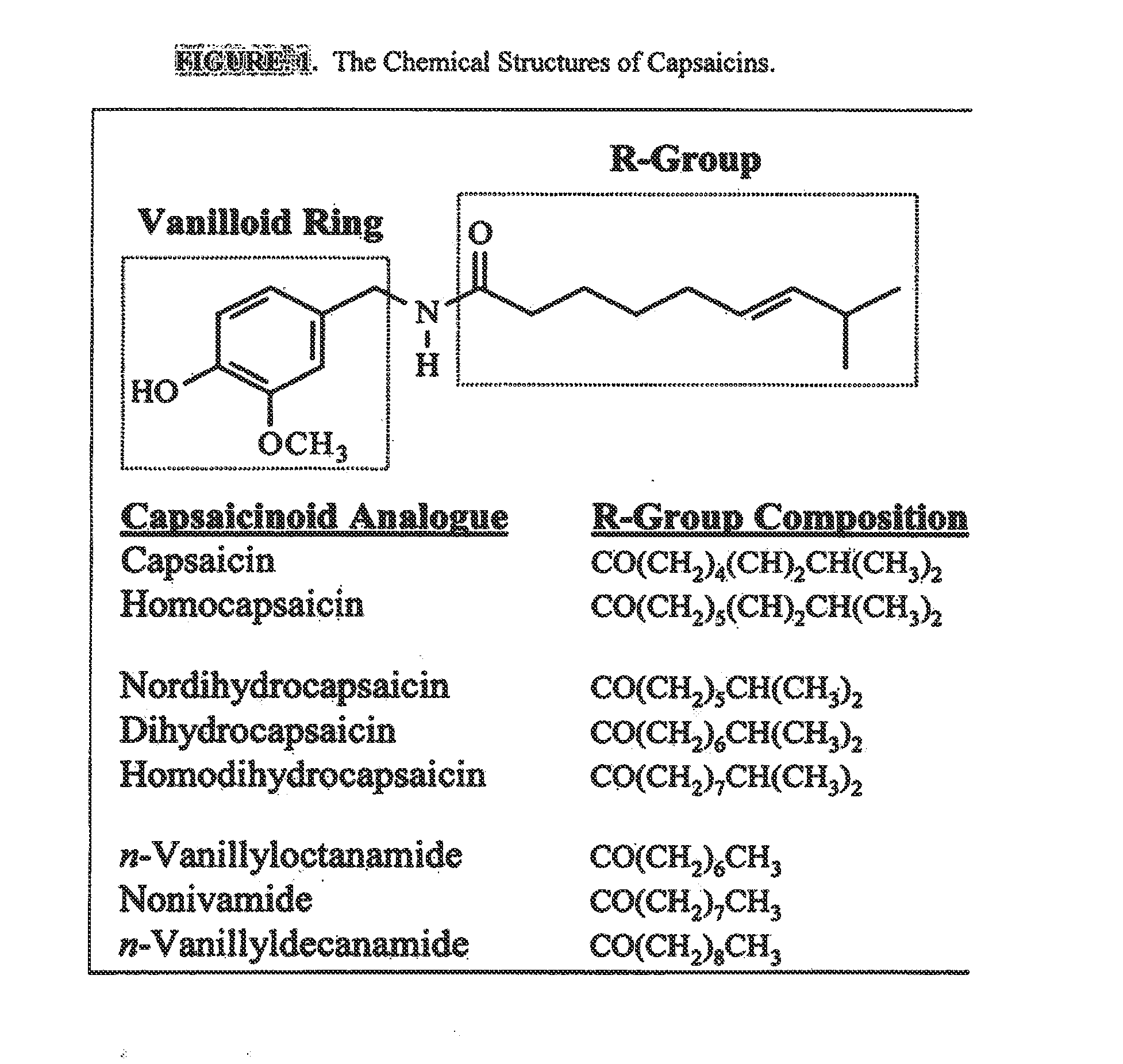
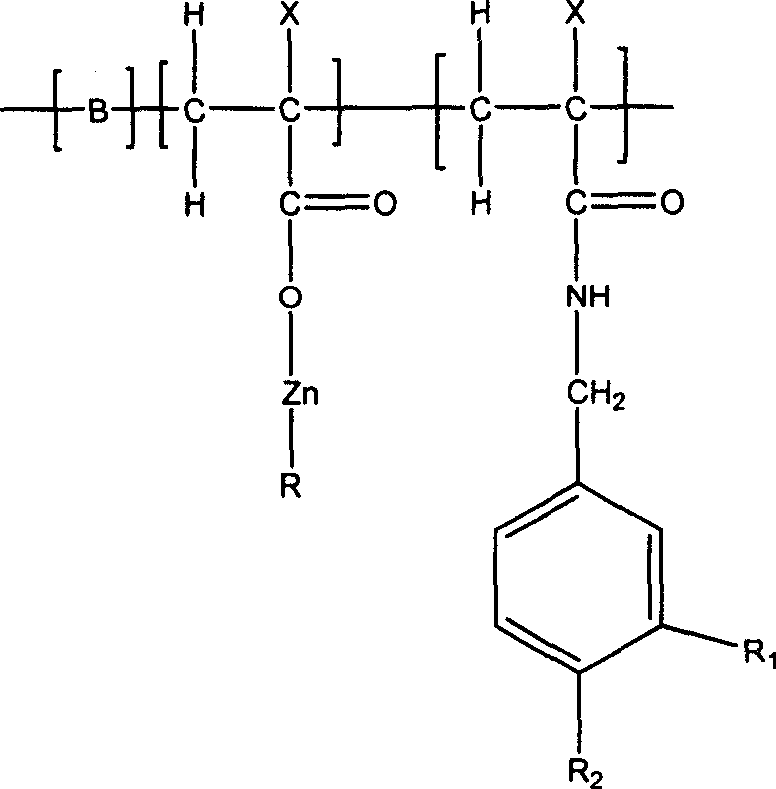
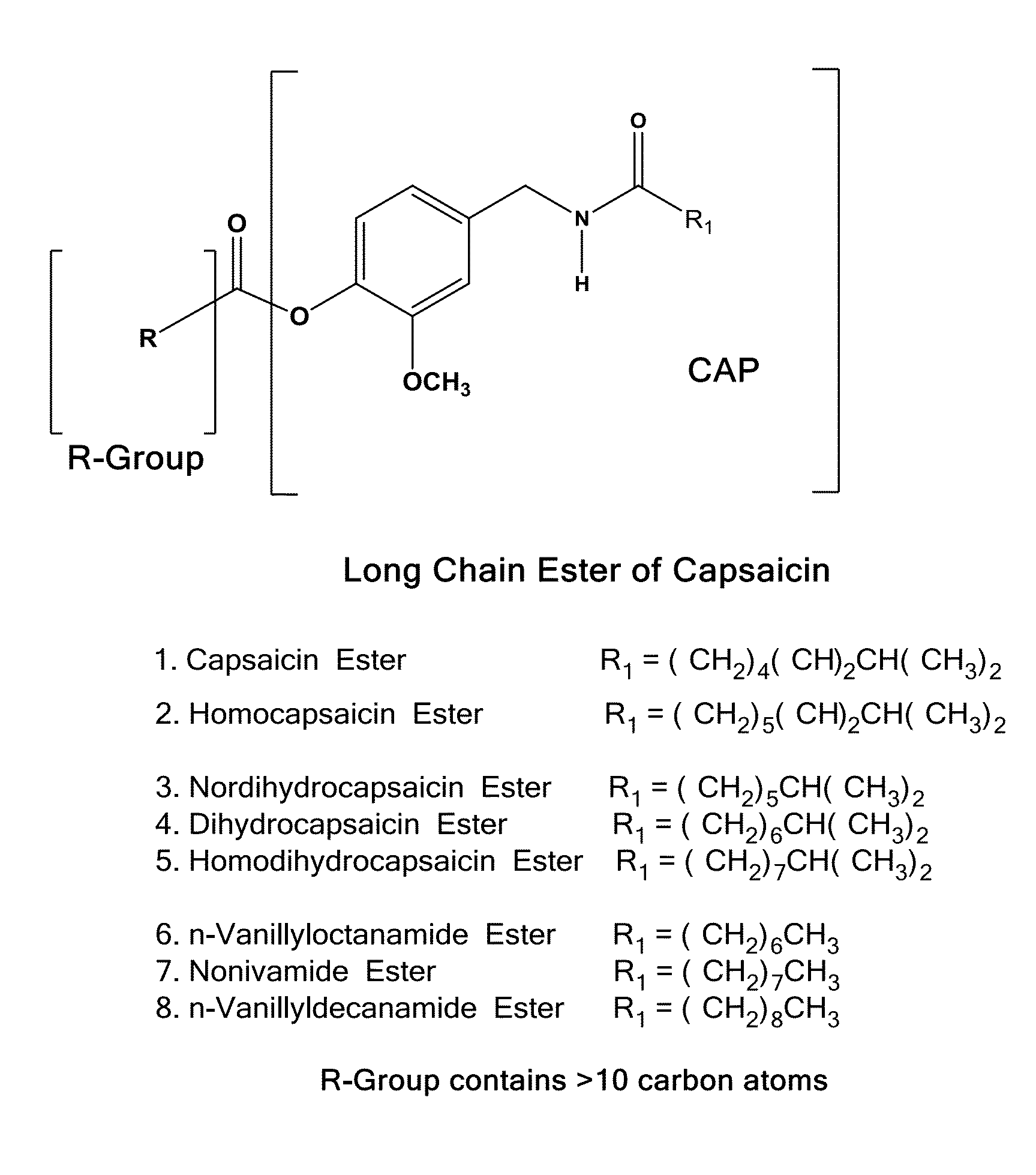
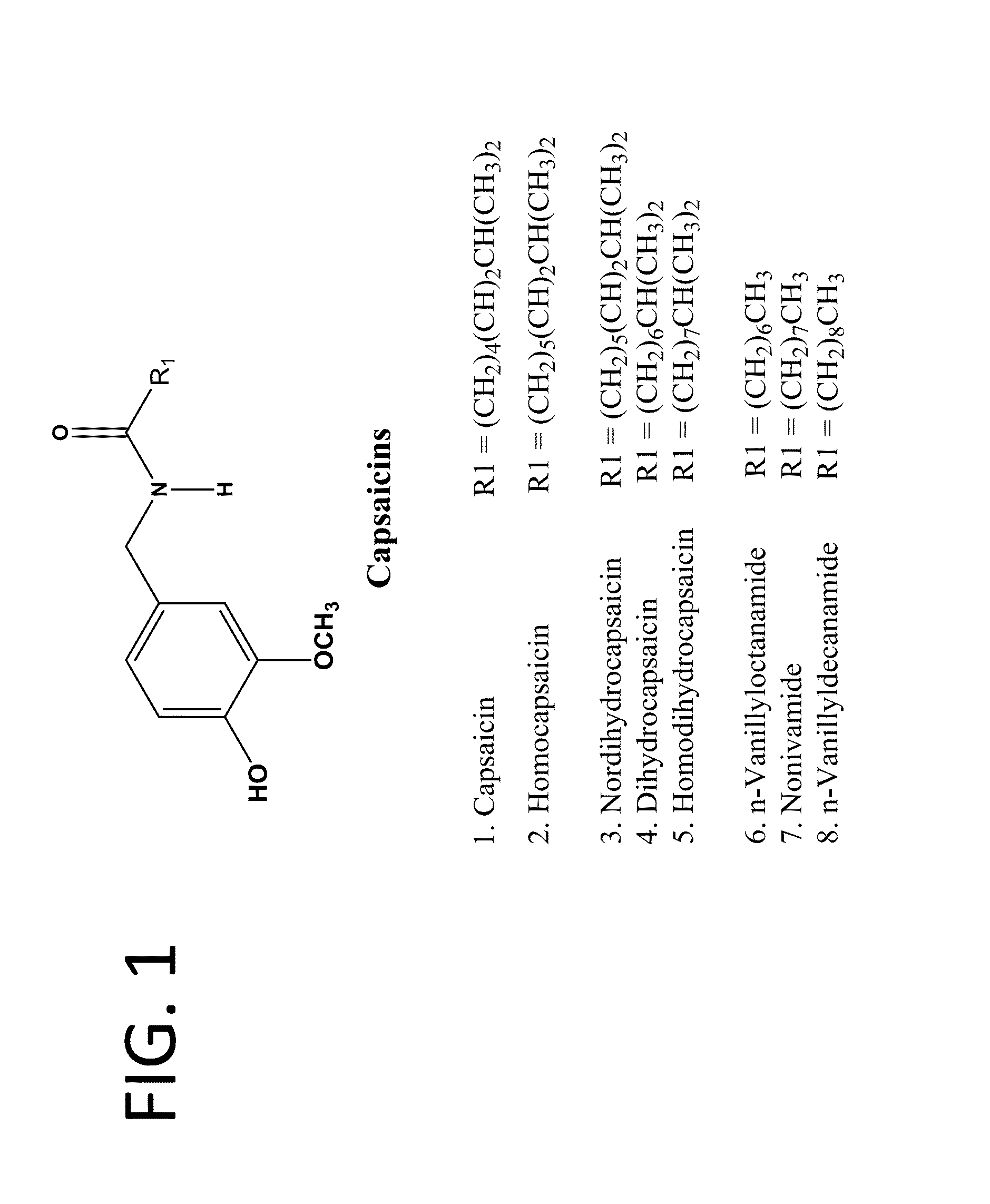
ActiveUS20080020996A1Reduce generationImprove lipophilicityAntibacterial agentsBiocideSolubilityIrritation
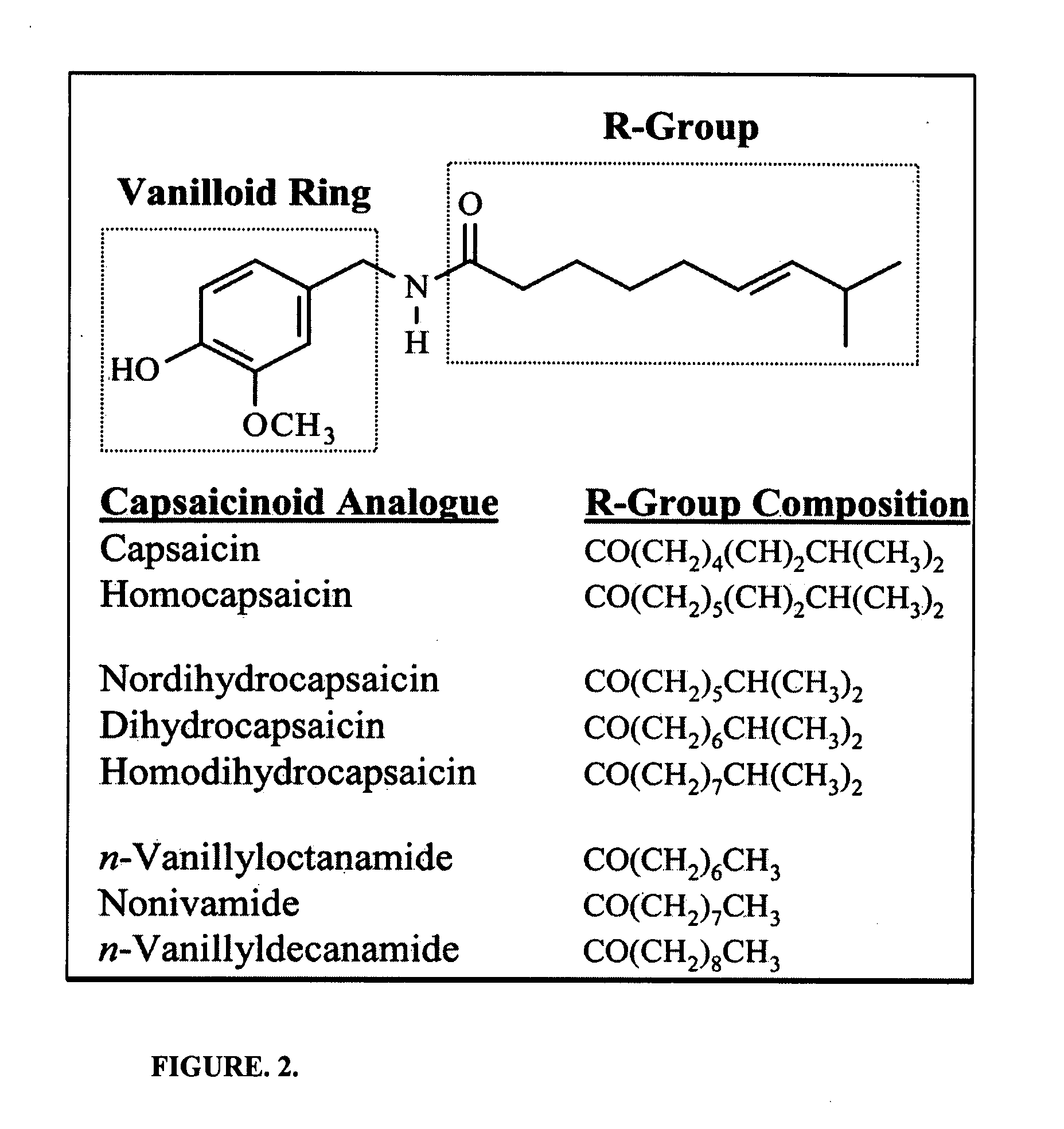
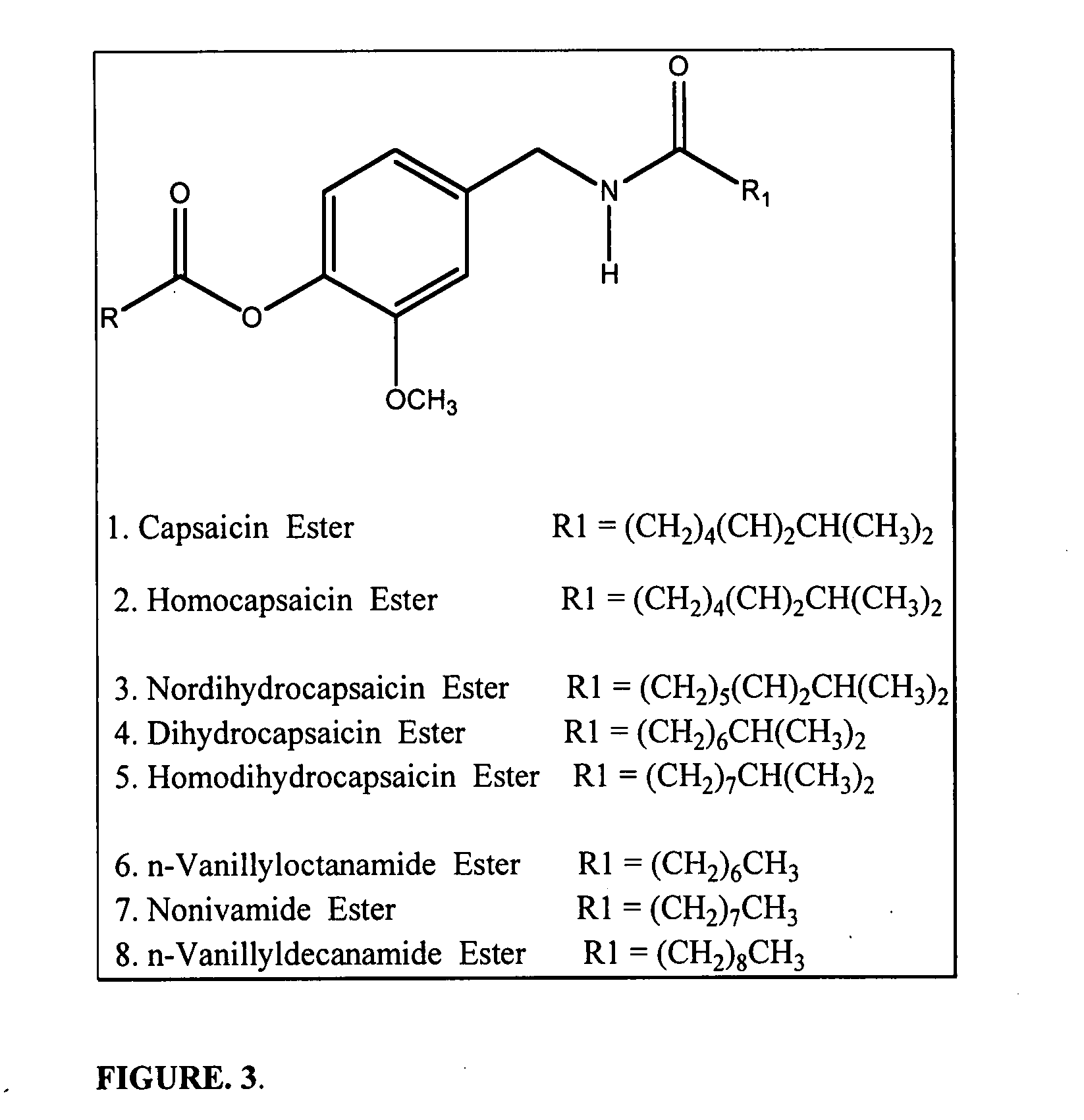
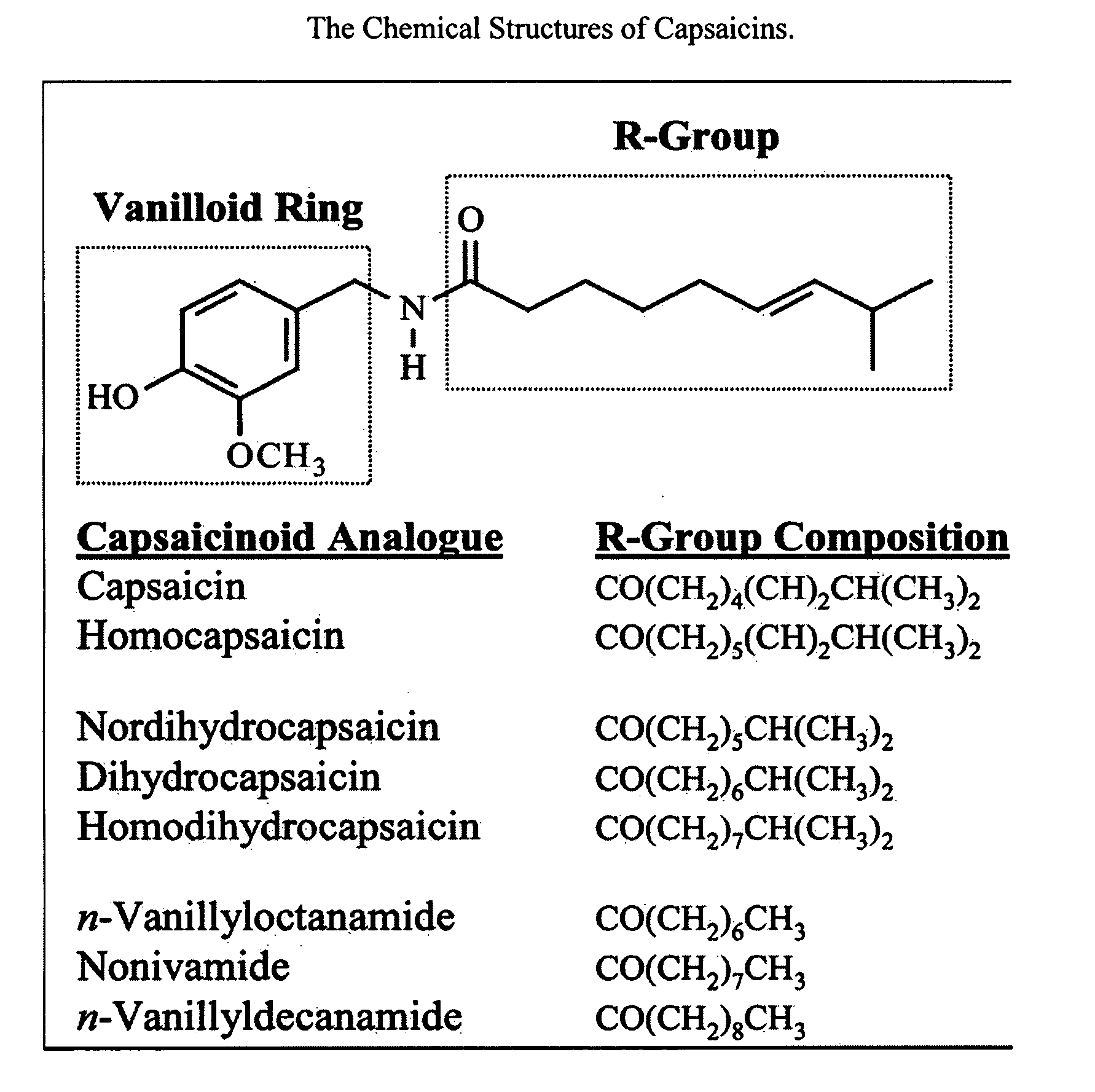
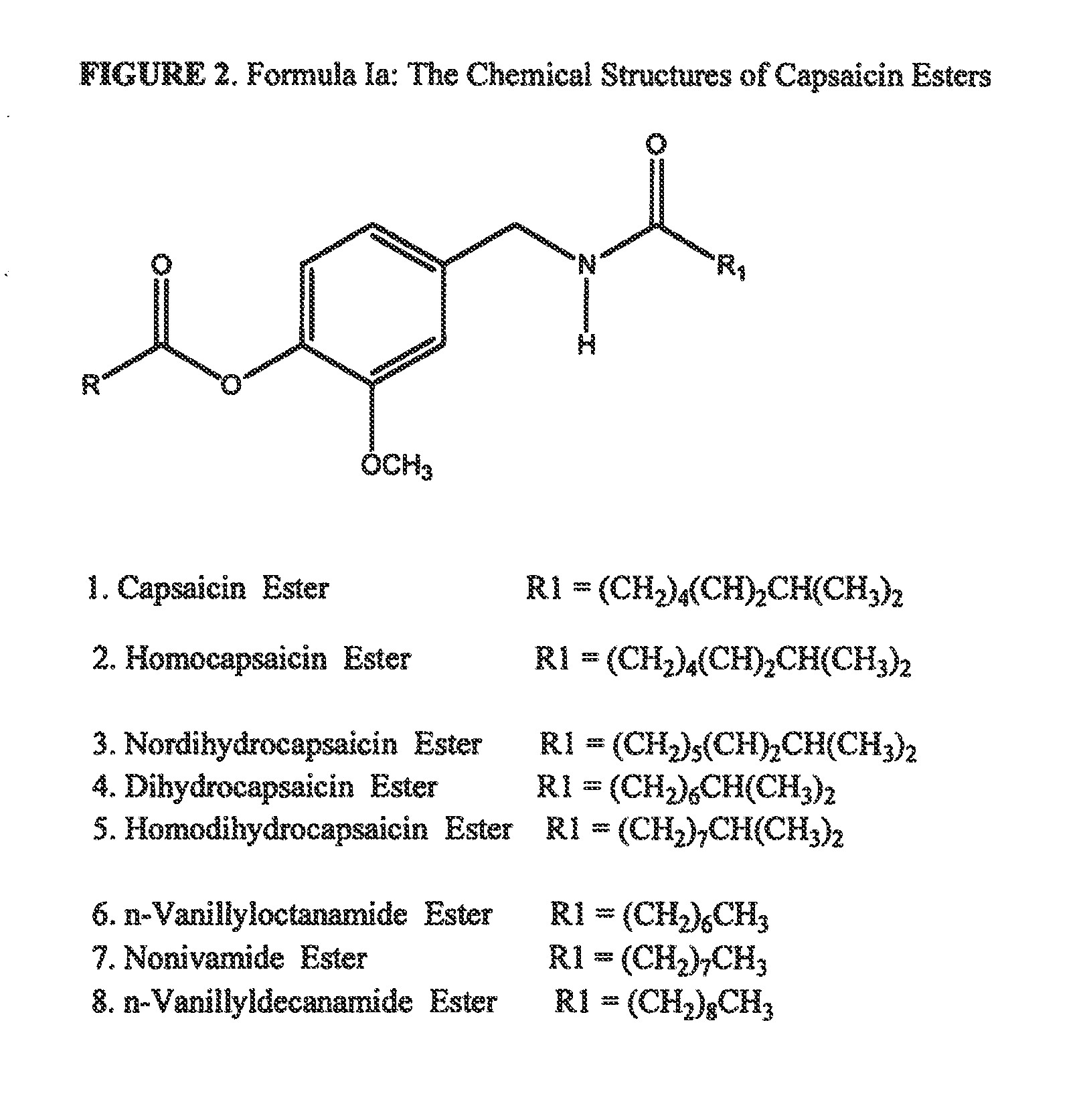
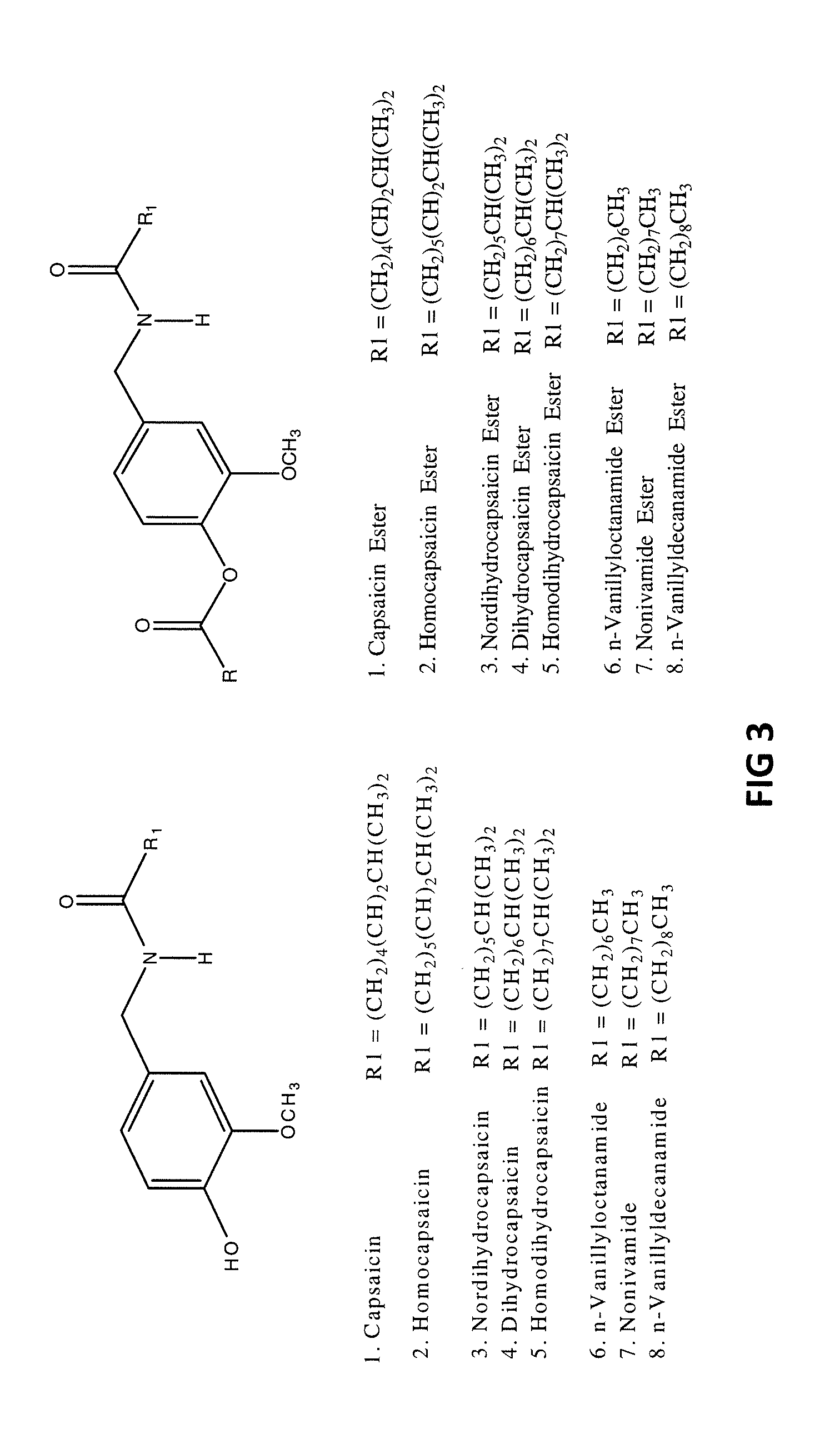
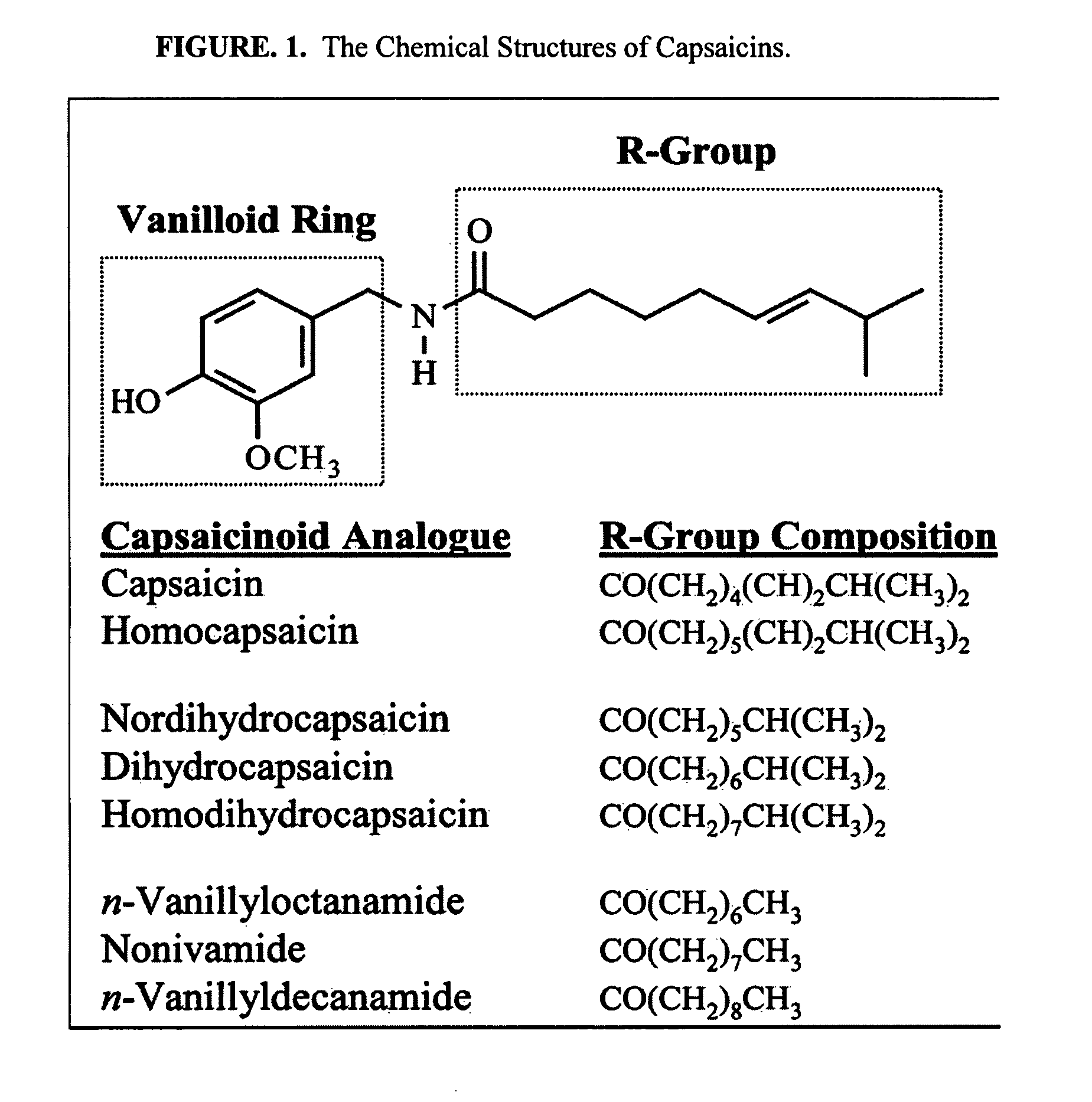
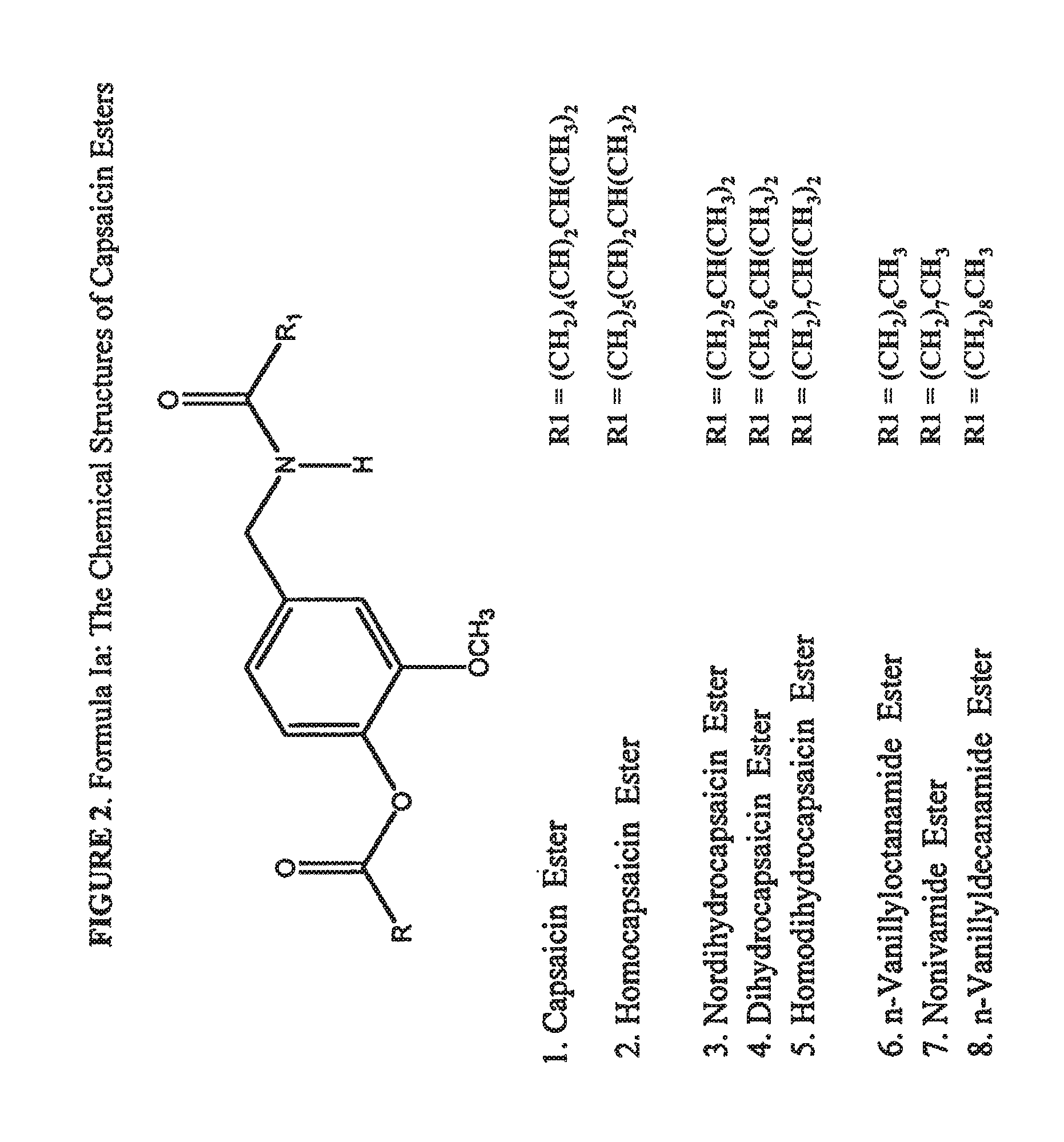
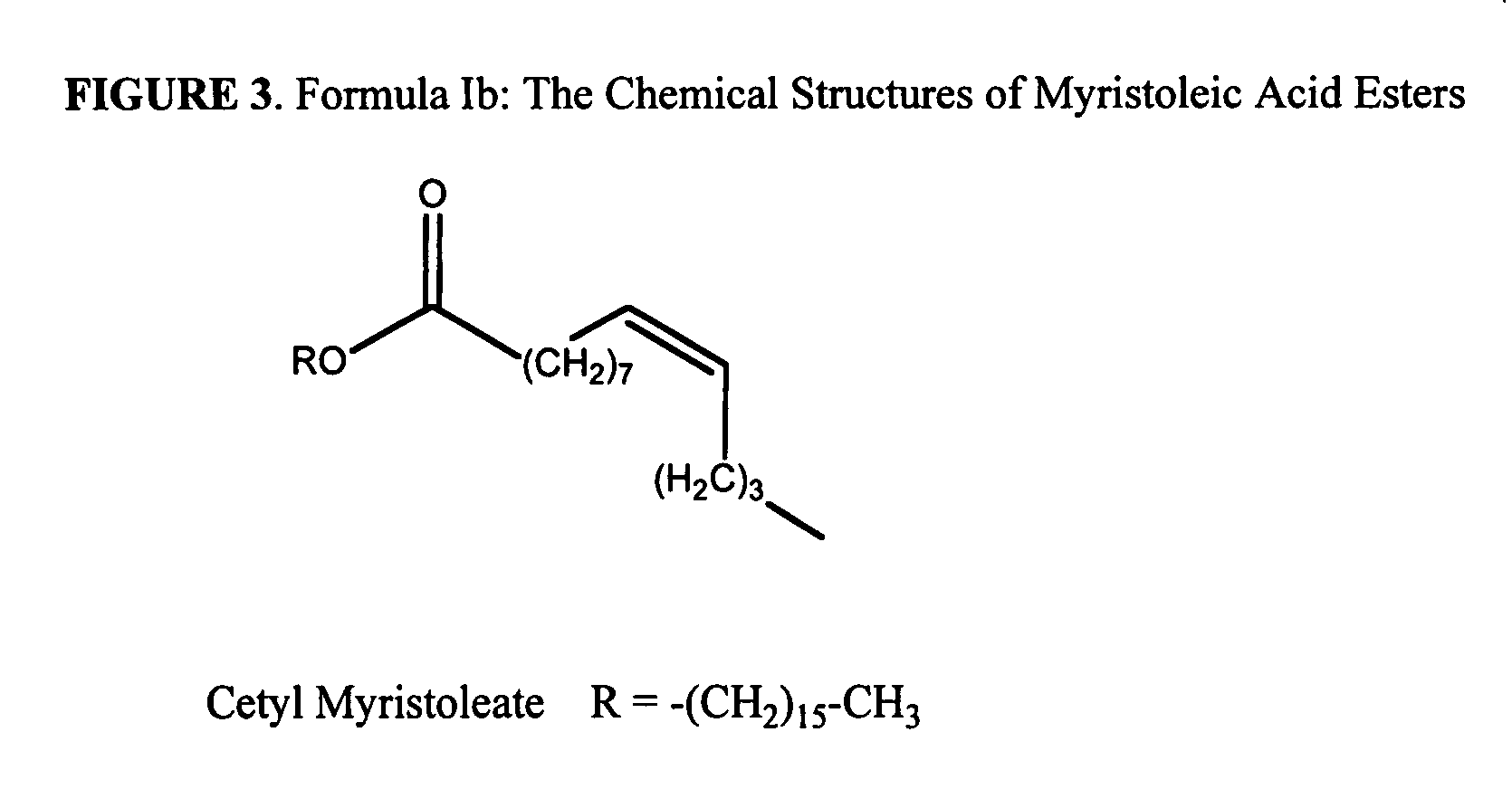
The present invention relates to the formulations of ester derivatives of capsaicin and ester derivatives of myristoleic acid. These derivatives are capable of reverting to the active parent compound following enzymatic or chemical hydrolysis. These derivatives have a higher lipophilicity, lipid solubility and less irritation to the skin than the parent compound, and hence are better able to be incorporated into certain pharmaceutical formulations, including cream and ointment pharmaceutical formulations. The pharmaceutical compositions of the present invention contain a compound of following formula (Ia):R—CO-CAP (Ia)wherein CAP refers to collectively the capsaicins represented in FIG. 1 and a compound of formula (Ib):MCO-O—R (Ib)wherein MCO refers to myristoleic acid.In formulae Ia and Ib, R is selected from alkyl groups of up to about 18 carbon atoms and aryl groups of up to about 18 carbon atoms and alkylene group of up to about 18 carbon atoms and an arylene group of up to about 18 carbon atoms. The alkyl, aryl and alkylene groups may be substituted or un-substituted, branched or straight chains. In addition, R may contain heteroatoms and may be straight chained or branched.The pharmaceutical compositions containing compounds of formulae Ia and Ib are useful for pain management in mammals in vivo and have been contemplated to be used in the treatment of various pains in humans.
Owner:TRINITY LAB INC
Esters of Capsaicin for Treating Pain
InactiveUS20110218180A1Improve lipophilicityNon-irritation to skinAntibacterial agentsBiocideSolubilityIrritation
The present invention relates to the formulations of ester derivatives of capsaicin and ester derivatives of myristoleic acid. These derivatives are capable of reverting to the active parent compound following enzymatic or chemical hydrolysis. These derivatives have a higher lipophilicity, lipid solubility and less irritation to the skin than the parent compound, and hence are better able to be incorporated into certain pharmaceutical formulations, including cream and ointment pharmaceutical formulations. The pharmaceutical compositions of the present invention contain a compound of following formula (Ia):R—CO—CAP (Ia)wherein CAP refers to collectively the capsaicins represented in FIG. 1 and a compound of formula (Ib):MCO—O—R (Ib)wherein MCO refers to myristoleic acid.In formulae Ia and Ib, R is selected from alkyl groups of up to about 18 carbon atoms and aryl groups of up to about 18 carbon atoms and alkylene group of up to about 18 carbon atoms and an arylene group of up to about 18 carbon atoms. The alkyl, aryl and alkylene groups may be substituted or un-substituted, branched or straight chains. In addition, R may contain heteroatoms and may be straight chained or branched.The pharmaceutical compositions containing compounds of formulae Ia and Ib are useful for pain management in mammals in vivo and have been contemplated to be used in the treatment of various pains in humans.
Owner:TRINITY LAB INC
Formulations for the treatment of pain
Formulations and methods are provided for the treatment of pain, and neuropathic pain in particular. The formulations are eutectic mixtures of a capsaicinoid and a local anesthetic agent and / or an anti-pruritic agent.
Owner:ACORDA THERAPEUTICS INC
Pain relief composition
InactiveUS7282224B1Long-termEffective and comfortable to applyBiocideOrganic active ingredientsGlucosamine SulfateMedicine
Disclosed is a pain relief composition comprising an effective amount of a nerve inhibiting component, including capsaicin, a capsaicinoid or a capsaicin analogue, which numbs or inhibits the nerve endings that signal pain, in combination with at least one of the following: an effective amount of an inflammation control component which is designed to reduce immediate pain and discourage future pain in the joints and muscles; an effective amount of a cooling component; an effective amount of a heat minimizing or blocking component; an effective amount of a circulation increasing component which effectuates better penetration of the actives to the skin and nerves; and an effective amount of a soothing and anti-inflammatory complex for the joints and / or muscles comprising Glucosamine sulfate or HCl, Zingiber officiniale (Ginger Root) extract, Methyl sulfonylmethane (MSM), Polygonum cuspidatum (Mexican Bamboo) extract, Aloe barbadensis leaf, and Salix alba (white willow) bark extract. Additionally, the composition includes an encapsulation or entrapment system for a timed release delivery.
Owner:GUTHY-RENKER
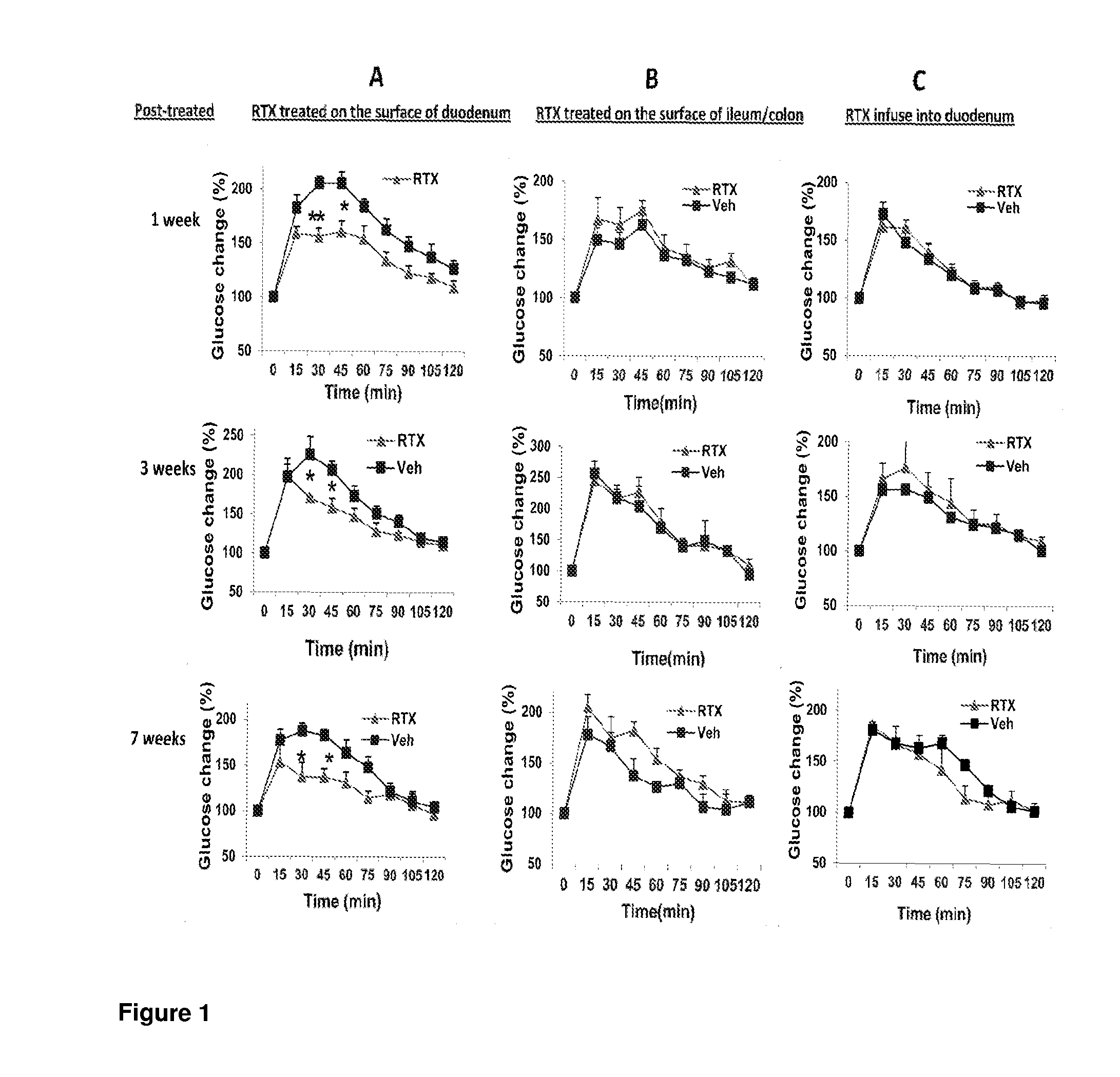
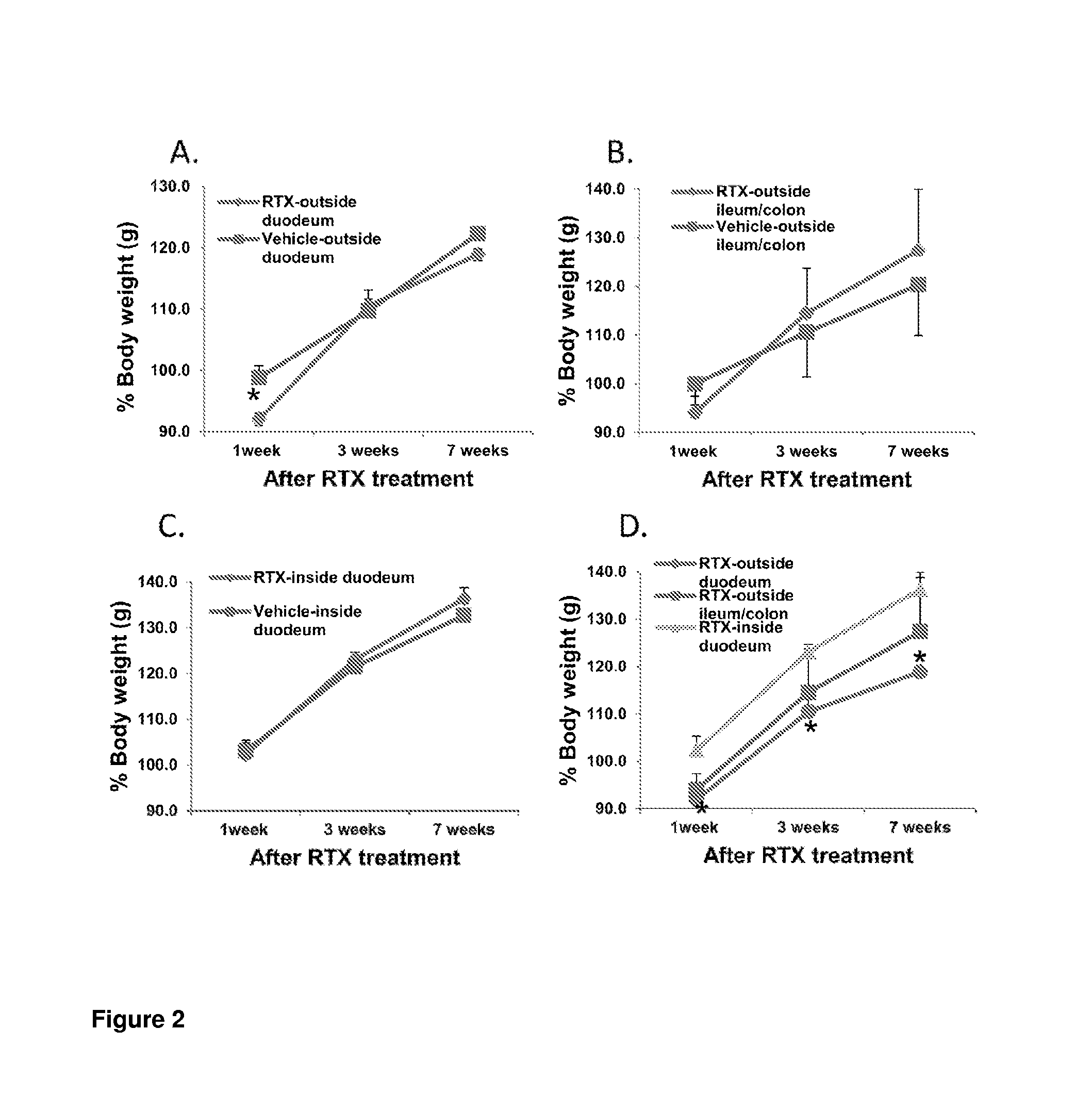
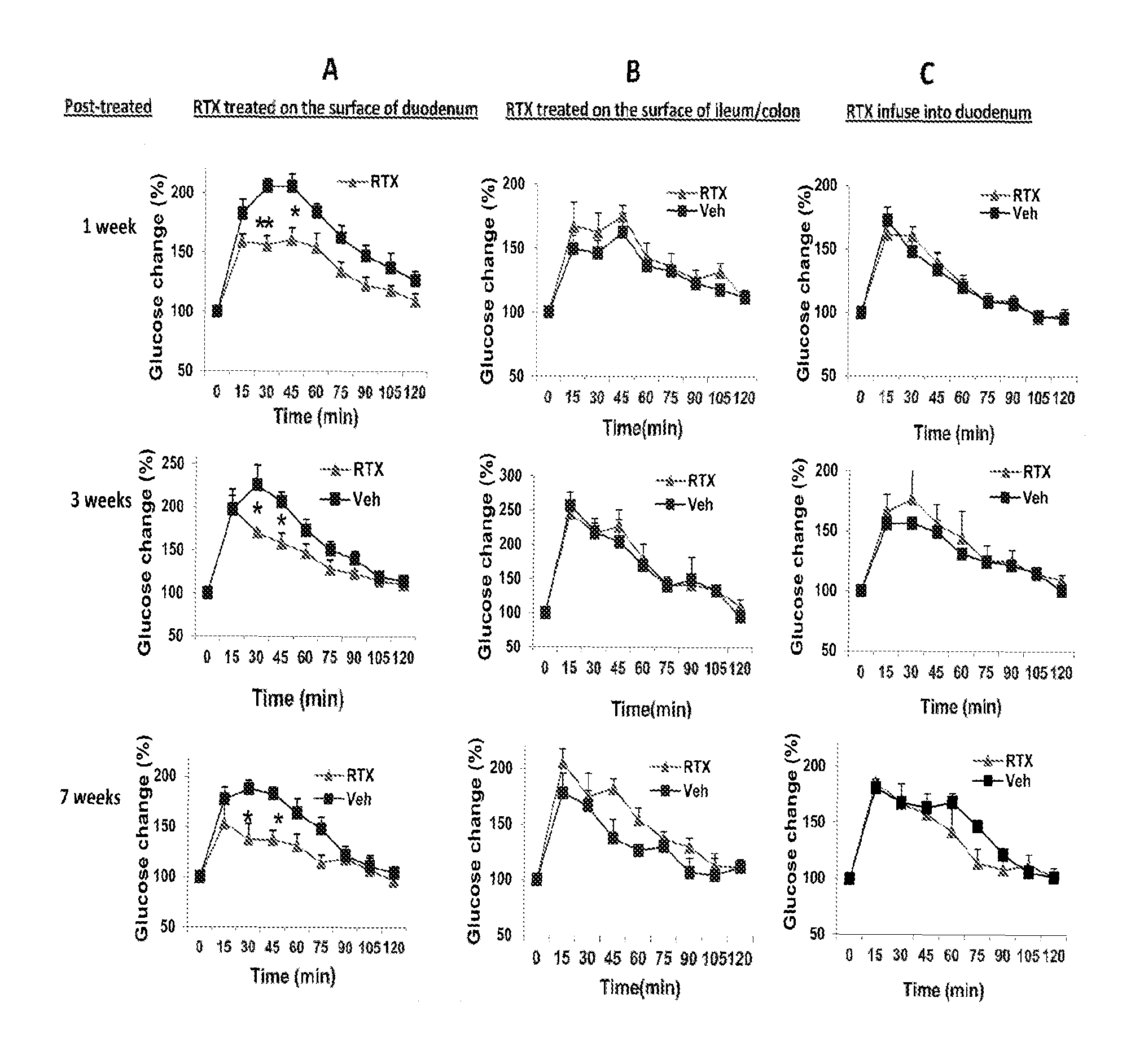
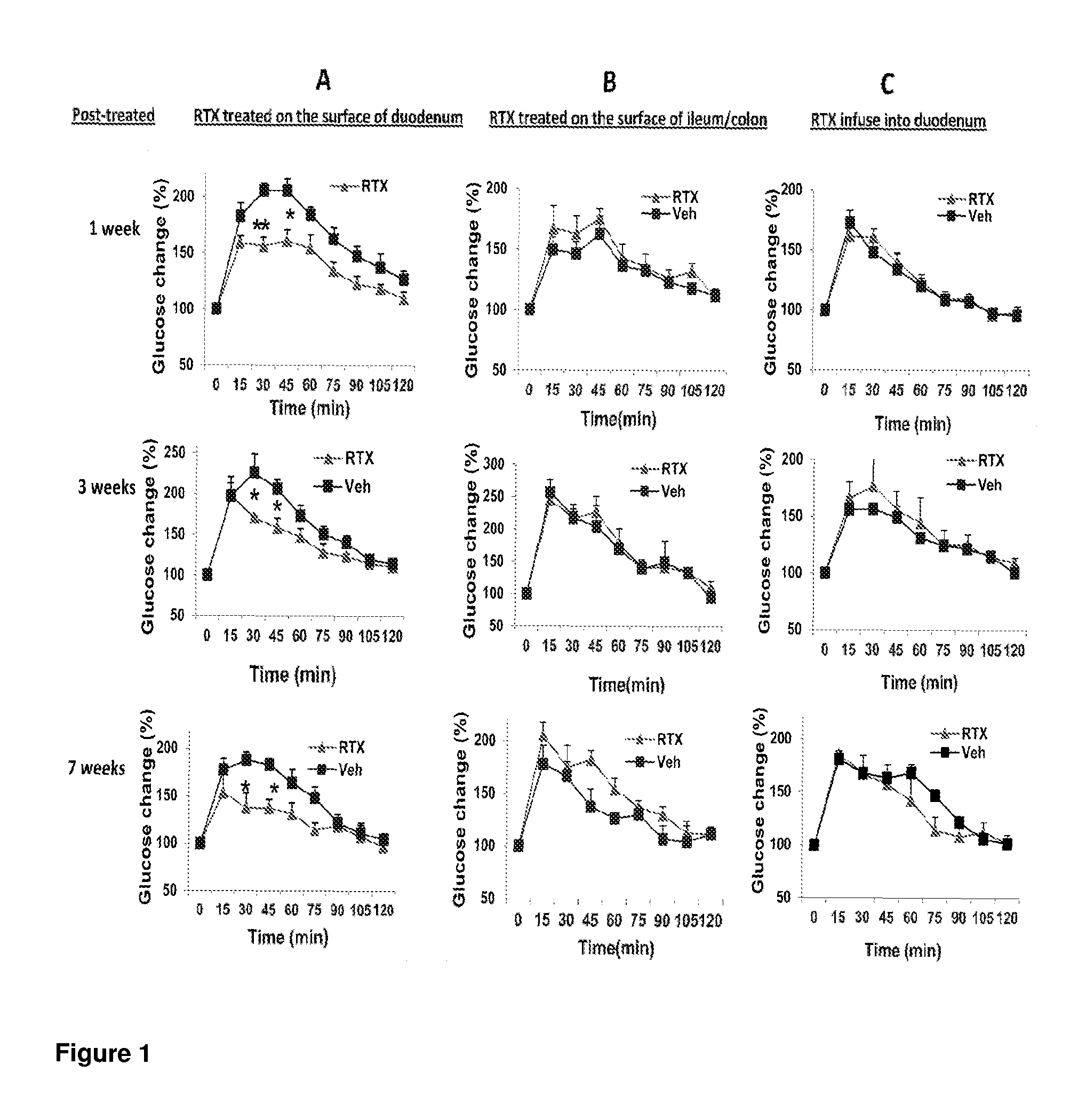
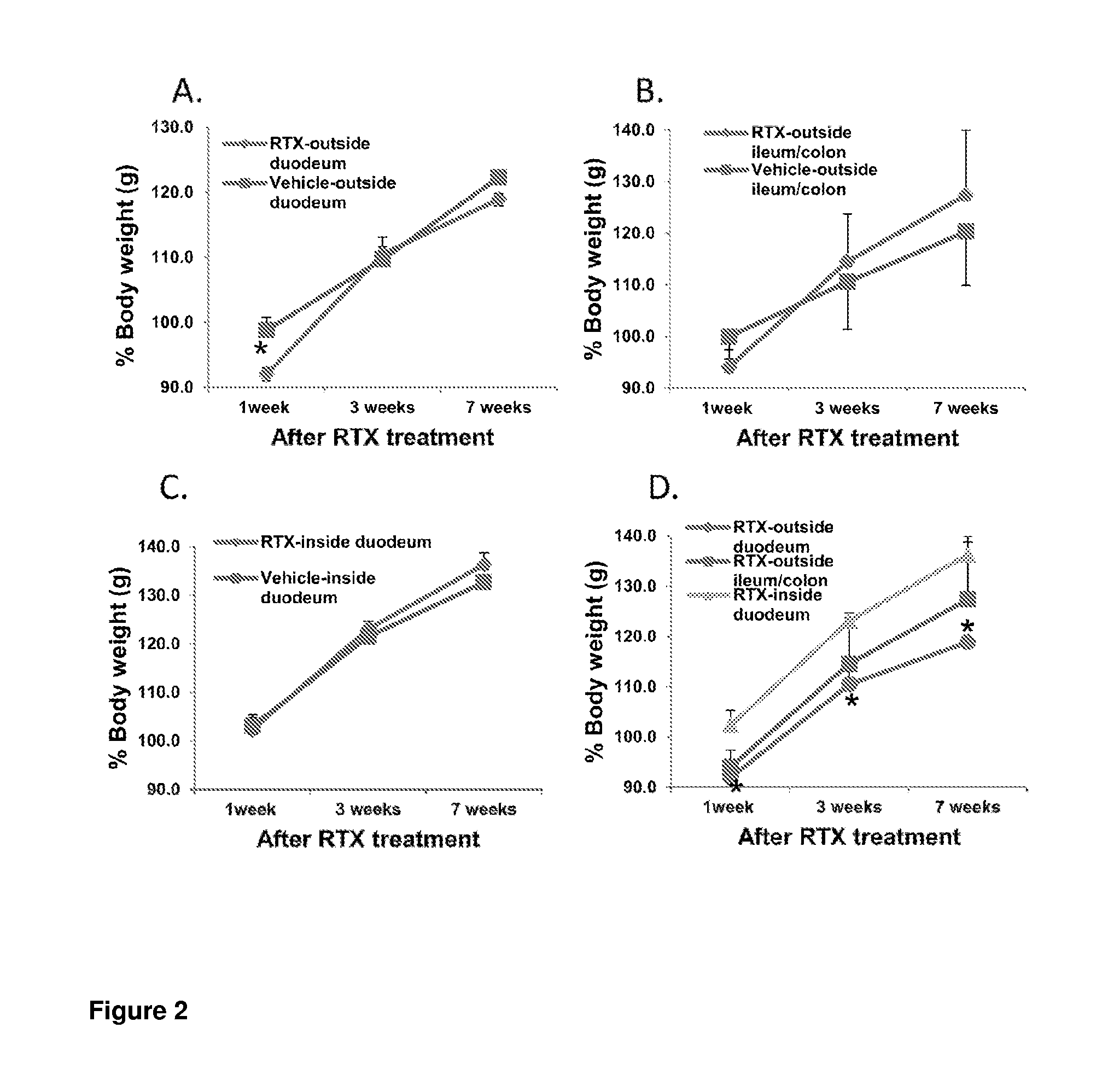
Treatments for diabetes mellitus and obesity
Methods of treatment in subjects suffering from diabetes mellitus or obesity are provided. The methods comprise the step of administering an active agent directly to the small intestine in the subject. In particular, the active agent may be administered directly to the duodenum in the subject. The active agents useful in the treatments described herein include analgesic agents and, in particular, antinociceptive agents such as capsaicin, resiniferatoxin, and their analogues.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Flame-retardant and environmentally-friendly polyurethane cable material and its preparation method
ActiveCN102977585AImprove flame retardant performanceExcellent non-flammabilityElastomerDecabromodiphenyl ether
The invention provides an flame-retardant and environmentally-friendly polyurethane cable material. The cable material is prepared from the following raw materials, by weight, 20-30 parts of chloroprene rubber, 60-80 parts of a polyurethane thermoplastic elastomer, 8-10 parts of acrylonitrile-butadiene rubber, 8-10 parts of chlorinated paraffin, 0.5-1.0 part of stearic acid, 1-3 parts of barium stearate, 4-6 parts of iron oxide, 0.5-1.0 part of ammonium trimolybdate, 30-50 parts of precipitated silica, 10-13 parts of modified argil, 0.5-1.0 part of capsaicin, 0.1-0.3 parts of an antioxidant AW, 0.5-1.5 parts of a promoter TMTD, 0.2-0.5 parts of sulfur, 8-10 parts of decabromodiphenyl oxide, 12-15 parts of antimony (III) oxide and 6-8 parts of zinc borate hydrate. The cable material has the advantages of excellent flame retardation, very less smoke in combustion, no generation of toxic gases or corrosive gases, good low temperature resistance, good oil resistance, and good abrasion resistance, and the cables processed through using the cable material can prevent the harms of mice and termites and simultaneously have the efficacies of low smoke, environmental protection and flame retardation.
Owner:蚌埠尚维知识产权运营有限公司
Pain relief composition
InactiveUS20080107747A1Minimize discomfortImmediate and long-lasting and cumulative long-termBiocideOintment deliveryCapsaicinEntrapment
Disclosed is a pain relief composition comprising an effective amount of a nerve inhibiting component, including capsaicin, a capsaicinoid or a capsaicin analogue, which numbs or inhibits the nerve endings that signal pain, in combination with at least one of the following: an effective amount of an inflammation control component which is designed to reduce immediate pain and discourage future pain in the joints and muscles; an effective amount of a cooling component; an effective amount of a heat minimizing or blocking component; an effective amount of a circulation increasing component which effectuates better penetration of the actives to the skin and nerves; and an effective amount of a soothing and anti-inflammatory complex for the joints and / or muscles comprising Glucosamine sulfate or HCl, Zingiber officiniale (Ginger Root) extract, Methyl sulfonylmethane (MSM), Polygonum cuspidatum (Mexican Bamboo) extract, Aloe barbadensis leaf, and Salix alba (white willow) bark extract. Additionally, the composition includes an encapsulation or entrapment system for a timed release delivery.
Owner:GUTHY-RENKER
Plant source bacteriostasis and detoxification deodorant and preparation method and application of plant source bacteriostasis and detoxification deodorant
InactiveCN103168803ASpeed up conversionGood antibacterial effectBiocideDispersed particle separationHordeum vulgareCapsaicin
The invention provides a plant source bacteriostasis and detoxification deodorant. The plant source bacteriostasis and detoxification deodorant is prepared by the following plant materials in parts by weight: 10-15 parts of tobacco stems, 12-20 parts of Ilex latifolia Thunb stems, 2-8 parts of Helianthus tuberosus Linn leaves, 5-15 parts of ginkgo biloba, 8-16 parts of capsaicin, 1-5 parts of tanshinone, 15-25 parts of orange peel, 2-8 parts of paeoniflorin, 1-5 parts of tea seed, 8-15 parts of ginger stems, 15-35 parts of wild chrysanthemum flower, 5-20 parts of garlic, 5-15 parts of ginger, 20-35 parts of sophora alopecuroides, 5-10 parts of pine needle oil, 15-30 parts of artemisia annua, 20-50 parts of green beans, 30-60 parts of barley and 20-35 parts of corn. The plant source bacteriostasis and detoxification deodorant provided by the invention has the advantages of high biological activity, strong broad-spectrum antibacterial property, fast insect and egg killing, good environment-friendly property, safety, high efficiency and the like. The invention also provides a preparation method and application of the plant source bacteriostasis and detoxification deodorant.
Owner:陈士进 +2
Natural pigment composition and preparation method thereof
The invention provides a natural pigment composition. The natural pigment comprises an emulsifier and two or more than two pigments of gardenia yellow pigment, curcumin, carithamine, citroxanthin, xanthophyll, riboflavin, capsaicin, monascus red pigment, bixa orettana pigment and gardenia blue. Natural pigments can prepare natural pigment compositions in different color systems and different tones in arbitrary proportions, such as yellow, orange, red, green and purple. Meanwhile, the natural pigment composition contains the emulsifier, so that the composition can be more uniform and more stable. Therefore, in the natural pigment composition provided by the invention, combinations of different natural pigments can overcome the defect of single pigment, for example, the light resistance of natural yellow pigment combined by the riboflavin, the curcumin and the like with poor light resistance and the gardenia yellow pigment with more stable light resistance is better than that of the riboflavin or the curcumin, thus the dosage can be reduced, and the cost can be reduced.
Owner:HENAN ZHONGDA HENGYUAN BIOTECH CO LTD
Administration of capsaicinoids
InactiveUS20040156931A1Eliminate side effectsRelieve painBiocideNervous disorderVascular dilatationCapsaicin
Disclosed in certain embodiments is a method for relieving pain at a site in a human or animal in need thereof, comprising administering by injection or infiltration, a dose of a capsaicinoid and coadministering a vasodilator.
Owner:ALGORX PHARMA INC
Methods and compositions for administration of TRPV1 agonists
Compositions are provided that contain a TRPV1 agonist, such as capsaicin, and a solvent system. Topical application of the composition results in rapid delivery of agonist to the dermis and epidermis. Method of using the compositions for reducing nociceptive nerve fiber function in subjects, and for treatment of capsaicin-responsive conditions are also provided.
Owner:GRT US HLDG INC
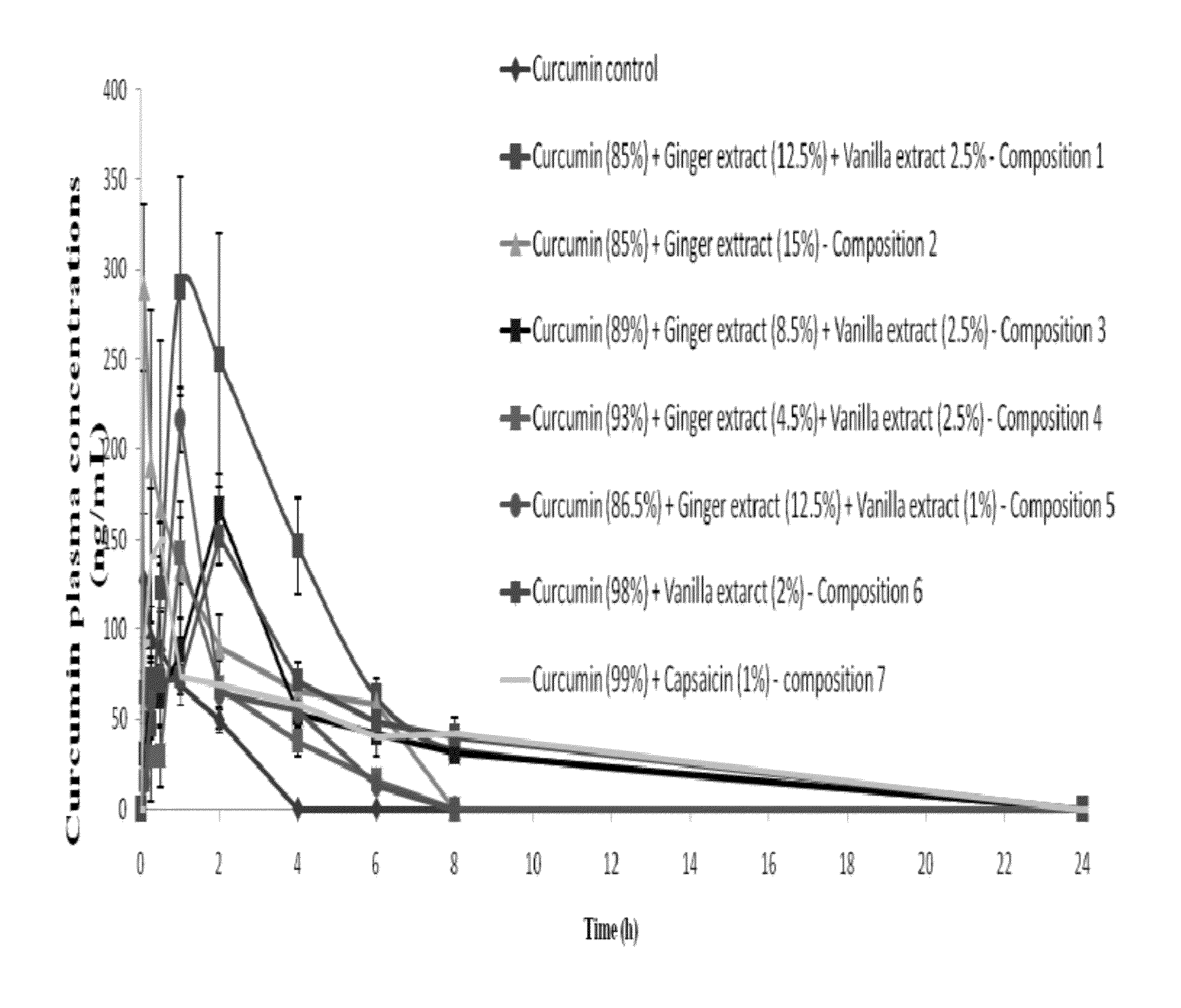
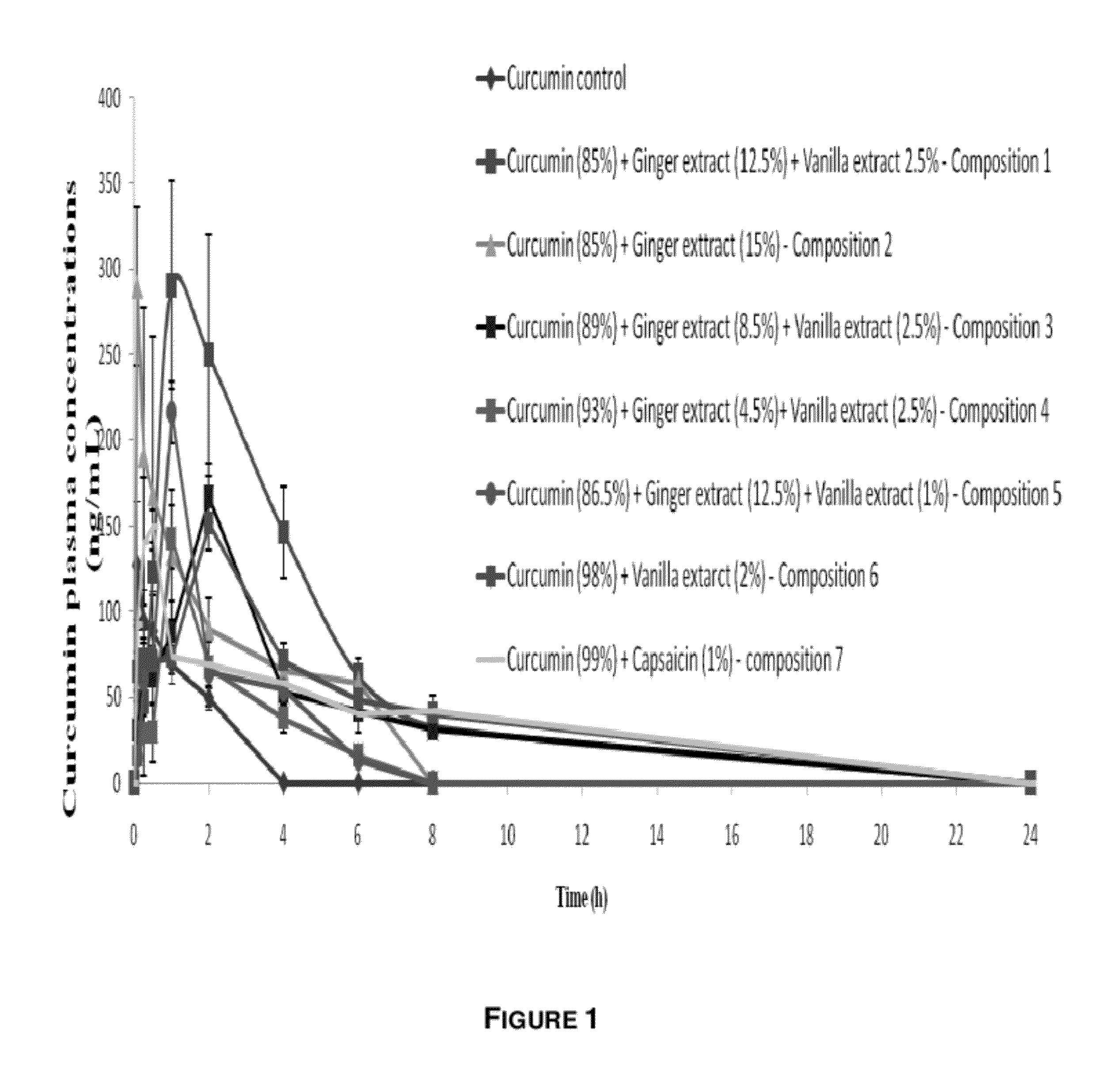
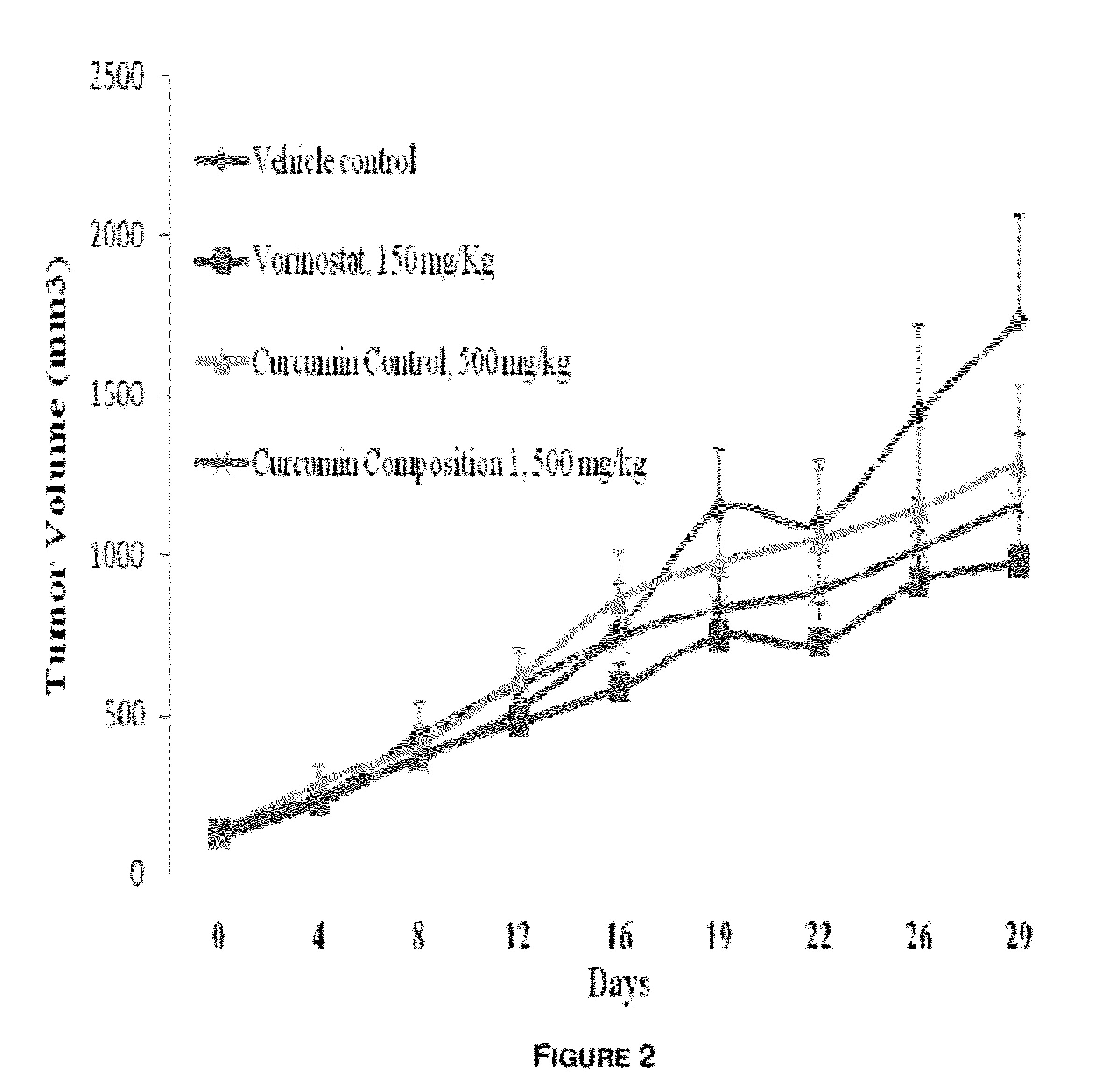
Synergistic Composition for Enhancing Bioavailability of Curcumin
InactiveUS20120058208A1Improve bioavailabilityPleasant smellBiocideKetone active ingredientsCapsaicinGingerol
The present disclosure relates to a composition to enhance the bioavailability of curcumin. In one embodiment, a composition comprising plant extracts of curcumin, vanilla and ginger, wherein the extracts of ginger and vanilla are rich in gingerol and vanillin respectively, is provided. In other embodiments, curcumin, and one or more items selected from the group of vanilla, ginger and capsaicin is provided.
Owner:SYNTHITE INDS
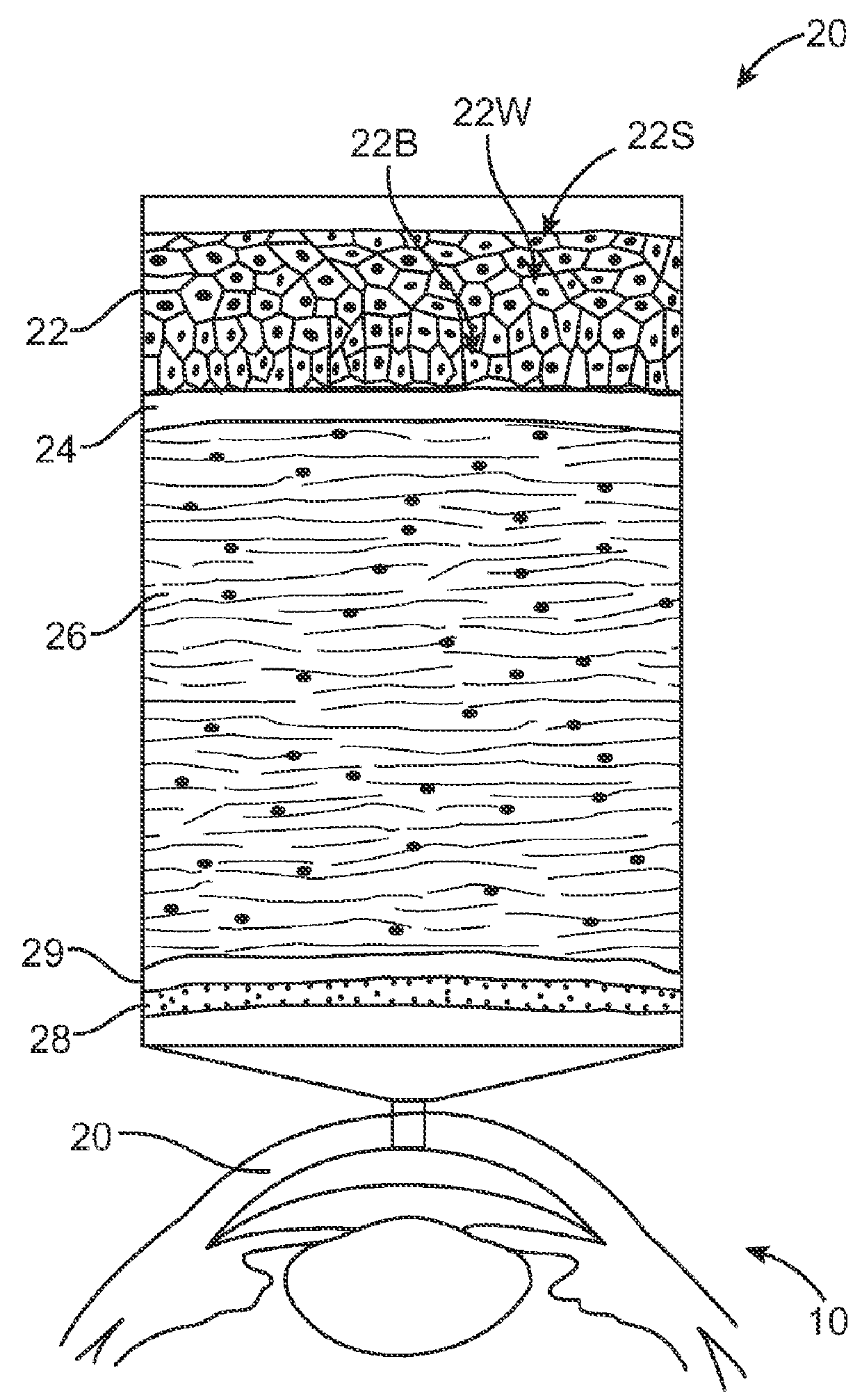
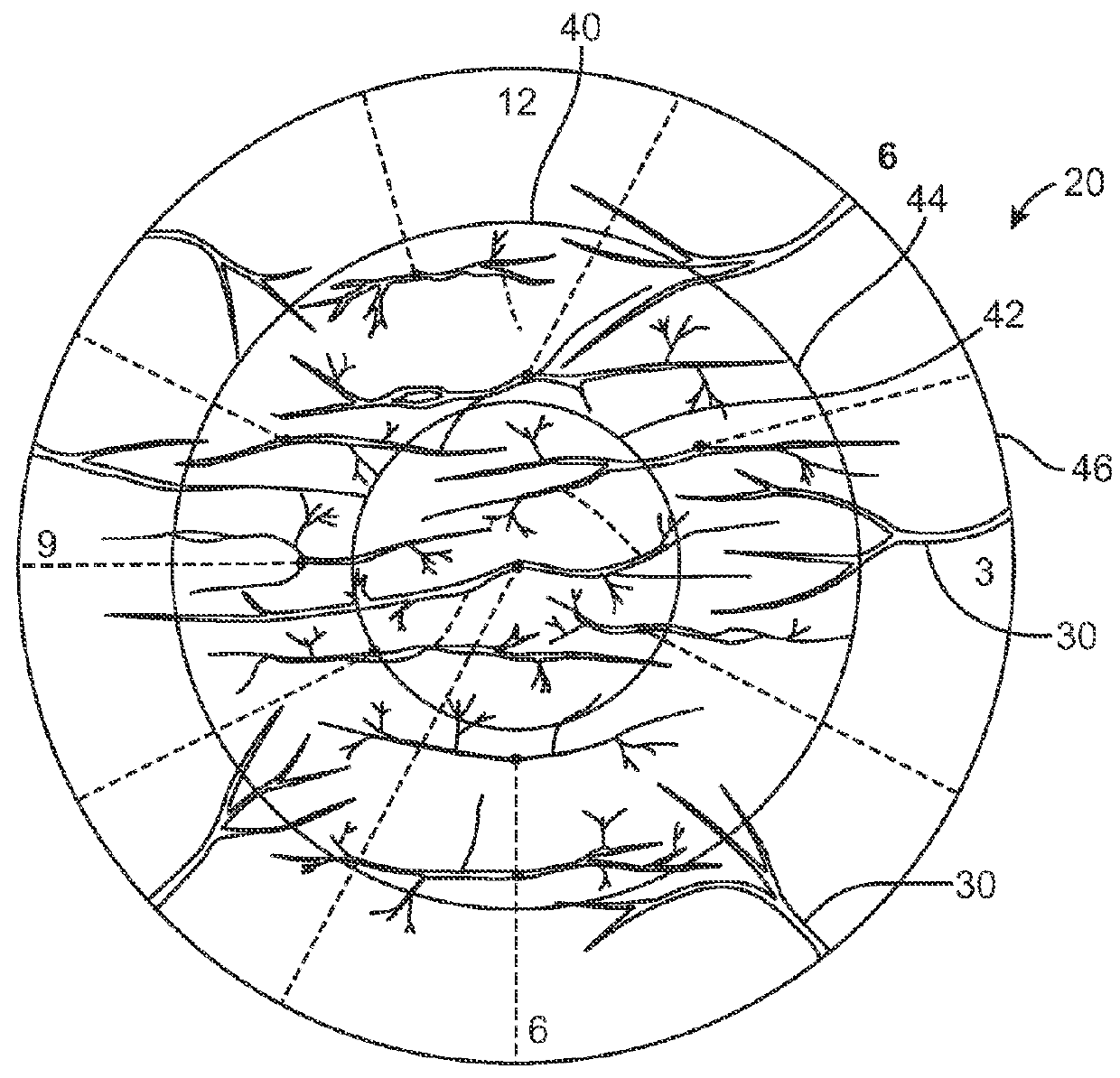
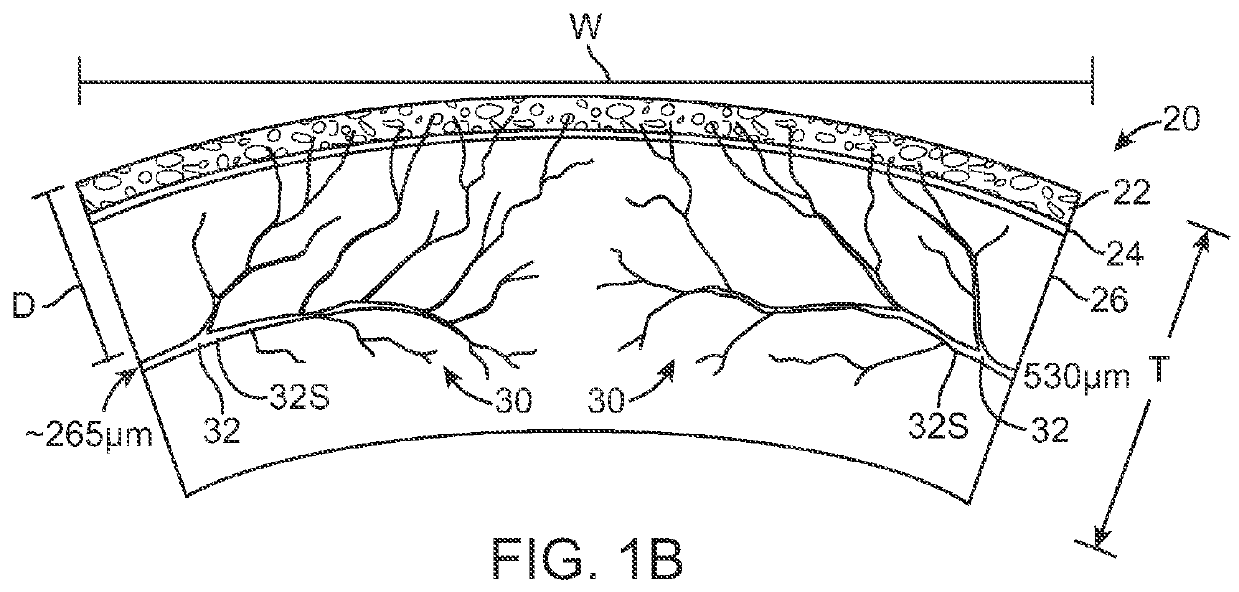
Corneal Denervation for Treatment of Ocular Pain
InactiveUS20130066283A1Relieve painReduce the amount of solutionLaser surgeryUltrasound therapyCorneal nerveCapsaicin
Methods and apparatus for the treatment of the eye to reduce pain can treat at least an outer region of the tissue so as to denervate nerves extending into the inner region and reduce the pain. For example, the cornea of the eye may comprise an inner region having an epithelial defect, and an outer portion of the cornea can be treated to reduce pain of the epithelial defect. The outer portion of the cornea can be treated to denervate nerves extending from the outer portion to the inner portion. The outer portion can be treated in many ways to denervate the nerve, for example with one or more of heat, cold or a denervating noxious substance such as capsaicin. The denervation of the nerve can be reversible, such that corneal innervation can return following treatment.
Owner:NEXISVISION
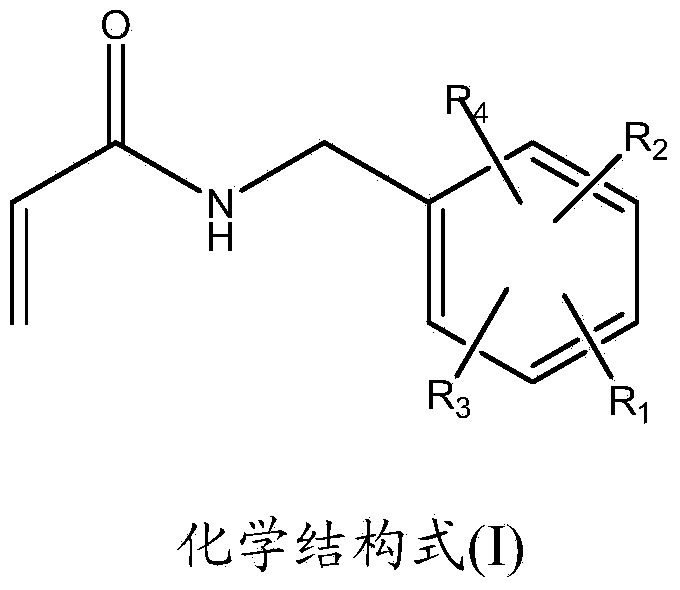
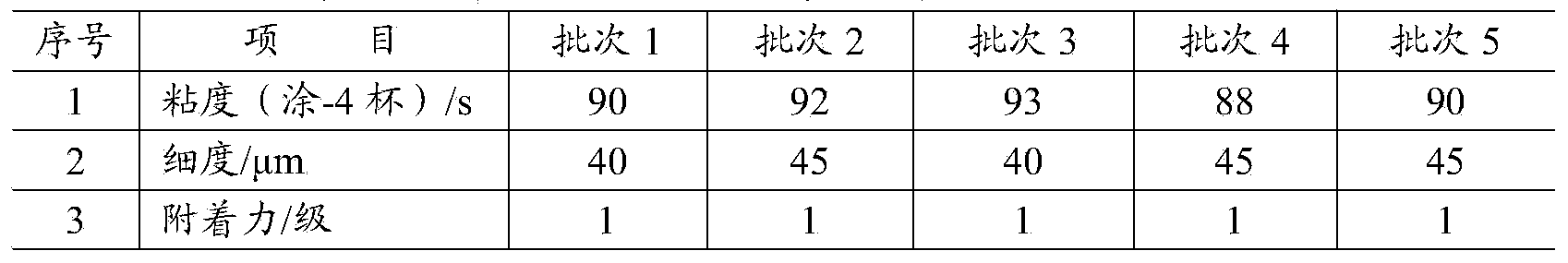
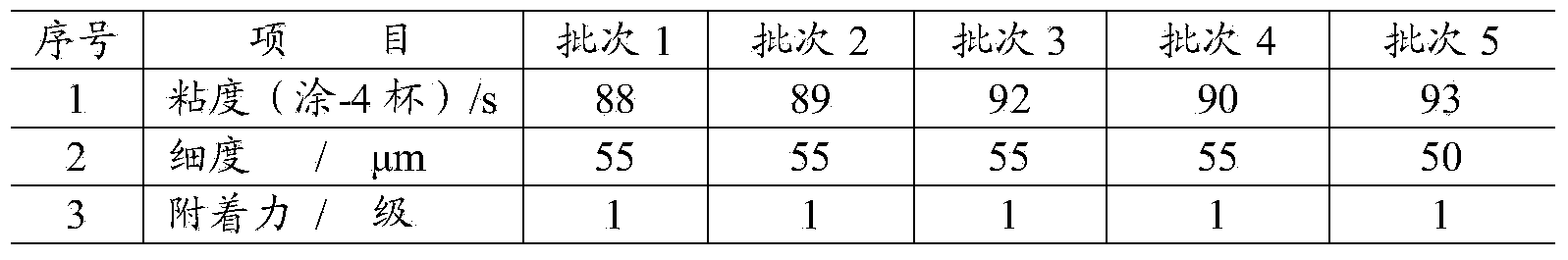
Monofunctional acrylamide compound with capsaicinoid functional structure and preparation method and application thereof
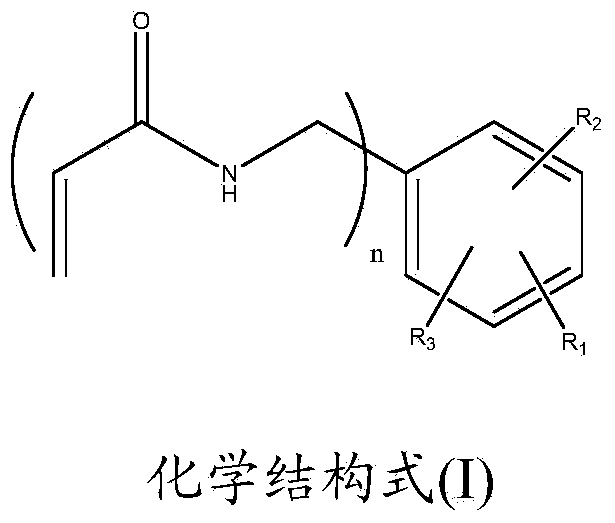
InactiveCN103951576AObvious Environmental AdvantageNo pollution in the processAntifouling/underwater paintsOrganic compound preparationChemical structureSynthesis methods
The invention relates to a new monofunctional acrylamide compound with a capsaicinoid functional structure and a preparation method and application thereof. The acrylamide compound has the chemical structural formula (I) in the specification, wherein in the formula, R1, R2, R3 or R4 represents alkyl, OH group, OCH3 and other substituent groups. The monofunctional acrylamide compound with the capsaicinoid functional structure contains not only a capsaicin important active group, but also active double-bonds capable of performing polymerization reaction, the synthesis method is simple and easy to operate, the yield is high, and the monofunctional acrylamide compound can make up for the defect that the mass production of natural capsaicin is limited by complicated extraction processes and the output of hot pepper and simultaneously make up for the insufficiency that the natural capsaicin can only be used as a pure additive. The monofunctional acrylamide compound with the capsaicinoid functional structure, provided by the invention, has very good application prospects in preparation of marine antifouling coatings or antibacterial and mildew-proof coatings.
Owner:OCEAN UNIV OF CHINA
Multifunctional acrylamide compound containing capsaicin-like functional structure and preparation method and application thereof
InactiveCN103951578AObvious Environmental AdvantageNo pollution in the processAntifouling/underwater paintsOrganic compound preparationChemical structureSynthesis methods
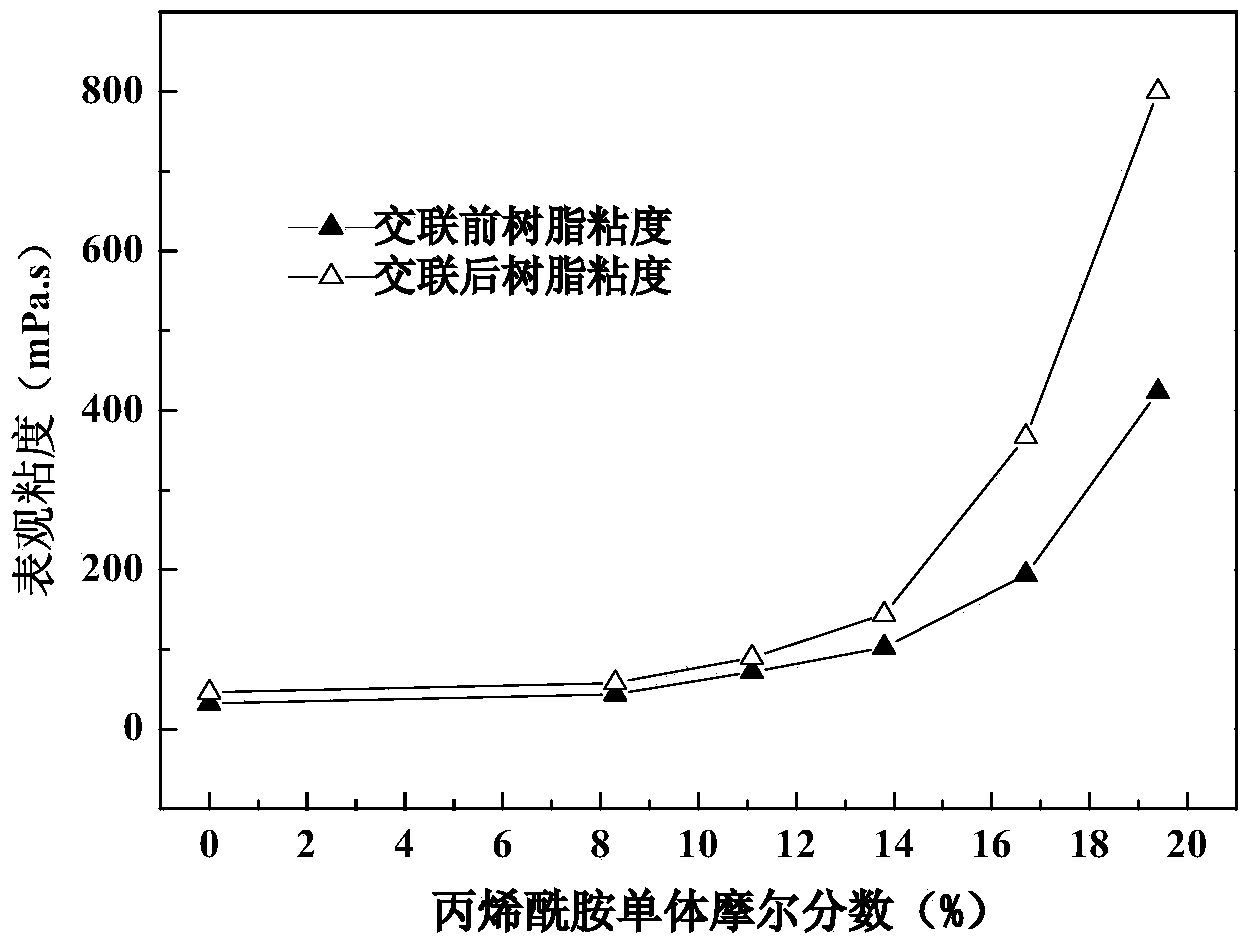
The invention relates to a novel multifunctional acrylamide compound containing a capsaicin-like functional structure and a preparation method and an application thereof. The compound has the chemical structure shown in the formula (I) in the specification, wherein R1, R2 or R3 represents an alkyl group, an OH group or an OCH3 substituent group. The acrylamide compound disclosed by the invention contains an important capsaicin active group as well as an active double bond capable of carrying out polymerization reaction. The synthesis method is simple and feasible, and the yield is high. The shortcomings that the amount of the natural capsaicin is limited by the complex extraction process and the pepper yield and the natural capsaicin is only used as an additive are made up. The acrylamide compound disclosed by the invention has very good application prospect in preparing the marine antifouling paint or antibacterial and antifungal coating and the viscosity of the polymer can be significantly increased when the acrylamide compound is used as a crosslinking agent.
Owner:OCEAN UNIV OF CHINA
Novel Pharmaceutical Compositions for Treating Chronic Pain and Pain Associated with Neuropathy
InactiveUS20130189354A1Reduced plasma concentrationEfficient managementOrganic active ingredientsBiocideGabapentinChronic pain
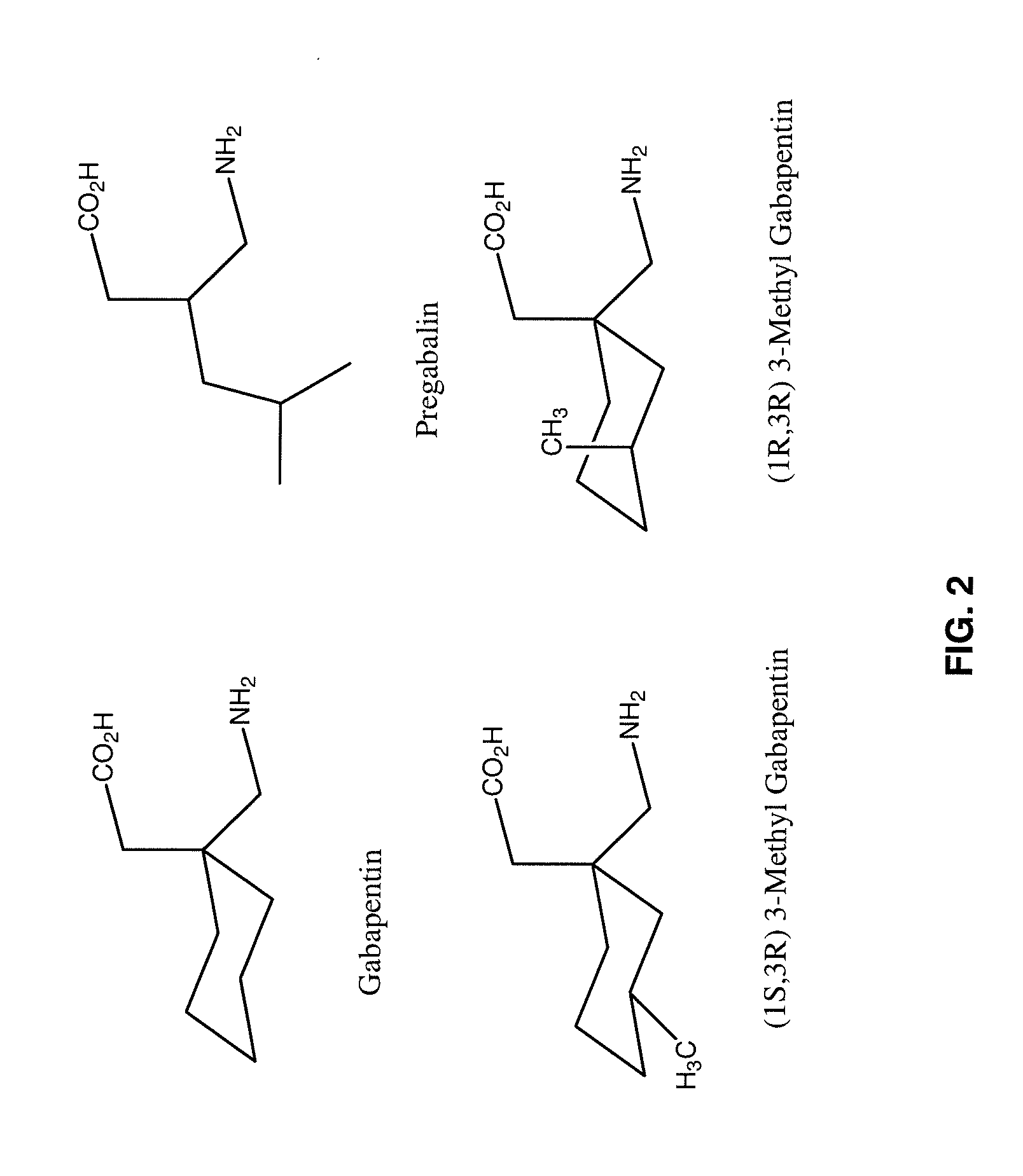
The present invention relates to compositions and methods for treating pain wherein the compositions comprise a combination of tramadol or a pharmaceutically acceptable salt thereof, magnesium or a pharmaceutically acceptable salt thereof; and gabapentin or pregabalin. The therapeutic combination can further contain capsaicin or an ester of capsaicin.
Owner:TRINITY LAB INC
Esters of capsaicin for treating pain
ActiveUS7943666B2Improve lipophilicityNon-irritation to skinAntibacterial agentsBiocideSolubilityIrritation
The present invention relates to the formulations of ester derivatives of capsaicin and ester derivatives of myristoleic acid. These derivatives are capable of reverting to the active parent compound following enzymatic or chemical hydrolysis. These derivatives have a higher lipophilicity, lipid solubility and less irritation to the skin than the parent compound, and hence are better able to be incorporated into certain pharmaceutical formulations, including cream and ointment pharmaceutical formulations. The pharmaceutical compositions of the present invention contain a compound of following formula (Ia):R—CO-CAP (Ia)wherein CAP refers to collectively the capsaicins represented in FIG. 1 and a compound of formula (Ib):MCO-O—R (Ib)wherein MCO refers to myristoleic acid.In formulae Ia and Ib, R is selected from alkyl groups of up to about 18 carbon atoms and aryl groups of up to about 18 carbon atoms and alkylene group of up to about 18 carbon atoms and an arylene group of up to about 18 carbon atoms. The alkyl, aryl and alkylene groups may be substituted or un-substituted, branched or straight chains. In addition, R may contain heteroatoms and may be straight chained or branched.The pharmaceutical compositions containing compounds of formulae Ia and Ib are useful for pain management in mammals in vivo and have been contemplated to be used in the treatment of various pains in humans.
Owner:TRINITY LAB INC
Special red sour soup and preparation method thereof
InactiveCN105054152AFull of nutritionImprove gastrointestinal functionFood preparationCelluloseAdditive ingredient
The invention discloses special red sour soup. The special red sour soup is prepared from the following components in parts by weight: 70-80 parts of fresh tomatoes, 15-25 of fresh chili pepper, 0.15-0.25 part of sticky rice, 0.1-0.2 part of ginger, 0.1-0.2 part of garlic, 1.5-3 parts of table salt, and 0.15-0.25 part of white spirit. Through improvements on the raw materials and the material ratio of the red sour soup, the fermented sour soup is rich in nutrients and contains nutritional ingredients such as VA, VB1, VB2, VC, Ca, P, Fe, carotene, capsaicin, cellulose and multiple amino acids, fermentation products and other nutritive substances in the sour soup enable the constitution of intestinal flora to have beneficial change and can improve gastrointestinal functions of a human body, recover flora balance in human intestinal tracts and form an antibacterial biological barrier to maintain human health, and meanwhile, the improved special red sour soup is easy to store and convenient to transport, thereby making the large-scale commercial production of the red sour soup possible.
Owner:贵州布依姑娘食品有限公司
Method for preparing antimicrobial polysulfone ultrafiltration membrane
ActiveCN102698619AImprove pollutionImprove hydrophilicitySemi-permeable membranesEscherichia coliHydrophilic monomer
The invention relates to a method for preparing an antimicrobial polysulfone ultrafiltration membrane, which comprises the following steps: preparing a reaction solution containing an initiator, a capsaicin and a hydrophilic monomer, putting a pretreated polysulfone ultrafiltration membrane into the reaction solution for reaction of 0.32-8 hours, and modifying the surface of the pretreated polysulfone ultrafiltration membrane to obtain the antimicrobial polysulfone ultrafiltration membrane. The capsaicin is acrylamide with the derivative structures of capsaicin, the hydrophilic monomer is acrylamide, acrylic acid, methacrylic acid, hydroxyethyl acrylate or p-vinyl benzenesulfonic acid containing olefinic double bonds and hydrophilic groups, and the reaction solution is the mixture of an organic solvent and water. The flux of the ultrafiltration membrane prepared by adopting the method is more than 100L / (m<2>.h), and the ultrafiltration membrane can retain more than 93% of bovine serum albumin and can resist more than 85% of colon bacillus and more than 80% of staphylococcus aureus. The method is easy to operate, and the antibacterial and pollution-resisting properties of the ultrafiltration membrane are improved significantly. The prepared ultrafiltration membrane can be applied to the fields of industrial wastewater treatment, seawater desalination, etc.
Owner:OCEAN UNIV OF CHINA
Capsaicin antifouling paint
InactiveCN1709997AHigh mechanical strengthStay alkaline for a long timeCoatingsCalcium hydroxideSodium Bentonite
The invention relates to a kind of capsicum alkali antifouling dope. Its materials weight proportions are: active acrylic resin 18-25%, calcareousness powderó±30-40%, calcareousness powderó�18-30%, calcium hydroxide 2-6%, capsicum alkali 0.05-0.10%, bentonite 9-14% and silica powder. It is a kind of antifouling dope which prevents sea biology from accreting with active acrylic resin as basic material, calcareousness powder and calcium hydroxide of different granularities, and capsicum alkali as driving agent. The quantity average molecular weight of active acrylic resin Mn = P800, the weight average weight Mw = 17100, and the dispersing intensity D = 1.75. Through seawater (pH = 7.5) simulating hanging board experimentation, the seeping rate of capsicum alkali of the dope stabilizes at 74 - 118 ª–gíñm-2d-1, its pH stabilizes at 9.5 - 10.0, and its effective antifouling period can get to over 3 years. The dope of the invention is non-toxic, highly effective, and low-cost.
Owner:心脑合一(上海)教育科技有限公司
Resin of zinc acrylate or copper acrylate containing capsaicin function group, its preparation and use
This is a kind of acrylic zinc or cuprum resin containing capsaicin functional group and its preparation method. Its characteristics are: firstly synthesize monomer containing capsaicin functional group, then synthesize acrylic resin containing functional group and carboxyl, finally make the acrylic resin and zinc hydroxide or cuprum hydroxide react to obtain acrylic zinc or cuprum resin containing capsaicin functional group. The resin of the invention can be used for preparing sea antifouling dope. In the seawater, the organic zinc or cuprum acrylic ester on the polymer linear main chain of the coat surface hydrolyzes, and send out organic zinc or cuprum; the hydrolyzed polymer main chain contains capsaicin functional group with highly efficient antifouling activeness, which makes the hydrolyzed polymer main chain still has sterilization activeness. So the dope can effectively prevent halobios from clinging on the surface of fishing tools, ships and seacoast establishments, and has good antifouling performance.
Owner:OCEAN UNIV OF CHINA
Treatments for diabetes mellitus and obesity
Methods of treatment in subjects suffering from diabetes mellitus or obesity are provided. The methods comprise the step of administering an active agent directly to the small intestine in the subject. In particular, the active agent may be administered directly to the duodenum in the subject. The active agents useful in the treatments described herein include analgesic agents and, in particular, antinociceptive agents such as capsaicin, resiniferatoxin, and their analogs.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Pharmaceutical Compositions Comprising Capsaicin Esters for Treating Pain and Cold Sores
InactiveUS20140134261A1Increase heart rateGood blood pressureBiocideHydroxy compound active ingredientsDiseaseAmyris
The present invention relates to pharmaceutical compositions comprising ester(s) of capsaicin and at least one other agent selected from salicylates, menthol, boswellic acids, DMSO, methyl sulfonylmethane, NSAIDs, corticosteroids, emu oil, opioid agonists and antagonists, NMDA antagonists, tramadol, hyaluronic acid, α2δ ligands, santalol, santalyl acetate, amyris alcohol, amyris acetate, aloe vera gel and aloe vera juice, for improved therapeutic properties. Further, the present invention relates to pharmaceutical compositions comprising high concentrations of ester(s) of capsaicin. Further, the present invention relates to a method of relieving pain due to various diseases in subjects by administering the pharmaceutical compositions of the invention. Further, the present invention relates to methods of relieving fever blisters due to cold sores in subjects by administering the pharmaceutical compositions comprising an ester of capsaicin and one other agent selected from santalol, santalyl acetate, amyris alcohol and amyris acetate.
Owner:TRINITY LAB INC
Seasoning processing technique for spicy capsaicin
The invention discloses a seasoning processing technique for spicy capsaicin, comprising the following steps of: soaking: soaking textured soybean protein by cold water, wherein the temperature of cold water is 10-15 DEG C and the soaking time is 4-6hours; dehydrating: dehydrating the soaked textured soybean protein till the water content is 45-50%, wherein the dehydration mode is natural drying dehydration or mechanical centrifugal dehydration; frying: slicing the hydrated textured soybean protein to obtain original capsaicin, and frying by a continuous automatic fryer, wherein the frying temperature is controlled at 145-170 DEG C, and the frying time is 70s; blending: adding seasoning in the fried capsaicin and blending. By strictly controlling the technical parameters of the seasoning processing technique, the produced product is even in color and luster, good in taste, more delicious after seasoning of a special formula is added, and is safe and healthy to eat, and only a food additive disodium 5'-ribonucleotide is added.
Owner:CHENGDU XIANGXIANGZUI FOOD
Application of lipid vehicles and use for drug delivery
InactiveUS20070003610A1Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticDiseaseAnticarcinogen
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com