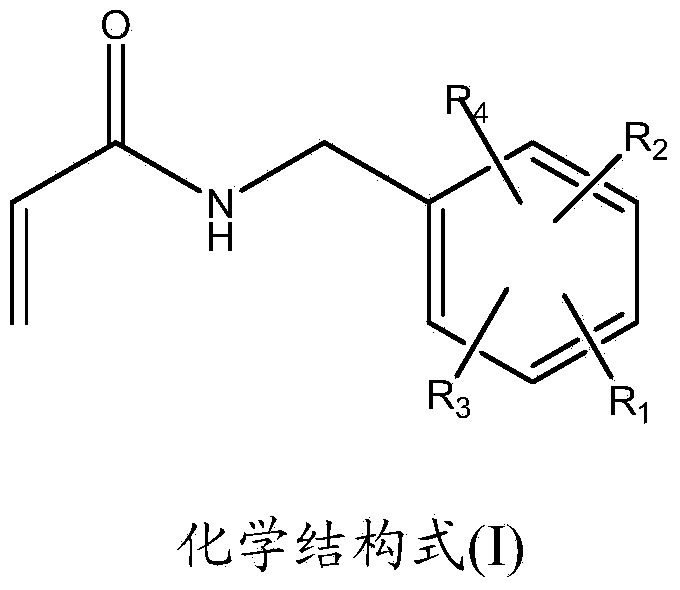
Monofunctional acrylamide compound with capsaicinoid functional structure and preparation method and application thereof
A technology of acrylamide and monofunctionality, which is applied in the field of acrylamide compounds with monofunctionality and capsaicin-like functional structure, which can solve the problems of unfeasible price on a large scale, low yield, and difficulty in large-scale promotion and use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Preparation of N-(3,5-dimethyl-4-hydroxybenzyl)acrylamide
[0080] Add 122g (1mol) of 2,6-xylenol and 151.5g (1.5mol) of N-methylol acrylamide (2,6-xylenol and N-methylol acrylamide molar ratio 1:1.5), then add 30 mL of concentrated sulfuric acid catalyst (36% by weight of 2,6-xylenol) and 115 mL of ethanol solvent (by weight of 2,6-xylenol) in turn 60%). The reaction was stirred in a water bath at a temperature of 35 °C for 15 hours, and a white precipitate was formed, which was filtered with suction. The obtained filter cake was repeatedly washed with water until neutral, and then recrystallized in absolute ethanol to obtain a white crystal.
[0081] The yield of white crystals in this example was 84.4%.
[0082] The melting point of the white crystals was 140-141°C according to melting point determination.
[0083] Infrared spectra were measured under the following conditions:
[0084] Instrument used: NICOLETAVATAR-360 infrared absorption spectrometer...
Embodiment 2
[0094] Example 2: Preparation of N-(4-hydroxy-3-methylbenzyl)acrylamide
[0095] In a 500 mL three-necked flask equipped with an electric stirring device, a condenser tube and a thermometer, add 74 mL of acetone (55% of the weight of o-cresol), 107 g (1 mol) of o-cresol, 101 g (1 mol) of N-methylolpropene amide (the molar ratio of o-cresol and N-methylol acrylamide is 1:1), then add 13g of anhydrous aluminum trichloride (12% of the weight of o-cresol) to the system, and heat in a water bath at 40°C Stir the reaction. The reaction was terminated after 36 hours, a large amount of solid was precipitated in the system, and the filter cake was washed repeatedly with dilute hydrochloric acid until neutral, and then recrystallized in absolute ethanol to obtain a white crystal.
[0096] According to the method described in Example 1, the following results were obtained:
[0097] The yield of white crystals was 80%, and the melting point was 141-142°C.
[0098] IR(KBr)υ / cm -1 : 330...
Embodiment 3
[0101] Example 3: Preparation of N-(2-hydroxy-4,5-methylenedioxybenzyl)acrylamide
[0102] Into a 1000mL three-necked flask equipped with an electric stirring device, a condenser tube and a thermometer, add 175mL of ethanol (100% by weight of 3,4-methylenedioxyphenol), 137g (1mol) of 3,4-methylenediol Oxyphenol, 101g (1mol) N-methylol acrylamide (3,4-methylenedioxyphenol and N-methylol acrylamide molar ratio 1:1), and then add 40mL of concentrated sulfuric acid to the system (54% by weight of 3,4-methylenedioxyphenol), heated and stirred in a water bath at 30°C for 24 hours, a large amount of solid was precipitated in the system, filtered, and the filter cake was repeatedly washed with water until neutral.
[0103] Subsequent recrystallization from absolute ethanol gave a white crystal.
[0104] According to the method described in Example 1, the following results were obtained:
[0105] The yield of white crystals was 90.2%, and the melting point was 128-129°C.
[0106] IR...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com