Multifunctional acrylamide compound containing capsaicin-like functional structure and preparation method and application thereof
An acrylamide and multifunctional technology, which is applied in the field of multifunctional acrylamide compounds containing capsaicinoid functional structure, can solve the problem that the price is unfeasible in scale, the steps of artificial total synthesis of capsaicin are cumbersome, and it is difficult to popularize on a large scale. use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
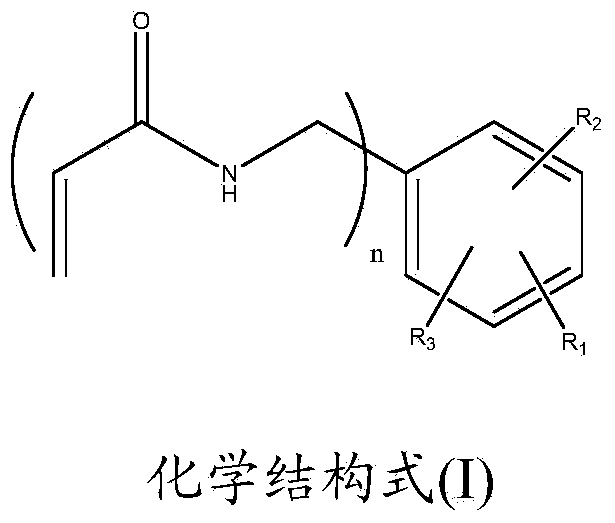
[0079] Embodiment 1: the preparation of N-(2-hydroxyl-3-acrylamide methyl-4,6-dimethylbenzyl)acrylamide
[0080] Add 61.0g (0.5mol) of 3,5-xylenol and 151.5g (1.5mol) of N-methylolacrylamide (3,5- The molar ratio of xylenol to N-methylolacrylamide is 1:3), and then add 10mL of concentrated sulfuric acid catalyst (30% of the weight of 3,5-xylenol) and 50mL of ethanol solvent (for 3,5-di 64.6% of cresol weight). After stirring and reacting in a water bath at 45° C. for 24 hours, a white precipitate was formed, which was filtered with suction, and the obtained filter cake was repeatedly washed with water until neutral, and then recrystallized in absolute ethanol to obtain a white crystal.
[0081] The yield was calculated to be 87.6% according to the following formula:
[0082] (m2 is the actual obtained product quality, n1 is the amount of substance substituted with benzene compound, and M2 is the molar molecular weight of the product)
[0083] The melting point of the whit...
Embodiment 2
[0096] Embodiment 2: Preparation of N-(2-hydroxyl-3-acrylamide methyl-4,5-dimethylbenzyl)acrylamide
[0097] Add 61.0g (0.5mol) of 3,4-xylenol and 252.5g (2.5mol) of N-methylolacrylamide (3,4- The molar ratio of xylenol to N-methylolacrylamide is 1:5), and then add 5 mL of concentrated sulfuric acid catalyst (15% of the weight of 3,4-xylenol) and 70 mL of ethanol solvent (for 3,4-di 90.5% of cresol weight). After stirring and reacting in a water bath at a temperature of 50° C. for 48 hours, a white precipitate was formed, which was filtered with suction, and the obtained filter cake was repeatedly washed with water until neutral, and then recrystallized in absolute ethanol to obtain a white crystal.
[0098] Measure according to the method described in embodiment 1 and obtain following result:
[0099] The yield of the white crystal product was 88.8%, and the melting point was 206-207°C.
[0100] IR (KBr) υ / cm-1: 691.4, (benzene ring-C=C): 1545.2, 1617.2, 1639.0; (C=O): 165...
Embodiment 3
[0105] Embodiment 3: Preparation of N-(3,6-dimethyl-4-acrylamidomethyl-2-hydroxybenzyl)acrylamide
[0106] Add 61.0g (0.5mol) of 2,5-xylenol and 151.5g (1.5mol) of N-methylolacrylamide (2,5- The molar ratio of xylenol to N-methylolacrylamide is 1:3), and then add 19 mL of concentrated sulfuric acid catalyst (57% of the weight of 2,5-xylenol) and 77 mL of n-butanol solvent (for 2, 99.5% by weight of 5-xylenol). After stirring and reacting in a water bath at a temperature of 28° C. for 20 h, a white precipitate was formed, which was filtered with suction, and the obtained filter cake was repeatedly washed with water until neutral, and then recrystallized in absolute ethanol to obtain a white crystal.
[0107] Measure according to the method described in embodiment 1 and obtain following result:
[0108] The yield of the white crystal product was 88.32%, and the melting point was 179-180°C.
[0109] IR (KBr) υ / cm-1: 712.7, (benzene ring-C=C): 1465.6, 1536.2, 1621.6; (C=O): 164...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com