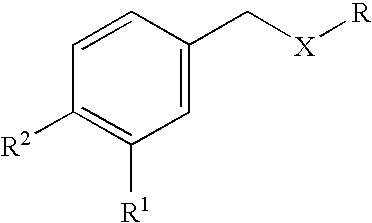
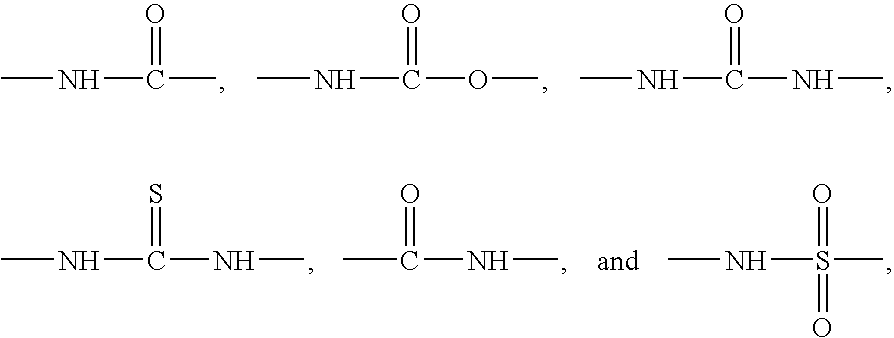
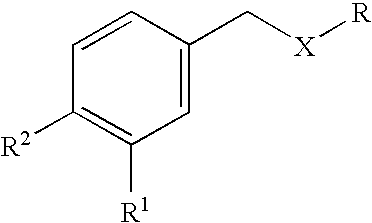
Formulations for the treatment of pain
a technology for pain and forms, applied in the field of pain management, can solve the problems of hyperactivity, significant and costly healthcare problems, and difficulty in treating chronic pain,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0041] Capsaicin (“Cap”) and lidocaine (“Lido”) were mixed in the various ratios set forth in Table 1. Some of the formulations spontaneously became partially liquid. All of the formulations were mildly heated to form a uniform solution. The solutions were allowed to crystallize and / or separate at room temperature. The mixtures were also analyzed by DSC.
TABLE 1ObservationsCompositionMelt / 48Cap:LidoImmediately24 hourshours96 hours100SolidSolidSolidSolid90:10SolidWet massWet massWet mass80:20SolidWet massWet massWet mass70:30Wet massWet massLiquidLiquid60:40Wet massWet massLiquidLiquid50:50Wet massWet massLiquidLiquid40:60Wet massWet massLiquidLiquid30:70SolidWet massWet massWet mass20:80SolidWet massWet massWet mass10:90SolidWet massWet massWet mass 0:100SolidSolidSolidSolid
example 2
[0042] Capsaicin, menthol, and lidocaine were mixed in the ratios given in Table 2. As in Example 1, some of the formulations spontaneously became partially liquid. All of the formulations were mildly heated to form a uniform solution. The solutions were allowed to crystallize and / or separate at room temperature.
TABLE 2ObservationsCompositionMelt / Cap:Menthol:LidoImmediately24 hours(over 2 weeks)100SolidSolidSolid10:45:45SolidWet massLiquid30:35:35SolidWet massLiquid40:30:30Wet massWet massLiquid50:25:25Wet massWet massLiquid60:20:20Wet massWet massWet mass70:15:15Wet massWet massWet mass90:5:5 Wet massWet massWet mass
example 3
[0043] Capsaicin (“Cap”) and tetracaine (“Tetra”) were mixed in the ratios set forth in Table 3. As in Example 1, some of the formulations spontaneously became partially liquid. All of the formulations were mildly heated to form a uniform solution. The solutions were allowed to crystallize and / or separate at room temperature.
TABLE 3ObservationsCompositionMelt / Cap:TetraImmediately24 hours(over 2 weeks)100SolidSolidSolid10:90SolidWet massLiquid30:70SolidWet massLiquid40:60Wet massWet massLiquid50:50Wet massWet massLiquid60:40Wet massWet massWet mass70:30Wet massWet massWet mass90:10Wet massWet massWet mass
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt. % | aaaaa | aaaaa |
| wt. % | aaaaa | aaaaa |
| wt. % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com