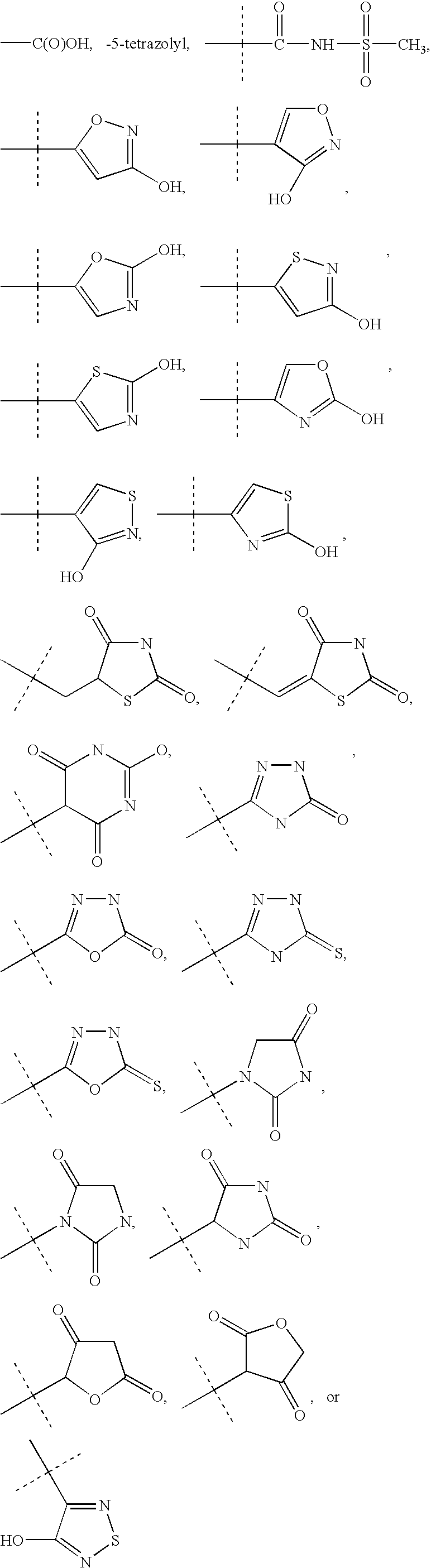
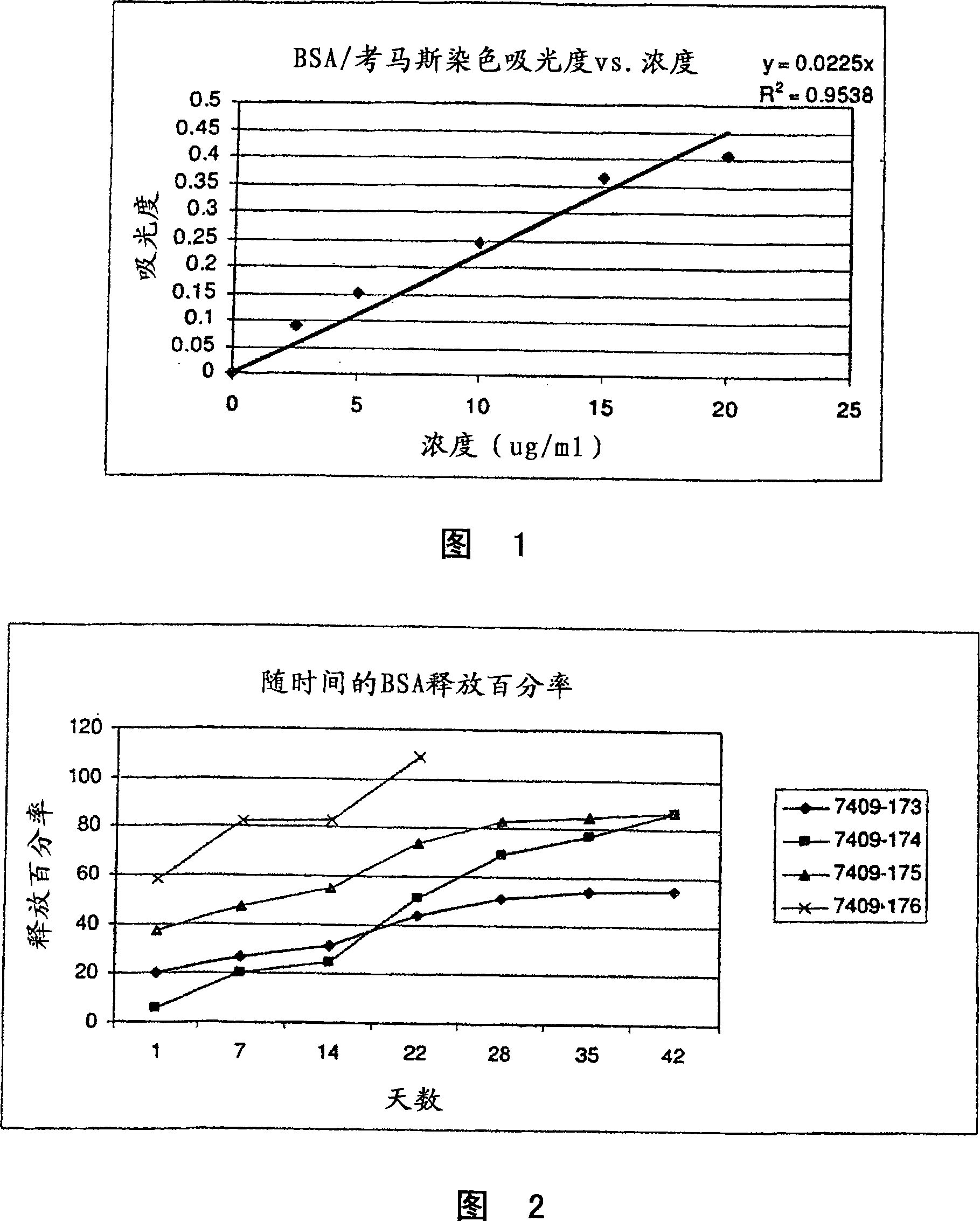
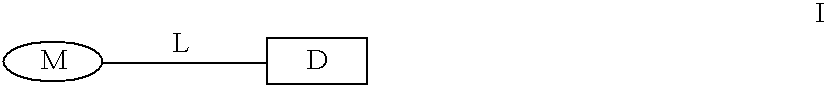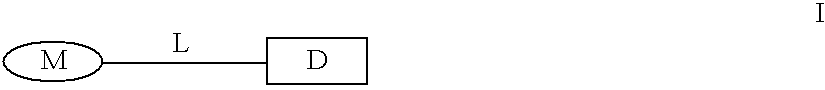Patents
Literature
46 results about "Nonsteroidal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A nonsteroidal compound is a drug that is not a steroid nor a steroid derivative. Nonsteroidal anti-inflammatory drugs (NSAIDs) are distinguised from corticosteroids as a class of anti-inflammatory agents.
Modified release multiple-units compositions of non-steroid anti-inflammatory drug substances (NSAIDs)
InactiveUS6599529B1Keep low levelQuick releasePowder deliveryNervous disorderNon steroid anti inflammatory drugTherapeutic effect
An oral pharmaceutical modified release multiple-units composition for the administration of a therapeutically and / or prophylactically effective amount of a non-steroid anti-inflammatory drug substance to obtain both a relatively fast onset of the therapeutic effect and the maintenance of a therapeutically active plasma concentration for a relatively long period of time is disclosed.
Owner:TAKEDA PHARMA AS +1
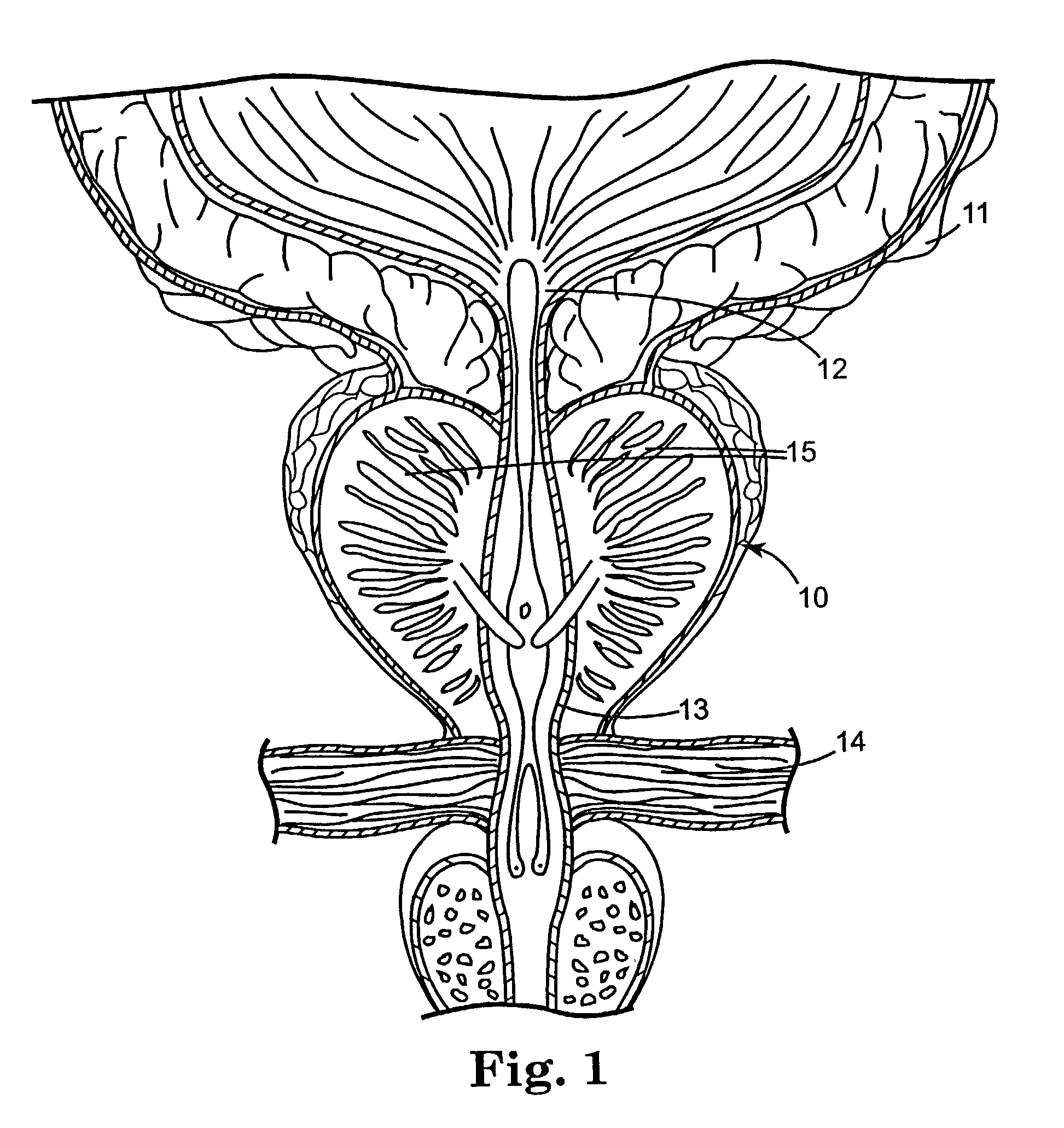
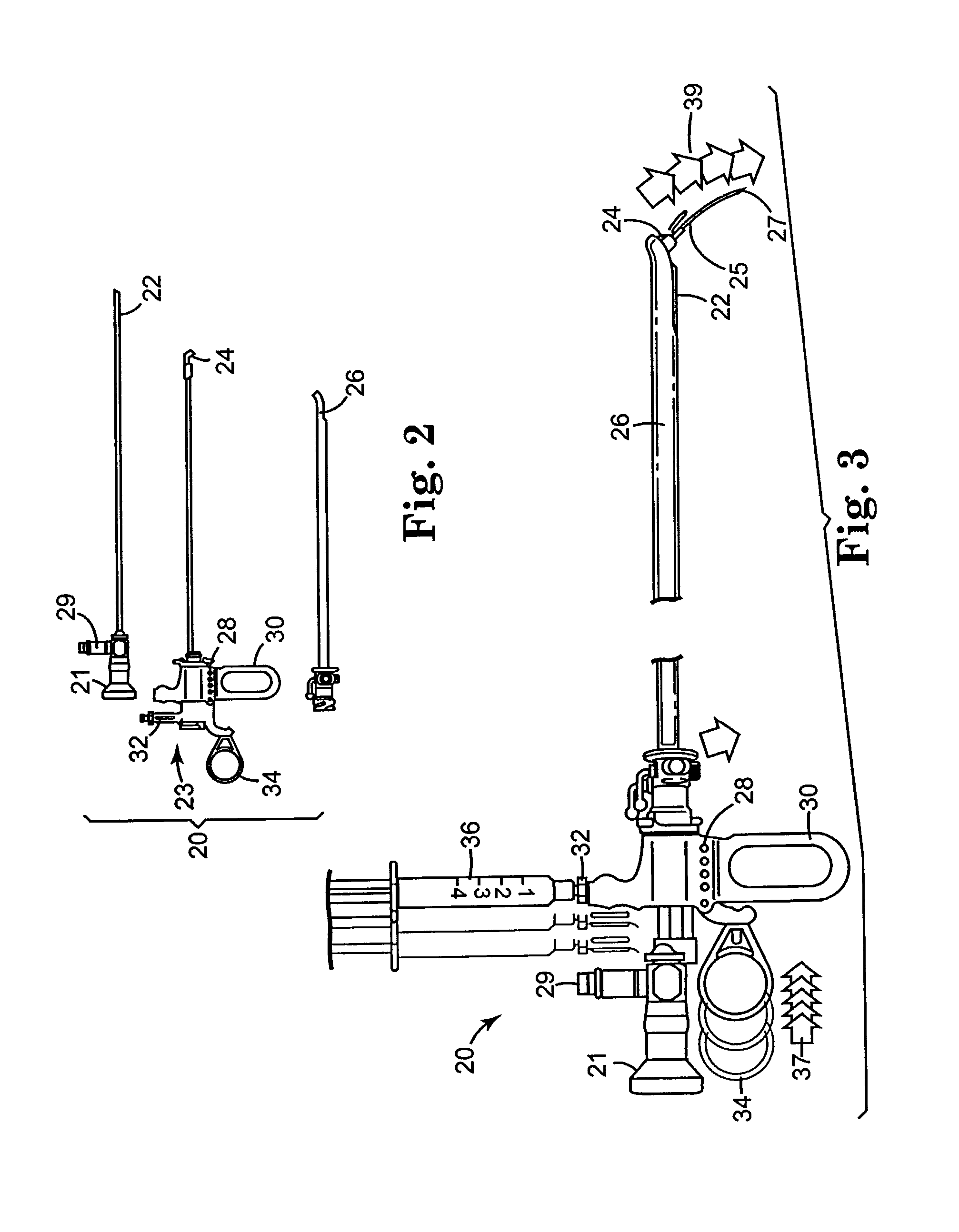
Regimen for treating prostate tissue and surgical kit for use in the regimen
InactiveUS7015253B2Decreasing prostate sizeSmall sizeBiocideHydroxy compound active ingredientsSteroidal antiandrogenRegimen
The present invention provides treatment regimens for treating diseased prostate tissue, including the steps of chemically ablating prostate tissue and coadministering an antiandrogen. In some embodiments, prostate tissue is chemically ablated by injection of ethanol, or an injectable gel comprising ethanol, into prostate tissue. Steroidal and non-steroidal antiandrogens are suitable antiandrogens. One suitable non-steroidal antiandrogen is bicalutamide. The treatment regimen is suitable for treatment of prostate tissue diseases including benign prostatic hyperplasia and prostatic carcinoma. The invention further provides a treatment regimen for treating benign prostatic hyperplasia, including the steps of damaging prostate tissue and coadministering an antiandrogen. Also provided by the present invention is a kit for treating a human male, including a means for necrosing prostate tissue, an antiandrogen drug, and a means for administering the antiandrogen drug. A kit including a first surgical device for delivering a chemoablation fluid to prostate tissue transurethrally, an antiandrogen drug such as bicalutamide, and a second surgical device for administering the antiandrogen drug, is further provided.
Owner:BOSTON SCI SCIMED INC
Vitamin D3 mimics
The present invention relates to non-secosteroidal compounds which activate and modulate the vitamin D receptor (VDR). Because compounds of the present invention display many of the beneficial properties of 1,25(OH)2D3, but with reduced calcium mobilization effects, they may be used advantageously to treat and prevent conditions that show vitamin D sensitivity. Such disease states typically show abnormal calcium regulatory, abnormal immune responsive, hyperproliferative, and / or neurodegenerative characteristics.
Owner:LIGAND PHARMA INC
Endometriosis treatment protocol
The endometriosis treatment protocol provides for administering to a female patient in need of treatment for endometriosis a pharmaceutical composition in a form suitable for vaginal or rectal delivery having a pharmaceutically effective amount of an aromatase inhibitor, which may be either a steroid or non-steroidal. The pharmaceutical composition may be formed as a vaginal suppository, a rectal suppository, a vaginal gel, a rectal gel, a vaginal cream or a rectal cream. The pharmaceutical composition may optionally have pharmaceutically effective amounts of progesterone and calcitriol, and may be administered in combination with an oral COX-2 inhibitor. Alternatively, the pharmaceutical composition comprises an aromatase inhibitor administered vaginally or rectally and is administered in combination with oral calcitriol and the oral COX-2 inhibitor. The aromatase inhibitor is either steroidal or non-steroidal.
Owner:SHIPPEN EUGENE R
Stable povidone-iodine compositions with steroids or non-steroidal Anti-inflammatories
Owner:TAKEDA PHARMA CO LTD
Multi-substitued selective androgen receptor modulators and methods of use thereof
InactiveUS7803970B2Reduce incidenceDecreasing regressionBiocideUrea derivatives preparationAging maleHyperplasia
Owner:UNIV OF TENNESSEE RES FOUND
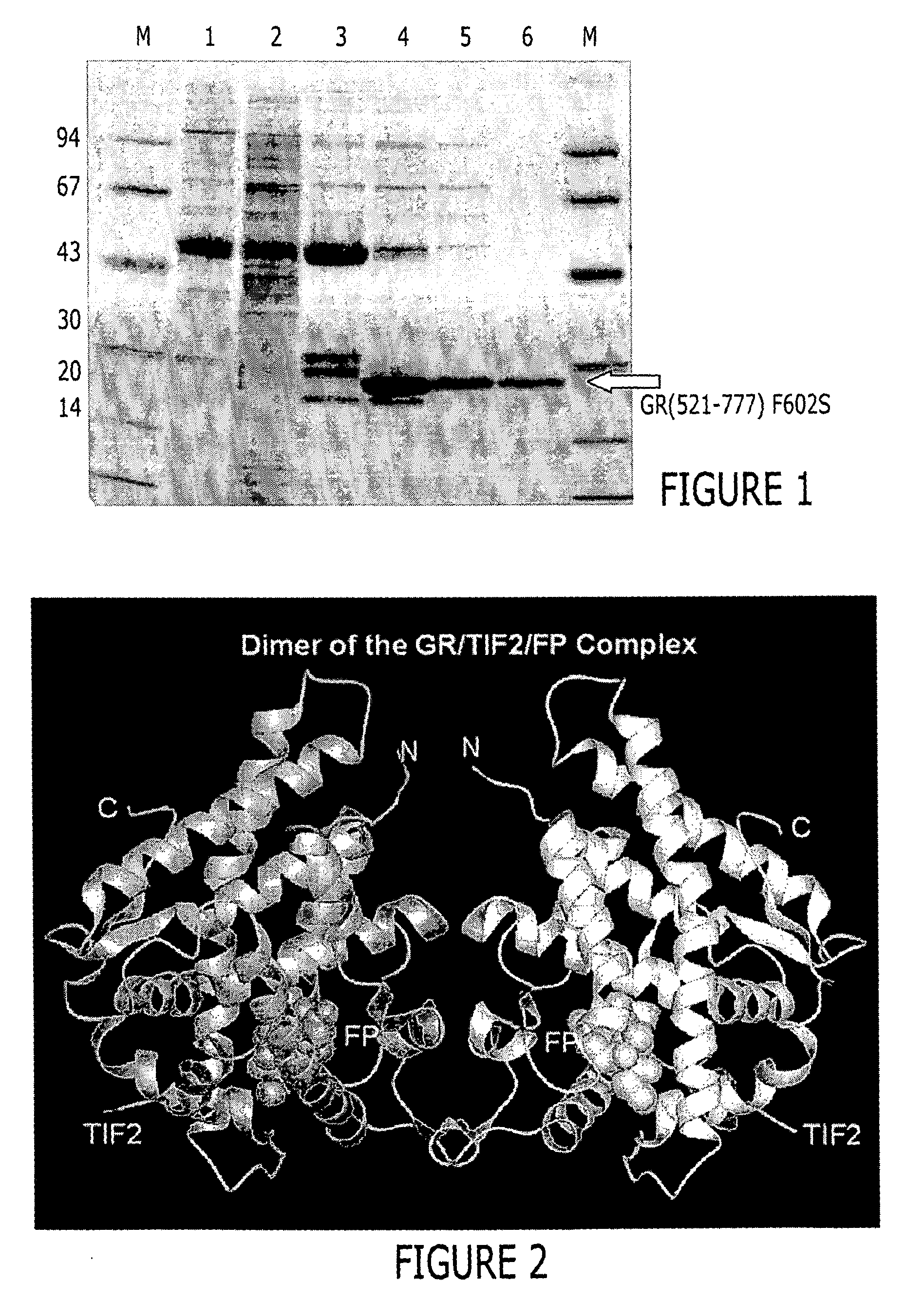
Structure of a glucocorticoid receptor ligand binding domain comprising an expanded binding pocket and methods employing same
InactiveUS20070020684A1Peptide/protein ingredientsBiological material analysisFluticasone propionatePR - Progesterone receptor
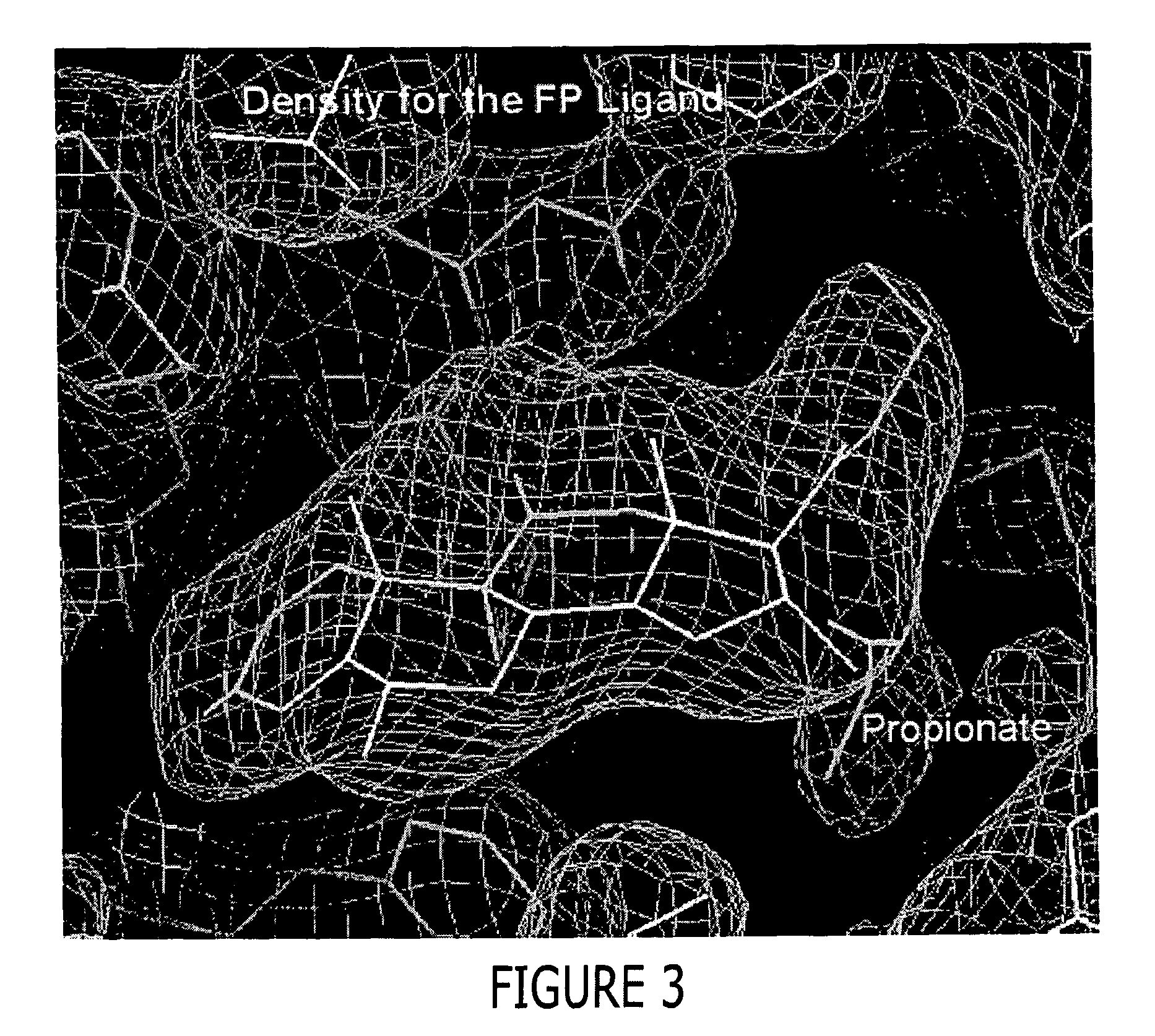
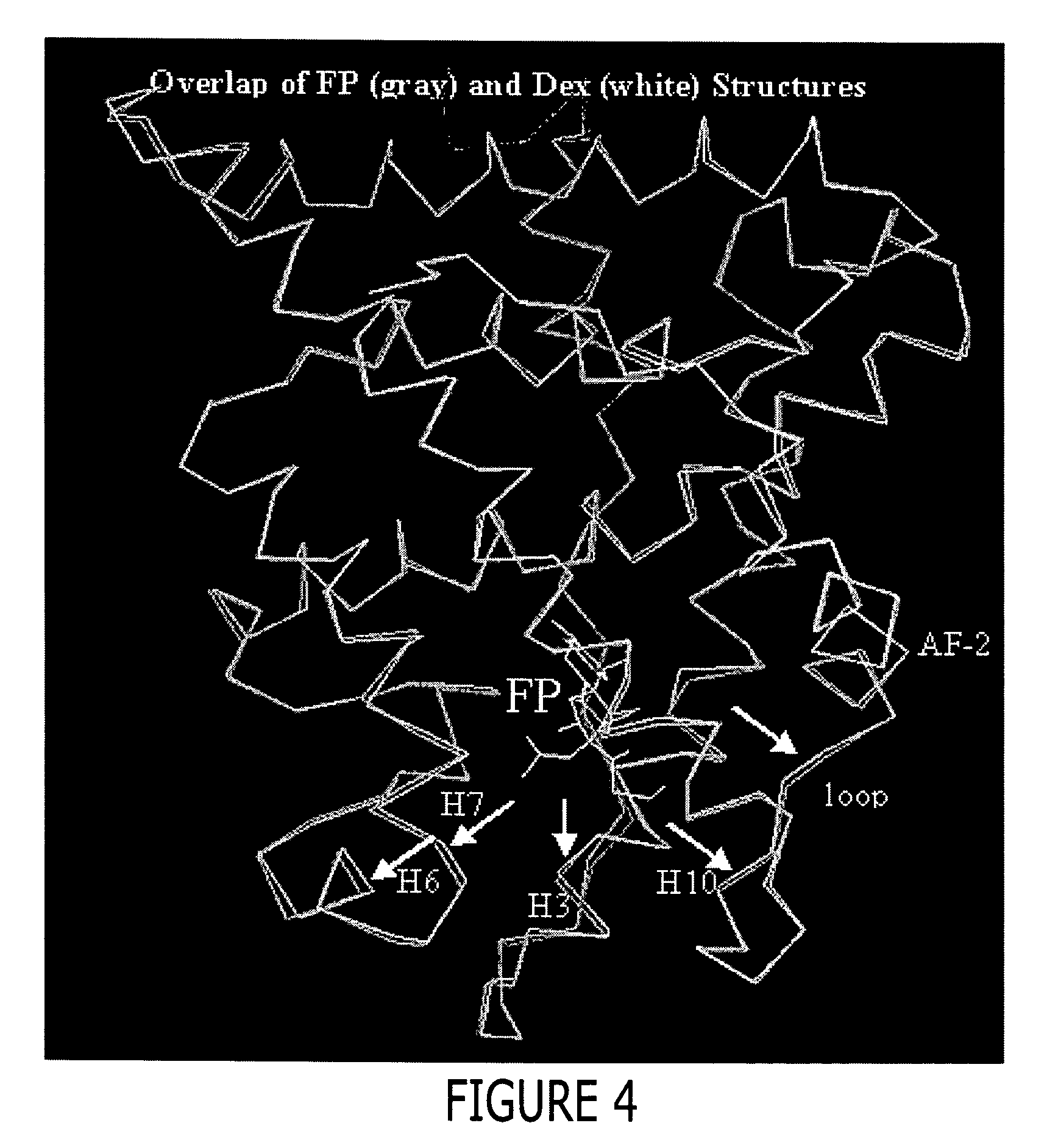
A solved three-dimensional crystal structure of a glucocorticord receptor (GR) α ligand binding domain polypeptide is disclosed, in the form of a crystalline glucocorticord receptor α ligand binding domain polypeptide in complex with the ligand fluticasone propionate (FP) and a peptide derived from the co-activator TIF2. The GR / FP / TIF2 structure includes an expanded binding pocket not seen in other GR structures. Methods of designing steroid and non-steroid modulators of the biological activity of GR and other nuclear receptors (NRs) are also disclosed. In another aspect of the present invention homology models of androgen receptor (AR), progesterone receptor (PR) and mineralcorticoid receptor (MR) are disclosed, as well as methods of forming homology models for other NRs. Methods of forming a soluble GR / FP / TIF2 complex are also disclosed.
Owner:SMITHKLINE BECKMAN CORP
Vitamin D receptor modulators
The present invention relates to novel, non-secosteroidal, hydroxyl substituted, carbon-linked diaryl compounds with vitamin D receptor (VDR) modulating activity that are less hypercalcemic than 1?,25 dihydroxy vitamin D3. These compounds are useful for treating bone disease and psoriasis.
Owner:ELI LILLY & CO
Thermostable carboxylesterase gene, coding protein and application thereof
The invention discloses a thermostable carboxylesterase gene, coding protein and application thereof, the esterase has amino acid sequence shown in SEQ ID NO:1. Compared with the prior art, the esterase containing EstW is thermostable esterase obtained from streptomycete in known mesophilic bacteria, can be widely applied to production of non-steroidal anti-inflammatory drugs or ferulic acid.
Owner:ANHUI NORMAL UNIV
Macromolecule-containing sustained release intraocular implants and related methods
InactiveCN101102733AExtended release timeSuccessful treatment outcomeOrganic active ingredientsSenses disorderTolerabilityCyclodextrin
Drug delivery systems suitable for administration into the interior of an eye of a person or animal are described. The present systems include one or more components which are effective in improving a release profile of a drug from the system, improving the stability of the drug, and improving the ocular tolerability of the drug. The present systems include one or more therapeutic agents in amounts effective in providing a desired therapeutic effect when placed in an eye, and an excipient component with reduced toxicity to retinal cells. The excipient component may include a cyclodextrin component that may be complexed with the therapeutic agents to provide advantages over existing intraocular drug delivery systems. The cyclodextrin component of the present systems have a reduced toxicity relative to benzyl alcohol or polysorbate 80. The drug delivery systems include one or more drug delivery elements such as microparticles, bioerodible implants, non-bioerodible implants, and combinations thereof. Methods of using and producing the drug delivery systems are also described.
Owner:ALLERGAN INC
Nitrosated and nitrosylated cyclooxygenase-2 inhibitors, compositions and methods of use
InactiveUS7166618B2Improving gastrointestinal property of COX-Promote wound healingBiocideSenses disorderSedating AntihistaminesHydrolase inhibitor
The present invention describes novel nitrosated and / or nitrosylated cyclooxygenase 2 (COX-2) inhibitors and novel compositions comprising at least one nitrosated and / or nitrosylated cyclooxygenase 2 (COX-2) inhibitor, and, optionally, at least one compound that donates, transfers or releases nitric oxide, stimulates endogenous synthesis of nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor or is a substrate for nitric oxide synthase, and / or optionally, at least one therapeutic agent, such as, steroids, nonsteroidal antiinflammatory compounds (NSAID), 5-lipoxygenase (5-LO) inhibitors, leukotriene B4 (LTB4) receptor antagonists, leukotriene A4 (LTA4) hydrolase inhibitors, 5-HT agonists, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) inhibitors, H2antagonists, antineoplastic agents, antiplatelet agents, decongestants, diuretics, sedating or non-sedating anti-histamines, inducible nitric oxide synthase inhibitors, opioids, analgesics, Helicobacter pylori inhibitors, proton pump inhibitors, isoprostane inhibitors, and mixtures thereof. The present invention also provides novel compositions comprising at least one parent COX-2 inhibitor and at least one nitric oxide donor, and, optionally, at least one therapeutic agent. The present invention also provides kits and methods for treating inflammation, pain and fever; for treating and / or improving the gastrointestinal properties of COX-2 inhibitors; for facilitating wound healing; for treating and / or preventing renal toxicity; and for treating and / or preventing other disorders resulting from elevated levels of cyclooxygenase-2.
Owner:NICOX SA
Patch containing nonsteroidal antinflammatory and analgesic agent
ActiveUS20060172002A1Excellent long-term storage stabilityImprove solubilityBiocideAntipyreticPolyethylene glycolNon steroidal anti inflammatory
An adhesive patch containing a non-steroidal anti-inflammatory agent, comprising a support and an adhesive layer laminated on this support, wherein the adhesive layer contains a non-steroidal anti-inflammatory agent having a carboxyl group or its salt, and polyethylene glycol having an average molecular weight of 1000 or more.
Owner:HISAMITSU PHARM CO INC
GABA enhancers in the treatment of diseases relating to reduced neurosteroid activity
The invention provides the use of a non-steroid compound which acts on the GABA receptor for the treatment of disorders relating to reduced neurosteroid activity. The non-steroid compounds may be GABA agonists, GABA uptake inhibitors or enhancers of GABAergic activity.
Owner:H LUNDBECK AS
Vitamin D receptor modulators
The present invention relates to novel, non-secosteroidal, diaryl compounds with vitamin D receptor (VDR) modulating activity that are less hypercalcemic than 1α,25 dihydroxy vitamin D3. These compounds are useful for treating bone disease and psoriasis.
Owner:ELI LILLY & CO
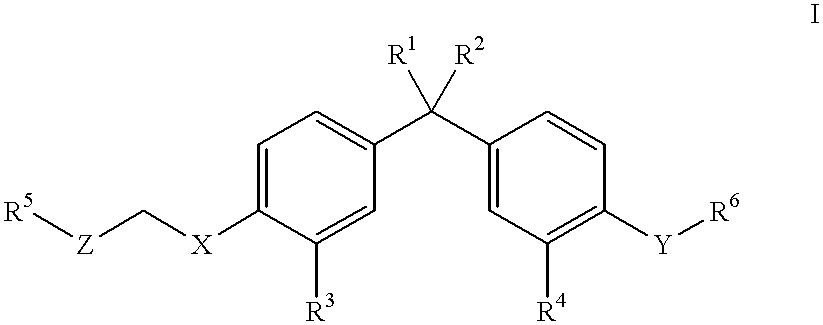
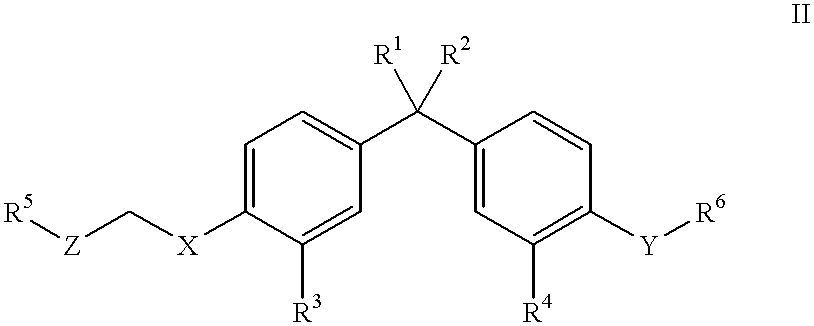
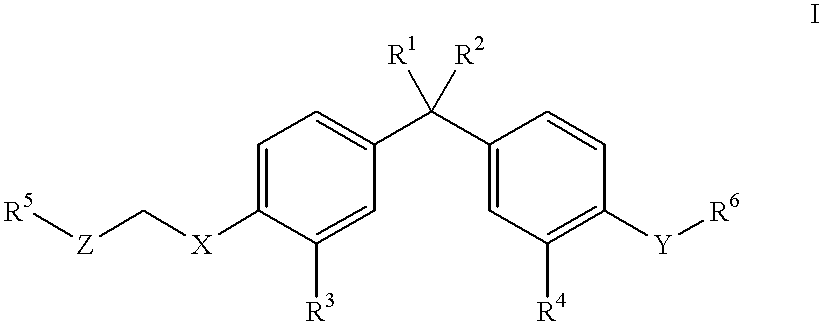
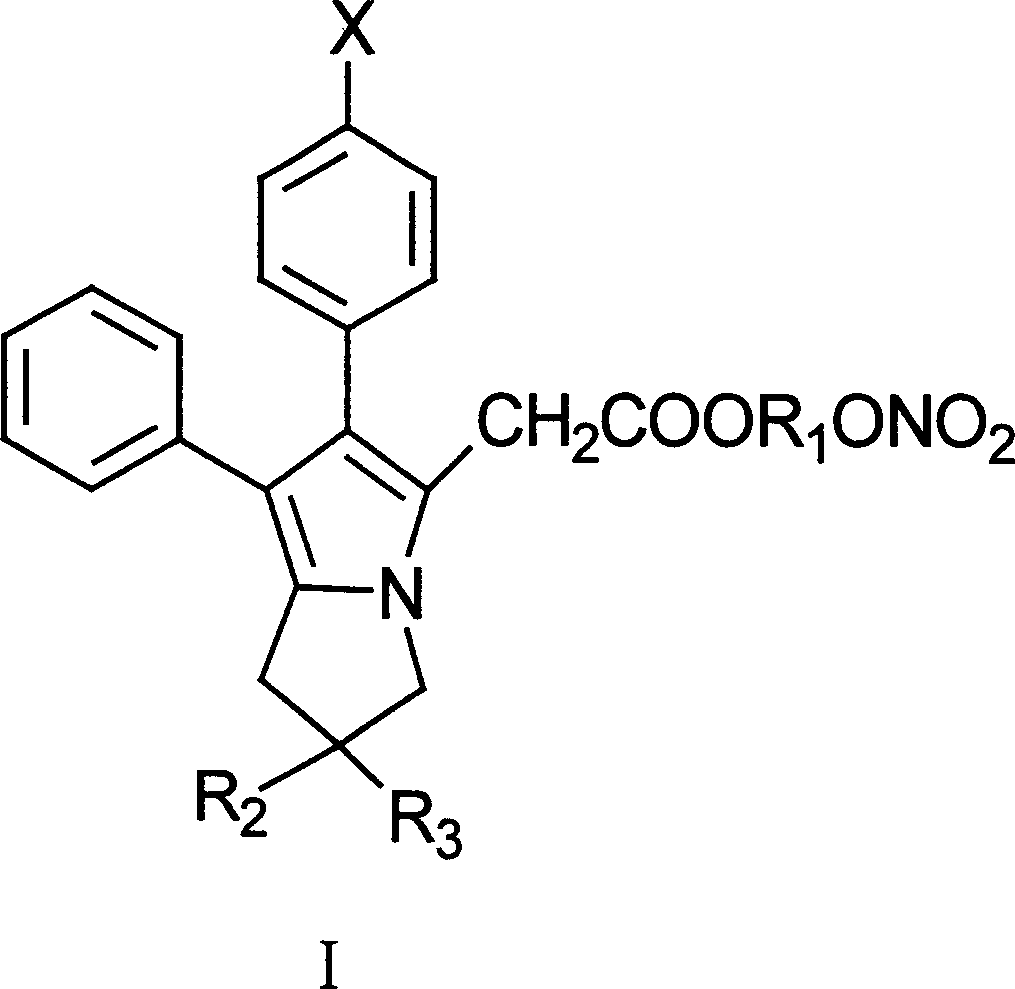
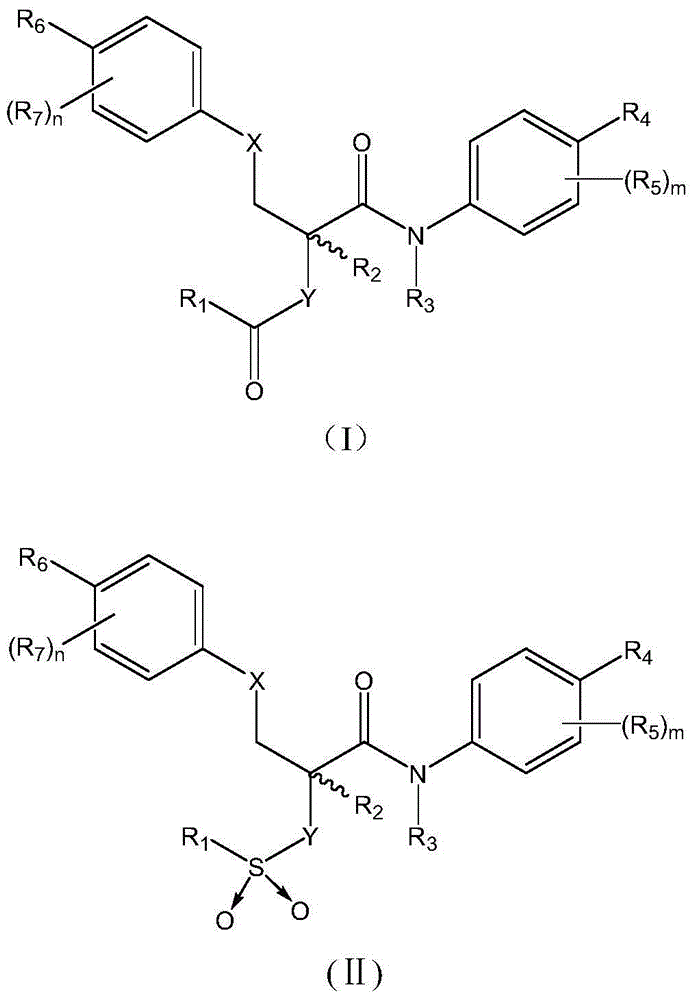
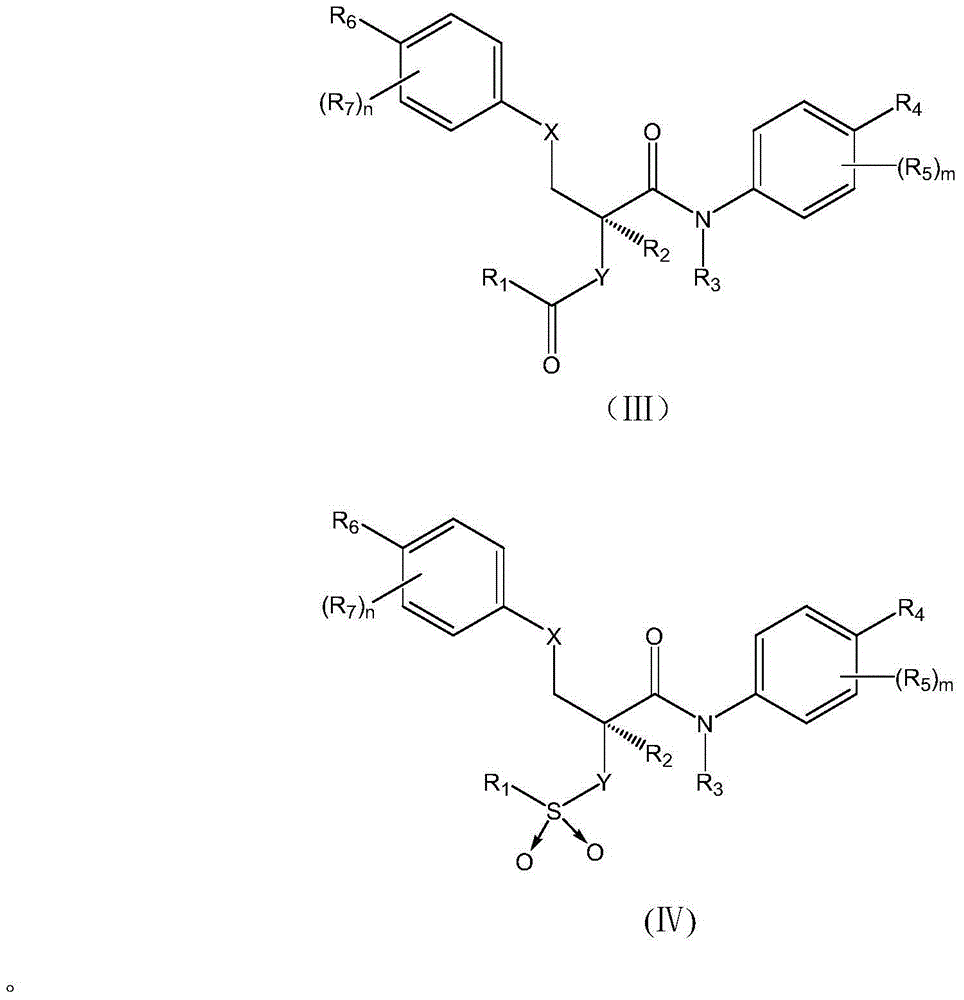
Antipyretic analgesic and anti-arthritis nonsteroidal compound and its pharmaceutical compositions
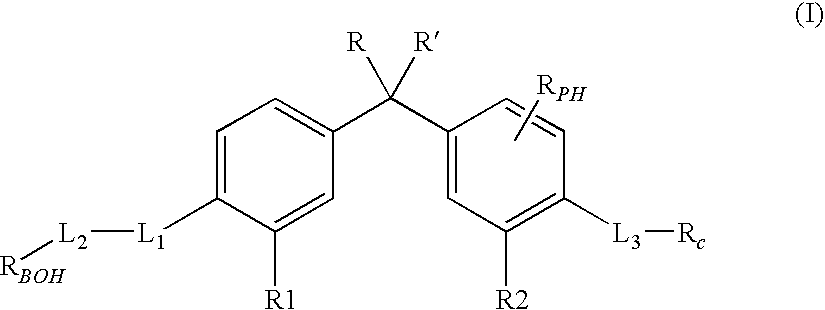
The invention relates a group of compounds having formula (I) and their use as medicaments for non-steroidal analgesic analgesia, anti-arthritis, and protecting gastrointestinal tract mucous membrane. The invention also discloses the pharmaceutical compositions containing the compounds of formula (I), wherein R1, R2, R3 and X are defined in the specification.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +1
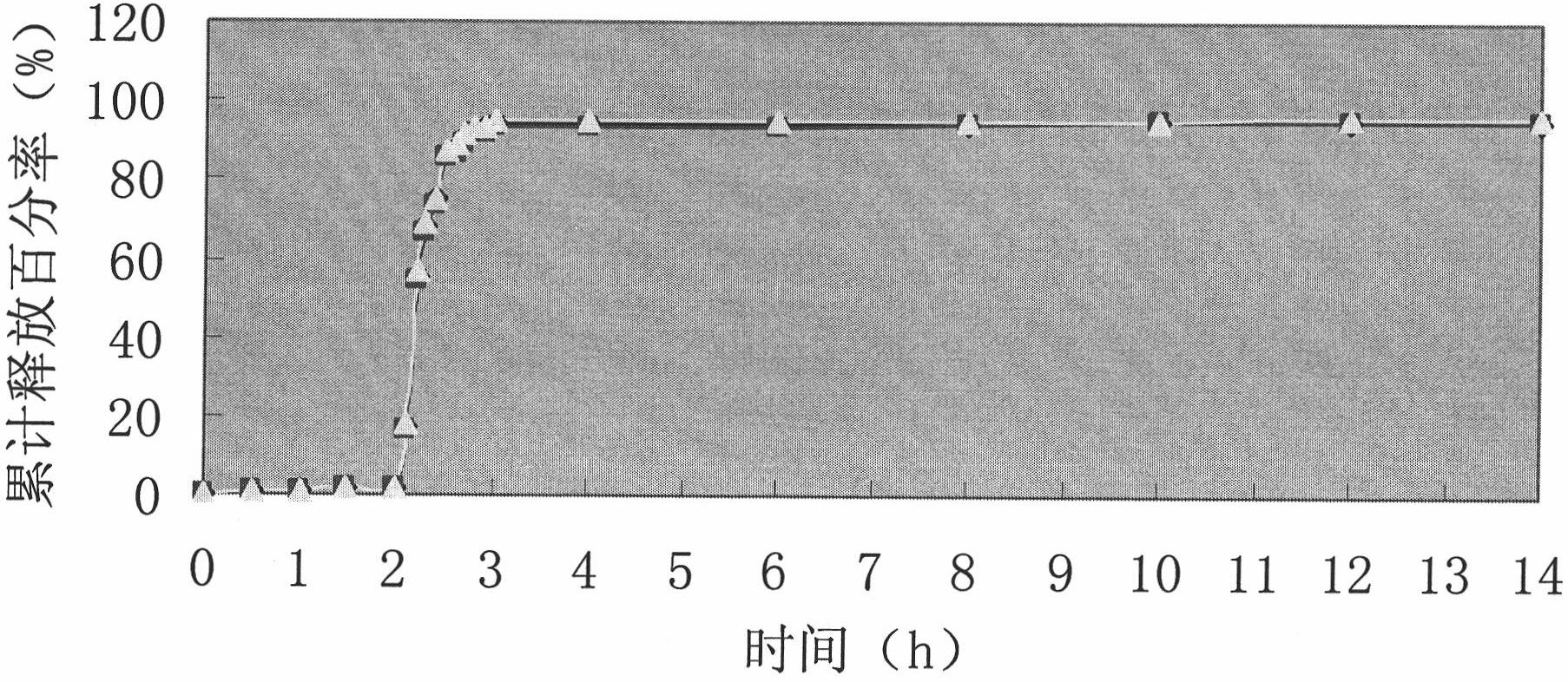
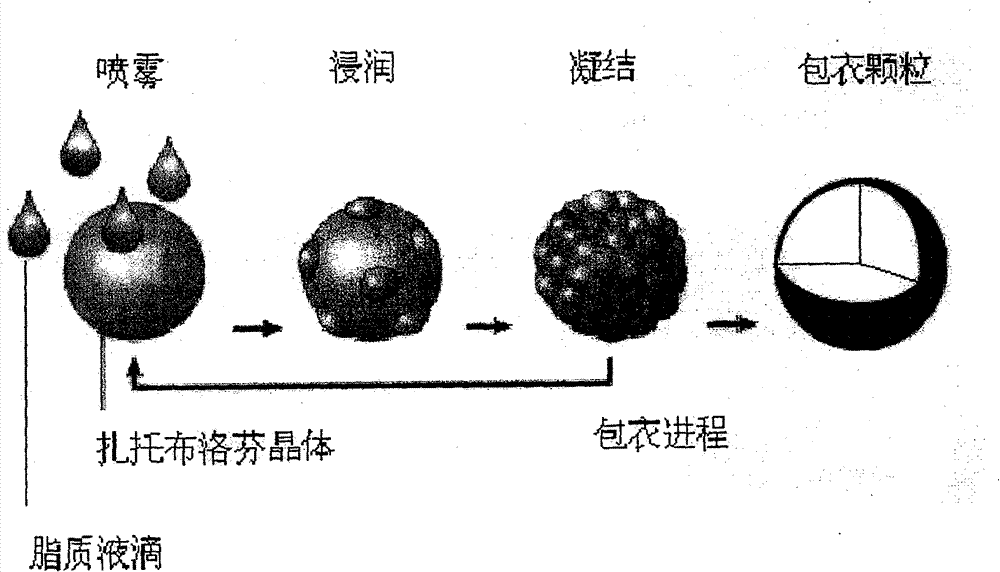
Enteric sustained-release preparation containing zaltoprofen and preparation method thereof
The invention discloses a new formulation of novel nonsteroidal antiinflammatory medicament-zaltoprofen, namely an enteric sustained-release preparation containing zaltoprofen. Matrix which is insoluble in water and is insoluble in gastrointestinal fluid is used as a sustained-release auxiliary material to prepare the enteric sustained-release preparation. The enteric sustained-release preparation prepared in the invention has no fast release phenomenon of the medicament, and the repeatability of in vitro medicament release action of the preparation with different batches is good, so as to bebeneficial to keep in-vivo plasma concentration of a patient stable after taking the medicament.
Owner:HEBEI AOXING GROUP PHARMA
Aromatic amide compound as well as preparation method and application thereof
The invention provides an aromatic amide compound which is a new selective nonsteroidal androgen receptor modulator, has an effect on modulating an androgen receptor, can be used for treating or preventing various diseases related with androgen, such as male androgen deficiency (ADAM) symptom, female androgen deficiency (ADIF) symptom, muscle wasting, osteoporosis, osteopenia, anemia, obesity, fatty liver, diabetes, dry eyes, muscle wasting caused by diseases such as cancers, AIDS, kidney diseases, burn injury and the like, and also can be used for sports and / or physical enhancers, animal growth promoters, feed additives and the like.
Owner:NINGBO XIJIAN PHARM TECH CO LTD
Compounds with anti-inflammatory activity
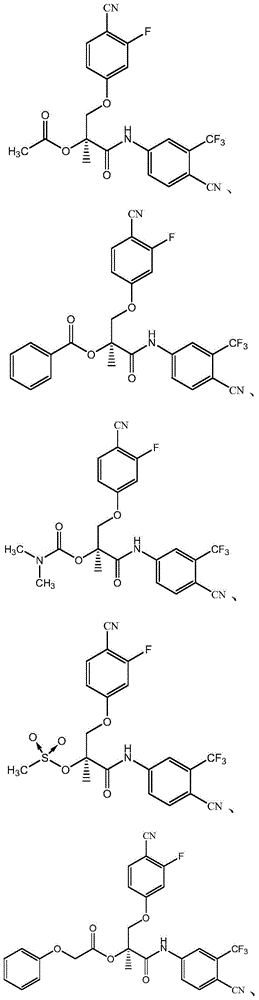
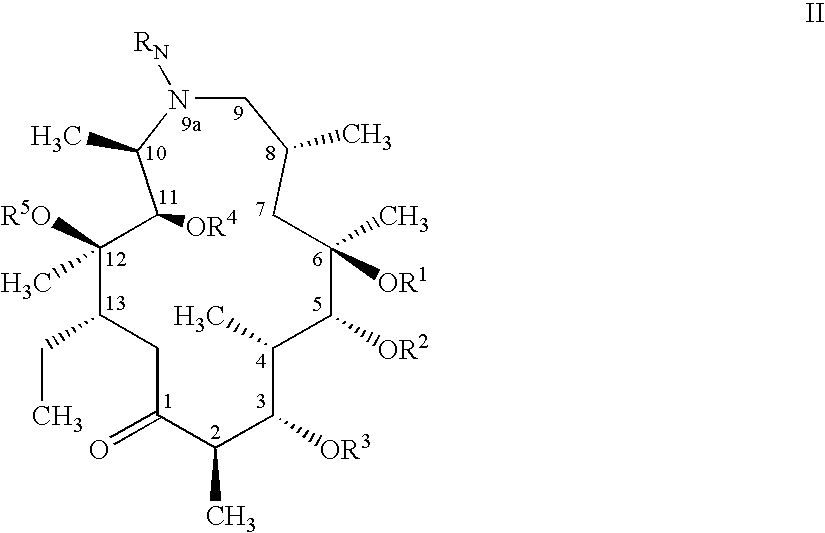
The present invention relates to new compounds represented by Formula I:wherein M represents a macrolide subunit of the substructure II:L represents the chain of the substructure III:—X1—(CH2)m-Q-(CH2)n—X2— IIID represents the steroid or nonsteroidal subunit derived from steroid or nonsteroidal (NSAID) drugs with anti-inflammatory activity;The present invention relates also to pharmaceutically acceptable salts and solvates of such prepared compounds, to process and intermediates for their preparation, as well as to the improved therapeutic action and the use in the treatment of inflammatory diseases and conditions in humans and animals.
Owner:GLAXOSMITHKLINE ISTRAZIVACKI CENTAR ZAGREB D O O
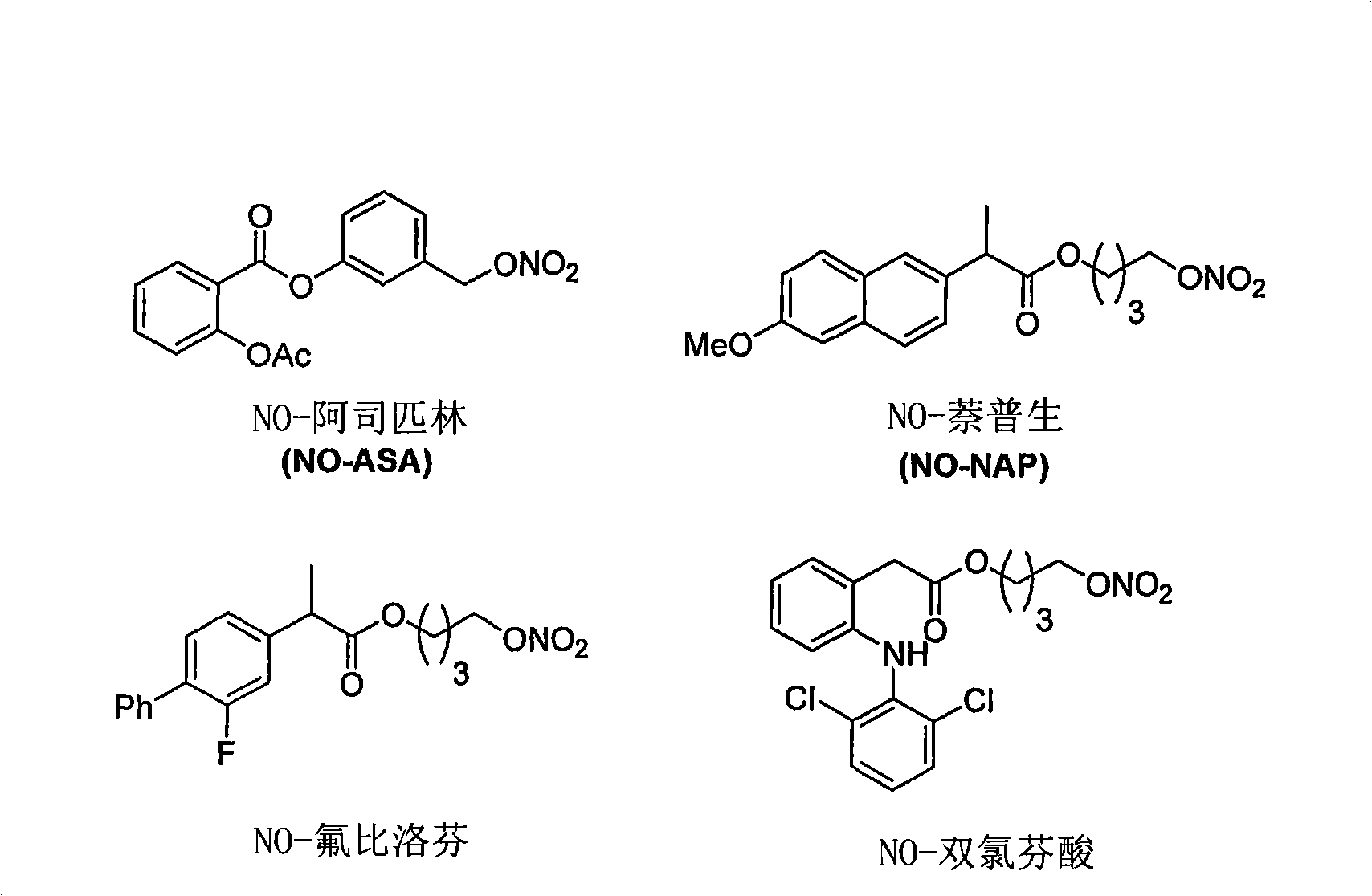
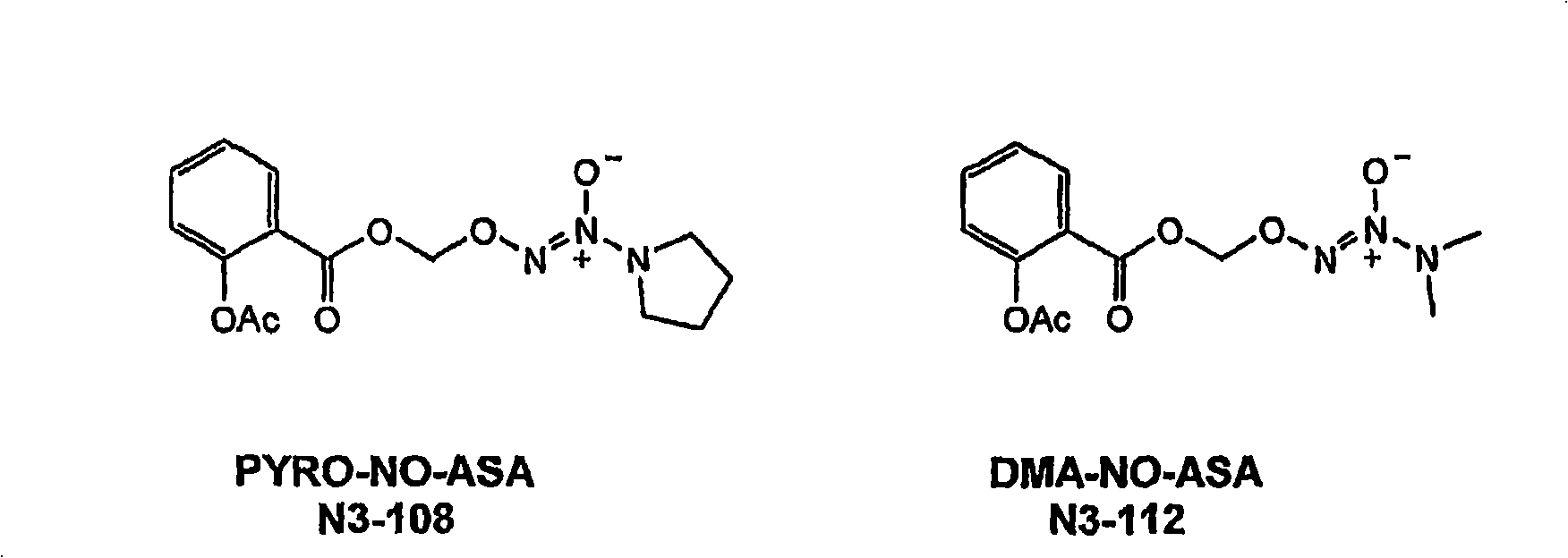
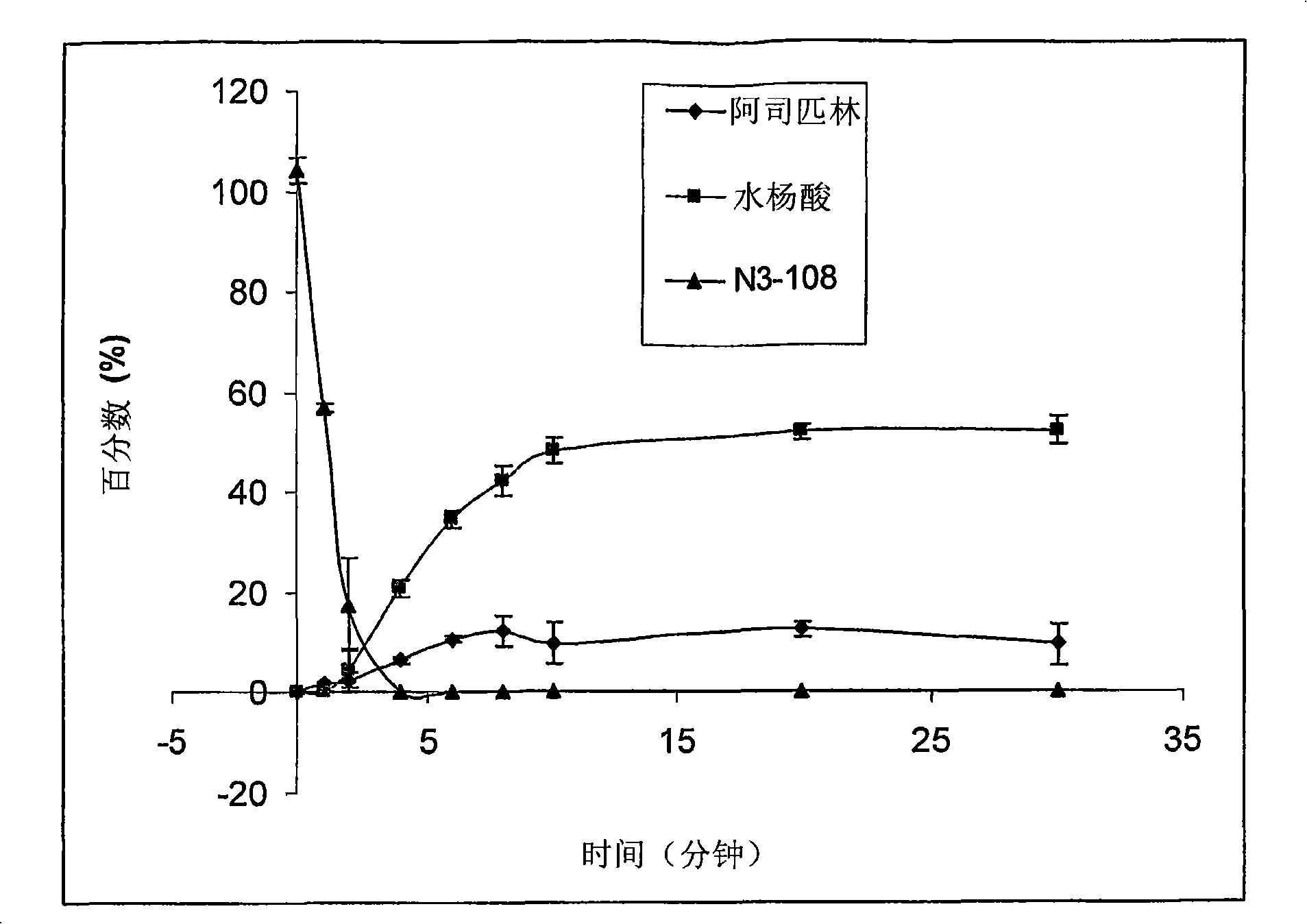
Development of prodrugs possessing a nitric oxide donor diazen-1-ium-1,2-diolate moiety using in vitro/in silico predictions
The present invention provides a method of using a physiologically-based pharmacokinetic model to select a prodrug molecule (NO-X) comprising a therapeutic agent X (e.g. nonsteroidal anti- inflammatory drug, (NSAID)) and an appropriate nitric oxide donor NO. The NSAID can be a non- selective or selective cyclooxygenase inhibitor or other biocompatible compound comprising a carboxyl group. The pharmacokinetic model uses in vitro and / or in silico data to estimate an optimal set of parameters that can predict whether a particular NO-X candidate is capable of producing desirable therapeutic effects, e.g. enhanced antiif lammatory activity, reduced intestinal, cardiac and renal toxicity. Accordingly, the present invention can greatly enhance proper selection of an appropriate candidate for drug development, thereby minimizing development time and conserving costs.
Owner:NOVOKIN BIOTECH
Modulators of glucocorticoid receptor, AP-1, and/or NF-kB activity and use thereof
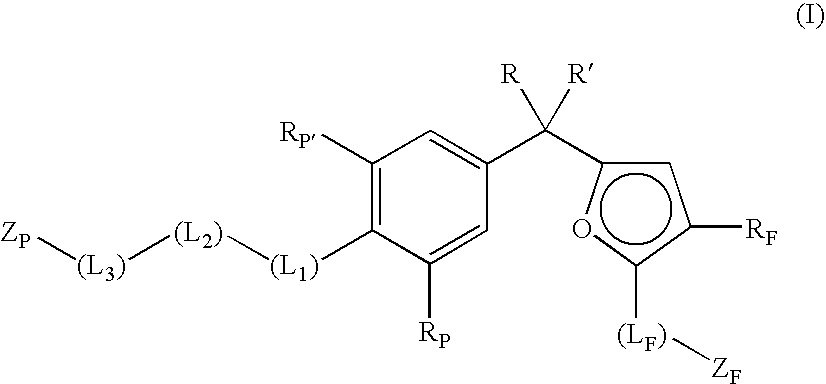
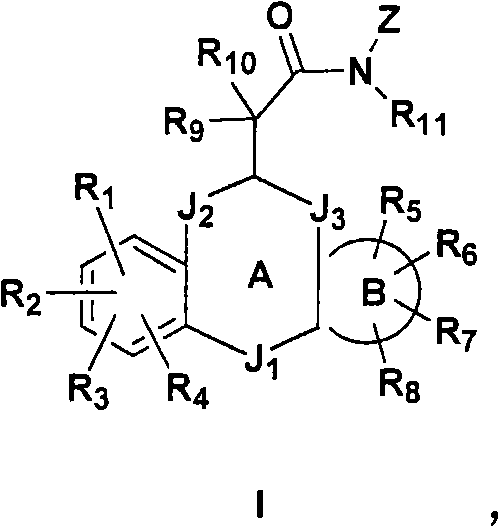
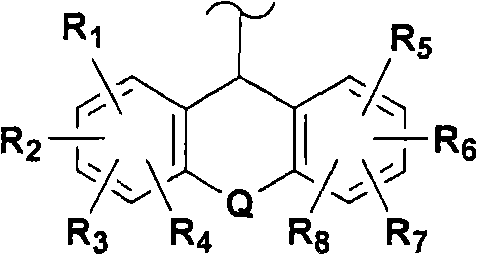
The present invention provides novel non-steroidal compounds which are useful in treating diseases associated with modulation of the glucocorticoid receptor, AP-1, and / or NF-kappaB activity, including inflammatory and immune diseases, having the structure of formula (I): an enantiomer, diastereomer, or tautomer thereof, or a prodrug ester thereof, or a pharmaceutically-acceptable salt thereof, in which: Z is heterocyclo or heteroaryl; A is a 5- to 8-membered carbocyclic ring or a 5- to 8-membered heterocyclic ring; B is a cycloalkyl, cycloalkenyl, aryl, heterocyclo, or heteroaryl ring, wherein each ring is fused to the A ring on adjacent atoms and optionally substituted by one to four groups which are the same or different and are independently selected from R5, R6, R7, and R8; J1, J2, and J3 are at each occurrence the same or different and are independently -A1QA2-; Q is a bond, O, S, S(O), or S(O)2; A1 and A2 are the same or different and are at each occurrence independently selected from a bond, C1-3alkylene, substituted C1-3alkylene, C2-4alkenylene, and substituted C2-4alkenylene, provided that A1 and A2 are chosen so that ring A is a 5- to 8-membered carbocyclic or heterocyclic ring; R1 to R11 are as defined herein. Also the present invention provides pharmaceutical compositions and methods of treating inflammatory- or immune-associated diseases and obesity and diabetes employing said compounds.
Owner:BRISTOL MYERS SQUIBB CO
Glucocorticoid receptor modulators to treat cervical cancer
Methods for treating a subject having a cancerous tumor are disclosed. The methods comprise administering to the subject an effective amount of a non-steroidal selective glucocorticoid receptor modulator (SGRM) and an effective amount of a chemotherapeutic agent. The tumor may be cervical cancer. The SGRM may be a fused azadecalin. In embodiments, the SGRM may be a heteroaryl ketone fused azadecalin or an octahydro fused azadecalin.
Owner:CORCEPT THERAPEUTICS INC
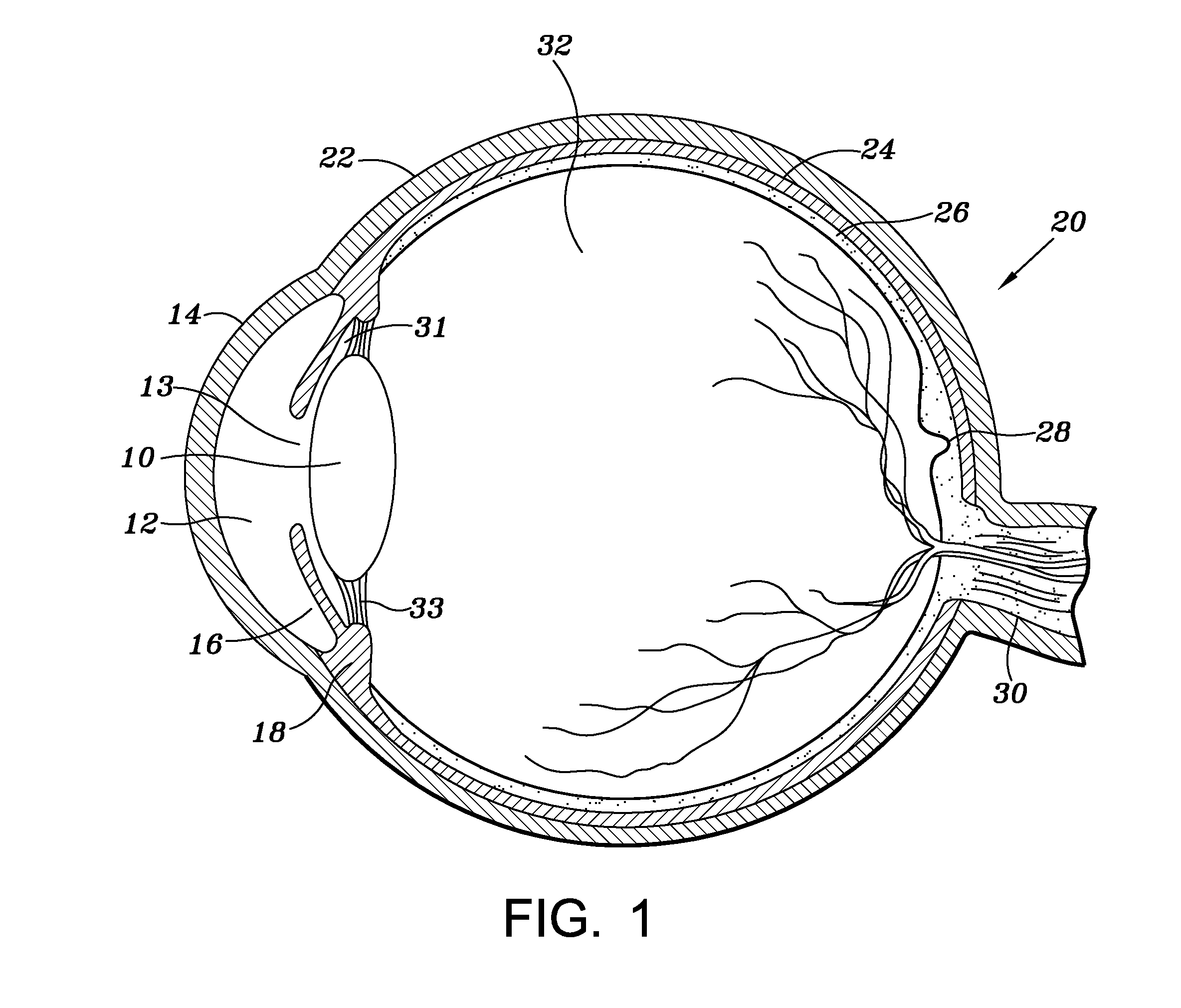
Ocular biodegradable drug implant and method of its use
A biodegradable drug implant includes a PLG matrix and a non-steroid anti inflammation drug, for example diclofenac sodium. The implant is inserted into the eye in the anterior chamber, for example in the ciliary sulcus, following eye surgery. A method for treating and or preventing inflammation of the eye following eye surgery includes placing the implant in the anterior portion of the eye.
Owner:LENSTEC BARBADOS
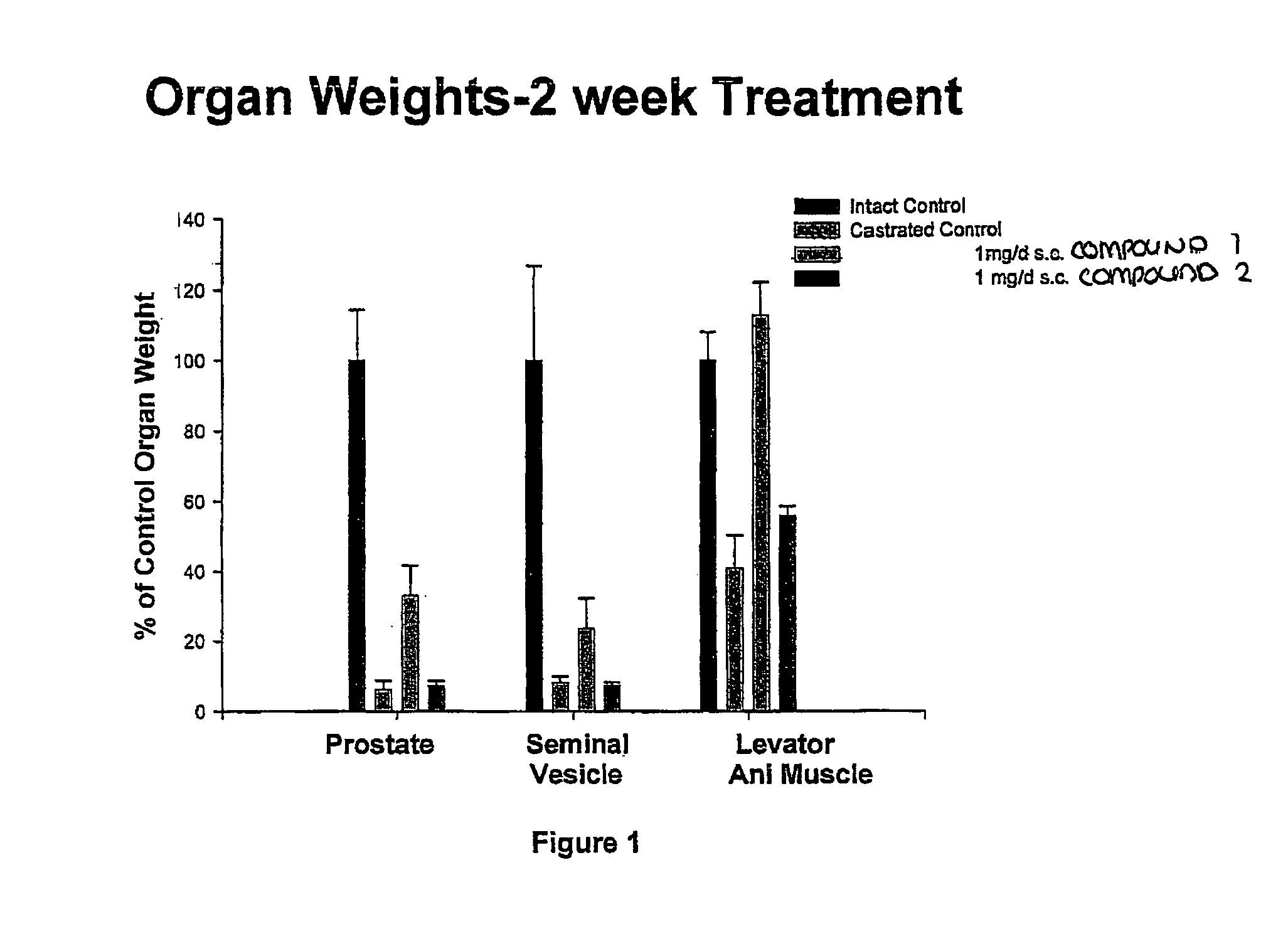
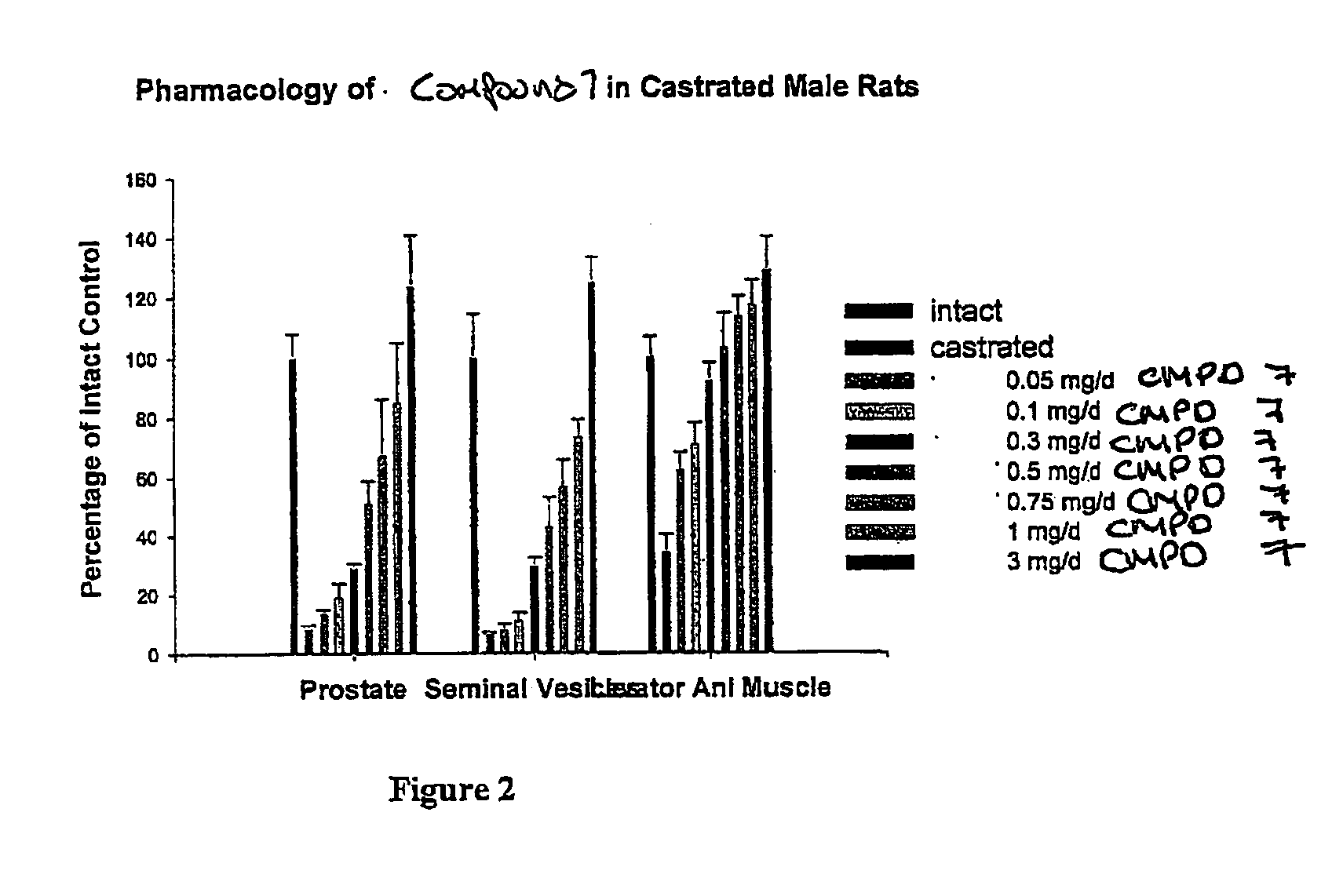
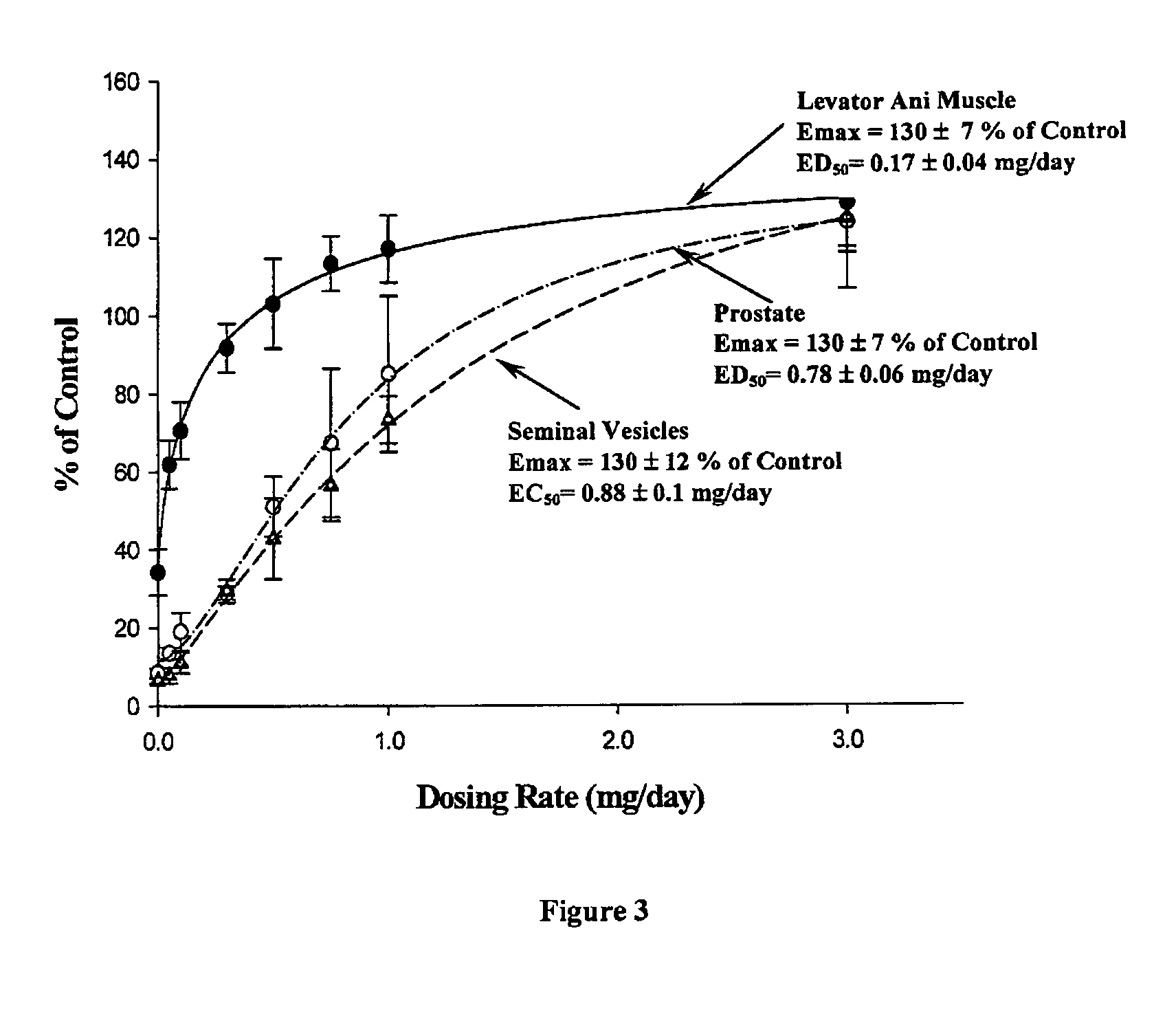
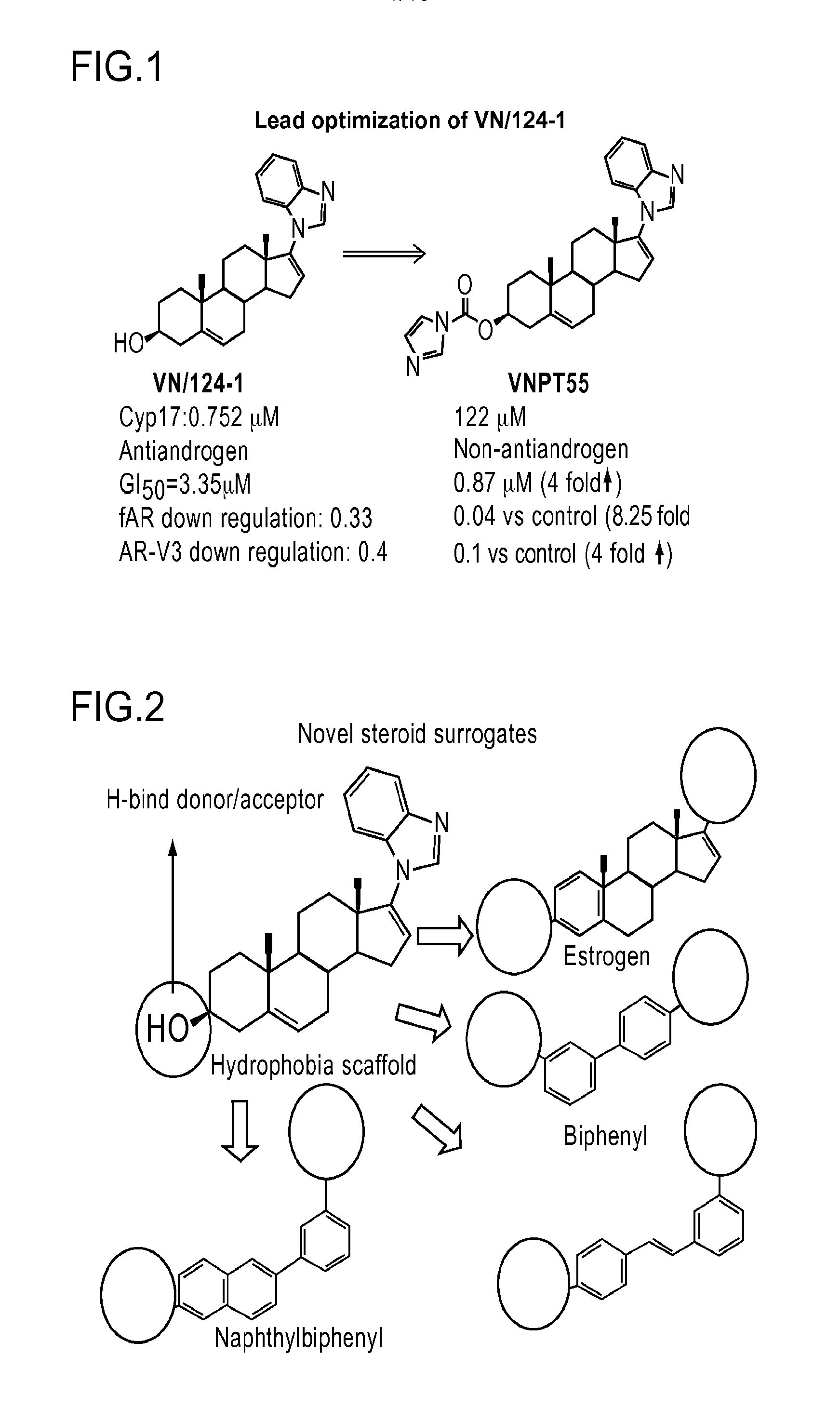
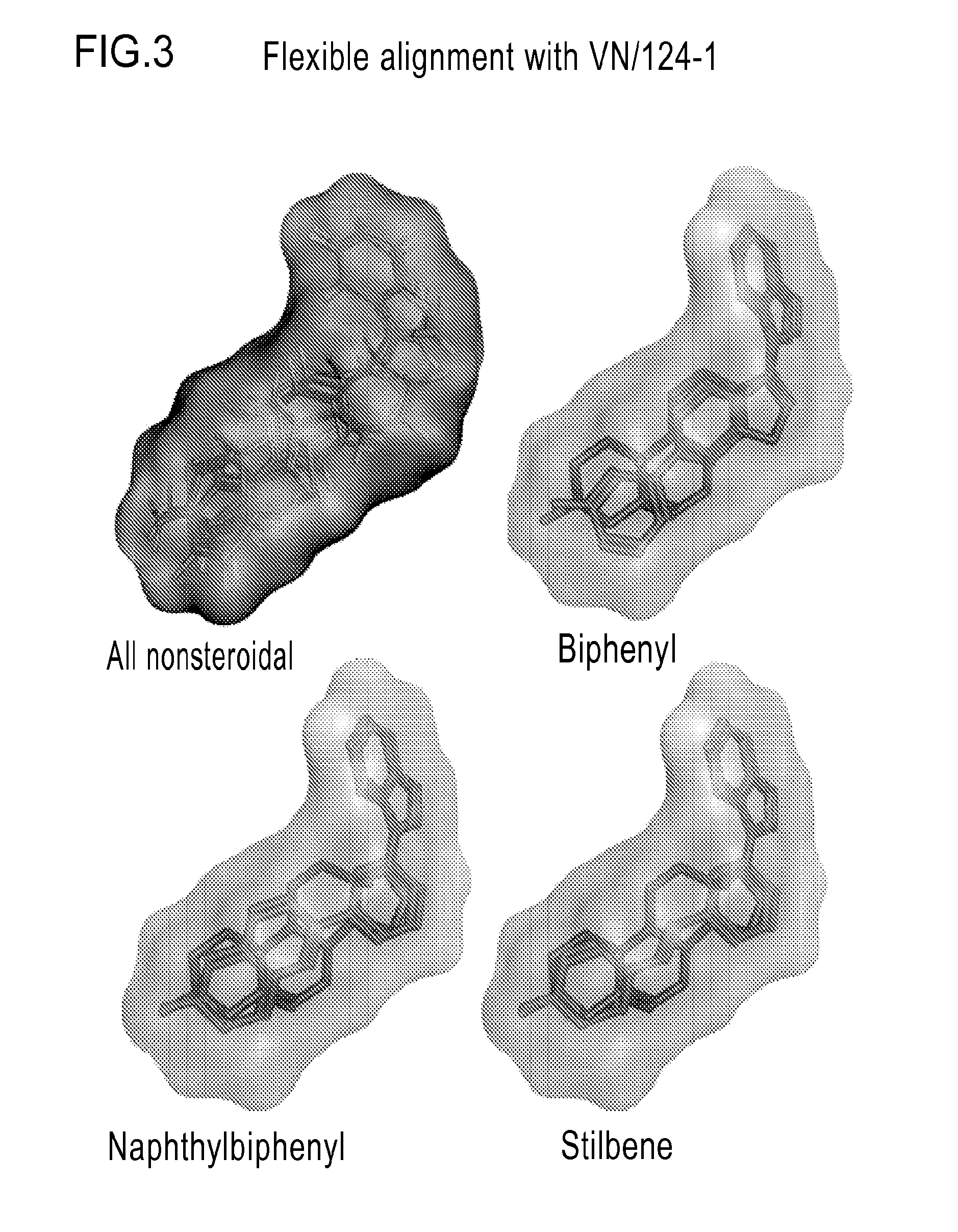
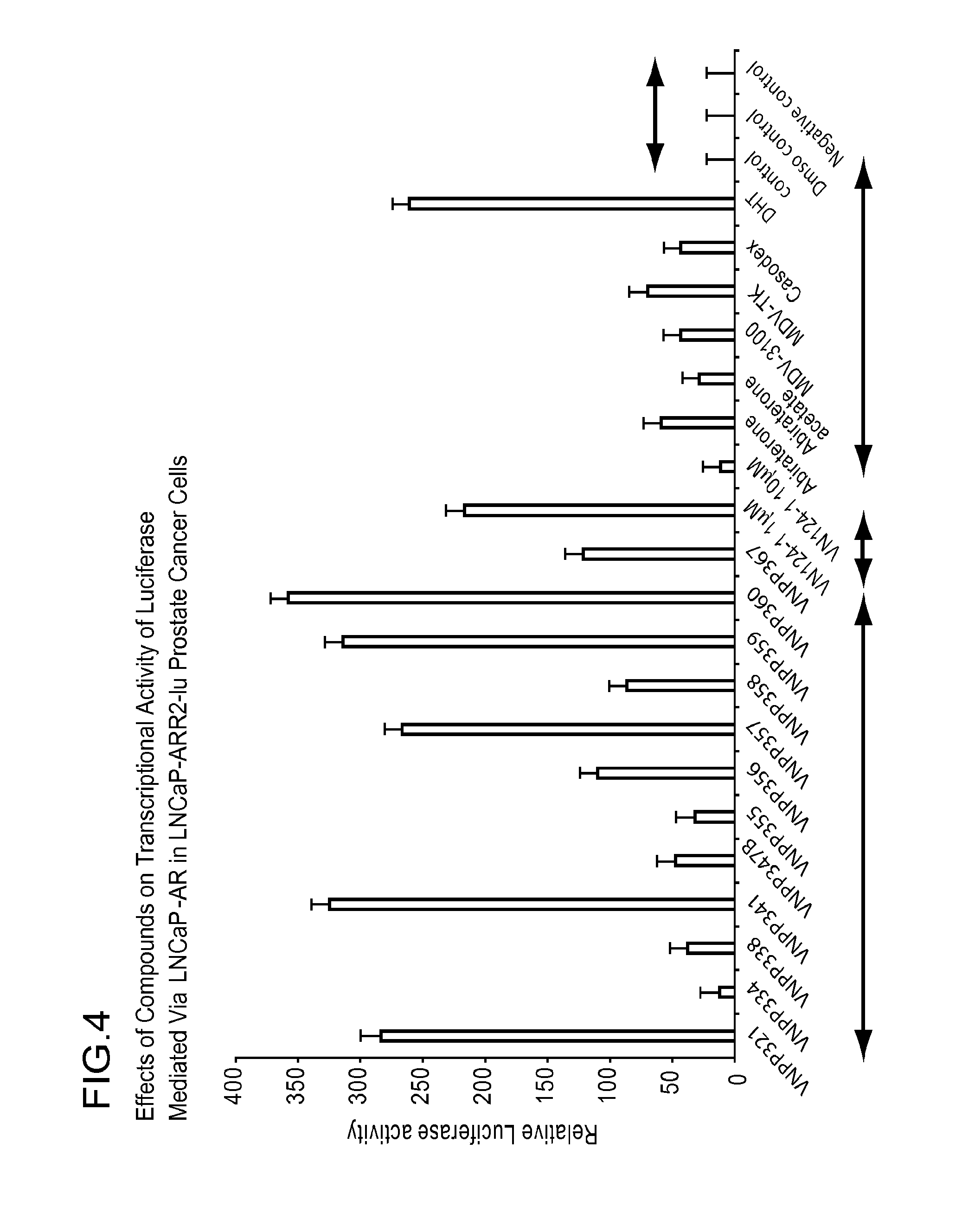
Nonsteroidal and steroidal compounds with potent androgen receptor down-regulation and anti prostate cancer activity
ActiveUS20160038476A1Improved pharmacokinetic propertiesHigh selectivityBiocideUrinary disorderCancer cellSterol
Nonsteroid and steroid compounds that cause down-regulation of the androgen receptor (AR), both full length and splice variant, induce apoptosis and inhibit proliferation of inhibiting proliferation and migration of androgen sensitive cancer cells. The steroid compounds and nonsteroid compounds may be agents for the prevention and / or treatment of cancer, including prostate cancer, castration resistant prostate cancer, bladder cancer, pancreatic cancer, hepatocellular carcinoma, benign prostatic hyperplasia (BPH), Kennedy's disease, androgenetic alopecia, breast cancer, androgen-insensitive syndrome, and spinal and bulbar muscular atrophy.
Owner:UNIV OF MARYLAND
Extended cycle multiphasic oral contraceptive method
InactiveCN101394845AReduce the number of timesAvoid irregular bleedingOrganic active ingredientsSexual disorderPhysiologyPlacebo
A multiphasic method of contraception comprising the steps of sequentially administering to a female of child bearing age a Phase I composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg norethiiidrpne acetate and an estrogen in an amount equivalent to about 5 to abo[mu]t 15 mcg of ethinyl estradiol for about 7 to about 14 days; a Phase II composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 25 meg of ethinyl estradiol for about 14 to about 22 days; a Phase m composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 15 to about 35 meg of ethinyl estradiol for about 20 to about 31 days; and an optional Phase IV composition containing (i) an estrogen in an amount equivalent to about 5 to about 20 meg of ethinyl estradiol, or (ii) a placebo or a non-steroidal component, or (iii) a combination of (i) and (ii), for about 2 to about 8 days. The ethinyl estradiol equivalent amount of estrogen in each of the successive Phases II and III is at least 5 meg greater than the ethinyl estradiol equivalent amount of estrogen in the immediately-preceding phase.
Owner:沃纳奇尔科特公司
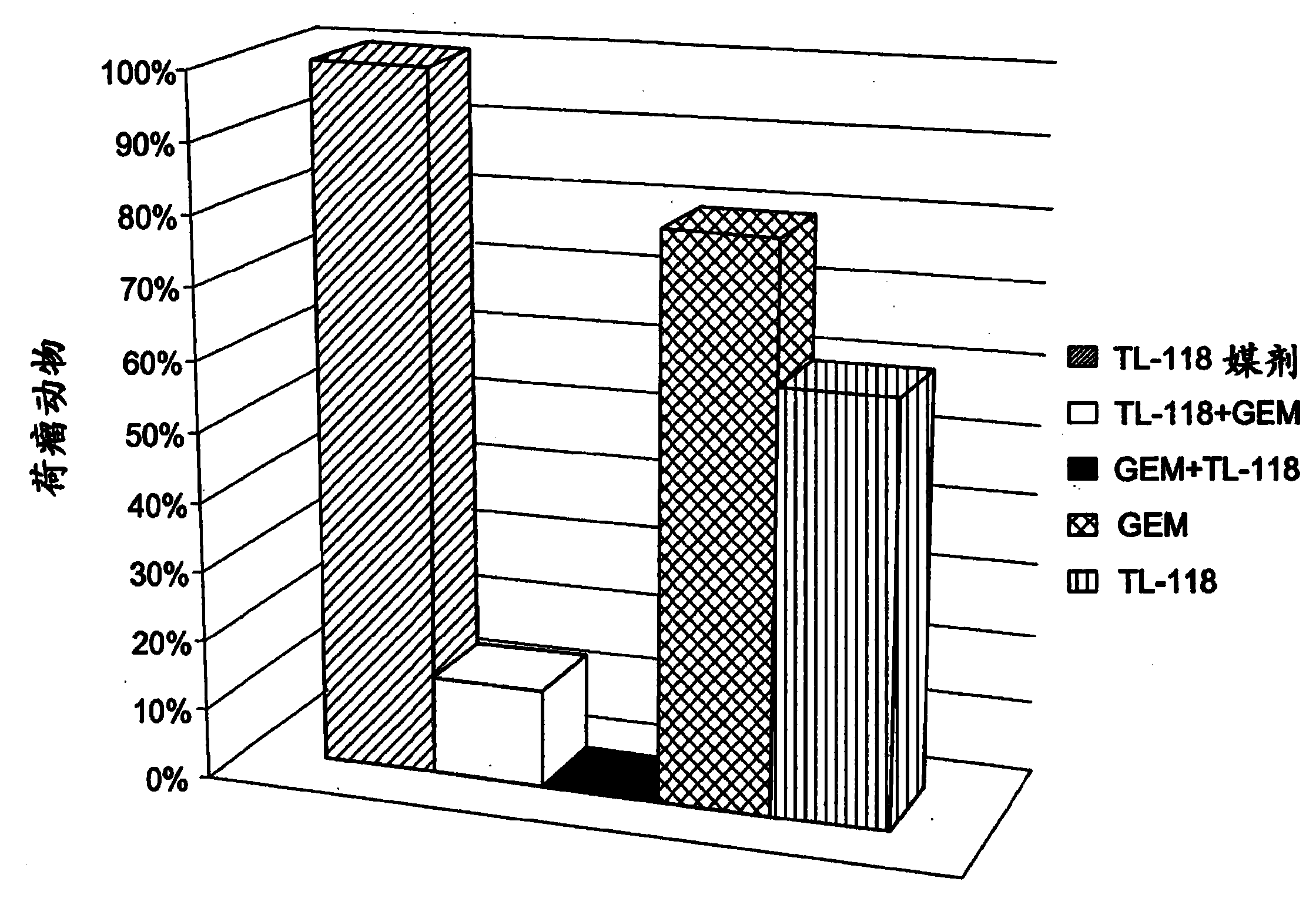
Combination therapy for the treatment of cancer
The present invention provides a combination comprising: a composition comprising at least one non-steroidal anti-inflammatory agent and at least one cytotoxic agent; and a composition comprising at least one ribonucleotide reductase inhibitor. The invention further includes kits comprising a combination of the invention and methods and uses of a combination of the invention for the treatment of cancer treatment of cancer, including solid tumors or tumor metastasis.
Owner:TILTAN PHARMA LTD
Phenyl-thiophene type vitamin D receptor modulators
Owner:ELI LILLY & CO
Vitamin D Receptor Modulators
InactiveUS20080200552A1Inhibition effectPrevent removalOrganic active ingredientsBiocideArylVitamin D+Metabolites
Owner:ELI LILLY & CO
Method for normalizing neutrophil to lymphocyte proportion in cancer patients by selective glucocorticoid receptor antagonists
PendingCN114650820AReduced responseReduce tumor burdenOrganic active ingredientsOrganic chemistryOncologyBlood vessel
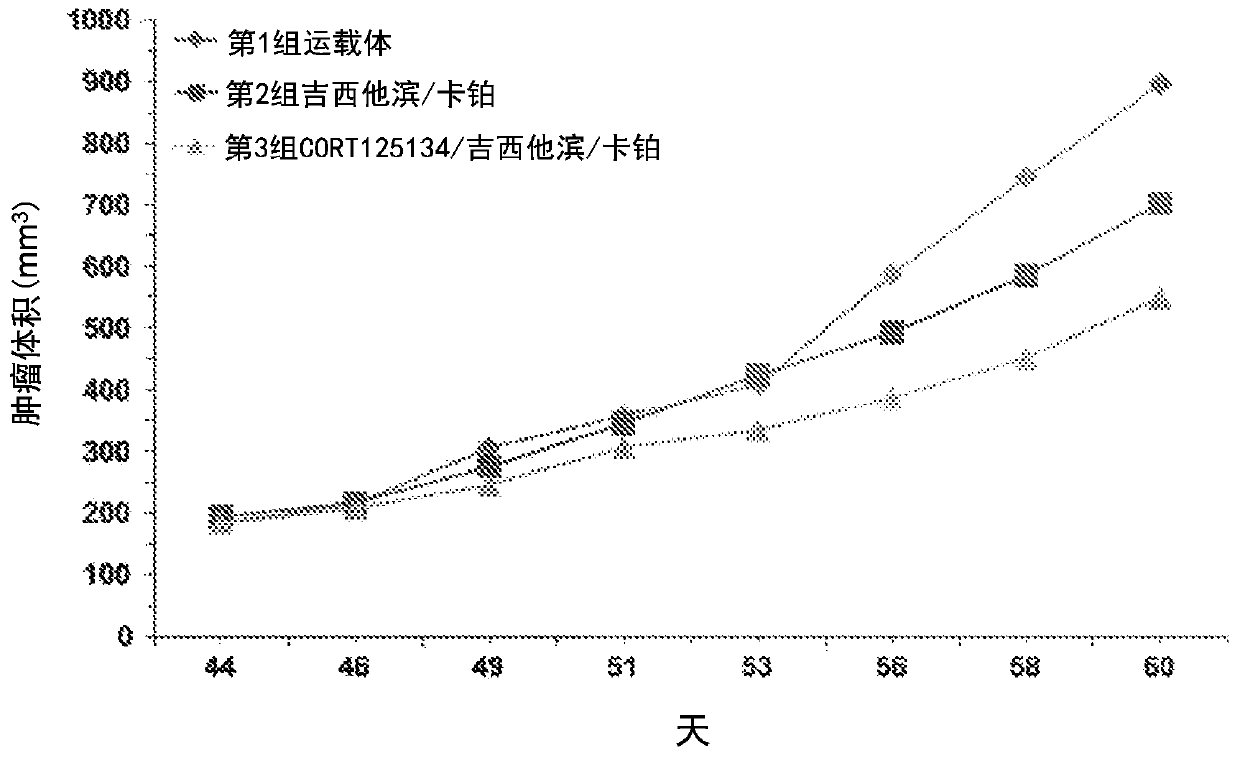
Disclosed herein is a method of treating a cancer patient having a neutrophil to lymphocyte ratio (NLR) greater than 3, comprising administering to such cancer patient a non-steroid glucocorticoid receptor antagonist (GRA) effective to reduce NLR in the patient. Methods include administering non-steroid GRA and anti-cancer treatments to such cancer patients that are effective to reduce NLR in the patient and enhance treatment in the cancer patient. The GRA may be administered orally. The non-steroid GRA may be a non-steroid compound comprising a heteroaryl ketone fused aza-decalin structure (such as relacolan) or an octahydro fused aza-decalin structure (such as acicolan). The anti-cancer treatment may include chemotherapy, immunotherapy, radiotherapy, administration of an anti-angiogenic agent, administration of a growth factor inhibitor, surgery. The method can enhance anti-cancer treatment, improve prognosis of cancer patients, improve survival rate of cancer patients, and provide beneficial clinical effects and advantages for patients.
Owner:CORCEPT THERAPEUTICS INC
Enteric sustained-release preparation containing zaltoprofen and preparation method thereof
The invention discloses a new formulation of novel nonsteroidal antiinflammatory medicament-zaltoprofen, namely an enteric sustained-release preparation containing zaltoprofen. Matrix which is insoluble in water and is insoluble in gastrointestinal fluid is used as a sustained-release auxiliary material to prepare the enteric sustained-release preparation. The enteric sustained-release preparation prepared in the invention has no fast release phenomenon of the medicament, and the repeatability of in vitro medicament release action of the preparation with different batches is good, so as to bebeneficial to keep in-vivo plasma concentration of a patient stable after taking the medicament.
Owner:HEBEI AOXING GROUP PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com