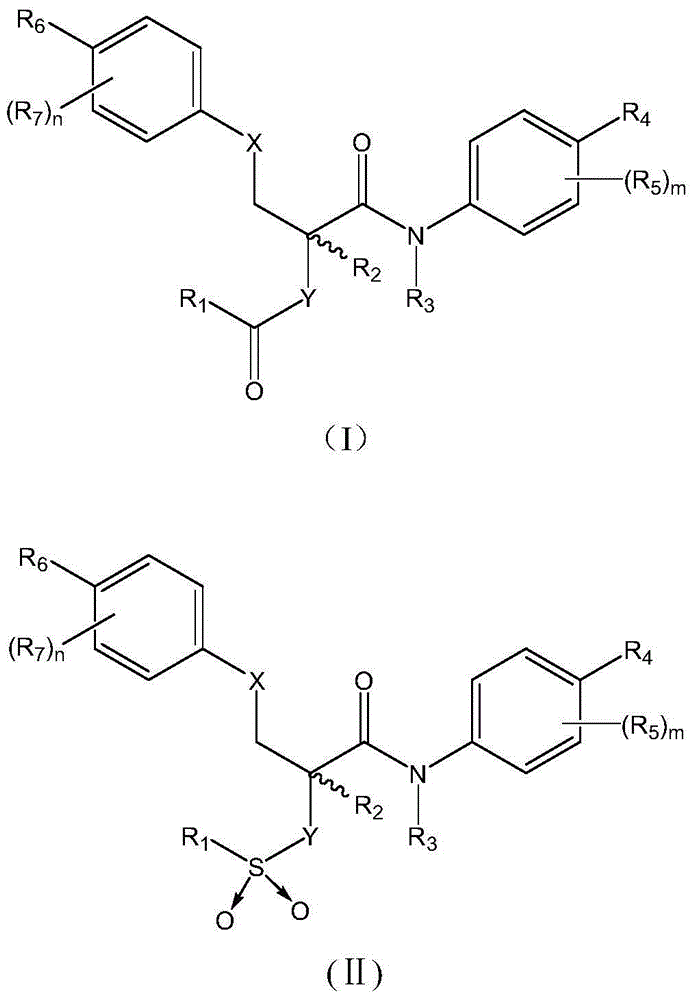
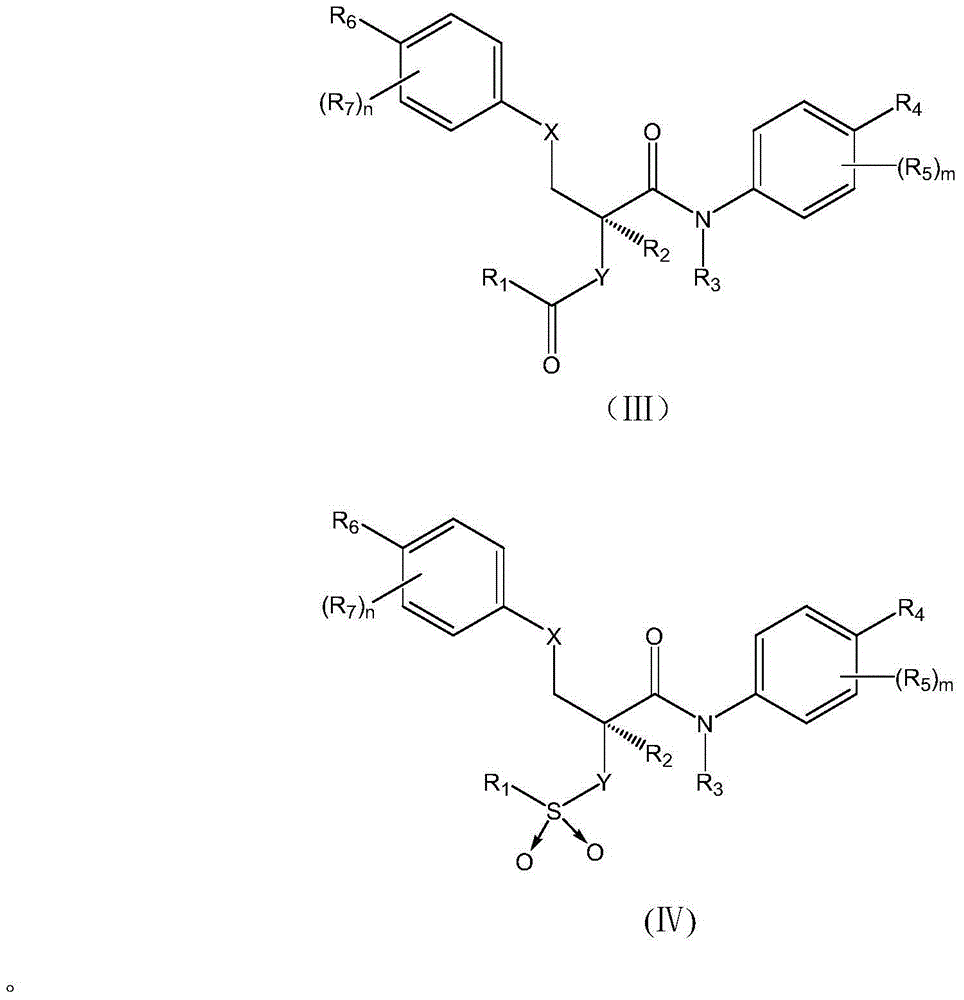
Aromatic amide compound as well as preparation method and application thereof
A technology of aromatic propionamides and compounds, applied in the field of drug synthesis, can solve the problems of limited effect, poor oral bioavailability of liver toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
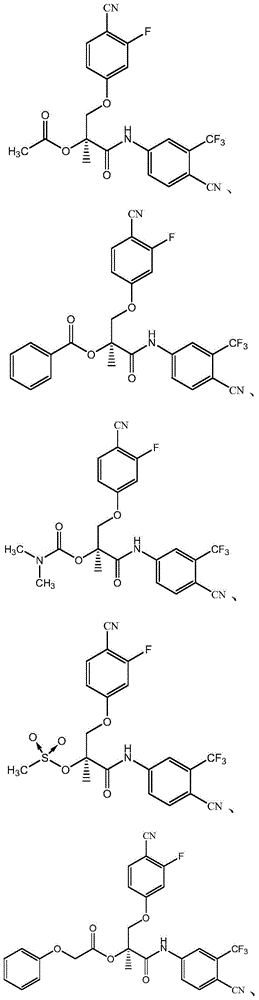
[0036] Example 1: Compound (S)-1-(4-cyano-3-(trifluoromethyl)anilino)-3-(4-cyano-3-fluorophenoxy)-2-methyl- Synthesis of 1-oxypropyl-2-acetate (C 21 h 15 f 4 N 3 o 4 )
[0037]
[0038] In a 50mL round bottom flask, add 0.4 grams of raw materials, 2.1 grams of acetic anhydride, and 10 mL of anhydrous pyridine as a solvent, reflux, stir, and the reaction time is 2.5-3.5 hours. The point chromatography plate determines the end point, thin layer chromatography: ethyl acetate: Hexane=1:1, the product point is relatively single, and there is no raw material point.
[0039] After the reaction was completed, it was cooled and sucked dry to obtain an oil. Silica gel column chromatography (dichloromethane: ethyl acetate = 98:2-95:5) was separated and purified to obtain a white powder, which was coaxed to dryness and weighed to obtain 0.4 g with a purity of about 98%.
[0040] NMR spectrum: 1 H NMR(400 MHz,DMSO-d6)δ10.50(s,1H,NH),8.30(s,1H,ArH),8.19-8.12(m,2H,ArH),7.87-7.83(m...
Embodiment 2
[0041] Example 2: Compound (S)-1-(4-cyano-3-(trifluoromethyl)anilino)-3-(4-cyano-3-fluorophenoxy)-2-methyl- Synthesis of 1-oxypropyl-2-benzoate (C 26 h 17 f 4 N 3 o 4 )
[0042]
[0043] Add 0.20 grams of raw materials, 0.5 grams of benzoic anhydride, and 10 mL of anhydrous pyridine as a solvent in a 100 mL round bottom flask, reflux, stir, and the reaction time is 9-10 hours. Point the chromatography plate to determine the end point, thin-layer chromatography: ethyl acetate :Hexane=1:1, the product point is relatively single, and there is no raw material point.
[0044] After the reaction was completed, it was cooled and drained to obtain an oil, which was separated and purified by silica gel column chromatography (dichloromethane:ethyl acetate=95:5) to obtain a white powder, which was coaxed to dryness and weighed to obtain 0.21 grams. The purity is about 98%.
[0045] NMR spectrum: 1 H NMR(400 MHz,DMSO-d6)δ10.60(s,1H,NH),8.29(s,1H,ArH),8.19-8.12(m,2H,ArH),7.98-7....
Embodiment 3
[0046] Example 3: Compound (S)-1-(4-cyano-3-(trifluoromethyl)anilino)-3-(4-cyano-3-fluorophenoxy)-2-methyl- Synthesis of 1-oxypropyl-2-methylcarbamate (C 22 h 18 f 4 N 4 o 4 )
[0047]
[0048] Add 1.50 g of raw materials and 20 mL of anhydrous DMF as a solvent in a 100 mL round-bottomed flask, and drop the temperature to 0°C, add 0.40 g of sodium hydride, stir for 2-3 hours, then add 0.88 g of dimethylcarbamoyl chloride, and raise the temperature to room temperature. Stir, the reaction time is 5-6 hours, point the chromatographic plate to determine the end point, thin-layer chromatography: dichloromethane: ethyl acetate = 9:1, the product point is relatively single, and there is no raw material point.
[0049] After the reaction was stopped, it was drained to obtain an oil, and the main product was separated by silica gel column chromatography (ethyl acetate: hexane=1:1), purified to obtain a white toner substance, coaxed to dryness, and weighed to obtain 0.99 g. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com