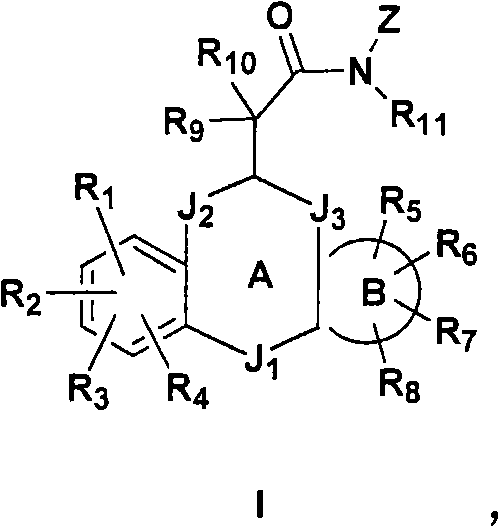
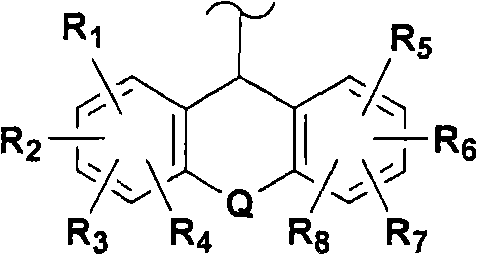
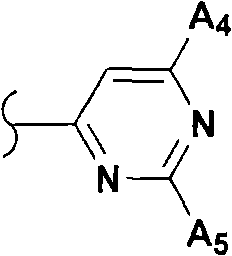
Modulators of glucocorticoid receptor, AP-1, and/or NF-kB activity and use thereof
一种对映异构体、互变异构体的技术,应用在非类固醇化合物领域,能够解决全身性使用限制等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1001]
[1002]To a solution of the product from Preparation 9 (34 mg, 0.14 mmol) in DMF (1.2 mL) was added sequentially triethylamine (0.049 mL, 0.35 mmol), HOAt (23 mg, 0.17 mmol), EDC (33 mg, 0.17 mmol) and The product of 4 was prepared (42 mg, 0.16 mmol). The resulting mixture was heated at 85°C for 16 hours, filtered through a 0.45 micron syringe tip filter and purified by preparative HPLC. The product was obtained as a white solid as TFA salt (30 mg, Y=35%). MS (E+) m / z: 490 (M+H); LC retention time: 3.24 minutes.
Embodiment 2 to 4
[1004] In the same manner as described for the preparation of the title compound of Example 1, the following Examples 2 to 4 were prepared using the amines of Preparations 1 to 3.
[1005]
Embodiment 5 to 9
[1007] In the same manner as described for the preparation of the title compound of Example 1, the following Examples 5 to 9 were prepared using the carboxylic acid product of Preparation 10 and the amine and 2-aminothiazole of Preparations 1 to 4.
[1008]
[1009]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com