Compositions and methods for modulating immune responses
A technology of immune response and composition, applied in the direction of drug combination, pharmaceutical formula, allergen antigen components, etc., can solve the problem of lack of support and usefulness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0097]Methods that have been developed to prepare microspheres for the delivery of coated agents are described in the literature, eg, as Doubrow, M., Ed., "Microcapsules and Nanoparticles in Medicine and Pharmacy," CRCPress, Boca Raton, 1992. The description of the method is also found in Mathiowitz and Langer (1987, J.Controlled Release 5, 13-22); Mathiowitz et al. (1987, Reactive Polymers 6, 275-283); and Mathiowitz et al. (1988, J.Appl . Polymer Sci.35, 755-774) and US Patent No. 5,213,812, US Patent No. 5,417,986, US Patent No. 5,360,610 and US Patent No. 5,384,133. The choice of method depends on the choice of polymer, size, shape and desired crystallinity, as described (e.g. Mathiowitz et al. (1990, Scanning Microscopy 4:329-340; 1992, J. Appl. Polymer Sci. .45, 125-134); and Benita et al. (1984, J. Pharm. Sci. 73, 1721-1724)).
[0098] In solvent evaporation, the polymer is dissolved in a volatile organic solvent, such as dichloromethane, as described, for example, in ...
Embodiment 1
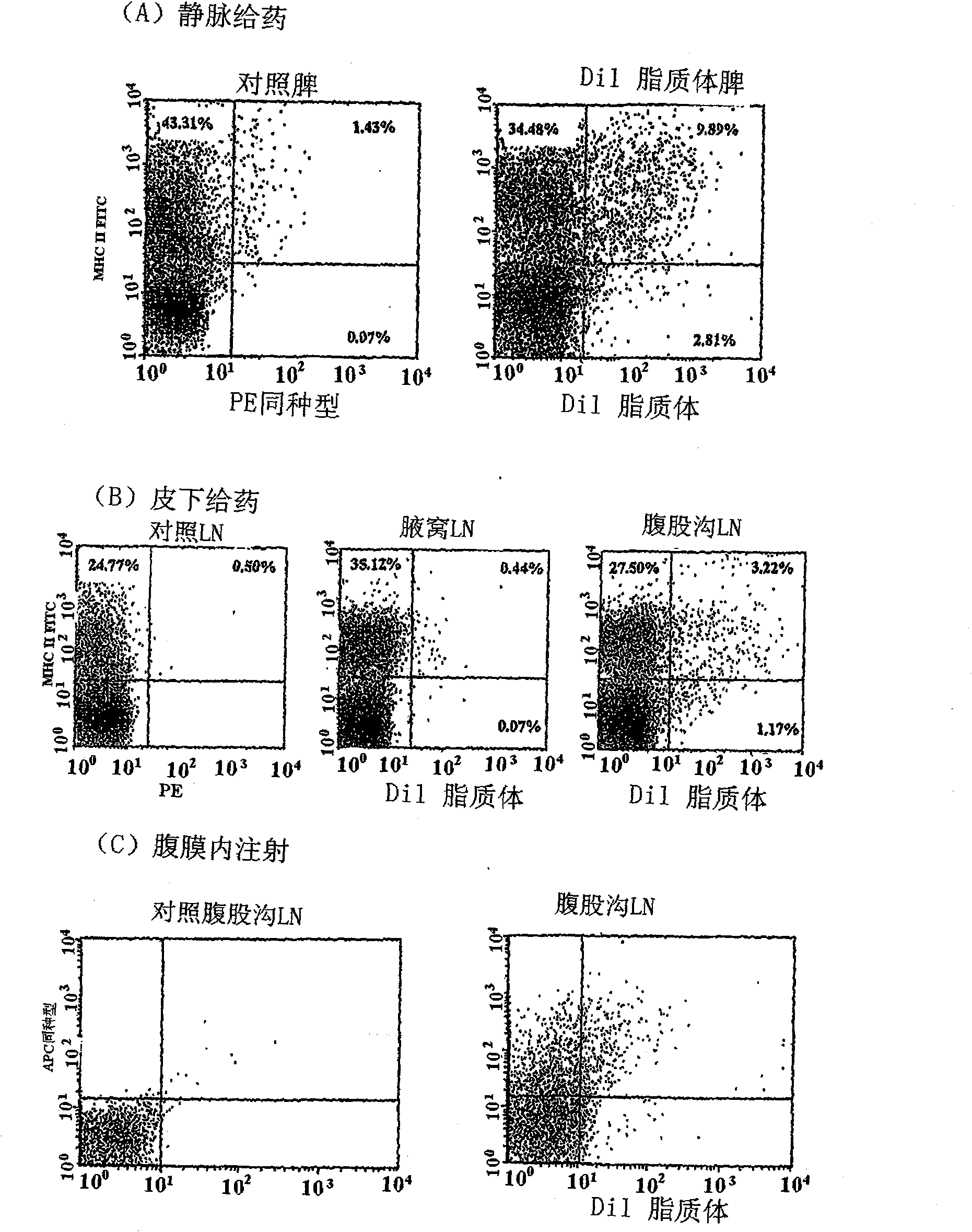
[0235] Antigen-specific inhibition of arthritis by liposomes
[0236] Materials and methods
[0237] Reagent
[0238] Fluorescein isothiocyanate (FITC), ovalbumin (OVA), methylated bovine serum albumin (mBSA) were purchased from Sigma-Aldrich (Missouri, USA). Penicillin, streptomycin, L-glutamine, sodium pyruvate and 2-mercaptoethanol were purchased from Invitrogen (California, USA). Complete Freund's adjuvant (CFA) was obtained from Sigma-Aldrich (Missouri, USA). Carboxyfluorescein diacetate succinimidyl ester (CFSE) was obtained from Molecular Probes (Oregon, USA). Phycoerythrin (PE)-labeled KJ1-26 antibody was purchased from BD Pharmingen (California, USA). Anti-mouse MHC II-FITC was purchased from Biolegend (California, USA). The CD4 epitope of OVA (sequence 323-339) was obtained from Auspep (Victoria, Australia). All other reagents were at least of analytical grade.
[0239] mouse
[0240] Male C57 / B16 and BALB / c mice were obtained from ARC, an OVA-specifi...
Embodiment 2
[0326] Antigen-associated liposomes or antigen-associated microspheres induce antigen-specific suppression by inducing regulatory T cells
[0327] Materials and methods
[0328] Reagent
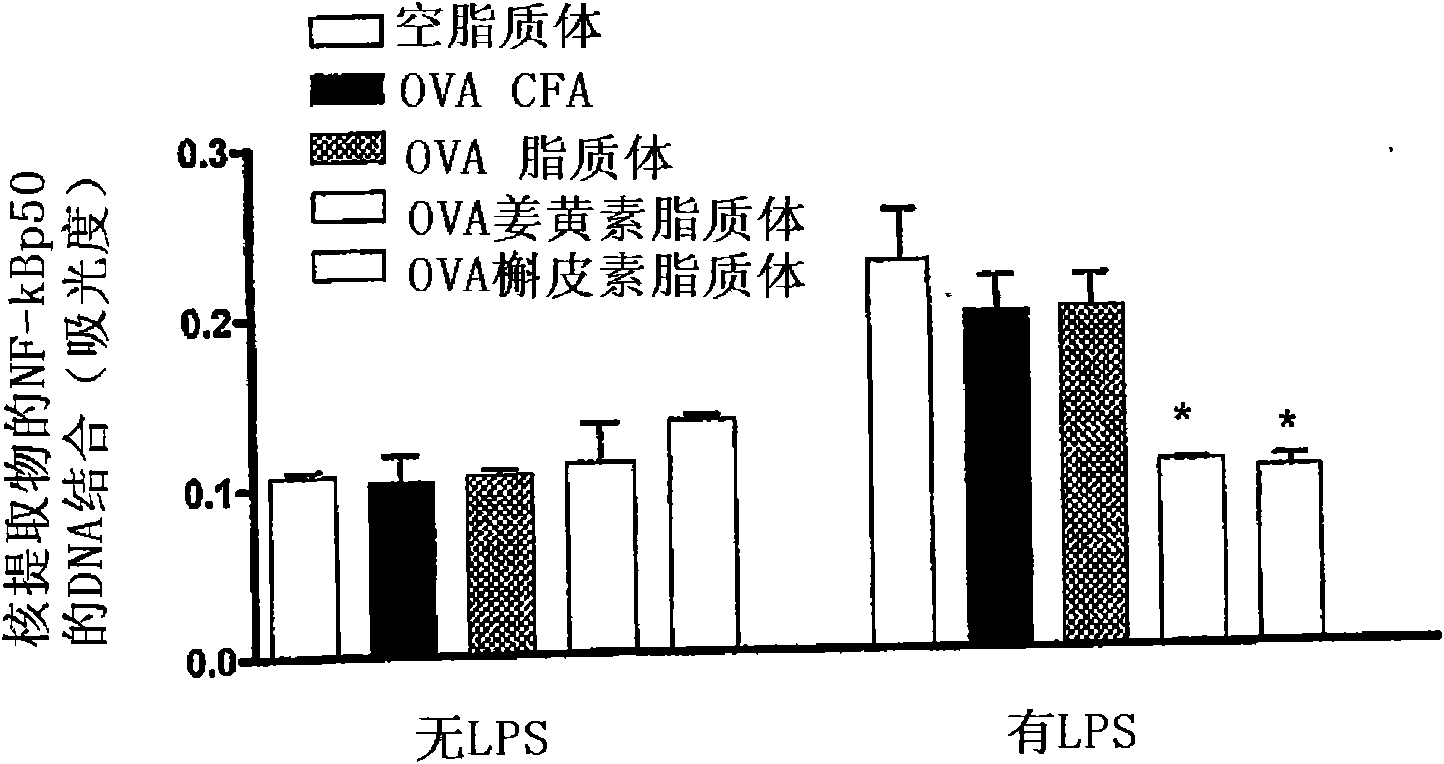
[0329] Poly(d,l-lactic-co-glycolic acid) (PLGA) microspheres were prepared by combining ovalbumin, curcumin, PLGA and polyvinyl alcohol (PVA), then mixing at 13500 rpm, followed by stirring at 800 rpm. After density centrifugation, the particles were dispersed in a 5% w / v sucrose solution, sized and lyophilized. Particles were reconstituted in PBS before use. OVA-curcumin liposomes were prepared as described in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com