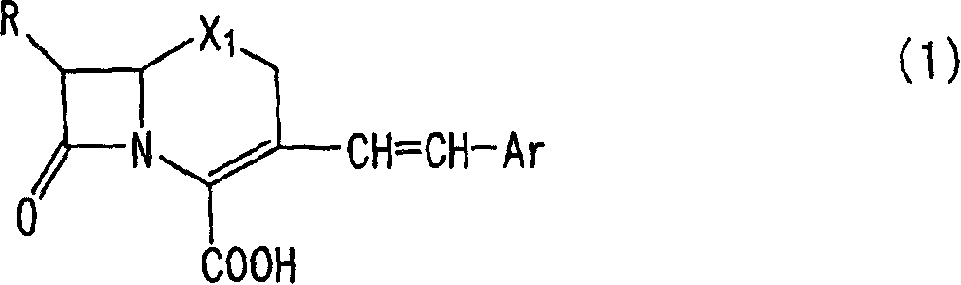Patents
Literature
364 results about "Aminothiazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
2-Aminothiazole is a heterocyclic amine featuring a thiazole core. It can also be considered a cyclic isothiourea. It possesses an odor similar to pyridine and is soluble in water, alcohols and diethyl ether. It is commonly used as a starting point for the synthesis of many compounds including sulfur drugs, biocides, fungicides, dyes and chemical reaction accelerators. 2-Aminothiazole can be used as a thyroid inhibitor in the treatment of hyperthyroidism and has antibacterial activity. Alternatively, its acid tartrate salt can be used. Recent studies using prion-infected neuroblastoma cell lines have suggested that aminothiazole may be used as a therapeutic drug for prion diseases.
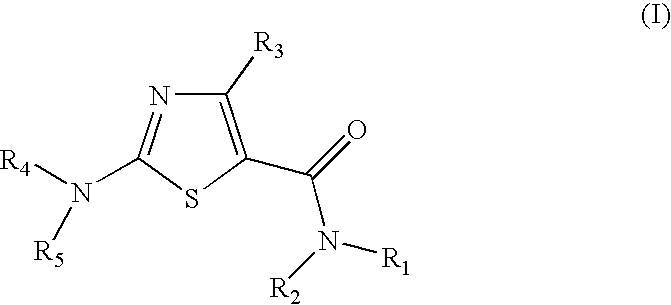
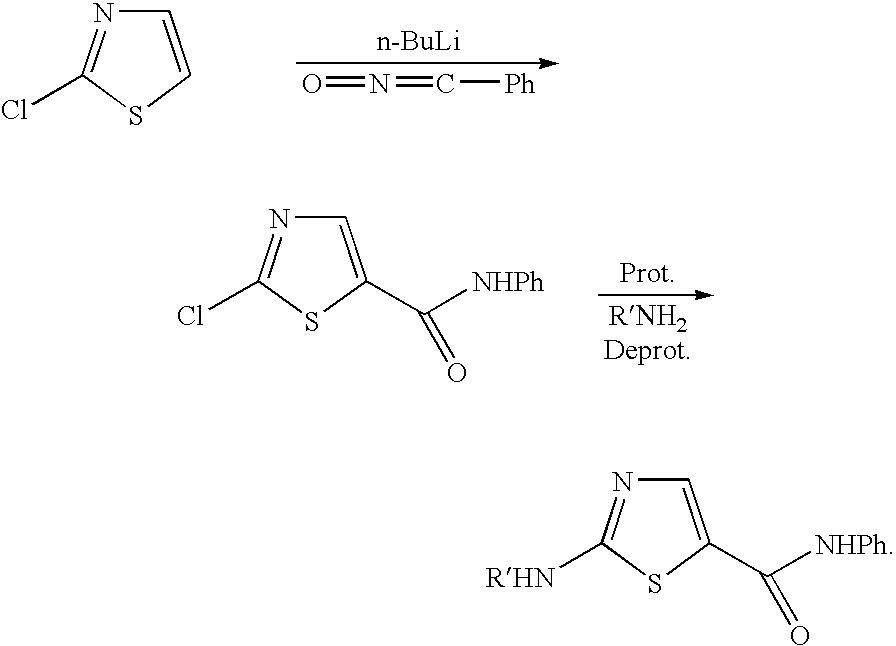
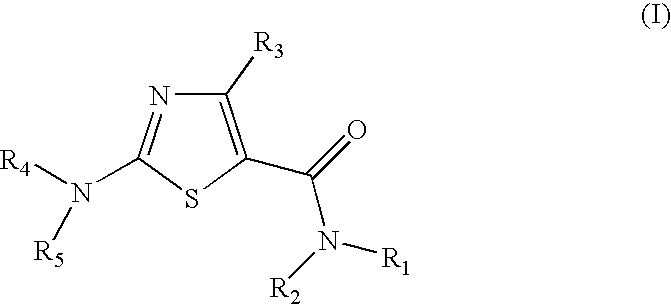
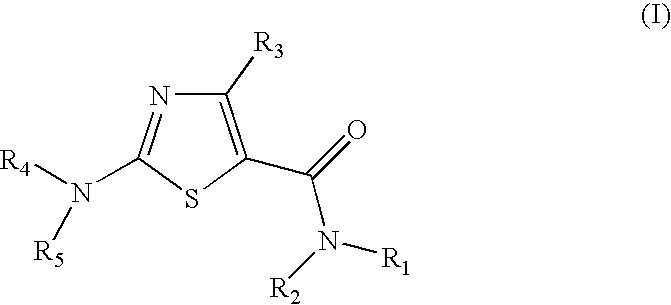
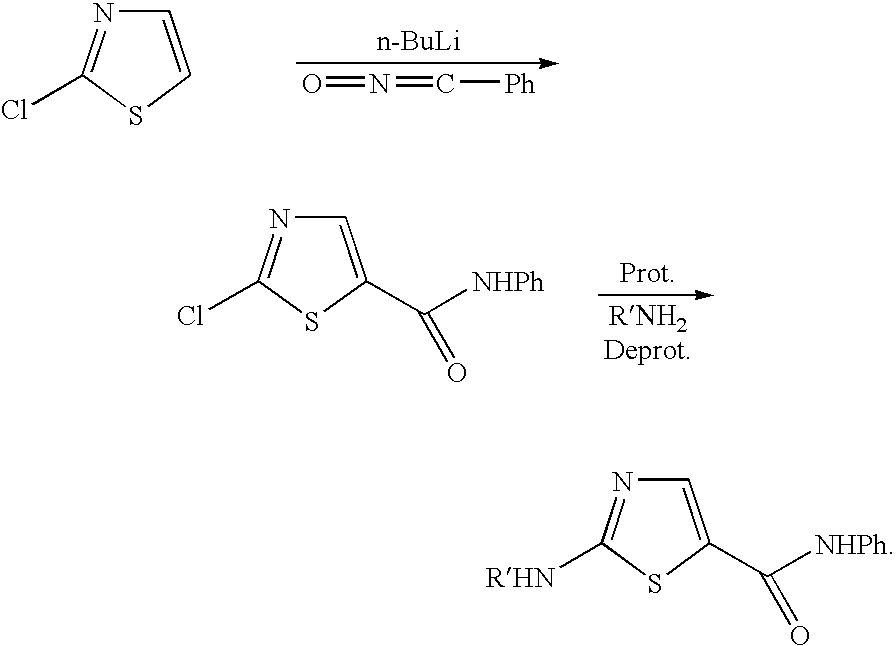
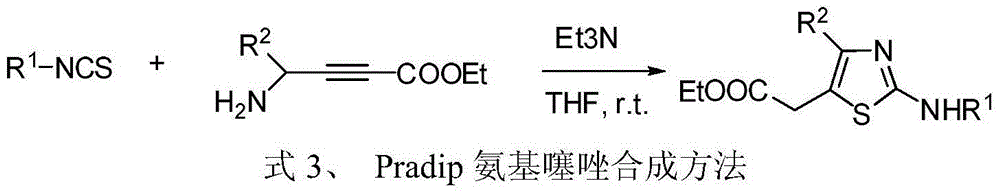
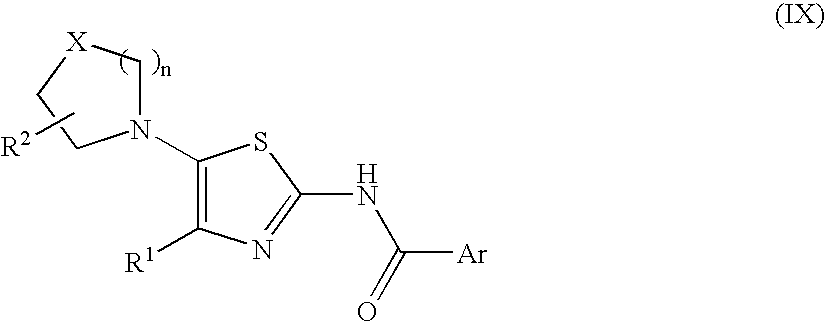
Process for preparing 2-aminothiazole-5-aromatic carboxamides as kinase inhibitors
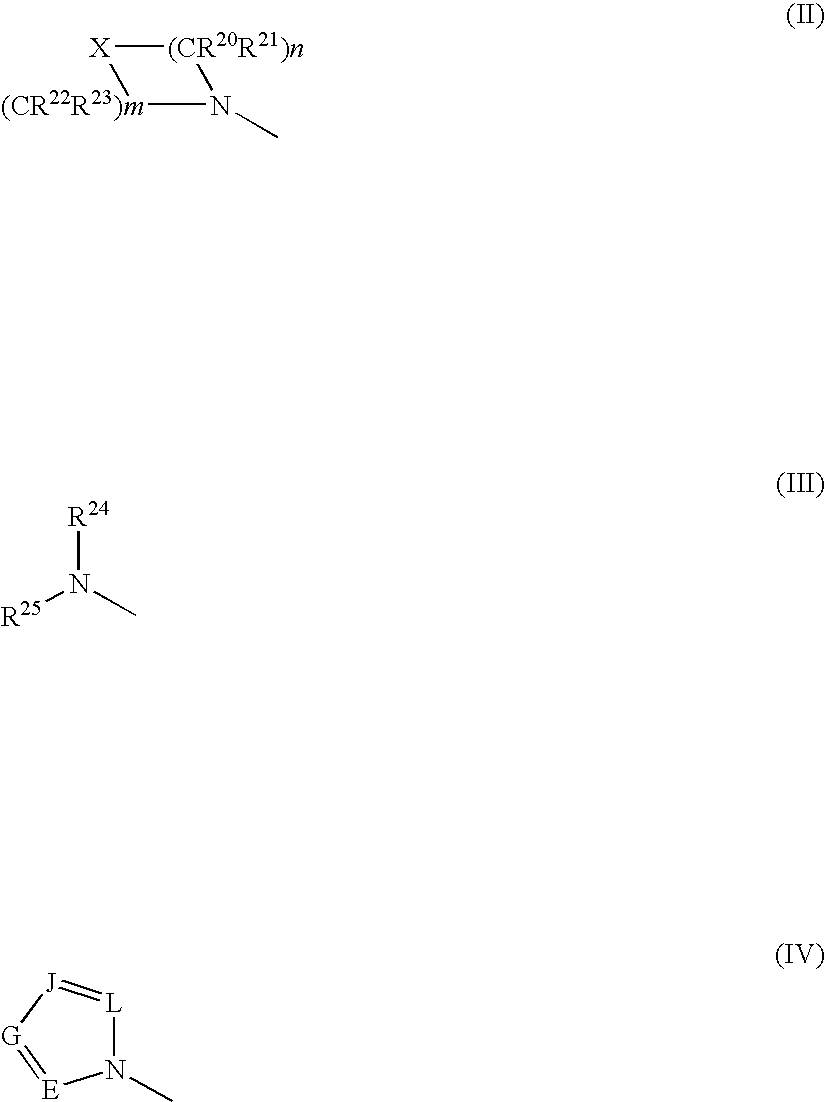
The invention relates to processes for preparing compounds having the formula (I) and crystalline forms thereof, wherein Ar is aryl or heteroaryl, L is an optional alkylene linker, and R2, R3, R4, and R5, are as defined in the specification herein, which compounds are useful as kinase inhibitors, in particular, inhibitors of protein tyrosine kinase and p38 kinase.
Owner:BRISTOL MYERS SQUIBB HLDG IRELAND UNLTD
Process for preparing 2-aminothiazole-5-aromatic carboxamides as kinase inhibitors
ActiveUS20060004067A1Efficient preparationHigh yieldOrganic active ingredientsBiocideArylProtein-Tyrosine Kinases
The invention relates to processes for preparing compounds having the formula, and crystalline forms thereof, wherein Ar is aryl or heteroaryl, L is an optional alkylene linker, and R2, R3, R4, and R5, are as defined in the specification herein, which compounds are useful as kinase inhibitors, in particular, inhibitors of protein tyrosine kinase and p38 kinase.
Owner:BRISTOL MYERS SQUIBB CO
Process for preparing 2-aminothiazole-5-aromatic carboxamides as kinase inhibitors
ActiveUS7491725B2Efficient preparationHigh yieldBiocideOrganic active ingredientsArylProtein-Tyrosine Kinases
The invention relates to processes for preparing compounds having the formula,and crystalline forms thereof, wherein Ar is aryl or heteroaryl, L is an optional alkylene linker, and R2, R3, R4, and R5, are as defined in the specification herein, which compounds are useful as kinase inhibitors, in particular, inhibitors of protein tyrosine kinase and p38 kinase.
Owner:BRISTOL MYERS SQUIBB CO
Process for preparing 2-aminothiazole-5-aromatic carboxamides as kinase inhibitors
InactiveUS20050215795A1Efficient preparationHigh yieldOrganic active ingredientsOrganic chemistryArylProtein-Tyrosine Kinases
The invention relates to processes for preparing compounds having the formula, and crystalline forms thereof, wherein Ar is aryl or heteroaryl, L is an optional alkylene linker, and R2, R3, R4, and R5, are as defined in the specification herein, which compounds are useful as kinase inhibitors, in particular, inhibitors of protein tyrosine kinase and p38 kinase.
Owner:BRISTOL MYERS SQUIBB CO
2-Acylaminothiazole derivative or salt thereof
ActiveUS20050153977A1Good effectIncrease the number ofBiocideOrganic chemistryPharmacologyIncreased platelets
Owner:ASTELLAS PHARMA INC
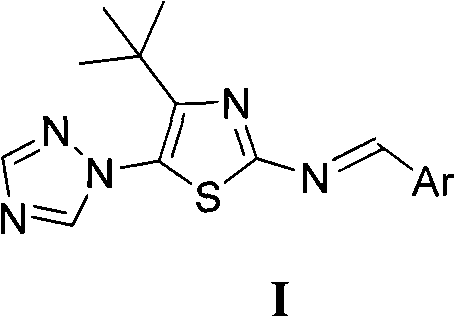
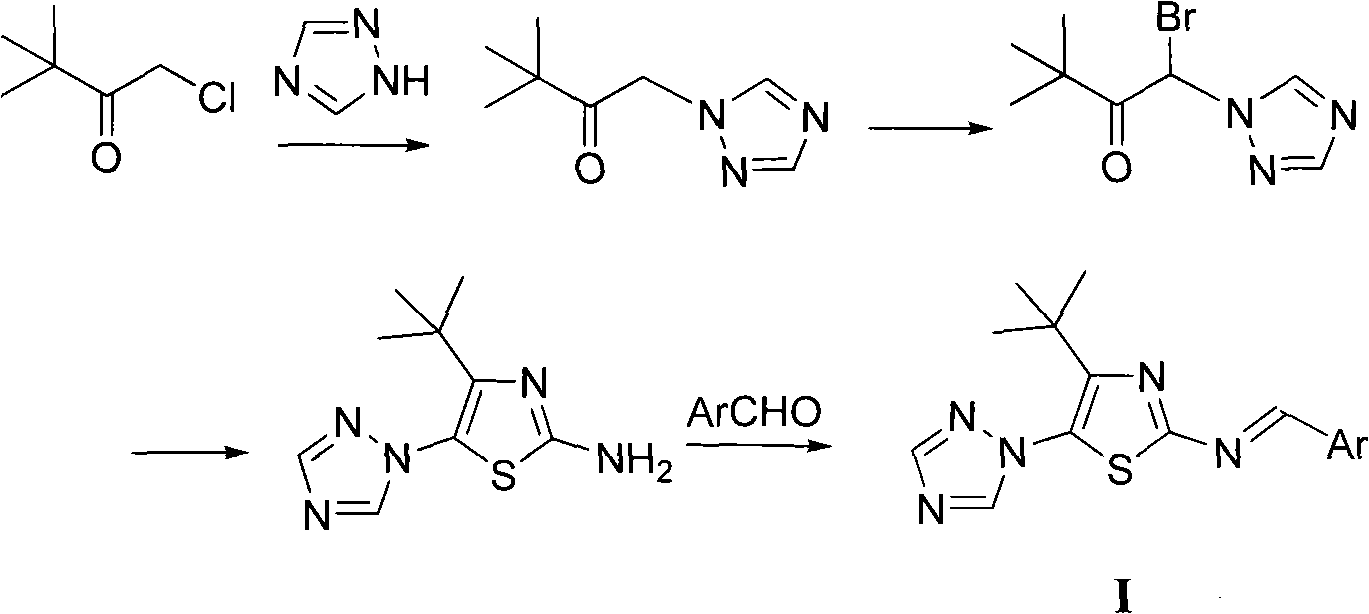
4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzylimino thiazole and preparation method and application thereof
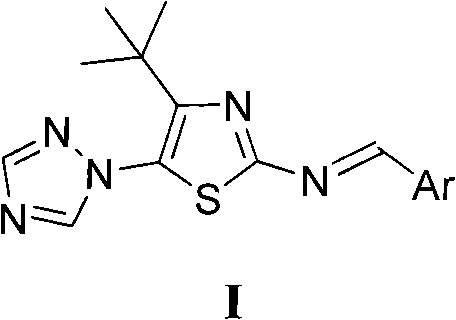
The invention discloses 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzylimino thiazole (I) having the chemical structural formula shown rightwards. A method for preparing the 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzylimino thiazole is as follows: the step of reflux reaction is carried out on the 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-aminothiazole and aryl aldehyde in benzene, thereby preparing the 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzylimino thiazole. The 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzylimino thiazole can be used for preparing bactericide.
Owner:HUNAN UNIV
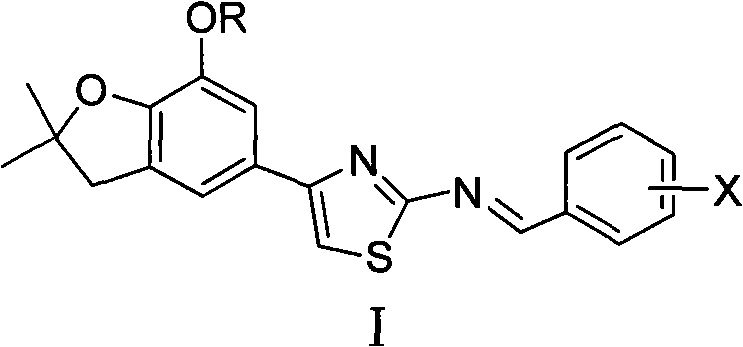
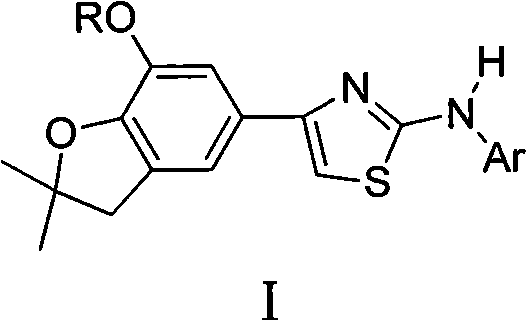
4-(benzofuran-5-yl)-2-benzal aminothiazole and application of 4-(benzofuran-5-base)-2-benzal aminothiazole as antineoplastic agent
The invention discloses 4-(benzofuran-5-yl)-2-benzal aminothiazole as shown in a chemical structural formula I. The preparation method of the 4-(benzofuran-5-yl)-2-benzal aminothiazole is as follows: 1-(7-hydroxy / alkoxy-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl) butanone is subject to bromination and reacts with thiourea to obtain 4-(7-hydroxy / alkoxy-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl)-2-aminothiazole which reacts with aromatic aldehyde to prepare the 4-(benzofuran-5-yl)-2-benzal aminothiazole. The 4-(benzofuran-5-yl)-2-benzal aminothiazole has good activity inhabiting activity on Hela cells, human liver cancer cells (Bel 7402 cells) and lung carcinoma cells (A549 cells) and can be used for preparing the antineoplastic agent.
Owner:HUNAN UNIV
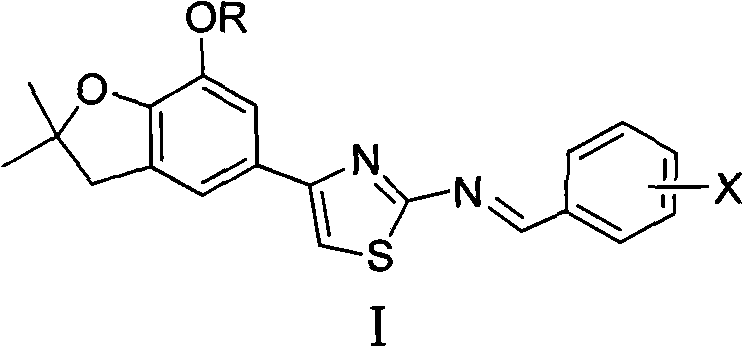
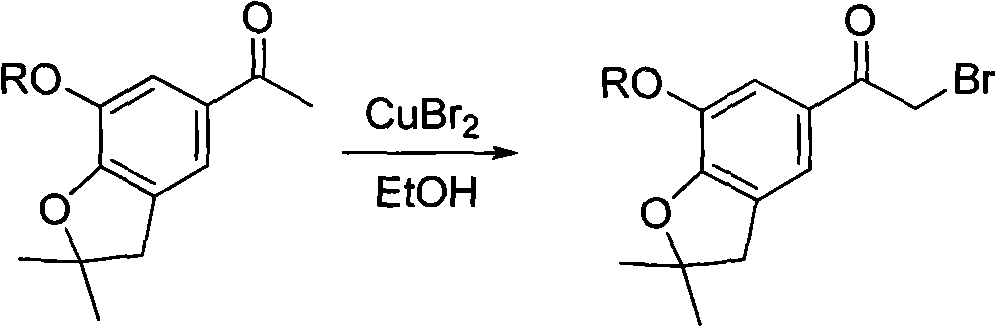
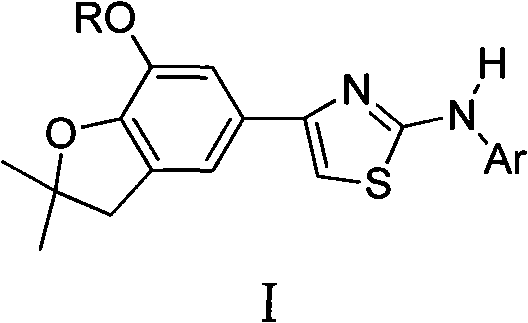
4-(benzofuran-5-yl)-2-aromatic aminothiazole and preparation method and application thereof
The invention discloses 4-(benzofuran-5-yl)-2-aromatic aminothiazole shown as a chemical structural formula I. The preparation method of the 4-(benzofuran-5-yl)-2-aromatic aminothiazole comprises the following steps of: heating and stirring 2-halogen-1-(7-hydroxyl / alkoxyl-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl) butanone and arylthiourea in acetone for reaction to obtain 4-(benzofuran-5-yl)-2-aromatic aminothiazole salt; and neutralizing the 4-(benzofuran-5-yl)-2-aromatic aminothiazole salt with stronger ammonia water to obtain the 4-(benzofuran-5-yl)-2-aromatic aminothiazole. The 4-(benzofuran-5-yl)-2-aromatic aminothiazole has high insecticidal activity and is applied to the preparation of pesticides.
Owner:HUNAN UNIV
Process for preparing 2-aminothiazole-5-carboxamides useful as kinase inhibitors
The invention is directed to processes for preparing 2-aminothiazole-5-carboxamides of formula I wherein R1, R2, R3, R4 and R5 are as defined as set forth in the specification herein.
Owner:BRISTOL MYERS SQUIBB CO
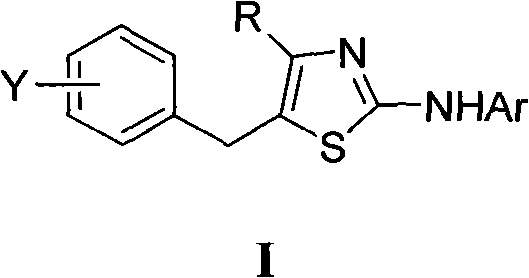
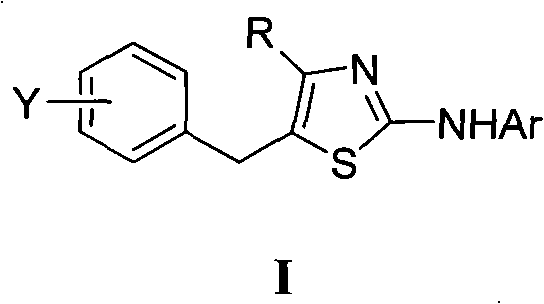
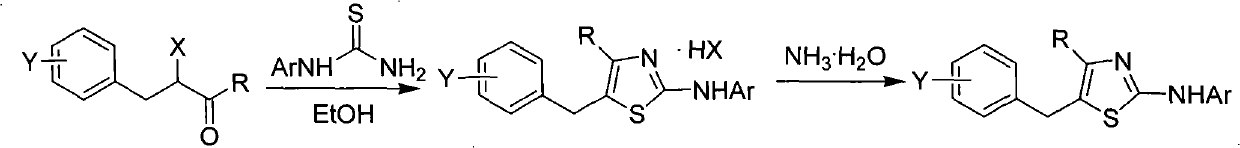
5-benzyl-4-alkyl-2-aminothiazole as well as preparation and application of 5-benzyl-4-alkyl-2-aminothiazole
InactiveCN102070556AHas antitumor activityOrganic active ingredientsOrganic chemistryChemical structureAryl
The invention discloses 5-benzyl-4-alkyl-2-aminothiazole as shown in a chemical structure formula I and pharmaceutically acceptable salts of the 5-benzyl-4-alkyl-2-aminothiazole as shown in the specification. A preparation method of the 5-benzyl-4-alkyl-2-aminothiazole is as follows: heating 2-haslogen-1-aryl alkyl ketone, alkyl thiourea and ethanol, stirring, and reacting for a certain time; filtering, drying to obtain5-benzyl-4-alkyl-2-aminothiazole salts; and filtering the obtained filter cake and neutralizing with ammonia water, and then obtaining 5-benzyl-4-alkyl-2-aminothiazole. The invention also discloses application of the 5-benzyl-4-alkyl-2-aminothiazole or salts thereof in preparing antitumor medicaments.
Owner:HUNAN UNIV
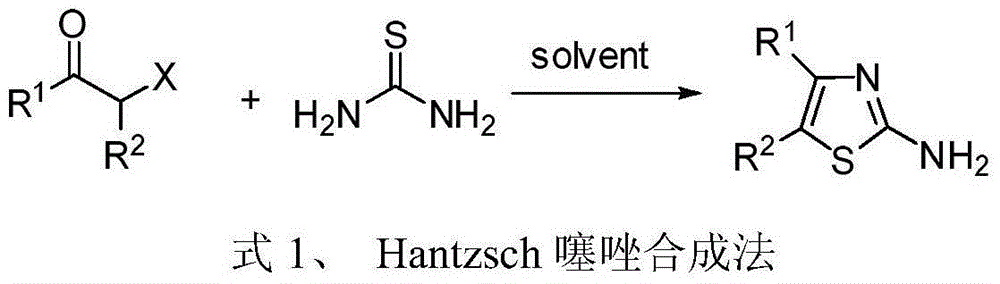
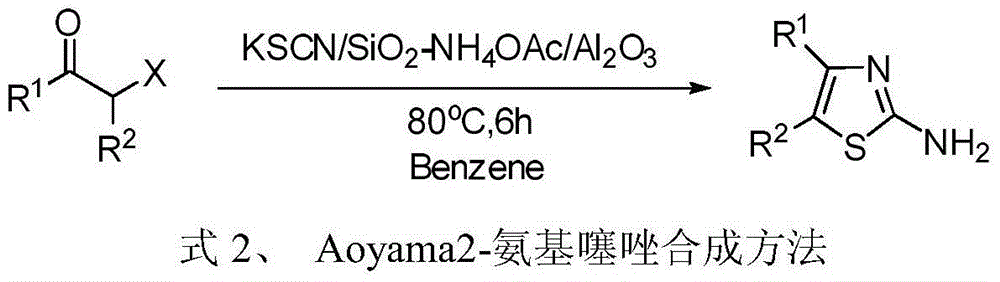
4, 5-disubstituted-2-aminothiazole compound and preparation method thereof
InactiveCN104151262ALow priceReduce pollutionOrganic chemistryAntineoplastic agentsRotary evaporatorPotassium thiocyanate
The invention discloses a 4, 5-disubstituted-2-aminothiazole compound. The structural formula is shown in the specification, wherein R1 is 4-tolyl, 4-chlorophenyl, 4-methoxyphenyl, 4-nitrobenzene or propoxy, and R2 is phenyl, 4-tolyl, 4-fluorophenyl, 4-methoxyphenyl, 2-furyl, isopropyl, 4-nitrophenyl or n-propyl. The invention simultaneously provides a preparation method of the 4, 5-disubstituted-2-aminothiazole compound. The preparation method comprises the following steps: enabling an olefin azide type compound and potassium thiocyanate to react at the temperature of 75-85 DEG C in the presence of a solvent and a metal catalyst, concentrating an obtained reaction solution, then extracting with water and ethyl acetate, washing an obtained organic layer, then drying and concentrating by a rotary evaporator; performing silica gel column chromatography on an obtained concentrate to obtain the 4, 5-disubstituted-2-aminothiazole compound.
Owner:ZHEJIANG UNIV
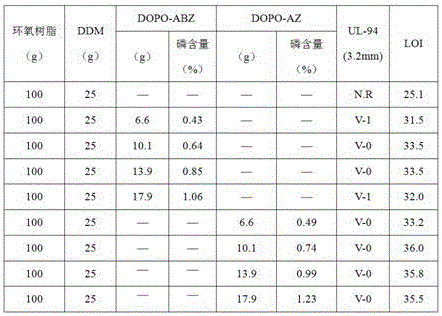
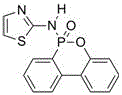
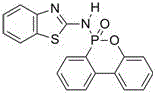
Flame retardant containing phosphorus, nitrogen and sulfur and used for epoxy resin and preparation method of flame retardant
InactiveCN106009040AThe synthesis process is simpleShort cycleGroup 5/15 element organic compoundsEpoxySulfur
The invention discloses a flame retardant containing phosphorus, nitrogen and sulfur and used for epoxy resin and a preparation method of the flame retardant. Firstly, DOPO and aminothiazole are taken as reaction raw materials and subjected to an Atherton-Todd reaction for preparation of the flame retardant containing phosphorus, nitrogen and sulfur. The process steps of the method are simple, the yield and the purity of the flame retardant are high, and the post-treatment process is simple and convenient. The flame retardant containing phosphorus, nitrogen and sulfur and prepared with the method is used for modifying cured epoxy resin and has good compatibility with epoxy resin, and the obtained cured flame-retardant epoxy resin has excellent flame retardancy; when the phosphorus content of the epoxy curing system reaches 0.43wt%-1.23wt%, the vertical burning grade can pass UL-94 V-0 level, and the limit oxygen index is as high as 36%.
Owner:FUJIAN NORMAL UNIV
Preparation method of cefdinir
ActiveCN101565427AEasy to recycleReduce pollutionAntibacterial agentsOrganic chemistryOrganic baseCarboxylic acid
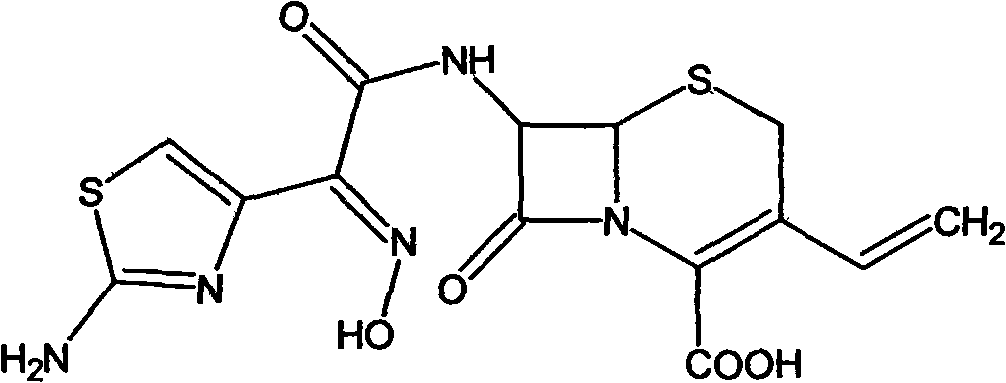
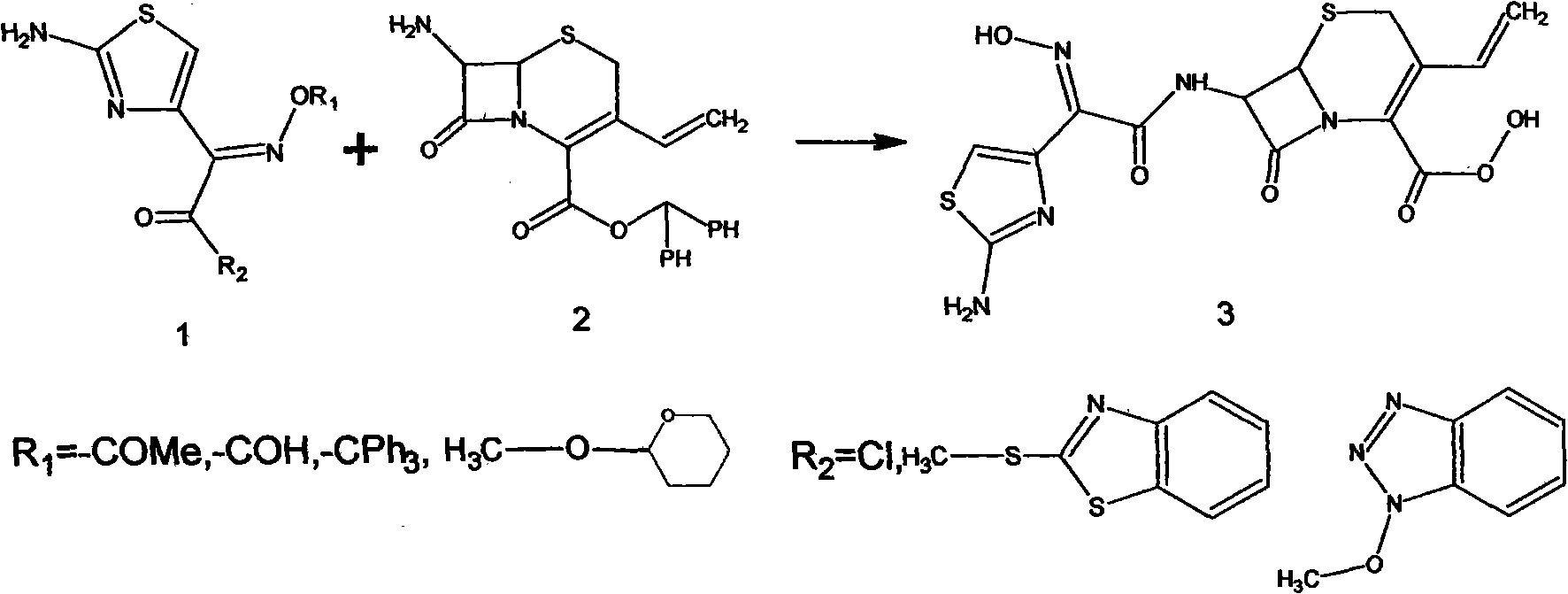
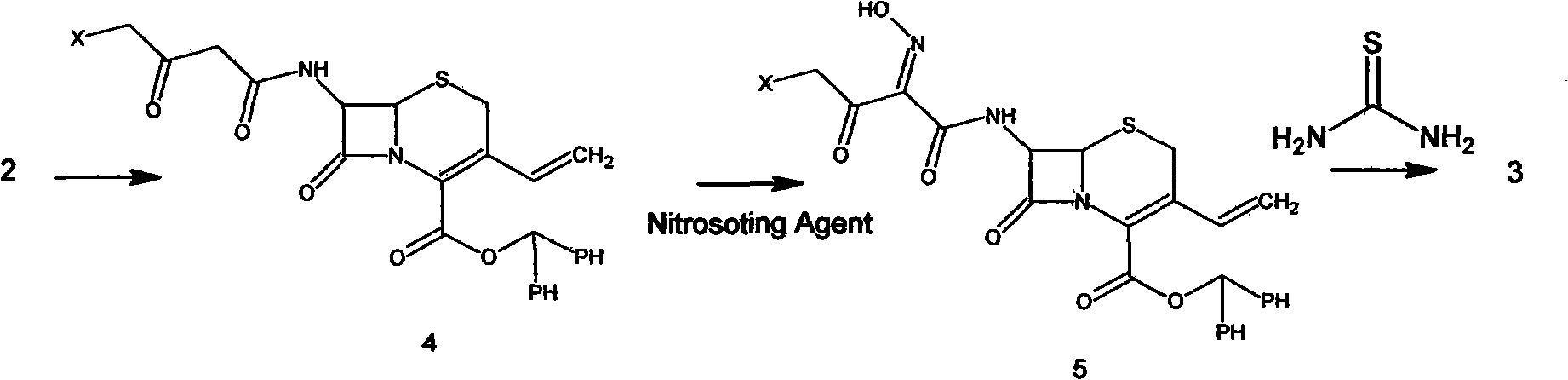
The invention relates to a preparation method of cefdinir, comprising the following steps: reacting 7-amino-3-vinyl-8-oxy-5-thia-1- nitric heterocyclic dicyclo[4.2.0]octyl-2-en-2-carboxylic acid with (Z)-2-(2-aminothiazole-4-yl)-2-acetoxy imino thioacetic acid (S-2-benzothiazole)ester in the presence of organic base at low temperature; extracting, adjusting p H value, preparing the intermediate of the cefdinir, removing the ester-group protective group of the intermediate of the cefdinir to obtain the cefdinir. The preparation method uses the low-temperature reaction technique, capable of increasing the reaction yield and reducing the impurities generated by the high temperature reaction. The hydrolysis and crystallization process is very easily controlled. The used alcohols, ketones or esters solvent is easily recovered, thus the production cost and the three-wastes drain are reduced, therefore the pollution to the environment is reduced. The preparation method of cefdinir is suitable for large-scale production.
Owner:ZHEJIANG ANGLIKANG PHARMA
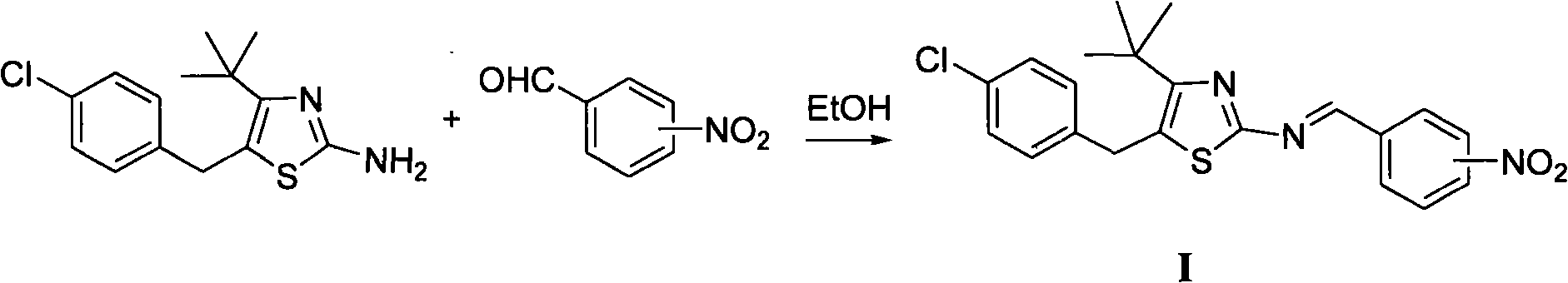
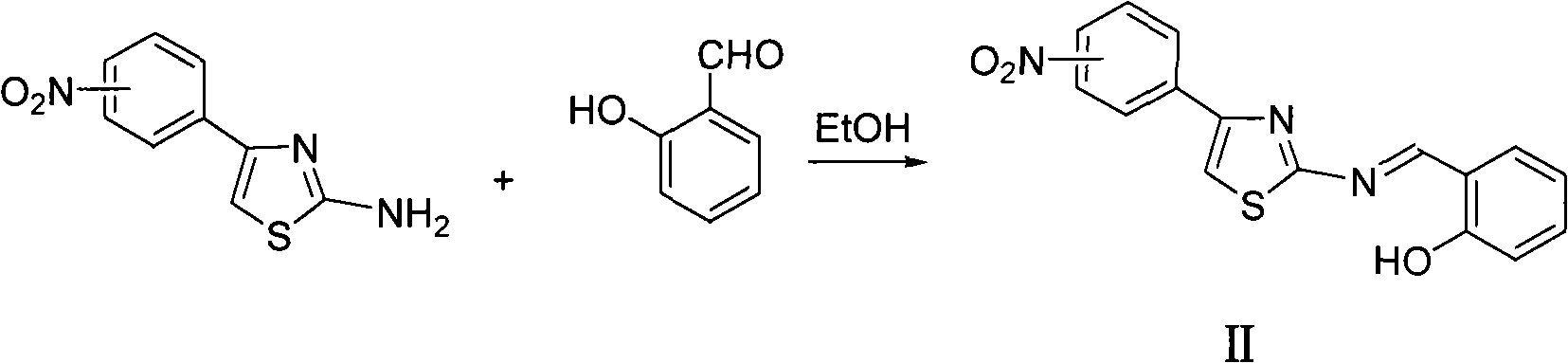
Thiazole schiff base containing nitryl, preparation and uses thereof
InactiveCN101492426AHas anti-inflammatory activityOrganic active ingredientsOrganic chemistrySalicylaldehydeStructural formula
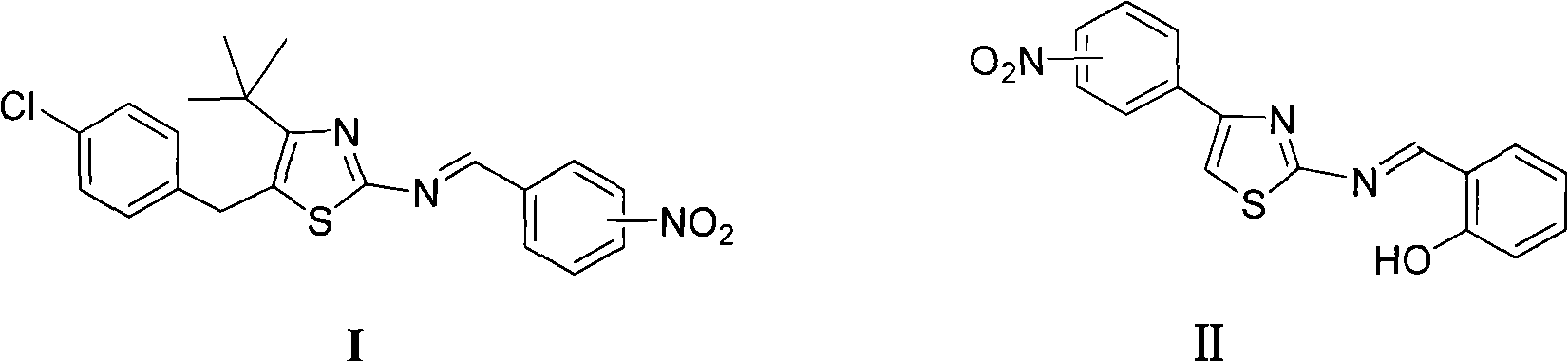
The invention discloses thiazole Schiff base (I, II) containing nitryl with a chemical structural formula on the right. A method for preparing the thiazole Schiff base containing the nitryl comprises the steps as follows: nitrobenzene formaldehyde takes reflux reaction with 5-(4-chlorobenzyl)-4-tert-butyl-2-amido thiazole in ethanol to prepare and obtain the thiazole Schiff base [I with chemical name of 5-(4-chlorobenzyl)-4-tert-butyl-2-(nitrobenzyl imino) thiazole] containing the nitryl; or salicylaldehyde takes reflux reaction with 4-(nitrophenyl)-2-amido thiazole in the ethanol to prepare and obtain the thiazole Schiff base [II with chemical name of 4-( nitrophenyl)-2-(2-hydroxy benzyl imino) thiazole] containing the nitryl. The thiazole Schiff base containing the nitryl can be used for preparing anti-inflammatory analgesics.
Owner:HUNAN UNIV
Polishing solution for cmp and polishing method using the polishing solution
ActiveUS20110275217A1Good water solubilitySatisfactory maintenance of dispersibilityOther chemical processesSemiconductor/solid-state device manufacturingMetallurgySilicon oxide
The polishing solution for CMP of the invention comprises abrasive grains, a first additive and water, wherein the first additive is at least 1,2-benzoisothiazole-3(2H)-one or 2-aminothiazole. The polishing method of the invention is a polishing method for a substrate having a silicon oxide film on the surface, and the polishing method comprises a step of polishing the silicon oxide film with a polishing pad while supplying the polishing solution for CMP between the silicon oxide film and the polishing pad.
Owner:RESONAC CORP
Beta-lactamase detecting reagent composition, detection kit and detection method
InactiveUS20060014230A1Quick checkOrganic chemistryMicrobiological testing/measurementΒ lactamasesNitrostyrol
The present invention provides a reagent composition for detecting β-lactamase including as a β-lactamase detection substrate 3-[2,4-dinitrostyryl]-7-(2-thienylacetamido]-3-cephem-4-carboxylic acid, or 7-[2-(2-aminothiazol-4-yl)-2-(1-carboxy-1-methylethoxy-imino)acetamido]-3-(2,4-dinitrostyryl)-3-cephem-4-carboxylic acid, and at least one β-lactamase inhibitor selected from the group consisting of clavulanic acid, aztreonam, ethylenediaminetetraacetic acid, and cloxacillin, which composition can detect β-lactamases rapidly and easily with high sensitivity. The present invention also provides a detection kit including the detecting reagent composition. Further, the present invention provides a β-lactamase detection method where a liquid specimen containing a target substance to be analyzed is brought into contact with the composition.
Owner:SHOWA YAKUHIN KAKO +1
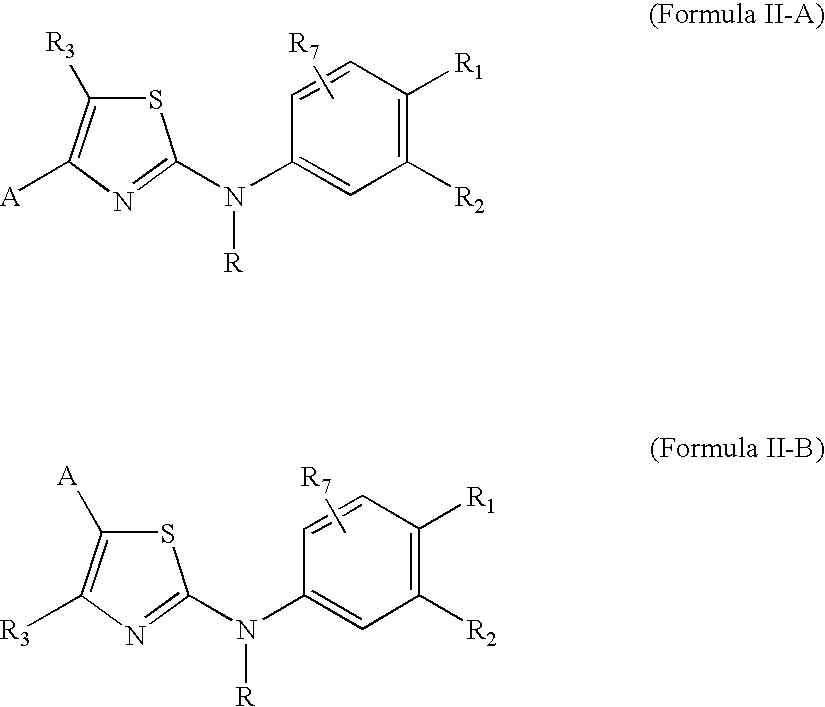
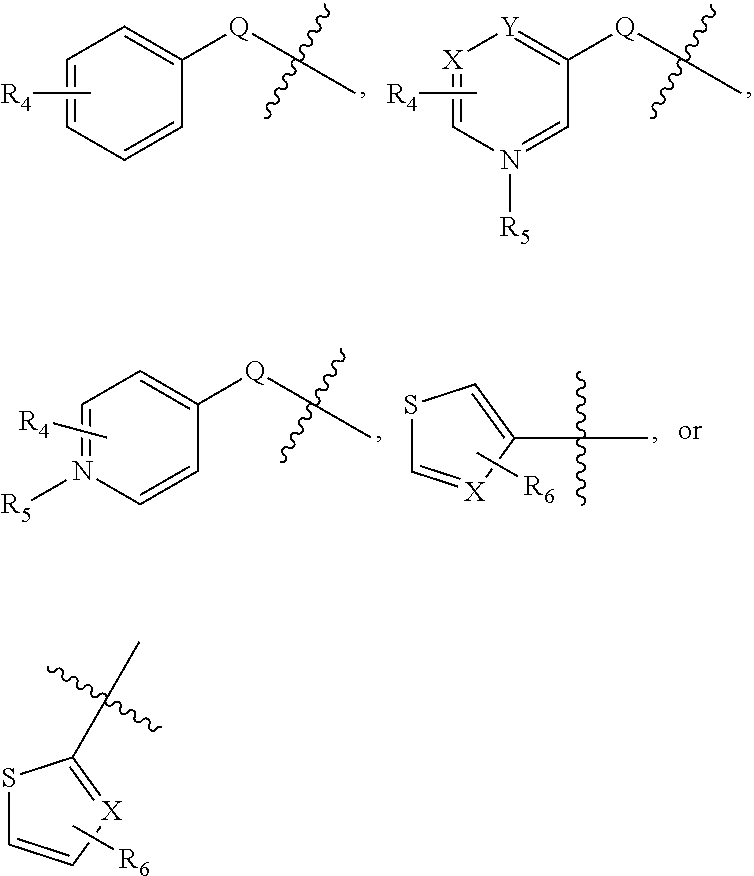
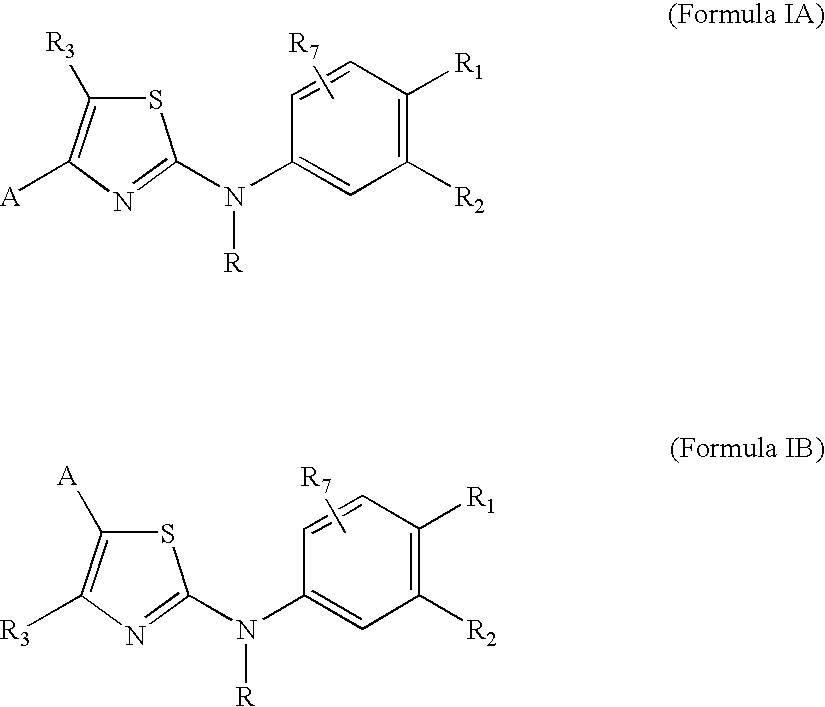
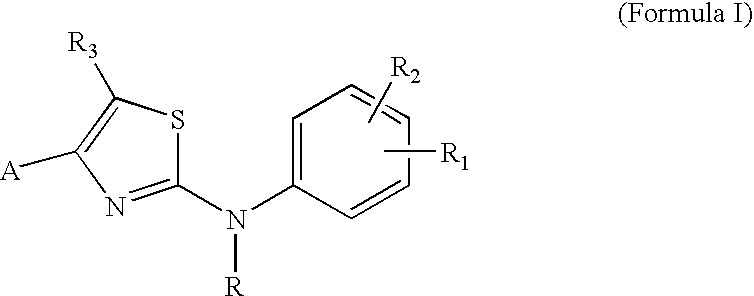
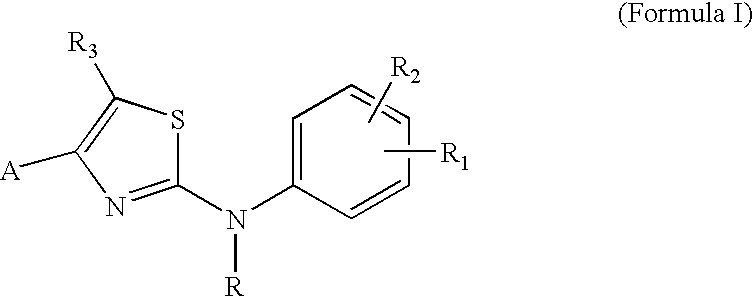
Substituted Aminothiazole Derivatives With Anti-HCV Activity
The invention provides amino-substituted aminothiazole compounds of Formula I in which A is a group of the formula: and the variables R and R1 to R7 are described herein. These compounds are useful as inhibitors of viral replication. Compositions containing such compounds, and methods of treating viral infections with these compounds, as well as to processes and intermediates useful for preparing such compounds are also provided by the invention.
Owner:ACHILLION PHARMA INC
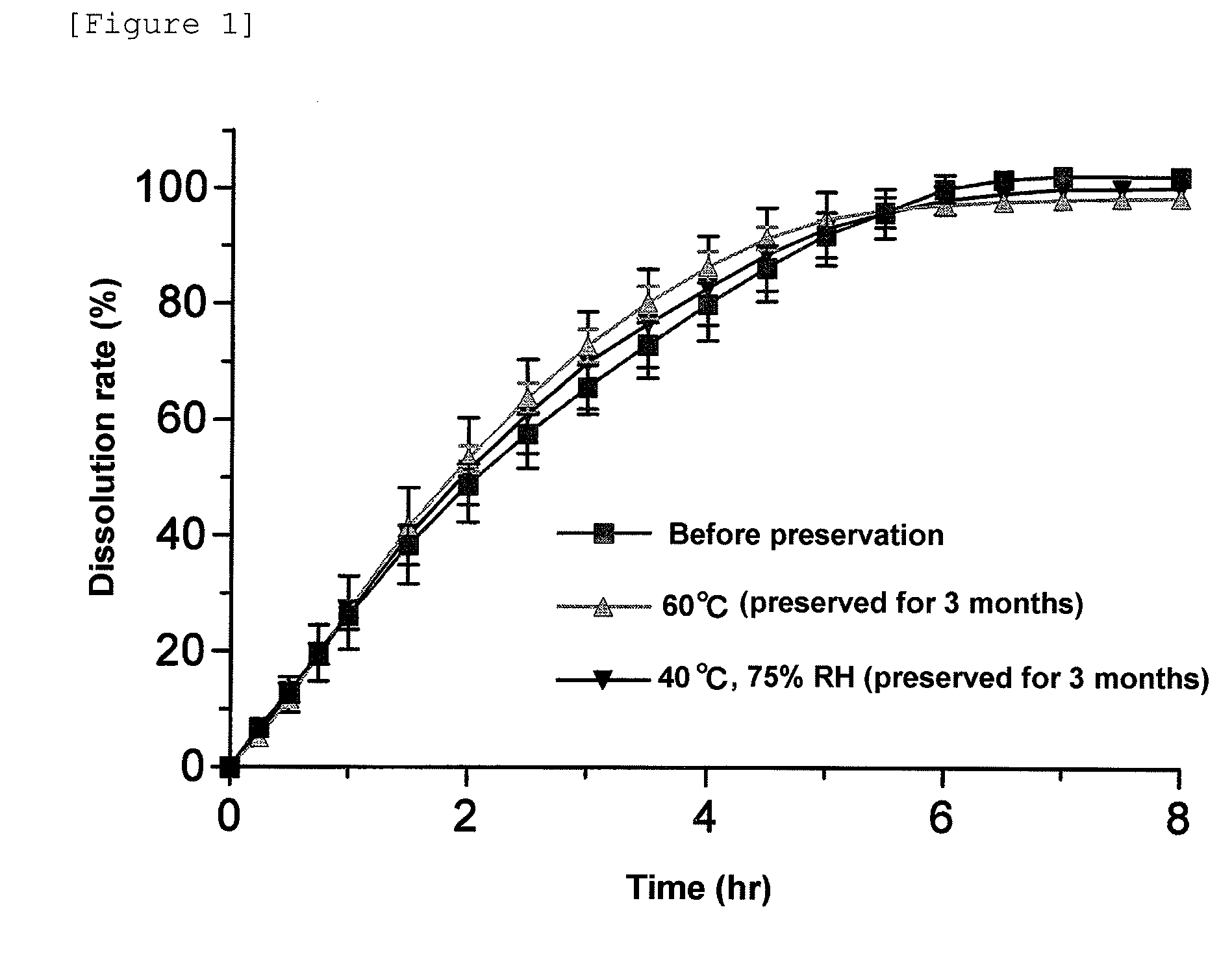
Pharmaceutical composition for modified release
InactiveUS20100144807A1Slow changeReduced stateOrganic active ingredientsBiocideSolubilityAcetic acid
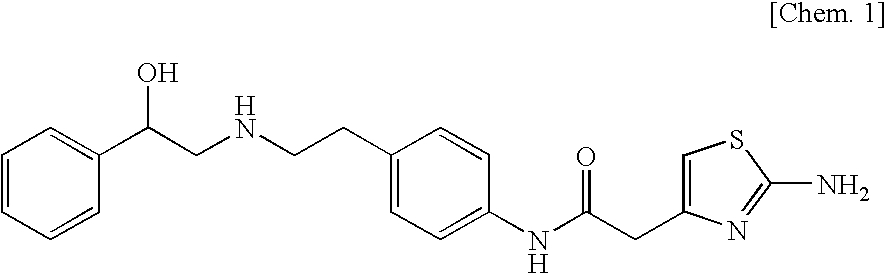
A pharmaceutical composition for modified release, comprising (1) (R)-2-(2-aminothiazol-4-yl)-4′-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]acetic acid anilide, or a pharmaceutically acceptable salt thereof, (2) at least one additive which ensures penetration of water into the pharmaceutical composition and which has a solubility such that the volume of water required for dissolving 1 g of the additive is 5 mL or less, and (3) a hydrogel-forming polymer having an average molecular weight of approximately 100,000 or more, or a viscosity of 12 mPa·s or more at a 5% aqueous solution at 25° C. is disclosed.
Owner:ASTELLAS PHARMA INC
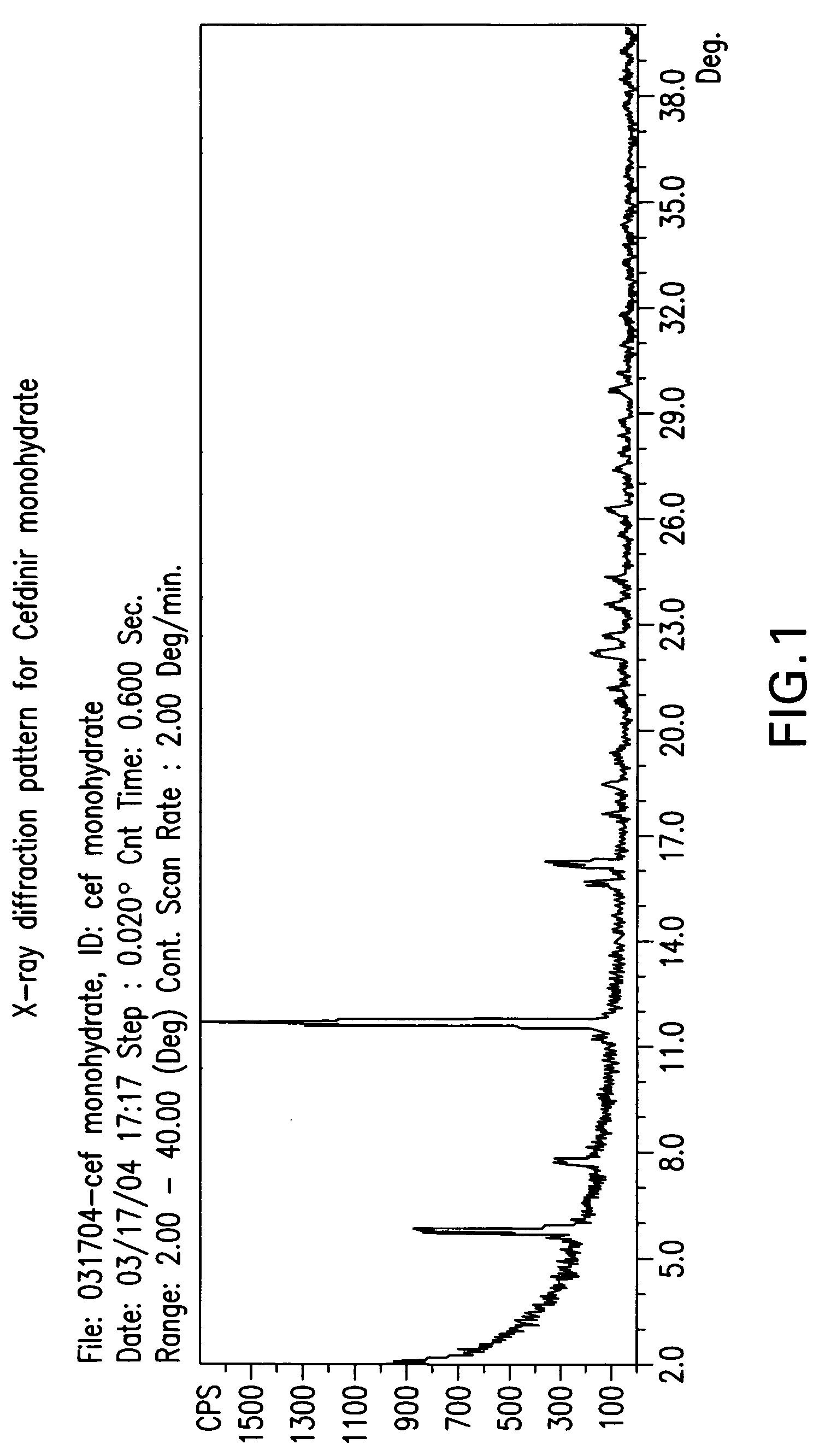
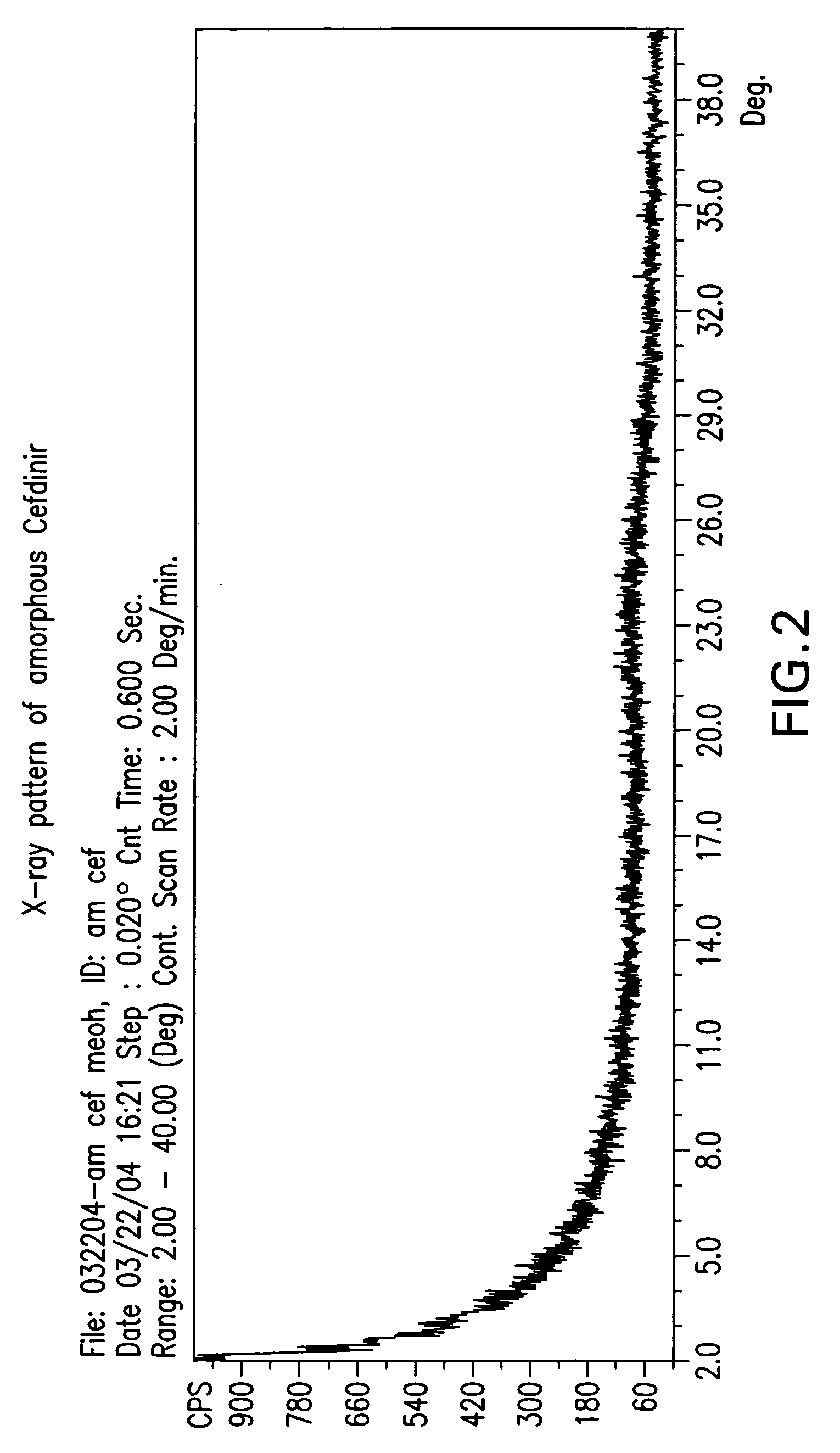
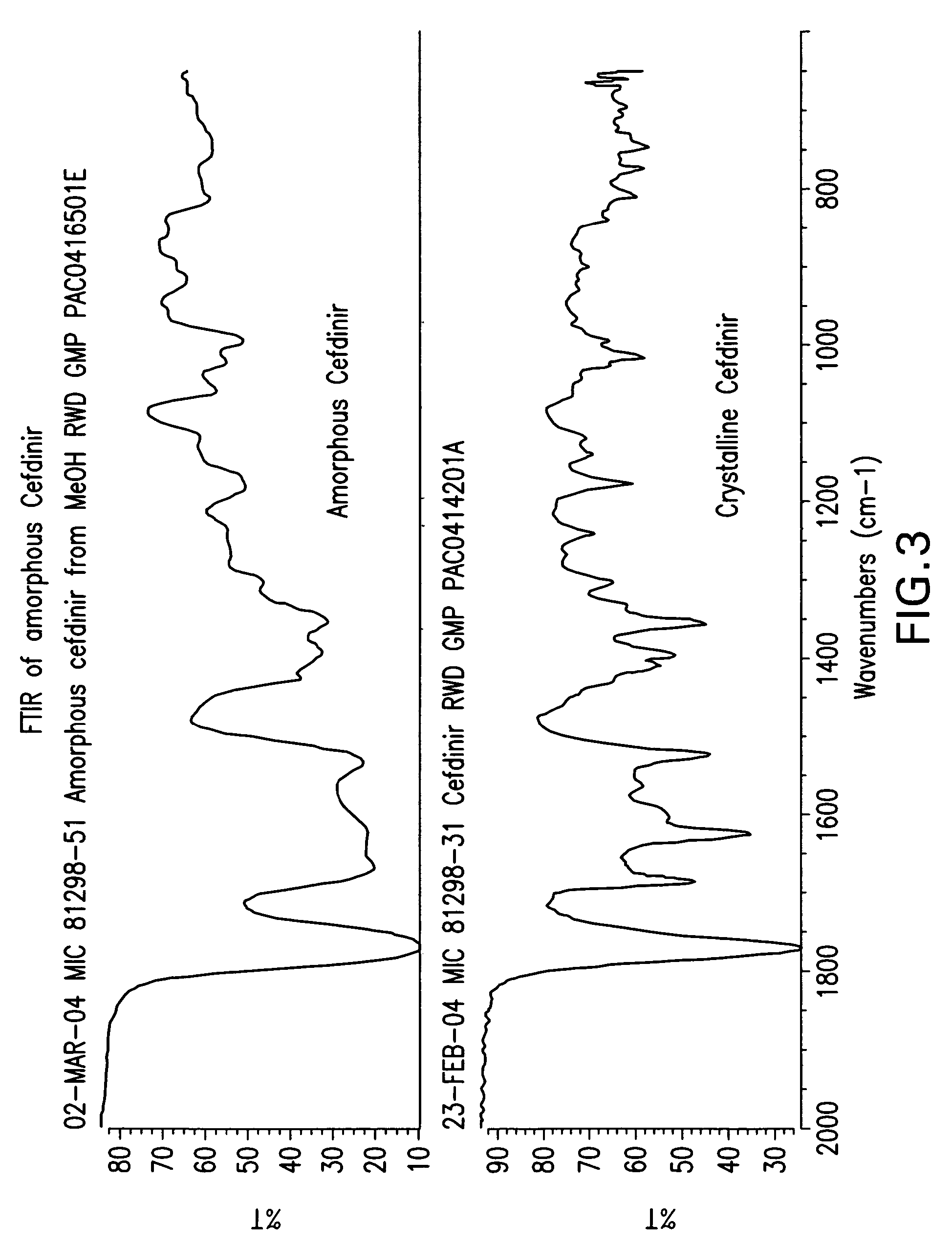
Stable amorphous cefdinir
The present invention relates to stable amorphous 7-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamide]-3-vinyl-3-cephem-4-carboxylic acid (syn isomer), methods for its preparation, and pharmaceutical compositions comprising stable amorphous 7-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamide]-3-vinyl-3-cephem-4-carboxylic acid (syn isomer).
Owner:ABBOTT LAB INC
2-Acylaminothiazole derivative or salt thereof
InactiveUS20060194844A1Function increaseHigh activityBiocideOrganic chemistryCell growthIncreased platelets
2-Acylaminothiazole derivatives or salts thereof which have a platelet increasing activity based on an excellent human c-mpl-Ba / F3 cell growth function and a function of accelerating formation of megakaryocytic colonies and which are useful for treating thrombocytopenia are provided.
Owner:ASTELLAS PHARMA INC
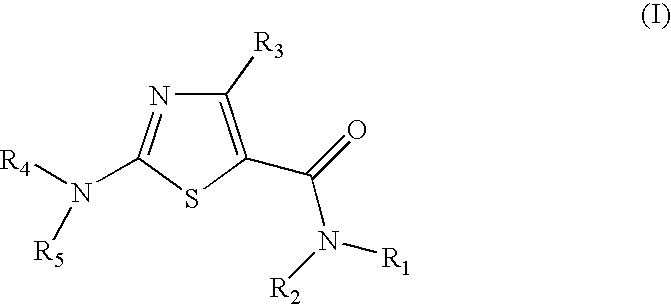
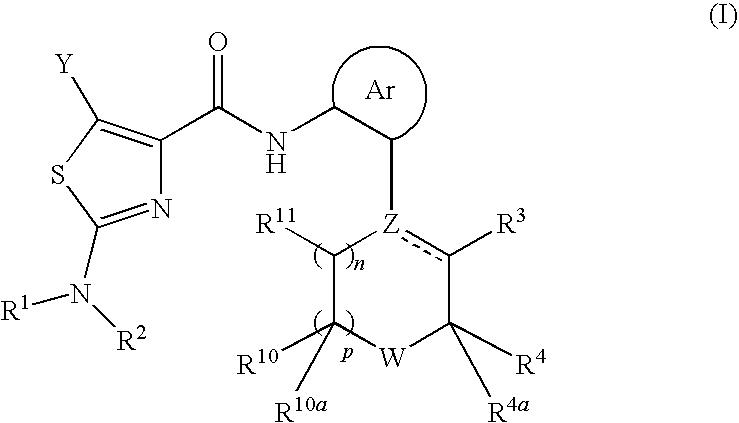
2-aminothiazole-4-carboxylic amides as protein kinase inhibitors
The present invention relates to novel Anilinopiperazine Derivatives of formula (I), compositions comprising the Anilinopiperazine Derivatives, and methods for using the Anilinopiperazine Derivatives for treating or preventing a proliferative disorder, an anti-proliferative disorder, inflammation, arthritis, a central nervous system disorder, a cardiovascular disease, alopecia, a neuronal disease, an ischemic injury, a viral disease, a fungal infection, or a disorder related to the activity of a protein kinase.
Owner:MERCK SHARP & DOHME LLC
Method for catalyzing gamma-valerolactone to be subjected to ring-opening polymerization by utilizing biomimetic catalyst
The invention provides a method for catalyzing gamma-valerolactone to be subjected to ring-opening polymerization by utilizing a biomimetic catalyst and belongs to the field of biomass high molecular materials. According to the method, the gamma-valerolactone is used as a monomer, the biomimetic catalyst is used as a catalyst and an alcohol compound is used as an initiator, and the gamma-valerolactone is subjected to the ring-opening polymerization in a solvent to obtain poly-gamma-valerolactone; the biomimetic catalyst is one or a mixture of more of a thioureaamide type dihydrogen bond donor catalyst, an aminothiazole type dihydrogen bond donor catalyst and a phenyl phosphate type dihydrogen bond donor catalyst. Compared with a traditional catalyst, the aminothiazole type biomimetic catalyst, the thioureaamide type biomimetic catalyst and the phenyl phosphate type biomimetic catalyst have no metal and the utilization amount is small; the catalysis effect is higher under the action of a dihydrogen bond.
Owner:NANJING UNIV OF TECH
3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof
InactiveCN103755697AHas anti-influenza drug neuraminidase activityOrganic chemistryAntiviralsThiazoleHydrogen
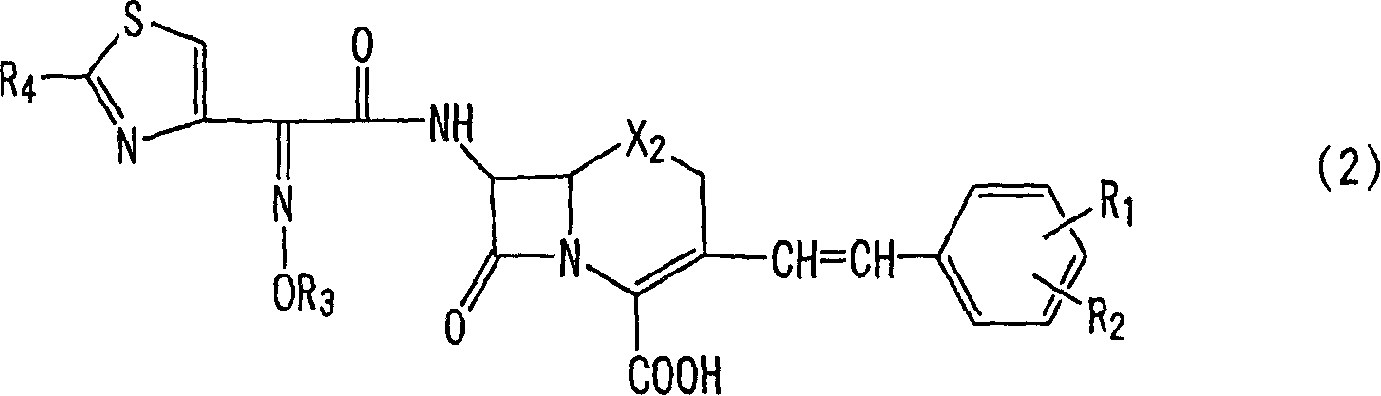
The invention relates to 3-[[2-(benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as shown in chemical structural formulas I and II or a salt thereof. In the chemical structural formulas, R, X<1> and X<2> are selected from hydrogen, deuterium, C1-C2 alkyl, C3-C4 linear-chain alkyl or branched-chain alkyl; X<3> is selected from hydroxyl, methoxyl and ethyoxyl; is selected from hydrogen, deuterium, C1-C2 alkyl, fluorine, fluorine or bromine; X4 and X6 are selected from hydrogen, deuterium, C1-C2 alkyl, fluorine, fluorine, bromine or nitryl; X5 and X7 are selected from hydrogen, deuterium and C1-C2 alkyl. The invention also provides an application of the 3-[[2-(benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone in preparation of an influenza virus neuraminidase inhibitor.
Owner:HUNAN UNIV
Reagent composition for detecting beta-lactamase, detection kit and detection method
InactiveCN1729299AMicrobiological testing/measurementBiological material analysisCarboxylic acidCephem
It is intended to provide a reagent composition for detecting beta-lactamase which contains 3-[2,4-dinitrostyryl]-7-(2-thienylacetamido)-3-cephem-4-carboxylic acid or 7-[2-(2-aminothiazol-4-yl)-2-(1-carboxy-1-methylethoxyimino)acetamido]-3-(2,4-dinitrostyryl)-3-cephem-4-carboxylic acid as a substrate for detecting beta-lactamase together with at least one beta-lactamase inhibitor selected from among clavulanic acid, aztreonam, ethylenediaminetetraacetic acid and cloxacillin and by which beta-lactamase can be quickly and easily identified at a high sensitivity. Moreover, a detection kit containing the above detection reagent composition and a method of detecting beta-lactamase comprising contacting a liquid specimen containing the subject to be analyzed with the above composition are provided.
Owner:昭和药品化工股份有限公司 +1
Novel cephem derivative
InactiveUS20130096299A1Potent antimicrobial spectrumHigh antibacterial activityAntibacterial agentsOrganic chemistryThiadiazolesPharmaceutical Substances
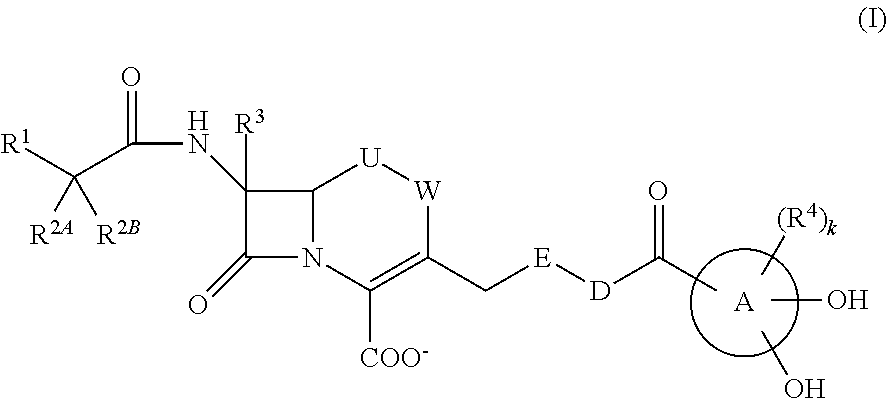
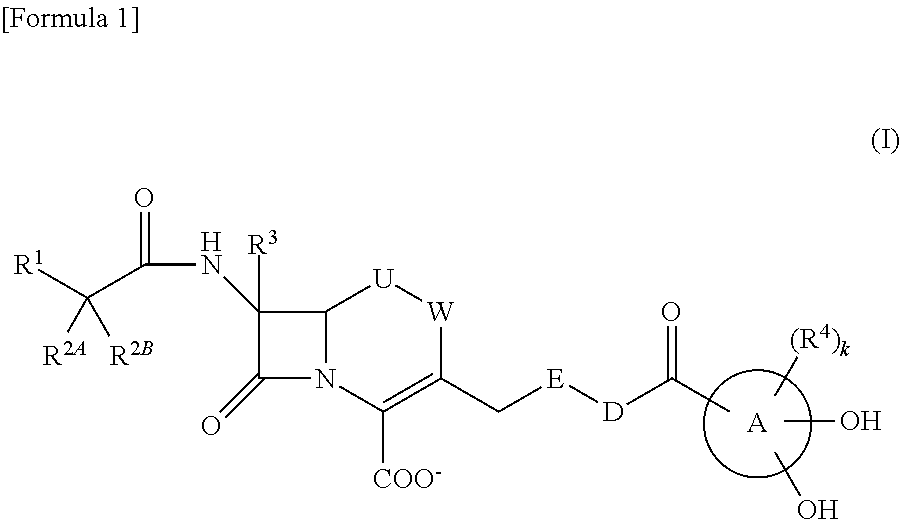
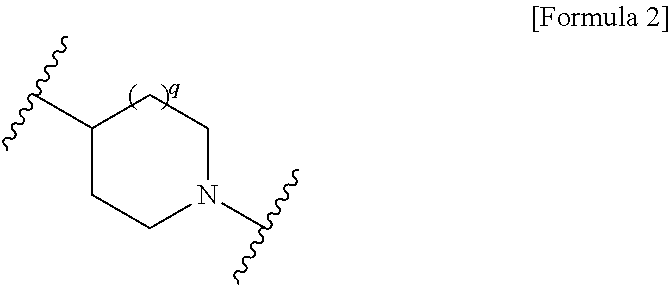
Provided is a cephem compound which has a wide antimicrobial spectrum, and in particular exhibit potent antimicrobial activity against beta-lactamase producing Gram negative bacteria, and pharmaceutical composition comprising the same.A Compound of the formula (I):whereinW and U are as defined in the specification;R1 is as defined in the specification;R2A and R2B are as defined in the specification, provided that R2A and R2B are not taken together to form an optionally substituted oxime group when R1 is aminothiazole or aminothiadiazole optionally protected at the amino group;ring A is a benzene ring or a 6-membered aromatic heterocyclic group having 1-3 nitrogen atoms;R3 is a hydrogen atom, —OCH3 or —NH—CH(═O);k is an integer from 0 to 2;R4 is as defined in the specification; andD and E are as defined in accordance with a) or b) described in the specification.
Owner:SHIONOGI & CO LTD
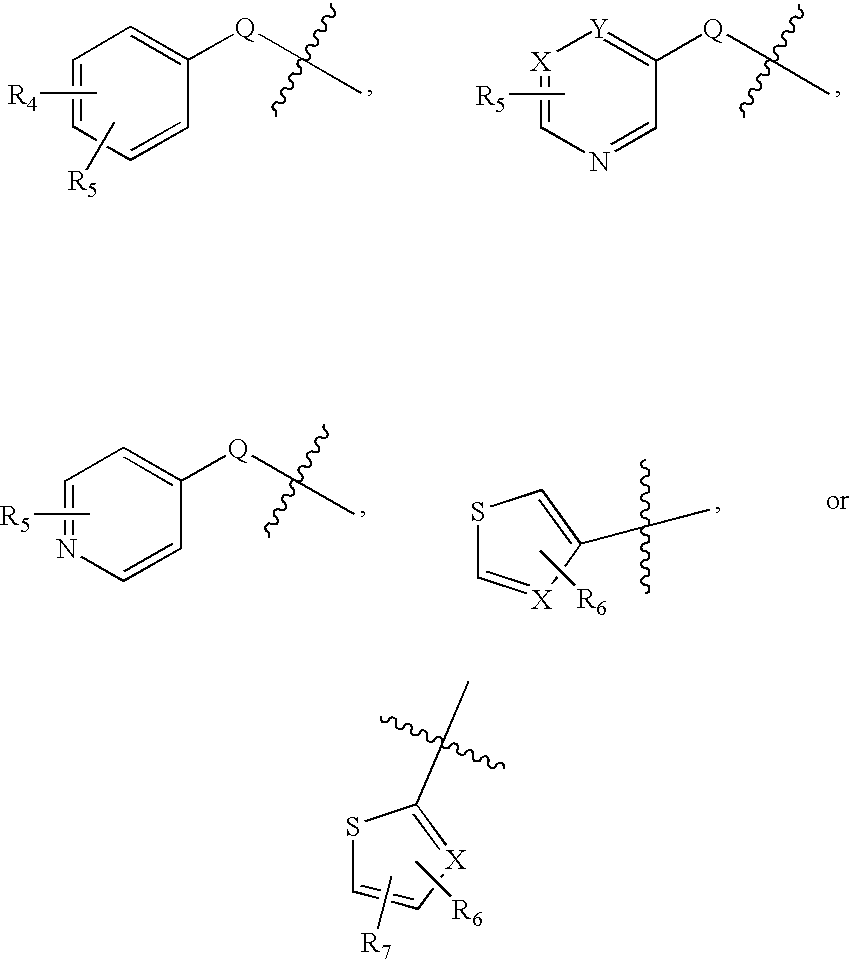
Substituted aminothiazole prodrugs of compounds with anti-HCV activity
The invention provides amino-substituted aminothiazole compounds of Formula I and Formula IIwhere A is a group of the formula:and the variables X, Y, R, and R1 to R7 are described herein. These compounds are prodrugs of compounds useful as inhibitors of viral replication. Compositions containing such compounds, and methods of treating viral infections with these compounds, as well as to processes and intermediates useful for preparing such compounds are also provided by the invention.
Owner:ACHILLION PHARMA INC
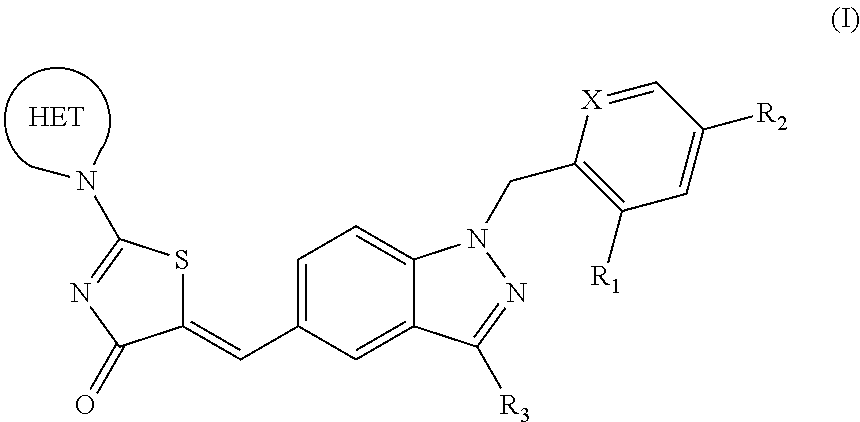
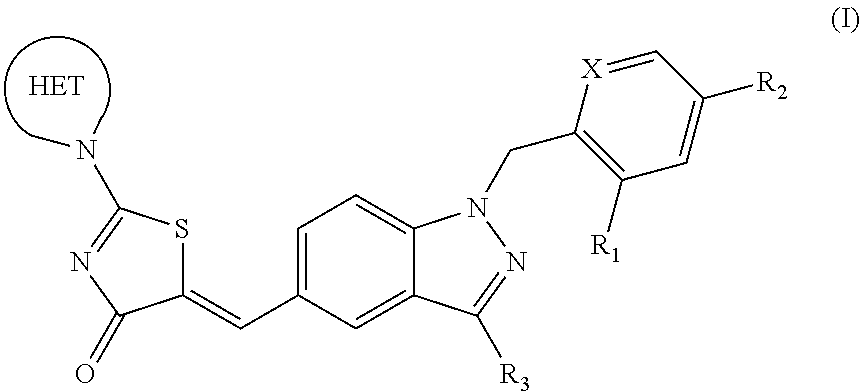
Substituted aminothiazolone indazoles as estrogen related receptor-alpha modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
4-aminothiazole derivs, their preparation and their use as inhibitors of cyclin-dependent kinases
This invention is directed to aminothiazole compounds of formula (I) wherein R<1> is a substituted or unsubstituted group selected from: C1-6-alkyl; C1-6-alkenyl; C1-6-alkynyl; C1-6-alkoxyl; C1-6-alcohol; carbocyclic or heterocyclic, monocyclic or fused or non-fused polycyclic, cycloalkyl; carbocyclic or heterocyclic, monocyclic or fused or non-fused polycyclic, aryl; carbonyl; ether; (C1-6-alkyl)-carbonyl; (C1-6-alkyl)-aryl; (C1-6-alkyl)-cycloalkyl; (C1-6-alkyl)-(C1-6-alkoxyl); aryl-(C1-6-alkoxyl); thioether; thiol; and sulfonyl; wherein when R<1> is substituted, each substituent independently is a halogen; haloalkyl; C1-6-alkyl; C1-6-alkenyl; C1-6-alkynyl; hydroxyl; C1-6-alkoxyl; amino; nitro; thiol; thioether; imine; cyano; amido; phosphonato; phosphine; carboxyl; thiocarbonyl; sulfonyl; sulfonamide; ketone; aldehyde; ester; oxygen; carbocyclic or heterocyclic, monocyclic or fused or non-fused polycyclic, cycloalkyl; or carbocyclic or heterocyclic, monocyclic or fused or non-fused polycyclic, aryl; and R<2 >is a carbocyclic or heterocyclic, monocyclic or fused or non-fused polycyclic, ring structure having a substituent at the position adjacent to the point of attachment, which ring structure is optionally further substituted, where each substituent of R<2> independently is a halogen; haloalkyl; C1-6-alkyl; C1-6-alkenyl; C1-6-alkynyl; hydroxyl; C1-6-alkoxyl; amino; nitro; thiol; thioether; imine; cyano; amido; phosphonato; phosphine; carboxyl; thiocarbonyl; sulfonyl; sulfonamide; ketone; aldehyde; ester; oxygen; carbocyclic or heterocyclic, monocyclic or fused or non-fused polycyclic, cycloalkyl; or carbocyclic or heterocyclic, monocyclic or fused or non-fused polycyclic, aryl; or a pharmaceutically acceptable salt of a compound of formula (I), or a prodrug or pharmaceutically active metabolite of a compound of formula (I) or pharmaceutically acceptable salt thereof, for inhibiting cyclin-dependent kinases (CDKs), such as CDK1, CDK2, CDK4, and CDK6.
Owner:AGOURON PHARMA INC
Process for preparing 2-aminothiazole-5-carboxamides useful as kinase inhibitors
The invention is directed to processes for preparing 2-aminothiazole-5-carboxamides of formula Iwherein R1, R2, R3, R4 and R5 are as defined as set forth in the specification herein.
Owner:BRISTOL MYERS SQUIBB CO
2-aminothiazole-4-carboxylic amides as protein kinase inhibitors
The present invention relates to novel Anilinopiperazine Derivatives of formula (I), compositions comprising the Anilinopiperazine Derivatives, and methods for using the Anilinopiperazine Derivatives for treating or preventing a proliferative disorder, an anti-proliferative disorder, inflammation, arthritis, a central nervous system disorder, a cardiovascular disease, alopecia, a neuronal disease, an ischemic injury, a viral disease, a fungal infection, or a disorder related to the activity of a protein kinase.
Owner:MERCK SHARP & DOHME LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

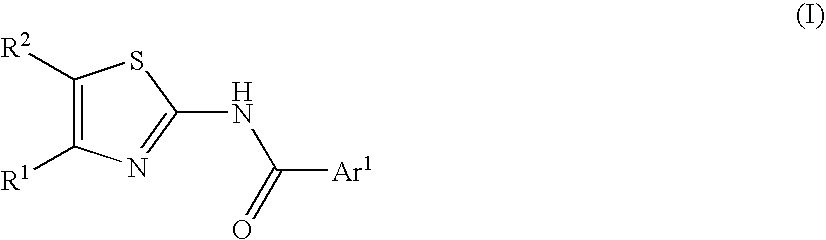
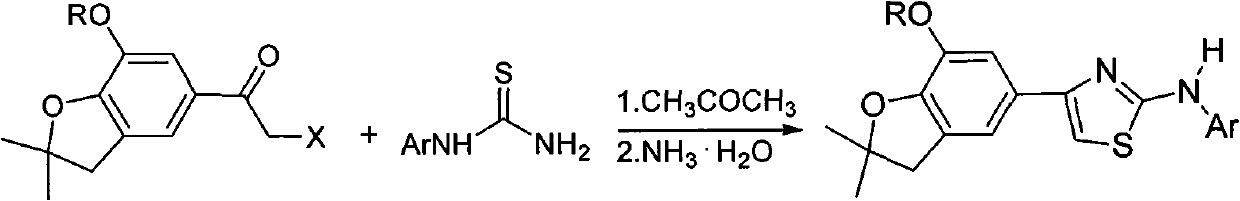

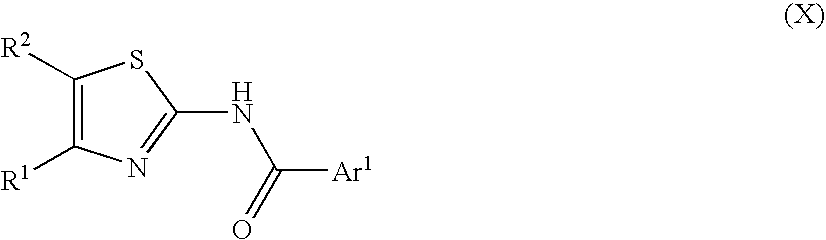
![3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof 3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/1ec64ec5-fc1c-433f-bd19-00bb4ff43c61/BDA0000456427430000021.PNG)
![3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof 3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/1ec64ec5-fc1c-433f-bd19-00bb4ff43c61/BDA0000456427430000022.PNG)
![3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof 3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/1ec64ec5-fc1c-433f-bd19-00bb4ff43c61/BDA0000456427430000023.PNG)