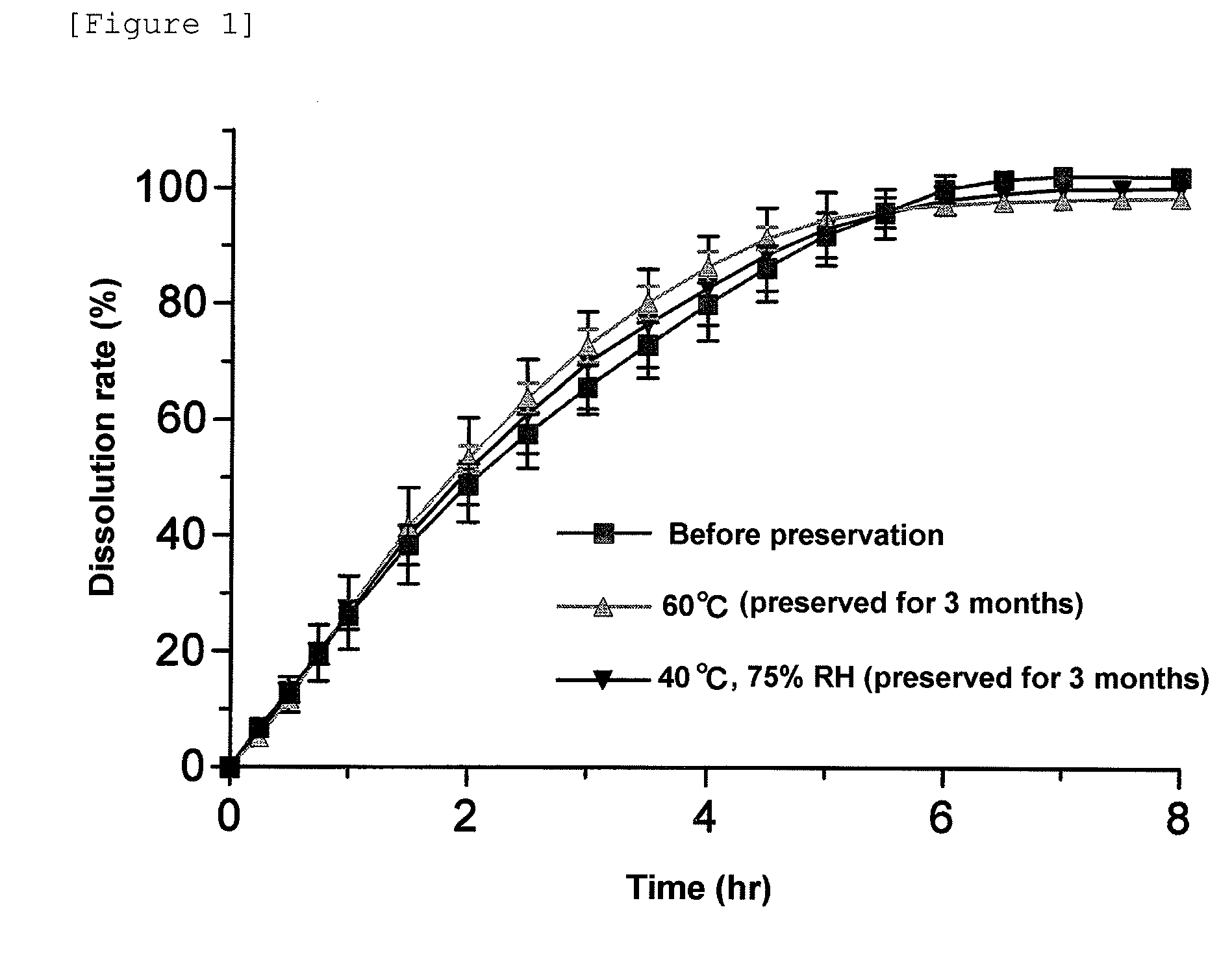
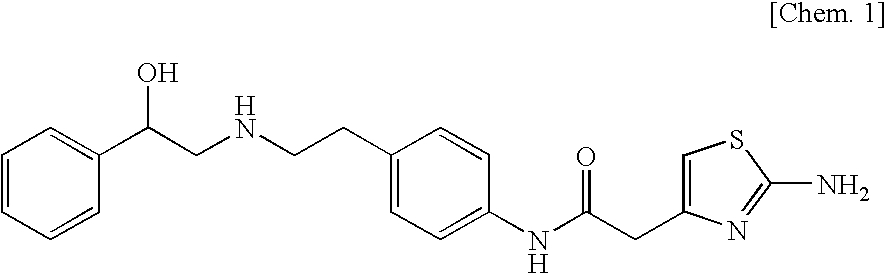
Pharmaceutical composition for modified release
a technology of pharmaceutical composition and modified release, which is applied in the direction of drug composition, biocide, metabolic disorder, etc., can solve the problems of unexpected variation in the data of drugcokinetic data according to the presence or absence of food intake, and achieve the reduction of cmax caused by food intake, significant relief of cmax, and reduction of changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0104]In a mortar, 10 g of compound A hydrochloride, 2.5 g of polyethylene oxide (Dow chemical; product name: WSR-N-60K; The same compound was used in the following Examples.), and 7.5 g of polyethylene glycol (Sanyo Chemical Industries, Ltd.; PEG 6000; The same compound was used in the following Examples.) were mixed well. The mixture was formed into tablets using Autograph (Shimadzu; The same apparatus was used in the following Examples.) to obtain a pharmaceutical composition for modified release of the present invention having a tablet weight of 400 mg.
example 2
[0105]In a mortar, 10 g of compound A hydrochloride, 3.5 g of polyethylene oxide, and 6.5 g of polyethylene glycol were mixed well, and the mixture was formed into tablets using Autograph to obtain a pharmaceutical composition for modified release of the present invention having a tablet weight of 400 mg.
example 3
[0106]In a mortar, 10 g of compound A hydrochloride, 6.25 g of polyethylene oxide, and 5 g of polyethylene glycol were mixed well, and the mixture was formed into tablets using Autograph to obtain a pharmaceutical composition for modified release of the present invention having a tablet weight of 425 mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com