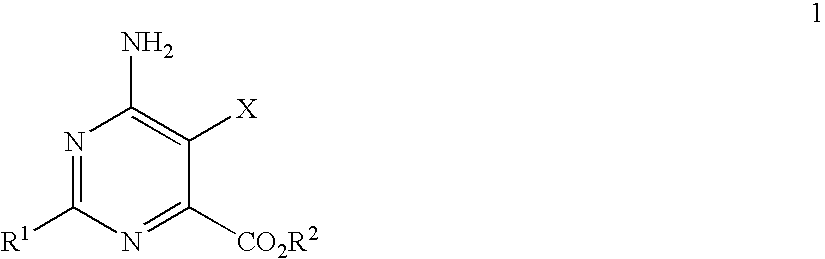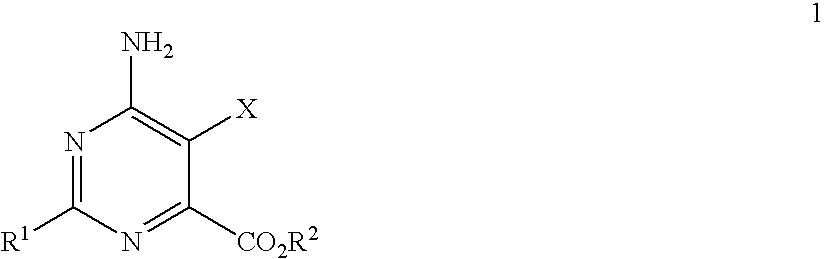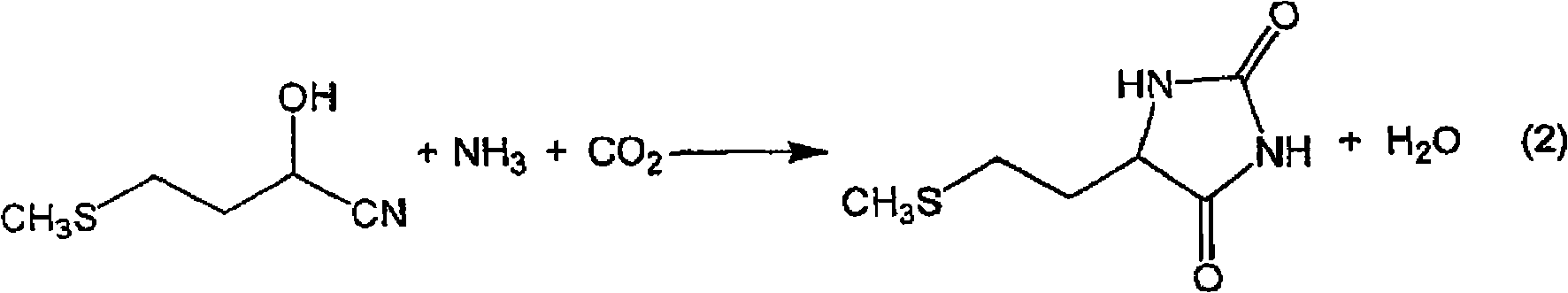Patents
Literature
3318 results about "Ethyl phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Triethyl phosphate is a chemical compound with the formula (C 2 H 5) 3 PO 4 or OP(OEt) 3. It is a colorless liquid. It is a colorless liquid. It is the triester of ethanol and phosphoric acid and can be called "phosphoric acid, triethyl ester".
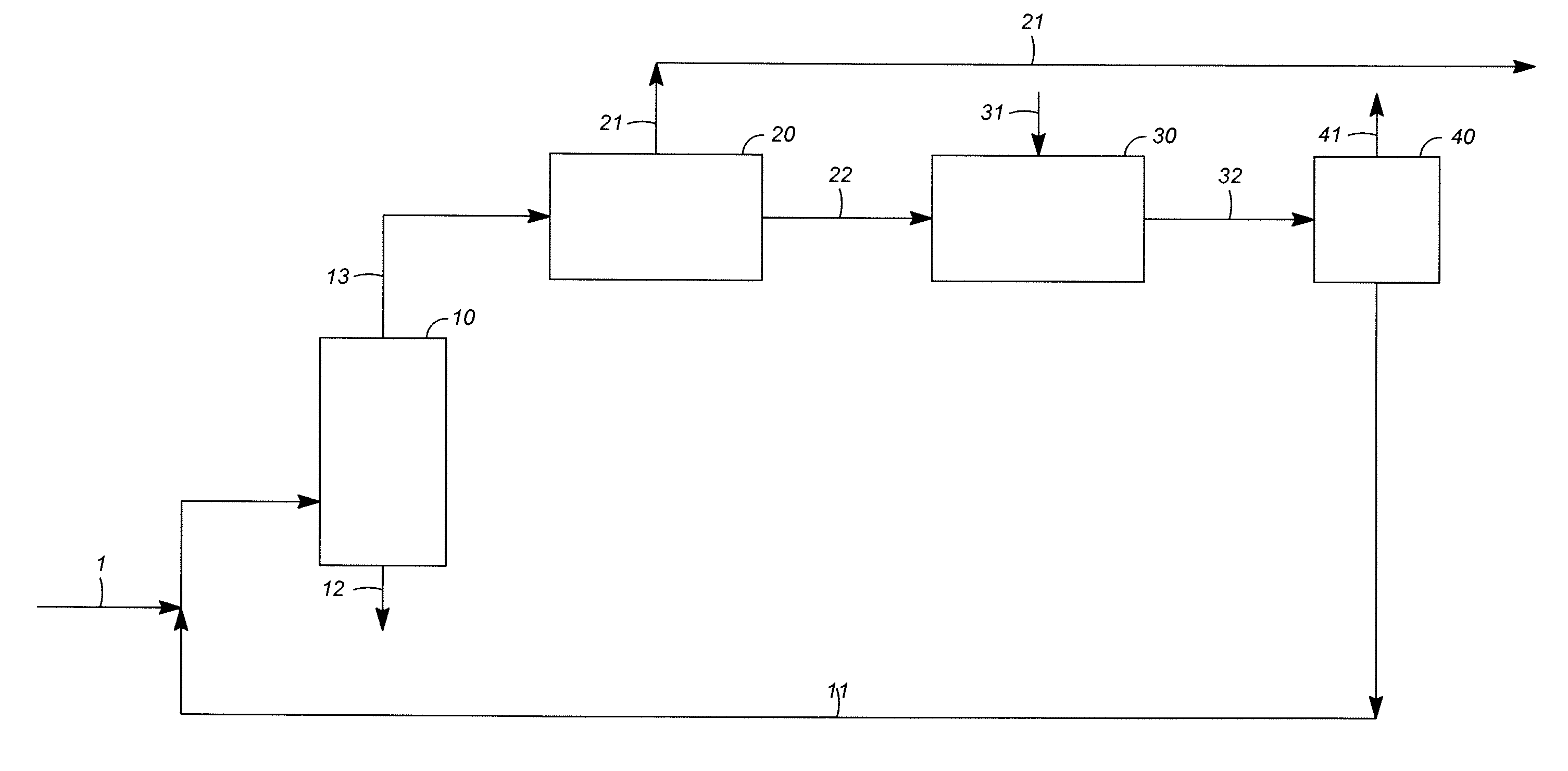
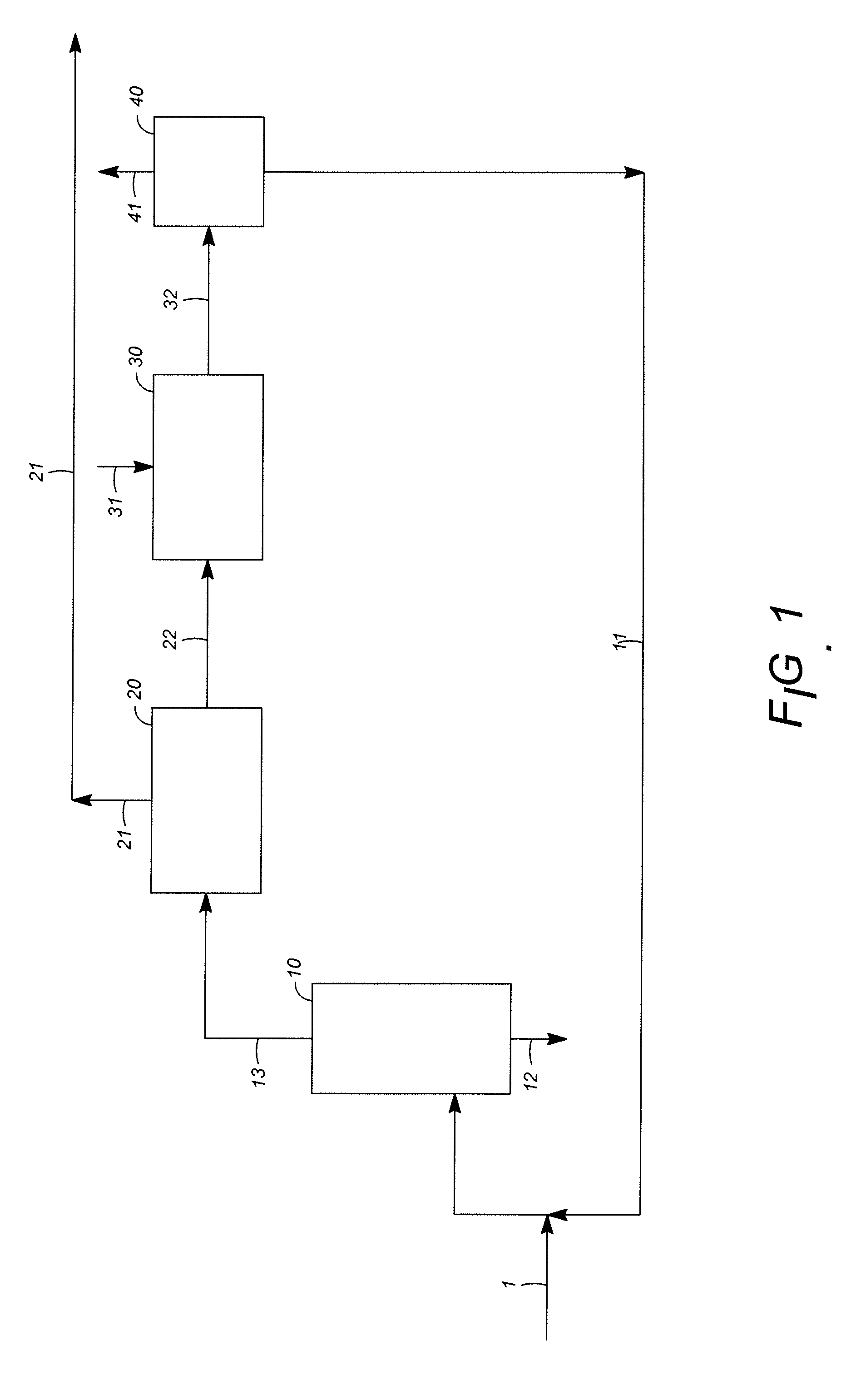
Orally administrable composition capable of providing enhanced bioavailability when ingested
InactiveUS6054136AImprove solubilityImprove bioavailabilityCosmetic preparationsToilet preparationsFatty acid esterIngestion
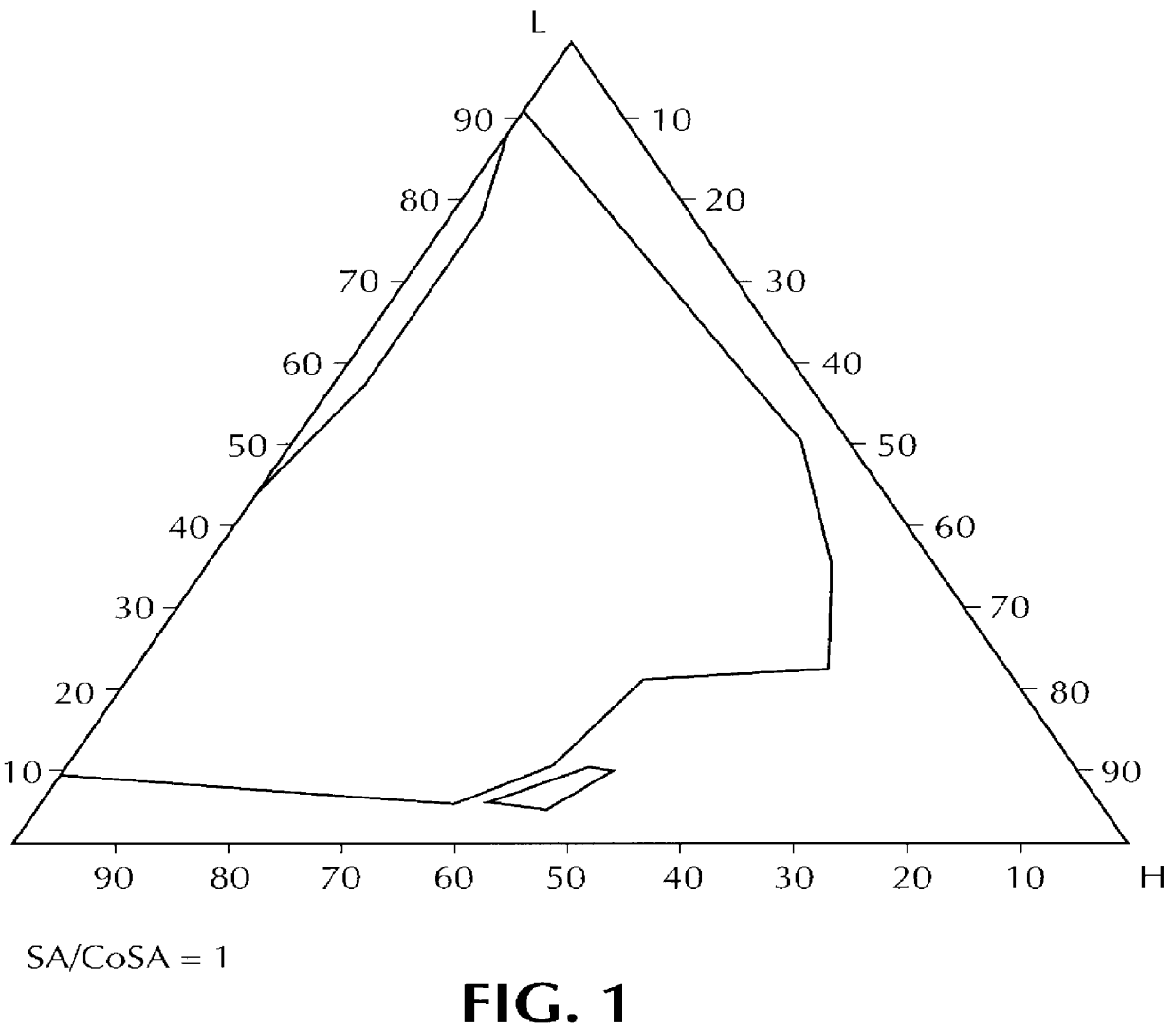
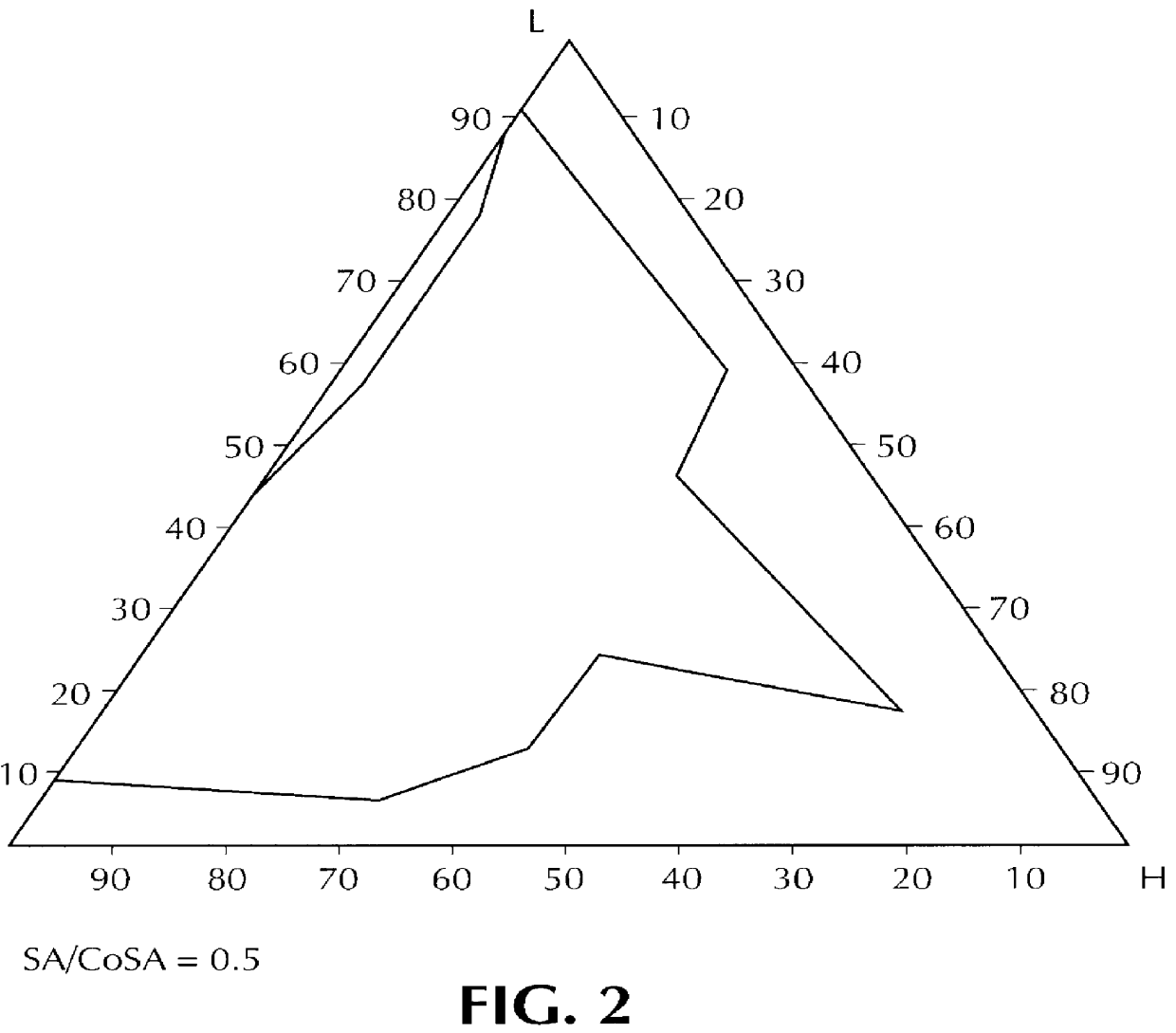
Composition for pharmaceutical or cosmetic use, capable of forming a microemulsion, comprising at least: an active principle, a lipophilic phase consisting of a mixture of fatty acid esters and glycerides, a surfactant (SA), a cosurfactant (CoSA), a hydrophilic phase, characterized: in that the lipophilic phase consists of a mixture of C8 to C18 polyglycolized glycerides having a hydrophilic-lipophilic balance (HLB) of less than 16, this lipophilic phase representing from 30 to 75% of the total weight of the composition; in that the surfactant (SA) is chosen from the group comprising saturated C8-C10 olyglycolized glycerides and oleic esters of polyglycerol, this surfactant having an HLB of less than 16; in that the cosurfactant (CoSA) is chosen from the group comprising lauric esters of propylene glycol, oleic esters of polyglycerol and ethyl diglycol; in that the SA / CoSA ratio is between 0.5 and 6; and in that the hydrophilic phase of the final microemulsion is supplied after ingestion by the physiological fluid of the digestive milieu.
Owner:GATTEFOSSE HLDG
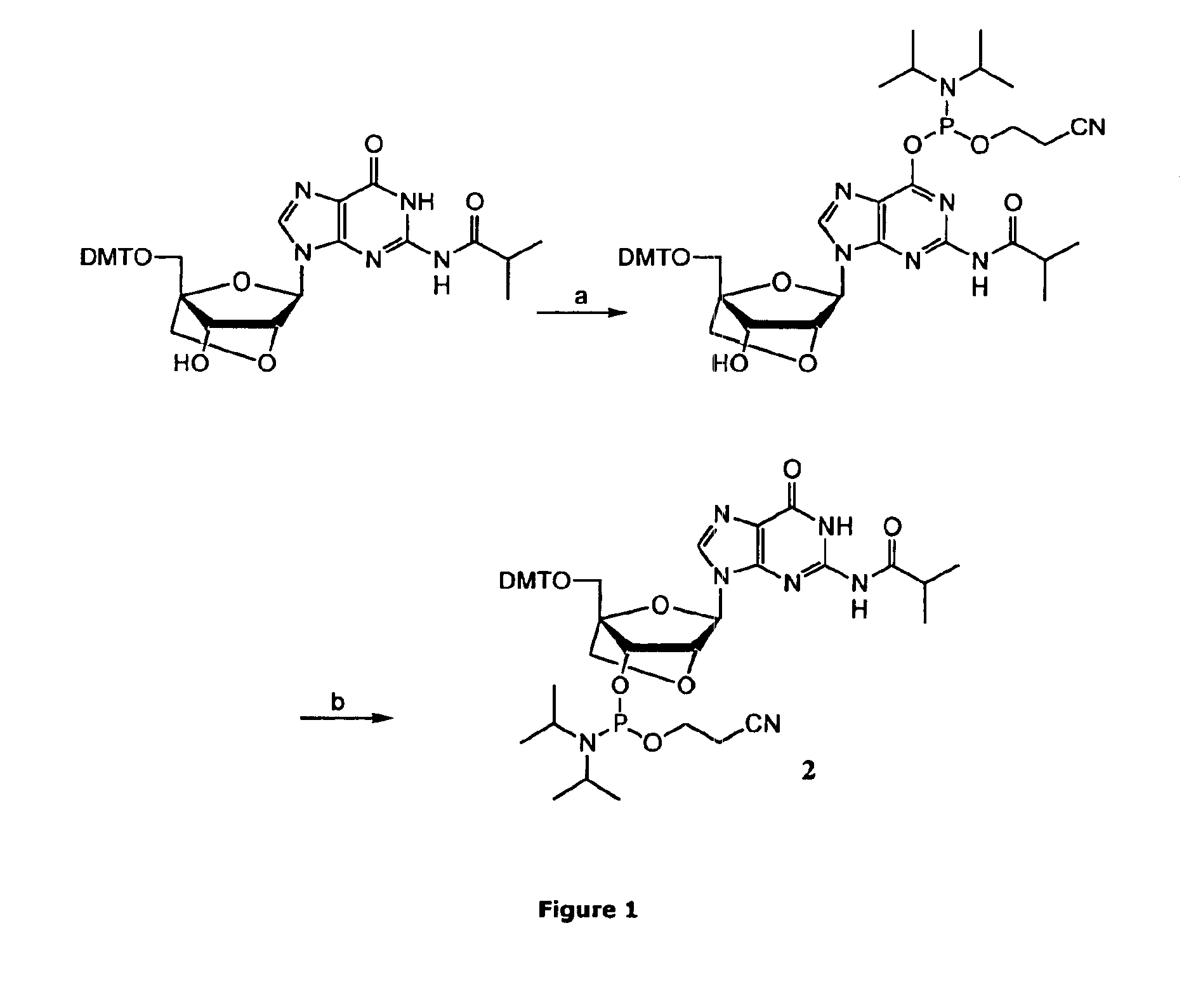
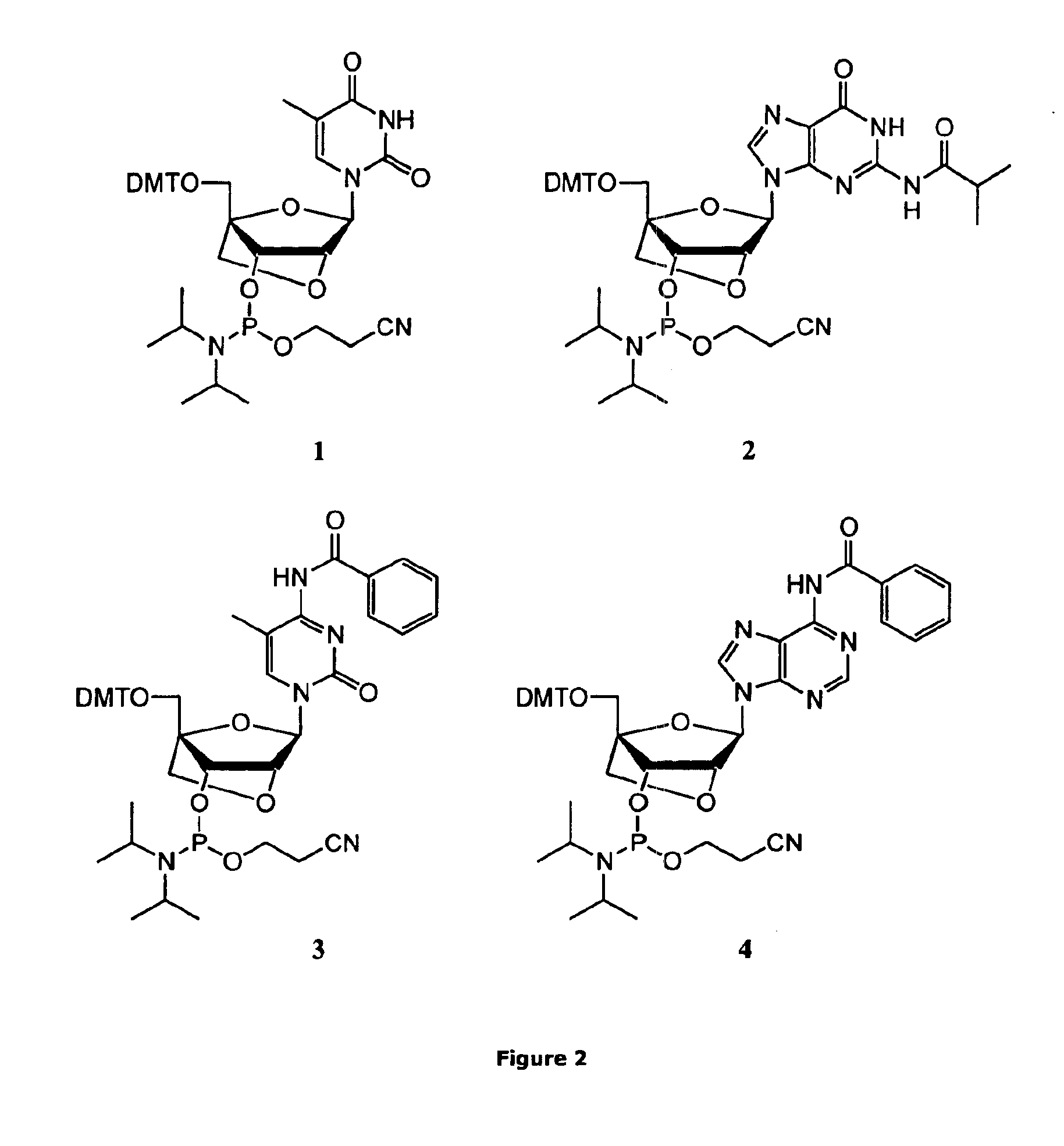
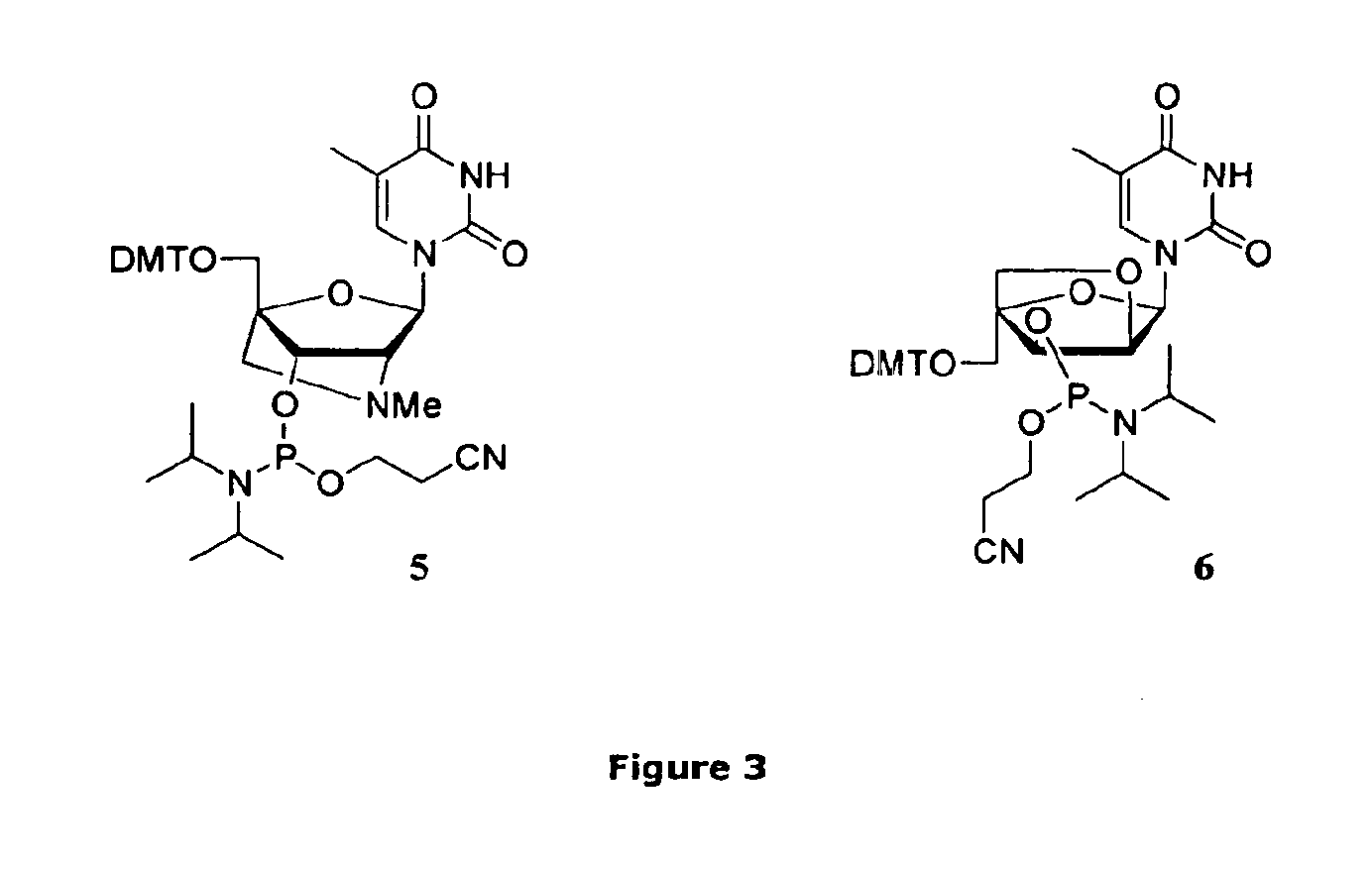
Method for preparation of LNA phosphoramidites
The present invention relates to large scale preparation of LNA phosphoramidites using a 2-cyanoethyl-N,N,N′,N′-tetra-substituted phosphoramidite and a nucleophilic activator, e.g. 2-cyanoethyl-N,N,N′,N′-tetraisopropylphosphoramidite and 4,5-dicyanoimidazole. The method is faster and more cost efficient that previously known methods.
Owner:SANTARIS PHARMA AS
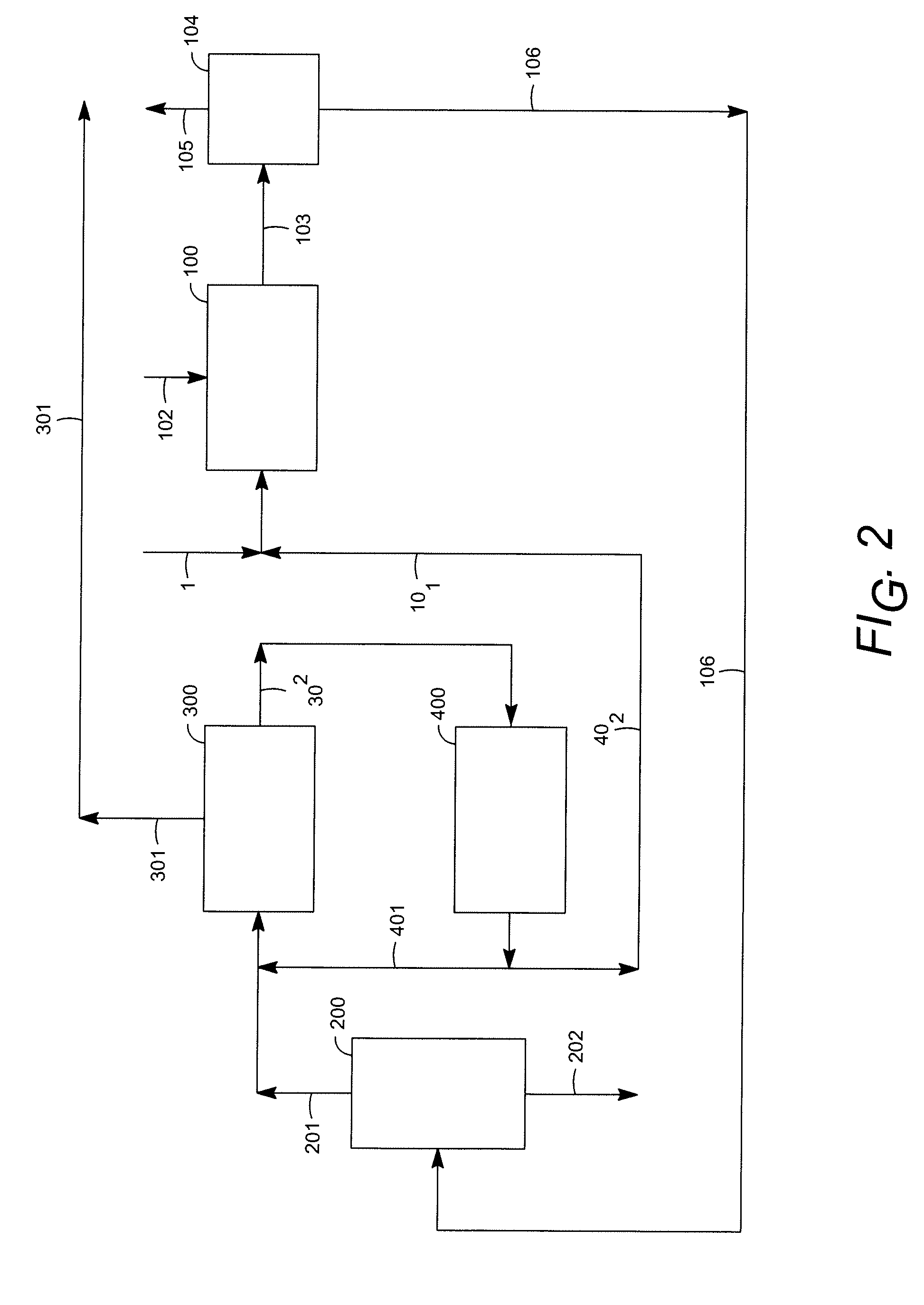
Dual layer tablet, method of making and use thereof
InactiveUS6863830B1Easy to optimizeComprehensive treatmentOrganic chemistryOther chemical processesPotassium persulfateLithium hypochlorite
A method for treating a recirculating water system which comprises introducing into said water system a multifunctional, multilayer tablet, wherein the multilayer tablet comprises a fast dissolving layer and a slow dissolving layer, wherein said fast dissolving layer releases a combination of active ingredients including a member selected from the group consisting of lithium hypochlorite, calcium hypochlorite, trichloroisocyanuric acid (TCCA), anhydrous sodium dichloroisocyanurate, sodium persulfate, potassium persulfate, potassium monopersulfate, sodium monopersulfate, and mixtures thereof, and at least one of a clarifier, chelating agent, sequesterant, algaestat, water softener, algaecide, corrosion inhibitor, scale inhibitor, flocculent, disintegrant, dispersant, colorant, dissolution control agent, fragrance, or surfactant and, wherein said slow dissolving layer includes a member selected from the group consisting of trichloroisocyanuric acid (TCCA), calcium hypochlorite, 1,3-dichloro-5,5-dimethylhydantoin (DCDMH), 1,3-dibromo-5,5-dimethylhydantoin (DBDMH), 1-bromo-3-chloro-5,5-dimethylhydantoin (BCDMH), 1,3-dichloro-5-ethyl-5-methylhydantoin (DCEMH), 1,3-dibromo-5-ethyl-5-methylhydantoin (DBEMH), 1-bromo-3-chloro-5-methyl-5-ethylhydantoin (BCEMH), and mixtures thereof, and at least one of a clarifier, chelating agent, sequesterant, algaestat, water softener, algaecide, corrosion inhibitor, scale inhibitor, flocculent, disintegrant, dispersant, colorant, dissolution control agent or surfactant.
Owner:BIO LAB
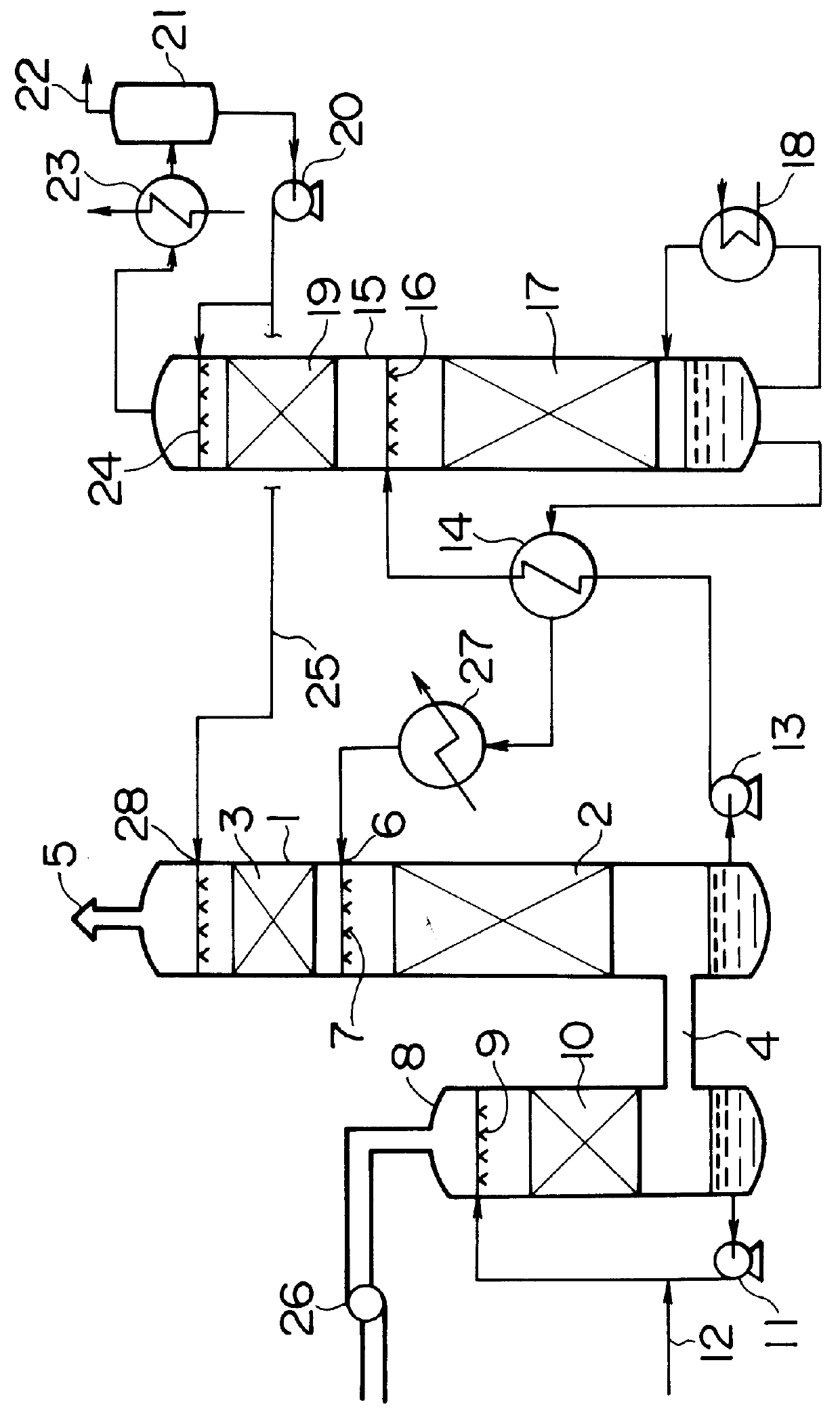
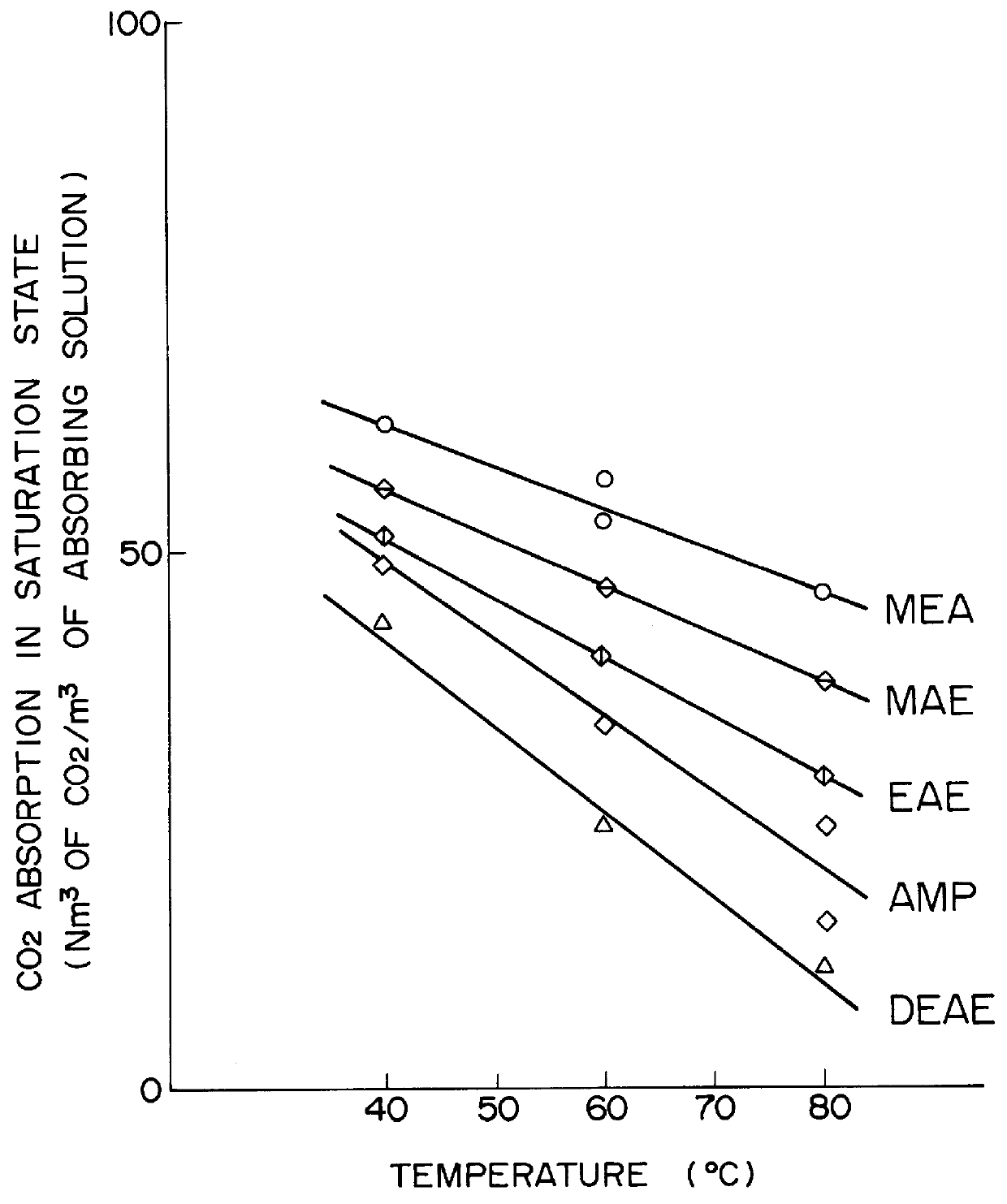
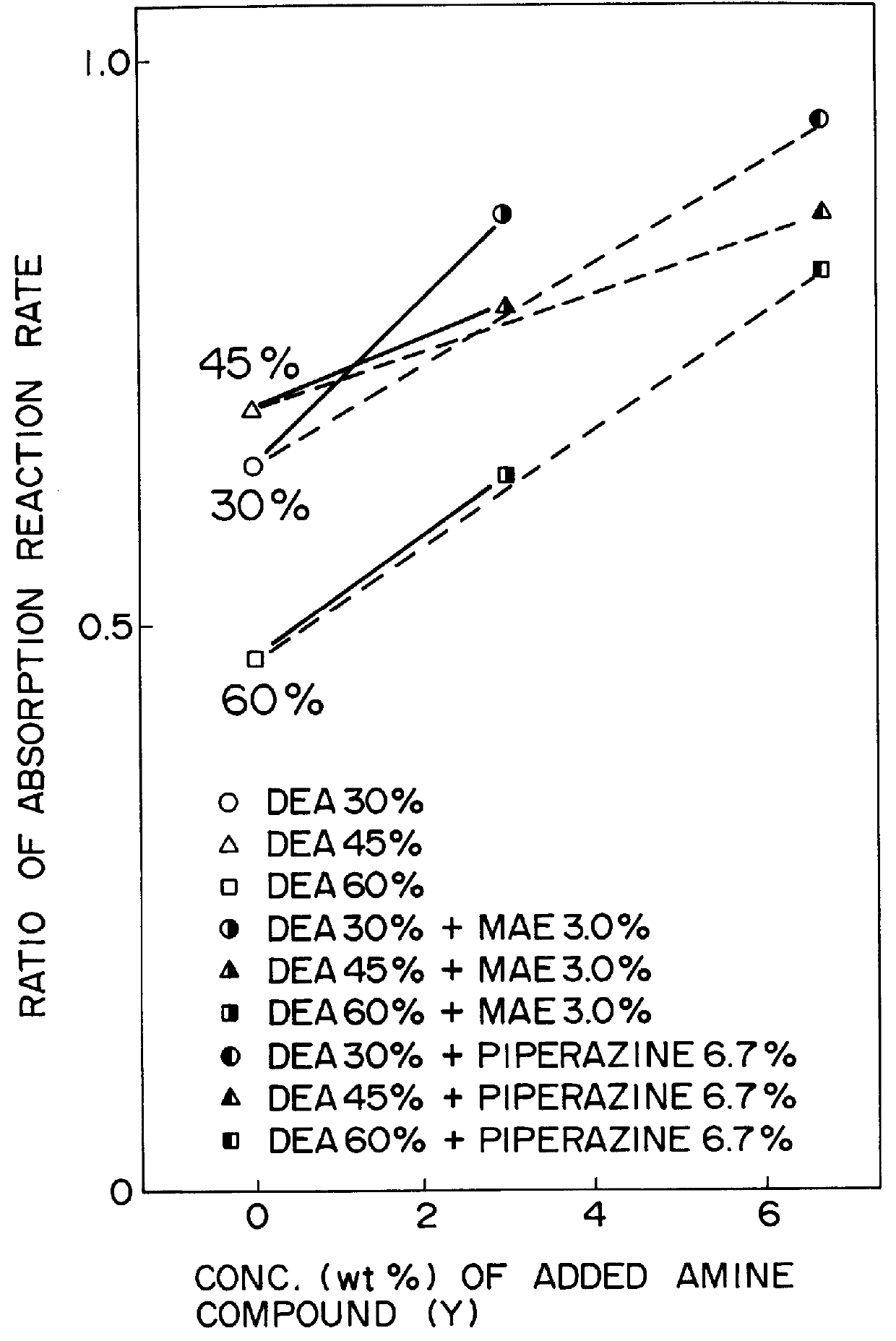
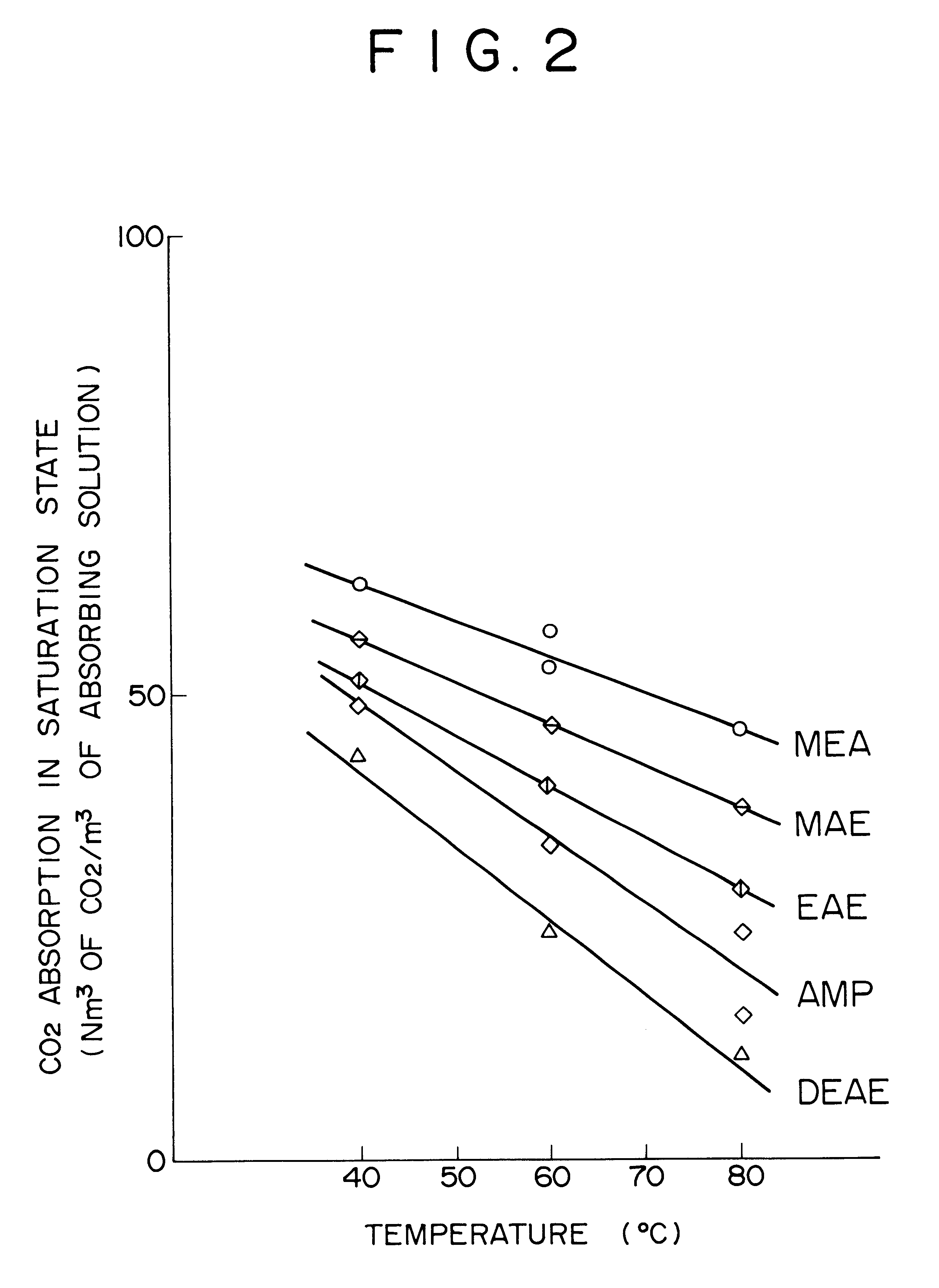
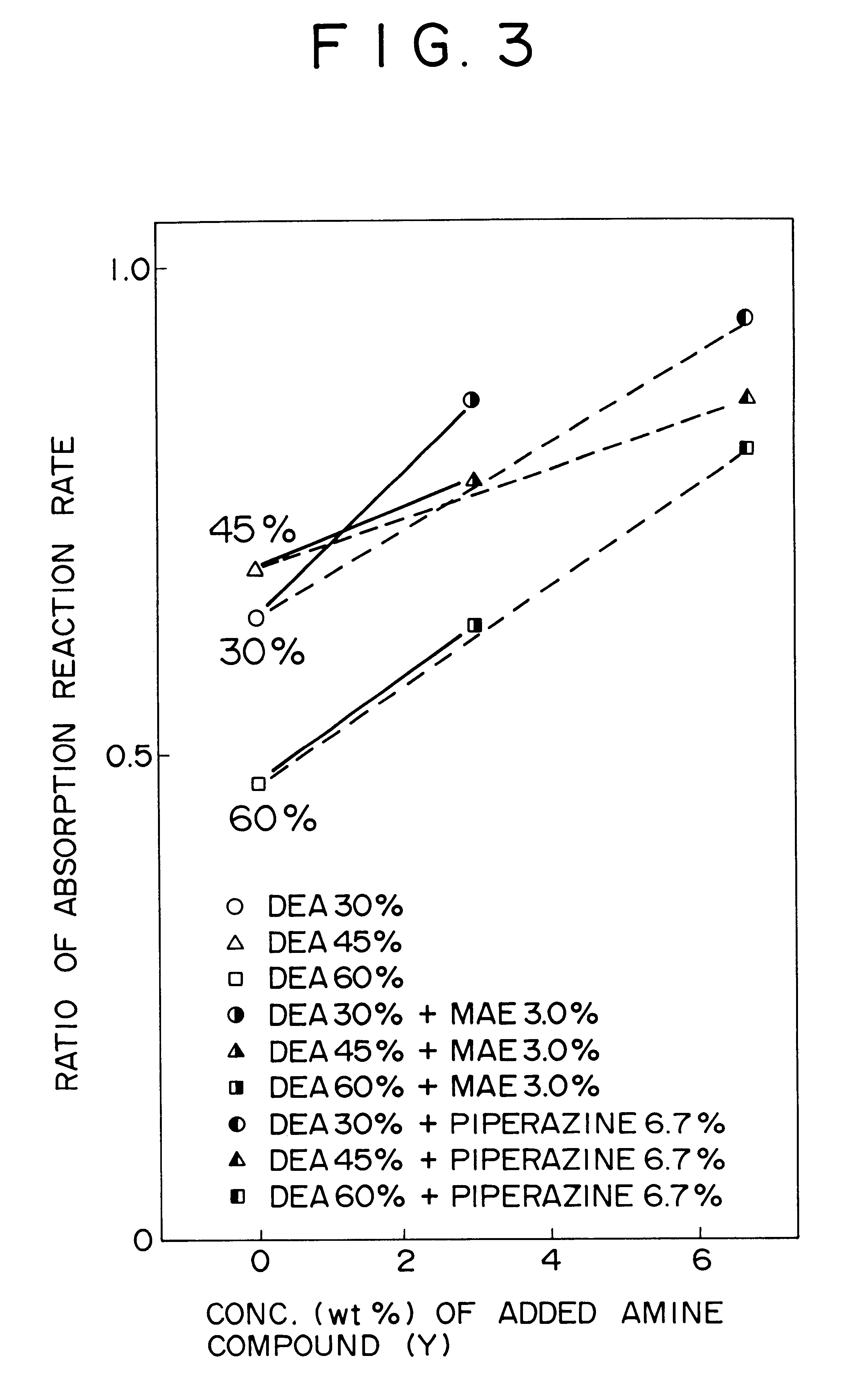
Method for removing carbon dioxide from combustion exhaust gas
InactiveUS6036931AHigh absorption rateImprove absorption rateDispersed particle separationHydrogen sulfides2-methylaminoethanolMorpholine
There are disclosed a method for removing CO2 from a combustion exhaust gas which comprises the step of bringing the combustion exhaust gas under atmospheric pressure into contact with an aqueous solution of a hindered amine selected from the group consisting of 2-amino-2-methyl-1-propanol, 2-methylaminoethanol, 2-ethylaminoethanol and 2-piperidineethanol; and another method for removing carbon dioxide from a combustion exhaust gas which comprises the step of bringing the combustion exhaust gas under atmospheric pressure into contact with a mixed aqueous solution of 100 parts by weight of an amine compound (X) selected from the group consisting of 2-amino-2-methyl-1,3-propanediol, 2-amino-2-methyl-1-propanol, 2-amino-2-ethyl-1,3-propanediol, t-butyldiethanolamine and 2-amino-2-hydroxymethyl-1,3-propanediol; and 1-25 parts by weight of an amine compound (Y) selected from the group consisting of piperazine, piperidine, morpholine, glycine, 2-methylaminoethanol, 2-piperidineethanol and 2-ethylaminoethanol.
Owner:THE KANSAI ELECTRIC POWER CO +1
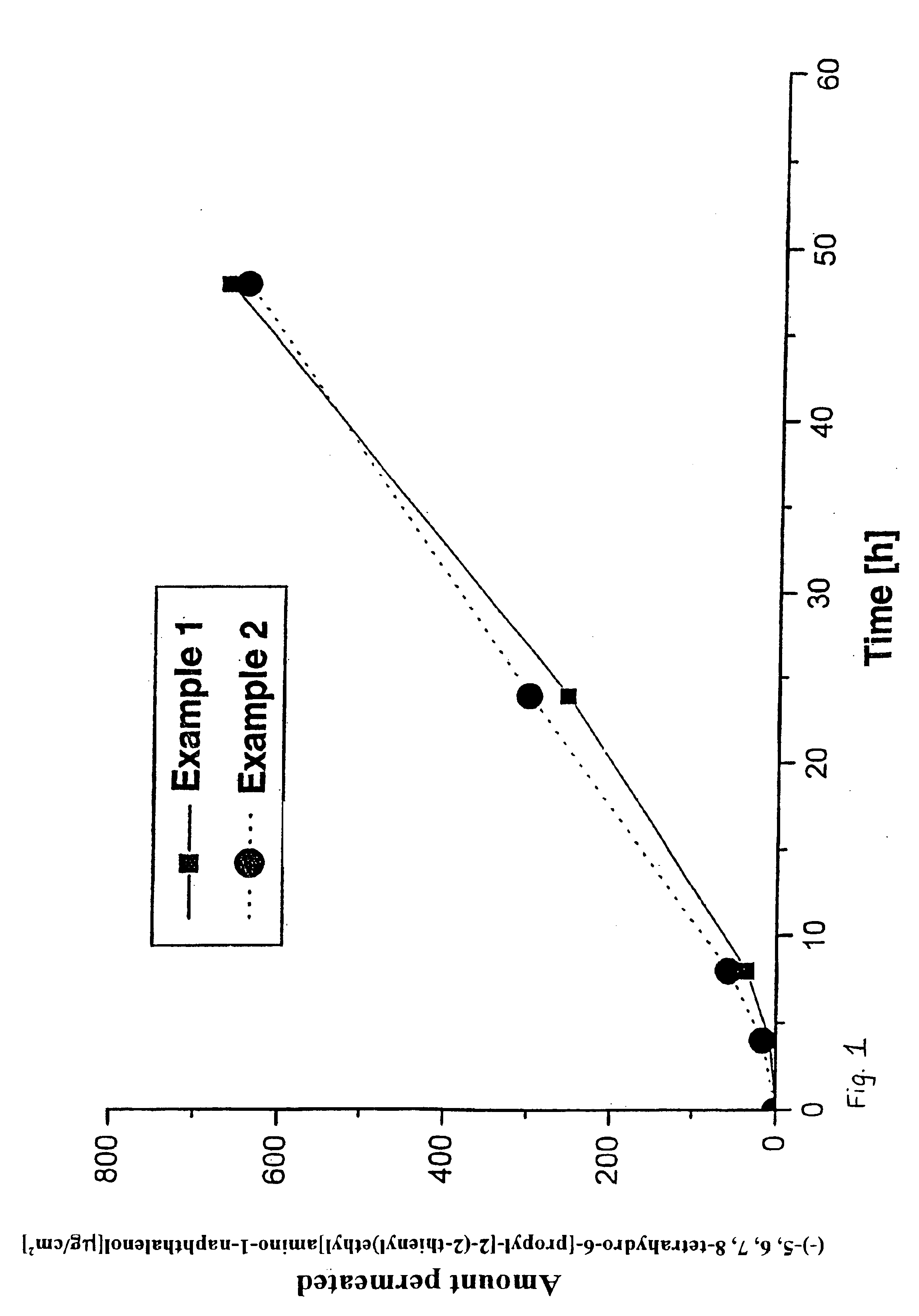
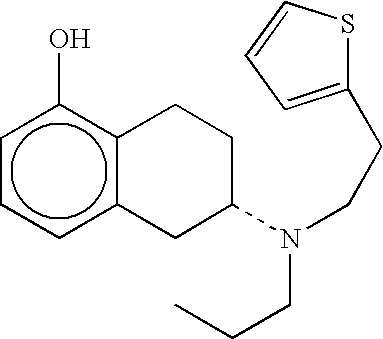
Transdermal therapeutic system which contains a d2 agonist and which is provided for treating parkinsonism, and a method for the production thereof
Owner:LTS LOHMANN THERAPIE-SYST AG
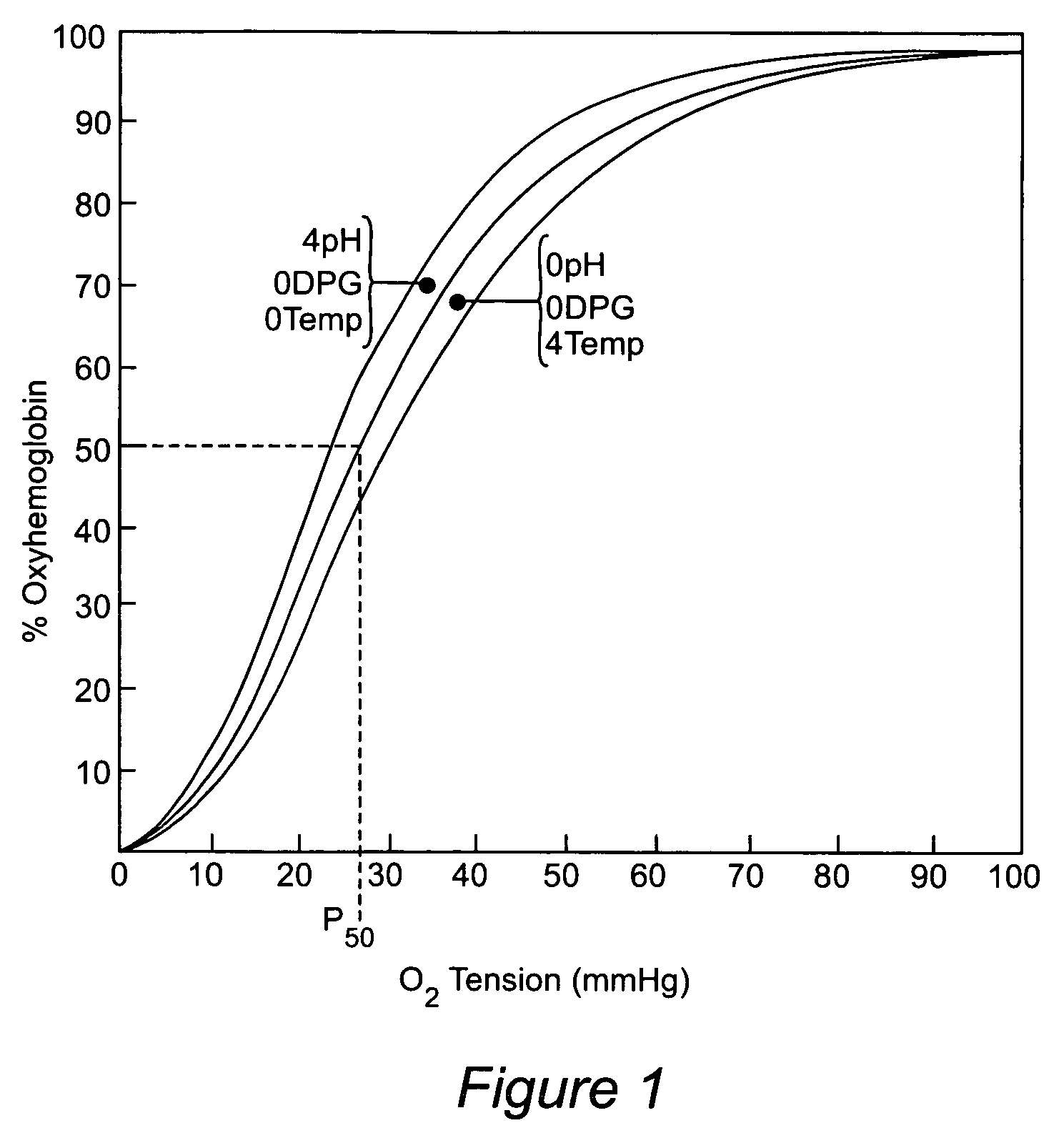
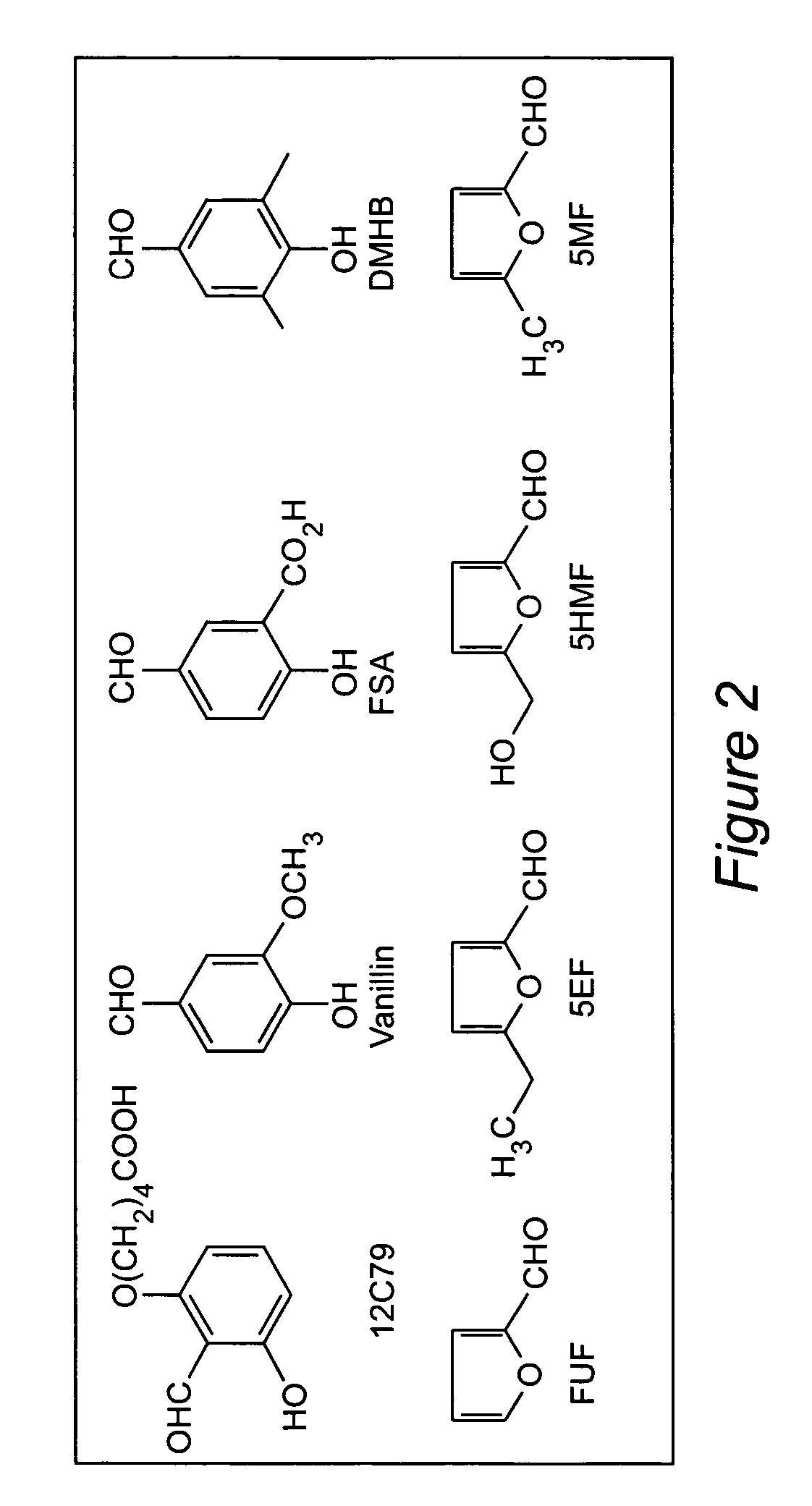
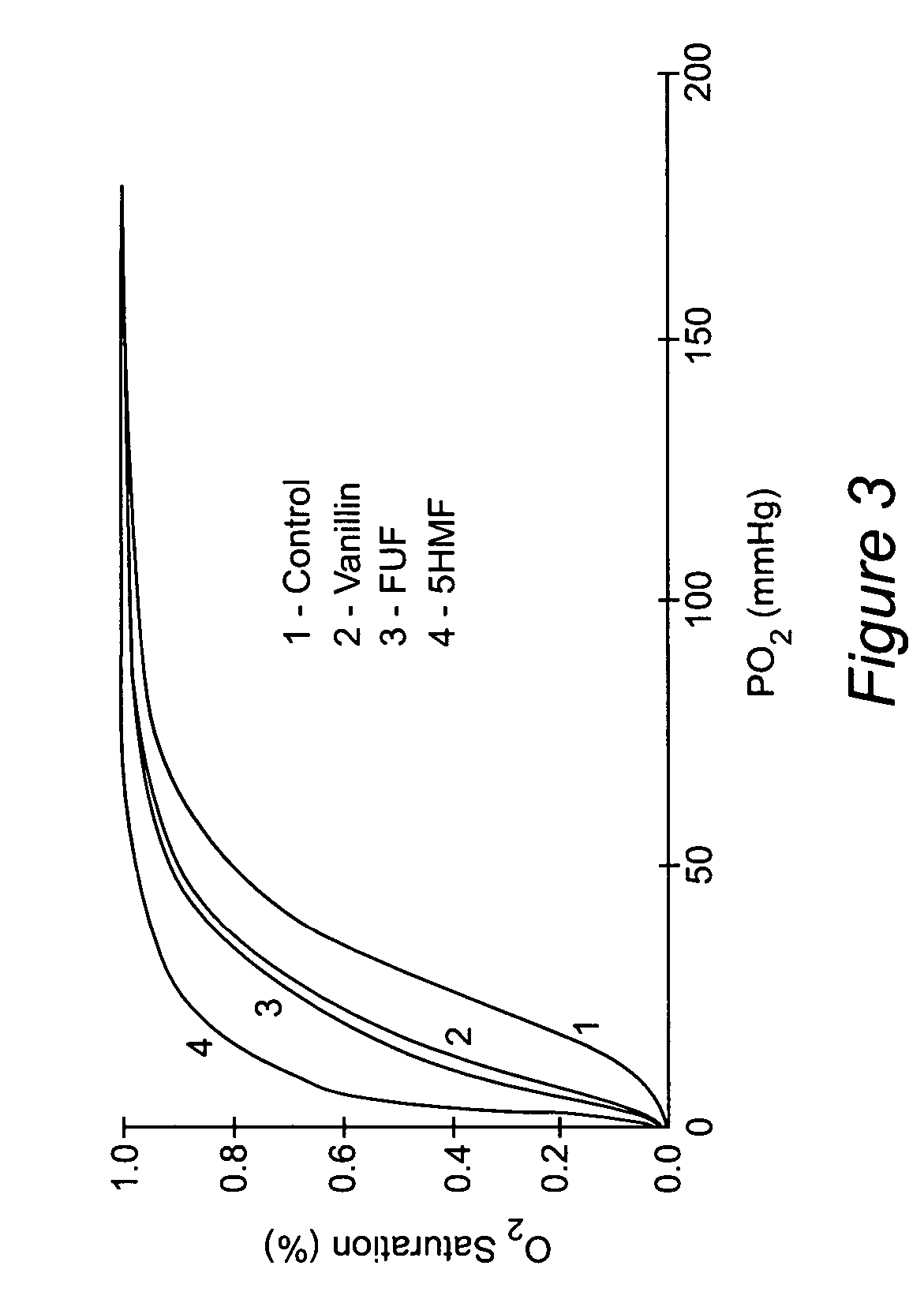
Anti-sickling agents
The provides compounds for the treatment of sickle-cell disease. In particular, the invention provides 5-membered heterocyclic anti-sickling agents that are highly effective and non-toxic, and methods for their use. The compounds include analogues and derivatives of naturally occurring occurring 5-hydroxymethyl-2-furfuraldehyde, 5-Ethyl-2-furfuraldehyde, 5-Methyl-2-furfuraldehyde, and 2-furfuraldehyde, and prodrug forms of the compound.
Owner:VIRGINIA COMMONWEALTH UNIV
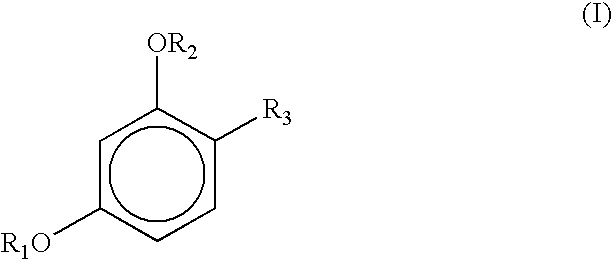
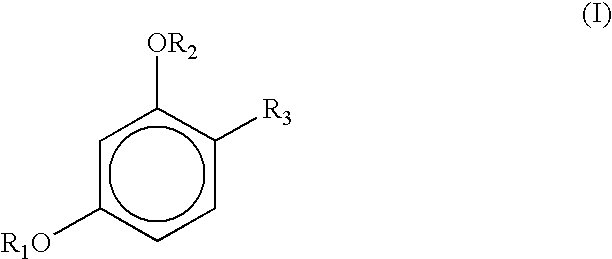
Stabilization of resorcinol derivatives in cosmetic compositions
InactiveUS6863897B2Improved storage/color stabilityImprove stabilityCosmetic preparationsToilet preparationsHydrogenEthyl group
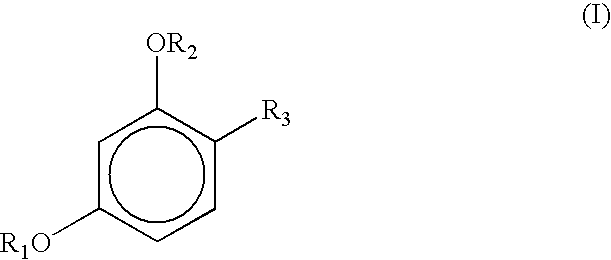
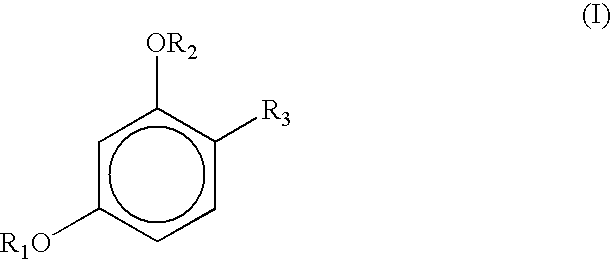
Cosmetic compositions containing a micronized metal oxide along with a 4-substituted resorcinol derivatives of the general formula I exhibit improved storage stability: where each R1 and R2, independently, represents a hydrogen atom, —CO—R (acyl group), —COO—R, CONHR; where R represents saturated or unsaturated, linear, branched or cyclic C1-C18 hydrocarbon groups; andR3 represents (1) an alkyl group, having from 1 to 18 carbon atoms, preferably having from 2 to 12 carbon atoms, with or without substitution of one or more hydrogen atoms of a linear alkyl group with a methyl or ethyl group; e.g., R3 constitutes linear or branched chain alkyls, or (2) a group of the general formula formula (II) Where X is preferably H, n is 0 to 3 and the dashed lines represents an optional double bond.
Owner:UNILVER HOME & PERSONAL CARE USA DIV OF CONOPCO
Method for removing carbon dioxide from combustion exhaust gas
InactiveUS6500397B1Improve absorption rateHigh absorption rateHydrogen sulfidesDispersed particle separation2-methylaminoethanolMorpholine
There are disclosed a method for removing CO2 from a combustion exhaust gas which comprises the step of bringing the combustion exhaust gas under atmospheric pressure into contact with an aqueous solution of a hindered amine selected from the group consisting of 2-amino-2-methyl-1-propanol, 2-methylaminoethanol, 2-ethylamino-ethanol and 2-piperidineethanol; and another method for removing carbon dioxide from a combustion exhaust gas which comprises the step of bringing the combustion exhaust gas under atmospheric pressure into contact with a mixed aqueous solution of 100 parts by weight of an amine compound (X) selected from the group consisting of 2-amino-2-methyl-1, 3-propanediol, 2-amino-2-methyl-1-propanol, 2-amino-2-ethyl-1, 3-propanediol, t-butyldiethanolamine and 2-amino-2-hydroxymethyl-1,3-propanediol; and 1-25 parts by weight of an amine compound (Y) selected from the group consisting of piperazine, piperidine, morpholine, glycine, 2-methylamino-ethanol, 2-piperidineethanol and 2-ethylaminoethanol.
Owner:THE KANSAI ELECTRIC POWER CO +1
Hydrogenation method for unsaturated block copolymers and hydrogenated unsaturated block copolymers
Use is made, for selectively hydrogenating the olefinic double bonds of block copolymers, at least one block of which comprises olefinic double bonds, using a catalyst based on a metal from Group VIII in a medium comprising an organic solvent for the copolymer and an ionic liquid as solvent for the catalyst, of a water-immiscible ionic liquid, preferably an ionic liquid for which the anion is the hexafluorophosphate anion and the cation is the 1-butyl-3-methylimidazolium (bmim+) or 1-ethyl-3-methylimidazolium (emim+) cation.Applied to poly(styrene)-b-poly(butadiene)-b-poly(methyl methacrylate) block copolymers, the poly(butadiene) block of which predominantly possesses a 1,4-microstructure, this process results in copolymers for which the degree of hydrogenation is at least equal to 50% and which exhibit a melting point of greater than 30 °C.
Owner:ATOFINA
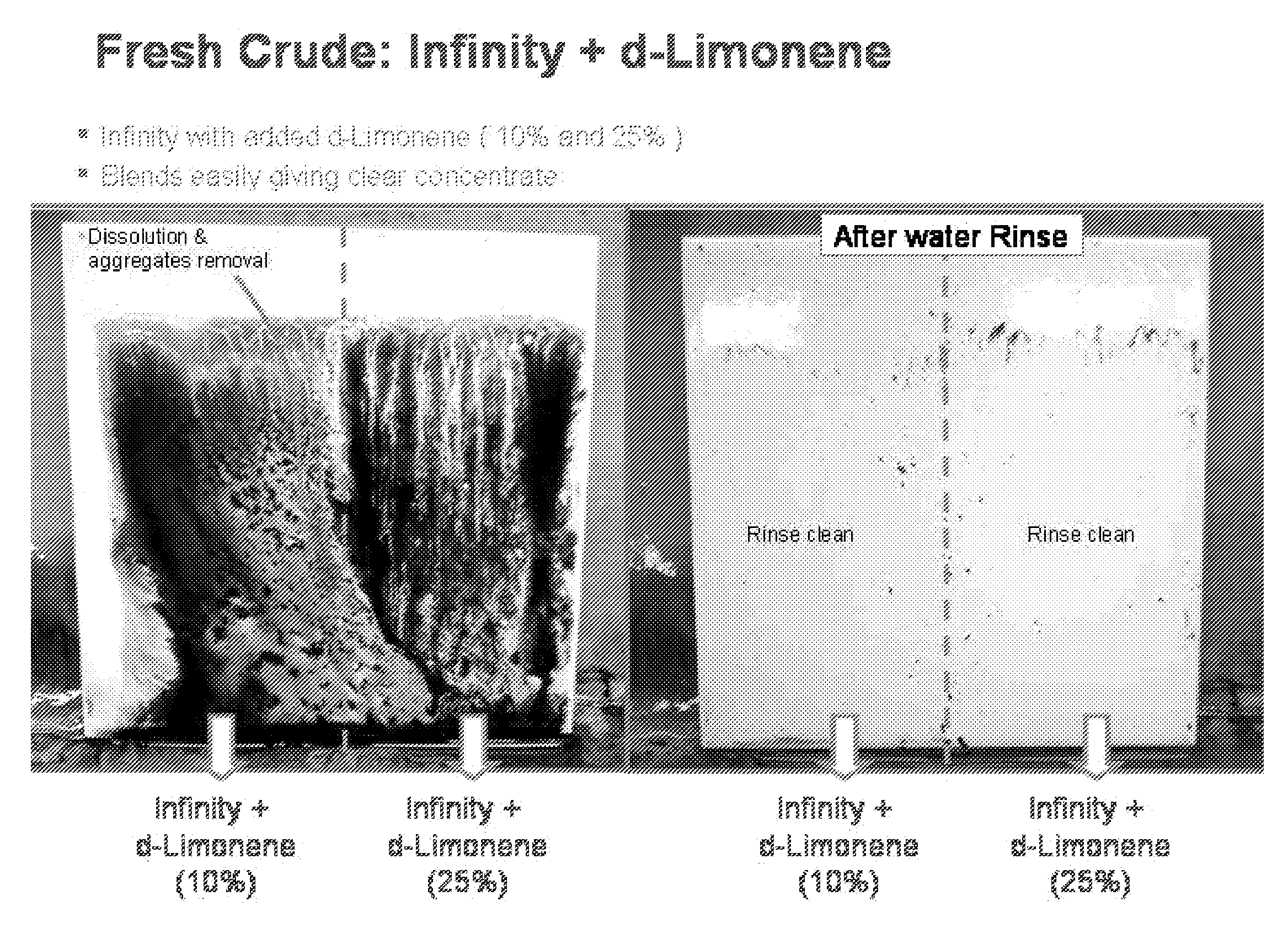
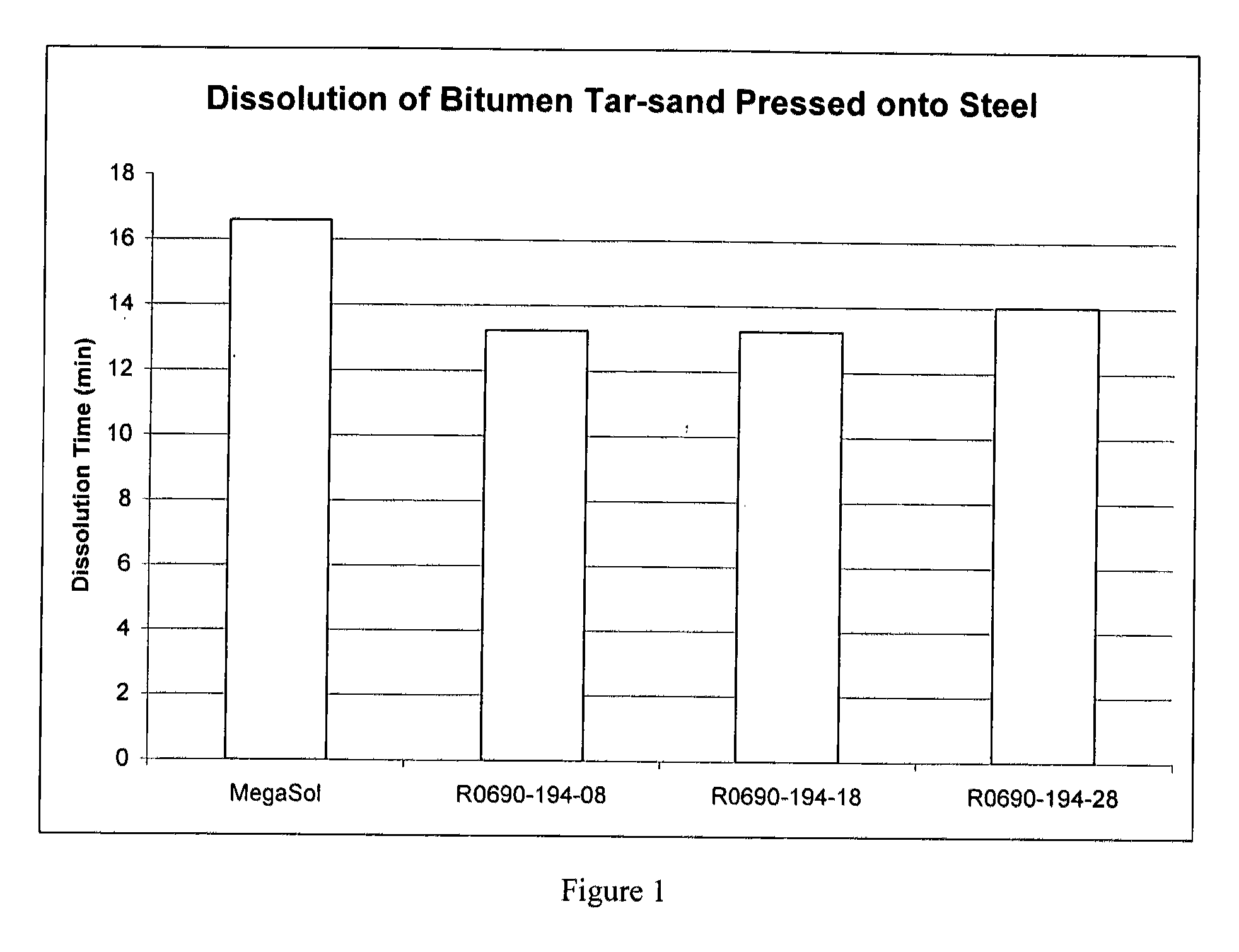
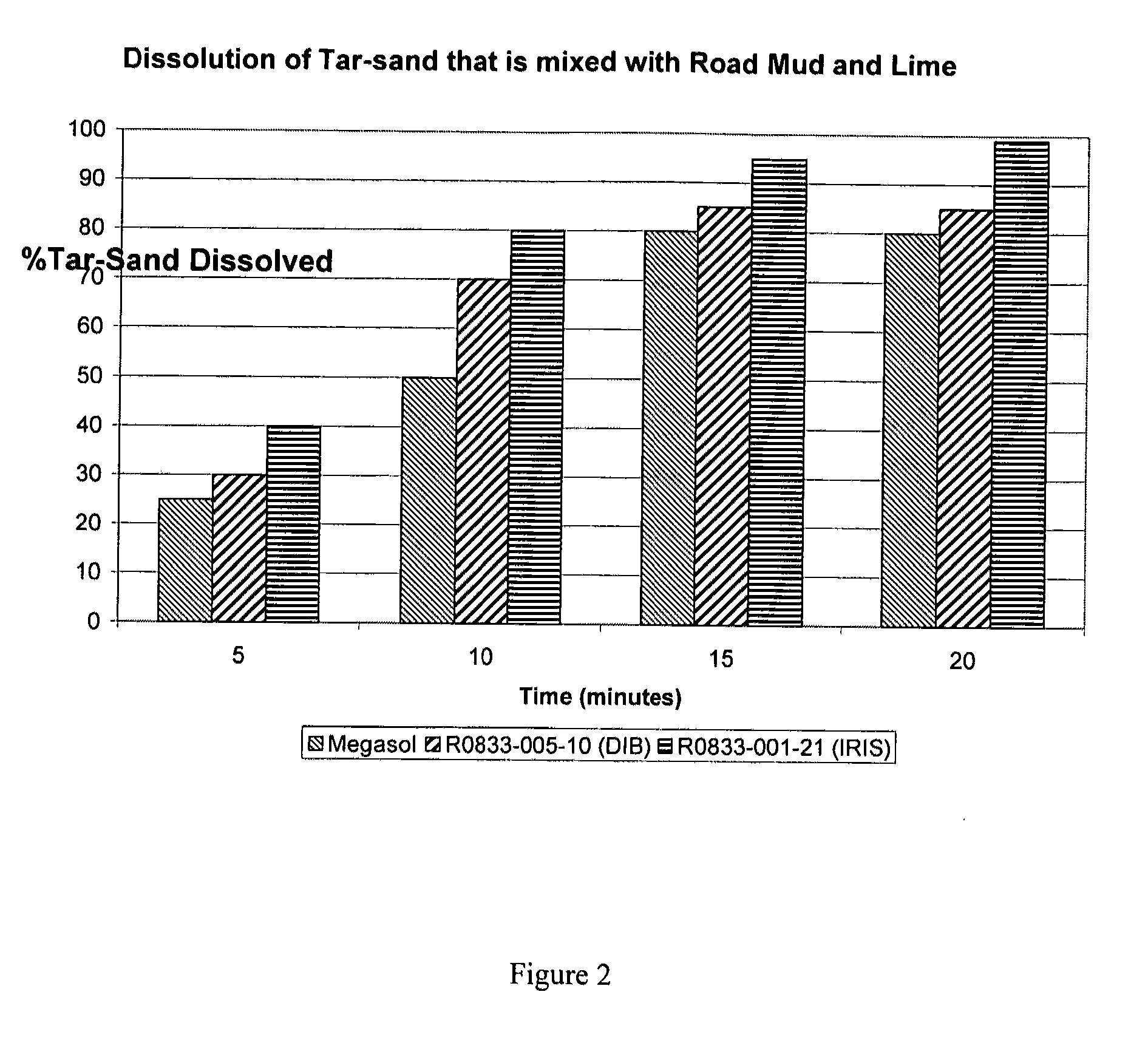
Dibasic esters utilized as terpene co-solvents, substitutes and/or carriers in tar sand/bitumen/asphaltene cleaning applications
ActiveUS20120149626A1Reduce concentrationImprove environmental conditionsOrganic detergent compounding agentsNon-ionic surface-active compoundsCarrier fluidFuel oil
A heavy oil cleaning composition comprising: a) a blend of dibasic esters comprising dialkyl methylglutarate and at least one of a dialkyl adipate or dialkyl ethylsuccinate; b) at least one terpene; and c) at least one surfactant. Also described are methods for delivering a solvent at reduced concentration comprising the steps of: a) obtaining a terpene-based solvent; and b) mixing the terpene-based solvent with a carrier fluid (the carrier fluid comprising a microemulsion of i) a blend of dibasic esters selected from the group consisting of dialkyl methylglutarate, dialkyl adipate, dialkyl ethylsuccinate, dialkyl succinate, dialkyl glutarate and any combination thereof, ii) at least one surfactant selected from the group consisting of a terpene alkoxylate, an alcohol alkoxylate and any combination thereof; and iii) water) in order to obtain a mixture to clean heavy oils.
Owner:RHODIA OPERATIONS SAS
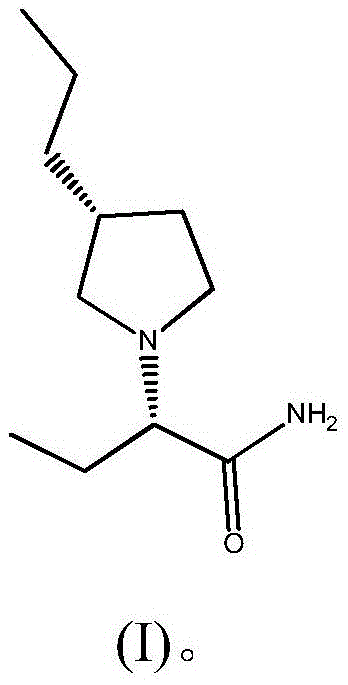
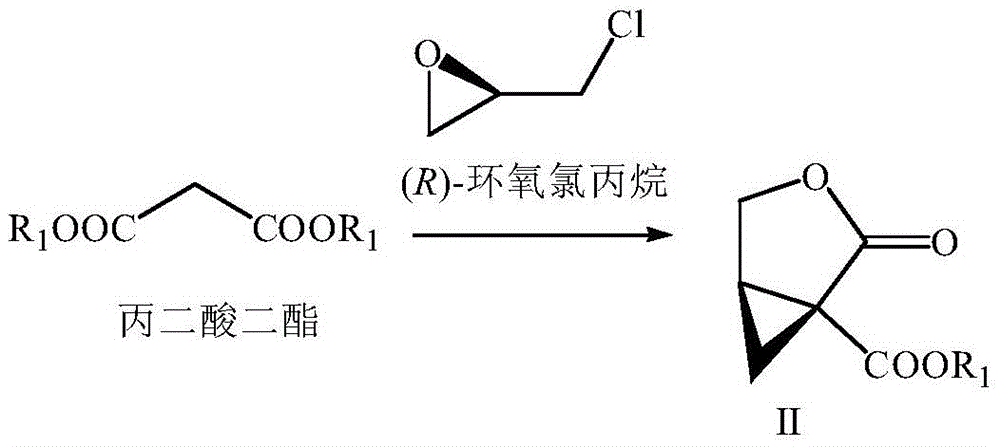
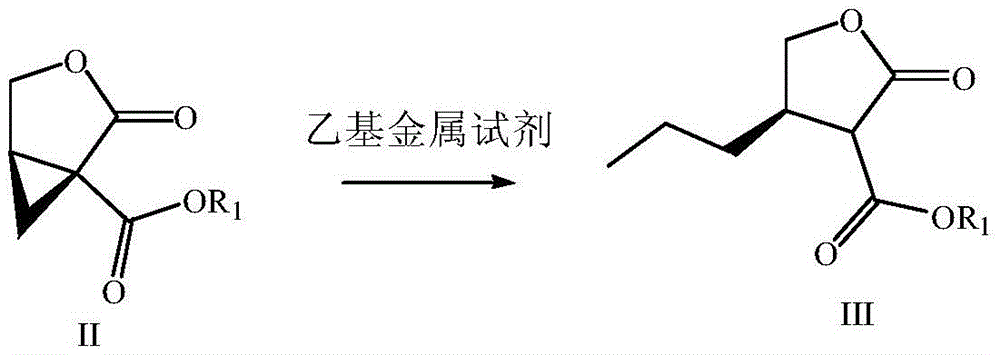
Preparation method of brivaracetam
The invention provides a preparation method of brivaracetam. The preparation method of brivaracetam comprises the following steps of in an alkaline reagent, reacting malonate ester and (R)-epichlorohydrin to obtain lactone (II), reacting the lactone (II) and an ethyl metal-based reagent to obtain an intermediate (III), removing carboxylase to obtain (R)-4-propyl-dihydrofuran-2-ketone (IV), performing ring-opening reaction under the action of a halogenated ring-opening reagent to obtain (R)-3-halogenarated methyl hexanoate or (R)-3- halogenarated methyl hexyl acetate (V), and reacting with (S)-2-aminobutanamide or an acceptable salt, so as to obtain the brivaracetam. The preparation method has the advantages that the high-purity brivaracetam (HPLC (high performance liquid chromatography: greater than 96%) and stereo rotary brivaracetam (chirality HPLC: greater than 98%) can be directly prepared; the silicagel column separation and purification or the chirality preparation column separation and purification is not used, so that the complicated separation and purification step is not performed, the cost is saved, and the preparation method is more suitable for industrial production.
Owner:佛山市隆信医药科技有限公司
Functional fiber and its manufacturing method
InactiveCN1693547AStrong aromaExtension of timeFilament/thread formingConjugated synthetic polymer artificial filamentsEmissivityPolymer science
The invention relates to a functional fiber and its manufacturing method, where the functional fiber has a skin-core structure, the weight ratio of skin to core 3 / 7 - 7 / 3; and the formula of the skin materials in weight percent comprises: skin polymer 91.6-97.6%, far infrared powder 2-8%, and coupling agent 0.1-0.4%; the skin polymer is one of PTT, PBT, PA-6, PET and PP with fusion index of 25-50; the coupling agent is one of gama-shink glycerol oxy propyl trimethoxy silane, gama-methyl propylene acyl oxy trimethoxy silane, N-beta-(amido ethyl)-gama amido propyl trimethoxy silane, and gama-amido propyl triethoxy silane; and the formula of the core-layer materials in weight percent comprises: core-layer polymer 88-99%, and flavor 1-12%; wherein the core-layer polymer is one of PE, PP and PET with melting point of 150-200 deg.C. The fiber of the invention has far infrared emissivity of 81-86%, very strong spicery and is a synthetic health functional fiber.
Owner:TIANJIN POLYTECHNIC UNIV
Propellantless pump sprays and pump foams containing an anionic copolymer of methacrylic acid and ethyl acrylate
InactiveUS6482394B1Improve flexural strengthImprove distributionCosmetic preparationsHair cosmeticsAnionic polymersEthyl acrylate
Hair treatment compositions in the form of pump sprays and pump foams that are free from propellant gases comprise at least one cationic and at least one anionic polymer having methacrylic and ethyl units.
Owner:BASF AG
Dual layer tablet, method of making and use thereof
InactiveUS20050040116A1Easy to optimizeComprehensive treatmentOrganic chemistryOther chemical processesPotassium persulfateLithium hypochlorite
A method for treating a recirculating water system which comprises introducing into said water system a multifunctional, multilayer tablet, wherein the multilayer tablet comprises a fast dissolving layer and a slow dissolving layer, wherein said fast dissolving layer releases a combination of active ingredients including a member selected from the group consisting of lithium hypochlorite, calcium hypochlorite, trichloroisocyanuric acid (TCCA), anhydrous sodium dichloroisocyanurate, sodium persulfate, potassium persulfate, potassium monopersulfate, sodium monopersulfate, and mixtures thereof, and at least one of a clarifier, chelating agent, sequesterant, algaestat, water softener, algaecide, corrosion inhibitor, scale inhibitor, flocculent, disintegrant, dispersant, colorant, dissolution control agent, fragrance, or surfactant and, wherein said slow dissolving layer includes a member selected from the group consisting of trichloroisocyanuric acid (TCCA), calcium hypochlorite, 1,3-dichloro-5,5-dimethylhydantoin (DCDMH), 1,3-dibromo-5,5-dimethylhydantoin (DBDMH), 1-bromo-3-chloro-5,5-dimethylhydantoin (BCDMH), 1,3-dichloro-5-ethyl-5-methylhydantoin (DCEMH), 1,3-dibromo-5-ethyl-5-methylhydantoin (DBEMH), 1-bromo-3-chloro-5-methyl-5-ethylhydantoin (BCEMH), and mixtures thereof, and at least one of a clarifier, chelating agent, sequesterant, algaestat, water softener, algaecide, corrosion inhibitor, scale inhibitor, flocculent, disintegrant, dispersant, colorant, dissolution control agent or surfactant.
Owner:BIO LAB
Multilayer imageable elements
InactiveUS6893783B2Increase the lengthPhotosensitive materialsRadiation applicationsN-phenylmaleimidePolymer science
Multilayer, positive working, thermally imageable elements are disclosed. The elements produce bakeable lithographic printing plates that are resistant to press chemistries. The elements have a substrate, an underlayer, and a top layer. The underlayer comprises a resin or resins having activated methylol and / or activated alkylated methylol groups, such as a resole resin, and a polymeric material that comprises, in polymerized form, (a) methacrylic acid; (b) N-phenylmaleimide, N-cyclohexylmaleimide, N-benzylmaleimide, or a mixture thereof; and (c) one or more monomers of the structure: [0001]in which:[0002]R1 is H or methyl; X is —(CH2)n—, where n is an integer from 2 to 12; —(CH2—CH2—O)p—CH2—CH2—, where p is an integer from 1 to 3; or —Si(R′)(R″)— where R′ and R″ are each independently methyl or ethyl; and m is 1, 2, or 3.
Owner:KODAK POLYCHROME GRAPHICS
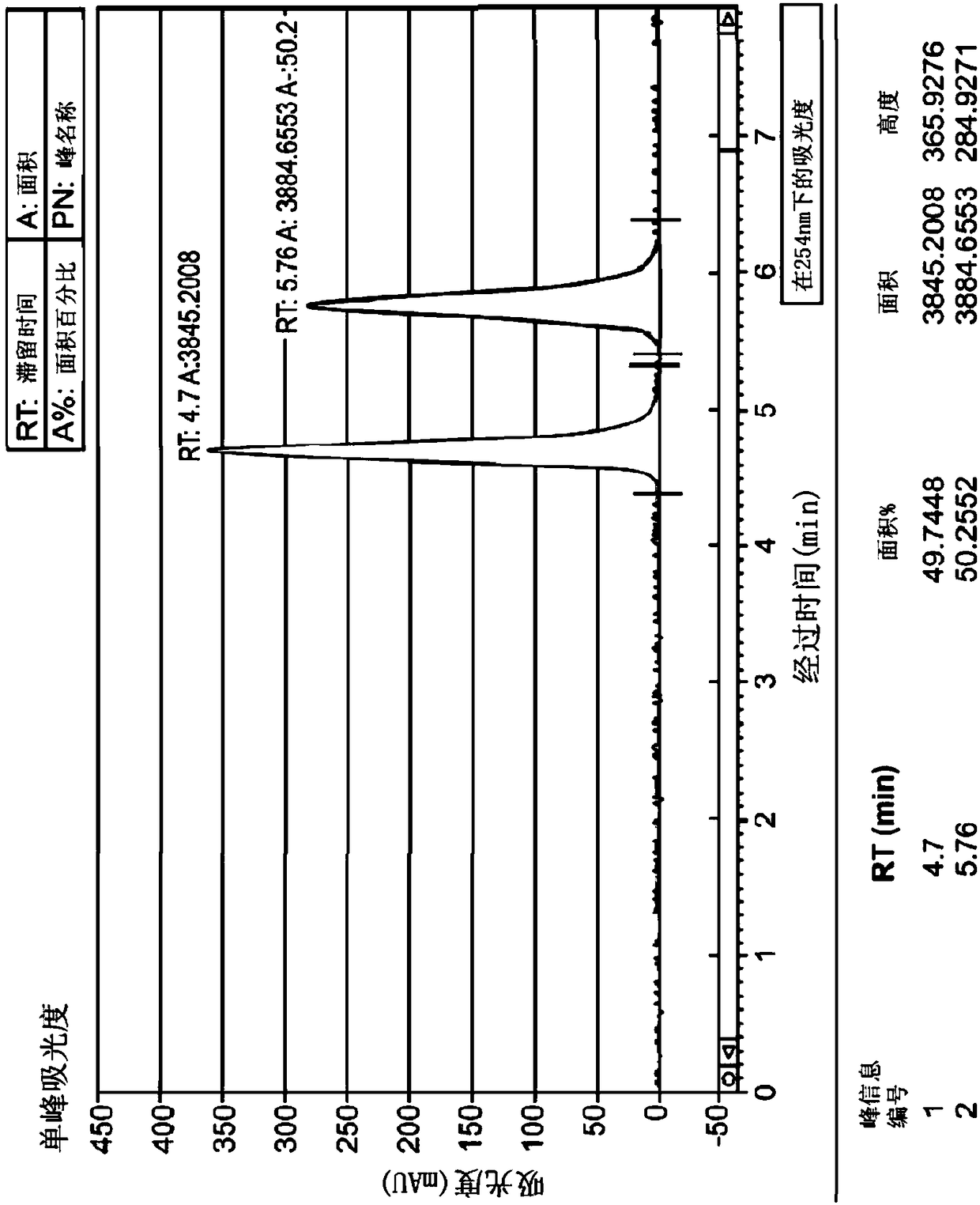
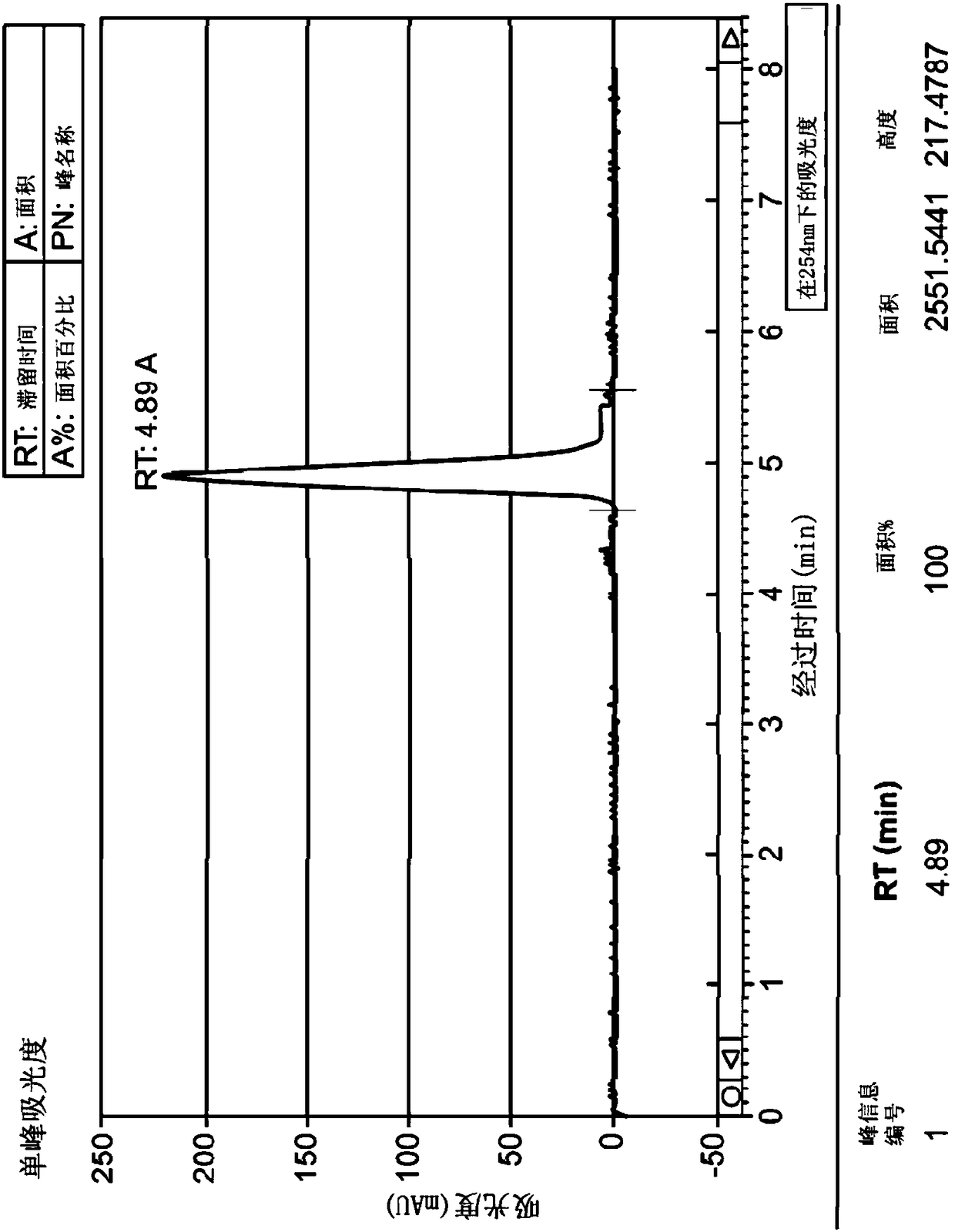
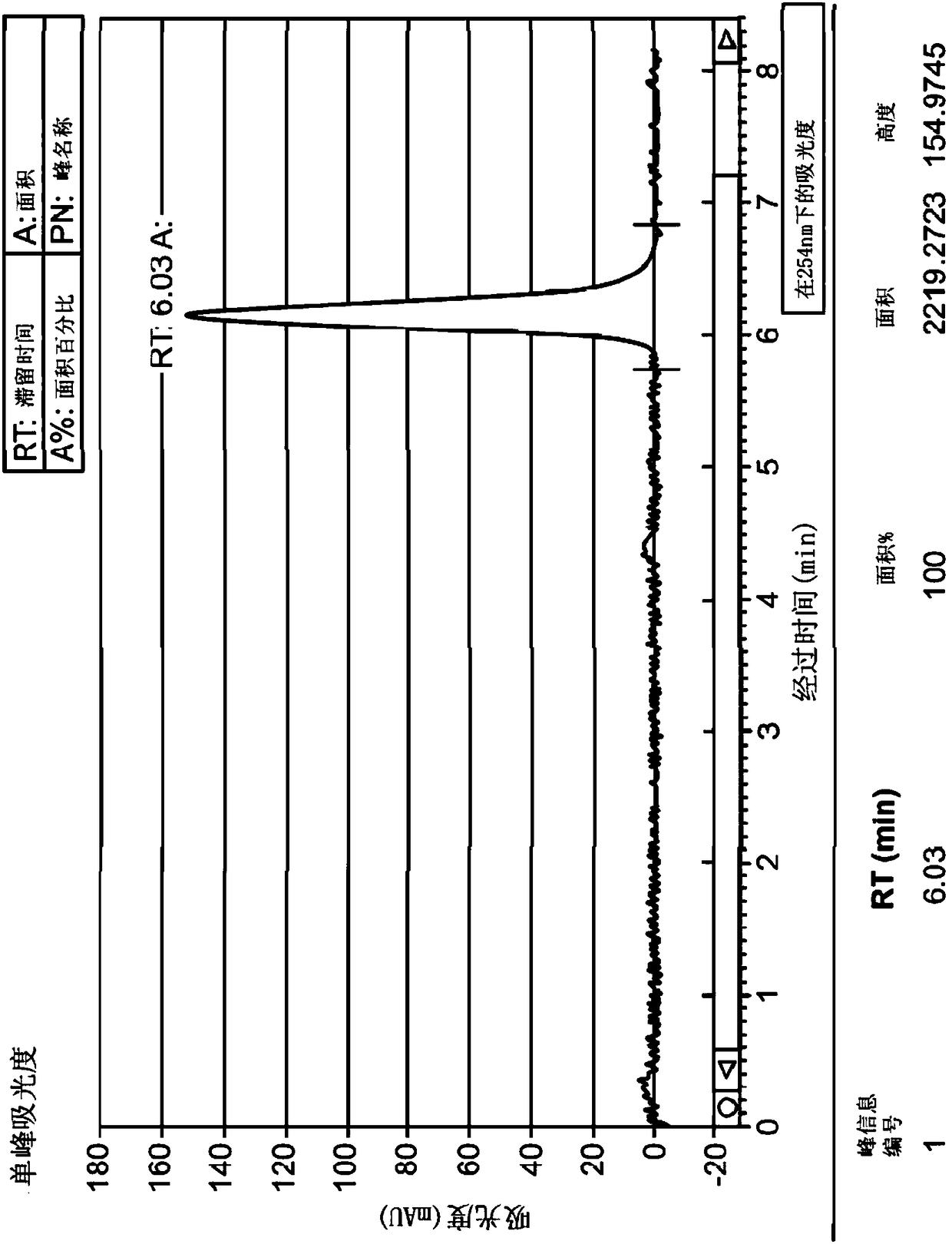
Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament
InactiveCN103893163AStrong inhibitory activitySelectiveOrganic active ingredientsNervous disorderMedicineMonoamine Oxidase A Gene
The invention belongs to the field of medicine, and in particular relates to a medical application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in a selective histone lysine-specific demethylase 1 (LSD1) inhibitor, especially the application in anti-tumor medicaments. Pharmacodynamic tests indicate that the 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate has a remarkable LSD1 inhibiting effect, and has selectivity to homologous proteins MAO-A (monoamine oxidase-A) and MAO-B.
Owner:CHINA PHARM UNIV
Stabilization of sunscreens in cosmetic compositions
InactiveUS6869598B2Promote oxidationImproved photooxidative stabilityCosmetic preparationsToilet preparationsSunscreen agentsHydrogen
Cosmetic compositions containing organic sunscreens along with a 4-substituted resorcinol derivatives of general formula I exhibit improved storage stability and oxidative stability: where each R1 and R2, independently, represents a hydrogen atom, —CO—R (acyl group), —COO—R CONHR; where R represents saturated or unsaturated, linear, branched or cyclic C1-C18 hydrocarbon groups; andR3 represents (1) an alkyl group, having from 1 to 18 carbon atoms, preferably having from 2 to 12 carbon atoms, with or without substitution of one or more hydrogen atoms of a linear alkyl group with a methyl or ethyl group; e.g., R3 constitutes linear or branched chain alkyls, or (2) a group of the general formula formula (II) Where X is preferably H, n is 0 to 3 and the dashed lines represents an optional double bond.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Anti-CTLA4 Antibody and Indolinone Combination Therapy for Treatment of Cancer
InactiveUS20090074787A1Organic active ingredientsAntibody ingredientsDiseaseReceptor tyrosine kinase inhibitor
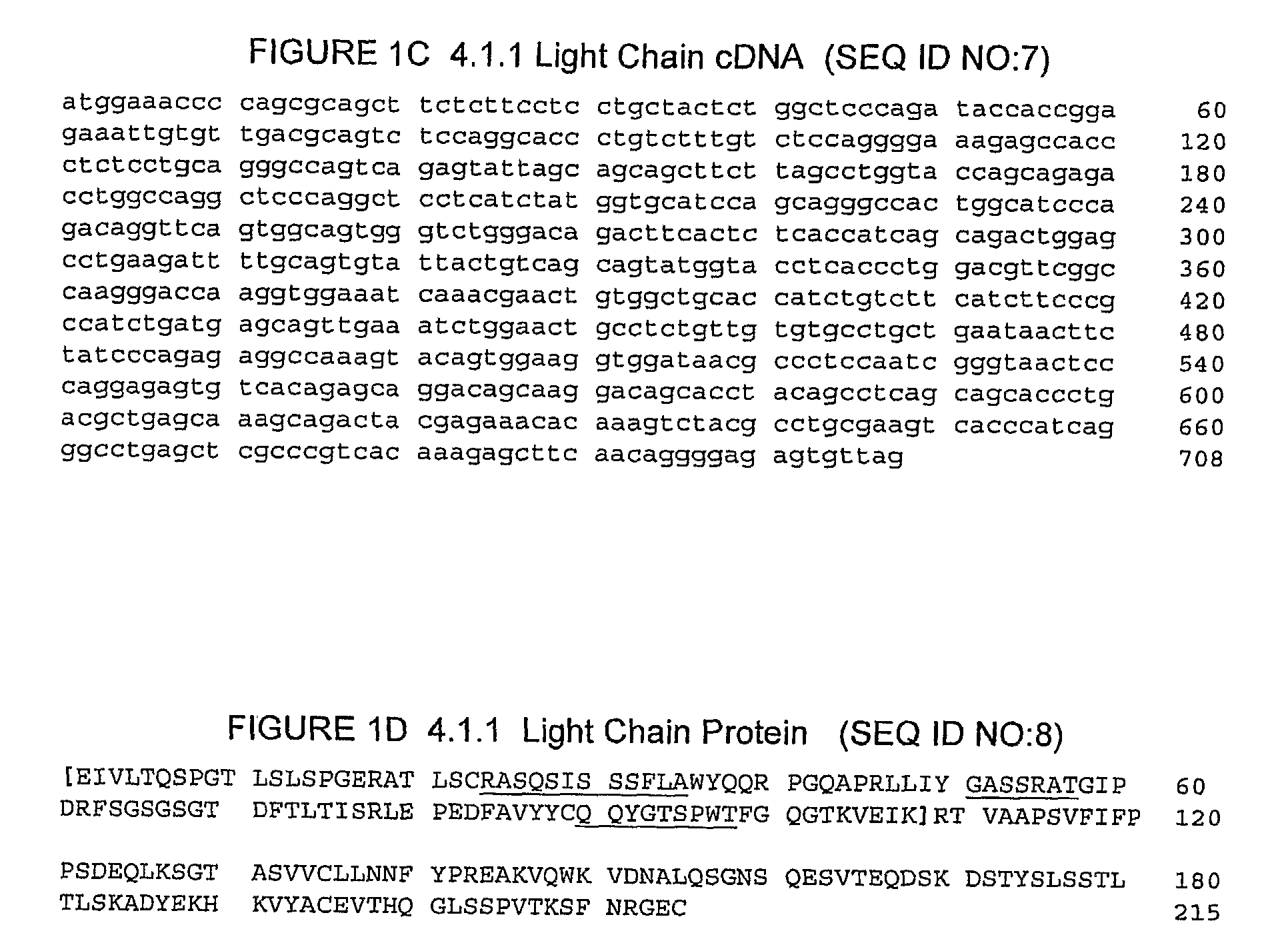
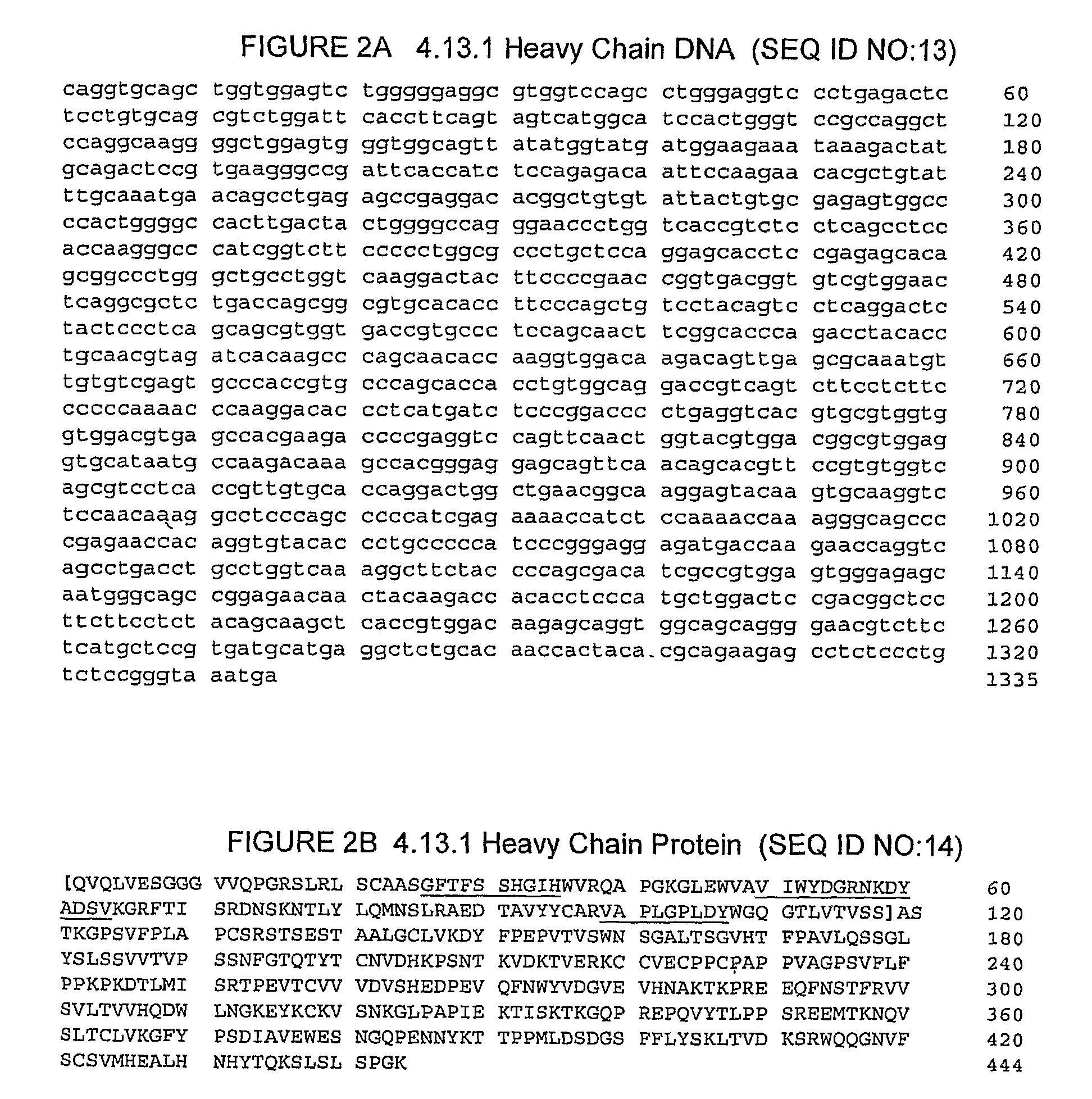
The invention relates to administration of an anti-CTLA4 antibody, particularly human antibodies to human CTLA4, such as those having amino acid sequences of antibodies 3.1.1, 4.1.1, 4.8.1, 4.10.2, 4.13.1, 4.14.3, 6.1.1, ticilimumab (also referred to as 11.2.1 or CP-675,206), 11.6.1, 11.7.1, 12.3.1.1, 12.9.1.1, and ipilimumab (also referred to as 10D1 or MDX-010), in combination with an indolinone receptor tyrosine kinase inhibitor (RTKI), e.g., N-[2-diethylamino]ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (compound 1), N-[2-(ethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (compound 2), and 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (compound 3), for treatment of cancer. The invention relates to administering a combination of an anti-CTLA4 antibody and an indolinone RTKI such as, inter alia, compound 1. The invention relates to neoadjuvant, adjuvant, first-line, second-line, and third-line therapy of cancer, whether localized or metastasized, and at any point(s) along the disease continuum (e.g., at any stage of the cancer).
Owner:PFIZER PFIZER PRODS
5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine
Compounds, compositions, and methods relating to 5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine or a pharmaceutically acceptable salt thereof are provided for the treatment of Type II diabetes and other diseases associated with poor glycemic control.
Owner:CYMABAY THERAPEUTICS
Herbicidal mixtures
Disclosed is a herbicidal mixture comprising (a) at least one herbicide compound selected from the pyrimidines of Formula 1, including all geometric and stereoisomers, N-oxides, and salts thereof:whereinR1 is cyclopropyl, 4-Br-phenyl or 4-Cl-phenyl;X is Cl or Br;R2 is H, C1-C14 alkyl, C2-C14 alkoxyalkyl, C3-C14 alkoxyalkoxyalkyl, C2-C14 hydroxyalkyl or benzyl; and(b) at least one additional herbicide or herbicide safener compound selected from the group consisting of (b1) ACCase inhibitors, (b2) AHAS inhibitors, (b3) photosystem II inhibitors, (b4) photosystem I electron diverters, (b5) PPO inhibitors, (b6) EPSP synthase inhibitors, (b7) GS inhibitors, (b8) VLCFA inhibitors, (b9) auxin mimics, (b10) auxin transport inhibitors, (b11) other herbicides selected from the group consisting of flamprop-M-methyl, flamprop-M-isopropyl, difenzoquat, DSMA, MSMA, bromobutide, flurenol, cinmethylin, cumyluron, dazomet, dymron, methyldymron, etobenzanid, fosamine-ammonium, isoxaflutole, asulam, clomazone, mesotrione, metam, oxaziclomefone, oleic acid, pelargonic acid and pyributicarb, (b12) herbicide safeners selected from the group consisting of benoxacor, 1-bromo-4-[(chloromethyl)sulfonyl]benzene, cloquintocet-mexyl, cyometrinil, dichlormid, 2-(dichloromethyl)-2-methyl-1,3-dioxolane, fenchlorazole-ethyl, fenclorim, flurazole, fluxofenim, furilazole, isoxadifen-ethyl, mefenpyr-diethyl, methoxyphenone, naphthalic anhydride and oxabetrinil, and their salts. Also disclosed is a method for controlling the growth of undesired vegetation comprising contacting the vegetation or its environment with a herbicidally effective amount of a mixture of the invention (e.g., as a composition described herein).
Owner:ARMEL GREGORY RUSSELL +8
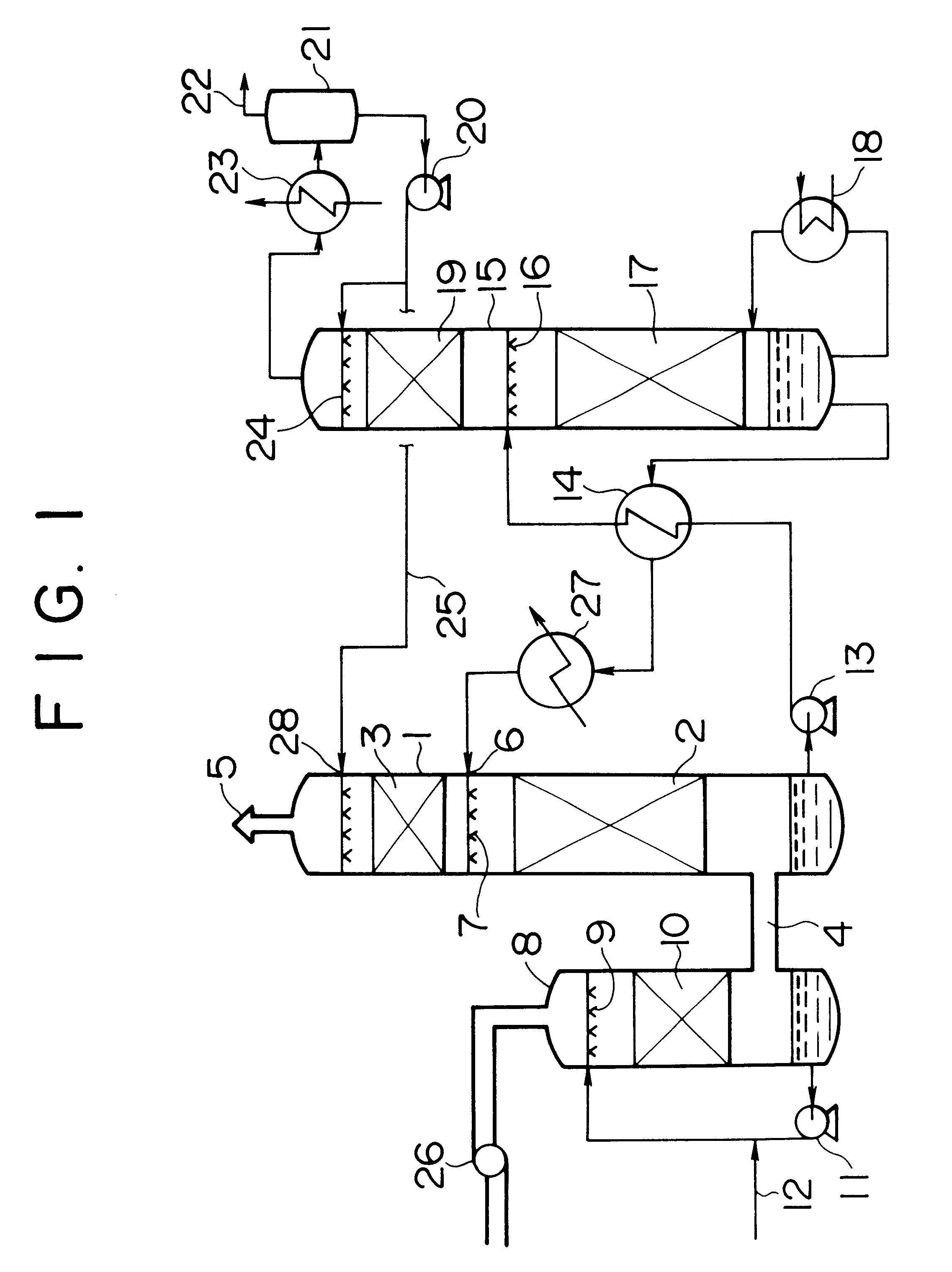
Process for producing methionine
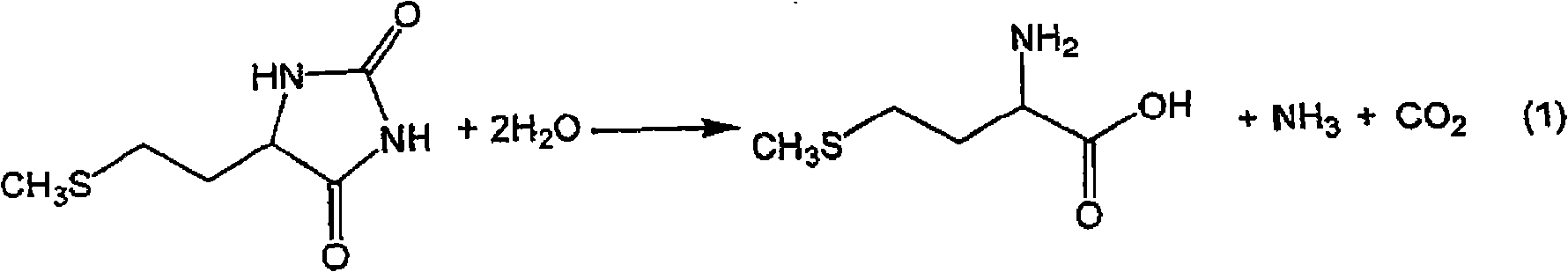
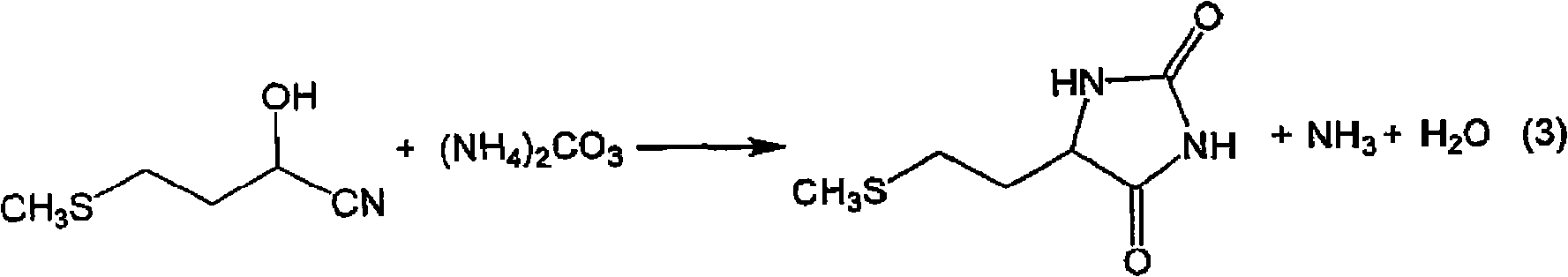
InactiveCN101602700AEfficient recyclingEfficient productionOrganic compound preparationSulfide preparationPotassiumSlurry
The present invention provides a process for producing methionine which is advantageous from the viewpoints of cost and wastewater disposal, and which comprises the following steps (1) to (5): (1) a reaction step comprising hydrolysis of 5-[2-(methylthio)ethyl]imidazolidine-2,4-dione in the presence of a basic potassium compound; (2) a first crystallization step comprising introduction of carbon dioxide into the reaction solution obtained in step (1) to precipitate methionine, and separation of the resultant slurry into a precipitate and a mother liquor; (3) a second crystallization step comprising concentration of the mother liquor obtained in step (2), mixing with a lower alcohol, introduction of carbon dioxide into the mixture to precipitate methionine and potassium hydrogen carbonate, and separation of the resultant slurry into a precipitate and a mother liquor; (4) a heating step comprising concentration of the mother liquor obtained in step (3) followed by a heating treatment at 150 to 200 DEG C; and (5) a third crystallization step comprising introduction of carbon dioxide into the heated mother liquor obtained in step (4) to precipitate methionine and potassium hydrogen carbonate, and separation of the resultant slurry into a precipitate and a mother liquor.
Owner:SUMITOMO CHEM CO LTD
Dental Treatment Compositions and Conformable Dental Treatment Trays Using the Same
ActiveUS20090087393A1Easy to optimizeHigh peroxide concentrationCosmetic preparationsHair cosmeticsHydration reactionDecomposition
The present invention is a formable dental treatment tray utilizing poly(2-ethyl-2-oxazoline) and a gelatinous active. Blending of the active is accomplished by mixing the Poly(2-ethyl-2-oxazoline) with an active ingredient, such as a peroxide like hydrogen peroxide, carbamide peroxide, sodium perborate, or sodium percarbonate, usually also with water or an appropriate organic solvent. Peroxide concentrations in these new gels can reach a 30% concentration of hydrogen peroxide while maintaining a shelf life of six months at room temperature without developing peroxide decomposition. The gels are applied to an appropriate backing and dried to a gelatinous state. In use, the active is hydrated and regains adhesiveness. Then the tray is pressed and formed around a user's dental arch to form the customizable tray. Multiple active ingredients may be used, with or without peroxide, for accomplishing desired treatment regimens.
Owner:CAO GROUP
Stabilizing polyalkylene carbonate resins
InactiveUS20040171721A1Simple processNot impair clean burning characteristicDecomposition4-Methylimidazole
Polyalkylene carbonate resins are stabilized against thermal and hydrolytic decomposition by the addition of a cyclic amine eliminating the need for complex chemical or other purifying techniques. Cyclic amines such as imidazole and substituted imidazoles (specifically 2-ethyl 4-methyl imidazole) were found to be effective at 5-30%, preferably 10-30%. Processes for producing stable flux containing coatings for the aluminum brazing industry are detailed. The modified polyalkylene carbonate resins have better adhesive properties than the unmodified resins while maintaining their low ash, clean burning characteristics.
Owner:ESEMPLARE PASCAL E
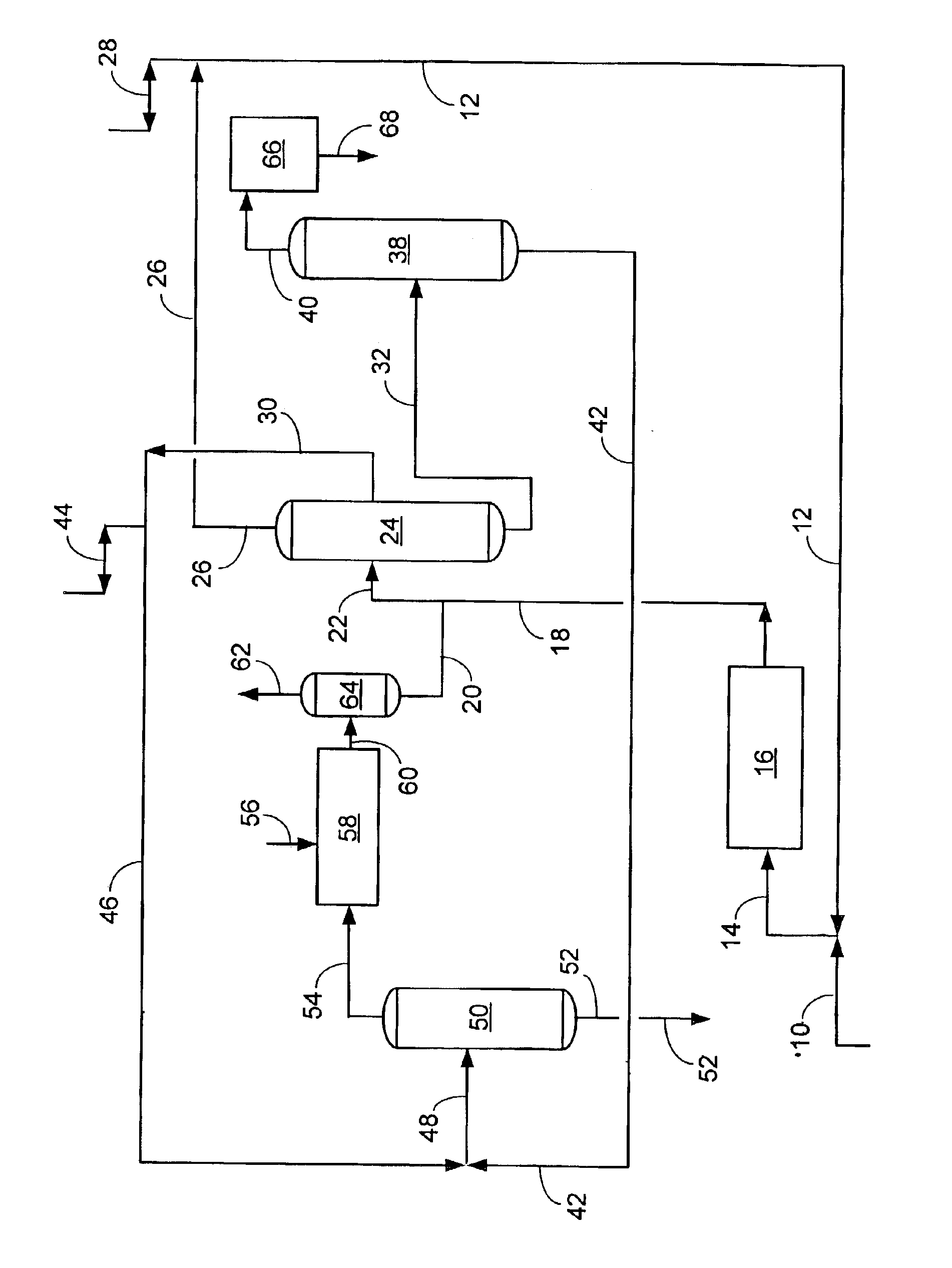
Process and apparatus for ethylbenzene production and transalkylation to xylene
InactiveUS6855854B1High selectivityHigh yieldHydrocarbon by isomerisationMolecular sieve catalystCarbon numberGas phase
The use of two transalkylation catalysts to react aromatic compounds of carbon number nine (and heavier carbon numbers) with benzene to form carbon number eight aromatics is disclosed. The two catalyst system preserves ethyl-group species on the heavier aromatics that would otherwise de-ethylate over most gas-phase transalkylation catalysts to form undesired ethane gas with benzene or toluene. Thus, by using a transalkylation step to save ethylbenzene, a greater yield of para-xylene or other carbon number eight aromatics may be achieved within an integrated complex. An apparatus and process for the two transalkylation catalyst system is disclosed with a liquid-phase unit and a gas-phase unit.
Owner:UOP LLC
(R)- and (S)-1-(3-(3-N,N-dimethylaminocarbonyl)phenoxyl-4-nitrophenyl)-1-ethyl-N,N'-bis (ethylene)phosphoramidate, compositions and methods for their use and preparation
ActiveCN108290911AOrganic active ingredientsOrganic chemistry methodsMedicinal chemistryPhosphoramidate
Provided herein are optically active compounds of the formulae (ii); and (III) and pharmaceutical compositions thereof. Also provided herein are processes of making these compounds and resolving the racemic mixture or the enrichment of same with in one of its enantiomers to provide (R)- and (S)-1-(3-(3-N,N-dimethylaminocarbonyl)phenoxyl-4-nitrophenyl)-1-ethyl-N,N'-bis(ethylene)phosphoramidate, andmethods of treating cancer comprising administering such compounds.
Owner:SHENZHEN ASCENTAWITS PHARM TECH CO LTD
Aqueous resin dispersion composition
InactiveUS6359030B1Excellent hot water resistanceMaintain good propertiesCoatingsKetone solventsMethyl ethyl ketone peroxide
An aqueous-dispersing composition of synthetic resin (A) having an intrinsic viscosity "eta" of from 0.3 to 2.0 measured at 35° C. in a methyl ethyl ketone solvent or a mixture of that aqueous dispersion and an aqueous dispersion of synthetic resin (B) having an intrinsic viscosity "eta" of not less than 0.6 which is larger by 0.3 or more than the intrinsic viscosity "eta" of the synthetic resin (A) when the intrinsic viscosity is measured at 35° C. in a methyl ethyl ketone solvent. The aqueous-dispersing composition and the mixture have improved film forming property and can give a coating film being excellent in chemical resistance, water resistance, mechanical properties and further hot water resistance.
Owner:DAIKIN IND LTD
Crystal form of Peimeiqusai disodium and its preparation
ActiveCN1778802AImprove controllabilitySimple and fast operationOrganic active ingredientsOrganic chemistryPhenacylPemetrexed disodium
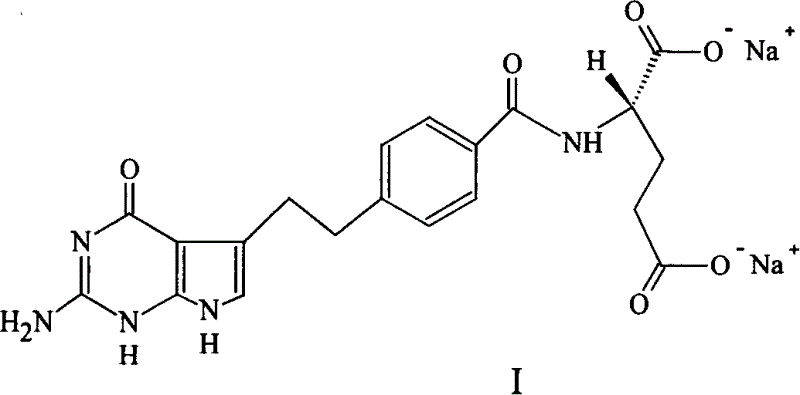
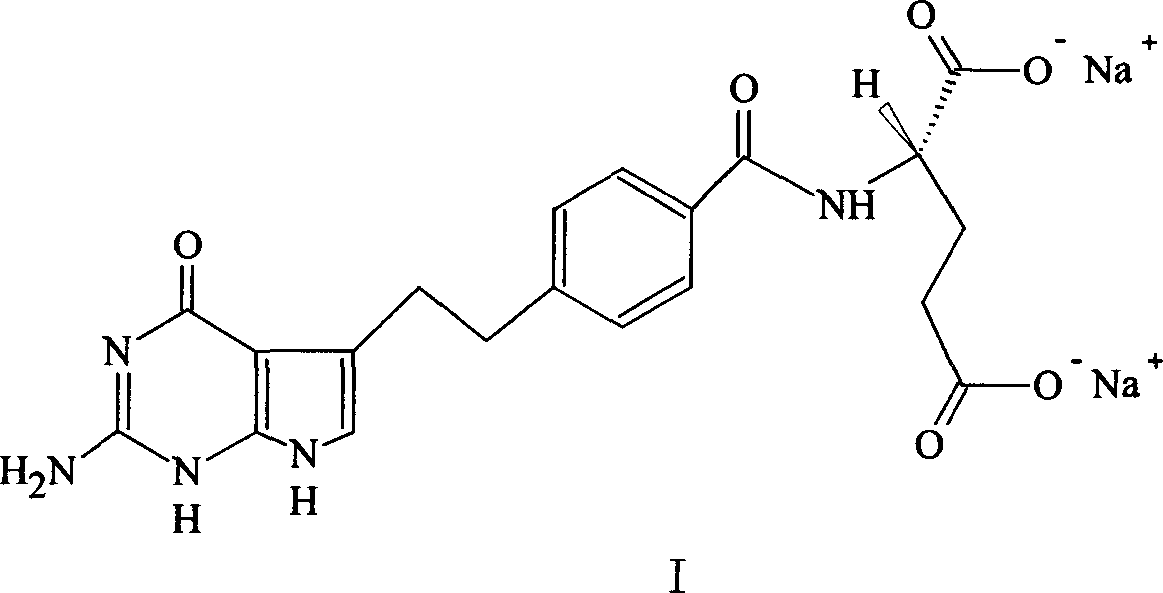
A crystal form of folic acid antagonistic N-(4-(2-(2-amino-4,7-methyl-4-oxo-1H-pyrrolo(2,3-d)pyrimidine-5-radical)ethyl)benzoyl)-L-glutamic acid disodium salt and its production are disclosed.
Owner:重庆凯林制药有限公司 +2
Preparation method of polydopamine modified alginic acid microspheres
InactiveCN102964610AImprove mechanical propertiesGood anti-swelling performanceMicroballoon preparationMicrocapsule preparationMicrosphereN-Hydroxysuccinimide
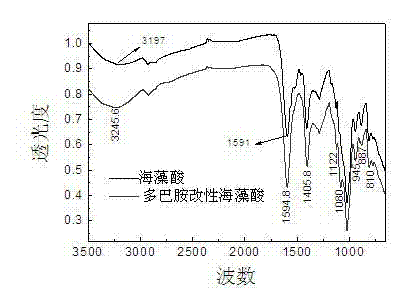
The invention discloses a preparation method of polydopamine modified alginic acid microspheres, which comprises the following steps of: under the protection of nitrogen, grafting dopamine to alginic acid by use of N-hydroxysuccinimide and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride; polymerizing the synthesized dopamine modified alginic acid under the slight alkaline condition to obtain a polydopamine modified alginic acid solution; dropwise adding the polydopamine modified alginic acid solution into a CaCl2 solution by a syringe; and aging to obtain polydopamine modified alginic acid microspheres. The method disclosed by the invention is simple in process, and the prepared modified alginic acid microspheres have higher swelling resistance and good mechanical properties.
Owner:TIANJIN UNIV
Synthesis path of Timisatem
InactiveCN1344712AReduce generationHigh yieldOrganic chemistryCardiovascular disorderActive componentEthyl group
The present invention relates to the preparation of Timisatan as one active component for antihypertensive medicine. The carboxylic ester derivative is first obtained through the nucleophilic substitution reaction between some intermediate and 4'-bromomethyl biphenyly-2-carboxylic ester, and then hydrolyzed to eliminnate protection radical and to obtain the destination product. In the technological process of the present invention, methyl radical and ethyl radical are used as protecting radical, and this facilitates and stabilizes the preparation of 4'-bromomethyl biphenylyl-2-methyl carboxylic ester and 4'-bromomethyl biphenylyl-2-ethyl carboxylic ester, and results in easy to control reaction with the intermediate, less impurity, easy elimination of protecting radical and high product yield and quality.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Energy-efficient process for para-xylene production
InactiveUS20070299289A1Increase productionLow cyclic lossHydrocarbon by isomerisationMolecular sieve catalystIsomerizationHydrocarbon
This invention is drawn to a process for producing and recovering one or more high-purity xylene isomers from a feed stream having a substantial content of C9 and heavier hydrocarbons. The feed stream is processed to de-ethylate heavy aromatics, fractionated and passed to a circuit comprising C8-aromatic isomer recovery and isomerization to recover the high-purity xylene isomer with lowered energy costs.
Owner:UOP LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament](https://images-eureka.patsnap.com/patent_img/7e706329-04e4-4b5b-b8c9-1a182cd2dbef/HSA0000102396500000011.PNG)
![Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament](https://images-eureka.patsnap.com/patent_img/7e706329-04e4-4b5b-b8c9-1a182cd2dbef/HSA0000102396500000012.PNG)
![Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament Application of 2-([1,1'-biphenyl]-4-yl)-2-oxoethyl 4-((3-chloro-4-methylphenyl) amino)-4-oxobutanoate in preparing an LSD1 (lysine-specific demethylase 1) inhibitor medicament](https://images-eureka.patsnap.com/patent_img/7e706329-04e4-4b5b-b8c9-1a182cd2dbef/HSA0000102396500000013.PNG)


![5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine 5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine](https://images-eureka.patsnap.com/patent_img/3ae644fc-9a10-45d9-a30b-421eab0a7beb/US07638541-20091229-D00001.png)
![5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine 5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine](https://images-eureka.patsnap.com/patent_img/3ae644fc-9a10-45d9-a30b-421eab0a7beb/US07638541-20091229-D00002.png)
![5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine 5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine](https://images-eureka.patsnap.com/patent_img/3ae644fc-9a10-45d9-a30b-421eab0a7beb/US07638541-20091229-D00003.png)