Patents
Literature
13783results about How to "Increase the length" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
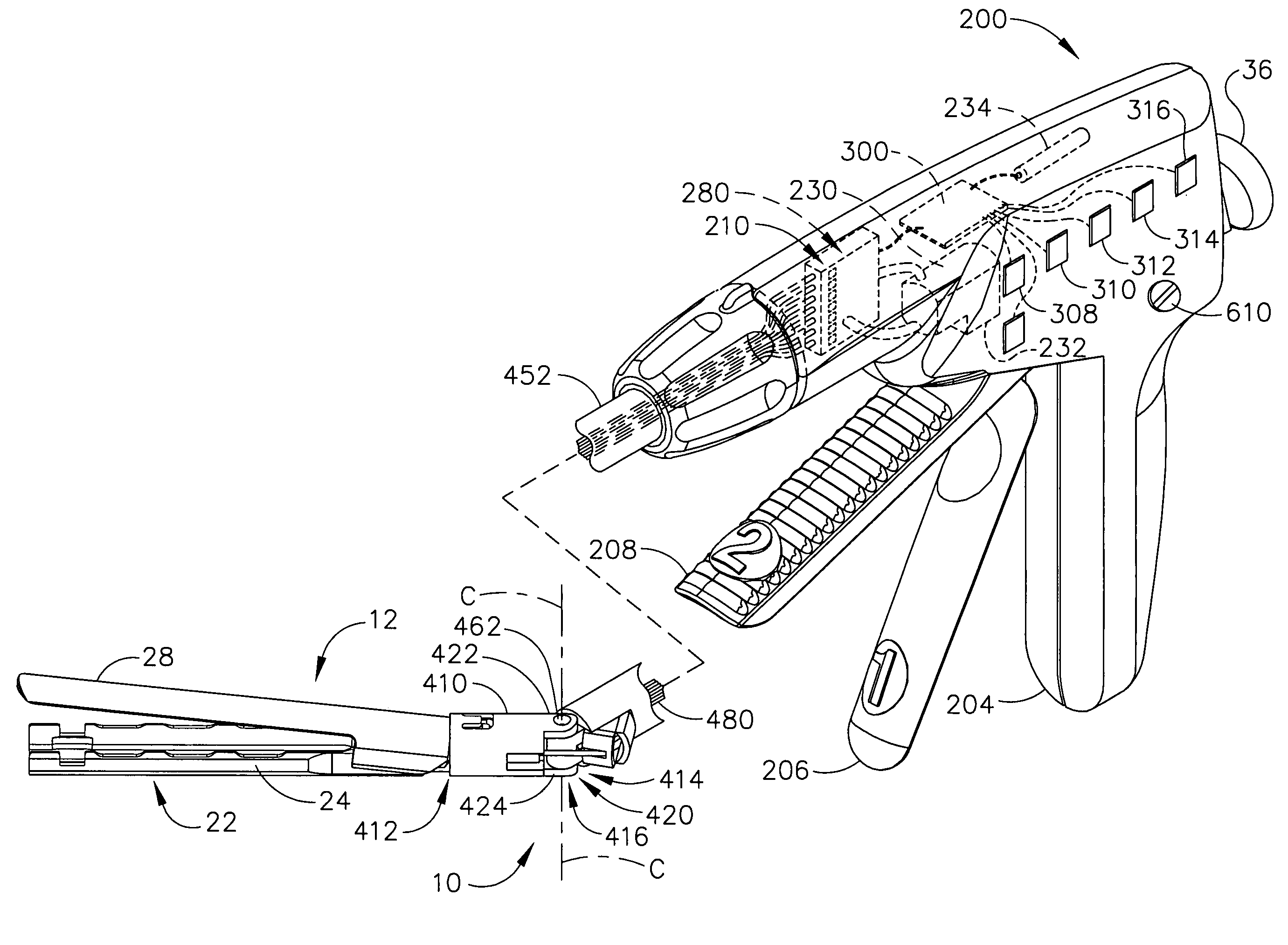
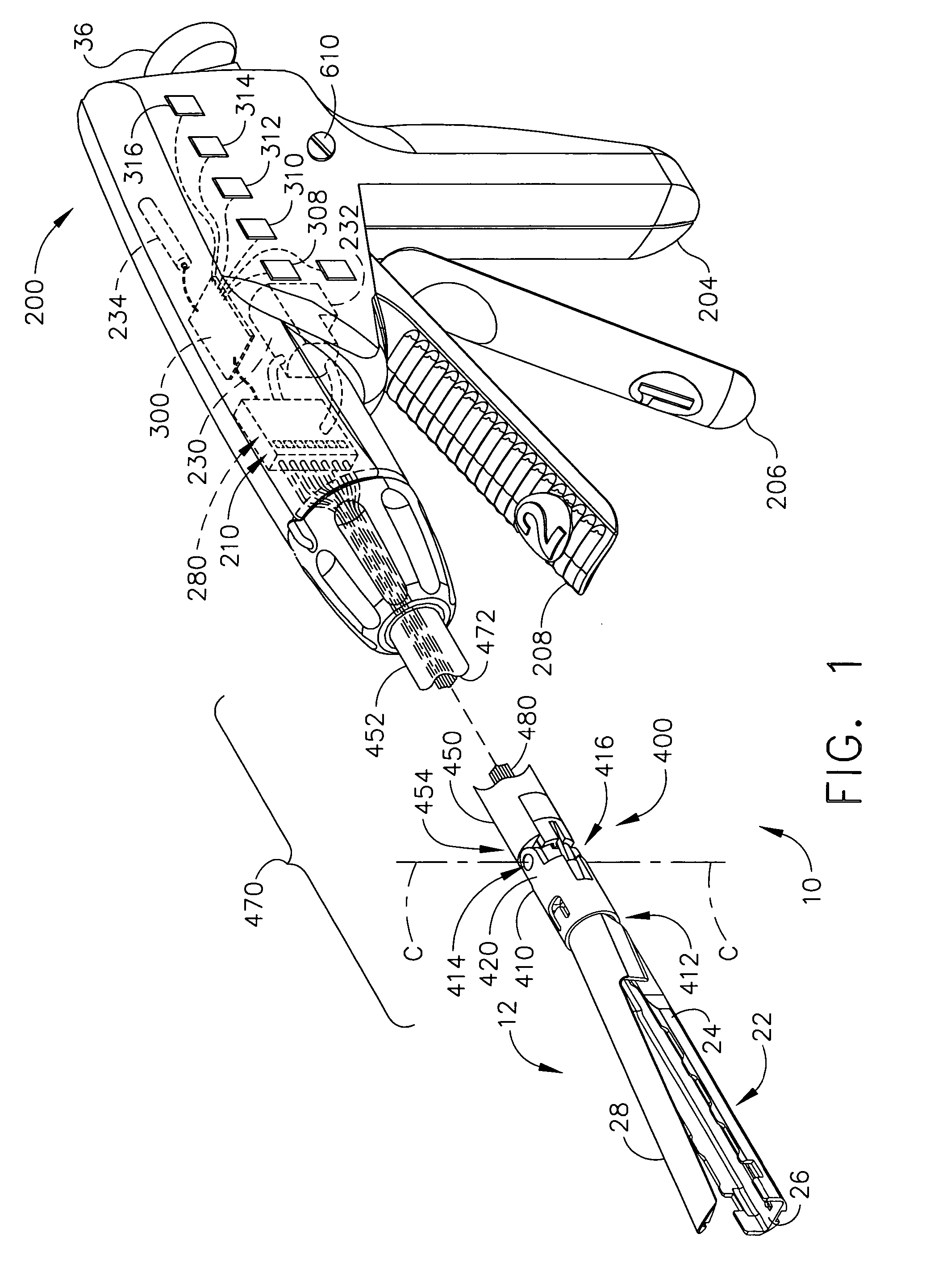
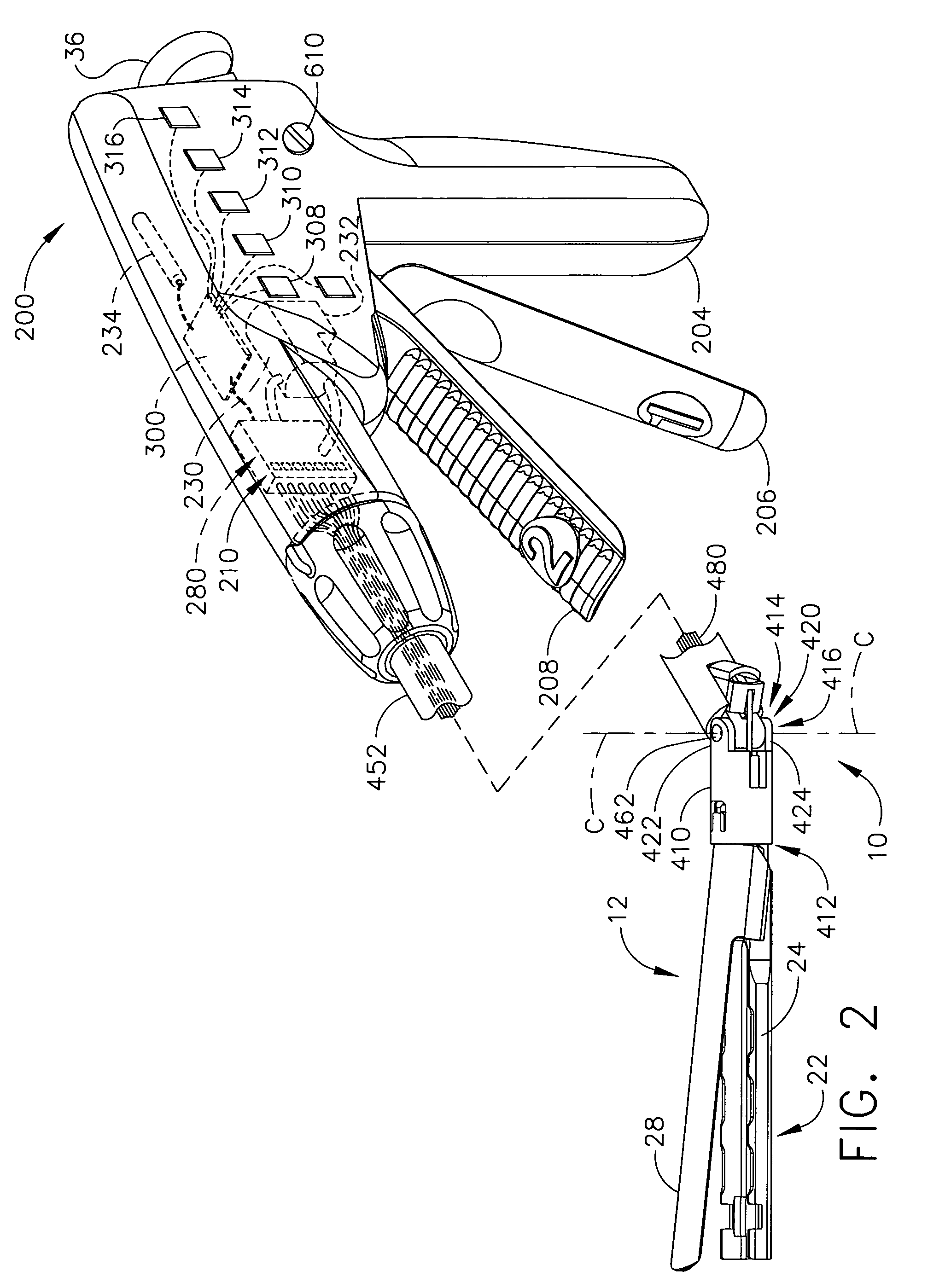
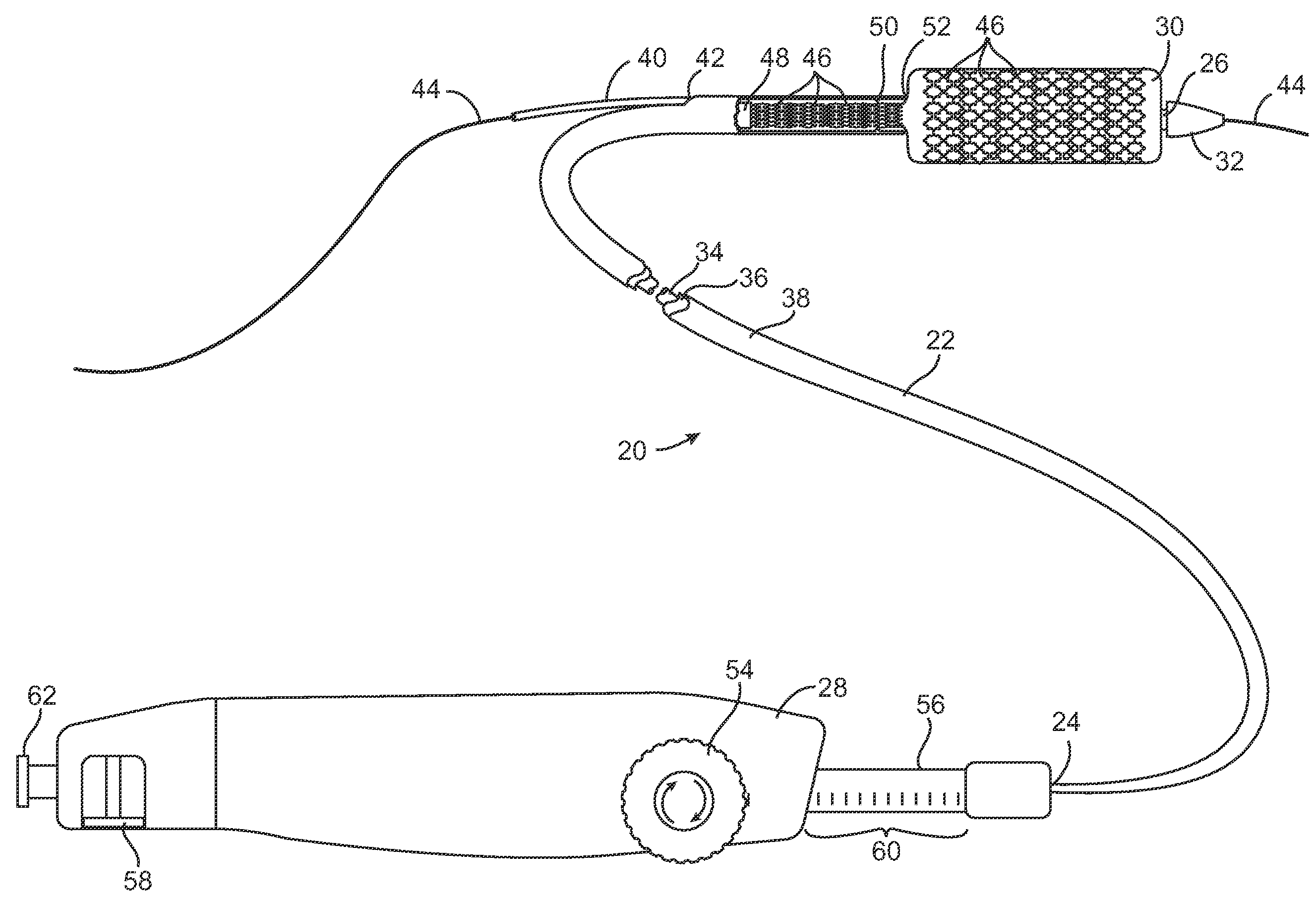
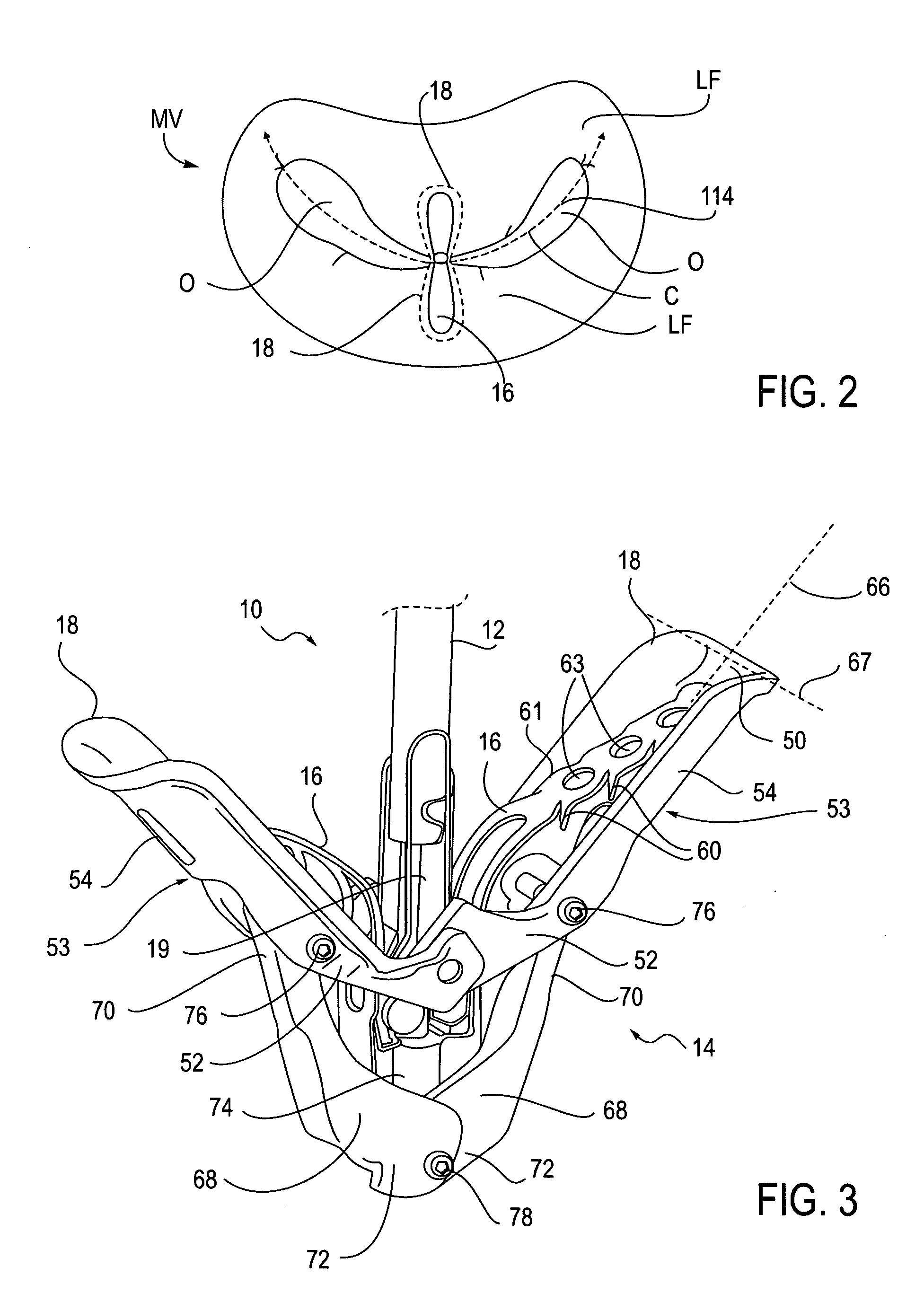
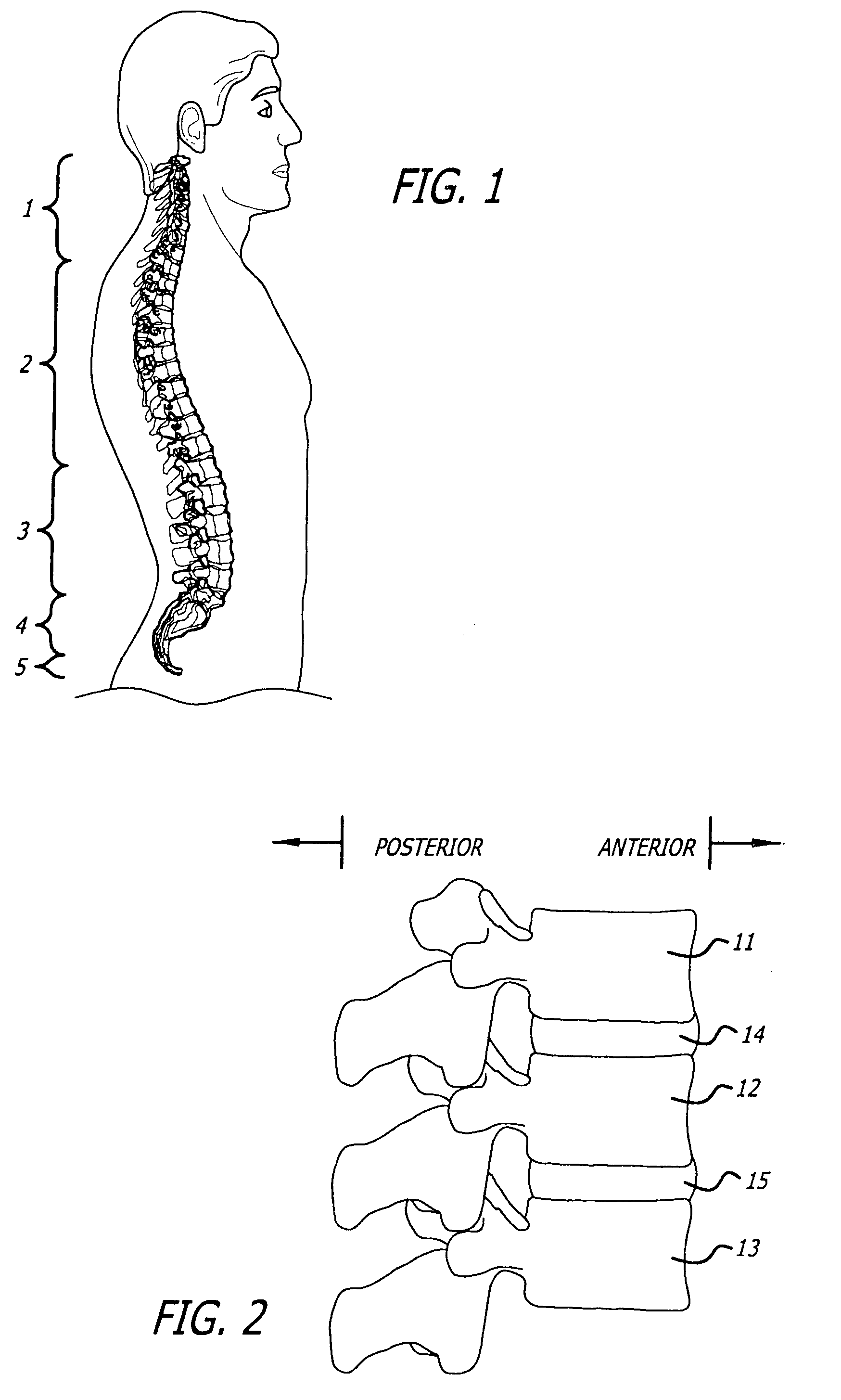
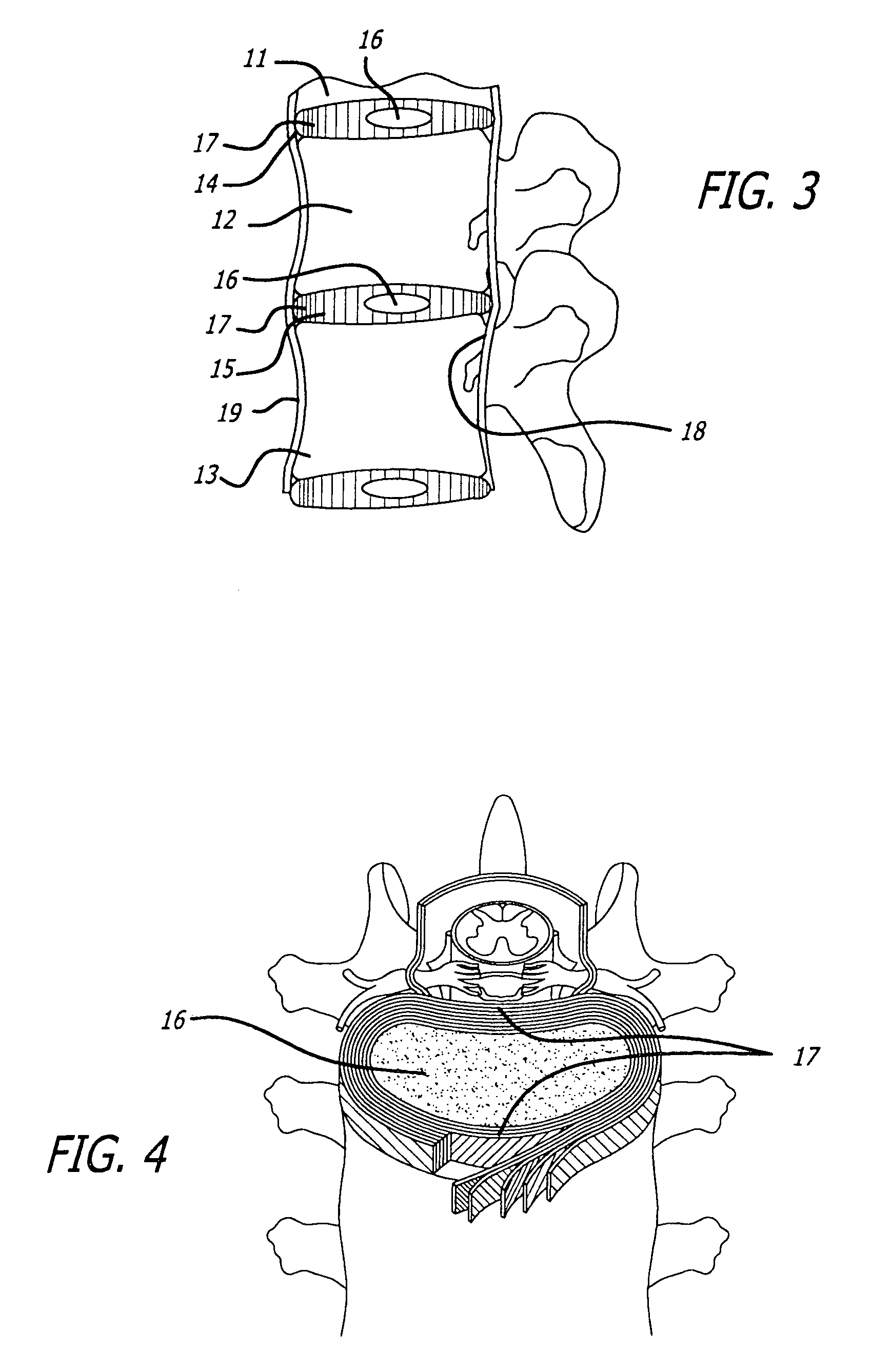
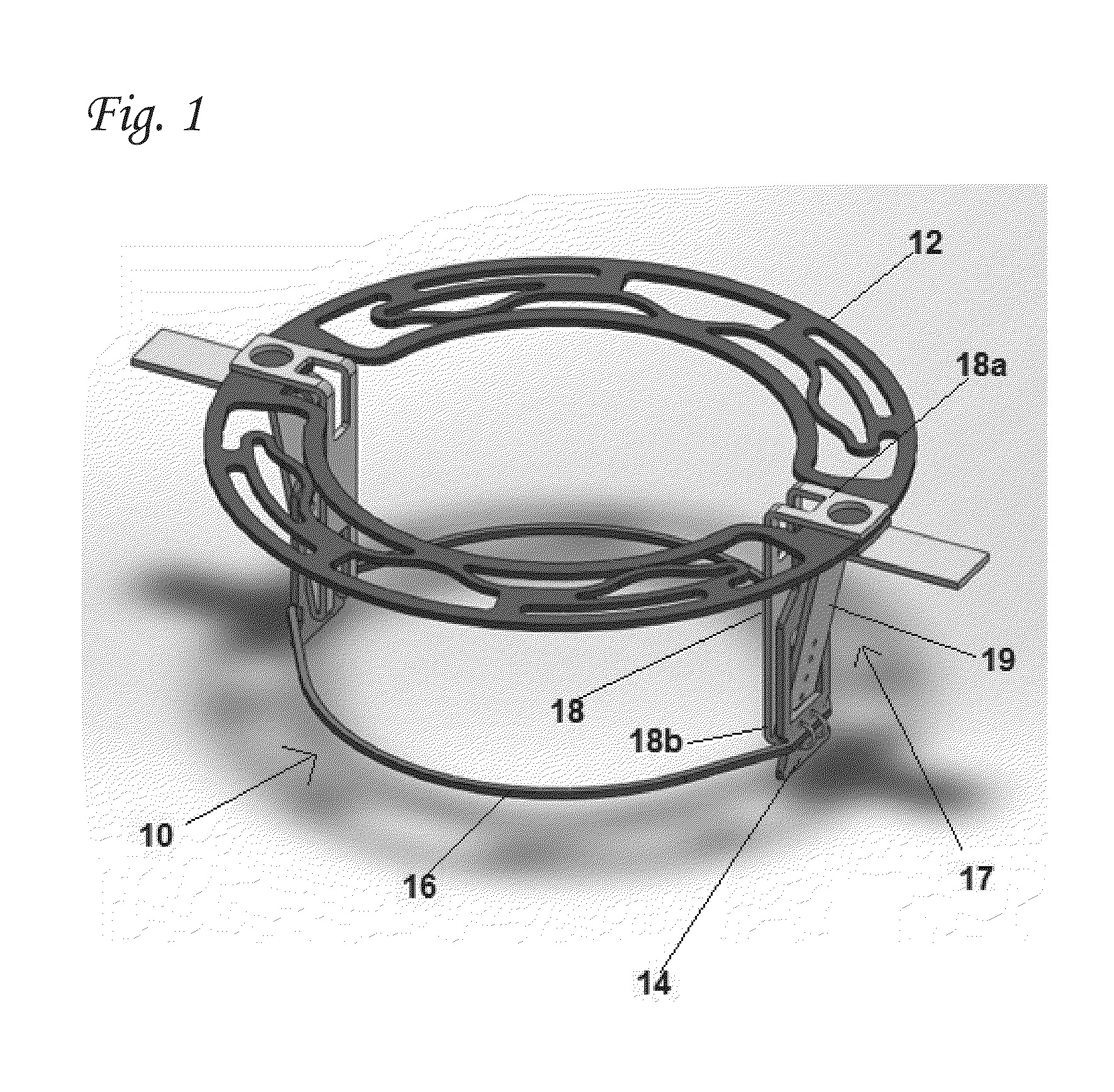
Articulation joint with improved moment arm extension for articulating an end effector of a surgical instrument
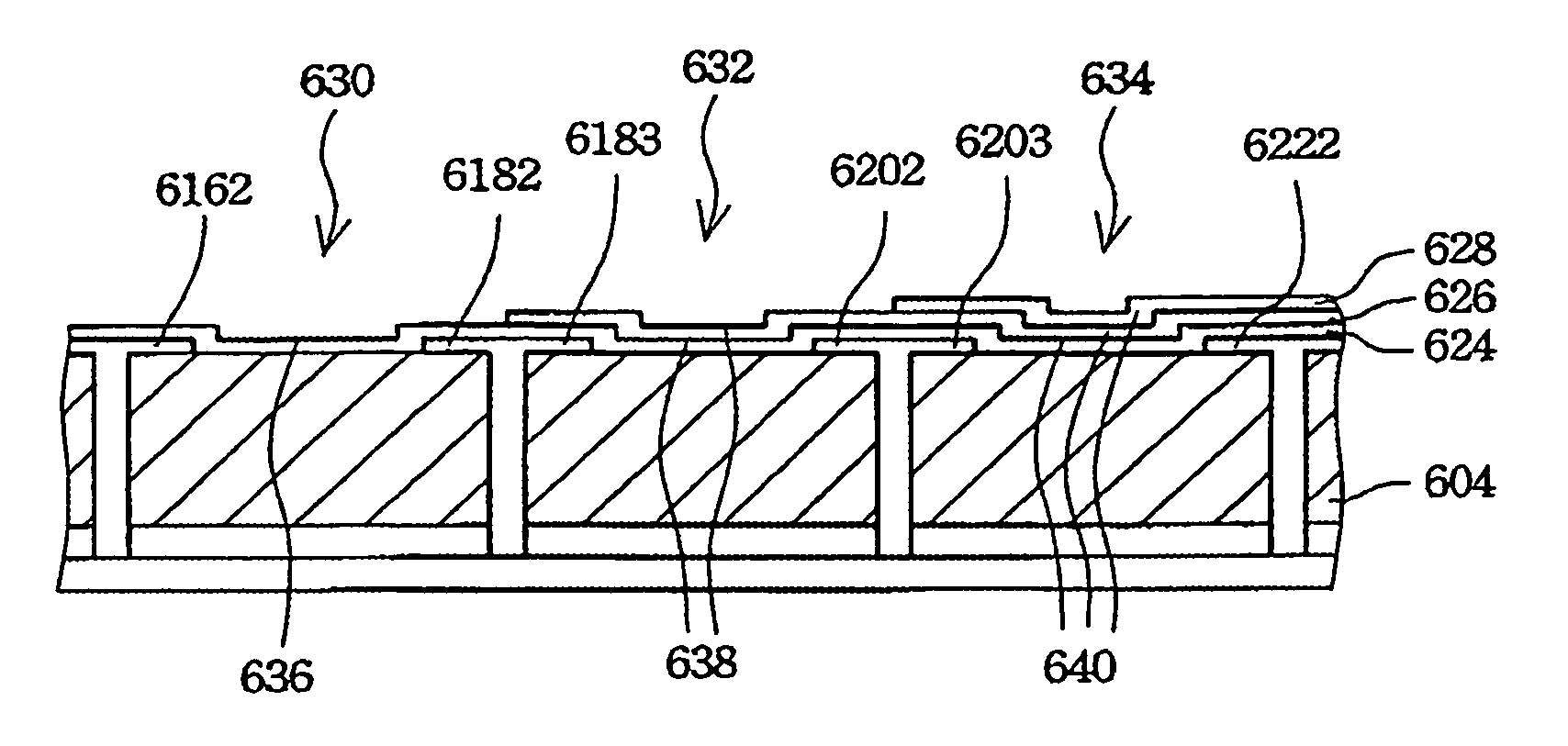
ActiveUS7673780B2High strengthIncrease the lengthSuture equipmentsStapling toolsCombined useEngineering
An articulation joint for use in connection with a surgical instrument that has a portion that must be passed through a trocar or similar structure and then articulated relative to another portion of the instrument received within the trocar. Various embodiments of the articulation joint provide structures for increasing the moment arm between the actuator and the pivot point between the proximal and distal tube segments interconnecting a handle assembly of the surgical instrument to a surgical implement such as an end effector of an endocutter.
Owner:ETHICON ENDO SURGERY INC
Analying polynucleotide sequences
InactiveUS6054270AStable duplexReduce impactSequential/parallel process reactionsSugar derivativesHybridization reactionSequence determination
This invention provides an apparatus and method for analyzing a polynucleotide sequence; either an unknown sequence or a known sequence. A support, e.g. a glass plate, carries an array of the whole or a chosen part of a complete set of oligonucleotides which are capable of taking part in hybridization reactions. The array may comprise one or more pair of oligonucleotides of chosen lengths. The polynucleotide sequence, or fragments thereof, are labelled and applied to the array under hybridizing conditions. Applications include analyses of known point mutations, genomic fingerprinting, linkage analysis, characterization of mRNAs, mRNA populations, and sequence determination.
Owner:OXFORD GENE TECH
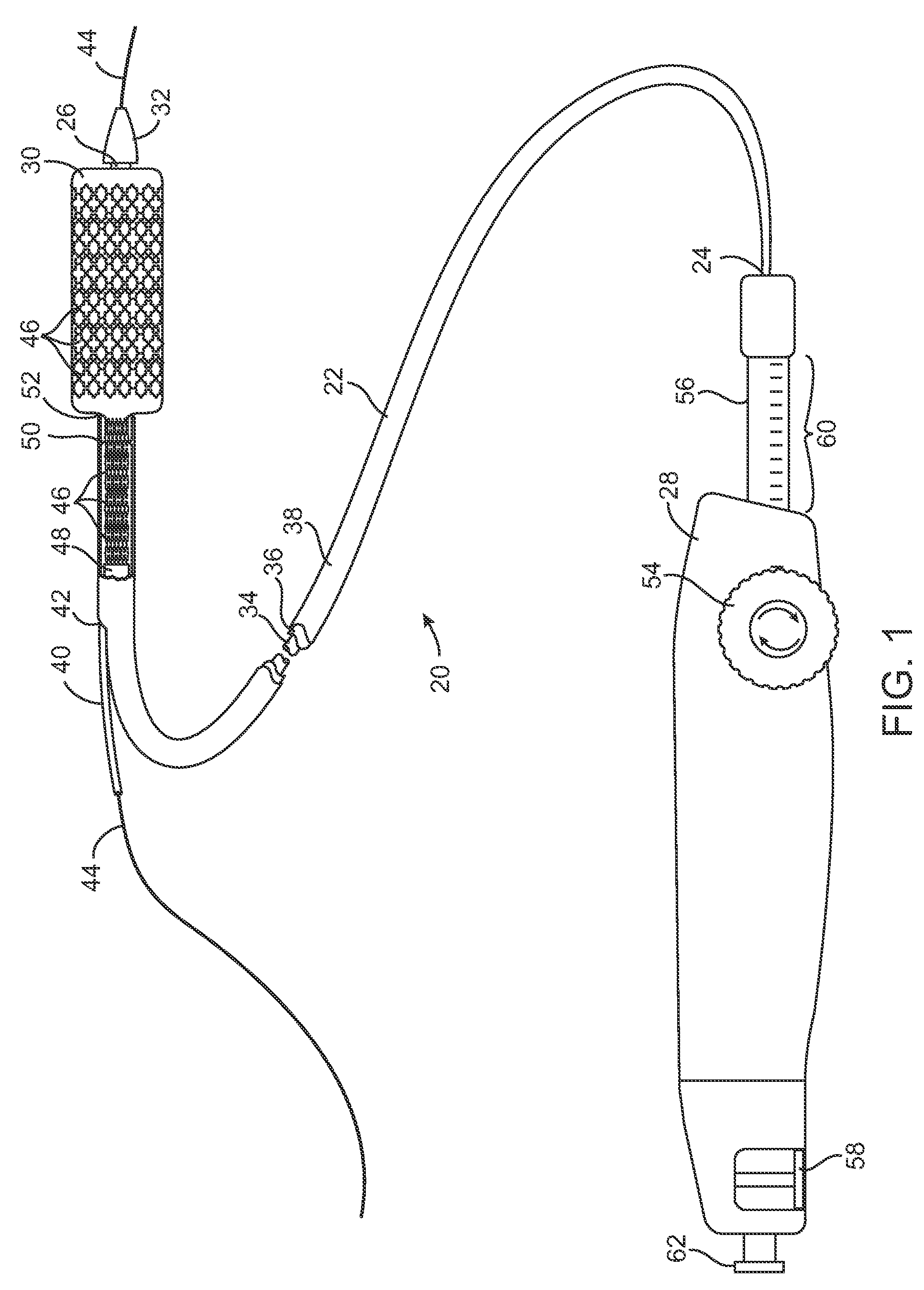
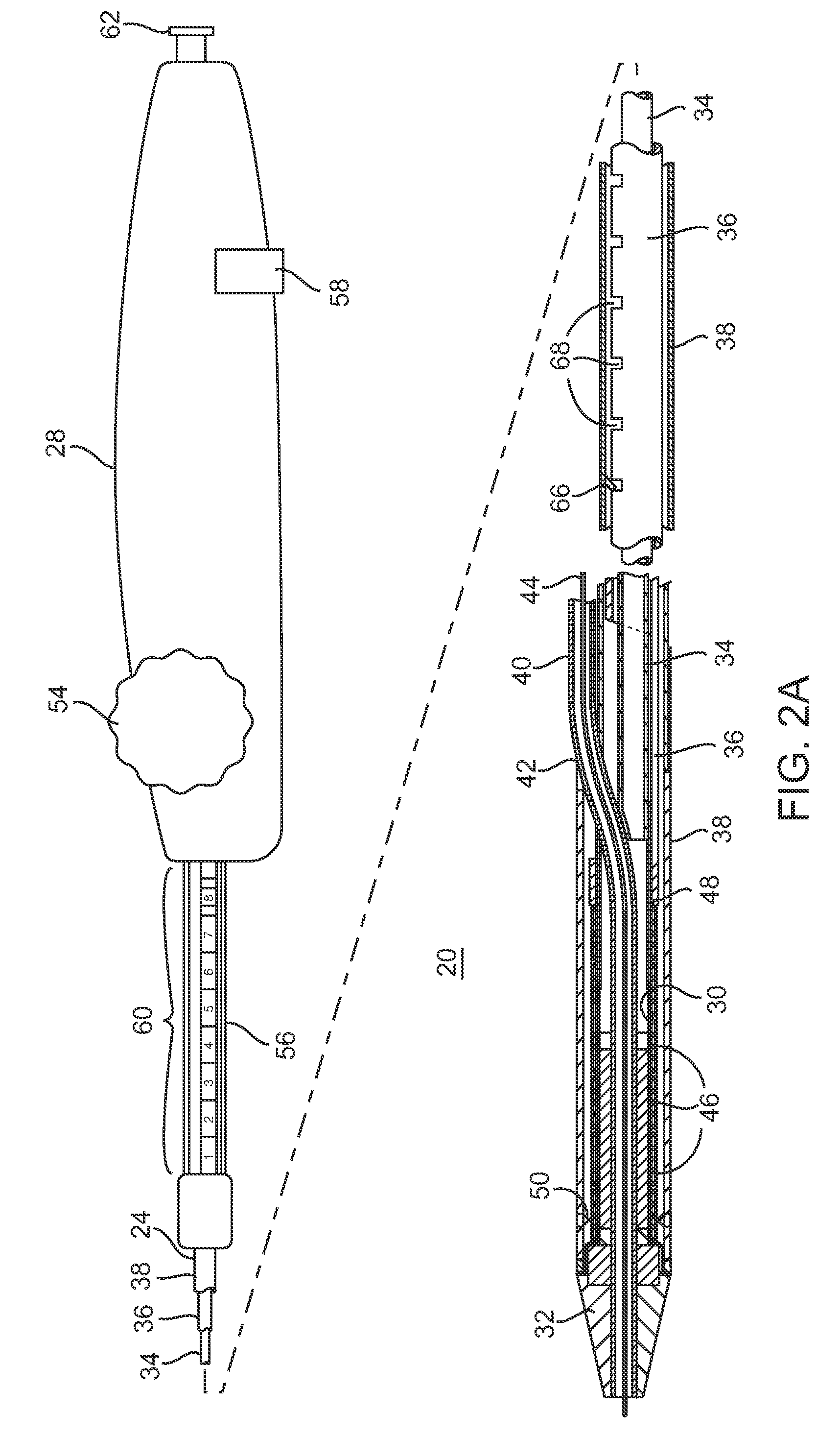
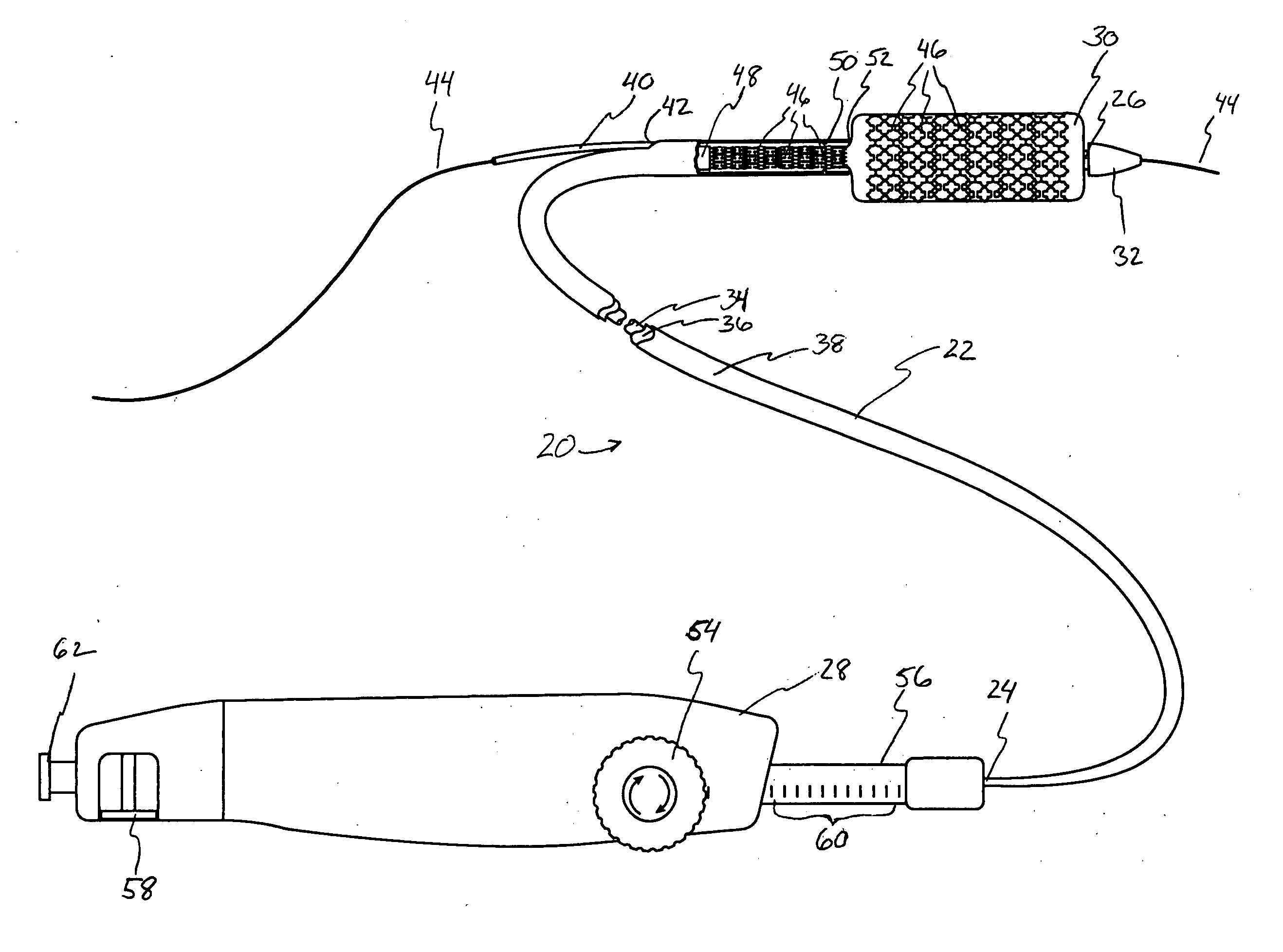
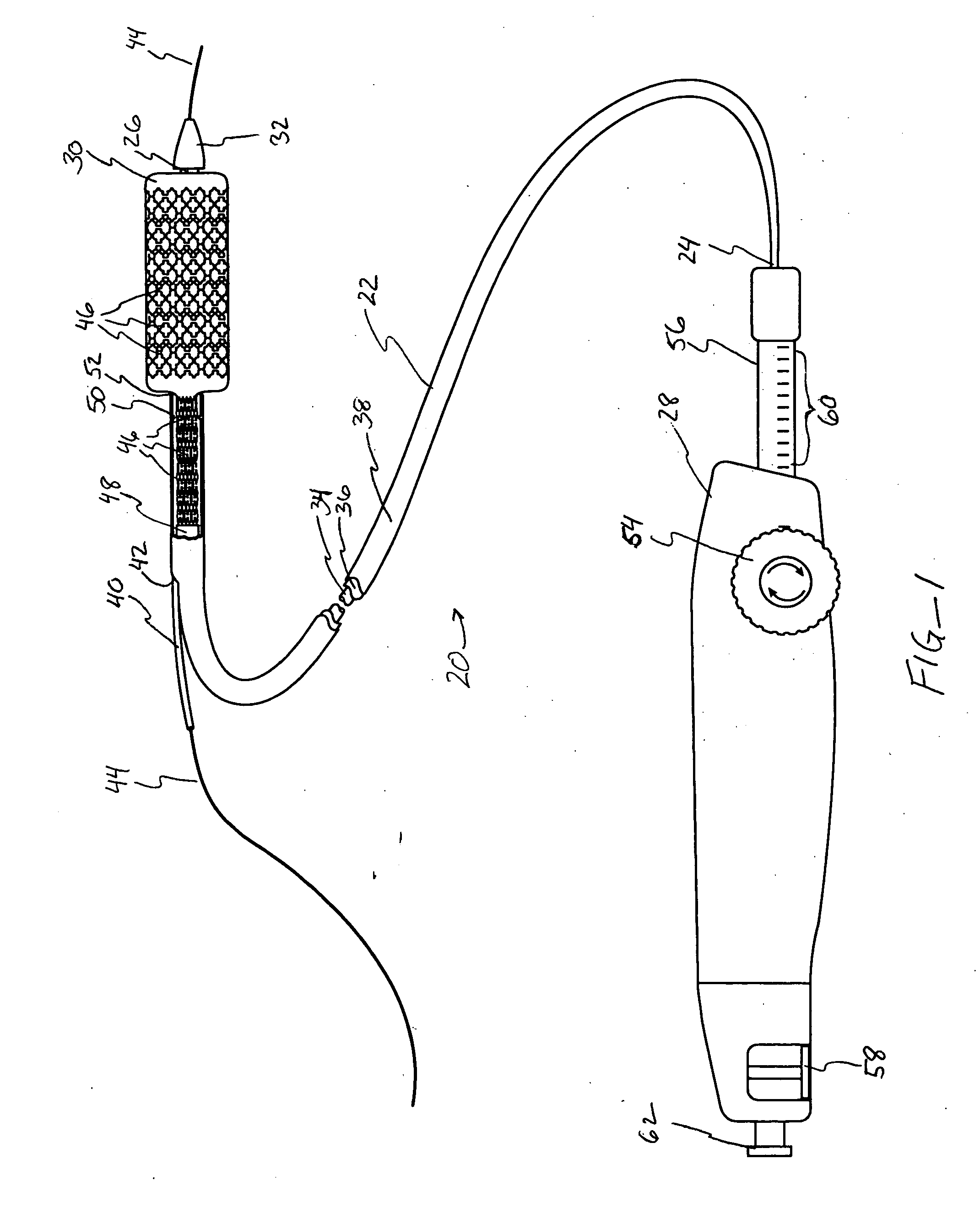
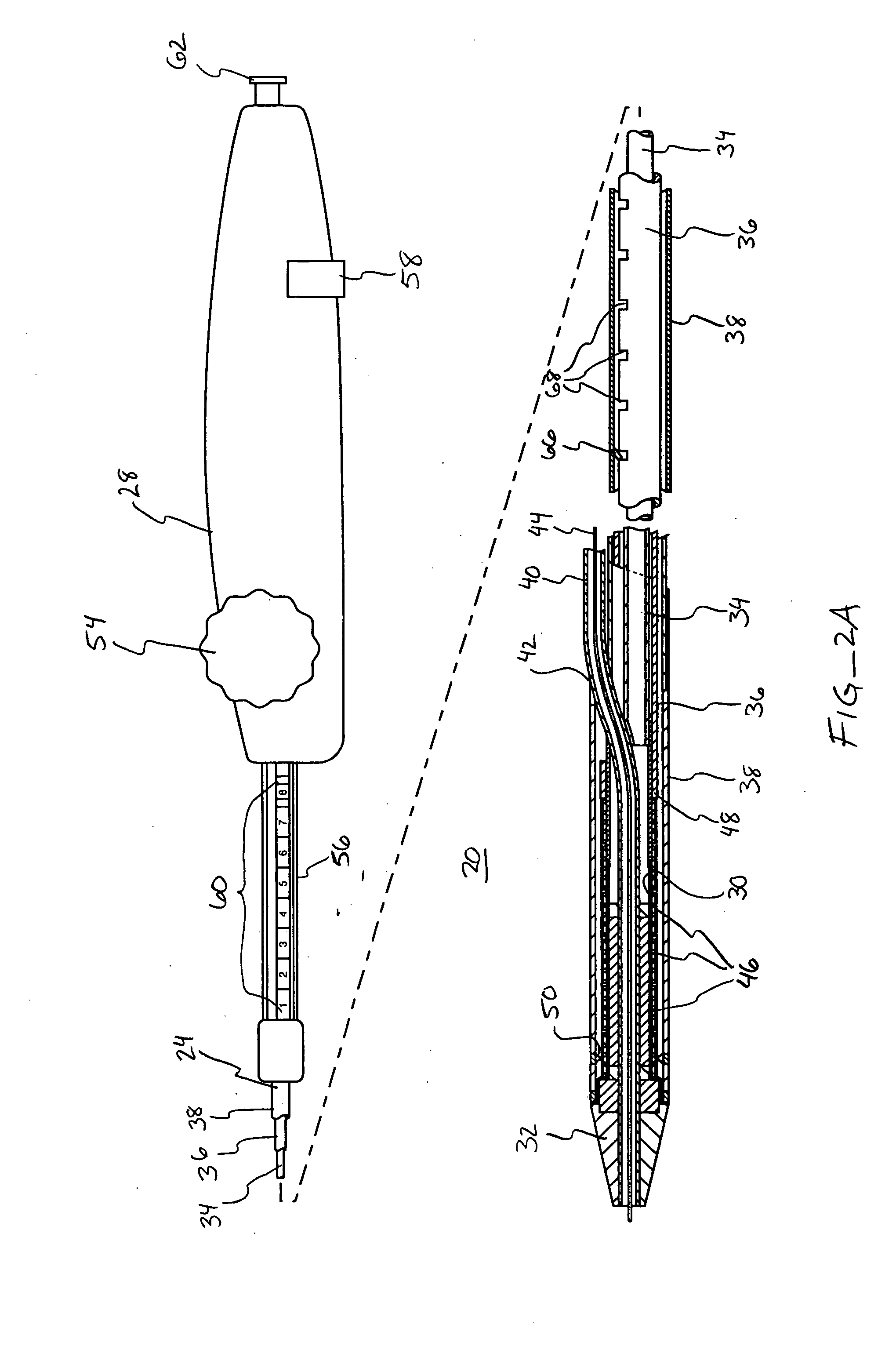
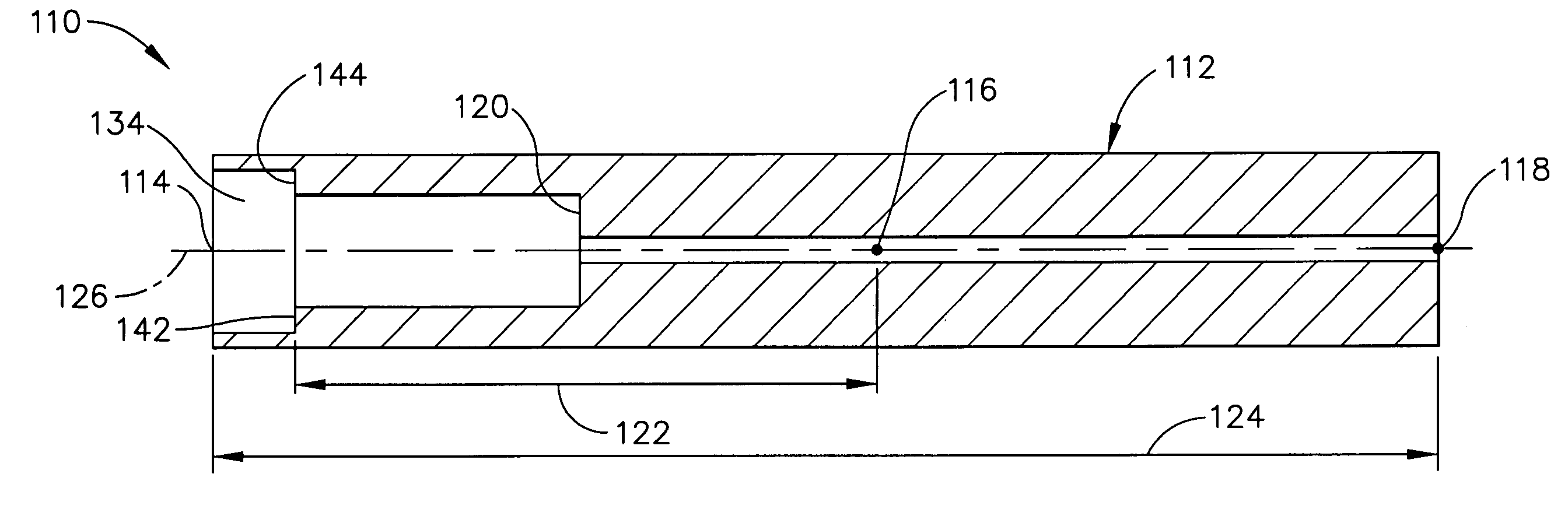
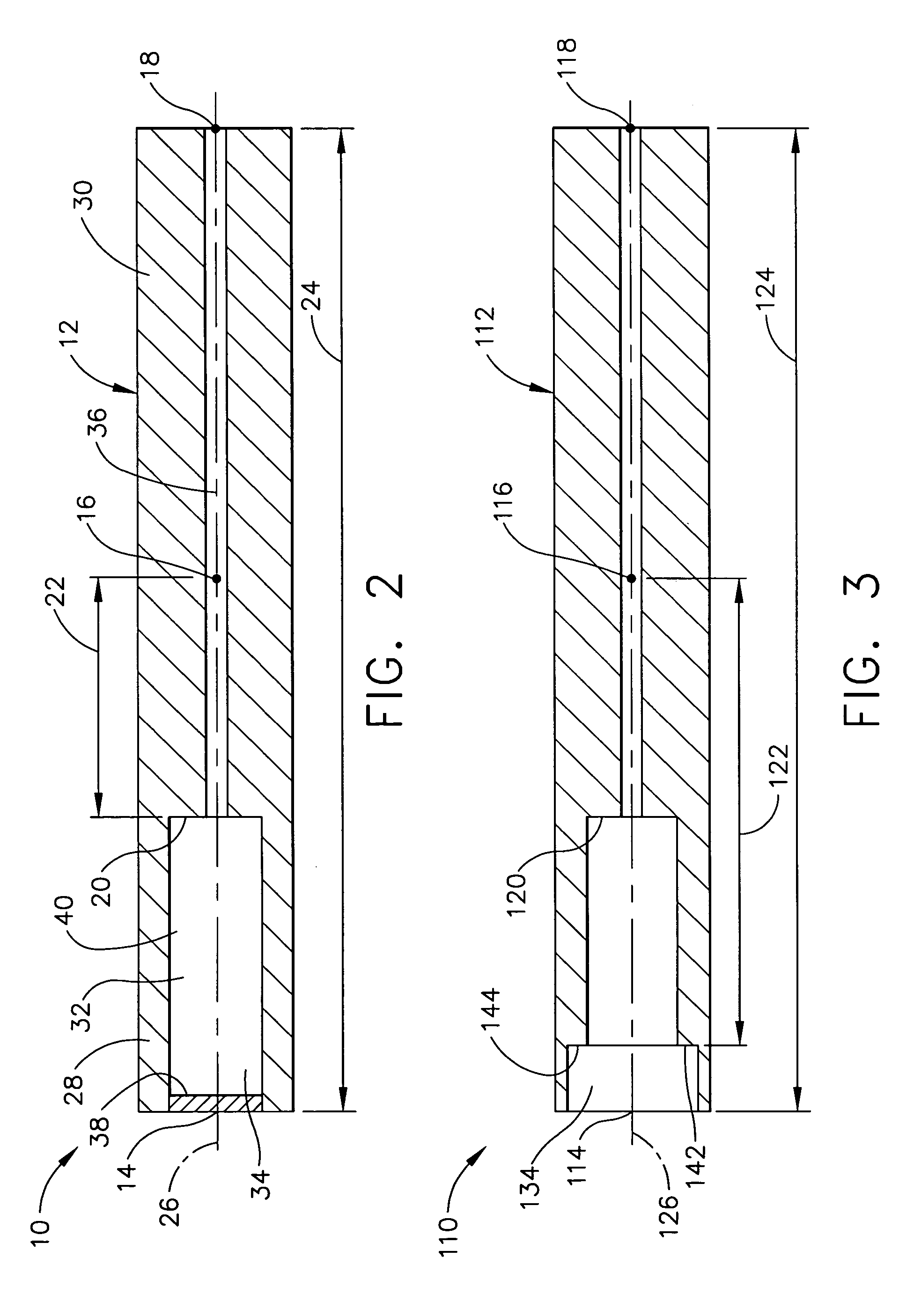
Devices and methods for controlling and indicating the length of an interventional element
Devices and methods are provided for controlling and indicating the deployed length of an interventional element on an interventional catheter. The interventional element may be a stent or series of stents, a balloon, or any other interventional element for which length control is necessary or desirable. Devices for controlling the length of the interventional element include gear driven actuators, motors, and other mechanisms. Devices for indicating length of an interventional element to the user include sensors, detents, visual displays and other mechanisms providing visual, audible, and tangible indications of length to the user. The control and indication devices preferably work in tandem to enable highly precise adjustment of interventional element length.
Owner:JW MEDICAL SYSTEMS LTD
Devices and methods for controlling and indicating the length of an interventional element
Devices and methods are provided for controlling and indicating the deployed length of an interventional element on an interventional catheter. The interventional element may be a stent or series of stents, a balloon, or any other interventional element for which length control is necessary or desirable. Devices for controlling the length of the interventional element include gear driven actuators, motors, and other mechanisms. Devices for indicating length of an interventional element to the user include sensors, detents, visual displays and other mechanisms providing visual, audible, and tangible indications of length to the user. The control and indication devices preferably work in tandem to enable highly precise adjustment of interventional element length.
Owner:JW MEDICAL SYSTEMS LTD
Ultrasonic surgical blade and instrument having a gain step
ActiveUS7163548B2Increase the lengthShorten half wave lengthCannulasTooth pluggers/hammersSurgical bladeWavelength
An ultrasonic surgical blade, and an instrument, having a gain step. The blade body has, in any half wave length of the ultrasonic-surgical-blade body, a first vibration antinode, a vibration node, a second vibration antinode, and a gain step. The gain step is located between the second vibration antinode and the first vibration antinode. The gain step is spaced apart from the vibration node by a gain-step distance greater than 5% of the distance between the second vibration antinode and the first vibration antinode. The instrument includes the blade, a handpiece having an ultrasonic transducer, and an ultrasonic transmission rod whose proximal end is operatively connected to the ultrasonic transducer and whose distal end activates the blade. In one option, the first vibration antinode is the distal tip, and the gain step is located between the vibration node and the distal tip, resulting in an increased active length of the blade.
Owner:CILAG GMBH INT
Electrophoretic displays using gaseous fluids
InactiveUS20080024429A1Reduce the impactIncrease the lengthStatic indicating devicesElectrophoresisDisplay device
An electrophoretic display comprises a pair of facing substrates, at least one of which is transparent, a plurality of particles and a gas between the substrates, and means for applying an electric field to cause the particles to move and thus change the electro-optic state of the display. The electric field means is arranged to increase the impulse applied to the display with increasing time since a reference time, or with increasing number of images written on the display. In another embodiment, an alternating current pulse is applied to the display, and the duration and / or amplitude of the alternating current pulse is increased with increasing time since a reference time.
Owner:E INK CORPORATION
Method and apparatus for delivering a virtual reality environment
InactiveUS20030046689A1Easy to getConvenient transactionTelevision system detailsDigital computer detailsPersonalizationVirtual intelligence
This invention involves a virtual environment created through the combination of technologies. The invention employs the knowledge and experience of a Personal Assistant or Host, created through Artificial Intelligence applications, which assists and guides the user of the environment to products and / or services that they will most likely be interested in purchasing or requiring. The intelligent assistant's choices are based on its experiences with the specific user. The intelligent assistant communicates with the user by means of a speech recognition and speech synthesis device. This invention is an easy to use virtual reality environment that takes advantage of existing technologies and global communications networks such as the Internet without requiring any given degree of computer literacy. This invention includes a virtual intelligent assistant for each user which adapts to its user as it provides individualized guidance. The intelligent assistant or avatar projects human-like features and behaviors appropriate to the preferences of its user and appears as a virtual person to the user.
Owner:MISSION THE
Structure of an optical interference display cell
A structure of an interference display cell is provided. The cell comprises a first plate and a second plate, wherein a support is located between the first plate and the second plate. The second plate is a deformable and reflective plate. An incident light from one side of the first plate is modulated and only specific frequency light reflects by the second plate. The frequency of the reflected light is related to the distance between the first plate and the second plate. The support has at least one arm. The arm's stress makes the arm hiking upward or downward. The distance between the first plate and the second plate is also changed. Therefore, the frequency of the reflected light is altered.
Owner:SNAPTRACK
Semiconductor processing apparatus with compact free radical source
InactiveUS20130337653A1Controllably generatingIncrease the lengthSemiconductor/solid-state device manufacturingChemical vapor deposition coatingEngineeringSemiconductor
A semiconductor processing apparatus (1), comprising: a substrate processing chamber (158), defining a substrate support location (156) at which a generally planar semiconductor substrate (300) is supportable; and at least one free radical source (200), including: a precursor gas source (250); an electric resistance heating filament (244); a sleeve (220) with a central sleeve axis (L), wherein said sleeve defines a reaction space (222) that accommodates the heating filament (244), and wherein said sleeve includes an inlet opening (224) via which the reaction space is fluidly connected to the precursor gas source (250), and an outlet opening (228) via which the reaction space is fluidly connected to the substrate processing chamber (158), said inlet and outlet openings (224, 228) being spaced apart along the central sleeve axis (L).
Owner:ASM IP HLDG BV
RF power transmission network and method
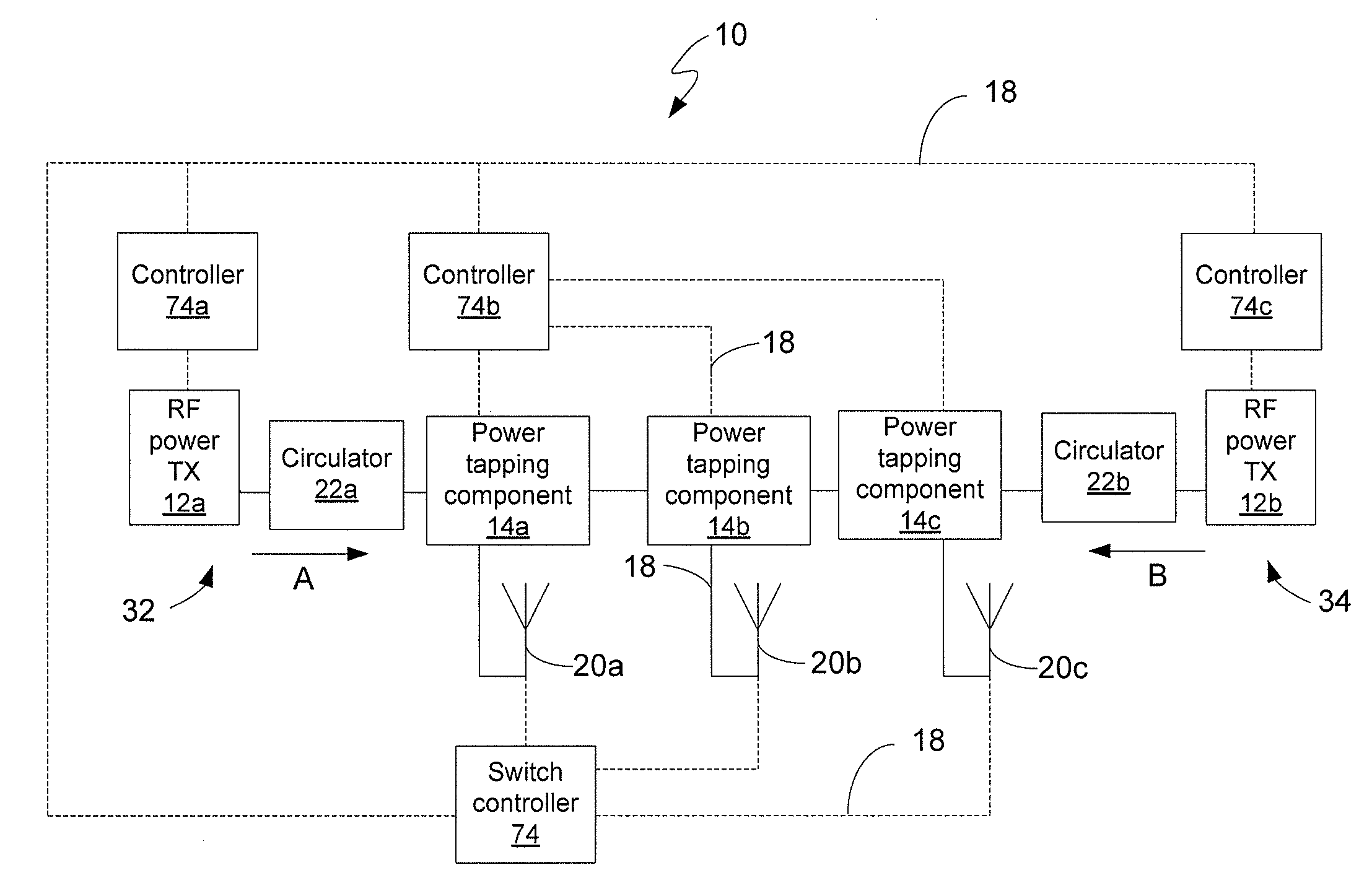
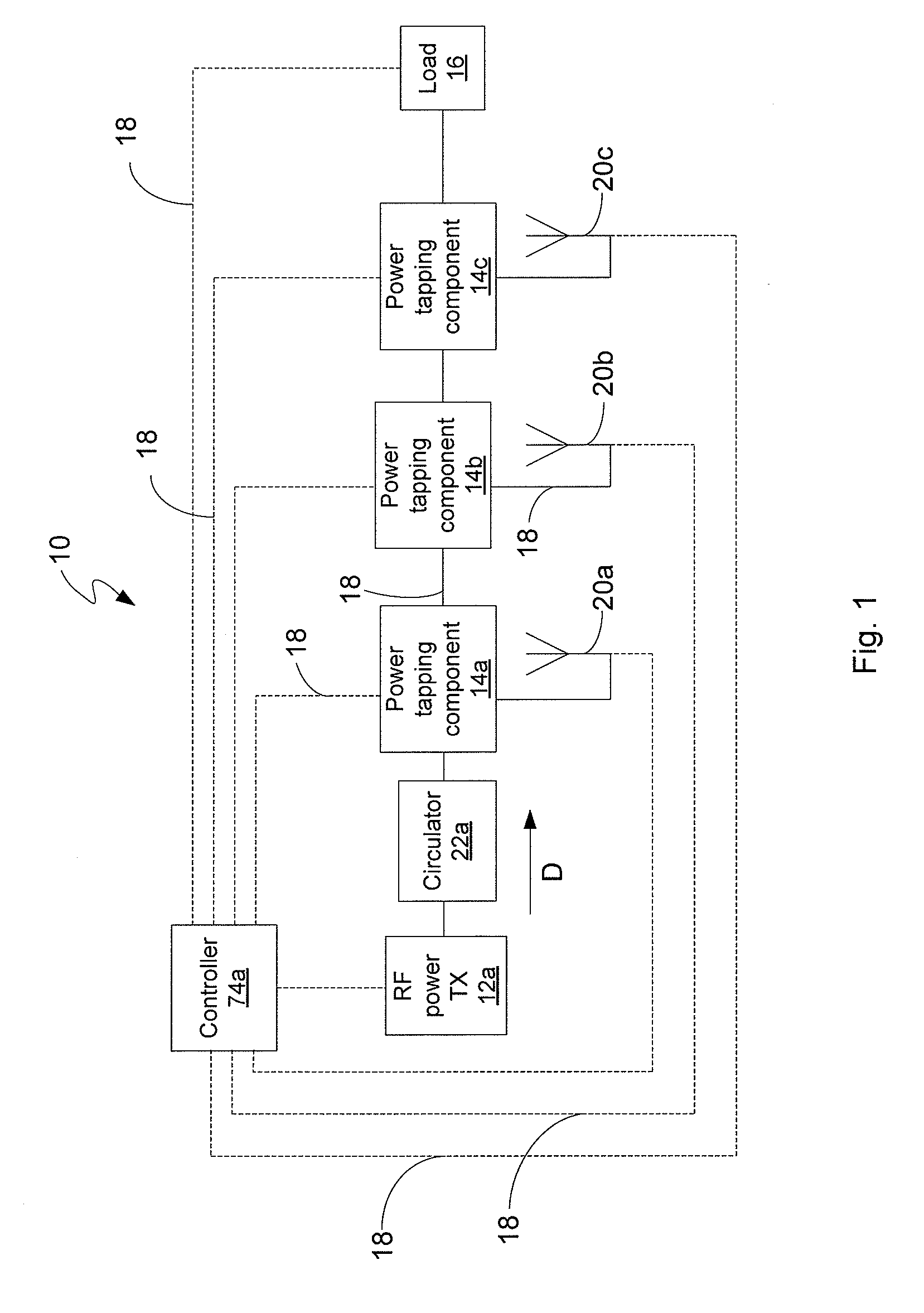
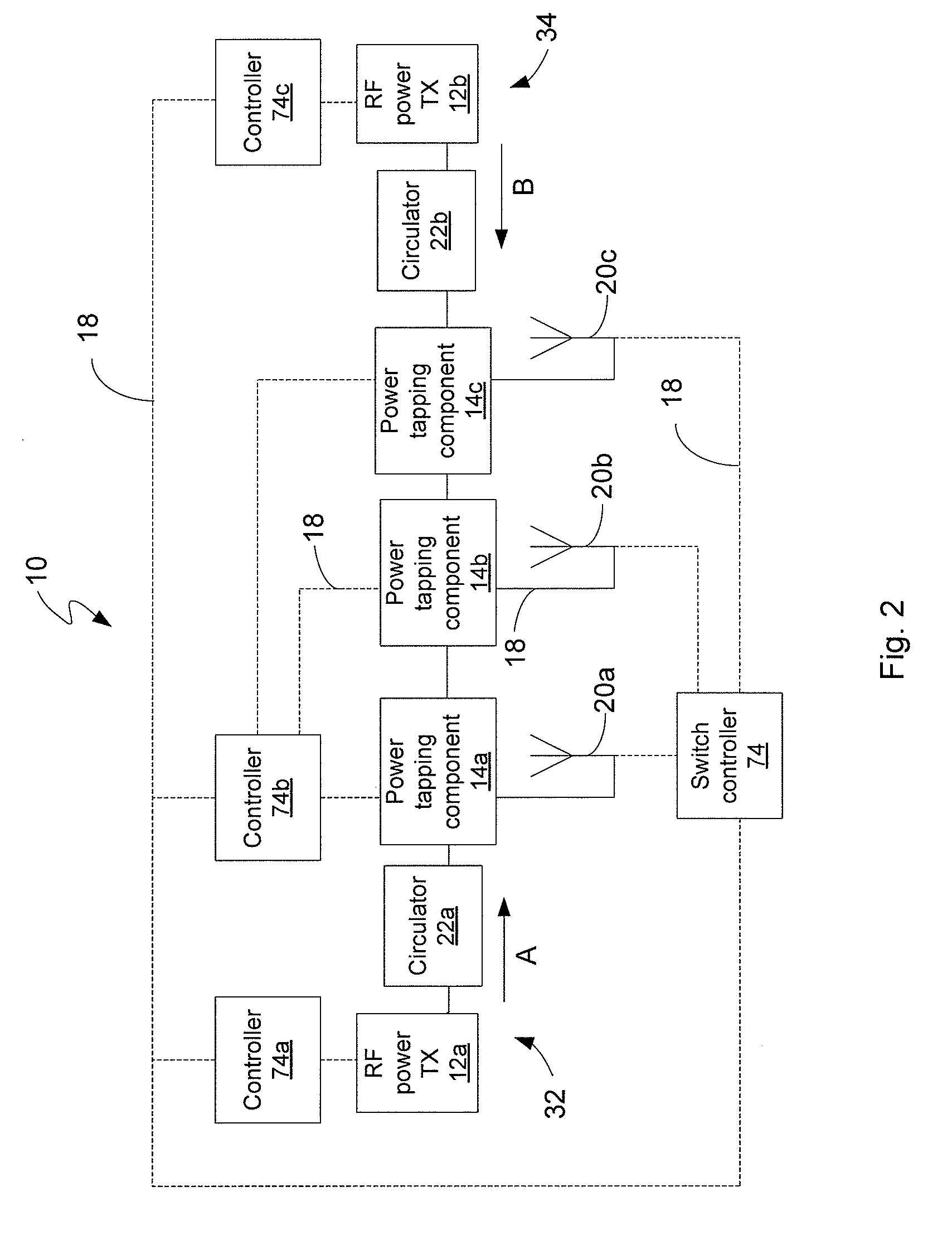
ActiveUS7639994B2Improve scalabilityIncrease the lengthResonant long antennasRepeater circuitsElectric powerRadio frequency power transmission
Disclosed is an RF power transmission network. The network includes at least one RF power transmitter, at least one power tapping component, and at least one load. The at least one RF power transmitter, the at least one power tapping component, and the at least one load are connected in series. The RF power transmitter sends power through the network. The power is radiated from the network to be received by a device to be charged, re-charged, or directly powered by the power.
Owner:POWERCAST
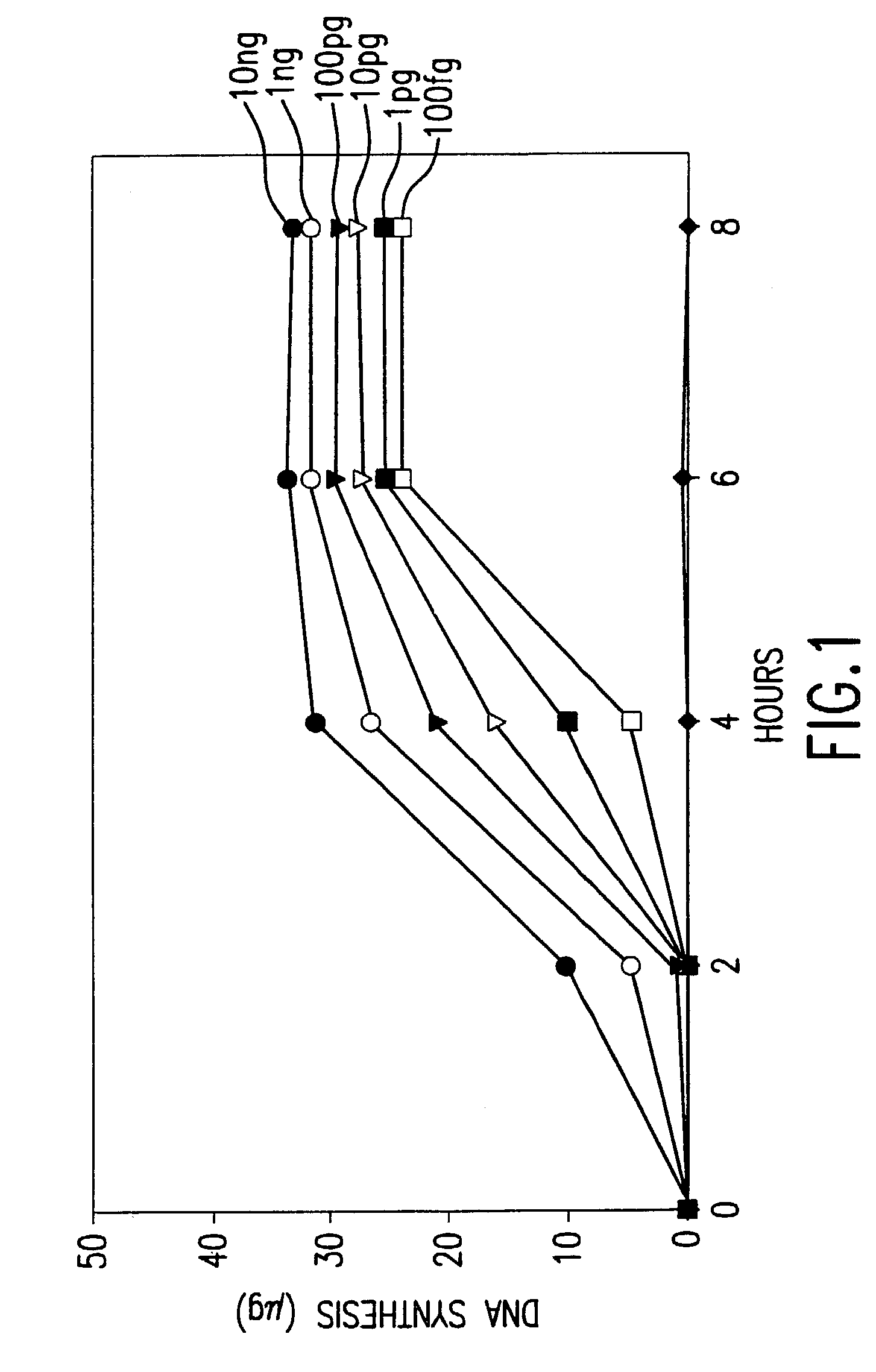
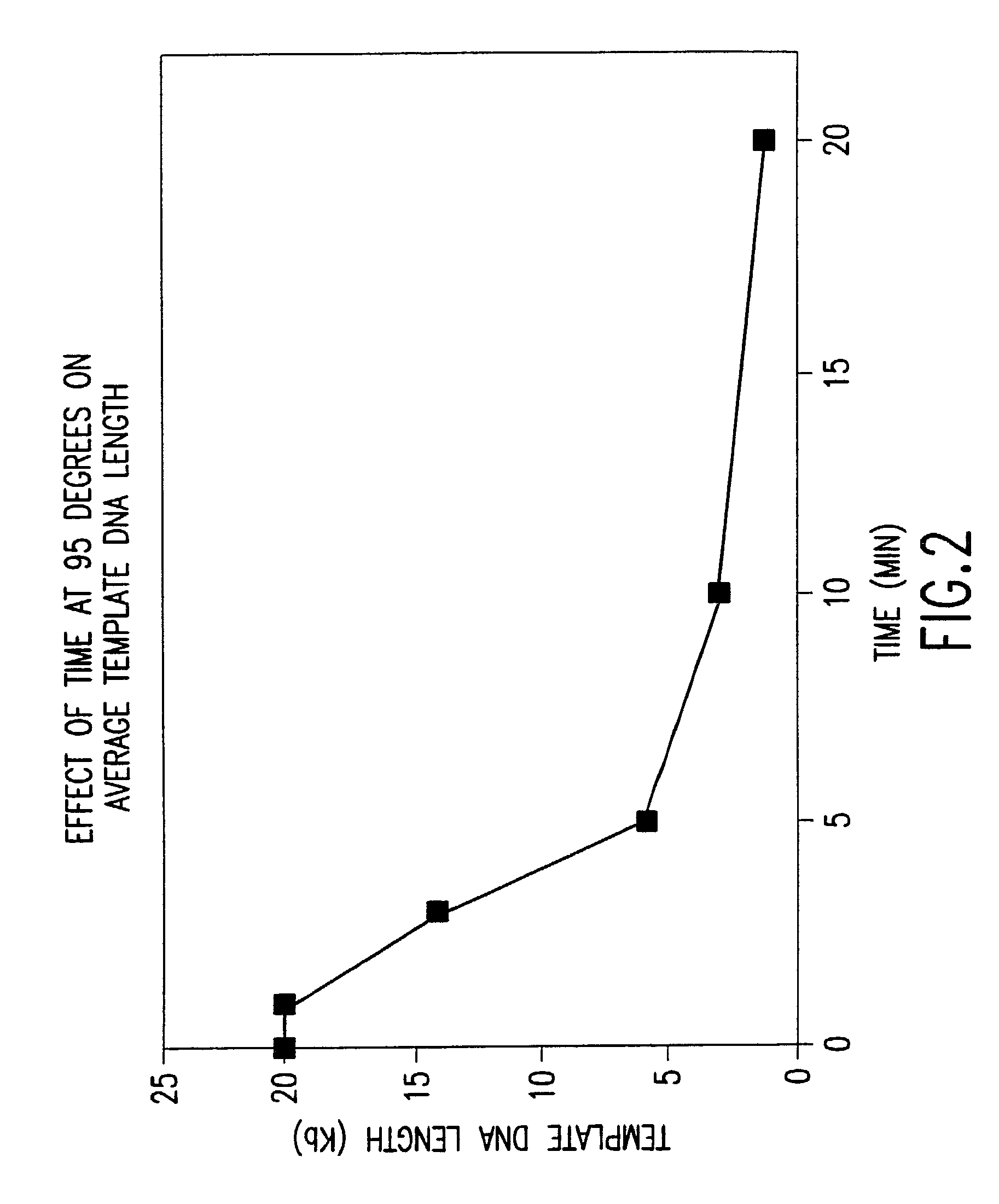
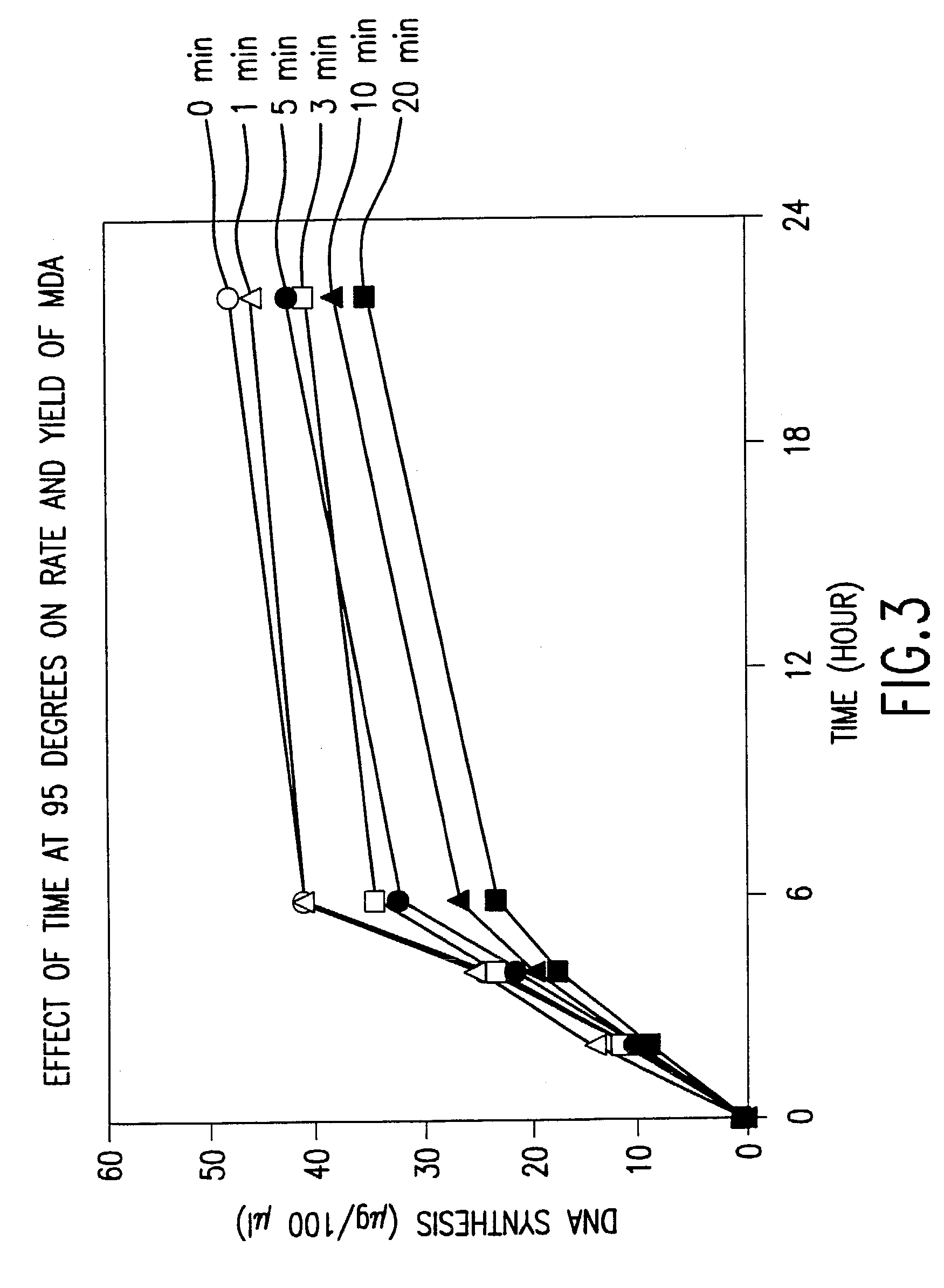
Method for nucleic acid amplification that results in low amplification bias
InactiveUS7297485B2Consistent outputQuick buildMicrobiological testing/measurementFermentationNucleic acid sequencingAmplification bias
Disclosed are compositions and methods for amplification of nucleic acid sequences of interest. It has been discovered that amplification reactions can produce amplification products of high quality, such as low amplification bias, if performed on an amount of nucleic acid at or over a threshold amount and / or on nucleic acids at or below a threshold concentration. The threshold amount and concentration can vary depending on the nature and source of the nucleic acids to be amplified and the type of amplification reaction employed. Disclosed is a method of determining the threshold amount and / or threshold concentration of nucleic acids that can be used with nucleic acid samples of interest in amplification reactions of interest. Because amplification reactions can produce high quality amplification products, such as low bias amplification products, below the threshold amount and / or concentration of nucleic acid, such below-threshold amounts and / or concentrations can be used in amplification reactions.
Owner:QIAGEN GMBH
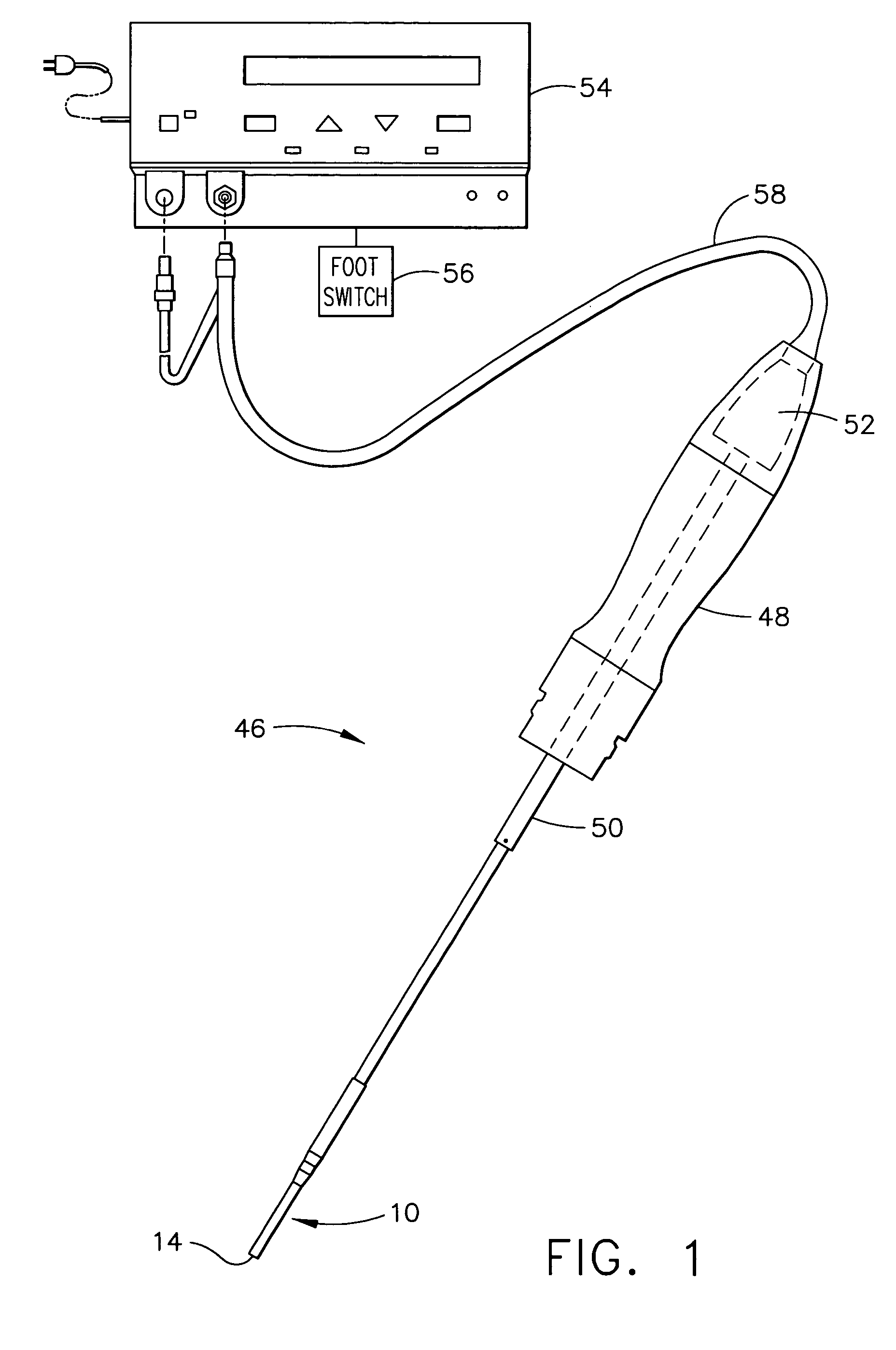
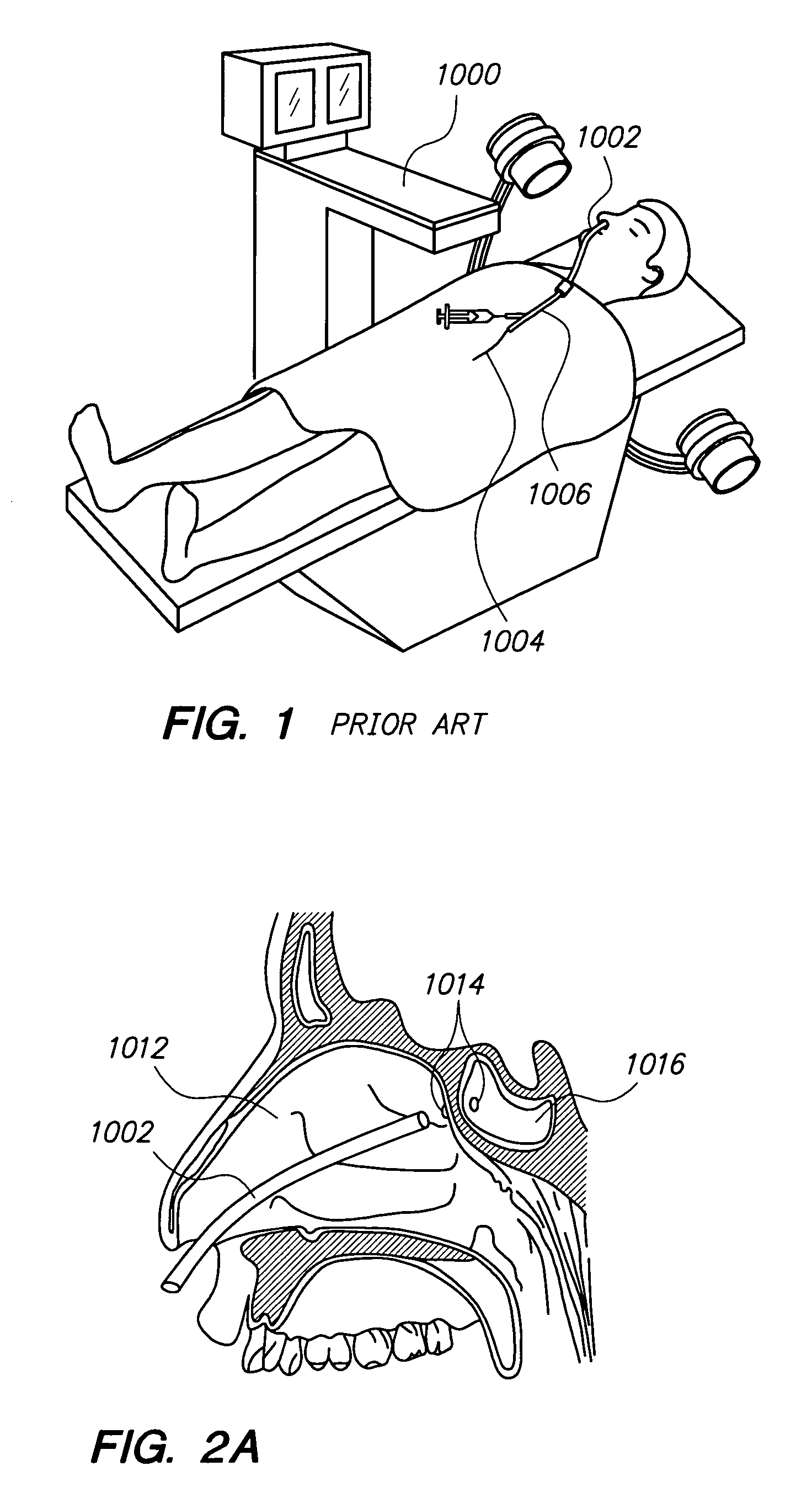
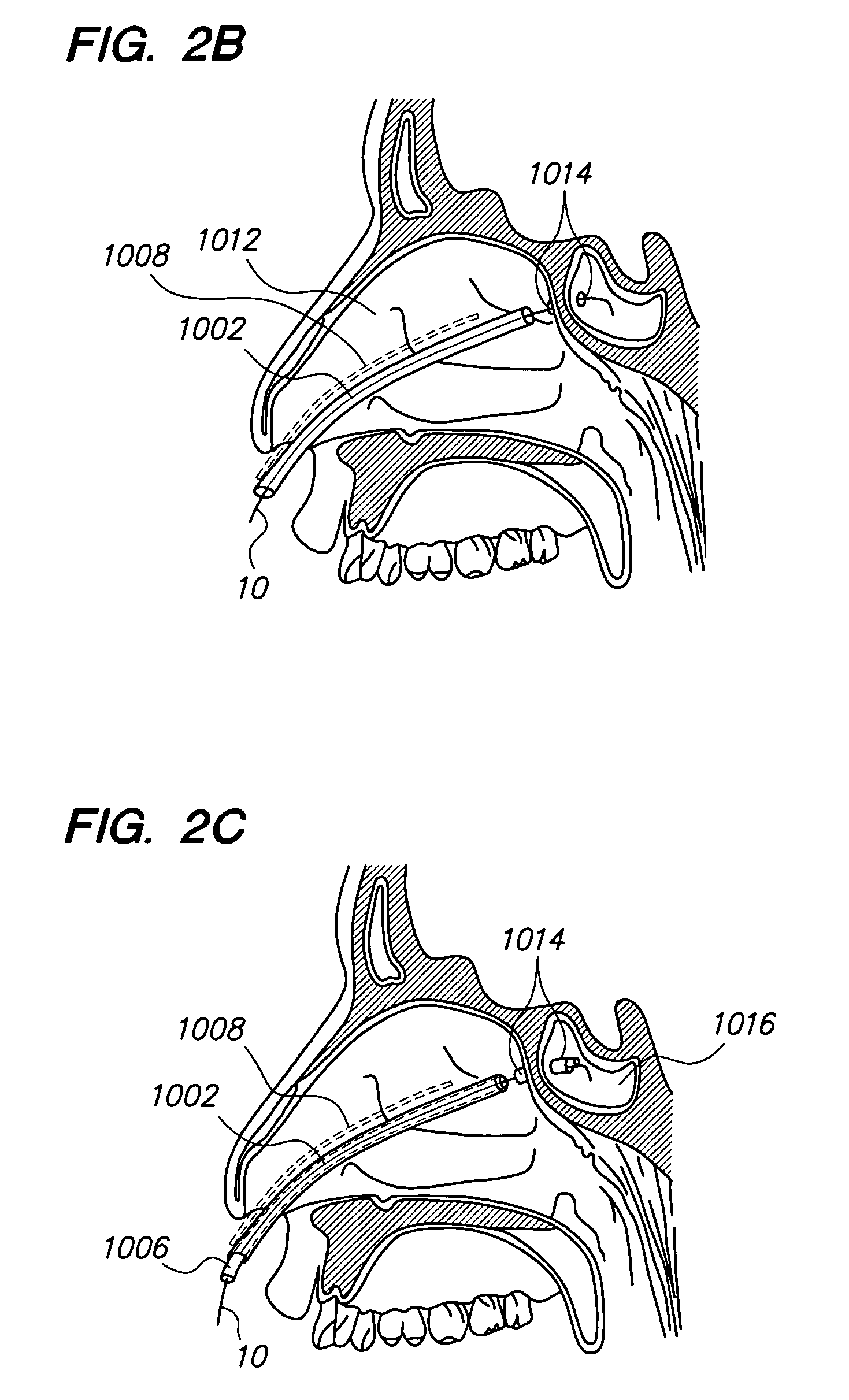
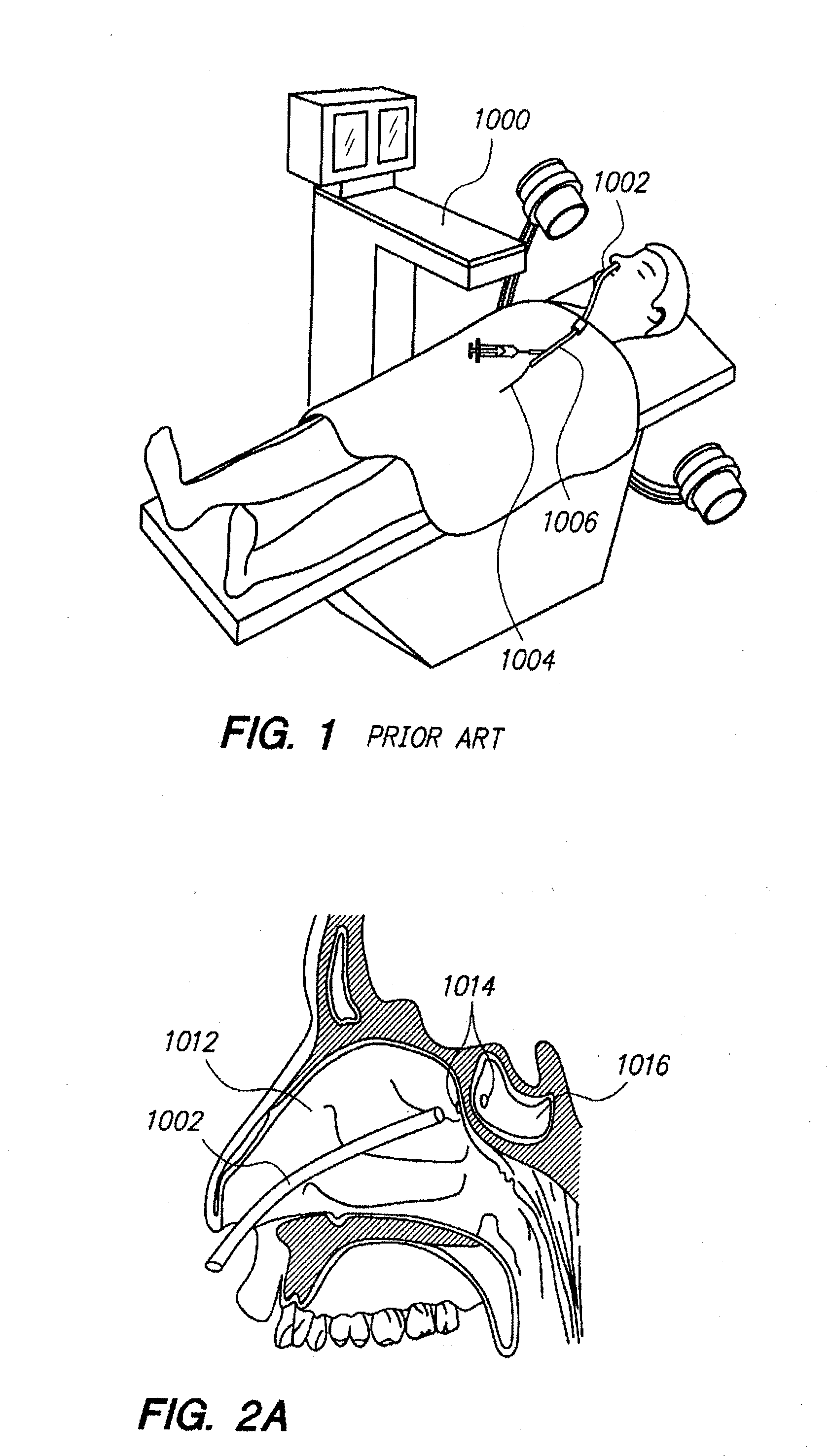
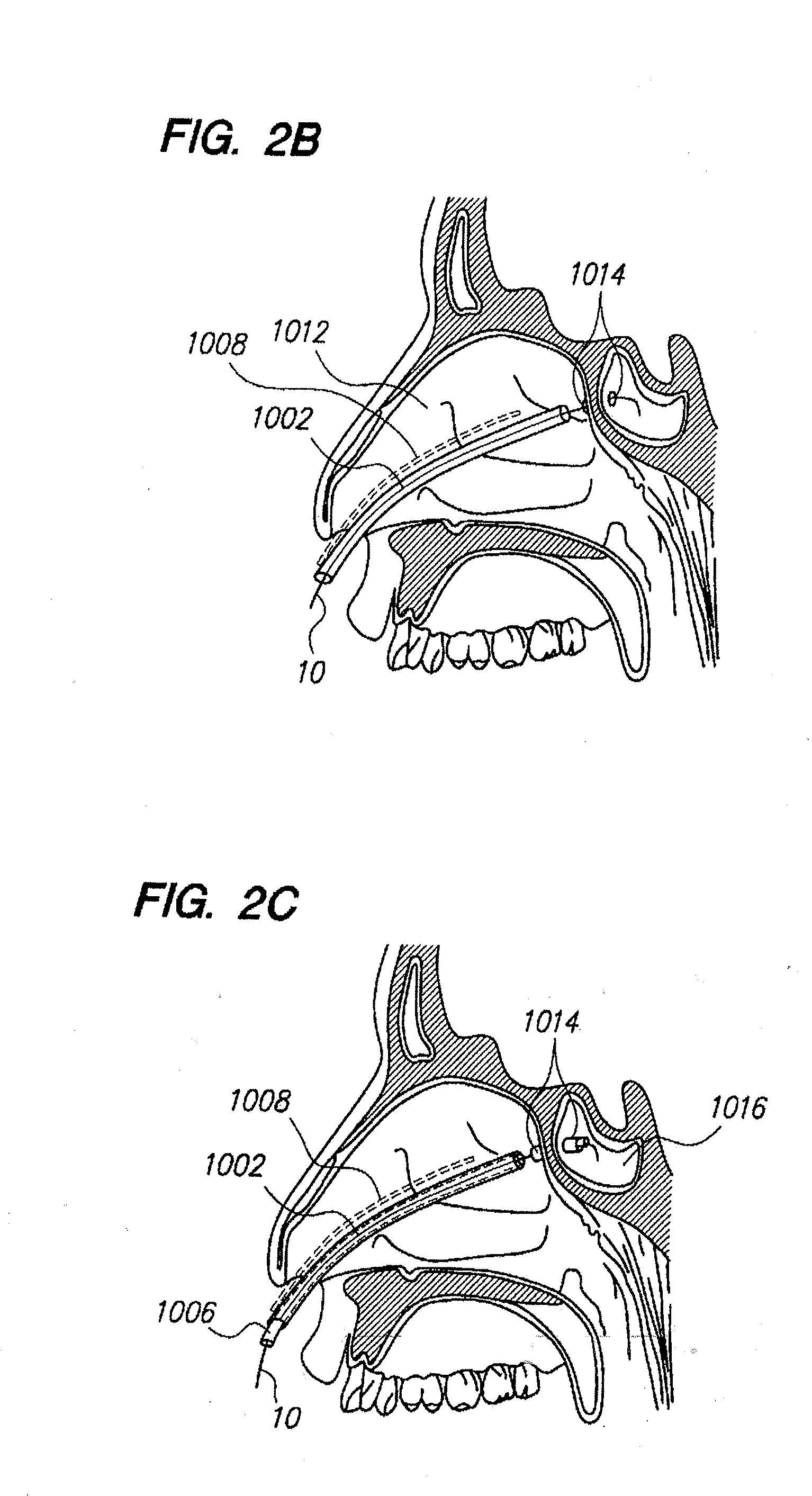
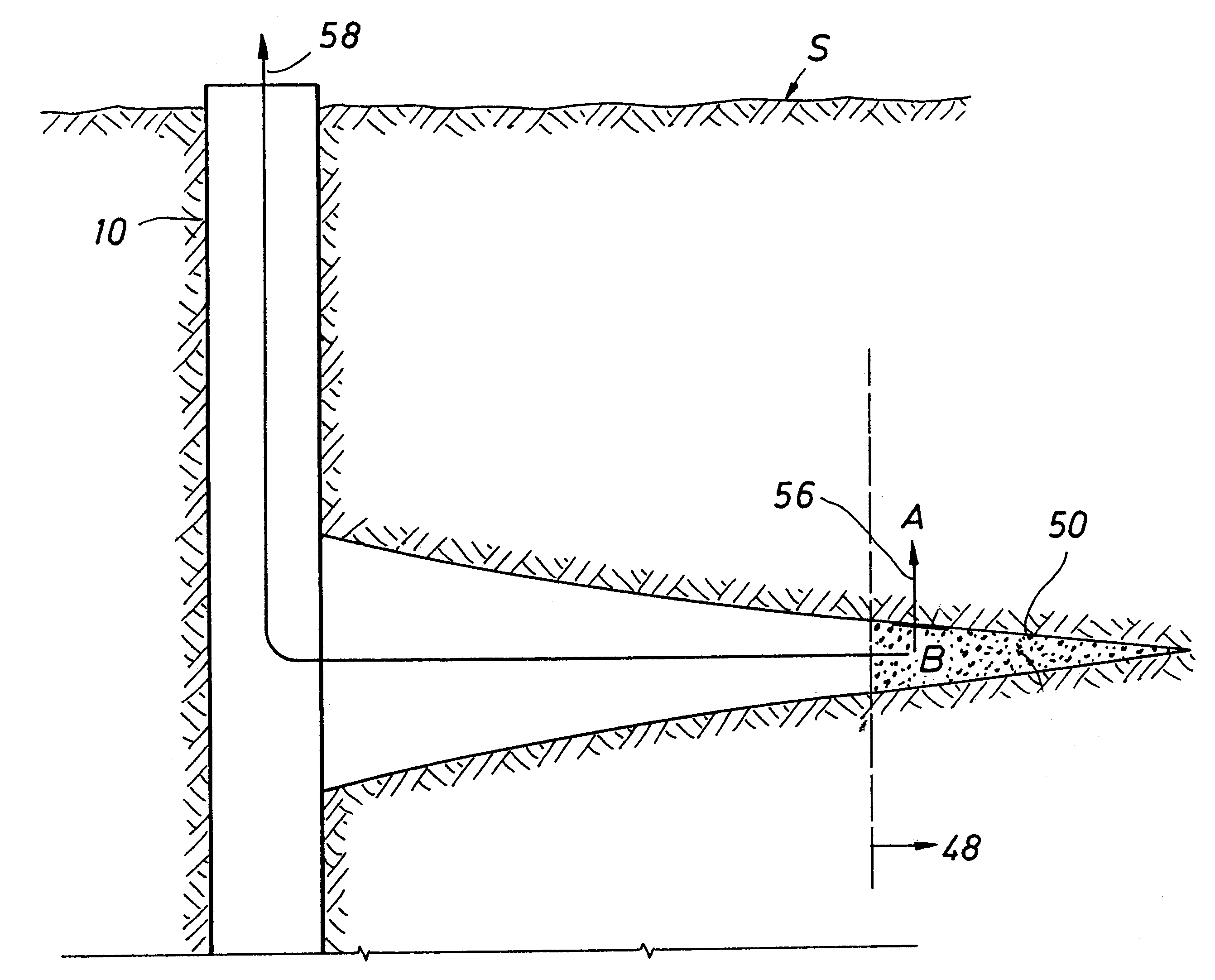
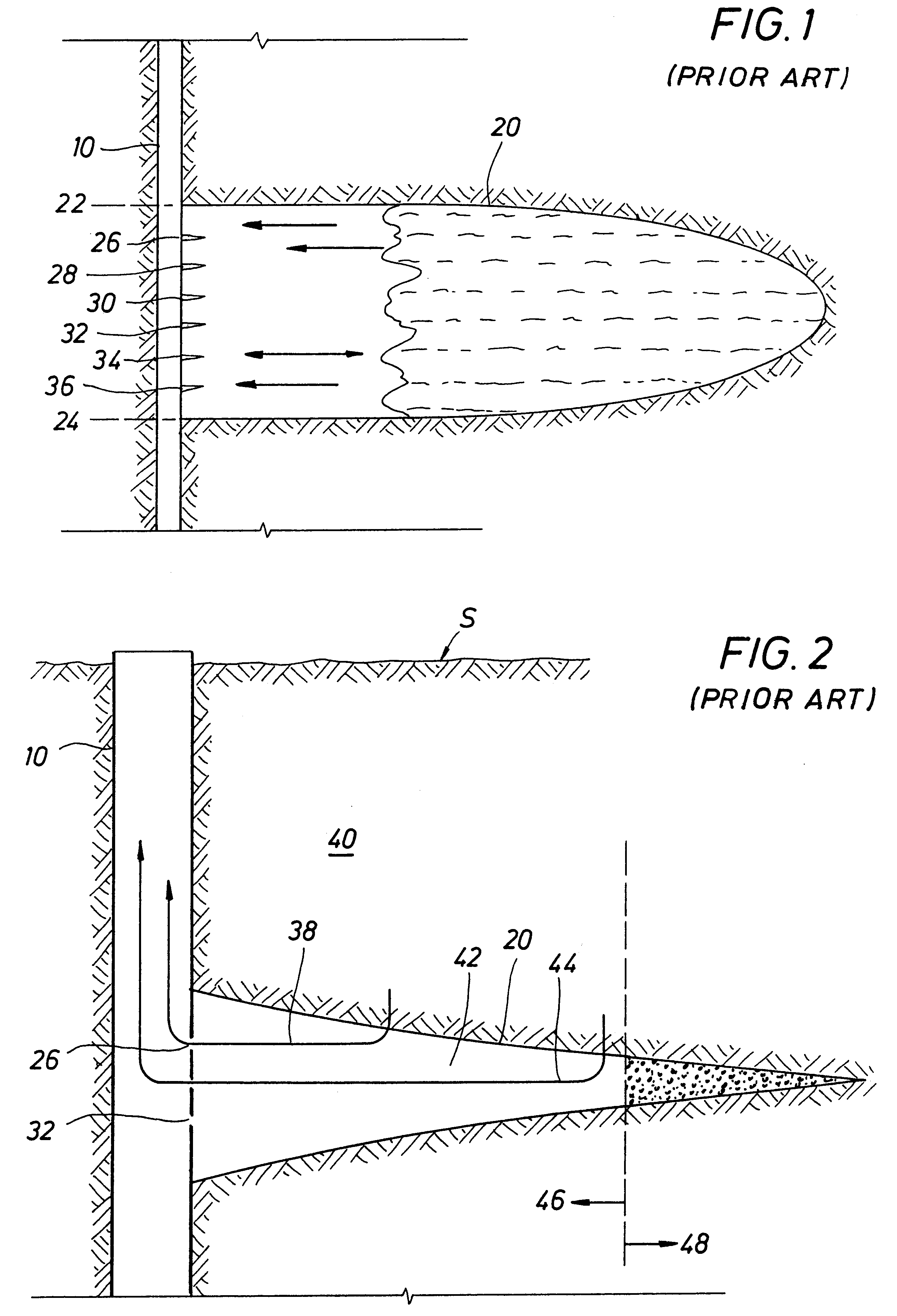
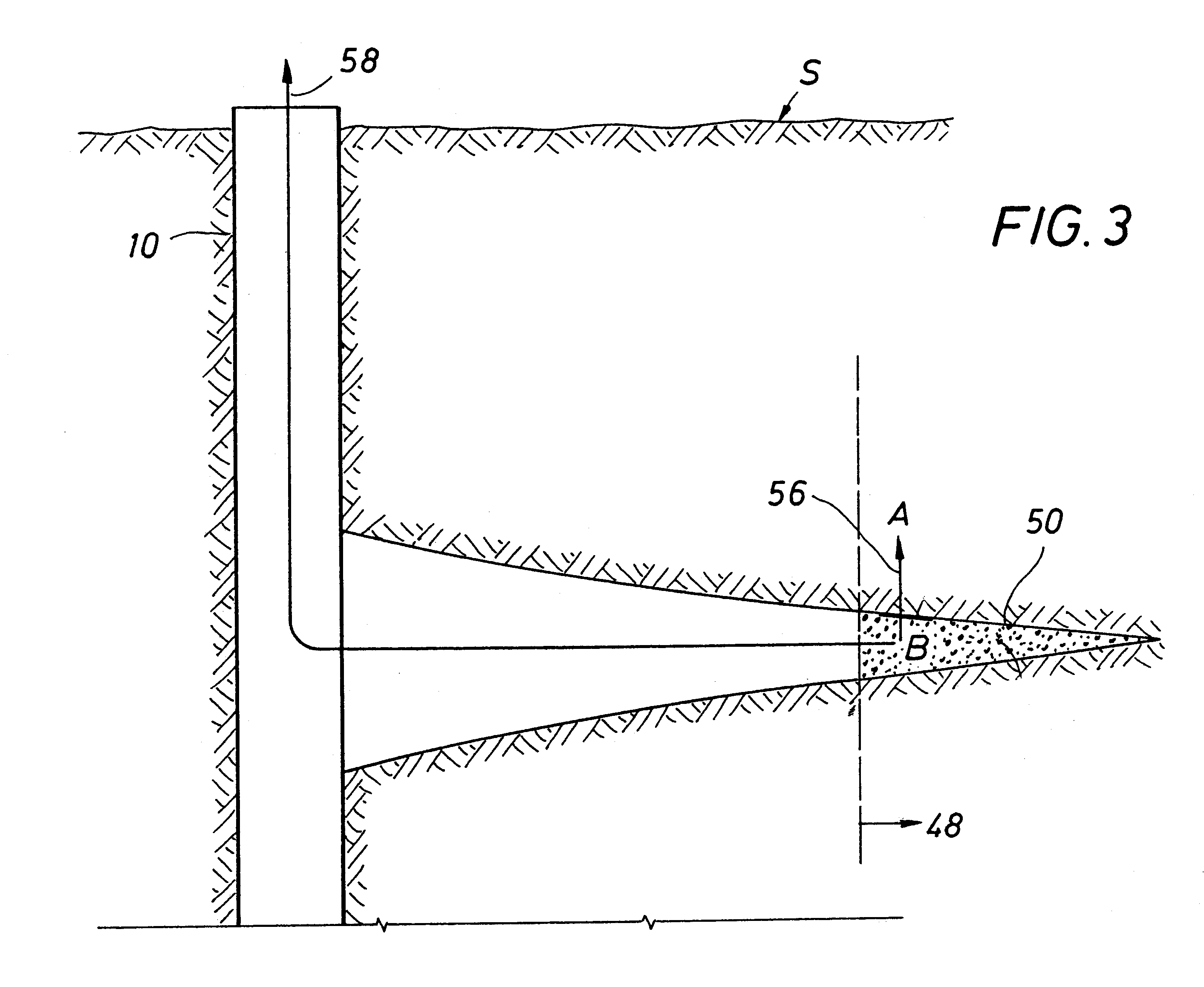
Methods and devices for facilitating visualization in a surgical environment
ActiveUS7559925B2Avoid insufficient lengthSufficient lightingMedical devicesEndoscopesMedicineLight emitting device
Owner:ACCLARENT INC
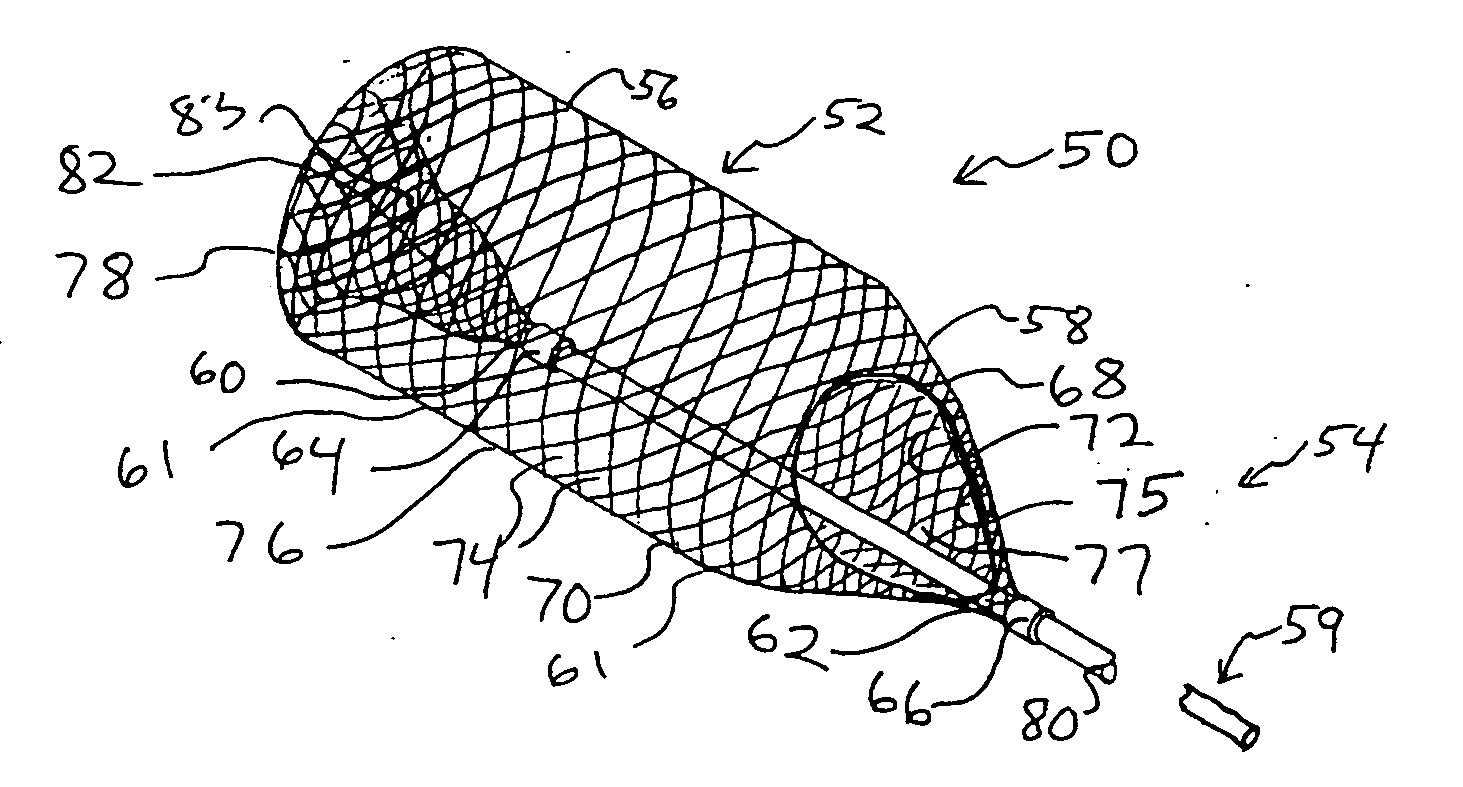
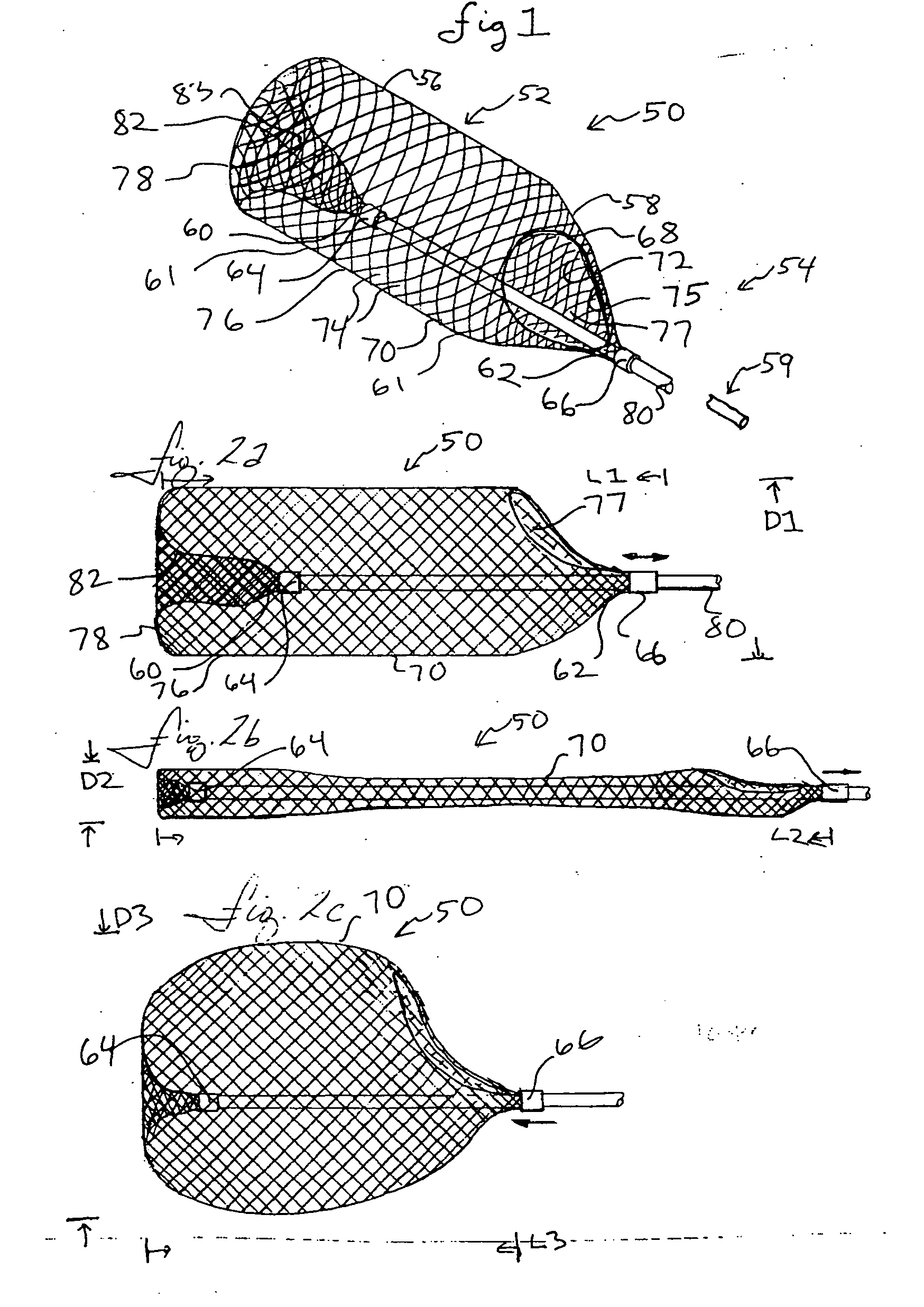
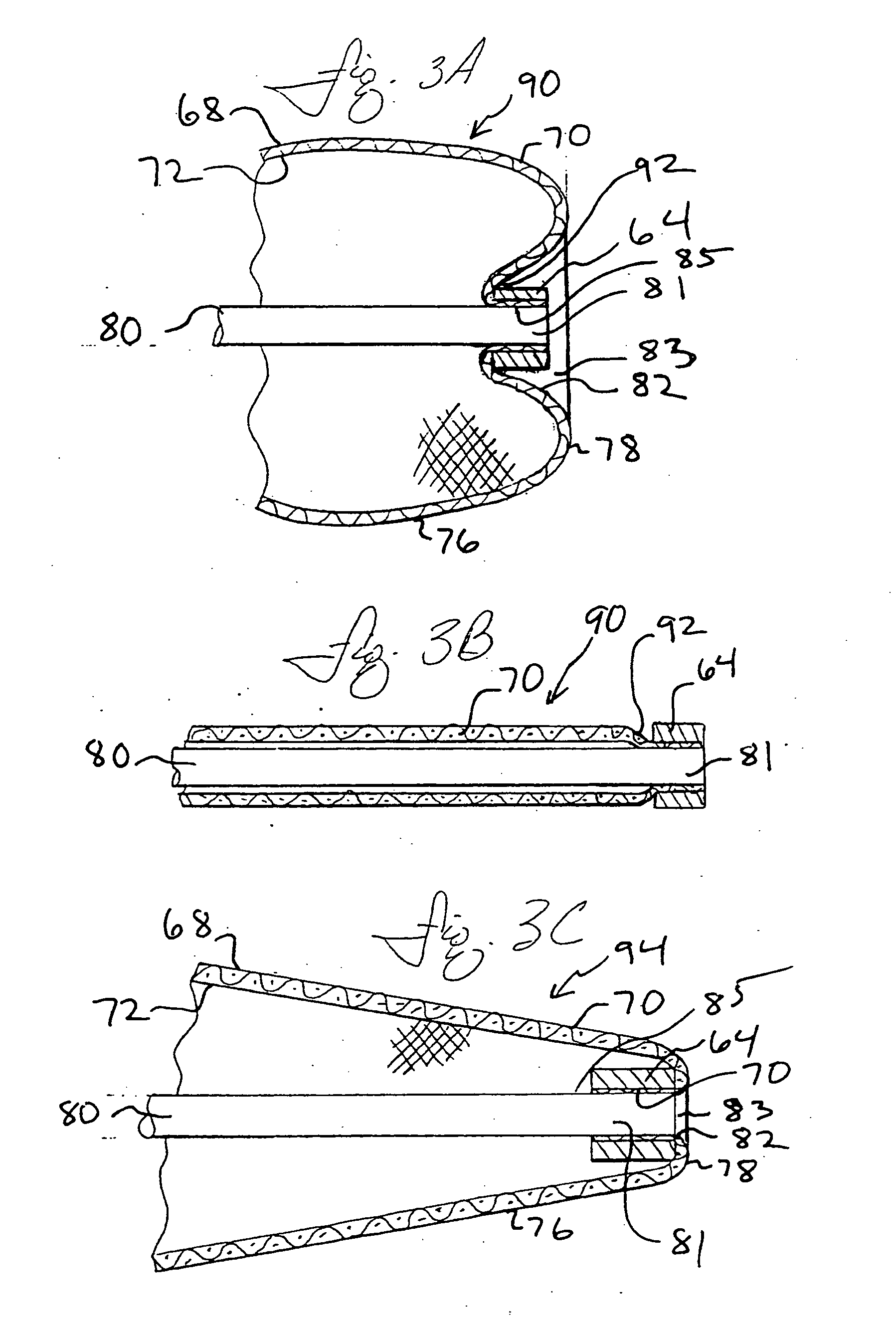
Everted filter device
ActiveUS20050283186A1Effective coverageProvide protectionBronchoscopesLaryngoscopesVascular filterThrombus
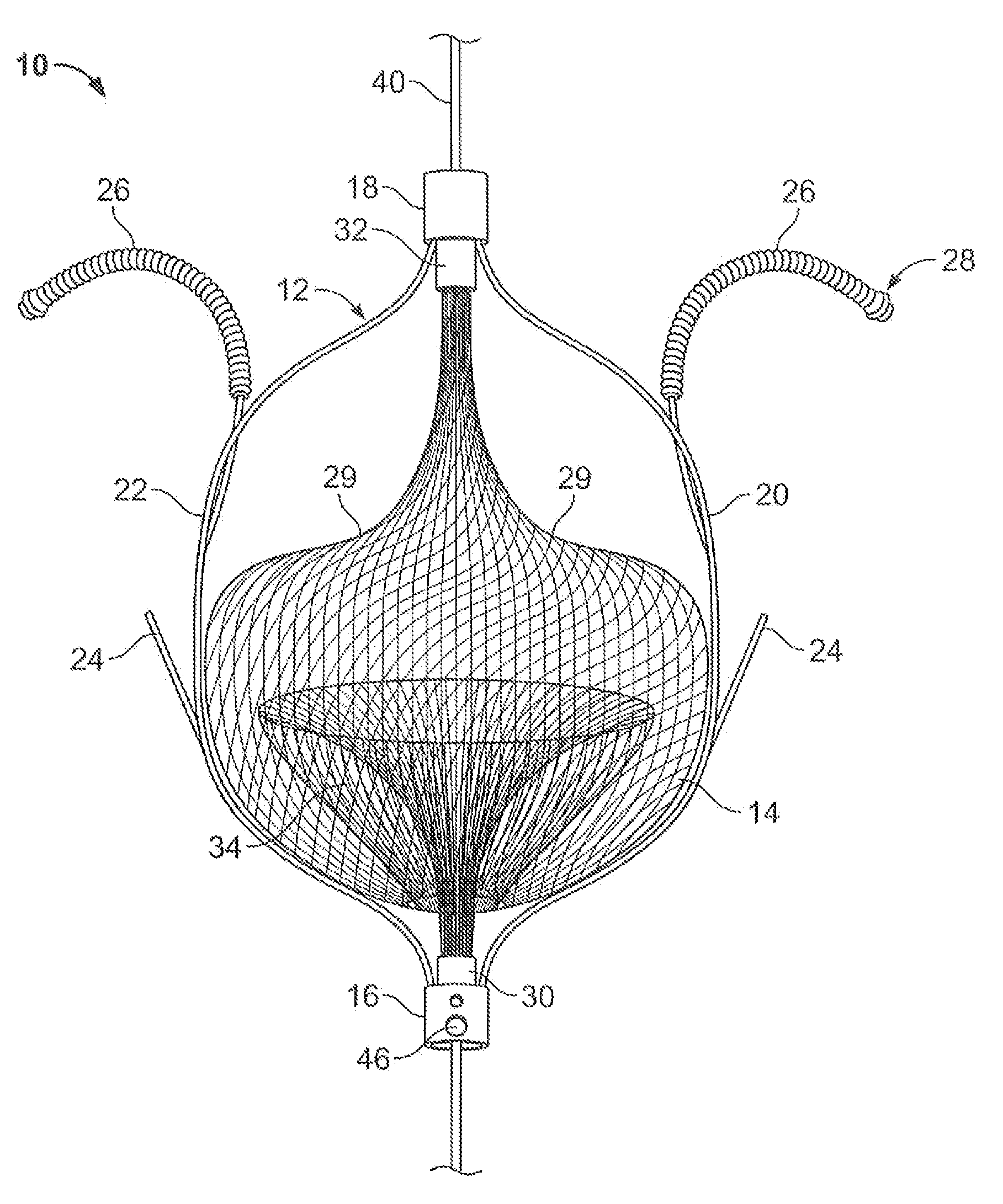
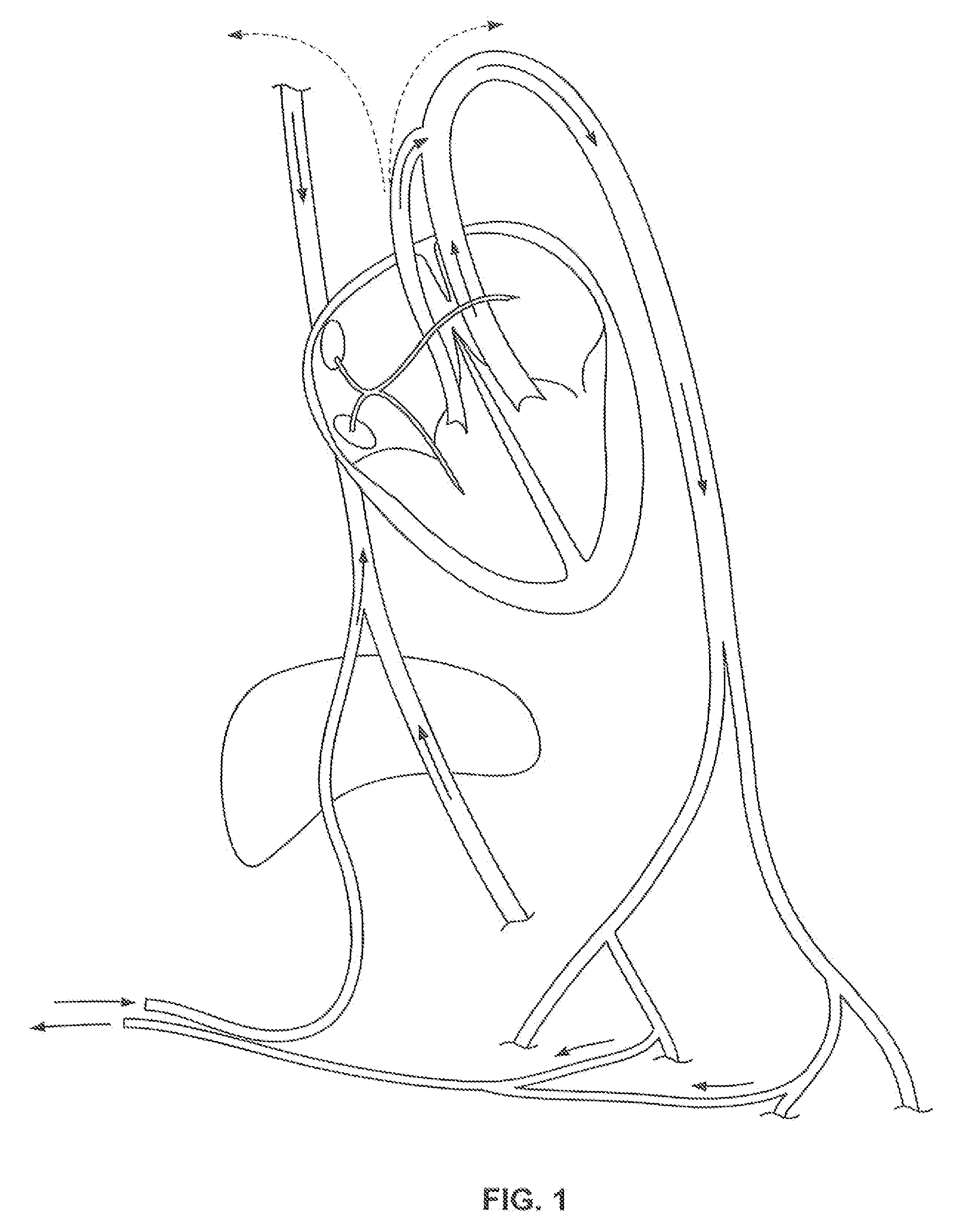
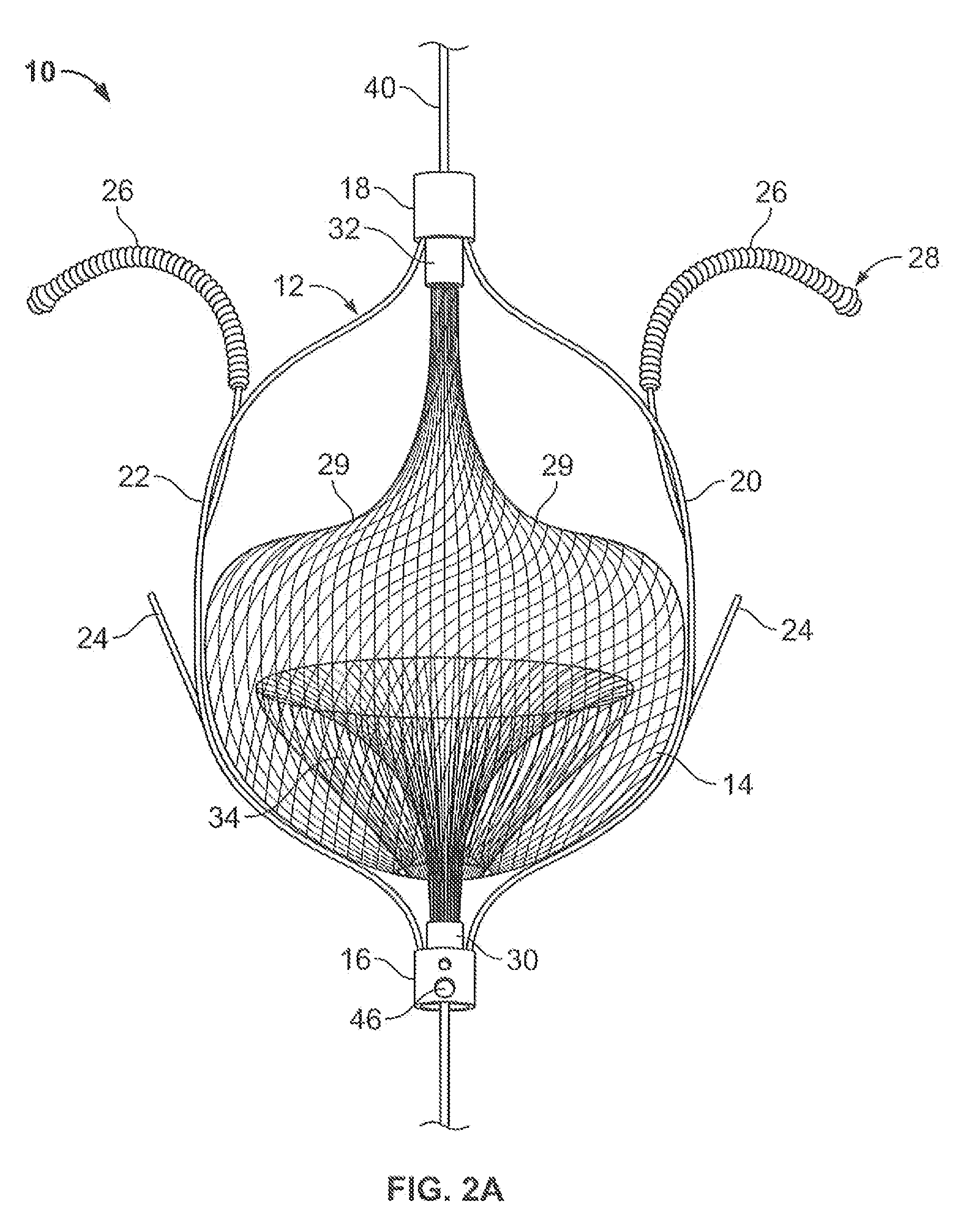
Everting filter devices and methods for using the devices, including using the devices as intra-vascular filters to filter thrombus, emboli, and plaque fragments from blood vessels. The filter devices include a filter body nominally tubular in shape and having a large proximal opening. The filter body can extend from a proximal first end region distally over the non-everted exterior surface of the filter, further extending distally to a distal-most region, then converging inwardly and extending proximally toward the filter second end region, forming a distal everted cavity. The degree of eversion of the filter can be controlled by varying the distance between the filter first end region near the proximal opening and the closed second end region. Bringing the filter first and second end regions closer together can bring filter material previously on the non-everted filter exterior to occupy the distal-most region. The everting process can also bring filter material previously in the distal-most position further into the distal everted cavity. The filter devices can be used to remove filtrate from body vessels, with the filtrate eventually occluding the distal-most region. The filter can then be further everted, bringing fresh, unoccluded filter material into place to provide additional filter capacity. Some everting filters have the capability of switching between occluding and filtering modes of operation, thereby allowing a treating physician to postpone the decision to use filtering or occluding devices until well after insertion of the device into the patient's body.
Owner:TYCO HEALTHCARE GRP LP
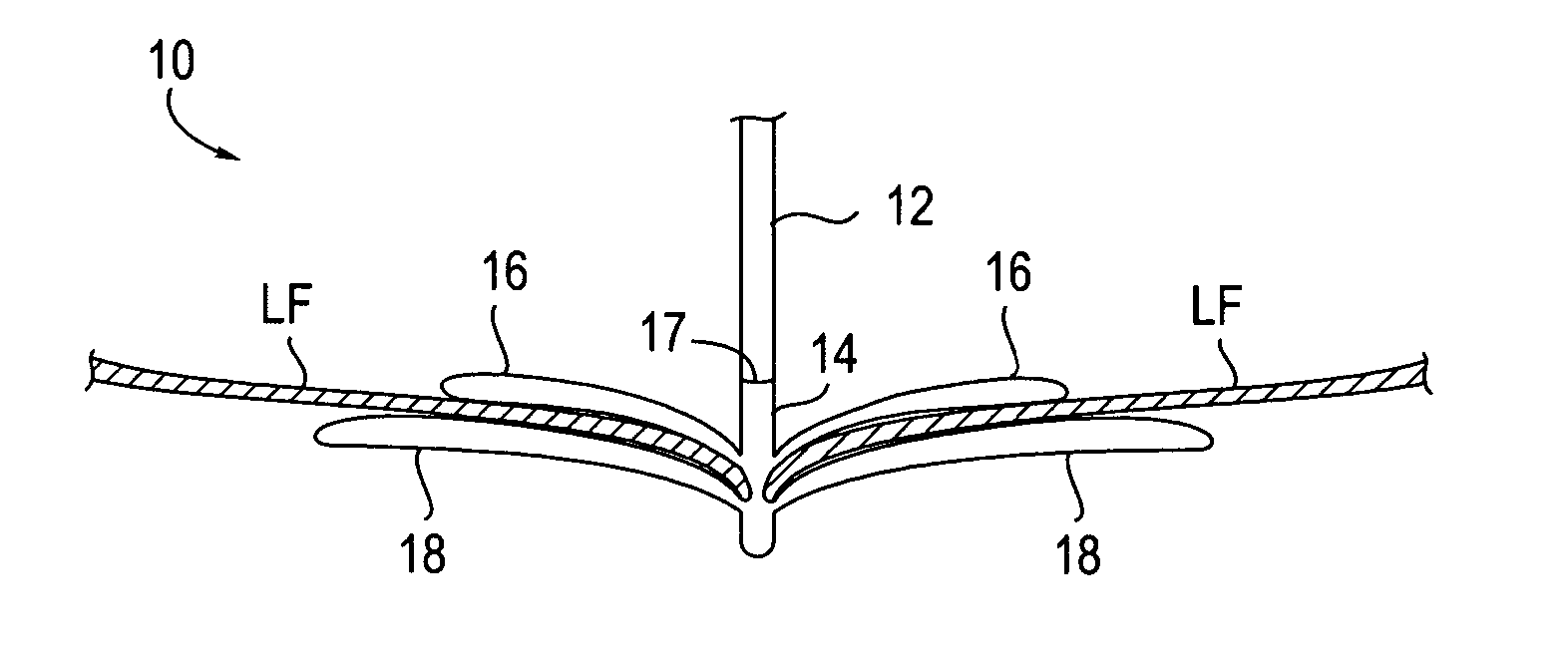
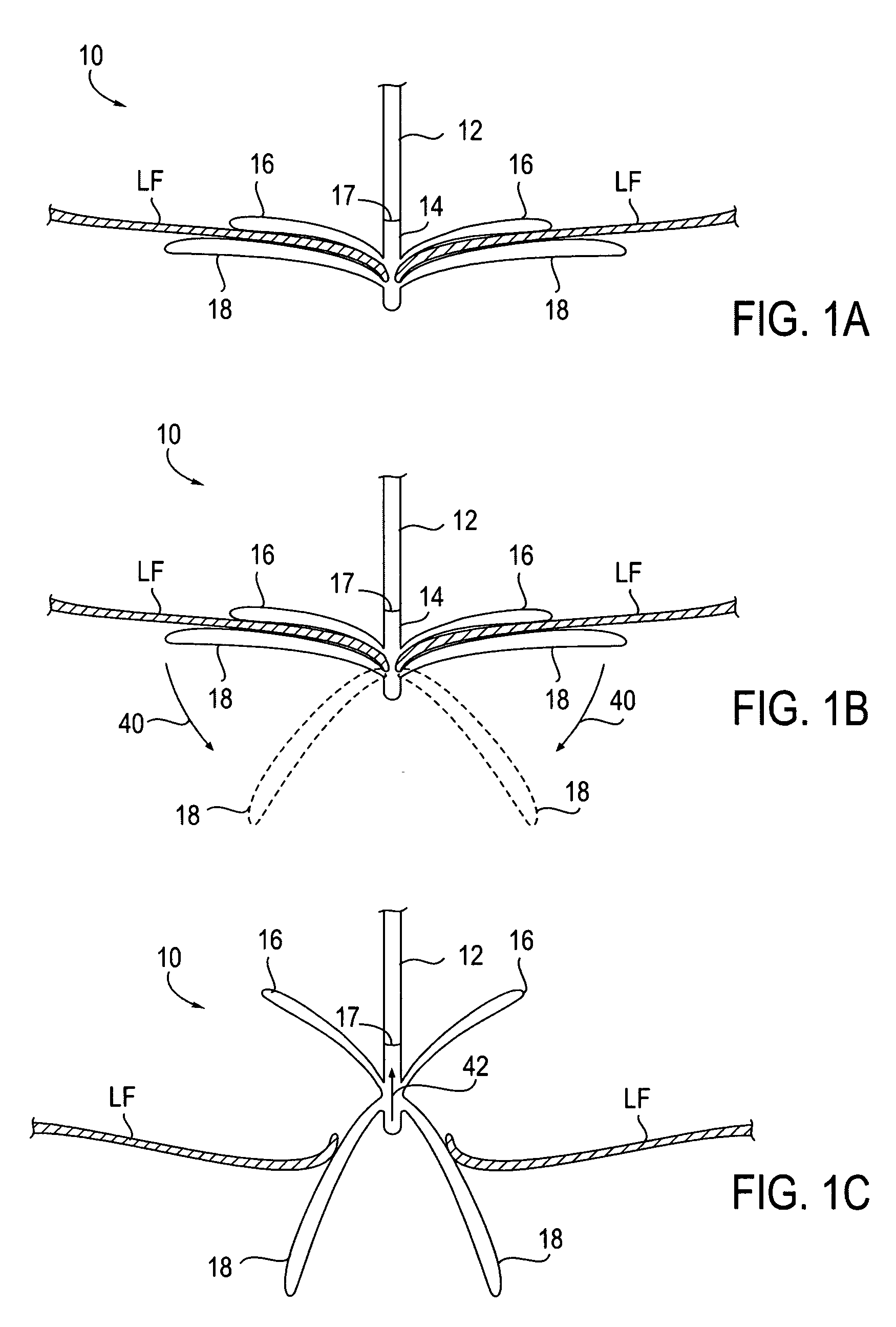
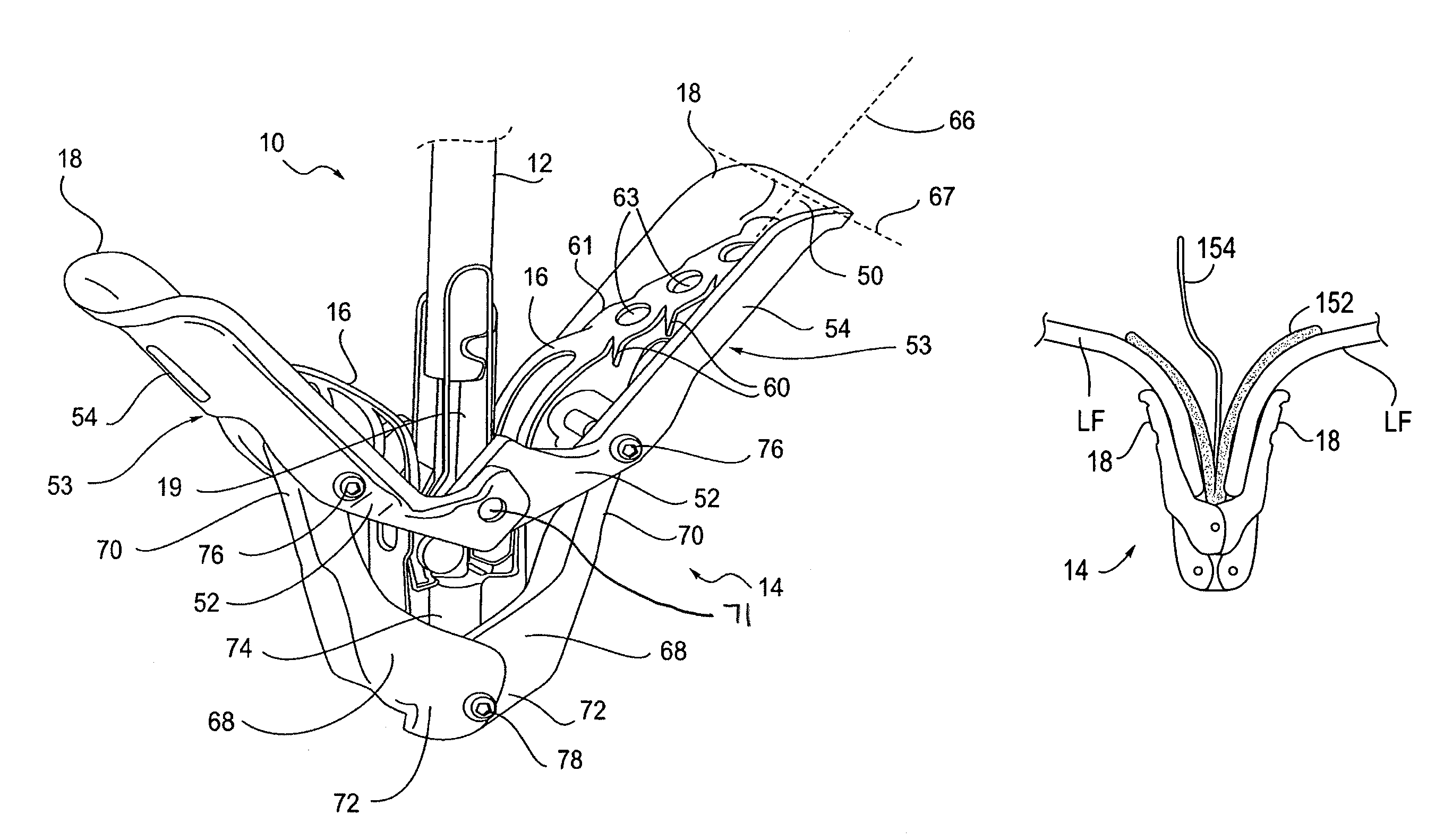
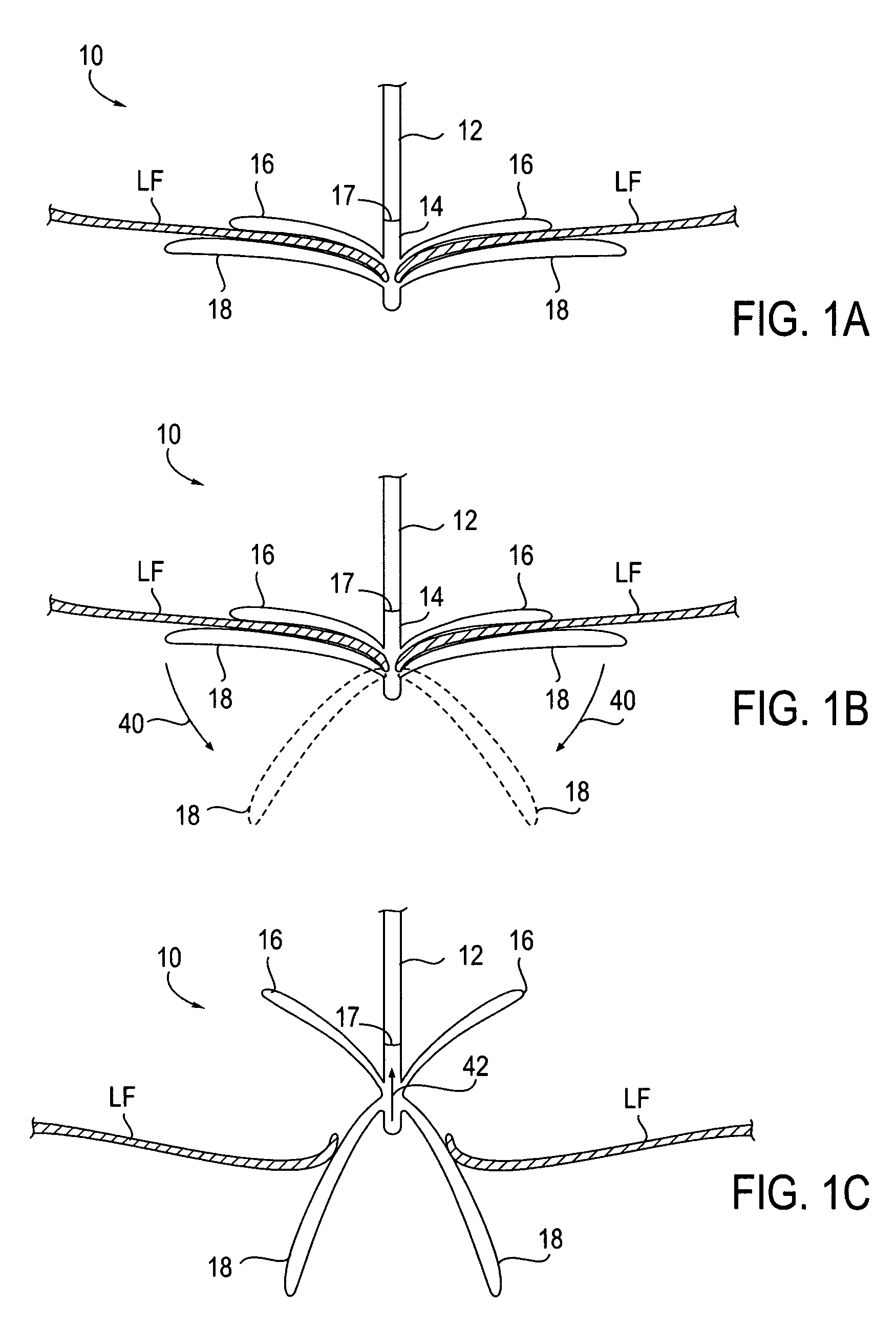
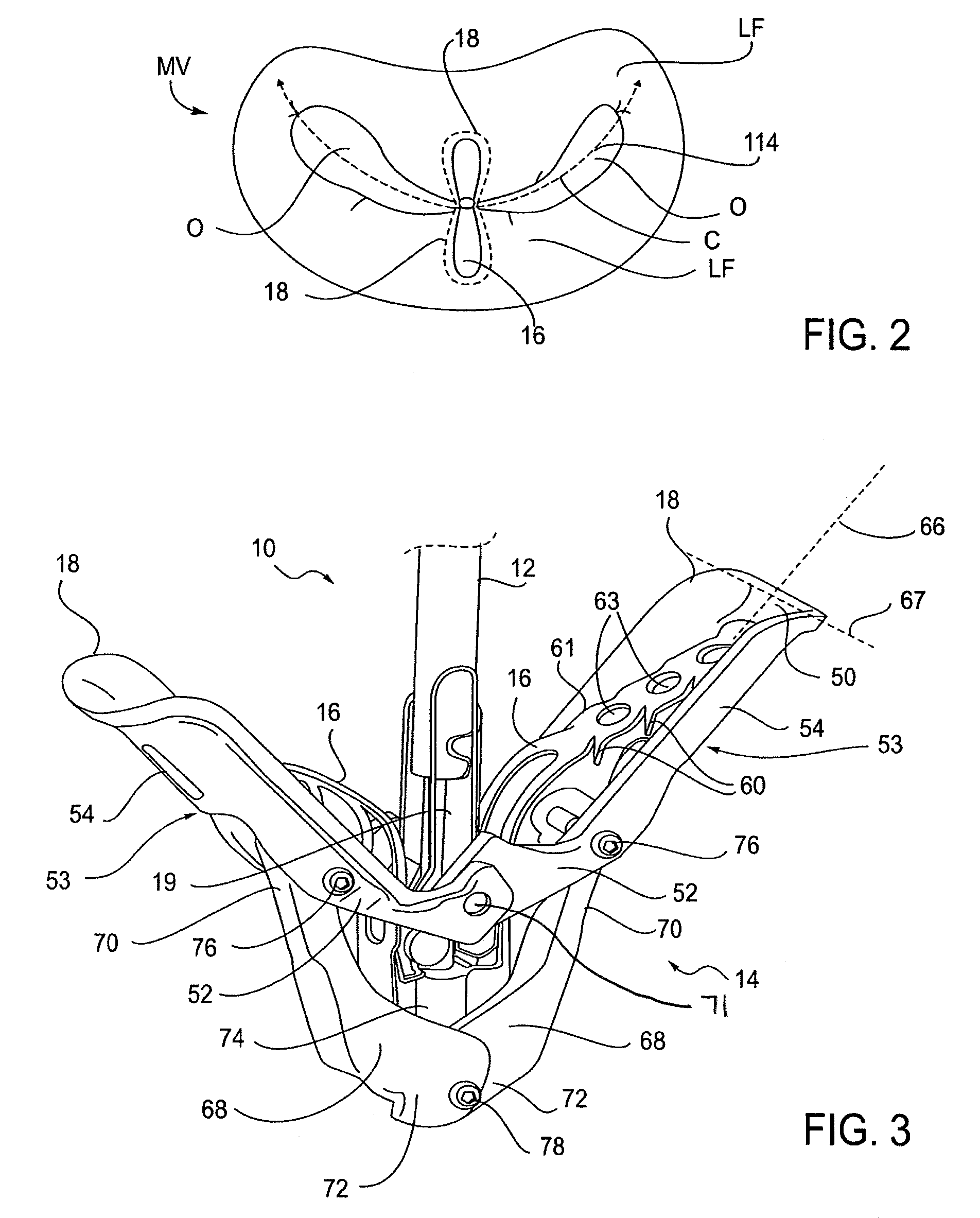
Fixation devices for variation in engagement of tissue
InactiveUS20060089671A1Robust graspReduce refluxHeart valvesSurgical veterinaryBiomedical engineeringBlood vessel
Owner:EVALVE
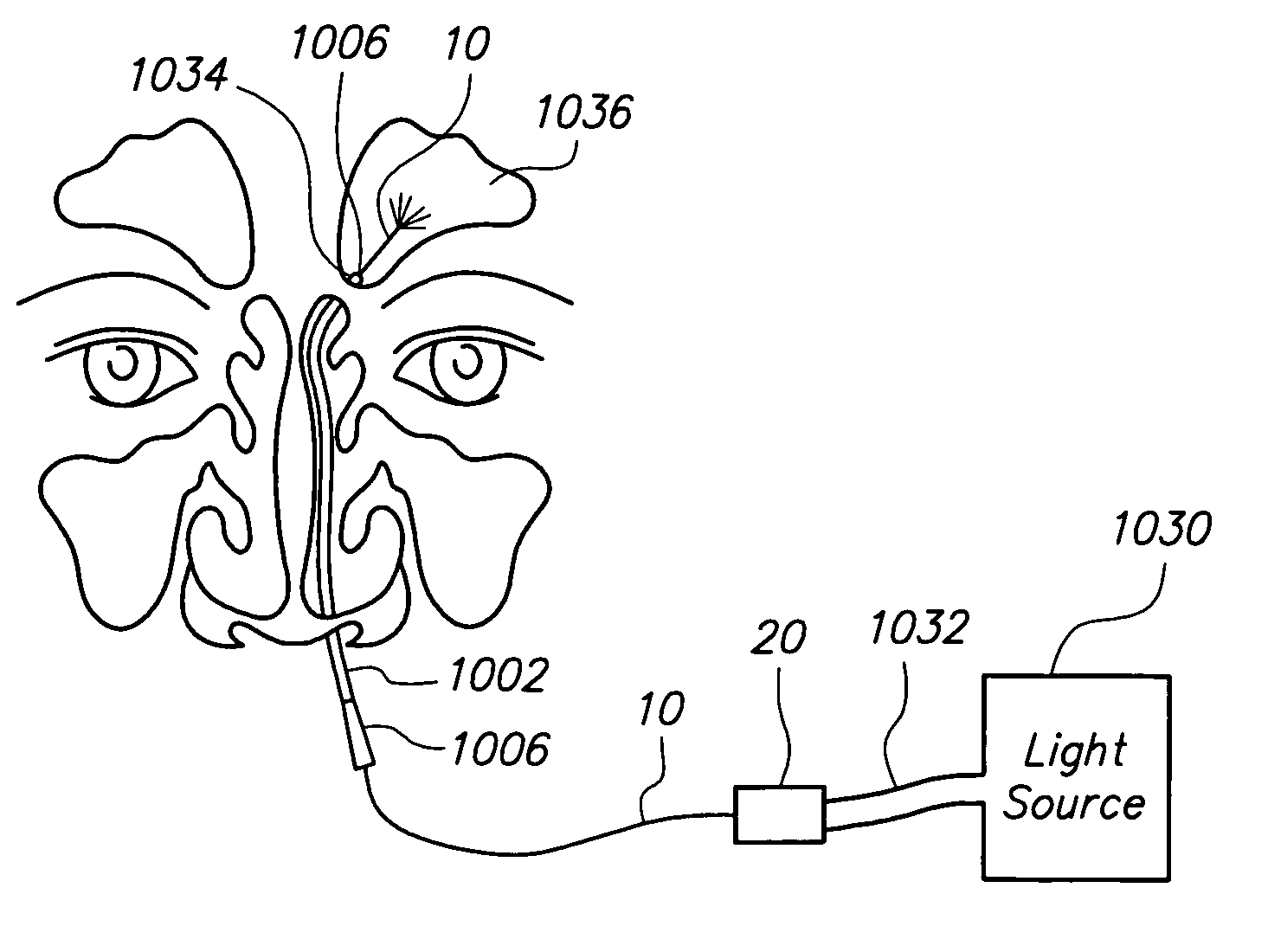
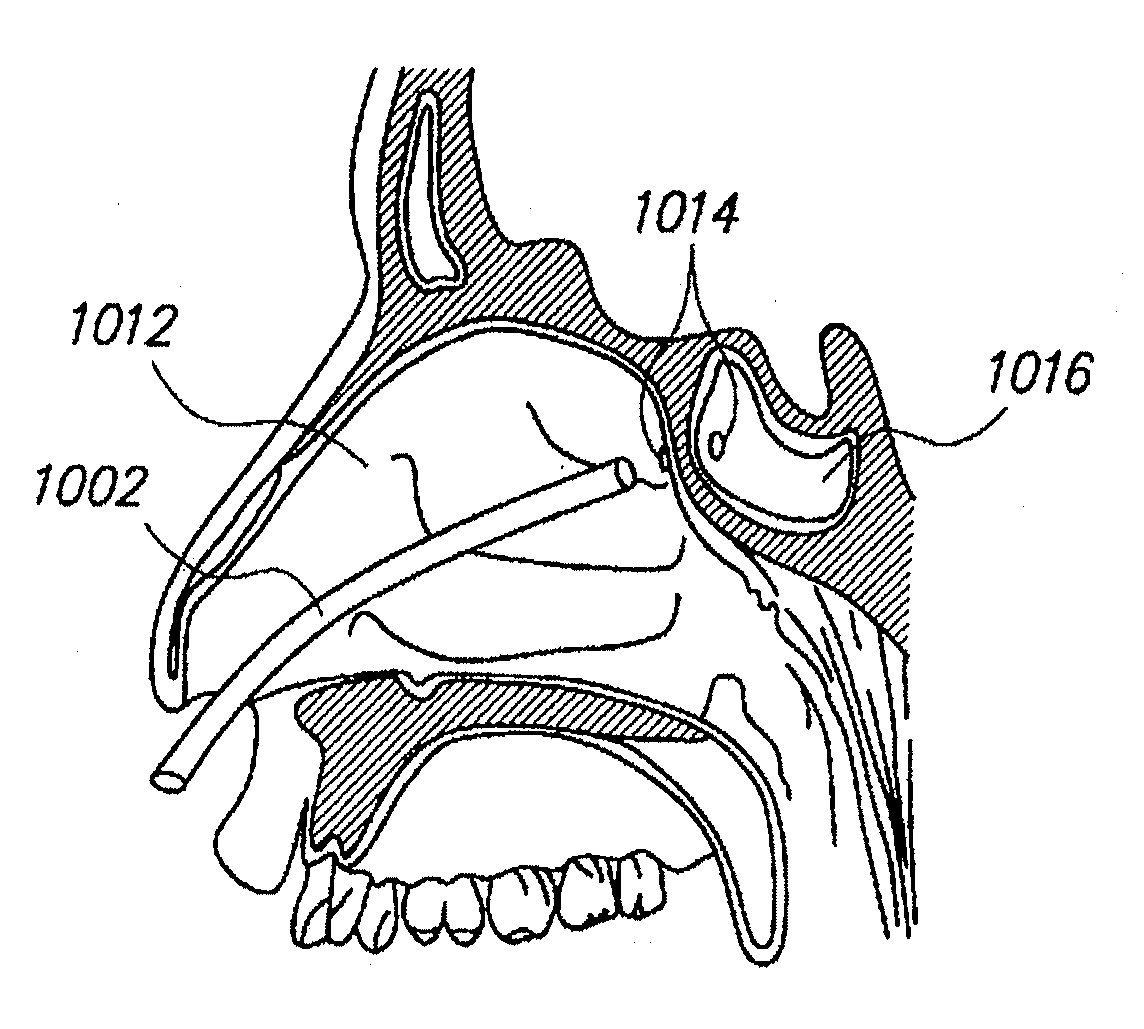
Sinus illumination lightwire device
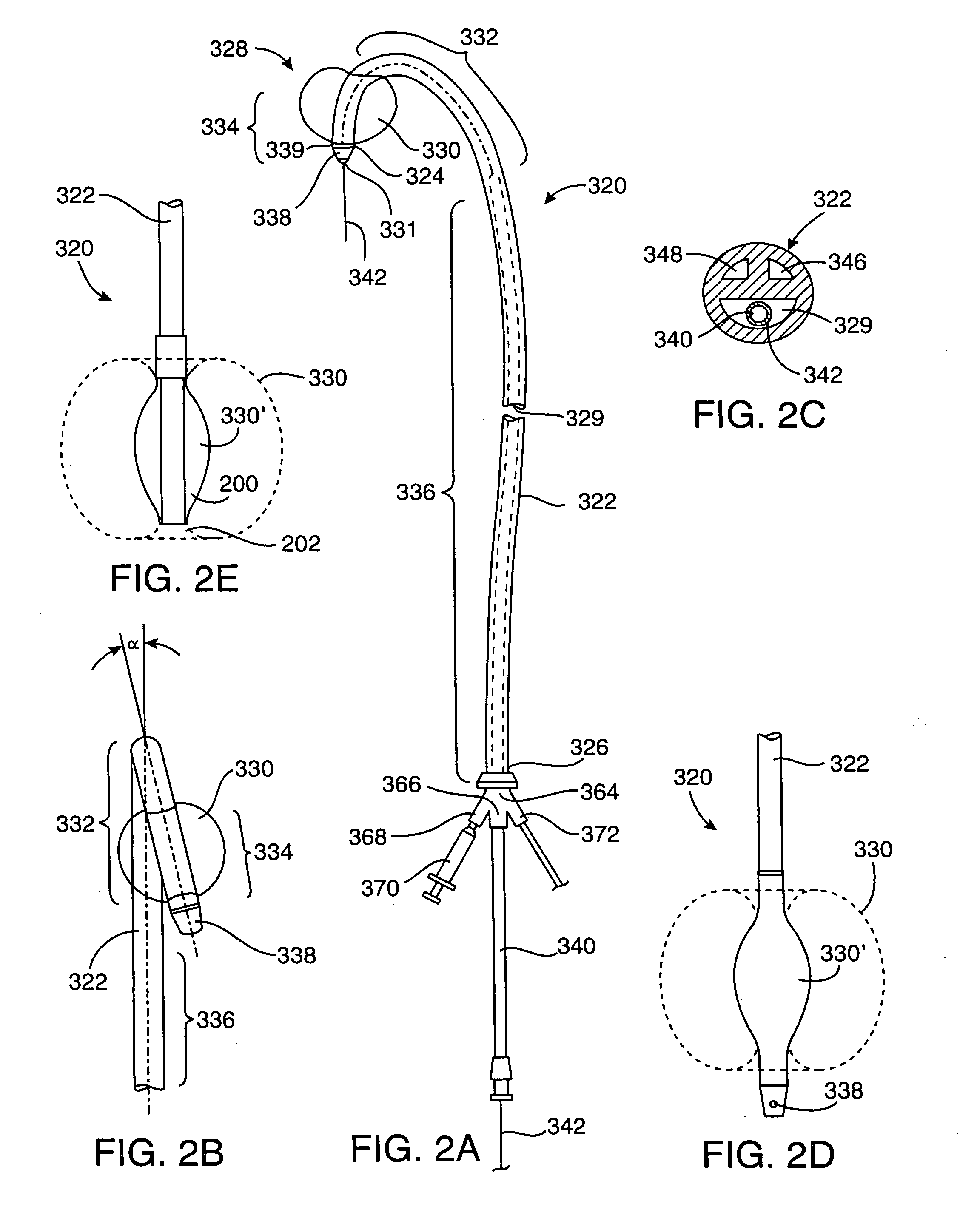
An illuminating wire medical device may include an elongate flexible housing and an illuminating fiber and a core wire extending through at least part of the housing, the core wire providing desired pushability and torquability. The illuminating device may further include a connector assembly cooperating with the illuminating fiber to accommodate changes in length and to absorb forces applied during use of the device.
Owner:ACCLARENT INC
Mechanical tissue device and method
InactiveUS20080119886A1Rigid enoughLength of device being increasedDilatorsOcculdersVeinVenous blood
The present invention relates generally to a device and method for preventing the undesired passage of emboli from a venous blood pool to an arterial blood pool. The invention relates especially to a device and method for treating certain cardiac defects, especially patent foramen ovales and other septal defects, through the use of an embolic filtering device capable of instantaneously deterring the passage of emboli from the moment of implantation. The device consists of a frame, and a braided mesh of sufficient dimensions to prevent passage of emboli through the mesh. The device is preferably composed of shape memory allow, such as Nitinol, which conforms to the shape and dimension of the defect to be treated.
Owner:SEPTRX
System and methods for performing endovascular procedures
InactiveUS20060058775A1Procedure is complicatedEasy to controlStentsGuide needlesExtracorporeal circulationAtherectomy
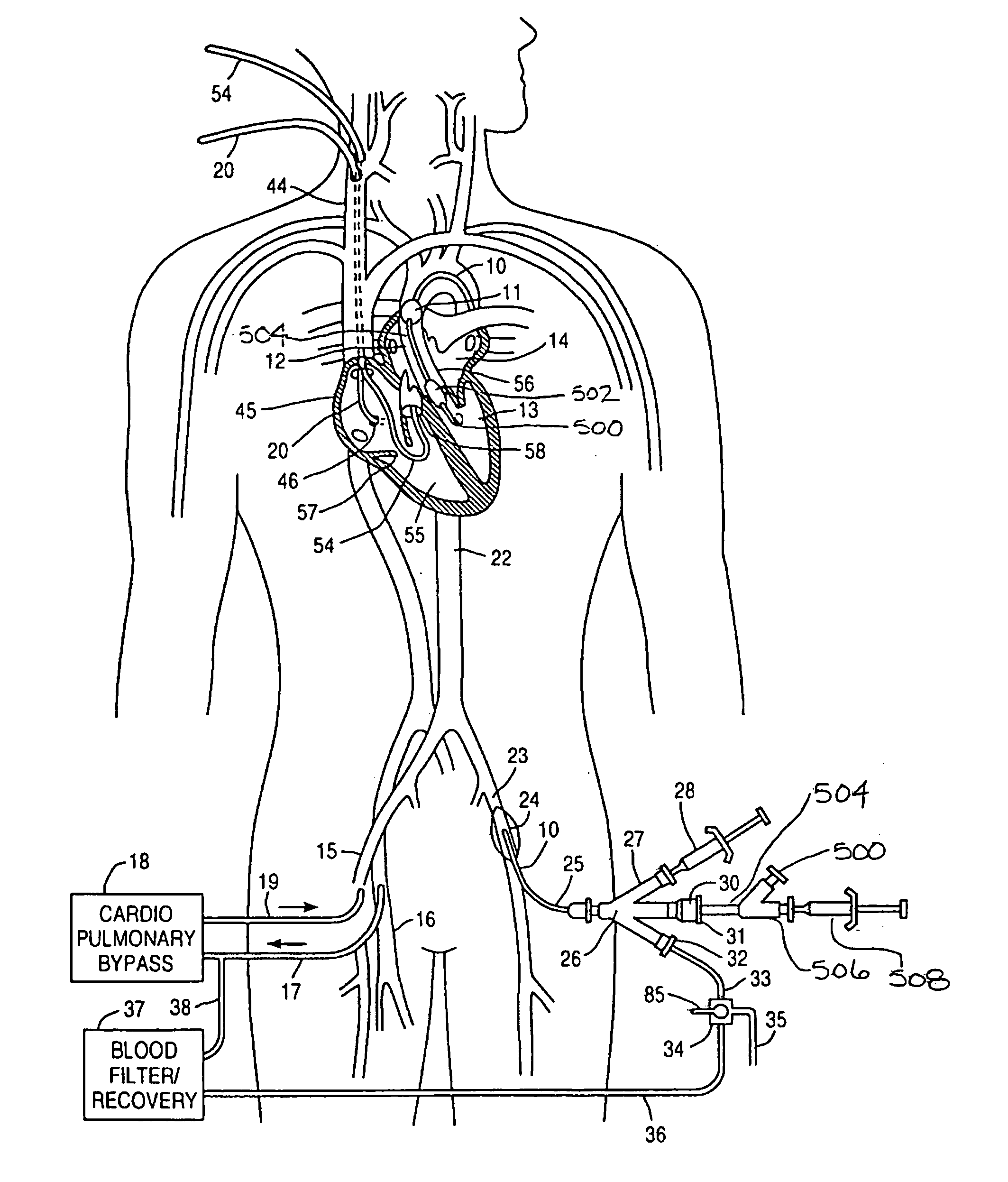
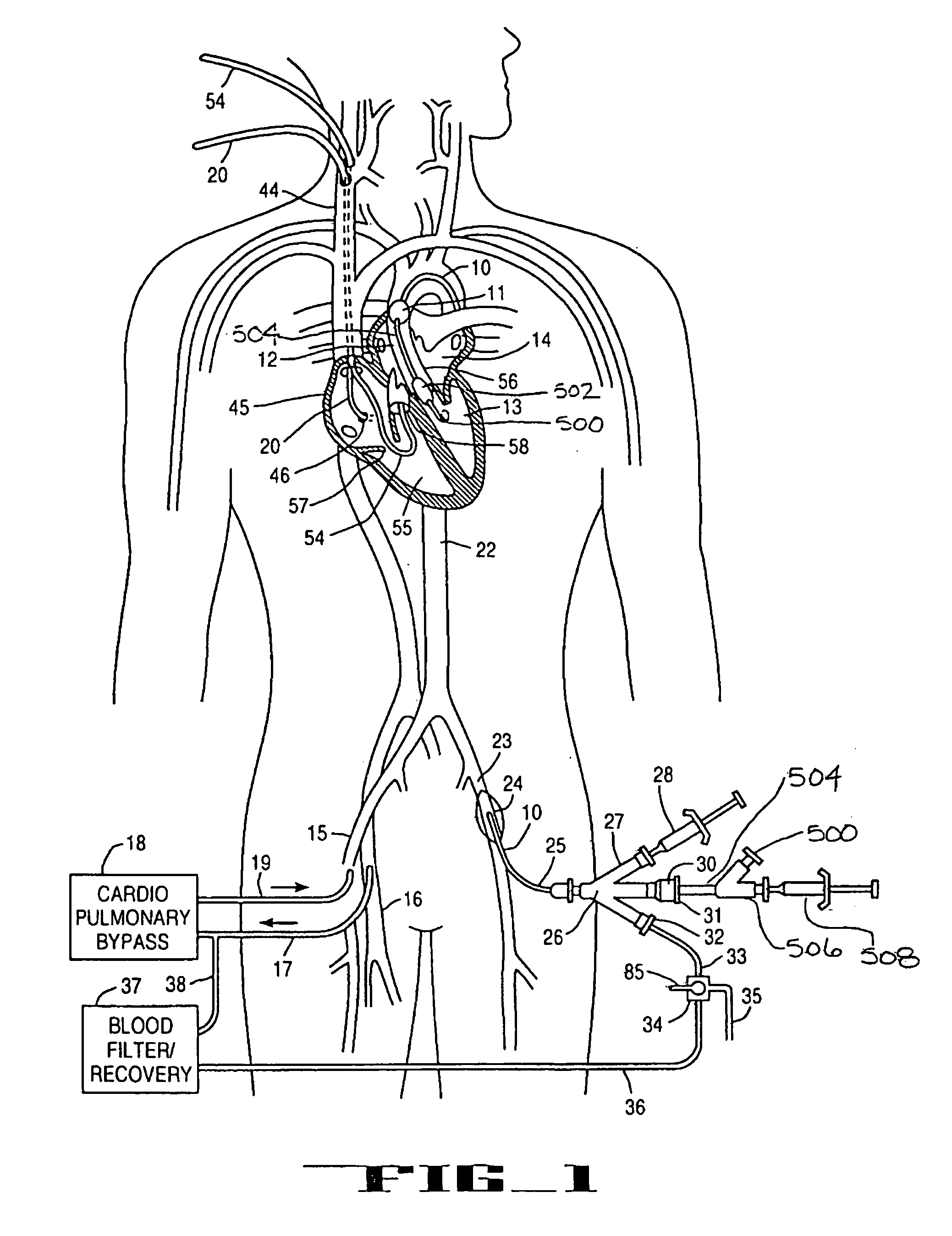
A system for inducing cardioplegic arrest and performing an endovascular procedure within the heart or blood vessels of a patient. An endoaortic partitioning catheter has an inflatable balloon which occludes the ascending aorta when inflated. Cardioplegic fluid may be infused through a lumen of the endoaortic partitioning catheter to stop the heart while the patient's circulatory system is supported on cardiopulmonary bypass. One or more endovascular devices are introduced through an internal lumen of the endoaortic partitioning catheter to perform a diagnostic or therapeutic endovascular procedure within the heart or blood vessels of the patient. Surgical procedures such as coronary artery bypass surgery or heart valve replacement may be performed in conjunction with the endovascular procedure while the heart is stopped. Embodiments of the system are described for performing: fiberoptic angioscopy of structures within the heart and its blood vessels, valvuloplasty for correction of valvular stenosis in the aortic or mitral valve of the heart, angioplasty for therapeutic dilatation of coronary artery stenoses, coronary stenting for dilatation and stenting of coronary artery stenoses, atherectomy or endarterectomy for removal of atheromatous material from within coronary artery stenoses, intravascular ultrasonic imaging for observation of structures and diagnosis of disease conditions within the heart and its associated blood vessels, fiberoptic laser angioplasty for removal of atheromatous material from within coronary artery stenoses, transmyocardial revascularization using a side-firing fiberoptic laser catheter from within the chambers of the heart, and electrophysiological mapping and ablation for diagnosing and treating electrophysiological conditions of the heart.
Owner:EDWARDS LIFESCIENCES LLC
Interference display unit
InactiveUS6995890B2Increase brightnessSimple and easy manufacturing processDecorative surface effectsOptical filtersEngineeringHeat treated
An interference display unit with a first electrode, a second electrode and posts located between the two electrodes is provided. The characteristic of the interference display unit is that the second electrode's stress is released through a thermal process. The position of the second electrode is shifted and the distance between the first electrode and the second electrode is therefore defined. A method for fabricating the structure described as follow. A first electrode and a sacrificial layer are sequentially formed on a substrate and at least two openings are formed in the first electrode and the sacrificial layer. A supporter is formed in the opening and the supporter may have at least one arm on the top portion of the supporter. A second electrode is formed on the sacrificial layer and the supporter and a thermal process is performed. Finally, The sacrificial layer is removed.
Owner:SNAPTRACK
Jettable compositions
ActiveUS20050171237A1Prevent long-term corrosionReaction is slowAdditive manufacturing apparatusLiquid surface applicatorsMeth-Oligomer
A fully curable jettable composition having a viscosity less than 30 cps at a temperature within the range of 15-180° C., more preferably at a temperature of 15-100° C., e.g. 60-80° C. the composition comprising: (A) at least one low viscosity reactive resin selected from the group consisting of compounds containing an oxetane ring, cycloaliphatic epoxy resins, tetrahydrofurans, hexahydropyrans and mono-functional (meth)acrylates, said resin having a molecular weight of not greater than 300 Daltons, e.g. 250 Daltons or less, and a viscosity at a temperature in the said range of less than 30 cps, e.g. 5 to 15 cps; (B) at least one higher viscosity resin selected from the group consisting of epoxy resins, compounds containing an oxetane ring and acrylates, which resin acts to thicken the low viscosity resin and strengthen a jetted deposit of the composition, the higher viscosity resin having: a viscosity greater than twice that of the low viscosity resin at the said temperature in the range stated above, and a functionality of greater than or equal to 2; (C) at least one curable toughener, preferably having a functionality of at least 2, such as hydroxy, epoxy, acrylic or other reactive functionalised polymer / oligomer (e.g. derived by functionalising poly(tetrahydrofuran), polycaprolactone, polycarbonate diol, or a dendrimeric polyol; (D) at least one initiator for the polymerisation of the resins, and (E) at least one stabiliser for delaying the curing of the resins of the composition; wherein the low viscosity resin is slower to react than the higher viscosity resin and acts to solvate the higher viscosity resin prior to curing and at least partly during curing and wherein at least 30% of the components A and B are cationically curable resins. The composition can be jetted from piezo electric printing heads under the control of a computer program to form a multi-layered article, e.g. a three dimensional article, in which the adjacent droplets merge and are cured homogeneously together.
Owner:3D SYST INC +1
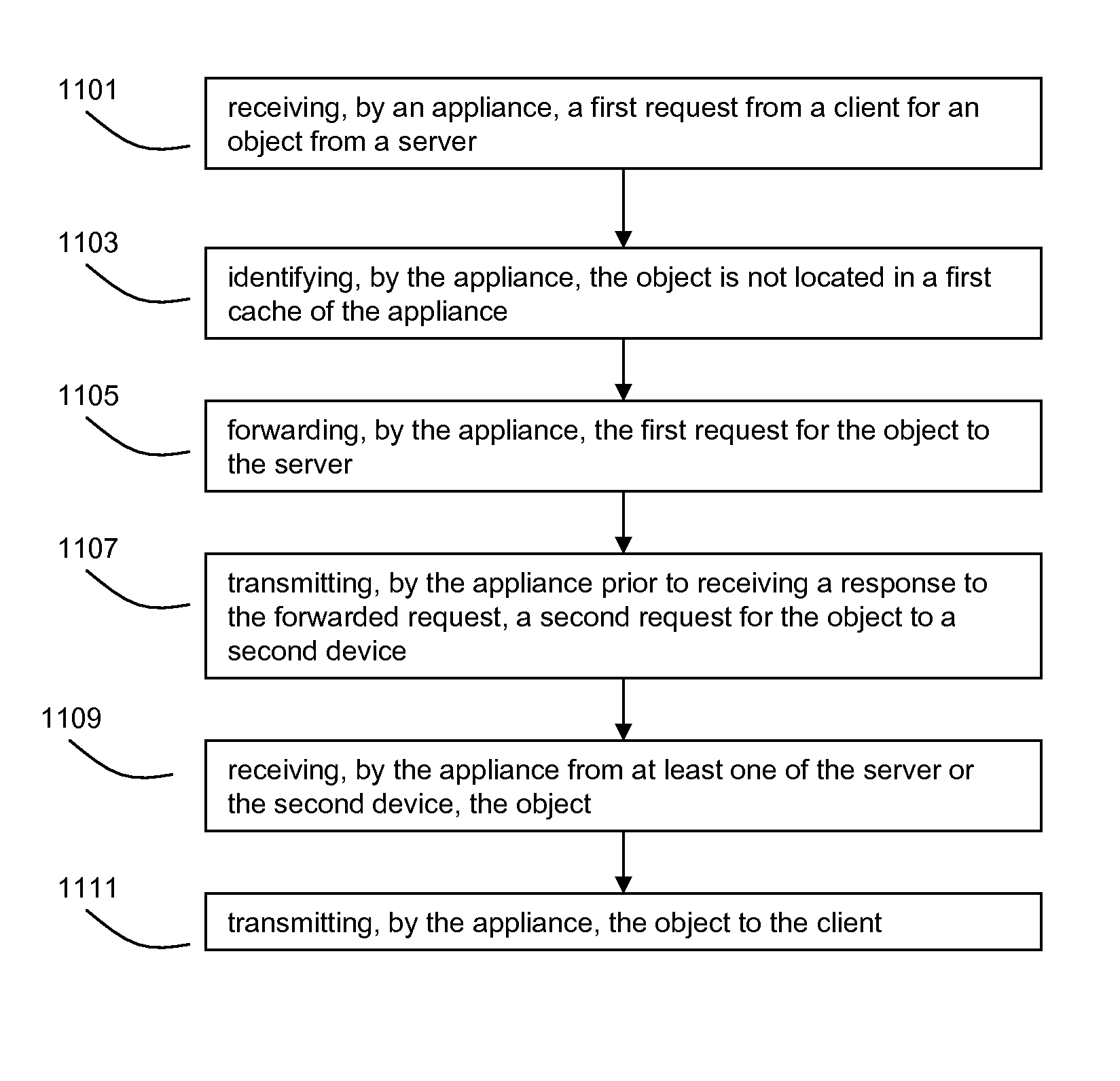
Systems and methods for providing dynamic ad hoc proxy-cache hierarchies
ActiveUS7865585B2Improve compression efficiently and speedIncrease the lengthMultiple digital computer combinationsTransmissionApplication specificDistributed computing
Systems and methods of storing previously transmitted data and using it to reduce bandwidth usage and accelerate future communications are described. By using algorithms to identify long compression history matches, a network device may improve compression efficiently and speed. A network device may also use application specific parsing to improve the length and number of compression history matches. Further, by sharing compression histories, compression history indexes and caches across multiple devices, devices can utilize data previously transmitted to other devices to compress network traffic. Any combination of the systems and methods may be used to efficiently find long matches to stored data, synchronize the storage of previously sent data, and share previously sent data among one or more other devices.
Owner:CITRIX SYST INC
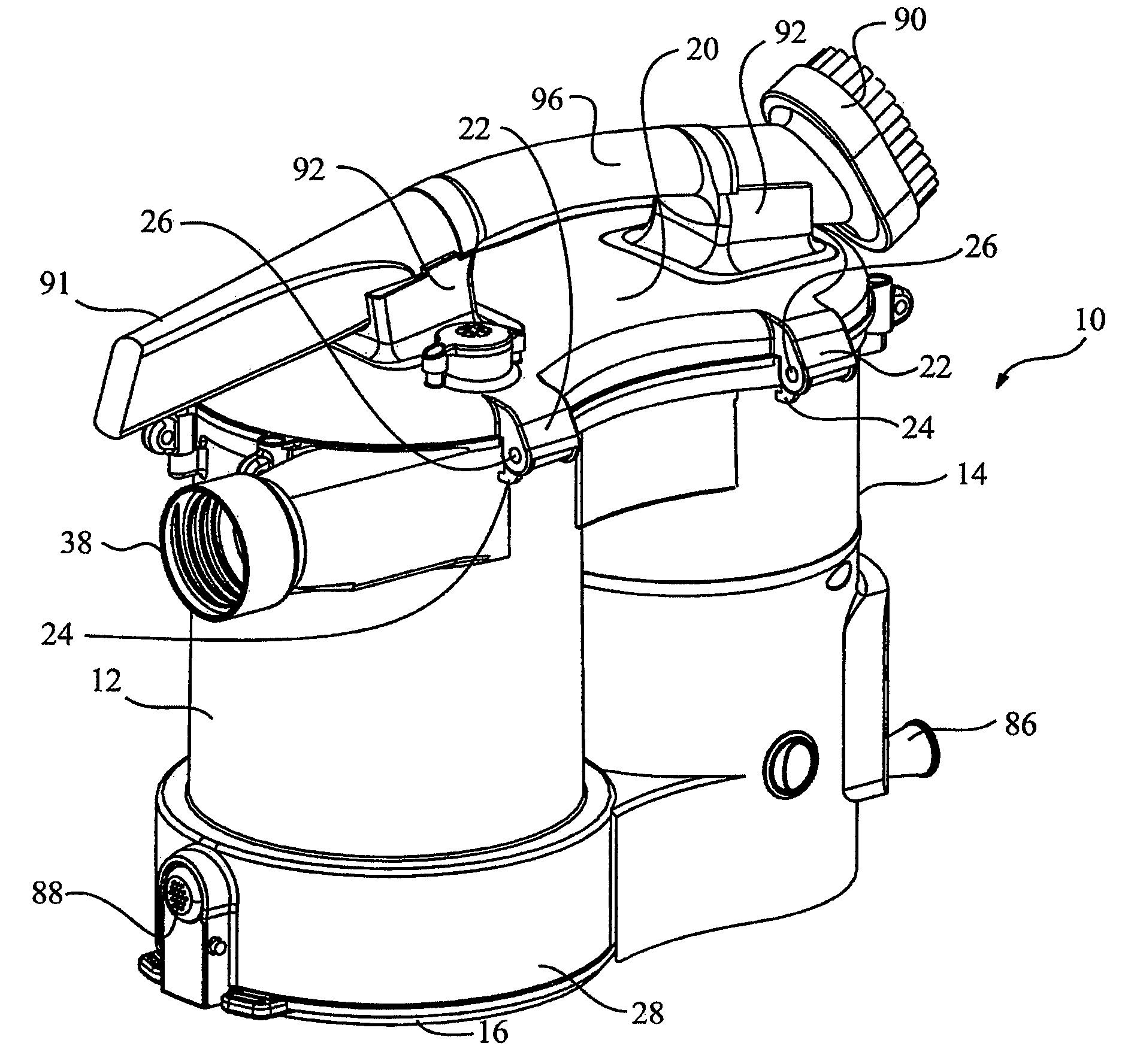
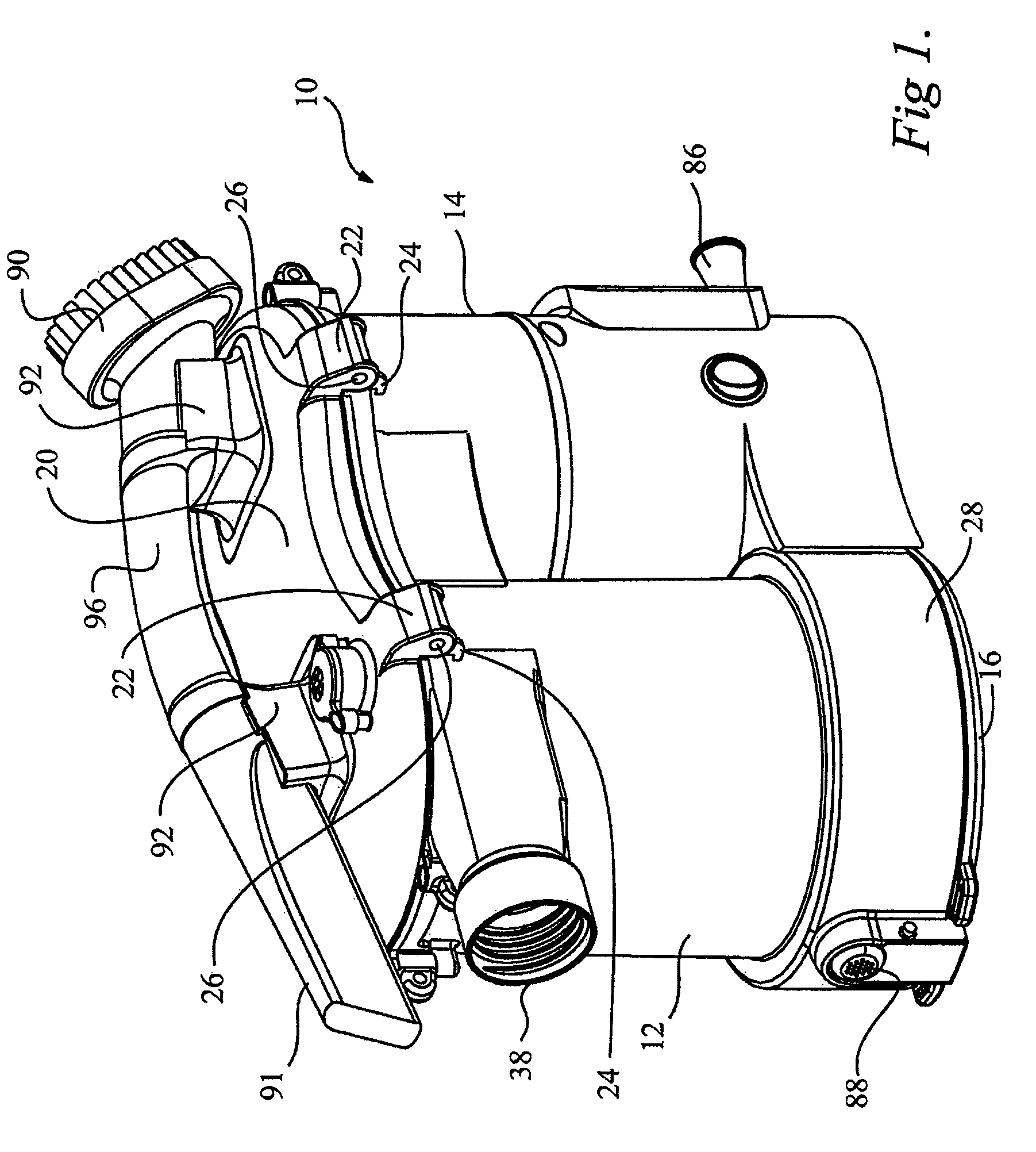
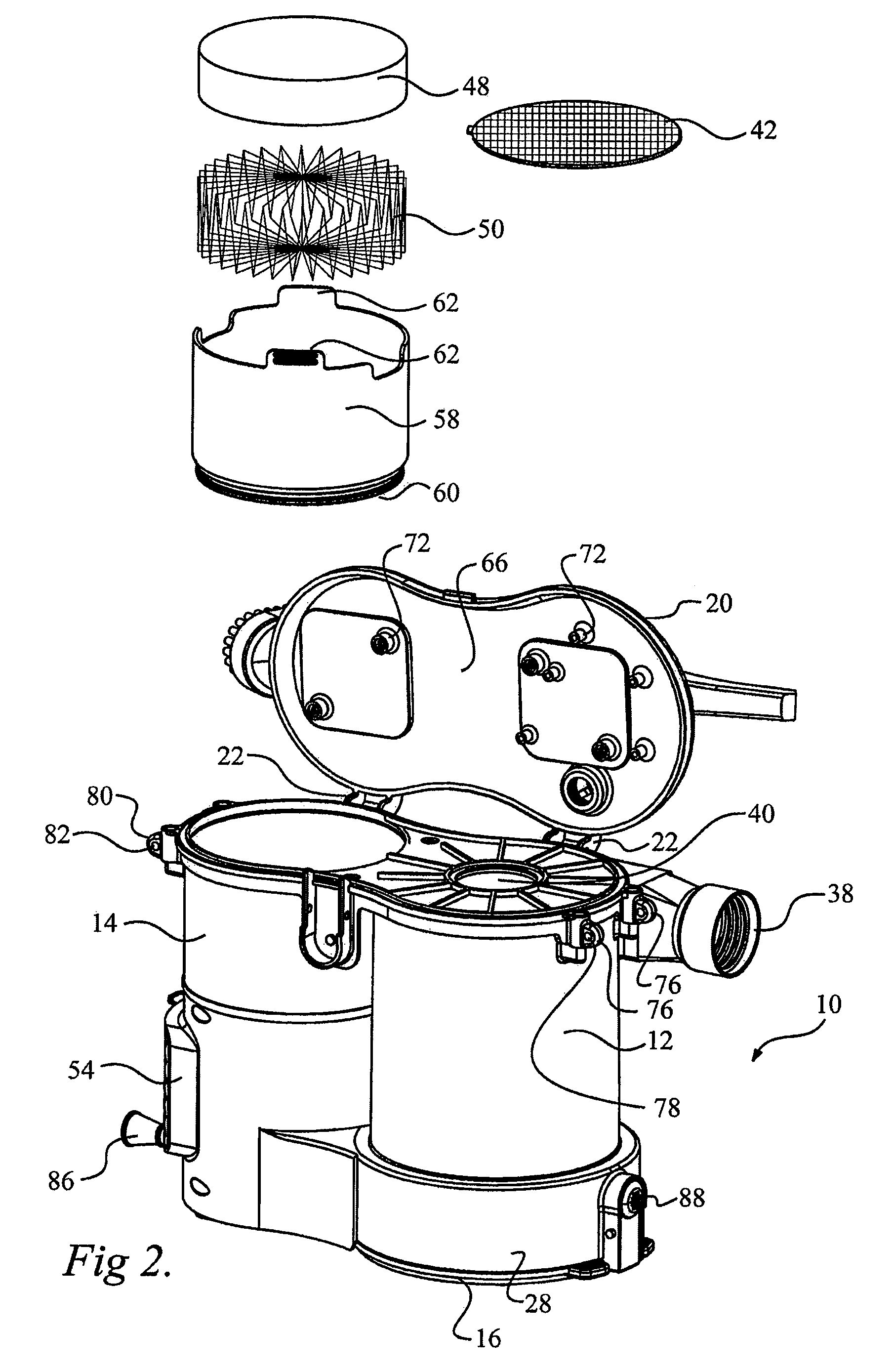
Surface cleaning apparatus
ActiveUS8146201B2Increasing overall height and linear extentMore compactCleaning filter meansBowling gamesSurface cleaningFiltration
A vacuum cleaner comprises adjacent housings, which contain the filtration and suction fan motor assembly of the vacuum cleaner.
Owner:OMACHRON INTPROP
Method for fabricating an interference display unit
InactiveUS20050168849A1Increase brightnessSimple and easy manufacturing processMirrorsDecorative surface effectsEngineeringHeat treated
An interference display unit with a first electrode, a second electrode and posts located between the two electrodes is provided. The characteristic of the interference display unit is that the second electrode's stress is released through a thermal process. The position of the second electrode is shifted and the distance between the first electrode and the second electrode is therefore defined. A method for fabricating the structure described as follow. A first electrode and a sacrificial layer are sequentially formed on a substrate and at least two openings are formed in the first electrode and the sacrificial layer. A supporter is formed in the opening and the supporter may have at least one arm on the top portion of the supporter. A second electrode is formed on the sacrificial layer and the supporter and a thermal process is performed. Finally, The sacrificial layer is removed.
Owner:SNAPTRACK
Methods And Devices For Sequencing Nucleic Acids In Smaller Batches
ActiveUS20100323350A1Reducing spectral crosstalkHigh strengthBioreactor/fermenter combinationsBiological substance pretreatmentsNucleotide sequencingComputational biology
The invention provides methods and compositions, including, without limitation, algorithms, computer readable media, computer programs, apparatus, and systems for determining the identity of nucleic acids in nucleotide sequences using, for example, data obtained from sequencing by synthesis methods. A plurality of smaller flow cells is employed, each with a relatively small area to be imaged, in order to provide greater flexibility and efficiency.
Owner:ISOPLEXIS
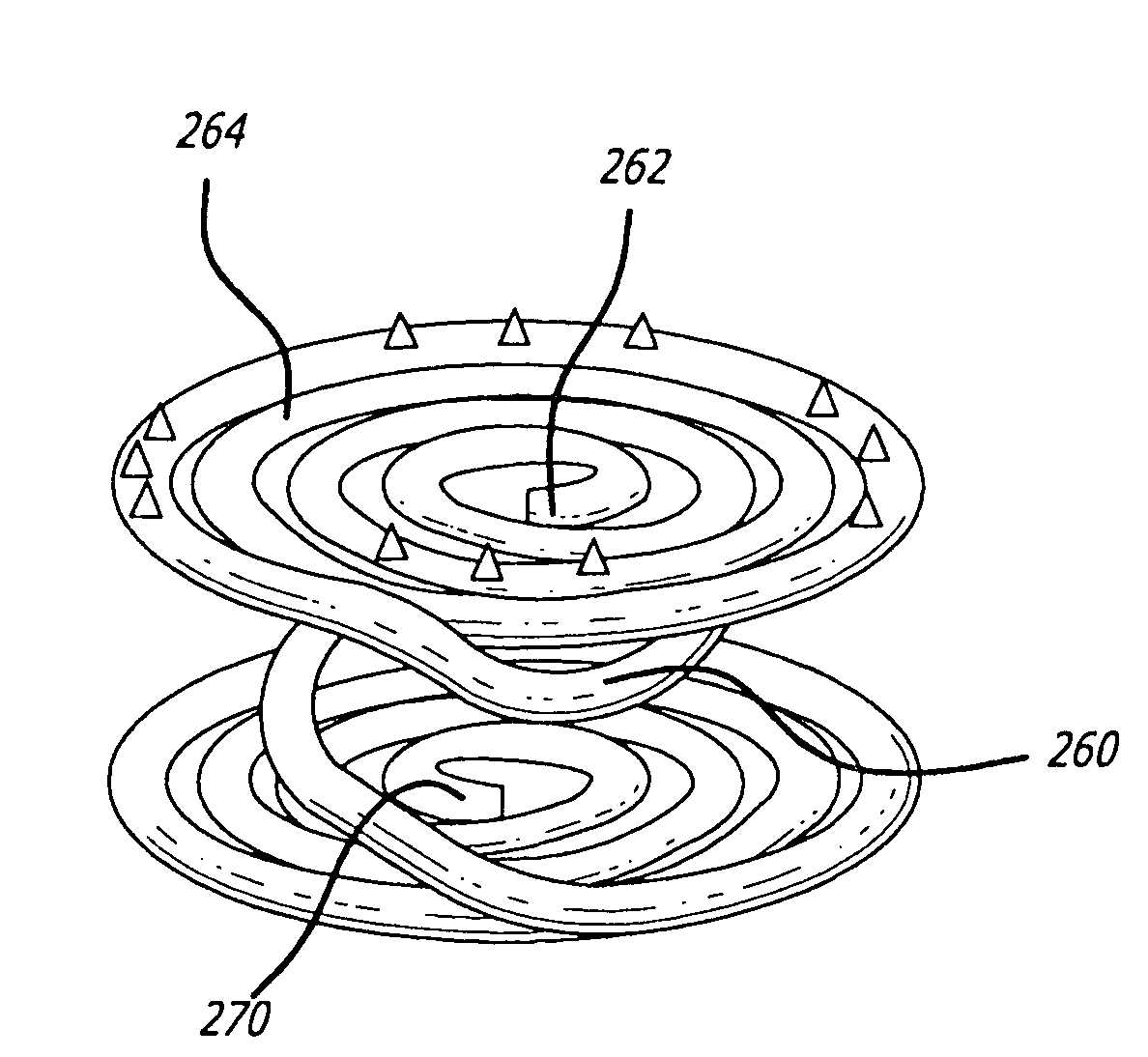
Prosthetic spinal discs
ActiveUS7309357B2Sufficient forceSmooth transitionMultiple spring combinationsSpinal implantsIntervertebral discEngineering
A prosthetic spinal disc uses a stiff spring or springs for resiliency between two plates that attach to adjacent vertebrae. When the disc has multiple springs, they may be adjacent, concentric or nested. Multiple springs may be spaced around the periphery of the plates. A foil metal bellows may surround the plates to prevent material from entering or exiting the space between the plates. Alternatively, the ends of the spring(s) may be machined with spikes to engage the vertebrae directly without plates.
Owner:INFINESSE CORP
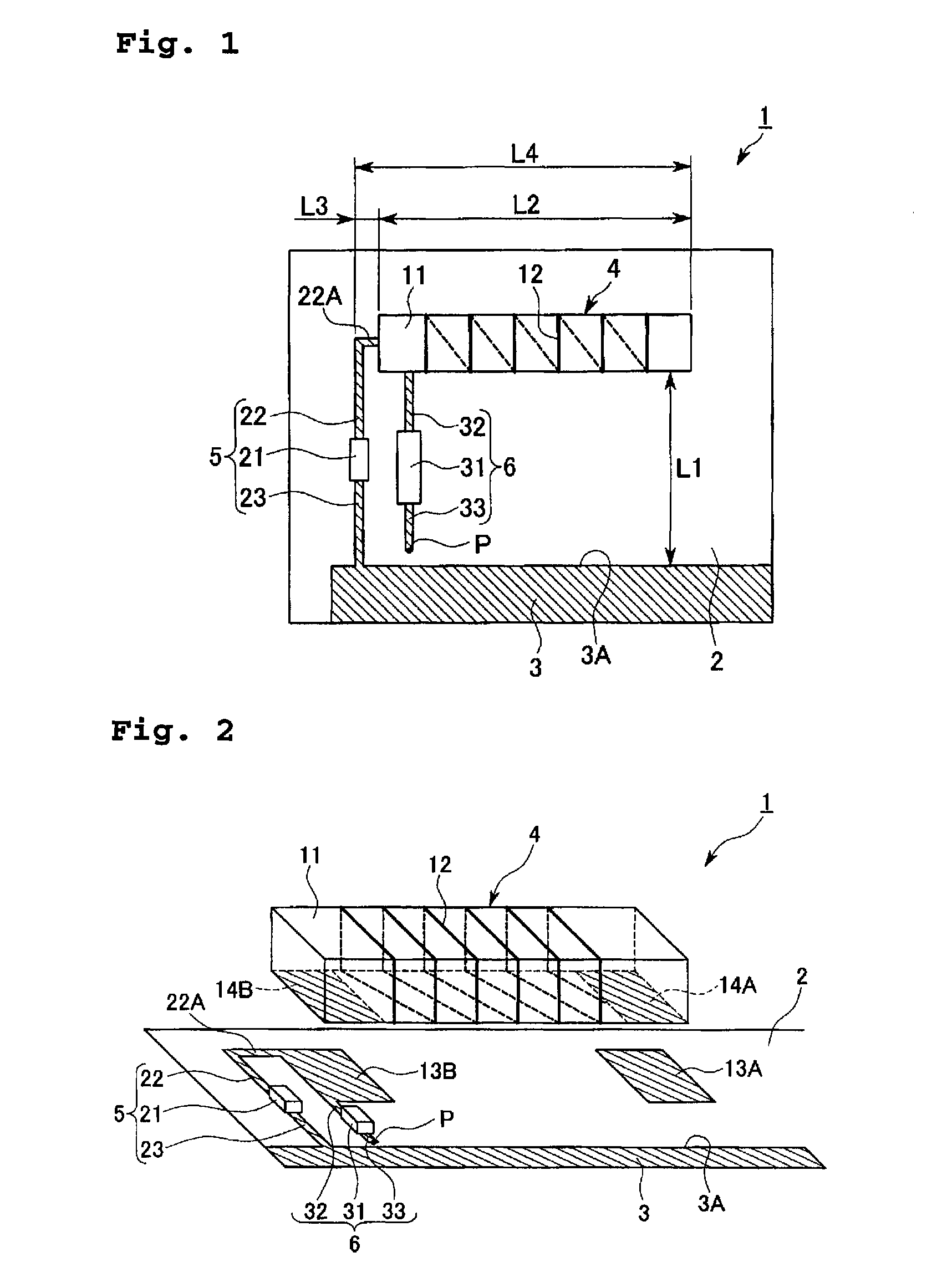
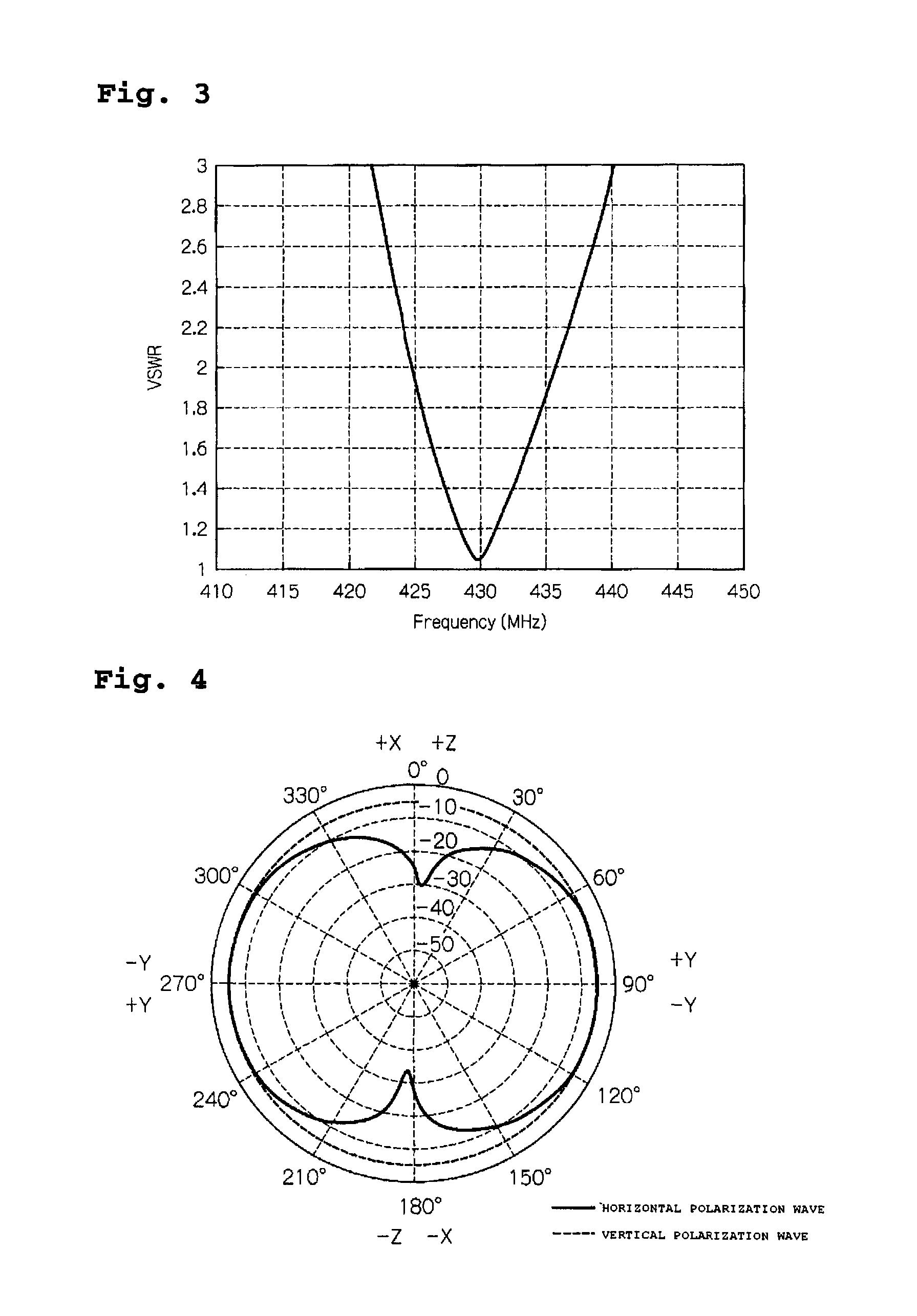
Antenna Device and Communication Apparatus
ActiveUS20070285335A1Length of conductor be increaseIncrease gainSimultaneous aerial operationsAntenna supports/mountingsInductorEngineering
There is provided an antenna device including a substrate, an earth section which is disposed on a portion of the substrate, a feed point which is disposed on the substrate, a loading section disposed on the substrate and constructed with a line-shaped conductor pattern which is formed in a longitudinal direction of an elementary body made of a dielectric material, an inductor section which connects one end of the conductor pattern to the earth section, and a feed point which feeds a current to a connection point of the one end of the conductor pattern and the inductor section, wherein a longitudinal direction of the loading section is arranged to be parallel to an edge side of the earth section.
Owner:MITSUBISHI MATERIALS CORP
Fixation devices for variation in engagement of tissue
InactiveUS7811296B2Robust graspReduce refluxHeart valvesSurgical veterinaryBiomedical engineeringBlood vessel
Owner:EVALVE
Carbon-Based Fine Structure Array, Aggregate of Carbon-Based Fine Structures, Use Thereof and Method for Preparation Thereof
ActiveUS20080095694A1Long and strong aggregateEasy alignmentMaterial nanotechnologyLayered productsFine structureStrong interaction
An aggregate of carbon-based fine structures in which a plurality of carbon-based fine structures are collected, wherein respective carbon-based fine structures are oriented in the same direction. The above aggregate of carbon-based fine structures is an aggregate of a plurality of carbon-based fine structures in a state they are pulled by one another with strong interaction, and has such a length that allows the improvement of the handleability and workability thereof.
Owner:NIPPON SANSO CORP
Method and apparatus for deliberate fluid removal by capillary imbibition
InactiveUS6283212B1Increase the lengthRateFluid removalDrilling compositionCapillary pressureWell stimulation
The present Invention relates to hydrocarbon well stimulation, and more particularly to methods and compositions to remove (or more generally to transfer) fluid introduced into the subsurface. For instance, preferred methods involve creating then exploiting a capillary pressure gradient at the fracture face to induce fluid flow from the fracture into the formation thereby increasing effective fracture length, and then improving fracture conductivity.
Owner:SCHLUMBERGER TECH CORP
Anchoring elements for intracardiac devices
InactiveUS20140200662A1Prevent leakageGood equipment stabilityAnnuloplasty ringsLateral extensionBiomedical engineering
An intracardiac device comprising a ring-shaped body and one or more anchoring or stabilizing elements attached to said body, said elements being selected from the group consisting of levered anchoring arms, elongate anchoring arms, and lateral extension elements, wherein said device is able to move between two conformations, a collapsed conformation suitable for insertion into a delivery catheter, and an open conformation, suitable for implantation at a cardiac valve annulus.
Owner:MVALVE TECH
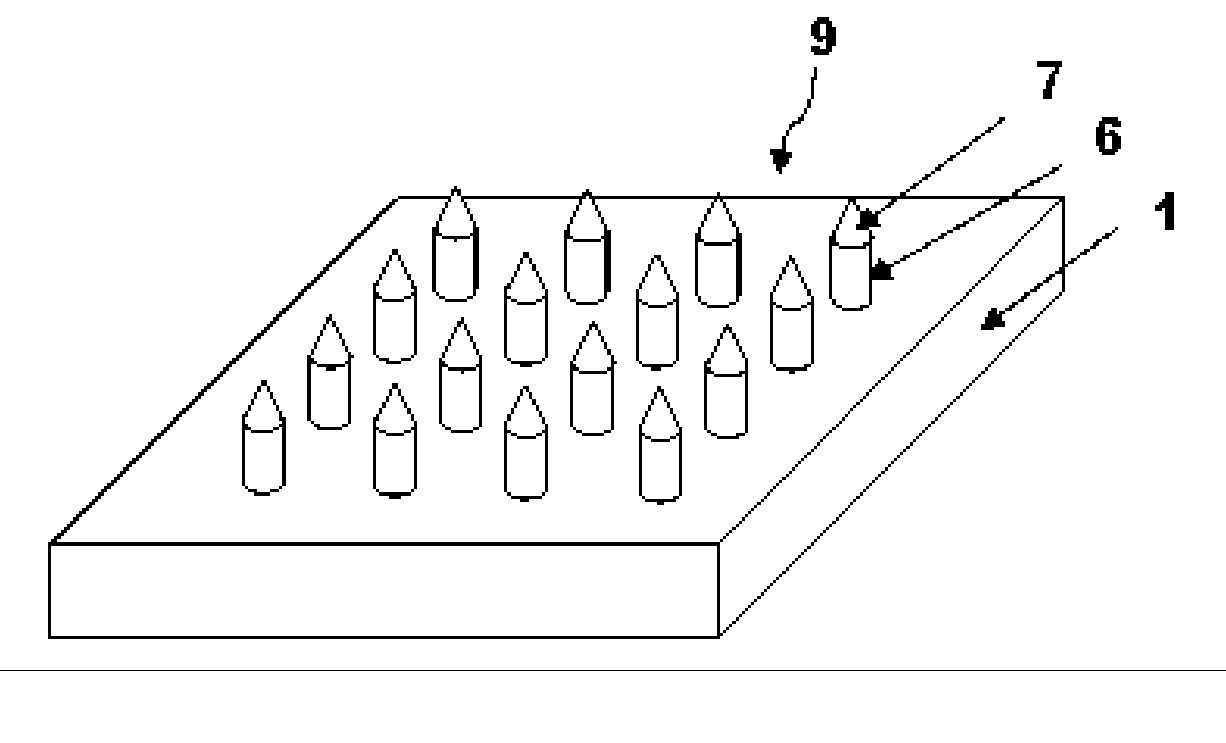
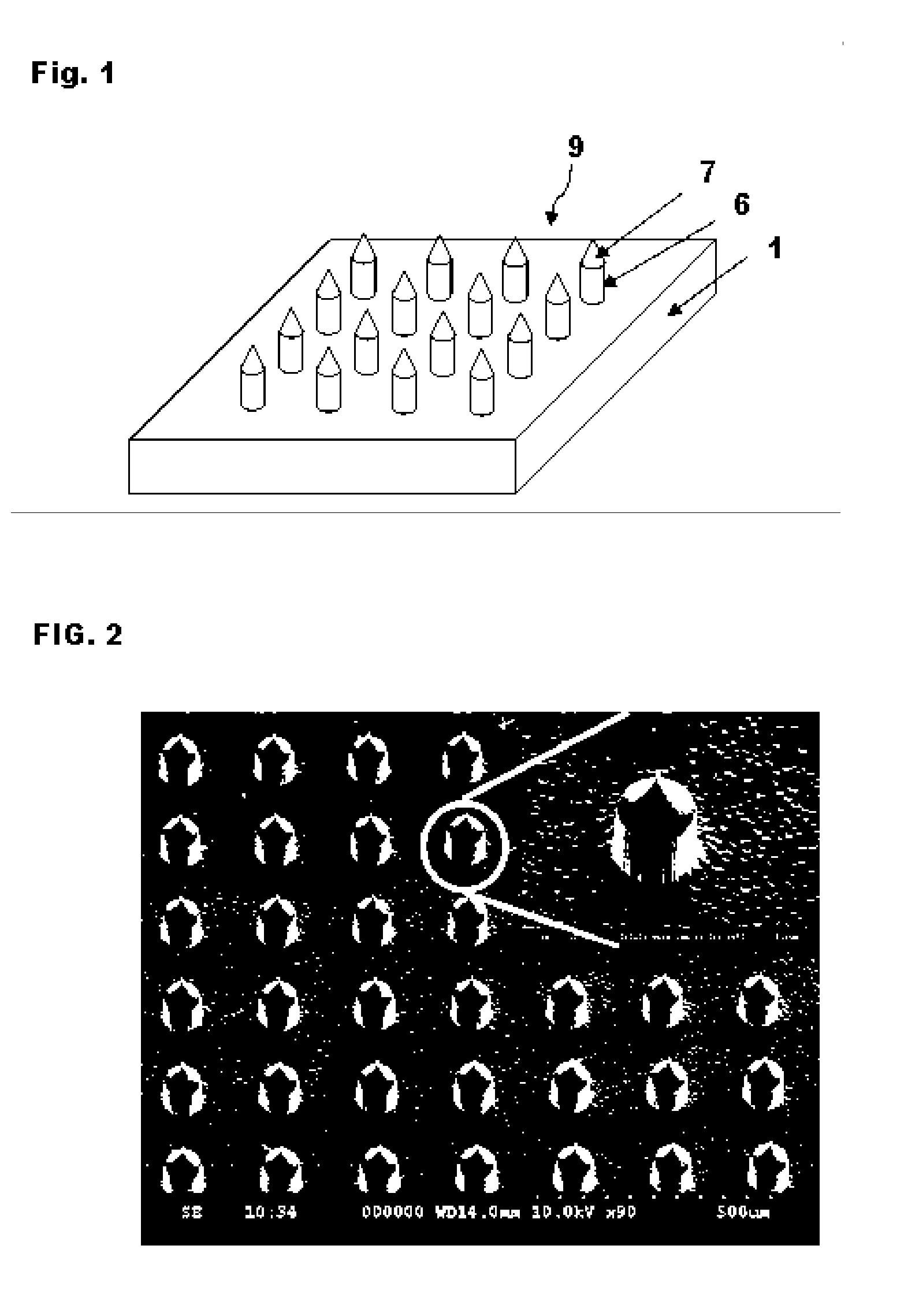
High-Aspect-Ratio Microdevices and Methods for Transdermal Delivery and Sampling of Active Substances
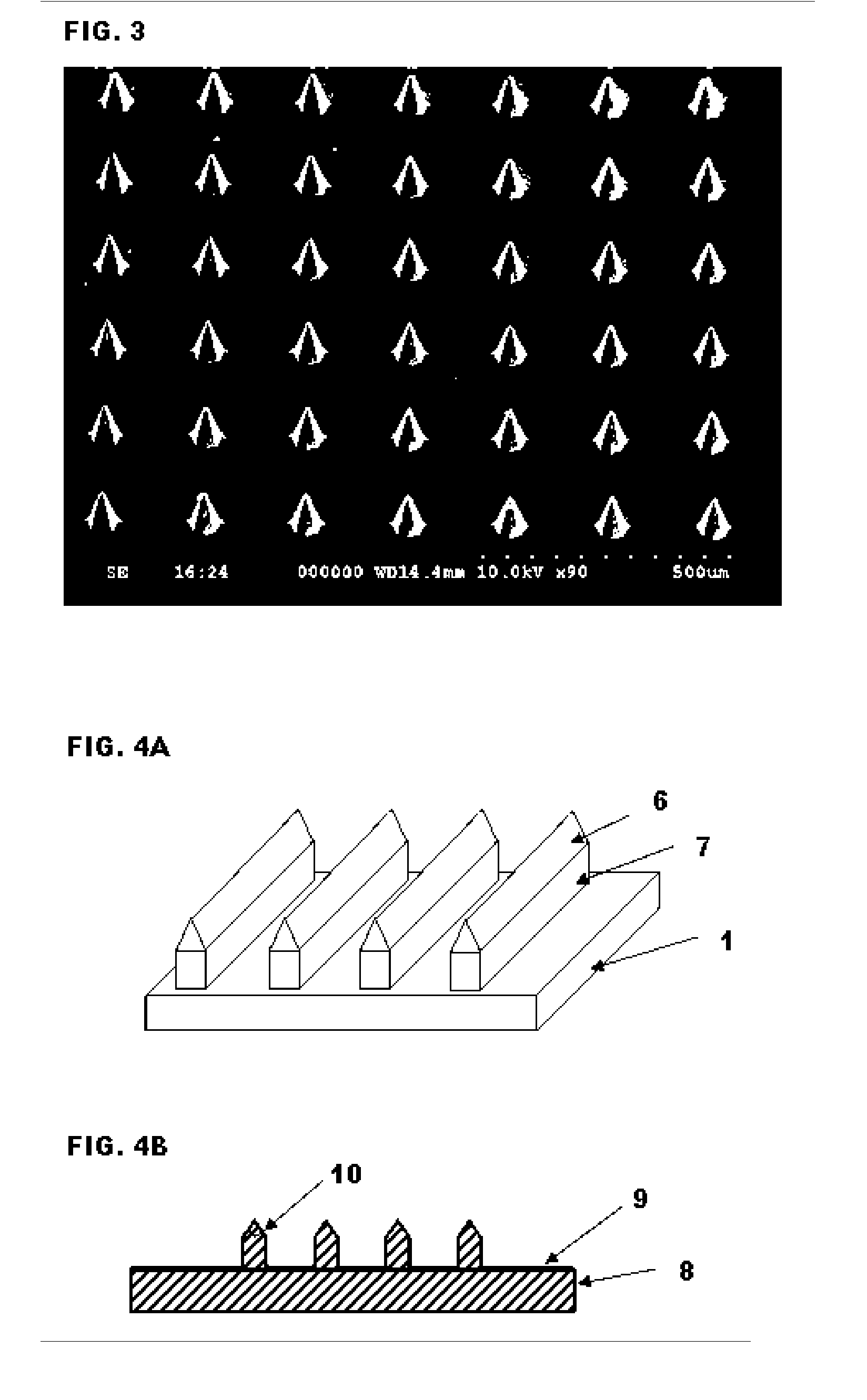
ActiveUS20050261632A1Increased needle lengthHigh selectivityNanoinformaticsMicroneedlesSensor arrayBody fluid
High-aspect-ratio microdevices comprising microneedles, microknives and microblades and their manufacturing methods are disclosed. These devices can be used for the delivery of therapeutic agents across the skin or other tissue barriers to allow chemical and biological molecules getting into the circulation systems. The microneedles can also be used to withdraw body fluids for analysis of clinically relevant species when the device has an integrated body fluid sensor or sensor array in the vicinity of microneedles.
Owner:XU BAI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com