Patents
Literature
39results about How to "Rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
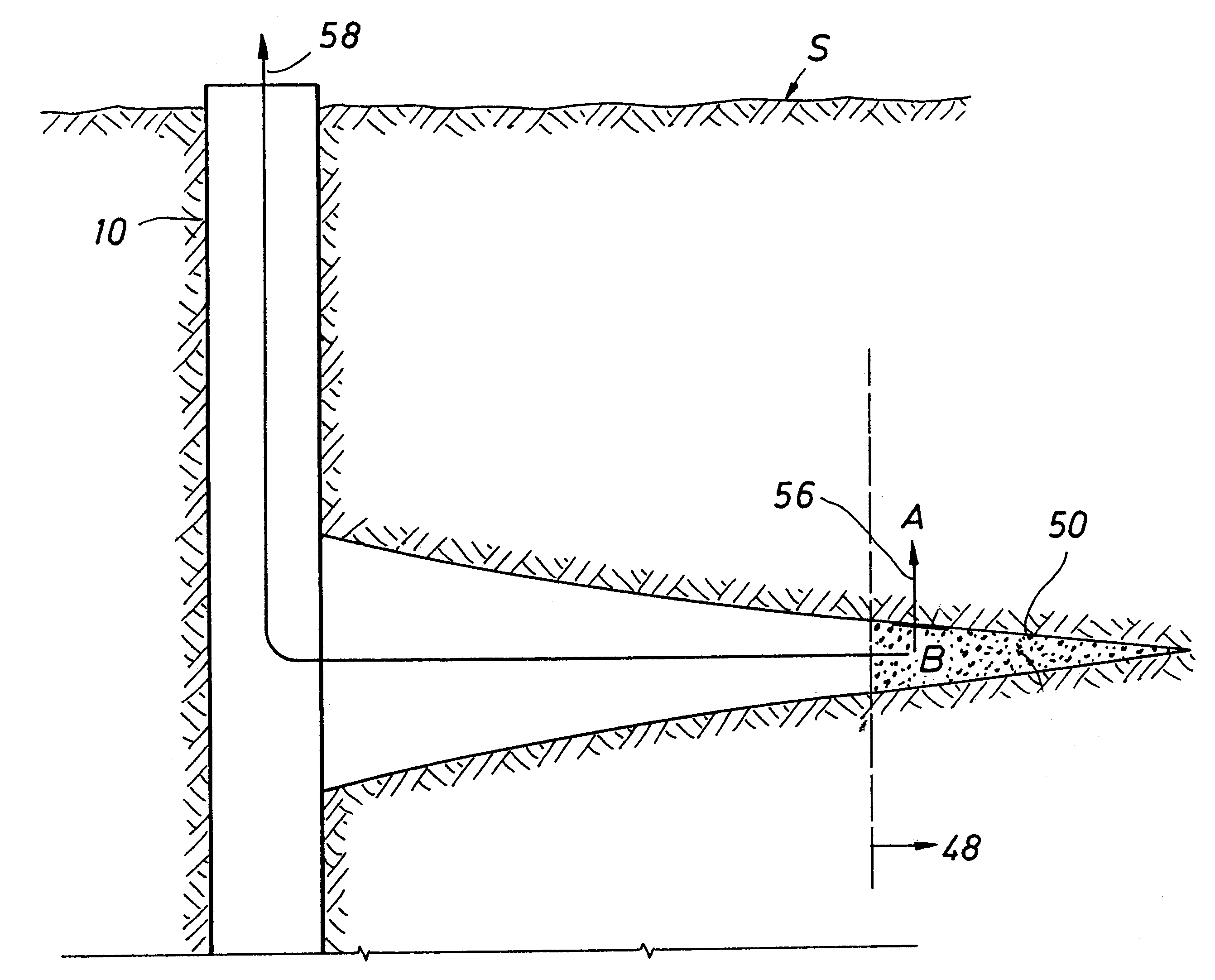
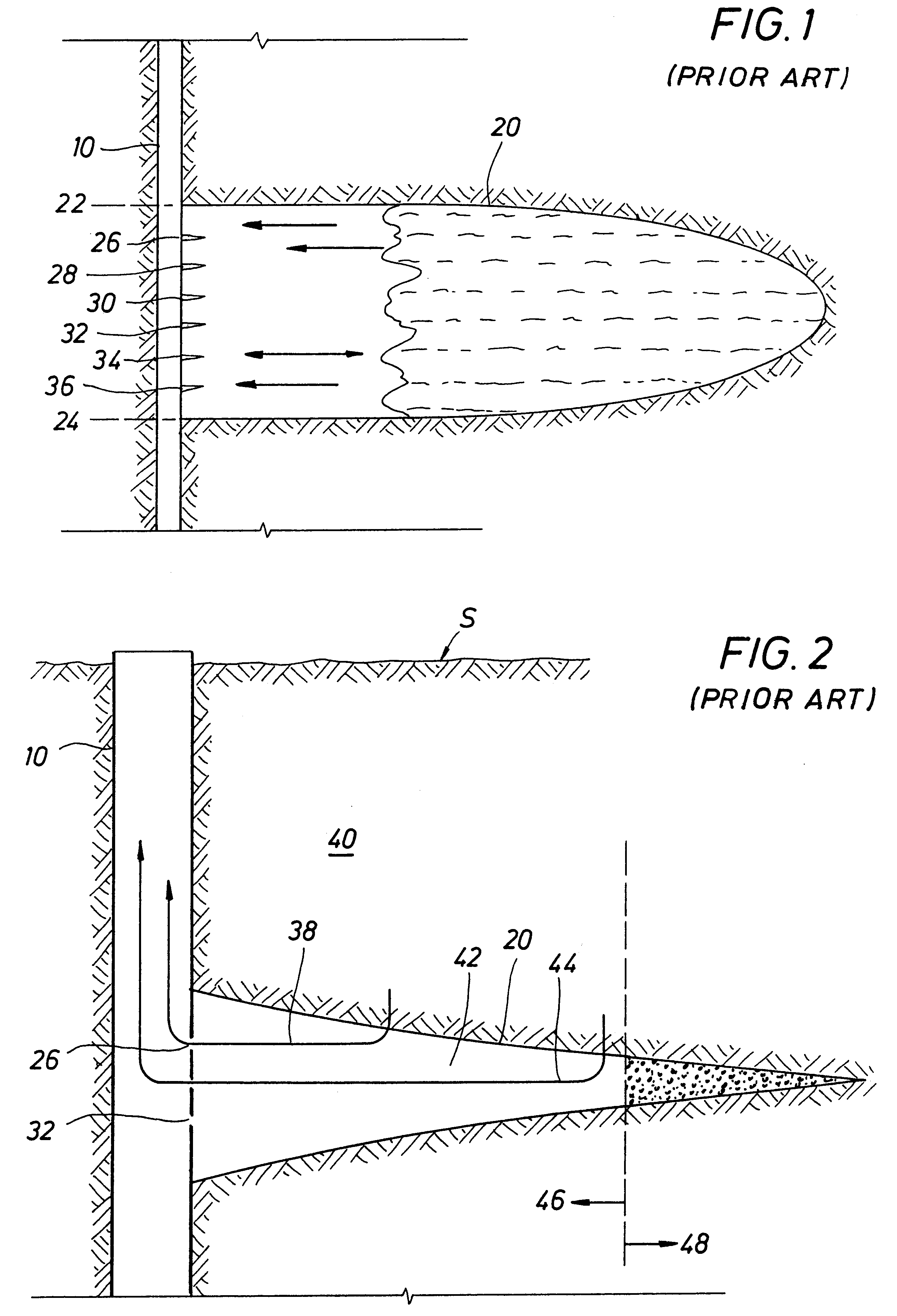
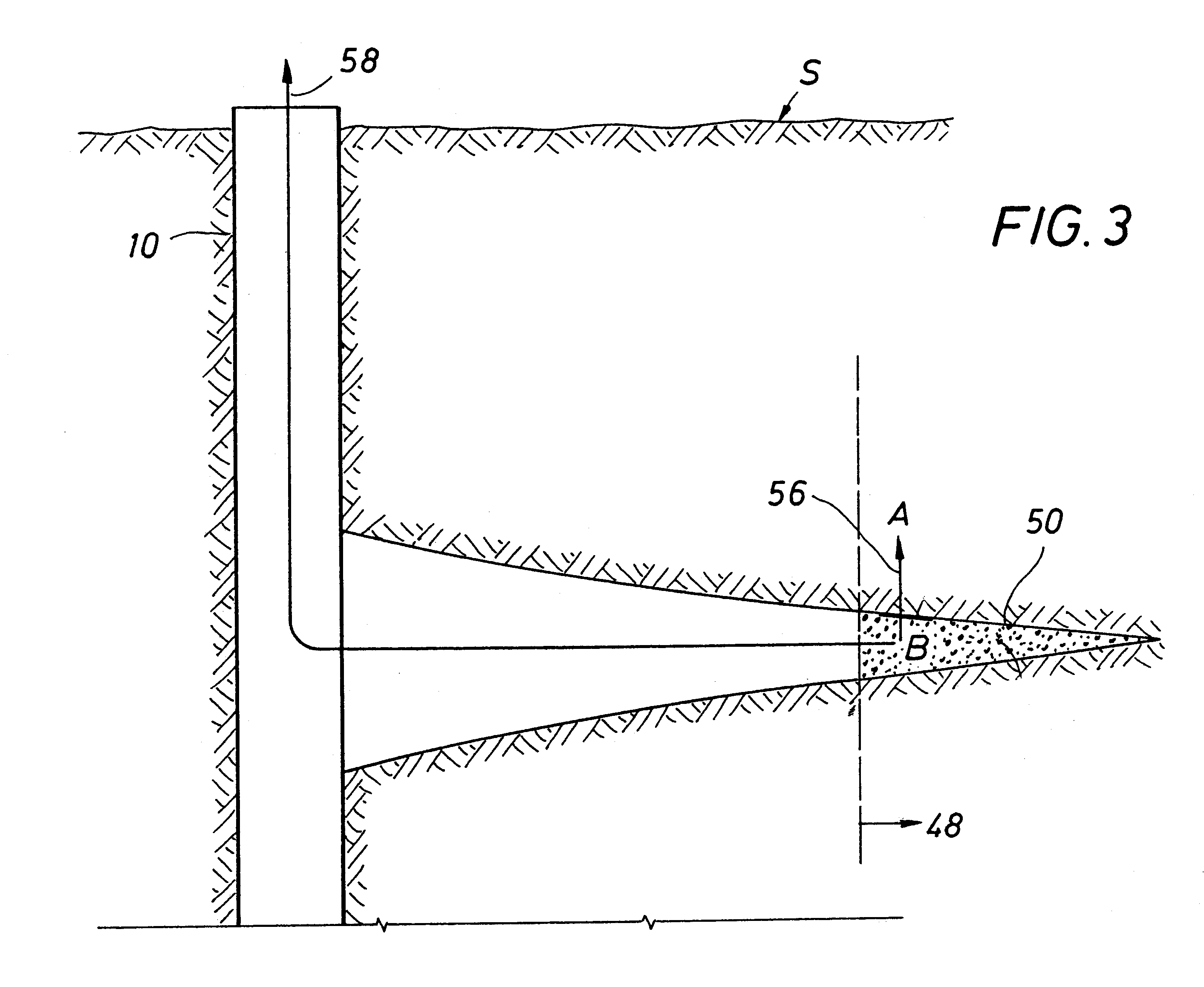
Method and apparatus for deliberate fluid removal by capillary imbibition
InactiveUS6283212B1Increase the lengthRateFluid removalDrilling compositionCapillary pressureWell stimulation
The present Invention relates to hydrocarbon well stimulation, and more particularly to methods and compositions to remove (or more generally to transfer) fluid introduced into the subsurface. For instance, preferred methods involve creating then exploiting a capillary pressure gradient at the fracture face to induce fluid flow from the fracture into the formation thereby increasing effective fracture length, and then improving fracture conductivity.
Owner:SCHLUMBERGER TECH CORP
Human sweat malodor counteractant composition and process for using same
InactiveUS6379658B1Increase in level of perceived pleasantnessMalodor-reducing concentrationBiocideCosmetic preparationsFuranPropionate
Described is a process and compositions for counteracting human sweat malodor. The compositions containing at least 10 weight percent of either 3,7-dimethyl-2,6-nonadienenitrile or 3,7-dimethyl-2,6-octadienenitrile. Additional ingredients may be added including napthyl methyl ether, methyl beta-naphthyl ketone, benzyl acetone, methanoinden propionates, methyl ionone, tetramethylnaphtho furan, ethylene glycol cyclic ester of dodecanedioic acid, 1-cyclohexadecen-6-one, 1-cycliheptadecen- 10-one, and corn mint oil. These compositions are applied to either fabric or a defined surface area of the human epidermis.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
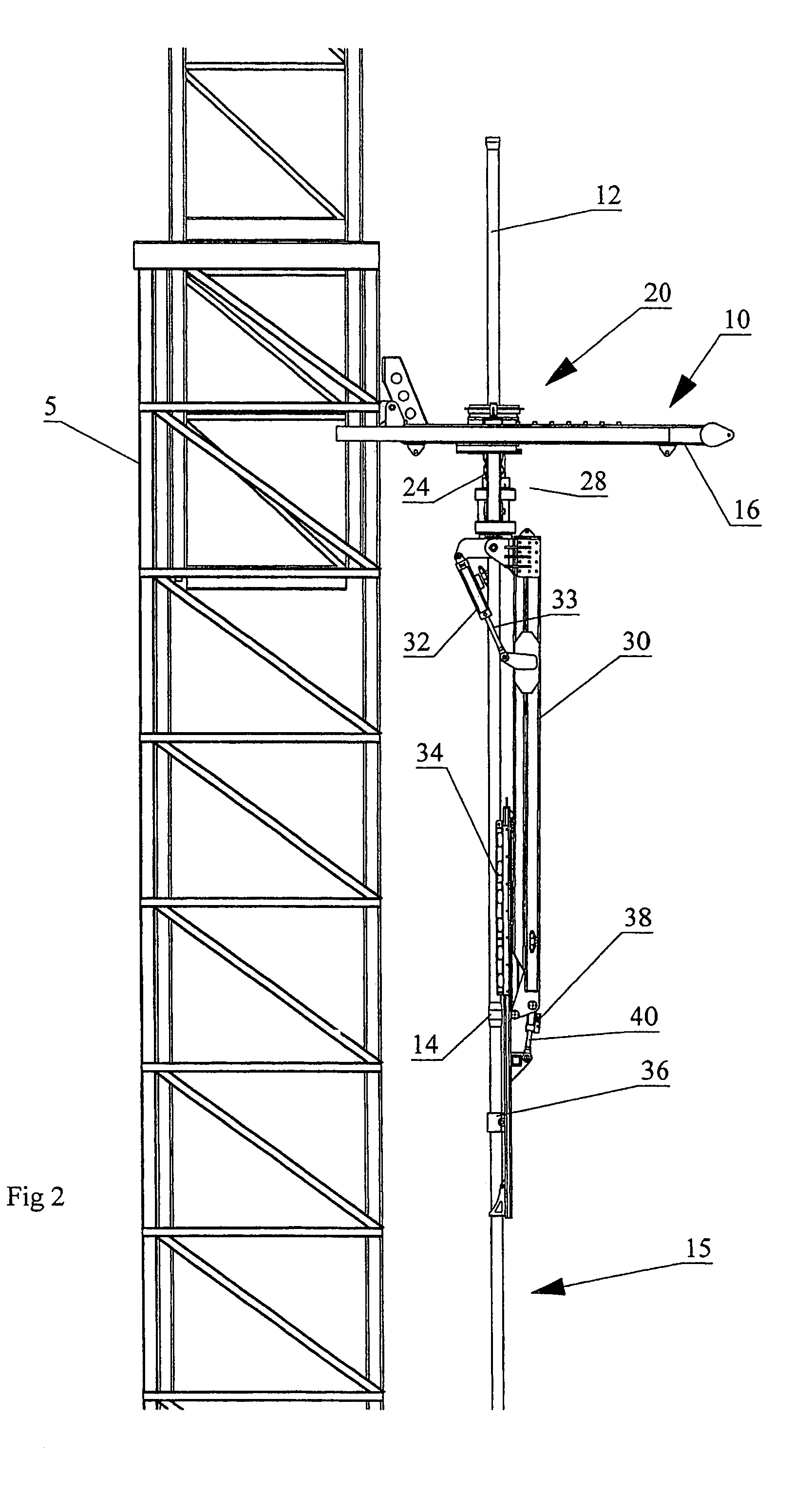
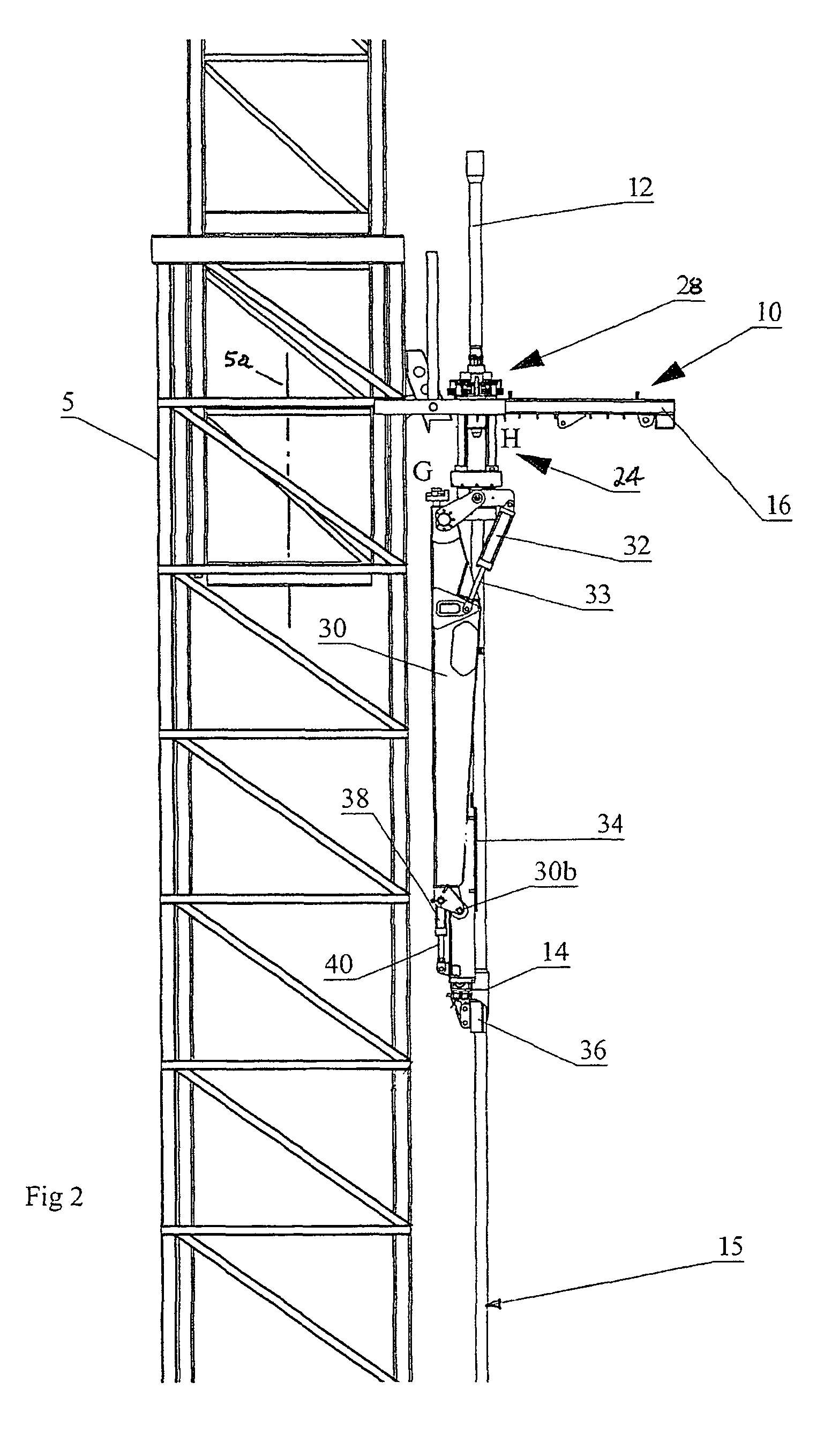
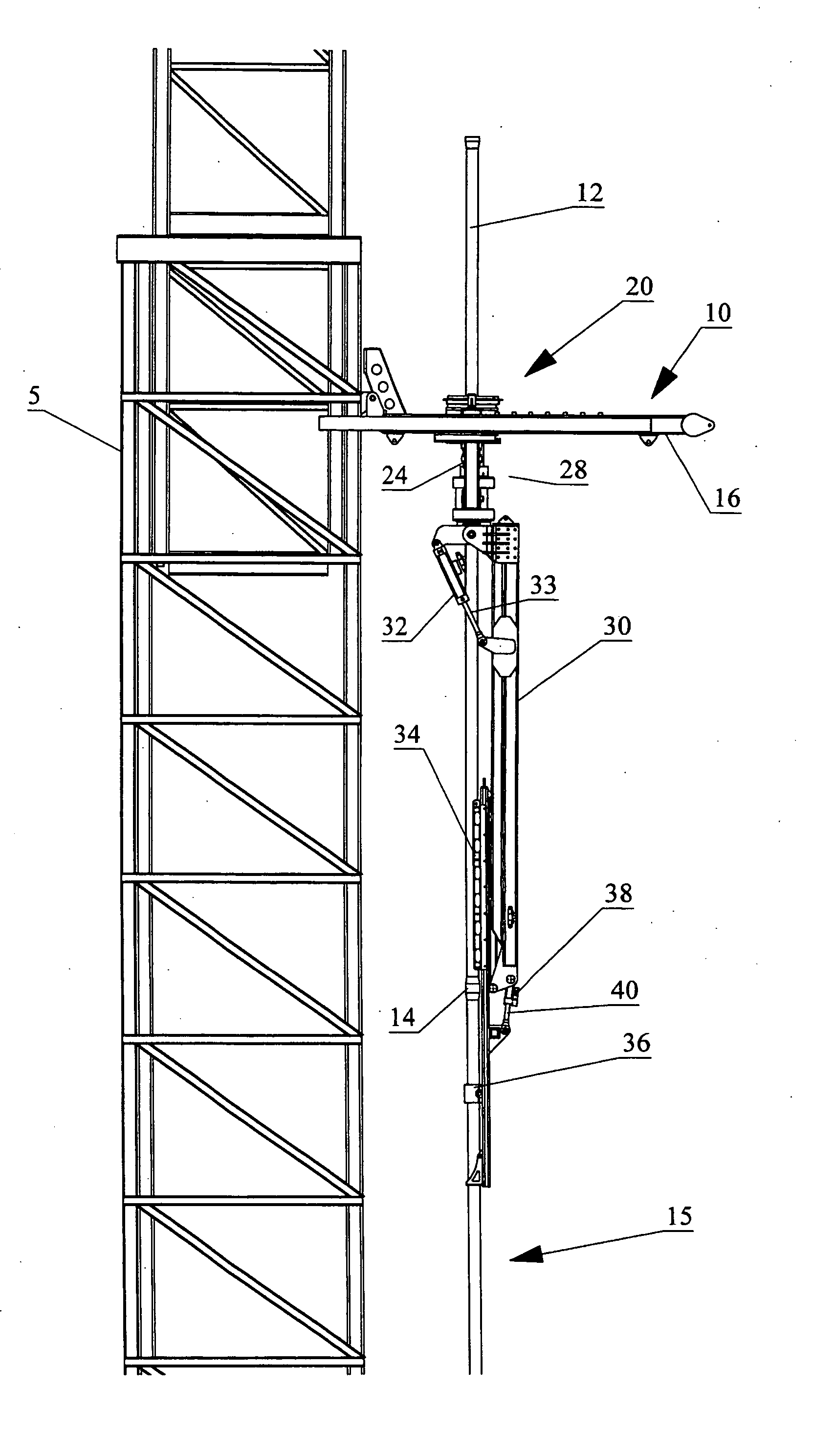
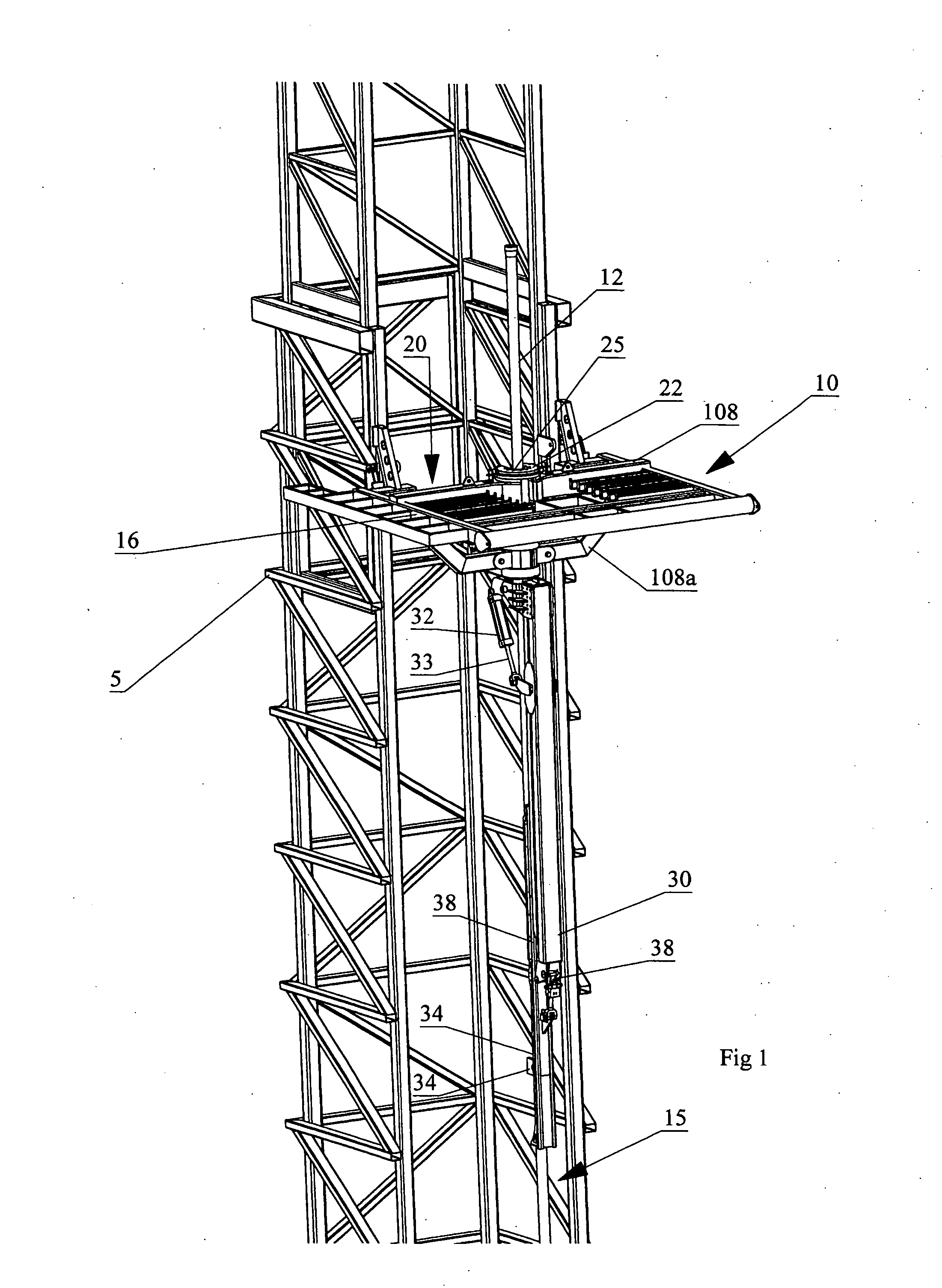
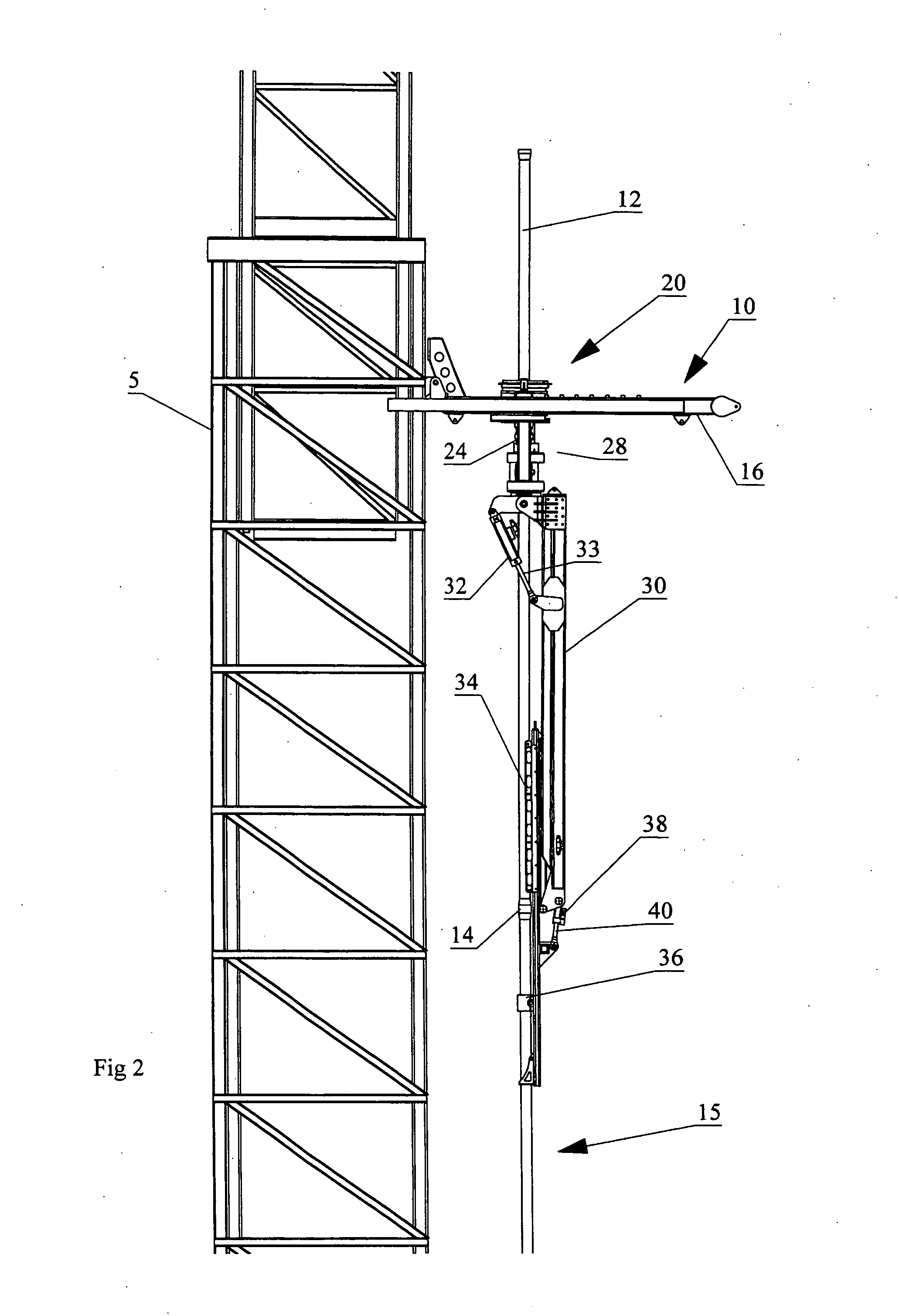
Apparatus for handling and racking pipes
An apparatus for handling pipes in a derrick and racking the pipes on a pipe racking assembly mounted on the derrick is provided to improve the stability of transferring pipes during a round trip operation. The apparatus includes a rotatable gate assembly mounted on the pipe racking assembly. The rotatable gate assembly includes a collar rotatably mounted to a first end of a shaft and an arm pivotably and rotatably mounted on a second end of the shaft. The collar defines a gate for securing an upper portion of the pipe stand. A first securing means mounted to a second end of the arm secures the mid-portion of the pipe stand and a second securing means mounted to the first securing means secures the lower portion of the pipe stand. After the arm secures the pipe stand from the derrick, a drive mechanism mounted to the shaft rotates the rotatable gate assembly such that the arm may rack the pipe on the pipe racking assembly.
Owner:WEATHERFORD CANADA
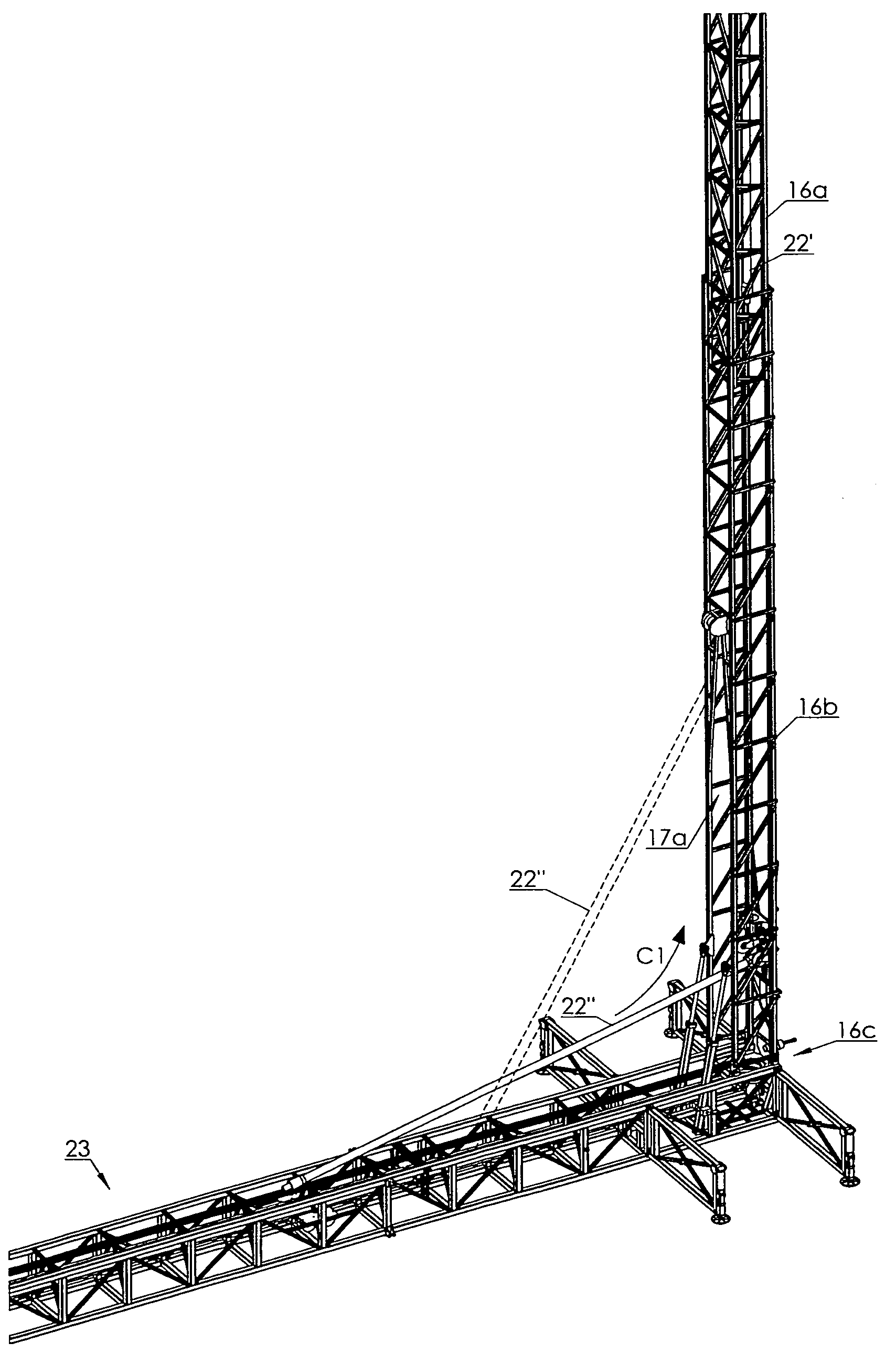
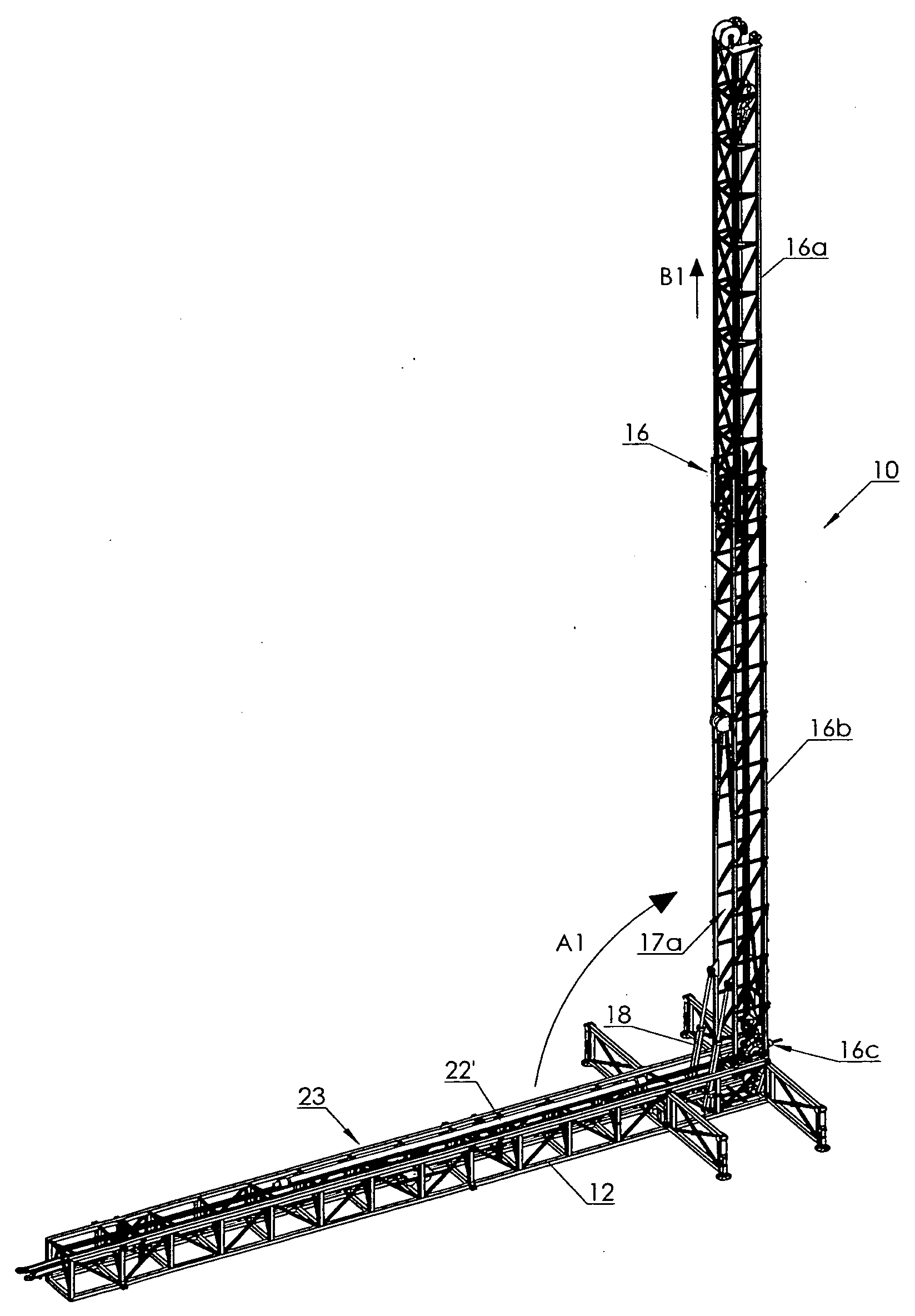
Vertical offline stand building and manipulating system
InactiveUS7967540B2RateImprove shelf efficiencyDrilling rodsFluid removalArchitectural engineeringPipe support
Owner:WEATHERFORD CANADA
Polycrystalline diamond thrust bearing and element thereof
ActiveUS20200032846A1Improve efficiencyImprove carrying capacityDrilling accessoriesShaftsPolycrystalline diamondThrust bearing
A thrust bearing assembly is provided, including a thrust ring defining a thrust face and an opposing thrust ring defining an opposing thrust face. At least one polycrystalline diamond element is coupled with the thrust face and defines an engagement surface. The opposing thrust ring includes a diamond reactive material. In operation, the engagement surface is in contact with the opposing thrust face. Also provided are methods of making, assembling, and using the same, as well as to systems and apparatus including the same.
Owner:XR RESERVE LLC
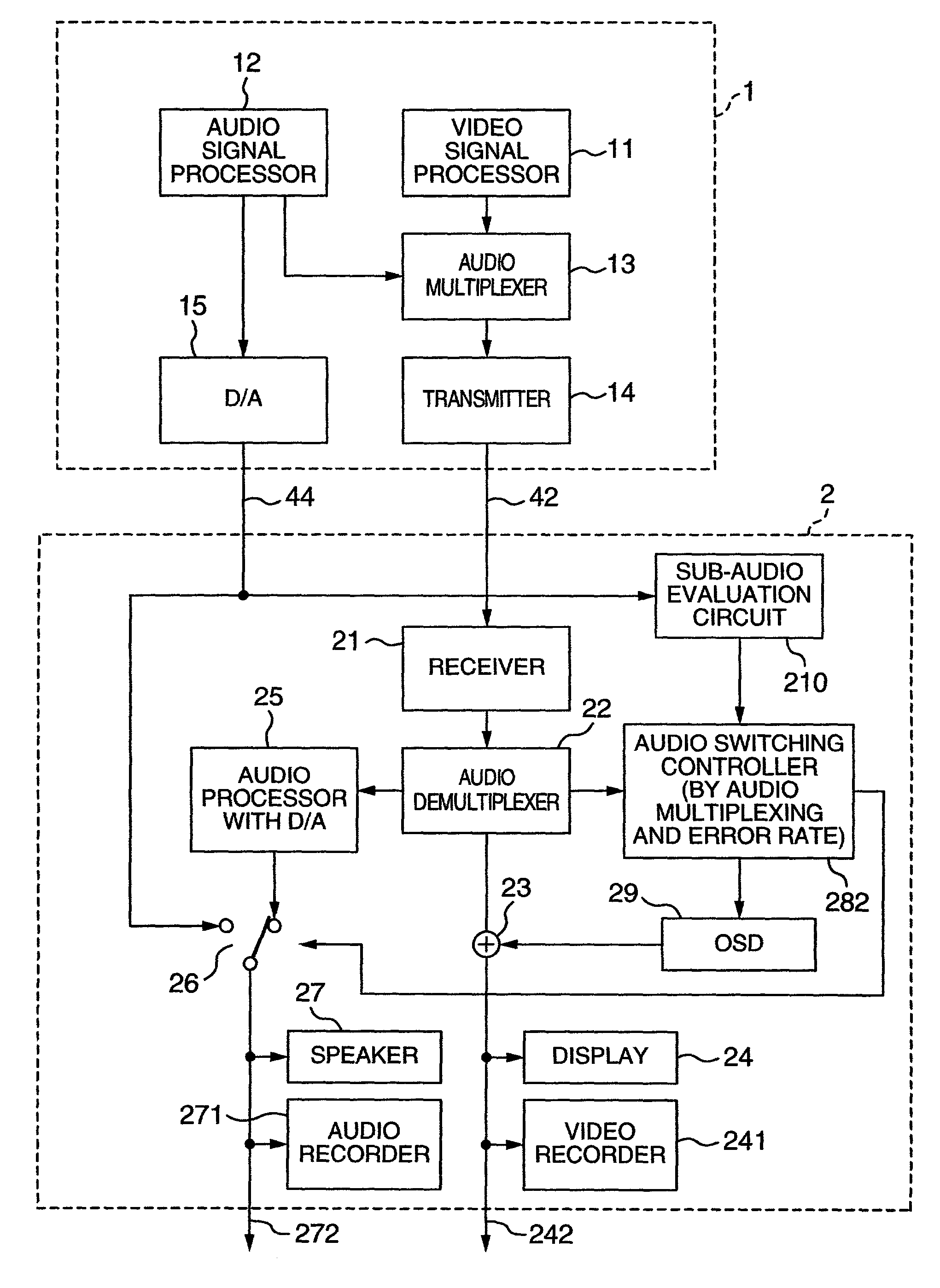
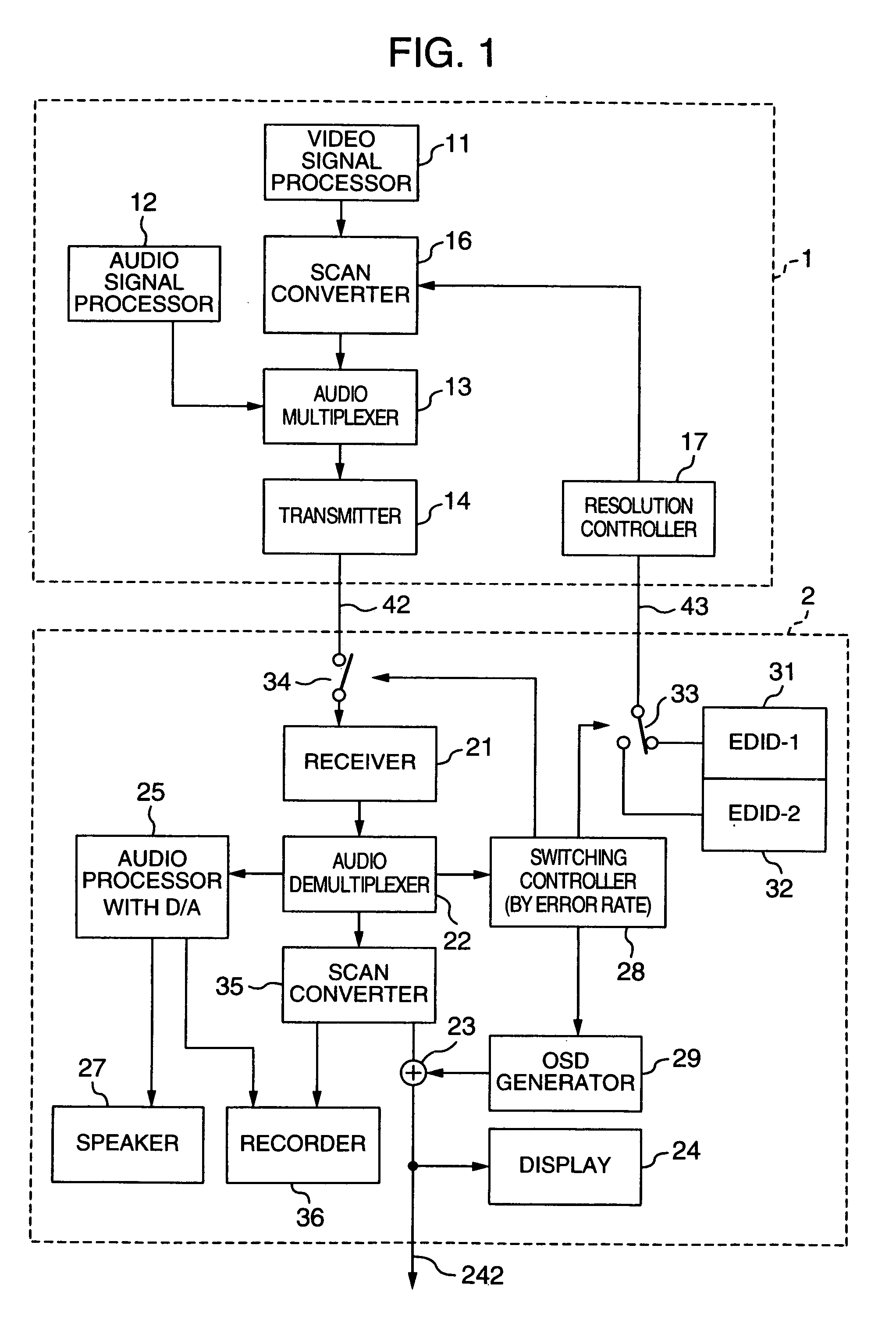
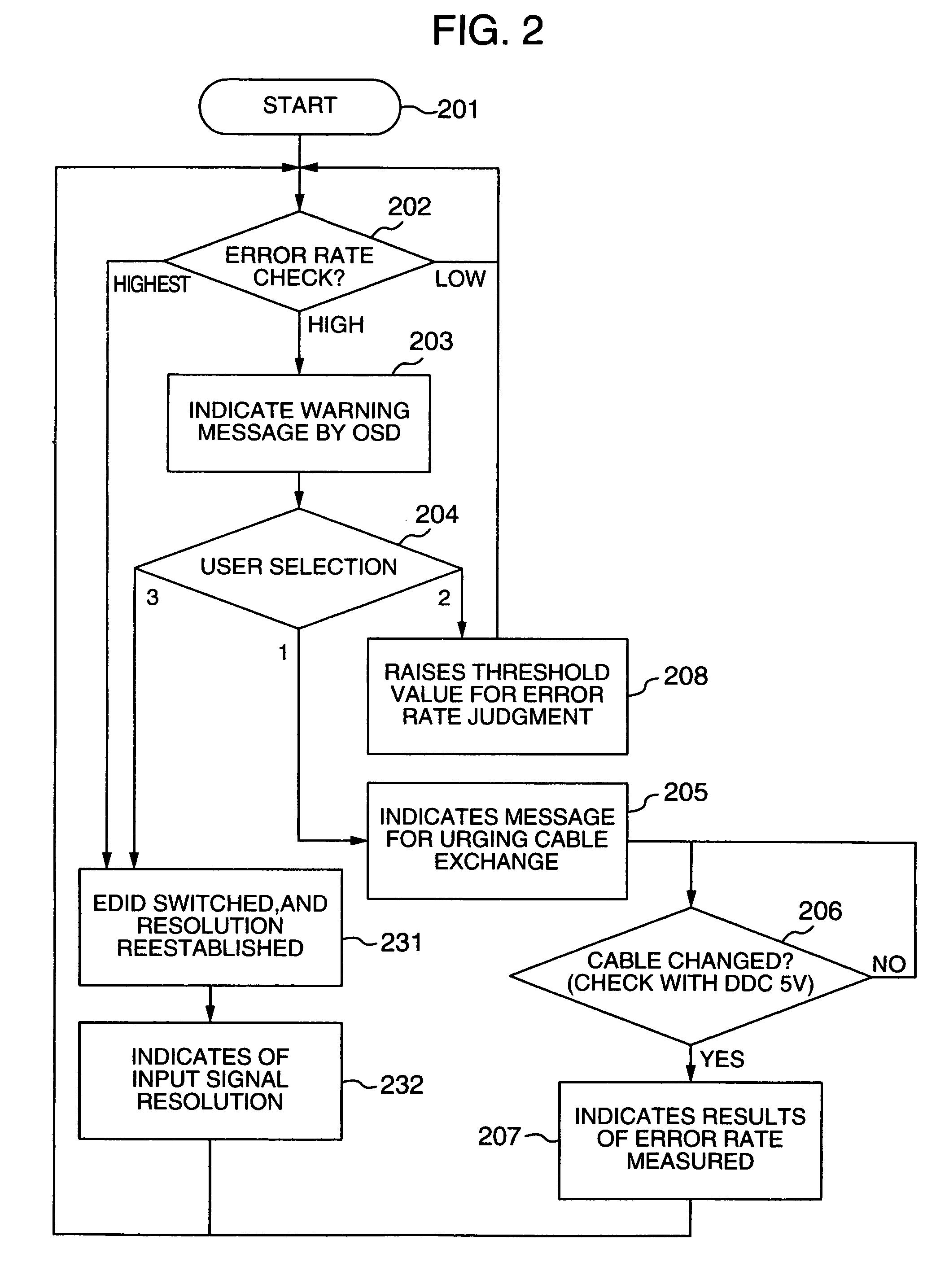
Video processing device
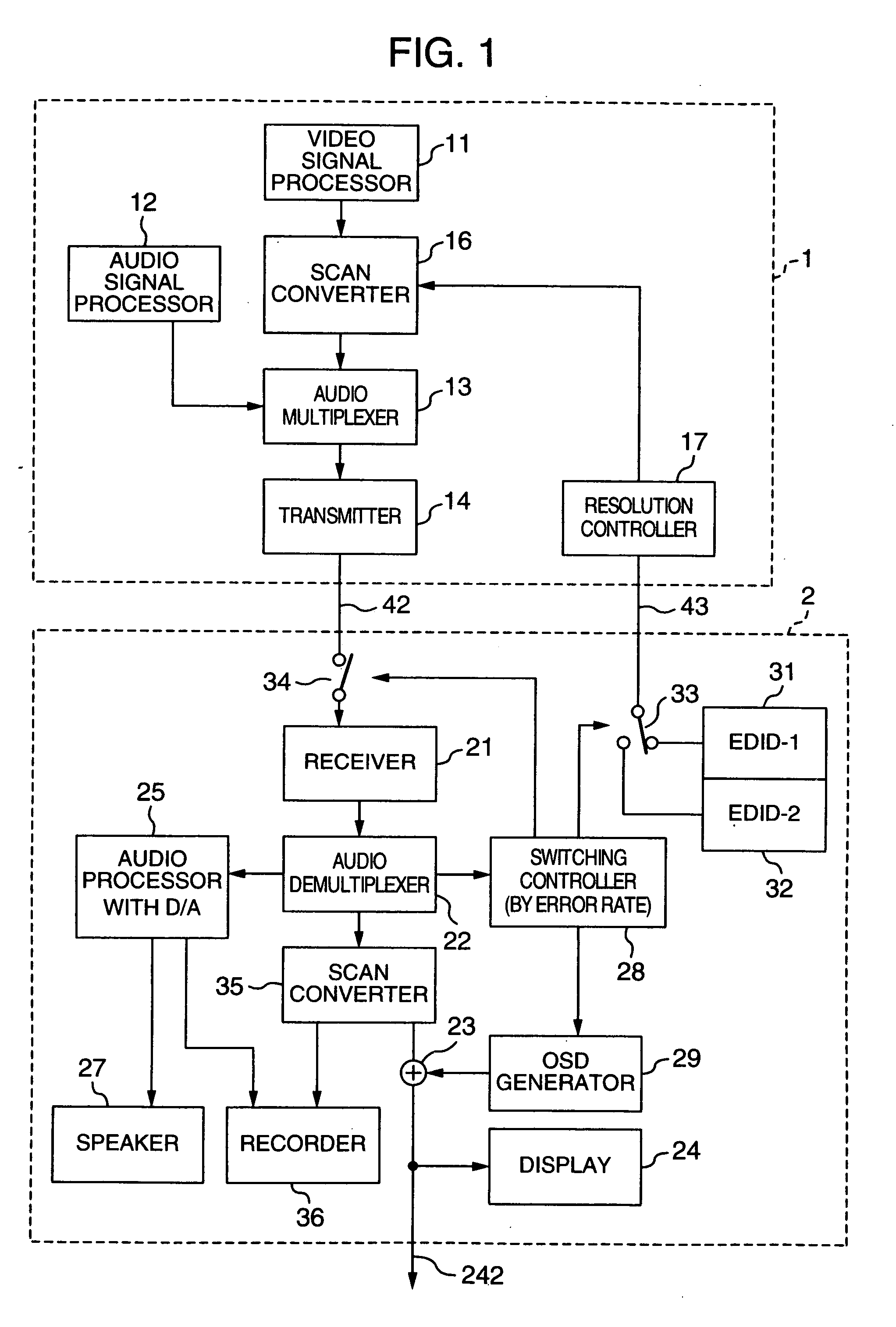
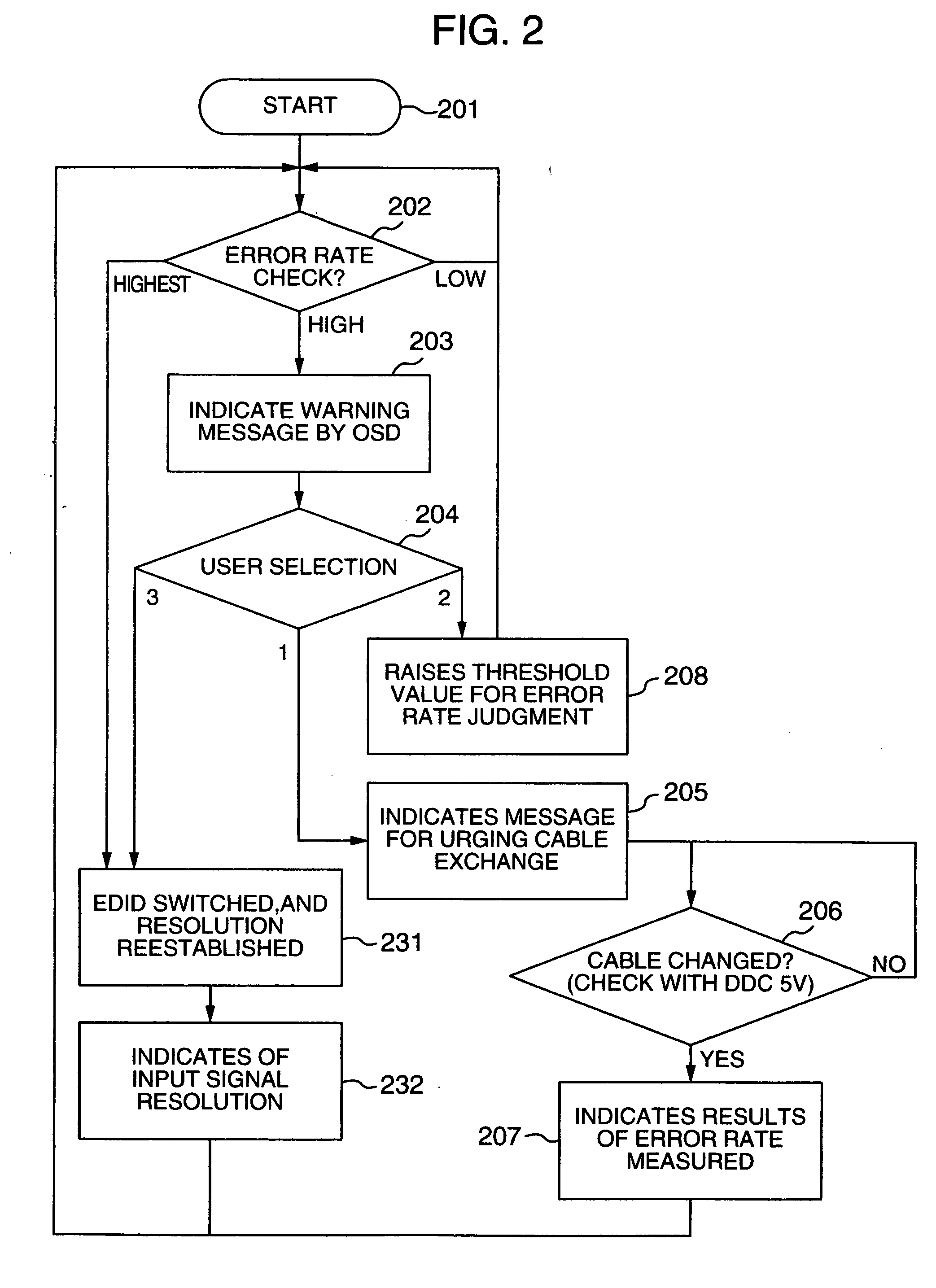
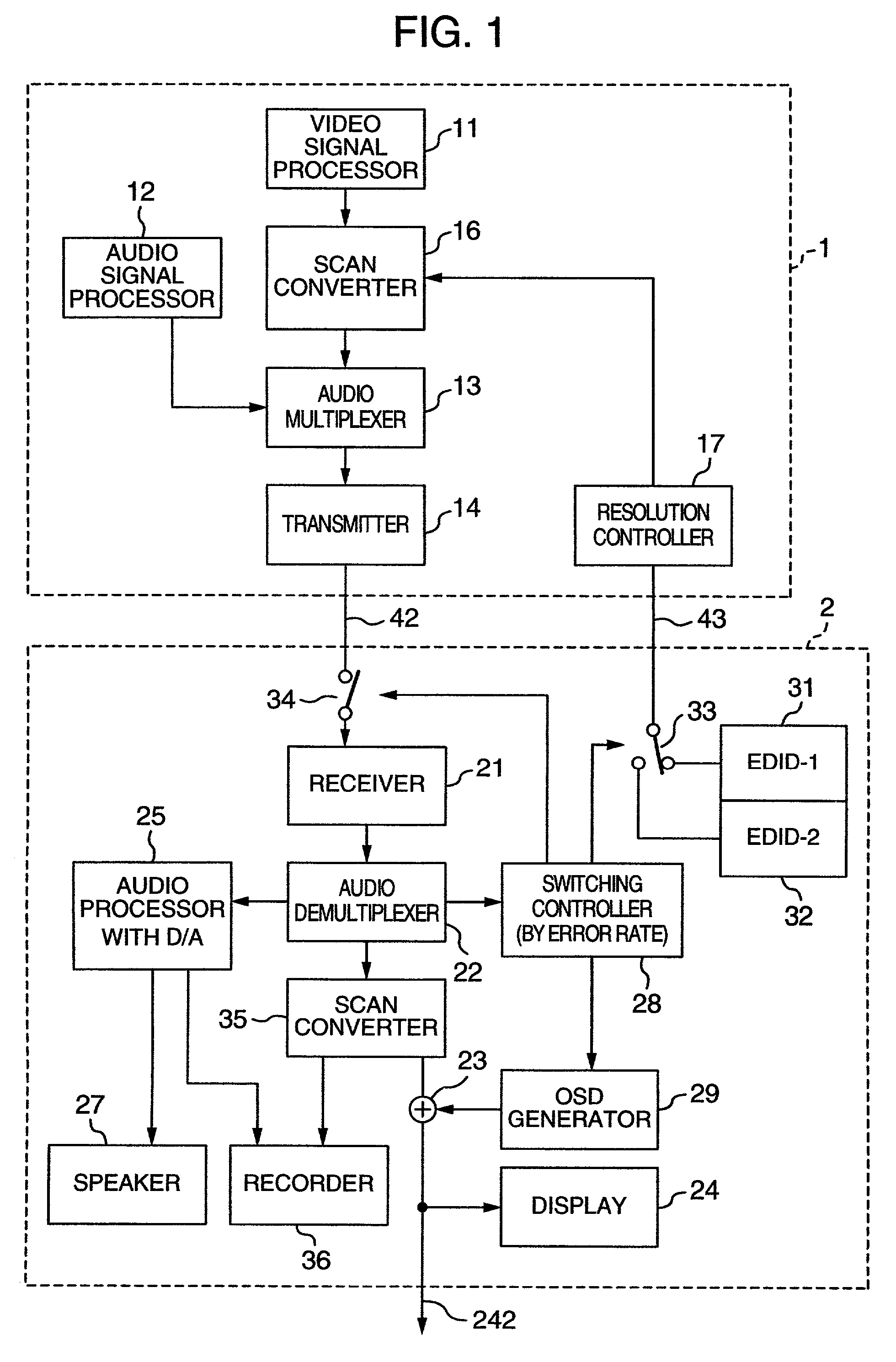
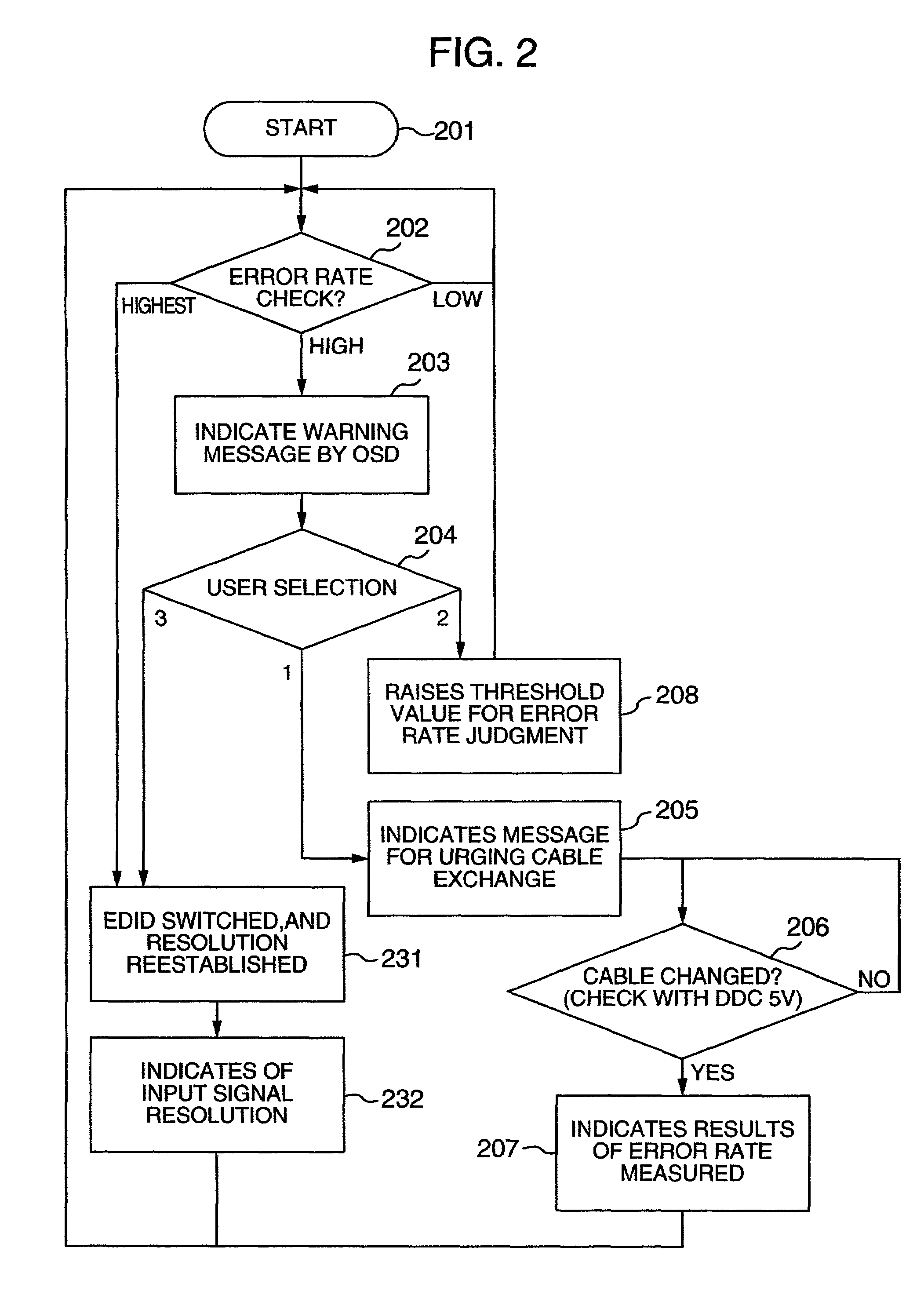
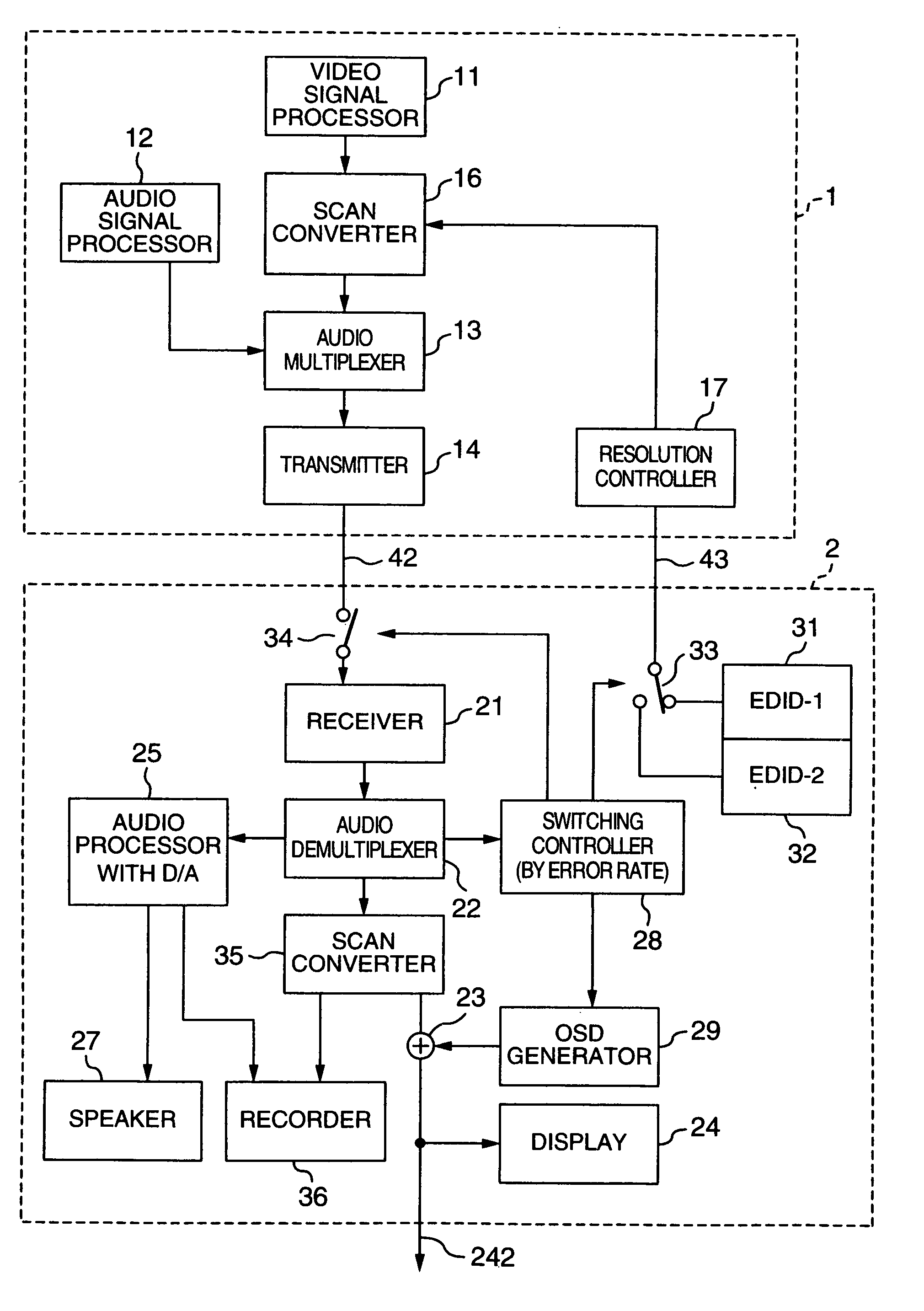
InactiveUS20060125959A1RateHigh error rateTelevision system detailsColor television detailsDigital videoVideo processing
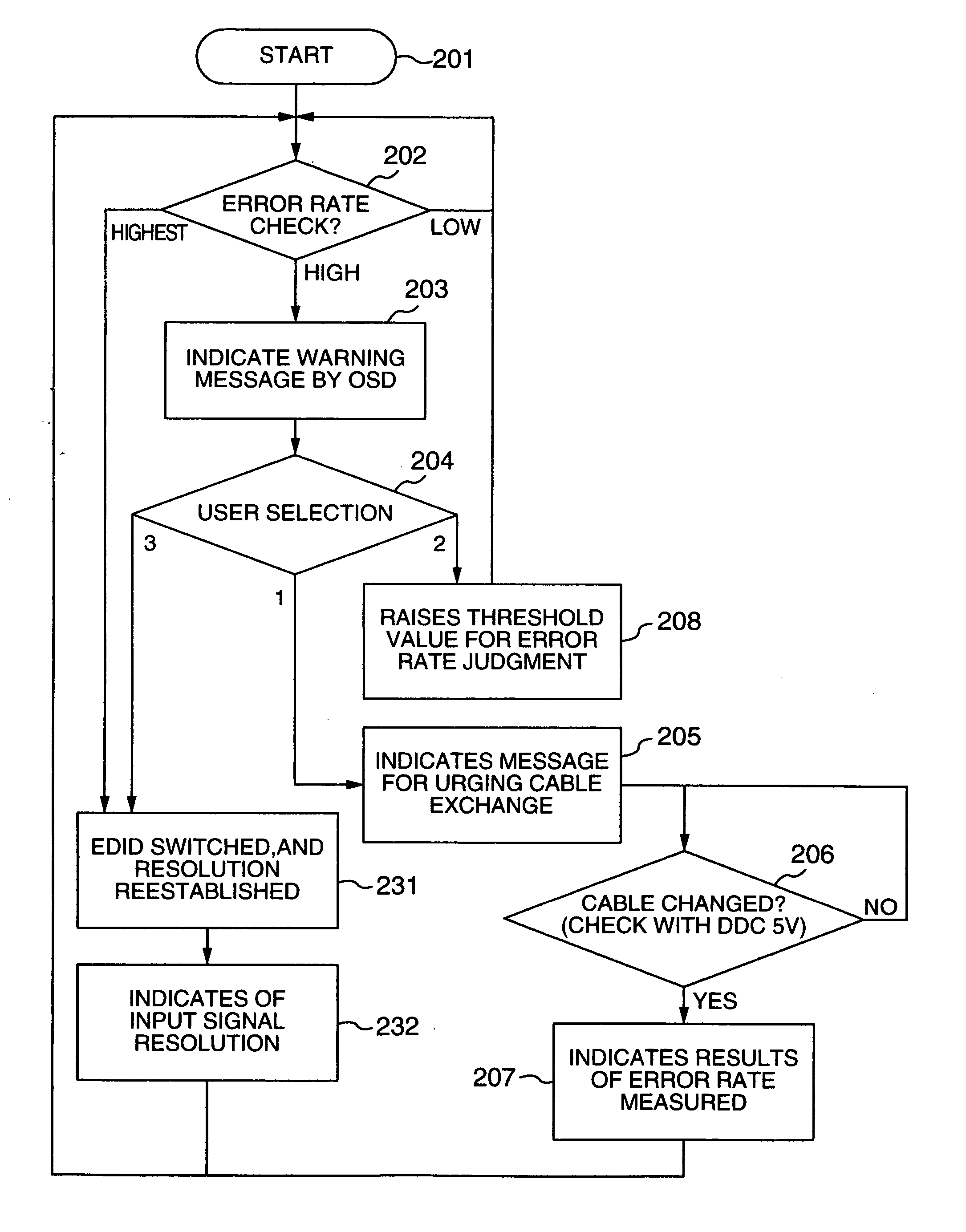
A video processing device includes a control circuit for detecting a physical quantity related to a size of noise contained in the input video signal, and an OSD (On Screen Display) circuit for providing a user with a warning screen when the physical quantity detected by the control circuit is larger than a prescribed value, obtaining an appropriate video signal even when a digital video signal has poor quality.
Owner:HITACHI LTD
Apparatus for handling and racking pipes
An apparatus and method for handling pipes in a derrick and racking the pipes on a pipe racking assembly mounted on the derrick. The apparatus includes a rotatable gate assembly rotatably mounted on the pipe racking assembly. The rotatable gate assembly includes a collar rotatably mounted to a first end of a rotatable pipe support. A pipe manipulator arm is pivotably mounted on a second end of the rotatable gate assembly. The collar defines a gate for securing an upper portion of the pipe stand. A pipe mount is mounted to a distal end of the arm for holding the pipe stand for transport between the derrick and the gate, and between the gate and the pipe rack. After the arm secures the pipe stand into the gate from the derrick, a drive mechanism rotates the rotatable gate assembly to a rack facing position from the derrick facing position such that the arm may transport the pipe between the gate and the pipe rack.
Owner:WEATHERFORD CANADA
Vertical offline stand building and manipulating system
ActiveUS20090185883A1RateImprove shelf efficiencyDrilling rodsFluid removalArchitectural engineeringPipe support
A vertical off-line stand building system includes a mast having a basal portion and an upper portion. An upper pipe elevator is mounted in the upper portion of the mast and is selectively translatable by a selecting actuable first hoist between upper raised and lowered positions. A lower pipe elevator is mounted in the basal portion of the mast and is selectively translatable by a selecting actuable second hoist between basal raised and lowered positions. A horizontal pipe support table is positioned adjacent the base of the mast. A horizontal pipe translator is mounted in the table for translating a pipe single longitudinally along the table so as to engage a first end of the pipe single with the lower pipe elevator when in its basal lowered position. The lower pipe elevator draws the first pipe into the mast through a front opening in the mast. The upper pipe elevator takes the first pipe and elevates it to the top of the mast. The lower pipe elevator raises the next pipe into the mast which is then threaded to the first pipe and the pipe stand then elevated by the upper pipe elevator so that the lower pipe elevator may add successive pipes to the pipe stand. The rear of the mast is entirely open so that the assembled pipe stand may be removed.
Owner:WEATHERFORD CANADA
Apparatus for handling and racking pipes
An apparatus for handling pipes in a derrick and racking the pipes on a pipe racking assembly mounted on the derrick is provided to improve the stability of transferring pipes during a round trip operation. The apparatus includes a rotatable gate assembly mounted on the pipe racking assembly. The rotatable gate assembly includes a collar rotatably mounted to a first end of a shaft and an arm pivotably and rotatably mounted on a second end of the shaft. The collar defines a gate for securing an upper portion of the pipe stand. A first securing means mounted to a second end of the arm secures the mid-portion of the pipe stand and a second securing means mounted to the first securing means secures the lower portion of the pipe stand. After the arm secures the pipe stand from the derrick, a drive mechanism mounted to the shaft rotates the rotatable gate assembly such that the arm may rack the pipe on the pipe racking assembly.
Owner:WEATHERFORD CANADA
Video processing device
InactiveUS7019791B2Small error rateReduce resolutionTelevision system detailsColor television detailsDigital videoVideo processing
A video processing device includes a control circuit for detecting a physical quantity related to a size of noise contained in the input video signal, and an OSD (On Screen Display) circuit for providing a user with a warning screen when the physical quantity detected by the control circuit is larger than a prescribed value, obtaining an appropriate video signal even when a digital video signal has poor quality.
Owner:HITACHI LTD
C-1 inhibitor prevents non-specific plasminogen activation by a prourokinase mutant without impeding fibrin-specific fibrinolysis
ActiveUS7837992B2Attenuation of rateHigh dose tolerancePeptide/protein ingredientsBlood disorderC1-inhibitorHigh doses
Owner:THROMBOLYTIC SCI +1
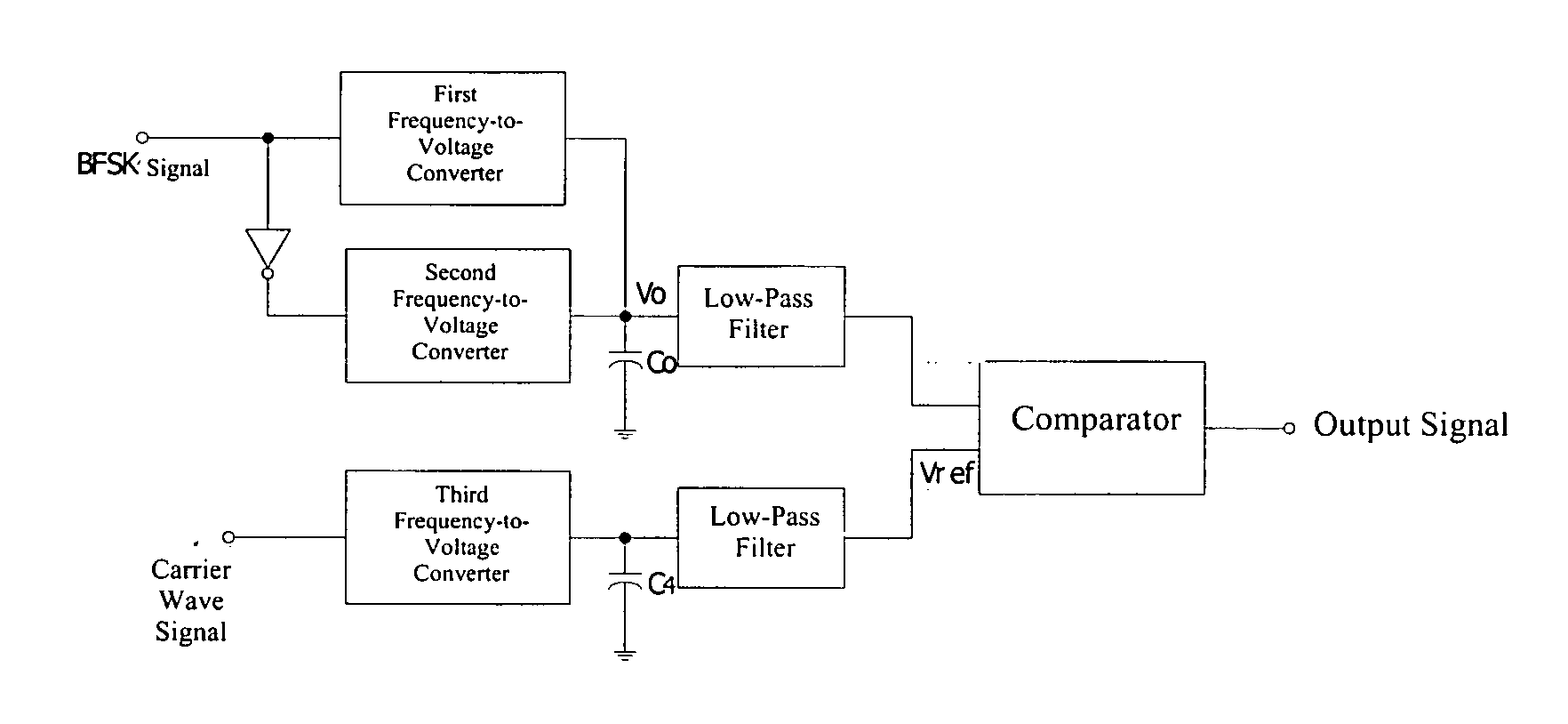
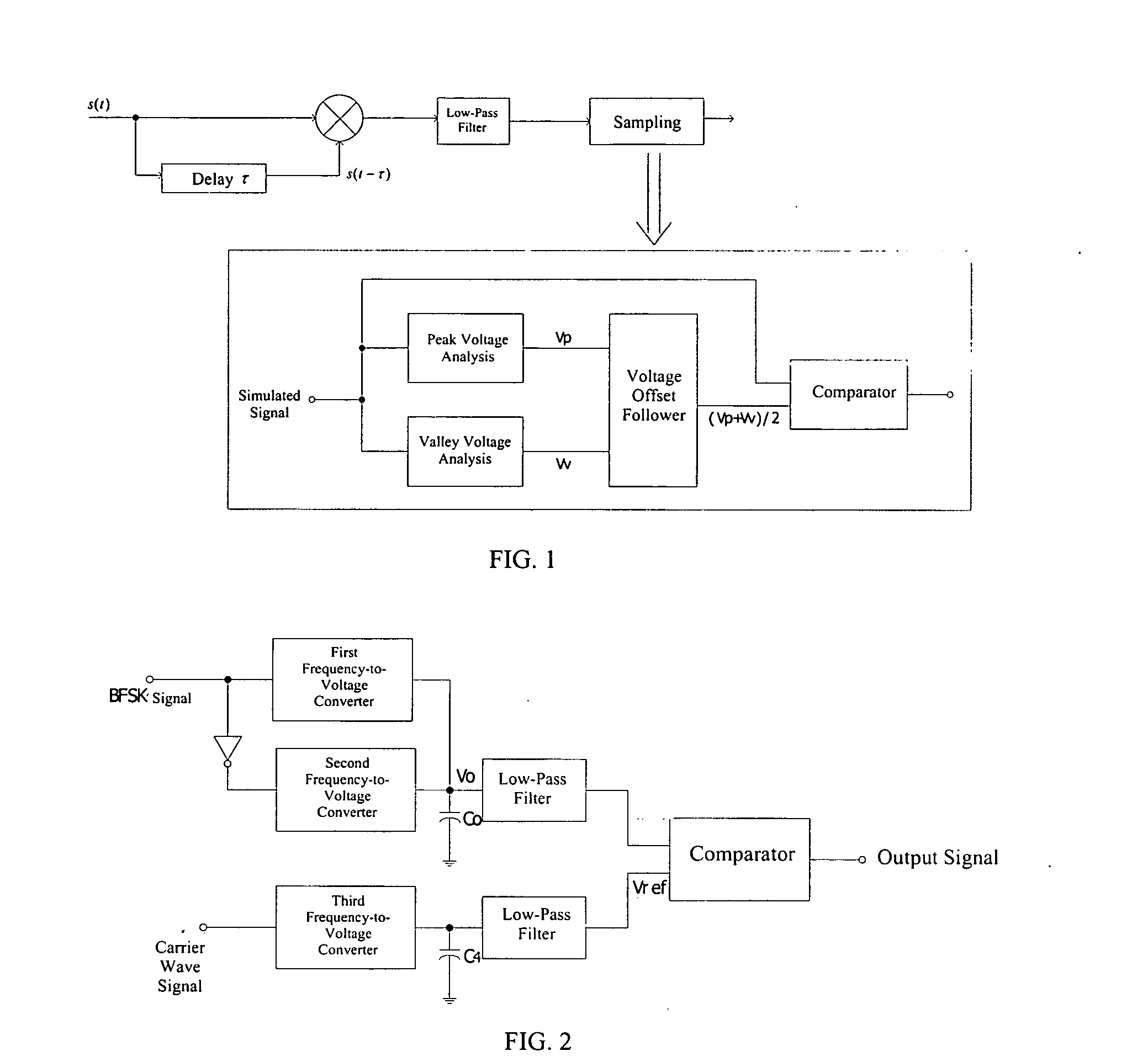
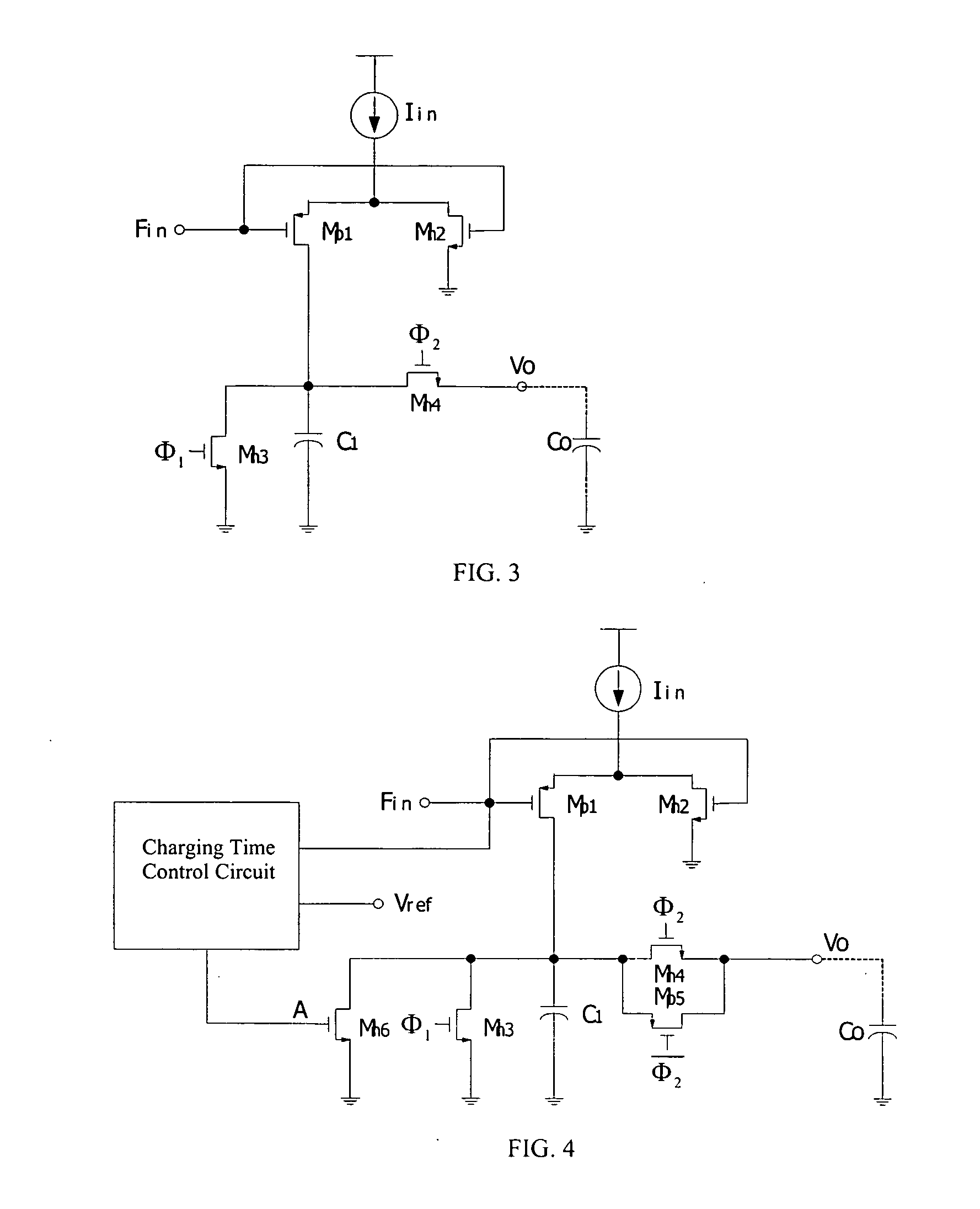
Binary frequency-shift keying demodulator and frequency-to-voltage converter
ActiveUS20050225383A1Reduce charge injection effectSimple and compact designDc-dc conversionAmplitude-modulated carrier systemsVoltage converterLow-pass filter
A BFSK demodulator comprises a three-channel frequency-to-voltage converter, an information signal inputting the first-channel frequency-to-voltage converter, a converted information signal inputting the second-channel frequency-to-voltage converter, wherein outputs of first and second channel frequency-to-voltage converter are connected with a capacitor, a output voltage signal produced by the first and second frequency-to-voltage converters and the capacitor, inputting into a positive terminal of the comparator after high frequency noise filtering through a first low-pass filter, a carrier signal inputs into the third-channel frequency-to-voltage converter; and an output from the third-channel frequency-to-voltage converter connected to a capacitor; and a reference voltage signal is produced after high frequency noise filtering through a second low-pass filter, producing a demodulated signal after comparing the information voltage signal with the reference voltage.
Owner:SPREADTRUM COMM (SHANGHAI) CO LTD
Polypeptides having DNA polymerase activity
ActiveUS7704713B2RateSugar derivativesMicrobiological testing/measurementBiotechnologyGenetic engineering
A polypeptide having a high fidelity DNA polymerase activity and thus being useful as a genetic engineering reagent; a gene encoding this polypeptide; a method of producing the polypeptide; and a method of amplifying a nucleic acid by using the polypeptide.
Owner:TAKARA HOLDINGS
Data label verification
ActiveUS20210158195A1Ensures correctnessConfidenceEnsemble learningProbabilistic networksData setSmall sample
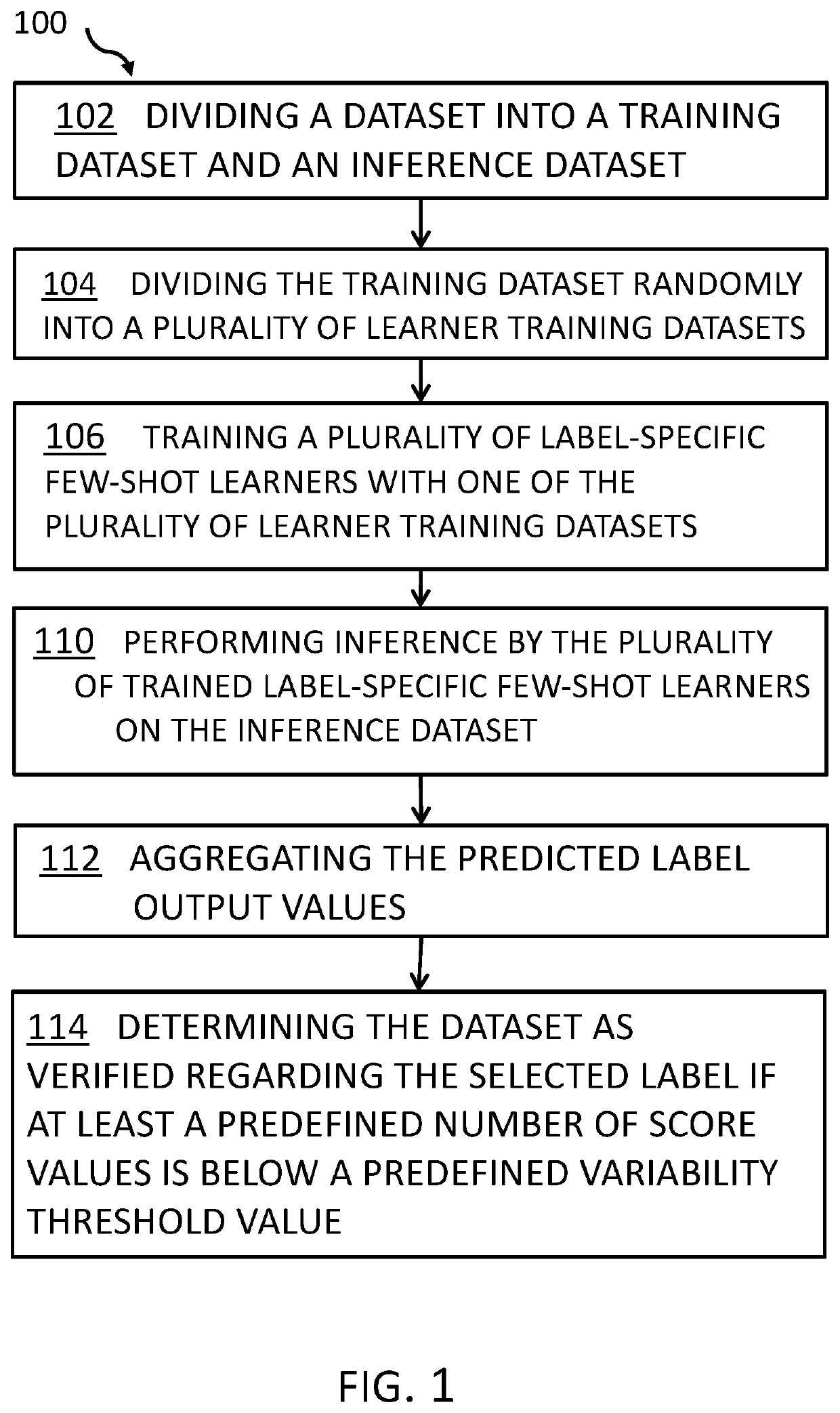
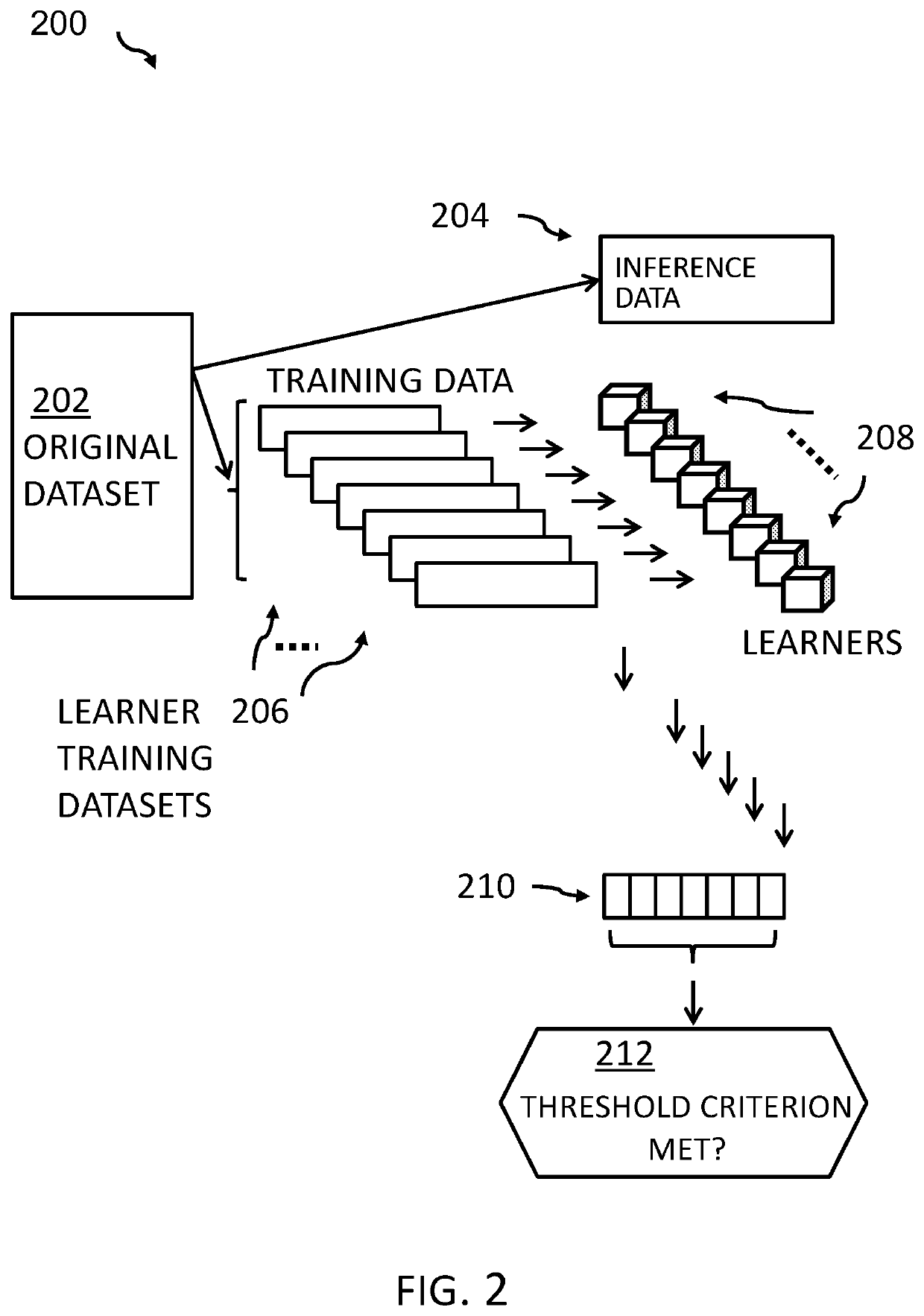
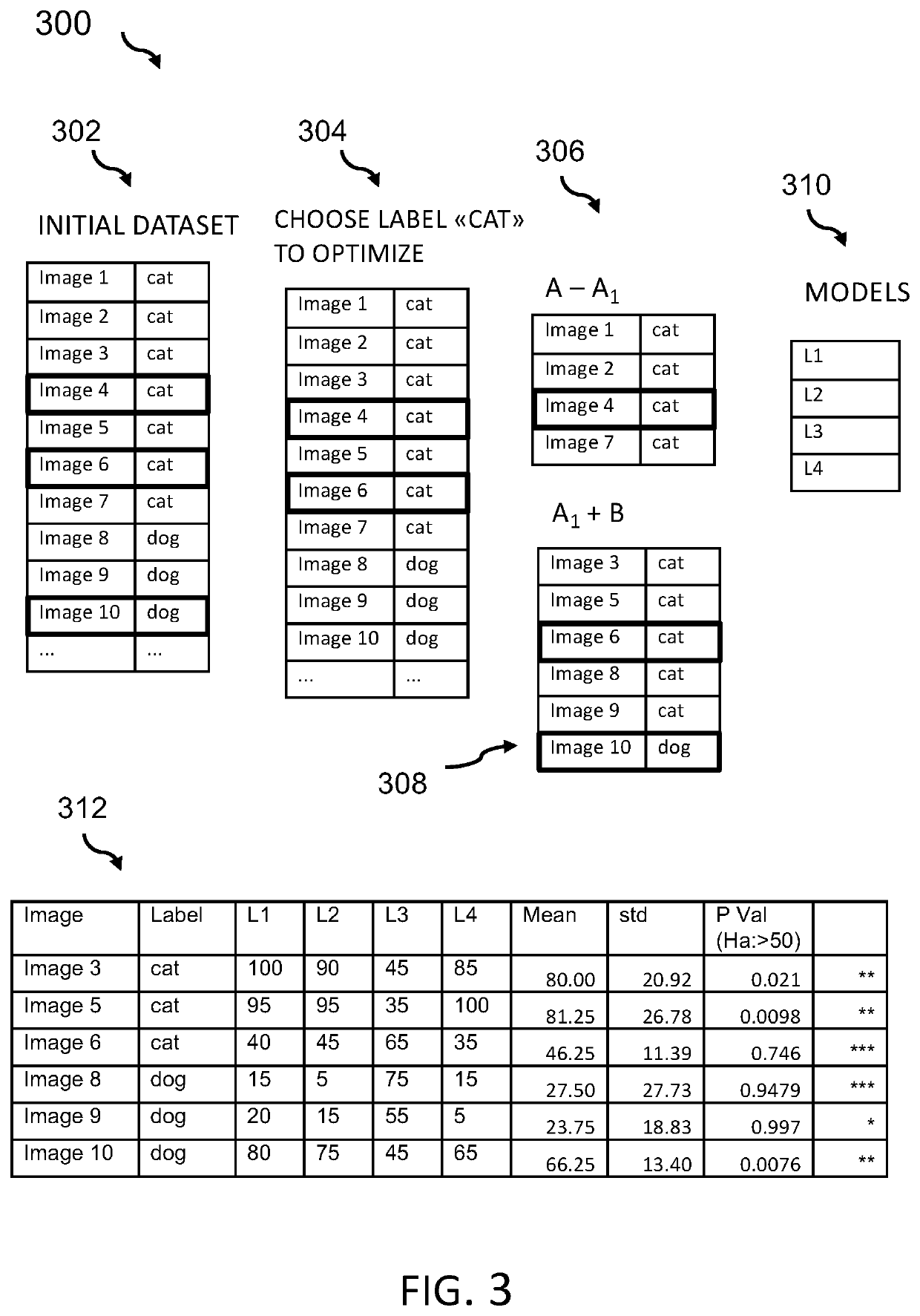
Aspects of the present invention disclose a method for verifying labels of records of a dataset. The records comprise sample data and a related label out of a plurality of labels. The method includes one or more processors dividing the dataset into a training dataset comprising records relating to a selected label and an inference dataset comprising records with sample data relating to the selected label and all other labels out of the plurality of labels. The method further includes dividing the training dataset into a plurality of learner training datasets that comprise at least one sample relating to the selected label. The method further includes training a plurality of label-specific few-shot learners with one of the learner training datasets. The method further includes performing inference by the plurality of trained label-specific few-shot learners on the inference dataset to generate a plurality of sets of predicted label output values.
Owner:IBM CORP
Video processing device
InactiveUS7289168B2RateHigh error rateTelevision system detailsColor television detailsDigital videoVideo processing
A video processing device includes a control circuit for detecting a physical quantity related to a size of noise contained in the input video signal, and an OSD (On Screen Display) circuit for providing a user with a warning screen when the physical quantity detected by the control circuit is larger than a prescribed value, obtaining an appropriate video signal even when a digital video signal has poor quality.
Owner:HITACHI LTD
Method and process of producing short chain fatty acids from waste stream containing phenolic lignin model compounds by controlled photocatalytic oxidation with titanium dioxide nanocatalyst in the presence of ultraviolet radiation
InactiveUS20110084231A1Hazard reductionEasy to controlMaterial nanotechnologyPhysical/chemical process catalystsPhotocatalytic degradationNano catalyst
A method of producing short chain carbon compounds from effluents that are rich in lignin-model compounds. The method is characterized by controlled photocatalytic degradation of lignin model compounds so as to produce short chain carbon compounds. The present invention provides converting recalcitrant and toxic organic compounds into chemicals which are of commercial value.
Owner:LALMAN JERALD A D +1
System for injecting fuel into a turbomachine
InactiveUS6854257B2Mitigate such drawbackSimplify the hydraulic circuitGas turbine plantsRotary piston pumpsCombustion chamberFuel tank
A system for injecting fuel into a turbomachine comprising N fuel injectors placed in a combustion chamber of the turbomachine and fed from a fuel tank, the injection system further comprising, interposed between the N injectors and the tank, a single pumping means for taking fuel from the tank and delivering N metered flow rates of fuel to the injectors.
Owner:SN DETUDE & DE CONSTR DE MOTEURS DAVIATION S N E C M A
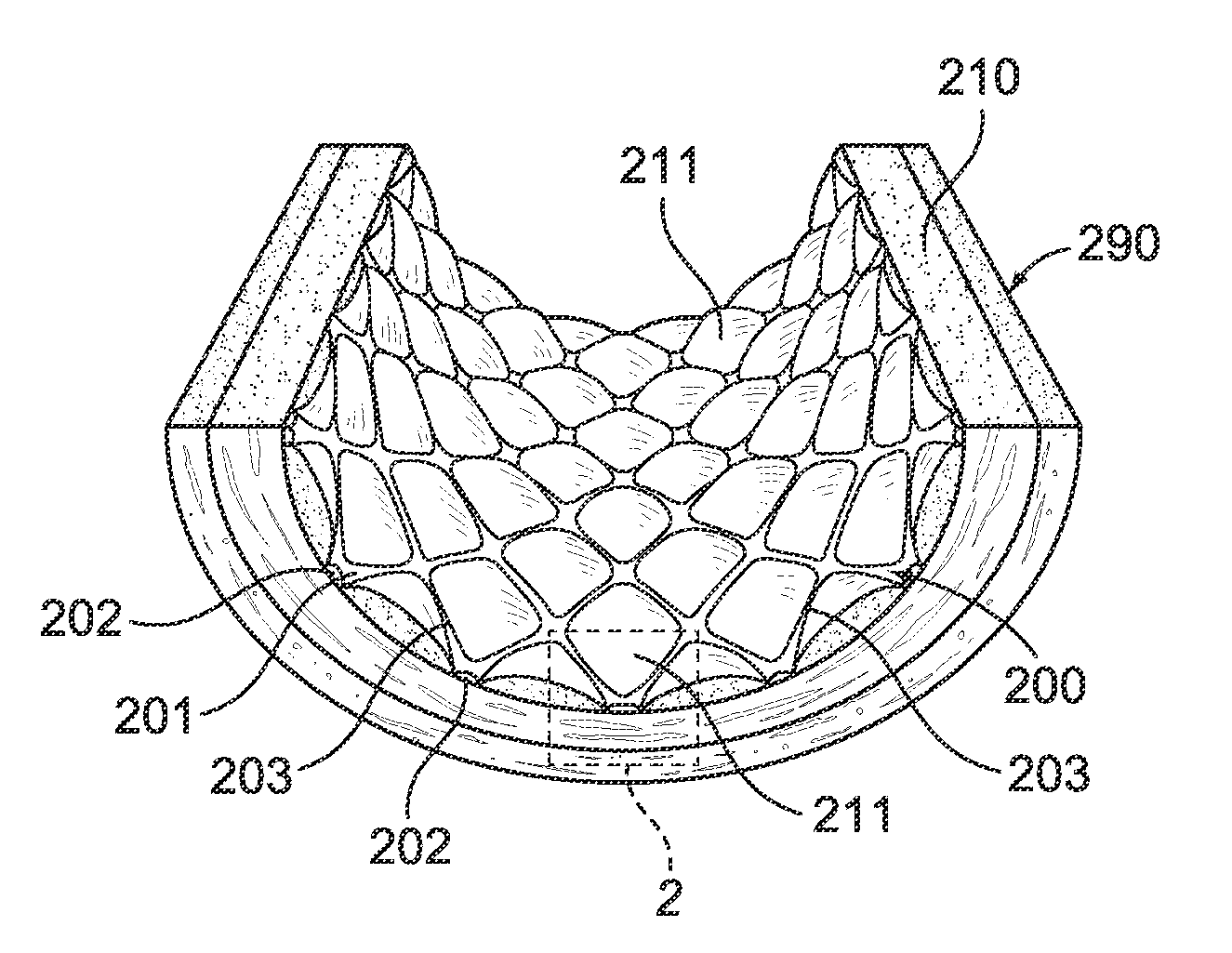
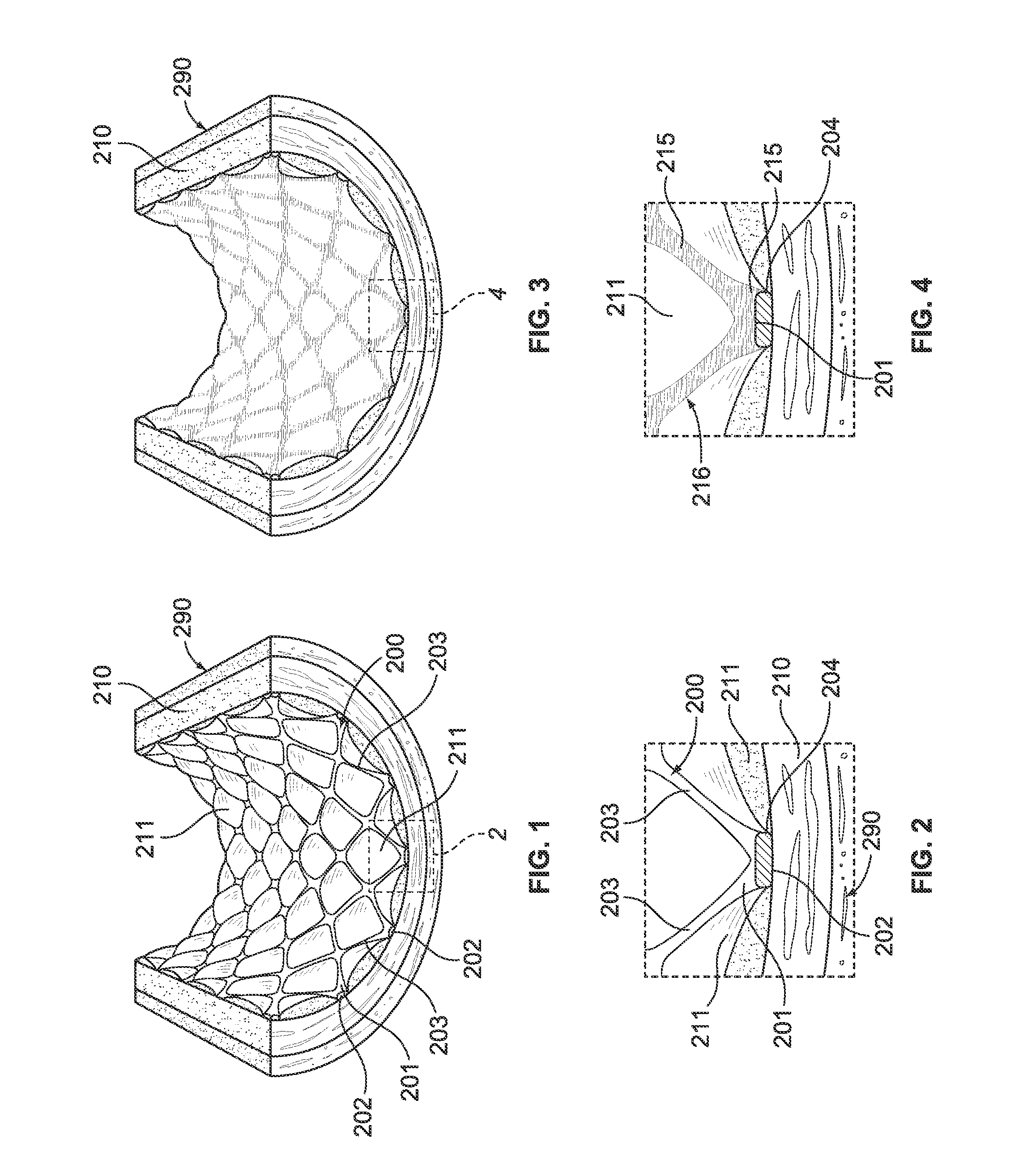
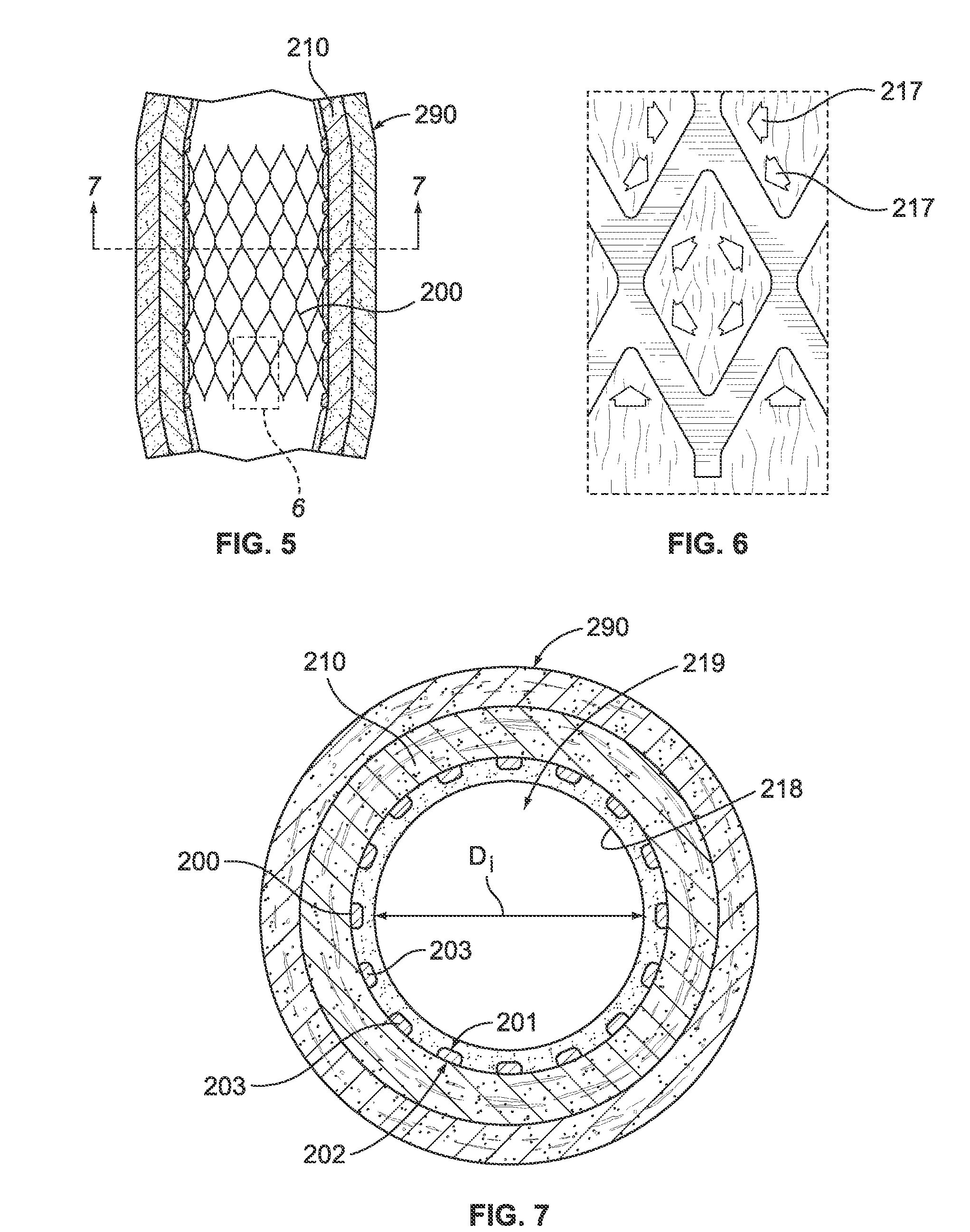
Grooved drug-eluting medical devices and method of making same
InactiveUS20120185037A1Promote rapid and healthy developmentRateStentsPharmaceutical containersMedicinePharmaceutical drug
The invention relates to methods and apparatus for manufacturing implantable medical devices, such as intravascular stents, wherein the medical device has a surface treated to promote the migration of endothelial cells onto the surface of the medical device. In particular, the surface of the medical device has at least one groove formed therein, the at least one groove may have a drug-eluting polymer disposed therein or a drug-eluting polymer coating may be provided on the surface of the medical device and grooves formed in the drug eluting polymer coating.
Owner:VACTRONIX SCI LLC
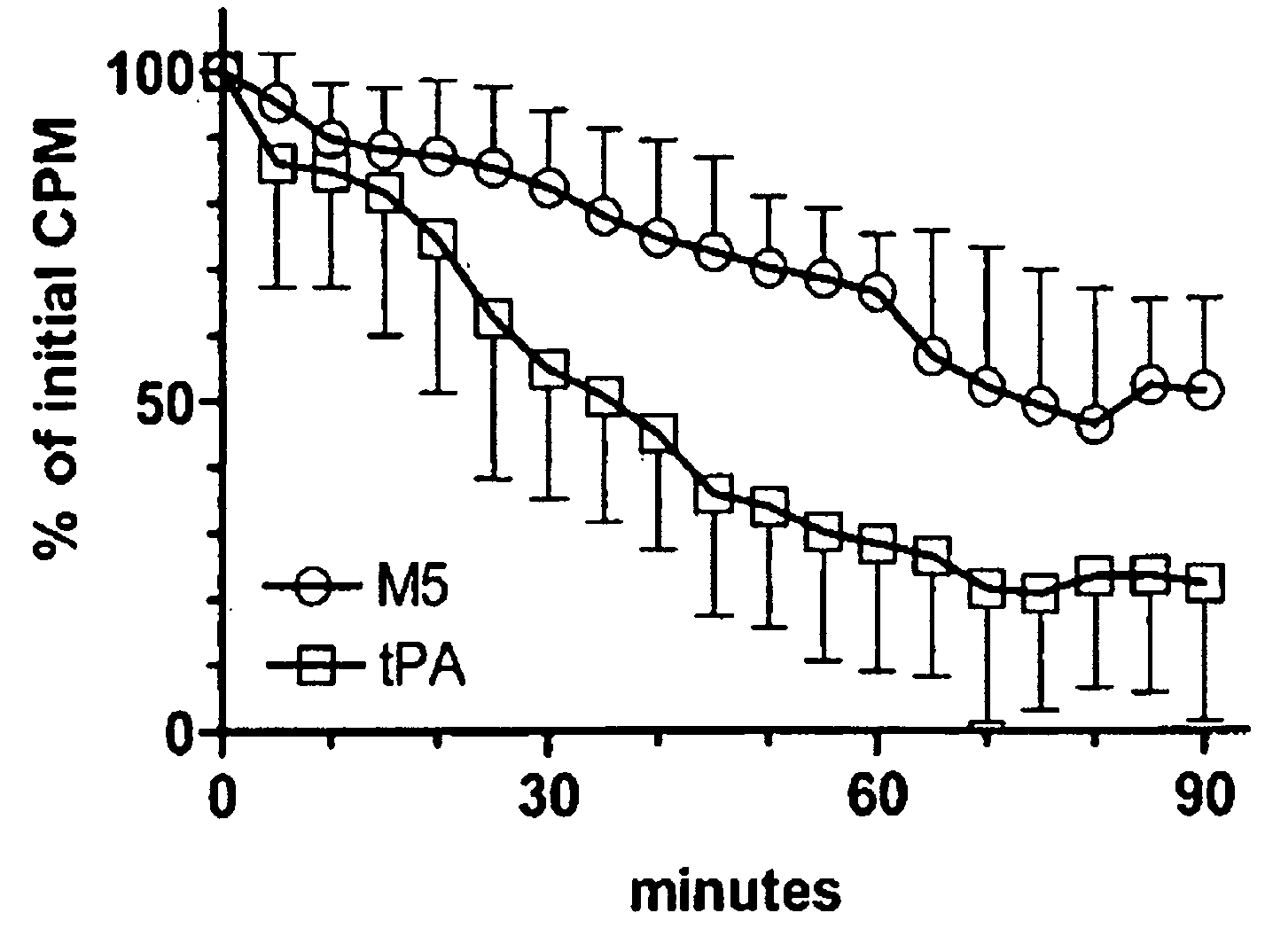
C-1 Inhibitor prevents non-specific plasminogen activation by a prourokinase mutant without impeding fibrin-specific fibrinolysis
InactiveUS20090010916A1High dose toleranceInhibition of activationPeptide/protein ingredientsBlood disorderTolerabilityC1-inhibitor
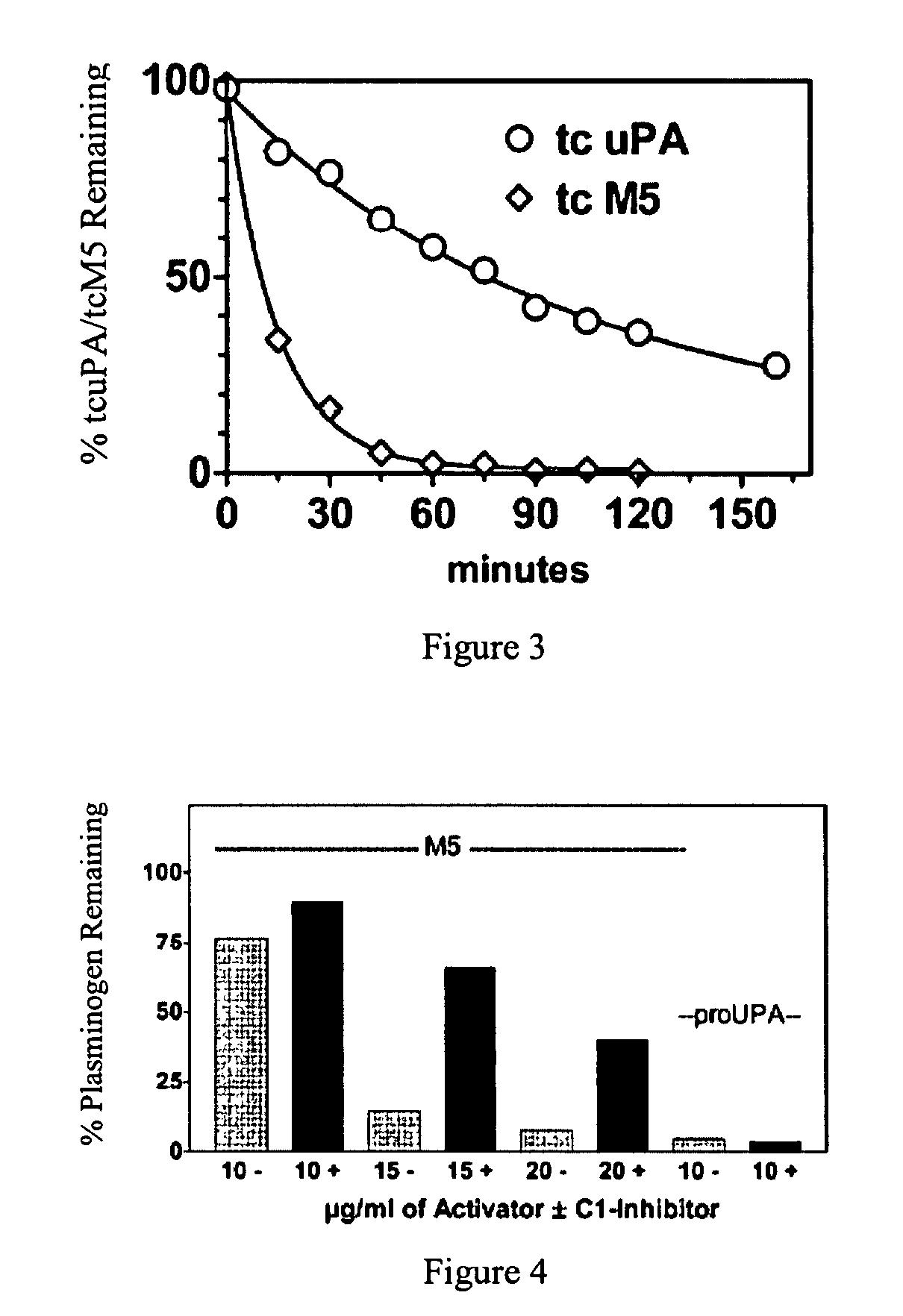
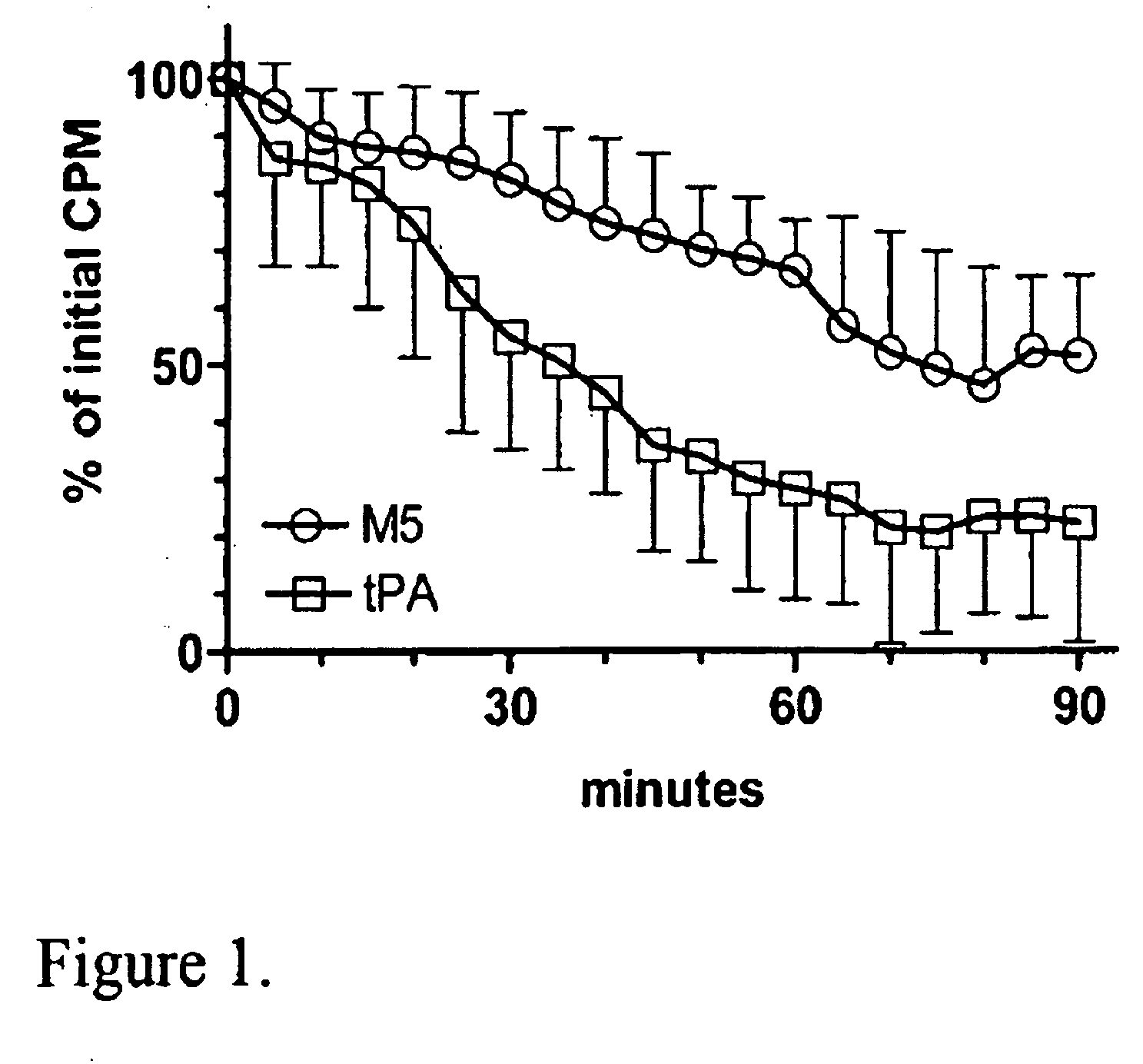
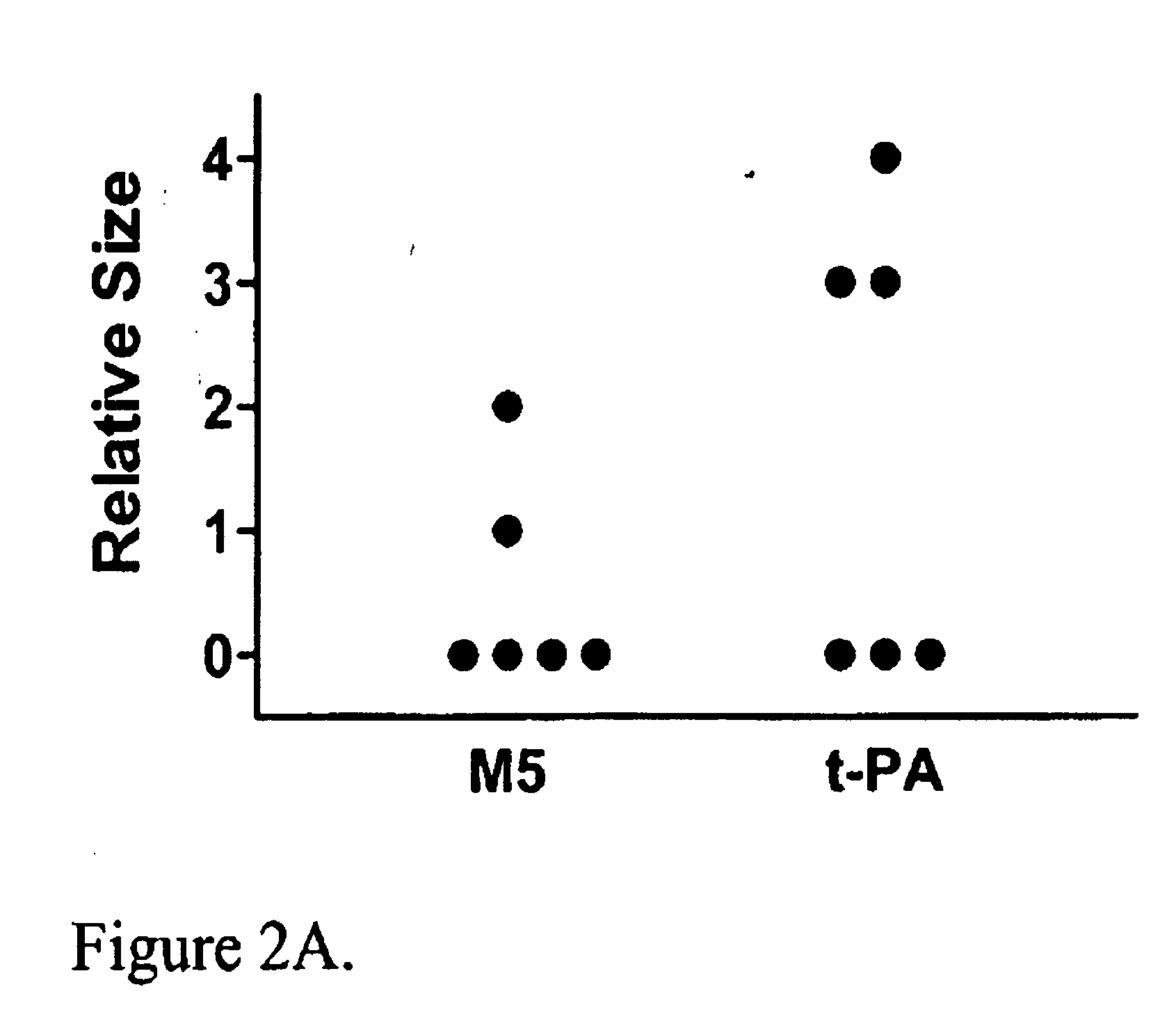
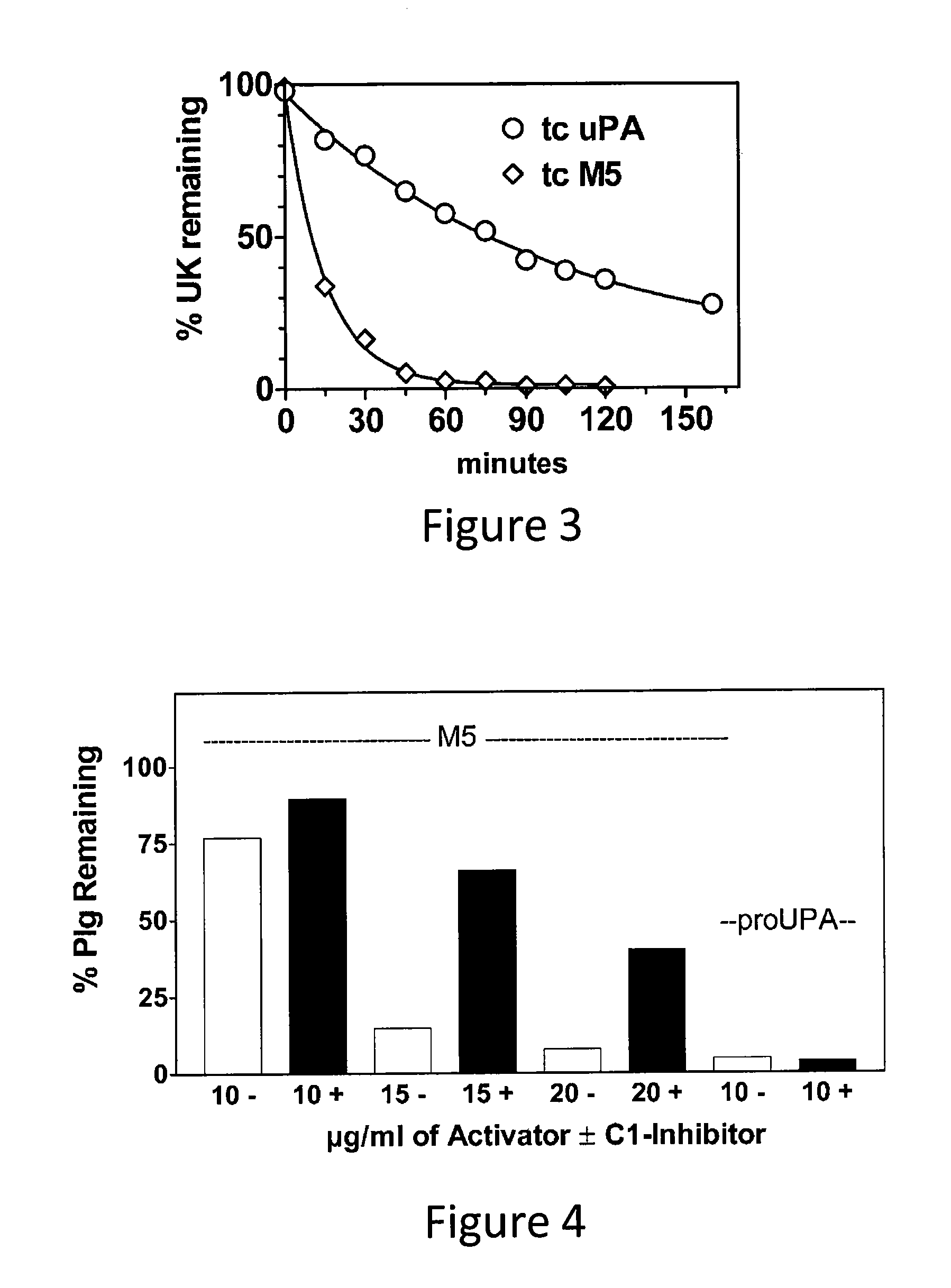
A mutant prourokinase plasminogen activator (M5) was developed to make prouPA less subject to spontaneous conversion to tcuPA in blood at therapeutic concentrations. Two-chain M5 was shown to form complexes with C1-inhibitor, which was the principal inhibitor of tcM5 in plasma. The effect of supplemental additions of C1-inhibitor on fibrinolysis and fibrinogenolysis by M5 was determined. Supplemental C1-inhibitor restored the stability of high-dose M5 and prevented fibrinogenolysis but not fibrinolysis, the rate of which was not compromised by the inhibitor. Due to higher dose tolerance of M5 in the presence of supplemental C1-inhibitor, the rate of fibrin-specific lysis reached that achievable by nonspecific fibrinolysis, which is the maximum possible for a plasminogen activator. Plasma C1-inhibitor stabilized M5 in plasma by inhibiting tcM5 which would otherwise greatly amplify non-specific plasminogen activation causing more tcM5 generation from M5. This unusual dissociation of inhibitory effects, whereby fibrinogenolysis and not fibrinolysis is inhibited, has significant implications for improving the safety and efficacy of fibrinolysis. Methods of reducing bleeding and non-specific plasminogen activation during fibrinolysis by administering M5 along with exogenous C1-inhibitor are disclosed.
Owner:THROMBOLYTIC SCI
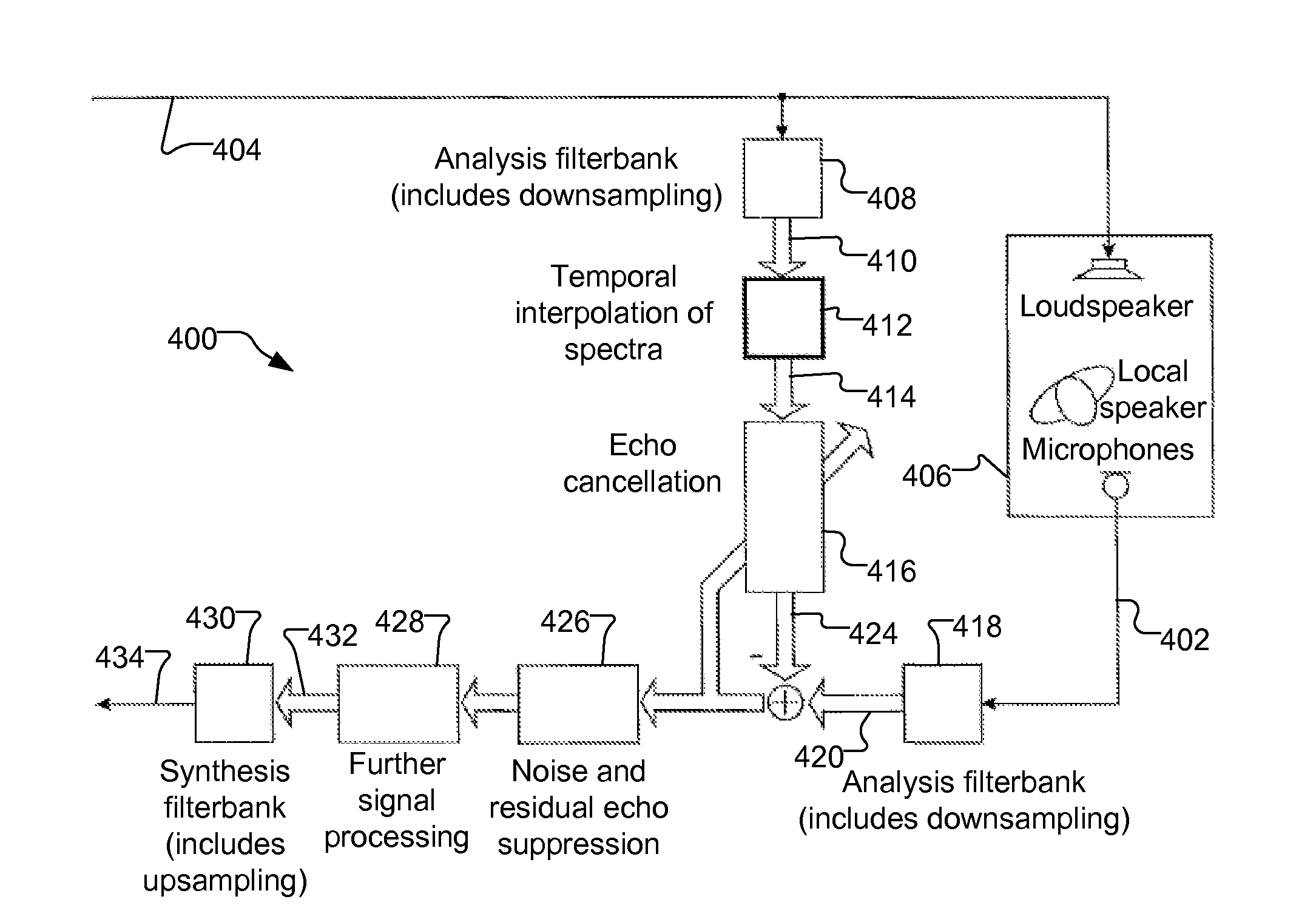
Temporal Interpolation of Adjacent Spectra
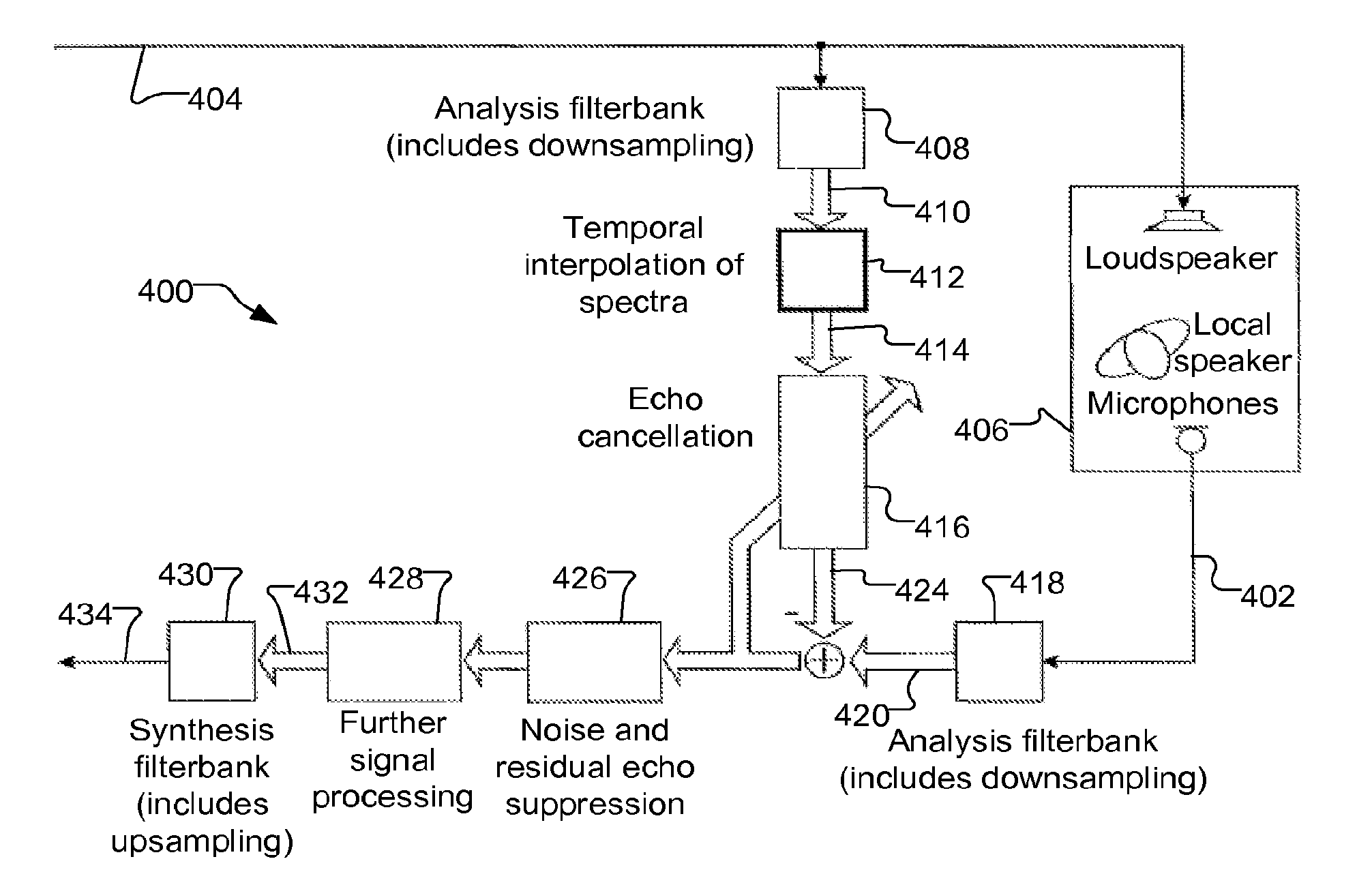
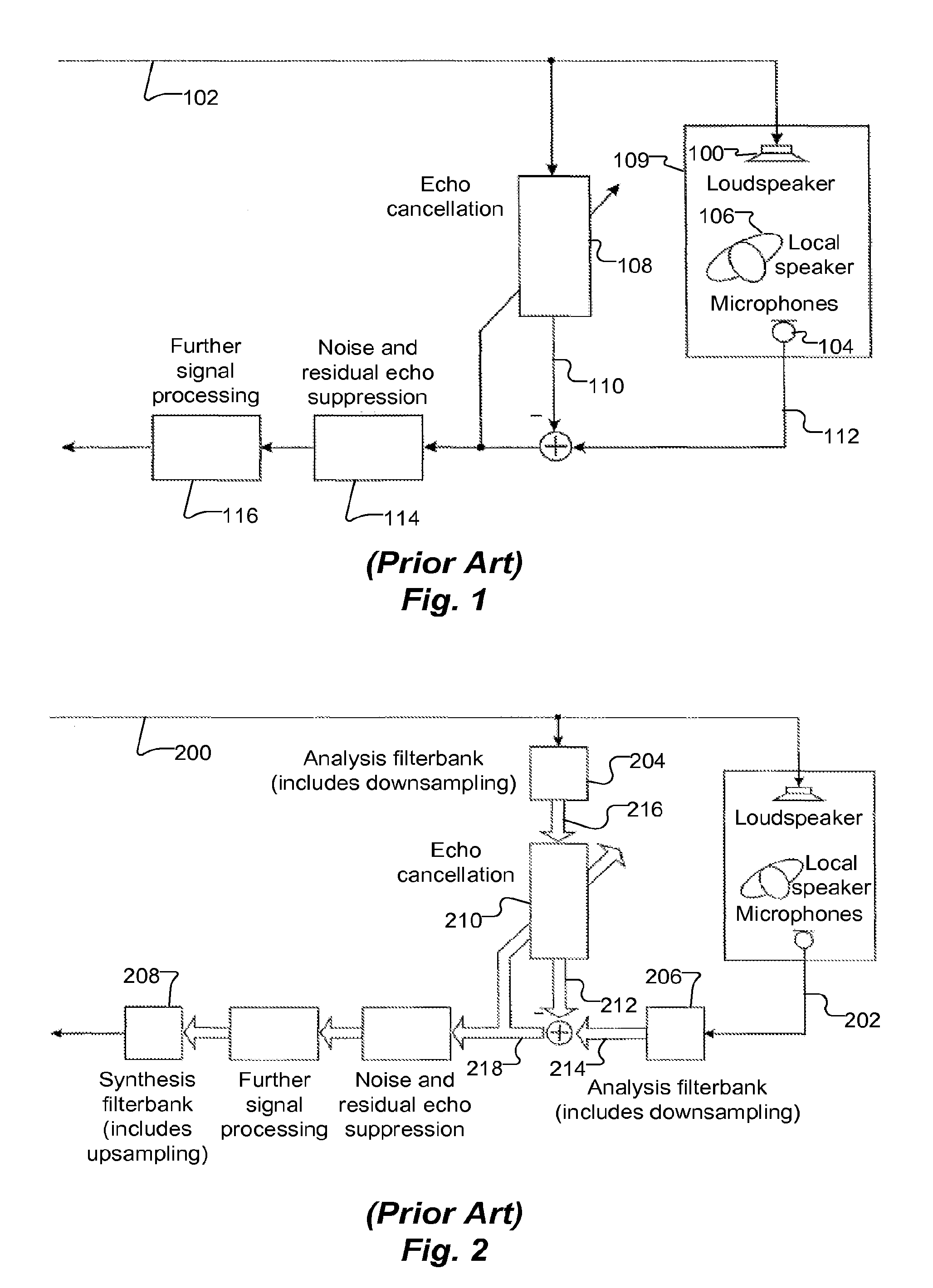
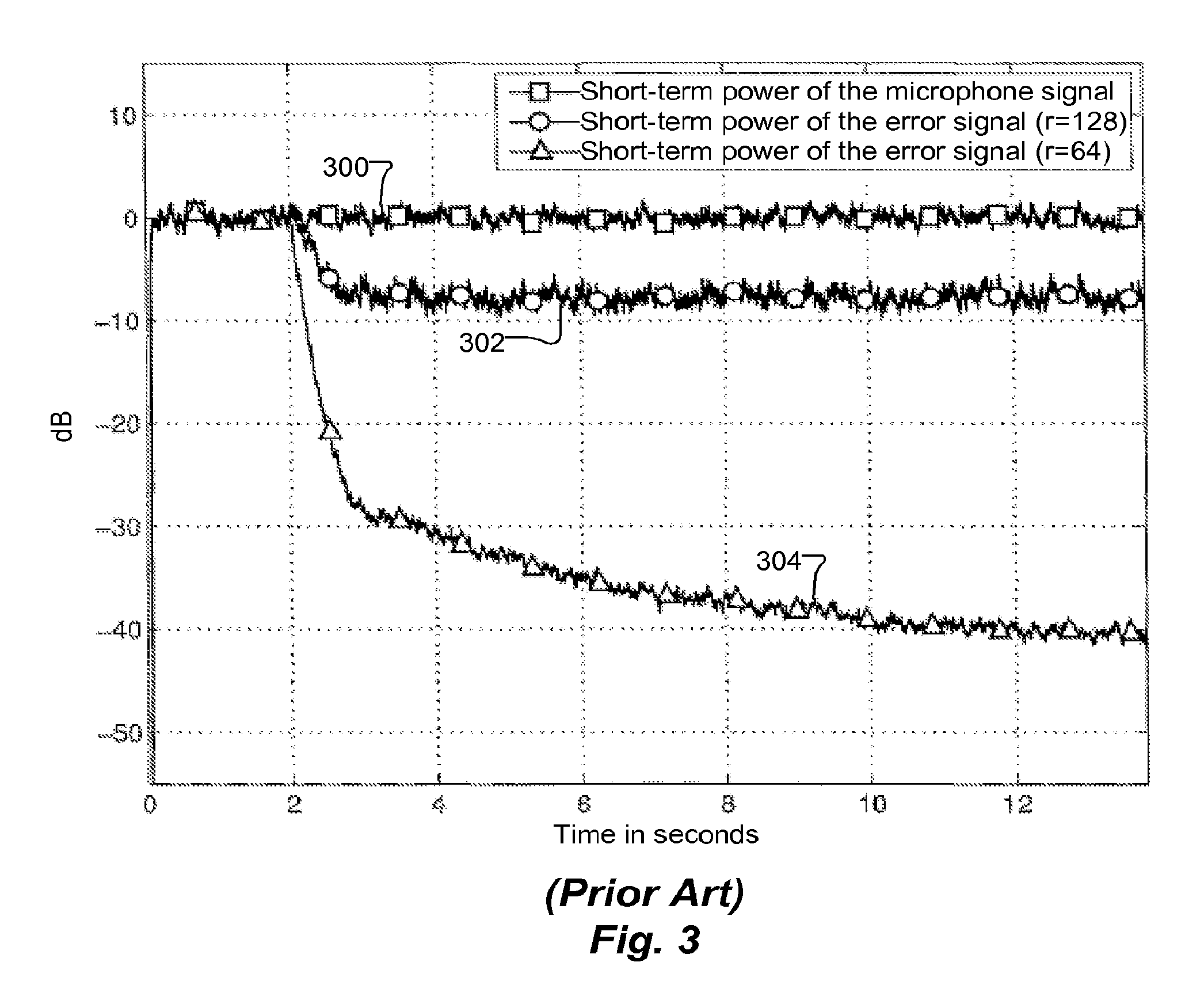
ActiveUS20130182868A1Doubling numberIncreasing computational requirementSpeech analysisSound producing devicesTime domainFrequency spectrum
Embodiments of the present invention exploit redundancy of succeeding FFT spectra and use this redundancy for computing interpolated temporal supporting points. An analysis filter bank converts overlapped sequences of an audio (ex. loudspeaker) signal from a time domain to a frequency domain to obtain a time series of short-time loudspeaker spectra. An interpolator temporally interpolates this time series. The interpolation is fed to an echo canceller, which computes an estimated echo spectrum. A microphone analysis filter bank converts overlapped sequences of an audio microphone signal from the time domain to the frequency domain to obtain a time series of short-time microphone spectra. The estimated echo spectrum is subtracted from the microphone spectrum. Further signal enhancement (filtration) may be applied. A synthesis filter bank converts the filtered microphone spectra to the time domain to generate an echo compensated audio microphone signal. Computational complexity of signal processing systems can, therefore, be reduced.
Owner:CERENCE OPERATING CO
Bone substitute material
ActiveUS20190209737A1Increase capacityImproves osteoblast differentiationSurgical adhesivesBone implantBiphasic calcium phosphateBone formation
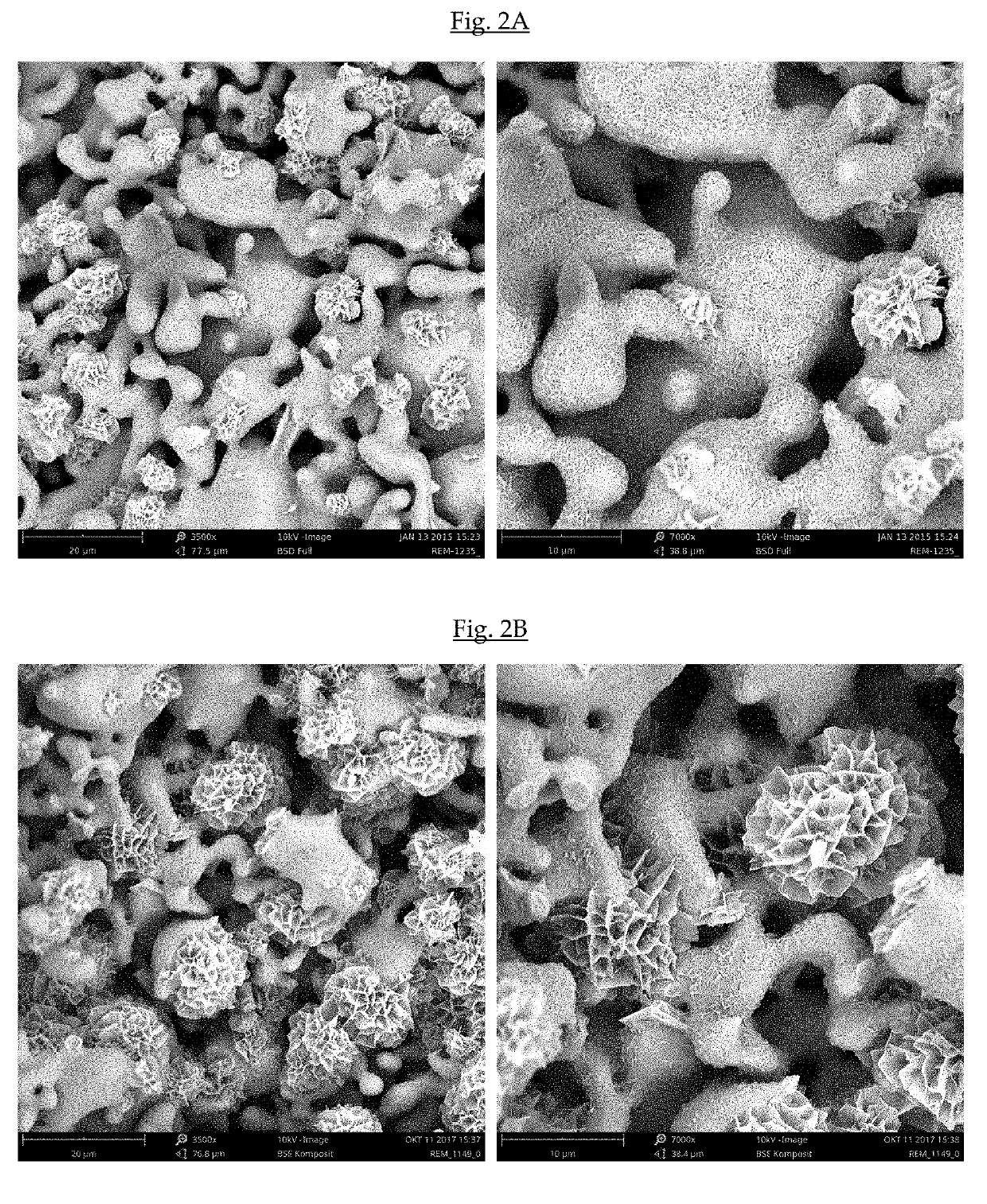
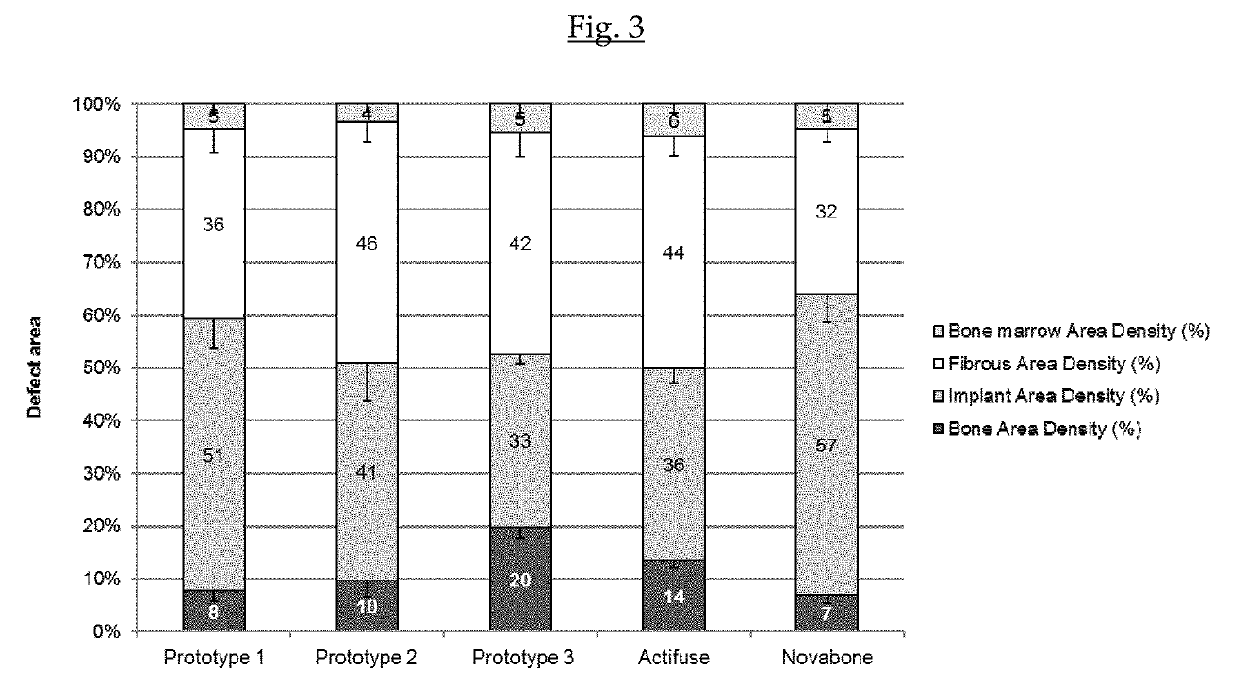
A biphasic calcium phosphate / hydroxyapatite (CAP / HAP) bone substitute material having a sintered CAP core and at least one closed epitactically grown layer of nanocrystalline HAP deposited on the external surface of the sintered CAP core, whereby the epitactically grown nanocrystals have the same size and morphology as human bone mineral, wherein the closed epitactically grown layer of nanocrystalline HAP deposited on the external surface of the sintered CAP core has a non-homogeneous external surface comprising individual clusters of flat crystal platelets consisting of epitactically grown HAP nanocrystals and coarse areas between the individual clusters, whereby the percentage of the coarse areas between the individual clusters as measured by SEM is at least 20% of the total surface, which material shows an increased capacity to induce bone formation, and a process of preparation thereof.
Owner:GEISTLICH PHARMA
C1-Inhibitor Prevents Non-Specific Plasminogen Activation by a Prourokinase Mutant without Impeding Fibrin-Specific Fibrinolysis
ActiveUS20110081334A1Attenuation of rateHigh dose tolerancePeptide/protein ingredientsBlood disorderC1-inhibitorHigh doses
A mutant prourokinase plasminogen activator (M5) was developed to make prouPA less subject to spontaneous activation during fibrinolysis. C1-inhibitor complexes with tcM5. The effect of C1-inhibitor on fibrinolysis and fibrinogenolysis by M5 was determined. Supplemental C1-inhibitor restores the stability of M5 but not that of prouPA. Clot lysis by M5 with supplemental C1-inhibitor showed no attenuation of the rate of fibrinolysis, whereas fibrinogenolysis was prevented by C1-inhibitor. Due to higher dose tolerance of M5 with C1-inhibitor, the rate of fibrin-specific lysis reached that achievable by nonspecific fibrinolysis without inhibitor. Plasma C1-inhibitor stabilized M5 in plasma by inhibiting tcM5 and thereby non-specific plasminogen activation. At the same time, fibrin-specific plasminogen activation remained unimpaired. This unusual dissociation of effects has significant implications for improving the safety and efficacy of fibrinolysis. Methods of reducing bleeding and non-specific plasminogen activation during fibrinolysis by administering M5 along with exogenous C1-inhibitor are disclosed.
Owner:THROMBOLYTIC SCI
Rapid thermal firing IR conveyor furnace having high intensity heating section
InactiveUS8571396B2Cooling effectRateDomestic stoves or rangesDrying solid materials with heatFiberProcess region
High reflectance element IR lamp module and method of firing multi-zone IR furnaces for solar cell processing comprising lamps disposed backed by a flat or configured plate of ultra-high reflectance ceramic material. Optionally, the high reflectance plate can be configured with ripples or grooves to isolate each lamp from adjacent lamps in the process zone. Furnace cooling air is exhausted and recycled upstream for energy conservation. Lamp spacing can be varied and power to each lamp individually controlled to provide infinite control of temperature profile in each heating zone. The high reflectance element may be constructed of dense ceramic fiber board, and then coated with high reflectance ceramic composition, and baked or fired to form the finished element.
Owner:TP SOLAR OF USA
Method for Producing Rubberized Concrete Using Waste Rubber Tires
ActiveUS20130030088A1High mechanical strengthHigh bonding strengthGaseous chemical processesLiquid-gas reaction of thin-film typePartial oxidationSuperplasticizer
Catalytic partial oxidation using a metal oxide catalyst surface treats crumb rubber that is recovered from waste rubber tires. Advantages of using catalytically oxidized crumb rubber relative to using non-catalytically oxidized crumb rubber in making the rubberized concrete, includes superior mechanical strength and water-repealing capability, lower oxidation temperature and shorter oxidation time, and accelerated hydration times. Rubber oil (a gas condensate) co-produced from the crumb rubber partial oxidation process is equal to or better than the commercial superplasticizers. Industrial scale partial oxidation employs a continuous flow tubular reactor where a crumb rubber / catalyst mixture is fed into the reactor co-currently with an air / nitrogen mixture.
Owner:SHIN CHUANG TECH CO LTD
System for injecting fuel into a turbomachine
InactiveUS20030094197A1Mitigate such drawbackSimplify the hydraulic circuitGas turbine plantsRotary piston pumpsCombustion chamberFuel tank
A system for injecting fuel into a turbomachine comprising N fuel injectors placed in a combustion chamber of the turbomachine and fed from a fuel tank, the injection system further comprising, interposed between the N injectors and the tank, a single pumping means for taking fuel from the tank and delivering N metered flow rates of fuel to the injectors.
Owner:SN DETUDE & DE CONSTR DE MOTEURS DAVIATION S N E C M A
Temporal Interpolation Of Adjacent Spectra
ActiveUS20130208905A1Doubling numberIncreasing computational requirementSpeech analysisSound producing devicesTime domainFrequency spectrum
Embodiments of the present invention exploit redundancy of succeeding FFT spectra and use this redundancy for computing interpolated temporal supporting points. An analysis filter bank converts overlapped sequences of an audio (ex. loudspeaker) signal from a time domain to a frequency domain to obtain a time series of short-time loudspeaker spectra. An interpolator temporally interpolates this time series. The interpolation is fed to an echo canceller, which computes an estimated echo spectrum. A microphone analysis filter bank converts overlapped sequences of an audio microphone signal from the time domain to the frequency domain to obtain a time series of short-time microphone spectra. The estimated echo spectrum is subtracted from the microphone spectrum. Further signal enhancement (filtration) may be applied. A synthesis filter bank converts the filtered microphone spectra to the time domain to generate an echo compensated audio microphone signal. Computational complexity of signal processing systems can, therefore, be reduced.
Owner:CERENCE OPERATING CO
Method for producing rubberized concrete using waste rubber tires
ActiveUS8536253B2High mechanical strengthHigh bonding strengthGaseous chemical processesLiquid-gas reaction of thin-film typePartial oxidationSuperplasticizer
Catalytic partial oxidation using a metal oxide catalyst surface treats crumb rubber that is recovered from waste rubber tires. Advantages of using catalytically oxidized crumb rubber relative to using non-catalytically oxidized crumb rubber in making the rubberized concrete, includes superior mechanical strength and water-repealing capability, lower oxidation temperature and shorter oxidation time, and accelerated hydration times. Rubber oil (a gas condensate) co-produced from the crumb rubber partial oxidation process is equal to or better than the commercial superplasticizers. Industrial scale partial oxidation employs a continuous flow tubular reactor where a crumb rubber / catalyst mixture is fed into the reactor co-currently with an air / nitrogen mixture.
Owner:SHIN CHUANG TECH CO LTD
Method for formation of iron sprayed coating and coated member
InactiveUS20160237543A1Improve adhesionImprove machinabilityMolten spray coatingCylinder headsSpray coatingFerric
A method for forming an iron sprayed coating on a substrate with droplets of a wire molten by an electric arc includes: a step of forming a first iron sprayed coating on the substrate, the wire for arc spraying being a first wire containing iron and 0.03 to 0.10% by mass of carbon and the compressed gas being inert gas; and a step of forming a second iron sprayed coating on the first iron sprayed coating, the wire for arc spraying being a second wire containing iron and 0.03 to 0.10% by mass of carbon and the compressed gas containing 10 to 21% by volume of oxygen gas.
Owner:TOYOTA JIDOSHA KK
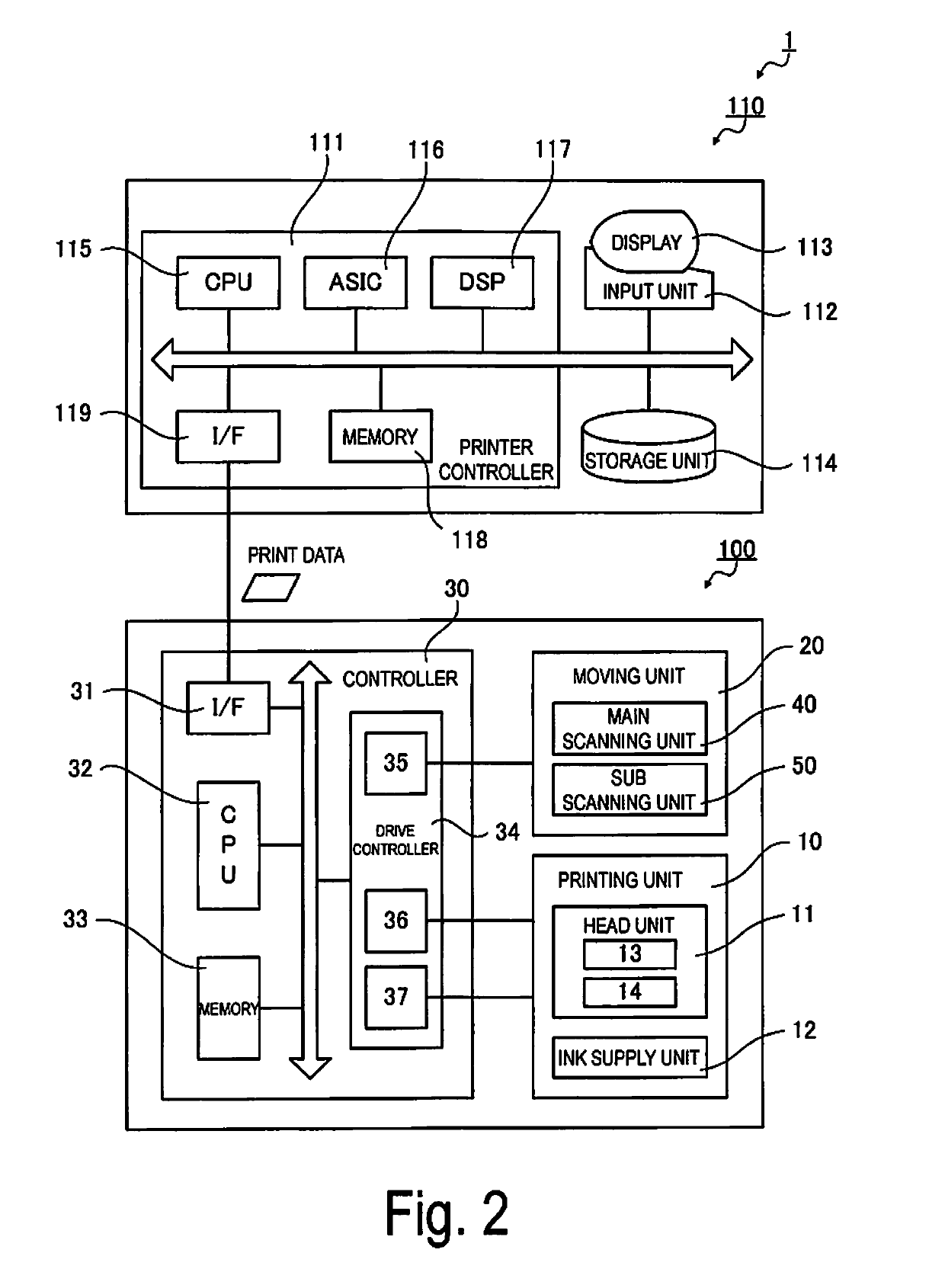
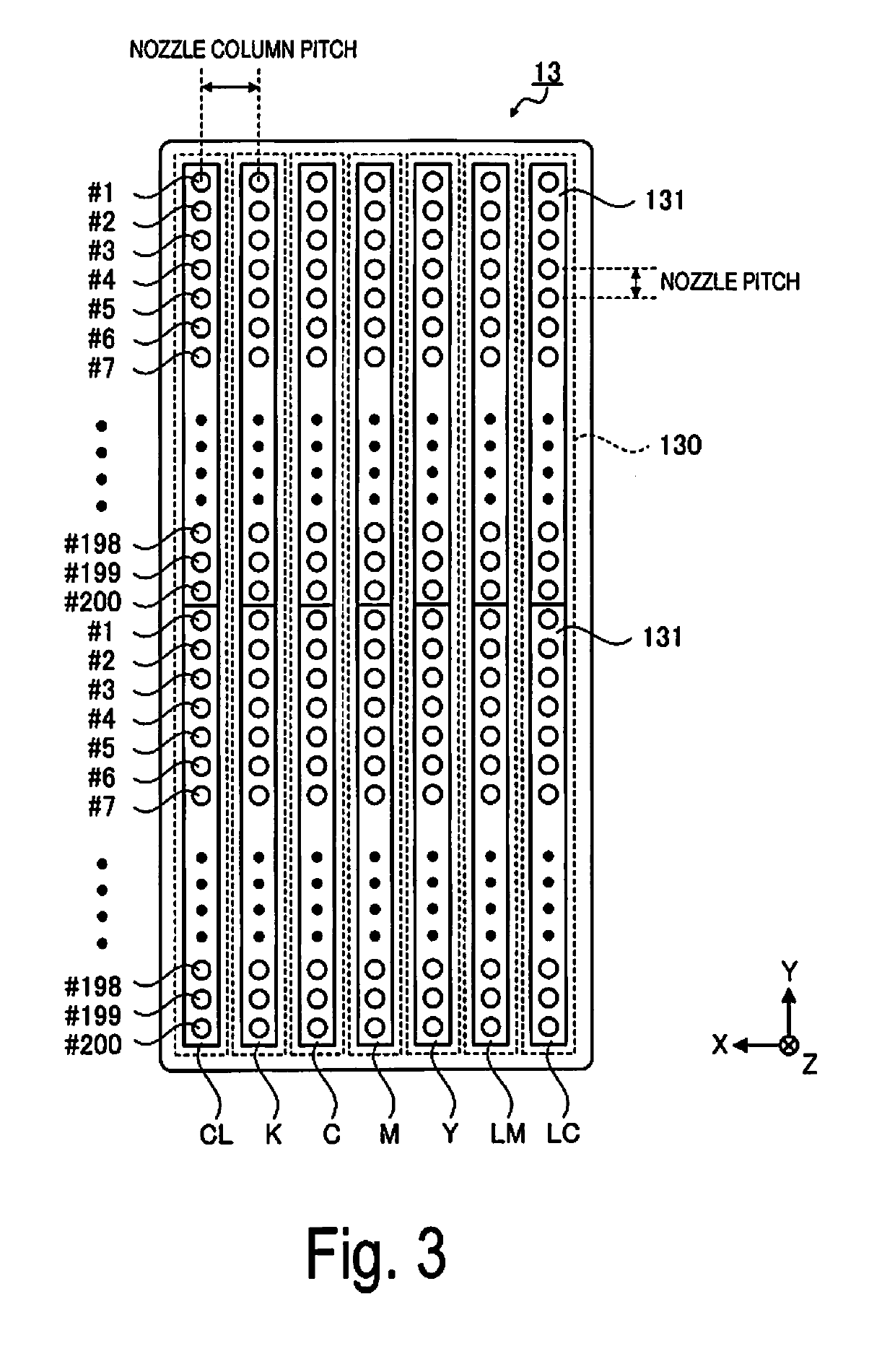
Image processing device, printing apparatus, image processing method, and non-transitory computer readable medium
ActiveUS10491759B2High rateLow degreeImage analysisCharacter and pattern recognitionImaging processingComputer graphics (images)
The disclosure includes an edge extracting unit configured to, based on image data corresponding to a print image, extract edge pixels forming an outline of a partial image, a dot formation specification determining unit configured to, based on the image data, determine dot formation specifications, and a print data generator configured to, based on the dot formation specifications, generate print data for causing execution of printing by a printing apparatus for printing the print image by discharging first ink on a printing medium and second ink that promotes spreading of the first ink on the printing medium to form the plurality of dots.
Owner:SEIKO EPSON CORP
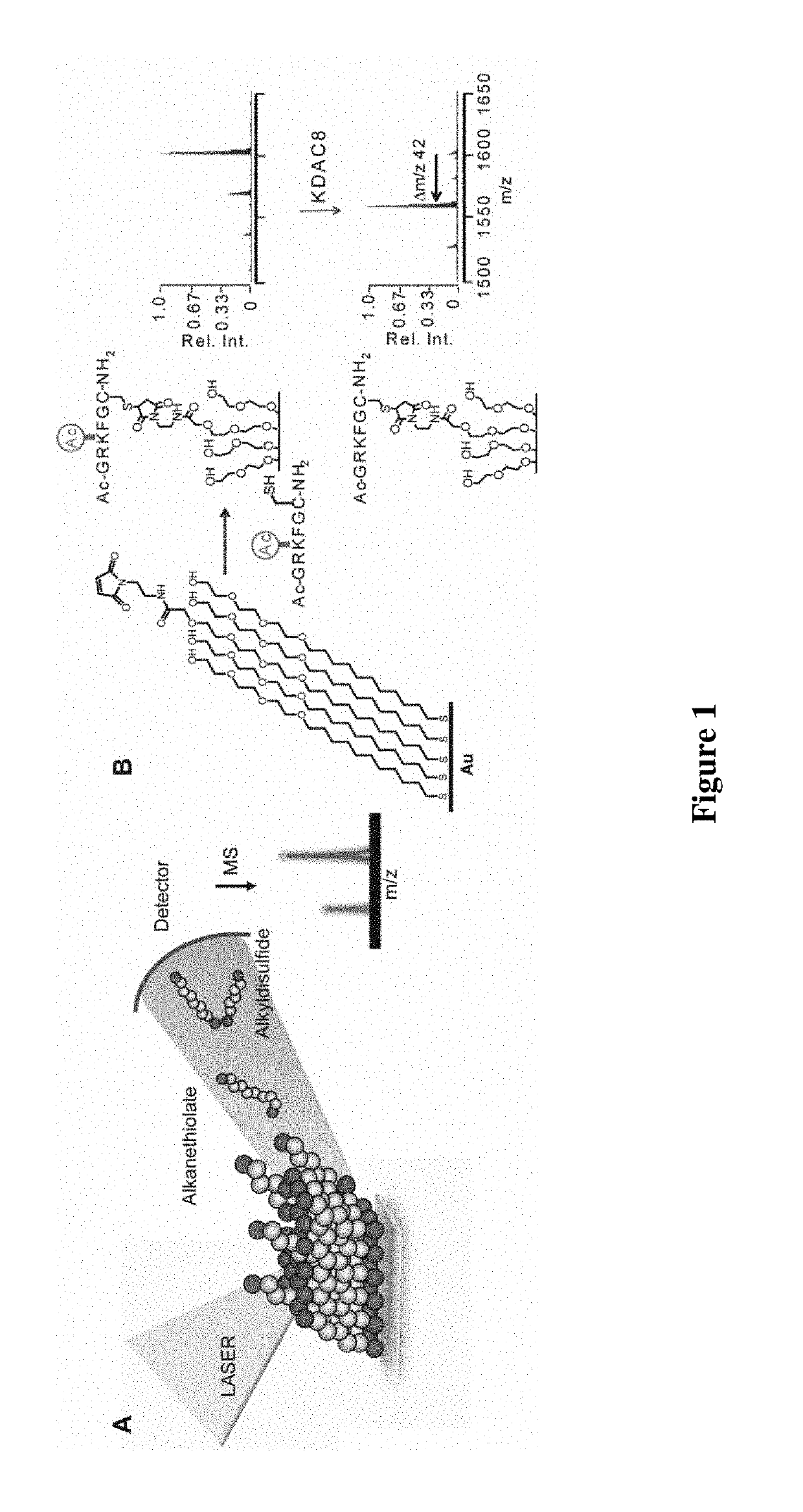
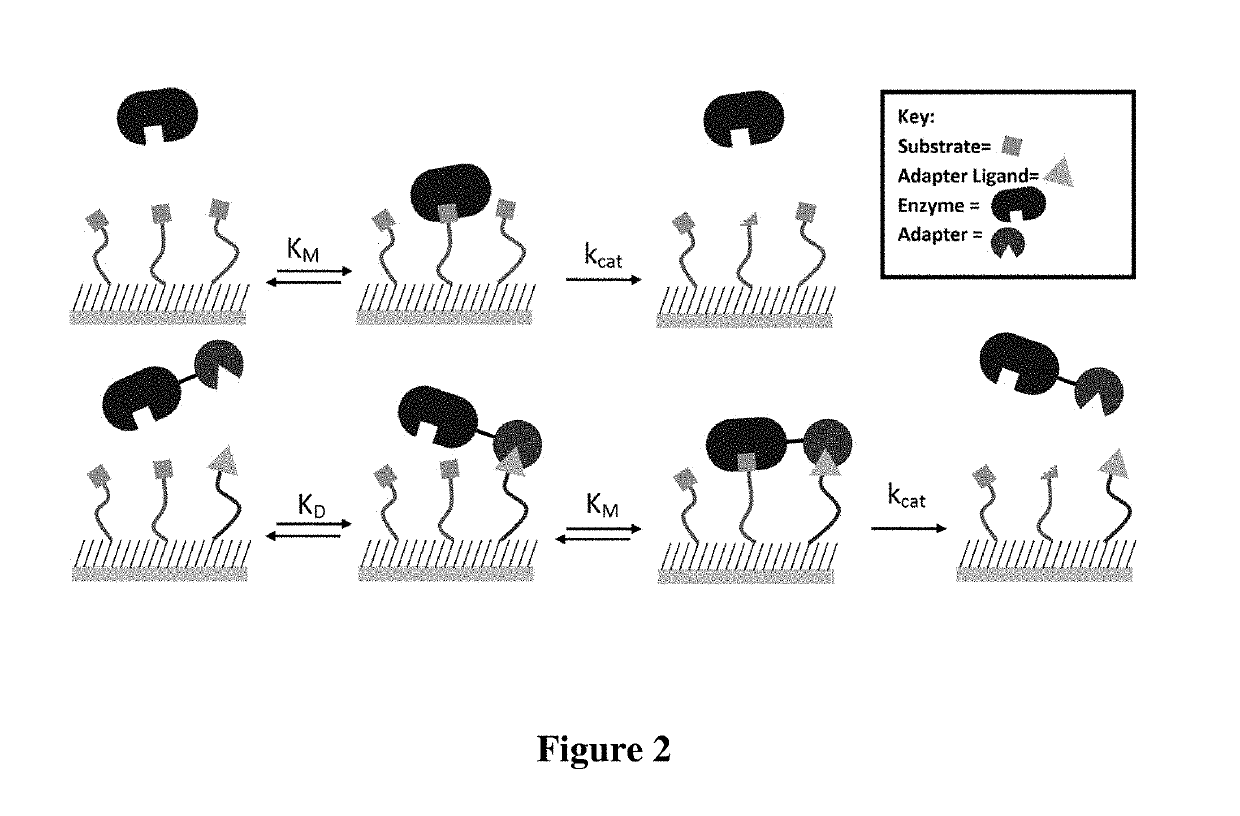
Enzyme coupled assay for quantification of protein and peptide binding by SAMDI mass spectrometry
ActiveUS10782287B2RateAccelerated rate for the enzyme-catalyzed reactionMass spectrometric analysisAssayMass Spectrometry-Mass Spectrometry
Owner:NORTHWESTERN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com