Crystal form of Peimeiqusai disodium and its preparation
A technology of pemetrexed disodium and dihydrogen, which is applied in the field of organic chemistry, can solve problems such as difficulty in controlling moisture content, increase production cost, increase equipment and the like, and achieves easy operation, strong controllability and good reproducibility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
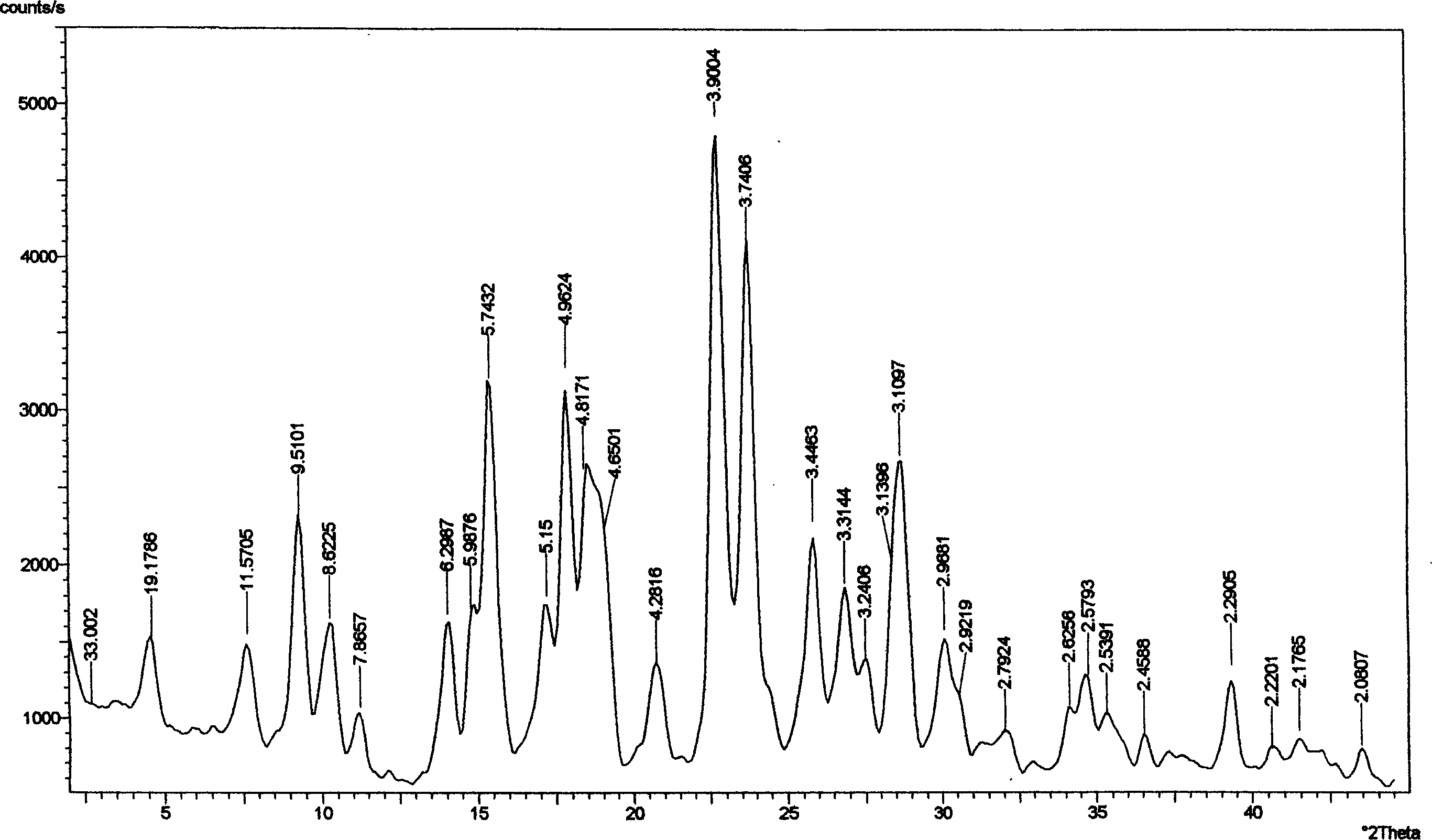
[0045] Preparation of Pemetrexed Disodium Trihydrate
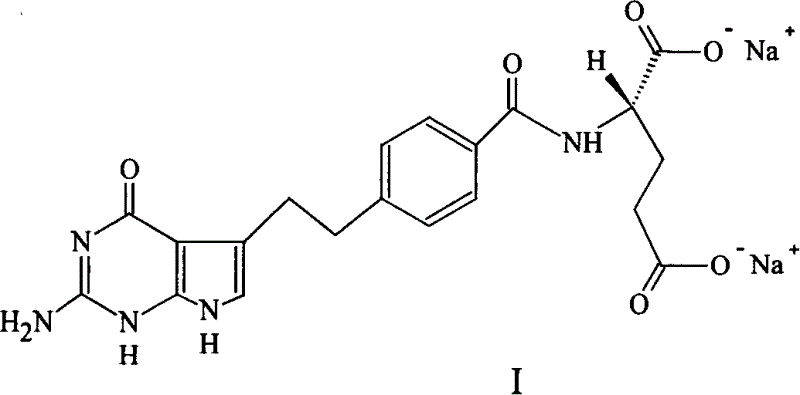
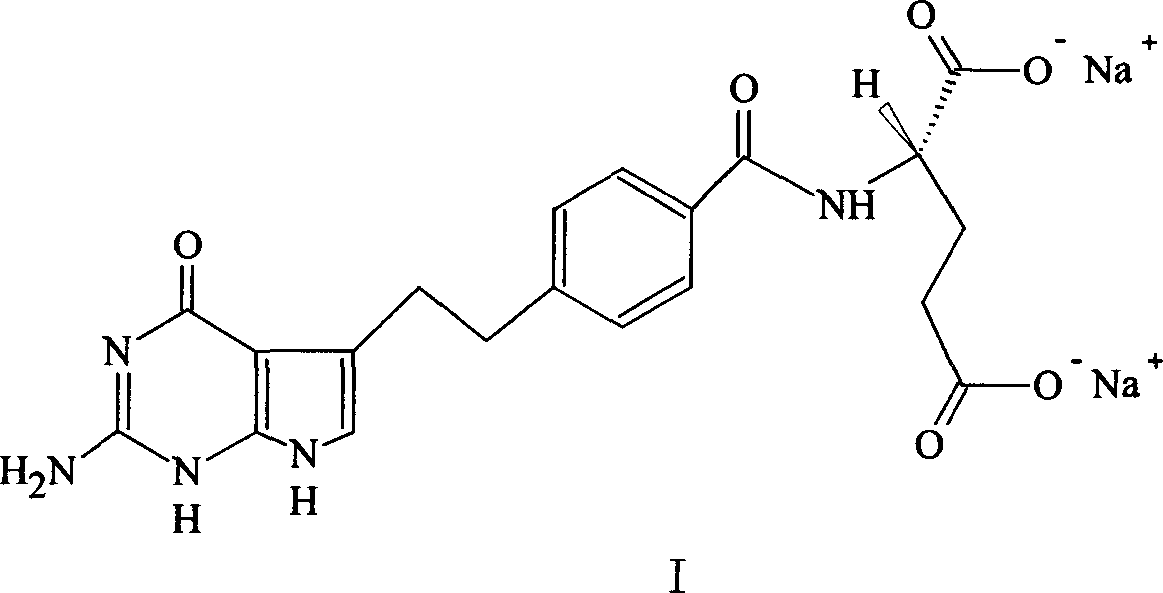
[0046] In a 3L reaction flask, N-[4-[2-(2-amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl Base] benzoyl]-L-glutamic acid (pemetrexed) 230.0g (0.538mol) was dissolved in 1.3L of 1mol / L sodium hydroxide solution, and then adjusted the pH of the mixture to 8 with 1mol / L hydrochloric acid solution Left and right, filter, and measure the volume of the filtrate to about 1.8L. Heat the filtrate to 45-50°C in a 20L reaction bottle, add ethanol, and adjust the feeding speed so that the temperature of the mixture is not lower than 40°C. When about 7L of ethanol is added, a black gum will precipitate in the system. Remove the precipitate by filtration; then add 2 L of ethanol to the filtrate, stir and cool, during which a large amount of off-white solids are precipitated; when the system cools down to room temperature, collect the solids by filtration and wash with ethanol. Measured by HPLC: 98.25%, further purificati...
Embodiment 2
[0049] Preparation of freeze-dried powder injection with pemetrexed disodium trihydrate
[0050] Pemetrexed Disodium Trihydrate 90.0g
[0051] Mannitol 90.0g
[0052] Water for injection 2000ml
[0053] Weigh 90.0 g of pemetrexed disodium trihydrate and 90.0 g of mannitol, add 2000 ml of water for injection, stir well to dissolve completely, measure the pH of the solution after cooling, adjust the pH of the solution to 7.0 with hydrochloric acid or sodium hydroxide if necessary ~8.5, then set the volume to 2250ml; add 0.05% activated carbon, stir for 10 minutes, filter, and then carry out sterilizing filtration to the filtrate; fill it into a freeze-drying bottle with 12.5ml / bottle, and freeze-dry; Enter the sterile filtered nitrogen, press the stopper, and press the aluminum cap.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com