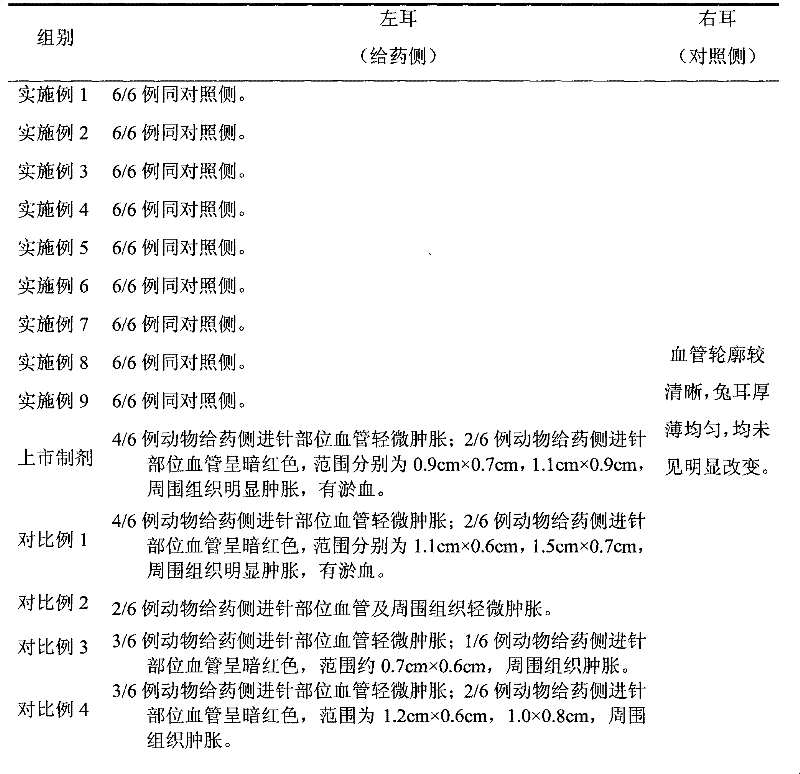
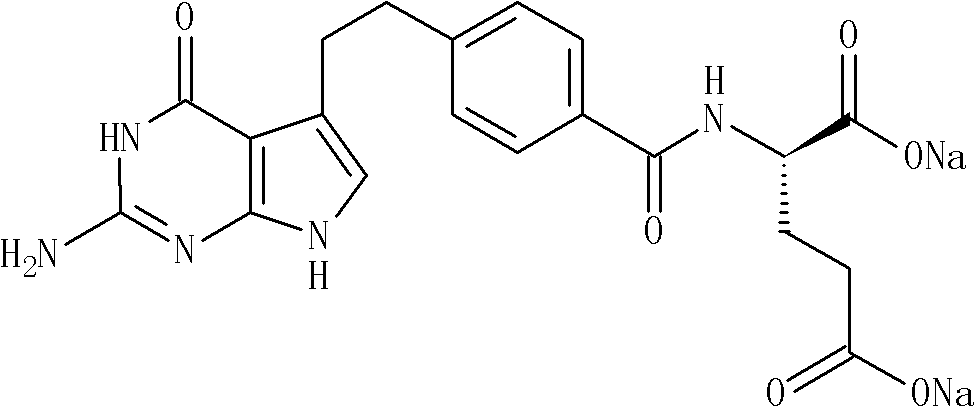
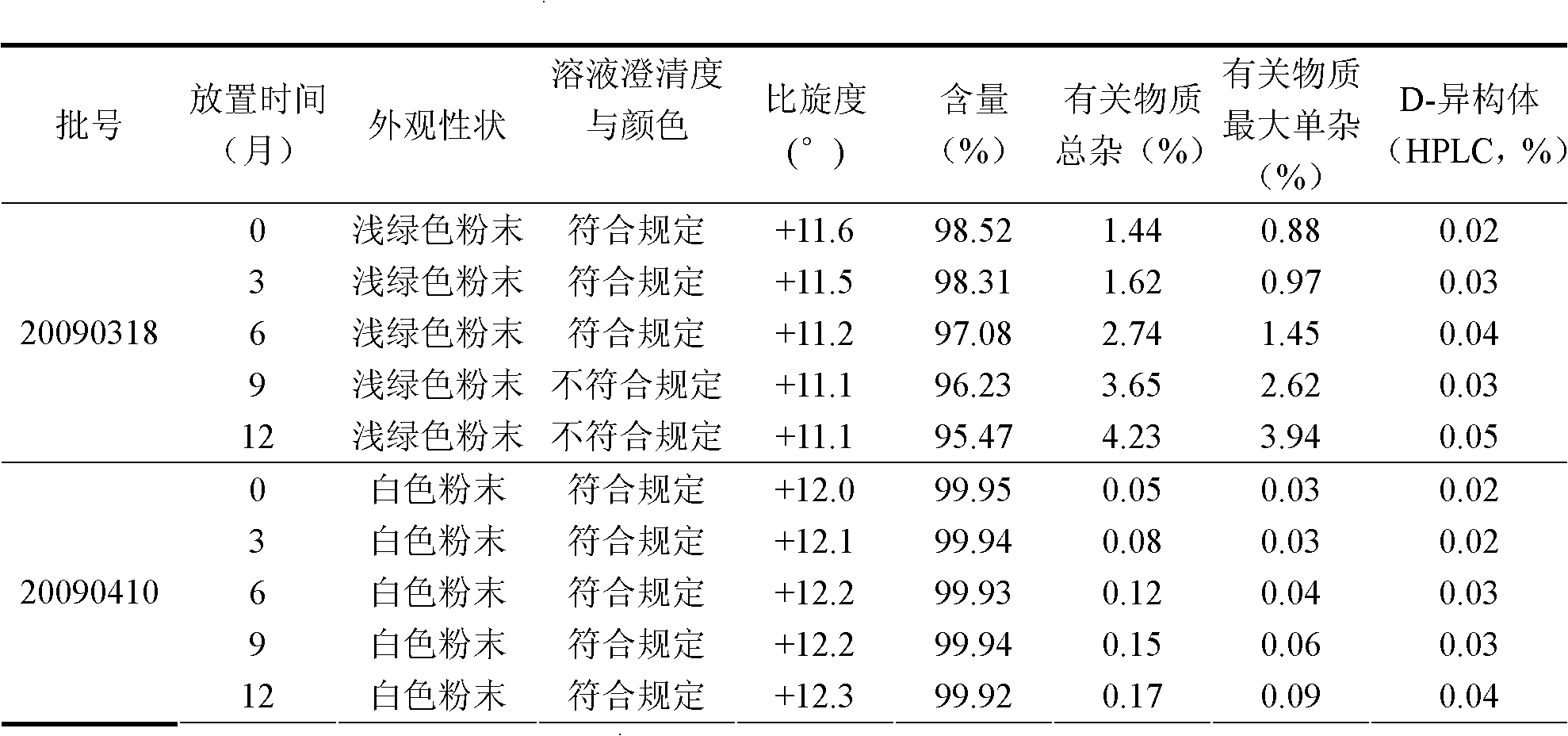
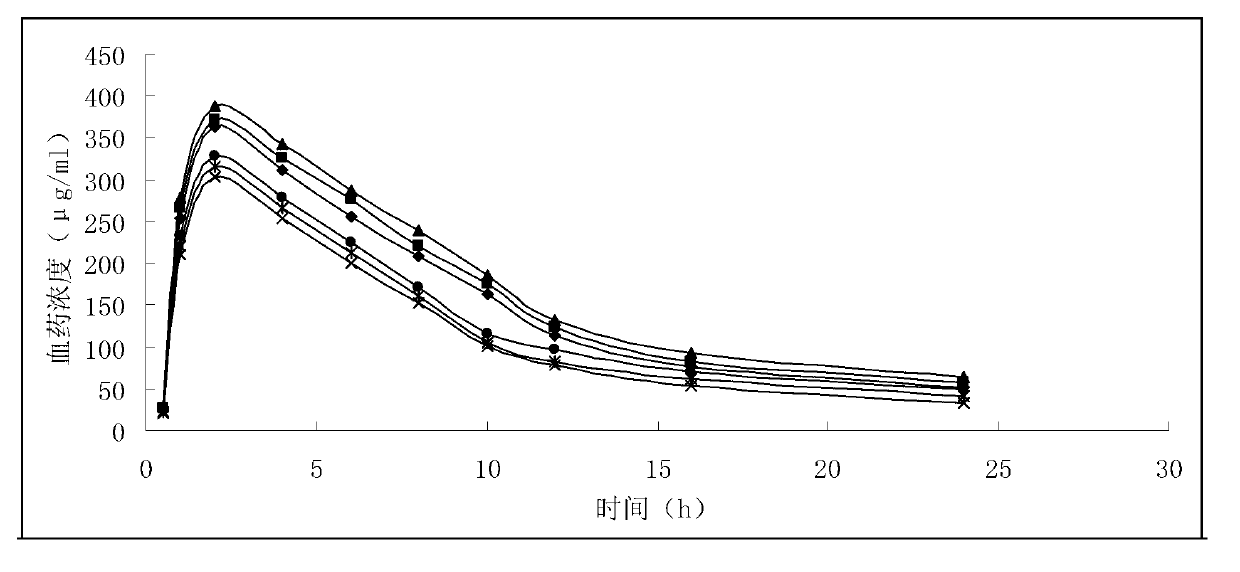
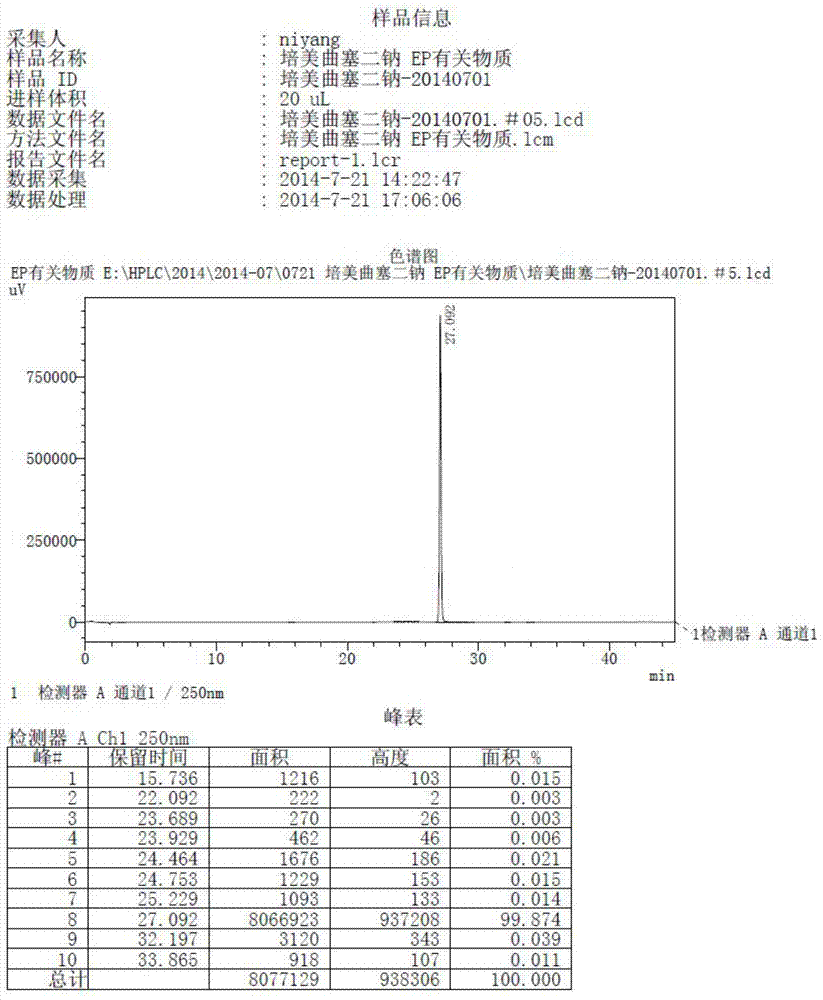
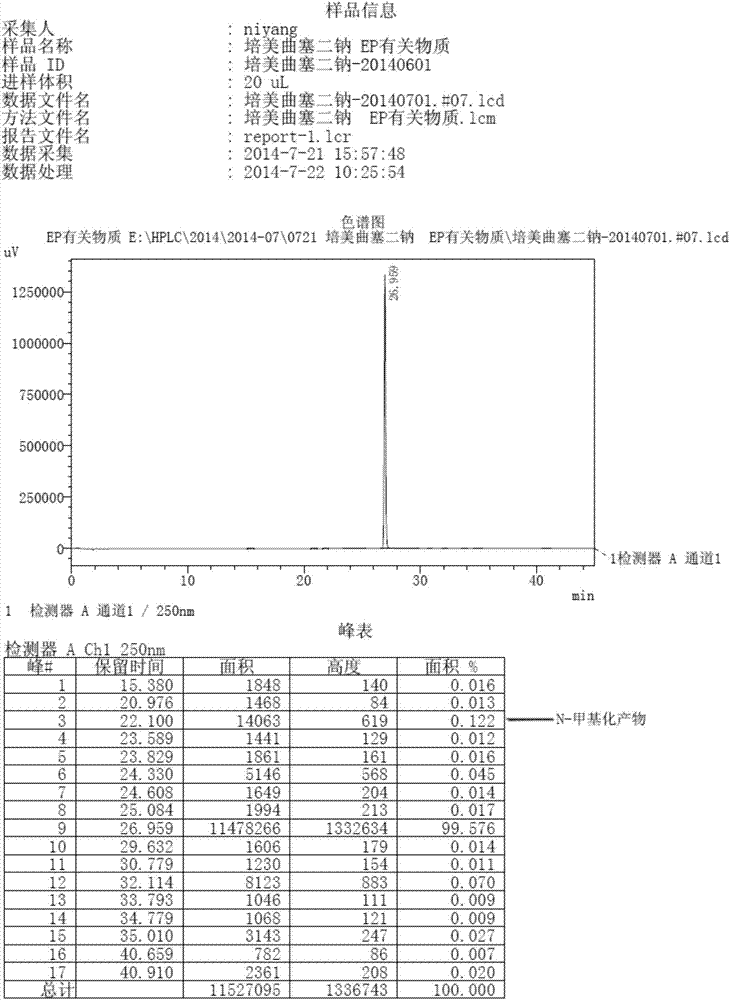
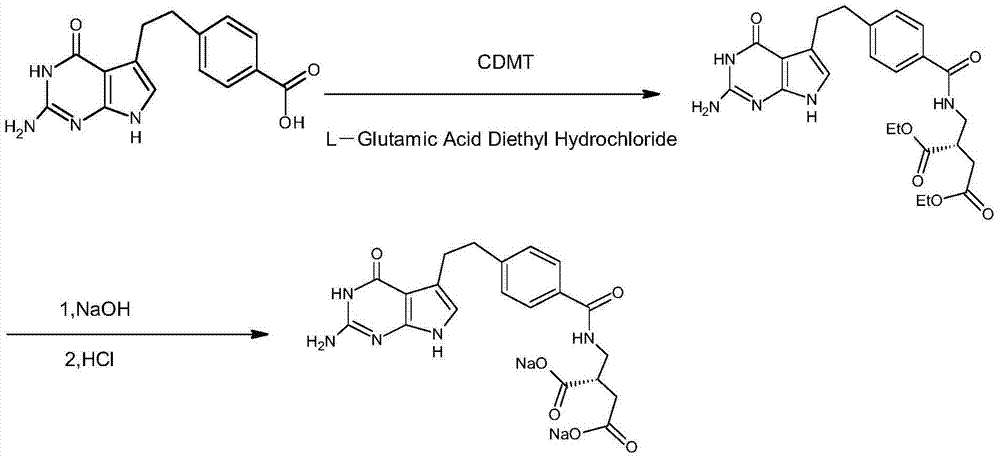
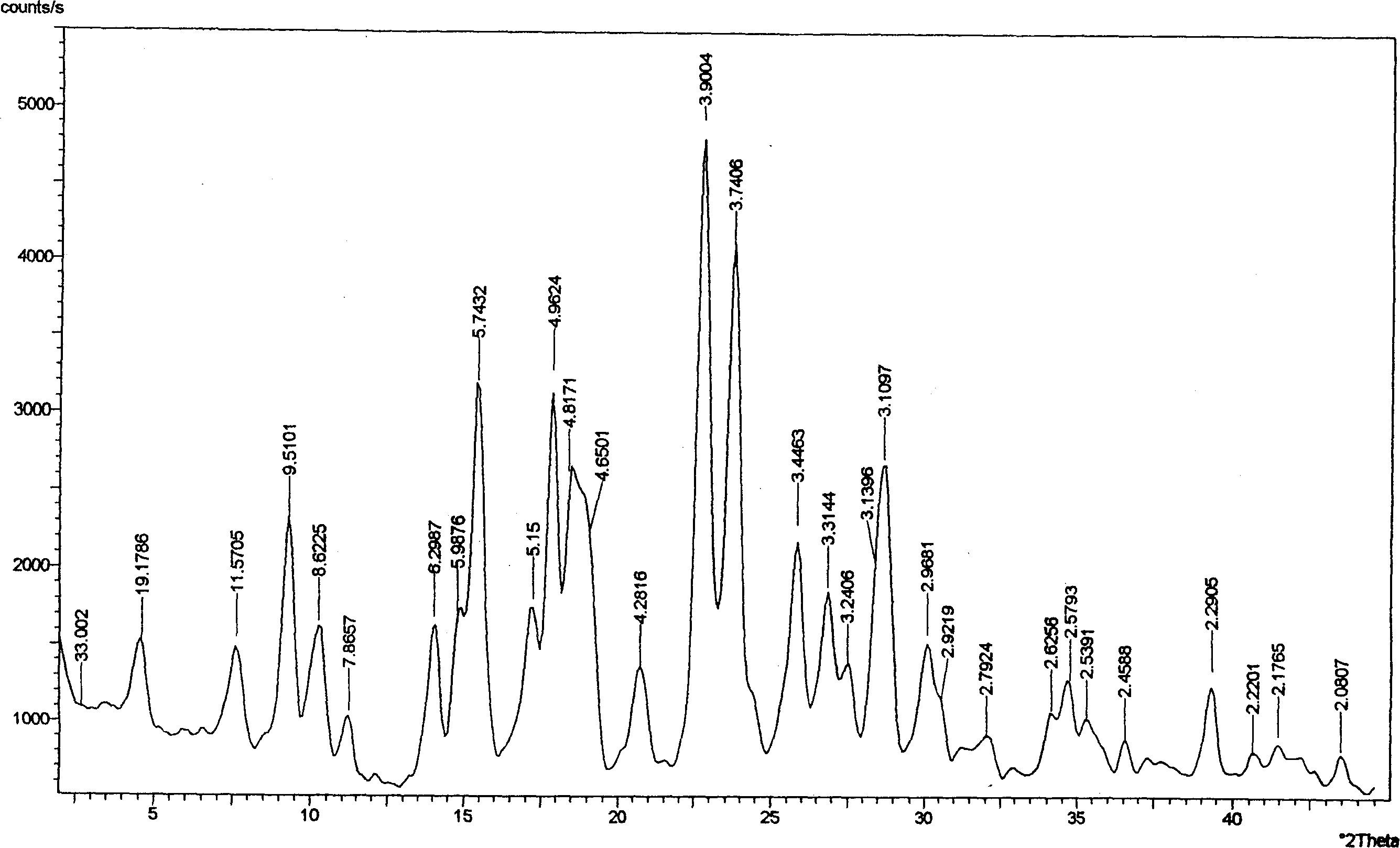
Patents
Literature
93 results about "Pemetrexed disodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
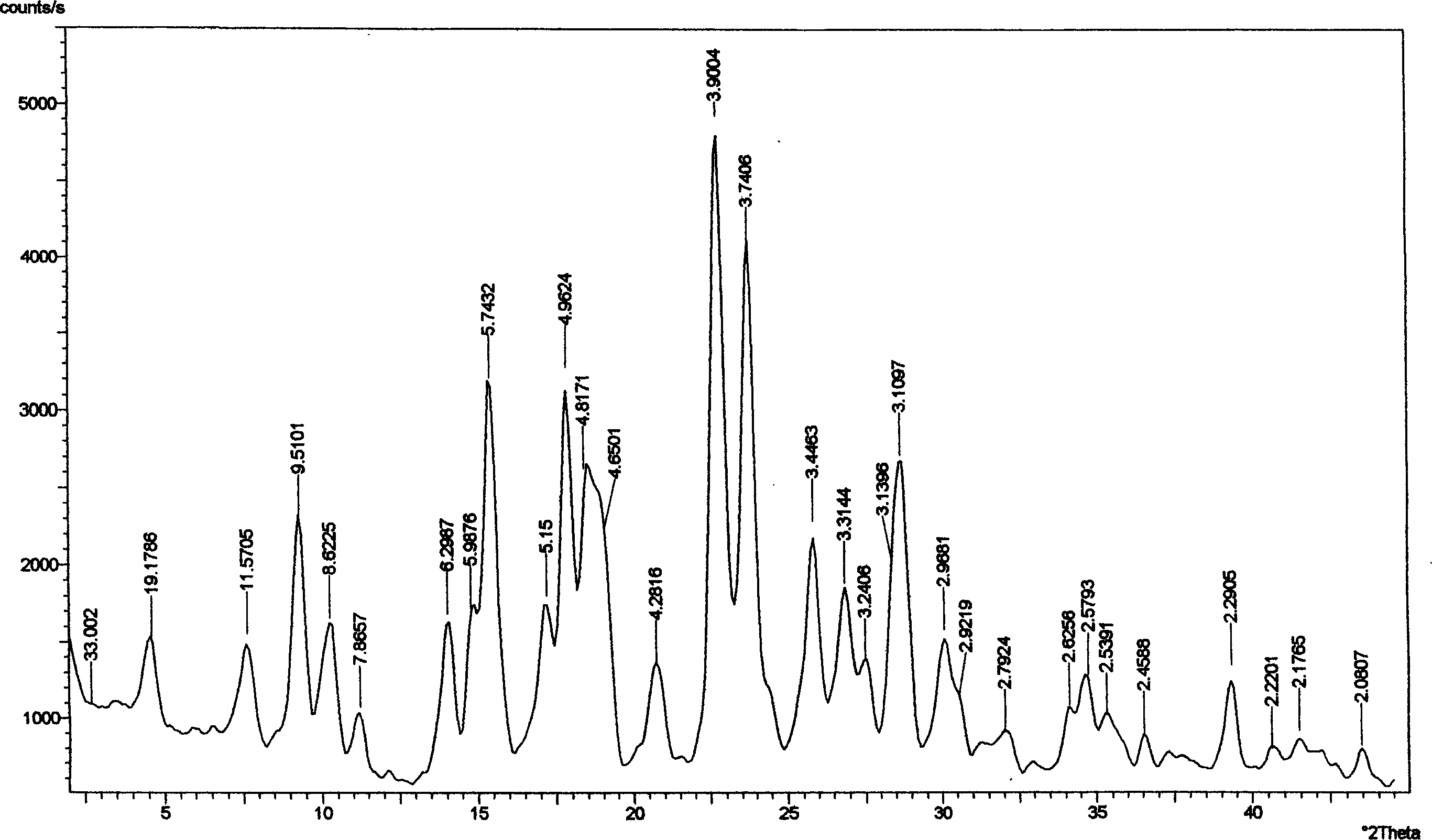
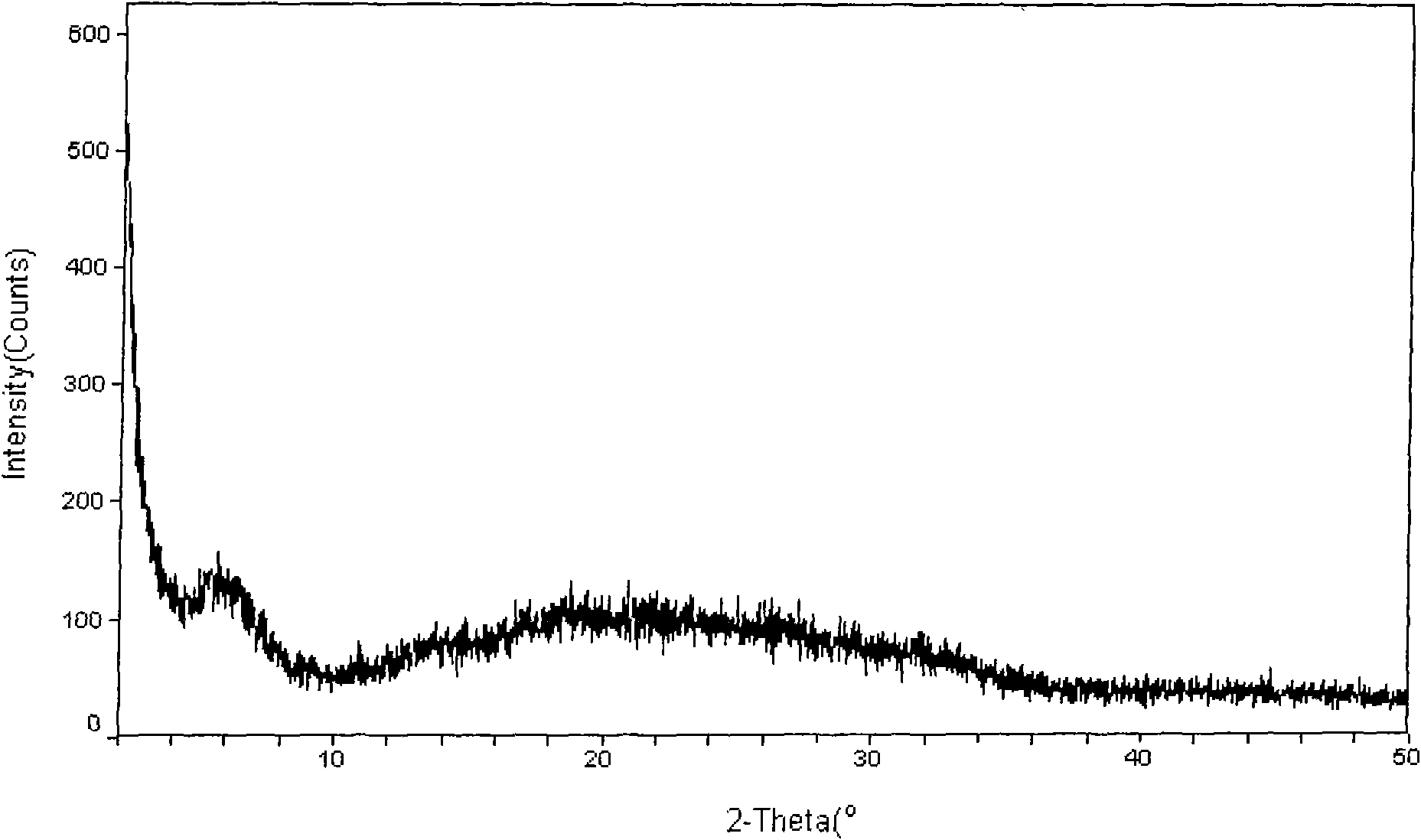
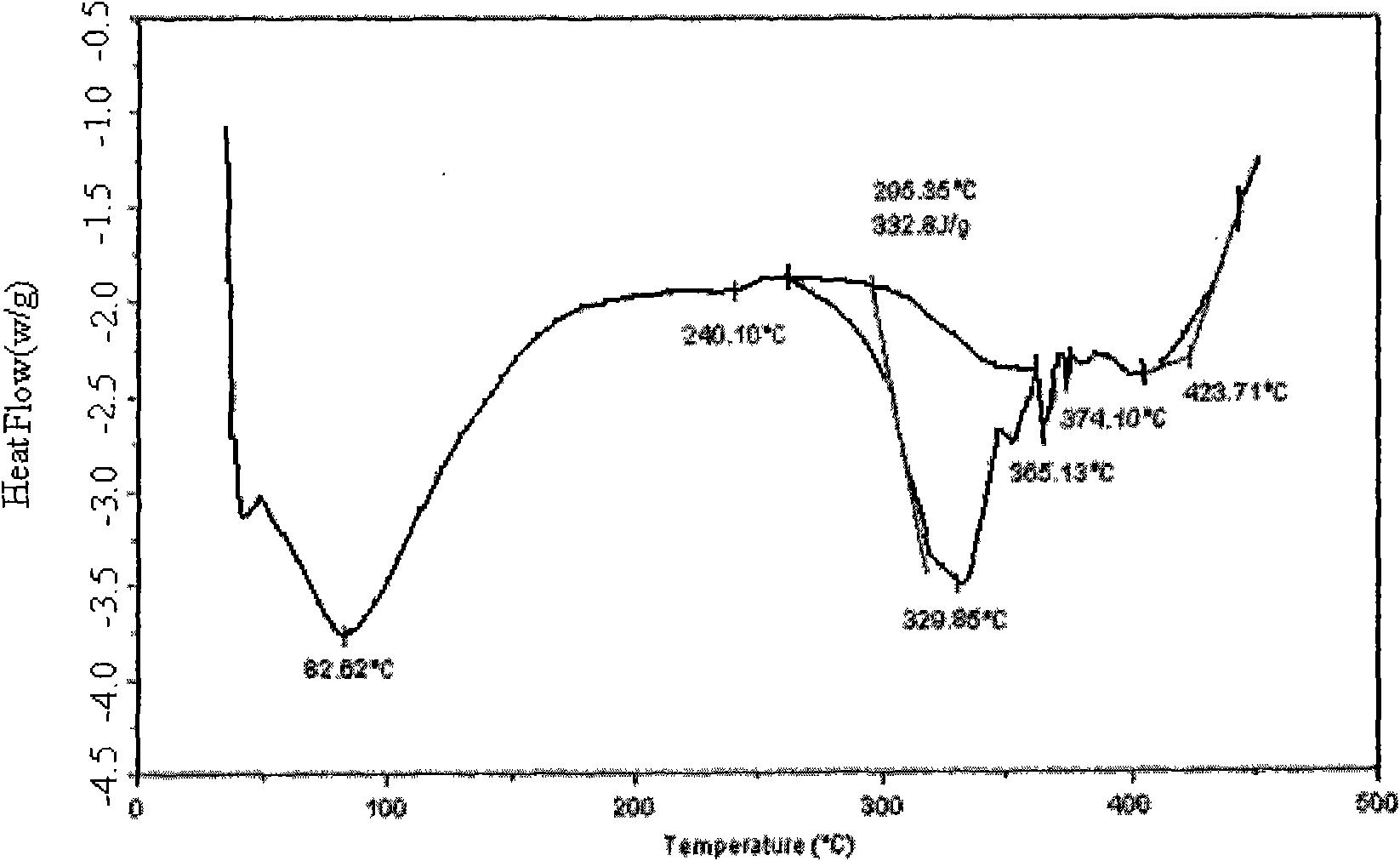
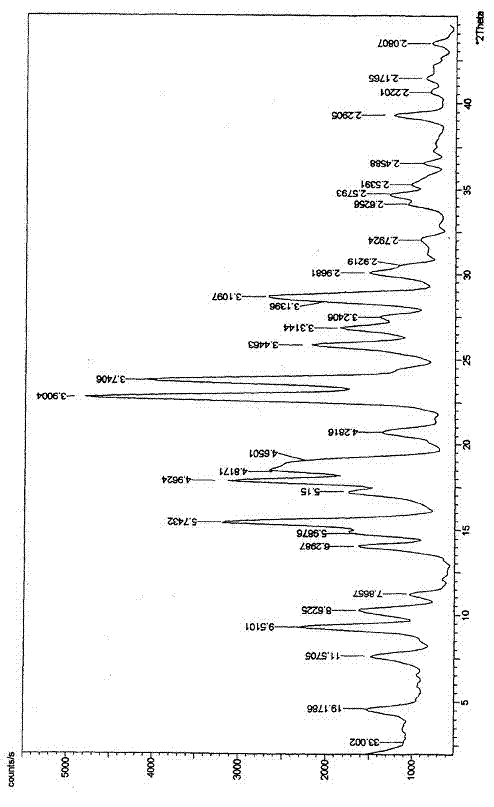
Crystal form of Peimeiqusai disodium and its preparation
ActiveCN1778802AImprove controllabilitySimple and fast operationOrganic active ingredientsOrganic chemistryPhenacylPemetrexed disodium
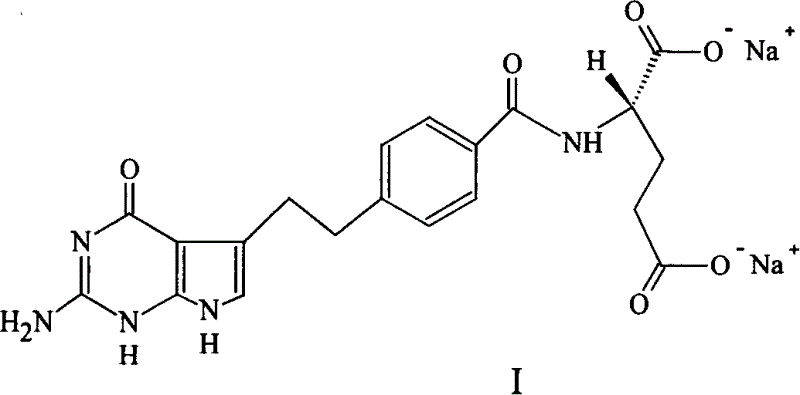
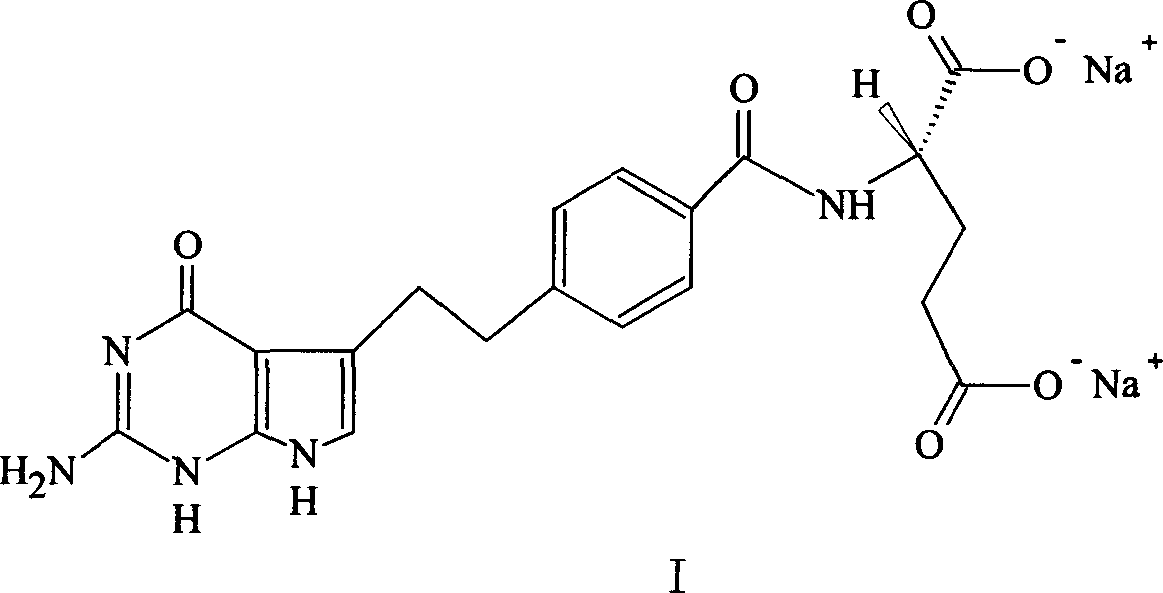
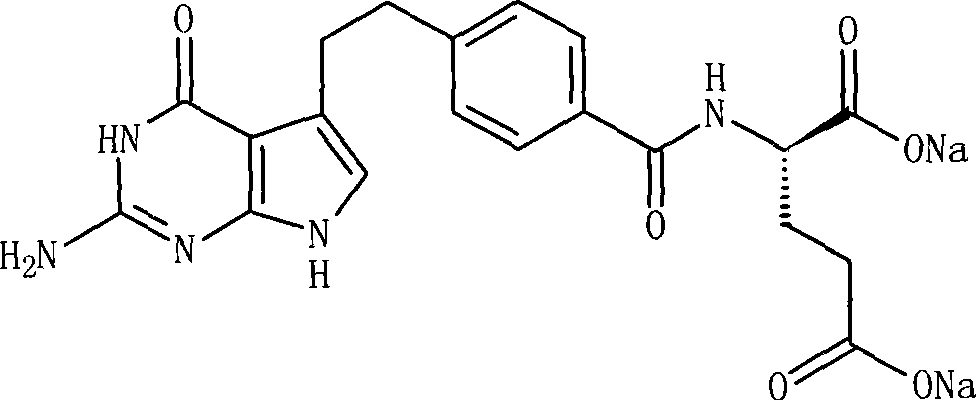
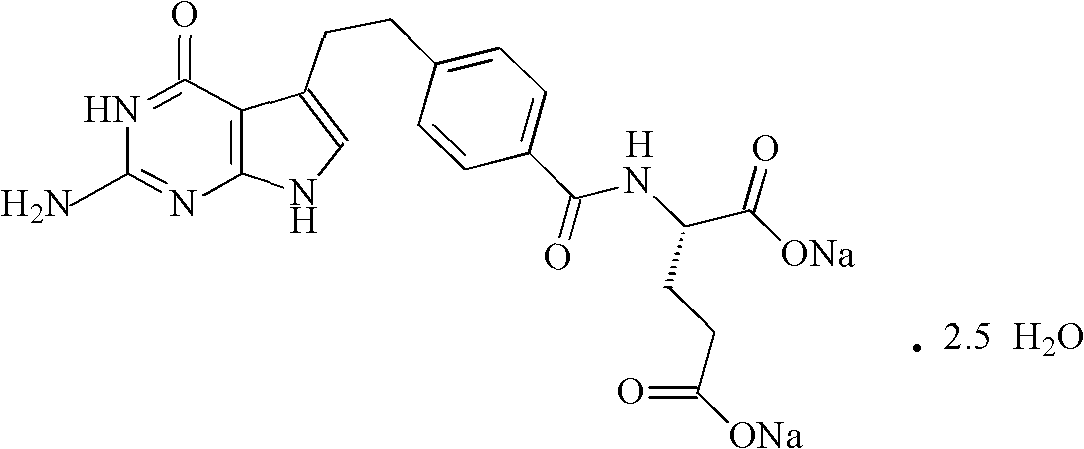
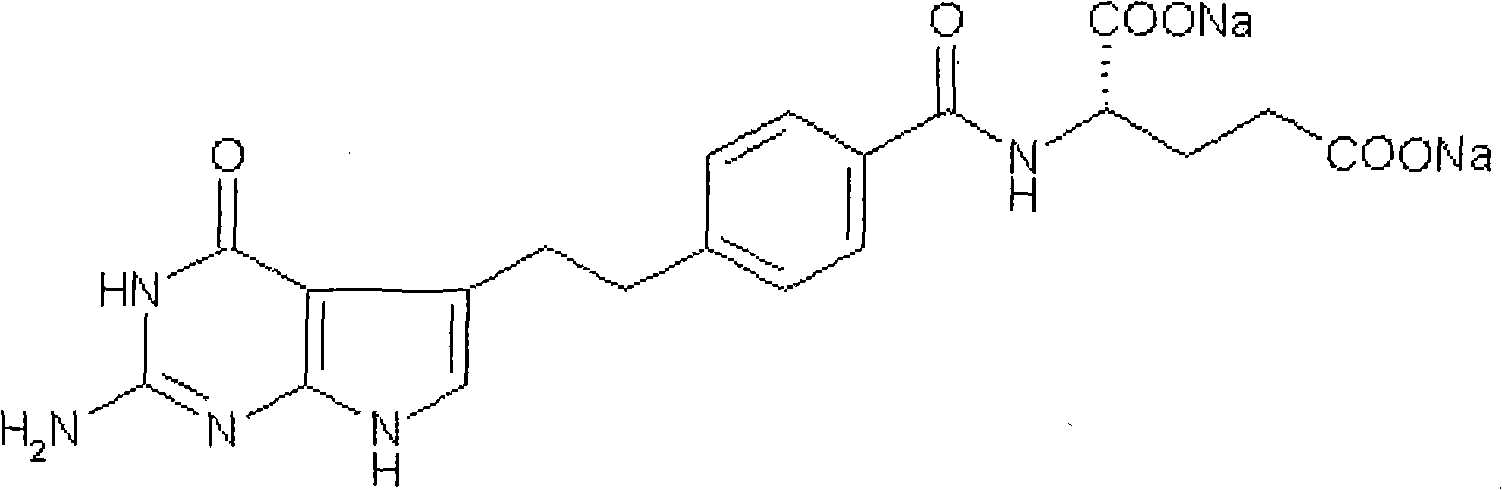
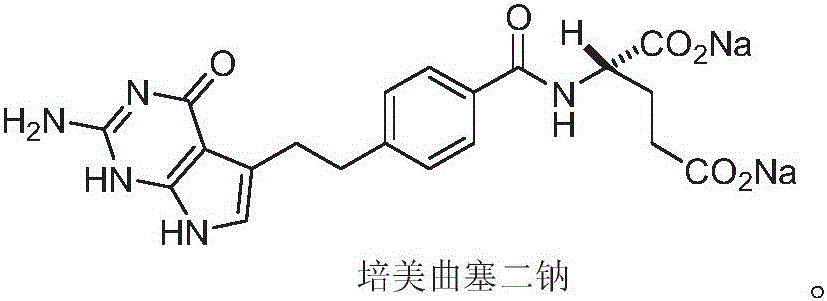
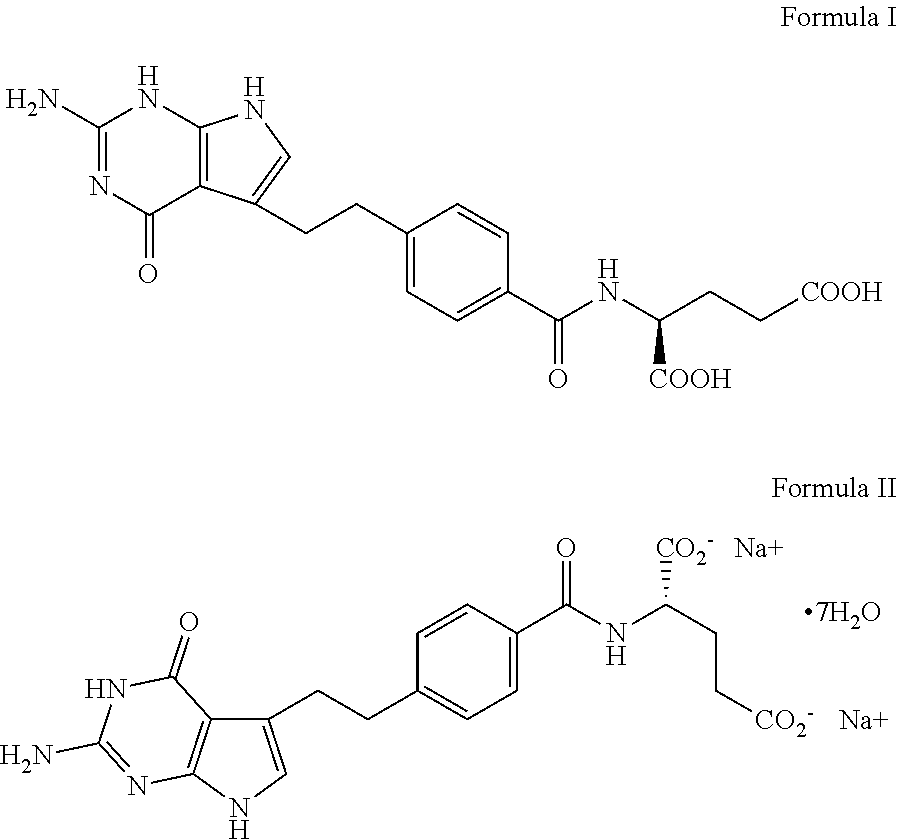
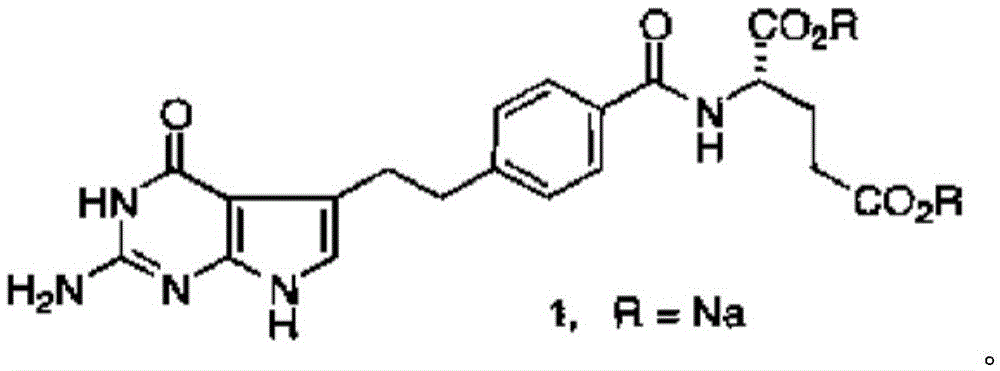
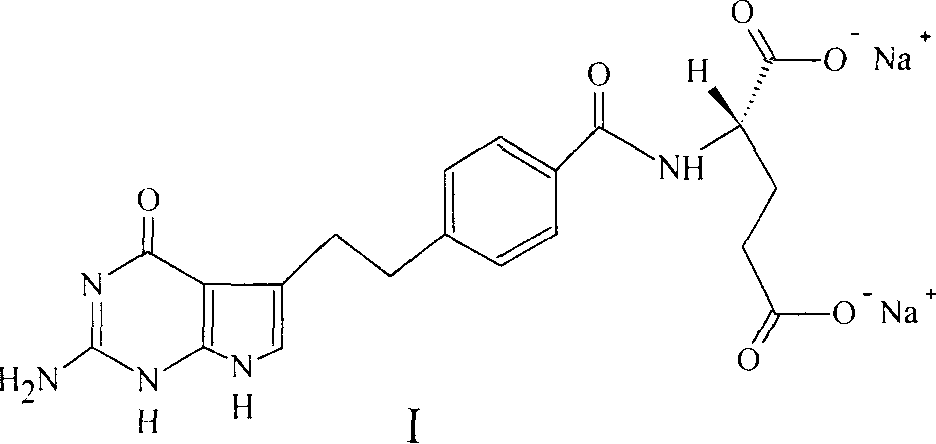
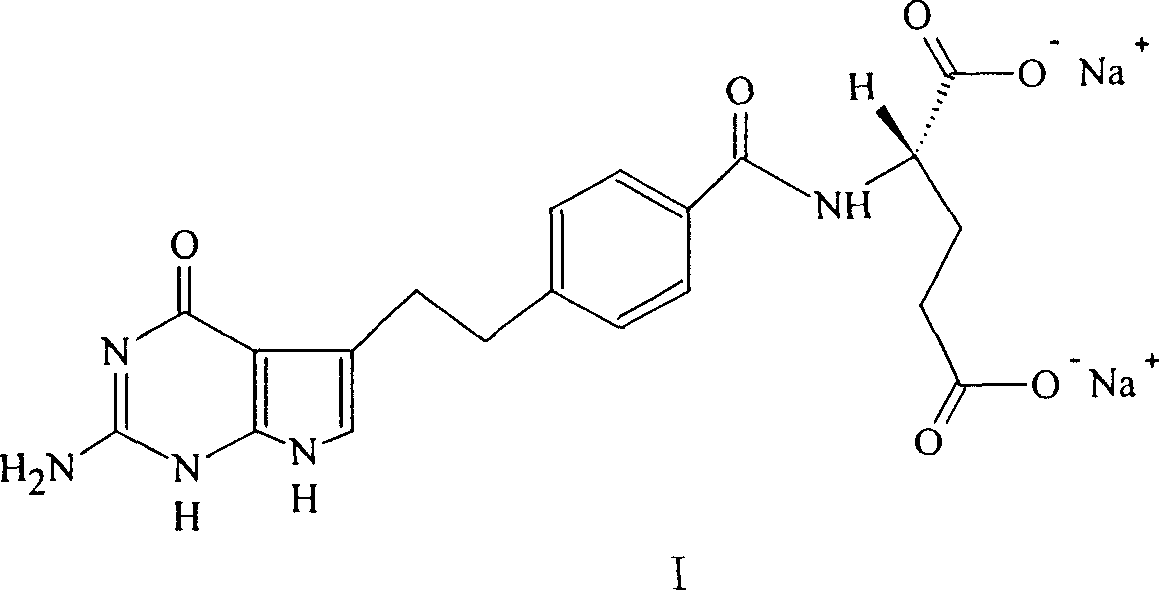
A crystal form of folic acid antagonistic N-(4-(2-(2-amino-4,7-methyl-4-oxo-1H-pyrrolo(2,3-d)pyrimidine-5-radical)ethyl)benzoyl)-L-glutamic acid disodium salt and its production are disclosed.
Owner:重庆凯林制药有限公司 +2
Pemetrexed disodium freeze-dried injection and preparation method thereof
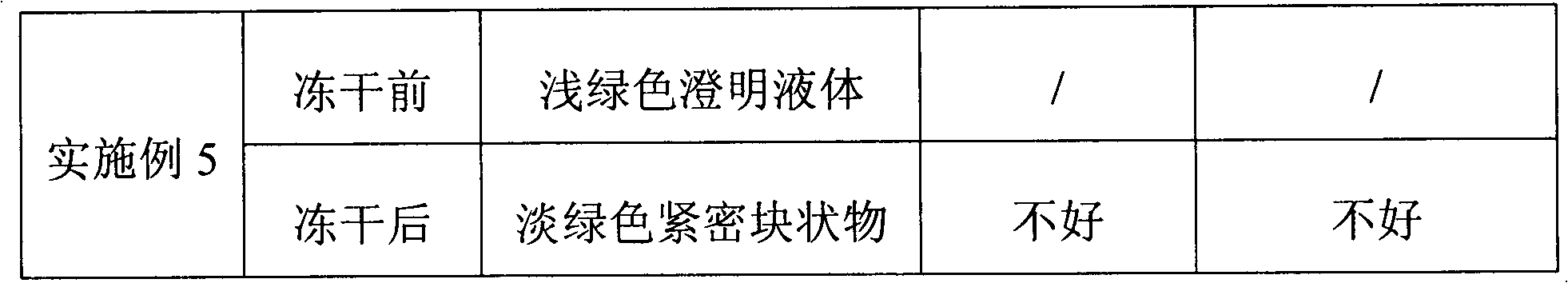
ActiveCN101411710AImprove stabilityLow content of related substancesPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLSulfite salt
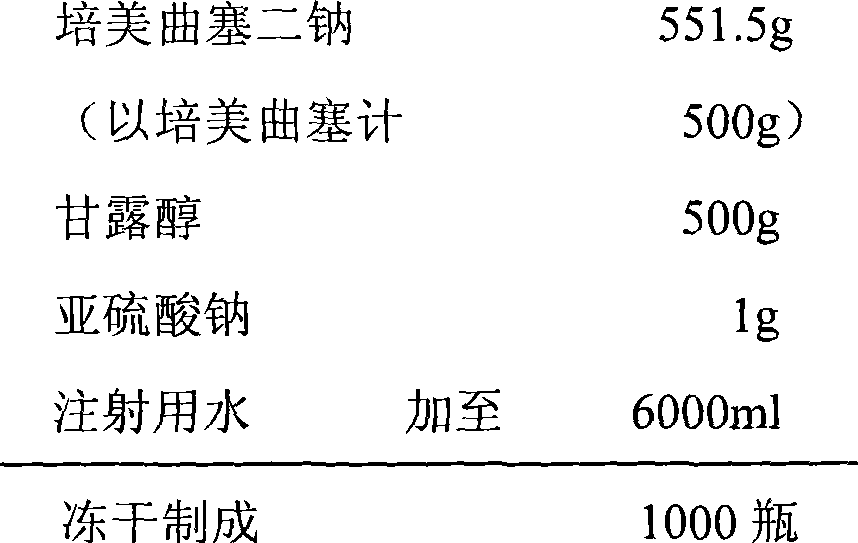
The invention relates to a pemetrexed disodium lyophilized powder injection, which consists of pemetrexed disodium, mannitol and sodium sulfite in the following weight portions: 50 portions of the pemetrexed disodium, 10 to 50 portions of mannitol, and 0.1 to 1 portions of sodium sulfite; and the pH value of the pemetrexed disodium lyophilized powder injection is between 7.0 and 8.0. The process for preparing the pemetrexed disodium lyophilized powder injection comprises the following steps: placing the mannitol in a sterile chamber; adding 80 percent of water for injection into the sterile chamber to dissolve the mannitol; adding the sodium sulfite to the mixture after the water for injection is cooled to a temperature of between 15 and 25 DEG C, and evenly stirring the solution for dissolving the sodium sulfite; then, adding the pemetrexed disodium into the solution, and stirring the solution to completely dissolve the pemetrexed disodium and evenly mixing the pemetrexed disodium, and adjusting the pH value of the solution to between 7.0 and 8.0; decarbidizing; after an intermediate compound passes examination, carrying out volume fixing, filtering, filling, partially stopping, traying, lyophilizing, nitrogen aerating, stopping and unboxing, sealing by a plastic-aluminum combined cap, and packaging after passes quality inspection to obtain the pemetrexed disodium lyophilized powder injection. The invention has the advantages of simple preparation process, convenience and practicality, good repeatability and low production cost, and can realize industrial large-scale production easily.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Pemetrexed disodium freeze-dried powder injection and preparation method thereof
ActiveCN102106833AReduce adverse effectsSimple prescriptionOrganic active ingredientsPowder deliveryActivated carbonFreeze-drying
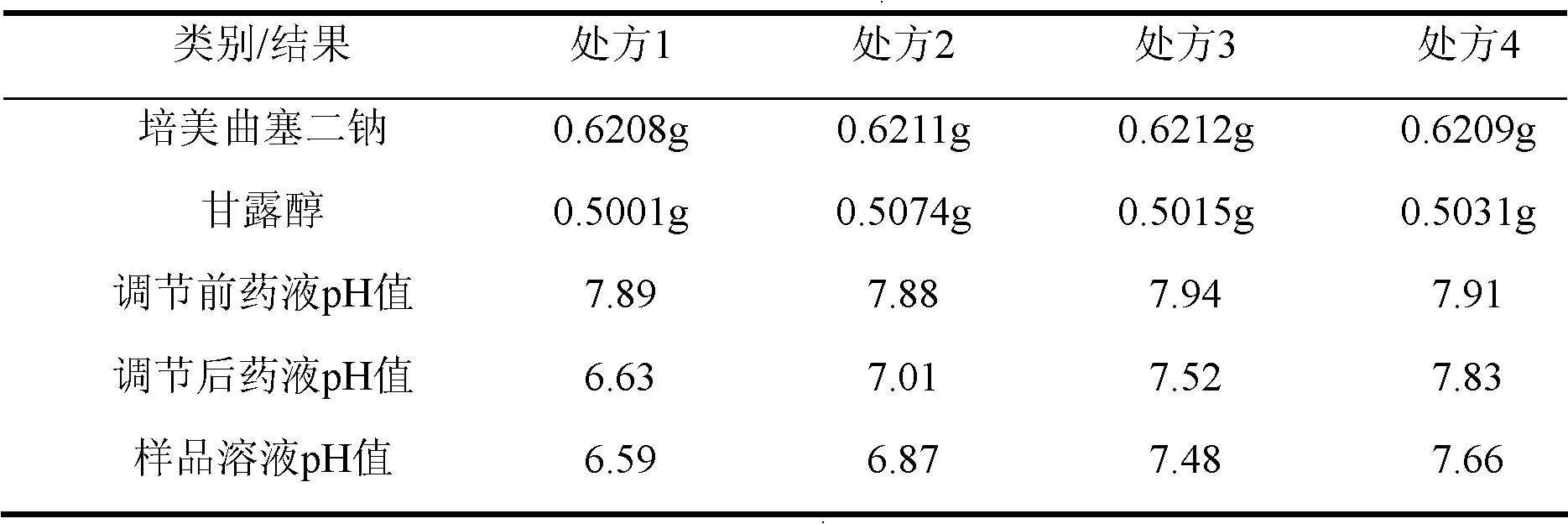
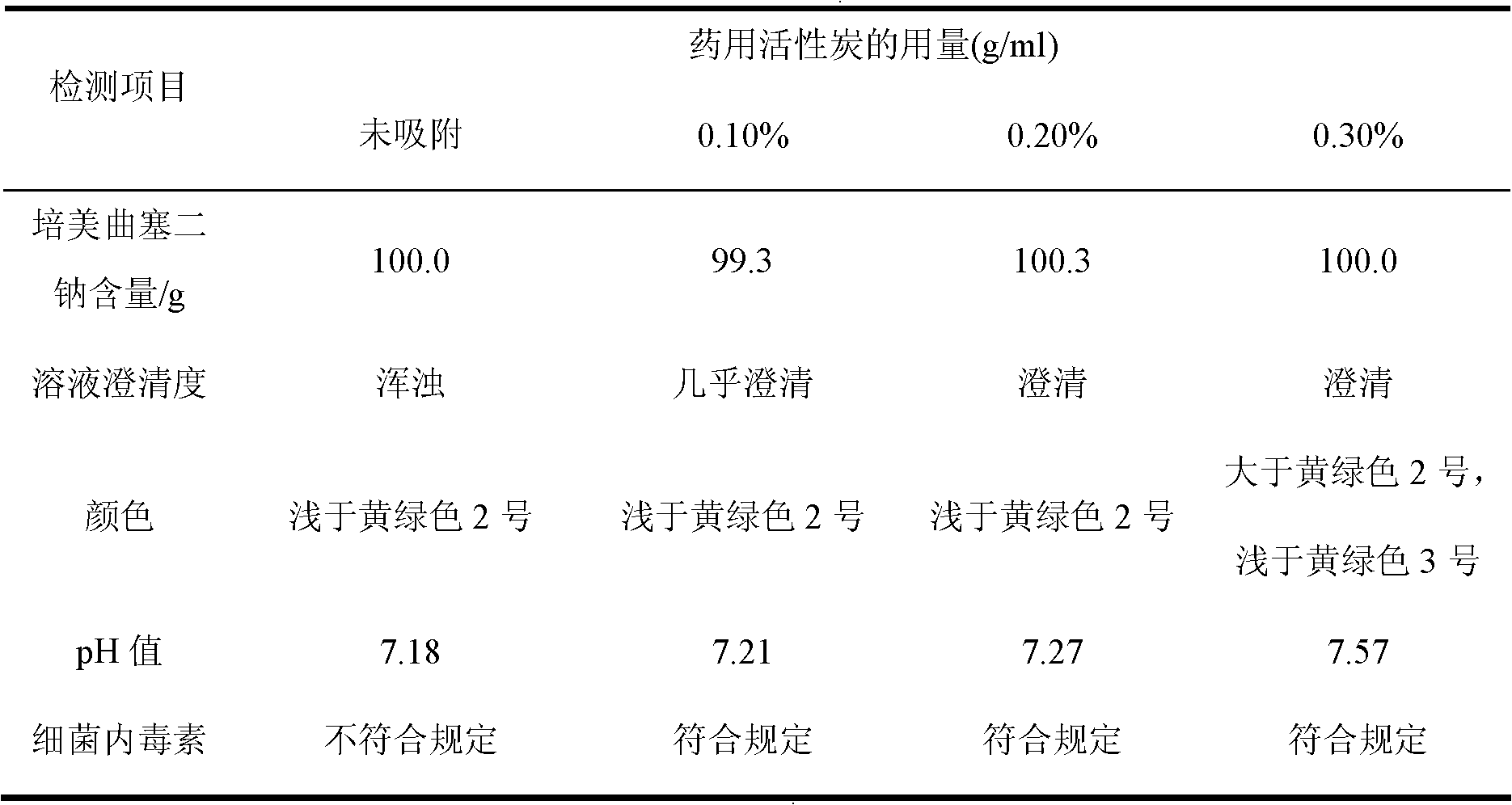
The invention belongs to the technical field of medication, and in particular relates to a pemetrexed disodium freeze-dried powder injection and a preparation method thereof. The pemetrexed disodium freeze-dried powder injection consists of pemetrexed disodium and mannitol, wherein the mass ratio of the mannitol to the pemetrexed disodium is (0.6-2.0):1. The preparation method comprises the following steps: adding injecting water into a liquid preparation tank; adding the pemetrexed disodium weighted according to the formula; stirring until the pemetrexed disodium completely dissolved; adding the mannitol; regulating the pH by utilizing a hydrochloric acid solution or a sodium hydroxide solution; adding activated carbon for decoloration; filtering to remove the carbon; finely filtering with a filter membrane; subpackaging; and freezing and drying. The pemetrexed disodium freeze-dried powder injection has excellent moldability; the appearance of the solution before freezing is clear; the frozen and dry product has good re-dissolubility; and the re-dissolved product has the advantages of good clarity, low impurity content, low moisture content, good stability and controllable quality.
Owner:HAINAN JINRUI PHARMA
Method for purifying high-purity pemetrexed disodium
The invention provides a method for purifying high-purity pemetrexed disodium. The method is realized by combining a salting-out method with an organic solvent / water mixed solvent crystallization method. The method provided by the invention is carried out at normal temperature; and by using the method, the quantities and contents of organic impurities and inorganic impurities in the purified products can be effectively controlled, the content of the organic impurities satisfies requirements on identification thresholds of the impurities of bulk drugs in China, and the quantity of the organic impurities is obviously reduced.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
A kind of pharmaceutical composition of pemetrexed disodium
InactiveCN102266298AIncrease painOrganic active ingredientsPowder deliveryPatient complianceNitrogen
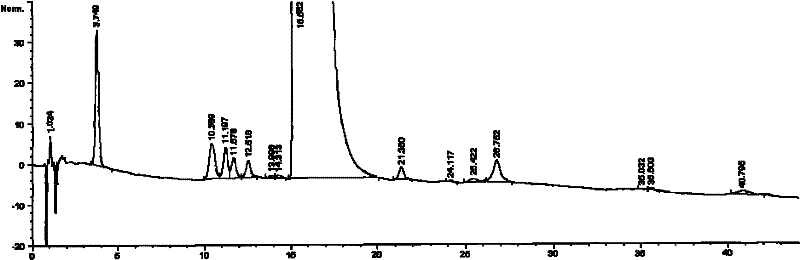
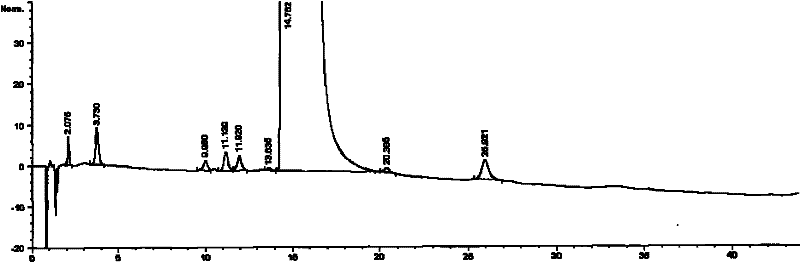
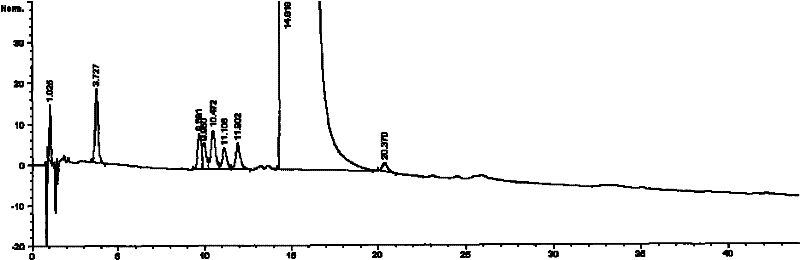
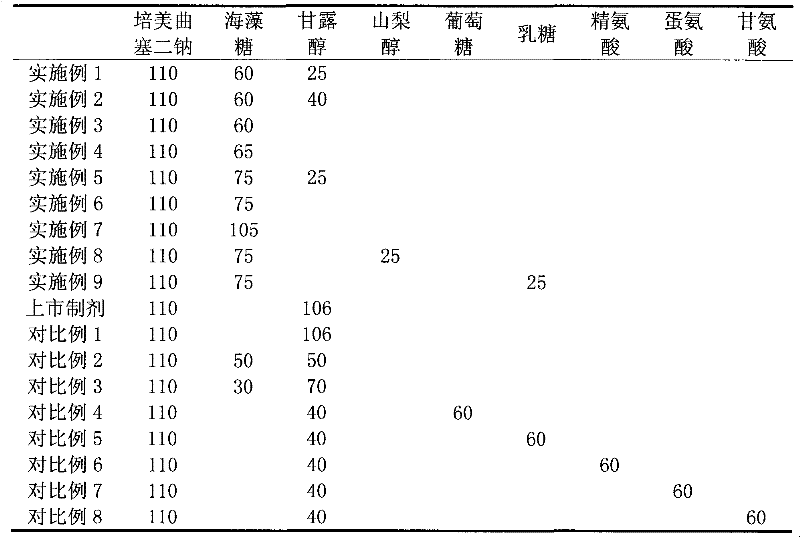
The invention relates to a freeze-dried pharmaceutical composition of pemetrexed disodium and a preparation method thereof. The invention provides a pemetrexed disodium freeze-dried composition containing pemetrexed disodium, trehalose and freeze-dried excipients, the parts by mass are 110 parts of pemetrexed disodium, 60-60 parts of trehalose 105 parts, 0-40 parts of freeze-dried excipients. The preparation process is: dissolving pemetrexed disodium, trehalose and lyophilized excipients in water, stirring until the solution is clear, adjusting the pH to 7.5-8.0 with sodium hydroxide or hydrochloric acid solution, and removing the pyrogen from the above clear solution and filter to sterilize, freeze-dry to make a sterile powdery solid pharmaceutical composition, seal the container under nitrogen protection, and pack to obtain a finished product. The pharmaceutical composition of the present invention can obviously improve local stimulation effects such as discomfort, pain and even inflammatory reaction during pemetrexed disodium infusion, and improve patient compliance.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Preparation method of pemetrexed disodium for injection
The invention provides a preparation method of pemetrexed disodium for injection, which comprises the following steps: (1) cleaning rubber plugs, and sterilizing; (2) cleaning and sterilizing vials; (3) disinfecting aluminum-plastic caps; (4) preparing solution; (5) filling medicinal solution passing tests into the vials, and half capping; (6) vacuum freeze drying; (7) pressing the rubber plugs, boxing, rolling the caps, and carrying out visual inspection; and (8) packaging, inspecting finished products, and storing after finished product inspection is passed.
Owner:SUZHOU ERYE PHARMA CO LTD
Industrialized production method of high-purity pemetrexed disodium
ActiveCN102086204AReduce Occupational InjuriesEasy to operateOrganic chemistryAcetonitrileSilica gel
The invention provides an industrialized production method of high-purity pemetrexed disodium, comprising the following steps of: (1) adding crude pemetrexed disodium into a reactor, adding water and stirring to dissolve at a temperature of 10-30 DEG C; (2) adding tetrahydrofuran or acetonitrile serving as a dissolvent into the reaction solution of the step (1), dissolving out a part of solids, adding kieselguhr or silica gel and stirring for 5-30 minutes; and (3) filtering the reaction solution of the step (2), adding dissolvent same as the dissolvent added in the step (2) into filtrate, crystallizing for 0.5-10 hours at a temperature of 10-30 DEG C, isolating solids, and drying for 0.5-10 hours at a temperature of 20-40 DEG C to obtain the high-purity pemetrexed disodium. By means of the production method, the shortcomings that in the prior art column chromatography, purification and heating are needed, the product purity is low, the operation is cumbersome and the industrialized production is difficult to realize are overcome; the production method is simple and convenient for operation, is easy to realize the industrialized production and has the advantages of few consumption of dissolvent, energy saving, environmental protection and low labor intensity; and the products have the advantages of white color, high purity, less than 0.05% of impurities in a single product and good stability.
Owner:NANJING HAIRUN PHARM CO LTD
Pemetrexed disodium liposome injection
InactiveCN103040748AInhibit aggregationLarge particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityMedicine
The invention discloses pemetrexed disodium liposome injection which is mainly made of pemetrexed disodium and a preparation method thereof, distearoyl phosphatidyl glycerol, dipalmitoyl phosphatidyl choline, PEG600, cholesterol and mannitol. The liposome injection has the advantages that the particle size of liposome is small, the liposome is uniformly distributed, the encapsulation efficiency is high, the leakage rate is low, the stability is good, the solubility of the pemetrexed disodium and the quality of injection products are improved, the toxic and side effect is reduced, and the curative effect is improved.
Owner:海南路易丹尼生物科技有限公司
Amorphous polymorph for pemetrexed disodium and preparation method thereof
The invention discloses an amorphous polymorph for pemetrexed disodium and a preparation method thereof. An X-ray powder diffraction pattern of the amorphous polymorph does not contain an identifiable diffraction peak form. For not containing crystal water, the amorphous polymorph for the pemetrexed disodium is simple to dry on production, does not need special equipment, has low production cost, and is suitable for industrial production.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
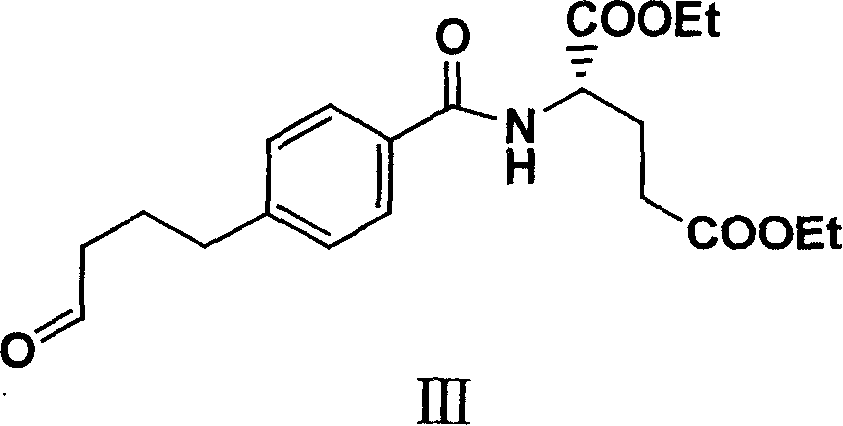
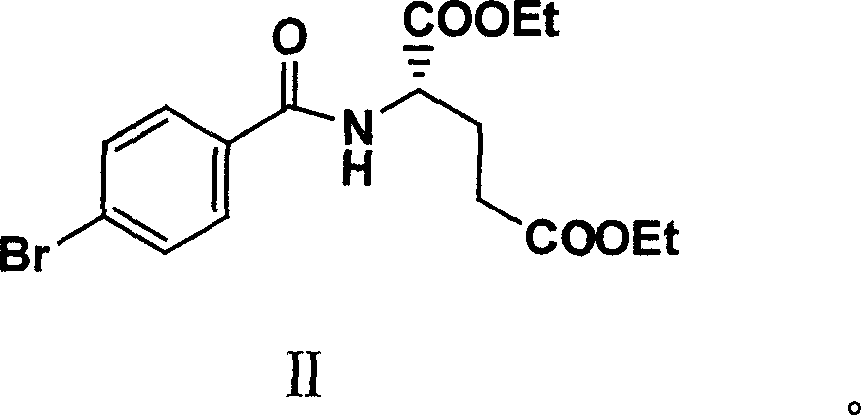
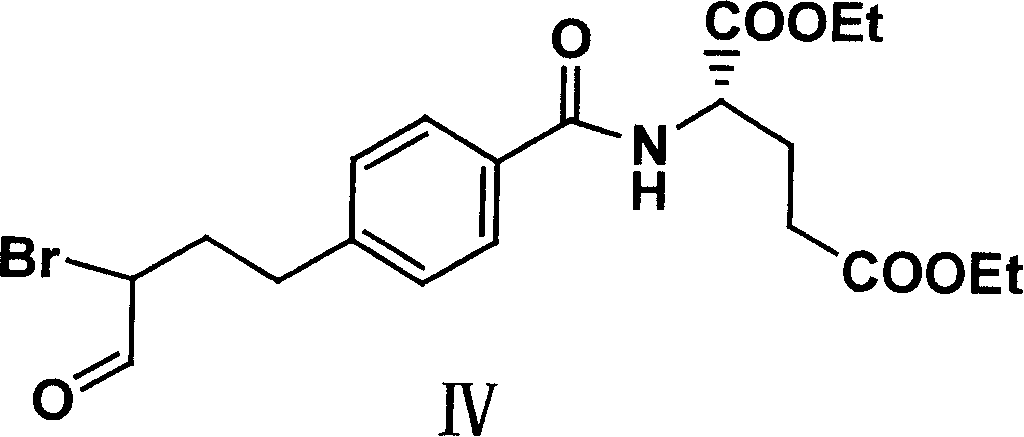
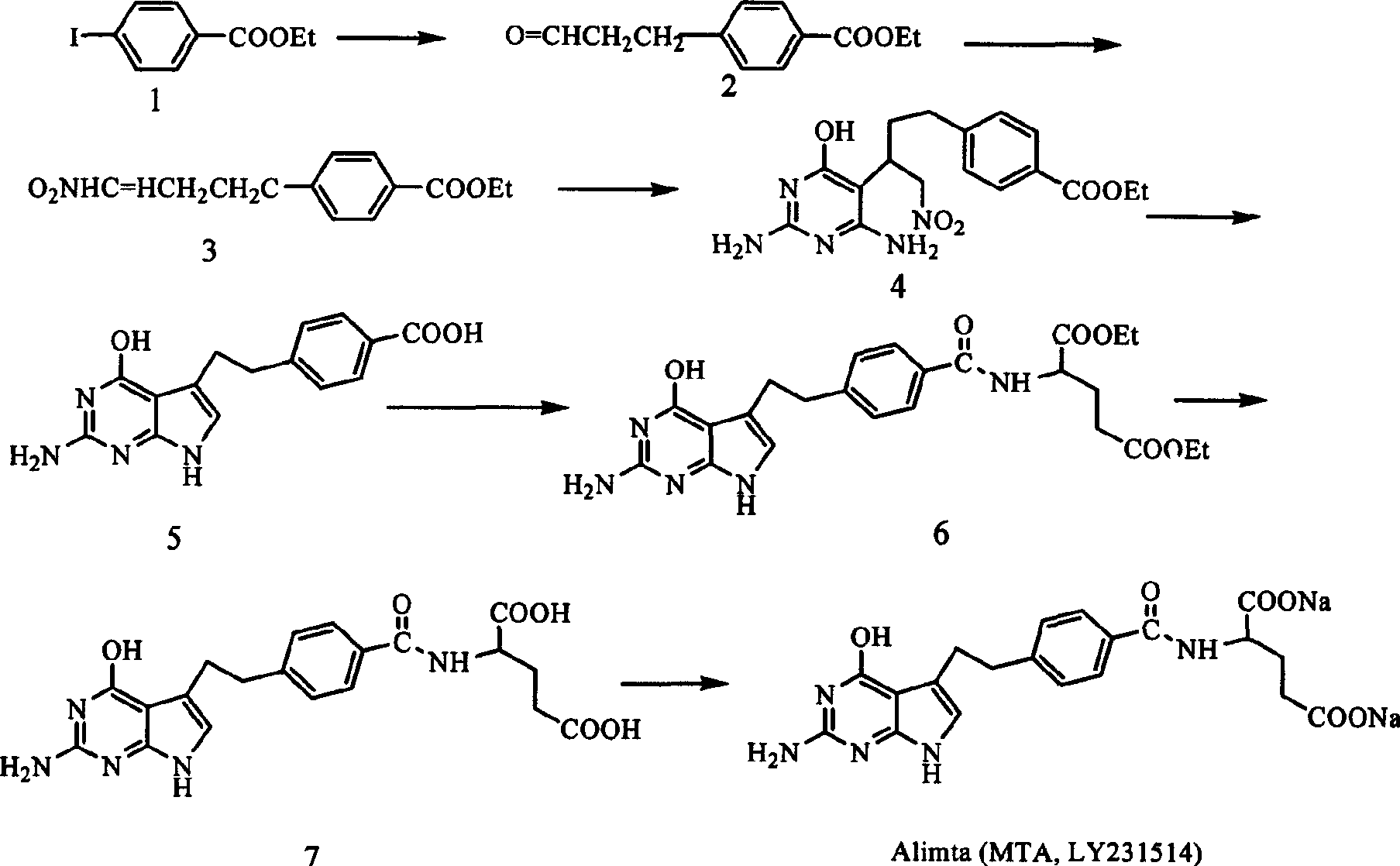
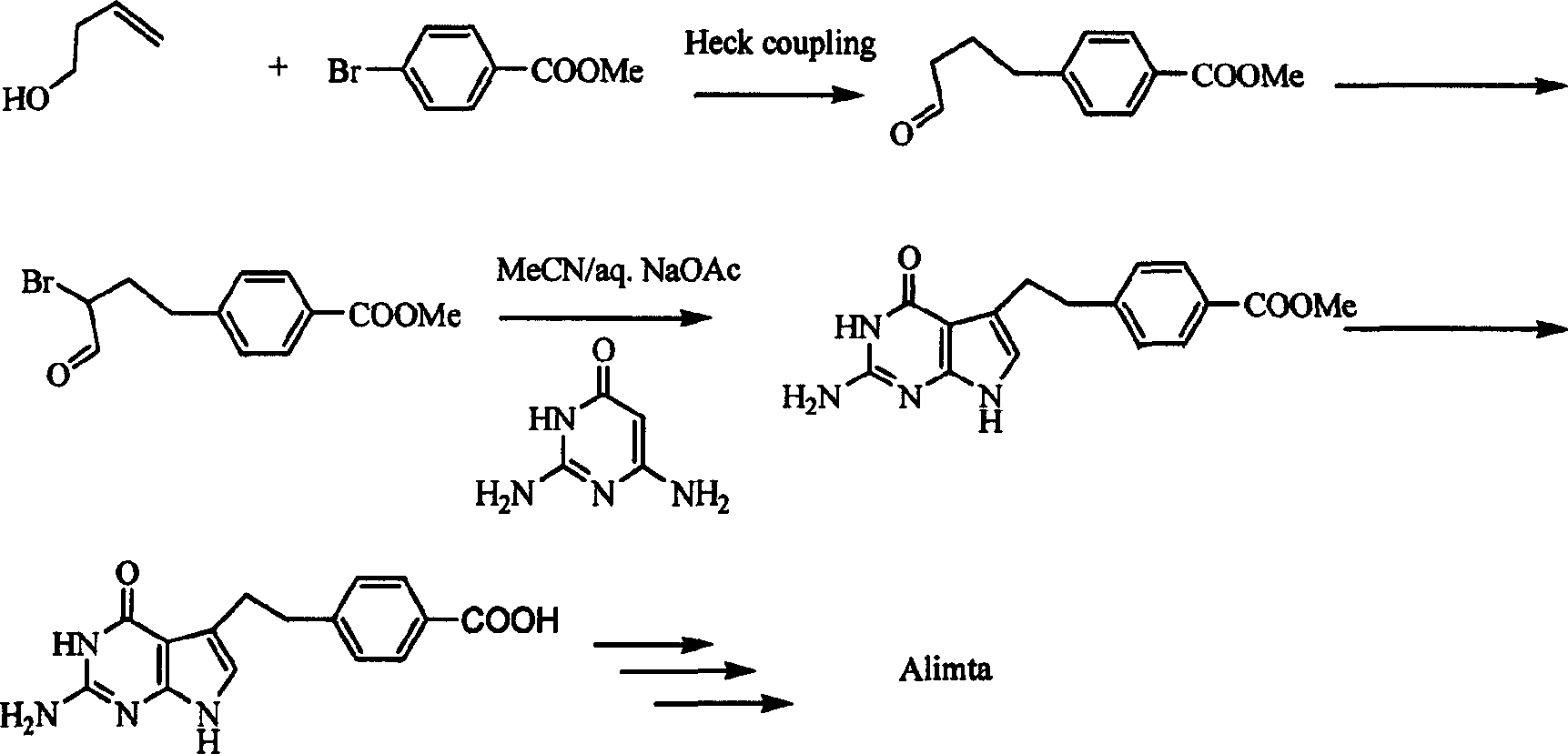
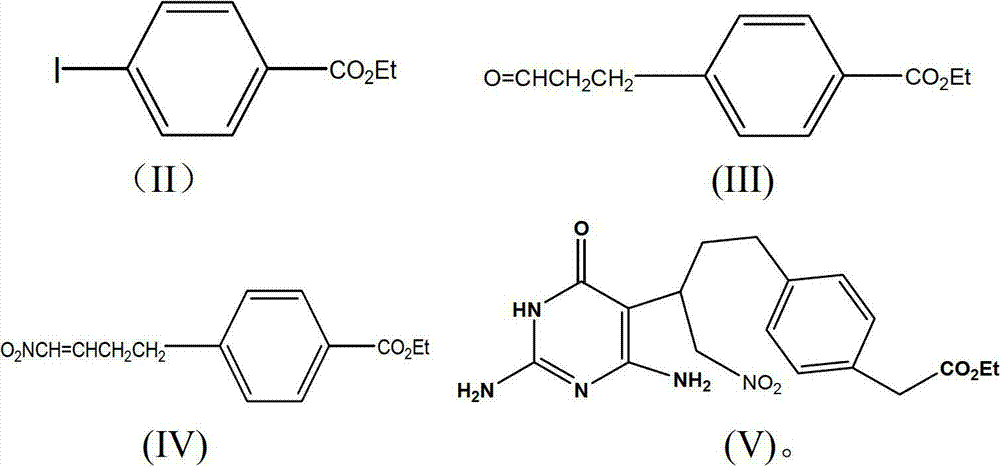
Diethyl 4(4-oxobutyl) benzoyl-L-glutamate and its preparation and use
InactiveCN1821219AHigh yieldMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationL glutamateHalide
The present invention relates to new compound diethyl 4-(4-oxobutyl) benzoyl-L-glutamate and its preparation process and application. The compound diethyl 4-(4-oxobutyl) benzoyl-L-glutamate in the chemical expression as shown is prepared through the reaction of compound diethyl 4-bromo benzoyl-L-glutamate and 3-butene-1-alcohol inside Nú¼N-dimethyl formamide solvent under the action of palladium acetate catalyst, weak alkali reagent, lithium halide and phase transfer catalyst in the protection of inert gas at 50-70 deg.c. The compound diethyl 4-(4-oxobutyl) benzoyl-L-glutamate is used in synthesizing diethyl 4-[(4-oxo-3-bromo) butyl] benzoyl-L-glutamate as the intermediate of Pemetrexed disodium. The present invention results in shortened Pemetrexed disodium synthesizing path and lowered production cost.
Owner:ZHEJIANG UNIV OF TECH
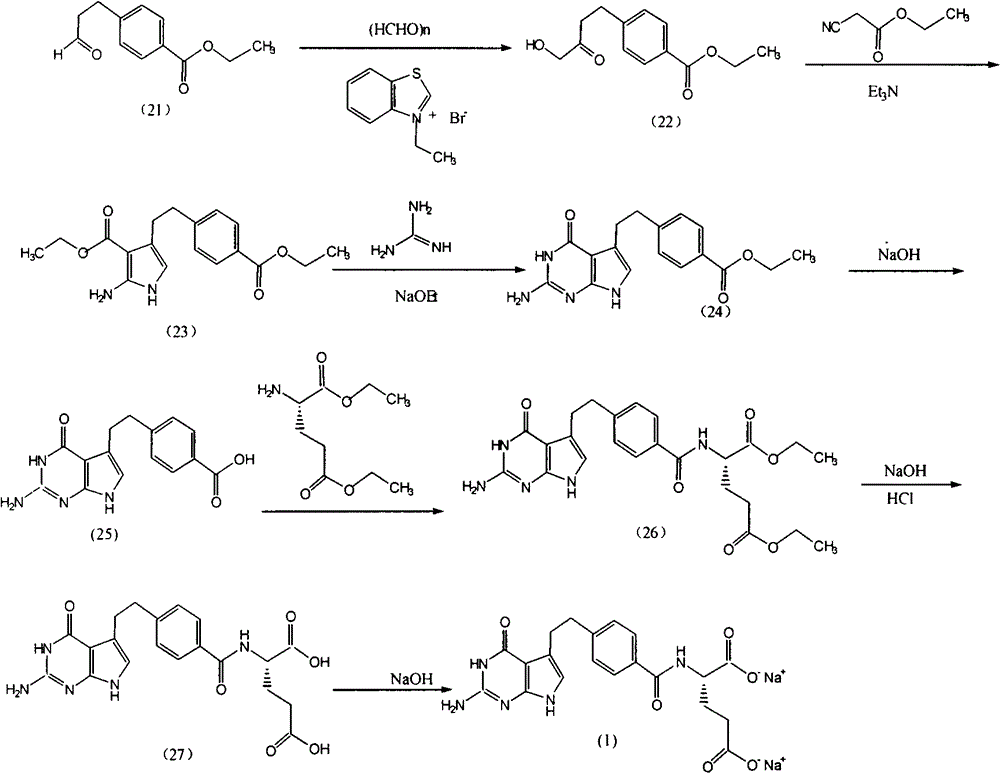
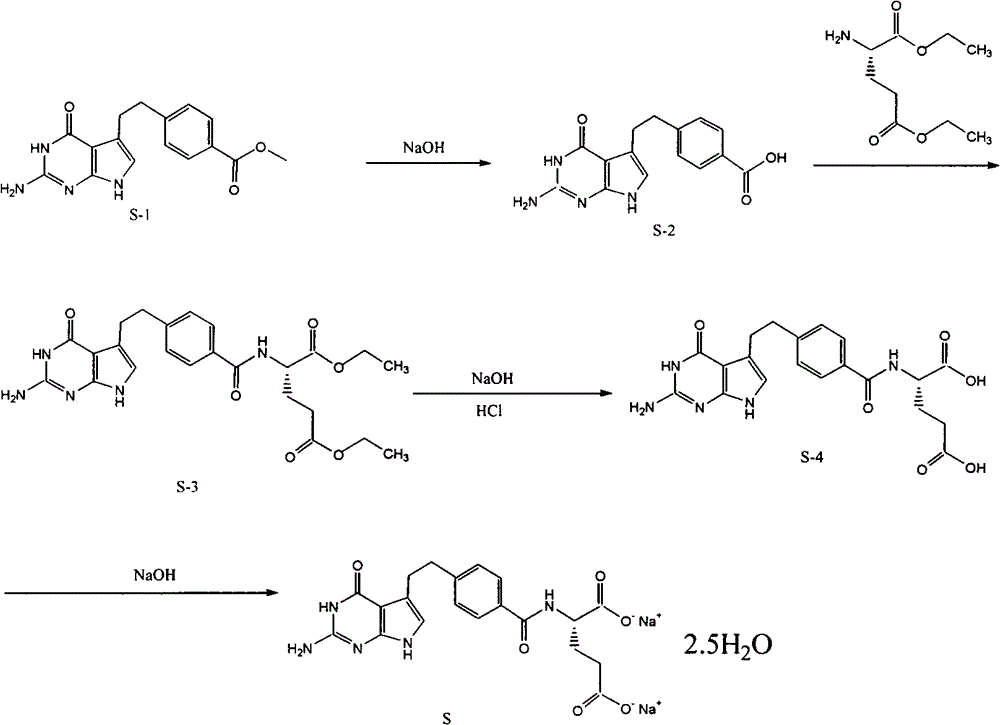
Preparation method of pemetrexed disodium
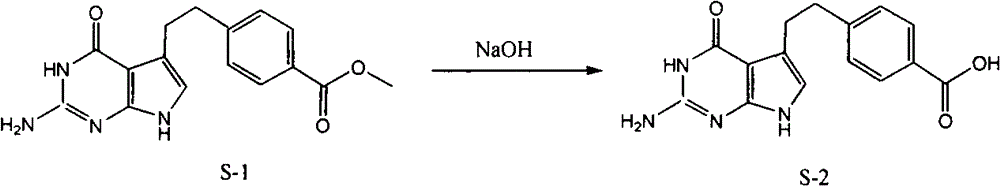
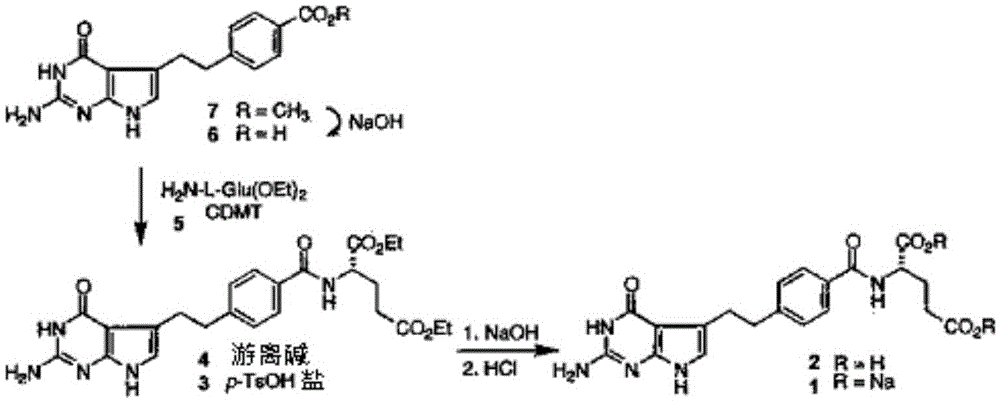
The invention provides a preparation method of pemetrexed disodium, which comprises the steps of performing hydrolysis reaction of 4-[2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl] methyl benzoate in sodium hydroxide to generate acid, then condensing with L-glutamic acid diethyl ester to obtain N-[4-[2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic acid diethyl ester toluenesulfonate, obtaining the crude pemetrexed disodium through saponification under the action of sodium hydroxide, and finally crystallizing in a mixed solvent at room temperature to obtain the end product. With the adoption of the technical scheme, the purification and purification of the crude pemetrexed disodium can be directly performed, so that the cost can be effectively lowered. As the steps are carried out at room temperature, the oxidation of the pemetrexed disodium caused by high temperature can be effectively avoided. The purity of the end product is high and meets the national requirement on purity of chemicals.
Owner:DEZHOU DEYAO PHARMA
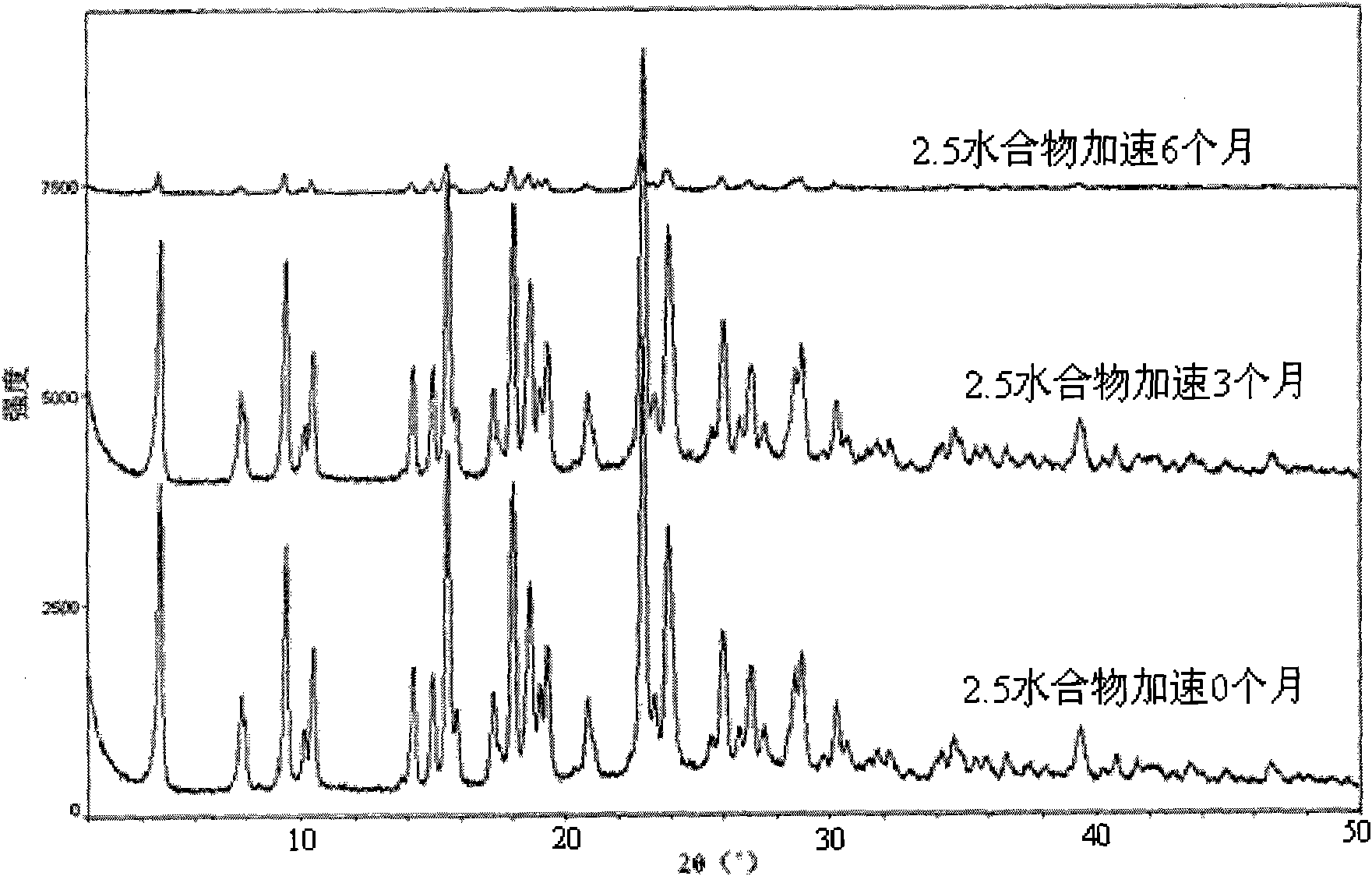
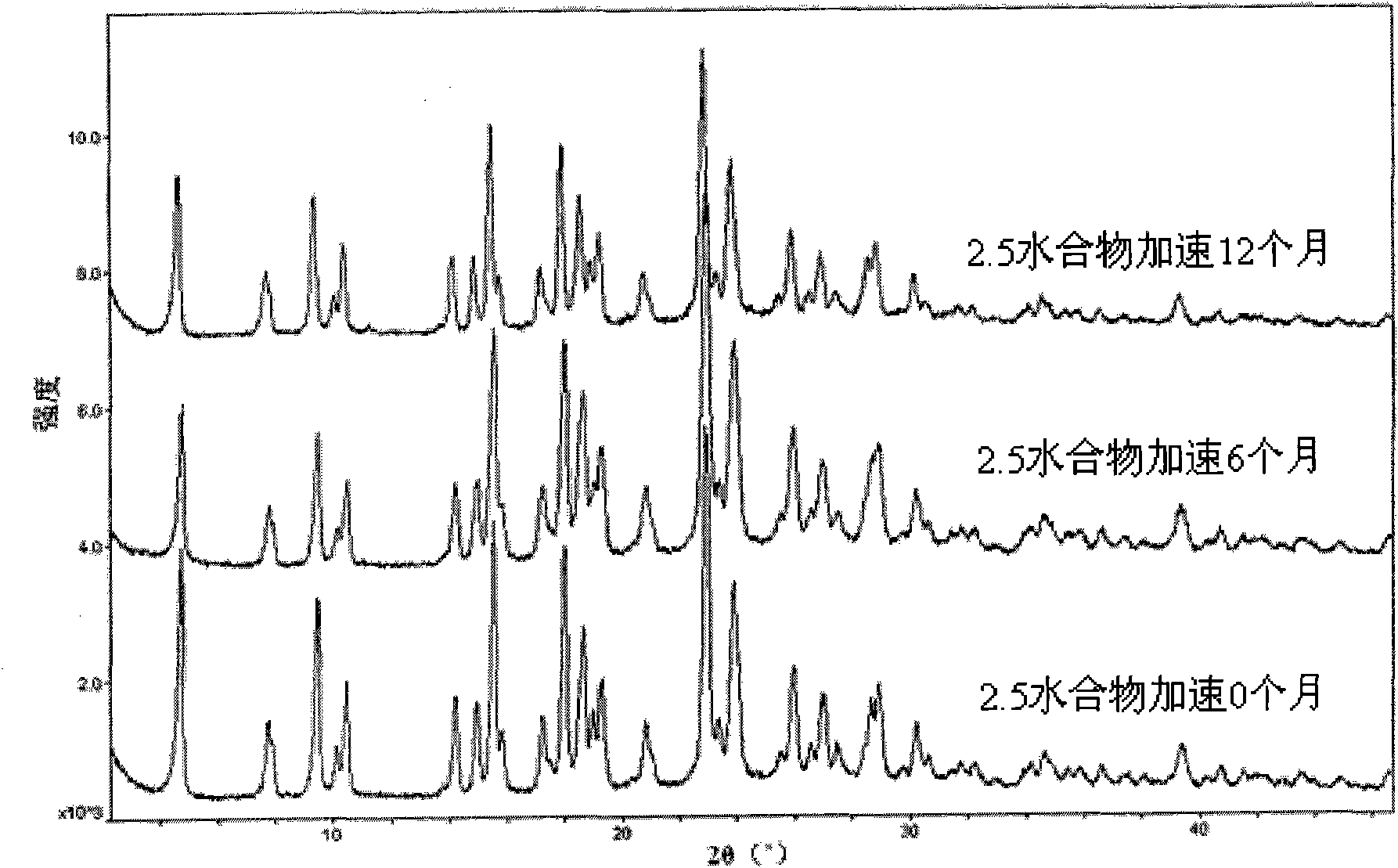
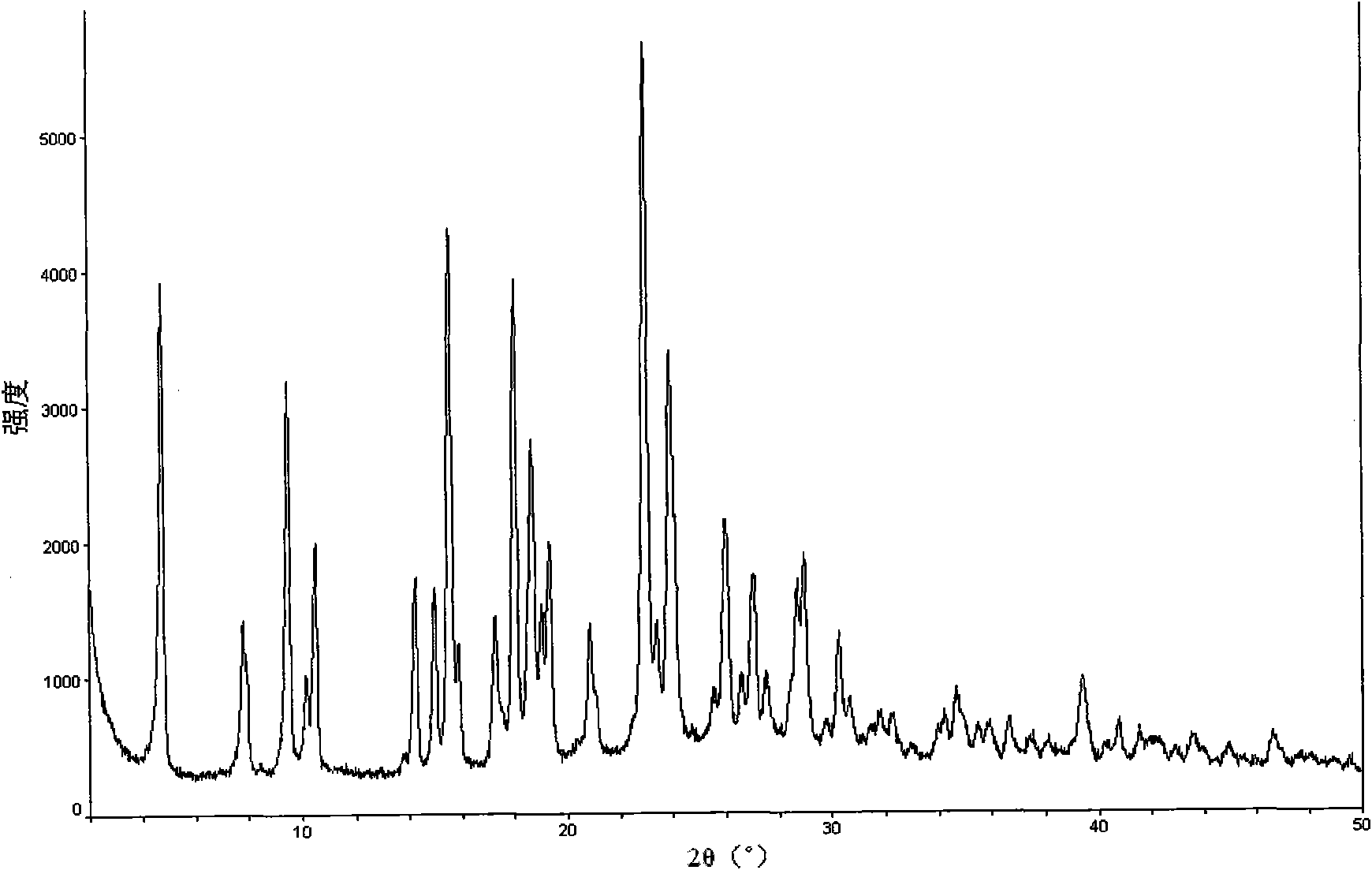
Method for preparing pemetrexed disodium 2.5 water crystal
The invention provides a method for preparing a pemetrexed disodium 2.5 water crystal. The method comprises the following steps of: (1) dissolving a raw material, namely pemetrexed disodium in water; (2) dropwise adding the aqueous solution of the pemetrexed disodium into an organic solvent which can be miscible with the water, and crystallizing; and (3) filtering, collecting a filter cake, and drying to obtain a target product. The preparation method is high in controllability, easy and convenient to operate, high in repeatability, high in production yield, and stable in crystal form and industrial production is easy to implement, and special equipment is not required.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
Pemetrexed disodium freeze-dried powder injection for injection and preparation method thereof
ActiveCN103432086AImprove stabilityAvoid degradationOrganic active ingredientsPowder deliveryDipotassium hydrogen phosphateFreeze-drying
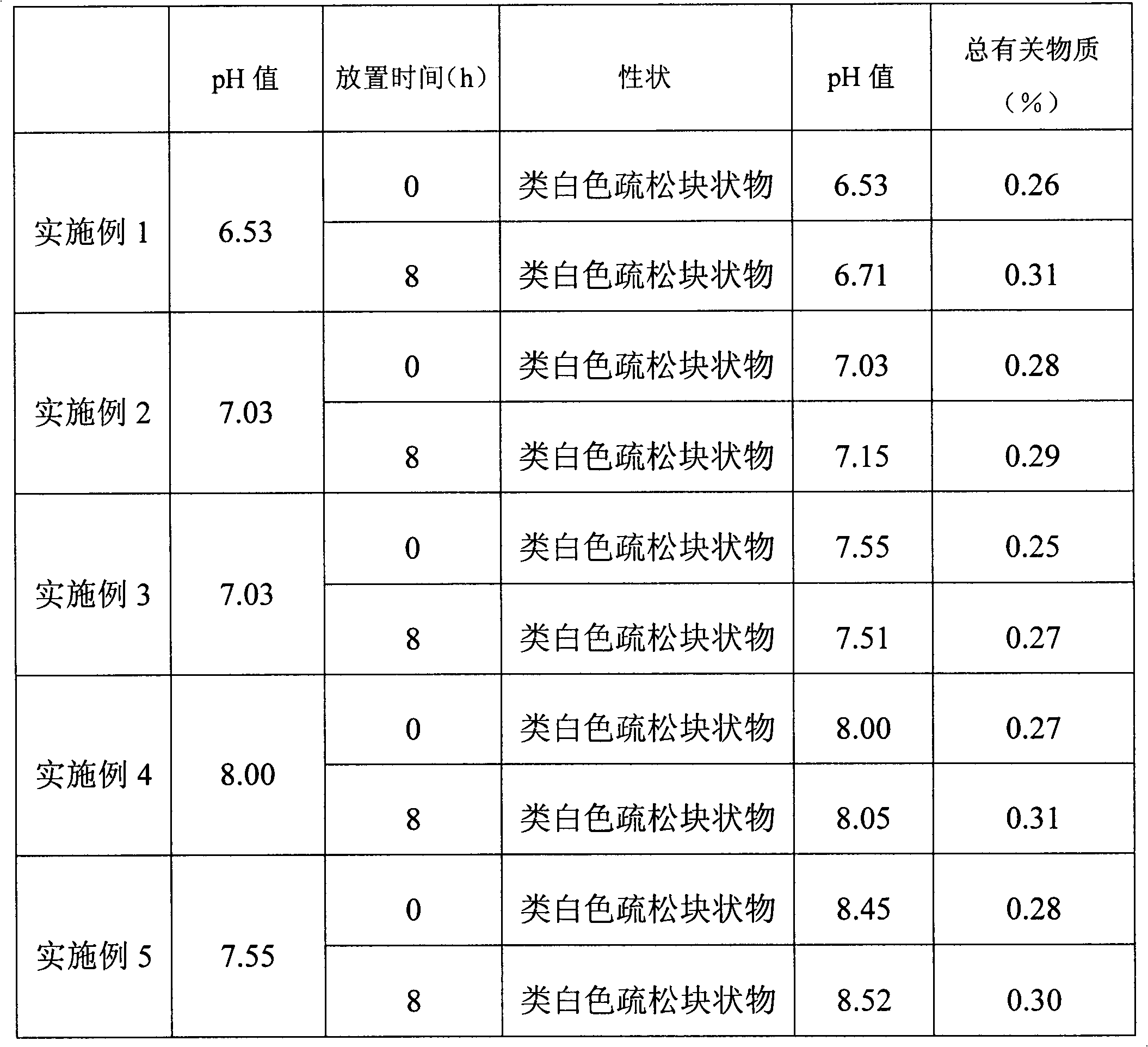
The invention discloses a pemetrexed disodium freeze-dried powder injection for injection and a preparation method thereof. The pemetrexed disodium freeze-dried powder injection contains disodium hydrogen phosphate and dipotassium hydrogen phosphate buffer salt, wherein the weight ratio of disodium hydrogen phosphate to dipotassium hydrogen phosphate is (8-10):1, and the pH value of liquid medicine is 6.5-7.5 before freeze-drying. The pemetrexed disodium freeze-dried powder injection disclosed by the invention enhances the pharmaceutical stability, has the advantages of less auxiliary material variety and usage amount, more safety and reliability and simple preparation process, and meets the requirement for mass production.
Owner:NANJING CHIA TAI TIANQING PHARMA
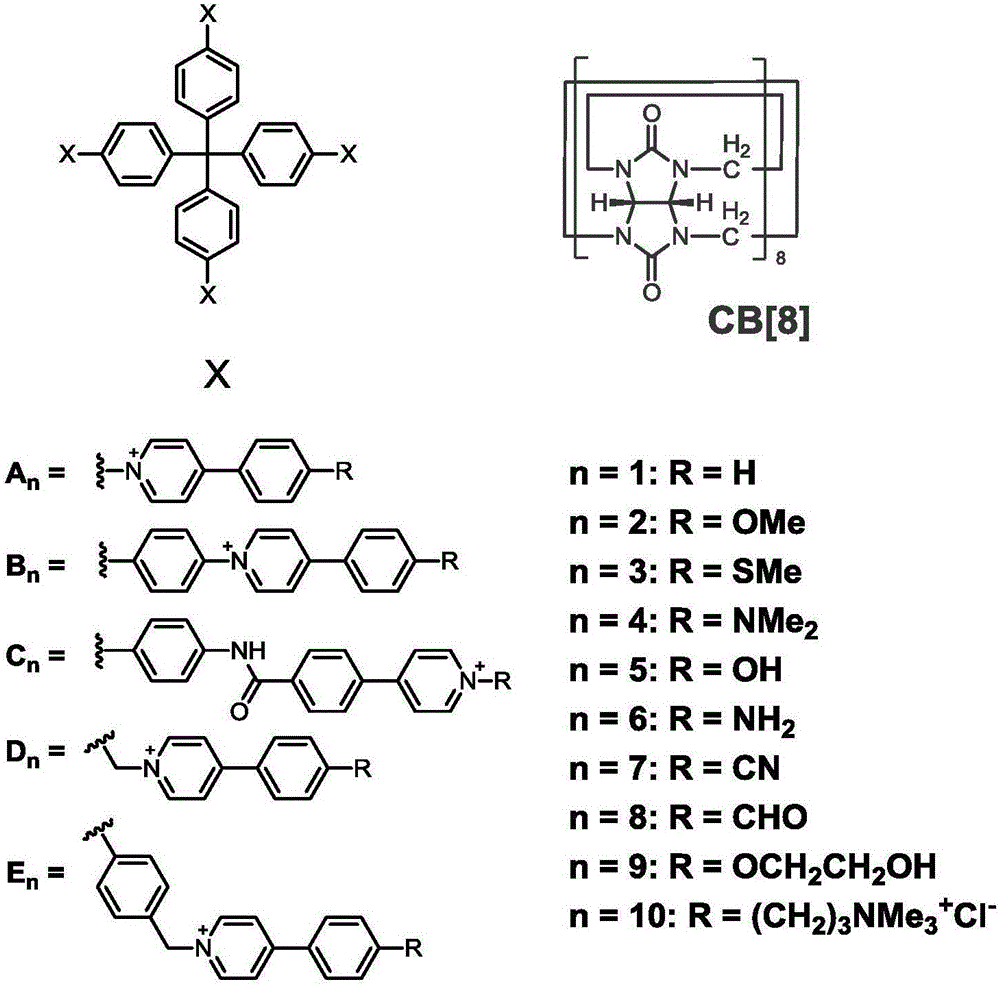
Nano drug delivery system inhibiting multidrug resistance breast cancer growth and preparation method and application thereof
InactiveCN105902498AHigh efficiency loadEfficient releaseOrganic active ingredientsPowder deliveryOncologyChemo therapy
The invention belongs to the field of pharmaceutical preparations and the technical field of breast cancers, and discloses a preparation method and application of a soluble nano drug delivery system inhibiting multidrug resistance breast cancer growth. According to the preparation method and application, water-soluble super-molecular organic framework (SOF) nano particles server as carriers for the first time, the system is obtained through self-assembly of tetrahedral molecules obtained through tetraphenylmethane derivatization and CB[8] in a water phase, and chemotherapy drugs such as neutral drug doxorubicin and anionic drug pemetrexed disodium, folate derivatives, targeted integrin specific ligands such as RDG peptide derivatives and infrared probe molecules such as IR-820 can be loaded simultaneously; the SOF nano-drug carrier has the release characteristic of pH dependence, is accumulated in tumor tissue at high concentration, has a specific growth inhibiting effect on tumor cells with drug resistance in the tumor tissue due to P-glycoprotein overexpression and can obviously increase the retention volume of a chemotherapy drug in multidrug resistance breast cancer tissue, effectively inhibit the multidrug resistance breast cancer growth and significantly improve the curative effect on a multidrug resistance breast cancer.
Owner:FUDAN UNIV
Injection composition of pemetrexed disodium and preparation method of injection composition
ActiveCN102846563AReduce moisture contentReduced stabilityPowder deliveryOrganic active ingredientsMedicineMannitol
The invention relates to an injection composition, and in particular relates to an injection composition of pemetrexed disodium and a preparation method of the injection composition. The injection composition is prepared from pemetrexed disodium, mannitol and beta-cyclodextrin. The injection composition of pemetrexed disodium is simple in preparation process and effectively reduces the preparation cost. The pemetrexed disodium freeze-dried powder prepared by the invention has the advantages of excellent dissolvability, few impurity content, low moisture content and good stability.
Owner:DEZHOU DEYAO PHARMA
Pemetrexed disodium key intermediate and its synthesis method, and method for synthesizing pemetrexed disodium from the said intermediate
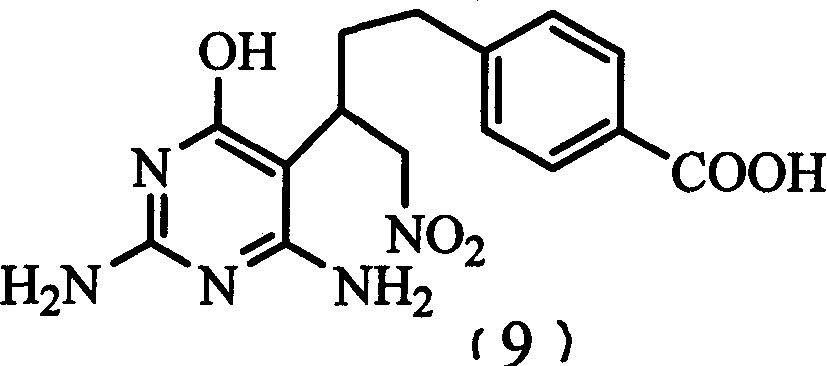
The invention relates to a disodium salt key point intermediate 1-nitro -2-(2, 6-diamino -4-(3H) oxo-metadiazine -5-group)-4 - (4-carboxyl phenyl)-1-butagas in the field of chemistry and medicine. It also discloses a method for preparing for the compound and the method for using the intermediate to synthesize the disodium salt.
Owner:SHANGHAI GOLDEN PHARMATECH +2
Method for synthesizing 4-(4-carbomethoxyphenyl) butyraldehyde
ActiveCN101591247AEasy to operateConvenient sourceOrganic compound preparationCarboxylic acid esters preparationOrganic solventBromine
The invention provides a method for synthesizing 4-(4-carbomethoxyphenyl) butyraldehyde, which comprises the following steps: condensing methyl 4-bromobenzoate and3-buten-1-ol; extracting the condensation product by organic solvent during post processing; adding silica gel to decolorize the product; removing the organic solvent by evaporation and then obtaining the 4-(4-carbomethoxyphenyl) butyraldehyde. A product obtained by the method has the yield of more than 80 percent and the GC detecting purity of more than 95 percent and can be directly used for the following brominating reaction for synthesizing pemetrexed disodium without any refinement. The method has the advantages of simple technological operation and low cost and easy acquisition of the used reagent and is suitable for industrial production.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
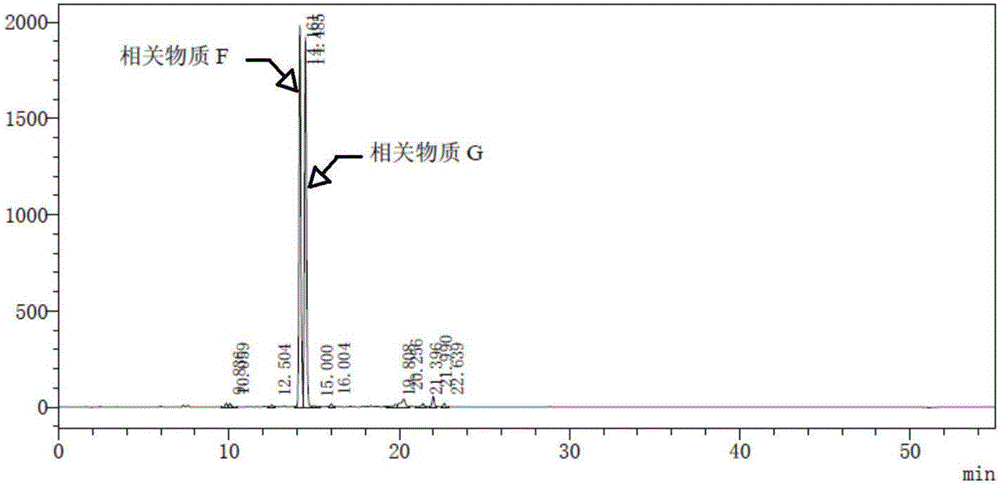
Related substances F and G of pemetrexed disodium as well as preparation and detection method thereof
ActiveCN106220634AImprove qualityImprove securityOrganic chemistryComponent separationDrug productPemetrexed disodium
The invention discloses related substances F and G of pemetrexed disodium as well as preparation and detection researches on the two substances. Remarkable economic and technical benefits can be made for improvement of the quality and the safety of medicines of pemetrexed disodium. The chemical formulae of the two substances are as shown in the specification.
Owner:SUZHOU LIXIN PHARMA
Stable ready-to-use pharmaceutical composition of pemetrexed
A stable ready-to-use pharmaceutical composition comprising pemetrexed or pharmaceutically acceptable salts thereof, wherein the composition is free from antioxidants, amino acids and chelating agents. Also provided is a process for preparing a stable ready-to-use pharmaceutical composition comprising the steps: i) purging inert gas into a parenterally acceptable aqueous solvent until the dissolved oxygen content of the solvent comes to less than 7 mg / L, preferably less than 3 mg / L; ii) adding pemetrexed disodium under stirring; iii) adjusting the pH of the resulting solution to between 4 to 9; iv) optionally adding additional aqueous solvent; wherein the composition is purged with inert gas throughout the entire process.
Owner:FRESENIUS KABI ONCOLOGY LTD
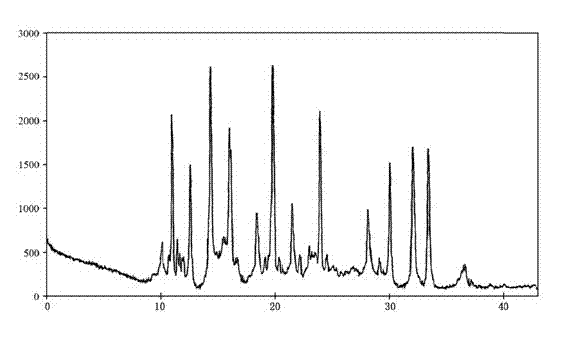
Method for analyzing and detecting pemetrexed disodium intermediate
ActiveCN103884784AEfficient separationQuality assuranceComponent separationMicrometerMonopotassium phosphate
The invention relates to a method for analyzing and detecting a pemetrexed disodium intermediate. The method is used for quality control of the pemetrexed disodium intermediate. According to the method, octadecylsilane chemically bonded silica serves as a chromatographic column of packing (C18, 4.6*250mm, 5 micrometers), a monopotassium phosphate solution and acetonitrile serve as a mobile phase, the detection wavelength is 249-259nm, and high performance liquid chromatography is performed for analysis and detection. According to the analysis and detection method, the pemetrexed disodium intermediate can be effectively separated from impurities thereof, and the method has the advantages of high separation degree, good linear relation, simple operation, high repeatability and durability and stable and reliable result.
Owner:SHANDONG NEWTIME PHARMA
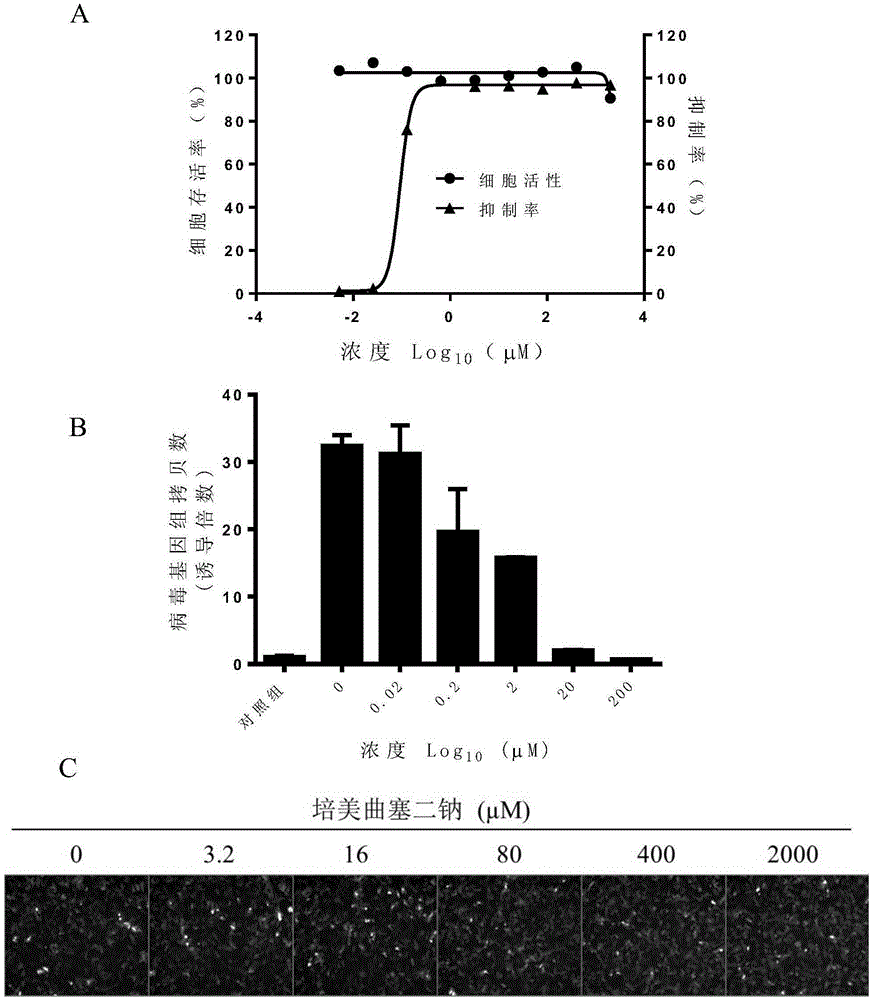
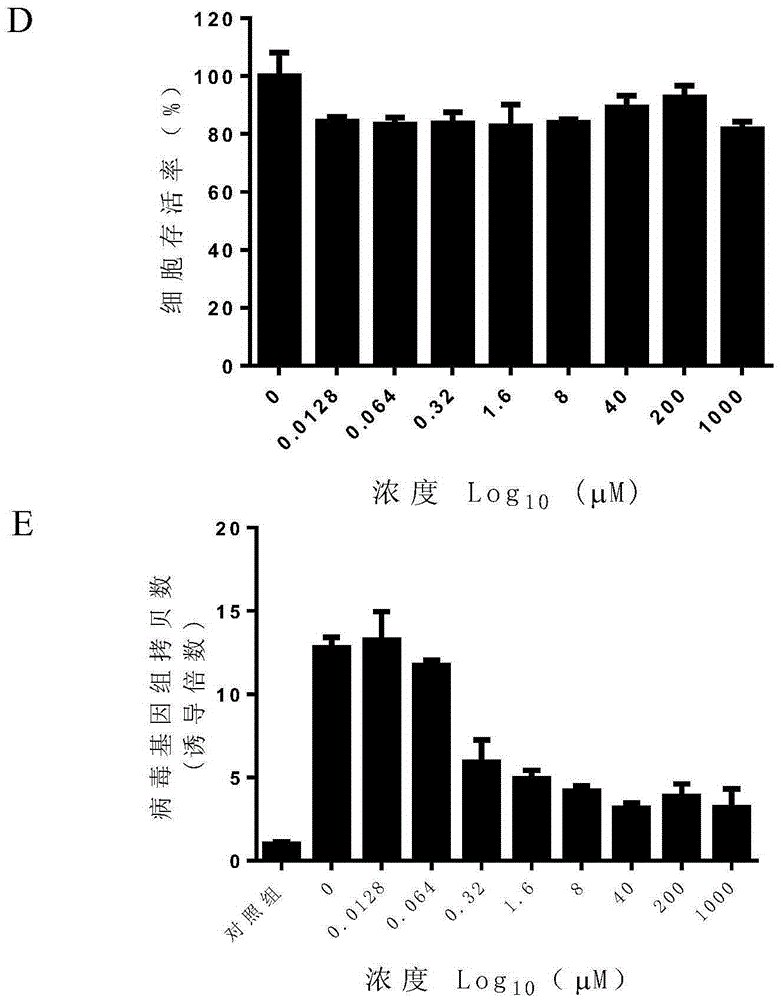
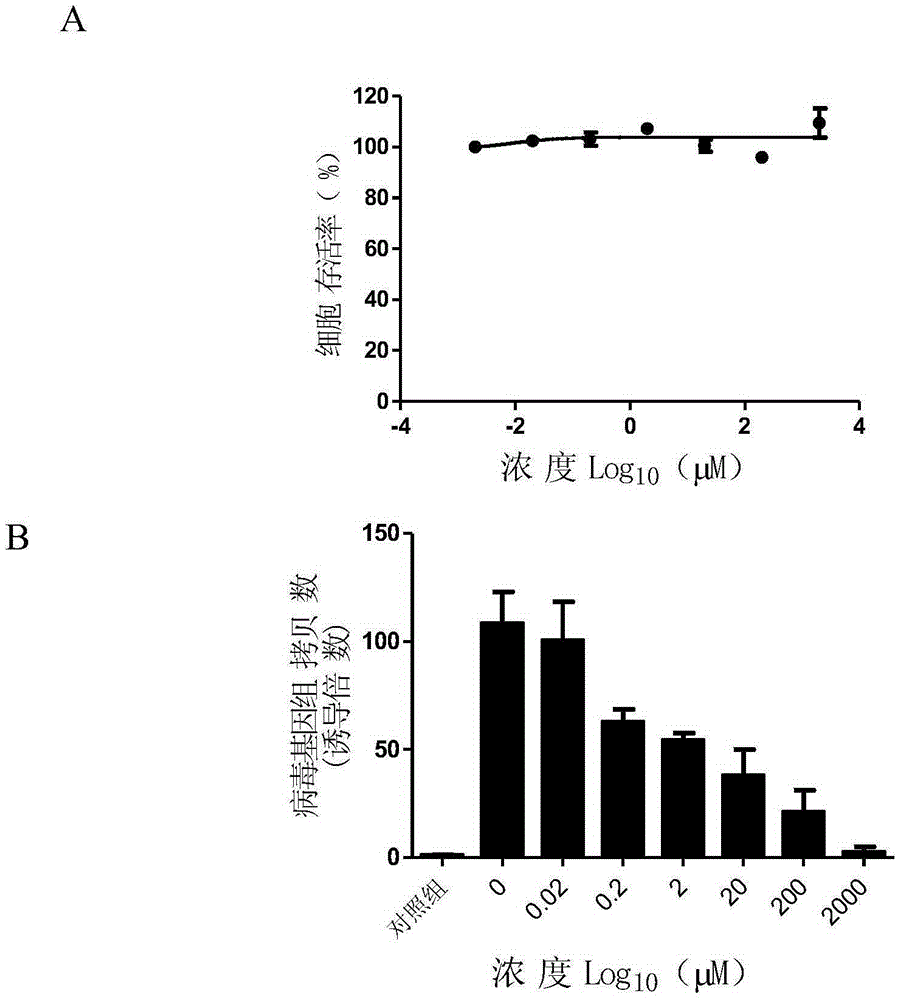
Application of pemetrexed disodium or pharmaceutically-acceptable salt thereof in preparing medicine for treating or preventing herpes virus infection
InactiveCN105412113ABroad spectrum of activity against multiple herpes virus infectionsGood activity against herpes virus infectionOrganic active ingredientsAntiviralsDiseaseHerpes simplex virus DNA
The invention discloses application of pemetrexed disodium or pharmaceutically-acceptable salt thereof in preparing medicine for treating or preventing herpes virus infection. According to the application, pemetrexed disodium of a completely non-cytotoxic concentration is selected and used for conducting a herpes virus resisting experiment, the result shows that the compound has significant herpes virus resisting activity in dose dependency, and pemetrexed disodium performs the herpes virus resisting effect by inhibiting virus reproduction. Detection of herpes viruses of different subtypes shows that pemetrexed disodium has broad-spectrum herpes virus resisting activity, is high in selection index, can be developed into medicine for treating or preventing herpes virus infection and has broad application prospects.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Pemetrexed disodium for injection and preparation method thereof
ActiveCN103040834AEnsure medication safetyAvoid Infusion PainOrganic active ingredientsOrganic chemistryUse medicationArginine
The invention discloses pemetrexed disodium for injection. The pemetrexed disodium for injection comprises the components in part by weight as follows: 50-60 parts of a pemetrexed disodium compound, 20-40 parts of mannitol and 2-4 parts of arginine. Besides, the invention further provides the pemetrexed disodium compound, the stability of the pemetrexed disodium compound is improved remarkably, the organic solvent residual volume is low, the quality can be controlled, and the pemetrexed disodium compound can be placed for a long time and cannot change easily, so that the medication safety for a patient is improved greatly.
Owner:SHANXI PUDE PHARMA CO LTD
Method for preparing improved intermediate for producing high-purity pemetrexed and method for producing high-purity pemetrexed using intermediate
InactiveCN105531276AHigh purityBest practiceOrganic active ingredientsOrganic chemistryDiethyl esterPemetrexed disodium
Owner:SAMYANG BIOPHARMLS CORP
Preparation process for synthesizing high-purity Pemedolac
ActiveCN102887902ALow costSolve the problem of low amplification yield of the original processOrganic chemistryBiochemical engineeringAlkali salt
The invention relates to the technical field of medicine, and specifically provides a preparation process for synthesizing high-purity Pemedolac. The invention solves the problem that the original process has very low enlarging yield by preparing a stable and high-purity intermediate sodium 3-(4-methoxycarbonyl)phenyl-1-hdyroxypropylsulphonate, establishes a fundamental base for preparing the high-purity Pemedolac at the same time, and further effectively controls the production of key impurities by using weak alkali salts of Pemedolac in a final purification stage; thus, the yield of the production process is high, and the quality of the product is extremely good; and the method is more convenient to prepare a high-purity anticancer drug pemetrexed disodium. At the same time, the preparation process provided by the invention has mild process conditions; the solvent is convenient to be recycled and applied mechanically; the preparation process provided by the invention is more energy-saving and environment-friendly. The structure of the Pemedolac is shown in the specification.
Owner:BEIJING LUNARSUN PHARMA
Preparation method of pemetrexed disodium
A preparation method of pemetrexed disodium is disclosed. The preparation method comprises the following steps: (1) dissolving 30-35 parts of 2-(3-(trifluoromethyl)phenyl) malonate and 15-20 parts ofN-(pyridin-5-yl methyl) pyridine-2-amine in a solvent, adding a catalyst, and carrying out a sealed stirring reaction under a microwave condition to obtain a crude product; and (2) removing the solvent from the crude product under reduced pressure, adding ethyl acetate, uniformly stirring, and carrying out silica gel column chromatography to obtain the pemetrexed disodium. According to the method,the 2-(3-(trifluoromethyl)phenyl) malonate and the N-(pyridin-5-yl methyl) pyridine-2-amine directly react under the conditions of the catalyst and microwaves, the reaction process is only one step,the reaction process is greatly shortened, and the yield of pemetrexed disodium is increased.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Preparation method of pemetrexed disodium key intermediate
ActiveCN103030641AHigh yieldImprove the efficiency of the loop closure reactionOrganic chemistryNef reactionNitromethane
The invention relates to a preparation method of a pemetrexed disodium key intermediate, more specifically to a high-yield preparation method of the pemetrexed disodium key intermediate. The preparation method comprises the following steps: (a) with a compound of a formula (I) as a raw material, carrying out a Heck reaction with allyl alcohol to obtain a compound of a formula (III); (b) removing one molecule of water by utilizing a dehydrating agent after condensing the compound of the formula (III) with nitromethane to obtain a compound of a formula (IV); (c) carrying out a Michael addition reaction on the compound of the formula (IV) to generate a compound of a formula (V); and (d) carrying out a Nef reaction on the compound of the formula (V) to obtain a compound of a formula (I). The preparation method of the pemetrexed disodium key intermediate has the advantages of having a short reaction process, not existing harsh reaction conditions, shortening the reaction time and being capable of remarkably improving the yield of products.
Owner:JIANGSU HANSOH PHARMA CO LTD
Pemetrexed methyl ester p-toluenesulfanate crystal form and preparation method thereof
ActiveCN102372719AGuaranteed normal productionQuality assuranceSulfonic acids salts preparationMedicinal chemistryPara-toluene sulfonate
The invention belongs to the pharmaceutical chemistry field and relates to a crystal form of a key intermediate of the folic acid inhibitor medicine pemetrexed disodium and a preparation method of the intermediate. The pemetrexed methyl ester p-toluenesulfanate provided by the invention has stable crystal form and high purity, thus the purity of pemetrexed methyl ester can be effectively increased, the problem of the complicated extraction of pemetrexed methyl ester can be solved, the pemetrexed methyl ester p-toluenesulfanate has positive influence on the increase of the quality of the subsequent product pemetrexed disodium and industrial production can be performed easily.
Owner:QILU PHARMA +1
Preparation method of pemetrexed disodium
InactiveCN104119346AHigh yieldSimplify the manufacturing processOrganic chemistryTransient stateOrganic solvent
The invention discloses a preparation method of pemetrexed disodium, which can directly hydrolyzing into salt after an organic solvent is removed, rather than salifying together with p-toluenesulfonic acid and / or purifying a product by adopting a crystallization method during the preparation process, thus omitting the step of salifying together with p-toluenesulfonic acid and / or crystallization through ethanol, effectively improving the total yield of drugs to 68-75%; in the preparation process, a peptide condensing agent is firstly prepared and is fully reacted with pemedolac in the organic solvent into transient-state acid ester, so that the generation of N-methylation impurities can be effectively inhibited in the reaction together with L-glutamic acid diethyl ester hydrochloride, the defect that the content of N-methylation impurities during the one-pot preparation process in the prior art exceeds 0.1% can be avoided, the advantages for opening up Europe and America markets can be brought, the defect that the raw materials are not reacted thoroughly to cause high cost can also be overcome; the HPLC (High Performance Liquid Chromatograph) of the finished pemetrexed disodium obtained by adopting the method is more than or equal to 99.8%, and the content of single impurities is less than 0.1%.
Owner:NINGBO MENOVO PHARMA
Crystal form of Peimeiqusai disodium and its preparation
Owner:重庆凯林制药有限公司 +2
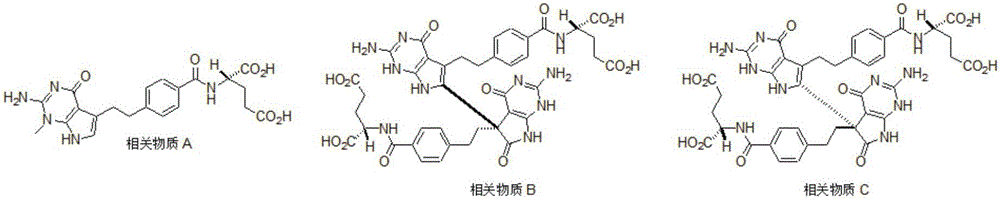
Synthesis method for intermediate of impurity A of pemetrexed disodium
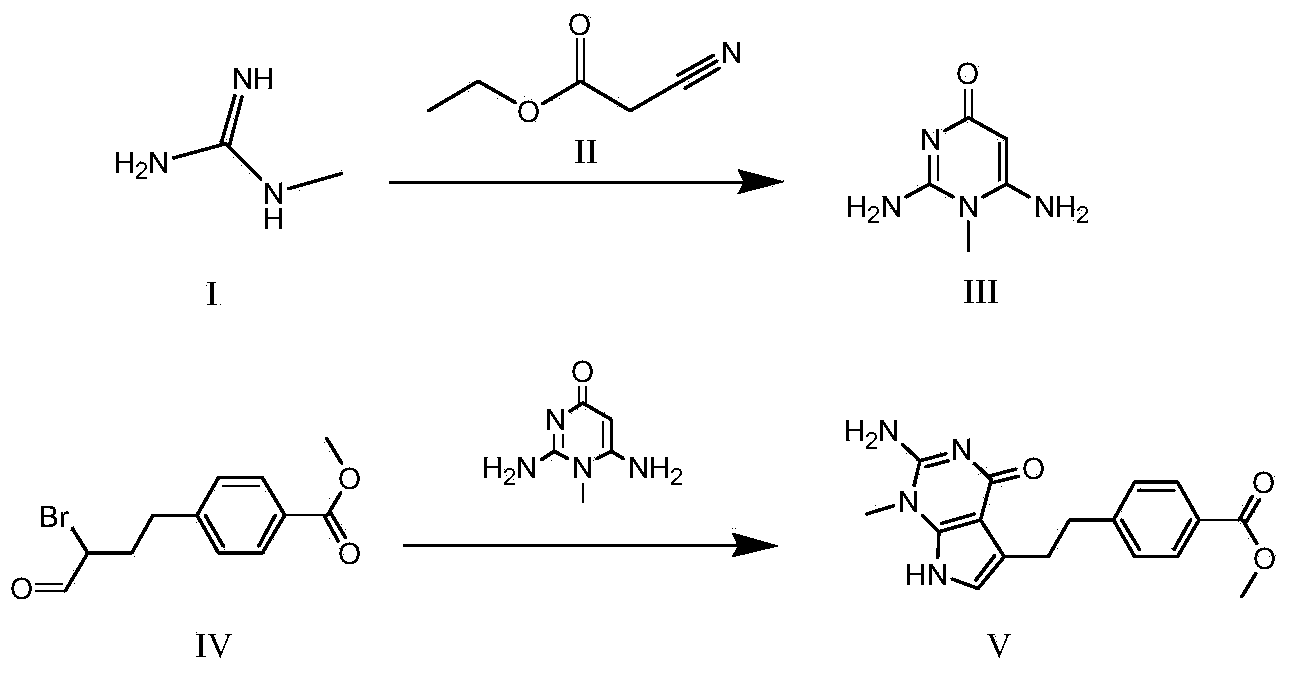
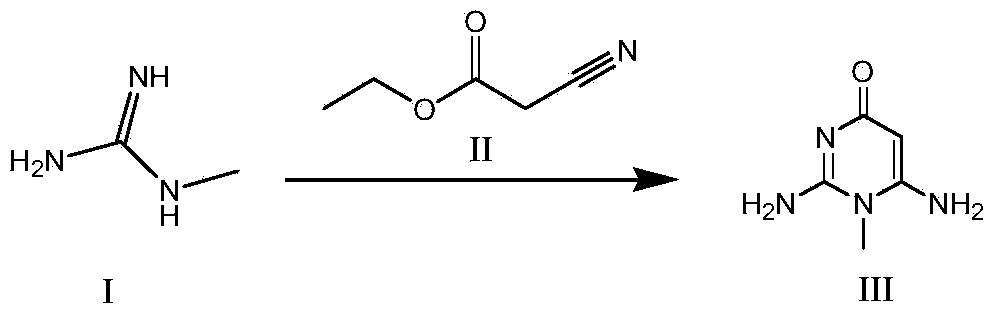
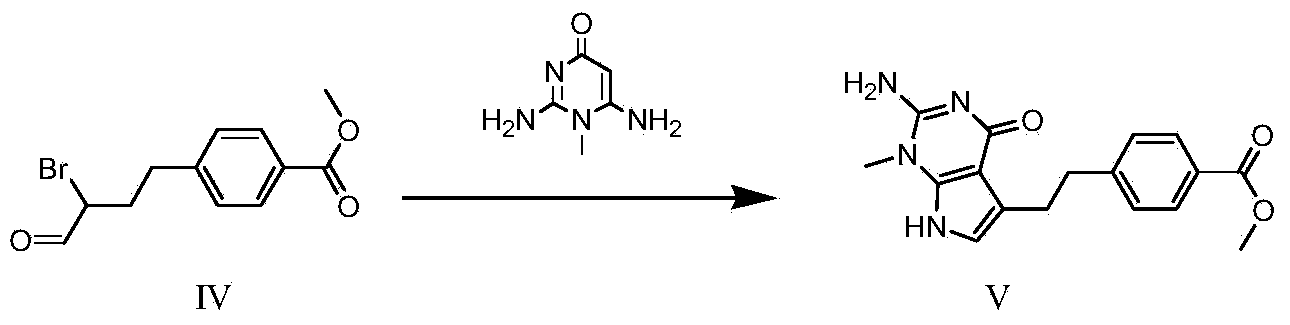
The invention discloses a synthesis method for an intermediate of an impurity A of pemetrexed disodium and belongs to the field of drug synthesis. (4-(2-(2-amino-1-methyl-4,7-dihydro-4-oxo-1H-pyrrolo(2,3-d)pyrimidine-5-yl)ethyl)benzoyl)-L-glutamic acid is an impurity A of pemetrexed disodium, and 4-(2-(2-amino-1-methyl-4,7-dihydro-4-oxo-1H-pyrrolo(2,3-d)pyrimidine-5-yl)ethyl)methyl benzoate is an important intermediate required for synthesis of the impurity A. The synthesis method of the intermediate comprises the following steps: carrying out a reaction on a compound I 1-methylguanidine and a compound II ethyl cyanoacetate to obtain a compound III 2,6-diamino-4-carbonyl-1-methylpyrimidine, and carrying out a reaction on a compound IV 4-(4-carbonyl-3 brombutyl)methyl benzoate and the compound III to obtain a compound V 4-(2-(2-amino-1-methyl-4,7-dihydro-4-oxo-1H-pyrrolo(2,3-d)pyrimidine-5-yl)ethyl)methyl benzoate.
Owner:SHANDONG BOYUAN PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com