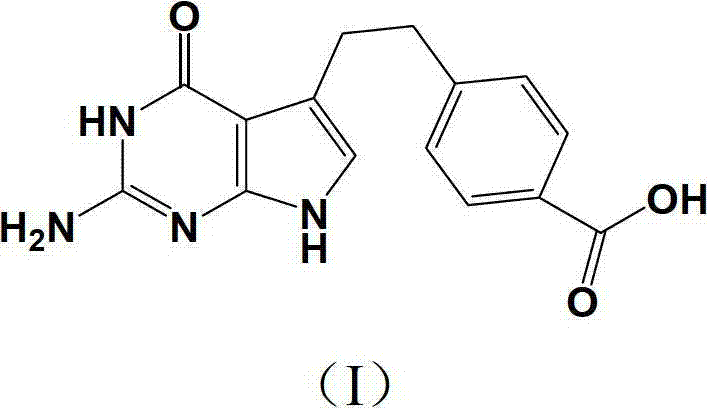
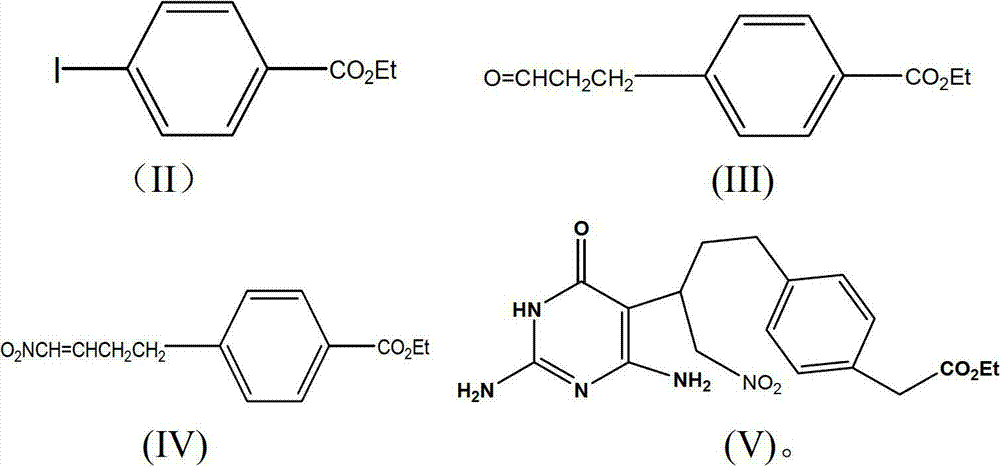
Preparation method of pemetrexed disodium key intermediate
A technology for pemetrexed disodium and intermediates, which is applied in the field of high-yield preparation of pemetrexed disodium intermediates, can solve the problems of low yield, poor product purity, difficult reaction control and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of 4-ethoxyformylphenylpropanal (compound of formula III)
[0023] The raw material 27.6g (0.1mol) ethyl p-iodobenzoate (compound of formula II), 8.4g (0.1mol) NaHCO 3 , 32.6g (0.1mol) tetrabutylamine bromide was added to the reaction flask, stirred, added N2 protection, added 8.7g (0.15mol) propenyl alcohol, N,N-dimethylformamide 150ml, 35 ~ 40 ℃ reaction After 20 hours, concentrate under reduced pressure, pour the residue into 100ml of water, extract the water layer 3 times with 50ml of petroleum ether, combine the organic layers, dry, filter, concentrate, and distill the residue under reduced pressure to collect fractions at 142-144°C at 670Pa to obtain 9.8 g of 4-ethoxyformylphenylpropionaldehyde (compound of formula III) is a transparent liquid, and the yield is 94.2%.
Embodiment 2
[0024] Example 2: Preparation of 1-nitro-4-(4-ethoxymethanesulfonylphenyl)-2-butene (compound of formula IV)
[0025] Add 5.2g (0.025mol) of 4-ethoxyformylphenylpropanal, 2.3g (0.025mol) of nitromethane, and 1.0g (0.0125mol) of triethylamine into the reaction flask, stir at room temperature for 12h, then add dichloromethane 30ml, cooled in an ice bath, dropwise added 2.6g (0.025mol) of methanesulfonyl chloride, 1.3g (0.0125mol) of aluminum oxide, 3.0g (0.0375mol) of triethylamine to the reaction solution, stirred for half an hour, and used 1N Wash with hydrochloric acid, then wash with water until neutral, dry, filter, and concentrate to obtain a light yellow oily substance 1-nitro-4-(4-ethoxymethanesulfonylphenyl)-2-butene (compound of formula IV) 6.1 g, yield 97.5%.
Embodiment 3
[0026] Example three: 1-nitro-2-(2,6-diamino-4-(3H) oxopyrimidin-5-yl)-4-(4-carboxyphenyl)-1-butane (formula V compound) preparation
[0027] 4.98g (0.02mol) 1-nitro-4-(4-ethoxymethanesulfonylphenyl)-2-butene, 2.28g (0.02mol) 2,6-diamino-4-(3H)oxy Pyrimidine was added to the reaction vessel, and then sodium methoxide / methanol solution (0.54g sodium methoxide, 10ml methanol) was added, and the reaction was carried out at a constant temperature of 50°C for 2h. Cool to room temperature, adjust the reaction solution to pH = 6 with acetic acid, filter and dry, the product is 1-nitro-2-(2,6-diamino-4-(3H)oxopyrimidin-5-yl) -4-(4-carboxyphenyl)-1-butane (compound of formula V), yield 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com