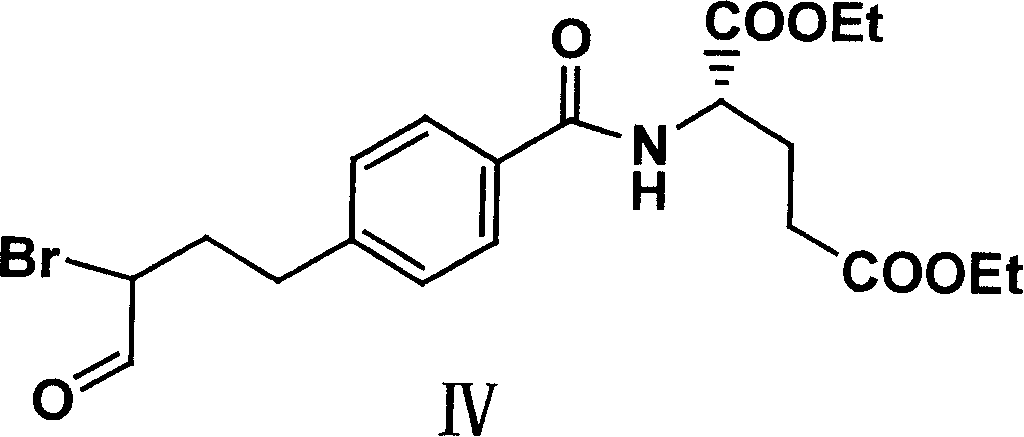
Diethyl 4(4-oxobutyl) benzoyl-L-glutamate and its preparation and use
A technology of diethyl glutamate and oxobutyl, which is applied in the field of new compound 4-benzoyl-L-diethyl glutamate, can solve problems that are not suitable for large-scale industrial production, long steps, and total Problems such as low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
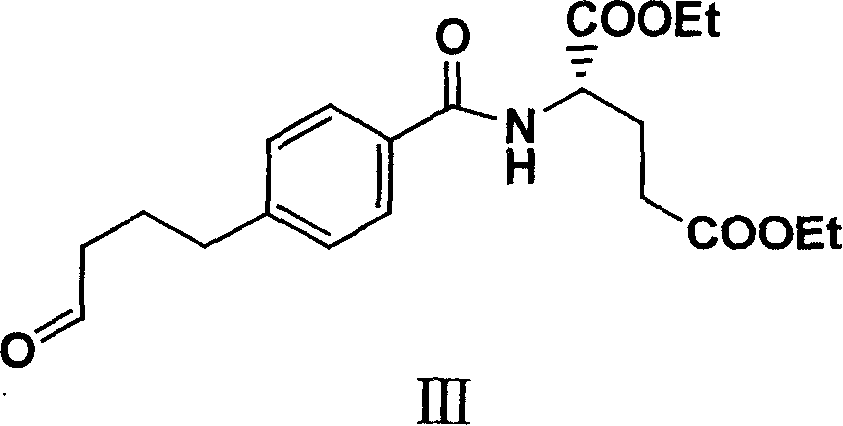
[0033] Example 1 Synthesis of 4-(4-oxobutyl)benzoyl-L-glutamic acid diethyl ester (III)
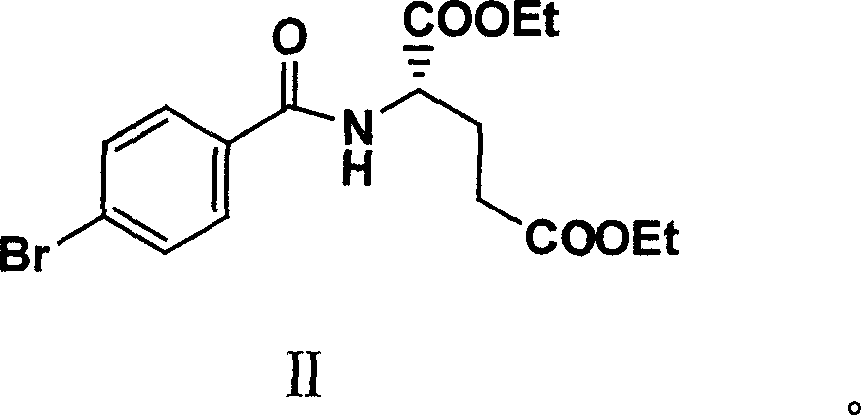
[0034] The amount ratio of the feed material is 4-bromobenzoyl-L-glutamic acid diethyl ester (II): lithium acetate: tetrabutylammonium bromide: lithium chloride: 3-butene-1-alcohol: palladium acetate =1:2:1.5:2.5:1.05:0.08.
[0035]In a 500ml three-necked reaction flask equipped with mechanical stirring, nitrogen inlet and outlet and a thermometer, add 4-bromobenzoyl-L-glutamic acid diethyl ester (II) (38.6g, 0.1mol), lithium acetate (13.2g , 0.2mol), tetrabutylammonium bromide (48.4g, 0.15mol), lithium chloride (10.6g, 0.25mol), N, N-dimethylformamide 300ml, stirred for 10 minutes, added palladium acetate (1.8 g), 3-buten-1-ol (7.6 g, 0.105 mol), heated up to 68-70° C. to react, followed the reaction by TLC. After the reaction was completed and cooled properly, it was filtered through a layer of diatomaceous earth, and the filter cake was washed three times with 50 mL of N, N-dimethylf...
Embodiment 2
[0038] Example 2 Synthesis of 4-(4-oxobutyl)benzoyl-L-glutamic acid diethyl ester (III)
[0039] The amount ratio of feeding material is 4-bromobenzoyl-L-glutamic acid diethyl ester (II): triethylamine: tetrabutylammonium bromide: lithium chloride: 3-butene-1-alcohol: acetic acid Palladium = 1:2:1.5:2.5:1.05:0.08.
[0040] The feeding amount of triethylamine is 20.2g, and the feeding amount and operation process of all the other raw materials are the same as in Example 1. 19.2 g of 4-(4-oxobutyl)benzoyl-L-glutamic acid diethyl ester (III) was obtained, the yield was 51%, the melting point was 86-88.2°C, and the physical and chemical data were the same as in Example 1.
Embodiment 3
[0041] Example 3 Synthesis of 4-(4-oxobutyl)benzoyl-L-glutamic acid diethyl ester (III)
[0042] The amount ratio of feeding material is 4-bromobenzoyl-L-glutamic acid diethyl ester (II): lithium acetate: tetrabutylammonium chloride: lithium chloride: 3-butene-1-alcohol: palladium acetate =1:2:1.5:2.5:1.05:0.08.
[0043] Tetrabutylammonium chloride charging amount is 41.9g, and all the other raw material charging amounts and operating process are with embodiment 1. 23.8 g of 4-(4-oxobutyl)benzoyl-L-glutamic acid diethyl ester (III) was obtained, the yield was 63.2%, the melting point was 86-87.9° C., and the physical and chemical data were the same as those in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com