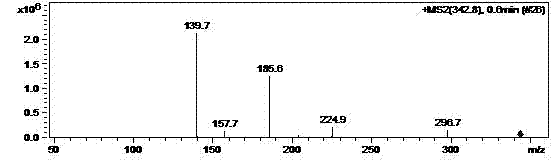
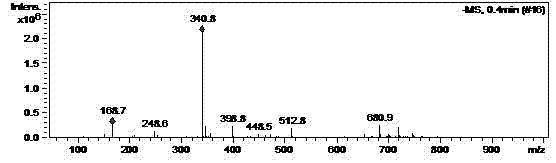
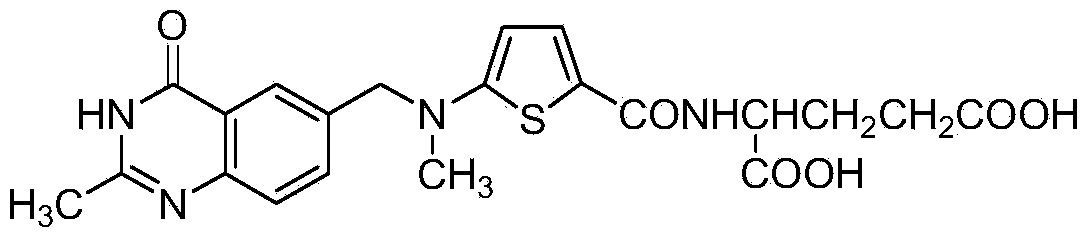
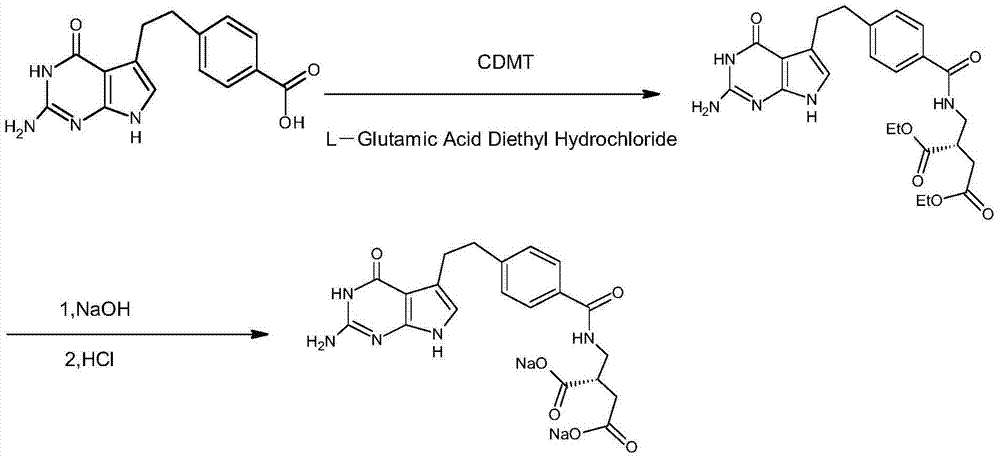
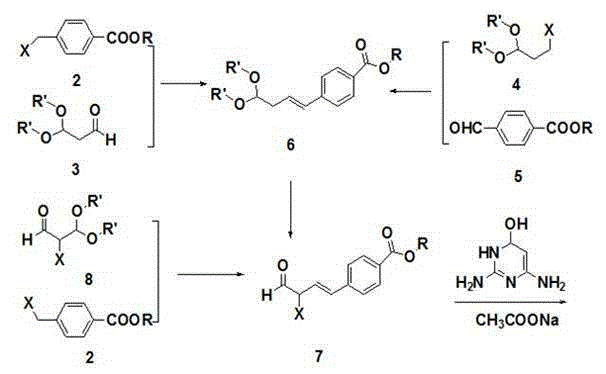
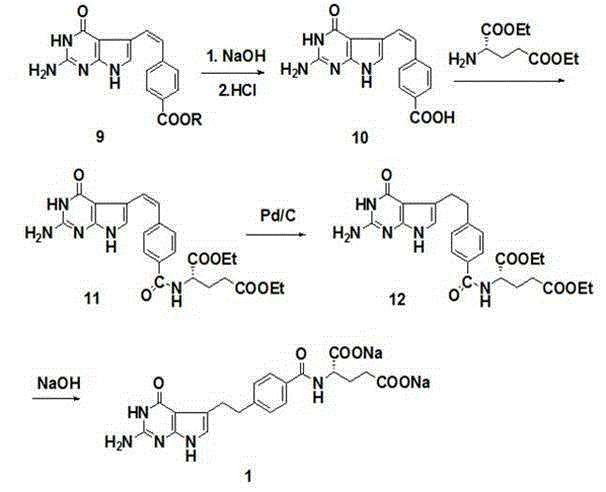
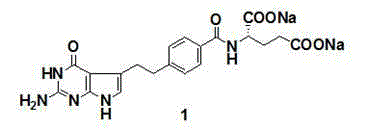
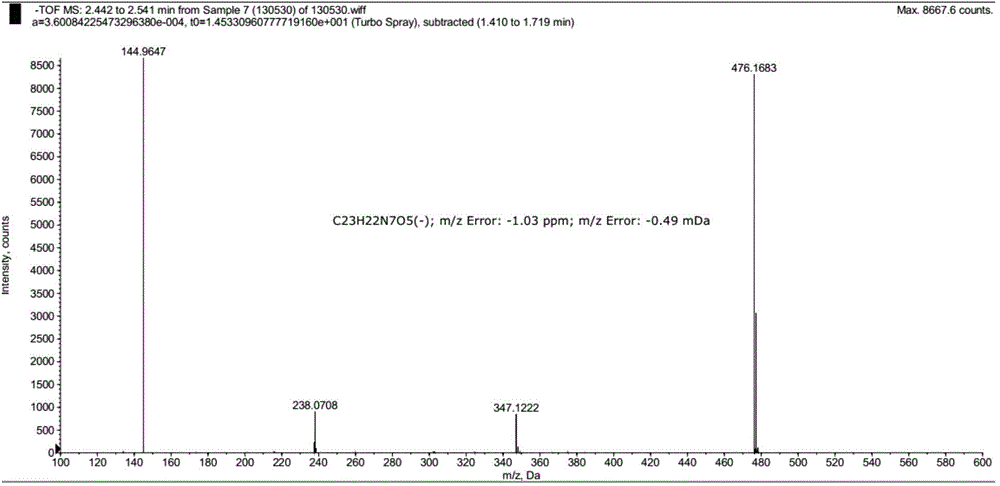
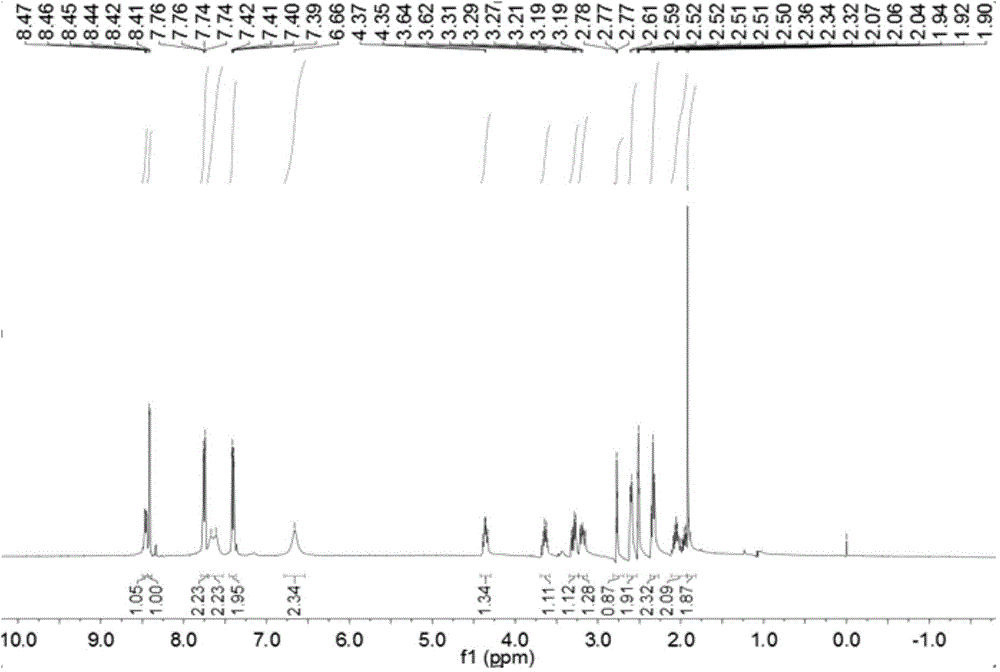
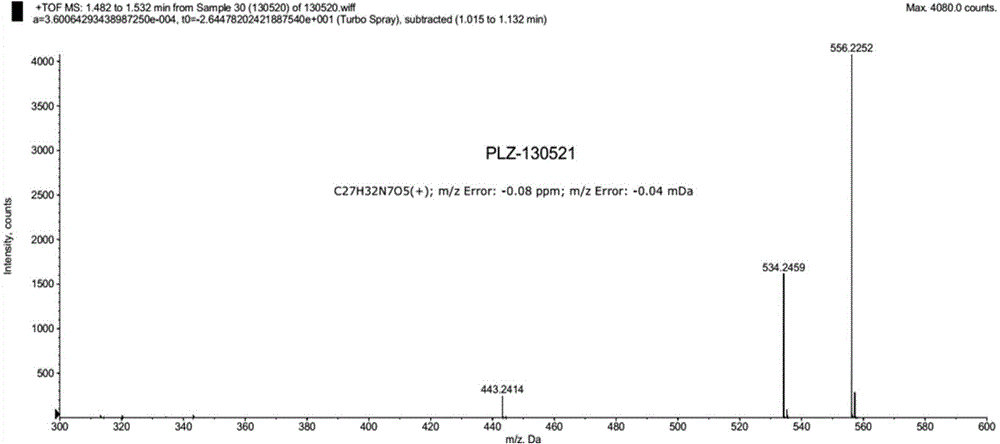
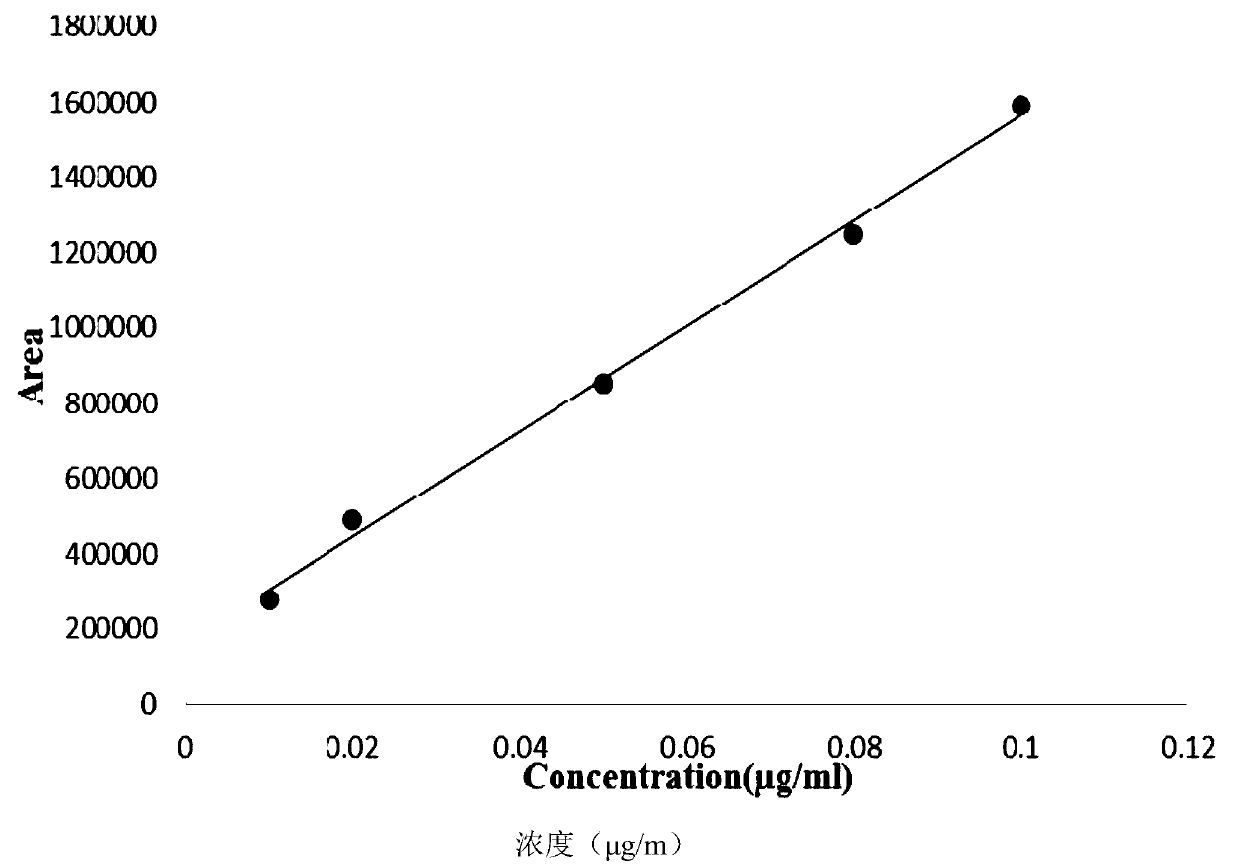
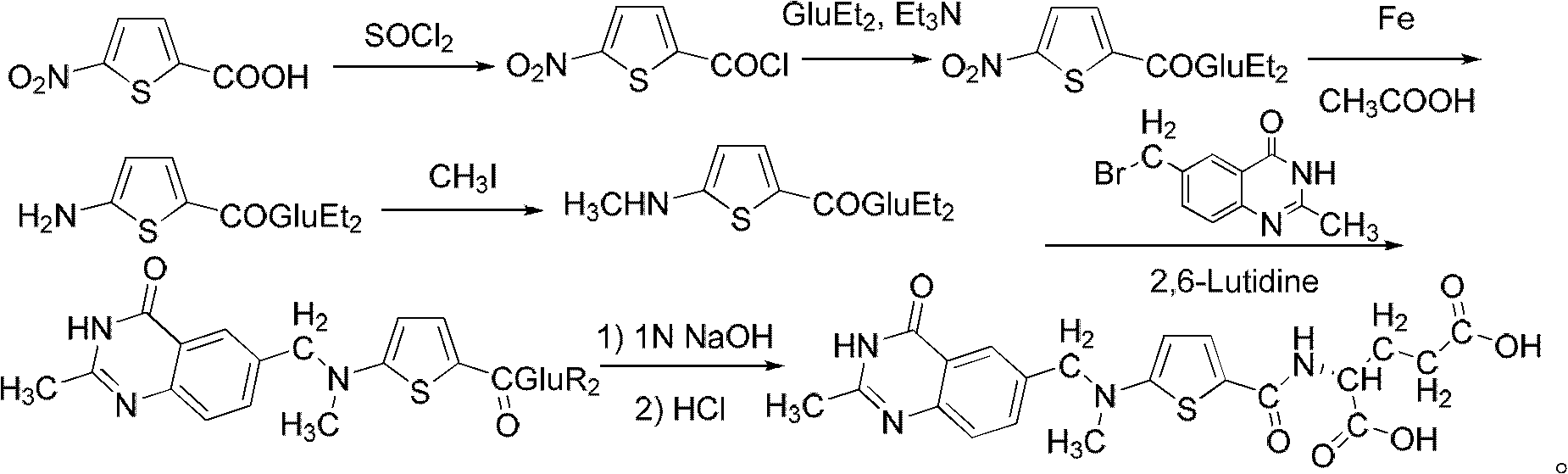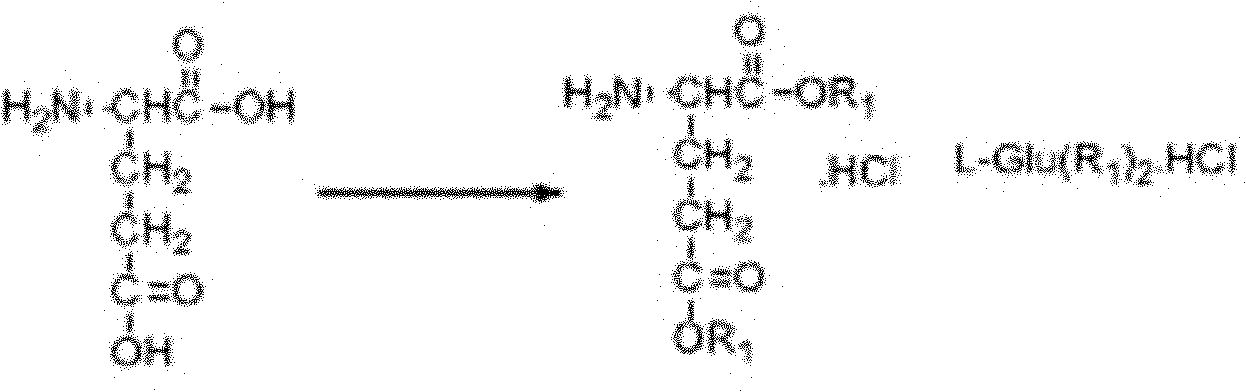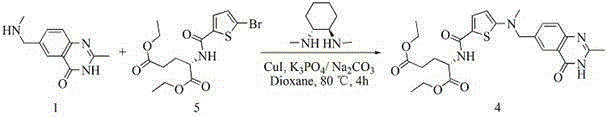Patents
Literature
34 results about "Glutamic acid diethyl ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
L-Glutamic acid diethyl ester hydrochloride 97% CAS Number 1118-89-4. Linear Formula C 2 H 5 OCOCH 2 CH 2 CH(NH 2)COOC 2 H 5 · HCl . Molecular Weight 239.70 . Beilstein Registry Number 3597595 . EC Number 214-270-4. MDL number MFCD00012509. PubChem Substance ID 24858729. SDS FTNMR (PDF) Similar Products.
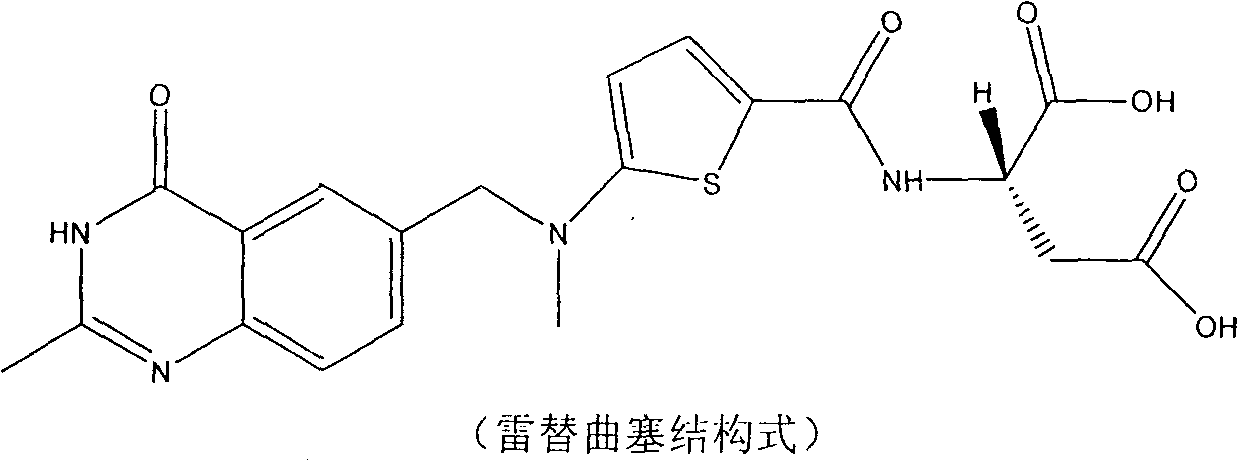
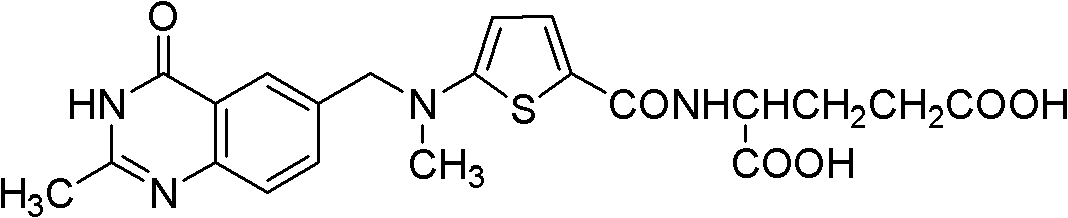
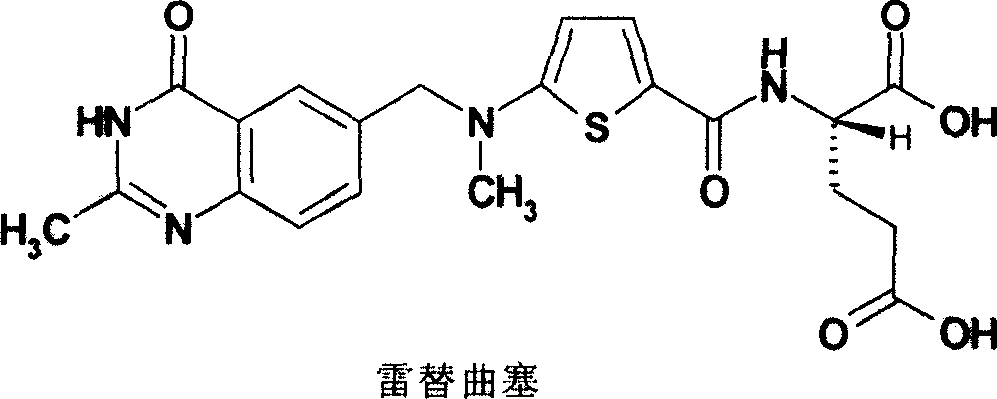
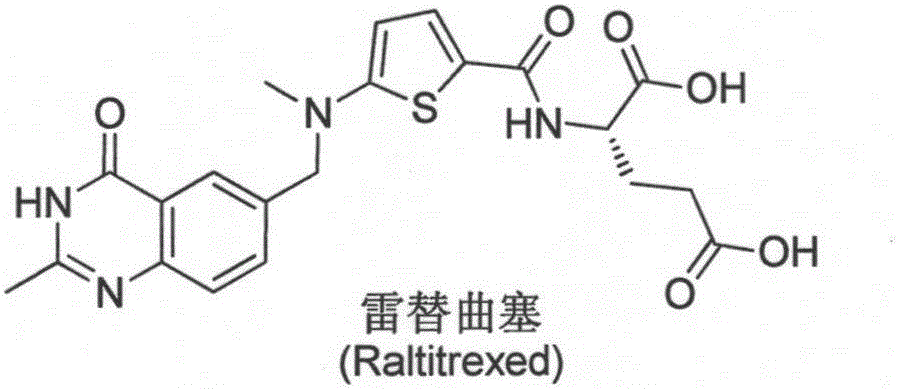
New synthesis technology of anti-cancer drug Raltitrexed
InactiveCN102127063ALow costOptimize the synthetic routeOrganic chemistryAntineoplastic agentsBenzoic acidTert-Butyloxycarbonyl protecting group
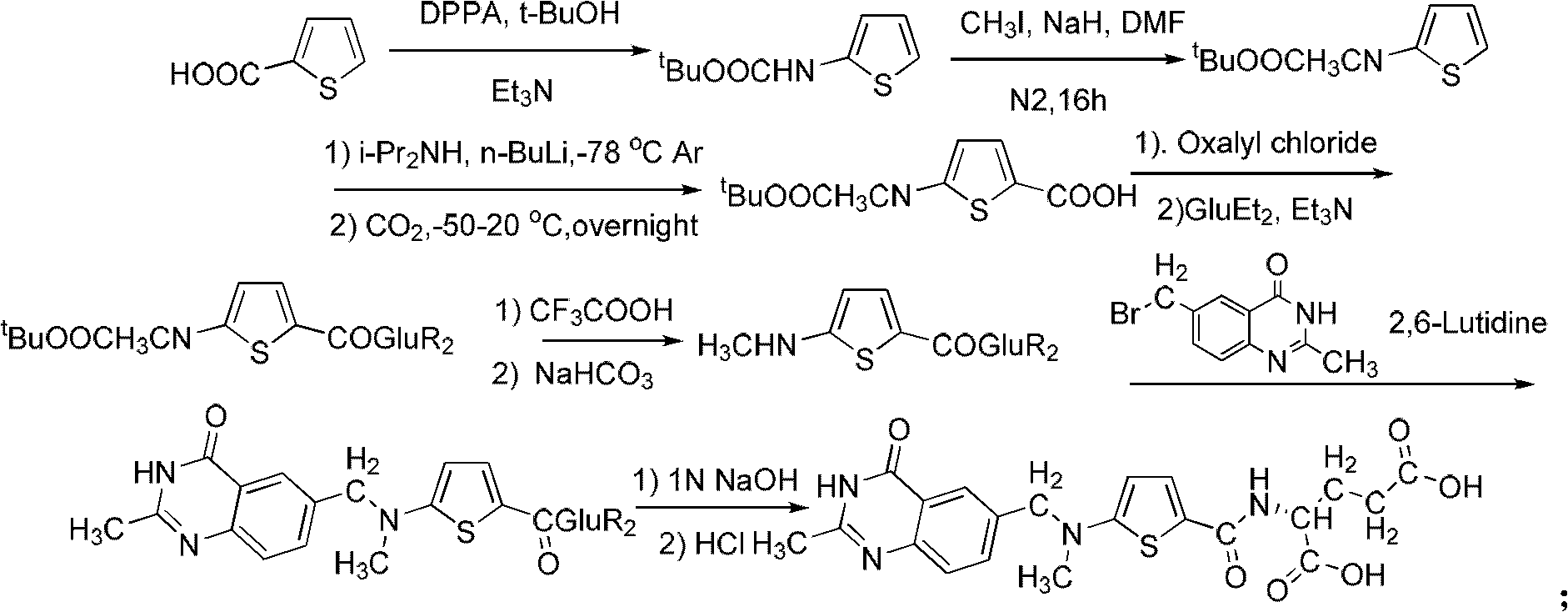
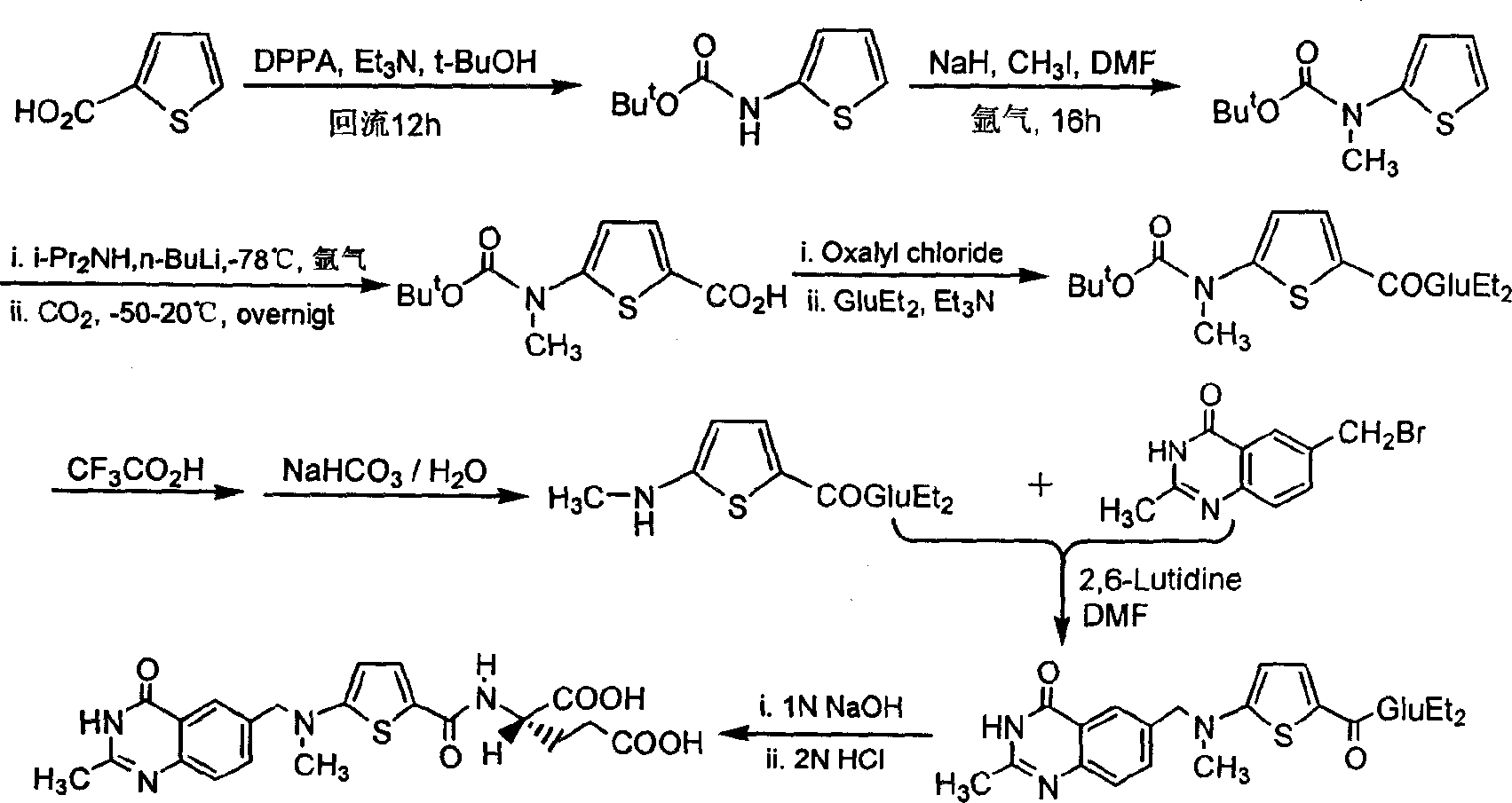
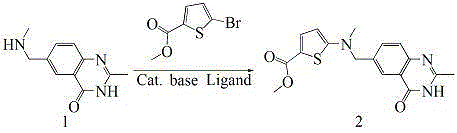
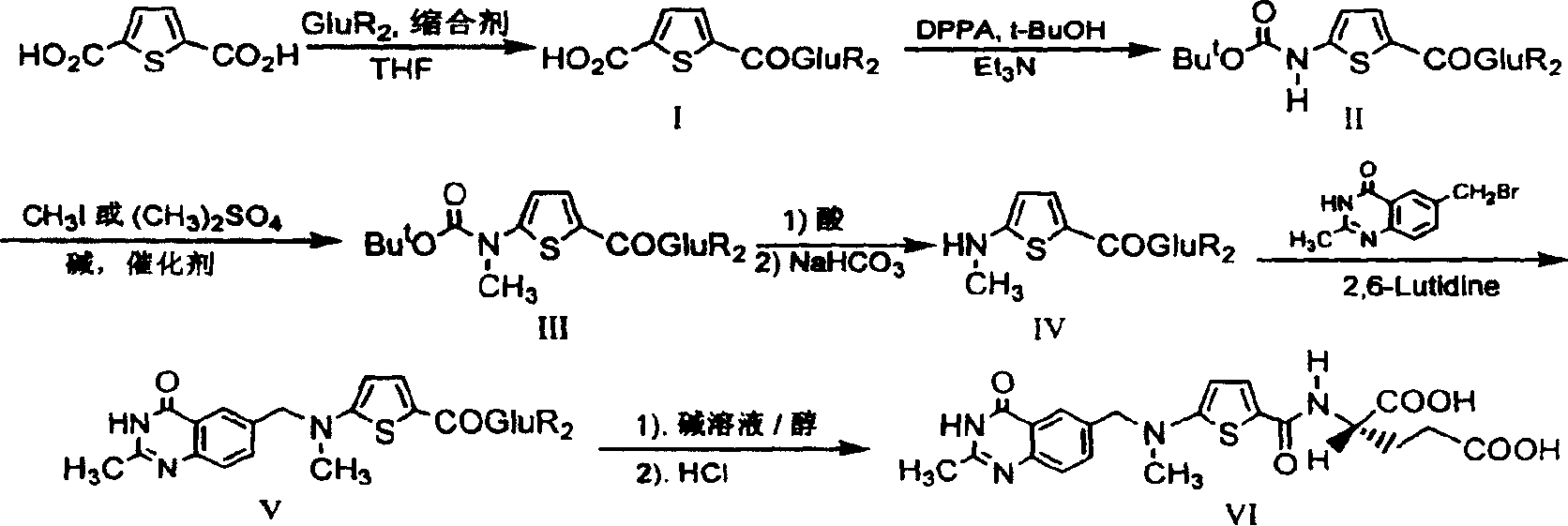
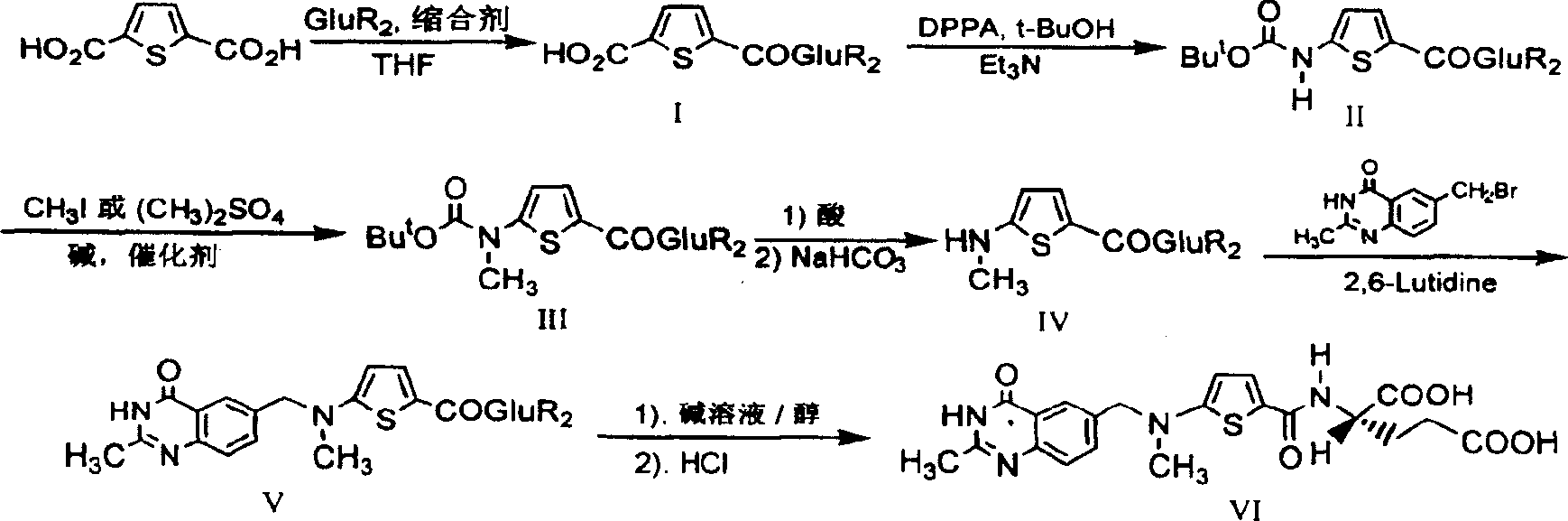
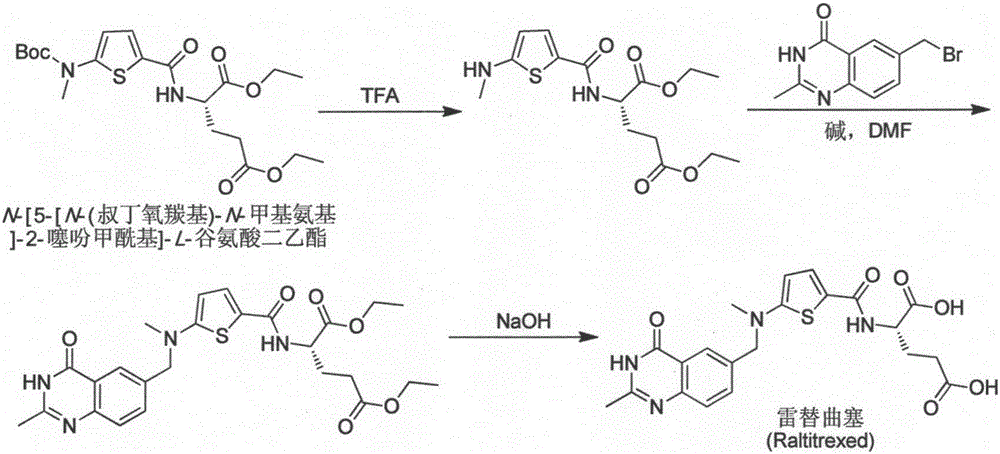
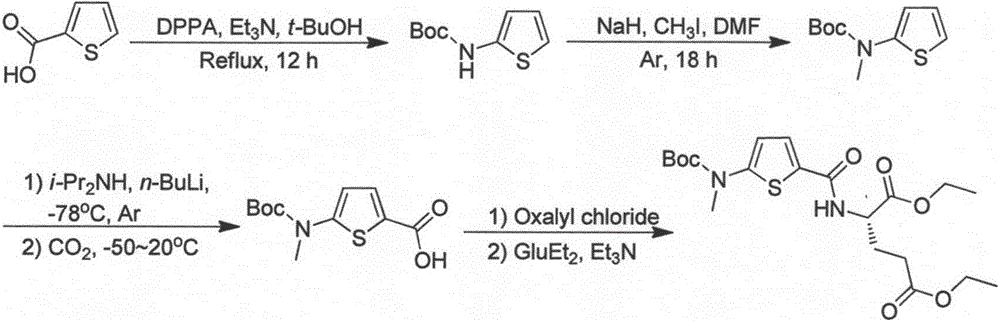
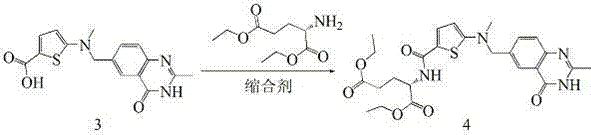
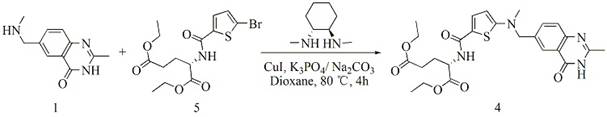
The invention relates to a new synthesis technology of anti-cancer drug Raltitrexed. The technology comprises the following steps: 1) using L-glutamic acid as raw material to perform esterification with alcohol under the action of halogenating agent and obtain L-glutamic acid diester hydrochloride; 2) using 2-amino-5-methyl-benzoic acid as raw material to prepare 6-bromomethyl-3,4-dihydro-2-methyl-4-oxo-6-quinazoline through cyclization, amination and bromination; 3) using 2-thienyl-propanedioic acid as raw material to prepare N-[5-[N-(tert-butoxycarbonyl)-N-methylamino]-2-thenoyl]-L-glutamic acid diethyl ester through nitrification, esterification, reduction, amino protection, N-methylation and device-esterification; 4) using L-glutamic acid diester hydrochloride and N-[5-[N-(tert-butoxycarbonyl)-N-methylamino]-2-thenoyl]-L-glutamic acid diethyl ester to prepare N-[5-(N-methylamino)-2-thenoyl]-L-glutamic acid diester through dehydrant condensation and deamination protection; and 5) using N-[5-(N-methylamino)-2-thenoyl]-L-glutamic acid diester and 6-bromomethyl-3,4-dihydro-2-methyl-4-oxo-6-quinazoline to perform condensation under the catalysis of alkali, recycling preparative chromatography, purifying, and performing de-esterification to obtain Raltitrexed.
Owner:深圳市普迈达科技有限公司
Synthesis of anticancer medicine Raltiprexed
InactiveCN1486985AShort processLow costOrganic chemistryAntineoplastic agentsTert-Butyloxycarbonyl protecting groupN methylation
The synthesis of anticancer medicine Raltitrexed with 2, 5-thienyl diformic acid and diethyl glutamate as initial material and through six reaction steps of monocondensation, rearrangement, N-methylation, e;limination of tert-butoxy carbonyl group, condensation with 6-bromomethyl-2-methyl-4-quinbolone and saponification. The present invention has total yield of 18.1%, higher than that of available synthesis line, less reaction steps, mild condition and simple operation, and is suitable for mass production.
Owner:CAPITAL NORMAL UNIVERSITY
Method for industrial preparation of raltitrexed and novel raltitrexed crystal form for pharmacy
The invention discloses a synthesis preparation technology of raltitrexed as an antitumor drug and a specific raltitrexed crystal form. The synthesis preparation technology comprises that N-(5-methylamino-2-thenoyl)-L-glutamate diethyl ester and 6-bromomethyl-3,4-dihydro-2-methyl-quinazoline-4-one undergo base catalysis and acid-binding synthesis reactions in the presence of an organic solvent to produce a C-N coupling product N-[5-[N-(3,4-dihydro-2-methyl-4-oxo-6-quinazoline)-methyl]-N-methyl]-2-thenoyl-L-glutamate diethyl ester; the C-N coupling product undergoes a hydrolysis reaction under the alkaline condition and the hydrolysis product is subjected to acid precipitation so that a raltitrexed crude product is obtained; and the raltitrexed crude product is subjected to recrystallization and the crystals are dried so that a high-purity raltitrexed raw drug in the specific crystal form is obtained. The specific crystal form is characterized definitely by an X-ray diffraction technology. The synthesis preparation technology utilizes extraction and crystallization processes to purify an intermediate and a product, is simple, economic and efficient, and is suitable for large-scale industrial preparation of raltitrexed.
Owner:NANJING YOKO PHARMA GRP CO LTD +1
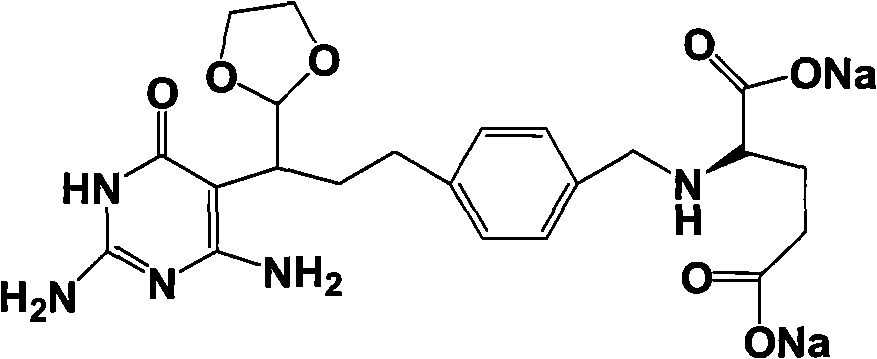
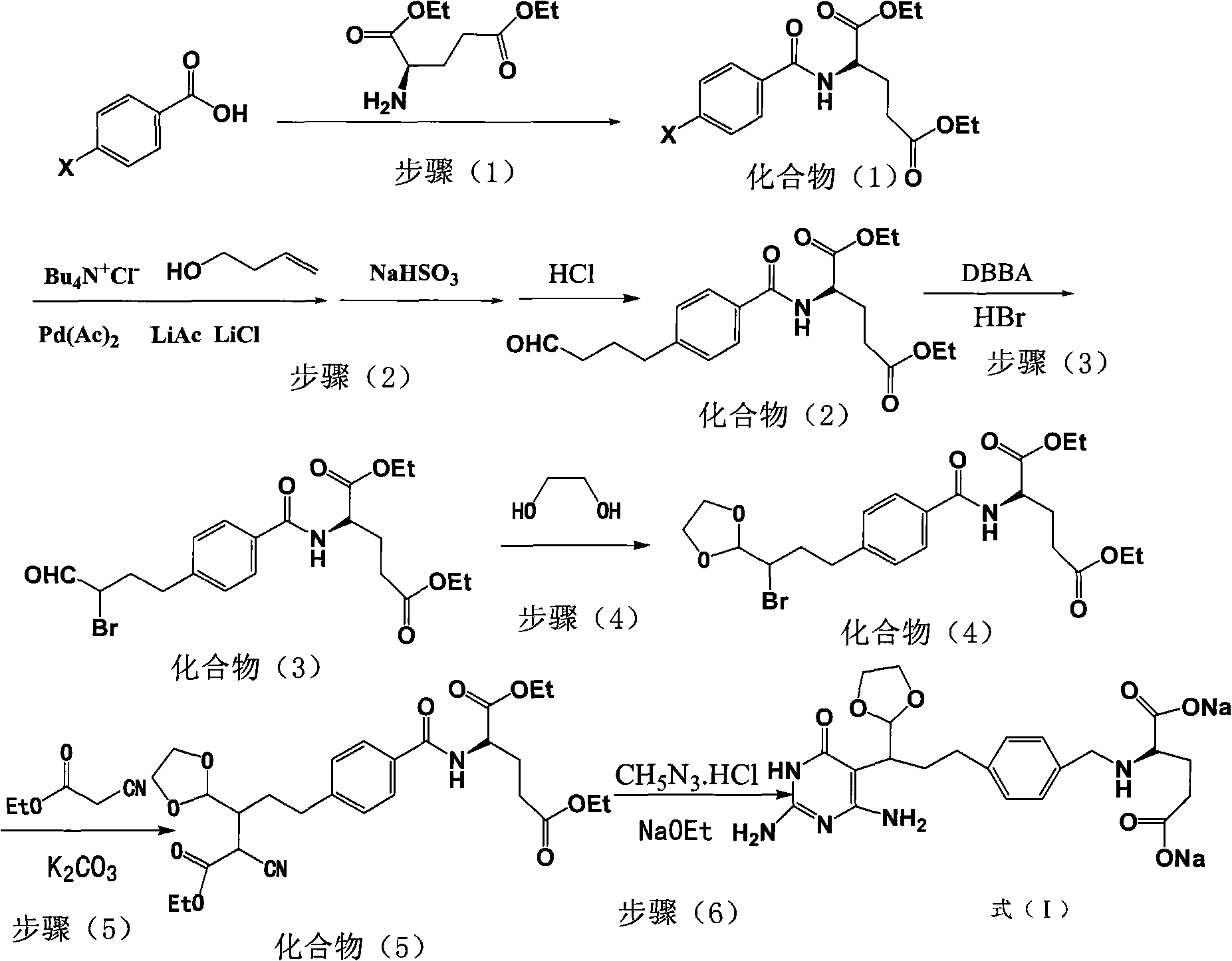
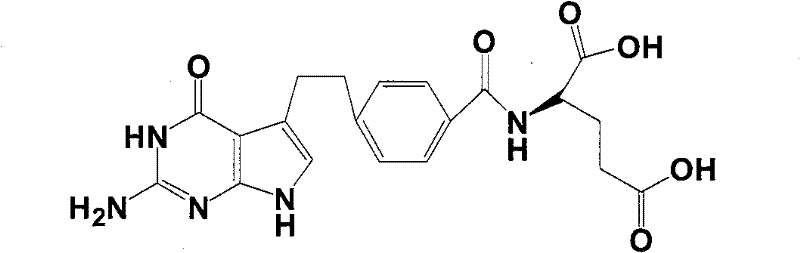
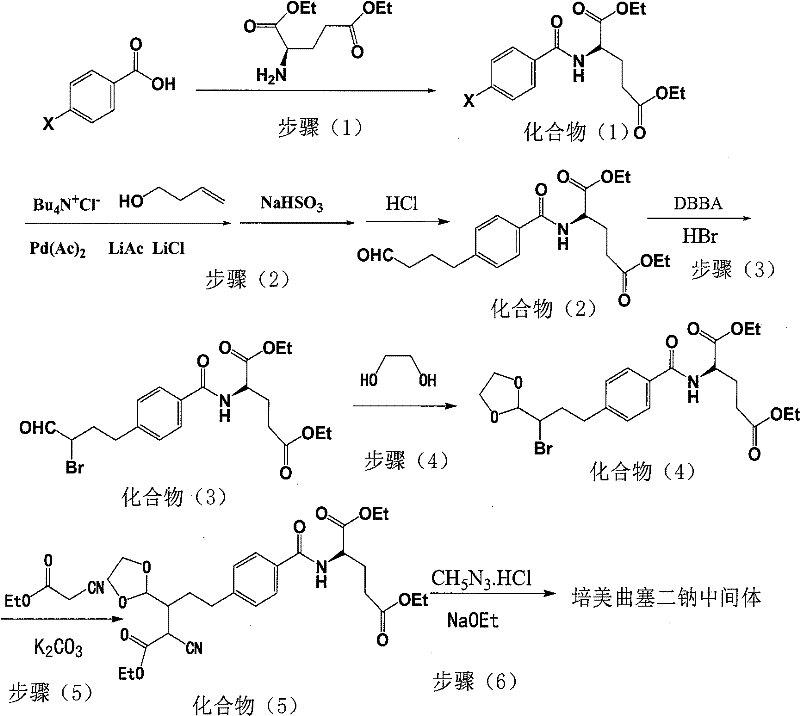
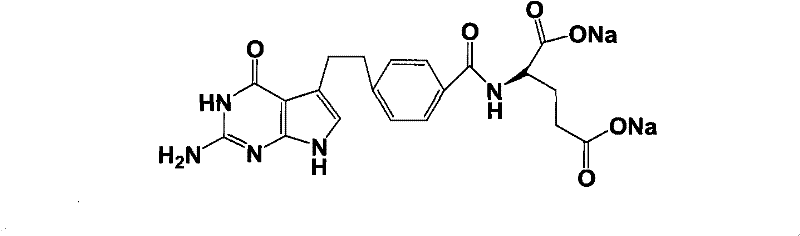
Intermediate of pemetrexed disodium, preparation method thereof and method for preparing pemetrexed disodium thereby
ActiveCN101560206AHigh yieldReduce manufacturing costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsBenzoic acidGlutaric acid
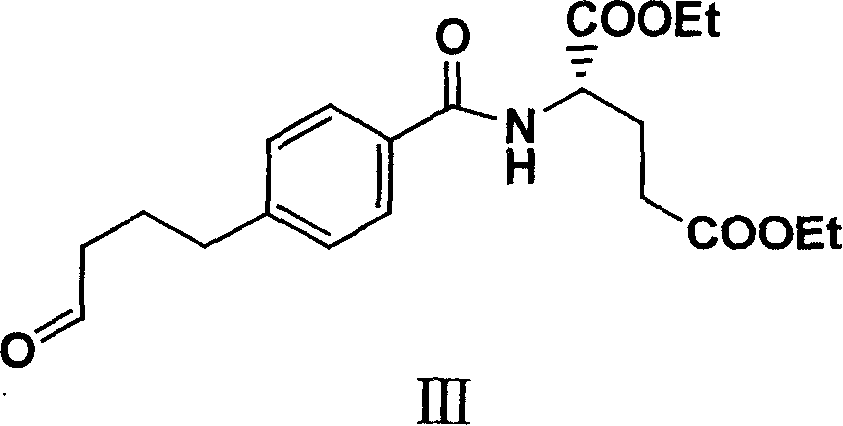
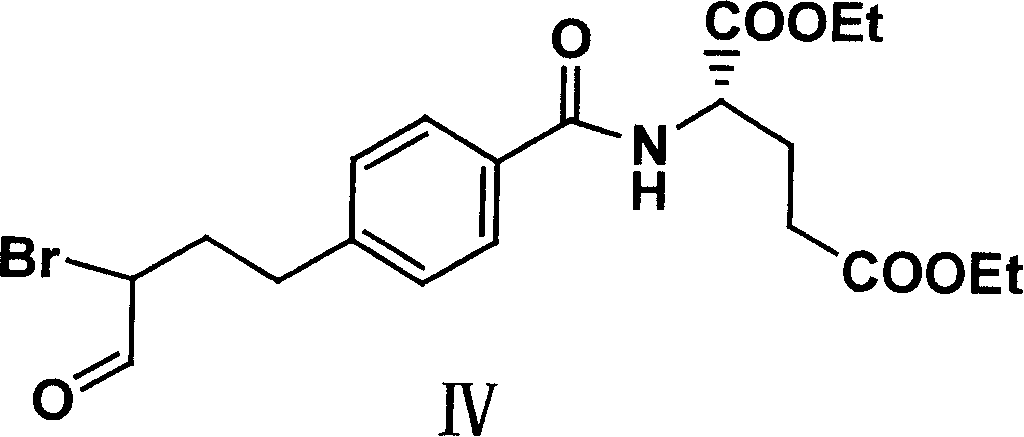
The invention relates to an intermediate of pemetrexed disodium, a preparation method thereof and a method for preparing pemetrexed disodium thereby; and the intermediate is (2-(4-(3-(2,4-diamino-6-oxy-1,6-dihydro-pyridine-5-group)-3-(1,3)dioxolane-2-group-propyl) benzylamine)sodium glutaric acid. The synthesis of the intermediate comprises the following steps: firstly, condensation reaction is conducted on 4-bromobenzoic acid or 4-iodobenzoic acid and L-glutamate diethylester, then Hack reaction is conducted, 4-bromo is replaced and 4-butyraldehyde is formed, then selective bromo replacement is conducted and the 4-butyladehyde is converted into 2-bromobutyraldehyde, and then condensation reaction of aldehyde and ethylene glycol is utilized for protecting the aldehyde, and pyrimidine ring is further synthesized, and finally the intermediate is obtained. Acid hydrolysis ring-closing reaction and sodium hydroxide salification are respectively conducted for once on the intermediate so as to obtain the pemetrexed disodium. The method for preparing pemetrexed disodium in the invention has high yield, low cost and easy operation and is applicable to industrialized production.
Owner:山东立新制药有限公司
Diethyl 4(4-oxobutyl) benzoyl-L-glutamate and its preparation and use
InactiveCN1821219AHigh yieldMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationL glutamateHalide
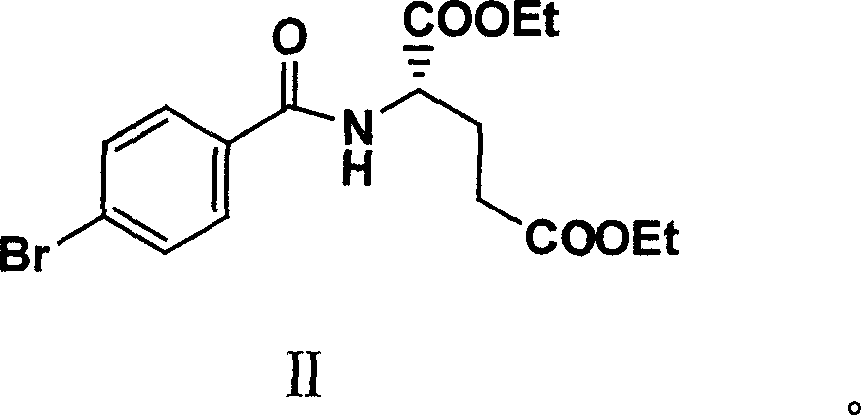
The present invention relates to new compound diethyl 4-(4-oxobutyl) benzoyl-L-glutamate and its preparation process and application. The compound diethyl 4-(4-oxobutyl) benzoyl-L-glutamate in the chemical expression as shown is prepared through the reaction of compound diethyl 4-bromo benzoyl-L-glutamate and 3-butene-1-alcohol inside Nú¼N-dimethyl formamide solvent under the action of palladium acetate catalyst, weak alkali reagent, lithium halide and phase transfer catalyst in the protection of inert gas at 50-70 deg.c. The compound diethyl 4-(4-oxobutyl) benzoyl-L-glutamate is used in synthesizing diethyl 4-[(4-oxo-3-bromo) butyl] benzoyl-L-glutamate as the intermediate of Pemetrexed disodium. The present invention results in shortened Pemetrexed disodium synthesizing path and lowered production cost.
Owner:ZHEJIANG UNIV OF TECH
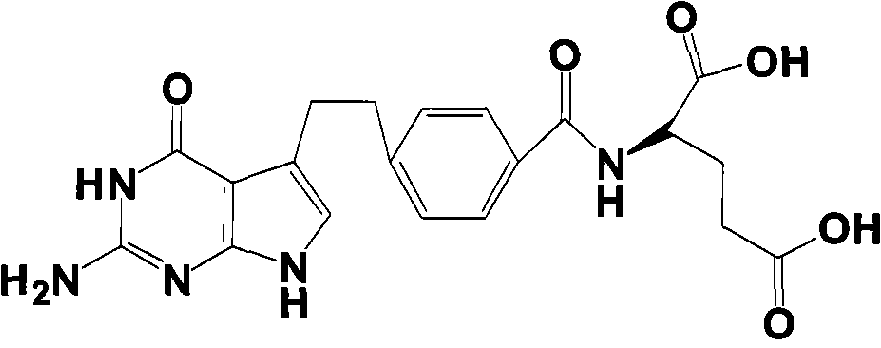
Preparation method of pemetrexed disodium
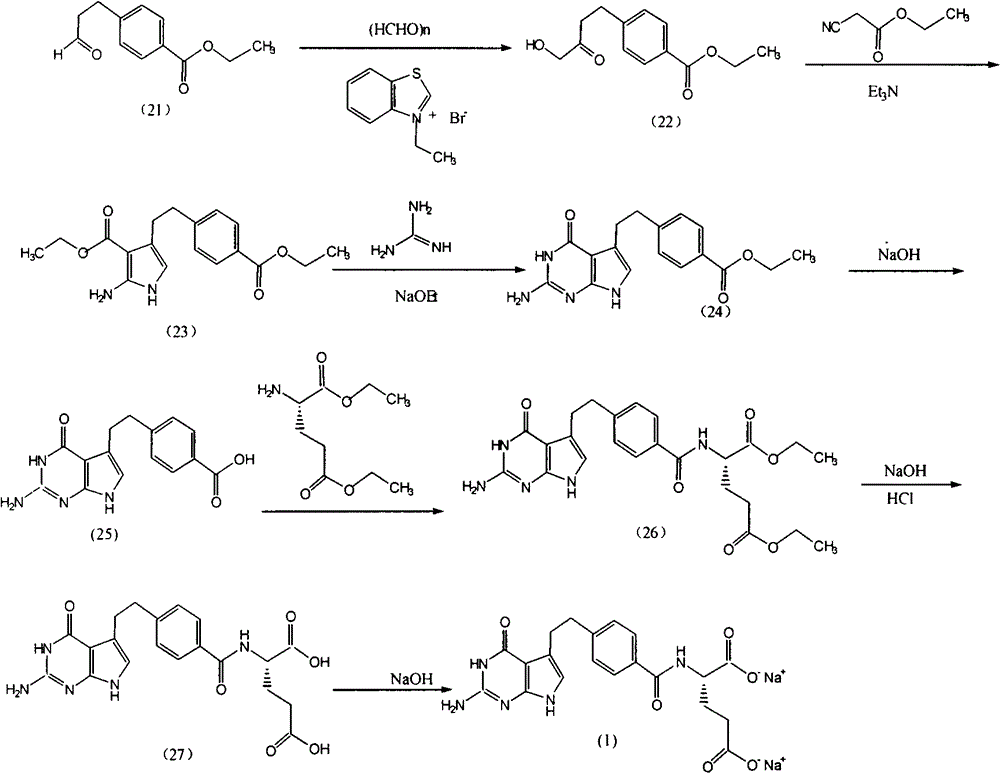
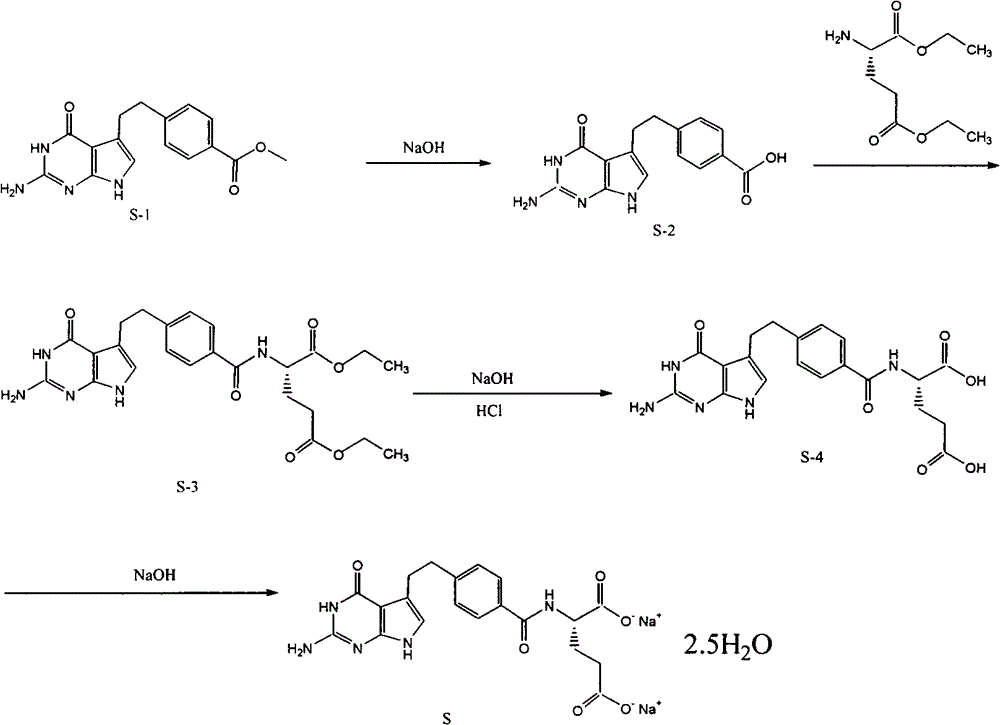
The invention provides a preparation method of pemetrexed disodium, which comprises the steps of performing hydrolysis reaction of 4-[2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl] methyl benzoate in sodium hydroxide to generate acid, then condensing with L-glutamic acid diethyl ester to obtain N-[4-[2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic acid diethyl ester toluenesulfonate, obtaining the crude pemetrexed disodium through saponification under the action of sodium hydroxide, and finally crystallizing in a mixed solvent at room temperature to obtain the end product. With the adoption of the technical scheme, the purification and purification of the crude pemetrexed disodium can be directly performed, so that the cost can be effectively lowered. As the steps are carried out at room temperature, the oxidation of the pemetrexed disodium caused by high temperature can be effectively avoided. The purity of the end product is high and meets the national requirement on purity of chemicals.
Owner:DEZHOU DEYAO PHARMA
Preparation method of Raltitrexed
The invention discloses a preparation method of Raltitrexed. The method comprises steps of: carrying out alkaline hydrolysis on N-[[5-[(1,4- dihydro-2-methyl-4-oxygen-6quinazoline) methyl] formoxyl-]-2-thiophene] formyl]-L-glutamic acid diethyl ester with existence of a certain proportion of alcoholic solvent; filtering out insoluble substances after the reaction; then extracting with an organic solvent; adjusting a pH of a water layer to 6-7 during neutralization to precipitate a small amount of viscous solid and stirring for 1-3 h; After complete solidification of a product, adjusting a pH to 2-3 to precipitate all of the product; dissolving a crude product with an organic solvent; filtering an depriving insoluble substances; adding water and crystallizing to obtain a finished product. The preparation method of the present invention well solves a problem, which commonly exists in a technology, of severe exceeding of standard of residue on ignition, has simple operations, produces high-quality product with high yield and is suitable for industrialized production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Improved process of preparing Raltitrexed
InactiveCN101088997AHigh yieldReduce consumptionOrganic active ingredientsOrganic chemistryDiethyl glutarateDiethyl ether
The improvement in the process of preparing Raltitrexed as antitumor medicine includes the reaction between N-(5-aminothienyl-2-formoxyl)-L-diethyl glutarate and methyl iodide at temperature raised to 100 deg.c in lucifugous condition with yield raised to 91 %; the replacement of sodium sulfide for iron powder as reductant to facilitate post-treatment and raise yield; the replacement of re-crystallization in mixed solvent of methanol and ether for column separation to expand the production capacity; and oxidizing 5-nitrothienyl-2-formaldehyde with chromic acid, rather than bromine, to eliminate toxicity and raise safety. The improved preparation process of Raltitrexed has high yield, great production capacity, low solvent consumption and high safety.
Owner:魏秀华
Synthesis methods of raltitrexed
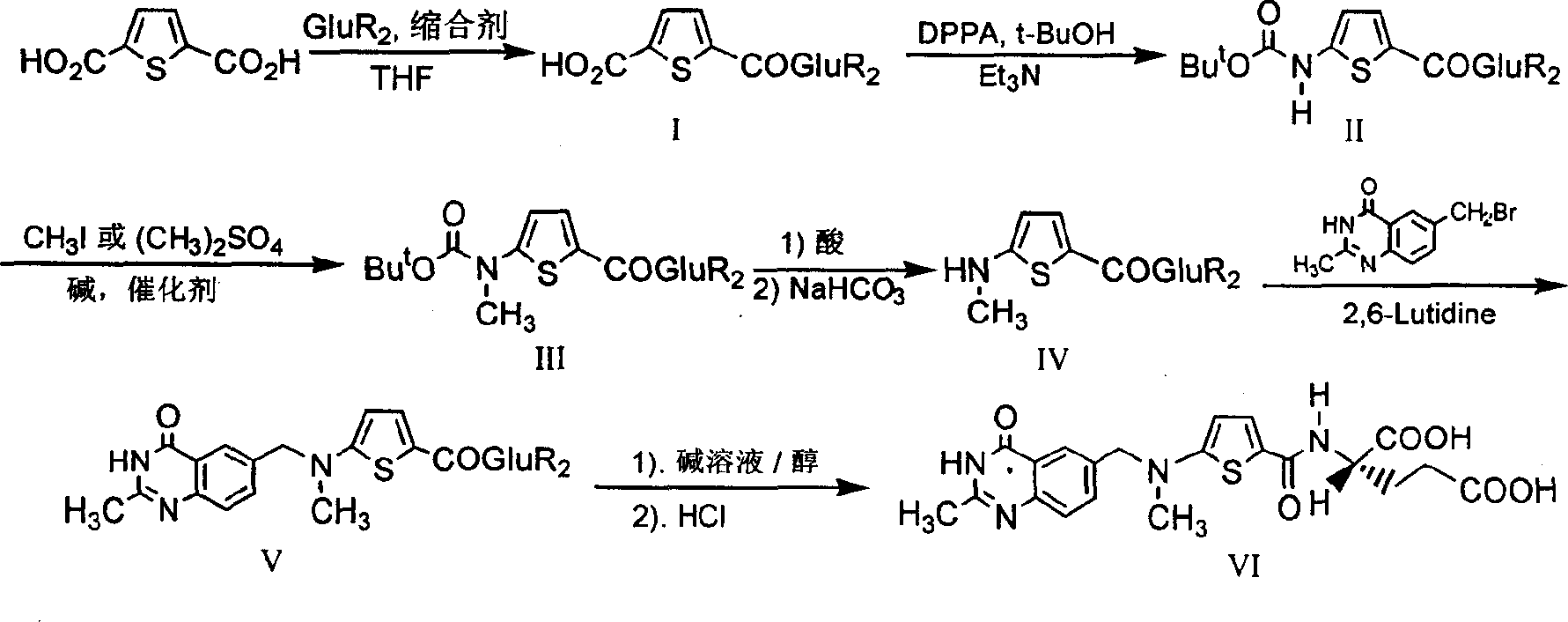
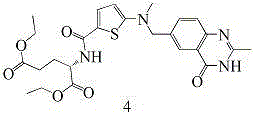
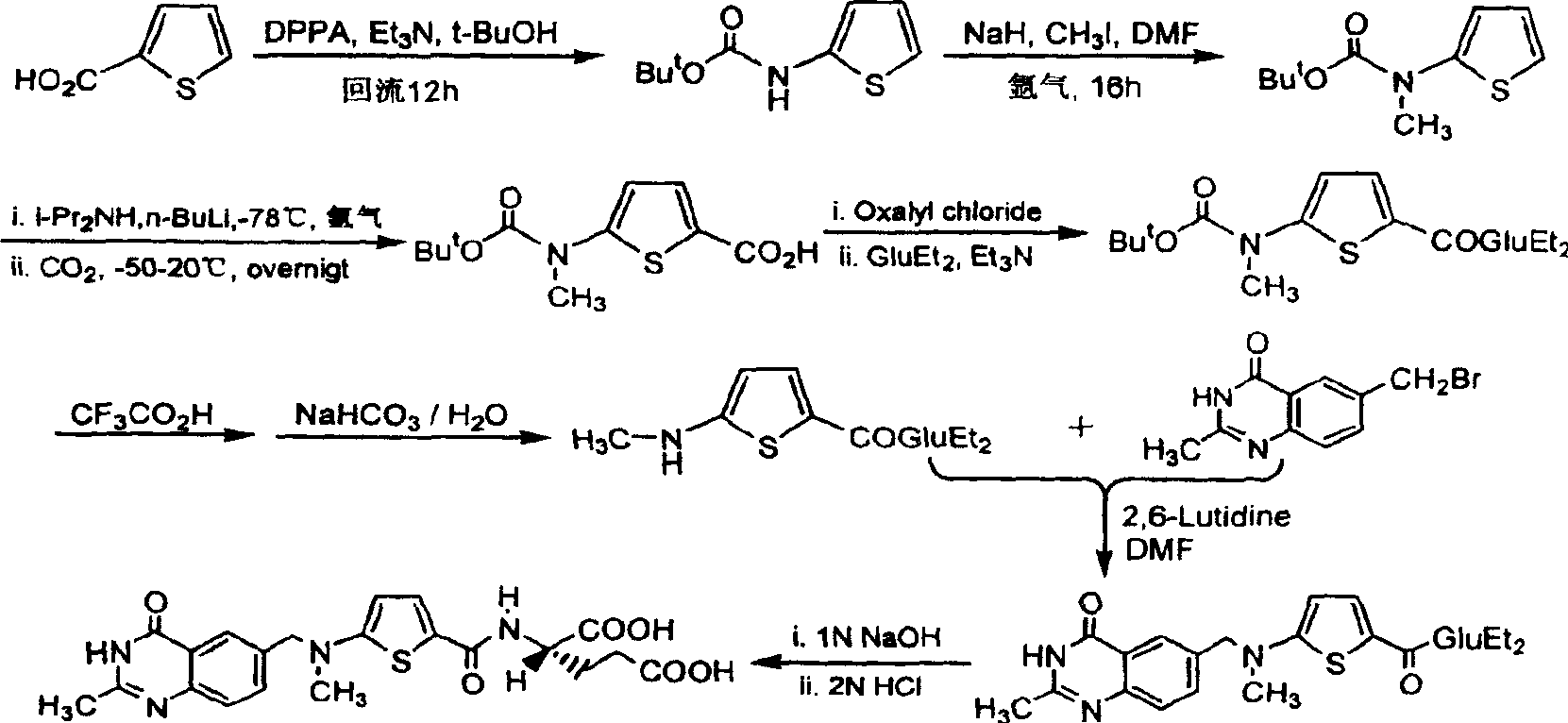
The invention provides two method for preparing a compound disclosed as Formula 4. The scheme 1 comprises the following steps: reacting the raw material methyl 5-bromothienyl-2-formate with 6-((methylamino)methyl)-3,4-dihydro-2-methyl-4-oxo-6-quinazoline (compound 1) to obtain methyl 2-[N-(2-methyl-4-oxoquinazolinyl-6-methyl)-N-methyl]-aminothienyl-2-formate, hydrolyzing in an alkaline water solution, and carrying out condensation reaction with diethyl L-glutamate to obtain the compound disclosed as Formula 4. The scheme 2 comprises the following steps: carrying out condensation on the raw material 5-bromothienyl-2-formic acid and di L-glutamate to obtain diethyl N-(5-bromothienyl-2-yl)glutamate, and reacting with a compound disclosed as Formula 1 to obtain the compound disclosed as Formula 4. The invention also provides a method for preparing raltitrexed, which comprises the following steps: hydrolyzing the compound disclosed as Formula 4 in an alkaline water solution, and acidifying with hydrochloric acid to obtain the raltitrexed. The synthesis route has the advantages of fewer reaction steps, low environmental pollution and high product yield, and is simple to operate.
Owner:SHANGHAI DINGYA PHARM CHEM CO LTD
Preparation method of chiral MOF supermolecule composite material and application of such material in identification of penicillamine enantiomer
InactiveCN110501397ASimple preparation processEasy to industrializeMaterial electrochemical variablesPenicillamineEnantiomer
The invention discloses a preparation method of a chiral MOF supermolecule composite material and an application of such material in efficient electrochemical identification of a penicillamine enantiomer and belongs to the technical field of nanomaterials, electrochemical chiral recognition and metal organic frame materials. The method mainly comprises the following steps: synthesizing a chiral L-glutamic acid bolaamphiphile L-HDGA by using hexadecyl diacid and diethyl glutamate, and then, preparing a spirally-arranged L-HDGA template with ethanol and water as solvents; and through a self-assembly method, preparing the composite material with ZIF 67 nanoparticles loaded on the surface of the L HDGA spiral structure. The application of the material in efficient electrochemical identification of the penicillamine enantiomer has the advantages of simple process, fast response and wide electrochemical identification; and the material has an application prospect.
Owner:UNIV OF JINAN
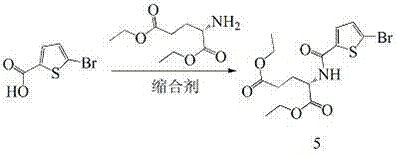
Method for preparing raltitrexed intermediate, i.e., N-(5-methylamino-2-thiophene formyl)-L-glutamate diethyl ester
InactiveCN102898415AMild reaction conditionsEasy to operateOrganic chemistryMethylene DichlorideL glutamate
The invention belongs to the technical field of medicine, and particularly disclose a method for preparing a raltitrexed intermediate, i.e., N-(5-methylamino-2-thiophene formyl)-L-glutamate diethyl ester. In the preparation method, N-(5-methylamino-2-thiophene formyl)-L-glutamate diethyl ester is taken as a raw material. The method is characterized by comprising the following steps of: dissolving the raw material into ethyl acetate, adding a condensing agent, reacting while stirring at the temperature of 30-60 DEG C, filtering, and concentrating under reduced pressure to obtain an oily matter; and dissolving the oily matter into absolute ethyl alcohol, adding sodium borohydride in batches, reacting at the temperature of 20-40 DEG C, adding water for stopping the reaction, concentrating under reduced pressure to dryness, adding water and methylene dichloride for laminating, drying an organic phase by using anhydrous magnesium sulfate to obtain a product. The method has milder reaction conditions, is easier to operate under the condition of not lowering the yield, and is more suitable for industrial production; and the problems of toxicity and safety are solved.
Owner:济南久创化学有限责任公司
Preparation method of Raltitrexed
The invention discloses a preparation method of Raltitrexed. The method comprises steps of: carrying out alkaline hydrolysis on N-[[5-[(1,4- dihydro-2-methyl-4-oxygen-6quinazoline) methyl] formoxyl-]-2-thiophene] formyl]-L-glutamic acid diethyl ester with existence of a certain proportion of alcoholic solvent; filtering out insoluble substances after the reaction; then extracting with an organic solvent; adjusting a pH of a water layer to 6-7 during neutralization to precipitate a small amount of viscous solid and stirring for 1-3 h; After complete solidification of a product, adjusting a pH to 2-3 to precipitate all of the product; dissolving a crude product with an organic solvent; filtering an depriving insoluble substances; adding water and crystallizing to obtain a finished product. The preparation method of the present invention well solves a problem, which commonly exists in a technology, of severe exceeding of standard of residue on ignition, has simple operations, produces high-quality product with high yield and is suitable for industrialized production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
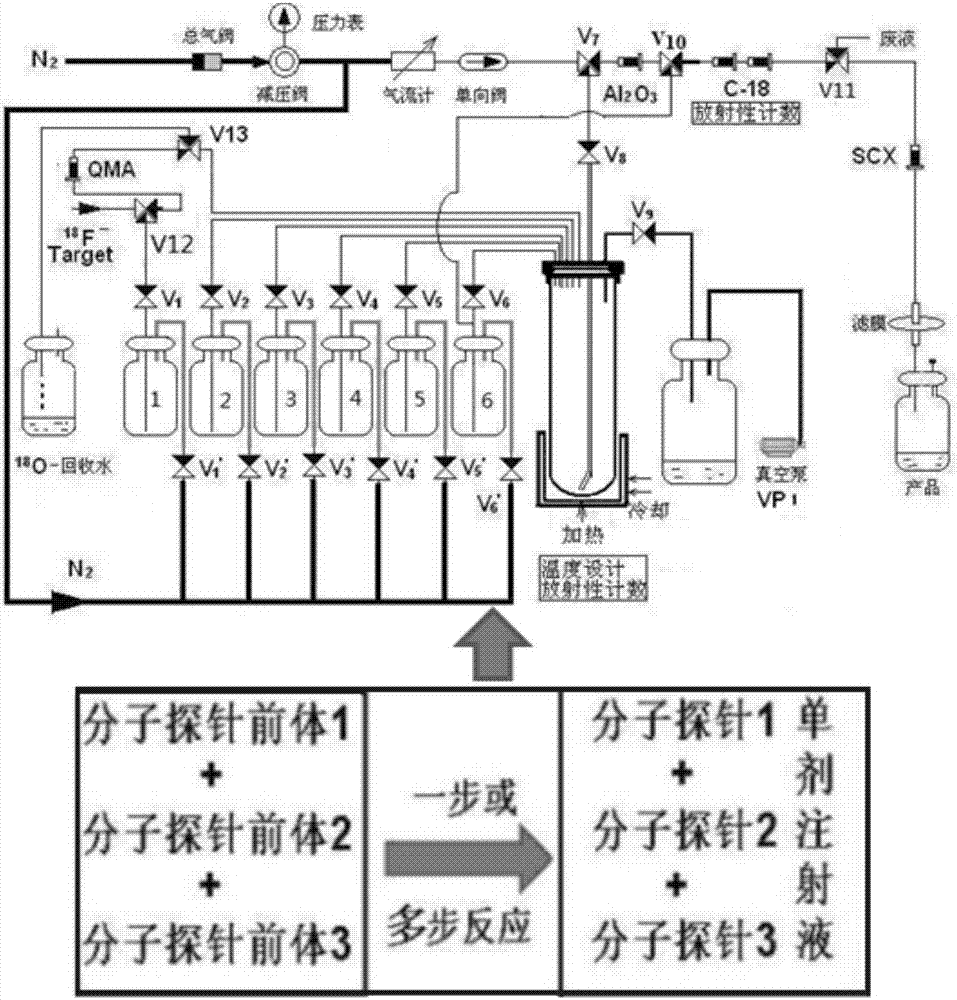
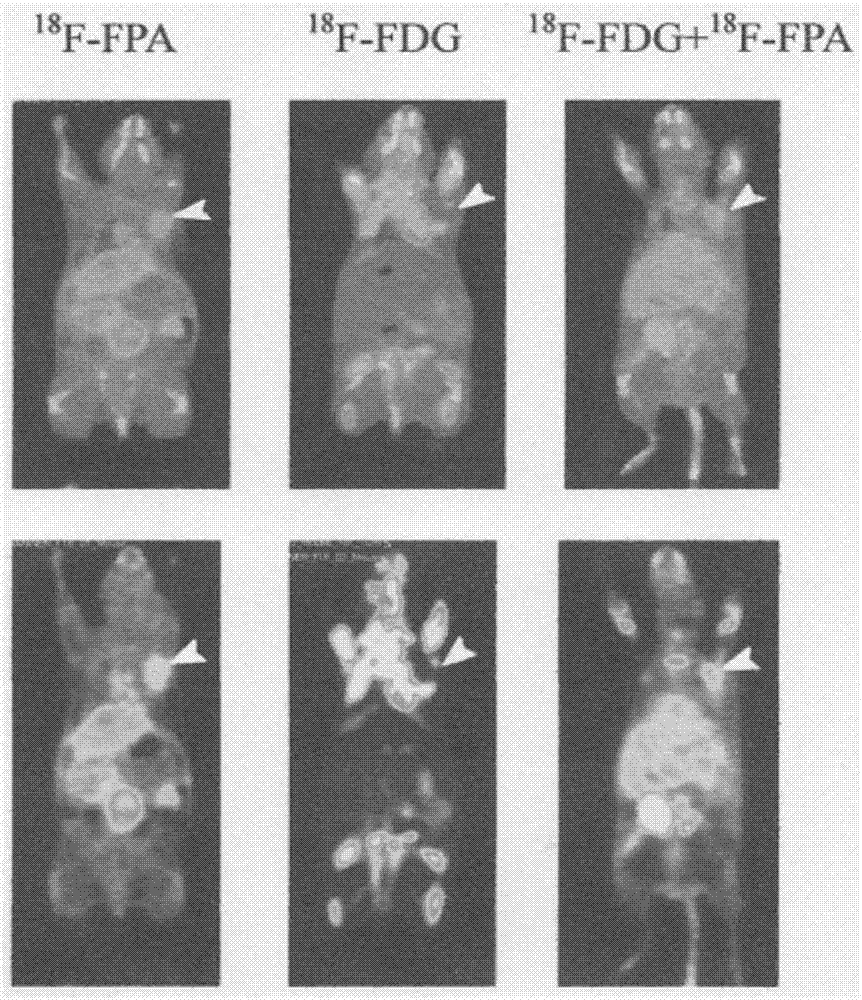
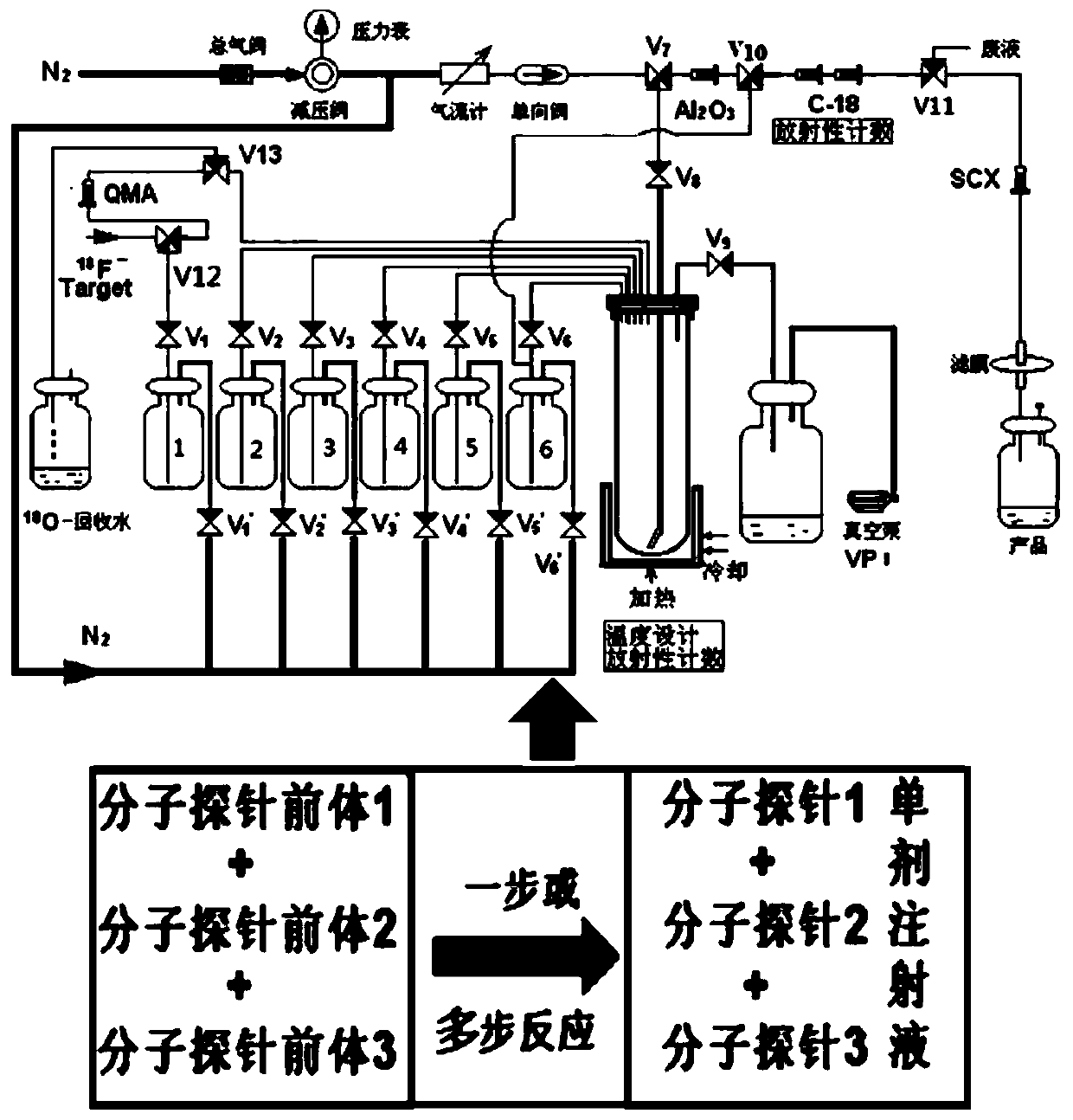
Radioactive composition as well as one-time radioactive synthesis method and application thereof
ActiveCN106902363ASolve key technical problemsWide applicabilityRadioactive preparation carriersDiseaseSynthesis methods
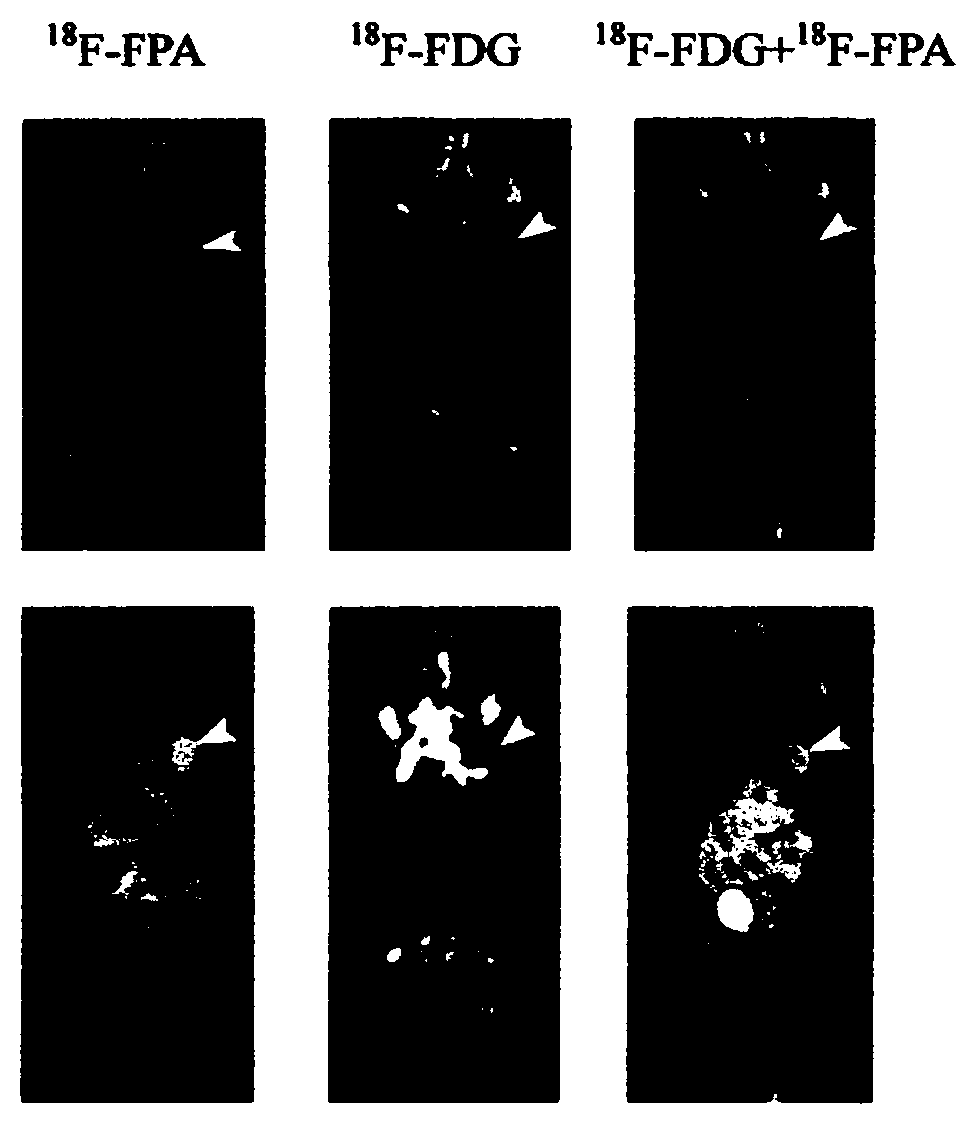
The invention relates to composition (a compound molecular probe preparation) containing various radioactive compounds such as <18>F-FDG (<18>F-fludeoxyglucose), <18>F-FPA (2-<18>F-fluoropropionic acid) and / or <18>F-NFPGlu ((N-2-<18>F-fluoropropionyl)-L-alpha-glutamic acid), a one-time radioactive synthesis method of the composition and an application of the composition in preparation of positron emission computed tomography drugs. The composition comprising <18>F-FDG+<18>F-FPA and <18>F-FDG+<18>F-NFPGlu or <18>F-FDG+<18>F-FPA+<18>F-NFPGlu as PET drugs can be obtained from precursors through nucleophilic fluorination reaction and a hydrolysis reaction, wherein the precursors comprise a mannose triflate and ethyl 2-bromopropionate mixture and a mannose triflate and (N-2-bromopropionyl)-L-alpha-diethyl glutamate mixture or a mixture of mannose triflate, ethyl 2-bromopropionate and (N-2-bromopropionyl)-L-alpha-diethyl glutamate. The composition can be used for differential diagnosis and therapeutic effect evaluation of tumor, cardiovascular and cerebrovascular diseases and nervous and mental diseases and can overcome limitation of <18>F-FDG.
Owner:GUANGDONG HUIXUAN PHARMA TECH
Preparation method of pemetrexed disodium
InactiveCN104119346AHigh yieldSimplify the manufacturing processOrganic chemistryTransient stateOrganic solvent
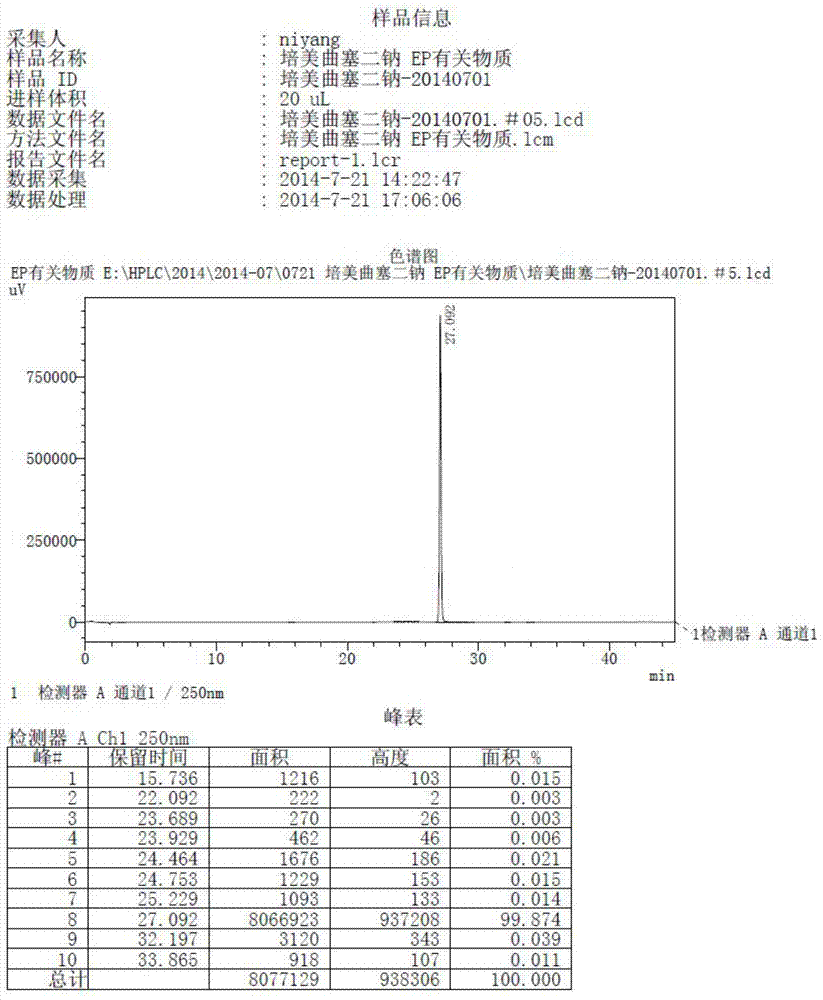
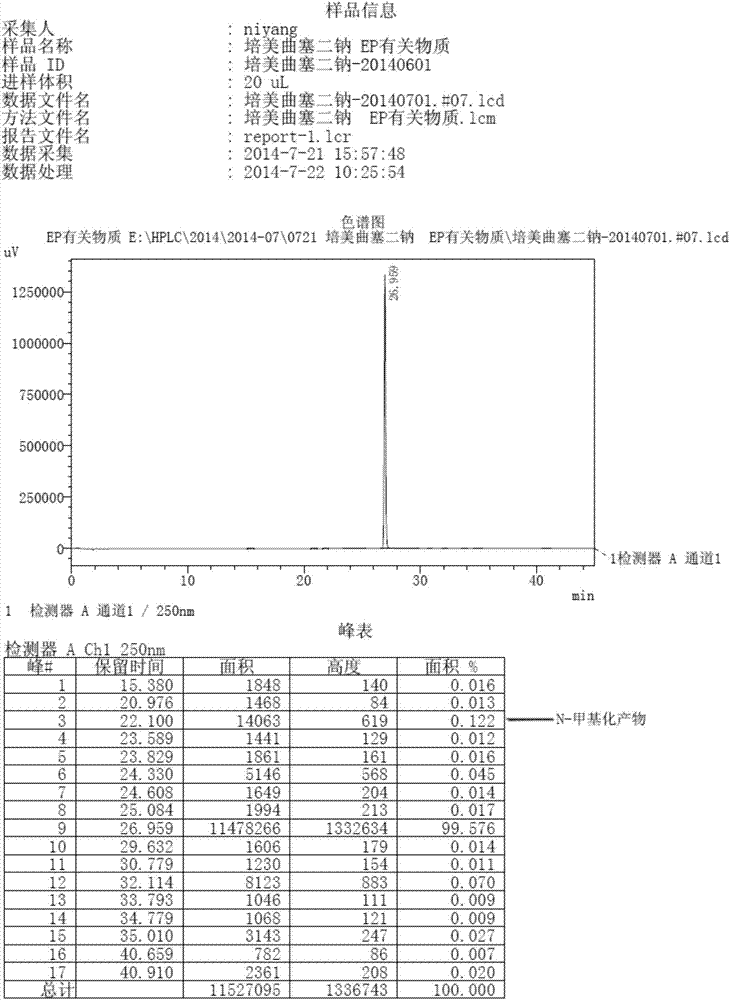
The invention discloses a preparation method of pemetrexed disodium, which can directly hydrolyzing into salt after an organic solvent is removed, rather than salifying together with p-toluenesulfonic acid and / or purifying a product by adopting a crystallization method during the preparation process, thus omitting the step of salifying together with p-toluenesulfonic acid and / or crystallization through ethanol, effectively improving the total yield of drugs to 68-75%; in the preparation process, a peptide condensing agent is firstly prepared and is fully reacted with pemedolac in the organic solvent into transient-state acid ester, so that the generation of N-methylation impurities can be effectively inhibited in the reaction together with L-glutamic acid diethyl ester hydrochloride, the defect that the content of N-methylation impurities during the one-pot preparation process in the prior art exceeds 0.1% can be avoided, the advantages for opening up Europe and America markets can be brought, the defect that the raw materials are not reacted thoroughly to cause high cost can also be overcome; the HPLC (High Performance Liquid Chromatograph) of the finished pemetrexed disodium obtained by adopting the method is more than or equal to 99.8%, and the content of single impurities is less than 0.1%.
Owner:NINGBO MENOVO PHARMA
Preparation method of pemetrexed disodium
ActiveCN105111214ARaw materials are easy to getWide variety of sourcesOrganic chemistryBenzoic acidPtru catalyst
The invention discloses a preparation method of pemetrexed disodium, which comprises the following steps: preparing 4-(4,4-dialkoxy-1-butenyl)benzoate 6 and 4-(3-formyl-3-halo-1-propenyl)benzoate 7, carrying out ring-closing reaction on the compound 7 and 2,4-diamido-6-hydroxypyrimidine under the action of an alkali to obtain a compound 9; carrying saponification on the compound 9, and acidifying to obtain a compound 10; carrying out condensation reaction on the compound 10 and diethyl L-glutamate to obtain a compound 11; carrying out catalytic hydrogenation reaction on the compound 11 under the action of a catalyst to obtain a compound 12; and carrying out hydrolysis reaction on the compound 12 under the action of an alkali to obtain the target product pemetrexed disodium 1. The method has the advantages of accessible raw materials, wide sources, low preparation cost and low price, is suitable for industrial production, and solves the problems of high preparation cost and difficulty for industrial production in the prior art.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Pralatrexate preparation method
InactiveCN104628728AAvoid high energy consumptionAvoid separationOrganic chemistryBenzoic acidPteroic acid
The present invention discloses a practical synthesis process of a new anticancer drug pralatrexate. According to the present invention, 10-propargyl-10-methoxycarbonyl-4-deoxy-4-amino-10-deaza pteroic acid methyl ester is adopted as a starting raw material, a saponification reaction is performed to obtain 4-(2-carboxy-1-(2,4-diaminopteridine-6-yl)pent-4-yn-2-yl)benzoic acid, the 4-(2-carboxy-1-(2,4-diaminopteridine-6-yl)pent-4-yn-2-yl)benzoic acid is subjected to decarboxylation to obtain 4-(1-(2,4-diaminopteridine-6-yl)pent-4-yn-2-yl)benzoic acid, the 4-(1-(2,4-diaminopteridine-6-yl)pent-4-yn-2-yl)benzoic acid reacts with L-diethyl glutamate to obtain 10-propargyl-10-deaza aminopterin diethyl ester, and finally a saponification reaction is performed to obtain the target product pralatrexate, wherein the green, high yield and convenient synthesis of the pralatrexate is completed, the final product can achieve the level from several grams to tens of grams, the purity is more than or equal to 90%, and the good industrial application prospects are provided.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
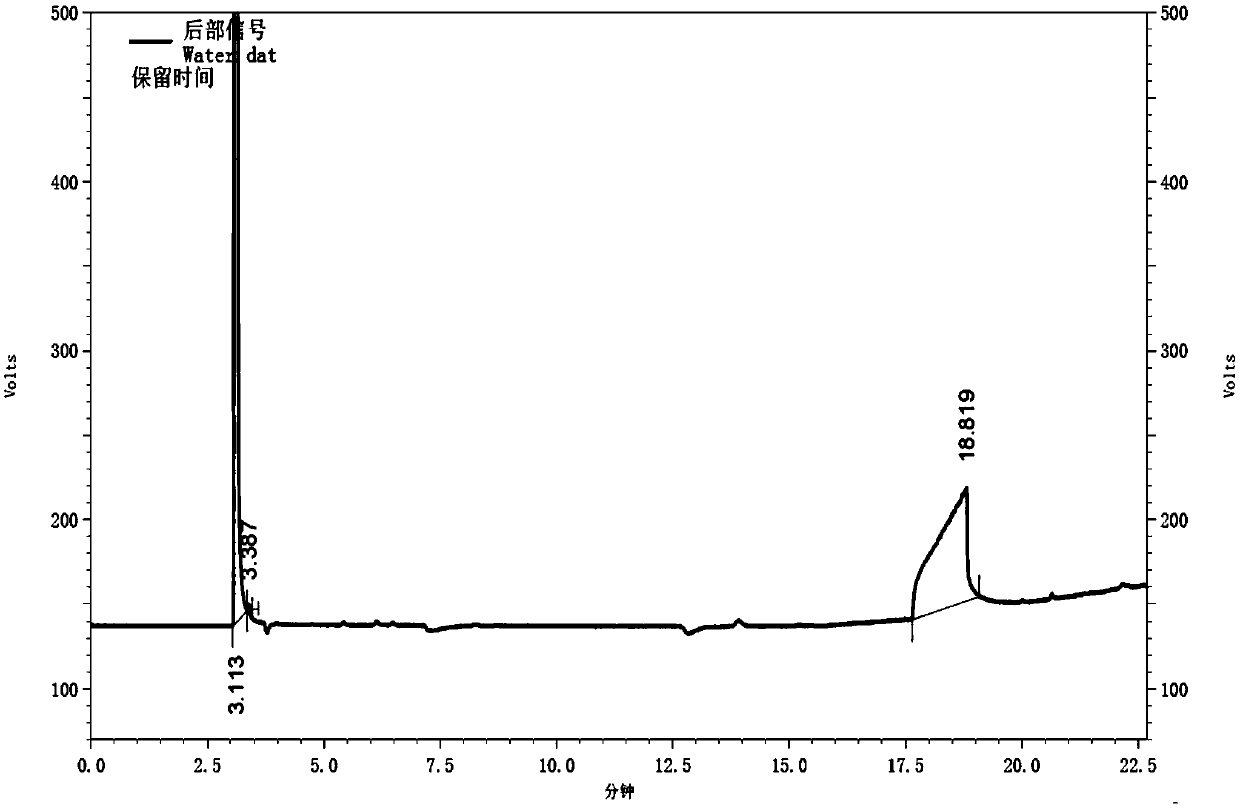
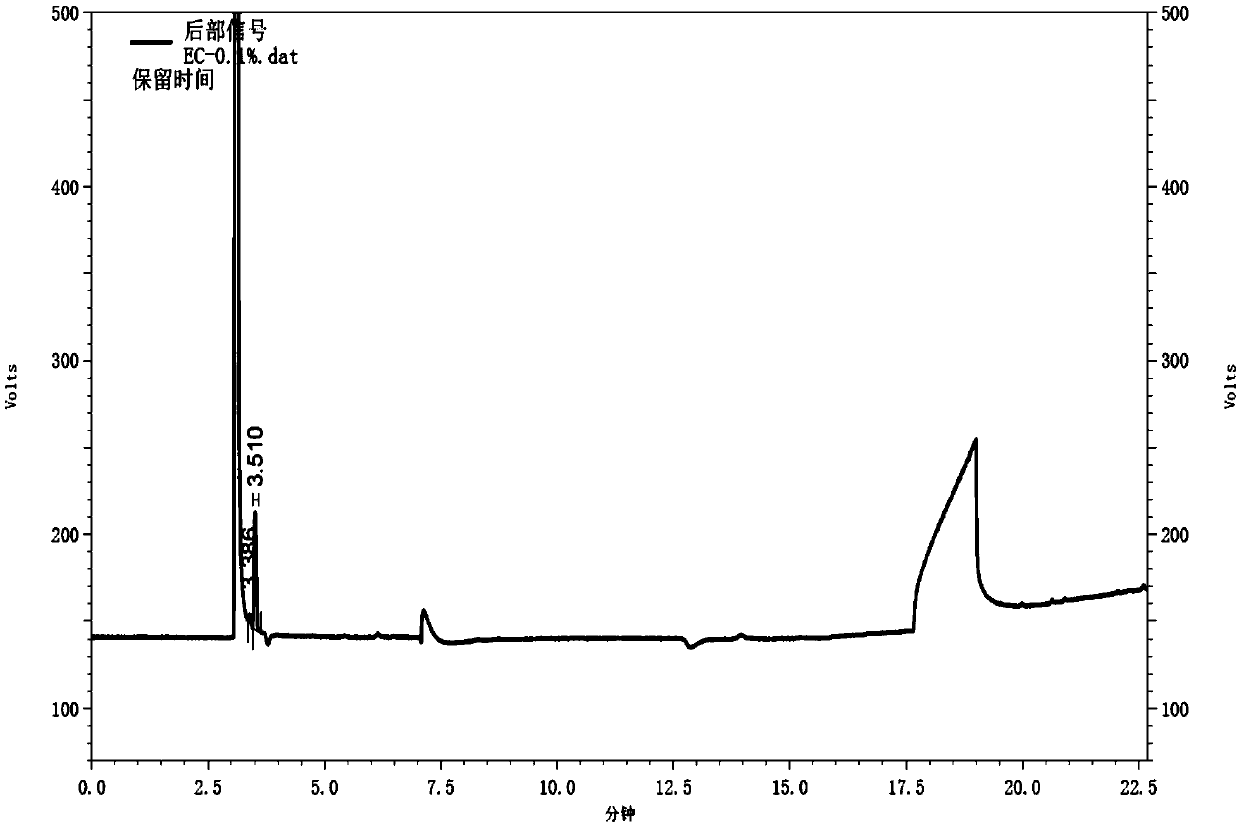
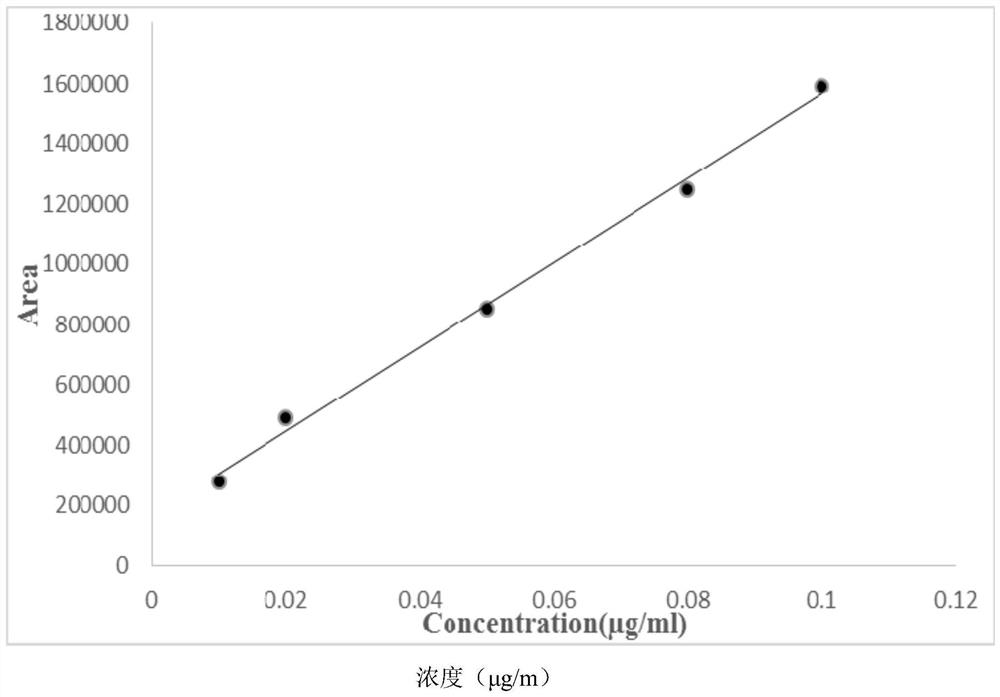
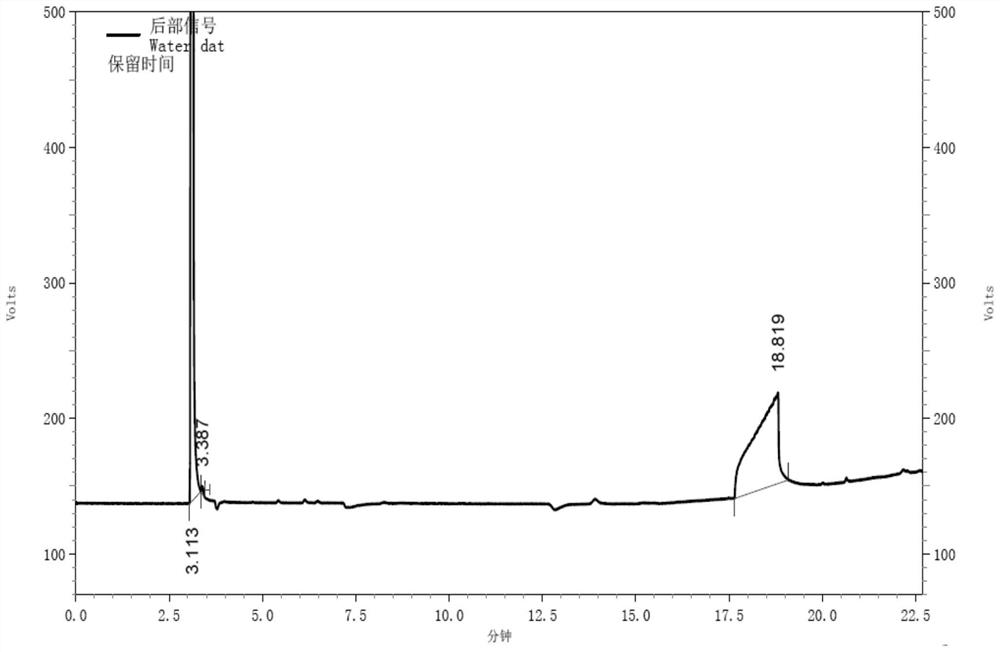
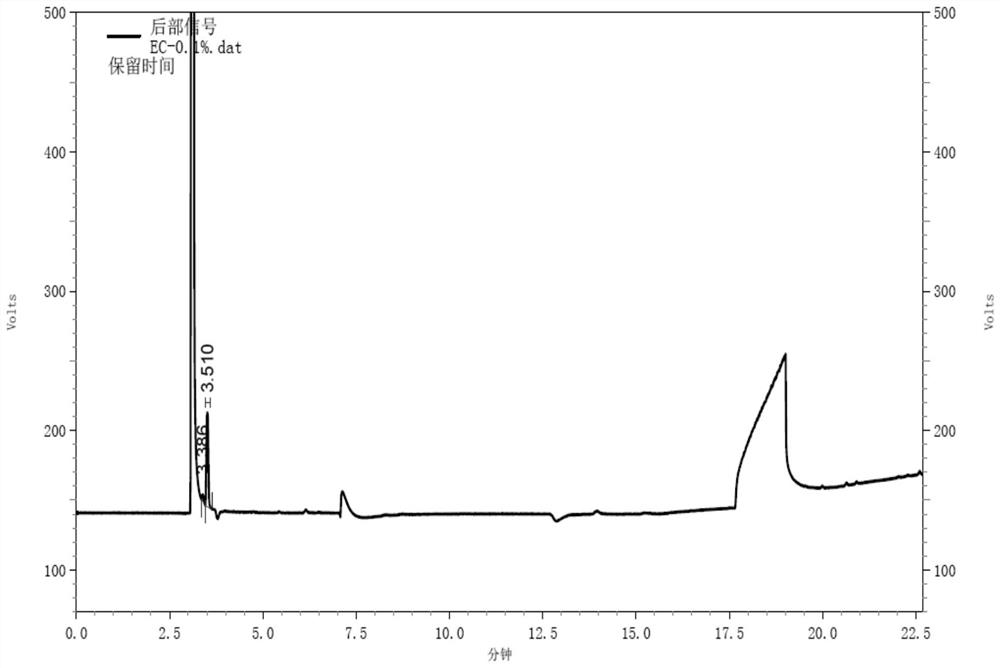
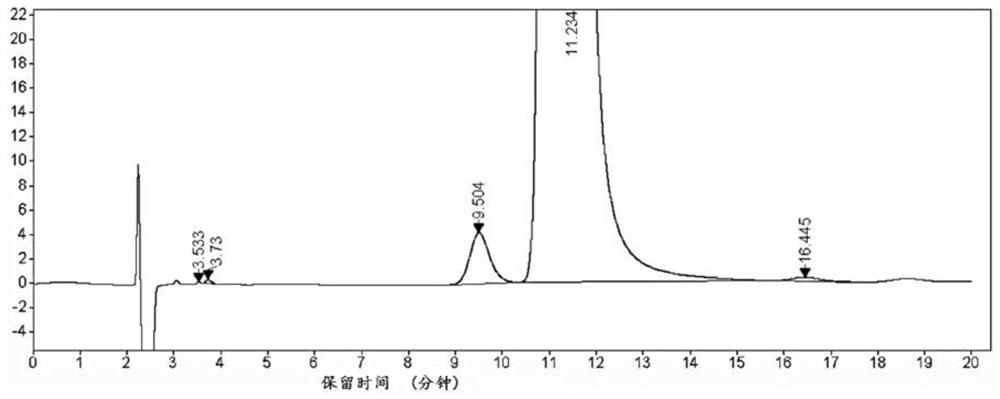
Gas chromatography detection method of chloroethane in L-glutamic acid diethyl ester hydrochloride
ActiveCN109633027AQuality improvementEasy to controlComponent separationGas phaseGlutamic acid diethyl ester
The invention discloses a gas chromatography detection method of chloroethane in L-glutamic acid diethyl ester hydrochloride. A L-glutamic acid diethyl ester hydrochloride sample is dissolved and thenheadspace sample injection is performed. A GC-ECD detection method is used to carry out qualitative and quantitative operation on the chloroethane in the L-glutamic acid diethyl ester hydrochloride and methodology validation is performed. The method has the advantages of high specificity, a rapid speed, sensitive performance and the like. In the invention, the method of carrying out qualitative and quantitative operation on the chloroethane in the L-glutamic acid diethyl ester hydrochloride through adopting the GC-ECD detection method is firstly established so that the quality of the L-glutamic acid diethyl ester hydrochloride can be conveniently controlled.
Owner:SHANDONG BOYUAN PHARM CO LTD
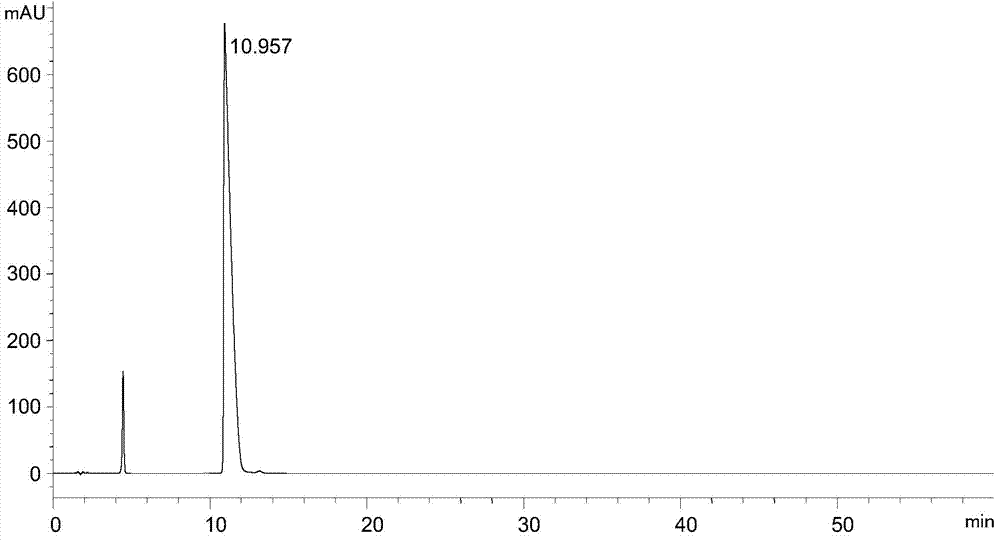
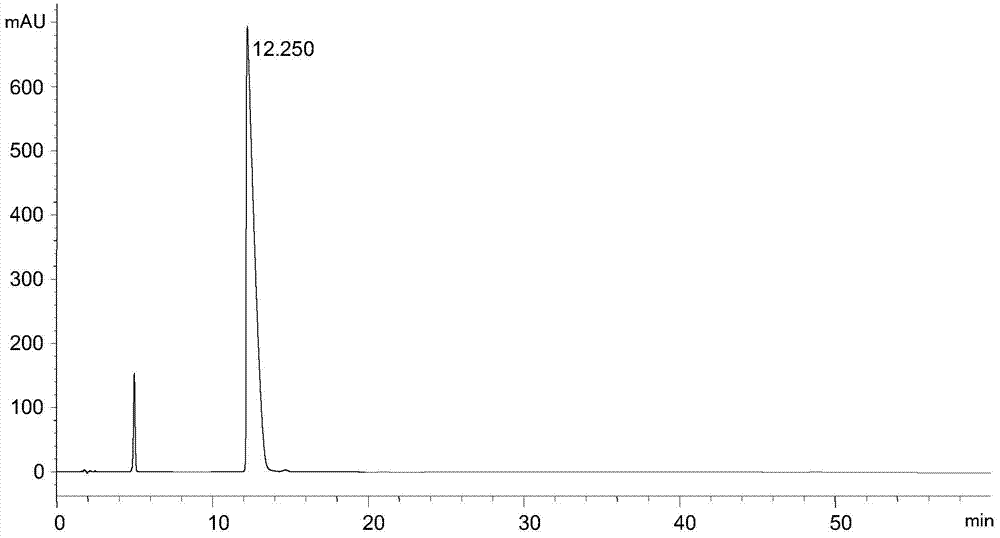
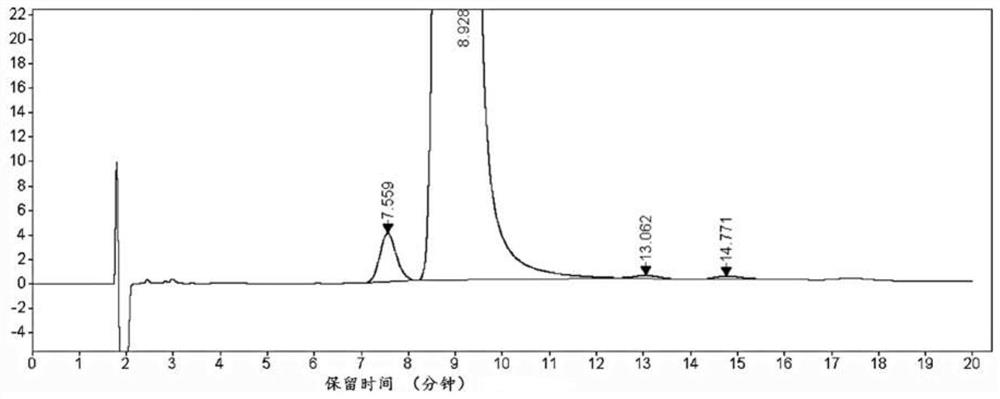
Analysis and detection method of L-diethyl glutamate
InactiveCN104515815AEfficient separationQuality assuranceComponent separationColumn temperatureSilica gel
The invention relates to an analysis and detection method of L-diethyl glutamate and is used in quality control of the L-diethyl glutamate. Analysis and detection are carried out through HPLC, of which chromatographic conditions are described as follows: a chromatographic column (C18, 4.6*150mm, 5[mu]m) is filled with octadecylsilane chemically bonded silica as a filling material; a mobile phase is a solution system prepared by mixing a buffer salt solution and acetonitrile and regulating a pH value to 7.0 with NaOH; a detection wavelength is 210 nm; and a column temperature is 22-32 DEG C. By means of the analysis and detection method, the L-diethyl glutamate can be effectively separated out from impurities thereof. The method is high in separation degree, is simple in operations, is good in repeatability and durability, and is stable and reliable in results.
Owner:SHANDONG NEWTIME PHARMA
Synthesis of anticancer medicine Raltiprexed
InactiveCN1216883CShort processLow costOrganic chemistryAntineoplastic agentsTert-Butyloxycarbonyl protecting groupN methylation
Owner:CAPITAL NORMAL UNIVERSITY
Synthesis method for Raltitrexed midbody
The invention discloses a synthesis method for a Raltitrexed midbody. The method comprises the following steps of a, pumping N-(5-amino-2-thenolyl)-L-glutamate diethyl ester and methyl iodide same in mole into a high flux-microchannel reactor from two feeding injection ports; b, placing the reactor in constant temperature water bath at 35-45 DEG C, enabling the reaction solution to react through the high-flux-microchannel reactor at a velocity of 5-13 mL / min, collecting the reaction solution at the outlet, cooling the reaction solution to a room temperature, adding water and ethyl acetate firstly in the reaction solution, and using ethyl acetate to extract the reaction solution twice, and merging organic layers; after performing alkaline hydrolysis on the water layer with anhydrous sodium carbonate, extracting the alkaline hydrolyzed water phase with ethyl acetate, merging the organic layer which is extracted by the reaction solution and the organic layer after the water layer is alkaline hydrolyzed; and drying the organic layers with anhydrous magnesium sulfate, filtering the dried organic layers, decompressing to remove the solvent, and thus obtaining the Raltitrexed midbody. The synthesis method provided by the invention is scientific and rational, the product yield and purity are high, and the method is suitable for promotion and application.
Owner:无锡紫杉药业股份有限公司
Preparation method of N-(5-(N-t-butyloxycarbonyl-N-methylamino)-2-thenoyl)-L-diethyl glutamate
InactiveCN105906605AHigh purityMild reaction conditionsOrganic chemistryOrganic solventTert-Butyloxycarbonyl protecting group
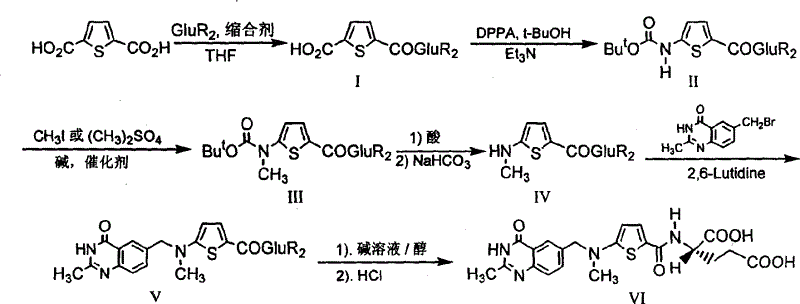
The invention provides a preparation method of N-(5-(N-t-butyloxycarbonyl-N-methylamino)-2-thenoyl)-L-diethyl glutamate. The preparation method is characterized by including dissolving N-(5-(N-t-butyloxycarbonyl amino)-2-thenoyl)-L-diethyl glutamate in organic solvent, adding alkali, then adding methylation reagent for methylation, filtering, concentrating filtrate to obtain a crude product, and purifying a pure product to obtain the N-(5-(N-t-butyloxycarbonyl-N-methylamino)-2-thenoyl)-L-diethyl glutamate. The preparation method is mild in reaction condition, easy to operate, high in yield, high in purity of reaction products and suitable for industrial production.
Owner:HONGGUAN BIO PHARMA CO LTD
The synthetic method of raltitrexed
The present invention provides two methods for preparing the compound of formula 4. Scheme 1 uses methyl 5-bromothiophene-2-formate as raw material, and 6-((methylamino)methyl)-3,4-dihydro-2- Methyl-4-oxo-6-quinazoline (compound 1) reacts to prepare 2-[N-(2-methyl-4-oxyquinazoline-6-methyl)-N-methyl]- Aminothiophene-2-formic acid methyl ester is then hydrolyzed in an aqueous alkali solution, and the compound of formula 4 is obtained through condensation reaction with L-diethyl glutamate; scheme two uses 5-bromothiophene-2-formic acid as raw material and N-(5-bromothiophene-2-yl) diethyl glutamate is obtained through condensation of L-glutamic acid diester, and reacts with formula 1 compound to make formula 4 compound; The present invention also provides a kind of preparation In the method of raltitrexed, the compound of formula 4 is hydrolyzed in an aqueous alkali solution, and then acidified with hydrochloric acid to prepare raltitrexed. The synthesis route adopted by the invention has few reaction steps, simple and convenient operation, little environmental pollution and high product yield.
Owner:SHANGHAI DINGYA PHARM CHEM CO LTD
Radioactive composition, method for its single radiosynthesis and use thereof
Owner:GUANGDONG HUIXUAN PHARMA TECH
Intermediate of pemetrexed disodium, preparation method thereof and method for preparing pemetrexed disodium thereby
ActiveCN101560206BHigh yieldReduce manufacturing costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsGlutaric acidL glutamate
Owner:山东立新制药有限公司
A kind of gas chromatographic detection method of ethyl chloride in L-glutamic acid diethyl ester hydrochloride
ActiveCN109633027BQuality improvementEasy to controlComponent separationGas liquid chromatographicGlutamic acid diethyl ester
The invention discloses a gas chromatography detection method for ethyl chloride in L-glutamic acid diethyl ester hydrochloride. It dissolves the L-glutamic acid diethyl ester hydrochloride sample and then injects the headspace sample, and adopts the GC-ECD detection method to qualitatively and quantitatively analyze the ethyl chloride in the L-glutamic acid diethyl ester hydrochloride. The method has been validated, and the method has the advantages of strong specificity, rapidity and sensitivity. The present invention first establishes the qualitative and quantitative method of ethyl chloride in L-glutamic acid diethyl ester hydrochloride using GC-ECD detection method, which is convenient to control the quality of L-glutamic acid diethyl ester hydrochloride .
Owner:SHANDONG BOYUAN PHARM CO LTD
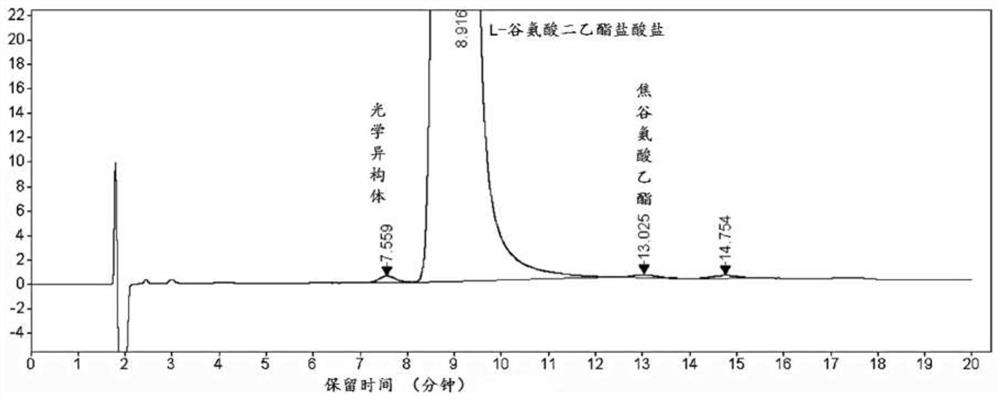
Separation and detection method of l-glutamic acid diethyl ester hydrochloride and its optical isomers
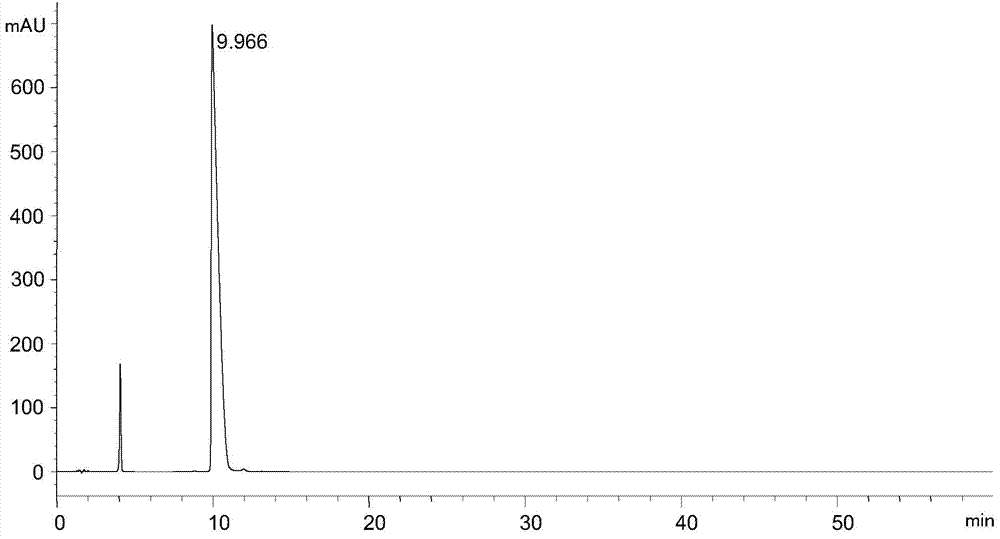
The invention discloses a separation and detection method of L-glutamic acid diethyl ester hydrochloride and its optical isomers. The separation and detection method adopts high performance liquid chromatography, the stationary phase is a silica gel chiral column, and the mobile phase is Reverse phase solvent: Perchloric acid in water - acetonitrile. The method of the invention has the characteristics of good separation, high sensitivity, strong specificity, etc., and can quickly and accurately perform quality control on L-diethyl glutamate hydrochloride.
Owner:YANGTZE RIVER PHARM GRP CO LTD
A kind of synthesis technique of raltitrexed key intermediate
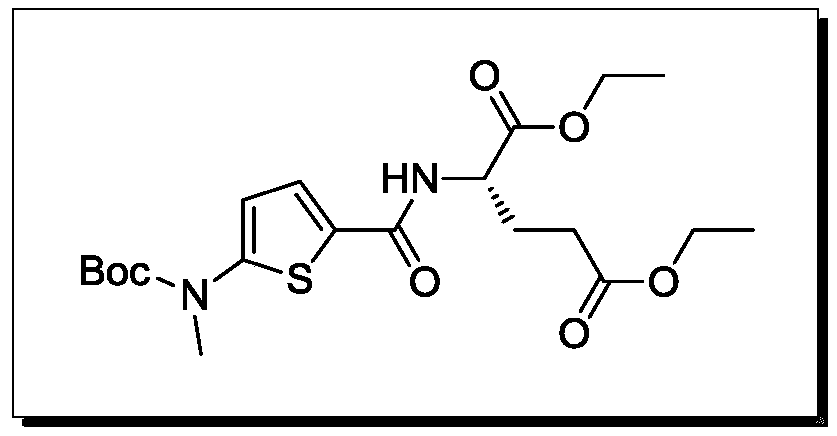
ActiveCN109734698BSignificant progressSimple processOrganic chemistryTert-Butyloxycarbonyl protecting groupRaltitrexed
The invention relates to the technical field of medicine preparation, in particular to a synthesis process of a Raltitrexed key intermediate. According to the synthesis process of the Raltitrexed keyintermediate, forming an active intermediate (isocyanate) through glutamate diethyl ester, the active intermediate and N-methyl-(2-thienyl) tert-butyl carbamate are directly reacted, and a target product N-[5-[N-(t-butyloxycarboryl)-N-methyl amino]-2-thenoyl]-L-glutamate diethyl ester is prepared by two steps. The synthesis process avoids difficulties of potential risks of part of racemization ofa chiral center caused by amidation reaction, low yield, complicated operation and purification and the like. The synthesis process of the Raltitrexed key intermediate is simple to operate, short in line, environmentally friendly and high in yield.
Owner:HONGGUAN BIO PHARMA CO LTD
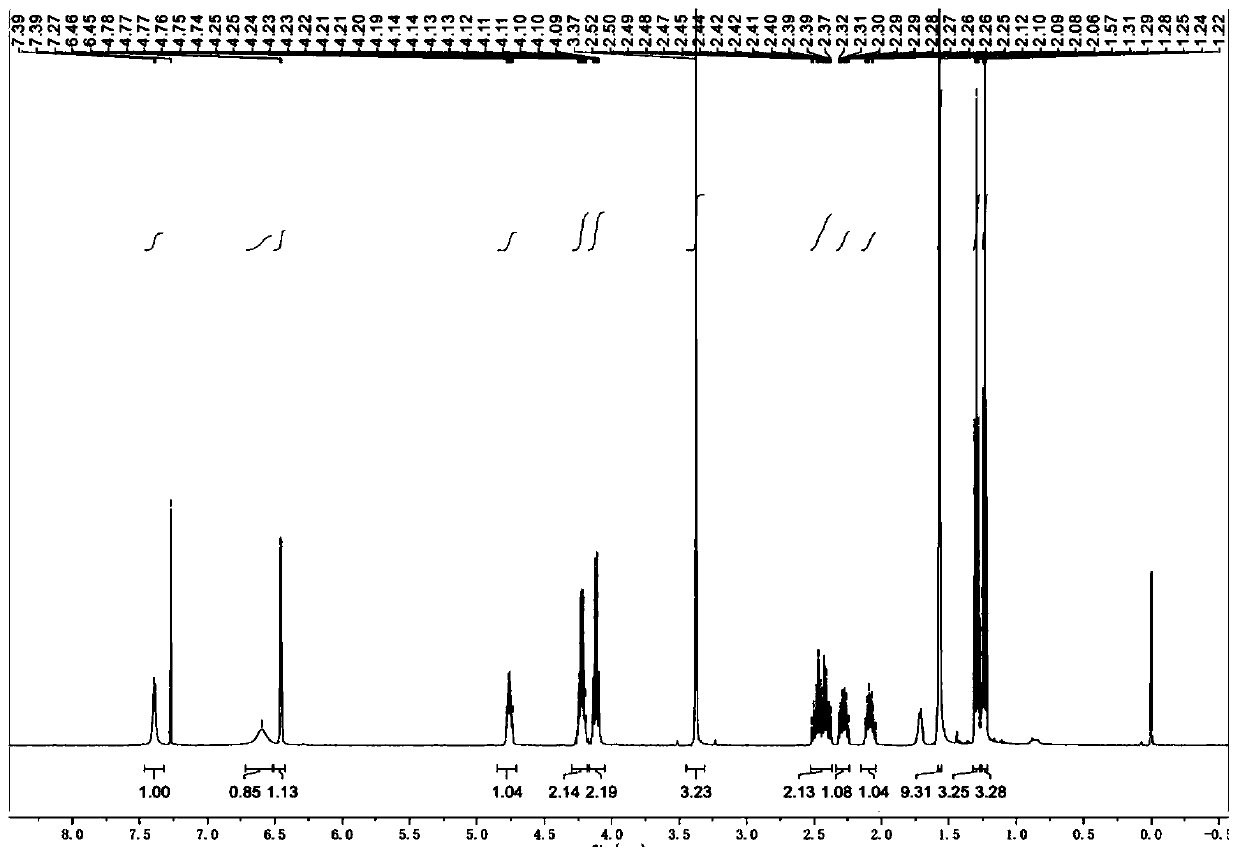
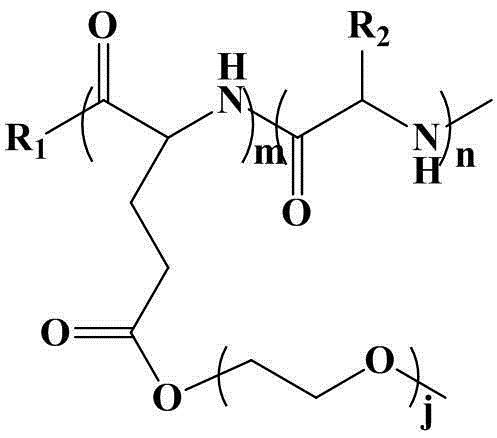
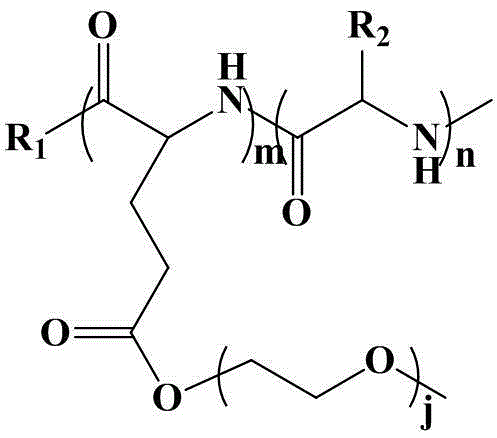
Poly (gamma-oligomerization ethylene glycol monomethyl ether-L-glutamic acid diethyl ester) - polyamino acid diblock copolymer and preparation method thereof
ActiveCN103059291BGood biocompatibilityPromote degradationPharmaceutical non-active ingredientsAmino acid side chainEnd-group
The invention provides a poly (gamma-oligomerization ethylene glycol monomethyl ether-L-glutamic acid diethyl ester) - polyamino acid diblock copolymer and a preparation method thereof, and further expands the application of polyamino acid materials to the field of biological medicine. A structural general formula of the diblock polyamino acid is shown in , wherein j is an integer from 1 to 10, m is an integer from 5 to 200, n is an integer from 5 to 200, R1 is an initiator end group, and R2 is an alpha-amino acid side chain group. The invention further provides a preparation method of poly (gamma-oligomerization ethylene glycol monomethyl ether-L-glutamic acid diethyl ester) - polyamino acid. The diblock copolymer not only is simple in preparation process and good in biocompatibility, but also has thermosensitive properties, and can be applied to the field of the biological medicine of targeted medicine delivery and the like.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Compound oil-absorbing material with flame retardant property and preparation method thereof
InactiveCN106433002AFlame retardantImprove flame retardant performanceQuinoxalineEthylhexyl palmitate
The invention discloses a compound oil-absorbing material with flame retardant property and a preparation method thereof. The compound oil-absorbing material is prepared by the following steps of: adopting polytetrafluoroethylene, dimethylaminoethyl acrylate, ethylhexyl palmitate, o-hydroxybenzoic acid phenyl ester and acetyl tributyl citrate as main components, adding polyvinylpyrrolidone, epoxidized sunflower oil, diphenyl silanediol, siliceous lime, nano microcrystalline ceramic powder, titanium dioxide, ramie fiber, L-glutamic acid diethyl ester hydrochloride, polyphenyl quinoxaline, hydroxygenkwanin, surface active agent and foaming agent, and adopting processes of grinding, sintering, ultrasonic treatment, polymerization, acid leaching, dry-process treatment, extrusion, foaming and granulation and the like. The compound oil-absorbing material disclosed by the invention is excellent in flame-retardant effect, has excellent oil-absorbing effect and mechanical oil-holding effect for crude oil, has no toxicity, can meet the industrial requirements and has better application prospect.
Owner:苏州佰思科节能环保科技有限公司
Radiosynthetic method of subglutamic acid pet imaging agent
Owner:GUANGDONG HUIXUAN PHARMA TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com