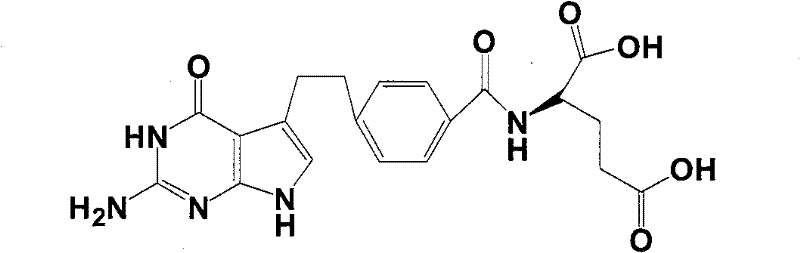
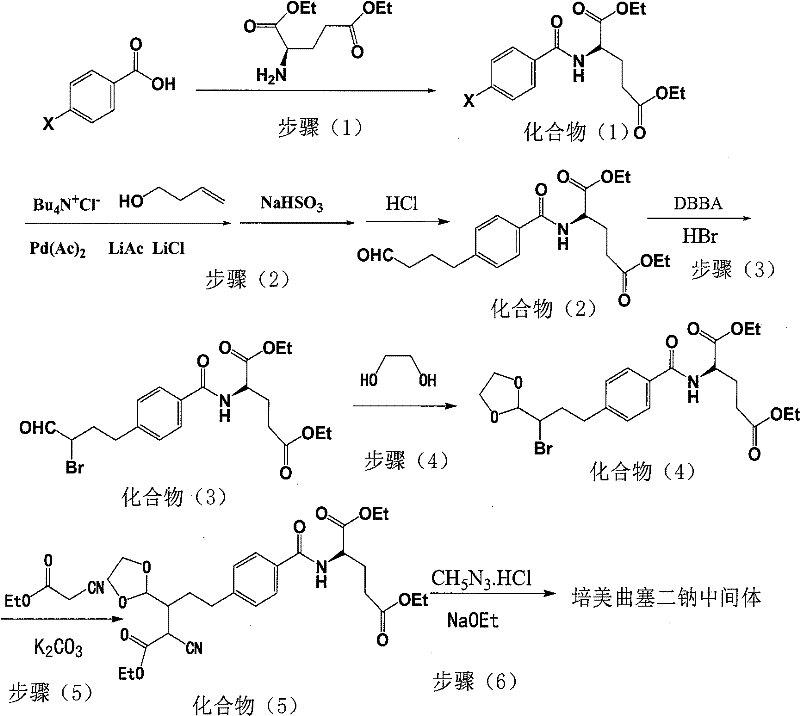
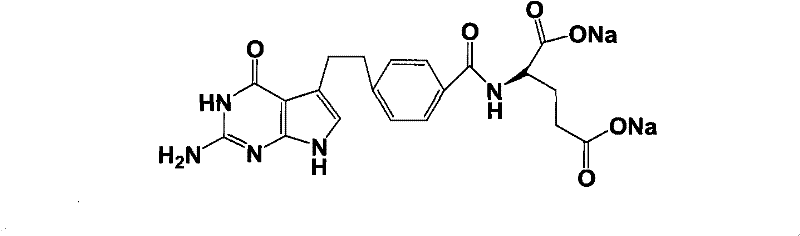
Intermediate of pemetrexed disodium, preparation method thereof and method for preparing pemetrexed disodium thereby
A technology of pemetrexed disodium and intermediates, applied in the field of organic synthesis, can solve the problems of low yield of pemetrexed disodium, unsatisfactory yield of closed-loop reaction, etc. Easy to oxidize, well designed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A preparation method of compound (1), the process is as follows:
[0040] Add 0.1mol of p-bromobenzoic acid and 0.1mol of L-diethyl glutamate into a 500ml reaction flask, then add 300ml of dichloromethane and 10g of CDI (carbonyldiimidazole) to obtain a reaction solution, and keep the reaction solution at -5-0°C After 3 hours of heat preservation reaction, the reaction solution was concentrated to dryness under reduced pressure, and recrystallized with ethyl acetate to obtain 31 g of off-white product 4-bromobenzoyl-L-glutamic acid diethyl ester, which is compound (1). The yield is 80%.
Embodiment 2
[0042] A kind of preparation method of compound (2), its process is as follows: get 1000ml there-necked flask, drop into compound (1) prepared in 0.5mol embodiment 1 successively, 20g lithium acetate, 20g lithium chloride, 12g tetrabutyl ammonium chloride and 600ml DMF, add 0.55mol 3-butenol and 18g palladium acetate under the protection of nitrogen, heat up to 80 ℃ ~ 85 ℃, keep warm for 2 hours, add water and dichloromethane to extract, recover palladium by filtering the organic phase, wash with water, distill under reduced pressure, add Sodium bisulfate aqueous solution, stirred, filtered, the resulting product was hydrolyzed into aldehyde by adding dilute hydrochloric acid, extracted with dichloromethane, concentrated to dryness, and 173.7g of oily 4-butyraldehyde-benzoyl-L-glutamic acid diethyl ester was obtained. It is compound (2), and the yield is 90%.
Embodiment 3
[0044] A kind of preparation method of compound (3), its process is as follows: get 1000ml there-necked flask, drop into compound (2) prepared in 0.5mol embodiment 2, 500ml THF and 0.55molDBBA successively, pass into appropriate hydrogen bromide gas as catalyst, in After reacting for 1 h under stirring at 0-5°C, tetrahydrofuran was distilled off under reduced pressure, washed with water, and extracted with dichloromethane. The organic phase was concentrated to dryness to obtain 203 g of light yellow oil 4-(2-bromobutyraldehyde)-benzoyl-L-glutamic acid diethyl ester as compound (3), with a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com