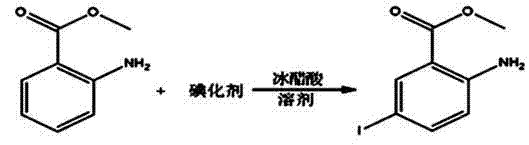
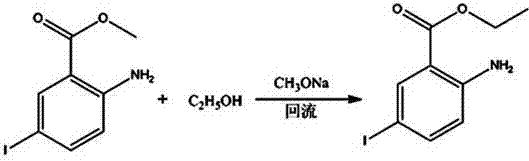
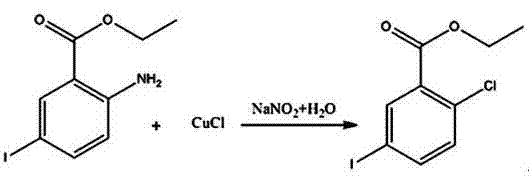
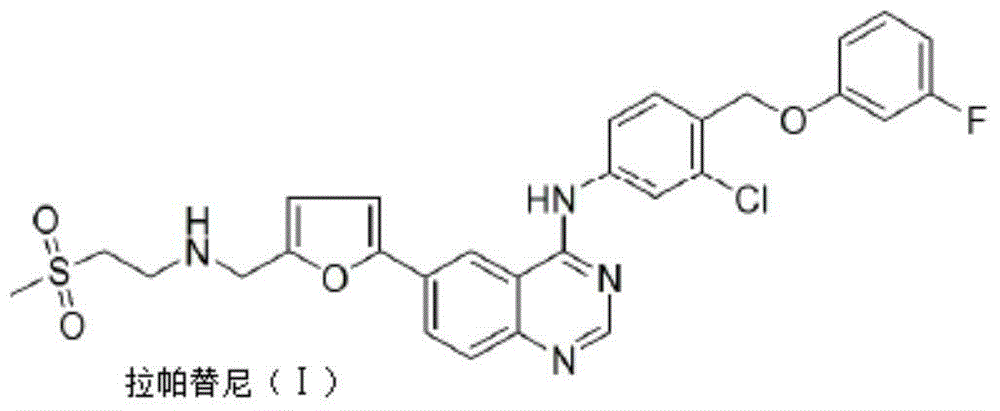
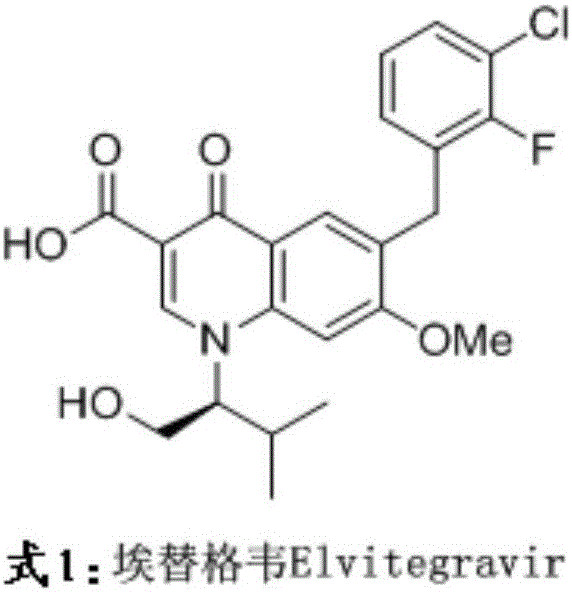
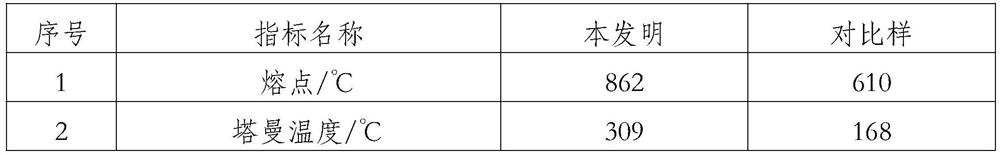
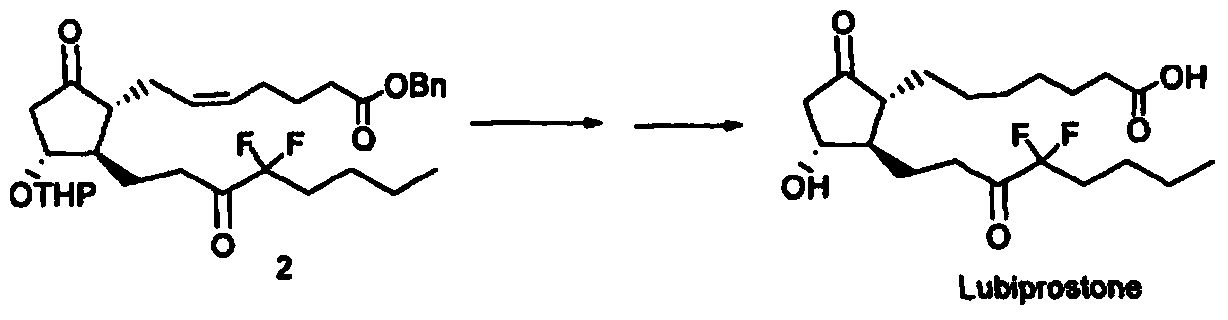Patents
Literature
46 results about "Iodobenzoic Acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
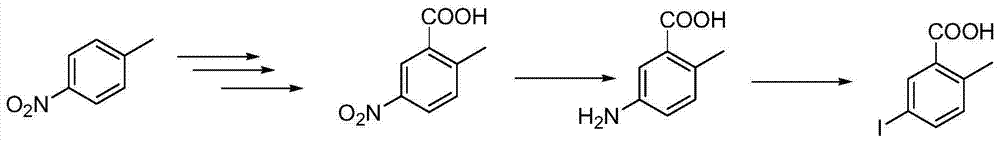
Method For Producing 2-Amino-5-Iodobenzoic Acid
InactiveUS20070219396A1Quality improvementHigh yieldOrganic compound preparationAmino-carboxyl compound preparationCompound (substance)Iodine
A method for producing 2-amino-5-iodobenzoic acid which comprises bringing 2-aminobenzoic acid (A) and molecular iodine (B) into reaction with each other in the liquid phase in the presence of an oxidizing agent. Hydrogen peroxide is preferable as the oxidizing agent. This method does not require a step for purifying 2-amino-5-iodobenzoic acid or a step for recovering iodine, and 2-amino-5-iodobenzoic acid having excellent quality can be produced economically advantageously with a great yield. The product can be advantageously used as an intermediate for drugs, an agricultural chemical and a raw material for functional chemicals.
Owner:MITSUBISHI GAS CHEM CO INC
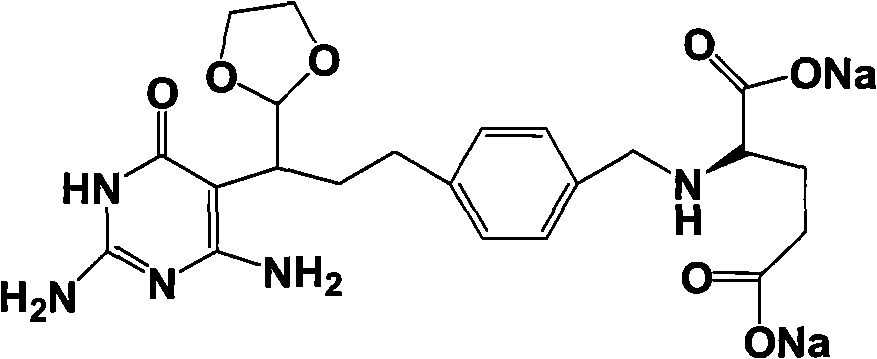
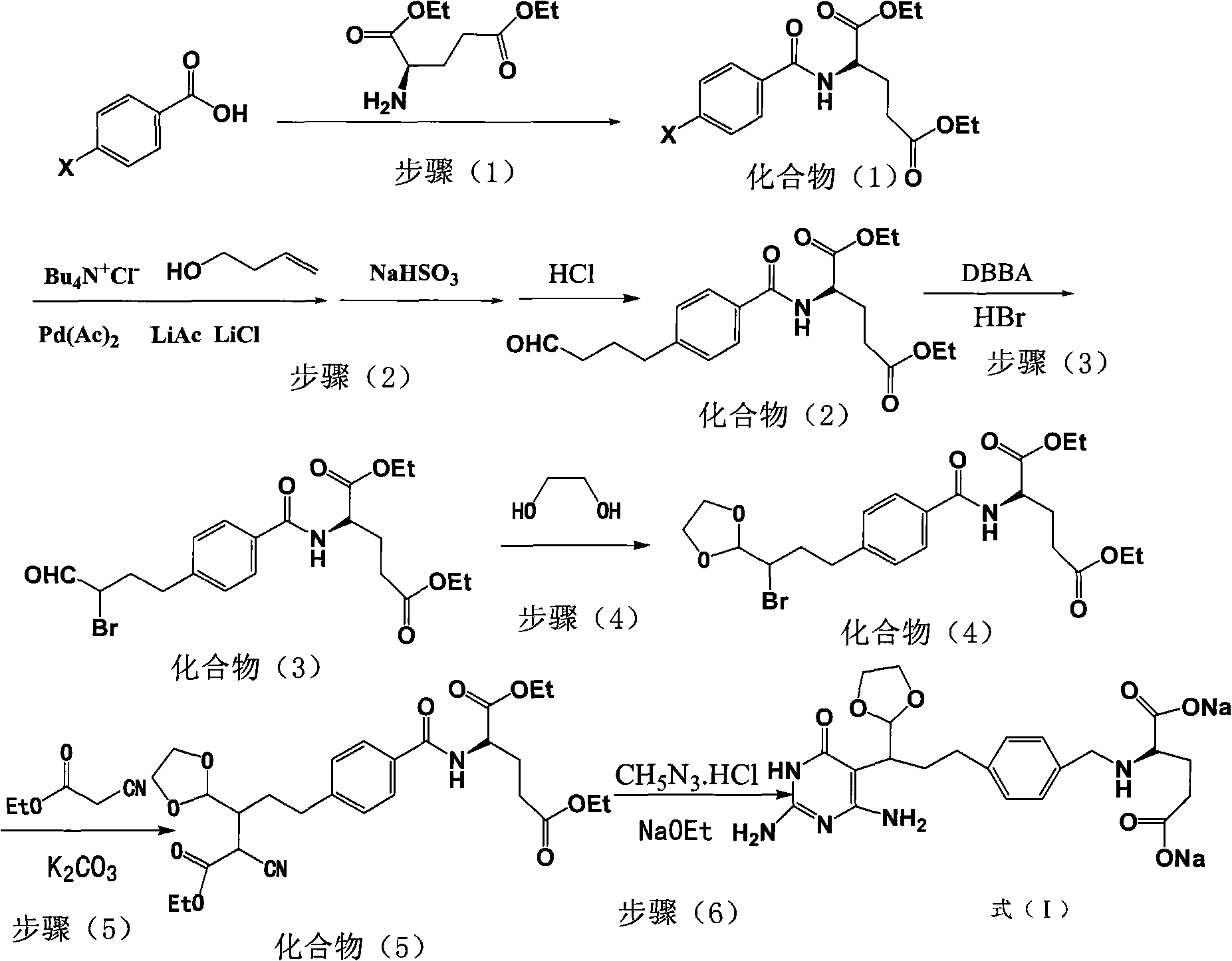
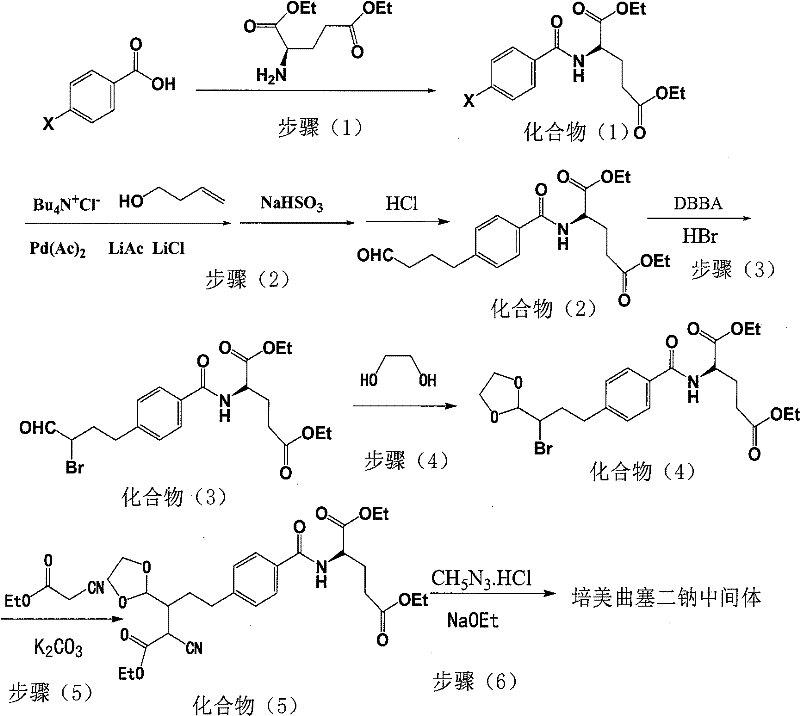
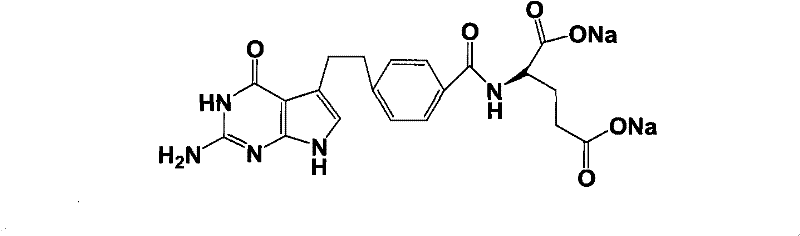
Intermediate of pemetrexed disodium, preparation method thereof and method for preparing pemetrexed disodium thereby
ActiveCN101560206AHigh yieldReduce manufacturing costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsBenzoic acidGlutaric acid
The invention relates to an intermediate of pemetrexed disodium, a preparation method thereof and a method for preparing pemetrexed disodium thereby; and the intermediate is (2-(4-(3-(2,4-diamino-6-oxy-1,6-dihydro-pyridine-5-group)-3-(1,3)dioxolane-2-group-propyl) benzylamine)sodium glutaric acid. The synthesis of the intermediate comprises the following steps: firstly, condensation reaction is conducted on 4-bromobenzoic acid or 4-iodobenzoic acid and L-glutamate diethylester, then Hack reaction is conducted, 4-bromo is replaced and 4-butyraldehyde is formed, then selective bromo replacement is conducted and the 4-butyladehyde is converted into 2-bromobutyraldehyde, and then condensation reaction of aldehyde and ethylene glycol is utilized for protecting the aldehyde, and pyrimidine ring is further synthesized, and finally the intermediate is obtained. Acid hydrolysis ring-closing reaction and sodium hydroxide salification are respectively conducted for once on the intermediate so as to obtain the pemetrexed disodium. The method for preparing pemetrexed disodium in the invention has high yield, low cost and easy operation and is applicable to industrialized production.
Owner:山东立新制药有限公司
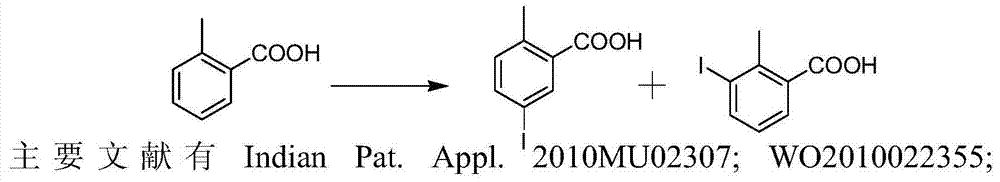
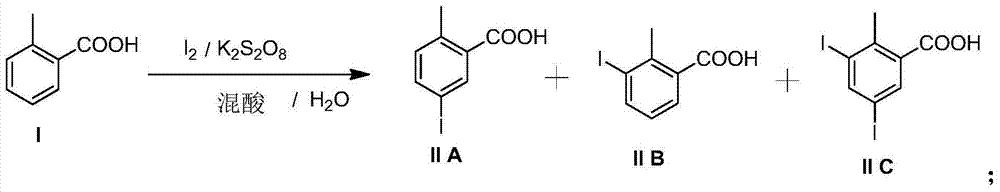
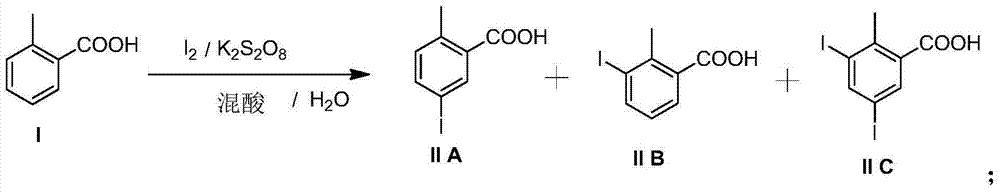
Preparation and recovery method of 2-methyl-5-iodobenzoic acid
The invention provides a preparation and recovery method of 2-methyl-5-iodobenzoic acid. The method comprises the steps: iodinating by using iodine / potassium periodate in a mixed acid solvent and taking otoluic acid as a raw material to obtain a mixture of 2-methyl-5-iodobenzoic acid, 2-methyl-3-iodobenzoic acid and 2-methyl-3, 5-diiodosalicylic acid; refining to obtain 2-methyl-5-iodobenzoic acid and a mother solution regenerant, wherein the mother solution regenerant is the mixture of 2-methyl-5-iodobenzoic acid, 2-methyl-3-iodobenzoic acid and 2-methyl-3, 5-diiodosalicylic acid; recovering otoluic acid and iodine ions through catalytic hydrogenation deiodination; oxidizing the iodine ions for recovering iodine. The whole process is circularly utilized. The preparation and recovery method has the advantages that the raw material is easy to obtain and low in price, the preparation process is simple in operation, the equipment requirement is low, and the method is suitable for large-scale industrial production.
Owner:ABA CHEM CORP
NON type chiral bisoxazoline ligand nd synthesis method and application thereof
ActiveCN111233852ASynthetic conditions are mildEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsBenzoic acidIobenzamic acid
The invention relates to an NON type chiral bisoxazoline ligand and a synthesis method and application thereof. The ligand has a bisoxazoline structure as shown in a general formula 1, the synthesis method of the NON type chiral bisoxazoline ligand comprises the following steps: taking an o-iodobenzoic acid compound as an initial raw material, performing acylating chlorination on a benzoisofuranylalkylene dicarboxylic acid skeleton prepared through multi-step reaction, performing condensation with chiral amino alcohol, and finally performing cyclization to obtain a ligand 1 which is used for catalytic synthesis of chiral fluorinated beta-ketone ester. Compared with the prior art, the synthesis method disclosed by the invention is simple and efficient, mild in synthesis condition and easy to operate and good in repeatability, and the corresponding metal complex shows good catalytic activity and stereoselectivity in the asymmetric fluorination reaction of beta-ketone ester.
Owner:SHANGHAI NORMAL UNIVERSITY
Preparation method of key intermediate of anti-hepatitis C drug ledipasvir
InactiveCN109678686ARaw materials are easy to getLow priceOrganic compound preparationCarboxylic acid esters preparationN-methylacetamideIobenzamic acid
The invention provides a preparation method of a key intermediate 1-(7-bromo-9,9-difluoro-9H-fluoren-2-yl)-2-chloroethanone. The method comprises the steps as follows: 2-amino-5-bromobenzoic acid is taken as a raw material, and subjected to diazotization, iodination,synthesis of 5-bromo-2-iodobenzoic acid, methylation, coupling reaction with phenylboronic acid, ester hydrolysis, acyl chlorination,intramolecular Friedel-Crafts alkylation, carbonyl reduction, iodization, fluorination and final reaction with 2-chloro-N-methoxy-N-methylacetamide to prepare the target product. The process adopts easily available starting raw materials, is low in price and free of hazardous process and has mild reaction conditions..
Owner:IANGSU COLLEGE OF ENG & TECH
Synthesis method of 2-halogen-5-iodobenzoic acid
ActiveCN110078613AReduce pollutionImprove product qualityOrganic compound preparationCarboxylic compound preparationBenzoic acidHalogen
The invention discloses a synthesis method of 2-halogen-5-iodobenzoic acid. The synthesis method comprises steps as follows: o-halogen benzoic acids are subjected to one-step iodo treatment in an iodio reagent, and 2-halogen-5-iodobenzoic acid is obtained. Aftertreatment comprises steps as follows: a reaction liquid is poured into a cold reductive water solution and quenched, a solvent is evaporated to dryness, recrystallization and filtration are performed, and a product can be obtained. The method is short in reaction route, simple to operate, environmentally friendly, safer and more economical and has broad application prospects.
Owner:杭州科耀医药科技有限公司
A synthetic method of 2-chloro-5-iodobenzoic acid
InactiveCN104193616ASimple processEasy to operateOxygen-containing compound preparationOrganic compound preparationSandmeyer reactionBenzoic acid
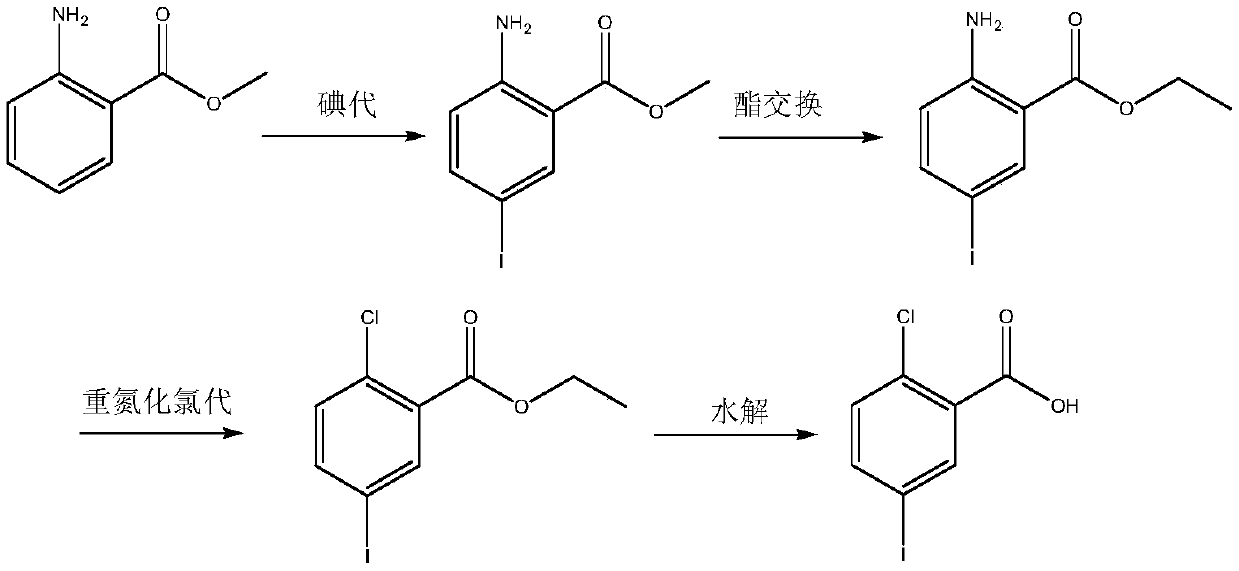
A synthetic method of 2-chloro-5-iodobenzoic acid is disclosed. The target product is obtained by subjecting methyl 2-aminobenzoate to iodination, substitution, a Sandmeyer reaction and hydrolysis under alkaline conditions. The method is characterized by modification or optimization of process steps and parameters based on traditional iodination, substitution, the Sandmeyer reaction and hydrolysis. The method is simple in process, easy in operation, safe in production process, free of pollution, and high in yield of each step. The purity of the product is 95-98%. The total yield of the product is 64-70%.
Owner:姜树林
Lapatinib preparation method
ActiveCN105111193AFew reaction stepsReduce manufacturing costOrganic chemistrySynthesis methodsKetone
The invention discloses a lapatinib preparation method. In the synthesis method, the initial raw materials of 2-amino-5-iodobenzoic acid and a cyclization reagent are used for preparing a midbody of 6-iodine-3,4-dihydroquinazoline-4-ketone (III), quinazoline sulfide (V) is generated through the midbody of 6-iodine-3,4-dihydroquinazoline-4-ketone (III) under the condition of sulpho-reagent and methine halide, and a target molecule is further synthesized. Due to reaction, the lapatinib yield of a final product is increased, generation of an unstable midbody of 4-chloroquinazoline product is avoided, meanwhile, use of corrosive phosphorus trichloride, phosphorus pentachloride, thionyl chloride, phosgene or phosphorus oxychloride and other chlorinating agents is avoided, and the lapatinib preparation method is suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
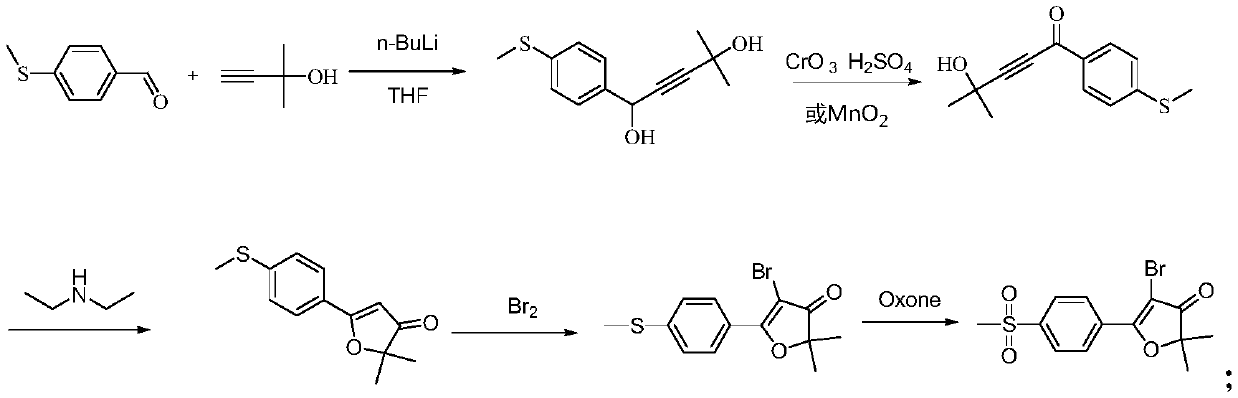
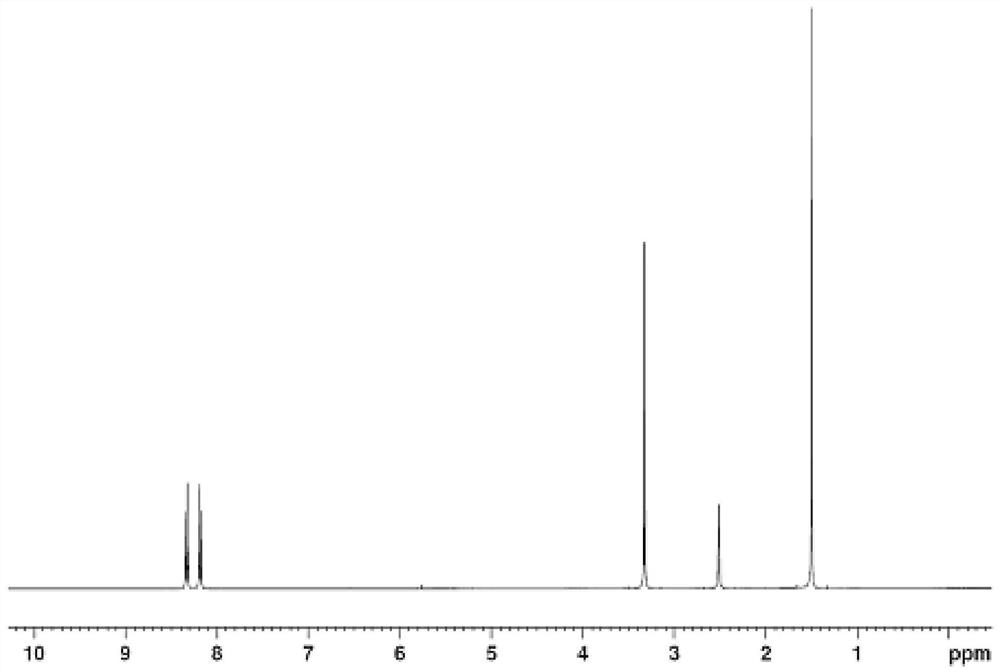
Synthesis method of 5-bromo-2, 2-dimethyl-5-(4-methylsulfonylphenyl) furan-3 (2H)-one
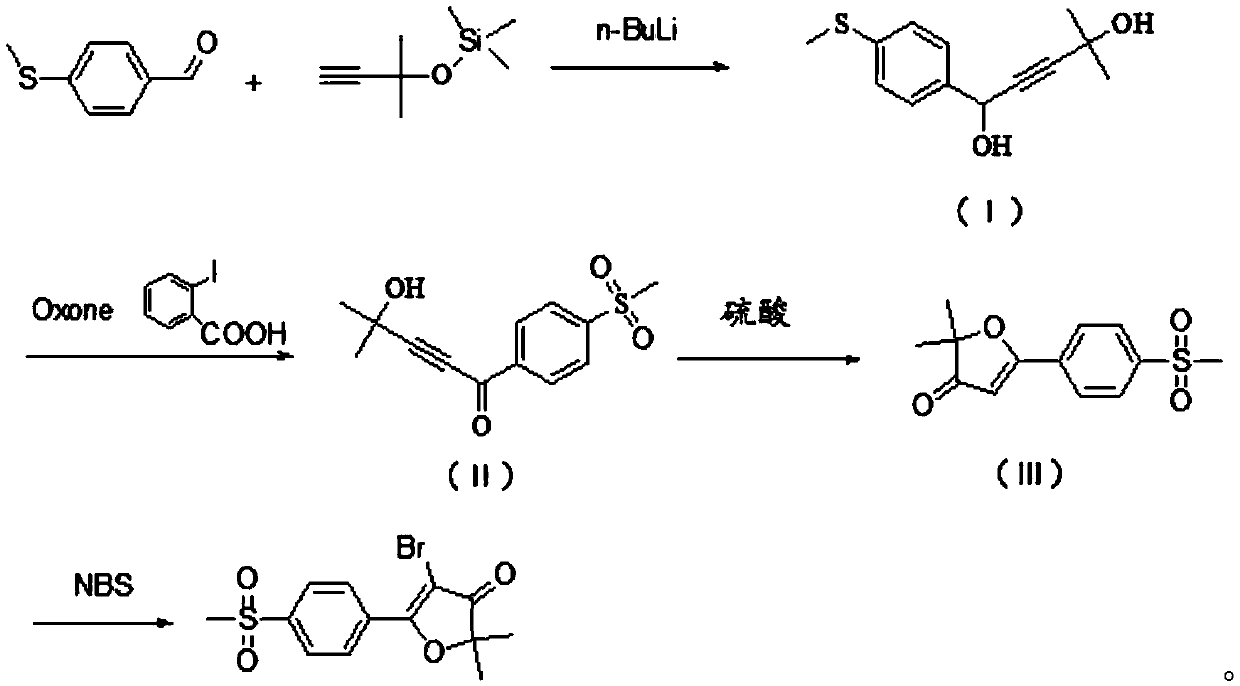
The invention discloses a synthesis method of 5-bromo-2, 2-dimethyl-5-(4-methylsulfonylphenyl) furan-3 (2H)-one, which belongs to the technical field of organic synthesis, and comprises the followingsteps: reacting 4-methylthiobenzaldehyde with a lithium salt of [(1, 1-dimethyl-2-propynyl) oxy] trimethylsilane to generate a compound shown in a formula (I); carrying out oxidation reaction on the compound shown in the formula (I), Oxone and o-iodobenzoic acid to generate a compound shown in a formula (II); reacting the compound shown in the formula (II) with sulfuric acid to generate a compoundshown in a formula (III); and reacting the compound shown in the formula (III) with NBS to obtain a target product. The method has the advantages of simple raw materials, low price, simple operation,short production period, no generation of chromium acidic wastewater, nitrogen-containing wastewater and the like, small environmental protection pressure, and easy realization of industrial production.
Owner:XIAN RUILIAN NEW MATERIAL CO LTD
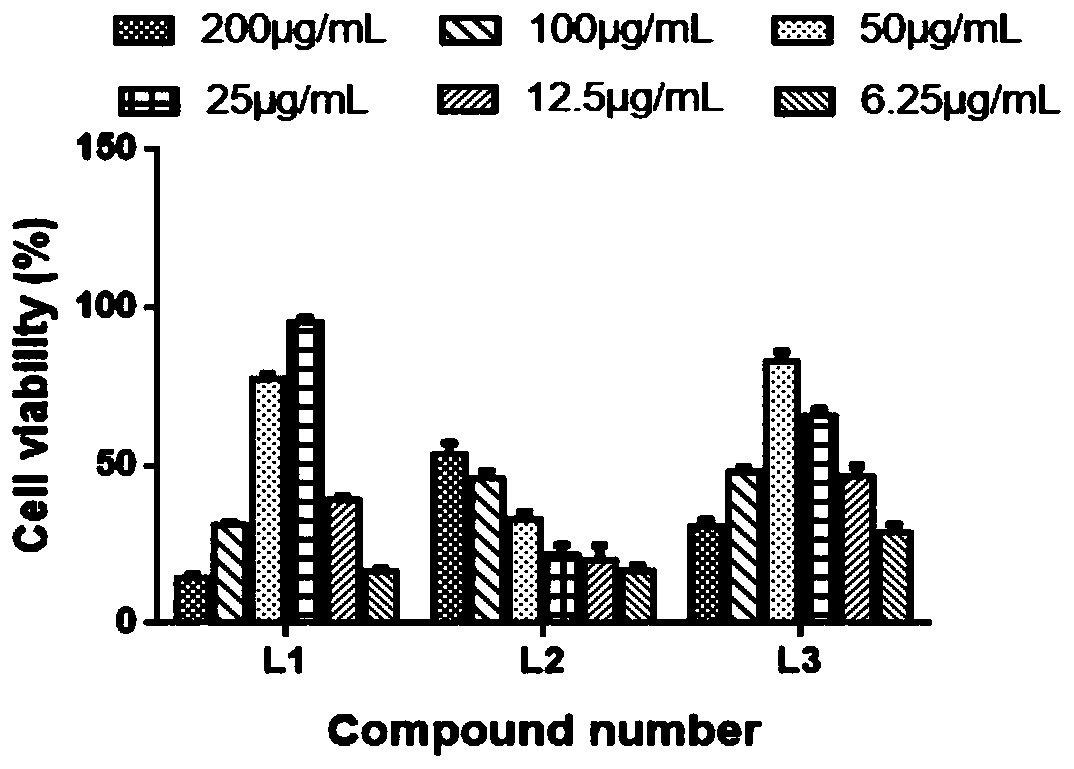
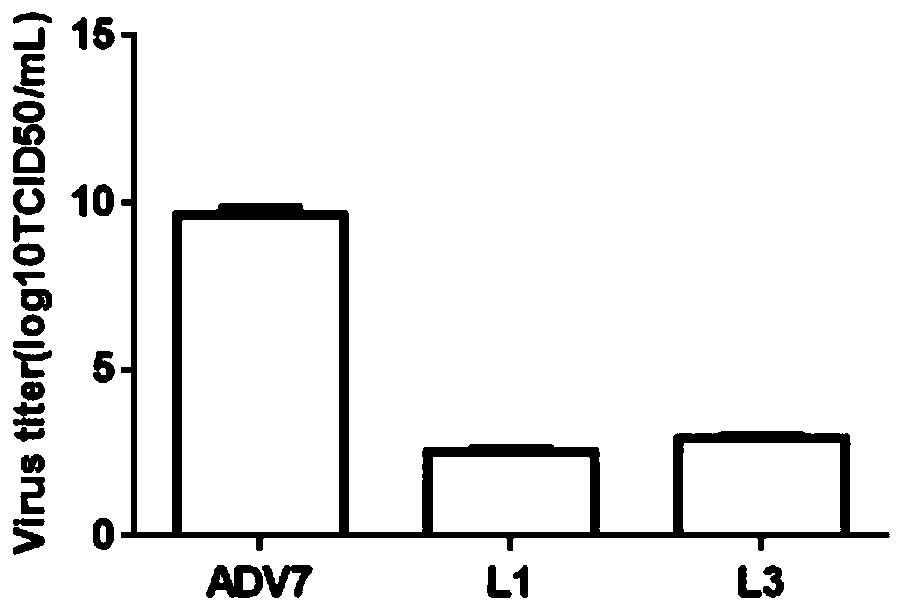
Monoiodobenzoic acid compound and application thereof in resisting ADV7 virus
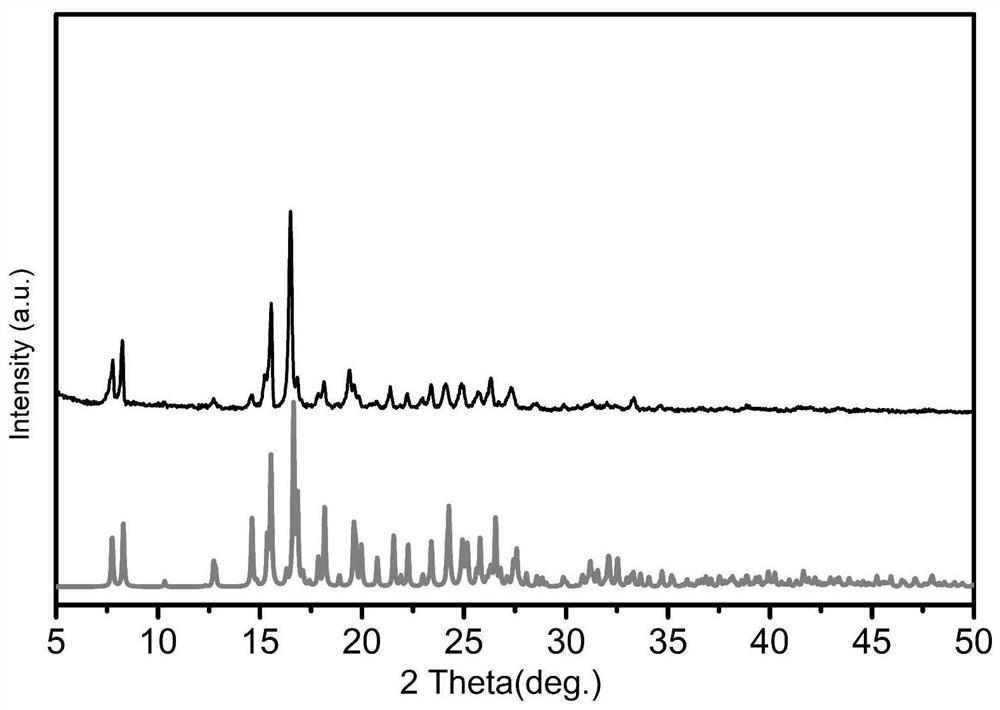
ActiveCN111012770AThe synthesis process is simpleEconomical and fastOrganic active ingredientsAntiviralsBenzoic acidCytopathic effect
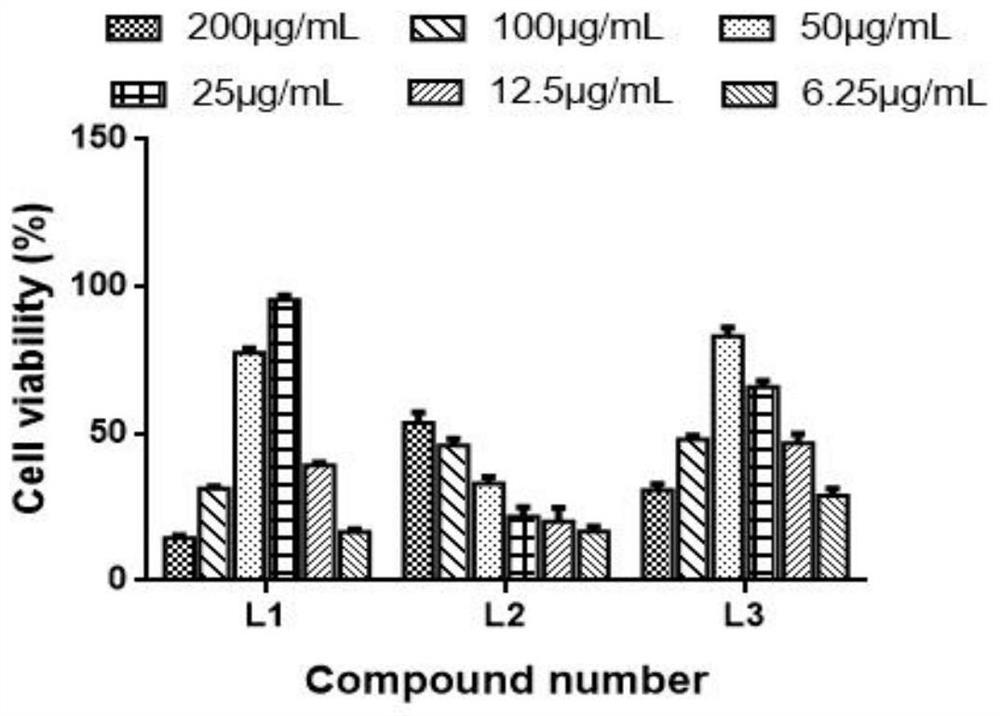
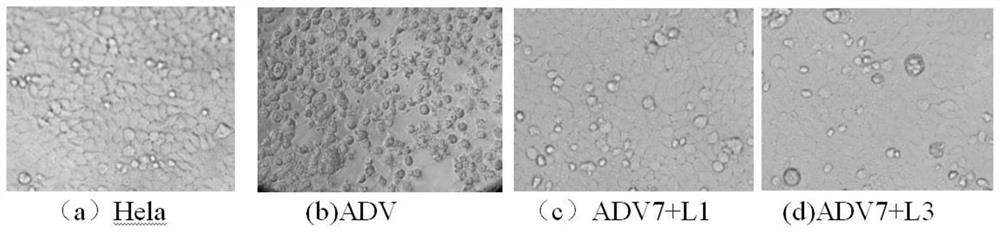
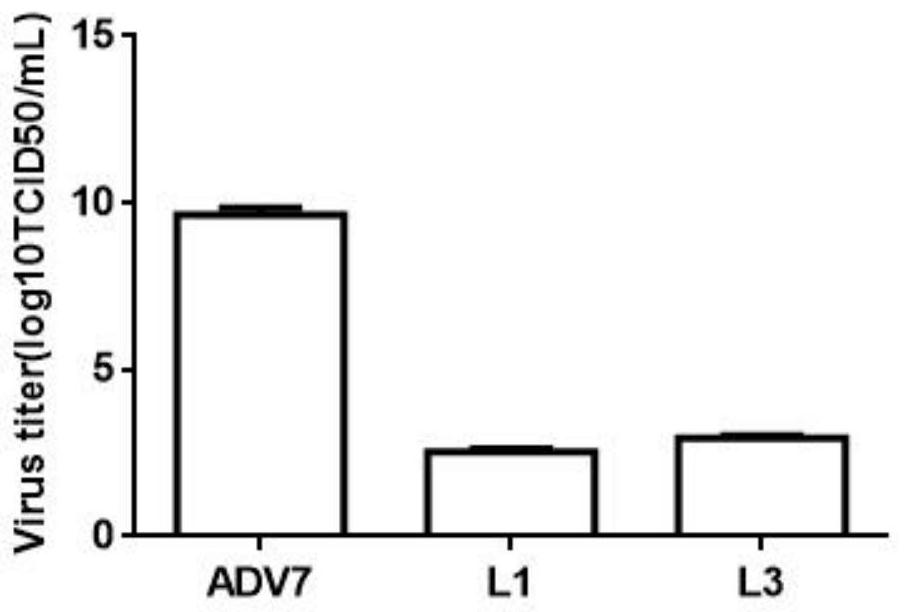
The invention discloses a monoiodobenzoic acid compound and an application thereof in resisting ADV7 virus. Through the research experiments on anti-ADV7 activity by monoiodobenzene comprising o-iodobenzoic acid (L1), m-iodobenzoic acid (L2) and p-iodobenzoic acid (L3), the compounds L1 and L3 inhibit cytopathic effect (CPE) generated by ADV7 on host cells Hela, enhance cell survival rate and reduce filial generation virus yield, and can be applied to preparation of anti-ADV7 virus drugs.
Owner:HUBEI UNIV OF TECH
Preparation method of 2,4-difluoro-5-iodobenzoic acid
ActiveCN106008195AMild temperatureSimple post-processingOrganic compound preparationCarboxylic compound preparationIodination reactionIodobenzoic Acids
The invention discloses a preparation method of 2,4-difluoro-5-iodobenzoic acid. 2,4-difluorobenzoic acid is taken as a starting material, sodium percarbonate and elemental iodine are taken as green iodination reagents, a mild iodination reaction is performed under an acidic condition, and a high-purity target product is obtained. The synthetic process is environment-friendly and suitable for large-scale industrial production, and aftertreatment is convenient.
Owner:苏州智高嘉华科技有限公司
Photoinduced nonlinear expansion coordination polymer and preparation method thereof
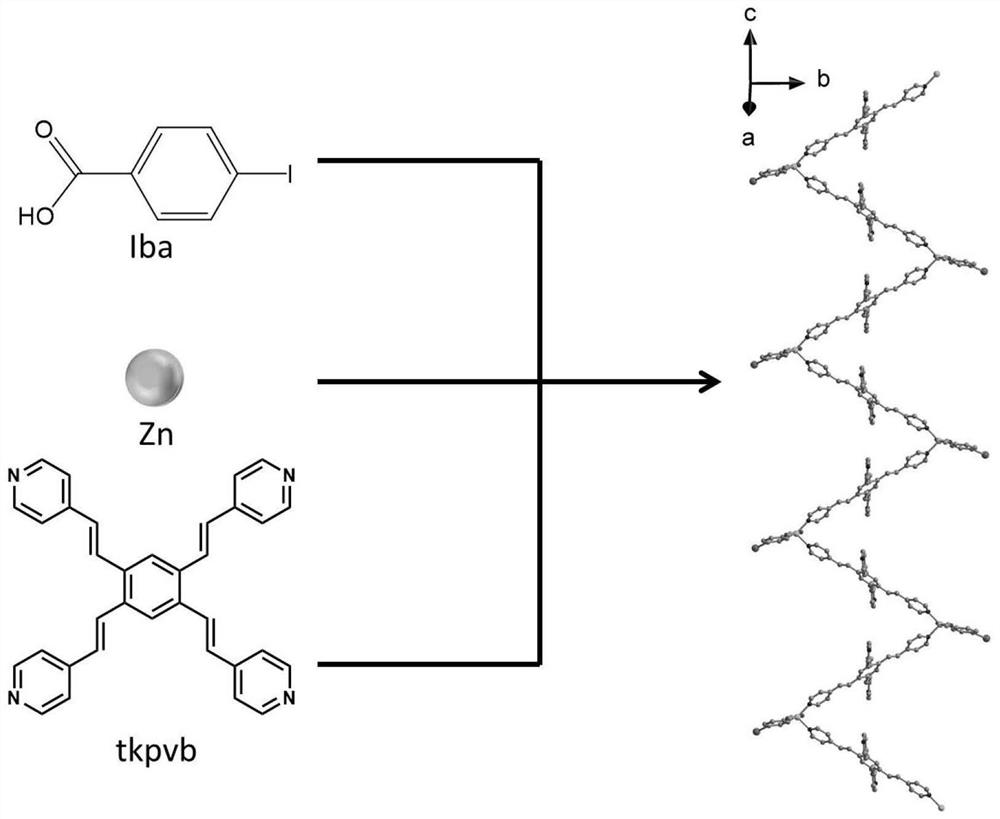
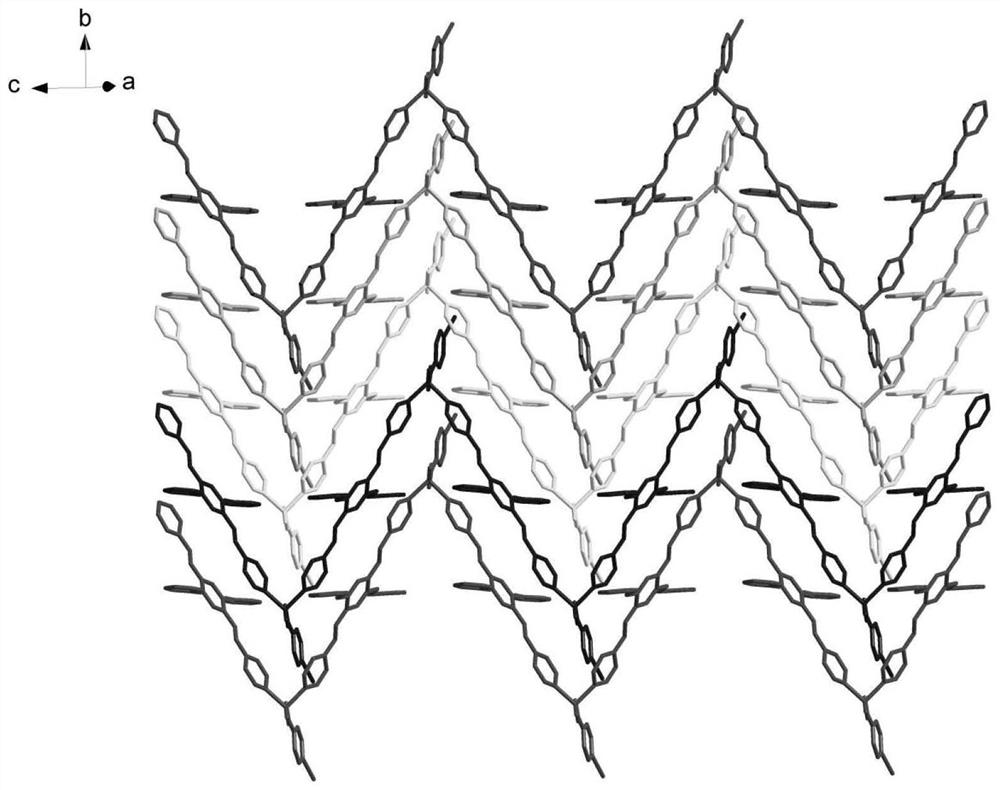
The invention belongs to the technical field of photosensitive materials, and particularly relates to a photoinduced nonlinear expansion coordination polymer and a preparation method thereof. The coordination polymer is a bright yellow bulk crystal, and the chemical formula of the coordination polymer is [Zn (iba) (tkpvb) Cl] n1, wherein iba represents p-iodobenzoic acid radical, tkpvb represents 1, 2, 4, 5-tetra ((E)-2-(4-pyridyl) vinyl) benzene, and n is equal to 3000-60000; crystal parameters are as follows: (1) the crystal system is a monoclinic system; (2) the space group is Cc; (3) the values of a, b, c and V are shown in the description, beta is equal to 127.430(4) degrees; (4) Z is equal to 4; (5) F (000) is equal to 1680, R1 is equal to 0.1363, wR2 is equal to 0.3788, and GOF is equal to 1.620; and iba represents a p-iodobenzoic acid radical, tkpvb represents 1, 2, 4, 5-tetra ((E)-2-(4-pyridyl) vinyl) benzene, and n1 is equal to 3000 to 60000. The preparation method of the coordination polymer is simple, the reaction condition is mild, and the light conversion rate is high; and meanwhile, addition reaction can be carried out under irradiation of light with different wavelengths, the light-induced nonlinear expansion performance of the material is shown, and a corresponding isomeride compound is obtained.
Owner:SUZHOU UNIV
Monoiodobenzoic acid compounds and their application in anti-adv7 virus
ActiveCN111012770BThe synthesis process is simpleEconomical and fastOrganic active ingredientsAntiviralsBenzoic acidPharmaceutical drug
The invention discloses a monoiodobenzoic acid compound and its application in anti-ADV7 virus. Through the mono-iodobenzoic acid (L1), meta-iodobenzoic acid (L2), p-iodobenzoic acid (L3) anti-ADV7 activity research experiments, compounds L1, L3 inhibit the cells produced by ADV7 on the host cell Hela Pathogenic effect (CPE), enhance cell survival rate, reduce progeny virus production, and can be used in the preparation of anti-ADV7 virus drugs.
Owner:HUBEI UNIV OF TECH
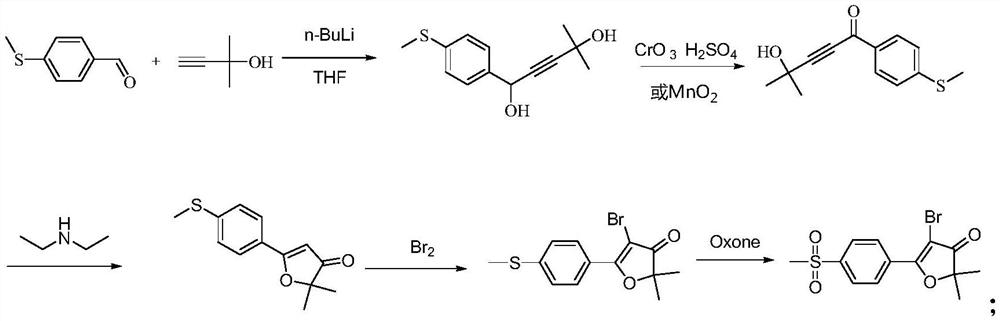
Synthesis of 5-bromo-2,2-dimethyl-5-(4-methylsulfonylphenyl)furan-3(2h)-one
The invention discloses a synthesis method of 5-bromo-2,2-dimethyl-5-(4-methylsulfonylphenyl)furan-3(2H)-one, which belongs to the technical field of organic synthesis and comprises the following steps : 4-methylthiobenzaldehyde reacts with lithium salt of [(1,1-dimethyl-2-propynyl)oxy] trimethylsilane to generate formula (I) compound; formula (I) compound Oxidation reaction with Oxone and o-iodobenzoic acid generates the compound of formula (II); the compound of formula (II) reacts with sulfuric acid to generate the compound of formula (III); the compound of formula (III) reacts with NBS to obtain the target product; the present invention The raw material is simple, the price is low, the operation is simple, the production cycle is short, and the generation of chromium-acid waste water and nitrogen-containing waste water is avoided, the environmental protection pressure is small, and industrial production is easy to realize.
Owner:XIAN RUILIAN NEW MATERIAL CO LTD
Intermediate of pemetrexed disodium, preparation method thereof and method for preparing pemetrexed disodium thereby
ActiveCN101560206BHigh yieldReduce manufacturing costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsGlutaric acidL glutamate
Owner:山东立新制药有限公司
Preparation method of 2-bromo-5-iodobenzoic acid
InactiveCN107673990AHigh yieldLow priceOrganic compound preparationAmino-carboxyl compound preparationIodobenzoic AcidsIodination reaction
The invention provides a preparation method of 2-bromo-5-iodobenzoic acid. The preparation method comprises the following steps: mixing 5-amino-2-bromobenzoic acid, inorganic acid, an organic solventand water to obtain 5-amino-2-bromobenzoic acid mixed liquid; (2) dropwise adding a nitrite aqueous solution into the 5-amino-2-bromobenzoic acid mixed liquid for carrying out diazo reaction to obtaina diazo salt system; (3) dropwise adding an iodizating agent aqueous solution into the diazo salt system for carrying out iodination reaction; (4) after the iodination reaction is finished, adding asodium hydrogen sulfite aqueous solution into the system for quenching to obtain the 2-bromo-5-iodobenzoic acid. According to the preparation method, the 5-amino-2-bromobenzoic acid is taken as a rawmaterial and is firstly subjected to diazotization, and diazotized diazo salt is then taken as a raw material and is subjected to the iodination reaction, so that the utilization rate of the 5-amino-2-bromobenzoic acid is greatly improved, and the yield of the 2-bromo-5-iodobenzoic acid is further increased.
Owner:SHANGHAI TWISUN BIO PHARM
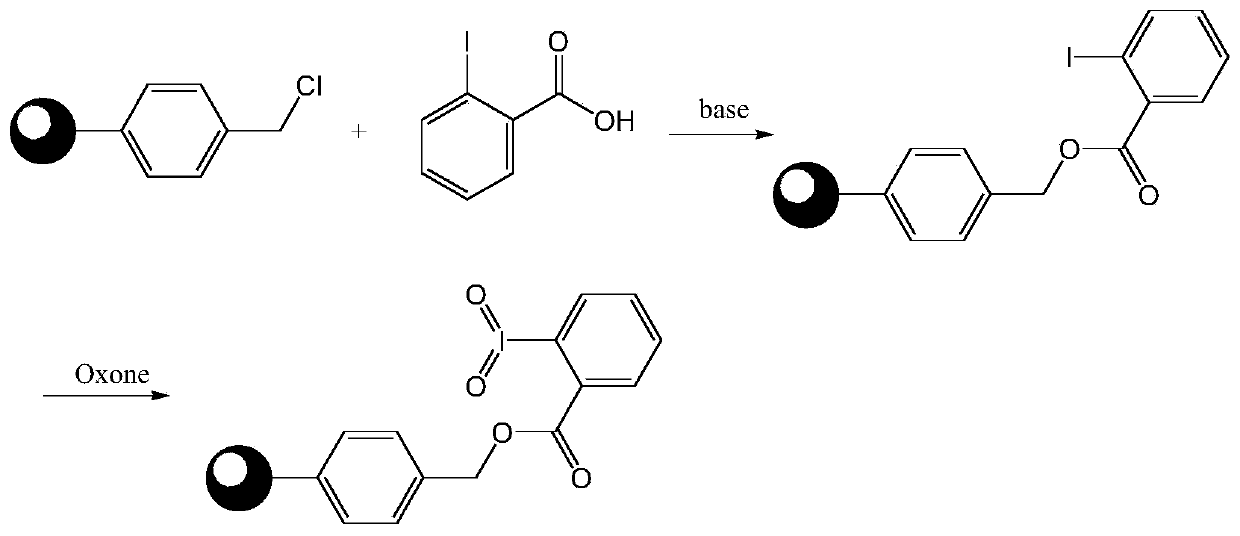
Macromolecule-loaded oxidizing agent containing iodine with high valence, and preparation method and application thereof
PendingCN111116333AEasy to manufactureReduce the impactOrganic compound preparationCarbonyl compound preparationBenzoic acidIobenzamic acid
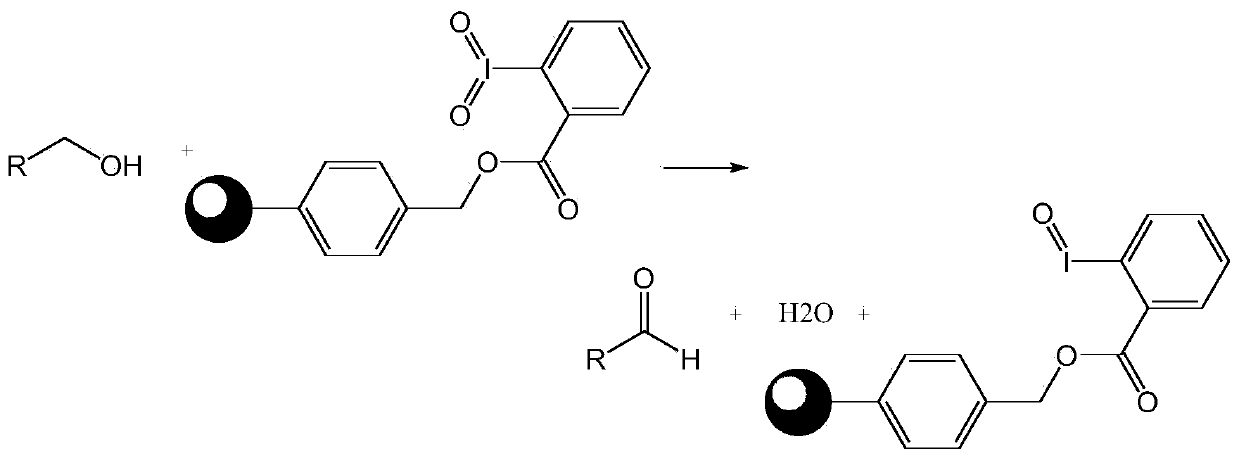
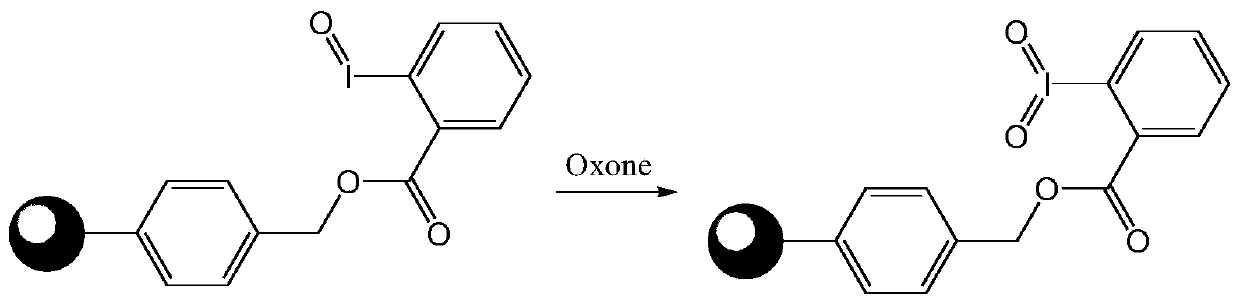
The invention discloses a macromolecule-loaded oxidizing agent containing iodine with high valence, and a preparation method and an application thereof. 2-iodobenzoic acid is used as a raw material and is loaded on chloromethyl polystyrene resin under an alkaline condition, and iodine is oxidized into high valence by using potassium monopersulfate. The macromolecule-loaded oxidizing agent containing iodine with high valence, prepared through the method can oxidize primary alcohol into aldehyde and, secondary alcohol into ketone, and a reaction byproduct is water; the reaction conditions are mild, the post-treatment is convenient, and the macromolecular-loaded oxidizing agent containing iodine with high valence can be separated from the reaction system only by simple filtration; and the macromolecule-loaded oxidizing agent containing iodine with high valence can be recycled for multiple times, so that the influence on the environment and the production cost are reduced. Besides, the macromolecular-loaded oxidizing agent containing iodine with high valence is simple and convenient to prepare, effectively solves the problems of stability and high cost of a micromolecular iodine-containing oxidizing agent, and has a wide application prospect in the field of fine chemical engineering.
Owner:PANASIA OLAUGHLIN BIO TECH WUHAN CO LTD
Preparation method for prostaglandin intermediate
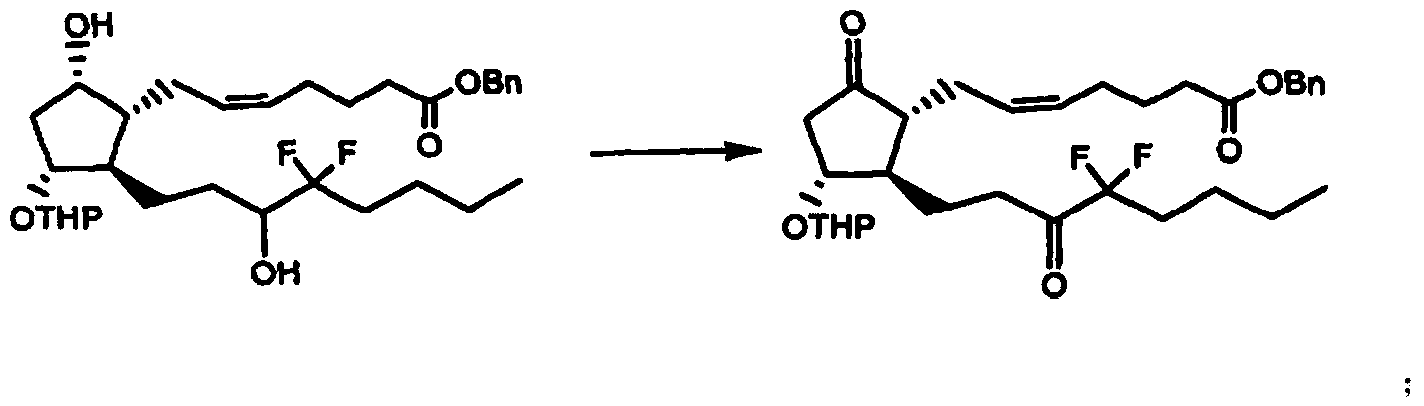
ActiveCN102050808BSimple and fast operationThe preparation method is mildGroup 4/14 element organic compoundsSilanesIodobenzoic Acids
The invention discloses a preparation method for a prostaglandin intermediate. The method comprises the step of: (1) mixing a compound represented by a formula II and a substitutive o-iodobenzoic acid type compound represented by a formula III to obtain a compound represented by a formula I, wherein R1 is C1-C4 straight chain or alkyl, phenyl or substitutive phenyl which contains branch chains; R2 is tetrahydropregnenolone (THP), silane radical substituted by trialkyl, alkyl acyl, benzoyl or benzoyl of which the benzene ring is provided with a substituent; and R3 is acetoxy.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Preparation method of o-iodobenzoic acid
InactiveCN112225662AEase of production and purchaseProduction and operation will not be affectedOxygen-containing compound preparationOrganic compound preparationBenzoic acidBiochemical engineering
The invention relates to a preparation method of o-iodobenzoic acid. According to the invention, methyl anthranilate is used as an initial raw material instead of anthranilic acid, so that the management of I type easily-prepared toxic raw materials of anthranilic acid is avoided; and according to product properties, methyl o-iodobenzoate is purified in a distillation manner by using a new purification method, so that the purity is high, and the safety is high.
Owner:苏州华鑫医药科技有限公司
Polyiodinated aromatic acid compound and application thereof in resisting adenovirus type 7
ActiveCN111116395AInhibition of replication proliferationInhibition of lesion effectOrganic active ingredientsOrganic chemistryBenzoic acidCytopathic effect
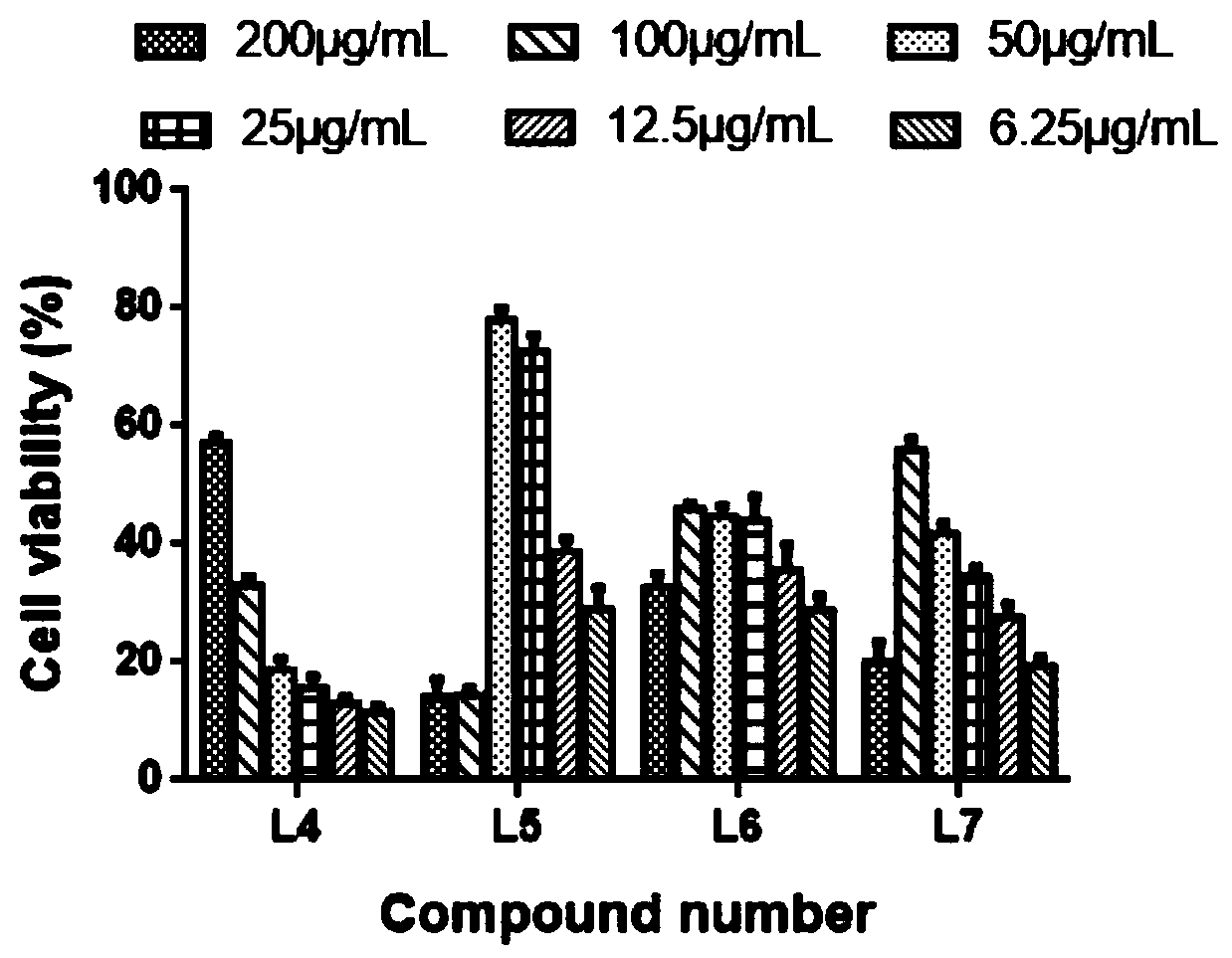
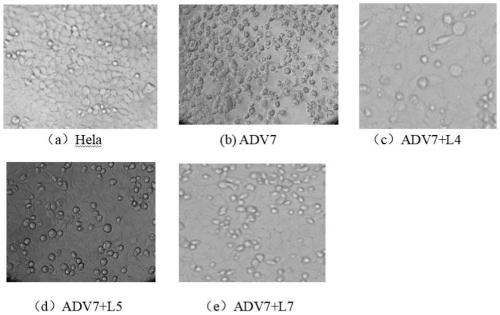
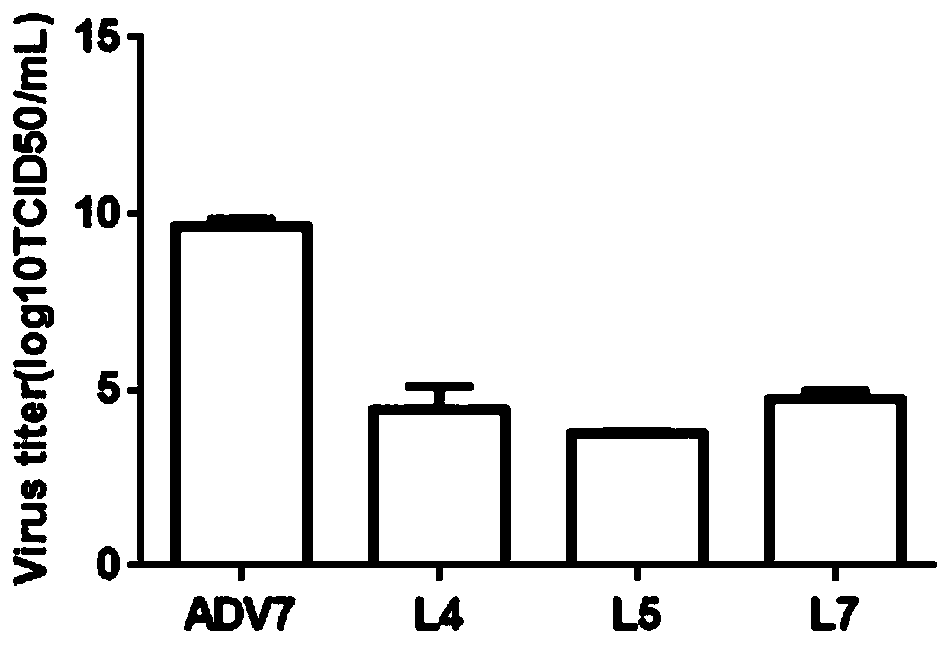
The invention discloses a polyiodinated aromatic acid compound and an application thereof in resisting adenovirus type 7. Anti-ADV7 virus activity research experiments of polyiodinated carboxylic acidcomprising 3-amino-4-iodobenzoic acid (L4), 3,4-diiodobenzoic acid (L5), 3,5- diiodosalicylic acid (L6) and 2,3,5-triiodobenoic acid (L7) show that the compounds L4, L5 and L7 inhibit cytopathic effect (CPE) generated by ADV7 on host cells Hela, the cell survival rate is increased, the yield of filial generation viruses is reduced, and the compounds can be applied to preparation of anti-ADV7 virus drugs.
Owner:HUBEI UNIV OF TECH
Quinazolinone derivatives and their preparation and application
InactiveCN101792440BSignificant PTP1B inhibitory activityImprove mechanical propertiesOrganic active ingredientsOrganic chemistryProtein Tyrosine Phosphatase 1BIodobenzoic Acids
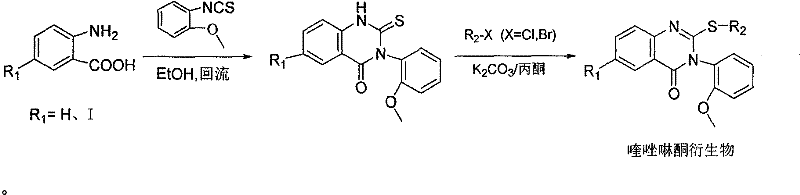
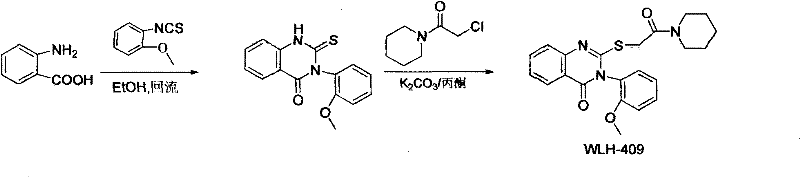
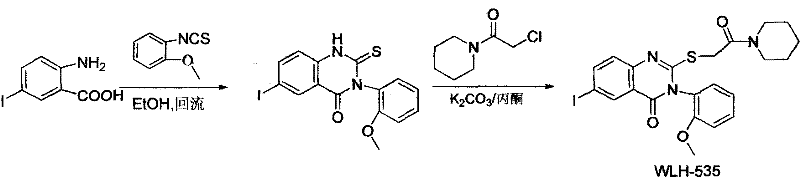
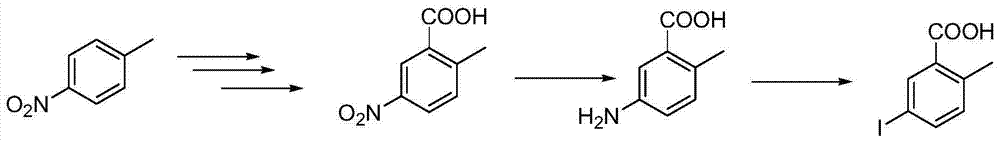
A class of quinazolinone derivatives and their preparation and application, the application refers to as a protein tyrosine phosphatase 1B (abbreviated PTP1B) inhibitor, belongs to the technical field of medicine and its preparation and application, with 2-amino-5 - Iodobenzoic acid or anthranilic acid as raw materials react with 2-methoxyphenylthioisocyanate to form a ring, and then undergo substitution reactions with various halogenated reagents to obtain a class of quinazolinone derivatives. The compound has significant PTP1B inhibitory activity, and is particularly suitable as a potential antidiabetic drug.
Owner:EAST CHINA NORMAL UNIV
Clozapine impurity as well as preparation method and application thereof
InactiveCN108129347AHigh purityHigh yieldOrganic compound preparationPreparing sample for investigationHalogenPhosphate
The invention discloses a clozapine impurity and a preparation method thereof. The preparation method comprises the following steps: carrying out a reaction by taking o-iodobenzoic acid and 4-chloro-2-nitroaniline as starting reactants and using one or more of halogen copper salts, carbonates and phosphates as catalysts so as to generate an intermediate SM1; mixing the intermediate SM1, oxalyl chloride and N-methyl piperazine, and using a one-pot method to synthesize an intermediate SM2; reducing the intermediate SM2 in an acid environment by using metal powder so as to obtain the clozapine impurity. The invention discloses a clozapine impurity compound and a preparation method thereof for the first time, and the technology of the preparation method of the impurity appears for the first time; furthermore, the method is simple to operate and easy in aftertreatment; the prepared clozapine impurity is high in purity and yield.
Owner:艾希尔(深圳)药物研发有限公司
Synthetic method of empagliflozin intermediate
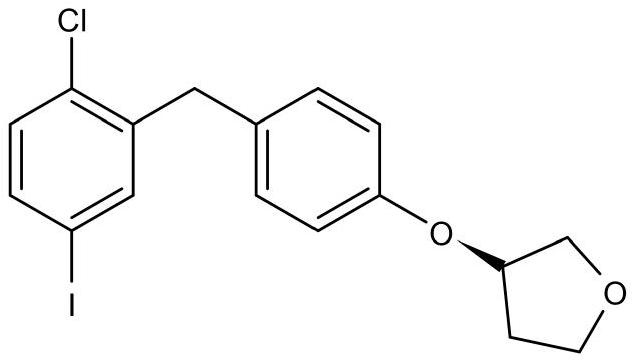
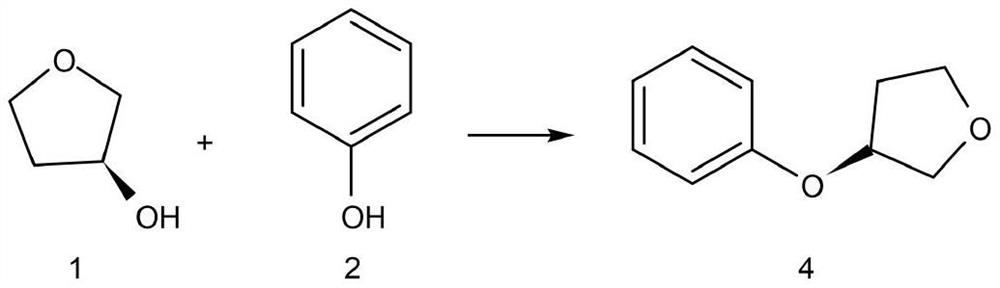
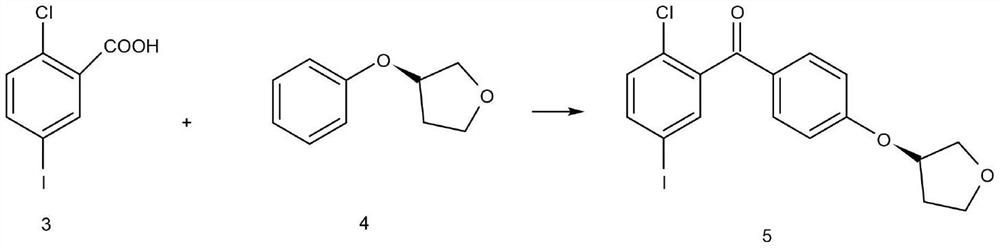
The invention discloses a synthesis method of an empagliflozin key intermediate. The preparation method comprises the following steps: generating (S)-3-phenoxy tetrahydrofuran from (S)-3-hydroxytetrahydrofuran and phenol under the action of BINAP and MTAD, then carrying out acylation reaction on the (S)-3-phenoxy tetrahydrofuran and 2-chloro-5-iodobenzoic acid to obtain 2-3 (5-iodo-2-chlorphenyl) [4-[[(3S)-tetrahydro-3-furyl] oxy] phenyl] ketone, and finally reducing to obtain the (S)-3-(4-(5-iodo-2-chlorobenzyl) phenoxy) tetrahydrofuran. Compared with the prior art, the method has the advantages of short reaction steps, mild conditions, simple post-treatment and high product yield.
Owner:山东鲁宁药业有限公司
Preparation and recovery method of 2-methyl-5-iodobenzoic acid
The invention provides a preparation and recovery method of 2-methyl-5-iodobenzoic acid. The method comprises the steps: iodinating by using iodine / potassium periodate in a mixed acid solvent and taking otoluic acid as a raw material to obtain a mixture of 2-methyl-5-iodobenzoic acid, 2-methyl-3-iodobenzoic acid and 2-methyl-3, 5-diiodosalicylic acid; refining to obtain 2-methyl-5-iodobenzoic acid and a mother solution regenerant, wherein the mother solution regenerant is the mixture of 2-methyl-5-iodobenzoic acid, 2-methyl-3-iodobenzoic acid and 2-methyl-3, 5-diiodosalicylic acid; recovering otoluic acid and iodine ions through catalytic hydrogenation deiodination; oxidizing the iodine ions for recovering iodine. The whole process is circularly utilized. The preparation and recovery method has the advantages that the raw material is easy to obtain and low in price, the preparation process is simple in operation, the equipment requirement is low, and the method is suitable for large-scale industrial production.
Owner:ABA CHEM CORP
Stable and medium-yield oxidant for oil production and preparation method of stable and medium-yield oxidant
PendingCN114854446AStable in natureStable physical and chemical propertiesHydrocarbon oils refiningSodium bicarbonateBenzoic acid
The invention discloses a stable and medium-yield oxidant for oil production and a preparation method thereof, and the stable and medium-yield oxidant comprises the following components in parts by weight: 3-6 parts of potassium permanganate, 20-30 parts of a 0.5 mol / L potassium hydrogen persulfate aqueous solution, 5-8 parts of sodium chlorate, 10-15 parts of 2-iodobenzoic acid, 3-5 parts of a stabilizer, 1-2 parts of an accelerant, 1-2 parts of a buffer agent, 10-15 parts of ammonium peroxydisulfate, 3-5 parts of 3-(2, 3-epoxypropoxy) propyltrimethoxysilane and 100-120 parts of a high-boiling-point solvent. 1-5 parts of a 3% sodium bicarbonate aqueous solution and 200 parts of pure water. Taking a strong oxidant potassium permanganate as a raw material to react with a potassium hydrogen persulfate aqueous solution and sodium chlorate to obtain a first preparation participant; according to the method, 2-iodobenzoic acid is used for preparing turbid liquid, the turbid liquid and a first participant are stirred, washed and dispersed and then cooled to separate out a product, the prepared oxidizing agent is stable in property, the viscosity of the turbid liquid is controlled through ionization, and the purity of the oxidizing agent in the preparation process is further controlled.
Owner:江苏品和石油科技有限公司
A kind of preparation method of 2-chloro-5-iodobenzoic acid
ActiveCN106748721BIncrease profitRaw materials are cheap and easy to getOrganic compound preparationAmino-carboxyl compound preparationNitrationIodobenzoic Acids
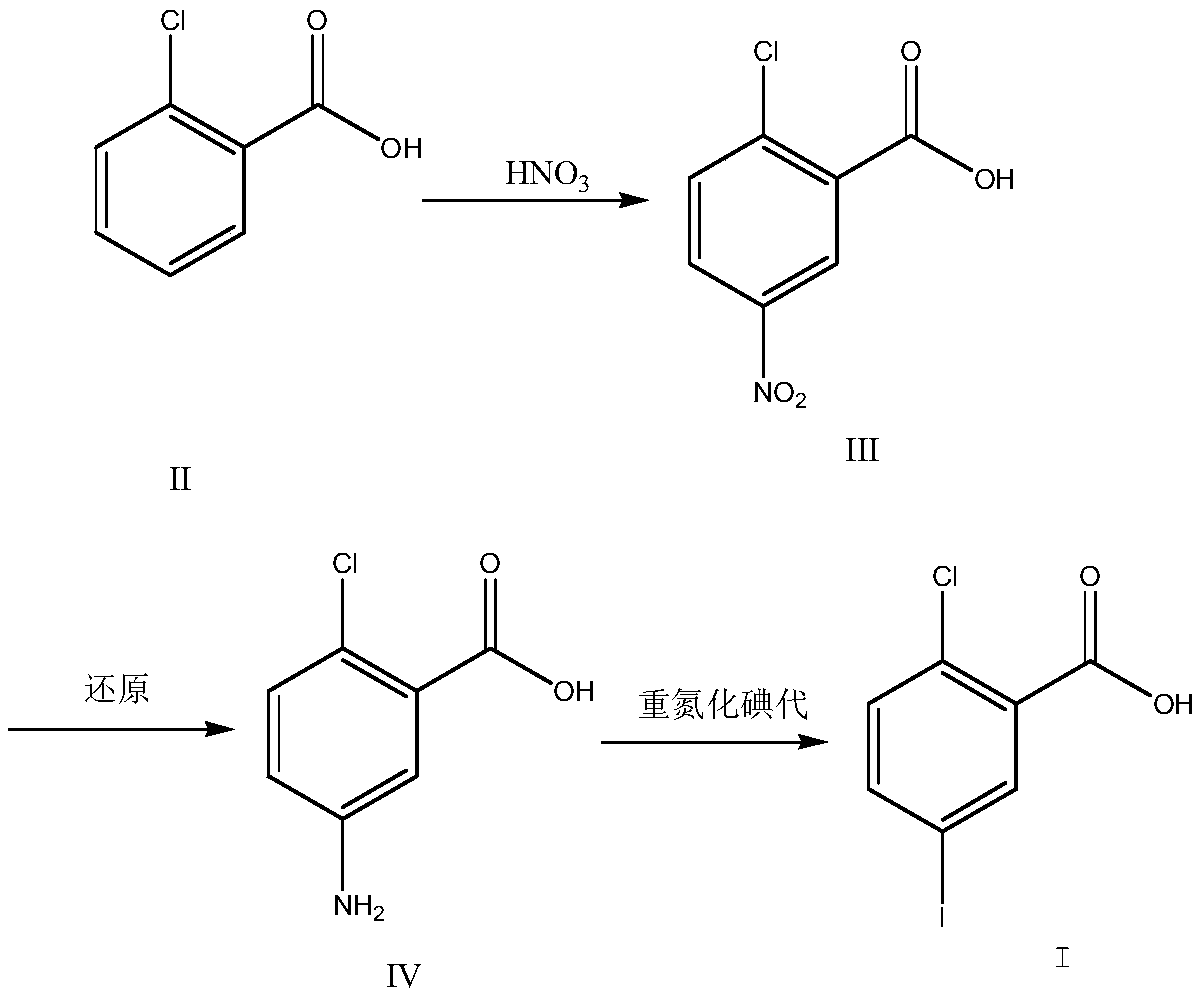
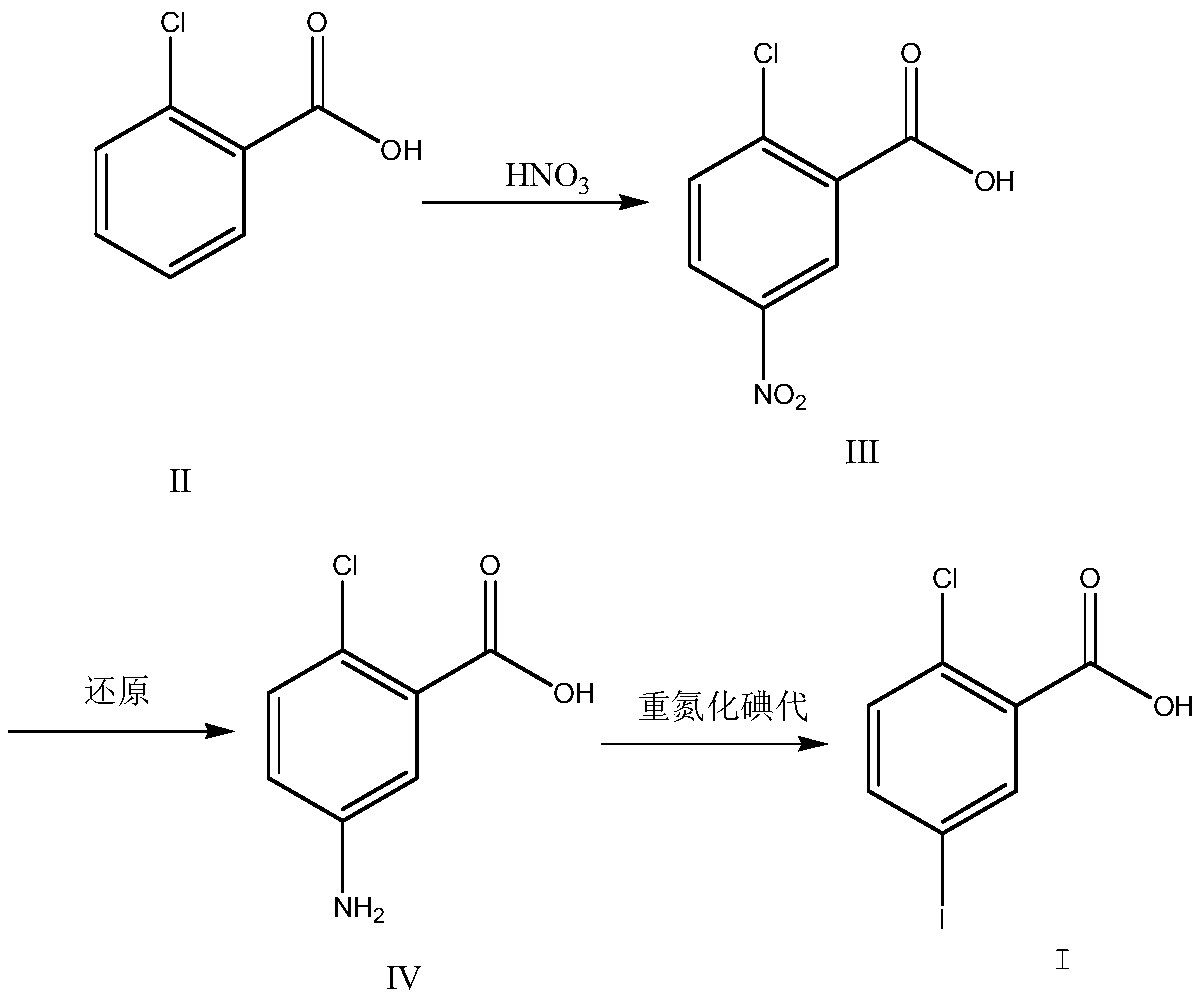
The invention discloses a preparation method for 2-chloro-5-iodobenzoic acid. The preparation method is characterized in that cheap o-chlorobenzoic acid is taken as a starting material to obtain 2-chloro-5-iodobenzoic acid through nitration, reduction and diazotization iodination. The method shortens reaction steps, increases the yield and is suitable for industrial production.
Owner:SHANDONG BOYUAN PHARM CO LTD
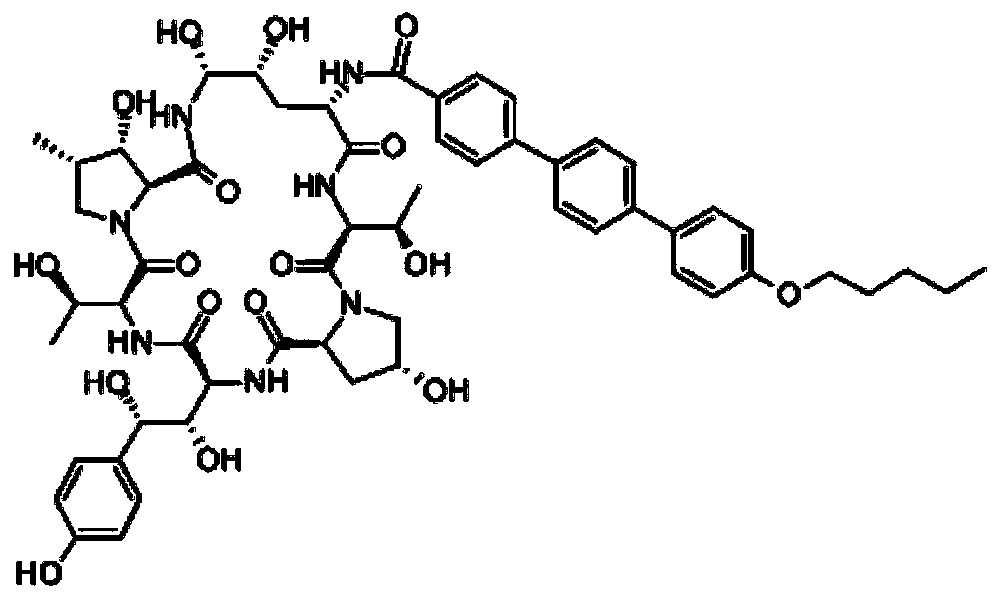
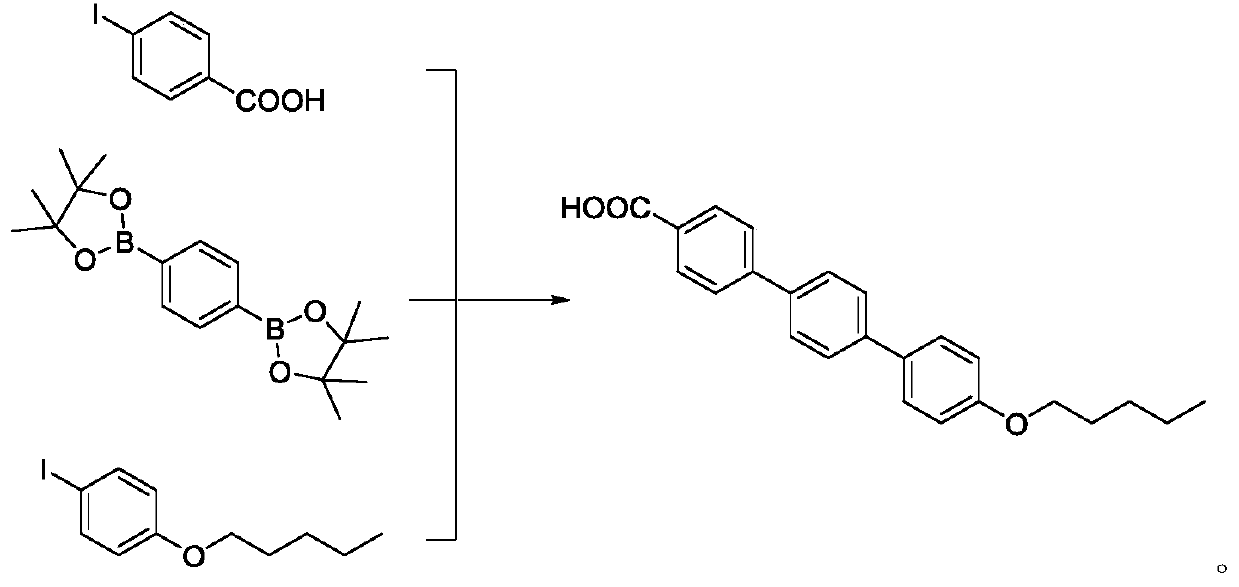
Preparation method of anidulafungin intermediate
InactiveCN109796325AEasy to operateSuitable for industrial productionOrganic compound preparationCarboxylic compound preparationCarboxylic acidSolvent
The invention belongs to the technical field of drug synthesis, and particularly relates to a preparation method of an anidulafungin intermediate. The preparation method includes the steps of (1), subjecting iodobenzoic acid and 1,4-indole-dibenzoboronic acid pinacol ester to reaction in a solvent in the presence of palladium and alkali; (2), adding 4-p-(pentyloxy) iodobenzene to reaction liquid of the step (1) to obtain 4''-(pentyloxy)-[1,1':4',1''-Terphenyl]-4-carboxylic acid, namely the anidulafungin intermediate. The preparation method is easy in operation and high in yield and capable ofmeeting requirements on industrial production.
Owner:南京天越星生物技术有限公司
Treating agent for biorefractory substances in high-salinity organic wastewater
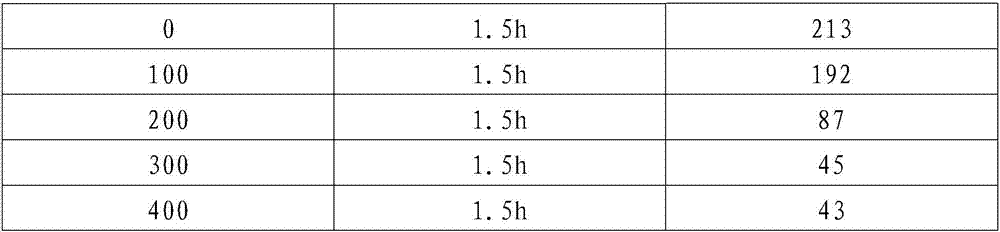
ActiveCN107055741AStrong oxidation abilityQuick breakdownWaste water treatment from quariesWater contaminantsIodobenzoic AcidsNuclear chemistry
The invention discloses a treating agent for biorefractory substances in high-salinity organic wastewater. The treating agent comprises a component A and a component B; the component A is prepared from, by weight, 20-50 parts of sulfuric acid, 10-20 parts of o-iodobenzoic acid, 10-20 parts of potassium bromate, 10-20 parts of potassium iodate and 20-50 parts of water; the component B is prepared from, by weight, 20-30 parts of sodium perchlorate, 10-20 parts of potassium perchlorate, 10-20 parts of potassium nitrate and 50-60 parts of water, wherein a weight ratio of the component A to the component B is 8:2. The treating agent is green, environmental friendly, high in oxidation performance and capable of quickly decomposing biorefractory organics in water to realize shape decline of COD values and also has an evident degradation effect on ammonia nitrogen in water. Therefore, the treating agent can be widely applied to removal of biorefractory organics in high-salinity wastewater of chemical engineering, coal chemical industry, oil extraction plants, refineries and the like and manifests technical progress.
Owner:CHENGDU KEHENG ENVIRONMENTAL PROTECTION TECH
Method for producing 2-amino-5-iodobenzoic acid
InactiveUS7378546B2Quality improvementHigh yieldOrganic compound preparationAmino-carboxyl compound preparationCompound (substance)Iodine
A method for producing 2-amino-5-iodobenzoic acid which comprises bringing 2-aminobenzoic acid (A) and molecular iodine (B) into reaction with each other in the liquid phase in the presence of an oxidizing agent. Hydrogen peroxide is preferable as the oxidizing agent. This method does not require a step for purifying 2-amino-5-iodobenzoic acid or a step for recovering iodine, and 2-amino-5-iodobenzoic acid having excellent quality can be produced economically advantageously with a great yield. The product can be advantageously used as an intermediate for drugs, an agricultural chemical and a raw material for functional chemicals.
Owner:MITSUBISHI GAS CHEM CO INC
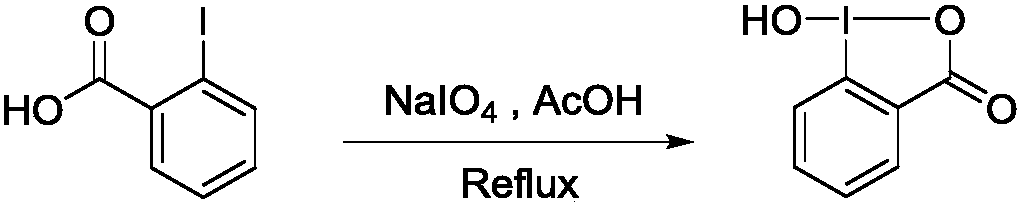
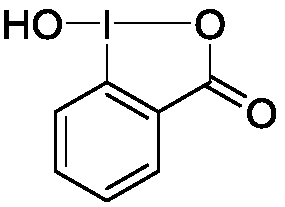
Preparation method of 1-hydroxyl-1,2-benziodoxol-3(1H)-one
InactiveCN107827868AMild reaction conditionsSimple processOrganic chemistryOrganic synthesisIodobenzoic Acids
The invention relates to a preparation method of 1-hydroxyl-1,2-benziodoxol-3(1H)-one. O-iodobenzoic acid, sodium periodate and glacial acetic acid are sequentially added to a 250 mL round-bottom flask and stirred until o-iodobenzoic acid is completely dissolved, and then reflux is performed; after condensed water (at the temperature of 25 DEG C) is introduced into a condensation pipe from bottomto top, the round-bottom flask is put in an oil bath pan at the temperature of 120 DEG C, magnetic stirring is performed, and the mixture reacts in a dark place for 4 h; after the reaction ends, the mixture is left to stand to be cooled to the room temperature, distilled water is added, the mixture is filtered, a rough product is collected and washed with icy water and acetone three times, a product is air-dried in the dark place and a white product of 1-hydroxyl-1,2-benziodoxol-3(1H)-one is obtained. The preparation method has mild reaction conditions, is simple in the technological process and high in selectivity, has higher yield reaching 93% and is environmentally friendly. The prepared product can be applied to the field of organic synthesis and the like.
Owner:HUBEI UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com