Clozapine impurity as well as preparation method and application thereof
A clozapine and impurity technology, applied in the field of pharmaceutical preparations, can solve the problems of difficult operation and treatment, reduce the effect, increase the burden on patients, etc., and achieve the effects of simple post-processing, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
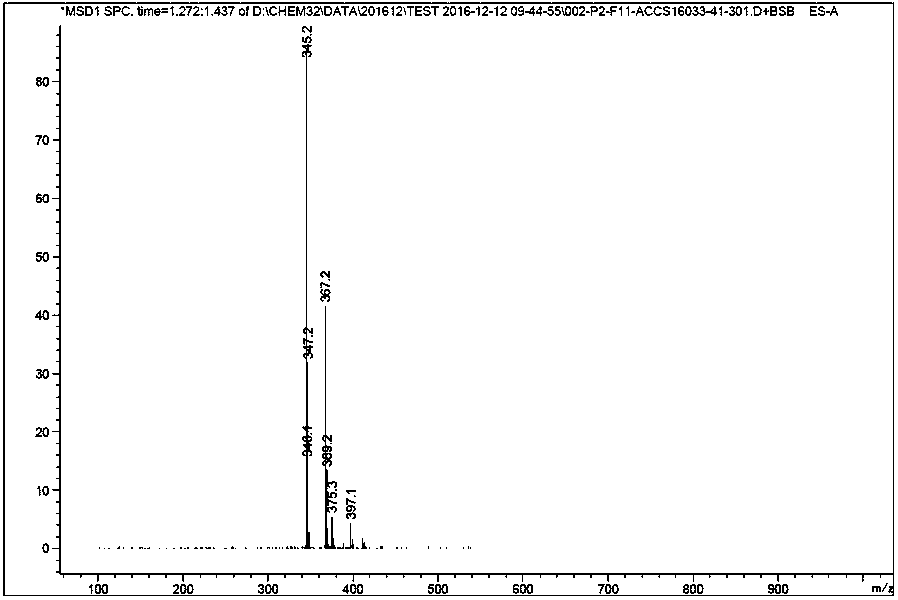
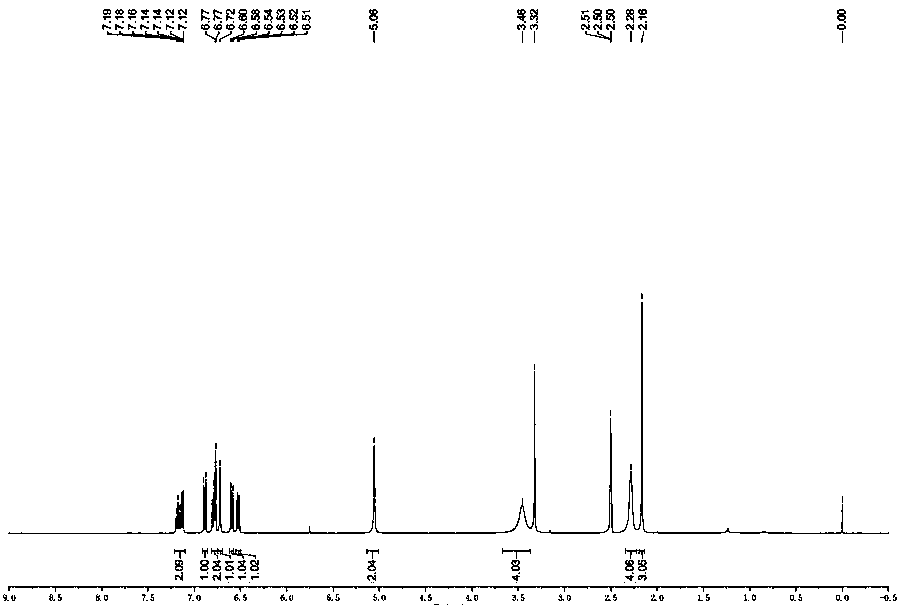
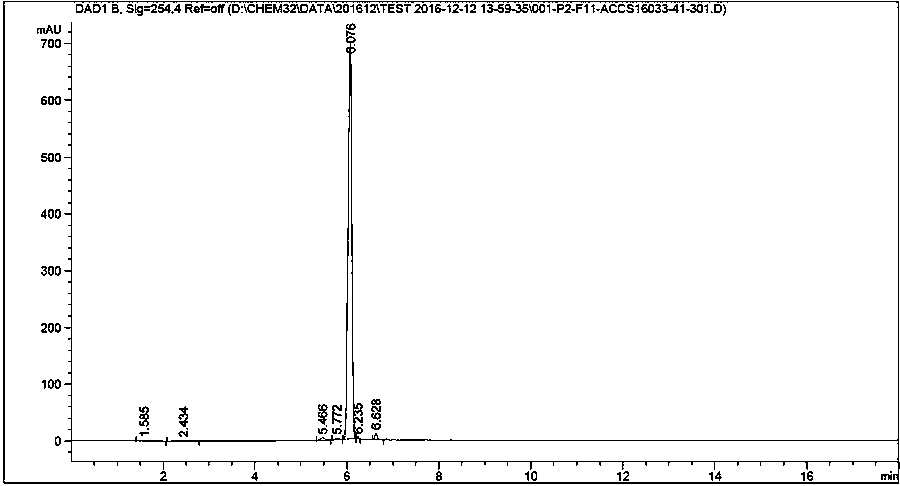
[0028] The preparation of embodiment clozapine impurity
[0029] Add 4-chloro-2-nitroaniline (1.0 g, 5.79 mmol, 1.0 eq), o-iodobenzoic acid (1.72 g, 6.95 mmol, 1.20 eq), K 2 CO 3 (1.6 g, 11.59 mmol, 2.0 eq), CuI (0.111 g, 0.58 mmol, and DMF20 mL), vacuumize, replace with argon three times, and finally stir and heat up to 120 °C under the protection of argon, react for 2 hours, and the reaction is complete , add water and stir, extract with n-butanol, concentrate the organic layer, and recrystallize from isopropanol to obtain 1.50 g of SM1 brown solid, with a yield of 88%.
[0030] Add SM1 (1.0 g, 3.42 mmol, 1.0 eq), oxalyl chloride (0.867 g, 6.83 mmol, 2.0 eq) and anhydrous dichloromethane (20 ml) into a 100 mL round bottom flask successively, stir at room temperature for 30 min, spin to dry the solvent, Excess oxalyl chloride was removed, and anhydrous dichloromethane (20ml) was added to the solid under ice bath, followed by triethylamine (1.38g, 13.67mmol, 4eq) and N-methy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com