Patents
Literature
58 results about "Clozapine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
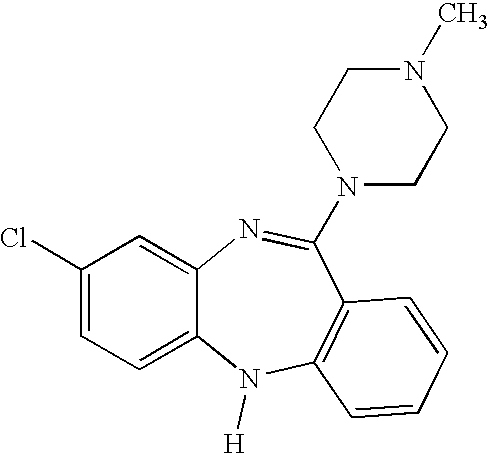
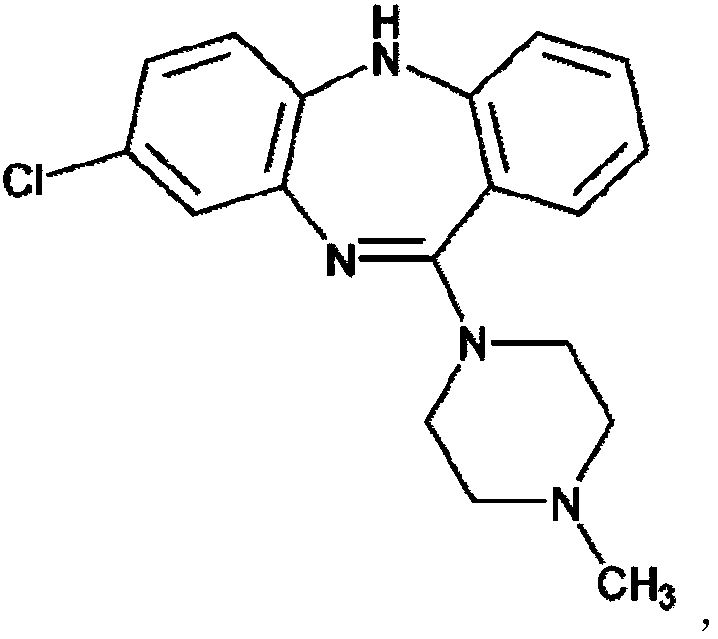
This medication is used to treat certain mental/mood disorders (schizophrenia, schizoaffective disorders). Clozapine is a psychiatric medication (anti-psychotic type) that works by helping to restore the balance of certain natural substances (neurotransmitters) in the brain. Clozapine decreases hallucinations and helps prevent suicide in people who are likely to try to harm themselves.
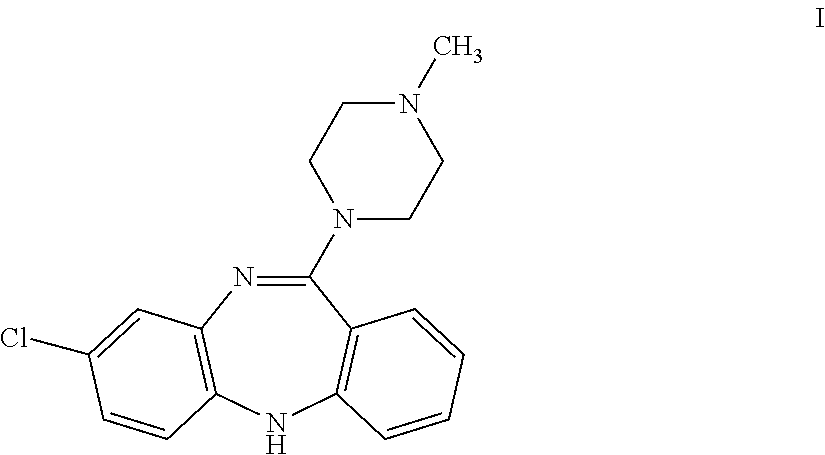
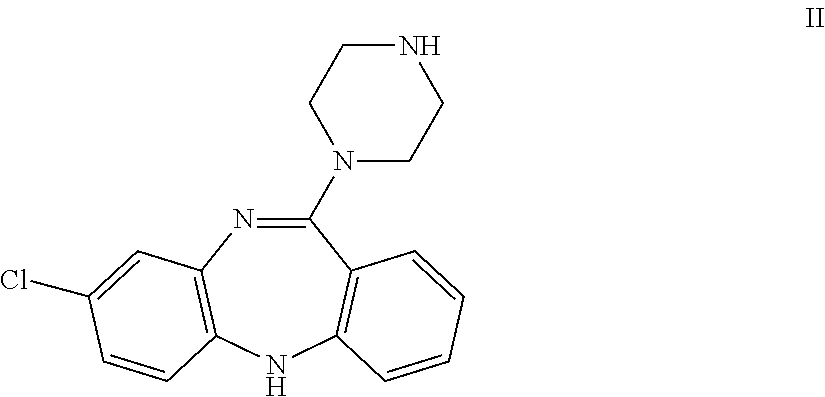
Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders
Disclosed herein are analogs of clozapine and pharmaceutically acceptable salts, esters, amides, or prodrugs thereof; methods of synthesizing the analogs; and methods of using the analogs for treating neuorpsychiatric disorders. In some embodiments, the analogs are amino substituted diaryl[a,d]cycloheptenes.
Owner:ACADIA PHARMA INC
Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders
Disclosed herein are analogs of clozapine and pharmaceutically acceptable salts, esters, amides, or prodrugs thereof; methods of synthesizing the analogs; and methods of using the analogs for treating neuorpsychiatric disorders. In some embodiments, the analogs are amino substituted diaryl[a,d]cycloheptenes.
Owner:ACADIA PHARMA INC
Amino bustituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders
Disclosed herein are analogs of clozapine and pharmaceutically acceptable salts, esters, amides, or prodrugs thereof; methods of synthesizing the analogs; and methods of using the analogs for treating neuorpsychiatric disorders. In some embodiments, the analogs are amino substituted diaryl[a,d]cycloheptenes.
Owner:ACADIA PHARMA INC
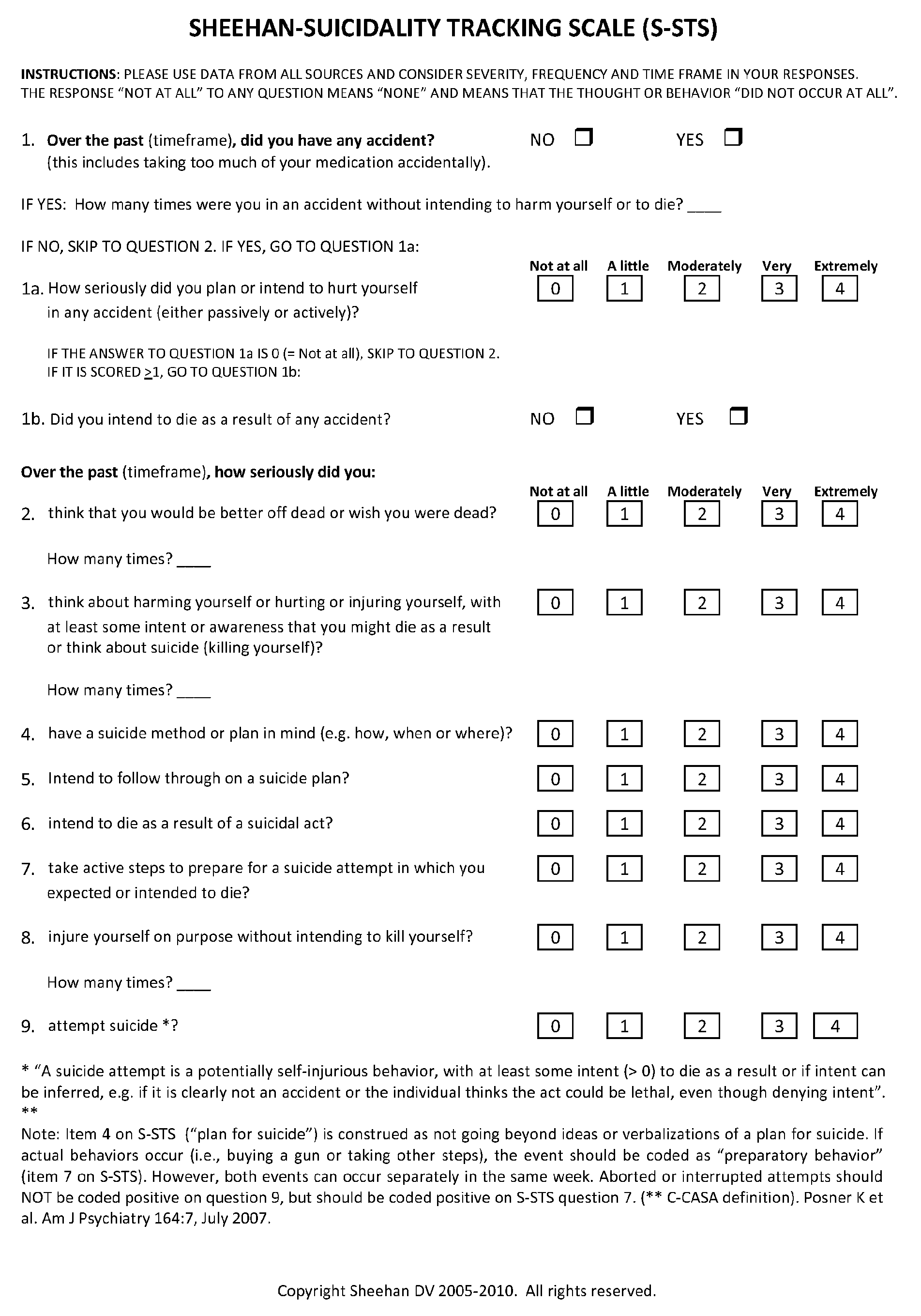
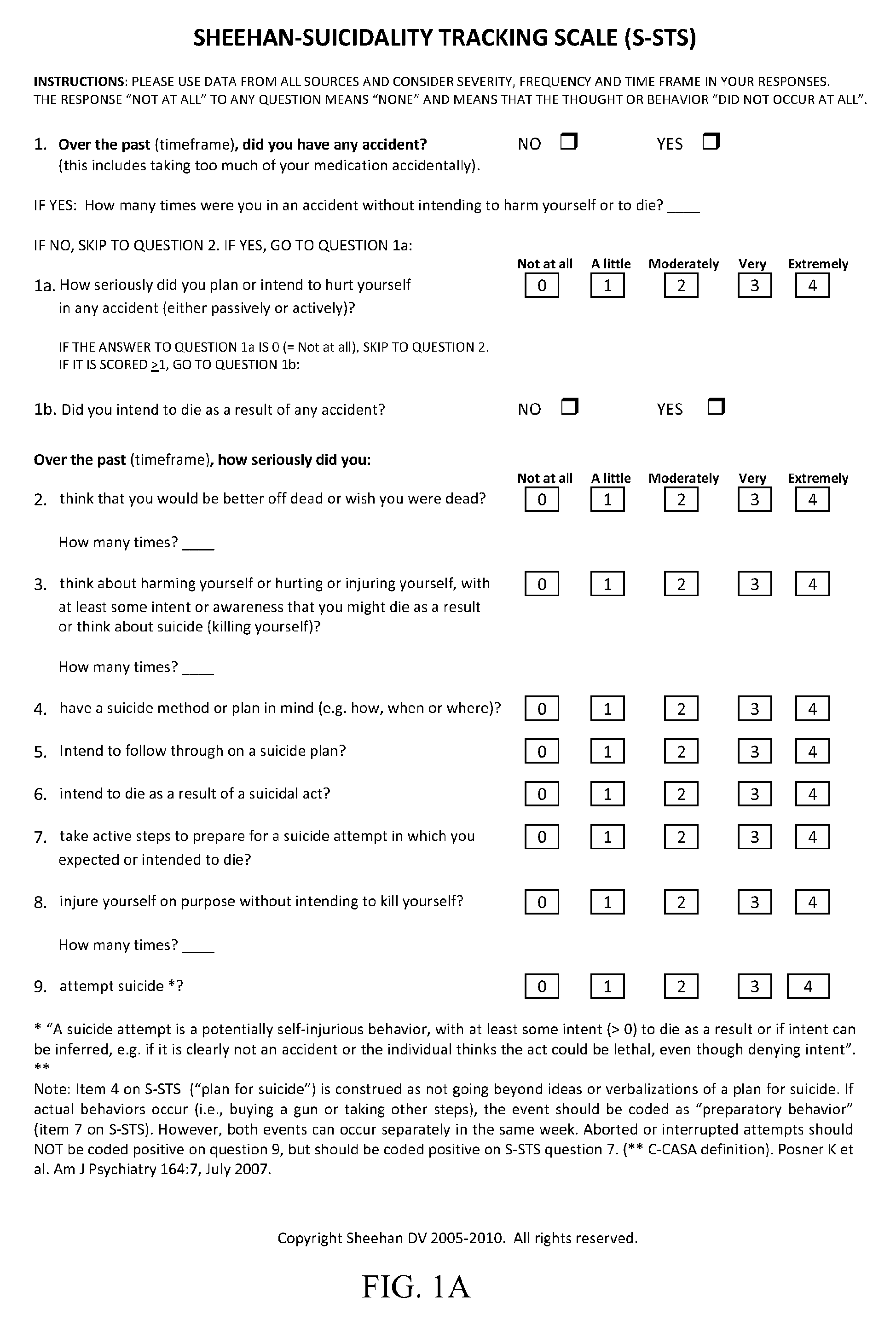
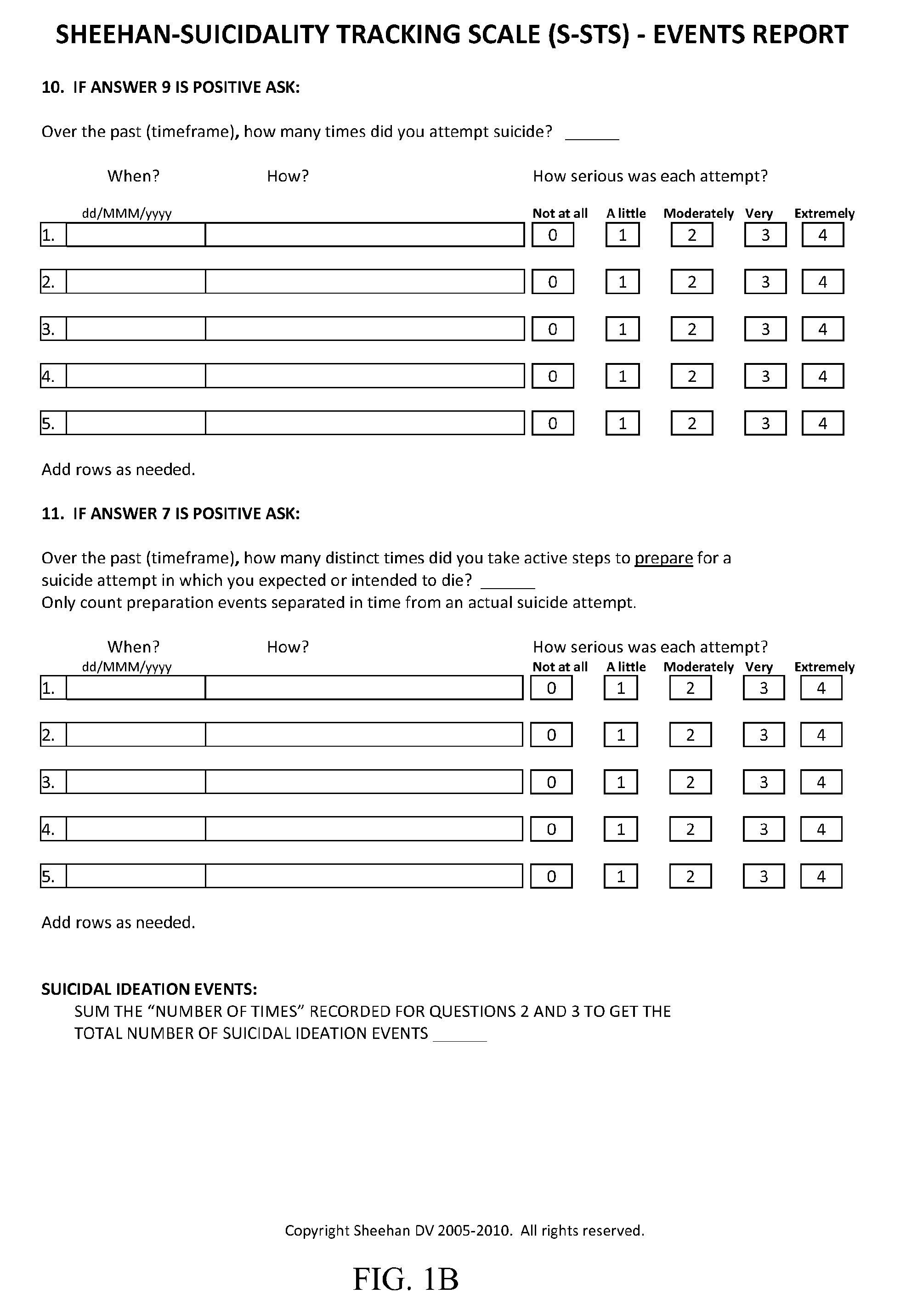
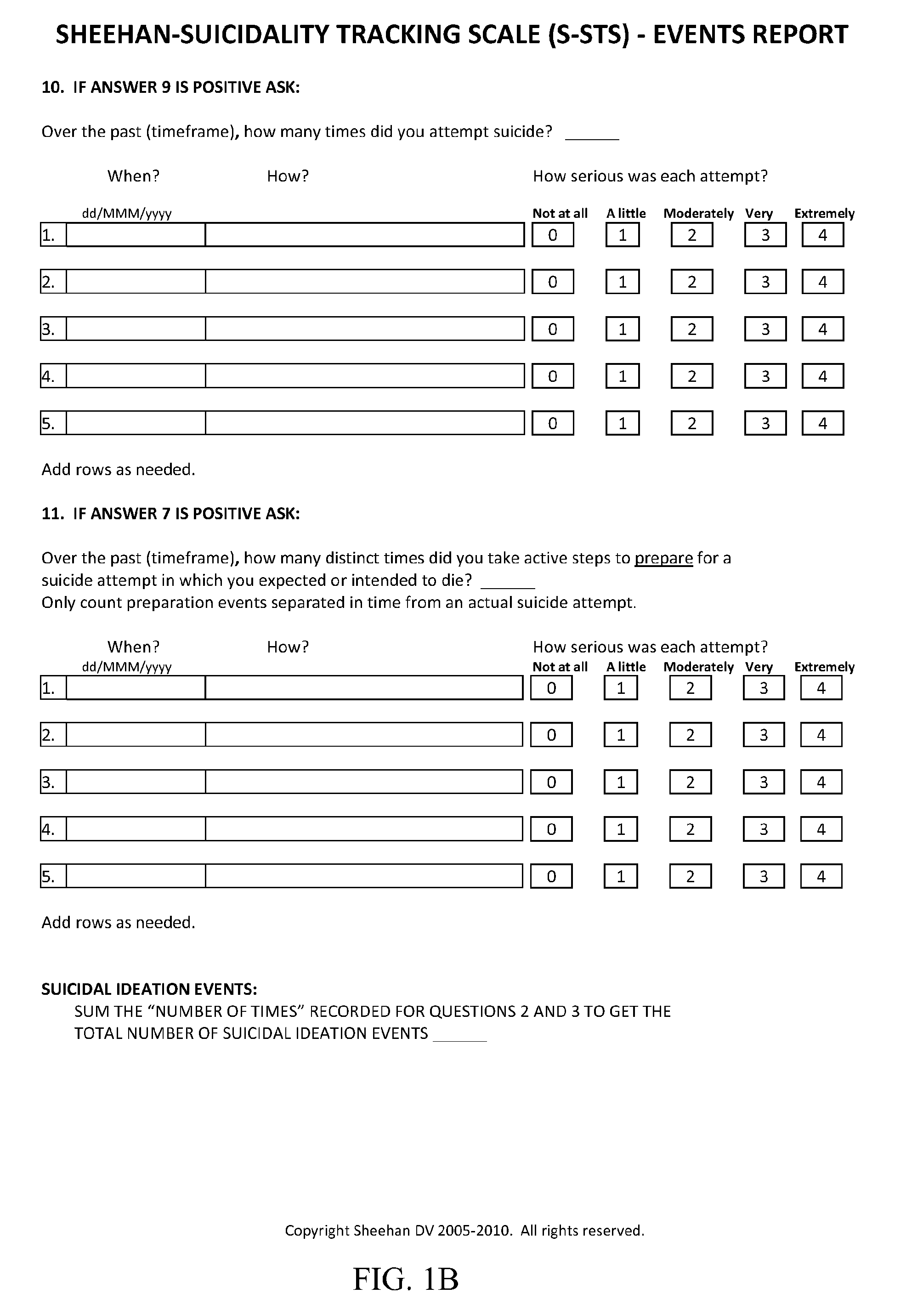
Treatment of suicidal ideation or behavior using inhibitors of nicotinic acetylcholine receptors
ActiveUS20120269906A1Reducing suicidalityIncreased riskBiocideNervous disorderLithium compoundMuscarinic acetylcholine receptor
The invention concerns methods of treating suicidal ideation or behavior in a subject in need thereof, comprising decreasing endogenous nicotinic acetylcholine receptor (nAChR) activity in the subject; therapeutic packages for treating suicidal ideation or behavior; and methods for determining the efficacy of a treatment for suicidal ideation or behavior. In some embodiments, the treatment methods comprise administering to the subject an effective amount of an inhibitor of a nAChR, such as a lithium compound, mecamylamine, clozapine, or asenapine.
Owner:UNIV OF SOUTH FLORIDA
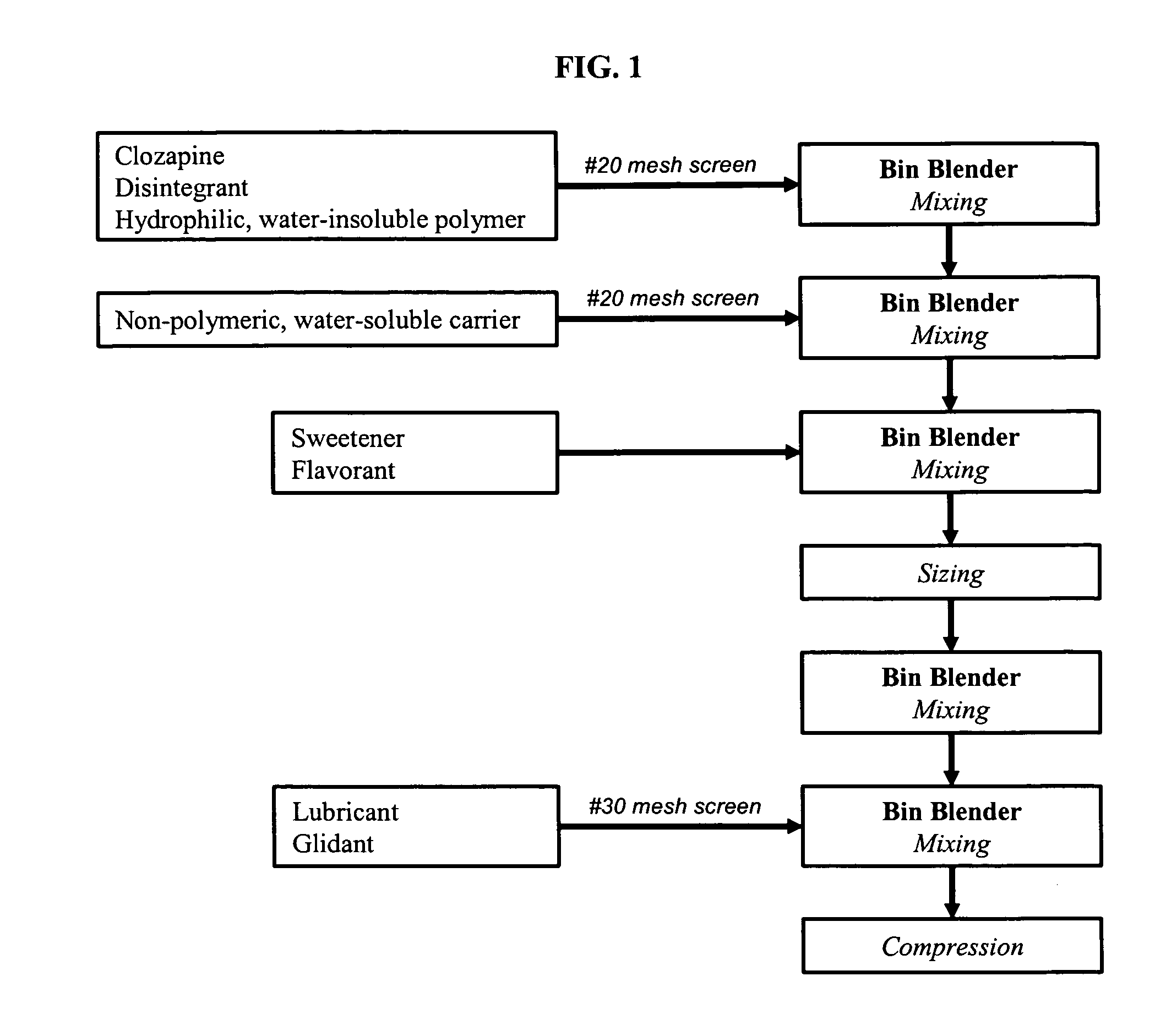
Oral-applied slow-releasing or release-controllable solid clozapine
InactiveCN1395931ASmall fluctuations in blood concentrationStable blood concentrationOrganic active ingredientsNervous disorderMedicineCurative effect
An oral-applied slow-releasing or release-controlalble solid clozapine for treating psychosis is prepared from clozepine, skeleton assistant material and filler (starch, hydroxypropyl cellulose, ethylcellulose, etc.). Its advantages are high curative effect, and stable concentration of medicine in blood.
Owner:DAYA PHARMA HUIZHOU
Leponex orally disintegrating tablet and preparation method thereof
InactiveCN1806805AGood dissolution uniformityMedication convenienceOrganic active ingredientsNervous disorderCarboxymethyl starchCellulose
The invention discloses a clozapine oral disintegrating tablet, which is prepared from 50.0-60.0 wt% of clozapine, 5.0-10.0.0wt% of sodium carboxymethylstarch, 30.0-40.0.0wt% of crystalline cellulose, 2.0-5.0wt% of miropowdered silica gel, 0.1-2.0wt% of magnesium stearate and 0.1-0.5 wt% of Aspartame through disintegrating and passing through 100 mesh sieve, mixing homogeneously, and carrying out powder direct pelleting method to obtain the composition.
Owner:马晶
Non-effervescent, orally disintegrating solid pharmaceutical dosage forms comprising clozapine and methods of making and using the same
Owner:BARR LAB
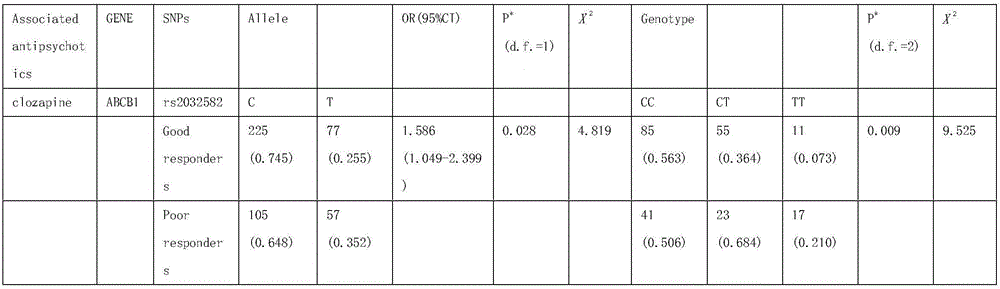
Kit for testing clozapine application effect based on rs2032582 and application method of kit
InactiveCN105861686AMicrobiological testing/measurementDNA/RNA fragmentationPharmacogenomicsSubfamily
The invention relates to a kit for testing clozapine application effect based on rs2032582 and an application method of the kit, and particularly relates to a method for testing single nucleotide polymorphism of ABCB1 (ATP binding cassette subfamily B member 1) NO.8 exon in the field of genes. Sequence of the nucleotide, which is isolated and comprises single nucleotide polymorphism locus, is as shown as SEQ ID NO:1; the single nucleotide polymorphism locus is located at NO.3169, rs2032582 (C->T). The method for testing the nucleotide includes the steps of firstly, testing the nucleotide at the locus NO.3169 of the sequence as shown as SEQ ID NO:1; secondly, testing whether there is of the single nucleotide polymorphism at the locus NO.3169 or not by means of a Taqman-MGB typing method. According to the kit, the application method thereof and the method for testing the nucleotide, a foundation for research on ABCB1 gene polymorphism and clinical medicine safety is laid, a theoretical basis for individualization of clinical medicine is provided, and a guidance for research and development of novel medicines based on pharmacogenomics idea is also provided.
Owner:SHANGHAI JIAO TONG UNIV
Treatment of suicidal ideation or behavior using inhibitors of nicotinic acetylcholine receptors
ActiveUS9180191B2Reducing suicidalityIncreased riskBiocideCosmetic preparationsLithium compoundClozapine
The invention concerns methods of treating suicidal ideation or behavior in a subject in need thereof, comprising decreasing endogenous nicotinic acetylcholine receptor (nAChR) activity in the subject; therapeutic packages for treating suicidal ideation or behavior; and methods for determining the efficacy of a treatment for suicidal ideation or behavior. In some embodiments, the treatment methods comprise administering to the subject an effective amount of an inhibitor of a nAChR, such as a lithium compound, mecamylamine, clozapine, or asenapine.
Owner:UNIV OF SOUTH FLORIDA
Clozapine tablets for treating schizophrenia and preparation method thereof
InactiveCN105769789ASignificant effectImprove efficiencyOrganic active ingredientsNervous disorderMedicineCurative effect
The invention relates to clozapine tablets for treating schizophrenia. The clozapine tablets are prepared from the following raw materials: 25 kilograms of clozapine, 28 kilograms of starch, 22 kilograms of dextrin, 2 kilograms of microcrystalline cellulose, 30 kilograms of ethanol of which the volume ratio is 50%, and 0.77 kilograms of magnesium stearate. The raw materials are taken and granulated according to the conventional method, and then one million tablets are prepared. The clozapine tablets disclosed by the invention are obvious in curative effect, and capable of treating both symptoms and root causes, and wide in application prospect.
Owner:仁和堂药业有限公司
Pharmaceutical composition for treating psychosis and preparation technology thereof
InactiveCN105327287ASignificant effectImprove efficiencyOrganic active ingredientsNervous disorderAlcoholCurative effect
The invention relates to a pharmaceutical composition for treating a psychosis. The pharmaceutical composition for treating the psychosis is prepared from 25 kg of clozapine, 28 kg of starch, 22 kg of dextrin, 2 kg of microcrystalline cellulose, 30 kg of ethyl alcohol of 50% (volume ratio) and 0.77 kg of magnesium stearate, the raw materials are taken, granulation is conducted according to a conventional method, and one million tablets are prepared. The pharmaceutical composition is significant in curative effect, treatment of the symptoms and the causes is achieved, and wide application prospect is achieved.
Owner:仁和堂药业有限公司
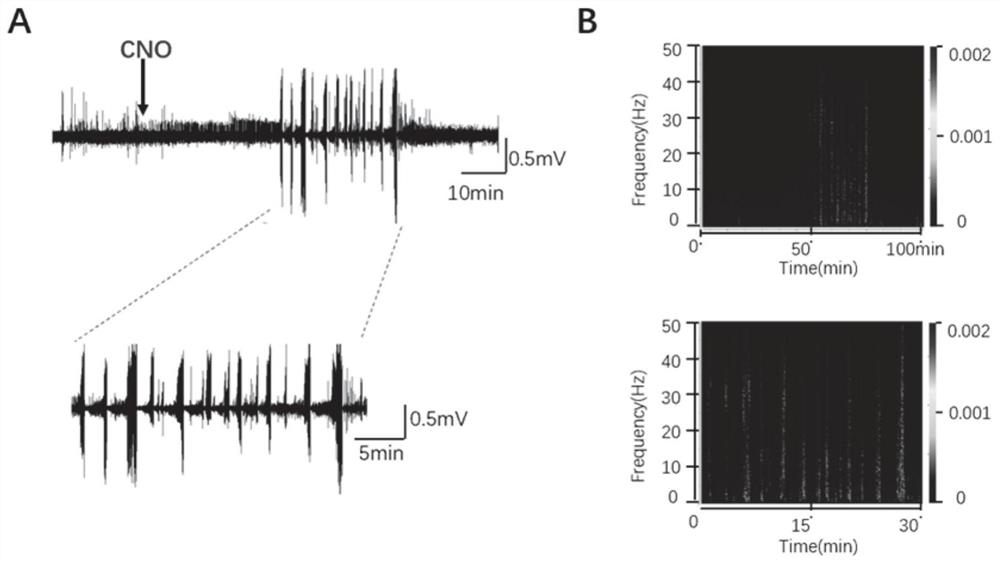
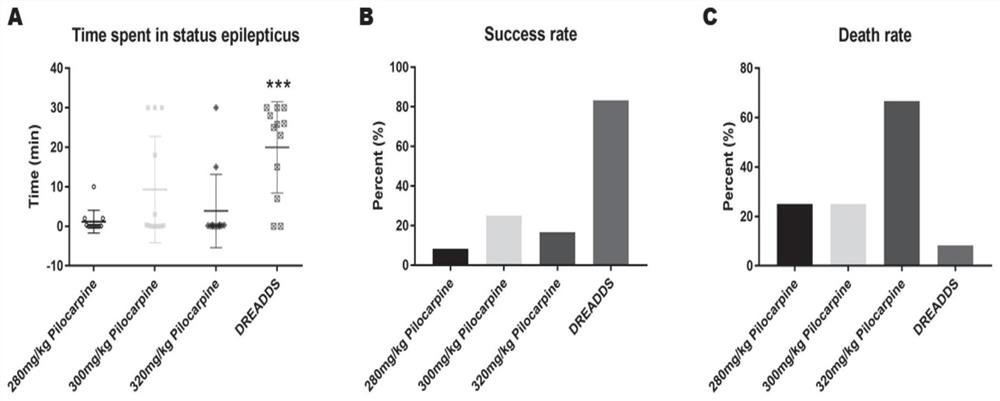
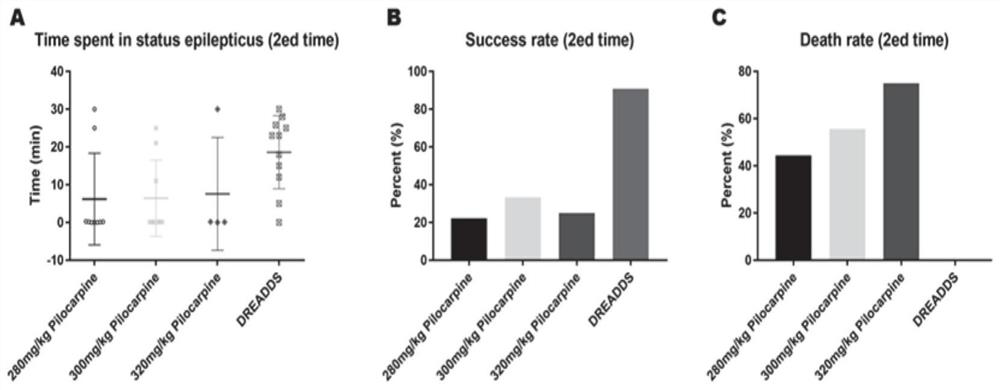
Chemical heredity epilepsy persistent state disease animal model and construction method and application thereof
InactiveCN111839800AInhibitory activityClearly reusableImplantable neurostimulatorsVeterinary instrumentsHippocampal regionMetabolite
The invention provides a chemical heredity epilepsy persistent state disease animal model and a construction method and application thereof. The epilepsy persistent state disease animal model is a mouse brain kernel group (a hippocampus CA1 region and a thalamus anterior nucleus VA region of a model I); injecting a brain stereotaxic virus (a chemical genetic virus rAAV-CaMKIIa-hM3D (Gq)-mCherry-WPREs-pA) in an apricot kernel BLA region and a thalamus anterior nucleus VA region on the outer side of a substrate of the model II, and embedding an electrode array in a mouse hippocampus CA3 region;after the mouse is recovered for one week, a metabolite CNO of clozapine is injected into the abdominal cavity so that the CNO is combined with a virus expression receptor, neurons are activated to induce epileptic persistent state attack, and the epilepsy persistent state disease animal model is obtained through behavioral observation and in-vivo multichannel local field potential recording judgment. The epilepsy persistent state disease animal model constructed by the method is stable in seizure duration, high in success rate, low in death rate and good in repeatability, and has important significance in researching the origin and formation mechanism of epilepsy persistent state, and screening and mechanism of drug-resistant epilepsy persistent state drugs.
Owner:THE FIRST AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIVERSITY
Pharmaceutical composition comprising sodium benzoate compound and clozapine, and uses thereof
ActiveUS10098861B1Reduce riskDelay moreOrganic active ingredientsPill deliverySodium benzoateClozapine
The present invention relates to a pharmaceutical composition comprising a sodium benzoate compound and clozapine; and a method for preventing, treating and / or reducing the risk of a neuropsychiatric disorder by administering such pharmaceutical composition.
Owner:SYNEURX INT
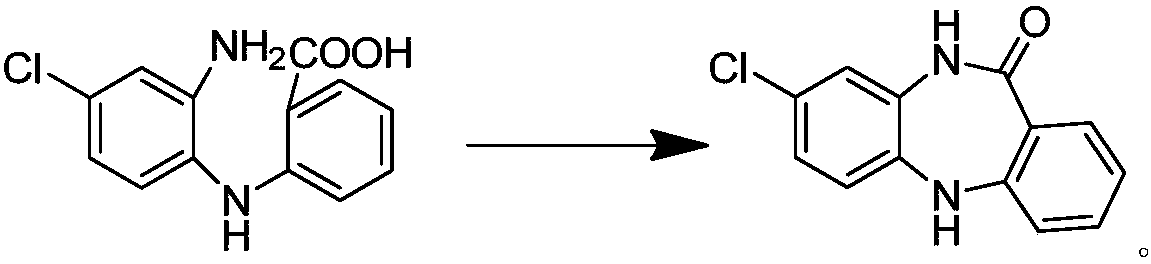
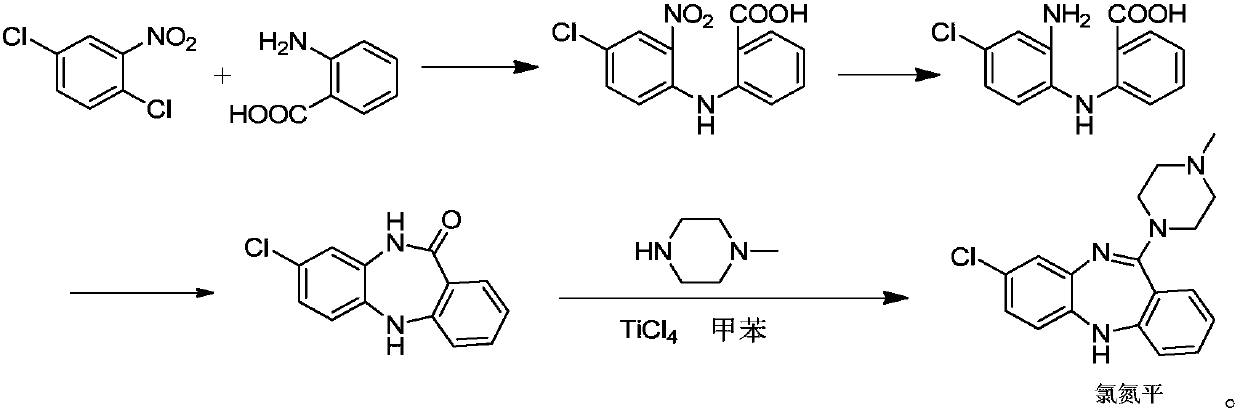
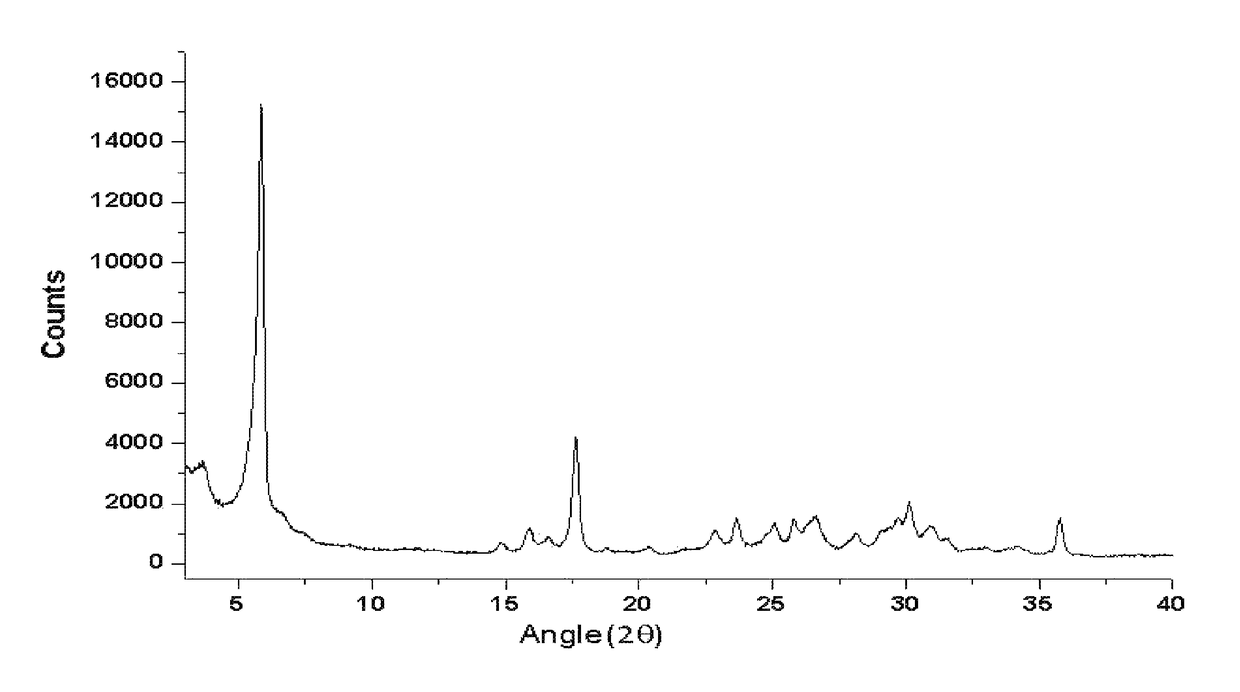
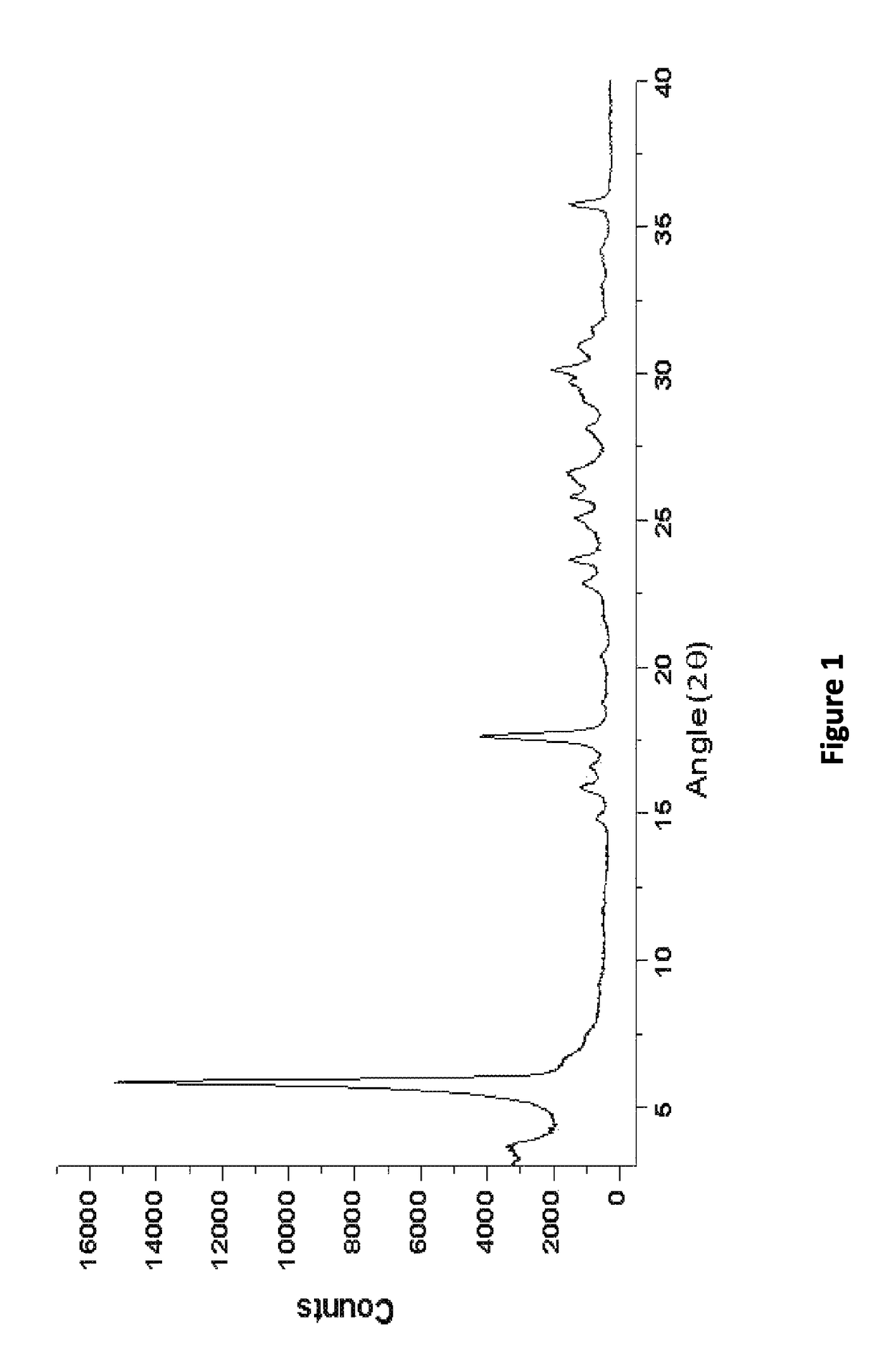
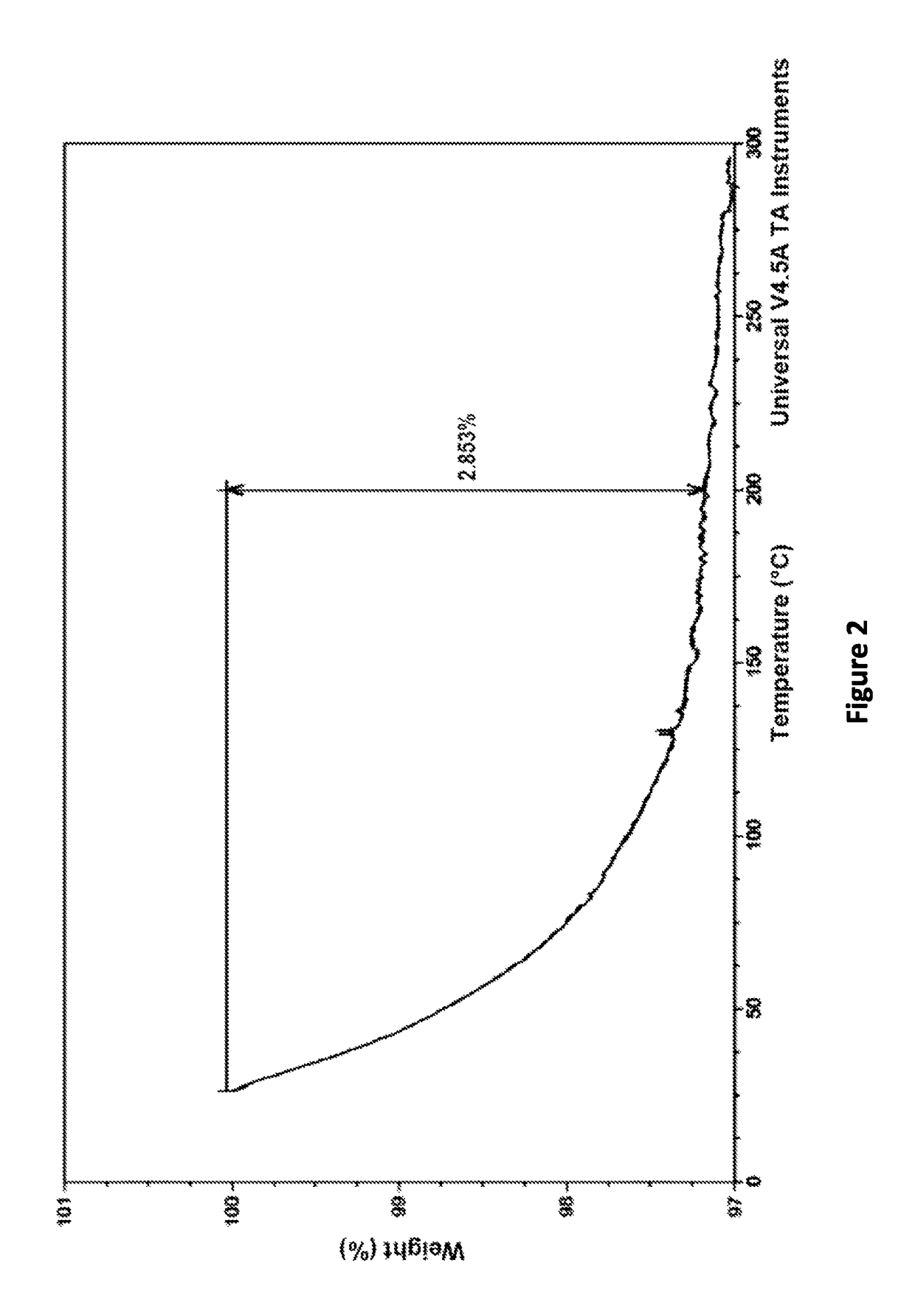
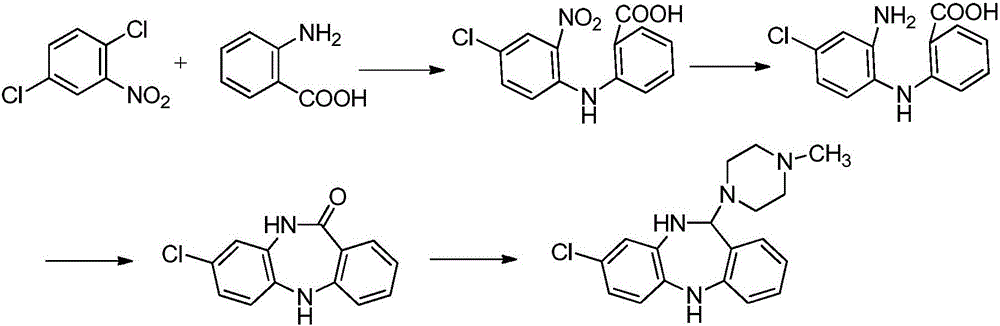
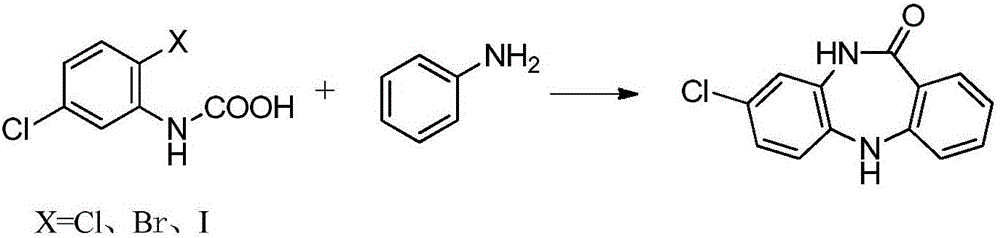
Key clozapine intermediate synthesis method
The invention discloses a key clozapine intermediate synthesis method and belongs to the field of drug synthesis. The key clozapine intermediate synthesis method comprises the steps that N-(2-halogenated-5-chlorphenyl) amino formic acid and aniline are used as starting raw materials, the aniline serves as a solvent, and 8-chlorine-5,10-dihydro-11H-dibenzo [b,e][1,4]-dinitrogen(shown in the description)-11-ketone is obtained through reaction under the effects of a catalyst and alkali. The key clozapine intermediate synthesis method has the positive and progressive advantages that the novel method for synthesis of the 8-chlorine-5,10-dihydro-11H-dibenzo [b,e][1,4]-dinitrogen(shown in the description)-11-ketone overcome lots of shortcomings in the prior art, adopts simple steps and only needs one-step reaction, the yield is up to 90% or above; the aniline is a reaction solvent and is also a raw material, can not only make reaction of the raw material (2-halogenated-3-pyridyl) carbamic acid complete, but also increase the yield of reaction, cost increase and environmental pollution brought by usage of other solvents are further avoided, the excessive aniline can be continuously used after being poste-treated and evaporated out, the cost is greatly saved, the environment is protected, and the key clozapine intermediate synthesis method has a good industrialized prospect.
Owner:CHANGZHOU UNIV
Leponex dispersible tablet and preparation method thereof
InactiveCN1806806AMedication convenienceImprove complianceOrganic active ingredientsNervous disorderCelluloseSilica gel
The invention discloses a clozapine dispersion tablet, which is prepared from 50.0-65.0 wt% of clozapine, 1.0-3.0.0wt% of sodium carboxymethylstarch, 25.0-40.0.0wt% of crystalline cellulose, 0.1-0.5.0wt% of Aspartame, 0.2-1.0.0wt% of magnesium stearate, 0.1-0.5wt% of sodium dodecyl sulfate, 2.0-4.0% of polyvinylpyrrolidone and 1.0-3.0% of miropowdered silica gel through disintegrating and passing through 100 mesh sieve, mixing homogenously, adjusting sheet weight, tabletting so as to obtain the dispersible tablets.
Owner:马晶
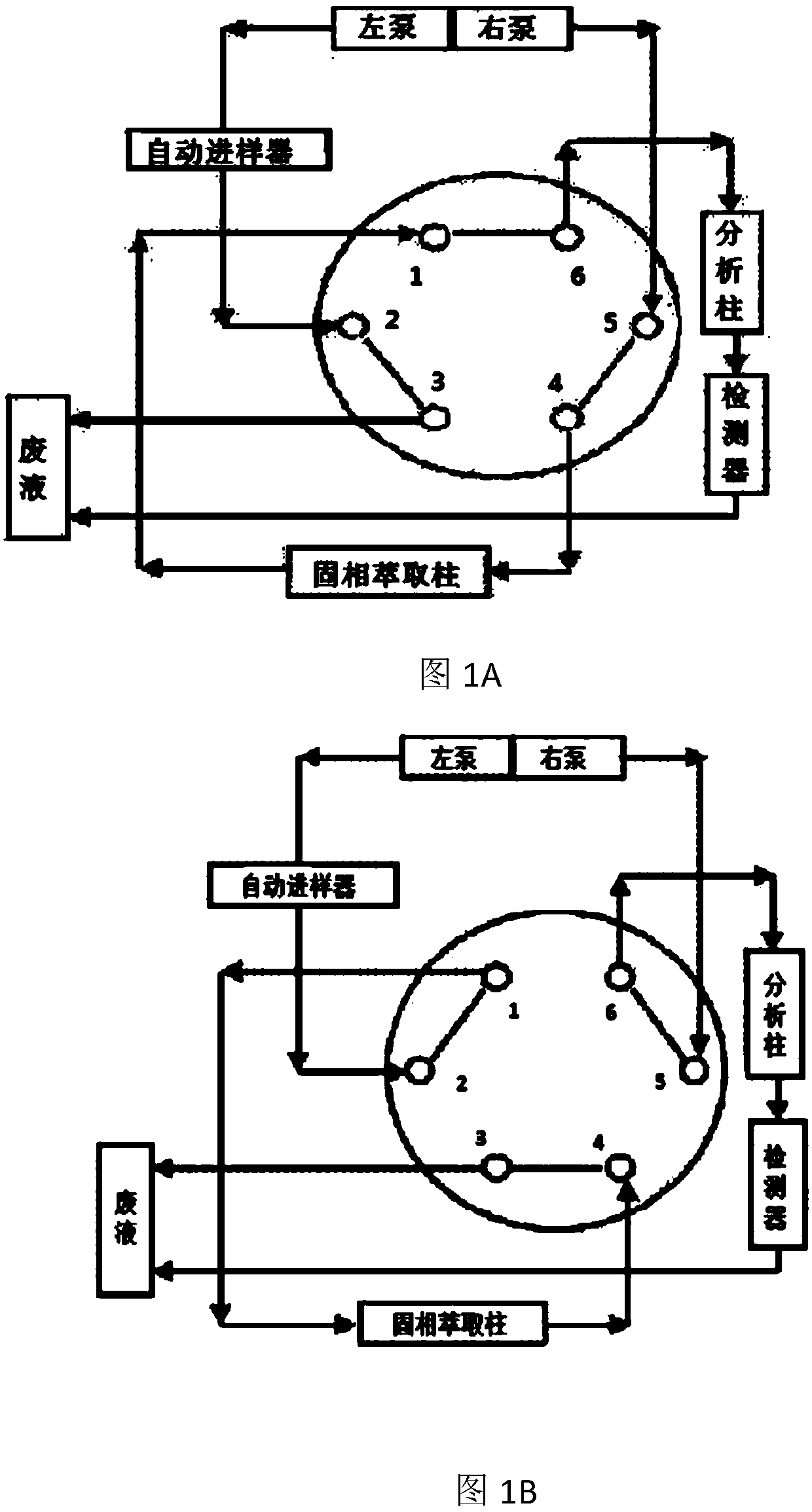
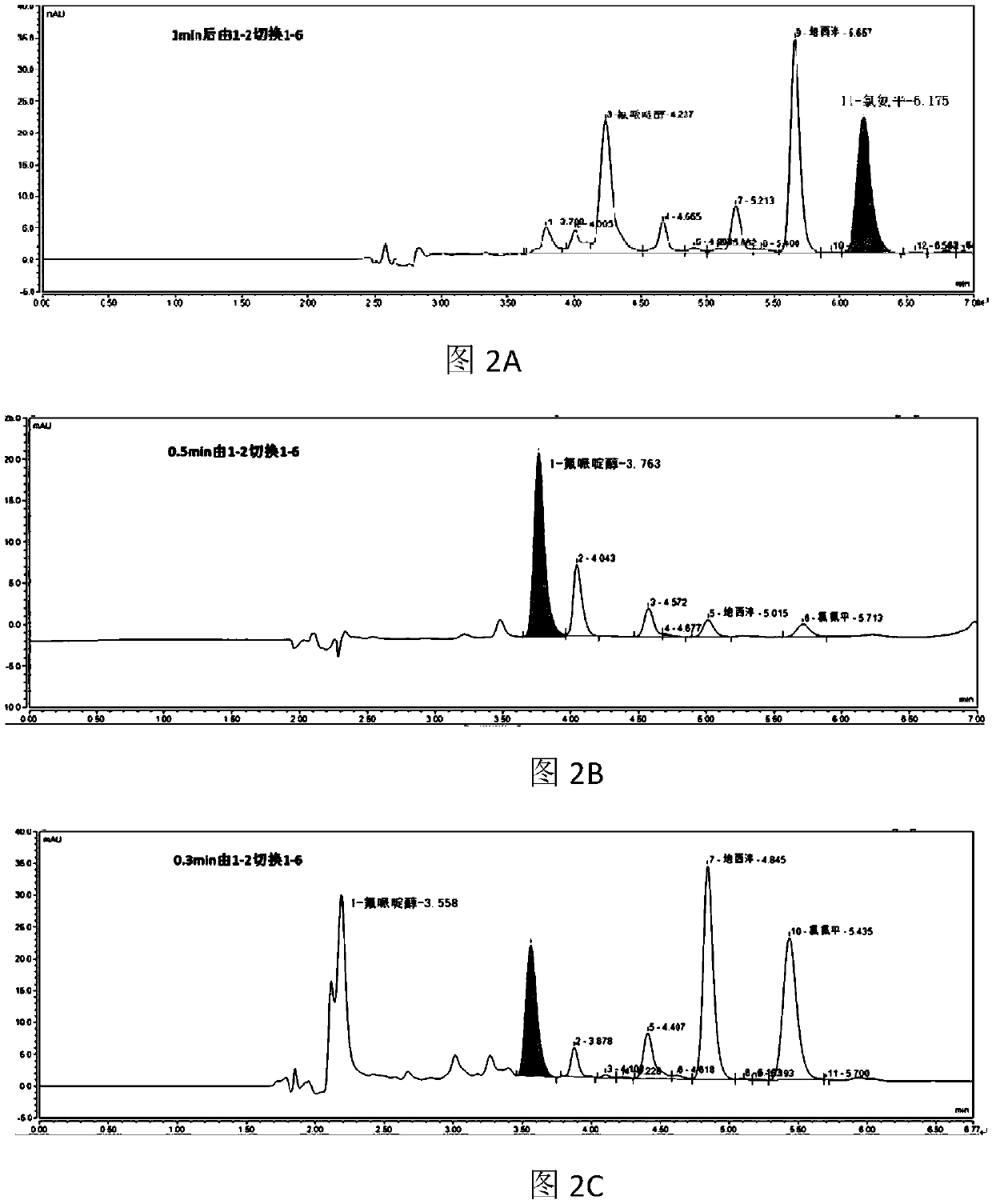
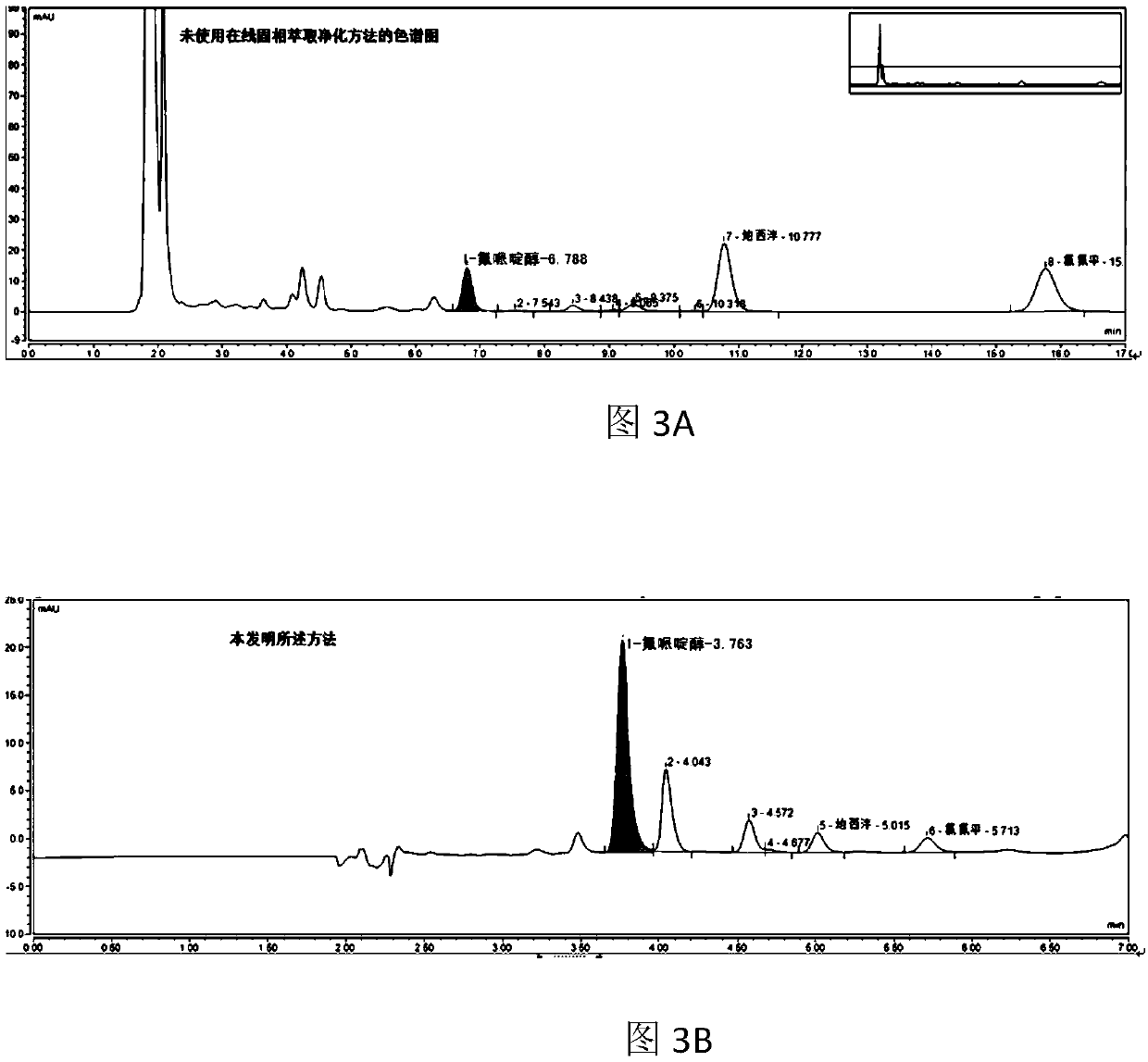
Online solid phase extraction method for detecting diazepam and clozapine in human body serum and system thereof
The invention provides an online solid phase extraction method for detecting diazepam and clozapine in human body serum and a system thereof. The invention aims to provide an online slid phase extraction technology and high-efficiency liquid phase chromatography coupling method, wherein the concentrations of two drugs of diazepam and clozapine in the human body serum can be simultaneously measured. Through methodology validation, the online solid phase extraction method and the system thereof have advantages of high detection accuracy, high detecting rate, high extraction recovery rate and high repeatability. Furthermore the invention provides an online solid phase extraction analysis system for detecting the diazepam and the clozapine in the human body serum.
Owner:苏州和合医学检验有限公司 +1
Certain tricyclic substituted diazabicyclo (3.2.1) octane derivatives
This invention encompasses compounds of the formula: where either R1 or R2 represents and the other represents hydrogen or straight or branched chain lower alkyl having 1-6 carbon atoms; and X is oxygen, methylene, or NH; Y is represents various inorganic and organic substituents; Z is hydrogen, amino or NHR6 where R6 is lowere alkyl having 1-6 carbon atoms; T is hydrogen, halogen, hydroxy, or lower alkoxy having 1-6 carbon atoms; and A is methylene, carbonyl or CHOH. These compounds are selective partial agonists or antagonists at brain monoamine receptor subtypes or prodrugs thereof and are useful in the diagnosis and treatment of affective disorders such as schizophrenia and depression as well as certain movement disorders such as Parkinsonism. Furthermore compounds of this invention may be useful in treating the extrapyramidal side effects associated with the use of conventional neurolepticagents. These compounds show unexpectedly atypical antipsychotic profiles (clozapine-like) in the animal models described in this patent.
Owner:NEUROGEN
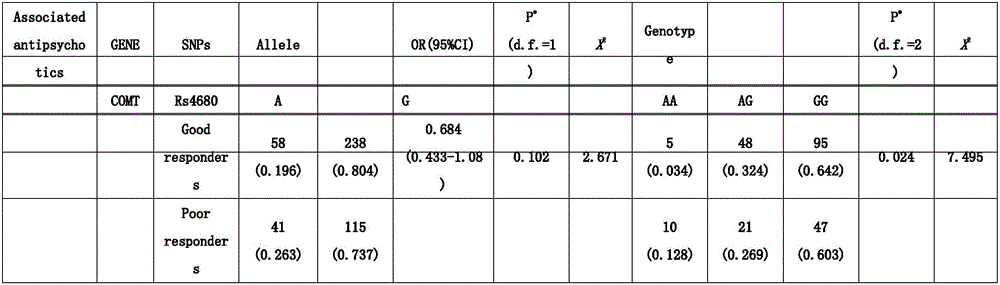
Kit for detecting medication effects of clozapine and risperidone by virtue of rs4680 polymorphism
InactiveCN105925679AMicrobiological testing/measurementDNA/RNA fragmentationCatechol-O-methyl transferaseNucleotide
The invention relates to a kit for detecting medication effects of clozapine and risperidone by virtue of rs4680 polymorphism, and belongs to the field of gene technology. The invention relates to separated nucleotide which contains a single-nucleotide polymorphism site, wherein the sequence of the nucleotide is as shown in SEQ ID NO: 1; the single-nucleotide polymorphism site is on the 854th position; and rs4680 is as shown from G to A. The invention relates to a method for detecting the nucleotide, wherein the method comprises the following steps: 1, detecting the nucleotide which has the sequence as shown in SEQ ID NO: 1 and is located on the 854th position; and 2, by virtue of a Taqman-MGB typing method, detecting whether single-nucleotide polymorphism exists on the 854th position or not. The kit disclosed by the invention lays a foundation for researching a relation between COMT (catechol-O-methyl transferase) gene polymorphism in China's people and clinical medication safety and provides a theoretical basis for the individualization of clinical medication; and meanwhile, the kit also provides a guidance basis to new drug research and development on the basis of pharmacogenomics concept.
Owner:SHANGHAI JIAO TONG UNIV
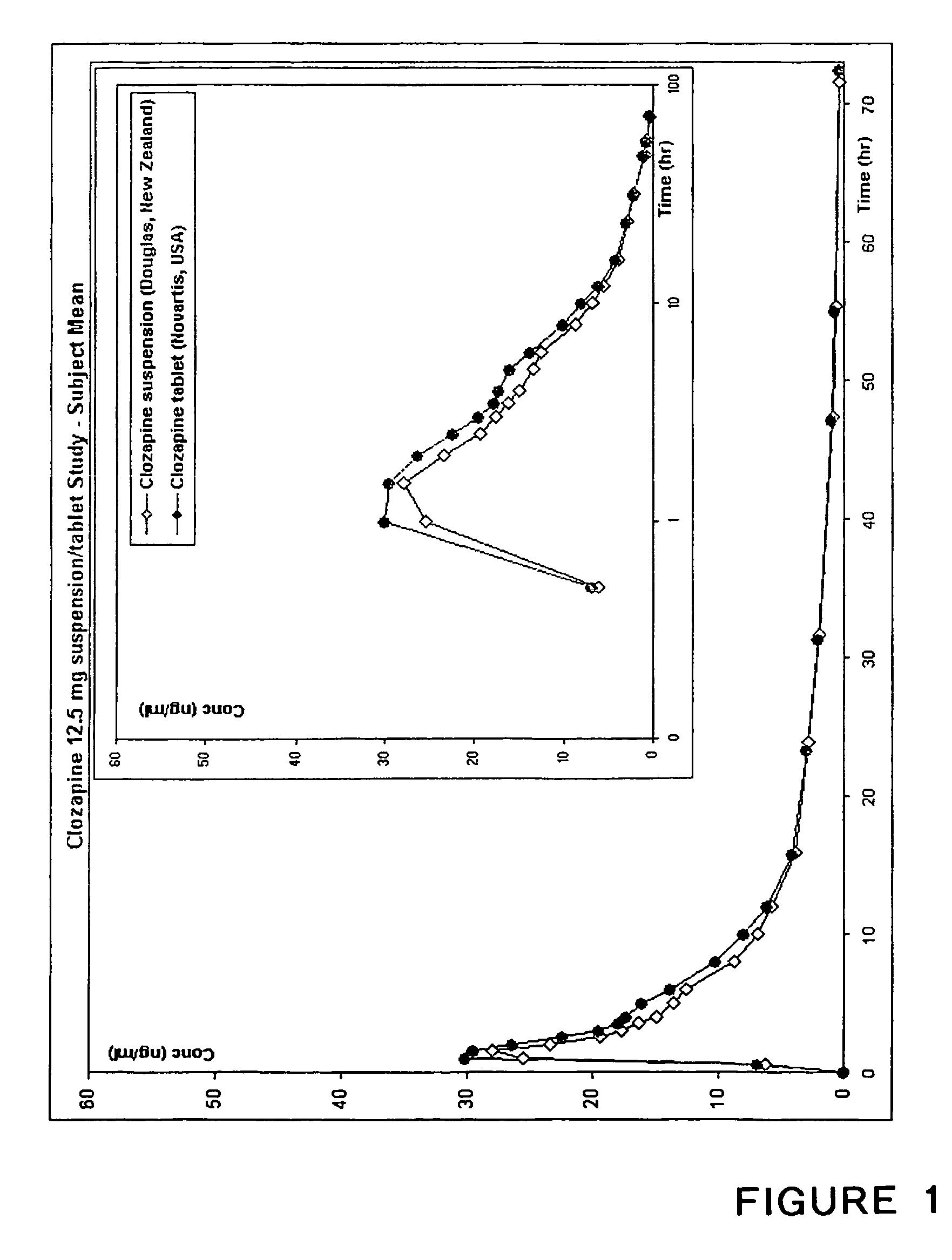
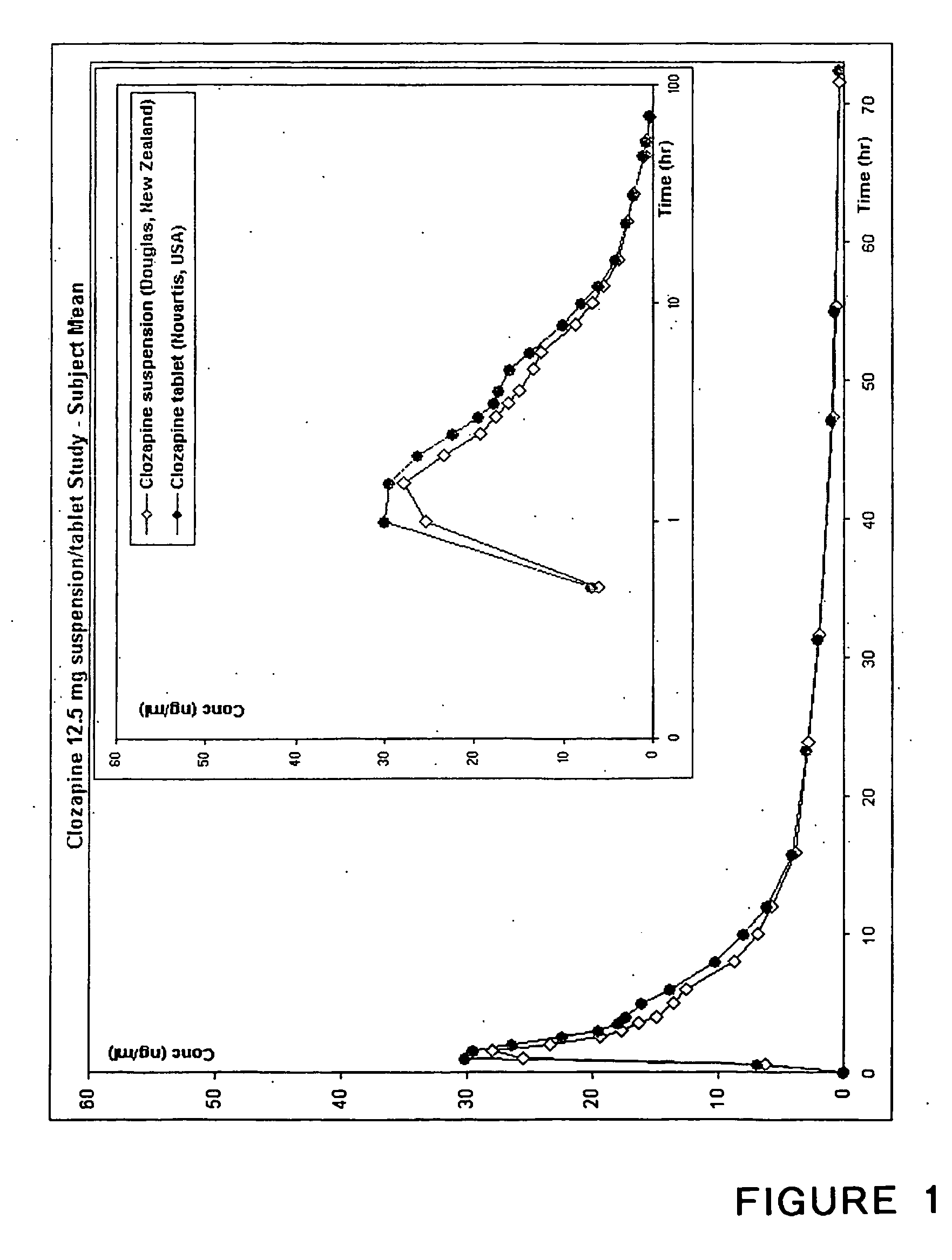
Stable clozapine suspension formulation
Owner:DOUGLAS PHARMA
Compositions and methods for the administration clozapine formulations which modulate body weight
InactiveUS20070093471A1Disintegrates quicklyEffect is exertedBiocidePill deliveryIn patientClozapine
Embodiments of the invention describe compositions and methods for the administration of fast disintegrating atypical antipsychotics which reduce weight in patients previously taking conventional formulation of atypical antipsychotics. In a preferred embodiment said fast dissolving atypical antipsychotic is FAZACLO.
Owner:AZUR PHARMA III
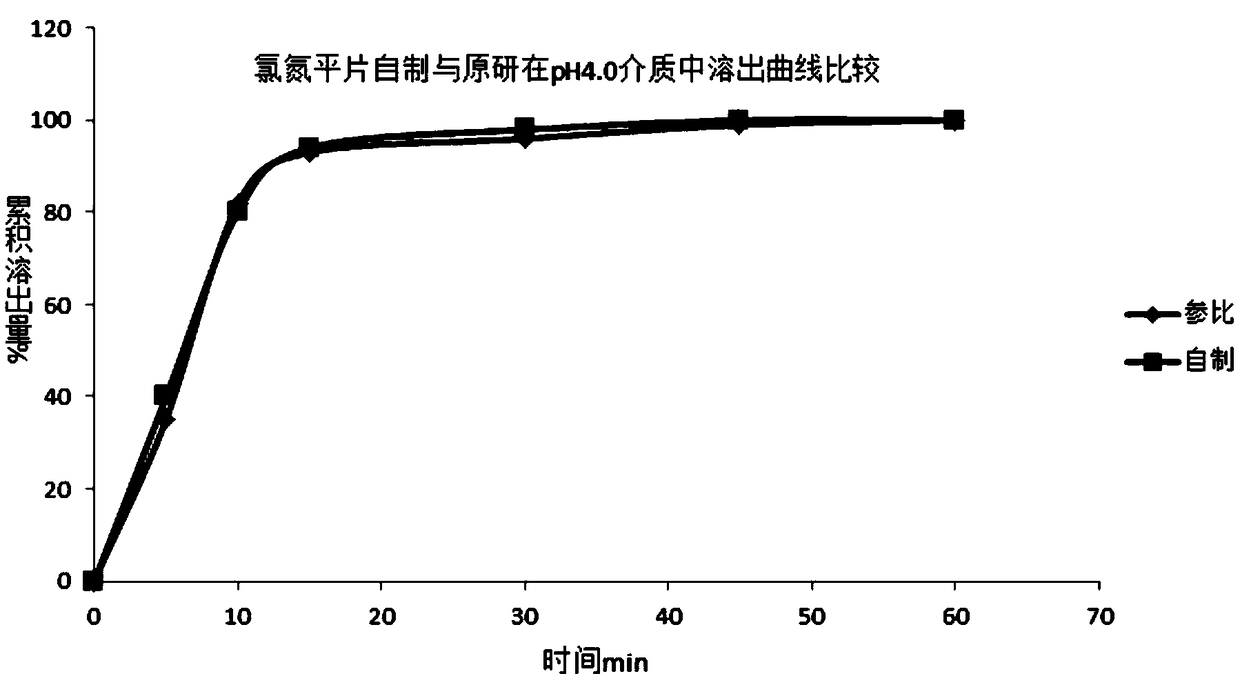
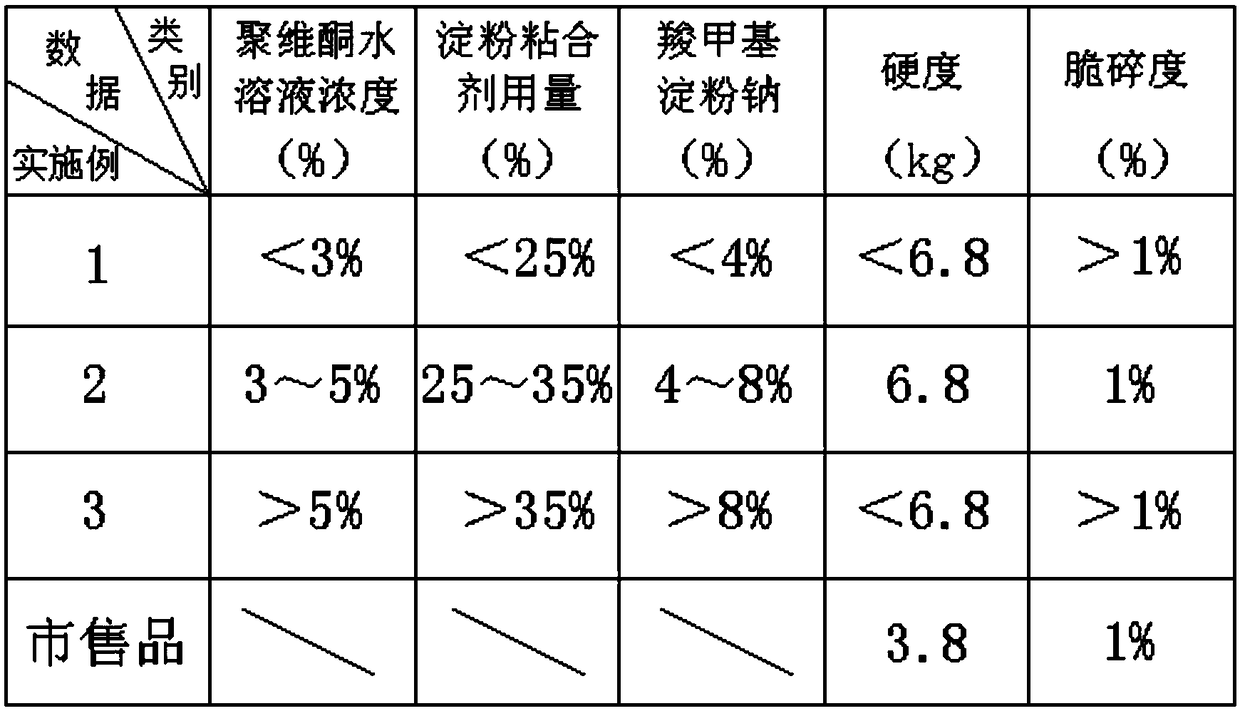
Clozapine tablet and preparation method thereof
InactiveCN108619103ASolve the problem of poor hardnessHigh similarityOrganic active ingredientsNervous disorderAdditive ingredientMedicine
The invention discloses a clozapine tablet and a preparation method thereof. The clozapine tablet is prepared from the main material ingredients in mass fraction: 25 parts of clozapine, 61.2 parts ofmicrocrystalline cellulose, 3.8 parts of lactose, the defined amount of povidone, 0.5 part of magnesium stearate, 1.4 parts of talcum powder and 0.4 part of silicon dioxide. The preparation method ofthe clozapine tablet comprises the following specific operation steps: step 1, sieving the clozapine by a 100-mesh sieve for standby application; step 2, weighing the clozapine, the microcrystalline cellulose and the lactose according to a formula in the claim 1 and jointly putting into a wet mixing granulator to be mixed evenly; step 3, weighing the defined amount of the povidone and adding waterto dissolve the povidone to be prepared into a water solution; step 4, adding the defined amount of binding agent into mixed powder in the last step to be prepared into an appropriate flexible material. According to the clozapine tablet disclosed by the invention, existing general auxiliary materials are utilized, and the problems that an existing formula has poor compressibility, tablet core hardness is poor, and the tablet split phenomenon happens in a conveying process are solved by optimizing the formula and a production technology.
Owner:南京双科医药开发有限公司
Genomic testing for effective therapies and determination of dosing strategy
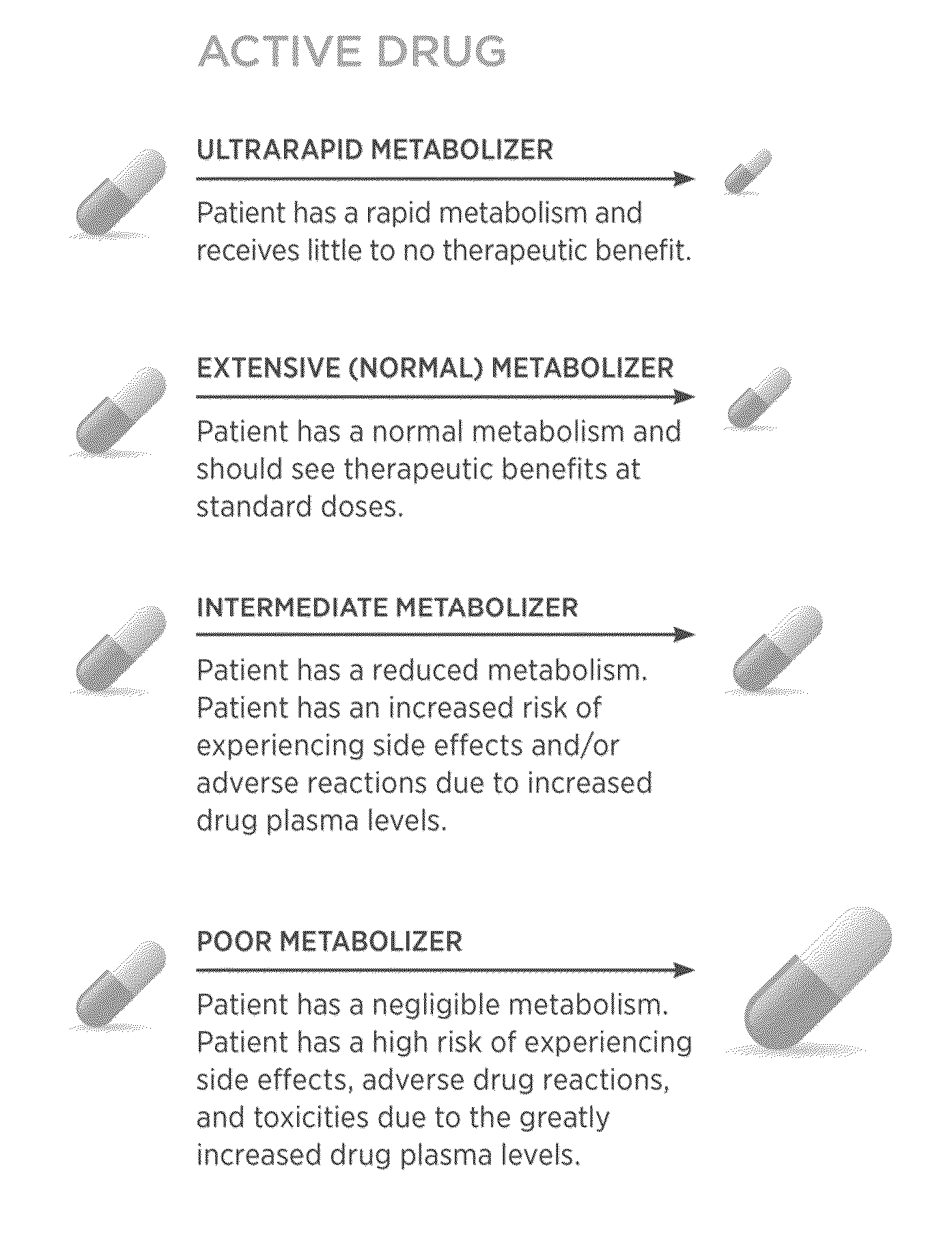
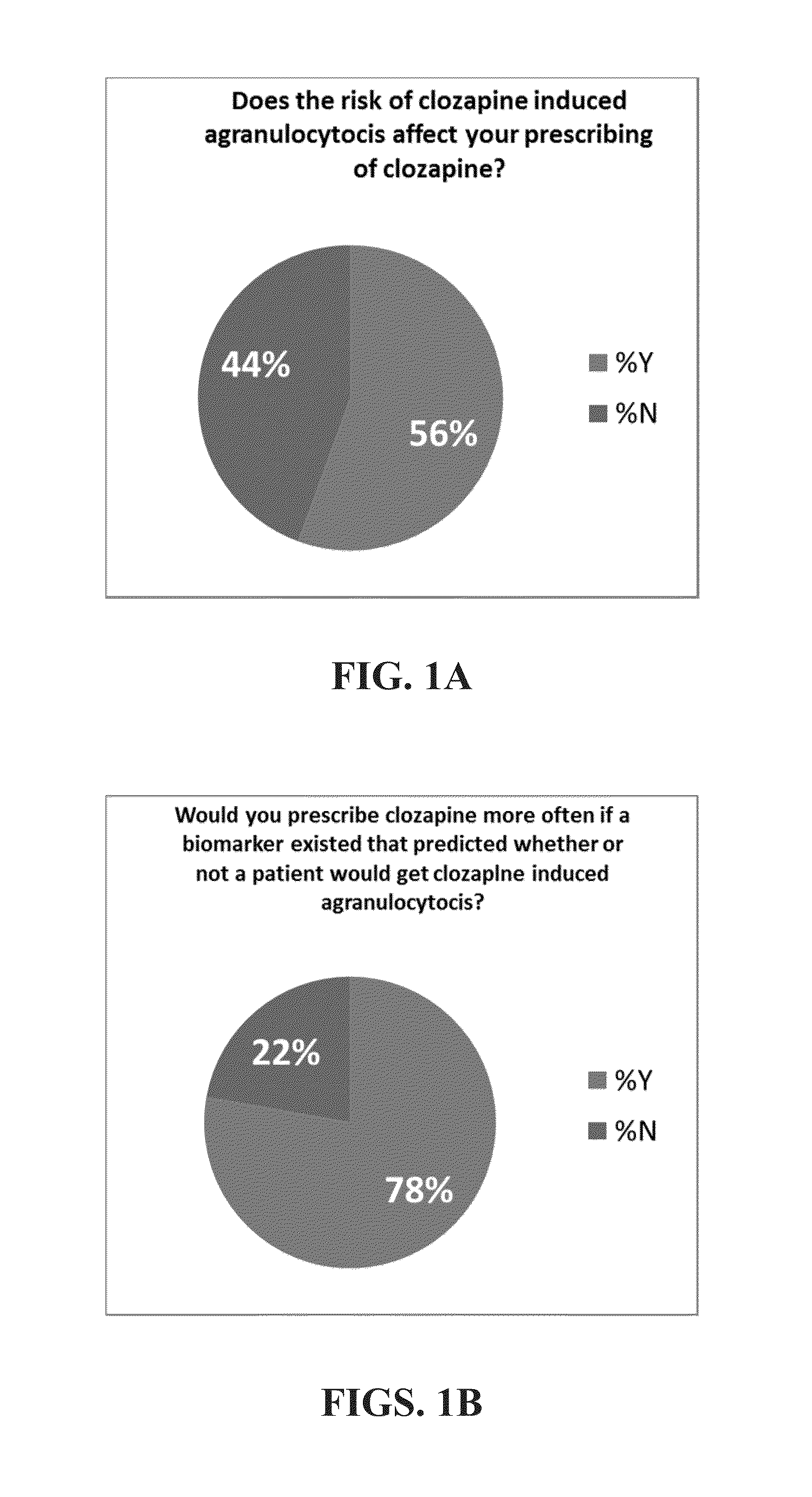
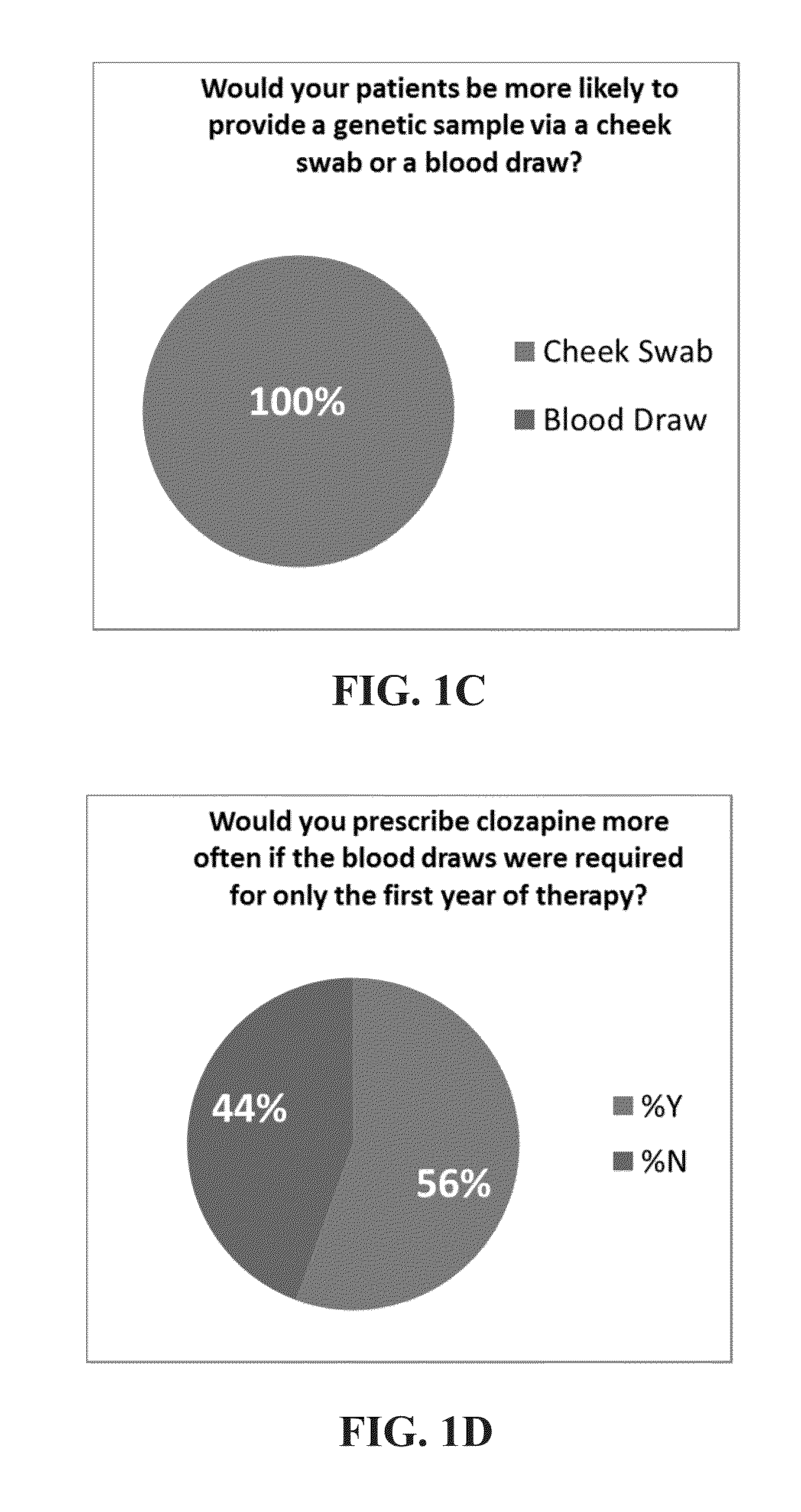
InactiveUS20150265628A1Good curative effectReduce chanceBiocideNervous disorderDosing regimenSide effect
Several embodiments disclosed herein relate to methods for calculating a dosing regimen that is tailored specifically to the genetic and metabolic profile of a particular patient. In some embodiments, the dosing regimen is related to administration of the antipsychotic drug clozapine, and the tailoring of the regimen reduces risk of adverse side effects.
Owner:COMPANION DX REFERENCE LAB
Clozapine tablet medicine composition and preparation method
ActiveCN109030684AExcellent Tablet PerformanceGood chemical stabilityOrganic active ingredientsNervous disorderHydrogen phosphateDiluent
The invention relates to a clozapine tablet medicine composition and a preparation method. The clozapine tablet medicine composition provided by the invention comprises clozapine, diluent, disintegrant, glidant, binder and lubricant. The lubricant is magnesium stearate. The diluent is selected from microcrystalline cellulose, lactose, calcium hydrogen phosphate and combination thereof. The invention also relates to the preparation method of the clozapine tablet medicine composition, a quality detection method of the clozapine tablet medicine composition and application of the clozapine tabletmedicine composition to preparation of medicine for preventing or treating schizophrenia. The clozapine tablet medicine composition provided by the invention presents excellent pharmaceutical performance, such as excellent chemical stability and excellent digestion performance.
Owner:HUNAN DONGTING PHARMA
Clozapine immunoassay
Novel conjugates and immunogens derived from clozapine and antibodies generated by these immunogens are useful in immunoassays for the quantification and monitoring of clozapine in biological fluids.
Owner:SALADAX BIOMEDICAL INC
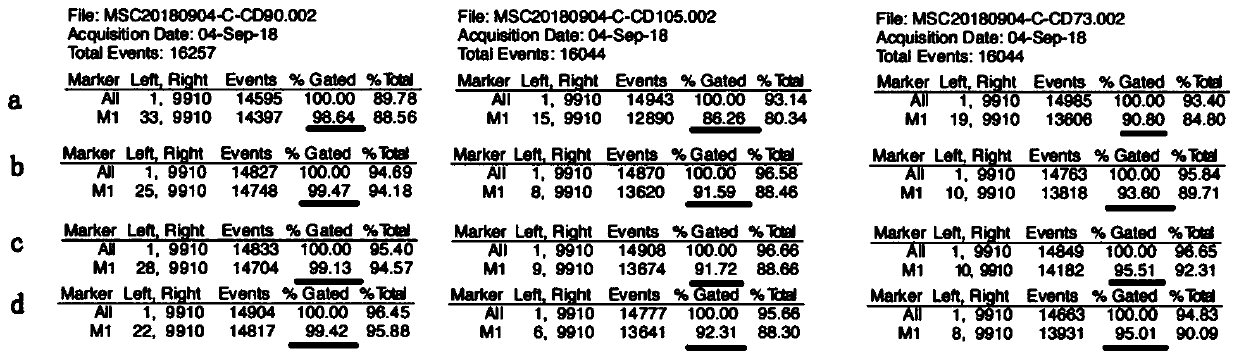
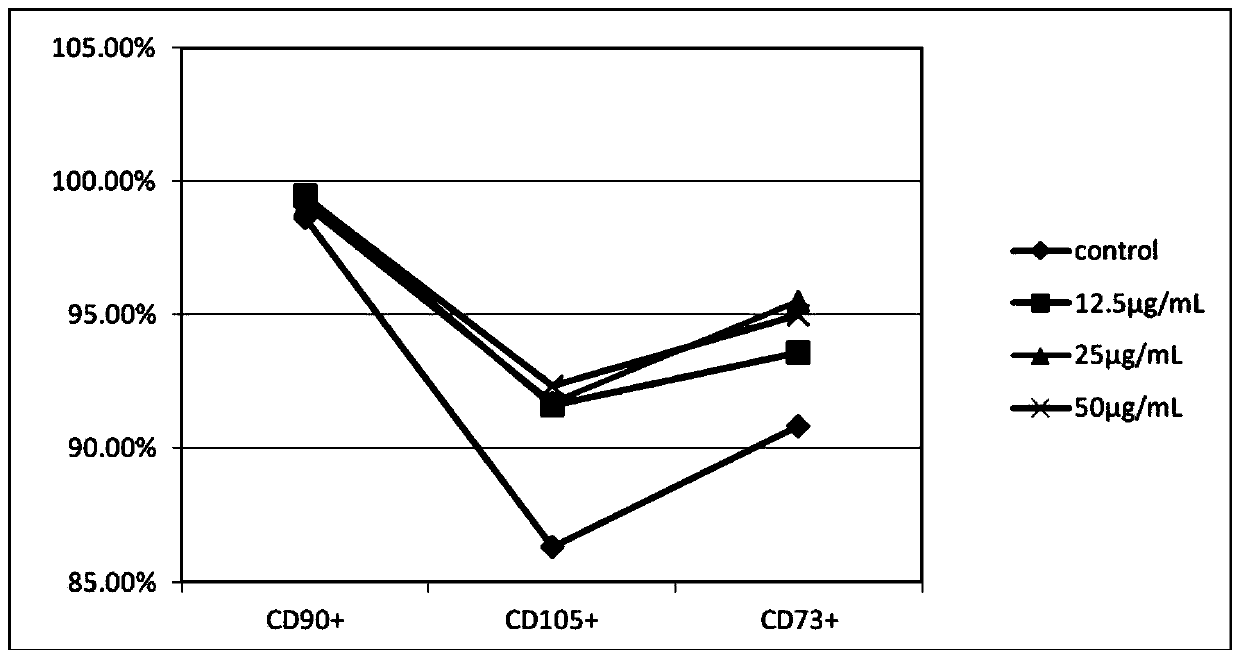
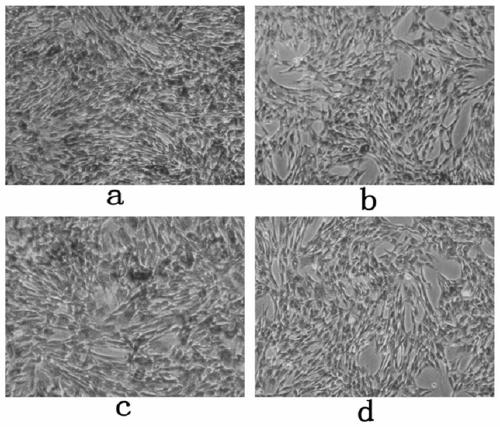
Application of clozapine in delaying senescence of cultured mesenchymal stem cells
ActiveCN109762784AGuaranteed efficacyNo toxicitySkeletal/connective tissue cellsMesenchymal stem cellBiochemistry
The invention discloses application of clozapine in delaying senescence of cultured mesenchymal stem cells, and belongs to the technical field of cell culture. A clozapine diluent is more convenient,safer and more efficient to use in the process of delaying the senescence of the mesenchymal stem cells, and the cost is low.
Owner:广州瑞铂茵健康科技有限公司
Stable clozapine suspension formulation
Owner:DOUGLAS PHARMA
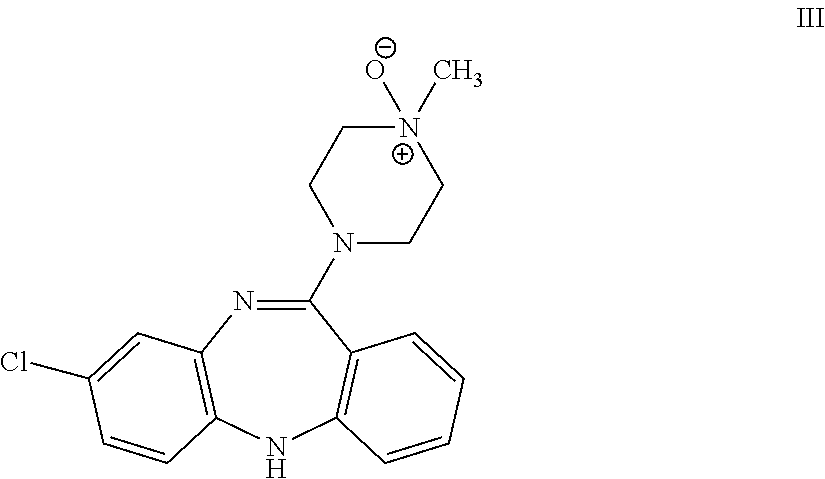
AMINO SUBSTITUTED DIARYL[a,d]CYCLOHEPTENE ANALOGS AS MUSCARINIC AGONISTS AND METHODS OF TREATMENT OF NEUROPSYCHIATRIC DISORDERS
Disclosed herein are analogs of clozapine and pharmaceutically acceptable salts, esters, amides, or prodrugs thereof; methods of synthesizing the analogs; and methods of using the analogs for treating neuorpsychiatric disorders. In some embodiments, the analogs are amino substituted diaryl[a,d]cycloheptenes.
Owner:ACADIA PHARMA INC
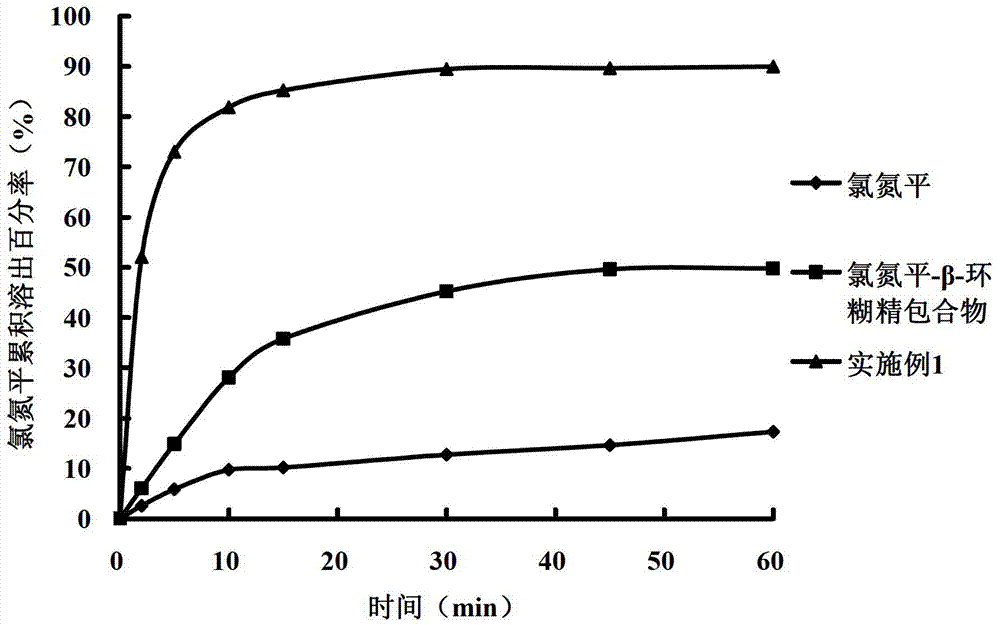
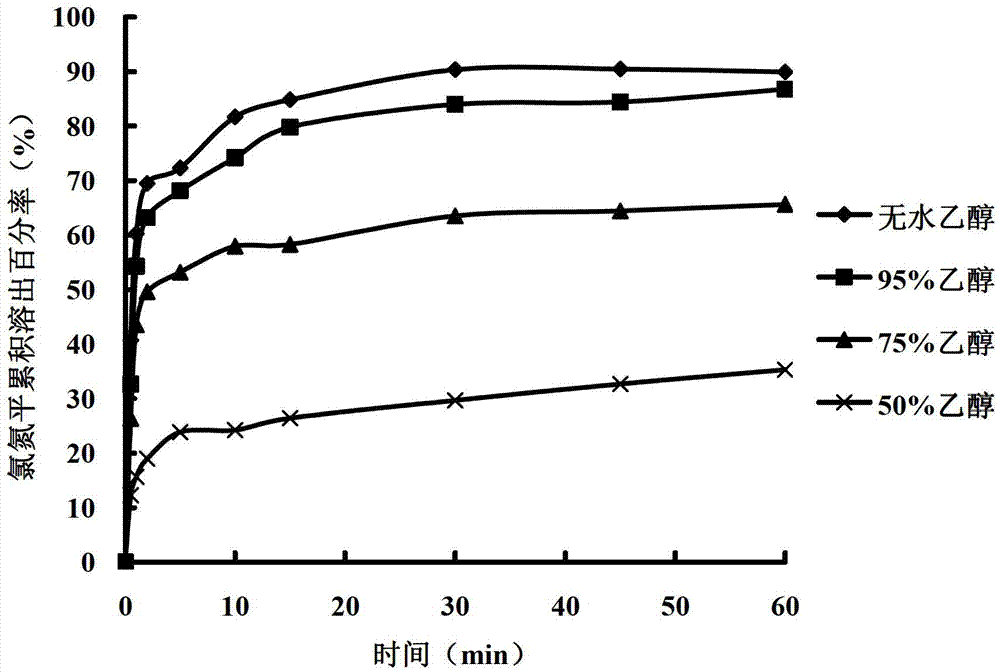
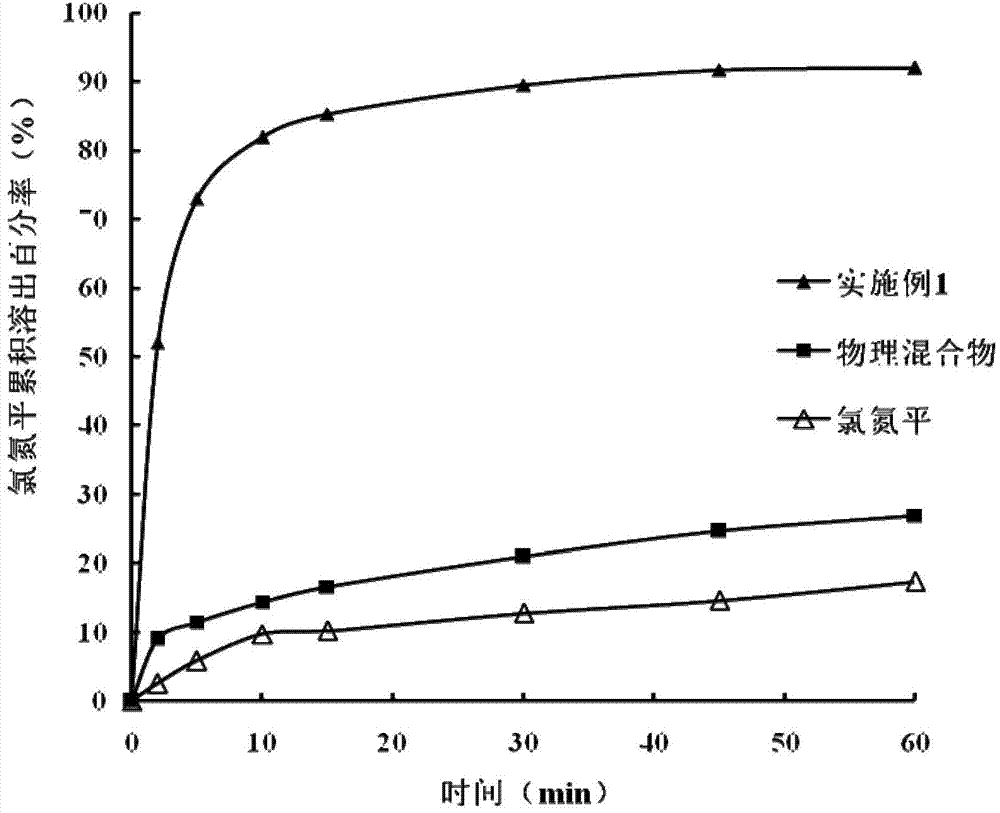
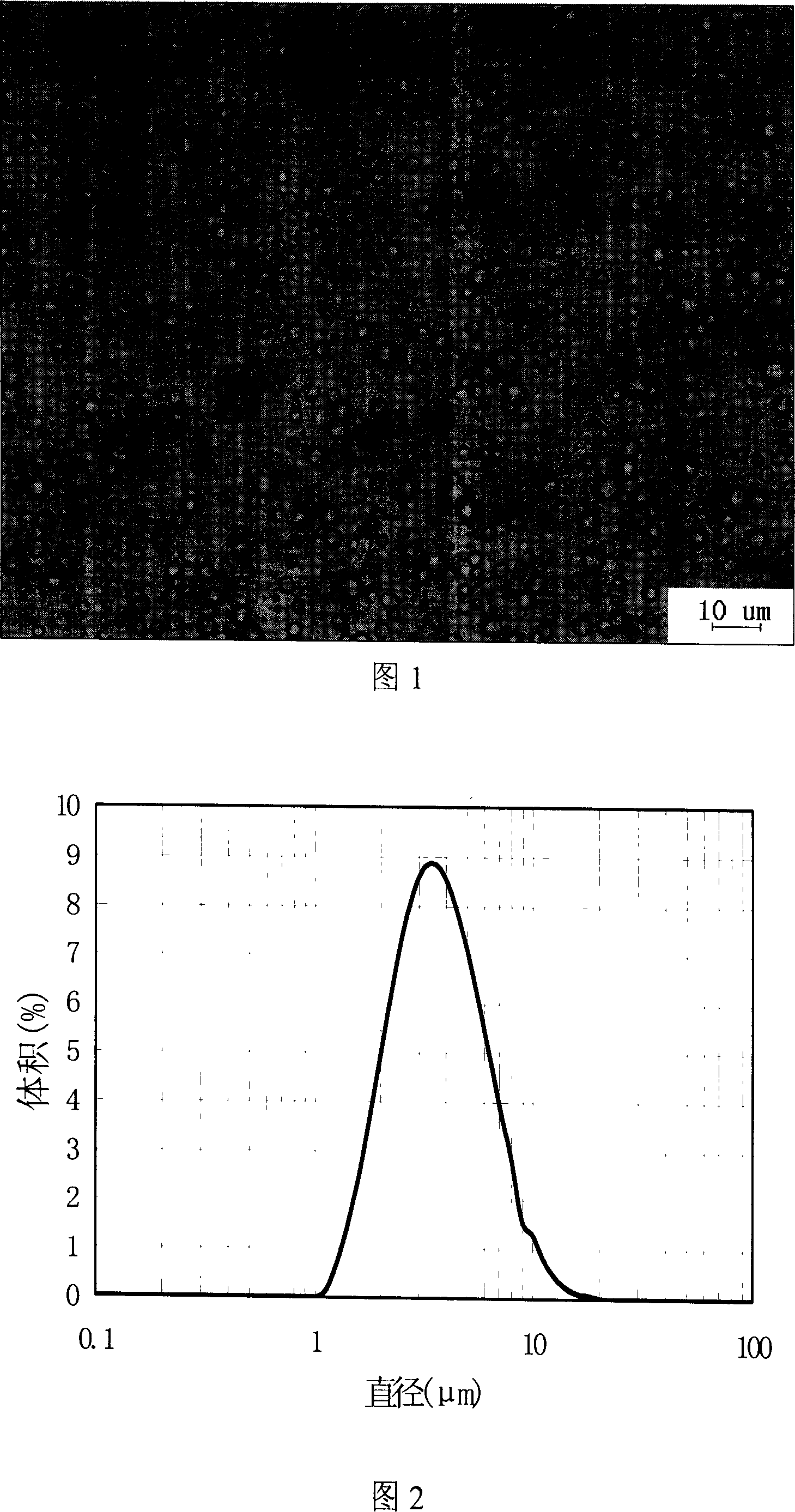
Clozapine cyclodextrin inclusion compound and preparation method thereof
The invention belongs to the field of pharmaceutic preparations and specifically relates to a clozapine cyclodextrin inclusion compound and a preparation method thereof. The preparation method is characterized by dissolving clozapine in a solvent, performing an inclusion by cyclodextrin, and drying an inclusion compound solution to obtain the clozapine cyclodextrin inclusion compound. The clozapine cyclodextrin inclusion compound is characterized in that the cyclodextrin is hydroxypropyl-beta-cyclodextrin, the molecule molar ratio between the clozapine and the hydroxypropyl-beta-cyclodextrin is (1:1)-(1:2), the solvent utilizes ethanol of 75-100% (v / v), and an ultrasound-solvent volatilization method is utilized for the inclusion. The preparation method has the advantages of being mild incondition, simple in process, easy to control and short in production cycle.
Owner:CHINA PHARM UNIV
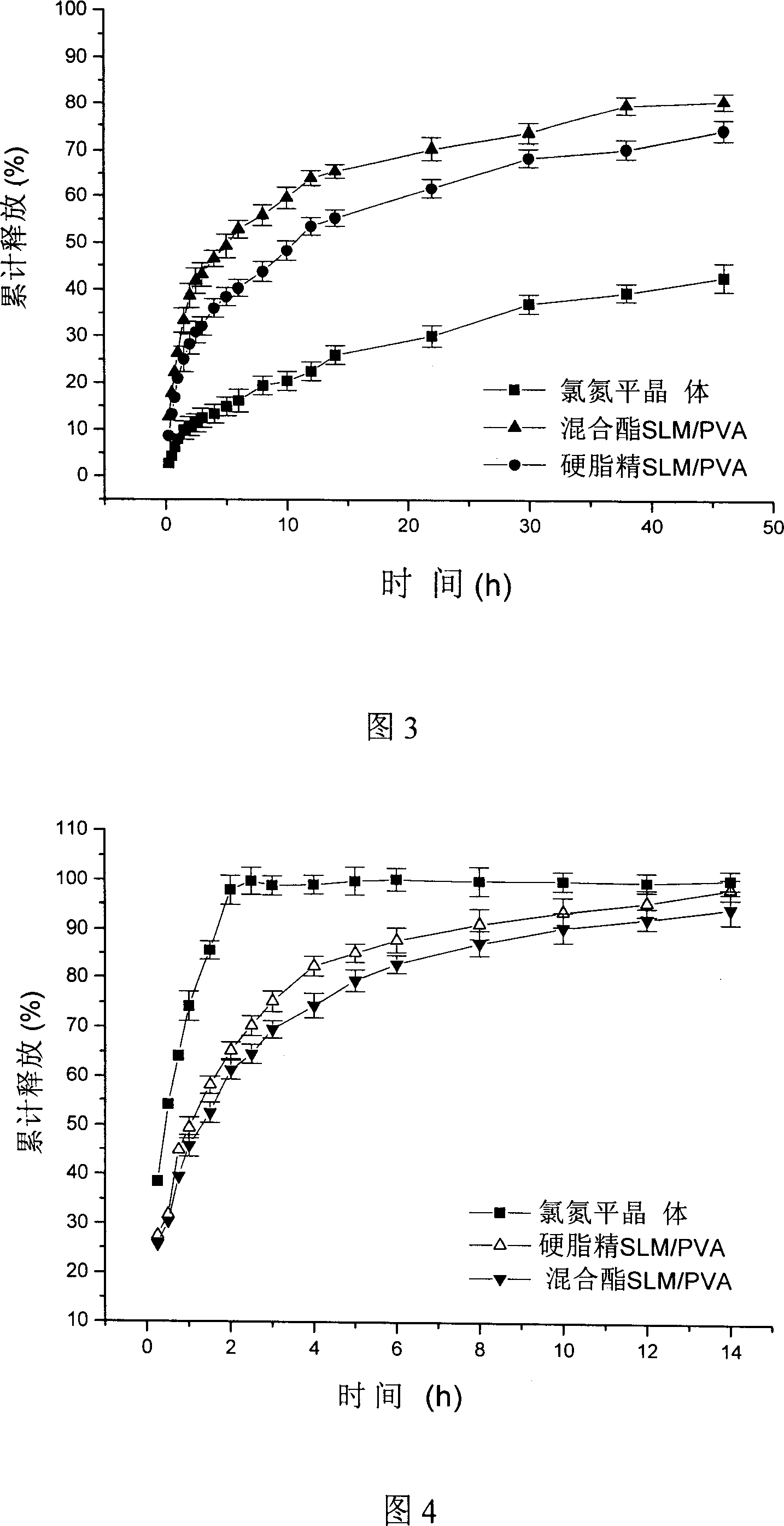
Sustained release system of clozapine solid liposome microparticle
InactiveCN1969861AControl releaseFacilitated releaseOrganic active ingredientsNervous disorderSaturated fatty acid esterMicroparticle
The invention discloses a clozapine solid liposome microparticle control slow-release system, which is characterized by the following: adopting high-fusing point and biological compatible solid liposome as carrier material such as fatty acid ester, fatty acid, fatty alcohol or their two composition; making surface activator or macromolecule as stabilizer such as lecithin, poloxamer series, polysorbate series, cholate and so on.
Owner:SOUTH CHINA UNIV OF TECH
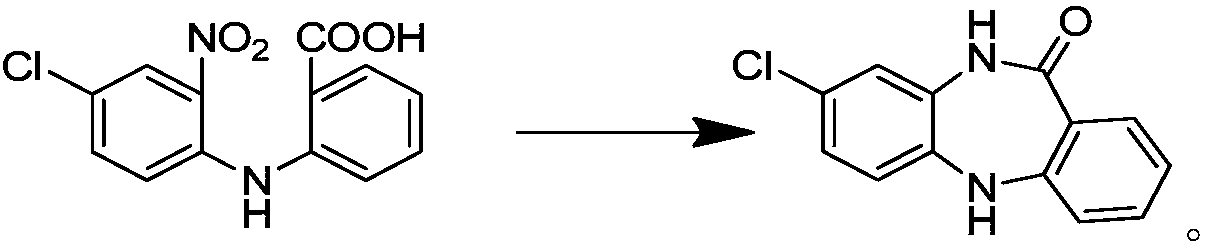
Synthetic method of key intermediate for preparing clozapine
The invention discloses a synthetic method of a key intermediate for preparing clozapine, and belongs to the field of drug synthesis. The method comprises the step that 2-((2-amino-4-chlorphenyl)amino)benzoic acid is taken as a raw material to be cyclized under backflow of a sulfuric acid water solution, and 8-chloro-5,10-dihydro-11H-dibenzo[b,e][1,4]-dinitro-11-ketone. The formula is shown in thedescription. The synthetic method utilizes the sulfuric acid water solution to conduct cyclization, and overcomes the multiple defects in the prior art, the reaction is simple, convenient and rapid,the impurities introduced by the raw material can be effectively removed, the product with the higher purity is obtained, and the waste liquid is easy to treat. Meanwhile, increasing cost and environmental pollution caused by using of other solvents are avoided, and the synthetic method has the good industrialized prospect.
Owner:唯智医药科技(北京)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders](https://images-eureka.patsnap.com/patent_img/c39d20f2-03bd-480c-bd6a-a01e1d9720ef/US20050192268A1-20050901-C00001.png)
![Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders](https://images-eureka.patsnap.com/patent_img/c39d20f2-03bd-480c-bd6a-a01e1d9720ef/US20050192268A1-20050901-C00002.png)
![Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders](https://images-eureka.patsnap.com/patent_img/c39d20f2-03bd-480c-bd6a-a01e1d9720ef/US20050192268A1-20050901-C00003.png)
![Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders](https://images-eureka.patsnap.com/patent_img/47854f59-05d8-419f-8cc7-8fcf2ee494c1/US20060194784A1-20060831-C00001.png)
![Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders](https://images-eureka.patsnap.com/patent_img/47854f59-05d8-419f-8cc7-8fcf2ee494c1/US20060194784A1-20060831-C00002.png)
![Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders](https://images-eureka.patsnap.com/patent_img/47854f59-05d8-419f-8cc7-8fcf2ee494c1/US20060194784A1-20060831-C00003.png)
![Amino bustituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders Amino bustituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders](https://images-eureka.patsnap.com/patent_img/0ab87a8e-7835-451b-8770-82c470c97c6a/US20060199798A1-20060907-C00001.png)
![Amino bustituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders Amino bustituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders](https://images-eureka.patsnap.com/patent_img/0ab87a8e-7835-451b-8770-82c470c97c6a/US20060199798A1-20060907-C00002.png)
![Amino bustituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders Amino bustituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders](https://images-eureka.patsnap.com/patent_img/0ab87a8e-7835-451b-8770-82c470c97c6a/US20060199798A1-20060907-C00003.png)





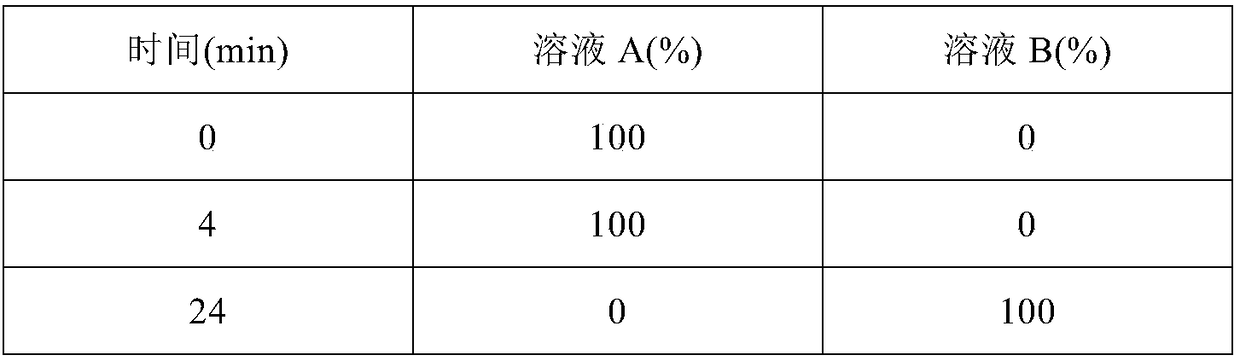
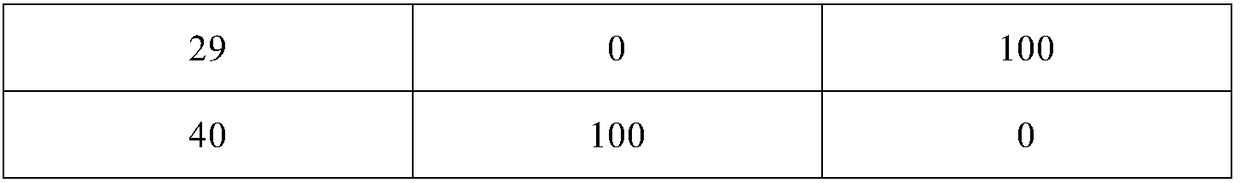
![AMINO SUBSTITUTED DIARYL[a,d]CYCLOHEPTENE ANALOGS AS MUSCARINIC AGONISTS AND METHODS OF TREATMENT OF NEUROPSYCHIATRIC DISORDERS AMINO SUBSTITUTED DIARYL[a,d]CYCLOHEPTENE ANALOGS AS MUSCARINIC AGONISTS AND METHODS OF TREATMENT OF NEUROPSYCHIATRIC DISORDERS](https://images-eureka.patsnap.com/patent_img/7f27281b-69f2-4cd4-961f-24f48a5deff4/US20090239840A1-20090924-D00001.png)
![AMINO SUBSTITUTED DIARYL[a,d]CYCLOHEPTENE ANALOGS AS MUSCARINIC AGONISTS AND METHODS OF TREATMENT OF NEUROPSYCHIATRIC DISORDERS AMINO SUBSTITUTED DIARYL[a,d]CYCLOHEPTENE ANALOGS AS MUSCARINIC AGONISTS AND METHODS OF TREATMENT OF NEUROPSYCHIATRIC DISORDERS](https://images-eureka.patsnap.com/patent_img/7f27281b-69f2-4cd4-961f-24f48a5deff4/US20090239840A1-20090924-C00001.png)
![AMINO SUBSTITUTED DIARYL[a,d]CYCLOHEPTENE ANALOGS AS MUSCARINIC AGONISTS AND METHODS OF TREATMENT OF NEUROPSYCHIATRIC DISORDERS AMINO SUBSTITUTED DIARYL[a,d]CYCLOHEPTENE ANALOGS AS MUSCARINIC AGONISTS AND METHODS OF TREATMENT OF NEUROPSYCHIATRIC DISORDERS](https://images-eureka.patsnap.com/patent_img/7f27281b-69f2-4cd4-961f-24f48a5deff4/US20090239840A1-20090924-C00002.png)