Patents
Literature
78 results about "Asenapine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
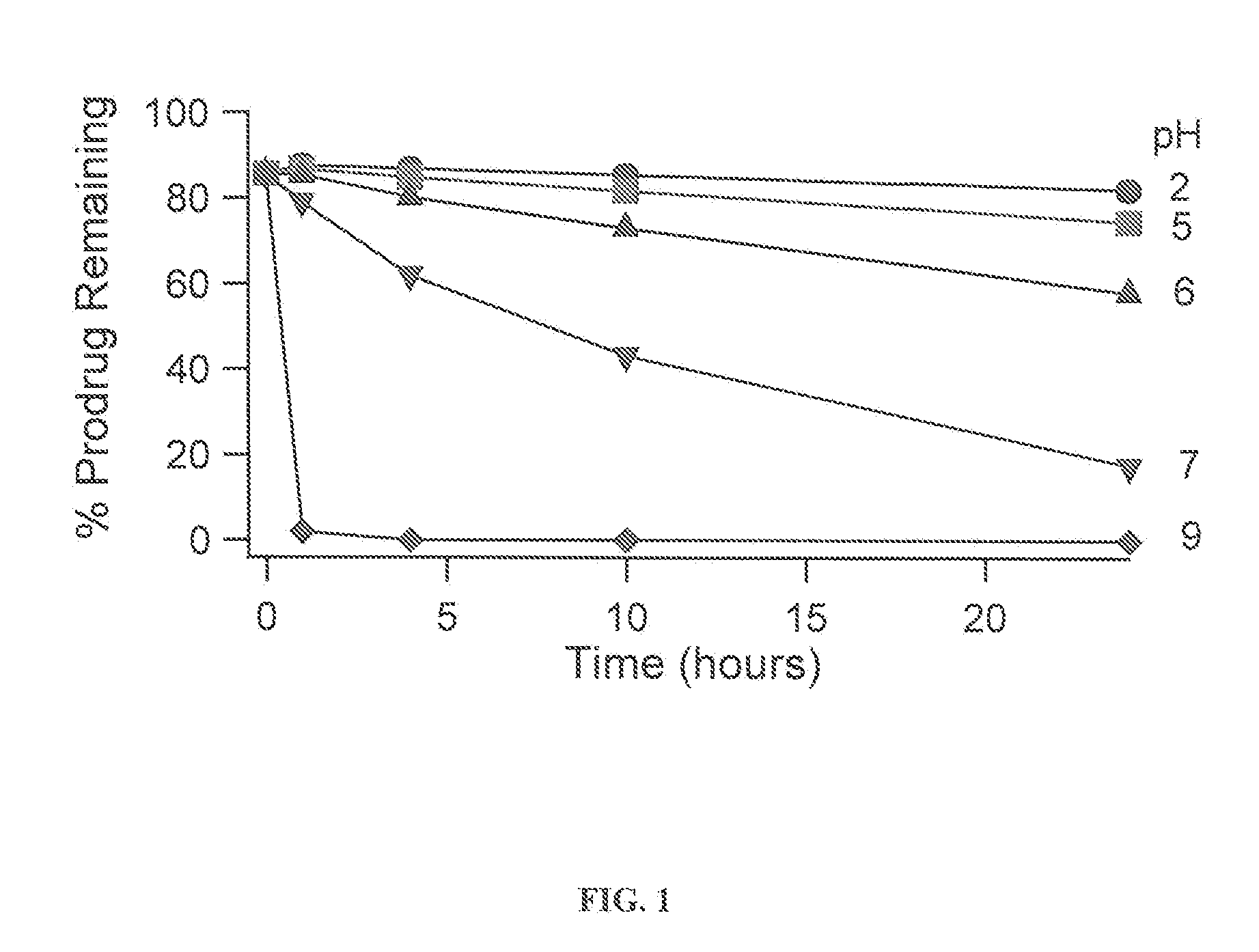
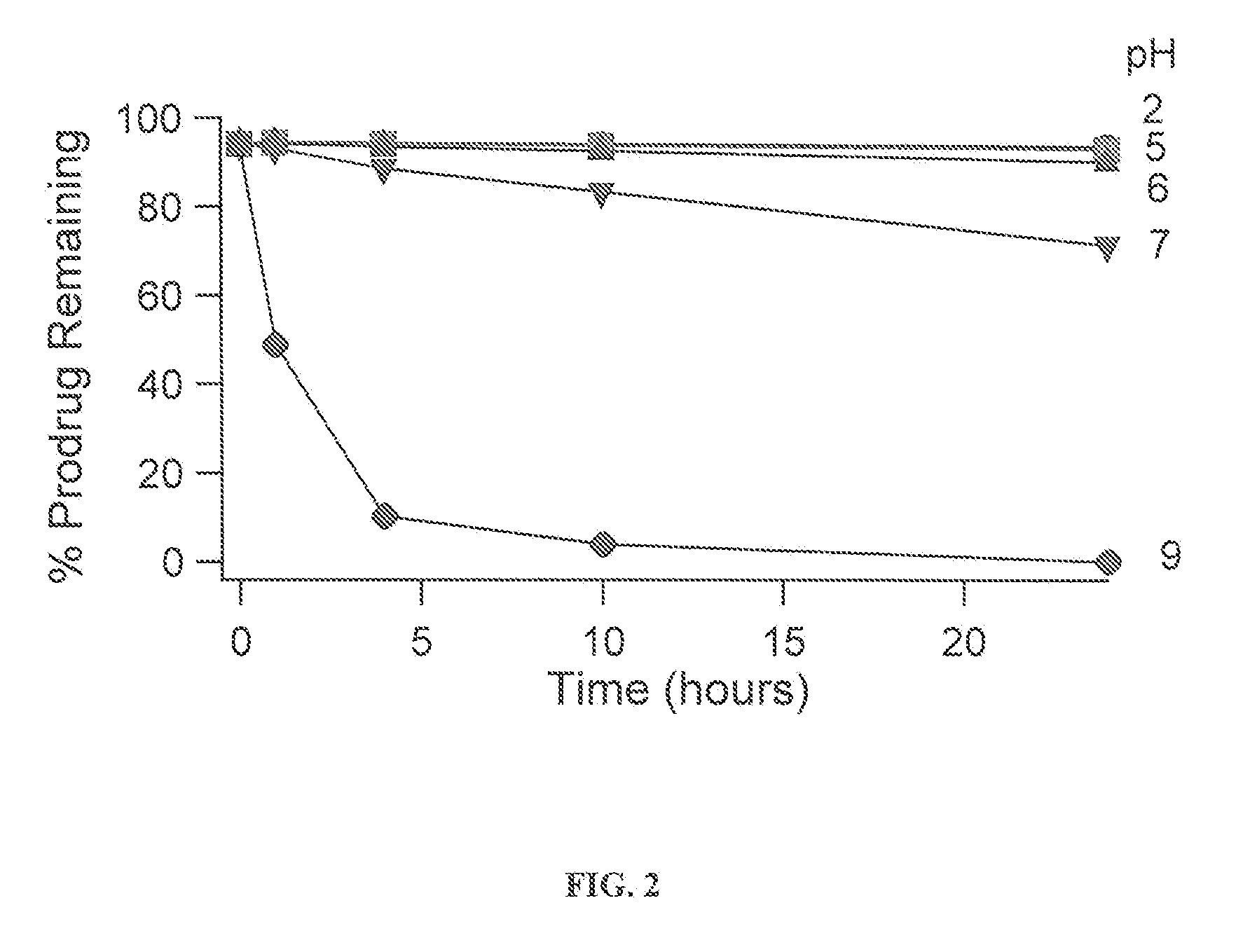
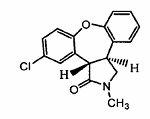
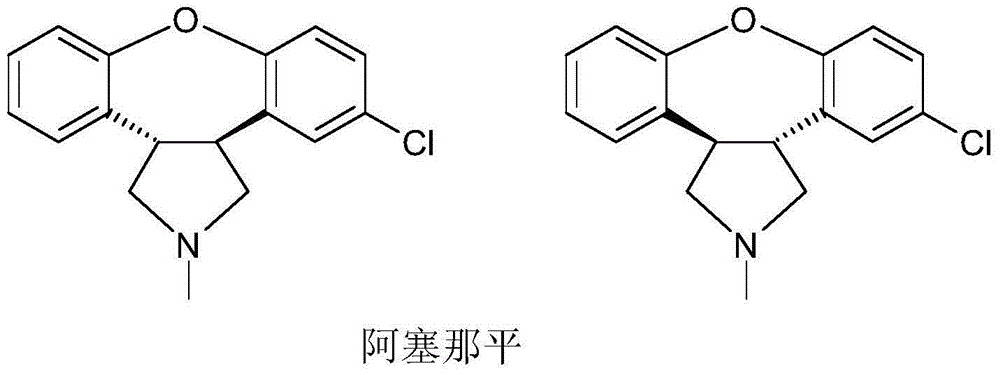
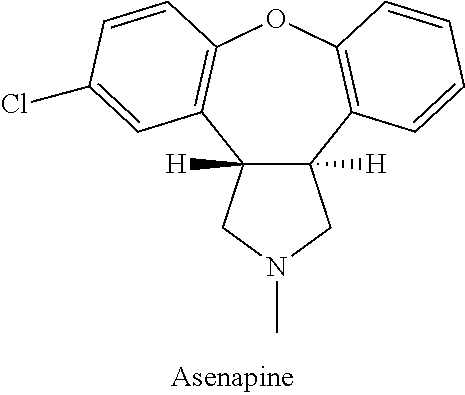
This medication is used to treat certain mental/mood disorders (such as schizophrenia, bipolar disorder).
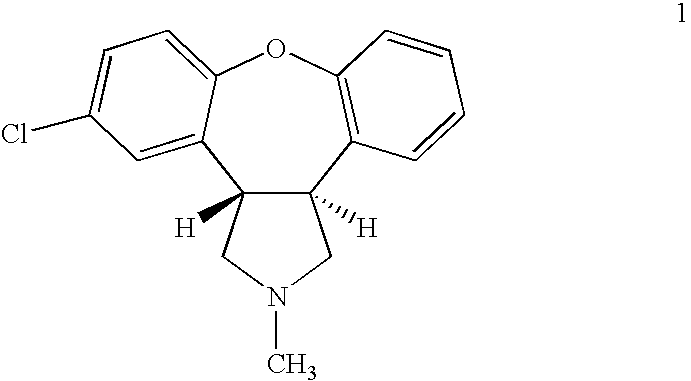
Asenapine Prodrugs
InactiveUS20110166194A1Minimize exposureMinimize diffusionBiocideNervous disorderBipolar type I disorderBipolar I disorder
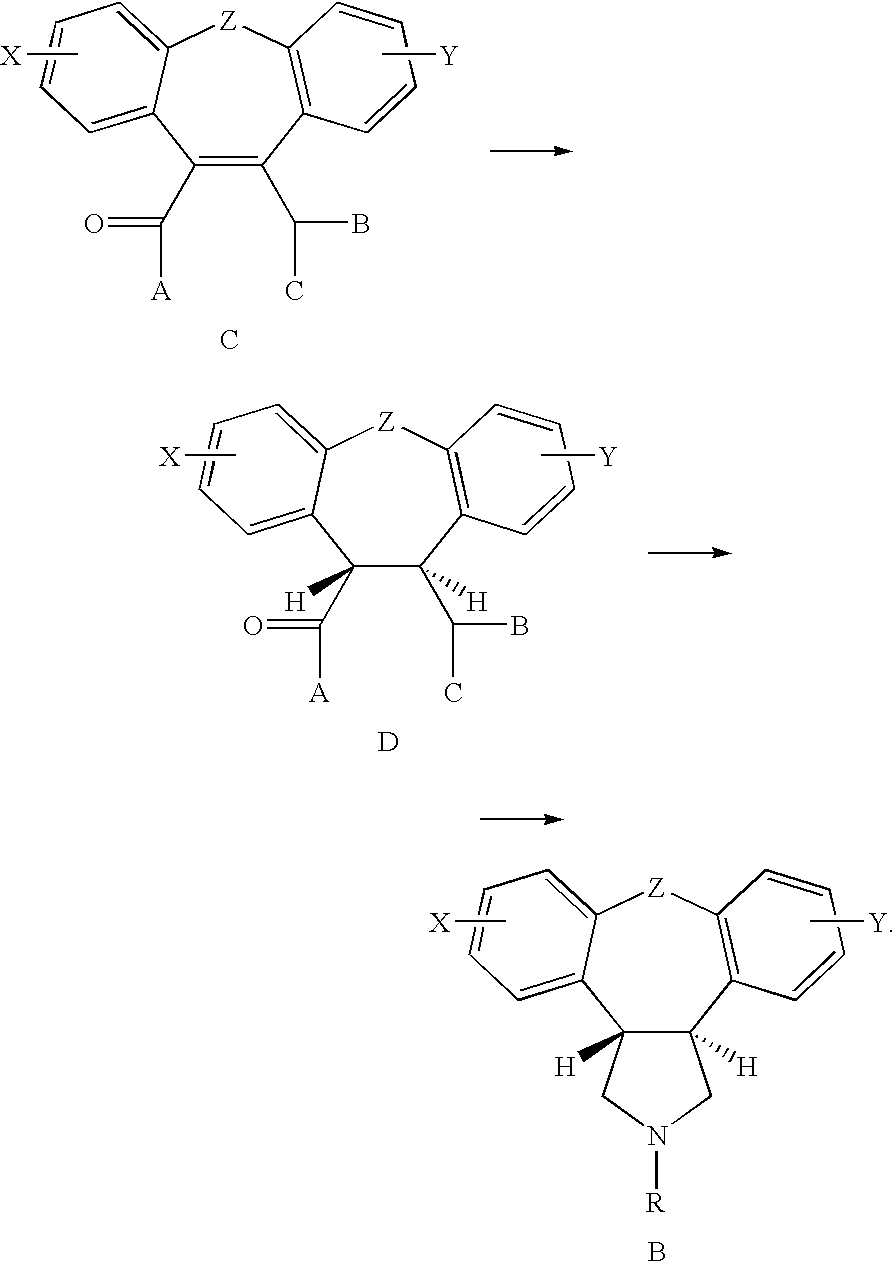
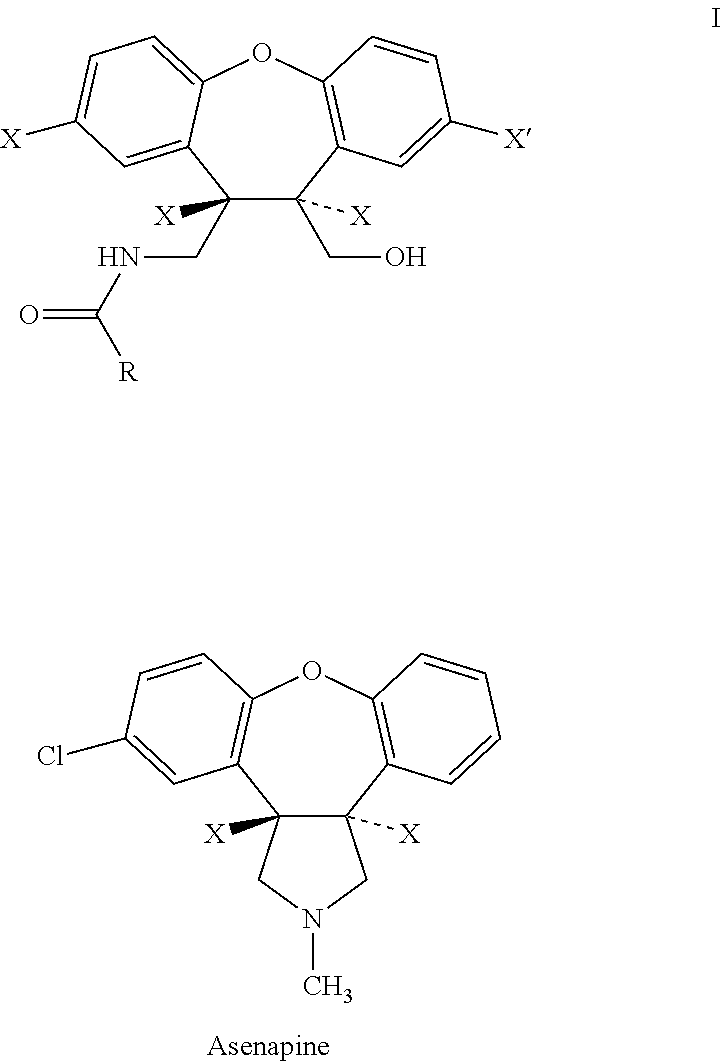
Compounds of Formula I and their use for the treatment of neurological and psychiatric disorders including schizophrenia and manic or mixed episodes associated with bipolar I disorder with or without psychotic features is disclosed:wherein R1-R8, G, N and A− are as defined in the written description.
Owner:ALKERMES INC
Patch
ActiveUS20150202183A1Suppressing plasma concentrationBiocideNervous disorderMedicineBiomedical engineering
Owner:HISAMITSU PHARM CO INC
Skin irritation suppressant and transdermal preparation
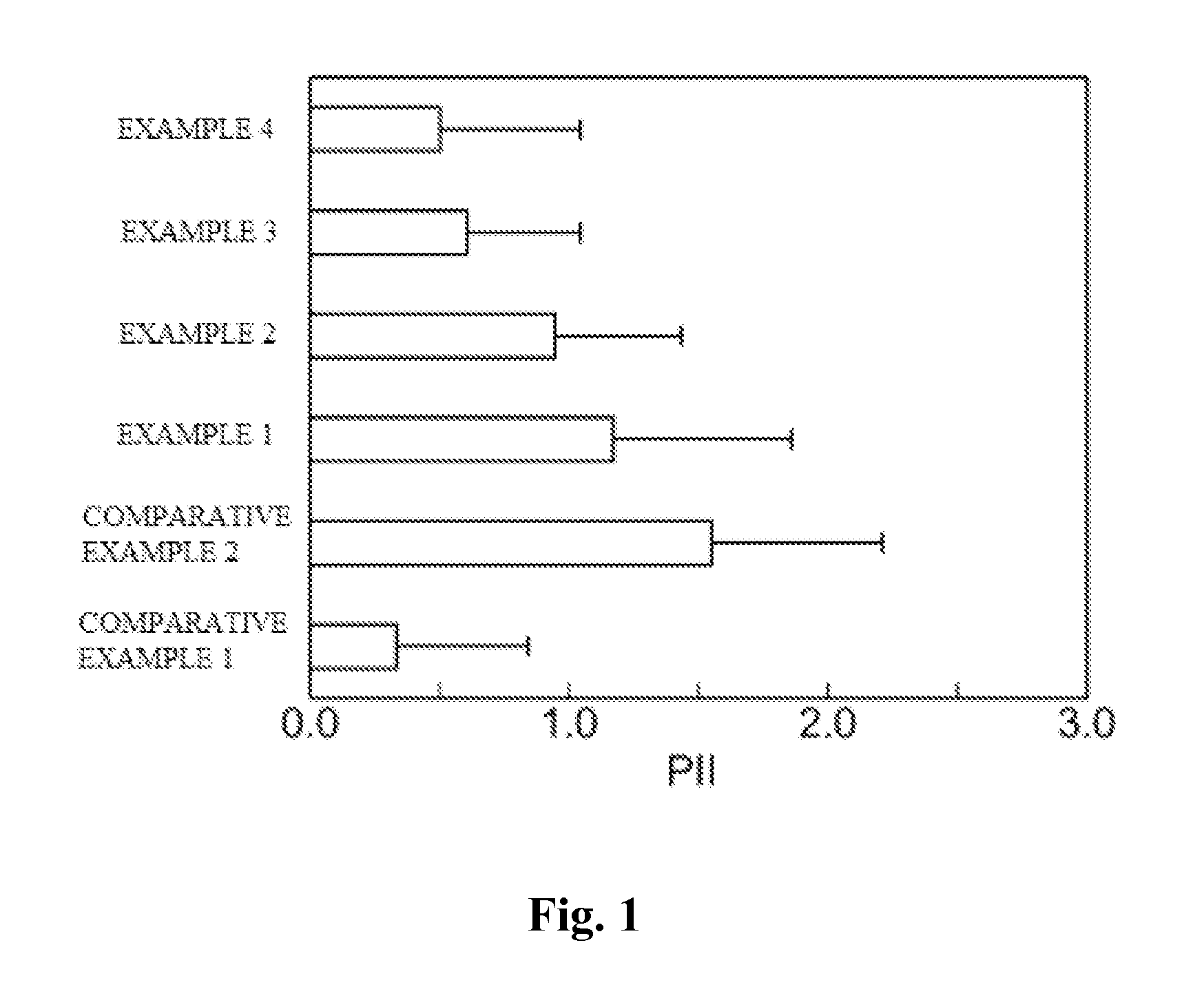
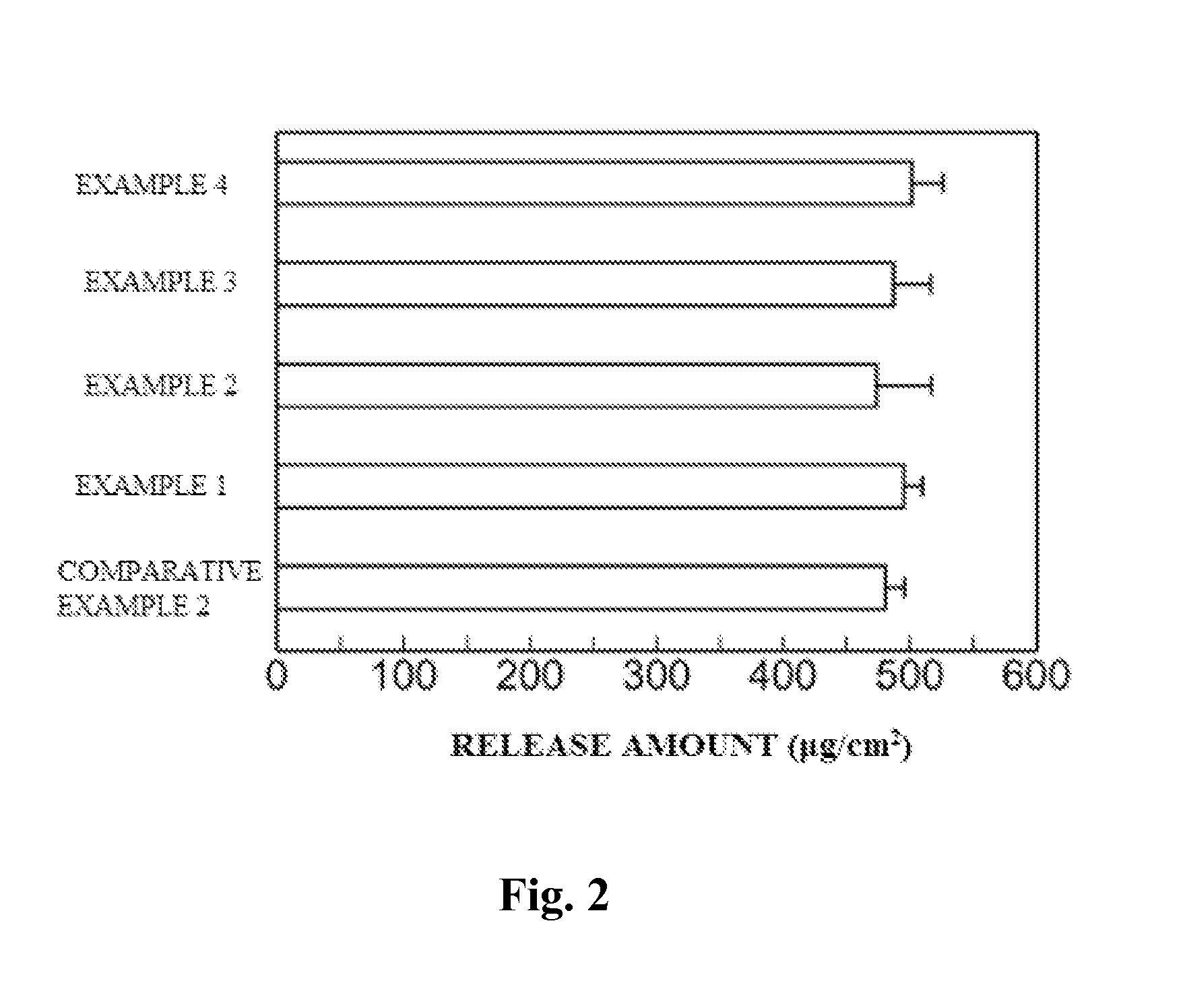
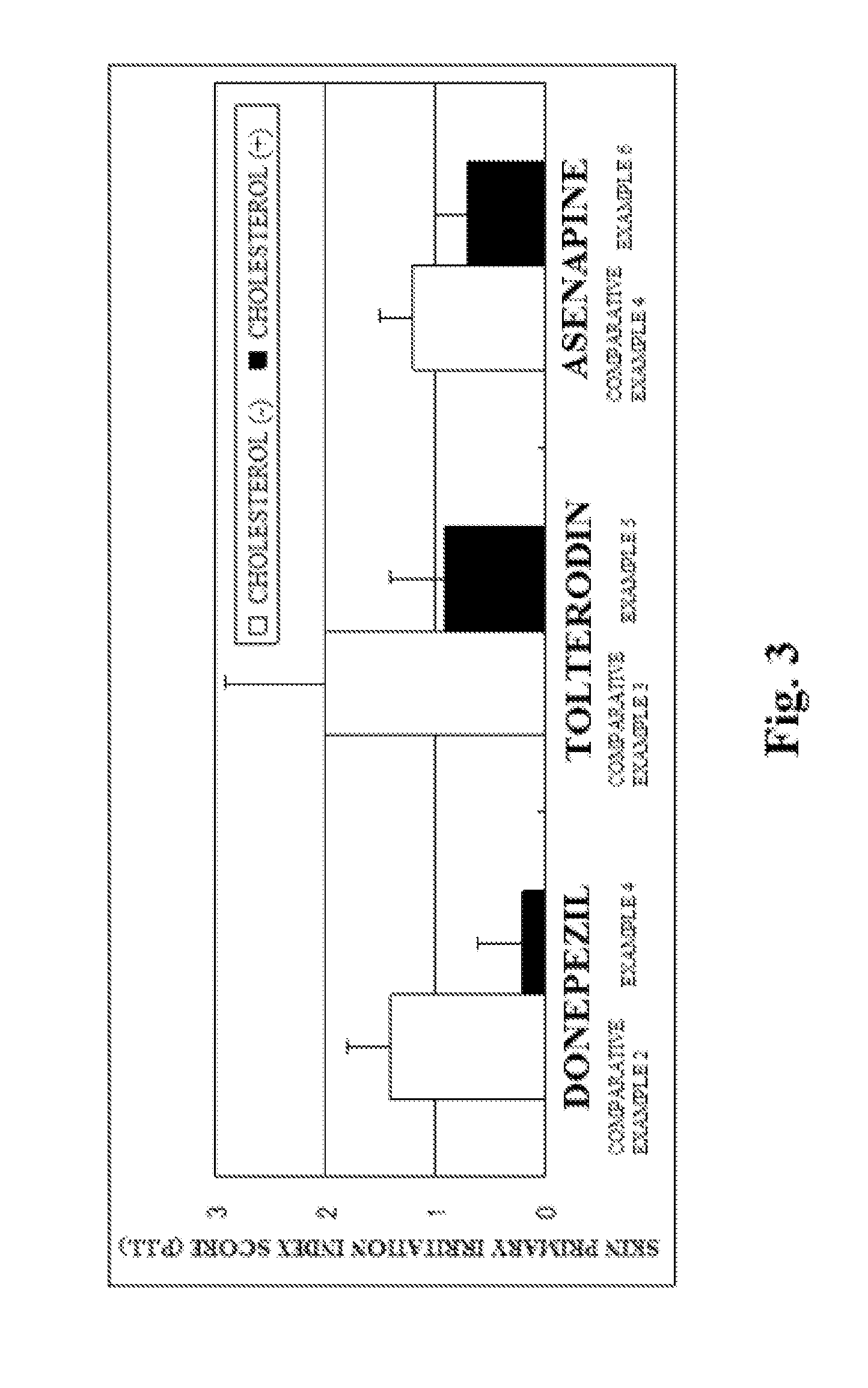
InactiveUS20130053357A1Sufficient reduction effectReduce skin effectOrganic active ingredientsBiocideCholesterol derivativeCitalopram
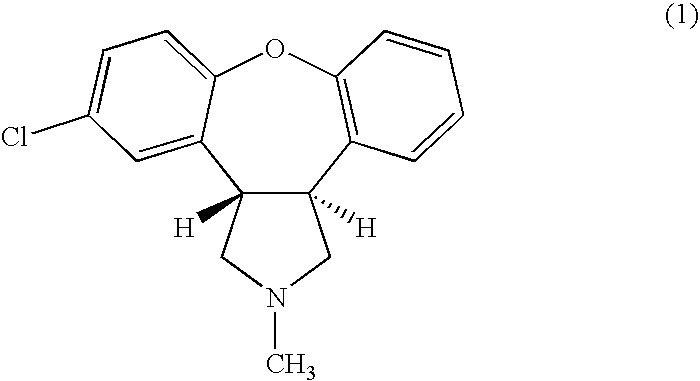
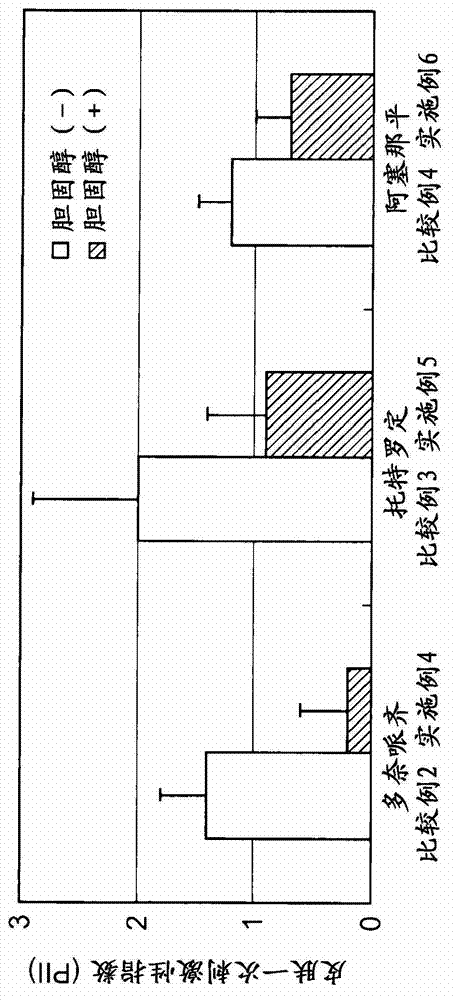
Provided is a skin irritation suppressant for transdermal preparations, having a sufficient reduction effect of skin irritation due to a drug. Also provided is a transdermal preparation comprising the skin irritation suppressant. One embodiment of the invention is a skin irritation suppressant for suppressing the skin irritation due to a drug and a pharmaceutical ingredient to be used in a transdermal preparation other than the drug, the skin irritation suppressant comprising a sterol compound selected from the group consisting of cholesterol, cholesterol derivatives and cholesterol analogs, and the drug is one or more basic drugs selected from the group consisting of tolterodine, asenapine, bisoprolol, risperidone, nicotine and citalopram, and their pharmaceutically acceptable salts.
Owner:HISAMITSU PHARM CO INC
Intranasal administration of asenapine and pharmaceutical compositions therefor
Asenapine or a pharmaceutically acceptable salt thereof can be administered intranasally, typically via an intranasal dosage formulation having a water-containing liquid carrier.
Owner:SYNTHON BV
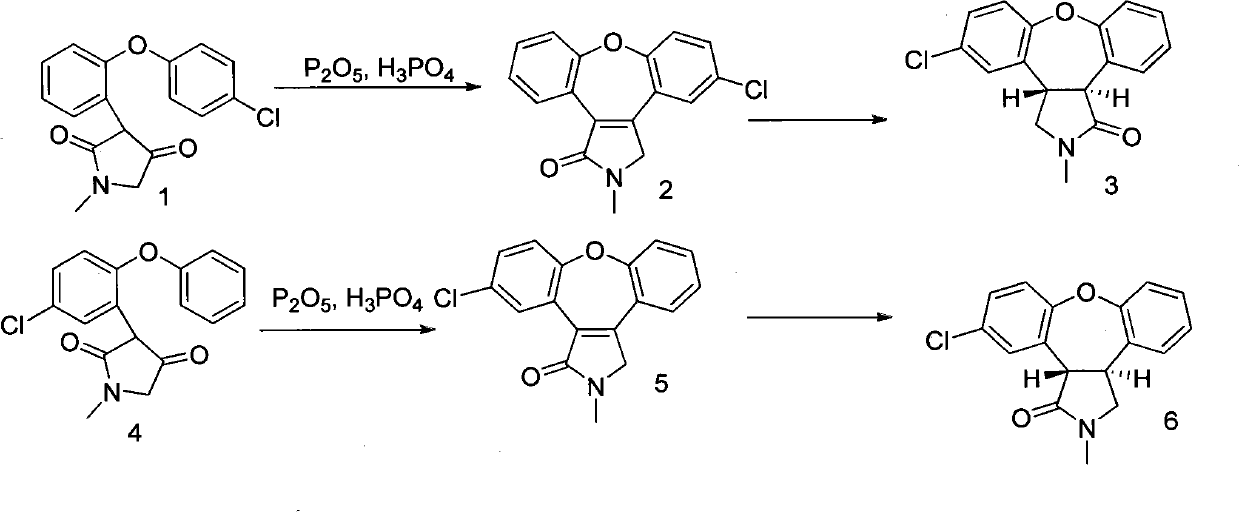
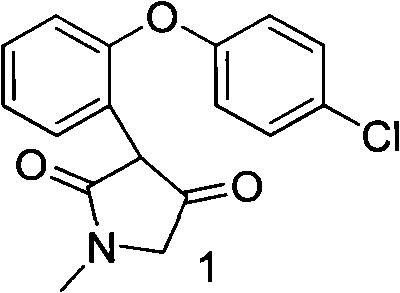
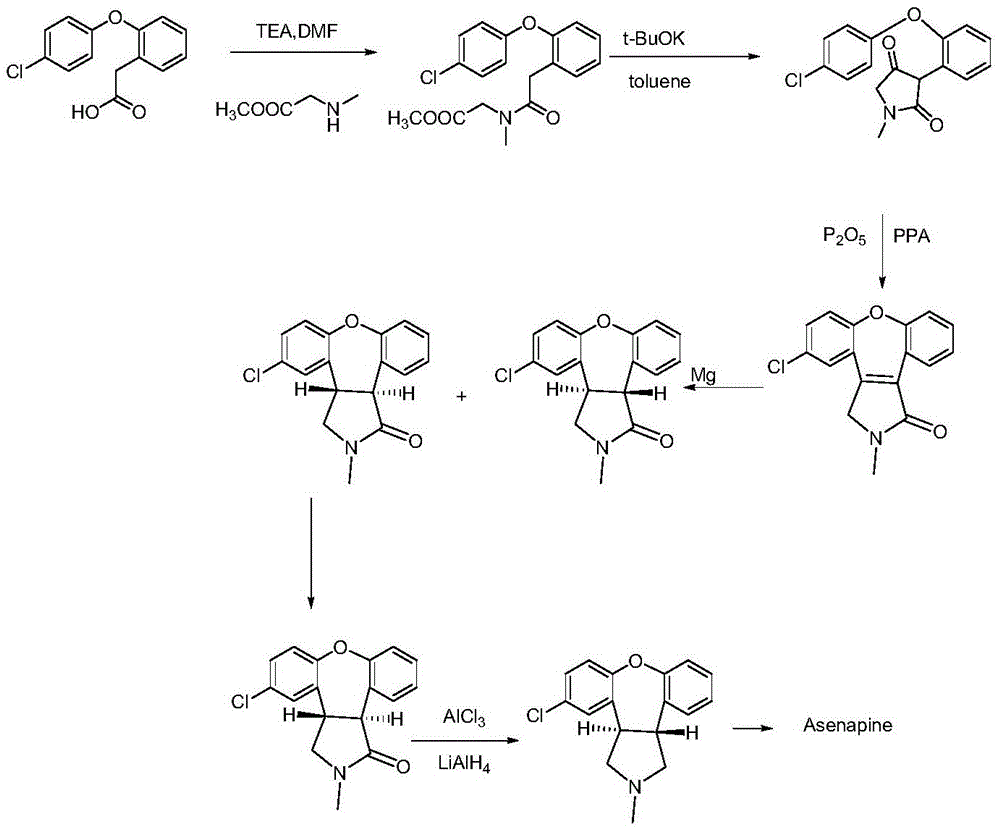
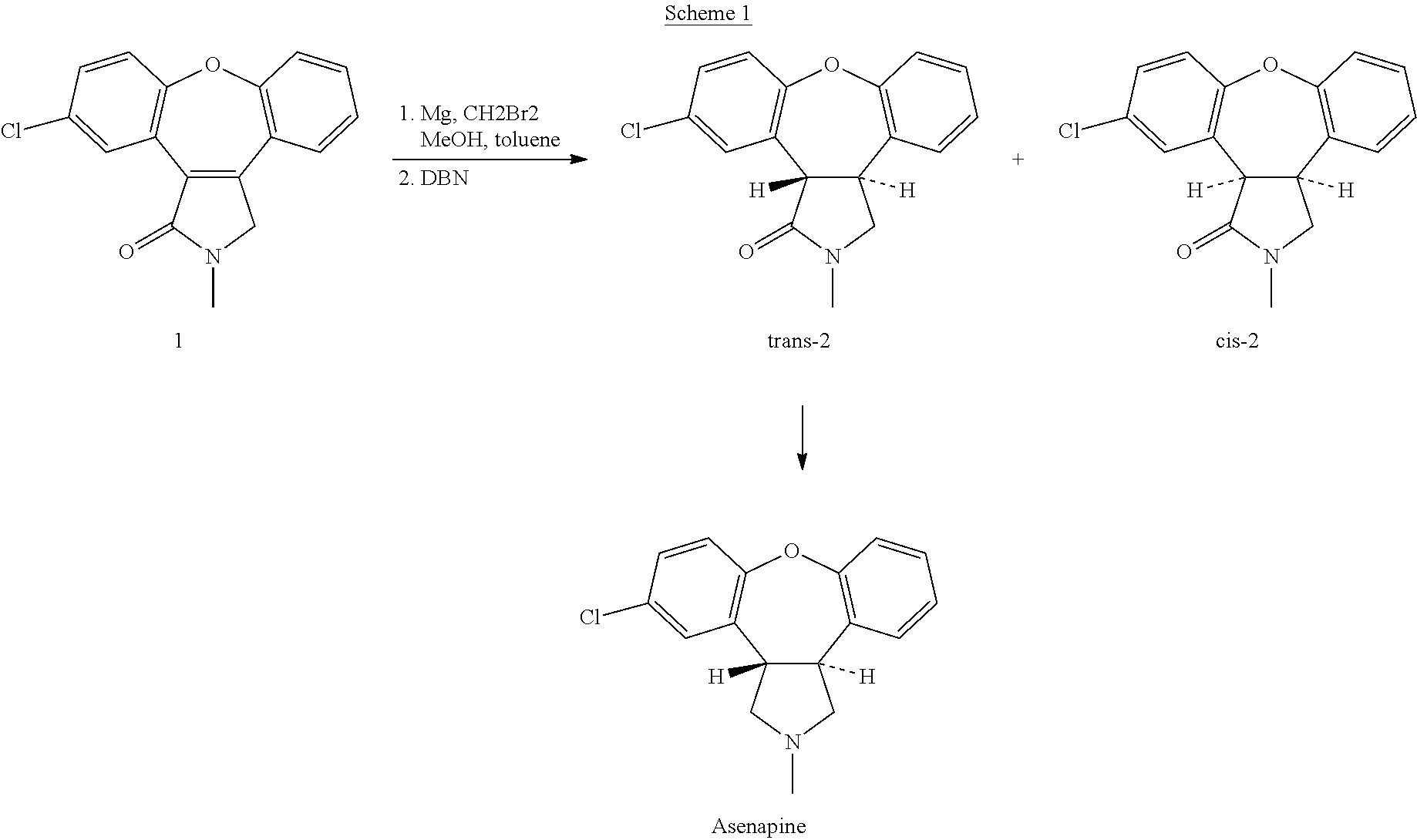
New process for synthesis of asenapine
ActiveCN102229613AReaction transposition effect is goodHigh yieldOrganic chemistryLoop closingMethyl group
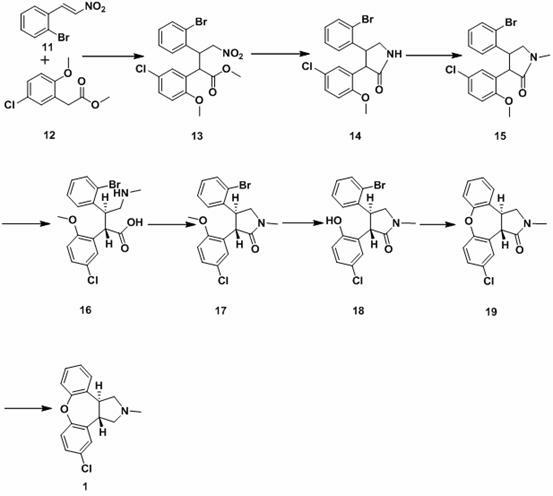
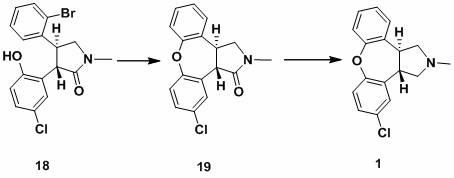
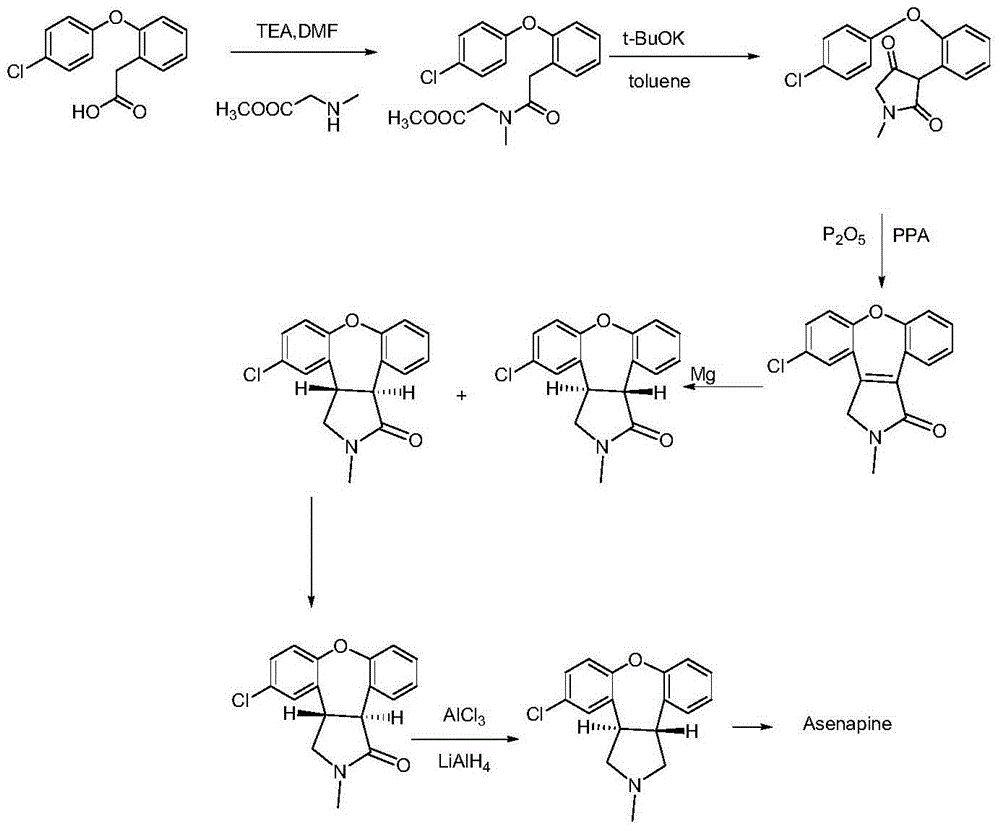
The invention discloses a process for synthesis of asenapine. The asenapine is prepared through adopting a compound (18) as a key intermediate and carrying out the following steps that: 1.1, the compound 18 is subjected to a Ullmann reaction under a alkaline condition through adopting copper powder as a catalyst to generate a ether (19); 1.2, the ether (19) is subjected to a carbonyl reduction to obtain the target compound of the asenapine (1). The process has the following advantages that: cheap and available 2-bromobenzaldehyde is adopted as an initial raw material and is subjected to acondensation, a addition, a reductive amination and a intramolecular cyclization reaction, a aminomethylation, a open loop transposition and then loop closing, a demethylation and a Ullmann loop closing reaction to synthesize of the asenapine (1); cis-trans-isomer is subjected to a delicate transposition to obtain a trans-product, such that the process is simplified and easy to be operated; the raw material is easy to be obtained and has cheap price; each reaction is a normal reaction, and reaction conditions are mild; a total yield is substantially improved; production cost is reduced; a purity of the product is more than 99% through a detection by HPLC.
Owner:安庆润科生物医药科技有限公司
Preparation method of asenapine intermediate
ActiveCN101851242ANovel process routeReasonable process conditionsOrganic chemistryTriflic acidKetone
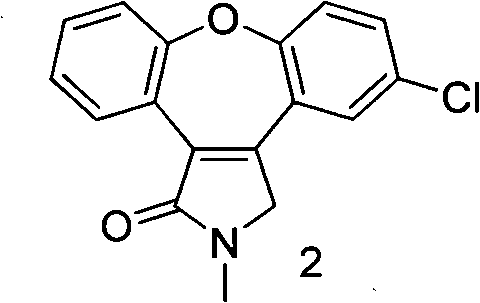
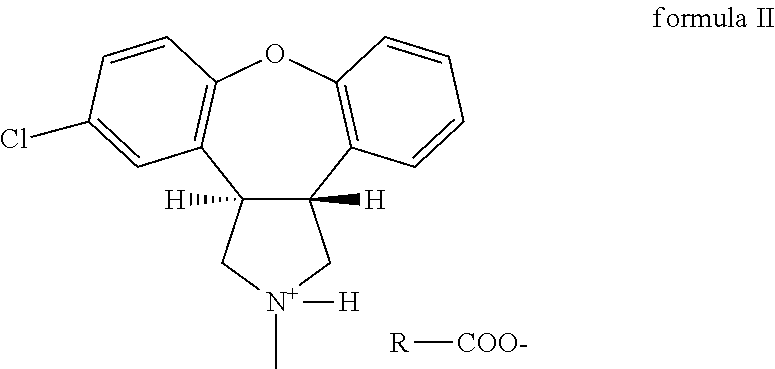
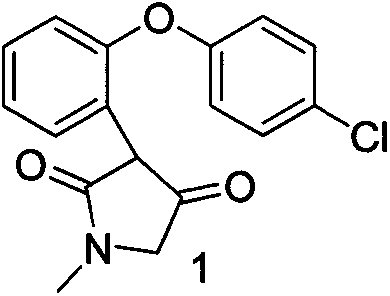
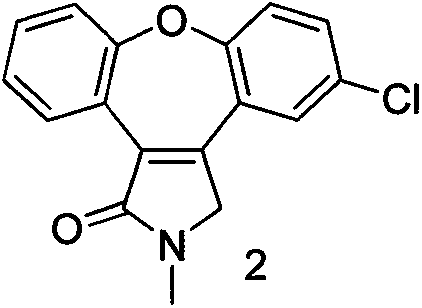
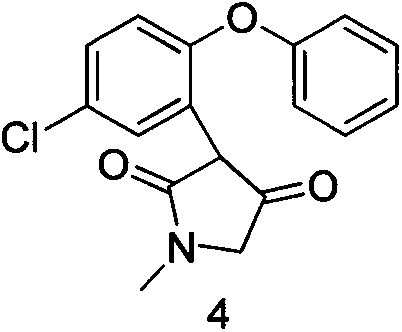
The invention discloses a novel preparation method of an asenapine intermediate, comprising the following steps of: carrying out cyclization in protonic acid including trifloromethanesulfonic acid by taking 3-(2-(4- chlorophenoxyl)-phenyl-4-hydroxyl-1-methyl-1H-pyrrole-2(5H)-ketone (compound 1) or 3-(5-chlorine-2-phenoxyl phenyl)-1-methyl-1H-pyrrole-2(4H)-ketone (compound 4) as a raw material to generate key intermediate compounds 2 and 4 of asenapine; and carrying out reduction reaction to obtain asenapine. The invention has the advantages of novel process route, high reaction yield and low production cost, and has greater application value and social economic effect.
Owner:SHANGHAI HAOYUAN MEDCHEMEXPRESS CO LTD
Spongy asenapine sublingual film agent with micropores and preparation method thereof
ActiveCN102657635ADissolve fastGuaranteed physical strengthOrganic active ingredientsNervous disorderWater insolubleWater soluble
The invention provides a spongy asenapine sublingual film agent with micropores and a preparation method thereof. The film agent comprises asenapine, water-soluble macromolecular materials and water-insoluble micro powder dispersed in the water-soluble macromolecular materials, wherein the molecular weight of at least one of the water-soluble macromolecular materials is 10000 to 200000 daltons, and the molecular weight of at least one of the water-soluble macromolecular materials is 200000 to 10000000 daltons. The film agent has a very remarkable effect, the film is quickly dissolved through the macromolecular material with low molecular weight, and the physical strength and the toughness of the film are ensured through the macromolecular material with high molecular weight, so that the aim of quick release is fulfilled and the strength of the film is ensured.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
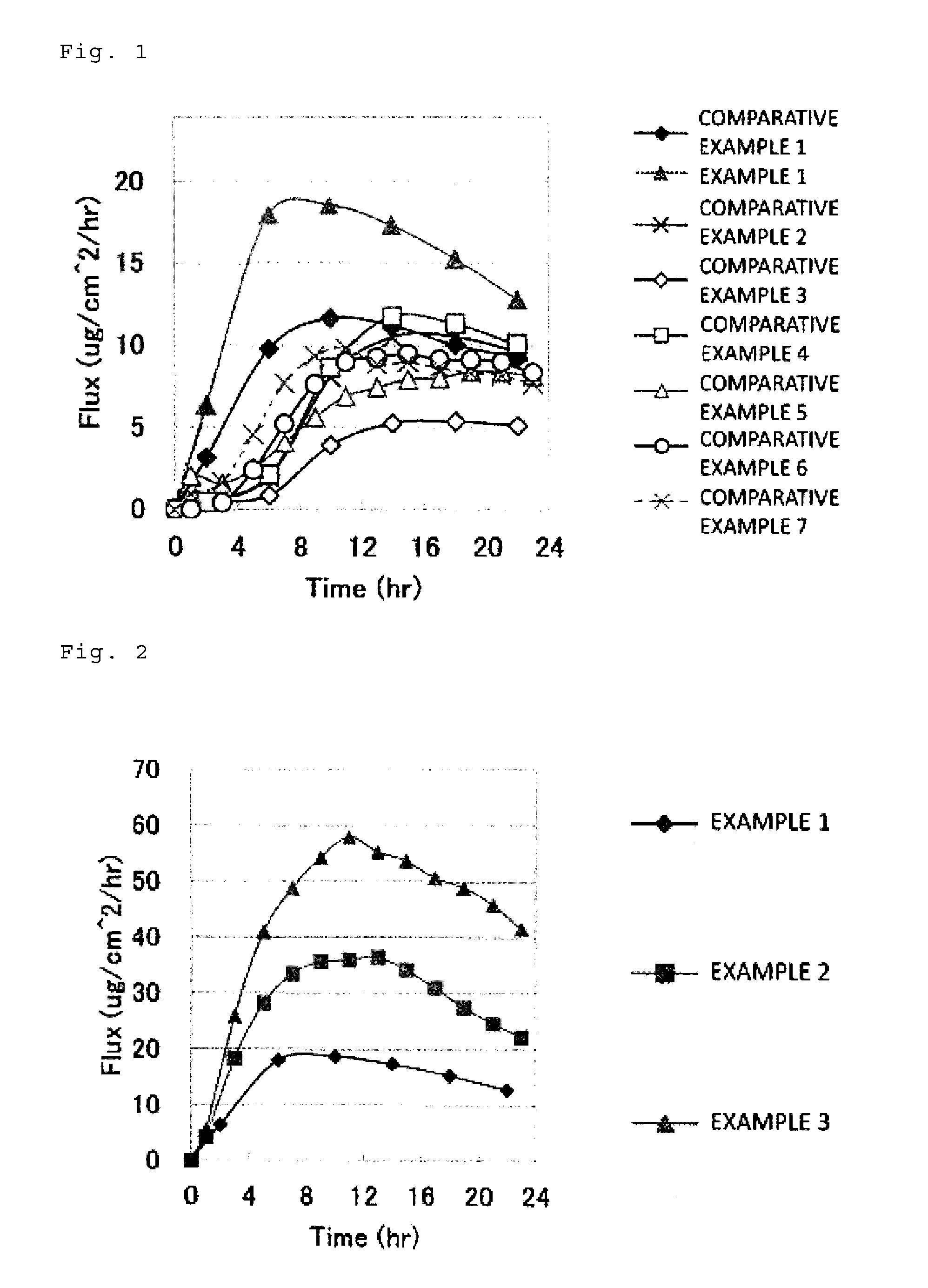
Transdermal therapeutic system containing asenapine
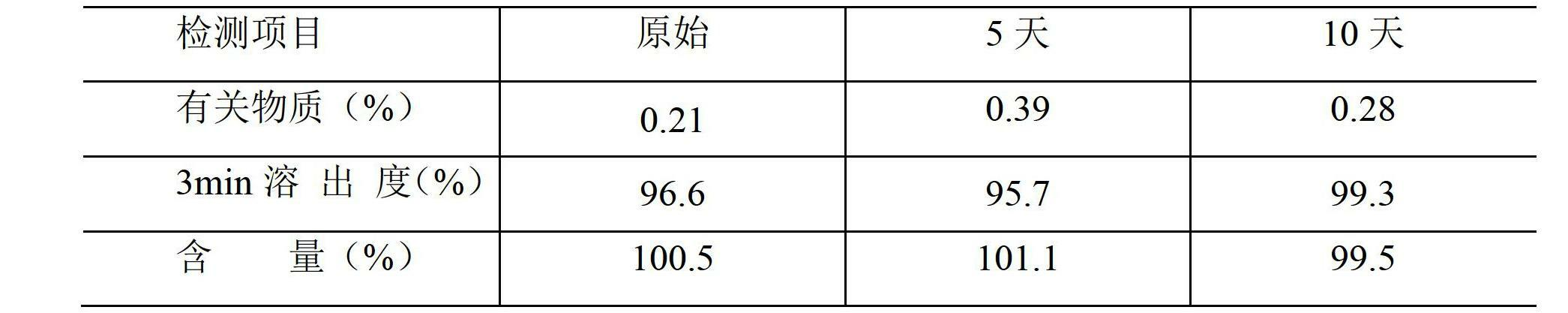
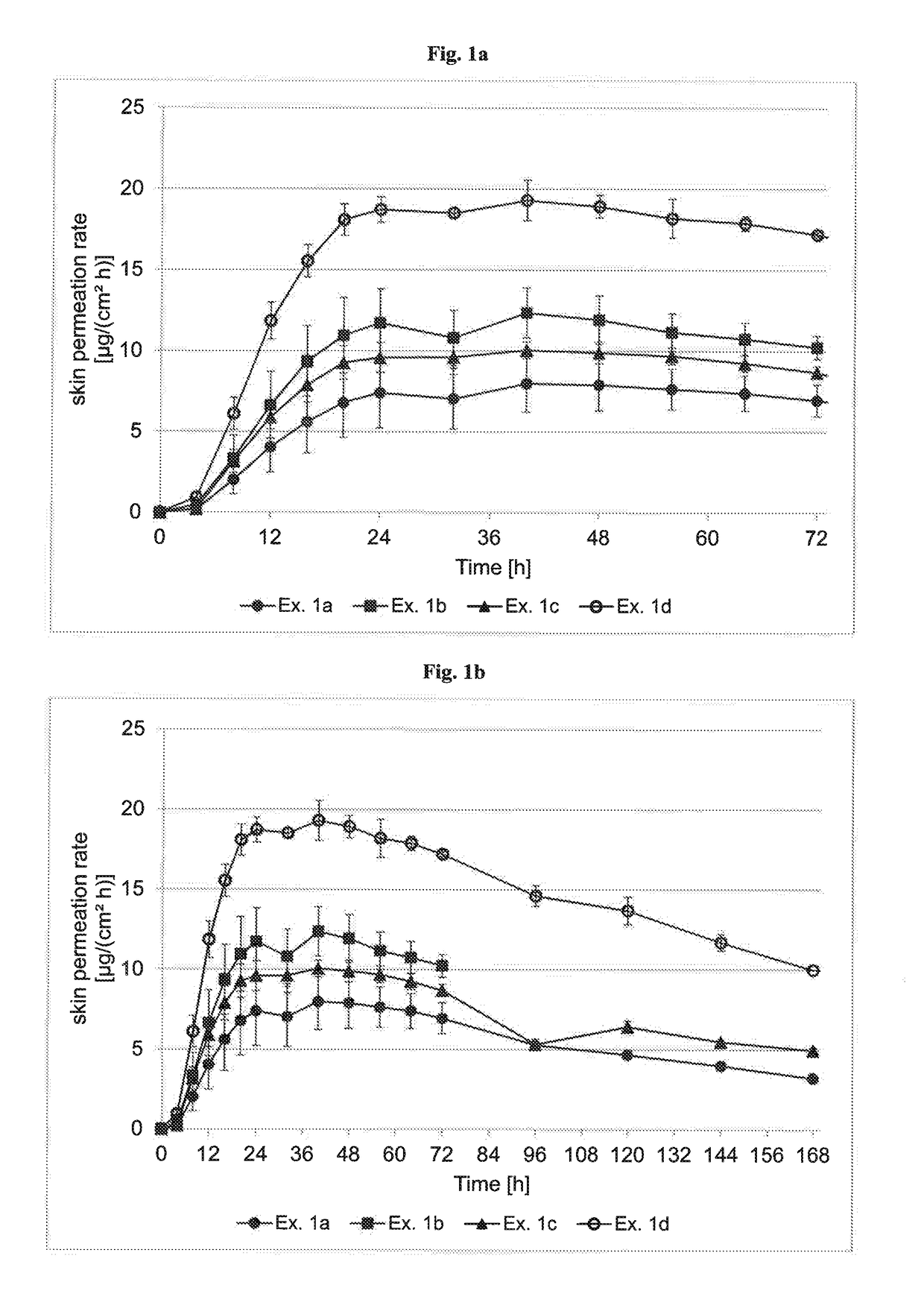
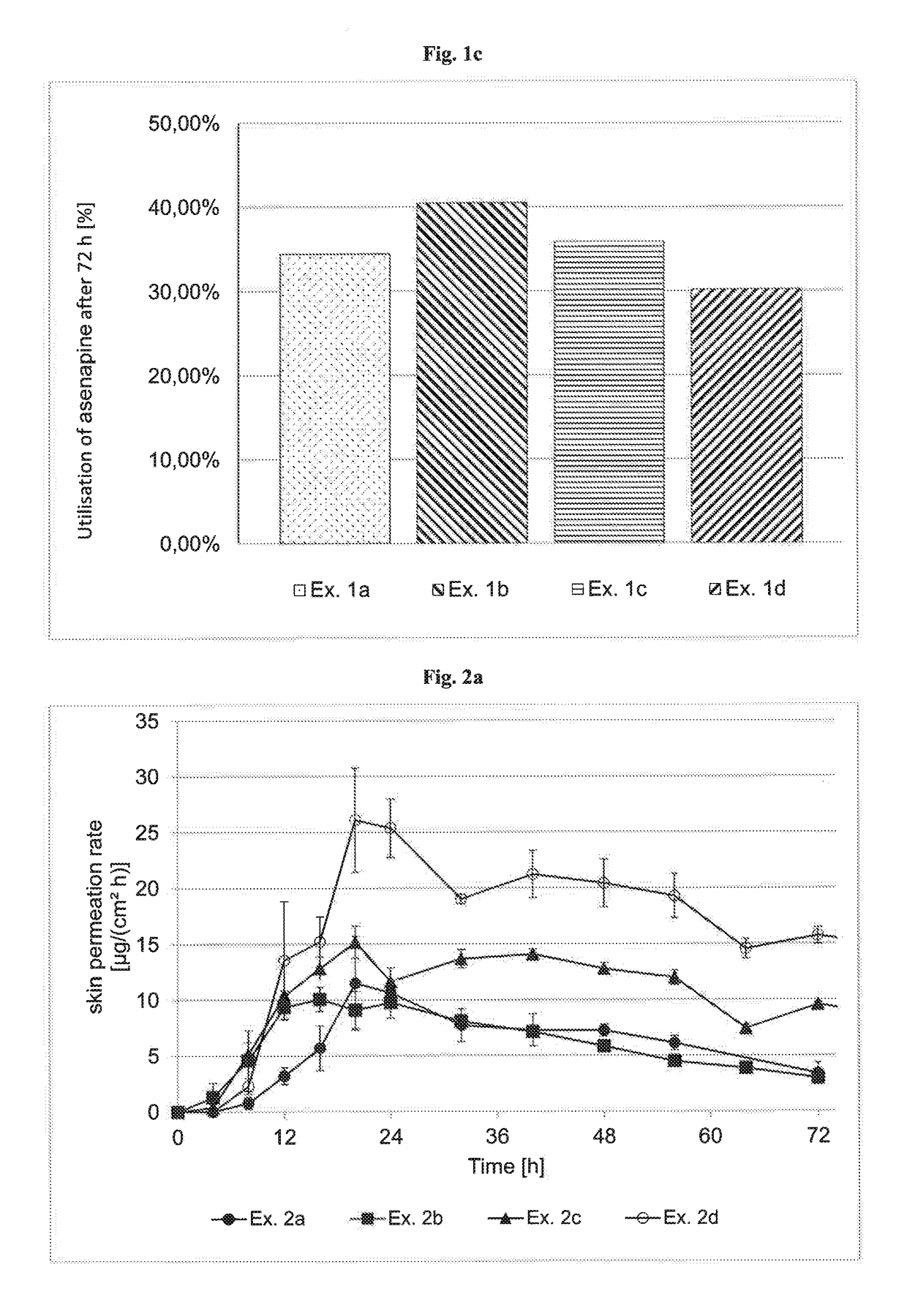
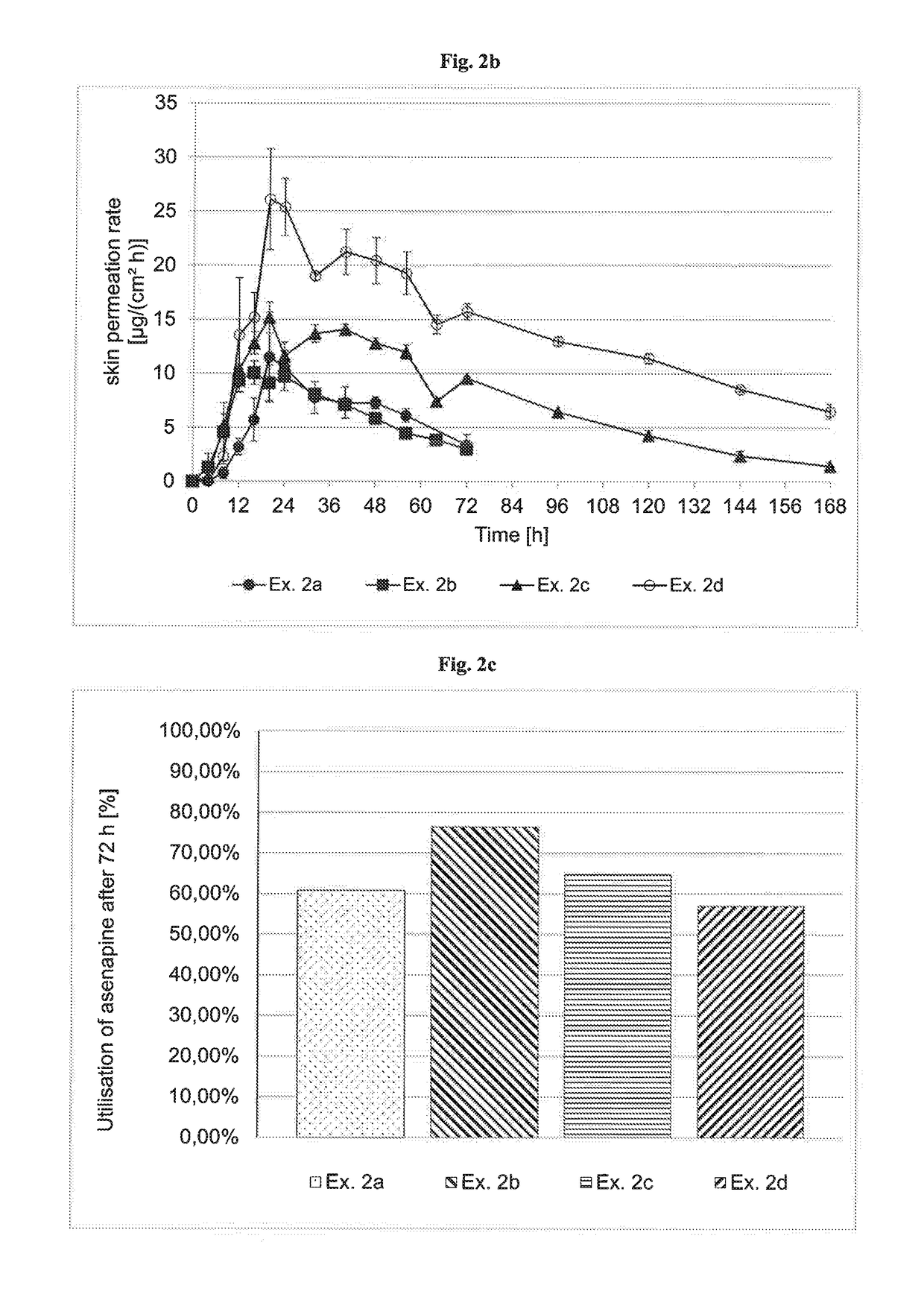
InactiveUS20180193283A1Reduce volatilityEasy and cost-efficient to manufactureNervous disorderPharmaceutical non-active ingredientsHuman patientAsenapine
The present invention relates to transdermal therapeutic systems (TTS) for the transdermal administration of asenapine comprising a self-adhesive layer structure containing a therapeutically effective amount of asenapine, such asenapine TTS for use in a method of treatment, processes of manufacture of such TTS as well as asenapine and transdermal therapeutic systems containing asenapine for use in a method of treatment and to a method of treating a human patient by transdermal administration of asenapine.
Owner:LTS LOHMANN THERAPIE-SYST AG
Preparation method of asenapine
The invention relates to a preparation method of a psychotropic medicine asenapine. The method comprises a step that asenapine is prepared through a reaction of a raw material trans-5-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-monobenzo[2,3:6,7]oxepino[4,5-C]pyrrole-1-one or trans-11-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxepino[4,5-C]pyrrole-1-one under the action of a reducing agent sodium bisaluminumhydride or a composite reducing agent composed of sodium bisaluminumhydride and other regents. The method has the advantages of mild and controllable reaction technology, low production cost, good product yield, realization of the large scale application in the industrial production, and good enforcement values and social and economic benefits.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
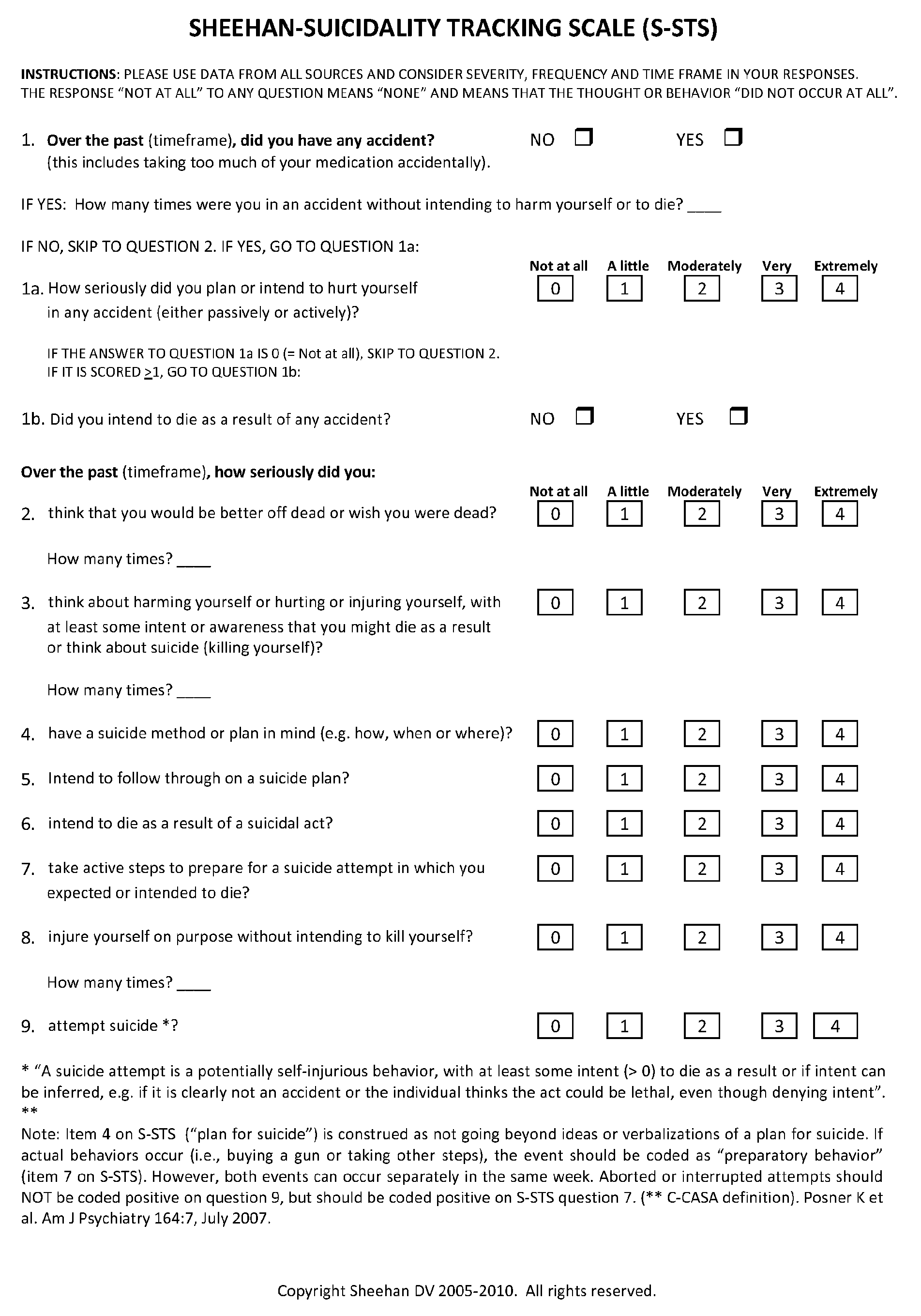
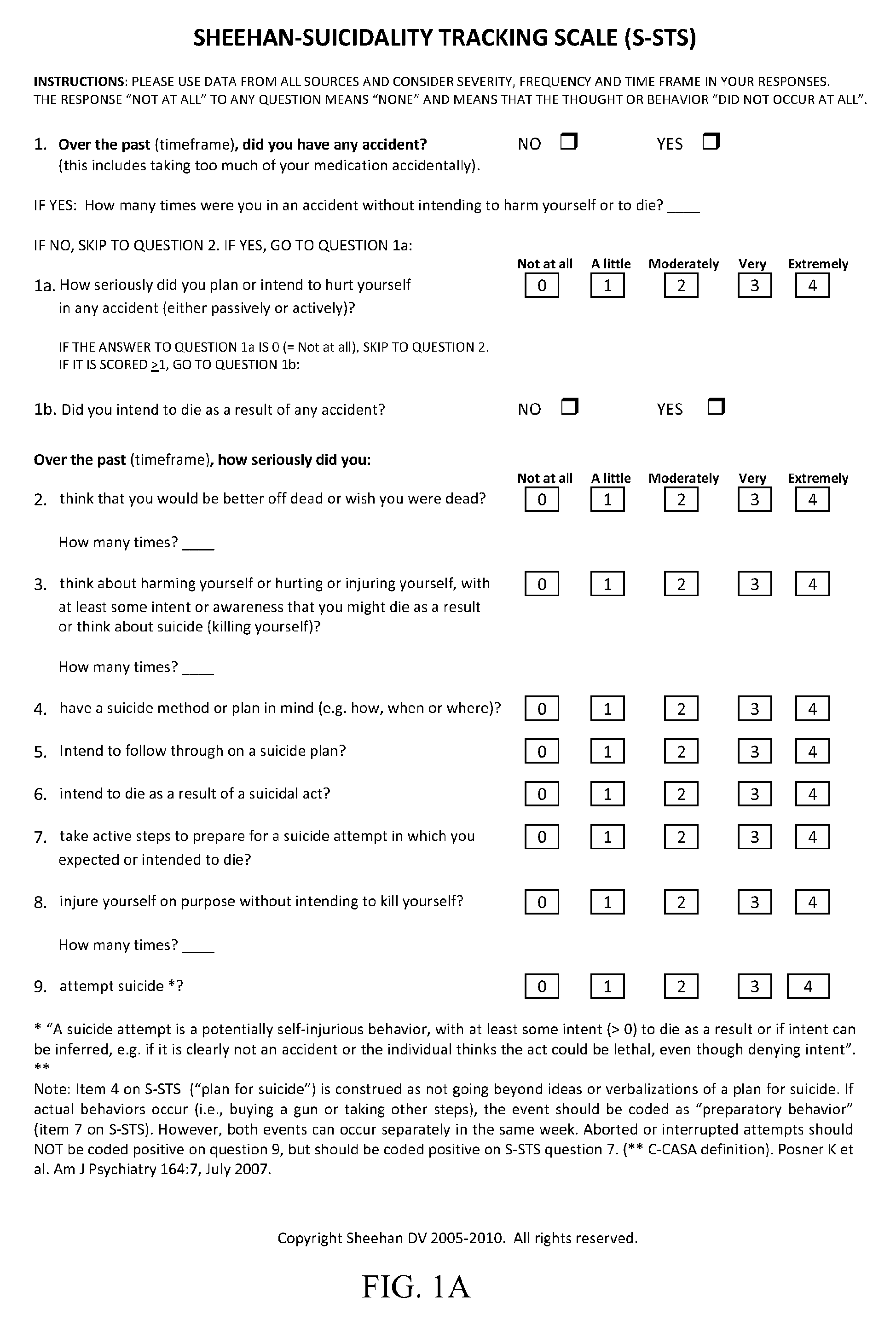
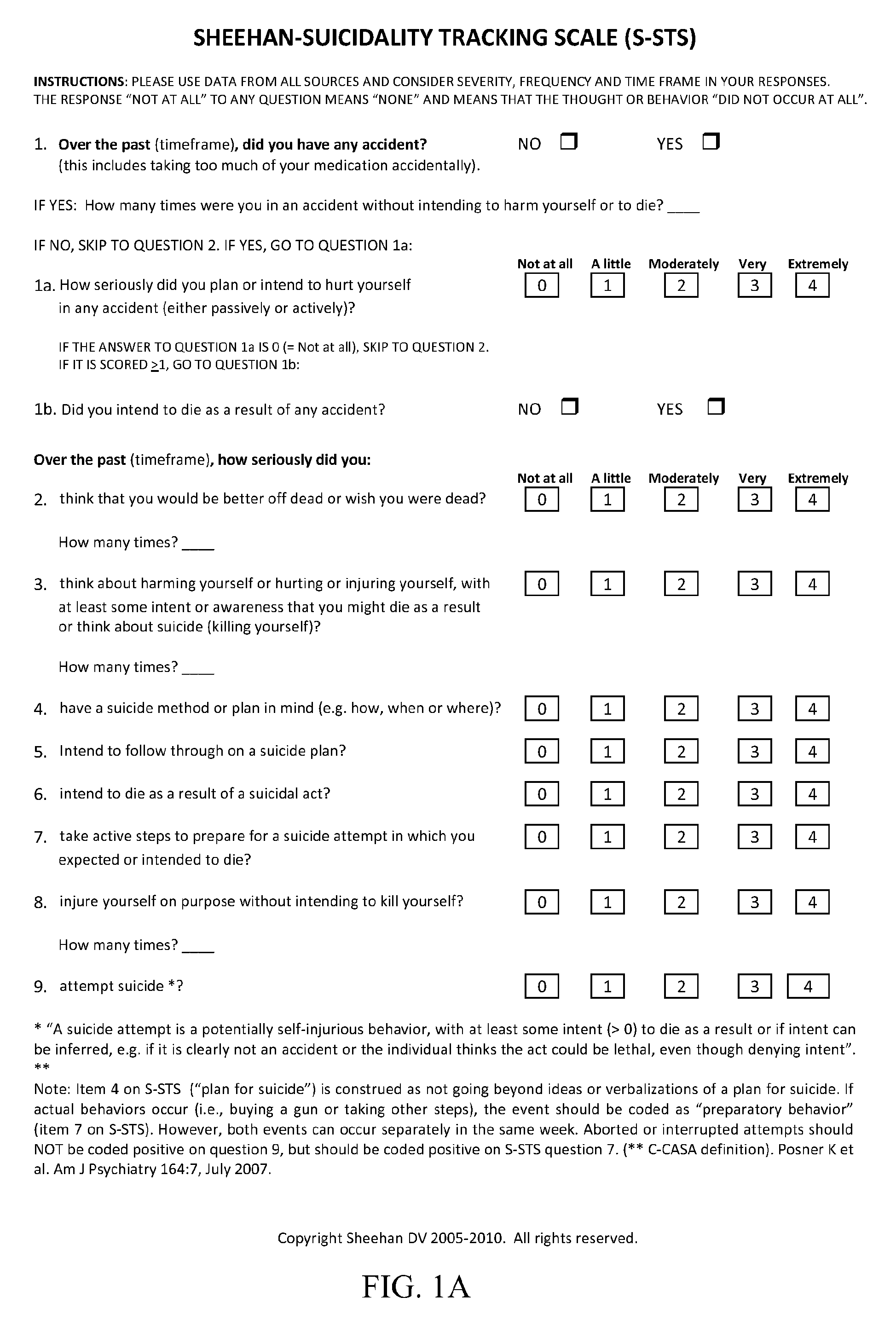
Treatment of suicidal ideation or behavior using inhibitors of nicotinic acetylcholine receptors
ActiveUS20120269906A1Reducing suicidalityIncreased riskBiocideNervous disorderLithium compoundMuscarinic acetylcholine receptor
The invention concerns methods of treating suicidal ideation or behavior in a subject in need thereof, comprising decreasing endogenous nicotinic acetylcholine receptor (nAChR) activity in the subject; therapeutic packages for treating suicidal ideation or behavior; and methods for determining the efficacy of a treatment for suicidal ideation or behavior. In some embodiments, the treatment methods comprise administering to the subject an effective amount of an inhibitor of a nAChR, such as a lithium compound, mecamylamine, clozapine, or asenapine.
Owner:UNIV OF SOUTH FLORIDA
Pharmaceutical compositions of asenapine
InactiveUS20170007537A1Improve bioavailabilityEasy to operateOrganic active ingredientsNervous disorderAsenapinePharmacology
The present invention relates to liquid compositions of asenapine with one or more pharmaceutically acceptable excipients. More particularly, the present invention relates to liquid spray compositions comprising asenapine for administration through oral mucosa.
Owner:HETERO RES FOUND
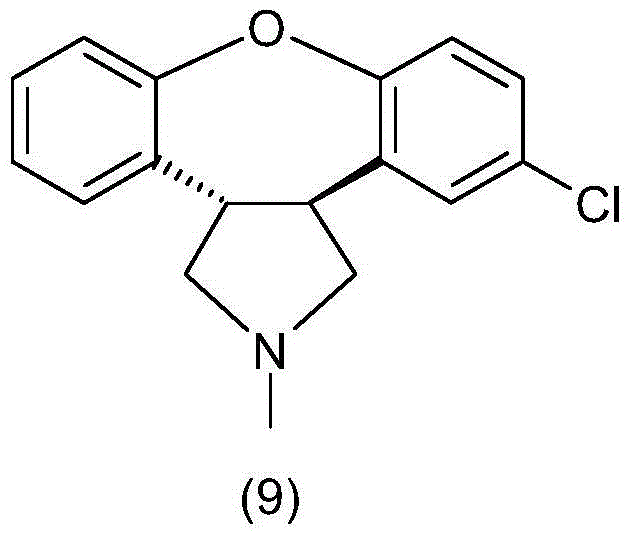
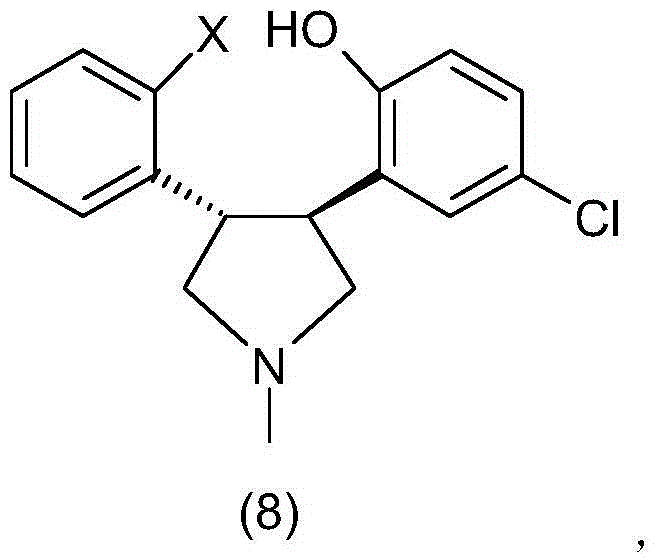
Preparation method of asenapine and intermediate used for preparing asenapine
InactiveCN104974167AMild reaction conditionsLow costOrganic chemistryBulk chemical productionCombinatorial chemistryAsenapine
The invention relates to a method for preparing asenapine shown in a general formula (9) and an intermediate shown in a general formula (7) and used for preparing asenapine. The general formulas are shown in the specification. The method comprises the step that asenapine shown in the general formula (9) is obtained through ring-closure reaction of a compound shown in a general formula (8).
Owner:YANGPU HG PHARMA
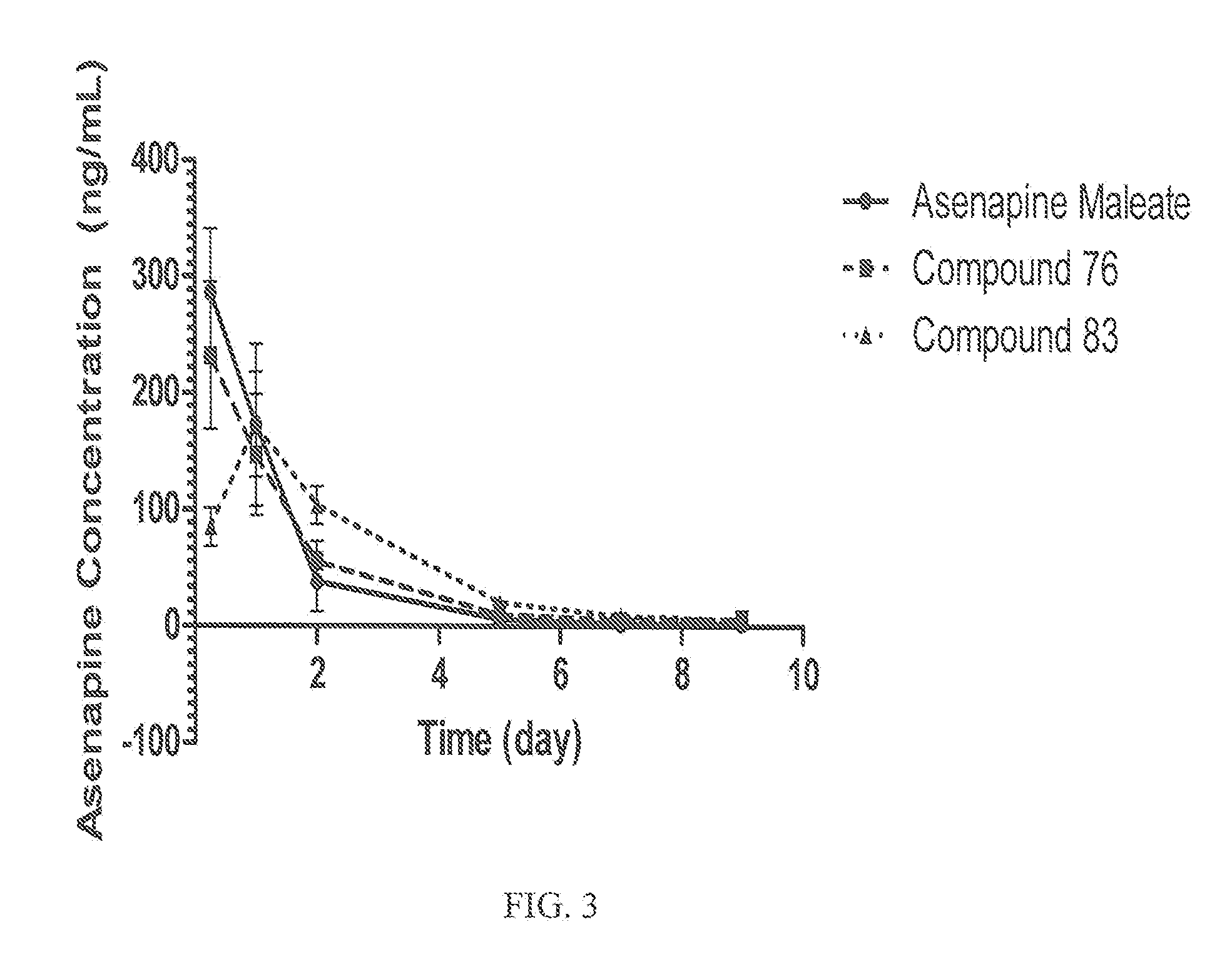
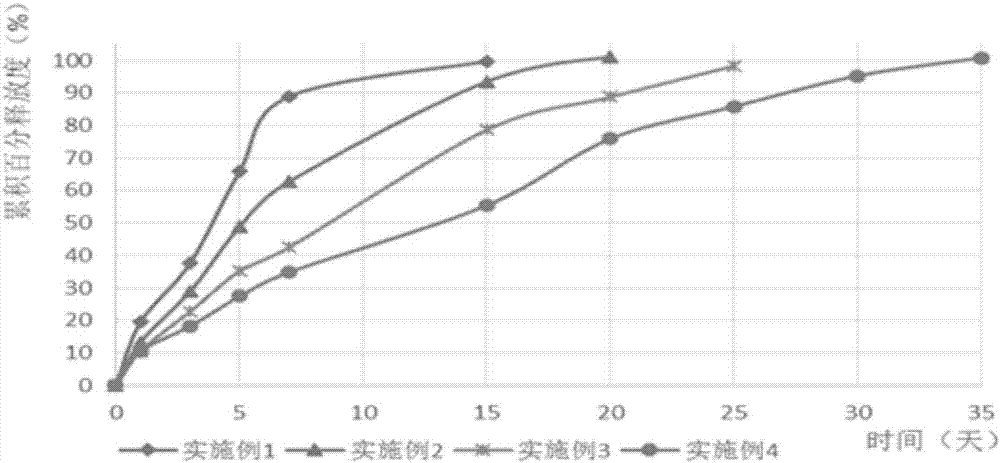
Injectable formulations containing asenapine and method of treatment using same
The present invention provides a formulation comprising asenapine hemipamoate suspended particles, which formulation can be administered via a Depot provided by an IM injection of the formulation, and which depot does not display a particle-size dependent release rate. The present invention provides also methods of treatment using the same.
Owner:FOREST LAB HLDG LTD
Skin irritation suppressant and transdermal preparation
InactiveCN102858372ALess irritatingOrganic active ingredientsNervous disorderCholesterol derivativePharmaceutical Adjuvants
Owner:HISAMITSU PHARM CO INC
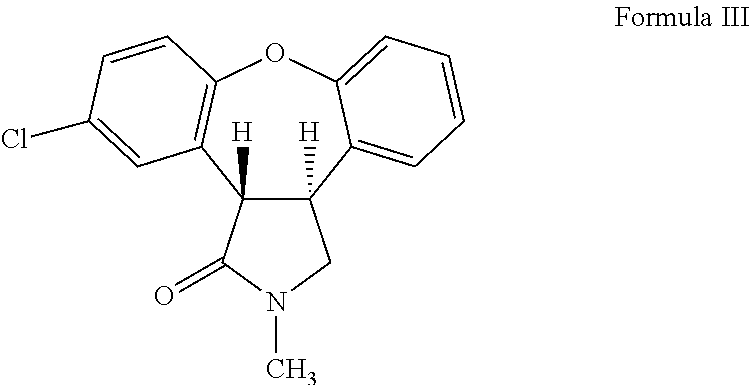
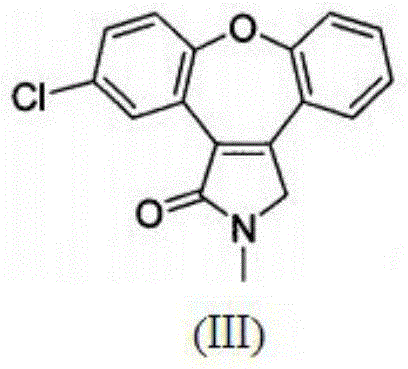
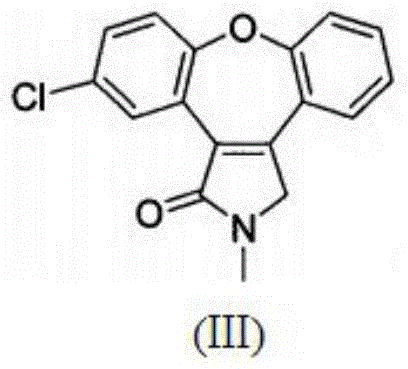
Preparation method of asenapine key intermediate
ActiveCN104098580AThe reaction steps are simpleRaw materials are easy to getOrganic chemistryChemical synthesisCombinatorial chemistry
The invention provides a chemical synthesis method, in particular to a preparation method of a key intermediate [formula (III)] compound of asenapine capable of serving as a schizophrenia drug. In an anhydrous system, the intermediate [formula (III)] compound is obtained through dehydration of an intermediate [formula (II)] compound and anhydride under the action of a catalyst. The invention further provides a novel method for preparing asenapine by the intermediate [formula (III)]. The provided method has the advantages of simple operation, high yield, environment-friendliness, lower cost, stable technology and the like.
Owner:成都明德至远医药科技有限公司
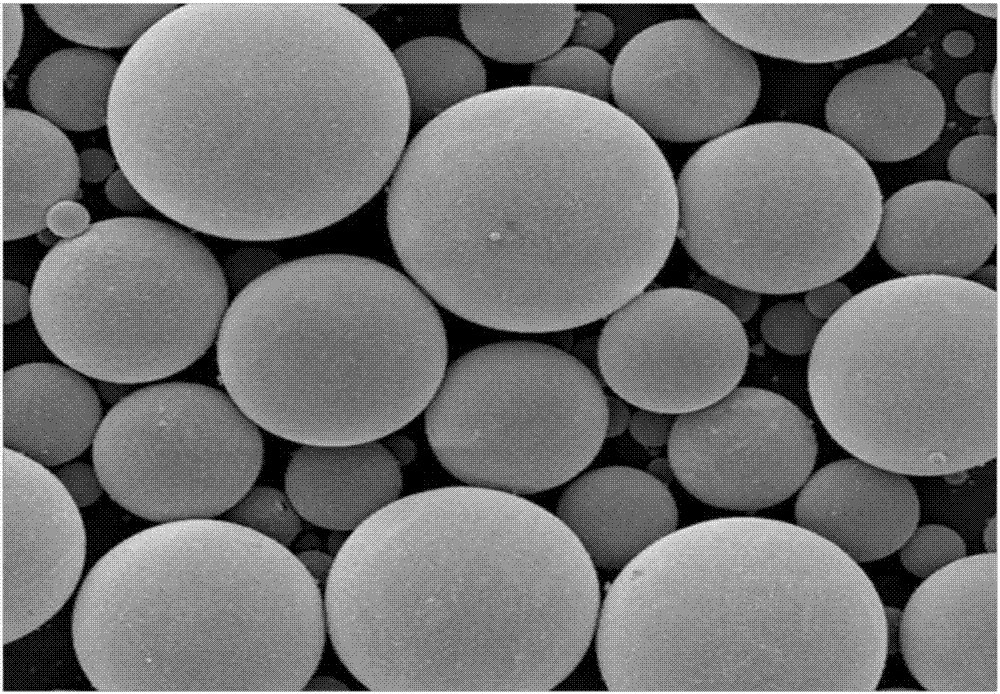
Asenapine microsphere agent and preparation method thereof
InactiveCN107137375AIncrease productivityLow burst rateOrganic active ingredientsNervous disorderPolyesterOrganic solvent
The invention discloses an asenapine slow-release microsphere agent and a preparation method thereof. The microsphere agent is prepared from asenapine or salts and polyesters thereof, wherein the weight percentage of the asenapine or salts thereof counted by asenapine is 5-50%, and the weight percentage of the polyesters is 50-95%. The preparation method comprises the following steps: (1) conducting weighing; (2) adding the polyester material into an organic solvent for completely dissolving the polyester material to obtain a settled solution, then dissolving or suspending the medicine asenapine or the salts thereof into the settled solution to be used as an oil phase; stirring or shearing the oil phase at high speed to form a homogeneous oil phase; and in case of need, adding a right amount of acid for salifying the asenapine; and (3) preparing the microsphere agent by a microdroplet generating device, or a spray drying method, or an atomizing extraction method. The microsphere agent provided by the invention has relatively high production efficiency, the D50 particle sizes of the prepared microspheres are all below 80mu m, are controllable and are concentrated in distribution, the drug-loading rate is as high as 45%, and the encapsulation rate is above 85%.
Owner:SHENZHEN FONCOO PHARMACEUTICAL CO LTD
Treatment of suicidal ideation or behavior using inhibitors of nicotinic acetylcholine receptors
ActiveUS9180191B2Reducing suicidalityIncreased riskBiocideCosmetic preparationsLithium compoundClozapine
The invention concerns methods of treating suicidal ideation or behavior in a subject in need thereof, comprising decreasing endogenous nicotinic acetylcholine receptor (nAChR) activity in the subject; therapeutic packages for treating suicidal ideation or behavior; and methods for determining the efficacy of a treatment for suicidal ideation or behavior. In some embodiments, the treatment methods comprise administering to the subject an effective amount of an inhibitor of a nAChR, such as a lithium compound, mecamylamine, clozapine, or asenapine.
Owner:UNIV OF SOUTH FLORIDA
Injectable formulations containing asenapine and method of treatment using same
Owner:FOREST LAB HLDG LTD
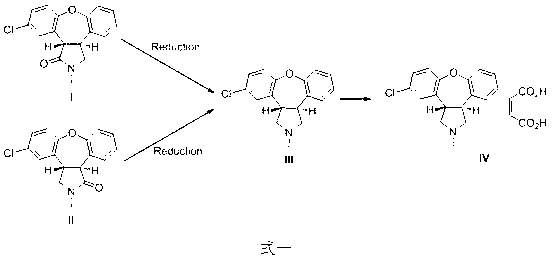
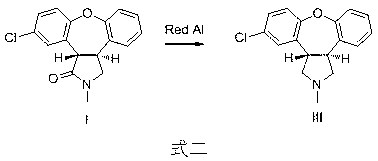
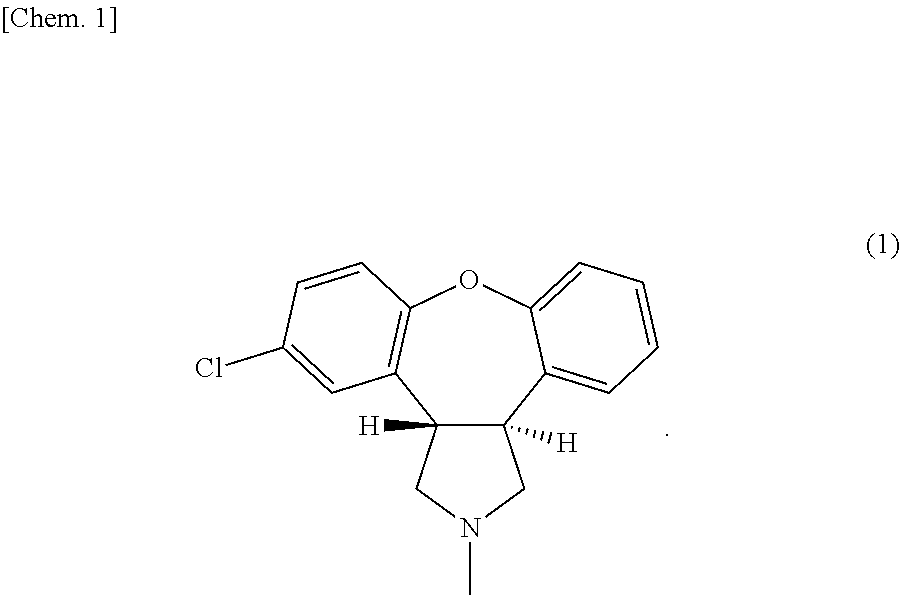
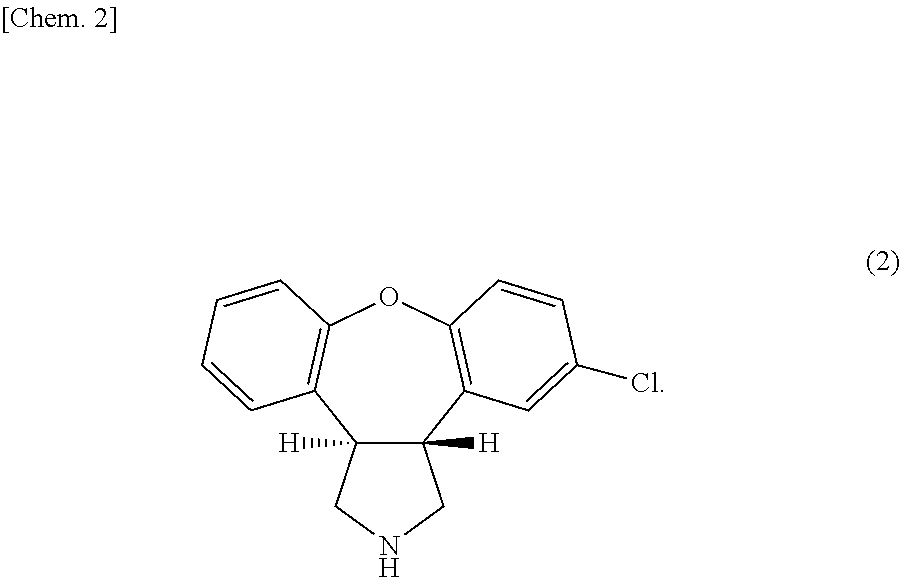
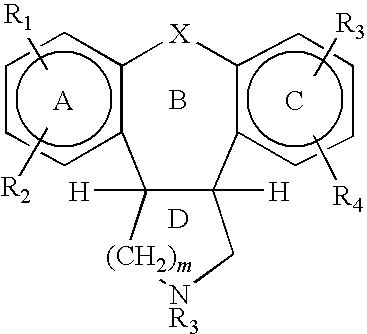
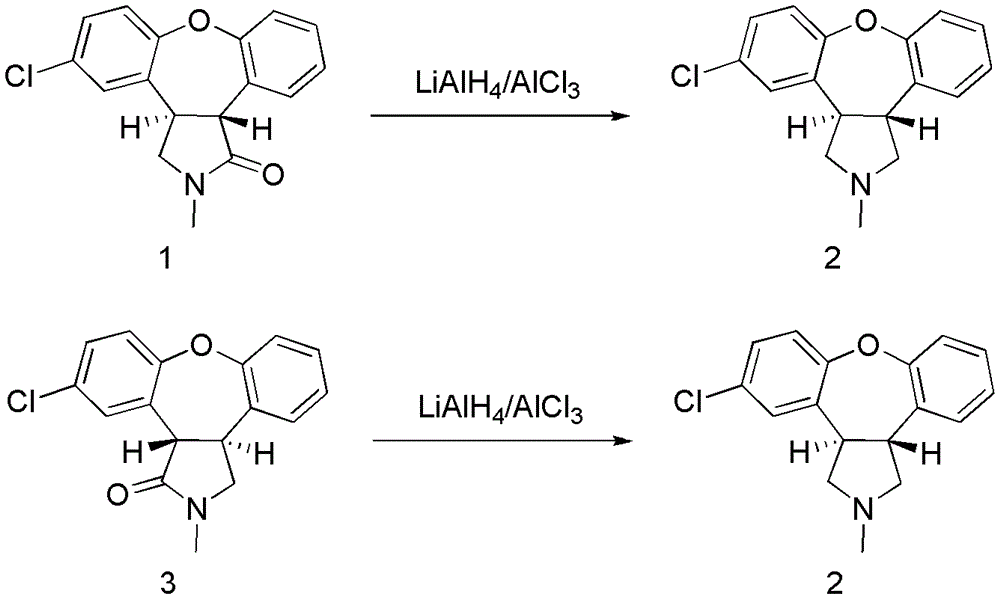
Preparation method of trans-5-chloro-2,3,3a,12b-tetrahydro-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrrole
The invention belongs to the field of pharmaceutical chemistry, and provides a preparation method of an asenapine key impurity (IV) trans-5-chloro-2,3,3a,12b-tetrahydro-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrrole. The trans-5-chloro-2,3,3a,12b-tetrahydro-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrrole is prepared from trans-5-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrrole used as the initial raw material. The impurity is a very key impurity for synthesizing asenapine, and is also an important intermediate for controlling the asenapine end product quality standard.
Owner:AVENTIS PHARMA HAINAN
A kind of preparation method of asenapine key intermediate
ActiveCN104098580BThe reaction steps are simpleRaw materials are easy to getOrganic chemistryChemical synthesisCombinatorial chemistry
The invention provides a chemical synthesis method, in particular to a preparation method of a key intermediate [formula (III)] compound of asenapine capable of serving as a schizophrenia drug. In an anhydrous system, the intermediate [formula (III)] compound is obtained through dehydration of an intermediate [formula (II)] compound and anhydride under the action of a catalyst. The invention further provides a novel method for preparing asenapine by the intermediate [formula (III)]. The provided method has the advantages of simple operation, high yield, environment-friendliness, lower cost, stable technology and the like.
Owner:成都明德至远医药科技有限公司
A kind of preparation method of asenapine
The invention relates to a preparation method of a psychotropic medicine asenapine. The method comprises a step that asenapine is prepared through a reaction of a raw material trans-5-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-monobenzo[2,3:6,7]oxepino[4,5-C]pyrrole-1-one or trans-11-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxepino[4,5-C]pyrrole-1-one under the action of a reducing agent sodium bisaluminumhydride or a composite reducing agent composed of sodium bisaluminumhydride and other regents. The method has the advantages of mild and controllable reaction technology, low production cost, good product yield, realization of the large scale application in the industrial production, and good enforcement values and social and economic benefits.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
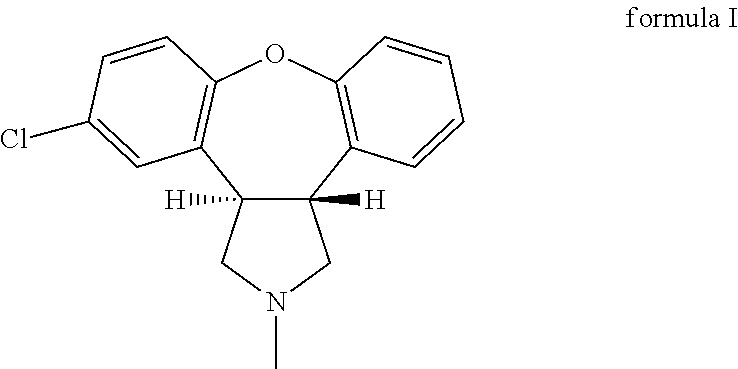
Process for the preparation of asenapine
The present invention is directed to novel compounds of formula (I) as well as to the process for their preparation. Novel compounds of formula (I) can be converted into asenapine through an efficient process. The invention also relates to novel intermediates used in this process and their use in the preparation of compounds of formula (I).
Owner:INKE SA (ES)
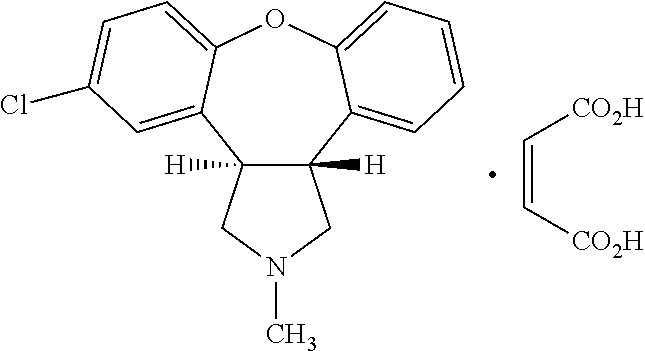
Asenapine compound
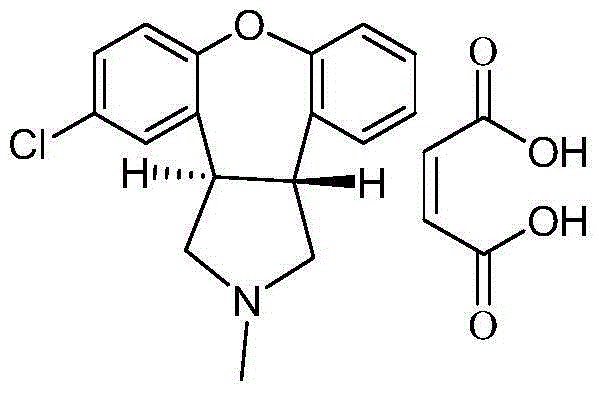
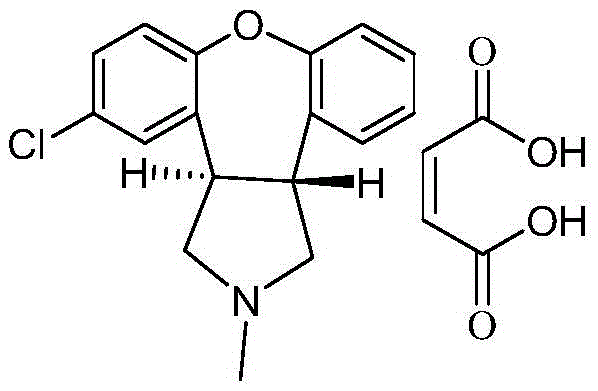
Belonging to the medical technology field, the invention in particular relates to a maleic acid asenapine compound and a preparation method thereof. The invention also relates to application of compositions containing the maleic acid asenapine compound in preparation of drugs treating antipsychotic drugs.
Owner:TIANJIN HANKANG PHARMA BIOTECH
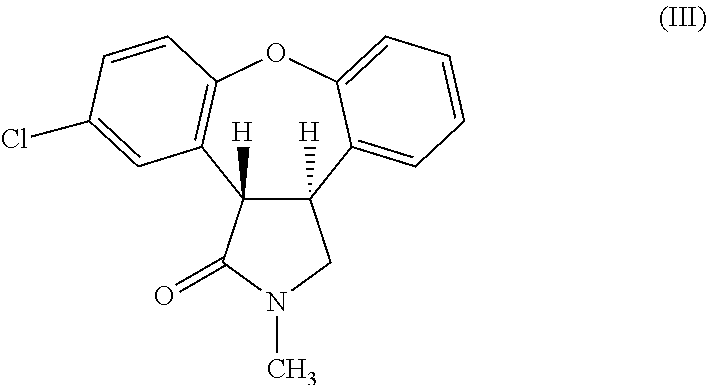
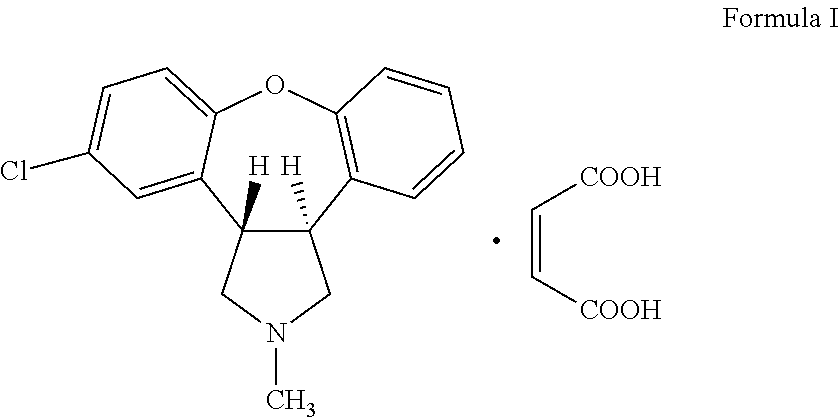
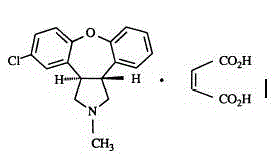
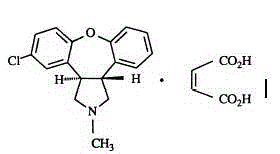
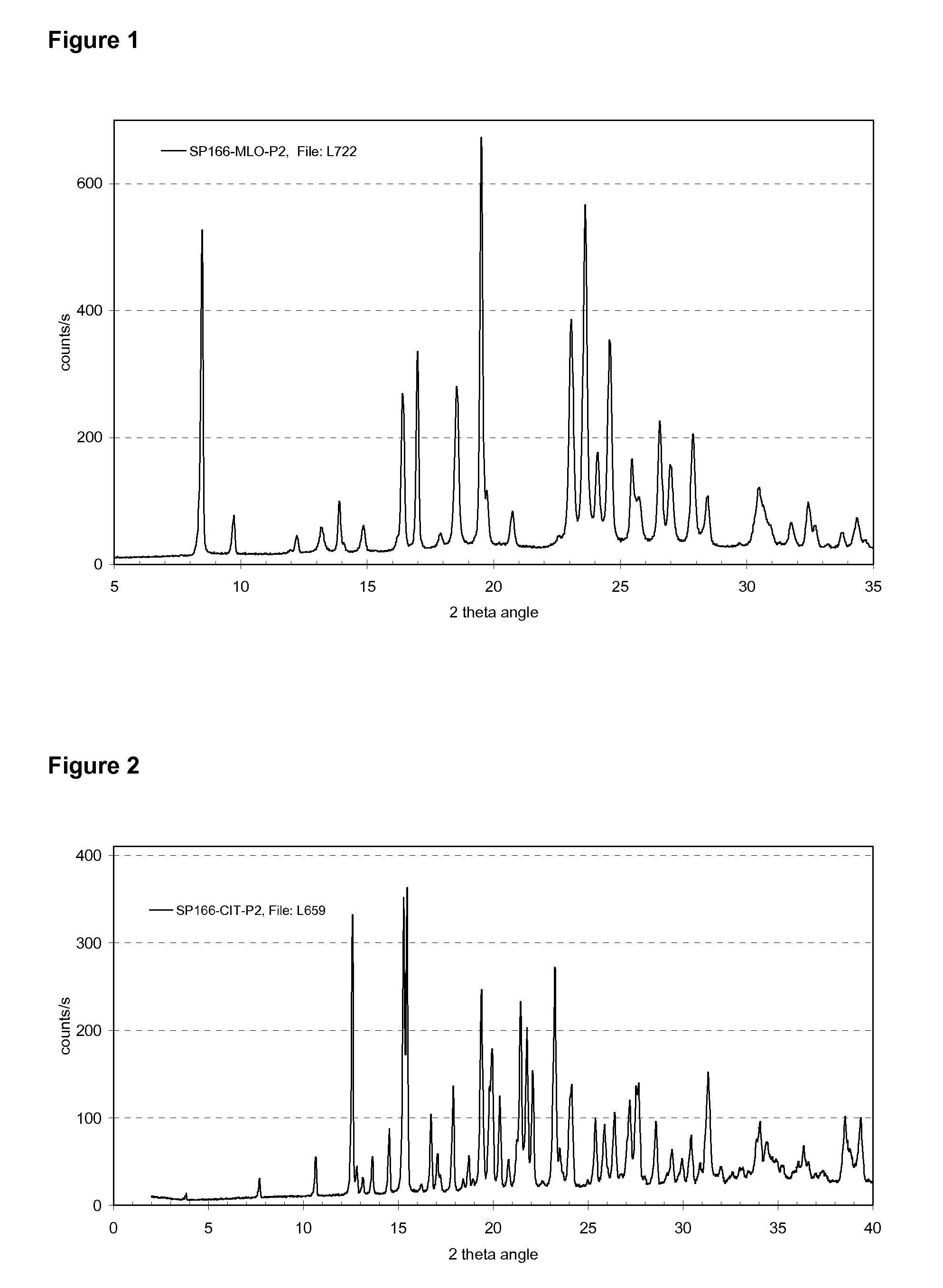
Crystalline salts of Asenapine with organic di-acids and tri-acids
InactiveUS9169262B2Avoid known polymorph issueImprove solubilityNervous disorderOrganic chemistryMedicinal chemistryAsenapine
Novel crystalline salts of Asenapine (I) with organic di-acids and tri-acids and to methods of their preparation are disclosed along with related pharmaceutical compositions and methods of treating psychotic diseases or disorders.
Owner:SANDOZ LTD
Preparation method of asenapine intermediate
Owner:SHANGHAI HAOYUAN MEDCHEMEXPRESS CO LTD
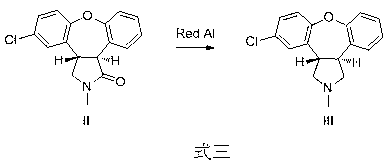
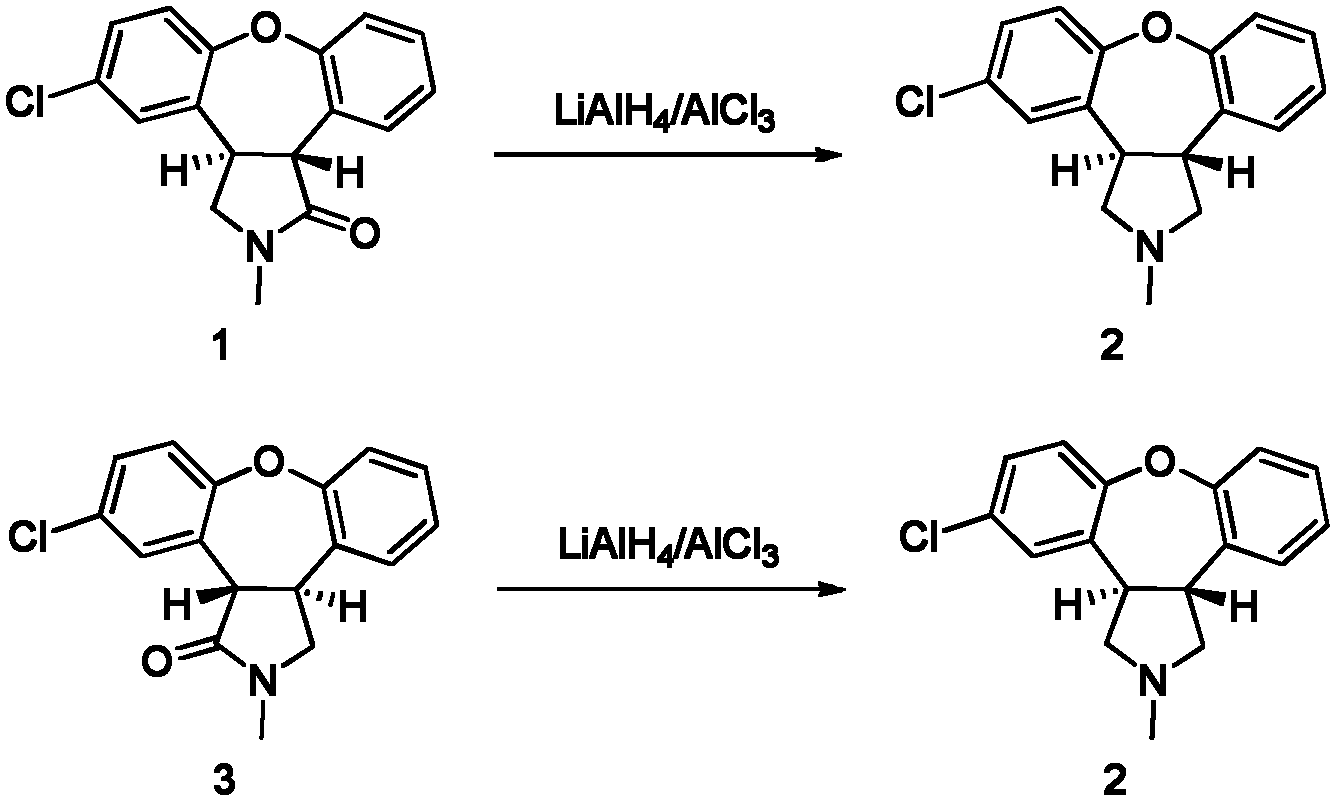
New refining method of 11-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrryl-1-one
The invention belongs to the field of pharmaceutical chemistry, and relates to a refining method of an asenapine important intermediate 11-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrryl-1-one. The refining method is simple to operate, can easily implement industrial production, and enhances the yield by nearly 20% relative to reported documents.
Owner:AVENTIS PHARMA HAINAN
Process for the preparation of asenapine intermediate
InactiveUS20140336391A1Good product selectionSpeed up the processOrganic chemistryAsenapineMagnesium
The present invention provides a process for the preparation of the asenapine intermediate of Formula (III) using a magnesium-methanol-acetic acid mixture.
Owner:RANBAXY LAB LTD
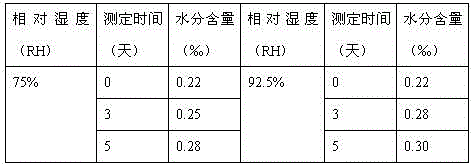
Purification method of asenapine
The invention discloses a method for purifying asenapine. The method carries out crystallization and purification through two different crystallization systems, and adopts a low-temperature drying method to purify asenapine. The purity of asenapine in the purified product reaches 99.9% % above, the total impurity content does not exceed 0.1%, and does not contain adsorbed water.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Preparation method of asenapine
InactiveCN103254202AHigh yieldReduce manufacturing costOrganic chemistryBiochemical engineeringCombinatorial chemistry
The invention discloses a preparation method of asenapine. According to the preparation method, the asenapine (III) is prepared from a compound I with formula 1 or a compound II with formula 2 under a vitride solution reduction condition. The method is high in yield and low in production cost due to the fact that vitride is much cheaper than borane; and moreover, vitride is safer and simpler to operate compared with the reagent used in the prior art, so that the asenapine is applicable to in-scale production.
Owner:甘肃皓天科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Preparation method of trans-5-chloro-2,3,3a,12b-tetrahydro-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrrole Preparation method of trans-5-chloro-2,3,3a,12b-tetrahydro-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrrole](https://images-eureka.patsnap.com/patent_img/ed58eb44-41ee-4095-a6c4-25392e607bef/2014100058924100002DEST_PATH_IMAGE002.PNG)
![Preparation method of trans-5-chloro-2,3,3a,12b-tetrahydro-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrrole Preparation method of trans-5-chloro-2,3,3a,12b-tetrahydro-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrrole](https://images-eureka.patsnap.com/patent_img/ed58eb44-41ee-4095-a6c4-25392e607bef/2014100058924100002DEST_PATH_IMAGE004.PNG)
![New refining method of 11-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrryl-1-one New refining method of 11-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrryl-1-one](https://images-eureka.patsnap.com/patent_img/a0d34cd3-1911-438d-b97a-286e94d53e67/2014100059823100002DEST_PATH_IMAGE001.PNG)
![New refining method of 11-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrryl-1-one New refining method of 11-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrryl-1-one](https://images-eureka.patsnap.com/patent_img/a0d34cd3-1911-438d-b97a-286e94d53e67/684948DEST_PATH_IMAGE002.PNG)