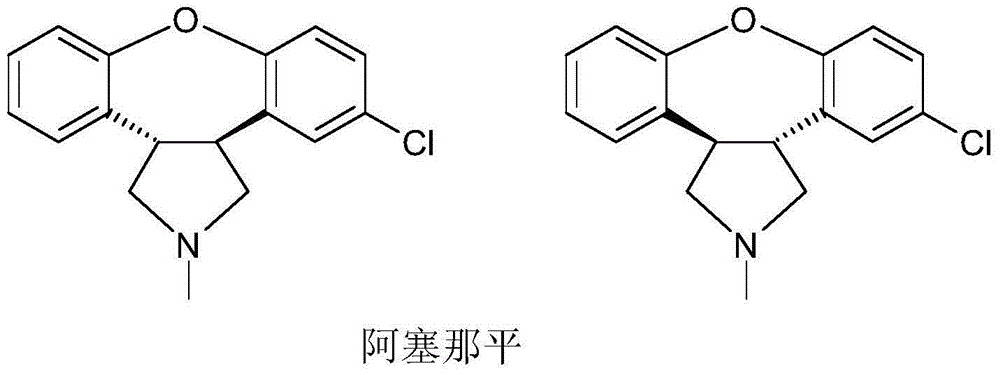
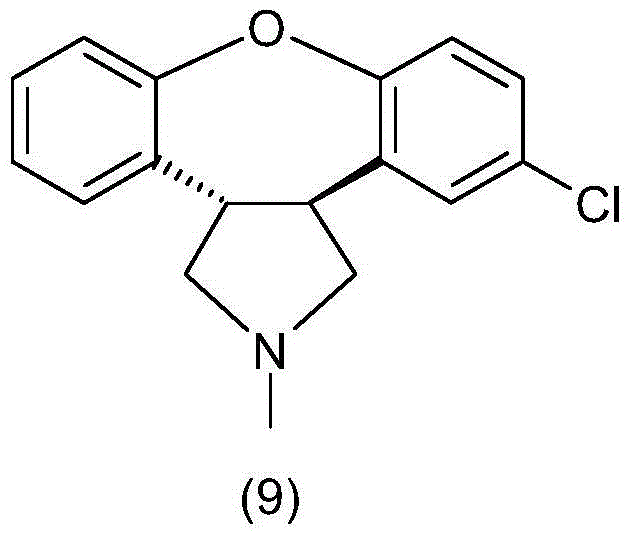
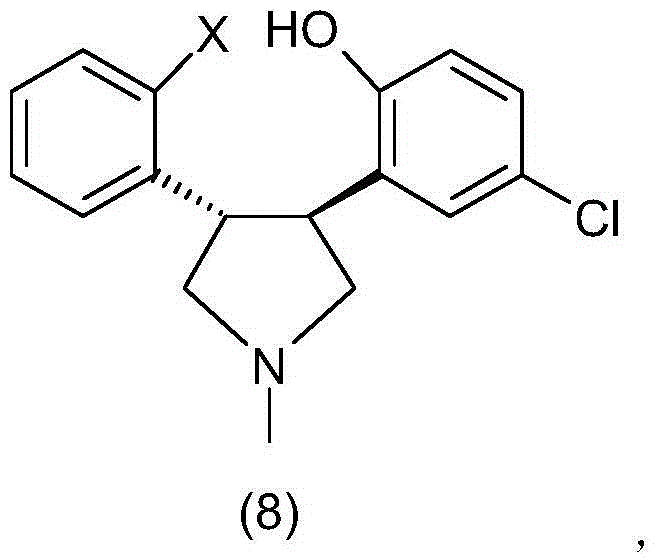
Preparation method of asenapine and intermediate used for preparing asenapine
A general formula and compound technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of difficult to obtain reactants, complicated post-processing, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0175] In order to make the present invention easier to understand, the present invention will be further described below in conjunction with specific examples. It should be understood that these examples are only used to illustrate the present invention and are not intended to limit the scope of the present invention. Moreover, the specific experimental methods not mentioned in the following examples are all carried out according to conventional experimental methods.
[0176] The raw materials and reagents used in the following examples were purchased from Shanghai Kaisai Chemical Co., Ltd.
Embodiment 1A
[0180] Synthesis of compounds represented by general formula (3A)
[0181] At room temperature, add absolute ethanol (300ml) into an eggplant-shaped bottle, stir, and add metallic sodium (1.38g, 60mmol) under nitrogen protection, and obtain sodium ethoxide after the sodium is completely dissolved.
[0182] The compound represented by general formula (1A) (34.1 g, 200 mmol) was added to a fresh ethanol solution of sodium ethoxide. The compound represented by general formula (2) (49.4 g, 252 mmol) was added under stirring. React at 35°C for 3-5h until the reaction is complete. Cool, filter with suction, wash the filter cake with ethanol (10 ml), and dry to obtain a white solid (59.3 g, yield: 85%).
[0183] 1 H NMR (400Hz, CDCl 3 ): δ3.841(s,3H);7.011-7.105(d,1H);7.150-7.234(m,2H);7.240-7.411(m,3H);7.591-7.605(d,1H);8.511(s ,1H).
[0184] MS: M+1=348.
Embodiment 2A
[0186] Synthesis of compounds represented by general formula (4A)
[0187] Acetonitrile (200ml) was added to an eggplant flask. Add the compound represented by the general formula (3A) (55.8g, 160mmol), TMSCN (30.0ml, 240mmol) (treat with hydrogen peroxide before use) and tetrabutylammonium fluoride (20.9g, 80mmol) in one go under stirring, React at 45°C for 1-2h until the reaction is complete, pour into a beaker filled with water (100ml), filter with suction, then soak with methanol (50ml), and filter with suction to obtain a white solid (49.0g, yield: 82%).
[0188] 1 H NMR (400Hz, CDCl 3 ): δ3.841(s,3H);4.201(d,2H);7.011-7.105(d,1H);7.150-7.234(m,2H);7.240-7.411(m,3H);7.591-7.605(d ,1H).
[0189] MS: M+1=373.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com